Abstract
Purpose:
Tumor-associated macrophages correlate with increased invasiveness, growth, and immunosuppression. Activation of the colony-stimulating factor-1 receptor (CSF-1R) results in proliferation, differentiation, and migration of monocytes/macrophages. This Phase 1 study evaluated the immunologic and clinical activity, and safety profile of CSF-1R inhibition with the monoclonal antibody LY3022855.
Patients and Methods:
Patients with advanced refractory metastatic breast cancer (MBC) or metastatic castration-resistant prostate cancer (mCRPC) were treated with LY3022855 intravenously in 6-week cycles in cohorts: A) 1.25 mg/kg every two weeks (Q2W); B) 1.0 mg/kg on Weeks 1, 2, 4, and 5; C) 100 mg once weekly; D)100 mg Q2W. mCRPC patients were enrolled in Cohorts A and B; MBC patients were enrolled in all cohorts. Efficacy was assessed by RECIST and Prostate Cancer Clinical Trials Working Group 2 criteria.
Results:
Thirty-four patients (22 MBC; 12 mCRPC) received ≥1 dose of LY3022855. At Day 8, circulating CSF-1 levels increased and pro-inflammatory monocytes CD14DIMCD16BRIGHT decreased. Best RECIST response was stable disease in five MBC patients (23%; duration 82–302 days) and three mCRPC patients (25%; duration 50–124 days). Two MBC patients (Cohort A) had durable stable disease >9 months and a third MBC patient had palpable reduction in a nontarget neck mass. Immune-related gene activation in tumor biopsies post-treatment was observed. Common any grade treatment-related adverse events were fatigue, decreased appetite, nausea, asymptomatic increased lipase, and creatine phosphokinase.
Conclusions:
LY3022855 was well tolerated and showed evidence of immune modulation. Clinically meaningful stable disease >9 months was observed in two MBC patients.
Keywords: LY3022855, CSF-1R inhibitor, CSF-1, metastatic breast cancer, metastatic castration-resistant prostate cancer
Introduction
Colony-stimulating factor-1 receptor (CSF-1R) is a tyrosine kinase receptor expressed selectively on macrophage and granulocyte cell lineages in normal individuals and on some cancer cells (1,2). Upon colony-stimulating factor-1 (CSF-1) or interleukin-34 (IL-34) binding to CSF-1R, downstream signaling molecules are phosphorylated and activated, resulting in the regulation of proliferation, differentiation, survival, and migration of monocytes/macrophages (3-5). In cancer, increased infiltration of macrophages within and surrounding the tumor mass correlates with increased tumor invasiveness and growth (6,7). Depleting tumor-associated macrophages (TAMs) results in decreased tumor growth in mice (8,9). While CSF-1R levels are infrequently increased in tumors compared with normal tissues, increased CSF-1 in sera is observed in cancer patients and is associated with a poor prognosis in multiple cancer types, including prostate and breast cancers (10-13). These data suggest targeting CSF-1R has the potential to limit cancer progression by disrupting TAM homeostasis.
Interactions between the innate and adaptive immune systems are critical for normal immune function, however, dysregulation of the innate axis in the tumor microenvironment can lead to a suppressive phenotype and may be a negative prognostic factor. For example, high numbers of TAMs inversely correlate with infiltration by CD8+ T-cells. Anti-CSF-1R treatments which limit TAM-dependent activities and enhance CD8+ T-cell infiltration, lead to decreases in tumor burden in mice (14). Breast and prostate cancer, two hormone driven malignancies, are often considered immunologically ‘cold’. Both cancers typically exhibit little CD8+ T cell infiltration and an abundance of TAMs, suggesting antagonizing CSF-1R could be an appealing target in these diseases. In addition, androgen inhibition, the backbone of metastatic prostate cancer therapy, has been shown to modulate macrophage activity. Androgen blockade before prostatectomy is associated with the induction of TAMs. In a murine prostate cancer model, androgen blockade increased CSF-1R expression resulting in an increase in protumorigenic macrophages which could be reversed with CSF-1R inhibition (15).
LY3022855 is a novel recombinant human monoclonal antibody of the immunoglobulin G, subclass 1 (IgG1) targeting CSF-1R. LY3022855 prevents ligands CSF-1 and IL-34 from binding to CSF-1R and consequently inhibits CSF-1R activation. CSF-1R activation is required for normal function and survival of tissue resident TAMs (16). Thus, by blocking CSF-1R activation, LY3022855 may inhibit monocyte proliferation and differentiation into macrophages. Preclinical work inhibiting CSF-1R in murine tumor models led to increased expression of genes related to the interferon gamma (IFNγ) response and reduced macrophage levels (17). Therefore, we hypothesized that CSF-1R targeted therapy will limit TAM-mediated tumorigenesis and enhance the anti-tumor effects of CD8+ T-cells, potentially conferring disease control. In this Phase 1 study of the CSF-1R directed antibody LY3022855, we sought a deeper understanding of the changes in immune cell function resulting from targeting a key TAM homeostatic molecule while also evaluating for preliminary evidence of activity and safety in patients with metastatic breast cancer (MBC) and metastatic castration-resistant prostate cancer (mCRPC).
Patients and Methods
Patients
Eligible patients were ≥18 years of age and had a confirmed diagnosis of MBC or mCRPC which was evaluable by radiologic testing either per Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 for MBC and mCRPC, and/or Prostate Cancer Clinical Trials Working Group 2 (PCWG2) guidelines for bone for mCRPC patients. Patients enrolled had experienced tumor progression on, or treatment intolerance to, ≥1 prior therapy for their cancer and had declined or were ineligible for standard treatment. Patients had to have adequate hematopoietic, hepatic, and renal function at baseline, and a performance status of ≤2 on the Eastern Cooperative Oncology Group (ECOG) scale. Patients with MBC could continue palliative hormone or trastuzumab therapy if hormone receptor-positive or HER2-positive, respectively. Patients with mCRPC were to continue ongoing androgen deprivation therapy (gonadotropin-releasing hormone agonist or antagonist) with castrate levels of serum testosterone <50 ng/dL determined within four weeks prior to starting treatment, and meet ≥1 criteria for progressive metastatic disease per PCWG2 guidelines at the time of study entry (18). Patients were excluded if they had symptomatic central nervous system malignancy or metastasis, active cardiac or other major illness, pregnancy, serologic markers of active hepatitis B or C infection, or had received prior treatment with agents targeting CSF-1 or CSF-1R.
Study Design
This multicenter, open-label, nonrandomized, Phase 1 study (NCT02265536) of intravenous LY3022855 was conducted at two centers: Memorial Sloan Kettering Cancer Center (MBC and mCRPC cohorts) and Cedars Sinai Medical Center (MBC cohorts). The study was performed in compliance with Declaration of Helsinki, good clinical practice (GCP) and International Conference on Harmonisation (ICH) guidelines and approved by the Institutional Review Board at the respective institutions. All patients included in the study signed the informed consent prior to joining the study. The study was designed with the primary objective of exploring the immunomodulatory activity of single-agent LY3022855 by assessing the changes from baseline over time of immune cells, cytokines, and biomarkers. Secondary objectives included assessing antitumor activity, measuring the pharmacokinetic (PK) serum concentrations of LY3022855, and evaluating the safety and toxicity profile of LY3022855. Exploratory objectives were to evaluate pharmacodynamic effects of LY3022855 on tissue biomarkers using flash-frozen tumor biopsies and to assess antitumor activity in bone per PCWG2 criteria (18).
Patients with MBC or mCRPC received LY3022855 in 6-week cycles in one of four dosage cohorts: Cohort A, 1.25 mg/kg every two weeks (Q2W); Cohort B, 1 mg/kg on Weeks 1, 2, 4, and 5 (WK1245); Cohort C, 100 mg weekly (QW); and Cohort D,100 mg Q2W. LY3022855 was evaluated for safety and to establish a maximum tolerated dose in an all solid tumor dose escalation trial (NCT01346358). Doses for patients in Cohorts A, B, C, and D were selected based on the pharmacokinetics, pharmacodynamics, and safety data in this study. Dose for patients in Cohort A was the lowest dose that showed a significant increase in the 2 ligands of CSF-1R (CSF-1, IL-34) and this dose had an acceptable safety profile. Dose for patients in Cohort B was expected to result in a cumulative dose of 4 mg/kg based on the JSCA study. It was a reasonable dose and schedule in terms of safety and was expected to enhance inhibition of CSF-1R, based on the preliminary pharmacodynamics data. Dose for patients in Cohort C was expected to result in enhanced target engagement, and dose for patients in Cohort D was selected because all patients who showed tumor shrinkage or stability in the JSCA study were treated on a Q2W schedule. MBC and mCRPC patients were enrolled in Cohort A and subsequently in Cohort B. Based upon the observed clinical activity in MBC patients treated in Cohort A, the study was amended to explore two further dose cohorts (C and D). After Cohort B was fully enrolled, MBC patients were assigned to Cohorts C and D on an alternating basis. Patients received LY3022855 as an intravenous infusion administered over a minimum duration of 30 minutes and a maximum duration of 4 hours.
Patients received LY3022855 for 6-week cycles until the patient either experienced disease progression or unacceptable toxicity. Patients with radiographic progression who were clinically stable and felt to be benefiting were considered for ongoing treatment upon discussion with the sponsor.
Immune response analyses
Human peripheral blood mononuclear cell (PBMC) samples were thawed and stained with a fixable Aqua viability dye (Invitrogen) and a cocktail of cell surface antibodies. For measurement of T cell activation/exhaustion status, fluorescent activated cell sorting (FACS) analysis was performed using the following cell surface markers: CD8-Qdot605 (Invitrogen, 3B5), CD4-Qdot 655 (Invitrogen, S3.5), PD-1-PE (BD, MIH4), LAG-3-FITC (Enzo, 17B4), ICOS-PE-Cy7 (eBioscience, ISA-3), and TIM-3-APC (R&D Systems, 344823). For multiplex cytokine measurements, validated V-PLEX Proinflammatory Panel 10-plex (human) kits (for IL-2, IL-4, IL-6, IL-8, IL-10, IL-12, IFNγ, TNFα, IL-1β, and IL-13) were used (MSD, Cat #K15049D-1). See Supplementary Materials and Methods for all FACS and cytokine assay details.
Safety and tumor assessments
Safety assessments were performed at baseline and pre-specified time points throughout the study. These assessments included documentation of adverse events (AEs), clinical laboratory evaluations, vital signs and other physical findings, electrocardiograms, and infusion-related reactions. Laboratory values and AEs were graded using the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE) version 4.0 and were coded using the Medical Dictionary for Regulatory Activities (MedDRA™). Treatement-emergent AEs (TEAEs) were any AEs that began on or after the first dose of the study drug, or any pre-existing condition that increased in CTCAE grade. Tumor assessment, by imaging, was performed at baseline and every six weeks thereafter, or as clinically indicated. Antitumor activity was assessed using RECIST version 1.1 (19) and irRECIST (20). For mCRPC patients, bone metastatic disease was assessed using PCWG2 criteria (18).
Pharmacokinetic analyses
Blood samples for serum concentration analysis of LY3022855 were collected pre-dose on: Cycle 1 days 1, 8, 22, and 36; Cycle 2 day 1; Cycle 3 days 1, 8, and 22; Cycle 5 Day 1. Post-dose samples were collected at 1, 4, 24, and 48 hours on Cycle 1 Day 1 and Cycle 3 Day 1. Serum samples were analyzed for LY3022855 using a validated ELISA method at Covance Laboratories Inc. located in Chantilly, Virginia, USA. The lower limit of quantification was 1562.5 ng/mL, and the upper limit of quantification was 50,000 ng/mL. The inter-assay accuracy (% relative error) during validation ranged from −11.9% to 5.2%. The inter-assay precision (% relative standard deviation) during validation ranged from 6.8% to 17.5%. LY3022855 was stable for up to 558 days when stored at −70°C. Pharmacokinetic analyses were conducted on patients who had received ≥1 dose of LY3022855 and had venous blood samples collected. Pharmacokinetic parameters for LY3022855 were computed by standard non-compartmental methods of analysis using Phoenix WinNonlin® Version 8.
Gene expression analyses
Gene expression analyses were performed to identify genes modulated by LY3022855 treatment. MBC tumor tissue biopsy samples were collected by core needle biopsy at baseline (within 14 days prior to dosing on Cycle 1 Day 1) and ≤14 days prior to dosing on Cycle 2 Day 1. Biopsy samples were flash-frozen and RNA was extracted (MolecularMD, ICON Laboratory Services). Differential gene expression pre- and post-LY3022855 treatment was assessed using NanoString gene analysis (nCounter PanCancer Immune profiling panel). Normalization and gene expression analysis was performed using ILAstring, an internally-developed automated workflow process. Standard NanoString files were input to ILAstring and nCounter. RNA data were normalized using the geometric mean of positive controls and housekeeping genes. For each gene across all samples, the maximum gene expression was determined and low-expressing genes (maximum <30, n = 12) and genes with low variance (variance p<200, n = 29) were removed from further analysis. The resulting genes (n = 689) were subjected to one-way ANOVA differential gene expression analysis (OmicSoft Array Studio 10.0.1.118; QIAGEN®, Cary, NC). Volcano plots were constructed (Tibco Spotfire Analyst 7.11.1; Tibco, Palo Alto, CA) and differentially-expressed genes were visualized via heatmap using ComplexHeatmap (21).
Statistical Analyses
To study the impact of LY3022855 on immune cell subsets and serum cytokines, the changes from baseline were summarized. For change from baseline analyses, baseline value was defined as the last reported measure on or before the first dose of LY3022855 (prior to the dose administration). For a change from baseline within a cycle, baseline value was defined as the measure prior to the first dose of that cycle, unless otherwise specified.
The safety population included patients who received ≥1 dose of LY3022855. The evaluable population included patients who completed one cycle of LY3022855 treatment, one baseline tumor biopsy, one post-treatment tumor biopsy, and one cycle of immune blood studies. Descriptive statistics were used to summarize baseline patient characteristics, safety, and tumor response. The objective response rate (ORR) was estimated by the proportion of enrolled patients who had a best overall response (BOR) of complete response (CR), or partial response (PR). Disease control rate (DCR) was estimated by the proportion of enrolled patients who had a BOR of CR, PR, or stable disease (SD).
Results
Patients
Twenty-two MBC patients were enrolled and all MBC patients received ≥1 dose of LY3022855(Figure 1A). In the MBC group, most patients (Cohort A, 83.3%; Cohort B, 40%; Cohort C, 100%; Cohort D, 83.3%) discontinued treatment due to progressive disease; 1 (16.7%) patient in Cohort A discontinued due to adverse event, 3 (60%) patients in Cohort B discontinued due to withdrawal by subject, and 1 (16.7%) patient in Cohort D died due to disease complication. Twelve mCRPC patients were enrolled, and all 12 received ≥1 dose of LY3022855 (Figure 1B). In the mCRPC group, most patients (Cohort A, 62.5%; Cohort B, 100%) discontinued treatment due to progressive disease; 2 (25.0%) patients in Cohort A discontinued due to physician decision, and 1 (12.5%) patient in Cohort B discontinued due to other reasons.
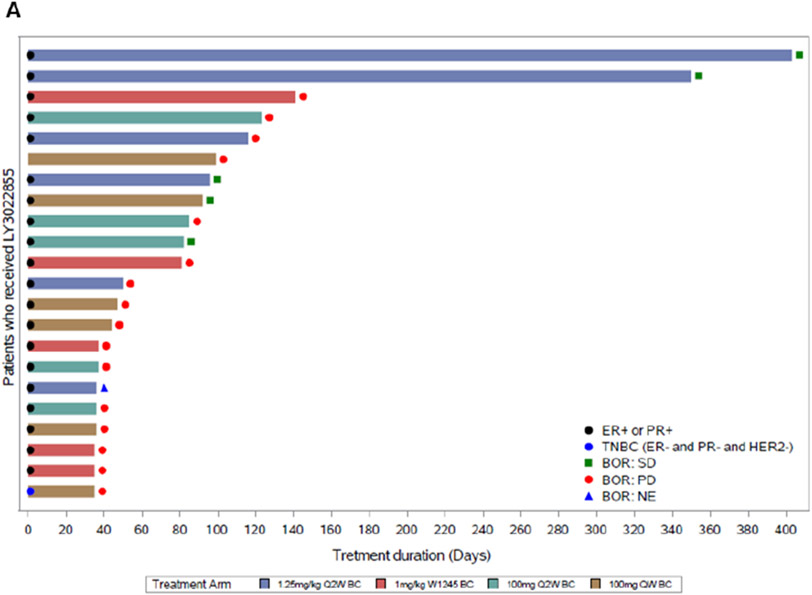
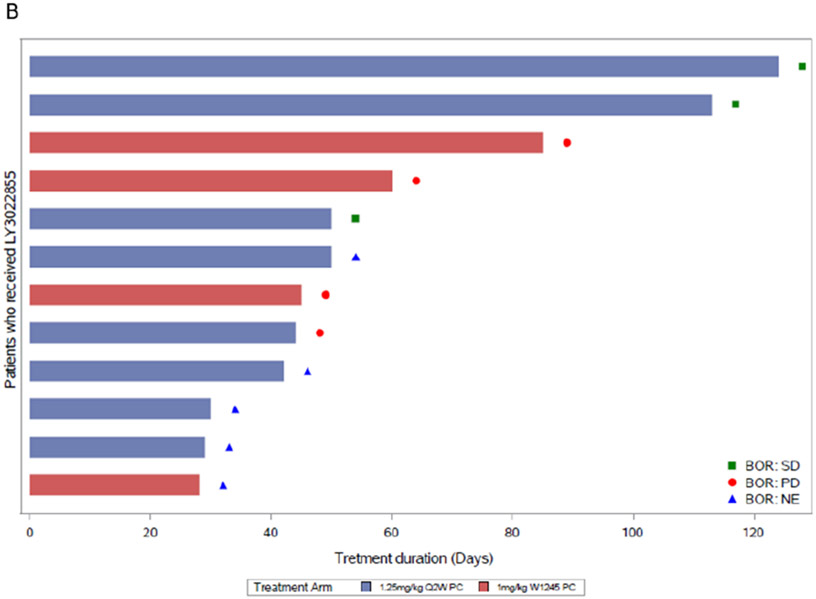
Figure 1.
Treatment duration and response for MBC (A) and mCRPC (B) patients in study (N = 34). Each horizontal bar represents a patient. Response is per RECIST 1.1 criteria. Best overall response was SD (MBC, n = 5; mCRPC, n = 3) and PD (MBC, n = 16; mCRPC, n = 4). One MBC patient and five mCRPC patients were not evaluable (NE). MBC tumors were estrogen receptor (ER) and/or progesterone receptor (PR) positive; one patient had triple negative breast cancer (TNBC). Abbreviations: BOR, best overall response; HER2, human epidermal growth factor receptor 2; N, total number of patients; n, number of patients in the specified category; PD, progressive disease; Q2W, every two weeks; QW, weekly; WK1245, weeks 1, 2, 3, 4, and 5; SD, stable disease.
Patient characteristics per dose, cohort, and disease type are listed in Table 1. The median age was 57 years (range, 32 to 81) for MBC patients and 73 years (range, 58 to 84) for mCRPC patients. In the MBC group, 9 patients (40.9%) had a baseline ECOG Performance Status of 0, and 11 (50%) patients had a baseline ECOG PS of 1. Twenty (91%) MBC patients were hormone receptor positive, with 3 (13.6%) MBC patients receiving concurrent hormone therapy and all received prior systemic therapy. Visceral disease was the main site of metastatic disease for MBC patients (19/22; 86%). In the mCRPC group, 4 patients (33.3%) had a baseline ECOG Performance Status of 0, and 7 (58.3%) had a baseline ECOG PS of 1. All mCRPC patients received prior systemic therapy. Five (42%) mCRPC patients had received prior taxane chemotherapy and all (100%) had prior abiraterone acetate and/or enzalutamide. Bone was the main site of metastatic disease for mCRPC patients (10/12; 83%).
Table 1:
Patient baseline demographics and disease characteristics
| MBC (N = 22) | mCRPC (N = 12) | |||||
|---|---|---|---|---|---|---|
|
n (%), except where indicated |
1.25 mg/kg Q2W n = 6 |
1 mg/kg WK1245 n = 5 |
100 mg Q2W n = 5 |
100 mg QW n = 6 |
1.25 mg/kg Q2W n = 8 |
1 mg/kg Wk1245 n = 4 |
| Sex | ||||||
| Female | 6 (100) | 5 (100) | 3 (60) | 6 (100) | – | – |
| Male | 0 | 0 | 2 (40) | 0 | 8 (100) | 4 (100) |
| Age, y | ||||||
| Median (range) | 54 (32 - 81) | 55 (37 - 68) | 60 (36 - 62) | 63 (39 - 78) | 69 (62 - 84) | 74 (58 - 78) |
| Race | ||||||
| n*a | 5 | 5 | 5 | 5 | 8 | 4 |
| Asian | 1 (20) | 0 | 1 (20) | 1 (20) | 0 | 0 |
| Black or African | 0 | 0 | 1 (20) | 0 | 0 | 2 (50) |
| American white | 4 (80) | 5 (100) | 3 (60) | 4 (80) | 8 (100) | 2 (50) |
| Ethnicity | ||||||
| Hispanic | 1 (17) | 0 | 0 | 0 | 0 | 0 |
| Not Hispanic or Latino | 5 (83) | 5 (100) | 5 (100) | 6 (100) | 8 (100) | 4 (100) |
| Weight, kg | ||||||
| Median (range) | 69 (56 - 101) | 61 (53 - 99) | 54 (44 - 98) | 68 (47 - 72) | 87 (65 - 99) | 88 (54 - 95) |
| ECOG PS | ||||||
| 0 | 3 (50) | 1 (20) | 1 (20) | 4 (67) | 1 (13) | 3 (75) |
| 1 | 3 (50) | 3 (60) | 3 (60) | 2 (33) | 6 (75) | 1 (25) |
| 2 | 0 | 1 (20) | 1 (20) | 0 | 1 (13) | 0 |
| Breast cancer hormone status | ||||||
| HR positive | 6 | 5 | 5 | 4 | – | – |
| Triple Negativeb | 0 | 0 | 0 | 1 | – | – |
| Unknown | 0 | 0 | 0 | 1 | – | – |
| Prior therapy | ||||||
| Surgery | 4 (67) | 5 (100) | 5 (100) | 5 (83) | 6 (75) | 3 (75) |
| Radiotherapy | 5 (83) | 4 (80) | 3 (60) | 6 (100) | 7 (88) | 3 (75) |
| Systemic therapy | 6 (100) | 5 (100) | 5 (100) | 6 (100) | 8 (100) | 4 (100) |
| Number of lines of systemic for locally advanced/metastatic disease | ||||||
| ≤4 regimens | 2 (33) | 0 | 1 (20) | 0 | 3 (38) | 2 (50) |
| ≥5 regimens | 4 (67) | 5 (100) | 3 (60) | 5 (83) | 4 (50) | 2 (50) |
| Site of metastatic disease c | ||||||
| Visceral | 4 (67) | 4 (80) | 5 (100) | 6 (100) | 2 (25) | 1 (25) |
| Bone | 3 (50) | 2 (40) | 4 (80) | 4 (67) | 7 (88) | 3 (75) |
| Lymph nodes | 4 (67) | 2 (40) | 2 (40) | 3 (50) | 5 (63) | 4 (100) |
| Breast | 3 (50) | 0 | 0 | 0 | 0 | 0 |
| Chest wall involvement | 0 | 1 (20) | 0 | 1 (17) | 0 | 0 |
| Patients on concurrent hormonal therapy | 2 (33) | 0 | 0 | 1 (17) | – | – |
Number of subjects with non-missing data, used as denominator.
Negative for all three hormone receptors (HER2, ER, and PR)
Patients can have ≥1 metastatic site
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group performance status; HR, hormone receptor; HER2, human epidermal growth factor receptor 2; PR, progesterone receptor; ER, estrogen receptor; N= number of patients in a group; n, number of patients in the specified category; QW, every week; Q2W, every two weeks; WK1245, weeks 1, 2, 3, 4, and 5
A subset of MBC patients shows clinical benefit after CSF-1R blockade
Efficacy analyses were performed for all patients who received ≥1 dose of LY3022855 (N = 34). No patient had an objective response (CR+PR) in either disease population studied (Supplementary Table S1). Patients without a valid response assessment ≥7 weeks after enrollment who had not already progressed were assigned a BOR of ‘not evaluable’.
For the MBC patients (n = 5 of 21 evaluable patients; 24%), the best overall response by RECIST was SD, with a duration of 82 to 302 days, and median progression free survival (PFS) across treatment arms was 1 to 3 months. Results observed by RECIST were consistent with results observed with irRECIST.
Two MBC patients (Patient A and Patient B) experienced prolonged SD, and a third MBC patient (Patient C) had a clinical response not captured on RECIST; all three patients received LY3022855 at a dose of 1.25 mg/kg Q2W (Cohort A). Patient A was a 32 year old female with HR+, HER2− breast cancer with bone and brain metastases who experienced durable SD for 10 months and whose treatment duration was 12 months. Patient B was an 81 year old female with HR+, HER2− MBC who experienced durable SD for 9 months and remained on study treatment for 13 months. Patient C was a 58 year old female with HR+, HER2− breast cancer with metastases to the bone, liver, and lymph nodes who had a palpable reduction in a non-target neck mass which was not well captured on RECIST (SD, −10.3%). She experienced SD for 3 months, and treatment duration was 96 days. The patient discontinued from the study due to an AE of confusion which, in the opinion of the investigator, was not related to the study drug.
For the mCRPC patients (n = 3 of 7 evaluable patients; 43%), the best overall response by RECIST was SD, with a duration of 50 to 124 days, and the median progression free survival (PFS) across treatment arms was 1 to 3 months. Results observed by RECIST were consistent with results observed with irRECIST.
An exploratory objective was to evaluate antitumor activity in bone in mCRPC patients by PCWG2 criteria for bone metastases, and to evaluate changes in known tumor markers in mCRPC and MBC. The BOR by PCWG2 for bone scan was SD (n = 4 of 9 evaluable patients; 44%); two patients in each dose cohort (1.25 mg/kg Q2W and 1.0 mg/kg WK1245). The remaining evaluable patients had PD as their best response on bone scan (Supplementary Table S1).
No correlation between the tumor biomarkers, Prostate Specific Antigen (PSA), carcinoma antigen (CA) 15-3, carcinoembryonic antigen (CEA), and clinical response were observed (Supplementary Figure S1).
Increasing exposure of LY3022855 resulted in greater CSF-1R pathway modulation
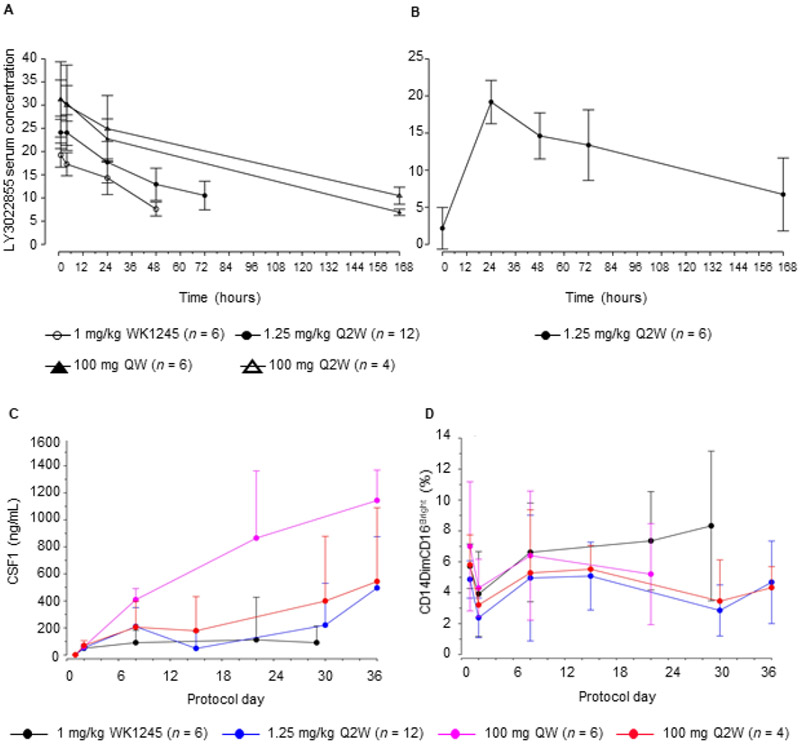
Pharmacokinetic data from 28 patients who received ≥1 dose of LY3022855, across four different dosing regimens, were evaluated to determine the effects on LY3022855 exposure on pharmacodynamic modulation. Non-compartmental PK parameters stratified by dosing regimen, infusion cycle, and day of sample collection are provided in Supplementary Table S2. Mean concentration-time profiles on Cycle 1 Day 1 and Day 29 are presented in Figures 2A and 2B, respectively.
Figure 2.
LY3022855 pharmacokinetics and target engagement. Mean (+/− standard deviation) serum concentration (ug/mL) versus time profiles for LY3022855 following intravenous administration in MBC and mCRPC patients who received ≥1 dose of LY3022855 on Cycle 1 Day 1 (A), and Cycle 1 Day 29 (B). (C) Time plots of circulating levels of CSF-1. (D) Increasing frequency of dosing with LY3022855 leads to greater target engagement and further reduction of pro-inflammatory macrophages as indicated by a reduction in circulating CD14dim and CD16bright macrophages. Abbreviations: n, number of subjects in the specified category; Q2W, every two weeks; QW, weekly; WK1245, weeks 1, 2, 3, 4, and 5.
Target engagement of LY3022855 was assessed by investigating circulating levels of CSF-1 and IL-34 as evidence of receptor blockade. We observed a rise in circulating levels of CSF-1 (Figure 2C) and IL-34 (Supplementary Figure S2) in line with LY3022855 PK. While an elevation of CSF-1 was observed for the duration of dosing for all dose regimens, this was reduced for the 1 mg/kg WK1245 dose compared to other doses. The highest change in CSF-1 was observed with LY3022855 administered at a dose regimen of 100 mg QW. A comparable change was observed for flat dosing regimens of 100 mg Q2W compared to the weight adjusted dose of 1.25 mg/kg Q2W. A similar trend was also observed for IL-34. To investigate the pharmacodynamic effect of CSF-1R blockade with LY3022855, we examined the level of circulating pro-inflammatory monocytes (CD14DIMCD16BR) which rely on CSF-1R signaling for their survival (22). All dose groups resulted in a reduction in the level of circulating CD14DIMCD16BR cells after dosing. The suppression of the number of these CD14DIMCD16BR cells was maintained over the monitoring period for all doses except 1 mg/kg WK1245 which saw initial suppression then a return to baseline ratios between doses (Figure 2D). Correlation of PK and pharmacodynamic data suggested the dosing of LY3022855 in this study was sufficient to modulate CSF-1R signaling and reduce cells dependent on CSF-1.
Clinical benefit is associated with minor changes in T-cell activation in peripheral blood immune cells
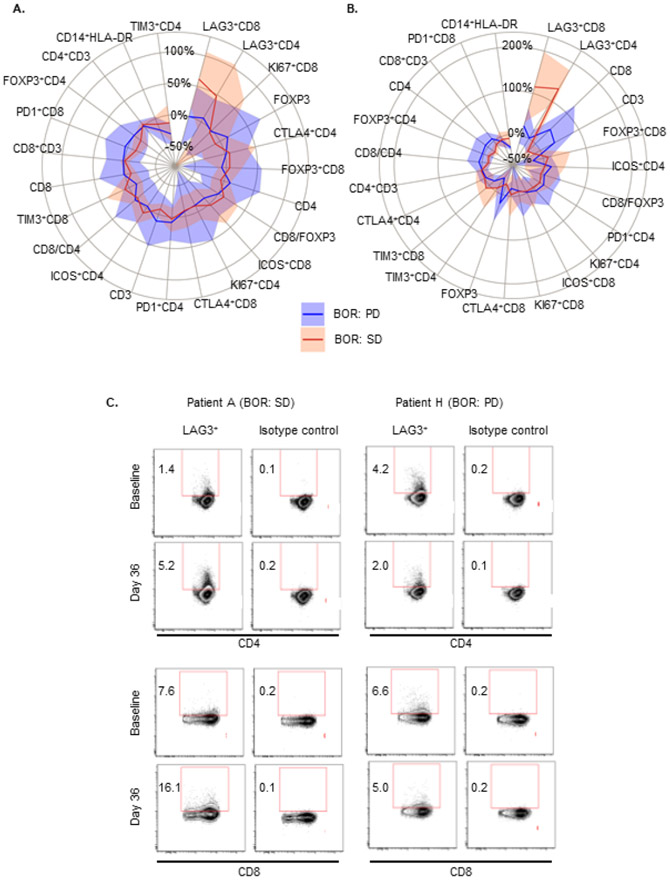
To evaluate immune function response in patients treated with LY3022855, changes in the distribution of peripheral blood immune cell subsets and activation marker phenotypes was assessed by FACS. Patient data was evaluated together and then additionally by clinical response per RECIST criteria. At Day 8, only minor changes were observed after the first dose (Supplementary Table S3) and were not associated with clinical benefit (Figure 3A and 3B). Patients with a BOR of SD (n = 8 of 24 patients analyzed) showed an increase in average percent change of activated LAG3+CD4+ and LAG3+CD8+ cells at Day 8 (Figure 3A) and Day 36 (Figure 3B and 3C) compared to patients with PD (n = 16 of 24 patients analyzed). Patients with SD had an average of ~100% increase of activated LAG3+CD4+ and LAG3+CD8+ T cells at Day 36 compared to baseline. By contrast, patients with PD had ~0% in this population (Figure 3B). For all other markers, changes in the expression of T cell surface markers did not consistently differentiate patients with SD compared with PD. In addition, immune function was also evaluated through the assessment of serum cytokines. Except for CSF-1 and IL-34, no major changes were observed in other cytokines and many remained below the limits of detection (Fig 2C and data not shown).
Figure 3.
Peripheral blood immune cell subset changes by response per RECIST. Percentage change from baseline to Cycle 1 Day 8 (A) and Cycle 1 Day 36 (B). Solid lines indicate the median percent change from baseline; shaded areas represent the median absolute deviation. Day 8: PD, n = 16; SD, n = 8; all markers had observations from all patients with the exception of CD14+HLA-DR where PD, n = 9, and SD, n = 8. Day 36: PD, n = 7; SD, n = 7; all markers had observations from all patients with the exception of CD14+HLA-DR where PD, n = 4, and SD, n = 6. (C). FACS analysis for LAG3+CD4 and LAG3+CD8 cell subsets at Cycle 1 Day 36 in peripheral blood from a patient who had a BOR of SD (Patient A) and PD (Patient H). Abbreviations: BOR, best overall response; PD, progressive disease; SD, stable disease.
Patients with clinical benefit display intra-tumor immune gene expression changes
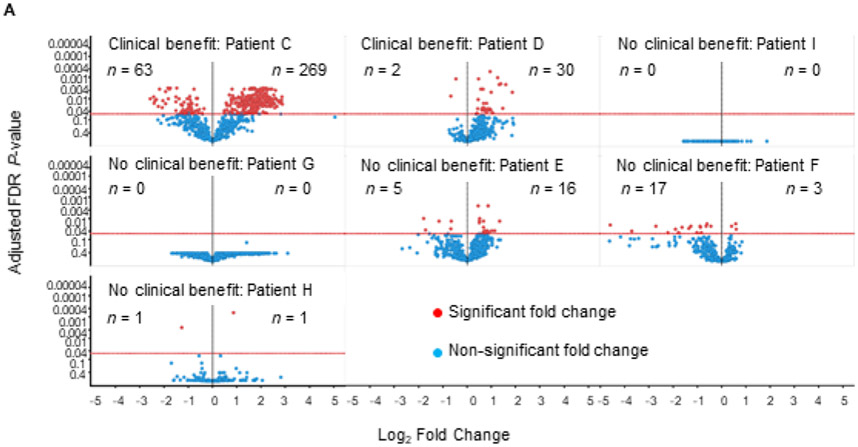
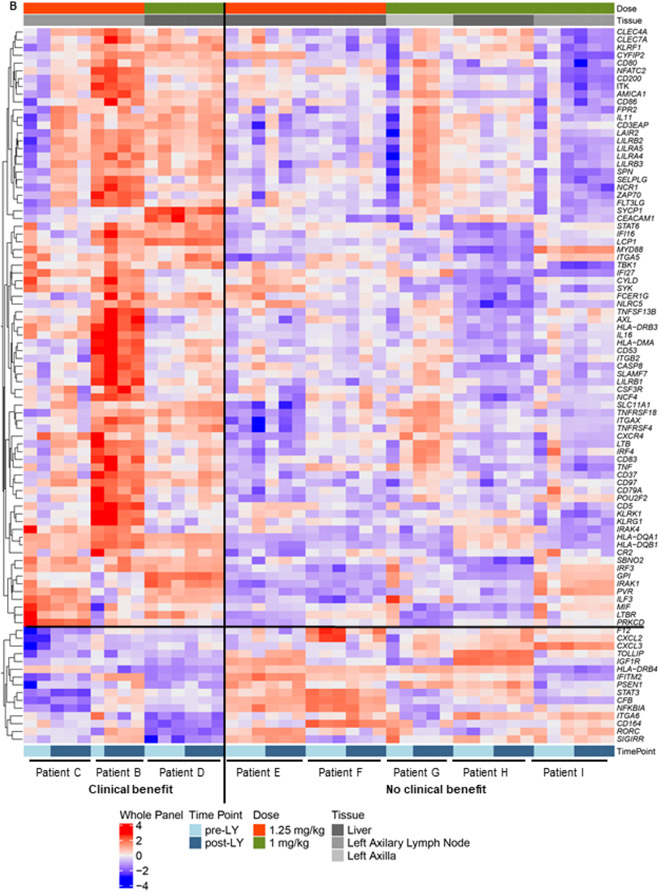
To evaluate the biologic effects of CSF-1R blockade we compared patients considered to have received clinical benefit and those who showed rapid progression. Patient matched (n = 8) MBC tumor biopsies pre- and post-LY3022855 treatment were evaluated for modulation of immune genes using NanoString technology. Samples were grouped into a “clinical benefit group” (n = 3) and a "no benefit" group (n = 5), where clinical benefit was defined as either a BOR of SD per RECIST, remaining on treatment >4 months, or a reduction in a non-target tumor mass. No benefit was defined as a BOR of PD per RECIST and remaining on treatment <4 months. Differential gene expression analysis was performed on patients with adequate pre- and post-treatment samples to enable statistical comparisons. Patients B and C were included in the “benefit group”; Patient A was unable to be captured as she did not have an adequate pre-treatment baseline biopsy for analysis. A trend towards increased immune activation in patients with clinical benefit was observed (Figure 4A). Patient C, who had a palpable reduction in a nontarget neck mass, displayed the most dramatic increase in immune related genes post-treatment (Figure 4A). Of note, the neck mass was not the biopsy site suggesting that a systemic immune response may have been initiated. Some of the genes are higher in the pre-treatment samples of responders compared with those of the non-responders. This may suggest the possibility that patients with higher expression of these genes are more likely to be responsive to this agent, although stringent analysis of a larger number of biopsy samples will be required to confirm this possibility.
Figure 4.
Differential gene expression of tumor biopsies pre- and post-LY3022855 treatment. (A) Volcano plots showing fold change in immune gene expression post-LY3022855 treatment. A significant increase in immune gene expression in tumor biopsies of patients with clinical benefit (Patients C and D) post-LY3022855 treatment was observed. Minimal changes in immune gene expression in tumor biopsies of patients with no clinical benefit (Patients I, G, E, F, and H) was observed. Patient B was excluded from this analysis because only one pretreatment sample was available. (B) Heatmap shows the distribution of the 92-gene signature differentially expressed across patient matched MBC tumor biopsies pre- and post-LY3022855 treatment. Patients with some clinical benefit tended to have relatively higher expression in the genes including and above PRKCD and relatively lower expression in the genes including and below F12. Abbreviations: LY, LY3022855, n, number of differentially expressed genes.
To gain a greater insight into genes consistently altered in subjects with clinical benefit, we compared the “clinical benefit” and “no benefit” groups using a Benjamini-Hochberg False Discovery Rate (FDR) of ≤05. We identified 92 significantly altered genes between patients with and without clinical benefit (Figure 4B). Consistent with the hypothesized mechanism of action for LY3022855/CSF1-R blockade, we noted many of these genes are expressed by cells of the myeloid and antigen presenting cell lineages (i.e. CD80, CD86, ITGAX). We also used the gene expression analysis to estimate changes in myeloid infiltrations. Although there was a trend (Supplementary Figure S3), we were not confident the sample numbers would provide sufficient confidence at making to draw a conclusion on reduced macrophage post therapy. We also attempted gene set pathway analysis; however, there was no change that reached significant levels of confidence in those limited differentially expressed genes, which were contained in the pre-specified gene set.
Relationship between confounding factors and clinical response
We explored the potential association between response (RECIST) and baseline characteristics of dose level, age, gender, and clinical site. Age, gender, and clinical site had no impact on response, however, a relationship between LY3022855 dose level and response was observed (Fisher exact p-value, 0.006; Supplementary Table S4). Stable disease was observed in patients treated with a higher LY3022855 dose (1.25 mg/kg Q2W, 100 mg Q2W, and 100 mg QW) but was not evident for any patient treated with the lower LY3022855 dose (1 mg/kg WK1245).
Treatment and safety
All 34 enrolled patients received ≥1 dose of LY3022855 and were included in the safety analysis. The median number of cycles of LY3022855 received per patient was one cycle with a median duration of therapy 7 weeks, range 2 to 58 weeks. At the time of data cutoff, the overall median relative dose intensity was 94%.
Treatment-emergent AEs (TEAEs) were reported for all patients and those reported in ≥20% of patients were fatigue, nausea, constipation, vomiting, anemia, diarrhea, pain, decreased appetite, and dyspnea. Asymptomatic increased blood creatinine phosphokinase (CK), increased lipase, and increased gamma-glutamyl transferase (GGT) occurred in ≥20% of patients.
The most common treatment-related TEAEs (those reported ≥10% of the patients) are presented in Table 2. Grade 3 treatment-related AEs reported in ≥1 MBC patients included increased blood CK and increased blood alkaline phosphatase (ALP). Grade 4 treatment-related AEs included increased GGT, increased aspartate aminotransferase (AST) and increased amylase. Grade 3 treatment-related TEAEs reported in ≥1 mCRPC patients included fatigue.
Table 2:
Treatment-related adverse events occurring in ≥10% of patients in the safety populationa
| Breast Cancer | Prostate Cancer | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1.25 mg/kg Q2W n = 6 |
1 mg/kg WK1245 n = 5 |
100 mg Q2W n = 5 |
100 mg QW n = 6 |
1.25 mg/kg Q2W n = 8 |
1 mg/kg WK1245 n = 4 |
Total N = 34 |
||||||||
| Preferred term | Any Grade |
≥Grade 3 |
Any Grade |
≥Grade 3 |
Any Grade |
≥Grade 3 |
Any Grade |
≥Grade 3 |
Any Grade |
≥Grade 3 |
Any Grade |
≥Grade 3 |
Any Grade |
≥Grade 3 |
| Fatigue | 1 (17) | 0 | 1 (20) | 0 | 2 (40) | 0 | 3 (50) | 0 | 4 (50) | 1 (13) | 2 (50) | 1 (25) | 13 (38) | 2 (6) |
| Decreased appetite | 2 (33) | 0 | 0 | 0 | 2 (40) | 0 | 1 (17) | 0 | 3 (38) | 0 | 1 (25) | 0 | 9 (27) | 0 |
| Nausea | 2 (33) | 0 | 0 | 0 | 2 (40) | 0 | 0 | 0 | 3 (38) | 1 (13) | 2 (50) | 0 | 9 (27) | 1 (3) |
| Increased lipase | 2 (33) | 0 | 0 | 0 | 3 (60) | 1 (20) | 2 (33) | 0 | 1 (13) | 0 | 0 | 0 | 8 (24) | 1 (3) |
| Increased blood CK | 1 (17) | 1 (17) | 0 | 0 | 2 (40) | 0 | 2 (33) | 1 (17) | 2 (25) | 0 | 0 | 0 | 7 (21) | 2 (6) |
| Increased GGT | 1 (17) | 0 | 1 (20) | 1 (20) | 2 (40) | 1 (20) | 2 (33) | 1 (17) | 0 | 0 | 0 | 0 | 6 (18) | 3 (9) |
| Increased ALT | 2 (33) | 0 | 0 | 0 | 1 (20) | 0 | 2 (33) | 1 (17) | 0 | 0 | 0 | 0 | 5 (15) | 1 (3) |
| Periorbital edema | 3 (50) | 0 | 1 (20) | 0 | 0 | 0 | 1 (17) | 0 | 0 | 0 | 0 | 0 | 5 (15) | 0 |
| Vomiting | 0 | 0 | 1 (20) | 0 | 2 (40) | 0 | 1 (17) | 0 | 1 (13) | 0 | 0 | 0 | 5 (15) | 0 |
| Increased AST | 2 (33) | 0 | 0 | 0 | 1 (20) | 0 | 1 (17) | 1 (17) | 0 | 0 | 0 | 0 | 4 (12) | 1 (3) |
The safety cohort includes patients who received ≥1 dose of LY3022855.
Data cutoff date: 10 May 2018.
Data are n (%)
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; CK, creatine phosphokinase; GGT, gamma-glutamyl transferase; n, number of patients in the specified category; N, total number of patients; Q2W, every two weeks; QW, weekly; WK1245, weeks 1, 2, 3, 4, and 5.
Treatment-related SAEs (all Grade 3) were reported by two MBC patients. One MBC patient experienced SAEs of stress induced cardiomyopathy and encephalopathy. The events of encephalopathy and stress-induced cardiomyopathy were resolved during the course of the study, and in the opinion of the investigator the event of encephalopathy was later revised to not-related to study treatment or protocol procedure. The second MBC patient experienced SAEs of bacteremia and pyrexia. The outcome of these SAEs were unknown at the time of data cutoff as the patient transitioned to hospice care and withdrew from follow-up. In the opinion of the investigator the event of bacteremia and pyrexia were revised to not-related to study treatment. No mCRPC patient experienced treatment-related SAEs.
Two MBC patients died while on study treatment or within 30 days following the last dose of LY3022855. Of these patients, one patient treated with 1 mg/kg (administered on Weeks 1, 2, 4, and 5) died 16 days after the last dose of LY3022855, which was reported to be most likely caused by complications of recently diagnosed venous thromboembolic event. Another patient treated with 100 mg QW died due to cancer progression. No mCRPC patient died while on study treatment or within 30 days following the last dose of LY3022855.
Laboratory assessments identified treatment-emergent AEs of increased ALT (2 patients, Grade 3), increased ALP (3 patients, Grade 3), increased AST (1 patient, Grade 4; 2 patients, Grade 3), increased CK (2 patients, Grade 3) and increased blood bilirubin (2 patients, Grade 3). Myoglobin was collected for CK >2.5 upper limit of normal, 2 patients met this parameter.
Immune related adverse events included 3 patients with diarrhea (2 Grade 1 and 1 Grade 2), 2 patients with a maculopapular rash (1 Grade 1 and 1 Grade 2), and 2 patients with pruritus (grade 1). Dermatologic toxicity was managed with topical steroids. No patients required systemic steroids for the management of these toxicities.
Discussion
Inhibiting CSF-1R with a novel monoclonal antibody (LY3022855) in this Phase 1 study of a treatment refractory population of patients with MBC or mCRPC demonstrated this agent had successful target engagement in the peripheral circulation. This was evidenced by increased CSF-1 and IL-34 levels in sera as well as a commensurate reduction in circulating monocytes. Further, myeloid cell-related gene expression changes in patients with clinical benefit suggested evidence of intra-tumoral immune modulation. Nevertheless, the single-agent clinical activity of LY3022855 was limited with a median PFS of 1 to 3 months across treatment arms and disease populations. Two MBC patients experienced SD with prolonged PFS for greater than 9 months suggestive of clinical benefit. Despite the limited sample set, changes in intra-tumor gene expression detected in patients deemed to have clinical benefit, suggests LY3022855 treatment might result in intra-tumor immune modulation which is associated with outcome. This is further exemplified by the comparative analysis showing increased activation of immune genes in tumor tissue and sustained activation of T cells in the periphery in patients who had benefit relative to those with early progression.
A number of small molecules or antibodies targeting CSF-1R are in clinical development (23). However, reports of monotherapy targeting this pathway, outside the non-malignant connective tissue disease diffuse-type tenosynovial giant cell tumors (24-26), has been limited (22,27-29). The monotherapy study we report here was an exploratory study aimed primarily at assessing the immune-modulatory effects of this novel antibody. Target engagement was indicated by the increased circulating levels of CSF-1 and IL-34 after venous administration of LY3022855, and with higher and more frequent dosing suppression of circulating CD14DIMCD16BR non-classical monocytes was observed, indicating LY3022855 blocked CSF-1R as expected.
While inhibition of CSF-1R with LY3022855 was indicated, the overall immune and clinical response in this patient cohort was limited. Although no patient had an objective response, 2 MBC patients with SD experienced prolonged PFS and there appeared to be a relationship between dose level and clinical response. In evaluable mCRPC patients with RECIST measurable disease, BOR was SD in 43%, however, most mCRPC patients with RECIST measurable disease also had bone metastases captured per PCWG2 on bone scintigraphy and progressed in bone. Per PCWG2 criteria for bone metastases, BOR was SD (44%) and PD (56%). The longest duration of SD per RECIST in the prostate cancer patients was 124 days, and no PSA responses were observed. Prostate responses were disappointing despite the pre-clinical rationale. This was a heavily pretreated patient population and perhaps the immune suppressive tumor microenvironment could not be altered by CSF-1R inhibition, or compensatory mechanisms were at play (30,31). The limited clinical activity we report here align with the monotherapy results obtained from clinical studies with emactuzumab and pexidartinib (22,27-29), suggesting targeting TAMs alone is not sufficient to induce a tumor response in advanced solid tumors.
In this patient cohort, changes in circulating peripheral immune cell subsets and serum cytokines after treatment were minimal. However, a significant increase in LAG3+CD4+ and LAG3+CD8+ cells at Day 8 was observed; this increase was associated with gene expression evidence of immune activation in these individuals. In addition, analysis of gene expression changes in tumor tissue after therapy indicated activation of immune genes in samples from MBC patients who displayed clinical benefit.
Overall, LY3022855 in patients with advanced refractory MBC or mCRPC exhibited PK properties generally consistent with typical IgG1 monoclonal antibodies. Total body clearance decreased with increasing dose, indicating potential target-mediated drug disposition kinetics. However, interpretation of these results is limited by the PK sampling period (up to 336 hours) and narrow dose range evaluated. Comparing fixed dosing regimens (100 mg QW and 100 mg Q2W) with body weight-dependent regimens (1.25 mg/kg Q2W and 1 mg/kg WK1245) revealed minimal effect of body weight on PK, with comparable single dose exposures observed between the 1.25 mg/kg WK1245 and 100 mg QW dose groups, supporting flat milligram dosing.
The safety data observed in this study were consistent with the safety profile expected for a CSF-1R inhibitor, with the most frequent treatment-related AEs consisting of fatigue, decreased appetite, nausea, and elevated liver enzymes. The most frequent AEs were generally predictable, manageable, and reversible with no apparent dose relationship and no new or unexpected safety findings.
Although small patient numbers in this study limits our ability to understand why some patients experienced prolonged SD with CSF-1R inhibition, while others did not, we did observe activation of immune genes in tumor tissue post-treatment. However, due to the limited availability of pre- and post-treatment tumor biopsies we only have indirect measures of a decrease in TAM at the tumor level. Given the limited availability of tissue samples we therefore conducted differential gene expression analyses on these metastatic biopsies. Genes differentially expressed between patients with clinical benefit and those with no benefit were identified. However, due to the small sample size of this comparison, it would be best to confirm these differences in a larger cohort to correct for any potential statistical errors (32). We must also note a small proportion of patients in this study were treated with concurrent hormonal therapy, while others were not, and this may be relevant for one of the HR+ MBC patients who had prolonged SD on trial.
In this study, there was a predominance of MBC patients with visceral metastatic disease which is associated with worse prognosis and lower likelihood of response to immune manipulation (33). Notably, with immune checkpoint blockade low response rates were observed with monotherapy in heavily pretreated breast cancer populations, but responses improved when administered first line therapy (34). For example, immunomodulatory treatments are effective as first line treatment and in combination with other chemotherapy regimens (per KEYNOTE-522 [NCT03036488] and Impassion130 [NCT02425891]) (35,36). Of note, the two MBC patients with durable SD in this study did not have active visceral disease and had received limited prior lines of therapy. Targeting TAMs early in the disease course in those MBC patients with limited prior therapy and non-visceral disease is of great interest.
In this study of a CSF-1R inhibitor, we observed durable clinical benefit with no significant toxicity in three patients with HR+MBC, including one young patient with taxane-resistant disease. Although there were few immunomodulatory changes detected in sera aside from increased CSF1 and IL-34 levels suggestive of a pharmacodynamic effect, there was an association with immune activation in metastatic biopsies after treatment in some patients who showed clinical benefit. This provides support that targeting TAM warrants further evaluation. Collectively, the clinical data would suggest the use of small molecules or antibodies targeting CSF-1R as monotherapy are unlikely to benefit the majority of patients, however, combination strategies to target tumor-associated macrophages and increase immune activation remain viable strategies in MBC and mCRPC, including concurrently targeting LAG3 as suggested by immune monitoring data in this study.
Supplementary Material
Statement of Translational Relevance.
The majority of breast and prostate cancers are immunologically cold and typically unresponsive to single agent checkpoint inhibitors. Therefore, there is interest in identifying alternative immunologic targets for the treatment of these cancers. Colony-stimulating factor-1Receptor (CSF-1R) and its ligand CSF-1Regulate the function and survival of tumor-associated macrophages which are involved in tumorigenesis and suppression of antitumor immunity. In this exploratory study, the immune response in sera and tumor tissue, antitumor activity, and safety of a CSF-1R directed antibody were evaluated in patients with advanced breast and prostate cancer. Circulating levels of CSF-1 increased across dose cohorts consistent with target engagement. At the tumor level there was a trend for increased immune activating genes in patients with clinical benefit. Two metastatic breast cancer patients displayed prolonged stable disease; however, no objective responses were observed in this pre-treated population. Targeting tumor-associated macrophages earlier in the disease course may be important.
Acknowledgements
We extend our gratitude to the patients and their families and caregivers for participating in this trial. This trial was funded by Eli Lilly and Company. Memorial Sloan Kettering Cancer Center (MSKCC) authors were supported in part by the NIH/NCI Cancer Center Support Grant P30 CA008748 and the Ludwig Center for Cancer Immunotherapy. C.A.K. is a member of the Parker Institute for Cancer Immunotherapy at MSKCC. We thank Tonya Quinlan for performing the PK analysis. Medical writing support was provided by Prudence Stanford, PhD, and Ira Ayene, PhD, and editorial support was provided by Dana Schamberger, MA, of Syneos Health, funded by Eli Lilly and Company.
Financial support:
Eli Lilly and Company; Memorial Sloan Kettering Cancer Center (MSKCC) authors were supported in part by the NIH/NCI Cancer Center Support Grant P30 CA008748. C.A. Klebanoff is a member of the Parker Institute for Cancer Immunotherapy at MSKCC
Footnotes
Conflict of Interest Disclosure Statement:
K.A. Autio reports grants from Eli Lilly and Company during the conduct of the study; and grants from Amgen, Astra Zeneca, Glaxo Smith Klein, Merck, and Pfizer outside the submitted work.
C.A. Klebanoff reports grants from Eli Lilly and Company during the conduct of the study; personal fees from Achilles Biotherapeutics, Aleta Biotherapeutics, Bellicum Pharmaceuticals, Obsidian Therapeutics, Klus Pharma, Roche-Genentech, and G1 Therapeutics; and grants from Kite/Gilead outside the submitted work.
D. Schaer was an employee of Eli Lilly and Company at the time of the study.
J. Kauh is a former employee of Eli Lilly and Company.
S.F. Slovin has nothing to declare.
M. Adamow has nothing to declare.
V.S. Blinder reports personal fees from Pfizer outside the submitted work.
M. Brahmachary is a former employee of Eli Lilly and Company and may have Eli Lilly and Company stock.
M. Carlsen is an employee of Eli Lilly and Company.
E. Comen reports consultancy fees from Pfizer, Novartis, Bristol Myers Squibb, COTA, Genentech-Roche, outside the scope of the submitted work.
D.C. Danila reports research support from US Department of Defense, American Society of Clinical Oncology, Prostate Cancer Foundation, Stand Up 2 Cancer, Janssen Research & Development, Astellas, Medivation, Agensys, Genentech, CreaTV; and is a consultant for Angle LLT, AxImmune, Janssen, Astellas, Pfizer, Medivation, Agensys, Bayer, and ScreenCell.
T.N. Doman is an employee and stockholder of Eli Lilly and Company.
J.C. Durack is an investor and scientific advisory board member of Adient Medical and an advisory board member receiving consultancy fees from Verix Health, both outside the scope of the submitted work.
J.J. Fox has nothing to declare.
J.S. Gluskin has nothing to declare.
D.M. Hoffman has nothing to declare.
S. Kang is an employee of Eli Lilly and Company.
P. Kang has nothing to declare.
J. Landa has nothing to declare.
P.F. McAndrew has nothing to declare.
S. Modi reports research support from Genentech, Daiichi Sankyo, Seattle Genetics, Novartis, and Synta pharmaceuticals; has received Honoria/advisory fees from Genentech, Daiichi Sankyo, Seattle Genetics, Macrogenics, Carrick, GSK, and Astra Zeneca; and is on the Speakers Bureau for Daiichi Sankyo, Astra Zeneca, and Genentech.
M.J. Morris reports personal fees from Blue Earth Diagnostics, and ORIC Pharmaceuticals; and institutional research support from Johnson & Johnson, Bayer, Sanofi, Progenics, Corcept, Genentech-Roche, and Janssen outside the submitted work.
R. Novosiadly is a shareholder of Eli Lilly and Company, and Bristol-Myers Squibb.
D.E. Rathkopf reports research funding from Janssen, Celgene, Ferring, Novartis, Taiho, Tracon, AstraZeneca, and Genentech-Roche; and is an uncompensated consultant for Janssen, Tracon, AstraZeneca, and Genentech-Roche.
R. Sanford has nothing to declare.
S.C. Chapman is an employee and stockholder of Eli Lilly and Company.
C.M. Tate is an employee and stockholder of Eli Lilly and Company.
D. Yu is an employee of Eli Lilly and Company.
P. Wong provided laboratory services for processing and testing of study clinical samples via a laboratory service agreement with Eli Lilly and Company during the conduct of the study; and has uncompensated relationships with Leap Therapeutics, and Sellas Life Sciences.
H.L. McArthur reports grants and personal fees from Bristol-Myers Squibb, Merck, AstraZeneca, and MedImmune; personal fees from Daiichi-Sankyo, Eli Lilly and Company, Pfizer, Genentech, Immunomedics, Puma Biotech, Amgen, Seattle Genetics, Genomic Health, and Spectrum Pharmaceuticals outside the submitted work.
Trial registration (clinicaltrials.gov): NCT02265536
References
- 1.Kacinski BM. CSF-1 and its receptor in ovarian, endometrial and breast cancer. Ann Medicine 1995;27(1):79–85. [DOI] [PubMed] [Google Scholar]
- 2.Sasmono RT, Oceandy D, Pollard JW, Tong W, Pavli P, Wainwright BJ, et al. A macrophage colony-stimulating factor receptor-green fluorescent protein transgene is expressed throughout the mononuclear phagocyte system of the mouse. Blood 2003;101(3):1155–63 doi 10.1182/blood-2002-02-0569. [DOI] [PubMed] [Google Scholar]
- 3.Bourette RP, Rohrschneider LR. Early events in M-CSF receptor signaling. Growth Factors 2000;17(3):155–66. doi 10.3109/08977190009001065 [DOI] [PubMed] [Google Scholar]
- 4.Lin H, Lee E, Hestir K, Leo C, Huang M, Bosch E, et al. Discovery of a cytokine and its receptor by functional screening of the extracellular proteome. Science 2008;320(5877):807–11 doi 10.1126/science.1154370. [DOI] [PubMed] [Google Scholar]
- 5.Pixley FJ, Stanley ER. CSF-1Regulation of the wandering macrophage: complexity in action. Trends Cell Biol 2004;14(11):628–38 doi 10.1016/j.tcb.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 6.Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Res 2006;66(2):605–12 doi 10.1158/0008-5472.Can-05-4005. [DOI] [PubMed] [Google Scholar]
- 7.Nowicki A, Szenajch J, Ostrowska G, Wojtowicz A, Wojtowicz K, Kruszewski AA, et al. Impaired tumor growth in colony-stimulating factor 1 (CSF-1)-deficient, macrophage-deficient op/op mouse: evidence for a role of CSF-1-dependent macrophages in formation of tumor stroma. Int J Cancer 1996;65(1):112–9 doi . [DOI] [PubMed] [Google Scholar]
- 8.Lin EY, Li JF, Gnatovskiy L, Deng Y, Zhu L, Grzesik DA, et al. Macrophages regulate the angiogenic switch in a mouse model of breast cancer. Cancer Res 2006;66(23):11238–46 doi 10.1158/0008-5472.Can-06-1278. [DOI] [PubMed] [Google Scholar]
- 9.Lin EY, Nguyen AV, Russell RG, Pollard JW. Colony-stimulating factor 1 promotes progression of mammary tumors to malignancy. J Exp Med 2001;193(6):727–40 doi 10.1084/jem.193.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ide H, Hatake K, Terado Y, Tsukino H, Okegawa T, Nutahara K, et al. Serum level of macrophage colony-stimulating factor is increased in prostate cancer patients with bone metastasis. Hum Cell 2008;21(1):1–6 doi 10.1111/j.1749-0774.2007.00042.x. [DOI] [PubMed] [Google Scholar]
- 11.Lawicki S, Szmitkowski M, Wojtukiewicz M. The pretreatment plasma level and diagnostic utility of M-CSF in benign breast tumor and breast cancer patients. Clin Chim Acta 2006;371(1–2):112–6 doi 10.1016/j.cca.2006.02.033. [DOI] [PubMed] [Google Scholar]
- 12.Mroczko B, Groblewska M, Wereszczynska-Siemiatkowska U, Okulczyk B, Kedra B, Laszewicz W, et al. Serum macrophage-colony stimulating factor levels in colorectal cancer patients correlate with lymph node metastasis and poor prognosis. Clin Chim Acta 2007;380(1–2):208–12 doi 10.1016/j.cca.2007.02.037. [DOI] [PubMed] [Google Scholar]
- 13.Zhu XD, Zhang JB, Zhuang PY, Zhu HG, Zhang W, Xiong YQ, et al. High expression of macrophage colony-stimulating factor in peritumoral liver tissue is associated with poor survival after curative resection of hepatocellular carcinoma. J Clin Oncol 2008;26(16):2707–16 doi 10.1200/jco.2007.15.6521. [DOI] [PubMed] [Google Scholar]
- 14.DeNardo DG, Brennan DJ, Rexhepaj E, Ruffell B, Shiao SL, Madden SF, et al. Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discov 2011;1(1):54–67 doi 10.1158/2159-8274.Cd-10-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Escamilla J, Schokrpur S, Liu C, Priceman SJ, Moughon D, Jiang Z, et al. CSF1 receptor targeting in prostate cancer reverses macrophage-mediated resistance to androgen blockade therapy. Cancer Res 2015;75(6):950–62 doi 10.1158/0008-5472.Can-14-0992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity 2014;41(1):49–61 doi 10.1016/j.immuni.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holmgaard RB, Brachfeld A, Gasmi B, Jones DR, Mattar M, Doman T, et al. Timing of CSF-1/CSF-1R signaling blockade is critical to improving responses to CTLA-4 based immunotherapy. Oncoimmunology 2016;5(7):e1151595 doi 10.1080/2162402x.2016.1151595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scher HI, Halabi S, Tannock I, Morris M, Sternberg CN, Carducci MA, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clinical Oncol 2008;26(7):1148–59 doi 10.1200/jco.2007.12.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45(2):228–47 doi 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 20.Nishino M, Giobbie-Hurder A, Gargano M, Suda M, Ramaiya NH, Hodi FS. Developing a common language for tumor response to immunotherapy: immune-related response criteria using unidimensional measurements. Clin Cancer Res 2013;19(14):3936–43 doi 10.1158/1078-0432.Ccr-13-0895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gu Z, Eils R, Schlesner M. Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics 2016;32(18):2847–9 doi 10.1093/bioinformatics/btw313. [DOI] [PubMed] [Google Scholar]
- 22.Ries CH, Cannarile MA, Hoves S, Benz J, Wartha K, Runza V, et al. Targeting tumor-associated macrophages with anti-CSF-1R antibody reveals a strategy for cancer therapy. Cancer Cell 2014;25(6):846–59 doi 10.1016/j.ccr.2014.05.016. [DOI] [PubMed] [Google Scholar]
- 23.Cannarile MA, Weisser M, Jacob W, Jegg AM, Ries CH, Ruttinger D. Colony-stimulating factor 1 receptor (CSF1R) inhibitors in cancer therapy. J Immunother Cancer 2017;5(1):53 doi 10.1186/s40425-017-0257-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cassier PA, Italiano A, Gomez-Roca CA, Le Tourneau C, Toulmonde M, Cannarile MA, et al. CSF1R inhibition with emactuzumab in locally advanced diffuse-type tenosynovial giant cell tumours of the soft tissue: a dose-escalation and dose-expansion phase 1 study. Lancet Oncol 2015;16(8):949–56 doi 10.1016/s1470-2045(15)00132-1. [DOI] [PubMed] [Google Scholar]
- 25.Tap WD, Gelderblom H, Palmerini E, Desai J, Bauer S, Blay JY, et al. Pexidartinib versus placebo for advanced tenosynovial giant cell tumour (ENLIVEN): a randomised phase 3 trial. Lancet 2019;394(10197):478–87 doi 10.1016/s0140-6736(19)30764-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tap WD, Wainberg ZA, Anthony SP, Ibrahim PN, Zhang C, Healey JH, et al. Structure-guided blockade of CSF1R kinase in tenosynovial giant-cell tumor. N Engl J Med 2015;373(5):428–37 doi 10.1056/NEJMoa1411366. [DOI] [PubMed] [Google Scholar]
- 27.Butowski N, Colman H, De Groot JF, Omuro AM, Nayak L, Wen PY, et al. Orally administered colony stimulating factor 1 receptor inhibitor PLX3397 in recurrent glioblastoma: an Ivy Foundation Early Phase Clinical Trials Consortium phase II study. Neuro Oncol 2016;18(4):557–64 doi 10.1093/neuonc/nov245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gomez-Roca CA, Italiano A, Le Tourneau C, Cassier PA, Toulmonde M, D'Angelo SP, et al. Phase I Study of emactuzumab single agent or in combination with paclitaxel in patients with advanced/metastatic solid tumors reveals depletion of immunosuppressive M2-like macrophages. Ann Oncol 2019. doi 10.1093/annonc/mdz163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Papadopoulos KP, Gluck L, Martin LP, Olszanski AJ, Tolcher AW, Ngarmchamnanrith G, et al. First-in-human study of AMG 820, a monoclonal anti-colony-stimulating factor 1 receptor antibody, in patients with advanced solid tumors. Clinical Can Res 2017;23(19):5703–10 doi 10.1158/1078-0432.Ccr-16-3261. [DOI] [PubMed] [Google Scholar]
- 30.Gyori D, Lim EL, Grant FM, Spensberger D, Roychoudhuri R, Shuttleworth SJ, et al. Compensation between CSF1R+ macrophages and Foxp3+ Treg cells drives resistance to tumor immunotherapy. JCI Insight 2018;3(11) doi 10.1172/jci.insight.120631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun W, Wei FQ, Li WJ, Wei JW, Zhong H, Wen YH, et al. A positive-feedback loop between tumour infiltrating activated Treg cells and type 2-skewed macrophages is essential for progression of laryngeal squamous cell carcinoma. Br J Cancer 2017;117(11):1631–43 doi 10.1038/bjc.2017.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Button KS, Ioannidis JPA, Mokrysz C, Nosek BA, Flint J, Robinson ESJ, et al. Power failure: why small sample size undermines the reliability of neuroscience. Nat Rev Neurosci 2013;14(5):365–76 doi 10.1038/nrn3475. [DOI] [PubMed] [Google Scholar]
- 33.Yardley DA. Visceral disease in patients with metastatic breast cancer: efficacy and safety of treatment with ixabepilone and other chemotherapeutic agents. Clin Breast Cancer 2010;10(1):64–73 doi 10.3816/CBC.2010.n.009. [DOI] [PubMed] [Google Scholar]
- 34.Hu ZI, McArthur HL. Immunotherapy in breast cancer: the new frontier. Curr Breast Cancer Rep 2018;10(2):35–40 doi 10.1007/s12609-018-0274-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schmid P, Cortes J, Dent R, Pusztai L, McArthur HL, Kuemmel S, et al. LBA8_PR KEYNOTE-522: Phase III study of pembrolizumab (pembro) + chemotherapy (chemo) vs placebo (pbo) + chemo as neoadjuvant treatment, followed by pembro vs pbo as adjuvant treatment for early triple-negative breast cancer (TNBC). Ann Oncol 2019;30(Supplement_5) doi 10.1093/annonc/mdz394.003. [DOI] [Google Scholar]
- 36.Schmid P, Adams S, Rugo HS, Schneeweiss A, Barrios CH, Iwata H, et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med 2018;379(22):2108–21 doi 10.1056/NEJMoa1809615. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.