Abstract
The International Commission on Radiological Protection (ICRP) has considered for over 60 years that the lens of the eye is among the most radiosensitive tissues, and has recommended dose limits for the lens to prevent occurrence of vision impairing cataracts (VICs). Epidemiological evidence that doses much lower than previously thought produce cataracts led ICRP to recommend reducing dose threshold for VICs and reducing an occupational equivalent dose limit for the lens in 2011, when only a single threshold of 0.5 Gy was recommended. On the basis of epidemiological evidence, ICRP assumed progression of minor opacities into VICs and no dose rate effect. This contrasts with previously recommended separate thresholds for minor opacities and VICs, and for different exposure scenarios. Progression was assumed based on similar risks of cataracts and cataract surgery in Japanese atomic bomb survivors. The absence of dose rate effect derived from the observed similar thresholds for protracted exposures in Chernobyl cleanup workers and in atomic bomb survivors. Since 2011, there has been an increasing body of epidemiological evidence relating to cataracts and other ocular diseases (i.e. glaucoma and macular degeneration), particularly at low doses and low dose rates. This review paper gives an overview of the scientific basis of the 2011 ICRP recommendation, discusses the plausibility of these two assumptions in the light of emerging scientific evidence, and considers the radiosensitivity of the lens among ocular structures.
Introduction
A cataract is a clouding or opacity of the normally transparent crystalline lens of the eye, and is an iconic age-related disease. Cataracts affect vision, especially during the daytime. Cataracts can be replaced with an artificial lens (typically with day surgery), but remain the first leading cause of visual impairment in the world.1 Cataracts are anatomically classified into nuclear, cortical, or posterior subcapsular (PSC) types.2 Ionizing radiation is a proven human cataractogen, and common radiation cataracts are PSC or cortical types (c.f. common senile cataracts being nuclear or cortical types).3,4
Radiation cataracts have long been recognized since the early days of radiation use. Following the discovery of X-rays in late 1895, the first case of radiation cataract was reported in experimental animals (rabbits) in 1897 and in humans in 1903.5,6 In Japanese atomic bomb (A-bomb) survivors, cataract studies preceded cancer studies by several years. In 1949, two papers on cataracts in A-bomb survivors and cyclotron workers were published simultaneously in Science,7,8 stimulating a surge of interest in radiation protection of the ocular lens.
In 1950, the International Commission on Radiological Protection (ICRP) listed cataracts as one of the “effects to be considered,” and assigned the ocular lens as one of critical organs.9 In 1954, ICRP recommended the first set of lens dose limits for workers and public and an effective depth of 3 mm for the lens.10 In 1977, ICRP classified cataracts as “non-stochastic effects” (renamed “deterministic effects” in 1990, then “tissue reactions” in 2007) with a dose threshold below which no effect would occur11–13: lens dose limits therefore aim to prevent vision impairing cataracts (VICs), but not minor opacities. Occupational and public lens dose limits, respectively, have so far undergone 8 and 6 revisions since 1954,14 among which the latest revision took place in April 2011 when ICRP recommended reducing an occupational equivalent dose limit for the lens from 150 mSv/year to 20 mSv/year averaged over defined periods of five years with no single year exceeding 50 mSv/year (Table 1).15 This triggered a resurgence of interest in radiation exposure of the lens and its effects. Various countries in Europe, Oceania and Asia have implemented the new ICRP occupational equivalent dose limit into national regulations, and extensive discussions toward regulatory implementations are ongoing in many other countries.16,17
Table 1.
ICRP equivalent dose limits and NCRP numeric protection criteria for the lens of the eye
| ICRP | NCRP | |||
|---|---|---|---|---|
| Past | Present | Past | Present | |
| Occupational exposure | 150 mSv/year (1980–2011) | 100 mSv/5 years (≤50 mSv/year) (2011–) | 150 mSv/year (1987–2016) | 50 mGy/year (2016–) |
| Public exposure | 50 mSv/year (1970–1990) | 15 mSv/year (1990–) | 15 mSv/year (1993–2016) | 15 mGy/year (2016–) |
| Threshold | >8 Sva | 0.5 Gy (2011–) | 4 Gyb | N.A. (2016–) |
| Radiation weighting | (1973–1990) | wR (1990–) | wR (1993–2016) | RBE (2016–) |
ICRP, International Commission on Radiological Protection;N.A., not available due to uncertainty; NCRP, US National Council on Radiation Protection and Measurements; , effective quality factor; RBE, relative biological effectiveness; VIC, vision impairing cataract; wR, radiation weighting factor.
For highly fractionated/protracted exposures:>8 Sv for VICs and 5 Sv for detectable opacities. For acute exposure: 5 (2–10) Sv for VICs and 0.5–2 Sv for detectable opacities. The “150 mSv/year limit” was obtained by rounding off “>160 mSv/year” calculated as >8 Sv divided by working life of 50 years.
For cataracts: 4 Gy for fractionated exposures and 2–10 Gy for acute exposure.
Since 2011, there has been an accumulating body of epidemiological evidence relating to cataracts and other ocular diseases (i.e. glaucoma and macular degeneration), particularly at low doses and low dose rates. This review paper provides an overview of the scientific basis of the 2011 ICRP recommendation, discusses the plausibility of its underlying assumptions in the light of emerging scientific evidence, and considers the radiosensitivity of the lens among ocular structures. This brief paper aims to give an update of the literature and cannot fully cover the range and complexity of this subject matter; relevant reviews3,4,14,18–20 will be helpful for a deeper understanding of earlier knowledge and discussions in this field.
Scientific basis for the ICRP threshold of 0.5 Gy for low-LET radiation
ICRP currently judges that cataracts are tissue reactions with a threshold of 0.5 Gy for low-linear energy transfer (LET) radiation and with no dose rate effect, and that VICs occur in 1% of exposed individuals at >20 years after exposure to 0.5 Gy where minor opacities progress to VICs.15 Among ~70 epidemiological papers available by 2010, the following three papers particularly played a major role in judging such a nominal threshold. A threshold for acute exposure was judged as 0.5 Gy mainly from two papers on prevalence of cataracts and cataract surgery both at 55–57 years after exposure in A-bomb survivors.21,22 A threshold for highly fractionated or protracted exposure was judged as <0.5 Gy mainly from one paper on cataracts at 12–14 years after exposure in Chernobyl clean-up workers.23 A threshold for chronic exposure was judged as uncertain due to lack of evidence. Collectively, threshold was judged as 0.5 Gy independent of dose rate.
Emerging epidemiological evidence
Cataracts
Shore assessed the impact of epidemiological papers published after the 2011 ICRP recommendation through early 2016 and concluded that no influential papers were published during that period of time.20 Indeed, no new papers on cataract prevalence in A-bomb survivors have been published since 2006.21 Provided below is summary of the recent findings from the two large cohorts that have been reported since mid 2016. One is the cohort of workers in the Mayak Production Association located in the Southern Urals in the vicinity of Ozyorsk city that started its operation in 1948 as the first Russian nuclear enterprise. The other is the cohort of the U.S. radiologic technologists (USRT). Table 2 compares characteristics of these two cohorts with A-bomb survivors.
Table 2.
Comparisons of cohorts in recent ophthalmological studies
| End points | Cohorts | ||
|---|---|---|---|
| Atomic bomb survivors | Mayak workers | Radiologic technologists | |
| Countries | Japan | Russia | US |
| Rounded number of eligible study participants | 900–10,000 | 21,000 | 70,000 |
| Male:female | one to 2 | three to 1 | one to 3 |
| Follow-up | ≤60 yearsa | ≤60 yearsb | ≤92 yearsc |
| Mean follow-up | ≤60 yearsa | >30 yearsb | >10 yearsc |
| Medical information | Biennial health exams | Annual health exams | Periodic questionnaire surveysd |
| Exposure scenarios | Acute | Chronic | Protracted |
| Dose evaluated | Eye absorbed dosee | Hp(10)f | Lens absorbed doseg |
| Mean dose | ~0.5 Gye | ~0.5 Svf | ~0.06 Gyg |
| Radiation cataracts in aggregate | Significantly increased | Significantly increased | Significantly increased |
| Radiation posterior subcapsular cataracts | Significantly increased | Significantly increased | N.A. |
| Radiation cortical cataracts | Significantly increased | Significantly increased | N.A. |
| Radiation nuclear cataracts | N.S. | Significantly increased | N.A. |
| Radiation cataract surgery | Significantly increased | N.S. | N.S. |
| Radiation glaucoma in aggregate | Significantly decreased | N.A. | N.S. |
| Radiation primary glaucoma | N.A. | N.S. | N.A. |
| Radiation primary open-angle glaucoma | N.A. | N.S. | N.A. |
| Radiation primary open-angle normal-tention glaucoma | Significantly increased | N.A. | N.A. |
| Radiation primary open-angle high-tention glaucoma | N.S. | N.A. | N.A. |
| Radiation primary angle-closure glaucoma | N.S. | N.A. | N.A. |
| Radiation diabetic retinopathy | Significantly increased | N.A. | N.A. |
| Radiation macular degeneration | N.S. | N.A. | N.S. |
NA, not available; NS, not significant.
Those exposed in 1945 were followed up through 2005.
Those first employed in 1948–1982 were followed up through 2008.
Those certified as radiologic technologists for ≥2 years in 1928–1982 were followed up through 2014.
Self-reporting but by medically literate individuals.
According to the Dosimetry System 2002 (DS02).24
According to the Mayak Worker Dosimetry System 2008 (MWDS–2008).25
According to the updated and improved dosimetry.26
In Mayak workers, the risk for cataracts in aggregate increased linearly with chronic cumulative effective dose from external γ-rays at ≥0.25 Sv, with the excess relative risk per unit effective dose (ERR/Sv) of 0.28 [95% confidence intervals (CIs): 0.20, 0.37].27 The risk for each of all three main cataract types also increased linearly with chronic cumulative effective dose from external γ-rays at ≥0.25 Sv with the ERR/Sv of 0.91 (95% CI: 0.67, 1.20) for PSC, 0.63 (95% CI: 0.49, 0.76) for cortical, and 0.47 (95% CI: 0.35, 0.60) for nuclear cataracts,28 indicating that radiosensitivity was highest for PSC, and progressively lower for cortical and nuclear cataracts. Females accounted for 25.4% of the cohort members, and the risk for each of all three cataract types was significantly higher in females than in males (ERR/Sv being 3.8-fold higher in females for PSC and 2.5-fold for cortical both with p < 0.001, 1.9-fold for nuclear with p = 0.018). This study was the first to suggest the significant gender difference in radiation cataracts (PSC cataracts in particular). This was also the first large study to suggest the significantly increased radiation risk for nuclear cataracts, in contrast to null results from other large studies, e.g. no significant dose response in A-bomb survivors21 and no significant elevation in risk in Chernobyl clean-up workers.23 An earlier population-based case–control study in commercial airline pilots reported that cosmic radiation exposure (dose reconstructed from pilots’ flight logs) significantly increases risk for nuclear cataracts and insignificantly decreases risk for PSC and cortical cataracts, but had various potential problems with selection factors, crude or absence of control for confounding factors, inadequate modeling of age as a confounder, and the small number of cases.29
In the USRT, the risk for self-reported cataracts in aggregate significantly increased linearly with the protracted cumulative 5 year lagged lens absorbed dose over the full dose range with the excess hazard ratio per unit lens absorbed dose (EHR/mGy) of 0.69 × 10–3 (95% CI: 0.27 × 10–3, 1.16 × 10–3), and remained significant at <100 mGy with EHR/mGy of 1.16 × 10–3 (95% CI: 0.11 × 10–3, 2.31 × 10–3).30 This study was the first to suggest that radiation exposure at low dose (at <100 mGy) and low dose rate (typically <5 mGy/h) causes cataract. A recent re-analysis of this cohort is the first to report excess absolute (additive) risk for radiation cataracts.31
In addition to these two cohorts, residents aged ≥45 years in natural high background radiation area in Yangjiang, Guangdong, China had a significantly increased risk for PSC opacities with the odds ratio (OR) of 4.05 (95% CI: 1.56, 10.46), but with a marginally significantly increased risk for cortical opacities (OR of 1.45, 95% CI: 0.99, 2.11), a non-significantly decreased risk for nuclear opacities (OR of 0.82, 95% CI: 0.60, 1.14) and a non-significant risk for all types of opacities in aggregate (OR of 0.99, 95% CI: 0.72, 1.37),32 where the lifetime chronic lens dose should be below a few hundred mSv (c.f., less than 100 mSv in control area).
As such, available epidemiological evidence tends to support lack of a clear dose rate effect. The lens cells stay inside the lens throughout life due to the lens capsule, and lens fiber cells have no cellular organelles. Mechanistically, the lens has little if any turnover of lens cells and its components (e.g. proteins, lipids).33 These make no dose rate effect biologically plausible. However, biological studies with animal models have shown either conventional sparing dose rate effects, no dose rate effect, or enhancing inverse dose rate effects,34–36 although the “low dose rates” in some of these experiments are not low from radiation protection viewpoints. So, we do not yet have enough evidence to make a decision either way.
Cataract surgery
A-bomb survivors exhibited a significantly increased risk for prevalence of cataract surgery at 55–57 years after exposure with insignificant threshold, and for incidence and prevalence of cataract surgery at 31–60 years after exposure with significant threshold.22,37,38 In contrast, risk for cataract surgery in Mayak workers (ERR/Gy of 0.09, 95% CI: –0.02, 0.22) and for self-reported cataract surgery in the USRT (EHR/Gy of 0.34, 95% CI: –0.19, 0.97) tended to increase, but not statistically significantly so.30,39 A significantly increased risk has thus far been observed only in A-bomb survivors, and it remains unclear whether such inconsistency is attributable to difference in dose rate, progression rate, age at exposure, nationality or follow-up period. In any case, the results should be interpreted with caution. Some VICs would require surgical intervention, but cataract surgery is a less specific surrogate for VICs than high-grade cataracts and an imperfect surrogate that may underestimate the prevalence of VICs. This is because various factors can influence the likelihood of cataract surgery, such as the size and location of the cataract, socioeconomic, medical-cost and health consciousness factors, visual acuity in the opposite eye, nature of work or avocational activities affecting the need for visual acuity, and amount of ultraviolet exposure.20 Nevertheless, cataract surgery is a better surrogate for VICs than low-grade opacities that dominate the existing examination studies.
Do minor opacities progress into vision impairing cataracts?
ICRP considered in 1969 and 1984 that minor opacities that do not interfere with vision do not progress in severity and may regress or disappear spontaneously, and that whether the lesion remains stationary or progressive depends on dose.40,41 ICRP had thus recommended thresholds separately for minor opacities and for VICs. In contrast, in the 2011 ICRP recommendation, minor opacities were judged to progress into VICs, mainly because in A-bomb survivors at 55–57 years after exposure, the risk for prevalence of cataract surgery [OR at 1 Sv of 1.39 (95% CI: 1.24, 1.55)] was similar to that for prevalence of cataracts [e.g. OR/Sv of 1.44 (95% CI: 1.19, 1.73) for PSC opacity].21,22 However, as discussed above, a significantly increased risk for cataract surgery has been observed only in A-bomb survivors. It is also intriguing to highlight that early A-bomb data show little progression in lenticular changes, e.g. 36% unchanged and 19% regressed at 6 years after exposure, and ~60% unchanged and ~30% regressed at 21 years after exposure.18 Taken together, in Fukushima nuclear workers, vacuoles in the PSC center (incipient PSC changes) tend to increase with time, but their regression has also been observed.17,42 In summary, available evidence does not tend to support progressive nature of radiation cataracts at low dose.
Why is the lens so radiosensitive?
Over the past six decades, ICRP has always considered that bone marrow, gonads and the eye lens are among the most radiosensitive tissues in the body.13,43
Mechanisms behind high sensitivity of the lens to low-LET radiation may involve abnormal proliferation and differentiation of lens epithelial cells (LECs), oxidative stress, and denaturation of lens crystalline proteins.33,44 Human LECs were found to contain a subset whose proliferation is stimulated by radiation and another subset sensitive to radiogenic premature senescence.45,46 Such radiation-stimulated LEC proliferation has been reported both in vitro in human cells45,47 and in vivo in experimental animals (mice and rabbits).48,49
The lens is more sensitive to high-LET radiation than other tissues. The mechanisms may involve low oxygen, high nitrogen, and cellular quiescence.34,35 Since 2016, the US National Council on Radiation Protection and Measurements (NCRP) has recommended the use of relative biological effectiveness for high-LET radiation for absorbed dose limits (numeric protection criteria), instead of a radiation weighting factor (wR) (Table 1),50–52 and a similar change has also been proposed by ICRP. Over the past few decades, ICRP has not provided the updated report on high-LET radiation cataracts.41,53 The implications of a possibly higher relative biological effectiveness for radiation protection need to be discussed.
In addition to little if any turnover of lens cells and its constituents as aforementioned, various unique features make the lens radiobiologically very intriguing. For instance, the lens does not develop primary tumors (neither spontaneously nor following radiation exposure), but there has been mounting evidence for involvement of tumor-related factors in cataractogenesis.54–56 Interestingly, the unique inverse dose rate effect within the very narrow dose rate range has also recently been reported for DNA damage response in the lens.36 There should be more unknown mechanisms operational in the lens. Clearly, continued efforts are needed for further biological and mechanistic developments, as those currently led by the European CONCERT-funded LDLensRad project.57
Integration of epidemiology and biology has long been discussed for cancer.58 This would also indeed be needed for cataracts,59 and development of risk-predictive biomathematical models60 would be important.
Is cataract a tissue reaction, a stochastic effect, or both?
ICRP considers that minor opacities are a linear function of dose, but VICs attributable to multiple minor opacities exhibit a threshold-type dose response.15 ICRP and NCRP have both classified cataracts as tissue reactions. Currently, ICRP recommends a nominal threshold of 0.5 Gy,15 but NCRP does not provide a quantitative estimate of a specific threshold because of large uncertainties and limitations in various studies (Table 1).50
Two papers21,22 that were used mainly to judge the ICRP threshold of 0.5 Gy for acute exposure reported insignificant thresholds for cataracts and cataract surgery in A-bomb survivors. Evidence for a significant threshold implies some degree of upward curvature in the dose response, but in one paper on cataract in Chernobyl clean-up workers23 that was used mainly to judge the ICRP threshold of 0.5 Gy for highly fractionated or protracted exposures, there was evidence for a significant threshold, albeit with little evidence for upward curvature. Likewise, in a subsequent paper on cataract surgery in A-bomb survivors,37 there was evidence for a significant threshold, but without linear-quadratic curvature. The discrepancy between the results of fitting threshold and linear-quadratic models to these data sets suggests methodological problems; the lack of C2 differentiability of the likelihood with respect to the threshold value means that asymptotic convergence of likelihood-based p-values and CIs of the threshold value is not guaranteed.4
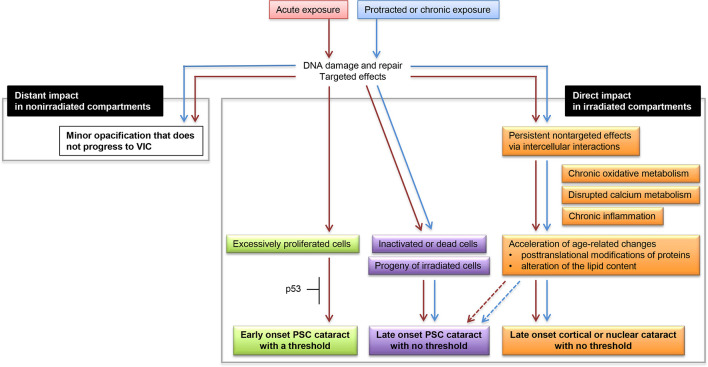
Epidemiological evidence tends to demonstrate that threshold for cataracts becomes less clear with longer follow-up. There may be early-onset cataracts with threshold (e.g. cataracts occurring within a decade post exposure) and late-onset cataracts with no threshold (e.g. cataracts occurring decades post exposure). In this respect, we previously proposed the etiologically different three types of radiation cataracts (early-onset PSC cataracts with threshold, late-onset PSC cataracts with no threshold, and late-onset cortical cataracts with no threshold).54 The updated model (Figure 1) now includes late-onset nuclear cataracts without threshold given a significantly increased risk for nuclear cataracts observed in Mayak workers,28 albeit with the caveat that all other major studies have had null results for nuclear cataracts.
Figure 1.
An updated hypothetical schematic of possible events that lead to ionizing radiation cataractogenesis. Red-colored arrows depict responses following acute exposure, and blue-colored arrows depict responses following protracted or chronic exposures. A previously proposed hypothetical schematic (Figure 2 in Hamada and Fujimichi54 was updated for the following three points: (1) “Late-onset cortical cataract with no threshold” was changed to “Late-onset cortical or nuclear cataract with no threshold” considering the recent evidence for a significantly increased risk for nuclear cataract28; (2) “denaturation of lens proteins” in “Acceleration of age-related changes” was changed to “posttranslational modifications of proteins” and “alteration of the lipid content” and (3) dotted arrows pointing from “Acceleration of age-related changes” to “Late-onset PSC cataract with no threshold” were added, both considering our recent proposal.33 PSC, posterior subcapsular. VIC, vision impairing cataract.
The lack of clear threshold and the involvement of tumor-related factors in cataractgenesis suggest the stochastic nature of cataractogenesis. It would be interesting to test whether an irradiated single lens stem cell can form a cloudy lens-like structure (i.e. a lentoid body),61 when such experiments become technically sound (not yet feasible).62
In summary, we do not yet know whether cataract is a tissue reaction, a stochastic effect or both, and more studies are clearly needed to address this issue.
The impact on ocular diseases other than cataracts
In 2016, NCRP recommended comprehensive evaluation of the overall effects of radiation on the eye.50 In this regard, ICRP considered in 1984 that ocular tissues other than the lens are relatively radioresistant based on data available before early 1980s,41 and has not provided the updated report since then. ICRP described in 2012 that ocular pathologies other than lens opacification occur after acute or fractionated exposures of between 5 and 20 Gy,15 but this dealt only with edema, atrophy and telangiectasia according to the previous report.41 Therefore, the low/moderate dose/dose rate radiation sensitivity of ocular structures other than lens remains almost entirely uncharacterized, and nether ICRP nor NCRP has discussed association between radiation exposure and various ocular diseases (other than cataracts) that are major causes of visual impairment, such as glaucoma, diabetic retinopathy (a typical ocular complication of diabetes) and macular degeneration (Table 3).
Table 3.
The top five causes of visual impairment
| Ranking | Ocular disease % | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Worlda | Japanb | Russiac | USAd | USAe | ||||||||||||||
| White | Black | Hispanic | White | Black | Hispanic | |||||||||||||
| 1 | Cataract | 48 | Glaucoma | 21 | Glaucoma | 29 | Macular degeneration | 54.4 | Cataract | 36.8 | Glaucoma | 28.6 | Cataract | 59.2 | Cataract | 50.9 | Cataract | 46.7 |
| 2 | Glaucoma | 12 | Diabetic retinopathy | 16 | Retinal impairment | 16 | Other | 25 | Glaucoma | 26 | Other | 28.6 | Macular degeneration | 22.9 | Other | 17 | Other | 18.5 |
| 3 | Macular degeneration | 9 | Retinitis pigmentosa | 12 | Optic nerve atrophy | 14 | Cataract | 8.7 | Other | 25.6 | Cataract | 14.3 | Other | 9.7 | Diabetic retinopathy | 14.5 | Macular degeneration | 14.1 |
| 4 | Corneal opacities | 5 | Macular degeneration | 10 | High myopia | 12 | Glaucoma | 6.4 | Diabetic retinopathy | 7.3 | Macular degeneration | 14.3 | Diabetic retinopathy | 4.9 | Glaucoma | 14.3 | Diabetic retinopathy | 13 |
| 5 | Diabetic retinopathy | 5 | Chorioretinal atrophy | 8 | Cataract | 11 | Diabetic retinopathy | 5.4 | Macular degeneration | 4.4 | Diabetic retinopathy | 14.3 | Glaucoma | 3.3 | Macular degeneration | 3.2 | Glaucoma | 7.6 |
In this light, subcohorts of A-bomb survivors exhibited a significantly increased risk for normal-tension glaucoma (a subtype of primary open-angle glaucoma), but an insignificantly decreased risk for high-tension primary glaucoma and macular degeneration.66–68 In A-bomb survivors, radiation dose was also positively associated with retinal degeneration and retinal arteriolosclerosis, and was negatively associated with the diameter of central retinal vein equivalent.68,69 On the other hand, risk was insignificant for self-reported glaucoma in aggregate and macular degeneration in USRT, and for primary glaucoma in Mayak workers (Table 2).70–72
A significantly increased risk for diabetic retinopathy has been reported in subcohorts of A-bomb survivors, with the small number of diabetic retinopathy cases.69 However, a genuine association between radiation exposure and diabetic retinopathy remains unclear, until the prevalence of diabetic retinopathy is analyzed by dose among the diabetics in the cohort, while adjusting, e.g. for length of time when diabetes was present.
Accordingly, normal-tension glaucoma is the only major ocular disease (other than cataracts) with a significantly increased risk suggested. Such an increased risk has so far been reported only in A-bomb survivors (a factor to be borne in mind is that normal-tension glaucoma is the most frequent type of glaucoma in the Japanese population unlike in other populations,72,73), and this result needs to be confirmed in other exposed cohorts, e.g. in Mayak workers.
Conclusions
A long-held tenet remains unchanged that the lens represents among the most radiosensitive tissues in the body and is the most radiosensitive ocular tissue. Radiation cataracts are no longer recognized as a typical tissue reaction with clear threshold of relatively high dose.
ICRP assumes progression of minor opacities into VICs and no dose rate effect. Available evidence tends to support the latter, but not necessarily the former at low dose and low dose rate. Whether a threshold exists for cataracts and whether cataracts are categorized as tissue reactions warrant further investigation.
Further biological and epidemiological developments, its integration, and continued assessment of implications are indispensable to evidence-based best expert judgments for radiation protection purposes.
Studies on cataracts and other ocular impacts are useful not only for radiation protection, but also for radiotherapy as typical normal tissue complications. Cataracts are also the unique effects significantly associated with radiation exposure in astronauts,74–76 but the US National Aeronautics and Space Administration has no longer conducted follow-up or new studies since 2012.76 So, epidemiological studies in cohorts on Earth will serve as an important scientific basis for estimating the risk in astronauts and other space travellers.
Footnotes
Acknowledgment: NH would like to thank the ICRR2019 Local Organizing Committee for an opportunity to serve as a keynote speaker and chair in the session on non-cancer effects at ICRR2019. The authors are grateful to BJR Guest Editors for an opportunity to contribute to this ICRR2019-themed Special Feature. The authors also wish to acknowledge the two referees for the detailed and helpful comments.
Funding: The authors received no specific funding for this work. The work of MPL was funded by the Intramural Research Program of the Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health.
Contributor Information
Nobuyuki Hamada, Email: hamada-n@criepi.denken.or.jp.
Tamara V. Azizova, Email: clinic@subi.su.
Mark P. Little, Email: mark.little@nih.gov.
REFERENCES
- 1.WHO . Blindness and visual impairment. 2002. Available from: http://www.who.int/news-room/fact-sheets/detail/blindness-and-visual-impairment [Accessed 20 October 2019].
- 2.Liu Y-C, Wilkins M, Kim T, Malyugin B, Mehta JS. Cataracts. The Lancet 2017; 390: 600–12. doi: 10.1016/S0140-6736(17)30544-5 [DOI] [PubMed] [Google Scholar]
- 3.Ainsbury EA, Bouffler SD, Dörr W, Graw J, Muirhead CR, Edwards AA, et al. Radiation cataractogenesis: a review of recent studies. Radiat Res 2009; 172: 1–9. doi: 10.1667/RR1688.1 [DOI] [PubMed] [Google Scholar]
- 4.Little MP. A review of non-cancer effects, especially circulatory and ocular diseases. Radiat Environ Biophys 2013; 52: 435–49. doi: 10.1007/s00411-013-0484-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chalupecky H. Uber die wirkung Der röntgenstrahlen auf das Auge und die haut. Zentralbl Augenheilkd 1897; 21: 234–71. [Google Scholar]
- 6.Rollins W, x-light Non. This effect of X-Light on the crystalline lens. The Boston Medical and Surgical Journal 1903; 148: 364–5. doi: 10.1056/NEJM190304021481404 [DOI] [Google Scholar]
- 7.Cogan DG, Martin SF, Kimura SJ. Atom bomb cataracts. Science 1949; 110: 654–5. doi: 10.1126/science.110.2868.654 [DOI] [PubMed] [Google Scholar]
- 8.Abelson PH, Kruger PG. Cyclotron-induced radiation cataracts. Science 1949; 110: 655–7. doi: 10.1126/science.110.2868.655 [DOI] [PubMed] [Google Scholar]
- 9.ICRP . International recommendations on radiological protection. Br J Radiol 1951; 24: 46–53. doi: 10.1259/0007-1285-24-277-46 [DOI] [PubMed] [Google Scholar]
- 10.ICRP . Recommendations of the International Commission on radiological protection. Br J Radiol 1955; 28(Suppl 6): 1–92. [PubMed] [Google Scholar]
- 11.ICRP . Recommendations of the International Commission on radiological protection. ICRP publication 26. Ann ICRP 1977; 1: 1–80. [PubMed] [Google Scholar]
- 12.ICRP . Recommendations of the International Commission on radiological protection. ICRP publication 60. Ann ICRP 1991; 21(1–3): 1–201. [PubMed] [Google Scholar]
- 13.ICRP . Recommendations of the International Commission on radiological protection. ICRP publication 103. Ann ICRP 2007; 37(2–4): 1–332. [DOI] [PubMed] [Google Scholar]
- 14.Hamada N, Fujimichi Y. Classification of radiation effects for dose limitation purposes: history, current situation and future prospects. J Radiat Res 2014; 55: 629–40. doi: 10.1093/jrr/rru019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stewart FA, Akleyev AV, Hauer-Jensen M, Hendry JH, Kleiman NJ, Macvittie TJ, et al. ICRP publication 118: ICRP statement on tissue reactions and early and late effects of radiation in normal tissues and organs--threshold doses for tissue reactions in a radiation protection context. Ann ICRP 2012; 41(1/2): 1–322. doi: 10.1016/j.icrp.2012.02.001 [DOI] [PubMed] [Google Scholar]
- 16.Yokoyama S, Hamada N, Hayashida T, Tsujimura N, Tatsuzaki H, Kurosawa T, et al. Current situations and discussions in Japan in relation to the new occupational equivalent dose limit for the lens of the eye. J Radiol Prot 2017; 37: 659–83. doi: 10.1088/1361-6498/aa73e8 [DOI] [PubMed] [Google Scholar]
- 17.Yokoyama S, Hamada N, Tsujimura N. Recent discussions toward regulatory implementation of the new occupational equivalent dose limit for the lens of the eye and related studies in Japan. Int J Radiat Biol 2019; 95: 1103–12. doi: 10.1080/09553002.2019.1605464 [DOI] [PubMed] [Google Scholar]
- 18.Hamada N, Fujimichi Y, Iwasaki T, Fujii N, Furuhashi M, Kubo E, et al. Emerging issues in radiogenic cataracts and cardiovascular disease. J Radiat Res 2014; 55: 831–46. doi: 10.1093/jrr/rru036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barnard SGR, Ainsbury EA, Quinlan RA, Bouffler SD. Radiation protection of the eye lens in medical workers—basis and impact of the ICRP recommendations. Br J Radiol 2016; 89: 20151034. doi: 10.1259/bjr.20151034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shore RE. Radiation and cataract risk: impact of recent epidemiologic studies on ICRP judgments. Mutation Research/Reviews in Mutation Research 2016; 770: 231–7. doi: 10.1016/j.mrrev.2016.06.006 [DOI] [PubMed] [Google Scholar]
- 21.Nakashima E, Neriishi K, Minamoto A. A reanalysis of atomic-bomb cataract data, 2000-2002: a threshold analysis. Health Phys 2006; 90: 154–60. doi: 10.1097/01.HP.0000175442.03596.63 [DOI] [PubMed] [Google Scholar]
- 22.Neriishi K, Nakashima E, Minamoto A, Fujiwara S, Akahoshi M, Mishima HK, et al. Postoperative cataract cases among atomic bomb survivors: radiation dose response and threshold. Radiat Res 2007; 168: 404–8. doi: 10.1667/RR0928.1 [DOI] [PubMed] [Google Scholar]
- 23.Worgul BV, Kundiyev YI, Sergiyenko NM, Chumak VV, Vitte PM, Medvedovsky C, et al. Cataracts among Chernobyl clean-up workers: implications regarding permissible eye exposures. Radiat Res 2007; 167: 233–43. doi: 10.1667/RR0298.1 [DOI] [PubMed] [Google Scholar]
- 24.Young RW, Kerr GD. Reassessment of the atomic bomb radiation dosimetry for Hiroshima and Nagasaki, dosimetry system 2002. Report of the joint US-Japan working group. Hiroshima, Japan: Radiation Effects Research Foundation; 2005. [Google Scholar]
- 25.Khokhryakov VV, Khokhryakov VF, Suslova KG, Vostrotin VV, Vvedensky VE, Sokolova AB, et al. Mayak worker dosimetry system 2008 (MWDS-2008): assessment of internal alpha-dose from measurement results of plutonium activity in urine. Health Phys 2013; 104: 366–78. [DOI] [PubMed] [Google Scholar]
- 26.Simon SL, Preston DL, Linet MS, Miller JS, Sigurdson AJ, Alexander BH, et al. Radiation organ doses received in a nationwide cohort of U.S. radiologic technologists: methods and findings. Radiat Res 2014; 182: 507–28. doi: 10.1667/RR13542.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Azizova TV, Bragin EV, Hamada N, Bannikova MV. Risk of cataract incidence in a cohort of Mayak PA workers following chronic occupational radiation exposure. PLoS One 2016; 11: e0164357. doi: 10.1371/journal.pone.0164357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Azizova TV, Hamada N, Grigoryeva ES, Bragin EV. Risk of various types of cataracts in a cohort of Mayak workers following chronic occupational exposure to ionizing radiation. Eur J Epidemiol 2018; 33: 1193–204. doi: 10.1007/s10654-018-0450-4 [DOI] [PubMed] [Google Scholar]
- 29.Rafnsson V, Olafsdottir E, Hrafnkelsson J, Sasaki H, Arnarsson A, et al. Cosmic radiation increases the risk of nuclear cataract in airline pilots: a population-based case-control study. Arch Ophthalmol 2005; 123: 1102–5. [DOI] [PubMed] [Google Scholar]
- 30.Little MP, Kitahara CM, Cahoon EK, Bernier M-O, Velazquez-Kronen R, Doody MM, et al. Occupational radiation exposure and risk of cataract incidence in a cohort of US radiologic technologists. Eur J Epidemiol 2018; 33: 1179–91. doi: 10.1007/s10654-018-0435-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Little MP, Cahoon EK, Kitahara CM, Simon SL, Hamada N, Linet MS.Occupational radiation exposure and excess additive risk of cataract incidence in a cohort of US radiologic technologists. Occup Environ Med 2020; 76. doi: 10.1136/oemed-2019-105902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Y, Akiba S, Sun Q. Surveys of lens opacities of residents living in high back ground radiation area in Yangjiang, Guangdong Province. Chin J Radiol Med Prot 2015; 35: 130–2. [Google Scholar]
- 33.Uwineza A, Kalligeraki AA, Hamada N, Jarrin M, Quinlan RA. Cataractogenic load – a concept to study the contribution of ionizing radiation to accelerated aging in the eye lens. Mutation Research/Reviews in Mutation Research 2019; 779: 68–81. doi: 10.1016/j.mrrev.2019.02.004 [DOI] [PubMed] [Google Scholar]
- 34.Hamada N, Sato T. Cataractogenesis following high-LET radiation exposure. Mutation Research/Reviews in Mutation Research 2016; 770: 262–91. doi: 10.1016/j.mrrev.2016.08.005 [DOI] [PubMed] [Google Scholar]
- 35.Hamada N. Ionizing radiation sensitivity of the ocular lens and its dose rate dependence. Int J Radiat Biol 2017; 93: 1024–34. doi: 10.1080/09553002.2016.1266407 [DOI] [PubMed] [Google Scholar]
- 36.Barnard SGR, McCarron R, Moquet J, Quinlan R, Ainsbury E. Inverse dose-rate effect of ionising radiation on residual 53BP1 foci in the eye lens. Sci Rep 2019; 9: 10418. doi: 10.1038/s41598-019-46893-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Neriishi K, Nakashima E, Akahoshi M, Hida A, Grant EJ, Masunari N, et al. Radiation dose and cataract surgery incidence in atomic bomb survivors, 1986–2005. Radiology 2012; 265: 167–74. doi: 10.1148/radiol.12111947 [DOI] [PubMed] [Google Scholar]
- 38.Nakashima E, Neriishi K, Minamoto A, Ohishi W, Akahoshi M. Radiation dose responses, thresholds, and false negative rates in a series of cataract surgery prevalence studies among atomic bomb survivors. Health Phys 2013; 105: 253–60. doi: 10.1097/HP.0b013e3182932e4c [DOI] [PubMed] [Google Scholar]
- 39.Azizova TV, Hamada N, Bragin EV, Bannikova MV, Grigoryeva ES. Risk of cataract removal surgery in Mayak PA workers occupationally exposed to ionizing radiation over prolonged periods. Radiat Environ Biophys 2019; 58: 139–49. doi: 10.1007/s00411-019-00787-0 [DOI] [PubMed] [Google Scholar]
- 40. ICRP . Radiosensitivity and spatial distribution of dose. In: ICRP Publication 14. Oxford, UK: Pergamon Press; 1969. [Google Scholar]
- 41.ICRP . Nonstochastic effects of ionizing radiation. ICRP publication 41. Ann ICRP 1984; 14: 1–33. [PubMed] [Google Scholar]
- 42.Hayashida T, Sasaki H, Sasaki H, Hamada N, Tatsuzaki H, Hatsusaki N, et al. Issues behind radiation management of workers at Fukushima nuclear power plant of Tokyo electric power company –From the viewpoint of radiation exposure of the ocular lens and the biological effects to the lens–. Jpn J Health Phys 2017; 52: 88–99. [Google Scholar]
- 43. ICRP . Recommendations of the International Commission on Radiological Protection. In: ICRP Publication 1. New York, USA: Pergamon Press; 1958. [Google Scholar]
- 44.Ainsbury EA, Barnard S, Bright S, Dalke C, Jarrin M, Kunze S, et al. Ionizing radiation induced cataracts: recent biological and mechanistic developments and perspectives for future research. Mutation Research/Reviews in Mutation Research 2016; 770: 238–61. doi: 10.1016/j.mrrev.2016.07.010 [DOI] [PubMed] [Google Scholar]
- 45.Fujimichi Y, Hamada N. Ionizing irradiation not only inactivates clonogenic potential in primary normal human diploid lens epithelial cells but also stimulates cell proliferation in a subset of this population. PLoS One 2014; 9: e98154. doi: 10.1371/journal.pone.0098154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hamada N. Ionizing radiation response of primary normal human lens epithelial cells. PLoS One 2017; 12: e0181530. doi: 10.1371/journal.pone.0181530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bahia S, Blais E, Murugkar S, Chauhan V, Kumarathasan P. Oxidative and nitrative stress-related changes in human lens epithelial cells following exposure to x-rays. Int J Radiat Biol 2018; 94: 366–73. doi: 10.1080/09553002.2018.1439194 [DOI] [PubMed] [Google Scholar]
- 48.Markiewicz E, Barnard S, Haines J, Coster M, van Geel O, Wu W, et al. Nonlinear ionizing radiation-induced changes in eye lens cell proliferation, cyclin D1 expression and lens shape. Open Biol 2015; 5: 150011. doi: 10.1098/rsob.150011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.von Sallmann L, Tobias CA, Anger HO, Welch C, Kimura SF, Munoz CM, et al. Effects of high-energy particles, x-rays, and aging on lens epithelium. Arch Ophthal 1955; 54: 489–514. doi: 10.1001/archopht.1955.00930020495003 [DOI] [PubMed] [Google Scholar]
- 50. NCRP . Guidance on radiation dose limits for the lens of the eye. NCRP Commentary No. 26. Maryland, USA: NCRP; 2016. [Google Scholar]
- 51.Dauer LT, Ainsbury EA, Dynlacht J, Hoel D, Klein BEK, Mayer D, et al. Guidance on radiation dose limits for the lens of the eye: overview of the recommendations in NCRP commentary No. 26. Int J Radiat Biol 2017; 93: 1015–23. doi: 10.1080/09553002.2017.1304669 [DOI] [PubMed] [Google Scholar]
- 52.Dauer LT, Hamada N, Blakely EA. National Council on radiation protection and measurements commentary number 26: impact of revised guidance on radiation protection for the lens of the eye. Journal of the American College of Radiology 2017; 14: 980–2. doi: 10.1016/j.jacr.2017.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.ICRP . Relative biological effectiveness (RBE), quality factor (Q), and radiation weighting factor (wR). ICRP Publication 92. Ann ICRP 2003; 33: 1–117. [DOI] [PubMed] [Google Scholar]
- 54.Hamada N, Fujimichi Y. Role of carcinogenesis related mechanisms in cataractogenesis and its implications for ionizing radiation cataractogenesis. Cancer Lett 2015; 368: 262–74. doi: 10.1016/j.canlet.2015.02.017 [DOI] [PubMed] [Google Scholar]
- 55.Foray N, Bourguignon M, Hamada N. Individual response to ionizing radiation. Mutation Research/Reviews in Mutation Research 2016; 770: 369–86. doi: 10.1016/j.mrrev.2016.09.001 [DOI] [PubMed] [Google Scholar]
- 56.De Stefano I, Tanno B, Giardullo P, Leonardi S, Pasquali E, Antonelli F, et al. The patched 1 tumor-suppressor gene protects the mouse lens from spontaneous and radiation-induced cataract. Am J Pathol 2015; 185: 85–95. doi: 10.1016/j.ajpath.2014.09.019 [DOI] [PubMed] [Google Scholar]
- 57.LDLensRad . Towards a full mechanistic understanding of low dose radiation induced cataracts [online];. 2017. Available from: https://www.researchgate.net/project/LDLensRad-the-European-CONCERT-project-starting-in-2017-Towards-a-full-mechanistic-understanding-of-low-dose-radiation-induced-cataracts [Accessed 20 October 2019].
- 58. NCRP . Health effects of low doses of radiation: perspectives on integrating radiation biology and epidemiology. NCRP Commentary No. 24. Maryland, USA: NCRP; 2015. [Google Scholar]
- 59.Dauer LT. Seeing through a glass darkly and taking the next right steps. Eur J Epidemiol 2018; 33: 1135–7. doi: 10.1007/s10654-018-0458-9 [DOI] [PubMed] [Google Scholar]
- 60.Sakashita T, Sato T, Hamada N. A biologically based mathematical model for spontaneous and ionizing radiation cataractogenesis. PLoS One 2019; 14: e0221579.. doi: 10.1371/journal.pone.0221579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hamada N. What are the intracellular targets and intratissue target cells for radiation effects? Radiat Res 2014; 181: 9–20. doi: 10.1667/RR13505.1 [DOI] [PubMed] [Google Scholar]
- 62.Qin Z, Zhang L, Lyu D, Li J, Tang Q, Yin H, et al. Opacification of lentoid bodies derived from human induced pluripotent stem cells is accelerated by hydrogen peroxide and involves protein aggregation. J Cell Physiol 2019; 234: 23750–62. doi: 10.1002/jcp.28943 [DOI] [PubMed] [Google Scholar]
- 63.Wako R, Yasukawa T, Kato A, Omori T, Ishida S, Ishibashi T, et al. Causes and prevalence of visual impairment in Japan. J Jpn Ophthalmol Soc 2014; 118: 495–501. [PubMed] [Google Scholar]
- 64.RNCEAB . Report by the Russian National Committee on elimination of avoidable blindness. 2014;.
- 65.Congdon N, O'Colmain B, Klaver CC, Klein R, Muñoz B, Friedman DS, et al. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol 2004; 122: 477–85. [DOI] [PubMed] [Google Scholar]
- 66.Kiuchi Y, Yokoyama T, Takamatsu M, Tsuiki E, Uematsu M, Kinoshita H, et al. Glaucoma in atomic bomb survivors. Radiat Res 2013; 180: 422–30. doi: 10.1667/RR3273.2 [DOI] [PubMed] [Google Scholar]
- 67.Itakura K, Takahashi I, Nakashima E, Yanagi M, Kawasaki R, Neriishi K, et al. Exposure to atomic bomb radiation and age-related macular degeneration in later life: the Hiroshima-Nagasaki atomic bomb Survivor study. Invest Ophthalmol Vis Sci 2015; 56: 5401–6. doi: 10.1167/iovs.15-16680 [DOI] [PubMed] [Google Scholar]
- 68.Kiuchi Y, Yanagi M, Itakura K, Takahashi I, Hida A, Ohishi W, et al. Association between radiation, glaucoma subtype, and retinal vessel diameter in atomic bomb survivors. Sci Rep 2019; 9: 8642. doi: 10.1038/s41598-019-45049-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Minamoto A, Taniguchi H, Yoshitani N, Mukai S, Yokoyama T, Kumagami T, et al. Cataract in atomic bomb survivors. Int J Radiat Biol 2004; 80: 339–45. doi: 10.1080/09553000410001680332 [DOI] [PubMed] [Google Scholar]
- 70.Little MP, Kitahara CM, Cahoon EK, Bernier MO, Velazquez-Kronen R, Doody MM, et al. Occupational radiation exposure and glaucoma and macular degeneration in the US radiologic technologists. Sci Rep 2018; 8: 10481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bragin EV, Azizova TV, Bannikova MV, Grigoryeva ES, Hamada N. Glaucoma incidence risk in a cohort of Mayak PA workers occupationally exposed to ionizing radiation. Sci Rep 2019; 9: 12469. doi: 10.1038/s41598-019-48915-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hamada N, Azizova TV, Little MP. Glaucomagenesis following ionizing radiation exposure. Mutation Research/Reviews in Mutation Research 2019; 779: 36–44. doi: 10.1016/j.mrrev.2019.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Iwase A, Suzuki Y, Araie M, Yamamoto T, Abe H, Shirato S, et al. The prevalence of primary open-angle glaucoma in Japanese: The Tajimi study. Ophthalmology 2004; 111: 1641–8. doi: 10.1016/S0161-6420(04)00665-7 [DOI] [PubMed] [Google Scholar]
- 74.Cucinotta FA, Manuel FK, Jones J, Iszard G, Murrey J, Djojonegro B, et al. Space radiation and cataracts in astronauts. Radiat Res 2001; 156: 460–6. doi: 10.1667/0033-7587(2001)156[0460:SRACIA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- 75.Chylack LT, Peterson LE, Feiveson AH, Wear ML, Manuel FK, Tung WH, et al. NASA study of cataract in astronauts (NASCA). Report 1: cross-sectional study of the relationship of exposure to space radiation and risk of lens opacity. Radiat Res 2009; 172: 10–20. doi: 10.1667/RR1580.1 [DOI] [PubMed] [Google Scholar]
- 76.Chylack LT, Feiveson AH, Peterson LE, Tung WH, Wear ML, Marak LJ, et al. NASCA report 2: longitudinal study of relationship of exposure to space radiation and risk of lens opacity. Radiat Res 2012; 178: 25–32. doi: 10.1667/RR2876.1 [DOI] [PubMed] [Google Scholar]