Abstract
Objective:
Qualitative and quantitative image analysis between Iopamidol-370 and Ioversol-320 in stents´ evaluation by coronary computed tomography angiography (CTA).
Methods:
Sixty-five patients with low-risk stable angina undergoing stent follow-up with coronary CTA were assigned to Iopamidol I-370 (n = 33) or Ioversol I-320 (n = 32) in this prospective, double-blind, non-inferiority, randomized trial. Stent lumen image quality was graded by 5-point Likert Scale. Lumen mean attenuation was measured at native coronary segments: pre-stent, post-stent, distal segments and at coronary plaques. Lumen attenuation increase (LAI) ratio was calculated for all stents. Heart rate (HR) variation, premature heart beats (PHB), heat sensation (HS), blooming and beam hardening were also assessed.
Results:
Image quality was similar between groups, with no significant difference (Likert score 4.48 ± 0.75 vs 4.54 ± 0.65, p = 0.5). There were similarities in LAI ratio between I-370 and I-320 (0.39 ± 0.42 vs 0.48 ± 0.44 HU, p = 0.08). Regarding lumen mean attenuation at native coronary segments, a significant difference was observed, with I-320 presenting lower values, including contrast mean attenuation in distal segments. After statistical multivariate analysis, three variables correlated with stent image quality: 1) stent diameter, 2) HR variation and 3) stent lumen LAI ratio.
Conclusions:
There was no significant difference between Iopamidol-370 mgI ml−1 and Ioversol-320 mgI ml−1 contrasts regarding overall stent lumen image quality, which was mainly influenced by stent diameter, HR and LAI ratio.
Advances in knowledge:
Coronary CTA allows adequate stents' visualization and image quality is influenced by stent diameter, HR variation and LAI ratio.
Stents' image quality showed no difference between different concentration contrasts (I-370 vs. I-320); however, higher concentration contrasts may provide an improved overall visualization, especially regarding coronary distal segments.
Introduction
The rate of percutaneous coronary intervention (PCI) in stable coronary artery disease (CAD) has decreased since the introduction of the Appropriateness Criteria for coronary revascularization in 20091 and also due to treatment improvements.2 However, PCI remains as the most frequently used method for revascularization, outnumbering surgery at a ratio around eight-fold. Each year, more than one million patients with CAD are treated with stent implantation.3
In those patients, coronary computed tomography angiography (CTA) is considered a reliable non-invasive diagnostic tool for stent evaluation, providing accurate information on stent lumen stenosis.4 The American Heart Association Guidelines also highlights the role of CTA for the evaluation of stent patency in a variety of clinical settings.5 Nevertheless, it is important to acknowledge the technical challenges in imaging the stent lumen by CTA. Artifacts generated by the incidence of X-rays over metal structures (blooming and beam hardening) may reduce CTA’s diagnostic accuracy, particularly in thick-strut or small diameter stents.
Among many studies that have attempted to address these issues, a recent one has demonstrated that using a higher-iodine concentration contrast (Iomeprol-400 vs Iodixanol-320) negatively affects coronary artery stent evaluation, yielding a worse image quality due to significantly higher beam hardening artifact burden.6 In daily routine, however, investigating the progression of CAD in untreated coronary segments is crucial, and for achieving optimal images in those territories, iodine concentrations higher than 320 mgI ml−1 are recommended.7 Indeed, the prevalence of higher concentration contrast usage (370 mgI ml−1 or above) reaches up to one-third in some large cohorts.8 Therefore, the current study aims to investigate a non-inferiority performance of contrast Iopamidol-370 mgI ml−1 (I-370) compared to Ioversol-320 mgI ml−1 (I-320) in the evaluation of coronary artery stents.
Methods and materials
Study design and randomization
This is a prospective, double-blind, non-inferiority, randomized trial which evaluated the enhancing effect of a 370 mgI ml−1 concentration contrast media in comparison with a less concentrated agent (320 mgI ml−1) in coronary artery stent evaluation. It is an unicenter study, performed at Sirio-Libanes Hospital, Sao Paulo – Brazil, from August 2015 to October 2016.
Enrolled population included adult patients with prior coronary stents, referred to CTA as part of their clinical care. Eligible patients were randomized in a 1:1 allocation ratio9 to receive either 370 mgI ml−1 (Isovue, Bracco Diagnostic Inc., Singen, Germany) – group I-370, or 320 mgI ml−1 (Optiray, Medtronic, São Paulo, Brazil) – group I-320. Exclusion criteria were as follows: prior surgical revascularization, impaired renal function (GFR <60 mL/min/1.73m2), history of hypersensitivity reactions to iodine contrast, pregnancy and contraindications to the use of nitroglycerine. Additionally, as severe obesity is known for degrading image quality in CTA, patients with body mass index (BMI) >40 kg/m2 were excluded as recommended by SCCT Guidelines.10 The Institutional Ethics Committee approved the study and all participants provided written informed consent.
CTA acquisition protocol
Patients with heart rate (HR) above 65 bpm received up to 100 mg oral and/or 15 mg i.v. metoprolol. All patients received 2.5 mg sublingual nitrate before scanning. An antecubital venous access in the right upper limb (18 gauge) was standard to allow 5 ml s−1 intravascular flow for all patients, followed by a 40 ml saline flush. Contrast volume was calculated individually considering the actual weight (1–2 mL/kg).
Scans were performed using a second-generation dual-source CT scanner (SOMATOM Definition Flash, Siemens Healthcare, Forchheim, Germany) with slice configuration of 256×0.6×0.3 mm, gantry rotation of 280 ms, tube potential of 120 kV, an automatic tube current selection algorithm (CAREDose 4D, Siemens Healthcare) and retrospective electrocardiogram gating (65–75% RR).
Data acquisition was performed during a single breath-hold, and the scan ranged from the level of the carina until the diaphragm. A test bolus protocol, with 10–15 ml of contrast, was used to determine optimal contrast arrival timing at the descending aorta. Radiation dose was recorded as effective dose (mSv) and size-specific dose estimate (mGy). Iterative reconstruction was applied using a pre-reconstruction filter for image smoothing and high-frequency noise reduction (Saphyre 2) for both groups, as previously described.11
Image evaluation
With the use of axial and multiplanar reconstructions, two experienced physicians (>5 years of experience) performed coronary CTA image evaluation (both qualitative and quantitative) in dedicated workstations (Aquarius versions 4.7–4.11, Terarecon Inc., San Mateo, CA, USA). Disagreements between readers were solved by consensus and used for final analysis. For image interpretation, window width of 1250 and window level of 450 HU were used.12 Information regarding stent type was unavailable, as their prior implant was performed in multiple sites.
Image quality and quantitative assessment
The primary efficacy measurement was image quality assessed by a 5-point Likert Scale: 5 = excellent (excellent attenuation of the vessel lumen and clear definition of its walls without artifacts), 4 = very good (very good attenuation of the vessel lumen, with defined walls and minimal image noise, without limitation to the diagnosis), 3 = good (slight limitation for vessel lumen attenuation and presence of more detectable noise, but without impairment of the vessel wall definition), 2 = regular (moderate limitation of vessel lumen attenuation and presence of noise that diminish image quality, with mild to moderate impairment of vessel wall definition and final diagnosis), 1 = poor (great limitation of the vessel lumen attenuation, excessive noise and poor definition of the vessel wall, with impossible diagnosis).
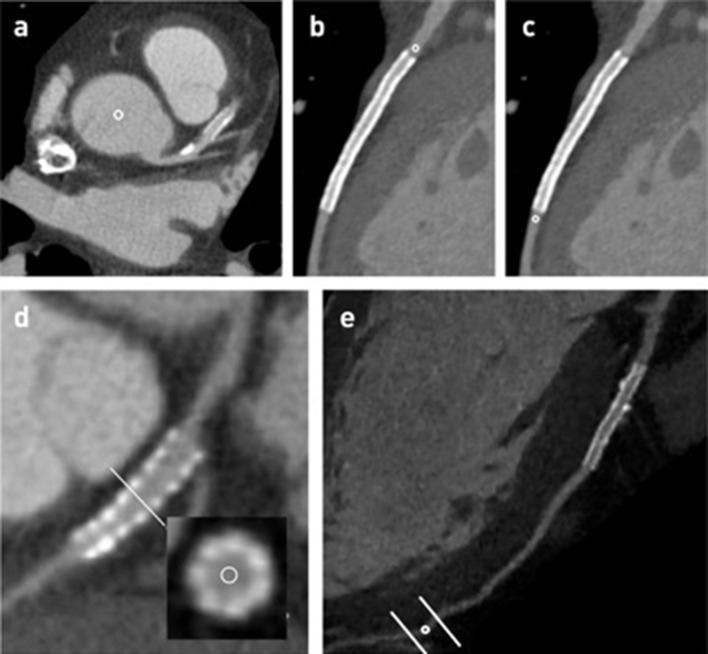
Quantitative evaluation was also performed to evaluate image quality. Lumen mean contrast attenuation measurements were performed through manually traced 1.0 mm2 regions of interest (ROI) at the aortic root, pre-stent coronary segments (defined as 1.0 mm above the stented segment), post-stent coronary segments (defined as 1.0 mm below the stented segment), stented segments, coronary plaques (defined as predominantly calcified with moderate luminal stenosis) and at distal coronary segments (defined as segments with 1.5 to 2.0 mm diameter) (Figure 1). All ROI measurements were performed in the reconstructed transversal plane. To assess high-attenuation stents, in all patients, a correction of the stent lumen mean attenuation was performed according to the pre-stent mean attenuation, as previously described (stent lumen attenuation–pre-stent lumen attenuation/pre-stent lumen attenuation), defined as LAI ratio.13
Figure 1.
Schematic ROI measurements in the aorta (a), pre-stent (b), post-stent (c), stent lumen (d) and distal coronary segment (e).
Contrast-to-noise ratio (CNR) and signal-to-noise ratio (SNR) were determined in both groups. The noise was obtained from the standard deviation (SD) of the stent lumen mean attenuation (HU), after ROI measurement. CNR was determined by dividing the mean attenuation difference between the stent lumen and myocardium measurements (obtained by individual ROIs) by the image noise. SNR was calculated by dividing stent lumen mean attenuation by the image noise.
Other measurements included the presence of stent blooming artifacts according to a 3-point scale, as previously described,14 and the presence of beam hardening. Stent lumen restenosis was assessed qualitatively (presence or absence) and confirmed by the corrected coronary opacification (CCO) difference (CCO pre-stent–CCO post-stent; CCO = coronary artery mean attenuation/descending aorta mean attenuation on the same axial section). Finally, the orthogonal wall thickness (OWT = stent external diameter - stent internal diameter / 2) was calculated in all stents, which correlates to the stent’s structure thickness and its true diameter after angioplasty, as previously reported.15
Study safety
Accordingly, any adverse event reported up to 30 minutes after the examination that had its causal relationship to the iodine contrast media was evaluated. Patient heat discomfort was assessed onsite using the Visual Analog Scale (VAS), a 0–10 point score (zero = no heat and ten = very intense heat).16
Statistical analysis
Descriptive data are expressed as mean ± standard deviation and frequency (percentage). To investigate the distribution of continuous variables, graphical evaluations were performed with QQ-plot and confirmed using the Shapiro-Wilk test. Comparisons between I-320 and I-370 groups were made by Student’s t-test (or Wilcoxon Rank-sum) and Chi-square (or Fisher’s exact) for continuous and categorical variables, respectively.
To investigate which variables (including age, sex, BMI, HR, stent diameter, OWT, tube current, contrast attenuation proximal to stent, LAI ratio, stent lumen SNR and CNR, and allocated protocol group) were associated with better image quality in the Likert scale, an ordinal logistic regression model was used. All variables that presented a level of significance <0.15 in the univariate analysis were included in the adjusted final model. Because multiple stents were examined per patient, a mixed-effects model was used for regression analysis to account for intrapatient correlation. All statistical analyses were performed using R v.3.5.3 (R Foundation for Statistical Computing, Vienna, Austria) and a P-value of <0.05 was considered statistically significant.
Results
Baseline characteristics
Sixty-five patients were randomized in the study (33 for group I-370 and 32 for I-320) with a total of 156 stents. Baseline patient and stent characteristics are shown in Tables 1 and 2, respectively. There were no clinical differences between groups. Mean acquisition HR was similar (55 ± 5 bpm for I-370 vs 57 ± 6 bpm for I-320, p = 0.24). Nearly half of stents were located at left anterior descending coronary artery (LAD) in both groups, being the vast majority ≥3 mm (Table 2). Neither stent size nor OWT were significantly different between groups. Visually, no patient presented stent stenosis, which was confirmed by CCO measurements.
Table 1.
Patient baseline characteristics
| I-370 (n = 33) |
I-320 (n = 32) |
p-value | |
|---|---|---|---|
| Demographics | |||
| Age, years | 64 ± 8 | 62 ± 11 | 0.58 |
| Male, n (%) | 30 (91) | 26 (81) | 0.44 |
| BMI, kg/m2 | 28 ± 3 | 27 ± 4 | 0.54 |
| Cardiovascular risk factors | |||
| Hypertension, n (%) | 18 (55) | 20 (63) | 0.69 |
| Dyslipidemia, n (%) | 11 (33) | 15 (47) | 0.39 |
| Diabetes, n (%) | 9 (27) | 7 (22) | 0.83 |
| Smoking, n (%) | 6 (18) | 3 (9) | 0.47 |
| FHx-CAD, n (%) | 20 (61) | 13 (41) | 0.17 |
| Acquisition parameters | |||
| HR, bpm | 55 ± 5 | 57 ± 6 | 0.24 |
| HR <65 bpm | 33 (100) | 31 (97) | 0.98 |
| PHB, n (%) | 2 (6) | 0 (0) | 0.49 |
| Tube voltage, kV | 120 | 120 | - |
| Tube current, mA | 404 ± 173 | 341 ± 67 | 0.33 |
| VAS for heat sensation | 5.7 ± 1.7 | 4.9 ± 2.1 | 0.11 |
| Dose-length product, mGy | 413.2 ± 133.8 | 409.9 ± 108.9 | 0.91 |
BMI, body mass index; FHX-CAD, family history of early coronary artery disease;HR, heart rate; PHB, premature heartbeats; VAS, visual analog scale; bpm, beats per minute.
Values are given as mean ± SD or absolute numbers and percentages.
Table 2.
Stent characteristics, quantitative analysis and image quality
| I-370 (n = 71) |
I-320 (n = 85) |
p-value | |
|---|---|---|---|
| Stent diameter, mm | 3.31 ± 0.53 | 3.34 ± 0.52 | 0.78 |
| Stent diameter ≥3 mm, n (%) | 62 (87) | 76 (89) | 0.88 |
| OWT, mm | 0.63 ± 0.22 | 0.59 ± 0.23 | 0.19 |
| Stent location | 0.65 | ||
| LAD, n (%) | 36 (51) | 38 (44) | |
| CX, n (%) | 17 (24) | 20 (24) | |
| RCA, n (%) | 18 (25) | 27 (32) | |
| CCO difference | 0.14 ± 0.17 | 0.17 ± 0.22 | 0.33 |
| Stent lumen quantitative analysis | |||
| Mean attenuation, HU | 523.7 ± 140 | 508.8 ± 149.5 | 0.56 |
| Myocardial attenuation, HU | 73.5 ± 8 | 70.6 ± 6.5 | 0.014 |
| Noise, HU | 41 ± 18 | 49.7 ± 20 | 0.002 |
| SNR | 16.9 ± 7.9 | 14.4 ± 10.8 | 0.006 |
| CNR | 14.2 ± 6.9 | 12.3 ± 9.4 | 0.006 |
| LAI ratio | 0.39 ± 0.42 | 0.48 ± 0.44 | 0.08 |
| Reference vessel | |||
| Aortic root, HU | 387.7 ± 53.8 | 343.2 ± 68.5 | <0.001 |
| Pre-stent, HU | 384.1 ± 63.6 | 348.1 ± 61.4 | <0.001 |
| Pre-stent <400, HU | 48 (67) | 48 (76) | 0.29 |
| Post-stent, HU | 329.1 ± 90.6 | 291.3 ± 103.1 | 0.001 |
| Stent lumen image quality | |||
| Likert ≥ 4, n (%) | 62 (87) | 77 (90) | 0.71 |
| Likert | 4.5 ± 0.8 | 4.5 ± 0.7 | 0.50 |
CCO, corrected coronary opacification; CNR, contrast-to-noise ratio; CX, left circumflex artery; LAD, left anterior descending coronary artery; LAI, lumen attenuation increase; RCA, right coronary artery; SNR, signal-to-noise ratio.
Values are given as mean ± SD or absolute numbers and percentages.
LAD, CX and RCA branches were attributed to the main artery.
No contrast extravasations occurred and no adverse reactions on the two iodine concentration contrast groups were reported. VAS heat sensation between groups was not different (5.8 ± 1.7 for I-370 vs 4.9 ± 2.1 for I-320, p = 0.11).
Image quality
There was no significant difference between groups I-370 and I-320 in the qualitative evaluation (Likert scale: Likert ≥ 4 in 90% for I-370 vs 87% for I-320, p = 0.71; and mean 4.5 ± 0.8 vs 4.5 ± 0.7, p = 0.5) (Table 2). Only one-third of stents presented mild blooming and 2% presented beam hardening artifacts, with no statistical difference between groups. There were 11 disagreements (<1%) between the two readers, all solved by consensus.
Although I-370 generated significantly higher mean attenuation values in pre-stent than I-320, in most cases of both groups this value was <400 HU (67% vs 76%, p = 0.29). Stent lumen SNR and CNR were higher in I-370, given its higher values for stent lumen mean attenuation and lower noise when compared to I-320 (Table 2).
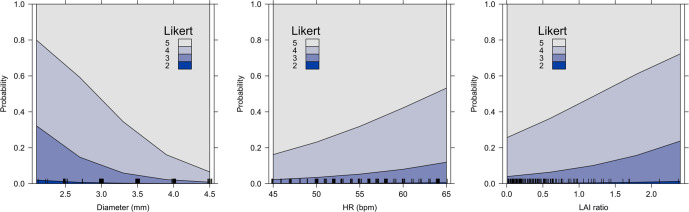
Variables that influenced image quality (univariate analysis) were stent diameter, tube current, HR and LAI ratio. In the multivariate analysis (Table 3), stent diameter, HR and LAI ratio remained significantly associated with image quality. Adjusted effects of these three variables on image quality can be seen in Figure 2.
Table 3.
Variables associated with image quality
| Variables | Univariate Ordinal Regression | Multivariate Ordinal Regression | ||
|---|---|---|---|---|
| Proportional Odds (95% CI) |
p-value | Proportional Odds (95% CI) |
p-value | |
| Age, years | 1.02 (0.98–1.04) | 0.30 | ||
| Male | 0.51 (0.18–1.30) | 0.16 | ||
| BMI, kg/m2* | 0.91 (0.82–1.00) | 0.06 | 1.15 (0.62–2.17) | 0.34 |
| HR, bpm* | 0.51 (0.37–0.69) | <0.001 | 0.64 (0.43–0.94) | 0.02 |
| I-370 protocol | 0.84 (0.44–1.60) | 0.61 | ||
| Diameter, mm | 7.93 (3.72–18.3) | <0.001 | 5.39 (2.38–13.2) | <0.001 |
| OWT | 0.84 (0.44–1.60) | 0.81 | ||
| Tube current, mA† | 0.82 (0.72–0.92) | 0.001 | 0.90 (0.76–1.07) | 0.25 |
| Mean attenuation proximal to stent, HU† | 1.06 (0.83–1.35) | 0.63 | ||
| LAI ratio | 0.18 (0.08–0.38) | <0.001 | 0.42 (0.18–0.99) | 0.04 |
| Stent lumen SNR, HU† | 1.81 (0.31–12.4) | 0.51 | ||
| Stent lumen CNR, HU† | 1.39 (0.19–12.3) | 0.74 | ||
BMI, body mass index; PHB, premature heartbeats; bpm, beats per minute.
Values are given as absolute numbers and ranges.
Only relevant variables were eligible for the multivariable regression model.
* every 5-unit increase; † every 50-unit increase.
Figure 2.
Effect plots of the association between stent diameter, LAI ratio and heart rate with image quality. Plot displays the stacked effect for the proportional odds from the multivariable ordinal logistic regression model, showing probabilities across the response categories.
Native coronary tree contrast enhancement analysis
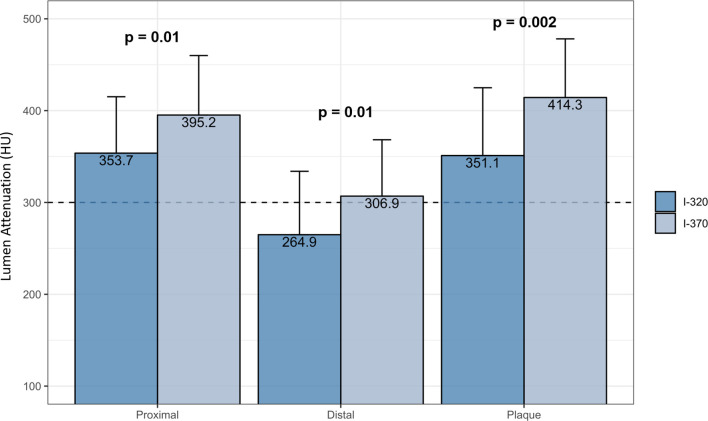
In coronary plaques, lumen mean attenuation was higher in group I-370 compared to I-320 (414.3 vs 351.1, p = 0.002). Group I-370 provided significantly higher lumen mean attenuation in distal segments compared to I-320 (306.9 ± 61.4 vs 264.9 ± 69.1, p = 0.01) (Figure 3).
Figure 3.
Lumen mean attenuation in coronary plaques, proximal (pre-stent) and distal coronary segments (diameter <2 mm) according to contrast type. Error bars indicate standard deviation of the mean attenuation.
Discussion
For the first time, this study demonstrated the non-inferiority of Iopamidol 370 mgI ml−1 in providing evaluable CT scans for assessment of coronary artery stents, as compared to Ioversol 320 mgI ml−1. Both contrasts provided adequate interpretation of stent lumen, with the majority presenting Likert scale 4 to 5. Stent size followed by HR and LAI ratio were the factors associated with image quality. Considering the native coronary tree, I-370 yielded a significantly higher mean attenuation, particularly in distal segments, than I-320.
A previous large trial with 234 patients showed that Ioversol-320 provides significantly higher stent evaluability than Iomeprol-400 given the lower number of severe beam hardening artifacts produced by the former.6 In the current study, the effects of I-370 and I-320 (injected at same flow rate) were similar, with no differences in image quality, number of artifacts or patient safety. As reported by those authors, contrast density in the adjacent coronary segment higher than 400 HU was one of the main predictors of poor stent lumen image quality. This explains in part our results once the pre-stent attenuation was less than 400HU in most cases of both I-370 and I-320 in our study. Indeed, regarding the quantitative assessment of image quality, in accordance with a previous report17 of improved assessment of stent restenosis,I-370 presented significantly less noise and higher SNR/CNR values than I-320. This difference in noise between contrasts might be due to beam hardening, which influence stent lumen mean attenuation, what may be linked to stents struts.18
Furthermore, Cademartiri et al19 observed that higher coronary attenuation significantly improved diagnostic performance in both proximal and distal segments, the greatest benefit was seen in distal segments. In agreement, Christensen et al20 found that attenuations greater than 300 HU would be more accurate for the evaluation of coronary distal segments. In the present study, the mean attenuation observed in distal segments (<2 mm) with I-320 was noticeably below than the desirable 300 HU to accurately evaluate them. These findings may have clinical implications. Firstly, stented patients are likely to have diffuse CAD, not sparing distal segments. Secondly, many evidences have highlighted the importance of the identification of high-risk plaque features and the extension of the CAD as markers of adverse prognosis. In such clinical scenarios, therefore, the use of low-iodine concentration contrast media may impair an adequate lumen interpretation particularly in distal segments.
In line with a significant number of studies, the factors associated with poor image quality in this study were stents´ lumen with a smaller diameter, higher HR variability and higher LAI ratio. Indeed, artifacts which arise from metallic stent struts (particularly in stents with a smaller lumen diameter) are likely to influence accuracy. Graaf et al,21 using 320-slice CTA evaluation in 53 patients with 89 metallic stents in vessels with diameter under 3 mm, demonstrated a reduced specificity of this method in assessing the incidence of stent lumen restenosis. A lower HR variability (<5 bpm during acquisition) also correlates with better image quality.6,20,22–24 Present reports regarding the prevalence of HR variability among different iodine concentration contrasts are conflicting. Some studies have demonstrated reduced HR variability when using lower concentration iodine contrasts.6,22,23 However, other studies20,25 demonstrated no significant difference between contrasts with different iodine concentrations regarding HR variability, what is in accordance with our findings. Finally, LAI ratio is useful to assess high-attenuation stents, which is a measurement mostly related to beam hardening artifacts.26,27 In fact, the lower LAI ratio, the better the image quality for this location (Figure 3) in quantitative analysis. In the current study, similar values of mean stent lumen attenuation were observed between the two contrasts evaluated (523.7 ± 140 vs. 508.8 ± 149.5, p = 0.56), which correlates with qualitative image analysis without difference among groups.
The following study limitations are acknowledged. Firstly, there was no information regarding stents’ type (technical specifications, polymer composition, diameter and geometrical structure), as most patients do not provide reliable information in clinical practice routine. Therefore, this trial could not account potential differences in stents’ type between contrast groups, what might have had an additional impact on image quality. However, in the study of Cui et al,28 true in vivo stent diameter relied on vessel shape and dilatation pressure during angioplasty (with possible retraction after placement), not necessarily corresponding to the nominal stent diameter provided by the manufacturer. Considering this, orthogonal stent wall thickness was calculated as in a previous study,15 whose measure is a reliable surrogate of the true in vivo stent wall thickness, and indirectly provides the actual diameter of the placed stent. Secondly, stent lumen restenosis was absent, which is aligned with its low prevalence in the literature.29,30 At last, a relatively small sample was included. Certainly, future studies with a larger patient population are needed to reinforce data about stent lumen attenuation and image quality.
Conclusions
There was no significant difference between Iopamidol 370 mgI ml−1 and Ioversol 320 mgI ml−1 contrasts regarding overall stent lumen image quality, which was mainly influenced by stent diameter, HR and LAI ratio. The contrast with higher iodine concentration may provide a better overall visualization given its effects on native coronary tree, with less noise, especially in distal coronary segments.
Footnotes
Acknowledgment: We thank Dr Theresa Cristina de Carvalho Costa (M.D) and Dr Renata Magalhães Pinheiro (M.D), residents at the Department of Cardiovascular Imaging at the time this study was designed, for assistance with patient inclusion. We also thank the nursing and biomedical team from Sirio-Libanes Hospital for their assistance in explaining the Consent Form to patients and masterfully performing the tests.
Competing interests: The authors declare that they have no competing interests.
Funding: This research did not receive any specific grant from funding agencies in the public, commercial, or not-forprofit sectors.
Patient consent: The Institutional Ethics Commitee approved the study and all patients provided written informed consent.
Ethics approval and consent to participate: This project was clearly described and outlined, with documentation presented in accordance with current legislation and institutional terms. It was registered at CEPesq as HSL 2015–91 and was approved according to the version sent (Stent Project and Free and Informed Consent Term, Version 2.0 of September 2015).
Availability of data and materials: All data generated or analyzed during this study are included in this published article.
Contributor Information
Annelisa Moura Garcia, Email: annelisam@gmail.com.
Antonildes N. Assunção-Jr, Email: antonildes.nassuncao@hsl.org.br.
Roberto Nery Dantas-Jr, Email: roberto.ndjunior@hsl.org.br.
Jose Rodrigues Parga, Email: jose.parga@hsl.org.br.
Fernando Ganem, Email: fernando.ganem@hsl.org.br.
REFERENCES
- 1.Patel MR, Dehmer GJ, Hirshfeld JW, Smith PK. Spertus JA. ACCF/SCAI/STS/AATS/AHA/ASNC 2009 appropriateness criteria for coronary revascularization. Circulation 2009; 119: 1330–52. [DOI] [PubMed] [Google Scholar]
- 2.Desai NR, Bradley SM, Parzynski CS, Nallamothu BK, Chan PS, Spertus JA, et al. Appropriate use criteria for coronary revascularization and trends in utilization, patient selection, and appropriateness of percutaneous coronary intervention. JAMA 2015; 314: 2045–53. doi: 10.1001/jama.2015.13764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blumenfeld O, Na'amnih W, Shapira‐Daniels A, Lotan C, Shohat T, Shapira OM. Trends in coronary revascularization and ischemic heart Disease–Related mortality in Israel. J Am Heart Assoc 2017; 6: 1–9. doi: 10.1161/JAHA.116.004734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dai T, Wang J-R, Hu P-F, rong WJ, fei HP. Diagnostic performance of computed tomography angiography in the detection of coronary artery in-stent restenosis: evidence from an updated meta-analysis. Eur Radiol 2018; 28: 1373–82. doi: 10.1007/s00330-017-5097-0 [DOI] [PubMed] [Google Scholar]
- 5., Mark DB, Berman DS, Budoff MJ, Carr JJ, Gerber TC, et al.American College of Cardiology Foundation Task Force on Expert Consensus Documents . ACCF/ACR/AHA/NASCI/SAIP/SCAI/SCCT 2010 expert consensus document on coronary computed tomographic angiography: a report of the American College of cardiology Foundation Task force on expert consensus documents. J Am Coll Cardiol 2010; 55: 2663–99. doi: 10.1016/j.jacc.2009.11.013 [DOI] [PubMed] [Google Scholar]
- 6.Andreini D, Pontone G, Mushtaq S, Bartorelli AL, Conte E, Bertella E, et al. Coronary stent evaluation with coronary computed tomographic angiography: comparison between low-osmolar, high-iodine concentration iomeprol-400 and iso-osmolar, lower-iodine concentration iodixanol-320. J Cardiovasc Comput Tomogr 2014; 8: 44–51. doi: 10.1016/j.jcct.2013.12.003 [DOI] [PubMed] [Google Scholar]
- 7.Abbara S, Blanke P, Maroules CD, Cheezum M, Choi AD, Han BK, et al. SCCT guidelines for the performance and acquisition of coronary computed tomographic angiography: a report of the Society of cardiovascular computed tomography guidelines Committee: endorsed by the North American Society for cardiovascular imaging (NASCI. J Cardiovasc Comput Tomogr [Internet] 2016; 10: 435–49Available from. [DOI] [PubMed] [Google Scholar]
- 8.Cha MJ, Kang DY, Lee W, Yoon SH, Choi YH, Byun JS, et al. Hypersensitivity reactions to iodinated contrast media: a multicenter study of 196 081 patients. Radiology 2019; 293: 117–24. doi: 10.1148/radiol.2019190485 [DOI] [PubMed] [Google Scholar]
- 9.No Title.. Available from: www.randomization.com.
- 10.Taylor AJ, Cerqueira M, Hodgson JM, Mark D, Min J, O'Gara P, et al.(eds);;e33 ACCF/SCCT/ACR/AHA/ASE/ASNC/NASCI/SCAI/SCMR 2010 appropriate use criteria for cardiac computed tomography. J Cardiovasc Comput Tomogr 2010; 4: 407.e1–407.e33. doi: 10.1016/j.jcct.2010.11.001 [DOI] [PubMed] [Google Scholar]
- 11.Yang J, Yang X, De Cecco CN, Duguay TM, Chen Z, Tesche C, et al. Iterative reconstruction improves detection of in-stent restenosis by high-pitch dual-source coronary CT angiography. Sci Rep 2017; 7: 1–8. doi: 10.1038/s41598-017-07499-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gao Y, Lu B, Hou ZH, Yu FF, Yin WH, Wang ZQ, et al. Coronary in-stent restenosis: assessment with corrected coronary opacification difference across coronary stents measured with CT angiography. Radiology 2015; 275: 403–12. doi: 10.1148/radiol.14140820 [DOI] [PubMed] [Google Scholar]
- 13.Horiguchi J, Fujioka C, Kiguchi M, Yamamoto H, Kitagawa T, Kohno S, et al. Prospective ECG-triggered axial CT at 140-kV tube voltage improves coronary in-stent restenosis visibility at a lower radiation dose compared with conventional retrospective ECG-gated helical CT. Eur Radiol 2009; 19: 2363–72. doi: 10.1007/s00330-009-1419-1 [DOI] [PubMed] [Google Scholar]
- 14.Scheffel H, Stolzmann P, Schlett CL, Engel L-C, Major GP, Károlyi M, et al. Coronary artery plaques: cardiac CT with model-based and adaptive-statistical iterative reconstruction technique. Eur J Radiol 2012; 81: e363–9. doi: 10.1016/j.ejrad.2011.11.051 [DOI] [PubMed] [Google Scholar]
- 15.Tan S, Soulez G, Diez Martinez P, Larrivée S, Stevens L-M, Goussard Y, et al. Coronary Stent Artifact Reduction with an Edge-Enhancing Reconstruction Kernel - A Prospective Cross-Sectional Study with 256-Slice CT. PLoS One 2016; 11: e0154292. doi: 10.1371/journal.pone.0154292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Svensson A, Ripsweden J, Rück A, Aspelin P, Cederlund K, Brismar BT. Heart rate variability and heat sensation during CT coronary angiography: Low-osmolar versus iso-osmolar contrast media. Acta Radiol 2010; 51: 722–6. doi: 10.3109/02841851.2010.488249 [DOI] [PubMed] [Google Scholar]
- 17.Schepis T, Koepfli P, Leschka S, Desbiolles L, Husmann L, Gaemperli O, et al. Coronary artery stent geometry and in-stent contrast attenuation with 64-slice computed tomography. Eur Radiol 2007; 17: 1464–73. doi: 10.1007/s00330-006-0502-0 [DOI] [PubMed] [Google Scholar]
- 18.Boas FE, Fleischmann D. Ct artifacts: causes and reduction techniques. Imaging Med 2012; 4: 229–40. doi: 10.2217/iim.12.13 [DOI] [Google Scholar]
- 19.Cademartiri F, Maffei E, Palumbo AA, Malagò R, La Grutta L, Meiijboom WB, et al. Influence of intra-coronary enhancement on diagnostic accuracy with 64-slice CT coronary angiography. Eur Radiol 2008; 18: 576–83. doi: 10.1007/s00330-007-0773-0 [DOI] [PubMed] [Google Scholar]
- 20.Christensen JD, Meyer LT, Hurwitz LM, Boll DT. Effects of iopamidol-370 versus iodixanol-320 on coronary contrast, branch depiction, and heart rate variability in dual-source coronary MDCT angiography. AJR Am J Roentgenol 2011; 197: W445–51. doi: 10.2214/AJR.10.6154 [DOI] [PubMed] [Google Scholar]
- 21.de Graaf FR, van Velzen JE, Witkowska AJ, Schuijf JD, van der Bijl N, Kroft LJ, et al. Diagnostic performance of 320-slice multidetector computed tomography coronary angiography in patients after coronary artery bypass grafting. Eur Radiol 2011; 21: 2285–96. doi: 10.1007/s00330-011-2192-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choi T-Y, Woo V, Gupta M, Sourayanezhad S, Li D, Mao SS, et al. Comparison of iodixanol 320 and iohexol 350 in image quality during 64-slice multidetector computed tomography: prospective randomized study. Int J Cardiol 2012; 158: 134–8. doi: 10.1016/j.ijcard.2012.04.038 [DOI] [PubMed] [Google Scholar]
- 23.Honoris L, Zhong Y, Chu E, Rosenthal D, Li D, Lam F, et al. Comparison of contrast enhancement, image quality and tolerability in coronary CT angiography using 4 contrast agents: a prospective randomized trial. Int J Cardiol 2015; 186: 126–8. doi: 10.1016/j.ijcard.2015.03.240 [DOI] [PubMed] [Google Scholar]
- 24.Becker CR, Vanzulli A, Fink C, de Faveri D, Fedeli S, Dore R, et al. Multicenter comparison of high concentration contrast agent Iomeprol-400 with iso-osmolar Iodixanol-320. Invest Radiol 2011; 46: 457–64. doi: 10.1097/RLI.0b013e31821c7ff4 [DOI] [PubMed] [Google Scholar]
- 25.Bamberg F, Becker A, Schwarz F, Marcus RP, Greif M, von Ziegler F, et al. Detection of hemodynamically significant coronary artery stenosis: incremental diagnostic value of dynamic CT-based myocardial perfusion imaging. Radiology 2011; 260: 689–98 10.1148/radiol.11110638 [DOI] [PubMed] [Google Scholar]
- 26.Mahnken AH. Ct imaging of coronary stents: past, present, and future. ISRN Cardiol 2012; 2012: 1–12. doi: 10.5402/2012/139823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stehli J, Fuchs TA, Singer A, Bull S, Clerc OF, Possner M, et al. First experience with single-source, dual-energy CCTA for monochromatic stent imaging. Eur Heart J Cardiovasc Imaging 2015; 16: 507–12. doi: 10.1093/ehjci/jeu282 [DOI] [PubMed] [Google Scholar]
- 28.Cui X, Li T, Li X, Zhou W. High-Definition computed tomography for coronary artery stents imaging: initial evaluation of the optimal reconstruction algorithm. Eur J Radiol 2015; 84: 834–9. doi: 10.1016/j.ejrad.2015.02.004 [DOI] [PubMed] [Google Scholar]
- 29.Carrabba N, Schuijf JD, de Graaf FR, Parodi G, Maffei E, Valenti R, et al. Diagnostic accuracy of 64-slice computed tomography coronary angiography for the detection of in-stent restenosis: a meta-analysis. J Nucl Cardiol 2010; 17: 470–8. doi: 10.1007/s12350-010-9218-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Andreini D, Pontone G, Mushtaq S, Bartorelli AL, Bertella E, Trabattoni D, et al. Coronary in-stent restenosis: assessment with CT coronary angiography. Radiology 2012; 265: 410–7. doi: 10.1148/radiol.12112363 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Availability of data and materials: All data generated or analyzed during this study are included in this published article.