Abstract
Objective
Particle radiobiology has contributed new understanding of radiation safety and underlying mechanisms of action to radiation oncology for the treatment of cancer, and to planning of radiation protection for space travel. This manuscript will highlight the significance of precise physical and biologically effective dosimetry to this translational research for the benefit of human health.
This review provides a brief snapshot of the evolving scientific basis for, and the complex current global status, and remaining challenges of hadron therapy for the treatment of cancer. The need for particle radiobiology for risk planning in return missions to the Moon, and exploratory deep-space missions to Mars and beyond are also discussed.
Methods
Key lessons learned are summarized from an impressive collective literature published by an international cadre of multidisciplinary experts in particle physics, radiation chemistry, medical physics of imaging and treatment planning, molecular, cellular, tissue radiobiology, biology of microgravity and other stressors, theoretical modeling of biophysical data, and clinical results with accelerator-produced particle beams.
Results
Research pioneers, many of whom were Nobel laureates, led the world in the discovery of ionizing radiations originating from the Earth and the Cosmos. Six radiation pioneers led the way to hadron therapy and the study of charged particles encountered in outer space travel. Worldwide about 250,000 patients have been treated for cancer, or other lesions such as arteriovenous malformations in the brain between 1954 and 2019 with charged particle radiotherapy, also known as hadron therapy. The majority of these patients (213,000) were treated with proton beams, but approximately 32,000 were treated with carbon ion radiotherapy. There are 3500 patients who have been treated with helium, pions, neon or other ions. There are currently 82 facilities operating to provide ion beam clinical treatments. Of these, only 13 facilities located in Asia and Europe are providing carbon ion beams for preclinical, clinical, and space research. There are also numerous particle physics accelerators worldwide capable of producing ion beams for research, but not currently focused on treating patients with ion beam therapy but are potentially available for preclinical and space research. Approximately, more than 550 individuals have traveled into Lower Earth Orbit (LEO) and beyond and returned to Earth.
Conclusion
Charged particle therapy with controlled beams of protons and carbon ions have significantly impacted targeted cancer therapy, eradicated tumors while sparing normal tissue toxicities, and reduced human suffering. These modalities still require further optimization and technical refinements to reduce cost but should be made available to everyone in need worldwide. The exploration of our Universe in space travel poses the potential risk of exposure to uncontrolled charged particles. However, approaches to shield and provide countermeasures to these potential radiation hazards in LEO have allowed an amazing number of discoveries currently without significant life-threatening medical consequences. More basic research with components of the Galactic Cosmic Radiation field are still required to assure safety involving space radiations and combined stressors with microgravity for exploratory deep space travel.
Advances in knowledge
The collective knowledge garnered from the wealth of available published evidence obtained prior to particle radiation therapy, or to space flight, and the additional data gleaned from implementing both endeavors has provided many opportunities for heavy ions to promote human health.
Introduction
The history of the discovery of X-rays, spontaneous radioactivity, and the electron, proton, α particle, β rays and neutrons are indelibly preserved in the record of Nobel Prizes in Physics and Chemistry (Figure 1) awarded to the pioneers of ground-based radiation research, including W.C. Roentgen, Pierre and Marie Curie, J.J. Thomson, and Sir James Chadwick (The Nobel Prize in Physics 1901–2000. NobelPrize.org. Nobel Media AB 2019. Tue. 17 Dec 2019. https://www.nobelprize.org/prizes/themes/the-nobel-prize-in-physics-1901-2000; https://www.nobelprize.org/prizes/lists/all-nobel-prizes-in-chemistry.) Victor Francis Hess’ high flying balloon studies in 1912 independently extended our understanding of sources of radiation when he revealed the existence of cosmic rays in the upper atmosphere of the Earth for which he was awarded the 1936 Nobel Prize in Physics.
Figure 1.
Research pioneers involved in the discovery of radiation. Wilhelm Conrad Roentgen discovered X-rays in 1895 and received the 1901 first Nobel Prize in Physics. Henry Becquerel, and Pierre and Marie Curie discovered radioactivity in 1897, and received the 1903 third Nobel Prize in Physics. John Joseph Thomson discovered the electron in 1897, and received the 1906 Nobel Prize in Physics, Ernest Rutherford discovered the proton, α particle and β rays in 1919 and was awarded the 1908 Nobel Prize in Physics, Sir James Chadwick discovered the neutron in 1932, and received the 1935 Nobel Prize in Physics, and Victor Francis Hess discovered cosmic rays in 1912 and received the 1936 Nobel Prize in Physics. Images are from (https://en.wikipedia.org/wiki/List_of_Nobel_laureates_in_Physics;https://en.wikipedia.org/wiki/List_of_Nobel_laureates_in_Chemistry).
The challenge to create a ground-based laboratory source of charged particle beams for research investigations led to Ernest Orlando Lawrence’s invention of the cyclotron which could accelerate protons to 80,000 volts, using less than 1000 volts. and the 1939 Nobel prize in Physics (Accelerators and Nobel Laureates. NobelPrize.org. Nobel Media AB 2019. Tue. 17 Dec 2019. https://www.nobelprize.org/prizes/themes/accelerators-and-nobel-laureates). This led to a new generation of radiation pioneers for hadron therapy (Figure 2). As accelerators of increasing diameter sizes became available, they permitted the evaluation of ion beams in physics laboratories to confirm the principles of Sir William Henry Bragg’s first reported Bragg Curve. This depth–dose profile of initially high energy ions, but with low dose energy absorption from stopping low energy charged ions, culminates in a Bragg Peak of ionizations at the maximum particle stopping depth of penetration. It led Robert R.Wilson to first propose the use of the Bragg peak for radiotherapy with protons,1 and that forged the path forward for hadron radiotherapy.
Figure 2.
Radiation pioneers for hadron therapy. Ernest O. Lawrence invented the cyclotron in 1931 and received the 1939 Nobel Prize in Physics. Sir William Henry Bragg first reported the Bragg Curve in 1903, Louis Harold Gray, the Father of Radiobiology, developed the Bragg-Gray equation and the concept of Relative Biological Effectiveness in 1940 and discovered the role of oxygen in radiation effects on tumor cells in 1952, and discovered the hydrated electron 1962. Robert R. Wilson first proposed the use of the Bragg peak for radiation therapy in 1946. John H. Lawrence was the Father of Nuclear Medicine and treated the first patient with proton beams in 1954, Cornelius A. Tobias, the Father of Heavy Ion Radiobiology, investigated the biological effects of protons and heavy ions. Images are from: Accelerators and Nobel Laureates. NobelPrize.org. Nobel Media AB 2019. Tue. 17 Dec 2019. https://www.nobelprize.org/prizes/themes/accelerators-and-nobel-laureatesand the University of California, Berkeley Lawrence and the Lawrence Berkeley National Laboratory photo archives.
Radiobiology (the study of radiation effects on living cells and tissues) was pioneered by Louis Harold Gray a physicist, who built neutron generators, and developed both the Bragg-Gray equation, and the concept of relative biological effectiveness (RBE). He established the Gray Laboratory at Mount Vernon Hospital in London in 1953 to focus on the role of oxygen in radiation effects on tumor cells. Gray observed that tumor cells were hypoxic, contributing to their X-ray radioresistance2 ; however, if irradiated with densely ionizing neutrons, the tumors were eradicated with less dependence on their oxygen status. Gray’s work advanced our understanding of the biological effects of densely ionizing radiations. He also developed pulse radiolysis and discovered the hydrated electron. These many significant contributions to the radiation sciences led in 1975 to the naming of the derived unit of ionizing radiation dose in the International System of Units (SI) as the gray (Gy), defined as the absorption of one joule of radiation energy per kilogram of matter. Hal Gray has therefore been considered the Father of Radiobiology.
First human trials with heavy ions
The dose penetrating absorption profile of neutrons was not ideal for sparing radiation damage to normal tissues overlying tumors, but the Bragg peak of stopping accelerated charged particles provided the additional targeting feature of increased dose absorption at depth. Ernest Orlando Lawrence and his brother John Hundale Lawrence dedicated the Donner Laboratory at the University of California, Berkeley in 1941 to apply physics, chemistry and the natural sciences to Biology and Medicine. John Lawrence and CorneliusTobias led early radiobiological investigations of protons, deuterons and helium ion beams, culminating in the first human exposure to accelerated protons in September 1954.3 Tobias is considered the Father of Heavy-Ion Radiobiology.
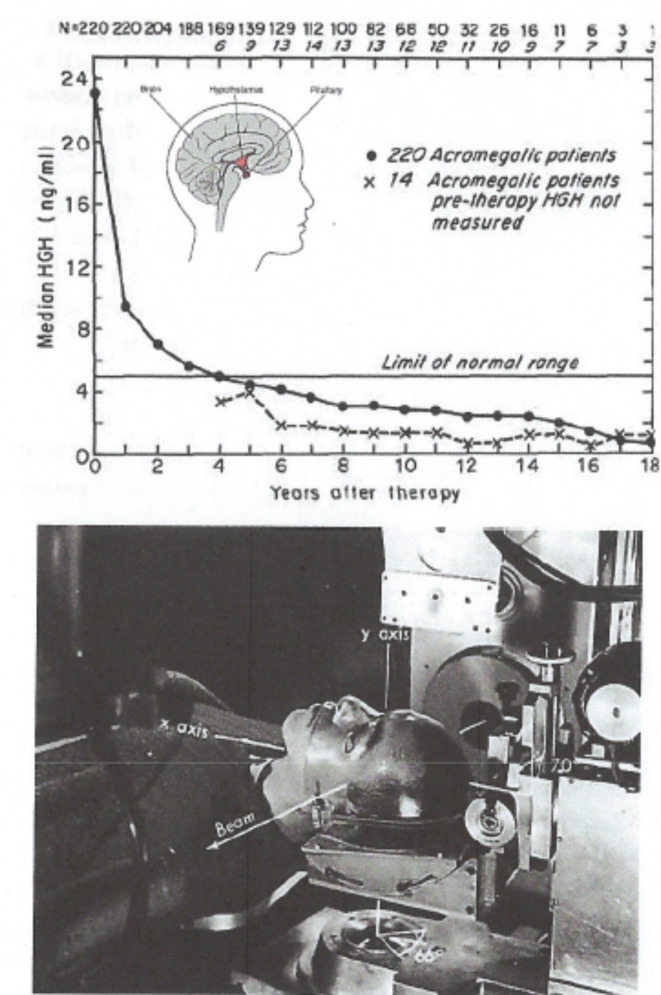
Radiosurgery of the pituitary gland was an early successful application of this new technology. The pituitary, located in the brain between the hypothalamus and the pineal gland, is about the size of a pea and hangs down from the brain by a thin stem of blood vessels and nerve cell projections that lies just over the roof of your mouth and controls multiple endocrine functions (Figure 3A insert). Acromegaly is a condition where an abnormal growth of the hands, feet, and face occurs, caused by overproduction of growth hormone by the pituitary gland, usually due to a benign adenoma. Initially 30 patients were treated with proton beams, but subsequently 820 patients were treated with high-energy plateau helium ions in 3–4 fractions over 5 days. An 18-year follow-up study that measured serum levels of human growth hormone (HGH) levels in a cohort of these patients has documented the successful control of the tumor and reduction of the abnormally high HGH levels, with focal necrosis or nerve injury in only 1% of the population4 (Figure 3B).
Figure 3.
Charged particle radiosurgery of the pituitary gland. (A) Late follow-up effects 18 years after treatment showing serum levels of human growth factor as evidence of tumor eradication. (B). Photograph of patient treatment set-up for helium-ion radiosurgery. Republished with permission of Karger Publisher, Basel, Switzerland from Levy, et al.,Copyright©19914; permission conveyed through Copyright Clearance Center, Inc.
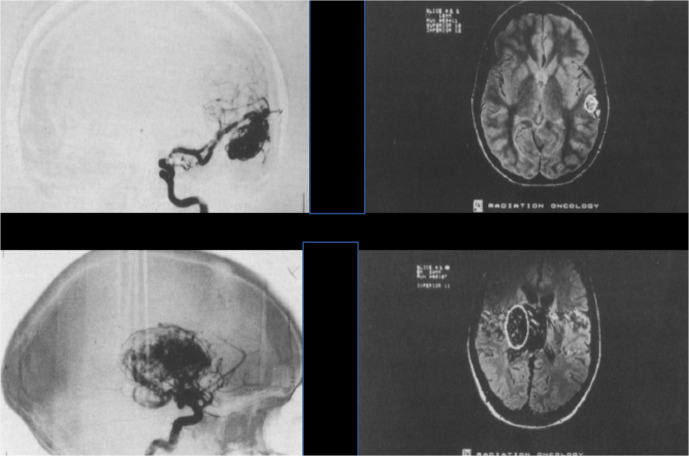
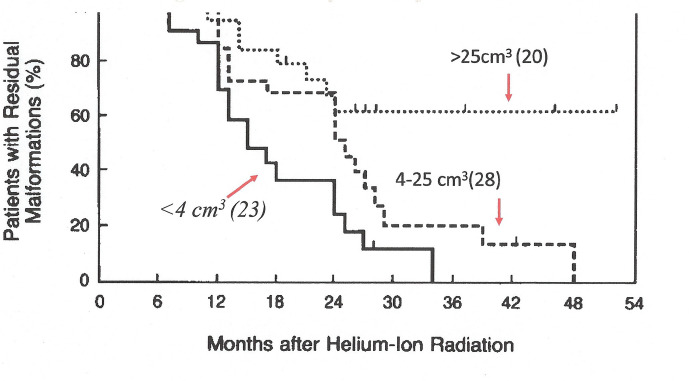
Intracranial arteriovenous malformations (AVMs) are a tangle of non-oncologic, abnormal and poorly formed blood vessels that can occur during fetal development or de novo anywhere in the body due to genetic susceptibility, but are particularly difficult to deal with in the brain.5 They have a higher rate of bleeding than normal vessels resulting in increased risk of strokes. The lesions can be small (e.g. 2.5 cm3 volume), or very large (45 cm3 volume)6 (Figure 4). Helium ion radiotherapy with a single 7.7–19.2 Gy dose of 225 MeV/u helium ions has been successfully used to eradicate lesions <4 cm3 within 3 years, and lesions 4–25 cm3 within 4 years. Lesions > 25 cm3 have been less successfully treated7 (Figure 5).
Figure 4.
Intracranial AVMs. (A) Top panels are images from a 26 year old female with a 2.5 cm2 AVM in her temporal lobe. (B) Bottom panels are images from a 21-year-old male with a 45 cm2 AVM in his basal ganglia and thalamus. Republished with permission of Elsevier Science and Technology Journals, from Phillips, et al., Copyright © 1991 Elsevier Sciences & Technology Journals.(6); permission conveyed through Copyright Clearance Center, Inc. AVM, arteriovenous malformations.
Figure 5.
Kaplan–Meier cumulative obliteration plots for 71 patients with intercranial AVM with angiography before and after treatment with a single 7.7–19.2 Gy dose of 225 MeV/n helium. Reprinted with permission of the Massachusetts Medical Society from Steinberg et al., Copyright ©1990 Massachusetts Medical Society7 ;permission conveyed through Copyright Clearance Center, Inc. AVM, arteriovenous malformations.
Recently, a set of choices was evaluated for treatment of unruptured AVM by individual practitionersusing either medical management, or interventional management (including surgical removal, embolization, SRS, or a combination of any of these). The study was closed to accrual early when it reached prespecified stopping rules, with results suggesting the superiority of the medical management group after 5 years of follow-up. At that time, 114 patients were assigned to interventional therapy, and 109 to medical management. However, the risk of death or stroke was significantly lower in the medical management group than in the interventional group (hazard ratio, 0.27; p < .0001). But studies with longer follow-up suggest that SRS has a superior outcome. Due to the current limitations in availability of heavy-ion beam facilities, helium-ion radiotherapy was not among the choices8–13 .
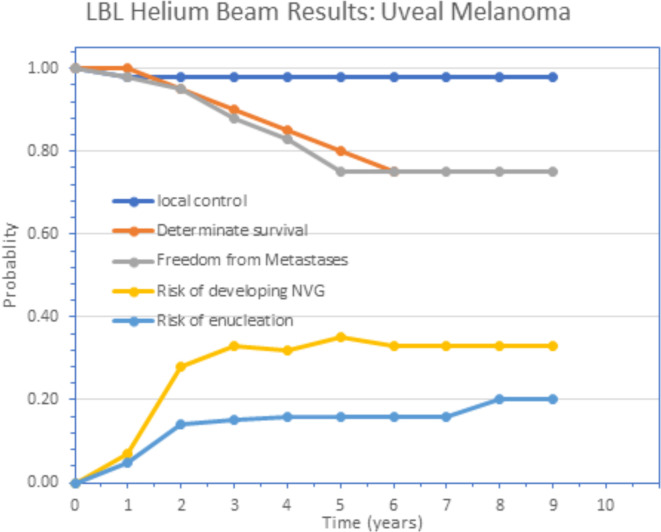
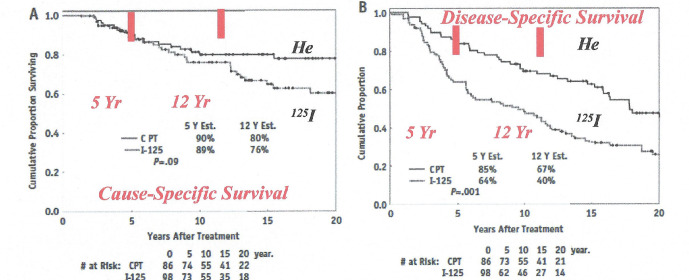
Uveal melanoma is a cancer (melanoma) of the eye involving the iris, ciliary body, or choroid (collectively referred to as the uvea). Tumors arise from the pigment cells (melanocytes) that reside within the uvea and give color to the eye. The first proton treatment of uveal melanoma was achieved by Gragoudas in 1975 at the Harvard Cyclotron (see review by Gragoudas14). Precision, helium ion high dose radiotherapy treatment of uveal melanoma was successfully implemented at the Lawrence Berkeley National Laboratory by Sanders et al,15 and Castro et al,16 with 97% success in eradicating the tumor and with retention of the eye with vision, but with some toxicities including cataract and neovascular glaucoma (Figure 6).17 The first Phase III randomized trial using charged particle beam radiotherapy comparing helium ion radiotherapy with 125Iodine plaque for choroidal and ciliary body melanoma was completed by Char et al.18 A recent 20 year follow-up of this Phase III randomized trial by Mishra et al,19 reported the cause- and disease-specific superiority of helium ion radiotherapy compared to plaque treatments (Figure 7).
Figure 6.
Nine-year actuarial follow-up of uveal melanoma patients treated with helium ions showing the probability of local control, freedom from distant metastases and determinate survival for the entire group of 307 patients, and the probability of enucleation or development of neovascular glaucoma following treatment. Republished with permission of Elsevier Science and Technology Journals, from Linstadt et al., Copyright ©199017; permission conveyed through Copyright Clearance Center, Inc.
Figure 7.
20-year Kaplan–Meier follow-up of Phase III randomized trial--Helium ion therapy vs 125Iodine plaque therapy for choroidal and ciliary body melanoma. (a) cause-specific survival (long rank:PZ.09), and (b) DFS (log rank: PZ:001). Cox multivariate regression model shows treatment is a significant predictor of DFS (adjusted: PZ.02); age and tumor diameter are independent predictors of cause specific survival and DFS. Republished with permission of Elsevier Science and Technology Journals, from Mishra et al., Copyright ©201519 ; permission conveyed through Copyright Clearance Center, Inc. DFS, disease-free survival.
Treatment with helium ions and technical optimization of beam delivery for neoplasms in critical locations such as the orbit, eye, skull base, head and neck, juxtaspinal area, retroperitoneum, biliary tract and pelvis have demonstrated outstanding improved outcomes with unparalleled local control and higher rates of survival. Mishra et al19 concluded that the hallmark of charged particle therapy with protons and helium ions is precise dose localization with tight margins to spare normal tissues.
Why heavier hadron beams?
The significantly increased benefits of using heavy ion beams heavier than helium was documented by many radiation biologists worldwide and drew the attention of radiation oncologists dealing with radioresistant tumors.20–24 Increased DNA damage in tumors and increased effectiveness against radioresistant hypoxic tumors became evident. Less repair of sublethal and potentially lethal damage in the cell cycle was confirmed. These observations led to shorter overall treatment regimens since there was no need to fractionate the dose to spare normal tissues, since the physics of the beam took care of that. The fact that elements of the target volume became radioactive and allowed verification of the treatment volume also proved useful for treatment verification.25–28
Based on this evidence, the NCI-funded clinical radiotherapy trials at the Lawrence Berkeley National Laboratory from 1975 to 1992 under the direction of Joseph R. Castro and Theodore L. Phillips from the University of California, San Francisco, CA and their team of clinicians, physicists and biologists. Preclinical biology established the radiobiological benefits of using neon and/or carbon ions, depending on the tumor size and depth in the body (see review of this history by Blakely & Chang29).
Development of particle therapy delivery methods
Computer-based technologies has evolved rapidly since the 1980’s, and continues today to detect and monitor radiation dose for dosimetry, to image the human body, and to prepare for implementation of personalized radiation treatment planning for individual patients in four dimensions (4D).30–34 The NCI-funded heavy charged particle program projects at LBNL contributed significantly to the early development of CT, MRI, and Positron Emission Tomography (PET) technologies to non-invasively image and guide the early development for successful particle treatment therapy treatment planning protocols. Novel approaches of using radioactive beams produced from fragmentation events in the primary ion treatment beam proved successful to verify the target volume in the treatment plans with clearly defined ion stopping regions. Tumors were localized in immobilized patients, and this allowed accurate transferring of information among these data sets to create detailed merged images.35–37
Heavy ion beams produced in tight narrow columns had to be broadened to accommodate the larger diameters needed to cover the initial larger Phase I/II tumor volumes that were being treated. A passive particle fragmentation method was initially introduced using heavy metal foils upstream of the target to broaden the diameter, with rotating spiral ridged heavy metal filters that broadened the width of the Bragg peak. Using this approach, the beam widths were broadened, each from 4 cm up to 10 cm. The BERKLET was an instrument built to actually measure beam fragments in two dimensions to evaluate dose deposition in terms of differential energy over distance (dE/DX) in the complex field of fragmented heavy ions.28 Biological effectiveness experiments to evaluate the quality of the ion beams examined in parallel studies with the BERKLET ion dosimeter revealed that passive particle scattering diminished the effectiveness of the ion beams.
As a result, alternative beam broadening methods were developed, including using large magnets to wobble the pristine beams.38 and then to raster scan pencil beams39 to “paint” the dose on each slice of the depth of the tumor volume in a raster fashion. The computer codes used to control the beam in the human therapy facility was an innovative system of the highest quality and led to an unblemished safety record in the human cancer therapy program. It also led to the development of one of the first and best 2D and 3D therapy planning programs for the use of ionizing radiation including charged particles in human therapy.
The differential-pencil beam algorithm (DPB) for charged-particle dose calculations is inherently 3D and takes into account multiple scattering effects in complex heterogeneous regions of the body. The DPB model was compared to Monte Carlo calculation. Petti et al,40 was the first one to suggest this technique for protons and heavier charged particles. Other groups and vendors have included DPB or similar algorithms in their software. The combined efforts of many computer scientists, biologists, biophysicists, mathematicians, accelerator physicists, graduate students and physicians were involved in integrating this approach. Many of the innovations and techniques begun at LBNL were incorporated into newer treatment planning programs for conformal X-ray therapy, stereotactic radiotherapy, and other charged particle therapy centers and throughout all of radiation oncology.
The development of rotating gantries to provide greater degrees of freedom for beam access to the patient was a remarkably innovative addition to ion therapy, first developed for protons (Loma Linda, PSI), and then for carbon ions (HIT, QST).
Ion beam radiobiology
Radiobiology contributed a wide range of biophysical, biological and clinical models that provided a considerable number of well-documented data sets of estimates of the relative biological effectiveness (RBE) that were dependent on the biological model used for the individual ion beams as a function of the atomic charge (Z), energy (E) and physical dose relative to a known low-LET radiation source. Comparisons were made of acute and late radiosensitivity and repair potentials. Biological variables that can alter radiation exposure outcomes were identified. Molecular characteristics were established that could be used to image discreet biological or clinical structures and functions.
Evolution of particle treatment planning
Current ion chambers used for particle dosimetry for measurement of the absorbed dose in clinical radiotherapy do not provide the biologically effective dose. Therefore, a key element of ion beam radiotherapy is the decision how to input the biological effectiveness measured by radiobiologists (relative to conventional radiation modalities) in order to implement treatment planning. Early proton clinical radiotherapy was assumed to be simpler than heavy charged particle radiotherapy since radiobiologists concluded reports of proton RBE values were diverse, with considerable uncertainty depending on the biological model, but that a representative RBE of 1.1 was reasonable to use until more explicit research became available,41–44 whereas helium and heavier ions had greater RBE values. There is now increasing evidence of elevated proton RBE values in the stopping proton peak just at the end of range of the particles that could be useful if considered in proton treatment planning.42 The only drawback has been the identification of the most representative proton RBE among the significant range of variability in the RBE values measured in the terminal end of the Bragg peak using a large number of biological end points.
The treatment planning system developed at LBNL included calculation of 2D dose distributions both in physical doses and biologically corrected doses with appropriate RBE values compiled from numerous in vitro and in vivo biological endpoints, as well as preparation of 3D dose-volume histograms for target volumes and critical structures.45–49 A thorough description of how the radiobiological input has contributed to ion beam therapy can be found in several review publications and books.29,50–53 For beam energies selected to achieve equivalent depths of penetration of ~10–14 cm, neon was found to have a greater high-LET dose component to reduce the oxygen effect (a greater oxygen gain factor), whereas carbon had a slightly better peak to plateau ratio of effective doses. But at higher beam energies and larger depths of penetration to ~10–24 cm neon and carbon had nearly equivalent effectiveness (Figure 8).
Figure 8.
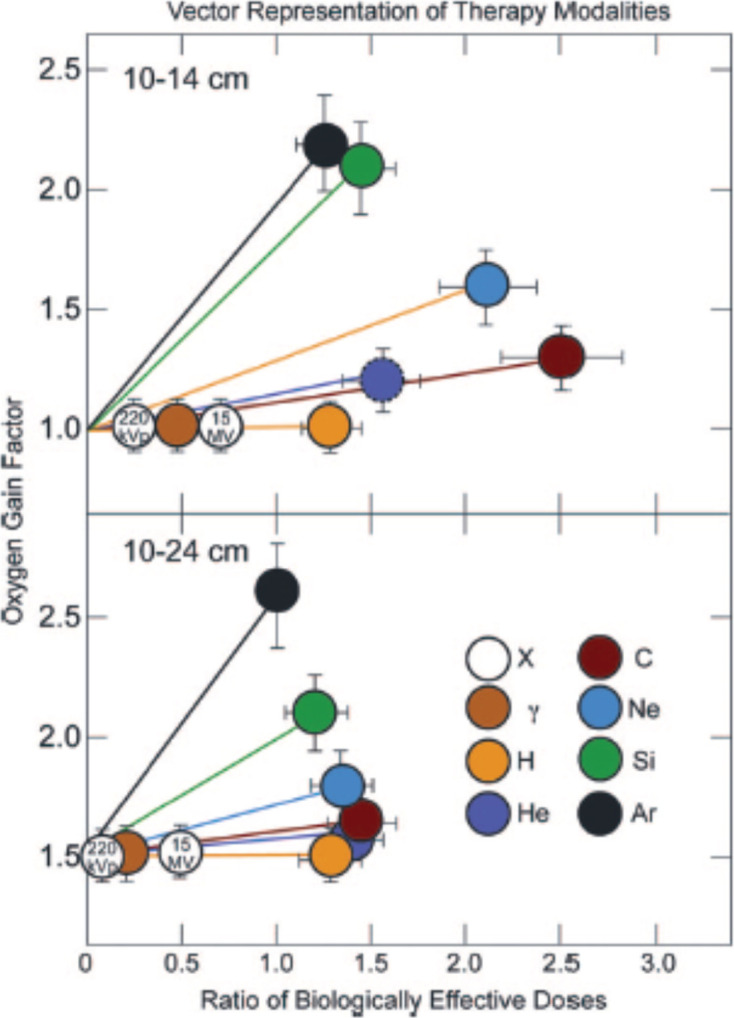
Vector representation of low LET and high LET particle therapy modalities for treatment of a small, shallow field (upper panel), and a large, deep field (lower panel). Republished with permission of Wolters Kluwer Health, Inc., from Blakely & Chang, Copyright ©200929; permission conveyed through Copyright Clearance Center, Inc. LET, linear energy transfer.
Clinical Phase I/II trials with heavy ions at Berkeley
A number of “first in human” Phase I exposures for a diverse array of tumors were completed including the first treatment dates of patients with Helium (June, 1975), Carbon (March, 1977), Neon (November, 1977), Argon (March, 1979), and Silicon (November, 1982).15,16,45,54–59 Neon ions were the heavy ions most extensively used to treat tumors at LBNL,58 although silicon and argon were tried in a few patients with superficial tumors.59
Treatment outcomes comparing neon, neutrons and conventional X-ray therapy in Berkeley
Treatment outcomes comparing the Phase I/II neon, neutron and conventional mega-voltage X-ray therapy indicated similarities between the neon and neutron results, with both indicating an approximately twofold improvement in local tumor control over conventional X-rays.58 By the end of 1988, a total of 239 patients had received a minimum neon physical dose of 1000 cGy (median follow-up for survivors 32 months). Compared with historical results, the 5 year actuarial disease-specific survival (DSSs) and local control (LC) rates suggested that neon treatment improved outcome for several types of tumors: a) advanced or recurrent macroscopic salivary gland carcinomas (DSSs 59%; LCs 61%); b) paranasal sinus tumors (DSSs 69%; LCs 69% for macroscopic disease); c) advanced soft tissue sarcomas (DSSs 56%, LCs 56% for macroscopic disease); d) macroscopic sarcomas of bone (DSSs 45%; LCs 59%); e) locally advanced prostate carcinomas (DSSs 90%; LCs 75%); and f) biliary tract carcinomas (DSSs 28%; LCs 44%). Treatment of malignant gliomas, pancreatic, gastric, esophageal, lung, and advanced or recurrent head and neck cancer were less successful. Many of these tumors were advanced, and results appeared to be no better than those achieved with conventional X-ray therapy.58
While neon ions were capable of controlling some tumors, particularly slow-growing salivary and soft tissue tumors, there was some evidence of significant late effects on normal tissues. In several studies comparing the outcome after neon or carbon ions indicated that the uniformity of dose distributions with carbon was more important than the higher-LET of neon ions. Subsequently, based on the biophysical and clinical outcomes,46,55,60 it was judged that carbon ions had the best biologically-corrected dose localization and should be used in future charged particle trials.
International clinical evidence
The worldwide number of patients treated with particle beams continues to increase with PTCOG (Particle Therapy Cooperative Group-www.ptcog.ch) reporting by December 2019 that it has compiled a grand total of approximately 250,000 patients treated with ion therapy, of which 213,000 patients were treated with protons. There are currently 82 operating proton facilities. An additional 32,000 patients were treated with carbon ions, and there are currently 13 operating carbon ion facilities. Several more proton and carbon facilities are under construction or are in various planning stages. A recent comprehensive review of the clinical outcomes for the many cancer tumor sites treated with CIRT from these facilities has been published.61 It would be impossible to summarize the clinical results from all of the proton and carbon operating facilities in this report, so instead a brief synopsis will be limited to some selected high-incidence tumor sites for which we have long-term (5–20 years) follow-up of carbon-ion results demonstrating improvements in local control and survival, and reports of any adverse normal tissue effects compared to more conventional therapeutic modalities.
In 2015, the longest operating carbon program in Japan reached the milestone of 20 years of clinical experience with carbon ion radiation therapy (CIRT) and launched a comprehensive assessment of their experience by an international team of experts. Overall, it was concluded that the National Institute of Radiation Sciences (NIRS) had pioneered a major paradigm shift for radiotherapy, and more generally for oncology. Besides improvements over the already favorable results achieved for some rare cancers, such as bone and soft tissue tumors, the most recent results supported the hypothesis that carbon ion radiotherapy improves outcomes for several common cancers with poor prognosis. Therefore, recommendations were made that more patients worldwide should have access to treatments based on CIRT.62 In fact, numerous Phase III randomized trials with CIRT for additional tumor sites are in progress worldwide (summarized in63–65). In addition, a list of international clinical protocols for Particle Therapy are available on the PTCOG (www.ptcog.ch) and a list of clinical trials is listed on the US NIHClinical Trials (www.clinicaltrials.gov) and the UMIN-CTR (www.umin.ac.jp/ctr/) websites.
Some examples of clinical results with carbon ion radiotherapy
Lung cancer
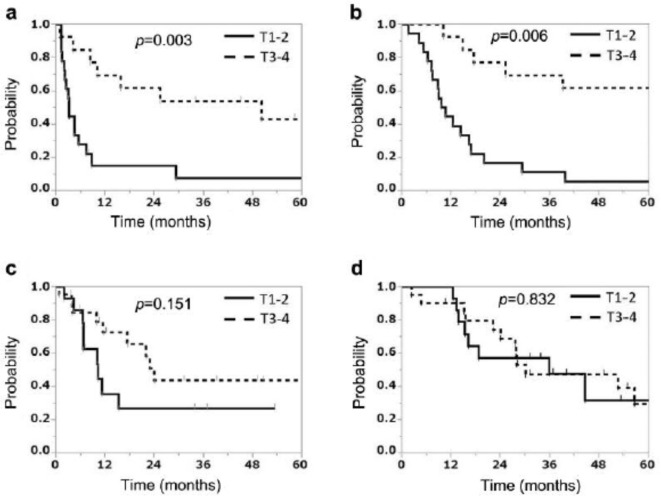
The efficacy and safety of CIRT alone at a single institution for 65 patients (median age = 73 years) with Stage III non-small-cell lung cancer (NSCLC) between 1997 and 2015 were retrospectively evaluated by Anzai et al.66 The median dose was 72.0 Gy (RBE). The median follow-up was 27.6 months (range = 1.6–207.7 months). 2-year LC, PFS, and OS rates were 73.9%, 38.6%, and 54.9%, respectively. Overall, 1 (2%), 4 (6%), and 1 (2%) patient developed Grade 4 (mediastinal hemorrhage), Grade 3 (radiation pneumonitis),and Grade 3 (bronchial fistula) toxicities, respectively. On univariate analysis, clinical T and N stage and CIRT timing were significant predictors of PFS and OS; clinical target volume was a significant predictor of PFS. The paper concludes that CIRT alone without any other therapy is effective with acceptable toxicity for Stage ΙΙΙ NSCLC. Anzai et al66 made a comparison of PFS rates and OS according to the T Stage of patients with advanced NSCLC treated with CIRT before and after 2005 which is illustrated in Figure 9. This study showed that patients staged T1-2 demonstrated significant differences in survival compared to patients in T3-4 stages treated before 2005, but not since 2005. This is probably because recent imaging techniques have excluded patients who have minimal distant metastases, especially patients with T1-2N2-3 disease.
Figure 9.
Progression-free survival rate (a) and overall survival rate (b) according to the T Stage of patients with stage III non-small-cell lung cancer who underwent carbon ion radiotherapy before January 2005. Progression-free survival rate (c) and overall survival rate (d) according to the T-stage of patients with Stage III non-small-cell lung cancer who received carbon ion radiotherapy after January 2005. Republished with permission of the IIAR Journal, from Anzai, et al., Copyright © 2020.66
Salivary gland tumor
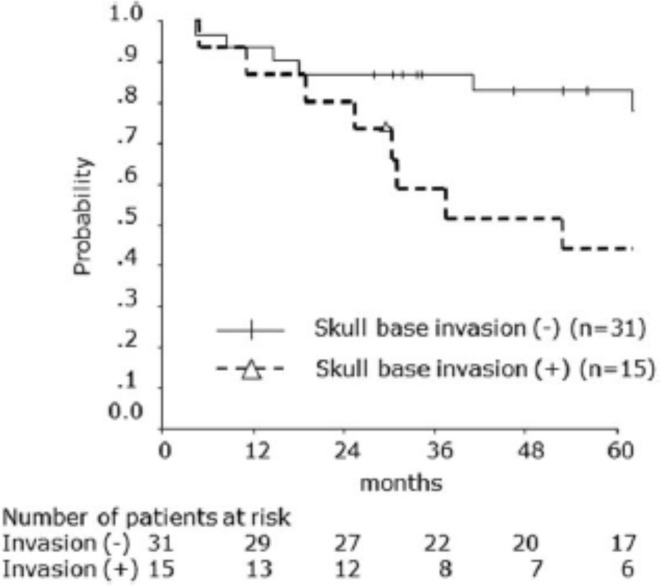
Malignant salivary gland tumors are divided into those involving the minor or the major salivary glands. There are three major salivary glands, two parotid glands near the ears, and the sublingual gland under the tongue. Locally advanced carcinoma of the parotid gland is another tumor that is effectively responsive to CIRT with acceptable toxicity levels. Koto et al 67 reported the outcomes of 46 patients receiving CIRT for either adenoid cystic carcinoma, adenocarcinoma, mucoepidermoid carcinoma or other carcinomas staged T2, T3, T4a, and T4b of the parotid gland. The CIRT was provided to 25 patients as the primary treatment, to 20 patients for local recurrences after surgery, and to 1 patient for residual tumor after surgery. During follow-up (median duration, 62 months), 5 year LC and OS rates were 74.5 and 70.1% respectively. The 5 year OS rates with and without skull base invasion were 44.0 and 83.1% respectively (Figure 10). Of the 30 patients without facial nerve palsy before C-ion RT, 25 showed no radiation-induced facial nerve palsy.
Figure 10.
Overall survival rates for carbon ion radiation therapy for locally advanced parotid gland carcinoma according to skull base invasion status. The 5 year overall survival rates with and without skull base invasion were 44.0 and 83.1%, respectively. Republished with permission of John/Wiley & Sons- Books, from Koto et al., Copyright ©201767 ; permission conveyed through Copyright Clearance Center, Inc.
In a recent study the Heidelberg Ion Beam Therapy (HIT) Facility68 reported on 59 patients with inoperable (or partially resected) locally advanced ACC of the minor salivary glands of the nasopharynx treated from 2009 to 2018 with primary radiotherapy (RT) with bimodal intensity-modulated RT (IMRT) combined with an active raster-scanned carbon-ion boost. Excellent survival results were reported for T1 to T3 tumors with moderate toxicity, while T4 tumors remain difficult to treat due to their close proximity to critical normal tissues.
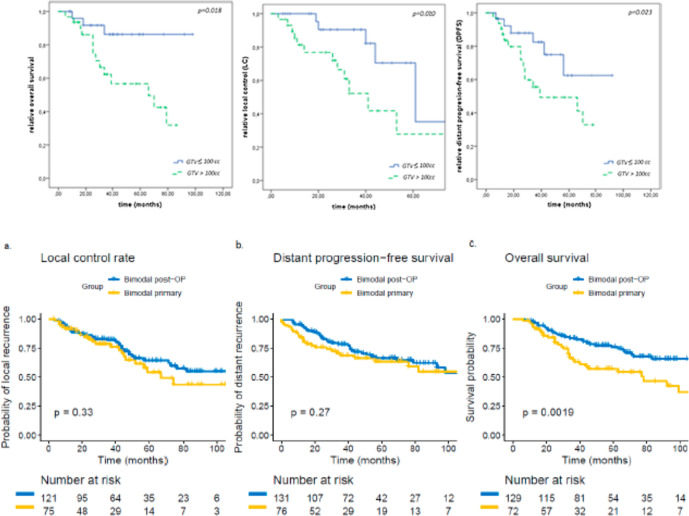
The median follow-up was 32 months. At last follow-up, 67% of the patients were still alive of whom 74% were free of progression. The 2 year LC, distant progression-free survival (DPFS) and OS were 83%, 81%, 87% and the estimated 5 year LC, DPFS and OS were 49%, 54%, 69%, respectively. LC was significantly inferior in patients with large tumor volumes (gross tumor volume, GTV > 100 cc, p = 0.020) (Figure 11A), and T4 tumors (p = 0.021).
Figure 11.
A.) Results of a combination treatment for adenoid cystic carcinoma of the minor salivary glands of the nasopharynx with intensity modulated radiotherapy and an active raster-scanning carbon ion boost. Kaplan-Meier curves for overall survival (OS), DPFS and LC in dependence of the gross tumor volume showing a significant disadvantage for patients with tumors >100 cc according to LC (p = 0.020), DPFS (p = 0.023) and OS (p = 0.018). Republished with permission of Elsevier Science & Technology Journals, from Akbaba et al., Copyright ©2019a68 ; permission conveyed through Copyright Clearance Center, Inc. (B.) Treatment outcome of 227 patients with sinonasal ACC 10 years after either primary (n = 90, 40%) or post-operative (n = 137, 60%; R2, n = 86, 63%) IMRT with doses between 48 and 56 Gy in 1.8 or 2 Gy fractions and active raster-scanning carbon ion boost with 18 to 24 Gy (RBE) in 3 Gy (RBE) fractions between 2009 and 2019 up to a median total dose of 80 Gy (EQD2, equivalent dose in 2 Gy single dose fractions, range 71–80 Gy) were reviewed. Kaplan–Meier estimates of LC (a., p = 0.33), DPFS (b., p = 0.27) and OS (c., p < 0.01) for primary and posto-perative bimodal radiotherapy. Republished with open access permission of mdpi.com, from Akbaba et al., Copyright ©2019b69 . ACC, adenoid cystic carcinoma; DPFS, distant progression-free survival; IMRT, intensity modulated radiation therapy; LC, local control; OS, overall survival; RBE, relative biological effectiveness.
In a subsequent larger 10-year follow-up study, 227 patients with sinonasal ACC were treated at the same institution with biomodal IMRT and active raster-scanned carbon ion boost.69 Patients received either a primary or post-operative fractionated IMRT combined with a fractionated active raster-scanning carbon ion boost between 2009 and 2019. Median follow-up was 50 months. In univariate and multivariate analysis, no significant difference in LC could be shown between the primary and post-operative treatment groups (p = 0.33) (Figure 11B). 3 year LC rates were 79% for primary bimodal RT and 82% for post-operative bimodal RT, respectively. T4 stage (p = 0.002) and solid histology (p = 0.005) were independent prognostic factors for decreased LC. Significant worse long-term treatment tolerance was observed for post-operatively irradiated patients with 17% vs 6% late Grade 3 toxicity (p < 0.001). The high rate of macroscopic tumor disease in the post-operative group makes the interpretation of the beneficial results in LC for primary RT difficult,
Cervical cancer
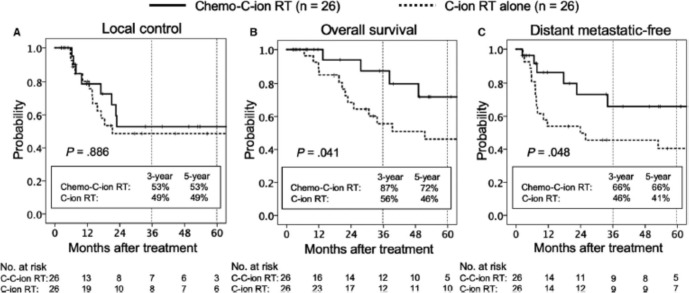
The long-term outcome of CIRT for adenocarcinoma (AC) of the uterine cervix is unknown. Recently, a pooled analysis of patients with locally advanced Stage IIB-IVA AC of the uterine cervix who underwent CIRT alone or combined with chemotherapy between September 2007 and December 2018 at a single institution has been reported.70 Patients received 74.4 Gy (RBE) with the long-term significance of concurrent weekly cisplatin (40 mg/m2 per week for up to 5 weeks), underwent no prior pelvic RT or systemic therapy, and had a performance status of 0–2. Propensity score matching was based on the year of diagnosis, regional lymph node metastasis, and stage. 26 patients were entered in each arm of the study. The median age and follow-up period were 57 (range 28–79) years and 34 (range, 2–126) months respectively. The 5 year OS rate was significantly better in the chemo plus CIRT group (72%) than in the C-ion RT alone group (46%, p = 0.041) (Figure 12). The 5 year distant metastatic-free rate was also significantly better in the chemo plus CIRT group (66%) than in the CIRT alone group (41%; p = 0.048). The incidence of grade >3 late toxicities was comparable between the two groups. Chemotherapy with cisplatin plus CIRT for locally advanced AC of the uterine cervix is associated with long-term survival benefit.
Figure 12.
Carbon ion with concurrent chemotherapy (cisplatin-40 mg/m2 per week for up to 5 weeks) for locally advanced cervical carcinoma. Kaplan–Meier curves of local control (A), overall survival (B), and distant metastatic-free rates (C) for all patients analyzed. Solid lines indicate carbon-ion radiotherapy with concurrent chemotherapy; dashed lines indicate carbon-ion radiotherapy alone. Number of patients at risk is show below the figure. Republished with permission of John Wiley & Sons - Books, from Okonogi et al., Copyright ©201970 ; permission conveyed through Copyright Clearance Center, Inc.
Prostate cancer
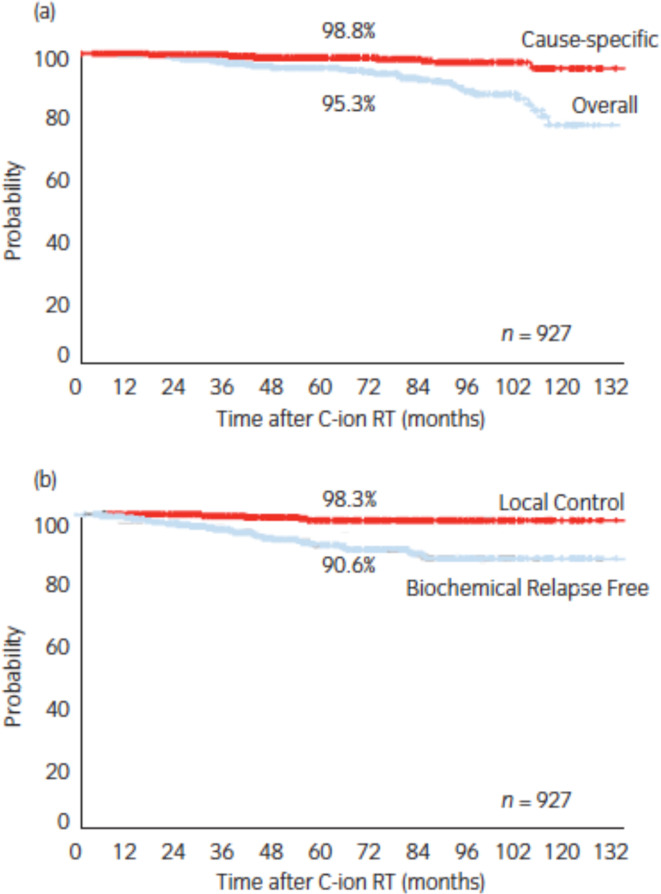
Prostate cancer was the single most-treated tumor receiving carbon ion radiotherapy at the NIRS in Chiba, Japan between June 1995 and March 2000.71Initially, two Phase I/II dose escalation studies of hypofractionated CIRT for both early- and advanced-stage prostate cancer patients were conducted to establish radiotherapy technique and to determine the optimal radiation dose. A second Phase II study was initiated in April 2000 using the shrinking field technique and the recommended dose fractionation (66 gray equivalents in 20 fractions over 5 weeks) obtained from the earlier two studies and was successfully completed in October 2003. The data from 175 patients in the Phase II study showed the importance of an appropriate use of androgen deprivation therapy according to tumor risk group. In November 2003, the Japanese Ministry of Health, Labor, and Welfare approved CIRT for prostate cancer as “Highly Advanced Medical Technology”. Ishikawa et al72 has reported on the outcome of about 1100 patients who received CIRT for prostate cancer between November 2003 and July 2011. At the last follow-up, 11 patients had died of recurrent prostate cancer and 36 of intercurrent diseases with no evidence of recurrence. The 5 year OS and cause-specific survival rates were 95.3 and 98.8%, respectively (Figure 13a). Biochemical relapses were observed in 63 (6.8%) of 927 patients, but local tumor control was achieved in all but 8 (0.8%) patients. Consequently, the 5 year biochemical elapse-free and LC rates were 90.6 and 98.3%,respectively (Figure 13b).
Figure 13.
(a) Overall and cause-specific survival and (b) local and biochemical disease control after carbon ion radiotherapy for prostate cancer. Republished with permission of John Wiley & Sons - Books, from Ishikawa, et al., Copyright ©201272; permission conveyed through Copyright Clearance Center, Inc.
Risk of treatment-induced cancers
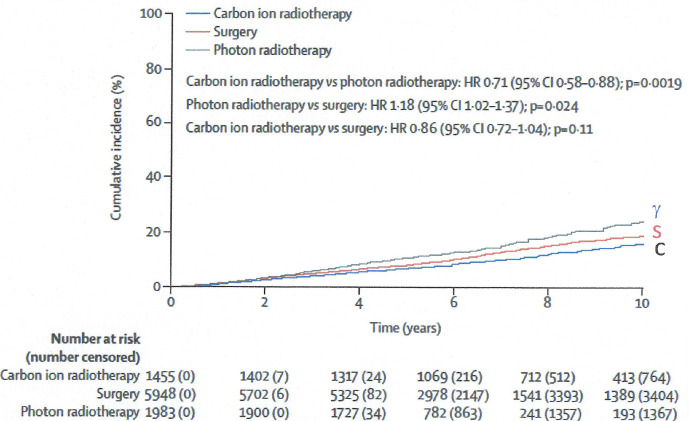
The risk of developing a new primary cancer from CIRT is understudied. Recently, a propensity score-weighted, retrospective, late follow-up cohort study of the risk of subsequent primary cancers after carbon ion radiotherapy, photon radiotherapy, or surgery for localized prostate cancer was published.73Currently, no other published studies evaluating risk of second cancers with a large size or randomized data are available for carbon ion radiotherapy in any setting. However, in single-arm trials from single institutions and in some retrospective studies, carbon ion radiotherapy has shown promising oncological outcomes with acceptable toxicities in selected patients with sarcomas, head and neck cancer, pancreatic cancer, and high-risk prostate cancer, among others, who have poor outcomes with conventional treatments. Nevertheless, the risk of subsequent primary cancers after carbon ion radiotherapy is concerning, especially in patients with long life expectancy. The cumulative incidence of subsequent primary cancers by treatment group (photon, surgery, or carbon) indicated that compared to photons or surgery, carbon ion therapy had statistically significant less cumulative cancer incidence at 10 years post-treatment (Figure 14).
Figure 14.
Cumulative incidence of subsequent primary cancers by treatment group after carbon ion radiotherapy, photon radiotherapy, or surgery for localized prostate cancer: a propensity score-weighted, retrospective, cohort study. Republished with permission of Elsevier Science & Technology Journals, from Mohamad et al., Copyright ©201973; permission conveyed through Copyright Clearance Center, Inc.
Particle beam issues requiring further research
A multidisciplinary field as diverse and fast growing as particle therapy has many ongoing scientific issues requiring further research. A few examples of these research opportunities include physics projects to reduce ion range uncertainties introduced by orbital interactions, tissue stopping powers, and beam fragmentation occurring as individual particles are slowing down in absorbing materials of the body. The myths and realities of this problem for protons are clearly described in a recent paper by Lomax.74
Lomax points out that X-ray CT imaging currently is the state-of-the-art method to obtain information on the attenuation of body tissues to X-rays, however proton stopping power must then be deduced using a calibration procedure which can lead to several mm of water equivalent range uncertainty. Unless however, one uses proton-based imaging. pCT (proton CT) has been developing for several years75–78 and can offer improved solutions to range uncertainty issues.
For ion therapy with heavier ions such as carbon, sources of range uncertainty are improved at lateral edges of the beams, but can become more complex at the distal edge where ion fragments of lower atomic number travel further than the stopping primary ions, and introduce additional dose beyond the primary Bragg peak. Ions heavier than neon, such as silicon, or argon produce high-LET events in the entrance plateau, thereby losing the sparing potential of the peak to plateau dose ratio of lower atomic numbered ions. The dose profile study of beam fragment mixtures by Traini et al 79 illustrates an ongoing approach to this issue. The question of whether or not there are interactions between damage produced by individual particle tracks remains to be further investigated.
Organ realignment for each dose fraction, and motion during treatment can also contribute to significant and non-reproducible range uncertainty. Numerous technical methods are under study to manage patient motion management.80
Optimization of a number of clinically important issues is still ongoing including identifying which is the most ideal RBE modeling approach for tumor cell eradication and for consideration of minimizing any cancer risk for each tumor site in the body, and what is the optimal dose fractionation and overall schedule. Many more Phase III trial designs are needed to provide Level 1 evidence for patient treatment by site with ion therapy following the lead of the Japanese and German programs, and international consensus and clarity to health insurance providers for institutional reimbursement. The estimated cost of building and operating accelerator facilities for ion therapy are expensive, but there are numerous international teams working to provide less expensive alternatives to some of the key expensive components, such as source, scanning, gantry, other patient alignment technologies.
Unique radiobiological response features
Over the more than 5 decades of molecular, cellular, tissue and organ radiobiological research that has been published, several reports of “unique” radiobiological response features have been attributed to “high-LET” radiations. These are phenomena that are either not qualitatively observed with conventional radiations with reduced radiation quality or are quantitatively different in their dose response to “low-LET” radiations. Table 1 provides a partial list of some of these unique features and a published citation for each. Many speculate that these response features are based on the body’s underlying response mechanisms of action to high-LET radiation particle-track-induced damage, compared to the more homogeneous distribution of initial damage from low-LET radiation modalities.
Table 1.
Some examples and reviews of unique features of the response to particle beam exposures
| Unique Response | Reference |
|---|---|
| Differential X-ray and particle beam phosphoproteome DNA damage response, cell-to-cell signaling & metabolic processes | Winter et al., 201781 |
| Carbon ions combined with a hedgehog inhibitor are more effective than X-rays in decreasing medulloblastoma and prostate cancer cell survival and metastasis | Koningset al., 201982 |
| Carbon ions were more effective than X-rays in decreasing motility genes in prostate and colon cancer cells | Suetens et al., 201583 |
| Carbon irradiations induced large interstitial chromosome deletions throughout the genome of T-cell lymphoma cells, but only infrequently after X-ray exposures | Blyth et al., 201584. |
| Carbon ion, but not X-ray irradiation suppresses migration and invasiveness of human pancreatic carcinoma cells MIAPa via Rac1 and RhoA degradation via Ub-mediated proteosomal degradation |
Fujita et al., 201585 |
| Photon irradiation enhances the phosphorylation of AKT, whereas carbon ion irradiation decreases it, leading to a reduction of HIF-1α resistance in normoxia | Harada et al., 201386 Ogata et al,201187 |
| Review of differences between photon and particle beam effects on hypofractionation, radiogenomics, combined chemo- and immune-therapies and late toxicities due to changes in the microenvironment | Durante et al. 201488 |
| Review of temporal and spatial dose variables on low-LET electron versus high-LET stopping protons on differential normal tissues and tumor effects | Mazal et al., 202089 |
| Review of available evidence indicating that particle beams are more effective than X-rays when used in combination with immunotherapy | Durante &Formenti, 202090 |
LET, linear energy transfer.
What makes particle radiation so effective are the features of the individual particle track structure with its dense ionization core, and peripheral δ ray extensions that protrude out radially as they each slow down passing through tissues, producing clustered damage and shattering and disrupting DNA molecules in bunches to release short DNA fragments and cause other molecular changes in cells and tissues. This kind of damage produces slower repair kinetics, and evidence of misrepair, genomic instabilities, microenvironmental changes and LET-dependent gene responses, as well as unique immune responses.91
Charged particle exposures in space travel
There is no more realistic venue for exposure to largely uncontrolled, and complex charged particle effects than what is encountered in space travel. Many comparisons have been made between the types and dose rates of radiation exposure encountered in ion beam therapy vs space travel.92,93 The clinical setting usually involves a controlled setting of partial body exposures to the tumor and some surrounding normal tissue of a single type of ion to a relatively high dose (>60 Gy) at usually 2 Gy/min, in one or a series of doses to a total dose limit linked to the surrounding normal tissue’s tolerance. In contrast, radiation exposures in space have been difficult to predict completely, since they arise from multiple radiation sources in the galactic cosmic ray spectrum according to an unpredictable solar year cycle.
More than 550 people have traveled into lower earth orbit (LEO) and beyond and returned to Earth.94 Radiation doses per astronaut from individual missions with a minimal number of Earth orbits such as occurred in the Mercury program, through to the 6 months or longer missions on the International Space Station (ISS) have increased total radiation exposures to crew members.
Space radiation exposures occur from a complex mixture of many radiation types and multiple orders of magnitude ranges of energies in an environment of chronic, low dose rates, of relatively low radiation qualities-predominantly from protons, with intermittent sprinklings of high-LET charged particle fluences. The two exposure scenarios could not be more different.
NASA bioastronautics roadmap
Today, the only permanent human outpost in space is the ISS, and current plans are to maintain this one-of-a-kind facility. NASA has received presidential approval for the return of males and females to the Moon by 2024, and Mars is a current planned destination if deemed safe for a manned mission in 2030 or beyond. The risks of space travel are complex but can be summarized into three broad categories: (1) physiological problems caused by microgravity, (2) psychological and medical problems caused by isolation, and (3) acute and late risks caused by exposure to space radiations. It is also important to point out that these three categories have many potential overlaps.
A comprehensive overview of the current status of space radiobiology is available.95 Internationally, there are also intense research programs ongoing in Europe96, and China.97
Risk of tumorigenesis
Ionizing radiation is a weak carcinogen compared to hazardous carcinogenic chemicals. However, the enhanced biological effectiveness of charged particles has long been a concern in the consideration of the use of these ions for health care, or as a late risk of space travel. However, neither the clinical ion therapy- or the space-cohorts has been reported to demonstrate an increased mortality from cancer due to their exposures to charged particles.98 It must be acknowledged though that space missions into deep space travel to Mars and beyond will involve greater space radiation risks, it is not yet known whether the higher doses preclude these manned-missions.
NASA radiation risk prediction model
The NASA Space Cancer Risk (NSCR)99 radiation risk prediction model was reviewed and approved by the National Research Council in 2012. It forms the basis for estimating crew risks for ISS missions and trade studies of future Exploration Class missions. It only considers the risk of carcinogenesis, and includes the GCR environment,100 trapped radiation environment, and radiation transport (HZETRN) for comprehensive dosimetry evaluation. It provides estimates of cancer incidence and mortality for age and gender-specific risks. The slope from age modification is 1.3:1.0 from age 35 to 55. The risk model utilizes astronaut healthy population characteristics (lifetime never-smokers) lowers space radiation risk compared to the U.S. avg. population of about 20%. It also includes new Quality Factors and improved uncertainty estimates.
The NSCR model utilizes data/information analyzed from: (1) the epidemiology of previous human cohorts exposed to radiation (BEIR-Biological Effect of Ionizing Radiation; UNSCEAR-United Nations Scientific Committee on the Effects of Atomic Radiation; and RERF-Radiation Effects Research Foundation), (2) from terrestrial radiation sources research (NIH/NCI-Terrestrial Cancer Research; DOE/DOD/DARPA-Radiation effects research; and International Research Activities, and finally (3) from NASA-funded space radiation research (the Human Research Program; the Space Radiation focused research, including information from animal models exposed to simulated space radiation environments at Brookhaven National Laboratory’s NASA Space Radiation Research Laboratory (NSRL)).
Uncertainties in risk assessment
To date, there is no evidence for space radiation-induced cancer in astronauts or cosmonauts.101–106 We do however have good measurements of the types and doses of radiation during transit to and from Mars with either a fly-by or a stay on the surface.107–109 But, to date, no human has yet experienced protracted exposure to the estimated space radiation types, doses, and dose rates from a Mars expedition. However, quantitative studies of tissue-specific cancer induction after low particle fluences are important to the understanding of cancer causation, e.g. by radiogenic targeted effect (TE) vs non-targeted effects (NTE). Animal experiments can help bridge this gap. In my own laboratory, we are using 1-ion, 2-ion, 3-ion and 6-ion mixed ion field whole-body murine exposures to investigate this important question. There is a need for renewed focus on the radiobiology of primary ion beam fragmentation into a mixture of potential interactive synergistic, or antagonistic effects from cell damage due to individual ions of lower atomic number, and on how these physical events can contribute to our understanding of enhanced RBE. Theoretical modeling of potential synergistic effects between ion beams is underway.110
New era for charged particle radiobiology
The human genome is mapped and being mined for tumor and normal tissue data on organ-specific radioresponses. We have powerful new genomic and proteomic tools available that have been able to help us focus on individualized medicine. New networks of gene and protein pathways have been identified. Gene expression profiles have been shown to change in a dose- and time-dependent fashion after exposure to particles of variable LET. Tailored 3D image-guided and intensity modulated physics is available. Theoretical biophysical modeling is guiding treatment optimization, but more work is needed to understand nano- and micro-dosimetric energy deposition effects.
Despite the guidance of the IAEA’s 2008 Technical Reports Series No. 461, RBE in Ion Beam Therapy, there are still issues remaining for standardizing and reporting carbon-ion treatment planning and prescribed doses. International standards for ion dose reporting currently do not exist and will hinder clinical comparisons. However, this may be resolved soon, with the anticipated release in 2020 of ICRU Report 93, Prescribing, Recording, and Reporting Light Ion Beam Therapy. The most controversial issue in the reporting of dose is not the physical absorbed dose, but the definition of the biologically- or clinically equivalent dose. Despite acknowledgment of higher proton RBE measurements at low energy, RBE values of 1.0–1.1 are in current use.
Three different carbon ion RBE strategies are currently in use. We need to establish the selection of the gold-standard for absolute dose calibration. The single best reference radiation for determination of the RBE needs to be selected. Guidance should be given as to the selection of the best biological systems for determination of the RBE, and the most appropriate theoretical model to use to fit the biological data, and to predict the biological and clinical outcome based on the ion beam characteristics.
Open questions remain regarding the optimal particle species for each clinical situation, whether or not using more than one ion in a treatment plan is optimal for a specific tumor site, and the time dependence of differential tissue effects. Optimal fractionation schemes, and overall duration of treatment are still under study for individual clinical cases, and the impact they might have on individual sensitivity, and acute and late effects, including cancer induction risks. Age- and sex-dependent normal tissue dose limits and volume effects are still incomplete as well as their effects on stem cells. More research is needed to elucidate the role of chemotherapy combined with ion therapy to tackle microscopic metastatic disease. Modeling of the radiochemistry of ion beam therapy would also contribute to clarification of the role of the reduced oxygen effect for high-LET radiations.
Conclusions
Ionizing radiations, including charged particle beams, are invisible (with rare exceptions, such as Cherenkov radiation or the northern or southern auroraborealis) and require shielding and monitoring to harness their potential therapeutic benefits and to limit their adverse health effects on Earth and in space travel. Results of several long-term follow-up (e.g. >5–20 years) studies of helium and carbon ion radiotherapy for the treatment of cancer or other non-oncological lesions in selected lesion sites demonstrate the increasing potential for improving human health conditions. Numerous Phase III trials are in progress or planned that will further elucidate the long-term cures and consequences of carbon ion therapy for an extensive list of tumor sites. Although the non-invasive feature of particle beams has demonstrated promise in clinical applications to eradicate targeted tissues, there are many remaining complex challenges of uncontrolled particle beam exposures confounded by exposure to simultaneous stressors such as microgravity for planning radiation protection in space travel.
Footnotes
Acknowledgment: The author would like to thank the International Commission on Radiation Units and Measurements (ICRU) for the honor of receiving the 20th Gray Medal. She also wishes to acknowledge several colleagues who contributed to making this manuscript possible. PY Chang was essential to my discussion of ideas and dialogue regarding concepts and details, and to the many experiments we completed as co-investigators. Her research team of James Bakke, Amber Grover, and the Toxicology Support Services team at SRI International were also essential to our in vivo particle studies. Collaborative research interactions with G Kraft, WKraft, S Ritter, JD Chapman, CLucke-Huhle, MR Raju, L Skarsgaard, BPalcic, and M Dosanjh were important to the development of many research concepts. My colleagues at the Lawrence Berkeley National Laboratory included CA Tobias, TCH Yang, R Roots, J-H Mao, A Kronenberg, A. Snijders, MH Barcellos-Hoff, FQH. Ngo. Support from K Bjornstad, C Rosen, M Parra and the Berkeley Lab animal care facility team was essential for our work. My colleagues, R Sachs and E Huang (UC Berkeley), M Roach, III, A Lazar, and B Faddegon (UCSF), R Schulte (Loma Linda University), M Hada, A Beitman, J Rhone, and P Saganti (Prairie View Texas A&M), and A Rusek, P Guida, M Sivertz, D Snyder and the animal facility at the NASA Space Radiation Laboratory at Brookhaven National Laboratory. Finally, I wish to dedicate this paper to my eternally patient husband George Zizka, and our daughter Maria Zizka and her husband Graham Bradley. The understanding and support of my family were essential to this work.
Funding: Supported by NIH/NCI #5P20CA183640, and NASA Grant # NNJ16HP22I, under DOE (BER)Contract No. DE-AC02-05CH11231.
REFERENCES
- 1.Wilson RR. Radiological use of fast protons. Radiology 1946; 47: 487–91. doi: 10.1148/47.5.487 [DOI] [PubMed] [Google Scholar]
- 2.Gray LH, Conger AD, Ebert M, Hornsey S, Scott OC. The concentration of oxygen dissolved in tissues at the time of irradiation as a factor in radiotherapy. Br J Radiol 1953; 26: 638–48. doi: 10.1259/0007-1285-26-312-638 [DOI] [PubMed] [Google Scholar]
- 3.Lawrence JH, Tobias CA. Radioactive isotopes and nuclear radiations in the treatment of cancer. Cancer Res 1956; 16: 185–93. [PubMed] [Google Scholar]
- 4.Levy RP, Fabrikant JI, Frankel KA, Phillips MH, Lyman JT, Lawrence JH, et al. Heavy-Charged-Particle radiosurgery of the pituitary gland: clinical results of 840 patients. Stereotact Funct Neurosurg 1991; 57(1-2): 22–35. doi: 10.1159/000099553 [DOI] [PubMed] [Google Scholar]
- 5.Tasiou A, Tzerefos C, Boccardi E, Karlsson B, Kitchen N, et al. Arteriovenous malformations: congenital or acquired lesions? World Neurosurg 2019;. [DOI] [PubMed] [Google Scholar]
- 6.Phillips MH, Kessler M, Chuang FY, Frankel KA, Lyman JT, Fabrikant JI, et al. Image correlation of MRI and CT in treatment planning for radiosurgery of intracranial vascular malformations. Int J Radiat Oncol Biol Phys 1991; 20: 881–9. doi: 10.1016/0360-3016(91)90036-4 [DOI] [PubMed] [Google Scholar]
- 7.Steinberg GK, Fabrikant JI, Marks MP, Levy RP, Frankel KA, Phillips MH, et al. Stereotactic heavy-charged-particle Bragg-peak radiation for intracranial arteriovenous malformations. N Engl J Med 1990; 323: 96–101. doi: 10.1056/NEJM199007123230205 [DOI] [PubMed] [Google Scholar]
- 8.Chan MD, Soltys SG, Halasz LM, Laack NN, Minniti G, Kirkpatrick JP. Management of unruptured AVMs: the pendulum Swings. Int J Radiat Oncol Biol Phys 2019; 105: 687–9. doi: 10.1016/j.ijrobp.2019.08.026 [DOI] [PubMed] [Google Scholar]
- 9.Ding D, Starke RM, Kano H, Mathieu D, Huang P, Kondziolka D, et al. Radiosurgery for Cerebral Arteriovenous Malformations in A Randomized Trial of Unruptured Brain Arteriovenous Malformations (ARUBA)-Eligible Patients: A Multicenter Study. Stroke 2016; 47: 342–9. doi: 10.1161/STROKEAHA.115.011400 [DOI] [PubMed] [Google Scholar]
- 10.Ding D, Yen C-P, Starke RM, Xu Z, Sheehan JP. Radiosurgery for ruptured intracranial arteriovenous malformations. J Neurosurg 2014; 121: 470–81. doi: 10.3171/2014.2.JNS131605 [DOI] [PubMed] [Google Scholar]
- 11.Karlsson B, Jokura H, Yang H-C, Yamamoto M, Martinez R, Kawagishi J, et al. The NASSAU (new assessment of cerebral arteriovenous malformations yet unruptured) analysis: are the results from the ARUBA trial also applicable to unruptured arteriovenous malformations deemed suitable for gamma knife surgery? Neurosurgery 2019; 85: E118–24. doi: 10.1093/neuros/nyy391 [DOI] [PubMed] [Google Scholar]
- 12.Meling TR, Patet G. What is the best therapeutic approach to a pediatric patient with a deep-seated brain AVM? Neurosurg Rev 2019; 42: 409–16. doi: 10.1007/s10143-019-01101-8 [DOI] [PubMed] [Google Scholar]
- 13.Mohr JP, Parides MK, Stapf C, Moquete E, Moy CS, Overbey JR, et al. Medical management with or without Interventional therapy for unruptured brain arteriovenous malformations (ARUBA): a multicentre, non-blinded, randomised trial. Lancet 2014; 383: 614–21. doi: 10.1016/S0140-6736(13)62302-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gragoudas ES. Proton beam irradiation of uveal melanomas: the first 30 years. The Weisenfeld lecture. Invest Ophthalmol Vis Sci 2006; 47: 4666–73. doi: 10.1167/iovs.06-0659 [DOI] [PubMed] [Google Scholar]
- 15.Saunders W, Castro JR, Chen GT, Collier JM, Zink SR, Pitluck S, et al. Helium-ion radiation therapy at the Lawrence Berkeley laboratory: recent results of a northern California Oncology group clinical trial. Radiat Res Suppl 1985; 8: S227–34. doi: 10.2307/3583532 [DOI] [PubMed] [Google Scholar]
- 16.Castro JR, Char DH, Petti PL, Daftari IK, Quivey JM, Singh RP, et al. 15 years experience with helium ion radiotherapy for uveal melanoma. Int J Radiat Oncol Biol Phys 1997; 39: 989–96. doi: 10.1016/S0360-3016(97)00494-X [DOI] [PubMed] [Google Scholar]
- 17.Linstadt D, Castro J, Char D, Decker M, Ahn D, Petti P, et al. Long-Term results of helium ion irradiation of uveal melanoma. Int J Radiat Oncol Biol Phys 1990; 19: 613–8. doi: 10.1016/0360-3016(90)90487-5 [DOI] [PubMed] [Google Scholar]
- 18.Char DH, Quivey JM, Castro JR, Kroll S, Phillips T. Helium ions versus iodine 125 brachytherapy in the management of uveal melanoma. A prospective, randomized, dynamically balanced trial. Ophthalmology 1993; 100: 1547–54. doi: 10.1016/s0161-6420(93)31446-6 [DOI] [PubMed] [Google Scholar]
- 19.Mishra KK, Quivey JM, Daftari IK, Weinberg V, Cole TB, Patel K, et al. Long-Term results of the UCSF-LBNL randomized trial: charged particle with helium ion versus iodine-125 plaque therapy for choroidal and ciliary body melanoma. Int J Radiat Oncol Biol Phys 2015; 92: 376–83. doi: 10.1016/j.ijrobp.2015.01.029 [DOI] [PubMed] [Google Scholar]
- 20.Barendsen GW. Differences between biological effects of high let and low let radiations in relation to their application in radiotherapy. Radiol Clin 1977; 46: 380–9. [PubMed] [Google Scholar]
- 21.Blakely EA, FQH N, Curtis SB, Tobias C. Heavy-Ion Radiobiology: Cellular Studies. In: Lett J. T, ed.Advances in Radiation Biology: Elseivier; 1984. pp. 295–389. [Google Scholar]
- 22.Raju MR. Heavy particle radiotherpy: Academic Press. 1980;.
- 23.Skarsgard LD. Radiobiology with heavy charged particles: a historical review. Phys Med 1998; 14 Suppl 1(Suppl 1): 1–19. [PubMed] [Google Scholar]
- 24.Todd PW. Heavy-Ion irradiation of human and Chinese hamster cells in vitro. Radiat Res 1975; 61: 288–97. doi: 10.2307/3574046 [DOI] [PubMed] [Google Scholar]
- 25.Chatterjee A, Alpen EL, Tobias CA, Llacer J, Alonso J. High energy beams of radioactive nuclei and their biomedical applications. Int J Radiat Oncol Biol Phys 1981; 7: 503–7. doi: 10.1016/0360-3016(81)90137-1 [DOI] [PubMed] [Google Scholar]
- 26.Chatterjee A, Saunders W, Alpen EI, Alonso J, Scherer J, Llacer J. Physical measurements with high-energy radioactive beams. Radiat Res 1982; 92: 230–44. doi: 10.2307/3576001 [DOI] [PubMed] [Google Scholar]
- 27.Chatterjee A, Takada E, Torikoshi M, Kanazawa M. Diagnostic imaging by energetic radioactive particle beams: applications in Bragg peak cancer therapy. Nucl Phys A 1997; A616(1-2): 478–89. doi: 10.1016/S0375-9474(97)00119-X [DOI] [PubMed] [Google Scholar]
- 28.Llacer J, Chatterjee A, Alpen EL, Saunders W, Andreae S, Jackson HC. Imaging by injection of accelerated radioactive particle beams. IEEE Trans Med Imaging 1984; 3: 80–90. doi: 10.1109/TMI.1984.4307660 [DOI] [PubMed] [Google Scholar]
- 29.Blakely EA, Chang PY. Biology of charged particles. Cancer J 2009; 15: 271–84. doi: 10.1097/PPO.0b013e3181b666c5 [DOI] [PubMed] [Google Scholar]
- 30.Beaton L, Bandula S, Gaze MN, Sharma RA. How rapid advances in imaging are defining the future of precision radiation oncology. Br J Cancer 2019; 120: 779–90. doi: 10.1038/s41416-019-0412-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seco J, Clasie B, Partridge M. Review on the characteristics of radiation detectors for dosimetry and imaging. Phys Med Biol 2014; 59: R303–47. doi: 10.1088/0031-9155/59/20/R303 [DOI] [PubMed] [Google Scholar]
- 32.Volz L, Kelleter L, Brons S, Burigo L, Graeff C, Niebuhr NI, et al. Experimental exploration of a mixed helium/carbon beam for online treatment monitoring in carbon ion beam therapy. Phys Med Biol 2020; 65: 055002. doi: 10.1088/1361-6560/ab6e52 [DOI] [PubMed] [Google Scholar]
- 33.Volz L, Piersimoni P, Bashkirov VA, Brons S, Collins-Fekete C-A, Johnson RP, et al. The impact of secondary fragments on the image quality of helium ion imaging. Phys Med Biol 2018; 63: 195016: 195016. doi: 10.1088/1361-6560/aadf25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Volz L, Piersimoni P, Johnson RP, Bashkirov VA, Schulte RW, Seco J. Improving single-event proton CT by removing nuclear interaction events within the energy/range detector. Phys Med Biol 2019; 64: 15NT01. doi: 10.1088/1361-6560/ab2671 [DOI] [PubMed] [Google Scholar]
- 35.Budinger TF, Lauterbur PC. Nuclear magnetic resonance technology for medical studies. Science 1984; 226: 288–98. doi: 10.1126/science.6385252 [DOI] [PubMed] [Google Scholar]
- 36.Chen GT, Castro JR, Quivey JM. Heavy charged particle radiotherapy. Annu Rev Biophys Bioeng 1981; 10: 499–529. doi: 10.1146/annurev.bb.10.060181.002435 [DOI] [PubMed] [Google Scholar]
- 37.Llacer J, Schmidt JB, Tobias CA. Characterization of fragmented heavy-ion beams using a three-stage telescope detector: measurements of 670-MeV/amu 20Ne beams. Med Phys 1990; 17: 151–7. doi: 10.1118/1.596536 [DOI] [PubMed] [Google Scholar]
- 38.Renner TR, Chu WT. Wobbler facility for biomedical experiments. Med Phys 1987; 14: 825–34. doi: 10.1118/1.596009 [DOI] [PubMed] [Google Scholar]
- 39.Castro JR, Petti PL, Daftari IK, Collier JM, Renner T, Ludewigt B, et al. Clinical gain from improved beam delivery systems. Radiat Environ Biophys 1992; 31: 233–40. doi: 10.1007/BF01214830 [DOI] [PubMed] [Google Scholar]
- 40.Petti PL, Lyman JT, Renner TR, Castro JR, Collier JM, Daftari IK, et al. Design of beam-modulating devices for charged-particle therapy. Med Phys 1991; 18: 513–8. doi: 10.1118/1.596655 [DOI] [PubMed] [Google Scholar]
- 41.Lühr A, von Neubeck C, Pawelke J, Seidlitz A, Peitzsch C, Bentzen SM, et al. "Radiobiology of Proton Therapy": Results of an international expert workshop. Radiother Oncol 2018; 128: 56–67. doi: 10.1016/j.radonc.2018.05.018 [DOI] [PubMed] [Google Scholar]
- 42.Paganetti H, Blakely E, Carabe-Fernandez A, Carlson DJ, Das IJ, Dong L, et al. Report of the AAPM TG-256 on the relative biological effectiveness of proton beams in radiation therapy. Med Phys 2019; 46: e53–78. doi: 10.1002/mp.13390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tang JT, Inoue T, Inoue T, Yamazaki H, Fukushima S, Fournier-Bidoz N, et al. Comparison of radiobiological effective depths in 65-MeV modulated proton beams. Br J Cancer 1997; 76: 220–5. doi: 10.1038/bjc.1997.365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tepper J, Verhey L, Goitein M, Suit HD. In vivo determinations of RBE in a high energy modulated proton beam using normal tissue reactions and fractionated dose schedules. Int J Radiat Oncol Biol Phys 1977; 2(11-12): 1115–22. doi: 10.1016/0360-3016(77)90118-3 [DOI] [PubMed] [Google Scholar]
- 45.Castro JR, Chen GT, Blakely EA. Current considerations in heavy charged-particle radiotherapy: a clinical research trial of the University of California Lawrence Berkeley laboratory, Northern California Oncology group, and radiation therapy Oncology Group. Radiat Res Suppl 1985; 8: S263–71. doi: 10.2307/3583536 [DOI] [PubMed] [Google Scholar]
- 46.Lyman J. Computer modeling of heavy charged particle beams. InProceedings of the International Workshop on Pion and Heavy-Ion Radiotherapy: Pre-clinical Studies. North Holland: Elsevier; 1983. [Google Scholar]
- 47.Lyman J. Heavy Charged Particle Dosimetry. In: editorAdvances in Dosimetry for Fast Neutrons and Heavy Charged Particles for Therapy Applications. Vienna: International Atomic Energy Agency (IAEA); 1982. [Google Scholar]
- 48.Lyman JT, Wolbarst AB. Optimization of radiation therapy, III: a method of assessing complication probabilities from dose-volume histograms. Int J Radiat Oncol Biol Phys 1987; 13: 103–9. doi: 10.1016/0360-3016(87)90266-5 [DOI] [PubMed] [Google Scholar]
- 49.Lyman JT, Wolbarst AB. Optimization of radiation therapy, IV: a dose-volume histogram reduction algorithm. Int J Radiat Oncol Biol Phys 1989; 17: 433–6. doi: 10.1016/0360-3016(89)90462-8 [DOI] [PubMed] [Google Scholar]
- 50.Blakely EA, Chang PY. Preclinical Radiobiology and Predictive Assays. In: Linz U, ed.Ion Beam Therapy--Fundamentals, Technology, Clinical Applications. Heidelberg: Springer; 2012. pp. 135–45. [Google Scholar]
- 51.Linz U. Linz U, ed.Ion Beams in Tumor Therapy: Chapman & Hall; 1995. [Google Scholar]
- 52.Skarsgard LD. Pion and Heavy Ion Radiotherapy: Pre-Clinical and Clinical Studies. New York: Elsevier Science Publishing Co; 1983. [Google Scholar]
- 53.Tsujii H, Kamada T, Shirai T, Noda K, Tsuji H, Karasawa K et al.Principles, practices and treatment planning. Tokyo: Springer; 2014. [Google Scholar]
- 54.Castro JR. Results of heavy ion radiotherapy. Radiat Environ Biophys 1995; 34: 45–8. doi: 10.1007/BF01210545 [DOI] [PubMed] [Google Scholar]
- 55.Castro JR, Linstadt DE, Bahary JP, Petti PL, Daftari I, Collier JM, et al. Experience in charged particle irradiation of tumors of the skull base: 1977-1992. Int J Radiat Oncol Biol Phys 1994; 29: 647–55. doi: 10.1016/0360-3016(94)90550-9 [DOI] [PubMed] [Google Scholar]
- 56.Castro JR, Saunders WM, Tobias CA, Chen GT, Curtis S, Lyman JT, et al. Treatment of cancer with heavy charged particles. Int J Radiat Oncol Biol Phys 1982; 8: 2191–8. doi: 10.1016/0360-3016(82)90569-7 [DOI] [PubMed] [Google Scholar]
- 57.Castro JR, Tobias CA, Saunders WM, Chen GT, Collier JM, Lyman JT, et al. Heavy particle experience in the treatment of human cancer. Prog Clin Biol Res 1983; 132D: 279–90. [PubMed] [Google Scholar]
- 58.Linstadt DE, Castro JR, Phillips TL. Neon ion radiotherapy: results of the phase I/II clinical trial. Int J Radiat Oncol Biol Phys 1991; 20: 761–9. [DOI] [PubMed] [Google Scholar]
- 59.Blakely E, Castro JR. Assessment of acute and late effects to High-LET radiation. Proceedings of the NIRS International Seminar on the Application of Heavy Ion Accelerator to Radiation Therapy of Cancer.editors. 1994;: 14–16 Chiba, Japan.. [Google Scholar]
- 60.Chapman JD, Blakely EA, Smith KC, Urtasun RC. Radiobiological characterization of the inactivating events produced in mammalian cells by helium and heavy ions. Int J Radiat Oncol Biol Phys 1977; 3: 97–102. doi: 10.1016/0360-3016(77)90234-6 [DOI] [PubMed] [Google Scholar]
- 61.Malouff TD, Mahajan A, Krishnan S, Beltran C, Seneviratne DS, Trifiletti DM. Carbon ion therapy: a modern review of an emerging technology. Front Oncol 2020; 10: 82. doi: 10.3389/fonc.2020.00082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kamada T, Tsujii H, Blakely EA, Debus J, De Neve W, Durante M, et al. Carbon ion radiotherapy in Japan: an assessment of 20 years of clinical experience. Lancet Oncol 2015; 16: e93–100. doi: 10.1016/S1470-2045(14)70412-7 [DOI] [PubMed] [Google Scholar]
- 63.Durante M, Orecchia R, Loeffler JS. Charged-Particle therapy in cancer: clinical uses and future perspectives. Nat Rev Clin Oncol 2017; 14: 483–95. doi: 10.1038/nrclinonc.2017.30 [DOI] [PubMed] [Google Scholar]
- 64.Lazar AA, Schulte R, Faddegon B, Blakely EA, Roach M. Clinical trials involving carbon-ion radiation therapy and the path forward. Cancer 2018; 124: 4467–76. doi: 10.1002/cncr.31662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rackwitz T, Debus J. Clinical applications of proton and carbon ion therapy. Semin Oncol 2019; 46: 226–32. doi: 10.1053/j.seminoncol.2019.07.005 [DOI] [PubMed] [Google Scholar]
- 66.Anzai M, Yamamoto N, Hayashi K, Nakajima M, Nomoto A, Ogawa K, et al. Safety and efficacy of Carbon-ion radiotherapy alone for stage III non-small cell lung cancer. Anticancer Res 2020; 40: 379–86. doi: 10.21873/anticanres.13963 [DOI] [PubMed] [Google Scholar]
- 67.Koto M, Hasegawa A, Takagi R, Ikawa H, Naganawa K, Mizoe J-E, et al. Definitive carbon-ion radiotherapy for locally advanced parotid gland carcinomas. Head Neck 2017; 39: 724–9. doi: 10.1002/hed.24671 [DOI] [PubMed] [Google Scholar]
- 68.Akbaba S, Ahmed D, Lang K, Held T, Mattke M, Hoerner-Rieber J, et al. Results of a combination treatment with intensity modulated radiotherapy and active raster-scanning carbon ion boost for adenoid cystic carcinoma of the minor salivary glands of the nasopharynx. Oral Oncol 2019; 91: 39–46. doi: 10.1016/j.oraloncology.2019.02.019 [DOI] [PubMed] [Google Scholar]
- 69.Akbaba S, Ahmed D, Mock A, Held T, Bahadir S, Lang K, et al. Treatment outcome of 227 patients with sinonasal adenoid cystic carcinoma (ACC) after intensity modulated radiotherapy and active Raster-Scanning carbon ion boost: a 10-year single-center experience. Cancers 2019; 11: 170501 11 2019. doi: 10.3390/cancers11111705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Okonogi N, Wakatsuki M, Kato S, Karasawa K, Miyasaka Y, Murata H, et al. A phase 1/2 study of carbon ion radiation therapy with concurrent chemotherapy for locally advanced uterine cervical squamous cell carcinoma (protocol 1302. Int J Radiat Oncol Biol Phys 2019; 104: 631–9. doi: 10.1016/j.ijrobp.2019.02.042 [DOI] [PubMed] [Google Scholar]
- 71.Ishikawa H, Tsuji H, Murayama S, Sugimoto M, Shinohara N, Maruyama S, et al. Particle therapy for prostate cancer: the past, present and future. Int J Urol 2019; 26: 971–9. doi: 10.1111/iju.14041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ishikawa H, Tsuji H, Kamada T, Akakura K, Suzuki H, Shimazaki J, et al. Carbon-ion radiation therapy for prostate cancer. Int J Urol 2012; 19: 296–305. doi: 10.1111/j.1442-2042.2012.02961.x [DOI] [PubMed] [Google Scholar]
- 73.Mohamad O, Tabuchi T, Nitta Y, Nomoto A, Sato A, Kasuya G, et al. Risk of subsequent primary cancers after carbon ion radiotherapy, photon radiotherapy, or surgery for localised prostate cancer: a propensity score-weighted, retrospective, cohort study. Lancet Oncol 2019; 20: 674–85. doi: 10.1016/S1470-2045(18)30931-8 [DOI] [PubMed] [Google Scholar]
- 74.Lomax AJ. Myths and realities of range uncertainty. Br J Radiol 2020; 93: 20190582. doi: 10.1259/bjr.20190582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Johnson RP. Review of medical radiography and tomography with proton beams. Rep Prog Phys 2018; 81: 016701. doi: 10.1088/1361-6633/aa8b1d [DOI] [PubMed] [Google Scholar]
- 76.Johnson RP, Bashkirov V, DeWitt L, Giacometti V, Hurley RF, Piersimoni P, et al. A fast experimental scanner for proton CT: technical performance and first experience with phantom scans. IEEE Trans Nucl Sci 2016; 63: 52–60. doi: 10.1109/TNS.2015.2491918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Plautz T, Bashkirov V, Feng V, Hurley F, Johnson RP, Leary C, et al. 200 MeV proton radiography studies with a hand phantom using a prototype proton CT scanner. IEEE Trans Med Imaging 2014; 33: 875–81. doi: 10.1109/TMI.2013.2297278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schultze B, Censor Y, Karbasi P, Schubert KE, Schulte RW. An improved method of total variation Superiorization applied to reconstruction in proton computed tomography. IEEE Trans Med Imaging 2020; 39: 294–307. doi: 10.1109/TMI.2019.2911482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Traini G, Mattei I, Battistoni G, Bisogni MG, De Simoni M, Dong Y, et al. Review and performance of the dose Profiler, a particle therapy treatments online monitor. Phys Med 2019; 65: 84–93. doi: 10.1016/j.ejmp.2019.07.010 [DOI] [PubMed] [Google Scholar]
- 80.Kanematsu N, Furukawa T, Hara Y, Inaniwa T, Iwata Y, Mizushima K, Yoshiyuki I, Kota M, et al. New technologies for carbon-ion radiotherapy — developments at the National Institute of radiological sciences, QST, Japan. Radiation Physics and Chemistry. 2019; 162: 90–5. doi: 10.1016/j.radphyschem.2019.04.038 [DOI] [Google Scholar]
- 81.Winter M, Dokic I, Schlegel J, Warnken U, Debus J, Abdollahi A, et al. Deciphering the acute cellular phosphoproteome response to irradiation with X-rays, protons and carbon ions. Mol Cell Proteomics 2017; 16: 855–72. doi: 10.1074/mcp.M116.066597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Konings K, Vandevoorde C, Belmans N, Vermeesen R, Baselet B, Walleghem MV, et al. The combination of particle irradiation with the hedgehog inhibitor GANT61 differently modulates the radiosensitivity and migration of cancer cells compared to X-ray irradiation. Front Oncol 2019; 9: 391. doi: 10.3389/fonc.2019.00391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Suetens A, Moreels M, Quintens R, Soors E, Buset J, Chiriotti S, et al. Dose- and time-dependent gene expression alterations in prostate and colon cancer cells after in vitro exposure to carbon ion and x-irradiation. J Radiat Res 2015; 56: 11–21. doi: 10.1093/jrr/rru070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Blyth BJ, Kakinuma S, Sunaoshi M, Amasaki Y, Hirano-Sakairi S, Ogawa K, et al. Genetic analysis of T cell lymphomas in carbon Ion-Irradiated mice reveals frequent interstitial chromosome deletions: implications for second cancer induction in normal tissues during carbon ion radiotherapy. PLoS One 2015; 10: e0130666. doi: 10.1371/journal.pone.0130666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fujita M, Imadome K, Shoji Y, Isozaki T, Endo S, Yamada S, et al. Carbon-Ion irradiation suppresses migration and invasiveness of human pancreatic carcinoma cells MIAPaCa-2 via Rac1 and RhoA degradation. Int J Radiat Oncol Biol Phys 2015; 93: 173–80. doi: 10.1016/j.ijrobp.2015.05.009 [DOI] [PubMed] [Google Scholar]
- 86.Harada R, Kawamoto T, Ueha T, Minoda M, Toda M, Onishi Y, et al. Reoxygenation using a novel CO2 therapy decreases the metastatic potential of osteosarcoma cells. Exp Cell Res 2013; 319: 1988–97. doi: 10.1016/j.yexcr.2013.05.019 [DOI] [PubMed] [Google Scholar]
- 87.Ogata T, Teshima T, Inaoka M, Minami K, Tsuchiya T, Isono M, et al. Carbon ion irradiation suppresses metastatic potential of human non-small cell lung cancer A549 cells through the phosphatidylinositol-3-kinase/Akt signaling pathway. J Radiat Res 2011; 52: 374–9. doi: 10.1269/jrr.10102 [DOI] [PubMed] [Google Scholar]
- 88.Durante M. New challenges in high-energy particle radiobiology. Br J Radiol 2014; 87: 20130626. doi: 10.1259/bjr.20130626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mazal A, Prezado Y, Ares C, de Marzi L, Patriarca A, Miralbell R, et al. Flash and minibeams in radiation therapy: the effect of microstructures on time and space and their potential application to protontherapy. Br J Radiol 2020; 93: 20190807. doi: 10.1259/bjr.20190807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Durante M, Formenti S. Harnessing radiation to improve immunotherapy: better with particles? Br J Radiol 2020; 93: 20190224. doi: 10.1259/bjr.20190224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Blakely EA, Lauriston S. Lauriston S. Taylor Lecture on radiation protection and measurements: what makes particle radiation so effective? Health Phys 2012; 103: 508–28. doi: 10.1097/HP.0b013e31826a5b85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cucinotta FA, Durante M. Cancer risk from exposure to galactic cosmic rays: implications for space exploration by human beings. Lancet Oncol 2006; 7: 431–5. doi: 10.1016/S1470-2045(06)70695-7 [DOI] [PubMed] [Google Scholar]
- 93.Durante M, Paganetti H. Nuclear physics in particle therapy: a review. Rep Prog Phys 2016; 79: 096702. doi: 10.1088/0034-4885/79/9/096702 [DOI] [PubMed] [Google Scholar]
- 94.Charles JB, Pietrzyk RA. A year on the International space Station: implementing a long-duration biomedical research mission. Aerosp Med Hum Perform 2019; 90: 4–11. doi: 10.3357/AMHP.5178.2019 [DOI] [PubMed] [Google Scholar]
- 95.Furukawa S, Nagamatsu A, Nenoi M, Fujimori A, Kakinuma S, Katsube T, et al. Space Radiation Biology for "Living in Space". Biomed Res Int 2020; 2020: 1–25. doi: 10.1155/2020/4703286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Walsh L, Schneider U, Fogtman A, Kausch C, McKenna-Lawlor S, Narici L, et al. Research plans in Europe for radiation health hazard assessment in exploratory space missions. Life Sci Space Res 2019; 21: 73–82. doi: 10.1016/j.lssr.2019.04.002 [DOI] [PubMed] [Google Scholar]
- 97.Pei W, Hu W, Chai Z, Zhou G. Current status of space radiobiological studies in China. Life Sci Space Res 2019; 22: 1–7. doi: 10.1016/j.lssr.2019.05.001 [DOI] [PubMed] [Google Scholar]
- 98.Reynolds RJ, Day SM. Mortality of US astronauts: comparisons with professional athletes. Occup Environ Med 2019; 76: 114–7. doi: 10.1136/oemed-2018-105304 [DOI] [PubMed] [Google Scholar]
- 99.Cucinotta FA, Kim M-H, Chapell L J. Space Radiation Cancer Risk Projections and Uncertainties - 2012. 2012;NASA/TP-2013-217375.
- 100.Badhwar GD, O'Neill PM. Galactic COSMIC radiation model and its applications. Adv Space Res 1996; 17: 7–17. doi: 10.1016/0273-1177(95)00507-B [DOI] [PubMed] [Google Scholar]
- 101.Cucinotta FA, Cacao E, Kim M-HY, Saganti PB. Non-Targeted effects lead to a PARIDIGM shift in risk assessment for a mission to the earth's moon or Martian moon PHOBOS. Radiat Prot Dosimetry 2019; 183(1-2): 213–8. doi: 10.1093/rpd/ncy264 [DOI] [PubMed] [Google Scholar]
- 102.Cucinotta FA, Chappell LJ, Kim M-HY, Wang M. Radiation carcinogenesis risk assessments for never-smokers. Health Phys 2012; 103: 643–51. doi: 10.1097/HP.0b013e318267b3ad [DOI] [PubMed] [Google Scholar]
- 103.Elgart SR, Little MP, Chappell LJ, Milder CM, Shavers MR, Huff JL, et al. Radiation exposure and mortality from cardiovascular disease and cancer in early NASA astronauts. Sci Rep 2018; 8: 8480. doi: 10.1038/s41598-018-25467-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kasaboski D, Hill H. Galactic Cosmis Radiation Risk in Human Space Missions. In: editors.65th International Astronautical Congress. Toronto, Canada: International Astronautical Federation; 2014. [Google Scholar]
- 105.Reynolds RJ, Bukhtiyarov IV, Tikhonova GI, Day SM, Ushakov IB, Gorchakova TYU. Contrapositive logic suggests space radiation not having a strong impact on mortality of US astronauts and Soviet and Russian cosmonauts. Sci Rep 2019; 9: 8583. doi: 10.1038/s41598-019-44858-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Reynolds RJ, Day SM. The effect of competing risks on astronaut and cosmonaut mortality. Life Sci Space Res 2018; 18: 35–41. doi: 10.1016/j.lssr.2018.05.006 [DOI] [PubMed] [Google Scholar]
- 107.Hassler DM, Zeitlin C, Wimmer-Schweingruber RF, Ehresmann B, Rafkin S, Eigenbrode JL, et al. Mars' surface radiation environment measured with the Mars science laboratory's curiosity rover. Science 2014; 343: 1244797. doi: 10.1126/science.1244797 [DOI] [PubMed] [Google Scholar]
- 108.Zeitlin C, Hassler DM, Cucinotta FA, Ehresmann B, Wimmer-Schweingruber RF, Brinza DE, et al. Measurements of energetic particle radiation in transit to Mars on the Mars science laboratory. Science 2013; 340: 1080–4. doi: 10.1126/science.1235989 [DOI] [PubMed] [Google Scholar]
- 109.Zeitlin C, Hassler DM, Ehresmann B, Rafkin SCR, Guo J, Wimmer-Schweingruber RF, et al. Measurements of radiation quality factor on Mars with the Mars science laboratory radiation assessment detector. Life Sci Space Res 2019; 22: 89–97. doi: 10.1016/j.lssr.2019.07.010 [DOI] [PubMed] [Google Scholar]
- 110.Huang EG, Wang R-Y, Xie L, Chang P, Yao G, Zhang B, et al. Simulating galactic COSMIC ray effects: synergy modeling of murine tumor prevalence after exposure to two one-ion beams in rapid sequence. Life Sci Space Res 2020; 25: 107–18. doi: 10.1016/j.lssr.2020.01.001 [DOI] [PubMed] [Google Scholar]