Abstract
There have been major advances in myeloma imaging over the past few years with focal lesions on imaging now forming part of the disease defining criteria. Whole body diffusion-weighted MRI (WB-MRI) is considered the most sensitive technique for the detection of focal active lesions. This pictorial review will focus on imaging the spectrum of myelomatous disorders on WB-MRI including diffusion and Dixon sequences. The typical imaging patterns of disease are demonstrated including in the contexts of staging, presumed solitary plasmacytoma, smouldering myeloma and examples of paramedullary and extramedullary disease. The utility of diffusion-weighted imaging in response assessment is a major advantage and this will be exemplified here.
Introduction
Multiple myeloma (MM) is a plasma cell proliferative disorder which is usually preceded by precursor stages termed monoclonal gammopathy of undetermined significance (MGUS) and smouldering MM (SMM). It is characterised by monoclonal plasma cells in bone marrow (or extramedullary), monoclonal protein in the blood or urine, and myeloma-related organ damage. The International Myeloma Working Group (IMWG) criterion now state that more than one focal lesion on MRI is a myeloma defining event.1,2 Therefore, imaging now takes a pivotal role in the diagnosis, work-up and follow-up of myeloma patients. Whole body MRI (WB-MRI) with diffusion-weighted imaging has emerged as the most sensitive technique for assessing patients with plasma cell disorders and MM. MRI may detect focal myelomatous disease early which permits appropriate treatment stratification with survival benefit.3,4 Additionally, the number of lesions has an impact on prognosis in terms of overall survival.5
Current guidelines, recognising that novel imaging techniques are not universally available, typically offer a range of imaging investigations, including low-dose whole body CT and FDG-PET/CT, while recognising the value of WB-MRI.1,6,7
Our group has previously published a detailed review article in this journal on advanced imaging in myeloma which covers the typical protocols, advantages and disadvantages of each imaging technique, as well as the evidence base for WB-MRI use.8 This pictorial review is complimentary to that article, providing more detailed examples of the typical patterns of myeloma on WB-MRI and changes with response.
Whole body MRI imaging protocol
Detailed international guidance on core and more comprehensive WB-MRI protocols for myeloma have been recently published.9 The core Myeloma Response Assessment and Diagnosis System (MY-RADS) protocol (Table 1) includes sagittal whole spine T1W and fat-suppressed T2W sequences, axial whole body (skull vertex to knees) b50 and b900 diffusion-weighted imaging (DWI)/apparent diffusion coefficient (ADC) map and axial whole body T1W Dixon sequences (in-phase (IP), out-of-phase (OP), water-only (WO) and fat-only (FO)). An inverted grey scale b900 maximum intensity projection (MIP) is useful for global tumour assessment and displaying disease in multidisciplinary meetings but should not be used in isolation since false-positives (e.g., T2 shine-through) and false-negatives can occur. All images in this review were acquired on a 1.5 T Siemens Aera with total acquisition time of 50 minutes.
Table 1.
Core MY-RADS8 whole body MRI protocol and the rationale for each imaging sequence. ADC, apparent diffusion coefficient; MIP, maximum intensity projection
| Core protocol | Rationale |
|---|---|
| 1. Sagittal T1W and T2W fat suppressed sequences of the whole spine | Anatomy, compression fractures, cord and neural compromise, T1 marrow signal, focal lesions |
| 2. Axial diffusion-weighted sequences (typical b values of 50 and 900 sec/mm2) 5 mm contiguous sections covering vertex to knees with corresponding ADC map | To assess for diffuse marrow and focal osseous and extraosseous increased cellularity indicating active versus inactive disease, crucial for staging and response assessment |
| 3. Axial T1W Dixon sequences (in-phase, out-of-phase, water-only and fat-only sequences) 5 mm contiguous sections covering vertex to knees | To assess for focal and diffuse osseous disease and to further characterise osseous and extraosseous structures with more anatomical detail |
| 4. Regional assessments if required | To further characterise known sites of disease, extramedullary disease or to assess for cord/ neural compromise |
| 5. Post-processing 3-dimensional MIP (and inverted MIP) reformats of the b900 sequence |
Knitting axial sequences together and generating MIP reformats to enable accurate and easier image reading and comparison between sequences and different studies |
Assessing for disease involvement
Normal bone marrow
The diffusion-weighted MRI sequences reflect cellular density. Normal background bone marrow has variable signal intensity on DWI that changes with age. Yellow bone marrow (e.g., femoral heads) has low signal intensity on the high b-values and ADC likely related to low cellular density and an abundance of fat cells, while red marrow has higher cellularity and water content contributing to higher signal intensity on high b-values and lower ADC (Table 2). With increasing age, red marrow converts to less cellular yellow marrow reflected by reduction in ADC values.10
Table 2.
Appearance of normal bone marrow, diffuse and focal active disease on the various whole-body MRI sequences
| MRI Sequence | |||||
|---|---|---|---|---|---|
| Pattern of Disease | T1 | FO | WO | b900 | ADC |
| Normal | SI > adjacent muscle or intervertebral disc | Similar SI to fat | Similar SI to fat | Varies with age | Varies with age & overlap of disease & normal but ADC typically <600–700 µm2/sec in untreated cases |
| Diffuse | Diffuse SI ≤ muscle or intervertebral disc | Diffuse low SI compared to extraosseous fat | Diffuse high SI compared to extraosseous fat | Diffuse high signal | Diffuse low signal (as above but typically >600–700 µm2/sec) |
| Focal activea | Focal SI ≤ muscle or intervertebral disc | Focal hypointense compared to background marrow | Focal hyperintense compared to background marrow | Focal high signal with corresponding abnormality on Dixon | Focal low signal |
FO, Fat only; SI, signal intensity; WO, water only.
Changes on both DWI and Dixon sequences are required to fulfil criteria for a focal active deposit.
Bone marrow involvement: Diffuse and focal disease
Myeloma lesions, either focal or diffuse, usually have signal intensity equal to or lower than that of muscle or non-degenerated intervertebral disc on T1W images (due to the replacement of marrow fat)11 and high signal on T2W fat-suppressed conventional MRI sequences.
WB-MRI may detect diffuse disease and focal lesions on a background of diffuse disease. The respective imaging characteristics of Dixon and diffusion sequences are summarised in Figures 1 and 2 and Table 2. Diffuse increased signal intensity on high b-value images throughout the marrow relative to normal muscle raises suspicion for diffuse disease but this must be confirmed with trephine biopsy (Figures 1b and 2b).9 There is some overlap in ADC values between normal bone marrow and diffuse disease but marrow ADC values above 600–700 µm2/sec in a non-treated, newly diagnosed MM patient have been suggested to increase confidence for the diagnosis of diffuse disease involvement.12,13
Figure 1.
Typical patterns on inverse grey scale b900 maximum intensity projection images of (a) normal bone marrow (age 55), (b) diffuse disease, (c) focal marrow disease and (d) focal on diffuse disease.
Figure 2.
MRI patterns of (a) normal bone marrow, (b) diffuse infiltration, (c) focal disease in the left iliac bone and (d) focal on diffuse myelomatous disease scattered throughout the pelvis, as demonstrated on axial FO Dixon, WO Dixon, b900 DWI and ADC map. Note in diffuse disease (b) the marrow is low signal on the FO, high signal compared to normal fatty marrow on the WO, high signal on b900 and low signal on ADC. Focal active disease (c) in the left iliac bone (arrows) is low signal compared to normal marrow on the FO, high signal on WO, high on b900 and low on ADC sequences. FO, fat only; WO, water only; b900, b value of 900 sec/mm2; ADC, apparent diffusion coefficient.
On the high b-value DWI images (800–900 sec/mm2), foci with signal intensity above surrounding marrow are considered suspicious but must be correlated with the corresponding ADC and Dixon sequences. DWI can be used to differentiate active and treated lesions with the former demonstrating high signal on DWI and low signal on ADC relative to adjacent muscle (Figures 1c and 2c), whereas treated lesions have high signal on both DWI and ADC (also known as T2 shine through).
Dixon sequences provide anatomical detail, complimentary to the functional diffusion sequences. They are considered important in reducing false-positive diagnoses of focal lesions on DWI.14 The separation of fat and water based on their chemical shift generates the four separate Dixon image sequences. Normal adult bone marrow typically consists of 50–90% fat and infiltration of marrow causes a reduction in the fat content.15 Focal myelomatous lesions are typically hypointense compared to background marrow on IP and FO sequences and hyperintense on the WO images (Figure 2).16 FO and WO images detect the most myeloma lesions compared to IP and OP images.17 Diffuse and focal-on-diffuse disease can be challenging to detect on Dixon sequences and focal lesions may become more apparent on follow-up response assessment imaging.
WB-MRI is significantly more sensitive than FDG-PET/CT for the detection of bone marrow disease although FDG-PET/CT is more specific.18 Potential false-positive high b-value focal lesions include bone marrow oedema from fractures, haemangiomas, infection, bone infarcts, focal fat poor marrow and artefacts around metal implants.9 Correlation with the Dixon sequences may improve specificity and the IMWG suggest that SMM patients with equivocal solitary small lesions should have a 3–6 month follow-up study.1,19
Staging MM
WB-MRI is the preferential imaging modality for staging MM.1,6,7 An example of staging WB-MRI is shown in Figure 3 and other staging examples in different circumstances are described in the following sections.
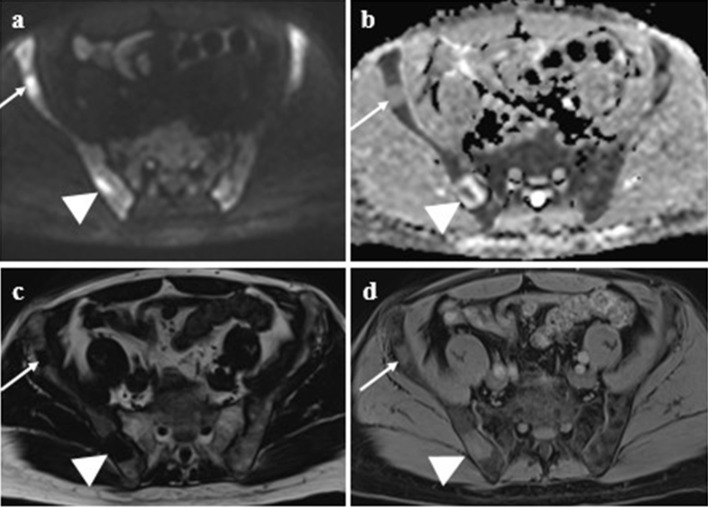
Figure 3.
A 76-year-old male staging WB-MRI. b900 inverted MIP image (a) demonstrates multifocal active disease with example sites (arrow) in the right ribcage with paramedullary disease (b–d) and right femoral shaft medullary disease (e–g) which is at potential fracture risk. Active disease has high signal on b900 DWI (b, e) and low signal on the ADC map (c, f) and high signal relative to normal background marrow on the WO Dixon sequences (d, g).
Paramedullary and extramedullary disease
WB-MRI may accurately visualise soft tissue disease adjacent to marrow disease (paramedullary) or separately at extraosseous sites (extramedullary disease). Extramedullary disease may be as solitary plasmacytoma formation or multifocal (Figures 4 and 5). Sites can include paraspinal or epidural locations, solid organs, nodes, retroperitoneum and skin.20
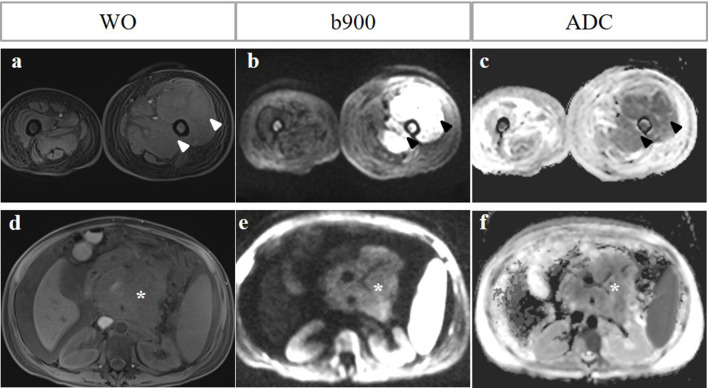
Figure 4.
Paramedullary versus extramedullary disease. A 51-year-old female with relapsed MM post-haematopoetic stem cell autograft. WO Dixon, b900 DWI and ADC map show paramedullary disease in the left mid femoral shaft with a large paramedullary mass (arrowheads, a-c). Note also extramedullary disease in the form of a retroperitoneal nodal mass and pancreatic involvement with associated ascites (asterisk, d–f). The signal intensity of the retroperitoneal mass of the b900 image is lower than expected as the patient had already commenced systemic therapy 7 days before the MRI.
Figure 5.
Examples of extramedullary disease in a series of different cases affecting the musculature - lesion in the left temporalis muscle restricted on ADC (a) and causing muscle thickening on in-phase Dixon (b); right adrenal gland and left kidney (arrows) on ADC (c) and left renal lesion on corresponding coronal T1 post-contrast image (d). A large mass in the right kidney upper pole and renal cortex (asterisk) on ADC (e) and b900 (f) biopsy proven as myeloma.
Solitary plasmacytoma
Solitary plasmacytoma usually occurs as a solitary bone plasmacytoma but in some cases may present as an extramedullary lesion. Whole-body imaging is important to exclude additional sites of disease which would constitute systemic MM (Figure 6).1 Recent IMWG guidance recommends WB-MRI as a first line, or alternatively, FDG-PET/CT, in presumed solitary plasmacytoma and extramedullary lesions. The risk of progression to MM or relapse is reported as 14–38% within 3 years,21 so yearly follow-up is recommended for the first 5 years, ideally with the same imaging technique used at initial diagnosis.1
Figure 6.
A 54-year-old male presenting with presumed T11 solitary plasmacytoma causing vertebral collapse and spinal cord compression (arrow; sagittal T1W spine, a). WB-MRI showed however further sites of focal active disease which are high signal on b900 DWI, low signal on ADC map and high signal on WO Dixon sequences at left posterior third rib (arrow; b–d) and right posterior iliac bone (arrow; e–g). Imaging findings are in keeping with multiple myeloma rather than solitary plasmacytoma changing clinical management from targeted radiotherapy to systemic therapy.
Smouldering multiple myeloma (SMM)
Early intervention in high-risk SMM is crucial as it delays progression to active disease and improves overall survival with MRI serving as a prognostic biomarker.22
Symptomatic disease warranting therapy is defined by more than one lesion measuring at least 5 mm. It is important to detect such active lesions on WB-MRI as this fulfils criteria for therapy (Figure 7). Equivocal lesions should be followed-up with WB-MRI in 3–6 months to detect any progression.1
Figure 7.
A 60-year-old female with smouldering multiple myeloma. WB-MRI detected focal active disease in the left sacral ala measuring 20 mm (arrows) showing high signal on b900 DWI (a) with low signal on ADC map (b) and FO Dixon (c) and intermediate signal on WO Dixon sequences (b–d). Note the background marrow also appears hypercellular-diffuse high b900 and low ADC marrow signal. The detection of a focal active lesion fulfils the criteria for a high-risk biomarker requiring therapy.
Treatment response assessment
Response assessment can be assessed by changes on diffusion-weighted imaging and Dixon sequences (Figures 8–10). In response to treatment, bone marrow starts to return to its usual fatty composition with a corresponding increase in ADC values and decreased signal on high b-value images (Figures 8 and 9A). Dixon sequences show increased signal on FO and decreased signal on WO sequences as compared to pre-treatment (Figure 9B). Reduction in size of focal lesions may be accompanied by intra- or peri-lesional fat formation. When diffuse disease responds to treatment, focal lesions may become apparent which were previously masked by the diffuse active disease (Figure 9A). Conversely, disease progression is suggested by the finding of new lesions greater than 5 mm, increasing size or high b-value signal intensity of focal lesions (Figure 10A and B), new diffuse disease, or conversion of focal to diffuse disease.9
Figure 8.
A 77-year-old male with multiple myeloma had restaging WB-MRI showing partially treated disease; lesion in the anterior right iliac blade (arrow) shows high signal on b900 DWI (a) and intermediate signal on ADC (b) in keeping with partially treated disease. The lesion in the posterior right iliac bone (arrowhead) has peripheral high ADC signal which appears to be treated but has a central low signal component in keeping with active disease. Both lesions on FO (c) and WO Dixon sequences (d) show residual low and high signal, respectively, compared to background marrow.
Figure 9.

(A). 70-year-old male had WB-MRI showing focal on diffuse active disease on initial imaging as demonstrated on b900 inverted MIP image (a), b900 DWI image (b) shows high signal diffusely and focally in the right sacrum (arrow) and diffuse low signal on ADC map (c). Post-treatment follow-up WB-MRI 6 months later shows improvement on MY-RADS Response Assessment Criteria (RAC) = RAC1 ‘Highly likely to be responding’ as reduction in b900 signal (d and e) and higher ADC signal >1400 (f) in the residual focal lesion in the right sacrum (arrow). (B). Treatment response demonstrated in figure 9A corresponds with typical changes on Dixon sequences pre- and post-treatment; reduction in signal intensity on WO Dixon sequence (a, b) and increase in signal on FO Dixon sequence (c, d). Note also a focal lesion in the right S1 level (arrow) becomes more apparent with treatment as previously masked by diffuse marrow involvement in addition to the focal disease.
Figure 10.
(A). Response assessment WB-MRI in a 75-year-old male with IgG multiple myeloma treated with VCD chemotherapy. Based on biochemical markers, patient achieved partial response but rising serum paraprotein was suspicious for relapse. b900 MIP images (a, f) show development of focal on diffuse disease. b900 axial images show new patchy increased signal in the pelvis post-treatment (b, d) although the intensity of a focal lesion in the left ilium appears improved. ADC signal is now diffusely low suggesting active disease (c, e). VCD, Velcade (bortezomib), cyclophosphamide and dexamethasone. (B). Corresponding changes on T1 sagittal lumbar spine and Dixon sequences from case in 10A. There is increasing vertebral body collapse and multifocal new lesions (a, f), and numerous new low and high signal lesions on FO (b, d) and WO (c, e) Dixon sequences, respectively.
More recently, there has been guidance on response assessment using WB-MRI. The Myeloma Response Assessment and Diagnosis System, or MY-RADS, uses response assessment categories of highly likely to be responding, likely to be responding, no change, likely to be progressing and highly likely to be progressing.9 This may act as a standardised decision support tool to guide change in management and patient follow up.
There is potential for fat fraction quantification in response evaluation. Fat fraction maps are calculated from Dixon sequences and may serve as a potential early biomarker for disease response. Response to treatment leads to an increase in the signal fat fraction (sFF) as the fatty marrow signal returns and early changes on sFF have been shown to predict response.23,24
False-positives (and incidental findings)
It is important to be aware that trephine tracts from biopsy and resultant blood products can show diffusion restriction and may mimic active disease (Figure 11).25 Please note that DWI has a role in the decision-making for a representative trephine sampling target site.9,25 Other causes for falsely high b-values include haemangiomas and bone marrow oedema related to fractures without an underlying focal lesion.
Figure 11.
Bone marrow biopsy tract and incidental findings: A right iliac trephine biopsy tract and blood products can show diffusion restriction and mimic focal active disease (arrow; b900 DWI and ADC map; a, b). Incidental descending colonic tumour (arrow) as a mass of hypercellularity on b900 DWI (c), subsequently confirmed as an annular mass with likely extramural venous invasion and regional lymphadenopathy on CT colonography (d).
Broad coverage of WB-MRI may also detect other abnormalities including synchronous malignancies (Figure 11).26 Review of all imaging sequences is important to ensure incidental findings that may influence management are not missed.
Granulocyte colony-stimulating factor (G-CSF) employed in MM treatment can make the bone marrow hypercellular with resultant diffusion restriction. This can mimic diffuse disease or obscure active lesions.17 Therefore, it is important to document and ideally avoid WB-MRI and FDG-PET/CT in the first few days after G-CSF therapy.
Conclusions and future directions
Whole-body diffusion-weighted MRI is the most sensitive imaging technique for detecting focal and diffuse marrow disease. It may be effectively used in defining disease, re-staging and assessing response to treatment, in addition to acting as a problem-solving tool.
In future, response assessment may be enhanced by combining diffusion-weighted imaging with fat fraction maps from the Dixon sequence. It is likely that WB-MRI will have an increasing role in response assessment and to guide management in non-responding patients. MRI-based radiomics features are being explored as biomarkers of response and outcome in myeloma.27 Hybrid FDG-PET/MR may represent an interesting area for future research in MM imaging. It may combine the superior sensitivity of WB-MRI in focal lesion detection with the improved treatment response assessment of FDG-PET/CT. Thus, there is the potential to include both anatomical and functional data. This technique may have a role in risk stratification.
Teaching points
Whole-body MRI is now incorporated into international standards for imaging patients with myeloma and includes anatomical and functional components.
Active disease demonstrates high signal on high b-value DWI sequences and low signal on ADC map relative to adjacent muscle, whereas treated lesions have high signal on both DWI and ADC map.
Similarly active lesions demonstrate high signal on water-only Dixon sequences and low signal on fat-only Dixon sequences.
Paramedullary and extramedullary disease is also well-demonstrated on WB-MRI.
MY-RADS provides standardisation of acquisition and reporting.
Footnotes
Acknowledgment: TB and AR acknowledge support from the NIHR Biomedical Research Centre to Imperial College London and Imperial CRUK centre. CM acknowledges CRUK and EPSRC support to the Cancer Imaging Centre at ICR and RMH in association with MRC and Department of Health C1060/A10334, C1060/A16464 and NHS funding to the NIHR Biomedical Research Centre, Clinical Research Facility in Imaging and the Cancer Research Network. The views expressed in this publication are those of the author(s) and not necessarily those of the NHS, the National Institute for Health Research or the Department of Health.
Contributor Information
Maira Hameed, Email: m.hameed@ic.ac.uk.
Amandeep Sandhu, Email: amandeep.sandhu2@nhs.net.
Neil Soneji, Email: neil.soneji@nhs.net.
Dimitri Amiras, Email: dimitri.amiras@nhs.net.
Andrea Rockall, Email: andrea.rockall@nhs.net.
Christina Messiou, Email: christina.messiou@icr.ac.uk.
Kathryn Wallitt, Email: kathryn.wallitt@nhs.net.
Tara D Barwick, Email: tara.barwick@nhs.net.
REFERENCES
- 1.Hillengass J, Usmani S, Rajkumar SV, Durie BGM, Mateos M-V, Lonial S, et al. International myeloma Working group consensus recommendations on imaging in monoclonal plasma cell disorders. Lancet Oncol 2019; 20: e302–12. doi: 10.1016/S1470-2045(19)30309-2 [DOI] [PubMed] [Google Scholar]
- 2.Rajkumar SV, Dimopoulos MA, Palumbo A, Blade J, Merlini G, Mateos M-V, et al. International myeloma Working group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol 2014; 15: e538–48. doi: 10.1016/S1470-2045(14)70442-5 [DOI] [PubMed] [Google Scholar]
- 3.Merz M, Hielscher T, Wagner B, Sauer S, Shah S, Raab MS, et al. Predictive value of longitudinal whole-body magnetic resonance imaging in patients with smoldering multiple myeloma. Leukemia 2014; 28: 1902–8. doi: 10.1038/leu.2014.75 [DOI] [PubMed] [Google Scholar]
- 4.Hillengass J, Fechtner K, Weber M-A, Bäuerle T, Ayyaz S, Heiss C, et al. Prognostic significance of focal lesions in whole-body magnetic resonance imaging in patients with asymptomatic multiple myeloma. Journal of Clinical Oncology 2010; 28: 1606–10. doi: 10.1200/JCO.2009.25.5356 [DOI] [PubMed] [Google Scholar]
- 5.Hillengass J, Ayyaz S, Kilk K, Weber M-A, Hielscher T, Shah R, et al. Changes in magnetic resonance imaging before and after autologous stem cell transplantation correlate with response and survival in multiple myeloma. Haematologica 2012; 97: 1757–60. doi: 10.3324/haematol.2012.065359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Overview | Myeloma: diagnosis and management | Guidance | NICE [Internet].. . 2016. Available from: https://www.nice.org.uk/guidance/ng35 [cited 20 March 2020].
- 7.Chantry A, Kazmi M, Barrington S, Goh V, Mulholland N, Streetly M, et al. Guidelines for the use of imaging in the management of patients with myeloma. Br J Haematol 2017; 178: 380–93. doi: 10.1111/bjh.14827 [DOI] [PubMed] [Google Scholar]
- 8.Barwick T, Bretsztajn L, Wallitt K, Amiras D, Rockall A, Messiou C. Imaging in myeloma with focus on advanced imaging techniques. Br J Radiol 2019; 92: 20180768. doi: 10.1259/bjr.20180768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Messiou C, Hillengass J, Delorme S, Lecouvet FE, Moulopoulos LA, Collins DJ, et al. Guidelines for acquisition, interpretation, and reporting of whole-body MRI in myeloma: myeloma response assessment and diagnosis system (MY-RADS. Radiology 2019; 291: 5–13. doi: 10.1148/radiol.2019181949 [DOI] [PubMed] [Google Scholar]
- 10.Lavdas I, Rockall AG, Castelli F, Sandhu RS, Papadaki A, Honeyfield L, et al. Apparent diffusion coefficient of normal abdominal organs and bone marrow from whole-body DWI at 1.5 T: the effect of sex and age. American Journal of Roentgenology 2015; 205: 242–50. doi: 10.2214/AJR.14.13964 [DOI] [PubMed] [Google Scholar]
- 11.Carroll KW, Feller JF, Tirman PFJ. Useful internal standards for distinguishing infiltrative marrow pathology from hematopoietic marrow at MRI. J. Magn. Reson. Imaging 1997; 7: 394–8. doi: 10.1002/jmri.1880070224 [DOI] [PubMed] [Google Scholar]
- 12.Messiou C, Collins DJ, Morgan VA, deSouza NM. Optimising diffusion weighted MRI for imaging metastatic and myeloma bone disease and assessing reproducibility. Eur Radiol 2011; 21: 1713–8. doi: 10.1007/s00330-011-2116-4 [DOI] [PubMed] [Google Scholar]
- 13.Koutoulidis V, Fontara S, Terpos E, Zagouri F, Matsaridis D, Christoulas D, et al. Quantitative diffusion-weighted imaging of the bone marrow: an adjunct tool for the diagnosis of a diffuse MR imaging pattern in patients with multiple myeloma. Radiology 2017; 282: 484–93. doi: 10.1148/radiol.2016160363 [DOI] [PubMed] [Google Scholar]
- 14.Dutoit JC, Verstraete KL, Whole-body MRI. Whole-Body MRI, dynamic contrast-enhanced MRI, and diffusion-weighted imaging for the staging of multiple myeloma. Skeletal Radiol 2017; 46: 733–50. doi: 10.1007/s00256-017-2609-6 [DOI] [PubMed] [Google Scholar]
- 15.Takasu M, Kaichi Y, Tani C, Date S, Akiyama Y, Kuroda Y, et al. Iterative decomposition of water and fat with echo asymmetry and least-squares estimation (ideal) magnetic resonance imaging as a biomarker for symptomatic multiple myeloma. PLoS One 2015; 10: e0116842. doi: 10.1371/journal.pone.0116842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singh S, Pilavachi E, Dudek A, Bray TJP, Latifoltojar A, Rajesparan K, et al. Whole body MRI in multiple myeloma: optimising image acquisition and read times. PLoS One 2020; 15: e0228424. doi: 10.1371/journal.pone.0228424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bray TJP, Singh S, Latifoltojar A, Rajesparan K, Rahman F, Narayanan P, et al. Diagnostic utility of whole body Dixon MRI in multiple myeloma: a multi-reader study. PLoS One 2017; 12: e0180562. doi: 10.1371/journal.pone.0180562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gariani J, Westerland O, Natas S, Verma H, Cook G, Goh V. Comparison of whole body magnetic resonance imaging (WBMRI) to whole body computed tomography (WBCT) or 18 F-fluorodeoxyglucose positron emission tomography/CT (18 F-FDG PET/CT) in patients with myeloma: Systematic review of diagnostic performance. Crit Rev Oncol Hematol 2018; 124: 66–72. doi: 10.1016/j.critrevonc.2018.02.012 [DOI] [PubMed] [Google Scholar]
- 19.Dimopoulos MA, Hillengass J, Usmani S, Zamagni E, Lentzsch S, Davies FE, et al. Role of magnetic resonance imaging in the management of patients with multiple myeloma: a consensus statement. Journal of Clinical Oncology 2015; 33: 657–64. doi: 10.1200/JCO.2014.57.9961 [DOI] [PubMed] [Google Scholar]
- 20.Tirumani SH, Shinagare AB, Jagannathan JP, Krajewski KM, Munshi NC, Ramaiya NH. Mri features of extramedullary myeloma. American Journal of Roentgenology 2014; 202: 803–10. doi: 10.2214/AJR.13.10856 [DOI] [PubMed] [Google Scholar]
- 21.Nahi H, Genell A, Wålinder G, Uttervall K, Juliusson G, Karin F, et al. Incidence, characteristics, and outcome of solitary plasmacytoma and plasma cell leukemia. population-based data from the Swedish myeloma register. Eur J Haematol 2017; 99: 216–22. doi: 10.1111/ejh.12907 [DOI] [PubMed] [Google Scholar]
- 22.Mateos M-V, Hernández M-T, Giraldo P, de la Rubia J, de Arriba F, Corral LL, et al. Lenalidomide plus dexamethasone for high-risk smoldering multiple myeloma. New England Journal of Medicine 2013; 369: 438–47. doi: 10.1056/NEJMoa1300439 [DOI] [PubMed] [Google Scholar]
- 23.Latifoltojar A, Hall-Craggs M, Bainbridge A, Rabin N, Popat R, Rismani A, et al. Whole-body MRI quantitative biomarkers are associated significantly with treatment response in patients with newly diagnosed symptomatic multiple myeloma following bortezomib induction. European Radiology. 2017;27(12):5325-5336. Messiou C, Kaiser M. Whole-Body Imaging in Multiple Myeloma. Magnetic Resonance Imaging Clinics of North America 2018; 26: 509–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Latifoltojar A, Hall-Craggs M, Rabin N, Popat R, Bainbridge A, Dikaios N, et al. Whole body magnetic resonance imaging in newly diagnosed multiple myeloma: early changes in lesional signal fat fraction predict disease response. Br J Haematol 2017; 176: 222–33. doi: 10.1111/bjh.14401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Messiou C, Kaiser M. Whole-Body imaging in multiple myeloma. Magn Reson Imaging Clin N Am 2018; 26: 509–25. doi: 10.1016/j.mric.2018.06.006 [DOI] [PubMed] [Google Scholar]
- 26.Wale A, Pawlyn C, Kaiser M, Messiou C, Frequency MC. Frequency, distribution and clinical management of incidental findings and extramedullary plasmacytomas in whole body diffusion weighted magnetic resonance imaging in patients with multiple myeloma. Haematologica 2016; 101: e142–4. doi: 10.3324/haematol.2015.139816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ekert K, Hinterleitner C, Baumgartner K, Fritz J, Horger M. Extended texture analysis of Non-Enhanced whole-body MRI image data for response assessment in multiple myeloma patients undergoing systemic therapy. Cancers 2020; 12: 761. doi: 10.3390/cancers12030761 [DOI] [PMC free article] [PubMed] [Google Scholar]