Abstract
Objectives:
To determine the performance of pretreatment structural and arterial spin labelling (ASL) MRI in predicting p53 mutation in patients with high-grade gliomas (HGGs).
Methods:
Pre-treatment structural and ASL MRI were performed in 57 patients with histologically confirmed HGGs and information of p53 status. Whole-lesion histogram analysis of cerebral blood flow (CBF) images of the enhancing tumour and the peritumoral oedema in the HGGs were performed. Visually AcceSAble Rembrandt Images features were used as qualitative analysis. The differences of ASL histogram parameters and Visually AcceSAble Rembrandt Images features between HGGs with or without p53 mutation were analyzed with post hoc correction for multiple comparisons. LASSO regression was performed to select the optimal features that could predict p53 mutation, followed by receiver operating characteristic analysis to determine the predictive efficacy.
Results:
A total of 33 HGGs with p53 mutation and 24 without p53 mutation were included. HGGs with mutant p53 showed lower CBFpercentile5 and CBFuniformity of the enhancing tumour (p < 0.05) and higher prevalence of the qualitative MRI feature of enhancing tumour crossing midline (ETCM) (p < 0.05) as compared with HGGs with wild-type p53. LASSO regression showed that the CBFuniformity of the enhancing tumour and ETCM were predictive features for p53 mutation. CBFuniformity showed an acceptable performance in predicting p53 mutation (area under the curve = 0.721), when combined with the feature of ETCM, its predictive efficacy was significantly improved (area under the curve = 0.814, p = 0.012).
Conclusion:
An integrated pre-treatment structural and ASL MRI can help to predict p53 mutation in HGGs.
Introduction
The 2016 WHO Classification of Tumours of the central nervous system highlights the integration of genetic profiles in the precise definition of distinct glioma entities.1 The tumour suppressor gene p53, as the most frequently mutated gene in human cancers, has attracted much interest.2 Indeed, p53 mutation plays a crucial role in glioma progression and resistance to temozolomide chemotherapy in high-grade glioma (HGGs).3 Recently, p53-based therapeutic regimens, such as gene therapy to rehabilitate p53 function or target therapy to eliminate mutant p53 provide alternative therapeutic options for HGGs with mutant p53 and hold great promise to improve the survival of those patients.4,5
To date, the most commonly used techniques for the detection of aberrant p53 in HGGs are histological analyses, which need histologic specimens that are obtained by surgical resection or stereotactic biopsy.6 Nonetheless, both methods have inherent limitations, such as procedure-related complications, interobserver variability and sampling errors.6 Thus, a non-invasive method to identify p53 mutation is preferable and sometimes mandatory, when the patient cannot receive surgery due to poor general condition or when the tumour involves or is adjacent to the important brain area.
MRI is the mainstay of imaging modalities for the pre-treatment diagnosis of brain tumours, due to its excellent soft tissue resolution and absence of radiation. Previously, anatomic MR images-based radiomic features have been found to be correlated with p53 phenotype in lower-grade gliomas (WHO stages II and III).7,8 Whereas, quantitative MRI features with the ability to predict p53 mutation in HGGs still need to be explored. Arterial spin labelling (ASL) is an advanced MRI technique that can assess cerebral blood flow (CBF) quantitatively without the need of contrast medium.9 As the p53 tumour suppressor pathway plays an essential role in the regulation of tumour angiogenesis,10,11 and can thereby affect the tumour blood flow; we hypothesised that the ASL imaging holds the potential to predict p53 mutation. Nonetheless, whether ASL imaging could be used to predict aberrant p53 in HGGs remains unknown so far.
In this study, we investigated the ability of whole-lesion histogram parameters of CBF derived from ASL combined with qualitative MRI features to predict p53 mutation in HGGs. The purpose of our study was to determine the performance of pre-treatment structural and ASL MRI in predicting p53 mutation in patients with HGGs.
Methods and materials
Patients
This study was approved by our institutional research ethics board (Guangdong 999 Brain Hospital), and written informed consent was obtained from all participants. Consecutive patients with brain tumours, who underwent MRI between March 2016 and March 2019, were identified from the Picture Archiving and Communication System of our institution (Guangdong 999 Brain Hospital). The inclusion criteria were: (a) patients with a pathological diagnosis of HGG according to the WHO 2016 classification of brain tumours, (b) patients with a pathologically confirmed status of p53 mutation, (c) patients who underwent structural and ASL MRI prior to surgery or biopsy. A total of 61 patients met the inclusion criteria. Patients were excluded if the image quality of structural or ASL MRI was ineligible for data analysis (n = 4). Finally, 57 patients were included in our study.
MRI
All patients included in our study underwent structural and ASL MRI. MRI was performed on a 3.0 T scanner (Signa HDxt, General Electric Medical System, Milwaukee, WI) with an eight-channel head coil. Structural MRI consisted of axial and sagittal fast spin echo (FSE) T2 weighted (T2W) imaging (repetition time/echo time [TR]/[TE], 4480 ms/120 ms; matrix size, 256 × 256; field of view [FOV], 240 × 240 mm2; slice thickness, 4 mm; slice gap, 0 mm; number of excitation, 1), axial FSE T1 weighted (T1W) imaging (TR/TE, 1900 ms/24 ms; inversion time [TI], 780 ms; matrix size, 256 × 256; FOV, 240 × 240 mm2 ; slice thickness, 4 mm; slice gap, 0 mm; number of excitation, 1), and axial T2W fluid-attenuated inversion recovery imaging (FLAIR) (TR/TE, 9480 ms/120 ms; TI, 2300 ms; matrix size, 256 × 256; FOV, 240 × 240 mm2 ; slice thickness, 4 mm; slice gap, 0 mm; number of excitation, 1). ASL MRI was performed by using 3D pseudocontinuous ASL (3D-pCASL) technique, as it can provide a higher signal-to-noise ratio and a reduced magnetisation transfer effect than pulsed ASL or continuous ASL.12 3D-pCASL images were acquired by using a spiral FSE sequence (TR/TE, 4599 ms/9.8 ms; matrix size, 512 × 512; FOV, 240 × 240 mm2; number of excitation, 3; slice thickness, 4 mm; slice gap, 0 mm; and post-labelling delay time, 1525 ms), and the total scan time was 4 min 27 s. The geometric parameters including slice thickness, slice gap and FOV of axial T1W, T2W and T2W FLAIR sequences are the same as those of ASL sequence. Furthermore, the imaging planes of axial T1W, T2W, T2W FLAIR and ASL were all aligned parallel to the splenium and genu line of the corpus callosum. As such, the structural MR images were matched with ASL images. After ASL MRI and intravenous injection of 0.1 mmol/kg gadopentetate dimeglumine (Magnevist, Bayer Healthcare, Germany), axial, sagittal and coronal contrast-enhanced FSE T1W imaging were performed using the same parameters as the pre-contrast T1W imaging.
p53 immunohistochemistry
Immunohistochemistry of p53 protein is a sensitive and specific marker to predict p53 gene mutation.13 All 57 patients included in our study received surgery after MRI, and immunohistochemical staining for p53 protein was performed using the resected specimens, as previously described.13 When the percentage of tumour cells with p53 positive was more than 10% in the section, the glioma was considered to be p53-mutated, as previously described.13
ASL data processing
First, all ASL data were transferred to the AW4.6 workstation (GE Healthcare) (Signa HDxt, General Electric Medical System, Milwaukee, WI, USA) to generate CBF maps. All the CBF images were registered to axial post-contrast T1W images and T2W FLAIR images respectively by using the Elastix software (http://elastix.isi.uu.nl/). Second, all CBF images, post-contrast T1W images and T2W FLAIR images of each patient were individually transferred to the open-source application ITK-SNAP (www.itk-snap.org). All the volume of interests (VOIs) were manually drawn by two authors (both with 8 years of experience in MRI diagnosis) who were blinded to the histological results independently on the post-contrast T1W images or T2W FLAIR images overlaid with the coregistered CBF images. The layer of CBF images was set to be semi-transparent with 50% opacity. The enhancement areas seen on the post-contrast T1W images were delineated on the corresponding slices of CBF images and defined as the VOIs of enhancing tumour. The hyperintense signal that represented peritumoral oedema on the T2W FLAIR images were manually outlined on the corresponding slices of CBF images and defined as the VOIs of peritumoral edema. Areas of necrosis, haemorrhage or non-tumour macrovessels that were evident on the post-contrast T1W images or T2W FLAIR images were excluded from the VOIs. Third, three-dimensional segmentation of the enhancing tumour or peritumoral oedema was completed automatically by the ITK-SNAP software, and the VOIs were saved. Finally, all CBF images and VOIs of the enhancing tumour and peritumoral edema for each lesion were transferred to an in-house histogram analysis software (Analysis Kit v. 2.1, GE Healthcare) for histogram analysis. 18 histogram features including maximum, minimum, mean, median, 5th–95th percentile, kurtosis (measure of the peakedness of the pixel histogram), skewness (measure of the asymmetry of the pixel histogram), uniformity (measure of the homogeneity of the distribution of grey levels), and entropy (measure of the randomness of the distribution of grey levels) were calculated. All histogram parameters included in our study are interpreted in detail in Supplementary Material 1. The schematic diagram for ASL data processing is shown in Figure 1.
Figure 1.
Schematic diagram for ASL data processing. ASL, arterial spin labelling; CBF, cerebral bloodflow; FLAIR, fluid-attenuated inversion recovery imaging; VOI, volume ofinterest; T2W, T2 weighted.
Assessment of qualitative MRI features
Qualitative MRI features of all patients were assessed by two authors (with 13 and 10 years of experience in MRI diagnosis, respectively) who were blinded to the histological results by consensus. 15 qualitative features that were easily assessed on structural MRI were selected from the MRI feature set of the Visually AcceSAble Rembrandt Images (VASARI) project (https://wiki.cancerimagingarchive.net/display/Public/VASARI+Research+Project), including side of tumour epicentre (unilateral or centre/bilateral), enhancement quality (marked/avidor minimal/mild), cysts(yes or no), definition of the enhancing margin (well-defined or poorly defined), definition of the non-enhancing margin (smooth or irregular), oedema crosses midline (yes or no), haemorrhage (yes or no), pial invasion (yes or no), ependymal invasion (yes or no), cortical involvement (yes or no), deep white matter invasion (yes or no), non-enhancing tumour crosses midline (yes or no), enhancing tumour crosses midline (yes or no), satellites (yes or no) and calvarial remodelling (yes or no). All qualitative VASARI features included in our study are described in detail in Supplementary Material 1.
Statistical analysis
The agreement of whole-lesion ASL histogram features evaluated by the two readers was calculated using the intraclass correlation coefficient (ICC). Data from the two readers were averaged for analysis. Interobserver variability for the assessment of VASARI features was analysed using κ statistics. The normality and equal variance of continuous variables was checked using the Shapiro–Wilk test and Levene’s F test, respectively. The differences of continuous variables between tumours with and without p53 mutation were analysed by using the independent Student’s t test or Mann–Whitney U test. Corrections for multiple comparisons were performed using the Benjamini-Hochberg procedure which controls the false discovery rate (FDR) (α = 0.05).14 Categorical variables between tumours with and without p53 mutation were analysed using the χ2 test or Fisher’s exact test. Followed by the univariate analyses, to avoid overfitting, the least absolute shrinkage and selection operator (LASSO) method, which is suitable for the regression of high-dimensional data,15,16 was performed to select the optimal features with higher distinguishing power and lower redundancy. The diagnostic performance of the independent or combined features to predict p53 mutation was evaluated by ROC analyses, and the area under the curve (AUC) were compared by using the Hanley and McNeill test. The optimal cut-off value was determined with the maximum Youden index. The sensitivity, specificity and accuracy were calculated. Statistical analyses were performed by using the SPSS (v. 26.0; SPSS, Chicago, IL) and R software (v. 3.2.4; R Foundation for Statistical Computing). A two-tailed p < 0.05 was considered to indicate a significant difference.
Results
Clinicopathological characteristics of patients
The demographics and clinical profiles of 57 patients included in our study are summarised in Table 1. According to the result of immunochemical staining for p53, 24 were classified into the wild-type p53 group and 33 into the mutant p53 group. The statistical results in Table 1 show that no significant differences were found in age, gender, histologic subtype, WHO grade and IDH status between the wild-type p53 group and mutant p53 group.
Table 1.
Demographics and clinical profiles of patients
| Clinical profiles | Total cases | Patients with wild-type p53 | Patients with mutant p53 | p-value |
|---|---|---|---|---|
| Patients | 57 | 24 | 33 | NA |
| Age (mean ± SD) | 39.3 ± 17.7 | 39.3 ± 17.9 | 39.3 ± 17.8 | 0.981 |
| Gender | 0.875 | |||
| Male | 41 (71.9%) | 17 (70.8%) | 24 (72.7%) | |
| Female | 16 (28.1%) | 7 (29.2%) | 9 (27.3%) | |
| Histologic subtype | 0.098 | |||
| Anaplastic pleomorphic xanthoastrocytoma | 1 (1.8%) | 1 (4.2%) | 0 (0.0%) | |
| Anaplastic oligodendroglioma | 2 (3.5%) | 1 (4.2%) | 1 (3.0%) | |
| Anaplastic astrocytoma | 11 (19.3%) | 6 (25.0%) | 5 (15.2%) | |
| Diffuse midline glioma | 7 (12.3%) | 5 (20.8%) | 2 (6.1%) | |
| Glioblastoma | 36 (63.2%) | 11 (45.8%) | 25 (75.8%) | |
| WHO grade | 0.189 | |||
| III | 14 (24.6%) | 8 (33.3%) | 6 (18.2%) | |
| IV | 43 (75.4%) | 16 (66.7%) | 27 (81.8%) | |
| IDH status | 1.000 | |||
| Wild-type | 47 (82.5%) | 20 (83.3%) | 27 (81.8%) | |
| Mutant | 10 (17.5%) | 4 (16.7%) | 6 (18.2%) | |
SD, standard deviation.
Numbers in parentheses are the percentages; NA = not applied; WHO: World Health Organization.
ASL histogram parameters
The ICC for interobserver measurement reproducibility for all ASL parameters generated from whole-lesion histogram analysis of the CBF images showed excellent agreement between the two readers (ICC: 0.843–0.978). The averages of all ASL parameters and their comparison between the wild-type p53 group and mutant p53 group are shown in Table 2. The 5th (CBFpercentile5), 15th (CBFpercentile15) and 25th percentile CBF (CBFpercentile25) of the enhancing tumour were significantly higher in the wild-type p53 group than in the mutant p53 group (p = 0.003, p = 0.047, p = 0.049, respectively). The uniformity of CBF (CBFuniformity) of the enhancing tumour was significantly higher in the wild-type p53 group than in the mutant p53 group (p = 0.005). After the Benjamini-Hochberg corrections, CBFuniformity and CBFpercentile5 remained statistically significant (corrected p-value = 0.045, 0.045, respectively), while CBFpercentile15 and CBFpercentile20 no longer showed statistically significant difference (corrected p-value = 0.221, 0.221, respectively). No significant differences were found among all other parameters of the enhancing tumour or peritumoral oedema (p > 0.05).
Table 2.
Histogram parameters of CBF in the enhancing tumour or peritumoral oedema of HGGs
| Parameters | Enhancing tumour | p-value | Corrected p-value | Peritumoral oedema | p-value | Corrected p-value | ||
|---|---|---|---|---|---|---|---|---|
| Wild-type p53 group (n = 24) | Mutant p53 group (n = 33) | Wild-type p53 group (n = 14) | Mutant p53 group (n = 17) | |||||
| CBFmax | 242.92 ± 20.95 | 243.18 ± 21.84 | 0.983 | 0.983 | 218.43 ± 42.08 | 215.65 ± 34.70 | 0.710 | 0.800 |
| CBFmin | 6.29 ± 15.21 | 7.24 ± 18.30 | 0.959 | 0.983 | 18.71 ± 27.88 | 4.41 ± 12.49 | 0.215 | 0.716 |
| CBFmean | 106.86 ± 26.84 | 96.41 ± 29.14 | 0.173 | 0.414 | 91.55 ± 40.86 | 78.45 ± 22.03 | 0.518 | 0.716 |
| CBFmedian | 103.51 ± 28.66 | 93.92 ± 27.51 | 0.207 | 0.414 | 90.04 ± 41.45 | 77.25 ± 23.15 | 0.570 | 0.716 |
| CBFkurtosis | 1.20 ± 1.83 | 0.43 ± 0.99 | 0.196 | 0.414 | 1.80 ± 3.66 | 1.23 ± 1.31 | 0.799 | 0.846 |
| CBFskewness | 0.21 ± 0.88 | 0.18 ± 0.46 | 0.833 | 0.937 | 0.45 ± 0.95 | 0.24 ± 0.52 | 0.476 | 0.716 |
| CBFuniformity | 0.60 ± 0.11 | 0.50 ± 0.13 | 0.005* | 0.045* | 0.59 ± 0.17 | 0.54 ± 0.18 | 0.466 | 0.716 |
| CBFentropy | 5.96 ± 0.54 | 5.89 ± 0.57 | 0.722 | 0.866 | 5.53 ± 0.47 | 5.54 ± 0.54 | 0.860 | 0.860 |
| CBFpercentile5 | 35.64 ± 30.19 | 17.95 ± 30.52 | 0.003* | 0.045* | 33.69 ± 41.49 | 20.98 ± 25.84 | 0.336 | 0.716 |
| CBFpercentile15 | 67.72 ± 25.92 | 50.26 ± 30.74 | 0.047* | 0.221 | 58.07 ± 36.86 | 46.21 ± 24.93 | 0.296 | 0.716 |
| CBFpercentile25 | 80.40 ± 27.37 | 63.92 ± 31.34 | 0.049* | 0.221 | 66.82 ± 40.78 | 57.07 ± 24.29 | 0.441 | 0.716 |
| CBFpercentile35 | 90.02 ± 28.05 | 79.25 ± 26.31 | 0.106 | 0.382 | 78.41 ± 38.03 | 64.13 ± 26.36 | 0.228 | 0.716 |
| CBFpercentile45 | 98.87 ± 28.29 | 89.33 ± 26.70 | 0.175 | 0.414 | 86.50 ± 40.61 | 72.68 ± 24.05 | 0.493 | 0.716 |
| CBFpercentile55 | 108.13 ± 29.08 | 99.08 ± 27.91 | 0.240 | 0.432 | 93.78 ± 42.60 | 81.08 ± 23.25 | 0.597 | 0.716 |
| CBFpercentile65 | 118.20 ± 29.70 | 109.97 ± 29.74 | 0.307 | 0.502 | 101.24 ± 44.43 | 89.30 ± 23.65 | 0.377 | 0.716 |
| CBFpercentile75 | 130.40 ± 31.37 | 122.52 ± 32.20 | 0.361 | 0.542 | 110.88 ± 45.79 | 98.66 ± 25.12 | 0.382 | 0.716 |
| CBFpercentile85 | 145.46 ± 32.81 | 138.71 ± 35.82 | 0.470 | 0.651 | 122.99 ± 45.65 | 111.60 ± 27.38 | 0.397 | 0.716 |
| CBFpercentile95 | 173.25 ± 33.99 | 168.15 ± 39.22 | 0.611 | 0.786 | 145.58 ± 42.98 | 135.84 ± 30.80 | 0.469 | 0.716 |
CBF, cerebral blood flow; HGG, high-grade glioma.
All numerical data are presented as the mean ± standard deviation. *p < 0.05
Qualitative VASARI features
The qualitative VASARI features of all patients stratified by p53 status are summarised in Table 3. The κ (κ) values between the two radiologists who independently assessed VASARI features ranged from 0.856 to 1 (mean, κ = 0.918), indicating substantial inter-reader agreement. The feature of enhanced tumour crossing midline (ETCM) was significantly different between the wild-type p53 group and mutant p53 group (0/24 vs 10/33, p = 0.009). No significant differences were found among all other qualitative MRI features between the two groups (p > 0.05).
Table 3.
Qualitative MRI features in the wild-type p53 group and mutant p53 group
| Feature | Wild-type p53 group | Mutant p53 group | p-value |
|---|---|---|---|
| Side of tumour epicentre | 0.100 | ||
| Unilateral | 18/24 (75.0%) | 31/33 (93.9%) | |
| Center/Bilateral | 6/24 (25.0%) | 2/33 (6.1%) | |
| Enhancement quality | 0.189 | ||
| Marked/Avid | 16/24 (66.7%) | 27/33 (81.8%) | |
| Minimal/Mild | 8/24 (33.3%) | 6/33 (18.2%) | |
| Cysts | 0.954 | ||
| Yes | 10/24 (41.7%) | 14/33 (42.4%) | |
| No | 14/24 (58.3%) | 19/33 (57.6%) | |
| Definition of the enhancing margin | 0.813 | ||
| Well-defined | 8/24 (33.3%) | 12/33 (36.4%) | |
| Poorly defined | 16/24 (66.7%) | 21/33 (63.6%) | |
| Definition of the non-enhancing margin | 0.919 | ||
| Smooth | 4/24 (16.7%) | 4/33 (12.1%) | |
| Irregular | 20/24 (83.3%) | 29/33 (87.9%) | |
| Oedema crosses midline | 1.000 | ||
| Yes | 2/24 (8.3%) | 3/33 (9.1%) | |
| No | 22/24 (91.7%) | 30/33 (90.9%) | |
| Haemorrhage | 0.088 | ||
| Yes | 5/24 (20.8%) | 14/33 (42.4%) | |
| No | 19/24 (79.2%) | 19/33 (57.6%) | |
| Pial invasion | 0.085 | ||
| Yes | 9/24 (37.5%) | 20/33 (60.6%) | |
| No | 15/24 (62.5%) | 13/33 (39.4%) | |
| Ependymal invasion | 0.502 | ||
| Yes | 2/24 (8.3%) | 6/33 (18.2%) | |
| No | 22/24 (91.7%) | 27/33 (81.8%) | |
| Cortical involvement | 1.000 | ||
| Yes | 22/24 (91.7%) | 30/33 (90.9%) | |
| No | 2/24 (8.3%) | 3/33 (9.1%) | |
| Deep WM invasion | 0.337 | ||
| Yes | 14/24 (58.3%) | 15/33 (45.5%) | |
| No | 10/24 (41.7%) | 18/33 (54.5%) | |
| nCET tumour crosses midline | 0.616 | ||
| Yes | 3/24 (12.5%) | 7/33 (21.2%) | |
| No | 21/24 (87.5%) | 26/33 (78.8%) | |
| Enhancing tumour crosses midline | 0.009* | ||
| Yes | 0/24 (0%) | 10/33 (30.3%) | |
| No | 24/24 (100%) | 23/33 (69.7%) | |
| Satellites | 0.346 | ||
| Yes | 4/24 (16.7%) | 9/33 (27.3%) | |
| No | 20/24 (83.3%) | 24/33 (72.7%) | |
| Calvarial remodelling | 1.000 | ||
| Yes | 3/24 (12.5%) | 4/33 (12.1%) | |
| No | 21/24 (87.5%) | 29/33 (87.9%) | |
*p <0.05nCET: non-contrast-enhancing tumour.
LASSO regression
The LASSO method was performed to select the most useful predictive features among the significant features determined by the univariate analyses. LASSO regression showed that CBFuniformity and ETCM were predictive features for p53 mutation (Figure 2).
Figure 2.
Feature selection using the LASSO regression model. LASSO, least absolute shrinkage and selection operator.
ROC analysis
CBFuniformity of the enhancing tumour and ETCM were included in the ROC analysis. The results of ROC analyses for CBFuniformity, ETCM, and CBFuniformity combined with ETCM in predicting mutant p53 are shown in Table 4 and Figure 3. The AUC of CBFuniformity was higher than ETCM (0.721 vs 0.652), despite no significance (p = 0.390). The AUC of CBFuniformity combined with ETCM was significantly higher than that of CBFuniformity or ETCM alone (0.814 vs 0.721, p = 0.012; 0.814 vs 0.652, p = 0.004). The optimal thresholds for predicting p53 mutation were CBFuniformity less than 0.506, and the sign of enhanced tumour crossing midline on post-contrast T1W images. Two representative cases in each group are shown in Figures 4–5.
Table 4.
ROC analyses of different parameters to predict mutant P53
| Parameters | Cut-off value | AUC (95% CI) |
Sensitivity (95% CI) |
Specificity (95% CI) |
Accuracy (95% CI) |
|---|---|---|---|---|---|
| CBFuniformity | 0.506 | 0.721 (0.585, 0.857) | 0.606 (0.422, 0.766) | 0.833 (0.618, 0.945) | 0.702 (0.566, 0.816) |
| ETCM | 0.500 | 0.652 (0.572, 0.731) | 0.303 (0.162, 0.489) | 1.000 (0.828, 1.000) | 0.596 (0.458, 0.724) |
| CBFuniformity +ETCM | 0.224 | 0.814 (0.702, 0.927) | 0.758 (0.574, 0.882) | 0.833 (0.618, 0.945) | 0.790 (0.661, 0.886) |
CBFuniformity +ETCM: CBFuniformitycombined with the sign of enhancing tumour crossing midline.AUC, area under the curve; CBF: Cerebral blood flow;CI, confidence interval; ETCM: Enhancing tumour crosses midline.
Figure 3.
ROC curves of single and combined ASL histogram feature and qualitative MRI feature. ASL, arterial spin labelling; CBF, cerebral bloodflow; ETCM, enhanced tumour crossing midline; ROC, receiver operatingcharacteristic.
Figure 4.
A 50-year-old male with histologically confirmed anaplastic astrocytoma without p53 mutation. (a) T2W FLAIR images and (b) post-contrast T1W images show a tumour located in the left frontal lobe without the sign of enhancing tumour crossing midline. (c) CBF image shows a relatively uniform blood perfusion of the enhancing tumour. (d) CBFuniformityof the enhancing tumour derived from the histogram is larger than the cut-off value (0.506). CBF, cerebral blood flow;FLAIR, fluid-attenuated inversion recovery imaging; T2W, T2 weighted.
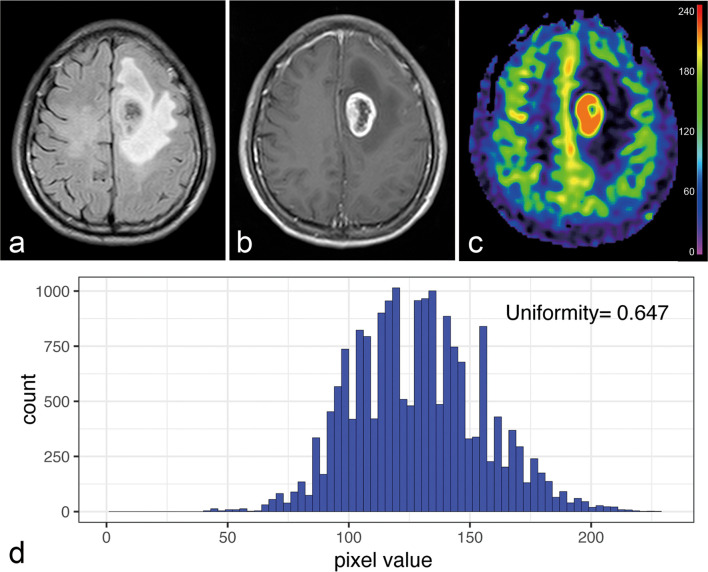
Figure 5.
A 59-year-old female with histologically confirmed glioblastoma with p53 mutation. (a) T2W FLAIR images and (b) post-contrast T1W images represents a tumour located in the right parietal lobe with the feature of enhancing tumour crossing midline. (c) CBF images show a relatively heterogeneous blood perfusion of the enhancing tumour. (d) CBFuniformityof the enhancing tumour generated from the histogram is less than the cut-off value (0.506). CBF, cerebral blood flow;FLAIR, fluid-attenuated inversion recovery imaging; T2W, T2 weighted.
Discussion
Our results showed that HGGs with mutant p53 had lower CBFuniformity of the enhancing tumour, and higher prevalence of the qualitative MRI feature (enhancing tumour crossing midline, ETCM) as compared with HGGs with wild-type p53. When combined with the sign of ETCM, CBFuniformity showed a high performance in predicting p53 mutation with an AUC of 0.814.
It is known that p53 mutation in HGGs is associated with tumour progression and resistance to temozolomide chemotherapy.3 Previously, only Ryoo et al have investigated the correlation between dynamic susceptibility contrast perfusion weighted imaging (DSC-PWI) based perfusion parameters and genomic aberrant of p53 in glioblastoma, but they found no significant correlation between the p53 status and averaged cerebral blood volume (CBV).17 This may contribute to the fact that averaged CBV cannot describe the heterogenous blood perfusion of the whole tumour in consideration of the high heterogeneity of malignant gliomas. To address this issue, we applied a whole-lesion histogram analysis of the CBF of the enhancing tumour and peritumoral oedema, as the volumetric histogram parameters could provide a more comprehensive and reliable assessment of blood perfusion characteristics of malignancies.18 ASL MRI-based CBF has a comparable ability with DSC-PWI-based CBF or CBV in the assessment of tumour perfusion.19,20 Furthermore, ASL MRI eliminates the need for administration of intravascular contrast, as the spin population of arterial water are labelled magnetically with an inversion pulse and serve as an endogenous tracer,21 which is desirable for patients with renal dysfunction or allergic history of contrast medium.
Our study showed that lower CBFuniformity could predict p53 mutation in HGGs. This phenomenon can be explained by the fact that p53 mutation promotes tumour angiogenesis,10 and the new blood vessel formation in HGGs was composed mainly of immature capillaries,22 which is highly heterogeneous, leading to the decreased uniformity of CBF. We also found that HGGs with mutant p53 showed lower fifth percentile CBF values in the enhancing tumour compared with HGGs with wild-type p53. This result may be explained by the fact that immature capillaries promoted by p53 mutation always have a very narrow lumen that does not carry any blood flow at all,22 which in turn leads to lower CBF values in HGGs with mutant p53. As regards peritumoral oedema, no significant differences of ASL metrics were found between HGGs with wild-type p53 and those with mutant p53 in our study. This result may be assumed to the complexity of the peritumoral oedema. It has been shown that peritumoral oedema of HGGs is a mixture of vasogenic oedema and tumour infiltration.23
Beside quantitative MR perfusion parameters, some qualitative MRI features have been reported to be able to predict p53 mutation.24,25 For example, Mut et al found that glioblastomas with high expression of p53 have some characteristic MRI features such as a ring enhancement and well-defined margin.24 In our study, we found that among all the qualitative MRI features, only the feature of ETCM was significantly different between HGGs with and without p53 mutations, and more ETCM was found in HGGs with p53 mutation. This finding is reasonable because p53 mutation promotes tumour growth and malignancy,26 leading to increased infiltration and aggressiveness of the tumour. Although the feature of ETCM only showed a relatively low predictive ability in distinguishing HGGs with wild-type p53 from those with mutant p53, when combined with CBFuniformity, its predictive efficacy was significantly improved. This finding suggests that the feature of ETCM can be used as a complementary index to improve the predictive ability of CBFuniformity.
Our study has some limitations. First, the sample size of HGGs was relatively small, as the patients were included from a single institution. To address the risk of overfitting, we used the LASSO method for the regression of high-dimensional data. It is generally accepted that the number of study cohort should be 15 to 20 multiples of the number of input variables for multivariate logistic regression to avoid overfitting. In this study, after LASSO regression, the number of the study cohort (n = 57) is 28.5 multiple of the number of input variables (n = 2), and thus overfitting has been avoided. Considering the non-invasiveness and easy accessibility of structural and ASL MRI, our study research is worth to be further validated by a multicentre study with a larger population cohort. Second, all the VOIs were drawn manually on the post-contrast T1W images or T2W FLAIR images overlaid with CBF images. Such manipulation is time-consuming and laborious. Moreover, DWI can provide more clear delineation of solid tumour components. Deep learning algorithms27 either based on structural MRI or DWI might be applied for fully automated lesion localisation and tumour segmentation in further studies.
In conclusion, our study shows that CBFuniformityof the enhancing tumour derived from pre-treatment ASL MRI could predict p53 mutation in HGGs. When combined with the qualitative MRI feature of enhanced tumour crossing midline, CBFuniformity could perform better in predicting p53 mutation. An integrated pre-treatment structural and ASL MRI could be used to predict p53 mutation in HGGs.
Footnotes
Funding: This study was supported by the National Natural Science Foundation of China (grant numbers: U1801681, 81801756), Key Areas Research and Development Program of Guangdong (grant number: 2019B020235001), Fundamental Research Funds for the Central Universities (grant number: 20ykpy102), Guangdong Province Universities and Colleges Pearl River Scholar Funded Scheme (2017), Guangdong Basic and Applied Basic Research Foundation (grant number: 2019A1515110189), Guangdong Natural Science Foundation (grant number: 2017A030313777), Medical science and Technology Research Fund of Guangdong Province (grant number: A2020074).
The authors Jiaji Mao and Dabiao Deng contributed equally to the work.
Contributor Information
Jiaji Mao, Email: maojj6@mail.sysu.edu.cn.
Dabiao Deng, Email: 276457685@qq.com.
Zehong Yang, Email: yangzeh2@mail.sysu.edu.cn.
Wensheng Wang, Email: wws161616@sina.com.
Minghui Cao, Email: caomh3@mail.sysu.edu.cn.
Yun Huang, Email: huangy279@mail2.sysu.edu.cn.
Jun Shen, Email: shenjun@mail.sysu.edu.cn.
REFERENCES
- 1.Reifenberger G, Wirsching H-G, Knobbe-Thomsen CB, Weller M. Advances in the molecular genetics of gliomas - implications for classification and therapy. Nat Rev Clin Oncol 2017; 14: 434–52. doi: 10.1038/nrclinonc.2016.204 [DOI] [PubMed] [Google Scholar]
- 2.Bykov VJN, Eriksson SE, Bianchi J, Wiman KG. Targeting mutant p53 for efficient cancer therapy. Nat Rev Cancer 2018; 18: 89–102. doi: 10.1038/nrc.2017.109 [DOI] [PubMed] [Google Scholar]
- 3.Messaoudi K, Clavreul A, Lagarce F. Toward an effective strategy in glioblastoma treatment. Part I: resistance mechanisms and strategies to overcome resistance of glioblastoma to temozolomide. Drug Discov Today 2015; 20: 899–905. doi: 10.1016/j.drudis.2015.02.011 [DOI] [PubMed] [Google Scholar]
- 4.Vassilev LT, Vu BT, Graves B, Carvajal D, Podlaski F, Filipovic Z, et al. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science 2004; 303: 844–8. doi: 10.1126/science.1092472 [DOI] [PubMed] [Google Scholar]
- 5.Hong B, van den Heuvel APJ, Prabhu VV, Zhang S, El-Deiry WS. Targeting tumor suppressor p53 for cancer therapy: strategies, challenges and opportunities. Curr Drug Targets 2014; 15: 80–9. doi: 10.2174/1389450114666140106101412 [DOI] [PubMed] [Google Scholar]
- 6.Reithmeier T, Lopez WO, Doostkam S, Machein MR, Pinsker MO, Trippel M, et al. Intraindividual comparison of histopathological diagnosis obtained by stereotactic serial biopsy to open surgical resection specimen in patients with intracranial tumours. Clin Neurol Neurosurg 2013; 115: 1955–60. doi: 10.1016/j.clineuro.2013.05.019 [DOI] [PubMed] [Google Scholar]
- 7.Li Y, Qian Z, Xu K, Wang K, Fan X, Li S, et al. Mri features predict p53 status in lower-grade gliomas via a machine-learning approach. Neuroimage Clin 2018; 17: 306–11. doi: 10.1016/j.nicl.2017.10.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang X, Tian Q, Wang L, Liu Y, Li B, Liang Z, et al. Radiomics strategy for molecular subtype stratification of Lower-Grade glioma: detecting IDH and TP53 mutations based on multimodal MRI. J Magn Reson Imaging 2018; 48: 916–26. doi: 10.1002/jmri.25960 [DOI] [PubMed] [Google Scholar]
- 9.Thamm T, Guo J, Rosenberg J, Liang T, Marks MP, Christensen S, et al. Contralateral hemispheric cerebral blood flow measured with arterial spin labeling can predict outcome in acute stroke. Stroke 2019; 50: 3408–15. doi: 10.1161/STROKEAHA.119.026499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Teodoro JG, Parker AE, Zhu X, Green MR. P53-Mediated inhibition of angiogenesis through up-regulation of a collagen prolyl hydroxylase. Science 2006; 313: 968–71. doi: 10.1126/science.1126391 [DOI] [PubMed] [Google Scholar]
- 11.Ravi R, Mookerjee B, Bhujwalla ZM, Sutter CH, Artemov D, Zeng Q, et al. Regulation of tumor angiogenesis by p53-induced degradation of hypoxia-inducible factor 1alpha. Genes Dev 2000; 14: 34–44. [PMC free article] [PubMed] [Google Scholar]
- 12.Alsop DC, Detre JA, Golay X, Günther M, Hendrikse J, Hernandez-Garcia L, et al. Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: a consensus of the ISMRM perfusion Study Group and the European Consortium for ASL in dementia. Magn Reson Med 2015; 73: 102–16. doi: 10.1002/mrm.25197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takami H, Yoshida A, Fukushima S, Arita H, Matsushita Y, Nakamura T, et al. Revisiting TP53 Mutations and Immunohistochemistry--A Comparative Study in 157 Diffuse Gliomas. Brain Pathol 2015; 25: 256–65. doi: 10.1111/bpa.12173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society: Series B 1995; 57. [Google Scholar]
- 15.Huang Y-Q, Liang C-H, He L, Tian J, Liang C-S, Chen X, et al. Development and validation of a Radiomics nomogram for preoperative prediction of lymph node metastasis in colorectal cancer. J Clin Oncol 2016; 34: 2157–64. doi: 10.1200/JCO.2015.65.9128 [DOI] [PubMed] [Google Scholar]
- 16.Sauerbrei W, Royston P, Binder H. Selection of important variables and determination of functional form for continuous predictors in multivariable model building. Stat Med 2007; 26: 5512–28. doi: 10.1002/sim.3148 [DOI] [PubMed] [Google Scholar]
- 17.Ryoo I, Choi SH, Kim J-H, Sohn C-H, Kim SC, Shin HS, et al. Cerebral blood volume calculated by dynamic susceptibility contrast-enhanced perfusion MR imaging: preliminary correlation study with glioblastoma genetic profiles. PLoS One 2013; 8: e71704–e04. doi: 10.1371/journal.pone.0071704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Just N. Improving tumour heterogeneity MRI assessment with histograms. Br J Cancer 2014; 111: 2205–13. doi: 10.1038/bjc.2014.512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morana G, Tortora D, Staglianò S, Nozza P, Mascelli S, Severino M, et al. Pediatric astrocytic tumor grading: comparison between arterial spin labeling and dynamic susceptibility contrast MRI perfusion. Neuroradiology 2018; 60: 437–46. doi: 10.1007/s00234-018-1992-6 [DOI] [PubMed] [Google Scholar]
- 20.Warmuth C, Gunther M, Zimmer C. Quantification of blood flow in brain tumors: comparison of arterial spin labeling and dynamic susceptibility-weighted contrast-enhanced MR imaging. Radiology 2003; 228: 523–32. doi: 10.1148/radiol.2282020409 [DOI] [PubMed] [Google Scholar]
- 21.Grade M, Hernandez Tamames JA, Pizzini FB, Achten E, Golay X, Smits M. A neuroradiologist's guide to arterial spin labeling MRI in clinical practice. Neuroradiology 2015; 57: 1181–202. doi: 10.1007/s00234-015-1571-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miyagami M, Katayama Y. Angiogenesis of glioma: evaluation of ultrastructural characteristics of microvessels and tubular bodies (Weibel-Palade) in endothelial cells and immunohistochemical findings with VEGF and p53 protein. Med Mol Morphol 2005; 38: 36–42. doi: 10.1007/s00795-004-0273-0 [DOI] [PubMed] [Google Scholar]
- 23.Provenzale JM, McGraw P, Mhatre P, Guo AC, Delong D. Peritumoral brain regions in gliomas and meningiomas: investigation with isotropic diffusion-weighted MR imaging and diffusion-tensor MR imaging. Radiology 2004; 232: 451–60. doi: 10.1148/radiol.2322030959 [DOI] [PubMed] [Google Scholar]
- 24.Mut M, Turba UC, Botella AC, Baskurt E, Lopes MBS, Shaffrey ME. Neuroimaging characteristics in subgroup of GBMs with p53 overexpression. J Neuroimaging 2007; 17: 168–74. doi: 10.1111/j.1552-6569.2007.00112.x [DOI] [PubMed] [Google Scholar]
- 25.Li W-bin, Tang K, Zhang W, Yan W, You G, Li S-wu, Li W-b TK, You G LS-w, et al. Relationship between magnetic resonance imaging and molecular pathology in patients with glioblastoma multiforme. Chin Med J 2011; 124: 2589–92. [PubMed] [Google Scholar]
- 26.Gillet E, Alentorn A, Doukouré B, Mundwiller E, van Thuijl HF, van Thuij H, Reijneveld JC, et al. Tp53 and p53 statuses and their clinical impact in diffuse low grade gliomas. J Neurooncol 2014; 118: 131–9. doi: 10.1007/s11060-014-1407-4 [DOI] [PubMed] [Google Scholar]
- 27.Laukamp KR, Thiele F, Shakirin G, Zopfs D, Faymonville A, Timmer M, et al. Fully automated detection and segmentation of meningiomas using deep learning on routine multiparametric MRI. Eur Radiol 2019; 29: 124–32. doi: 10.1007/s00330-018-5595-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.