Abstract
Objectives:
The isotope bone scan (IBS) is the gold-standard imaging modality for detecting skeletal metastases as part of prostate cancer staging. However, its clinical utility for assessing skeletal metastatic burden is limited due to the need for subjective interpretation. We designed and tested a novel custom software tool, the Metastatic Bone Scan Tool (MetsBST), aimed at improving interpretation of IBSs, and compared its performance with that of an established software programme.
Methods:
We used IBS images from 62 patients diagnosed with prostate cancer and suspected bone metastases to design and implement MetsBST in MATLAB by defining thresholds used to identify the texture and size of metastatic bone lesions. The results of MetsBST were compared with those of the commercially available automated Bone Scan Index (aBSI) with regression analysis.
Results:
There was strong agreement between the MetsBST and aBSI results (R2 = 0.9189). In a subregional analysis, MetsBST quantified the extent of metastatic disease in multiple bone sites in patients receiving multimodality therapy (radium-223 and external beam radiotherapy) to illustrate the differences in bone metastatic response to different treatments.
Conclusion:
The results of MetsBST and the commercial software aBSI were highly consistent. MetsBST introduces novel clinical utility by its ability to differentiate between the responses of different bone metastases to multimodality therapies.
Advances in knowledge:
MetsBST reduces the variability in assessment of tumour burden caused by subjective interpretation. Therefore, it is a useful aid to physicians reporting nuclear medicine scans, and may improve decision-making in the treatment of metastatic prostate cancer.
Introduction
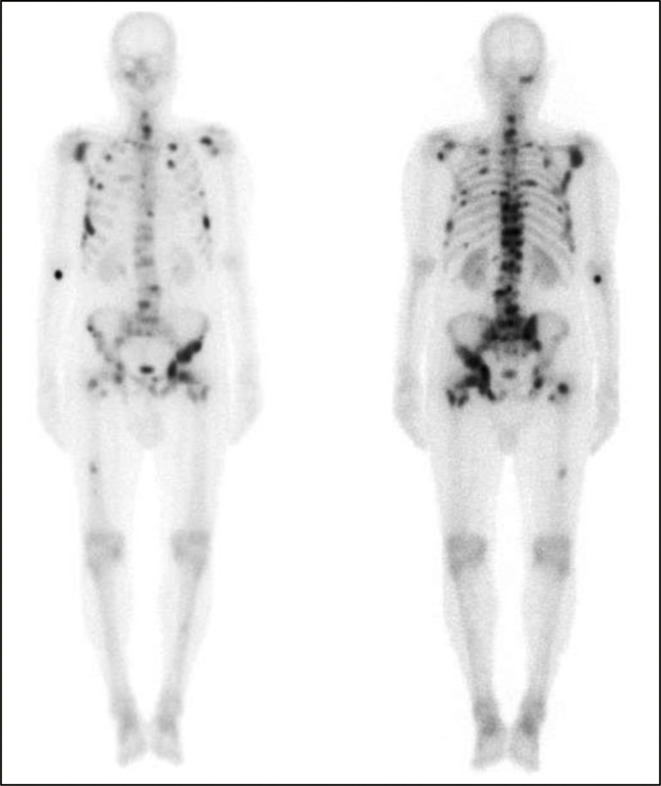
More than 90% of males with metastatic castration-resistant prostate cancer (mCRPC) have bone metastases which can result in significant morbidity due to a variety of complications, including pain, pathological fractures, spinal cord compression, and bone marrow failure.1,2 Isotope bone scans (IBSs), using technetium (99mTc) as the tracer, are the most commonly applied and standardised modality for imaging of skeletal metastases in prostate cancer patients. IBSs are used in clinical practice for staging and monitoring the progression of bone disease burden (Figure 1).
Figure 1.
Whole-body IBS: anterior (left) and posterior (right) views showing widespread bony metastases involving the pelvis, femora, ribs, spine, sternum, and skull. IBS, isotope bone scan.
Although IBS has limited sensitivity (79%) and specificity (82%) for detection of bone metastases,3,4 it is still the most frequently used method for diagnosis to assess skeletal involvement in prostate cancer. A validated end point in mCRPC trials is bone scan progression, which relies on the appearance of new lesions, as proposed by the prostate cancer working Group 3 (PCWG).5 The PCWG 3 aims to determine criteria for bone progression to make clinical trial results comparable. However, the difficulty in establishing bone metastatic burden at baseline and assessing disease progression and response to treatment has impeded the ability of clinical trials to report conclusive results.6 This situation has paradoxically become more complicated with the introduction of newer functional imaging techniques.4,6,7
IBS has been shown to be an independent predictor of overall survival (OS) benefit from local radiotherapy to the prostate in patients with de novo metastatic prostate cancer in the STAMPEDE trial (ARM H). The volume of bony metastatic disease (>3 metastases = no OS benefit from local radiotherapy), as identified on IBS, has been shown to be predictive of response and OS in patients receiving radical dose radiotherapy to the prostate in the metastatic setting.8 According to the definition used in the CHAARTED trial, the metastatic burden classified ‘high-volume’ (high metastatic burden) was defined by the presence of visceral metastases or ≥4 bone metastases with ≥1 outside the vertebral bodies or pelvis; all other assessable patients were considered to have ‘low-volume’ (low metastatic burden).8,9 Therefore, it is expected that IBSs [despite the increasing use of whole-body MRI (WBMRI) or prostate-specific membrane antigen positron emission tomography (PSMA-PET) scanning], will continue to influence decisions made regarding therapeutic interventions for patients presenting with de novo metastatic disease.
Currently, the standard IBS assessment relies on describing the sites and the extent of tumour burden in bone and counting the number of metastatic lesions.3 This semi-quantitative method of IBS interpretation is subjective, which may limit its clinical utility.2,3 Assessment of the degree of tumour burden in bone is considered a limiting factor in both clinical decision-making and outputs from clinical trials.7 As therapeutic options in the management of bony metastases increase, more accurate and quantitative analysis of IBS is needed to help understand responses to new treatments such as novel hormonal agents and bone-targeted therapies, e.g. Radium-223.
Recently, an automated software-based programme aiding interpretation of IBSs, the automated Bone Scan Index (aBSI), was identified as a highly reliable quantitative treatment response biomarker for quantifying bone metastases.10 A large randomised clinical trial, recruited at 241 sites in 37 countries, validated the potential predictive value of aBSI.3 Univariate analysis revealed that aBSI could be a good predictor of OS. While aBSI can aid interpretation of the overall response, to date, no tool provides information on the degree of response in different regions of the skeleton and assess differences between bone metastases, e.g. by comparing uptake intensity and anatomical site-specific response. Therefore, there is an unmet need to improve the interpretation of IBSs to aid clinicians with assessment of treatment effectiveness and decision-making to introduce new therapies. For instance, patients can exhibit progression radiologically in a number of different ways: (i) new sites of disease apparent on IBS; (ii) increased activity in existing sites; (iii) oligometastatic progression with 1–5 new bone sites; or (iv) oligoprogression in an existing site or sites in the absence of new disease. Improved IBS interpretation and evaluation in these clinical scenarios could give added information to guide the introduction of targeted therapies, such as stereotactic radiotherapy to a progressing lesion or the use of a systemic radionuclide, e.g. Radium-223.
In this study, we describe the development and testing of a MATLAB-based functional bone analysis tool. MATLAB is high-level interactive computing language that provides quantitative analysis and visualisation tools which can be used in multiple applications, including the analysis of different nuclear medicine images.11 The overall aim is to aid interpretation of IBSs in relation to the response of prostate cancer bone metastases to therapy. We outline: (1) the development of the code and (2) comparison with a commercially available software programme. In addition, we propose avenues of early clinical use of the code in standard and clinical trials of metastatic prostate cancer patients.
Methods and materials
Patients
62 patients diagnosed with prostate cancer who had undergone IBS from April 2017 to May 2019 due to suspected bone metastatic disease were selected for this study. A pragmatic decision was made to test IBSs of a variety of patients at different stages in their prostate cancer treatment pathway. Three different patient cohorts were included: (i) prostate cancer patients with no metastases seen on IBS (n = 16); (ii) mCRPC patients with multiple bone metastases (n = 16); and (iii) metastatic castration-sensitive prostate cancer (mCSPC) patients (n = 30) who were at the androgen-sensitive stage of their disease and concurrently receiving external beam radiotherapy (EBRT) and radium-223 to the prostate and pelvic areas as part of a prospective clinical trial (ADRRAD Trial, EudraCT number: 2014-000273-39). The ADRRAD patients received standard ADT (LHRH-agonist or LHRH-antagonist) initially for a minimum of 3 months (up to a maximum of 12 months) before commencing treatment with radium-223 and radiotherapy to the prostate and pelvic nodes. The patients then received 74 Gy in 37 fractions of volumetric-modulated arc radiotherapy [VMAT], one treatment every weekday, and six cycles of radium-223 (55 kBq/kg, one i.v. bolus every 4 weeks for 6 months), commencing at the time of radiotherapy.
Isotope bone scans
Patients were examined with a dual detector γ camera (GE Optima or Siemens Symbia). All digital images from IBS were checked for quality to ensure that both anterior and posterior views of each patient were saved in the standard Digital Imaging and Communications in Medicine (DICOM) format. Each IBS was acquired 2–3 h post-intravenous administration of 600–800 MBq of99mTc-hydroxymethylene diphosphonate (99mTc-HDP; Curium Pharma, UK). Whole-body images with anterior and posterior views (matrix 256 × 1024) were digitally obtained with a low-energy, high-resolution collimator and energy discrimination (15–20%) of a window centred on 140 keV of 99mTc.
Automated bone scan index
The aBSI (EXINI Diagnostics AB, Sweden) was used to quantify the extent of bone metastasis in the prostate cancer patients in this study. The bone scan index (BSI) is a form of IBS interpretation estimating the quantitative bone metastasis burden in prostate cancer patients, originally reported in 1998 as an imaging biomarker. Subsequently, aBSI was developed using a computer-assisted diagnostic software programme, which makes the evaluation of metastatic spread more objective.12 The automated BSI tool has been described elsewhere in detail.13 Briefly, different anatomical regions of the skeleton are segmented, and hot spots detected. Then, features describing the hot spots are calculated and classified, using artificial neural networks as metastatic lesions based on the hotspot features. The automated BSI is calculated as the sum of mass fractions of the skeleton of all metastatic hotspots.
Code development
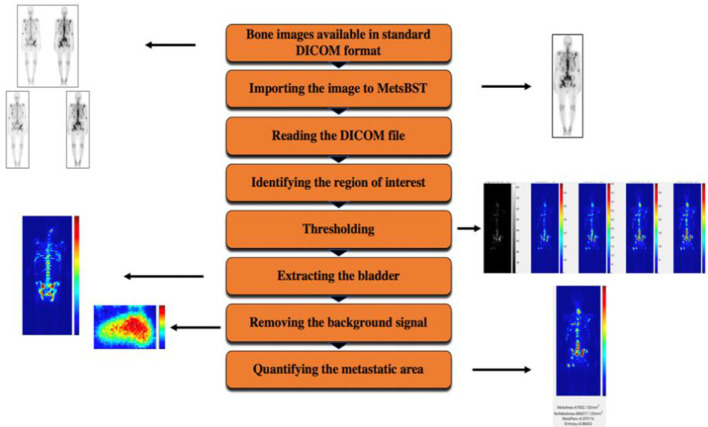
The Metastatic Bone Scan Tool (MetsBST) was designed and implemented in MATLAB (MathWorks Inc., 2019a). The image-processing workflow of MetsBST included automated importing of DICOM images, user defined region of interest (ROI) identification, bladder extraction, background signal removal (i.e. limiting the analysis to patients’ skeleton), thresholding and metastasis size and texture quantification (Figure 2). The code and its application are comprehensively illustrated in Supplementary Material 1.
Figure 2.
A schematic diagram of the design and workflow of the MetsBST. MetsBST, Metastatic Bone Scan Tool.
DICOM image data processing and thresholding
After reading the DICOM files using the graphical user interface (Supplementary Material 1), users are prompted to choose two points to define the ROI. Depending on the input, a user could choose to analyse any anatomical ROI. Images are then analysed using image thresholding techniques. Different thresholds were tested to assess the variable uptake occurring in different areas. These thresholds were implemented to remove unnecessary detail from the image and help clarify the appearance of information that can be visually difficult to detect. The range of pixel values was not standardised, i.e. every IBS had a different maximum intensity depending on whether the bone was normal or affected by metastatic disease. Therefore, for visual presentation, different thresholds were tested incrementally from 10% up to 90% of maximum intensity to collect consistent readouts and to obtain relevant values for IBS tested in aBSI. Fixed absolute thresholds were also tested, and the results were compared to aBSI. The thresholds were identified by setting all intensity values below maximum intensity to 0. Images were displayed, and the visual differences in the four thresholding schemes were captured.
Bladder extraction and background signal removal
Due to renal excretion of 99mTc-HDP, uptake appearing in the bladder area is included in the metastatic area. To account for this, MetsBST was used to extract data corresponding to the bladder based on size and location by defining the ROI around the uptake shown in the bladder. All uptake appearing in the bladder was excluded from the interpretation, which then showed the percentage of metastasis only in bone. An additional step taken during the processing of IBSs was the removal of unnecessary information (background signal) from the defined ROIs. The background signal from the IBS images was identified and contoured. Then, the final image was displayed with different colours range as specified by the chosen thresholds (Figure 3) representing different intensities of bone metastases. Hotspots were classified as high-intensity (red), medium-intensity (yellow), low-intensity (green) or no metastases (blue).
Figure 3.
Whole-body IBS images of the anterior (left) and posterior (right) showing areas of metastases displayed with the MetsBST. Areas of uptake in correlation with IBS are visualised with different colours representing the intensity of bone metastases: high (red), medium (yellow), low (green), and no metastases (blue). IBS, isotope bone scan; MetsBST, metastatic bone scan tool.
Comparison
The standard assessment of IBS for the presence or absence of metastasis in bone is based on clinical reports describing the extent of the bone tumour burden. Patient clinical data, including previous history of trauma and localisation of bone pain, were available to the physicians for reporting purposes. The reports of all images were re-evaluated to identify patients who had fractures and superscans. The extent of bone metastases on each IBS was calculated as a percentage using both aBSI and MetsBST for assessing their comparability. It was computed in aBSI by dividing the area of the metastasis by areas of the anatomical region in which the metastasis is located and multiplying by a coefficient reflecting the regional proportion of the total skeletal mass. In MetsBST, the extent of bone metastases was calculated by setting a counter from the number of bone pixels above the specified threshold and another counter with the number of pixels below that level. The ratio of normal bone to bone with metastases is then calculated (MetsBST values (%)). Based on the values obtained by aBSI and MetsBST, a cut-off value was chosen to classify the IBSs as normal (‘no bone metastases’) or abnormal (‘bone metastases’).
Validation
All available scans were analysed by aBSI, and MetsBST was used to objectively assess the extent of bone metastasis in the same IBSs tested in aBSI. The relationship between the extent of bone metastases (%) obtained from aBSI and that from MetsBST was analysed using linear regression (R2). The best threshold showing a good relationship between the values for IBSs tested in MetsBST and those tested in aBSI for the same group of patients was selected.
Results
The characteristics of the 62 patients included in this study are described inTable 1. The prostate cancer patients with no metastases at baseline had IBSs carried out as they exhibited high-risk features (Gleason >4 +3,>T3 disease, PSA >20). The mCRPC patients with multiple bone metastases had a history of multiple lines of treatment, including chemotherapy, surgery, hormone therapy, EBRT and radium-223, and had baseline and follow-up IBSs. The mCSPC patients, from a Phase I/II open-label clinical ADRRAD trial for new presentation of T1-4 N0/1 M1B prostate adenocarcinoma with >3 bone metastases, had baseline IBSs. 18 patients in the third group had subsequent follow-up IBSs available for analysis.
Table 1.
Demographics and characteristics of prostate cancer patients (N = 62).
| Prostate cancer (no metastasis) |
Castration-resistant prostate cancer (mCRPC) |
Castration-sensitive prostate cancer (mCSPC) |
||||
|---|---|---|---|---|---|---|
| (n = 16) | (n = 16) | (n = 16) | ||||
| IBSs | Baseline | Follow-up | Baseline | Follow-up | Baseline | Follow-up |
| Number of IBSs | 16 | 0 | 16 | 16 | 16 | 18 |
| Age (years) | ||||||
| Mean | 68 | 72 | 66 | |||
| Range | 49–82 | 52–81 | 57–80 | |||
| Treatment received | ||||||
| Hormone therapy | - | 16 (100%) | 18 (100%) | |||
| Surgery | - | 2 (12.5%) | 0 (0%) | |||
| External beam radiotherapy | - | 10 (62.5%) | 18 (100%) | |||
| Radium-223 | - | 16 (100%) | 18 (100%) | |||
ISBs, isotope bone scans.
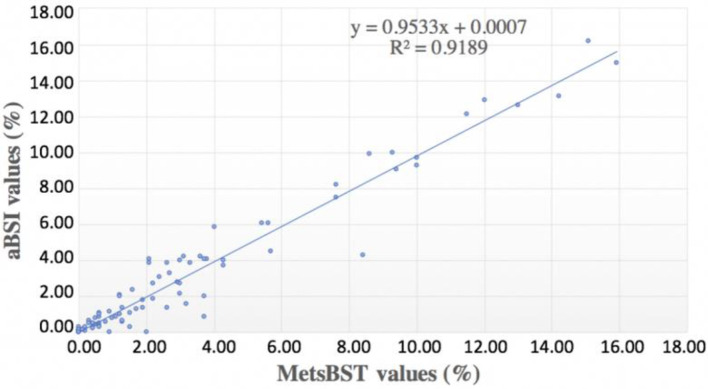
All patients had IBSs in DICOM format and, therefore, were eligible for analysis in aBSI and MetsBST. In MetsBST, the different thresholds and fixed thresholds were tested to identify the extent of bone metastases. The analysis showed that, for visualisation, a fixed threshold (maximum intensity) of 300 produced the most similar visualisation of metastatic regions. Additionally, an absolute cut-off value of 100 for identifying metastatic bone regions gave the best comparable quantitative readouts to aBSI in all patients except one individual patient that had a superscan. Patients with extensive osteoblastic metastatic disease with reduced renal/soft tissue activity (superscans) were excluded from the analysis as both MetsBST and aBSI fail in the setting of very high tumour burden. Each IBS showed anterior and posterior views of the whole skeleton, including the skull, ribs, vertebrae, pelvis, and extremities. The fixed threshold for the three different groups tested by MetsBST yielded consistent readings that correlated with the extent of bone metastases. There was good correspondence between the values for IBSs tested in MetsBST and those tested in aBSI (R2 = 0.9189; Figure 4). As shown in Figure 5, there was high conformity between the two tools in visualisation of bone metastases.
Figure 4.
The correspondence between IBS readings tested in the MetsBST and those tested in the aBSI. aBSI, automated bone scan index; IBS, isotope bone scan; MetsBST, metastatic bone scan tool.
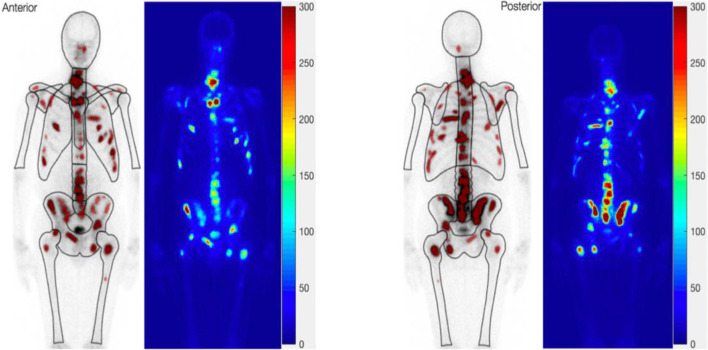
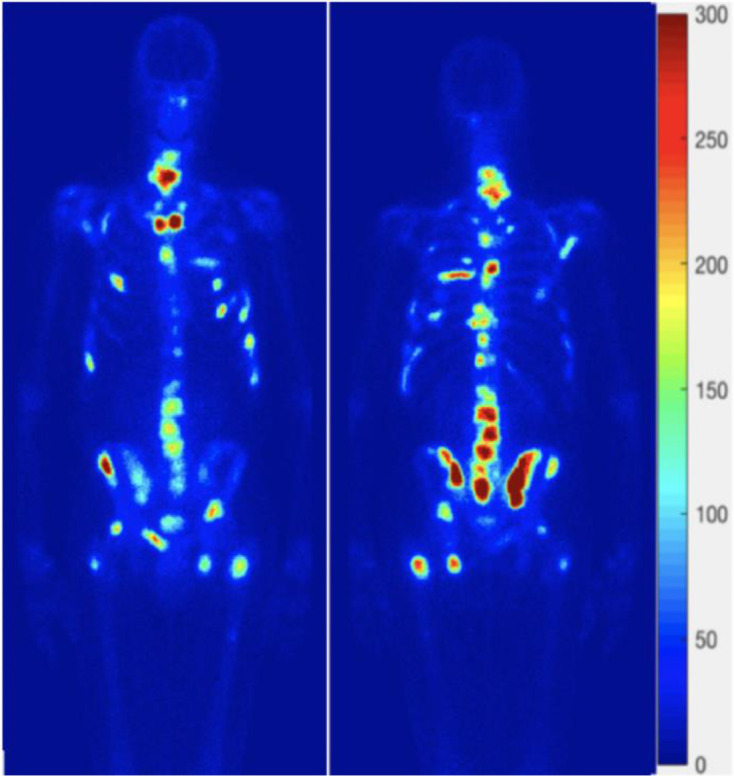
Figure 5.
An example showing consistence in the appearance of bone metastases in the aBSI (image on the left of each of the anterior and posterior views) and the MetsBST (image on the right of each view) using the fixed threshold (300) in IBSs. aBSI, automated bone scan index; IBS, isotope bone scan; MetsBST, metastatic bone scan tool.
All IBSs with values <1 were identified as normal scans (i.e. no metastases), while the IBSs with values >1 were classified as having bone metastases. All 16 prostate cancer patients with no metastases in this study had baseline aBSI and MetsBST values of <1 (no presence of bone metastases). Of the 16 mCRPC patients with extensive bone disease at baseline, 8 patients showed an increase in aBSI and MetsBST values in IBSs post-treatment, whereas the other 8 patients exhibited a decrease. Of the 18 mCSPC patients with baseline IBSs and subsequent follow-up IBSs, 15 showed a reduction or stability in aBSI and MetsBST readings of IBSs (Figure 6), and 3 showed an increase. Compared to subjective assessment of IBS in the 34 patients (16 mCRPC patients and 18 mCSPC patients) who had baseline and subsequent follow-up IBSs, the changes in MetsBST were concordant with the subjective evaluation of IBSs over time, whether response, stable disease or progressive disease.
Figure 6.
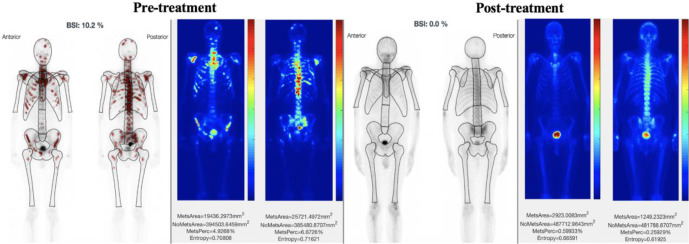
An example of post-treatment change in the extent of metastases obtained from the aBSI and MetsBST for IBSs. Whole-body IBS images of the anterior and posterior views pre- and post-treatment are shown for a 65-year-old prostate cancer patient with bone metastases. IBS images tested in aBSI (pre-treatment, left image) and MetsBST (pre-treatment, right image) show the same areas of metastases within the spine anteriorly and posteriorly. Post-treatment IBS images tested in both aBSI (post-treatment, left image) and MetsBST (post-treatment, right image) show an almost complete response anteriorly and posteriorly. aBSI, automated bone scan index; IBS, isotope bone scan; MetsBST, metastatic bone scan tool.
Clinical applicability: improving the assessment of bone metastases by subregion analysis
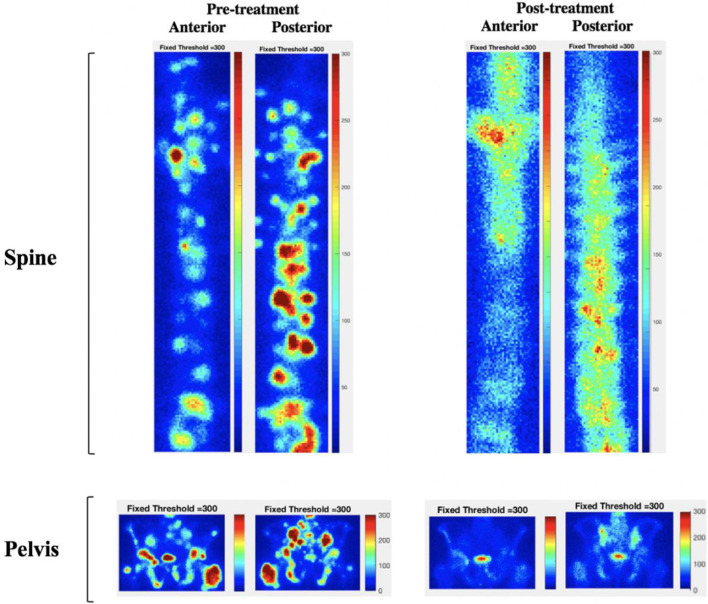
MetsBST can also be used to assess different anatomical areas of disease in the IBS and to investigate site-specific response to therapy. For the locally led ADRRAD trial, we identified two separate ROIs: (1) in-field regions (the prostate and pelvic areas that received the combination of radium-223 and EBRT); and (2) out-of-field regions (the spine area that only received radium-223 and no EBRT). An area of interest in each patient was selected, and the value for each scan-selected ROI using a region-specific threshold algorithm was generated. The different responses in both areas at baseline and post-treatment were calculated. MetsBST was used to ascertain if there was a differential response in bone areas in mCSPC patients with baseline IBSs and subsequent follow-up IBSs. In 12 out of the 18 mCSPC patients (66%), there was complete or almost complete resolution of identifiable disease on the IBS in the pelvic region, and of the 18 patients with bone metastases in the spine (out-of-field), 4 patients (22%) exhibited a complete response (Figure 7).
Figure 7.
Differential responses to therapy in bone metastases in the pelvis (in-field) and the spine (out-offield) shown in the whole-body 99mTc-HDP bone scan of a 57-year-old prostate cancer patient with multiple sites of bone metastases pre- and post-treatment. The patient received six cycles of radium-223 to the whole body, in addition to EBRT to the prostate and pelvis. Post-treatment, there was almost complete resolution of identifiable disease in the pelvic region that received the combined treatments, while some sites of uptake were less conspicuous or not seen in the spine. EBRT, external beam radiotherapy.
Discussion
Significant advances have been made in imaging for metastatic prostate cancer, with increasing use of PSMA-PET and WBMRI adding increased sensitivity and anatomical delineation of metastases.4 Despite this, bone scans will remain an affordable method that allows treatment decisions to be made based on the volume of disease present. Novel imaging tools may not be affordable to or available in many countries. Therefore, simple tools such as aBSI and MetsBST may enable clinicians to monitor responses to novel therapies, including hormonal agents, bone-targeted therapies and stereotactic radiotherapy, more economically.
Subjective assessment is the most commonly used method for evaluating images for diagnostic purposes.14,15 Scintigraphic imaging with 99mTc-HDP is the most frequently used to evaluate high-risk prostate cancer patients with suspected skeletal metastases.16,17 However, subjective variation in the interpretation of IBS to assess the degree of tumour burden in bone is considered a limiting factor for both clinical decision-making and clinical trials.7 The increasing interest in the use of objective and quantitative methods for IBS data is an exciting development in the field of nuclear medicine.
We developed and tested MetsBST on different prostate cancer cohorts, and validated the tool by comparing its results with those of the aBSI. The strong correspondence between the values for IBSs tested in MetsBST and those tested in aBSI demonstrate that the former can accurately detect and quantify individual bone metastases from IBS, enabling quantitative assessment of the extent of the tumour burden in bone at baseline and follow-up treatment. The intensity threshold chosen for analysing IBS using MetsBST provided consistent measurement of bone metastases, allowing for differentiation between prostate cancer groups and between multiple bone areas in the same patients.
We have developed a tool that can aid interpretation of IBSs when multiple therapies are used. MetsBST can assess differences between bone metastases by comparing intensity and sites, and investigate differential responses to radiation in multiple bone areas (Figure 7). Compared to aBSI, MetsBST allows interpretation of responses in specific anatomical sites, i.e. sites received combined treatments compared to those receiving a single treatment. For patients receiving radium-223 and EBRT in the ADRRAD trial, MetsBST provided early signs that combination therapy can improve local responses in bony metastatic disease, and may identify synergistic effects between radium-223 and EBRT. This way of interpreting individual or localised bone responses could aid trials investigating stereotactic radiotherapy in oligometastatic or oligoprogressive prostate cancer. In our clinical trial, WBMRI scans have been prospectively collected to monitor response, and further studies will explore responses by comparing MRI and IBS and assessing the impact of using MetsBST in prognostication of response in mCSPC patients.
MetsBST was developed to enable reliable detection and quantification of the extent of bone disease, by minimising the variability inherent in subjective manual assessment of IBS images; therefore, allowing for more accurate and objective evaluation of changes in IBS over time. With the emergence of more expensive and less widely available imaging modalities, such as WBMRI and PSMA-PET,17,18 for assessing the extent of bone disease, MetsBST may serve as an attractive alternative for monitoring metastatic prostate cancer patients in clinical trials and everyday practice. Incorporating the use of MetsBST into clinical practice as an adjunct to the reports of nuclear medicine physicians may allow a more objective analysis of changes in IBSs.
Single-photon emission computed tomography/computed tomography (SPECT/CT) is increasingly used for imaging bone metastases in metastatic prostate cancer. This has the ability to improve the sensitivity and specificity to planar bone scans, in both detection of metastases in comparison to osteoarthritic changes19 and assessment of bony metastatic burden.20 These techniques may have additional benefits in patients with low tumour burden, with the caveats including prolongation of study time.
A number of techniques are available to assess and evaluate longitudinal responses to bone metastases in prostate cancer patients. These techniques include 68Ga-PSMA, 18F-choline and 18F-sodium fluoride (18F-NaF) PET/CT, all of which provide highly accurate results in the assessment of bone metastases in this setting. In addition, SPECT/CT has recently been used to quantitatively assess bone metastases by documenting standardised uptake values (SUV).19 Whilst this approach can add additional information, it is significantly more labour-intensive than the MetsBST tool described in this paper. SPECT/CT SUVmax approaches require identification of high intensity lesions at the time of scanning and cannot be applied to data sets retrospectively (as in larger clinical trial datasets) as well as to datasets with no axial/CT imaging. The MetsBST tool requires no additional three-dimensional imaging for analysis. Notwithstanding the differences between the use of two-dimensional planar images and three-dimensional SPECT/CT imaging, investigation of both these approaches could shed additional light on the bone microenvironmental changes occurring in patients, including sclerosis, reduced vascularisation and other factors which may contribute to the diversity of responses of different anatomical metastases.
Conclusion
MetsBST produces results that are highly correlated with the results of aBSI. It is a useful and objective tool to predict outcomes in prostate cancer patients, and generates highly reproducible results. It adds new functionalities for quantifying the extent of metastatic disease in multiple bone sites. Our results suggest that MetsBST can be a valuable tool for interpretation of IBS in mCRPC and mCSPC patients treated with radium‐223. It has the potential to distinctly reduce the variability in assessment of tumour burden caused by subjective interpretation. An additional and novel component of this tool is its ability to differentiate between responses in different bones which may help in treatment selection, providing a platform for further studies involving potential synergy between radium-223 and EBRT as a putative combination for de novo metastatic prostate cancer. Further studies exploring the use of this tool will take place in metastatic prostate cancer patients undergoing a variety of treatments, including chemotherapy (docetaxel/cabazitaxel), novel hormonal agents (abiraterone/enzalutamide) and radium-223.
Footnotes
Acknowledgements: We highly acknowledge the ministry of higher education in Saudi Arabia through the Saudi Arabian Cultural Bureau (SACB) in London, United Kingdom, for fully funding Ali Alshehri’s study and research at Queen's University Belfast. We are also grateful for funding support to our wider group from Prostate Cancer UK, The Movember Foundation, Friends of the Cancer Centre, and Queen’s University Foundation.
Contributor Information
ALI H.D. Alshehri, Email: a.alila.a@hotmail.com.
Sarah O.S. Osman, Email: S.Osman@qub.ac.uk.
Kevin M. Prise, Email: k.prise@qub.ac.uk.
Caoimhghin Campfield, Email: Caoimhghin.Campfield@belfasttrust.hscni.net.
PG Turner, Email: Philip.Turner@belfasttrust.hscni.net.
Suneil FRCR PhD Jain, Email: s.jain@qub.ac.uk.
Joe M. O’Sullivan, Email: joe.osullivan@qub.ac.uk.
Aidan J. Cole, Email: a.cole@qub.ac.uk.
REFERENCES
- 1.Kirby M, Hirst C, Crawford ED. Characterising the castration-resistant prostate cancer population: a systematic review. Int J Clin Pract 2011; 65: 1180–92. doi: 10.1111/j.1742-1241.2011.02799.x [DOI] [PubMed] [Google Scholar]
- 2.Scher HI, Morris MJ, Stadler WM, Higano C, Basch E, Fizazi K, et al. Trial design and objectives for castration-resistant prostate cancer: updated recommendations from the prostate cancer clinical trials Working group 3. J Clin Oncol 2016; 34: 1402–18. doi: 10.1200/JCO.2015.64.2702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Armstrong AJ, Anand A, Edenbrandt L, Bondesson E, Bjartell A, Widmark A, et al. Phase 3 assessment of the automated bone scan index as a prognostic imaging biomarker of overall survival in men with metastatic castration-resistant prostate cancer: a secondary analysis of a randomized clinical trial. JAMA Oncol 2018; 4: 944–51. doi: 10.1001/jamaoncol.2018.1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Turpin A, Girard E, Baillet C, Pasquier D, Olivier J, Villers A, et al. Imaging for metastasis in prostate cancer: a review of the literature. Front Oncol 2020; 10: 55. doi: 10.3389/fonc.2020.00055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anand A, Heller G, Scher HI, Morris MJ. Translating prostate cancer Working Group (PCWG) criteria into a quantitative progression biomarker in metastatic castration resistant prostate cancer (mCRPC. JCO 2017; 35(15_suppl): 5068. doi: 10.1200/JCO.2017.35.15_suppl.5068 [DOI] [Google Scholar]
- 6.Mota JM, Armstrong AJ, Larson SM, Fox JJ, Morris MJ. Measuring the unmeasurable: automated bone scan index as a quantitative endpoint in prostate cancer clinical trials. Prostate Cancer Prostatic Dis 2019; 22: 522–30. doi: 10.1038/s41391-019-0151-4 [DOI] [PubMed] [Google Scholar]
- 7.Saad F. Reliably quantifying bone metastases in prostate Cancer-Are we finally there? JAMA Oncol 2018; 4: 951–2. doi: 10.1001/jamaoncol.2018.1069 [DOI] [PubMed] [Google Scholar]
- 8.Parker CC, James ND, Brawley CD, Clarke NW, Hoyle AP, Ali A, et al. Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): a randomised controlled phase 3 trial. Lancet 2018; 392: 2353–66. doi: 10.1016/S0140-6736(18)32486-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sweeney CJ, Chen Y-H, Carducci M, Liu G, Jarrard DF, Eisenberger M, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med 2015; 373: 737–46. doi: 10.1056/NEJMoa1503747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dennis ER, Jia X, Mezheritskiy IS, Stephenson RD, Schoder H, Fox JJ, et al. Bone scan index: a quantitative treatment response biomarker for castration-resistant metastatic prostate cancer. J Clin Oncol 2012; 30: 519–24. doi: 10.1200/JCO.2011.36.5791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lyra M, Ploussi A, Georgantzoglou A. MATLAB as a Tool in Nuclear Medicine Image Processing. In: Ionescu C, ed.MATLAB: A Ubiquitous tool for the practical engineer. London, UK: Intech Open; 2011. https://www.intechopen.com/books/matlab-a-ubiquitous-tool-for-the-practical-engineer/matlab-as-a-tool-in-nuclear-medicine-image-processing. [Google Scholar]
- 12.Song H, Jin S, Xiang P, Hu S, Jin J. Prognostic value of the bone scan index in patients with metastatic castration-resistant prostate cancer: a systematic review and meta-analysis. BMC Cancer 2020; 20: 238. doi: 10.1186/s12885-020-06739-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ulmert D, Kaboteh R, Fox JJ, Savage C, Evans MJ, Lilja H, et al. A novel automated platform for quantifying the extent of skeletal tumour involvement in prostate cancer patients using the bone scan index. Eur Urol 2012; 62: 78–84. doi: 10.1016/j.eururo.2012.01.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wesolowski CA, Yahil A, Puetter RC, Babyn PS, Gilday DL, Khan MZ. Improved lesion detection from spatially adaptive, minimally complex, Pixon reconstruction of planar scintigraphic images. Comput Med Imaging Graph 2005; 29: 65–81. doi: 10.1016/j.compmedimag.2004.08.003 [DOI] [PubMed] [Google Scholar]
- 15.Ardenfors O, Svanholm U, Jacobsson H, Sandqvist P, Grybäck P, Jonsson C. Reduced acquisition times in whole body bone scintigraphy using a noise-reducing Pixon®-algorithm-a qualitative evaluation study. EJNMMI Res 2015; 5: 48. doi: 10.1186/s13550-015-0127-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mitsui Y, Shiina H, Yamamoto Y, Haramoto M, Arichi N, Yasumoto H, et al. Prediction of survival benefit using an automated bone scan index in patients with castration-resistant prostate cancer. BJU Int 2012; 110(11 Pt B): E628–34. doi: 10.1111/j.1464-410X.2012.11355.x [DOI] [PubMed] [Google Scholar]
- 17.Lecouvet FE, Talbot JN, Messiou C, Bourguet P, Liu Y, de Souza NM, et al. Monitoring the response of bone metastases to treatment with magnetic resonance imaging and nuclear medicine techniques: a review and position statement by the European organisation for research and treatment of cancer imaging group. Eur J Cancer 2014; 50: 2519–31. doi: 10.1016/j.ejca.2014.07.002 [DOI] [PubMed] [Google Scholar]
- 18.Vapiwala N, Hofman MS, Murphy DG, Williams S, Sweeney C. Strategies for evaluation of novel imaging in prostate cancer: putting the horse back before the CART. J Clin Oncol 2019; 37: 765–9. doi: 10.1200/JCO.18.01927 [DOI] [PubMed] [Google Scholar]
- 19.Tabotta F, Jreige M, Schaefer N, Becce F, Prior JO, Nicod Lalonde M. Quantitative bone SPECT/CT: high specificity for identification of prostate cancer bone metastases. BMC Musculoskelet Disord 2019; 20: 619. doi: 10.1186/s12891-019-3001-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Umeda T, Koizumi M, Fukai S, Miyaji N, Motegi K, Nakazawa S, et al. Evaluation of bone metastatic burden by bone SPECT/CT in metastatic prostate cancer patients: defining threshold value for total bone uptake and assessment in radium-223 treated patients. Ann Nucl Med 2018; 32: 105–13. doi: 10.1007/s12149-017-1224-x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.