Abstract
Background
Numerous case reports and case series have described brain Magnetic Resonance Imaging (MRI) findings in Coronavirus disease 2019 (COVID-19) patients with concurrent posterior reversible encephalopathy syndrome (PRES).
Purpose
We aim to compile and analyze brain MRI findings in patients with COVID-19 disease and PRES.
Methods
PubMed and Embase were searched on April 5th, 2021 using the terms “COVID-19”, “PRES”, “SARS-CoV-2” for peer-reviewed publications describing brain MRI findings in patients 21 years of age or older with evidence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and PRES.
Results
Twenty manuscripts were included in the analysis, which included descriptions of 30 patients. The average age was 57 years old. Twenty-four patients (80%) required mechanical ventilation. On brain MRI examinations, 15 (50%) and 7 (23%) of patients exhibited superimposed foci of hemorrhage and restricted diffusion respectively.
Conclusions
PRES is a potential neurological complication of COVID-19 related disease. COVID-19 patients with PRES may exhibit similar to mildly greater rates of superimposed hemorrhage compared to non-COVID-19 PRES patients.
Abbreviations: COVID-19, Coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus; PRES, posterior reversible encephalopathy syndrome; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; MRI, magnetic resonance imaging; SWI, susceptibility-weighted imaging
Keywords: COVID-19, PRES, MRI brain
1. Introduction
The novel Coronavirus disease 2019 (COVID-19) caused by the severe acute respiratory syndrome coronavirus (SARS-CoV-2) can affect multiple organ systems in addition to its primary effect on the respiratory system.1 Several central nervous system (CNS) complications have been described, including intracranial hemorrhage, acute infarction, cerebral vein thrombosis, and CNS inflammatory disorders.2., 3., 4. Recently, posterior reversible encephalopathy syndrome (PRES) has also been described in association with COVID-19 infections, with more cases emerging as the pandemic progresses. The clinical presentation of PRES varies from headache, altered mental status, seizures, and visual loss, while MRI demonstrates white matter edema predominating in the posterior parietal and occipital cerebrum.5 Currently, the brain MRI findings of PRES in the setting of COVID-19 infection is limited to case reports and case series. Utilizing the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines,6 we performed a systematic review in order to collect and evaluate the brain MRI findings in COVID-19 associated PRES.
2. Methods
2.1. Data sources and searches
A systematic search of the PubMed and Embase databases was performed to identify published manuscripts that described brain MRI findings in patients with COVID-19 disease and PRES. Search terms included: “COVID”, “PRES”, and “SARS-CoV-2”. Literature searches were performed on April 5th, 2021.
2.2. Study selection and data extraction
We included articles published in peer-reviewed journals if they described brain MRI findings in patients older than 21 years of age with SARS-CoV-2 infection and a provided diagnosis of PRES. Duplicate manuscripts from literature search results were removed prior to the screening process. Three reviewers (E.G., J.S., R.Y.) examined titles and abstracts from the literature searches to identify potential manuscripts for inclusion. Articles included after initial screening were reviewed for final inclusion by two neuroradiologists (E.G., J.S.).
2.3. Data synthesis and analysis
Data extraction from each manuscript was performed by two board-certified radiologists, one with a certificate of added qualification in neuroradiology (E.G.) and the other with specialty training in neuroradiology (J.S.). Data extracted from each article included: authors, title, country of origin, number of patients with brain MRI imaging findings described, age and gender of the patients, clinical presentation, clinical parameters, and specific findings on brain MRI exams. One patient (Table 3 , patient #2) was described in two different manuscripts.7., 8.
Table 3.
Manuscripts describing Brain MRI findings in COVID-19 patients with PRES.
| Patient no./sex/age(y) | First author | Clinical presentation |
Clinical intervention |
MRI brain findings |
Clinical Outcome | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Blood pressure | Visual changes | Altered mental status | Seizures | Highest level O2 therapy | Immunotherapy status | Findings | Hemorrhage | Diffusion restriction | |||
| P1/M/64 | Doo FX et al.28 | NR | NR | Present | Present | Mechanical ventilation | Tocilizumab | Parietooccipital edema with small foci of hemorrhage. | Present | NR | NR |
| P2/F/64 | Princiotta et al., and Colombo et al.7., 8. | Hypertensive | Present | Present | Absent | Mechanical ventilation | NR | MRI brain consistent with PRES | Present | NR | Discharged |
| P3/M/58 | Kishfy L et al.29 | Hypertensive | Absent | Present | NR | Mechanical ventilation | Tocilizumab | T2 FLAIR hyperintensity involving subcortical occipital and temporal white matter. Convexity subarachnoid hemorrhage. No abnormal contrast enhancement. | Present | Absent | Discharged |
| P4/F/67 | Kishfy L et al.29 | Hypertensive | Absent | Present | NR | Mechanical ventilation | NR | T2 FLAIR hyperintense foci in right occipital subcortical white matter and left cerebellum. SWI with petechial hemorrhage. No abnormal contrast enhancement. | Present | Absent | Discharged |
| P5/F/63 | Conte G et al.30 | Hypertensive | Present | NR | Present | Mechanical ventilation | Anakinra | Partially confluent, tumefactive white matter lesions, with posterior predominance. Left precentral subarachnoid blood products. Perivascular contrast enhancement within white matter. | Present | Present | Discharged |
| P6/M/59 | Rogg J et al.12 | Hypertensive | NR | Present | NR | Mechanical ventilation | NR | Extensive FLAIR hyperintensity within subcortical greater than deep white matter, internal and external capsules, and cerebellar white matter. No contrast enhancement. | NR | Absent | Death |
| P7/NR/NR | Dias DA et al.31 | NR | NR | Present | NR | Mechanical ventilation | NR | Bilateral parieto-occipital vasogenic edema with superimposed blood products. Foci of restricted diffusion attributed to blood products. | Present | Absent | NR |
| P8/M/64 | Parauda SC et al.32 | Hypertensive | Present | Present | Present | Mechanical ventilation | NR | T2 FLAIR hyperintensities occipital white matter, and within left thalamus and internal capsule. | Absent | Absent | Discharged |
| P9/M/73 | Parauda SC et al.32 | Hypertensive | NR | Present | Present | Mechanical ventilation | NR | Confluent T2 hyperintensity bilateral parietooccipital white matter. | Absent | Absent | Discharged |
| P10/F/65 | Parauda SC et al.32 | Hypertensive | NR | Present | Absent | Mechanical ventilation | NR | Non enhancing bilateral occipital subcortical white matter T2 hyperintensities. | NR | Absent | Discharged |
| P11/F/74 | Parauda SC et al.32 | Hypertensive | NR | Present | Absent | Mechanical ventilation | Tocilizumab | T2 hyperintensities in bilateral parietooccipital lobes with restricted diffusion and SWI hypointense foci. | Present | Present | Discharged |
| P12/F/27 | Agarwal A et al.33 | NR | NR | Present | NR | Mechanical ventilation | NR | Subcortical vasogenic edema in occipital subcortical white matter. | NR | Absent | Death |
| P13/F/24* | López Pérez V et al.34 | Hypertensive | NR | Present | Present | Mechanical ventilation | Tocilizumab | FLAIR hyperintensity in bilateral parietal and parasagittal fronal regions. | NR | NR | Discharged |
| P14/F/64 | D'Amore F et al.35 | NR | Present | Present | NR | NR | NR | Vasogenic edema and occipital hemorrhages. | Present | NR | NR |
| P15/F/33 | Ghosh R et al.36 | Normotensive | Present | Absent | NR | NR | NR | FLAIR hyperintensity bilateral occipital subcortical white matter. | Absent | Absent | Discharged |
| P16/F/25* | Sripadma PV et al.37 | Hypertensive | NR | NR | Present | Mechanical ventilation | NR | Bilateral parietal-occipital T2 hyperintensities with bilateral small hemorrhages. | Present | Absent | Discharged |
| P17/F/61 | Anand P et al.38 | Hypertensive | NR | Present | Present | Mechanical ventilation | Anakinra | T2 hyperintensities in bilateral parietooccipital lobes. Susceptibility in right fontal lobe. | Present | Absent | Discharged |
| P18/F/52 | Anand P et al.38 | Hypertensive | Absent | Present | Present | Mechanical ventilation | NR | T2 hyperintensities within bilateral parietal, occipital, frontal, temporal white matter. Punctate hemorrhages in temporal and occipital lobes. | Present | Absent | Discharged |
| P19/M/48 | Franceschi AM et al.11 | Hypertensive | NR | Present | NR | Mechanical ventilation | NR | Vasogenic edema in the posterior parieto-occipital regions with subacute blood products. SWI with petechial hemorrhages diffusely throughout the corpus callosum. | Present | Present | NR |
| P20/F/67 | Franceschi AM et al.11 | Hypertensive | NR | Present | NR | NR | NR | Multiple areas of restricted diffusion and edema, greatest within the parietooccipital regions, and within the right frontal lobe, basal ganglia, and cerebellar hemispheres. Extensive superimposed hemorrhages in the parietooccipital region with abnormal contrast enhancement. | Present | Present | Discharged |
| P21/M/46 | Ordoñez-Boschetti L et al.39 | Normotensive | Absent | Present | NR | Mechanical ventilation | NR | T2 FLAIR hyperintensities in frontal and occipital white matter. | NR | Absent | Discharged |
| P22/M/74 | Gómez-Enjuto S et al.40 | Hypertensive | Present | Present | Present | NR | Carfilzomib | T2 FLAIR hyperintensities frontalparietal and occipital subcortical areas. | NR | NR | Discharged |
| P23/M/54 | Colombo A et al.8 | Hypertensive | Present | Absent | Present | Mechanical ventilation | NR | MRI brain consistent with PRES | NR | NR | Discharged |
| P24/F/63 | Colombo A et al.8 | Hypertensive | Present | Present | Present | Mechanical ventilation | IL-1 antagonist | MRI brain consistent with PRES | NR | NR | Discharged |
| P25/M/64 | Colombo A et al.8 | Normotensive | Present | Present | Present | Mechanical ventilation | NR | Symmetric white matter alterations, mainly occipital lobes. No enhancement. | Present | NR | Discharged |
| P26/M/68 | Colombo A et al.8 | Hypertensive | Present | Present | Absent | Mechanical ventilation | NR | MRI brain consistent with PRES | NR | NR | Discharged |
| P27/F/57 | Colombo A et al.8 | Hypertensive | Present | Absent | Present | Non-invasive ventilation | NR | MRI brain consistent with PRES | NR | NR | Discharged |
| P28/F/43 | Santos de Lima F et al.41 | Normotensive | Absent | Present | Present | Mechanical ventilation | NR | 1st MRI-Area of hyperintensity in the splenium. 2nd MRI- extensive T2/FLAIR hyperintensity in bilateral cererbal hemispheres with mild sulcal enhancement, and gyroform restricted diffusion in right temporooccipito-parietal region. Lesion in the splenium resolved. 3rd MRI-progression of white signal abnormality. | NR | Present | Discharged |
| P29/M/55 | Wijeratne T et al.42 | Hypertensive | Absent | Present | Absent | NR | NR | Bilateral parietal-occipital T2 FLAIR hyperintensities. Diffuse petechial hemorrhages in basal ganglia and deep white matter. Small foci of restricted diffusion in deep white matter. | Present | Present | Discharged |
| P30/M/70 | Talluri K et al.43 | Hypertensive | Absent | Present | Absent | Mechanical ventilation | Tocilizumab | Cortical and subcortical FLAIR signal in bilateral occipital lobes and paramedian frontal and parietal lobes. Restricted diffusion bilateral occipital lobes, posterior thalami and left temporal lobe. | NR | Present | Death |
NR = Not recorded. Patient #2 was described in two separate manuscripts. * = Patients were pregnant.
3. Results
3.1. Manuscript searches
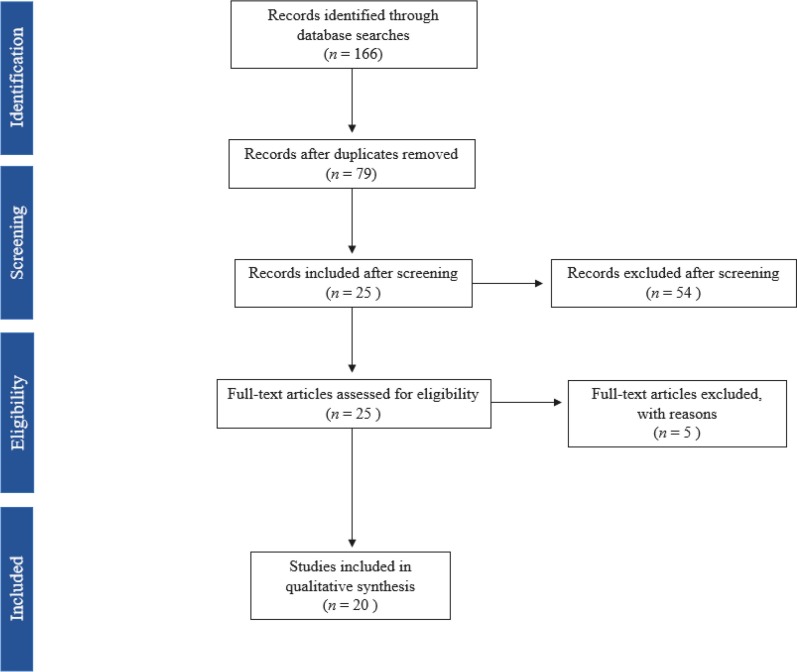
We identified 166 articles (Fig. 1 ). After removal of duplicates, 79 articles remained for screening. After screening, 25 articles were fully assessed for eligibility. Of the 25 articles evaluated for eligibility, 5 articles were excluded secondary to: 4 articles not describing brain MRI findings, and 1 with discordant description of brain MRI findings and the provided figure. This resulted in the inclusion of 20 manuscripts (Table 1 ). These included: 11 case reports, 8 case series, and a letter to an editor. The manuscripts originated from 7 different countries (United States: 9, Italy: 4, India: 2, Spain: 2, Australia: 1, Brazil: 1, and Mexico: 1).
Fig. 1.
Literature search results.
Table 1.
Manuscripts describing Brain MRI findings in COVID-19 patients with PRES.
| Manuscripts describing MRI findings in Covid-19 patients with PRES | |
|---|---|
| Articles | 20 |
| Journals | 16 |
| Countries | 7 |
| USA | 9 |
| Italy | 4 |
| India | 2 |
| Spain | 2 |
| Australia | 1 |
| Brazil | 1 |
| Mexico | 1 |
| Patients with MR imaging of the brain described | 30 |
| Case report | 11 |
| Case series | 8 |
| Letter to editor | 1 |
3.2. Patients
Thirty patients with COVID-19 and PRES with described brain MRI findings were included in the analysis (Table 2 ). The mean age was 57 years (range 24–74), and 16 were female. Of the clinical presentations associated with PRES, there were 22 patients with hypertensive episodes, 14 with visual changes, 25 with altered mental status and 14 with seizure. Twenty-four (80%) of the patients required mechanical ventilation, and 9 patients (30%) received immunotherapy. In most cases, patients exhibited symptoms of COVID-19 related illness prior to hospitalization and development of PRES as an inpatient. Eight manuscripts provided descriptions of CSF results, most of which were within normal limits. Only two manuscripts mentioned if CSF was tested for the SARS-CoV-2 virus. Only 2 manuscripts mentioned that patients were given pressors (patients 17 and 18), and three were administrated steroid therapy (patients 15, 22, 29).
Table 2.
Patient characteristics and brain MRI findings in COVID-19 patients with PRES.
| COVID-19 patients with PRES (n = 30) | |||
|---|---|---|---|
| Demographics | Age | ||
| Range | 24–74 | ||
| Mean | 57 | ||
| Median | 63 | ||
| n | % | ||
| Male | 13 | 43% | |
| Female | 16 | 53% | |
| Gender not reported | 1 | 3% | |
| Clinical presentation | >1 hypertensive episode | 22 | 73% |
| Visual changes | 14 | 45% | |
| Altered mental status | 25 | 83% | |
| Seizure | 14 | 42% | |
| Clinical intervention | Mechanical ventilation | 24 | 80% |
| Immunotherapy | 9 | 30% | |
| Imaging findings | Hemorrhage | 15 | 50% |
| Diffusion restriction | 7 | 23% | |
| Clinical outcome | Death | 3 | 10% |
| Discharged | 23 | 77% | |
3.3. Imaging findings
MRI brain findings varied from briefs statements such as, “MRI brain consistent with PRES”, to more comprehensive explanations detailing findings on different pulse sequences. Most manuscripts did not provide MRI protocols utilized, nor field strength of the MRI magnet used. Most manuscripts did not specify whether gradient echo or susceptibility-weighted imaging sequences were used for detecting blood products, and was only provided in ten cases. Eight cases (patients 3, 4, 5, 7, 17, 19, 20, 29) utilized SWI, and two cases (patients 2, 14) utilized gradient echo sequences. On brain MRI, 15 patients (50%) had foci of hemorrhage superimposed on described findings of PRES, and 7 patients (23%) had superimposed foci of restricted diffusion. The described brain MRI findings within the manuscripts precluded adequate tabulation of the distribution of FLAIR signal abnormalities typical of PRES. Additionally, few articles mentioned whether or not there was abnormal intracranial contrast enhancement.
4. Discussion
This first systematic review of brain MRI findings in COVID-19 patients with PRES utilizing PRISMA guidelines shows that PRES patients who have COVID-19 may have similar to mildly increased rates of hemorrhage compared to PRES patients who do not have COVID-19.
Numerous neurological and neuroimaging manifestations of COVID-19 infection have been described including intracranial hemorrhage, acute strokes, leukoencephalopathy, and CNS inflammatory disorders. As the pandemic continues, more and more case reports and case series are describing an association of COVID-19 disease with clinical and imaging findings typical of PRES. While the complex pathogenesis and possible neurotropism of the SARS-CoV-2 virus is still being elucidated, there is growing evidence that the intracranial complications of COVID-19 disease may at least in part be due to the cytokine release syndrome (a.k.a. ‘cytokine storm’).9., 10. Typical laboratory findings of cytokine release syndrome include decreased T-cells and natural killer cells, and an increase in interleukin 6,11 among elevation of other inflammatory markers.9 PRES is commonly linked to alterations in the blood-brain barrier secondary to loss of autoregulation or endothelial dysfunction.11 The massive release of cytokines and inflammatory markers that can occur in COVID-19 patients, may result in breakdown of the blood-brain barrier and endothelial injury that leads to PRES.11., 12.
In the absence of established diagnostic criteria, PRES is often diagnosed in the context of clinical and imaging findings after other possibilities have been excluded.13 Typical clinical presentations include either seizure, headache, visual disturbance, or altered mental status and often occurs in the setting of elevated blood pressures.14 Numerous additional causes have been associated with PRES including: eclampsia, immunosuppressive drugs, as well as an array of additional drugs and diseases.14 On imaging, typical PRES is characterized by vasogenic edema preferentially involving the parietooccipital regions, whereas atypical PRES can involve the frontal lobes, basal ganglia, brainstem, and cerebellum (Fig. 2).14 Three hemispheric pattern variants can be observed (holohemispheric, superior frontal sulcal, and primary parietal-occipital), which resemble brain watershed zones.15 Predominate involvement of the basal ganglia, brain stem, and deep white matter with less hemispheric involvement can lead to a challenging diagnosis of PRES.15 Catheter cerebral angiogram or MRA time-of-flight imaging reveals vasculopathy with diffuse vasoconstriction, focal vasoconstriction/vasodilatation, and usually resolves on repeat imaging.15 Reports of perfusion patterns in PRES have varied including descriptions of hyperperfusion and hypoperfusion.15
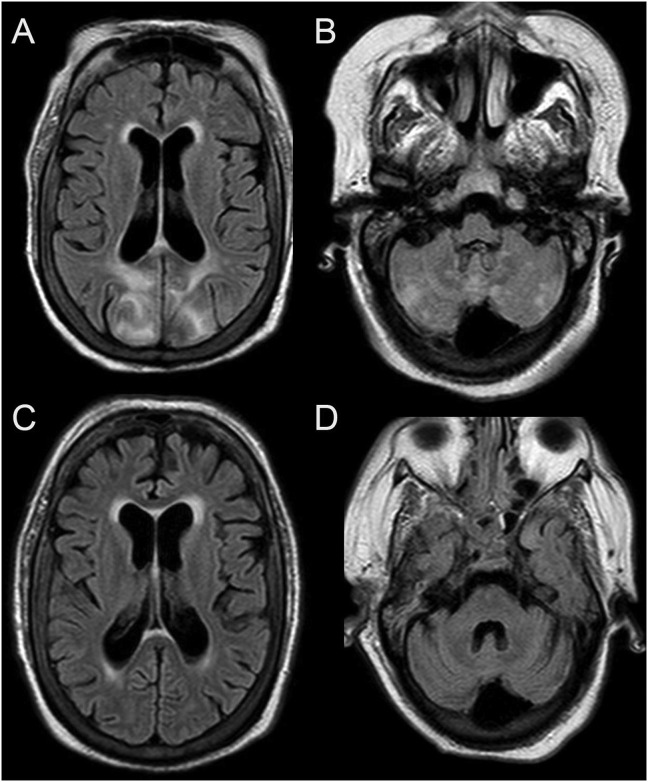
Fig. 2.
PRES in a patient with COVID-19.
Brain MRI images in a 61-year-old female with COVID-19 and respiratory failure requiring mechanical ventilations. Axial T2/FLAIR images (A and B) demonstrate T2/FLAIR hyperintensities predominately with the bilateral parietooccipital regions. Foci of T2/FLAIR hyperintensities were also present with the bilateral cerebellum. Imaging findings were consistent with PRES. A follow-up Brain MRI exam (C and D) approximately 3 weeks later demonstrated resolution of the parenchymal abnormalities. There was no evidence of restricted diffusion or hemorrhagic foci on the first Brain MRI exam.
The key difference between PRES associated with COVID-19 and PRES in other settings appears to be the similar to mildly higher rate of hemorrhage, while the rate of restricted diffusion is approximately the same. Prior reports described hemorrhage in PRES patients occurring in approximately 15–17% of patients,15., 16. and a more recent report demonstrated 36% of hemorrhage in PRES patients.17 T2-star weighted imaged was utilized in two of these manuscripts.16., 17. In a more recent report that utilized SWI, hemorrhage was present in 64.5% of patients with PRES.18 In our analysis, MRI techniques for detecting hemorrhage was described in only ten patients (SWI was used in eight patients) resulting in a limitation to our study. Foci of restricted diffusion can in occur 16–17% of cases19, and we observed 23%. In our analysis, too few manuscripts commented on the absence or presence of intracranial contrast enhancement, precluding adequate comparison with non-COVID-19 PRES patients.
PRES is typically associated with a favorable outcome with proper medical management.14 However, the presence of hemorrhage or restricted diffusion is associated with worse clinical outcomes.17 Of the cases we reviewed, 3 patients (10%) expired. Our study is limited in evaluating outcomes in COVID-19 patients with PRES due to a small sample size, and a formal meta-analysis with a larger patient sample would be needed to compare mortality rates between PRES patients with and without COVID-19.
The majority of patients in our study required mechanical ventilation, indicative of severe disease, and 9 of the patients received immunotherapy. Guidelines from the national institutes of health recommend use of baricitinib and tocilizumab in certain hospitalized patients: 1) hospitalized patients requiring oxygen through a high-flow device or noninvasive ventilation, and with increasing oxygen need and systemic inflammation after a recent hospitalization, and 2) hospitalized patients requiring mechanical ventilation or ECMO, and are within 24 h of ICU admission. Larger studies are needed to investigate whether immunomodulators cause PRES in COVID-19 patients.20
Our study is limited by reliance on descriptions rather than direct interpretation of brain MRI findings, and the presumed diagnosis of PRES. This precludes adequate review of the distribution of parenchymal abnormalities which can be variable in PRES. Insofar as there may be overlap in the imaging findings of PRES and COVID-19 related neuroimaging findings, such as COVID-19 related leukoencephalopathy or acute hemorrhagic leukoencephalities, this may be a significant source of bias. Recent reports4., 21., 22., 23. have described confluent white matter T2 hyperintensities in patients with severe COVID-19 disease and prolonged mechanical ventilation, in some cases with superimposed foci of restricted diffusion and microhemorrhages, possibly due to hypoxemia.4 In two studies the leukoencephalopathy was reported to have a posterior predominance.21., 24. Microhemorrhages have also been described as a common finding in patients with COVID-19.25 Recent reports of acute hemorrhagic leukoencephalitis in COVID-19 patients, have described multifocal white matter lesions with hemorrhages and foci of restricted diffusion.26., 27. Distinction between PRES, COVID-19 related leukoencephalopathy, and acute hemorrhagic leukoencephalitis may be relevant clinically as it may alter clinical management. Further studies may be helpful in confidently distinguishing between these entities on brain MRI.
The medical community's understanding of COVID-19 is rapidly changing as the pandemic evolves. While our study aimed to incorporate all cases of COVID-19 and PRES, there may be some cases that were missed or will subsequently be published. Additional and larger studies will be needed to further understand the interplay between COVID-19 and PRES.
5. Conclusion
PRES is a potential neurological complication of COVID-19 related disease. COVID-19 patients with PRES may exhibit similar to mildly greater rates of hemorrhage compared to non-COVID-19 PRES patients.
References
- 1.Abobaker A., Raba A.A., Alzwi A. Extrapulmonary and atypical clinical presentations of COVID-19. J Med Virol. 2020;92(11):2458–2464. doi: 10.1002/jmv.26157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hughes C., Nichols T., Pike M., Subbe C., Elghenzai S. Cerebral venous sinus thrombosis as a presentation of COVID-19. Eur J Case Rep Intern Med. 2020;7(5) doi: 10.12890/2020_001691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oxley T.J., Mocco J., Majidi S., et al. Large-vessel stroke as a presenting feature of Covid-19 in the young. N Engl J Med. Apr 2020 doi: 10.1056/NEJMc2009787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Radmanesh A., Derman A., Lui Y.W., et al. COVID-19 -associated diffuse leukoencephalopathy and microhemorrhages. Radiology. 2020 doi: 10.1148/radiol.2020202040. May. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sudulagunta S.R., Sodalagunta M.B., Kumbhat M., Settikere Nataraju A. Posterior reversible encephalopathy syndrome(PRES) Oxf Med Case Reports. 2017;(4) doi: 10.1093/omcr/omx011. Apr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moher D., Liberati A., Tetzlaff J., Altman D.G., Group P Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7) doi: 10.1371/journal.pmed.1000097. Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Princiotta Cariddi L., Tabaee Damavandi P., Carimati F., et al. Reversible encephalopathy syndrome (PRES) in a COVID-19 patient. J Neurol. Nov 2020;267(11):3157–3160. doi: 10.1007/s00415-020-10001-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colombo A., Martinelli Boneschi F., Beretta S., et al. Posterior reversible encephalopathy syndrome and COVID-19: a series of 6 cases from Lombardy, Italy. eNeurologicalSci. 2021;22:100306. doi: 10.1016/j.ensci.2020.100306. Mar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ye Q., Wang B., Mao J. The pathogenesis and treatment of the `cytokine storm' in COVID-19. J Infect. 2020;80(6):607–613. doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mehta P., DF McAuley, Brown M., et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. 03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Franceschi A.M., Ahmed O., Giliberto L., Castillo M. Hemorrhagic posterior reversible encephalopathy syndrome as a manifestation of COVID-19 infection. AJNR Am J Neuroradiol. 2020;41(7):1173–1176. doi: 10.3174/ajnr.A6595. 07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rogg J., Baker A., Tung G. Posterior reversible encephalopathy syndrome (PRES): another imaging manifestation of COVID-19. Interdiscip Neurosurg. Dec 2020;22 doi: 10.1016/j.inat.2020.100808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fischer M., Schmutzhard E. Posterior reversible encephalopathy syndrome. J Neurol. Aug 2017;264(8):1608–1616. doi: 10.1007/s00415-016-8377-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Osborn A.G. 1st ed. xi. Amirsys Pub; 2013. Osborn's brain: imaging, pathology, and anatomy. 1272 p. [Google Scholar]
- 15.Bartynski W.S. Posterior reversible encephalopathy syndrome, part 1: fundamental imaging and clinical features. AJNR Am J Neuroradiol. Jun 2008;29(6):1036–1042. doi: 10.3174/ajnr.A0928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hefzy H.M., Bartynski W.S., Boardman J.F., Lacomis D. Hemorrhage in posterior reversible encephalopathy syndrome: imaging and clinical features. AJNR Am J Neuroradiol. Aug 2009;30(7):1371–1379. doi: 10.3174/ajnr.A1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schweitzer A.D., Parikh N.S., Askin G., et al. Imaging characteristics associated with clinical outcomes in posterior reversible encephalopathy syndrome. Neuroradiology. Apr 2017;59(4):379–386. doi: 10.1007/s00234-017-1815-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McKinney A.M., Sarikaya B., Gustafson C., Truwit C.L. Detection of microhemorrhage in posterior reversible encephalopathy syndrome using susceptibility-weighted imaging. AJNR Am J Neuroradiol. May 2012;33(5):896–903. doi: 10.3174/ajnr.A2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McKinney A.M., Short J., Truwit C.L., et al. Posterior reversible encephalopathy syndrome: incidence of atypical regions of involvement and imaging findings. AJR Am J Roentgenol. Oct 2007;189(4):904–912. doi: 10.2214/AJR.07.2024. [DOI] [PubMed] [Google Scholar]
- 20.Therapeutic Management of Hospitalized Adults With COVID-19. Updated July 8, 2021. Accessed July 19, 2021.
- 21.Sachs J.R., Gibbs K.W., Swor D.E., et al. COVID-19-associated Leukoencephalopathy. Radiology. 2020;296(3):E184–E185. doi: 10.1148/radiol.2020201753. 09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lang M., Buch K., Li M.D., et al. Leukoencephalopathy associated with severe COVID-19 infection: sequela of hypoxemia? AJNR Am J Neuroradiol. 2020;41(9):1641–1645. doi: 10.3174/ajnr.A6671. 09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Freeman C.W., Masur J., Hassankhani A., Wolf R.L., Levine J.M., Mohan S. Coronavirus disease (COVID-19)-related disseminated leukoencephalopathy: a retrospective study of findings on brain MRI. AJR Am J Roentgenol. 2021;216(4):1046–1047. doi: 10.2214/AJR.20.24364. 04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Radmanesh A., Raz E., Zan E., Derman A., Kaminetzky M. Brain imaging use and findings in COVID-19: a single academic center experience in the epicenter of disease in the United States. AJNR Am J Neuroradiol. 2020;41(7):1179–1183. doi: 10.3174/ajnr.A6610. 09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gulko E., Oleksk M.L., Gomes W., et al. MRI brain findings in 126 patients with COVID-19: initial observations from a descriptive literature review. AJNR Am J Neuroradiol. 2020;41(12):2199–2203. doi: 10.3174/ajnr.A6805. 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yong M.H., YFZ Chan, Liu J., et al. A rare case of acute hemorrhagic leukoencephalitis in a COVID-19 patient. J Neurol Sci. 2020;416:117035. doi: 10.1016/j.jns.2020.117035. 09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Varadan B., Shankar A., Rajakumar A., et al. Acute hemorrhagic leukoencephalitis in a COVID-19 patient-a case report with literature review. Neuroradiology. May 2021;63(5):653–661. doi: 10.1007/s00234-021-02667-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Doo F.X., Kassim G., Lefton D.R., Patterson S., Pham H., Belani P. Rare presentations of COVID-19: PRES-like leukoencephalopathy and carotid thrombosis. Clin Imaging. Jan 2021;69:94–101. doi: 10.1016/j.clinimag.2020.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kishfy L., Casasola M., Banankhah P., et al. Posterior reversible encephalopathy syndrome (PRES) as a neurological association in severe Covid-19. J Neurol Sci. 2020;414:116943. doi: 10.1016/j.jns.2020.116943. 07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Conte G., Avignone S., Carbonara M., et al. COVID-19-associated PRES-like encephalopathy with perivascular gadolinium enhancement. AJNR Am J Neuroradiol. 2020;41(12):2206–2208. doi: 10.3174/ajnr.A6762. 04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dias D.A., de Brito L.A., Neves L.O., RGS Paiva, Barbosa Júnior O.A., JWL Tavares-Júnior. Hemorrhagic PRES: an unusual neurologic manifestation in two COVID-19 patients. Arq Neuropsiquiatr. 2020;78(11):739–740. doi: 10.1590/0004-282X20200184. 03. [DOI] [PubMed] [Google Scholar]
- 32.Parauda S.C., Gao V., Gewirtz A.N., et al. Posterior reversible encephalopathy syndrome in patients with COVID-19. J Neurol Sci. Sep 2020;416 doi: 10.1016/j.jns.2020.117019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Agarwal A., Pinho M., Raj K., et al. Neurological emergencies associated with COVID-19: stroke and beyond. Emerg Radiol. Dec 2020;27(6):747–754. doi: 10.1007/s10140-020-01837-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.López Pérez V., Cora Vicente J., Echevarría Granados C., Salcedo Vázquez M.L., Estol F., Tebar Cuesta M.Y. Postpartum consciousness disturbance: can covid-19 cause posterior reversible encephalopathy syndrome? Rev Esp Anestesiol Reanim (Engl Ed) Nov 2020;67(9):511–515. doi: 10.1016/j.redar.2020.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.D'Amore F., Vinacci G., Agosti E., et al. Pressing issues in COVID-19: probable cause to seize SARS-CoV-2 for its preferential involvement of posterior circulation manifesting as severe posterior reversible encephalopathy syndrome and posterior strokes. AJNR Am J Neuroradiol. 2020;41(10):1800–1803. doi: 10.3174/ajnr.A6679. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ghosh R., Lahiri D., Dubey S., Ray B.K., Benito-León J. Hallucinatory palinopsia in COVID-19-induced posterior reversible encephalopathy syndrome. J Neuroophthalmol. 2020;40(4):523–526. doi: 10.1097/WNO.0000000000001135. 12. [DOI] [PubMed] [Google Scholar]
- 37.Sripadma P.V., Rai A., Wadhwa C. Postpartum Atypical posterior reversible encephalopathy syndrome in a COVID-19 patient - an obstetric emergency. J Stroke Cerebrovasc Dis. 2020;29(12) doi: 10.1016/j.jstrokecerebrovasdis.2020.105357. Dec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anand P., Lau K.H.V., Chung D.Y., et al. Posterior reversible encephalopathy syndrome in patients with coronavirus disease 2019: two cases and a review of the literature. J Stroke Cerebrovasc Dis. Nov 2020;29(11) doi: 10.1016/j.jstrokecerebrovasdis.2020.105212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ordoñez-Boschetti L., Torres-Romero C.M., Ortiz de Leo M.J. Associated posterior reversible encephalopathy syndrome (PRES) to SARS-CoV-2. Case report. Neurologia (Engl Ed) 2020;35(9):696–698. doi: 10.1016/j.nrl.2020.08.001. 2020 Nov - Dec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gómez-Enjuto S., Hernando-Requejo V., Lapeña-Motilva J., et al. Verapamil as treatment for refractory status epilepticus secondary to PRES syndrome on a SARS-Cov-2 infected patient. Seizure. 2020;08(80):157–158. doi: 10.1016/j.seizure.2020.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Santos de Lima F., Klein S., El Ammar F., et al. Rapid development of seizures and PRES in a COVID-19 patient. Epilepsy Behav Rep. 2021;15:100436. doi: 10.1016/j.ebr.2021.100436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wijeratne T., Wijeratne C., Karimi L., Sales C., Crewther S.G. Case report: posterior reversible leukoencephalopathy syndrome (PRES) as a Biologically predictable neurological association in severe COVID-19. First reported case from australia and review of internationally published cases. Front Neurol. 2020;11:600544. doi: 10.3389/fneur.2020.600544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Talluri K., Lall N., Moreno M.A., Nichols L., Bande D. Posterior reversible encephalopathy syndrome in a patient with SARS-CoV-2 infection treated with tocilizumab. Cureus. Feb 2021;13(2) doi: 10.7759/cureus.13475. [DOI] [PMC free article] [PubMed] [Google Scholar]