Abstract
A strong association between perinatal viral infections and neurodevelopmental disorders has been established. Both the direct contact of the virus with the developing brain and the strong maternal immune response originated by viral infections can impair proper neurodevelopment. Coronavirus disease 2019 (COVID-19), caused by the highly-infectious severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is currently responsible for a large global outbreak and is a major public health issue. While initial studies focused on the viral impact on the respiratory system, increasing evidence suggest that SARS-CoV-2 infects other organs and tissues including the mature brain. While studies continue to determine the neuropathology associated to COVID-19, the consequences of SARS-CoV-2 infection to the developing brain remain largely unexplored. The present review discusses evidence suggesting that SARS-CoV-2 infection may have persistent effects on the course of pregnancy and on brain development. Studies have shown that several proinflammatory mediators which are increased in the SARS-CoV-2-associated cytokine storm, are also modified in other viral infections known to increase the risk of neurodevelopmental disorders. In this sense, further studies should assess the genuine effects of SARS-CoV-2 infection during pregnancy and delivery along with an extended follow-up of the offspring, including neurocognitive, neuroimaging, and electrophysiological examination. It also remains to be determined whether and by which mechanisms SARS-CoV-2 intrauterine and early life infection could lead to an increased risk of developing neuropsychiatric disorders, such as autism (ASD) and schizophrenia (SZ), in the offspring.
This article is part of the special Issue on ‘Cross Talk between Periphery and the Brain’.
Keywords: Brain, Development, SARS-CoV-2, Autism, Schizophrenia, Pregnancy
1. Introduction
Coronavirus disease 2019 (COVID-19), caused by the highly-infectious severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is currently responsible for a large global outbreak and is a major public health issue, having caused the death of millions of people worldwide. Even though the main risk factors for COVID-19-related poor outcomes include older age, male gender, cardiovascular conditions, hypertension and diabetes (Williamson et al., 2020), the disease is associated with unpredictable and variable outcomes independently of patient profile. In addition, the worrying emergence of new viral variants is leading to increased complications in patients outside the main risk groups. Clearly, there is an urgent need for studies to unravel the pathophysiology of the diverse disease symptoms and the possible late consequences associated with infection. Initial studies focused on the viral impact on the respiratory system, but increasing evidence suggest that SARS-CoV-2 infects other organs and tissues. In particular, the brain was recently shown to be targeted by the virus (Song et al., 2021), and neurological manifestations were shown to affect over 30% of patients (Mao et al., 2020).
Although the clinical studies performed to this date have focused mainly on the neurological consequences of SARS-CoV-2 infection in non-pregnant adults, it is expected that only in the United States approximately 120.000 pregnant women have been infected with SARS-CoV-2 until October 2021 (CDC, 2020). This number is probably even bigger, since many patients experience only mild symptoms and others may even not have themselves tested. Despite initial studies suggested that COVID-19 is associated with low birth-weight infants and increased risk of preterm deliveries (Lokken, Huebner, et al., 2021; Lokken, Taylor, et al., 2021; Zambrano et al., 2020), whether SARS-CoV-2 infection has the potential to negatively affect intrauterine development remains controversial. Several studies suggest that mother-to-child transmission of SARS-CoV-2 can occur in utero, intrapartum, or early in the postnatal period (Fenizia et al., 2020; Hosier et al., 2020; Patanè et al., 2020; Sukhikh et al., 2021). Although most neonates testing positive for the virus do not develop severe respiratory symptoms (Raschetti et al., 2020), studies addressing the consequences of this infection to the developing brain are lacking. Importantly, due to the short timeframe available for patient follow-up and to the lack of studies in animal models, the true long-term impacts to the brains of infants born from SARS-CoV-2-infected mothers are still unknown.
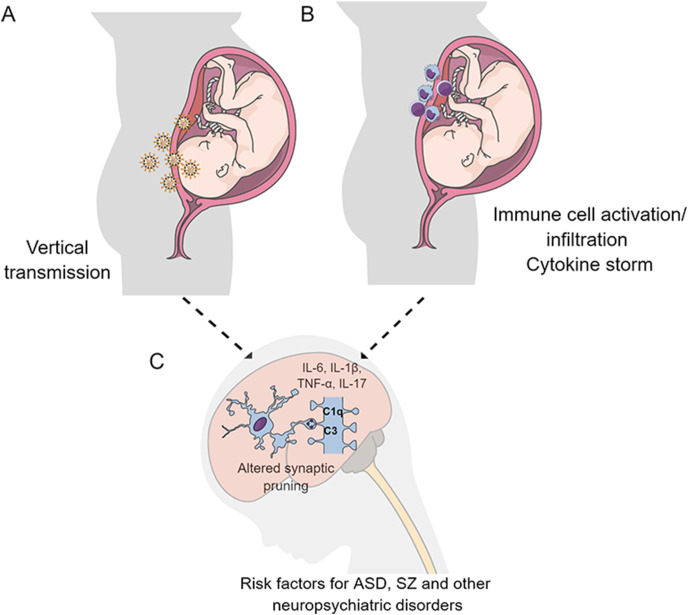
Here, we review evidence suggesting that SARS-CoV-2 infection may influence the outcome of pregnancies and interfere with the developing fetus. Most importantly, we discuss the possibility that either the direct exposure of the offspring to SARS-CoV-2 or the maternal immune response originated by the virus, in cases of infections during pregnancy, may have persistent effects on the immature brain. Since neurodevelopmental disorders do not manifest until late childhood or adolescence, further studies are warranted to determine whether this pandemic will result in an increased diagnosis for these diseases (see Fig. 1 ). We suggest that, while further studies are performed in this area of research, post-pandemic infants born from mothers diagnosed with COVID-19 are carefully followed by healthcare workers.
Fig. 1.
Possible mechanisms associated with deleterious consequences of SARS-CoV-2 infection during pregnancy. As previously shown for other viral infections, the direct infection of the fetal brain (A) or maternal immune response associated with infection (B) may have persistent effects on the developing brain (C). Cytokine storm syndrome, characterized by high levels of proinflammatory mediators is experienced by COVID-19 patients. Several proinflammatory markers associated with the cytokine storm were shown to influence synapse pruning and susceptibility of neurodevelopmental disorders, including autism spectrum disorder (ASD) and schizophrenia (SZ).
2. Clinical outcomes of SARS-CoV-2 during pregnancy and evidence of vertical transmission
Pregnancy is characterized by physiological immunosuppression and increased susceptibility to respiratory infections (Parker et al., 2016). The first report of poor clinical outcome in pregnant women infected with SARS occurred nearly two decades ago (Wong et al., 2004), but the control of viral spread and the limited number of cases (Chowell et al., 2004) precluded the performance of long-term prospective studies to evaluate whether neurodevelopment damages were associated to infection. Since the worldwide outbreak of the novel coronavirus, the genuine effects of SARS-CoV-2 infection on pregnant women and offspring remain unclear. Concerning the influence of infection on the course of pregnancy, an increased risk of preterm delivery during the acute course of COVID-19 disease was reported (Pierce-Williams et al., 2020; Woodworth et al., 2020; Zambrano et al., 2020). A small cohort study performed in women infected during late pregnancy found that known risk factors for COVID-19 severity in the general population, such as obesity, increased the need of a premature C-section to alleviate respiratory difficulty (Lokken et al., 2020). Fetal growth restrictions (Sukhikh et al., 2021), low birth weight of at-term babies (Patanè et al., 2020) and occasional miscarriages and stillbirths have also been reported following COVID-19 diagnosis in pregnant patients (Baud et al., 2020; Sukhikh et al., 2021). However, in other cohorts no increased risk of premature delivery or impaired fetal growth were described (Santana and Luis, 2021). These findings suggest that preexisting health conditions among other still unknown factors can influence the outcome of COVID-19 in pregnant patients and their offspring (Jamieson and Rasmussen, 2021).
The transplacental transmission of SARS-CoV-2 is a matter of debate. Recent reports have shown that the two known receptors for SARS-CoV-2, angiotensin-converting enzyme (ACE2) and transmembrane protease serine 2, are widely expressed in the placenta and in the maternal-fetal interface (M. Li et al., 2020), suggesting that, as with any respiratory virus, mother-to-child transmission of SARS-CoV-2 could occur. Accordingly, some case studies have demonstrated the presence of SARS-CoV-2 genome in placentas from women infected during the second and third trimester of pregnancy (Hosier et al., 2020; Patanè et al., 2020; Sukhikh et al., 2021), with increased placental infiltration of inflammatory cells, mostly phagocytic cells (Kirtsman et al., 2020; Schoenmakers et al., 2020). In one of these cases, an uneventful and healthy pregnancy was complicated immediately after the patient acquired COVID-19 at 21 weeks of pregnancy. Combined analysis of placental tissue, umbilical cord blood and child blood indicated the occurrence of vertical viral transmission, possibly causing maternal vascular malperfusion, which led to premature birth (26th week) and child death (Sukhikh et al., 2021). In another study, viral genome has been detected not only in some of the analyzed at-term placentas but also in umbilical cords as well as in vaginal mucosa and milk specimens (Fenizia et al., 2020). Importantly, a case report of vertical transmission showed placental infection at high viral loads as well as viremia and neurological symptoms in the newborn (Vivanti et al., 2020). These findings contrast with one initial study performed in Wuhan with two pregnant subjects, showing no evidence of SARS-CoV-2 infection in all products of conception and in newborns (Fan et al., 2021), and a 29 pregnant women cohort study, in which no evidence of viral proteins or RNA or morphological alterations were found in the placenta and nasopharyngeal swabs from neonates (Santana and Luis, 2021). In addition, a systematic review of 51 studies involving a total of 336 newborns, found positive throat swabs in ∼5% of babies with SARS-CoV-2 being detected in only a small percentage of tissues in the maternal-fetus interface. These findings suggest that the transmission could have occurred during or shortly after labour (Tolu, Ezeh, and Feyissa, 2021).
In conclusion, the findings accumulated so far suggest that the risk of in utero SARS-CoV-2 transmission is low but possible, and may be influenced by other factors, such as the stage of pregnancy when infection occurs (first, second or third trimester), preexistence of other risk factors or placental vulnerability, maternal viremia, infection by different viral variants, among others. Indeed, a large systematic review found a 2–3.7% probability of SARS-CoV2 vertical transmission for infections during the third trimester (Kotlyar et al., 2021). It is important to note that most studies involved patients in the final stages of pregnancy (third trimester), and thus no assessment can be made regarding the rates and consequences of vertical transmission in early pregnancy and potential risk for fetal morbidity and mortality.
3. Viral infections and neurodevelopmental disorders
The immature brain is extremely sensitive to external and internal environmental signals, and a developmental origin has been proposed for several different neurological and neuropsychiatric disorders (Bale et al., 2010; Estes and McAllister, 2016; Ozaki et al., 2020). Although conditions such as major depressive and bipolar disorders have already been associated with early life infections in humans (Agid et al., 1999; Clair and Michelle, 2015), autistic spectrum disorders (ASD) and schizophrenia (SZ) are the diseases that show a most tight link to intrauterine and/or early-life stress (Brown, 2012; Parboosing et al., 2013; Simanek and Meier, 2015). Both ASD and SZ are usually only diagnosed several years after birth and appear to be influenced by the cumulative impact of genetic and environmental factors, such as maternal infections (Estes and McAllister, 2016; van Loo and Martens, 2007). Although it remains unknown how the transient inflammatory milieu associated with viral infections could elicit such persistent and deleterious effects on brain functioning, some potential mechanisms have been proposed, such as: 1) functional reprogramming of innate immune cells, a process called innate immune memory in the fetal brain; 2) epigenetic changes in key genes for brain development; and 3) permanent impairments to the process of synaptic refinement, known as synaptic pruning, which is mainly performed by microglial cells and is crucial for proper brain maturation and network wiring. Whether SARS-CoV-2 is deleterious to the brain during development by interfering with any of these processes remains to be established.
3.1. Possible mechanisms linking SARS-CoV-2 to ASD development
ASD are a range of conditions characterized by impaired social and communication abilities as well as repetitive and stereotyped behaviors. The first evidence linking infectious agents to ASD development arose decades ago and involved congenital rubella infection. Since then, several other pathogens, especially viruses, were shown to cause a similar outcome (Meltzer and Van de Water, 2017). These viruses have been proposed to trigger ASD either due to direct infection of the fetal brain after they cross the placental barrier, or indirectly, by modulating maternal immune response, which could affect the fetus (Libbey et al., 2005).
Studies have reported that IL-1β, IL-6 and IL-17 are key molecules for infection-induced ASD (Al-Ayadhi and Mostafa, 2012; Ashwood et al. 2011a, 2011b; Shuid et al., 2021), being the injection of either of these cytokines in animal models sufficient to trigger ASD-like behavior (Favrais et al., 2011; T. Smith et al., 2007). Concerning the SARS-CoV-2 infection, one recent study found a concordant profile of inflammatory markers, particularly IL-6, IL-17 and tumor necrosis factor alpha (TNF-α), in peripheral blood of pregnant women as well as in the umbilical cord blood (Fenizia et al., 2020). Additionally, upregulation of IL-6 and TNF-α expression was found in SARS-CoV-2-positive placentas compared to control. Accordingly, other reports showed increased serum levels of IL-6, IL-10, and TNF-α in SARS-CoV-2-negative neonates born from infected mothers (Dong et al., 2020; Fenizia et al., 2020; Liu et al., 2020). One attractive hypothesis for the mechanism by which exposure to inflammatory mediators induces ASD is through the activation of microglia.
Microglia are the main brain-resident phagocytic cells involved in the communication of systemic inflammation to the brain. These cells seem to play a key role in ASD development since the pharmacological inhibition of microglial activation in infected pregnant mice prevented the development of ASD-like behavior in the offspring (Chu et al., 2010; Zhan et al., 2014). So far, the impact of SARS-CoV-2 on microglial activation is practically unknown. Microglial activation has already been found in SARS-CoV-2-infected adult patients (Matschke et al., 2020), and recently a case study found gliosis in periventricular and subcortical areas in brain images from a SARS-CoV-2-positive newborn (Vivanti et al., 2020). It is important to highlight that innate immune memory in the brain is possibly associated with epigenetic modulation. Interestingly, genes of pathogen-response pathways, known to control microglial activation, such as TNF-α, IL-6 and interferon regulatory factor 8 (IRF8), were shown to be epigenetically modulated in both ASD patients and animal models (Gilmore et al., 2005; Matcovitch-Natan et al., 2016; Pratt et al., 2013). Then, it is possible that the transient inflammatory response to which fetus’ brains are exposed in utero during maternal infection with SARS-CoV-2 (as well as with other infectious agents) leads to the development of innate immune memory. However, whether SARS-CoV-2 itself and/or maternal inflammatory mediators cross the placental barrier and act directly on the fetal brain remains unknown.
Microglia have a central role both in physiological and pathological synaptic refinement (Paolicelli and Ferretti, 2017). Studies in animal models of ASD and post-mortem analysis of ASD brains have shown an overabundance of cortical neurons and connections, which was associated with abnormal synapse pruning impairments during the first stages of postnatal brain development (Hutsler and Zhang, 2010; Marchetto et al., 2017; Tang et al., 2014). Also, it has already been shown that a transient reduction in microglial cells during early postnatal stages leads to increased synapse number and development of ASD-like behavior in adult rodents (Chen et al., 2010; Zhan et al., 2014). Since microglial cells may assume different phenotypes, each of them associated to specific responses and cytokine profiles (Wolf et al., 2017), it is possible that infection induces an inflammatory amoeboid phenotype, which fails to perform its physiological role of synapse engulfment and elimination.
Evidence that environmental pathogens could interfere with synapse pruning to induce ASD symptoms come from genetic studies, which showed that ASD patients show decreased DNA methylation in genes coding for complement system proteins (Nardone et al., 2014), involved both in the immune response and in synapse pruning (Perry and O'Connor, 2008). Based on the relationship between the maternal inflammatory response to viral infections during pregnancy and possible brain injury, it remains possible that SARS-CoV-2 infection during critical stages of fetuses' neurodevelopment contributes to the development of ASD later in life.
3.2. Possible mechanisms linking SARS-CoV-2 to SZ development
SZ is a neuropsychiatric disorder that manifests during adolescence or early adulthood, and in most patients is associated with hallucinations, disorganized speech, poor social interaction and cognitive impairments. Epidemiological studies suggest that early-life exposure to infectious agents and systemic inflammation are associated to SZ development later in life (Brown and Derkits, 2010; G M Khandaker et al., 2013; Golam M Khandaker et al., 2012; Miller et al., 2011). Animal models of infection and maternal immune activation inspired by these clinical studies have recapitulated several behavioral and neurochemical abnormalities observed in SZ patients (Patterson, 2009). Post-mortem analysis of SZ patients’ brains revealed intense microgliosis (Bayer et al., 1999; Doorduin et al., 2009) and there is considerable evidence of upregulation in genes involved with inflammatory response, in both SZ animal models and patients (Calabrò et al., 2015; Cocchi et al., 2016; Inta et al., 2017). Soluble molecular markers that positively correlate to the birth of SZ-patients include IL-8, TNF-α (Buka et al., 2001; Buka et al., 2001), and IL-6 (Misiak et al., 2021; Zhou et al., 2021), whereas IL-1β levels was not associated with increased risk of psychosis (Buka et al., 2001; Buka et al., 2001). Studies in animal models of maternal immune activation showed that a single injection of IL-6 on day 12.5 of mouse pregnancy causes SZ-like behavior in the offspring (S. E. P. Smith et al., 2007). In the case of SARS-CoV-2 infection, potential risk for neurodevelopmental disorders in neonates is supported by recent reports showing that infected pregnant women had higher levels of IL-6, IL-8 and TNF-α in the materno-fetal interface (Fenizia et al., 2020), and gliosis in fetal brain (Vivanti et al., 2020). The cellular and molecular mechanisms by which in utero or neonatal exposure to infection contributes to SZ risk are still unknown. Of note, olfactory function is altered in SZ patients, correlating with the intensity of negative symptoms and with a worse prognosis (Cohen et al., 2012; Z.-T. Li et al., 2020). Expression of ACE2 and TMPRSS2 has been reported in the nasal epithelium of human fetuses at gestation weeks 11 and 14 (Nampoothiri et al., 2020), and the loss of sense of smell is one well-known early symptom of COVID-19. Whether SARS-CoV-2 is capable of causing long-lasting effect on the developing brain and/or sensory system, increasing the risk of SZ development, as well as the associated cellular and molecular mechanisms remains to be established.
As for ASD development, abnormal synaptic pruning has emerged as an important event in SZ pathophysiology (Neniskyte and Gross, 2017), but in case of SZ, an increased synaptic pruning instead of a deficit in this process seems to be involved. C1q, a complement system protein, is implicated in the tagging of synapses that will be later engulfed by microglial cells (Severance et al., 2014). Complement activation was seen in plasma of critically ill patients with COVID-19. Pfister and colleagues (2021) detected the deposition of C1q, C3 and C5b-9 complement proteins in human renal tissue, the second organ most commonly affected by COVID-19 (Pfister et al., 2020). In this line, a study found that SZ patients’ mothers also showed significantly elevated serum levels of C1q, and IgG directed against several infectious disease agents (Severance et al., 2014), suggesting that SARS-CoV-2 infection could ultimately lead to increased synapse pruning mediators, potentially favoring SZ development.
The human brain continues to develop throughout childhood and well into early adulthood (de Graaf-Peters and Hadders-Algra, 2006). The onset of SZ symptoms typically occurs between the ages of 15 and 25, a critical age where intense synaptic pruning is taking place in the adolescent prefrontal cortex (PFC). This late stage of synapse refinement is thought to be required for impulse control necessary for adult social and cognitive behavior (Casey et al., 2008). Since SZ is associated with reduced grey-matter volume (Cannon et al., 2002) and reduced number of synaptic structures on neurons in the PFC (Garey et al., 1998; Glantz and Lewis, 2000), the development of this disorder has been linked to excessive synapse pruning in this brain region. In addition, the effects of infection in increasing the risk for SZ development are not confined to the perinatal period, since infections by neurotropic viruses during childhood were also shown to influence susceptibility to this disease (Golam M Khandaker et al., 2012, 2014). Of note, children with inflammatory multisystem syndrome temporally associated with SARS-CoV-2, as well as infected adults, showed neurologic manifestations and neuroimaging alterations (Abdel-Mannan et al., 2020; Riphagen et al., 2020; Whittaker et al., 2020). These findings demonstrate that epidemiological studies evaluating both the offspring from women infected during pregnancy as well as SARS-CoV-2-infected children must be followed to assess whether the virus can influence future neuropsychiatric outcomes.
4. Concluding remarks
A strong association between neurodevelopmental disorders and intrauterine and/or early life viral infections has been established. Emerging findings support the capacity of SARS-CoV-2 neuroinvasion and its association with the development of neurological complications. While studies continue to unravel the mechanisms of SARS-CoV-2 neuropathology and late consequences of infection in adult patients, the possible consequences of infection during pregnancy should not be ignored or left aside. Currently, only studies performed in small cohorts have assessed the outcomes of SARS-CoV-2 infection to the course of pregnancy and fetal development, and most studies are limited to patients infected during late stages of gestation.
The pandemic dimension is an unrivaled opportunity to extend our understanding about the effect of maternal infection on neurodevelopment. While worldwide vaccination and social isolation will contribute to block viral dissemination, millions of newborn infants have already been exposed to a virus with unknown consequences. In this sense, further studies should assess the genuine effects of SARS-CoV-2 infection during pregnancy and delivery along with an extended follow-up of the offspring, including neurocognitive, neuroimaging, and electrophysiological examination. In addition, it remains to be determined whether and by which mechanism SARS-CoV-2 intrauterine and early life infections could lead to an increased risk of developing ASD, SZ and/or other neuropsychiatric disorders in the offspring.
Acknowledgements
This work was supported by grants from Brazilian funding agencies: Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (CPF, FLFD, ATP and JRC), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CPF, ATP and JRC), Institutos Nacionais de Pesquisa - Inovação em Medicamentos e Identificação de Novos Alvos Terapêuticos (CPF), Rio Network of Innovation in nanosystems for the health - Nanohealth/FAPERJ (E-26/010.000983/2019), Instituto D'Or de Pesquisa e Ensino – IDOR, Swedish Foundation for International Cooperation in Research and Higher Education (CAPES/STINT, 88881.465507/2019–01) and La Caixa Bank Foundation, Spain (grant number: LCF/PR/HR17/52150011). We thank Jadilma Araujo Ferreira for technical support and Ana Claudia Rangel for competent lab and project management.
References
- Abdel-Mannan Omar, et al. Neurologic and radiographic findings associated with COVID-19 infection in children. JAMA Neurol. 2020;77(11):1440–1445. doi: 10.1001/jamaneurol.2020.2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agid O., et al. Environment and vulnerability to major psychiatric illness: a case control study of early parental loss in major depression, bipolar disorder and schizophrenia. Mol. Psychiatr. 1999;4(2):163–172. doi: 10.1038/sj.mp.4000473. [DOI] [PubMed] [Google Scholar]
- Al-Ayadhi Laila Yousef, Ahmed Mostafa Gehan. Elevated serum levels of interleukin-17A in children with autism. J. Neuroinflammation. 2012;9:158. doi: 10.1186/1742-2094-9-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashwood Paul, et al. Associations of impaired behaviors with elevated plasma chemokines in autism spectrum disorders. J. Neuroimmunol. 2011;232(1–2):196–199. doi: 10.1016/j.jneuroim.2010.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashwood Paul, et al. Elevated plasma cytokines in autism spectrum disorders provide evidence of immune dysfunction and are associated with impaired behavioral outcome. Brain Behav. Immun. 2011;25(1):40–45. doi: 10.1016/j.bbi.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale Tracy L., et al. Early life programming and neurodevelopmental disorders. Biol. Psychiatr. 2010;68(4):314–319. doi: 10.1016/j.biopsych.2010.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baud David, et al. Second-trimester miscarriage in a pregnant woman with SARS-CoV-2 infection. J. Am. Med. Assoc. 2020;323(21):2198–2200. doi: 10.1001/jama.2020.7233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer T.A., Buslei R., Havas L., Falkai P. Evidence for activation of microglia in patients with psychiatric illnesses. Neurosci. Lett. 1999;271(2):126–128. doi: 10.1016/s0304-3940(99)00545-5. [DOI] [PubMed] [Google Scholar]
- Brown Alan S. Epidemiologic studies of exposure to prenatal infection and risk of schizophrenia and autism. Dev. Neurobiol. 2012;72(10):1272–1276. doi: 10.1002/dneu.22024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown Alan S., Derkits Elena J. Prenatal infection and schizophrenia: a review of epidemiologic and translational studies. Am. J. Psychiatr. 2010;167(3):261–280. doi: 10.1176/appi.ajp.2009.09030361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buka S.L., Tsuang M.T., Torrey E.F., Klebanoff M.A., Wagner R.L., et al. Maternal cytokine levels during pregnancy and adult psychosis. Brain Behav. Immun. 2001;15(4):411–420. doi: 10.1006/brbi.2001.0644. [DOI] [PubMed] [Google Scholar]
- Buka S.L., Tsuang M.T., Torrey E.F., Klebanoff M.A., Bernstein D., et al. Maternal infections and subsequent psychosis among offspring. Arch. Gen. Psychiatr. 2001;58(11):1032–1037. doi: 10.1001/archpsyc.58.11.1032. [DOI] [PubMed] [Google Scholar]
- Calabrò Marco, et al. Genes involved in pruning and inflammation are enriched in a large mega-sample of patients affected by schizophrenia and bipolar disorder and controls. Psychiatr. Res. 2015;228(3):945–949. doi: 10.1016/j.psychres.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon Tyrone D., et al. Cortex mapping reveals regionally specific patterns of genetic and disease-specific gray-matter deficits in twins discordant for schizophrenia. Proc. Natl. Acad. Sci. U. S. A. 2002;99(5):3228–3233. doi: 10.1073/pnas.052023499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey B.J., Jones Rebecca M., Hare Todd A. The adolescent brain. Ann. N. Y. Acad. Sci. 2008;1124:111–126. doi: 10.1196/annals.1440.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC . Centers for Disease Control and Prevention; 2020. CDC COVID Data Tracker; pp. 7–11.https://covid.cdc.gov/covid-data-tracker/#vaccinations-pregnant-women%0Ahttps://covid.cdc.gov/covid-data-tracker/#datatracker-home%0Ahttps://covid.cdc.gov/covid-data-tracker/#cases_casesper100k November 14) [Google Scholar]
- Chen Shau-Kwaun, et al. Hematopoietic origin of pathological grooming in Hoxb8 mutant mice. Cell. 2010;141(5):775–785. doi: 10.1016/j.cell.2010.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowell Gerardo, et al. Model parameters and outbreak control for SARS. Emerg. Infect. Dis. 2004;10(7):1258–1263. doi: 10.3201/eid1007.030647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu Yunxiang, et al. Enhanced synaptic connectivity and epilepsy in C1q knockout mice. Proc. Natl. Acad. Sci. U. S. A. 2010;107(17):7975–7980. doi: 10.1073/pnas.0913449107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clair St, Michelle C., et al. Childhood adversity subtypes and depressive symptoms in early and late adolescence. Dev. Psychopathol. 2015;27(3):885–899. doi: 10.1017/S0954579414000625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocchi Enrico, Drago Antonio, Serretti Alessandro. Hippocampal pruning as a new theory of schizophrenia etiopathogenesis. Mol. Neurobiol. 2016;53(3):2065–2081. doi: 10.1007/s12035-015-9174-6. [DOI] [PubMed] [Google Scholar]
- Cohen Alex S., Brown Laura A., Auster Tracey L. Olfaction, ‘olfiction,’ and the schizophrenia-spectrum: an updated meta-analysis on identification and acuity. Schizophr. Res. 2012;135(1–3):152–157. doi: 10.1016/j.schres.2011.12.005. [DOI] [PubMed] [Google Scholar]
- de Graaf-Peters, Victorine B., Hadders-Algra Mijna. Ontogeny of the human central nervous system: what is happening when? Early Hum. Dev. 2006;82(4):257–266. doi: 10.1016/j.earlhumdev.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Dong Lan, et al. Possible vertical transmission of SARS-CoV-2 from an infected mother to her newborn. J. Am. Med. Assoc. 2020;323(18):1846–1848. doi: 10.1001/jama.2020.4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doorduin Janine, et al. Neuroinflammation in schizophrenia-related psychosis: a PET study. J. Nucl. Med. : Off. Publ, Soc Nucl. Med. 2009;50(11):1801–1807. doi: 10.2967/jnumed.109.066647. [DOI] [PubMed] [Google Scholar]
- Estes Myka L., McAllister A Kimberley. Maternal immune activation: implications for neuropsychiatric disorders. Science. 2016;353(6301):772–777. doi: 10.1126/science.aag3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Cuifang, et al. Perinatal transmission of 2019 coronavirus disease-associated severe acute respiratory syndrome coronavirus 2: should we worry? Clin. Infect. Dis. : Off. Publ. Infect. Dis. Soc. Am. 2021;72(5):862–864. doi: 10.1093/cid/ciaa226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favrais Géraldine, et al. Systemic inflammation disrupts the developmental program of white matter. Ann. Neurol. 2011;70(4):550–565. doi: 10.1002/ana.22489. [DOI] [PubMed] [Google Scholar]
- Fenizia Claudio, et al. Analysis of SARS-CoV-2 vertical transmission during pregnancy. Nat. Commun. 2020;11(1):5128. doi: 10.1038/s41467-020-18933-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garey L.J., et al. Reduced dendritic spine density on cerebral cortical pyramidal neurons in schizophrenia. J. Neurol. Neurosurg. Psychiatr. 1998;65(4):446–453. doi: 10.1136/jnnp.65.4.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore John H., Jarskog L Fredrik, Vadlamudi Swarooparani. Maternal poly I:C exposure during pregnancy regulates TNF alpha, BDNF, and NGF expression in neonatal brain and the maternal-fetal unit of the rat. J. Neuroimmunol. 2005;159(1–2):106–112. doi: 10.1016/j.jneuroim.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Glantz L.A., Lewis D.A. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch. Gen. Psychiatr. 2000;57(1):65–73. doi: 10.1001/archpsyc.57.1.65. [DOI] [PubMed] [Google Scholar]
- Hosier Hillary, et al. SARS-CoV-2 infection of the placenta. J. Clin. Invest. 2020;130(9):4947–4953. doi: 10.1172/JCI139569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutsler, Jeffrey J., Zhang Hong. Increased dendritic spine densities on cortical projection neurons in autism spectrum disorders. Brain Res. 2010;1309:83–94. doi: 10.1016/j.brainres.2009.09.120. [DOI] [PubMed] [Google Scholar]
- Inta Dragos, et al. Microglia activation and schizophrenia: lessons from the effects of minocycline on postnatal neurogenesis, neuronal survival and synaptic pruning. Schizophr. Bull. 2017;43(3):493–496. doi: 10.1093/schbul/sbw088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson Denise J., Rasmussen Sonja A. American journal of obstetrics and gynecology; 2021. An Update on Coronavirus Disease 2019 (COVID-19) and Pregnancy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandaker, Golam M., et al. Childhood infection and adult schizophrenia: a meta-analysis of population-based studies. Schizophr. Res. 2012;139(1–3):161–168. doi: 10.1016/j.schres.2012.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandaker, Golam M., et al. Childhood epstein-Barr virus infection and subsequent risk of psychotic experiences in adolescence: a population-based prospective serological study. Schizophr. Res. 2014;158(1–3):19–24. doi: 10.1016/j.schres.2014.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandaker G.M., Zimbron J., Lewis G., Jones P.B. Prenatal maternal infection, neurodevelopment and adult schizophrenia: a systematic review of population-based studies. Psychol. Med. 2013;43(2):239–257. doi: 10.1017/S0033291712000736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirtsman Maksim, et al. Probable congenital SARS-CoV-2 infection in a neonate born to a woman with active SARS-CoV-2 infection. CMAJ (Can. Med. Assoc. J.) : Can. Med. Assoc. J. = Journal de l’Association medicale canadienne. 2020;192(24):E647–E650. doi: 10.1503/cmaj.200821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotlyar Alexander M., et al. Vertical transmission of coronavirus disease 2019: a systematic review and meta-analysis. Am. J. Obstet. Gynecol. 2021;224(1):35–53. doi: 10.1016/j.ajog.2020.07.049. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Mengmeng, et al. The SARS-CoV-2 receptor ACE2 expression of maternal-fetal interface and fetal organs by single-cell transcriptome study. PLoS One. 2020;15(4) doi: 10.1371/journal.pone.0230295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Ze-Tian, et al. Early-onset schizophrenia showed similar but more severe olfactory identification impairment than adult-onset schizophrenia. Front. Psychiatr. 2020;11:626. doi: 10.3389/fpsyt.2020.00626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libbey Jane E., Thayne L Sweeten, William M McMahon, Robert S Fujinami. Autistic disorder and viral infections. J. Neurovirol. 2005;11(1):1–10. doi: 10.1080/13550280590900553. [DOI] [PubMed] [Google Scholar]
- Liu Pin, et al. The immunologic status of newborns born to SARS-CoV-2-infected mothers in Wuhan, China. J. Allergy Clin. Immunol. 2020;146(1):101–109.e1. doi: 10.1016/j.jaci.2020.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lokken Erica M., Gray Taylor G., et al. American journal of obstetrics and gynecology; 2021. Higher Severe Acute Respiratory Syndrome Coronavirus 2 Infection Rate in Pregnant Patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lokken Erica M., Huebner Emily M., et al. American journal of obstetrics and gynecology; 2021. Disease Severity, Pregnancy Outcomes, and Maternal Deaths Among Pregnant Patients with Severe Acute Respiratory Syndrome Coronavirus 2 Infection in Washington State. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lokken Erica M., et al. Clinical characteristics of 46 pregnant women with a severe acute respiratory syndrome coronavirus 2 infection in Washington state. Am. J. Obstet. Gynecol. 2020;223(6) doi: 10.1016/j.ajog.2020.05.031. 911.e1-911.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Ling, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):1–9. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetto Maria C., et al. Altered proliferation and networks in neural cells derived from idiopathic autistic individuals. Mol. Psychiatr. 2017;22(6):820–835. doi: 10.1038/mp.2016.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matcovitch-Natan Orit, et al. Microglia development follows a stepwise program to regulate brain homeostasis. Science. 2016;353(6301):aad8670. doi: 10.1126/science.aad8670. [DOI] [PubMed] [Google Scholar]
- Matschke Jakob, et al. Neuropathology of patients with COVID-19 in Germany: a post-mortem case series. Lancet Neurol. 2020;19(11):919–929. doi: 10.1016/S1474-4422(20)30308-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer Amory, Judy Van de Water The role of the immune system in autism spectrum disorder. Neuropsychopharmacology : Off. Publ. Am. College of Neuropsychopharmacol. 2017;42(1):284–298. doi: 10.1038/npp.2016.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller Brian J., et al. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol. Psychiatr. 2011;70(7):663–671. doi: 10.1016/j.biopsych.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misiak Błażej, et al. Immune-inflammatory markers and psychosis risk: a systematic review and meta-analysis. Psychoneuroendocrinology. 2021;127:105200. doi: 10.1016/j.psyneuen.2021.105200. [DOI] [PubMed] [Google Scholar]
- Nampoothiri Sreekala, et al. The hypothalamus as a hub for SARS-CoV-2 brain infection and pathogenesis. 2020. http://biorxiv.org/content/early/2020/06/19/2020.06.08.139329.abstract bioRxiv: 2020.06.08.139329.
- Nardone S., et al. DNA methylation analysis of the autistic brain reveals multiple dysregulated biological pathways. Transl. Psychiatry. 2014;4(9):e433. doi: 10.1038/tp.2014.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neniskyte Urte, Gross Cornelius T. Errant gardeners: glial-cell-dependent synaptic pruning and neurodevelopmental disorders. Nat. Rev. Neurosci. 2017;18(11):658–670. doi: 10.1038/nrn.2017.110. [DOI] [PubMed] [Google Scholar]
- Ozaki Kana, et al. Maternal immune activation induces sustained changes in fetal microglia motility. Sci. Rep. 2020;10(1):21378. doi: 10.1038/s41598-020-78294-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolicelli Rosa C., Ferretti Maria T. Function and dysfunction of microglia during brain development: consequences for synapses and neural circuits. Front. Synaptic Neurosci. 2017;9:9. doi: 10.3389/fnsyn.2017.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parboosing Raveen, et al. Gestational influenza and bipolar disorder in adult offspring. JAMA Psychiatr. 2013;70(7):677–685. doi: 10.1001/jamapsychiatry.2013.896. [DOI] [PubMed] [Google Scholar]
- Parker Samantha E., et al. Upper respiratory infection during pregnancy and neurodevelopmental outcomes among offspring. Neurotoxicol. Teratol. 2016;57:54–59. doi: 10.1016/j.ntt.2016.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patanè Luisa, et al. Vertical transmission of coronavirus disease 2019: severe acute respiratory syndrome coronavirus 2 RNA on the fetal side of the placenta in pregnancies with coronavirus disease 2019-positive mothers and neonates at birth. Am. J. Obstetr. Gynecol. MFM. 2020;2(3):100145. doi: 10.1016/j.ajogmf.2020.100145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson Paul H. Immune involvement in schizophrenia and autism: etiology, pathology and animal models. Behav. Brain Res. 2009;204(2):313–321. doi: 10.1016/j.bbr.2008.12.016. [DOI] [PubMed] [Google Scholar]
- Perry V Hugh, Vincent O'Connor. C1q: the perfect complement for a synaptic feast? Nat. Rev. Neurosci. 2008;9(11):807–811. doi: 10.1038/nrn2394. [DOI] [PubMed] [Google Scholar]
- Pfister Frederick, et al. Complement activation in kidneys of patients with COVID-19. Front. Immunol. 2020;11:594849. doi: 10.3389/fimmu.2020.594849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce-Williams, Rebecca A.M., et al. Clinical course of severe and critical coronavirus disease 2019 in hospitalized pregnancies: a United States cohort study. Am. J. Obstetr. Gynecol. MFM. 2020;2(3):100134. doi: 10.1016/j.ajogmf.2020.100134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt Lorelei, Ni Li, Ponzio Nicholas M., Miller Jonakait G. Maternal inflammation promotes fetal microglial activation and increased cholinergic expression in the fetal basal forebrain: role of interleukin-6. Pediatr. Res. 2013;74(4):393–401. doi: 10.1038/pr.2013.126. [DOI] [PubMed] [Google Scholar]
- Raschetti Roberto, et al. Synthesis and systematic review of reported neonatal SARS-CoV-2 infections. Nat. Commun. 2020;11(1):5164. doi: 10.1038/s41467-020-18982-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riphagen Shelley, et al. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020;395:1607–1608. doi: 10.1016/S0140-6736(20)31094-1. 10237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santana Blasco, Luis, et al. Maternal and perinatal outcomes and placental pathologic examination of 29 SARS-CoV-2 infected patients in the third trimester of gestation. J. Obstet. Gynaecol. Res. 2021 doi: 10.1111/jog.14784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenmakers Sam, et al. Severe acute respiratory syndrome coronavirus 2 placental infection and inflammation leading to fetal distress and neonatal multi-organ failure in an asymptomatic woman. J. Pediatr. Infect. Dis. Soc. 2020 doi: 10.1093/jpids/piaa153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severance Emily G., et al. Maternal complement C1q and increased odds for psychosis in adult offspring. Schizophr. Res. 2014;159(1):14–19. doi: 10.1016/j.schres.2014.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuid, Ahmad Naqib, et al. Association between viral infections and risk of autistic disorder: an overview. Int. J. Environ. Res. Publ. Health. 2021;18(6) doi: 10.3390/ijerph18062817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simanek Amanda M., Meier Helen C.S. Association between prenatal exposure to maternal infection and offspring mood disorders: a review of the literature. Curr. Probl. Pediatr. Adolesc. Health Care. 2015;45(11):325–364. doi: 10.1016/j.cppeds.2015.06.008. [DOI] [PubMed] [Google Scholar]
- Smith Stephen E.P., et al. Maternal immune activation alters fetal brain development through interleukin-6. J. Neurosci. : Off. J. Soc. Neurosci. 2007;27(40):10695–10702. doi: 10.1523/JNEUROSCI.2178-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith Tristram, et al. Designing research studies on psychosocial interventions in autism. J. Autism Dev. Disord. 2007;37(2):354–366. doi: 10.1007/s10803-006-0173-3. [DOI] [PubMed] [Google Scholar]
- Song Eric, et al. Neuroinvasion of SARS-CoV-2 in human and mouse brain. J. Exp. Med. 2021;218(3) doi: 10.1084/jem.20202135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukhikh Gennady, et al. Vertical transmission of SARS-CoV-2 in second trimester associated with severe neonatal pathology. Viruses. 2021;13(3) doi: 10.3390/v13030447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Guomei, et al. Loss of MTOR-dependent macroautophagy causes autistic-like synaptic pruning deficits. Neuron. 2014;83(5):1131–1143. doi: 10.1016/j.neuron.2014.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolu Lemi Belay, Ezeh Alex, Garumma Tolu Feyissa Vertical transmission of severe acute respiratory syndrome coronavirus 2: a scoping review. PLoS One. 2021;16(4) doi: 10.1371/journal.pone.0250196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Loo K.M.J., Martens G.J.M. Genetic and environmental factors in complex neurodevelopmental disorders. Curr. Genom. 2007;8(7):429–444. doi: 10.2174/138920207783591717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivanti, Alexandre J., et al. Transplacental transmission of SARS-CoV-2 infection. Nat. Commun. 2020;11(1):3572. doi: 10.1038/s41467-020-17436-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittaker Elizabeth, et al. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. J. Am. Med. Assoc. 2020;324(3):259–269. doi: 10.1001/jama.2020.10369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson Elizabeth J., et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584(7821):430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf Susanne A., Boddeke H.W.G.M., Kettenmann Helmut. Microglia in physiology and disease. Annu. Rev. Physiol. 2017;79:619–643. doi: 10.1146/annurev-physiol-022516-034406. [DOI] [PubMed] [Google Scholar]
- Wong Shell F., et al. Pregnancy and perinatal outcomes of women with severe acute respiratory syndrome. Am. J. Obstet. Gynecol. 2004;191(1):292–297. doi: 10.1016/j.ajog.2003.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodworth Kate R., et al. Birth and infant outcomes following laboratory-confirmed SARS-CoV-2 infection in pregnancy - SET-NET, 16 jurisdictions, march 29-October 14, 2020. MMWR (Morb. Mortal. Wkly. Rep.) 2020;69(44):1635–1640. doi: 10.15585/mmwr.mm6944e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambrano Laura D., et al. Update: characteristics of symptomatic women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status - United States, January 22-October 3, 2020. MMWR (Morb. Mortal. Wkly. Rep.) 2020;69(44):1641–1647. doi: 10.15585/mmwr.mm6944e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan Yang, et al. Deficient neuron-microglia signaling results in impaired functional brain connectivity and social behavior. Nat. Neurosci. 2014;17(3):400–406. doi: 10.1038/nn.3641. [DOI] [PubMed] [Google Scholar]
- Zhou Xin, Tian Bo, Han Hai-Bin. Serum interleukin-6 in schizophrenia: a system review and meta-analysis. Cytokine. 2021;141:155441. doi: 10.1016/j.cyto.2021.155441. [DOI] [PubMed] [Google Scholar]