Graphical abstract
Keywords: SARS-CoV-2, COVID-19, Emerging Variants, Pathogenesis, Immune Responses, Therapy, Vaccine
Abbreviations: SARS-CoV-2, Severe Acute Respiratory Syndrome coronavirus-2; COVID-19, Coronavirus disease of 2019; IL, Interleukin; TGF, Transforming growth factor; RBD, Receptor binding-domain; NSPs, Nonstructural proteins; HAT, Human airway trypsin-like protease; RdRp, RNA-dependent RNA polymerases; ICU, Intensive care unit; AKI, Acute kidney injury; CRS, Cytokine release syndrome; Th17, T helper 17; Ang II, Angiotensin II; RAS, Renin-angiotensin system; MIP1α, Macrophage inflammatory protein 1α; VTM, Viral transport medium; FNR, False‐negative rate; CT, Cycle threshold; BALF, Broncho alveolar lavage fluid; LAMP, Loop-mediated isothermal amplification; CRISPR, Clustered regularly interspaced short palindromic repeats; CT, Computed tomography; MRI, Magnetic resonance imaging; GGOs, Ground-glass opacities; LF, Lactoferrin; FDA, Food and drug administration; DENV, Dengue virus; WNV, West Nile virus; HIV, Human immunodeficiency virus; JEV, Japanese encephalitis virus; MSC, Mesenchymal Stem Cells; cGMP, Good Manufacturing Practice; IBV, infectious bronchitis virus; NCD, Newcastle disease virus; VLPs, Virus-like particles; NP, Nasopharyngeal; OP, Oropharyngeal; ACE-2, Angiotensin-converting enzyme 2
Abstract
More than a year after the SARS-CoV-2 pandemic, the Coronavirus disease 19 (COVID-19) is still a major global challenge for scientists to understand the different dimensions of infection and find ways to prevent, treat, and develop a vaccine. On January 30, 2020, the world health organization (WHO) officially announced this new virus as an international health emergency. While many biological and mechanisms of pathogenicity of this virus are still unclear, it seems that cytokine storm resulting from an immune response against the virus is considered the main culprit of the severity of the disease. Despite many global efforts to control the SARS-CoV-2, several problems and challenges have been posed in controlling the COVID-19 infection. These problems include the various mutations, the emergence of variants with high transmissibility, the short period of immunity against the virus, the possibility of reinfection in people improved, lack of specific drugs, and problems in the development of highly sensitive and specific vaccines. In this review, we summarized the results of the current trend and the latest research studies on the characteristics of the structure and genome of the SARS-CoV- 2, new mutations and variants of SARS-CoV-2, pathogenicity, immune response, virus diagnostic tests, potential treatment, and vaccine candidate.
1. Introduction
SARS-CoV-2 is the third member of the coronavirus family, which is highly pathogenic in the human population and causes COVID-19 in the upper respiratory tract that has been hitherto responsible globally for more than 220,563,227 confirmed cases of infection and more than 4,565,483 deaths [1]. The coronaviridae family contains 27–34 kilobases positive-sense and single-strand RNA, which are the largest genomes among RNA viruses, but unlike other RNA viruses, it has been proven that coronaviridae family possesses the proof-reading capability [2]. Coronaviruses include four genera: alpha-CoV, beta-CoV, gamma-CoV, and delta-CoV. These genera can cause an infection in humans and animals with a high recombination rate, causing various diseases, including respiratory syndrome, renal, enteric, and neurological disorders. Seven pathogenic strains which can cause mild to severe infections in humans belong to coronaviridae family [3], [4].
On January 30th, the World Health Organization (WHO) declared the 2019-nCoV (novel coronavirus) outbreak as a global concern. On February 11th 2020, the 2019-nCoV was officially renamed SARS-CoV-2. Eventually, the WHO recognized these virus outbreaks as a pandemic in March 2020 [5]. As it has been reported that some initial infected people had been exposed to the seafood market in Wuhan, China, in December 2019, some scientists are pro and con of SARS-CoV-2 as a zoonotic origin virus [6]. However, due to the lack of sufficient evidence, the first reservoir of SARS-CoV remains unknown. SARS-CoV-2 is highly contagious, and transmits through droplets, direct contact, and contact with infected objects [7]. The genetic similarity of SARS-CoV-2 with MERS-CoV and SARS-CoV is 50% and 79%, respectively [8], [9]. The primary target of SARS-CoV-2 is the lower respiratory tract. Although some common flu-like illness symptoms of COVID-19 such as dry cough, fever, fatigue, aches, and pains are similar to the symptoms of two older coronaviruses, some new signs such as loss of taste or smell, myocarditis, dyspnea, gastrointestinal disorders, neurological symptoms, and cutaneous manifestations are introduced day by day [10], [11], [12]. SARS-CoV-2 can cause asymptomatic to mild respiratory infections, pneumonia, as well as serious health problems such as acute respiratory distress syndrome (ARD) after increasing activated T lymphocytes and mononuclear macrophages macrophage-related proinflammatory cytokines production, “cytokine storm”, such as interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-α), and IL-1β, IL-2, IL-4, IL-7, IL-10, IL-17, IFN-ϒ, Transforming growth factor-β (TGF-β), and IL-8. Chemokine responses and inflammatory cell infiltration occur seven to ten days after the beginning of symptoms, leading to increased risk of vascular hyperpermeability, multi-organ failure, and eventually, death following uncontrolled conditions [13], [14], [15], [16]. Recently, some unique symptoms have been reported in infected patients with SARS-CoV-2: rhinorrhea, sore throat, diarrhea, vomiting, smell and loss of taste, myocarditis, and sneezing [17], [18], [19]. In a cohort study carried out by Xiaobo Yang. et al. [20], it has been reported that pyrexia is the typical symptom of SARS-CoV-2 pneumonia; however, this sign was not detected in all cases. As COVID-19 can damage leucocytes, leucopenia was detected in more than 80 % of severe patients. COVID19 in adult patients is more severe than in younger individuals due to the increased epithelial expression of ACE receptors in the respiratory and gastrointestinal tract [21] (Fig. 2). COVID19 in pediatrics has often no symptoms or has mild symptoms and, therefore, has a critical role in spreading the SARS-CoV-2 infection [22]. Although the immunity profile against COVID-19 is not wholly determined, it seems that innate immunity has an essential role in the antiviral activity and mild or severe symptoms of COVID-19 [23].
Fig. 2.
The Immunopathogenesis of SARS-CoV-2 (COVID-19) in pediatric and adults lung that causes mild or asymptotic infection in children, and severe lung injury and extracellular manifestation in adult and elderly patients. Due to having a low level of SARS-Cov-2 receptors, decreased proinflammatory cytokines in respiratory epithelial cells, increased level of type I interferons and strong innate immune response, children have shown a mild or asymptomatic COVID-19 infection. By comparison, adults due to increased level of SARS-Cov-2 receptors and proinflammatory cytokines in respiratory epithelial cells, following the damage to epithelial cells, fluid accumulation, respiratory failure, cytokine storm, viremia, and systemic infection experience severe respiratory infection and extra pulmonary manifestations.
Unfortunately, despite growing studies, there is no effective drug or treatment for COVID-19 pandemic disease, and patients are being cured by supportive or unspecific treatments [24]. Most vaccine leaders are currently developing vaccines for SARS-CoV-2. Hitherto, eight vaccines have been approved for full use, eleven authorized in early or limited use, and many candidate vaccines are in various phases of vaccine design [25], [26]. Although research on various aspects of nature and pathogenicity of the virus is ongoing, the lack of sufficient information and mutations of the virus genome and new variants have made a barrier to reach specific treatment options and the potency of vaccine development [27]. Thus, we here review the literature on the structure, genome organization, and variants of SARS-CoV-2, its pathogenicity, the most important clinical features, new mutations and variants of SARS-CoV-2, host-cell interactions, immune responses, diagnostic tests, potential treatments, and vaccines candidates to help improve the symptoms of COVID-19 infection to reach specific treatment options, as well as a vaccine.
2. SARS-CoV-2 structure and genome organization
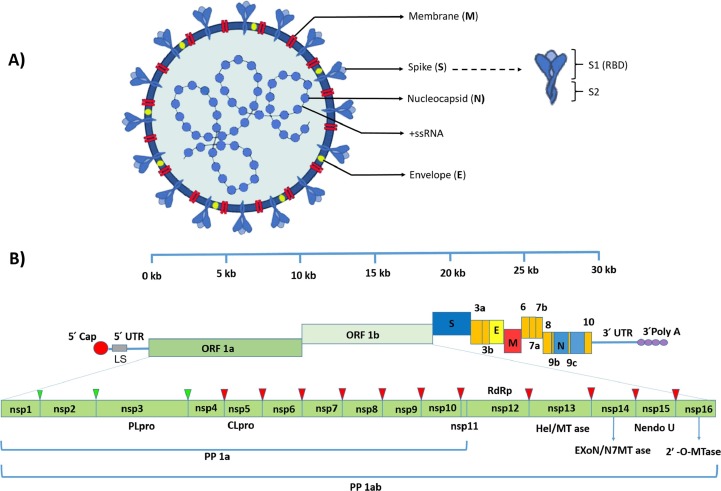
Understanding the SARS-CoV-2 structure and genome organization has a pivotal role in determining the mechanisms of viral replication, infectivity, and proteins that induce host immune responses that could help find the potential drugs for treatment and vaccine development for the prevention of COVID-19 [28], [29], [30]. It has been demonstrated that the isolated positive-sense single-stranded RNA with a genome size of 29.9 kB of SARS-CoV-2 has 88% similarity to the sequence of viruses isolated from infected bats with SARS-like coronaviruses [31], [32]. The SARS-CoV-2 genome contains 12 functional open reading frames (ORFs) expressing 12 proteins from 11 protein-coding genes [33], [34] (Fig. 1 ). After entry of SARS-CoV-2 into host cells, the viral genome act as messenger RNA (mRNA) and is translated by host ribosomes. Sixteen nonstructural proteins (NSPs) are encoded via two large polyproteins, pp1a/pp1ab, from 5′ two-third end of the CoVs genome. Two viral proteases named 3c-like protease and papain-like protease are responsible for processing these polypeptides to produce NSPs. Four structural proteins and accessory proteins are encoded by open reading frames (ORFs) from the 3′ one-third end of the genome from a set of nested subgenomic RNAs (sgRNAs) beginning from a negative-sense RNA intermediate. About 60 to 140 nm in diameter SARS-CoV-2 virion structure consists of four structural proteins, including spike surface glycoprotein (S), nucleocapsid protein (N), membrane protein (M), and envelope protein (E), essential for viral assembly and establishing an infection [35], [36], [37] (Fig. 1). Accessory proteins are not necessary for virus replication but are involved in the viral pathogenicity by modulating the interferon signaling pathway [38].
Fig. 1.
Schematic diagram of SARS-CoV-2 structure and genomic organization. A. SARS-CoV-2 virion structure consists of four structural proteins, including spike (S), envelope (E), membrane (M) in the envelope, and nucleocapsid (N) protein that encapsulates the virus positive-sense single-stranded RNA (+ssRNA) genome. Spike protein contains two subunits including S1 and S2, which act as a receptor-binding and cell fusion domain. B. The positive single-stranded mRNA from 5′ to 3′ contain 5′ cap, leader sequence (LS), 5′ UTR, ORF1a, ORF1b, Spike (S), ORF3a, ORF3b, Envelope (E), Membrane (M), ORF6, ORF7a, ORF7b, ORF8, Nucleocapsid (N), ORF9, ORF10, 3′ UTR and poly-A tail respectively. Two-thirds of the 5′ end of the genome, including ORF1a and ORF1b, encodes two large polyproteins comprising pp1a and pp1ab that are cleaved into 16 non-structural proteins at the nsp1 to nsp3 cleavage sites by papain-like cysteine protease (PLpro) and nsp4 to nsp16 cleavage sites by a 3C-like serine protease (3CLpro) (showed by the green and red triangles respectively). Nsp12 to nsp16 encodes the RNA-dependent RNA polymerase (RdRp), Helicase (Hel/MTase) and Exonuclease (ExonN), Endonuclease (Nendo U) and 2′ -O-RNA methyltransferase (2‘-O-MTase) respectively. The 3′ one-third end of the SARS-CoV-2 genome encodes the structural (S, E, M, and N) and accessory proteins. Accessory proteins sequences are displayed in orange color. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
The 150 kDa trimer crown-like spikes protein is cleaved into S1 and S2 subunits. The type of cell and cellular tropism is determined by S1 part, while S2 is responsible for viral fusion in host cells. CoVs proteins classified in class I fusion proteins require protease cleavage to be functional. A single region named receptor-binding domain (RBD), located in the S1 subunit, is independently responsible for host-cell interaction in beta coronaviruses [39], [40]. Using the cellular proteases by CoVs is related to the strain of the virus and the cell type. One or various types of proteases like human airway trypsin-like protease (HAT), transmembrane protease serine protease-2, 4 (TMPRSS-2,4), cathepsins, furin, and trypsin can promote CoV S proteins cleavage; however, the roles of TMPRSS-2 and Furin in cleavage of SARS-CoV 2 are demonstrated [41], [42], [43]. S protein in some CoVs plays an essential role in virus-cell fusion. It can mediate syncytia forming, considered a major strategy to virus cell-to-cell transmission, and impairs the function of neutralizing antibodies. S protein has been considered an ideal option for vaccine design. Antibodies can disturb the protein S-cell binding and also neutralize the virus [44].
The highly phosphorylated N protein, which has the highest expression in host cells, is structurally bound to the genome and plays an indispensable role in the replication cycle process. Membrane protein M, which is the most prevalent structural protein, forms the shape of the envelope and helps the completion of the virus assembly process by binding to nucleocapsid N. IgG antibodies against SARS-CoV-2 N protein can be detectable in serum four days after onset of the disease. The E protein is a structural protein that acts as an ion channel [45], [46].
Many functions associated with NSPs have remained unclear; however, several features are relevant to viral replication and subgenomic RNAs (e.g., NSP15). The Nsp14 with exonuclease activity can eliminate the nucleoside analogs (NAs) incorporated into viral RNA and reduce their efficacy. Due to the critical role of Viral RNA-dependent RNA polymerases (RdRp) along with other major enzymes in the RNA virus’s replication cycle and virus pathogenesis, they have been seemed to be a superior choice to design efficient drugs to prevent or treat several viral diseases. With over 25 approvals, the class of NAs is the most promising treatment for a wide range of diseases. However, in coronaviruses, exonuclease domains (ExoN) are a serious barrier to reach appropriate NA drugs because resistance emerges against many of these antivirals. Despite this impairing mechanism, NAs can still be considered an efficient choice of medicines in CoVs, because of their highly conserved RdRps, and their special structural conservation of nucleotide-binding sites. The ExoN interaction with Nsp12, Nsp7, and Nsp8 promotes proofreading activity. Owing to the insignificant structural and functional differences between SARS- CoV and SARS-CoV-2 RdRps, the last analysis for virus structure and function may be helpful for future studies [47], [48].
It is worth mentioning that some significant dissimilarities between the novel coronavirus and other CoVs genomes, such as Spike proteins, ORF8, and ORF3a proteins, may make differences in the ability of SARS-CoV-2 transmission and pathogenicity [35]. Khailany et al. [49] found 116 mutations in the genome of SARS-CoV-2. The most prevalent mutation was seen in 29095C > T in the N gene, 8782C > T in the ORF1ab gene, and 28144 T > C in the ORF8 gene. The result of phylogenetic tree analysis has shown that the genetic sequences of coronavirus can be mutated because of the immunological pressure among people around the world. It presumably makes the virus easier to transmit. However, many aspects of SARS-CoV-2 pathogenicity and genome function are still unclear [50].
3. SARS-CoV-2 mutations and variants
Mutations in the virus genome have an important influence on diagnosing and treating infection and vaccine development, as well as virus prevention. Since S protein of SARS-CoV-2 is the primary target in vaccine design, mutations of this gene in regions that interact with the host cell's angiotensin-converting enzyme 2 (ACE-2) receptor can reduce vaccines' effectiveness against the virus. Although SARS-CoV-2, unlike many other RNA viruses, has a low sequence diversity due to the proofreading activity, genetic recombination that is caused by nsp14 3′-to-5′ exoribonuclease (Nsp14-ExoN) activity is a common event in the replication of the virus [51], [52]. Hence, the generation of virus diversity and emerging variants should be monitored in vaccinated and non-vaccinated positive cases.
A clade is a group of different virus lineages that forms from evolves over time. From the beginning of the SARS-CoV-2 pandemic, two major lineages of the virus, L and S, were identified based on two single nucleotide polymorphisms in the ORF1ab and ORF8 the virus genome [53]. Then, with further analysis on more genome sequences of the virus, they were divided into three variants A, B, and C, based on amino acid changes and outbreaks [54]. Before the naming of SARS-CoV-variants by the WHO, there were some databases and softwares by different nomenclature for real-time tracking of SARS-CoV-2 evolution and clades distribution by assessing the whole genomic sequences of virus in global such as Nextstrain, GISAID, PANGOLIN, and other websites such as CoVariants [55], [56], [57], [58]. According to these databases, Nextstrain had identified 11 major clades (19A, 19B, and 20A–20I) as of January 2021 [55], GISAID had identified eight global clades (S, O, L, V, G, GH, GR, and GV) [56], and PANGOLIN had identified six major lineages (A, B, B.1, B.1.1, B.1.177, B.1.1.7) as of February 2021 [57]. Using these tools could be useful for the assessment of the novel variant distribution and the impact of mutations on virus transmissibility, Clinical features, and also on the performance of antiviral drugs and the risk of escape from vaccines [59] (Table 1 ).
Table 1.
Variants of SARS-CoV-2 based on three nomenclature methods, location and date of detection, and the location of mutations in the genome of the virus.
| GISAID clades |
PANGO lineages | Nextstrain clades | Notable and elevated risk variants | WHO label | First detection |
Mutations | ||
|---|---|---|---|---|---|---|---|---|
| Location | Date | |||||||
| S | A.1–A.6 | 19B | “reference sequence” hCoV-19/Wuhan/WIV04/2019 (WIV04) | Shenzhen China | Jan 2020 | C8782T,T28144C includes NS8-L84S | ||
| L |
B.3-B.7, B.9, B.10, B.13–B.16 | 19A | Wuhan China |
Dec 2019 | C241,C3037,A23403,C8782,G11083,G26144,T28144 | |||
| O | France | Jan 2020 | ||||||
| V | B.2 | South Korea | Feb 2020 | G11083T,G26144T NSP6-L37F + NS3-G251V | ||||
| G |
B.2 |
B.1.5–B.1.72 |
20A |
20A/S:S477N (EU2) |
Australia, western Europe | Jun-Oct 2020 Outbreak [63], [64], [67] |
S:S477N, N:M234I, N:A376T, ORF1b:A176S, ORF1b:V767L, ORF1b:K1141R, ORF1b:E1184D | |
|
G/484 K.V3 20A/S.484 K (B.1.525) |
Eta (VOI) |
North America, Europe, Asia, Africa, and Australia | late 2020 | S:A67V, S:E484K, D614G,S:Q677H, S:F888L, N:A12G deletion of S: 69, 70, 144 deletion of ORF1a (3675–3677) |
||||
| 20A/S:439 K | emerged twice independently in Europe | Apr 2020 [63], [65], [76] |
S:S439K ORF1a:I2501T deletion of S: 69 |
|||||
| 20A/S:98F | Belgium | Jul 2020 | S:S98F, N:P199L, ORF3a:Q38R, ORF3a:G172R ORF3a:V202L |
|||||
| A.23.1 | Uganda | Oct 2020 [77] |
S:F157L, S:V367F, S:Q613H, S:P681R | |||||
|
B.1.9, B.1.13, B.1.22, B.1.26, B.1.37 |
21A/S:154 K |
B.1.617.1 G/452R.V3 |
Kappa (VOI) |
India | October 2020 | S) L452R, E484Q, D614G, P681R, E154K, Q1071H) | ||
| 21A/S:478 K |
B.1.617.2 G/452R.V3 |
Delta (VOC) |
India | October 2020 | S(L452R, D614G, P681R, T19R, R158G, T478K, D950N), | |||
| GH |
B.1.3–B.1.66 |
20C 21F Derived from 20A, |
CAL.20C 20C/S.452R (B.1.427/B.1.429) |
Epsilon (VOI) |
United States [78], Southern California | October 2020 [64] |
S:S13I, S:W152C, S:L452R, ORF1a:I4205V, ORF1b:D1183Y | |
| 20C/S.484 K, (B.1.526) |
Iota (VOI) |
USA, particularly in New York state |
Late 2020 | S:L5F, S:T95I, S:D253G, S:E484K, S:A701V, ORF1b:Q1011H, ORF3a:P42L N:P199L, N:M234I, ORF8:T11I deletion of ORF1a (3675–3677) |
||||
|
20G (“20C-US”) Derived from 20C |
S:677H.Robin1 |
USA dominant in Wisconsin |
Jan '21 [69] |
S:Q677H, N:P67S, N:P199L, N:D377Y, ORF1a:T265I, ORF1a:L3352F, ORF1b:N1653D, ORF1b:R2613C | ||||
| 20G/S:677P.Pelican | USA. dominant in Louisiana | early 2021 [69] |
S:Q677P, N:P67S, N:P199L, ORF1a:T265I ORF1a:L3352F, ORF1b:N1653D ORF1b:R2613C |
|||||
| 21H | B.1.621 |
Mu (VOI) |
Colombia | Jan 2021 | T95I, Y144S, Y145N, R346K, E484K N501Y, D614G, P681H, D950N |
|||
|
|
20H Derived from 20C |
(B.1.351) 20H:501Y.V2 |
Beta (VOC) | South Africa |
Dec 2020 [79] |
S:N501Y, K417N, E484K, S:L18F and S:D80A deletion of ORF1a (3675–3677) deletion at Spike positions 242-245 mutation in Nucleocapsid:N:T205I |
||
| GR |
B.1.1 |
20B | B.1.1.207 | Nigeria | Mar 2020 | P681H | ||
| 20B/S:1122L (B.1.1302) |
Sweden, Norway and Denmark | Jul 2020 | S:V1122L | |||||
| 20B/S:626S (B1.1.277) |
Norway, Denmark, UK | Jul 2020 | S:A626S | |||||
| 20 J |
20 J/S:501Y.V3 (B.1.1.28.1) (P.1) |
Gamma (VOC) |
Brazil, Japan [72] |
January 2021 | S:N501Y, S:E484K, S:D614G S:K417T S: L18F, S: K417N, S: H655Y. deletion of ORF1a (3675–3677) N:P80R N:R203K, N:G204R |
|||
|
20D Derived from 20B |
20B/S.484 K (P.2) |
Zeta (VOI) |
Brazil | January 2021 | S:E484K, N:A119S, N:M234I, ORF1a:L3458V , ORF1a:L3930F | |||
|
20B/S:265C |
P.3 |
Theta (VOI) |
Philippines |
Jan-2021 | S (E484K, N501Y, D614G, P681H, E1092K, H1101Y, V1176F) ORF1ab (L3201P, D3681E, L3930F, P4715L), ORF8 (K2Q), N (R203K,G204R) |
|||
| 20D |
GR/452Q.V1 C.37 |
Lambda (VOI) |
Peru | Jun 2021 | G75V, T76I, R246N, DEL247/253, L452Q, F490S, D614G, T859N | |||
|
20F Derived from 20B |
Australia | Jun-Oct 2020 outbreak | S:S477N |
|||||
|
20I Derived from 20B |
GRY (B.1.1.7) 20I/S:501Y.V1 |
Alpha (VOC) |
United Kingdom [80] |
18 Dec 2020 | S:N501Y, S:A570D 69–70del, P681H (adjacent to the furin cleavage site). deletion of ORF8 (3675–3677) and ORF1a(Nsp6) Q27 |
|||
| GV | B.1.177 | 20E (EU1) Derived from 20A, | 20I/501Y.V1 | Spain [81] |
early summer of 2020 | S:A222V ORF10:V30L N:A220V |
||
From December 2019 to October 30, 2021, more than 3,215,645 SARS-CoV-2 genomic sequences have been reported by different countries [60]. A phylogenetic analysis of SARS-CoV-2 genomes showed that they are under evolutionary selection in the human host across different regions of the world [54] and these mutants can daily transport to other provinces and countries by passengers. Comparative analysis of these SARS-CoV-2 genomes has shown that there are thousands of missense mutations, including synonymous and deletions or insertions in the non-coding, in-frame, or stop codon regions of variants. For example, Koyama et al.'s study on 10,022 SARS-CoV-2 genomes from 68 countries revealed 65,776 variants with 5775 distinct variants and 2969 missense mutations [61]. Studies also showed that the most frequent mutations were in S, ORF1ab, ORF8, and N genes [49]. Nagy A. et al.’s study showed that mutations in the ORF8, NSP6, ORF3a, NSP4, and N regions were correlated with mild outcomes. Mutations in the spike (S), RdRP, 3′-5′ exonuclease, ORF3a, NSP2, and N were associated with inferior outcomes, and mutations in the S and NSP7 leading to severe outcomes with low prevalence [62]. There are many most prevalent forms of SARS-CoV-2 variants due to their mutations that lead to more transmissibility of these strains for example the D614G mutation [52], S477N in EU2 variant [63], L452R in 20C [64], and S439K in 20A variant [63], [65] that are located in the receptor-binding domain (RDB) of Spike protein and important to ACE-2 binding and antibody recognition, that may increase ACE-2 binding leading to higher levels of virus RNA [66], the resistance to multiple antibodies [67], escaped neutralization and enhanced virion infectivity [64], [68]. D614 G mutation emerges early during the pandemic, and viruses containing G614 are called the “G clade” by GISAID, due to the replacement of glycine (G) instead of aspartic acid (D) in the 614th amino acid [52]. Meanwhile, S:677H mutation in 20G (“20C-US”) and P681H in B.1.1.7 variants that are close to the furin binding pocket (polybasic site) and important for S1/S2 cleavage [69], as well as the presence of a proline in the S:Q677P mutation in 20G, could influence the cleavage of S1/S2 [69]. However, studies have shown that mutations near a furin cleavage site in the spike protein could cause mild symptoms in infected individuals [70].
According to the WHO, SARS-CoV-2 was characterized into two variants, including Variants of Concern (VOC) and Variants under Investigation (VUI) or Variants of interest (VOI), based on the significant genomic changes and emergency in increasing the risk to global public health (Table 1). Hitherto WHO has detected four Variants of Concern (VOC) including B.1.1.7, B.1.351 [71], P.1 [72], and B.1.617.2, which are recently labeled as Greek Alphabet Alpha, Beta, Gamma, and Delta, respectively. Also, eight Variants of interest (VOI), including B.1.427/B.1.429, P.2, B.1.525, P.3, B.1.526, B.1.617.1, C.37, and B.1.621 are detected and labeled by Greek Alphabet from Epsilon to Mu [60], [73] (Table 1).
However, despite the high transmissibility and mild symptoms of infection by mutated variants, access to herd immunity seems closer if people at high risk are vaccinated [74]. Since mutations may lead to the escape from immune recognition, understanding the molecular evolution of SARS-CoV-2 is pivotal, and the emergence of variants with different mutations should be taken into account during the development of vaccines. When seeing emerging variants of the virus, several points should be considered including, identifying mutations of the new variant to survey their effect on the diagnosis of the virus, its rate of spread and infectivity compared to previous variants, as well as the effect of new mutations on the efficacy of the produced vaccines [75].
4. Pathogenesis
In the initial phase of COVID-19 infection, following the SARS-CoV-2 infiltration to the lung parenchyma, the S glycoprotein of the virus binds to the ACE-2 receptor of the host cell. By interacting the RBD of subunit S1 with ACE-2 and cleavage at S1 and S2 by transmembrane protease serine protease-2 (TMPRSS2) and Furin, the S2 subunit mediates the fusion of virus and endosomal cell membrane and release the viral genome into the cytoplasm and continue the replication and cell-to-cell spreading [41], [42]. Furthermore, studies have demonstrated that the SARS-CoV-2 spike protein integrin-binding motif (arginine-glycine-aspartate [RGD]) binds to α5β1integrins, and ACE-2/α5β1 facilitates entry into host cells [82], [83]. By entering the peripheral blood in this phase, the virus causes viremia in humans. In the second phase (Pulmonary phase), with the onset of the inflammatory response, apoptosis of alveolar cells, endothelial and epithelial permeability, edema, fluid accumulation, thrombus (Adhesion, activation, and aggregation of platelets) occur. Consequently, by releasing the pro-inflammatory molecules, vasoconstriction, neutrophil recruitment, Neutrophil extracellular traps (NETosis) [84], [85], cell necrosis, and tissue damage, the pulmonary symptoms including impaired gas exchange, hypoxemia (Saturation of oxygen (SpO2) < 94%), impaired carbon dioxide excretion, respiratory failure, and acute respiratory distress syndrome (ARDS) occur [86], [87]. Finally, in the third phase (hyperinflammation phase), symptoms are exacerbated by systemic inflammation and inflammatory-induced damage to distant organs due to the increased plasma pro-inflammatory cytokines ‘Cytokine storm’ [88] (Fig. 2 ).
After that, all organs that express ACE-2 and TMPRSS2, including the gastrointestinal tract, kidneys, heart, and lungs, can be the potential target for virus attack [89]. It is suggested that the virus begins a second attack, which resulted in the severity condition in patients between 7 and 14 days after symptom onset. During this period, the notable reduction in lymphocyte count and the increase in the production of the inflammatory cytokines exacerbate the severity of the infection (Fig. 2).
According to the Centers for Disease Control and Prevention (CDC) report, although all individuals are susceptible to the SARS-CoV-2, the infectivity ratio in the 18–29 age group is about three times higher than in 5 to 17 age reference group and one times higher than in other adults. The rate of hospitalization and death rate increases by nearly 2 and 3 times for each age group by 10 years compared to the reference group, respectively. For example, the death rates in the age group of 18–29, 30–39, 40–49, 50–64, and 65–74 are 15, 45, 130, 400, and 1100 times higher than the reference age group, respectively [90], [91]. According to the studies, the COVID-19 severe cases ratio, Crude case fatality rate (CFR), and Case fatality rate in patients are 15.7%, 1.4–6.9%, and 11%, respectively [16].
The common symptoms of COVID-19 include sore throat, cough, fever, fatigue, headache, malaise, Myalgia, Chest distress, gastrointestinal disorders, diarrhea, loss of taste or smell, breathing difficulties, myocarditis, neurological symptoms, and cutaneous manifestations. Severe cases include pneumonia, cardiovascular implications, heart failure, viral sepsis [92], bacterial co-infection [93], severe acute respiratory syndrome, and death, especially in high-risk groups and comorbid conditions such as chronic obstructive pulmonary disease (COPD), Hypertension, type 2 diabetes, and myocardial infarction [10], [17], [79], [94], [95], [96], [97].
In addition to severe pneumonia, studies have shown the correlation between virus pathogenesis with host immune responses and cytokine storm. Cytokine storm is potentially responsible for multi-organ damage due to the COVID-19 infection [6], [98]. Data from China has shown that cardiac involvement is a common feature of hospitalized COVID-19 patients (20% to 30 %) and causes about 40% of deaths exclusively or related to respiratory failure [99]. It is suggested that similar to SARS-CoV and SARS-CoV-2 interferon-mediated responses, production of TNFα, and activation of TGF-β signaling associated with respiratory dysfunction and hypoxemia give rise to myocardial dysfunction in COVID-19 infection [100], [101]. Arrhythmia is also reported as a common manifestation in patients; however, its pathophysiology is not still proven. Siripanthong B et al.’s study showed that arrhythmia is related to transferring a significant number of patients to the intensive care unit (ICU) [102].
Due to expressing a significant amount of ACE-2, which is considered the main receptor of SARS-CoV-2 [103], in some cases, SARS-CoV-2 also affected the urogenital tract and testis and resulted in acute kidney injury (AKI) and testicle inflammation (Orchitis). It is proven that there is a positive correlation between ACE-2 mRNA and GFR and a negative correlation between ACE-2 mRNA and serum creatinine. However, the rate of patients showing renal injury caused by COVID-19 is relatively low. A study conducted on 116 COVID-19 patients showed no severe renal impairment in these patients [104].
The high expression of ACE-2 mRNA in infected patients has been reported in digestive organs (duodenum, colon, and small intestine), causing diarrhea and other gastrointestinal symptoms. In several studies, a significant increase in ACE2 mRNA expression has been reported in the gallbladder, testes, and kidneys [105]. Older children and even young adults might be affected by COVID-19, and may experience abdominal pain and diarrhea of unknown cause [106].
The interaction between ACE-2 and SARS-CoV-2 receptors leads to an elevation of free angiotensin II (Ang II) in serum. Consequently, Ang II accumulation can stimulate the release of inflammatory cytokines such as soluble IL-6, TNF-alpha, and IL-6 amplifiers, causing an increase in NFκB activation and eventually releasing the vast amounts of proinflammatory cytokines [107], [108]. There is a correlation between pulmonary ACE-2 dysfunction and severe lung damage, thus it is suggested that the downregulation of virus-induced ACE-2 is important for COVID-19 infection pathology. ACE-2, as the renin-angiotensin system (RAS) regulator, cleaves the angiotensin II to produce the angiotensin 1–7 involved in anti-inflammation, anti-fibrosis, anti-thrombosis, and ROS neutralization [109]. The SARS virus S1 protein binding via the RBD domain to the ACE-2 receptor of host cells induces the cleavage of ACE-2 receptors by A disintegrin and metalloprotease 17 (ADAM-17). Therefore, the increased number of viruses and binding the spike to the ACE-2 receptor lower the ACE-2 level, and consequently decreased the conversion of angiotensin 2 to angiotensin 1–7. Elevated levels of angiotensin −2 eventually lead to vasoconstriction, muscle pain, lack of blood supply to tissues, inflammation, endothelial tissue damage, thrombosis, and lung damage [110].
The decrease in ACE-2 function might intervene in RAS, leading to disturbance in the balance of fluid-electrolyte and blood pressure system and the inflammation increase in the respiratory system. Pro-inflammatory cytokines and chemokine's such as IL-6, interferon gamma-induced protein 10 (IP-10), macrophage inflammatory protein 1α (MIP1α), MIP1β, and monocyte chemoattractant protein 1 (MCP1), recruit immune cells (T cells, macrophage, and monocyte) to the site of infection resulted in more production of IFNγ by T cells and promoted severe inflammation. Studies on the clinical features of COVID-19 have shown that ICU patients have higher plasma levels of pro-inflammatory cytokines, including IL2, IL6, IL7, IL10, granulocyte-colony stimulating factor (GSCF), CRP, IP10, MCP1, MIP1α, TNFα, and also have the lowest hyperactivated peripheral CD4 and CD8 T cells [111], [112], [113], [114].
Although the increased expression of angiotensin-converting enzyme-2 (ACE-2) with increasing age has lung protective effect via restricting angiotensin-2 mediated pulmonary capillary leak and inflammation, severe COVID-19 infection in adults is correlated with the high level of ACE-2 and SARS-CoV-2 loads. The mild or asymptotic infection in children may be due to several factors including, low levels of ACE-2 and TMPRSS2 receptor for SARS-CoV-2 in their respiratory tract [115], [116], enhanced viral sensing, strong innate immune response, and high level of type I interferon (IFN) concentration in respiratory epithelial cells that fight the virus faster and more effectively compared to that of adults [117]. Children unlike adult patients do not exhibit severe infections due to dysfunctional over-active innate immune responses and repressed adaptive immunity. However, children with pre-existing illnesses are high-risk groups for COVID-19 infection [118]. Also, trained immunity in children due to routine live vaccines, including measles, mumps, rubella (MMR), polio, and BCG vaccination [119], [120], [121], [122], and protective Th2 immunity by cytokines, including IL-4, IL-5, and IL-13 as well as the ability to reproduce the pulmonary epithelium can control COVID-19 infection at the site of virus entry [123] (Fig. 2). Studies showed that the incidence of COVID-19 cases in countries that routinely used the BCG vaccine in neonates was less than in countries where it is not used. This may be due to the enhancement of non-specific protection by heterologous lymphocyte activation and the initiation of innate immune memory [124] (Fig. 2).
Many patients have neurological symptoms, including dizziness, headache, vomiting, and delirium. Studies have shown that coronaviruses possess neurotropism, and can enter the CNS through different routes such as trans-synaptic pathways. Infected neurons related to the control of the cardio-respiratory system by coronaviruses in the brain stem may promote respiratory failure [125]. Mao et al.’s study has shown neurologic manifestations in 36.4% of patients [126]. Meanwhile, Garg RK et al. revealed that encephalopathy is common in COVID-19 patients older than 50 years old [127]. Additionally, disseminated intravascular coagulation, rhabdomyolysis, Testicle inflammation (Orchitis), and liver injury (high level of liver biomarkers ALT, AST) are the other identified manifestations induced by COVID-19 infection. Clinical neurological features, including encephalopathy, Cerebrovascular disease, Cerebral hemorrhage, have been reported in COVID-19 [128]. The use of some anticoagulation drugs can help us improve outcomes in severe patients [129]. Furthermore, in some cases of COVID-19, cutaneous manifestations are reported. However, more investigations are required to prove this relationship and the mechanism leading to this symptom [130].
5. Immune responses
5.1. Innate immune response
The innate immune system that is activated following virus replication by increasing the JAK-STAT signal pathway increases the expression of IFN-stimulated genes (ISGs) and reduces the spread of the virus by antigen-presenting macrophage. However, in most cases, the virus escapes the innate immune barrier due to the long incubation period and inhibition of interferon production by the N protein of SARS-CoV [108], [131]. Studies also showed that dysregulated innate immune response is associated with lethal pneumonia in young COVID 19 patients [113]. However, a review study revealed that the adaptive immunity contributes to the control of SARS-CoV-2 in both mild and severe cases of COVID-19 [132].
Immune responses play a crucial role in CoVs disease severity [27]. In SARS-CoV-2, like two older CoVs, the increased level of inflammatory cytokines and chemokines in the first wave of infection is due to the accumulation of Natural Killer (NK) cells, as well as plasmacytoid (p) DCs, macrophages, CD4 + T cells, and NKT cells in the lungs and related to the emergence of cytokine release syndrome (CRS), followed by respiratory failure and ARDS in the hyper inflammation phase [133]. Furthermore, severely infected patients by beta coronaviruses showed an elevated concentration of serum C-reactive protein (CRP). IL-6 binds to the membrane-bound IL-6 receptor (mIL-6R) affects both acquired and innate immune systems (lymphocytes B and T, macrophages, neutrophils, and natural killer cells) via cis signaling, leading to CRS. On the other hand, in trans-signaling, IL-6 binds to the soluble form of IL-6R (sIL-6R) which can contribute to making cytokines storm including the release of IL-8, IL-6 and monocyte chemoattractant protein–1 (MCP-1), reduction of epithelial-cadherin expression on endothelial cells, and the release of vascular endothelial growth factor (VEGF), as a vascular permeability and ARDS mediators. The ample secretion of these factors eventually results in respiratory failure. Meanwhile, the third type of IL-6 signaling that was recently introduced as “trans-presentation” related to T helper 17 (Th17) cells may cause ARDS [134].
Like other viruses, CoVs have some strategies to escape from the host innate immune system to enter and infect target cells. By forming a double vesicle, CoVs can disturb the recognition of dsRNA by pattern recognition receptors (PRRs) [135]. Additionally, Nucleocapcid, and accessory proteins encoded by ORF3b and ORF6 are responsible for blocking the IFN production cascade via several mechanisms [136], [137]. These inhibitory functions promote viral replication and lead to disease severity at the initial stages of infection. CoVs also mimic the capping machinery of target cells via nsp14 and nsp16 in order to avoid immune system recognition [138]. Nsp3 also produces two functional proteins, macrodomain-x, and PLpro, involved in the immune system avoidance process [45], [139], [140].
5.2. Adaptive immune response
Adaptive immunity is mediated by activating T-dependent B cells by helper T cells, producing antibodies against the virus, and killing the virus by cytotoxic T cells [141]. The dysfunctional immune response can lead to lung injury by increasing immune cell accumulation in the lungs and producing pro-inflammatory cytokines [142], [27], [143]. Circulating cytokines can also damage other organs. Studies have demonstrated a strong correlation between neutralizing antibody (nAb) titer and disease severity and anti-spike IgG levels. COVID-19 ICU patients exhibited high nAb titers compared to milder symptoms or asymptomatic patients [144]. Furthermore, non-neutralizing antibodies promote the severity of the infection, and multi-organs damage via antibody-dependent enhancement (ADE) [139], [145]. On the other hand, in a functional immune response through the attraction of virus-specific T cells, lung damage recovery and virus clearance could occur before spreading. In addition to this, neutralizing antibody activity causes efficient phagocytosis by resident macrophages. It is worth mentioning that the detection of IL-1β, which is significantly secreted during pyroptosis, an inflammatory type of programmed cell death, in COVID 19 patients is good evidence to show that SARS-CoV 2 induces pyroptosis in host cells similar to SARS-CoV and other cytopathic viruses [27], [146].
A significant decrease in blood lymphocyte cells, which is considered as one of the main pathological features of COVID-19 infection is strongly related to clinical severity [147]. However, because of the correlation between the decreased lymphocyte level (Lymphopenia) and the mortality rate in the 2009 H1N1 pandemic, we cannot identify lymphopenia as a specific key feature for COVID-19 infection [148]. Neutrophil/lymphocyte ratio elevation, which is remarkably common in COVID-19 patients (around 80%), may result from the migration of immune cells and the infiltration of lymphocytes into the respiratory system [27], [149]. According to the current evidence-based studies conducted on MERS, SARS, and SARS-CoV-2, Th1 plays a crucial role in managing these infections, hence cellular immunity provided by T cells plays an essential role in gaining a successful vaccine [150], [151]. Based on the previous studies on the coronavirus's ability to infect monocytes, T cells, dendritic cells as well as the reduced ability of dendritic cells to control infection due to age-related changes, it is suggested that the novel SARS-CoV-2 may be able to infect dendritic cells [152]. There is a potential theory for understanding the immunopathogenesis of COVID-19, suggesting that dendritic cells may not be able to activate T lymphocytes, and it might cause the exhaustion and apoptosis of T cells. Although T cell response is impaired during SARS-CoV infection, coronavirus-specific memory T cells were found in recovered patients after two years [134], [153]. In patients infected with SARS-CoV-2, impaired maturation of NK compartment or immigration of the mature circulating NK cells into peripheral tissues or the lungs have been reported. Furthermore, the NKG2A immune checkpoint is elevated on NK cells and CD8+ T cells from COVID-19 patients. NKG2A hinders cell cytotoxicity via attaching to the non-classical HLA-E molecule [154]. Moreover, two studies indicated reduced frequencies of Treg cells in severe COVID-19 cases. Since Treg cells have been observed to clear ARDS inflammation in mouse models, a loss of Tregs might assist the progression of COVID-19 lung immunopathology. M2 macrophages (wound-healing) may be activated to repair inflamed tissues in ARDS but, Th2 shifting probably inhibits the wound-healing through secretion of inhibitory cytokine [155].
S1 receptor-binding domain (RBD), N-terminal domain (NTD), and S2 region are the major targets for the current developed neutralizing antibodies (nAbs) against SARS-CoV-2. It has been reported that some patients may not produce long-lasting antibodies against SARS-CoV-2. However, there is currently no conclusive evidence whether they will be re-infected or they will be at the recurrence risk of the original infection [156]. Studies have shown that despite some overlapped neutralizing epitopes among SARS-CoV-2 and other CoVs, SARS-CoV does not have a robust mechanism to inhibit antibody neutralization; mutations in SARS-CoV-2 S proteins during its spread may make it resistant to some monoclonal antibodies [157], [158].
6. COVID-19 diagnostic tests
Clinical diagnosis of COVID-19 for the management of the current outbreak is mainly based on the combination of epidemiological history, clinical criteria along with RT-PCR assay, the sign of pneumonia on chest CT scan/X‐ray and lastly, detection of SARS‐CoV‐2‐specific immunoglobulin IgM/IgG from patients’ sera by enzyme-linked immunosorbent assay (ELISA) [159], [160], [161].
6.1. Real‐time reverse transcriptase‐polymerase chain reaction (RT‐PCR)
Within five to six days of the onset of symptoms, the number of COVID-19 cases has demonstrated high viral loads in their upper and lower respiratory tracts [13], [162]. A nasopharyngeal (NP) and oropharyngeal (OP) swabs are used to extract the SARS-CoV-2 RNA before both Real‐time RT‐PCR under the biosafety level 2 practices and proper use of personal protective equipment (PPE) condition [32], [163], [164]. For the late detection of severe COVID-19 patients during the intubation procedure, sputum sampling or bronchoalveolar lavage should be used because of the highest viral loads [165].
The preferred testing method is the Real‐time RT‐PCR test for two sequence regions N (as a screening) and ORF1b (as a confirmatory) that are highly conserved amongst sarbecoviruses (SARS-related coronavirus) [166]. CDC has recommended two nucleocapsid protein targets (N1 and N2), and WHO has recommended the E gene for first-line screening followed by the RdRp gene as a confirmatory test due to the higher sensitivity and specificity [167]. Studies have shown that mutations in the N gene (C29200T) and E gene (C26340T) affect the SARS-CoV-2 detection [168], [169]. Several factors have been recommended to describe the difference or the high false‐negative rate (FNR) as high as 30% to 50% of Real‐time RT‐PCR testing. These factors include the number and site of primers, point mutation in the N gene, viral load in different anatomic sites of the patients, sampling methods, sample transport condition, and laboratory practice standard, which mostly contribute to high FNR [170].
Deep sequencing molecular methods such as next-generation sequencing and metagenomic next-generation sequencing will continue to determine future mutations of SARS-CoV-2, however, they are currently impracticable for diagnosing COVID-19 [171]. Meanwhile, some studies developed new methods to diagnose the SARS-CoV-2 virus RNA, such as loop-mediated isothermal amplification (LAMP) using a visual, colorimetric detection method [172] and Clustered regularly interspaced short palindromic repeats (CRISPR) technology [173], [174].
6.2. Serological test
Serological methods have concentrated on detecting serum antibodies SARS-CoV-2 envelope spike (S) proteins (high sensitivity), and N protein (low sensitivity). Seroconversion usually happens after two weeks of symptom onset [175]. IgG tests work better than IgM and reveal better sensitivity when the samples are taken at least three months after the beginning of symptoms [176], [177]. Yanan Wang et al. have shown that the positive results of virus-specific IgM were up to over 80% between 2 and 8 weeks after the onset of symptom, then decreased quickly to under 30% in the third month. [177]. Generally, immunoassays for rapid detection of SARS-CoV-2 antibodies are used for lateral flow assays or population-level screening for detecting antibodies (IgM and IgG) against COVID-19. Serology detection is only utilized to determine the immune status of patients without symptoms and diagnose/confirm late COVID-19 cases or to assess the immunity of health care workers as the outbreak progress [166], [178].
6.3. Hematological and inflammatory parameters
Assessment of the hematological laboratory data of COVID-19 positive patients showed that these patients had significantly higher levels of hematocrit (HCT) and mean platelets volume (MPV), and neutrophils (Neutrophilia) due to cytokine storm. These patients have significantly lower levels of Hemoglobin (Hb) [179] due to inhibition of erythropoietin production by cytokines [180]. Also, they have bone marrow's incapability to engender adequate RBCs to transport oxygen. In addition, COVID-19 positive patients have Lymphopenia, eosinopenia, monocytes, and basophil because of the immune suppression, excessive inflammation, and immune-mediated apoptosis of lymphocytes [111], [181]. Studies also showed that the levels of inflammatory parameters, including erythrocyte sedimentation rate (ESR) and CRP, D dimer, creatinine, and procalcitonin were significantly higher in severe COVID-19 patients compared to nonsevere patients [10], [96], [112].
6.4. Chest computed tomography (CT)
The positive load of Real‐time RT‐PCR reduces from the third week and finally becomes undetectable. Nonetheless, the Ct values of critical patients are lower than those of mild patients, and PCR positivity may continue for up to three weeks after the onset of the disease when most mild COVID-19 patients will yield negative results. However, a positive PCR result only shows the detection of viral RNA, which does not certainly show the presence of a viable virus [175]. Repeated testing may be essential if patients have clinical symptoms of viral pneumonia and a history of exposure, Radiological findings, including chest computed tomography (CT) or magnetic resonance imaging (MRI) scan accordant with COVID-19 pneumonia [182]. For cases with a high clinical suspicion of COVID-19 infection with negative RT-PCR test results, a combination of repeated RT-PCR tests and chest CT scans can help clinicians to a rapid and accurate diagnosis [183]. Typical chest CT findings included two-sided pulmonary parenchymal ground-glass opacities (GGOs), consolidation, seldom with rounded morphology, and a peripheral distribution [184], [185], [186], [187].
7. COVID-19 treatment strategies
However, with no specific medicine for COVID-19 patients up to now, there are several suggested potential therapies, including antiviral drugs, anti-inflammatory agents, convalescent plasma, Cellular therapy (Mesenchymal stem cell, DCs, and NK cells), Oxygen therapy, traditional herbal medicines, vitamins and micronutrients, and other options [188] (Table 2 ). The efficacy of these potential drug options has been investigated by researchers. As there is an urgent need for treatment due to the rapid outbreak of SARS-CoV-2, many scientists around the globe have launched clinical trials to find an efficient drug. In the case of co-infection, antibiotics and antifungals should be received by patients [79].
Table 2.
Summary of the proposed therapeutic approaches under investigation for COVID-19.
| COVID-19 Therapy strategies |
Treatment |
Procedure and effectiveness of the drug |
Reference |
|---|---|---|---|
|
Anti-virial therapy |
Viral entry inhibitor Umifenovir (Arbidol) nafamostat mesylate (Futhan) hrsACE-2 ATN-161 |
Arbidol, an antiviral compound for prophylaxis and treatment of influenza, is a potential antiviral drug for treating SARS-CoV-2 by blocking trimerization of the spike glycoprotein and inhibiting host cell attachment. Zhen Zhu et al. showed that Arbidol monotherapy is superior to lopinavir/ritonavir in treating COVID-19. | [268], [269] |
|
Nafamostat is an active synthetic serine protease inhibitor against TMPRSS2 and suppresses TMPRSS2-dependent host cell entry |
[270], [271] | ||
| hrsACE-2 inhibits SARS-CoV-2 from entering the host cells. | [189], [190] | ||
| ATN-161 is an integrin-binding peptide that, by interacting between the SARS-CoV-2 spike protein and α5β1 integrin inhibits the virus entry to host cells. | [191], [192] | ||
|
Interferons: Interferon-alpha (IFNα) and Beta (IFNβ) |
Although the early administration of interferon can reduce viral load and improve COVID-19 symptoms, interferon therapy has not shown any significant benefits in the late phase. Also, it has some adverse effects if administered later. The combination of ribavirin and interferon can stop the polymerase activity. | [98], [171], [206] | |
|
protease inhibitors (Lopinavir/ritonavir) |
These drugs, which are used as HIV protease inhibitors, can be a potential treatment for COVID-19 infection, and some studies have shown that the use of these drugs has no significant effect. | [200], [272] | |
|
Chloroquine and hydroxychloroquine |
Although chloroquine and its derivatives are used to treat malaria, autoimmune diseases and some viral infections, and can inhibit endosomal:lysosomal trafficking, maturation of virus fusion protein, studies have shown that they are inefficive against COVID-19 and due to cardiovascular toxicity and other safety concerns, the risks are not worth the benefits. | [193], [194], [199] | |
|
RNA synthesis inhibitor (Remdesivir) |
Remdesivir is a nucleoside analog that, by intervention with viral RdRp, inhibits viral replication. Remdesivir is an FDA-approved antiviral drug and globally used for COVID-19 treatment in the early of infection which decreases recovery time for patients hospitalized with COVID-19. | [208], [209], [210] | |
|
RNA Interference (RNAi) |
siRNA and miRNA |
Highly conserved genes of SARS-CoV-2, including nsp5, RdRP, and N protein, can be a potential target of siRNA for COVID-19 treatment. Bioinformatics data showed that several miRNAs could have antiviral properties by targeting the ORF1ab and the S genes. Studies showed that MiR-200c and miR-98-5p have antiviral activity by inhibiting the ACE-2 and TMPRSS2 mRNAs, respectively. Also, miR-155, an inflammation-associated miRNA, is overexpressed in SARS-CoV-2 infected cell lines, and elimination of this miRNA may decrease pulmonary injuries of COVID-19 infection. |
[264], [267], [273], [274], [275], [276] |
|
Anti-inflammatory agents |
Glucocorticoids (Dexamethasone) |
Glucocorticoids may modulate inflammation-mediated lung injury and reduce. low doses of Dexamethasone reduced 28-day mortality in the presence of receiving respiratory support, also may benefit in presence of chronic obstructive pulmonary disease (COPD), septic shock, or ARDS. | [227], [228], [229] |
|
corticosteroids (Methylprednisolone) |
Some studies showed that low doses of methylprednisolone effectively improved the prognosis of patients with severe COVID-19 pneumonia if used before the onset of ARDS. |
[234], [235] |
|
|
Cytokine inhibitors IL-6 inhibitors (Tocilizumab) TNF-α inhibitors (Infliximab) |
Tocilizumab and Sarilumab are recombinant- monoclonal antibodies against the IL-6 receptor. Although several studies have shown that Tocilizumab does not prevent death in acute patients, TCZ appears to be an effective treatment option in COVID‐19 patients with a risk of cytokine storms. interleukin-6 inhibitors may provide important benefits in patients with severe COVID-19 |
[224], [277] |
|
| Seven-day administration of infliximab has successfully treated inflammatory bowel disease (IBD) patients with active disease and COVID-19 pneumonia. | [278], [279] | ||
|
Complement inhibitors C5 inhibitors (Eculizumab) C3 inhibitors (AMY-101) |
Eculizumab is a humanized monoclonal antibody functioning as a terminal complement C5 inhibitor. A case study showed that C3 inhibition holds potential as a novel anti-inflammatory therapy in COVID-19. |
[280], [281], [282] |
|
|
Antiplatelets: Aspirin and Clopidogrel Ticagrelor and Prasugrel |
Aspirin and Clopidogrel reduce platelet activation, and Ticagrelor and Prasugrel by nuclear factor-kB (NF-kB) attenuation decrease inflammatory response and virus propagation. |
[238] |
|
|
Anticoagulants: Heparin Vitamin K Antagonists Dabigatran, Apixaban, Rivaroxaban, Edoxaban |
Heparin, by blocking the viral S protein, inhibits virus attachment and S1-S2 cleavage. Also, it acts as an anti-inflammatory, anticoagulant, and venous thromboembolism (VTE) prophylaxis agent. |
[238] |
|
|
Immunotherapy |
Convalescent plasma therapy |
Transfusion of convalescent plasma with higher antibody titers to SARS‐CoV‐2 compared with lower antibody titers within three days of their COVID-19 diagnosis, as strategies to improve cytokine storm symptoms, significantly reduced the mortality in hospitalized COVID‐19 patients. |
[98], [260], [283], [284] |
|
Cellular therapy |
Mesenchymal stem cell treatment |
Mesenchymal Stem Cells (MSC) have immune-regulatory and anti-inflammatory activities. They can promote the differentiation of abnormal T cells and macrophages into T regulatory cells (Treg) and anti-inflammatory macrophages, and inhibit pro-inflammatory cytokine production and repair the damaged tissues in severe COVID-19 pneumonia patients, particularly for the patients in critically severe conditions. |
[285], [286], [287] |
| Dendritic cell (DC) and monocyte-based therapy | In this method, after collecting the PBMCs from vulnerable patients and isolating monocytes from PBMCs, monocytic-DCs are pulsed with SARS-CoV-2 peptides. Then isolated autologous T cells are stimulated and are primed with pulsed monocytic-DCs, and eventually, these personalized T cells are infused into the papulations as prevention or treatment against COVID-19. DC-based therapy is a potential treatment for COVID-19 ARDS. | [245], [246] | |
| Natural killer cells (NK), | NK cells derived from healthy individuals and toll-like receptor activators such as PUL-042 CpG could be used as a prophylactic treatment in the early stage of COVID-19 infection. NK cells and TLR activity must be inhibited to decrease hyper inflammation and prevent multi-organ complications in the hyper inflammation stage. | [242], [243], [244] | |
|
Toll-lik receptor (TLR) activator (PUL-042 CpG) | |||
|
Oxygen therapy |
mechanical ventilation |
A ventilator's early employment for mild COVID-19 patients could prevent progressing to more severe lung injury. Mechanical ventilation is lifesaving in severe respiratory failure. By increasing the esophageal pressure swings above15 cmH2O, intubation should be performed as soon as possible to decrease the risk of lung injury. Tobias Herold et al. showed that elevated levels of IL-6 and CRP predict the need for mechanical ventilation in COVID-19. |
[288], [289], [290] |
| Extracorporeal membrane oxygenation (ECMO) | ECMO is an artificial lung for patients who suffer from refractory hypoxemia, and artificial ventilation is insufficient to sustain blood oxygenation levels. | [36] | |
|
Adjunctive therapy |
General Management |
Supportive treatments, ample bed rest, maintaining a good water-electrolyte balance and monitoring vital signs (temperature, heart rate, oxygen saturation, blood pressure, pulse rate, respiratory rate) | [291] |
|
Traditional Chinese medicines |
Glycyrrhizin, isolated from liquorice roots and Hesperetin, has been showing inhibiting SARS-CoV infection by binding to ACE-2 and suppressing the cleavage activity of the 3C-like pro- tease (3CLpro) respectively that could be effective for the treatment of COVID-19. Also, studies showed that Quercetin is a plant flavonol with an anti-SARS-CoV effect by inhibiting the viral 3-chymotrypsin-like cysteine protease (3CLpro) activity and blocking the entry of the virus into host cells. Baicalein is a flavonoid in the roots of Scutellaria baicalensis and Scutellaria lateriflora that has a role as an antioxidant and anti-inflammatory effect. One study has found that Baikaline inhibits cell damage caused by SARS-CoV-2 and improves cell morphology in vitro and might be a promising therapeutic drug for the treatment of COVID-19. |
[240], [264], [292], [293], [294], [295] |
|
| Vitamins and Micronutrients | Vitamins B, C, D, Zinc, Copper (Cu), glutathione, and Lactoferrin (LF) might have a potential role in prophylaxis and treatment of SARS-CoV-2 infection. | [296], [297], [298], [299], [300], [301], [302] |
7.1. Anti-viral therapy
Human recombinant soluble ACE-2 (hrsACE-2) is a decoy biomolecule that, by neutralizing the virus, can inhibit SARS-CoV-2 from entering the host cells, decrease the viral load in early-stage of infection [189], and improves lung injury of COVID-19 patients [190]. Also, ATN-161 is a recombinant integrin-binding peptide that by interacting between the SARS-CoV-2 spike protein and α5β1 integrin and α5β1/ACE-2 inhibits the virus entry to host cells invitro [191] and potentially can inhibit SARS-CoV-2 entry as a therapeutic agent for COVID-19 infection [192].
According to previous studies, chloroquine and hydroxychloroquine used for malaria and also autoimmune diseases have shown some antiviral activities in viruses such as dengue virus (DENV), West Nile virus (WNV), human immunodeficiency virus (HIV), Japanese encephalitis virus (JEV), and influenza virus [193], [194]. Chloroquine and hydroxychloroquine can inhibit viral entry by blocking the proteolytic process and receptor glycosylation. The results of in- vitro studies on these drugs are auspicious. There is no evidence about the efficacy of chloroquine for SARS and MERS treatment [195]. However, it is suggested that chloroquine can inhibit virus fusion, replication, assembly, release, and other crucial processes through the change of cell surface pH [188], [196], [197]. Some studies proposed the combination therapy of hydroxychloroquine with azithromycin to control the cytokine storm in COVID-19 patients [198] and the Food and drug administration (FDA) suggested emergency use of chloroquine and hydroxychloroquine over a period of time to decline symptoms in infected COVID-19 patients, however, after a while, studies did not show efficiency against COVID-19, it revoked the law declaring that their risks outweigh the benefits [199] (Table 2).
Lopinavir/ritonavir is a combination of protease inhibitors used in HIV treatment and thought to be a potential COVID-19 infection therapy [200]. However, Lim J et al.’s case study showed that using the LPV/RTV reduced the clinical symptoms of COVID-19 infection [201]. There were no significant differences between patients who received standard care and the others who received lopinavir/ritonavir [202], [203]. Andrea Giacomelli et al.’s study revealed that early administration of lopinavir/ritonavir plus hydroxychloroquine does not alter the clinical course of SARS‐CoV‐2 infection [204] (Table 2).
Ribavirin is an antiviral medication that, together with IFN-α is used to treat RSV, HCV, and some viral hemorrhagic fevers [205]. Studies have shown that the combination of ribavirin and interferon would stop the polymerase activity; however, their significant adverse effects seem to be a barrier to their prescription [206], [207]. Remdesivir is a nucleoside analog that intervenes with RNA-dependent RNA polymerase (RdRp), resulting in the inhibition of viral replication [208]. The FDA approves Remdesivir for COVID-19 treatment. Many studies have shown that Remdesivir can reduce the recovery time of hospitalized patients with COVID-19 [209], [210] and is suitable for viral prophylaxis and in the early stages of virus replication, However, it is needed to monitor renal and liver function in patients [211], [212] (Table 2). On 20 November, WHO released a conditional recommendation against the use of remdesivir in hospitalized COVID-19 patients and announced that there is no evidence that remdesivir can improve survival and other outcomes in hospitalized COVID-19 patients [213]. Studies have shown that using remdesivir is not associated with clinical benefits, and does not result in a substantial increase in adverse effects [214], [215], [216], [217], [218].
7.2. Anti-inflammatory agents
Since cytokine storm is a critical feature of exacerbation of COVID-19 infection and consequently ARDS, immunomodulators, and cytokine antagonists, have been considered a potential treatment [98]. In September 2020, WHO recommended the use of interleukin-6 inhibitors and Corticosteroids that may have important benefits in severe COVID-19 patients [219], [220]. Researchers found that the use of tocilizumab and Sarilumab, recombinant humanized anti-human -interleukin-6 receptor monoclonal antibodies (IL-6R Ab), has significantly improved therapeutic effects on patients who experienced cytokine storm during COVID-19 infection [221]. Tocilizumab can bind to the IL-6 receptor with high affinity, thus preventing IL-6 itself from binding to its receptor, rendering it incapable of immune damage to target cells, and alleviating the inflammatory responses [222]. Although several studies have shown that Tocilizumab does not prevent death in acute patients, TCZ appears to be an effective treatment option in COVID‐19 patients with a risk of cytokine storms. [223], [224], [225]. Recent studies have shown that dexamethasone, a glucocorticoid hormone that is produced in the adrenal cortex and acts as an anti-inflammatory drug has a promising result in treating severe cases of COVID-19 [226]. Only low doses of this drug are prescribed to patients admitted to the ICU in a severe condition requiring oxygen, which can be life-saving for this group of patients and not for patients who do not require respiratory support [227], [228], [229]. Dexamethasone by binding to glucocorticoid receptor (GR) on the cell membrane and traveling to the nucleus via binding to specific sites of DNA inhibits the transcription of pro-inflammatory cytokines such as IL-1, IL-2, IL-6, IL-8, TNF, IFN-ϒ and activation of anti-inflammatory cytokine genes such as IL-10 [230], [231]. Glucocorticoids based on their concentration in the blood could have both stimulatory and inhibitory effects on the immune response [232].
Dexamethasone has more and quick antiinflammatory effects to control the cytokine storm-mediated damage of COVID-19 patient lung tissue, compared to other corticosteroids, including hydrocortisone, prednisone, or methylprednisolone [233]. Some studies however showed that Low dose and Short-course methylprednisolone effectively improved the prognosis of patients with severe COVID-19 pneumonia if used before the onset of ARDS [234]. A study showed that the use of systemic glucocorticoids did not reduce mortality and prolonged the duration of hospitalization of non-severe but advanced COVID-19 patients [235]. Also, since studies determined that the complement complex is associated with SARS‐CoV‐2 pathogenesis [236], complement pathway inhibitors, including C5 and C3 inhibitors, could be an attractive therapeutic approach for the treatment of COVD-19 [237]. Meanwhile, Antiplatelets by platelet activation reducing or nuclear factor-kB (NF-kB) attenuation could have decreased inflammatory response and virus propagation [238] (Table 2).
Type 1 interferons (IFN–I) are a subgroup of interferon proteins, including IFN-α (alpha), IFN-β (beta), etc., secreted by various cell types, including endothelial cells, dendritic cells, fibroblasts, macrophages, lymphocytes, and others, during the recognition of viral components by pattern recognition receptors (PRR). IFN–I, by stimulating the interferon-stimulated genes (ISG) in macrophages and NK cells, elicit an anti-viral immunity in the early stage of infection in patients with mild to moderate COVID-19. Therefore, the early use of interferon can decrease viral load and improve COVID-19 signs in some cases [239], [240]. However, interferon therapy has not shown any notable benefits at other stages compared to a placebo and has some adverse effects if administered later [98], [171] (Table 2).
Coronaviruses potently suppress type I IFN responses [241]. Since NK cells as cytotoxic lymphocytes are a crucial component of the innate immune system and are critical in response to many viral infections [242], and the reduction of circulating NK cells in the primary infection in high-risk individuals as well as more recruit of NK cells to the lungs, are observed in hyper inflammation phase these cells may be responsible for the progression and severity of COVID-19 [242], [243]. Studies have shown that the use of umbilical cord blood-derived or isolated NK cell from healthy donor PBMCs may be helpful to the treatment of COVID-19 in the early of infection. Meanwhile, the activation of pathogen recognition receptors, including toll-like receptors 2, 6, and 9 (TLR2/6/9) by PUL-042 CpG oligodeoxynucleotides (CpG ODNs) in the early stage of COVID-19 infection, particularly on DCs and B cells, leading to innate immune stimulation can be employed as prophylactic or therapeutic [244]. In the hyperinflammation level of COVID-19 infection in vulnerable patients in which the host immune system remains active over a prolonged period, the inhibition of TLR and NK cell pathway can help to prevent or dampen hyperinflammation and multi-organ complications [244] (Table 2).
7.3. Cellular therapy
Dendritic cells (DCs) and macrophages have a crucial role in innate immunity and produce inflammatory cytokines and chemokines. DC-based therapy is a potential treatment for COVID-19 ARDS. At first, in this method, after collecting the PBMCs from vulnerable patients and isolating the monocytes, monocytic-DCs are pulsed with SARS-CoV-2 peptides. Then the isolated autologous T cells are stimulated and primed by pulsed monocytic-DCs. Eventually, these personalized T cells are infused into the papulations as a prophylactic or therapy of COVID-19 infection [245], [246] (Table 2).
Interestingly, several studies have stated that Mesenchymal Stem Cells (MSC) are isolated from a different tissue, including peripheral blood, umbilical cord blood (hUC-MSCs) [247], bone marrow (BM-MSCs) [248], and adipose tissues (AT-MSCs) [249] have immune-regulatory and anti-inflammatory activities and are safe and effective for the treatment of COVID-19 pneumonia patients, especially in patients with ARDS. They can stimulate the differentiation of abnormal T cells and macrophage into T regulatory cells (Treg) and anti-inflammatory macrophages, as well as inhibiting pro-inflammatory cytokine production, thereby improving lung injury and ARDS and enhancing tissue repair and reducing lung fibrosis of patients [250], [251], [252] (Table 2).
7.4. Immunotherapy
Human antibodies in convalescent plasma block the virus entry into the target cells by inhibiting the spike protein binding to ACE-2 and thus limits viral amplification [253], [254]. FDA issued an Emergency Use Authorization (EUA) for convalescent plasma to treat COVID-19 [255], and some studies suggested a benefit of high-titer plasma in hospitalized patients with COVID-19 in the early stage of infection (within three days of their COVID-19 diagnosis) [256], [257], [258], [259]. However, some studies demonstrated no difference in seven-day mortality between the patients who received high-titer and those who received low-titer plasma and showed that the data are insufficient to recommend the convalescent plasma for COVID-19 treatment [255]. Also, plasma exchange and blood filtration are considered as strategies to improve cytokine storm symptoms [98], [260] (Table 2).
7.5. RNA interference (RNAi)
RNA interference process (RNAi) involves endogenous (originating in the many eukaryote cells) or exogenous (viral or recombinant) RNA molecules that either destroy the target mRNA or inhibit its translation. Small interfering RNA (siRNA) is a 20–25 base pairs in length double-stranded non-coding RNA molecules, which can regulate the expression of genes by RNA interference (RNAi) phenomenon [261]. Studies showed that among SARS-CoV-2 genes, nsp5 sequence encoding 3CLpro and RdRP, the key enzyme for replicating the virus, and N protein genes, are highly conserved that have been determined as a potential target of siRNA for COVID-19 treatment [262], [263], [264] (Table 2).
The miRNAs are a group of small non-coding RNAs with twenty-two nucleotides of length that are transcribed from the nucleus genome and play a significant role in regulating the eukaryotic gene. miRNA can be used as a therapeutic agent for the COVID-19 treatment. Khan et al.’s bioinformatics study showed that several miRNAs could have antiviral properties by targeting the ORF1ab and the S genes. Other studies have shown that miR-200c by inhibiting ACE-2 mRNA [265] and miR-98-5p by regulating TMPRSS2 [266] have antiviral activity. Also, Wyler et al. ’s study showed that among the small RNA profiling in SARS-CoV-2 infectied cell lines, the inflammation-associated miR-155 was strongly overexpressed, and elimination of this miRNA could reduce the pulmonary injuries of COVID-19 patients [267] (Table 2).
7.6. Vitamins and micronutrients
It is suggested that the deficiency in some vitamins and micronutrients such as vitamins B, C, D, and zinc may reduce the function of the immune system. Furthermore, losing significant weight could impact the severity of infection, the number of hospitalization days, and response to therapy. For these reasons, improving the nutrition and diet patterns could effectively strengthen the immune system and reduce the side effects induced by infection [303].
Due to the improving of the innate and specific immune system and decreasing pro-inflammatory cytokines, Vitamin B could potentially be used to prophylaxis and treat SARS-CoV-2 infection [297], [298]. The rapid release of vast amounts of cytokines and free radicals increases oxidative stress during ARDS and, consequently, multi-organ damage and death. Thus, high doses of an antioxidant such as vitamin C could be considered as a protection and a treatment against COVID-19 [304]. Like many other micronutrients, Vitamin D has an immunomodulatory function and can potentially inhibit the release of inflammatory cytokines during infection [299]. Severe vitamin D deficiency (<25 nmol/L) is associated with disease progression and increased mortality in older, housebound individuals COVID-19 patients [305], [306], [307]. The assessment of 25 randomized control trials revealed that vitamin D has a protective impact against respiratory infections [303]. A Meta-analysis study reported that regular uptake of vitamin D could reduce the risk of acute respiratory infection and the severity of diseases induced by SARS-COV-2 [300]. Although Hastie et al. reported that no significant association was found between 25 (OH) D and the risk of COVID-19 infection, and vitamin D seems not to be an effective option to improve the patients’ condition [308]. Copper (Cu) can potentially boost the innate and adaptive immune system against viral infection by involving natural killer cells, macrophages, B cells, and T helper cells. Cu can kill many types of viruses, such as poliovirus and human immunodeficiency virus type 1 (HIV-1) [301]. Lactoferrin (LF), which is found in mammalian milk, has shown antiviral activity. Binding to HSPGs in the host cell surface, LF inhibited murine coronavirus and Hcov-NL63 from entering into the host cells. LF also has anti-inflammatory and immunomodulatory activities, which can be potentially helpful in alleviating the symptom of COVID 19 infection [296].
Impaired glucose metabolism in obese patients, and type 2 diabetes, as well as hypertension as in most other diseases are the important risk factors for COVID-19 [309]. It seems that consuming a healthy diet with more fibers, vegetables, antioxidant grains, and unsaturated fats along with taking vitamin supplements can significantly improve the prevention and recovery process of many types of disease, including COVID-19 [310], [311], [312].
8. Vaccine development against SARS-CoV-2
Typically, a vaccine takes eight to ten years to develop [313]. The optimistic timeline for developing the COVID-19 vaccine in the body was estimated at least 12 to 18 months [314]. Vaccines have to pass several trial phases to ensure that they are efficient and safe. The development of a vaccine for human use is in processes that comply with current Good Manufacturing Practice (cGMP) to ensure vaccines' consistent quality and safety. Once sufficient preclinical in vitro and in vivo testing data are available and initial batches of the vaccine have been produced by Current Good Manufacturing Practices (cGMP) quality, clinical trials might be initiated. Typically, clinical development of vaccine candidates begins with a small number (20–80) in phase I trials, followed by phase II trials on a large number of volunteers (100–300) to determine the initial proof of vaccine formulation efficacy and dose, and finally the efficacy and quality of a vaccine requiring a proven evaluation in a broader cohort phase III trials on many people (1000–3000). If efficiency is confirmed, a vaccine might be fully licensed or approved for emergency use by regulatory agencies. Vaccines then enter phase four of Clinical Trial for Post-Market Surveillance and adverse events reporting as long as they are on the market. Urgently respond to epidemics based on developing the vaccine technology can differ. For example, since the technology of H1N1 influenza vaccine was previously well developed in eggs and cell-based platforms, the influenza vaccine relatively developed rapidly. However, in the case of previous SARS coronavirus, there has been an experience designing a vaccine for SARS in 2003 using protein S, and a number of vaccines such as mRNA flu vaccines by Moderna (mRNA-1010) and Pfizer/BioNTech [315] have entered the human clinical phase that rapidly contributed to the development of COVID-19 vaccines [44], [316].
As of the 5th of September 2020, the global COVID-19 vaccine R & D landscape includes 206 vaccine candidates, of which; eight approved for full use and entered into phase four, seven were authorized in early or limited use, 53 candidate vaccines are in the clinical phase 1, and 45 candidate vaccines are in phase 2 evaluation on humans, 33 candidate vaccines are at the final stages of testing (phase 3) and more than 75 vaccines are in preclinical evaluation (Table 3 ) [25], [26].
Table 3.
Summary of the Clinical-phase COVID-19 vaccine candidates.
| Platform | target | Vaccine name | Vaccine characteristics | Efficacy/Dose/routes of administration/storage | stage of clinical evaluation/regulatory status | Reference |
|---|---|---|---|---|---|---|
| Inactivated vaccines | Whole virion | WIBP-CorV | Inactivated SARS-CoV-2 Vaccine. Developed by the Wuhan Institute of Biological Products. |
72.51% efficacy |
Phase 1/2 were designed by Wuhan Institute of Biological Products/Sinopharm (ChiCTR2000031809). Phase 3 trials are undergoing in several countries. China approved the Wuhan vaccine for general use. Limited used in the United Arab Emirates. |
[355] |
| BBIBP-CorV | Inactivated SARS-CoV-2 Vaccine. Developed by Beijing Institute of Biological Products; China National Pharmaceutical Group (Sinopharm). An unpublished study showed that the vaccine was only modestly weaker against B.1.351 |
79.34% efficacy/ 2 doses, 3 weeks apart/ Muscle injection/ |
Phases 1/2 by Beijing Institute of Biological Products/Sinopharm (ChiCTR2000032459). Phase 3 trial began in the United Arab Emirates in July, and in Morocco and Peru the following month (ChiCTR2000034780). Approved in China, Bahrain and the United Arab Emirates. Authorized for emergency use in several other countries. Accepted by WHO for Emergency Use Listing (EUL). Entered Phase four Clinical Trial |
[356], [357], [358] |
||
| CoronaVac, known as PiCoVacc | Formalin-inactivated and alum-adjuvanted candidate vaccine. Developed by Sinovac Biotech in china. |
50.65% efficacy in Brazil trial and 91.25% in Turkey trial/ 2 doses, 2 weeks apart/ Muscle injection/ Refrigerated |
Phases 1/2 by Sinovac Biotech (NCT04383574) (NCT04352608). Phase 3 trial (NCT04617483). Phase 3 trial in Turkey (NCT04582344), Indonesia (NCT04508075) and Brazil (NCT04456595). Approved in China and authorized for emergency use in several other countries. Accepted by WHO for Emergency Use Listing (EUL). Entering Phase four Clinical Trial |
[359], [360] | ||
| Covaxin also known as BBV152, BBV154 |
BBV152 is an inactivated SARS-CoV-2 Vaccine. Developed by Indian company Bharat Biotech. BBV154 is a novel adenovirus vectored, intranasal vaccine for COVID-19 |
78% efficacy, Efficacy against Severe COVID-19 disease is 100 % injection into the deltoid muscle/ 2 doses, 4 weeks apart/ Storage at least a week at room temperature |
Phases 1/2 trials induce tolerable safety outcomes and enhanced immune responses (NCT04471519) Authorized for emergency use in India and several other countries. Expression of interest (EOI) data submitted to WHO on 19 April for EUL. |
[361], [362] |
||
|
Live attenuated vaccines |
Whole virion | COVI-VAC | Codon deoptimized live attenuated vaccines that are RNA molecules encoding the rewritten genes of viruses, introducing hundreds of mutations. Developed by Indian company Codagenix |
Single dose/Intranasal | Pre-Clinical by Codagenix/Serum Institute of India and Indian Immunologicals Ltd/Griffith University. Phase 1 trial was launched in the United Kingdom in January (NCT04619628). |
[319] |
|
MV-014–212 (Meissa Vaccine) |
Live attenuated vaccine against respiratory syncytial virus (RSV) that expresses the spike (S) protein of SARS-CoV-2. Developed by Meissa Vaccines company |
single-dose/intranasal | Pre-Clinical by Meissa Vaccines company Phase 1 trial was begun in the United States, Kansas, on April 12, 2021 (NCT04798001). |
[320], [321] | ||
| BCG vaccine | BCG-CORONA | World Health Organization (WHO) reported that the BCG vaccine may be effective in preventing acute respiratory tract infections in elderly patients, other respiratory infections and sepsis. | One dose (0.1 ml) of the licensed BCG vaccine. |
Phase 3 BRACE trial in Australia (NCT04327206) phase 3 BCG-CORONA trial in the US (NTC04632537), Egypt (NCT0350931), Netherlands (NCT04328411), and South Africa (NCT04379336). |
[124], [363], [364], [365] | |
| Recombinant Viral proteins | S protein |
Novavax NVX-CoV2373 |
NVX-CoV2373 is a Subunit Protein vaccine, including a full-length recombinant SARS CoV-2 glycoprotein nanoparticle vaccine adjuvant with Matrix-M1. Developed by Novavax company in the USA. |
96% efficacy against original coronavirus, 86% against B.1.1.7, and 49% against B.1.351 2 doses, 3 weeks apart /Muscle injection /Stable in refrigerator |
Phases 1/2 were generally well-tolerated and elicited robust antibody responses (NCT04533399) Phases 1/2 by the biOTech company (NCT04368988). phase 3 trial in the US and Mexico (NCT04611802) phase 3 trial in the UK (NCT04583995) Accepted by WHO for EUL Entering Phase four Clinical Trial |
[366], [367] |
| EpiVacCorona | Recombinant peptide vaccine for blocking the spike 1 protein receptor-binding domain (S1-RBD) of SARS CoV-2. Developed by Federal Budgetary Research Institution State Research Center of Virology and Biotechnology in Russia. |
2 doses (0.5 ml 2) 3 weeks apart/Muscle injection/Stable in refrigerator for up to two years |
Phases 1/2 trial Being safe and immunogenic vaccine. Containing low reactogenicity. NCT04527575 Approved in Turkmenistan and |
[368] | ||
| ZF2001 | Recombinant peptide vaccine for RBD section of the spike protein of SARS CoV-2 Developed by China's Anhui Zhifei Longcom Biopharmaceutical and the Institute of Microbiology of the Chinese Academy of Sciences. |
3 doses, 4 weeks apart/ Muscle injection |
Phase 1 (NCT04445194) and Phase 2 (NCT04466085), (NCT04550351), Phase 3 trial (NCT04833101) Phase 4 trial (NCT04833101) trials were designed in China. Emergency used in China and Uzbekistan. |
[369] |
||
| CoVLP | Plant-based COVID-19 vaccine in combination with adjuvants (AS03 or CpG1018) to boost the immune system’s response to the viral proteins. Virus genes delivers into leaves, and the plant cells (called Nicotiana benthamiana,) then create protein shells that mimic viruses. Developed by Medicago in Canada |
2 doses, 3 weeks apart/ Muscle injection/ Stable in refrigerator |
In phase 1 two doses of CoVLP (3.75 μg + AS03), lead to strong humoral and cellular responses (NCT04450004) Phase 2/3 started on November 19, 2020 (NCT04636697) |
[370] | ||
| COVID-19 VLP-based vaccine | Virus-like particle, based on RBD displayed on virus-like particles | Pre-Clinical in Switzerland by Saiba GmbH The vaccine was able to induce high levels of antibodies within a week in test animals |
[371] | |||
| Recombinant viral vectors | S protein |
CanSino, Convidecia also known as Ad5-nCoV |
Non-Replicating Viral Vector (Ad5-nCoV) is based on their adenovirus type 5 vector platform (deletions in the E1 and E3 early genes) expresses the full-length spike glycoprotein. Developed by CanSino Biologics company. |
65.28% efficacy/ Single-dose/ Muscle injection Refrigerated. |
Phase 1 in Wuhan, Hubei, China (NCT04313127) phase II trial (NCT04341389) phase III trial (NCT04540419) Phase 3 by CanSino Biologics in (Tianjin, China), (NCT04526990) Approved in China. Emergency use in other countries. WHO's accepted for EUL Enter Phase four Clinical Trial |
[372], [373], [374] |
|
COVID-19 Vaccine Janssen (JNJ-78436735; Ad26.COV2.S) |
Is based on the human adenovirus Ad26 expresses the full-length spike protein. Developed by Johnson & Johnson in the Netherlands and the US. |
72% in the United States, 68% in Brazil, and 64% in South Africa 1 dose/Muscle injection/ Storage up to two years frozen at –4° F (–20° C), and up to three months refrigerated at 36–46° F (2–8° C). |
phase 1/2 trials in the US (NCT04436276) and Japan (NCT04509947) phase 3 trials (NCT04505722) and (NCT04614948) Authorized for emergency use in the United States, the European Union, Canada, and other countries. WHO's accepted for EUL Enter Phase four Clinical Trial |
[343] | ||
| Sputnik V or Gam-Covid-Vac | Sputnik V is a combination of two different Adenoviruses, Ad26 and Ad5. Developed by Gamaleya Institute, in Russia. The vaccine is early use in Sputnik Light is a one-dose version of the Gamaleya vaccine that is newly authorized in Russia. |
91.6% efficacy 2 doses with 3 weeks apart/ muscle injection/Freezer storage |
phase 1 trial (NCT04436471) phase 2 trial (NCT04437875) Phase 3 trial at 25 hospitals and polyclinics in Moscow, Russia on Sept 7 and Nov 24, 2020. (NCT04530396). It is authorized for emergency use in Russia and dozens of other countries. WHO's Emergency Use Listing (EUL) will be decided after all data are submitted and inspections completed |
[341], [375], [376] |
||
| AstraZeneca (AZD1222) also known as Vaxzevria and Covishield in India | Non-Replicating Recombinant Viral Vector. Developed by Oxford-AstraZeneca in the UK using a chimpanzee adenovirus vaccine vector (ChAdOx1). |
76% in a U.S. study/ 2 doses/3 weeks interval Muscle injection/ Stable in the refrigerator for at least 6 months |
phase 1/2 clinical trials in South Africa (NCT04324606) phase 2/3 trials in the UK (NCT04400838) Phase III trial in Brazil (NCT04444674) Approved in Brazil and authorized for emergency use in dozens of countries. WHO's accepted for Emergency Use Listing (EUL) Enter Phase four Clinical Trial |
[377], [378] |
||
| LV-SMENP-DC | LV-SMENP-DC is a dendritic cells (DCs) modified with a lentiviral vector expressing synthetic minigene SMENP and immune-modulatory genes to modify DCs and to activate CTLs by LV-DC presenting SARS-CoV-2 specific antigens. | Phases 1 and 2 by Shenzhen Geno-Immune Medical Institute, in China (NCT04276896) |
[379] |
|||
| Covid-19/aAPC vaccine | Pathogen-specific aAPC (artificial antigen-presenting cell), modified with a lentiviral vector expressing synthetic minigene based on multiple viral genes to express the viral proteins and immune-modulatory genes to modify the aAPC and to activate T cells | Phase 1 by Shenzhen Geno-Immune Medical Institute, (NCT04299724) |
[380] |
|||
| Coroflu | Coroflu, a Non-Replicating Influenza A virus Vector based on FluGen’s flu vaccine candidate known as M2SR (lacks the M2 gene), is a self-limiting version of the influenza virus, which is modified by insertion of the SARS-CoV-2 gene sequence of the spike protein. CoroFlu also expresses the influenza virus hemagglutinin protein antigen and gets immune responses to both coronavirus and influenza. |
intra-nasal delivery | Pre-clinical development Developed by University of Wisconsin-Madison | FluGen | Bharat Biotech |
[381] |
||
| nucleic acid vaccines (RNA or DNA Vaccines) | S protein | Comirnaty known as tozinameran or BNT162 mRNA | BNT162 mRNA vaccine, encapsulates the nucleic acid in special 80 nm ionizable, glycol-lipid nanoparticles Developed by BioNTech - Pfizer; Fosun Pharma. |
91.3% efficacy/ 2 doses, Muscle injection/storage at –25 °C to –15 °C |
Phase I/II trials are ongoing in Germany (NCT04380701) and China (NCT04523571) Phase 2/3 by BioNTech (Mainz, Germany) and Pfizer (New York, NY, USA) (NCT04368728) The WHO and FDA authorized Pfizer’s COVID-19 vaccine for emergency use. Enter Phase four Clinical Trial |
[382], [383], [384] |
|
Spikevax, (mRNA-1273, Moderna Biotech) |
mRNA-1273 vaccine is a part of the spike protein genetic code embedded in special lipid-based nanoparticles. Developed by Moderna, BARDA, NIAID in the USA. |
More than 90% efficacy/ 2 doses, through muscle injection/storage 30 days with refrigeration, 6 months at –20 °C |
phase I trial (NCT04283461) Phase 2 trial (NCT04405076) phase 3 trial (NCT04470427) WHO's accepted for Emergency Use Listing (EUL) Enter Phase four Clinical Trial |
[385] | ||
| INO-4800 |
INO-4800 DNA is a plasmid vaccine candidate that contains the plasmid pGX9501, which encodes for the entire length of the Spike glycoprotein of SARS-CoV-2. Developed by Inovio company. In the cells by translating this optimized DNA into proteins, the immune system activates to generate a robust targeted T cell and antibody response. |
2 doses (1.0 mg)/ Administered intradermally by Electroporation/ stable at room temperature for more than a year |
A preclinical study in animals showed induction of functional antibody responses and T-cell responses following immunization. Phase 1 on April 3, 2020 in Center for Pharmaceutical Research, Kansas City. Mo.; University of Pennsylvania, Philadelphia (NCT04336410) Safety, tolerability and immunogenic were 100% (38/38) in vaccinated subjects. Phase 2 trials in the United States as well as China and South Korea Approved in Switzerland and authorized for emergency use in many others |
[338], [386], [387], [388] |
||
| ZyCoV-D | ZyCoV-D is a DNA-based vaccine (plasmid) encoding for the membrane proteins of the virus. |
delivered by a skin patch/three doses at an interval of 28 days/ Stable at room temperature for three months |
Phase 3 trial , developed by Zydus Cadila in Indian CTRI/2021/01/030416 |
[389], [390] |
||
| AG0302-COVID-19 | Is a DNA-based vaccine, developed by Osaka University and Takara Bio in Japan | 2.0 mg twice at 2-week intervals |
Phases 2/3 trial (NCT04655625) |
[391] |
The SARS-CoV-2 spike (S) glycoprotein is a central focus in vaccine development. S protein contains amino-terminal S1 as an RBD and carboxyl-terminal S2 as membrane fusion and induction of neutral neutralizing (VN) antibodies that can inhibit binding to the host receptor [160]. Other SARS-CoV proteins, including M protein and N protein, can also induce VN antibodies and contain T-cell epitopes, respectively that may also be vaccine targets [317], [318].
Different types of SARS-CoV-2 Vaccines under development, include live attenuated vaccines, Inactivated vaccines, recombinant viral vector vaccines, recombinant viral proteins (Subunit Protein and virus-like particle vaccines), and nucleic acid vaccines (mRNA or Plasmid). Live attenuated vaccines contain weakened whole viruses by a temperature-sensitive mutant with restricted replication to the upper respiratory tract and reverse genetics to construct additional targeted attenuating mutations, to create a protective immune response, but cannot cause illness in healthy people. However, although live attenuated vaccines have a strong and lasting immune response, they are not suitable for people with immune deficiency since the phenotypic or genotypic reversion is possible [316]. COVI-VAC is an Indian live attenuated vaccine against SARS-CoV-2 in which virus genes have been rewritten with hundreds of mutations (Table 3) [319].
Meissa intranasal COVID-19 Vaccine (MV-014–212) is a recombinant live attenuated human respiratory syncytial virus (RSV) that expresses a chimeric SARS-CoV-2 spike protein (composed of ectodomain and transmembrane domains of SARS-CoV-2 spike, and the cytoplasmic tail of the RSV fusion protein) and can stimulate both mucosal (nasal IgA) and systemic (IgG) antibodies against SARS-CoV-2. In addition to preventing infection, this vaccine, unlike other vaccines can block the transmission of the virus and provides a long-lasting immunity against SARS-CoV-2. MV-014–212 can reduce the SARS-CoV-2 peak shedding in the monkey's nose by more than 200-fold [320], [321].
Inactivated vaccines contain whole viruses that have been killed with chemicals (Formalin or beta‐propiolactone), UV light, and heat which cannot cause disease, even in immunosuppressed individuals. Since, inactivated vaccines do not have a durable and long-lasting immune response as live attenuated vaccines, they usually require repeated doses or booster doses, and often adjuvant such as aluminum salts should be added to the vaccine. Meanwhile, formalin inactivation compared to other inactivation methods is associated with a harmful Th2 skewing immune response [322]. The Covaxin, CoronaVac, BBIBP-CorV, and WIBP-CorV are inactivated vaccines approved or authorized for emergency use in several other countries (Table 3). Jara A et al.’s study in Chile on the effectiveness of an inactivated CoronaVac on 10.2 million persons aged 16 years and older has shown that the adjusted vaccine effectiveness for prevention of Covid-19, hospitalization, ICU admission, and prevention of COVID-19–related death has been 65.9%, 87.5%, 90.3%, and 86.3%, respectively [323].
Recombinant viral vector vaccines expressing SARS-CoV S protein include recombinant adenovirus (Ad) vectors, vaccinia virus, measles, Newcastle disease virus (NCD), and vesicular stomatitis virus (VSV) [318], [324]. However, recombinant viral vector vaccines are safe and induce high cellular and humoral immune responses, possibly present pre-existing immunity. The Convidecia (Ad5-nCoV), Janssen Janssen (Ad26.COV2.S), Sputnik V (Ad26 and Ad5), Oxford-AstraZeneca AZD1222 (previously ChAdOx1), are recombinant viral vectors that were approved or authorized for emergency use in other countries. Also, Coroflu (Influenza A virus Vector), and Dendritic cells (DCs) or aAPC (artificial antigen-presenting cell) modified with a lentiviral vector expressing synthetic minigene, are vaccine candidates in phase 1 clinical trial and preclinical stage, respectively (Table 3).
Recombinant Viral protein vaccines include subunits or virus-like particle vaccines. Subunit vaccines are highly safe and can induce cellular and humoral immune responses, but these vaccines have a high cost, lower immunogenicity, and require repeated doses and adjuvants. Virus-like particles (VLPs) are self-assembled viral protein complexes and lack the viral genome. VLPs are safe and efficient tools for studying the pathogenic mechanisms of virus infection and evaluating the efficiency of the vaccine [46]. S protein or RBD subunit vaccines as well as replicating or non-replicating vector vaccines expressing S protein or the RBD provide antigenic proteins to motivate antibody and T-cell immune responses [317], [318], [324] (Table 3).
Nucleic acid vaccines include DNA plasmids such as INO-4800, ZyCoV-D, and AG0302 or mRNA vaccines such as BNT162 mRNA (BioNTech - Pfizer) and mRNA-1273 (Moderna) encode S or RBD proteins. Although DNA vector vaccines may be safe, stable, and produced rapidly, their immune responses in humans are lower, and repeated doses may cause toxicity. Meanwhile, mRNA vaccines are easier to design, transiently expressed, and quickly induce strong immune responses and do not integrate into the host genome, but are highly unstable under physiological conditions [325]. Delivery of the mRNA vaccine is enhanced by using lipid nanoparticles for intramuscular (IM) or intradermal administration [317], [326]. Adding S protein to synthetic nucleic acid vaccines causes booster vaccines, and these vaccines, as mentioned before, are candidate vaccines based on criteria.
BioNTech - Pfizer, Moderna, and Johnson & Johnson are the developed COVID-19 vaccines that FDA authorized for emergency use [327]. In addition to these vaccines, WHO has approved AstraZeneca, Sputnik V, Sinopharm-BBIBP, Novavax, and CoronaVac vaccines for Emergency Use Listing (EUL). Most candidate vaccines are represented on the induction of antibodies and cell-mediated immune responses in the animal models [328]. The protective monoclonal antibodies (mAbs) against the target viral proteins can be essential to map the epitopes that guide vaccine design and provide passive immunoprophylaxis for infected patients by COVID-19 [329] (Table 3). Deborah Steensels et al.’s study on antibody response against SARS-CoV-2 RBD following the second dose vaccination with BioNTech - Pfizer and Moderna has shown that the antibody titers after vaccination by Moderna were higher than BNT162b2. The high ability of the Moderna vaccine to induce humeral immunity may be due to the high mRNA content of the modern vaccine and the longer time interval between the first and second doses of the modern vaccine (4 weeks vs. 3 weeks). Lack of evaluation of cellular immunity and neutralizing antibodies has been one of the limitations of this study [330].
There are several studies on the efficiency of COVID-19 vaccines against emerging variants. Studies have shown that the Moderna COVID-19 vaccine protects neutralizing activity against Alpha (previously known as B.1.1.7 or 501Y.V1) and Beta (previously known as B.1.351 or 501Y.V2) Variants [331]. The beta variant has a more significant reduction to both mAbs and convalescent sera than Alpha [332]. Mutation N501Y within the RBD increases the binding affinity to ACE-2 and, in combination with P681H mutation, increases the viral fusion [333] and virus transmission [334]. A study by Jamie Lopez Bernal et al. showed that the efficacy of the Pfizer vaccine in individuals after the second dose was 93.7% against the alpha strain and 88% against the B.1.617.2 (Delta) strain [335].
The convalescent plasma of patients infected with non-501Y.V3 variants could neutralize the gamma variant (formerly known as 20 J/501Y.V3 , P.1 or B.1.1.28.1) 6-fold reduced than non-501Y.V3 isolates compared to non-501Y.V3 isolates [336]. Souza WMd et al.’s study showed that plasma from inactivated CoronaVac vaccine neutralizes 501Y.V3 isolates less effectively than non-501Y.V3. [336]. Pragya Yadav et al.’s study has shown that convalescent sera of the COVID-19 patients infected with Indian Delta Variant (previously known as B.1.617) and recipients of BBV152 (Covaxin) were able to neutralize this variant [337]. Andrade, V.M., et al.’s study showed that the INO-4800 vaccine could induce neutralizing antibodies against Alpha and Gamma variants, with reduced levels detected against Beta variant [338].
There are some controversies reported on the adenoviral-based SARS COV-2 vaccine. Shabir A. et al.’s study showed that the AstraZeneca AZD1222 (ChAdOx1 nCoV-19 vaccine) does not protect against a predominant variant B.1.351, and mild-to-moderate COVID-19 infection developed in 19 of 750 vaccine recipients (2.5%). The efficacy of the ChAdOx1 nCoV-19 vaccine against Beta (B.1.351) variant was 10.4% in participants infected with this variant [339]. However, because AstraZeneca only needs to be refrigerated rather than mRNA vaccines that need frozen, it can be used far more widely. Katherine R. W. Emary et al.’s study showed that the efficacy of the AstraZeneca vaccine against the Alpha variant was similar to other lineages [340]. Also, Jamie Lopez Bernal et al.’s study revealed that the effectiveness of the ChAdOx1 nCoV-19 vaccine after the second dose was 74% and 67% for the alpha and delta strains, respectively [335]. As for the Sputnik V, Ikegame S et al.’s study has shown that Sputnik V is unable to neutralize vesicular stomatitis virus (VSV) encoding S from Beta variant of SARS COV-2 instead of VSV-G (rcVSV-CoV2-S: B.1.351.) and moderately reduced activity against S from Alpha in a clonal HEK-293 T ACE-2 TMPRSS2 cell line [341]. Wibmer CK et al.’s study showed that Beta has 93% resistance to South African COVID-19 donor mAbs and convalescent plasma samples [342]. Jerald Sadoff et al.’s study showed that the efficiency of Janssen vaccine (Ad26.COV2.S) (5 × 1010 viral particles in a single dose) against Beta variant in moderate to severe and severe–critical COVID-19 is 64.0% and 81.7%, respectively at least 28 days after administration [343]. Meanwhile, Galit Alter et al. in their study revealed that median pseudovirus neutralizing antibody titers and Median binding antibody titers 71 days following vaccination with Ad26.COV2.S vaccine was 5.0- and 3.3-fold lower and 2.9- and 2.7-fold lower against the Beta and Gamma variants, respectively [344].
In the case of the adenoviral-based platform SARS COV-2 vaccines, studies have shown that in rare cases AstraZeneca and Johnson & Johnson vaccines cause blood clots and cerebral and intestinal venous thrombosis with thrombocytopenia syndrome (TTS) which is termed Vaccine-Induced Thrombotic Thrombocytopenia (VITT) or autoimmune heparin-induced thrombocytopenia (HIT) (low platelets count, <150,000 per microliter) [345] and induced by platelet-activating antibodies against platelet factor 4 (PF4) [346], which mimics thrombocytopenia. This side effect has been reported in four cases per million people vaccinated with AstraZeneca [347], [348] and nine cases per 1 million doses for the Janssen COVID-19 vaccine [349], [350]. As previously mentioned, binding of SARS-CoV-2 S1 protein (in both natural viral infection and DNA spike protein vaccines) to ACE-2 receptor by cleavage and reduction of ACE-2 in the host cell leads to a decrease in the conversion of angiotensin 2 to angiotensin 1–7 and an increase in the level of angiotensin 2, which ultimately leads to vasoconstriction, thrombosis, and tissue damage [110]. This process is associated with high viral copy numbers or high viral capsid proteins expressed by DNA vaccines that can damage the alveolar endothelial and lead to the circulation of viral capsid proteins to the ACE-2 + endothelia which are more commonly found in the brain [351]. In the case of AstraZeneca and Johnson vaccines, these side effects are probably due to the high copy number of adenovirus particles used per dose of the vaccine (5 × 1010 viral particles in a single dose). On the other hand, after the first exposure to a vaccine by decreasing the vaccine antigen over time for producing the higher-affinity antibody and immune memory in the affinity maturation process, just those B cells population that has fit interaction with antigenic epitopes are stimulated, that needs long time interval between the primary series and booster of vaccine [352]. For example, as for AstraZeneca, it was revealed that longer prime-boost interval increased the vaccine efficacy from 55·1% in a short interval (<6 weeks) to 81·3% in a long boost interval (≥12 weeks) [353]. However, using a single dose of vaccine or a further increase in the distance between two doses may lead to the formation of antibody-selected variants due to competition between neutralizing antibodies and the virus. Therefore, using a highly stable single dose of vaccine or, using a fewer viral particles in each dose or other vaccines as a booster dose can significantly improve the chances of successful vaccination.
Regarding intranasal vaccines such as covaxin (BBV154), COVI-VAC, and Coroflu, and Meissa Vaccine (MV-014–212), it should be mentioned that these vaccines, in addition to the ease of administration, can stimulate broad immune responses, including neutralizing IgG, mucosal IgA, and T cell responses. Moreover, due to mucosal immunity in the nasal mucosa, these vaccines have an essential role in blocking both COVID-19 infection and SARS COV-2 transmission [354].
9. Conclusion
Most people infected with COVID-19 will have only a mild illness. The elderly and those with chronic health conditions remain the most vulnerable to severe illness derive from COVID-19. More than one year and six months after the pandemic of COVID-19, the number of cases and mortality rates are still rising in the world due to asymptomatic transmission. COVID-19 pandemic continuity depends on many things, including researchers’ work for effective treatments, vaccine development and the public’s effort to slow its spread by social distancing and wearing masks [392]. Although there is no specific treatment for COVID-19, several clinical trials of antiviral drugs are underway. Nevertheless, more studies in the SARS-CoV-2 structure and valid trials with a larger sample size are required. According to new studies, some promising evidence has been observed for the use of Corticosteroids and interleukin-6 inhibitors. However, Dexamethasone does not prevent COVID-19 and even increases the susceptibility of viral infections by weakening the immune system. There was also no evidence of the efficacy of chloroquine, hydroxychloroquine, lopinavir, ritonavir and remdesivir. Other medicines, including favipiravir, cytokine inhibitors, interferons, Cytosorb, Stem cells, anticoagulants, and monoclonal antibodies, have had conflicting results. However, improving the immune system by proper nutrition, consuming vitamins and micronutrients can help us protect ourselves against SARS-CoV-2 infection. Hitherto, more than 206 vaccine projects are in various stages of development, and several vaccines are expected to be licensed by the end of 2021. However, it is possible that due to the possibility of genetic changes in the Coronaviruses, a new strain of the virus will develop as a result of the virus circulating around the world. Therefore, after the large-scale production of vaccines, effectiveness against emerging variants, then transport, storage, availability, and distribution of the vaccines are the main vaccine properties that should be considered in assessing vaccine efficacy. Also, control of new SARS-CoV-2 variants will be obtained from updated vaccines. The increased risk of SARS-Cov-2 infection can be attributed to the high transmissibility of delta strain and its escape from vaccine-induced immunity, as well as the increased exposure of vaccinated people to unvaccinated individuals in public places. Altogether, by adherence to COVID-19 public health guidelines, using effective drugs, quick and extensive vaccination of all people, first the elderly or people with underlying diseases, using a booster dose, and recruiting vaccinated and young people in activities that require more community participation can effectively interrupt the transmission of the virus and reduce disease burden.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors would like to express their gratitude to the Hepatitis and AIDS department of Pasture Institute of Iran and the virology department of Iran University of Medical Sciences for their kind assistance and support.
References
- 1.WHO, WHO Coronavirus (COVID-19) Dashboard, 2021. https://covid19.who.int/.
- 2.Zhang L., Liu Y. Potential interventions for novel coronavirus in China: A systematic review. J. Med. Virol. 2020;92(5):479–490. doi: 10.1002/jmv.25707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brian D.A., Baric R.S. Coronavirus genome structure and replication. Curr. Top. Microbiol. Immunol. 2005;287:1–30. doi: 10.1007/3-540-26765-4_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ou X., Liu Y., Lei X., Li P., Mi D., Ren L., Guo L., Guo R., Chen T., Hu J. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat. Commun. 2020;11(1):1–12. doi: 10.1038/s41467-020-15562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cucinotta D., Vanelli M. WHO declares COVID-19 a pandemic. Acta bio-medica: Atenei Parmensis. 2020;91(1):157–160. doi: 10.23750/abm.v91i1.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rothan H.A., Byrareddy S.N. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J. Autoimmun. 2020;102433 doi: 10.1016/j.jaut.2020.102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harrison A.G., Lin T., Wang P. Mechanisms of SARS-CoV-2 Transmission and Pathogenesis. Trends Immunol. 2020;41(12):1100–1115. doi: 10.1016/j.it.2020.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8..J. Borges do Nascimento, N. Cacic, H.M. Abdulazeem, T.C. von Groote, U. Jayarajah, I. Weerasekara, M.A. Esfahani, V.T. Civile, A. Marusic, A. Jeroncic, Novel coronavirus infection (COVID-19) in humans: a scoping review and meta-analysis, Journal of clinical medicine 9(4) (2020) 941. [DOI] [PMC free article] [PubMed]
- 9.Petrosillo N., Viceconte G., Ergonul O., Ippolito G., Petersen E. COVID-19, SARS and MERS: are they closely related? Clin. Microbiol. Infect. Off. Publicat. European Soc. Clin. Microbiol. Infect. Dis. 2020;26(6):729–734. doi: 10.1016/j.cmi.2020.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang J.-J., Dong X., Cao Y.-Y., Yuan Y.-D., Yang Y.-B., Yan Y.-Q., Akdis C.A., Gao Y.-D. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020;75(7):1730–1741. doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- 11.Xu X.-W., Wu X.-X., Jiang X.-G., Xu K.-J., Ying L.-J., Ma C.-L., Li S.-B., Wang H.-Y., Zhang S., Gao H.-N., Sheng J.-F., Cai H.-L., Qiu Y.-Q., Li L.-J. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ. 2020;368 doi: 10.1136/bmj.m606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McGonagle D., Bridgewood C., Ramanan A.V., Meaney J.F.M., Watad A. COVID-19 vasculitis and novel vasculitis mimics. Lancet Rheumatol. 2021;3(3):e224–e233. doi: 10.1016/S2665-9913(20)30420-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zou L., Ruan F., Huang M., Liang L., Huang H., Hong Z., Yu J., Kang M., Song Y., Xia J. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N. Engl. J. Med. 2020;382(12):1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tang Y., Liu J., Zhang D., Xu Z., Ji J., Wen C. Cytokine Storm in COVID-19: The Current Evidence and Treatment Strategies. Front. Immunol. 2020;11(1708) doi: 10.3389/fimmu.2020.01708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen G., Wu D., Guo W., Cao Y., Huang D., Wang H., Wang T., Zhang X., Chen H., Yu H. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Investig. 2020;130(5):2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Y.-Y., Li B.-R., Ning B.-T. The comparative immunological characteristics of SARS-CoV, MERS-CoV, and SARS-CoV-2 coronavirus infections. Front Immunol. 2020;11:2033. doi: 10.3389/fimmu.2020.02033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi S., Qin M., Shen B., Cai Y., Liu T., Yang F., Gong W., Liu X., Liang J., Zhao Q., Huang H., Yang B., Huang C. Association of Cardiac Injury With Mortality in Hospitalized Patients With COVID-19 in Wuhan. China, JAMA Cardiology. 2020;5(7):802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.D'Amico F., Baumgart D.C., Danese S., Peyrin-Biroulet L. Diarrhea During COVID-19 Infection: Pathogenesis, Epidemiology, Prevention, and Management. Clin Gastroenterol Hepatol. 2020;18(8):1663–1672. doi: 10.1016/j.cgh.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pan L., Mu M., Yang P., Sun Y., Wang R., Yan J., Li P., Hu B., Wang J., Hu C., Jin Y., Niu X., Ping R., Du Y., Li T., Xu G., Hu Q., Tu L. Clinical Characteristics of COVID-19 Patients With Digestive Symptoms in Hubei, China: A Descriptive, Cross-Sectional, Multicenter Study. Am. J. Gastroenterol. 2020;115(5):766–773. doi: 10.14309/ajg.0000000000000620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang X., Yu Y., Xu J., Shu H., Xia J.a., Liu H., Wu Y., Zhang L., Yu Z., Fang M., Yu T., Wang Y., Pan S., Zou X., Yuan S., Shang Y. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir. Med. 2020;8(5):475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lingappan K., Karmouty-Quintana H., Davies J., Akkanti B., Harting M.T. Understanding the age divide in COVID-19: why are children overwhelmingly spared? American J. Physiol.-Lung Cellular Mol. Physiol. 2020;319(1):L39–L44. doi: 10.1152/ajplung.00183.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kronbichler A., Kresse D., Yoon S., Lee K.H., Effenberger M., Shin J.I. Asymptomatic patients as a source of COVID-19 infections: A systematic review and meta-analysis. Int. J. Infect. Dis. 2020;98:180–186. doi: 10.1016/j.ijid.2020.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee J.S., Shin E.-C. The type I interferon response in COVID-19: implications for treatment. Nat. Rev. Immunol. 2020;20(10):585–586. doi: 10.1038/s41577-020-00429-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cascella M., Rajnik M., Aleem A., Dulebohn S.C., Di Napoli R. Stat Pearls; Treasure Island (FL): 2021. Features, Evaluation, and Treatment of Coronavirus (COVID-19) [PubMed] [Google Scholar]
- 25.World Health Organization, DRAFT landscape of COVID-19 candidate vaccines, 2020. https://www.who.int/docs/default-source/a-future-for-children/novel-coronavirus_landscape_covid-19.pdf?sfvrsn=4d8bd201_1.
- 26.J.C.a.S.-L.W. Carl Zimmer, Coronavirus Vaccine Tracker, 2021. https://www.nytimes.com/interactive/2020/science/coronavirus-vaccine-tracker.html.
- 27.Tay M.Z., Poh C.M., Rénia L., MacAry P.A., Ng L.F. The trinity of COVID-19: immunity, inflammation and intervention. Nat. Rev. Immunol. 2020;20(6):363–374. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Y. Chen, C. Yiu, K. Wong, Prediction of the SARS-CoV-2 (2019-nCoV) 3C-like protease (3CLpro) structure: virtual screening reveals velpatasvir, ledipasvir, and other drug repurposing candidates [version 2; peer review: 3 approved], F1000Research 9(129) (2020). [DOI] [PMC free article] [PubMed]
- 29.Walls A.C., Park Y.-J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell. 2020;181(2):281–292.e6. doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gordon D.E., Jang G.M., Bouhaddou M., Xu J., Obernier K., White K.M., O’Meara M.J., Rezelj V.V., Guo J.Z., Swaney D.L., Tummino T.A., Hüttenhain R., Kaake R.M., Richards A.L., Tutuncuoglu B., Foussard H., Batra J., Haas K., Modak M., Kim M., Haas P., Polacco B.J., Braberg H., Fabius J.M., Eckhardt M., Soucheray M., Bennett M.J., Cakir M., McGregor M.J., Li Q., Meyer B., Roesch F., Vallet T., Mac Kain A., Miorin L., Moreno E., Naing Z.Z.C., Zhou Y., Peng S., Shi Y., Zhang Z., Shen W., Kirby I.T., Melnyk J.E., Chorba J.S., Lou K., Dai S.A., Barrio-Hernandez I., Memon D., Hernandez-Armenta C., Lyu J., Mathy C.J.P., Perica T., Pilla K.B., Ganesan S.J., Saltzberg D.J., Rakesh R., Liu X., Rosenthal S.B., Calviello L., Venkataramanan S., Liboy-Lugo J., Lin Y., Huang X.-P., Liu Y., Wankowicz S.A., Bohn M., Safari M., Ugur F.S., Koh C., Savar N.S., Tran Q.D., Shengjuler D., Fletcher S.J., O’Neal M.C., Cai Y., Chang J.C.J., Broadhurst D.J., Klippsten S., Sharp P.P., Wenzell N.A., Kuzuoglu-Ozturk D., Wang H.-Y., Trenker R., Young J.M., Cavero D.A., Hiatt J., Roth T.L., Rathore U., Subramanian A., Noack J., Hubert M., Stroud R.M., Frankel A.D., Rosenberg O.S., Verba K.A., Agard D.A., Ott M., Emerman M., Jura N., von Zastrow M., Verdin E., Ashworth A., Schwartz O., d’Enfert C., Mukherjee S., Jacobson M., Malik H.S., Fujimori D.G., Ideker T., Craik C.S., Floor S.N., Fraser J.S., Gross J.D., Sali A., Roth B.L., Ruggero D., Taunton J., Kortemme T., Beltrao P., Vignuzzi M., García-Sastre A., Shokat K.M., Shoichet B.K., Krogan N.J. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature. 2020;583(7816):459–468. doi: 10.1038/s41586-020-2286-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tang X., Wu C., Li X., Song Y., Yao X., Wu X., Duan Y., Zhang H., Wang Y., Qian Z. On the origin and continuing evolution of SARS-CoV-2. Natl. Sci. Rev. 2020;7(6):1012–1023. doi: 10.1093/nsr/nwaa036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang W., Xu Y., Gao R., Lu R., Han K., Wu G., Tan W. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020;323(18):1843–1844. doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dos Santos W.G. Impact of virus genetic variability and host immunity for the success of COVID-19 vaccines. Biomed. Pharmacother. 2021;111272 doi: 10.1016/j.biopha.2021.111272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Michel C.J., Mayer C., Poch O., Thompson J.D. Characterization of accessory genes in coronavirus genomes. Virol. J. 2020;17(1):131. doi: 10.1186/s12985-020-01402-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu W., Li H. COVID-19: attacks the 1-beta chain of hemoglobin and captures the porphyrin to inhibit human heme metabolism. Preprint revised on. 2020;10(04) [Google Scholar]
- 36.Guo Y.-R., Cao Q.-D., Hong Z.-S., Tan Y.-Y., Chen S.-D., Jin H.-J., Tan K.-S., Wang D.-Y., Yan Y. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak–an update on the status. Mil. Med. Res. 2020;7(1):1–10. doi: 10.1186/s40779-020-00240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bar-On Y.M., Flamholz A., Phillips R., Milo R. SARS-CoV-2 (COVID-19) by the numbers. Elife. 2020;9 doi: 10.7554/eLife.57309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu D.X., Fung T.S., Chong K.K.-L., Shukla A., Hilgenfeld R. Accessory proteins of SARS-CoV and other coronaviruses. Antiviral Res. 2014;109:97–109. doi: 10.1016/j.antiviral.2014.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Letko M., Marzi A., Munster V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat. Microbiol. 2020;5(4):562–569. doi: 10.1038/s41564-020-0688-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yuan M., Wu N.C., Zhu X., Lee C.-C.D., So R.T., Lv H., Mok C.K., Wilson I.A. A highly conserved cryptic epitope in the receptor binding domains of SARS-CoV-2 and SARS-CoV. Science. 2020;368(6491):630–633. doi: 10.1126/science.abb7269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.V’kovski P., Kratzel A., Steiner S., Stalder H., Thiel V. Coronavirus biology and replication: implications for SARS-CoV-2. Nat. Rev. Microbiol. 2021;19(3):155–170. doi: 10.1038/s41579-020-00468-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shang J., Wan Y., Luo C., Ye G., Geng Q., Auerbach A., Li F. Cell entry mechanisms of SARS-CoV-2. Proc. Natl. Acad. Sci. 2020;117(21):11727–11734. doi: 10.1073/pnas.2003138117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., Müller M.A., Drosten C., Pöhlmann S. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181(2):271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Amanat F., Krammer F. SARS-CoV-2 Vaccines: Status Report. Immunity. 2020;52(4):583–589. doi: 10.1016/j.immuni.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Astuti I. Ysrafil, Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2): An overview of viral structure and host response. Diabetes Metab Syndr. 2020;14(4):407–412. doi: 10.1016/j.dsx.2020.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang J., Zeng H., Gu J., Li H., Zheng L., Zou Q. Progress and prospects on vaccine development against SARS-CoV-2. Vaccines. 2020;8(2):153. doi: 10.3390/vaccines8020153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim Y., Jedrzejczak R., Maltseva N.I., Wilamowski M., Endres M., Godzik A., Michalska K., Joachimiak A. Crystal structure of Nsp15 endoribonuclease NendoU from SARS-CoV-2. Protein Sci. Publicat. Protein Soc. 2020;29(7):1596–1605. doi: 10.1002/pro.3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shannon A., Le N.T., Selisko B., Eydoux C., Alvarez K., Guillemot J.C., Decroly E., Peersen O., Ferron F., Canard B. Remdesivir and SARS-CoV-2: Structural requirements at both nsp12 RdRp and nsp14 Exonuclease active-sites. Antiviral Res. 2020;178 doi: 10.1016/j.antiviral.2020.104793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Khailany R.A., Safdar M., Ozaslan M. Genomic characterization of a novel SARS-CoV-2. Gene Rep. 2020;19 doi: 10.1016/j.genrep.2020.100682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rokni M., Ghasemi V., Tavakoli Z. Immune responses and pathogenesis of SARS-CoV-2 during an outbreak in Iran: Comparison with SARS and MERS. Rev. Med. Virol. 2020;30(3) doi: 10.1002/rmv.2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gribble J., Stevens L.J., Agostini M.L., Anderson-Daniels J., Chappell J.D., Lu X., Pruijssers A.J., Routh A.L., Denison M.R. The coronavirus proofreading exoribonuclease mediates extensive viral recombination. PLoS Pathog. 2021;17(1) doi: 10.1371/journal.ppat.1009226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Korber B., Fischer W.M., Gnanakaran S., Yoon H., Theiler J., Abfalterer W., Hengartner N., Giorgi E.E., Bhattacharya T., Foley B., Hastie K.M., Parker M.D., Partridge D.G., Evans C.M., Freeman T.M., de Silva T.I., Angyal A., Brown R.L., Carrilero L., Green L.R., Groves D.C., Johnson K.J., Keeley A.J., Lindsey B.B., Parsons P.J., Raza M., Rowland-Jones S., Smith N., Tucker R.M., Wang D., Wyles M.D., McDanal C., Perez L.G., Tang H., Moon-Walker A., Whelan S.P., LaBranche C.C., Saphire E.O., Montefiori D.C. Tracking Changes in SARS-CoV-2 Spike: Evidence that D614G Increases Infectivity of the COVID-19 Virus. Cell. 2020;182(4):812–827.e19. doi: 10.1016/j.cell.2020.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tang X., Wu C., Li X., Song Y., Yao X., Wu X., Duan Y., Zhang H., Wang Y., Qian Z., Cui J., Lu J. On the origin and continuing evolution of SARS-CoV-2. Natl. Sci. Rev. 2020;7(6):1012–1023. doi: 10.1093/nsr/nwaa036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Forster P., Forster L., Renfrew C., Forster M. Phylogenetic network analysis of SARS-CoV-2 genomes. Proc. Natl. Acad. Sci. 2020;117(17):9241–9243. doi: 10.1073/pnas.2004999117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nextstrain: Genomic Epidemiology of Novel Coronavirus - Global Subsampling 2020, 2021. https://nextstrain.org/sars-cov-2.
- 56.GISAID- Global Initiative on Sharing All Influenza Data, Clade and Lineage Nomenclature Aids in Genomic Epidemiology Studies of Active hCoV-19 Viruses, 2021. https://www.gisaid.org/references/statements-clarifications/clade-and-lineage-nomenclature-aids-in-genomic-epidemiology-of-active-hcov-19-viruses/.
- 57.pangolin: Software package for assigning SARS-CoV-2 genome sequences to global lineages, 2021. https://github.com/cov-lineages/pangolin.
- 58.CoVariants. Enabled by data from GISAID, Powered by Nextstrain, 2021. https://covariants.org/.
- 59.Alm E., Broberg E.K., Connor T., Hodcroft E.B., Komissarov A.B., Maurer-Stroh S., Melidou A., Neher R.A., O’Toole Á., Pereyaslov D. Geographical and temporal distribution of SARS-CoV-2 clades in the WHO European Region, January to June 2020. Eurosurveillance. 2020;25(32):2001410. doi: 10.2807/1560-7917.ES.2020.25.32.2001410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Julia L Mullen, Ginger Tsueng, Alaa Abdel Latif, Manar Alkuzweny, Marco Cano, Emily Haag, Jerry Zhou, Mark Zeller, Emory Hufbauer, Nate Matteson, Kristian G Andersen, Chunlei Wu, Andrew I Su, Karthik Gangavarapu, Laura D Hughes, SARS-CoV-2 (hCoV-19) Mutation Reports, 2020. https://outbreak.info/.
- 61.Koyama T., Platt D., Parida L. Variant analysis of SARS-CoV-2 genomes. Bull. World Health Organ. 2020;98(7):495. doi: 10.2471/BLT.20.253591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nagy Á., Pongor S., Győrffy B. Different mutations in SARS-CoV-2 associate with severe and mild outcome. Int. J. Antimicrob. Agents. 2021;57(2) doi: 10.1016/j.ijantimicag.2020.106272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen J., Wang R., Wang M., Wei G.-W. Mutations Strengthened SARS-CoV-2 Infectivity. J. Mol. Biol. 2020;432(19):5212–5226. doi: 10.1016/j.jmb.2020.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Z. Liu, L.A. VanBlargan, P.W. Rothlauf, L.-M. Bloyet, R.E. Chen, S. Stumpf, H. Zhao, J.M. Errico, E.S. Theel, A.H. Ellebedy, D.H. Fremont, M.S. Diamond, S.P.J. Whelan, Landscape analysis of escape variants identifies SARS-CoV-2 spike mutations that attenuate monoclonal and serum antibody neutralization, bioRxiv (2020) 2020.11.06.372037.
- 65.E.C. Thomson, L.E. Rosen, J.G. Shepherd, R. Spreafico, A. da Silva Filipe, J.A. Wojcechowskyj, C. Davis, L. Piccoli, D.J. Pascall, J. Dillen, S. Lytras, N. Czudnochowski, R. Shah, M. Meury, N. Jesudason, A. De Marco, K. Li, J. Bassi, A. O’Toole, D. Pinto, R.M. Colquhoun, K. Culap, B. Jackson, F. Zatta, A. Rambaut, S. Jaconi, V.B. Sreenu, J. Nix, R.F. Jarrett, M. Beltramello, K. Nomikou, M. Pizzuto, L. Tong, E. Cameroni, N. Johnson, A. Wickenhagen, A. Ceschi, D. Mair, P. Ferrari, K. Smollett, F. Sallusto, S. Carmichael, C. Garzoni, J. Nichols, M. Galli, J. Hughes, A. Riva, A. Ho, M.G. Semple, P.J.M. Openshaw, J.K. Baillie, S.J. Rihn, S.J. Lycett, H.W. Virgin, A. Telenti, D. Corti, D.L. Robertson, G. Snell, The circulating SARS-CoV-2 spike variant N439K maintains fitness while evading antibody-mediated immunity, bioRxiv (2020) 2020.11.04.355842.
- 66.Grubaugh N.D., Hanage W.P., Rasmussen A.L. Making Sense of Mutation: What D614G Means for the COVID-19 Pandemic Remains Unclear. Cell. 2020;182(4):794–795. doi: 10.1016/j.cell.2020.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.C. Gaebler, Z. Wang, J.C.C. Lorenzi, F. Muecksch, S. Finkin, M. Tokuyama, M. Ladinsky, A. Cho, M. Jankovic, D. Schaefer-Babajew, T.Y. Oliveira, M. Cipolla, C. Viant, C.O. Barnes, A. Hurley, M. Turroja, K. Gordon, K.G. Millard, V. Ramos, F. Schmidt, Y. Weisblum, D. Jha, M. Tankelevich, J. Yee, I. Shimeliovich, D.F. Robbiani, Z. Zhao, A. Gazumyan, T. Hatziioannou, P.J. Bjorkman, S. Mehandru, P.D. Bieniasz, M. Caskey, M.C. Nussenzweig, Evolution of Antibody Immunity to SARS-CoV-2, bioRxiv (2020) 2020.11.03.367391.
- 68.Plante J.A., Liu Y., Liu J., Xia H., Johnson B.A., Lokugamage K.G., Zhang X., Muruato A.E., Zou J., Fontes-Garfias C.R. Spike mutation D614G alters SARS-CoV-2 fitness. Nature. 2021;592(7852):116–121. doi: 10.1038/s41586-020-2895-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.E.B. Hodcroft, D.B. Domman, D.J. Snyder, K. Oguntuyo, M.V. Diest, K.H. Densmore, K.C. Schwalm, J. Femling, J.L. Carroll, R.S. Scott, M.M. Whyte, M.D. Edwards, N.C. Hull, C.G. Kevil, J.A. Vanchiere, B. Lee, D.L. Dinwiddie, V.S. Cooper, J.P. Kamil, Emergence in late 2020 of multiple lineages of SARS-CoV-2 Spike protein variants affecting amino acid position 677, medRxiv (2021) 2021.02.12.21251658.
- 70.Jin X., Xu K., Jiang P., Lian J., Hao S., Yao H., Jia H., Zhang Y., Zheng L., Zheng N., Chen D., Yao J., Hu J., Gao J., Wen L., Shen J., Ren Y., Yu G., Wang X., Lu Y., Yu X., Yu L., Xiang D., Wu N., Lu X., Cheng L., Liu F., Wu H., Jin C., Yang X., Qian P., Qiu Y., Sheng J., Liang T., Li L., Yang Y. Virus strain from a mild COVID-19 patient in Hangzhou represents a new trend in SARS-CoV-2 evolution potentially related to Furin cleavage site. Emerging Microbes Infect. 2020;9(1):1474–1488. doi: 10.1080/22221751.2020.1781551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.European Centre for Disease Prevention and Control. Risk related to spread of new SARS-CoV-2 variants of concern in the EU/EEA, first update – 21 January 2021. ECDC: Stockholm; 2021.
- 72.R.M. Coutinho, F.M.D. Marquitti, L.S. Ferreira, M.E. Borges, R.L.P. da Silva, O. Canton, T.P. Portella, S.P. Lyra, C. Franco, A.A.M. da Silva, R.A. Kraenkel, M.A.d.S.M. Veras, P.I. Prado, Model-based estimation of transmissibility and reinfection of SARS-CoV-2 P.1 variant, medRxiv (2021) 2021.03.03.21252706. [DOI] [PMC free article] [PubMed]
- 73.Tracking SARS-CoV-2 variants, 2021. https://www.who.int/en/activities/tracking-SARS-CoV-2-variants/.
- 74.Erol A. Are the emerging SARS-COV-2 mutations friend or foe? Immunol. Lett. 2021;230:63. doi: 10.1016/j.imlet.2020.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Raman R., Patel K.J., Ranjan K. COVID-19: Unmasking Emerging SARS-CoV-2 Variants, Vaccines and Therapeutic Strategies. Biomolecules. 2021;11(7):993. doi: 10.3390/biom11070993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.T.N. Starr, A.J. Greaney, A. Addetia, W.W. Hannon, M.C. Choudhary, A.S. Dingens, J.Z. Li, J.D. Bloom, Prospective mapping of viral mutations that escape antibodies used to treat COVID-19, bioRxiv (2020) 2020.11.30.405472. [DOI] [PMC free article] [PubMed]
- 77.D.L. Bugembe, M.V.T. Phan, I. Ssewanyana, P. Semanda, H. Nansumba, B. Dhaala, S. Nabadda, Á.N. O’Toole, A. Rambaut, P. Kaleebu, M. Cotten, A SARS-CoV-2 lineage A variant (A.23.1) with altered spike has emerged and is dominating the current Uganda epidemic, medRxiv (2021) 2021.02.08.21251393. [DOI] [PMC free article] [PubMed]
- 78.W. Zhang, B.D. Davis, S.S. Chen, J.M. Sincuir Martinez, J.T. Plummer, E. Vail, Emergence of a Novel SARS-CoV-2 Variant in Southern California, JAMA (2021). [DOI] [PMC free article] [PubMed]
- 79.Singhal T. A review of coronavirus disease-2019 (COVID-19) The Indian J. Pediatrics. 2020;87(4):281–286. doi: 10.1007/s12098-020-03263-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.E. Volz, S. Mishra, M. Chand, J.C. Barrett, R. Johnson, L. Geidelberg, W.R. Hinsley, D.J. Laydon, G. Dabrera, Á. O’Toole, R. Amato, M. Ragonnet-Cronin, I. Harrison, B. Jackson, C.V. Ariani, O. Boyd, N.J. Loman, J.T. McCrone, S. Gonçalves, D. Jorgensen, R. Myers, V. Hill, D.K. Jackson, K. Gaythorpe, N. Groves, J. Sillitoe, D.P. Kwiatkowski, S. Flaxman, O. Ratmann, S. Bhatt, S. Hopkins, A. Gandy, A. Rambaut, N.M. Ferguson, Transmission of SARS-CoV-2 Lineage B.1.1.7 in England: Insights from linking epidemiological and genetic data, medRxiv (2021) 2020.12.30.20249034.
- 81.E.B. Hodcroft, M. Zuber, S. Nadeau, K.H.D. Crawford, J.D. Bloom, D. Veesler, T.G. Vaughan, I. Comas, F.G. Candelas, T. Stadler, R.A. Neher, Emergence and spread of a SARS-CoV-2 variant through Europe in the summer of 2020, medRxiv (2020) 2020.10.25.20219063.
- 82.Sigrist C.J., Bridge A., Le Mercier P. A potential role for integrins in host cell entry by SARS-CoV-2. Antiviral Res. 2020;177 doi: 10.1016/j.antiviral.2020.104759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang Y., Liu M., Gao J. Enhanced receptor binding of SARS-CoV-2 through networks of hydrogen-bonding and hydrophobic interactions. Proc. Natl. Acad. Sci. 2020;117(25):13967–13974. doi: 10.1073/pnas.2008209117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Manfredi A.A., Ramirez G.A., Rovere-Querini P., Maugeri N. The Neutrophil’s Choice: Phagocytose vs Make Neutrophil Extracellular Traps. Front Immunol. 2018;9(288) doi: 10.3389/fimmu.2018.00288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chen R.-F., Chang J.-C., Yeh W.-T., Lee C.-H., Liu J.-W., Eng H.-L., Yang K.D. Role of vascular cell adhesion molecules and leukocyte apoptosis in the lymphopenia and thrombocytopenia of patients with severe acute respiratory syndrome (SARS) Microbes Infect. 2006;8(1):122–127. doi: 10.1016/j.micinf.2005.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Krishnan A., Hamilton J.P., Alqahtani S.A., Woreta T.A. COVID-19: An overview and a clinical update. World J. Clin. Cases. 2021;9(1):8. doi: 10.12998/wjcc.v9.i1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Machhi J., Herskovitz J., Senan A.M., Dutta D., Nath B., Oleynikov M.D., Blomberg W.R., Meigs D.D., Hasan M., Patel M., Kline P., Chang R.C.-C., Chang L., Gendelman H.E., Kevadiya B.D. The Natural History, Pathobiology, and Clinical Manifestations of SARS-CoV-2 Infections. J Neuroimmune Pharmacol. 2020;15(3):359–386. doi: 10.1007/s11481-020-09944-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tsang H.F., Chan L.W.C., Cho W.C.S., Yu A.C.S., Yim A.K.Y., Chan A.K.C., Ng L.P.W., Wong Y.K.E., Pei X.M., Li M.J.W., Wong S.-C.C. An update on COVID-19 pandemic: the epidemiology, pathogenesis, prevention and treatment strategies. Expert Review of Anti-infective Therapy. 2021;19(7):877–888. doi: 10.1080/14787210.2021.1863146. [DOI] [PubMed] [Google Scholar]
- 89.Cevik M., Kuppalli K., Kindrachuk J., Peiris M. Virology, transmission, and pathogenesis of SARS-CoV-2. BMJ. 2020;371 doi: 10.1136/bmj.m3862. [DOI] [PubMed] [Google Scholar]
- 90.Fehr A.R., Perlman S. Coronaviruses: an overview of their replication and pathogenesis. Methods Mol. Biol. 2015;1282:1–23. doi: 10.1007/978-1-4939-2438-7_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.CDC and Prevention, Risk for COVID-19 Infection, Hospitalization, and Death By Age Group, 2021. https://www.cdc.gov/coronavirus/2019-ncov/covid-data/investigations-discovery/hospitalization-death-by-age.html.
- 92.Li H., Liu L., Zhang D., Xu J., Dai H., Tang N., Su X., Cao B. SARS-CoV-2 and viral sepsis: observations and hypotheses. Lancet. 2020;395(10235):1517–1520. doi: 10.1016/S0140-6736(20)30920-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bengoechea J.A., Bamford C.G. SARS-CoV-2, bacterial co-infections, and AMR: the deadly trio in COVID-19? EMBO Mol. Med. 2020;12(7) doi: 10.15252/emmm.202012560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pan F., Yang L., Li Y., Liang B., Li L., Ye T., Li L., Liu D., Gui S., Hu Y., Zheng C. Factors associated with death outcome in patients with severe coronavirus disease-19 (COVID-19): a case-control study. Int J Med Sci. 2020;17(9):1281–1292. doi: 10.7150/ijms.46614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jordan R.E., Adab P., Cheng K.K. Covid-19: risk factors for severe disease and death. BMJ. 2020;368 doi: 10.1136/bmj.m1198. [DOI] [PubMed] [Google Scholar]
- 96.Chen T., Wu D., Chen H., Yan W., Yang D., Chen G., Ma K., Xu D., Yu H., Wang H., Wang T., Guo W., Chen J., Ding C., Zhang X., Huang J., Han M., Li S., Luo X., Zhao J., Ning Q. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368 doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Guo T., Fan Y., Chen M., Wu X., Zhang L., He T., Wang H., Wan J., Wang X., Lu Z. Cardiovascular Implications of Fatal Outcomes of Patients With Coronavirus Disease 2019 (COVID-19) JAMA Cardiol. 2020;5(7):811–818. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ye Q., Wang B., Mao J. The pathogenesis and treatment of theCytokine Storm'in COVID-19. J. Infect. 2020;80(6):607–613. doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chen L., Li X., Chen M., Feng Y., Xiong C. The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS-CoV-2. Cardiovasc. Res. 2020;116(6):1097–1100. doi: 10.1093/cvr/cvaa078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Akhmerov A., Marbán E. COVID-19 and the Heart. Circ. Res. 2020;126(10):1443–1455. doi: 10.1161/CIRCRESAHA.120.317055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Babapoor-Farrokhran S., Gill D., Walker J., Rasekhi R.T., Bozorgnia B., Amanullah A. Myocardial injury and COVID-19: Possible mechanisms. Life Sci. 2020;253 doi: 10.1016/j.lfs.2020.117723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Siripanthong B., Nazarian S., Muser D., Deo R., Santangeli P., Khanji M.Y., Cooper L.T., Jr., Chahal C.A.A. Recognizing COVID-19-related myocarditis: The possible pathophysiology and proposed guideline for diagnosis and management. Heart Rhythm. 2020;17(9):1463–1471. doi: 10.1016/j.hrthm.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Karmakar D., Lahiri B., Ranjan P., Chatterjee J., Lahiri P., Sengupta S. Road Map to Understanding SARS-CoV-2 Clinico-Immunopathology and COVID-19 Disease Severity. Pathogens. 2021;10(1):5. doi: 10.3390/pathogens10010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lopes-Pacheco M., Silva P.L., Cruz F.F., Battaglini D., Robba C., Pelosi P., Morales M.M., Caruso Neves C., Rocco P.R.M. Pathogenesis of Multiple Organ Injury in COVID-19 and Potential Therapeutic Strategies. Front. Physiol. 2021;12(29) doi: 10.3389/fphys.2021.593223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Xiao F., Tang M., Zheng X., Liu Y., Li X., Shan H. Evidence for Gastrointestinal Infection of SARS-CoV-2. Gastroenterology. 2020;158(6):1831–1833.e3. doi: 10.1053/j.gastro.2020.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jiang L., Tang K., Levin M., Irfan O., Morris S.K., Wilson K., Klein J.D., Bhutta Z.A. COVID-19 and multisystem inflammatory syndrome in children and adolescents. Lancet. Infect. Dis. 2020;20(11):e276–e288. doi: 10.1016/S1473-3099(20)30651-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chakraborty C., Sharma A.R., Bhattacharya M., Sharma G., Lee S.S., Agoramoorthy G. COVID-19: Consider IL-6 receptor antagonist for the therapy of cytokine storm syndrome in SARS-CoV-2 infected patients. J Med Virol. 2020;92(11):2260–2262. doi: 10.1002/jmv.26078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Li G., Fan Y., Lai Y., Han T., Li Z., Zhou P., Pan P., Wang W., Hu D., Liu X. Coronavirus infections and immune responses. J. Med. Virol. 2020;92(4):424–432. doi: 10.1002/jmv.25685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lee C., Choi W.J. Overview of COVID-19 inflammatory pathogenesis from the therapeutic perspective. Arch. Pharmacal Res. 2021:1–18. doi: 10.1007/s12272-020-01301-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Labò N., Ohnuki H., Tosato G. Vasculopathy and Coagulopathy Associated with SARS-CoV-2 Infection. Cells. 2020;9(7):1583. doi: 10.3390/cells9071583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., Liu S., Zhao P., Liu H., Zhu L. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respiratory Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Prompetchara E., Ketloy C., Palaga T. Immune responses in COVID-19 and potential vaccines: Lessons learned from SARS and MERS epidemic. Asian Pac J. Allergy Immunol. 2020;38(1):1–9. doi: 10.12932/AP-200220-0772. [DOI] [PubMed] [Google Scholar]
- 114.Hu B., Huang S., Yin L. The cytokine storm and COVID-19. J. Med. Virol. 2021;93(1):250–256. doi: 10.1002/jmv.26232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Schuler B.A., Christian Habermann A., Plosa E.J., Taylor C.J., Jetter C., Kapp M.E., Benjamin J.T., Gulleman P., Nichols D.S., Braunstein L.Z., Hackett A., Koval M., Guttentag S.H., Blackwell T.S., Webber S.A., Banovich N.E., Kropski J.A., Sucre J.M.S. Age-determined expression of priming protease TMPRSS2 and localization of SARS-CoV-2 infection in the lung epithelium. J. Clin. Investig. 2021;131(1) doi: 10.1172/JCI140766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dioguardi M., Cazzolla A.P., Arena C., Sovereto D., Caloro G.A., Dioguardi A., Crincoli V., Laino L., Troiano G., Lo Muzio L. Innate Immunity in Children and the Role of ACE2 Expression in SARS-CoV-2 Infection. Pediatric Rep. 2021;13(3):363–382. doi: 10.3390/pediatric13030045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Loske J., Röhmel J., Lukassen S., Stricker S., Magalhães V.G., Liebig J., Chua R.L., Thürmann L., Messingschlager M., Seegebarth A., Timmermann B., Klages S., Ralser M., Sawitzki B., Sander L.E., Corman V.M., Conrad C., Laudi S., Binder M., Trump S., Eils R., Mall M.A., Lehmann I. Pre-activated antiviral innate immunity in the upper airways controls early SARS-CoV-2 infection in children. Nat. Biotechnol. 2021 doi: 10.1038/s41587-021-01037-9. [DOI] [PubMed] [Google Scholar]
- 118.Dhochak N., Singhal T., Kabra S.K., Lodha R. Pathophysiology of COVID-19: Why Children Fare Better than Adults? Indian J. Pediatrics. 2020;87(7):537–546. doi: 10.1007/s12098-020-03322-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.R.J. Arts, S.J. Moorlag, B. Novakovic, Y. Li, S.-Y. Wang, M. Oosting, V. Kumar, R.J. Xavier, C. Wijmenga, L.A. Joosten, BCG vaccination protects against experimental viral infection in humans through the induction of cytokines associated with trained immunity, Cell host & microbe 23(1) (2018) 89-100. e5. [DOI] [PubMed]
- 120.Kleinnijenhuis J., Quintin J., Preijers F., Benn C.S., Joosten L.A., Jacobs C., Van Loenhout J., Xavier R.J., Aaby P., Van Der Meer J.W. Long-lasting effects of BCG vaccination on both heterologous Th1/Th17 responses and innate trained immunity. J. Innate Immun. 2014;6(2):152–158. doi: 10.1159/000355628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Netea M.G., Domínguez-Andrés J., Barreiro L.B., Chavakis T., Divangahi M., Fuchs E., Joosten L.A.B., van der Meer J.W.M., Mhlanga M.M., Mulder W.J.M., Riksen N.P., Schlitzer A., Schultze J.L., Stabell Benn C., Sun J.C., Xavier R.J., Latz E. Defining trained immunity and its role in health and disease. Nat. Rev. Immunol. 2020;20(6):375–388. doi: 10.1038/s41577-020-0285-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.López-Martin I., Andrés Esteban E., García-Martínez F.J. Relationship between MMR vaccination and severity of Covid-19 infection. Survey among primary care physicians. Medicina Clínica (English Edition) 2021;156(3):140–141. doi: 10.1016/j.medcle.2020.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Steinman J.B., Lum F.M., Ho P.P.-K., Kaminski N., Steinman L. Reduced development of COVID-19 in children reveals molecular checkpoints gating pathogenesis illuminating potential therapeutics. Proc. Natl. Acad. Sci. 2020;117(40):24620–24626. doi: 10.1073/pnas.2012358117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mohapatra P.R., Mishra B., Behera B. BCG vaccination induced protection from COVID-19. Indian J. Tuberculosis. 2021;68(1):119–124. doi: 10.1016/j.ijtb.2020.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Steardo L., Steardo L., Jr, Zorec R., Verkhratsky A. Neuroinfection may contribute to pathophysiology and clinical manifestations of COVID-19. Acta Physiol. 2020 doi: 10.1111/apha.13473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Nardo A.D., Schneeweiss-Gleixner M., Bakail M., Dixon E.D., Lax S.F., Trauner M. Pathophysiological mechanisms of liver injury in COVID-19. Liver Int. 2021;41(1):20–32. doi: 10.1111/liv.14730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Garg R.K., Paliwal V.K., Gupta A. Encephalopathy in patients with COVID-19: a review. J. Med. Virol. 2021;93(1):206–222. doi: 10.1002/jmv.26207. [DOI] [PubMed] [Google Scholar]
- 128.Al-Sarraj S., Troakes C., Hanley B., Osborn M., Richardson M.P., Hotopf M., Bullmore E., Everall I. Invited Review: The spectrum of neuropathology in COVID-19. Neuropathol. Appl. Neurobiol. 2021;47(1):3–16. doi: 10.1111/nan.12667. [DOI] [PubMed] [Google Scholar]
- 129.Zaim S., Chong J.H., Sankaranarayanan V., Harky A. COVID-19 and Multiorgan Response. Curr Probl Cardiol. 2020;45(8) doi: 10.1016/j.cpcardiol.2020.100618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sachdeva M., Gianotti R., Shah M., Bradanini L., Tosi D., Veraldi S., Ziv M., Leshem E., Dodiuk-Gad R.P. Cutaneous manifestations of COVID-19: Report of three cases and a review of literature. J. Dermatol. Sci. 2020;98(2):75–81. doi: 10.1016/j.jdermsci.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mason R.J. Pathogenesis of COVID-19 from a cell biology perspective. European Respiratory J. 2020;55(4) doi: 10.1183/13993003.00607-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sette A., Crotty S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell. 2021;184(4):861–880. doi: 10.1016/j.cell.2021.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhang C., Wu Z., Li J.-W., Zhao H., Wang G.-Q. The cytokine release syndrome (CRS) of severe COVID-19 and Interleukin-6 receptor (IL-6R) antagonist Tocilizumab may be the key to reduce the mortality. Int. J. Antimicrob. Agents. 2020;105954 doi: 10.1016/j.ijantimicag.2020.105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Moore J.B., June C.H. Cytokine release syndrome in severe COVID-19. Science. 2020;368(6490):473–474. doi: 10.1126/science.abb8925. [DOI] [PubMed] [Google Scholar]
- 135.Li G., Fan Y., Lai Y., Han T., Li Z., Zhou P., Pan P., Wang W., Hu D., Liu X., Zhang Q., Wu J. Coronavirus infections and immune responses. J. Med. Virol. 2020;92(4):424–432. doi: 10.1002/jmv.25685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lu X., Pan J.A., Tao J., Guo D. SARS-CoV nucleocapsid protein antagonizes IFN-β response by targeting initial step of IFN-β induction pathway, and its C-terminal region is critical for the antagonism. Virus Genes. 2011;42(1):37–45. doi: 10.1007/s11262-010-0544-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kopecky-Bromberg S.A., Martínez-Sobrido L., Frieman M., Baric R.A., Palese P. Severe acute respiratory syndrome coronavirus open reading frame (ORF) 3b, ORF 6, and nucleocapsid proteins function as interferon antagonists. J. Virol. 2007;81(2):548–557. doi: 10.1128/JVI.01782-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hall M.D., Anderson J.M., Anderson A., Baker D., Bradner J., Brimacombe K.R., Campbell E.A., Corbett K.S., Carter K., Cherry S., Chiang L., Cihlar T., de Wit E., Denison M., Disney M., Fletcher C.V., Ford-Scheimer S.L., Götte M., Grossman A.C., Hayden F.G., Hazuda D.J., Lanteri C.A., Marston H., Mesecar A.D., Moore S., Nwankwo J.O., O’Rear J., Painter G., Singh Saikatendu K., Schiffer C.A., Sheahan T.P., Shi P.-Y., Smyth H.D., Sofia M.J., Weetall M., Weller S.K., Whitley R., Fauci A.S., Austin C.P., Collins F.S., Conley A.J., Davis M.I. Report of the National Institutes of Health SARS-CoV-2 Antiviral Therapeutics Summit. J. Infect. Dis. 2021;224:S1–S21. doi: 10.1093/infdis/jiab305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Cao X. COVID-19: immunopathology and its implications for therapy. Nat. Rev. Immunol. 2020;20(5):269–270. doi: 10.1038/s41577-020-0308-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Arya R., Kumari S., Pandey B., Mistry H., Bihani S.C., Das A., Prashar V., Gupta G.D., Panicker L., Kumar M. Structural insights into SARS-CoV-2 proteins. J. Mol. Biol. 2021;433(2) doi: 10.1016/j.jmb.2020.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Swain S.L., McKinstry K.K., Strutt T.M. Expanding roles for CD4+ T cells in immunity to viruses. Nat. Rev. Immunol. 2012;12(2):136–148. doi: 10.1038/nri3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Saxena S.K. Springer Nature; 2020. Coronavirus Disease 2019 (COVID-19): Epidemiology, Pathogenesis, Diagnosis, and Therapeutics. [Google Scholar]
- 143.Bourgonje A.R., Abdulle A.E., Timens W., Hillebrands J.L., Navis G.J., Gordijn S.J., Bolling M.C., Dijkstra G., Voors A.A., Osterhaus A.D. Angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 and the pathophysiology of coronavirus disease 2019 (COVID-19) The Journal of pathology. 2020;251(3):228–248. doi: 10.1002/path.5471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Legros V., Denolly S., Vogrig M., Boson B., Siret E., Rigaill J., Pillet S., Grattard F., Gonzalo S., Verhoeven P., Allatif O., Berthelot P., Pélissier C., Thiery G., Botelho-Nevers E., Millet G., Morel J., Paul S., Walzer T., Cosset F.L., Bourlet T., Pozzetto B. A longitudinal study of SARS-CoV-2-infected patients reveals a high correlation between neutralizing antibodies and COVID-19 severity. Cell. Mol. Immunol. 2021;18(2):318–327. doi: 10.1038/s41423-020-00588-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Negro F. Is antibody-dependent enhancement playing a role in COVID-19 pathogenesis? Swiss Med. Weekly. 2020;150(1516) doi: 10.4414/smw.2020.20249. [DOI] [PubMed] [Google Scholar]
- 146.Yang M. Cell pyroptosis, a potential pathogenic mechanism of 2019-nCoV infection. Available at SSRN. 2020;3527420 [Google Scholar]
- 147.Qin C., Zhou L., Hu Z., Zhang S., Yang S., Tao Y., Xie C., Ma K., Shang K., Wang W., Tian D.S. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2020. Dysregulation of Immune Response in Patients With Coronavirus 2019 (COVID-19) in Wuhan, China; pp. 762–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Xiang L., Hu X., Zhang J., She J., Li M., Zhou T. Immunodepression induced by influenza A virus (H1N1) in lymphoid organs functions as a pathogenic mechanism. Clin. Exp. Pharmacol. Physiol. 2020;47(10):1664–1673. doi: 10.1111/1440-1681.13358. [DOI] [PubMed] [Google Scholar]
- 149.Tan L., Wang Q., Zhang D., Ding J., Huang Q., Tang Y.-Q., Wang Q., Miao H. Lymphopenia predicts disease severity of COVID-19: a descriptive and predictive study. Signal Transduct. Targeted Therapy. 2020;5(1):1–3. doi: 10.1038/s41392-020-0148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Alosaimi B., Hamed M.E., Naeem A., Alsharef A.A., AlQahtani S.Y., AlDosari K.M., Alamri A.A., Al-Eisa K., Khojah T., Assiri A.M. MERS-CoV infection is associated with downregulation of genes encoding Th1 and Th2 cytokines/chemokines and elevated inflammatory innate immune response in the lower respiratory tract. Cytokine. 2020;126 doi: 10.1016/j.cyto.2019.154895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Weiskopf D., Schmitz K.S., Raadsen M.P., Grifoni A., Okba N.M., Endeman H., van den Akker J.P., Molenkamp R., Koopmans M.P., van Gorp E.C. Phenotype and kinetics of SARS-CoV-2-specific T cells in COVID-19 patients with acute respiratory distress syndrome. Sci. Immunol. 2020;5(48) doi: 10.1126/sciimmunol.abd2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Borges R.C., Hohmann M.S., Borghi S.M. Dendritic cells in COVID-19 immunopathogenesis: insights for a possible role in determining disease outcome. Int. Rev. Immunol. 2021;40(1–2):108–125. doi: 10.1080/08830185.2020.1844195. [DOI] [PubMed] [Google Scholar]
- 153.Diao B., Wang C., Tan Y., Chen X., Liu Y., Ning L., Chen L., Li M., Liu Y., Wang G. Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID-19) Front Immunol. 2020;11:827. doi: 10.3389/fimmu.2020.00827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Yaqinuddin A., Kashir J. Innate immunity in COVID-19 patients mediated by NKG2A receptors, and potential treatment using Monalizumab, Cholroquine, and antiviral agents. Med. Hypotheses. 2020;109777 doi: 10.1016/j.mehy.2020.109777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Rossi F., Tortora C., Argenziano M., Di Paola A., Punzo F. Cannabinoid Receptor Type 2: A Possible Target in SARS-CoV-2 (CoV-19) Infection? Int. J. Mol. Sci. 2020;21(11):3809. doi: 10.3390/ijms21113809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Jiang S., Hillyer C., Du L. Neutralizing Antibodies against SARS-CoV-2 and Other Human Coronaviruses. Trends Immunol. 2020;41(5):355–359. doi: 10.1016/j.it.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Liu Z., Xia S., Wang X., Lan Q., Xu W., Wang Q., Jiang S., Lu L. Inefficiency of Sera from Mice Treated with Pseudotyped SARS-CoV to Neutralize 2019-nCoV Infection. Virologica Sinica. 2020;35(3):340–343. doi: 10.1007/s12250-020-00214-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Hassan A.O., Case J.B., Winkler E.S., Thackray L.B., Kafai N.M., Bailey A.L., McCune B.T., Fox J.M., Chen R.E., Alsoussi W.B., Turner J.S., Schmitz A.J., Lei T., Shrihari S., Keeler S.P., Fremont D.H., Greco S., McCray P.B., Jr., Perlman S., Holtzman M.J., Ellebedy A.H., Diamond M.S. A SARS-CoV-2 Infection Model in Mice Demonstrates Protection by Neutralizing Antibodies. Cell. 2020;182(3):744–753.e4. doi: 10.1016/j.cell.2020.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Loeffelholz M.J., Tang Y.-W. infections, Laboratory diagnosis of emerging human coronavirus infections–the state of the art. Emerging microbes. 2020;9(1):747–756. doi: 10.1080/22221751.2020.1745095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zhou P., Yang X.-L., Wang X.-G., Hu B., Zhang L., Zhang W., Si H.-R., Zhu Y., Li B., Huang C.-L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.WHO, Laboratory testing for coronavirus disease 2019 (COVID-19) in suspected human cases: interim guidance, 2 March 2020, World Health Organization, Geneva, 2020.
- 162.Pan Y., Zhang D., Yang P., Poon L.L., Wang Q. Viral load of SARS-CoV-2 in clinical samples. Lancet. Infect. Dis. 2020;20(4):411–412. doi: 10.1016/S1473-3099(20)30113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Centers for Disease Control Prevention (CDC), Interim guidelines for collecting, handling, and testing clinical specimens from patients under investigation (PUIs) for 2019 novel coronavirus (2019-nCoV). 2020, 2020.
- 164.Druce J., Garcia K., Tran T., Papadakis G., Birch C. Evaluation of swabs, transport media, and specimen transport conditions for optimal detection of viruses by PCR. J. Clin. Microbiol. 2012;50(3):1064–1065. doi: 10.1128/JCM.06551-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.M. Cevik, K. Kuppalli, J. Kindrachuk, M. Peiris, Virology, transmission, and pathogenesis of SARS-CoV-2, bmj 371 (2020). [DOI] [PubMed]
- 166.Tang Y.-W., Schmitz J.E., Persing D.H., Stratton C.W. Laboratory diagnosis of COVID-19: current issues and challenges. J. Clin. Microbiol. 2020;58(6) doi: 10.1128/JCM.00512-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Corman V.M., Landt O., Kaiser M., Molenkamp R., Meijer A., Chu D.K., Bleicker T., Brünink S., Schneider J., Schmidt M.L. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Eurosurveillance. 2020;25(3):2000045. doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Vanaerschot M., Mann S.A., Webber J.T., Kamm J., Bell S.M., Bell J., Hong S.N., Nguyen M.P., Chan L.Y., Bhatt K.D. Identification of a polymorphism in the N gene of SARS-CoV-2 that adversely impacts detection by reverse transcription-PCR. J. Clin. Microbiol. 2021;59(1) doi: 10.1128/JCM.02369-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ziegler K., Steininger P., Ziegler R., Steinmann J., Korn K., Ensser A. SARS-CoV-2 samples may escape detection because of a single point mutation in the N gene. Eurosurveillance. 2020;25(39):2001650. doi: 10.2807/1560-7917.ES.2020.25.39.2001650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.N. Sinnott-Armstrong, D.L. Klein, B. Hickey, Evaluation of Group Testing for SARS-CoV-2 RNA, medRxiv (2020) 2020.03.27.20043968.
- 171.Hadjadj J., Yatim N., Barnabei L., Corneau A., Boussier J., Smith N., Péré H., Charbit B., Bondet V., Chenevier-Gobeaux C., Breillat P., Carlier N., Gauzit R., Morbieu C., Pène F., Marin N., Roche N., Szwebel T.-A., Merkling S.H., Treluyer J.-M., Veyer D., Mouthon L., Blanc C., Tharaux P.-L., Rozenberg F., Fischer A., Duffy D., Rieux-Laucat F., Kernéis S., Terrier B. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science. 2020;369(6504):718–724. doi: 10.1126/science.abc6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Zhang Y., Odiwuor N., Xiong J., Sun L., Nyaruaba R.O., Wei H., Tanner N.A. Rapid molecular detection of SARS-CoV-2 (COVID-19) virus RNA using colorimetric LAMP. MedRxiv. 2020 [Google Scholar]
- 173.Broughton J.P., Deng X., Yu G., Fasching C.L., Servellita V., Singh J., Miao X., Streithorst J.A., Granados A., Sotomayor-Gonzalez A., Zorn K., Gopez A., Hsu E., Gu W., Miller S., Pan C.-Y., Guevara H., Wadford D.A., Chen J.S., Chiu C.Y. CRISPR–Cas12-based detection of SARS-CoV-2. Nat. Biotechnol. 2020;38(7):870–874. doi: 10.1038/s41587-020-0513-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Qiu M., Li P. CRISPR/Cas-based Diagnostics and Gene Therapy. BIO Integration. 2021;2 [Google Scholar]
- 175.Sethuraman N., Jeremiah S.S., Ryo A. Interpreting diagnostic tests for SARS-CoV-2. JAMA. 2020;323(22):2249–2251. doi: 10.1001/jama.2020.8259. [DOI] [PubMed] [Google Scholar]
- 176.Kontou P.I., Braliou G.G., Dimou N.L., Nikolopoulos G., Bagos P.G. Antibody tests in detecting SARS-CoV-2 infection: a meta-analysis. Diagnostics. 2020;10(5):319. doi: 10.3390/diagnostics10050319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Wang Y., Li J., Li H., Lei P., Shen G., Yang C. Persistence of SARS-CoV-2-specific antibodies in COVID-19 patients. Int. Immunopharmacol. 2021;90 doi: 10.1016/j.intimp.2020.107271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Venter M., Richter K. Towards effective diagnostic assays for COVID-19: a review. J. Clin. Pathol. 2020;73(7):370–377. doi: 10.1136/jclinpath-2020-206685. [DOI] [PubMed] [Google Scholar]
- 179.Algassim A.A., Elghazaly A.A., Alnahdi A.S., Mohammed-Rahim O.M., Alanazi A.G., Aldhuwayhi N.A., Alanazi M.M., Almutairi M.F., Aldeailej I.M., Kamli N.A., Aljurf M.D. Prognostic significance of hemoglobin level and autoimmune hemolytic anemia in SARS-CoV-2 infection. Ann. Hematol. 2021;100(1):37–43. doi: 10.1007/s00277-020-04256-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Jelkmann W.E., Fandrey J., Frede S., Pagel H. Implications for the anemia involved in inflammatory states. Annals of the New York Academy of Sciences; 1994. Inhibition of erythropoietin production by cytokines; pp. 300–311. [PubMed] [Google Scholar]
- 181.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., Wang B., Xiang H., Cheng Z., Xiong Y., Zhao Y., Li Y., Wang X., Peng Z. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan. China, JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Pan Y., Guan H., Zhou S., Wang Y., Li Q., Zhu T., Hu Q., Xia L. Initial CT findings and temporal changes in patients with the novel coronavirus pneumonia (2019-nCoV): a study of 63 patients in Wuhan, China. European Radiol. 2020;30(6):3306–3309. doi: 10.1007/s00330-020-06731-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Lee E.Y., Ng M.-Y., Khong P.-L. COVID-19 pneumonia: what has CT taught us? Lancet. Infect. Dis. 2020;20(4):384–385. doi: 10.1016/S1473-3099(20)30134-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Ye Z., Zhang Y., Wang Y., Huang Z., Song B. Chest CT manifestations of new coronavirus disease 2019 (COVID-19): a pictorial review. Eur. Radiol. 2020;30(8):4381–4389. doi: 10.1007/s00330-020-06801-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Chung M., Bernheim A., Mei X., Zhang N., Huang M., Zeng X., Cui J., Xu W., Yang Y., Fayad Z.A. CT imaging features of 2019 novel coronavirus (2019-nCoV) Radiology. 2020;295(1):202–207. doi: 10.1148/radiol.2020200230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Li Y., Xia L. Coronavirus disease 2019 (COVID-19): role of chest CT in diagnosis and management. Am. J. Roentgenol. 2020;214(6):1280–1286. doi: 10.2214/AJR.20.22954. [DOI] [PubMed] [Google Scholar]
- 187.Ai T., Yang Z., Hou H., Zhan C., Chen C., Lv W., Tao Q., Sun Z., Xia L. Correlation of Chest CT and RT-PCR Testing for Coronavirus Disease 2019 (COVID-19) in China: A Report of 1014 Cases. Radiology. 2020;296(2):E32–E40. doi: 10.1148/radiol.2020200642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Ziaie S., Koucheck M., Miri M., Salarian S., Shojaei S., Haghighi M., Sistanizad M. Review of therapeutic agents for the treatment of COVID-19. J. Cellular Mol. Anesthesia. 2020;5(1):32–36. [Google Scholar]
- 189.V. Monteil, H. Kwon, P. Prado, A. Hagelkrüys, R.A. Wimmer, M. Stahl, A. Leopoldi, E. Garreta, C.H. Del Pozo, F. Prosper, Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2, Cell 181(4) (2020) 905-913. e7. [DOI] [PMC free article] [PubMed]
- 190.Zhang H., Penninger J.M., Li Y., Zhong N., Slutsky A.S. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020;46(4):586–590. doi: 10.1007/s00134-020-05985-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.B. Beddingfield, N. Iwanaga, W. Zheng, C.J. Roy, T.Y. Hu, J. Kolls, G. Bix, The Integrin Binding Peptide, ATN-161, as a Novel Therapy for SARS-CoV-2 Infection, bioRxiv (2020) 2020.06.15.153387. [DOI] [PMC free article] [PubMed]
- 192.Luan J., Lu Y., Gao S., Zhang L. A potential inhibitory role for integrin in the receptor targeting of SARS-CoV-2. J. Infect. 2020;81(2):318–356. doi: 10.1016/j.jinf.2020.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Savarino A., Boelaert J.R., Cassone A., Majori G., Cauda R. Effects of chloroquine on viral infections: an old drug against today's diseases. Lancet. Infect. Dis. 2003;3(11):722–727. doi: 10.1016/S1473-3099(03)00806-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Lamballerie X.D., Boisson V., Reynier J.-C., Enault S., Charrel R.N., Flahault A., Roques P., Grand R.L. On chikungunya acute infection and chloroquine treatment. Vector-Borne Zoonotic Diseases. 2008;8(6):837–840. doi: 10.1089/vbz.2008.0049. [DOI] [PubMed] [Google Scholar]
- 195.Sanders J.M., Monogue M.L., Jodlowski T.Z., Cutrell J.B. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;323(18):1824–1836. doi: 10.1001/jama.2020.6019. [DOI] [PubMed] [Google Scholar]
- 196.Carrière F., Longhi S., Record M. The endosomal lipid bis (monoacylglycero) phosphate as a potential key player in the mechanism of action of chloroquine against SARS-COV-2 and other enveloped viruses hijacking the endocytic pathway. Biochimie. 2020;179:237–246. doi: 10.1016/j.biochi.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M., Shi Z., Hu Z., Zhong W., Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30(3):269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Gautret P., Lagier J.-C., Parola P., Meddeb L., Mailhe M., Doudier B., Courjon J., Giordanengo V., Vieira V.E., Dupont H.T. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents. 2020;56(1) doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Food and Drug Administration, Letter revoking EUA for chloroquine phosphate and hydroxychloroquine sulfate, 6/15/2020, 2020. https://www.fda.gov/media/138945/download.
- 200.Chan J.F.-W., Yao Y., Yeung M.-L., Deng W., Bao L., Jia L., Li F., Xiao C., Gao H., Yu P. Treatment with lopinavir/ritonavir or interferon-β1b improves outcome of MERS-CoV infection in a nonhuman primate model of common marmoset. J. Infect. Dis. 2015;212(12):1904–1913. doi: 10.1093/infdis/jiv392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Lim J., Jeon S., Shin H.Y., Kim M.J., Seong Y.M., Lee W.J., Choe K.W., Kang Y.M., Lee B., Park S.J. Case of the Index Patient Who Caused Tertiary Transmission of COVID-19 Infection in Korea: the Application of Lopinavir/Ritonavir for the Treatment of COVID-19 Infected Pneumonia Monitored by Quantitative RT-PCR. J. Korean Med. Sci. 2020;35(6):e79. doi: 10.3346/jkms.2020.35.e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Cao B., Wang Y., Wen D., Liu W., Wang J., Fan G., Ruan L., Song B., Cai Y., Wei M., Li X., Xia J., Chen N., Xiang J., Yu T., Bai T., Xie X., Zhang L., Li C., Yuan Y., Chen H., Li H., Huang H., Tu S., Gong F., Liu Y., Wei Y., Dong C., Zhou F., Gu X., Xu J., Liu Z., Zhang Y., Li H., Shang L., Wang K., Li K., Zhou X., Dong X., Qu Z., Lu S., Hu X., Ruan S., Luo S., Wu J., Peng L., Cheng F., Pan L., Zou J., Jia C., Wang J., Liu X., Wang S., Wu X., Ge Q., He J., Zhan H., Qiu F., Guo L., Huang C., Jaki T., Hayden F.G., Horby P.W., Zhang D., Wang C. A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe Covid-19. N. Engl. J. Med. 2020;382(19):1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Horby P.W., Mafham M., Bell J.L., Linsell L., Staplin N., Emberson J., Palfreeman A., Raw J., Elmahi E., Prudon B. Lopinavir–ritonavir in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. The Lancet. 2020;396(10259):1345–1352. doi: 10.1016/S0140-6736(20)32013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Giacomelli A., Pagani G., Ridolfo A.L., Oreni L., Conti F., Pezzati L., Bradanini L., Casalini G., Bassoli C., Morena V. Early administration of lopinavir/ritonavir plus hydroxychloroquine does not alter the clinical course of SARS-CoV-2 infection: a retrospective cohort study. J. Med. Virol. 2021;93(3):1421–1427. doi: 10.1002/jmv.26407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Nyström K., Waldenström J., Tang K.-W., Lagging M. Ribavirin: pharmacology, multiple modes of action and possible future perspectives. Future Virol. 2019;14(3):153–160. [Google Scholar]
- 206.Khalili J.S., Zhu H., Mak N.S.A., Yan Y., Zhu Y. Novel coronavirus treatment with ribavirin: Groundwork for an evaluation concerning COVID-19. J. Med. Virol. 2020;92(7):740–746. doi: 10.1002/jmv.25798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Hung I.F.-N., Lung K.-C., Tso E.Y.-K., Liu R., Chung T.W.-H., Chu M.-Y., Ng Y.-Y., Lo J., Chan J., Tam A.R., Shum H.-P., Chan V., Wu A.K.-L., Sin K.-M., Leung W.-S., Law W.-L., Lung D.C., Sin S., Yeung P., Yip C.C.-Y., Zhang R.R., Fung A.Y.-F., Yan E.Y.-W., Leung K.-H., Ip J.D., Chu A.W.-H., Chan W.-M., Ng A.C.-K., Lee R., Fung K., Yeung A., Wu T.-C., Chan J.W.-M., Yan W.-W., Chan W.-M., Chan J.F.-W., Lie A.K.-W., Tsang O.T.-Y., Cheng V.C.-C., Que T.-L., Lau C.-S., Chan K.-H., To K.K.-W., Yuen K.-Y. Triple combination of interferon beta-1b, lopinavir–ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. The Lancet. 2020;395(10238):1695–1704. doi: 10.1016/S0140-6736(20)31042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Grein J., Ohmagari N., Shin D., Diaz G., Asperges E., Castagna A., Feldt T., Green G., Green M.L., Lescure F.-X. Compassionate use of remdesivir for patients with severe Covid-19. N. Engl. J. Med. 2020;382(24):2327–2336. doi: 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.World Health Organization, Therapeutics and COVID-19: living guideline, 20 November 2020, 2020. https://apps.who.int/iris/handle/10665/336729. (Accessed WHO/2019-nCov/remdesivir/2020.1.
- 210.Beigel J.H., Tomashek K.M., Dodd L.E., Mehta A.K., Zingman B.S., Kalil A.C., Hohmann E., Chu H.Y., Luetkemeyer A., Kline S., Lopez de Castilla D., Finberg R.W., Dierberg K., Tapson V., Hsieh L., Patterson T.F., Paredes R., Sweeney D.A., Short W.R., Touloumi G., Lye D.C., Ohmagari N., Oh M.-D., Ruiz-Palacios G.M., Benfield T., Fätkenheuer G., Kortepeter M.G., Atmar R.L., Creech C.B., Lundgren J., Babiker A.G., Pett S., Neaton J.D., Burgess T.H., Bonnett T., Green M., Makowski M., Osinusi A., Nayak S., Lane H.C., Members A.-S.G. Remdesivir for the Treatment of Covid-19 - Final Report. New England J. Med. 2020;383(19):1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Wang Y., Zhang D., Du G., Du R., Zhao J., Jin Y., Fu S., Gao L., Cheng Z., Lu Q. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. The Lancet. 2020;395(10236):1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.A. Vitiello, F. Ferrara, R.L. Porta, Remdesivir and COVID-19 infection, therapeutic benefits or unnecessary risks?, Irish Journal of Medical Science (1971 -) (2021). [DOI] [PMC free article] [PubMed]
- 213.W.S.t. consortium, H. Pan, R. Peto, Q.A. Karim, M. Alejandria, A.M. Henao-Restrepo, C.H. García, M.-P. Kieny, R. Malekzadeh, S. Murthy, M.-P. Preziosi, S. Reddy, M.R. Periago, V. Sathiyamoorthy, J.-A. Røttingen, S. Swaminathan, a.r.f.t.c. as the members of the Writing Committee, i.o.t. article, Repurposed antiviral drugs for COVID-19 –interim WHO SOLIDARITY trial results, medRxiv (2020) 2020.10.15.20209817.
- 214.Shrestha D.B., Budhathoki P., Syed N.-I.-H., Rawal E., Raut S., Khadka S. Remdesivir: A potential game-changer or just a myth? A systematic review and meta-analysis. Life Sci. 2021;264 doi: 10.1016/j.lfs.2020.118663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Beigel J.H., Tomashek K.M., Dodd L.E., Mehta A.K., Zingman B.S., Kalil A.C., Hohmann E., Chu H.Y., Luetkemeyer A., Kline S., Lopez de Castilla D., Finberg R.W., Dierberg K., Tapson V., Hsieh L., Patterson T.F., Paredes R., Sweeney D.A., Short W.R., Touloumi G., Lye D.C., Ohmagari N., Oh M.D., Ruiz-Palacios G.M., Benfield T., Fätkenheuer G., Kortepeter M.G., Atmar R.L., Creech C.B., Lundgren J., Babiker A.G., Pett S., Neaton J.D., Burgess T.H., Bonnett T., Green M., Makowski M., Osinusi A., Nayak S., Lane H.C. Remdesivir for the Treatment of Covid-19 - Final Report. N Engl J Med. 2020;383(19):1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Wang Y., Zhang D., Du G., Du R., Zhao J., Jin Y., Fu S., Gao L., Cheng Z., Lu Q., Hu Y., Luo G., Wang K., Lu Y., Li H., Wang S., Ruan S., Yang C., Mei C., Wang Y., Ding D., Wu F., Tang X., Ye X., Ye Y., Liu B., Yang J., Yin W., Wang A., Fan G., Zhou F., Liu Z., Gu X., Xu J., Shang L., Zhang Y., Cao L., Guo T., Wan Y., Qin H., Jiang Y., Jaki T., Hayden F.G., Horby P.W., Cao B., Wang C. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet (London, England) 2020;395(10236):1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.K. Ansems, F. Grundeis, K. Dahms, A. Mikolajewska, V. Thieme, V. Piechotta, M.I. Metzendorf, M. Stegemann, C. Benstoem, F. Fichtner, Remdesivir for the treatment of COVID-19, The Cochrane database of systematic reviews 8(8) (2021) Cd014962. [DOI] [PMC free article] [PubMed]
- 218.A. Izcovich, R.A. Siemieniuk, J.J. Bartoszko, L. Ge, D. Zeraatkar, E. Kum, A.M. Khamis, B. Rochwerg, T. Agoritsas, D.K. Chu, S.L. McLeod, R.A. Mustafa, P.O. Vandvik, R. Brignardello-Petersen, Adverse effects of remdesivir, hydroxychloroquine, and lopinavir/ritonavir when used for COVID-19: systematic review and meta-analysis of randomized trials, medRxiv (2020) 2020.11.16.20232876.
- 219.Siemieniuk R.A., Bartoszko J.J., Ge L., Zeraatkar D., Izcovich A., Kum E., Pardo-Hernandez H., Qasim A., Martinez J.P.D., Rochwerg B., Lamontagne F., Han M.A., Liu Q., Agarwal A., Agoritsas T., Chu D.K., Couban R., Cusano E., Darzi A., Devji T., Fang B., Fang C., Flottorp S.A., Foroutan F., Ghadimi M., Heels-Ansdell D., Honarmand K., Hou L., Hou X., Ibrahim Q., Khamis A., Lam B., Loeb M., Marcucci M., McLeod S.L., Motaghi S., Murthy S., Mustafa R.A., Neary J.D., Rada G., Riaz I.B., Sadeghirad B., Sekercioglu N., Sheng L., Sreekanta A., Switzer C., Tendal B., Thabane L., Tomlinson G., Turner T., Vandvik P.O., Vernooij R.W., Viteri-García A., Wang Y., Yao L., Ye Z., Guyatt G.H., Brignardello-Petersen R. Drug treatments for covid-19: living systematic review and network meta-analysis. BMJ. 2020;370 doi: 10.1136/bmj.m2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Rochwerg B., Agarwal A., Siemieniuk R.A., Agoritsas T., Lamontagne F., Askie L., Lytvyn L., Leo Y.-S., Macdonald H., Zeng L., Amin W., Burhan E., Bausch F.J., Calfee C.S., Cecconi M., Chanda D., Du B., Geduld H., Gee P., Harley N., Hashimi M., Hunt B., Kabra S.K., Kanda S., Kawano-Dourado L., Kim Y.-J., Kissoon N., Kwizera A., Mahaka I., Manai H., Mino G., Nsutebu E., Preller J., Pshenichnaya N., Qadir N., Sabzwari S., Sarin R., Shankar-Hari M., Sharland M., Shen Y., Ranganathan S.S., Souza J.P., Stegemann M., De Sutter A., Ugarte S., Venkatapuram S., Dat V.Q., Vuyiseka D., Wijewickrama A., Maguire B., Zeraatkar D., Bartoszko J.J., Ge L., Brignardello-Petersen R., Owen A., Guyatt G., Diaz J., Jacobs M., Vandvik P.O. A living WHO guideline on drugs for covid-19. BMJ. 2020;370 doi: 10.1136/bmj.m3379. [DOI] [PubMed] [Google Scholar]
- 221.Valencia D.N. Brief review on COVID-19: the 2020 pandemic caused by SARS-CoV-2. Cureus. 2020;12(3) doi: 10.7759/cureus.7386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Xu X., Han M., Li T., Sun W., Wang D., Fu B., Zhou Y., Zheng X., Yang Y., Li X., Zhang X., Pan A., Wei H. Effective treatment of severe COVID-19 patients with tocilizumab. Proc. Natl. Acad. Sci. 2020;117(20):10970–10975. doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Lan S.-H., Lai C.-C., Huang H.-T., Chang S.-P., Lu L.-C., Hsueh P.-R. Tocilizumab for severe COVID-19: a systematic review and meta-analysis. Int. J. Antimicrob. Agents. 2020;56(3) doi: 10.1016/j.ijantimicag.2020.106103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Stone J.H., Frigault M.J., Serling-Boyd N.J., Fernandes A.D., Harvey L., Foulkes A.S., Horick N.K., Healy B.C., Shah R., Bensaci A.M. Efficacy of tocilizumab in patients hospitalized with Covid-19. N. Engl. J. Med. 2020;383(24):2333–2344. doi: 10.1056/NEJMoa2028836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Radbel J., Narayanan N., Bhatt P.J. Use of Tocilizumab for COVID-19-Induced Cytokine Release Syndrome: A Cautionary Case Report. Chest. 2020;158(1):e15–e19. doi: 10.1016/j.chest.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Ahmed M.H., Hassan A. Dexamethasone for the Treatment of Coronavirus Disease (COVID-19): a Review. SN Compr. Clin. Med. 2020:1–10. doi: 10.1007/s42399-020-00610-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Moon C. Dexamethasone to the rescue. Nat. Rev. Immunol. 2020;20(8):463. doi: 10.1038/s41577-020-0394-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Group R.C. Dexamethasone in hospitalized patients with Covid-19. N. Engl. J. Med. 2021;384(8):693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.C.Z. Katherine J Wu, Jonathan Corum, Coronavirus Drug and Treatment Tracker, 2021. https://www.nytimes.com/interactive/2020/science/coronavirus-vaccine-tracker.html.
- 230.Chikanza I.C. Mechanisms of corticosteroid resistance in rheumatoid arthritis: a putative role for the corticosteroid receptor β isoform. Ann. N. Y. Acad. Sci. 2002;966(1):39–48. doi: 10.1111/j.1749-6632.2002.tb04200.x. [DOI] [PubMed] [Google Scholar]
- 231.Newman S., Flower R., Croxtall J. Dexamethasone suppression of IL-1β-induced cyclooxygenase 2 expression is not mediated by lipocortin-1 in A549 cells. Biochem. Biophys. Res. Commun. 1994;202(2):931–939. doi: 10.1006/bbrc.1994.2019. [DOI] [PubMed] [Google Scholar]
- 232.Singh A.K., Majumdar S., Singh R., Misra A. Role of corticosteroid in the management of COVID-19: A systemic review and a Clinician's perspective. Diabetes Metab. Syndr. 2020;14(5):971–978. doi: 10.1016/j.dsx.2020.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Shaikh S., Verma H., Yadav N., Jauhari M., Bullangowda J. Applications of Steroid in Clinical Practice: A Review. ISRN Anesthesiology. 2012;2012 [Google Scholar]
- 234.Ruiz-Irastorza G., Pijoan J.-I., Bereciartua E., Dunder S., Dominguez J., Garcia-Escudero P., Rodrigo A., Gomez-Carballo C., Varona J., Guio L. Second week methyl-prednisolone pulses improve prognosis in patients with severe coronavirus disease 2019 pneumonia: An observational comparative study using routine care data. PLoS ONE. 2020;15(9) doi: 10.1371/journal.pone.0239401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Hu Z., Lv Y., Xu C., Sun W., Chen W., Peng Z., Chen C., Cui X., Jiao D., Cheng C., Chi Y., Wei H., Hu C., Zeng Y., Zhang X., Yi Y. Clinical Use of Short-Course and Low-Dose Corticosteroids in Patients With Non-severe COVID-19 During Pneumonia Progression. Front. Public Health. 2020;8(355) doi: 10.3389/fpubh.2020.00355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Kulasekararaj A.G., Lazana I., Large J., Posadas K., Eagleton H., Lord Villajin J., Zuckerman M., Gandhi S., Marsh J.C.W. Terminal complement inhibition dampens the inflammation during COVID-19. Br. J. Haematol. 2020;190(3):e141–e143. doi: 10.1111/bjh.16916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Holter J.C., Pischke S.E., de Boer E., Lind A., Jenum S., Holten A.R., Tonby K., Barratt-Due A., Sokolova M., Schjalm C., Chaban V., Kolderup A., Tran T., Tollefsrud Gjølberg T., Skeie L.G., Hesstvedt L., Ormåsen V., Fevang B., Austad C., Müller K.E., Fladeby C., Holberg-Petersen M., Halvorsen B., Müller F., Aukrust P., Dudman S., Ueland T., Andersen J.T., Lund-Johansen F., Heggelund L., Dyrhol-Riise A.M., Mollnes T.E. Systemic complement activation is associated with respiratory failure in COVID-19 hospitalized patients. Proc. Natl. Acad. Sci. 2020;117(40):25018–25025. doi: 10.1073/pnas.2010540117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Godino C., Scotti A., Maugeri N., Mancini N., Fominskiy E., Margonato A., Landoni G. Antithrombotic therapy in patients with COVID-19?-Rationale and Evidence. Int. J. Cardiol. 2021;324:261–266. doi: 10.1016/j.ijcard.2020.09.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Mantlo E., Bukreyeva N., Maruyama J., Paessler S., Huang C. Antiviral activities of type I interferons to SARS-CoV-2 infection. Antiviral Res. 2020;179 doi: 10.1016/j.antiviral.2020.104811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Li H., Liu S.-M., Yu X.-H., Tang S.-L., Tang C.-K. Coronavirus disease 2019 (COVID-19): current status and future perspectives. Int. J. Antimicrob. Agents. 2020;55(5) doi: 10.1016/j.ijantimicag.2020.105951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Vijay R., Perlman S. Middle East respiratory syndrome and severe acute respiratory syndrome. Current Opin. Virol. 2016;16:70–76. doi: 10.1016/j.coviro.2016.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Market M., Angka L., Martel A.B., Bastin D., Olanubi O., Tennakoon G., Boucher D.M., Ng J., Ardolino M., Auer R.C. Flattening the COVID-19 curve with natural killer cell based immunotherapies. Front Immunol. 2020;11(1512) doi: 10.3389/fimmu.2020.01512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Zheng M., Gao Y., Wang G., Song G., Liu S., Sun D., Xu Y., Tian Z. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell. Mol. Immunol. 2020;17(5):533–535. doi: 10.1038/s41423-020-0402-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Bezemer G.F.G., Garssen J. TLR9 and COVID-19: A Multidisciplinary Theory of a Multifaceted Therapeutic Target. Front. Pharmacol. 2021;11:601685. doi: 10.3389/fphar.2020.601685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Golchin A. Cell-Based Therapy for Severe COVID-19 Patients: Clinical Trials and Cost-Utility. Stem Cell Rev. Rep. 2021;17(1):56–62. doi: 10.1007/s12015-020-10046-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Hossein-Khannazer N., Shokoohian B., Shpichka A., Aghdaei H.A., Timashev P., Vosough M. Novel therapeutic approaches for treatment of COVID-19. J. Mol. Med. 2020;98:789–803. doi: 10.1007/s00109-020-01927-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Shu L., Niu C., Li R., Huang T., Wang Y., Huang M., Ji N., Zheng Y., Chen X., Shi L., Wu M., Deng K., Wei J., Wang X., Cao Y., Yan J., Feng G. Treatment of severe COVID-19 with human umbilical cord mesenchymal stem cells. Stem Cell Res. Ther. 2020;11(1):361. doi: 10.1186/s13287-020-01875-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Tang L., Jiang Y., Zhu M., Chen L., Zhou X., Zhou C., Ye P., Chen X., Wang B., Xu Z., Zhang Q., Xu X., Gao H., Wu X., Li D., Jiang W., Qu J., Xiang C., Li L. Clinical study using mesenchymal stem cells for the treatment of patients with severe COVID-19. Front. Med. 2020;14(5):664–673. doi: 10.1007/s11684-020-0810-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Sánchez-Guijo F., García-Arranz M., López-Parra M., Monedero P., Mata-Martínez C., Santos A., Sagredo V., Álvarez-Avello J.-M., Guerrero J.E., Pérez-Calvo C., Sánchez-Hernández M.-V., Del-Pozo J.L., Andreu E.J., Fernández-Santos M.-E., Soria-Juan B., Hernández-Blasco L.M., Andreu E., Sempere J.M., Zapata A.G., Moraleda J.M., Soria B., Fernández-Avilés F., García-Olmo D., Prósper F. Adipose-derived mesenchymal stromal cells for the treatment of patients with severe SARS-CoV-2 pneumonia requiring mechanical ventilation. A proof of concept study, EClinicalMedicine. 2020;25:10454. doi: 10.1016/j.eclinm.2020.100454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Golchin A., Seyedjafari E., Ardeshirylajimi A. Mesenchymal stem cell therapy for COVID-19: present or future. Stem Cell Rev. Rep. 2020;16(3):427–433. doi: 10.1007/s12015-020-09973-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Can A., Coskun H. The rationale of using mesenchymal stem cells in patients with COVID-19-related acute respiratory distress syndrome: What to expect. Stem Cells Translat. Med. 2020;9(11):1287–1302. doi: 10.1002/sctm.20-0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Alzahrani F.A., Saadeldin I.M., Ahmad A., Kumar D., Azhar E.I., Siddiqui A.J., Kurdi B., Sajini A., Alrefaei A.F., Jahan S. The Potential Use of Mesenchymal Stem Cells and Their Derived Exosomes as Immunomodulatory Agents for COVID-19 Patients. Stem Cells International. 2020;2020:8835986. doi: 10.1155/2020/8835986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Gniffke E.P., Harrington W.E., Dambrauskas N., Jiang Y., Trakhimets O., Vigdorovich V., Frenkel L., Sather D.N., Smith S.E.P. Plasma From Recovered COVID-19 Patients Inhibits Spike Protein Binding to ACE2 in a Microsphere-Based Inhibition Assay. J. Infect. Dis. 2020;222(12):1965–1973. doi: 10.1093/infdis/jiaa508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Liu L., Wang P., Nair M.S., Yu J., Rapp M., Wang Q., Luo Y., Chan J.F.-W., Sahi V., Figueroa A. Potent neutralizing antibodies against multiple epitopes on SARS-CoV-2 spike. Nature. 2020;584(7821):450–456. doi: 10.1038/s41586-020-2571-7. [DOI] [PubMed] [Google Scholar]
- 255.Pau A.K., Aberg J., Baker J., Belperio P.S., Coopersmith C., Crew P., Grund B., Gulick R.M., Harrison C., Kim A., Lane H.C., Masur H., Sheikh V., Singh K., Yazdany J., Tebas P. Convalescent Plasma for the Treatment of COVID-19: Perspectives of the National Institutes of Health COVID-19 Treatment Guidelines Panel. Ann. Intern. Med. 2021;174(1):93–95. doi: 10.7326/M20-6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.M.J. Joyner, J.W. Senefeld, S.A. Klassen, J.R. Mills, P.W. Johnson, E.S. Theel, C.C. Wiggins, K.A. Bruno, A.M. Klompas, E.R. Lesser, K.L. Kunze, M.A. Sexton, J.C. Diaz Soto, S.E. Baker, J.R.A. Shepherd, N. van Helmond, N.S. Paneth, D. Fairweather, R.S. Wright, R.E. Carter, A. Casadevall, t.U.E.C.-P. Consortium, Effect of Convalescent Plasma on Mortality among Hospitalized Patients with COVID-19: Initial Three-Month Experience, medRxiv (2020) 2020.08.12.20169359.
- 257.Joyner M.J., Bruno K.A., Klassen S.A., Kunze K.L., Johnson P.W., Lesser E.R., Wiggins C.C., Senefeld J.W., Klompas A.M., Hodge D.O., Shepherd J.R.A., Rea R.F., Whelan E.R., Clayburn A.J., Spiegel M.R., Baker S.E., Larson K.F., Ripoll J.G., Andersen K.J., Buras M.R., Vogt M.N.P., Herasevich V., Dennis J.J., Regimbal R.J., Bauer P.R., Blair J.E., van Buskirk C.M., Winters J.L., Stubbs J.R., van Helmond N., Butterfield B.P., Sexton M.A., Diaz Soto J.C., Paneth N.S., Verdun N.C., Marks P., Casadevall A., Fairweather D., Carter R.E., Wright R.S. Safety Update: COVID-19 Convalescent Plasma in 20,000 Hospitalized Patients. Mayo Clin. Proc. 2020;95(9):1888–1897. doi: 10.1016/j.mayocp.2020.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Joyner M.J., Wright R.S., Fairweather D., Senefeld J.W., Bruno K.A., Klassen S.A., Carter R.E., Klompas A.M., Wiggins C.C., Shepherd J.R.A., Rea R.F., Whelan E.R., Clayburn A.J., Spiegel M.R., Johnson P.W., Lesser E.R., Baker S.E., Larson K.F., Ripoll J.G., Andersen K.J., Hodge D.O., Kunze K.L., Buras M.R., Vogt M.N.P., Herasevich V., Dennis J.J., Regimbal R.J., Bauer P.R., Blair J.E., Van Buskirk C.M., Winters J.L., Stubbs J.R., Paneth N.S., Verdun N.C., Marks P., Casadevall A. Early safety indicators of COVID-19 convalescent plasma in 5000 patients. J. Clin. Investig. 2020;130(9):4791–4797. doi: 10.1172/JCI140200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Stratton C.W., Tang Y.-W., Lu H. Pathogenesis-directed therapy of 2019 novel coronavirus disease. J. Med. Virol. 2021;93(3):1320–1342. doi: 10.1002/jmv.26610. [DOI] [PubMed] [Google Scholar]
- 260.Leng Z., Zhu R., Hou W., Feng Y., Yang Y., Han Q., Shan G., Meng F., Du D., Wang S. Transplantation of ACE2-mesenchymal stem cells improves the outcome of patients with COVID-19 pneumonia. Aging Dis. 2020;11(2):216. doi: 10.14336/AD.2020.0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Ghosh S., Firdous S.M., Nath A. siRNA could be a potential therapy for COVID-19. EXCLI J. 2020;19:528–531. doi: 10.17179/excli2020-1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Liu C., Zhou Q., Li Y., Garner L.V., Watkins S.P., Carter L.J., Smoot J., Gregg A.C., Daniels A.D., Jervey S., Albaiu D. Research and Development on Therapeutic Agents and Vaccines for COVID-19 and Related Human Coronavirus Diseases. ACS Cent. Sci. 2020;6(3):315–331. doi: 10.1021/acscentsci.0c00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Wang Z., Ren L., Zhao X., Hung T., Meng A., Wang J., Chen Y.G. Inhibition of severe acute respiratory syndrome virus replication by small interfering RNAs in mammalian cells. J. Virol. 2004;78(14):7523–7527. doi: 10.1128/JVI.78.14.7523-7527.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Kalhori M.R., Saadatpour F., Arefian E., Soleimani M., Farzaei M.H., Aneva I.Y., Echeverría J. The Potential Therapeutic Effect of RNA Interference and Natural Products on COVID-19: A Review of the Coronaviruses Infection. Front. Pharmacol. 2021;12(116) doi: 10.3389/fphar.2021.616993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Lu D., Chatterjee S., Xiao K., Riedel I., Wang Y., Foo R., Bär C., Thum T. MicroRNAs targeting the SARS-CoV-2 entry receptor ACE2 in cardiomyocytes. J. Mol. Cell. Cardiol. 2020;148:46–49. doi: 10.1016/j.yjmcc.2020.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Matarese A., Gambardella J., Sardu C., Santulli G. miR-98 regulates TMPRSS2 expression in human endothelial cells: key implications for COVID-19. Biomedicines. 2020;8(11):462. doi: 10.3390/biomedicines8110462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.W. Emanuel, M. Kirstin, F. Vedran, D. Asija, G.L. Theresa, A. Roberto, K. Filippos, K. David, A. Salah, B. Christopher, R. Anja, L. Ivano, I. Andranik, M. Tommaso, D.G. Simone, P.J. Patrick, M.M. Alexander, N. Daniela, S. Matthias, A. Altuna, R. Nikolaus, D. Christian, L. Markus, Bulk and single-cell gene expression profiling of SARS-CoV-2 infected human cell lines identifies molecular targets for therapeutic intervention, bioRxiv (2020) 2020.05.05.079194.
- 268.Zhu Z., Lu Z., Xu T., Chen C., Yang G., Zha T., Lu J., Xue Y. Arbidol monotherapy is superior to lopinavir/ritonavir in treating COVID-19. J. Infect. 2020;81(1):e21–e23. doi: 10.1016/j.jinf.2020.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Vankadari N. Arbidol: A potential antiviral drug for the treatment of SARS-CoV-2 by blocking trimerization of the spike glycoprotein. Int. J. Antimicrob. Agents. 2020;56(2) doi: 10.1016/j.ijantimicag.2020.105998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Yamamoto M., Kiso M., Sakai-Tagawa Y., Iwatsuki-Horimoto K., Imai M., Takeda M., Kinoshita N., Ohmagari N., Gohda J., Semba K. The anticoagulant nafamostat potently inhibits SARS-CoV-2 S protein-mediated fusion in a cell fusion assay system and viral infection in vitro in a cell-type-dependent manner. Viruses. 2020;12(6):629. doi: 10.3390/v12060629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Hoffmann M., Schroeder S., Kleine-Weber H., Müller M.A., Drosten C., Pöhlmann S. Nafamostat Mesylate Blocks Activation of SARS-CoV-2: New Treatment Option for COVID-19. Antimicrob Agents Chemother. 2020;64(6) doi: 10.1128/AAC.00754-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Cao B., Wang Y., Wen D., Liu W., Wang J., Fan G., Ruan L., Song B., Cai Y., Wei M. A trial of lopinavir–ritonavir in adults hospitalized with severe Covid-19. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Li Y., Garner L.V., Watkins S.P., Carter L.J., Smoot J., Gregg A.C., Daniels A.D., Jervey S., Albaiu D. Research and development on therapeutic agents and vaccines for COVID-19 and related human coronavirus diseases. ACS Cent. Sci. 2020;6(3):315–331. doi: 10.1021/acscentsci.0c00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Mu J., Xu J., Zhang L., Shu T., Wu D., Huang M., Ren Y., Li X., Geng Q., Xu Y., Qiu Y., Zhou X. SARS-CoV-2-encoded nucleocapsid protein acts as a viral suppressor of RNA interference in cells, Science China. Life Sci. 2020;63(9):1413–1416. doi: 10.1007/s11427-020-1692-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Khan M., Sany M., Us R., Islam M., Islam A.B.M.M. Epigenetic regulator miRNA pattern differences among SARS-CoV, SARS-CoV-2, and SARS-CoV-2 world-wide isolates delineated the mystery behind the epic pathogenicity and distinct clinical characteristics of pandemic COVID-19. Front. Genet. 2020;11:765. doi: 10.3389/fgene.2020.00765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Mirzaei R., Mahdavi F., Badrzadeh F., Hosseini-Fard S.R., Heidary M., Jeda A.S., Mohammadi T., Roshani M., Yousefimashouf R., Keyvani H., Darvishmotevalli M., Sani M.Z., Karampoor S. The emerging role of microRNAs in the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Int. Immunopharmacol. 2021;90:107204. doi: 10.1016/j.intimp.2020.107204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Luo P., Liu Y., Qiu L., Liu X., Liu D., Li J. Tocilizumab treatment in COVID-19: A single center experience. J. Med. Virol. 2020;92(7):814–818. doi: 10.1002/jmv.25801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Bezzio C., Manes G., Bini F., Pellegrini L., Saibeni S. Infliximab for severe ulcerative colitis and subsequent SARS-CoV-2 pneumonia: a stone for two birds. Gut. 2021;70(3):623–624. doi: 10.1136/gutjnl-2020-321760. [DOI] [PubMed] [Google Scholar]
- 279.Robinson P.C., Richards D., Tanner H.L., Feldmann M. Accumulating evidence suggests anti-TNF therapy needs to be given trial priority in COVID-19 treatment. Lancet Rheumatol. 2020;2(11):e653–e655. doi: 10.1016/S2665-9913(20)30309-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Risitano A.M., Mastellos D.C., Huber-Lang M., Yancopoulou D., Garlanda C., Ciceri F., Lambris J.D. Complement as a target in COVID-19? Nat. Rev. Immunol. 2020;20(6):343–344. doi: 10.1038/s41577-020-0320-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Mastaglio S., Ruggeri A., Risitano A.M., Angelillo P., Yancopoulou D., Mastellos D.C., Huber-Lang M., Piemontese S., Assanelli A., Garlanda C., Lambris J.D., Ciceri F. The first case of COVID-19 treated with the complement C3 inhibitor AMY-101. Clinical Immunol. 2020;215 doi: 10.1016/j.clim.2020.108450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Rother R.P., Rollins S.A., Mojcik C.F., Brodsky R.A., Bell L. Discovery and development of the complement inhibitor eculizumab for the treatment of paroxysmal nocturnal hemoglobinuria. Nat. Biotechnol. 2007;25(11):1256–1264. doi: 10.1038/nbt1344. [DOI] [PubMed] [Google Scholar]
- 283.Stratton C.W., Tang Y.W., Lu H. Pathogenesis-directed therapy of 2019 novel coronavirus disease. J. Med. Virol. 2020;93(3):1320–1342. doi: 10.1002/jmv.26610. [DOI] [PubMed] [Google Scholar]
- 284.Duan K., Liu B., Li C., Zhang H., Yu T., Qu J., Zhou M., Chen L., Meng S., Hu Y. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc. Natl. Acad. Sci. 2020;117(17):9490–9496. doi: 10.1073/pnas.2004168117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Rajarshi K., Chatterjee A., Ray S. Combating COVID-19 with mesenchymal stem cell therapy. Biotechnol. Rep, 2020;26 doi: 10.1016/j.btre.2020.e00467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Liu S., Peng D., Qiu H., Yang K., Fu Z., Zou L. Mesenchymal stem cells as a potential therapy for COVID-19. Stem Cell Res. Ther. 2020;11:1–4. doi: 10.1186/s13287-020-01678-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Shetty A.K. Mesenchymal Stem Cell Infusion Shows Promise for Combating Coronavirus (COVID-19)- Induced Pneumonia. Aging Dis. 2020;11(2):462–464. doi: 10.14336/AD.2020.0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Marini J.J., Gattinoni L. Management of COVID-19 Respiratory Distress. JAMA. 2020;323(22):2329–2330. doi: 10.1001/jama.2020.6825. [DOI] [PubMed] [Google Scholar]
- 289.Herold T., Jurinovic V., Arnreich C., Lipworth B.J., Hellmuth J.C., von Bergwelt-Baildon M., Klein M., Weinberger T. Elevated levels of IL-6 and CRP predict the need for mechanical ventilation in COVID-19. J. Allergy Clin. Immunol. 2020;146(1):128–136.e4. doi: 10.1016/j.jaci.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Mahase E. Covid-19: most patients require mechanical ventilation in first 24 hours of critical care. BMJ. 2020;368 doi: 10.1136/bmj.m1201. [DOI] [PubMed] [Google Scholar]
- 291.Hanna R., Dalvi S., Sălăgean T., Pop I.D., Bordea I.R., Benedicenti S. Understanding COVID-19 pandemic: molecular mechanisms and potential therapeutic strategies. An evidence-based review. J. Inflamm. Res. 2021;14:13. doi: 10.2147/JIR.S282213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Song J., Zhang L., Xu Y., Yang D., Yang S., Zhang W., Wang J., Tian S., Yang S., Yuan T. The comprehensive study on the therapeutic effects of baicalein for the treatment of COVID-19 in vivo and in vitro. Biochem. Pharmacol. 2021;183 doi: 10.1016/j.bcp.2020.114302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Khan N., Chen X., Geiger J.D. Possible Therapeutic Use of Natural Compounds Against COVID-19. J Cell Signal. 2021;2(1):63–79. [PMC free article] [PubMed] [Google Scholar]
- 294.Derosa G., Maffioli P., D'Angelo A., Di Pierro F. A role for quercetin in coronavirus disease 2019 (COVID-19) Phytother. Res. 2021;35(3):1230–1236. doi: 10.1002/ptr.6887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Lu H., Huo N., Wang G., Li H., Nie L., Xu X. Clinical observation of therapeutic effect of compound glycyrrhizin on SARS. China Pharm. 2003;10:34–36. [Google Scholar]
- 296.Chang R., Ng T.B., Sun W.Z. Lactoferrin as potential preventative and adjunct treatment for COVID-19. Int. J. Antimicrob. Agents. 2020;56(3) doi: 10.1016/j.ijantimicag.2020.106118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Mikkelsen K., Apostolopoulos V. Nutrition and immunity. Springer; 2019. Vitamin B1, B2, B3, B5, and B6 and the Immune System; pp. 115–125. [Google Scholar]
- 298.Michele C.A., Angel B., Valeria L., Teresa M., Giuseppe C., Giovanni M., Ernestina P., Mario B. Vitamin supplements in the Era of SARS-Cov2 pandemic. GSC Biolog. Pharm. Sci. 2020;11(2):007–019. [Google Scholar]
- 299.Ebadi M., Montano-Loza A.J. Perspective: improving vitamin D status in the management of COVID-19. Eur. J. Clin. Nutr. 2020;74(6):856–859. doi: 10.1038/s41430-020-0661-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Panarese A., Shahini E. Covid-19, and vitamin D. Alimentary Pharmacol. Therapeut. 2020;51(10):993. doi: 10.1111/apt.15752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Raha S., Mallick R., Basak S., Duttaroy A.K. Is copper beneficial for COVID-19 patients? Med. Hypotheses. 2020;109814 doi: 10.1016/j.mehy.2020.109814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Tan C.W., Ho L.P., Kalimuddin S., Cherng B.P.Z., Teh Y.E., Thien S.Y., Wong H.M., Tern P.J.W., Chandran M., Chay J.W.M., Nagarajan C., Sultana R., Low J.G.H., Ng H.J. Cohort study to evaluate the effect of vitamin D, magnesium, and vitamin B(12) in combination on progression to severe outcomes in older patients with coronavirus (COVID-19) Nutrition. 2020;79–80 doi: 10.1016/j.nut.2020.111017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Zabetakis I., Lordan R., Norton C., Tsoupras A. COVID-19: The Inflammation Link and the Role of Nutrition in Potential Mitigation. Nutrients. 2020;12(5):1466. doi: 10.3390/nu12051466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Carr A.C., Rowe S. The Emerging Role of Vitamin C in the Prevention and Treatment of COVID-19. Nutrients. 2020;12(11):3286. doi: 10.3390/nu12113286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Alzaman N.S., Dawson-Hughes B., Nelson J., D’Alessio D., Pittas A.G. Vitamin D status of black and white Americans and changes in vitamin D metabolites after varied doses of vitamin D supplementation. American J. Clin. Nutrit. 2016;104(1):205–214. doi: 10.3945/ajcn.115.129478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Yancy C.W. COVID-19 and African Americans. JAMA. 2020;323(19):1891–1892. doi: 10.1001/jama.2020.6548. [DOI] [PubMed] [Google Scholar]
- 307.I.Z. Pugach, S. Pugach, Strong Correlation Between Prevalence of Severe Vitamin D Deficiency and Population Mortality Rate from COVID-19 in Europe, medRxiv (2020) 2020.06.24.20138644. [DOI] [PMC free article] [PubMed]
- 308.Hastie C.E., Mackay D.F., Ho F., Celis-Morales C.A., Katikireddi S.V., Niedzwiedz C.L., Jani B.D., Welsh P., Mair F.S., Gray S.R., O’Donnell C.A., Gill J.M.R., Sattar N., Pell J.P. Vitamin D concentrations and COVID-19 infection in UK Biobank. Diabetes Metabolic Syndrome Clin. Res. Rev. 2020;14(4):561–565. doi: 10.1016/j.dsx.2020.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Smith S.M., Boppana A., Traupman J.A., Unson E., Maddock D.A., Chao K., Dobesh D.P., Brufsky A., Connor R.I. Impaired glucose metabolism in patients with diabetes, prediabetes, and obesity is associated with severe COVID-19. J. Med. Virol. 2021;93(1):409–415. doi: 10.1002/jmv.26227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Dietz W., Santos-Burgoa C. Obesity and its Implications for COVID-19 Mortality, Obesity. (Silver Spring Md.) 2020;28(6):1005. doi: 10.1002/oby.22818. [DOI] [PubMed] [Google Scholar]
- 311.Butler M.J., Barrientos R.M. The impact of nutrition on COVID-19 susceptibility and long-term consequences. Brain Behav Immun. 2020;87:53–54. doi: 10.1016/j.bbi.2020.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Laviano A., Koverech A., Zanetti M. Nutrition support in the time of SARS-CoV-2 (COVID-19) Nutrition. 2020;74:110834. doi: 10.1016/j.nut.2020.110834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Pronker E.S., Weenen T.C., Commandeur H., Claassen E.H., Osterhaus A.D. Risk in vaccine research and development quantified. PLoS ONE. 2013;8(3) doi: 10.1371/journal.pone.0057755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Verch T., Trausch J.J., Shank-Retzlaff M. Principles of vaccine potency assays. Bioanalysis. 2018;10(3):163–180. doi: 10.4155/bio-2017-0176. [DOI] [PubMed] [Google Scholar]
- 315.M. Vaidya, Race to develop mRNA flu vaccines will contend with a longer review than Covid-19, 2021. https://www.clinicaltrialsarena.com/analysis/mrna-flu-vaccine-safety/.
- 316.W. Shang, Y. Yang, Y. Rao, X.J.n.V. Rao, The outbreak of SARS-CoV-2 pneumonia calls for viral vaccines, 5(1) (2020) 1-3. [DOI] [PMC free article] [PubMed]
- 317.Rauch S., Jasny E., Schmidt K.E., Petsch B. New vaccine technologies to combat outbreak situations. Front. Immunol. 2018;9:1963. doi: 10.3389/fimmu.2018.01963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Enjuanes L., DeDiego M.L., Álvarez E., Deming D., Sheahan T., Baric R. Vaccines to prevent severe acute respiratory syndrome coronavirus-induced disease. Virus Res. 2008;133(1):45–62. doi: 10.1016/j.virusres.2007.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Bendel D. Safety and Immunogenicity of COVI-VAC, a Live Attenuated Vaccine. Against COVID-19. 2020 https://clinicaltrials.gov/ct2/show/ NCT04619628. [Google Scholar]
- 320.M.F. Tioni, R. Jordan, A.S. Pena, A. Garg, D. Wu, S.I. Phan, X. Cheng, J. Greenhouse, T. Orekov, D. Valentin, S. Kar, L. Pessaint, H. Andersen, C.C. Stobart, M.H. Bloodworth, R.S. Peebles, Y. Liu, X. Xie, P.-Y. Shi, M.L. Moore, R.S. Tang, One mucosal administration of a live attenuated recombinant COVID-19 vaccine protects nonhuman primates from SARS-CoV-2, bioRxiv (2021) 2021.07.16.452733.
- 321.O. Medzihradsky, Safety and Immunogenicity of an Intranasal RSV Vaccine Expressing SARS-CoV-2 Spike Protein (COVID-19 Vaccine) in Adults, 2021. https://clinicaltrials.gov/ct2/show/NCT04798001?term=NCT04798001&draw=2&rank=1.
- 322.Tsunetsugu-Yokota Y., Ato M., Takahashi Y., Hashimoto S.-I., Kaji T., Kuraoka M., Yamamoto K.-I., Mitsuki Y.-Y., Yamamoto T., Oshima M. Formalin-treated UV-inactivated SARS coronavirus vaccine retains its immunogenicity and promotes Th2-type immune responses. Japanese J. Infect. Dis. 2007;60(2/3):106. [PubMed] [Google Scholar]
- 323.Jara A., Undurraga E.A., González C., Paredes F., Fontecilla T., Jara G., Pizarro A., Acevedo J., Leo K., Leon F., Sans C., Leighton P., Suárez P., García-Escorza H., Araos R. Effectiveness of an Inactivated SARS-CoV-2 Vaccine in Chile. N. Engl. J. Med. 2021;385(10):875–884. doi: 10.1056/NEJMoa2107715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Schindewolf C., Menachery V.D. Middle east respiratory syndrome vaccine candidates: cautious optimism. Viruses. 2019;11(1):74. doi: 10.3390/v11010074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Prüβ B.M. Current State of the First COVID-19 Vaccines. Vaccines. 2021;9(1):30. doi: 10.3390/vaccines9010030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Hodgson J. The pandemic pipeline. Nat. Biotechnol. 2020;38(5):523–532. doi: 10.1038/d41587-020-00005-z. [DOI] [PubMed] [Google Scholar]
- 327.COVID-19 Vaccines Authorized for Emergency Use, 2021. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/covid-19-vaccines. [PMC free article] [PubMed]
- 328.Saif L.J. Vaccines for COVID-19: perspectives, prospects, and challenges based on candidate SARS, MERS, and animal coronavirus vaccines. Euro Med. J. 2020;200324 10.33590. [Google Scholar]
- 329.Ju B., Zhang Q., Ge J., Wang R., Sun J., Ge X., Yu J., Shan S., Zhou B., Song S., Tang X., Yu J., Lan J., Yuan J., Wang H., Zhao J., Zhang S., Wang Y., Shi X., Liu L., Zhao J., Wang X., Zhang Z., Zhang L. Human neutralizing antibodies elicited by SARS-CoV-2 infection. Nature. 2020;584(7819):115–119. doi: 10.1038/s41586-020-2380-z. [DOI] [PubMed] [Google Scholar]
- 330.Steensels D., Pierlet N., Penders J., Mesotten D., Heylen L. Comparison of SARS-CoV-2 Antibody Response Following Vaccination With BNT162b2 and mRNA-1273. JAMA. 2021 doi: 10.1001/jama.2021.15125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Moderna, Moderna COVID-19 Vaccine Retains Neutralizing Activity Against Emerging Variants First Identified in the U.K. and the Republic of South Africa, 2021. https://investors.modernatx.com/news-releases/news-release-details/moderna-covid-19-vaccine-retains-neutralizing-activity-against.
- 332.P. Wang, L. Liu, S. Iketani, Y. Luo, Y. Guo, M. Wang, J. Yu, B. Zhang, P.D. Kwong, B.S. Graham, J.R. Mascola, J.Y. Chang, M.T. Yin, M. Sobieszczyk, C.A. Kyratsous, L. Shapiro, Z. Sheng, M.S. Nair, Y. Huang, D.D. Ho, Increased Resistance of SARS-CoV-2 Variants B.1.351 and B.1.1.7 to Antibody Neutralization, bioRxiv (2021) 2021.01.25.428137.
- 333.T. Meng, H. Cao, H. Zhang, Z. Kang, D. Xu, H. Gong, J. Wang, Z. Li, X. Cui, H. Xu, H. Wei, X. Pan, R. Zhu, J. Xiao, W. Zhou, L. Cheng, J. Liu, The insert sequence in SARS-CoV-2 enhances spike protein cleavage by TMPRSS, bioRxiv (2020) 2020.02.08.926006.
- 334.N. Teruel, O. Mailhot, R.J. Najmanovich, Modelling conformational state dynamics and its role on infection for SARS-CoV-2 Spike protein variants, bioRxiv (2021) 2020.12.16.423118. [DOI] [PMC free article] [PubMed]
- 335.Lopez Bernal J., Andrews N., Gower C., Gallagher E., Simmons R., Thelwall S., Stowe J., Tessier E., Groves N., Dabrera G., Myers R., Campbell C.N.J., Amirthalingam G., Edmunds M., Zambon M., Brown K.E., Hopkins S., Chand M., Ramsay M. Effectiveness of Covid-19 Vaccines against the B.1.617.2 (Delta) Variant. N. Engl. J. Med. 2021;385(7):585–594. doi: 10.1056/NEJMoa2108891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Souza WMd A.M., Sesti-Costa R., Coimbra L.D., Toledo-Teixeira D.A.d. Levels of SARS-CoV-2 Lineage P.1 Neutralization by Antibodies Elicited after Natural Infection and Vaccination. The Lancet PPR290427. 2012 [Google Scholar]
- 337.P. Yadav, G.N. Sapkal, P. Abraham, R. Ella, G. Deshpande, D.Y. Patil, D. Nyayanit, N. Gupta, R.R. Sahay, A.M. Shete, S. Panda, B. Bhargava, V.K. Mohan, Neutralization of variant under investigation B.1.617 with sera of BBV152 vaccinees, bioRxiv (2021) 2021.04.23.441101. [DOI] [PubMed]
- 338.V.M. Andrade, A. Christensen-Quick, J. Agnes, J. Tur, C. Reed, R. Kalia, I. Marrero, D. Elwood, K. Schultheis, M. Purwar, E. Reuschel, T. McMullan, P. Pezzoli, K. Kraynyak, A. Sylvester, M.P. Mammen, P. Tebas, J.J. Kim, D.B. Weiner, T.R.F. Smith, S.J. Ramos, L.M. Humeau, J.D. Boyer, K.E. Broderick, INO-4800 DNA Vaccine Induces Neutralizing Antibodies and T cell Activity Against Global SARS-CoV-2 Variants, bioRxiv (2021) 2021.04.14.439719. [DOI] [PMC free article] [PubMed]
- 339.Madhi S.A., Baillie V., Cutland C.L., Voysey M., Koen A.L., Fairlie L., Padayachee S.D., Dheda K., Barnabas S.L., Bhorat Q.E., Briner C., Kwatra G., Ahmed K., Aley P., Bhikha S., Bhiman J.N., Bhorat A.a.E., du Plessis J., Esmail A., Groenewald M., Horne E., Hwa S.-H., Jose A., Lambe T., Laubscher M., Malahleha M., Masenya M., Masilela M., McKenzie S., Molapo K., Moultrie A., Oelofse S., Patel F., Pillay S., Rhead S., Rodel H., Rossouw L., Taoushanis C., Tegally H., Thombrayil A., van Eck S., Wibmer C.K., Durham N.M., Kelly E.J., Villafana T.L., Gilbert S., Pollard A.J., de Oliveira T., Moore P.L., Sigal A., Izu A. Efficacy of the ChAdOx1 nCoV-19 Covid-19 Vaccine against the B.1.351 Variant. N. Engl. J. Med. 2021;384(20):1885–1898. doi: 10.1056/NEJMoa2102214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.K.R. Emary, T. Golubchik, P.K. Aley, C.V. Ariani, B. Angus, S. Bibi, B. Blane, D. Bonsall, P. Cicconi, S. Charlton, Efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 variant of concern 202012/01 (B. 1.1. 7): an exploratory analysis of a randomised controlled trial, The Lancet 397(10282) (2021) 1351-1362. [DOI] [PMC free article] [PubMed]
- 341.S. Ikegame, M.N.A. Siddiquey, C.-T. Hung, G. Haas, L. Brambilla, K.Y. Oguntuyo, S. Kowdle, A.E. Vilardo, A. Edelstein, C. Perandones, J.P. Kamil, B. Lee, Qualitatively distinct modes of Sputnik V vaccine-neutralization escape by SARS-CoV-2 Spike variants, medRxiv (2021) 2021.03.31.21254660.
- 342.C.K. Wibmer, F. Ayres, T. Hermanus, M. Madzivhandila, P. Kgagudi, B.E. Lambson, M. Vermeulen, K. van den Berg, T. Rossouw, M. Boswell, V. Ueckermann, S. Meiring, A. von Gottberg, C. Cohen, L. Morris, J.N. Bhiman, P.L. Moore, SARS-CoV-2 501Y.V2 escapes neutralization by South African COVID-19 donor plasma, bioRxiv (2021) 2021.01.18.427166. [DOI] [PubMed]
- 343.Sadoff J., Le Gars M., Shukarev G., Heerwegh D., Truyers C., de Groot A.M., Stoop J., Tete S., Van Damme W., Leroux-Roels I. Safety and immunogenicity of the Ad26.COV2.S COVID-19 vaccine candidate: interim results of a phase 1/2a, double-blind, randomized, placebo-controlled trial. N. Engl. J. Med. 2020.09.23:20199604. [Google Scholar]
- 344.Alter G., Yu J., Liu J., Chandrashekar A., Borducchi E.N., Tostanoski L.H., McMahan K., Jacob-Dolan C., Martinez D.R., Chang A., Anioke T., Lifton M., Nkolola J., Stephenson K.E., Atyeo C., Shin S., Fields P., Kaplan I., Robins H., Amanat F., Krammer F., Baric R.S., Le Gars M., Sadoff J., de Groot A.M., Heerwegh D., Struyf F., Douoguih M., van Hoof J., Schuitemaker H., Barouch D.H. Immunogenicity of Ad26.COV2.S vaccine against SARS-CoV-2 variants in humans. Nature. 2021;596(7871):268–272. doi: 10.1038/s41586-021-03681-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Kupferschmidt K., Vogel G. Vaccine link to serious clotting disorder firms up. Science. 2021;372(6539):220–221. doi: 10.1126/science.372.6539.220. [DOI] [PubMed] [Google Scholar]
- 346.Greinacher A., Thiele T., Warkentin T.E., Weisser K., Kyrle P.A., Eichinger S. Thrombotic Thrombocytopenia after ChAdOx1 nCov-19 Vaccination. N Engl J Med. 2021;384(22):2092–2101. doi: 10.1056/NEJMoa2104840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Mahase E. AstraZeneca vaccine: Blood clots are “extremely rare” and benefits outweigh risks, regulators conclude. BMJ. 2021;373 doi: 10.1136/bmj.n931. [DOI] [PubMed] [Google Scholar]
- 348.Torjesen I. Covid-19: Risk of cerebral blood clots from disease is 10 times that from vaccination, study finds. BMJ. 2021;373 doi: 10.1136/bmj.n1005. [DOI] [PubMed] [Google Scholar]
- 349.MacNeil J.R., Su J.R., Broder K.R., Guh A.Y., Gargano J.W., Wallace M., Hadler S.C., Scobie H.M., Blain A.E., Moulia D., Daley M.F., McNally V.V., Romero J.R., Talbot H.K., Lee G.M., Bell B.P., Oliver S.E. Updated Recommendations from the Advisory Committee on Immunization Practices for Use of the Janssen (Johnson & Johnson) COVID-19 Vaccine After Reports of Thrombosis with Thrombocytopenia Syndrome Among Vaccine Recipients - United States, April 2021. MMWR Morb. Mortal Wkly Rep. 2021;70(17):651–656. doi: 10.15585/mmwr.mm7017e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Mahase E. Covid-19: US suspends Johnson and Johnson vaccine rollout over blood clots. BMJ. 2021;373 doi: 10.1136/bmj.n970. [DOI] [PubMed] [Google Scholar]
- 351.Magro C.M., Mulvey J., Kubiak J., Mikhail S., Suster D., Crowson A.N., Laurence J., Nuovo G. Severe COVID-19: A multifaceted viral vasculopathy syndrome. Ann. Diagnostic Pathology. 2021;50 doi: 10.1016/j.anndiagpath.2020.151645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Pichichero M.E. Vaccine-induced immunologic memory and pace of pathogenesis: predicting the need for boosters. Expert Rev. Vacc. 2008;7(9):1299–1303. doi: 10.1586/14760584.7.9.1299. [DOI] [PubMed] [Google Scholar]
- 353.Voysey M., Clemens S.A.C., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., Angus B., Baillie V.L., Barnabas S.L., Bhorat Q.E. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomised trials. The Lancet. 2021;397(10277):881–891. doi: 10.1016/S0140-6736(21)00432-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.T.L. Bricker, T.L. Darling, A.O. Hassan, H.H. Harastani, A. Soung, X. Jiang, Y.-N. Dai, H. Zhao, L.J. Adams, M.J. Holtzman, A.L. Bailey, J.B. Case, D.H. Fremont, R. Klein, M.S. Diamond, A.C.M. Boon, A single intranasal or intramuscular immunization with chimpanzee adenovirus vectored SARS-CoV-2 vaccine protects against pneumonia in hamsters, bioRxiv (2020) 2020.12.02.408823. [DOI] [PMC free article] [PubMed]
- 355.Xia S., Duan K., Zhang Y., Zhao D., Zhang H., Xie Z., Li X., Peng C., Zhang Y., Zhang W., Yang Y., Chen W., Gao X., You W., Wang X., Wang Z., Shi Z., Wang Y., Yang X., Zhang L., Huang L., Wang Q., Lu J., Yang Y., Guo J., Zhou W., Wan X., Wu C., Wang W., Huang S., Du J., Meng Z., Pan A., Yuan Z., Shen S., Guo W., Yang X. Effect of an Inactivated Vaccine Against SARS-CoV-2 on Safety and Immunogenicity Outcomes: Interim Analysis of 2 Randomized Clinical Trials. JAMA. 2020;324(10):951–960. doi: 10.1001/jama.2020.15543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Wang H., Zhang Y., Huang B., Deng W., Quan Y., Wang W., Xu W., Zhao Y., Li N., Zhang J., Liang H., Bao L., Xu Y., Ding L., Zhou W., Gao H., Liu J., Niu P., Zhao L., Zhen W., Fu H., Yu S., Zhang Z., Xu G., Li C., Lou Z., Xu M., Qin C., Wu G., Gao G.F., Tan W., Yang X. Development of an Inactivated Vaccine Candidate, BBIBP-CorV, with Potent Protection against SARS-CoV-2. Cell. 2020;182(3):713–721.e9. doi: 10.1016/j.cell.2020.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.B. Huang, L. Dai, H. Wang, Z. Hu, X. Yang, W. Tan, G.F. Gao, Neutralization of SARS-CoV-2 VOC 501Y.V2 by human antisera elicited by both inactivated BBIBP-CorV and recombinant dimeric RBD ZF2001 vaccines, bioRxiv (2021) 2021.02.01.429069.
- 358.Xia S., Zhang Y., Wang Y., Wang H., Yang Y., Gao G.F., Tan W., Wu G., Xu M., Lou Z., Huang W., Xu W., Huang B., Wang H., Wang W., Zhang W., Li N., Xie Z., Ding L., You W., Zhao Y., Yang X., Liu Y., Wang Q., Huang L., Yang Y., Xu G., Luo B., Wang W., Liu P., Guo W., Yang X. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: a randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet. Infect. Dis. 2021;21(1):39–51. doi: 10.1016/S1473-3099(20)30831-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Z. Wu, Y. Hu, M. Xu, Z. Chen, W. Yang, Z. Jiang, M. Li, H. Jin, G. Cui, P. Chen, L. Wang, G. Zhao, Y. Ding, Y. Zhao, W. Yin, Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine (CoronaVac) in healthy adults aged 60 years and older: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial, The Lancet Infectious Diseases. [DOI] [PMC free article] [PubMed]
- 360.Gao Q., Bao L., Mao H., Wang L., Xu K., Yang M., Li Y., Zhu L., Wang N., Lv Z. Development of an inactivated vaccine candidate for SARS-CoV-2. Science. 2020;369(6499):77–81. doi: 10.1126/science.abc1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Ella R., Vadrevu K.M., Jogdand H., Prasad S., Reddy S., Sarangi V., Ganneru B., Sapkal G., Yadav P., Abraham P., Panda S., Gupta N., Reddy P., Verma S., Kumar Rai S., Singh C., Redkar S.V., Gillurkar C.S., Kushwaha J.S., Mohapatra S., Rao V., Guleria R., Ella K., Bhargava B. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBV152: a double-blind, randomised, phase 1 trial. Lancet. Infect. Dis. 2021;21(5):637–646. doi: 10.1016/S1473-3099(20)30942-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.B. Biotech, Bharat Biotech and ICMR Announce Interim Results from Phase 3 trials of COVAXIN®; Demonstrates overall Interim Clinical Efficacy of 78% and 100% efficacy against Severe COVID-19 disease 2021. https://www.bharatbiotech.com/images/press/covaxin-phase3-clinical-trials-interim-results.pdf.
- 363.Madsen A.M.R., Schaltz-Buchholzer F., Benfield T., Bjerregaard-Andersen M., Dalgaard L.S., Dam C., Ditlev S.B., Faizi G., Johansen I.S., Kofoed P.E., Kristensen G.S., Loekkegaard E.C.L., Mogensen C.B., Mohamed L., Ostenfeld A., Oedegaard E.S., Soerensen M.K., Wejse C., Jensen A.K.G., Nielsen S., Krause T.G., Netea M.G., Aaby P., Benn C.S. Using BCG vaccine to enhance non-specific protection of health care workers during the COVID-19 pandemic: A structured summary of a study protocol for a randomised controlled trial in Denmark. Trials. 2020;21(1):799. doi: 10.1186/s13063-020-04714-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Junqueira-Kipnis A.P., Dos Anjos L.R.B., Barbosa L.C.S., da Costa A.C., Borges K.C.M., Cardoso A., Ribeiro K.M., Rosa S.B.A., Souza C.C., das Neves R.C., Saraiva G., da Silva S.M., Silveira E.A., Rabahi M.F., Conte M.B., Kipnis A. BCG revaccination of health workers in Brazil to improve innate immune responses against COVID-19: A structured summary of a study protocol for a randomised controlled trial. Trials. 2020;21(1):881. doi: 10.1186/s13063-020-04822-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Yitbarek K., Abraham G., Girma T., Tilahun T., Woldie M. The effect of Bacillus Calmette-Guérin (BCG) vaccination in preventing severe infectious respiratory diseases other than TB: Implications for the COVID-19 pandemic. Vaccine. 2020;38(41):6374–6380. doi: 10.1016/j.vaccine.2020.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Keech C., Albert G., Cho I., Robertson A., Reed P., Neal S., Plested J.S., Zhu M., Cloney-Clark S., Zhou H., Smith G., Patel N., Frieman M.B., Haupt R.E., Logue J., McGrath M., Weston S., Piedra P.A., Desai C., Callahan K., Lewis M., Price-Abbott P., Formica N., Shinde V., Fries L., Lickliter J.D., Griffin P., Wilkinson B., Glenn G.M. Phase 1–2 Trial of a SARS-CoV-2 Recombinant Spike Protein Nanoparticle Vaccine. New England J. Med. 2020;383(24):2320–2332. doi: 10.1056/NEJMoa2026920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Callaway E., Mallapaty S. Novavax offers first evidence that COVID vaccines protect people against variants. Nature. 2021;590(7844):17. doi: 10.1038/d41586-021-00268-9. [DOI] [PubMed] [Google Scholar]
- 368.Ryzhikov A., Ryzhikov E., Bogryantseva M., Usova S., Danilenko E., Nechaeva E., Pyankov O., Pyankova O., Gudymo A., Bodnev S. A single blind, placebo-controlled randomized study of the safety, reactogenicity and immunogenicity of the “EpiVacCorona” Vaccine for the prevention of COVID-19, in volunteers aged 18–60 years (phase I-II) Russian J. Infect. Immunity. 2021;11(2):283–296. [Google Scholar]
- 369.S. Yang, Y. Li, L. Dai, J. Wang, P. He, C. Li, X. Fang, C. Wang, X. Zhao, E. Huang, C. Wu, Z. Zhong, F. Wang, X. Duan, S. Tian, L. Wu, Y. Liu, Y. Luo, Z. Chen, F. Li, J. Li, X. Yu, H. Ren, L. Liu, S. Meng, J. Yan, Z. Hu, L. Gao, G.F. Gao, Safety and immunogenicity of a recombinant tandem-repeat dimeric RBD-based protein subunit vaccine (ZF2001) against COVID-19 in adults: two randomised, double-blind, placebo-controlled, phase 1 and 2 trials, The Lancet Infectious Diseases. [DOI] [PMC free article] [PubMed]
- 370.B.J. Ward, P. Gobeil, A. Séguin, J. Atkins, I. Boulay, P.-Y. Charbonneau, M. Couture, M.-A. D’Aoust, J. Dhaliwall, C. Fi2nkle, K. Hager, A. Mahmood, A. Makarkov, M. Cheng, S. Pillet, P. Schimke, S. St-Martin, S. Trépanier, N. Landry, Phase 1 trial of a Candidate Recombinant Virus-Like Particle Vaccine for Covid-19 Disease Produced in Plants, medRxiv (2020) 2020.11.04.20226282.
- 371.Zha L., Chang X., Zhao H., Mohsen M.O., Hong L., Zhou Y., Chen H., Liu X., Zhang J., Li D. Development of a Vaccine against SARS-CoV-2 Based on the Receptor-Binding Domain Displayed on Virus-Like Particles. Vaccines. 2021;9(4):395. doi: 10.3390/vaccines9040395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Gordon C.J., Tchesnokov E.P., Feng J.Y., Porter D.P., Götte M. The antiviral compound remdesivir potently inhibits RNA-dependent RNA polymerase from Middle East respiratory syndrome coronavirus. J. Biol. Chem. 2020;295(15):4773–4779. doi: 10.1074/jbc.AC120.013056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Zhu F.-C., Li Y.-H., Guan X.-H., Hou L.-H., Wang W.-J., Li J.-X., Wu S.-P., Wang B.-S., Wang Z., Wang L., Jia S.-Y., Jiang H.-D., Wang L., Jiang T., Hu Y., Gou J.-B., Xu S.-B., Xu J.-J., Wang X.-W., Wang W., Chen W. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: a dose-escalation, open-label, non-randomised, first-in-human trial. Lancet. 2020;395(10240):1845–1854. doi: 10.1016/S0140-6736(20)31208-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Zhu F.-C., Guan X.-H., Li Y.-H., Huang J.-Y., Jiang T., Hou L.-H., Li J.-X., Yang B.-F., Wang L., Wang W.-J., Wu S.-P., Wang Z., Wu X.-H., Xu J.-J., Zhang Z., Jia S.-Y., Wang B.-S., Hu Y., Liu J.-J., Zhang J., Qian X.-A., Li Q., Pan H.-X., Jiang H.-D., Deng P., Gou J.-B., Wang X.-W., Wang X.-H., Chen W. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2020;396(10249):479–488. doi: 10.1016/S0140-6736(20)31605-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Logunov D.Y., Dolzhikova I.V., Shcheblyakov D.V., Tukhvatulin A.I., Zubkova O.V., Dzharullaeva A.S., Kovyrshina A.V., Lubenets N.L., Grousova D.M., Erokhova A.S., Botikov A.G., Izhaeva F.M., Popova O., Ozharovskaya T.A., Esmagambetov I.B., Favorskaya I.A., Zrelkin D.I., Voronina D.V., Shcherbinin D.N., Semikhin A.S., Simakova Y.V., Tokarskaya E.A., Egorova D.A., Shmarov M.M., Nikitenko N.A., Gushchin V.A., Smolyarchuk E.A., Zyryanov S.K., Borisevich S.V., Naroditsky B.S., Gintsburg A.L. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet. 2021;397(10275):671–681. doi: 10.1016/S0140-6736(21)00234-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Logunov D.Y., Dolzhikova I.V., Zubkova O.V., Tukhvatullin A.I., Shcheblyakov D.V., Dzharullaeva A.S., Grousova D.M., Erokhova A.S., Kovyrshina A.V., Botikov A.G. Safety and immunogenicity of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: two open, non-randomised phase 1/2 studies from Russia. Lancet. 2020;396(10255):887–897. doi: 10.1016/S0140-6736(20)31866-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.M.N. Ramasamy, A.M. Minassian, K.J. Ewer, A.L. Flaxman, P.M. Folegatti, D.R. Owens, M. Voysey, P.K. Aley, B. Angus, G. Babbage, S. Belij-Rammerstorfer, L. Berry, S. Bibi, M. Bittaye, K. Cathie, H. Chappell, S. Charlton, P. Cicconi, E.A. Clutterbuck, R. Colin-Jones, C. Dold, K.R.W. Emary, S. Fedosyuk, M. Fuskova, D. Gbesemete, C. Green, B. Hallis, M.M. Hou, D. Jenkin, C.C.D. Joe, E.J. Kelly, S. Kerridge, A.M. Lawrie, A. Lelliott, M.N. Lwin, R. Makinson, N.G. Marchevsky, Y. Mujadidi, A.P.S. Munro, M. Pacurar, E. Plested, J. Rand, T. Rawlinson, S. Rhead, H. Robinson, A.J. Ritchie, A.L. Ross-Russell, S. Saich, N. Singh, C.C. Smith, M.D. Snape, R. Song, R. Tarrant, Y. Themistocleous, K.M. Thomas, T.L. Villafana, S.C. Warren, M.E.E. Watson, A.D. Douglas, A.V.S. Hill, T. Lambe, S.C. Gilbert, S.N. Faust, A.J. Pollard, J. Aboagye, K. Adams, A. Ali, E.R. Allen, L. Allen, J.L. Allison, F. Andritsou, R. Anslow, E.H. Arbe-Barnes, M. Baker, N. Baker, P. Baker, I. Baleanu, D. Barker, E. Barnes, J.R. Barrett, K. Barrett, L. Bates, A. Batten, K. Beadon, R. Beckley, D. Bellamy, A. Berg, L. Bermejo, E. Berrie, A. Beveridge, K. Bewley, E.M. Bijker, G. Birch, L. Blackwell, H. Bletchly, C.L. Blundell, S.R. Blundell, E. Bolam, E. Boland, D. Bormans, N. Borthwick, K. Boukas, T. Bower, F. Bowring, A. Boyd, T. Brenner, P. Brown, C. Brown-O'Sullivan, S. Bruce, E. Brunt, J. Burbage, J. Burgoyne, K.R. Buttigieg, N. Byard, I. Cabera Puig, S. Camara, M. Cao, F. Cappuccini, M. Carr, M.W. Carroll, P. Cashen, A. Cavey, J. Chadwick, R. Challis, D. Chapman, D. Charles, I. Chelysheva, J.-S. Cho, L. Cifuentes, E. Clark, S. Collins, C.P. Conlon, N.S. Coombes, R. Cooper, C. Cooper, W.E.M. Crocker, S. Crosbie, D. Cullen, C. Cunningham, F. Cuthbertson, B.E. Datoo, L. Dando, M.S. Datoo, C. Datta, H. Davies, S. Davies, E.J. Davis, J. Davis, D. Dearlove, T. Demissie, S. Di Marco, C. Di Maso, D. DiTirro, C. Docksey, T. Dong, F.R. Donnellan, N. Douglas, C. Downing, J. Drake, R. Drake-Brockman, R.E. Drury, S.J. Dunachie, C.J. Edwards, N.J. Edwards, O. El Muhanna, S.C. Elias, R.S. Elliott, M.J. Elmore, M.R. English, S. Felle, S. Feng, C. Ferreira Da Silva, S. Field, R. Fisher, C. Fixmer, K.J. Ford, J. Fowler, E. Francis, J. Frater, J. Furze, P. Galian-Rubio, C. Galloway, H. Garlant, M. Gavrila, F. Gibbons, K. Gibbons, C. Gilbride, H. Gill, K. Godwin, K. Gordon-Quayle, G. Gorini, L. Goulston, C. Grabau, L. Gracie, N. Graham, N. Greenwood, O. Griffiths, G. Gupta, E. Hamilton, B. Hanumunthadu, S.A. Harris, T. Harris, D. Harrison, T.C. Hart, B. Hartnell, L. Haskell, S. Hawkins, J.A. Henry, M. Hermosin Herrera, D. Hill, J. Hill, G. Hodges, S.H.C. Hodgson, K.L. Horton, E. Howe, N. Howell, J. Howes, B. Huang, J. Humphreys, H.E. Humphries, P. Iveson, F. Jackson, S. Jackson, S. Jauregui, H. Jeffers, B. Jones, C.E. Jones, E. Jones, K. Jones, A. Joshi, R. Kailath, J. Keen, D.M. Kelly, S. Kelly, D. Kelly, D. Kerr, L. Khan, B. Khozoee, A. Killen, J. Kinch, L.D.W. King, T.B. King, L. Kingham, P. Klenerman, J.C. Knight, D. Knott, S. Koleva, G. Lang, C.W. Larkworthy, J.P.J. Larwood, R. Law, A. Lee, K.Y.N. Lee, E.A. Lees, S. Leung, Y. Li, A.M. Lias, A. Linder, S. Lipworth, S. Liu, X. Liu, S. Lloyd, L. Loew, R. Lopez Ramon, M. Madhavan, D.O. Mainwaring, G. Mallett, K. Mansatta, S. Marinou, P. Marius, E. Marlow, P. Marriott, J.L. Marshall, J. Martin, S. Masters, J. McEwan, J.L. McGlashan, L. McInroy, N. McRobert, C. Megson, A.J. Mentzer, N. Mirtorabi, C. Mitton, M. Moore, M. Moran, E. Morey, R. Morgans, S.J. Morris, H.M. Morrison, G. Morshead, R. Morter, N.A. Moya, E. Mukhopadhyay, J. Muller, C. Munro, S. Murphy, P. Mweu, A. Noé, F.L. Nugent, K. O'Brien, D. O'Connor, B. Oguti, V. Olchawski, C. Oliveira, P.J. O'Reilly, P. Osborne, L. Owen, N. Owino, P. Papageorgiou, H. Parracho, K. Parsons, B. Patel, M. Patrick-Smith, Y. Peng, E.J. Penn, M.P. Peralta-Alvarez, J. Perring, C. Petropoulos, D.J. Phillips, D. Pipini, S. Pollard, I. Poulton, D. Pratt, L. Presland, P.C. Proud, S. Provstgaard-Morys, S. Pueschel, D. Pulido, R. Rabara, K. Radia, D. Rajapaska, F. Ramos Lopez, H. Ratcliffe, S. Rayhan, B. Rees, E. Reyes Pabon, H. Roberts, I. Robertson, S. Roche, C.S. Rollier, R. Romani, Z. Rose, I. Rudiansyah, S. Sabheha, S. Salvador, H. Sanders, K. Sanders, I. Satti, C. Sayce, A.B. Schmid, E. Schofield, G. Screaton, C. Sedik, S. Seddiqi, R.R. Segireddy, B. Selby, I. Shaik, H.R. Sharpe, R. Shaw, A. Shea, S. Silk, L. Silva-Reyes, D.T. Skelly, D.J. Smith, D.C. Smith, N. Smith, A.J. Spencer, L. Spoors, E. Stafford, I. Stamford, L. Stockdale, D. Stockley, L.V. Stockwell, M. Stokes, L.H. Strickland, A. Stuart, S. Sulaiman, E. Summerton, Z. Swash, A. Szigeti, A. Tahiri-Alaoui, R. Tanner, I. Taylor, K. Taylor, U. Taylor, R. te Water Naude, A. Themistocleous, M. Thomas, T.M. Thomas, A. Thompson, K. Thompson, V. Thornton-Jones, L. Tinh, A. Tomic, S. Tonks, J. Towner, N. Tran, J.A. Tree, A. Truby, C. Turner, R. Turner, M. Ulaszewska, R. Varughese, D. Verbart, M.K. Verheul, I. Vichos, L. Walker, M.E. Wand, B. Watkins, J. Welch, A.J. West, C. White, R. White, P. Williams, M. Woodyer, A.T. Worth, D. Wright, T. Wrin, X.L. Yao, D.-A. Zbarcea, D. Zizi, Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial, The Lancet 396(10267) (2020) 1979-1993 [380] M. Voysey, S.A.C. Clemens, S.A. Madhi, L.Y. Weckx, P.M. Folegatti, P.K. Aley, B. Angus, V.L. Baillie, S.L. Barnabas, Q.E. Bhorat, S. Bibi, C. Briner, P. Cicconi, A.M. Collins, R. Colin-Jones, C.L. Cutland, T.C. Darton, K. Dheda, C.J.A. Duncan, K.R.W. Emary, K.J. Ewer, L. Fairlie, S.N. Faust, S. Feng, D.M. Ferreira, A. Finn, A.L. Goodman, C.M. Green, C.A. Green, P.T. Heath, C. Hill, H. Hill, I. Hirsch, S.H.C. Hodgson, A. Izu, S. Jackson, D. Jenkin, C.C.D. Joe, S. Kerridge, A. Koen, G. Kwatra, R. Lazarus, A.M. Lawrie, A. Lelliott, V. Libri, P.J. Lillie, R. Mallory, A.V.A. Mendes, E.P. Milan, A.M. Minassian, A. McGregor, H. Morrison, Y.F. Mujadidi, A. Nana, P.J. O’Reilly, S.D. Padayachee, A. Pittella, E. Plested, K.M. Pollock, M.N. Ramasamy, S. Rhead, A.V. Schwarzbold, N. Singh, A. Smith, R. Song, M.D. Snape, E. Sprinz, R.K. Sutherland, R. Tarrant, E.C. Thomson, M.E. Török, M. Toshner, D.P.J. Turner, J. Vekemans, T.L. Villafana, M.E.E. Watson, C.J. Williams, A.D. Douglas, A.V.S. Hill, T. Lambe, S.C. Gilbert, A.J. Pollard, M. Aban, F. Abayomi, K. Abeyskera, J. Aboagye, M. Adam, K. Adams, J. Adamson, Y.A. Adelaja, G. Adewetan, S. Adlou, K. Ahmed, Y. Akhalwaya, S. Akhalwaya, A. Alcock, A. Ali, E.R. Allen, L. Allen, T.C.D.S.C. Almeida, M.P.S. Alves, F. Amorim, F. Andritsou, R. Anslow, M. Appleby, E.H. Arbe-Barnes, M.P. Ariaans, B. Arns, L. Arruda, P. Azi, L. Azi, G. Babbage, C. Bailey, K.F. Baker, M. Baker, N. Baker, P. Baker, L. Baldwin, I. Baleanu, D. Bandeira, A. Bara, M.A.S. Barbosa, D. Barker, G.D. Barlow, E. Barnes, A.S. Barr, J.R. Barrett, J. Barrett, L. Bates, A. Batten, K. Beadon, E. Beales, R. Beckley, S. Belij-Rammerstorfer, J. Bell, D. Bellamy, N. Bellei, S. Belton, A. Berg, L. Bermejo, E. Berrie, L. Berry, D. Berzenyi, A. Beveridge, K.R. Bewley, H. Bexhell, S. Bhikha, A.E. Bhorat, Z.E. Bhorat, E. Bijker, G. Birch, S. Birch, A. Bird, O. Bird, K. Bisnauthsing, M. Bittaye, K. Blackstone, L. Blackwell, H. Bletchly, C.L. Blundell, S.R. Blundell, P. Bodalia, B.C. Boettger, E. Bolam, E. Boland, D. Bormans, N. Borthwick, F. Bowring, A. Boyd, P. Bradley, T. Brenner, P. Brown, C. Brown, C. Brown-O'Sullivan, S. Bruce, E. Brunt, R. Buchan, W. Budd, Y.A. Bulbulia, M. Bull, J. Burbage, H. Burhan, A. Burn, K.R. Buttigieg, N. Byard, I. Cabera Puig, G. Calderon, A. Calvert, S. Camara, M. Cao, F. Cappuccini, J.R. Cardoso, M. Carr, M.W. Carroll, A. Carson-Stevens, Y.d.M. Carvalho, J.A.M. Carvalho, H.R. Casey, P. Cashen, T. Castro, L.C. Castro, K. Cathie, A. Cavey, J. Cerbino-Neto, J. Chadwick, D. Chapman, S. Charlton, I. Chelysheva, O. Chester, S. Chita, J.-S. Cho, L. Cifuentes, E. Clark, M. Clark, A. Clarke, E.A. Clutterbuck, S.L.K. Collins, C.P. Conlon, S. Connarty, N. Coombes, C. Cooper, R. Cooper, L. Cornelissen, T. Corrah, C. Cosgrove, T. Cox, W.E.M. Crocker, S. Crosbie, L. Cullen, D. Cullen, D.R.M.F. Cunha, C. Cunningham, F.C. Cuthbertson, S.N.F. Da Guarda, L.P. da Silva, B.E. Damratoski, Z. Danos, M.T.D.C. Dantas, P. Darroch, M.S. Datoo, C. Datta, M. Davids, S.L. Davies, H. Davies, E. Davis, J. Davis, J. Davis, M.M.D. De Nobrega, L.M. De Oliveira Kalid, D. Dearlove, T. Demissie, A. Desai, S. Di Marco, C. Di Maso, M.I.S. Dinelli, T. Dinesh, C. Docksey, C. Dold, T. Dong, F.R. Donnellan, T. Dos Santos, T.G. dos Santos, E.P. Dos Santos, N. Douglas, C. Downing, J. Drake, R. Drake-Brockman, K. Driver, R. Drury, S.J. Dunachie, B.S. Durham, L. Dutra, N.J.W. Easom, S. van Eck, M. Edwards, N.J. Edwards, O.M. El Muhanna, S.C. Elias, M. Elmore, M. English, A. Esmail, Y.M. Essack, E. Farmer, M. Farooq, M. Farrar, L. Farrugia, B. Faulkner, S. Fedosyuk, S. Felle, S. Feng, C. Ferreira Da Silva, S. Field, R. Fisher, A. Flaxman, J. Fletcher, H. Fofie, H. Fok, K.J. Ford, J. Fowler, P.H.A. Fraiman, E. Francis, M.M. Franco, J. Frater, M.S.M. Freire, S.H. Fry, S. Fudge, J. Furze, M. Fuskova, P. Galian-Rubio, E. Galiza, H. Garlant, M. Gavrila, A. Geddes, K.A. Gibbons, C. Gilbride, H. Gill, S. Glynn, K. Godwin, K. Gokani, U.C. Goldoni, M. Goncalves, I.G.S. Gonzalez, J. Goodwin, A. Goondiwala, K. Gordon-Quayle, G. Gorini, J. Grab, L. Gracie, M. Greenland, N. Greenwood, J. Greffrath, M.M. Groenewald, L. Grossi, G. Gupta, M. Hackett, B. Hallis, M. Hamaluba, E. Hamilton, J. Hamlyn, D. Hammersley, A.T. Hanrath, B. Hanumunthadu, S.A. Harris, C. Harris, T. Harris, T.D. Harrison, D. Harrison, T.C. Hart, B. Hartnell, S. Hassan, J. Haughney, S. Hawkins, J. Hay, I. Head, J. Henry, M. Hermosin Herrera, D.B. Hettle, J. Hill, G. Hodges, E. Horne, M.M. Hou, C. Houlihan, E. Howe, N. Howell, J. Humphreys, H.E. Humphries, K. Hurley, C. Huson, A. Hyder-Wright, C. Hyams,S. Ikram, A. Ishwarbhai, M. Ivan, P. Iveson, V. Iyer, F. Jackson, J. De Jager, S. Jaumdally, H. Jeffers, N. Jesudason, B. Jones, K. Jones, E. Jones, C. Jones, M.R. Jorge, A. Jose, A. Joshi, E.A.M.S. Júnior, J. Kadziola, R. Kailath, F. Kana, K. Karampatsas, M. Kasanyinga, J. Keen, E.J. Kelly, D.M. Kelly, D. Kelly, S. Kelly, D. Kerr, R.d.Á. Kfouri, L. Khan, B. Khozoee, S. Kidd, A. Killen, J. Kinch, P. Kinch, L.D.W. King, T.B. King, L. Kingham, P. Klenerman, F. Knapper, J.C. Knight, D. Knott, S. Koleva, M. Lang, G. Lang, C.W. Larkworthy, J.P.J. Larwood, R. Law, E.M. Lazarus, A. Leach, E.A. Lees, N.-M. Lemm, A. Lessa, S. Leung, Y. Li, A.M. Lias, K. Liatsikos, A. Linder, S. Lipworth, S. Liu, X. Liu, A. Lloyd, S. Lloyd, L. Loew, R. Lopez Ramon, L. Lora, V. Lowthorpe, K. Luz, J.C. MacDonald, G. MacGregor, M. Madhavan, D.O. Mainwaring, E. Makambwa, R. Makinson, M. Malahleha, R. Malamatsho, G. Mallett, K. Mansatta, T. Maoko, K. Mapetla, N.G. Marchevsky, S. Marinou, E. Marlow, G.N. Marques, P. Marriott, R.P. Marshall, J.L. Marshall, F.J. Martins, M. Masenya, M. Masilela, S.K. Masters, M. Mathew, H. Matlebjane, K. Matshidiso, O. Mazur, A. Mazzella, H. McCaughan, J. McEwan, J. McGlashan, L. McInroy, Z. McIntyre, D. McLenaghan, N. McRobert, S. McSwiggan, C. Megson, S. Mehdipour, W. Meijs, R.N.Á. Mendonça, A.J. Mentzer, N. Mirtorabi, C. Mitton, S. Mnyakeni, F. Moghaddas, K. Molapo, M. Moloi, M. Moore, M.I. Moraes-Pinto, M. Moran, E. Morey, R. Morgans, S. Morris, S. Morris, H.C. Morris, F. Morselli, G. Morshead, R. Morter, L. Mottal, A. Moultrie, N. Moya, M. Mpelembue, S. Msomi, Y. Mugodi, E. Mukhopadhyay, J. Muller, A. Munro, C. Munro, S. Murphy, P. Mweu, C.H. Myasaki, G. Naik, K. Naker, E. Nastouli, A. Nazir, B. Ndlovu, F. Neffa, C. Njenga, H. Noal, A. Noé, G. Novaes, F.L. Nugent, G. Nunes, K. O'Brien, D. O'Connor, M. Odam, S. Oelofse, B. Oguti, V. Olchawski, N.J. Oldfield, M.G. Oliveira, C. Oliveira, A. Oosthuizen, P. O'Reilly, P. Osborne, D.R.J. Owen, L. Owen, D. Owens, N. Owino, M. Pacurar, B.V.B. Paiva, E.M.F. Palhares, S. Palmer, S. Parkinson, H.M.R.T. Parracho, K. Parsons, D. Patel, B. Patel, F. Patel, K. Patel, M. Patrick-Smith, R.O. Payne, Y. Peng, E.J. Penn, A. Pennington, M.P. Peralta Alvarez, J. Perring, N. Perry, R. Perumal, S. Petkar, T. Philip, D.J. Phillips, J. Phillips, M.K. Phohu, L. Pickup, S. Pieterse, J. Piper, D. Pipini, M. Plank, J. Du Plessis, S. Pollard, J. Pooley, A. Pooran, I. Poulton, C. Powers, F.B. Presa, D.A. Price, V. Price, M. Primeira, P.C. Proud, S. Provstgaard-Morys, S. Pueschel, D. Pulido, S. Quaid, R. Rabara, A. Radford, K. Radia, D. Rajapaska, T. Rajeswaran, A.S.F. Ramos, F. Ramos Lopez, T. Rampling, J. Rand, H. Ratcliffe, T. Rawlinson, D. Rea, B. Rees, J. Reiné, M. Resuello-Dauti, E. Reyes Pabon, C.M. Ribiero, M. Ricamara, A. Richter, N. Ritchie, A.J. Ritchie, A.J. Robbins, H. Roberts, R.E. Robinson, H. Robinson, T.T. Rocchetti, B.P. Rocha, S. Roche, C. Rollier, L. Rose, A.L. Ross Russell, L. Rossouw, S. Royal, I. Rudiansyah, S. Ruiz, S. Saich, C. Sala, J. Sale, A.M. Salman, N. Salvador, S. Salvador, M. Sampaio, A.D. Samson, A. Sanchez-Gonzalez, H. Sanders, K. Sanders, E. Santos, M.F.S. Santos Guerra, I. Satti, J.E. Saunders, C. Saunders, A. Sayed, I. Schim van der Loeff, A.B. Schmid, E. Schofield, G. Screaton, S. Seddiqi, R.R. Segireddy, R. Senger, S. Serrano, R. Shah, I. Shaik, H.E. Sharpe, K. Sharrocks, R. Shaw, A. Shea, A. Shepherd, J.G. Shepherd, F. Shiham, E. Sidhom, S.E. Silk, A.C. da Silva Moraes, G. Silva-Junior, L. Silva-Reyes, A.D. Silveira, M.B.V. Silveira, J. Sinha, D.T. Skelly, D.C. Smith, N. Smith, H.E. Smith, D.J. Smith, C.C. Smith, A. Soares, T. Soares, C. Solórzano, G.L. Sorio, K. Sorley, T. Sosa-Rodriguez, C.M.C.D.L. Souza, B.S.D.F. Souza, A.R. Souza, A.J. Spencer, F. Spina, L. Spoors, L. Stafford, I. Stamford, I. Starinskij, R. Stein, J. Steven, L. Stockdale, L.V. Stockwell, L.H. Strickland, A.C. Stuart, A. Sturdy, N. Sutton, A. Szigeti, A. Tahiri-Alaoui, R. Tanner, C. Taoushanis, A.W. Tarr, K. Taylor, U. Taylor, I.J. Taylor, J. Taylor, R. te Water Naude, Y. Themistocleous, A. Themistocleous, M. Thomas, K. Thomas, T.M. Thomas, A. Thombrayil, F. Thompson, A. Thompson, K. Thompson, A. Thompson, J. Thomson, V. Thornton-Jones, P.J. Tighe, L.A. Tinoco, G. Tiongson, B. Tladinyane, M. Tomasicchio, A. Tomic, S. Tonks, J. Towner, N. Tran, J. Tree, G. Trillana, C. Trinham, R. Trivett, A. Truby, B.L. Tsheko, A. Turabi, R. Turner, C. Turner, M. Ulaszewska, B.R. Underwood, R. Varughese, D. Verbart, M. Verheul, I. Vichos, T. Vieira, C.S. Waddington, L. Walker, E. Wallis, M. Wand, D. Warbick, T. Wardell, G. Warimwe, S.C. Warren, B. Watkins, E. Watson, S. Webb, A. Webb-Bridges, A. Webster, J. Welch, J. Wells, A. West, C. White, R. White, P. Williams, R.L. Williams, R. Winslow, M. Woodyer, A.T. Worth, D. Wright, M. Wroblewska, A. Yao, R. Zimmer, D. Zizi, P. Zuidewind, Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK, The Lancet 397(10269) (2021) 99-111. [DOI] [PMC free article] [PubMed]
- 378.M. Voysey, S.A.C. Clemens, S.A. Madhi, L.Y. Weckx, P.M. Folegatti, P.K. Aley, B. Angus, V.L. Baillie, S.L. Barnabas, Q.E. Bhorat, S. Bibi, C. Briner, P. Cicconi, A.M. Collins, R. Colin-Jones, C.L. Cutland, T.C. Darton, K. Dheda, C.J.A. Duncan, K.R.W. Emary, K.J. Ewer, L. Fairlie, S.N. Faust, S. Feng, D.M. Ferreira, A. Finn, A.L. Goodman, C.M. Green, C.A. Green, P.T. Heath, C. Hill, H. Hill, I. Hirsch, S.H.C. Hodgson, A. Izu, S. Jackson, D. Jenkin, C.C.D. Joe, S. Kerridge, A. Koen, G. Kwatra, R. Lazarus, A.M. Lawrie, A. Lelliott, V. Libri, P.J. Lillie, R. Mallory, A.V.A. Mendes, E.P. Milan, A.M. Minassian, A. McGregor, H. Morrison, Y.F. Mujadidi, A. Nana, P.J. O’Reilly, S.D. Padayachee, A. Pittella, E. Plested, K.M. Pollock, M.N. Ramasamy, S. Rhead, A.V. Schwarzbold, N. Singh, A. Smith, R. Song, M.D. Snape, E. Sprinz, R.K. Sutherland, R. Tarrant, E.C. Thomson, M.E. Török, M. Toshner, D.P.J. Turner, J. Vekemans, T.L. Villafana, M.E.E. Watson, C.J. Williams, A.D. Douglas, A.V.S. Hill, T. Lambe, S.C. Gilbert, A.J. Pollard, M. Aban, F. Abayomi, K. Abeyskera, J. Aboagye, M. Adam, K. Adams, J. Adamson, Y.A. Adelaja, G. Adewetan, S. Adlou, K. Ahmed, Y. Akhalwaya, S. Akhalwaya, A. Alcock, A. Ali, E.R. Allen, L. Allen, T.C.D.S.C. Almeida, M.P.S. Alves, F. Amorim, F. Andritsou, R. Anslow, M. Appleby, E.H. Arbe-Barnes, M.P. Ariaans, B. Arns, L. Arruda, P. Azi, L. Azi, G. Babbage, C. Bailey, K.F. Baker, M. Baker, N. Baker, P. Baker, L. Baldwin, I. Baleanu, D. Bandeira, A. Bara, M.A.S. Barbosa, D. Barker, G.D. Barlow, E. Barnes, A.S. Barr, J.R. Barrett, J. Barrett, L. Bates, A. Batten, K. Beadon, E. Beales, R. Beckley, S. Belij-Rammerstorfer, J. Bell, D. Bellamy, N. Bellei, S. Belton, A. Berg, L. Bermejo, E. Berrie, L. Berry, D. Berzenyi, A. Beveridge, K.R. Bewley, H. Bexhell, S. Bhikha, A.E. Bhorat, Z.E. Bhorat, E. Bijker, G. Birch, S. Birch, A. Bird, O. Bird, K. Bisnauthsing, M. Bittaye, K. Blackstone, L. Blackwell, H. Bletchly, C.L. Blundell, S.R. Blundell, P. Bodalia, B.C. Boettger, E. Bolam, E. Boland, D. Bormans, N. Borthwick, F. Bowring, A. Boyd, P. Bradley, T. Brenner, P. Brown, C. Brown, C. Brown-O'Sullivan, S. Bruce, E. Brunt, R. Buchan, W. Budd, Y.A. Bulbulia, M. Bull, J. Burbage, H. Burhan, A. Burn, K.R. Buttigieg, N. Byard, I. Cabera Puig, G. Calderon, A. Calvert, S. Camara, M. Cao, F. Cappuccini, J.R. Cardoso, M. Carr, M.W. Carroll, A. Carson-Stevens, Y.d.M. Carvalho, J.A.M. Carvalho, H.R. Casey, P. Cashen, T. Castro, L.C. Castro, K. Cathie, A. Cavey, J. Cerbino-Neto, J. Chadwick, D. Chapman, S. Charlton, I. Chelysheva, O. Chester, S. Chita, J.-S. Cho, L. Cifuentes, E. Clark, M. Clark, A. Clarke, E.A. Clutterbuck, S.L.K. Collins, C.P. Conlon, S. Connarty, N. Coombes, C. Cooper, R. Cooper, L. Cornelissen, T. Corrah, C. Cosgrove, T. Cox, W.E.M. Crocker, S. Crosbie, L. Cullen, D. Cullen, D.R.M.F. Cunha, C. Cunningham, F.C. Cuthbertson, S.N.F. Da Guarda, L.P. da Silva, B.E. Damratoski, Z. Danos, M.T.D.C. Dantas, P. Darroch, M.S. Datoo, C. Datta, M. Davids, S.L. Davies, H. Davies, E. Davis, J. Davis, J. Davis, M.M.D. De Nobrega, L.M. De Oliveira Kalid, D. Dearlove, T. Demissie, A. Desai, S. Di Marco, C. Di Maso, M.I.S. Dinelli, T. Dinesh, C. Docksey, C. Dold, T. Dong, F.R. Donnellan, T. Dos Santos, T.G. dos Santos, E.P. Dos Santos, N. Douglas, C. Downing, J. Drake, R. Drake-Brockman, K. Driver, R. Drury, S.J. Dunachie, B.S. Durham, L. Dutra, N.J.W. Easom, S. van Eck, M. Edwards, N.J. Edwards, O.M. El Muhanna, S.C. Elias, M. Elmore, M. English, A. Esmail, Y.M. Essack, E. Farmer, M. Farooq, M. Farrar, L. Farrugia, B. Faulkner, S. Fedosyuk, S. Felle, S. Feng, C. Ferreira Da Silva, S. Field, R. Fisher, A. Flaxman, J. Fletcher, H. Fofie, H. Fok, K.J. Ford, J. Fowler, P.H.A. Fraiman, E. Francis, M.M. Franco, J. Frater, M.S.M. Freire, S.H. Fry, S. Fudge, J. Furze, M. Fuskova, P. Galian-Rubio, E. Galiza, H. Garlant, M. Gavrila, A. Geddes, K.A. Gibbons, C. Gilbride, H. Gill, S. Glynn, K. Godwin, K. Gokani, U.C. Goldoni, M. Goncalves, I.G.S. Gonzalez, J. Goodwin, A. Goondiwala, K. Gordon-Quayle, G. Gorini, J. Grab, L. Gracie, M. Greenland, N. Greenwood, J. Greffrath, M.M. Groenewald, L. Grossi, G. Gupta, M. Hackett, B. Hallis, M. Hamaluba, E. Hamilton, J. Hamlyn, D. Hammersley, A.T. Hanrath, B. Hanumunthadu, S.A. Harris, C. Harris, T. Harris, T.D. Harrison, D. Harrison, T.C. Hart, B. Hartnell, S. Hassan, J. Haughney, S. Hawkins, J. Hay, I. Head, J. Henry, M. Hermosin Herrera, D.B. Hettle, J. Hill, G. Hodges, E. Horne, M.M. Hou, C. Houlihan, E. Howe, N. Howell, J. Humphreys, H.E. Humphries, K. Hurley, C. Huson, A. Hyder-Wright, C. Hyams, S. Ikram, A. Ishwarbhai, M. Ivan, P. Iveson, V. Iyer, F. Jackson, J. De Jager, S. Jaumdally, H. Jeffers, N. Jesudason, B. Jones, K. Jones, E. Jones, C. Jones, M.R. Jorge, A. Jose, A. Joshi, E.A.M.S. Júnior, J. Kadziola, R. Kailath, F. Kana, K. Karampatsas, M. Kasanyinga, J. Keen, E.J. Kelly, D.M. Kelly, D. Kelly, S. Kelly, D. Kerr, R.d.Á. Kfouri, L. Khan, B. Khozoee, S. Kidd, A. Killen, J. Kinch, P. Kinch, L.D.W. King, T.B. King, L. Kingham, P. Klenerman, F. Knapper, J.C. Knight, D. Knott, S. Koleva, M. Lang, G. Lang, C.W. Larkworthy, J.P.J. Larwood, R. Law, E.M. Lazarus, A. Leach, E.A. Lees, N.-M. Lemm, A. Lessa, S. Leung, Y. Li, A.M. Lias, K. Liatsikos, A. Linder, S. Lipworth, S. Liu, X. Liu, A. Lloyd, S. Lloyd, L. Loew, R. Lopez Ramon, L. Lora, V. Lowthorpe, K. Luz, J.C. MacDonald, G. MacGregor, M. Madhavan, D.O. Mainwaring, E. Makambwa, R. Makinson, M. Malahleha, R. Malamatsho, G. Mallett, K. Mansatta, T. Maoko, K. Mapetla, N.G. Marchevsky, S. Marinou, E. Marlow, G.N. Marques, P. Marriott, R.P. Marshall, J.L. Marshall, F.J. Martins, M. Masenya, M. Masilela, S.K. Masters, M. Mathew, H. Matlebjane, K. Matshidiso, O. Mazur, A. Mazzella, H. McCaughan, J. McEwan, J. McGlashan, L. McInroy, Z. McIntyre, D. McLenaghan, N. McRobert, S. McSwiggan, C. Megson, S. Mehdipour, W. Meijs, R.N.Á. Mendonça, A.J. Mentzer, N. Mirtorabi, C. Mitton, S. Mnyakeni, F. Moghaddas, K. Molapo, M. Moloi, M. Moore, M.I. Moraes-Pinto, M. Moran, E. Morey, R. Morgans, S. Morris, S. Morris, H.C. Morris, F. Morselli, G. Morshead, R. Morter, L. Mottal, A. Moultrie, N. Moya, M. Mpelembue, S. Msomi, Y. Mugodi, E. Mukhopadhyay, J. Muller, A. Munro, C. Munro, S. Murphy, P. Mweu, C.H. Myasaki, G. Naik, K. Naker, E. Nastouli, A. Nazir, B. Ndlovu, F. Neffa, C. Njenga, H. Noal, A. Noé, G. Novaes, F.L. Nugent, G. Nunes, K. O'Brien, D. O'Connor, M. Odam, S. Oelofse, B. Oguti, V. Olchawski, N.J. Oldfield, M.G. Oliveira, C. Oliveira, A. Oosthuizen, P. O'Reilly, P. Osborne, D.R.J. Owen, L. Owen, D. Owens, N. Owino, M. Pacurar, B.V.B. Paiva, E.M.F. Palhares, S. Palmer, S. Parkinson, H.M.R.T. Parracho, K. Parsons, D. Patel, B. Patel, F. Patel, K. Patel, M. Patrick-Smith, R.O. Payne, Y. Peng, E.J. Penn, A. Pennington, M.P. Peralta Alvarez, J. Perring, N. Perry, R. Perumal, S. Petkar, T. Philip, D.J. Phillips, J. Phillips, M.K. Phohu, L. Pickup, S. Pieterse, J. Piper, D. Pipini, M. Plank, J. Du Plessis, S. Pollard, J. Pooley, A. Pooran, I. Poulton, C. Powers, F.B. Presa, D.A. Price, V. Price, M. Primeira, P.C. Proud, S. Provstgaard-Morys, S. Pueschel, D. Pulido, S. Quaid, R. Rabara, A. Radford, K. Radia, D. Rajapaska, T. Rajeswaran, A.S.F. Ramos, F. Ramos Lopez, T. Rampling, J. Rand, H. Ratcliffe, T. Rawlinson, D. Rea, B. Rees, J. Reiné, M. Resuello-Dauti, E. Reyes Pabon, C.M. Ribiero, M. Ricamara, A. Richter, N. Ritchie, A.J. Ritchie, A.J. Robbins, H. Roberts, R.E. Robinson, H. Robinson, T.T. Rocchetti, B.P. Rocha, S. Roche, C. Rollier, L. Rose, A.L. Ross Russell, L. Rossouw, S. Royal, I. Rudiansyah, S. Ruiz, S. Saich, C. Sala, J. Sale, A.M. Salman, N. Salvador, S. Salvador, M. Sampaio, A.D. Samson, A. Sanchez-Gonzalez, H. Sanders, K. Sanders, E. Santos, M.F.S. Santos Guerra, I. Satti, J.E. Saunders, C. Saunders, A. Sayed, I. Schim van der Loeff, A.B. Schmid, E. Schofield, G. Screaton, S. Seddiqi, R.R. Segireddy, R. Senger, S. Serrano, R. Shah, I. Shaik, H.E. Sharpe, K. Sharrocks, R. Shaw, A. Shea, A. Shepherd, J.G. Shepherd, F. Shiham, E. Sidhom, S.E. Silk, A.C. da Silva Moraes, G. Silva-Junior, L. Silva-Reyes, A.D. Silveira, M.B.V. Silveira, J. Sinha, D.T. Skelly, D.C. Smith, N. Smith, H.E. Smith, D.J. Smith, C.C. Smith, A. Soares, T. Soares, C. Solórzano, G.L. Sorio, K. Sorley, T. Sosa-Rodriguez, C.M.C.D.L. Souza, B.S.D.F. Souza, A.R. Souza, A.J. Spencer, F. Spina, L. Spoors, L. Stafford, I. Stamford, I. Starinskij, R. Stein, J. Steven, L. Stockdale, L.V. Stockwell, L.H. Strickland, A.C. Stuart, A. Sturdy, N. Sutton, A. Szigeti, A. Tahiri-Alaoui, R. Tanner, C. Taoushanis, A.W. Tarr, K. Taylor, U. Taylor, I.J. Taylor, J. Taylor, R. te Water Naude, Y. Themistocleous, A. Themistocleous, M. Thomas, K. Thomas, T.M. Thomas, A. Thombrayil, F. Thompson, A. Thompson, K. Thompson, A. Thompson, J. Thomson, V. Thornton-Jones, P.J. Tighe, L.A. Tinoco, G. Tiongson, B. Tladinyane, M. Tomasicchio, A. Tomic, S. Tonks, J. Towner, N. Tran, J. Tree, G. Trillana, C. Trinham, R. Trivett, A. Truby, B.L. Tsheko, A. Turabi, R. Turner, C. Turner, M. Ulaszewska, B.R. Underwood, R. Varughese, D. Verbart, M. Verheul, I. Vichos, T. Vieira, C.S. Waddington, L. Walker, E. Wallis, M. Wand, D. Warbick, T. Wardell, G. Warimwe, S.C. Warren, B. Watkins, E. Watson, S. Webb, A. Webb-Bridges, A. Webster, J. Welch, J. Wells, A. West, C. White, R. White, P. Williams, R.L. Williams, R. Winslow, M. Woodyer, A.T. Worth, D. Wright, M. Wroblewska, A. Yao, R. Zimmer, D. Zizi, P. Zuidewind, Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK, The Lancet 397(10269) (2021) 99-111. [DOI] [PMC free article] [PubMed]
- 379.Lung-Ji Chang, Phase I/II Multicenter Trial of Lentiviral Minigene Vaccine (LV-SMENP) of Covid-19 Coronavirus, 2020. https://clinicaltrials.gov/ct2/show/NCT04276896ClinicalTrials.gov.
- 380.Lung-Ji Chang, Safety and Immunity of Covid-19 aAPC Vaccine, ClinicalTrials.gov, 2020.
- 381.U.o. Wisconsin–Madison, UW–Madison, FluGen, Bharat Biotech to develop CoroFlu, a coronavirus vaccine, 2020. https://news.wisc.edu/uw-madison-flugen-bharat-biotech-to-develop-coroflu-a-coronavirus-vaccine/.
- 382.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J.L., Pérez Marc G., Moreira E.D., Zerbini C. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Walsh E.E., Frenck R.W., Falsey A.R., Kitchin N., Absalon J., Gurtman A., Lockhart S., Neuzil K., Mulligan M.J., Bailey R., Swanson K.A., Li P., Koury K., Kalina W., Cooper D., Fontes-Garfias C., Shi P.-Y., Türeci Ö., Tompkins K.R., Lyke K.E., Raabe V., Dormitzer P.R., Jansen K.U., Şahin U., Gruber W.C. Safety and Immunogenicity of Two RNA-Based Covid-19 Vaccine Candidates. N. Engl. J. Med. 2020;383(25):2439–2450. doi: 10.1056/NEJMoa2027906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Mulligan M.J., Lyke K.E., Kitchin N., Absalon J., Gurtman A., Lockhart S., Neuzil K., Raabe V., Bailey R., Swanson K.A., Li P., Koury K., Kalina W., Cooper D., Fontes-Garfias C., Shi P.Y., Türeci Ö., Tompkins K.R., Walsh E.E., Frenck R., Falsey A.R., Dormitzer P.R., Gruber W.C., Şahin U., Jansen K.U. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature. 2020;586(7830):589–593. doi: 10.1038/s41586-020-2639-4. [DOI] [PubMed] [Google Scholar]
- 385.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., Diemert D., Spector S.A., Rouphael N., Creech C.B. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Tebas P., Yang S., Boyer J.D., Reuschel E.L., Patel A., Christensen-Quick A., Andrade V.M., Morrow M.P., Kraynyak K., Agnes J., Purwar M., Sylvester A., Pawlicki J., Gillespie E., Maricic I., Zaidi F.I., Kim K.Y., Dia Y., Frase D., Pezzoli P., Schultheis K., Smith T.R.F., Ramos S.J., McMullan T., Buttigieg K., Carroll M.W., Ervin J., Diehl M.C., Blackwood E., Mammen M.P., Lee J., Dallas M.J., Brown A.S., Shea J.E., Kim J.J., Weiner D.B., Broderick K.E., Humeau L.M. Safety and immunogenicity of INO-4800 DNA vaccine against SARS-CoV-2: A preliminary report of an open-label, Phase 1 clinical trial. EClinicalMedicine. 2021;31 doi: 10.1016/j.eclinm.2020.100689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.A. Patel, J. Walters, E.L. Reuschel, K. Schultheis, E. Parzych, E.N. Gary, I. Maricic, M. Purwar, Z. Eblimit, S.N. Walker, D. Guimet, P. Bhojnagarwala, A. Doan, Z. Xu, D. Elwood, S.M. Reeder, L. Pessaint, K.Y. Kim, A. Cook, N. Chokkalingam, B. Finneyfrock, E. Tello-Ruiz, A. Dodson, J. Choi, A. Generotti, J. Harrison, N.J. Tursi, V.M. Andrade, Y. Dia, F.I. Zaidi, H. Andersen, M.G. Lewis, K. Muthumani, J.J. Kim, D.W. Kulp, L.M. Humeau, S. Ramos, T.R.F. Smith, D.B. Weiner, K.E. Broderick, Intradermal-delivered DNA vaccine provides anamnestic protection in a rhesus macaque SARS-CoV-2 challenge model, bioRxiv (2020) 2020.07.28.225649.
- 388.Smith T.R.F., Patel A., Ramos S., Elwood D., Zhu X., Yan J., Gary E.N., Walker S.N., Schultheis K., Purwar M., Xu Z., Walters J., Bhojnagarwala P., Yang M., Chokkalingam N., Pezzoli P., Parzych E., Reuschel E.L., Doan A., Tursi N., Vasquez M., Choi J., Tello-Ruiz E., Maricic I., Bah M.A., Wu Y., Amante D., Park D.H., Dia Y., Ali A.R., Zaidi F.I., Generotti A., Kim K.Y., Herring T.A., Reeder S., Andrade V.M., Buttigieg K., Zhao G., Wu J.-M., Li D., Bao L., Liu J., Deng W., Qin C., Brown A.S., Khoshnejad M., Wang N., Chu J., Wrapp D., McLellan J.S., Muthumani K., Wang B., Carroll M.W., Kim J.J., Boyer J., Kulp D.W., Humeau L.M.P.F., Weiner D.B., Broderick K.E. Immunogenicity of a DNA vaccine candidate for COVID-19. Nat. Commun. 2020;11(1):2601. doi: 10.1038/s41467-020-16505-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.D.R. Mittal, Novel Corona Virus-2019-nCov vaccine by intradermal route in healthy subjects. , 2020. http://ctri.nic.in/Clinicaltrials/showallp.php?mid1=51254&EncHid=&userName=ZyCoV-D.
- 390.Kaur S.P., Gupta V. COVID-19 Vaccine: A comprehensive status report. Virus Res. 2020;288:198114. doi: 10.1016/j.virusres.2020.198114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Phase II/III Study of COVID-19 DNA Vaccine (AG0302-COVID19), ClinicalTrials.gov, 2020.
- 392.Si R., Yao Y., Zhang X., Lu Q., Aziz N. Investigating the Links Between Vaccination Against COVID-19 and Public Attitudes Toward Protective Countermeasures: Implications for Public Health Front. Public Health. 2021;9(1040) doi: 10.3389/fpubh.2021.702699. [DOI] [PMC free article] [PubMed] [Google Scholar]