Abstract
Sleep is punctuated by transient elevations of vigilance level called arousals or awakenings depending on their durations. Understanding the dynamics of brain activity modifications during these transitional phases could help to better understand the changes in cognitive functions according to vigilance states. In this study, we investigated the activity of memory‐related areas (hippocampus and orbitofrontal cortex) during short (3 s to 2 min) arousing reactions detected from thalamic activity, using intracranial recordings in four drug‐resistant epilepsy patients. The average power of the signal between 0.5 and 128 Hz was compared across four time windows: 10 s of preceding sleep, the first part and the end of the arousal/awakening, and 10 s of wakefulness. We observed that (a) in most frequency bands, the spectral power during hippocampal arousal/awakenings is intermediate between wakefulness and sleep whereas frontal cortex shows an early increase in low and fast activities during non‐rapid‐eye‐movement (NREM) sleep arousals/awakenings; (b) this pattern depends on the preceding sleep stage with fewer modifications for REM than for non‐REM sleep arousal/awakenings, potentially reflecting the EEG similarities between REM sleep and wakefulness; (c) a greater activation at the arousing reaction onset in the prefrontal cortex predicts longer arousals/awakenings. Our findings suggest that hippocampus and prefrontal arousals/awakenings are progressive phenomena modulated by sleep stage, and, in the neocortex, by the intensity of the early activation. This pattern of activity could underlie the link between sleep stage, arousal/awakening duration and restoration of memory abilities including dream recall.
Keywords: arousal, dream, frontal lobe, hippocampus, humans, intra‐cranial electoencephalography, sleep, wakefulness
The average duration of intra‐sleep awakenings is a good predictor of awakenings and dream recall, suggesting that encoding and consolidation memory processes may be restored as a function of time during the awakening process. In this study, using intracranial recordings in epilepsy patients, we show that the wake‐like activity restoration during arousals/awakenings in the hippocampus and the prefrontal cortex is a progressive phenomenon modulated by the preceding sleep stage and the duration of the arousal/awakening, which depends, in the neocortex, on the intensity of the initial arousing reaction. These results may explain part of the variability in dream recall, REM sleep‐related and longer awakenings allowing to recover wake‐like activity in memory‐related regions.
1. INTRODUCTION
The vanishing of conscious executive and perceptive abilities (Bastuji & Garcia‐Larrea, 1999) as well as of explicit encoding of information into memory (Ruch & Henke, 2020) is a central component of the changes in cognitive functioning at the passing from wake to sleep state. Its cerebral correlates are not yet fully understood, nor are those associated with the restoration of these abilities at awakening. Transient periods of activation such as arousals (ASDA, 1992) and short awakenings provide a useful model to get insight about how the brain raises its level of alertness. Notably, arousals (lasting 3–15 s) are usually not associated with a conscious perception nor are they encoded in memory, whereas awakenings, lasting longer, may be consciously perceived, encoded in long‐term memory, and reported by the sleeper in the morning (Campbell & Webb, 1981; Koulack & Goodenough, 1976). During these short time windows (from 3 s to a few minutes) the changes in brain activity would allow the recovery of consciousness and/or the ability to encode the content of the mind (may it be the dream content, the trigger of the awakening, or the awakening itself) in long‐term memory (Koulack & Goodenough, 1976). Several experimental results suggest that a minimum of about 1–2 min of wakefulness is required for the sleeper to recover consciousness or memory encoding abilities at awakening (Campbell & Webb, 1981; Eichenlaub, Bertrand, Morlet, & Ruby, 2014; Vallat et al., 2017).
In the search for a better understanding of the changes in memory processes according to vigilance state, the hippocampus seems to be the first region of the brain to be investigated. This structure is indeed involved in memory processes during wakefulness, but also during sleep through its implication in sleep‐related memory consolidation phenomena (Albouy et al., 2008; Squire & Zola‐Morgan, 1991; Walker & Stickgold, 2004). The different aspects of memory underpinned by the hippocampus, that is, encoding, consolidation and retrieval, are thought to be theoretically at least partially exclusive, and associated with distinct electrophysiological patterns of activity (Axmacher, Elger, & Fell, 2008; Douchamps, Jeewajee, Blundell, Burgess, & Lever, 2013). In agreement with these theories, several results suggest that the hippocampus might be unable to encode a new information into memory during sleep, although simple items presented during sleep may lead to memory traces (Aarons, 1976; Andrillon, Pressnitzer, Leger, & Kouider, 2017; Mizuseki & Miyawaki, 2017; Suzuki, 2006). Thus, the investigation of the dynamic changes of the hippocampus, and of related areas such as the prefrontal cortex, activity during arousals and intra‐sleep awakenings seems to be relevant to improve our understanding of how memory encoding/retrieval is restored upon awakening.
However, the electrophysiological investigation of hippocampal activity during sleep in human must overcome two major difficulties. The first is related to the fact that only deep stereo‐electro‐encephalographic recordings (S‐EEG) allow access to the hippocampus which cannot be explored with scalp EEG. The second difficulty is that S‐EEG recordings are performed as part of a pre‐surgical evaluation in drug‐resistant epilepsy patients whose hippocampus is often involved in epileptic activity, especially during non‐rapid‐eye‐movement (NREM) sleep (Lambert et al., 2018). However, intracranial recording of apparently normal hippocampus is sometimes required during pre‐surgical evaluation, in particular to exclude bi‐temporal epilepsy, leading the unique opportunity of studying healthy hippocampal activity in human. Thanks to a large intracranial EEG recordings database (Epilepsy Unit of Lyon University Hospital), we were able to identify four patients presenting both a strictly normal hippocampal activity during wakefulness and sleep and a recording of the thalamic activity enabling precise scoring of vigilance stages and arousals (Magnin, Bastuji, Garcia‐Larrea, & Mauguiere, 2004; Peter‐Derex, Magnin, & Bastuji, 2015). Prefrontal (orbitofrontal) cortex was also explored in all of them, allowing to investigate neocortical activity.
Previously, the analysis of S‐EEG recordings from several neocortical areas allowed us to identify a high heterogeneity of brain activity during the first 3 s of arousals, which depended on various factors such as the on‐going sleep stage at the time of the arousal, the stimulus which had triggered arousal, homeostatic factors and overall the brain area considered (Peter‐Derex et al., 2015). In the present study, we aimed to explore more specifically the hippocampus and to focus not only on the first seconds of the arousal but also on several time windows at the beginning, in the middle and at the end of both arousals and short awakenings. These analyses allowed us to characterize the dynamics of hippocampal and neocortical activity in the first tens of seconds of wakefulness after an awakening from sleep. We investigated the hippocampus and the prefrontal cortex activity during NREM and REM sleep thalamic arousal reactions, and compared this activity with that of wakefulness, NREM, and REM sleep in order to assess (a) the dynamics of restoration of the hippocampus typical wake activity, which is supposed to be associated with the restoration of its memory functions, as compared with neocortex activity during arousals (b) if the modification of hippocampus activity during arousal/awakenings depends on the preceding sleep stage as can be expected from our previous results (Peter‐Derex et al., 2015), which may suggest functional differences between NREM and REM sleep awakenings (c) if hippocampal and neocortical activity are modulated by the duration of the arousing reaction, with greater activation during long as compared with short events.
2. MATERIALS AND METHODS
2.1. Patients
Four patients (3 men and 1 woman, mean age 33 years, range 19–45 years) suffering from focal refractory temporal lobe epilepsy were included in this study (Table 1). To delineate the extent of the epileptogenic zone and plan a tailored surgical treatment, depth EEG electrodes were implanted according to the stereotactic technique of Talairach and Bancaud (Bancaud & Talairach, 1973; Guenot et al., 2001). The choice of cortical anatomic targets was guided by data from non‐invasive investigations (clinical history, video‐scalp‐EEG monitoring, morphologic MRI, [18F]‐fluorodeoxyglucose position emission tomography). Three of the four patients underwent bitemporal exploration in order to rule out bitemporal seizures. The thalamus, and more specifically the medial pulvinar nucleus (PuM), was a target of stereotactic implantation because it might be an important relay in the propagation of epileptic discharges, given its reciprocal connections with temporal cortical areas (Rosenberg et al., 2006). Explorations of temporal neocortical areas and PuM was possible using a single multicontact electrode, so that thalamic exploration did not increase the risk of the procedure by requiring an additional electrode track specifically devoted to the PuM activity recording. Anti‐seizure drugs were tapered down in order to increase the occurrence probability of spontaneous seizures. In agreement with the French legislation relative to invasive investigations with a direct individual benefit, patients were fully informed about electrode implantation and stereotactic EEG recordings. They gave written informed consent for the use of recordings for research purposes. The procedure was approved by the national Ethics Committee (Comité de Protection des Personnes CPP 09‐CHUG‐12, no 0907).
TABLE 1.
Clinical and demographic characteristics of the patients (MRI, magnetic resonance imaging)
| Patient | Age | Gender | Epilepsy diagnosis | MRI | Treatment (mg) (at the time of the night recording) | Implanted hippocampus (selected for analyses) |
|---|---|---|---|---|---|---|
| 1 | 35 | M | Left mesio‐temporal | Left temporal polymicrogyria |
Levetiracetam (250 Lacosamide (50) Clobazam (10) |
L + R |
| 2 | 19 | M | Right temporal pole | Normal | None | R |
| 3 | 33 | M | Right temporal neocortex | Right superior temporal gyrus dysplasia | Clobazam (10) | L + R |
| 4 | 45 | F | Left mesio‐temporal | Normal |
Lacosamide (300) Lamotrigine (350) |
L + R |
2.2. Electrode implantation and anatomical localization of recording sites
The electrode implantation procedure was carried out using multiple contact electrodes introduced into the brain perpendicular to the midsagittal plane with a stereotactic frame. Each platinum‐iridium electrode had a diameter of 0.8 mm, and contained 5–15 recording contacts (2 mm long, spaced by 1.5 mm; Dixi, Besançon, France). The stereotactic implantation procedure was derived from the one first described by Bancaud and Talairach (1973) and is detailed in Ostrowsky et al. (2002). Anatomical localization of the cortical electrodes contacts was counterchecked using postimplantation MRI. We focused our analyses on electrodes leads located inside the hippocampus and in the orbitofrontal cortex which was explored in all patients (ipsilateral to the hippocampus selected for our study in three patients). In each patient, stereotactic coordinates electrode contacts were reported within the anatomical model of normal brain proposed by the McConnell Brain Imaging Center of the Montréal Neurological Institute (MNI), McGill University (http://www.bic.mni.mcgill.ca/brainweb/). Superimposition of cortical contacts on the MNI brain template was performed using a MATLAB routine (Figure 1). The placement of the contacts within the thalamus was assessed using Morel's atlas of the human thalamus (Morel, Magnin, & Jeanmonod, 1997). Thalamic contacts were in the PuM in all patients but one. In patient 2, the electrode was more anterior and reached the lateral posterior and ventral lateral posterior nuclei (Figure 1).
FIGURE 1.
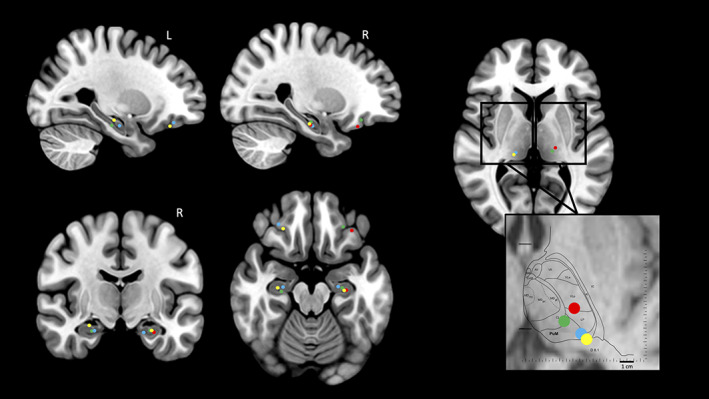
Recording contact locations represented on MNI brain template and Morel's thalamic atlas. The reconstruction of hippocampus, orbito‐frontal cortex, and thalamus contacts locations on the MNI brain template is presented for the four patients. For the thalamus, left and right side are merged in the zoom window which shows the location of the different nuclei on the Morel's atlas. R, right; L, left. Each color refers to a patient: Patient 1: blue circle; patient 2: red circle; patient 3: green circle; patient 4: yellow circle. Thalamic nuclei: PuM, medial pulvinar; CL, central lateral; L, lateral posterior; VLp, ventral lateral posterior. The two horizontal lines indicates the positions of the anterior and posterior commissures. D8.1: horizontal plane 8.1 mm dorsal to the horizontal inter‐commissural plane
2.3. Data acquisition
Night sleep recordings under stereo‐EEG (S‐EEG) video monitoring were conducted in the patient's own room at least 5 days after electrode implantation. S‐EEG signals were obtained using both referential and bipolar montages with Micromed software (Treviso, Italy) (sampling frequency: 256 Hz; band pass filter: 0.53–128 Hz; 32 dB/octave). The reference electrode was chosen for each patient on an implanted contact located in the skull. Blinks and saccades were recorded with two electro‐oculograms (EOG) electrodes placed on the supero‐lateral and infero‐lateral right canthus. EOG, electrocardiogram (EKG), and video were recorded continuously during the night.
2.4. Sleep scoring and intra‐sleep awakening reaction detection
Sleep stages were visually scored using 30 s epochs by one of the authors (L.P.‐D.) trained in scoring intra‐cerebral sleep, using AASM criteria adapted to intra‐cerebral recordings and thalamic activity especially for REM sleep (Berry et al., 2017; Magnin et al., 2004). Intra Sleep Awakening Reaction (ISAR) were defined as an abrupt EEG frequency shift which may include theta, alpha, and/or frequency greater than 16 Hz except spindles, following 10 s of stable sleep, lasting 3–15 s for arousals according to the ASDA standard definition and more than 15 s for awakenings, and ending with sleep (ASDA, 1992). We investigated awakenings up to 2 min long since this time duration was pointed out in previous work as being related to the restoration of metacognitive abilities such as awareness or explicit memory recall (Campbell & Webb, 1981; Eichenlaub et al., 2014; Eichenlaub, Bertrand, et al., 2014). ISAR visual detection (performed by M.E. and countercheck by L.P.‐D.) was based on thalamic activity, as we had previously shown that the pattern of thalamic activation during arousals was easily detectable and highly reproducible as it manifests as an abrupt shift to high‐frequency activity (Peter‐Derex et al., 2015). For each arousal, onset and end were visually marked. As medial pulvinar nucleus is connected on the one hand to the reticular thalamus and to brainstem nuclei which belong to the ascending reticular activation system and on the other hand to limbic mesial temporal structures, the choice of this structure was particularly relevant to explore arousing reactions although we had already observed that clear arousing reactions could be also seen in other thalamic nuclei such as the lateral posterior nuclei (Benarroch, 2015; Peter‐Derex et al., 2015). In one patient (No. 4), scalp EEG recording was also available, which allowed us to quantify sensitivity and specificity of thalamus‐based arousal detection as compared with scalp EEG‐based detection. S‐EEG channel contacts selected for power analysis were located inside the hippocampus and the orbitofrontal cortex. All contacts considered in the present work were located outside the seizure onset zone and/or any structural brain lesion. In addition, all recordings were systematically reviewed by a board‐certified neurophysiologist (L.P.‐D.) to confirm that the EEG activity recorded in these contacts was free of epileptic interictal abnormalities, whatever the sleep stage considered.
2.5. Signal analyses
Using a MATLAB script S‐EEG files were segmented into pieces including the whole ISAR and 10 s of the preceding sleep. The spectral analysis of the SEEG signal of the hippocampus and of the orbitofrontal cortex was performed on bipolar derivation (adjacent contacts of the S‐EEG electrode) with Morlet wavelet transform between 0.5 and 128 Hz (step = 1 sample; frequency resolution = 0.5 Hz; ω [width of the wavelet, determines the temporal and spectral resolution] = 7 Hz; σ [the length of the used wavelets in standard deviations of the implicit Gaussian kernel] = 3 s).
2.5.1. Temporal windows of interest
First analysis
This analysis was designed to assess the possible difference in the spectral content of the hippocampus signal between the four following time windows (Figure 2a). Such analysis entailed that the “bodies” of the ISAR time windows (see below) could be of different durations, but it allowed to assess a possible global evolution of the signal between the beginning and the end of the ISAR for the ISARs of all durations (i.e., ≥3 s).
Baseline‐tw: This time window corresponded to the 10 s of sleep (N2 or REM) immediately preceding the ISAR [according to the ASDA definition (ASDA, 1992)].
Onset‐tw: It was a 5 s time window, starting 2 s before and ending 3 s after the onset of the thalamic arousal (T0). This choice resulted from (a) previous observation of early delta frequency increase in several cortical areas 1–2 s before thalamic activation and asynchrony between cortical areas during arousals (Nobili et al., 2011; Peter‐Derex et al., 2015), (b) the ASDA rules stating that the minimal duration for an arousal is 3 s (ASDA, 1992). This allowed us to make sure that we were able to investigate the very beginning of the arousal in every studied area and that we did not include any early arousing activity in the baseline time window. As a result, the baseline started −12 s and ended −2 s before the onset of the thalamic arousal.
Body‐tw: This time window started at T0 + 3 s and ended at arousal offset. The duration of this window was consequently variable between arousals/awakenings reactions and its longest possible duration was set at 1 min 57 s, that is, starting at T0 + 3 s and ending at T0 + 2 min. It shortest duration was set at 2 s as we wanted to study frequencies over 0.5 Hz.
Wake‐tw: A period of 10 s of daytime wakefulness was randomly chosen for each patient among a 30 min period of quiet wakefulness selected during the evening before the recorded night, at least 30 min away from sleep onset, while patients were sitting in their bed, eyes open, watching TV. These nonoverlapping 10 s segments were used to compare arousals with stabilized wakefulness (each segment was used no more than one time), and were merged for that purpose to the Baseline‐Onset‐Body segments.
FIGURE 2.
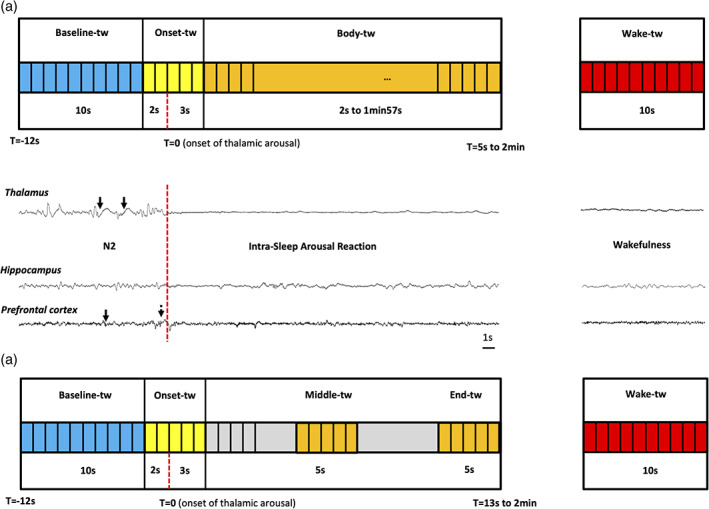
Temporal windows of interest for analyses of intra‐sleep arousal reactions (ISAR). Baseline time window (baseline‐tw, 10 s) is presented in blue. It corresponds to the ongoing sleep stage. Onset time window (onset‐tw, 5 s) is represented in yellow. It starts 2 s before the onset of the thalamic‐detected arousal reaction and ends 3 s after. Wake time window (wake‐tw, 10 s) is represented in red. For the first analysis (a), body time window (body‐tw, variable duration, min 2 s, max 1 min 57 s) is represented in orange. For the second analysis (b), the 5 s long middle‐tw (5 s in the middle of the ISAR) and end‐tw (5 last seconds of the ISAR) are represented in orange. The vertical red dashed line represents the onset of the thalamic‐detected arousal reaction. In the example presented in the middle of the figure (patient #1), the intra‐sleep arousal reaction occurs in N2 (see the thalamic and prefrontal cortex spindles, black arrows). A slow wave is observed in the prefrontal cortex just before the thalamic arousal onset (dotted black arrow)
Second analysis
This analysis was designed to assess the possible difference in the spectral content of the hippocampus signal in 5 s time windows at the beginning, the middle and the end of the ISAR (Figure 2(b)). Such analysis enabled to have a more precise idea of the evolution of the signal across time and to explore more specifically the last seconds of the ISAR (before falling asleep again) but it entailed that such analysis was only possible for the ISAR of 13 s or longer.
Baseline‐tw: This time window corresponded to the 10s of sleep (N2 or REM) immediately preceding the ISAR (according to the ASDA definition ASDA, 1992).
Onset‐tw: It was a 5 s time window, starting 2 s before and ending 3 s after the onset of the thalamic ISAR (T0).
Middle‐tw: This 5 s time window was centered on the middle of the ISAR that is, at equal distance from T0 and the end of the ISAR.
End‐tw: This 5 s time window started at the end of the ISAR minus 5 s and ended at the ISAR offset.
2.5.2. Frequency bands of interest
The mean spectral power of the signal during each time window was calculated for each ISAR in the following frequency bands (FB): low‐delta [0.5–1 Hz], delta [1.5–3.5 Hz], theta [4–7.5 Hz], alpha [8–11 Hz], sigma [12–16 Hz], beta [17–40 Hz], and gamma [60–128 Hz]. The choice of these frequency bands was supported by previous works about neocortex and hippocampus activity during sleep and wakefulness (Frauscher et al., 2018; von Ellenrieder et al., 2020). They notably enable to investigate rhythms typical of sleep like slow activity (slow delta and higher delta including the “rhythmic slow activity” described in the hippocampus during REM and wakefulness) but also spindles and gamma oscillations (Bodizs et al., 2001; Brazier, 1968; Cantero, Atienza, Madsen, & Stickgold, 2004; Birgit Frauscher et al., 2015; Montplaisir, Leduc, Laverdiere, Walsh, & Saint‐Hilaire, 1981; Moroni et al., 2007; Peter‐Derex, Comte, Mauguiere, & Salin, 2012).
2.5.3. Normalization
A normalization of the signal was performed to ensure that the power in the different frequency bands of interest, time windows and sleep stages could be compared within and across subjects. For each segment and each frequency band, we extracted the mean and SD of the wake window. We used them to apply the geometric Z‐score along all the segments. The geometric mean was preferred to the arithmetic mean because it is less sensitive to extreme values and better suited to the distribution (normal log) of our data. Geometric Z‐score was calculated in MATLAB using the following formula
where, geometric mean: μ = ; geometric standard deviation: ; xs corresponds to each power value in the sleep and ISAR time windows; and xw corresponds to each power value in the wake windows.
Afterward, average signal power during each time window of interest was extracted for each patient, each sleep stage, and each frequency band of interest.
2.6. Statistical analyses
Statistical analyses were conducted using R. Our aim was to model spectral power (in several frequency bands) of the hippocampus and the orbitofrontal cortex S‐EEG signal between different sleep stages (N2 and REM sleep), time windows (Baseline, Onset, Body and Wake or Baseline, Onset, Midde, End, and Wake) and ISAR type (arousal and short awakenings) within a subject. To take into account possible variability of power in the frequency bands of interest and in the sleep stage, ISAR type and time window effects between subjects, we used a Linear mixed‐effects model (lme4 package, Linear Mixed Effects version 4; Bates et al., 2015). We accounted for the heterogeneity of power values by defining them as effects with a random intercepts and slopes, thus instructing the model to correct for any systematic differences between these variability. To confirm the need for mixed, nested model, a likelihood ratio test, Akaike information criterion (AIC) and Bayesian information criterion (BIC) were used to test model fit before and after sequential addition of random effects. To optimize our model, we checked the normality of the model residual.
We analyzed the influence of four possible fixed effects on power: (a) sleep stage (two levels: N2 and REM sleep); (b) frequency band (seven levels: [0.5–1] Hz, [1.5–3.5] Hz, [4–7.5] Hz, [8–11] Hz, [11.5–16] Hz, [17–40] Hz, and [60–128] Hz); (c) time window (four or five levels: Baseline, Onset, Body and Wake or Baseline, Onset, Midde, End, Wake), and (d) ISAR type (two levels: arousal (3–15 s) and short awakening (15 s to 2 min)). We ran a type III analysis of variance. Wald Chi‐square tests were used (Car package, version 3.0‐0; Fox and Weisberg, 2011) for fixed effects in linear mixed‐effects models.
For post hoc tests we used the Lsmean package (Lsmean version 2.20‐23; Searle et al., 1980). For most of the post hoc tests, we contrasted signal power in each specific frequency band and sleep stage in two conditions (Win2–Win1 where Win1 and Win2 are two time windows). We used a complex contrast to highlight the ISAR duration effect according to time window factor for each sleep stage and frequency band (Win2 – Win1)awakening – (Win2 – Win1)arousals. We computed the correlation between the intensity of the initial change in spectral power (contrast onset – baseline) and the duration of the ISAR with a Pearson's correlation test. The significance threshold was set p < .05 and we take the correlation of the model parameters into account. Dealing with General Linear Hypothesis, we use the model error (from the variance–covariance matrix of the main parameters of a fitted model). The random effect model that we used allowed us to model power variability between different sleep stage, time windows and ISAR type within a subject. The fixed effect represents the mean effect across all subjects after residual variability was removed and takes the correlation of the model parameters into account.
3. RESULTS
Clinical characteristics of the patients are described in Table 1. All patients suffered from temporal lobe epilepsy. The hippocampus selected for the study was contralateral to the epileptic hemisphere in three patients, homolateral but only late involved in the seizure propagation in the remaining patient.
3.1. Intra sleep awakening reactions detection
Six hundred and seventeen thalamic ISAR were selected during the four whole nights analyzed: 379 in N2, 38 in N3, and 200 in REM sleep (respectively, in patient 1:121, 16 and 39; in patient 2:105, 7 and 65; in patient 3:30, 3 and 62 and in patient 4:123, 12 and 34). Examples of NREM and REM arousals observed in scalp EEG and S‐EEG are presented in the Figure S1. The low number of ISAR in N3 prevented us from performing analyses in this stage, and no ISAR was selected for analysis in N1 because of the difficulty to recognize this sleep stage in S‐EEG recordings, especially given local aspects of sleep during transition states with possible discrepancies between thalamic and cortical activity and between cortical areas (Magnin et al., 2010). The following results therefore relate to N2 and REM sleep ISAR. Examples of hippocampal EEG activity during ISAR selected upon thalamic activity in N2 and REM sleep are presented in Figure 3a. In patient 4, availability of scalp EEG recordings allowed us to quantify the sensitivity (99%) and the specificity (86%) of thalamus‐based detection of arousals as compared with scalp EEG‐based detection. Most selected ISAR lasted between 3 and 25 s, with a median duration of 11.1 s (interquartile range 15.1 s) and a mean ± SD duration of 20.6 ± 25.5 s for N2 and REM ISAR together (median 10.7 s in N2 and 12.5 s in REM). Time‐frequency representations of ISAR according to their duration are shown in Figure 3b.
FIGURE 3.
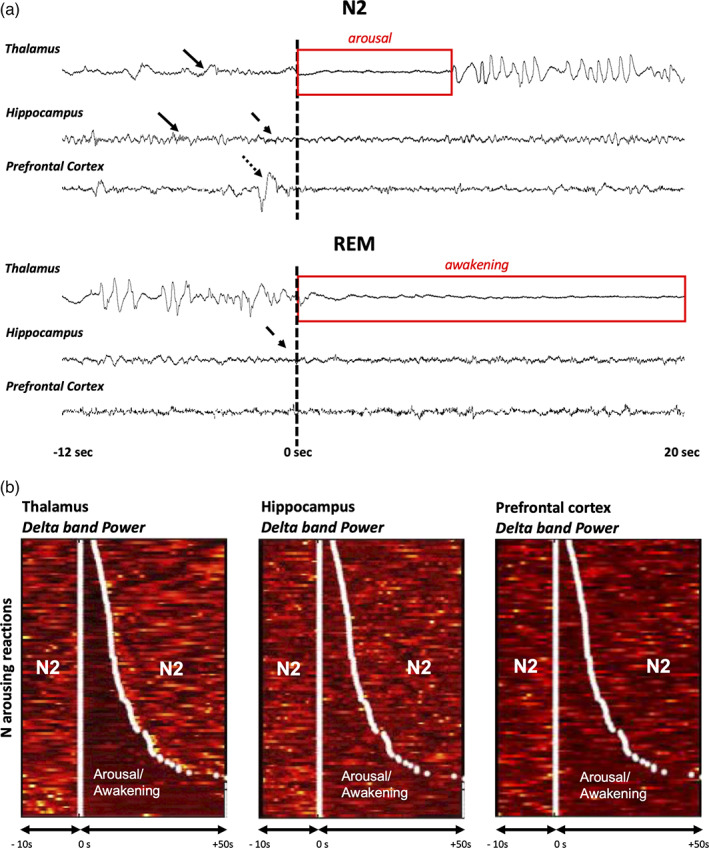
Examples ISAR in the hippocampus and in the prefrontal cortex: raw data and time‐frequency analysis. (a) Examples of ISAR detected using the thalamic activity, in the thalamus, the hippocampus and the prefrontal cortex of patient #2, during N2 (arousal, top) and REM sleep (awakening, bottom). T = 0 s corresponds to the onset of the arousal/awakening in the thalamus. In N2, sleep spindles are observed in the thalamus and the hippocampus structures (black arrows). In REM, the typical high delta activity is observed in the thalamus. Note the discrete asynchrony between the three structures: in the N2 example, the increase in high frequencies in the hippocampus (dashes black arrow) and a high amplitude slow wave in the prefrontal cortex (dotted black arrow) precede the ISAR onset of the thalamus; in the REM example, the increase in high frequencies in the hippocampus (dashes black arrow) is observed before the ISAR onset in the thalamus, while few modifications are observed in the prefrontal cortex. (b) Time frequency‐representation of ISAR ordered by duration in the same patient in N2. The signal power in the delta band is represented in the thalamus, in the hippocampus, and in the prefrontal cortex. N arousals are ordered by duration so that the shortest is at the top and the longest is at the bottom. White points delimit the onset and the end of the arousals according to the scoring performed on the thalamus lead. The figure shows that the strong decrease in the thalamus delta power, used to delimit ISAR, is also visible in the hippocampus and in the prefrontal cortex, and exhibits overall a similar timing
3.2. Hippocampus activity during thalamic intra‐sleep awakening reactions
3.2.1. Hippocampus signal during N2 (and to a lesser extent REM) ISAR differs from both wakefulness and baseline sleep
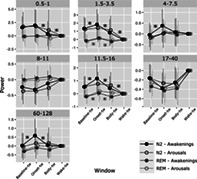
For this first analysis, 320 N2 ISAR (206 arousals, 114 short awakenings) and 157 REM ISAR (85 arousals and 72 short awakenings), lasting more than 5 s, were selected. Signal power in each frequency bands of interest during wake and ISAR from N2 and REM sleep is presented in Figure 4a. A significant interaction between frequency band, time window and sleep stage was found (Chi2 = 168.6; p < .0001).
FIGURE 4.
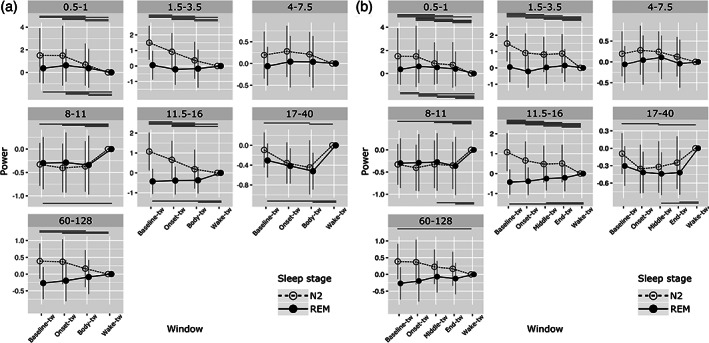
Evolution of spectral power of the signal during sleep, ISAR, and wakefulness in the hippocampus. Mean power (±SD) in different frequency bands (from low delta to gamma). (a) First analysis, during selected temporal windows: baseline‐tw, onset‐tw and body‐tw, and wake‐tw. (b) Second analysis, during selected temporal windows: baseline‐tw, onset‐tw, middle‐tw, end‐tw, and wake‐tw. Transparent circle: N2 sleep; Black circle: REM sleep. Significant differences between time windows (adjusted p‐values) are presented on the figure with horizontal lines located at the top (N2) or the bottom (REM) of each frequency‐band graph (detailed statistical analyses are presented in Tables S1 and S2)
Overall, spectral composition of EEG signal in the hippocampus during sleep, ISAR and wakefulness was quite similar in REM sleep (except for low delta), whereas it differed in many frequency bands, especially low delta, delta, sigma and gamma, in N2. Results of Post hoc comparisons are depicted in Figure 4a and statistical data are detailed in Table S1). We found that, for N2 ISAR, signal power in low delta [0.5–1 Hz], delta [1.5–3.5 Hz], and sigma [11.5–16 Hz] frequency bands was significantly lower during arousal reactions (onset and body) than during preceding sleep (except for low delta, which did not significantly differed from baseline), and remained significantly higher than during wakefulness. Gamma [60–128 Hz] power was higher during baseline N2 than during wakefulness and decreased significantly at the end of the ISAR as compared with baseline, whereas no or few differences were observed for theta [4–7.5 Hz], alpha [8–11 Hz], and beta [17–40 Hz] frequency bands, except for alpha power which was lower during both sleep, onset‐tw, and body‐tw than during wake, and beta power which decreased in the second part of the ISAR (body‐tw) as compared with baseline sleep and wake. For REM ISAR, signal power in low delta [0.5–1 Hz] band remained significantly higher during both baseline sleep, onset and body‐tw than during wakefulness. In alpha, sigma and beta bands, signal power was lower during both baseline sleep and, for beta, body‐tw, as compared with wakefulness whereas the very onset of the ISAR was similar to wakefulness. No significant differences between the four temporal windows were observed for delta, theta, and gamma bands.
3.2.2. Dynamics of hippocampal activity along the ISAR, from sleep back to sleep
In order to further explore intra‐ISAR dynamics, we performed an analysis with five time windows including the onset, the middle and the last part of arousing reactions. For this second analysis, only 211 ISAR longer than 13 s were selected: 16 N2 arousals, 114 N2 short awakenings, 9 REM arousals, and 72 REM short awakenings. As previously, a significant interaction between frequency band, time window and sleep stage was found (Chi2 = 107.4; p < .001). Results of post hoc comparisons are depicted Figure 4b and statistical data are detailed in Table S2). Overall, we observed, for N2 ISAR, a decrease in low delta signal power from the onset to the middle part of the ISAR and a stagnation in the end‐part was observed, but power during this end‐part remained significantly higher than during wakefulness. For delta and sigma, signal power was lower than during sleep and higher than during wakefulness for each intra‐ISAR time window. For theta band, signal power remained stable across temporal windows, and for alpha band it remained overall at lower values than during wakefulness, without any evident dynamics within the ISAR. For beta band, only the middle‐tw exhibited a lower signal power than both baseline‐tw and wake‐tw. Finally, a progressive decrease in signal power in gamma band was observed from the beginning to the end of the ISAR but only baseline and wakefulness significantly differed, with higher gamma power during sleep. For REM ISAR, signal power in low delta was higher during baseline sleep and during each intra‐ISAR time window than during wakefulness and even higher during onset‐tw than during baseline sleep and middle‐tw. No difference between the five temporal time windows was observed for delta, theta, and gamma bands, and few differences were observed for alpha, sigma and beta bands with overall a stable intra‐arousal profile.
3.3. Prefrontal cortex activity during thalamic intra‐sleep awakening reactions
3.3.1. Prefrontal signal during ISAR differs from both wakefulness and baseline sleep in N2 only
Like in the hippocampus, a significant interaction between frequency band, time window and sleep stage was found (Chi2 = 285.9; p < .0001). Results of post hoc comparisons are depicted in Figure 5a and statistical data are detailed in Table S3. For N2 ISAR, an early increase in signal power was observed between baseline sleep and ISAR onset, significant for delta but also for theta, sigma and gamma band. Then, a decrease in signal power in low delta, delta, sigma bands was found between the onset and the end of the arousal and between the end of the arousal and wakefulness. For all these frequency bands, power values were higher during baseline sleep than during wakefulness. No differences between time windows were observed for alpha and beta frequencies. For REM ISAR, no differences between time windows were observed, except for a slight increase in delta frequencies during the ISAR onset as compared with baseline sleep (ns, p = .053) and to the end of the arousal (p = .017).
FIGURE 5.
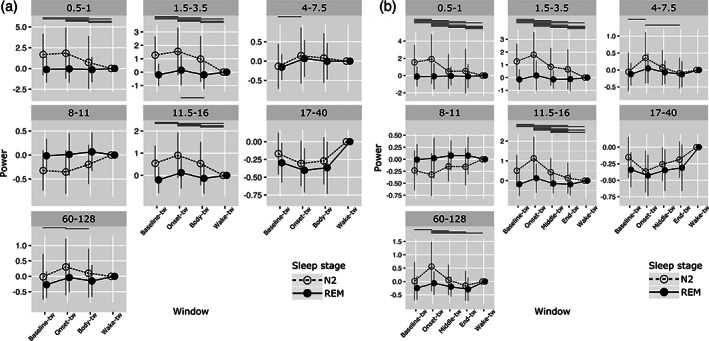
Evolution of spectral power of the signal during sleep, ISAR, and wakefulness in the prefrontal cortex. Mean power (±SD) in different frequency bands (from low delta to gamma). (a) First analysis, during selected temporal windows: baseline‐tw, onset‐tw and body‐tw, and wake‐tw. (b) Second analysis, during selected temporal windows: baseline‐tw, onset‐tw, middle‐tw, end‐tw and wake‐tw. Transparent circle: N2 sleep; Black circle: REM sleep. Significant differences between time windows (adjusted p‐values) are presented on the figure with horizontal lines located at the top (N2) or the bottom (REM) of each frequency‐band graph (detailed statistical analyses are presented in Tables S3 and S4)
3.3.2. Dynamic of frontal activity along the ISAR, from sleep back to sleep
A significant interaction between frequency band, time window and sleep stage was also found for the five time windows analysis (Chi2 = 179.4; p < .0001). Results of post hoc comparisons are depicted Figure 5b and statistical data are detailed in Table S4. This complementary analysis confirmed the profile described above, and added information about the very last part of the arousal in N2 ISAR. It showed that, for low delta and delta, whereas a progressive decrease in signal power between onset and middle time windows was found, no further decrease was observed for the last time window, with rather a nonsignificant re‐increase (low delta) or a stagnation (delta) in power values. These values remained higher than during wakefulness. In contrast, for all higher frequency bands (theta to gamma), signal power during the last time window did not differ from wakefulness. For REM ISAR, no difference between the five time windows was observed.
3.4. ISAR duration depends on the intensity of the first seconds of the arousal reaction in the frontal cortex
In order to compare the dynamics of EEG signal in the hippocampus and in the prefrontal cortex between short (≤15 s, i.e., arousal) and long (>15 s) ISAR, we explored the interaction between type of ISAR (arousal or short awakening), frequency band, sleep stage and time window. This interaction was significant in the prefrontal cortex only (Chi2 = 40.0; p = .01). Visual examination of results presented in Figure 6 prompted us to contrast more specifically the onset time window with baseline sleep and with the body time window, as well as the body time window with the wake time window. In N2, the early increase in low and fast frequencies at the beginning of the ISAR was more intense for long than for short ISAR (delta, p = .046; theta, p = .0008; sigma, p < .0001; gamma, p < .0001) and the decrease in signal power in these frequency bands was also greater during the second part of long than short ISAR (low delta, p < .0001; delta, p < .0001; sigma, p < .0001, gamma, p = .0003). Interestingly, the difference between the last part of long ISAR and wake was lower than the difference between the last part of short ISAR and wake in delta and sigma bands (low delta, p = .0002; delta, p < .0001; sigma, p = .0003). In REM, long and short ISAR differed in delta and sigma band, where the increase in signal power between baseline sleep and onset time window and the decrease between onset and body time window were higher for long than for short events (p = .003 and p = .001, respectively, for delta and p = .011 and p = .023, respectively, for sigma). A highly significant linear positive correlation between the intensity of the initial change in spectral power and ISAR duration was found in N2 for theta and sigma bands (r = .23; 95% CI [0.12; 0.33], p = <.0001 and r = 0.24; 95% CI [0.13; 0.34], p = <.0001, respectively).
FIGURE 6.
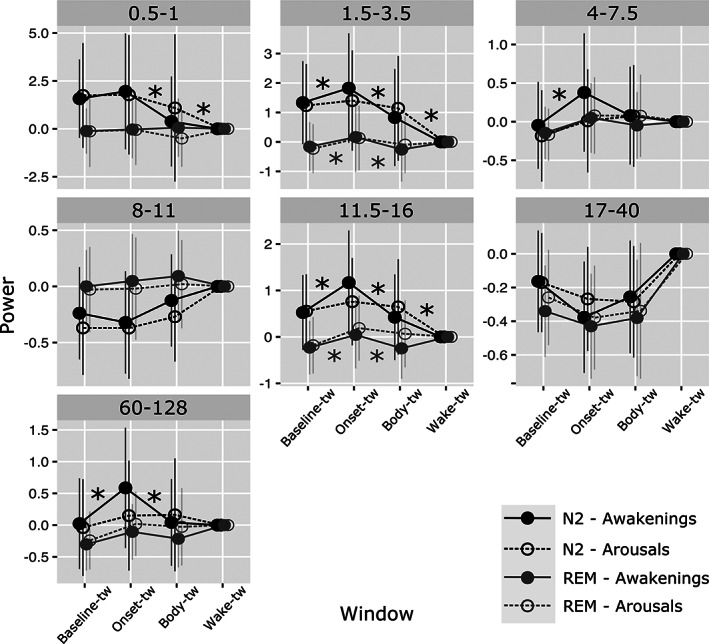
Evolution of spectral power of the signal during sleep, ISAR, and wakefulness in the prefrontal cortex according to the duration of ISAR. Signal power in selected time windows (baseline‐tw, onset‐tw and body‐tw, and wake‐tw) is shown in the prefrontal cortex for arousals (3–15 s, transparent circles and dotted lines) and awakening (15 s to 2 min, full circles, continuous line) in N2 (black) and REM (gray) sleep. Statistically significant differences between arousals and short awakenings for contrast between baseline‐tw and onset‐tw, between onset‐tw and body‐tw, and between body and wake‐tw are indicated with stars. In N2, and in a less extent in REM, longer ISAR are associated with a greater early increase and greater secondary decrease in low and fast activities, with activity during the second part of the arousing reaction being closer to wake‐like activity than is the case for short ISAR
4. DISCUSSION
In the present study, using combined thalamic, hippocampal, and neocortical deep recordings, we provide for the first time a detailed analysis of the hippocampus and prefrontal cortex activation dynamics during arousing reactions detected in the thalamus in human. We demonstrate that (a) in most frequency bands, the spectral power of the signal during arousing reactions is intermediate between wakefulness and sleep in the hippocampus whereas an early increase in low and fast activities is observed in frontal cortex for N2 arousals/awakenings; (b) the signal power evolves according to time reflecting a progressive rather than a clear‐cut phenomenon; (c) the EEG signal during the arousing reactions depends on the preceding sleep stage with fewer modifications in REM than in N2 in hippocampus and prefrontal cortex, potentially reflecting the similarities in the EEG signal between REM and wakefulness in these two structures; (d) the intra‐ISAR dynamics in the prefrontal cortex is associated with the arousal reaction duration, with greater activation at the very onset of longest versus shortest ISAR.
4.1. Awakening as a progressive and local process
We found that EEG activity during N2 arousals and awakenings in the hippocampus and the prefrontal cortex differs from both sleep and wakefulness in most frequency bands and that the pattern of activation exhibits specificities associated to each anatomical structure. The study of transitions between vigilance states in animals and humans, using electrophysiological and intracranial studies, have brought strong arguments in favor of progressive rather than clear‐cut switches from one state to another, underpinned by both spatial (“local wake” or “local sleep” in different areas) and temporal (mixed wake‐like and sleep‐like activity within the same structure) heterogeneities (Magnin et al., 2010; Nobili et al., 2011; Peter‐Derex et al., 2015; Sarasso, Pigorini, et al., 2014; Vyazovskiy et al., 2014). With respect to sleep onset, an asynchrony between cortical regions, between the cortex and the thalamus, and between the cortex and the hippocampus has been reported (Caderas, Niedermeyer, Uematsu, Long, & Nastalski, 1982; Magnin et al., 2010; Sarasso, Proserpio, et al., 2014). Imaging and EEG studies in humans have suggested that awakening was also a progressive process associated with the sequential re‐activation of brain structures and the persistence of sleep slow oscillations, all of these components underlying the phenomenon of sleep inertia (Balkin et al., 2002; Marzano, Ferrara, Moroni, & De Gennaro, 2011; Vallat, Meunier, Nicolas, & Ruby, 2019). However, few S‐EEG studies have specifically studied the sleep‐to‐wake transition except in pathological situations such as parasomnia (Flamand et al., 2018; Terzaghi et al., 2009). Regarding physiological awakening from NREM sleep, Magnin et al. did not report thalamo‐cortical nor intra‐cortical asynchrony, but it is possible that the dimension of activation (which was used to quantify signal complexity and to differentiate vigilance states) was not suitable to explore brief events such as arousals and short awakenings (Magnin et al., 2010). Local phenomena may however occur during arousing processes as Nobili et al. reported short‐lasting local activations during NREM sleep in the motor cortex (blockage of slow waves and increase in alpha‐beta frequencies) whereas other areas, especially the dorsolateral prefrontal cortex, exhibited an increase in slow wave activity (Nobili et al., 2011). In a previous study on arousals recorded in the thalamus and in the cortex, we also observed local arousals and found that the level of “activation,” as assessed by the spectral composition of the signal, differed between the arousals and wakefulness (Peter‐Derex et al., 2015).
In the present study, we confirm the local specificities in arousals, including the particular “mixed slow wave and fast frequencies” activity observed at the onset of the arousal in the prefontal neocortex only (Peter‐Derex et al., 2015), and extent our observations to the hippocampus, where short (<2 min) arousing reactions exhibit some asynchrony with thalamus arousals and are not associated with full recovery of wakefulness activity. This is particularly the case for N2, as specific sleep oscillations such as slow oscillations and delta waves decrease as a function of time during ISAR but do not reach wakefulness values. The progressive rather than abrupt exit from sleep could be expected given the transitional nature of arousal. However, we demonstrate in this study for the first time that this pattern [not entirely consistent with the ASDA definition of an abrupt shift (ASDA, 1992)] is observed in structures whose physiological activity is extremely rarely explored in humans. The fact that wakefulness activity is not reestablished instantaneously in the hippocampus may be associated with the observation that arousals and most short awakenings are not recalled at awakening (Baker, Maloney, & Driver, 1999; Goodenough, Sapan, Cohen, Portnoff, & Shapiro, 1971; Lauderdale, Knutson, Yan, Liu, & Rathouz, 2008; Winser, McBean, & Montgomery‐Downs, 2013). These results are also consistent with studies showing that long awakenings are more often recalled than shorter ones (Campbell & Webb, 1981) and with others works showing that the duration of intra‐sleep awakenings is related to dream recall (Eichenlaub, Bertrand, et al., 2014; Eichenlaub, Nicolas, et al., 2014; Vallat et al., 2017)
4.2. Short awakenings as dynamic events
The difference between wakefulness and ISAR in hippocampus and prefrontal cortex activity may not only reflect an “inertia” effect, but also the fact that arousals and short awakenings are, by definition, unstable, heterogeneous and transient processes: they reflect both sleep‐to‐wake and wake‐back‐to‐sleep phenomena. Indeed, we observed an intra‐ISAR dynamics, as demonstrated by the differences observed in several frequency bands between the onset, the middle and/or the end of the arousing reaction. Interestingly, this dynamics differed between frequency bands and between structures. For example, in frontal cortex, a strong decrease in spectral power was observed in many frequency bands (low and fast activities) between the onset and the middle of N2 ISAR but the last part was marked by a stagnation (or even a nonsignificant re‐increase for low delta) in signal power. In hippocampus N2 ISAR, the arousing reaction was associated with an early decrease in some specific sleep oscillations (delta and sigma). In contrast, a decrease during the second part of the ISAR (body‐tw for gamma and beta power) was found in fast activities as compared with baseline whereas this decrease was not observed at the arousal onset. This decrease in gamma power in the hippocampus might be due to a decrease in ripples, which characterize the activity of the hippocampus during NREM and are believed to play a key role in long term memory consolidation processes (Axmacher et al., 2008; Cantero et al., 2004; Clemens et al., 2007; Staresina et al., 2015). Such differences in temporal dynamics between distinct oscillations within a single brain structure may be associated with functional specificities of transitional stages. It can be hypothesized that the functional impairment associated with sleep fragmentation is not only the consequence of a decrease in the sleep time spent in different stages but also of an increased duration of these transitional states where neither wakefulness nor sleep activity (nor stage‐specific related functions) are fully re‐instored (Bonnet & Arand, 2003; Stepanski, Lamphere, Badia, Zorick, & Roth, 1984).
4.3. Sleep stage matters
Regarding full awakening, sleep inertia has been reported to be more pronounced in N3 than in REM sleep than in N2/N1 (Tassi & Muzet, 2000; Vallat et al., 2019). In the present study, we also observed that the hippocampus and the prefrontal cortex activity during transitions between sleep and wakefulness depends on the ongoing sleep stage. In particular, few differences in S‐EEG signal were observed for REM sleep ISAR between baseline sleep, arousing reactions, and full wakefulness. This could suggest that no arousing reaction occurred in the hippocampus or in the frontal cortex at the time of thalamic arousals; however, the observation of significant changes in some frequency bands (increase in low delta frequency at the onset of the arousal in the hippocampus and decrease in delta between the first and the last part of the arousal in the prefrontal cortex) indicates that the modification in thalamic activity was associated with some subtle changes in hippocampo‐frontal structures activity. This findings echoes our previous work about cortical heterogeneity in arousing patterns, as we observed very few changes in frontal area between baseline REM sleep and arousals whereas, at the same time, obvious modifications (especially in alpha band) were found in posterior areas (Peter‐Derex et al., 2015). The fact that, unlike what was observed for NREM sleep arousals, both prefrontal cortex and hippocampus activities during REM sleep arousals were not very different from wake and REM sleep activity may be explained by the fact that the spectral composition of the signal is more similar between REM and wakefulness than between NREM and wakefulness. This finding is in line with previous works about the prefrontal and hippocampus activity according to vigilance stages, explored with intracranial recordings although, unlike in our study, no direct comparison between stages was reported (von Ellenrieder et al., 2020). Specifically, a particular slow oscillation has been described during both wakefulness and REM in the hippocampus, and referred as a “rhythmic slow activity” (1.5–3 Hz) which may be the counterpart of the theta activity observed in rodents (Bodizs et al., 2001; Brazier, 1968; Clemens et al., 2009; Moroni et al., 2007; Watrous et al., 2013). This delta activity has been associated with specific cognitive functions such as navigation and memory formation (Brazier, 1968; Lega, Jacobs, & Kahana, 2012; Watrous et al., 2013). Gamma oscillations are also observed in both REM and wakefulness (Mari, Zelmann, Andrade‐Valenca, Dubeau, & Gotman, 2012). We found that only alpha to beta frequencies differed significantly between REM and wakefulness, with higher spectral power during the latter state. This proximity between REM sleep and wakefullness activity in the hippocampus, also found in the prefrontal cortex, may facilitate the transition between these two stages in these structures. This may contribute to the difference in dream recall between NREM and REM awakenings (Eichenlaub, Bertrand, et al., 2014; Eichenlaub, Nicolas, et al., 2014; Nielsen, 2000; Stickgold, Malia, Fosse, Propper, & Hobson, 2001) by allowing during arousals from REM sleep a faster reinstatement of encoding functions thought to be impaired during sleep (Diekelmann & Born, 2010; Tononi & Cirelli, 2014).
4.4. Awakening duration depends on the very first seconds of the arousing reaction
Every studied ISAR ended with a come back to sleep. However, the time needed to reach sleep again differed between arousals and awakenings. While no clear difference between short and long ISAR could be seen in the hippocampus, the duration of the ISAR was associated with the intensity of the early arousing reaction in the prefrontal cortex and the strength of the decrease in slow and fast oscillations during the second part of the event, with longer events being associated with the restoration of an activity closer to wakefulness. This result may appear as paradoxical but it refers to the complex nature of arousal (Halasz, Terzano, Parrino, & Bodizs, 2004). The early delta increase observed during the first seconds of the arousal may correspond to a slow reactive grapho‐element (K‐complex or delta burst) which is mixed with high frequency activities (Halasz & Ujszaszi, 1991; Latreille et al., 2020). As the arousal reaction evolves, delta power decrease and get closer to wakefulness values. At the same time, a decrease in sigma band, corresponding to spindles which are suppressed during arousals, is observed. Thus, the very beginning of the arousing reaction in the prefrontal cortex led to different trajectories back to sleep with a positive correlation between the intensity of this initial activation and the duration of the ISAR in N2. ISAR duration, reflecting the time needed to restore sleep, may be related to the relative balance between arousal and anti‐arousal processes engaged from the very onset of the arousing reaction. Individual differences in brain reactivity to auditory stimuli during sleep, as assessed by the amplitude of cognitive evoked potentials, have been reported between high and low dream recallers: a higher reactivity would enable a longer awakening duration and thus allow for a greater brain “re‐activation” necessary for encoding and consolidating dream content (Vallat et al., 2017).
4.5. Limitations
Several limitations should be kept in mind when interpreting the present findings. First, the results were obtained in patients with epilepsy taking anti‐seizure drugs with more disrupted sleep than healthy individuals as evidenced by the high number of ISAR detected in our study. Although we applied very strict criteria for hippocampus and orbitofrontal channels selection, we cannot definitely rule out the fact that the underlying epileptic condition may have influenced arousing processes on a quantitative (arousal threshold) or qualitative (spectral composition of the signal) way. However, all works about electrophysiological activity in the hippocampus during wakefulness and sleep have used such a methodology as intracranial recordings are obviously only performed for clinical purposes (Bodizs et al., 2001; Brazier, 1968; Moroni et al., 2007; Sarasso, Proserpio, et al., 2014). Moreover, our results are in line with noninvasive scalp EEG works about sleep–wake transition, which demonstrated a progressive restoration of wakefulness activity (Marzano et al., 2011; Vallat et al., 2019). Last, our findings about wake and sleep activity in the hippocampus and the frontal cortex are consistent with results reported by many other groups in patients taking different types of anti‐seizure drugs, which argues for the fact that main EEG features of vigilance states are robust (Bodizs et al., 2001; Brazier, 1968; Frauscher et al., 2018; Moroni et al., 2007; von Ellenrieder et al., 2020). Another limitation is the small number of patients, which is the counterpart of our strict selection criteria: however, the high number of arousals/awakenings allowed us to overcome this limitation. We also acknowledge that we investigated the hippocampus activity during thalamic arousals without assumptions about the presence or not of a concomitant detectable scalp EEG arousal (we provide nonetheless in one patient data and figures which suggest a good correspondence between the thalamic and scalp EEG arousals). As arousals have been shown to be local phenomena (Nobili et al., 2011; Peter‐Derex et al., 2015) it is not clear what is best, the use of scalp EEG or of S‐EEG activity from selected cortical brain area to detect arousals. There is currently no ideal brain structure to identify ISAR or gold standard strictly and precisely defining arousals at the local and global level. However, the particular connection of the medial pulvinar thalamus with subcortical, cortical and limbic structures makes the thalamus a good candidate for a pertinent structure to detect arousal in the context of hippocampus activity study (Benarroch, 2015; Gattass, Soares, & Lima, 2018). Finally, the fact that this study was retrospective did not allow us to standardize arousals regarding the trigger (all arousals were “spontaneous,” that is, we do not know what internal or external stimulation had triggered them) and to explore the cognitive correlates of our findings, regarding memory of arousing reactions for example.
5. CONCLUSIONS
As a conclusion, thanks to the unique opportunity of simultaneous recordings of the thalamus and of healthy hippocampus and prefrontal cortex in humans, we found that awakening from sleep in the hippocampus and orbitofrontal cortex is a progressive and dynamic process, modulated by the stage of sleep and, in the frontal cortex, by the intensity of the initial arousing response. Such findings may explain variability in dream recall, REM sleep‐related, and longer awakenings allowing the hippocampus activity to get closer to wakefulness activity and thus to recover encoding and consolidation functions. These results are in line with dream recall theories regarding the key role of brain reactivity and awakening length in memory formation (De Gennaro et al., 2010; Eichenlaub, Bertrand, et al., 2014; Eichenlaub, Nicolas, et al., 2014; Ruby et al., 2013; Vallat et al., 2017). Further studies will be necessary to confirm this hypothesis. Notably, the investigation in the same study of the activity in memory‐related brain areas, and of functional connectivity between these areas, along with subjective perception and memory of arousals/awakenings and of dreams would help to understand which particular activity is critical to these cognitive processes.
CONFLICT OF INTEREST
The authors have declared no conflicts of interest for this article.
AUTHOR CONTRIBUTION
Perrine Ruby: Research design, data analysis, writing‐editing. Romain Bouet: Research design, data analysis, writing‐editing. Laure Peter‐Derex: Research design, data analysis, writing‐editing. Mickël Eskinazi: Data analysis, writing‐editing. Sylvain Rheims: Data collection, writing‐editing.
ETHICS STATEMENT
The procedure was approved by the national Ethics Committee (Comité de Protection des Personnes CPP 09‐CHUG‐12, no 0907). Patients were fully informed and gave written informed consent for the use of intra‐cerebral recordings for research purposes.
Supporting information
Table S1 Comparison of spectral power between four time windows (first analysis) in the hippocampus. Table S1A: N2 sleep intra sleep awakening reactions; Table S1B: REM sleep intra sleep awakening reactions
Table S2 Comparison of spectral power between five time windows (second analysis) in the hippocampus. Table S2A: N2 sleep intra sleep awakening reactions; Table S2B: REM sleep intra sleep awakening reactions
Table S3 Comparison of spectral power between four time windows (first analysis) in the prefrontal cortex. Table S3A: N2 sleep intra sleep awakening reactions; Table S3B: REM sleep intra sleep awakening reactions
Table S4 Comparison of spectral power between four time windows (second analysis) in the prefrontal cortex. Table S4A: N2 sleep intra sleep awakening reactions; Table S4B: REM sleep intra sleep awakening reactions
Figure S1 Examples of arousals observed in intracranial and scalp derivations (patient 4) in N2, N3, and REM sleep. Three intracranial channels are showed (top); they record from the thalamus, the hippocampus and the orbitofrontal‐cortex. Below are showed right‐hemispheric scalp channels (contro‐lateral to the epileptic focus which prevented scalp exploration of the left side given the high number of intracranial electrodes on this side and the infectious risk), the EOG and EKG. The red square indicates the thalamic arousal. In N2, the arousal consists in a desynchronized fast activity observed in every intracranial channels and in scalp; note the mixed slow waves in all channels except for the thalamus. In N3, the onset of the arousal is clearly seen in the thalamus where the fast activity contrasts with the preceding slow waves, whereas mixed slow and fast activities are observed at the same time in the hippocampus, the orbitofrontal cortex and scalp channels (black arrow). In REM, the very particular slow activity in the thalamus allows for a clear temporal delimitation of the arousal, which is also observed in the hippocampus and orbitofrontal cortex (increase in fast frequencies) as well as scalp channels (alpha activity). Note the electromyographic artifacts (dotted black arrow) in the scalp EEG where the arousal seems to start 1 s before the thalamic arousal.
Ruby, P. , Eskinazi, M. , Bouet, R. , Rheims, S. , & Peter‐Derex, L. (2021). Dynamics of hippocampus and orbitofrontal cortex activity during arousing reactions from sleep: An intracranial electroencephalographic study. Human Brain Mapping, 42(16), 5188–5203. 10.1002/hbm.25609
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Aarons, L. (1976). Sleep‐assisted instruction. Psychological Bulletin, 83(1), 1–40. [PubMed] [Google Scholar]
- Albouy, G. , Sterpenich, V. , Balteau, E. , Vandewalle, G. , Desseilles, M. , Dang‐Vu, T. , … Maquet, P. (2008). Both the hippocampus and striatum are involved in consolidation of motor sequence memory. Neuron, 58(2), 261–272. 10.1016/j.neuron.2008.02.008 [DOI] [PubMed] [Google Scholar]
- Andrillon, T. , Pressnitzer, D. , Leger, D. , & Kouider, S. (2017). Formation and suppression of acoustic memories during human sleep. Nature Communications, 8(1), 179. 10.1038/s41467-017-00071-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- ASDA . (1992). EEG arousals: Scoring rules and examples: A preliminary report from the sleep disorders atlas task force of the American sleep disorders association. Sleep, 15(2), 173–184. [PubMed] [Google Scholar]
- Axmacher, N. , Elger, C. E. , & Fell, J. (2008). Ripples in the medial temporal lobe are relevant for human memory consolidation. Brain: A Journal of Neurology, 131, 1806–1817. 10.1093/brain/awn103 [DOI] [PubMed] [Google Scholar]
- Baker, F. C. , Maloney, S. , & Driver, H. S. (1999). A comparison of subjective estimates of sleep with objective polysomnographic data in healthy men and women. Journal of Psychosomatic Research, 47(4), 335–341. 10.1016/s0022-3999(99)00017-3 [DOI] [PubMed] [Google Scholar]
- Balkin, T. J. , Braun, A. R. , Wesensten, N. J. , Jeffries, K. , Varga, M. , Baldwin, P. , … Herscovitch, P. (2002). The process of awakening: A PET study of regional brain activity patterns mediating the re‐establishment of alertness and consciousness. Brain: A Journal of Neurology, 125, 2308–2319. 10.1093/brain/awf228 [DOI] [PubMed] [Google Scholar]
- Bancaud, J. , & Talairach, J. (1973). Methodology of stereo EEG exploration and surgical intervention in epilepsy. Revue d'oto‐Neuro‐Ophtalmologie, 45(4), 315–328. [PubMed] [Google Scholar]
- Bastuji, H. , & Garcia‐Larrea, L. (1999). Evoked potentials as a tool for the investigation of human sleep. Sleep Medicine Reviews, 3(1), 23–45. [DOI] [PubMed] [Google Scholar]
- Bates, D. , Mächler, M. , Bolker, B. , & Walker, S. (2015). Fitting linear mixed‐effects models using lme4. Journal of Statistical Software, 67(1), 1–48. [Google Scholar]
- Benarroch, E. E. (2015). Pulvinar: Associative role in cortical function and clinical correlations. Neurology, 84(7), 738–747. 10.1212/WNL.0000000000001276 [DOI] [PubMed] [Google Scholar]
- Berry, R. B. , Brooks, R. , Gamaldo, C. E. , Harding, S. M. , Lloyd, R. , Marcus, C. L. , & Vaughn, B. V. (2017). The AASM manual for the scoring of sleep and associated events: Rules, terminology and technical specifications, version 2.4. Darien, Illinois: American Academy of Sleep Medicine. [Google Scholar]
- Bodizs, R. , Kantor, S. , Szabo, G. , Szucs, A. , Eross, L. , & Halasz, P. (2001). Rhythmic hippocampal slow oscillation characterizes REM sleep in humans. Hippocampus, 11(6), 747–753. 10.1002/hipo.1090 [DOI] [PubMed] [Google Scholar]
- Bonnet, M. H. , & Arand, D. L. (2003). Clinical effects of sleep fragmentation versus sleep deprivation. Sleep Medicine Reviews, 7(4), 297–310. [DOI] [PubMed] [Google Scholar]
- Brazier, M. A. (1968). Studies of the EEG activity of limbic structures in man. Electroencephalography and Clinical Neurophysiology, 25(4), 309–318. 10.1016/0013-4694(68)90171-5 [DOI] [PubMed] [Google Scholar]
- Caderas, M. , Niedermeyer, E. , Uematsu, S. , Long, D. M. , & Nastalski, J. (1982). Sleep spindles recorded from deep cerebral structures in man. Clinical EEG (Electroencephalography), 13(4), 216–225. 10.1177/155005948201300402 [DOI] [PubMed] [Google Scholar]
- Campbell, S. S. , & Webb, W. B. (1981). The perception of wakefulness within sleep. Sleep, 4(2), 177–183. [PubMed] [Google Scholar]
- Cantero, J. L. , Atienza, M. , Madsen, J. R. , & Stickgold, R. (2004). Gamma EEG dynamics in neocortex and hippocampus during human wakefulness and sleep. NeuroImage, 22(3), 1271–1280. 10.1016/j.neuroimage.2004.03.014 [DOI] [PubMed] [Google Scholar]
- Clemens, Z. , Molle, M. , Eross, L. , Barsi, P. , Halasz, P. , & Born, J. (2007). Temporal coupling of parahippocampal ripples, sleep spindles and slow oscillations in humans. Brain: A Journal of Neurology, 130(Pt 11), 2868–2878. 10.1093/brain/awm146 [DOI] [PubMed] [Google Scholar]
- Clemens, Z. , Weiss, B. , Szucs, A. , Eross, L. , Rasonyi, G. , & Halasz, P. (2009). Phase coupling between rhythmic slow activity and gamma characterizes mesiotemporal rapid‐eye‐movement sleep in humans. Neuroscience, 163(1), 388–396. 10.1016/j.neuroscience.2009.06.044 [DOI] [PubMed] [Google Scholar]
- De Gennaro, L. , Marzano, C. , Moroni, F. , Curcio, G. , Ferrara, M. , & Cipolli, C. (2010). Recovery sleep after sleep deprivation almost completely abolishes dream recall. Behavioural Brain Research, 206(2), 293–298. 10.1016/j.bbr.2009.09.030 [DOI] [PubMed] [Google Scholar]
- Diekelmann, S. , & Born, J. (2010). The memory function of sleep. Nature Reviews. Neuroscience, 11(2), 114–126. 10.1038/nrn2762 [DOI] [PubMed] [Google Scholar]
- Douchamps, V. , Jeewajee, A. , Blundell, P. , Burgess, N. , & Lever, C. (2013). Evidence for encoding versus retrieval scheduling in the hippocampus by theta phase and acetylcholine. The Journal of Neuroscience, 33(20), 8689–8704. 10.1523/JNEUROSCI.4483-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenlaub, J.‐B. , Bertrand, O. , Morlet, D. , & Ruby, P. (2014). Brain reactivity differentiates subjects with high and low dream recall frequencies during both sleep and wakefulness. Cerebral Cortex (New York, N.Y.: 1991), 24(5), 1206–1215. 10.1093/cercor/bhs388 [DOI] [PubMed] [Google Scholar]
- Eichenlaub, J. B. , Nicolas, A. , Daltrozzo, J. , Redoute, J. , Costes, N. , & Ruby, P. (2014). Resting brain activity varies with dream recall frequency between subjects. Neuropsychopharmacology, 39(7), 1594–1602. 10.1038/npp.2014.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flamand, M. , Boudet, S. , Lopes, R. , Vignal, J.‐P. , Reyns, N. , Charley‐Monaca, C. , … Szurhaj, W. (2018). Confusional arousals during non‐rapid eye movement sleep: Evidence from intracerebral recordings. Sleep, 41(10). 10.1093/sleep/zsy139 [DOI] [PubMed] [Google Scholar]
- Frauscher, B. , Bernasconi, N. , Caldairou, B. , von Ellenrieder, N. , Bernasconi, A. , Gotman, J. , & Dubeau, F. (2015). Interictal hippocampal spiking influences the occurrence of hippocampal sleep spindles. Sleep, 38(12), 1927–1933. 10.5665/sleep.5242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frauscher, B. , von Ellenrieder, N. , Zelmann, R. , Dolezalova, I. , Minotti, L. , Olivier, A. , … Gotman, J. (2018). Atlas of the normal intracranial electroencephalogram: Neurophysiological awake activity in different cortical areas. Brain: A Journal of Neurology, 141(4), 1130–1144. 10.1093/brain/awy035 [DOI] [PubMed] [Google Scholar]
- Fox, J. , & Weisberg, S. (2011). An R companion to applied regression, Thousand Oaks, CA: SAGE Publications. [Google Scholar]
- Gattass, R. , Soares, J. G. M. , & Lima, B. (2018). Modulation of Pulvinar neuronal activity by arousal. Advances in Anatomy, Embryology, and Cell Biology, 225, 49–51. 10.1007/978-3-319-70046-5_10 [DOI] [PubMed] [Google Scholar]
- Goodenough, D. R. , Sapan, J. , Cohen, H. , Portnoff, G. , & Shapiro, A. (1971). Some experiments concerning the effects of sleep on memory. Psychophysiology, 8(6), 749–762. 10.1111/j.1469-8986.1971.tb00512.x [DOI] [PubMed] [Google Scholar]
- Guenot, M. , Isnard, J. , Ryvlin, P. , Fischer, C. , Ostrowsky, K. , Mauguiere, F. , & Sindou, M. (2001). Neurophysiological monitoring for epilepsy surgery: The Talairach SEEG method. StereoElectroEncephaloGraphy. Indications, results, complications and therapeutic applications in a series of 100 consecutive cases. Stereotactic and Functional Neurosurgery, 77, 29–32. 10.1159/000064595 [DOI] [PubMed] [Google Scholar]
- Halasz, P. , Terzano, M. , Parrino, L. , & Bodizs, R. (2004). The nature of arousal in sleep. Journal of Sleep Research, 13(1), 1–23. 10.1111/j.1365-2869.2004.00388.x [DOI] [PubMed] [Google Scholar]
- Halasz, P. , & Ujszaszi, J. (1991). Spectral features of evoked microarousals. New York, NY: Raven Press. [Google Scholar]
- Koulack, D. , & Goodenough, D. R. (1976). Dream recall and dream recall failure: An arousal‐retrieval model. Psychological Bulletin, 83, 975–984. [Google Scholar]
- Lambert, I. , Roehri, N. , Giusiano, B. , Carron, R. , Wendling, F. , Benar, C. , & Bartolomei, F. (2018). Brain regions and epileptogenicity influence epileptic interictal spike production and propagation during NREM sleep in comparison with wakefulness. Epilepsia, 59(1), 235–243. 10.1111/epi.13958 [DOI] [PubMed] [Google Scholar]
- Latreille, V. , von Ellenrieder, N. , Peter‐Derex, L. , Dubeau, F. , Gotman, J. , & Frauscher, B. (2020). The human K‐complex: Insights from combined scalp‐intracranial EEG recordings. NeuroImage, 213, 116748. 10.1016/j.neuroimage.2020.116748 [DOI] [PubMed] [Google Scholar]
- Lauderdale, D. S. , Knutson, K. L. , Yan, L. L. , Liu, K. , & Rathouz, P. J. (2008). Self‐reported and measured sleep duration: How similar are they? Epidemiology, 19(6), 838–845. 10.1097/EDE.0b013e318187a7b0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lega, B. C. , Jacobs, J. , & Kahana, M. (2012). Human hippocampal theta oscillations and the formation of episodic memories. Hippocampus, 22(4), 748–761. 10.1002/hipo.20937 [DOI] [PubMed] [Google Scholar]
- Magnin, M. , Bastuji, H. , Garcia‐Larrea, L. , & Mauguiere, F. (2004). Human thalamic medial pulvinar nucleus is not activated during paradoxical sleep. Cerebral Cortex (New York, N.Y.: 1991), 14(8), 858–862. 10.1093/cercor/bhh044 [DOI] [PubMed] [Google Scholar]
- Magnin, M. , Rey, M. , Bastuji, H. , Guillemant, P. , Mauguiere, F. , & Garcia‐Larrea, L. (2010). Thalamic deactivation at sleep onset precedes that of the cerebral cortex in humans. Proceedings of the National Academy of Sciences of the United States of America, 107(8), 3829–3833. 10.1073/pnas.0909710107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mari, F. , Zelmann, R. , Andrade‐Valenca, L. , Dubeau, F. , & Gotman, J. (2012). Continuous high‐frequency activity in mesial temporal lobe structures. Epilepsia, 53(5), 797–806. 10.1111/j.1528-1167.2012.03428.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzano, C. , Ferrara, M. , Moroni, F. , & De Gennaro, L. (2011). Electroencephalographic sleep inertia of the awakening brain. Neuroscience, 176, 308–317. 10.1016/j.neuroscience.2010.12.014 [DOI] [PubMed] [Google Scholar]
- Mizuseki, K. , & Miyawaki, H. (2017). Hippocampal information processing across sleep/wake cycles. Neuroscience Research, 118, 30–47. 10.1016/j.neures.2017.04.018 [DOI] [PubMed] [Google Scholar]
- Montplaisir, J. , Leduc, L. , Laverdiere, M. , Walsh, J. , & Saint‐Hilaire, J. M. (1981). Sleep spindles in the human hippocampus: Normal or epileptic activity? Sleep, 4(4), 423–428. [DOI] [PubMed] [Google Scholar]
- Morel, A. , Magnin, M. , & Jeanmonod, D. (1997). Multiarchitectonic and stereotactic atlas of the human thalamus. The Journal of Comparative Neurology, 387(4), 588–630. [DOI] [PubMed] [Google Scholar]
- Moroni, F. , Nobili, L. , Curcio, G. , De Carli, F. , Fratello, F. , Marzano, C. , … Ferrara, M. (2007). Sleep in the human hippocampus: A stereo‐EEG study. PLoS One, 2(9), e867. 10.1371/journal.pone.0000867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen, T. A. (2000). A review of mentation in REM and NREM sleep: "covert" REM sleep as a possible reconciliation of two opposing models. The Behavioral and Brain Sciences, 23(6), 851–866. 10.1017/s0140525x0000399x [DOI] [PubMed] [Google Scholar]
- Nobili, L. , Ferrara, M. , Moroni, F. , De Gennaro, L. , Russo, G. L. , Campus, C. , … De Carli, F. (2011). Dissociated wake‐like and sleep‐like electro‐cortical activity during sleep. NeuroImage, 58(2), 612–619. 10.1016/j.neuroimage.2011.06.032 [DOI] [PubMed] [Google Scholar]
- Ostrowsky, K. , Magnin, M. , Ryvlin, P. , Isnard, J. , Guenot, M. , & Mauguiere, F. (2002). Representation of pain and somatic sensation in the human insula: A study of responses to direct electrical cortical stimulation. Cerebral Cortex (New York, N.Y.: 1991), 12(4), 376–385. [DOI] [PubMed] [Google Scholar]
- Peter‐Derex, L. , Comte, J.‐C. , Mauguiere, F. , & Salin, P. A. (2012). Density and frequency caudo‐rostral gradients of sleep spindles recorded in the human cortex. Sleep, 35(1), 69–79. 10.5665/sleep.1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter‐Derex, L. , Magnin, M. , & Bastuji, H. (2015). Heterogeneity of arousals in human sleep: A stereo‐electroencephalographic study. NeuroImage, 123, 229–244. 10.1016/j.neuroimage.2015.07.057 [DOI] [PubMed] [Google Scholar]
- Rosenberg, D. S. , Mauguiere, F. , Demarquay, G. , Ryvlin, P. , Isnard, J. , Fischer, C. , … Magnin, M. (2006). Involvement of medial pulvinar thalamic nucleus in human temporal lobe seizures. Epilepsia, 47(1), 98–107. 10.1111/j.1528-1167.2006.00375.x [DOI] [PubMed] [Google Scholar]
- Ruby, P. , Blochet, C. , Eichenlaub, J. B. , Bertrand, O. , Morlet, D. , & Bidet‐Caulet, A. (2013). Alpha reactivity to first names differs in subjects with high and low dream recall frequency. Frontiers in Psychology, 4, 419. 10.3389/fpsyg.2013.00419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruch, S. , & Henke, K. (2020). Learning during sleep: A dream comes true? Trends in Cognitive Sciences, 24(3), 170–172. 10.1016/j.tics.2019.12.007 [DOI] [PubMed] [Google Scholar]
- Sarasso, S. , Pigorini, A. , Proserpio, P. , Gibbs, S. A. , Massimini, M. , & Nobili, L. (2014). Fluid boundaries between wake and sleep: Experimental evidence from stereo‐EEG recordings. Archives Italiennes de Biologie, 152(2‐3), 169–177. 10.12871/0002982920142311 [DOI] [PubMed] [Google Scholar]
- Sarasso, S. , Proserpio, P. , Pigorini, A. , Moroni, F. , Ferrara, M. , De Gennaro, L. , … Nobili, L. (2014). Hippocampal sleep spindles preceding neocortical sleep onset in humans. NeuroImage, 86, 425–432. 10.1016/j.neuroimage.2013.10.031 [DOI] [PubMed] [Google Scholar]
- Searle, S. R. , Speed, F. M. , & Milliken, G. A. (1980). Population marginal means in the linear model: An alternative to least squares means. The American Statistician, 34(4), 216–221. [Google Scholar]
- Squire, L. R. , & Zola‐Morgan, S. (1991). The medial temporal lobe memory system. Science (New York, N.Y.), 253(5026), 1380–1386. [DOI] [PubMed] [Google Scholar]
- Staresina, B. P. , Bergmann, T. O. , Bonnefond, M. , van der Meij, R. , Jensen, O. , Deuker, L. , … Fell, J. (2015). Hierarchical nesting of slow oscillations, spindles and ripples in the human hippocampus during sleep. Nature Neuroscience, 18(11), 1679–1686. 10.1038/nn.4119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanski, E. , Lamphere, J. , Badia, P. , Zorick, F. , & Roth, T. (1984). Sleep fragmentation and daytime sleepiness. Sleep, 7(1), 18–26. 10.1093/sleep/7.1.18 [DOI] [PubMed] [Google Scholar]
- Stickgold, R. , Malia, A. , Fosse, R. , Propper, R. , & Hobson, J. A. (2001). Brain‐mind states: I. longitudinal field study of sleep/wake factors influencing mentation report length. Sleep, 24(2), 171–179. [DOI] [PubMed] [Google Scholar]
- Suzuki, W. A. (2006). Encoding new episodes and making them stick. Neuron, 50(1), 19–21. 10.1016/j.neuron.2006.03.029 [DOI] [PubMed] [Google Scholar]
- Tassi, P. , & Muzet, A. (2000). Sleep inertia. Sleep Medicine Reviews, 4(4), 341–353. 10.1053/smrv.2000.0098 [DOI] [PubMed] [Google Scholar]
- Terzaghi, M. , Sartori, I. , Tassi, L. , Didato, G. , Rustioni, V. , LoRusso, G. , … Nobili, L. (2009). Evidence of dissociated arousal states during NREM parasomnia from an intracerebral neurophysiological study. Sleep, 32(3), 409–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tononi, G. , & Cirelli, C. (2014). Sleep and the price of plasticity: From synaptic and cellular homeostasis to memory consolidation and integration. Neuron, 81(1), 12–34. 10.1016/j.neuron.2013.12.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallat, R. , Lajnef, T. , Eichenlaub, J.‐B. , Berthomier, C. , Jerbi, K. , Morlet, D. , & Ruby, P. M. (2017). Increased evoked potentials to arousing auditory stimuli during sleep: Implication for the understanding of dream recall. Frontiers in Human Neuroscience, 11, 132. 10.3389/fnhum.2017.00132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallat, R. , Meunier, D. , Nicolas, A. , & Ruby, P. (2019). Hard to wake up? The cerebral correlates of sleep inertia assessed using combined behavioral, EEG and fMRI measures. NeuroImage, 184, 266–278. 10.1016/j.neuroimage.2018.09.033 [DOI] [PubMed] [Google Scholar]
- von Ellenrieder, N. , Gotman, J. , Zelmann, R. , Rogers, C. , Nguyen, D. K. , Kahane, P. , … Frauscher, B. (2020). How the human brain sleeps: Direct cortical recordings of Normal brain activity. Annals of Neurology, 87(2), 289–301. 10.1002/ana.25651 [DOI] [PubMed] [Google Scholar]
- Vyazovskiy, V. V. , Cui, N. , Rodriguez, A. V. , Funk, C. , Cirelli, C. , & Tononi, G. (2014). The dynamics of cortical neuronal activity in the first minutes after spontaneous awakening in rats and mice. Sleep, 37(8), 1337–1347. 10.5665/sleep.3926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker, M. P. , & Stickgold, R. (2004). Sleep‐dependent learning and memory consolidation. Neuron, 44(1), 121–133. 10.1016/j.neuron.2004.08.031 [DOI] [PubMed] [Google Scholar]
- Watrous, A. J. , Lee, D. J. , Izadi, A. , Gurkoff, G. G. , Shahlaie, K. , & Ekstrom, A. D. (2013). A comparative study of human and rat hippocampal low‐frequency oscillations during spatial navigation. Hippocampus, 23(8), 656–661. 10.1002/hipo.22124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winser, M. A. , McBean, A. L. , & Montgomery‐Downs, H. E. (2013). Minimum duration of actigraphy‐defined nocturnal awakenings necessary for morning recall. Sleep Medicine, 14(7), 688–691. 10.1016/j.sleep.2013.03.018 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Comparison of spectral power between four time windows (first analysis) in the hippocampus. Table S1A: N2 sleep intra sleep awakening reactions; Table S1B: REM sleep intra sleep awakening reactions
Table S2 Comparison of spectral power between five time windows (second analysis) in the hippocampus. Table S2A: N2 sleep intra sleep awakening reactions; Table S2B: REM sleep intra sleep awakening reactions
Table S3 Comparison of spectral power between four time windows (first analysis) in the prefrontal cortex. Table S3A: N2 sleep intra sleep awakening reactions; Table S3B: REM sleep intra sleep awakening reactions
Table S4 Comparison of spectral power between four time windows (second analysis) in the prefrontal cortex. Table S4A: N2 sleep intra sleep awakening reactions; Table S4B: REM sleep intra sleep awakening reactions
Figure S1 Examples of arousals observed in intracranial and scalp derivations (patient 4) in N2, N3, and REM sleep. Three intracranial channels are showed (top); they record from the thalamus, the hippocampus and the orbitofrontal‐cortex. Below are showed right‐hemispheric scalp channels (contro‐lateral to the epileptic focus which prevented scalp exploration of the left side given the high number of intracranial electrodes on this side and the infectious risk), the EOG and EKG. The red square indicates the thalamic arousal. In N2, the arousal consists in a desynchronized fast activity observed in every intracranial channels and in scalp; note the mixed slow waves in all channels except for the thalamus. In N3, the onset of the arousal is clearly seen in the thalamus where the fast activity contrasts with the preceding slow waves, whereas mixed slow and fast activities are observed at the same time in the hippocampus, the orbitofrontal cortex and scalp channels (black arrow). In REM, the very particular slow activity in the thalamus allows for a clear temporal delimitation of the arousal, which is also observed in the hippocampus and orbitofrontal cortex (increase in fast frequencies) as well as scalp channels (alpha activity). Note the electromyographic artifacts (dotted black arrow) in the scalp EEG where the arousal seems to start 1 s before the thalamic arousal.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


