Abstract
Repetitive TMS (rTMS) with a frequency of 5–10 Hz is widely used for language mapping. However, it may be accompanied by discomfort and is limited in the number and reliability of evoked language errors. We, here, systematically tested the influence of different stimulation frequencies (i.e., 10, 30, and 50 Hz) on tolerability, number, reliability, and cortical distribution of language errors aiming at improved language mapping. 15 right‐handed, healthy subjects (m = 8, median age: 29 yrs) were investigated in two sessions, separated by 2–5 days. In each session, 10, 30, and 50 Hz rTMS were applied over the left hemisphere in a randomized order during a picture naming task. Overall, 30 Hz rTMS evoked significantly more errors (20 ± 12%) compared to 50 Hz (12 ± 8%; p <.01), whereas error rates were comparable between 30/50 and 10 Hz (18 ± 11%). Across all conditions, a significantly higher error rate was found in Session 1 (19 ± 13%) compared to Session 2 (13 ± 7%, p <.05). The error rate was poorly reliable between sessions for 10 (intraclass correlation coefficient, ICC = .315) and 30 Hz (ICC = .427), whereas 50 Hz showed a moderate reliability (ICC = .597). Spatial reliability of language errors was low to moderate with a tendency toward increased reliability for higher frequencies, for example, within frontal regions. Compared to 10 Hz, both, 30 and 50 Hz were rated as less painful. Taken together, our data favor the use of rTMS‐protocols employing higher frequencies for evoking language errors reliably and with reduced discomfort, depending on the region of interest.
Keywords: brain stimulation, error rate, noninvasive, pain, picture naming, speech mapping, TMS, tolerability, virtual lesion
The use of repetitive transcranial magnetic stimulation (rTMS) for language mapping is still hampered by a limited sensitivity and specificity, by an overall poor reliability and by stimulation‐associated discomfort. We, here, found that increasing the stimulation intensity up to 30 and 50 Hz can improve language mapping results as compared to the most commonly used protocol of 10 Hz rTMS.
Abbreviations
- ANG
angular gyrus
- ANOVA
analysis of variance
- aSMG
anterior supramarginal gyrus
- aSTG
anterior superior temporal gyrus
- CPS
cortical parcellation system
- DCS
direct cortical stimulation
- dPoG
dorsal postcentral gyrus
- dPrG
dorsal precentral gyrus
- FDR
false‐discovery rate
- fMRI
functional magnetic resonance imaging
- ICC
intraclass correlation coefficient
- MEP
motor‐evoked potential
- MIT
motor inhibition threshold
- mITG
middle inferior temporal gyrus
- mMFG
middle middle frontal gyrus
- mMTG
middle middle temporal gyrus
- mPoG
middle postcentral gyrus
- mPrG
middle precentral gyrus
- mSFG
middle superior frontal gyrus
- MSO
maximum stimulator output
- mSTG
middle superior temporal gyrus
- NRS
numeric rating scale
- nTMS
neuronavigated transcranial magnetic stimulation
- opIFG
opercular inferior frontal gyrus
- pITG
posterior inferior temporal gyrus
- pMFG
posterior middle frontal gyrus
- pMTG
posterior middle temporal gyrus
- pSFG
posterior superior frontal gyrus
- pSMG
posterior supramarginal gyrus
- pSTG
posterior superior temporal gyrus
- RMT
resting motor threshold
- rTMS
repetitive transcranial magnetic stimulation
- SPL
superior parietal lobe
- trIFG
triangular inferior frontal gyrus
1. INTRODUCTION
During the last years, a lot of effort has been made to investigate the human language network and particularly the cortical distribution of language‐relevant areas at the individual level. This information is of high importance because of the large interindividual anatomical and functional variability of language (Ojemann, Ojemann, Lettich, & Berger, 1989), especially when, for example, considering resection of potentially functional tissue in patients with brain tumors in language‐eloquent regions. Here, intraoperative direct cortical stimulation (DCS) still represents the current gold‐standard for language mapping. Neuro‐navigated transcranial magnetic stimulation (nTMS), however, represents a noninvasive alternative allowing language mapping already in a preoperative setting as well as in healthy subjects. By using a “virtual lesion” approach similar to DCS, repetitive TMS (rTMS) can evoke short lasting speech‐errors by stimulation over different, functionally linked cortical areas (e.g., Epstein, 1998; Pascual‐Leone, Cohen, & Hallett, 1992; Tarapore et al., 2013). Preoperative nTMS motor mappings have been shown to highly agree with results obtained from DCS (Forster et al., 2011; Picht et al., 2013; Tarapore et al., 2013; Weiss Lucas, Nettekoven, & Neuschmelting, 2020). In contrast, rTMS language mapping may have a high sensitivity (~90%) in comparison to DCS but reports on the specificity highly vary (24–98%; Ille et al., 2015a, 2015b; Krieg et al., 2014; Picht, 2014; Picht et al., 2013; Tarapore et al., 2013).
For rTMS language mapping a stimulation frequency of 5–10 Hz is most commonly used and widely accepted (Krieg et al., 2017). Nevertheless, stimulation with these frequencies often elicits side‐effects like direct nerve stimulation, which can lead to undesired jaw muscle contractions, discomfort and even pain depending on the individual susceptibility and the exact stimulation location (Tarapore et al., 2016a, 2016b). In addition to their relatively poor reliability (Sollmann et al., 2013), the number of evoked speech and language errors is quite low, with overall error rates ranging from 14% to 22% (e.g., Hauck et al., 2015b; Hauck et al., 2019; Krieg et al., 2016) and with a rare occurrence of arrests and anomias, which range amongst the most frequent types of language disruption induced by the gold standard technique of DCS (Rosengarth et al., 2021). Therefore, language mapping by rTMS still needs to be improved, for example, by varying parameters like the stimulation frequency. Recent studies found that higher stimulation frequencies of up to 20 Hz led to an increase in the number of naming errors (Hauck et al., 2015b; Rogić, Deletis, & Fernández‐Conejero, 2014; Sollmann et al., 2013, 2015). However, the value of rTMS‐frequencies higher than 20 Hz, that is, of up to 50 Hz, for online mapping of language function is yet unclear.
We, therefore, aimed to test whether the rate of speech‐errors and the test–retest reliability can be increased by applying frequencies of 30 and 50 Hz rTMS and whether these frequencies are better tolerated than the commonly used protocol, that is, 10 Hz.
2. MATERIALS AND METHODS
2.1. Subjects and study design
We examined 15 healthy, right‐handed subjects (8 males, median age: 29 yrs, range: 25–41 years) with no prior history of neurological diseases and without TMS contraindications (no previous medical history of seizures or migraine). All subjects had German as a mother tongue, except of one subject with mother tongue‐like German language skills. The study was carried out according to the declaration of Helsinki (1969, last revision 2008) and was approved by the local ethics committee (ID 12‐011). Written informed consent was obtained from all subjects.
Due to prolonged headache occurring after the first session, two subjects did not accomplish both stimulation sessions, one of which reported a history of habitual cephalgia post‐hoc. The other 13 subjects (7 males, median age: 29 years, range: 25–41 years) participated in two sessions separated by 2–5 days. In each session, a language mapping was performed using short trains of rTMS, applied over the facial motor representation and functionally associated, language‐relevant areas of the left hemisphere (cf., Weiss Lucas et al., 2019). Three different protocols, that is, 10, 30, and 50 Hz, were applied in a pseudo‐randomized order. At the end of each rTMS sequence, the maximum level of pain/discomfort was assessed on a subjective 0–10 numeric rating scale (NRS).
2.2. MRI acquisition
Structural MR images, which were used for navigation of the TMS system, were obtained from each subject prior to the first TMS session on a 3T MR scanner (Trio, Siemens, Erlangen, Germany). High resolution T1 volumes were acquired using the following parameters: TR = 2,250 ms, TE = 3.93 ms, flip angle = 90°, voxel size 1.0 × 1.0 × 1.0 mm3, FOV = 256 mm2, 176 sagittal slices.
2.3. TMS
Neuronavigated TMS was conducted using a figure‐of‐eight shaped stimulation coil with a diameter of 70 mm and biphasic stimulation waveform (system version 4.3, Nexstim Plc., Helsinki, Finland). Therefore, the head of the subject was co‐registered with the corresponding high‐resolution anatomical MR image. EMG muscle recordings included the right abductor pollicis brevis and the anterior (subapical) bottom of the tongue (cf., Weiss et al., 2013).
2.3.1. Definition of rTMS intensity
First, the “hotspot,” that is, the stimulation site yielding the largest MEP amplitude, for both the hand area and the tongue area as well as the respective resting motor threshold (RMT) were determined. Then, the “motor inhibition threshold” (MIT, cf., Weiss Lucas et al., 2019) was assessed, which was defined as the minimum stimulation intensity that led to a visible interference with the voluntary movement of interest, that is, stopping or discoordination of either thumb abduction or tongue twisting, in at least 3 out of 5 stimulations. Effects were visually rated by two independent observers, both regarding the effect on the respective task execution (i.e., smoothness of movement) and on the online EMG. The MIT was separately determined for 10, 30, and 50 Hz. The stimulation intensity for language mapping was adjusted to the MIT for the tongue. We, here, chose the tongue rather than the hand area due to the close proximity of its functional representation to the perisylvian area (i.e., the regions intended for language mapping). Therefore, we expected a comparatively good estimation of the stimulation intensity needed to interfere with language brain functions. For safety reasons (Rossi, Hallett, Rossini, & Pascual‐Leone, 2009), the MIT and thus the stimulation intensity for language mapping was limited to 130% of the tongue RMT.
The RMT and MIT are given in the %‐maximum stimulator output (%‐MSO) and in the electric field strength (V/m). The %‐MSO is the most common measure for the stimulation intensity. Electric field models (given in V/m), however, serve as a better approximate of the biological TMS effects, which are influenced by the coil orientation and tilt, and vary depending on the respective coil location (due to variations in the cranial anatomy, e.g., the scalp to cortex distance).
2.3.2. Language mapping parameters
The rTMS intensity was kept stable during each session and rTMS‐protocol. Stimulation was performed at the individual, protocol‐specific MIT for the tongue. The stimulation parameters were (a) 10 Hz, 1.5 s train duration, (b) 30 Hz, 1 s train duration, and (iii) 50 Hz, 0.5 s train duration (cf., Weiss Lucas et al., 2019). Of note, comparable pulse numbers were chosen for 30 and 50 Hz rTMS (i.e., 25 vs. 30 pulses). The intertrain interval was 5 s. This interval as well as the different lengths and durations of the trains were chosen in order to maximize the stimulation effect while considering the risk and safety guidelines for rTMS (Rossi et al., 2009; Wassermann, 1998).
The stimulation was performed during a picture naming task (cf., Keil et al., 2020; Nettekoven, Reck, Goldbrunner, Grefkes, & Weiß Lucas, 2018). All subjects were trained on the data set until stable naming performance was reached before the stimulation. Pictures were discarded in case of a delay or misnaming. Naming of the pictures should always be introduced by the German (lead‐in) phrase “Das ist ein(e)” [“this is a(n)”]. Before rTMS, a baseline of the picture naming performance was assessed. The picture presentation duration was 500 ms and rTMS trains were given upon the onset of the respective picture (picture‐to‐trigger interval 0 ms; Krieg et al., 2017; Krieg et al., 2014). Hence, all stimulation protocols were active during the entire presentation period of the picture.
2.3.3. Language mapping procedure
Stimulation was performed over the left hemisphere. The stimulated cortical area comprised (a) inferior frontal gyrus, (b) dorsal parts of the medial frontal gyrus, (c) lateral parts of the precentral gyrus, (d) inferior parietal lobule, and (e) superior and medial temporal gyrus (cf., Weiss Lucas et al., 2019). In case of significant pain (NRS > 5/10) the stimulation was interrupted at the respective stimulation site so that not all of the mentioned areas could always be tested.
The orientation of the TMS‐induced electric field was strictly posterior–anterior over the frontoparietal region. However, over the temporal lobe, the orientation was perpendicular to the Sylvian fissure. In order to achieve a systematic mapping of the above‐mentioned regions (a‐e), standard grids with spacing of 5 mm (implemented in the software of the TMS device) were projected onto the brain region to be mapped, and were repositioned up to three times per session to cover the individual map extent. In each session, all grid units were stimulated in a pseudo‐sequential order. To increase the resting time of distinct cortical areas, the space between two consecutive TMS trains was kept to a minimum of 15 mm.
2.4. Data analysis
The experiments were video‐taped. The post‐hoc video analysis was performed by two independent investigators, trained and supervised by a clinical linguist (co‐author K. J.). Both raters were blind to stimulation site and frequency. In case of differing rating results, the decision was taken by the linguist. Naming performance during rTMS was always compared to baseline. Of note, delays of the lead‐in phrase (cf., definition below) were always analyzed in a row, separated from all other error categories, after listening to at least 20 sentence onsets of the respective, analyzed subject consecutively (in order to account for interindividual variations of normality in terms of reaction times/sentence onset timings). Overall, speech and language errors were assigned to the following categories, according to a rule system developed by authors K. J., J. P., and C. W. L.:
arrest: complete inability to produce language
anomia: ability to produce lead‐in phrase without the ability to name the object
delay‐(lead‐in) phrase: increased response latency/delayed onset of the lead‐in phrase (relative to the individual overall performance), naming of target item without delay/hesitation in respect of the lead‐in phrase
delay‐item: delayed/increased response latency within or after the lead‐in phrase (lead‐in phrase starts promptly)
dysarthria/speech motor disorder (SMD): changes in intelligibility of speech (throughout lead‐in phrase and target item), due to deviations in for example, speed, tone, and accuracy
speech dysfluencies: for example, iteration of syllables, stuttering
phonemic paraphasia: incorrect phonemic realization of target item only (lead‐in phrase unaffected; including phonemic neologisms)
semantic paraphasia: substitution of the target item with a semantically related or associated word
Errors associated with muscle contraction or painful sensations leading to obvious discomfort of the subject were discarded from the analysis. Total error rates were calculated relative to the number of stimuli per session. Error rates for the different error categories were calculated relative to the absolute amount of errors. The cortical distribution of errors was analyzed according to the cortical parcellation system (CPS, Figure 1, Corina et al., 2005).
FIGURE 1.
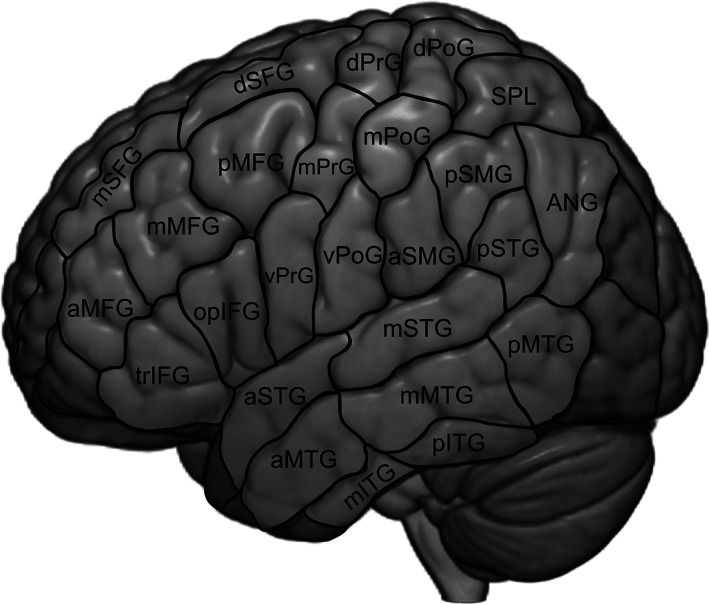
Cortical parcellation system (CPS): ANG, angular gyrus; aSMG, anterior supramarginal gyrus; aSTG, anterior superior temporal gyrus; dPoG, dorsal postcentral gyrus; dPrG, dorsal precentral gyrus; mMFG, middle middle frontal gyrus; mITG, middle inferior temporal gyrus; mMTG, middle middle temporal gyrus; mPoG, middle postcentral gyrus; mPrG, middle precentral gyrus; mSFG, middle superior frontal gyrus; mSTG, middle superior temporal gyrus; opIFG, opercular inferior frontal gyrus; pMFG, posterior middle frontal gyrus; pITG, posterior inferior temporal gyrus; pMTG, posterior middle temporal gyrus; pSFG, posterior superior frontal gyrus; pSMG, posterior supramarginal gyrus; pSTG, posterior superior temporal gyrus; SPL –superior parietal lobe; trIFG, triangular inferior frontal gyrus; vPoG, ventral postcentral gyrus; vPrG, ventral precentral gyrus
2.5. Statistical tests
Differences between frequencies and sessions were tested via a repeated‐measures analysis of variance (RM‐ANOVA) followed by a post‐hoc t‐test (MIT, percentage of errors [total]). The Greenhouse‐Geisser alpha‐correction was used in case of a violation of the nonsphericity assumption. Wilcoxon signed rank test was used in case of not normally distributed paired data (pain level, RMT, percentage of errors [categories]). False discovery rate (FDR)‐correction was used to correct p‐values for multiple comparisons (Benjamini & Hochberg, 1995). To test for correlations between pain level, stimulation intensity and error rate Spearman's Rho was calculated, separately for each session and frequency.
To assess the test–retest reliability the intraclass correlation coefficient (ICC) was calculated. ICC values below .5 are considered to reflect poor reliability, .5–.74 moderate reliability, and ≥.75 high reliability (Portney & Watkins, 2000). For estimation of mean values as well as statistical comparisons between sessions and frequencies, ICC values were transformed to Fisher z‐scores using Matlab (version 2014a, The MathWorks Inc., Natick, MA). Apart from the power analysis (calculated in R version 3.6.3 using the {pwr} package), all statistical tests were performed using SPSS 22 (Statistical Package for the Social Sciences, IBM, New York, NY).
3. RESULTS
3.1. Tolerability
Overall, rTMS was tolerated by most of the subjects (mean NRS scores, Table 1). Two subjects dropped out of the study due to prolonged headache after the first session. Of note, one of the two subjects, which were excluded due to headache, reported afterward to suffer from frequent cephalgia, which is a known risk factor for nontolerance of rTMS (Teo et al., 2014), which we regard a major reason for the comparatively high drop‐out rate (i.e., 2/15 ≈ 13 %) in our study. Moreover, mapping was not completed due to pain in the first session in two subjects for stimulation with 10 and 30 Hz, respectively. However, these subjects completed the second session.
TABLE 1.
Maximum pain level (mean NRS ± SD, ICC [CI: confidence interval])
| 10 Hz | 30 Hz | 50 Hz | |
|---|---|---|---|
| Session 1 | 6.92 ± 2.07 | 5.27 ± 2.13 | 5.42 ± 2.41 |
| Session 2 | 6.12 ± 2.00 | 5.42 ± 2.48 | 5.35 ± 2.66 |
| Mean | 6.52 ± 1.96 | 5.35 ± 2.19 | 5.38 ± 2.43 |
| ICC |
.847 [CI: .572–.951] |
.796 [CI: .457–.933] |
.842 [CI: .560–.949] |
In contrast to a relatively high intraindividual reliability of the subjective pain ratings across sessions found for all frequencies (ICCs, 10 Hz: .847, 30 Hz: .796, 50 Hz: .842), we observed a large intersubject variability (Table 1). Overall, pain ratings were significantly higher for 10 versus 30 Hz and 10 versus 50 Hz, but only in the first session (p ≤.05, FDR‐corrected). Moreover, pain ratings were significantly higher in Session 1 versus Session 2 for 10 and 30 Hz (p ≤.05, FDR‐corrected; Figure 2).
FIGURE 2.
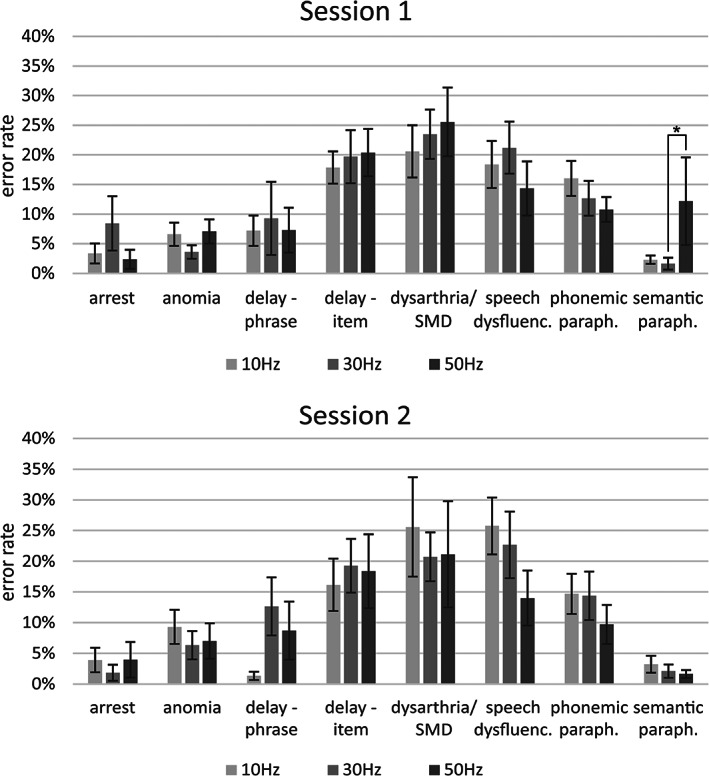
Number of errors within the different error categories relative to the total amount of errors (sum of all categories), mean ± SEM, *p ≤.05, FDR‐corrected
3.2. MIT
MITs for the hand and tongue area are shown in Table 2 (see Table S1 for RMT), indicating that least stimulation intensities are needed to interrupt repetitive thumb/tongue movements when using high rTMS‐frequencies (i.e., 30 and 50 Hz).
TABLE 2.
Motor inhibition threshold (MIT) of the hand and tongue area (mean ± SD)
| Session 1 | Session 2 | Mean | |||
|---|---|---|---|---|---|
| Hand | 10 Hz | %‐MSO | 34.38 ± 4.82 | 34.00 ± 5.21 | 34.19 ± 4.85 |
| V/m | 62.31 ± 13.21 | 61.62 ± 14.64 | 61.96 ± 3.29 | ||
| 30 Hz | %‐MSO | 30.85 ± 4.88 | 30.62 ± 4.11 | 30.73 ± 4.34 | |
| V/m | 56.85 ± 11.82 | 52.31 ± 9.20 | 54.58 ± 9.40 | ||
| 50 Hz | %‐MSO | 26.69 ± 5.12 | 29.92 ± 4.27 | 29.81 ± 4.62 | |
| V/m | 54.69 ± 12.25 | 50.15 ± 8.55 | 52.42 ± 9.63 | ||
| Tongue | 10 Hz | %‐MSO | 40.38 ± 5.33 | 39.69 ± 4.63 | 40.04 ± 3.97 |
| V/m | 77.08 ± 14.14 | 77.92 ± 24.34 | 77.50 ± 18.10 | ||
| 30 Hz | %‐MSO | 33.15 ± 5.10 | 32.62 ± 4.37 | 32.88 ± 4.46 | |
| V/m | 66.08 ± 17.76 | 59.38 ± 10.07 | 62.73 ± 11.75 | ||
| 50 Hz | %‐MSO | 33.08 ± 4.37 | 32.31 ± 4.29 | 32.69 ± 4.10 | |
| V/m | 63.92 ± 15.53 | 58.46 ± 9.85 | 61.19 ± 10.72 |
Note: MITs are expressed by %‐MSO (in gray) as well as by V/m, representing the maximum electric field strength.
The RM‐ANOVA for the MITs in %‐MSO with the factors SESSION, REGION, and FREQUENCY showed significant main effects for the factors REGION (F 1,12 = 14.472, p ≤.05) and FREQUENCY (F 2,24 = 134.173, p ≤.001) as well as a significant interaction effect for REGION × FREQUENCY (F 2,24 = 9.484, p ≤.01). Post‐hoc Student's t‐tests revealed significantly lowest MITs for 50 Hz, followed by 30 and 10 Hz for all comparisons (p ≤.01, FDR‐corrected), except for 30 versus 50 Hz for the tongue area. MITs were significantly higher for the tongue than for the hand area for 10 (p ≤.001), 30 (p ≤.1), and 50 Hz (p ≤.05, FDR‐corrected). Between sessions, MITs were not significantly different as also reflected by mostly high ICCs (Table 3).
TABLE 3.
Intraclass correlation coefficients for the MITs in %‐MSO (gray) and V/m
| 10 Hz | 30 Hz | 50 Hz | ||
|---|---|---|---|---|
| MIT hand | %‐MSO | .869 [CI: .627–.958] | .848 [CI: .575–.951] | .917 [CI: .571–.974] |
| V/m | .817 [CI: .503–.940] | .575 [CI: .061–.848] | .663 [CI: .201–.883] | |
| MIT tongue | %‐MSO | .263 [CI: −.314–.698] | .765 [CI: .392–.922] | .795 [CI: .455–.933] |
| V/m | .654 [CI: .187–.880] | .326 [CI: −.250–.731] | .358 [CI: −.215–.748] |
Abbreviation: CI, confidence interval.
Likewise, the RM‐ANOVA for the MITs in V/m with the factors SESSION, REGION, and FREQUENCY showed significant main effects for the factors REGION (F 1,12 = 25.408, p ≤.001) and FREQUENCY (F 2,24 = 36.550, p ≤.001). A statistical trend was evident for the factor SESSION (F 1,12 = 3.880, p ≤.1). Moreover, a significant interaction effect was found for REGION X FREQUENCY (F 2,24 = 6.272, p ≤.01). Post‐hoc Student's t‐tests revealed lowest MITs for 50 Hz, followed by 30 and 10 Hz for all comparisons (p ≤.05, FDR‐corrected, see Table 2). Furthermore, MITs were significantly higher for the tongue than for the hand area for all frequencies (p ≤.01, FDR‐corrected). In contrast to the analysis based on %‐MSO, the MIT in V/m showed a statistical trend toward a slightly higher MIT in the first than in the second session across frequencies and regions (63.49 ± 12.88 V/m vs. 59.97 ± 10.14 V/m, p ≤.1). In agreement with this finding, the ICCs of the MIT revealed also a lower reliability of the electric field strength between sessions when the analysis is based on V/m compared to %‐MSO (see Table 3).
We observed weak to moderate, however statistically insignificant positive correlations of the stimulation intensities/MITs with the subjective pain ratings (which underlie a considerable intersubject variability; cf., Results and Tolerability) for nearly all of the frequencies and sessions with r ranging from −.036 (30 Hz, Session 2) to .487 (50 Hz, Session 1).
3.3. Speech and language errors
3.3.1. Relative number of errors—total
In Table 4, the amount of total errors (sum of all categories) relative to the total number of trains (Table S2) per session and frequency are shown.
TABLE 4.
Percentage of total errors (relative to the number of trains, mean ± SD)
| 10 Hz | 30 Hz | 50 Hz | Mean | |
|---|---|---|---|---|
| Session 1 | 21 ± 18% | 24 ± 18% | 14 ± 9% | 19 ± 13% |
| Session 2 | 15 ± 8% | 16 ± 9% | 11 ± 9% | 14 ± 7% |
| Mean | 18 ± 11% | 20 ± 12% | 12 ± 8% |
The two‐factorial ANOVA revealed significant main effects for the factors SESSION (F 1,12 = 5.841, p =.033) and FREQUENCY F 2,24 = 7.433, p =.003). However, there was no interaction effect between sessions and frequencies. Post‐hoc Student's t‐test revealed significantly more errors in Session 1 than in Session 2 (p ≤.05, across frequencies). When testing for differences between frequencies (across sessions), we found a significantly higher number of errors for 30 than for 50 Hz (p ≤.05, FDR‐corrected). In contrast, the numbers of errors were comparable between all other paired frequencies.
Overall, the number of errors was poorly reliable for 10 Hz (ICC = .315, CI = ‐.261 – .726) and 30 Hz (ICC = .427, CI = ‐.136 – .782), whereas 50 Hz showed a moderate reliability (ICC = .597, CI = .095 – .857). Of note, there was no significant correlation between the error rate and the subjective pain ratings or stimulation intensities (p ≥.1).
3.3.2. Relative number of errors—error categories
The highest percentages of errors (relative to the sum of all categories) were found for the categories “delay‐term,” “dysarthria/SMD,” and “speech dysfluencies” in both sessions (see Figure S1 for errors relative to the total number of trains). The lowest amount of errors was evident for “anomia,” “arrest,” and “semantic paraphasia.” There were no statistical differences between sessions or frequencies within the different error categories, except for significantly more semantic paraphasias for 50 versus 30 Hz in Session 1 (p ≤.05, FDR‐corrected).
The ICCs for the different error categories and frequencies are shown in Table 5 (see Table S3 for ICCs of errors relative to total number of trains).
TABLE 5.
Intraclass correlation coefficients for the relative number or errors (light gray: moderate reliability)
| 10 Hz | 30 Hz | 50 Hz | |
|---|---|---|---|
| Arrest | .449 | .059 | −.094 |
| Anomia | .003 | −.105 | .319 |
| Delay‐item | .635 | .674 | .545 |
| Delay‐phrase | .248 | .555 | .535 |
| Dysarthria/SMD | .001 | .218 | .182 |
| Speech dysfluencies | .323 | .400 | .749 |
| Phonemic paraphasia | .033 | .571 | .228 |
| Semantic paraphasia | −.187 | .100 | .022 |
3.3.3. Cortical distribution of speech and language errors
Figure 3 depicts the cortical distribution of the relative number of (total) speech and language errors. In Session 1, highest error rates could be found for 10 and 30 Hz in motor‐related areas (e.g., vPRG and mPRG) and the IFG as well as the anterior temporal cortex. Similar results could be observed for Session 2, but with a general reduction of error rates across parcels.
FIGURE 3.
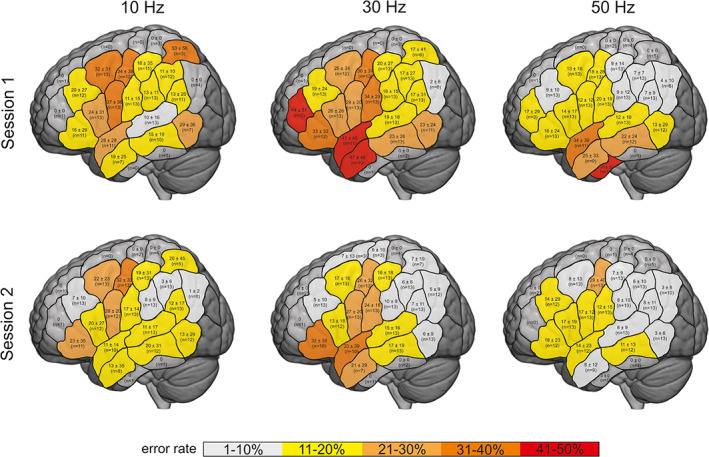
Total errors relative to total number of trains within the parcels of the CPS (mean ± SD)
Figure 4 shows the reliability within the different parcels. A statistical trend indicated a better reliability across parcels for 30 Hz (ICC = .249 ± .337) vs. 10 Hz (ICC = .027 ± .292; p <.1, FDR‐corrected; Figure 4). In contrast, there was no difference for 50 Hz (ICC = .346 ± .531) versus 10 or 30 Hz. Overall, reliability showed a high intra‐ and interindividual variability and was rather low across parcels. However, reliability seemed to increase along with the frequency, for example, within the IFG.
FIGURE 4.
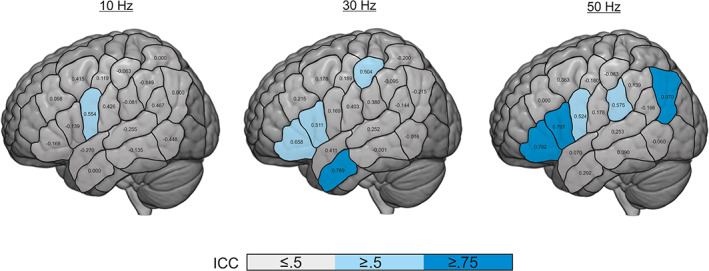
Intraclass correlation coefficient (ICC) of the error rates (≤.5: poor reliability, .5–.74: moderate reliability, ≥.75 high reliability; Portney & Watkins, 2000)
4. DISCUSSION
Currently recommended rTMS‐protocols for language mapping (i.e., 5–10 Hz) suffer from low specificity as well as low reliability and are accompanied by unfavorable side‐effects limiting its applicability. We, here, found that higher‐frequent rTMS (i.e., 30 and 50 Hz) is capable of inducing a similar amount of speech and language errors while being significantly less painful. Moreover, higher‐frequent rTMS seems to evoke language errors more reliably, particularly in inferior frontal and anterior temporal regions, which are considered highly relevant network nodes in language processing.
4.1. Tolerability
The mean pain intensity found here was comparable to other studies reporting pain ratings of 5–6/10 when applying frequencies of 5–7 Hz over temporal regions, whereas pain ratings were substantially lower (2–3/10) over parietal regions (Hauck et al., 2015a, 2015b; Krieg et al., 2016; Sollmann et al., 2015, 2016; Tarapore et al., 2016a, 2016b). Thus, pain ratings, here, might rather reflect pain evoked by stimulation over inferior frontal and temporal regions, which are more prone to pain than, for example, parietal regions.
Since it has been previously described that the discomfort associated with rTMS correlates with the magnetic field intensity (Borckardt et al., 2006), one could suggest that increasing the stimulation frequency might lead to a better tolerability. However, here, the better tolerability for higher frequencies cannot solely be explained by lower magnetic field/stimulation intensities. Less stimulation intensity was needed to disrupt tongue movements with increasing frequencies and, therefore, less intensity was used for language mapping. However, there was no significant correlation between stimulation intensities and pain ratings, which is in line with previous, retrospective, multicentric data from brain tumors patients. Tarapore et al. (2016a) also found no significant correlation between the electric field strength and pain (r =.108; p =.27). This rather contra‐intuitive finding might mainly result from the strong dependency of pain perception on individual characteristics, such as gender, age, or ethnic group affiliation, interacting in complex ways (cf., Fillingim, 2017 for review) — reflected also by the high intersubject variability of pain ratings despite their high intraindividual reliability in our study. In our view, the strength of correlation coefficients might have suffered from the strong individuality of pain perception. The sample size of the study, however, was only sufficient to detect strong correlations (i.e., r >.64) at the chosen statistical threshold (i.e., p ≤.05), with an acceptable statistical power ≥70%. Therefore, the results of the correlation analyses (particularly regarding pain ratings) should be interpreted with caution (cf. Limitations).
Although the pain rating was highly reliable between sessions, subjects reported lower pain levels in the second session (for 10 and 30 Hz), which might be explained by a different expectation of the subjects after the first session. Nevertheless, in a clinical context, results regarding the first session seem more important since patients usually only receive one preoperative mapping, which should be as comfortable as possible. In the first session, the frequency had a substantial impact on the tolerability with lower pain levels for protocols employing higher frequencies. Thus, the 10 Hz protocol does not seem to be most advisable in this context. Of note, pain levels in our cohort of healthy subjects were moderately higher compared to patient data reported in the literature (i.e., NRS score of 6.5 in our study versus NRS score of 5 reported by Tarapore et al., 2016a). In this context, one could hypothesize that the tolerability of patients might be positively influenced by the expectation of a direct benefit of the mapping (regarding the postoperative outcome).
4.2. Error rate
The overall amount of errors was within the range of other studies (11–24%, see for example, Hauck et al., 2019; Hauck et al., 2015a, 2015b; Krieg et al., 2016). However, our data did not reveal a clear superiority for one of the rTMS‐frequencies regarding the number of speech and language errors. Although 30 Hz seemed to disrupt language function more often than 50 Hz, both of the protocols employing higher frequencies were comparable to 10 Hz, thus indicating that there is no continuous or even linear relationship between stimulation frequencies and rTMS‐evoked language disruptions. Likewise, the overall reliability of error rates was limited, i.e., low to moderate, irrespective of the stimulation frequency. Although the preclinical study setting does not allow to draw definitive conclusions regarding the validity of the obtained results (cf. Limitations), discussing rTMS frequency‐dependent error rate (and reliability) in the context of modern language processing models might facilitate the interpretation (cf. parcel‐wise error rate).
The higher amount of errors in the first compared to the second session cannot be explained by higher pain ratings and thereby possibly related distractions in the first session (no correlation between pain and amount of errors), but the better performance (i.e., less errors) in the second session might rather be influenced by a learning effect, given the short intersession‐interval and the use of the same picture set in both sessions. Although in clinical routine, patients are usually investigated only once (prior to surgery), it should be kept in mind that repeated measurements might be biased by a possibly reduced susceptibility of cortical language functions to rTMS due to a learning effect. In brain tumor patients, however, this effect might be less pronounced, especially in patients with cognitive impairments. A study by Schwarzer et al. (2018) found a higher incidence of TMS‐induced as well as spontaneous errors in patients with severe cognitive impairments. This effect was not age‐dependent. The compliance of the patients, as well as other factors like alertness are a crucial prerequisite for language mapping using an overt picture naming task. This aspect should be kept in mind during patient selection. Moreover, the duration of the session should reflect a reasonable balance between repeated, confirmatory stimulations over potentially critical cortex regions on the one hand and a limited overall mapping duration on the other hand. Achieving this balance should help to maintain the compliance/alertness of the patients and to avoid learning effects, altogether aiming at best‐available data quality.
For the different error categories, it was previously suggested that higher frequencies (5 – 20 Hz) lead to a higher number of speech arrests (Hauck et al., 2015a; Pascual‐Leone, Gates, & Dhuna, 1991; Sollmann, Fuss‐Ruppenthal, Zimmer, Meyer, & Krieg, 2018). According to our results, however, the different rTMS‐frequencies overall did not evoke different types of speech and language errors. For instance, the relative rates of speech arrests were rather low compared to data from DCS studies (representing the gold standard; see for example, Rosengarth et al., 2021) throughout all rTMS frequencies. In contrast, we observed a large over‐representation of performance errors, which might be linked to unintended, secondary rTMS effects (further discussed below). In terms of reproducibility, one of the most robust error categories, was the category delay‐item/delay‐term (commonly also referred to as “hesitation errors”). This is somewhat surprising since these errors are considered as not trustworthy and are sometimes even not included into the analysis (Corina et al., 2010; Lioumis et al., 2012; Sollmann et al., 2018). Although we tried to reach high stability of the data (e.g., by defining objective rules), performance and phonological errors still showed a rather low reproducibility as also observed in other studies (Hauck et al., 2015a, 2015b; Sollmann et al., 2013). This finding might be influenced by the possible interference of rTMS not only with cortical functions, but also with cranial nerve fibers in the induced electric field. Such direct rTMS effects can lead to unintended (though not always obvious) jaw and face muscle contractions, possibly contributing to expressive speech disturbance. Due to their lower reliability, performance and phonological errors should be interpreted with caution, especially when used in the context of surgery planning.
Rater‐independent methods for, for example, automatic detection of speech/naming onsets would be highly welcome to reduce investigator bias and to streamline the workflow, but have not yet been sufficiently validated to become openly available or to achieve market maturity, and were, therefore, not applied in this study (cf., Limitations).
4.2.1. Parcel‐wise error rate
Studies exploring the validity of rTMS language maps by comparing them to intraoperative DCS report good results regarding the sensitivity and negative predictive value of rTMS (Ille et al., 2015a, 2015b; Krieg et al., 2014; Picht, 2014; Tarapore et al., 2013). Altogether, the mapping of language‐negative cortical regions seems to be highly reliable (Ille et al., 2015a, 2015b; Krieg et al., 2014; Picht, 2014; Tarapore et al., 2013). In contrast, rTMS language mapping lacks from a high specificity and a good positive‐predictive value and is rather variable across cortical language areas. Nevertheless, convergence of TMS and DCS language maps has been shown to be relatively high for the opercular regions, whereas the convergence was lower in posterior regions (Tarapore et al., 2013). Moreover, depending on the cut‐off for defining a language‐positive site (Ille et al., 2015a, 2015b), rTMS seems to be too sensitive, that is, too many false‐positive sites are found (Tarapore et al., 2013). In this context, the validity of rTMS mappings was shown to underly a considerable influence by the stimulation frequency. However, to date no recommendation regarding the optimal stimulation frequency for distinct cortical parcels can be inferred from the literature, due to the strong variability of results. For instance, Sollmann et al. (2015) suggested 20 Hz as optimal in 2/5 subjects for mapping Broca's area and in 3/5 subjects for Wernicke's area, whereas in a more recent study they found that stimulation with 5 Hz rTMS achieves optimal results for Broca's area and 10 Hz for Wernicke's area (Sollmann et al., 2018).
Taken together, our results show a predominant and rather reliable disruption of speech/language functions associated with rTMS over the primary motor (and premotor) cortex for 10 Hz rTMS, in contrast to inferior frontal and anterior temporal regions which were more evident at 30 and 50 Hz rTMS (cf. Figures 3 and 4). Of note, the parcel yielding an at least moderate reliability for 10 Hz rTMS represents the primary motor representation of the face/tongue. Accordingly, primary motor and premotor regions range amongst parcels with highest error rates and ICC values, across all frequencies. This might be explained by the broad evidence that the motor system is generally much more robust, that is, less prone to inter‐ and intrasubject variabilities of function localization, than other parts of the language network (Mueller et al., 2013).
Unlike using 10 Hz rTMS, not only (pre‐) motor areas but also inferior frontal and anterior temporal regions seemed to be rather reliably susceptible to 30 and 50 Hz rTMS. Being part of the ventral stream in the dual‐stream model proposed by Hickok and Poeppel (2007) (the exact functional roles and components of which are still a matter of vivid discussion), the anterior temporal lobe is commonly regarded as a network node for conceptual‐semantic functions (cf. Fridriksson, Yourganov, Bonilha, Basilakos, & Den Ouden, 2016), thus playing an important role for picture naming task execution. This translates also to the relative rates of error categories in our study, where naming delays (supposedly reflecting problems of, for example, semantic retrieval) ranged amongst the most frequent error categories. Likewise, inferior frontal regions being part of the (para‐) limbic system connect to both the dorsal stream (via association fibers like the arcuate fasciculus) and the ventral stream (via the uncinate fasciculus; e.g., Hau et al., 2017). Accordingly, inferior frontal regions represent key players in language processing, covering both articulatory/phonological functions attributable to the dorsal stream (suggested to mediate auditory speech‐to‐articulation transformation) as well as semantic and morphological functions representing ventral stream components. For instance, semantic dementia has been shown in patients with atrophy of the anterior frontal lobe or its connections (Agosta et al., 2010), whereas naming difficulties have been attributed to lesions of the uncinate fasciculus (Papagno et al., 2011).
In this regard, a more model‐based look on our results might lead to the conclusion that the results of higher‐frequent rTMS (≥30 Hz) reflect a more specific interaction with semantic and phonological/morphological network nodes compared to 10 Hz rTMS, beyond the overall relatively robust interference of rTMS with (pre‐) motor functions corresponding, for example, to dysarthria. Evidence from MEG and EEG studies associates the alpha‐frequency band of 8–12 Hz predominantly with active movements (and movement planning; cf., e.g., Ramos‐Murguialday & Birbaumer, 2015), whereas cognition‐related language functions such as, for example, semantic retrieval and phonological composition, correspond to the beta band (17–25 Hz) and to the low gamma band (26–50 Hz; e.g., Hirata et al., 2010; Hirata et al., 2004). Although the exact neurophysiological mechanisms leading to immediate, rTMS‐induced disruption of cortical processing are not yet fully understood, our conclusion might therefore be supported by the assumption that using a stimulation frequency surpassing the natural frequency band of brain oscillations (attributed to the function and region to be disrupted) might increase the probability of TMS pulses to match with the appropriate timing to interfere with cortical processing. However, further confirmation of this integrative conclusion is needed, for example, using TMS‐EEG to explore rTMS frequency‐dependent effects on stimulation‐induced neurophysiological processes in the addressed cortical areas and the related network components.
Regarding the translation of our results into a possible recommendation for clinical routine mappings in brain tumor patients, our data support the call for an adaptation of the rTMS parameters depending on the region/functional network component of interest. For instance, 30 Hz rTMS seems comparatively well suited for disrupting functions located in inferior frontal regions (IFG) as well as in the anterior portions of the temporal lobe (aSTG, aMTG). Of note, 50 Hz rTMS of the IFG revealed almost similar numbers and comparatively high reliability of speech and language errors in our study whilst being overall better tolerated, thus qualifying as an interesting alternative especially for the inferior frontal cortex. Such region‐dependent protocol adaptation could be particularly helpful in brain tumor patients.
Overall, similar error rates could be evoked for 10 and 30 Hz, whereas 50 Hz revealed lowest error rates. It has been suggested that discrepancies regarding the efficacy of lower‐ or higher‐frequency rTMS‐protocols might arise from the use of different number of pulses applied at the specific frequencies (Sollmann et al., 2018). Indeed, the use of different number of pulses within one TMS‐burst (10 Hz: 15, 30 Hz: 30, and 50 Hz: 25) and the different stimulation durations might have influenced language mapping results in our study. Here, stimulation parameters were chosen as a trade‐off between the amount of energy applied during one TMS‐burst and the safety guidelines for TMS. However, different number of pulses or train durations might be necessary depending on the stimulated region. Therefore, further adaptations of the stimulation parameters depending on the region of interest need to be investigated. Despite the expected advantages of region‐dependent protocol adjustments throughout the mapping procedure, it should still be considered that the practicality suffers from frequent changes of the parameter settings. This problem might be less pronounced in clinical mappings, where the stimulated area is often limited to the peritumoral region(s).
5. LIMITATIONS
The use of different stimulation intensities (adjusted to the respective MIT) limits the comparability between rTMS‐protocols, for example, with respect to the subjective pain ratings or error rates. Using the same, that is, higher stimulation intensities as for 10 Hz in protocols employing higher frequencies might have led to more errors, but also more discomfort for 30 and 50 Hz. However, this was not applicable due to safety reasons. Vice versa, using lower stimulation intensities for the 10 Hz protocol might have been rather ineffective and would have probably caused even lower number of speech and language errors (Sollmann et al., 2015). The effect of different stimulation intensities within subjects and rTMS‐protocols still needs to be tested for different frequencies. It should also be highlighted that the results of this study — for example, higher incidence of rTMS‐induced language disruption using a distinct frequency like 30 Hz — cannot be readily translated to a higher accuracy of the respective rTMS protocol in a clinical setting. This question can only be appropriately answered in the framework of a prospective clinical study, in direct comparison with the gold standard technique of DCS during awake surgery.
Moreover, the small number of subjects is a clear limitation of this study. However, since we here used a within‐subject design (and pair‐wise statistics), the number of subjects seems to be suitable to detect a medium or large effect size. Likewise, results are influenced by the high interindividual variability between subjects. Especially over critical language regions, like the IFG, only a small number of stimuli could be applied due to the high sensitivity to stimulation induced pain in these regions. Here, more subjects are needed to increase the comparability of results in future studies. Likewise, to enable an adequate interpretation of the results, it should be noted that the statistical power of the correlation analyses was inappropriate to detect weak or moderate correlations (i.e., r <.6; cf. Discussion, Tolerability).
Of note, the use of observer‐independent methods for automatic detection of speech/naming onsets (category “delay”) might reduce the investigator bias and, thus, improve the methodological quality of language mapping analyses. Both automatic waveform analysis of the voice recordings (Seynaeve et al., 2020), as well as the use of an accelerometer to detect vocalization‐related larynx vibrations (Vitikainen, Mäkelä, Lioumis, Jousmäki, & Mäkelä, 2015) have been suggested to overcome this limitation of the current standard language mapping procedure. However, such tools are not yet available, also due to inherent methodological constraints linked to, that is, background noise (e.g., produced by the TMS stimulator/cooling system), and bias introduced by nontargeted sounds/words such as cough, filler words, and alternative namings.
6. CONCLUSION
Taken together, our data favor the use of ≥30 Hz for evoking speech and language errors in systematic cortical mappings. Here, overall, less pain and more reliable language mapping can be achieved than by the commonly used 10 Hz protocol. While articulation execution seems to be rather susceptible to rTMS independent of the applied frequency, the error rate and reliability maps obtained from ≥30 rTMS correspond better to semantic and phonological processing.
Translating our results into clinical practice, it seems advisable to adjust the stimulation protocol with respect to the region (and the corresponding network function) of interest, that is, the tumor location, aiming at robust, and reliable mapping results. This requires a reasonable number of stimulation repetitions (over distinct cortical sites), balanced against overall mapping duration to limit learning effects, and ensure sufficient alertness and coping.
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
Supporting information
Appendix S1. Supporting Information.
ACKNOWLEDGMENT
The authors would like to thank the healthy subjects who participated in this study and the Max Planck Institute for Metabolism Research (former Max Planck Institute for Neurological Research) for the MR supply. The navigated TMS system used in this study was funded by the German Research Foundation (DFG, INST 1850/50‐1). Carolin Weiss Lucas received funding from the University of Cologne, Faculty of Medicine (Grant: Gerok 8/2016).
Open access funding enabled and organized by Projekt DEAL.
Nettekoven, C. , Pieczewski, J. , Neuschmelting, V. , Jonas, K. , Goldbrunner, R. , Grefkes, C. , & Weiss Lucas, C. (2021). Improving the efficacy and reliability of rTMS language mapping by increasing the stimulation frequency. Human Brain Mapping, 42(16), 5309–5321. 10.1002/hbm.25619
Funding information Deutsche Forschungsgemeinschaft, Grant/Award Number: INST 1850/50‐1; Universität zu Köln, Grant/Award Number: Gerok 8/2016
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on reasonable request from the corresponding author (Carolin Weiss Lucas).
REFERENCES
- Agosta, F. , Henry, R. G. , Migliaccio, R. , Neuhaus, J. , Miller, B. L. , Dronkers, N. F. , … Gorno‐Tempini, M. L. (2010). Language networks in semantic dementia. Brain, 133, 286–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini, Y. , & Hochberg, Y. (1995). Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. Series B (Methodological), 57, 289–300. [Google Scholar]
- Borckardt, J. J. , Smith, A. R. , Hutcheson, K. , Johnson, K. , Nahas, Z. , Anderson, B. , … George, M. S. (2006). Reducing pain and unpleasantness during repetitive transcranial magnetic stimulation. The Journal of ECT, 22, 259–264. [DOI] [PubMed] [Google Scholar]
- Corina, D. P. , Gibson, E. K. , Martin, R. , Poliakov, A. , Brinkley, J. , & Ojemann, G. A. (2005). Dissociation of action and object naming: Evidence from cortical stimulation mapping. Human Brain Mapping, 24, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corina, D. P. , Loudermilk, B. C. , Detwiler, L. , Martin, R. F. , Brinkley, J. F. , & Ojemann, G. (2010). Analysis of naming errors during cortical stimulation mapping: Implications for models of language representation. Brain and Language, 115, 101–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein, C. M. (1998). Transcranial magnetic stimulation: Language function. Journal of Clinical Neurophysiology, 15, 325–332. [DOI] [PubMed] [Google Scholar]
- Fillingim, R. B. (2017). Individual differences in pain: Understanding the mosaic that makes pain personal. Pain, 158(Suppl 1), S11–s18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster, M. T. , Hattingen, E. , Senft, C. , Gasser, T. , Seifert, V. , & Szelényi, A. (2011). Navigated transcranial magnetic stimulation and functional magnetic resonance imaging: Advanced adjuncts in preoperative planning for central region tumors. Neurosurgery, 68, 1317–1324 discussion 1324–1315. [DOI] [PubMed] [Google Scholar]
- Fridriksson, J. , Yourganov, G. , Bonilha, L. , Basilakos, A. , Den Ouden, D. B. & Rorden, C. (2016). Revealing the dual streams of speech processing. Proceedings of the National Academy of Sciences of the United States of America, 113, 15108–15113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hau, J. , Sarubbo, S. , Houde, J. C. , Corsini, F. , Girard, G. , Deledalle, C. , … Petit, L. (2017). Revisiting the human uncinate fasciculus, its subcomponents and asymmetries with stem‐based tractography and microdissection validation. Brain Structure & Function, 222, 1645–1662. [DOI] [PubMed] [Google Scholar]
- Hauck, T. , Probst, M. , Zimmer, C. , Ringel, F. , Meyer, B. , Wohlschlaeger, A. , Krieg, S.M. , 2019. Language function shows comparable cortical patterns by functional MRI and repetitive nTMS in healthy volunteers. 13, 1071‐1092. [DOI] [PubMed] [Google Scholar]
- Hauck, T. , Tanigawa, N. , Probst, M. , Wohlschlaeger, A. , Ille, S. , Sollmann, N. , … Krieg, S. M. (2015a). Stimulation frequency determines the distribution of language positive cortical regions during navigated transcranial magnetic brain stimulation. BMC Neuroscience, 16, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauck, T. , Tanigawa, N. , Probst, M. , Wohlschlaeger, A. , Ille, S. , Sollmann, N. , … Krieg, S. M. (2015b). Task type affects location of language‐positive cortical regions by repetitive navigated transcranial magnetic stimulation mapping. PLoS One, 10, e0125298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickok, G. , & Poeppel, D. (2007). The cortical organization of speech processing. Nature Reviews. Neuroscience, 8, 393–402. [DOI] [PubMed] [Google Scholar]
- Hirata, M. , Goto, T. , Barnes, G. , Umekawa, Y. , Yanagisawa, T. , Kato, A. , … Yoshimine, T. (2010). Language dominance and mapping based on neuromagnetic oscillatory changes: Comparison with invasive procedures. Journal of Neurosurgery, 112, 528–538. [DOI] [PubMed] [Google Scholar]
- Hirata, M. , Kato, A. , Taniguchi, M. , Saitoh, Y. , Ninomiya, H. , Ihara, A. , … Yoshimine, T. (2004). Determination of language dominance with synthetic aperture magnetometry: Comparison with the Wada test. NeuroImage, 23, 46–53. [DOI] [PubMed] [Google Scholar]
- Ille, S. , Sollmann, N. , Hauck, T. , Maurer, S. , Tanigawa, N. , Obermueller, T. , … Krieg, S. M. (2015a). Impairment of preoperative language mapping by lesion location: A functional magnetic resonance imaging, navigated transcranial magnetic stimulation, and direct cortical stimulation study. Journal of Neurosurgery, 123, 314–324. [DOI] [PubMed] [Google Scholar]
- Ille, S. , Sollmann, N. , Hauck, T. , Maurer, S. , Tanigawa, N. , Obermueller, T. , … Krieg, S. M. (2015b). Combined noninvasive language mapping by navigated transcranial magnetic stimulation and functional MRI and its comparison with direct cortical stimulation. Journal of Neurosurgery, 123, 212–225. [DOI] [PubMed] [Google Scholar]
- Keil, P. , Nettekoven, C. , Weiss, K. , Lichtenstein, T. , Goldbrunner, R. , Giese, D. , & Weiss Lucas, C. (2020). Accelerated Clustered Sparse Acquisition to Improve Functional MRI for Mapping Language Functions. Journal of Neurological Surgery Part A: Central European Neurosurgery, 81, 95–104. [DOI] [PubMed] [Google Scholar]
- Krieg, S. M. , Lioumis, P. , Makela, J. P. , Wilenius, J. , Karhu, J. , Hannula, H. , … Picht, T. (2017). Protocol for motor and language mapping by navigated TMS in patients and healthy volunteers; workshop report. Acta Neurochirurgica, 159, 1187–1195. [DOI] [PubMed] [Google Scholar]
- Krieg, S. M. , Sollmann, N. , Tanigawa, N. , Foerschler, A. , Meyer, B. , & Ringel, F. (2016). Cortical distribution of speech and language errors investigated by visual object naming and navigated transcranial magnetic stimulation. Brain Structure & Function, 221, 2259–2286. [DOI] [PubMed] [Google Scholar]
- Krieg, S. M. , Tarapore, P. E. , Picht, T. , Tanigawa, N. , Houde, J. , Sollmann, N. , … Nagarajan, S. (2014). Optimal timing of pulse onset for language mapping with navigated repetitive transcranial magnetic stimulation. NeuroImage, 100, 219–236. [DOI] [PubMed] [Google Scholar]
- Lioumis, P. , Zhdanov, A. , Mäkelä, N. , Lehtinen, H. , Wilenius, J. , Neuvonen, T. , … Mäkelä, J. P. (2012). A novel approach for documenting naming errors induced by navigated transcranial magnetic stimulation. Journal of Neuroscience Methods, 204, 349–354. [DOI] [PubMed] [Google Scholar]
- Mueller, S. , Wang, D. , Fox, M. D. , Yeo, B. T. , Sepulcre, J. , Sabuncu, M. R. , … Liu, H. (2013). Individual variability in functional connectivity architecture of the human brain. Neuron, 77, 586–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nettekoven, C. , Reck, N. , Goldbrunner, R. , Grefkes, C. , & Weiß Lucas, C. (2018). Short‐ and long‐term reliability of language fMRI. NeuroImage, 176, 215–225. [DOI] [PubMed] [Google Scholar]
- Ojemann, G. , Ojemann, J. , Lettich, E. , & Berger, M. (1989). Cortical language localization in left, dominant hemisphere. An electrical stimulation mapping investigation in 117 patients. Journal of Neurosurgery, 71, 316–326. [DOI] [PubMed] [Google Scholar]
- Papagno, C. , Miracapillo, C. , Casarotti, A. , Romero Lauro, L. J. , Castellano, A. , Falini, A. , … Bello, L. (2011). What is the role of the uncinate fasciculus? Surgical removal and proper name retrieval. Brain, 134, 405–414. [DOI] [PubMed] [Google Scholar]
- Pascual‐Leone, A. , Cohen, L. G. , & Hallett, M. (1992). Cortical map plasticity in humans. Trends in Neurosciences, 15, 13–14. [DOI] [PubMed] [Google Scholar]
- Pascual‐Leone, A. , Gates, J. R. , & Dhuna, A. (1991). Induction of speech arrest and counting errors with rapid‐rate transcranial magnetic stimulation. Neurology, 41, 697–702. [DOI] [PubMed] [Google Scholar]
- Picht, T. (2014). Current and potential utility of transcranial magnetic stimulation in the diagnostics before brain tumor surgery. CNS Oncology, 3, 299–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picht, T. , Krieg, S. M. , Sollmann, N. , Rösler, J. , Niraula, B. , Neuvonen, T. , … Ringel, F. (2013). A comparison of language mapping by preoperative navigated transcranial magnetic stimulation and direct cortical stimulation during awake surgery. Neurosurgery, 72, 808–819. [DOI] [PubMed] [Google Scholar]
- Portney, L. G. , & Watkins, M. P. (2000). Foundations of clinical research: Applications to practice. New Jersey: Prentice Hall. [Google Scholar]
- Ramos‐Murguialday, A. , & Birbaumer, N. (2015). Brain oscillatory signatures of motor tasks. Journal of Neurophysiology, 113, 3663–3682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogić, M. , Deletis, V. , & Fernández‐Conejero, I. (2014). Inducing transient language disruptions by mapping of Broca's area with modified patterned repetitive transcranial magnetic stimulation protocol. Journal of Neurosurgery, 120, 1033–1041. [DOI] [PubMed] [Google Scholar]
- Rosengarth, K. , Pai, D. , Dodoo‐Schittko, F. , Hense, K. , Tamm, T. , Ott, C. , 2021. A Novel Language Paradigm for Intraoperative Language Mapping: Feasibility and Evaluation. 10. [DOI] [PMC free article] [PubMed]
- Rossi, S. , Hallett, M. , Rossini, P. M. , Pascual‐Leone, A. , & Safety of TMS Consensus Group (2009). Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clinical Neurophysiology, 120, 2008–2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzer, V. , Bährend, I. , Rosenstock, T. , Dreyer, F. R. , Vajkoczy, P. , & Picht, T. (2018). Aphasia and cognitive impairment decrease the reliability of rnTMS language mapping. Acta Neurochirurgica, 160, 343–356. [DOI] [PubMed] [Google Scholar]
- Seynaeve, L. , Baby, D. , Van Hamme, H. , De Vleeschouwer, S. , Dupont, P. , & Van Paesschen, W. (2020). Automated speech analysis to improve TMS‐based language mapping: Algorithm and proof of concept. Brain Stimulation, 13, 267–269. [DOI] [PubMed] [Google Scholar]
- Sollmann, N. , Fuss‐Ruppenthal, S. , Zimmer, C. , Meyer, B. , & Krieg, S. M. (2018). Investigating stimulation protocols for language mapping by repetitive navigated transcranial magnetic stimulation. Frontiers in Behavioral Neuroscience, 12, 197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sollmann, N. , Hauck, T. , Hapfelmeier, A. , Meyer, B. , Ringel, F. , & Krieg, S. M. (2013). Intra‐ and interobserver variability of language mapping by navigated transcranial magnetic brain stimulation. BMC Neuroscience, 14, 150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sollmann, N. , Hauck, T. , Tussis, L. , Ille, S. , Maurer, S. , Boeckh‐Behrens, T. , … Krieg, S. M. (2016). Results on the spatial resolution of repetitive transcranial magnetic stimulation for cortical language mapping during object naming in healthy subjects. BMC Neuroscience, 17, 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sollmann, N. , Ille, S. , Obermueller, T. , Negwer, C. , Ringel, F. , Meyer, B. , & Krieg, S. M. (2015). The impact of repetitive navigated transcranial magnetic stimulation coil positioning and stimulation parameters on human language function. European Journal of Medical Research, 20, 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarapore, P. E. , Findlay, A. M. , Honma, S. M. , Mizuiri, D. , Houde, J. F. , Berger, M. S. , & Nagarajan, S. S. (2013). Language mapping with navigated repetitive TMS: Proof of technique and validation. NeuroImage, 82, 260–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarapore, P. E. , Picht, T. , Bulubas, L. , Shin, Y. , Kulchytska, N. , Meyer, B. , … Krieg, S. M. (2016a). Safety and tolerability of navigated TMS for preoperative mapping in neurosurgical patients. Clinical Neurophysiology, 127, 1895–1900. [DOI] [PubMed] [Google Scholar]
- Tarapore, P. E. , Picht, T. , Bulubas, L. , Shin, Y. , Kulchytska, N. , Meyer, B. , … Krieg, S. M. (2016b). Safety and tolerability of navigated TMS in healthy volunteers. Clinical Neurophysiology, 127, 1916–1918. [DOI] [PubMed] [Google Scholar]
- Teo, W. P. , Kannan, A. , Loh, P. K. , Chew, E. , Sharma, V. K. , & Chan, Y. C. (2014). Poor tolerance of motor cortex rTMS in chronic migraine. Journal of Clinical and Diagnostic Research, 8, Mm01–Mm02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitikainen, A. M. , Mäkelä, E. , Lioumis, P. , Jousmäki, V. , & Mäkelä, J. P. (2015). Accelerometer‐based automatic voice onset detection in speech mapping with navigated repetitive transcranial magnetic stimulation. Journal of Neuroscience Methods, 253, 70–77. [DOI] [PubMed] [Google Scholar]
- Wassermann, E. M. (1998). Risk and safety of repetitive transcranial magnetic stimulation: Report and suggested guidelines from the international workshop on the safety of repetitive transcranial magnetic stimulation, June 5–7, 1996. Electroencephalography and Clinical Neurophysiology, 108, 1–16. [DOI] [PubMed] [Google Scholar]
- Weiss, C. , Nettekoven, C. , Rehme, A. K. , Neuschmelting, V. , Eisenbeis, A. , Goldbrunner, R. , & Grefkes, C. (2013). Mapping the hand, foot and face representations in the primary motor cortex ‐ retest reliability of neuronavigated TMS versus functional MRI. NeuroImage, 66, 531–542. [DOI] [PubMed] [Google Scholar]
- Weiss Lucas, C. , Kallioniemi, E. , Neuschmelting, V. , Nettekoven, C. , Pieczewski, J. , Jonas, K. , … Julkunen, P. (2019). Cortical inhibition of face and jaw muscle activity and discomfort induced by repetitive and paired‐pulse TMS during an overt object naming task. Brain Topography, 32, 418–434. [DOI] [PubMed] [Google Scholar]
- Weiss Lucas, C. , Nettekoven, C. , Neuschmelting, V. , Oros‐Peusquens, A. M. , Stoffels, G. , Rehme, A. K. , & Grefkes, C. (2020). Invasive versus non‐invasive mapping of the motor cortex. Human Brain Mapping, 41, 3970–3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting Information.
Data Availability Statement
The data that support the findings of this study are available on reasonable request from the corresponding author (Carolin Weiss Lucas).


