Abstract
Recently, the medications used for the severe form of the coronavirus disease-19 (COVID-19) therapy are of particular interest. In this sense, it has been supposed that anti-VEGF compounds would be good candidates in the face of “cytokine storm” and intussuscepted angiogenesis due to having an appreciable anti-inflammatory effect. Therefore, they can be subjected to therapeutic protocols to manage acute respiratory distress syndrome (ARDS). Since the compelling evidence emphasized that VEGFs contribute to the inflammatory process and play a mainstay role in disease pathogenesis, in this review, we aimed to highlight the VEGF's plausible participation in the cytokine storm exacerbation in COVID-19. Next, the recent clinical advances regarding the anti-VEGF medications, including humanized monoclonal antibody, immunosuppressant, a tyrosine kinase inhibitor, and a cytokine inhibitor, have been addressed in the setting of COVID-19 treatment in critically ill patients. Together, retrieving the increased level of VEGF subsets, as well as antagonizing VEGF related receptors, could be helpful for the treatment of COVID-19, especially in those suffering from ARDS.
Keywords: Anti-VEGF therapy, COVID-19, Critically Ill Patients, Inflammation, SARS-CoV-2, Therapeutic Target
1. Introduction
Nowadays, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the causative factor of coronavirus disease 2019 (COVID-19), is defined as a new emerging pathogenic virus with highly transmissible potential, accounting as a great threat to global public health. As of July 2021, SARS-CoV-2 has infected more than 193 million people, with more than 4 million death worldwide [1]. Despite developing different platforms of vaccines, evolving of mutant variants is considered a serious concern, making pharmacological intervention a helpful adjuvant. To the best of our knowledge, inflammation is considered the most common pathological event and one of the main consequences of respiratory tract infection induced by SARS-CoV-2 [2], [3]. In this regard, recent studies showed that the levels of inflammatory mediators such as interleukin 6 (IL-6), -IL-8, IL-1β, tumor necrosis factor-alpha (TNF-α) [4], [5], [6], granulocyte colony-stimulating factor (G-CSF) [7], [8], interferon γ-induced protein 10 (IP-10), macrophagechemoattractant protein-1 (MCP-1) [9], and macrophage inflammatory proteins (MIP) are overexpressed in COVID-19 positive patients [10]. These exaggerated inflammatory responses are called ‘cytokine storm’, which directly links with the COVID-19 severity [10]. Moreover, the cytokine storm phenomenon and subsequent events are considered the leading cause of acute respiratory distress syndrome (ARDS) progression and 28-day mortality in COVID-19 patients [11], [12].
Vascular endothelial growth factor (VEGF), which belongs to the platelet-derived growth factor supergene family, has been shown to play a significant role in regulating of both physiological and pathological vasculogenesis, angiogenesis, and lymphangiogenesis under various conditions [13], [14], [15]. Five VEGF family members were identified, including VEGF-A (also known as VEGF), VEGF-B, VEGF-C, VEGF-D, and placenta growth factor (PlGF), which are involved in the COVID-19 pathogenesis.
It has also been well-established that the release of viral infection-induced pro-inflammatory cytokines, as well as pro-angiogenic factors, mainly from the alveolar macrophages, neutrophils, and lung epithelial cells, promotes endotheliitis [10]. On the other hand, coagulopathy events can occur during the acute phase of the severe SARS-CoV-2 infection. Herein, we aimed to establish a new insight on the negative role of VEGF overproduction, involving in overwhelming inflammatory responses in COVID-19 ill patients. Moreover, the therapeutic potential of the anti-VEGF agents with direct/indirect impacts was elegantly declared to apply for the treatment in this era.
2. The role of VEGF in COVID-19 pathogenesis
2.1. VEGF and COVID-19 severity
Following the suppression of angiotensin-converting enzyme 2 (ACE2) induced by SARS-CoV-2, the VEGF-VEGF receptor (VEGFR) system is also dysregulated, and subsequently, the modulatory effect on the VEGF activation is hampered. Therefore, it can be assumed that these biological modulations are more likely to be attributed to the impairment of the renin-angiotensin and the kallikrein-kinin axis to weaken the immune system further when exposing to the viral infection [16]. Given that a fraction of the SARS-CoV-2 positive patients experiences the severe (15%), the mortality rate in critically ill patients with ARDS is higher than patients with the severe form [16]. Thereby, exploring the causal effectors involved in disease pathogenesis, particularly in inflammatory responses, would be more imperative to appear practical therapeutic approaches. In consistence with this, it has been documented that some of the VEGF subsets, as primary factors for thrombosis and coagulopathy progression, are upregulated in COVID-19 positive patients [16], which is supposed to be another reason for the reasonable correlation between VEGF elevated levels and COVID-19 induced ARDS. In addition, recent studies have shown that SARS-CoV-2 leads to accumulating the angiocentric mixed inflammatory cells in post-infection respiratory failure [17].
2.2. Endothelial dysfunction following the inflammatory responses
Intriguingly, COVID-19-induced endothelial dysfunction also participates in disease severity, followed by vascular damage, including disseminated clots formation, vasoconstriction, and angiogenesis [18]. In turn, inflammatory-induced thrombotic microangiopathies are also accompanied by hypoxia and elevated pulmonary vascular resistance in COVID-19 patients [19]. Autopsy specimens prepared from the pulmonary vasculature of these patients further confirmed this finding. In addition to the respiratory system, aberrant angiogenesis has also been detected in other vital organs of the COVID-19 patients [20]. It is worth noting that the close relationship between inflammation and angiogenesis leads to exacerbating the inflammatory phase in the COVID-19 patients. Besides, it has been shown that toll-like receptors, as predominant factors involved in the inflammation and numerous pathologies, are also highly expressed in the endothelial cells (ECs), which can accelerate the release of inflammatory cytokines and pro-angiogenic factors (e.g., VEGF) into the target organs [21], [22]. Given the overexpression of VEGF following the hyperactivity of pro-inflammatory cytokines and related effectors derived from the neutrophils and epithelial cells, it can subsequently lead to the expression of EC adhesion molecules (e.g., intercellular adhesion molecule 1 (ICAM-1), vascular cell adhesion molecule 1 (VCAM-1), and E-selectin) via stimulation of the inflammatory transcription factor, namely nuclear factor-κB (NF-κB) [23].
2.3. VEGF mode of actions during COVID-19 progression
VEGF may play a substantial role in the pathogenesis of COVID-19 for multiple reasons: (1) the pulmonary edema, (2) decline oxygen saturation (sO2), and (3) vascular remodeling, in part, due to disturbance of the alveolar-capillary membrane integrity, leading to fibrin deposition and development of the ARDS-related fibroproliferative phase. Moreover, VEGF can facilitate the virus transmission from the lung to the blood circulation via the impairment of glycocalyx, as a peri-cellular matrix in the lung tissue [16]. Notably, VEGF also has the potential to instigate neuroinflammation in the brain of COVID-19 non-survivors following the induction of inflammatory responses, as well as the disruptive effect on the blood–brain barrier [24]. It can be proposed that inhibition of VEGF could lead to vascular normalization and reduce the virus spreading throughout the body fluids.
Since the alveolar epithelial type 2 (AE2) cells are considered a major source of VEGF in adults can play an elaborated role in the maintenance of homeostasis (in physiological levels) and injury progression in lung tissue (due to over-expression) [25]. Even so, it has been reported that in patients with early ARDS (in the exudative phase), the levels of intrapulmonary VEGF are primarily reduced due to both AE2 injury and proteases-mediated VEGF degradation while the plasma levels of VEGF are dramatically elevated [26].
3. Biological functions of various VEGFs
Among various VEGF members, VEGF-A is defined as a key vasodilating and permeability factor involved in angiogenesis, exhibiting a pro/anti-angiogenic property by VEGFR 1/2 activation [14]. In detail, VEGFR-1 (sFlt-1) activation appears to be as an endogenous VEGF inhibitor, while VEGFR-2 (KDR/Flk-1) presents an intense tyrosine kinase activity towards pro-angiogenic signals [14]. VEGF-C and –D mainly stimulate VEGFR-3 to participate in lymphangiogenesis (Flt-4) [27]. It has also been well-established that VEGFA-stimulated VEGFR1/2 is considered as one of the critical processes for modulating multiple biological functions, such as ECs proliferation, migration, and vascular permeability [28], [29]. To note, VEGFA/VEGFR2 system can also recruit the TSAd adapter protein complex to simultaneously regulate VEGFA-induced proto-oncogene tyrosine-protein kinase Src activation, as well as vascular permeability in ECs [29]. In the setting of COVID-19, VEGFA is also over-expressed in the lung tissue of the non-survivor individuals [17]. Beyond the ACE2, other proteins like the neuropilin-1 receptor (NRP-1), as a co-receptor, also participates in SARS-CoV-2′s spike protein cell entry. Remarkably, it has been shown that manipulating the VEGF-A165a subtype/b1 domain of NRP-1 signaling, which is upregulated in the transcriptional levels during COVID-19, can affect disease transmission in asymptomatic subjects [30].
4. Modulatory effects of pharmacologic agents on VEGF/VEGFR
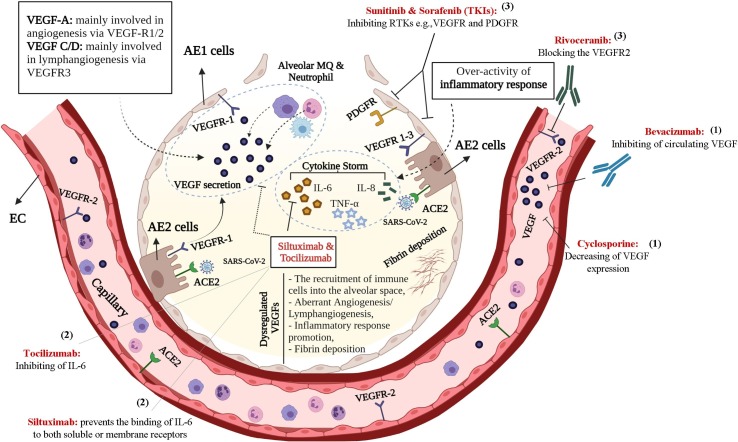
Bevacizumab, a humanized anti-VEGF monoclonal antibody, is being used to treat various types of cancer, including metastatic colorectal and renal carcinoma, lung, pancreatic, and breast cancers [31]. Mechanistically, bevacizumab inhibits VEGF-mediated angiogenesis by exclusively targeting VEGF in blood circulation to impede the cancer cells’ growth and subsequently confines the blood supply to tumor tissue. In this regard, the therapeutic potential of bevacizumab in COVID-19-induced pneumonia and ARDS is currently under intense investigation. Other classes with secondary anti-VEGF properties refer to sunitinib and sorafenib. These tyrosine kinase inhibitors (TKIs) blockade both cytosolic VEGF and platelet-derived growth factor (PDGF) receptor [32]. However, there are limited data regarding their presumable therapeutic impact against either COVID-19 or non-COVID-19-induced ARDS. Rivoceranib, an oral anti-angiogenesis inhibitor, competitively and selectively can inhibit the VEGFR-2, as well [33]. Cyclosporine, an immunosuppressant agent with a possible anti-VEGF effect, also serves a vascular protective role accompanied by anti-angiogenic and anti-apoptotic properties on ECs in low concentrations [34]. In addition, it has been reported that cyclosporine could down-regulate VEGF through a cAMP-mediated signaling pathway in a dose-dependent manner [35]. The characteristics of different anti-VEGF agents are summarized in Table 1 . In addition, in Fig. 1 , the mechanism of the anti-VEGF drugs was expressly illustrated, which can directly/indirectly affect VEGF kinetics and dynamics through three main pathways, including 1. Direct VEGFs inhibition, 2. Direct IL-6 inhibition with a secondary anti-VEGF impact, and 3. VEGFRs blockage.
Table 1.
The Characteristic of Medications Used for VEGF Regulation.
| Anti- VEGF Agent | Targets | Relevant Functions | Anti-VEGF effects | Anticipated effect in the COVID-19 |
|---|---|---|---|---|
| Bevacizumab (mAb) | VEGF | Circulating VEGF inhibition | Primary | In severe cases of COVID-19, adding bevacizumab to standard of care improves oxygenation (PaO2/FiO2 ratios) and reduced ventilation support. |
| Siltuximab (mAb) | IL-6/VEGF | Preventing the binding of IL-6 to its soluble or membrane receptors | Secondary | Siltuximab improves clinical status in patients with ARDS secondary to COVID-19. |
| Tocilizumab (mAb) | IL-6 receptor/VEGF | Antagonizing the IL-6 | Secondary | In severe cases of COVID-19, adding tocilizumab to standard of care decreases the need for mechanical ventilation and is associated with better survival. |
| Sunitinib Sorafenib (Multi-TKIs) | TK Receptor | Blockage of VEGF and PDGF | Secondary | In patients with rapidly progressing COVID-19 respiratory failure on ventilatory support, siltuximab may improve survival and cytokine hyperinflammation |
| Apatinib/Rivoceranib (TKI) | TK Receptor | Selectively VEGFR-2 inhibition | Primary | Although TKIs have not been studied in COVID-19 yet, given the selective inhibitory effects of these drugs on VEGFR-2, they may be useful in patients with severe COVID-19 in a cytokine hyperinflammation phase. |
| Cyclosporine | Cytokines | Modulates the T-cell activation and down-regulates VEGF production through cAMP-mediated signaling pathway | Secondary | Adding cyclosporine to the low-dose steroids appears to be beneficial in hospitalized patients with COVID-19 induced pneumonia. |
Abbreviations: Interleukin-6, IL-6; mAb, Monoclonal antibody; PDGF, Platelet Endothelial Growth Factor; TKI, tyrosine kinases receptor inhibitors; VEGF, Vascular Endothelial Growth Factor, PaO2/FiO2, ratio of arterial oxygen partial pressure to fractional inspired oxygen; ARDS, Acute respiratory distress syndrome.
Fig. 1.
A schematic representation of anti-VEGF compounds effect against over-expression of VEGF-induced ARDS following SARS-CoV-2 infection. These agents exert therapeutic role through three main manners including: (1) Direct VEGF inhibition, (2) Direct IL-6 cytokine inhibition with a secondary anti-VEGF effect by preventing IL-6 binding to both soluble and membrane receptors, (3) VEGFR blockage. Abbreviations: ACE2, Angiotensin-Converting Enzyme 2; AE 1/2 cells, Alveolar Epithelial Type 1/2 cells; Type 1; ECs, Endothelial cells; MQ, Macrophage; PDGFR, Platelet-Derived Growth Factor Receptor; RTKs, Receptor Tyrosine Kinases; TKIs, Tyrosine Kinase Inhibitors; VEGF, vascular endothelial growth factor; VEGFR, VEGF receptor. This is an original figure created with Biorender.
5. Presumable adverse effects induced by VEGF inhibitors
As mentioned, neovascularization is essential for both tumors growth and the spread of blood-borne metastases. In hence, VEGF inhibitors would be effective in quenching the angiogenesis in tumor vessels [36]. Notably, unlike tumor vessels, which warrants VEGF for survival, it is assumed that normal vasculature is independent of VEGF for survival, stability, and normal function [37]. On the other hand, given that angiogenesis is an essential stage of wound healing, the compounds that affect either vascular growth or angiogenesis can potentially disturb wound healing and cell migration, as well. In this respect, anti-VEGF agents with shorter half-life are associated with faster recovery from this complication [38], [39].
Given that VEGF is involved in the normal functioning of various organs, e.g., the liver, kidney, neurons, and blood vessels (regulation of blood pressure), it can be predicted that the inhibition of this factor subsequently leads to some clinical complications in patients receiving these medications [40], [41], [42]. Preclinical studies have also shown that following systemic administration of these drugs for 1–3 weeks, vascular regression in the pancreas, thyroid, adrenal cortex, pituitary, small intestine, adipose tissue, and trachea could be observed in a dose-dependent and reversible manner [36], [43], [44], [45].
In the systemic administration, one of the well-document undesirable effects of these drugs is hypertension [46], which is prevalent in up to 32% of patients and may occur at any time upon the initiation of the medication. If this complication occurs, treatment can be continued without maintaining or dropping the dose, and antihypertensive drugs would merely be added to the therapeutic schedule. This effect is probably mediated by decreased levels of nitric oxide production or blocking the VEGF effects, particularly in the kidneys [47], [48]. Another complication reported with VEFF inhibitors refers to the nephrotic syndrome and proteinuria, which can be observed in up to 23% of the patients [49], [50]. Proteinuria is usually asymptomatic and disappears when treatment is discontinued. Nevertheless, frequent renal dysfunction induced by anti-VEGF drugs is rare. The underlying mechanism of this complication is supposed to be through inhibition of VEGFR-2 in glomerular vascular endothelial cells and disruption of the filtration barrier [42], [51].
Gastrointestinal (GI) perforation is an uncommon but life-threatening complication that can occur following anti-VEGF therapy [46]. In patients with colorectal cancer treated with bevacizumab, GI perforation occurred in 1.5% of patients [49]. This complication is more common at the beginning of treatment, likely due to the regression of blood vessels in the intestinal villi [43]. GI, cerebral, and vaginal bleeding have also been reported in patients taking these drugs [52], [53]. Mild forms (epistaxis) are common and can resolve without any medical treatment. Fortunately, severe and fatal bleeding from these drugs is less common.
Furthermore, some cardiovascular events such as thromboembolism, myocardial infarction, and angina have also been reported following the treatment with these drugs [54]. It seems that aging and a previous history of these events increase the risk of experiencing this complication. Both venous and arterial thrombosis have been reported with these drugs, which probably due to inhibiting the effects of VEGF on endothelial cell survival and maintaining vascular integrity, inhibiting the production of nitric oxide and prostaglandin I2, as well as increasing hematocrit and blood viscosity by enhancing erythropoietin production [55], [56]. Moreover, cardiac complications may present with reduced cardiac function, myocardial ischemia or infarction, and decreased cardiac output in patients taking sunitinib and sorafenib [57].
Another side effect reported with these drugs is reversible leukoencephalopathy, followed by cerebral edema, vasospasm, and blood–brain barrier disruption [58], [59], [60]. Prescribing these drugs for three weeks has been associated with increased blood TSH levels [43]. Thyroid dysfunction and hypothyroidism due to regression of arteries around the follicles have been reported in patients taking anti-VEGF drugs, which may be involved in the development of fatigue in these patients [61], [62].
6. Relevant clinical researches
Given that ARDS, a serious lung injury and respiratory failure, plays a crucial role in COVID-19 mortality rates in critical care settings, the causal effectors, and involved signaling pathways, should be considered for target-based drug design with a predominant advantageous in dealing with COVID-19 treatment. As mentioned earlier, it has been documented that the VEGF levels are elevated following the COVID-19-induced hypoxia, overexpression of airway epithelial cells, and systemic inflammation [63]. Thereby, anti-VEGF medications could have a therapeutic potential in better managing of acute lung injury (ALI) and ARDS, particularly in hospitalized patients.
Given the substantial role of VEGF members in the development of both angiogenesis and lymphangiogenesis, it has also been documented that these factors can remarkably fluctuate in COVID-19 severe form. For instance, the results of a retrospective single-center study revealed that the elevated levels of VEGF-D in patients who experienced the severe and critical forms of the disease make it a novel prognostic and diagnostic value (VEGF-D AUC = 0.836 vs. d-dimer AUC = 0.755). In this study, of 24 enrolled patients, 14 (58.3%) and 10 (41.7%) subjects were identified as severe and critically ill patients, respectively. Among various inflammatory biomarkers, a striking increase of VEGF-D levels was observed in critical patients, which was also directly correlated with sequential organ failure assessment (SOFA) score (VEGF-D in severe patients = 29 vs. VEGF-D in critical patients = 62.9) [16]. In a multi-center study, it has been revealed that the levels of VEGF-A and the soluble levels of Flt-1/VEGFR1 significantly enhanced, which indicated the disease severity while VEGF-D reduced in sera of COVID-19 patients required mechanical ventilation (MV) in comparison with healthy and non-mechanical ventilated individuals [64], [65].
Notably, bevacizumab has been recruited in ongoing clinical trials for severe or critically ill patients with COVID-19 [66], [67], [68]. To date, the results of only one of these studies have been published. In a two-center clinical trial (in China and Italy), bevacizumab 500 mg was administered in combination with the standard of care in patients with severe COVID-19 pneumonia. The patients were followed up for 28 days [69]. This study demonstrated improved patients' oxygenation, which was monitored by the PaO2/FiO2 ratio [69].
Furthermore, in those patients following the receiving bevacizumab, a significant reduction of lesions areas in computerized tomography (CT) scan was detected, and the increased number of circulating lymphocytes along with a considerable dropping in C-reactive protein levels were observed. Moreover, bevacizumab could significantly reduce the duration of MV in comparison to the control group [69]. Overall, it has been recommended that bevacizumab, as a safe and well-tolerated medication with no profound adverse effect, would be practical for a severe form of COVID-19 with a direct impact on VEGF inhibition [69]. However, there is no comparison with the control group for further validation of the results, which is a major limitation in this study.
According to the recent evidence, patients with severe COVID-19 exhibited elevated levels of IL-6, a well-known inducer of VEGF production [70], [71], [72]. In this line, there are some medications with primary inhibition of IL-6 and secondary anti-VEGF properties purposed as a proper therapeutic candidate in this setting. For instance, siltuximab can prevent the binding of IL-6 to either its soluble or membrane receptors and exhibits an anti-VEGF property, as well [73]. In a randomized clinical trial (RCT), COVID-19 positive patients requiring MV support received siltuximab with a standard of care. In these patients, intravenous siltuximab (IV, 11 mg/kg) was infused during two separated doses (at the initial and 72 h later). The patients were followed up for a mean duration of 33 days. Notably, an overwhelming inflammatory response, as well as a remarkable reduction in time for invasive MV, were observed in patients with the severe form of COVID-19. Consequently, obtained results illustrated that siltuximab could decrease both hyper-inflammation responses and the rate of 28 day-mortality in hospitalized patients. Therefore, siltuximab may have further benefits against the early-stage progression of respiratory failure in invasive MV patients.
An ongoing RCT has also been designed to evaluate the efficacy and safety of siltuximab compared to glucocorticosteroids in 200 hospitalized individuals with COVID-19 pneumonia. Some critical factors, including duration of intensive care unit (ICU), hospital stay, MV, resolution of fever, mortality rate, and the rate of secondary invasive bacterial and fungal infections, are recorded. The patients are to be followed up for consecutive 29 days. [74]. The results of this trial will better clarify the potential efficacy and safety of siltuximab in COVID-19 treatment. Another agent with an IL-6 receptor-blocking property is related to tocilizumab. In line with this, a systematic review revealed that the administration of tocilizumab on over 5776 patients showed inadequate data on clinical effectiveness and safety of this medication [75]. However, according to the obtained results of a recent meta-analysis published in May 2021, the administration of tocilizumab could profoundly diminish the need for invasive MV and short-term mortality benefits in hospitalized COVID-19 positive patients [76]. As shown in Table 2 , several ongoing clinical trials have been designed to investigate the efficacy of IV or oral routes of cyclosporine administration on COVID-19 related clinical severity and symptoms, length of stay at both ICU and hospital, and mortality rate. An overview of different registered RCTs to evaluate the efficacy and safety of anti-VEGF medications in the management of COVID-19 were also represented in Table 2.
Table 2.
Studies of anti-VEGF for prevention and treatment of new coronavirus pneumonia (COVID-19).*
| Supplement | ID | Study type | Study time | Recruiting Status |
Type of disease | Treatment/Prevention | N number | Population' age (years) |
Intervention Group(s) | Primary outcomes | Secondary outcomes | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bevacizumab | NCT04305106 | RCT | March 2020 until now | Recruiting | COVID-19 | Treatment | 140 | 18–80 years old | Bevacizumab 7.5 mg/kg body weight IV infusion | The time from randomization to clinical improvement or improvement of two points on a seven-category ordinal scale or discharge from the hospital | _ | [77] |
| Bevacizumab | NCT04275414 | RCT | February 2020 to May 2021 | completed | COVID-19 | Treatment | 27 | 18–80 years old | Group 1: Bevacizumab 500 mg + normal saline (NS) 100 ml, ivdrip ≥ 90 min + standard care Group 2: standard care |
PaO2/FiO2 ratio at 24 h and 7 days | Improvement of oxygen-support status, the change of areas of pulmonary lesions were shown on chest CT or X-ray, Blood lymphocyte counts, Level of CRP, All-cause mortality, Discharge. | [78] |
| Bevacizumab | NCT04344782 | RCT | April 2020 Until now |
Recruiting | COVID-19 | Treatment | 130 | N/K | Group 1: IV infusion of Bevacizumab 7.5 mg/kg in 100 ml saline Group 2: standard care |
% surviving patients without need for MV | SaO2, PaO2, PaO2/FiO2, and CT-scan score on day 14, dyspnea, overall survival, admission to ICU, incidence of MV and ADR, and VEGF plasma concentration on day 28 | [79] |
| Siltuximab | NCT04329650 | RCT | April 2020 Until now |
Recruiting | COVID-19 | Treatment | 20 | ≥18 | Group 1: IV single-dose of 11 mg/Kg of siltuximab Group 2: IV 250 mg/24 h of methylprednisolone during 3 days followed by 30 mg/24 h during 3 days |
% patients requiring ICU admission at any time of the study | Days of ICU stay, the time of resolution of fever, % patients with worsening O2sat, duration of hypoxemia, % patients using and duration of mechanical ventilation, duration of hospitalization, all-cause mortality rate, % patients with serious adverse events, % patients with invasive bacterial or fungal infections, % patients with hypersensitivity reactions, % patients with gastrointestinal perforation, % patients with secondary severe infections, Changes from baseline in plasma leukocyte, hemoglobin, platelet, creatinine, total bilirubin levels, chest Rx and plasma biomarkers (PCR, lymphocytes, ferritin, d-dimer and LDH), % patients with ALT ≥ 3 times ULN | [80] |
| Bevacizumab | NCT04822818 | RCT | March 2021 Until now |
Not yet recruiting | COVID-19 | Treatment | 174 | ≥18 | Group 1: 7.5 mg/kg (with a maximum of 750 mg) on day 1 and standard of care Group 2: standard of care |
time to recovery (WHO Progression scale) | Clinical status (OMS Progression scale), Overall survival, Ventilator free days, High flow free days, Time to oxygen supply weaning, Changes in VEGF plasma levels, Adverse Event | [81] |
| Cyclosporine | NCT04540926 | RCT | September 2020 Until now |
Not yet recruiting | COVID-19 pneumonia patients | treatment | 200 | ≥18 | oral Cyclosporine at a dose of 1–2 mg /kg/day, for 7 days | Number of days to clinical improvement until hospital discharge or death | [82] | |
| Cyclosporine | NCT04412785 | RCT (single arm) | June 2020 Until now |
Recruiting | Moderate COVID-19 | Treatment | 20 | ≥18 | 9 mg/kg/day oral divided q12h, For IV 3 mg/kg/day continuous IV infusion for up to 14 days, as tolerated. | Safety-oxygen, ICU transfer and ventilation, changes in absolute lymphocyte count, creatinine clearance, secondary bacterial infections. | The clearance of SARS-CoV-2 from respiratory secretions, D-dimer levels, ferritin, and IL-6 levels. | [83] |
| Cyclosporine | NCT04492891 | RCT | July 2020 Until now |
Recruiting | COVID-19 | treatment | 75 | 18–90 years old | Group 1: Cyclosporine 2.5 mg/kg PO BID 7 days and standard of care, group 2: standard of care | WHO COVID-19 clinical severity scale | [84] | |
| Cyclosporine | NCT04392531 | RCT | May 2020 Until now |
Recruiting | COVID-19 | Treatment | 120 | ≥18 | Group 1: Cyclosporine and standard of care, group 2: standard of care | Severity Category | Mortality Rate, ICU and hospital length of stay, Fio2 Needs, Adverse events, Change in CRP, ferritin, LDH, CPK, D Dimer, IL-6, Viral Load and specific antibodies | [85] |
Due to the recently published systematic review on the effect of Tocilizumab and COVID-19, Tocilizumab registered trials are not reviewed in this table. N/K: not known, ICU: intensive care, ADR: adverse drug reaction, MV: mechanical ventilation, SaO2: oxygen saturation, paO2: partial pressure of oxygen, paO2/FiO2: arterial oxygen partial to fractional inspired oxygen.
7. Future prospective
In this article, we theoretically attempted to purpose various anti-VEGF agents as the potential candidates for the management of severe forms of COVID-19 by considering their related mechanism of actions toward the inflammatory mediators. However, there are still numerous unanswered queries and great challenges in developing modulation of anti-VEGF therapy, including the risk of secondary infections, the possibility of the administration in patients with predisposing conditions and/or co-morbidities, drug interactions with existing standard therapeutic protocol, and the optimum duration of treatment. Thereby, further multi-center clinical studies with a large sample size are needed to determine the effectiveness of these compounds in critically ill patients, as well as severe forms of COVID-19. On the other hand, it would not be possible to argue about the pros and cons of anti-VEGF comparative advantages as an adjuvant or alternative therapy over the steroidal anti-inflammatory drugs, e.g., dexamethasone, which has met plenty of clinical trials. As a latter concern, both anti-VEGF medications and glucocorticoids could reduce the clearance of the virus and can disrupt the performance of repair proteins, such as lipoxins, by inhibiting cytokine effectors. Besides, both drug classes potentially increase the risk of secondary fungal infections (caused by mycorrhizal fungi and aspergillus) and other secondary infections due to the immunosuppressive impact of these compounds. Therefore, patient phenotyping, health-oriented individualized precision medicine, and identification of the appropriate phase of the disease for intervention are of great value. For this purpose, the possible response of the patients could be determined through transcriptomics, metabolomics, and lipidomics analysis. Designing such comparative studies can presumably warrant the effectiveness of these drugs’ application in the clinical care settings.
8. Conclusion
To date, the corroborative evidence suggests that retrieving the increased level of VEGF subsets, as well as antagonizing VEGF related receptors, could have a substantial clinical significance in the treatment of COVID-19, especially in those suffering from ARDS, making it necessary to design further RCTs for recruitment of divergent anti-VEGF compounds in critically ill patients.
Funding
This study did not receive any funding.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Tsang H.F., Chan L.W.C., Cho W.C.S., Yu A.C.S., Yim A.K.Y., Chan A.K.C., Ng L.P.W., Wong Y.K.E., Pei X.M., Li M.J.W. An update on COVID-19 pandemic: the epidemiology, pathogenesis, prevention and treatment strategies. Expert Rev. Anti-infective Therapy. 2021;19(7):877–888. doi: 10.1080/14787210.2021.1863146. [DOI] [PubMed] [Google Scholar]
- 2.Cao W., Li T. COVID-19: towards understanding of pathogenesis. Cell Res. 2020;30(5):367–369. doi: 10.1038/s41422-020-0327-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tay M.Z., Poh C.M., Rénia L., MacAry P.A., Ng L.F. The trinity of COVID-19: immunity, inflammation and intervention. Nat. Rev. Immunol. 2020;20(6):363–374. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Costela-Ruiz V.J., Illescas-Montes R., Puerta-Puerta J.M., Ruiz C., Melguizo-Rodríguez L. SARS-CoV-2 infection: The role of cytokines in COVID-19 disease. Cytokine Growth Factor Rev.. 2020;54:62–75. doi: 10.1016/j.cytogfr.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li L., Li J., Gao M., Fan H., Wang Y., Xu X., Chen C., Liu J., Kim J., Aliyari R., Zhang J., Jin Y., Li X., Ma F., Shi M., Cheng G., Yang H. Interleukin-8 as a Biomarker for Disease Prognosis of Coronavirus Disease-2019 Patients. Front. Immunol. 2021;11 doi: 10.3389/fimmu.2020.602395. 602395–602395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen L.Y.C., Biggs C.M., Jamal S., Stukas S., Wellington C.L., Sekhon M.S. Soluble interleukin-6 receptor in the COVID-19 cytokine storm syndrome. Cell Rep. Med. 2021;2(5) doi: 10.1016/j.xcrm.2021.100269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Banji D., Alqahtani S.S., Banji O.J.F., Machanchery S., Shoaib A. Calming the inflammatory storm in severe COVID-19 infections: Role of biologics- A narrative review. Saudi Pharm. J. 2021;29(3):213–222. doi: 10.1016/j.jsps.2021.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mehta P., Porter J.C., Manson J.J., Isaacs J.D., Openshaw P.J., McInnes I.B., Summers C., Chambers R.C. Therapeutic blockade of granulocyte macrophage colony-stimulating factor in COVID-19-associated hyperinflammation: challenges and opportunities. Lancet Respirat. Med. 2020;8(8):822–830. doi: 10.1016/S2213-2600(20)30267-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Y., Wang J., Liu C., Su L., Zhang D., Fan J., Yang Y., Xiao M., Xie J., Xu Y., Li Y., Zhang S. IP-10 and MCP-1 as biomarkers associated with disease severity of COVID-19. Mol. Med. 2020;26(1):97. doi: 10.1186/s10020-020-00230-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.García L.F. Immune response, inflammation, and the clinical spectrum of COVID-19. Front. Immunol. 2020;11:1441. doi: 10.3389/fimmu.2020.01441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coperchini F., Chiovato L., Croce L., Magri F., Rotondi M. The cytokine storm in COVID-19: An overview of the involvement of the chemokine/chemokine-receptor system. Cytokine Growth Factor Rev. 2020;53:25–32. doi: 10.1016/j.cytogfr.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gibson P.G., Qin L., Puah S.H. COVID-19 acute respiratory distress syndrome (ARDS): clinical features and differences from typical pre-COVID-19 ARDS. Med. J. Australia. 2020;213(2) doi: 10.5694/mja2.50674. 54-56. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shibuya M. VEGF-VEGFR signals in health and disease. Biomol. Therapeut. 2014;22(1):1. doi: 10.4062/biomolther.2013.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shibuya M. Vascular endothelial growth factor and its receptor system: physiological functions in angiogenesis and pathological roles in various diseases. J. Biochem. 2013;153(1):13–19. doi: 10.1093/jb/mvs136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pain C.E., Felsenstein S., Cleary G., Mayell S., Conrad K., Harave S., Duong P., Sinha I., Porter D., Hedrich C.M. Novel paediatric presentation of COVID-19 with ARDS and cytokine storm syndrome without respiratory symptoms. Lancet Rheumatol. 2020;2(7):e376–e379. doi: 10.1016/S2665-9913(20)30137-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kong Y., Han J., Wu X., Zeng H., Liu J., Zhang H. VEGF-D: a novel biomarker for detection of COVID-19 progression. Crit. Care. 2020;24(1):1–4. doi: 10.1186/s13054-020-03079-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ackermann M., Verleden S.E., Kuehnel M., Haverich A., Welte T., Laenger F., Vanstapel A., Werlein C., Stark H., Tzankov A. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N. Engl. J. Med. 2020;383(2):120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huertas A., Montani D., Savale L., Pichon J., Tu L., Parent F., Guignabert C., Humbert M. Endothelial cell dysfunction: a major player in SARS-CoV-2 infection (COVID-19)? Eur. Respiratory Soc. 2020 doi: 10.1183/13993003.01634-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sugiyama M.G., Gamage A., Zyla R., Armstrong S.M., Advani S., Advani A., Wang C., Lee W.L. Influenza virus infection induces platelet-endothelial adhesion which contributes to lung injury. J. Virol. 2016;90(4):1812–1823. doi: 10.1128/JVI.02599-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Price L.C., McCabe C., Garfield B., Wort S.J. Thrombosis and COVID-19 pneumonia: the clot thickens! Eur. Respiratory Soc. 2020 doi: 10.1183/13993003.01608-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ranchoux B., Antigny F., Rucker-Martin C., Hautefort A., Péchoux C., Bogaard H.J., Dorfmüller P., Remy S., Lecerf F., Planté S. Endothelial-to-mesenchymal transition in pulmonary hypertension. Circulation. 2015;131(11):1006–1018. doi: 10.1161/CIRCULATIONAHA.114.008750. [DOI] [PubMed] [Google Scholar]
- 22.Khakpour S., Wilhelmsen K., Hellman J. Vascular endothelial cell Toll-like receptor pathways in sepsis. Innate immunity. 2015;21(8):827–846. doi: 10.1177/1753425915606525. [DOI] [PubMed] [Google Scholar]
- 23.Kim I., Moon S.-O., Kim S.H., Kim H.J., Koh Y.S., Koh G.Y. Vascular endothelial growth factor expression of intercellular adhesion molecule 1 (ICAM-1), vascular cell adhesion molecule 1 (VCAM-1), and E-selectin through nuclear factor-κB activation in endothelial cells. J. Biol. Chem. 2001;276(10):7614–7620. doi: 10.1074/jbc.M009705200. [DOI] [PubMed] [Google Scholar]
- 24.Yin X.-X., Zheng X.-R., Peng W., Wu M.-L., Mao X.-Y. Vascular Endothelial Growth Factor (VEGF) as a Vital Target for Brain Inflammation during the COVID-19 Outbreak. ACS Chem. Neurosci. 2020 doi: 10.1021/acschemneuro.0c00294. [DOI] [PubMed] [Google Scholar]
- 25.Barratt S., Medford A., Millar A. Vascular endothelial growth factor in acute lung injury and acute respiratory distress syndrome. Respiration. 2014;87(4):329–342. doi: 10.1159/000356034. [DOI] [PubMed] [Google Scholar]
- 26.Thickett D.R., Armstrong L., Millar A.B. A role for vascular endothelial growth factor in acute and resolving lung injury. Am. J. Respir. Crit. Care Med. 2002;166(10):1332–1337. doi: 10.1164/rccm.2105057. [DOI] [PubMed] [Google Scholar]
- 27.Shibuya M. Vascular endothelial growth factor (VEGF) and its receptor (VEGFR) signaling in angiogenesis: a crucial target for anti-and pro-angiogenic therapies. Genes Cancer. 2011;2(12):1097–1105. doi: 10.1177/1947601911423031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karaman S., Leppänen V.-M., Alitalo K. Vascular endothelial growth factor signaling in development and disease. Development. 2018;145(14):dev151019.. doi: 10.1242/dev.151019. [DOI] [PubMed] [Google Scholar]
- 29.Jin Y., Ji W., Yang H., Chen S., Zhang W., Duan G. Endothelial activation and dysfunction in COVID-19: from basic mechanisms to potential therapeutic approaches. Signal Transduct. Targeted Therapy. 2020;5(1):1–13. doi: 10.1038/s41392-020-00454-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moutal A., Martin L.F., Boinon L., Gomez K., Ran D., Zhou Y., Stratton H.J., Cai S., Luo S., Gonzalez K.B. SARS-CoV-2 Spike protein co-opts VEGF-A/Neuropilin-1 receptor signaling to induce analgesia. Pain. 2021;162(1):243. doi: 10.1097/j.pain.0000000000002097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pavlidis E.T., Pavlidis T.E. Role of bevacizumab in colorectal cancer growth and its adverse effects: a review. World J. Gastroenterol.: WJG. 2013;19(31):5051. doi: 10.3748/wjg.v19.i31.5051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stein M.N., Flaherty K.T. CCR drug updates: sorafenib and sunitinib in renal cell carcinoma. Clin. Cancer Res. 2007;13(13):3765–3770. doi: 10.1158/1078-0432.CCR-06-2844. [DOI] [PubMed] [Google Scholar]
- 33.Y.-K. Kang, M.-H. Ryu, Y.S. Hong, C.-M. Choi, T.W. Kim, B.-Y. Ryoo, J.E. Kim, S.-W. Kim, J.R. Weis, G.W. Gilcrease, Phase I Study of Rivoceranib, a Selective VEGFR-2 Angiogenesis Inhibitor, Administered Daily in Patients With Advanced Solid Tumors, (2021). [DOI] [PMC free article] [PubMed]
- 34.Alvarez-Arroyo M.V., Yagüe S., Wenger R.M., Pereira D.S., Jiménez S., González-Pacheco F.R., Castilla M.A., Deudero J.J.P., Caramelo C. Cyclophilin-mediated pathways in the effect of cyclosporin A on endothelial cells: role of vascular endothelial growth factor. Circ. Res. 2002;91(3):202–209. doi: 10.1161/01.res.0000027562.91075.56. [DOI] [PubMed] [Google Scholar]
- 35.Rafiee P., Heidemann J., Ogawa H., Johnson N.A., Fisher P.J., Li M.S., Otterson M.F., Johnson C.P., Binion D.G. Cyclosporin A differentially inhibits multiple steps in VEGF induced angiogenesis in human microvascular endothelial cells through altered intracellular signaling. Cell Commun. Signal. 2004;2(1):3. doi: 10.1186/1478-811X-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Inai T., Mancuso M., Hashizume H., Baffert F., Haskell A., Baluk P., Hu-Lowe D.D., Shalinsky D.R., Thurston G., Yancopoulos G.D. Inhibition of vascular endothelial growth factor (VEGF) signaling in cancer causes loss of endothelial fenestrations, regression of tumor vessels, and appearance of basement membrane ghosts. Am. J. Pathol. 2004;165(1):35–52. doi: 10.1016/S0002-9440(10)63273-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Longo R., Sarmiento R., Fanelli M., Capaccetti B., Gattuso D., Gasparini G. Anti-angiogenic therapy: rationale, challenges and clinical studies. Angiogenesis. 2002;5(4):237–256. doi: 10.1023/a:1024532022166. [DOI] [PubMed] [Google Scholar]
- 38.Bao P., Kodra A., Tomic-Canic M., Golinko M.S., Ehrlich H.P., Brem H. The role of vascular endothelial growth factor in wound healing. J. Surg. Res. 2009;153(2):347–358. doi: 10.1016/j.jss.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scappaticci F.A., Fehrenbacher L., Cartwright T., Hainsworth J.D., Heim W., Berlin J., Kabbinavar F., Novotny W., Sarkar S., Hurwitz H. Surgical wound healing complications in metastatic colorectal cancer patients treated with bevacizumab. J. Surg. Oncol. 2005;91(3):173–180. doi: 10.1002/jso.20301. [DOI] [PubMed] [Google Scholar]
- 40.Lambrechts D., Carmeliet P. VEGF at the neurovascular interface: therapeutic implications for motor neuron disease. Biochimica et Biophysica Acta (BBA) – Mol. Basis Dis. 2006;1762(11–12):1109–1121. doi: 10.1016/j.bbadis.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 41.DeLeve L.D., Wang X., Hu L., McCuskey M.K., McCuskey R.S. Rat liver sinusoidal endothelial cell phenotype is maintained by paracrine and autocrine regulation, American Journal of Physiology-Gastrointestinal and Liver. Physiology. 2004;287(4):G757–G763. doi: 10.1152/ajpgi.00017.2004. [DOI] [PubMed] [Google Scholar]
- 42.Eremina V., Sood M., Haigh J., Nagy A., Lajoie G., Ferrara N., Gerber H.-P., Kikkawa Y., Miner J.H., Quaggin S.E. Glomerular-specific alterations of VEGF-A expression lead to distinct congenital and acquired renal diseases. J. Clin. Investig. 2003;111(5):707–716. doi: 10.1172/JCI17423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kamba T., Tam B.Y., Hashizume H., Haskell A., Sennino B., Mancuso M.R., Norberg S.M., O'Brien S.M., Davis R.B., Gowen L.C. VEGF-dependent plasticity of fenestrated capillaries in the normal adult microvasculature. Am. J. Physiol.-Heart Circulat. Physiol. 2006;290(2):H560–H576. doi: 10.1152/ajpheart.00133.2005. [DOI] [PubMed] [Google Scholar]
- 44.Baffert F., Thurston G., Rochon-Duck M., Le T., Brekken R., McDonald D.M. Age-related changes in vascular endothelial growth factor dependency and angiopoietin-1-induced plasticity of adult blood vessels. Circ. Res. 2004;94(7):984–992. doi: 10.1161/01.RES.0000125295.43813.1F. [DOI] [PubMed] [Google Scholar]
- 45.Mancuso M.R., Davis R., Norberg S.M., O’Brien S., Sennino B., Nakahara T., Yao V.J., Inai T., Brooks P., Freimark B. Rapid vascular regrowth in tumors after reversal of VEGF inhibition. J. Clin. Investig. 2006;116(10):2610–2621. doi: 10.1172/JCI24612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hurwitz H., Saini S. Bevacizumab in the treatment of metastatic colorectal cancer: safety profile and management of adverse events. Semin. Oncol. 2006:S26–S34. doi: 10.1053/j.seminoncol.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 47.Horowitz J.R., Rivard A., van der Zee R., Hariawala M., Sheriff D.D., Esakof D.D., Chaudhry G.M., Symes J.F., Isner J.M. Vascular endothelial growth factor/vascular permeability factor produces nitric oxide–dependent hypotension: evidence for a maintenance role in quiescent adult endothelium. Arterioscler. Thromb. Vasc. Biol. 1997;17(11):2793–2800. doi: 10.1161/01.atv.17.11.2793. [DOI] [PubMed] [Google Scholar]
- 48.Hood J.D., Meininger C.J., Ziche M., Granger H.J. VEGF upregulates ecNOS message, protein, and NO production in human endothelial cells. Am. J. Physiol.-Heart Circulat. Physiol. 1998;274(3):H1054–H1058. doi: 10.1152/ajpheart.1998.274.3.H1054. [DOI] [PubMed] [Google Scholar]
- 49.Hurwitz H., Fehrenbacher L., Novotny W., Cartwright T., Hainsworth J., Heim W., Berlin J., Baron A., Griffing S., Holmgren E. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N. Engl. J. Med. 2004;350(23):2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 50.Kabbinavar F.F., Schulz J., McCleod M., Patel T., Hamm J.T., Hecht J.R., Mass R., Perrou B., Nelson B., Novotny W.F. Addition of bevacizumab to bolus fluorouracil and leucovorin in first-line metastatic colorectal cancer: results of a randomized phase II trial. J. Clin. Oncol. 2005;23(16):3697–3705. doi: 10.1200/JCO.2005.05.112. [DOI] [PubMed] [Google Scholar]
- 51.Schrijvers B.F., Flyvbjerg A., De Vriese A.S. The role of vascular endothelial growth factor (VEGF) in renal pathophysiology. Kidney Int. 2004;65(6):2003–2017. doi: 10.1111/j.1523-1755.2004.00621.x. [DOI] [PubMed] [Google Scholar]
- 52.Xiao B., Wang W., Zhang D. Risk of bleeding associated with antiangiogenic monoclonal antibodies bevacizumab and ramucirumab: a meta-analysis of 85 randomized controlled trials. OncoTargets Therapy. 2018;11:5059. doi: 10.2147/OTT.S166151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sonpavde G., Bellmunt J., Schutz F., Choueiri T.K. The double edged sword of bleeding and clotting from VEGF inhibition in renal cancer patients. Curr. Oncol. Rep. 2012;14(4):295–306. doi: 10.1007/s11912-012-0237-9. [DOI] [PubMed] [Google Scholar]
- 54.Skillings J., Johnson D., Miller K., Kabbinavar F., Bergsland E., Holmgren E., Holden S., Hurwitz H., Scappaticci F. Arterial thromboembolic events (ATEs) in a pooled analysis of 5 randomized, controlled trials (RCTs) of bevacizumab (BV) with chemotherapy. J. Clin. Oncol. 2005;23(16_suppl) 3019–3019. [Google Scholar]
- 55.Zachary I. Signaling mechanisms mediating vascular protective actions of vascular endothelial growth factor. Am. J. Physiol.-Cell Physiol. 2001;280(6):C1375–C1386. doi: 10.1152/ajpcell.2001.280.6.C1375. [DOI] [PubMed] [Google Scholar]
- 56.Tam B.Y., Wei K., Rudge J.S., Hoffman J., Holash J., Park S.-K., Yuan J., Hefner C., Chartier C., Lee J.-S. VEGF modulates erythropoiesis through regulation of adult hepatic erythropoietin synthesis. Nat. Med. 2006;12(7):793–800. doi: 10.1038/nm1428. [DOI] [PubMed] [Google Scholar]
- 57.Haas N.B., Manola J., Ky B., Flaherty K.T., Uzzo R.G., Kane C.J., Jewett M., Wood L., Wood C.G., Atkins M.B. Effects of adjuvant sorafenib and sunitinib on cardiac function in renal cell carcinoma patients without overt metastases: results from ASSURE, ECOG 2805. Clin. Cancer Res. 2015;21(18):4048–4054. doi: 10.1158/1078-0432.CCR-15-0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Allen J.A., Adlakha A., Bergethon P.R. Reversible posterior leukoencephalopathy syndrome after bevacizumab/FOLFIRI regimen for metastatic colon cancer. Arch. Neurol. 2006;63(10):1475–1478. doi: 10.1001/archneur.63.10.1475. [DOI] [PubMed] [Google Scholar]
- 59.Govindarajan R., Adusumilli J., Baxter D.L., El-Khoueiry A., Harik S.I. Reversible posterior leukoencephalopathy syndrome induced by RAF kinase inhibitor BAY 43–9006. J. Clin. Oncol.: Off. J. Am. Society Clin. Oncol. 2006;24(28) doi: 10.1200/JCO.2006.08.4608. e48–e48. [DOI] [PubMed] [Google Scholar]
- 60.Connolly R.M., Doherty C.P., Beddy P., O’Byrne K. Chemotherapy induced reversible posterior leukoencephalopathy syndrome. Lung Cancer. 2007;56(3):459–463. doi: 10.1016/j.lungcan.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 61.Clemons J., Gao D., Naam M., Breaker K., Garfield D., Flaig T.W. Thyroid dysfunction in patients treated with sunitinib or sorafenib. Clin. Genitourinary Cancer. 2012;10(4):225–231. doi: 10.1016/j.clgc.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Buda-Nowak A., Kucharz J., Dumnicka P., Kuzniewski M., Herman R.M., Zygulska A.L., Kusnierz-Cabala B. Sunitinib-induced hypothyroidism predicts progression-free survival in metastatic renal cell carcinoma patients. Med. Oncol. 2017;34(4):68. doi: 10.1007/s12032-017-0928-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.M. Turkia, COVID-19, vascular endothelial growth factor (VEGF) and iodide, Vascular Endothelial Growth Factor (VEGF) and Iodide (June 3, 2020) (2020).
- 64.Rovas A., Osiaevi I., Buscher K., Sackarnd J., Tepasse P.-R., Fobker M., Kühn J., Braune S., Göbel U., Thölking G., Gröschel A., Pavenstädt H., Vink H., Kümpers P. Microvascular dysfunction in COVID-19: the MYSTIC study. Angiogenesis. 2021;24(1):145–157. doi: 10.1007/s10456-020-09753-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pine A.B., Meizlish M.L., Goshua G., Chang C.-H., Zhang H., Bishai J., Bahel P., Patel A., Gbyli R., Kwan J.M. Circulating markers of angiogenesis and endotheliopathy in COVID-19. Pulmonary Circulat. 2020;10(4) doi: 10.1177/2045894020966547. 2045894020966547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.ClinicalTrials.gov Register [Internet], identifier (NCT number): NCT04305106, Available from: https://clinicaltrials.gov/ct2/show/NCT04305106?term=Bevacizumab&cond=COVID-19&draw=2&rank=1.
- 67.ClinicalTrials.gov Register [Internet], identifier (NCT number): NCT04275414, Available from: https://clinicaltrials.gov/ct2/show/NCT04275414?term=Bevacizumab&cond=COVID-19&draw=2&rank=3.
- 68.ClinicalTrials.gov Register [Internet], identifier (NCT number): NCT04344782, Available from: https://clinicaltrials.gov/ct2/show/NCT04344782?term=Bevacizumab&cond=COVID-19&draw=2&rank=2.
- 69.Pang J., Xu F., Aondio G., Li Y., Fumagalli A., Lu M., Valmadre G., Wei J., Bian Y., Canesi M. Efficacy and tolerability of bevacizumab in patients with severe Covid-19. Nat. Commun. 2021;12(1):1–10. doi: 10.1038/s41467-021-21085-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46(5):846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mazzoni A., Salvati L., Maggi L., Capone M., Vanni A., Spinicci M., Mencarini J., Caporale R., Peruzzi B., Antonelli A. Impaired immune cell cytotoxicity in severe COVID-19 is IL-6 dependent. J. Clin. Investig. 2020;130(9) doi: 10.1172/JCI138554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tzeng H.-E., Tsai C.-H., Chang Z.-L., Su C.-M., Wang S.-W., Hwang W.-L., Tang C.-H. Interleukin-6 induces vascular endothelial growth factor expression and promotes angiogenesis through apoptosis signal-regulating kinase 1 in human osteosarcoma. Biochem. Pharmacol. 2013;85(4):531–540. doi: 10.1016/j.bcp.2012.11.021. [DOI] [PubMed] [Google Scholar]
- 73.Sahebnasagh A., Avan R., Saghafi F., Mojtahedzadeh M., Sadremomtaz A., Arasteh O., Tanzifi A., Faramarzi F., Negarandeh R., Safdari M. Pharmacological treatments of COVID-19. Pharmacol. Rep. 2020:1–33. doi: 10.1007/s43440-020-00152-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.ClinicalTrials.gov Register [Internet], identifier (NCT number): NCT04329650, Available from: https://clinicaltrials.gov/ct2/show/NCT04329650?term=Siltuximab&cond=Covid19&draw=2&rank=1.
- 75.Cortegiani A., Ippolito M., Greco M., Granone V., Protti A., Gregoretti C., Giarratano A., Einav S., Cecconi M. Rationale and evidence on the use of tocilizumab in COVID-19: a systematic review. Pulmonology. 2020 doi: 10.1016/j.pulmoe.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Snow T.A.C., Saleem N., Ambler G., Nastouli E., Singer M., Arulkumaran N. Tocilizumab in COVID-19: a meta-analysis, trial sequential analysis, and meta-regression of randomized-controlled trials. Intensive Care Med. 2021 doi: 10.1007/s00134-021-06416-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.ClinicalTrials.gov Register [Internet], identifier (NCT number): NCT04305106, Available from: https://clinicaltrials.gov/ct2/show/NCT04305106.
- 78.ClinicalTrials.gov Register [Internet], identifier (NCT number): NCT04275414, Available from: https://clinicaltrials.gov/ct2/show/NCT04275414.
- 79.ClinicalTrials.gov Register [Internet], identifier (NCT number): NCT04344782, Available from: https://clinicaltrials.gov/ct2/show/NCT04344782.
- 80.ClinicalTrials.gov Register [Internet], identifier (NCT number): NCT04329650, Available from: https://clinicaltrials.gov/ct2/show/NCT04329650.
- 81.ClinicalTrials.gov Register [Internet], identifier (NCT number): NCT04822818, Available from: https://clinicaltrials.gov/ct2/show/NCT04822818.
- 82.ClinicalTrials.gov Register [Internet], identifier (NCT number): NCT04540926, Available from: https://clinicaltrials.gov/ct2/show/NCT04540926.
- 83.ClinicalTrials.gov Register [Internet], identifier (NCT number): NCT04412785, Available from: https://clinicaltrials.gov/ct2/show/NCT04412785.
- 84.ClinicalTrials.gov Register [Internet], identifier (NCT number): NCT04492891, Available from: https://clinicaltrials.gov/ct2/show/NCT04492891.
- 85.ClinicalTrials.gov Register [Internet], identifier (NCT number): NCT04392531, Available from: https://clinicaltrials.gov/ct2/show/NCT04392531.