Abstract
Pre-mRNA editing involving the conversion of adenosine to inosine is mediated by adenosine deaminases that act on RNA (ADAR1 and ADAR2). ADARs contain multiple double-stranded RNA(dsRNA)-binding domains in addition to an adenosine deaminase domain. An adenosine deaminase acting on tRNAs, scTad1p (also known as scADAT1), cloned from Saccharomyces cerevisiae has a deaminase domain related to the ADARs but lacks dsRNA-binding domains. We have identified a gene homologous to scADAT1 in the region of Drosophila melanogaster Adh chromosome II. Recombinant Drosophila ADAT1 (dADAT1) has been expressed in the yeast Pichia pastoris and purified. The enzyme has no activity on dsRNA substrates but is a tRNA deaminase with specificity for adenosine 37 of insect alanine tRNA. dADAT1 shows greater similarity to vertebrate ADARs than to yeast Tad1p, supporting the hypothesis of a common evolutionary origin for ADARs and ADATs. dAdat1 transcripts are maternally supplied in the egg. Zygotic expression is widespread initially and later concentrates in the central nervous system.
Adenosine and cytosine have exocyclic amino groups that participate in Watson-Crick base pairing during transcription and translation. RNA editing enzymes have been discovered that deaminate specific adenosine residues to inosine (4, 32, 38, 40) or specific cytosine residues to uridine in RNA molecules (11, 39). This can result in the incorporation of different amino acids at edited positions or the formation of a smaller protein due to the generation of a stop codon. Two closely related adenosine deaminases acting on RNA (ADARs) have been identified in vertebrates (3) that catalyze the deamination of specific adenosine residues to inosine in pre-mRNAs (for a review, see reference 22). These enzymes have homologous adenosine deaminase domains (32) and also contain multiple double-stranded RNA(dsRNA)-binding domains (47). ADARs recognize and deaminate specific adenosines within exons that form duplexes with flanking intronic sequences in pre-mRNA (20, 32). ADAR activity is ubiquitous and has been found in all metazoans tested and in most tissues (52). The abundance of inosine in polyA+ RNA has been estimated to be 1 in 17,000 nucleotides in brain and less in other mammalian tissues, correlating with ADAR expression levels (35). Inosine in edited transcripts directs the incorporation of cytosine during first-strand synthesis of cDNA (5), and RNA editing events have usually been identified in cDNA sequences in which guanosine replaces a genomically encoded adenosine (8, 45). Pre-mRNAs encoding subunits of the glutamate-gated ion channels (for a review, see reference 42) and the G protein-coupled serotonin 2C receptor (8) undergo RNA editing of their sequences by this mechanism. Proteins encoded by edited mRNAs often have functional properties that differ from the genomically encoded versions.
Inosine was first observed as a noncanonical base occurring in a number of tRNAs (21). Inosine in tRNAs is generated by deamination of genomically encoded adenosine (2). Inosine occurs at position 34 in the anticodon in a number of different tRNAs (for a review, see reference 17). Eukaryotic alanine tRNA (tRNAAla), containing the anticodon IGC, is the only class of tRNA that undergoes deamination of adenosine to inosine at position 37 adjacent to the anticodon (17). This inosine is subsequently methylated by an as-yet-uncharacterized enzyme (2). Recently, it has been found that inosine at position 34 and methylinosine at position 37 are major epitopes for anti-PL-12 myositis autoantibodies (6). An adenosine deaminase acting on tRNA (ADAT1) catalyzing the site-specific deamination of adenosine at position 37 in yeast tRNAAla has been cloned and characterized (15). This ADAT1 from Saccharomyces cerevisiae, named scTad1p (or scADAT1) and encoded by the TAD1 gene, is homologous to the ADARs throughout its sequence and has three characteristic adenosine deaminase motifs containing residues thought to chelate zinc and contribute to catalysis in the active site of the enzymes (9). Gerber et al. (15) have proposed that ADARs involved in pre-mRNA editing have a common evolutionary origin with ADATs involved in the modification of tRNAAla at position 37. These adenosine deaminase domains are also related to a cytosine deaminase involved in mRNA editing (APOBEC) and to cytidine deaminases involved in the deamination of free nucleosides (9).
Embryonic nuclear extracts from Drosophila melanogaster contain an ADAR-like activity that converts adenosine to inosine in the antigenome RNA of hepatitis D virus (10). Editing of mRNAs has been proposed to occur in Drosophila, based on the detection of variant cDNAs having adenosine-to-guanosine substitutions in mRNAs encoded by the 4f-rnp gene (37), the cacophony (cac) gene (36, 44), and the paralytic (para) gene (18). We describe here the cloning and characterization of a gene encoding an adenosine deaminase from D. melanogaster that is similar to vertebrate ADARs but lacks dsRNA-binding domains. Characterization of a purified recombinant protein shows that this protein is a tRNA-specific adenosine deaminase. This protein, dADAT1, has a higher degree of amino acid sequence homology to the ADARs than the previously characterized yeast ADAT1. These findings support an evolutionary relationship between pre-mRNA editing and tRNA modification.
MATERIALS AND METHODS
Oligonucleotides used in this study.
The oligonucleotides used in this study are as follows: DRSB (5′ GGATCCGGAACAAAGTGCATTG 3′), DRSH (5′ AAGCTTAAATGTCCTACAATCGA 3′), hADAT181R (5′ CGTTCCATCGGGCCATCTTGTCAC 3′), hADAT585R (5′ TCTGGAATAATCTGAAGAGTCCAC 3′), λgt10 (5′ AGCAAGTTCAGCCTGGTTAAGT 3′), λgt10d (5′ CGAGCTGCTCTATAGACTGCTG 3′), DRS2 (5′ CCGCAATTTCCTTAACAG 3′), DRS3 (5′ CGGCATGGGAATCATTCAGGATGA 3′), ADAT1 (5′ CTTTGTTCCGCATCCAAGCG 3′), and (dC)13 adapter (5′ GACTCGAGTCGACATCGCCCCCCCCCCCCC 3′).
Isolation of cDNA clones and sequencing.
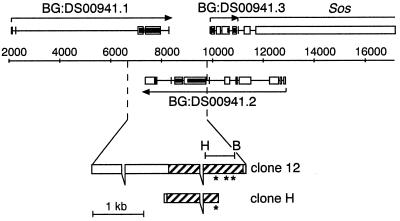
The P1 clone DS00941, sequenced by the Berkeley Drosophila Genome Project (BDGP), carries an open reading frame (ORF) (BG:DS00941.2) with homology to the deaminase domains of mammalian ADAR1 and ADAR2. A 600-nucleotide fragment encoding this homologous domain was amplified from genomic DNA by PCR. The PCR primers (DRSB and DRSH) were designed to introduce the restriction sites BamHI and HindIII at the 5′ and 3′ ends, respectively, of the PCR product which was subcloned into the polylinker of pBluescript KS(−) (Stratagene) at these sites (see Fig. 1). This 600-nucleotide fragment was sequenced with a Dye Terminator Cycle Sequencing Ready Reaction kit (Perkin-Elmer) and used to screen a λgt10 (3- to 12-h embryo) cDNA library. Nitrocellulose filters were hybridized overnight at 65°C as previously described (50) with minor modifications. The hybridization buffer contained 6× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate), 5× Denhardt's solution, 0.5% sodium dodecyl sulfate (SDS), sonicated salmon sperm DNA (100 mg/ml), and approximately 2 × 105 cpm of denatured probe per ml. The filters were washed twice for 15 min in 2× SSC and 0.1% SDS at room temperature, followed by two 15-min washes at 68°C in 0.1% SSC and 0.5% SDS. One full-length positive clone (clone 12) was obtained (see Fig. 1). A second shorter clone (clone H) isolated from a λZAP (4- to 8-h embryo) cDNA library (Stratagene) was also analyzed (see Fig. 1). Both clones were sequenced with a Dye Terminator Cycle Sequencing Ready Reaction kit on an Applied Biosystems 373A sequencer.
FIG. 1.
Structure of the dAdat1 gene and encoded protein. (A) Genes and predicted ORFs carried in the region from 5 to 15 kb from the centromere distal end of the Drosophila P1 genomic clone DS00941 sequenced by the BDGP. The telomere is to the left and the centromere is to the right. This schematic is derived from a CloneView analysis (19) of the P1 sequence, which can be viewed at the BDGP web site (http://www.fruitfly.org). Sos is a previously characterized gene, whereas the genes indicated as BG:DS00941.N are predicted from the genomic sequence. Predicted ORFs are shown as black bars and predicted exons are shown as white boxes. Bases 9078 to 9776 in this genomic clone showed the initial homology to an ADAR-like adenosine deaminase domain. cDNA clones are shown in the enlarged view beneath, and the endpoints of clone 12 on the genomic sequence are indicated. Exons and introns are drawn to scale, and the coding sequence is hatched. The cDNAs are depicted in a reverse orientation to maintain colinearity with DS00941. Asterisks indicate the locations of adenosine deaminase motifs I, II, and III.
A human homologue to the dAdat1 gene was identified in the Washington University expressed sequence tag (EST) database in a BLAST search with S. cerevisiae ADAT1. This clone (embl/AA161179/HSAA61179), isolated from a Stratagene human neuron library, was obtained from the IMAGE consortium (26). Further sequencing of this 1.9-kb cDNA clone indicated that the sequence in the database was from the 5′ end of the clone and that the region encoding the amino terminus was missing. In order to obtain additional 5′ coding sequence, PCR amplification was performed on a human fetal brain cDNA library (Clontech) with λgt10d and an hADAT reverse primer, hADAT585R. Individual gel-purified PCR products from the first round of amplification were amplified again with a nested vector primer, λgt10, and an hADAT-specific primer, hADAT181R. The resulting PCR products were sequenced directly to complete the coding sequence. A BLAST search with this new sequence identified three new overlapping ESTs (embl/AI417361/AI41, emnew/AI598171/AI5, and embl/AA0854484/HSAA).
5′ end mapping of dAdat1.
Rapid amplification of cDNA ends was performed as previously described (14). One microgram of embryo poly(A)+ mRNA from D. melanogaster (Clontech) was mixed with DRS3, which is a dAdat1-specific reverse primer, and reverse transcribed with 200 U of SuperScript II (RNase H−) reverse transcriptase (Gibco BRL) at 42°C for 50 min. Excess primer was then removed with a QIAquick nucleotide removal kit (Qiagen) and a poly(G) tail was added to the 3′ end of the first cDNA strand with 40 U of terminal deoxynucleotide transferase (Pharmacia) for 15 min at 37°C. The tailed cDNA was amplified with a (dC)13 adapter primer and the reverse primer ADAT1. A further round of amplification was carried out with the primer DRS2 and the adapter primer. The final PCR product was subcloned in the T/A cloning vector pGEM-Teasy (Promega) and sequenced with a Dye Terminator Cycle Sequencing Ready Reaction kit on an Applied Biosystems 373A sequencer with vector-specific primers.
Expression of epitope-tagged recombinant dADAT1 protein in Pichia pastoris and protein purification.
The coding sequence of dAdat1 missing the first methionine and the stop codon (2 to 394 amino acids) was amplified by PCR from clone 12 with primers containing NheI restriction sites at their 5′ termini. The PCR product was subsequently subcloned in the T/A cloning vector pGEM-Teasy (Promega). The resulting clone, pTE-ADAT, was sequenced, digested with NheI, and subcloned into the SpeI site in pSK-FLIS6 (33) to express a recombinant protein with the FLAG epitope tag (Sigma) at the N terminus and a histidine hexamer at the C terminus. This subclone was digested with NotI and used for gene replacement of the AOX1 locus in P. pastoris GS115 (Invitrogen). Twenty-five-milliliter cultures of His+ transformants were grown in buffered minimal methanol medium and used to make small-scale liquid nitrogen extracts for expression monitoring. Expression of recombinant dADAT1 was monitored by immunoblot analysis with anti-dADAT1 polyclonal antiserum (1:1,000) raised against the deaminase domain of dADAT1 and with an α-FLAG M2 monoclonal antibody (1:5,000) (Sigma).
For protein purification, all manipulations were carried out at 4°C. The large-scale protein preparation was performed as previously described (16, 33). The buffer used was buffer A-80 (50 mM Tris-HCl [pH 7.9], 80 mM KCl, 10% glycerol, 0.1 mM dithiothreitol, 0.5 mM phenylmethylsulfonyl fluoride (PMSF), 0.7 mg of pepstatin per ml, and 0.4 mg of leupeptin per ml). Twenty-five milliliters of extract supernatant containing 5 to 10 mg of soluble protein per ml was mixed with Ni2+-nitrolotriacetic acid agarose (Ni2+-NTA) (Qiagen) that had been preequilibrated with buffer A-80 according to the manufacturer's instructions, poured into a column, washed, and eluted with buffer A-80 containing 250 mM imidazole. Immunoblot analysis of the column fractions with an α-FLAG M2 monoclonal antibody revealed a band of 48 kDa.
Fractions containing recombinant dADAT1 from the Ni2+-NTA column were pooled, further purified by chromatography on an α-FLAG M2 antibody matrix (Sigma), and eluted with FLAG peptide (Sigma). A 150-μl bed volume of anti-FLAG M2 matrix was poured in a minicolumn (diameter, 0.7 cm) and equilibrated with TKG-150 buffer (50 mM Tris-HCl [pH 7.9], 150 mM KCl, 10% glycerol, 0.1 mM EDTA [pH 8.0], 0.02% NP-40, 0.1 mM dithiothreitol, 0.5 mM PMSF, 0.7 μg of pepstatin per ml, and 0.4 μg of leupeptin per ml). The column was washed with 5 ml of TKG-250 (TKG buffer with 250 mM KCl) and 4 ml of TKG-50 (TKG buffer with 50 mM KCl). The protein was eluted with TKG-50 containing 50 μg of FLAG-peptide per ml, and 200-μl fractions were collected. Fractions were frozen in liquid nitrogen and stored at −70°C. Ten microliters of the fractions was analyzed by electrophoresis on an SDS–12% polyacrylamide gel, and proteins were stained with Coomassie blue R-250 (Bio-Rad) (see Fig. 3A). For immunoblot analysis with the rabbit anti-dADAT1 serum, 5 μl of the fractions was electrophoresed on an SDS–10% polyacrylamide gel (see Fig. 3B).
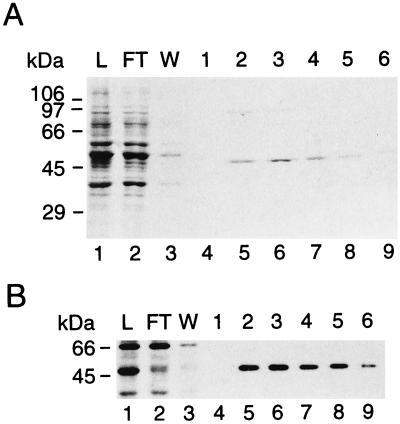
FIG. 3.
Purification of recombinant Drosophila adenosine deaminase from P. pastoris. (A) Fractions from a FLAG affinity column eluted with a FLAG peptide were electrophoresed on an SDS–10% polyacrylamide gel, and proteins were stained with Coomassie blue. L, load; FT, flowthrough, W, wash. Molecular masses are indicated on the left. (B) Immunoblot analysis of fractions from the FLAG affinity column with antiserum raised against Drosophila ADAT1. Molecular masses are indicated on the left. Numbers at the top of the lanes are eluate fraction numbers; numbers at the bottom are lane numbers.
Antiserum preparation.
The 600-nucleotide PCR fragment used to screen cDNA libraries encoded the deaminase domain of dADAT1. This PCR fragment was also subcloned into the histidine tag expression vector pTrcHisB (Invitrogen) with the BamHI and HindIII restriction sites (pTrcHisB-DM) to generate a fusion protein with six histidines at the N terminus. Escherichia coli containing plasmid pTrcHisB-DM was grown in Superbroth and induced at an optical density at 600 nm of 1 with 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) and allowed to grow for an additional 4 h at 37°C. The bacteria were harvested by centrifugation, and the overexpressed protein was purified under denaturing conditions as described by the manufacturer (Qiagen) and chromatographed over an Ni2+-NTA affinity column. The fusion protein was eluted in 5 ml of buffer C (0.1 M sodium phosphate, 0.01 M Tris-HCl [pH 6.3], 250 mM imidazole, and 1 M urea). Aliquots of the fractions from the Ni2+-NTA affinity column were analyzed by electrophoresis on an SDS–12% polyacrylamide gel, and proteins were stained with Coomassie blue. The fusion protein had a molecular mass of approximately 30 kDa.
A New Zealand White rabbit was injected intradermally with approximately 100 μg of the recombinant fusion protein. The antigen was emulsified with Specol adjuvant (Central Veterinary Institute, Lelystad, The Netherlands). The rabbit was given a boost every 4 weeks, and blood was collected from the dorsal ear vein 2 weeks after each boost.
tRNA adenosine deaminase assay.
tRNA was transcribed in vitro with T7 RNA polymerase and [α-33P]ATP and purified as previously described (2, 15). The tRNA adenosine deaminase assays were performed at 30°C for 1 h with recombinant dADAT1. The assay was carried out under optimized conditions as previously described (16); in particular, 200 fmol of α-33P-labelled tRNAs was incubated with 1 μl of purified recombinant dADAT1 for 45 min at 30°C. One nanogram of scTad1p was used as a positive control. To measure specific activity on Bombyx or yeast substrates, 200 fmol of tRNA (4 nM) was used in a 50-μl reaction mixture with 1 ng of dADAT1 (0.04 nM). The activity unit is as defined by Auxilien et al. (2) and 1 U of enzyme activity converts 10−6 mol of adenosine to inosine per h under these conditions.
UV cross-linking experiments.
The cross-linking experiments were performed as previously described (30). The reaction mixture was the same as for the activity assays. Forty-five nanograms of purified recombinant dADAT1 was incubated with 200 fmol of substrate tRNA for 15 min at room temperature in a 10-μl final volume. The reactions were irradiated to 250 mJ in a Stratalinker-1800 (Stratagene) and subsequently digested with 250 ng of RNase A for 30 min at 37°C. A total of 2.5 μl of 4× SDS loading buffer (24) was added directly to the samples. Samples were electrophoresed on an SDS–12% polyacrylamide gel. Gels were fixed, dried, and exposed overnight on a PhosphorImager screen (Molecular Dynamics). For cross-linking studies with recombinant scTad1p, either 50 or 100 ng of protein was incubated with the tRNAs.
tRNA genes.
Yeast and Bombyx mori substrates are described by Gerber et al. (15). mut 5 was a gift from Henri Grosjean and is as previously described (2).
Whole-mount in situ hybridization.
Digoxigenin-labelled sense (T3 RNA polymerase and antisense (T7 RNA polymerase) RNA transcripts of dAdat1 from clone 12 were generated with a Boehringer Mannheim Dig-labelling kit according to the manufacturer's instructions and hybridized to Drosophila embryos overnight following standard protocols (49). Hybridized transcripts were detected with an alkaline phosphatase-conjugated anti-digoxigenin Fab fragment (Boehringer Mannheim), with nitroblue tetrazolium (Sigma) and 5-bromo-4-chloro-3-indolylphosphate (BCIP) (Sigma) as chromogenic substrates. Embryos were whole mounted in Canada balsam (Serva) and photographed with an Olympus 35-mm camera on an Olympus Provus microscope with differential interference contrast optics.
Nucleotide sequence accession numbers.
The nucleotide sequence of dAdat1 encoded by clone 12 has been submitted to the GenBank database; the accession number is AF192530. The hADAT sequence was submitted previously (28), and the accession number is AF125188.
RESULTS
Characterization of a Drosophila gene encoding an adenosine deaminase domain.
Homology searches with hADAR1 identified an ORF encoding an ADAR-like adenosine deaminase domain in a 3-kb subclone of D. melanogaster P1 genomic clone DS00941 that had been sequenced by the BDGP (Fig. 1). The sequence conservation in the predicted adenosine deaminase domain included the three motifs containing a histidine and two cysteine residues, proposed to be involved in zinc coordination at the active site, as well as a conserved glutamate residue in motif I which is also believed to participate in the deamination reaction (23, 34). The coding sequence in DS00941 did not contain any potential dsRNA-binding domain (47).
The genomic sequence from DS00941 was used to design PCR primers to amplify a 600-bp DNA fragment encoding this putative adenosine deaminase sequence from genomic DNA (Fig. 1), which was then used to screen embryonic cDNA libraries. Low-stringency Southern blots with this fragment indicated that the gene is single copy (results not shown). One long cDNA clone (clone 12) and another shorter clone (clone H) were isolated, confirming that this ORF is part of a transcribed sequence.
The P1 clone DS00941 is now fully sequenced by the BDGP and is part of a 2.9-Mb stretch of contiguous sequence in the Adh region on the left arm of Drosophila chromosome II. The gene encoding the adenosine deaminase domain is the second predicted gene product from the centromere distal end of this 82-kb P1 clone and is referred to as BG:DS00941.2 (1). The relationship between the isolated cDNAs and the structure of the BG:DS00941.2 transcript as predicted from the genomic sequence by Drosophila GRAIL and Genefinder programs are indicated in Fig. 1, together with the 600-nucleotide fragment that was used as a probe to isolate the cDNAs. Comparison of cDNA sequences with the genomic DNA sequence revealed that the cDNA clones are interrupted by a short intron of 57 bp within the protein coding sequence and by a second short intron of 54 bp within the 3′ untranslated region of the longer cDNA clone. Clone H begins at base pair 574 of clone 12 and terminates at a more proximal polyadenylation site. Two different polyadenylation sites are used, one located 30 bp after the ORF and a second site located 1.8 kb more 3′, which produces a transcript with a long 3′ region in which no additional ORFs can be identified. Both polyadenylation sites are preceded by a polyadenylation consensus sequence.
Clone 12 encodes an ORF (from nucleotides 23 to 1207) encoding a protein of 394 amino acids (Fig. 2) that contains a putative ADAR-type adenosine deaminase domain but lacks a dsRNA-binding domain. The predicted adenosine deaminase domain shows 38% identity and 47% similarity to human ADAR2, whereas the S. cerevisiae Tad1p has 25% identity and 37% similarity to human ADAR2 (Wisconsin Package; best fit, gap weight 8 and length weight 2) (13). The predicted Drosophila adenosine deaminase domain shows 29% identity and 40% similarity to the S. cerevisiae Tad1 protein.
FIG. 2.
Multiple sequence alignment showing the Drosophila adenosine deaminase encoded by clone 12, compared to vertebrate ADARs and yeast scTad1p and spTad1p. The adenosine deaminase motifs containing cysteine and histidine residues that are proposed zinc ligands (#) and a glutamate residue (Δ) that is thought to be involved in proton transfer in the deaminase active site are indicated. A potential RNA recognition region flanking the third cysteine of the deaminase motif is underlined. The human ADAT (hADAT) has 93 residues more than dADAT1 between deaminase motifs II and III, and this number of amino acids has been removed from hADAT between the sequences EDQP and SAKV after residue 157 (∗). The DDBJ, EMBL, and GenBank accession numbers of the sequences are as follows: human hADAR1, U10439; human hADAR2a (a shorter protein lacking an Alu element inserted between deaminase motifs II and III), U82120; S. cerevisiae scTad1p, AJ007297; S. pombe spTad1p, AL021748; dADAT1, AF192530; and hADAT, AF125188. The alignment was generated with the Wisconsin GCG Pileup program with a gap penalty of 3 and a gap extension penalty of 1 and edited by using Lineup. The order in which sequences are presented is determined by the program according to their similarity to each other.
This was the first gene of the ADAR family identified in Drosophila and we considered it a candidate enzyme for catalyzing the proposed editing events in the cac and para pre-mRNAs (18, 36, 44). Since the sequence of the encoded deaminase domain resembles vertebrate ADAR2 more than yeast Tad1p, we initially considered the possibility that the ORF was the 3′ end of a longer mRNA that would also encode dsRNA-binding domains. We therefore directed considerable effort to search for cDNAs that could encode a longer protein. Other embryonic cDNA libraries were screened, and despite the isolation of additional positive clones, none contained ORFs longer than that of clone 12. Neither amplification of 5′ ends from embryonic cDNA libraries nor 5′ rapid amplification of DNA ends with poly(A)+ RNA from embryos, larvae, or adults gave any indication of an RNA significantly longer than clone 12 that could encode additional amino acids on the 5′ end. Northern blot analysis of poly(A)+ RNA from embryos with a fragment encoding the deaminase domain used as a probe revealed two bands, one of approximately 4.3 kb and a shorter one of 3 kb, which is the same size as clone 12 (data not shown). We therefore conclude that the 4.3-kb band must encode an mRNA with an extended 3′ untranslated region. The genomic sequence upstream of the start of clone 12 contains no good consensus for a TATA box, but there is a consensus for an initiator (Inr) element (27) 15 nucleotides from the predicted start codon.
A rabbit polyclonal antiserum was raised against the deaminase domain. The BamHI-HindIII fragment encoding part of the deaminase domain was subcloned into the E. coli histidine tag expression vector pTrcHisB to obtain a fusion protein with six histidines at the N terminus. This protein was purified and used for polyclonal antibody production in rabbits. Antiserum raised against the adenosine deaminase domain recognized a protein of 43.5 kDa, which is the size predicted from the coding sequence in clone 12, on immunoblots of Drosophila embryonic nuclear extracts (data not shown).
BG:DS00941.2 encodes a Drosophila ADAT that deaminates adenosine 37 in a silk moth tRNA-alanine substrate.
The methylotrophic yeast P. pastoris was used to overexpress the protein encoded by clone 12 so as to determine its function. Previous work has shown this yeast to be excellent for the production of active ADARs and ADATs (15, 16, 33). The coding sequence of clone 12 was subcloned into the P. pastoris expression vector pSK-FLIS6 to express a fusion protein with a FLAG epitope at the amino terminus and six histidine residues at the carboxy terminus. This construct was transformed into P. pastoris by standard methods. Extracts from transformed cells contain a protein with a molecular mass of 48 kDa detectable on immunoblots with α-FLAG M2 antibody. This molecular mass is consistent with the length of the coding sequence of clone 12 and the N- and C-terminal epitope tags (results not shown).
Extracts were made from 300-ml cultures grown in methanol medium. Soluble extract was mixed with Ni2+-NTA, poured into a column, and eluted with 250 mM imidazole. This eluate was applied to an affinity column bearing the α-FLAG M2 monoclonal antibody, and proteins were eluted with FLAG peptide. Figure 3A shows SDS-polyacrylamide gel electrophoresis of fractions from a FLAG affinity column in which the proteins have been stained with Coomassie blue. A protein of 48 kDa is present in the eluate fractions, and this protein is recognized by the antibody raised against the deaminase domain (Fig. 3B). Approximately 40 μg of pure dADAT1 was obtained per 300 ml of starting culture.
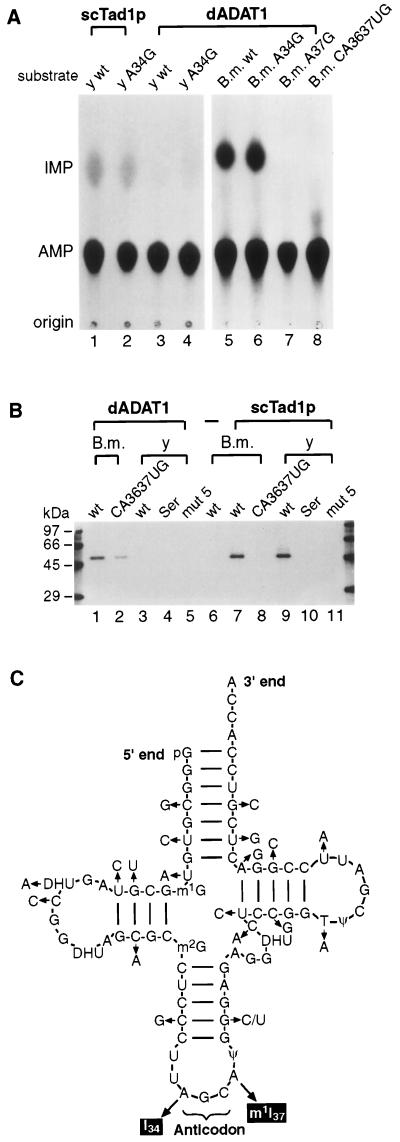
Peak fractions containing the 48-kDa protein from the FLAG affinity column were tested for adenosine deaminase activity on in vitro-synthesized [α-33P]ATP-labelled tRNAAla from the insect B. mori (2, 46, 51) or from S. cerevisiae. scTad1p has been shown to specifically deaminate the adenosine at position 37 in yeast and B. mori tRNAAla (15). The recombinant 48-kDa Drosophila protein converts adenosine to inosine in tRNAAla from B. mori with a specific activity of 8.4 U/mg (Fig. 4A, lane 5). Mutated B. mori tRNAAla in which the adenosine at position 34 has been changed to guanosine has the same level of adenosine-to-inosine conversion as the wild-type substrate (lane 6). However, a change from adenosine to guanosine at position 37 (lane 7) or a double change of cytosine 36 to uridine and adenosine 37 to guanosine (lane 8) eliminates all adenosine-to-inosine conversion in the Bombyx tRNA substrate, indicating that it is adenosine 37 and not adenosine 34 that is deaminated. In contrast, yeast tRNAAla is not an efficient substrate for the Drosophila enzyme that has a specific activity of 0.08 U/mg with this substrate (Fig. 4A, lane 3). The location of the inosine formed in the tRNAAla substrates was confirmed by performing reverse transcription PCR on the reaction products and sequencing the resulting cDNAs (data not shown). Therefore, the Drosophila clone encodes a protein that is clearly a functional homolog of scTad1p, and we have named it Drosophila ADAT1 (dADAT1).
FIG. 4.
Recombinant Drosophila adenosine deaminase binds to and modifies tRNAAla. (A) tRNA-specific adenosine deaminase assay with peak fractions from an α-FLAG M2 antibody column. Different tRNAAla species were labelled with [α-33P]ATP. The extracts used were recombinant scTad1p (lanes 1 and 2) and recombinant dADAT1 (lanes 3 to 8). The substrates used are indicated above each lane. Abbreviations: y, yeast; B.m., B. mori; wt, wild-type tRNAAla; A34G, G34 instead of A34; A37G, G37 instead of A37; CA3637UG, substrate has a double change of C36 to U36 and A37 to G37. (B) UV cross-linking of dADAT1 and scTad1p to [α-33P]ATP-labelled tRNA. The reaction mixtures were irradiated, digested with RNase A, and electrophoresed on an SDS–12% polyacrylamide gel. Lanes 1 to 5 each contained 45 ng of recombinant dADAT1, and lanes 7 to 11 each contained 100 ng of recombinant scTad1p. Lane 6 had no protein. The abbreviations for the substrates are the same as in panel A. Ser, tRNASer; mut5, yeast tRNAAsp with tRNAArg anticodon loop (2). (C) Sequence differences between tRNAAla from yeast and B. mori. The 19-base differences between the two tRNAAla species are indicated. The silk gland-specific tRNAAla from B. mori contains U instead of C at position 40.
The Drosophila enzyme cannot bind efficiently to the yeast tRNAAla. UV cross-linking of dADAT1 to [α-33P]ATP-labelled tRNAAla substrates from B. mori or yeast confirm that dADAT1 binds much more efficiently to the tRNA from B. mori (Fig. 4B, lanes 1 and 3). Surprisingly, dADAT1 also binds to a Bombyx tRNA substrate in which positions 36 and 37 are mutated (Fig. 4B, lane 2) although less tightly than to the wild type. The scTad1 protein deaminates both the yeast tRNA (Fig. 4A) and the B. mori tRNA efficiently (15), and UV cross-linking experiments confirm that scTad1p binds efficiently to both tRNAAla from B. mori (Fig. 4B, lane 7) and from yeast (lane 9).
We also tested whether purified recombinant dADAT1 could deaminate adenosine to inosine on extended dsRNA, but no conversion was observed (results not shown), suggesting that dADAT1 has no role in pre-mRNA editing.
Expression of dAdat1 transcripts in Drosophila embryos.
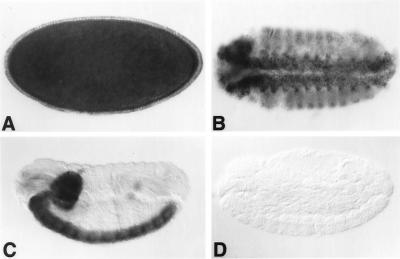
Digoxigenin-labelled sense or antisense dAdat1 RNA probes derived from clone 12 were hybridized to Drosophila embryos. As expected, a signal could be visualized only with the antisense dAdat1 probe. The expression patterns observed with antisense probes from clone H and clone 12 were identical, and those obtained with clone 12 are shown in Fig. 5. A strong overall staining was detected at the blastoderm stage (Fig. 5A), suggesting that dAdat1 mRNA is maternally provided, since no zygotic transcripts are expressed at this early stage. Zygotic expression was very strong during germ band extension, especially in the mesoderm and neuroectoderm (Fig. 5B). From germ band retraction onwards, high levels of transcripts were confined to the central nervous system and transcript levels remained high in the entire brain and ventral nerve cord throughout late embryogenesis (Fig. 5C).
FIG. 5.
Expression of dAdat1 transcripts in Drosophila embryos. Embryos were hybridized with digoxigenin-labelled antisense probe to dADAT1 clone 12. (A) Embryo at cellular blastoderm formation, showing maternally supplied transcript. (B) Ventral view of an embryo during germ band retraction, showing widespread expression of dAdat1 transcripts in mesoderm, ectoderm, ventral nerve cord, and supraesophageal ganglion. (C) Lateral view of a later-stage embryo, showing expression confined to the ventral nerve cord and supraesophageal ganglion. (D) Lateral view of late embryo hybridized with sense probe. All embryos have their anterior to the left, and photographs were taken at ×60 magnification.
Isolation of a human ADAT gene and comparison with Drosophila Adat1 reveals metazoan ADATs with adenosine deaminase domains surprisingly similar to ADARs involved in pre-mRNA editing.
Database searches with S. cerevisiae ADAT1 identified a partial sequence of a potential human homolog in the Washington University EST database. The clone from which this sequence derived was obtained, and additional sequence information for the amino-terminal half of this protein was determined (see Materials and Methods). The resulting sequence revealed an ORF with homology over its entire coding sequence to Drosophila Adat1, which is not surprising considering that tRNAAla species from B. mori and humans are very similar (6). While this paper was in preparation, Maas et al. (28) reported the characterization of the human ADAT1 and showed that this protein deaminates adenosine 37 of human tRNAAla. The amino acid sequence of this human ADAT is included in the multiple sequence alignment shown in Fig. 2. Both human and Drosophila ADATs are more similar to adenosine deaminase domains of vertebrate ADARs than to the yeast ADATs.
To examine phylogenetic relationships among adenosine deaminase domains, ADAR and ADAT sequences were recovered from databases and multiple sequence alignments were performed with the Wisconsin Genetics Computer Group (GCG) Pileup program (13). The most consistent alignments involve residues close to the histidine and cysteine residues of deaminase motifs I, II, and III (Fig. 2). All ADAT proteins are approximately the same size and there is homology among the proteins over the full length of the sequences that is observed when lower gap insertion and extension penalties are used in the Pileup program. Aligned sequences were truncated to remove dsRNA-binding domain sequences from the ADAR genes, and multiple sequence alignments of adenosine deaminase domains were used to generate phylogenetic trees with the PAUP (phylogenetic analysis using parsimony) (48) program in the Wisconsin GCG program package (13).
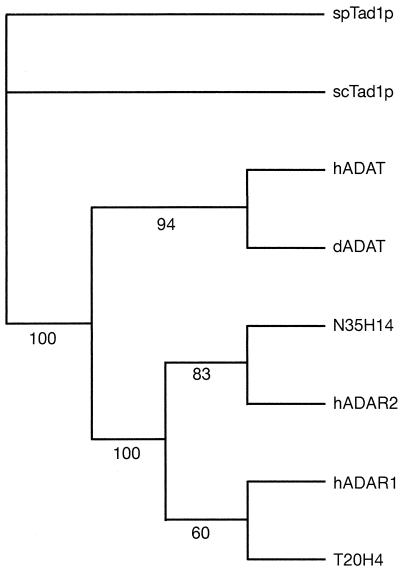
Many adenosine deaminase domain trees generated from Pileup alignments made with different gap insertion and extension penalties have topologies similar to that shown in Fig. 6. Human ADAT and Drosophila ADAT1 are closely related and their relationship to S. cerevisiae Tad1p and Schizosaccharomyces pombe Tad1p pair is distant. The ADAR genes are always found to branch from the ADAT line. Among the ADARs, human ADAR2 and the putative Drosophila ADAR N35H14 (accession no. AL035207) are closely related, while the branching order of the other two ADARs is less consistent. In order to obtain the best possible alignment with fewer gaps and with aligned residues representing true homologies, a region around the conserved deaminase motifs was selected and aligned more stringently to produce the tree shown in Fig. 6.
FIG. 6.
Phylogenetic tree of ADAR and ADAT adenosine deaminase domain sequences. The sequence region from the TGXKC block before deaminase motif I to the PIYL conservation after deaminase motif III (see Fig. 2) was taken for each sequence and used to generate a more-stringent Pileup alignment (gap insertion penalty, 8; extension penalty, 2). This alignment was used to generate the tree and bootstrap values shown with PAUP in the Wisconsin GCG software package (48). GenBank database accession numbers for sequences used here are as follows: Drosophila ADAR sequence N35H14, AL035207; and C. elegans ADAR sequence T20H4.4, Q22618.
DISCUSSION
An enzymatic activity capable of converting adenosine to inosine in dsRNA is present in D. melanogaster (10). To find a gene encoding this activity, we performed a homology search with hADAR1. An ORF was found that had high sequence homology to the deaminase domain of hADAR1. We isolated this gene and showed that it encoded an adenosine deaminase that specifically converts adenosine to inosine at position 37, adjacent to the anticodon in B. mori tRNAAla(IGC). We have named this gene dAdat1 because of its homology to the yeast scADAT1.
The dAdat1 gene is located 2 to 7 kb centromere distally to Son of sevenless (Sos) (7, 43) in the Adh region on the left arm of chromosome II. It is within a 2.9-Mb stretch of contiguous sequence that has been correlated with extensive sets of deletions and point mutations (1). dAdat1 is flanked centromere distally by a predicted carbonate dehydratase gene (BG:DS00941.1) and centromere proximally by a predicted proteasome subunit gene (BG:DS00941.3). The predicted carbonate dehydratase coding sequence is fully contained within the 3′ untranslated region of the longer dAdat1 cDNA clone 12. dAdat1 and the predicted proteasome subunit gene are both present on genomic fragments that contained Sos and 10 kb of upstream DNA that was transformed into Drosophila to identify the correct mRNA for Sos by genetic rescue (7, 43).
Drosophila ADAT1 binds and deaminates tRNAAla from B. mori 100-fold more efficiently than the tRNAAla from S. cerevisiae (data not shown). The yeast Tad1p deaminates A37 more effectively in yeast tRNAAla than in the tRNAAla from B. mori (15). Each ADAT shows preference for tRNAAla from the same or more closely related organisms.
The tRNAAla from B. mori (Fig. 3C) that was used as a substrate for dADAT1 in this study is a tRNA expressed specifically in the silk moth salivary gland, where alanine is incorporated into the silk fibroin protein (46, 51). Wild-type and mutant forms of this tRNA substrate have been used in earlier studies of adenosine-to-inosine conversion and other modifications in tRNAAla (2, 17). This salivary gland-specific tRNAAla differs by one base at position 40 in the anticodon arm from the constitutive silk moth tRNAAla, which also has an inosine at position 37 (Fig. 4C). One gene encoding a tRNAAla has been characterized in Drosophila (12), but it is not known whether some Drosophila tRNAs have tissue-specific expression similar to that found for the silk moth tRNAAla genes (46). It is not known if the conversion of adenosine to inosine at position 37 in tRNAAla is always complete, and the degree of conversion could vary in different tissues.
The significance of adenosine-to-inosine conversion adjacent to the anticodon in tRNAAla(IGC) is not known. Yeast tad1 mutants are viable and contain unmodified adenosine 37 in their tRNAAla species (15). No deletion in dAdat1 is available that only removes dAdat1 and not neighboring genes. Almost all the lethal mutations that have been found near Sos have been assigned to genes that were previously known or that have been identified in the genomic sequence (1). None of the known lethal mutations are in dAdat1. The effect of loss of function in Drosophila Adat1 therefore cannot yet be assessed.
Transcripts of dAdat1 are produced by nurse cells and loaded into the egg before fertilization (Fig. 5). Many mRNAs encoding proteins that will be required for transcription and translation during the early stages of embryonic development are provided in this way. Zygotic cells throughout the embryo contain dAdat1 mRNA in germ band-extended embryos, but most of the expression in later germ band-retracted embryos is in the central nervous system. Much of the mesoderm and ectoderm is larval tissue that will be histolyzed at metamorphosis; thus, it is possible that early dAdat1 expression is sufficient for the remaining lifetime of these cells. The high level of expression in the nervous system is intriguing. It is possible that dAdat1 activity is ubiquitous, even though the transcript expression pattern observed by in situ hybridization is concentrated in the nervous system. Considering the high degree of homology between dADAT1 and the ADAR enzymes, it is curious that the embryonic expression of dAdat1 is found in the central nervous system and is reminiscent of rRED2 expression, which is confined to the brain (31).
It is surprising that Drosophila ADAT1 shows a greater similarity to ADARs involved in pre-mRNA editing than to the yeast Tad1 proteins, considering that these proteins have very different substrates (Fig. 2). Based on functional groupings, dADAT1 might have been expected to resemble S. cerevisiae Tad1p more than vertebrate ADARs. A human ADAT gene also exhibits similarity to dAdat1 and to vertebrate ADARs. It is possible that this similarity between dADAT1 and ADARs reflects a role for dADAT1 in editing pre-mRNA. However, such an activity is unlikely, since recombinant dADAT1 protein cannot convert adenosine to inosine in dsRNA substrates. An ADAR-like gene (N35H14) with two dsRNA-binding domains has been sequenced by the European Drosophila Genome Project and independently cloned from Drosophila (R. Reenan, personal communication). The protein encoded by this gene converts adenosine to inosine in dsRNA substrates (M. O'Connell and R. Reenan, unpublished results) and is a strong candidate for catalyzing editing in the cac and para pre-mRNAs (18, 36, 44).
One major question is how the ADAR family of enzymes recognize their target adenosines in pre-mRNA. The dsRNA-binding domains show little sequence specificity, recognizing continuous dsRNA structures (41). With the emergence of the homologous ADAT family of enzymes having a deaminase domain that binds to specific tRNA, it seems probable that the adenosine deaminase domain of ADARs contributes more than was previously anticipated to RNA binding and hence to substrate recognition. It has previously been shown that adenosine deaminase domains alone are not sufficient for editing by ADARs in vitro (25, 29), but it has not been determined if the deaminase domain alone can bind to RNA. A domain of ADAR corresponding to the region of ADAT homology can now be expressed in recombinant form. Detailed RNA-binding studies can then be performed in vitro to determine if indeed the deaminase domain alone can bind to pre-mRNA substrates and contribute to the target specificity of these enzymes.
ADATs are likely to have a higher affinity for RNA substrates than an isolated ADAR adenosine deaminase domain, since ADARs probably depend on their dsRNA-binding domains for RNA affinity. It is interesting therefore to compare ADAR and ADAT sequences around the putative zinc-chelating residues. The number of residues between deaminase motifs I and II is well conserved, whereas the number of residues between deaminase motifs II and III differs (22). Structural adjustments near deaminase motif III could be associated with changes in the target specificity of adenosine deaminase domains. A cluster of positively charged residues is well conserved between dADAT1, hADAT1, and the yeast ADATs in the region amino terminal to deaminase motif III (Fig. 2) (28). This cluster of positively charged residues could be a region of the protein that contacts RNA.
This region of sequence similarity among ADATs has also been noted by Maas et al. (28) in an alignment in which all hADAT residues between deaminase motifs II and III were included. Due to the variability in the number of residues between motifs II and III, sequence alignments that include this region can have different gaps and therefore highlight different blocks of homology. In addition to the ADAT signature sequence near deaminase motif III, Maas et al. (28) observed an ADAR specific signature sequence 19 residues amino terminal to the ADAT signature sequence. However, visual inspection of many different computer-generated alignments suggests that this ADAR-specific signature could be a variant of the ADAT signature sequence which has been moved 19 residues further from deaminase motif III. Residues 178 to 189 of hADAR1 (LRTKvenGEgTi) (Fig. 2), which are conserved among ADARs, resemble residues 176 to 187 of dADAT1 (LRTKpgrGErT1), which are conserved among ADATs. It is possible that the evolution of ADATs to ADARs involved an insertion close to motif III that could have affected target sequence recognition.
It is possible that an ADAT signature sequence is only visible close to the active site of the enzyme, as other tRNA-protein contacts may involve multiple weakly conserved sites over the entire protein and therefore be difficult to recognize. One or more of the 19 differences between tRNAAla from S. cerevisiae and B. mori have a more significant effect on the activity of dADAT1 than on Tad1p, suggesting that these proteins may not make all RNA contacts in precisely the same manner.
Pre-mRNA editing may have evolved when an original ADAT acquired dsRNA-binding domains and a new set of targets in pre-mRNA. An interesting interpretation of sequence homologies and of the phylogenetic tree shown in Fig. 6 would be the following. (i) Tad1p does not encode an essential function in yeast, and the sequence conservation with metazoan ADATs is correspondingly low and focused on the zinc-chelating residues in the deaminase domain. (ii) ADARs evolved from ADATs after the divergence of fungal and metazoan lines. (iii) Double-stranded RNA-binding domains may have been added to an ADAT gene after a gene duplication event, and such a protein might have retained ADAT function for a period until it evolved to recognize a more ADAR-like range of RNA targets. Other evolutionary scenarios can be envisaged, however; Drosophila and human ADAT genes appear to be more closely related than yeast TAD1 to a precursor gene that gave rise to the ADAR family. Studies on the recognition of tRNA substrates by Drosophila or human ADAT1 should help to explain how pre-mRNA editing evolved and how target sequences are recognized in pre-mRNA editing.
ACKNOWLEDGMENTS
We are grateful to Hyouta Himeno, Hirosaki University, for yeast tRNA-Ser; to H. Grosjean for tRNA substrates; to M. Affolter and A. Jarman for cDNA libraries; and to R. Reenan for sharing unpublished results.
This work was supported by the Medical Research Council, the University of Basel, the Swiss National Science Foundation, and the Louis-Jeantet Foundation for Medicine. A.P.G. was the recipient of a predoctoral fellowship from the Boehringer Ingelheim Fonds, and R.L. was supported by a grant from the European Union (via the Bundesamt für Bildung und Wissenschaft, Bern, Switzerland).
REFERENCES
- 1.Ashburner M, Misra S, Roote J, Lewis S E, Blazej R, Davis T, Doyle C, Galle R, George R, Harris N, Hartzell G, Harvey D, Hong L, Houston K, Hoskins R, Johnson G, Martin C, Moshrefi A, Palazzolo M, Reese M G, Spradling A, Tsang G, Wan K, Whitelaw K, Kimmel B, et al. An exploration of the sequence of a 2.9-Mb region of the genome of Drosophila melanogaster. The Adh region. Genetics. 1999;153:179–219. doi: 10.1093/genetics/153.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Auxilien S, Crain P F, Trewyn R W, Grosjean H. Mechanism, specificity and general properties of the yeast enzyme catalysing the formation of inosine 34 in the anticodon of transfer RNA. J Mol Biol. 1996;262:437–458. doi: 10.1006/jmbi.1996.0527. [DOI] [PubMed] [Google Scholar]
- 3.Bass B L, Nishikura K, Keller W, Seeburg P H, Emeson R B, O'Connell M A, Samuel C E, Herbert A. A standardized nomenclature for adenosine deaminases that act on RNA. RNA. 1997;3:947–949. [PMC free article] [PubMed] [Google Scholar]
- 4.Bass B L, Weintraub H. A developmentally regulated activity that unwinds RNA duplexes. Cell. 1987;48:607–613. doi: 10.1016/0092-8674(87)90239-x. [DOI] [PubMed] [Google Scholar]
- 5.Bass B L, Weintraub H, Cattaneo R, Billeter M A. Biased hypermutation of viral RNA genomes could be due to unwinding/modification of double-stranded RNA. Cell. 1989;56:331. doi: 10.1016/0092-8674(89)90234-1. [DOI] [PubMed] [Google Scholar]
- 6.Becker H F, Corda Y, Mathews M B, Fourrey J L, Grosjean H. Inosine and N1-methylinosine within a synthetic oligomer mimicking the anticodon loop of human tRNA(Ala) are major epitopes for anti-PL-12 myositis autoantibodies. RNA. 1999;5:865–875. doi: 10.1017/s1355838299990118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonfini L, Karlovich C A, Dasgupta C, Banerjee U. The Son of sevenless gene product: a putative activator of Ras. Science. 1992;255:603–606. doi: 10.1126/science.1736363. [DOI] [PubMed] [Google Scholar]
- 8.Burns C M, Chu H, Rueter S M, Hutchinson L K, Canton H, Sanders-Bush E, Emeson R B. Regulation of serotonin-2C receptor G-protein coupling by RNA editing. Nature. 1997;387:303–308. doi: 10.1038/387303a0. [DOI] [PubMed] [Google Scholar]
- 9.Carter C W. Nucleoside deaminases for cytidine and adenosine: comparison with deaminases acting on RNA. In: Grosjean H, Benne R, editors. Modification and editing of RNA. Washington, D.C.: ASM Press; 1998. pp. 363–375. [Google Scholar]
- 10.Casey J L, Gerin J L. Hepatitis D virus RNA editing: specific modification of adenosine in the antigenomic RNA. J Virol. 1995;69:7593–7600. doi: 10.1128/jvi.69.12.7593-7600.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen S H, Habib G, Yang C Y, Gu Z W, Lee B R, Weng S A, Silberman S R, Cai S J, Deslypere J P, Rosseneu M, Gotto A M J, Li W H, Chan L. Apolipoprotein B-48 is the product of a messenger RNA with an organ-specific in frame stop codon. Science. 1987;238:363–366. doi: 10.1126/science.3659919. [DOI] [PubMed] [Google Scholar]
- 12.DeLotto R, Schedl P. A Drosophila melanogaster transfer RNA gene cluster at the cytogenetic locus 90BC. J Mol Biol. 1984;179:587–605. doi: 10.1016/0022-2836(84)90157-8. [DOI] [PubMed] [Google Scholar]
- 13.Devereux J, Haeberli P, Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984;12:387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frohmann M A, Dush M K, Martin G R. Rapid production of full-length cDNAs from rare transcripts: amplification using a single gene-specific oligonucleotide primer. Proc Natl Acad Sci USA. 1988;85:8998–9002. doi: 10.1073/pnas.85.23.8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gerber A, Grosjean H, Melcher T, Keller W. Tad1p, a yeast tRNA-specific adenosine deaminase, is related to the mammalian pre-mRNA editing enzymes ADAR1 and ADAR2. EMBO J. 1998;17:4780–4789. doi: 10.1093/emboj/17.16.4780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gerber A, O'Connell M A, Keller W. Two forms of human double-stranded RNA-specific editase 1 (hRED1) generated by the insertion of an Alu cassette. RNA. 1997;3:453–463. [PMC free article] [PubMed] [Google Scholar]
- 17.Grosjean H, Auxilien S, Constantinesco F, Simon C, Corda Y, Becker H F, Foiret D, Morin A, Jin Y X, Fournier M, Fourrey J L. Enzymatic conversion of adenosine to inosine and to N1-methylinosine in transfer RNAs: a review. Biochimie. 1996;78:488–501. doi: 10.1016/0300-9084(96)84755-9. [DOI] [PubMed] [Google Scholar]
- 18.Hanrahan C J, Palladino M J, Bonneau L J, Reenan R A. RNA editing of a Drosophila sodium channel gene. Ann N Y Acad Sci. 1998;868:51–66. doi: 10.1111/j.1749-6632.1999.tb11273.x. [DOI] [PubMed] [Google Scholar]
- 19.Helt G A, Lewis S, Loraine A E, Rubin G M. BioViews: Java-based tools for genomic data visualization. Genome Res. 1998;8:291–305. doi: 10.1101/gr.8.3.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Higuchi M, Single F N, Köhler M, Sommer B, Sprengel R, Seeburg P H. RNA editing of AMPA receptor subunit GluR-B: a base-paired intron-exon structure determines position and efficiency. Cell. 1993;75:1361–1370. doi: 10.1016/0092-8674(93)90622-w. [DOI] [PubMed] [Google Scholar]
- 21.Holley R W, Everett G A, Madison J T, Zamir A. Nucleotide sequences in yeast alanine transfer RNA. J Biol Chem. 1965;240:2122–2127. [PubMed] [Google Scholar]
- 22.Keller W, Wolf J, Gerber A. Editing of messenger RNA precursors and of tRNAs by adenosine to inosine conversion. FEBS Lett. 1999;452:71–76. doi: 10.1016/s0014-5793(99)00590-6. [DOI] [PubMed] [Google Scholar]
- 23.Kim U, Wang Y, Sanford T, Zeng Y, Nishikura K. Molecular cloning of cDNAs for double-stranded RNA adenosine deaminase, a candidate enzyme for nuclear RNA editing. Proc Natl Acad Sci USA. 1994;91:11457–11461. doi: 10.1073/pnas.91.24.11457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laemmli U K. Cleavage of structural proteins during the assembly of the head of the bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 25.Lai F, Drakas R, Nishikura K. Mutagenic analysis of double-stranded RNA adenosine deaminase, a candidate enzyme for RNA editing of glutamate-gated ion channel transcripts. J Biol Chem. 1995;270:17098–17105. doi: 10.1074/jbc.270.29.17098. [DOI] [PubMed] [Google Scholar]
- 26.Lennon G G, Auffray C, Polymeropoulos M, Soares M B. The I. M. A. G. E. consortium: an integrated molecular analysis of genomes and their expression. Genomics. 1996;33:151–152. doi: 10.1006/geno.1996.0177. [DOI] [PubMed] [Google Scholar]
- 27.Lo K, Smale S T. Generality of a functional initiator consensus sequence. Gene. 1996;182:13–22. doi: 10.1016/s0378-1119(96)00438-6. [DOI] [PubMed] [Google Scholar]
- 28.Maas S, Gerber A P, Rich A. Identification and characterization of a human tRNA-specific adenosine deaminase related to the ADAR family of pre-mRNA editing enzymes. Proc Natl Acad Sci USA. 1999;96:8895–9000. doi: 10.1073/pnas.96.16.8895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maas S, Melcher T, Herb A, Seeburg P H, Keller W, Krause S, Higuchi M, O'Connell M A. Structural requirements for RNA editing in glutamate receptor pre-mRNA by recombinant double-stranded RNA adenosine deaminase. J Biol Chem. 1996;271:12221–12226. doi: 10.1074/jbc.271.21.12221. [DOI] [PubMed] [Google Scholar]
- 30.Martin G, Keller W. Mutational analysis of mammalian poly(A) polymerase identifies a region for primer binding and a catalytic domain, homologous to the family X polymerases, and to other nucleotidyltransferases. EMBO J. 1996;15:2593–2603. [PMC free article] [PubMed] [Google Scholar]
- 31.Melcher T, Maas S, Herb A, Sprengel R, Higuchi M, Seeburg P H. RED2, a brain specific member of the RNA-specific adenosine deaminase family. J Biol Chem. 1996;271:31795–31798. doi: 10.1074/jbc.271.50.31795. [DOI] [PubMed] [Google Scholar]
- 32.Melcher T, Maas S, Herb A, Sprengel R, Seeburg P H, Higuchi M. A mammalian RNA editing enzyme. Nature. 1996;379:460–464. doi: 10.1038/379460a0. [DOI] [PubMed] [Google Scholar]
- 33.O'Connell M A, Gerber A, Keegan L P. Purification of native and recombinant double-stranded RNA-specific adenosine deaminases. Methods (Orlando) 1998;15:51–62. doi: 10.1006/meth.1998.0605. [DOI] [PubMed] [Google Scholar]
- 34.O'Connell M A, Krause S, Higuchi M, Hsuan J J, Totty N F, Jenny A, Keller W. Cloning of cDNAs encoding mammalian double-stranded RNA-specific adenosine deaminase. Mol Cell Biol. 1995;15:1389–1397. doi: 10.1128/mcb.15.3.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paul M, Bass B L. Inosine exists in mRNA at tissue-specific levels and is most abundant in brain mRNA. EMBO J. 1998;17:1120–1127. doi: 10.1093/emboj/17.4.1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peixoto A A, Smith L A, Hall J C. Genomic organization and evolution of alternative exons in a Drosophila calcium channel gene. Genetics. 1997;145:1003–1013. doi: 10.1093/genetics/145.4.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Petschek J P, Mermer M J, Scheckelhoff M R, Simone A A, Vaughn J C. RNA editing in Drosophila 4f-rnp gene nuclear transcripts by multiple A-to-G conversions. J Mol Biol. 1996;259:885–890. doi: 10.1006/jmbi.1996.0365. [DOI] [PubMed] [Google Scholar]
- 38.Polson A G, Crain P F, Pomerantz S C, McCloskey J A, Bass B L. The mechanism of adenosine to inosine conversion by the double-stranded RNA unwinding/modifying activity: a high-performance liquid chromatography-mass spectrometry analysis. Biochemistry. 1991;30:11507–11514. doi: 10.1021/bi00113a004. [DOI] [PubMed] [Google Scholar]
- 39.Powell L M, Wallis S C, Pease R J, Edwards Y H, Knott T J, Scott J. A novel form of tissue-specific RNA processing produces apolipoprotein-48 in intestine. Cell. 1987;50:831–840. doi: 10.1016/0092-8674(87)90510-1. [DOI] [PubMed] [Google Scholar]
- 40.Rebagliati M R, Melton D A. Antisense RNA injections in fertilized frog eggs reveal an RNA duplex unwinding activity. Cell. 1987;48:599–605. doi: 10.1016/0092-8674(87)90238-8. [DOI] [PubMed] [Google Scholar]
- 41.Ryter J M, Schultz S C. Molecular basis of double-stranded RNA-protein interactions: structure of a dsRNA-binding domain complexed with dsRNA. EMBO J. 1998;17:7505–7513. doi: 10.1093/emboj/17.24.7505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seeburg P H. The role of RNA editing in controlling glutamate receptor channel properties. J Neurochem. 1996;66:1–5. doi: 10.1046/j.1471-4159.1996.66010001.x. [DOI] [PubMed] [Google Scholar]
- 43.Simon M A, Bowtell D D, Dodson G S, Laverty T R, Rubin G M. Ras1 and a putative guanine nucleotide exchange factor perform crucial steps in signalling by the sevenless protein tyrosine kinase. Cell. 1991;67:701–716. doi: 10.1016/0092-8674(91)90065-7. [DOI] [PubMed] [Google Scholar]
- 44.Smith L A, Wang X J, Peixoto A A, Neumann E K, Hall L M, Hall J C. A Drosophila calcium channel α1 subunit gene maps to a genetic locus associated with behavioural and visual defects. J Neurosci. 1996;16:7868–7879. doi: 10.1523/JNEUROSCI.16-24-07868.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sommer B, Köhler M, Sprengel R, Seeburg P H. RNA editing in brain controls a determinant of ion flow in glutamate-gated channels. Cell. 1991;67:11–19. doi: 10.1016/0092-8674(91)90568-j. [DOI] [PubMed] [Google Scholar]
- 46.Sprague K U, Hagenbuchle O, Zuniga M C. The nucleotide sequence of two silk gland alanine tRNAs: implications for fibroin synthesis and for initiator tRNA structure. Cell. 1977;11:561–570. doi: 10.1016/0092-8674(77)90074-5. [DOI] [PubMed] [Google Scholar]
- 47.St. Johnston D, Brown N H, Gall J G, Jantsch M. A conserved double-stranded RNA-binding domain. Proc Natl Acad Sci USA. 1992;89:10979–10983. doi: 10.1073/pnas.89.22.10979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Swofford D L. PAUP: phylogenetic analysis using parsimony. Champaign, Ill: Illinois Natural History Survey; 1993. [Google Scholar]
- 49.Tautz D, Pfeifle C. A non-radioactive in situ hybridization method for the localization of specific RNAs in Drosophila embryos reveals translational control of the segmentation gene hunchback. Chromosoma. 1989;98:81–85. doi: 10.1007/BF00291041. [DOI] [PubMed] [Google Scholar]
- 50.Ullrich A, Berman C H, Dull T J, Gray A, Lee J M. Isolation of the human insulin-like growth factor 1 gene using a single synthetic DNA probe. EMBO J. 1984;3:361–364. doi: 10.1002/j.1460-2075.1984.tb01812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Underwood D C, Knickerbocker H, Gardner G, Condliffe D P, Sprague K U. Silk gland-specific tRNA(Ala) genes are tightly clustered in the silkworm genome. Mol Cell Biol. 1988;8:5504–5512. doi: 10.1128/mcb.8.12.5504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wagner R W, Yoo C, Wrabetz L, Kamholz J, Buchhalter J, Hassan N F, Khalili K, Kim S U, Perussia B, McMorris F A, Nishikura K. Double-stranded RNA unwinding and modifying activity is detected ubiquitously in primary tissues and cell lines. Mol Cell Biol. 1990;10:5586–5590. doi: 10.1128/mcb.10.10.5586. [DOI] [PMC free article] [PubMed] [Google Scholar]