Abstract
Objectives
We aimed to evaluate the (a) potential predictors of first biological disease-modifying anti-rheumatic drug (bDMARD) failure and (b) factors associated with failure of multiple therapies in psoriatic arthritis (PsA).
Materials and methods
We enrolled consecutive PsA patients attending our unit and undergoing bDMARDs during 2004–2020. Disease characteristics, previous/ongoing treatments, comorbidities, and follow-up duration were recorded. Disease activity and functional and clinimetric scores were recorded at baseline and yearly and were compared between switchers and non-switchers, and within switchers according to the reasons for switching. Effectiveness was evaluated over time with descriptive statistics; multivariate Cox and logistic regression models were used to evaluate predictors of response and failure of multiple bDMARDs. Kaplan–Meier curves were used to assess differences in time-to-first bDMARD discontinuation. Infections and adverse events were recorded.
Results
Two hundred sixty-four patients were included (117 (44.32%) females, mean age 56 years, mean PsA duration 15 years); 117 (44.32%) switched bDMARDs at least once. Switchers were mostly females, with higher Psoriasis Area and Severity Index and worse Health Assessment Questionnaire at baseline. Mean time-to-first bDMARD discontinuation was 72 months; 2-year and 5-year retention rates were 75% and 60%, respectively. Survival curves for anti-TNFα/anti-IL12/23/anti-IL17 were similar (p = 0.66). Main reasons for switching were inefficacy (67.52%) and adverse events (25.7%). Female sex was associated with a higher risk of first bDMARD discontinuation (HR = 2.39; 95% CI: 1.50–3.81) and failure of multiple bDMARDs (OR = 1.99; 95% CI: 1.07–3.69); initiating therapy before 2015 was protective (HR = 0.40; 95% CI: 0.22–0.73).
Conclusions
Survival rate was good for anti-TNFα and other bDMARDs. Female sex was a predictor of first bDMARD discontinuation, unlike mechanism of action, comorbidities, and BMI.
|
Key Points • Drug survival in PsA patients was confirmed be greater for the first bDMARD administered. • In case of failure of the first bDMARD, switching/swapping proved a good treatment option, as reflected by a persistent satisfactory effectiveness with second-line bDMARDs and so subsequent switches. • Female sex may constitute a predisposing risk factor for flare and therapeutic switches. • Discontinuation or switching of biologics due to mechanism of action, comorbidities tolerability and BMI did not seem to impact first bDMARD withdrawal. |
Supplementary Information
The online version contains supplementary material available at 10.1007/s10067-021-05799-0.
Keywords: Biological therapy, Disease activity, Interleukins, Psoriatic arthritis, Survival analysis
Introduction
Psoriatic arthritis (PsA) is a chronic inflammatory disease characterized by articular and skin involvement [1]. Enthesitis, dactylitis, spine involvement, and extra-articular manifestations (e.g., uveitis, inflammatory bowel disease, and other comorbidities such as metabolic disorders and cardiovascular diseases) are typical occurrences in PsA [1–6]. The reported prevalence of PsA in the general population is about 1%, and the disease affects up to 30–40% (range 6–42%) of patients with psoriasis [7]. The disease often causes substantial functional impairment and decreased quality of life, if not diagnosed early and treated appropriately [8]. Non-steroidal anti-inflammatory drugs (NSAIDs) are commonly used to reduce the symptoms. Conventional synthetic disease-modifying anti-rheumatic drugs (csDMARDs) are recommended to treat the peripheral manifestations of the disease, thus improving clinical response and slowing disease progression. The European League Against Rheumatism (EULAR), the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA), and the Italian Society of Rheumatology (SIR) guidelines suggest to treat non-responsive patients with csDMARDs and those with an aggressive form with biological disease-modifying anti-rheumatic drugs (bDMARDs) [9–11]. Until the 2000s, PsA patients were treated with traditional csDMARDs, often with unsatisfactory results as it relates to disease control and radiographic progression [8]. The advent of biologics has changed the natural history of PsA by significantly improving quality of life and reducing damage progression. Biologics are currently recommended for the treatment of PsA in patients who respond inadequately to first-line treatment with NSAIDs and/or csDMARDs [9–11]. Randomized, placebo-controlled trials (RCTs) on currently available anti-tumor necrosis factor (TNF) α (infliximab, etanercept, adalimumab, certolizumab pegol, golimumab, biosimilars) agents have shown clinical improvement in the majority of PsA patients [12–18]. However, around 30% of PsA patients fail to respond to the first anti-TNFα and others experience adverse events, hence the question of whether anti-TNFα switching may be clinically beneficial [12–18]. Although anti-TNFα biologics are central to bDMARD treatment recommendations for PsA, new therapeutic alternatives have been approved, namely interleukin (IL)12/23 inhibitors (e.g., ustekinumab) and IL17 inhibitors (e.g., secukinumab, ixekizumab) as well as targeted synthetic disease-modifying anti-rheumatic drugs such as phosphodiesterase 4 inhibitor (apremilast) and Janus-activating kinase (Jak) inhibitor (tofacitinib) [19–23]. Notably, abatacept (cytotoxic T lymphocyte–associated antigen-4 immunoglobulin (CTLA-4-Ig)) has also been effective in patients with PsA [24]. bDMARDs have shown good tolerability and efficacy owing to a very high selectivity of therapeutic targets, a major step forward in PsA treatment [25].
However, according to the nationwide registries of drug continuation rate such as BIOBADASER (Spanish Registry of Adverse Events of Biological Therapies in Rheumatic Diseases), BSRBR (the British Society for Rheumatology Biologics Register), DANBIO (Danish Database for Biological Therapies in Rheumatology), and NOR-DMARD (Norwegian DMARD Registry), the treatment rate is considerable, with drug survival in the 63–82% range [26–30].
Our monocentric study evaluated PsA patients followed at the Rheumatology Unit of Padova University Hospital and undergoing long-term treatment with bDMARDs targeting TNFα (infliximab, etanercept, adalimumab, certolizumab pegol, golimumab), IL12/23 (ustekinumab), and IL17 (secukinumab), which have been approved for PsA treatment, in this chronological order. The aims of our study were to evaluate the (i) predictors of first bDMARD failure, including their mechanism of action; (ii) factors associated with failure of multiple therapies, including the frequency and the reasons for switching/swapping; and (iii) retention rate for anti-TNFα ustekinumab and secukinumab.
Materials and methods
Study population
This retrospective cohort study was conducted in a single tertiary center, Spondyloarthritis (SpA) Clinic of the Rheumatology Unit, Department of Medicine DIMED of Padova University Hospital (Italy). Consecutive patients classified as PsA according to CASPAR criteria [31] and initiating treatment with bDMARDs for a moderate or severe disease according to the EULAR/GRAPPA/SIR guidelines [9–11], during the period 2004–2020, were eligible. The bDMARDs investigated were infliximab, etanercept, adalimumab, certolizumab pegol, golimumab, ustekinumab, and secukinumab. Approval for the study was obtained from our institution’s ethics committee (n. 52,723), and all participants provided informed consent according to the principles of the Declaration of Helsinki. The dose and administration intervals of each bDMARD were predetermined in accordance with the manufacturer’s instructions. The dose or frequency of bDMARDs was not escalated arbitrarily. Infliximab was infused at 3 mg/kg at weeks 0, 2, and 6 and every 8 weeks thereafter. Depending on efficacy, patients would then receive gradual increments of 100 mg up to a maximum of 400 mg administered at 4- to 8-week intervals. The average dosage after 6 months was about 4.5 mg/kg every 8 weeks. Etanercept was administered twice weekly with an initial 25-mg subcutaneous dosage, often followed by 50 mg once weekly. Adalimumab was administered as a 40-mg subcutaneous dose every other week. Golimumab was administered by subcutaneous injection, 50 mg once every fourth week. Certolizumab was administered 400 mg subcutaneously, initially at weeks 2 and 4, followed by 200 mg every 2 weeks. Ustekinumab was administered as a 45-mg or 90-mg dose—according to body mass index (BMI)—at baseline, at 4 weeks, and every 12 weeks. Secukinumab was administered subcutaneously at a dosage of 150 mg or 300 mg as needed—according to the decision of the treating rheumatologist and the national registration indications of the drug—for severe psoriasis or multi-drug failure at weeks 0, 1, 2, 3, and 4 and every 4 weeks thereafter.
Clinical and laboratory evaluation
Demographic, clinical, and laboratory data were collected at the time of bDMARD initiation and yearly, including the number of swollen and painful joints (swollen joint count (SJC), tender joint count (TJC)) out of 66/68 number of joints according to the American College of Rheumatology (ACR), Leeds Enthesitis Index (LEI), Psoriasis Area and Severity Index (PASI), visual analogue scale (VAS) pain and global health, C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), Health Assessment Questionnaire (HAQ), and Disease Activity in PSoriatic Arthritis (DAPSA) [32]. Medical records were reviewed for information on patient age, disease duration, family history, smoking status, BMI, concomitant medications, and comorbidities (measured as Charlson Comorbidity Index [33]). Baseline characteristics were compared between switchers (≥ 1 switch/swap) and non-switchers. Switchers were patients who switched from the first prescribed bDMARD to one of the aforementioned bDMARDs. Non-switchers were patients who continued the first bDMARD. The physician decided the eligibility of an individual to switch biological medication. The duration of biological therapy, date of, and reasons (inefficacy, adverse events, infections) for switching/swapping were recorded.
Statistical analysis
Data processing and statistical analyses were performed using SPSS, version 21.0 (SPSS, Chicago, IL, USA). Continuous variables are presented as medians and interquartile range and were compared using the Mann–Whitney U test. Categorical variables were analyzed using a chi-square test or Fisher’s exact test. Kaplan–Meier curves were used to assess differences in time-to-first bDMARD discontinuation according to the targeted cytokine. Survival of the second-line biological therapy was compared between swap and switch by log-rank test. Moreover, a Cox regression model with survival of the second-line biological therapy as outcome was built, in order to adjust for the effect of switch/swap as reason of the therapeutic change (loss of effectiveness/primary ineffectiveness/other reasons). A multivariable Cox proportional-hazard (PH) model was built to evaluate the influence of mechanism of action and negative prognostic factors for drug response on time-to-first bDMARD discontinuation. The following covariates were examined in this first model: drug mechanism of action (anti-TNFα/anti-IL12/23/anti-IL17), age, female sex, baseline comorbidities (measured by Charlson Comorbidity Index), BMI, baseline PASI, baseline HAQ, baseline DAPSA, polyarticular arthritis, and bDMARD initiation before 2015 (time frame reflects the unavailability of biological drugs with different mechanisms of action). Furthermore, a multivariable logistic regression model was built to assess the association between negative prognostic factors for drug response (independent variables) and failure of multiple bDMARDs (“multi-failure,” outcome). In this second model, the following covariates were examined: mechanism of action (anti-TNFα or anti-IL12/23 or anti-IL17), age, sex, and bDMARD initiation before 2015. p values ≤ 0.05 were considered significant.
Results
Our study included 264 patients, 117 (44.32%) females, mean age of 56 (46–65) years, and mean PsA duration of 15 (10–22) years. One hundred forty-seven (55.68%) patients were non-switchers, and 117 (44.32%) were switchers. Switchers were mostly females (p < 0.001), with a lower PASI (p = 0.047), a higher prevalence of polyarticular arthritis (p = 0.048), and worse HAQ (p = 0.046) at baseline vs. non-switchers (Table 1). Mean time-to-first bDMARD discontinuation was 72 ± 58 months. Among 117 switchers, 54 (46.15%) patients underwent only one bDMARD switch, while 63 (53.84%) patients underwent ≥ 2 switches.
Table 1.
Baseline characteristics of the monocentric cohort at the start of bDMARD therapy, comparison between switchers and non-switchers
| Characteristics | Non-switchers (N = 147) | Switchers (N = 117) | p value |
|---|---|---|---|
| Female sex | 48 (32.65%) | 69 (58.97%) | < 0.0001 |
| Age (years) | 56.0 (46.0–65.0) | 57.0 (49.0–65.0) | 0.06 |
| Psoriatic arthritis duration (years) | 15.0 (10.0–22.0) | 15 (10.0–21.0) | 0.94 |
| Polyarticular arthritis | 35 (23.81%) | 42 (35.89%) | 0.048 |
| Mono/oligoarticular arthritis | 61 (41.50%) | 54 (46.15%) | 0.82 |
| Axial involvement | 42 (28.57%) | 38 (32.48%) | 0.95 |
| Psoriasis duration (years) | 25.0 (17.0–36.0) | 24.0 (15.0–34.0) | 0.67 |
| Family history | 9 (6.12%) | 14 (11.96%) | 0.07 |
| Psoriasis | 127 (86.39%) | 102 (87.18%) | 0.73 |
| Inflammatory bowel disease | 1 (0.68%) | 3 (2.56%) | 0.22 |
| Uveitis | 4 (2.72%) | 0 (0%) | 0.11 |
| Tender joints (66/68 joint count) | 5.0 (2.0–8.0) | 4.0 (2.0–8.0) | 0.44 |
| Swollen joints (66/68 joint count) | 2.0 (0.0–4.0) | 2.5 (0.0–6.0) | 0.60 |
| VAS pain 0–10 | 7.0 (5.3–8.0) | 7.0 (5.0–7.6) | 0.90 |
| VAS global health 0–10 | 6.0 (4.5–7.0) | 6.5 (5.0–8.0) | 0.35 |
| CRP (mg/L) | 6.0 (3.0–15.5) | 5.5 (2.9–12.0) | 0.37 |
| ESR (mm/h) | 17.0 (8.0–34.0) | 19.0 (9.0–36.0) | 0.42 |
| DAPSA | 20.2 (15.1–27.9) | 18.9 (15.3–25.7) | 0.67 |
| Leeds Enthesitis Index (0–6) | 0.0 (0.0–1.0) | 0.0 (0.0–1.0) | 0.69 |
| Dactylitis (presence/absence) | 18 (12.24%) | 15 (12.85%) | 0.23 |
| HAQ | 0.5 (0.25–1.0) | 0.62 (0.25–1.5) | 0.046 |
| PASI 0–72 | 1.0 (0.3–3.0) | 1 (0.0–2.7) | 0.047 |
| Smoking | |||
| Non-smokers | 91 (61.90%) | 83 (70.94%) | 0.46 |
| Ever smokers | 56 (38.10%) | 34 (29.06%) | |
| BMI | 25.3 (22.9–27.7) | 25.4 (23.4–27.6) | 0.86 |
| Updated Charlson Comorbidity Index | 1 (0–3) | 1 (0–7) | 0.79 |
| Association therapy with a csDMARDs | 56 (38.10%) | 39 (33.33%) | 0.61 |
| First-line biological drug | |||
| Anti-TNFα | 120 (81.63%) | 106 (90.59%) | 0.75 |
| Ustekinumab | 15 (10.20%) | 8 (6.84%) | 0.88 |
| Secukinumab | 12 (8.16%) | 3 (2.56%) | 0.68 |
Significant results are highlighted in bold. Categorical variables are shown as number (%). Continuous variables are shown as medians and interquartile range. p ≤ 0.05 (between non-switchers vs. switchers)
VAS visual analogue scale, CRP C-reactive protein, ESR erythrocyte sedimentation rate, DAPSA Disease Activity in PSoriatic Arthritis, HAQ Health Assessment Questionnaire, PASI Psoriasis Area and Severity Index, BMI body mass index, csDMARDs conventional synthetic disease-modifying anti-rheumatic drugs, TNFα tumor necrosis factor α
The anti-TNFα was the bDMARD used in the majority of patients (n = 226, 85.61%), followed by ustekinumab (n = 23, 8.71%) and secukinumab (n = 15, 5.68%). In the period of observation, there were no patients undergoing ixekizumab, guselkumab, apremilast, abatacept, and tofacitinib.
Of the 117 switchers, 45 (38.46%) were initially on etanercept, 38 (32.48%) on adalimumab, 19 (16.24%) on infliximab, 8 (6.84%) on ustekinumab, 2 (1.71%) on golimumab, 2 (1.71%) on certolizumab, and 3 (2.56%) on secukinumab. Of the 147 non-switchers, 60 (40.82%) were initially on adalimumab, 48 (32.65%) on etanercept, 15 (10.2%) on ustekinumab, 12 (8.16%) on secukinumab, 8 (5.44%) on infliximab, 3 (2.04%) on golimumab, and 1 (0.68%) on certolizumab.
Overall, the survival rate of first bDMARD was good both in patients treated with anti-TNFα and in those undergoing anti-IL12/23 or anti-IL17 (75% at 2 years and 60% at 5 years). The group of patients undergoing anti-TNFα achieved a survival rate of about 50% after 10 years of treatment. We compared the survival rate for the main 3 anti-TNFα drugs administered (adalimumab, etanercept, and infliximab): the former 2 showed a higher probability of survival rate over 50% even after 10 years of treatment, whereas the survival rate of the former as first biologic was slightly below 50% after 10 years.
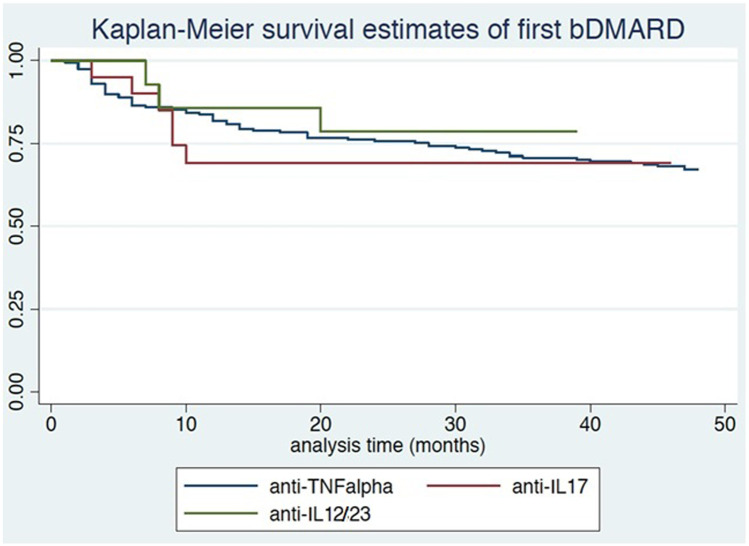
Survival curves for anti-TNFα (ustekinumab and secukinumab) did not diverge significantly (log-rank test = 0.83; p = 0.66; Fig. 1). There was no difference, in terms of survival for the second biologic, between patients who swapped and those who switched (log-rank test = 2.10, p = 0.147). The same was observed with Cox regression even after adjusting for the reason behind the therapeutic change (data not shown). We found no differences among all anti-TNFα biologics (infliximab, etanercept, adalimumab, certolizumab pegol, and golimumab) in terms of first or multiple bDMARD discontinuation (data not shown).
Fig. 1.
Kaplan–Meier survival curves of first bDMARD according to its mechanism of action
The principal reasons for bDMARD discontinuation were drug inefficacy (79 patients, 67.52%) and adverse events (38 patients, 25.7%). In more detail, these included lack of efficacy on arthritis (25 patients, 21.37%), lack of efficacy on cutaneous psoriasis (6 patients, 5.13%), loss of efficacy on arthritis (38 patients, 32.48%), loss of efficacy on cutaneous psoriasis (10 patients, 8.55%), recurrent or serious infections (10 patients; 8.55%), infusion reactions (14 patients, 11.97%), new onset of neoplasia (7 patients, 5.98%), severe comorbidities (2 patients, 1.71%), and biohumoral blood alterations (5 patients, 4.27%). No patient discontinued therapy due to non-compliance or pregnancy. In the comparison between switchers due to drug inefficacy and switchers due to adverse events, we observed a higher prevalence of psoriasis (p = 0.041) and dactylitis (p = 0.043) and a higher PASI (p = 0.045) in the former and a higher prevalence of comorbidities (p = 0.046) in the latter (Table 2). A detailed description of the principal reasons for discontinuation of each anti-TNFα biologic, anti-IL12/23, and anti-IL17 was reported in Supplementary Table 1.
Table 2.
Baseline characteristics of switchers at the start of bDMARD therapy, according to the reason for subsequent switching
| Characteristics | Inefficacy (N = 79) | Adverse event (N = 38) | p value |
|---|---|---|---|
| Female sex | 43 (54.43%) | 26 (68.42%) | 0.65 |
| Age (years) | 58.0 (49.0–64.0) | 56.0 (48.0–66.0) | 0.07 |
| Psoriatic arthritis duration (years) | 15.0 (10.0–22.0) | 16 (10.0–20.0) | 0.91 |
| Polyarticular arthritis | 23 (29.11%) | 9 (23.68%) | 0.68 |
| Mono/oligoarticular arthritis | 36 (45.57%) | 18 (47.37%) | 0.86 |
| Axial involvement | 34 (43.08%) | 14 (36.84%) | 0.91 |
| Psoriasis duration (years) | 21.0 (14.0–30.0) | 24.0 (11.0–33.0) | 0.82 |
| Family history | 7 (8.86%) | 7 (18.42%) | 0.06 |
| Psoriasis | 75 (94.93%) | 27 (71.05%) | 0.041 |
| Inflammatory bowel disease | 1 (1.27%) | 2 (5.26%) | 0.16 |
| Uveitis | 0 (0%) | 0 (0%) | 0.10 |
| Tender joints (66/68 joint count) | 4.0 (2.0–7.0) | 5.0 (2.0–9.0) | 0.34 |
| Swollen joints (66/68 joint count) | 1.0 (0.0–3.0) | 2.0 (0.0–5.0) | 0.20 |
| VAS pain 0–10 | 7.0 (5.0–8.0) | 7.0 (5.0–7.5) | 0.88 |
| VAS global health 0–10 | 6.5 (4.5–8.0) | 6.5 (5.1–7.0) | 0.45 |
| CRP (mg/L) | 5.0 (3.0–11.5) | 7.0 (3.0–12) | 0.07 |
| ESR (mm/h) | 20.0 (10.0–30.0) | 16.0 (10.0–29.0) | 0.13 |
| DAPSA | 19.0 (14.0–27.0) | 22.0 (18.0–26.0) | 0.53 |
| Leeds Enthesitis Index (0–6) | 0.0 (0.0–1.0) | 0.0 (0.0–0.0) | 0.75 |
| Dactylitis (presence/absence) | 13 (16.46%) | 2 (5.26%) | 0.043 |
| HAQ | 1.0 (0.0–1.0) | 1 (0.0–2.0) | 0.061 |
| PASI 0–72 | 1.0 (0.0–3.0) | 0 (0.0–1.0) | 0.045 |
| Smoking | |||
| Non-smokers | 47 (59.49%) | 32 (84.21%) | 0.055 |
| Ever smokers | 32 (40.51%) | 6 (15.79%) | |
| BMI | 26 (24–284) | 25.0 (22.0–27.0) | 0.76 |
| Updated Charlson Comorbidity Index | 1 (1–1) | 1 (1–3) | 0.046 |
| Association therapy with a csDMARDs | 26 (32.91%) | 13 (34.21%) | 0.62 |
| First-line biological drug | |||
| Anti-TNFα | 71 (89.87%) | 35 (92.11%) | 0.61 |
| Ustekinumab | 7 (88.60%) | 1 (2.63%) | 0.55 |
| Secukinumab | 1 (1.27%) | 2 (5.26%) | 0.92 |
Significant results are highlighted in bold. Categorical variables are shown as number (%). Continuous variables are shown as medians and interquartile range. p ≤ 0.05 (between inefficacy vs. adverse event)
VAS visual analogue scale, CRP C-reactive protein, ESR erythrocyte sedimentation rate, DAPSA Disease Activity in PSoriatic Arthritis, HAQ Health Assessment Questionnaire, PASI Psoriasis Area and Severity Index, BMI body mass index, csDMARDs conventional synthetic disease-modifying anti-rheumatic drugs, TNFα tumor necrosis factor α
The whole population achieved a significant decrease in tender/swollen joint count, VAS pain, VAS global health, PASI, LEI, HAQ, ESR, CRP, and DAPSA during a 15-year follow-up, with a trend of greater reduction of disease activity and improvement of functional index values in non-switchers vs. switchers (Table 3).
Table 3.
Clinical, disease activity, and serological parameters of non-switchers (n = 147) and switchers (n = 117) among PsA patients during 15 years of follow-up (FU)
| Years to follow-up | p | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | ||
| No. of patients | |||||||||||||||||
| 264 | 253 | 221 | 210 | 180 | 156 | 129 | 103 | 98 | 79 | 67 | 55 | 46 | 28 | 13 | 10 | ||
| SJ | |||||||||||||||||
| Non-switchers | 2 (0–4) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | < 0.01 |
| Switchers | 2.5 (0–6) | 0 (0–2) | 0 (0–1) | 0 (0–1) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–2) | 0 (0–2) | 0 (0–2) | 0 (0–2.5) | 0 (0–0) | < 0.01 |
| TJ | |||||||||||||||||
| Non-switchers | 5 (2–8) | 1 (0–3) | 0 (0–2) | 0 (0–2) | 0 (0–2) | 0 (0–1) | 0 (0–1) | 0 (0–1) | 0 (0–1) | 0 (0–2) | 0 (0–1) | 0 (0–1) | 0 (0–1) | 0 (0–1) | 0 (0–1) | 0 (0–0) | < 0.001 |
| Switchers | 4 (2–8) | 2 (1–6) | 2 (0–5) | 1 (0–4) | 1 (0–4) | 1 (0–3) | 1 (0–2) | 0 (0–2) | 1 (0–2.3) | 0 (0–2.25) | 2 (0–3.5) | 2.5 (0–5.3) | 2 (0–8) | 1 (0–3) | 2 (1.8–6.3) | 0 (0–2) | < 0.001 |
| VAS pain | |||||||||||||||||
| Non-switchers | 7 (5.3–8) | 3 (2–5) | 3 (1.5–4) | 3 (1.8–4.5) | 3 (1.5–4.5) | 3 (1.5–4.5) | 2 (1.0–3.7) | 2.8 (1.0–4.0) | 2.4 (1.0–4.0) | 2.0 (1.0–3.0) | 2.0 (1.0–3.0) | 3.0 (2.0–3.2) | 2.5 (1.5–3.0) | 2.0 (2.0–3.0) | 3.0 (2.0–4.0) | 2 (1.8–4.0) | < 0.001 |
| Switchers | 7.0 (5.0–7.5) | 5.0 (3.0–7.0) | 5.0 (3.0–7.0) | 4.3 (2.5–7.0) | 4.0 (2.7–6.3) | 3.5 (2.0–6.0) | 3.5 (2.5–5.0) | 3.0 (2.5–5.0) | 4.0 (2.9–5.7) | 3.5 (2.0–5.6) | 4.0 (3.0–6.6) | 4.0 (2.0–6.1) | 5.0 (3.0–7.0) | 4.0 (1.0–6.0) | 5.5 (3.8–7.3) | 3.0 (2.0–5.0) | < 0.001 |
| VAS global health | |||||||||||||||||
| Non-switchers | 6.0 (4.5–7.0) | 29.5 (1.5–4.5) | 25 (15–38.5) | 2.0 (1.0–4.0) | 2.5 (1.2–4.0) | 2.5 (1.5–4.0) | 2.0 (1.0–4.0) | 2.1 (1.0–3.9) | 2.0 (1.0–3.0) | 2.0 (1.0–3.0) | 2.2 (1.0–3.0) | 2.1 (1.5–3.0) | 2.0 (1.0–3.0) | 2.0 (2.0–3.0) | 3.0 (2.0–3.9) | 2.5 (2.0–3.3) | < 0.001 |
| Switchers | 6.5 (5.0–8.0) | 4.5 (28.8–6.5) | 4.0 (2.5–6.5) | 4.0 (2.5–6.0) | 3.5 (2.0–5.6) | 3.0 (2.0–5.5) | 3.0 (1.9–5.6) | 3.0 (1.5–4.5) | 3.0 (2.1–5.3) | 3.25 (2.0–4.85) | 4.0 (2.7–6.3) | 3.5 (2.5–5.0) | 4.0 (2.0–5.5) | 3.0 (2.2–5.0) | 4.0 (2.8–6.0) | 2.5 (1.5–3.0) | < 0.001 |
| LEI | |||||||||||||||||
| Non-switchers | 0 (0–1) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | < 0.05 |
| Switchers | 0 (0–1) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | < 0.05 |
| PASI | |||||||||||||||||
| Non-switchers | 1.0 (0.3–3.0) | 0 (0.0–1.0) | 0 (0.0–1.0) | 0 (0.0–1.0) | 0.2 (0.0–1.0) | 0 (0.0–1.0) | 0 (0.0–1.0) | 0 (0.0–1.0) | 0.3 (0.0–1.0) | 0.20 (0.0–1.0) | 0 (0.0–0.8) | 0 (0.0–0.8) | 0 (0.0–0.8) | 0.5 (0.0–0.8) | 0.2 (0.0–1.0) | 0 (0.0–0.3) | < 0.001 |
| Switchers | 1.0 (0.0–2.7) | 0 (0.0–1.2) | 0 (0–1) | 0 (0–1) | 0 (0–1.4) | 0 (0–1) | 0 (0–1) | 0 (0–1) | 0 (0–1) | 0.1 (0.0–1.5) | 0.1 (0.0–2.0) | 0 (0.0–0.9) | 0 (0–1) | 0 (0–0.5) | 0 (0–0.5) | 0 (0–0.4) | < 0.001 |
| ESR | |||||||||||||||||
| Non-switchers | 17 (8–34) | 8 (5–15.8) | 9 (5–19) | 7 (5–16.3) | 10 (7–15) | 10 (6–16.5) | 10 (7–15) | 10 (8–14) | 10 (8–15) | 10 (8–15) | 10 (10–15) | 10 (7–12) | 10 (5–18.8) | 7 (4–15.5) | 7 (4–9.8) | 6.5 (4–10.8) | < 0.001 |
| Switchers | 19 (9–36) | 11 (9–20) | 12 (7–20) | 10 (8–20) | 10 (7–21.5) | 10 (8–23) | 10 (7–16) | 10 (7–19.8) | 10 (7–23.3) | 10 (8–17.75) | 10 (7.5–31.5) | 10 (8–20.8) | 10 (7.5–20) | 10 (5.5–21) | 10 (4–21) | 10 (7.8–23.8) | < 0.05 |
| PCR | |||||||||||||||||
| Non-switchers | 6 (2.9–15.5) | 3.05 (2–5.1) | 3 (1.9–5.3) | 2.9 (1.5–5.0) | 2.9 (2.0–5.0) | 2.9 (1.1–5.0) | 3.7 (2.0–5.0) | 3.0 (2.0–5.0) | 3.5 (2.3–5.0) | 3.0 (2.15.5.0) | 2.9 (2.0–5.0) | 2.9 (2.0–5.0) | 2.9 (1.4–4.5) | 2.9 (0.9–2.9) | 2.9 (1.0–3.5) | 1.3 (0.9–5.6) | < 0.001 |
| Switchers | 5.5 (2.9–12.0) | 4.0 (2.9–6.0) | 4.0 (2.9–6.0) | 3.0 (2.9–6.0) | 3.0 (2.4–5.0) | 3.0 (2.2–5.0) | 2.9 (2.0–4.9) | 2.9 (2.1–5.0) | 3.0 (2.9–5.1) | 5.0 (2.9–6.0) | 3.4 (2.9–5.0) | 3.3 (2.2–7.9) | 2.9 (1.5–5.0) | 3.7 (2.2–7.0) | 3.0 (1.3–5.3) | 3.7 (3.3–7.9) | < 0.05 |
| HAQ | |||||||||||||||||
| Non-switchers | 0.5 (0.3–1.0) | 0.5 (0.3–0.8) | 0.3 (0.3–0.6) | 0.3 (0.3–0.6) | 0.3 (0.3–0.6) | 0.3 (0.3–0.6) | 0.3 (0.3–0.6) | 0.3 (0.3–0.3) | 0.3 (0.3–0.4) | 0.25 (0.25–0.25) | 0.3 (0.3–0.3) | 0.3 (0.3–0.3) | 0.3 (0.3–0.5) | 0.3 (0.3–0.3) | 0.3 (0.3–0.3) | 0.3 (0.3–0.3) | < 0.01 |
| Switchers | 0.62 (0.3–1.5) | 0.6 (0.3–1.4) | 0.7 (0.3–1.0) | 0.6 (0.3–0.8) | 0.6 (0.3–0.8) | 0.5 (0.3–0.6) | 0.3 (0.3–0.6) | 0.5 (0.3–0.6) | 0.4 (0.3–0.6) | 0.63 (0.25–0.63) | 0.5 (0.3–0.6) | 0.5 (0.3–0.6) | 0.5 (0.3–0.6) | 0.3 (0.3–0.6) | 0.3 (0.3–0.6) | 0.3 (0.3–0.4) | < 0.01 |
| DAPSA | |||||||||||||||||
| Non-switchers | 20.2 (15.1–27.9) | 8 (5.2–12.3) | 6.8 (3.8–10.6) | 7.0 (4.3–10.7) | 6.9 (3.9–11.2) | 6.3 (4.0–9.7) | 6.1 (3.2–8.4) | 6.1 (2.9–8.8) | 5.5 (2.8–9.7) | 5.7 (3.34–8.22) | 5.5 (2.0–8.0) | 6.1 (4.2–8.0) | 5.7 (3.5–7.6) | 5.1 (4.1–7.3) | 5.9 (4.3–10.6) | 5.6 (4.1–8.9) | < 0.001 |
| Switchers | 18.9 (15.3–25.7) | 13.7 (8.3–20.2) | 12.4 (7.4–19.8) | 11.1 (6.1–19.4) | 10.1 (6.1–16.2) | 8.6 (5.4–14.9) | 8.2 (5.9–13.4) | 7.8 (5.4–12.9) | 9.5 (6.9–13.8) | 8.12 (4.45–14.18) | 10.2 (7.3–16.4) | 12.5 (5.9–19.7) | 12.2 (6.1–21.5) | 10.4 (4.1–16.6) | 12.9 (9.8–25.6) | 7.1 (4.1–10.0) | < 0.001 |
Significant results are highlighted in bold. Continuous variables are shown as medians and interquartile range. Values were computed by means of a chi-square test (for proportion) or the Mann–Whitney U test (for continuous data); p ≤ 0.05 T15 vs. T0
VAS visual analogue scale, ESR erythrocyte sedimentation rate, DAPSA Disease Activity in PSoriatic Arthritis, HAQ Health Assessment Questionnaire, PASI Psoriasis Area and Severity Index, LEI Leeds Enthesitis Index
The Cox PH model showed that female sex was independently associated with a higher risk of first bDMARD discontinuation (HR = 2.39; 95% CI: 1.50–3.81), while initiating therapy before 2015 was protective (HR = 0.40; 95% CI: 0.22–0.73). Other independent variables, including mechanism of action (HR = 0.76; 95% CI: 0.30–1.74 for secukinumab; HR = 0.53; 95% CI 0.15–1.86 for ustekinumab; reference: anti-TNFα), age (HR = 1.00; 95% CI: 0.99–1.03), baseline DAPSA (HR = 0.98; 95% CI: 0.96–1.00), PASI (HR = 0.95; 95% CI: 0.86–1.04), HAQ (HR = 1.29; 95% CI: 0.91–1.83), BMI (HR = 1.02; 95% CI: 0.98–1.07), polyarticular arthritis (HR = 1.23; 95% CI: 0.94–1.52), and comorbidities (HR = 1.10; 95% CI: 0.92–1.31), were not associated with the outcome “multi-failure” (Table 4). In the logistic regression model, only female sex was significantly associated with failure of multiple bDMARDs (OR = 1.99, 95% CI: 1.07–3.69) whereas bDMARD mechanism of action, age, and treatment initiation before 2015 were not independently associated with the outcome (Table 5).
Table 4.
Cox regression model with first bDMARD discontinuation as outcome
| Independent variables | First bDMARD discontinuation | |
|---|---|---|
| Multivariable analysis | ||
| OR (95% CI) | p | |
| Anti-IL17 as first drug | 0.76 (0.29, 1.94) | 0.567 |
| Anti-IL12/23 as first drug | 0.53 (0.15, 1.86) | 0.325 |
| Female sex | 2.38 (1.49, 3.81) | < 0.001 |
| Age | 1.01 (0.99, 1.03) | 0.322 |
| BMI | 1.02 (0.98, 1.07) | 0.329 |
| PASI baseline | 0.95 (0.86, 1.04) | 0.263 |
| DAPSA baseline | 0.98 (0.96, 1.01) | 0.153 |
| HAQ baseline | 1.29 (0.91, 1.83) | 0.155 |
| Charlson Comorbidity Index | 1.09 (0.92, 1.31) | 0.299 |
| Polyarticular arthritis | 1.23 (0.94, 1.52) | 0.151 |
| bDMARD initiation < 2015 | 0.41 (0.22, 0.73) | 0.003 |
Significant results are highlighted in bold. p ≤ 0.05
bDMARD biological disease-modifying anti-rheumatic drug, IL interleukin, BMI body mass index, PASI Psoriasis Area and Severity Index, HAQ Health Assessment Questionnaire, DAPSA Disease Activity Index for Psoriatic Arthritis, CI confidence interval
Table 5.
Multivariable logistic regression model with failure of multiple (≥ 2) bDMARD therapies as outcome
| Independent variables | Failure of multiple (≥ 2) bDMARD therapies | |
|---|---|---|
| Multivariable analysis | ||
| OR (95% CI) | p | |
| Anti-IL17 as first drug | − 1.91 (− 4.06, 0.24) | 0.082 |
| Anti-IL12/23 as first drug | − 0.64 (− 2.31, 1.02) | 0.447 |
| Female sex | 0.69 (0.07, 1.31) | 0.030 |
| Age | 0.02 (0.01, 0.04) | 0.229 |
| bDMARD initiation < 2015 | 0.09 (0.72, 0.91) | 0.060 |
Significant result is highlighted in bold. p ≤ 0.05
bDMARD biological disease-modifying anti-rheumatic drug, IL interleukin, CI confidence interval
Discussion
Our study evaluated 264 PsA patients who had been undergoing biologics for a maximum during 2004–2020. Clinical and demographic characteristics of our study population were in line with the main nationwide registries BIOBADASER, BSRBR, DANBIO, and NOR-DMARD [26–30]. One hundred and seventeen (44.32%) PsA patients treated with a first bDMARD (anti-TNFα, ustekinumab or secukinumab) switched to another. Loss of efficacy, lack of efficacy, and adverse events were the main reasons for switching to another anti-TNFα or swapping for another biologic altogether [34–37]. Notably, the main reason for switching in our study was drug inefficacy (67.52%). The different mechanisms of action (anti-TNFα, anti-IL12/23, anti-IL17) were not associated with a higher probability of switching. Observational studies have reported a sustained clinical response at 5 years, with satisfactory infliximab and adalimumab survival rates and higher etanercept survival [12]. Survival rate of patients receiving anti-TNFα treatment appears to be greater in PsA vs. rheumatoid arthritis (RA) patients [26, 38, 39]. In our patients, the survival rate of the first bDMARD was 60% at 5 years and 75% at 2 years both in patients treated with anti-TNFα and in those undergoing anti-IL12/23 or anti-IL17. A survival rate > 50% at 10 years was observed among patients undergoing anti-TNFα. The persistence of treatment with first bDMARD was in line with the current literature [12, 38, 39] whereas the percentage of switchers (44.32%) was higher vs. nationwide registries, in which 20–35% of SpA patients had switched first TNFα inhibitors, though in those latter studies, the duration of follow-up was shorter (mean 1–5 years vs. 2–15 years). Overall, drug survival rate of the second anti-TNFα appears to be lower vs. the first anti-TNFα [40]. In the BIOBADASER registry, among 4706 patients with chronic arthritis—including RA, PsA, and ankylosing spondylitis (AS)—10% had been treated with more than one anti-TNFα over a 4-year period [41]; 88% of PsA patients continued with their first anti-TNFα drug for 12 months vs. 83% of RA patients [26]. Recently, a Norwegian study found that 77.3% of PsA vs. 65.4% of RA patients continued with their first anti-TNFα for 12 months [42]. A BSRBR observational study found that 31% of 566 PsA patients switched treatment and were followed up for a mean duration of 2.3 years [43]. Similarly, a French single-center study found that 64% of rheumatic patients continued their first anti-TNFα treatment for 12 months [38]. Conversely, the DANBIO registry reported that 39% of 1422 patients, initiated anti-TNFα, then switched to a second biologic over a 10-year follow-up period, as corroborated by our findings [29]. However, nationwide registries provide scarce data and few observational studies have investigated anti-TNFα switching in patients with PsA (e.g., 5-year estimated drug survival for first-time switchers was 51% in a Southern Sweden cohort study) [44]. A real-life French study reported switching rates of 26–32% in patients with SpA [45]. Overall, drug survival rate of the second anti-TNFα appears to be lower vs. that of the first anti-TNFα [40, 46]. As previously reported in the literature, we also found a decrease of disease activity parameters in our patients in treatment with bDMARDs with a stronger trend in non-switchers [26–30]. We observed no differences as regards survival rate to the second biological drug, between patients who swapped/switched biological therapy, a finding in line with EULAR recommendations for therapeutic switching and evidence on efficacy [9]. Moreover, we were able to confirm previous reports of a low incidence of serious and/or recurrent infections—one of the most feared causes of drug withdrawal [47]. In fact, only 8.55% of our patients required a switch due to recurrent infections. Behrens et al. [48] underlined that available evidence on the efficacy and safety of anti-TNFα monotherapy vs. add-on methotrexate therapy showed little or no improvement with combination therapy, though the use of concomitant methotrexate appears to prolong anti-TNFα drug survival by reducing the development of TNFα inhibitor antibodies. Likewise, the percentage of patients treated with combined therapy in our study (bDMARDs + csDMARDs) was similar in non-switchers (38.10%) and in switchers (33.33%). Therefore, concurrent csDMARD use, and specifically methotrexate, did not yield better response vs. monotherapy.
Our study investigated potential predictors of switching in first- and second-line biologics.
To the best of our knowledge, few studies have formally explored possible predictors of drug discontinuation in patients with SpA and PsA treated with anti-TNFα [43]. Kristensen et al. [18] suggested that concomitant use of methotrexate and elevated C-reactive protein levels was associated with treatment continuation using anti-TNFα drugs. Gomez-Reino et al. [41] reported that older age was a predictor of drug discontinuation, while Heiberg et al. [42] found that higher baseline disease activity and female sex were associated with treatment continuation.
We were able to corroborate previous reports in the literature pertaining to a higher discontinuation rate of first- and second-line biologics among females [37, 49, 50]. In fact, Iannone et al. [51] reported that male PsA patients showed a 50% risk of discontinuation and were 60% more likely to achieve long-term stable minimal disease activity. Female PsA patients more frequently present a polyarticular pattern, often compounded by fibromyalgia, which amplifies the perception of pain and fatigue and therefore negatively impacts self-reported assessment of disease activity [52]. Furthermore, concomitant fibromyalgia may constitute a challenge to the therapeutic strategy in female PsA patients, as fibromyalgia-associated symptoms can alter the assessment of clinical response to treatments over time. We also observed that switchers more frequently had polyarticular arthritis, probably due to a more severe disease, without differences related to reason for switching (inefficacy or adverse event), although this subtype did not appear to be a significantly negative prognostic factor for time-to-first bDMARD discontinuation. These findings corroborate a previous report by literature [49]. Conversely, the mono-oligoarticular subtype and axial disease did not appear to influence treatment response and drug discontinuation. We also found a trend towards better drug survival in patients who initiated bDMARDs before 2015, probably reflecting the limited number of available therapies with specific mechanism of action. We hypothesized that another possible reason may be that before 2015, patients were usually switched to biological drugs following multiple csDMARD failure and after a longer disease course. Thus, even a partial response could be deemed successful and physicians would maintain the same treatment longer. The presence of comorbidities was higher among switchers though it was not defined as a predictor of higher withdrawal rates. Although current smoking was also found to be an independent predictor of discontinuation of biological therapies [40], we were not able to confirm this association despite about 34% of our patients being current or previous smokers. Interestingly, we observed a high prevalence of psoriasis and higher PASI at the time of bDMARD initiation among switchers and in cases where bDMARD discontinuation was due to a lack of efficacy.
The strengths of our study were the evaluation of predictors of switching of bDMARDs and the collection of data about efficacy and reasons for switching in a real-life setting. Some of our limitations were the retrospective nature of the study design; the choice of first bDMARD influenced by factors such as the treating physician’s preference and the timing of the availability of the novel mechanism of action; the severity bias of enrolled PsA patients, who attended a tertiary center and therefore could present a more difficult-to-treat and aggressive disease; and the small sample size that does not allow to evaluate fine differences between different anti-TNFα drugs and between anti-TNFα and other bDMARDs such as anti-IL17 and anti-IL12/23.
Conclusions
The drug survival in PsA patients was greater for the first biologic administered, which could arise from a better drug selection tailored to each patient’s prevalent clinical manifestations and comorbidities according to the EULAR/GRAPPA/SIR recommendations.
Overall, almost half of PsA patients treated with a first bDMARDs switched to another during the 15 years of follow-up. At 2 years and 5 years, the survival rate of the first bDMARD was from really optimal to good in over 50% of the PsA patients, without a significant difference in patients undergoing anti-TNFα, anti-IL12/23, and anti-IL17 biological agents. In case of failure of the first bDMARD, switching/swapping proved to be a good treatment option, as reflected by a persistently satisfactory effectiveness with second-line bDMARDs and so subsequent switches. Discontinuation or switching of first bDMARD due to tolerability issues or infections occurred rarely in PsA with respect to RA.
Furthermore, female sex may constitute a predisposing risk factor for flare and therapeutic switches. Discontinuation or switching of biologics due to mechanism of action, comorbidities tolerability, and BMI did not seem to impact first bDMARD withdrawal. Lack of efficacy does not appear to be a frequent occurrence in PsA vs. other rheumatic diseases such as RA.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contribution
All the authors made substantive intellectual contributions to the study, reviewed the article, and gave their final approval for the version being submitted.
Funding
Open access funding provided by Università degli Studi di Padova within the CRUI-CARE Agreement.
Declarations
Conflicts of interest
RR and AD has received honoraria and speaker fees from Novartis, AbbVie, Pfizer, MSD, and Janssen. The other authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Mariagrazia Lorenzin and Augusta Ortolan contributed equally to this work.
References
- 1.Gladman D, Antoni C, Mease P, Clegg D, Nash P. Psoriatic arthritis: epidemiology, clinical features, course, and outcome. Ann Rheum Dis. 2005;64(Suppl 2):ii14–7. doi: 10.1136/ard.2004.032482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ramonda R, Lo Nigro A, Modesti V, Nalotto L, Musacchio E, Iaccarino L, et al. Atherosclerosis in psoriatic arthritis. Autoimmun Rev. 2011;10(12):773–778. doi: 10.1016/j.autrev.2011.05.022. [DOI] [PubMed] [Google Scholar]
- 3.Ortolan A, Lorenzin M, Tadiotto G, Russo FP, Oliviero F, Felicetti M, et al. Metabolic syndrome, non-alcoholic fatty liver disease and liver stiffness in psoriatic arthritis and psoriasis patients. Clin Rheumatol. 2019;38(10):2843–2850. doi: 10.1007/s10067-019-04646-7. [DOI] [PubMed] [Google Scholar]
- 4.Ramonda R, Puato M, Punzi L, Rattazzi M, Zanon M, Balbi G, et al. Atherosclerosis progression in psoriatic arthritis patients despite the treatment with tumor necrosis factor-alpha blockers: a two-year prospective observational study. Joint Bone Spine. 2014;81(5):421–425. doi: 10.1016/j.jbspin.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 5.Ortolan A, Ramonda R, Lorenzin M, Pesavento R, Spinazzè A, Felicetti M et al (2020) Subclinical atherosclerosis evolution during 5 years of anti-TNF-alpha treatment in psoriatic arthritis patients. Clin Exp Rheumatol. Online ahead of print. [DOI] [PubMed]
- 6.Ortolan A, Lorenzin M, Felicetti M, Ramonda R (2020) Do obesity and overweight influence disease activity measures in axial spondyloarthritis? A systematic review and meta-analysis. Arthritis Care Res (Hoboken). 10.1002/acr.24416. Online ahead of print. [DOI] [PubMed]
- 7.Scotti L, Franchi M, Marchesoni A, Corrao G. Prevalence and incidence of psoriatic arthritis: a systematic review and meta-analysis. Semin Arthritis Rheum. 2018;48(1):28–34. doi: 10.1016/j.semarthrit.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 8.Mease PJ, Armstrong AW. Managing patients with psoriatic disease: the diagnosis and pharmacologic treatment of psoriatic arthritis in patients with psoriasis. Drugs. 2014;74:423–441. doi: 10.1007/s40265-014-0191-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gossec L, Baraliakos X, Kerschbaumer A, de Wit M, McInnes I, Dougados M, et al. EULAR recommendations for the management of psoriatic arthritis with pharmacological therapies: 2019 update. Ann Rheum Dis. 2020;79(6):700–712. doi: 10.1136/annrheumdis-2020-217159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coates LC, Kavanaugh A, Mease PJ, Soriano ER, Acosta L, Felquer M, et al. Group for research and assessment of psoriasis and psoriatic arthritis 2015 treatment recommendations for psoriatic arthritis. Arthritis Rheumatol. 2016;68(5):1060–1071. doi: 10.1002/art.39573. [DOI] [PubMed] [Google Scholar]
- 11.Marchesoni A, Olivieri I, Salvarani C, Pipitone N, D’Angelo S, Mathieu A, et al. Recommendations for the use of biologics and other novel drugs in the treatment of psoriatic arthritis: 2017 update from the Italian Society of Rheumatology. Clin Exp Rheumatol. 2017;35(6):991–1010. [PubMed] [Google Scholar]
- 12.Saougou I, Markatseli TE, Papagoras C, Voulgari PV, Alamanos Y, Drosos AA. Sustained clinical response in psoriatic arthritis patients treated with anti-TNF agents: a 5-year open-label observational cohort study. Semin Arthritis Rheum. 2011;40:398–406. doi: 10.1016/j.semarthrit.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 13.Mease PJ, Kivitz AJ, Burch FX, Siegel EL, Cohen SB, Ory P, et al. Etanercept treatment of psoriatic arthritis: safety, efficacy, and effect on disease progression. Arthritis Rheum. 2004;50:2264–2272. doi: 10.1002/art.20335. [DOI] [PubMed] [Google Scholar]
- 14.Antoni C, Kavanaugh A, Kirkham B, Tutuncu Z, Burmester GR, Schneider U, et al. Sustained benefits of infliximab therapy for dermatologic and articular manifestations of psoriatic arthritis: results from the Infliximab Multinational Psoriatic Arthritis Controlled Trial (IMPACT) Arthritis Rheum. 2005;52:1227–1236. doi: 10.1002/art.20967. [DOI] [PubMed] [Google Scholar]
- 15.Mease PJ, Gladman DD, Ritchlin CT, Ruderman EM, Steinfeld SD, Choy EH, Sharp JT, Ory PA, Perdok RJ, Weinberg MA, Adalimumab Effectiveness in Psoriatic Arthritis Trial Study Group Adalimumab for the treatment of patients with moderately to severely active psoriatic arthritis: results of a double-blind, randomized, placebo-controlled trial. Arthritis Rheum. 2005;52:3279–3289. doi: 10.1002/art.21306. [DOI] [PubMed] [Google Scholar]
- 16.Mease PJ, Fleischmann R, Deodhar AA, Wollenhaupt J, Khraishi M, Kielar D, et al. Effect of certolizumab pegol on signs and symptoms in patients with psoriatic arthritis: 24-week results of a phase 3 double-blind randomised placebo-controlled study (RAPID-PsA) Ann Rheum Dis. 2014;73:48–55. doi: 10.1136/annrheumdis-2013-203696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kavanaugh A, McInnes I, Mease P, Krueger GG, Gladman D, Gomez-Reino J, et al. Golimumab, a new human tumor necrosis factor alpha antibody, administered every four weeks as a subcutaneous injection in psoriatic arthritis: twenty-four-week efficacy and safety results of a randomized, placebo-controlled study. Arthritis Rheum. 2009;60:976–986. doi: 10.1002/art.24403. [DOI] [PubMed] [Google Scholar]
- 18.Kristensen LE, Gülfe A, Saxne T, Geborek P. Efficacy and tolerability of anti-tumour necrosis factor therapy in psoriatic arthritis patients: results from the South Swedish Arthritis Treatment Group register. Ann Rheum Dis. 2008;67:364–369. doi: 10.1136/ard.2007.073544. [DOI] [PubMed] [Google Scholar]
- 19.Sakkas LI, Zafiriou E, Bogdanos DP. Mini review: New treatments in psoriatic arthritis focus on the IL-23/17 axis. Focus on the IL-23/17 axis. Front Pharmacol. 2019;10:872. doi: 10.3389/fphar.2019.00872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McInnes IB, Kavanaugh A, Gottlieb AB, Puig L, Rahman P, Ritchlin C, Brodmerkel C, Li S, Wang Y, Mendelsohn AM, Doyle MK, PSUMMIT 1 Study Group Efficacy and safety of ustekinumab in patients with active psoriatic arthritis: 1 year results of the phase 3, multicentre, double-blind, placebo-controlled PSUMMIT 1 trial. Lancet. 2013;382:780–789. doi: 10.1016/S0140-6736(13)60594-2. [DOI] [PubMed] [Google Scholar]
- 21.Mease PJ, Kavanaugh A, Reimold A, Tahir H, Rech J, Hall S, Geusens P, Pellet P, Delicha EM, Pricop L, Mpofu S, FUTURE 1 study group Secukinumab provides sustained improvements in the signs and symptoms of psoriatic arthritis: final 5-year results from the phase 3 FUTURE 1 Study. ACR Open Rheumatol. 2020;2(1):18–25. doi: 10.1002/acr2.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kavanaugh A, Mease PJ, Gomez-Reino JJ, Adebajo AO, Wollenhaupt J, Gladman DD, et al. Treatment of psoriatic arthritis, in a phase 3 randomised, placebo-controlled trial with apremilast, an oral phosphodiesterase 4 inhibitor. Ann Rheum Dis. 2014;73:1020–1026. doi: 10.1136/annrheumdis-2013-205056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gladman DD, Orbai AM, Gomez-Reino J, Chang-Douglass S, Leoncini E, Burton HE, et al. Network meta-analysis of tofacitinib, biologic disease-modifying antirheumatic drugs, and apremilast for the treatment of psoriatic arthritis. Curr Ther Res Clin Exp. 2020;93(100601):2020. doi: 10.1016/j.curtheres.2020.100601.eCollection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mease P, Genovese MC, Gladstein G, Kivitz AJ, Ritchlin C, Tak PP, et al. Abatacept in the treatment of patients with psoriatic arthritis: results of a six-month, multicenter, randomized, double-blind, placebo-controlled, phase II trial. Arthritis Rheum. 2011;63(4):939–948. doi: 10.1002/art.30176. [DOI] [PubMed] [Google Scholar]
- 25.Lubrano E, Perrotta FM. Beyond TNF inhibitors: new pathways and emerging treatments for psoriatic arthritis. Drugs. 2016;76(6):663–673. doi: 10.1007/s40265-016-0557-4. [DOI] [PubMed] [Google Scholar]
- 26.Carmona L, Gomez-Reino JJ, Group B Survival of TNF antagonists in spondylarthritis is better than in rheumatoid arthritis. Data from the Spanish registry BIOBADASER. Arthritis Res Ther. 2006;8(3):R72. doi: 10.1186/ar1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kristensen LE, Karlsson JA, Englund M, Petersson IF, Saxne T, Geborek P. Presence of peripheral arthritis and male sex predicting continuation of anti-tumor necrosis factor therapy in ankylosing spondylitis: an observational prospective cohort study from the South Swedish Arthritis Treatment Group Register. Arthritis Care Res (Hoboken) 2010;62(10):1362–1369. doi: 10.1002/acr.20258. [DOI] [PubMed] [Google Scholar]
- 28.Soliman MM, Ashcroft DM, Watson KD, Lunt M, Symmons DP, Hyrich KL, British Society for Rheumatology Biologics Register Impact of concomitant use of DMARDs on the persistence with anti-TNF therapies in patients with rheumatoid arthritis: results from the British Society for Rheumatology Biologics Register. Ann Rheum Dis. 2011;70(4):583–589. doi: 10.1136/ard.2010.139774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Glintborg B, Ostergaard M, Krogh NS, Andersen MD, Tarp U, Loft AG, et al. Clinical response, drug survival, and predictors thereof among 548 patients with psoriatic arthritis who switched tumor necrosis factor alpha inhibitor therapy: results from the Danish Nationwide DANBIO Registry. Arthritis Rheum. 2013;65(5):1213–1223. doi: 10.1002/art.37876. [DOI] [PubMed] [Google Scholar]
- 30.Harrold LR, Stolshek BS, Rebello S, Collier DH, Mutebi A, Wade SW, et al. Impact of prior biologic use on persistence of treatment in patients with psoriatic arthritis enrolled in the US Corrona registry. Clin Rheumatol. 2017;36(4):895–901. doi: 10.1007/s10067-017-3593-x. [DOI] [PubMed] [Google Scholar]
- 31.Taylor W, Gladman D, Helliwell P, Marchesoni A, Mease P, Mielants H, CASPAR Study Group Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum. 2006;54(8):2665–2673. doi: 10.1002/art.21972. [DOI] [PubMed] [Google Scholar]
- 32.Mease PJ. Measures of psoriatic arthritis: Tender and Swollen Joint Assessment, Psoriasis Area and Severity Index (PASI), Nail Psoriasis Severity Index (NAPSI), Modified Nail Psoriasis Severity Index (mNAPSI), Mander/Newcastle Enthesitis Index (MEI), Leeds Enthesitis Index (LEI), Spondyloarthritis Research Consortium of Canada (SPARCC), Maastricht Ankylosing Spondylitis Enthesis Score (MASES), Leeds Dactylitis Index (LDI), Patient Global for Psoriatic Arthritis, Dermatology Life Quality Index (DLQI), Psoriatic Arthritis Quality of Life (PsAQOL), Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F), Psoriatic Arthritis Response Criteria (PsARC), Psoriatic Arthritis Joint Activity Index (PsAJAI), Disease Activity in Psoriatic Arthritis (DAPSA), and Composite Psoriatic Disease Activity Index (CPDAI) Arthritis Care Res (Hoboken) 2011;63(Suppl 11):S64–85. doi: 10.1002/acr.20577. [DOI] [PubMed] [Google Scholar]
- 33.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 34.Lorenzin M, Ortolan A, de Hooge M, Frallonardo P, Piccoli A, Cozzi F, et al. Lengthening the time intervals between doses of biological agents in psoriatic arthritis patients: a single-center retrospective study. Int J Immunopathol Pharmacol. 2015;28(4):479–487. doi: 10.1177/0394632015599446. [DOI] [PubMed] [Google Scholar]
- 35.Ip K, Hartley L, Solanki K, White D. Retention on anti-tumour necrosis factor therapy: the Waikato experience. N Z Med J. 2015;128(1415):34–40. [PubMed] [Google Scholar]
- 36.Aaltonen K, Heinonen A, Joensuu J, Parmanne P, Karjalainen A, Varjolahti-Lehtinen T, et al. Effectiveness and drug survival of TNF-inhibitors in the treatment of psoriatic arthritis: a prospective cohort study. Semin Arthritis Rheum. 2017;46(6):732–739. doi: 10.1016/j.semarthrit.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 37.Lorenzin M, Ortolan A, Frallonardo P, Oliviero F, Punzi L, Ramonda R. Predictors of response and drug survival in ankylosing spondylitis patients treated with infliximab. BMC Musculoskelet Disord. 2015;16:166. doi: 10.1186/s12891-015-0620-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Soubrier A, Bele-Philippe P, Cortet B, Ramdane-Sebbane N, Bacle-Boutry MA, Lemeunier L, et al (2015). Treatment response, drug survival and safety of anti-tumour necrosis factor α therapy in 193 patients with psoriatic arthritis: a twelve-year “real life” experience. Joint Bone Spine 82(1):31-37. http://www.sciencedirect.com/science/article/pii/S1297319X14001912. 10.1016/j.jbspin.2014.08.001 [DOI] [PubMed]
- 39.Costa L, Perricone C, Chimenti M, Del Puente A, Caso P, Peluso R, et al. Switching between biological treatments in psoriatic arthritis: a review of the evidence. Drugs R D. 2017;17(4):509–522. doi: 10.1007/s40268-017-0215-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fagerli KM, Lie E, van der Heijde D, Heiberg MS, Kalstad S, Rødevand E, et al. Switching between TNF inhibitors in psoriatic arthritis: data from the NOR-DMARD study. Ann Rheum Dis. 2013;72:1840–1844. doi: 10.1136/annrheumdis-2012-203018. [DOI] [PubMed] [Google Scholar]
- 41.Gomez-Reino JJ, Carmona L, BIOBADASER Group Switching TNF antagonists in patients with chronic arthritis: an observational study of 488 patients over a four-year period. Arthritis Res Ther. 2006;8(1):R29. doi: 10.1186/ar1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heiberg MS, Koldingsnes W, Mikkelsen K, Rodevand E, Kaufmann C, Mowinckel P, et al. The comparative one-year performance of anti-tumor necrosis factor alpha drugs in patients with rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis: results from a longitudinal, observational, multicenter study. Arthritis Rheum. 2008;59:234–240. doi: 10.1002/art.23333. [DOI] [PubMed] [Google Scholar]
- 43.Saad AA, Ashcroft DM, Watson KD, Hyrich KL, Noyce PR, Symmons DP. Persistence with anti-tumour necrosis factor therapies in patients with psoriatic arthritis: observational study from the British Society of Rheumatology Biologics Register. Arthritis Res Ther. 2009;11:R52. doi: 10.1186/ar2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kristensen LE, Lie E, Jacobsson LT, Christensen R, Mease PJ, Bliddal H, et al. Effectiveness and feasibility associated with switching to a second or third TNF inhibitor in patients with psoriatic arthritis: a cohort study from Southern Sweden. J Rheumatol. 2016;43(1):81–87. doi: 10.3899/jrheum.150744. [DOI] [PubMed] [Google Scholar]
- 45.Paccou J, Solau-Gervais E, Houvenagel E, Salleron J, Luraschi H, Philippe P, et al. Efficacy in current practice of switching between anti-tumour necrosis factor- α agents in spondyloarthropathies. Rheumatology (Oxford) 2011;50(4):714–720. doi: 10.1093/rheumatology/keq377. [DOI] [PubMed] [Google Scholar]
- 46.Reddy SM, Crean S, Martin AL, Burns MD, Palmer JB. Real-world effectiveness of anti-TNF switching in psoriatic arthritis: a systematic review of the literature. Clin Rheumatol. 2016;35(12):2955–2966. doi: 10.1007/s10067-016-3425-4. [DOI] [PubMed] [Google Scholar]
- 47.Modesti V, Ramonda R, Ortolan A, Lorenzin M, Lo Nigro A, Frallonardo P, et al. Infection relapse in spondyloarthritis treated with biological drugs: a single-centre study. Scand J Rheumatol. 2012;41(6):490–491. doi: 10.3109/03009742.2012.698393. [DOI] [PubMed] [Google Scholar]
- 48.Behrens F, Cañete JD, Olivieri I, van Kuijk AW, McHugh N, Combe B. Tumour necrosis factor inhibitor monotherapy vs combination with MTX in the treatment of PsA: a systematic review of the literature. Rheumatology (Oxford) 2015;54:915–926. doi: 10.1093/rheumatology/keu415. [DOI] [PubMed] [Google Scholar]
- 49.Iannone F, Lopriore S, Bucci R, Scioscia C, Anelli MG, Notarnicola A, et al. Two-year survival rates of anti-TNF-a therapy in psoriatic arthritis (PsA) patients with either polyarticular or oligoarticular PsA. Scand J Rheumatol. 2015;44:192–199. doi: 10.3109/03009742.2014.962081. [DOI] [PubMed] [Google Scholar]
- 50.Ramos Pinheiro R, Brasileiro A, Brito A, Barreto P, Pinheiro S (2016) SAT0412 Biological therapy in psoriatic arthritis (PSA): differences between switchers and non-switchers. Ann Rheum Dis 75(Suppl 2):819. http://ard.bmj.com/content/75/Suppl_2/819.1.abstract. 10.1136/annrheumdis-2016-eular.3019
- 51.Iannone F, Nivuori M, Fornaro M, Venerito V, Cacciapaglia F, Lopalco G. Comorbid fibromyalgia impairs the effectiveness of biologic drugs in patients with psoriatic arthritis. Rheumatology (Oxford) 2020;59(7):1599–1606. doi: 10.1093/rheumatology/kez505. [DOI] [PubMed] [Google Scholar]
- 52.Iannone F, Santo L, Anelli MG, Bucci R, Semeraro A, Quarta L, D’Onofrio F, Marsico A, Carlino G, Casilli O, Cacciapaglia F, Zuccaro C, Falappone PC, Cantatore FP, Muratore M, Lapadula G. Golimumab in real-life settings: 2 years drug survival and predictors of clinical outcomes in rheumatoid arthritis, spondyloarthritis, and psoriatic arthritis. Semin Arthritis Rheum. 2017;47(1):108–114. doi: 10.1016/j.semarthrit.2017.01.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.