Abstract
Introduction
Swedish National Diabetes Registry data show a correlation of improved glycemic control in people with type 1 diabetes (T1D) with increased use of diabetes technologies over the past 25 years. However, novel technologies are often associated with a high initial outlay. The aim of the present study was to evaluate the long-term cost-effectiveness of the advanced hybrid closed-loop (AHCL) MiniMed 780G system versus intermittently scanned continuous glucose monitoring (isCGM) plus self-injection of multiple daily insulin (MDI) or continuous subcutaneous insulin infusion (CSII) in people with T1D in Sweden.
Methods
Outcomes were projected over patients’ lifetimes using the IQVIA CORE Diabetes Model (v9.0). Clinical data, including changes in glycated hemoglobin (HbA1c) and hypoglycemia rates, were sourced from observational studies and a randomized crossover trial. Modeled patients were assumed to receive the treatments for their lifetimes, with HbA1c kept constant following the application of treatment effects. Costs were accounted from a societal perspective and expressed in Swedish krona (SEK). Utilities and days off work estimates were taken from published sources.
Results
The MiniMed 780G system was associated with an improvement in life expectancy of 0.16 years and an improvement in quality-adjusted life expectancy of 1.95 quality-adjusted life years (QALYs) versus isCGM plus MDI or CSII. These clinical benefits were due to a reduced incidence and a delayed time to onset of diabetes-related complications. Combined costs were estimated to be SEK 727,408 (EUR 72,741) higher with MiniMed 780G, with treatment costs partially offset by direct cost savings from the avoidance of diabetes-related complications and indirect cost savings from the avoidance of lost workplace productivity. The MiniMed 780G system was associated with an incremental cost-effectiveness ratio of SEK 373,700 per QALY gained.
Conclusions
Based on a willingness-to-pay threshold of SEK 500,000 per QALY gained, the MiniMed 780G system was projected to be cost-effective versus isCGM plus MDI or CSII for the treatment of T1D in Sweden.
Keywords: Cost-effectiveness, Type 1 diabetes, Sweden, Advanced hybrid closed-loop
Key Summary Points
| Why carry out this study? |
| The clinical burden of type 1 diabetes in Sweden is growing, and novel treatments could help to keep individuals with the disease within glycemic targets and thereby lower the incidence of costly long-term diabetes-related complications. |
| However, the benefits of novel efficacious interventions must be judged against the costs of introducing such therapies as healthcare budgets come under increasing strain worldwide. |
| The present study therefore aimed to evaluate the long-term cost-effectiveness of a novel advanced hybrid closed-loop (AHCL) system (the MiniMed 780G system) versus intermittently scanned continuous glucose monitoring (isCGM) plus multiple daily insulin (MDI) or continuous subcutaneous insulin infusion (CSII) in people with type 1 diabetes in Sweden. |
| What was learned from the study? |
| Outcomes projected over patients’ lifetimes indicated that the MiniMed 780G system was associated with improved life expectancy and quality-adjusted life expectancy and increased costs versus is CGM plus MDI or CSII from a societal perspective in Sweden, resulting in an incremental cost-effectiveness ratio of SEK 373,700 per quality-adjusted life year gained. |
| Based on long-term projections, the MiniMed 780G system was considered a cost-effective treatment option in people with type 1 diabetes in Sweden. |
Introduction
Type 1 diabetes is characterized by a loss of beta-cell function and consequent insulin deficiency, and represents one of the most common endocrine disorders in the world, particularly in pediatric populations [1]. In Sweden, the clinical burden of type 1 diabetes is growing, with the disease affecting more than 45,000 people in 2020, a marked increase since records began in 1996 [2]. Prolonged heightened blood glucose levels (measured via glycated hemoglobin [HbA1c]) can have a substantial impact on the incidence of diabetes-related complications over both the short and long term, as evidenced by the landmark Diabetes Control and Complications Trial (DCCT) and the Epidemiology of Diabetes Interventions and Complications (EDIC) study [3, 4]. Diabetes-related complications are associated with a significant economic burden in terms of both direct treatment costs and indirect costs relating to lost workplace productivity. Maintaining glycemic control within target ranges therefore represents the key goal for people with type 1 diabetes and for healthcare payers, as this reduces the incidence of long-term complications and avoids short-term complications such as hypoglycemia as well as the associated cost burdens. With healthcare systems worldwide coming under increasing budgetary strain, novel interventions for type 1 diabetes are often subjected to health economic analyses to evaluate whether they represent value for money for the healthcare payer and for society overall.
Type 1 diabetes requires lifelong treatment and management to avoid substantial morbidity and early mortality. Traditional treatments for type 1 diabetes have relied on self-injection of multiple daily insulin doses (MDI), which requires regular user-administered self-monitoring of blood glucose (SMBG) testing, and these therapies often represent the first-line treatment option for type 1 diabetes. However, innovations in diabetes technologies can help to relieve the burden of self-injection and SMBG testing on the user while offering improvements in glycemic control and subsequent reductions in long-term diabetes-related complications and hypoglycemic events [5–13]. Currently available diabetes technologies incorporate both a continuous glucose monitor (CGM, either intermittently scanned [isCGM] or real-time [rtCGM]), which can replace the need for SMBG testing, and insulin pumps for continuous subcutaneous insulin infusion (CSII), which can replace multiple daily self-injections. Combinations of CGM and CSII in sensor-augmented pumps (SAPs) can also embed predictive low-glucose management (PLGM) functions that, due to the communication between the two devices, allows partially automated insulin delivery based on blood glucose levels, improving glycemic control and reducing the risk of hypoglycemia [14, 15]. Mean HbA1c levels have been decreasing in the Swedish population with type 1 diabetes since 1996, and this trend correlates with an increased use of diabetes technologies over this time period [2]. However, the reported mean HbA1c level of people with type 1 diabetes in 2020 was 7.7%, which is above optimal levels [2, 16]. Novel diabetes technologies that can lower HbA1c levels while providing benefits for patients’ quality of life could therefore offer an attractive alternative to traditional MDI or older technological therapy.
Recently, advanced hybrid closed-loop (AHCL) systems have been developed that allow automated insulin administration via CSII in response to fluctuations in blood glucose levels measured by the CGM. The MiniMed 780G represents an AHCL system that automatically adjusts basal insulin delivery, as well as providing safe correction bolus doses as required, via an algorithm that updates insulin delivery every 5 min. This system was evaluated versus SAP therapy with PLGM in a recent study [17]. That randomized, open-label, crossover study, performed at two centers and conducted in automated-insulin-delivery-naïve participants with type 1 diabetes (aged between 7–80 years; n = 60), found that time in range (TIR) was improved with AHCL versus SAP plus PLGM, with higher use of the automated mode and no increase in hypoglycemia [17]. The automated features of AHCL systems could also relieve the burden of insulin administration from people with type 1 diabetes, thereby offering quality-of-life benefits and reduced fear of hypoglycemia [18].
Given the scope for improvement in type 1 diabetes care in Sweden, and the potential benefits offered by AHCL systems, the present study aimed to evaluate the long-term cost-effectiveness of the AHCL MiniMed 780G system compared with isCGM plus either MDI or CSII in the Swedish setting, and thereby estimate whether this system can offer both clinical benefits and value for money.
Methods
Model Overview
The IQVIA CORE Diabetes Model (version 9.0) was used to project clinical and cost outcomes over modeled patients’ lifetimes, in line with guidance on the cost-effectiveness of interventions for diabetes [19]. This model is a validated, web-based, non-product-specific diabetes analysis tool designed to project the long-term health outcomes of novel interventions for the treatment of type 1 and type 2 diabetes [20–22]. The model incorporates numerous submodels, each with a semi-Markov structure, that use time-, state-, time-in-state-, and diabetes-type-dependent probabilities derived from published sources. Relevant model outputs include life expectancy, quality-adjusted life expectancy (expressed in quality-adjusted life years [QALYs]), cumulative incidence and time to onset of diabetes-related complications, direct costs arising from the treatment of diabetes-related complications, indirect costs arising from lost workplace productivity, incremental cost-effectiveness ratios (ICERs) based on both direct costs and combined (direct and indirect) costs, as well as cost-effectiveness scatterplots and acceptability curves.
Clinical Data
Baseline cohort characteristics, including age, duration of diabetes, proportion male, HbA1c, systolic and diastolic blood pressure, total cholesterol, high-density lipoprotein (HDL) cholesterol, and body mass index (BMI) were sourced from the FUTURE study, a 12-month, prospective, observational, multicenter, real-world study investigating the impact of isCGM on glycemic control (Table 1) [23]. A total of 1913 people with type 1 diabetes for > 3 months were included, with 78% receiving MDI therapy. The mean age of the cohort was 45.8 years, with a duration of diabetes of 23 years, a proportion male of 53.9%, and a mean HbA1c of 7.8%.
Table 1.
Baseline characteristics, treatment effects, and adverse event rates applied in the base case analysis
| Parameter | MiniMed 780G | isCGM plus MDI or CSII |
|---|---|---|
| Baseline cohort characteristics, mean (SD) | ||
| Age, years | 45.8 (15.3) | |
| Diabetes duration, years | 22.8 (13.7) | |
| Male, % | 53.9 | |
| HbA1c, % | 7.8 (0.0) | |
| Treatment effects and adverse event rates | ||
| HbA1c, % | − 0.5% | 0.0% |
| Severe hypoglycemic event rate, per 100 patient-years | 0.0 | 63.9 |
| Diabetic ketoacidosis event rate, per 100 patient-years | 0.0 | 0.0 |
HbA1c glycated hemoglobin, SD standard deviation
Treatment effects for the MiniMed 780G system and isCGM plus MDI or CSII were sourced from a recent randomized crossover trial and the FUTURE study, respectively (Table 1) [17, 23]. In the MiniMed 780G arm, HbA1c was reduced by 0.5%, and the rate of severe hypoglycemic events (requiring the assistance of a third party) was set to zero, in line with the results from the randomized trial [17]. In the isCGM plus MDI or CSII arm, no changes from baseline in HbA1c were applied, and the rate of severe hypoglycemia (requiring the assistance of a third party) was set to 63.9 events per 100 patient-years, in line with results from the FUTURE study [23]. Following the application of treatment effects in the first year of the analysis, HbA1c was assumed to remain constant for the remainder of each patient’s lifetime.
Changes in other physiological parameters, such as blood pressure and serum lipid levels, were not applied in lieu of treatment-specific data, and baseline values were assumed to follow the model’s default progression equations based on published sources. No changes in BMI were applied, and this parameter was assumed to remain constant over the duration of the analyses. The rate of diabetic ketoacidosis was set to zero in both treatment arms. This approach allowed the impacts of different levels of glycemic control and hypoglycemic events (the two parameters evaluated in the clinical data sources) to be assessed.
Cost Data and Utilities
Costs were accounted from a Swedish societal perspective and expressed in Swedish krona (SEK). Selected cost outcomes were also expressed in euros (EUR), applying an exchange rate of SEK 1.0 = EUR 0.1. All analyses were performed using a first-order Monte Carlo approach, with future clinical and cost benefits discounted at 3.0% per annum, in line with pharmacoeconomic guidance for the Swedish setting [24]. Direct costs captured the costs of treating diabetes-related complications and the costs of patient management, which were taken from published sources (Table 2) [25–34]. Indirect costs arising from lost workplace productivity were based on the days off work estimates published by Sørensen and Ploug and the 2019 average salaries for men and women in Sweden [35, 36]. Indirect costs were only accrued while simulated individuals were below the set retirement age (64 years) [37].
Table 2.
Direct costs associated with the treatment of diabetes-related complications applied in the analyses
| Complication | Cost, SEK | References |
|---|---|---|
| Myocardial infarction, year of event | 99,979 | [28] |
| Myocardial infarction, years 2 + | 2346 | [28] |
| Angina, year of onset | 118,565 | [28] |
| Angina, years 2 + | 5242 | [28] |
| Congestive heart failure, year of onset | 78,395 | [28] |
| Congestive heart failure, years 2 + | 7416 | [28] |
| Stroke, year of event | 185,948 | [29] |
| Stroke, years 2 + | 40,537 | [29] |
| Stroke, death within 30 days | 87,436 | [30] |
| Peripheral vascular disease, onset | 92,941 | [28] |
| Peripheral vascular disease, years 2 + | 5173 | [28] |
| Hemodialysis, onset | 538,887 | [30] |
| Hemodialysis, years 2 + | 538,887 | [30] |
| Peritoneal dialysis, onset | 538,887 | [30] |
| Peritoneal dialysis, years 2 + | 538,887 | [30] |
| Kidney transplant, first year | 308,275 | [30] |
| Kidney transplant, years 2 + | 52,128 | [30] |
| Non-severe hypoglycemia | 55 | [26, 31] |
| Severe hypoglycemia (not requiring medical assistance) | 288 | [32] |
| Severe hypoglycemia (requiring medical assistance) | 4641 | [32] |
| Diabetic ketoacidosis | 26,442 | [30] |
| Laser treatment | 7181 | [30] |
| Cataract operation | 17,581 | [30] |
| Cataract operation, years 2 + | 0 | [30] |
| Blindness, first year | 9799 | [33] |
| Blindness, years 2 + | 4183 | [33] |
| Neuropathy, year of onset | 6351 | [25] |
| Neuropathy, years 2 + | 6351 | [25] |
| Amputation, procedure | 279,550 | [34] |
| Amputation, prosthesis | 19,863 | [29] |
| Gangrene treatment | 222,381 | [34] |
| Infected foot ulcer | 95,906 | [34] |
| Uninfected foot ulcer | 85,587 | [34] |
| Cost after healed ulcer | 8559 | [34] |
| Cost of healed ulcer (history of amputation) | 8559 | [34] |
SEK Swedish krona
Pharmacy costs captured the costs of the MiniMed 780G device, the isCGM, basal and bolus insulin, the CSII pump, cannula, and reservoir, as well as the SMBG testing apparatus and training to use the insulin pump. Resource use, including the doses of insulin applied in the MDI arm and the cost of isCGM plus MDI or CSII, was based on a weighted average of patients in the FUTURE study (77.8% receiving MDI and 22.2% receiving CSII) [23]. Annual costs for each treatment arm were calculated based on resource use and pharmacy costs, and totaled SEK 75,644.75 for treatment with MiniMed 780G and SEK 29,971.05 for treatment with isCGM plus MDI or CSII.
Health-state utilities and event-based disutilities associated with diabetes-related complications were taken from published sources [38–46]. In the MiniMed 780G arm, a treatment-specific utility increase of 0.0552 was applied to capture the improvement in quality of life associated with reduced fear of hypoglycemic events in patients using SAPs, based on the results of the INTERPRET study (which noted a decrease of 6.9 in the hypoglycemic fear survey) and a quality-of-life translation instrument developed by Currie et al. [47–49]. For the isCGM plus MDI or CSII arm, no significant improvement in the hypoglycemic fear survey score was reported in the FUTURE study, and therefore no utility improvement was assumed [23].
Sensitivity Analyses
Modeling the long-term outcomes of diabetes from short-term data is associated with uncertainty. A series of sensitivity analyses were therefore performed to evaluate the robustness of the base case findings. These included evaluating the effect of over- or underestimating the HbA1c benefit with MiniMed 780G by increasing and decreasing the benefit in the 780G treatment arm in several steps between − 0.8% to − 0.4%, and testing the effect of a better-controlled cohort by lowering the baseline HbA1c from 7.8% to 7.5% in the MiniMed 780G arm (based on the findings of the US Pivotal trial) [17].
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Results
Base Case Analysis
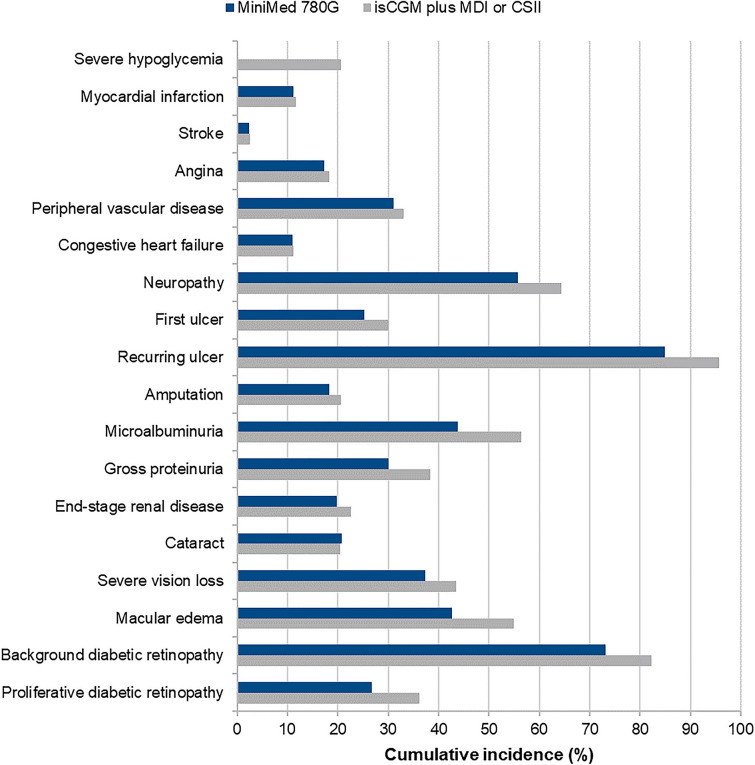
Long-term projections indicated that the MiniMed 780G system was associated with an improvement in life expectancy of 0.16 years and an improvement in quality-adjusted life expectancy of 1.95 QALYs versus isCGM plus MDI or CSII (Table 3). The improved clinical outcomes were a result of a reduced incidence and an increased time to onset of diabetes-related complications with MiniMed 780G, as well as quality-of-life benefits arising from reduced fear of hypoglycemia (Fig. 2).
Table 3.
Base case analysis results
| Health outcomes | MiniMed 780G | isCGM plus MDI or CSII | Difference |
|---|---|---|---|
| Discounted life expectancy, years | 19.27 (0.20) | 19.11 (0.19) | + 0.16 |
| Discounted quality-adjusted life expectancy, QALYs | 14.25 (0.15) | 12.31 (0.13) | + 1.95 |
| Discounted direct costs, SEK | 2,593,226 (58,912) | 1,754,941 (59,956) | + 838,285 |
| Discounted direct costs, EUR | 259,323 (5,891) | 175,494 (5,996) | + 83,829 |
| Discounted combined costs, SEK | 3,414,589 (95,866) | 2,687,181 (95,819) | + 727,408 |
| Discounted combined costs, EUR | 341,459 (9,587) | 268,718 (9,582) | + 72,741 |
| ICER based on direct costs | SEK 430,663 per QALY gained | ||
| ICER based on combined costs | SEK 373,700 per QALY gained | ||
Values are means (standard deviations)
CSII continuous subcutaneous insulin infusion, EUR euros, ICER incremental cost-effectiveness ratio, isCGM intermittently scanned continuous glucose monitor, MDI self-injection of multiple daily insulin doses, QALYs quality-adjusted life years, SEK Swedish krona
Fig. 2.
Cumulative incidence of diabetes-related complications in the base case analysis. CSII continuous subcutaneous insulin infusion, isCGM intermittently scanned continuous glucose monitor, MDI self-injection of multiple daily insulin doses
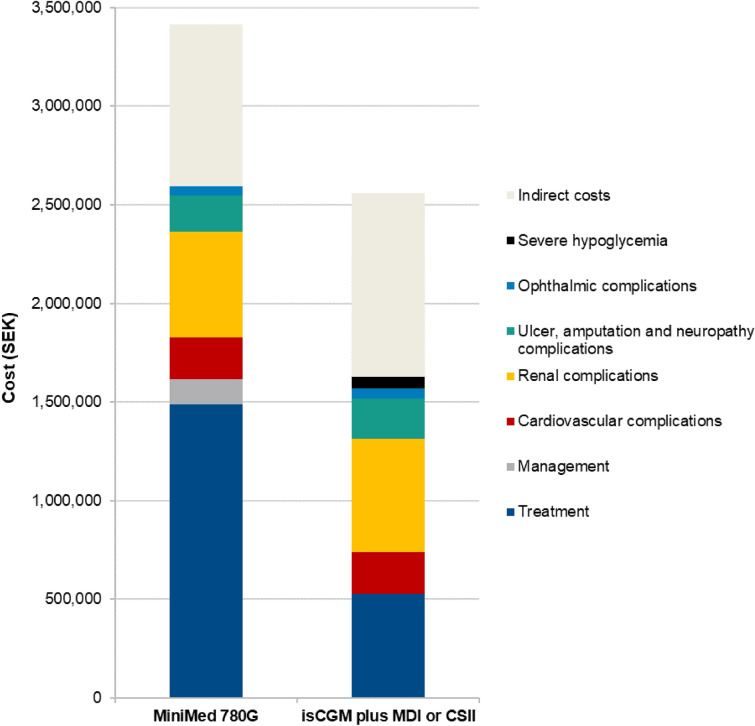
Total direct costs were estimated to be SEK 838,285 (EUR 83,829) higher with the MiniMed 780G system compared with isCGM plus MDI or CSII over a patient’s lifetime, due to the higher acquisition cost of the device and the increased survival and further treatment of individuals over the long term (Fig. 1). However, higher treatment costs were partially offset by cost savings arising from the avoidance of diabetes-related complications, most notably renal complications (mean cost savings of SEK 36,704 [EUR 3,670] per person). Moreover, indirect costs were estimated to be SEK 110,877 (EUR 11,088) lower with the MiniMed 780G system compared with isCGM plus MDI or CSII, leading to combined (direct and indirect) cost increases of SEK 727,408 (EUR 72,741) with the MiniMed 780G system over a patient’s lifetime.
Fig. 1.
Total costs accumulated in the base case analysis over a patient’s lifetime. CSII continuous subcutaneous insulin infusion, isCGM intermittently scanned continuous glucose monitor, MDI self-injection of multiple daily insulin doses, SEK Swedish krona
Estimation of long-term clinical outcomes indicated that both life expectancy and quality-adjusted life expectancy were improved with the MiniMed 780G system compared with isCGM plus MDI or CSII, at an increased cost from a Swedish societal perspective (Table 3). The MiniMed 780G system was therefore associated with an ICER of SEK 373,700 per QALY gained, based on combined costs, versus isCGM plus MDI or CSII. Based on a willingness-to-pay threshold of SEK 500,000 per QALY gained in Sweden (as recommended by the Swedish Agency for Health Technology Assessment [SBU] for high-cost interventions), the MiniMed 780G system was considered a cost-effective treatment option compared with isCGM plus MDI or CSII for the treatment of type 1 diabetes [50].
Sensitivity Analyses
Sensitivity analyses showed that the results of the base case findings were robust to changes in baseline HbA1c and treatment effects (Table 4). Reducing the HbA1c benefit with the MiniMed 780G system to − 0.4% resulted in reduced clinical benefits and cost savings, with the ICER increasing to SEK 387,755 per QALY gained. Conversely, increasing the HbA1c benefit to − 0.6%, − 0.7%, and − 0.8% resulted in increased clinical benefits and cost savings with 780G, yielding ICERs of SEK 358,016, 346,607, and 332,476 per QALY gained, respectively. Reducing baseline HbA1c to 7.5% in the 780G arm (as opposed to 7.8% in the base case analysis) led to increased quality-adjusted life expectancy benefits with the AHCL system, but also increased costs due to the greater survival and further treatment of patients over the long term. The MiniMed 780G system was therefore associated with an ICER of SEK 250,547 per QALY gained versus isCGM plus MDI or CSII.
Table 4.
Sensitivity analyses results
| Analysis | Discounted quality-adjusted life expectancy, QALYs | Discounted combined costs, SEK | ICER based on combined costs, SEK per QALY gained | ||||
|---|---|---|---|---|---|---|---|
| MiniMed 780G | isCGM plus MDI or CSII | Difference | MiniMed 780G | isCGM plus MDI or CSII | Difference | ||
| Base case | 14.25 | 12.31 | + 1.95 | 3,414,589 | 2,687,181 | + 727,408 | 373,700 |
| HbA1c reduction of − 0.8% with 780G | 14.36 | 12.31 | + 2.05 | 3,369,655 | 2,687,181 | + 682,474 | 332,476 |
| HbA1c reduction of − 0.7% with 780G | 14.33 | 12.31 | + 2.02 | 3,387,534 | 2,687,181 | + 700,353 | 346,607 |
| HbA1c reduction of − 0.6% with 780G | 14.30 | 12.31 | + 1.99 | 3,399,454 | 2,687,181 | + 712,272 | 358,016 |
| HbA1c reduction of − 0.4% with 780G | 14.22 | 12.31 | + 1.92 | 3,429,771 | 2,687,181 | + 742,590 | 387,755 |
| Baseline HbA1c of 7.5% in the 780G arm | 16.24 | 12.31 | + 3.94 | 3,673,633 | 2,687,181 | + 986,452 | 250,547 |
CSII continuous subcutaneous insulin infusion, HbA1c glycated hemoglobin, ICER incremental cost-effectiveness ratio, isCGM intermittently scanned continuous glucose monitor, MDI self-injection of multiple daily insulin doses, QALY quality-adjusted life year, SEK Swedish krona
Discussion
Demonstrating the cost-effectiveness of novel diabetes technologies, particularly AHCL systems that are associated with a high initial outlay, is crucial for increasing the uptake of efficacious treatment options for type 1 diabetes. From a societal perspective in Sweden, the MiniMed 780G system was found to be a cost-effective treatment option compared with isCGM plus MDI or CSII in people with type 1 diabetes. Greater reductions in HbA1c and severe hypoglycemic events yielded improved life expectancy and quality-adjusted life expectancy over the long term, a result of the reduced incidence and increased time to onset of diabetes-related complications with the MiniMed 780G system (Fig. 2). Increased treatment costs were partially offset by cost savings from the avoidance of diabetes-related complications and associated lost workplace productivity. The MiniMed 780G system was therefore projected to offer clinical benefits for people with type 1 diabetes while providing value for money for healthcare payers in Sweden.
The selection of isCGM plus either MDI or CSII as a comparator was based on the uptake of CGM and CSII in Sweden. While CGM was in use in 84% of the population with type 1 diabetes in Sweden in 2020, similar levels of uptake have not been observed for insulin pumps, with only 26% of the population covered [2]. Therefore, many patients still rely on MDI when responding to prompts from the CGM, and a comparator combining both approaches was considered appropriate. Baseline cohort characteristics and insulin dose resource use from the FUTURE study, which investigated the impact of isCGM on glycemic control, were also chosen to reflect the current state of type 1 diabetes therapy in Sweden, with a high proportion utilizing CGM but fewer people using insulin pumps [23]. Moreover, these data from the National Diabetes Register indicate that there is scope for improving the treatment of type 1 diabetes in Sweden, with insulin pumps currently underutilized. Given the improvements in HbA1c associated with improved diabetes care and greater use of effective technologies, the support and reimbursement of novel cost-effective technologies should continue. The present study has demonstrated that a next-generation AHCL device is a cost-effective treatment option for people with type 1 diabetes in Sweden. Prescribing such a device could therefore help to further improve diabetes care, considering the high mean levels of HbA1c and low insulin pump uptake still observed in people with type 1 diabetes in Sweden [2, 16].
Fear of hypoglycemia was a key factor in the present analysis. Fear of hypoglycemia is common in populations with type 1 diabetes, and can affect quality of life both directly and indirectly through an increased burden of self-management [51, 52]. A history of severe hypoglycemic events, as well as certain situations (such as driving or being in the workplace, particularly an industrial setting), can also drive an increased fear of future events, and can act as a barrier to physical activity or other activities, further limiting patients’ quality of life [51, 53, 54]. The psychological burden of fear of hypoglycemia should also be considered, as this can affect both adult patients and parents or caregivers of younger individuals with type 1 diabetes [55, 56]. Given the reductions in hypoglycemia observed with the AHCL system in the recent trial and applied in the present analysis, the application of an additional utility for avoidance of fear of hypoglycemia was considered appropriate to capture not only the physical effects of hypoglycemia but also the associated psychological distress [17].
A limitation of the analysis, inherent to all long-term cost-effectiveness analyses, is the projection of long-term outcomes from short-term clinical data. That acknowledged, this is one of the essential tenets of long-term health economic modeling, and it remains one of the best available options for informing decision making in the absence of long-term clinical trial data, with guidelines for computer modeling of diabetes interventions recommending the projection of outcomes over patients’ lifetimes [19]. Moreover, every effort was made to limit uncertainty by using a published and validated health economic model and conducting sensitivity analyses around model inputs [20–22]. The use of data from a crossover trial could also be viewed as a weakness of the analysis. However, no randomized head-to-head study comparing an AHCL system with CGM in combination with either MDI or previous-generation devices has been published, and the crossover trial therefore represented the most robust evidence source currently available. Future clinical studies should focus on elucidating potential benefits for glycemic control and hypoglycemia in head-to-head comparisons of diabetes technologies, with subsequent health economic analyses utilizing these data to provide further measurements of cost-effectiveness for novel devices.
The inability to include TIR data could also be seen as a limitation. Published evidence has indicated that the MiniMed 780G system is associated with improved TIR and reduced hyperglycemia versus SAP plus PLGM and a previous HCL system, respectively [17, 57]. In that sense, the exclusion of these data was conservative from the perspective of the 780G system. Nonetheless, as TIR becomes more central to country-specific recommendations for treatments for type 1 diabetes, novel modeling methods should be developed to utilize these data and to help further inform decision makers.
Conclusions
Long-term projections indicate that the AHCL MiniMed 780G system is likely to represent a cost-effective treatment option versus isCGM plus MDI or CSII in people with type 1 diabetes in Sweden.
Acknowledgements
Funding
This study, the Rapid Service Fee and the Open Access fee were supported by funding from Medtronic International Trading Sàrl, Geneva, Switzerland.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author’s Contributions
JJ, MIB, ALH, SdP and OC contributed to the study conception and design. Material preparation, data collection and analysis were performed by MIB, ALH, SdP and OC. The manuscript was written by SJPM with input from all authors. All authors read and approved the final manuscript.
Disclosures
Johan Jendle has received speaker’s and or consultant fees from Abbott, Ascensia, AstraZeneca, Boehringer Ingelheim, Eli-Lilly, Medtronic, Nordic Infucare, Novo Nordisk and Sanofi. Maria Ida Buompensiere, Simona de Portu and Ohad Cohen are employees of Medtronic International Trading Sàrl. Astrid Ledgaard Holm is an employee of Medtronic Denmark. Samuel Joseph Paul Malkin is an employee of Ossian Health Economics and Communications GmbH, which received consulting fees to support preparation of the manuscript.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1.Patterson CC, Karuranga S, Salpea P, et al. Worldwide estimates of incidence, prevalence and mortality of type 1 diabetes in children and adolescents: results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 2019;157:107842. [DOI] [PubMed]
- 2.Svensson A-M, Eliasson B, Linder E, et al. Nationella Diabetesregistret [National Diabetes Register]—nationwide results 1996–2020. 2021. https://www.ndr.nu/pdfs/NationWideResults_1996-2019.pdf. Accessed 7 May 2021.
- 3.Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 4.Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) Research Group Effect of intensive diabetes therapy on the progression of diabetic retinopathy in patients with type 1 diabetes: 18 years of follow-up in the DCCT/EDIC. Diabetes. 2015;64:631–642. doi: 10.2337/db14-0930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burckhardt M-A, Smith GJ, Cooper MN, Jones TW, Davis EA. Real-world outcomes of insulin pump compared to injection therapy in a population-based sample of children with type 1 diabetes. Pediatr Diabetes. 2018;19(8):1459–1466. doi: 10.1111/pedi.12754. [DOI] [PubMed] [Google Scholar]
- 6.Moreno-Ferández J, García-Seco JA, Herrera-Moraleda M, Seco AM, Muñoz-Rodríguez JR. Real-world outcomes of insulin pump compared to multiple daily injection therapy in adult type 1 diabetes mellitus patients in a Mediterranean scenario. Int J Diabetes Dev Ctries. 2020 doi: 10.1007/s13410-020-00887-4. [DOI] [Google Scholar]
- 7.McGill JB, Ahmann A. Continuous glucose monitoring with multiple daily insulin treatment: outcome studies. Diabetes Technol Ther. 2017;19(Suppl 3):S3–S12. doi: 10.1089/dia.2017.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahmadi SS, Westman K, Pivodic A, et al. The association between HbA1c and time in hypoglycemia during CGM and self-monitoring of blood glucose in people with type 1 diabetes and multiple daily insulin injections: a randomized clinical trial (GOLD-4) Diabetes Care. 2020;43(9):2017–2024. doi: 10.2337/dc19-2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beck RW, Riddlesworth T, Ruedy K, et al. Effect of continuous glucose monitoring on glycemic control in adults with type 1 diabetes using insulin injections: the DIAMOND randomized clinical trial. JAMA. 2017;317(4):371–378. doi: 10.1001/jama.2016.19975. [DOI] [PubMed] [Google Scholar]
- 10.Hirsch IB, Bode BW, Garg S, et al. Continuous subcutaneous insulin infusion (CSII) of insulin aspart versus multiple daily injection of insulin aspart/insulin glargine in type 1 diabetic patients previously treated with CSII. Diabetes Care. 2005;28(3):533–538. doi: 10.2337/diacare.28.3.533. [DOI] [PubMed] [Google Scholar]
- 11.Steineck I, Ranjan A, Nørgaard K, Schmidt S. Sensor-augmented insulin pumps and hypoglycemia prevention in type 1 diabetes. J Diabetes Sci Technol. 2017;11(1):50–58. doi: 10.1177/1932296816672689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zucchini S, Scipione M, Balsamo C, et al. Comparison between sensor-augmented insulin therapy with continuous subcutaneous insulin infusion or multiple daily injections in everyday life: 3-day analysis of glucose patterns and sensor accuracy in children. Diabetes Technol Ther. 2011;13(12):1187–1193. doi: 10.1089/dia.2011.0080. [DOI] [PubMed] [Google Scholar]
- 13.Bosi E, Choudhary P, de Valk HW, et al. Efficacy and safety of suspend-before-low insulin pump technology in hypoglycaemia-prone adults with type 1 diabetes (SMILE): an open-label randomised controlled trial. Lancet Diabetes Endocrinol. 2019;7:462–472. doi: 10.1016/S2213-8587(19)30150-0. [DOI] [PubMed] [Google Scholar]
- 14.Abraham MB, Nicholas JA, Smith GJ, et al. Reduction in hypoglycemia with the predictive low-glucose management system: a long-term randomized controlled trial in adolescents with type 1 diabetes. Diabetes Care. 2018;41(2):303–310. doi: 10.2337/dc17-1604. [DOI] [PubMed] [Google Scholar]
- 15.Forlenza GP, Li Z, Buckingham BA, et al. Predictive low-glucose suspend reduces hypoglycemia in adults, adolescents, and children with type 1 diabetes in an at-home randomized crossover study: results of the PROLOG trial. Diabetes Care. 2018;41:2155–61. [DOI] [PubMed]
- 16.Lind M, Pivodic A, Svensson A-M, Ólafsdóttir AF, Wedel H, Ludvigsson J. HbA1c level as a risk factor for retinopathy and nephropathy in children and adults with type 1 diabetes: Swedish population based cohort study. BMJ. 2019;366:14894. doi: 10.1136/bmj.l4894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Collyns OJ, Meier RA, Betts ZL, et al. Improved glycemic outcomes with Medtronic MiniMed advanced hybrid closed-loop delivery: results from a randomized crossover trial comparing automated insulin delivery with predictive low glucose suspend in people with type 1 diabetes. Diabetes Care. 2021;44(4):969–75. [DOI] [PubMed]
- 18.Fidler C, Christensen TE, Gillard S. Hypoglycemia: an overview of fear of hypoglycemia, quality-of-life, and impact on costs. J Med Econ. 2011;14(5):646–655. doi: 10.3111/13696998.2011.610852. [DOI] [PubMed] [Google Scholar]
- 19.American Diabetes Association Consensus Panel Guidelines for computer modeling of diabetes and its complications. Diabetes Care. 2004;27:2262–2265. doi: 10.2337/diacare.27.9.2262. [DOI] [PubMed] [Google Scholar]
- 20.Palmer AJ, Roze S, et al. The CORE diabetes model: projecting long-term clinical outcomes, costs and cost-effectiveness of interventions in diabetes mellitus (types 1 and 2) to support clinical and reimbursement decision-making. Curr Med Res Opin. 2004;20(Suppl 1):S5–26. doi: 10.1185/030079904X1980. [DOI] [PubMed] [Google Scholar]
- 21.Palmer AJ, Roze S, Valentine WJ, et al. Validation of the CORE Diabetes Model against epidemiological and clinical studies. Curr Med Res Opin. 2004;20(Suppl 1):S27–40. doi: 10.1185/030079904X2006. [DOI] [PubMed] [Google Scholar]
- 22.McEwan P, Foos V, Palmer JL, Lamotte M, Lloyd A, Grant D. Validation of the IMS CORE Diabetes Model. Value Health. 2014;17(6):714–724. doi: 10.1016/j.jval.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 23.Charleer S, De Block C, Van Huffel L, et al. Quality of life and glucose control after 1 year of nationwide reimbursement of intermittently scanned continuous glucose monitoring in adults living with type 1 diabetes (FUTURE): a prospective observational real-world cohort study. Diabetes Care. 2020;43(2):389–397. doi: 10.2337/dc19-1610. [DOI] [PubMed] [Google Scholar]
- 24.International Society for Pharmacoeconomics and Outcomes Research (ISPOR). Pharmacoeconomic guidelines around the world—Sweden. 2020. https://www.tools.ispor.org/PEguidelines/countrydet.asp?c=21&t=1. Accessed 10 May 2021.
- 25.TLV. Tariff database. 2020. https://www.tlv.se/beslut/sok-i-databasen.html. Accessed 12 May 2021.
- 26.Socialstyrelsen. Viktlister for NordDRG. Prospektiva kliniklvikter 2020. https://www.socialstyrelsen.se/utveckla-verksamhet/e-halsa/klassificering-och-koder/drg/viktlistor/. Accessed 12 May 2021.
- 27.Karolinska Universitetssjukhus. Prislista för utomlänsvård samt för utlandspatienter inom EU 2017 [Price list for outpatient care and for international patients within the EU 2017]. 2017. https://www.docplayer.se/25866982-Prislista-for-utomlansvard-samt-for-utlandspatienter-inom-eu-2017.html. Accessed 12 May 2021.
- 28.Gerdtham UG, Clarke P, Hayes A, Gudbjornsdottir S. Estimating the cost of diabetes mellitus-related events from inpatient admissions in Sweden using administrative hospitalization data. Pharmacoeconomics. 2009;27(1):81–90. doi: 10.2165/00019053-200927010-00008. [DOI] [PubMed] [Google Scholar]
- 29.Ghatnekar O, Carlsson K. Kostnader för insjuknande i stroke år 2009. En incidensbaserad studie. [Costs for stroke incidence in 2009. An incidence-based study]. Lund: Institutet för hälsooch sjukvårdsekonomi (IHE), Lund Universitet [Lund: Institute of Health and Medical Economics (IHE), Lund University]; 2012. p. 2.
- 30.Henriksson F. Applications of economic models in healthcare: the introduction of pioglitazone in Sweden. Pharmacoeconomics. 2002;20(Suppl 1):43–53. doi: 10.2165/00019053-200220001-00005. [DOI] [PubMed] [Google Scholar]
- 31.Geelhoed-Duijvestijn PH, Pedersen-Bjergaard U, Weitgasser R, Lahtela J, Jensen MM, Östenson CG. Effects of patient-reported non-severe hypoglycemia on healthcare resource use, work-time loss, and wellbeing in insulin-treated patients with diabetes in seven European countries. J Med Econ. 2013;16(12):1453–1461. doi: 10.3111/13696998.2013.852098. [DOI] [PubMed] [Google Scholar]
- 32.Jönsson L, Bolinder B, Lundkvist J. Cost of hypoglycemia in patients with type 2 diabetes in Sweden. Value Health. 2006;9(3):193–198. doi: 10.1111/j.1524-4733.2006.00100.x. [DOI] [PubMed] [Google Scholar]
- 33.Persson U, Willis M, Odegaard K. A case study of ex ante, value-based price and reimbursement decision-making: TLV and rimonabant in Sweden. Eur J Health Econ. 2010;11(2):195–203. doi: 10.1007/s10198-009-0166-1. [DOI] [PubMed] [Google Scholar]
- 34.Prompers L, Huijberts M, Schaper N, et al. Resource utilisation and costs associated with the treatment of diabetic foot ulcers. Prospective data from the Eurodiale Study. Diabetologia. 2008;51(10):1826–1834. doi: 10.1007/s00125-008-1089-6. [DOI] [PubMed] [Google Scholar]
- 35.Sørensen J, Ploug UJ. The cost of diabetes-related complications: registry-based analysis of days absent from work. Econ Res Int. 2013;618039.
- 36.Statistics Sweden. Salary dispersion by sector and sex 2019. 2020. http://www.scb.se/en/finding-statistics/statistics-by-subject-area/labour-market/wages-salaries-and-labour-costs/salary-structures-whole-economy/pong/tables-and-graphs/salary-dispersion-by-sector-and-sex/. Accessed 12 May 2021.
- 37.Statistics Sweden. Sverige har högst pensionsålder i EU [Sweden has the highest retirement age in the EU]. 2016. https://www.scb.se/hitta-statistik/artiklar/2016/Sverige-har-hogst-pensionsalder-i-EU/. Accessed 12 May 2021.
- 38.Clarke P, Gray A, Holman R. Estimating utility values for health states of type 2 diabetic patients using the EQ-5D (UKPDS 62) Med Decis Mak. 2002;22(4):340–349. doi: 10.1177/027298902400448902. [DOI] [PubMed] [Google Scholar]
- 39.Bagust A, Beale S. Modelling EuroQol health-related utility values for diabetic complications from CODE-2 data. Health Econ. 2005;14:217–230. doi: 10.1002/hec.910. [DOI] [PubMed] [Google Scholar]
- 40.Wasserfallen JB, Halabi G, Saudan P, et al. Quality of life on chronic dialysis: comparison between haemodialysis and peritoneal dialysis. Nephrol Dial Transplant. 2004;19:1594–1599. doi: 10.1093/ndt/gfh175. [DOI] [PubMed] [Google Scholar]
- 41.Fenwick EK, Xie J, Ratcliffe J, et al. The impact of diabetic retinopathy and diabetic macular edema on health-related quality of life in type 1 and type 2 diabetes. Investig Ophthalmol Vis Sci. 2012;53:677–684. doi: 10.1167/iovs.11-8992. [DOI] [PubMed] [Google Scholar]
- 42.Kiberd BA, Jindal KK. Screening to prevent renal failure in insulin dependent diabetic patients: an economic evaluation. BMJ. 1995;311:1595–1599. doi: 10.1136/bmj.311.7020.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee WJ, Song KH, Noh JH, et al. Health-related quality of life using the EuroQol 5D questionnaire in Korean patients with type 2 diabetes. J Korean Med Sci. 2012;27:255–260. doi: 10.3346/jkms.2012.27.3.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lauridsen JT, Lønborg J, Gundgaard J, Jensen HH. Diminishing marginal disutility of hypoglycaemic events: results from a time trade-off survey in five countries. Qual Life Res. 2014;23(9):2645–2650. doi: 10.1007/s11136-014-0712-x. [DOI] [PubMed] [Google Scholar]
- 45.Marrett E, Radican L, Davies MJ, Zhanget Q. Assessment of severity and frequency of self-reported hypoglycemia on quality of life in patients with type 2 diabetes treated with oral antihyperglycemic agents: a survey study. BMC Res Notes. 2011;4:251. doi: 10.1186/1756-0500-4-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Evans M, Khunti K, Mamdani M, et al. Health-related quality of life associated with daytime and nocturnal hypoglycaemic events: a time trade-off survey in five countries. Health Qual Life Outcomes. 2013;11:90. doi: 10.1186/1477-7525-11-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nørgaard K, Scaramuzza A, Bratina N, Lalić N, Jarosz-Chobot P, Kocsis G, et al. Sensor-augmented pump therapy in real-life: patients reported outcomes results of the INTERPRET observational study. Abstract 1058. 48th EASD Annual Meeting; 2012 Oct 1–5; Berlin, Germany.
- 48.Nørgaard K, Scaramuzza A, Bratina N, Lalić NM, Jarosz-Chobot P, Kocsis G, et al. Routine sensor-augmented pump therapy in type 1 diabetes: the INTERPRET study. Diabetes Technol Ther. 2013;15(4):273–280. doi: 10.1089/dia.2012.0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Currie CJ, Morgan CL, Poole CD, Sharplin P, Lammert M, McEwan P. Multivariate models of health-related utility and the fear of hypoglycaemia in people with diabetes. Curr Med Res Opin. 2006;22(8):1523–1534. doi: 10.1185/030079906X115757. [DOI] [PubMed] [Google Scholar]
- 50.Swedish Agency for Health Technology Assessment (SBU). Hälsoekonomiska utvärderingar [Health economic evaluations]. 2017. https://www.sbu.se/globalassets/ebm/metodbok/sbushandbok_kapitel11.pdf. Accessed 11 June 2021.
- 51.Wild D, von Maltzahn R, Brohan E, Christensen T, Clauson P, Gonder-Frederick L. A critical review of the literature on fear of hypoglycemia in diabetes: Implications for diabetes management and patient education. Patient Educ Couns. 2007;68(1):10–15. doi: 10.1016/j.pec.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 52.Gonder-Frederick L. Fear of hypoglycemia: a review. Diabet Hypoglycemia. 2013;5:3–11.
- 53.Dømgaard M, Bagger M, Rhee NA, Burton CM, Thorsteinsson B. Individual and societal consequences of hypoglycemia: a cross-sectional survey. Postgrad Med. 2015;127(5):438–445. doi: 10.1080/00325481.2015.1045815. [DOI] [PubMed] [Google Scholar]
- 54.Brennan MC, Brown JA, Ntoumanis N, Leslie GD. Barriers and facilitators of physical activity participation in adults living with type 1 diabetes: a systematic scoping review. Appl Physiol Nutr Metab. 2020 doi: 10.1139/apnm-2020-0461. [DOI] [PubMed] [Google Scholar]
- 55.Herbert LJ, Clary L, Owen V, Monaghan M, Alvarez V, Streisand R. Relations among school/daycare functioning, fear of hypoglycaemia and quality of life in parents of young children with type 1 diabetes. J Clin Nurs. 2015;24(9–10):1199–1209. doi: 10.1111/jocn.12658. [DOI] [PubMed] [Google Scholar]
- 56.Macaulay GC, Boucher SE, Yogarajah A, Galland BC, Wheeler BJ. Sleep and night-time caregiving in parents of children and adolescents with type 1 diabetes mellitus—a qualitative study. Behav Sleep Med. 2020;18(5):622–636. doi: 10.1080/15402002.2019.1647207. [DOI] [PubMed] [Google Scholar]
- 57.Bergenstal RM, Nimri R, Beck RW, et al. A comparison of two hybrid closed-loop systems in adolescents and young adults with type 1 diabetes (FLAIR): a multicentre, randomised, crossover trial. Lancet. 2021;397(10270):208–219. doi: 10.1016/S0140-6736(20)32514-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.