Key Summary Points
| Why carry out this study? |
| Despite the very widespread clinical use of metformin, there is a lack of systematic evidence to guide optimal selection of the various formulations available. |
| What was learned from the study? |
| Updated data have now become available to add to our systematic review and meta-analysis. |
| Incorporation of these new data does not substantively alter the conclusions of our meta-analysis. |
| We showed that long-acting metformin formulations have equal efficacy in glycaemic control compared to immediate-release metformin, with additional benefits of reduced low-density lipoprotein cholesterol concentrations with extended-release metformin and reduced gastrointestinal side effects with delayed-release metformin. |
Editorial
Metformin is not a new drug, yet its use continues to expand for an ever-increasing range of indications. Metformin is safe, relatively low cost, easy to store, and simple to administer, making it an attractive option for patients and providers alike. Between 2007 and 2017, the number of prescriptions issued for metformin in the UK doubled to more than 20 million [1]. However when metformin scripts are issued, there are surprisingly few robust sources of evidence on which to make good clinical decisions.
To tackle this evidence gap, we recently performed a systematic review and meta-analysis of all studies that compared different formulations of metformin, i.e. immediate release (IR), sustained release (also known as long-acting or extended release; XR), or delayed release (DR) [2]. Delayed release metformin is a new formulation, with all global rights owned by Anji Pharmaceuticals; it is currently available only to participants in clinical studies. Studies were included in our analysis if they involved a head-to-head comparison of one or more different formulations. Overall, data were available from 15 different studies that randomised a total of 3765 participants.
Our results showed that in terms of glycaemic control, there were no significant differences between the different formulations. This alone is valuable evidence for the clinician who seeks to optimise the management of diabetes. However, metformin’s use now extends beyond diabetes care, making evaluation of differences in other parameters increasingly important. For example metformin XR was significantly more effective than IR in lowering low-density lipoprotein (LDL) cholesterol, which may be an important benefit in patients with significant obesity or other metabolic disease.
A key influence on real-world efficacy of any drug is tolerability. A drug with few unpleasant side effects is much more likely to produce good results outside of the highly regulated world of clinical trials, because patients are more likely to actually take it. An important element of our study was therefore to assess whether there are significant differences between metformin formulations in terms of tolerability, in particular the likelihood of experiencing gastrointestinal side effects. Gastrointestinal effects are a major barrier to tolerating metformin therapy, affecting 20–30% of patients taking metformin. Around 1 in 20 patients will experience effects bothersome enough for them to stop taking metformin altogether [3]. Longer-acting formulations are often recommended to patients who experience gastrointestinal side effects with immediate release metformin, based on the idea that there may be fewer unwanted effects with these preparations. Our data support the idea that metformin DR is associated with a significantly reduced likelihood of gastrointestinal side effects than metformin IR (OR 0.45, 95% CI 0.30–0.66, p < 0.0001). When metformin XR is compared to metformin IR, there is also a reduction in the likelihood of adverse gastrointestinal side effects, although this does not reach our pre-specified threshold for statistical significance.
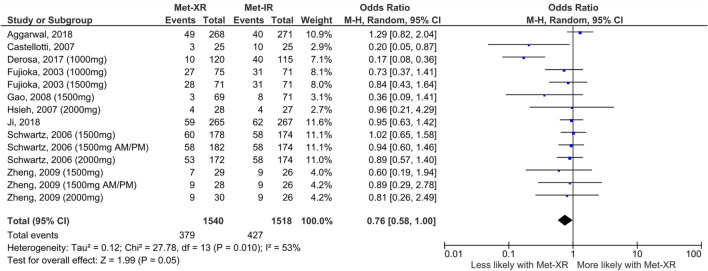
Shortly after the online publication of our study, further data regarding the likelihood of gastrointestinal side effects with metformin XR versus IR were made available to us by the data holders of the 2003 study by Fujioka et al. [4]. This study was included in the original review, but only a subset of the full data regarding frequency of gastrointestinal side effects had been published in the original manuscript. We had previously requested the full data directly from the authors but were not successful in contacting them, perhaps unsurprisingly 17 years after the original publication. Re-analysing our data to include the previously unpublished full results of the study by Fujioka et al. edges the meta-analysis result further towards significance, but it remains just below the pre-specified threshold and therefore there is no change to the original conclusions of our meta-analysis. It is entirely possible that if even more data were available from other studies, there might be a significantly reduced likelihood of gastrointestinal side effects with metformin XR compared IR; however, this is not possible to determine on the basis of available data.
These data are an interesting addition to our study and underscore the surprising paucity of studies that have specifically addressed comparisons between metformin formulations. Given that metformin is among the top five most commonly prescribed drugs in the USA [5], there is a clear research gap regarding both the relative benefits of each available metformin formulation and the health economic implications of prescribing each. Our meta-analysis goes some way towards helping to clarify the comparisons and we hope will thus be useful to both prescribers and consumers of metformin (Fig. 1).
Fig. 1.
The effect of metformin formulation upon gastrointestinal side effects comparing met-XR versus met-IR. This is an updated version of Fig. 5a within our previous publication [2], now including additional data from the study by Fujioka et al. [4] that has not previously been published but was subsequently made available outside of our pre-specified search strategy by kind permission of Merck Healthcare KGaA. The addition of this extra data does not alter the substantive conclusions of the meta-analysis previously presented. There is a lower likelihood of experiencing gastrointestinal side effects with metformin XR than IR (OR 0.76, CI 0.58–1.00), but this does not reach the pre-specified threshold for significance (p = 0.05)
Acknowledgements
Funding
JT-A and SO are both funded by the British Heart Foundation (RG/17/12/33167) and the Medical Research Council (UK) (MC_UU_00014/4). A further Medical Research Council grant (MR/T016701/1) also funded JT-A. RR acknowledges the support of the British Heart Foundation (RE/18/5/34216). CA is supported by an Action Medical Research grant (UK) (GN2788) and by a Medical Research Council New Investigator Grant (UK) (MR/T016701/1). No funding was received for the publication of this article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authors’ Contributions
JT-A and IG were responsible for data mining and analysis, statistical analysis and drafting of the manuscript. SO and RR were responsible for the study concept and design and drafting of the manuscript. CA was responsible for statistical analysis, study concept and design and drafting of the manuscript.
Prior Presentation
This editorial is based on work that has been previously presented/published [2].
Disclosures
Catherine Aiken, Jane Tarry-Adkins, Imogen Grant, Rebecca Reynolds and Susan Ozanne have nothing to disclose.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors and therefore ethical approval was not required.
Data Availability
All data generated or analyzed during this study are included in our previously published article/as supplementary information files [2].
Footnotes
This Editorial is based on a previous published article (Tarry-Adkins JL et al. Efficacy and Side Effect Profile of Different Formulations of Metformin: A Systematic Review and Meta-Analysis. Diabetes Ther. 2021)
References
- 1.NHS Digital. Increase in prescriptions for diabetes exceeds rise in overall prescribing. https://digital.nhs.uk/news-and-events/news-archive/2017-news-archive/increase-in-prescriptions-for-diabetes-exceeds-rise-in-overall-prescribing. Accessed 1 July 2021.
- 2.Tarry-Adkins JL, Grant ID, Ozanne SE, Reynolds RM, Aiken CE. Efficacy and side effect profile of different formulations of metformin: a systematic review and meta-analysis. Diabetes Ther. 2021 doi: 10.1007/s13300-021-01058-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dujic T, Zhou K, Donnelly LA, Tavendale R, Palmer CN, Pearson ER. Association of organic cation transporter 1 with intolerance to metformin in type 2 diabetes: a GoDARTS study. Diabetes. 2015;64(5):1786–1793. doi: 10.2337/db14-1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fujioka K, Pans M, Joyal S. Glycemic control in patients with type 2 diabetes mellitus switched from twice-daily immediate-release metformin to a once-daily extended-release formulation. Clin Ther. 2003;25(2):515–529. doi: 10.1016/S0149-2918(03)80093-0. [DOI] [PubMed] [Google Scholar]
- 5.Agency for Healthcare Research and Quality Rockville MD. Medical expenditure panel survey (MEPS). 2018. https://www.ahqr.gov/data/meps.html. Accessed 1 July 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in our previously published article/as supplementary information files [2].