Abstract
In this study, we prepared hydrocolloid gels in which flaxseed gum (FSG), konjac glucomannan (KGM), and agar (AG) were blended in different ratios for use as a viscoelastic food. The prepared hydrogels’ physicochemical properties were analyzed concerning their water solubility index (WSI), swelling power (SL), frequency sweep results, and microstructures. As the FSG ratio decreased, the WSI value of the compound gel tended to increase. However, it showed a tendency to have a relatively high SP value and a low tan δ value according to a specific KGM/FSG/AG mixing ratios (8:2:1.5 and 6:4:1.5). Through microstructure analysis, the FKA821.5 sample showed a relatively small, monodispersed gel building structure, correlated with the rheological results. In conclusion, the FKA821.5 gel was determined to have good water retention capacity and high structural strength. These results are expected to increase the applicability of FSG-based gelling agents in the food industry.
Keywords: Flaxseed gum (FSG), Konjac glucomannan (KGM), Agar (AG), Water retention, Gel strength, Microstructure
Introduction
Many natural polymers, including mucilages, have been successfully used in food applications due to their health benefits, biodegradability, suitable mechanical properties, and non-toxicity (Valencia et al., 2019). Natural mucilages are composed of polysaccharides with numerous sugar units, which are linked together to form huge polymers with large molecules. Some naturally derived mucilages can form a three-dimensional interconnected network known as a “gel” (Rana et al., 2015).
Soluble flaxseed gum (FSG), which is abundant in the hull of the flaxseed (Linum usitatissimum L.), is a heterogeneous polysaccharide composed of arabinose, xylose, glucose, rhamnose, galactose, fucose, and galacturonic acid (Fedeniuk and Biliaderis, 1994). Flaxseed gum is a hydrocolloid with excellent water retention ability due to its marked swelling capacity and high adhesiveness in aqueous solution (Chen et al. 2004a; 2004b; Erskine and Jones, 1957; Fedeniuk and Biliaderis, 1994). FSG can also be easily extracted from the fiber-rich whole seed by soaking in solvents such as water and ethanol (Cui et al., 1994; Diederichsen et al., 2006; Naran et al., 2008; Warr et al., 2003; Warrand et al., 2005a;2005b). However, FSG is limited in its use as a gelling agent in food because of its weak gelling properties (Chen et al., 2006; Cui and Mazza, 1996).
Konjac glucomannan (KGM), a neutral polysaccharide derived from the tuber of Amorphophallus konjac c. Koch, consists of β‐1,4‐linked D‐mannose and D‐glucose in a ratio of 1.6:1 (Kato et al., 1970). KGM is a thermally stable gelling agent, and its gelation mechanism is closely related to the formation of the network structure of junction zones by hydrogen bonding. Recently, a number of studies have investigated the synergistic effect of the KGM gelling ability by its interaction with many types of polysaccharides, including κ‐carrageenan, xanthan, and gellan gum (Brenner et al., 2015; Engle et al., 2012; Fitzpatrick et al., 2013; Fitzsimons et al., 2008; Harding et al., 2011; Ma et al., 2019; Penroj et al., 2005).
Agar (AG), a gel-forming polysaccharide, can be extracted from red algae with linear polymers of alternating units of 1,3-linked β-D-galactopyranose and 1,4-linked 3,6-anhydro-α-L-galactopyranose (Araki, 1956; Arnott et al., 1974; Dea et al., 1972). The gelation of agar occurs as a result of the agarose content produced by hydrogen bonding, and its mechanism is associated with a double-coil helix transition (Armisen et al., 2000). Agar is produced by the linking of bundles of right-hand double helixes, which result from a double-coil helix transition (Norton et al., 1986; Schafer and Stevens, 1995). The interaction of these helixes occurs at junction zones, which can form a three-dimensional network (Arnott et al., 1974). The resulting AG has properties such as high rigidity, high water retention, and structural stability. Therefore, the rheological properties of AG can be adjusted, as AG is easily mixed with other carbohydrates and proteins (Barrangou et al., 2006; Ng and McKinley, 2008; Norziah et al., 2006; Rhein-Knudsen et al., 2017; Rocha et al., 2014; Sasaki and Kohyama, 2011).
In this study, as a potential strategy for the use of FSG as a food replacement, a simple FSG extraction process was proposed, and hydrogels were prepared by mixing FSG with KGM and AG. Then, the hydrogels that were mixed at different FSG, KGM, and AG ratios were tested to determine their WSI and SP values, viscoelastic properties, and microstructures. The results of this study are expected to expand the applicability of FSG as a gelling agent in the food industry.
Materials and methods
Materials
Flaxseed was purchased from Handsherb Co. (Yeongcheon, Korea). KGM powder was purchased from Haenafood Co. (Seoul, Korea). Agar powder was obtained from Woori-ga Co. (Yangju, Korea).
Extraction of flaxseed gum
The separation process was optimized to obtain gum material from raw flaxseed according to the method described by (Cui et al., 1994), with some modifications. Choosing the mechanical extraction method of the gum solution from flaxseed is of great importance in technical issues such as the extraction of other substances (Anna, 2012). Therefore, we finally have decided to use hot water extraction from whole seeds. As shown in Fig. 1, after washing the flaxseeds with deionized water to remove any dirt, the flaxseeds (3.3%, w/w) were added to hot distilled water (90 °C) and boiled continuously while gently stirring for 10 min. The gum solution from the heat-treated mixture was then filtered through a sieve with a 14 mesh Tyler Screen (NAVIMRO, Korea) and precipitated in three volumes of 95% ethanol. The sedimented gum solution (pH 7.0) was obtained by centrifugation and dried at 65 °C overnight. The dried gum was then pulverized into fine particles (0.1 mm) with a mixer. The FSG yield was determined using the following Equation:
| 1 |
where “Dried mucilage” represents the total mass of the water-soluble portion of FSG in g (dry weight) after drying, and “Seed” is the mass of the FSG in g (dry weight). The FSG separation yield was approximately 15%.
Fig. 1.
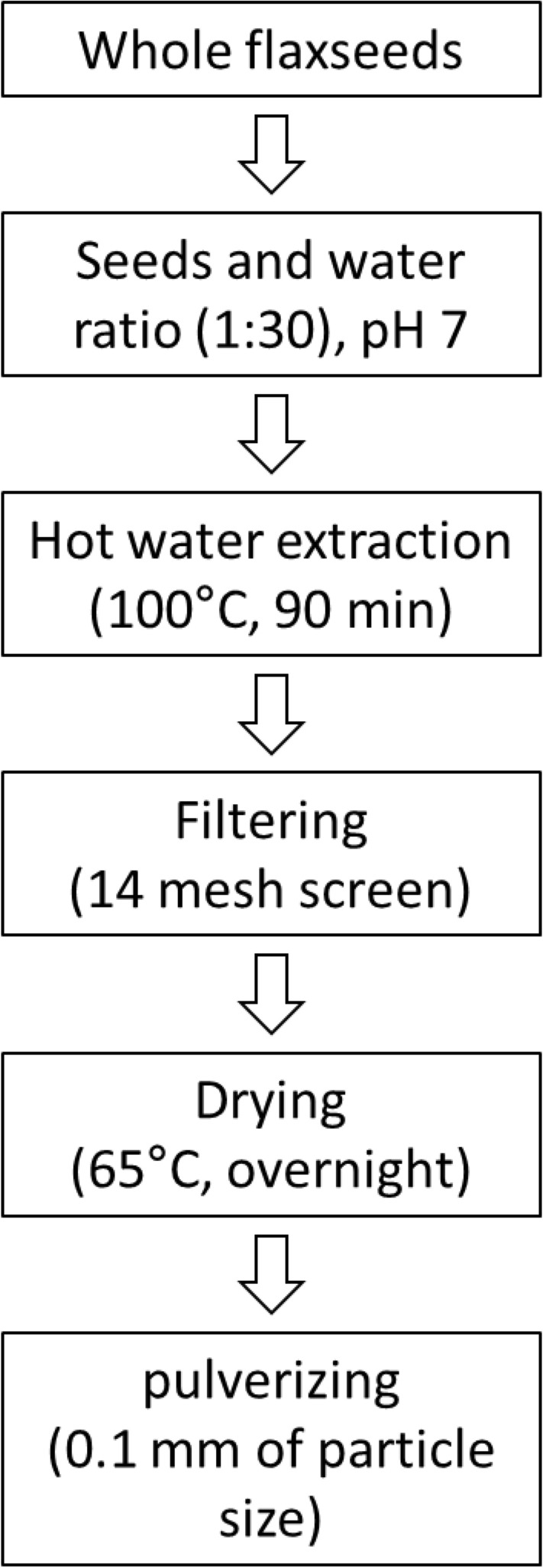
Simple extraction process of flaxseed gum
Component analysis in the flaxseed gum solution
Component analysis including carbohydrate, crude protein, crude oil, ash, and dietary fiber of flaxseed gum solution was carried out by SGS Korea Co. (Seoul, Korea). The sample used for the analysis was prepared by the aforementioned gum extraction method.
Preparation of the hydrogel samples
Mixed polysaccharide solutions containing FSG, KGM, and AG were prepared at total concentrations of 2.1–2.2 g/100 mL. The different ratios of FSG/KGM/AG included: 10:0:0.5, 8:2:0.5, 6:4:0.5, 4:6:0.5, 2:8:0.5, 0:10:0.5, 10:0:0.5, 8:2:0.5, 6:4:0.5, 4:6:0.5, 2:8:0.5, 0:10:0.5, 10:0:0.5, 8:2:0.5, 6:4:0.5, 4:6:0.5, 2:8:0.5, and 0:10:1.5, which were denoted as FKA1000.5, FKA820.5, FKA640.5, FKA460.5, FKA280.5, FKA0100.5, FKA1001, FKA821, FKA641, FKA461, FKA281, FKA0101, FKA1001.5, FKA821.5, FKA641.5, FKA461.5, FKA281.5, and FKA0101.5, respectively. The hydrogel solutions with a constant concentration of 1.2 g/50.0 mL were prepared by thoroughly dissolving FSG powder, KGM powder, and AG powder in distilled water by stirring at 95 °C. The gel samples were then cooled to room temperature and refrigerated at 4 °C overnight in a silicone mold (2.2 cm-wide × 2.2 cm-long × 2.5 cm-high) having a volume of about 12 mL. The compound gels were kept at room temperature for 2 h before use in the experiment.
Water solubility index and swelling power
The WSI and SP values of the blended gel samples were obtained using the procedure described by Jiang et al. (2019) with slight modification. Each sample (0.115 g) was mixed with distilled water (30 mL) in sealed centrifuge tubes, and incubated in a water bath at 90 °C for 30 min. Each sample was then centrifuged at 20,000 g for 30 min. The supernatant was then transferred to a Petri dish and heated in an air oven at 65 °C overnight. The WSI and SP values were calculated using Eqs. (2) and (3), respectively.
| 2 |
| 3 |
In Eq. 2, W1 and W2 are the weight of an empty Petri dish and the weight of the Petri dish containing the dried residue, respectively. In Eq. (3), W3 and W4 are the weight of the tube and the weight of the tube containing the sediment, respectively.
Frequency sweep tests
Analysis of the rheological properties of the samples was performed using a HAAKE RheoStress 1 Rheometer (Thermo Fisher Scientific, Waltham, MA, USA). The shear stress amplitude was fixed at 1 Pa. Oscillatory frequency sweeps were performed from 0.01 Hz to 1 Hz. The storage modulus (G′), loss modulus (G″), and tan δ value, which is equal to G″/G′, were determined as functions of the frequency at 25 °C.
Scanning electron microscopy (SEM) observation
The microstructural morphologies of the hydrogels were examined using a JEOL-5410 LV scanning electron microscope (JEOL, Tokyo, Japan) for which the micrograph magnifications were × 300.
Statistical analyses
The experimental data analysis was performed using SPSS Statistics 25 software. One-way analysis of variance, least significant difference and Tukey’s post-hoc tests were used to evaluate the data. A statistical significance of p < 0.05 was accepted. All experiments were conducted in triplicate. The results are expressed as the mean ± standard deviation (SD).
Results and discussion
Nutritional composition of flaxseed gum
Table 1 shows the nutrient composition of SFG obtained from whole flaxseed. The crude protein content (10.92% ± 0.6%) was lower than that reported for whole flaxseed (≈18%) (Edel et al., 2015). The crude fat content was significantly lower than that accumulated by the reported flaxseed (≈42%) (1.08% ± 0.2%). Total sugar (83.46% ± 1.5%) and water-soluble dietary fiber (40.46% ± 0.7%) were higher than that reported for whole flaxseed, which had approximately 28.9% and 27.3%, respectively. This content was expected to vary depending on the cultivar, moisture content, hull removal procedure, and gum extraction method.
Table 1.
Composition of representative nutrients of the extracted flaxseed mucilage
| Compound | Dry basis (%) |
|---|---|
| Carbohydrate | 83.46 ± 1.5 |
| Crude protein | 10.92 ± 0.6 |
| Crude fat | 1.08 ± 0.2 |
| Ash | 2.08 ± 0.1 |
| Dietary fiber | 40.46 ± 0.7 |
Morphology
Figure 2 shows the morphologies of the hydrocolloid gels composed of FSG, KGM, and AG in different ratios. As the proportion of agar is gradually increased, the gels become able to maintain a cuboid shape. The sample groups with AG ratios of 1.0 and 1.5 maintained similar cubic shapes (Fig. 2G–L). However, the sample groups with AG ratios of 0 and 0.5 could not maintain their structure and collapse (Fig. 2A–F). In addition, as the ratio of FSG increased, the compound gel did not form a rigid structure, and it showed a tendency to collapse in the sample group with AG ratios of 0 and 0.5 (Fig. 2A–G). Taken together, we found that AG is a major factor that has an influence on the formation of the rigid gel structure among the polysaccharides since all sample groups with an AG ratio of 1.0 or higher formed and maintained a constant structure. In addition, we found that FSG had the less effect on the formation and maintenance of a rigid structure than KGM, in that the samples with a higher FSG ratio in a fixed AG ratio showed the formation of a more complex structure of the compound gel. Based on these results, we prepared gels with different ratios of FSG and KGM at AG ratios of 0.5, 1.0, and 1.5 to perform rheological and microstructural analyses of the compound gels with physical structure differences, and we investigated their characteristics.
Fig. 2.
Photos of hydrocolloid gels composed of different ratios of FSG, KGM, and AG [(A) FKA280, (B) FKA550, (C) 20, (D) FKA280.5, (E) FKA550.5, (F) FKA820.5, (G) FKA281, (H) FKA551, (I) FKA821, (J) FKA281.5, (K) FKA551.5, (L) FKA821.5]
Water solubility index and swelling power
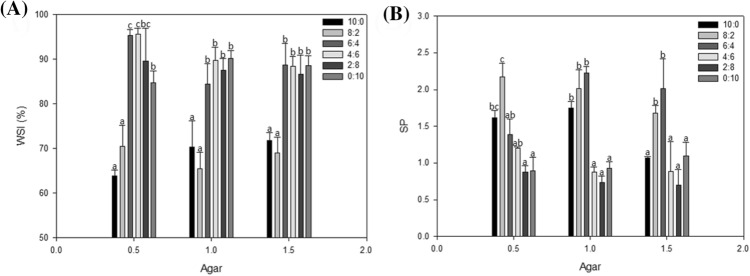
To analyze the physicochemical properties of the compound gels containing different ratios of FSG, KGM, and AG, we determined the WSI and SP values, the results of which are shown in Fig. 3. According to Eq. (2), WSI is the percent concentration of the dry weight of the supernatant to the total dry weight of the test sample. In other words, the WSI value indicates how soluble the gel sample is in water. All of the gel samples with respective FSG and KGM ratios of 10:0 and 8:2 (FKA1000.5, FKA1001, FKA1001.5, FKA820.5, FKA821, and FKA821.5) exhibited significantly (p < 0.05) lower water solubility than the other samples (Fig. 3A). In addition, the test gels tended to have lower solubility in water as the proportion of FSG was increased. These results might be explained by the difference in the composition of the hydroxyl groups in KGM and FSG (Connolly et al., 2010). The hydroxyl groups of D-mannose and D-glucose in KGM have very strong polarity, so they can form relatively stronger hydrogen bonds with water molecules (Connolly et al., 2010; Devaraj et al., 2019). On the other hand, FGS, which contains 75% arabinoxylans and 25% rhamnogalacturonans having a carboxyl group, was found to show relatively low solubility (Guo et al., 2019).
Fig. 3.
Physicochemical properties of the hydrogels: (A) water solubility index and (B) swelling power. Data of different alphabets were different with statistical significant (p < 0.05)
The SP, which includes the WSI, is obtained using Eq. (3), in which SP is the reciprocal of the percent concentration of the dry weight of the sediment relative to the weight of the sediment. In other words, SP indicates how much gel can combine with water without being able to be removed by physical means. As shown in Fig. 3B, the samples (FKA821, FKA641, FKA821.5, and KFA641.5) with respective FSG and KGM ratios of 8:2 and 6:4 at AG ratios of 1 and 1.5 presented high SP values relative to the other samples. At a 0.5 AG ratio, the 8: 2 gel sample (FKA820.5) showed the highest SP value. The swelling ability of gels is known to be related to factors such as the polymer–solvent interaction, network density, and structure (Sangnark and Noomhorm, 2003). The blending of KGM, FSG, and AG is also known to form a hydrocolloid gel by interlinking polymer chains (Burey et al., 2008), and in this study, we found that the relatively high SP value appeared according to the specific KGM/FSG/AG mixing ratio (FKA820.5, FKA821, FKA641, FKA821.5, and KFA641.5).
Oscillatory rheological behavior
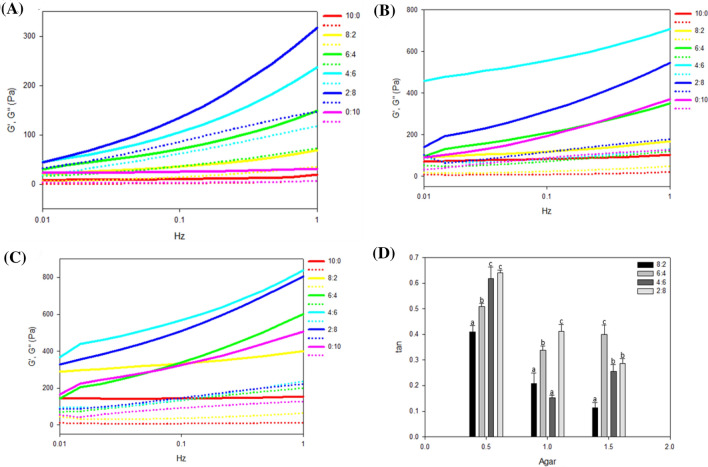
Generally, dynamic gel rheology exhibits a more direct correlation with microstructures than static rheology because gel materials can be measured almost in the at-rest state without disturbing the basic structures of the hydrocolloid gel (Cross, 1965). Figure 4 show the viscoelastic behavior of the compound gel solutions based on the results of frequency sweep tests. The frequency spectrum of the measured system indicates the characteristics of the microstructure of the gel system (Russel et al., 1989), the results of which are discussed in the next section with respect to their correlation with the actual microstructural images obtained by SEM. Figure 4 shows the frequency sweep curves of the compound gels, which reveal that all the gel samples had a higher storage modulus (G′) than loss modulus (G″). This means that the elastic component was predominant relative to the viscous component in the system. As the AG ratio increased from 0.5 to 1.5, the overall G′ value tended to increase, which is considered to be due to the AG’s tendency to form a solid structure and thus affect the gel system. In addition, we examined the rheological properties of the gel according to the ratio of FSG. In the test groups with an AG ratio of 0.5, both the G′ and G″ values tended to increase with decreases in the FSG ratio (Fig. 4A). In the test groups with AG ratios of 1 and 1.5, the samples with FSG and KGM ratios of 4:6 and 2:8 (FKA461, FKA281, FKA461.5, and FKA281.5) showed relatively high G′ values (p < 0.05), which differed significantly from the G′ values of the other samples (Fig. 4B, C). The value of the loss tangent (tan δ), which is equal to G″/G′, reflects the dynamic viscoelastic behavior, and Fig. 4D shows the tan δ value of all of the samples at 0.1 Hz. In principle, when the tan δ value is less than 1, the system tends to exhibit elastic behavior rather than viscous behavior. Given the oscillatory rheological behavior of all of the test samples that, were found to have tan δ values lower than 1, they could all be gelled to form a solid structure. In the test groups in which the AG ratio was 0.5, the tan δ value increased as the FSG ratio decreased. However, when the AG ratios were 1.0 and 1.5, the tan δ values were low in the gel samples with FSG and KGM ratios of 4:6 (FKA 461) and 8:2 (FKA 821.5). These findings indicate that this gel can exhibit relatively high elasticity given the appropriate blending ratios of FSG, KGM, and AG.
Fig. 4.
Frequency sweep curves of FKA hydrogels. Storage modulus (G′) and loss modulus (G″) of FKA hydrogels at AG ratios of (A) 0.5, (B) 1, and (C) 1.5. The frequency ranged from 0.01 Hz to 1 Hz. (D) Loss tangent (tan δ) of FSG/KGM/AG compound gels at 0.1 Hz. Data of different alphabets in the same column were different with statistical significant (p < 0.05). The solid lines and dashed lines in (A)–(C) meant G′ and G″, respectively
Scanning electron microscopy
SEM imaging can be used to observe the three-dimensional local microstructure of a hydrogel. Based on our SEM observations, we confirmed the synergistic interaction of FSG, KGM, and AG. The elasticity of the gel is related closely to the formation of the flexible network structure of the polymer (Cao and Mezzenga, 2020). As shown in Fig. 5, the microstructure of FKA821.5 had a smaller pore size and a denser structure than the other samples. In addition, this gel sample showed relatively uniform gel building blocks connected to junction zones, which are an association of polymer molecules over a portion of their lengths (BeMiller, 2008). These results, which are consistent with the results of the physicochemical analysis, suggest that FKA821.5 had high elasticity and water holding capacity. In addition, as the ratio of KGM was increased under the proposed conditions, the arrangement of the hydrogel microstructure appeared irregular. In conclusion, FKA821.5 at the proposed ratios was found to have a synergistic effect that yields solid properties, such as rigid and elastic structures.
Fig. 5.

SEM images of the microstructures of hydrogel samples: (A) FKA1001.5, (B) FKA821.5, (C) FKA281.5, and (D) FKA0101.5. Samples are shown at 300× magnification
Acknowledgements
This research was supported by the Technology Development Program (S2777815), funded by the Ministry of SMEs and Startups (MSS, Korea).
Author Contributions
JH Conceptualization; Writing—original draft; Formal analysis; Writing—review and editing. JY Methodology; Writing—review and editing. JK Writing—review and editing. YJC Conceptualization; Writing—review and editing.
Declarations
Conflict of interest
Declare any conflicts of interest, or state that there are none to declare.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jisoo Yang and Junghoon Kim contributed equally to the work.
Contributor Information
Young Jin Choi, Email: choiyj@snu.ac.kr.
Jungwoo Hahn, Email: wjddnek1@snu.ac.kr.
References
- Andrea LE, Michel A, Grant NP. Stability of bioactives in flaxseed and flaxseed-fortified foods. Food Research International. 2015;77(2):140–155. [Google Scholar]
- Anna Z. Laws of flaxseed mucilage extraction. Food Hydrocolloids. 2012;26(1):197–204. doi: 10.1016/j.foodhyd.2011.04.022. [DOI] [Google Scholar]
- Araki C. Structure of the agarose constituent of agar-agar. Bulletin of the Chemical Society of Japan. 1956;29:543–544. doi: 10.1246/bcsj.29.543. [DOI] [Google Scholar]
- Armisen R, Galatas F, Phillips G, Willians P. Handbook of hydrocolloids. UK: Woodhead Publishing; 2000. [Google Scholar]
- Arnott S, Fulmer A, Scott W, Dea I, Moorhouse R, Rees D. The agarose double helix and its function in agarose gel structure. Journal of Molecular Biology. 1974;90:269–284. doi: 10.1016/0022-2836(74)90372-6. [DOI] [PubMed] [Google Scholar]
- Barrangou LM, Daubert CR, Foegeding EA. Textural properties of agarose gels. I. Rheological and Fracture Properties. Food Hydrocolloids. 2006;20:184–195. doi: 10.1016/j.foodhyd.2005.02.019. [DOI] [Google Scholar]
- Bemiller JN. Gums and related polysaccharides. glyc: 1513 (2008)
- Brenner T, Tuvikene R, Fang Y, Matsukawa S, Nishinari K. Rheology of highly elastic iota-carrageenan/kappa-carrageenan/xanthan/konjac glucomannan gels. Food Hydrocolloids. 2015;44:136–144. doi: 10.1016/j.foodhyd.2014.09.016. [DOI] [Google Scholar]
- Burey P, Bhandari B, Howes T, Gidley M. Hydrocolloid gel particles: formation, characterization, and application. Critical Reviews in Food Science and Nutrition. 2008;48:361–377. doi: 10.1080/10408390701347801. [DOI] [PubMed] [Google Scholar]
- Cao Y, Mezzenga R. Design principles of food gels. Nature Food. 2020;1:106–118. doi: 10.1038/s43016-019-0009-x. [DOI] [PubMed] [Google Scholar]
- Chen H, Xu S, Wang Z. Separation and purification of acidic polysaccharides and neutral polysaccharides in flaxseed gum. Food and Fermentation Industries. 2004;1:96–100. [Google Scholar]
- Chen H-H, Xu S-Y, Wang Z. Rheological properties of flaxseed gum. Journal of Wuxi University of Light Industry 1 (2004)
- Chen H-H, Xu S-Y, Wang Z. Gelation properties of flaxseed gum. Journal of Food Engineering. 2006;77:295–303. doi: 10.1016/j.jfoodeng.2005.06.033. [DOI] [Google Scholar]
- Connolly ML, Lovegrove JA, Tuohy KM. Konjac glucomannan hydrolysate beneficially modulates bacterial composition and activity within the faecal microbiota. Journal of Functional Foods. 2010;2:219–224. doi: 10.1016/j.jff.2010.05.001. [DOI] [Google Scholar]
- Cross MM. Rheology of non-Newtonian fluids: a new flow equation for pseudoplastic systems. Journal of Colloid Science. 1965;20:417–437. doi: 10.1016/0095-8522(65)90022-X. [DOI] [Google Scholar]
- Cui W, Mazza G. Physicochemical characteristics of flaxseed gum. Food Research International. 1996;29:397–402. doi: 10.1016/0963-9969(96)00005-1. [DOI] [Google Scholar]
- Cui W, Mazza G, Biliaderis C. Chemical structure, molecular size distributions, and rheological properties of flaxseed gum. Journal of Agricultural and Food Chemistry. 1994;42:1891–1895. doi: 10.1021/jf00045a012. [DOI] [Google Scholar]
- Dea I, McKinnon A, Rees D. Tertiary and quaternary structure in aqueous polysaccharide systems which model cell wall cohesion: reversible changes in conformation and association of agarose, carrageenan and galactomannans. Journal of Molecular Biology. 1972;68:153–172. doi: 10.1016/0022-2836(72)90270-7. [DOI] [PubMed] [Google Scholar]
- Devaraj RD, Reddy CK, Xu B. Health-promoting effects of konjac glucomannan and its practical applications: A critical review. International Journal of Biological Macromolecules. 2019;126:273–281. doi: 10.1016/j.ijbiomac.2018.12.203. [DOI] [PubMed] [Google Scholar]
- Diederichsen A, Raney JP, Duguid SD. Variation of mucilage in flax seed and its relationship with other seed characters. Crop Science. 2006;46:365–371. doi: 10.2135/cropsci2005.0146. [DOI] [Google Scholar]
- Edel AL, Rodriguez-Leyva D, Maddaford TG, Caligiuri SPB, Austria JA, Weighell W, Guzman R, Aliani M, Pierce GN. Dietary flaxseed independently lowers circulating cholesterol and lowers It beyond the effects of cholesterol-lowering medications alone in patients with peripheral artery disease. The Journal of Nutrition. 145(4):749-757 (2015) [DOI] [PubMed]
- Engle KM, Mei T-S, Wasa M, Yu J-Q. Weak coordination as a powerful means for developing broadly useful C-H functionalization reactions. Accounts of Chemical Research. 2012;45:788–802. doi: 10.1021/ar200185g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erskine A, Jones J. The structure of linseed mucilage. Part I. Canadian Journal of Chemistry. 1957;35:1174–1182. doi: 10.1139/v57-158. [DOI] [Google Scholar]
- Fedeniuk RW, Biliaderis CG. Composition and physicochemical properties of linseed (Linum usitatissimum L.) mucilage. Journal of Agricultural and Food Chemistry. 1994;42:240–247. doi: 10.1021/jf00038a003. [DOI] [Google Scholar]
- Fitzpatrick P, Meadows J, Ratcliffe I, Williams PA. Control of the properties of xanthan/glucomannan mixed gels by varying xanthan fine structure. Carbohydrate Polymers. 2013;92:1018–1025. doi: 10.1016/j.carbpol.2012.10.049. [DOI] [PubMed] [Google Scholar]
- Fitzsimons SM, Tobin JT, Morris ER. Synergistic binding of konjac glucomannan to xanthan on mixing at room temperature. Food Hydrocolloids. 2008;22:36–46. doi: 10.1016/j.foodhyd.2007.01.023. [DOI] [Google Scholar]
- Guo Q, Du J, Jiang Y, Goff HD, Cui SW. Pectic polysaccharides from hawthorn: Physicochemical and partial structural characterization. Food Hydrocolloids. 2019;90:146–153. doi: 10.1016/j.foodhyd.2018.10.011. [DOI] [Google Scholar]
- Harding SE, Smith IH, Lawson CJ, Gahler RJ, Wood S. Studies on macromolecular interactions in ternary mixtures of konjac glucomannan, xanthan gum and sodium alginate. Carbohydrate Polymers. 2011;83:329–338. doi: 10.1016/j.carbpol.2010.06.035. [DOI] [Google Scholar]
- Jiang Y, Reddy CK, Huang K, Chen L, Xu B. Hydrocolloidal properties of flaxseed gum/konjac glucomannan compound gel. International Journal of Biological Macromolecules. 2019;133:1156–1163. doi: 10.1016/j.ijbiomac.2019.04.187. [DOI] [PubMed] [Google Scholar]
- Kato K, Watanabe T, Matsuda K. Studies on the chemical structure of konjac mannan: Part II. Isolation and characterization of oligosaccharides from the enzymatic hydrolyzate of the mannan. Agricultural and Biological Chemistry. 1970;34:532–539. [Google Scholar]
- Ma S, Zhu P, Wang M, Wang F, Wang N. Effect of konjac glucomannan with different molecular weights on physicochemical properties of corn starch. Food Hydrocolloids. 2019;96:663–670. doi: 10.1016/j.foodhyd.2019.06.014. [DOI] [Google Scholar]
- Naran R, Chen G, Carpita NC. Novel rhamnogalacturonan I and arabinoxylan polysaccharides of flax seed mucilage. Plant Physiology. 2008;148:132–141. doi: 10.1104/pp.108.123513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng TS, McKinley GH. Power law gels at finite strains: The nonlinear rheology of gluten gels. Journal of Rheology. 2008;52:417–449. doi: 10.1122/1.2828018. [DOI] [Google Scholar]
- Norton I, Goodall D, Austen K, Morris E, Rees D. Dynamics of molecular organization in agarose sulphate. Biopolymers. 1986;25(6):1009–1029. doi: 10.1002/bip.360250604. [DOI] [Google Scholar]
- Norziah M, Foo S, Karim AA. Rheological studies on mixtures of agar (Gracilaria changii) and κ-carrageenan. Food Hydrocolloids. 2006;20:204–217. doi: 10.1016/j.foodhyd.2005.03.020. [DOI] [Google Scholar]
- Penroj P, Mitchell J, Hill S, Ganjanagunchorn W. Effect of konjac glucomannan deacetylation on the properties of gels formed from mixtures of kappa carrageenan and konjac glucomannan. Carbohydrate Polymers. 2005;59:367–376. doi: 10.1016/j.carbpol.2004.10.007. [DOI] [Google Scholar]
- Rana V, Kamboj S, Sharma R, Singh K. Modification of gums: synthesis techniques and pharmaceutical benefits. Handbook of Polymers for Pharmaceutical Technologies: Biodegradable Polymers. 2015;3:299–364. doi: 10.1002/9781119041450.ch10. [DOI] [Google Scholar]
- Rhein-Knudsen N, Ale MT, Ajalloueian F, Yu L, Meyer AS. Rheological properties of agar and carrageenan from Ghanaian red seaweeds. Food Hydrocolloids. 2017;63:50–58. doi: 10.1016/j.foodhyd.2016.08.023. [DOI] [Google Scholar]
- Rocha CM, Souza HK, Magalhães NF, Andrade CT, Gonçalves MP. Rheological and structural characterization of agar/whey proteins insoluble complexes. Carbohydrate Polymers. 2014;110:345–353. doi: 10.1016/j.carbpol.2014.04.015. [DOI] [PubMed] [Google Scholar]
- Russel W, Saville D, Schowalter W. Rheology. Colloidal Dispersions, Cambridge University Press, Cambridge. 456–506 (1989)
- Sangnark A, Noomhorm A. Effect of particle sizes on functional properties of dietary fibre prepared from sugarcane bagasse. Food Chemistry. 2003;80:221–229. doi: 10.1016/S0308-8146(02)00257-1. [DOI] [Google Scholar]
- Sasaki T, Kohyama K. Effect of non-starch polysaccharides on the in vitro digestibility and rheological properties of rice starch gel. Food Chemistry. 2011;127:541–546. doi: 10.1016/j.foodchem.2011.01.038. [DOI] [PubMed] [Google Scholar]
- Schafer SE, Stevens ES. A reexamination of the double-helix model for agarose gels using optical rotation. Biopolymers. 1995;36(1):103–108. doi: 10.1002/bip.360360109. [DOI] [Google Scholar]
- Valencia GA, Zare EN, Makvandi P, Gutiérrez TJ. Self-Assembled Carbohydrate Polymers for Food Applications: A Review. Comprehensive Reviews in Food Science and Food Safety. 2019;18:2009–2024. doi: 10.1111/1541-4337.12499. [DOI] [PubMed] [Google Scholar]
- Warr J, Michaud P, Picton L, Muller G, Courtois B, Ralainirina R, Courtois J. Large-scale purification of water-soluble polysaccharides from flaxseed mucilage, and isolation of a new anionic polymer. Chromatographia. 2003;58:331–335. [Google Scholar]
- Warrand J, Michaud P, Picton L, Muller G, Courtois B, Ralainirina R. Courtois Journal of Structural investigations of the neutral polysaccharide of Linum usitatissimum L. seeds mucilage. International Journal of Biological Macromolecules. 2005;35:121–125. doi: 10.1016/j.ijbiomac.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Warrand J, Michaud P, Picton L, Muller G, Courtois B, Ralainirina R, Courtois J. Contributions of intermolecular interactions between constitutive arabinoxylans to the flaxseeds mucilage properties. Biomacromolecules. 2005;6:1871–1876. doi: 10.1021/bm049249p. [DOI] [PubMed] [Google Scholar]