Abstract
Background
Planktonic bacteria can be inadvertently introduced during breast surgery procedures, which are hypothesized to lead to complications such as infection, capsular contracture, breast implant-associated anaplastic large cell lymphoma, and a prolonged local inflammatory response. The utilization of antimicrobial solutions such as triple antibiotic solution (TAB) and/or 10% povidone-iodine (PI) in breast pocket irrigation or implant soaking has been proposed to reduce planktonic bacterial attachment and potential complications.
Objectives
A series of in vitro assessments were performed to evaluate the antimicrobial utility of TAB and PI, either alone or in combination, against planktonic bacteria.
Methods
Planktonic gram-positive and gram-negative bacterial strains were exposed to TAB and PI ± TAB for up to 10 minutes in a bacterial time-kill assay. The efficacy of various dilutions of PI as well as the effects of serum protein on PI efficacy were also investigated.
Results
TAB was ineffective at the timeframes tested (≤10 minutes) when utilized alone; however, when utilized with PI, significant log reduction of all tested planktonic species was achieved. PI alone was also effective, even including dilute concentrations (eg, 0.5% PI), although the presence of serum proteins required higher concentrations of PI (eg, 2.5%) to eradicate the bacterial load.
Conclusions
Our data suggest PI-containing solutions may be preferred over either saline or TAB without PI for primary breast pocket irrigation and implant soaking in primary breast surgeries as a means to significantly reduce planktonic bacteria. These data provide an impetus for surgeons to re-evaluate the efficacy of TAB solution in these clinical settings.
Bacterial colonization can occur with any implantable device. In primary augmentation and reconstructive breast surgeries, silicone-based breast implants and tissue expanders are commonly utilized and planktonic bacteria can be inadvertently introduced during the surgical procedure1 due to electrostatic charge from opening the implant package,2 transfer from the existing local skin and/or breast tissue microbiome,3,4 or introduction through breast ductal tissue.5 Hypothesized complications associated with bacterial bioburden at the breast implant-tissue interface include infection, capsular contracture, breast implant-associated anaplastic large cell lymphoma, and a prolonged local inflammatory response.6-11 With an increasing emphasis on the potential contributions of bioburden to these complications, aseptic techniques as reported in the 14-point plan have been touted to help reduce bacterial contamination on and around implants.1 Point #8 (ie, perform pocket irrigation with triple antibiotic solution [TAB] or Betadine) is particularly germane. The utilization of antimicrobial solutions such as TAB or 10% povidone-iodine (PI) is intended to minimize bioburden either on the implant itself or in the surrounding tissue on mammary pocket creation in primary breast surgeries.12
There is a prevalent and long history of antimicrobial solution utilization in breast surgery, particularly in breast pocket irrigation and more recently for implant soaking.13,14 For the latter, soaking the implant in an antimicrobial solution is meant to address potential bacterial attraction due to an electrostatic charge imparted on opening the sterile package.2,4,15,16 A variety of antimicrobial solutions have been utilized,13,14 including but not limited to bacitracin and other individual antibiotics; 17 TAB as per Adams; 18,19 PI (Betadine) at full strength (100%), half strength (50%),20,21 or diluted in combination with TAB; 17,22 stabilized hypochlorous acid (0.025% HOCl); 23-25 and chlorhexidine.13,26 The present study focuses on assessing the antimicrobial efficacy of TAB and PI either alone or in combination, because these were the most commonly utilized solutions highlighted in recent surveys of plastic surgeons.13,14 More recently, the effectiveness of many of these antimicrobial solutions was revisited,22,25,26 which supported our decision to exclude the other solutions from our study.
Given the warning introduced in 2001 by the US Food and Drug Administration on the utilization of PI in conjunction with silicone breast implants and tissue expanders due to concerns that it would degrade the silicone implant shell and lead to premature device failure, there has been minimal emphasis on evaluating PI efficacy in the intervening 2 decades. The seminal work reported by Adams and colleagues17,18 provides guidelines for utilization of PI and/or TAB for pocket irrigation. However, TAB without PI is generally favored in clinical practice for implant soaking,14 and there are few, if any, guidelines for this practice despite fairly widespread utilization. Additionally, concerns about cytotoxicity of PI under in vitro conditions27-30 has led some clinicians to dilute it to “tea-color” in clinical utilization to mitigate bioburden while being cautious about potentially damaging healthy tissue. Lack of proper standardization and the anecdotal nature of PI utilization among clinicians warrant the need for proper investigation of PI concentrations and the time needed for optimal efficacy for both breast pocket irrigation and implant soaking. As such, we have also investigated the efficacy of dilute PI concentrations in this study.
A series of in vitro assessments to evaluate the utility of TAB and PI, either alone or in combination, for both breast pocket irrigation and implant soaking prior to placement was conducted in this study. The following bacterial species reported to be present in breast capsule flora were selected for testing: gram-positive Staphylococcus aureus, Staphylococcus epidermidis,31 and Cutibacterium acnes (formerly known as Propionibacterium acnes),4,32,33 as well as gram-negative Ralstonia pickettii7 and Pseudomonas aeruginosa.31 Given that primary breast surgeries account for initial exposure of the breast pocket or implant to bacteria, the strong likelihood is that only planktonic bacteria would be encountered in these situations. As such, this study assessed the in vitro efficacy of antimicrobial solutions on eradicating the planktonic form of these bacterial species. Exposure times were limited to the preferred upper limit (≤10 minutes) to make utilization practical in the operating room. This report will serve as part 1 of a series of 2 companion manuscripts to inform the utilization of antimicrobial solutions on target procedures. The first manuscript will target primary breast surgeries where planktonic bacteria can contaminate the implant/tissue expander surface. The second manuscript will address revision or tissue expander-implant exchange surgeries where biofilm can be present in the breast pocket capsule.36
METHODS
All bacterial strains were purchased from American Type Culture Collection (ATCC, Manassas, VA). Refer to Table 1 for the specific ATCC strains utilized in this study.
Table 1.
Planktonic Bacterial Strains Tested
| Species | Strain |
|---|---|
| Staphylococcus aureus | ATCC 29213 |
| Ralstonia pickettii | ATCC 27511 |
| Staphylococcus epidermidis | ATCC 35984 |
| Cutibacterium acnes; former designation: Propionibacterium acnes | ATCC 6919 |
| Pseudomonas aeruginosa | ATCC 27312 |
ATCC, American Type Culture Collection
Aliquots of the strains were stored as 20% glycerol stocks at −80°C. Every 2 weeks, fresh agar streak plates were prepared. For daily utilization, a fresh overnight culture was prepared from a single colony of the designated species taken from an agar streak plate. Consumables in these studies were utilized in sterile form, where applicable. Agar, growth media, buffers, and other solutions utilized in the studies were prepared according to manufacturers’ recommendations and/or following internal protocols. PI (Betadine; NDC code 67618-150-01, Lot No. 80558-16, Medline Industries, Northfield, IL) was utilized as received or aseptically diluted with sterile 0.9% saline to obtain various PI dilutions. For clarity, the diluted concentrations of PI (eg, 50% PI) refer to the diluted percentage of stock Betadine solution, not the percentage of povidone-iodine (ie, 50% PI = 50% Betadine = 5% povidone-iodine). TAB was prepared as per Adams:17 0.2% w/v cefazolin sodium salt (Cat. No. 14325, Lot No. CZSS3756455, ChemImpex, Wood Dale, IL, 92.9%), 0.016% w/v gentamicin sulfate salt (Cat. No. G1264-5g, Lot No. SLBL4466V, Sigma, St. Louis, MO), and 100 IU/mL bacitracin (Cat. No. 226100050, Lot No. A0371439, Acros Organics, Waltham, MA, 60 IU/mg) in sterile saline. The 50% PI with TAB was prepared by mixing equal volumes of 100% PI and TAB. Human serum was obtained from Millipore Sigma, Burlington, MA (Cat. No. H4522). Dey-Engley (D/E) broth (Cat. No. 281910; Becton Dickinson Difco, Franklin Lakes, NJ) was utilized for neutralization of the various antimicrobial test articles and prepared per the manufacturer’s instructions.
C. acnes was cultured under anaerobic conditions throughout the experiment, and both the starting culture utilized for inoculation and the tryptic soy agar (TSA) plates utilized for enumeration were incubated for 48 hours due to the low growth rate of this species. Given the short timepoints, treatments against C. acnes were performed in aerobic conditions, as with other species. All other species were grown aerobically following standard conditions as recommended by ATCC.
Time-Kill Assay Setup
Assays to determine the efficacy of the antimicrobial solutions against planktonic bacteria were performed in microtiter plates (96-well and 24-well plates as noted below). For the 96-well plate setup (all treatments that did not contain TAB), 190 µL of the antimicrobial solutions was added to wells of the plate at the start of the assay. Then 10 µL of the bacterial inoculum was added and the suspension mixed well, resulting in a starting inoculum of approximately 0.5 to 5×107 CFUs. After 1, 5, or 10 minutes of treatment, aliquots of the suspensions (60 µL) were transferred to wells containing D/E neutralizer (240 µL) and mixed well. The neutralized samples were then plated on 1.5% TSA plates (with or without serial dilution) and enumerated utilizing standard plate counts. In this study, all colonies were counted and reported from the enumeration plates; however, it should be recognized that the reliable detection limit for standard plate counts is 10 CFU per plate, or 1.8 log using the plating technique of 150 μL of the neutralized treatment per plate.
Assays utilizing surgical solutions containing TAB were conducted in a 24-well plate given that a 400-fold dilution of the treatment in D/E broth was required for proper neutralization. Bacterial suspensions (inoculum) containing approximately 109 CFU/mL were prepared either directly from the overnight cultures of bacteria (R. pickettii and P. aeruginosa; C. acnes: 2-day-old culture) or by concentrating the overnight cultures (S. aureus and S. epidermidis). Then 950 µL of the antimicrobial solutions was added to the wells at the start of the assay, after which 50 µL of the bacterial inoculum was added and mixed well, resulting in a starting inoculum of approximately 0.5 to 5×108 CFUs. After 1, 5, or 10 minutes of treatment, 5-µL aliquots of the suspensions were transferred to the wells containing D/E neutralizer (2 mL). The neutralized samples were then plated on 1.5% TSA plates (with or without serial dilution) and enumerated. For each of the assays described above, there were 6 sample replicates within each experiment and the experiment was performed 3 times.
For evaluation of the effects of serum on PI efficacy, human serum with 50 g/L of serum proteins was prepared by diluting commercial human serum with saline. This was done to simulate potential dilution of PI in a freshly dissected breast pocket by protein-rich tissue exudate and blood. All other serum concentrations were prepared by diluting the serum stock with saline immediately prior to utilization. For assay setup, appropriate concentrations of serum were added to wells of the 96-well plate (50 µL/well) followed by addition of the microbial inoculum (50 µL/well). After 5 minutes, 100 µL/well of the designated PI solution was added to each well and the suspension mixed and incubated for 5 minutes. The individual samples were then neutralized in D/E broth and enumerated as described above. Each treatment condition was evaluated with 6 sample replicates and 3 experimental replicates. Data were collected from multiple in vitro experiments performed between July 2017 and August 2019.
RESULTS
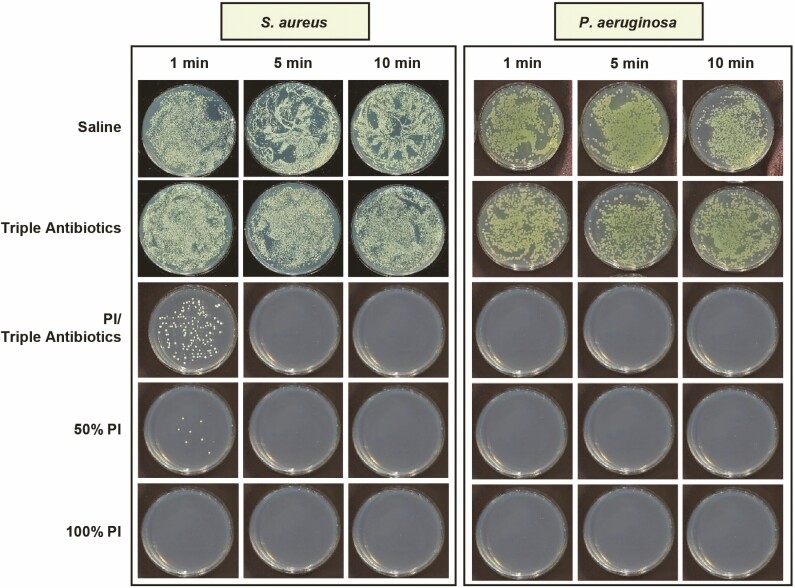
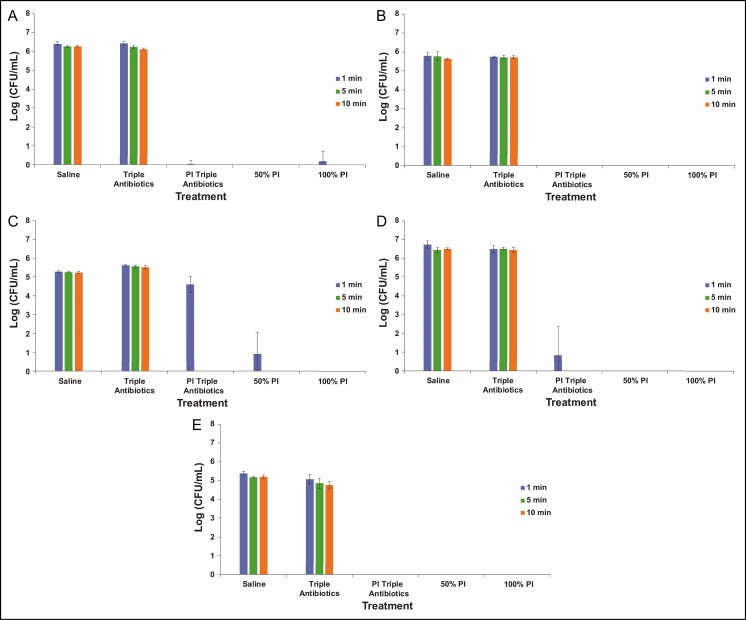
The bacterial time-kill assay was designed to evaluate the efficacy of antimicrobial solutions analogous to the clinical utilization of these solutions, with a high ratio of antimicrobial solution to bacteria (ie, 20:1 v/v). Following treatment, bacterial survival was qualitatively visualized on TSA plates and quantified utilizing enumeration. Examples of bacteria survival on TSA plates are shown in Figure 1, with S. aureus and P. aeruginosa chosen as representative gram-positive and gram-negative species, respectively, regarding residual bacterial survival following treatment. Neither saline nor TAB appeared to be effective at eradicating planktonic bacteria across all 5 species (data not shown for S. epidermidis, C. acnes, or R. pickettii) at any of the 3 treatment times evaluated because bacterial survival was clearly observed. In contrast, both full-strength (100%) and half-strength (50%) PI with or without TAB exhibited potent antibacterial properties against all 5 planktonic bacteria species tested when exposure time was greater than 1 minute. This qualitative assessment reflects the results of the enumeration of bacterial survival (Figure 2). TAB was ineffective at eradicating planktonic bacteria when administered alone within the timeframes tested (≤10 minutes), that is, less than 1 log reduction compared with the saline-treated control. When utilized in combination with PI, TAB exhibited significant log reduction in bacterial survival within 1 minute for the non-Staphylococcus bacterial species, whereas it required at least 5 minutes for both S. aureus and S. epidermidis. PI at both full strength and half strength was generally effective at significantly reducing all bacteria within 1 minute for all species. Some variability was noted with S. aureus survival after 1 minute of treatment; however, this variability is within the detection limit of standard plate counts (eg, 10 CFU/plate, or 1.8 log utilizing the methodology reported here).
Figure 1.
Qualitative visualization of planktonic bacterial survival as exhibited with representative gram-positive (Staphylococcus aureus) or gram-negative (Pseudomonas aeruginosa) species in tryptic soy agar plates following treatment with saline, triple antibiotic solution (TAB), 50% PI (±TAB), or 100% PI for 1, 5, or 10 minutes. Note: PI refers to 10% povidone-iodine or full-strength Betadine.
Figure 2.
Enumeration of planktonic bacterial survival for gram-negative Pseudomonas aeruginosa (A) and Ralstonia pickettii (B) as well as gram-positive Staphylococcus aureus (C), Staphylococcus epidermidis (D), and Cutibacterium acnes (E) following treatment with saline, triple antibiotic solution (TAB), 50% PI (±TAB), or 100% PI for 1, 5, or 10 minutes. Data are represented as mean bacterial survival ± standard deviation in log (CFU/mL) from 3 experimental replicates and 6 treatment replicates per experiment. Note: PI refers to 10% povidone-iodine or full-strength Betadine.
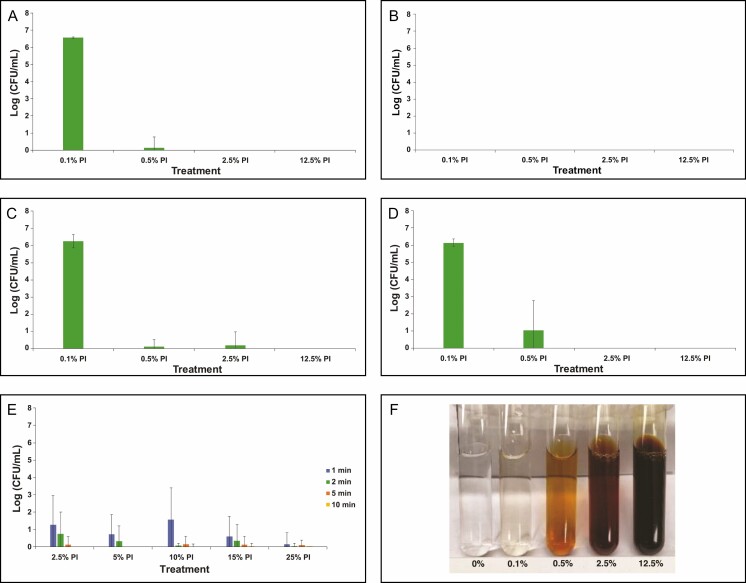
In addition to high-strength PI, we evaluated dilutions of PI ranging from 0.1% to 12.5% (Figure 3A-E), aligning with reports of “tea-colored PI” utilization in clinical settings. Based on the color of the dilutions utilized in this study, this is likely to be in the range of 0.5% and 2.5% PI (Figure 3F). Given the results of the high concentration experiments (Figure 2), we only tested dilute PI efficacy at 5 minutes of treatment time. All of the evaluated dilutions of PI (down to 0.1%) exhibited eradication of planktonic R. pickettii and C. acnes. This suggests that these 2 species are quite sensitive to PI. The PI treatment at 0.1% was not as efficacious against S. aureus, S. epidermidis, and P. aeruginosa. Instead, higher concentrations were required to show full efficacy, with at least 0.5% PI being required for S. aureus, P. aeruginosa, and S. epidermidis. Increased variability in bacterial survival within the detection limit was seen with S. epidermidis, suggesting that it may be the most PI-tolerant planktonic species evaluated in this study.
Figure 3.
Enumeration of planktonic bacterial survival for gram-negative Pseudomonas aeruginosa (A) and Ralstonia pickettii (B) as well as gram-positive Staphylococcus aureus (C), Staphylococcus epidermidis (D), and Cutibacterium acnes (E) following treatment with dilute concentrations of PI (0.1, 0.5%, 2.5%, and 12.5%) for 5 minutes. Data are represented as mean bacterial survival ± standard deviation in log (CFU/mL) from 3 experimental replicates and 6 treatment replicates per experiment. (F) Color palette for different dilutions of PI shown for reference. Note: PI refers to 10% povidone-iodine or full-strength Betadine.
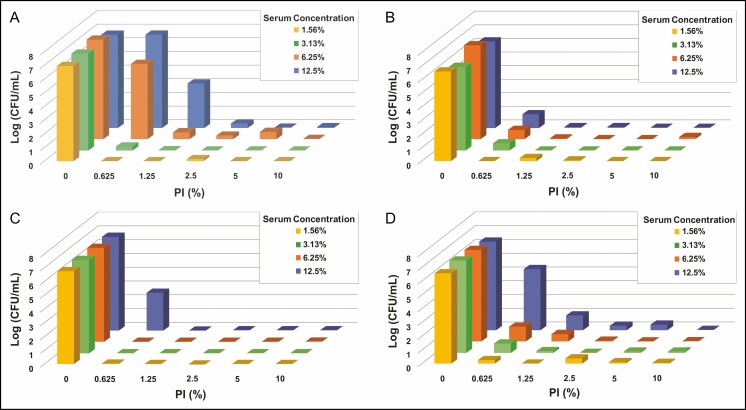
Because serum proteins may affect antimicrobial efficacy, bacterial time-kill tests were also performed in the presence of serum proteins. Tests were conducted with 4 bacterial species of interest: 2 gram-positive bacteria, S. epidermidis and S. aureus, and 2 gram-negative bacteria, P. aeruginosa and R. pickettii. C. acnes was not evaluated in these experiments given the high efficacy observed for PI against this species. Given the results of the dilute PI study, PI concentrations were kept between 0.5% and 10%. The concentration of serum ranged between 1.5% and 12.5% to cover the potential range of protein levels present in the breast pocket as reported in the literature.24,25 Results for all species tested suggested that in the presence of 1.5% to 12.5% serum, there can be a diminished effect on PI efficacy if diluted lower than 2.5% (Figure 4).
Figure 4.
Enumeration of planktonic bacterial survival for gram-negative Pseudomonas aeruginosa (A) and Ralstonia pickettii (B) as well as gram-positive Staphylococcus aureus (C) and Staphylococcus epidermidis (D) under varying concentrations of both human serum and PI with treatment for 5 minutes. The range of tested concentrations for PI (0%-10%) and serum (1.56%-12.5%) are represented on the x-axis and y-axis, respectively. Data are represented as mean bacterial survival in log (CFU/mL) in the z-axis from 3 experimental replicates and 6 treatment replicates per experiment. Note: PI refers to 10% povidone-iodine or full-strength Betadine.
DISCUSSION
To evaluate the efficacy of antimicrobial solutions against planktonic bacteria inadvertently introduced on the implant surface in primary breast implant surgeries, we have developed an in vitro experimental setup and tested TAB and PI (±TAB) at various concentrations. Although a variety of antimicrobial solutions have been reported in the literature for utilization in these applications (antibiotics and antiseptics), there has not been much work conducted to study their optimal concentration and time needed for killing planktonic bacteria as reported here.
A number of investigators have reported in recent ASPS surveys that over one-half of plastic surgeons soak their implants in TAB solution and/or PI.13,14 In addition, these recent surveys suggest that many surgeons are in favor of utilizing TAB for breast pocket irrigation without the addition of PI.14 Some studies as reviewed by Lynch et al34 suggest that utilization of Adams’s TAB solution in pocket irrigation is effective at reducing infection and capsular contracture rates compared with saline alone or no irrigation; however, a recent study by Culbertson and colleagues22 suggests that TAB alone may require a longer exposure time (at least 18 hours) to be completely bactericidal compared with PI-containing antimicrobial solutions. In the Culbertson study,22 antimicrobial neutralization was not performed, so residual antimicrobial effects could be seen at longer timepoints. Our selection of 1, 5, and 10 minutes of exposure time to evaluate the effectiveness of these antimicrobial solutions was empiric yet representative of the time that could be allocated within the surgical procedure for antimicrobial irrigation or implant soaking without necessarily prolonging surgery and increasing its associated costs. In addition, neutralization of the antimicrobial with D/E broth allows us to understand the baseline antimicrobial activity of these solutions in these short timeframes. Our in vitro results suggest that at these short exposure times (≤10 minutes), TAB solutions alone do not exhibit significant log reduction of the strains of planktonic bacteria that were tested (Figures 1 and 2). Whereas TAB alone appears to be minimally effective under these conditions, TAB with PI appears to be effective when exposed for at least 5 minutes, largely due to the addition of PI, which is consistent with clinical results previously reported.35 The lack of efficacy for TAB in our study is likely due to the short exposure times utilized and the fact that there are no growth media components in the treatments to promote metabolic activity. The antibiotics in TAB all work through active metabolic uptake of the bacteria, which is minimal in the absence of media and during short time periods. Given that PI has a strong chemical mechanism of action (ie, not requiring metabolic activity), it is not surprising that both half-strength and full-strength PI were effective at significantly reducing bacterial survival of all tested strains. Results for S. aureus utilizing combined TAB and PI showed limited efficacy at 1 minute and high efficacy for half-strength PI, which is interesting given that the concentration of PI is the same in both treatments (Figure 2C). However, when developing the assay, we have observed some variability at the 1-minute treatment time point, which is further justification for soaking implants for longer time periods (eg, 5-10 minutes) to ensure complete eradication of planktonic cells.
We also studied the efficacy of dilute PI given the practice by some surgeons of diluting it. Dilutions of PI were tested at concentrations that are more relevant to real-life clinical situations such as implant soaking or primary augmentation surgeries where utilization of lower PI concentrations may be considered. In these situations, the implant surface and/or the breast pocket should have minimal colonization of planktonic bacteria, and therefore utilization of lower PI concentrations may be appropriate. We aimed to assess the minimum threshold concentration required for eradication of various gram-positive and gram-negative bacteria. A wide range of dilutions was tested ranging from 0.1% to 12.5% (Figure 3), which also aligns with reports of “tea-colored” dilutions of PI. Based on our results, R. pickettii and C. acnes were the most susceptible to PI. S. aureus, S. epidermidis, and P. aeruginosa were shown to be more resistant to eradication by PI, with S. epidermidis showing the most resistance to eradication with at least 2.5% PI required to show maximum log reduction in bacterial survival. Therefore, it appears that for implant soaking and irrigation of primary breast pockets, significant dilution of PI is possible while still maintaining general antimicrobial efficacy at an exposure time of 5 minutes, which is in stark contrast to what was observed in our biofilm-associated bacteria study,36 where dilution of PI lower than one-eighth to quarter-strength significantly reduced its efficacy at 5 minutes of exposure. Interestingly, our results with 5 minutes of exposure time to diluted PI (refer to Figure 3C) are similar to those reported by Berkelman and co-workers37 for efficacy of dilute PI against S. aureus at 4 minutes of exposure time, at least for concentrations greater than 1% PI (ie, 1:100 dilution of stock PI as reported by Berkelman37). In this study, the authors show that PI diluted lower than 0.1% started to lose its efficacy against planktonic S. aureus when exposure time was 1 minute or less; in addition, their data also suggest loss of efficacy at PI concentrations greater than 25% after 1 minute of exposure or less. Based on these confounding results at higher PI concentrations compared with our results at 5 minutes, further research may be warranted with respect to how the dilution of PI, the nature of the diluent, and exposure time affects PI efficacy and bioavailability of free iodine. Regardless, the Berkelman37 results as well as the data reported here suggest that contact time of PI should be longer, because brief exposure of PI for 1 minute or less either negatively impacted efficacy and/or exhibited significant variability in bacterial survival in response to PI.
For primary augmentation and reconstructive surgeries, the implant is placed in a potentially protein-rich tissue exudate environment unless hemostasis is completely controlled. The presence of serum proteins in the breast pocket or implant surface could have a pronounced effect on the efficacy of an antimicrobial solution (eg, PI). To address this concern, the effects of serum protein presence on PI efficacy were evaluated. Results for all 4 species tested suggest that in the presence of 1% to 12.5% serum, there can be a diminished effect on efficacy if PI is diluted lower than 2.5%, especially if serum protein concentrations are greater than 6.25% (Figure 4). We concluded that dilute concentrations of PI may still be effective at eradicating planktonic bacteria in newly created pockets, assuming appropriate hemostasis is achieved to limit the presence of blood serum proteins. As such, when evaluating the appropriate concentration of PI to utilize, one should consider the fact that the presence of blood serum proteins may negatively affect its efficacy. Of further relevance is the notion that the presence of any microbial survivors on the implant surface may lead to implant-associated complications post-surgical placement. Once inside the body, and if not eliminated by the host immune system, residual bacteria may cause further complications.
It is important to note some limitations associated with this study. The study was conducted utilizing only 5 strains of bacteria known to be relevant to the clinical setting in breast surgery procedures. Additional pertinent bacterial species such as Escherichia coli and Mycobacterium fortuitum could also be tested in future studies. In addition, only a subset of antimicrobial solutions was tested, with the focus on TAB and PI solutions. Additional solutions such as stabilized hypochlorous acid could be evaluated in future studies. This study also investigated defined endpoints for exposure to antimicrobial solutions with the active agent neutralized from further activity at these timepoints; therefore, the results do not preclude that TAB may be more efficacious when left in the breast pocket for longer contact times as has been suggested by other studies.22,38 Furthermore, the assay setup in this study is a simplified 2-dimensional in vitro testing platform, which did not utilize surrogate silicone surfaces in lieu of breast implant materials; however, a recently published study utilized smooth silicone coupons and demonstrated very similar results regarding PI and TAB efficacy against S. aureus and S. epidermidis, lending credence to the applicability of our results to silicone surfaces.38 Given these limitations, the correlation between the results of these in vitro studies and clinical outcomes is not known and could be further investigated through appropriate in vivo preclinical studies and clinical work. However, these in vitro findings are supported by the outcomes reported for utilizing PI in pocket irrigation in recent clinical studies. Pat McGuire39 performed a risk factor analysis for capsular contracture in patients from the prospective Continued Access Reconstruction/Revision Expansion trial and noted that intraoperative utilization of antibiotic-based pocket irrigation instead of PI was a risk factor for increased capsular contracture in the primary reconstruction cohort. A retrospective review by Giordano and colleagues35 also indicated that utilizing PI combined with antibiotic irrigation reduced the rate of capsular contracture in cosmetic breast augmentation procedures.
CONCLUSIONS
In summary, our results clearly demonstrate that at the short exposure times tested, undiluted PI (1% free iodine concentration) had the most consistent bactericidal effect against both gram-positive and gram-negative organisms in our in vitro study. Dilutions of PI were also shown to be effective against all planktonic bacterial organisms tested at an exposure time of 5 minutes. As such, the results suggest that PI dilutions may still maintain strong effectiveness in primary breast surgery or implant-soaking conditions. The results in our study are supported by a recent report by Culbertson et al22 that recommends the utilization of PI-containing antimicrobial solutions for breast pocket irrigation. With respect to the limited efficacy noted in our results with TAB solutions, even though the concentrations of antibiotics utilized in TAB are significantly higher (>100×) than the minimal bactericidal concentration, the exposure times utilized in this study may not be long enough to observe the expected bactericidal effect. This may help explain the mixed clinical results reported with the utilization of TAB irrigation in breast surgeries.40,41 Taken together, these data suggest that PI-containing solutions may be preferred over the utilization of either saline or TAB solution without PI for breast pocket irrigation and implant soaking. It is important to note that the in vitro results from this study may not translate directly into clinical results and outcomes; however, it provides impetus to re-evaluate the efficacy of TAB solution and consider utilization of PI in clinical settings for implant soaking and primary breast pocket irrigation.
Acknowledgments
The authors acknowledge Nina Bionda, PhD; Alison V. Moran, MS; and Elizabeth J. Bevels of iFyber LLC, Ithaca, NY, for their contributions to study design, collection, analysis, and interpretation of the data.
Disclosures
Drs Leung and Hariri are employees of Allergan Aesthetics, an AbbVie company (Irvine, CA), and Drs Strickland and Lantz are employees of iFyber LLC (Ithaca, NY). Dr Jewell has received honoraria from Allergan plc and serves as a consultant for Allergan Aesthetics, an AbbVie company, and New Beauty magazine. Ms Jewell declared no potential conflicts of interest with respect to the research, authorship, and publication of this article. Allergan Aesthetics, an AbbVie company, participated in the development of the study design and in the analysis and interpretation of the data. iFyber LLC participated in the study design, collection, analysis, and interpretation of the data. None of the authors received honorarium or other form of financial support related to the development of this article.
Funding
This study was sponsored by Allergan Aesthetics, an AbbVie company (Irvine, CA). Assistance in generation of the figures was provided to the authors by Peloton Advantage (Parsippany, NJ) and was funded by Allergan plc.
REFERENCES
- 1. Adams WP Jr, Culbertson EJ, Deva AK, et al. Macrotextured breast implants with defined steps to minimize bacterial contamination around the device: experience in 42,000 implants. Plast Reconstr Surg. 2017;140(3):427-431. [DOI] [PubMed] [Google Scholar]
- 2. Scheflan M. Electrostatic field around breast implants: can we further reduce biofilms? Paper presented at: The Aesthetic Meeting (ASAPS); April 27, 2018, New York, NY.
- 3. Hieken TJ, Chen J, Hoskin TL, et al. The microbiome of aseptically collected human breast tissue in benign and malignant disease. Sci Rep. 2016;6:30751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Thornton JW, Argenta LC, McClatchey KD, Marks MW. Studies on the endogenous flora of the human breast. Ann Plast Surg. 1988;20(1):39-42. [DOI] [PubMed] [Google Scholar]
- 5. Collis N, Mirza S, Stanley PR, Campbell L, Sharpe DT. Reduction of potential contamination of breast implants by the use of ‘nipple shields.’ Br J Plast Surg. 1999;52(6):445-447. [DOI] [PubMed] [Google Scholar]
- 6. Burkhardt BR, Dempsey PD, Schnur PL, Tofield JJ. Capsular contracture: a prospective study of the effect of local antibacterial agents. Plast Reconstr Surg. 1986;77(6):919-932. [PubMed] [Google Scholar]
- 7. Hu H, Johani K, Almatroudi A, et al. Bacterial biofilm infection detected in breast implant-associated anaplastic large-cell lymphoma. Plast Reconstr Surg. 2016;137(6):1659-1669. [DOI] [PubMed] [Google Scholar]
- 8. Netscher DT, Weizer G, Wigoda P, Walker LE, Thornby J, Bowen D. Clinical relevance of positive breast periprosthetic cultures without overt infection. Plast Reconstr Surg. 1995;96(5):1125-1129. [DOI] [PubMed] [Google Scholar]
- 9. Pajkos A, Deva AK, Vickery K, Cope C, Chang L, Cossart YE. Detection of subclinical infection in significant breast implant capsules. Plast Reconstr Surg. 2003;111(5):1605-1611. [DOI] [PubMed] [Google Scholar]
- 10. Pittet B, Montandon D, Pittet D. Infection in breast implants. Lancet Infect Dis. 2005;5(2):94-106. [DOI] [PubMed] [Google Scholar]
- 11. Tamboto H, Vickery K, Deva AK. Subclinical (biofilm) infection causes capsular contracture in a porcine model following augmentation mammaplasty. Plast Reconstr Surg. 2010;126(3):835-842. [DOI] [PubMed] [Google Scholar]
- 12. Frois AO, Harbour PO, Azimi F, et al. The role of antibiotics in breast pocket irrigation and implant immersion: a systematic review. Plast Reconstr Surg Glob Open. 2018;6(9):e1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chopra K, Gowda AU, McNichols CHL, Brown EN, Slezak S, Rasko Y. Antimicrobial prophylaxis practice patterns in breast augmentation: a national survey of current practice. Ann Plast Surg. 2017;78(6):629-632. [DOI] [PubMed] [Google Scholar]
- 14. Epps MT, Langsdon S, Pels TK, et al. Pocket irrigation and technique during reconstructive surgery: an American Society of Plastic Surgery survey of current practice. Ann Plast Surg. 2019;82(6S Suppl 5):S427-S432. [DOI] [PubMed] [Google Scholar]
- 15. Khoo LS, Stevens HP. Preventing electrostatic contamination of breast implants: an effective and simple intraoperative method. Aesthet Surg J. 2017;37(6):731-733. [DOI] [PubMed] [Google Scholar]
- 16. Reischies FMJ, Krause R, Holzer J, et al. What can we learn from sonication results of breast implants? PLoS One. 2017;12(8):e0182267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Adams WP Jr, Conner WC, Barton FE Jr, Rohrich RJ. Optimizing breast-pocket irrigation: the post-betadine era. Plast Reconstr Surg. 2001;107(6):1596-1601. [DOI] [PubMed] [Google Scholar]
- 18. Adams WP Jr, Conner WC, Barton FE Jr, Rohrich RJ. Optimizing breast pocket irrigation: an in vitro study and clinical implications. Plast Reconstr Surg. 2000;105(1):334-338; discussion 339. [DOI] [PubMed] [Google Scholar]
- 19. Adams WP Jr, Rios JL, Smith SJ. Enhancing patient outcomes in aesthetic and reconstructive breast surgery using triple antibiotic breast irrigation: six-year prospective clinical study. Plast Reconstr Surg. 2006;117(1):30-36. [PubMed] [Google Scholar]
- 20. Jewell ML, Adams WP Jr. Betadine and breast implants. Aesthet Surg J. 2018;38(6):623-626. [DOI] [PubMed] [Google Scholar]
- 21. Wiener TC. Betadine and breast implants: an update. Aesthet Surg J. 2013;33(4):615-617. [DOI] [PubMed] [Google Scholar]
- 22. Culbertson EJ, Felder-Scott C, Deva AK, Greenberg DE, Adams WP. Optimizing breast pocket irrigation: the breast implant-associated anaplastic large cell lymphoma (BIA-ALCL) era. Aesthet Surg J. 2020;40(6):619-625. [DOI] [PubMed] [Google Scholar]
- 23. Brindle CT, Porter S, Bijlani K, et al. Preliminary results of the use of a stabilized hypochlorous acid solution in the management of Ralstonia pickettii biofilm on silicone breast implants. Aesthet Surg J. 2018;38(suppl_2):S52-S61. [DOI] [PubMed] [Google Scholar]
- 24. Haws MJ, Gingrass MK, Porter RS, Brindle CT. Surgical breast pocket irrigation with hypochlorous acid (HOCl): an in vivo evaluation of pocket protein content and potential HOCl antimicrobial capacity. Aesthet Surg J. 2018;38(11):1178-1184. [DOI] [PubMed] [Google Scholar]
- 25. Hu H, Sleiman J, Johani K, Vickery K. Hypochlorous acid versus povidone-iodine containing irrigants: which antiseptic is more effective for breast implant pocket irrigation? Aesthet Surg J. 2018;38(7):723-727. [DOI] [PubMed] [Google Scholar]
- 26. Zhadan O, Becker H. Surgical site irrigation in plastic surgery. Aesthet Surg J. 2018;38(3):265-273. [DOI] [PubMed] [Google Scholar]
- 27. Balin AK, Pratt L. Dilute povidone-iodine solutions inhibit human skin fibroblast growth. Dermatol Surg. 2002;28(3): 210-214. [DOI] [PubMed] [Google Scholar]
- 28. Lineaweaver W, McMorris S, Soucy D, Howard R. Cellular and bacterial toxicities of topical antimicrobials. Plast Reconstr Surg. 1985;75(3):394-396. [DOI] [PubMed] [Google Scholar]
- 29. Liu JX, Werner JA, Buza JA III, Kirsch T, Zuckerman JD, Virk MS. Povidone-iodine solutions inhibit cell migration and survival of osteoblasts, fibroblasts, and myoblasts. Spine (Phila Pa 1976). 2017;42(23):1757-1762. [DOI] [PubMed] [Google Scholar]
- 30. Müller G, Kramer A. Biocompatibility index of antiseptic agents by parallel assessment of antimicrobial activity and cellular cytotoxicity. J Antimicrob Chemother. 2008;61(6):1281-1287. [DOI] [PubMed] [Google Scholar]
- 31. Klein GM, Phillips BT, Dagum AB, Bui DT, Khan SU. Infectious loss of tissue expanders in breast reconstruction: are we treating the right organisms? Ann Plast Surg. 2017;78(2):149-152. [DOI] [PubMed] [Google Scholar]
- 32. Rieger UM, Mesina J, Kalbermatten DF, et al. Bacterial biofilms and capsular contracture in patients with breast implants. Br J Surg. 2013;100(6):768-774. [DOI] [PubMed] [Google Scholar]
- 33. Walker JN, Hanson BM, Pinkner CL, et al. Insights into the microbiome of breast implants and periprosthetic tissue in breast implant-associated anaplastic large cell lymphoma. Sci Rep. 2019;9(1):10393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lynch JM, Sebai ME, Rodriguez-Unda NA, Seal S, Rosson GD, Manahan MA. Breast pocket irrigation with antibiotic solution at implant insertion: a systematic review and meta-analysis. Aesthetic Plast Surg. 2018;42(5):1179-1186. [DOI] [PubMed] [Google Scholar]
- 35. Giordano S, Peltoniemi H, Lilius P, Salmi A. Povidone-iodine combined with antibiotic topical irrigation to reduce capsular contracture in cosmetic breast augmentation: a comparative study. Aesthet Surg J. 2013;33(5):675-680. [DOI] [PubMed] [Google Scholar]
- 36. Jewell ML, Bionda N, Moran AV, et al. In vitro evaluation of common antimicrobial solutions used for breast pocket irrigation—part 2: efficacy against biofilm-associated bacteria. Aesthet Surg J. 2021;41(11):1252–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Berkelman RL, Holland BW, Anderson RL. Increased bactericidal activity of dilute preparations of povidone-iodine solutions. J Clin Microbiol. 1982;15(4):635-639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ngaage LM, Elegbede A, Brao K, et al. The efficacy of breast implant irrigant solutions: a comparative analysis using an in vitro model. Plast Reconstr Surg. 2020;146(2):301-308. [DOI] [PubMed] [Google Scholar]
- 39. McGuire P, Reisman NR, Murphy DK. Risk factor analysis for capsular contracture, malposition, and late seroma in subjects receiving Natrelle 410 form-stable silicone breast implants. Plast Reconstr Surg. 2017;139(1):1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Campbell CA. The role of triple-antibiotic saline irrigation in breast implant surgery. Ann Plast Surg. 2018;80(6S Suppl 6):S398-S402. [DOI] [PubMed] [Google Scholar]
- 41. Drinane JJ, Bergman RS, Folkers BL, Kortes MJ. Revisiting triple antibiotic irrigation of breast implant pockets: a placebo-controlled single practice cohort study. Plast Reconstr Surg Glob Open. 2013;1(7):e55. [DOI] [PMC free article] [PubMed] [Google Scholar]