Abstract
Background
Biofilm-associated bacteria have been observed in both breast implant revision and tissue expander-implant exchange surgeries. The utilization of antimicrobial solutions in breast surgery, especially those containing triple antibiotics (TAB) and/or 10% povidone-iodine (PI), may help reduce existing biofilm-associated bacteria, which is particularly important in a mature breast pocket that may contain residual bacteria from a previously colonized implant surface or, theoretically, bacteria that may arrive postoperatively through hematogenous spread.
Objectives
A series of in vitro assessments was performed to evaluate the antimicrobial utility of TAB and PI, either alone or in combination, against preformed biofilm-associated bacteria.
Methods
Preformed biofilm-associated gram-positive and gram-negative bacterial strains were exposed to TAB and PI ± TAB for up to 30 minutes in a bacterial time-kill assay. Efficacy of various dilutions of PI and the effects of serum protein on PI efficacy were also investigated.
Results
TAB was ineffective at the timeframes tested when utilized alone; when utilized in conjunction with PI, significant log reduction of all biofilm-associated bacterial species tested was achieved when treated for at least 5 minutes. PI alone at a concentration of 25% or higher was also effective, although its efficacy was negatively affected by increasing serum protein concentration only for Staphylococcus epidermidis.
Conclusions
Our data indicate that PI-containing solutions significantly reduce biofilm-associated bacteria, suggesting potential utility for breast pocket irrigation during revision or exchange surgeries. Care should be taken to minimize excessive dilution of PI to maintain efficacy.
In breast implant revision and tissue expander-implant exchange surgeries, mature multi-layered bacteria can be present on the preexisting implant or in the surrounding tissue capsule.1-5 This may occur when free-floating planktonic bacteria colonize extensively to form a 3-dimensional structure and evolve into biofilms. Biofilm is usually polymicrobial in nature and encapsulated by an excreted exopolysaccharide that conveys a protective mechanism to the underlying bacteria and binds firmly to the underlying surface.6 It is therefore appropriate to assume that in a revision surgery, a new breast implant could be placed into a contaminated pocket with high levels of preexisting bacteria.7,8 Addressing biofilm-associated bacteria is significantly more challenging compared with planktonic bacteria and may require longer exposure times and higher concentrations of antimicrobial solutions. When bacterial contamination inside the breast pocket is not mitigated effectively at the time of revision surgery, infection may persist, and it is hypothesized to lead to serious complications such as capsular contracture, breast implant-associated anaplastic large cell lymphoma, and a prolonged local inflammatory response.3,6,7,9-12 These complications may take on greater importance when textured breast implants and tissue expanders are utilized because these products have been linked to the potential for increased bioburden and biofilm formation.4
The utilization of antimicrobial solutions, especially those containing triple antibiotics (TAB) and/or 10% povidone-iodine (PI; Betadine), for breast pocket irrigation during revision surgeries, has been common practice for many surgeons over the past few decades. This technique is intended to minimize the presence of preexisting biofilm-associated bacteria in the capsule prior to placement of a new implant.13 A variety of antimicrobial solutions have been utilized in secondary breast surgery procedures.14-16 Bacitracin and other individual antibiotics; 17 combinations of antibiotics such as TAB containing bacitracin, cefazolin, and gentamicin; 18,19 PI at full strength and half strength; 20,21 PI diluted in combination with TAB; 17,22 stabilized hypochlorous acid (0.025% HOCl); 23-25 and chlorhexidine14,26 have all been evaluated. The present study focuses on investigating the efficacy of PI or TAB, either alone or in combination, to significantly reduce biofilm-associated bacteria. More recently, the effectiveness of many of the aforementioned antimicrobial solutions was revisited, and the results supported our decision to exclude the other solutions from our current study.22,25,26
The presence of biofilm-associated bacteria in the breast pocket can be significantly more challenging to address. As such, this type of contamination requires a thorough investigation for treatment parameters such as effective concentrations of antimicrobial solutions as a function of time. Because PI has been associated with cytotoxicity, some clinicians utilize dilute “tea-colored” PI to mitigate bacterial contamination while minimizing cytotoxic effects.27-30 However, there has been little investigation into the efficacy of dilute PI to mitigate preformed biofilm. Lack of proper guidelines on diluting PI could lead to incomplete kill of biofilm-associated bacteria during revision surgeries, which could have deleterious downstream effects such as the induction of a persistent host inflammatory reaction.6 In addition to the broader challenge of addressing biofilm-associated bacteria with dilute PI, one must consider the potential negative impact of the presence of blood serum proteins on PI efficacy. In a revision surgery or tissue expander-breast implant exchange surgery, the implant can be placed in a protein-rich environment, where the extravasation of serum proteins from a capsulectomy could affect the efficacy of antimicrobial irrigation solutions; prior work suggests that this could be the case.31-33 Based on this information, identification of proper treatment guidelines for the utilization of PI also relies heavily on understanding how variables such as dilution factor and serum content affect its overall efficacy.
In this study, the anti-biofilm properties of TAB and PI solutions were evaluated against 5 species of bacteria implicated in breast implant infections. The following bacterial species reported to be present in breast tissue capsule flora were selected for testing, including gram-positive Staphylococcus aureus, Staphylococcus epidermidis,34 and Cutibacterium acnes (formerly known as Proprionibacterium acnes).35-37C. acnes was of particular interest because this bacterial species has been associated with the potential to elicit aberrant T-cell responses38,39 and T-cell transformation has been linked to breast implant-associated anaplastic large cell lymphoma.40-42 In addition, relevant gram-negative bacteria Ralstonia pickettii3 and Pseudomonas aeruginosa34 were also selected. This study assessed the efficacy of various concentrations of TAB and PI solutions against biofilm-associated phenotypes of these gram-positive and gram-negative bacterial species utilizing an in vitro screening platform. This report serves as part 2 of a series of companion publications in which the first part focuses on planktonic bacteria with relevance to primary breast surgeries and contamination of breast implant or tissue expander surfaces.45 The current manuscript discusses treatment parameters to address potential infections presented during revision surgeries and tissue expander-breast implant exchange surgeries where a mature capsule has formed prior to placement of the new breast implant and biofilm-associated bacteria may be present.
METHODS
All bacterial strains were purchased from American Type Culture Collection (ATCC, Manassas, VA). Refer to Table 1 for the specific ATCC strains employed in this study.
Table 1.
Biofilm-Associated Bacterial Strains Tested
| Species | Strain |
|---|---|
| Staphylococcus aureus | ATCC 29213 |
| Ralstonia pickettii | ATCC 27511 |
| Staphylococcus epidermidis | ATCC 35984 |
| Cutibacterium acnes; former designation: Propionibacterium acnes | ATCC 6919 |
| Pseudomonas aeruginosa | ATCC 27312 |
ATCC, American Type Culture Collection
Aliquots of the strains were stored as 20% glycerol stocks at −80°C. Every 2 weeks, fresh agar streak plates were prepared, and for daily utilization, a fresh overnight culture was prepared from a single colony of the designated species taken from an agar streak plate. Consumables utilized in these studies were employed in sterile form, where applicable. Agar, growth media, buffers, and other solutions utilized in the studies were prepared according to manufacturers’ recommendations and/or following internal protocols. Then PI (Betadine; NDC code 67618-150-01, Lot No. 80558-16; Medline Industries, Northfield, IL) was utilized as received or aseptically diluted with sterile 0.9% saline to obtain various PI dilutions. For clarity, the diluted concentrations of PI (eg, 50% PI) refers to the diluted percentage of stock Betadine solution, not the percentage of povidone-iodine (ie, 50% PI = 50% Betadine = 5% povidone-iodine). TAB was prepared as per Adams:17 0.2% w/v cefazolin sodium salt (Cat. No. 14325, Lot No. CZSS3756455; ChemImpex, Wood Dale, IL, 92.9%), 0.016% w/v gentamicin sulfate salt (Cat. No. G1264-5 g, Lot No. SLBL4466V; Sigma, St. Louis, MO), and 100 IU/mL bacitracin (Cat. No. 226100050, Lot No. A0371439; Acros Organics, Waltham, MA, 60 IU/mg) in sterile saline. The 50% PI with TAB was prepared by mixing equal volumes of 100% PI and TAB. Human serum was obtained from Millipore Sigma (Burlington, MA, Cat. No. H4522). Dey-Engley (D/E) broth (Cat. No. 281910; Becton Dickinson Difco, Franklin Lakes, NJ) was utilized for neutralization of the various antimicrobial test articles and was prepared per the manufacturer’s instructions.
C. acnes was cultured under anaerobic conditions throughout the experiment, and both the starting culture employed for inoculation and the tryptic soy agar (TSA) plates employed for enumeration were incubated for 48 hours due to the low growth rate of this species. Given the short timepoints, treatments against C. acnes biofilms were performed in aerobic conditions, as with other species. All other species were grown aerobically following standard conditions as recommended by the ATCC.
Time-Kill Assay Setup
Assays to determine the efficacy of the antimicrobial solutions against biofilm-associated bacteria were performed in polystyrene 96-well microtiter plates. Briefly, biofilms were established on the bottom of the microtiter plate wells by inoculating each well with 100 µL of an overnight culture diluted in tryptic soy broth to a final bacterial concentration of approximately 107 CFU/mL. Biofilms were grown for 48 hours in static conditions at 37°C and in a humidified atmosphere. Fresh tryptic soy broth media was replaced at 24 hours. At the 48-hour mark, contents of the wells were aspirated to remove loosely associated and planktonic bacteria, leaving only biofilm-associated bacteria prior to treatment with antimicrobial solutions. Within a single 96-well plate, a single antimicrobial solution type was assessed, and a number of treatment time points were evaluated, ranging from 1 to 30 minutes. To the preformed biofilms, 300 µL of the antimicrobial solution was added and then aspirated at the designated treatment time, followed by the addition of neutralizer (D/E broth, 300 µL/well) for 10 minutes. The neutralizer was then aspirated, and fresh solution of D/E added to the wells. Note that in this assay, the majority of the treatment is removed through aspiration, allowing for TAB to be evaluated and appropriately neutralized, unlike the planktonic assay previously reported,45 which required a 24-well plate setup to allow for proper neutralization of the TAB treatment (ie, 400× dilution). Viable bacteria were recovered through indirect sonication, wherein the 96-well plate was placed on a water bath sonicator equipped with a metal lid. Bacterial survivors were enumerated through serial dilution and plating on 1.5% TSA plates. Each condition was evaluated in 3 independent experiments and 8 sample replicates within the experiment. In this study, all colonies were counted and included in the reported results; however, it is important to note that the reliable detection limit for standard plate counts is 10 CFU per plate, which corresponds to 1.8 log when plating 150 μL of the neutralized sample, as done in this assay.
For experiments including serum, biofilms were grown as described above, followed by addition of serum and the designated treatment (ie, varying concentrations of PI). A stock of human serum was prepared utilizing commercially available human serum and diluting with saline so that the final concentration of proteins was 50 g/L. To the wells containing preformed biofilms, 75 µL/well of the designated concentration of serum was added and after 5 minutes, 225 µL/well of the designated PI solution was added. The biofilms were treated with the serum-PI suspension for 5 minutes. Recovery and enumeration of surviving bacteria was performed as described above. Each condition was evaluated in 3 independent experiments and 8 sample replicates were included for each treatment and for each experimental replicate. Data were collected from multiple in vitro experiments performed between July 2017 and August 2019.
RESULTS
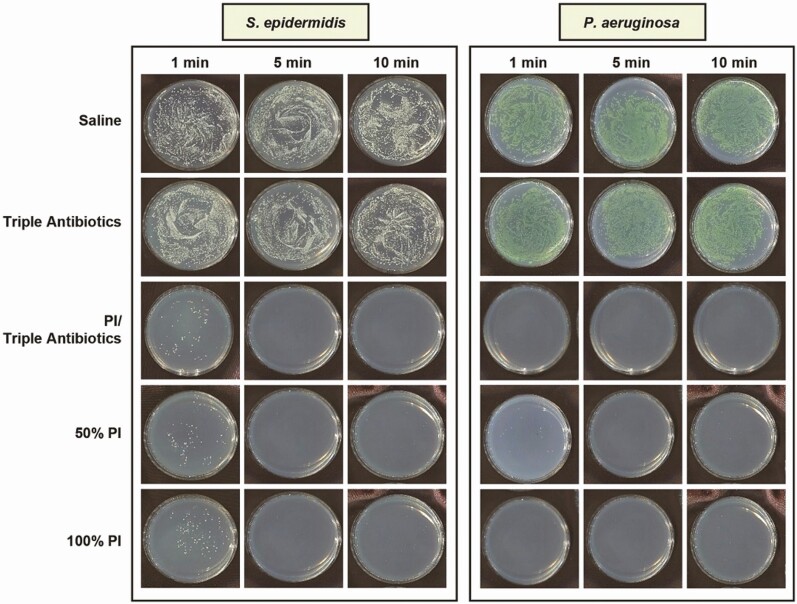
The time-kill assay studies were designed to evaluate the efficacy of antimicrobial solutions against biofilm-associated bacteria using an in vitro model system that approximates their clinical use, where there is a high ratio of antimicrobial solution to bacteria. For this study, eradication was defined as reduction of bacteria beyond the reliable limit of detection for standard plate counts, which, as stated earlier, is 10 CFU/plate or 1.8 log utilizing the methodology reported in the Methods section. As such, bacterial survival at or less than 1.8 log would be considered eradication of the biofilm-associated bacteria. Following treatment, bacterial survival was qualitatively observed and quantified utilizing enumeration. Examples of bacteria survival on TSA plates are shown in Figure 1, with S. epidermidis and P. aeruginosa chosen as representative gram-positive and gram-negative species, respectively, regarding residual bacterial survival following treatment. Neither saline nor TAB appeared to be effective at eradicating biofilm-associated bacteria across all 5 species (data not shown for S. aureus, C. acnes, or R. pickettii) at any of the 5 time points evaluated (data for 20 and 30 minutes of exposure not shown). Both full-strength (100%) and half-strength (50%) PI with or without TAB exhibited efficacy at eradicating biofilm-associated bacteria following exposure of at least 1 minute, except for the Staphylococci species, where some residual bacterial survival was observed.
Figure 1.
Qualitative visualization of biofilm-associated bacterial survival as exhibited with representative gram-positive (Staphylococcus epidermidis) or gram-negative (Pseudomonas aeruginosa) species in tryptic soy agar plates following treatment with saline, triple antibiotic solution (TAB), 50% PI (±TAB), or 100% PI for 1, 5, or 10 minutes. Data not shown for 20- and 30-minute treatment times. Note: PI refers to 10% povidone-iodine or full-strength Betadine.
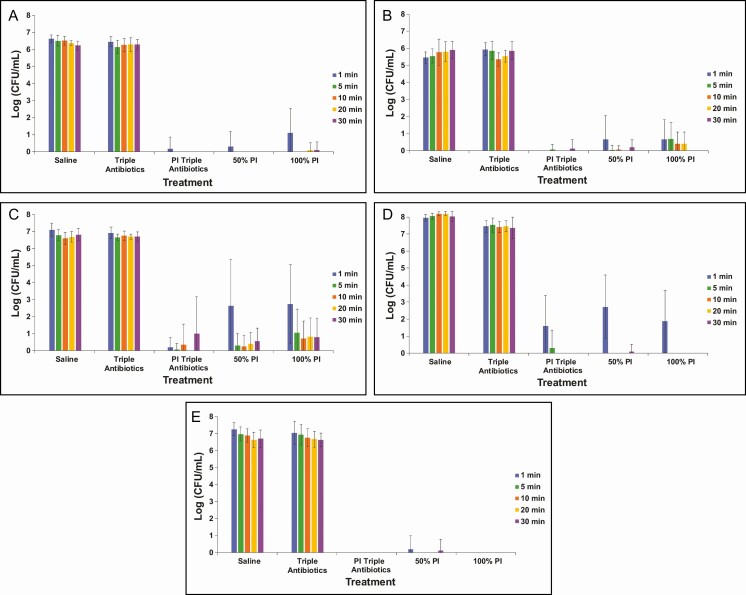
This qualitative assessment reflects the results seen with bacterial enumeration (Figure 2). TAB was ineffective at all timeframes tested (≤30 minutes), which was comparable with the saline control treatment. This suggests that TAB, when administered alone, was ineffective at significantly reducing biofilm-associated bacteria for all 5 species evaluated within the tested treatment times. The combination treatment of 50% PI-TAB eradicated biofilm within 5 minutes for all bacterial species but required only approximately 1 minute to eradicate the non-Staphyloccocus bacterial species (ie, C. acnes, P. aeruginosa, and R. pickettii) biofilm. Both full-strength and half-strength PI were generally effective at significantly reducing biofilm-associated bacterial species within 5 minutes of exposure, although some variability within the limit of detection was observed with S. aureus and R. pickettii in this timeframe.
Figure 2.
Enumeration of biofilm-associated bacterial survival for gram-negative Pseudomonas aeruginosa (A) and Ralstonia pickettii (B) as well as gram-positive Staphylococcus aureus (C), Staphylococcus epidermidis (D), and Cutibacterium acnes (E) following treatment with saline, triple antibiotic solution (TAB), 50% PI (±TAB), or 100% PI for 1, 5, 10, 20, or 30 minutes. Data are represented as mean bacterial survival ± standard deviation in log (CFU/mL) from 3 experimental replicates and 8 treatment replicates per experiment. Note: PI refers to 10% povidone-iodine or full-strength Betadine.
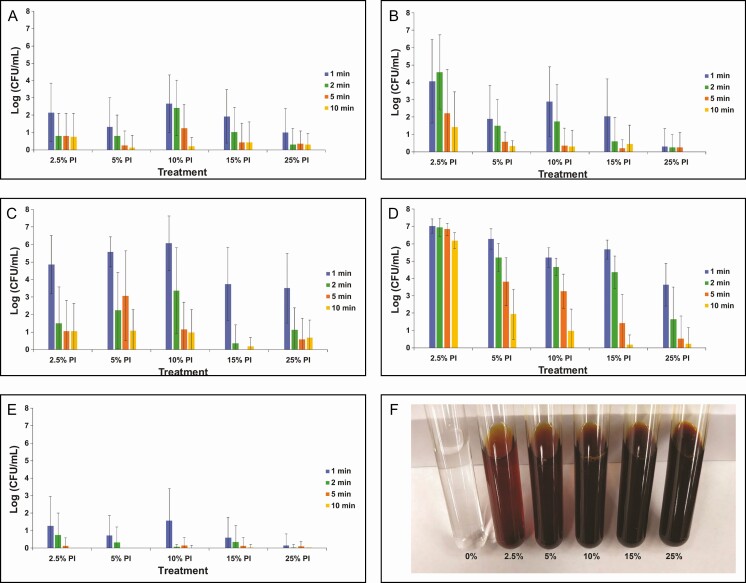
Given its effectiveness at concentrations of 50% (half-strength) or higher, the efficacy of dilute concentrations of PI between 2.5% and 25% was evaluated with treatment times between 1 and 10 minutes (Figure 3A-E). For reference, the color of diluted PI at 2.5%, 5%, 10%, 15%, and 25%, respectively, is shown in Figure 3F. Dilute concentrations of PI (down to 2.5%) exhibited significant reduction in biofilm-associated bacteria after at least 2 minutes of exposure for C. acnes and at least 5 minutes of exposure for P. aeruginosa. This suggests that these 2 species are quite susceptible to PI treatment. R. pickettii appeared to require at least 15% PI to show significant log reduction after at least 2 minutes of treatment. Very dilute concentrations of PI were not as effective for the 2 Staphylococcus strains (S. epidermidis and S. aureus). For example, at the 5-minute treatment time, 10% PI or higher was required to eradicate S. aureus biofilm and 25% PI was required to eradicate S. epidermidis biofilm, suggesting that S. epidermidis may be the most tolerant species to PI, which is consistent with the results of our study with planktonic bacteria.45
Figure 3.
Enumeration of biofilm-associated bacterial survival for gram-negative Pseudomonas aeruginosa (A) and Ralstonia pickettii (B) as well as gram-positive Staphylococcus aureus (C), Staphylococcus epidermidis (D), and Cutibacterium acnes (E) following treatment with dilute concentrations of PI (2.5%, 5%, 10%, 15%, and 25%) for 1, 2, 5, or 10 minutes. Data are represented as mean bacterial survival ± standard deviation in log (CFU/mL) from 3 experimental replicates and 8 treatment replicates per experiment. (F) Color palette for different dilutions of PI shown for reference. Note: PI refers to 10% povidone-iodine or full-strength Betadine.
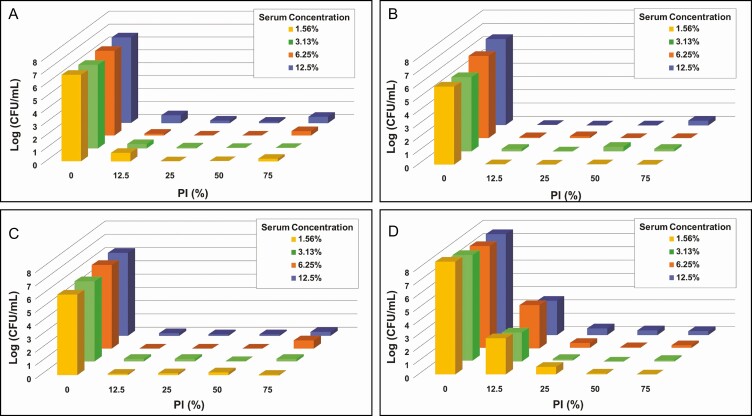
Antimicrobial efficacy of PI may be affected by the presence of proteins, so a number of experiments were performed in the presence of serum proteins utilizing 4 bacterial species of interest: 2 gram-positive bacteria, S. epidermidis and S. aureus, and 2 gram-negative bacteria, P. aeruginosa and R. pickettii. C. acnes was not evaluated in these experiments given the high efficacy observed for PI against this species. PI concentrations between 12.5% and 75% were evaluated to ensure that the full range of log reduction in biofilm-associated bacteria could be detected. The concentration of serum ranged between 1.5% and 12.5% to cover the potential range of protein levels present in the breast pocket as reported in the literature.24,25 For P. aeruginosa, R. pickettii, and S. aureus, diluting to one-eighth strength PI (12.5%) did not appear to have a pronounced negative effect on PI efficacy. However, there was a reduction in PI efficacy against S. epidermidis when PI was diluted lower than quarter-strength (25% PI). Of the 4 bacterial species evaluated, only efficacy against S. epidermidis appeared to be affected by the presence of serum and only at the lowest concentration of PI evaluated, that is, 12.5% PI (Figure 4).
Figure 4.
Enumeration of biofilm-associated bacterial survival for gram-negative Pseudomonas aeruginosa (A) and Ralstonia pickettii (B) as well as gram-positive Staphylococcus aureus (C), Staphylococcus epidermidis (D) under varying concentrations of both human serum and PI with treatment for 5 minutes. The ranges of tested concentrations for PI (0%-75%) and serum (1.56%-12.5%) are represented on the x-axis and y-axis, respectively. Data are represented as mean bacterial survival in log (CFU/mL) in the z-axis from 3 experimental replicates and 8 treatment replicates per experiment. Note: PI refers to 10% povidone-iodine or full-strength Betadine.
DISCUSSION
In situations where a mature capsule has already formed around a breast implant or tissue expander, biofilm-associated bacteria may be present at the implant-tissue interface. Bacteria may be introduced through various means, such as from inadvertent contamination during the original implantation, bacterial co-localization due to hematogenous spread, and/or bacterial transfer from the breast microbiome, all of which could lead to the development of biofilm on or around the implant.43,44 As such, plastic surgeons often utilize antimicrobial solutions for breast pocket irrigation during revision or exchange surgeries. According to recent American Society of Plastic Surgeons surveys,15,16 over one-half of all plastic surgeons perform breast pocket irrigation in reconstructive procedures with either TAB or PI, with TAB being generally favored.15 However, little consistency regarding clinical utilization parameters such as specific concentrations of antimicrobial solutions and treatment times have been documented. The current study evaluates the effect of different antimicrobial solution concentrations and exposure times on various bacterial species utilizing a series of in vitro assays.
Our initial selection of treatment times for these antimicrobial solutions was up to 30 minutes given the more challenging nature of biofilm-associated bacteria. However, given the minimal difference observed at the longer exposure times, subsequent analyses focused on shorter times (≤10 minutes), which is also consistent with an acceptable amount of time for intraoperative procedures such as breast pocket irrigation. Within this short exposure timeframe, PI-containing solutions were quite effective in terms of log reduction of biofilm-associated bacteria when treated for at least 5 minutes (Figures 1 and 2). Additionally, we studied the efficacy of diluted PI given the practice of diluting it by some surgeons. We set out to determine the minimum threshold concentration of PI required for significant log reduction of biofilm-associated bacteria. Due to the increased complexity of bacterial biofilms compared with its planktonic form and its associated tolerance against antimicrobial solutions, we observed that at 5 minutes of exposure, 25% (quarter-strength) to 50% (half-strength) PI was required to retain good antimicrobial effects (Figures 2 and 3). The relatively strong efficacy observed with utilization of PI is expected when considering its mechanism of action. The active component of PI is iodine, and at the highest concentration utilized in this study (100% PI), the free iodine is approximately 1%. Iodine quickly dissociates from the povidone-iodine complex, penetrating the cells and rapidly reacting with a multitude of molecular targets, including proteins, nucleotides, and fatty acids, ultimately causing bacterial death. Treatment with PI can also elicit cytotoxic effects on healthy cells27-30 and, as such, some clinicians dilute PI to avoid these effects while mitigating bioburden. However, as noted in our results and in contrast to the results of our planktonic bacteria study,45 diluting PI lower than quarter-strength will likely minimize its efficacy against biofilm-associated bacteria.
In addition, for secondary procedures (ie, implant revision with capsulectomy or tissue expander-implant exchange), the implant is placed in a potentially protein-rich environment unless hemostasis is completely controlled. In combination with the potential for preformed biofilm within the capsule, interaction of serum proteins could have a pronounced negative effect on the efficacy of an antimicrobial solution (eg, PI), which has been suggested by others.31-33 The presence of blood serum proteins in the breast pocket can neutralize the efficacy of PI. In this study, we aimed to determine the efficacy of dilute PI as a function of serum concentration. Our goal was to establish the minimum concentration of PI that could significantly reduce biofilm-associated bacteria while standing up to the neutralizing effects of serum proteins. Given that Staphylococcus epidermidis is one of the most common microorganisms seen in the breast microbiome and biofilm-associated bacteria tend to be polymicrobial in nature, the in vitro data suggest that PI efficacy may not be maintained if diluted to concentrations lower than 25% in the presence of between 1% and 12.5% serum (Figure 4). What is also relevant to note is that in the absence of serum (see Figure 3), quarter-strength (25%) PI also significantly reduces the biofilm-associated microbial load for all species if treatment time is at least 5 minutes. Taken together, this suggests that at PI concentrations relevant for significant log reduction of biofilm-associated bacteria (>25%), the effect of serum proteins at levels likely to be present in the breast pocket may be less pronounced. It is also important to note that these reported studies are in vitro experiments that do not necessarily capture the whole picture of the clinical situation. The goal here was to provide general guidance with respect to how both PI dilution and the presence of serum proteins affect anti-biofilm efficacy. Furthermore, there is a difference between a high level of observed efficacy and implant and/or surgical pocket “sterilization” utilizing an antimicrobial treatment. Any level of bioburden left on the implant or within the breast pocket has the potential to develop into an infection after surgery; however, significantly reducing the number of bacteria will certainly limit this potential as the natural immune response ensues. All of these are important considerations that should be followed-up with further studies utilizing in vivo preclinical models and clinical studies.
As highlighted in a recent American Society of Plastic Surgeons survey on utilization patterns around breast pocket irrigation in reconstructive breast surgery,15 approximately 60% of plastic surgeons utilize TAB. Various clinical studies, as summarized in Lynch et al46, suggest that irrigation with Adams’s TAB is effective at reducing infection and capsular contracture rates compared with saline or no irrigation. However, nearly 75% of surveyed plastic surgeons utilized a dwell time of 2 minutes or less for their pocket irrigation solution in reconstructive procedures.15 Our in vitro study results show that short exposure times (eg, ≤10 minutes) do not necessarily support TAB (containing bacitracin, cefazolin, and gentamicin) as an effective treatment for significant log reduction of biofilm-associated bacteria for the strains that were tested. This makes sense because the individual components of TAB (ie, bacitracin, cefazolin, and gentamicin) generally require longer exposure times and often only affect metabolically active bacteria. For example, bacitracin is a polypeptide antibiotic that affects transport of key building blocks to the growing cell wall, so, over time, the presence of this antibiotic will stunt the formation of the cell wall.47 Similarly, cefazolin exerts its effects by preventing peptidoglycan production, ultimately leading to cell lysis.48 Additionally, gentamicin works by binding to the ribosome and inhibiting protein synthesis.49
Taken together, it suggests that bactericidal activity through antibiotics will not manifest without bacterial metabolic activity and/or proliferation, which requires an extended period of treatment time (ie, generally measured in hours and days, not minutes). Even though the concentrations of antibiotics in TAB utilized in our in vitro assay are significantly higher (>100×) than the minimal bactericidal concentration, the exposure times evaluated in this study are likely not long enough to obtain the desired bactericidal effect. This is further confounded when the bacteria are in a metabolically quiescent state, as is the case with biofilm-associated bacteria. Therefore, given that the mechanism of action for TAB requires metabolic activity, it is not surprising that TAB alone did not show efficacy in our in vitro assay, but clinically, local delivery of TAB may still be important because some antibiotic will remain in the surgical pocket to protect against any remaining bacteria over time. A prospective clinical study looking at the utilization of TAB irrigation by Adams and colleagues19 suggested a reduction in rates of capsular contracture. However, there was no active evacuation of the TAB solution following pocket irrigation, which may contribute to the outcomes. Additionally, a recent update by the same group supports the assertion that continuous exposure to TAB may be important because their in vitro data suggest that TAB alone may require a longer exposure time (eg, at least 18 hours for R. pickettii) to be completely bactericidal against planktonic bacteria compared with PI-containing solutions.22 Another recent study looking at TAB efficacy against S. aureus and S. epidermidis shows 1 log reduction after 30 minutes of exposure.50 Combined, these results suggest that a much longer exposure time may be required to significantly reduce biofilm-associated bacteria compared with planktonic bacteria. Given the varying clinical utilization patterns and protocols, TAB effectiveness may vary greatly, depending on the amount of residual antibiotic solution that may persist in the pocket. There are no published studies on the persistence of antimicrobial irrigation fluid within the pocket, which could vary depending on pocket size given varying dissection techniques. This may help explain the mixed clinical results reported by others on the utilization of TAB irrigation in breast surgeries.51-53 Because our study did not look specifically at prolonged exposure of bacteria to TAB, it is unclear whether longer term exposure would be more effective and may warrant further study considering that irrigation fluid may remain in the surgical site. Although TAB alone appears to be minimally effective under our test conditions, it is important to note that the combination treatment of TAB with PI appears to be effective. The data suggest that PI is a crucial component for antimicrobial efficacy, either alone or in combination with Adams’s TAB, with the latter possibly being more important for longer term antimicrobial action post-surgery.
There are some limitations associated with this study. Biofilms tend to be polymicrobial in nature, and our in vitro assays only studied a single bacterial species at a time. In addition, these in vitro assays were performed with 48-hour-old biofilm-associated bacteria, which may not reflect what would occur with mature biofilm that may be present on or surrounding the implanted device in revision or exchange surgeries. This study was conducted utilizing 5 strains of bacteria known to be relevant to the clinical setting, so additional bacterial species could be tested in future studies. In addition, the focus of these studies was on evaluating TAB and PI solutions; there has been increasing interest in the utilization of stabilized hypochlorous acid for pocket irrigation and this could be evaluated in a separate study. Furthermore, the in vitro assay setup in this study is a simplified 2-dimensional testing platform, which employed polystyrene instead of silicone surfaces. The role of surface texturization as well as its effects on protein binding have been evaluated in various studies32,33,54 and suggest that the presence of proteins such as fibrinogen and type I or type III collagen may have a pronounced effect on bacterial biofilm formation. In our study, data suggest that interaction of serum proteins with PI could negatively impact its efficacy. Therefore, future studies could interrogate the complex interplay between implant surface properties, serum protein binding, and possible inactivation of antimicrobial solutions such as PI. As such, the correlation between the results of these in vitro studies and clinical outcomes is not known and could be further investigated through appropriate in vivo preclinical studies and clinical work. However, these in vitro findings are supported by the outcomes reported for utilizing PI in pocket irrigation in prospective clinical studies. Clinical studies from Burkhardt and colleagues55,56 demonstrated a significant reduction in grade III/IV capsular contracture with utilization of antibiotic-containing solutions or foams that could have prolonged contact with surrounding tissue as well as PI irrigation compared with saline controls. More recently, Pat McGuire performed a risk factor analysis for capsular contracture in patients from the prospective Continued Access Reconstruction/Revision Expansion trial and noted that intraoperative utilization of antibiotic-based pocket irrigation instead of PI was a risk factor for increased capsular contracture in the primary reconstruction cohort.57
CONCLUSIONS
In summary, we acknowledge that there are multiple approaches for breast implant pocket irrigation designed to mitigate the presence of biofilm within the capsule, including saline, TAB, PI diluted in combination with TAB, and PI. In this report, we aimed to evaluate the efficacy of TAB and various dilutions of PI in significantly reducing biofilm-associated bacteria as a function of time. Based on the results of this study, 10% povidone-iodine (full-strength Betadine) appears effective in killing both gram-positive and gram-negative biofilm-associated bacteria ranging from full-strength down to 25% PI (quarter-strength or 2.5% povidone-iodine). Our findings are supported by a recent report by Culbertson et al22 that recommends the utilization of PI-containing antimicrobial solutions for breast pocket irrigation and suggests that up to 18 hours of exposure to TAB alone does not consistently eradicate all bacterial species tested. Saline, which was utilized as a control in the study, showed minimal bactericidal effect, suggesting that its value for breast pocket irrigation to mitigate bioburden load is questionable and may simply be beneficial only for removing nonbacterial components like wound fluid or blood. The data also suggest that TAB without PI may not be the ideal choice for perioperative utilization as a breast pocket irrigant. Our data suggest that PI-containing solutions over either saline or TAB without PI allow for significant log reduction of biofilm-associated bacteria. In addition, care should be taken to minimize excessive dilution of PI to maintain efficacy against biofilm-associated bacteria.
Acknowledgments
The authors acknowledge Aaron D. Strickland, PhD, and Ellen E. Lantz, PhD, of iFyber LLC, Ithaca, NY, for their contributions to study design, collection, analysis, and interpretation of the data.
Disclosures
Drs Leung and Hariri are employees of Allergan Aesthetics, an AbbVie company (Irvine, CA), and Drs Bionda, Bevels, and Moran are employees of iFyber LLC. Dr Jewell has received honoraria from Allergan plc and serves as a consultant for Allergan plc and New Beauty magazine. Allergan Aesthetics, an AbbVie company, participated in the development of the study design and in the analysis and interpretation of the data. iFyber LLC (Ithaca, NY) participated in the study design, collection, analysis, and interpretation of the data. All authors received no honorarium or other form of financial support related to the development of this article.
Funding
This study was sponsored by Allergan Aesthetics, an AbbVie company (Irvine, CA). Assistance in generation of the figures was provided to the authors by Peloton Advantage (Parsippany, NJ) and was funded by Allergan Aesthetics, an AbbVie company. All authors received no honorarium or other form of financial support related to the development of this article.
REFERENCES
- 1. Ahn CY, Ko CY, Wagar EA, Wong RS, Shaw WW. Microbial evaluation: 139 implants removed from symptomatic patients. Plast Reconstr Surg. 1996;98(7):1225-1229. [DOI] [PubMed] [Google Scholar]
- 2. Danino MA, Efanov JI, Dimitropoulos G, et al. Capsular biofilm formation at the interface of textured expanders and human acellular dermal matrix: a comparative scanning electron microscopy study. Plast Reconstr Surg. 2018;141(4):919-928. [DOI] [PubMed] [Google Scholar]
- 3. Hu H, Johani K, Almatroudi A, et al. Bacterial biofilm infection detected in breast implant-associated anaplastic large-cell lymphoma. Plast Reconstr Surg. 2016;137(6):1659-1669. [DOI] [PubMed] [Google Scholar]
- 4. Jacombs A, Tahir S, Hu H, et al. In vitro and in vivo investigation of the influence of implant surface on the formation of bacterial biofilm in mammary implants. Plast Reconstr Surg. 2014;133(4):471e-480e. [DOI] [PubMed] [Google Scholar]
- 5. Poppler L, Cohen J, Dolen UC, et al. Histologic, molecular, and clinical evaluation of explanted breast prostheses, capsules, and acellular dermal matrices for bacteria. Aesthet Surg J. 2015;35(6):653-668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Deva AK, Adams WP Jr, Vickery K. The role of bacterial biofilms in device-associated infection. Plast Reconstr Surg. 2013;132(5):1319-1328. [DOI] [PubMed] [Google Scholar]
- 7. Pajkos A, Deva AK, Vickery K, Cope C, Chang L, Cossart YE. Detection of subclinical infection in significant breast implant capsules. Plast Reconstr Surg. 2003;111(5):1605-1611. [DOI] [PubMed] [Google Scholar]
- 8. Virden CP, Dobke MK, Stein P, Parsons CL, Frank DH. Subclinical infection of the silicone breast implant surface as a possible cause of capsular contracture. Aesthetic Plast Surg. 1992;16(2):173-179. [DOI] [PubMed] [Google Scholar]
- 9. del Pozo JL, Auba C. Role of biofilms in breast implant associated infections and capsular contracture. Adv Exp Med Biol. 2015;831:53-67. [DOI] [PubMed] [Google Scholar]
- 10. Netscher DT, Weizer G, Wigoda P, Walker LE, Thornby J, Bowen D. Clinical relevance of positive breast periprosthetic cultures without overt infection. Plast Reconstr Surg. 1995;96(5):1125-1129. [DOI] [PubMed] [Google Scholar]
- 11. Pittet B, Montandon D, Pittet D. Infection in breast implants. Lancet Infect Dis. 2005;5(2):94-106. [DOI] [PubMed] [Google Scholar]
- 12. Tamboto H, Vickery K, Deva AK. Subclinical (biofilm) infection causes capsular contracture in a porcine model following augmentation mammaplasty. Plast Reconstr Surg. 2010;126(3):835-842. [DOI] [PubMed] [Google Scholar]
- 13. Frois AO, Harbour PO, Azimi F, et al. The role of antibiotics in breast pocket irrigation and implant immersion: a systematic review. Plast Reconstr Surg Glob Open. 2018;6(9):e1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chopra K, Gowda AU, McNichols CHL, Brown EN, Slezak S, Rasko Y. Antimicrobial prophylaxis practice patterns in breast augmentation: a national survey of current practice. Ann Plast Surg. 2017;78(6):629-632. [DOI] [PubMed] [Google Scholar]
- 15. Epps MT, Langsdon S, Pels TK, et al. Pocket irrigation and technique during reconstructive surgery: an American Society of Plastic Surgery survey of current practice. Ann Plast Surg. 2019;82(6S Suppl 5):S427-S432. [DOI] [PubMed] [Google Scholar]
- 16. Gowda AU, Chopra K, Brown EN, Slezak S, Rasko Y. Preventing breast implant contamination in breast reconstruction: a national survey of current practice. Ann Plast Surg. 2017;78(2):153-156. [DOI] [PubMed] [Google Scholar]
- 17. Adams WP Jr, Conner WC, Barton FE Jr, Rohrich RJ. Optimizing breast-pocket irrigation: the post-betadine era. Plast Reconstr Surg. 2001;107(6):1596-1601. [DOI] [PubMed] [Google Scholar]
- 18. Adams WP Jr, Conner WC, Barton FE Jr, Rohrich RJ. Optimizing breast pocket irrigation: an in vitro study and clinical implications. Plast Reconstr Surg. 2000;105(1):334-338; discussion 339. [DOI] [PubMed] [Google Scholar]
- 19. Adams WP Jr, Rios JL, Smith SJ. Enhancing patient outcomes in aesthetic and reconstructive breast surgery using triple antibiotic breast irrigation: six-year prospective clinical study. Plast Reconstr Surg. 2006;117(1):30-36. [PubMed] [Google Scholar]
- 20. Jewell ML, Adams WP Jr. Betadine and breast implants. Aesthet Surg J. 2018;38(6):623-626. [DOI] [PubMed] [Google Scholar]
- 21. Wiener TC. Betadine and breast implants: an update. Aesthet Surg J. 2013;33(4):615-617. [DOI] [PubMed] [Google Scholar]
- 22. Culbertson EJ, Felder-Scott C, Deva AK, Greenberg DE, Adams WP. Optimizing breast pocket irrigation: the breast implant-associated anaplastic large cell lymphoma (BIA-ALCL) era. Aesthet Surg J. 2020;40(6):619-625. [DOI] [PubMed] [Google Scholar]
- 23. Brindle CT, Porter S, Bijlani K, et al. Preliminary results of the use of a stabilized hypochlorous acid solution in the management of ralstonia pickettii biofilm on silicone breast implants. Aesthet Surg J. 2018;38(suppl_2):S52-S61. [DOI] [PubMed] [Google Scholar]
- 24. Haws MJ, Gingrass MK, Porter RS, Brindle CT. Surgical breast pocket irrigation with hypochlorous acid (HOCl): an in vivo evaluation of pocket protein content and potential HOCl antimicrobial capacity. Aesthet Surg J. 2018;38(11):1178-1184. [DOI] [PubMed] [Google Scholar]
- 25. Hu H, Sleiman J, Johani K, Vickery K. Hypochlorous acid versus povidone-iodine containing irrigants: which antiseptic is more effective for breast implant pocket irrigation? Aesthet Surg J. 2018;38(7):723-727. [DOI] [PubMed] [Google Scholar]
- 26. Zhadan O, Becker H. Surgical site irrigation in plastic surgery. Aesthet Surg J. 2018;38(3):265-273. [DOI] [PubMed] [Google Scholar]
- 27. Balin AK, Pratt L. Dilute povidone-iodine solutions inhibit human skin fibroblast growth. Dermatol Surg. 2002;28(3):210-214. [DOI] [PubMed] [Google Scholar]
- 28. Lineaweaver W, McMorris S, Soucy D, Howard R. Cellular and bacterial toxicities of topical antimicrobials. Plast Reconstr Surg. 1985;75(3):394-396. [DOI] [PubMed] [Google Scholar]
- 29. Liu JX, Werner JA, Buza JA III, Kirsch T, Zuckerman JD, Virk MS. Povidone-iodine solutions inhibit cell migration and survival of osteoblasts, fibroblasts, and myoblasts. Spine (Phila Pa 1976). 2017;42(23):1757-1762. [DOI] [PubMed] [Google Scholar]
- 30. Müller G, Kramer A. Biocompatibility index of antiseptic agents by parallel assessment of antimicrobial activity and cellular cytotoxicity. J Antimicrob Chemother. 2008;61(6):1281-1287. [DOI] [PubMed] [Google Scholar]
- 31. Müller R, Eidt A, Hiller KA, et al. Influences of protein films on antibacterial or bacteria-repellent surface coatings in a model system using silicon wafers. Biomaterials. 2009;30(28):4921-4929. [DOI] [PubMed] [Google Scholar]
- 32. Walker JN, Pinkner CL, Lynch AJL, et al. Deposition of host matrix proteins on breast implant surfaces facilitates staphylococcus epidermidis biofilm formation: in vitro analysis. Aesthet Surg J. 2020;40(3):281-295. [DOI] [PubMed] [Google Scholar]
- 33. Walker JN, Pinkner CL, Pinkner JS, Hultgren SJ, Myckatyn TM. The detection of bacteria and matrix proteins on clinically benign and pathologic implants. Plast Reconstr Surg Glob Open. 2019;7(2):e2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Klein GM, Phillips BT, Dagum AB, Bui DT, Khan SU. Infectious loss of tissue expanders in breast reconstruction: are we treating the right organisms? Ann Plast Surg. 2017;78(2):149-152. [DOI] [PubMed] [Google Scholar]
- 35. Rieger UM, Mesina J, Kalbermatten DF, et al. Bacterial biofilms and capsular contracture in patients with breast implants. Br J Surg. 2013;100(6):768-774. [DOI] [PubMed] [Google Scholar]
- 36. Thornton JW, Argenta LC, McClatchey KD, Marks MW. Studies on the endogenous flora of the human breast. Ann Plast Surg. 1988;20(1):39-42. [DOI] [PubMed] [Google Scholar]
- 37. Walker JN, Hanson BM, Pinkner CL, et al. Insights into the microbiome of breast implants and periprosthetic tissue in breast implant-associated anaplastic large cell lymphoma. Sci Rep. 2019;9(1):10393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Squaiella-Baptistão CC, Teixeira D, Mussalem JS, Ishimura ME, Longo-Maugéri IM. Modulation of Th1/Th2 immune responses by killed Propionibacterium acnes and its soluble polysaccharide fraction in a type I hypersensitivity murine model: induction of different activation status of antigen-presenting cells. J Immunol Res. 2015;2015:132083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tchaptchet S, Kirberg J, Freudenberg N, Schamel WW, Galanos C, Freudenberg MA. Innate, antigen-independent role for T cells in the activation of the immune system by Propionibacterium acnes. Eur J Immunol. 2010;40(9):2506-2516. [DOI] [PubMed] [Google Scholar]
- 40. Alcalá R, Llombart B, Lavernia J, Traves V, Guillén C, Sanmartín O. Skin involvement as the first manifestation of breast implant-associated anaplastic large cell lymphoma. J Cutan Pathol. 2016;43(7):602-608. [DOI] [PubMed] [Google Scholar]
- 41. Leberfinger AN, Behar BJ, Williams NC, et al. Breast implant-associated anaplastic large cell lymphoma: a systematic review. JAMA Surg. 2017;152(12):1161-1168. [DOI] [PubMed] [Google Scholar]
- 42. Rastogi P, Deva AK, Prince HM. Breast implant-associated anaplastic large cell lymphoma. Curr Hematol Malig Rep. 2018;13(6):516-524. [DOI] [PubMed] [Google Scholar]
- 43. Reischies FMJ, Krause R, Holzer J, et al. What can we learn from sonication results of breast implants? PLoS One. 2017;12(8):e0182267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Adams WP Jr, Culbertson EJ, Deva AK, et al. Macrotextured breast implants with defined steps to minimize bacterial contamination around the device: experience in 42,000 implants. Plast Reconstr Surg. 2017;140(3):427-431. [DOI] [PubMed] [Google Scholar]
- 45. Jewell ML, Hariri S, Lantz EE, Jewell HL, Strickland AD, Leung BK. In vitro evaluation of common antimicrobial solutions used for breast implant soaking and breast pocket irrigation—part 1: efficacy against planktonic bacteria. Aesthet Surg J. 2021;41(11):1242–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lynch JM, Sebai ME, Rodriguez-Unda NA, Seal S, Rosson GD, Manahan MA. Breast pocket irrigation with antibiotic solution at implant insertion: a systematic review and meta-analysis. Aesthetic Plast Surg. 2018;42(5):1179-1186. [DOI] [PubMed] [Google Scholar]
- 47. O’Donnell JA, Gelone SP, Safdar A. Topical antibacterials. In: Bennett JE, Dolin R, Blaser MJ, eds. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. Vol. 1. 8th ed. Philadelphia, PA: Elsevier Saunders; 2015:452-462. [Google Scholar]
- 48. Deck DH, Winston LG. Beta-lactam and other cell wall- and membrane-active antibiotics. In: Katzung BG, Trevor AJ, eds. Basic and Clinical Pharmacology. 13th ed. New York, NY: McGraw Hill Education; 2015:1216. [Google Scholar]
- 49. Borovinskaya MA, Pai RD, Zhang W, et al. Structural basis for aminoglycoside inhibition of bacterial ribosome recycling. Nat Struct Mol Biol. 2007;14(8):727-732. [DOI] [PubMed] [Google Scholar]
- 50. Ngaage LM, Elegbede A, Brao K, et al. The efficacy of breast implant irrigant solutions: a comparative analysis using an in vitro model. Plast Reconstr Surg. 2020;146(2):301-308. [DOI] [PubMed] [Google Scholar]
- 51. Campbell CA. The role of triple-antibiotic saline irrigation in breast implant surgery. Ann Plast Surg. 2018;80(6S Suppl 6):S398-S402. [DOI] [PubMed] [Google Scholar]
- 52. Drinane JJ, Bergman RS, Folkers BL, Kortes MJ. Revisiting triple antibiotic irrigation of breast implant pockets: a placebo-controlled single practice cohort study. Plast Reconstr Surg Glob Open. 2013;1(7):e55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Giordano S, Peltoniemi H, Lilius P, Salmi A. Povidone-iodine combined with antibiotic topical irrigation to reduce capsular contracture in cosmetic breast augmentation: a comparative study. Aesthet Surg J. 2013;33(5):675-680. [DOI] [PubMed] [Google Scholar]
- 54. Barr SP, Hill EW, Bayat A. Novel proteomic assay of breast implants reveals proteins with significant binding differences: implications for surface coating and biocompatibility. Aesthet Surg J. 2018;38(9):962-969. [DOI] [PubMed] [Google Scholar]
- 55. Burkhardt BR, Dempsey PD, Schnur PL, Tofield JJ. Capsular contracture: a prospective study of the effect of local antibacterial agents. Plast Reconstr Surg. 1986;77(6):919-932. [PubMed] [Google Scholar]
- 56. Burkhardt BR, Eades E. The effect of Biocell texturing and povidone-iodine irrigation on capsular contracture around saline-inflatable breast implants. Plast Reconstr Surg. 1995;96(6):1317-1325. [DOI] [PubMed] [Google Scholar]
- 57. McGuire P, Reisman NR, Murphy DK. Risk factor analysis for capsular contracture, malposition, and late seroma in subjects receiving Natrelle 410 form-stable silicone breast implants. Plast Reconstr Surg. 2017;139(1):1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]