Abstract
Background
Rejuvenation of the under-eye area is a popular facial aesthetic treatment option.
Objectives
This study evaluated the safety and effectiveness of VYC-15L for the correction of moderate or severe infraorbital hollowing.
Methods
This was a randomized, controlled, single-blind study with a primary endpoint defined as the proportion of participants with ≥1-grade improvement at Month 3 assessed by an evaluating investigator employing the Allergan Infraorbital Hollow Scale. Three-dimensional imaging was conducted to assess infraorbital volume up to Month 12. Procedure pain and injection-site responses (ISRs) were documented, and safety was monitored throughout the study.
Results
At Month 3, the difference between treatment (83.1%) and control (15.6%) was 67.5% (95% CI = 52.9 to 82.0, P < 0.0001). 3D imaging showed a mean volume increase from baseline of 0.733 mL (left) and 0.777 mL (right) at Month 12. Mean pain scores were ≤1.7 (scale of 0 to 10). Most ISRs with initial treatment were mild/moderate and resolved in ≤1 week, including tenderness (49.5%), bruising (42.7%), and swelling (41.7%). Thirty-four participants had treatment-emergent adverse events (TEAEs), of which 14 (10.3%) had treatment-related TEAEs, including bruising (3.8%) and swelling/edema (2.9%), which resolved in ≤2 weeks. Three participants had swelling/edema starting >30 days posttreatment; 2 resolved in ≤4 days, 1 by 45 days. No treatment-related serious AEs were reported.
Conclusions
VYC-15L was safe and effective for the correction of moderate or severe infraorbital hollowing and lasted through 1 year.
Level of Evidence: 2

The periorbital area is often the first to reveal the impact of aging and is among the first facial areas for which patients seek aesthetic treatments.1,2 The eyes are an essential element of facial expression; therefore, the perception of shadowing or “dark circles” under the eye (lower eyelid) attributable to even mild changes in contour can convey a look of unhappiness or fatigue.3 The use of hyaluronic acid (HA) gel for volume correction of the infraorbital area (IOA) is joining the rising trend in non-invasive facial aesthetic procedures and yields a high rate of patient satisfaction.4-9 The IOA is a treatment area where even subtle corrective actions can provide noticeable benefit; however, the treatment approach varies from patient to patient. Therefore, an understanding of the periorbital anatomy, appropriate injection technique, and product selection is essential for a successful treatment outcome.
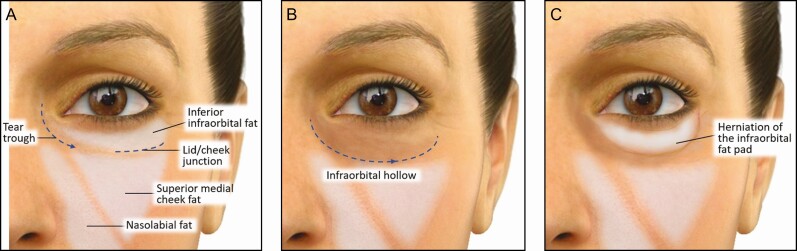
The anatomy of the IOA includes a natural trough, commonly called the tear trough (TT). The TT is a subtle depression that extends from the medial canthus to the medial third of the lower eyelid (Figure 1A).10 From the lower lid’s medial-third, the lid-cheek junction (or palpebromalar groove) can be traced below/parallel to the infraorbital rim. Compared with the cheek skin below it, the TT area’s skin is thinner with a much softer texture. At the subcutaneous level, the TT and lid-cheek junction correspond with the palpebral and orbital portions of the orbicularis muscle and have a characteristic cleft between the 2 portions of the orbicularis muscle. In addition, the malar fat pad adjoins the same muscular junction, providing further differentiation to the distinct areas above and below the lid-cheek junction.
Figure 1.
Characteristics of the infraorbital hollow. (A) Orbital bone and deep midfacial fat pads support the tear trough contour and provide a continuous plane with the lid-cheek junction. (B) Orbital bone resorption and atrophy or loss of mid-facial fat pad volume contribute to the infraorbital hollow, extending to span the lid-cheek junction. (C) Independent of these effects, fat herniation of the infraorbital fat pad can also occur above the hollow, which differentiates a surgical vs nonsurgical correction.
In the context of age-related changes, a primary reason for deepening (or hollowing) of the trough and its extension laterally with the palpebromalar groove is orbital bone resorption and atrophy or loss of mid-facial fat pad volume (Figure 1B). Laxity of regional soft tissue structures can include anterior migration (or prolapse) of the infraorbital fat, an important feature that should be employed to differentiate a patient who is a candidate for surgical vs nonsurgical correction (Figure 1C).11-14 Independent of age-related regional changes, a naturally prominent TT may reflect a mostly familial or cultural bony configuration that results in a relative contour deficit causing perceived shadowing or hollowing. Because the skin overlying the IOA is thin and even transparent in some patients, this area is less forgiving of contour irregularities; therefore, filler product selection is an especially important consideration for optimal results.15
VYC-15L (Juvéderm Volbella XC; Allergan Aesthetics, an AbbVie Company, Irvine, CA) is a 15-mg/mL HA filler with 0.3% w/w lidocaine.16 A propriety mix of higher- and lower-molecular-weight HA and low elastic modulus (G’ ~160 Pa) improves moldability (spreading, modeling, and shaping) and ease of flow during injection, characteristics that make this gel especially suitable for treatment of the perioral area, lips, and TT.8,17-21 This study evaluated the effectiveness and safety of VYC-15L treatment for the correction of moderate to severe bilateral infraorbital hollows in treated participants for over 1 year.
METHODS
Study Design
This was a prospective, multicenter, evaluator-blinded, randomized, and controlled study conducted between January 2018 and August 2019 at 15 US sites to evaluate the safety and effectiveness of VYC-15L used to correct infraorbital hollowing. Eligible participants were males and females (aged ≥22 years) with a Grade 2 (moderate) or Grade 3 (severe) score assessed by the investigator employing the validated 5-point photonumeric Allergan Infraorbital Hollow Scale (AIHS) and considered amenable to improvement to an AIHS grade of 0 or 1 (none or minimal). Both eyes must have qualified but did not need to have the same score (Supplemental Figure 1).22 Participants were excluded from the study if they had atrophic skin in the TT region or large lower lid fat pads that would mask improvement; had steatoblepharon (anterior prolapse of eyelid fat pad); had hyperpigmentation in the IOA (excluding dark under-eye circles not due to hyperpigmentation); had a cornea that projected farther forward than the most anteriorly projected part of the cheek; had received permanent facial implants; had received a blepharoplasty, facelift, or browlift; had undergone fat injections above the subnasale; or had undergone volume augmentation with dermal fillers in the malar area, temples, or around the eyes within 12 months before enrollment. Participants were randomized in a 3:1 ratio to VYC-15L treatment or no-treatment control group and 1:1 to receive first treatment in the right or left side (the same order employed for all treatments). The randomization schedule was generated using an established program that was internally developed with SAS. The study-specific parameters were a 3:1 ratio for treatment group, a 1:1 ratio for left/right side for each treatment group, and block size.
The VYC-15L treatment group received initial treatment and an optional touch-up after 1 month. Touch-up was recommended if, based on the treating investigator’s assessment, an AIHS score of 1 had not been achieved in either infraorbital hollow. However, the decision to proceed with touch-up treatment was made jointly by the participant and treating investigator (TI). All participants were followed up to 12 months to assess safety and effectiveness. Repeat treatment was offered at 12 months. A no-treatment control group maintained parallel assessment visits for a 3-month control period, after which they were offered VYC-15L treatment (including optional touch-up after 1 month) and followed for safety for 9 months. All assessments were conducted by blinded evaluating investigators (EIs) independent of the TIs. This study conformed to the ethical guidelines of the 1975 Declaration of Helsinki, was conducted in compliance with good clinical practice, and obtained informed consent prior to treatment. IRB approval was obtained from Copernicus Group IRB (Cary, NC) and Duke University Health Systems IRB for Clinical Investigations (Durham, NC). This study is registered at clinicaltrials.gov (NCT# 03418545).
VYC-15L Treatment
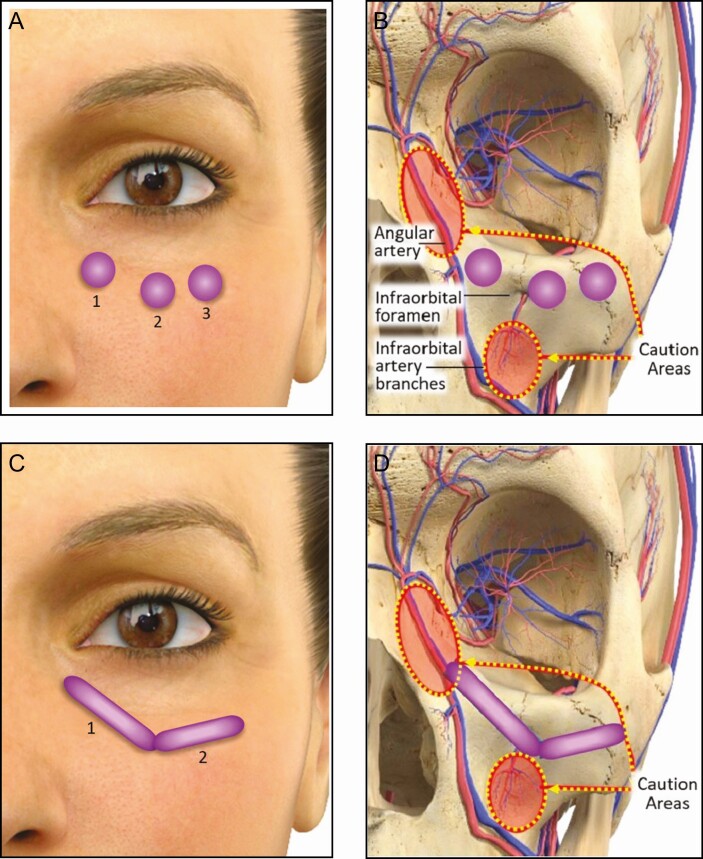
Prior to treatment, the IOAs were prepared according to each site’s practice, which typically consists of washing the area with soap and water and swabbing it with alcohol or other antiseptics. Following anesthetic administration for pain management, VYC-15L was administered at the supraperiosteal or submuscular plane along the infraorbital hollow length and extending approximately 2 cm below the inferior orbital rim as needed to achieve a smooth contour. The injection area encompassed the medial, central, and lateral IOA, which was aligned with the goal of improving the hollow and not treating or hiding the presence of herniated orbital fat. After palpating the border of the inferior orbital rim for areas of caution (ie, infraorbital foramen, infraorbital artery branches, and angular artery), injections were made by either a depot technique using a 32 G 1/2-inch needle (Figure 2A and B) or a retrograde linear technique using a 27 G 1½-inch cannula (TSK Steriglide, Vancouver, British Columbia, Canada) (Figure 2C and D) followed by gentle massage to distribute the product evenly. Intraseptal injection was avoided by not pushing through any resistance encountered when using the cannula/needle and ensuring tip placement at all times. Injection technique and total volume were determined by the TI, but volume was not to exceed 2.2 mL per side for initial and touch‐up treatments combined. The TI also evaluated product attributes, including ease of product injection (0 = difficult to 10 = easy) and product moldability (0 = stiff to 10 = moldable).
Figure 2.
Placement of VYC-15L using a needle or cannula. After palpating the orbital rim and making note of caution areas (infraorbital foramen, infraorbital artery branches, and angular artery), the product is placed just below the orbital rim at the submuscular/supraperiosteal plane by either a depot injection technique using a 32 G ½-inch needle (A, B) or a retrograde linear injection technique using a 27 G 1½-inch cannula (C, D).
Effectiveness Assessments
The rate of responders, defined as a ≥1-point improvement from baseline on the AIHS in both IOAs, was assessed by a blinded EI at Months 1, 3 (primary endpoint), 6, 9, and 12 post initial treatment and at Month 1 post repeat treatment. The change from baseline IOA volume (mL) was assessed for the VYC-15L treatment group by 3-dimensional (3D) facial digital imaging using a VECTRA M3 device (Canfield Scientific, Parsippany, NJ) at Months 1, 3, 6, 9, and 12 post initial treatment and at Month 1 post repeat treatment. Month 12 image was the baseline for the Month 1 post repeat treatment volumetric change. The no-treatment control group was assessed at baseline and Months 1 and 3. The area for 3D assessment was defined by landmark perimeter points on each side of the face (Supplemental Figure 2).
Safety Assessments
Procedural pain at each treatment session was assessed by the participant using an 11-point scale of 0 (no pain) to 10 (worst pain imaginable), and the incidence, severity, and duration of injection-site responses (ISRs) were collected following each treatment in a 30-day electronic participant diary. The presence of Tyndall effect was assessed by the EI 30 days after each treatment. A Snellen visual acuity test, confrontational visual field test, and ocular motility test were performed on day of treatment (before and 30 minutes posttreatment) and 14 days after each treatment. In addition, ocular motility was measured throughout the study. Adverse events (AEs) were monitored throughout the study.
Analysis Populations
All participants who were randomized to the VYC-15L group and received at least 1 treatment and at least 1 posttreatment primary effectiveness assessment were included in the modified intent-to-treat (mITT) population. All participants who received at least 1 VYC-15L treatment were included in the safety population. For the primary effectiveness analysis, a P value with normal approximation at the 5% level was employed to test if the AIHS responder rate at Month 3 in the VYC-15L treatment group was significantly greater than that in the no-treatment control group at Month 3 (95% CIs by Wald test were included).
RESULTS
Participant Demographics and Baseline Characteristics
The majority of participants were female (91.9%) with a mean age of 47 years (range, 23-68 years), Caucasian (80.0%), not of Hispanic ethnicity (86.7%), with a mean (standard deviation) BMI of 25.3 (4.7) kg/m2, and a baseline AHIS Grade 3 (60.0 %) (Table 1). A total of 140 participants were randomized 3:1 to VYC-15L (n = 105) or no-treatment control (n = 35), and 135 participants (VYC-15L, n = 103; no-treatment control, n = 32) were included in the primary effectiveness analysis. The preclusion of 5 participants’ data from analysis was due to non-compliance with mITT definition: 2 in the VYC-15L group (1 screen failure/randomized in error and 1 withdrawal by participant) and 3 in the control group (1 withdrawal by participant and 2 lost to follow-up). Among those included in the initial treatment population, 64 (60.9%) received a touch-up treatment under 1 or both eyes at Day 30, and 37 (35.2%) received repeat treatment at Month 12.
Table 1.
Participant Demographics and Baseline Characteristics (mITT Population)
| Parameter | No-treatment control (N = 32) | VYC-15L (N = 103) | Total (N = 135) |
|---|---|---|---|
| Gender, no. (%) | |||
| Female | 31 (96.9) | 93 (90.3) | 124 (91.9) |
| Male | 1 (3.1) | 10 (9.7) | 11 (8.1) |
| Age, y, median (min, max) | 40.0 (23, 59) | 47.0 (23, 68) | 47.0 (23, 68) |
| Race/ethnicity, n (%) | |||
| White | 27 (84.4) | 81 (78.6) | 108 (80.0) |
| Black/African American | 2 (6.3) | 16 (15.5) | 18 (13.3) |
| Asian | 2 (6.3) | 1 (1.0) | 3 (2.2) |
| American Indian or Native Alaskan | 1 (3.1) | 3 (2.9) | 4 (3.0) |
| Multiplea | 0 (0.0) | 2 (1.9) | 2 (1.5) |
| Ethnicity, no. (%) | |||
| Not Hispanic or Latino | 25 (78.1) | 92 (89.3) | 117 (86.7) |
| Hispanic or Latino | 7 (21.9) | 11 (10.7) | 18 (13.3) |
| BMI, kg/m2, median (min, max) | 23.1 (18.0, 37.1) | 24.6 (18.2, 37.7) | 24.3 (18.0, 37.7) |
| Fitzpatrick skin type, no. (%) | |||
| I, II | 11 (34.4) | 35 (34.0) | 46 (34.1) |
| III, IV | 18 (56.3) | 51 (49.5) | 69 (51.1) |
| V, VI | 3 (9.4) | 17 (16.5) | 20 (14.8) |
| Baseline AIHS score (highest of left/right), n (%) | |||
| 2, Moderate | 14 (43.8) | 40 (38.8) | 54 (40.0) |
| 3, Severe | 18 (56.3) | 63 (61.2) | 81 (60.0) |
AIHS, Allergan Infraorbital Hollow Scale; mITT, modified intent-to-treat; VYC-15L, Juvéderm Volbella XC.
a1, White/Asian; 1, White/African American.
Treatment Administration
Total median treatment volumes for initial treatment and touch-up were 1.5 mL (0.7 mL each side) and 1.0 mL (0.5 mL each side), respectively, and 1.3 mL (0.65 mL each side) for repeat treatment. All injections were placed in the supraperiosteal or submuscular plane, just deep to the orbicularis oculi. The most common injection techniques employed included tunneling (56.2%), fanning (47.6%), and serial puncture (27.6%). The investigators gave high ratings for ease of VYC-15L injection and moldability, with average ratings of 9.2 and 9.5, respectively. Injection parameters are summarized in Table 2.
Table 2.
Key Injection Parameters (as-Treated, Repeat Treatment, and Optional Treatment Populations)
| Initial treatment | Repeat treatment | Optional treatmenta | |||
|---|---|---|---|---|---|
| Initial | Touch-up | Initial | Touch-up | ||
| Participants, no. | 105 | 64 | 37 | 29 | 15 |
| Median volume, mLb (min, max) | 1.5 (0.3, 2.2) | 1.0 (0.1, 2.2) | 1.3 (0.3, 2.2) | 1.4 (0.7, 2.2) | 0.8 (0.2, 2.2) |
| Injection instrument, no. (%) | |||||
| 27G 1½-inch cannula | 59 (56.2) | 6 (56.3) | 18 (48.6) | 17 (58.6) | 3 (20.0) |
| 32G ½-inch needle | 46 (43.8) | 28 (43.8) | 16 (43.2) | 12 (41.4) | 12 (80.0) |
| Both | 0 (0.0) | 1 (1.6) | 3 (8.1) | 0 (0.0) | 0 (0.0) |
| Pretreatment anesthesia, no. (%) | |||||
| Topicalc | 60 (57.1) | 38 (59.4) | 11 (29.7) | 18 (62.1) | 8 (53.6) |
| Locald | 35 (33.3) | 5 (39.1) | 18 (48.6) | 9 (31.0) | 2 (13.3) |
| Ice | 24 (22.9) | 18 (28.1) | 11 (29.7) | 4 (13.8) | 1 (6.7) |
| Planes of injection, no. (%) | |||||
| Submuscular/supraperiosteal | 105 (100.0) | 64 (100.0) | 37 (100.0) | 29 (100.0) | 15 (100.0) |
| Other | 7 (6.7) | 6 (9.4) | 5 (13.5) | 2 (6.9) | 1 (6.7) |
| Injection technique, no. (%) | |||||
| Tunneling | 59 (56.2) | 39 (60.9) | 22 (59.5) | 15 (51.7) | 9 (60.0) |
| Fanning | 50 (47.6) | 28 (43.8) | 15 (40.5) | 12 (41.4) | 3 (20.0) |
| Serial puncture | 29 (27.6) | 9 (29.7) | 11 (29.7) | 10 (34.5) | 8 (53.3) |
| Bolus | 12 (11.4) | 5 (7.8) | 10 (27.0) | 5 (17.2) | 2 (13.3) |
| Other | 1 (1.0) | 1 (1.6) | 1 (2.7) | 0 (0.0) | 0 (0.0) |
| Product characteristic scores, mean (min, max) | |||||
| Ease of injectione | 9.2 (4, 10) | 8.9 (4, 10) | 9.5 (7, 10) | ||
| Moldabilityf | 9.5 (5, 10) | 9.0 (4, 10) | 9.3 (5, 10) | ||
aControl group participants who opted for treatment after the control period.
bTotal for both sides.
cIncluded lidocaine, prilocaine, betacaine, benzocaine.
dLidocaine with epinephrine.
eScale of 0 (most difficult) to 10 (easiest).
fScale of 0 (stiff) to 10 (moldable).
Effectiveness
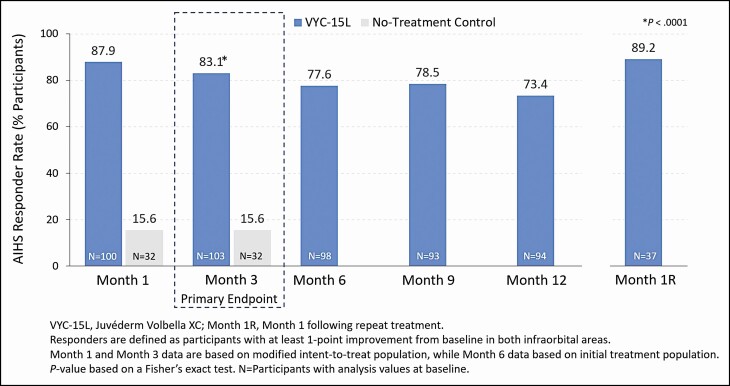
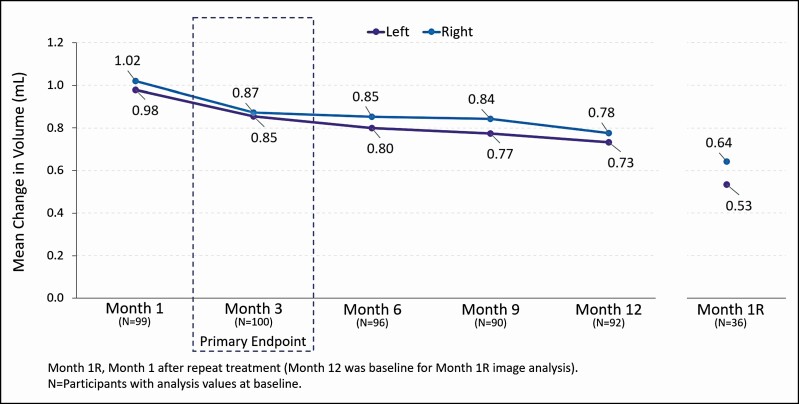
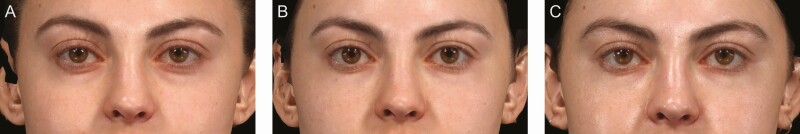
At Month 3, the responder rate for the VYC-15L treatment group (83.1% [95% CI = 75.8 to 90.4]) was statistically greater than for the no-treatment control group (15.6% [95% CI = 3.0 to 28.2]), with a difference of 67.5% (95% CI = 52.9 to 82.0, P < 0.0001) (Figure 3). Assessment by 3D imaging showed a mean change from baseline volume of 0.857 mL for the left IOA and 0.872 mL for the right IOA at Month 3, and 0.733 mL (left) and 0.777 mL (right) at Month 12 (Figure 4). For those who received a repeat treatment after Month 12, the mean change from baseline volume was 0.534 mL (left) and 0.642 mL (right) at Month 1 following repeat treatment. Representative photographs of participants who achieved a 1- and 2-grade improvement on AIHS with VYC-15L treatment are shown in Figure 5.
Figure 3.
Allergan Infraorbital Hollow Scale (AIHS) responder rate (as-treated and repeat treatment populations).
Figure 4.
Mean change from baseline in 3D infraorbital hollow volume (as-treated and repeat treatment populations).
Figure 5.
This female participant, age 24 years, achieved a 1-point improvement on the Allergan Infraorbital Hollow Scale (AIHS) following treatment with VYC-15L (A) at baseline with AIHS score = 2 (moderate, both sides), (B) at Month 3 with AIHS score = 1 (minimal, both sides), and (C) at Month 12 with AIHS score = 1 (minimal, both sides) following initial treatment volume of 1.0 mL (left side) and 1.5 mL (right side) total.
Safety
Procedural Pain and Injection Site Responses
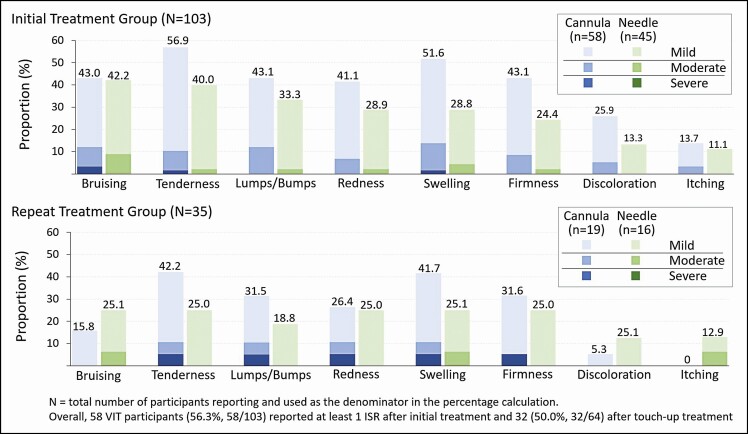
The mean procedural pain scores reported immediately after treatment were ≤1.7 among initial, touch-up, and repeat treatment groups. The majority of ISRs were mild in intensity and resolved within 1 week (Figure 6). Following initial and touch-up treatments, 56.3% (58/103) and 50.0% (32/64) of participants, respectively, reported at least 1 ISR, which most frequently included tenderness to touch, bruising, and swelling. For those who received a repeat treatment after Month 12, 34.3% (12/35) reported at least 1 ISR after repeat treatment, which most frequently included tenderness to touch, pain after injection, firmness, and swelling.
Figure 6.
Incidence and intensity of injection-site responses (ISRs) in initial and repeat treatment groups. VIT, VYC-15L initial treatment group.
Presence of Tyndall Effect and Visual Function Assessments
In the initial treatment group, bilateral Tyndall effect was observed in 6 participants at Month 1. In the repeat treatment group, Tyndall effect was observed in 7 additional participants at Month 1 following repeat treatment (3 of them bilaterally). One participant in the no-treatment control group, who was not yet treated, was also assessed with Tyndall effect. There were no significant changes in vision function related to treatment.
Adverse Events
A total of 34 participants had treatment-emergent AEs (TEAEs), of which 14 (10.3%) had treatment-related TEAEs, which were mild in intensity and resolved without sequelae (Table 3). In the initial treatment group, the most common treatment-related TEAEs (occurring in ≥2% of participants) were injection site bruising (3.8%) and swelling/edema (2.9%); most began ≤7 days of injection and resolved in ≤2 weeks. There were no treatment-related TEAEs after repeat treatment. Of note, 3 participants (2.9%) had 3 treatment-related late-onset (>30 days posttreatment) TEAEs, which included swelling or edema; 2 resolved in ≤4 days, 1 lasted 45 days and was resolved with oral antibiotic treatment. During the study, there were no delayed-onset granulomas. There were no deaths, unanticipated adverse device effects, treatment-related serious AEs, or AEs of special interest.
Table 3.
Summary of AEs and TEAEs
| Initial treatment | Repeat treatment | Optional treatment | |
|---|---|---|---|
| Initial + Touch-up (N = 105) | (N = 37) | Initial + Touch-up (N = 29) | |
| All TEAEs, no. (%) | 28 (26.7) | 1 (2.7) | 6 (20.7) |
| Treatment-related TEAEs | 10 (9.5) | 0 | 4 (13.8) |
| At injection site | 7 (6.7) | 0 | 4 (13.8) |
| Not at injection site | 3 (2.9) | 0 | 1 (3.4) |
| All SAEsa, no. (%) | 1 (1.0) | 1 (2.7) | 0 |
| Treatment-related | 0 | 0 | |
| AESIsb, no. (%) | 2 (1.9) | 0 | 0 |
| Treatment related | 0 | 0 | |
| Discontinued due to TEAE, no. (%) | 0 | 0 | 0 |
| Deaths | 0 | 0 | 0 |
AE, adverse event; AESI, adverse event of special interest; SAE, serious adverse event; TEAE, treatment-emergent adverse event.
aUnrelated SAEs were 1 breast infection and 1 tooth fracture.
bUnrelated AESIs were 1 blurred vision in right eye due to new contact lenses and 1 astigmatism.
DISCUSSION
The study’s primary endpoint was met, with an 83.1% (95% CI, 75.8 to 90.4) responder rate in the VYC-15L initial treatment group at Month 3, which was 67.5% (95% CI = 52.9 to 82.0, P < 0.0001) greater than in the control group (15.6% [95% CI = 3.0 to 28.2]) (Figure 3). Further, 73.4% of the initial treatment group retained that level of improvement up to Month 12. In contrast to the durability of VYC-15L in the lip, where response rate declined from rates as high as 95.5% at Month 1 to ≤61.8% at Month 12, increased durability in the TTs may be attributable to less dynamic movement of the IOA.17,23-25 The mean change in volume assessed by 3D imaging (0.857 mL left side and 0.872 mL right side) aligned with the AIHS score improvement through 1 year following treatment (Figure 4).
When the AIHS responder rate at Month 3 was categorized by baseline AIHS severity, total treatment volume administered, and injection instrument (needle or cannula), the results demonstrate efficacy in all circumstances. Among participants with a baseline AIHS grade of moderate or severe, the responder rate was 87.2% and 80.6%, respectively. Among participants who received equal to or less than median or greater than median treatment volume, the responder rate was 90.2% and 76.0%, respectively, and slightly higher responder rates were achieved with a cannula (92.9%) compared with a needle (71.1%) (Table 4). Treatment results achieved by both needle and cannula in this study are comparable with a prospective single-arm study (N = 73, 63% baseline AIHS grade of 3/4) in which 92.0% of VYC-15L–treated participants injected with a needle achieved ≥1-grade improvement from baseline on the AIHS at Month 6.4
Table 4.
Responder Rate Categorized by Baseline AIHS Baseline Severity, Median Treatment Volume, and Injection Instrument (mITT)
| VYC-15L (N = 103) | |
|---|---|
| Baseline AIHS, no. (%) | |
| Moderate | 34/39 (87.2) |
| Severe | 50/62 (80.6) |
| Treatment volume, no. (%) | |
| ≤Mediana | 46/51 (90.2) |
| >Median | 38/50 (76.0) |
| Injection instrument | |
| 27G 1½-inch cannula | 52/56 (90.2) |
| 32G ½-inch needle | 32/45 (71.1) |
AIHS, Allergan Infraorbital Hollow Scale; mITT, modified intent-to-treat; VYC-15L, Juvéderm Volbella XC.
aMedian total volume injected for initial and touch-up treatments combined was 1.0 mL (range, 0.2-2.2 mL) for the left side and 1.0 mL (range, 0.1-2.2 mL) for the right side, with a total median of 1.5 mL (0.7 mL each side) for initial treatment and 1.0 mL (0.5 mL each side) for touch-up treatment.
The total median injection volume (both sides combined) for initial treatment and touch-up was 1.5 and 1.0 mL, respectively, and 1.3 mL total for the repeat treatment after Month 12 to restore effectiveness. The most frequently used pretreatment anesthetics were topical anesthetic (eg, lidocaine, prilocaine, betacaine) and local anesthetic (eg, lidocaine + epinephrine) injection. Procedural pain, which was rated immediately after treatment, was minimal, and most ISRs were mild to moderate in intensity and resolved within 1 week (Figure 6). The pattern of ISRs with repeat treatment was similar to those with initial and touch-up treatment. Interestingly, the incidence of bruising was lower, although the population receiving repeat treatment was smaller (n = 35), an observation that may reflect the more frequent utilization of local anesthetic (containing epinephrine) in that population. Of note, ISRs associated with cannula injections reflected a slightly higher incidence than those administered by needle and included tenderness to touch, bruising, and swelling.
Although the presentation of Tyndall effect in 6 participants at the Month 1 visit and 7 additional participants at Month 1 following repeat treatment was a source of concern, it was also questionable because injection depths were primarily at the supraperiosteal or submuscular plane. The refractive phenomenon that causes the appearance of blue-gray discoloration obeys the same principles that govern why veins appear blue under the skin although they transport red blood.26 Because the evaluators were specifically looking for anything resembling this effect, it is possible that discoloration caused by underlying vasculature could have been mistakenly identified as Tyndall effect, especially in participants with fair skin. Three (3) of the 6 in the initial treatment group recorded as having Tyndall effect did not present with it at the 30-day optional touch-up visit (AND did not receive touch-up) just prior to the formal Month 1 assessment. Among the 7 repeat treatment participants, 2 were recorded as having Tyndall effect at their 30-day optional touch-up visit (did not receive touch-up) but did not present with it at the Month 1 following repeat treatment assessment.
The safety profile of VYC-15L injection in the IOA is comparable with previous studies evaluating VYC-15L and other HA-based fillers in the treatment of infraorbital hollows and reflects the AEs typically associated with injection of soft tissue fillers, including localized, transient, mild to moderate erythema, bruising, and edema.4-9,15,27,28 Moreover, the safety profile observed in this study is consistent with data obtained from multiple prospective trials evaluating treatment of the lips and perioral area, in which AE profiles included transient mild to moderate edema and bruising as commonly observed AEs.21,23,24,29
Achieving an optimal outcome begins with patient selection and thoughtful treatment plans, which require a thorough evaluation of the patient’s anatomy and corrective needs and is based on informed consent. Treatment considerations include the choice of FDA-approved products, products with the most suitable rheological properties, and the choice of techniques employed to administer the product. Risks associated with treatment to the IOA include injury to the superficial arteries of the periorbital area. Recommendations for prevention and management are based primarily on expert opinion and consensus reports, which are important for injectors to understand but are beyond the scope of this manuscript.30-32
An important limitation of this study design was that treatment was isolated to the infraorbital hollow area (the area of interest) for FDA registration to expand the indication for VYC-15L. Treatment of the infraorbital hollow without consideration of adjoining facial areas does not reflect the clinical practice treatment approach. Although hollowing of the IOA is due, in part, to infraorbital fat volume loss, for some patients, the primary breach in surface contour may be the collateral result of malar fat ptosis.13 A global treatment approach takes into consideration the effect of adjoining facial areas. Without first correcting volume loss in the adjoining midface and cheek area, study TIs likely employed a larger product volume in the IOA than would typically be used in clinical practice to correct baseline volume deficits characterized as moderate (43.8%) and severe (56.3%) on the AIHS. Another possible limitation of this study was the short duration of follow-up (1 month) after repeat treatment, which precluded continued evaluation of the treatment effect’s durability.
CONCLUSIONS
Treatment with VYC-15L was safe and effective for the correction of infraorbital hollows in participants with baseline volume deficits characterized as moderate or severe using the AIHS. Results lasted through 1 year, and repeat treatment required less product to restore correction compared with initial and touch-up volumes. Similar effectiveness was achieved regardless of injection instrument (needle or cannula), and the safety profile was favorable for VYC-15L injections administered by both instruments.
Disclosures
Dr Fabi is a consultant, investigator, and advisory board member for Allergan Aesthetics, an AbbVie Company (Irvine, CA). Drs Zoumalan and Yoelin are investigators for Allergan Aesthetics, an AbbVie Company. Dr Fagien is a consultant and investigator for Allergan Aesthetics, an AbbVie Company. Drs Sartor and Chawla are employees of AbbVie Inc (North Chicago, IL) and may own stock in the company.
Funding
This research was supported by Allergan Aesthetics, an AbbVie Company (Irvine, CA). Writing assistance and editorial support was provided by Erika von Grote, PhD, an employee of AbbVie. Employees of AbbVie participated in the research, interpretation of data, review of the manuscript, and the decision to submit for publication.
Supplementary Material
REFERENCES
- 1. DeFatta RJ, Williams EF 3rd. Evolution of midface rejuvenation. Arch Facial Plast Surg. 2009;11(1):6-12. [DOI] [PubMed] [Google Scholar]
- 2. Narurkar V, Shamban A, Sissins P, Stonehouse A, Gallagher C. Facial treatment preferences in aesthetically aware women. Dermatol Surg. 2015;41 Suppl 1:S153-S160. [DOI] [PubMed] [Google Scholar]
- 3. Michaud T, Gassia V, Belhaouari L. Facial dynamics and emotional expressions in facial aging treatments. J Cosmet Dermatol. 2015;14(1):9-21. [DOI] [PubMed] [Google Scholar]
- 4. Niforos F, Acquilla R, Ogilvie P, et al. A prospective, open-label study of hyaluronic acid-based filler with lidocaine (VYC-15L) treatment for the correction of infraorbital skin depressions. Dermatol Surg. 2017;43(10):1271-1280. [DOI] [PubMed] [Google Scholar]
- 5. Hall MB, Roy S, Buckingham ED. Novel use of a volumizing hyaluronic acid filler for treatment of infraorbital hollows. JAMA Facial Plast Surg. 2018;20(5):367-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Diwan Z, Trikha S, Etemad-Shahidi S, Alli Z, Rennie C, Penny A. A prospective study on safety, complications and satisfaction analysis for tear trough rejuvenation using hyaluronic acid dermal fillers. Plast Reconstr Surg Glob Open. 2020;8(4):e2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sharad J. Treatment of the tear trough and infraorbital hollow with hyaluronic acid fillers using both needle and cannula. Dermatol Ther. 2020;33(3):e13353. [DOI] [PubMed] [Google Scholar]
- 8. Lera M. Treatment of infraorbital skin depressions using the hyaluronic acid filler VYC-15L based on the MD codes approach: a retrospective analysis. J Clin Exp Dermatol Res. 2020;11:526. [Google Scholar]
- 9. Peng HP, Peng JH. Treating the tear trough-eye bag complex: treatment targets, treatment selection, and injection algorithms with case studies. J Cosmet Dermatol. 2020;19(9):2237-2245. [DOI] [PubMed] [Google Scholar]
- 10. Haddock NT, Saadeh PB, Boutros S, Thorne CH. The tear trough and lid/cheek junction: anatomy and implications for surgical correction. Plast Reconstr Surg. 2009;123(4):1332-1340. [DOI] [PubMed] [Google Scholar]
- 11. Rohrich RJ, Arbique GM, Wong C, Brown S, Pessa JE. The anatomy of suborbicularis fat: implications for periorbital rejuvenation. Plast Reconstr Surg. 2009;124(3):946-951. [DOI] [PubMed] [Google Scholar]
- 12. Stutman RL, Codner MA. Tear trough deformity: review of anatomy and treatment options. Aesthet Surg J. 2012;32(4):426-440. [DOI] [PubMed] [Google Scholar]
- 13. Glaser DA, Lambros V, Kolodziejczyk J, Magyar A, Dorries K, Gallagher CJ. Relationship between midface volume deficits and the appearance of tear troughs and nasolabial folds. Dermatol Surg. 2018;44(12):1547-1554. [DOI] [PubMed] [Google Scholar]
- 14. Wong CH, Hsieh MKH, Mendelson B. The tear trough ligament: anatomical basis for the tear trough deformity. Plast Reconstr Surg. 2012;129(6):1392-1402. [DOI] [PubMed] [Google Scholar]
- 15. Goldberg RA, Fiaschetti D. Filling the periorbital hollows with hyaluronic acid gel: initial experience with 244 injections. Ophthalmic Plast Reconstr Surg. 2006;22(5):335-341; discussion 341. [DOI] [PubMed] [Google Scholar]
- 16.Juvéderm Volbella XC [package insert]. Irvine, CA: Allergan; 2020.
- 17. Geronemus RG, Bank DE, Hardas B, Shamban A, Weichman BM, Murphy DK. Safety and effectiveness of VYC-15L, a hyaluronic acid filler for lip and perioral enhancement: one-year results from a randomized, controlled study. Dermatol Surg. 2017;43(3):396-404. [DOI] [PubMed] [Google Scholar]
- 18. Lim HK, Suh DH, Lee SJ, Shin MK. Rejuvenation effects of hyaluronic acid injection on nasojugal groove: prospective randomized split face clinical controlled study. J Cosmet Laser Ther. 2014;16(1):32-36. [DOI] [PubMed] [Google Scholar]
- 19. Goodman GJ, Swift A, Remington BK. Current concepts in the use of Voluma, Volift, and Volbella. Plast Reconstr Surg. 2015;136(5 Suppl):139S-148S. [DOI] [PubMed] [Google Scholar]
- 20. Devgan L. Abstract: Juvéderm Volbella for use in periorbital volumization. Plast Reconstr Surg Glob Open. 2017;5(9 Suppl):192-193. [Google Scholar]
- 21. Rivkin A, Weinkle SH, Hardas B, et al. Safety and effectiveness of repeat treatment with VYC-15L for lip and perioral enhancement: results from a prospective multicenter study. Aesthet Surg J. 2019;39(4):413-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Donofrio L, Carruthers J, Hardas B, et al. Development and validation of a photonumeric scale for evaluation of infraorbital hollows. Dermatol Surg. 2016;42(Suppl 1): S251-S258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Eccleston D, Murphy DK. Juvéderm(®) Volbella™ in the perioral area: a 12-month prospective, multicenter, open-label study. Clin Cosmet Investig Dermatol. 2012;5:167-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Raspaldo H, Chantrey J, Belhaouari L, et al. Lip and perioral enhancement: a 12-month prospective, randomized, controlled study. J Drugs Dermatol. 2015;14(12): 1444-1452. [PubMed] [Google Scholar]
- 25. Glaser DA, Kenkel JM, Paradkar-Mitragotri D, Murphy DK, Romagnano L, Drinkwater A. Duration of effect by injection volume and facial subregion for a volumizing hyaluronic acid filler in treating midface volume deficit. Dermatol Surg. 2015;41(8):942-949. [DOI] [PubMed] [Google Scholar]
- 26. Funt D, Pavicic T. Dermal fillers in aesthetics: an overview of adverse events and treatment approaches. Clin Cosmet Investig Dermatol. 2013;6:295-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wollina U. Improvement of tear trough by monophasic hyaluronic acid and calcium hydroxylapatite. J Clin Aesthet Dermatol. 2014;7(10):38-43. [PMC free article] [PubMed] [Google Scholar]
- 28. Philipp-Dormston WG, Hilton S, Nathan M. A prospective, open-label, multicenter, observational, postmarket study of the use of a 15 mg/mL hyaluronic acid dermal filler in the lips. J Cosmet Dermatol. 2014;13(2):125-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Calvisi L, Gilbert E, Tonini D. Rejuvenation of the perioral and lip regions with two new dermal fillers: the Italian experience with Vycross™ Technology. J Cosmet Laser Ther. 2017;19(1):54-58. [DOI] [PubMed] [Google Scholar]
- 30. Urdiales-Gálvez F, Delgado NE, Figueiredo V, et al. Treatment of soft tissue filler complications: expert consensus recommendations. Aesthetic Plast Surg. 2018;42(2):498-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jitaree B, Phumyoo T, Uruwan S, Sawatwong W, McCormick L, Tansatit T. The feasibility determination of risky severe complications of arterial vasculature regarding the filler injection sites at the tear trough. Plast Reconstr Surg. 2018;142(5):1153-1163. [DOI] [PubMed] [Google Scholar]
- 32. Hufschmidt K, Bronsard N, Foissac R, et al. The infraorbital artery: clinical relevance in esthetic medicine and identification of danger zones of the midface. J Plast Reconstr Aesthet Surg. 2019;72(1):131-136. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.