Learning objectives.
By reading this article, you should be able to:
-
•
Describe the normal physiology of intestinal blood flow and perioperative factors affecting it.
-
•
Discuss the modifiable and non-modifiable risk factors associated with increased rates of anastomotic leakage.
-
•
Outline strategies to address modifiable risk factors in the perioperative period.
-
•
Explain the rationale underpinning the perioperative management of patients undergoing intestinal anastomosis.
-
•
Detail the options available for managing patients with a diagnosed anastomotic leak.
Key points.
-
•
Colorectal anastomotic leakage significantly increases morbidity and mortality.
-
•
Factors related to the patient, anaesthesia and surgery influence the risk of anastomotic leakage.
-
•
There are modifiable and non-modifiable risk factors, but the exact cause of anastomotic leakage is often multifactorial.
-
•
Effective optimisation of modifiable risk factors depends on multidisciplinary team working within high quality, integrated care processes.
-
•
Early recognition and management of anastomotic leakage is critical to minimise serious consequences.
Intestinal surgery is performed for a variety of pathologies including cancer, inflammatory bowel disease (IBD) and trauma. Anastomotic leakage (AL) is the most common complication associated with intestinal surgery. It can cause considerable morbidity and mortality and depending on its severity, has the potential to cause sepsis and may even result in recurrence of cancer. Despite recent advances in perioperative care pathways and improved surgical techniques, the incidence of AL is as high as 20%.1 The aetiology of AL is heterogeneous and involves multiple complex factors before, during and after surgery. Pre-existing comorbidities, perioperative medical conditions and anaesthesia and surgical technique can all influence the risk of AL. It is essential to understand the pathophysiology and practice meticulous multidisciplinary perioperative care in order to decrease its risk and associated adverse outcomes. In this article we summarise the consequences, risk factors and measures available to minimise the risk of AL, with a focus on anastomoses involving the large intestine in adults.
Definition of anastomotic leak
There are multiple definitions for intestinal AL, many of which comprise both clinical and radiological criteria.2 The Association of Surgeons of Great Britain and Ireland (ASGBI) define AL as a leak of luminal contents from a surgical join between two hollow viscera. This definition is not site-specific and does not indicate severity. The International Study Group of Rectal Cancer (ISGRC) defines AL as a defect in the integrity of the intestinal wall at the anastomotic site leading to a communication between the intra- and extraluminal compartments.3 The ISGRC also proposed a severity grading system, which has subsequently been validated. Grade A requires no therapeutic intervention, Grade B requires active intervention (e.g. systemic antimicrobials or drainage of localised abscess) and Grade C requires repeat laparotomy.
Incidence
The reported incidence of AL varies between 2% and 20% depending on how it is defined, the site of the anastomosis and the population being studied.1 In terms of anatomical site, extraperitoneal anastomoses are twice as likely to leak as intraperitoneal anastomoses; anastomoses involving the rectum confer the highest risk for developing AL: the incidences are 5–20% for ileorectal, 5–15% for colorectal, 3–6% for colocolic and 2–5% for ileocolic anastomoses, respectively. Anastomotic leakage is also more common after emergency surgery (a 2- to 3-fold increased incidence compared with elective surgery).
Prediction, diagnosis and consequences of AL
Prediction
Preoperative prediction of AL may aid decisionmaking when considering the formation of a protecting stoma. The colon leakage score (CLS) was developed to predict the risk of a leak before surgery by assessing 11 weighted patient and operative factors for left-sided colonic resection. The Dutch leakage score (DULK) comprises 13 factors, whereas the modified DULK score is based on four (ventilatory frequency >20 bpm, clinical deterioration, abdominal pain other than wound pain and plasma C-reactive protein [CRP] >250 mg L−1). The DULK and modified DULK both aid in prediction of clinically relevant postoperative anastomotic leaks.2 The DULK has a negative predictive value of 97% but a positive predictive value of less than 20%.2 The modified DULK has similar predictive values but a slightly higher specificity. It is simple to implement and uses only four clinical variables, which allows it to be incorporated into daily postoperative bedside monitoring for early detection of AL.
Diagnosis
There is no gold standard tool for diagnosing AL. Clinical symptoms and signs, laboratory tests, surgical drain fluid analysis and radiological investigations have been suggested for early diagnosis.3
-
•
Timing of AL: The median time to AL is frequently reported as being 7 days after surgery. Nonetheless, AL can occur several weeks after surgery. Early AL (between 5 and 7 days) more commonly presents with peritonitis, whereas late AL (after 7 days) often presents with an intra-abdominal abscess. When enhanced recovery after surgery (ERAS) pathways are used, patients are often discharged from hospital before the median time of AL. A number of patients will develop AL after discharge, and it remains the most common cause for readmission after colorectal surgery.
-
•
Clinical features: The clinical picture varies from no significant signs to septic shock. Development of pyrexia, pelvic pain, paralytic ileus, diarrhoea, oliguria, or increased white cell count (WCC) in the first 3 to 4 postoperative days should be considered as warning signs. Pus or enteric contents may be present in the drain.
-
•
Biomarkers: Several biomarkers such as CRP, WCC, procalcitonin, interleukin 6 (IL-6), serum lactate and peritoneal fluid amylase have been studied as possible predictors of AL. Currently, there is no single biomarker with high sensitivity and positive predictive value. Nonetheless, third or fourth postoperative day CRP concentrations >150 mg L−1 may suggest AL.2 Although routine use is not yet established, in high-risk patients, biomarkers can help guide diagnostic investigations such as CT imaging.
-
•
Radiological investigations: CT is commonly used to diagnose AL with a sensitivity of around 70%. Contrast tomography imaging may not be possible in critically ill or haemodynamically unstable patients who are unsuitable for transfer to the radiology department.
Consequences
Anastomotic leakage is associated with significant consequences for both the patient and healthcare providers (Table 1). Consequences are determined by several factors including site, severity, pre-existing comorbidities and the provision of clinical care (see Fig. 1).
Table 1.
Consequences of anastomotic leak
Hospital:
|
Surgical:
|
Oncologic:
|
Medical:
|
Physiological and psychosocial:
|
Immunological:
|
Financial:
|
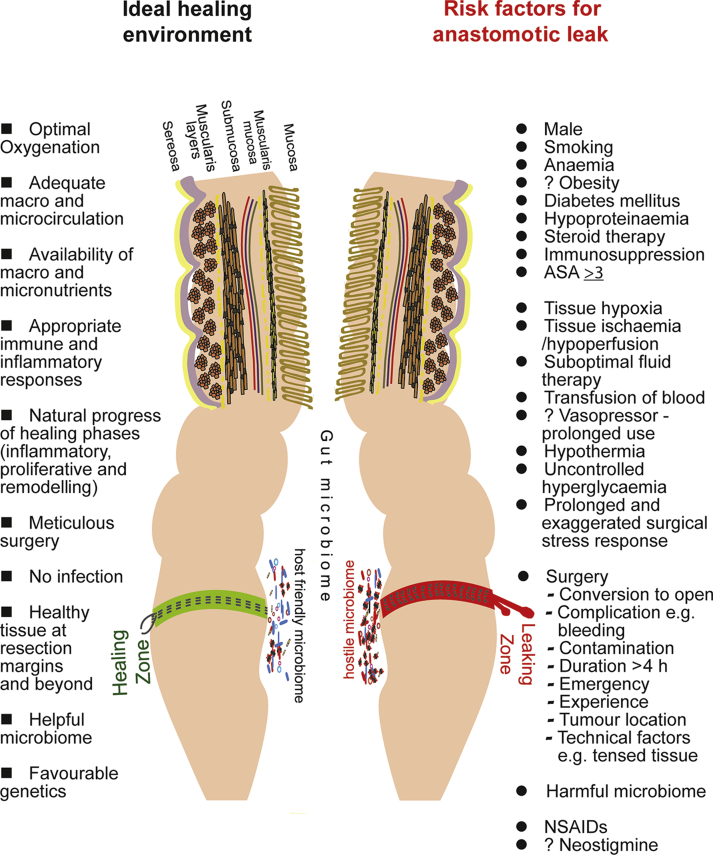
Fig 1.
Ideal healing environment and major risk factors for anastomotic leak.
Physiology of intestinal anastomosis formation
Anastomotic healing process
Although the classical phases of tissue healing are the same, intestinal tissue healing differs from cutaneous wound healing because it takes place in a distinct environment so that different factors are involved (e.g. shear stress from peristalsis and luminal contents, the presence of a microbiome, blood supply fluctuations, inability of healthcare professionals to assess the progress). Intestinal anastomotic healing relies on a delicate balance between physiological, molecular and biochemical processes in an optimal tissue environment (Fig. 1). These processes overlap and consist of:
-
•
Haemostasis (begins immediately)
-
•Inflammatory phase (up to 1 week)
-
-Leucocyte, monocyte and platelet response leads to the release of growth factors and cytokines
-
-
-
•Proliferative phase (3 days–3 weeks)
-
-Collagen is synthesised by fibroblasts and smooth muscle cells and angiogenesis occurs
-
-
-
•Remodelling (3 weeks to a few months)
-
-Epithelial repair and differentiation of cell layers occurs
-
-
Each intestinal wall layer plays a role during healing; the submucosa is responsible for the tensile strength of the wall, the serosa provides a matrix for fibroblasts and the mucosa is important in maintaining the homeostasis that facilitates anastomotic healing. Measurement of the hydroxyproline content at the anastomotic healing area, bursting pressure (intraluminal pressure at which leakage or rupture of anastomosis occurs) and histological examination of healing tissue are some of the methods that have been used to evaluate the healing process in animals.4 These experimental methods require access to anastomosed and perianastomotic tissue, which makes them unsuitable for human clinical practice. During surgery, anastomotic strength can be assessed with an air or dye leak test and endoscopic examination.
Intestinal blood flow and oxygenation
The intestines have a unique macro- and microcirculatory arrangement and metabolic requirements. Watershed areas (e.g. the splenic flexure and rectosigmoid junction) where collateral circulation is not well developed, anastomoses are more vulnerable during ischaemia. Anastomosis involving the rectum are more susceptible to changes in blood flow because of its dual blood supply from splanchnic and systemic arterial systems. An arterial plexus in the intestinal submucosa branches into capillaries that deliver oxygen and other nutrients to the mucosa and muscularis layers via parallel networks. Owing to its higher metabolic requirements, 70–80% of the intestinal blood flow travels to the mucosa under normal circumstances. Autoregulatory mechanisms permit redistribution of blood flow from outer layers of the intestine to the mucosa during low perfusion states. The physiological range for autoregulation of intestinal blood flow is not known.
The countercurrent arrangement of arteriole and venule within the villi results in oxygen diffusion from the arterial to venous side as the blood travels towards the luminal side of mucosa. The Po2 drops precipitously along the radial axis from the intestinal submucosa to the lumen of the colon, which functions at a Pao2 of 1.33 kPa.5 During normal health, adaptive mechanisms allow epithelium on the luminal side to function at low environmental Pao2.5 Hypoxia of anastomosed tissue impairs healing through its adverse effects on inflammatory and immune repsonses.6 Although production of growth factors (e.g. vascular endothelial growth factor) is upregulated in low oxygen environments, their processing mechanisms may not function well in the presence of cellular hypoxia. Experimental animal studies suggest a hypoxic environment in the colon decreases hydroxyproline content (a major component of protein collagen) of healing intestinal tissue, which renders it more vulnerable to AL.
Gut microbiome
The gut's natural microbiome and its metabolites have several physiobiological functions including immunological responses, epithelial regeneration (e.g. butyrate producing bacteria) and healing of intestinal tissue (e.g. Lactobacillus spp.).7 Microbial dysbiosis, referred to as altered microbial numbers, diversity, composition and activity have been implicated in the pathogenesis of AL. Experimental and clinical studies suggest significant changes in the anastomotic tissue-associated microbiome (e.g. a decrease in obligate anaerobes) as a result of changes in local environmental conditions.7 Many internal and external perioperative factors may cause harmful effects on healing by causing microbial dysbiosis and disturbing microbial–host and microbial–microbial harmony, which can be improved by targeted therapeutic measures.7 These measures aim to maintain local environment and re-establish ‘desired’ microbiome in the colon (Table 2).7
Table 2.
Factors affecting the gut microbiome, microbial mechanisms for anastomotic leak and measures to modify these
Factors affecting
|
Microbial mechanisms for AL:
|
Measures to modify microbiome:
|
Perioperative risk factors
Inconsistency in research methodologies has resulted in variable and often contradictory perioperative risk factors being identified.1,2 Despite this, it is worth remembering that AL may occur in the presence of no identifiable risk factors. When present, risk factors can be categorised as modifiable or non-modifiable. Early identification of modifiable risk factors should be integrated into ERAS programmes and prehabilitation.
Prehabilitation
Although a prehabilitation programme may provide an opportunity to address several of the modifiable risk factors for AL such as smoking, malnutrition, anaemia, alcohol intake and obesity and improving functional capacity and physiological well-being, there is little evidence to date showing that the use of a prehabilitation programme is associated with a reduced incidence of AL.
Enhanced recovery after surgery
Originally, ERAS programmes were developed for use in colonic resection surgery with the goal of reducing postoperative complications and improving recovery times. As a major cause of morbidity is AL, it is perhaps not surprising that many of the ERAS elements (https://erassociety.org/guidelines/list-of-guidelines/) are aimed at reducing AL. There is no definitive consensus about the relative effectiveness of a regimen of no preoperative bowel preparation compared with oral antibiotics alone, or combined mechanical bowel preparation (MBP) and oral antibiotics. However, for rectal surgery MBP is often advocated particularly when a diverting stoma is planned. Prophylactic i.v. antibiotics should be given within 60 min before the skin incision for colorectal surgery. Reduced fasting times can contribute towards optimal fluid management. Carbohydrate loading before surgery can reduce insulin resistance after surgery, prevent postoperative hyperglycaemia, help maintain protein balance and promote an early return to gut function. This could minimise any potential negative changes to the gut microbiome. Maintenance of normal body temperature is recommended as perioperative hypothermia has detrimental effects on healing of anastomoses.
Lines of evidence proving the association between ERAS protocols and reduced AL rates are scarce. Nonetheless, one large multicentre prospective cohort study demonstrated that AL incidence in their ERAS group was 6% compared with 7.8% in the non-ERAS group (odds ratio [OR]=0.75; 95% confidence interval [CI], 0.58–1.08).8 Patients with the highest rate of compliance to an ERAS protocol had less risk of AL compared with those with lowest adherence to ERAS components (OR=0.48; 95% CI, 0.28–0.81; P=0.007).8
Role of quality improvement programmes
In the UK, the Perioperative Quality Improvement Programme (PQIP) (www.pqip.org.uk) aims to reduce the burden of perioperative complications including AL by improving perioperative care processes and implementation of best practices with the involvement of multidisciplinary team composed of clinicians, nurses, healthcare managers and patients. PQIP and the National emergency laparotomy audit (NELA) collect hospital data through local collaborators and support the quality improvement activities. In the USA, the Agency for Healthcare Research and Quality (AHRQ) collaborates with hospitals and surgical professional organisations to provide evidence-based guidance to improve perioperative care of colorectal surgical patients. However, the impact of these programmes on reducing the rates of AL and minimising its consequences remains to be demonstrated.
Modifiable preoperative risk factors
Anaemia
Up to 50% of patients undergoing colorectal surgery are anaemic. This may be a consequence of gastrointestinal blood loss, nutritional deficiencies or an inflammatory-mediated reduction in nutrient absorption. Severe anaemia (haemoglobin [Hb] <94 g L−1) has been identified as an independent risk factor for AL.9 Anaemia is the most common reason for perioperative allogenic blood transfusion, which itself is a risk factor for anastomotic complications. Patient blood management strategies should be implemented before surgery if required.
Nutrition
Preoperative weight loss >10% and hypoalbuminaemia (serum albumin < 3.5g L−1) are risk factors for AL.1 The risk correlates with the degree of hypoproteinaemia. The systemic stress response to surgery is known to decrease serum albumin after surgical trauma. This impairs the wound healing process and may cause intestinal tissue oedema by decreasing plasma oncotic pressure. Nutrition assessment and optimisation should be re-evaluated throughout the perioperative period. This strategy has been shown to reduce the risks in non-malnourished patients.
Obesity
Dietary advice and increased activity before surgery can aid weight loss. Time for these changes may be limited in patients awaiting surgery for cancer or IBD. Any weight loss before surgery must be calculated and pragmatic to prevent the risks associated with malnutrition. In one meta-analysis for laparoscopic surgery, the risk of AL and overall postoperative mortality rate were increased in patients with obesity.10 Increased mesocolon thickness, visceral adiposity and increased abdominal pressure may pose technical challenges in creating the anastomoses and keeping them free of tension. Patients with obesity are at risk of increased blood loss, prolonged duration of surgery and conversion from laparoscopic to open surgery.10 Smaller studies have failed to replicate these results for right-sided colorectal surgery and for patients undergoing surgery for IBD. Visceral obesity (waist circumference or waist to hip ratio) is more predictive than BMI.2
Diabetes mellitus
Diabetes is associated with several comorbidities including obesity, atherosclerosis and increased susceptibility to infection. A recent meta-analysis found the relative risk of AL in patients with diabetes was 1.562 (95% CI, 1.197–2.036).11 Patients with diabetes who are also obese or taking steroids have additional risks.11 The risk of death is four times higher in diabetic patients who develop AL compared with in non-diabetic patients.2 Although the relationship between preoperative HbA1c and AL is not clear, postoperative hyperglycaemia (blood glucose >10 mmol L−1) is common in both prediabetic (HbA1c 5.7–6.4%) and diabetic patients HbA1c >6.5 %). Intra- and postoperative hyperglycemia require correction with insulin therapy as both are associated with adverse outcomes.
Smoking
Anastomoses are more likely to leak in current (relative risk [RR]=3.18; 95% CI, 1.44–7.00) and ex-smokers after colorectal surgery.12 The risk from smoking appears to be dose-dependent. Smoking affects all phases of healing as smokers are prone to developing atherosclerosis, microvascular thrombosis, immunosuppression and cellular hypoxia. To reverse the detrimental effects on healing, a minimum of 4–6 weeks' abstinence has been suggested.12 The effects of electronic cigarettes on the incidence of AL are not known and require further investigation.
Alcohol intake
Increased alcohol intake (>3 units day−1) has been associated with an increased incidence of AL.12 This may be because of the systemic effects of alcohol including malnutrition (anaemia, hypoproteinaemia and electrolyte imbalances), immunosuppression, cardiac dysfunction and impaired haemostasis. A period of abstinence (4–6 weeks) is recommended to help reverse the immune-related adverse effects of alcohol.
Non-modifiable preoperative risk factors
Sex
Men are more likely to suffer AL than females, particularly after rectal surgery.1,2 There is a suggestion that the technical difficulty associated with undertaking surgery in the narrower male pelvis could account for the increased rates of rectal AL.
Age
Although age is not a contraindication to forming an anastomosis formation, in one study patients aged >60 yrs undergoing anterior resection surgery had double the average risk for AL.2 However, these data should be interpreted with caution because older people are more likely to have other co-existing morbidities and risk factors.
Tumour characteristics
Tumours within 5 cm from the anal verge, >3 cm in size and those that have metastasised are risk factors for AL.1,2
History of pelvic radiotherapy
Whether used as neoadjuvant treatment for rectal carcinoma or another unrelated malignancy, radiotherapy significantly increases the risk of AL.1
Steroids
Steroids suppress anastomotic healing by various mechanisms including decreased activation and infiltration of macrophages and polymorphonuclear leucocytes, inhibition of growth factors and collagen synthesis. Perioperative steroid use is common in patients with inflammatory bowel and autoimmune diseases and for optimisation in some patients with chronic pulmonary disease. Perioperative and long-term use of corticosteroids has been associated with increased rates of AL and other infectious complications after open or laparoscopic surgery for cancer or benign conditions.13 The definition, dose and duration of steroid use varies amongst studies.13 In contrast to long-term preoperative steroid therapy, a single dose of steroid (e.g. dexamethasone 4–8 mg i.v.) before the surgical incision may be beneficial for early anastomotic healing period by inducing connective tissue growth factor, which is important for the formation of extracellular matrix and angiogenesis. However, more multicentre studies are required to investigate this including the mechanisms of any benefits and interactions with other drugs affecting anastomotic healing such as non-steroidal anti-inflammatory drugs (NSAIDs).
Immunotherapy and chemotherapeutic agents
The evidence suggesting a link between infliximab and increased rates of AL is inconclusive. Patients taking bevacizumab are advised to stop treatment 6 weeks before elective surgery and delay initiation of therapy for at least 28 days after surgery.
Urgency
Emergency surgery is an independent predictive risk factor for all colorectal resections.2 Patients presenting for emergency general surgery are often systemically unwell, and their risk may be compounded by other risk factors such as suboptimal nutrition (e.g. hypoproteinaemia), shock, faecal peritonitis, bowel obstruction, pre-existing medical problems, advanced age and blood loss.2
Intraoperative anaesthetic risk factors
Anaesthetic technique
There is no evidence to suggest that AL is related to the choice of TIVA or inhalational anaesthesia either for open or laparoscopic or robotic-assisted colorectal surgery.
Tissue oxygenation and ventilatory targets
Intestinal tissue oxygenation is critical for the healing of anastomoses. This depends on partial pressure of oxygen, cardiac output and local tissue blood flow. During the perioperative period, increased oxygen demands, surgery-related vasoconstriction, microvascular thrombosis and tissue oedema predispose anastomotic and perianastomotic areas to hypoxia. Experimental studies demonstrated that tissue hypoxia leads to AL by various mechanisms including impairment of collagen synthesis and cross-linking.6 Perianastomotic tissue Pao2 has been shown to predict AL in experimental and clinical studies.14,15 In an experimental animal study, Shandall and colleagues showed anastomoses performed with a perianastomotic Po2 (Pto2) >7.33 kPa healed well, whereas below a ‘critical level’ of 3.3 kPa a severe leak developed in all anastomoses.14 Nonetheless, the findings from animal studies cannot be extrapolated to humans as morphological, functional and physiological variables differ significantly among species.
Studies in humans have shown a correlation between decreasing tissue oxygenation and increased risk of AL.6 Sheridan and colleagues15 demonstrated a linear relationship between Pao2 and colon Po2 at different colonic sites during the intraoperative period whilst breathing Fio2 0.33. The colon serosal Pao2 (Pto2) averaged 32% of Pao2. Anastomoses constructed in tissue with an oxygen tension <2.6 kPa had a significantly increased risk of subsequent leakage.15 The optimal Fio2 to reduce the risk of AL during the perioperative period remains unknown. Increasing Fio2 from 0.3 to 1.0 doubled colonic Po2 in healthy and perianastomotic (2 cm proximal to anastomosis) areas.16 Some studies have shown a reduction in the risk of AL risk with Fio2 in the range of 0.8. It is possible that higher Fio2 may be more useful for anastomoses involving the rectum, but more robust data are required to clarify the role of high Fio2 on recovery of intestinal function, postoperative anastomotic leak, wound infection and pulmonary complications. Until further data are available, it is advisable to maintain Spo2 >93% and Fio2 between 0.8 and 0.3. In one study, the risk of AL was 4.2 times higher with colonic haemoglobin oxygen saturation (Sto2) ≤90%; the mean Sto2 was 93% in patients who did not develop AL.17 Sto2 does not reflect microperfusion and partial pressure of oxygen.
Carbon dioxide may also affect anastomoses by influencing tissue oxygenation via its effects on cardiac output, mesenteric vascular resistance and peritoneal blood flow. One study (with intraoperative Fio2 0.8) demonstrated that mild hypercapnia (end tidal CO2 6.66 kPa) significantly increased colonic tissue oxygenation when compared with normocapnia (Pe′co2 4.66 kPa).18 The median colonic Po2 was 14.23 kPa in patients with mild hypercapnia compared with 7.07 kPa in normocapnic patients). This measurement was taken in healthy colon distant from the anastomotic site.18 The effects of mild hypercarbia during the postoperative period are not known.
Intestinal haemodynamics and blood flow
Adequate blood flow in the perianastomotic region is critical for the viability of the anastomosis. Several perioperative factors affect intestinal blood flow including fluid therapy, anaesthetic technique, vasopressor usage and intra-abdominal pressure. Technical factors such as pneumoperitoneum, mobilisation of the bowel, stretching and twisting of mesentery, ligation of blood vessels and suture or staple tension can compromise circulation around the anastomotic site.
The relationship between perioperative arterial pressure or other commonly used systemic cardiovascular monitoring goals (e.g. stroke volume variation [SVV], pulse pressure variation [PPV], cardiac output) and AL is not clear. The optimal intra- and postoperative BP for each individual patient should be based upon their preoperative BP in health. There is no robust evidence describing what degree of deviation from preoperative BP may be detrimental to intestinal anastomotic healing. A preoperative diastolic BP >90 mmHg or intraoperative decrease of >40% in diastolic BP from baseline value have been shown to be independent risk factors for AL.19 This study included patients undergoing open and laparoscopic surgery, and most received patient-controlled epidural analgesia in the postoperative period, which was not standardised.19 Individualised haemodynamic goals set within standardised institutional haemodynamic protocols are advised. Such an approach has already been shown to reduce AL after oesophageal cancer surgery.20
Visual inspection of the serosal surface, appropriate bleeding at incision sites and peristalsis may suggest viable bowel. These clinical features can be deceptive and cannot be relied upon entirely. Assessment of perfusion at the anastomotic site facilitates decision making when deciding on the level of bowel transection. A meta-analysis demonstrated that real-time assessment with intraoperative use of fluorescence perfusion angiography (FPA) reduced colorectal anastomosis leakage significantly.21 The use of PFA led to a change in the anastomotic site in 9.7% of 2220 patients. Other anastomotic vascularisation assessment methods exist, but the evidence supporting them is lacking. They include laser Doppler flowmetry, near-infrared or visible light spectrophotometry, intravital microscopy, mucosal Ph and luminal microdialysis.
Fluids
Intestinal capillaries are fenestrated. The intestines have a large extracellular compartment and are more susceptible to oedema formation in comparison with lung tissue. Changes in microvascular permeability, portal venous pressure, plasma albumin concentration and intestinal lymphatic function or flow result in an imbalance in intestinal capillary Starling forces.
Experimsental studies have shown that giving large volumes of crystalloids results in lower bursting pressures, reduced hydroxyproline content of healing intestinal anastomosed tissue and increased submucosal oedema at the anastomotic site. These changes weaken the mechanical strength of anastomoses. Intestinal oedema results in impaired oxygen delivery, which leads to tissue hypoxia and acidosis. In a porcine model,22 goal-directed colloid therapy with hydroxyethyl starch (HES) significantly increased tissue oxygen tension and microcirculatory perfusion in healthy and perianastomotic colon in comparison with both goal-directed and restricted Hartmann's fluid regimens. This was despite no significant differences in cardiac output and capillary pulmonary wedge pressure amongst the groups.22
The effects of restrictive (e.g. <1.75 L day−1), standard (fixed ml kg−1 h−1), zero balance (fluid therapy which cause zero weight gain) and liberal (e.g. >2.75 L day−1) fluid strategies on AL rates are controversial. Lack of clear definitions, protocol standardisation and small study populations make it difficult to generalise findings from studies comparing these regimens. On balance, the evidence suggests that fluid excess after bowel anastomosis is harmful. Patients who were given more than 8 L i.v fluid during the 72 h perioperative period had a statistically significant increased risk of developing AL (OR=3.20; 95% CI, 1.10–9.31; P=0.049).23 In patients who underwent primary colonic anastomosis because of traumatic injury, multivariate analysis showed 10.5 L crystalloids given over the first 72 h was independently associated with anastomotic breakdown (OR=5.26; 95% CI, 1.14–24.39; P=0.033).24 Most consensus guidelines advise avoiding excessive fluids.
Goal-directed fluid therapy (GDFT) remains contentious in the era of laparoscopic surgery and ERAS. A meta-analysis of seven RCTs25 examined the role of transoesophageal Doppler-guided GDFT (345 patients vs 365 control patients in the conventional fluid therapy group) on the rate of AL in adult patients undergoing elective laparoscopic or open colorectal surgery. Intraoperative GDFT did not affect the incidence of anastomotic dehiscence (RR=0.90; 95% CI, 0.43–1.90; P=0.79). This meta-analysis highlights the fact that many fluid therapy research studies do not report the AL complication rate or differentiate between studies that used an ERAS or conventional pathway.25 Another meta-analysis of six RCTs comparing conventional fluid therapy with GDFT within ERAS found no significant difference in AL between groups (OR=0.66; 95% CI, 0.29–1.49; P=0.31).26 GDFT studies included a variable number and type of interventions in the ERAS group, and also different monitoring tools were used in different studies. In the presence of other ERAS interventions, GDFT may not influence the risk of AL particularly in low-risk groups. In addition, GDFT may not be useful for low-risk patients undergoing laparoscopic surgery.
It is unclear whether or how the type of fluid used affects the outcome of intestinal anastomoses. In a randomised trial of balanced 6% HES (130/0.4, Volulyte) compared with balanced crystalloid (Hartmann's solution) for SVV-guided GDFT in high-risk patients undergoing elective (open or laparoscopic) colorectal surgery, there was no difference in the incidence of AL.27 The fluid balance at 24 h from the start of surgery in the crystalloid group was 4226 ml (range 3251–5779 ml) compared with 3610 ml (range 2443–4519 ml) in the colloid group. There was no difference between the groups with regard to vasopressor use and serum concentrations of inflammatory markers such as IL-6 and CRP.27 In contrast, Joosten and colleagues28 recently reported a higher incidence of AL in patients receiving crystalloids compared with those receiving colloids (eight vs zero patients, P=0.046). In this double-blinded RCT, a 100 ml bolus of either crystalloid or colloid was delivered using closed-loop systems to achieve targeted stroke volume and SVV.28
Levy and colleagues29 and demonstrated that lower systemic oxygen delivery (o2) is common despite volume optimisation in patients undergoing laparoscopic colorectal surgery and is associated with AL.29 Of the 18 patients with a o2 of <400 ml min−1 m−2, four (22%) developed AL compared with one (6%) of the 57 patients with a o2 of >400 ml min−1 m−2 (P=0.01).29
In summary, GDFT may not benefit patients who have no significant comorbidities and who are undergoing laparoscopic surgery within an ERAS pathway. Patients with multiple comorbidities or those who have complicated surgery (e.g. laparoscopic converted to open), undergo emergency surgery or deviate from ERAS pathways (e.g. unable to resume oral intake) require careful monitoring of their fluid management to mitigate the intestinal effects of fluid imbalance on anastomotic healing. Further research is warranted to explore the effects of various fluid management strategies on intestinal oxygen delivery and its effects on the incidence of AL.
Vasopressors
Effects of vasopressors on intestinal circulation are determined by the drug used, dose, duration, indication, blood volume, cardiovascular effects and baseline intestinal vascular resistance. Interestingly, intestinal mucosal perfusion remains relatively constant despite reductions in regional blood flow caused by autoregulatory mechanisms. In theory, vasopressors may lead to splanchnic vasoconstriction and result in intestinal ischaemia and hypoxia. Conversely, prolonged untreated hypotension may compromise perfusion and oxygen delivery in injured and anastomosed tissue.
There are limited data to support a correlation between perioperative use of vasopressors and AL. A retrospective review of 223 patients undergoing a variety of gastrointestinal anastomosis admitted to ICU after surgery revealed vasopressor use was associated with AL (P=0.02).30 This risk was independent of the Acute Physiology and Chronic Health Evaluation II (APACHE II) score and was higher with prolonged duration of vasopressor use.30 Multivariable analysis revealed that vasopressor exposure was associated with AL with an OR of 3.26 (95% CI, 1.13–9.39). Of note, only 26 (of 223) patients received any form of vasopressor. Several other clinical studies in ‘fluid-optimised’ patients undergoing gastrointestinal and pancreatic surgery have found no association between the use of vasopressors and impairment of the intestinal microcirculation or risk of AL.20
Another important question is how the effects of vasopressors on intestinal haemodynamics and oxygenation vary with different fluid regimens. In animals receiving restrictive fluid therapy (3 ml kg h−1), low to moderate dose noradrenaline used to increase MAP to 65–75 mmHg resulted in insignificant changes in intestinal blood flow, tissue Pao2 and pH; however, the study was of short duration (4 h) and no bowel resection was performed. Further work is required.
Analgesic techniques
Multimodal techniques are often employed to achieve optimal analgesia, including neural blockade (e.g. epidural, intrathecal, regional), systemic (e.g. opioids, NSAIDs, adjuvant analgesics) and oral analgesics. These techniques have evolved with minimally invasive surgery (MIS) and the introduction of ERAS. With the exceptions of epidural analgesia and NSAIDs, there is limited evidence regarding the association between AL and other analgesic techniques.
Epidural analgesia
Effects of epidural analgesia on intestinal haemodynamics may be beneficial or detrimental depending on several factors including baseline sympathetic activity, spread of sympathetic block, local anaesthetic used (volume and concentration), method of delivery (bolus or infusion), blood volume and associated use of vasopressor. Theoretically, if a block is limited to mesenteric sympathetic activity (T8–L1), arteriolar and venous dilatation should increase both intestinal macro- and microcirculatory perfusion. Haemorrhage, sepsis, inflammation and increased intra-abdominal pressure may further complicate the effects of epidural anaesthesia on intestinal circulation and oxygen delivery.
The effects of epidural analgesia on intestinal motility in patients undergoing intestinal surgery are of interest. Case reports in the 1980s suggested epidural anaesthesia or analgesia may contribute to an increased AL risk by increasing colonic motility, which may result in tonic contraction and shortening of the rectum. In contrast, many experimental and clinical studies have demonstrated beneficial effects of epidural analgesia on early return of intestinal function (e.g. shorter time to pass flatus and early oral intake), which may be beneficial to healing intestinal tissue.
The effect of epidural analgesia on AL rates remains controversial despite one meta-analysis concluding that the technique does not influence the outcome of anastomoses.31 The majority of studies often include small numbers, lack standardisation of management of breakthrough pain, utilise variable evaluating tools, suggest different strategies to manage hypotension, implement variable ERAS protocols and code complications differently. In the past decade, retrospective studies from large databases have found no significant impact on the incidence of AL with the use of epidural analgesia for open or laparoscopic surgical patients. Current ERAS protocols do not recommend the routine use of epidural analgesia for patients undergoing laparoscopic surgery, and the decision to use them in open surgery should be individualised to the patients' needs.
Hypotensive episodes, excessive i.v. fluids and increased vasopressor requirements have been associated with negative effects on intestinal anastomoses. The role of epidural analgesia in ERAS pathways is controversial. It impairs early mobilisation, can make the timing of thromboprophylaxis complex and often results in incomplete analgesia.
Non-steroidal anti-inflammatory drugs
Non-steroidal anti-inflammatory drugs may predispose to AL by several proposed mechanisms including impaired leucocyte function, decreased production of vascular endothelial growth factor, reduced angiogenesis and impaired collagen production and cross-linking. Studies and metanalyses have reported conflicting results with regard to the association of NSAIDs and risk of AL.2,32 The risk depends on the location of anastomosis in addition to class of NSAIDs and its duration of use and indication (e.g. cancer vs IBD surgical patients).32 Small bowel anastomoses are affected to a greater extent than colonic anastomosis.32
One metanalysis in 2019 reported that the use of an NSAID after surgery was associated with an overall increased risk of AL (OR=1.58; 95% CI, 1.23–2.03; P=0.0003).31 Non-selective NSAIDs were associated with an increased risk (OR=1.79; 95% CI, 1.47–2.18; P=<0.00001), but there was no increased risk of AL with selective NSAIDs.31 The authors advised caution against NSAID use after colonic and rectal anastomoses. However, a later metanalysis of nine studies involving cancer patients (10,868 patients – 70.7% low rectal resection and 28.1% colon resection) only concluded the overall AL rate was not increased in patients using NSAIDs for postoperative analgesia compared with non-users (RR=1.23; 95% CI, 0.81–1.86; P=0.34).33 Subgroup analysis showed neither non-selective NSAID use nor cyclo-oxygenase-2 (COX-2) selective NSAID use caused an increase in AL.33
Non-steroidal anti-inflammatory drugs are an important component of multimodal analgesia. Nonetheless, careful consideration should be given to their use in patients undergoing colorectal anastomosis.
Other analgesia options
Intrathecal and systemic opioids, regional techniques (transverse abdominis plane, paravertebral, or wound and peritoneal infiltration blocks/catheters) and systemic adjuvant analgesics (e.g. i.v. lidocaine infusion) are used for postoperative analgesia. There is no strong evidence to suggest any specific choice with regard to AL outcome after laparoscopic surgery.
Choice of reversal agent
Some case reports have suggested an association between the use of neostigmine and an increased incidence of AL. The suggested mechanisms are increased bowel motility and intraluminal pressure with an associated decrease in blood flow.
The lack of effect on intestinal motility associated with sugammadex has prompted interest in its use in colorectal surgery, particularly if anastomoses are planned. Current evidence demonstrates only a reduction in time to first bowel movement with sugammadex when compared with the combination of neostigmine and glycopyrrolate.
Intraoperative surgical risk factors
Minimally invasive surgery
Historical data demonstrate no difference in AL rates between laparoscopic and open techniques.2 A recent large population-based propensity matched retrospective study, reported a significantly lower incidence of AL after MIS (laparoscopic and robotic) in comparison with open surgery. MIS reduced AL rates for both left colectomy (OR=0.775; 95% CI, 0.710–0.845; P=<0.001) and right colectomy (OR=0.770; 95% CI, 0.713–0.830; P=<0.001).34
Abdominal sepsis
Intraoperative contamination of bowel contents is an independent risk factor for the development of AL.2 Surgeons commonly face this clinical scenario during emergency surgery. Determining if the calculated risk is acceptable or a bowel diversion strategy is more appropriate can be challenging and involves a multidisciplinary approach.
Other surgical factors
The effects of preoperative oral antibiotics, surgical approach, anastomosis technique, blood loss and blood transfusion, duration of surgery, diverting stoma, drainage issues, intraoperative tests for perfusion and leakage, individual surgeon experience, institution caseload and use of adjuvants to strengthen anastomosis are detailed elsewhere and are not discussed further here.1,2,35
Postoperative risk factors
Preoperative and intraoperative risk factors, if not optimised, continue into the postoperative period. Postoperative complications including anaemia, hyperglycaemia and hypoproteinaemia are likely to increase the risk of AL. Hypoxia and hypotension during the postoperative period are detrimental for healing. Postoperative compliance to ERAS components such as early enteral feeding and preventing fluid excess are likely to have positive effects on anastomotic healing. Colorectal surgical patients are at high risk of other postoperative medical complications. The association between complications such as pneumonia, paralytic ileus, renal failure and AL are not clear.
Management of the patient with AL
Anaesthetists have an important role to facilitate diagnosis and provide critical care in patients with AL while in the high dependency unit, ICU and operating room.
Clinical management depends on the severity.2 A Grade A leak is diagnosed radiologically in asymptomatic patients. Grade B leak is characterised by a localised collection resulting in low grade sepsis and peritonitis. Grade C leak results in generalised peritonitis with systemic effects. Antibiotics and CT-guided percutaneous drainage depending on size of collection are required for Grade B leak whereas emergency laparotomy is indicated for Grade C leak.
Conclusions
Anastomotic leakage remains a common and devastating complication after colorectal surgery despite advances in care pathways, surgery and anaesthesia. Identification of risk factors and formulating strategies to prevent this frequent postoperative complication is of paramount importance. A collaborative perioperative approach is essential when addressing the multifactorial causes of AL. Anaesthetists have great potential to affect perioperative care with the support of national quality improvement initiatives at every phase of perioperative care, whether that is in identifying and optimising modifiable risk factors, facilitating diagnosis of AL or providing high-quality care to patients with AL.
Declaration of interests
The authors declare that they have no conflict of interest.
Biographies
Santosh Patel MD FRCA PG Dip (Med Edu) is currently working as a consultant anaesthetist at Tawam hospital, Al ain, UAE. He has previously held clinical and academic posts in India, the UK and the USA. His major clinical and research interests are anaesthesia for colorectal surgery, neuraxial drug errors, patient safety and medical education.
Alastair Duncan FRCA is a consultant at Manchester University NHS Foundation Trust. His major clinical and research interests are hepatobiliary surgery, perioperative medicine, patient safety and medical education.
Matrix codes: 1A01, 2A03, 3A03
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bjae.2021.06.001.
MCQs
The associated MCQs (to support CME/CPD activity) will be accessible at www.bjaed.org/cme/home by subscribers to BJA Education.
Supplementary Material
The following are the Supplementary data to this article:
References
- 1.Kingham T.P., Pachter H.L. Colonic anastomotic leak: risk factors, diagnosis, and treatment. J Am Coll Surg. 2009;208:269–278. doi: 10.1016/j.jamcollsurg.2008.10.015. [DOI] [PubMed] [Google Scholar]
- 2.McDermott F.D., Heeney A., Kelly M.E., Steele R.J., Carlson G.L., Winter D.C. Systematic review of preoperative, intraoperative and postoperative risk factors for colorectal anastomotic leaks. Br J Surg. 2015;102:462–479. doi: 10.1002/bjs.9697. [DOI] [PubMed] [Google Scholar]
- 3.Daams F., Wu Z., Lahaye M.J., Jeekel J., Lange J.F. Prediction and diagnosis of colorectal anastomotic leakage: a systematic review of literature. World J Gastrointest Surg. 2014;6:14–26. doi: 10.4240/wjgs.v6.i2.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bosmans J.W., Jongen A.C., Bouvy N.D., Derikx J.P. Colorectal anastomotic healing: why the biological processes that lead to anastomotic leakage should be revealed before conducting intervention studies. BMC Gastroenterol. 2015;1:1–6. doi: 10.1186/s12876-015-0410-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zheng L., Kelly C.J., Colgan S.P. Physiologic hypoxia and oxygen homeostasis in the healthy intestine. A review in the theme: cellular responses to hypoxia. Am J Physiol Cell Physiol. 2015;309:350–360. doi: 10.1152/ajpcell.00191.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Makanyengo S.O., Carroll G.M., Goggins B.J., Smith S.R., Pockney P.G., Keely S. Systematic review on the influence of tissue oxygenation on gut microbiota and anastomotic healing. J Surg Res. 2020;249:186–196. doi: 10.1016/j.jss.2019.12.022. [DOI] [PubMed] [Google Scholar]
- 7.Foppa C., Ng S.C., Montorsi M., Spinelli A. Anastomotic leak in colorectal cancer patients: new insights and perspectives. Eur J Surg Oncol. 2020;46:943–954. doi: 10.1016/j.ejso.2020.02.027. [DOI] [PubMed] [Google Scholar]
- 8.Ripollés-Melchor J., Ramírez-Rodríguez J.M., Casans-Francés R. Association between use of enhanced recovery after surgery protocol and postoperative complications in colorectal surgery: the Postoperative Outcomes within Enhanced Recovery after Surgery Protocol (POWER) study. JAMA Surg. 2019;154:725–736. doi: 10.1001/jamasurg.2019.0995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Rooijen S.J., Huisman D., Stuijvenberg M. Intraoperative modifiable risk factors of colorectal anastomotic leakage: why surgeons and anesthesiologists should act together. Int J Surg. 2016;36:183–200. doi: 10.1016/j.ijsu.2016.09.098. [DOI] [PubMed] [Google Scholar]
- 10.Fung A., Trabulsi N., Morris M. Laparoscopic colorectal cancer resections in the obese: a systematic review. Surg Endosc. 2017;31:2072–2088. doi: 10.1007/s00464-016-5209-y. [DOI] [PubMed] [Google Scholar]
- 11.Lin X., Li J., Chen W. Diabetes and risk of anastomotic leakage after gastrointestinal surgery. J Surg Res. 2015;196:294–301. doi: 10.1016/j.jss.2015.03.017. [DOI] [PubMed] [Google Scholar]
- 12.Sørensen L.T., Jørgensen T., Kirkeby L.T., Skovdal J., Vennits B., Wille-Jørgensen P. Smoking and alcohol abuse are major risk factors for anastomotic leakage in colorectal surgery. Br J Surg. 1999;86:927–931. doi: 10.1046/j.1365-2168.1999.01165.x. [DOI] [PubMed] [Google Scholar]
- 13.Eriksen T.F., Lassen C.B., Gögenur I. Treatment with corticosteroids and the risk of anastomotic leakage following lower gastrointestinal surgery: a literature survey. Colorectal Dis. 2014;16:154–160. doi: 10.1111/codi.12490. [DOI] [PubMed] [Google Scholar]
- 14.Shandall A., Lowndes R., Young H.L. Colonic anastomotic healing and oxygen tension. Br J Surg. 1985;72:606–609. doi: 10.1002/bjs.1800720808. [DOI] [PubMed] [Google Scholar]
- 15.Sheridan W.G., Lowndes R.H., Young H.L. Tissue oxygen tension as a predictor of colonic anastomotic healing. Dis Colon Rectum. 1987;11:867–871. doi: 10.1007/BF02555426. [DOI] [PubMed] [Google Scholar]
- 16.Kimberger O., Fleischmann E., Brandt S. Supplemental oxygen, but not supplemental crystalloid fluid, increases tissue oxygen tension in healthy and anastomotic colon in pigs. Anesth Analg. 2007;105:773–779. doi: 10.1213/01.ane.0000277490.90387.96. [DOI] [PubMed] [Google Scholar]
- 17.Salusjärvi J.M., Carpelan-Holmström M.A., Louhimo J.M., Kruuna O., Scheinin T.M. Intraoperative colonic pulse oximetry in left-sided colorectal surgery: can it predict anastomotic leak? Int J Colorect Dis. 2018;33:333–336. doi: 10.1007/s00384-018-2963-4. [DOI] [PubMed] [Google Scholar]
- 18.Fleischmann E., Herbst F., Kugener A. Mild hypercapnia increases subcutaneous and colonic oxygen tension in patients given 80% inspired oxygen during abdominal surgery. Anesthesiology. 2006;104:944–949. doi: 10.1097/00000542-200605000-00009. [DOI] [PubMed] [Google Scholar]
- 19.Post I.L., Verheijen P.M., Pronk A., Siccama I., Houweling P.L. Intraoperative blood pressure changes as a risk factor for anastomotic leakage in colorectal surgery. Int J Colorect Dis. 2012;27:765–772. doi: 10.1007/s00384-011-1381-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klevebro F., Boshier P.R., Low D.E. Application of standardized hemodynamic protocols within enhanced recovery after surgery programs to improve outcomes associated with anastomotic leak and conduit necrosis in patients undergoing esophagectomy. J Thorac Dis. 2019;11:692–701. doi: 10.21037/jtd.2018.11.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chan D.K., Lee S.K., Ang J.J. Indocyanine green fluorescence angiography decreases the risk of colorectal anastomotic leakage: systematic review and meta-analysis. Surgery. 2020;168:1128–1137. doi: 10.1016/j.surg.2020.08.024. [DOI] [PubMed] [Google Scholar]
- 22.Kimberger O., Arnberger M., Brandt S. Goal-directed colloid administration improves the microcirculation of healthy and perianastomotic colon. Anesthesiology. 2009;110:496–504. doi: 10.1097/ALN.0b013e31819841f6. [DOI] [PubMed] [Google Scholar]
- 23.Boesen A.K., Maeda Y., Rørbaek Madsen M. Perioperative fluid infusion and its influence on anastomotic leakage after rectal cancer surgery: implications for prevention strategies. Colorect Dis. 2013;15:522–527. doi: 10.1111/codi.12321. [DOI] [PubMed] [Google Scholar]
- 24.Schnüriger B., Inaba K., Wu T., Eberle B.M., Belzberg H., Demetriades D. Crystalloids after primary colon resection and anastomosis at initial trauma laparotomy: excessive volumes are associated with anastomotic leakage. J Trauma. 2011;70:603–610. doi: 10.1097/TA.0b013e3182092abb. [DOI] [PubMed] [Google Scholar]
- 25.Rollins K.E., Mathias N.C., Lobo D.N. Meta-analysis of goal-directed fluid therapy using transoesophageal Doppler monitoring in patients undergoing elective colorectal surgery. BJS Open. 2019;3:606–616. doi: 10.1002/bjs5.50188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang Y.B., Yin K.Y., Zhang X.P., Peng M.Q. Is goal-directed fluid therapy beneficial for gastrointestinal surgery within an enhanced recovery program? A systematic review and meta-analysis. Signa Vitae. 2020;16:1–9. [Google Scholar]
- 27.Yates D.R., Davies S.J., Milner H.E., Wilson R.J. Crystalloid or colloid for goal-directed fluid therapy in colorectal surgery. Br J Anaesth. 2014;112:281–289. doi: 10.1093/bja/aet307. [DOI] [PubMed] [Google Scholar]
- 28.Joosten A., Delaporte A., Ickx B. Crystalloid versus colloid for intraoperative goal-directed fluid therapy using a closed-loop system: a randomized, double-blinded, controlled trial in major abdominal surgery. Anesthesiology. 2018;128:55–66. doi: 10.1097/ALN.0000000000001936. [DOI] [PubMed] [Google Scholar]
- 29.Levy B.F., Fawcett W.J., Scott M.J., Rockall T.A. Intra-operative oxygen delivery in infusion volume-optimized patients undergoing laparoscopic colorectal surgery within an enhanced recovery programme: the effect of different analgesic modalities. Colorel Dis. 2012;14:887–892. doi: 10.1111/j.1463-1318.2011.02805.x. [DOI] [PubMed] [Google Scholar]
- 30.Zakrison T., Nascimento B.A., Jr., Tremblay L.N. Perioperative vasopressors are associated with an increased risk of gastrointestinal anastomotic leakage. World J Surg. 2007;31:1627–1634. doi: 10.1007/s00268-007-9113-4. [DOI] [PubMed] [Google Scholar]
- 31.Holte K., Kehlet H. Epidural analgesia and risk of anastomotic leakage. Reg Anesth Pain Med. 2001;26:111–117. doi: 10.1053/rapm.2001.21241. [DOI] [PubMed] [Google Scholar]
- 32.Modasi A., Pace D., Godwin M., Smith C., Curtis B. NSAID administration post colorectal surgery increases anastomotic leak rate: systematic review/meta-analysis. Surg Endosc. 2019;33:879–885. doi: 10.1007/s00464-018-6355-1. [DOI] [PubMed] [Google Scholar]
- 33.Arron M.N., Lier E.J., de Wilt J.H., Stommel M.W., van Goor H., ten Broek R.P. Postoperative administration of non-steroidal anti-inflammatory drugs in colorectal cancer surgery does not increase anastomotic leak rate; a systematic review and meta-analysis. Eur J Surg Oncol. 2020;46:2167–2173. doi: 10.1016/j.ejso.2020.07.017. [DOI] [PubMed] [Google Scholar]
- 34.Wei D., Johnston S., Goldstein L., Nagle D. Minimally invasive colectomy is associated with reduced risk of anastomotic leak and other major perioperative complications and reduced hospital resource utilization as compared with open surgery: a retrospective population-based study of comparative effectiveness and trends of surgical approach. Surg Endosc. 2020;34:610–621. doi: 10.1007/s00464-019-06805-y. [DOI] [PubMed] [Google Scholar]
- 35.Sciuto A., Merola G., De Palma G.D. Predictive factors for anastomotic leakage after laparoscopic colorectal surgery. World J Gastroenterol. 2018;24:2247–2260. doi: 10.3748/wjg.v24.i21.2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.