Abstract
Severe acute respiratory syndrome-coronavirus-2 (COVID-19) virus uses Angiotensin-Converting Enzyme 2 (ACE2) as a gateway for their entry into the human body. The ACE2 with cleaved products have emerged as major contributing factors to multiple physiological functions and pathogenic complications leading to the clinical consequences of the COVID-19 infection Decreased ACE2 expression restricts the viral entry into the human cells and reduces the viral load. COVID-19 infection reduces the ACE2 expression and induces post-COVID-19 complications like pneumonia and lung injury. The modulation of the ACE2-Ang (1–7)-Mas (AAM) axis is also being explored as a modality to treat post-COVID-19 complications. Evidence indicates that specific food components may modulate the AAM axis. The variations in the susceptibility to COVID-19 infection and the post-COVID its complications are being correlated with varied dietary habits. Some of the food substances have emerged to have supportive roles in treating post-COVID-19 complications and are being considered as adjuvants to the COVID-19 therapy. It is possible that some of their active ingredients may emerge as the direct treatment for the COVID-19.
Keywords: ACE-2 expression, COVID-19, Post-COVID complications, Food components
Introduction
After having experienced the first wave of COVID-19, the second wave has posed unprecedented challenges of the variants of the SARC-CoV-2 virus and post-COVID-19 disease complications [1]. Although much has been understood regarding SARS-CoV-2 transmission, the pathophysiology of COVID-19 still remains to be thoroughly understood [2]. The global data indicates that the majority of the COVID-19 patients experience mild to moderate symptoms. However, only a small portion of the patient population progresses up to critical illness [3]. A considerable number of patients, including those with mild symptoms of COVID-19, experience certain symptoms of post-COVID-19 complications after their initial recovery [4]. Several such short- and long-term complications like pulmonary fibrosis [5], mental disorders [6, 7], and retinopathy [8] are being extensively reported.
The severity of COVID-19 infection and associated mortality rate vary considerably across different populations and countries. The probable factors contributing to such variations include seasonal, environmental, or nutritional differences [9, 10]. It has been speculated that certain dietary habits predominant in the Asian countries might have contributed to lesser severity and low death rates compared to those of the European and American countries [10]. Recently, Rodriguez and Pierce [11] reviewed the impact the nutritional status in COVID-19 and suggested a possibility that food habits can modulate infectious disease and the inflammatory processes associated with it positively or negatively by altering the immune system during COVID-19. Although inconclusive the impact of vitamin D and zinc status in COVID-19 patients regarding viral transmission and its clinical symptoms has distinctly emerged in various studies. It has been concluded by Rodriguez and Pierce [11] that those nutritional interventions may have effects on the incidence of COVID-19 infection and mortality rates [11]. Other factors responsible for these variations in the severity and mortality rate include obesity, old age, diabetes, and cardiovascular diseases [12]. ACE2 protein can be found in two forms, membrane ACE2 (mACE2) and soluble (sACE2). Important, the soluble form of ACE2 is also active and it has been reported that SARS-CoV2 can be blocked by the recombinant soluble ACE2, which might represent a therapeutic option; in this respect, a pilot trial (NCT04335136) has been launched. Gender differences have also been reported in relation to the ACE2 expression. Males to have a higher level of tissue ACE2 [14], while serum activities seem to be higher in females [13, 14]. Further, there are evidences that show that ACE2 expression is age dependent [15].
Certain food items reduce the Angiotensin-converting enzyme (ACE) activity and exert antioxidant effects. However, the COVID-19 virus uses ACE2 as a gateway to human cells, and there is a need to explore if any food components affect the expression and effects of ACE2. Diet-dependent variations in the ACE2 expressions may provide insights into the variations in the COVID-19 severity, complications, and related death rates [10]. Recently our group reported several phytoconstituents interacting with the ACE2, which may contribute to the treatment of COVID-19 [16]. Similarly, it is proposed that certain food components may be useful in the treatment of COVID-19 [17]. The COVID-19 pathogenesis and its complications are based on the immune-inflammatory cascade, cytokine storm, pneumonia, and genetic predispositions [18–20]. The differences in the ACE2 expressions have been reported between different countries, and these correlate with genetic variations [21, 22]. Many food components are reported to be useful for the treatment of COVID-19, and these may act through altering ACE2 expression and its activity. Even the expression and activity of ACE2 change rapidly in response to certain food items [23–28] Hence, it can be speculated that the dietary habits and dietary components may affect a person’s susceptibility to COVID-19 infection and the severity of the post-COVID-19 complications. Through this review, we have tried to explore whether any food components alter the ACE2 levels and thereby may have a role in the treatment of COVID-19 and its consequences.
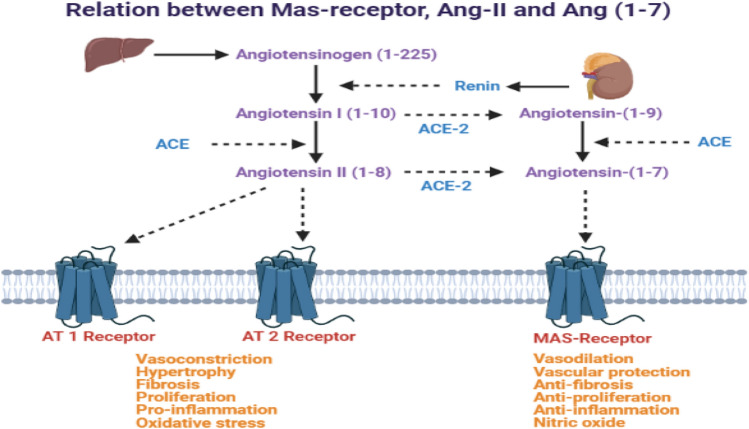
COVID-19 virus enters the host cell infection by binding virus-specific ‘S’ spikes to the human ACE-2 [19]. Ang-II mediates its actions via AT1 and AT2 receptors, whereas Ang (1–7) acts via MAS1 protooncogene GPCR receptors (Fig. 1). The entry in the body occurs by endosome formation through transmembrane protease serine 2 (TMPRSS2). After the entry of the virus inside the cell, viral polyproteins are synthesized that encode for the replicase-transcriptase complex. The virus then synthesizes RNA via its RNA-dependent RNA polymerase (RdRp) and subsequently structural proteins of the new viruses are formed and after assembly viral particles are released [20]. Because of high virus titer, strong cytokine surge and inflammatory response are induced in lungs and other organs, where ACE2 is highly expressed, leading to high morbidity and mortality.
Fig. 1.
Role of ACE2-Ang (1–7)-Mas axis in the physiology and pathophysiology
In the human body, alveolar epithelial type II cells mainly express ACE2 (almost 83% of type II cells) and serve as a reservoir for viral invasion [29]. Apart from these cells, other cells expressing ACE2 include the heart, kidney, endothelium, brain, and intestine [30, 31]. The expression of ACE2 has also been noted at different anatomical sites like oral and nasal mucosa, nasopharynx, stomach, skin, lymph nodes, thymus, bone marrow, spleen, and liver [30–33]. New SARS-CoV-2 variants more frequently affect the intestinal mucosa. The expression of ACE2 is of great significance in the susceptibility of the patient to infection and severity of the post-COVID-19 complications. Higher ACE2 expression provides more sites of entry and hence facilitates the COVID-19 infection [34, 35]. The COVID-19 higher viral load induces more severe COVID-19 symptoms and increases the mortality rate [36, 37]. The occurrence and severity of COVID-19 are more in the older patients; however, it is noteworthy that the ACE2 expression is reduced with the older age [38]. Apart from old age, male gender and hypertension also strongly correlate with the severity of COVID-19 [39, 40]. Conversely, higher ACE2 expression as such is observed in younger age, female gender, normotensive state, and even Asian smokers. These populations suffer less from the COVID-19 and its consequences. Additionally, the immunocompromised patients are not necessarily at higher risk of COVID-19 infection. Considering the strong affinity of the COVID-19 virus to ACE2 and its contagiousness, it has been thought that the levels of ACE2 expression may not significantly alter either the rates of initial infection or the viral clearance [41]. However, more recent reports confirm the vital role of ACE2 in the pathogenesis and consequences of COVID-19 in clinical studies (http://ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=48763&EncHid=&userName=Remedium).
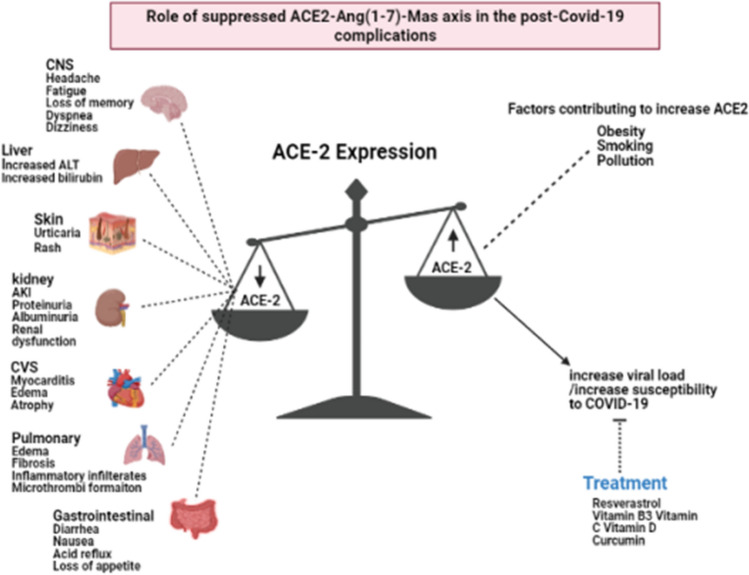
The physiological role of ACE2, Angiotensin (1–7) and its receptor – MAS1 protooncogene GPCR is depicted in Fig. 1. Apart from these roles, alterations in the expression of ACE2 plays a crucial role in the pathogenesis of COVID-19 infection and its consequences. Overexpression of human ACE2 enhances disease severity in a mouse model of COVID-19 infection, demonstrating that the viral entry into cells is a critical step. It has also been found that ACE2 level varies as per demographic area and diet as well. ACE2 downregulation by COVID-19 may reduce the further viral entry into cells, thereby limiting viral spread. However, such reduction of ACE2 expression may lead to the development of post-COVID-19 complications. The COVID-19 infection consequently reduces the ACE-2 expression. Such reduction in the ACE2 is considered to be responsible for the post-COVID-19 complications like acute lung injury rather than the COVID-19 viral load or cytotoxic actions of this virus. Therefore, increasing ACE2 expression would most likely help to prevent secondary fibrotic changes following COVID-19 pneumonia along with other consequences [41]. The role of altered activity of ACE2-Ang (1–7)-Mas axis is represented in the Fig. 2.
Fig. 2.
Role of ACE2 in COVID-19 complications
Pulmonary complications
ACE2 and transmembrane serine protease-2 (TMPRSS2) are the two receptors present in the respiratory tract's epithelial cells, which support the spread of viral infections. The ACE2 expression is increased in the lungs of COVID-19 patients suffering from other comorbidities [42]. On the other hand, cardiopulmonary diseases are associated with a decreased ACE2 activity [43]. The role of ACE in cardiopulmonary diseases has been substantiated through multiple preclinical and clinical studies. A cohort study in 576 hypertensive patients has concluded that Angiotensin-converting enzyme inhibitors (ACEIs) increase the increased risk of pneumonia [44]. Ang-II receptor blockers (ARBs) and ACE inhibitors also increase ACE2 in the heart and brain [45]. Chronic administration of ACE inhibitors increases ACE2 expression in pneumocytes [46]. Such observations prove a direct relationship between the cardiopulmonary diseases, their treatment with ACEIs/ARBs, and susceptibility to the COVID-19 infections. It has been recently found that administration of RAAS inhibitors worsens the symptoms of COVID-19 while increasing the expression of ACE 2 in the lungs [46]. However, the ACEIs/ARBs were not found to possess any therapeutic advantages in reducing the COVID-19 infection, its progression or consequences [47]. Reports are stating that cytokine storm leads to an increased ACE2 expression in the lungs [48]. The COVID-19 patients suffer more from cellular exudates in the small airways and alveoli [49, 50].
Extra pulmonary complications
Renal complications
ACE-2 is expressed in podocytes and proximal straight tubular cells. Hence, SARS-CoV-2 could directly infect the human kidney and induce cytopathic effects in renal cells, contributing to AKI and the spread of the virus in the body [51]. Renal dysfunction could result from both direct coronavirus infection and the cytokine storm due to abnormal host immune response [52]. The COVID-19 patients with severe symptoms may develop various levels of renal damage and even AKI. Such occurrence of kidney damage has been reported in multiple clinical studies. As per an estimate by Guan et al., the AKI incidence in the COVID-19 patients is only 0.5% [53]. However, a recent study on 59 SARS-CoV-2 patients has reported massive albuminuria in 20 patients on the first days of the admission and proteinuria in 37 patients during their hospitalization [54]. This study further noted that 35.5% patients developed AKI, and 12.3% of them succumbed to death [55]. Still, the incidence of AKI in SARS-CoV-2infection is lower than in SARS and MERS [56]. ACE-2 is the main binding site for SARS-CoV-2, and it is expressed in lung tissue and other vital organs, including the kidneys.
COVID-19 may induce renal impairment through direct viral infection via ACE-2 binding. It is plausible that the higher renal tropism of COVID-19 due to increased affinity of the S1 domain for ACE-2 worsens virus-induced cytopathic changes and disruption of the RAS homeostasis [57]. The expression level of ACE-2 in the kidneys is crucial in the pathogenesis and progression of renal dysfunction in several diseases [58]. Decreased ACE-2 expression and activity were implicated in different acute and chronic kidney disease models. It was associated with the loss of RAS homeostasis and worsened pathological changes in renal parenchyma [59]. Even in the archived renal biopsy samples of diabetics, a decreased expression of ACE2 messenger RNA was evident [60].
Cardiovascular complications
ACE2 is localized to the endothelial and smooth muscle cells of intramyocardial vessels and cardiac myocytes [61]. ACE2 internalization by COVID-19 may potentially result in the loss of ACE2 at the cell surface and voids a key pathway for the cell to degrade Ang-II and generate the Ang-(1–7). Possible pathophysiology of SARS-CoV-2–related to myocarditis is proposed by Siripanthong et al. [62]. In patients with Cardiovascular diseases (CVD), the loss of ACE2 by COVID-19-induced internalization may exacerbate acute and chronic CVD [63]. Myocardial damage, inflammatory cell infiltration, oedema of the myocardium, fibrosis and cardiac muscle atrophy are reported in the COVID-19 [64]. Acute myocardial injury and associated increased risk of mortality is a routine finding in the patients with COVID-19 [65]. Activation of AAM axis induces relaxation of coronary vessels, reduces oxidative stress, suppresses myocardial remodelling, and improves post-ischemic cardiac work [66]. According to recent studies, a majority of patients with severe COVID-19 also have hypertension [67, 68]. An increased activity of RAAS might pre-exist in these individuals irrespective of the infection. The downregulation of ACE2 with a concomitant increase in Ang-II in COVID-19 patients leads to RAAS over-activation. Further, the reduced protection of Ang (1–7) may worsen cardiac injuries. Higher ACE2 expression contributes to heart injury even though it slows the disease progresses [69].
Central nervous system-related complications
ACE2 predominantly expresses in the glial cells and contributes to the neural regulation of multiple physiological functions, including neurogenesis, metabolic activities, and stress response [33, 70]. The neurological symptoms reported in the COVID-19 patients include fatigue, memory loss, disturbance in sleep, and dyspnoea [71]. As per the Center for Disease Control and Prevention (CDC) report, almost 35% of the mild COVID-19 cases continue to suffer from post-COIVD-19 symptoms [72]. The most persistent post-COVID-19 symptoms that may last for more than six weeks include fatigue (92%), memory ad attention disturbances (74%), headache (65%), weakness (68%), and dizziness (64%) [71]. Other symptoms like fatigue and dyspnoea affecting patients' quality of life have been reported by others [73, 74].
Gastrointestinal tract (GIT)-related complications
The intestine is more vulnerable to COVID-19 infections [64]. A significant occurrence of GIT symptoms like anorexia, nausea-vomiting, diarrhea, and abdominal pain have been commonly reported in COVID-19 patients (84.85). In addition, the ACE2 receptors are highly expressed in the colonic tissue [75] and feces [76] of COVID-19 patients. All such findings suggest that the abundance of ACE2 in the gut contributes to the pathogenesis of post-COVID-19 GIT complications.
Hepatic complications
ACE2 is expressed in both liver cells and bile duct cells, contributing to the liver damage associated with COVID-19 infection [77]. It is evident that many patients with COVID-19 have an increased Serum glutamate pyruvate transaminase (SGPT) and Serum glutamate oxaloacetate transaminase (SGOT) levels [78, 79]. Even the autopsies of COVID-19 patients have revealed cellular infiltration, fatty depositions, and necrosis in the liver [64]. Different detection techniques including single-cell RNA-seq analysis and immunohistochemistry have revealed that only cholangiocytes were positive for ACE2 expression whereas hepatocytes, the endothelial lining of sinusoids, and Kupffer cells were negative for ACE2 [80–82]. In congruence with this, certain COVID-19 patients had elevated levels of Gamma-glutamyltranspeptidase (GGT) reflecting the cholangiocyte damage [83]. Overall, these findings suggest that the acute hepatic injury may be due to the drug treatment given in COVID-19 rather than the infection itself. Further investigations are warranted to determine the role of ACE2 in the COVID-19-induced cholangiocyte [69].
Skin-related complications
The skin cutaneous manifestations of COVID-19 include itching, reddening, rashes and chickenpox-like vesicles. In the COVID-19 patients, the cutaneous symptoms like chilblain-like lesions are most commonly reported [84]. Similarly, certain other symptoms like necrosis, maculopapular rash, vesicular lesions, and livedoid lesions have also been identified in COVID patients. The chilblain-like lesions were frequent in less-severe COVID-19 whereas the livedoid/ necrotic lesions were common in the severe COVID-19 [85].
Food and herbals affecting ACE2
The factors contributing to the variations in the susceptibility to COVID-19 and its consequences also include dietary habits [10]. The impact of certain food items on the ACE activity is well-reported. Considering the role of ACE2 in COVID-19, a meticulous exploration of the food items and their components on the ACE2 activity. Such food items may prove to be important adjuvants to the treatment of COVID-19 [86].
Following are some of the chemical constituents from the food items that can be considered for the prevention and treatment of post-COVID-19 complication:
Terpenes
Carvacrol (C10H14O)
Carvacrol (C10H14O), is a phenolic monoterpenoid found in the essential oils of plant origin [87, 88]. It is safe for consumption and approved by the US-FDA and Council of Europe for its use in food, confectionery, and alcoholic beverages [89]. In a mice model of autoimmune encephalomyelitis, carvacrol reduces pro-inflammatory mediators (IL-6, IL-17, and IFN-γ), and increases anti-inflammatory cytokines (IL-4, IL-10, and TGF-β) [90]. The in-silico studies have shown that carvacrol has an affinity for the receptor-binding domain of S protein of COVID-19 and can inhibit the binding of the virus to ACE2. It may also inhibit the replication and maturation of the COVID-19 virus [91, 92]. The capability of carvacrol to avoid ACE2 binding of the virus, antiviral properties, and antibacterial properties further strengthen its candidature as an important adjuvant to the treatment of COVID-19 infections [89]. It possesses drug-like physicochemical and pharmacokinetic properties; however, detailed investigations on its efficacy and safety are warranted to prove its usefulness as a therapeutic intervention in treating COVID-19 infection and its consequences [93].
Lemon oil
Lemon oil biologically obtained from Citrus limon. ACE2 expression is significantly down-regulated in human colorectal adenocarcinoma (HT-29) cells by applying lemon oil [94]. D-limonene, the main component of lemon oil, inhibits the production of IL-2, IL-4, IL-13, IFN-γ, and TNF-α from CD3+CD4+ T cells and IL-2, TNF-α, and IFN-γ in CD3+CD8+T cells [95]. The immunomodulatory, antiviral, and anti-inflammatory effects of limonene might also prove beneficial against COVID-19 [96]. The in silico evaluations of limonene indicate its potential to inhibit the ACE2 [97]. These predictions have been substantiated through cell culture studies. Limonene decreased the ACE-2 expression HT-29 cell line [94]. Thus, the lemon oil and its components may prove valuable natural anti-COVID-19 compounds to be added to the therapeutic arsenal [94].
Geranium oil
This volatile oil is obtained derived from Pelargonium graveolens leaves [98]. It is widely used in perfumery, cosmetics and aromatherapy. Geranium oil possesses immunomodulatory properties and us considered to cleanses the lymphatic system. Clinically this oil is used in treating GIT ailments and urolithiasis. Geranium oil significantly down-regulated the expression of ACE-2 while producing moderate cytotoxic effects in Human colorectal adenocarcinoma (HT-29) cells. GC–MS analysis of Geranium oil indicated 22 compounds, out of which citronellol and geraniol had the highest concentration. Further investigations demonstrated the decreased expression of ACE-2 in HT-29 cells upon treatment with citronella and geraniol. Out of 22 compounds in geranium oil including geraniol, citronellol, and neryl acetate as the major compounds which downregulate the ACE2 expression in epithelial cells [94].
Polyphenols
Resveratrol
Resveratrol, a polyphenol is exhaustively reported for potential protective effect against a number of conditions like respiratory illness, cancer, and cardiovascular disease [99]. Miyazaki et al. reported that resveratrol, through activation of SIRT1, down-regulated the expression of AT1R in the aorta of C57/B6 mice [100]. In another study, resveratrol suppressed AngII-mediated fibrosis by inducing SIRT1-mediated manganese superoxide dismutase (Mn-SOD) in TO-2 hamsters [101]. Also, resveratrol is reported to increase the expression of ACE2, AT2R, and MasR and decrease the pro-fibrotic protein expression in Ang-II actuated Vascular smooth muscle cells (VSMCs) [102]. In SD rats, resveratrol increased the expression of ACE2, AT2R, and MAS in the liver, which might have a role in preventing Non-alcoholic fatty liver disease (NAFLD) [103]. It has been observed that upon feeding Ang (1–7) and resveratrol along with a high-fat diet to male FVB/N mice, both increased the expression of ACE2 and SIRT1 [104]. In another study, Moran et al. provided evidence that resveratrol induced the expression of ACE2 in apolipoprotein-deficient mice (ApoE-/-Ace2-/y) on account of SIRT1[105]. Few computation studies have reported a considerable affinity of resveratrol with S1: ACE2 complex and postulated about the probable potential of resveratrol in preventing the entry of COVID 19 into the host cells [106, 107].
Curcumin
A polyphenol from the rhizome of turmeric (Curcuma longa) is known to possess significant antiviral potential [108]. Already, curcumin has been proved to impede the growth of SARS-coronavirus [109], and the genomic semblance of SARS-CoV-2 with MERS coronavirus [110] and SARS-coronavirus (> 80%) collectively imply the prospect of the efficacy of curcumin against COVID-19. Administration of curcumin is associated with decreased expression of the AT1 receptor and elevated expression of both the AT2 receptor and ACE2 in male SD rats. These findings also indicated that curcumin modulated the myocardial fibrosis via ACE2 and reduced the expression of TGF-beta1 [111]. An in-silico investigation projected that curcumin interacts with several amino acids (Ala 348, Asn 394, Glu402, His 378, His 401, and Tyr385) of ACE2 that are involved in the binding of ACE2 with the S1 subunit of SARS-CoV-2[107]. This might have significant implications on the entry of SARS-Cov2 inside host cells. SARS-CoV-2 preferentially infects and propagates alveolar type II cells that are the precursor of gas exchanging [112], and curcumin has reportedly protected alveolar type II cells of the inflammatory lung [113]. It could be assumed that curcumin has the potential to be effective against SARS Cov-2 but, its limited oral bioavailability is a snag in its handiness. However, it can be managed with the help of novel drug delivery systems [114]. Curcumin possesses significant antioxidant and anti-inflammatory potentials [115] and can reduce the pulmonary fibrosis [116], hence it has a promising role in treating COVID-19.
Ginger
Ginger is widely popular because of its ethnomedicinal and condimental uses. The 6-gingerol inhibited the AngII induced AT1 receptor activation in HEK293/Gα15/AT1 cells. Sini decoction, formulation ginger, and other herbal drugs significantly improved acute inflammatory lung injury by reducing ACE and AT1R expression and ACE2/Ang (1–7)/Mas axis modulation [117]. In silico studies have disclosed that gingerol has a strong binding affinity with RBD domain S protein of SARS-CoV-2, and interaction of gingerol with RNA-dependent RNA polymerase RdRp was comparable with remdesivir [118].
The phytoconstituents having a binding affinity towards spike protein include 10-paradol, 8-paradol, scopoletin, 10-shogaol, 8-gingerol, and 10-gingerol. Administration of nasal purge of ginger oil may be considered for in vitro studies as well as clinical trials for minimizing the severity and transmission of COVID-19. The in silico evaluation has revealed that the components of ginger interact with the active sites of the receptor-binding domain of COVID-19 spike protein and ACE-2 to exert antiviral effects against COVID-19 [119].
Flavonoids
Quercetin
Quercetin exists in diet as glycosides and rutinosides [120]. A recent study using supercomputer-based in silico drug-docking has revealed that quercetin inhibits interaction between ACE-2 and COVID-19 virus spike protein [121]. It might exert protection against COVID-19, particularly its antioxidant, anti-inflammatory [122], and antiviral properties [123]. Quercetin modulates several key elements such as PI-3 kinase, RNA-dependent RNA polymerase, reverse transcriptase, TNF, TLR3, HA2 subunit of glycoprotein hemagglutinin, glycoprotein D. that are crucial for the replication and packaging of viruses [124].
An in-vitro study proved that quercetin (IC50 4.48 μM) and its metabolites inhibited recombinant human (rh) ACE2 activity at physiologically relevant concentrations [120]. A Tibetan remedy called Tsantan Sumtang, quercetin is one of the constituents that significantly reduced chronic hypoxia-induced right ventricular tissue remodeling and fibrosis through up regulation of the ACE2-Ang1-7-Mas axis [125]. Quercetin inhibited the Akt activation, up-regulated Fas and caveolin 1 expression, and hindered the advancement of bleomycin-induced pulmonary fibrosis in aged mice [126]. In another study, sphingosine kinase 1 (SphK1)/sphingosine-1-phosphate lyase (S1PL) pathway inhibition by quercetin prevented the progress of pulmonary fibrosis [127].
Vitamins
Vitamin A
A metabolite of vitamin A- All-trans retinoic acid (atRA) is biologically active. A study performed by Zhong et al. demonstrated that chronic all-trans retinoic acid (atRA) induces expressions of ACE2 at both gene and protein level thereby reducing the blood pressure and myocardial damage in spontaneously hypertensive rats. This indicates the possibility of using atRA in the potential prevention and treatment of hypertension [128]. All-trans retinoic acid up-regulates the ACE2 expression in the heart and kidney, ensuing the reduced blood pressure and attenuated cardiomyocyte injury in spontaneously hypertensive rats [128]. The well-reported role of Vitamin A in preventing the fibrosis of the lungs and liver [129, 130] indicates its possible role in treating post-COVID-19 complications [131].
Vitamin C
Vitamin C or ascorbic acid is involved in enzymatic processes, antioxidative defense, and collagen synthesis [132]. It stimulates the INF formation, induces lymphocyte multiplication and stimulates the phagocytic capacity of neutrophils [133]. One of the first clinical trials on the efficacy of vitamin C in COVID-19 patients (n = 148) who received 24 g per day for 7 days had a decreased need for ventilation, needed lesser vasopressor therapy, and had reduced organ failure. Patients receiving this dose needed less ICU length of stay and had a reduced mortality. High-dose intravenous vitamin C as well as additional bolus doses had been used to treat moderate to severe cases of COVID-19 in China. In critically ill patients, additional bolus doses of vitamin C were needed [134]. Though ineffective in preventing the COVID-19 infection, vitamin C decreased the period of the disease, reduced the duration of indoor confinement, and ameliorated symptoms [135].
Vitamin D
A meta-analysis by Martineau et al. deduced that daily or weekly administration of vitamin D protects from the risk of acute respiratory tract infection [136]. Even in preclinical findings, vitamin D protected rats from the lipopolysaccharide-induced lung injury by decreasing ACE expression, reducing Ang-II production, and up-regulating the expression of ACE2 [137]. Likewise, vitamin D receptor gene knocked down mice exhibited severe acute lung injury and had higher mortality in the lipopolysaccharide-induced sepsis model [138]. Vitamin D also blocked the RAAS cascade by reducing the expression of renin [139]. Retrospective investigations have revealed that the serum levels of vitamin D were significantly lower in COVID-19 patients, correlated with critical pulmonary symptoms, a longer period of infection, and a higher death risk in COVID-19 patients [140]. Further, a randomized, double-blind pilot study has proved that a large dose of Calcifediol in COVID-19 patients significantly decreased the severity of the diseases and mitigated the need for intensive care [141]. It is also speculated that old age is associated with reduced vitamin D levels, and this population mostly suffers from the COVID-19.
Organosulphur compounds
Garlic
The Organic sulfur compounds (OSCs) are the principle bioactive constituents of garlic and account for garlic’s pungent odor and pharmacological effects [142]. To date, a little over thirty OSCs have been identified in garlic and categorized into two groups, L-cysteine sulfoxides and γ-glutamyl-l-cysteine peptides [143]. Amongst the OSCs, allicin (S-allyl-l-cysteine sulfoxide) is plentiful in both fresh and dry garlic. It transforms to allicin(diallylthiosulfinate), having antiviral activity, antioxidant, anti-inflammatory, immunomodulatory, and other pharmacological properties [144, 145].
A few randomized clinical studies have investigated the effect of garlic extract on viral infections and immunity. These studies concluded that the administration of garlic extract is associated with a reduction in the severity of cold and flu symptoms and decreased duration of viral infection [144, 146]. Recent computational studies have indicated that allicin-derived OSCs may inhibit the Mpro of SARS-CoV-2 through hydrogen bonding [147]. The allyl disulfide and allyl trisulfide present in the garlic essential oil is predicted to strongly inhibit the ACE2 protein expression as well as proteases of SARS-CoV-2 [148]. In addition, garlic and its OSCs have immune-boosting properties. OSCs decrease the stimulation of pro-inflammatory cytokines, the signature hallmark of SARS-CoV-2, via down-regulation of the leptin-mediated pathway [149]. Thus, garlic essential oil is an important component of food containing natural antivirus agents and may be considered in the treatment of COVID-19.
Proteins/peptides
Rapeseed proteins
Rapeseed is a common name for the plant Brassica oleifera. Several studies have reported the antiviral, antioxidant [150], and ACE inhibiting [151] properties of the peptides and proteins of rapeseed. Oral administration of rapeseed peptide, namely LY, RALP, and GHS, to spontaneously hypertensive rats for 5 weeks decreased the systolic blood pressure. Furthermore, it reduced the expression of ACE and renin in the myocardium. Also, myocardial expression of ACE2, Ang (1–7), and Mas receptors were up-regulated by these peptides at the gene and protein levels. Moreover, LY reduced the expression of Ang-II in cardiac tissue [151]. These findings indicate the advantages of rapeseed proteins in reducing the post-COVID-19 complications.
Linoleic acid (LA)
Polyunsaturated fatty acids (PUFA) are essential for the architecture and functionality of cell membrane and various biological processes, including cell signaling, synthesis of key inflammatory mediators (prostaglandins, leukotrienes, protectins, resolvins, etc.), and regulation of gene expression [152]. A metabolomic profiling study done on sera of COVID-19 patients observed the dysregulated lipid metabolism after SARS-CoV-2 [153]. Another study on Human coronavirus 229E (HCoV-229E) infected cells observed a significant elevation in linoleic acid and arachidonic acid. However, the growth of the HCov-229E virus was significantly suppressed by exogenous supplementation of LA or AA, and similar results were obtained with MERS-CoV [154]. Studies on SARS-CoV-2 S glycoprotein reported that S trimer apo S cryo-EM structures have about 60–75% open conformation with one of the RBD rotated up for ACE2 binding preferentially [155]. In contrast, the binding of linoleic acid with S trimer stabilized the closed conformation by the lockdown of hydrophilic anchor and compaction of RBD trimer resulting in reorganization of receptor-binding motif needed for ACE2 interaction. Therefore, the LA-binding pocket on S protein is a potential target for synthesizing small molecules that could stabilize the closed conformation of S protein and interfere with ACE2 [156].
Broccoli
Broccoli produces sulforaphane (Isothiocyanates) from glucoraphanin in response to stress. The stimulation of the Nrf2 signaling pathway by sulforaphane aids the transcription factor interaction with Antioxidant response elements (AREs). Consequently, it up-regulates the transcription of a group of genes associated with cell protection through antioxidant defense and cellular detoxification [157]. Sulforaphane is an activator of Nrf2 and has been proved to block the effects of Ang-II-mediated via ROS [158]. Further, sulforaphane is reported to produce anti-inflammatory effects by inhibiting the JNK/AP-1/NF-κB pathway. Therefore, sulforaphane and other nutraceuticals may be recommended to have therapeutic relevance against COVID-19 [159].
Liquorice
Liquorice contains saponins, flavonoids, and coumarins. The characteristic yellow color and sweet aromatic flavor are because of flavonoids and glycyrrhizin, respectively. Glycyrrhizin (GL) is a triterpenoid saponin obtained as calcium, potassium, and ammonium salts of 18β-glycyrrhizic acid (glycyrrhizic or glycyrrhizic acid and a glycoside of Glycyrrhetinic acid [GA]). Both GL and GA exert antiviral effects against the SARS-coronavirus in in vitro studies. GL administration in Vero cells infected with patient plasma samples resulted in a significant reduction in virus absorption and replication rate [160]. Chen et al. reported a similar result of GL in the Vero-E6 cell line, but encouraging outcomes were not observed in the fRhK4 cell line [161]. Given the circumstances, another important fact is the role of serine protease TMPRSS2 along with ACE2 in infecting the cells with the virus [162]. TMPRSS2 is of considerable importance in both influenza and coronavirus infections [163]. Intriguingly, GA regulated the expression of TMPRSS2 via mineralocorticoid receptor [164], which may describe the wide-range antiviral effects of GL [165]. Another significant property of GL and GA is the immunomodulation by inhibition of Toll-like Receptor-4 (TLR4). TLR4 is associated with endotoxin storm as a consequence of the suppression of the AAM axis [164].
The Traditional Chinese Medicine, Sini decoction contains three herbs, namely aconite (Aconitumcarmichaelii), licorice (Glycyrrhizaglabra), and ginger rhizome (Zingiber officinale). Reportedly, Sini decoction produced a significant reduction in E. coli-induced acute lung injury through a decrease in inflammatory mediators in lung tissue, and it also down-regulated the expression of ACE and AngII type 1 receptor (AT1R). Further, Sini decoction stimulated the AAM axis [117]. Additionally, liquorice extract has been reported to stimulate the release of interferon-1ß in upper and lower respiratory tract cells, which might be important in protecting against inflammation induced by COVID-19 [166]. Therefore, liquorice might reduce the ACE2 expression in the lung and still be able to decrease lung inflammation.
White button mushroom (WBM)
Reportedly, White button mushroom (WBM), commonly consumed in Asia–Pacific, Europe and US, inhibited dihydrotestosterone (DHT)-mediated Androgen receptor (AR) activation. TMPRSS2 is reportedly induced by androgen in prostate cancer. Wang et al., proved that WBM interferes with AR mediated TMPRSS2 expression and reduces the concentration of pro-inflammatory cytokines in serum [167].
Non-vegetarian components of food
Egg ovotransferrin‐derived ACE inhibitory peptide IRW
Recent in silico modeling studies have projected IRW (Ile-Arg-Try), an egg white protein ovotransferrin, as a novel ACE inhibitory tripeptide [168]. IRW possesses anti‐inflammatory, antioxidant, and modulatory effects on the TNF-mediated expression of cytokines and adhesion molecules through suppression of NF-κB in endothelial cells [169]. RPYL (a lactoferrin‐derived peptide) was reported to inhibit Ang II-induced vasoconstriction in the rabbit carotid artery [169]. Also, IRW significantly upregulated the expression of ACE2 and decreased the pro-inflammatory mediators in the kidney tissue of SHR [170]. Such reports highlight the importance of these bioactive peptides in the treatment of COVID-19 [171].
Tuna protein-derived peptides
Tuna (Katsuwonus pelamis) is a common component of human diets. Tuna protein hydrolysates have multiple etiological activities including ACE inhibition [172]. Tuna skeletal myosin heavy chain protein contains an antiviral peptide EEAGGATAAQIEM (E-M). Molecular docking simulation demonstrated that Gly143 and Gln189 of this protein might play an important role in the interactions of peptide E-M and SARS Cov -2 protein Mpro. The peptide E-M can block SARS-CoV-2 attachment to host cells by interfering with the interaction of COVID-19 virus with receptor ACE2. All these findings indicate that tuna may contribute significantly as an adjuvant to COVID-19 treatment [173]. The role of various food components and their chemical constituents is summarized in Table 1.
Table 1.
Impact of food components on the ACE2-Ang (1–7)-MAS axis (AAM) and their applications in the treatment of post-COVID-19 complications
| Food component | Active ingredients | Effect on COVID-19 | References |
|---|---|---|---|
| Terpenes | |||
| Thyme and oregano | Carvacrol | Interference of ACE2 receptors in the host | Kulkarni [92], Kumar [91] |
| Lemon oil | Limonene | Downregulated ACE2 expression in epithelial cells and Stoppage of SARS-CoV-2/COVID-19 entry into the human body | Abdelli [97] |
| Geranium oil | Citronellol, geraniol, and neryl acetate | Prevention of the invasion of SARS-CoV-2/COVID-19 into the human body | Senthil Kumar [94] |
| Polyphenols | |||
| Resveratrol | Resveratrol | increase the expression of ACE2, AT2R and MASR | Kim [102] |
| Turmeric | Curcumin | Inhibition of binding of spike glycoprotein to ACE2 receptors | Mason [112] |
| Zinger | 10-paradol, 8-paradol, scopoletin, 10-shogaol, 8-gingerol, 10-gingerol | Reduction of viral load. Decreased shedding of COVID-19 virus in the nasal passages | Haridas [119] |
| Flavanoid | |||
| Dill, fennel leaf, onion, oregano, chili pepper | Quercetin | Binding to S protein & inhibition of rhACE2 activity | Sargiacomo [121] |
| Vitamins | |||
| Beef liver, cod liver oil, sweet potatoes, carrots, black-eyed peas, spinach, broccoli | Vitamin A | Upregulation of ACE2 receptor | Zhong [128] |
| Citrus fruits | Vitamin C | Prevention of the development of “cytokine storm” decreased the period of infection, reduced the duration of indoor confinement, and ameliorated symptoms | Hoang [135] |
| Vitamin D | Calcitriol | Increase in the ACE2 level | Xu [137] |
| Organosulphur compounds | |||
| Garlic | Allyldisulfide and allyltrisulfide | Strongly inhibit the ACE2 protein expression and COVID-19 proteases | Thuy [148] |
| Proteins/Peptides | |||
| Rapeseed protein | LY, RALP, and GHS | Increased ACE2 expression, angiotensin-(1–7) and Mas receptor levels | Rong He [151] |
| Vegetarian food components | |||
| Essential free fatty acid (FFA) | Linoleic acid (LA) | SARS-cov-2 spike (S) glycoprotein receptor-binding domains (rbds) bind tightly and specifically with essential free fatty acid (FFA) linoleic acid (LA) in three composite binding pockets | Christine Toelzer [156] |
| Broccoli | Sulforaphane | anti-inflammatory effects by inhibiting JNK/AP-1/NF-κB pathway | Horowitz [159] |
| Liquorice | Glycyrrhizin | reduces the ACE2 expression in the lung and still be able to decrease lung inflammation | Feng [166] |
| Non-vegetarian food components | |||
| Egg ovotransferrin‐derived ACE inhibitory peptide | IRW | Increase in ACE2 and decrease in pro-inflammatory genes expression | Majumder [171] |
| Tuna | Peptides and selenonein | Blockade of COVID-19 viral attachment to host cells though ACE2 binding | Zhipeng Yu [173] |
Conclusion
The ACE2-Ang (1–7)-MAS axis has emerged as an important mediator of the post-COVID-19 complications. The disparity of the susceptibility to COVID-19 infection and to the post-Covid complication is now being correlated not only to the genetic constitution but also to the dietary habits. The ACE2-Ang (1–7)-MAS axis plays a cardinal role in the pathogenesis of COVID-19 and its complications. The viral entry into human body depends on the ACE2 expression levels. Whereas, reduced ACE2 expression is related to post-COVID-19 complications. Many foods and their components interact with the main pathway through which COVID-19 enters human body and induce post-COVID-19 complications. This review proposes that such food components can be considered as adjuvants to the COVID-19 therapy.
Author contributions
RKG and SA conceptualized the topic and supervised the compilation; SS and SK explored the literature and sorted the data, SS and CRP compiled the manuscript; RKG, CRP, and SA supervised compilation process and edited the manuscript.
Funding
Funding information is not applicable/No funding was received.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Informed consent
Not applicable.
Research involving human and animal rights
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.COVID research: a year of scientific milestones : nature.com search. https://www.nature.com/search?q=COVID+research%3A+a+year+of+scientific+milestones. Accessed 26 May 2021 [DOI] [PubMed]
- 2.Cevik M, Kuppalli K, Kindrachuk J, Peiris M. Virology, transmission, and pathogenesis of SARS-CoV-2. BMJ. 2020 doi: 10.1136/bmj.m3862. [DOI] [PubMed] [Google Scholar]
- 3.Lu, R, Zhao X, Li J et al (2020) Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. The Lancet 395(10224):565–574. 10.1016/s0140-6736(20)30251-8 [DOI] [PMC free article] [PubMed]
- 4.COVID-19 (coronavirus): long-term effects - Mayo Clinic. https://www.mayoclinic.org/diseases-conditions/coronavirus/in-depth/coronavirus-long-term-effects/art-20490351. Accessed 26 May 2021
- 5.Spagnolo P, Balestro E, Aliberti S, et al. Pulmonary fibrosis secondary to COVID-19: a call to arms? Lancet Respir Med. 2020;8:750–752. doi: 10.1016/S2213-2600(20)30222-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rajkumar RP. COVID-19 and mental health: a review of the existing literature. Asian J Psychiatr. 2020;52:102066. doi: 10.1016/j.ajp.2020.102066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xiong J, Lipsitz O, Nasri F, et al. Impact of COVID-19 pandemic on mental health in the general population: a systematic review. J Affect Disord. 2020;277:55–64. doi: 10.1016/j.jad.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Invernizzi A, Torre A, Parrulli S, et al. Retinal findings in patients with COVID-19: results from the SERPICO-19 study. EClinicalMedicine. 2020 doi: 10.1016/j.eclinm.2020.100550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kissler SM, Tedijanto C, Goldstein E, et al. Projecting the transmission dynamics of SARS-CoV-2 through the postpandemic period. Science. 2020;80(368):860–868. doi: 10.1126/science.abb5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bousquet J, Anto JM, Iaccarino G, et al. Is diet partly responsible for differences in COVID-19 death rates between and within countries? Clin Transl Allergy. 2020;10:16–18. doi: 10.1186/s13601-020-00323-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodriguez-Leyva D, Pierce GN. The impact of nutrition on the COVID-19 pandemic and the impact of the COVID-19 pandemic on nutrition. Nutrients. 2021;13(6):1752. doi: 10.3390/nu13061752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fernández-Atucha A, Izagirre A, Fraile-Bermúdez AB, Kortajarena M, Larrinaga G, Martinez-Lage P, Gil J. Sex differences in the aging pattern of renin–angiotensin system serum peptidases. Biol Sex Differ. 2017 doi: 10.1186/s13293-017-0128-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yao S, Song J, Gao J, Lin P, Yang M, Zahid KR, Yan Y, Cao C, Ma P, Zhang H, Li Z. Cognitive function and serum hormone levels are associated with gray matter volume decline in female patients with prolactinomas. Front Neurol. 2018;29(8):742. doi: 10.3389/fneur.2017.00742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sama IE, Ravera A, Santema BT et al (2020) Circulating plasma concentrations of angiotensin-converting enzyme 2 in men and women with heart failure and effects of renin-angiotensin-aldosterone inhibitors. Eur Heart J 41(19):1810–1817. 10.1093/eurheartj/ehaa373 [DOI] [PMC free article] [PubMed]
- 15.Guzik 2017 | Statin | Stroke. https://www.scribd.com/document/435477686/guzik2017-pdf. Accessed 26 May 2021
- 16.Goyal RK, Majeed J, Tonk R, et al. Current targets and drug candidates for prevention and treatment of SARS-CoV-2 (COVID-19) infection. Rev Cardiovasc Med. 2020;21:365–3984. doi: 10.31083/j.rcm.2020.03.118. [DOI] [PubMed] [Google Scholar]
- 17.Patel B, Sharma S, Nair N, et al. Therapeutic opportunities of edible antiviral plants for COVID-19. Mol Cell Biochem. 2021;476:2345–2364. doi: 10.1007/s11010-021-04084-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Song P, Li W, Xie J, et al. Cytokine storm induced by SARS-CoV-2. Clin Chim Acta. 2020;509:280–287. doi: 10.1016/j.cca.2020.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ye Q, Wang B, Mao J. The pathogenesis and treatment of the ‘Cytokine Storm’’ in COVID-19’. J Infect. 2020;80:607–613. doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sinha P, Matthay MA, Calfee CS. Is a “cytokine Storm” relevant to COVID-19? JAMA Intern Med. 2020;180:1152–1154. doi: 10.1001/jamainternmed.2020.3313. [DOI] [PubMed] [Google Scholar]
- 21.Li MY, Li L, Zhang Y, Wang XS. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect Dis Poverty. 2020;9:1–7. doi: 10.1186/s40249-020-00662-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Al-Eitan LN, Alahmad SZ (2021) Pharmacogenomics of genetic polymorphism within the genes responsible for SARS-CoV-2 susceptibility and the drug-metabolising genes used in treatment. Rev Med Virol 31(4):e2194. 10.1002/rmv.2194 [DOI] [PMC free article] [PubMed]
- 23.Quiles JL, Rivas-García L, Varela-López A, et al. Do nutrients and other bioactive molecules from foods have anything to say in the treatment against COVID-19? Environ Res. 2020 doi: 10.1016/j.envres.2020.110053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gheblawi M, Wang K, Viveiros A, et al. Angiotensin-converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system: celebrating the 20th anniversary of the discovery of ACE2. Circ Res. 2020;126:1456–1474. doi: 10.1161/CIRCRESAHA.120.317015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sparks MA, Crowley SD, Gurley SB, et al. Classical renin-angiotensin system in kidney physiology. Compr Physiol. 2014;4:1201–1228. doi: 10.1002/cphy.c130040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tipnis SR, Hooper NM, Hyde R, et al. A human homolog of angiotensin-converting enzyme: cloning and functional expression as a captopril-insensitive carboxypeptidase. J Biol Chem. 2000;275:33238–33243. doi: 10.1074/jbc.M002615200. [DOI] [PubMed] [Google Scholar]
- 27.Deshotels MR, Xia H, Sriramula S, et al. Angiotensin II mediates angiotensin converting enzyme type 2 internalization and degradation through an Angiotensin II type I receptor-dependent mechanism. Hypertension. 2014;64:1368–1375. doi: 10.1161/HYPERTENSIONAHA.114.03743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bader M, Alenina N, Young D, et al. The meaning of mas. Hypertension. 2018;72:1072–1075. doi: 10.1161/HYPERTENSIONAHA.118.10918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao Y, Zhao Z, Wang Y, et al. Single-cell RNA expression profiling of ACE2, the receptor of SARS-CoV-2. BioRxiv. 2020 doi: 10.1101/2020.01.26.919985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang H, Penninger JM, Li Y, et al. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020;46:586–590. doi: 10.1007/s00134-020-05985-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patel VB, Zhong JC, Grant MB, Oudit GY. Role of the ACE2/angiotensin 1–7 axis of the renin-angiotensin system in heart failure. Circ Res. 2016;118:1313–1326. doi: 10.1161/CIRCRESAHA.116.307708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis - PubMed. https://pubmed.ncbi.nlm.nih.gov/15141377/. Accessed 27 May 2021 [DOI] [PMC free article] [PubMed]
- 33.Xia H, Lazartigues E. Angiotensin-converting enzyme 2 in the brain: properties and future directions. J Neurochem. 2008;107:1482–1494. doi: 10.1111/j.1471-4159.2008.05723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mourad JJ, Levy BI. Interaction between RAAS inhibitors and ACE2 in the context of COVID-19. Nat Rev Cardiol. 2020;17:313. doi: 10.1038/s41569-020-0368-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zheng YY, Ma YT, Zhang JY, Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020;17:259–260. doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fajnzylber J, Regan J, Coxen K, et al. SARS-CoV-2 viral load is associated with increased disease severity and mortality. Nat Commun. 2020;11:1–9. doi: 10.1038/s41467-020-19057-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu Y, Yan LM, Wan L, et al. Viral dynamics in mild and severe cases of COVID-19. Lancet Infect Dis. 2020;20:656–657. doi: 10.1016/S1473-3099(20)30232-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.How ACE2 Reduction Associated with Aging Affects COVID-19 Severity. https://www.hcplive.com/view/how-ace2-reduction-associated-with-aging-affects-covid19-severity. Accessed 27 May 2021
- 39.Xudong X, Junzhu C, Xingxiang W, et al. Age- and gender-related difference of ACE2 expression in rat lung. Life Sci. 2006;78:2166–2171. doi: 10.1016/j.lfs.2005.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shibata S, Arima H, Asayama K, et al. Hypertension and related diseases in the era of COVID-19: a report from the Japanese Society of Hypertension Task Force on COVID-19. Hypertens Res. 2020;43:1028–1046. doi: 10.1038/s41440-020-0515-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chaudhry F, Lavandero S, Xie X, et al. Manipulation of ACE2 expression in COVID-19. Open Hear. 2020;7:1424. doi: 10.1136/openhrt-2020-001424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pinto BGG, Oliveira AER, Singh Y, et al. ACE2 expression is increased in the lungs of patients with comorbidities associated with severe COVID-19. MedRxiv. 2020 doi: 10.1101/2020.03.21.20040261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sharma RK, Stevens BR, Obukhov AG, et al. ACE2 (Angiotensin-Converting Enzyme 2) in cardiopulmonary diseases: ramifications for the control of SARS-CoV-2. Hypertension. 2020;76:651–661. doi: 10.1161/HYPERTENSIONAHA.120.15595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Woodhead M (2014) The Relationship between Angiotensin Converting Enzyme Inhibitors (ACEIs), Statins and Pneumonia. Turk Toraks Derg 15:39–41. 10.5152/ttd.2014.12515
- 45.Kreutz R, Algharably EAEH, Azizi M, et al. Hypertension, the renin-angiotensin system, and the risk of lower respiratory tract infections and lung injury: implications for covid-19. Cardiovasc Res. 2020;116:1688–1699. doi: 10.1093/cvr/cvaa097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wysocki J, Lores E, Ye M, et al. Kidney and Lung ACE2 Expression after an ACE Inhibitor or an Ang II Receptor Blocker: Implications for COVID-19. J Am Soc Nephrol. 2020;31:1941–1943. doi: 10.1681/ASN.2020050667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Iaccarino G, Borghi C, Cicero AFG, et al. Renin-angiotensin system inhibition in cardiovascular patients at the time of COVID19: much ado for nothing? A statement of activity from the directors of the board and the scientific directors of the Italian society of hypertension. High Blood Press Cardiovasc Prev. 2020;27:105–108. doi: 10.1007/s40292-020-00380-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Girija ASS, Shankar EM, Larsson M. Could SARS-CoV-2-induced hyperinflammation magnify the severity of coronavirus disease (CoViD-19) leading to acute respiratory distress syndrome? Front Immunol. 2020;11:1206. doi: 10.3389/fimmu.2020.01206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wichmann D, Sperhake JP, Lütgehetmann M, et al. Autopsy findings and venous thromboembolism in patients With COVID-19: a prospective cohort study. Ann Intern Med. 2020;173:268–277. doi: 10.7326/M20-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Diao B, Wang C, Wang R, et al. Human kidney is a target for novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. MedRxiv. 2020 doi: 10.1101/2020.03.04.20031120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Qian J-Y, Wang B, Liu B-C. Acute kidney injury in the 2019 novel coronavirus disease. Kidney Dis. 2020;6:318–323. doi: 10.1159/000509086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guan W, Ni Z, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/nejmoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Naicker S, Yang CW, Hwang SJ, et al. The novel coronavirus 2019 epidemic and kidneys. Kidney Int. 2020;97:824–828. doi: 10.1016/j.kint.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cheng Y, Luo R, Wang K, et al. Kidney impairment is associated with in-hospital death of COVID-19 patients. Kidney Int. 2020 doi: 10.1101/2020.02.18.20023242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stasi A, Castellano G, Ranieri E, et al. SARS-CoV-2 and viral sepsis: immune dysfunction and implications in kidney failure. J Clin Med. 2020;9:4057. doi: 10.3390/jcm9124057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Perico L, Benigni A, Remuzzi G. Should COVID-19 concern nephrologists? Why and to what extent? The emerging impasse of angiotensin blockade. Nephron. 2020;144:213–221. doi: 10.1159/000507305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Soler MJ, Wysocki J, Batlle D. ACE2 alterations in kidney disease. Nephrol Dial Transplant. 2013;28:2687–2697. doi: 10.1093/ndt/gft320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fang F, Liu GC, Zhou X, et al. Loss of ACE2 exacerbates murine renal ischemia-reperfusion injury. PLoS ONE. 2013 doi: 10.1371/journal.pone.0071433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gilbert RE, Caldwell L, Misra PS, et al. Overexpression of the severe acute respiratory syndrome coronavirus-2 receptor, angiotensin-converting enzyme 2, in diabetic kidney disease: implications for kidney injury in novel coronavirus disease 2019. Can J Diabetes. 2021;45:162–166.e1. doi: 10.1016/j.jcjd.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Donoghue M, Hsieh F, Baronas E, et al. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1–9. Circ Res. 2000 doi: 10.1161/01.res.87.5.e1. [DOI] [PubMed] [Google Scholar]
- 62.Siripanthong B, Nazarian S, Muser D, et al. Recognizing COVID-19–related myocarditis: the possible pathophysiology and proposed guideline for diagnosis and management. Hear Rhythm. 2020;17:1463–1471. doi: 10.1016/j.hrthm.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yousif MHM, Dhaunsi GS, Makki BM, et al. Characterization of Angiotensin-(1–7) effects on the cardiovascular system in an experimental model of Type-1 diabetes. Pharmacol Res. 2012;66:269–275. doi: 10.1016/j.phrs.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 64.Gu J, Korteweg C. Pathology and pathogenesis of severe acute respiratory syndrome. Am J Pathol. 2007;170:1136–1147. doi: 10.2353/ajpath.2007.061088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Srivastava K. Association between COVID-19 and cardiovascular disease. Int J Cardiol Heart Vasc. 2020 doi: 10.1016/j.ijcha.2020.100583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jiang F, Yang J, Zhang Y, et al. Angiotensin-converting enzyme 2 and angiotensin 1–7: novel therapeutic targets. Nat Rev Cardiol. 2014;11:413–426. doi: 10.1038/nrcardio.2014.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Studies find hypertension most prevalent comorbidity in patients hospitalized for COVID-19 (2020). https://www.healio.com/news/cardiology/20200910/hypertension-may-affect-outcomes-in-covid19
- 68.COVID-19 and hypertension: what we know and don’t know - American College of Cardiology (2020). https://www.acc.org/latest-in-cardiology/articles/2020/07/06/08/15/covid-19-and-hypertension
- 69.Ni W, Yang X, Yang D, et al. Role of angiotensin-converting enzyme 2 (ACE2) in COVID-19. Crit Care. 2020 doi: 10.1186/s13054-020-03120-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Alenina N, Bader M. ACE2 in brain physiology and pathophysiology: evidence from transgenic animal models. Neurochem Res. 2019;44:1323–1329. doi: 10.1007/s11064-018-2679-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tabacof L, Tosto-Mancuso J, Wood J, et al. Post-acute COVID-19 syndrome negatively impacts health and wellbeing despite less severe 1 acute infection 2 3. MedRxiv. 2020 doi: 10.1101/2020.11.04.20226126. [DOI] [Google Scholar]
- 72.Tenforde MW, Kim SS, Lindsell CJ, et al. Symptom duration and risk factors for delayed return to usual health among outpatients with COVID-19 in a multistate health care systems network — United States, March–June 2020. MMWR Morb Mortal Wkly Rep. 2020;69:993–998. doi: 10.15585/mmwr.mm6930e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Carfì A, Bernabei R, Landi F. Persistent symptoms in patients after acute COVID-19. JAMA J Am Med Assoc. 2020;324:603–605. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Moreno-Pérez O, Merino E, Leon-Ramirez JM, et al. Post-acute COVID-19 syndrome. Incidence and risk factors: a Mediterranean cohort study. J Infect. 2021;82:378–383. doi: 10.1016/j.jinf.2021.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xiao F, Tang M, Zheng X, et al. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. 2020;158:1831–1833.e3. doi: 10.1053/j.gastro.2020.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wu Y, Guo C, Tang L, et al. Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. Lancet Gastroenterol Hepatol. 2020;5:434–435. doi: 10.1016/S2468-1253(20)30083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Prins GH, Olinga P. Potential implications of COVID-19 in non-alcoholic fatty liver disease. Liver Int. 2020;40:2568. doi: 10.1111/liv.14484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA J Am Med Assoc. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hamming I, Timens W, Bulthuis MLC, et al. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zou X, Chen K, Zou J, et al. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med. 2020;14:185–192. doi: 10.1007/s11684-020-0754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chai X, Hu L, Zhang Y, et al. Specific ACE2 expression in cholangiocytes may cause liver damage after 2019-nCoV infection. BioRxiv. 2020 doi: 10.1101/2020.02.03.931766. [DOI] [Google Scholar]
- 83.Fan Z, Chen L, Li J, et al. Clinical features of COVID-19-related liver functional abnormality. Clin Gastroenterol Hepatol. 2020;18:1561–1566. doi: 10.1016/j.cgh.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jia JL, Kamceva M, Rao SA, Linos E. Cutaneous manifestations of COVID-19: a preliminary review. J Am Acad Dermatol. 2020;83:687–690. doi: 10.1016/j.jaad.2020.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Galván Casas C, Català A, Carretero Hernández G, et al. Classification of the cutaneous manifestations of COVID-19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol. 2020;183:71–77. doi: 10.1111/bjd.19163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Avery A. Can diet influence the COVID-19 mortality rate? Kompass Nutr Diet. 2021;1:16–18. doi: 10.1159/000512841. [DOI] [Google Scholar]
- 87.Butt MS, Sultan MT. Nigella sativa: reduces the risk of various maladies. Crit Rev Food Sci Nutr. 2010;50:654–665. doi: 10.1080/10408390902768797. [DOI] [PubMed] [Google Scholar]
- 88.Fachini-Queiroz FC, Kummer R, Estevão-Silva CF, et al. Effects of thymol and carvacrol, constituents of Thymus vulgaris L. essential oil, on the inflammatory response. Evid Based Complement Altern Med. 2012 doi: 10.1155/2012/657026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ultee A, Bennik MHJ, Moezelaar R. The phenolic hydroxyl group of carvacrol is essential for action against the food-borne pathogen Bacillus cereus. Appl Environ Microbiol. 2002;68:1561–1568. doi: 10.1128/AEM.68.4.1561-1568.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mahmoodi M, Amiri H, Ayoobi F, et al. Carvacrol ameliorates experimental autoimmune encephalomyelitis through modulating pro- and anti-inflammatory cytokines. Life Sci. 2019;219:257–263. doi: 10.1016/j.lfs.2018.11.051. [DOI] [PubMed] [Google Scholar]
- 91.Kumar A, Choudhir G, Shukla SK, et al. Identification of phytochemical inhibitors against main protease of COVID-19 using molecular modeling approaches. J Biomol Struct Dyn. 2020 doi: 10.1080/07391102.2020.1772112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kulkarni SA, Nagarajan SK, Ramesh V, et al. Computational evaluation of major components from plant essential oils as potent inhibitors of SARS-CoV-2 spike protein. J Mol Struct. 2020;1221:128823. doi: 10.1016/j.molstruc.2020.128823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Javed H, Meeran MFN, Jha NK, Ojha S. Carvacrol, a plant metabolite targeting viral protease (Mpro) and ACE2 in host cells can be a possible candidate for COVID-19. Front Plant Sci. 2021;11:2237. doi: 10.3389/fpls.2020.601335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Senthil Kumar KJ, Vani MG, Wang CS, et al. Geranium and lemon essential oils and their active compounds downregulate angiotensin-converting enzyme 2 (ACE2), a SARS-CoV-2 spike receptor-binding domain, in epithelial cells. Plants. 2020;9:1–12. doi: 10.3390/plants9060770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lappas CM, Lappas NT. D-Limonene modulates T lymphocyte activity and viability. Cell Immunol. 2012 doi: 10.1016/j.cellimm.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 96.Nagoor Meeran MF, Seenipandi A, Javed H, et al. Can limonene be a possible candidate for evaluation as an agent or adjuvant against infection, immunity, and inflammation in COVID-19? Heliyon. 2021;7:e05703. doi: 10.1016/j.heliyon.2020.e05703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Abdelli I, Hassani F, Bekkel Brikci S, Ghalem S. In silico study the inhibition of angiotensin converting enzyme 2 receptor of COVID-19 by Ammoides verticillata components harvested from Western Algeria. J Biomol Struct Dyn. 2021;39:3263–3276. doi: 10.1080/07391102.2020.1763199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Narnoliya LK, Jadaun JS, Singh SP (2019) The phytochemical composition, biological effects and biotechnological approaches to the production of high-value essential oil from geranium. In: Essential Oil Research. Springer International Publishing, pp 327–352
- 99.Horne JR, Vohl MC. Biological plausibility for interactions between dietary fat, resveratrol, ACE2, and SARS-CoV illness severity. Am J Physiol - Endocrinol Metab. 2020;318:E830–E833. doi: 10.1152/ajpendo.00150.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Miyazaki R, Ichiki T, Hashimoto T, et al. SIRT1, a longevity gene, downregulates angiotensin II type 1 receptor expression in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2008;28:1263–1269. doi: 10.1161/ATVBAHA.108.166991. [DOI] [PubMed] [Google Scholar]
- 101.Tanno M, Kuno A, Yano T, et al. Induction of manganese superoxide dismutase by nuclear translocation and activation of SIRT1 promotes cell survival in chronic heart failure. J Biol Chem. 2010;285:8375–8382. doi: 10.1074/jbc.M109.090266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kim EN, Kim MY, Lim JH, et al. The protective effect of resveratrol on vascular aging by modulation of the renin–angiotensin system. Atherosclerosis. 2018;270:123–131. doi: 10.1016/j.atherosclerosis.2018.01.043. [DOI] [PubMed] [Google Scholar]
- 103.Tiao MM, Lin YJ, Yu HR, et al. Resveratrol ameliorates maternal and post-weaning high-fat diet-induced nonalcoholic fatty liver disease via renin-angiotensin system. Lipids Health Dis. 2018 doi: 10.1186/s12944-018-0824-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Andrade JMO, Paraíso AF, de Oliveira MVM, et al. Resveratrol attenuates hepatic steatosis in high-fat fed mice by decreasing lipogenesis and inflammation. Nutrition. 2014;30:915–919. doi: 10.1016/j.nut.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 105.Moran CS, Biros E, Krishna SM, et al. Resveratrol inhibits growth of experimental abdominal aortic aneurysm associated with upregulation of angiotensin-converting enzyme 2. Arterioscler Thromb Vasc Biol. 2017;37:2195–2203. doi: 10.1161/ATVBAHA.117.310129. [DOI] [PubMed] [Google Scholar]
- 106.Wahedi HM, Ahmad S, Abbasi SW. Stilbene-based natural compounds as promising drug candidates against COVID-19. J Biomol Struct Dyn. 2020 doi: 10.1080/07391102.2020.1762743. [DOI] [PubMed] [Google Scholar]
- 107.Maurya VK, Kumar S, Prasad AK, et al. Structure-based drug designing for potential antiviral activity of selected natural products from Ayurveda against SARS-CoV-2 spike glycoprotein and its cellular receptor. VirusDisease. 2020;31:179–193. doi: 10.1007/s13337-020-00598-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Praditya D, Kirchhoff L, Brüning J, et al. Anti-infective properties of the golden spice curcumin. Front Microbiol. 2019;10:912. doi: 10.3389/fmicb.2019.00912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wen CC, Kuo YH, Jan JT, et al. Specific plant terpenoids and lignoids possess potent antiviral activities against severe acute respiratory syndrome coronavirus. J Med Chem. 2007;50:4087–4095. doi: 10.1021/jm070295s. [DOI] [PubMed] [Google Scholar]
- 110.Liu Z, Ying Y. The inhibitory effect of curcumin on virus-induced cytokine storm and its potential use in the associated severe pneumonia. Front Cell Dev Biol. 2020;8:479. doi: 10.3389/fcell.2020.00479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pang XF, Zhang LH, Bai F, et al. Attenuation of myocardial fibrosis with curcumin is mediated by modulating expression of angiotensin II AT1/AT2 receptors and ACE2 in rats. Drug Des Devel Ther. 2015;9:6043–6054. doi: 10.2147/DDDT.S95333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mason RJ. Pathogenesis of COVID-19 from a cell biology perspective. Eur Respir. 2020 doi: 10.1183/13993003.00607-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Almatroodi SA, Alrumaihi F, Alsahli MA, et al. Curcumin, an active constituent of turmeric spice: implication in the prevention of lung injury induced by benzo(a) pyrene (BAP) in rats. Molecules. 2020 doi: 10.3390/molecules25030724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sauraj, Vinay kumar, Kumar B, et al (2020) Redox responsive xylan-SS-curcumin prodrug nanoparticles for dual drug delivery in cancer therapy. Mater Sci Eng C 107:110356 10.1016/j.msec.2019.110356 [DOI] [PubMed]
- 115.Menon VP, Sudheer AR. Antioxidant and anti-inflammatory properties of curcumin. Adv Exp Med Biol. 2007;595:105–125. doi: 10.1007/978-0-387-46401-5_3. [DOI] [PubMed] [Google Scholar]
- 116.Punithavathi D, Venkatesan N, Babu M. Curcumin inhibition of bleomycin-induced pulmonary fibrosis in rats. Br J Pharmacol. 2000;131:169–172. doi: 10.1038/sj.bjp.0703578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Liu J, Chen Q, Liu S, et al. Sini decoction alleviates E. coli induced acute lung injury in mice via equilibrating ACE-AngII-AT1R and ACE2-Ang-(1–7)-Mas axis. Life Sci. 2018;208:139–148. doi: 10.1016/j.lfs.2018.07.013. [DOI] [PubMed] [Google Scholar]
- 118.Kumar Verma A, Kumar V, Singh S, et al. Repurposing potential of Ayurvedic medicinal plants derived active principles against SARS-CoV-2 associated target proteins revealed by molecular docking, molecular dynamics and MM-PBSA studies. Biomed Pharmacother. 2021;137:111356. doi: 10.1016/j.biopha.2021.111356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Haridas M, Sasidhar V, Nath P, et al. Compounds of Citrus medica and Zingiber officinale for COVID-19 inhibition: in silico evidence for cues from Ayurveda. Futur J Pharm Sci. 2021;7:1–9. doi: 10.1186/s43094-020-00171-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Liu X, Raghuvanshi R, Ceylan FD, Bolling BW. Quercetin and Its metabolites inhibit recombinant human angiotensin-converting enzyme 2 (ACE2) activity. J Agric Food Chem. 2020;68:13982–13989. doi: 10.1021/acs.jafc.0c05064. [DOI] [PubMed] [Google Scholar]
- 121.Sargiacomo C, Sotgia F, Lisanti MP. COVID-19 and chronological aging: senolytics and other anti-aging drugs for the treatment or prevention of corona virus infection? Aging (Albany NY) 2020;12:6511–6517. doi: 10.18632/AGING.103001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Huang R, Zhong T, Wu H. Quercetin protects against lipopolysaccharide-induced acute lung injury in rats through suppression of inflammation and oxidative stress. Arch Med Sci. 2015;11:427–432. doi: 10.5114/aoms.2015.50975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Veckenstedt A, Pusztai R. Mechanism of antiviral action of quercetin against cardiovirus infection in mice. Antiviral Res. 1981;1:249–261. doi: 10.1016/0166-3542(81)90015-2. [DOI] [PubMed] [Google Scholar]
- 124.Saakre M, Mathew D, Ravisankar V. Perspectives on plant flavonoid quercetin-based drugs for novel SARS-CoV-2. Beni-Suef Univ J Basic Appl Sci. 2021;10(1):1–3. doi: 10.1186/s43088-021-00107-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Dang Z, Su S, Jin G, et al. Tsantan Sumtang attenuated chronic hypoxia-induced right ventricular structure remodeling and fibrosis by equilibrating local ACE-AngII-AT1R/ACE2-Ang1-7-Mas axis in rat. J Ethnopharmacol. 2020;250:112470. doi: 10.1016/j.jep.2019.112470. [DOI] [PubMed] [Google Scholar]
- 126.Hohmann MS, Habiel DM, Coelho AL, et al. Quercetin enhances ligand-induced apoptosis in senescent idiopathic pulmonary fibrosis fibroblasts and reduces lung fibrosis in vivo. Am J Respir Cell Mol Biol. 2019;60:28–40. doi: 10.1165/rcmb.2017-0289OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhang X, Cai Y, Zhang W, Chen X. Quercetin ameliorates pulmonary fibrosis by inhibiting SphK1/S1P signaling. Biochem Cell Biol. 2018;96:742–751. doi: 10.1139/bcb-2017-0302. [DOI] [PubMed] [Google Scholar]
- 128.Zhong JC, Huang DY, Yang YM, et al. Upregulation of angiotensin-converting enzyme 2 by all-trans retinoic acid in spontaneously hypertensive rats. Hypertension. 2004;44:907–912. doi: 10.1161/01.HYP.0000146400.57221.74. [DOI] [PubMed] [Google Scholar]
- 129.Tsuchida T, Friedman SL. Mechanisms of hepatic stellate cell activation. Nat Rev Gastroenterol Hepatol. 2017;14:397–411. doi: 10.1038/nrgastro.2017.38. [DOI] [PubMed] [Google Scholar]
- 130.Bin QJ, Fan QQ, Xing L, et al. Vitamin A-decorated biocompatible micelles for chemogene therapy of liver fibrosis. J Control Release. 2018;283:113–125. doi: 10.1016/j.jconrel.2018.05.032. [DOI] [PubMed] [Google Scholar]
- 131.Stephensen CB, Lietz G. Vitamin A in resistance to and recovery from infection: relevance to SARS-CoV2. Br J Nutr. 2021 doi: 10.1017/S0007114521000246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhang L, Liu Y. Potential interventions for novel coronavirus in China: a systematic review. J Med Virol. 2020;92:479–490. doi: 10.1002/jmv.25707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhang J, Xie B, Hashimoto K. Current status of potential therapeutic candidates for the COVID-19 crisis. Brain Behav Immun. 2020;87:59–73. doi: 10.1016/j.bbi.2020.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sahebnasagh A, Saghafi F, Avan R, et al. The prophylaxis and treatment potential of supplements for COVID-19. Eur J Pharmacol. 2020;887:173530. doi: 10.1016/j.ejphar.2020.173530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hoang X, Shaw G, Fang W, Han B. Possible application of high-dose vitamin C in the prevention and therapy of coronavirus infection. J Glob Antimicrob Resist. 2020;23:256–262. doi: 10.1016/j.jgar.2020.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Martineau AR, Jolliffe DA, Hooper RL, et al. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ. 2017 doi: 10.1136/bmj.i6583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Xu J, Yang J, Chen J, et al. Vitamin D alleviates lipopolysaccharide-induced acute lung injury via regulation of the renin-angiotensin system. Mol Med Rep. 2017;16:7432–7438. doi: 10.3892/mmr.2017.7546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kong J, Zhu X, Shi Y, et al. VDR attenuates acute lung injury by blocking Ang-2-Tie-2 pathway and renin-angiotensin system. Mol Endocrinol. 2013;27:2116–2125. doi: 10.1210/me.2013-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Yuan W, Pan W, Kong J, et al. 1,25-Dihydroxyvitamin D3 suppresses renin gene transcription by blocking the activity of the cyclic AMP response element in the renin gene promoter. J Biol Chem. 2007;282:29821–29830. doi: 10.1074/jbc.M705495200. [DOI] [PubMed] [Google Scholar]
- 140.Sulli A, Gotelli E, Casabella A, et al. Vitamin d and lung outcomes in elderly covid-19 patients. Nutrients. 2021;13:1–13. doi: 10.3390/nu13030717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Entrenas Castillo M, Entrenas Costa LM, Vaquero Barrios JM, et al. Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: a pilot randomized clinical study. J Steroid Biochem Mol Biol. 2020;203:105751. doi: 10.1016/j.jsbmb.2020.105751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Omar SH, Al-Wabel NA. Organosulfur compounds and possible mechanism of garlic in cancer. Saudi Pharm J. 2010;18:51–58. doi: 10.1016/j.jsps.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Yamaguchi Y, Kumagai H. Characteristics, biosynthesis, decomposition, metabolism and functions of the garlic odour precursor, S-allyl-l-cysteine sulfoxide (Review) Exp Ther Med. 2019;19:1528–1535. doi: 10.3892/etm.2019.8385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Rouf R, Uddin SJ, Sarker DK, et al. Antiviral potential of garlic (Allium sativum) and its organosulfur compounds: a systematic update of pre-clinical and clinical data. Trends Food Sci Technol. 2020;104:219–234. doi: 10.1016/j.tifs.2020.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Borlinghaus J, Albrecht F, Gruhlke MCH, et al. Allicin: chemistry and biological properties. Molecules. 2014;19:12591–12618. doi: 10.3390/molecules190812591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Percival SS. Aged garlic extract modifies human immunity. J Nutr. 2016;146:433S–436S. doi: 10.3945/jn.115.210427. [DOI] [PubMed] [Google Scholar]
- 147.Khubber S, Hashemifesharaki R, Mohammadi M, Gharibzahedi SMT. Garlic (Allium sativum L.): a potential unique therapeutic food rich in organosulfur and flavonoid compounds to fight with COVID-19. Nutr J. 2020;19(1):1–3. doi: 10.1186/s12937-020-00643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Thuy BTP, My TTA, Hai NTT, et al. Investigation into SARS-CoV-2 resistance of compounds in garlic essential oil. ACS Omega. 2020;5:8312–8320. doi: 10.1021/acsomega.0c00772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Donma MM, Donma O. The effects of allium sativum on immunity within the scope of COVID-19 infection. Med Hypotheses. 2020;144:109934. doi: 10.1016/j.mehy.2020.109934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Chmielewska A, Kozłowska M, Rachwał D, et al. Canola/rapeseed protein–nutritional value, functionality and food application: a review. Crit Rev Food Sci Nutr. 2020 doi: 10.1080/10408398.2020.1809342. [DOI] [PubMed] [Google Scholar]
- 151.He R, Wang Y, Yang Y, et al. Rapeseed protein-derived ACE inhibitory peptides LY, RALP and GHS show antioxidant and anti-inflammatory effects on spontaneously hypertensive rats. J Funct Foods. 2019;55:211–219. doi: 10.1016/j.jff.2019.02.031. [DOI] [Google Scholar]
- 152.Choque B, Catheline D, Rioux V, Legrand P. Linoleic acid: between doubts and certainties. Biochimie. 2014;96:14–21. doi: 10.1016/j.biochi.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 153.Shen B, Yi X, Sun Y, et al. Proteomic and metabolomic characterization of COVID-19 patient sera. Cell. 2020;182:59–72.e15. doi: 10.1016/j.cell.2020.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Yan B, Chu H, Yang D, et al. Characterization of the lipidomic profile of human coronavirus-infected cells: implications for lipid metabolism remodeling upon coronavirus replication. Viruses. 2019;11:73. doi: 10.3390/v11010073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Walls AC, Park YJ, Tortorici MA, et al. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181:281–292.e6. doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Toelzer C, Gupta K, Yadav S, et al. Unexpected free fatty acid binding pocket in the cryo-EM structure of SARS-CoV-2 spike protein. Science. 2020 doi: 10.1101/2020.06.18.158584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sakurai H, Morishima Y, Ishii Y, et al. Sulforaphane ameliorates steroid insensitivity through an Nrf2-dependent pathway in cigarette smoke-exposed asthmatic mice. Free Radic Biol Med. 2018;129:473–485. doi: 10.1016/j.freeradbiomed.2018.10.400. [DOI] [PubMed] [Google Scholar]
- 158.Ma A, Gao L, Wafi AM, et al. Overexpression of central ACE2 (angiotensin-converting enzyme 2) attenuates the pressor response to chronic central infusion of ang II (angiotensin II): a potential role for Nrf2 (nuclear factor [erythroid-derived 2]-like 2) Hypertension. 2020 doi: 10.1161/HYPERTENSIONAHA.120.15681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Horowitz RI, Freeman PR. Three novel prevention, diagnostic, and treatment options for COVID-19 urgently necessitating controlled randomized trials. Med Hypotheses. 2020;143:109851. doi: 10.1016/j.mehy.2020.109851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Cinatl J, Morgenstern B, Bauer G, et al. Glycyrrhizin, an active component of liquorice roots, and replication of SARS-associated coronavirus. Lancet. 2003;361:2045–2046. doi: 10.1016/S0140-6736(03)13615-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.inatl J, Morgenstern B, Bauer G et al (2003) Glycyrrhizin, an active component of liquorice roots, and replication of SARS-associated coronavirus. Lancet 361(9374):2045. 10.1016/s01406736(03)13615-x [DOI] [PMC free article] [PubMed]
- 162.Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Shen LW, Mao HJ, Wu YL, et al. TMPRSS2: a potential target for treatment of influenza virus and coronavirus infections. Biochimie. 2017;142:1–10. doi: 10.1016/j.biochi.2017.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Murck H. Symptomatic protective action of glycyrrhizin (Licorice) in COVID-19 infection? Front Immunol. 2020;11:1239. doi: 10.3389/fimmu.2020.01239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Hoever G, Baltina L, Michaelis M, et al. Antiviral activity of glycyrrhizic acid derivatives against SARS-coronavirus. J Med Chem. 2005;48:1256–1259. doi: 10.1021/jm0493008. [DOI] [PubMed] [Google Scholar]
- 166.Feng Yeh C, Wang KC, Chiang LC et al (2013) Water extract of licorice had anti-viral activity against human respiratory syncytial virus in human respiratory tract cell lines. J Ethnopharmacol. 10.1016/j.jep.2013.04.040 [DOI] [PMC free article] [PubMed]
- 167.Chen S, Wang X, Ha D, Yoshitake R (2021) White button mushroom (Agaricusbisporus) interrupts tissue AR-TMPRSS2 expression and attenuates pro-inflammatory cytokines in C57BL/6 mice: implication for COVID-19 dietary intervention. Res Sq [Preprint]. 2021 Mar 25:rs.3.rs-244245. 10.21203/rs.3.rs-244245/v1. Update in: NPJ Sci Food. 2021 Aug 2;5(1):20 [DOI] [PMC free article] [PubMed]
- 168.Majumder K, Wu J. A new approach for identification of novel antihypertensive peptides from egg proteins by QSAR and bioinformatics. Food Res Int. 2010;43:1371–1378. doi: 10.1016/j.foodres.2010.04.027. [DOI] [Google Scholar]
- 169.Majumder K, Chakrabarti S, Davidge ST, Wu J. Structure and activity study of egg protein ovotransferrin derived peptides (IRW and IQW) on endothelial inflammatory response and oxidative stress. J Agric Food Chem. 2013;61:2120–2129. doi: 10.1021/jf3046076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Fernández-Musoles R, Castelló-Ruiz M, Arce C, et al. Antihypertensive mechanism of lactoferrin-derived peptides: angiotensin receptor blocking effect. J Agric Food Chem. 2014;62:173–181. doi: 10.1021/jf404616f. [DOI] [PubMed] [Google Scholar]
- 171.Majumder K, Liang G, Chen Y, et al. Egg ovotransferrin-derived ACE inhibitory peptide IRW increases ACE2 but decreases proinflammatory genes expression in mesenteric artery of spontaneously hypertensive rats. Mol Nutr Food Res. 2015;59:1735–1744. doi: 10.1002/mnfr.201500050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Li Y, Wang B, Zhang H, et al. High-level expression of angiotensin converting enzyme inhibitory peptide Tuna AI as tandem multimer in Escherichia coli BL21 (DE3) Process Biochem. 2015;50:545–552. doi: 10.1016/j.procbio.2015.01.017. [DOI] [Google Scholar]
- 173.Yu Z, Kan R, Ji H, et al. Identification of tuna protein-derived peptides as potent SARS-CoV-2 inhibitors via molecular docking and molecular dynamic simulation. Food Chem. 2021;342:128366. doi: 10.1016/j.foodchem.2020.128366. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.