Abstract
Objective:
The current study was undertaken to evaluate the seasonal dependency and prevalence of gastrointestinal roundworms (nematodes) infecting domestic fowls (Gallus gallus domesticus) in Kashmir.
Materials and Methods:
From August 2017 through July 2019, the investigation was undertaken during each of the four seasons. We tested 400 guts obtained from varied places around the Kashmir valley for nematode infestation. The nematodes found within the digestive tract were collected and identified using a variety of identification keys under the microscope. Statistical Package for the Social Sciences was used to analyze the data (version 20). Chi-square (χ2) test was carried out to analyze the sample data.
Results:
196 hosts were infected with various nematodes, indicating an overall prevalence of 49% (196/400). The findings revealed that the prevalence of Ascaridia galli was 32.97% (61/185) in the first year and 35.34% (76/215) in the second year. Heterakis gallinarum had a prevalence rate of 20.80% (38/185) in the first year and 24.18% (52/215) in the second year, whereas Capillaria spp. had a prevalence rate of 10.81% (20/185) in the first year and 12.55% (27/215) in the second year. The overall prevalence of A. galli was determined to be 34.25% in both years (August 2017–July 2019), with a mean intensity of 4.86. Summer months had the highest parasitic load. Heterakis gallinarum had a prevalence rate of 22.5% and a mean intensity of 26.83. Summer was shown to have the most considerable parasitic burden. Capillaria spp. had an overall prevalence of 11.75% and a mean intensity of 4.59; autumn had the highest parasite load. The most abundant species was identified as A. galli. It was shown that there is a significant (p < 0.01) link between seasonality and helminth parasite prevalence.
Conclusion:
The study’s findings indicate that these gastrointestinal nematodes are ubiquitous throughout the year, but are particularly abundant in the summer and fall seasons among domestic poultry in the study area. This study on the prevalence of gastrointestinal nematodes in Gallus gallus domesticus demonstrates the seasonality of infection rates and also offers various methods and techniques for framing effective strategies for controlling these helminthes to maximize profit from backyard chicken farming.
Keywords: Domestic fowl, Kashmir, nematodes, parasitic load, prevalence
Introduction
Poultry has been recognized for thousands of years to provide meat and eggs, which are considered the two primary sources of animal protein for humans. India has a large poultry population of 498 million birds, which is growing at an average annual pace of 8%–10%. India is the third largest producer of eggs and the sixth largest producer of broiler meat [1]. Poultry production is constrained by a number of constraints, the most significant of which are illnesses, including bacterial, viral, and parasite infections [2]. Domestic chickens often consume a variety of foods, including grains (cereals), fruits, and insects that may contain the eggs or larval stages of certain helminth parasites, predisposing them to various parasitic illnesses, most notably gastrointestinal parasites [3].
Gastrointestinal parasites are an important factor in the decline of domestic fowl welfare [4]. Helminth parasites are generally seen in unfenced poultry around the world. The reason for the frequent recurrence of roundworm infections in an unfenced poultry system is mostly due to close contact with their feces, which ensures the completion of the nematodes’ direct life cycle via the effective fecal–oral transmission route [5]. As a result, numerous studies conducted in various parts of the world have revealed a high prevalence of chicken contamination with gastrointestinal helminths; in this context, helminths are regarded as a significant cause of bad health and decreased poultry yield [6].
Roundworms are a significant group among helminth parasites of poultry birds, in terms of both species and the amount of damage they inflict. Ascaridia, Heterakis, and Capillaria are the three major genera of roundworms that infect domestic chickens [7]. Throughout the year, many types of gastrointestinal parasites are prevalent in backyard poultry [8]. Although researchers in the Kashmir valley, such as Dar and Tanvir et al. [9], Tanveer et al. [10], and Salam [11], have conducted extensive work on helminth parasites of birds, there is still a knowledge gap about certain roundworms that infect domestic fowls in the region. Capillaria sp. is one such species that is listed in our study. We present 2-year prevalence and mean intensity data with seasonal change for roundworms, including Capillaria spp. that has not been documented previously in this location. Thus, the purpose of this study was to determine the prevalence rate and seasonal distribution of nematodes infecting domestic fowls in the Kashmir region, which would aid in developing subsequent control measures and preventing economic losses to our indigenous chicken business.
Materials and Methods
Study area and methods
From August 2017 to July 2019, the study was conducted in Jammu and Kashmir’s Kashmir province. At an elevation of 1,583 meters above sea level in the Himalayas between 34°20ʹ–34°36ʹ N latitudes and 74°82ʹ–74°85ʹ E longitudes [12], Kashmir Valley’s climate is moderate; it is usually cool in the spring and fall, slightly hot in the summer, and cold in the winter. For a period of 2 years, a total of 400 guts from local backyard chickens were collected from various marketplaces throughout the Kashmir valley. Our survey sample size was determined using the following formula [13]:
We take here P = 0.5, Zα = 1.96, and d = 0.05. This gives the sample size for our study as n ~ 384 and we chose n = 400.
Parasite processing and identification
The gut samples were transported to the Parasitology Research Laboratory at the University of Kashmir’s Department of Zoology. Routine examinations of the collected samples for the presence of gastrointestinal parasites were carried out in accordance with the approach outlined by Fowler [14]. The recovered nematodes were initially stored in normal saline, completely cleaned, and then fixed in hot 70% ethanol. The obtained nematodes were kept in glycerin alcohol following fixation. Lactophenol was utilized to rapidly clear nematodes and Kaiser’s glycerin jelly was used to mount the worms. The prepared slides were examined closely under a light microscope at a magnification of 100× and identified using a variety of keys and books [10,15].
Definitions
In this study, the prevalence was estimated by Thrusfield’s [16] equation:
P = 100 × number of infected chickens/total number of observed chickens
The abundance is calculated as follows:
A = number of parasite species isolated/total number of observed chickens
Mean intensity = total number of parasites/total number of hosts infected.
Data analysis
The data were tabulated and analyzed using basic statistical techniques such as percentages, graphs, and chi-square test. p < 0.05 was considered significant at the 5% level of significance; p < 0.01 was considered significant at the 1% level of significance; and p > 0.05 was judged as statistically non-significant.
Results
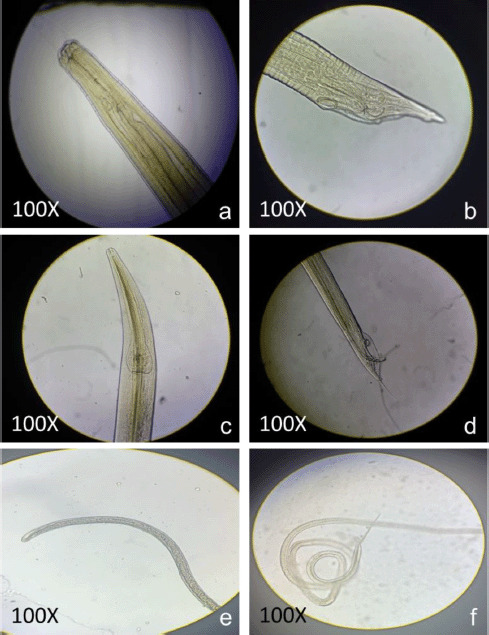
A total of 400 gastrointestinal tracts were analyzed for nematodes during the investigation. Three nematode species, Ascaridia galli, Heterakis gallinarum, and Capillaria spp., were isolated from the diseased guts. For A. galli, the overall prevalence rate was 34.25% with a mean intensity of 4.86; for H. gallinarum, the prevalence rate was 22.5% with a mean intensity of 26.83; and for Capillaria spp., the prevalence rate was 11.75% with a mean intensity of 4.59. Ascaridia galli was isolated from the duodenum and H. gallinarum from the diseased gut’s caecum. Capillaria spp. was isolated from the host’s small intestine and caecum. Summer was the peak season for parasitic load in A. galli and H. gallinarum. Autumn was the season with the largest worm burden for Capillaria spp. Figure 1 shows the month-by-month mean intensity, while Tables 1 and 2 detail the quantitative structure of A. galli, H. gallinarum, and Capillaria spp. The prevalence of the collected roundworm species by season is shown in Table 3. The front and posterior ends of collected nematodes are shown in Figure 2 to differentiate these round worms.
Figure 1. Overall mean intensity of A. galli, H. gallinarum, and Capillaria spp.

Table 1. Quantitative structure of A. galli, H. gallinarum, and Capillaria infectivity in domestic fowls for the year 2017–2018.
| Helminth | No. of hosts examined | No. of hosts infected | No. of individuals recovered | Prevalence rate | Mean intensity | Abundance | Index of infection |
|---|---|---|---|---|---|---|---|
| A. galli | 185 | 61 | 284 | 32.97% | 4.65 | 1.53 | 0.50 |
| H. gallinarum | 185 | 38 | 1,032 | 20.54% | 27.15 | 5.57 | 1.14 |
| Capillaria spp. | 185 | 20 | 106 | 10.81% | 5.3 | 0.57 | 0.06 |
Table 2. Quantitative structure of A. galli, H. gallinarum, and Capillaria infectivity in domestic fowls for the year 2018–2019.
| Helminth | No. of hosts examined | No. of hosts infected | No. of individuals recovered | Prevalence rate | Mean intensity | Abundance | Index of infection |
|---|---|---|---|---|---|---|---|
| A. galli | 215 | 76 | 382 | 35.34% | 5.02 | 1.77 | 0.62 |
| H. gallinarum | 215 | 52 | 1,383 | 24.18% | 26.59 | 6.43 | 1.55 |
| Capillaria spp | 215 | 27 | 110 | 12.55% | 4.07 | 0.51 | 0.06 |
Table 3. Season-wise prevalence of gastrointestinal nematodes in Gallus gallus domesticus.
| Season | Total no. of hosts | No. of infected hosts | Prv % | No. infected hosts with particular parasitic spp. (% prevalence) | |||||
|---|---|---|---|---|---|---|---|---|---|
| A. galli | H. gallinarum | Capillaria | |||||||
| Spring | 103 | 44 | 42.7 | 34 | 33.00 | 20 | 19.41 | 10 | 9.70 |
| Summer | 103 | 66 | 64.07 | 47 | 45.63 | 31 | 30.09 | 17 | 16.50 |
| Autumn | 99 | 66 | 64.07 | 40 | 40.40 | 30 | 30.30 | 18 | 18.18 |
| Winter | 95 | 20 | 21.05 | 16 | 16.84 | 9 | 9.47 | 2 | 2.10 |
| Total | 400 | 196 | 49 | 137 | 34.25 | 90 | 22.5 | 47 | 11.75 |
|
χ
2
p |
29.469<0.01 | 15.438 <0.01 |
14.089 <0.01 |
14.021 <0.01 |
|||||
Figure 2. Anterior and posterior ends of collected nematodes. a) Anterior end of A.galli, b) posterior end of A.galli, c) anterior end of H. gallinarum, d) posterior end of H.gallinarum, e) anterior end of Capillaria, and f) posterior end of Capillaria.
Discussion
During the study period, the overall prevalence of infection was found to be 49%, signifying that roundworm infection is a common problem in the region and is more or less similar to the prevalence of 45.66%, as reported by Jaiswal et al. [17]. In comparison to the current study, Sreedevi et al. [18] reported a higher frequency (63.21%) in India, and El-Dakhly et al. [19] reported a prevalence of 55.79% in Egypt. Jegede et al. [20] found a significantly lower rate (42.5%) for backyard hens in Nigeria, whereas Baboolal et al. [21] reported a rate of 10.5% for broiler chickens in Trinidad. The high frequency found in domestic fowls may be related to the type of production system, their constant contact with intermediate hosts, free-ranging management, study techniques, and parasite control strategies used in the studied areas and under the studied climatic conditions [22,23].
The birds produced from backyard poultry systems receive little or no supplemental feeds and receive no veterinary treatment; these hens are constantly scavenging and exposed to various infectious helminth stages and its intermediate hosts [24]. Ascaridia galli was the most abundant nematode species encountered in the study (34.25%), followed by H. gallinarum (22.5%) and Capillaria spp. (11.75%). Das et al. [8] identified A. galli as the most frequent nematode parasite in Meghalaya, India. Nevertheless, numerous reports indicate that H. gallinarum is the most frequent nematode [25]. Both Salam [11] and Eshetu et al. [26] reported a nearly same prevalence (35.35% and 35.6%) of A. galli in domestic fowls in Kashmir.
Sarba et al. [27] found a significant incidence of A. galli (69.8%) and a low prevalence of H. gallinarum (13.5%) in Ethiopian backyard chickens. Heterakis gallinarum is the most prevalent nematode in infected poultry intestinal caeca. This may be attributed to their fully developed digestive system, which provides them with a better opportunity to build a positive host–parasite interaction. Heterakis gallinarum infection will expose chickens to the protozoan Histomonas meleagridis [19]. In comparison to our investigation, Katoch et al. [28] reported a nearly identical prevalence rate (24.0%) of H. gallinarum in Jammu, India. We found a prevalence rate of 11.7% for Capillaria spp., which was greater than the percentage reported by Katoch et al. [28] in Jammu, India. There was a significant (p < 0.001) correlation between seasonality and prevalence of gastrointestinal nematodes. Summer and fall had the highest prevalence rates, while winters had the lowest. Fotedar and Khateeb [29] likewise reported a high prevalence of helminth infection in September and a low prevalence in December and January, noting that the prevalence and mean worm load decreased when temperature and rainfall decreased. Das et al. [8] found that infection levels were highest in summer and lowest in winter in Meghalaya. High mean temperature and relative humidity may explain the pattern of infection seen during hot and rainy months, as these conditions are favorable for the development and survival of larval/immature stages of various parasites and insects, the latter of which act as vectors/carriers for helminths, resulting in an increased availability of infective stages for the host [30]. Winters in the valley are typically snow-covered, and domestic fowls are fed indoors; also, the low winter temperature slows down the growth of parasites and their larval stages both inside the host and in the environment [31].
Magwisha et al. [23] observed that nematode infection prevalence and intensity disparities might be attributable to climatic variables (temperature and humidity) in that area. According to Shukla and Mishra [32], A. galli is the most prevalent parasite in both domestic and exotic chicken species. Jordan and Pattison [33], Luka and Ndams [34], and Sonune [35] identified A. galli as the most prevalent and important chicken helminth. The observations of Hassouni and Belghyti [36] in Morocco, Permin et al. [37] in Denmark, Ashenafi and Eshetu [38] in Ethiopia, Nithiuthai et al. [39] in Bangkok, Phiri et al. [40] in central Zambia, Mwale and Masika [41] in South Africa, and Asumang et al. [42] in Ghana coincide with our study Khan et al. [25] also reported a high incidence of H. gallinarum in Pakistan, Ybañez et al. [43] in the Philippines, Singh and Nama [4] in Jodhpur, and Worku and Bedanie [44] in Ethiopia. Because the results are consistent with those of numerous others, the discrepancies may be attributed to the area’s environmental factors and host feeding behavior. Temperature and humidity levels affect larval development/maturation and facilitate the transmission and ingestion of infested droppings.
Conclusion
The study demonstrates unequivocally that helminth infection is prevalent in domestic fowls and confirms the significant frequency of the worms A. galli and H. gallinarum in the Kashmir region. Additionally, the study revealed an increase in the prevalence of Capillaria spp. As a result of this study, future researchers will be able to design control strategies for these roundworms based on their dispersion patterns. Increased attention should be paid to poultry management and maintenance of domestic chickens that are often free-ranging. In conclusion, additional studies highlighting and controlling various elements of parasitism in poultry and increasing domestic fowl production in the region should be conducted.
List of Abbreviations
A. galli, Ascaridia galli; H. gallinarum, Heterakis gallinarum, spp., species; p-value, probability value.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Author contributions
Ishrat Ara conceived of the study, analyzed the data, conducted laboratory work, and authored the report. Humira Rashid Khan assisted in data collection, laboratory work, and manuscript preparation. Syed Tanveer and Bilal Bhat contributed to the preparation and critical review of this work, as well as the statistical analysis.
References
- [1].United States Department of Agriculture (USDA) International egg and poultry review. U S Department of Agriculture (USDA) 2011;14:34. [Google Scholar]
- [2].Ojok L. Diseases as important factor affecting increased poultry production in Uganda. Der Tropenlandwirt J Agric Trop Subtrop. 1993;(94):37–44. [Google Scholar]
- [3].Oniye SJ, Audu PA, Adebote DA, Kwaghe BB, Ajanusi OJ, Nfor MB. Survey of helminth parasites of laughing dove (Streptopelia senegalensis) in Zaria Nigeria. Afr J Nat Sci. 2001;4:65–6. [Google Scholar]
- [4].Singh H, Nama P. Incidence of endohelminth parasites in the alimentry canal of domestic fowl (Gallus domesticus), butchered at Pipar city, Jodhpur. Int J Res Anal Rev. 2018;5:130–3. [Google Scholar]
- [5].Wongrak K, Daş G, Moors E, Sohnrey B, Gauly M. Establishment of gastro-intestinal helminth infections in free-range chickens: a longitudinal on farm study. Berl Münch Tierarztl Wochenschr. 2014;127:305–13. [PubMed] [Google Scholar]
- [6].Ajala MK, Nwagu BI, Otchere EO. Socio-economics of free-range poultry production among agropastoral women in Giwa local government area of Kaduna state, Nigeria. Niger Vet J. 2007;28(3):11–8. https://doi.org/10.4314/nvj.v28i3.3562. [Google Scholar]
- [7].Matur BM, Dawam NN, Malann YD. Gastrointestinal helminth parasites of local and exotic chickens slaughtered in Gwagwalada, Abuja (FCT), Nigeria. New York Sci J. 2010;3.5:96–9. [Google Scholar]
- [8].Das M, Laha R, Doley S. Gastrointestinal parasites in backyard poultry of subtropical hilly region of Meghalaya. J Entomol Zool Stud. 2020;8(5):1301–5. [Google Scholar]
- [9].Dar JA, Tanvir S. Prevalence of cestode parasites in free-range backyard chickens (Gallus gallus domestics) of Kashmir, India. Agric Biol J North Am. 2013;4(1):67–70. https://doi.org/10.5251/abjna.2013.4.1.67.70. [Google Scholar]
- [10].Tanveer S, Ahad S, Chishti MZ. Morphological characterization of nematodes of the genera Capillaria, Acuaria, Amidostomum, Streptocara, Heterakis, and Ascaridia isolated from intestine and gizzard of domestic birds from different regions of the temperate Kashmir valley. J Parasitol Dis. 2015;39:745–60. doi: 10.1007/s12639-013-0401-7. https://doi.org/10.1007/s12639-013-0401-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Salam ST. Ascariasis in backyard chicken–prevalence, pathology and control. Int J Recent Sci Res. 2015;6(4):3361–5. [Google Scholar]
- [12].Ahangar IA, Saksena DN, Farooq MM, Afzal AM. Crustacean community in Anchar lake, Kashmir. Bull Environ Pharm Life Sci. 2012;1(7):18–21. [Google Scholar]
- [13].Cochran W. 3rd. New York, NY: Wiley; 1977. Sampling techniques. [Google Scholar]
- [14].Fowler NG. Poultry diseases, F. T.W. 2nd. Londal: Bailliere Tindall; 1990. How to carry out a field investigation; pp. 370–400. [Google Scholar]
- [15].Soulsby EJL. 7th. London, UK: Bailliere, and Tindall; 1982. Helminthes, arthropods, and protozoa of domestic animals; pp. 83–115. [Google Scholar]
- [16].Michael T. Oxford, UK: John Wiley & Sons; 2018. Veterinary epidemiology. [Google Scholar]
- [17].Jaiswal K, Mishra S, Bee A. Prevalence of gastrointestinal helminth parasites in Gallus gallus domesticus in Lucknow, UP, India. Adv Zoo Bot. 2020;8(5):422–30. https://doi.org/10.13189/azb.2020.080506. [Google Scholar]
- [18].Sreedevi C, Jyothisree C, Devi VR, Annapurna P, Jeyabal L. Seasonal prevalence of gastrointestinal parasites in desi fowl (Gallus gallus domesticus) in and around Gannavaram, Andhra Pradesh. J Parasit Dis. 2016;40(3):656–61. doi: 10.1007/s12639-014-0553-0. https://doi.org/10.1007/s12639-014-0553-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].El-Dakhly KM, El-Seify MA, Mohammed ES, Elshahawy IS, Fawy SA, Omar MA. Prevalence and distribution pattern of intestinal helminths in chicken and pigeons in Aswan, Upper Egypt. Trop Anim Health Prod. 2019;51(3):713–8. doi: 10.1007/s11250-018-1725-1. https://doi.org/10.1007/s11250-018-1725-1. [DOI] [PubMed] [Google Scholar]
- [20].Jegede OC, Asadu IA, Opara M, Obeta SS, Olayemi DO. Gastrointestinal parasitism in local and exotic breeds of chickens reared in Gwagwalada Guinea Savannah zone of Nigeria. Sokoto J Vet Sci. 2015;13(3):25–30. https://doi.org/10.4314/sokjvs.v13i3.5. [Google Scholar]
- [21].Baboolal V, Suratsingh V, Gyan L, Brown G, Offiah NV, Adesiyun AA, et al. The prevalence of intestinal helminths in broiler chickens in Trinidad. Vet Arhiv. 2012;82(6):591–7. [Google Scholar]
- [22].Yadav AK, Tandon DV. Helminth parasitism of domestic fowl (Gallus domesticus L.) in a subtropical high-rainfall area of India. Beitr Trop Landwirtsch Veterinarmed. 1991;29(1):97–104. [PubMed] [Google Scholar]
- [23].Magwisha HB, Kassuku AA, Kyvsgaard NC, Permin A. A comparison of the prevalence and burdens of helminth infections in growers and adult free-range chickens. Trop Anim Health Prod. 2002;34(3):205–14. doi: 10.1023/a:1015278524559. https://doi.org/10.1023/A:1015278524559. [DOI] [PubMed] [Google Scholar]
- [24].Yousfi F, Senouci K, Medjoual I, Djellil H, Slimane TH. Gastrointestinal helminths in the local chicken Gallus gallus domesticus (Linnaeus, 1758) in traditional breeding of North-Western Algeria. Biod J. 2013;4(1):229–34. [Google Scholar]
- [25].Khan L, Qureshi AW, Shah MN, Feroz K, Mansoor A, Draz O. Prevalence and identification of nematodes in chickens from district Charsadda, KPK, Pakistan. J Biol Environ Sci. 2016;9(1):274–82. [Google Scholar]
- [26].Eshetu Y, Mulualem E, Ibrahim H, Berhanu A, Aberra K. Study of gastro-intestinal helminths of scavenging chickens in four rural districts of Amhara region, Ethiopia. Revue Sci Tech. 2001;20(3):791–6. doi: 10.20506/rst.20.3.1310. https://doi.org/10.20506/rst.20.3.1310. [DOI] [PubMed] [Google Scholar]
- [27].Sarba EJ, Bayu MD, Gebremedhin EZ, Motuma K, Leta S, Abdisa K, et al. Gastrointestinal helminths of backyard chickens in selected areas of West Shoa Zone Central, Ethiopia. Vet Parasitol Reg Stud Rep. 2019;15:100265. doi: 10.1016/j.vprsr.2019.100265. https://doi.org/10.1016/j.vprsr.2019.100265. [DOI] [PubMed] [Google Scholar]
- [28].Katoch R, Yadav A, Godara R, Khajuria JK, Borkataki S, Sodhi SS. Prevalence and impact of gastrointestinal helminths on body weight gain in backyard chickens in subtropical and humid zone of Jammu, India. J Parasit Dis. 2012;36(1):49–52. doi: 10.1007/s12639-011-0090-z. https://doi.org/10.1007/s12639-011-0090-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Fotedar DN, Khateeb NG. Occurrence and seasonal variation of helminth parasites of domestic fowl in Kashmir. Indian J Helmin. 1986;38(1):49–54. [Google Scholar]
- [30].Dube S, Zindi P, Mbanga J, Dube C. A study of scavenging poultry gastrointestinal and ecto-parasites in rural areas of Matebeleland Province, Zimbabwe. Int J Poult Sci. 2010;9(9):911–5. [Google Scholar]
- [31].Sheikh BA, Sofi TA, Ahmad F. Prevalence of helminth parasites in Gallus domesticus from Figurez valley. Agric Adv. 2015;4(11):129–37. https://doi.org/10.14196/aa.v4i11.2046. [Google Scholar]
- [32].Shukla S, Mishra P. Gatrointestinal helminthes parasites of local chickens samples from tribal areas of Madhya Pradesh. Int J Life Sci. 2013;1(4):284–7. [Google Scholar]
- [33].Jordan FTM, Pattison M. 4th. London: W.B Sauder company Ltd; 1996. Poultry diseases; pp. 283–6. [Google Scholar]
- [34].Luka SA, Ndams IS. Gastrointestinal parasites of domestic chickens Gallus gallus domesticus Linnaeus 1758 in Samaru Zaria Nigeria. Sci World J. 2007;2(1):27–9. https://doi.org/10.4314/swj.v2i1.51723. [Google Scholar]
- [35].Sonune MB. Analysis of gastrointestinal parasites of poultry birds around Chikhli, Buldana (MS) India. Sci Res Rep. 2012;2(3):274–6. [Google Scholar]
- [36].Hassouni T, Belghyti D. Distribution of gastrointestinal helminths in chicken farms in the Gharb region—Morocco. Parasitol Res. 2006;99(20):181–3. doi: 10.1007/s00436-006-0145-8. https://doi.org/10.1007/s00436-006-0145-8. [DOI] [PubMed] [Google Scholar]
- [37].Permin A, Bisgaard M, Frandsen F, Pearman M, Kold J, Nansen P. Prevalence of gastrointestinal helminths in different poultry production systems. Br Poult Sci. 1999;40(4):439–43. doi: 10.1080/00071669987179. https://doi.org/10.1080/00071669987179. [DOI] [PubMed] [Google Scholar]
- [38].Ashenafi H, Eshetu Y. Study on gastrointestinal helminths of local chickens in central Ethiopia. Rev Méd Vét. 2004;155:504–7. [Google Scholar]
- [39].Nithiuthai S, Sudchit C, Woraporn S. Study of gastro-intestinal helminthes in native chicken and the efficacy of mebendazole against the helminth parasites. Wetchasan Sattawaphaet. 2003:65–72. [Google Scholar]
- [40].Phiri IK, Phiri AM, Ziela M, Chota A, Masuku M, Monrad J. Prevalence and distribution of gastrointestinal helminths and their effects on weight gain in free-range chickens in Central Zambia. Trop Anim Health Prod. 2007;39(4):309–15. doi: 10.1007/s11250-007-9021-5. https://doi.org/10.1007/s11250-007-9021-5. [DOI] [PubMed] [Google Scholar]
- [41].Mwale , M , Masika PJ. Point prevalence study of gastro-intestinal parasites in village chickens of Centane district, South Africa. Afr J Agri Res. 2011;6(9):2033–8. https://doi.org/10.5897/AJAR09.495. [Google Scholar]
- [42].Asumang P, Delali JA, Wiafe F, Kamil Z, Balali GI, Gobe VAD, Prevalence of gastrointestinal parasites in local exotic breeds of chickens in Pankrono–Kumasi. Ghana. J Parasitol Res. 2019:1–7. doi: 10.1155/2019/5746515. http://doi.org/10.1155/2019/5746515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Ybañez RH, Resuelo KJ, Kintanar AP, Ybañez AP. Detection of gastrointestinal parasites in small-scale poultry layer farms in Leyte, Philippines. Vet World. 2018;11(11):1587–91. doi: 10.14202/vetworld.2018.1587-1591. https://doi.org/10.14202/vetworld.2018.1587-1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Worku Y, Bedanie T. Identification of helminths parasites, species richness and their effects on hematological components in chicken kept under scavenging chicken production system in and around Bishoftu, Ethiopia. J Vet Sci Technol. 2019;10(577):2. [Google Scholar]


