Abstract
Objective:
The avian influenza virus (AIV) subtype H9N2 circulating in Indonesia has raised increasing concern about its impact on poultry and its public health risks. In this study, the H9N2 virus from chicken poultry farms in Java was isolated and characterized molecularly.
Materials and Methods:
Thirty-three pooled samples of chicken brain, cloacal swab, trachea, and oviduct were taken from multiple chickens infected with AIV in five regions of Java, Indonesia. The samples were isolated from specific pathogenic-free embryonated eggs that were 9 days old. Reverse transcription polymerase chain reaction and sequencing were used to identify H9N2 viruses.
Results:
This study was successful in detecting and characterizing 13 H9N2 isolates. The sequencing analysis of hemagglutinin genes revealed a 96.9%–98.8% similarity to the H9N2 AIV isolated from Vietnam in 2014 (A/muscovy duck/Vietnam/LBM719/2014). According to the phylogenetic analysis, all recent H9N2 viruses were members of the lineage Y280 and clade h9.4.2.5. Nine of the H9N2 isolates studied showed PSKSSR↓GLF motifs at the cleavage site, while four had PSKSSR↓GLF. Notably, all contemporary viruses have leucine (L) at position 216 in the receptor-binding region, indicating that the virus can interact with a human-like receptor.
Conclusion:
This study described the features of recent H9N2 viruses spreading in Java’s poultry industry. Additionally, H9N2 infection prevention and management must be implemented to avoid the occurrence of virus mutations in the Indonesian poultry industry.
Keywords: Avian influenza, HA gene, layer, phylogenetic analysis, sequencing, H9N2
Introduction
Since the early 1980s, Indonesia’s poultry sector has risen dramatically. The poultry industry in Indonesia is projected to be worth more than 34 billion US dollars. According to CASARED, there are approximately 67.9 million birds on 99,000 farms in Indonesia that use a commercially oriented production method [1,2].
In the last two decades, avian influenza virus (AIV) infection has become a common problem for the global poultry industry [3,4], including Indonesia. These viruses are categorized into two groups. The first group is an highly pathogenic AIV, for example, the H5N1 subtype which can cause mortality up to 100%. This subtype has become a major concern for human and animal health in Indonesia, especially the entire province of Java, since 2003 [5], and vaccinations have been routinely administered against this subtype. The second group was infected with various types of domestic and wild birds with mild to moderate infection called low pathogenic avian influenza virus (LPAIV) [6]. H9N2 belongs to this group and causes significant economic losses associated with increased mortality and decreased egg production [7]. Coinfection of bacteria such as Mycoplasma gallisepticum and Escherichia coli or other infections from other viruses such as infectious bronchitis (IB) and newcastle disease (ND) can increase the mortality of chickens infected with H9N2 [8]. Currently, this subtype is endemic to poultry in several African, Asian, and Middle Eastern countries [9].
There is a dearth of evidence on the characteristics of circulating AIVs subtype H9. The bulk of these virus sequences are classified as G1 (clade h9.4.1) or Y280 (h9.4.2 of clade). The h9.4.1 lineage includes viruses from India, Iran, Israel, and Pakistan, whereas the h9.4.2 lineage had all strains found in China. Prior to 2007, H9N2 was classified as clade h9.4.2.1–h9.4.2.4. After 2010, the clade H9.4.2.5 represented by Guangxi/55/2005 became well established and began spreading rapidly throughout the country. Furthermore, all h9.4.2.5 viruses discovered between 2013 and 2015 possessed human-like receptor specificity, implying the possibility of cross-species transmission [10].
Based on the duck sera’s susceptibility to recombinant hemagglutinins and neuraminidases in indirect enzyme-linked immunosorbent assay and dot blot studies, the H9N2 subtype was believed to exist in Java, Indonesia, duck population [11]. This is the first report of H9N2 outbreaks in Java, and surveillance will be required to confirm the existence of H9N2 subtypes via virus isolation and molecular characterization. In this work, we detected and examined H9N2 viruses from several regions of Java.
Materials and Methods
The study was carried out in April 2017–March 2018. The materials and methods used in this study are describe below.
Ethical approval
The current study follows the requirements established by the Indonesian Law on Animal Health Research (UU/18/2009, article 80. Ethical approval was not required for this investigation because no live animals were used.
Samples
A total of 33 commercial poultry chickens were sampled in 5 provinces of Java, Indonesia (Banten, n = 4; Central Java, n = 2; East Java, n = 9; West Java, n = 17; and Yogyakarta, n = 1) with a history of widespread depression, respiratory and neurological disorders, and decreased egg production. The poultry flocks comprised birds ranging in age from 25 to 79 weeks. Three moribunds or dead hens were randomly selected from each group and brought to the laboratory. The chicken brain, cloacal swab, trachea, and oviduct were taken and pooled for virus isolation. The samples collected are listed in Table 1.
Table 1. Collected samples for identification of AIV H9N2 in five provinces of Java, Indonesia.
| Name of sample | District | Province | Age (weeks) | Collection date | Population |
|---|---|---|---|---|---|
| Chicken/Banten-01/17 | Tangerang | Banten | 64 | 20-Apr-17 | >3,000 |
| Chicken/Banten-02/17 | Tangerang | Banten | 56 | 20-Apr-17 | >3,000 |
| Chicken/WestJava-01/17 | Cianjur | West Java | 26 | 24-Apr-17 | >5,000 |
| Chicken/Banten-03/17 | Tangerang | Banten | 35 | 29-Apr-17 | >3,000 |
| Chicken/WestJava-02/17 | Bogor | West Java | 49 | 03-May-17 | >4,000 |
| Chicken/WestJava-03/17 | Sukabumi | West Java | 45 | 03-May-17 | >10,000 |
| Chicken/WestJava-04/17 | Bogor | West Java | 38 | 21-May-17 | >3,000 |
| Chicken/WestJava-05/17 | Bogor | West Java | 38 | 21-May-17 | >3,000 |
| Chicken/WestJava-06/17 | Bogor | West Java | 35 | 21-May-17 | >3,000 |
| Chicken/WestJava-07/17 | Sukabumi | West Java | 54 | 15-Jun-17 | >7,000 |
| Chicken/WestJava-08/17 | Sukabumi | West Java | 50 | 15-Jun-17 | >7,000 |
| Chicken/EastJava-01/17 | Situbondo | East Java | 33 | 16-Jun-17 | >3,000 |
| Chicken/ EastJava-02/17 | Kediri | East Java | 64 | 01-Jul-17 | >3,000 |
| Chicken/WestJava-09/17 | Subang | West Java | 25 | 05-Jul-17 | >6,000 |
| Chicken/ WestJava-10/17 | Bogor | West Java | 52 | 21-Jul-17 | >4,000 |
| Chicken/EastJava-03/17 | Surabaya | East Java | 48 | 22-Jul-17 | >3,000 |
| Chicken/EastJava-04/17 | Situbondo | East Java | 44 | 03-Aug-17 | >2,500 |
| Chicken/EastJava-05/17 | Situbondo | East Java | 52 | 03-Aug-17 | >2,500 |
| Chicken/WestJava-11/17 | Subang | West Java | 64 | 19-Aug-17 | >8,000 |
| Chicken/WestJava-12/17 | Cianjur | West Java | 54 | 21-Aug-17 | >10,000 |
| Chicken/EastJava-06/17 | Situbondo | East Java | 52 | 30-Aug-17 | >3,000 |
| Chicken/EastJava-07/17 | Situbondo | East Java | 72 | 30-Aug-17 | >3,000 |
| Chicken/EastJava-08/17 | Situbondo | East Java | 47 | 30-Aug-17 | >3,000 |
| Chicken/WestJava-13/17 | Subang | West Java | 41 | 01-Sep-17 | >5,000 |
| Chicken/WestJava-14/17 | Subang | West Java | 49 | 01-Sep-17 | >5,000 |
| Chicken/WestJava-15/17 | Subang | West Java | 36 | 01-Sep-17 | >5,000 |
| Chicken/Banten-04/17 | Tangerang | Banten | 31 | 03-Sep-17 | >3,000 |
| Chicken/EastJava-09/17 | Blitar | East Java | 36 | 05-Sep-17 | >6,000 |
| Chicken/Yogyakarta-01/17 | Bantul | Yogyakarta | 79 | 19-Sep-17 | >3,000 |
| Chicken/WestJava-16/17 | Sukabumi | West Java | 30 | 10-Oct-17 | >6,000 |
| Chicken/WestJava-17/17 | Sukabumi | West Java | 30 | 10-Oct-17 | >6,000 |
| Chicken/CentralJava-01/17 | Surakarta | Central Java | 26 | 17-Oct-17 | >2,500 |
| Chicken/CentralJava-02/17 | Semarang | Central Java | 29 | 21-Oct-17 | >3,000 |
Virus isolation
Propagation of the sample was carried out according to conventional laboratory protocols. The swab or tissue was suspended in sterile phosphate buffer saline with penicillin (200 μg/ml) and streptomycin (100 μg/ml) in a 2:10–20 ratio and centrifuged at 1,000× g for 10 min at 4°C. The supernatant was filtered via a 0.22 μm membrane filter and inoculated into 9-day-old specific pathogenic-free (SPF) embryonated eggs via the allantoic route. The eggs were incubated at 37°C for 48–72 h and daily embryo death was monitored. Eggs that died before 24 h were discarded because the virus considered was non-specific. Harvested allantoic fluid was used in the hemagglutination (HA) experiment. Three serial passages were conducted prior to the selection of negative samples [12].
Detection of viral RNA using reverse transcription-polymerase chain reaction (RT-PCR) test
Allantoic fluid was extracted using a total ribonucleic acid (RNA) mini kit (Geneaid, Taiwan). MyTaqTM OneStep RT-PCR kit (Bioline®, Taunton) was used to test the RNA for H9N2 viruses by RT-PCR utilizing H9 and N2-specific primers (Table 2). The following thermal profile was used to amplify the genes: 48°C for 20 min, 95°C for 2 min, followed by 40 cycles of 95°C for 10 sec, 52°C for 10 sec, and 72°C for 2 min. At the conclusion of the amplification, the final stage was carried out at 72°C for 10 min. Electrophoresis was used to visualize the amplified samples. The usual size marker is 100 bp (Geneaid, Taipei, Taiwan). Observations were conducted with the aid of a UV transilluminator. Additionally, real-time polymerase chain reaction (RT-PCR) assays for H5N1, Newcastle disease virus (NDV), and infectious bronchitis virus (IBV) were performed [17].
Table 2. Oligonucleotide primers used for the detection of influenza viruses (H5 and H9), NDV, and IBV by RT-PCR assay.
| Target gene fragment | Primer | Sequence (5′-3′) | Amplicon size (base pair) | Reference |
|---|---|---|---|---|
| H9 | H9-F | ATCGGCTGTTAATGGAATGTGTT | 221 | [13] |
| H9-R | TGGGCGTCTTGAATAGGGTAA | |||
| N2 | N2-F | CTCCAATAGACCCGTACTAT | 460 | [14] |
| N2-R | CCTGAAGTCCCACAAAATAC | |||
| H5 | H5-F | ACAAAGCTCTATCAAAACCCAAC | 499 | [13] |
| H5-R | TACCCATACCAACCATCTACCAT | |||
| ND | ND-F | TGGAGCCAAACCGCGCACCTGCGG | 766 | [15] |
| ND-R | GAGGATGTTGGCAGCAT | |||
| IB | IB-F | GCTTTT GAG CCT AGC GTT | 149 | [16] |
| IB-R | GCCATGTTGTCACTGTCTATT | |||
| HA | HA9-F | CAAGATGGAAGTAGTATCACT | 1,683 | [10] |
| HA9-R | TTGCCAATTATATACAAATGT |
Sequencing
Following good RT-PCR results for the H9 gene, the HA gene was amplified and sequenced using the MyTaq One-Step RT-PCR kit (Bioline) with the HA9-F and HA9-R primers (Table 2). Electrophoresis was used to separate the PCR products, and the desired band was purified for sequencing (by First BASE Laboratories Sdn Bhd, Malaysia). Determination of the nucleotide and amino acid sequences for the HA gene from a recently isolated strain using Bioedit v.7 and alignment with ClustalW. MEGA 7 was used to create the phylogenetic tree of contemporary viruses using the neighbor-joining method [18] with 1,000 alignment repeats [19]. Clades were defined using the genetic distance between members and the topology of the phylogenetic tree. We studied the molecular properties of amino acid sequence derivation from 13 nucleotide sequences. The nucleotide sequences of hemagglutinin genes were compared between the isolates from this investigation, isolated studies with prototype H9N2 strains, and isolated studies with H9N2 subtype vaccine strain China (chicken/Guangdong/SS/94, chicken/Shanghai/F/98, and chicken/Shandong/6/96).
This study investigated several critical areas, including receptor-binding sites (RBS) on the left edge of the binding pocket, the right edge of the binding pocket, and the cleavage site. Additionally, certain amino acid residues (54, 80, 106, 109, 113, 123, 125, 129, 130, 135, 137, 146, 147, 149, 150, 152, 164, 165, 178, 179, 182, 183, 188, 189, 194, and 216) were evaluated for their association with H9N2 virus antigenicity. The amino acid sequences of the HA genes from the 13 most recent H9N2 viruses have been uploaded to http://www.cbs.dtu.dk/services/NetNGlyc/. to analyze probable N-glycosylation sites.
Results
Isolation and molecular identification of recent viruses
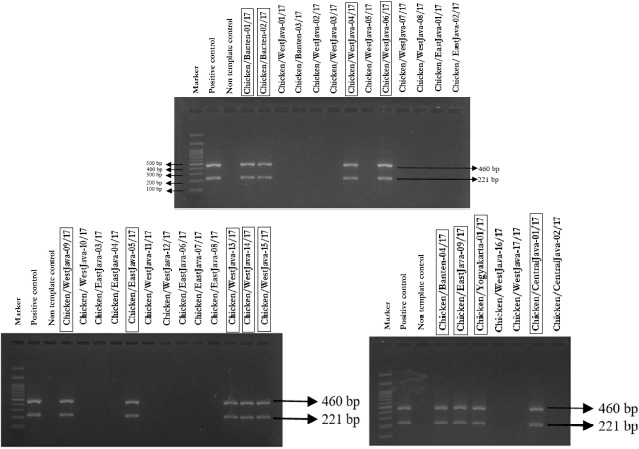
The result revealed that 18 samples were HA-positive after three passaged in embryonated chicken eggs with mean log 2, 4, and 8 HA units (Table 3), while the findings of RT-PCR showed only 13 samples were positive for H9N2 AIV (Fig. 1). Six of them were single infections of H9N2 AIV, while the seven positive samples were multiple infections by many coinfected viruses such as H5N1 (15.4%), NDV (38.5%), or IBV (7.6%). (7.6%). Regarding the distribution of positive H9N2 cases across different months, we observed that two samples (15.4%) were positive in April, two (15.4%) in May, one (7.7%) in July, one (7.7%) on August, six (48.2%) in September, and one (7.7%) in October 2017.
Table 3. Results of isolation and identification using RT-PCR.
| Name of Sample | HA test (HAU) |
RT-PCR | |||
|---|---|---|---|---|---|
| H9N2 | H5 | NDV | IBV | ||
| Chicken/Banten-01/17 | 28 | + | - | - | - |
| Chicken/Banten-02/17 | 25 | + | - | + | - |
| Chicken/WestJava-01/17 | 28 | - | + | - | - |
| Chicken/Banten-03/17 | - | - | - | - | - |
| Chicken/WestJava-02/17 | 25 | - | - | + | - |
| Chicken/WestJava-03/17 | - | - | - | - | - |
| Chicken/WestJava-04/17 | 27 | + | - | - | - |
| Chicken/WestJava-05/17 | - | - | - | - | - |
| Chicken/WestJava-06/17 | 24 | + | - | - | + |
| Chicken/WestJava-07/17 | - | - | - | - | - |
| Chicken/WestJava-08/17 | - | - | - | - | - |
| Chicken/EastJava-01/17 | - | - | - | - | - |
| Chicken/ EastJava-02/17 | - | - | - | - | - |
| Chicken/WestJava-09/17 | 24 | + | + | - | - |
| Chicken/ WestJava-10/17 | 25 | - | - | + | - |
| Chicken/EastJava-03/17 | - | - | - | - | - |
| Chicken/EastJava-04/17 | - | - | - | - | - |
| Chicken/EastJava-05/17 | 27 | + | - | + | - |
| Chicken/WestJava-11/17 | - | - | - | - | - |
| Chicken/WestJava-12/17 | 28 | - | - | + | - |
| Chicken/EastJava-06/17 | - | - | - | - | - |
| Chicken/EastJava-07/17 | 24 | - | - | + | - |
| Chicken/EastJava-08/17 | - | - | - | - | - |
| Chicken/WestJava-13/17 | 26 | + | - | + | - |
| Chicken/WestJava-14/17 | 27 | + | - | - | - |
| Chicken/WestJava-15/17 | 25 | + | + | - | - |
| Chicken/Banten-04/17 | 28 | + | - | - | - |
| Chicken/EastJava-09/17 | 25 | + | - | - | - |
| Chicken/Yogyakarta-01/17 | 27 | + | - | + | - |
| Chicken/WestJava-16/17 | - | - | - | - | - |
| Chicken/WestJava-17/17 | - | - | - | - | - |
| Chicken/CentralJava-01/17 | 28 | + | - | + | - |
| Chicken/CentralJava-02/17 | - | - | - | - | - |
Figure 1. Visualization of the PCR product for the H9 (221 bp) and N2 (460 bp) genes of recent samples on an agarose gel. Electrophoresis results showed that from 33 research samples only 13 samples were positive for the H9 and N2 genes.
Sequence and phylogenetic analysis
The RT-PCR experiment detected the presence of a complete HA gene with an amplicon size of 1,683 bp in the allantoic fluid (Fig. 2). The HA gene’s 13 most recent nucleotide sequences were deposited in GenBank with accession numbers MG957200, MG957201, MG957202, MG957203, MG957204, MG957205, MG957206, MG957207, MG957208, MG957209, MG957210, MG957211, and MG957212. A phylogenetic tree analysis based on the HA gene was created on the sequences of 13 recent isolates in our investigation and prior AIV H9N2 viruses [20]. Phylogenetic analysis revealed that all recent viruses belonged to clade h9.4.2.5 (Fig. 3), and a comparison of the 13 isolates’ HA sequences at the nucleotide level revealed 96.2%–100% identity with the lineage Y280 reference strain (Table 4). When compared to Chinese strains (Guangdong/SS/94, Shandong/6/96, and Shanghai/F/98), a reduced similarity rate (86.6%–92.3%) at the nucleotide level was identified. The present isolates had a nucleotide similarity of 96.9%–98.8% to the most recently circulating strains in Vietnam (muscovy duck/Vietnam/LBM719/2014) and China (chicken/Henan/LY-36/2013 and chicken/Zhejiang/HE6/2009).
Figure 2. Visualization of PCR products on the HA gene of 13 H9N2 positive isolates on an agarose gel. Electrophoresis results showed that all isolates of VAI H9N2 were successfully amplified with a size of 1,683 bp.

Figure 3. Phylogenetic tree of the HA gene of AIV H9N2 viruses. Recent Indonesian H9N2 viruses used in this study are represented with ▲. The region of HA was analyzed using MEGA version 7. The neighbor-joining bootstrap analysis (1,000 replicates) used the maximum composite likelihood method.

Table 4. Comparison of nucleotide sequence similarity from the 13 study isolates with several other H9N2 subtype isolates available in GenBank (%).
| 13 recent isolates | Clade | ||||||
|---|---|---|---|---|---|---|---|
| h9.1a | h9.2b | h9.3c | h9.4.1d | h9.4.2e | |||
| 13 recent isolates | 96.2–100 | ||||||
| Clade | h9.1a | 68.9–71.6 | |||||
| h9.2b | 71–73.6 | 76 | |||||
| h9.3c | 72.6–75.1 | 79.2 | 77.6 | ||||
| h9.4.1d | 77.8–80.3 | 75.1 | 67.6 | 83.7 | |||
| h9.4.2e | 91.5–93.6 | 75.9 | 69.2 | 79.5 | 83.6 | ||
h9.1 represented by A/turkey/California/189/66.
h9.2 represented by A/shorebird/Delaware/9/96/189/66.
h9.3 represented by A/duck/Hong_Kong/Y439/97.
h9.4.1 represented by A/quail/Hong_Kong/G1/97.
9.4.2 represented by A/duck/Hong_Kong/Y280/97.
Analysis of deduced amino acid sequences
The characteristics of LPAIV seen from the sequence of HA segments reveal a monobasic amino acid motif at the cleavage site (PSRSSR↓GLF) in 9 out of 13 study isolates, while 4 other isolates, A/chicken/Banten-01/17 (Accession No. MG957200), A/chicken/Banten-02/17 (Accession No. MG957201), A/chicken/Banten-04/17 (Accession No. MG957202), and A/chicken/EastJava-05/17 (Accession No. MG957210), showed a non-synonymous substitution of the amino acid (R317K) at this cleavage site to make PSKSSR↓GLF. All isolates in the present study showed H173N and Q217M mutations at receptor-binding sites while comparing the reference strains in GenBank. The E180A substitution was found in only five of our isolates, while the remaining seven isolates contain the E180T substitution (Table 5).
Table 5. Receptor-binding pockets, cleavage sites, and antigenic site of several isolates of H9N2 studies compared with several different clade precursors (*).
| Virus | Receptor-binding site | Left edge of the binding pocket | Right edge of the binding pocket | Cleavage site | Antigenic site | Accession Number | |
|---|---|---|---|---|---|---|---|
| Site I | Site II | ||||||
| Turkey/California/189/66 (h9.1) | P.W.T.H.E.L.Y | N.D.Q.Q.G.R | G.T.S.R.A | P.A.V.S.S.R↓G.L.F | T.K.P | N.D.L | AF156390 |
| Shorebird/Delaware/9/96 (h9.2) | P.W.T.H.E.L.Y | N.G.Q.Q.G.R | G.T.S.K.A | P.A.A.S.N.R↓G.L.F | T.K.P | N.D.L | AF156386 |
| Duck/Hong_Kong/Y439/97 (h9.3) | P.W.T.H.E.L.Y | N.D.Q.Q.G.R | G.T.S.R.A | P.A.A.S.N.R↓G.L.F | T.K.P | N.N.L | AF156377 |
| Quail/Hong_Kong/G1/97 (h9.4.1) | P.W.T.H.E.L.Y | N.D.L.Q.G.R | G.I.S.R.A | P.A.R.S.S.R↓G.L.F | T.K.P | G.N.L | AF156378 |
| Duck/Hong_Kong/Y280/97 (h9.4.2) | P.W.T.N.T.L.Y | N.G.L.Q.M.R | G.T.S.K.A | P.A.R.S.S.R↓G.L.F | S.K.P | D.N.L | AF156376 |
| Muscovy_duck/Vietnam/LBM719/2014 (h9.4.2.5) | P.W.T.N.A.L.Y | N.G.L.M.G.R | G.T.S.K.A | P.S.R.S.S.R↓G.L.F | S.K.P | D.N.L | LC028176 |
| Chicken/Banten-01/17 (h9.4.2.5) | P.W.T.N.A.L.Y | N.G.L.M.G.R | G.T.S.K.A | P.S.K.S.S.R↓G.L.F | S.K.P | D.N.L | MG957200 |
| Chicken/Banten-02/17 (h9.4.2.5) | P.W.T.N.A.L.Y | N.G.L.M.G.R | G.T.S.K.A | P.S.K.S.S.R↓G.L.F | S.K.P | D.N.L | MG957201 |
| Chicken/Banten-04/17 (h9.4.2.5) | P.W.T.N.A.L.Y | N.G.L.M.G.R | G.T.S.K.A | P.S.K.S.S.R↓G.L.F | S.K.P | D.N.L | MG957202 |
| Chicken/WestJava-04/17 (h9.4.2.5) | P.W.T.N.T.L.Y | N.G.L.M.G.R | G.T.S.K.A | P.S.R.S.S.R↓G.L.F | S.K.P | D.N.L | MG957203 |
| Chicken/WestJava-06/17 (h9.4.2.5) | P.W.T.N.T.L.Y | N.G.L.M.G.R | G.T.S.K.A | P.S.R.S.S.R↓G.L.F | S.K.P | D.N.L | MG957204 |
| Chicken/WestJava-09/17 (h9.4.2.5) | P.W.T.N.T.L.Y | N.G.L.M.G.R | G.T.S.K.A | P.S.R.S.S.R↓G.L.F | S.K.P | D.N.L | MG957205 |
| Chicken/WestJava-13/17 (h9.4.2.5) | P.W.T.N.T.L.Y | N.G.L.M.G.R | G.T.S.K.A | P.S.R.S.S.R↓G.L.F | S.K.P | D.N.L | MG957206 |
| Chicken/WestJava-14/17 (h9.4.2.5) | P.W.T.N.T.L.Y | N.G.L.M.G.R | G.T.S.K.A | P.S.R.S.S.R↓G.L.F | S.K.P | D.N.L | MG957207 |
| Chicken/WestJava-15/17 (h9.4.2.5) | P.W.T.N.A.L.Y | N.G.L.M.G.R | G.T.S.K.A | P.S.R.S.S.R↓G.L.F | S.K.P | D.N.L | MG957208 |
| Chicken/CentralJava-01/17 (h9.4.2.5) | P.W.T.N.T.L.Y | N.G.L.M.G.R | G.T.S.K.A | P.S.R.S.S.R↓G.L.F | S.K.P | D.N.L | MG957209 |
| Chicken/EastJava-05/17 (h9.4.2.5) | P.W.T.N.A.L.Y | N.G.L.M.G.R | G.T.S.K.A | P.S.K.S.S.R↓G.L.F | S.K.P | D.N.L | MG957210 |
| Chicken/EastJava-09/17 (h9.4.2.5) | P.W.T.N.A.L.Y | N.G.L.M.G.R | G.T.S.K.A | P.S.K.S.S.R↓G.L.F | S.K.P | D.N.L | MG957211 |
| Chicken/Yogyakarta-01/17 (h9.4.2.5) | P.W.T.N.T.L.Y | N.G.L.M.G.R | G.T.S.K.A | P.S.K.S.S.R↓G.L.F | S.K.P | D.N.L | MG957212 |
(*) Positions 92, 143, 145, 173, 180, 184, and 185 (receptor binding site); 214–219 (left-edge of binding pocket, at position); 128–132 (right-edge of binding pocket); 315–323 (cleavage site); Antigenic site, divided into two namely site I at positions 125, 147 and 152; site II at positions 135, 183 and 216. Placement of residues according to H9 numbering.
The antigenic epitope of HA of H9N2 is mapped in two parts: site I and site II [21]. The antigenic site of the HA gene in all isolates obtained the same site I motif on the position residues of 125, 147, and 152, respectively, serine (S), lysine (K), and proline (P). While in site II, there are two kinds of motives at positions 135, 183, and 216. The first motif is aspartic acid (D), asparagine (N), and leucine (L), owned by 11 isolates in this study (Table 5). Unlike the others, N183D substitution occurs in two isolates from West Java, namely A/chicken/WestJava-04/17 (Accession No. MG957203) and A/chicken/WestJava-06/17 (Accession No. MG957204). One non-synonymous amino acid substitution (K164N) occurred in A/chicken/Banten-01/17 (Accession No. MG957200), A/chicken/Banten-02/17 (Accession No. MG957201), A/chicken/Banten-04/17 (Accession No. MG957202), and A/chicken/EastJava-05/17 (Accession No. MG957210) (Fig. 4).
Figure 4. Multiple alignments of HA amino acid sequences in the field strains. Non-synonymous amino acid substitutions K164N and N183D had occurred in some isolates (in the black square).

The potential glycosylation site of the HA gene analysis from 13 study isolates revealed 6 sites with N-X-T / S motif (where X is any amino acid except proline) in the HA1 gene section with positions in 11–13 (NST), 123–125 (NVS), 200–202 (NRT), 280–282 (NTT), 287–289 (NVS), and 295–297 (NCS) (Table 6).
Table 6. Comparison of the PGS in HA proteins of 13 H9N2 isolates.
| Isolates | PGS | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 11–13 | 123–125 | 127–130 | 178–180 | 188–190 | 200–202 | 280–282 | 287–289 | 295–297 | |
| Chicken/Banten-01/17 | N.S.T | N.V.S | – | – | – | N.R.T | N.T.T | N.V.S | N.C.S |
| Chicken/Banten-02/17 | N.S.T | N.V.S | – | – | – | N.R.T | N.T.T | N.V.S | N.C.S |
| Chicken/Banten-04/17 | N.S.T | N.V.S | – | – | – | N.R.T | N.T.T | N.V.S | N.C.S |
| Chicken/WestJava-04/17 | N.S.T | N.V.S | – | – | – | N.R.T | N.T.T | N.V.S | N.C.S |
| Chicken/WestJava-06/17 | N.S.T | N.V.S | – | – | – | N.R.T | N.T.T | N.V.S | N.C.S |
| Chicken/WestJava-09/17 | N.S.T | N.V.S | – | – | – | N.R.T | N.T.T | N.V.S | N.C.S |
| Chicken/WestJava-13/17 | N.S.T | N.V.S | – | – | – | N.R.T | N.T.T | N.V.S | N.C.S |
| Chicken/WestJava-14/17 | N.S.T | N.V.S | – | – | – | N.R.T | N.T.T | N.V.S | N.C.S |
| Chicken/WestJava-15/17 | N.S.T | N.V.S | – | – | – | N.R.T | N.T.T | N.V.S | N.C.S |
| Chicken/CentralJava-01/17 | N.S.T | N.V.S | – | – | – | N.R.T | N.T.T | N.V.S | N.C.S |
| Chicken/EastJava-05/17 | N.S.T | N.V.S | – | – | – | N.R.T | N.T.T | N.V.S | N.C.S |
| Chicken/EastJava-09/17 | N.S.T | N.V.S | – | – | – | N.R.T | N.T.T | N.V.S | N.C.S |
| Chicken/Yogyakarta-01/17 | N.S.T | N.V.S | – | – | – | N.R.T | N.T.T | N.V.S | N.C.S |
Discussion
Detection of H5N1 AIV from H9N2 positive cases indicated continuous co-circulation of the two subtypes in commercial chicken flocks. These study results agreed with previous studies [22] and provided a possible explanation for increased infectivity of H9N2 AIV over time due to genetic reassortment with the H5N1 subtype [23,24].
The H9N2 virus has spread widely in poultry throughout the Asian region, providing the fact that the virus is potentially rapidly evolving with virulence rising [25,26,27]. It is similar to the report in Java. Lesser than a year ago, H9N2 AIV was found in all provinces of layer farms in Java. The presence of H9N2 in commercial farms of Java may indicate some defect in applying biosecurity [28].
The motif of the cleavage site of the recent isolate study was different from those of clades representative isolates. All H9N2 AIV fit the characteristic of LPAI. The proteases (such as trypsin), which are only secreted by the respiratory organs and intestines, will recognize a single arginine (R) residue [29].
The h9.4.2 was categorized as h9.4.2.1–h9.4.2.6 [30]. The recent H9N2 viruses isolated in this study belonged to h9.4.2.5 of the H9N2 clades. These results indicate that clade h9.4.2.5 dominated the predominant virus in Java.
While vaccination is the optimum technique for preventing AIV, earlier research indicates that inadequate protective immunization may result in an antigenic drift of the H9N2 virus [31]. To assess if a vaccination strain should be updated in response to the virus that is circulated in the field, an antigenic link between the commercial vaccine strain and the emerging recent virus should be evaluated [32].
The receptor-binding specificity was associated with amino acids in the RBS of HA proteins. In general, AIVs specifically recognize sialylα2,3-galactose receptors, while the human virus recognizes the sialyl-α2,6-galactose receptor [33]. Hemaglutinine proteins of H9N2 viruses isolated from chickens in a recent study had histidine (H), glutamic acid (E), glutamine (Q), and glutamine (Q) at 173, 180, 216, and 217 positions at the RBS [34]. The amino acid substitution in this study had previously appeared in Chinese isolates (A/chicken/Guangdong /LGQ02/2014). The presence of asparagine (N) at 173 positions is owned by the AIV subtype H9N2 belonging to clade h9.4.2, whereas methionine (M) at position 217 can only be encountered in clade h9.4.2.5.
Leucine (L) at position 216 was found mainly in the HA protein of H2 and H3 viruses isolated from humans. In a previous study, Leucine (L) at 216 caused a van der Waals bond with the C-6 atom to form a sialyl-α2,6-galactose linkage. HA having leucine (L) at position 216 tends to bind to sialyl-α2,6-galactose, while Q in that position tends to bind to sialyl-α2,3-galactose. The substitution of Q to L in position 216 allows the H9N2 virus to replicate more efficiently in human cell culture [34]. The substitution of Q to L at position 216 is one of the genetic changes that occurs during the adaptation of avian influenza strains in pigs [35]. The existence of a backyard poultry system exacerbates the condition. Infected poultry without any clinical symptoms may increase the likelihood of exposure in humans [25].
The N183D mutation with Q216L at antigenic site II is involved in inhibiting viral interactions with epitope-specific antibodies and can result in mutant virus escape; both of these residues are identical to those in the most closely related vaccine. The 127 amino acid residue is another critical location in the HA H9N2 gene [21]. In a recent investigation, all H9N2 viruses from Java showed residual S127, which was identical to that of the H5 subtype [36]. Recent research [37] identified strains of H9 variations having the S127N mutation, which may increase the pathogenicity of SPF hens, while 13 isolates lacked the mutation.
In general, N-linked glycosylation has a substantial effect on the virus’ biological features. It may be impacted by the N-X-S/T-X amino acid structure [38]. Additionally, earlier research has demonstrated that alterations in glycosylation residues near HA protein cleavage sites might influence viral virulence [39]. Previous research has established that N-linked glycosylation of the HA protein has an effect on host innate immune system evasion and survival, as seen in human influenza virus, H1 and H3 subtypes [40]. Changes in the glycosylation status of the antigenic protein of the influenza virus HA region alter virus binding to antibodies, whereas changes in the glycosylation site at numerous places have no effect on the structure and function of HA [41]. There was a potential glycosylation site (PGS) within the antigenic area, NVS, between locations 123 and 125 in this study. Previous research with H1N1 viruses revealed that the presence of K123N mutations results in immune evasion via glycosylation [42].
Conclusion
In five regions of Java, the H9N2 virus was isolated from chickens. The H9N2 virus that recently circulated in Java is classified as clade h9.4.2.5 and lineage Y280. Additionally, all recent viruses have a genetic link with viruses from China and Vietnam.
List of Abbreviations
AIV: Avian influenza viruses, HA: Hemagglutination, HAU; Hemagglutination unit, IBV: Infectious bronchitis virus, LPAIV: Low pathogenic avian influenza virus, ND: Newcastle disease, PBS: Phosphate buffer saline, RBS: Receptor-binding site, RNA: Ribonucleic acid, RT-PCR: Reverse transcription polymerase chain reaction, SPF: Specific pathogenic-free.
Acknowledgments
The authors express their gratitude to the University of Indonesia for sponsoring this study under the PUTI Grant NKB-583/UN2.RST/HKP.05.00/2020. The authors also express their gratitude to Drs. Lily Natalia, Adin Priadi, and Sudarisman of PT Medika Satwa Laboratories in Bogor, Indonesia, for their assistance with laboratory facilities.
Conflict of interests
There was no conflict of interests in all parts of this study.
Authors’ contribution
CMHN designed the study, collecting of data, and evaluated the data under the supervision of RDS, ONP, and AS. Data and papers were interpreted and produced by OSMS. RSK collected samples and assembled the resource materials. CMHN, RDS, ONP, OSMS, RSK, and AS approved the final text.
References
- [1].Kurscheid J, Millar J, Abdurrahman M, Ambarawati IG, Suadnya W, Yusuf RP, et al. Knowledge and perceptions of highly pathogenic avian influenza (HPAI) among poultry traders in live bird markets in Bali and Lombok, Indonesia. PLoS One. 2015;10(10):e0139917. doi: 10.1371/journal.pone.0139917. https://doi.org/10.1371/journal.pone.0139917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].CASERED (Indonesian Centre of Agricultural Socio-Economic Research And Development) Rome, Italy: FAO; Socio-economic impact assessment of the avian influenza crisis in poultry production systems in Indonesia, with particular focus on independent smallholders. Final report for FAO’s tcp/ras/3010.2004; p. 87. [Google Scholar]
- [3].Umar S, Sarfraz S, Mushtaq A, Attique M. Emerging threat of H9N2 viruses in poultry of Pakistan and vaccination strategy. World’s Poult Sci J. 2016;72(2):343–52. https://doi.org/10.1017/S0043933916000179. [Google Scholar]
- [4].Yang WT, Yang GL, Shi SH, Liu YY, Huang HB, Jiang YL, et al. Protection of chickens against H9N2 avian influenza virus challenge with recombinant Lactobacillus plantarum expressing conserved antigens. J Appl Microbiol Biotechnol. 2017;101(11):4593–603. doi: 10.1007/s00253-017-8230-8. https://doi.org/10.1007/s00253-017-8230-8. [DOI] [PubMed] [Google Scholar]
- [5].Njoto EN, Scotch M, Bui CM, Adam DC, Chughtai AA, MacIntyre CR. Phylogeography of H5N1 avian influenza virus in Indonesia. Transbound Emerg Dis. 2018;(5):1339–47. doi: 10.1111/tbed.12883. https://doi.org/10.1111/tbed.12883. [DOI] [PubMed] [Google Scholar]
- [6].Alexander DJ. A review of avian influenza in different bird species. Vet Microbiol. 2000;74(1–2):3–13. doi: 10.1016/s0378-1135(00)00160-7. https://doi.org/10.1016/S0378-1135(00)00160-7. [DOI] [PubMed] [Google Scholar]
- [7].Swayne DE, Halvorson DA. 12th. Hoboken, NJ: Blackwell Publishing; 2003. Influenza. Diseases of poultry; pp. 135–60. [Google Scholar]
- [8].Zhang P, Tang Y, Liu X, Peng D, Liu W, Liu H, et al. Characterization of H9N2 influenza viruses isolated from vaccinated flocks in an integrated broiler chicken operation in eastern China during a 5 year period (1998–2002) J Gen Virol. 2008;89(12):3102–12. doi: 10.1099/vir.0.2008/005652-0. https://doi.org/10.1099/vir.0.2008/005652-0. [DOI] [PubMed] [Google Scholar]
- [9].Nagy A, Mettenleiter TC, Abdelwhab EM. A brief summary of the epidemiology and genetic relatedness of avian influenza H9N2 virus in birds and mammals in the Middle East and North Africa. Epidemiol Infect. 2017;145(16):3320–33. doi: 10.1017/S0950268817002576. https://doi.org/10.1017/S0950268817002576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Shen HQ, Yan ZQ, Zeng FG, Liao CT, Zhou QF, Qin JP, et al. Isolation and phylogenetic analysis of hemagglutinin gene of H9N2 influenza viruses from chickens in South China from 2012 to 2013. J Vet Sci. 2015;16(3):317–24. doi: 10.4142/jvs.2015.16.3.317. https://doi.org/10.4142/jvs.2015.16.3.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Tarigan S, Indriani R, Sumarningsih. Endemicity of avian influenza in ducks living around commercial layer farms. Indonesian J Anim Vet Sci. 2015;20(4):285–96. [Google Scholar]
- [12].Beard CW. Serological procedures. In: Purchase HG, Arp LH, Domermuth CH, Pearson JE, editors. A laboratory manual for the isolation and identification of avian pathogens. 3rd. Dubuque, Iowa: Kendall/Hunt Publishing Company; 1989. pp. 192–200. [Google Scholar]
- [13].Chaharaein B, Omara AR, Aini I, Yusoff K, Hassan SS. Detection of H5, H7 and H9 subtypes of avian influenza viruses by multiplex reverse transcription-polymerase chain reaction. J Els Microbial Res. 2009;164(2):174–9. doi: 10.1016/j.micres.2007.01.001. https://doi.org/10.1016/j.micres.2007.01.001. [DOI] [PubMed] [Google Scholar]
- [14].Abid M, Yaqub T, Mehboob A, Shabbir MZ. Characterization and phylogenetic analysis of avian influenza virus subtype H9N2 in Pakistan. Hosts Viruses. 2017;4(4):62–9. https://doi.org/10.17582/journal.hv/2017/4.4.62.69. [Google Scholar]
- [15].Mazumder AC, Khatun S, Nooruzzaman M, Chowdhury EH, Das PM, Islam MR. Isolation and identification of Newcastle disease viruses from field outbreaks in chickens and pigeons. Bangladesh Vet. 2013;29:41–8. https://doi.org/10.3329/bvet.v29i2.14341. [Google Scholar]
- [16].Callison SA, Hilt DA, Boynton TO, Sample BF, Robison R, Swayne DE, et al. Development and evaluation of a real-time Taqman RT-PCR assay for the detection of infectious bronchitis virus from infected chickens. J Virol Methods. 2006;138(1-2):60–5. doi: 10.1016/j.jviromet.2006.07.018. https://doi.org/10.1016/j.jviromet.2006.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].El-Hamid HS, Ellakany AH, Ahmed RE, Ahmed RG. Molecular characterization of H9N2 avian influenza viruses isolated from commercial broiler chickens in Egypt during 2014–2015. Alexan J Vet Sci. 2018;56(2):54–61. https://doi.org/10.5455/ajvs.220473. [Google Scholar]
- [18].Tamura K, Dudley J, Nei M, Kumar S. MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol. 2007;24:1596–9. doi: 10.1093/molbev/msm092. https://doi.org/10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- [19].Edgar RC. MUSCLE: a multiple sequence alignment method with reduced time and space complexity. BMC Bioinformatics. 2004;5:113. doi: 10.1186/1471-2105-5-113. https://doi.org/10.1186/1471-2105-5-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Liu H, Liu X, Cheng J, Peng D, Jia L, Huang Y. Phylogenetic analysis of the hemagglutinin genes of twenty-six avian influenza viruses of subtype H9N2 isolated from chickens in China during 1996–2001. Avian Dis. 2003;47:116–27. doi: 10.1637/0005-2086(2003)047[0116:PAOTHG]2.0.CO;2. https://doi.org/10.1637/0005-2086(2003)047[0116:PAOTHG]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- [21].Kaverin NV, Rudneva IA, Ilyushina NA, Lipatov AS, Krauss S, Webster RG. Structural differences among hemagglutinins of influenza a virus subtypes are reflected in their antigenic architecture: analysis of H9 escape mutants. J Virol. 2004;78:240–9. doi: 10.1128/JVI.78.1.240-249.2004. https://doi.org/10.1128/JVI.78.1.240-249.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kayali G, Kandeil A, El-Shesheny R, Kayed AS, Gomaa MM, Maatouq AM, et al. Active surveillance for avian influenza virus, Egypt, 2010–2012. Emerg Infect Dis. 2014;20(4):542–51. doi: 10.3201/eid2004.131295. https://doi.org/10.3201/eid2004.131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Monne I, Hussein AH, Fusaro A, Valastro V, Hamoud MM, Khalefa RA, et al. H9N2 influenza a virus circulates in H5N1 endemically infected poultry population in Egypt. Influenza Other Respir Viruses. 2013;7(3):240–3. doi: 10.1111/j.1750-2659.2012.00399.x. https://doi.org/10.1111/j.1750-2659.2012.00399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Webster RG, Bean WJ, Gorman OT, Chambers TM, Kawaoka Y. Evolution and ecology of influenza a viruses. Microbiol Rev. 1992;56(1):152–79. doi: 10.1128/mr.56.1.152-179.1992. https://doi.org/10.1128/mr.56.1.152-179.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Iqbal M, Yaqub T, Reddy K, McCauley JW. Novel genotypes of H9N2 influenza a viruses isolated from poultry in Pakistan containing NS genes similar to highly pathogenic H7N3 and H5N1 viruses. PLoS One. 2009;4:e5788. doi: 10.1371/journal.pone.0005788. https://doi.org/10.1371/journal.pone.0005788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Naeem K, Siddique N, Ayaz M, Jalalee MA. Avian influenza in Pakistan: outbreaks of low and high pathogenicity avian influenza in Pakistan during 2003–2006. Avian Dis. 2007;51:189–93. doi: 10.1637/7617-042506R.1. https://doi.org/10.1637/7617-042506R.1. [DOI] [PubMed] [Google Scholar]
- [27].Butt AM, Siddique S, Tahir S, Nasrullah I, Hussain M, Idrees M, et al. Comparative sequence, antigenic and phylogenetic analysis of avian influenza (H9N2) surface proteins isolated in Pakistan between 1999 and 2008. J Infection Dev Ctries. 2011;5:413–24. doi: 10.3855/jidc.1372. https://doi.org/10.3855/jidc.1372. [DOI] [PubMed] [Google Scholar]
- [28].Ismail ZM, El-Deeb AH, El-Safty MM, Hussein HA. Enhanced pathogenicity of low-pathogenic H9N2 avian influenza virus after vaccination with infectious bronchitis live attenuated vaccine. Vet World. 2018;11(7):977–85. doi: 10.14202/vetworld.2018.977-985. https://doi.org/10.14202/vetworld.2018.977-985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Neumann G, Kawaoka Y. Host range restriction and pathogenicity in the context of influenza pandemic. Emerg Infect Dis. 2006;12:881–6. doi: 10.3201/eid1206.051336. https://doi.org/10.3201/eid1206.051336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Jiang W, Liu S, Hou G, Li J, Zhuang Q, Wang S, et al. Chinese and global distribution of H9 subtype avian influenza viruses. PloS One. 2012;7(12):e52671. doi: 10.1371/journal.pone.0052671. https://doi.org/10.1371/journal.pone.0052671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Li C, Yu K, Tian G, Yu D, Liu L, Jing B. Evolution of H9N2 influenza viruses from domestic poultry in Mainland China. Virology. 2005;340:70–83. doi: 10.1016/j.virol.2005.06.025. https://doi.org/10.1016/j.virol.2005.06.025. [DOI] [PubMed] [Google Scholar]
- [32].Lu XH, Renshaw M, Tumpey TM, Kelly GD, Primmer JH, Katz JM. Immunity to influenza a H9N2 viruses induced by infection and vaccination. J Virol. 2001;75:4896–901. doi: 10.1128/JVI.75.10.4896-4901.2001. https://doi.org/10.1128/JVI.75.10.4896-4901.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Suzuki Y. Sialobiology of influenza: molecular mechanism of host range variation of influenza viruses. Biol Pharm Bull. 2005;28:399–408. doi: 10.1248/bpb.28.399. https://doi.org/10.1248/bpb.28.399. [DOI] [PubMed] [Google Scholar]
- [34].Matrosovich MN, Scott K, Robert GW. H9N2 Influenza a viruses from poultry in Asia have human virus-like receptor specificity. Virology. 2001;281:156–62. doi: 10.1006/viro.2000.0799. https://doi.org/10.1006/viro.2000.0799. [DOI] [PubMed] [Google Scholar]
- [35].Wan H, Perez DR. Amino acid 226 in the hemagglutinin of H9N2 influenza viruses determines cell tropism and replication in human airway epithelial cells. J Virol. 2007;81:5181–91. doi: 10.1128/JVI.02827-06. https://doi.org/10.1128/JVI.02827-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Yan LC, Juan P, Qin FL, Shuai W, Guo ZZ, Xing LZ, et al. Antigenic and genetic characterization of H9N2 swine influenza viruses in China. J Gen Virol. 2007;88:2035–41. doi: 10.1099/vir.0.82783-0. https://doi.org/10.1099/vir.0.82783-0. [DOI] [PubMed] [Google Scholar]
- [37].Nobusawa E, Aoyama T, Kato H, Suzuki Y, Tateno Y, Nakajima K. Comparison of complete amino acid sequences and receptor-binding properties among 13 serotypes of hemagglutinins of influenza a viruses. Virology. 1991;182:475–85. doi: 10.1016/0042-6822(91)90588-3. https://doi.org/10.1016/0042-6822(91)90588-3. [DOI] [PubMed] [Google Scholar]
- [38].Land A, Braakman I. Folding of the human immunodeficiency virus type 1 envelope glycoprotein in the endoplasmic reticulum. Biochimie. 2001;83(8):783–90. doi: 10.1016/s0300-9084(01)01314-1. https://doi.org/10.1016/S0300-9084(01)01314-1. [DOI] [PubMed] [Google Scholar]
- [39].Deshpande KL, Fried VA, Ando M, Webster RG. Glycosylation affects the cleavage of an H5N2 influenza virus hemagglutinin and regulates virulence. Proc Natl Acad Sci USA. 1987;84(1):36–40. doi: 10.1073/pnas.84.1.36. https://doi.org/10.1073/pnas.84.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Chen RA, Lai HZ, Li L, Liu YP, Pan WL, Zhang WY, et al. Genetic variation and phylogenetic analysis of hemagglutinin genes of H9 avian influenza viruses isolated in China during 2010–2012. Vet Microbiol. 2013;165:312–8. doi: 10.1016/j.vetmic.2013.04.005. https://doi.org/10.1016/j.vetmic.2013.04.005. [DOI] [PubMed] [Google Scholar]
- [41].Tate MD, Job ER, Deng Y, Gunalan V, Sebastian M, Patrick CR. Playing hide and seek: how glycosylation of the influenza virus hemagglutinin can modulate the immune response to infection. Viruses. 2014;6(3):1294–316. doi: 10.3390/v6031294. https://doi.org/10.3390/v6031294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].O’Donnell CD, Vogel L, Wright A, Das SR, Wrammert J, Li GM, et al. Antibody pressure by a human monoclonal antibody targeting the 2009 pandemic H1N1 virus hemagglutinin drives the emergence of a virus with increased virulence in mice. mBio. 2012;3:e00120–31. doi: 10.1128/mBio.00120-12. https://doi.org/10.1128/mBio.00120-12. [DOI] [PMC free article] [PubMed] [Google Scholar]


