Abstract
Introduction
Immune response following viral infections has been suggested as a probable mechanism leading to subacute thyroiditis (SAT). A few cases of SAT following SARS-CoV-2 infection have been described since the outbreak of the pandemic in 2019. Cases of SAT after vaccination against influenza have also been reported. We describe two female patients with thyroiditis after vaccination against SARS-CoV-2.
Presentation of cases
The first patient presented with fever and pain in the thyroid area typical of SAT two weeks after vaccination with the BNT162B2 mRNA (Pfizer-BioNTech) COVID-19 vaccine. The second patient presented with biochemical and imaging features consistent with silent thyroiditis three weeks after vaccination with the ChAdOx1-S (AstraZeneca) vaccine. Both patients were asymptomatic prior to vaccination and PCR of nasopharyngeal swab for SARS-CoV-2 and other respiratory viruses associated with SAT was negative. Serology testing for measles, mumps, rubella, CMV and EBV viruses was suggestive of immunity. Antibody titre against spike S protein of SARS-CoV-2 was measured for both patients and was indicative of adequate post vaccination antibody response. Two months after initial assessment, both patients were euthyroid and asymptomatic.
Conclusions
Subacute as well as silent thyroiditis may rarely occur after vaccination against COVID-19. Further research is needed to investigate the prevalence and pathogenesis of thyroid dysfunction following vaccination against COVID-19.
Keywords: Subacute thyroiditis, Silent thyroiditis, COVID-19, Vaccine, SARS-CoV-2
Highlights
-
•
Thyroid function abnormalities are common in COVID-19 patients.
-
•
Thyroiditis is a rare complication of influenza, HPV and HBV vaccination.
-
•
We report two cases of thyroiditis after vaccination against COVID-19.
1. Introduction
Since the outbreak of the COVID-19 pandemic, the effect of SARS-CoV-2 infection on thyroid function has been rigorously studied. As more than 234 million confirmed COVID-19 cases have been reported so far and more than 6 billion vaccine doses have been administered worldwide, it has become evident that thyroid dysfunction is commonly associated with SARS-CoV-2 infection, resulting in diagnostic and management challenges [1].
A frequently encountered thyroid abnormality, particularly in those patients with severe disease, is the “non-thyroidal illness” (or “sick euthyroid syndrome”), a condition characterized by low serum thyroid stimulating hormone (TSH) and thyroid hormone levels that does not require any specific treatment. In addition, at least 22 cases of subacute thyroiditis (SAT), a self-limited inflammatory disease of the thyroid, have been reported to date in COVID-19 patients [2]. Clinical presentation, epidemiological evidence and several case reports suggest an association of SAT with preceding viral infections [3,4]. Genetic predisposition may also play a role, as suggested by the association with Human Leukocyte Antigen–B35 (HLAB35) and the report of familial SAT cases [5,6]. Notably, vaccines against influenza, hepatitis B, H1N1, HPV, have also been associated with SAT [7]. A total of ten SAT cases after SARS-CoV-2 vaccination (BNT162B2 SARS-CoV-2 and CoronaVac) have been recently reported [[8], [9], [10], [11], [12], [13]].
We present a case of SAT as well as the first case of silent thyroiditis following SARS-CoV-2 vaccination and we briefly review the relevant literature.
2. Case presentation
2.1. Patient 1
A 51-year-old female presented with nausea, mild anterior neck pain and fever up to 38,2 °C. The symptoms had started 11 days prior to presentation and 4 days after receiving the first dose of the BNT162B2 SARS-CoV-2 (Pfizer-BioNTech) vaccine. She was previously healthy with no history of thyroid disease. The patient mentioned contact with COVID-19 case in her family four months prior to presentation; however at that time she had no symptoms suggestive of COVID-19 infection and a negative SARS-CoV-2 real-time reverse transcription polymerase chain reaction (rRT-PCR) in a nasopharyngeal specimen had been obtained after a 14-day quarantine. The thyroid gland was tender on palpation. There were no signs of hyperthyroidism such as tachycardia or fine tremor.
Thyroid function tests revealed hyperthyroxinemia with suppressed serum TSH and elevated free thyroxine levels (fT4) (Table 1). Testing for thyrotropin receptor (TRAb), thyroid peroxidase (TPOAb) and thyroglobulin (TGAb) antibodies was negative. Erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) levels were markedly elevated (Table 1). Thyroid scintigraphy with 99mTc-pertechnetate showed markedly decreased thyroid uptake, consistent with thyroiditis (Fig. 1). Nasopharyngeal swab for molecular detection of SARS-CoV-2 and a respiratory multiplex virus PCR (including Adenovirus, Human Rhinovirus/Enterovirus, Respiratory Syncytial Virus, Coronavirus HKU1-NL63-229E&OC43, Influenza A-A/H1-A/H1-2009-A/H3–B, Parainfluenza 1-2-3-4, Human Metapneumovirus) was negative. Serological tests were positive for antibody (Ab) against receptor-binding domain (RBD) and negative for Ab against the SARS-CoV-2 nucleocapsid (N) protein (Table 1), thus indicating vaccine induced antibody production without evidence of prior SARS-CoV-2 infection. Virus serologies for measles, mumps and rubella, CMV and EBV suggested immunity.
Table 1.
Laboratory results.
| Patient 1 | Patient 2 | Reference range | |
|---|---|---|---|
| Baseline | |||
| TSH | 0.08 | <0.03 | 0.38–5.33 mIU/mL |
| fT4 | 24.84 | 20.47 | 7.72–17.63 pmol/L |
| TT3 | 2.3 | 2.22 | 0.86–2.39 nmol/L |
| TPOAb | 0.6 | 777.4 | 0–9 IU/mL |
| TGAb | <0.9 | 275.3 | 0–4 IU/mL |
| TRAb | <0.1 | 0.2 | <1 IU/L |
| ESR | 103 | 17 | 0–30 mm/h |
| CRP | 135 | 1 | <6 mg/L |
| SARS-CoV-2 IgG II Quant | 1271.3 | 245.4 | <50 AU/mL |
| Follow up | 8th week | 8th week | |
| TSH | 1.93 | 2.88 | 0.38–5.33 mIU/mL |
| fT4 | 10.55 | 9.27 | 7.72–17.63 pmol/L |
| TT3 | NA | 1.64 | 0.86–2.39 nmol/L |
| TPOAb | 0.5 | 665.3 | 0–9 IU/mL |
| TGAb | <0.9 | 246.3 | 0–4 IU/mL |
| ESR | 17 | 16 | 0–30 mm/h |
| CRP | 2 | NA | <6 mg/L |
Abbrevations: TGAb, anti-thyroglobulin antibody; TPOAb, thyroid peroxidise antibody; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; TT3, total triiodothyronine; fT4, free thyroxine; NA, not assessed; TRAB, TSH receptor antibodies; TSH, thyroid-stimulating hormone; SARS-CoV-2 IgG II Quant: IgG antibodies against the spike receptor-binding domain (RBD) of SARS-CoV-2.
Fig. 1.
Scintigraphy image of patient 1 showing decreased uptake of 99mTc-pertechnetate by the thyroid gland.
The patient's history, physical examination findings and laboratory test results, were consistent with subacute thyroiditis probably associated with the preceding vaccination. Treatment with 16 mg of methylprednisolone once daily p.o. was initiated with rapid resolution of fever and neck pain two days after initiation. The glucocorticoid dose was gradually reduced and two months after initial assessment the patient was clinically and biochemically euthyroid and asymptomatic.
2.2. Patient 2
A 39-year-old female patient was referred for the evaluation of abnormal thyroid function tests revealed during a routine laboratory test that was ordered by her primary care physician. She had no history of thyroid disease and serum TSH was normal six months prior to presentation. Her mother had hypothyroidism due to Hashimoto thyroiditis. The patient did not report any fever, neck pain or symptoms of upper respiratory infection in the preceding weeks. There was no exposure to iodine or drugs known to affect thyroid function. She had been vaccinated with the ChAdOx1-S [recombinant] (AstraZeneca) vaccine against SARS-CoV-2 three weeks before thyroid hormone testing was performed. Physical examination revealed no abnormal findings.
Thyroid function tests revealed suppressed TSH (<0.003 U/mL), increased fT4 and normal total triiodothyronine (TT3) concentrations (Table 1). Inflammatory markers were normal and TPOAb titer was elevated (Table 1). Thyroid scintigraphy showed decreased uptake and thyroid ultrasound showed diffuse hypoechoic echotexture of the thyroid gland with reduced blood flow (Fig. 2). Nasopharyngeal swab testing for SARS-CoV-2 and the same respiratory multiplex virus PCR as for patient 1 was negative. Serological tests were positive for Ab against RBD, indicating immune response to the vaccination. No specific treatment was initiated, and two months later thyroid function tests had returned to normal.
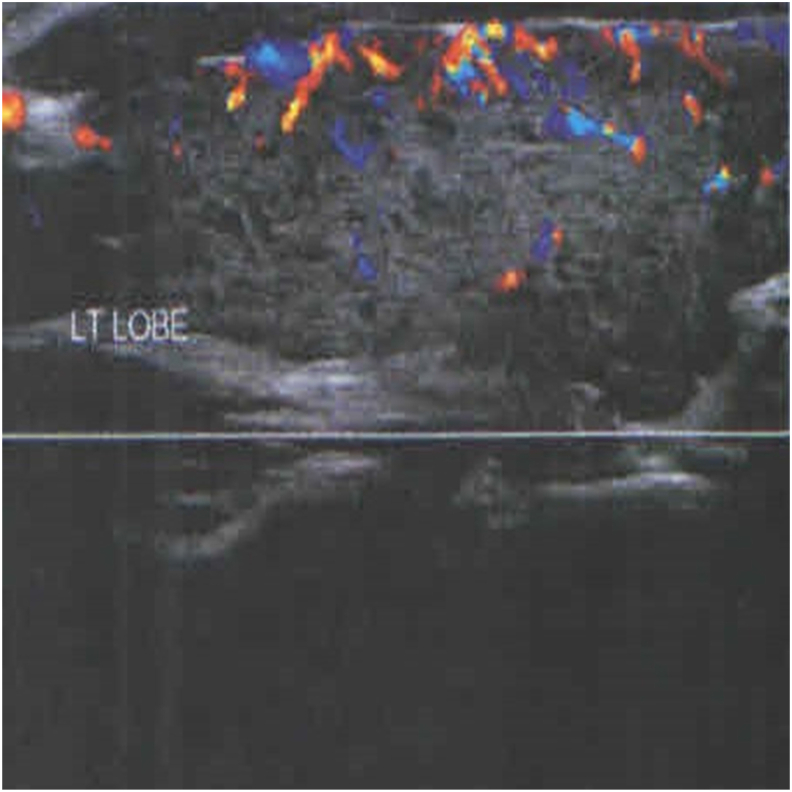
Fig. 2.
Thyroid ultrasound image of patient 2; profoundly hypoechoic left lobe with decreased blood flow.
3. Discussion
A multitude of autoimmune disorders, including autoimmune thyroid disease, have been described following infection with SARS-CoV-2 [14]. Immune-mediated complications after SARS-CoV-2 infection are induced by immune system hyper-stimulation and molecular mimicry between the human proteome and SARS-CoV-2 components [14,15]. The pathogenesis of COVID-19 induced thyroid dysfunction might involve either direct viral infection or abnormal inflammatory-immune responses. Abundant expression of the SARS-CoV-2 receptor (angiotensin-converting enzyme 2) mRNA in thyroid cells suggests that the thyroid gland could be a target organ of COVID-19 infection [16]. At least 22 cases of SAT associated with COVID-19 infection have been reported so far and thyrotoxicosis has been reported in up to 20.2% of patients hospitalized for COVID-19 in a single-center retrospective study [2,17]. Approximately half of these patients had subclinical thyrotoxicosis and low fT3, suggesting that non-thyroidal illness may explain the hormonal findings in most of these cases. In another study, 8 patients with COVID-19 and any thyroid dysfunction were followed up for 55 days; in 75% (6/8) of these cases, TSH concentrations were low or suppressed and ultrasound and scintigraphic features were suggestive of inflammatory/destructive thyroiditis [18].
Autoimmune endocrine disorders (including thyroiditis) have been associated with exposure to several vaccines or adjuvants (molecules that potentiate antigen specific immune response). The autoimmune/inflammatory syndrome induced by adjuvants (ASIA) has been used to describe post-vaccination autoimmune conditions since 2011 [19]. As described in a recent review article by Bragazzi et al., subacute thyroiditis has been reported after influenza (8 cases), HBV (one case) as well as H1N1 (one case) vaccination [20]. Pelegrino et al. reported 41 cases of thyroiditis following human papilloma (HPV) vaccination using information from a HPV vaccine database in the US [21].
Since the outbreak of the COVID-19 pandemic massive efforts have been made to produce safe and effective vaccines against the new coronavirus; to this date at least 23 different vaccines have been developed worldwide according to World Health Organisation data. The first mass vaccination programme against COVID-19 started in early December 2020 and it is estimated that more than 45% of the world population has received at least one dose of a COVID-19 vaccine up to October 2021 [1]. A total of 10 cases of subacute thyroiditis after vaccination against COVID-19 have been reported as of the date of this report. Four of them have been reported after the inactivated virus COVID-19 vaccine CoronaVac® [9,11]. Moreover, two cases of SAT have been reported after the BNT162B2 SARS-CoV 2 (Pfizer-BioNTech) mRNA vaccine [8,10], three cases after the ChAdOx1 nCoV-19 vaccine (AstraZeneca) and one case after the Spikevax (Moderna Biotech, Spain) vaccine [7,12,13]. To our knowledge, no case of silent thyroiditis after COVID-19 vaccination has been reported. In all these cases, association of SAT with the administered vaccines was suggested by the patient's history (no preceding symptoms of viral infection) and the timing of symptom onset after the vaccination (Table 2). In most (7/10) cases, symptoms started after the first dose. Onset of symptoms ranged from 4 to 21 days after the vaccination. Most patients were female (8/10 cases) and their age ranged from 26 to 55 years old. In 7/10 cases, investigation included a negative nasopharyngeal swab testing for SARS-CoV-2 in order to exclude viral infection.
Table 2.
Previously reported cases of subacute thyroiditis after vaccination against COVID-19.
| Case 1 [9] | Case 2 [9] | Case 3 [9] | Case 4 [11] | Case 5 [10] | Case 6 [8] | Case 7 [13] | Case 8 [7] | Case 9 [12] | Case 10 [12] | |
|---|---|---|---|---|---|---|---|---|---|---|
| Age | 35 | 34 | 37 | 67 | 57 | 42 | 55 | 75 | 26 | 49 |
| Sex | Female | Female | Female | Male | Female | Female | Female | Male | Female | Female |
| Type of vaccine | CoronaVac® | CoronaVac® | CoronaVac® | CoronaVac® | BNT162B2 | BNT162B2 | ChAdOx1-S | ChAdOx1-S | ChAdOx1-S | Spikevax |
| Time of symptoms onset | 4 days after 1st dose | 4 days after 1st dose | 7 days after 2nd dose | 17 days after 2nd dose | 1 day after 2nd dose | 5 days after 2nd dose | 21 days after 1st dose | 14 days after 1st dose | 2 days after 1st dose | 7 days after 1st dose |
| Symptoms | Neck pain, palpiyations | Neck pain, fatigue, weight loss | Neck pain | Fever, weight loss, mild neck pain | Neck pain and swelling | Sore throat, palpitations | Neck pain and swelling, sore throat, headache, palpitations | Neck pain and tenderness, shortness of breath, intermittent palpitations, insomnia and generalized anxiety | Fever, neck pain | Sore throat, headaches and difficulty in concentrating |
| Thyroid function testsa | ↓TSH, ↑fT3 | ↓TSH, ↓fT4 ↑fT3 |
↓TSH, ↑fT3 | ↓TSH, ↑fT4, ↑fT3 | ↓TSH, ↑fT4 | ↓TSH | ↓TSH, ↑fT4 | ↓TSH, ↑fT4, ↑fT3 | ↑fT3 | Euthyroid |
| TPOAb | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative |
| TGAb | Negative | Negative | Negative | Negative | Negative | Negative | NA | Negative | Negative | Negative |
| TRAbs | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative |
| Thyroid scintigraphy | NA | NA | NA | NA | NA | NA | NA | ↓ uptake | NA | NA |
| Nasopharyngeal swab testing for SARS-CoV-2 | Negative | Negative | Negative | Negative | Negative | Negative | Negative | NA | NA | NA |
| Antibodies to the spike protein of SARS-CoV-2 | NA | NA | NA | NA | NA | NA | NA | ↑↑ | NA | NA |
| Outcome in reassessment | Euthyroidism | Thyrotoxicosis | Thyrotoxicosis | Euthyroidism | NA | Thyrotoxicosis | Hypothyroidism | Euthyroidism | Euthyroidism | Euthyroidism |
Abbrevations: TGAb, anti-thyroglobulin antibody; TPOAb, thyroid peroxidise antibody; fT3, free triiodothyronine; fT4, free thyroxine; NA, not assessed; TRAB, TSH receptor antibodies; TSH, thyroid-stimulating hormone.
Only abnormal laboratory results are noted.
We herein present two cases of subacute thyroiditis with a case -described for the first time-of silent thyroiditis following SARS-CoV-2 vaccination. Investigation included measurement of antibodies against the spike receptor-binding domain of SARS-CoV-2, used to assess immune response to the vaccination. In addition, we arranged nasopharyngeal swab sampling and performed respiratory multiplex virus PCR as well as viral antibody screen against viruses that have been associated with SAT to exclude the possibility of a mild preceding viral infection that might have been associated with the thyroiditis in our patients.
Autoimmune endocrine disorders after vaccination, may develop as a result of either molecular mimicry or the use of adjuvants in vaccine excipients. Among the listed excipients of the COVID-19 vaccines, aluminum is used as an adjuvant in CoronaVac® vaccine and polysorbate 80 (E 433) as an excipient in the ChAdOx1-S [recombinant] vaccine. The latter is an ingredient of the MF59 adjuvant that has been used in SARS, MERS and influenza vaccines [22]. The immunomodulating properties of the lipid nanoparticles used in the mRNA vaccines are not well known. The fact that SAT has been described after different types of vaccines against COVID-19 (viral vectors or mRNA vaccines) regardless of the presence of adjuvants in the excipients, suggests that molecular mimicry might play a significant role in the pathophysiology of these autoimmune responses. SARS-CoV-2 antibodies may promote a mild and transient subacute thyroiditis by reacting with cellular antigens located on the thyroid. Due to structural similarity between the SARS-CoV-2 spike protein and the thyroid peroxidase, SARS-CoV-2 antibodies against spike protein may also cross-react with TPO antibodies [23].
We report two cases of thyroiditis after vaccination against COVID-19, including the first case of silent thyroiditis after the ChAdOx1-S vaccine. Several medical associations (e.g. Brazilian Society of Endocrinology and Metabolism, British Thyroid Association) have recently published statements regarding the management of patients with thyroid disease such as hypothyroidism, hyperthyroidism, thyroid eye disease and thyroid cancer during the COVID-19 pandemic [24,25]. Importantly, stable pre-existing thyroid disease is not considered a contraindication for COVID-19 vaccination. The established benefits of vaccination far outweigh the risk of rare, mild-to-moderate side effects, like subacute or silent thyroiditis. However, in the context of vaccine safety monitoring, clinicians should be aware that thyroiditis might be a probably underreported adverse effect of COVID-19 vaccines. Further research is needed to investigate the prevalence and the mechanisms of thyroiditis after COVID-19 vaccination.
Author contribution
Athanasios Siolos: Conceptualization, writing-original draft. Konstantina Gartzonika: Writing – review & editing Stelios Tigas: Conceptualization, supervision, writing – review & editing.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
The authors declare no competing interest relevant to the contents of this article.
Acknowledgements
NA.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.metop.2021.100136.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.WHO COVID-19 dashboard. World Health Organization; Geneva: 2020. https://covid19.who.int/ (last cited: October 5th, 2021) [Google Scholar]
- 2.Caron P. Thyroiditis and SARS-CoV-2 pandemic: a review. Endocrine. 2021;72(2):326–331. doi: 10.1007/s12020-021-02689-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stasiak M., Lewiński A. New aspects in the pathogenesis and management of subacute thyroiditis. Rev Endocr Metab Disord. 2021 May 5:1–13. doi: 10.1007/s11154-021-09648-y. Epub ahead of print. PMID: 33950404; PMCID: PMC8096888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martino E., Buratti L., Bartalena L. High prevalence of subacute thyroiditis during summer season in Italy. J Endocrinol Invest. 1987;10(3):321–323. doi: 10.1007/BF03348138. [DOI] [PubMed] [Google Scholar]
- 5.Nyulassy S., Hnilica P., Buc M., Guman M., Hirschová V., Stefanovic J. Subacute (de Quervain's) thyroiditis: association with HLA-Bw35 antigen and abnormalities of the complement system, immunoglobulins and other serum proteins. J Clin Endocrinol Metab. 1977;45(2):270–274. doi: 10.1210/jcem-45-2-270. [DOI] [PubMed] [Google Scholar]
- 6.Kramer A.B., Roozendaal C., Dullaart R.P. Familial occurrence of subacute thyroiditis associated with human leukocyte antigen-B35. Thyroid. 2004;14(7):544–547. doi: 10.1089/1050725041517048. [DOI] [PubMed] [Google Scholar]
- 7.Ratnayake G.M., Dworakowska D., Grossman A.B. Can COVID-19 immunisation cause subacute thyroiditis? [published online ahead of print, 2021 Jul 17] Clin Endocrinol. 2021 doi: 10.1111/cen.14555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stephanie Franquemont D.O., Galvez Juan. MD subacute thyroiditis after mRNA vaccine for covid-19 journal of the endocrine society. April-May 2021;ume 5(Issue Supplement_1) A956–A957. [Google Scholar]
- 9.İremli B.G., Şendur S.N., Ünlütürk U. Three cases of subacute thyroiditis following SARS-CoV-2 vaccine: postvaccination ASIA syndrome. J Clin Endocrinol Metab. 2021;106(9):2600–2605. doi: 10.1210/clinem/dgab373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schimmel J., Alba E.L., Chen A., Russell M., Srinath R. Letter to the editor: thyroiditis and thyrotoxicosis after the SARS-CoV-2 mRNA vaccine. Thyroid. 2021;31(9):1440. doi: 10.1089/thy.2021.0184. [DOI] [PubMed] [Google Scholar]
- 11.Şahin Tekin M., Şaylısoy S., Yorulmaz G. Subacute thyroiditis following COVID-19 vaccination in a 67-year-old male patient: a case report [published online ahead of print, 2021 Jul 1] Hum Vaccines Immunother. 2021:1–3. doi: 10.1080/21645515.2021.1947102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bornemann C., Woyk K., Bouter C. Case report: two cases of subacute thyroiditis following SARS-CoV-2 vaccination. Front Med. 2021;8:737142. doi: 10.3389/fmed.2021.737142. Published 2021 Aug 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oyibo S.O. Subacute thyroiditis after receiving the adenovirus-vectored vaccine for coronavirus disease (COVID-19) Cureus. 2021;13(6) doi: 10.7759/cureus.16045. Published 2021 Jun 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dotan A., Muller S., Kanduc D., David P., Halpert G., Shoenfeld Y. The SARS-CoV-2 as an instrumental trigger of autoimmunity. Autoimmun Rev. 2021;20(4):102792. doi: 10.1016/j.autrev.2021.102792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oldstone M.B. Molecular mimicry: its evolution from concept to mechanism as a cause of autoimmune diseases. Monoclon Antibodies Immunodiagn Immunother. 2014;33(3):158–165. doi: 10.1089/mab.2013.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rotondi M., Coperchini F., Ricci G. Detection of SARS-COV-2 receptor ACE-2 mRNA in thyroid cells: a clue for COVID-19-related subacute thyroiditis. J Endocrinol Invest. 2021;44(5):1085–1090. doi: 10.1007/s40618-020-01436-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lania A., Sandri M.T., Cellini M., Mirani M., Lavezzi E., Mazziotti G. Thyrotoxicosis in patients with COVID-19: the THYRCOV study. Eur J Endocrinol. 2020;183(4):381–387. doi: 10.1530/EJE-20-0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muller I., Cannavaro D., Dazzi D. SARS-CoV-2-related atypical thyroiditis. Lancet Diabetes Endocrinol. 2020;8(9):739–741. doi: 10.1016/S2213-8587(20)30266-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shoenfeld Y., Agmon-Levin N. ASIA' - autoimmune/inflammatory syndrome induced by adjuvants. J Autoimmun. 2011;36(1):4–8. doi: 10.1016/j.jaut.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 20.Bragazzi N.L., Hejly A., Watad A., Adawi M., Amital H., Shoenfeld Y. ASIA syndrome and endocrine autoimmune disorders. Best Pract Res Clin Endocrinol Metabol. 2020;34(1):101412. doi: 10.1016/j.beem.2020.101412. [DOI] [PubMed] [Google Scholar]
- 21.Pellegrino P., Perrone V., Pozzi M. The epidemiological profile of ASIA syndrome after HPV vaccination: an evaluation based on the Vaccine Adverse Event Reporting Systems. Immunol Res. 2015;61(1–2):90–96. doi: 10.1007/s12026-014-8567-3. [DOI] [PubMed] [Google Scholar]
- 22.Liang Z., Zhu H., Wang X. Adjuvants for coronavirus vaccines. Front Immunol. 2020;11:589833. doi: 10.3389/fimmu.2020.589833. Published 2020 Nov 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vojdani A., Vojdani E., Kharrazian d. Reaction of human monoclonal antibodies to SARS-CoV-2 proteins with tissue antiogens: implications for autoimmune diseases. Front Immunol. 2021;11089 doi: 10.3389/fimmu.2020.617089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.BTA/SFE statement regarding issues specific to thyroid dysfunction during the COVID-19 pandemic. https://www.endocrinology.org/media/3573/management-of-thyroid-dysfunction-during-covid-19.docx Available online:
- 25.Martins J.R.M., Villagelin D.G.P., Carvalho G.A. Management of thyroid disorders during the COVID-19 outbreak: a position statement from the Thyroid Department of the Brazilian Society of Endocrinology and Metabolism (SBEM) [published online ahead of print, 2021 Apr 12] Arch Endocrinol Metab. 2021 doi: 10.20945/2359-3997000000352. 2359-3997000000352. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.