Abstract
Fyn is a prototype Src-family tyrosine kinase that plays specific roles in neural development, keratinocyte differentiation, and lymphocyte activation, as well as roles redundant with other Src-family kinases. Similar to other Src-family kinases, efficient regulation of Fyn is achieved through intramolecular binding of its SH3 and SH2 domains to conserved regulatory regions. We have investigated the possibility that the tyrosine kinase regulatory protein Cbl provides a complementary mechanism of Fyn regulation. We show that Cbl overexpression in 293T embryonic kidney and Jurkat T-lymphocyte cells led to a dramatic reduction in the active pool of Fyn; this was seen as a reduction in Fyn autophosphorylation, reduced phosphorylation of in vivo substrates, and inhibition of transcription from a Src-family kinase response element linked to a luciferase reporter. Importantly, a Fyn mutant (FynY528F) relieved of intramolecular repression was still negatively regulated by Cbl. The Cbl-dependent negative regulation of Fyn did not appear to be mediated by inhibition of Fyn kinase activity but was correlated with enhanced protein turnover. Consistent with such a mechanism, elevated levels of Fyn protein were observed in cell lines derived from Cbl−/− mice compared to those in wild-type controls. The effects of Cbl on Fyn were not observed when the 70ZCbl mutant protein was analyzed. Taken together, these observations implicate Cbl as a component in the negative regulation of Fyn and potentially other Src-family kinases, especially following kinase activation. These results also suggest that protein degradation may be a general mechanism for Cbl-mediated negative regulation of activated tyrosine kinases.
Activation of protein tyrosine kinases in response to engagement of cell surface receptors by their ligands is a common mechanism to control cellular events such as proliferation and differentiation. Autophosphorylation of tyrosine kinases and subsequent phosphorylation of key intracellular signaling proteins are the earliest detectable events following activation of a variety of receptors and are required for transcriptional events that ultimately mediate cellular responses (71, 72). Therefore, precise regulation of tyrosine kinases is critical to avoid inappropriate cellular responses to extracellular cues.
The protein product of the c-cbl proto-oncogene has emerged as a prominent component of tyrosine kinase-mediated signal transduction cascades (reviewed in references 33 and 42). Phosphorylation of the Cbl protein follows the engagement of a variety of cell surface receptors that either possess a cytoplasmic tyrosine kinase domain or are noncovalently linked to cytoplasmic or membrane-anchored tyrosine kinases. Furthermore, Cbl associates with a number of cellular signaling proteins, including tyrosine kinases themselves. The C-terminal portion of Cbl (Cbl-C) is primarily responsible for mediating protein-protein interactions. This region encodes a large proline-rich segment (amino acids 481 to 690) which contains multiple potential SH3 domain-binding sites that mediate the interactions of Cbl with proteins such as Grb2 and Nck (14, 18, 37, 38, 58). Cbl-C also encompasses the major sites of Cbl tyrosine phosphorylation (Y700, Y731, and Y774), which function as docking sites for SH2 domain-containing proteins, such as Vav guanine nucleotide exchange factor, the Crk family of adapter proteins, and the p85 subunit of phosphatidylinositol 3-kinase (16, 42). Thus, the Cbl-C region mediates both constitutive associations with SH3 domain-containing proteins and activation-induced associations with SH2 domain-containing proteins.
The N-terminal portion of Cbl has been highly conserved throughout evolution, implying that it is important for Cbl function. This region includes a RING finger domain and a more N-terminal region (Cbl-N, amino acids 1 to 357), which corresponds to sequences that are retained in the v-cbl oncogene. Cbl-N is sufficient to transform NIH 3T3 cells (5, 27). Recent studies have demonstrated that Cbl-N associates directly and selectively with a number of autophosphorylated tyrosine kinases (reviewed in reference 42), and is therefore referred to as the tyrosine kinase-binding (TKB) domain. The TKB domain has recently been shown to consist of a four-helix domain, an EF hand, and an incomplete SH2 domain, which together create a phosphotyrosine-binding platform (41).
Genetic and biochemical analyses have implicated Cbl as a negative regulator of tyrosine kinases. Initial evidence for this idea came from studies of vulval development in Caenorhabditis elegans. A Cbl homologue, SLI-1, was identified in a screen for negative regulators of the LET-23 receptor, a homologue of the mammalian epidermal growth factor receptor (EGFR) (78). A single-amino-acid substitution within the TKB domain homology region of SLI-1, Gly 315 Glu, abolished its ability to function as a negative regulator of LET-23 (78). Similarly D-Cbl, the Drosophila homologue of Cbl, was shown to negatively regulate R7 photoreceptor development, which is also mediated through an EGFR signaling pathway (39). Further in vivo evidence for the negative regulatory role of Cbl comes from the phenotype of Cbl−/− mice, which exhibit hypercellularity in the lymphoid organs and enhanced branching of the mammary gland ducts (45). These analyses are complemented by a number of in vitro studies that have firmly established that Cbl can function as a negative regulator of the receptor tyrosine kinases EGFR, platelet-derived growth factor receptor α and β (PDGFRα and PDGFRβ), as well as non-receptor tyrosine kinases of the ZAP70/Syk family (6, 30, 34, 43). A naturally occurring Cbl mutant, 70ZCbl, is unable to exert an inhibitory effect on tyrosine kinases and is in fact highly oncogenic when overexpressed in NIH 3T3 cells (3). 70ZCbl is identical to wild-type Cbl with the exception of a 17-amino-acid deletion (amino acids 366 to 382), suggesting that the RING finger or surrounding areas are critical for Cbl function. Indeed, RING finger mutations markedly reduced the inhibitory effect of Cbl on EGFR and Syk (49a, 73).
The mechanism by which Cbl mediates its negative regulatory effects on tyrosine kinases is still unclear. Interestingly, studies with COS cells have revealed that coexpression with Cbl resulted in a reduction of Syk protein levels, concurrent with a loss of the active pool of this kinase (34). Recent studies suggest a related mechanism of Cbl-induced negative regulation of receptor tyrosine kinases. Overexpression of Cbl resulted in enhanced ligand-induced ubiquitination and subsequent degradation of PDGFRα and EGFR, and monocytes from Cbl−/− mice showed reduced ligand-induced ubiquitination and turnover of the CSF-1 receptor (29–31, 43, 73). Taken together, these results suggest that Cbl may act to terminate signals from activated tyrosine kinases by promoting their removal from the cell surface and enhancing their degradation.
All of the tyrosine kinase targets of Cbl examined thus far associate with Cbl in a strictly activation-dependent manner. In contrast, Cbl associates with several members of the Src family of tyrosine kinases prior to cellular activation (14, 17, 18, 35, 51, 54, 56, 67, 70). The Src family of kinases is composed of at least nine members that are involved in the regulation of diverse cellular functions (11, 32). N-terminal myristoylation, which is required for biological activity, targets all Src-family kinases to the inner face of the cell membrane, positioning them for signal transduction downstream of transmembrane receptors (55). Gene-targeting experiments have implicated Src-family kinases as being critical in the development and activation of lymphocytes and other hematopoietic cell lineages, as well as in the regulation of osteoclast function and in neural development (11, 32). To date, the role of Cbl as a potential regulator of Src-family kinases has not been assessed.
The Src-family kinases exhibit a conserved primary structure comprising an N-terminal myristoylation signal, adjacent SH3 and SH2 domains, a kinase domain, and a negative regulatory tyrosine within the C-terminal tail which is phosphorylated by the C-terminal Src kinase (Csk) (11, 32). Given their widespread expression and the multitude of cellular processes in which they play important roles, regulation of Src kinases has been an area of intense research. The importance of precise regulation of these enzymes is further underscored by the fact that mutational activation of their kinase activity often converts them into potent oncogenes (12).
A general paradigm of Src-family kinase regulation has emerged from the large body of mutational analysis, together with the recent crystallization of the Src, Hck, and Lck proteins (60, 75, 77). According to this model, Src-family kinases are maintained in an inactive or closed conformation by intramolecular binding of the SH3 domain to the SH2-kinase linker region, in conjunction with the SH2 domain binding to a phosphorylated tyrosine residue in the C-terminal tail (60, 75). It is hypothesized that activation signals communicated by noncovalently associated transmembrane receptors lead to the unfolding of the molecule, concurrently promoting the active conformation of the kinase and releasing the SH2 and SH3 domains for protein-protein interactions. Consistent with this model, certain inactivating point mutations in the SH3 or SH2 domains can significantly enhance kinase activity (59). Furthermore, expression of high-affinity SH3 domain-binding ligands (e.g., the Nef protein of human immunodeficiency virus type 1 HIV-1) or mutations within the SH2-kinase linker that abolish intramolecular SH3 domain binding increase the kinase activity of Hck, Src, and Lck (7, 21, 44). Similarly, deletion or substitution of the negative regulatory tyrosine within the carboxyl tail results in enhanced kinase activity and oncogenic activation of all Src-family kinases examined (23, 24).
The current paradigm of Src-family kinase regulation does not address a number of important biological issues. Given that Src-family kinases are constantly present in a milieu of accessible SH3 domain-binding ligands, what mechanisms prevent their activation in the absence of extracellular signals? Additionally, once activated, how are Src-family kinases either returned to their basal state or eliminated? It is quite likely that cells use additional regulatory mechanisms that, in conjunction with the intramolecular associations, fine-tune the levels of active versus repressed species of Src-family kinases. Given the ability of Cbl to associate with a number of Src-family kinases (2, 14, 17, 18, 48, 51, 54, 56, 67, 70) and its evolutionarily conserved role as a negative regulator of tyrosine kinases, we have explored the hypothesis that Cbl may provide one regulatory mechanism that complements the intramolecular regulation of Src-family kinases. This hypothesis was tested by using the Fyn tyrosine kinase, since Cbl-Fyn complexes have been demonstrated in vivo and Cbl is known to be an excellent substrate for Fyn (16). Furthermore, Fyn is known to be physiologically important in a number of cellular functions. For example, Fyn-deficient mice exhibit defects in neural development and keratinocyte differentiation, suggesting a role for Fyn in these cells (9, 63, 76). While lymphocyte development and function were essentially normal in Fyn−/− or Lyn−/− animals but defective in Lck−/− animals, more severe defects were observed when the Fyn and Yes genes (64), or the Fyn and Lck genes (22, 50), were both disrupted. These findings suggest an important role for Fyn in lymphocyte activation that is functionally redundant with that of other Src-family kinases.
Here, we provide direct evidence that Cbl functions as a negative regulator of Fyn. Using a transient-overexpression system in 293T cells, we demonstrate that Cbl can function as a negative regulator of both the wild-type Fyn and its activated mutant, FynY528F, which has been relieved of intramolecular repression. This inhibition is achieved by enhancing the rate of Fyn protein turnover. The results obtained with 293T cells were confirmed by expressing Cbl and Fyn proteins in Jurkat T-lymphocytic cells, and increased Fyn protein levels were detected in cell lines derived from Cbl−/− mice compared to control cells. These results lead us to suggest that one mechanism for down regulating the activity of Fyn is Cbl-dependent degradation of the activated tyrosine kinase. This mechanism is likely to complement the basal repression achieved via intramolecular interactions. Given the prominent association of Cbl with other Src-family members and the conservation of structure in this family of proteins, Cbl is likely to function as a general negative regulator for Src-family kinases.
MATERIALS AND METHODS
Cells.
293T is a human embryonic kidney epithelial cell line expressing the simian virus 40 (SV40) large T antigen. The cells were maintained in Dulbecco's modified Eagle's medium (DMEM; Life Technologies, Gaithersburg, Md.) containing 10% fetal bovine serum (FBS), 20 mM HEPES, 1 mM sodium pyruvate, 1 mM nonessential amino acids, 100 U of penicillin per ml, and 100 U of streptomycin per ml (all from Life Technologies). JMC-T, an SV40 large T-antigen-expressing derivative of the Jurkat-JMC T-lymphocytic cell line, was maintained in RPMI 1640 with 10% FBS as described previously (35).
Generation of Cbl-deficient cell lines.
T-cell lines were established from Cbl-deficient mice and normal littermates (45) by intraperitoneal injection of newborn mice with Moloney murine leukemia virus supernatants provided by J. W. Hartley and H. C. Morse III (National Institutes of Health, Bethesda, Md.). The T-cell lymphomas were subsequently cultured by plating in 24-well dishes at 107/ml in RPMI 1640 supplemented with 10% FBS, 20 mM HEPES, 1 mM sodium pyruvate, 1 mM nonessential amino acids, 100 U of penicillin per ml, and 100 U of streptomycin per ml.
Primary embryo fibroblasts were established from 13.5-day-old embryos by using standard procedures and culture conditions (20). The cells were maintained in alpha minimal essential medium supplemented with 10% FBS, 20 mM HEPES, 1 mM sodium pyruvate, 1 mM nonessential amino acids, 100 U of penicillin per ml, and 100 U of streptomycin per ml.
Antibodies.
The antibodies used in this work were mouse monoclonal antibody (MAb) 4G10 (antiphosphotyrosine) (15) (a gift of Brian Druker, Oregon Health Sciences University, Portland, Oreg.), MAb SPV-T3b (anti-CD3ɛ) (62), MAb 12CA5 (anti-influenza virus hemagglutinin [HA] epitope tag) (74), MAb 6B10.2 (anti-ζ chain) (Santa Cruz Biotechnology, Santa Cruz, Calif.), the FYN3 rabbit polyclonal anti-Fyn antibody (Santa Cruz Biotechnology), rabbit polyclonal anti-Cbl antibody C15 (Santa Cruz Biotechnology), and a rabbit anti-p44/42 mitogen-activated protein (MAP) kinase antibody 9102 (New England BioLabs, Beverly, Mass.).
Expression constructs and site-directed mutagenesis.
The pSRαNeo-CD8-ζ chimera construct, encoding human CD8 extracellular and transmembrane domains fused to the T-cell receptor ζ cytoplasmic tail, and pSRαNeo-HA-Cbl and pAlterMAX HA-Cbl constructs, encoding HA-tagged wild-type Cbl protein, have been described previously (36). The pSRαNeo-HA-CblΔ472-540 (from which the sequences encoding amino acids 472 to 540 are absent) and pSRαNeo-HA-CblΔ540-645 (from which the sequences encoding amino acids 540 to 645 are absent) constructs were created by subcloning Cbl cDNA sequences from the corresponding pJZenNeo expression constructs (4). The SRE-luciferase reporter construct containing the Egr-1 serum response element linked to the firefly luciferase gene (1) was provided by K. Alexandropoulos (Department of Pharmacology, Columbia University, New York, N.Y.). The cDNA for murine FynT was obtained from R. Perlmutter (Howard Hughes Medical Institute, University of Washington, Seattle) and was subcloned into the pAlterMAX expression vector at the EcoRI restriction site. Constructs encoding Fyn proteins with inactivating amino acid substitutions in the SH3 (P134V) or SH2 (R176K) domain or in both domains (P134V/R176K) (57) were provided by A. Shaw (Washington University School of Medicine, St. Louis, Mo.) and were directionally cloned into pAlterMAX by using the XhoI and SmaI sites. The pAlterMAX-FynTY528F mutant was generated by site-directed mutagenesis of the pAlterMAX-FynT construct, using the Altered Sites-II mammalian mutagenesis system (Promega Corp., Madison, Wis.) as specified by the manufacturer. The mutagenic oligonucleotide was 5′-CAC CGG GCT GAA ACT GGG GCT CT-3′. All constructs were verified by DNA sequencing.
Transient transfection of cell lines and preparation of lysate.
293T cells were seeded into 100-mm tissue culture dishes 12 to 16 h prior to transfection, such that the cells were 10 to 20% confluent at the time of transfection. The cells were transfected by a modified version of the calcium phosphate method (10). Where appropriate, input doses of the cytomegalovirus promoter were equalized among transfections by using the pAlterMAX vector, and 10 μg of pBluescript plasmid DNA (Stratagene, La Jolla, Calif.) was added to each transfection reaction mixture as a carrier. The amounts of the specific DNA constructs used for each experiment are indicated in the appropriate figure legends. Culture medium was replaced 12 to 14 h following the addition of DNA precipitates, and cells were harvested 48 h posttransfection. JMC-T cells were transfected with the indicated amounts of expression plasmids by the electroporation method as previously described (34). The cells were lysed 48 h posttransfection. Cell lysates were prepared with Triton X-100 lysis buffer (50 mM Tris [pH 7.5], 150 mM sodium chloride, 0.5% Triton X-100) supplemented with 1 mM phenylmethylsulfonyl fluoride, 1 μg each of leupeptin, pepstatin, antipain, and chymostatin per ml, 1 mM sodium orthovanadate, and 10 mM sodium fluoride. The concentration of lysate proteins was determined by the Bradford protein assay (Bio-Rad Laboratories, Hercules, Calif.) with bovine serum albumin as the standard.
Immunoprecipitation and immunoblotting.
The procedures for immunoprecipitation and immunoblotting were described previously (54). The amount of lysate protein and the type of antibodies used for each experiment are indicated in the relevant figure legends. Resolved proteins were transferred to polyvinylidene difluoride (PVDF) membranes (Immobilon-P; Millipore, Bedford, Mass.). Following incubation of membranes with the indicated primary antibodies, horseradish peroxidase-conjugated protein A or horseradish peroxidase-conjugated anti-mouse antibodies (Cappel/Organon Teknika Corp., West Chester, Pa.) were used as secondary reagents. Detection was performed by enhanced chemiluminescence with the Renaissance Western Blot Chemiluminescence Reagent Plus kit (NEN Life Science Products, Inc., Boston, Mass.). Where indicated, membranes were stripped and reprobed with additional antibodies as previously described (54). Figures were prepared by direct scanning of films with a Hewlett Packard ScanJet 4c scanner and Corel Draw version 6 software. Fyn protein levels were quantified with the ScionImage program (version beta 3b) and are expressed relative to the signal observed with the smallest amount of input DNA for a particular construct.
Pulse-chase analysis of protein turnover.
293T cells in 100-mm tissue culture dishes were transfected with the appropriate DNA constructs by using the calcium phosphate technique. At 48 h posttransfection, the cells were rinsed with methionine-free DMEM and methionine starved for 1 h at 37°C by incubation in methionine-free DMEM supplemented with 2% dialyzed FBS. The cells were then pulse-labeled for 45 min at 37°C by incubation with 250 μCi of EXPRE35S35S labeling mix (NEN/DuPont, Boston, Mass.) per ml. They were washed in DMEM, cultured in chase medium (DMEM, 10% FBS, 3 mg of l-methionine per ml) for the indicated times, and lysed as described above. Anti-Fyn immunoprecipitations were performed on 600 μg of protein from cells expressing Fyn alone; 715 μg of protein was used from cells expressing Fyn plus Cbl, FynY528F, or FynY528F plus Cbl; and 225 μg of protein from each transfectant was used for anti-CD8ζ immunoprecipitates. Bound proteins were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a PVDF membrane. Radiolabeled proteins were detected by autoradiography with BIOMAX-MR film (Eastman Kodak Co., Rochester, N.Y.). The signals for radiolabeled proteins were quantified with the ScionImage program (version beta 3b). Finally, the membrane was immunoblotted with anti-Fyn or anti-ζ antibodies to evaluate steady-state protein levels of Fyn and CD8-ζ.
Luciferase assays.
293T cells were transfected by the calcium phosphate method with an SRE-luciferase reporter construct and the appropriate Cbl and Fyn constructs, as described in the figure legends. At 48 h posttransfection, the cells were lysed with cell culture lysis reagent (Promega Corp.) and lysate protein concentrations were determined by the Bradford assay. SRE-luciferase activity was determined by using a Monolight 3010C luminometer (Analytical Bioluminescence Laboratory Inc., Cockeysville, Md.) and Luciferin reagent (Promega Corp.). For Jurkat JMC-T cells, the cells were transfected with the appropriate constructs by electroporation as described previously (34). Cells were cultured for 24 h and then seeded in V-bottom 96-well plates in replicates of five for each stimulation condition (2 × 105 cells/well). The cells were stimulated for 6 h at 37°C with medium alone, anti-CD3 antibody (1:2,000 dilution of SpV:T3b ascites), or 50 ng of phorbol myristate acetate (PMA) per ml plus 1 μg of ionomycin per ml (Sigma Chemical Co., St. Louis, Mo.). Following stimulation, cell lysates were prepared and the SRE-luciferase activity present in each lysate sample was determined as described above.
In vitro kinase assay.
The kinase activity of the Fyn pool associated with Cbl or 70ZCbl was determined by immunoprecipitation of Cbl proteins with the 12CA5 anti-HA MAb. Bound proteins were washed five times in lysis buffer before being resuspended in kinase assay buffer (50 mM HEPES [pH 7.5], 0.01% Brij 35, 15 mM MgCl2, 0.06% β-mercaptoethanol, 5 μg of Raytide substrate [Oncogene Research Products, Cambridge, Mass.]). The reaction was started by the addition of an ATP mix containing 0.05 mM unlabeled ATP and 10 μCi of [γ32P]ATP (6,000 Ci/mmol; NEN/DuPont). The reaction was allowed to proceed for 30 min at 30°C and was stopped by the addition of 2.5 volumes of 20% phosphoric acid. Half the reaction mixture was applied to P81 cation-exchange chromatography paper (Watman, England). The cation-exchange paper was washed five times in 0.5% phosphoric acid, allowed to dry, and counted with a Packard 1600TR liquid scintillation counter. A nonphosphorylatable Raytide substrate (in which the tyrosine has been replaced by leucine) (Oncogene Research Products) was used as a negative control.
RESULTS
In vivo association of Cbl with Fyn.
Previous studies have shown that both the SH3 and SH2 domains of Src-family kinases can bind to Cbl in vitro (14, 17, 18, 55). Furthermore, Cbl is basally associated with Fyn and other Src-family kinases, and this association increases upon cell surface receptor stimulation (2, 54, 70). These results suggest that both the SH3 and SH2 domains of Src-family kinases contribute to the in vivo association of the kinases with Cbl; however, this has not been demonstrated experimentally. Therefore, we first attempted to define the regions of Cbl and Fyn that are required for their in vivo association.
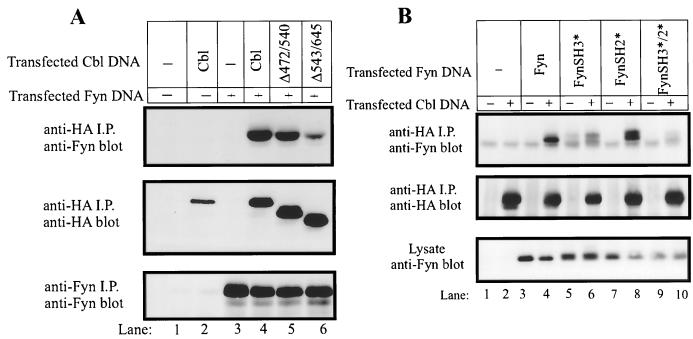
For this purpose, 293T cells were mock transfected or transfected with Fyn, with or without the indicated HA-tagged Cbl mutants. At 48 h posttransfection, the cells were lysed and HA-tagged Cbl proteins were immunoprecipitated. Immunoprecipitates were resolved by SDS-PAGE and immunoblotted with anti-Fyn antibodies to detect the coimmunoprecipitated Fyn protein (Fig. 1A, top panel). As expected, Fyn was found in complex with Cbl when the two proteins were coexpressed in 293T cells (lane 4). In this transient-expression system, approximately 25% of the expressed Fyn protein could be detected in association with Cbl, as determined by coimmunoprecipitation experiments (data not shown). Fyn protein was not detected in anti-HA immunoprecipitates of mock-transfected cells (lane 1) or cells transfected with HA-Cbl or Fyn alone (lanes 2 and 3, respectively), demonstrating the specificity of the association. A mutant form of Cbl, in which a segment of the proline-rich region had been deleted (Δ472–543), was also able to associate with Fyn (lane 5). Significantly, a second proline-rich region deletion mutant (Δ543–640) exhibited reduced ability to associate with Fyn (lane 6). The region of Cbl deleted in this mutant does not contain any known Fyn phosphorylation sites (16), and when this mutant was coexpressed with Fyn, its phosphorylation was not reduced (data not shown). These observations strongly suggest that one mechanism for in vivo association of Cbl with Fyn involves the Fyn SH3 domain binding to one or more motifs within the distal part of the Cbl proline-rich region.
FIG. 1.
Role of the Cbl proline-rich region and Fyn SH3 domain in Cbl-Fyn association in vivo. (A) 293T cells in 100-mm tissue culture dishes were transfected with the AprM8 expression vector encoding Fyn (0.5 μg), with or without pSRαneo expression vectors (0.5 μg) encoding HA-tagged Cbl constructs. Cell lysates were prepared 48 h after transfection, and 1-mg aliquots of lysate were subjected to immunoprecipitation (i.p.) with anti-HA or anti-Fyn antibodies as indicated. Immune complexes were resolved by SDS-PAGE, transferred to a PVDF membrane, and immunoblotted with anti-HA (middle panel) or anti-Fyn (top and bottom panels) antibodies. (B) 293T cells were transfected with the indicated pAlterMAX-Fyn constructs (0.1 μg), with or without pAlterMAX-HA-Cbl (1 μg). Anti-HA immunoprecipitations (i.p.) were carried out with 200 μg of cell lysate protein. Immune complexes or 15 μg of whole-cell lysate proteins were resolved by SDS-PAGE, transferred to a PVDF membrane, and blotted with the indicated antibodies.
To test whether the Fyn SH3 domain was required for the Fyn-Cbl interaction, we analyzed the in vivo association of various Fyn mutants with wild-type Cbl (Fig. 1B). Wild-type HA-Cbl was transfected into 293T cells together with Fyn constructs bearing single-amino-acid substitutions which inactivate the ligand-binding capability of the SH3 domain (FynSH3*), the SH2 domain (FynSH2*), or both (FynSH3*/2*). Anti-HA immunoprecipitates of these transfectants were immunoblotted with an anti-Fyn antibody to assess the ability of the various Fyn mutants to associate with Cbl (Fig. 1B, top panel). While inactivation of the SH2 domain alone did not detectably affect the association of Fyn with Cbl (compare lane 8 with lane 4), inactivation of the SH3 domain markedly reduced the Fyn-Cbl association (compare lane 6 with lane 4). Association of the FynSH3*/SH2* double mutant with Cbl was minimal (lane 10). These results further establish the predominance of the Fyn SH3 domain/Cbl proline-rich region interaction as a molecular mechanism for in vivo Fyn-Cbl association. However, the SH2 domain of Fyn appears to play a role, albeit minor, in the Fyn-Cbl association.
Fyn or FynY528F activity and protein levels are reduced by Cbl coexpression.
A prediction of the Src-family kinase repression model, invoking SH3 and SH2 domain-mediated intramolecular associations, is that engagement of these domains by their cognate ligands will result in activation of the kinase. Results from coexpression of HIV Nef protein and a fragment of the cellular SH3-binding protein Sin support this prediction (1, 7, 44). Since a Fyn-Cbl complex is easily detectable in resting lymphocytes, in which Fyn is presumably inactive, we wished to determine the effect of Cbl coexpression on Fyn activity. Initially, we compared the effect of Cbl on wild-type Fyn with that on the FynY528F mutant. In this mutant, the negative regulatory carboxyl tail tyrosine 528 has been mutated to phenylalanine, and the protein is thereby relieved of its intramolecular negative regulation and is constitutively active (24).
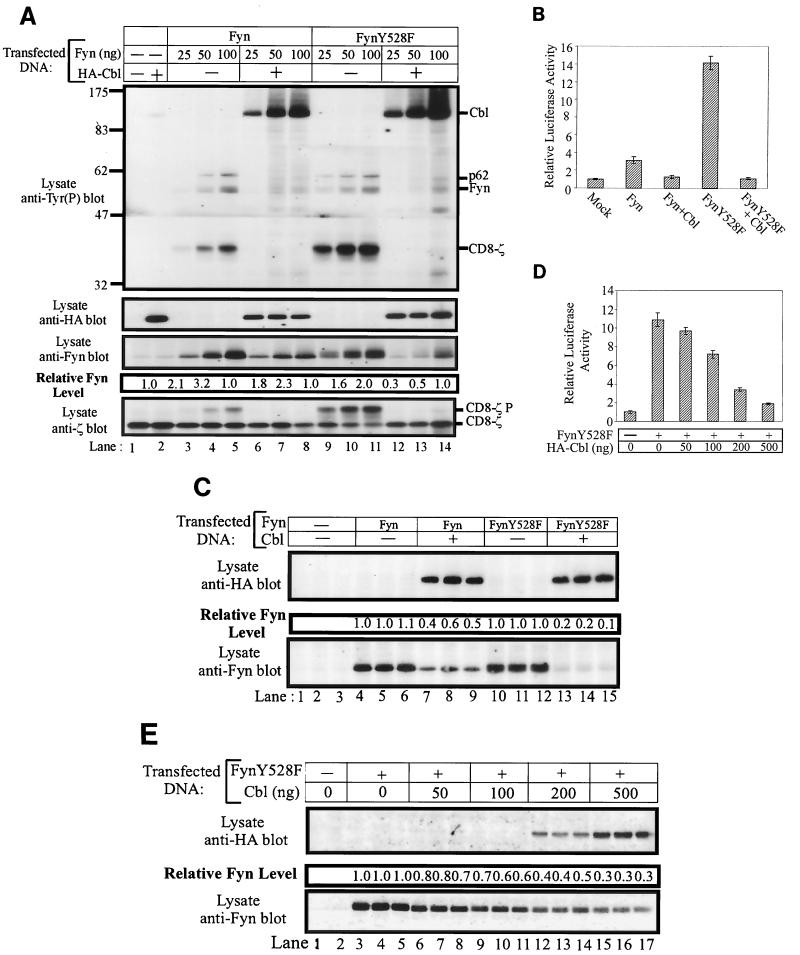
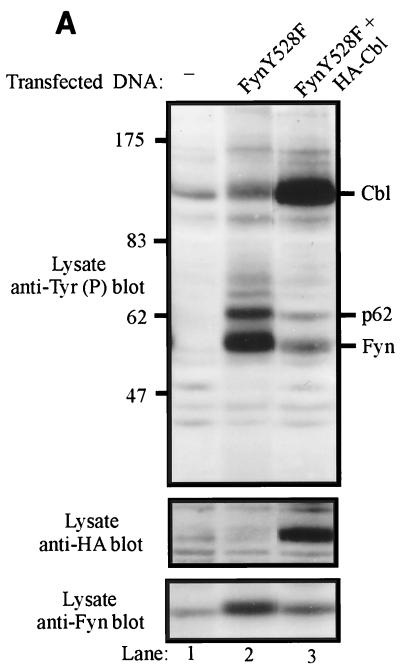
The effect of Cbl on Fyn was tested by transfecting increasing amounts of Fyn expression plasmids into 293T cells, in the presence or absence of a constant amount of Cbl plasmid. To provide an in vivo substrate for Fyn, a CD8-ζ chain chimera construct was also cotransfected. Lysates of these cells were prepared at 48 h posttransfection and analyzed by antiphosphotyrosine immunoblotting (Fig. 2A, top panel). Relatively little tyrosine phosphorylation of cellular proteins or the CD8-ζ chimera was observed in lysates of mock-transfected cells (lane 1) or cells transfected with Cbl alone (lane 2). As anticipated, transfection of increasing amounts of Fyn or FynY528F expression plasmid led to the expression of increasing amounts of Fyn protein (third panel from top, lanes 3 to 5 and 9 to 11). Expression of Fyn or FynY528F proteins resulted in prominent tyrosine phosphorylation of three species (top panel, lanes 3 to 5 and 9 to 11): transfected Fyn, the CD8-ζ chimera, and a 62-kDa endogenous substrate (p62) whose identity is unknown. Phosphorylation of all three proteins increased as Fyn expression levels increased. Coexpression of a fixed amount of Cbl along with Fyn or FynY528F resulted in prominent Fyn-dependent phosphorylation of the expressed Cbl protein. In contrast, tyrosine phosphorylation of Fyn itself, the CD8-ζ chimera, and p62 were dramatically reduced (top panel, compare lanes 3 to 5 with 6 to 8 and lanes 9 to 11 with 12 to 14). While levels of the CD8-ζ protein did not appear to be affected by Cbl expression (bottom panel, lanes 3 to 5 and 6 to 8), protein levels of both Fyn and FynY528F were reduced by Cbl overexpression (third panel from top, lanes 3 to 8 and 9 to 14). Densitometric analysis indicated that FynY528F protein levels were reduced 2- to 3.3-fold by Cbl coexpression while a less pronounced effect was observed on wild-type Fyn (0.3-fold).
FIG. 2.
Overexpression of Cbl decreases the phosphorylation of in vivo Fyn substrates, reduces the steady-state pools of Fyn proteins, and inhibits the Fyn- or FynY528F-dependent transactivation of an SRE-luciferase reporter. (A) 293T cells were transfected with the indicated amounts of pAlterMAX-Fyn or FynY528F and 0.5 μg of pSRαneo-CD8-ζ, with or without the pAlterMAX-HA-Cbl expression construct (1 μg). Cell lysates were prepared 48 h posttransfection, and 15-μg aliquots of cell lysate were resolved by SDS-PAGE, transferred to a PVDF membrane, and immunoblotted with the indicated antibodies. Fyn protein levels were determined as described in Materials and Methods. This experiment was performed three times, with similar results in each case. (B) 293T cells were transfected with the SRE-luciferase reporter construct (5 μg), together with pSRαneo-CD8-ζ (0.5 μg), pAlterMAX-Fyn or FynY528F (0.1 μg), and pAlterMAX-HA-Cbl (1 μg) expression plasmids as indicated. At 48 h posttransfection, the cells were lysed and equal amounts of protein were used to assay luciferase activity. Results are shown as the mean and 1 standard deviation (SD) based on five replicate transfections, and are expressed relative to the luciferase activity of the mock transfectant, which was arbitrarily set at 1. (C) Fyn and Cbl protein expression from panel B was determined by separating 7.5-μg aliquots of three replicates by SDS-PAGE, transferring them to a PVDF membrane, and immunoblotting with the appropriate antibodies. Relative Fyn protein levels are shown above the Fyn immunoblot. (D) 293T cells were transfected with pAlterMAX-FynY528F (0.1 μg), pSRαneo-CD8-ζ (0.5 μg), and the SRE-luciferase (5 μg) constructs, along with the indicated amounts of pAlterMAX-HA-Cbl. At 48 h posttransfection, the cells were lysed and SRE-luciferase activity was measured as in panel B. Results are shown as the mean and 1 SD based on five replicate transfections and are expressed relative to the luciferase activity of the mock transfectant, which was arbitrarily set at 1. (E) Aliquots (15 μg) of the cell lysates used in panel D were resolved by SDS-PAGE, transferred to a PVDF membrane, and immunoblotted with either anti-HA (top panel) or anti-Fyn (bottom panel) antibodies. The relative Fyn protein levels are shown above the Fyn immunoblot. Tyr(P), phosphotyrosine.
The effect of Cbl on Fyn tyrosine kinase-dependent cellular signaling was then assessed in a reporter assay with the SRE from the early growth response gene 1 promoter linked to the firefly luciferase gene (1). Src-family kinases enhance gene transcription regulated via this element (53), and the SRE-luciferase reporter assay has been used as a measure of Src-family kinase activation in vivo (1).
293T cells were transfected with Fyn or FynY528F, alone or in combination with Cbl, or were mock transfected. Each transfection was performed in replicates of five, and luciferase activity was determined 48 h posttransfection. As previously observed with other Src-family kinases (1), expression of wild-type Fyn led to a small but reproducible increase in SRE-luciferase activity compared to mock-transfected cells while FynY528F expression induced a 14-fold increase in SRE-luciferase activity (Fig. 2B). Significantly, both Fyn- and FynY528F-induced SRE-luciferase activity could be suppressed by Cbl coexpression. Expression of Cbl alone had no effect on SRE-luciferase activity (data not shown; see Fig. 3C). Lysates from this experiment were also analyzed by immunoblotting to determine the relative levels of Fyn expression. As observed in Fig. 2A, Cbl expression caused a reduction in the protein levels of both Fyn and FynY528F that mirrored the reduction in SRE-luciferase activity (Fig. 2C, bottom panel).
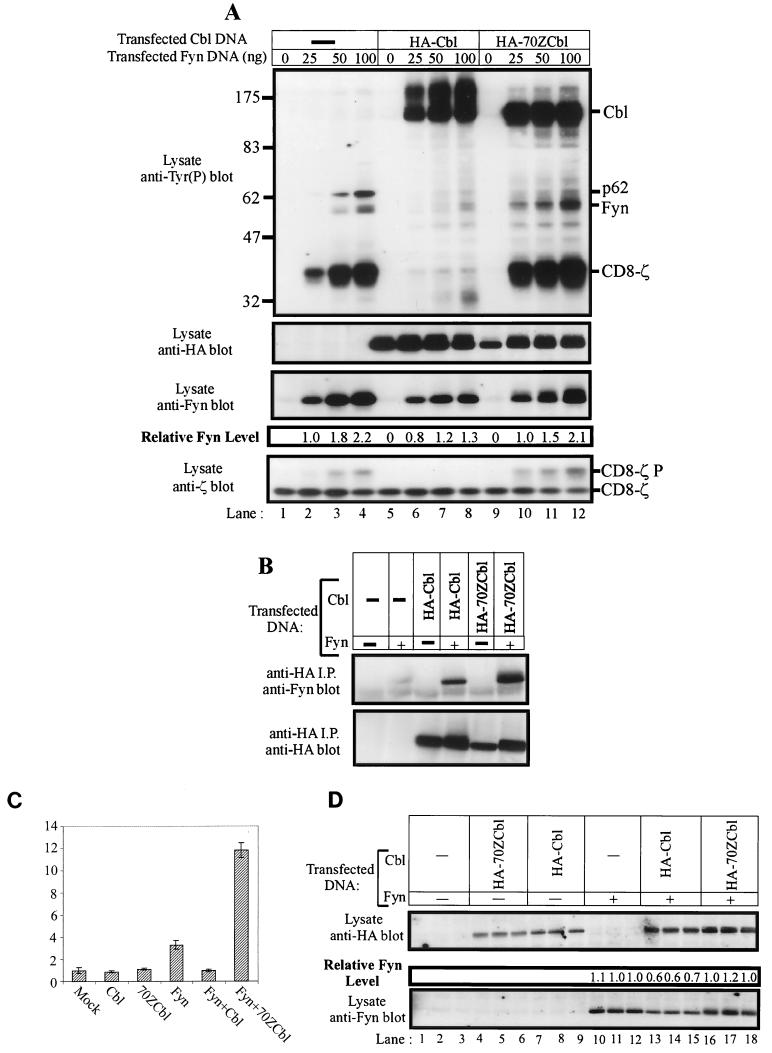
FIG. 3.
70ZCbl enhances Fyn autophosphorylation, phosphorylation of in vivo substrates, and SRE-luciferase transactivation. (A) The indicated amounts of pAlterMax-Fyn and 0.5 μg of pSRαneo-CD8-ζ were transfected into 293T cells, either alone or in the presence of 1 μg of pAlterMax-HA-Cbl or pAlterMAX-HA- 70ZCbl. Aliquots (10 μg) of cell lysates were separated by SDS-PAGE, transferred to a PVDF membrane, and immunoblotted with the indicated antibodies. Relative Fyn protein levels were determined as described in Materials and Methods. (B) Lysates (250 μg) from panel A (lanes 1, 4, 5, 8, 9, and 12) were subjected to anti-HA immunoprecipitation (i.p.). Bound proteins were separated by SDS-PAGE, transferred to a PVDF membrane, and blotted with either anti-HA or anti-Fyn antibodies. (C) 293T cells were transfected with the SRE-luciferase reporter construct (5 μg), pSRαneo-CD8-ζ (0.5 μg), and the indicated combinations of pAlterMAX-Fyn (0.1 μg), pAlterMAX-HA-Cbl, or pAlterMAX-HA-70ZCbl (1 μg) expression plasmids. At 48 h posttransfection, the cells were lysed and equal amounts of protein were used to assay luciferase activity. Luciferase activity was determined, and the results were expressed relative to the luciferase activity of the mock transfectant, which was arbitrarily set at 1. Results represent the mean and 1 SD of five replicate transfections. (D) Aliquots (10 μg) of the cell lysates used in panel C were resolved by SDS-PAGE, transferred to a PVDF membrane, and immunoblotted with either anti-HA (top panel) or anti-Fyn (bottom panel) antibodies. Relative Fyn protein levels are shown above the anti-Fyn immunoblot. Tyr(P), phosphotyrosine.
Finally, a titration experiment was performed in which 293T cells were transfected with FynY528F alone or with increasing amounts of the pAlterMAX-Cbl expression construct. The expression of FynY528F produced an 11-fold increase in SRE-luciferase activity relative to the mock transfectant (Fig. 2D). Coexpression of increasing amounts of wild-type Cbl with FynY528F led to a dose-dependent reduction in SRE-luciferase activity (Fig. 2D). Analysis of cell lysates by anti-Fyn immunoblotting revealed that Fyn protein levels decreased (Fig. 2E, bottom panel) as Cbl protein levels increased (Fig. 2E, top panel), again mirroring the reduction in SRE-luciferase activity. This series of experiments indicates that coexpression of Cbl results in a decrease in the activity of the Src-family kinase Fyn in vivo and, surprisingly, that the reduction in Fyn activity is accompanied by a reduction in steady-state Fyn protein levels. Thus, in contrast to previously examined SH3 domain ligands that induce Fyn activation, Cbl causes Fyn suppression. Furthermore, this effect was more pronounced with FynY528F, suggesting that the effect of Cbl is independent of the intramolecular mechanism of Fyn regulation.
An oncogenic mutant form of Cbl is incapable of inhibiting Fyn activity in vivo.
We next performed a series of experiments in which the effect of wild-type Cbl on Fyn was compared to that of 70ZCbl. 70ZCbl is a mutant with a 17-amino-acid (amino acids 366 to 382) deletion near the N-terminal boundary of the RING finger domain. This form of Cbl is acutely transforming when overexpressed in NIH 3T3 cells and deregulates endogenous tyrosine kinase-mediated signaling cascades in a number of systems (3, 4, 6, 69).
Wild-type Cbl or 70ZCbl was expressed in 293T cells either alone or with increasing amounts of pAlterMax-Fyn expression construct, as indicated in Fig. 3A. As noted above, phosphorylation of the CD8-ζ chimera and the p62 substrate increased as the amount of Fyn construct transfected was increased (lanes 1 to 4). Coexpression of Cbl induced a significant reduction in the amount of phosphorylated Fyn signals and in the phosphotyrosine signals of CD8-ζ and p62 (lanes 6 to 8). Reduced Fyn protein levels again accompanied the decrease in phosphotyrosine signal (top and third panels, compare lanes 2 to 4 with lanes 6 to 8). Conversely, coexpression of 70ZCbl caused an increase in the level of CD8-ζ and Fyn tyrosine phosphorylation. This was particularly obvious at the smallest amounts of input Fyn construct (compare lanes 2 and 10). Phosphorylation of the p62 substrate was still reduced by 70ZCbl coexpression. A possible explanation for this finding is that binding of p62 substrate via the Fyn SH3 domain may be required for phosphorylation to take place. Therefore, overexpression of 70ZCbl would be able to reduce the amount of Fyn available to interact with p62 via the Fyn SH3 domain with a concomitant reduction in phosphorylation of p62. Significantly, no reduction in Fyn protein levels was observed following 70ZCbl coexpression. To ensure that the observed differences were not due to the inability of 70ZCbl to bind Fyn, a coimmunoprecipitation experiment was performed on the lysates used in the experiment in Fig. 3A (lanes 1, 4, 5, 8, 9, and 12). Cbl protein was immunoprecipitated with an anti-HA antibody, and bound proteins were analyzed by anti-Fyn or anti-HA immunoblotting (Fig. 3B). Fyn was found to be associated with both Cbl and 70ZCbl. Indeed, more Fyn was associated with 70ZCbl, most probably reflecting the larger amounts of Fyn protein present in the transfectants. Thus, the region deleted from 70ZCbl is not required for Cbl interaction with the Fyn protein. However, unlike wild-type Cbl, 70ZCbl does not negatively regulate Fyn; instead, it may even enhance its activity.
The effect of 70ZCbl on Fyn was then tested by the SRE-luciferase reporter assay. 293T cells were transfected with Cbl, 70ZCbl, or Fyn alone or the indicated combinations of constructs. Expression of Cbl or 70ZCbl in isolation did not significantly alter the basal SRE-luciferase activity compared to mock-transfected cells (Fig. 3C). Expression of Fyn caused an increase in SRE-luciferase activity of approximately threefold, which was abolished by coexpression of wild-type Cbl. Strikingly, coexpression of 70ZCbl with Fyn resulted in a significant enhancement of SRE-luciferase activity above that observed with Fyn alone (3.3- and 11.8-fold, respectively). The reduction in SRE-luciferase activity caused by Cbl coexpression paralleled the reduction of Fyn protein levels in the same lysates, while 70ZCbl did not seem to significantly affect Fyn levels (Fig. 3D). These data strongly suggest that wild-type Cbl protein negatively regulates the in vivo activity of Fyn, while 70ZCbl, a known transforming mutant, enhances Fyn activity.
The Cbl-dependent decrease in Fyn protein levels is the result of enhanced turnover.
An important observation made during the previous series of experiments was that Cbl-mediated negative regulation of Fyn coincided with a reduction in Fyn protein levels. This effect was unlikely to have been a transfection artifact, since no effect on Fyn protein levels was observed when equivalent amounts of the 70ZCbl expression construct were used. A likely explanation for the Cbl-dependent decrease in Fyn or FynY528F protein levels was that Cbl facilitated their degradation; however, a potential effect of Cbl on Fyn protein synthesis could not be excluded based on the results at hand. To differentiate between these possibilities, a metabolic pulse-chase analysis was performed in the 293T expression system.
293T cells were transfected with Fyn or FynY528F, with or without Cbl. Following processing for a pulse-chase analysis, anti-Fyn immunoprecipitates were prepared from the cell lysates, resolved by SDS-PAGE, and transferred to a PVDF membrane. Radiolabeled Fyn protein was visualized by autoradiography (Fig. 4A, top panels), and the membrane was subsequently immunoblotted with anti-Fyn antibodies to assess the steady-state amounts of Fyn protein (bottom panels).
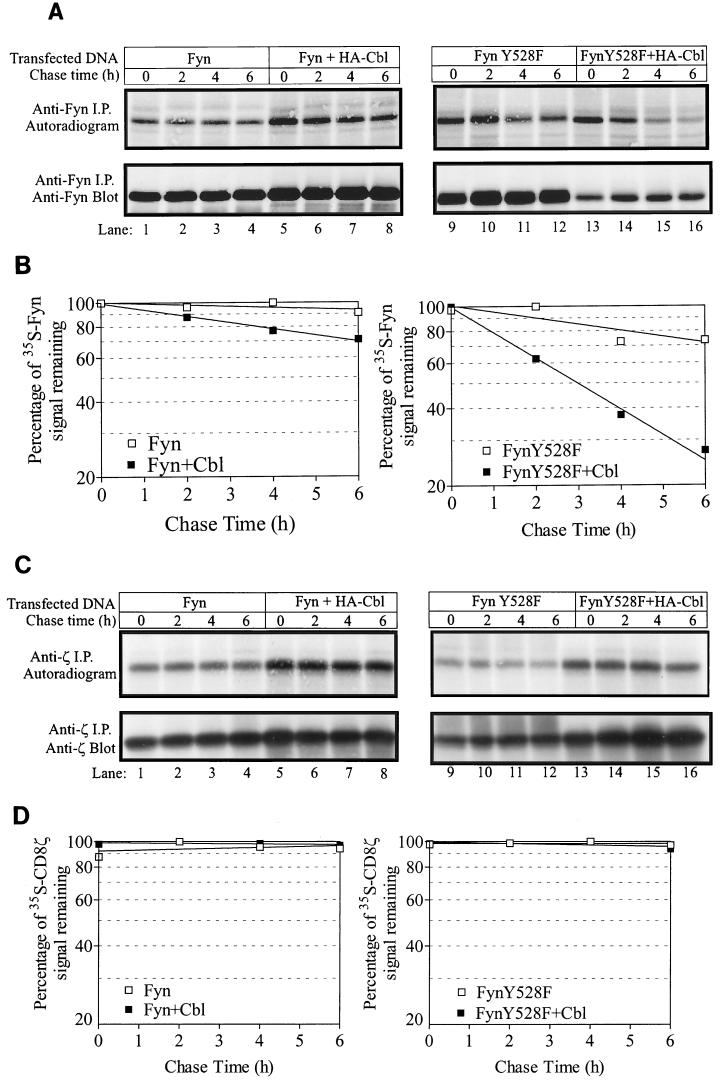
FIG. 4.
Cbl overexpression enhances the turnover of Fyn and FynY528F protein as determined by metabolic pulse-chase analysis but has no effect on the half-life of CD8-ζ. 293T cells in 100-mm tissue culture dishes were transfected with pSRαneo-CD8-ζ (0.5 μg), pAlterMAX-Fyn (0.1 pg), or FynY528F (0.1 μg) with or without the pAlterMAX-HA-Cbl expression construct (1 μg). At 48 h posttransfection, the cells were methionine starved for 1 h and then were pulse-labeled with [35S]methionine for 45 min. The pulse was chased with culture medium supplemented with unlabeled methionine for the indicated times, and cell lysates were prepared. (A) Anti-Fyn immunoprecipitates (i.p.) of cell lysates were resolved by SDS-PAGE and transferred to a PVDF membrane. Labeled Fyn protein was visualized by exposing the membrane to X-ray film for 48 h (top panel). The membrane was subsequently immunoblotted with an anti-Fyn antibody (bottom panel). (B) The signals in panel A were quantified as described in Materials and Methods, and the values at various chase times are expressed as a percentage relative to the highest 35S-Fyn signal. The line of best fit was calculated with the Prism program. A representative experiment is shown. This experiment was performed three times, with similar results in each case. (C) CD8-ζ protein was immunoprecipitated (i.p.) from lysates prepared by the same method as in panel A, and the labeled protein was visualized by autoradiography following transfer to PVDF (top panel, 16-h exposure). The membrane was then immunoblotted with a ζ-specific antibody (bottom panel). (D) Signals in panel C were quantified and expressed as for panel B.
Comparable 35S-Fyn signals were observed at time zero (no chase) in all transfectants (Fig. 4A, top panels, compare lanes 1, 5, 9, and 13), indicating that Cbl did not act at the level of transcription or translation. Labeled wild-type Fyn protein appeared very stable, with only a small decrease in 35S signal observed during the chase period. Cells transfected with both Fyn and Cbl exhibited a time-dependent decrease in 35S signal, representing a 30% loss of the signal over the chase period (Fig. 4B). A discernible reduction in 35S-FynY528F signal was observed when the protein was expressed alone or in combination with Cbl; however, Cbl coexpression resulted in a significant enhancement in the rate of signal loss (Fig. 4B, t1/2 = 12 h without Cbl and 3 h with Cbl). As a control, the CD8-ζ chain was also immunoprecipitated from lysates of 35S-labeled cells. Since Cbl does not stably associate with CD8-ζ (data not shown), this protein is suitable for detection of any nonspecific effects of Cbl such as the rate at which unlabeled amino acids quench the 35S signal during the chase period. Unlike Fyn proteins, expression of Cbl had no effect on 35S-CD8-ζ over the course of the chase period (Fig. 4C and D). Given this finding, we conclude that Cbl enhances the turnover of Fyn protein, thereby accounting for the observed decrease in Fyn protein levels observed upon coexpression with Cbl.
Cbl-mediated negative regulation of Fyn is not the result of reduced kinase activity.
One possible interpretation of the above results is that Fyn degradation is a secondary phenomenon and that Cbl mediates its effect by directly inhibiting the tyrosine kinase activity of Fyn. We attempted to address this question by performing in vitro kinase assays to determine if Cbl expression had any effect on Fyn kinase activity. This experiment was complicated by the fact that coexpression of Cbl with Fyn consistently resulted in a reduction in the steady-state pool of Fyn protein. The problem was circumvented by analyzing the kinase activity of the pool of Fyn directly associated with Cbl or 70ZCbl. We reasoned that if Cbl directly affected Fyn kinase activity, the pool associated with Cbl should have significantly reduced activity compared to that associated with 70ZCbl if comparable amounts of Fyn protein are analyzed.
The assay was performed by transfecting 293T cells with Cbl, 70ZCbl, or Fyn expression constructs, alone or in the indicated combinations. Cell lysates were prepared 48 h posttransfection, and anti-HA immunoprecipitations were performed. The immunoprecipitates were divided equally, with half of each sample being subjected to a kinase assay and the other half being analyzed by immunoblotting. The kinase activity was assessed by measuring the incorporation of 32P into the synthetic tyrosine kinase substrate Raytide. When Cbl, 70ZCbl, or Fyn proteins were singly transfected into 293T cells, minimal kinase activity was detected in anti-HA immunoprecipitates (Fig. 5A). However, when Cbl or 70ZCbl was cotransfected along with Fyn, significant kinase activity was detected in anti-HA immunoprecipitates. When a nonphosphorylatable Raytide substrate was used as a specificity control, no Cbl- or 70ZCbl-associated kinase activity was observed (data not shown).
FIG. 5.
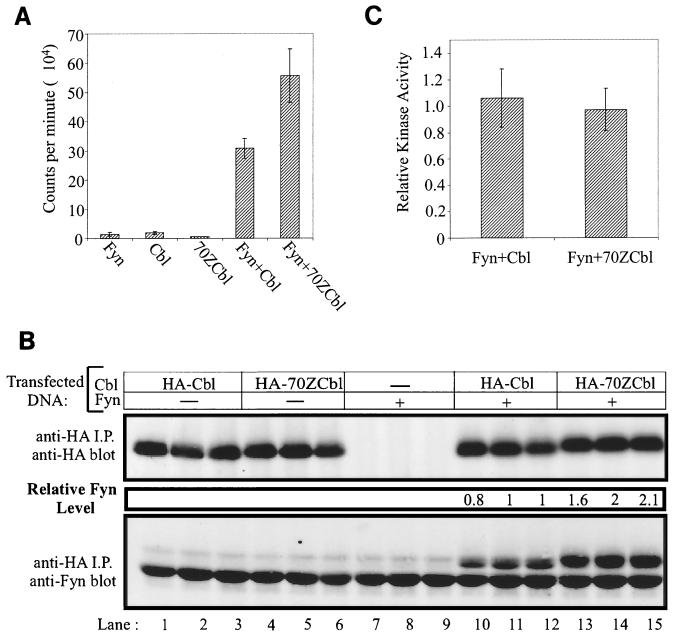
Cbl-associated Fyn kinase activity does not differ from 70ZCbl-associated Fyn activity. (A and B) 293T cells were transfected with either 1 μg of pAlterMAX-HA-Cbl or pAlterMAX-HA-70ZCbl alone or in combination with 0.05 μg of pAlterMAX Fyn. The cells were lysed 48 h after transfection, and Cbl proteins were immunoprecipitated from 900 μg of lysate with the 12CA5 anti-HA antibody. Following washing, the immunoprecipitates (i.p.) were divided equally and used for either an in vitro kinase assay (A) or immunoblot analysis (B). (A) Kinase activity associated with Cbl or 70ZCbl was determined by incubating the bound proteins in kinase buffer containing a synthetic substrate (Raytide) and [γ32P]ATP. 32P incorporation into the Raytide substrate was quantified with a Packard 1600TR liquid scintillation counter. Results are expressed as the mean and 1 SD of three replicates. (B) Fyn associated with Cbl or 70ZCbl was visualized by immunoblotting and quantified as described in Materials and Methods. Data from triplicate samples are shown for each transfection condition. (C) Fyn kinase activities in panel A were normalized for the Fyn protein levels observed in panel B. This experiment was conducted twice, with similar results observed in each case.
The kinase activity of Fyn associated with Cbl was approximately half that of Fyn associated with 70ZCbl (Fig. 5A, mean = 30,839 counts for Fyn plus Cbl and 55,677 counts for Fyn plus 70ZCbl). The amount of Fyn protein associated with Cbl or 70ZCbl was determined by immunoblotting anti-HA immunoprecipitates with anti-Fyn antibodies. This analysis revealed that approximately twofold more Fyn was associated with 70ZCbl than with wild-type Cbl (Fig. 5B). When kinase activity was normalized for Fyn protein levels, no significant difference in Fyn activity associated with wild-type Cbl versus 70ZCbl was observed (Fig. 5C). This experiment strongly suggests that the negative regulatory effect of Cbl on the Src-family kinase Fyn is not mediated by a direct inhibition of Fyn kinase activity.
Coexpression of Cbl reduces FynY528F protein levels in Jurkat T lymphocytes.
Fyn and Lck are critical in the initiation of signaling events following T-cell receptor engagement (72). Notably, Cbl mRNA levels are higher in hematopoietic cells than in other cell types, with the highest levels being observed in thymocytes (28). This suggests a particularly important role for Cbl in these cells. Therefore, we wished to determine if Cbl-mediated negative regulation of Fyn, observed in 293T cells, could also be demonstrated in lymphoid cells.
A derivative of the Jurkat T-lymphocytic cell line that expresses SV40 large T antigen (Jurkat JMC-T) was electroporated with the FynY528F expression vector alone or in combination with Cbl. Total-cell lysates were prepared 48 h posttransfection and analyzed by antiphosphotyrosine immunoblotting. Transfection of the FynY528F expression construct led to detection of a prominent tyrosine-phosphorylated band of approximately 55 to 60 kDa (Fig. 6A, top panel, lane 2); anti-Fyn reprobing of this membrane showed that this band corresponded to FynY528F (bottom panel, lane 2). Cotransfection of Cbl with FynY528F led to a marked reduction in the level of FynY528F phosphorylation (top panel, lane 3). This reduction in phosphorylation correlated with a reduction in the FynY528F protein level (bottom panel, compare lanes 2 and 3).
FIG. 6.
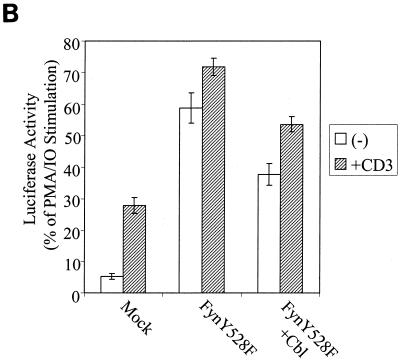
Enhanced Cbl-dependent degradation of transfected FynY528F protein in Jurkat T-lymphocytes correlates with reduced SRE-luciferase activity. (A) Jurkat JMC-T cells were electroporated with pAlterMAX-FynY528F (5 μg) together with either the pAlterMAX vector or pAlterMAX-HA-Cbl expression plasmid (20 μg). At 48 h posttransfection, cell lysates were prepared, and proteins from 3 × 105 cell equivalents were resolved by SDS-PAGE, transferred to a PVDF membrane, and immunoblotted with antiphosphotyrosine Tyr(P) (top panel), anti-HA (middle panel), or anti-Fyn (bottom panel) antibodies. (B) Cells were transfected as in panel A, with the addition of SRE-luciferase reporter construct (10 μg). At 24 h posttransfection, the cells were washed and either left unstimulated (−) or stimulated by addition of anti-CD3 antibody or a combination of PMA and ionomycin (IO) for 6 h. The SRE-luciferase activity was determined, and the data are expressed as a percentage of the maximal stimulation observed in correspondingly transfected cells cultured with PMA and ionomycin. Each error bar represents the mean and 1 SD based on five replicates.
To assess the effect of Cbl on distal signaling events mediated by FynY528F expression in JMC-T cells, the SRE-luciferase reporter assay was used. The indicated constructs were transfected into JMC-T cells by electroporation. After 24 h, the cells were mock treated or were stimulated either with an anti-CD3 antibody or a combination of PMA and ionomycin. After 6 h of stimulation, the cells were lysed and the SRE-luciferase activity was determined (Fig. 6B). The results are expressed as a percentage of the maximal transcriptional activation achieved upon cell stimulation with PMA and ionomycin.
Mock-transfected cells exhibited low basal SRE-luciferase activity, which increased approximately sixfold following anti-CD3 stimulation. Overexpression of FynY528F in JMC-T cells resulted in a dramatic elevation of the basal level of SRE-luciferase activity, similar to results obtained with 293T cells (Fig. 2B and D). Following anti-CD3 antibody stimulation of JMC-T cells, only a small increase in SRE-luciferase activity was observed; this result is consistent with the constitutive kinase activity of FynY528F. Coexpression of Cbl along with FynY528F resulted in a significant reduction in the level of SRE-luciferase activity in unstimulated as well as in anti-CD3-stimulated cells. Taken together, these results demonstrate that the Cbl-mediated enhancement of Fyn protein degradation and the reduction in Fyn signaling function is not limited to 293T cells but can be reproduced in a lymphoid-cell environment.
Cbl-deficient cell lines have increased Fyn protein levels.
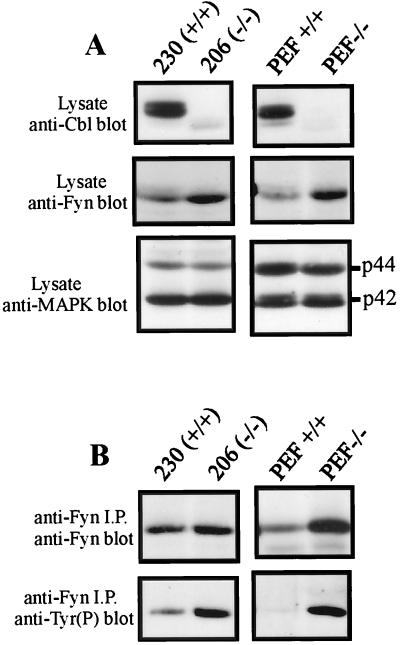
Finally, cell lines derived from Cbl−/− mice were examined for evidence of Fyn deregulation. T-cell lines were generated from Cbl knockout mice (45) or normal littermate controls by intraperitoneal injection of newborn mice with Moloney murine leukemia virus, followed by in vitro culture of the resulting lymphomas. The cell lines were designated 230 (derived from a wild-type mouse) and 206 (derived from a Cbl−/− mouse). Immortalized fibroblast lines were also established by long-term in vitro culture of embryonic fibroblasts derived from the appropriate mice (designated PEF+/+ and PEF−/−). Immunoblotting of equal amounts of total cellular protein with an anti-Cbl antibody revealed a prominent band of the expected size in cells derived from wild-type mice and its absence in cells derived from Cbl−/− mice (Fig. 7A, top panels).
FIG. 7.
Fyn phosphorylation and protein levels are increased in Cbl−/− cell lines. (A) Total-cell lysates from the Cbl−/− T lymphoma cell line 206 and control line 230 (150 μg) were separated by SDS-PAGE and transferred to a PVDF membrane. Similarly, total cellular protein (200 μg) from the Cbl−/− fibroblast line PEF−/− or the control line PEF+/+ was immunoblotted with anti-Cbl (top panel), anti-Fyn (middle panel), or anti-MAP kinase (MAPK) (bottom panel) antibody. (B) Fyn protein was immunoprecipitated (i.p.) from T-cell or fibroblast lysates, resolved by SDS-PAGE, and transferred to a PVDF membrane. The membrane was then probed with anti-Fyn antibody, stripped, and reprobed with antiphosphotyrosine [Tyr(P)] antibody.
Significantly, anti-Fyn immunoblots indicated that both the T-cell and fibroblast cell lines derived from Cbl−/− mice had higher levels of endogenous Fyn protein than did the control cell lines (Fig. 7A, center panels). This result was not nonspecific or due to an error in protein estimation: reimmunoblotting of the membranes with an anti-MAP kinase-specific antibody revealed equivalent signals in lysates of wild-type and Cbl−/− cells (Fig. 7A, bottom panels). Furthermore, the phosphotyrosine content of Fyn proteins immunoprecipitated from the Cbl−/− lines was significantly higher than that of Fyn in the wild-type cell lines (Fig. 7B, bottom panels). These results provide evidence complementary to Cbl overexpression analysis and further strengthen the idea that Cbl plays a role in regulation of Fyn protein levels.
The Fyn SH3 domain and Cbl's TKB domain both participate in Cbl-dependent negative regulation of Fyn, apparently in a redundant manner.
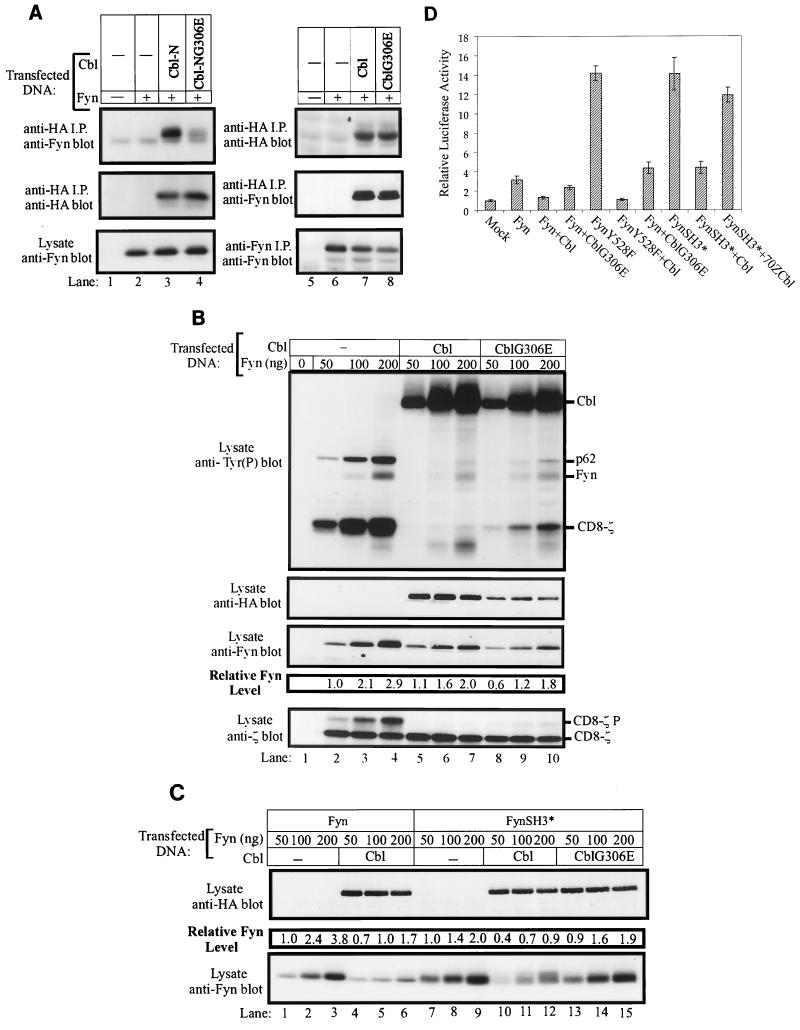
The TKB domain of Cbl is capable of directly interacting with a number of tyrosine kinases in a phosphotyrosine-dependent manner, and this interaction is critical for Cbl-dependent negative regulation of ZAP70/Syk, PDGFR, and EGFR tyrosine kinases (30, 34, 36, 43, 68). To determine whether the TKB domain of Cbl is capable of interacting with Fyn, we transfected 293T cells with Fyn together with constructs encoding either HA-Cbl-N or its TKB domain-inactive mutant, HA-Cbl-NG306E. Anti-HA immunoprecipitates were assessed for Cbl-associated Fyn (Fig. 8A). The association of Fyn with Cbl-N could be readily detected (lane 3). In contrast, the TKB domain mutant Cbl-NG306E failed to associate with Fyn (lane 4). These observations indicated that the TKB of Cbl domain can associate with Fyn. Significantly, full-length wild-type Cbl and its G306E mutant protein were found to associate with wild-type Fyn (Fig. 8A). Thus, a functional TKB domain is not required to mediate a stable interaction between the wild-type forms of Fyn and Cbl.
FIG. 8.
A functional TKB domain is required for Cbl-induced negative regulation of the FynSH3* mutant protein. (A) 293T cells in 100-mm tissue culture dishes were transiently transfected with pAlterMAX-Fyn expression construct (0.1 μg) along with the pAlterMAX constructs encoding HA-Cbl-N or HA-CblNG306E or constructs encoding HA-Cbl or HA-CblG306E (1 μg). HA-tagged proteins were immunoprecipitated (i.p.) from 200 μg of cell lysate with the 12CA5 antibody, and immune complexes were resolved by SDS-PAGE, transferred to a PVDF membrane, and immunoblotted with anti-Fyn (top panel) or anti-HA (middle panel) antibodies. Fyn expression levels were evaluated by either anti-Fyn immunoblotting of 15 μg of total-cell lysate (lanes 1 to 4) or immunoblots of anti-Fyn immunoprecipitates from 100 μg of cell lysate (lanes 5 to 8). (B) 293T cells were transiently transfected with pAlterMAX vector (lane 1) or the indicated amounts of pAlterMAX-Fyn construct and 0.5 μg of pSRαneo-CD8-ζ expression construct, without or with the pAlterMAX-HA-Cbl or pAlterMAX-HA-CblG306E construct (1 μg). At 48 h after transfection, the cells were lysed and 15-μg aliquots were resolved by SDS-PAGE, transferred to a PVDF membrane, and immunoblotted with the indicated antibodies. Fyn protein levels were determined as described in Materials and Methods. Tyr(P), phosphotyrosine. (C) 293T cells were transfected with the indicated amounts of pAlterMAX-Fyn or pAlterMAX-FynSH3* expression constructs, along with 1 μg of pAlterMAX vector, pAlterMAX-HA-Cbl, or pAlterMAX-HA-CblG306E expression plasmids. Cell lysates were treated as in panel B and immunoblotted with either anti-HA or anti-Fyn antibodies as indicated. Fyn protein levels were determined as described in Materials and Methods. (D) 293T cells were transfected with the SRE-luciferase reporter (5 μg) and the indicated pAlterMAX Fyn constructs (0.05 μg), without or with pAlterMAX-HA-Cbl or pAlterMAX-HA-CblG306E expression plasmid (1 μg). Luciferase activity was determined, and the results were expressed relative to the luciferase activity of the mock transfectant, which was arbitrarily set at 1. Results represent the mean and 1 SD of five replicate transfections.
The functional role of the Cbl TKB domain in the negative regulation of Fyn was then tested by coexpressing CblG306E with Fyn, and comparing its effect to that of wild-type Cbl (Fig. 8B). Coexpression of CblG306E with Fyn induced a marked decrease in the pool of autophosphorylated Fyn, as well as a marked decrease in phosphorylation of CD8-ζ and p62 (top panel, compare lanes 8 to 10 with lanes 2 to 4). This effect was similar to that induced by wild-type Cbl (top panel, compare lanes 5 to 7 with lanes 8 to 10). Furthermore, Fyn levels were comparably decreased by coexpressing Fyn with either Cbl or CblG306E (third panel from top, compare lanes 5 to 7 and 8 to 10 with lanes 2 to 4). Since an inactivating amino acid substitution in the SH3 domain of Fyn significantly reduced the ability of Fyn to associate with Cbl (Fig. 1B, lane 6), we focused on the role of SH3-mediated Fyn-Cbl interactions as a potential explanation for the phenotype of CblG306E.
293T cells were transfected with increasing amounts of the FynSH3* mutant alone or in combination with constant amounts of Cbl or CblG306E expression vector (Fig. 8C). As expected, coexpression of Cbl with wild-type Fyn caused a reduction in wild-type Fyn protein levels (Fig. 8C, bottom panel, compare lanes 1 to 3 with lanes 4 to 6). Strikingly, coexpression of Cbl also led to a significant reduction in FynSH3* protein levels (Fig. 8C, bottom panel, compare lanes 7 to 9 with lanes 10 to 12). These results suggested that a mechanism distinct from Fyn SH3-Cbl binding was responsible for recruiting Cbl as a negative regulator to the FynSH3* protein. Consistent with the possibility that the Cbl TKB domain-mediated interaction accounted for this functional effect, CblG306E failed to decrease FynSH3* protein levels (Fig. 8C, bottom panel, compare lanes 7 to 9 with lanes 13 to 15).
To further establish the distinct yet redundant roles of the TKB domain of Cbl and the SH3 domain of Fyn in mediating Cbl-dependent regulation of Fyn, additional analyses were performed by the SRE-luciferase reporter assay. 293T cells were transfected with wild-type Fyn, FynY528F, or FynSH3*, either alone or in combination with Cbl or Cbl G306E (Fig. 8D). Expression of the FynSH3* mutant resulted in a high level of SRE-luciferase activity, comparable to that in cells expressing the FynY528F mutant. This is consistent with previous results in which mutation of the Fyn SH3 domain was found to enhance the kinase activity of the protein (9). A significant reduction in SRE-luciferase activity was observed when wild-type Cbl was expressed in combination with either FynY528F or FynSH3* (Fig. 8D). In contrast, CblG306E expression did not reduce the SRE-luciferase activity in cells transfected with the FynSH3* mutant, but it was capable of inhibiting the activity in cells expressing FynY528F. Taken together, these results strongly indicate that both Fyn SH3 domain binding to the Cbl proline-rich region and Cbl TKB domain binding to an unidentified motif(s) in Fyn mediate interactions leading to negative regulation of Fyn via protein degradation.
DISCUSSION
All nine members of the Src tyrosine kinase family contain conserved structural domains that are required for the regulation of kinase activity and mediate interactions with signaling proteins following activation. Mutational analyses and crystal-structure studies of Src-family kinases have demonstrated that kinase activity is regulated by intramolecular binding of the SH3 domain to the SH2-kinase linker and of the SH2 domain to the negative regulatory phosphorylation site near the C terminus. The importance of these interactions in the repression of kinase activity is highlighted by the fact that mutation of the SH3 or SH2 domains or their intramolecular binding sites results in unregulated kinase activity (32). While the intramolecular model provides an elegant mechanism by which Src kinases are maintained in an inactive form, the processes involved in the activation and subsequent inactivation of Src-family kinases have not been determined. Given that Cbl associates with multiple members of the Src kinase family (2, 14, 17, 18, 48, 51, 54, 56, 67, 70) and given its ability to function as an activation-dependent negative regulator of other tyrosine kinases (6, 30, 34, 43), we investigated the potential role of Cbl as a negative regulator of a prototype Src-family kinase, Fyn.
The previously studied tyrosine kinase targets of Cbl, such as ZAP70, Syk, PDGFR, and EGFR, require activation-induced phosphorylation to physically interact with Cbl (6, 30, 34, 43). In contrast, earlier analyses with lymphocytic and other cell types indicated that stable complexes of Cbl with Fyn, Lck, Src, and Lyn can be found prior to cellular activation (2, 14, 17, 18, 48, 51, 54, 56, 67, 70). In vitro studies suggested that a likely mechanism for this basal association was the ability of the SH3 domains of Src-family kinases to interact with proline-rich sequences in Cbl (14, 17, 18, 54, 56). Using Cbl mutants in which parts of the proline-rich region were deleted and an inactivating point mutation of the Fyn SH3 domain, we demonstrated that this mechanism predominates in vivo. Nonetheless, Fyn SH2 domain binding to one or more phosphotyrosines on Cbl also appeared to contribute to the Fyn-Cbl interaction. Consistent with the Fyn SH2 domain mediating an association of Fyn with Cbl, the three dominant phosphorylation sites in Cbl (amino acid residues Y700, Y731, and Y774) have been found to provide binding sites for Src-family kinase SH2 domains (16). The combined SH2- and SH3-dependent mechanisms mediate a relatively stable association of Cbl with Fyn, with approximately 25% of the expressed Fyn protein being complexed to Cbl under our experimental conditions (data not shown). It should be noted that our results do not allow us to conclude whether the various mechanisms of physical interaction function concurrently or whether distinct pools of Fyn-Cbl complexes use different interactions. Together, these data clearly suggest a complex mechanism of physical interaction between Cbl and Src-family kinases.
The fact that the SH3 domain of a Src-family kinase (Fyn) mediated complex formation with a cellular polypeptide (Cbl) and that this complex was readily detectable in vivo raised an apparent paradox in view of the current model of Src-family kinase regulation. Based on the intramolecular-repression model of Src-family kinase regulation, ligand binding to the SH3 domain is predicted to displace it from the SH2-kinase linker and to lead to kinase activation. Indeed, in vitro and in vivo analyses of the interaction of the HIV Nef protein with Hck and of Src coexpressed with a fragment of Sin protein support this prediction (1, 7, 44). An obvious question was whether binding of Cbl to the SH3 domain of Fyn would lead to activation of the kinase or whether Cbl might use binding via the SH3 domain to promote negative regulation of Fyn. Our results demonstrate that association of Fyn with Cbl leads to a dramatic reduction in the active pool of Fyn. This conclusion was based on multiple criteria: Cbl dose-dependent reduction in the overall levels of autophosphorylated Fyn, decreased phosphorylation of endogenous (p62) and exogenously introduced (CD8-ζ) in vivo substrates of Fyn, and reduced transcriptional activation of a Src-family kinase-responsive promoter element. Thus, while displacement of the SH3 domain from the SH2-kinase linker can lead to activation of Src-family kinases, dissociation followed by SH3 domain-dependent association of Fyn with Cbl reduces kinase activity.
Identification of a role for Cbl in the negative regulation of the Src-family kinase Fyn raises questions about its relative biological significance, in comparison to the previously established model of intramolecular regulation. While more detailed studies are needed to definitively answer this important question, our results support a complementary role for the two mechanisms. Intramolecular regulation of Src kinases can be disrupted by mutation of the negative regulatory C-terminal tyrosine (Y528 in Fyn), resulting in the constitutive activation of kinase activity. We used the FynY528F mutant to ask if Cbl-dependent negative regulation of Fyn could still be observed. The results presented here clearly demonstrate that FynY528F not only retains its susceptibility to the negative regulatory effects of Cbl but also is more susceptible to this effect than is the wild-type Fyn. These results suggest that Cbl-mediated negative regulation would be important in situations where Fyn intramolecular associations have been disrupted, as is predicted to occur upon activation of cell surface receptors.
An unexpected finding was the observation that the Cbl-dependent reduction in autophosphorylated Fyn and inhibition of distal signaling events was consistently accompanied by a reduction in Fyn protein levels. The effect of Cbl on Fyn levels correlated with its impact on Fyn kinase activity: both effects were more pronounced for the FynY528F mutant than for wild-type Fyn. Significantly, coexpression of the 70ZCbl mutant with Fyn did not affect Fyn activity or alter Fyn protein levels, implying that the effect of Cbl on Fyn protein levels was not a transfection artifact. These results suggested that Cbl may regulate Fyn activity by mediating the degradation of activated Fyn protein. This hypothesis was tested by pulse-chase analysis, which directly demonstrated that the Cbl-dependent decrease in Fyn protein levels resulted from enhanced Fyn-specific protein turnover. Cbl-mediated reduction of Fyn protein levels was also observed in Jurkat T-lymphocytic cells, indicating that Cbl-dependent degradation of Fyn is likely to occur widely. Complementing these analyses based on Cbl overexpression, we also demonstrated that fibroblast and T-lymphoma cell lines derived from Cbl−/− mice express higher levels of Fyn protein than do cell lines derived from wild-type mice. Significantly, the observed half-life of FynY528F was shorter than that of wild-type Fyn following coexpression of Cbl in 293T cells, implying that the catalytically active pool of Fyn is targeted for degradation. Consistent with this suggestion, the E6AP-mediated degradation of Blk (see below for further details) required a catalytically active kinase (47). Together, these results provide strong support for a role for Cbl in regulating Fyn protein levels in vivo. At present it is not clear whether the other two Cbl-family members, Cbl-b and Cbl-3, also exert an effect on Src-family kinases. Given the high structural similarity of the TKB and RING finger domains among the Cbl-family members, such a role is not unlikely. However, direct analysis is required to determine if Cbl-b and Cbl-3 provide redundant and/or complementary roles to Cbl in the regulation of Src-family kinases.
The possibility that the reduction in Fyn protein levels was a secondary effect of Cbl-mediated inhibition of Fyn kinase activity was also tested. While the total amount of Fyn kinase activity associated with Cbl was smaller than that associated with 70ZCbl, normalization of the data for Fyn protein levels revealed that there was no significant difference in Fyn specific activity. This result is consistent with those published previously in which Cbl was found in complex with catalytically active c-Src in osteoclast and macrophage cell lines (49, 66). Together, these results lead us to conclude that Cbl does not significantly affect Fyn kinase activity and that the most likely explanation for the observed negative regulation of Fyn activity by Cbl is that Cbl enhances the rate of Fyn degradation.
A correlation between an increase in the kinase activity of Src-family members and a concurrent reduction in protein levels has been observed in a number of experimental systems. For example, the specific kinase activity of Fyn is increased in peripheral blood mononuclear cells of asymptomatic HIV patients compared to that in uninfected controls or patients with unrelated diseases (52). Notably, while the kinase activity of Fyn is increased, Fyn protein levels are markedly reduced (52). Similarly, a dramatic reduction in the protein levels of c-Src, Fyn, and Lyn has been observed in Csk-deficient cells concurrent with increased kinase activity (25, 46). Finally, it has been reported that stimulation of rat fibroblasts with a combination of PDGF and transforming growth factor β1 led to degradation of c-Src (19). Since transforming growth factor β1 treatment alone has no effect on Src protein levels, these results imply that activation of Src by PDGF was important for Src degradation (19).
These observations, in conjunction with the results presented in this paper, implicate protein degradation as one mechanism for downregulation of activated Src-family kinases. Cbl enhances the level of ubiquitination and rate of degradation of EGFR and PDGFRα (30, 43, 73). The RING finger and surrounding sequences are required for Cbl-mediated enhancement of EGFR degradation, since 70ZCbl or other Cbl proteins bearing mutations in this area fail to exert this effect and are incapable of mediating in vitro ubiquitination of the EGFR (30, 73). Indeed, 70ZCbl did not affect Fyn protein levels in our 293T transient-transfection system, suggesting the possibility that the mechanism by which Cbl enhances the rate of Fyn degradation is also mediated via a ubiquitin-dependent process. Such a possibility is supported by a recent report of the degradation of the Src-family kinase Blk via a ubiquitin-dependent mechanism (47). Oda et al. found that E6AP, an E3 ubiquitin ligase, could associate with and enhance the rate of Blk degradation upon pervanadate treatment of cells (47). Furthermore, multiubiquitinated forms of Blk were detected, and degradation of Blk was blocked by a proteasome inhibitor, MG132 (47). Significantly, while the present paper was under review, Cbl was reported to interact with a ubiquitin-conjugating enzyme (E2) via its RING finger domain and to function as an E3 ubiquitin ligase by promoting the transfer of ubiquitin from E2 to a substrate protein (26). These recent observations suggest a model in which Cbl may either function as an E3 and allow a RING finger-associated E2 to ubiquitinate activated Src-family kinases or function primarily as a scaffold to juxtapose Cbl-associated E2 with Src-family kinase-associated E3 to promote ubiquitination. Further analysis is required to clarify the role of the ubiquitin/proteasome pathway in Cbl-dependent Src-family kinase degradation and to determine whether Cbl-dependent degradation requires E6AP or other ubiquitin ligases. Taken together, our results indicate that Cbl-mediated degradation may complement the known mechanisms of Src-family kinase regulation, namely, dephosphorylation of the tyrosine residue within the catalytic loop and phosphorylation of the negative regulatory tyrosines by Csk (13, 65). Thus, precise regulation of Src-family kinases is likely to encompass multiple mechanisms working in concert.
While our model predicts that the major function of Cbl is the downregulation of Src-kinase activity by degradation, others have suggested that Cbl is downstream of c-Src and is required for signal transduction. The major defect in Src−/− mice is osteopetrosis, caused by a deficiency in bone resorption by osteoclasts, suggesting that Src kinase activity is required for osteoclast function (61). Phosphorylation of Cbl in osteoclast cell lines is Src dependent, and reduction of c-Src or Cbl protein levels by antisense oligonucleotides inhibits in vitro bone resorption (66). Similarly, Cbl phosphorylation is markedly reduced in Hck/Fgr/Lyn−/− macrophages following ligation of integrins by fibonectin, and treatment of wild-type cells with Cbl antisense oligonucleotides blocks cell spreading (40). Thus, Cbl may play two distinct roles in Src-family kinase-mediated signaling: downregulation of Src activity by enhancing degradation and simultaneous action as an adapter for SH2-containing proteins, thereby positively contributing to the transmission of the signal.
Finally, our studies demonstrate that Cbl-mediated Src-family kinase regulation is likely to be even more complex than Cbl-mediated regulation of other tyrosine kinases. The Cbl TKB domain is required for negative regulation of EGFR and PDGFR, as well as the ZAP70/Syk family of tyrosine kinases (6, 8, 30, 34, 43). This, coupled with the observation that the Cbl TKB domain bound wild-type Fyn, suggested that this domain would be required for regulation of Fyn by Cbl. Surprisingly, inactivation of the Cbl TKB domain alone did not affect the ability of Cbl to reduce the kinase-active pool of Fyn. A similar effect was noted in NIH 3T3 cells, where overexpression of wild-type Cbl as well as CblG306E led to inhibition of DNA synthesis induced by a constitutively active form of Src (8). This result suggested that Fyn SH3 domain binding to Cbl may be the only interaction required for negative regulation of Fyn by Cbl. However, the FynSH3* mutant was still negatively regulated by Cbl. By using a combination of the Cbl TKB domain mutant and an SH3 domain mutant of Fyn, we demonstrated that either domain is sufficient to mediate Cbl-induced negative regulation of an activated form of Fyn. These observations suggest that one or more phosphotyrosine motifs on Fyn are recognized by the TKB domain of Cbl, which may in turn enhance the susceptibility of the activated kinase to Cbl-mediated regulation. It should be noted that the Fyn SH3* mutant has enhanced kinase activity with respect to wild-type Fyn and so may contain phosphotyrosine motifs not found in the wild-type protein. Therefore, at present we are unable to rule out the possibility that the TKB domain effect observed is relevant only to constitutively active Src-family kinases.
In conclusion, our results implicate Cbl as an important component of the negative regulation of the Src-family kinase Fyn and provide evidence that Cbl-dependent regulation is achieved via enhanced degradation of Fyn. Given that Cbl is known to associate with many members of the Src kinase family, and given the structural conservation among the Src-family members, Cbl is likely to be a general negative regulator of Src-family kinases. Additionally, these results implicate kinase degradation as a general mechanism by which Cbl functions as a negative regulator of tyrosine kinases.
ACKNOWLEDGMENTS
This work was supported in part by grants to H.B. from the National Institutes of Health (CA64503, CA75075, and CA76118) and the American Cancer Society (CIM-89513). C.E.A. is a U.S. Department of Defense Breast Cancer Research Program Postdoctoral Fellow, N.L.L. is a recipient of a U.S. Department of Defense Breast Cancer Research Program Career Development Award, and M. L. L. is a Breast Cancer Research Fellow of the Massachusetts Department of Public Health.
We thank R. Perlmutter (Howard Hughes Medical Institute, University of Washington, Seattle) and A. Shaw (Washington University School of Medicine, St. Louis, Mo.) for Fyn constructs, K. Alexandropoulos (Department of Pharmacology, Columbia University, New York, N.Y.) for the SRE-luciferase construct, and B. Druker (Division of Hematology and Medical Oncology, Oregon Health Sciences University, Portland, Oreg.) for the 4G10 antibody.
REFERENCES
- 1.Alexandropoulos K, Baltimore D. Coordinate activation of c-Src by SH3- and SH2-binding sites on a novel p130Cas-related protein, Sin. Genes Dev. 1996;10:1341–1355. doi: 10.1101/gad.10.11.1341. [DOI] [PubMed] [Google Scholar]
- 2.Anderson S M, Burton E A, Koch B L. Phosphorylation of Cbl following stimulation with interleukin-3 and its association with Grb2, Fyn, and phosphatidylinositol 3-kinase. J Biol Chem. 1997;272:739–745. doi: 10.1074/jbc.272.2.739. [DOI] [PubMed] [Google Scholar]
- 3.Andoniou C E, Thien C B, Langdon W Y. Tumour induction by activated abl involves tyrosine phosphorylation of the product of the Cbl oncogene. EMBO J. 1994;13:4515–4523. doi: 10.1002/j.1460-2075.1994.tb06773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andoniou C E, Thien C B, Langdon W Y. The two major sites of cbl tyrosine phosphorylation in abl-transformed cells select the crkL SH2 domain. Oncogene. 1996;12:1981–1989. [PubMed] [Google Scholar]
- 5.Blake T J, Shapiro M, Morse H C, Langdon W Y. The sequences of the human and mouse c-cbl proto-oncogenes show v-cbl was generated by a large truncation encompassing a proline-rich domain and a leucine zipper-like motif. Oncogene. 1991;6:653–657. [PubMed] [Google Scholar]
- 6.Bonita D P, Miyake S, Lupher M L, Jr, Langdon W Y, Band H. Phosphotyrosine binding domain-dependent upregulation of the platelet-derived growth factor receptor alpha signaling cascade by transforming mutants of Cbl: implications for Cbl's function and oncogenicity. Mol Cell Biol. 1997;17:4597–4610. doi: 10.1128/mcb.17.8.4597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Briggs S D, Sharkey M, Stevenson M, Smithgall T E. SH3-mediated Hck tyrosine kinase activation and fibroblast transformation by the Nef protein of HIV-1. J Biol Chem. 1997;272:17899–17902. doi: 10.1074/jbc.272.29.17899. [DOI] [PubMed] [Google Scholar]
- 8.Broome M A, Galisteo M L, Schlessinger J, Courtneidge S A. The proto-oncogene c-Cbl is a negative regulator of DNA synthesis initiated by both receptor and cytoplasmic tyrosine kinases. Oncogene. 1999;18:2908–2912. doi: 10.1038/sj.onc.1202873. [DOI] [PubMed] [Google Scholar]
- 9.Calautti E, Missero C, Stein P L, Ezzell R M, Dotto G P. fyn tyrosine kinase is involved in keratinocyte differentiation control. Genes Dev. 1995;9:2279–2291. doi: 10.1101/gad.9.18.2279. [DOI] [PubMed] [Google Scholar]
- 10.Chen C, Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987;7:2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chow L M, Veillette A. The Src and Csk families of tyrosine protein kinases in hemopoietic cells. Semin Immunol. 1995;7:207–226. doi: 10.1006/smim.1995.0026. [DOI] [PubMed] [Google Scholar]
- 12.Cooper J A, Howell B. The when and how of Src regulation. Cell. 1993;73:1051–1054. doi: 10.1016/0092-8674(93)90634-3. [DOI] [PubMed] [Google Scholar]
- 13.Cooper J A, Simon M A, Kussick S J. Signaling by ectopically expressed Drosophila Src64 requires the protein-tyrosine phosphatase corkscrew and the adapter downstream of receptor kinases. Cell Growth Differ. 1996;7:1435–1441. [PubMed] [Google Scholar]
- 14.Donovan J A, Wange R L, Langdon W Y, Samelson L E. The protein product of the c-cbl protooncogene is the 120-kDa tyrosine-phosphorylated protein in Jurkat cells activated via the T cell antigen receptor. J Biol Chem. 1994;269:22921–22924. [PubMed] [Google Scholar]
- 15.Druker B J, Mamon H J, Roberts T M. Oncogenes, growth factors, and signal transduction. N Engl J Med. 1989;321:1383–1391. doi: 10.1056/NEJM198911163212007. [DOI] [PubMed] [Google Scholar]
- 16.Feshchenko E A, Langdon W Y, Tsygankov A Y. Fyn, Yes, and Syk phosphorylation sites in c-Cbl map to the same tyrosine residues that become phosphorylated in activated T cells. J Biol Chem. 1998;273:8323–8331. doi: 10.1074/jbc.273.14.8323. [DOI] [PubMed] [Google Scholar]
- 17.Fournel M, Davidson D, Weil R, Veillette A. Association of tyrosine protein kinase Zap-70 with the protooncogene product p120c-cbl in T lymphocytes. J Exp Med. 1996;183:301–306. doi: 10.1084/jem.183.1.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fukazawa T, Reedquist K A, Trub T, Soltoff S, Panchamoorthy G, Druker B, Cantley L, Shoelson S E, Band H. The SH3 domain-binding T cell tyrosyl phosphoprotein p120. Demonstration of its identity with the c-cbl protooncogene product and in vivo complexes with Fyn, Grb2, and phosphatidylinositol 3-kinase. J Biol Chem. 1995;270:19141–19150. doi: 10.1074/jbc.270.32.19141. [DOI] [PubMed] [Google Scholar]
- 19.Fukuda K, Kawata S, Tamura S, Matsuda Y, Inui Y, Igura T, Inoue S, Kudara T, Matsuzawa Y. Altered regulation of Src tyrosine kinase by transforming growth factor beta 1 in a human hepatoma cell line. Hepatology. 1998;28:796–804. doi: 10.1002/hep.510280329. [DOI] [PubMed] [Google Scholar]
- 20.Goldman A. Isolation and culture of fibroblasts. In: Spector D L, Goldman R D, Leinwand L A, editors. Cells: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1998. pp. 4.1–4.7. [Google Scholar]
- 21.Gonfloni S, Williams J C, Hattula K, Weijland A, Wierenga R K, Superti-Furga G. The role of the linker between the SH2 domain and catalytic domain in the regulation and function of Src. EMBO J. 1997;16:7261–7271. doi: 10.1093/emboj/16.24.7261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Groves T, Smiley P, Cooke M P, Forbush K, Perlmutter R M, Guidos C J. Fyn can partially substitute for Lck in T lymphocyte development. Immunity. 1996;5:417–428. doi: 10.1016/s1074-7613(00)80498-7. [DOI] [PubMed] [Google Scholar]
- 23.Hirai H, Varmus H E. SH2 mutants of c-src that are host dependent for transformation are trans-dominant inhibitors of mouse cell transformation by activated c-src. Genes Dev. 1990;4:2342–2352. doi: 10.1101/gad.4.12b.2342. [DOI] [PubMed] [Google Scholar]
- 24.Hunter T. A tail of two src's: mutatis mutandis. Cell. 1987;49:1–4. doi: 10.1016/0092-8674(87)90745-8. [DOI] [PubMed] [Google Scholar]
- 25.Imamoto A, Soriano P. Disruption of the csk gene, encoding a negative regulator of Src family tyrosine kinases, leads to neural tube defects and embryonic lethality in mice. Cell. 1993;73:1117–1124. doi: 10.1016/0092-8674(93)90641-3. [DOI] [PubMed] [Google Scholar]
- 26.Joazeiro C A P, Wing S S, Huang H, Leverson J D, Hunter T, Liu Y. The tyrosine kinase negative regulator c-Cbl as a RING-type, E2-dependent ubiquitin-protein ligase. Science. 1999;286:309–312. doi: 10.1126/science.286.5438.309. [DOI] [PubMed] [Google Scholar]
- 27.Langdon W Y, Hartley J W, Klinken S P, Ruscetti S K, Morse H C. v-cbl, an oncogene from a dual-recombinant murine retrovirus that induces early B-lineage lymphomas. Proc Natl Acad Sci USA. 1989;86:1168–1172. doi: 10.1073/pnas.86.4.1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Langdon W Y, Hyland C D, Grumont R J, Morse H C. The c-cbl proto-oncogene is preferentially expressed in thymus and testis tissue and encodes a nuclear protein. J Virol. 1989;63:5420–5424. doi: 10.1128/jvi.63.12.5420-5424.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee P S W, Wang Y, Domingez M G, Yeung Y G, Murphy M A, Bowtell D D L, Stanley E R. The Cbl protoncoprotein stimulates CSF-1 receptor multiubiquitination and endocytosis, and attenuates macrophage proliferation. EMBO J. 1999;18:3616–3628. doi: 10.1093/emboj/18.13.3616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levkowitz G, Waterman H, Zamir E, Kam Z, Oved S, Langdon W Y, Beguinot L, Geiger B, Yarden Y. c-Cbl/Sli-1 regulates endocytic sorting and ubiquitination of the epidermal growth factor receptor. Genes Dev. 1998;12:3663–3674. doi: 10.1101/gad.12.23.3663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lill, N. L., P. Douillard, R. A. Awwad, S. Ota, S. Miyake, N. Meissner-Lula, V. W. Hsu, and H. Band. The evolutionarily conserved amino terminal region of Cbl is sufficient to enhance down-regulation of the epidermal growth factor receptor. J. Biol. Chem., in press. [DOI] [PubMed]
- 32.Lowell C A, Soriano P. Knockouts of Src-family kinases: stiff bones, wimpy T cells, and bad memories. Genes Dev. 1996;10:1845–1857. doi: 10.1101/gad.10.15.1845. [DOI] [PubMed] [Google Scholar]
- 33.Lupher M L, Jr, Rao N, Eck M J, Band H. The Cbl protooncoprotein: a negative regulator of immun receptor signal transduction. Immunol Today. 1999;20:375–382. doi: 10.1016/s0167-5699(99)01484-x. [DOI] [PubMed] [Google Scholar]
- 34.Lupher M L, Jr, Rao N, Lill N L, Andoniou C E, Miyake S, Clark E A, Druker B, Band H. Cbl-mediated negative regulation of the syk tyrosine kinase. A critical role for cbl phosphotyrosine-binding domain binding to syk phosphotyrosine 323. J Biol Chem. 1998;273:35273–35281. doi: 10.1074/jbc.273.52.35273. [DOI] [PubMed] [Google Scholar]
- 35.Lupher M L, Jr, Reedquist K A, Miyake S, Langdon W Y, Band H. A novel phosphotyrosine-binding domain in the N-terminal transforming region of Cbl interacts directly and selectively with ZAP-70 in T cells. J Biol Chem. 1996;271:24063–24068. doi: 10.1074/jbc.271.39.24063. [DOI] [PubMed] [Google Scholar]
- 36.Lupher M L, Jr, Songyang Z, Shoelson S E, Cantley L C, Band H. The Cbl phosphotyrosine-binding domain selects a D(N/D)XpY motif and binds to the Tyr292 negative regulatory phosphorylation site of ZAP-70. J Biol Chem. 1997;272:33140–33144. doi: 10.1074/jbc.272.52.33140. [DOI] [PubMed] [Google Scholar]
- 37.Meisner H, Conway B R, Hartley D, Czech M P. Interactions of Cbl with Grb2 and phosphatidylinositol 3′-kinase in activated Jurkat cells. Mol Cell Biol. 1995;15:3571–3578. doi: 10.1128/mcb.15.7.3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meisner H, Czech M P. Coupling of the proto-oncogene product c-Cbl to the epidermal growth factor receptor. J Biol Chem. 1995;270:25332–25335. doi: 10.1074/jbc.270.43.25332. [DOI] [PubMed] [Google Scholar]
- 39.Meisner H, Daga A, Buxton J, Fernandez B, Chawla A, Banerjee U, Czech M P. Interactions of Drosophila Cbl with epidermal growth factor receptors and role of Cbl in R7 photoreceptor cell development. Mol Cell Biol. 1997;17:2217–2225. doi: 10.1128/mcb.17.4.2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meng F, Lowell C A. A beta 1 integrin signaling pathway involving Src-family kinases, Cbl and PI-3 kinase is required for macrophage spreading and migration. EMBO J. 1998;17:4391–4404. doi: 10.1093/emboj/17.15.4391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meng W, Sawasdikosol S, Burakoff S J, Eck M J. Structure of the amino-terminal domain of Cbl complexed to its binding site on ZAP-70 kinase. Nature. 1999;398:84–90. doi: 10.1038/18050. [DOI] [PubMed] [Google Scholar]
- 42.Miyake S, Lupher M L, Jr, Andoniou C E, Lill N L, Ota S, Douillard P, Rao N, Band H. The Cbl protooncogene product: from an enigmatic oncogene to center stage of signal transduction. Crit Rev Oncog. 1997;8:189–218. doi: 10.1615/critrevoncog.v8.i2-3.30. [DOI] [PubMed] [Google Scholar]
- 43.Miyake S, Lupher M L, Jr, Druker B, Band H. The tyrosine kinase regulator Cbl enhances the ubiquitination and degradation of the platelet-derived growth factor receptor alpha. Proc Natl Acad Sci USA. 1998;95:7927–7932. doi: 10.1073/pnas.95.14.7927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moarefi I, LaFevre-Bernt M, Sicheri F, Huse M, Lee C H, Kuriyan J, Miller W T. Activation of the Src-family tyrosine kinase Hck by SH3 domain displacement. Nature. 1997;385:650–653. doi: 10.1038/385650a0. [DOI] [PubMed] [Google Scholar]
- 45.Murphy M A, Schnall R G, Venter D J, Barnett L, Bertoncello I, Thien C B, Langdon W Y, Bowtell D D. Tissue hyperplasia and enhanced T-cell signalling via ZAP-70 in c-Cbl-deficient mice. Mol Cell Biol. 1998;18:4872–4882. doi: 10.1128/mcb.18.8.4872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nada S, Yagi T, Takeda H, Tokunaga T, Nakagawa H, Ikawa Y, Okada M, Aizawa S. Constitutive activation of Src family kinases in mouse embryos that lack Csk. Cell. 1993;73:1125–1135. doi: 10.1016/0092-8674(93)90642-4. [DOI] [PubMed] [Google Scholar]
- 47.Oda H, Kumar S, Howley P M. Regulation of the Src family kinase Blk through E6AP-mediated ubiquitination. Proc Natl Acad Sci USA. 1999;96:9557–9562. doi: 10.1073/pnas.96.17.9557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Odai H, Sasaki K, Iwamatsu A, Hanazono Y, Tanaka T, Mitani K, Yazaki Y, Hirai H. The proto-oncogene product c-Cbl becomes tyrosine phosphorylated by stimulation with GM-CSF or Epo and constitutively binds to the SH3 domain of Grb2/Ash in human hematopoietic cells. J Biol Chem. 1995;270:10800–10805. doi: 10.1074/jbc.270.18.10800. [DOI] [PubMed] [Google Scholar]
- 49.Ojaniemi M, Martin S S, Dolfi F, Olefsky J M, Vuori K. The proto-oncogene product of p120cbl links c-Src and phosphatidylinositol 3-kinase to the integrin signaling pathway. J Biol Chem. 1997;272:3780–3787. doi: 10.1074/jbc.272.6.3780. [DOI] [PubMed] [Google Scholar]
- 49a.Ota, S., N. Rao, M. L. Lupher, Jr., C. E. Andoniou, and H. Band. The ring finger domain of Cbl is essential for negative regulation of the Syk tyrosine kinase. J. Biol. Chem., in press. [DOI] [PubMed]
- 50.Page S T, van Oers N S, Perlmutter R M, Weiss A, Pullen A M. Differential contribution of Lck and Fyn protein tyrosine kinases to intraepithelial lymphocyte development. Eur J Immunol. 1997;27:554–562. doi: 10.1002/eji.1830270229. [DOI] [PubMed] [Google Scholar]
- 51.Panchamoorthy G, Fukazawa T, Miyake S, Soltoff S, Reedquist K, Druker B, Shoelson S, Cantley L, Band H. p120cbl is a major substrate of tyrosine phosphorylation upon B cell antigen receptor stimulation and interacts in vivo with Fyn and Syk tyrosine kinases, Grb2 and Shc adaptors, and the p85 subunit of phosphatidylinositol 3-kinase. J Biol Chem. 1996;271:3187–3194. doi: 10.1074/jbc.271.6.3187. [DOI] [PubMed] [Google Scholar]
- 52.Phipps D J, Yousefi S, Branch D R. Increased enzymatic activity of the T-cell antigen receptor-associated fyn protein tyrosine kinase in asymptomatic patients infected with the human immunodeficiency virus. Blood. 1997;90:3603–3612. [PubMed] [Google Scholar]
- 53.Qureshi S A, Cao X M, Sukhatme V P, Foster D A. v-Src activates mitogen-responsive transcription factor Egr-1 via serum response elements. J Biol Chem. 1991;266:10802–10806. [PubMed] [Google Scholar]
- 54.Reedquist K A, Fukazawa T, Druker B, Panchamoorthy G, Shoelson S E, Band H. Rapid T-cell receptor-mediated tyrosine phosphorylation of p120, an Fyn/Lck Src homology 3 domain-binding protein. Proc Natl Acad Sci USA. 1994;91:4135–4139. doi: 10.1073/pnas.91.10.4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Resh M D. Myristylation and palmitylation of Src family members: the fats of the matter. Cell. 1994;76:411–413. doi: 10.1016/0092-8674(94)90104-x. [DOI] [PubMed] [Google Scholar]
- 56.Ribon V, Saltiel A R. Insulin stimulates tyrosine phosphorylation of the proto-oncogene product of c-Cbl in 3T3-L1 adipocytes. Biochem J. 1997;324:839–845. doi: 10.1042/bj3240839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Richard S, Yu D, Blumer K J, Hausladen D, Olszowy M W, Connelly P A, Shaw A S. Association of p62, a multifunctional SH2- and SH3-domain-binding protein, with src family tyrosine kinases, Grb2, and phospholipase C gamma-1. Mol Cell Biol. 1995;15:186–197. doi: 10.1128/mcb.15.1.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rivero-Lezcano O M, Sameshima J H, Marcilla A, Robbins K C. Physical association between Src homology 3 elements and the protein product of the c-cbl proto-oncogene. J Biol Chem. 1994;269:17363–17366. [PubMed] [Google Scholar]
- 59.Seidel-Dugan C, Meyer B E, Thomas S M, Brugge J S. Effects of SH2 and SH3 deletions on the functional activities of wild-type and transforming variants of c-Src. Mol Cell Biol. 1992;12:1835–1845. doi: 10.1128/mcb.12.4.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sicheri F, Moarefi I, Kuriyan J. Crystal structure of the Src family tyrosine kinase Hck. Nature. 1997;385:602–609. doi: 10.1038/385602a0. [DOI] [PubMed] [Google Scholar]
- 61.Soriano P, Montgomery C, Geske R, Bradley A. Targeted disruption of the c-src proto-oncogene leads to ostepetrosis in mice. Cell. 1991;64:693–702. doi: 10.1016/0092-8674(91)90499-o. [DOI] [PubMed] [Google Scholar]
- 62.Spits H, Keizer G, Borst J, Terhorst C, Hekman A, de Vries J E. Characterization of monoclonal antibodies against cell surface molecules associated with cytotoxic activity of natural and activated killer cells and cloned CTL lines. Hybridoma. 1983;2:423–437. doi: 10.1089/hyb.1983.2.423. [DOI] [PubMed] [Google Scholar]
- 63.Stein P L, Lee H M, Rich S, Soriano P. pp59fyn mutant mice display differential signaling in thymocytes and peripheral T cells. Cell. 1992;70:741–750. doi: 10.1016/0092-8674(92)90308-y. [DOI] [PubMed] [Google Scholar]
- 64.Stein P L, Vogel H, Soriano P. Combined deficiencies of Src, Fyn, and Yes tyrosine kinases in mutant mice. Genes Dev. 1994;8:1999–2007. doi: 10.1101/gad.8.17.1999. [DOI] [PubMed] [Google Scholar]
- 65.Sun G, Sharma A K, Budde R J. Autophosphorylation of Src and Yes blocks their inactivation by Csk phosphorylation. Oncogene. 1998;17:1587–1595. doi: 10.1038/sj.onc.1202076. [DOI] [PubMed] [Google Scholar]
- 66.Tanaka S, Amling M, Neff L, Peyman A, Uhlmann E, Levy J, Baron R. c-Cbl is downstream of c-Src in a signalling pathway necessary for bone resorption. Nature. 1996;383:528–531. doi: 10.1038/383528a0. [DOI] [PubMed] [Google Scholar]
- 67.Tanaka S, Neff L, Baron R, Levy J B. Tyrosine phosphorylation and translocation of the c-cbl protein after activation of tyrosine kinase signaling pathways. J Biol Chem. 1995;270:14347–14351. doi: 10.1074/jbc.270.24.14347. [DOI] [PubMed] [Google Scholar]
- 68.Thien C B, Langdon W Y. EGF receptor binding and transformation by v-cbl is ablated by the introduction of a loss-of-function mutation from the Caenorhabditis elegans sli-1 gene. Oncogene. 1997;14:2239–2249. doi: 10.1038/sj.onc.1201193. [DOI] [PubMed] [Google Scholar]
- 69.Thien C B, Langdon W Y. Tyrosine kinase activity of the EGF receptor is enhanced by the expression of oncogenic 70Z-cbl. Oncogene. 1997;15:2909–2919. doi: 10.1038/sj.onc.1201468. [DOI] [PubMed] [Google Scholar]
- 70.Tsygankov A Y, Mahajan S, Fincke J E, Bolen J B. Specific association of tyrosine-phosphorylated c-Cbl with Fyn tyrosine kinase in T cells. J Biol Chem. 1996;271:27130–27137. doi: 10.1074/jbc.271.43.27130. [DOI] [PubMed] [Google Scholar]
- 71.van der Geer P, Hunter T, Lindberg R A. Receptor protein-tyrosine kinases and their signal transduction pathways. Annu Rev Cell Biol. 1994;10:251–337. doi: 10.1146/annurev.cb.10.110194.001343. [DOI] [PubMed] [Google Scholar]
- 72.Wange R L, Samelson L E. Complex complexes: signaling at the TCR. Immunity. 1996;5:197–205. doi: 10.1016/s1074-7613(00)80315-5. [DOI] [PubMed] [Google Scholar]
- 73.Waterman H, Levkowitz G, Alroy I, Yarden Y. The RING finger of c-Cbl mediates desensitization of the epidermal growth factor receptor. J Biol Chem. 1999;274:22151–22154. doi: 10.1074/jbc.274.32.22151. [DOI] [PubMed] [Google Scholar]
- 74.Wilson I A, Niman H L, Houghten R A, Cherenson A R, Connolly M L, Lerner R A. The structure of an antigenic determinant in a protein. Cell. 1984;37:767–778. doi: 10.1016/0092-8674(84)90412-4. [DOI] [PubMed] [Google Scholar]
- 75.Xu W, Harrison S C, Eck M J. Three-dimensional structure of the tyrosine kinase c-Src. Nature. 1997;385:595–602. doi: 10.1038/385595a0. [DOI] [PubMed] [Google Scholar]
- 76.Yagi T, Nada S, Watanabe N, Tamemoto H, Kohmura N, Ikawa Y, Aizawa S. A novel negative selection for homologous recombinants using diphtheria toxin A fragment gene. Anal Biochem. 1993;214:77–86. doi: 10.1006/abio.1993.1459. [DOI] [PubMed] [Google Scholar]
- 77.Yamaguchi H, Hendrickson W A. Structural basis for activation of human lymphocyte kinase Lck upon tyrosine phosphorylation. Nature. 1996;384:484–489. doi: 10.1038/384484a0. [DOI] [PubMed] [Google Scholar]
- 78.Yoon C H, Lee J, Jongeward G D, Sternberg P W. Similarity of sli-1, a regulator of vulval development in C. elegans, to the mammalian proto-oncogene c-cbl. Science. 1995;269:1102–1105. doi: 10.1126/science.7652556. [DOI] [PubMed] [Google Scholar]