Abstract
Background:
Metabolic syndrome increases the risk of cardiovascular disease in adults. Antecedents likely begin in childhood and whether childhood exposure to air pollution plays a contributory role is not well understood.
Objectives:
To assess whether children’s exposure to air pollution is associated with markers of risk for metabolic syndrome and oxidative stress, a hypothesized mediator of air pollution-related health effects.
Methods:
We studied 299 children (ages 6–8) living in the Fresno, CA area. At a study center visit, questionnaire and biomarker data were collected. Outcomes included hemoglobin A1c (HbA1c), urinary 8-isoprostane, systolic blood pressure (SBP), and BMI. Individual-level exposure estimates for a set of four pollutants that are constituents of traffic-related air pollution (TRAP) – the sum of 4-, 5-, and 6-ring polycyclic aromatic hydrocarbon compounds (PAH456), NO2, elemental carbon, and fine particulate matter (PM2.5) – were modeled at the primary residential location for 1-day lag, and 1-week, 1-month, 3-month, 6-month, and 1-year averages prior to each participant’s visit date. Generalized additive models were used to estimate associations between each air pollutant exposure and outcome.
Results:
The study population was 53% male, 80% Latinx, 11% Black and largely low-income (6% were White and 3% were Asian/Pacific Islander). HbA1c percentage was associated with longer-term increases in TRAP; for example a 4.42 ng/m3 increase in 6-month average PAH456 was associated with a 0.07% increase (95% CI: 0.01, 0.14) and a 3.62 μg/m3 increase in 6-month average PM2.5 was associated with a 0.06% increase (95% CI: 0.01, 0.10). The influence of air pollutants on blood pressure was strongest at 3 months; for example, a 6.2 ppb increase in 3-month average NO2 was associated with a 9.4 mmHg increase in SBP (95% CI: 2.8, 15.9). TRAP concentrations were not significantly associated with anthropometric or adipokine measures. Short-term TRAP exposure averages were significantly associated with creatinine-adjusted urinary 8-isoprostane.
Discussion:
Our results suggest that both short- and longer-term estimated individual-level outdoor residential exposures to several traffic-related air pollutants, including ambient PAHs, are associated with biomarkers of risk for metabolic syndrome and oxidative stress in children.
Keywords: children, metabolic syndrome, HbA1c, oxidative stress, traffic-related air pollution, polycyclic aromatic hydrocarbons
1. INTRODUCTION
Metabolic syndrome is a cluster of conditions that increases the risk of cardiovascular disease, type 2 diabetes mellitus, and all-cause mortality. Insulin resistance, abdominal obesity, dyslipidemia, and hypertension are several of the known risk factors that contribute to the syndrome (Huang 2009). Metabolic syndrome is now recognized as a worldwide public health problem (Alberti et al. 2009) leading to calls for research on potentially modifiable risk factors, including air pollution (Hutcheson and Rocic 2012). Evidence has been accumulating that risk factors likely associated with adult metabolic syndrome are also impacted in children through exposure to air pollutants. Such risk factors include diabetes, obesity and systolic blood pressure (Faienza et al. 2016; Lim and Thurston 2019). If modifiable environmental risk factors for metabolic syndrome can be identified, especially in high-risk populations, then strategies at the community and individual level – known and yet to be developed – to reduce childhood exposures to these factors should be prioritized for implementation. Here we present an examination of air pollution among children as one such modifiable environmental risk factor for several indices of metabolic syndrome.
The Children’s Health and Air Pollution Study (CHAPS) in the San Joaquin Valley (SJV) of California is a research project investigating the adverse health effects of early childhood exposure to air pollution. The SJV has some of the worst air pollution in the U.S., a large Hispanic/Latinx population, and a high rate of poverty. Compared with other ethnic groups, Latinx children and adolescents in the United States are disproportionately affected by obesity (Ogden et al. 2012). Babey et al. showed that a high proportion of Latinx adults living in the San Joaquin Valley have pre-diabetes or type 2 diabetes (Babey et al. 2016).
We have collected extensive air pollution exposure data for many years in Fresno and more recently in Bakersfield, the two most populous cities in the SJV, including ambient polycyclic aromatic hydrocarbon (PAH) concentrations (Noth et al. 2011, 2016, 2020). The spatial variability in ambient PAHs in Fresno is primarily due to traffic and rail lines. PAHs are putative endocrine disruptors, which have been associated with obesity and metabolic dysregulation, and thus are of particular interest (Zhang et al. 2016).
Capitalizing on our extensive air pollution exposure data, we conducted a study of the potential effects of PAHs and other traffic-related air pollutants on anthropometric measures and biomarkers of metabolic dysfunction in young children enrolled in CHAPS. Our overall paradigm was that oxidative stress induced by exposure to traffic-related air pollution, especially ambient PAHs, leads to systemic inflammation that contributes to abnormal fat and glucose metabolism and thereby increases risk of obesity and diabetes. The measures and biomarkers we examined were anthropometry to assess childhood obesity (BMI-percentile, percent body fat and waist-to-height ratio), glycosolated hemoglobin (HbA1c) as a measure of glucose dysregulation, adipokines involved with both glucose and fat metabolism (leptin and adiponectin), 8-isoprostane as a measure of oxidative stress, and blood pressure. This set of measurements provides an approach to the assessment of metabolic syndrome risk in children. Here we report the results of a cross-sectional analysis of the associations between residential concentrations of traffic-related air pollutants and markers of metabolic dysfunction among the CHAPS children (ages 6 to 8 years).
2. MATERIALS AND METHODS
2.1. Study population and recruitment
We partnered with the Fresno Unified School District (FUSD) to recruit children ages 6 to 8 years who were enrolled in FUSD in 2015–2017. In 2017, FUSD had a student population of 70,725, with 88.9% of children classified as socioeconomically disadvantaged (California Department of Education 2017). Recruiting through the public elementary school system allowed us to recruit a group of predominantly low-income children, distributed spatially across Fresno.
Since FUSD schools operate primarily as neighborhood schools, in order to ensure appropriate residential exposure contrasts between study participants, Kindergarten-6th (K-6) grade elementary schools in FUSD were ranked by traffic density and recruitment efforts used these traffic-density rankings to achieve heterogeneity of traffic-related air pollution exposure among study participants. Traffic density was assessed using California Department of Transportation (Caltrans) Annual Average Daily Traffic (AADT) volumes traveling in both directions, accessed in 2015 and using a 300-meter rate of decay from roadways (Margolis et al. 2009). Of the 65 K-6 schools in FUSD, we randomly sampled schools across traffic density strata for recruitment until we reached our desired sample size of 299 children. This sample size was based on power calculations using the association of ambient polycyclic aromatic hydrocarbons (PAHs) and %HbA1c in a previous sample of Fresno children. A sample size of 200 children aged 7–9 would detect a change of 0.0022 %HbA1c with a power of 0.8.
Children ages 6 to 8 in the selected schools were sent home with flyers containing information about the study. In total, we recruited at 55 of the K-6 FUSD schools. Interested parents contacted the study center to assess their child’s eligibility (age 6–8, residence in Fresno or Clovis for at least the past 3 months, residence within 20 km of the central air quality monitoring site, no plans to move from the Fresno/Clovis area in the next 2 years, English- or Spanish-speaking, and no cancer, HIV, or autoimmune disease). Of our cohort of 299 children, n=288 (96.3%) came from the sampled K-6 FUSD schools. However, due to word-of-mouth, some parents outside the selected FUSD schools contacted the study center to have their children participate; as long as the child met all eligibility criteria described above, they were invited to participate in the study.
If interested and eligible, families were invited to visit the study center at UCSF Fresno. All study protocols were approved by the Institutional Review Boards at the University of California, Berkeley; the University of California, San Francisco-Fresno (UCSF Fresno); and Stanford University. Written, informed permission was obtained from each accompanying parent or guardian and written child assent for participation was also obtained.
To minimize participant burden and thereby maximize study enrollment and participation, appointments at the study center were not constrained to one time of day and the children were not required to fast prior to their visits and blood draws. Our selection of study biomarkers and our overall analytic approach accommodates the potential diurnal variation in some biomarkers and a non-fasting state.
2.2. Study center visit
At the study center visit, which occurred over a two-year period from May 2015 to May 2017, each participant’s parent or guardian was interviewed using a detailed, structured health and general history questionnaire, and for each child participant, anthropometric measurements were taken, blood pressure was measured, and a non-fasting blood sample and urine sample were obtained.
The questionnaire was programmed using CASIC Builder™ (West Portal Software Corporation) for direct data entry and administered by trained office interview staff. The questionnaire was offered to participants’ parents or guardians in either English or Spanish and assessed participant demographics, including sex, age and race/ethnicity, in addition to parental socioeconomic indicators such as annual household income, parental education levels, parental employment, and home ownership.
The question about race and ethnicity, taken from National Cooperative Inner-City Asthma Study (NCICAS), was “How would you describe [CHILD’S NAME]’s race, nationality, or ethnic background?” Response categories were adapted and upcoded to Hispanic/Latinx, Black, Non-Hispanic White, Asian/Pacific Islander and American Indian/Alaska Native. Parents were permitted to provide up to four different race/ethnicity responses. For purposes of this analysis, any child coded as Hispanic/Latinx was defined as Latinx, and among those remaining, the first other listed race was used.
The question about annual household income was “For the last calendar year, what was your household income from all sources, before taxes?” with the response categories of <$15,000, more than $15,000 to $30,000, more than $30,000 to $50,000, more than $50,000 to $100,000, and more than $100,000.
2.3. Outcome measurement
The physiological and biochemical indicators measured in our 6–8 year old children were chosen to a) reflect potential risk of development of metabolic syndrome in adulthood and b) feasibility of measurement. The components of the metabolic syndrome in adults are hypertension, insulin resistance, overweight/obesity (especially central adiposity), and hyperlipidemia (low high-density lipoprotein (HDL) and high triglycerides). We chose to measure blood pressure, HbA1c, BMI, percent body fat, waist circumference, and HDL in our participants. We chose not to measure either fasting blood glucose or triglycerides because of the logistical challenges of obtaining fasting blood samples on young children; HbA1c and HDL measurements do not have to be made on fasting blood samples. Unfortunately, due to logistical constraints, we only were able to obtain HDL measurements in a relatively small subset of the 299 participants and so did not include these measurements in the current analysis.
2.3.1. Anthropometry
All anthropometric measures (height, weight, and waist circumference) followed the National Health and Nutrition Examination Survey (NHANES) anthropometry protocols (CDC 2011a). Child barefoot standing height was measured (to the nearest 0.1 cm) using a stadiometer (SECA, model CE 0123), weight was measured (to the nearest 0.1 kg) using a digital weight scale (Tanita Class III, model BWB-800A), and waist circumference was measured (to the nearest 0.1 mm) using a retractable steel measuring tape. Each of these measures was replicated at least twice, and repeated a third time if the first two measures differed by more than a predetermined amount (>0.5 cm for standing height, >0.3 kg for weight, and >1.0 cm for waist circumference). The two measures with the smallest difference were averaged. The averaged waist circumference (cm) and height (cm) were used to calculate waist-height ratio (WHR), a measure of central adiposity.
Definitions of underweight, normal weight, overweight, and obesity in children are not directly comparable with the definitions in adults. Instead, standardized BMI-for-age percentiles were calculated for each child using a CDC SAS macro that compares averaged height (cm), averaged weight (kg), sex, and age in months to CDC growth charts (CDC 2016). To assess childhood obesity, we used the age-and sex-specific 5th, 85th, and 95th percentiles of the 2000 CDC growth charts as cutoff criteria as follows: (1) BMI < 5th percentile: underweight; (2) BMI 5th to < 85th percentiles: normal weight; (3) BMI 85th to < 95th percentiles: overweight; (4) BMI ≥ 95th percentile: obese. In order to better measure adiposity among children who have very high BMIs, the continuous outcome we analyzed was the child’s BMI relative to the 95th percentile of the 2000 CDC growth charts for sex and age. This value (BMI-percentile95) represents the percent above (or below) the threshold for obesity in children, defined as a BMI-percentile95 ≥ 100, such that a child with a BMI-percentile95 < 100 would not be obese, whereas a child with a BMI-percentile95 = 120 would have a BMI equal to 1.2 times the 95th percentile BMI for their sex and age and be considered severely obese. The decision to use BMI-percentile95 follows the CDC recommendation to use this measure for a study population with a large proportion of children with severe obesity (BMI-percentile95 ≥ 120). Ten percent of our cohort had severe obesity.
Body composition (reactance and resistance) was measured using a bioelectrical impedance analyzer (RJL Systems, model Quantum IV). Reactance and resistance were each measured three times, and the two measures with the smallest difference were averaged for further analysis. Fat-free mass (FFM) and percent body fat were then calculated using average measures in previously described formulas (Goran et al. 1993).
2.3.2. Blood pressure
Blood pressure was measured three times following the NHANES protocol (CDC 2011b) using an appropriate child cuff with an automatic blood pressure monitor (OMRON Model #: HEM-705CP). For each measure (systolic blood pressure (SBP) and diastolic blood pressure (DBP)), we slightly modified the NHANES protocol (CDC 2011b) by averaging the two values with the smallest difference to use in data analyses.
2.3.3. Biomarkers of metabolic function and oxidative stress
Blood specimens were collected by venipuncture by a trained phlebotomist, with serum collected in serum separator tubes and whole blood collected in EDTA vacutainers (Becton, Dickinson and Company, Franklin Lakes, NJ). The samples for HbA1c were picked up at room temperature within 24 hours of draw and assayed by a commercial laboratory (LabCorp). The samples for the leptin and adiponectin assays were shipped overnight on a gel pack to the Nadeau laboratory at Stanford, where following centrifugation, separated components were divided into serum and clot aliquots that were stored at −80°C. When ready to be assayed, serum samples were shipped frozen to the Holland laboratory at UC Berkeley.
Urine collected to assay 8-isoprostane and creatinine was shipped overnight on a gel pack to the Nadeau laboratory. If urine could not be shipped out within 24 hours, it was frozen before shipping overnight. The Nadeau laboratory stored all urine at −80°C, and when it was ready to be assayed, urine samples were shipped frozen to the Holland laboratory.
Serum adiponectin and leptin were measured in the banked serum samples using enzyme-linked immunoassay (ELISA) kits (RayBiotech Life, Norcross, GA) as previously described (Volberg et al. 2013). Briefly, the manufacturer-recommended protocol was used with two exceptions: 1) the standard curve for adiponectin was narrowed to smaller values for better resolution while 2) the standard curve was widened for leptin. These changes were necessary to tailor the ELISAs towards the adipokine levels observed in this population. The minimum detectable concentrations for adiponectin and leptin ELISAs were 10 pg/mL and 6 pg/mL, respectively. All samples were run in duplicate and the values were averaged. The intra- and inter-plate coefficients of variance (CV) were 4% and 12%, respectively, for adiponectin and 3% and 15%, respectively, for leptin.
Urinary total 8-isoprostane was measured in the banked samples using an ELISA kit (Oxford Biomedical Research, Rochester Hills, MI) as previously described (Tran et al. 2017). Briefly, urine samples were pre-treated with beta-glucuronidase (Oxford Biomedical Research, Rochester Hills, MI) prior to running the ELISA. The limit of detection (LOD) for 8-isoprostane concentration was 0.08 ng/mL. Undetected oxidative stress measures were replaced with the LOD divided by the square root of 2. Additional quality assurance/quality control (QA/QC) provisions included repeats of 5% of samples and blanks, and internal lab controls with good reproducibility of 8-isoprostane (coefficient of variation <7%). Creatinine levels in the urine samples were analyzed using a urinary creatinine ELISA kit (Oxford Biomedical Research, Rochester Hills, MI). Samples were randomized across plates and the coefficient of variation for creatinine was less than 3%. All 8-isoprostane concentrations were adjusted to account for urinary dilution by dividing 8-isoprostane concentrations (ng/mL) by creatinine levels (mg/dL) with results reported in ng/mg creatinine.
2.4. Air pollution exposure assessment
The ultimate goal of our air pollution exposure assessment was to model pollutant exposures for each study participant. To achieve this goal, we used a combined field monitoring and modeling approach to estimate individual-level air pollution exposure estimates for the 299 participants, as it is not feasible to conduct personal sampling for multiple pollutants on young children for up to 9 years of exposures. In the sections below we provide some detail on the field monitoring (section 2.4.1), exposure model building (section 2.4.2), and exposure assignment (section 2.4.3). Greater detail on the monitoring and model building can be found elsewhere (Noth et al. 2011, 2016, 2020; Tager et al. 2005).
2.4.1. Air pollution measurement data
We collected air pollution measurements using both continuous daily pollutant concentrations measured at fixed air monitoring stations in Fresno and daily concentrations obtained from periodic spatially intensive sampling campaigns.
Continuous daily concentrations were collected for four different pollutants. Hourly, quality-assured, ambient pollutant (NO2 and PM2.5) concentrations and meteorological data collected at the local air pollution control district’s Fresno central site monitoring station (Garland) and three other sites in Fresno were obtained from the U.S. Environmental Protection Agency’s (EPA) Air Quality System (AQS). Black carbon (BC) was determined from Aethalometer™ (model AE42; Magee Scientific, Berkeley, CA) measurements of the optical absorption of PM2.5 ambient aerosol at 880 nm, and particle-bound PAHs were monitored with the PAS2000 (EcoChem Analytics, League City, TX). The PAS2000 uses a photoelectric aerosol sensor to measure the levels of particle-bound ambient PAH with three or more rings (pPAH). We used data collected from these real-time continuous monitors from 2002 through 2017. The air pollution data were subject to rigorous checks for quality assurance. These included range and persistence checks, comparison of values at nearby monitoring sites, and consistency with historical temporal and/or diurnal patterns for each pollutant. Completeness was uniformly assessed using a 75% criterion.
During the periodic, spatially intensive sampling campaign in 2014–2015, daily filter samples were collected and subsequently analyzed for NO2, elemental carbon (EC) and 37 individual PAHs and oxygenated PAHs, as described elsewhere (Noth et al. 2011, 2016, 2020). These sampling sites were selected to represent traffic (road and rail), industrial and residential sources of pollutants. PAH analyses were performed by gas chromatography/mass spectrometry (HP 6890/5972 or Agilent 7820/5977E) in the selected ion-monitoring mode with a 30m (5%-Phenyl)- methylpolysiloxane column (Agilent HP-5MS). In this analysis, we used the sum of the measured concentrations of 4-, 5-, and 6-ring PAH compounds (fluoranthene, benz[a]anthracene, chrysene, benzo[a]pyrene, benzo[b]fluoranthene, benzo[k]flouoranthene, benzo[ghi]perylene, indeno[1,2,3-cd]pyrene, and dibenz[a,h]anthracene) (Noth et al. 2011, 2016, 2020). We refer to this sum as PAH456.
2.4.2. Air pollution exposure models
We used two methods to model outdoor air pollution concentrations – interpolation (for PM2.5) and regression modeling (for all other pollutants we considered). We estimated outdoor concentrations of PM2.5 through interpolation from daily fixed site data using inverse distance-squared weighting. Up to four sites were included in the model with a maximum allowed radius from each participant’s residence of 50 km. The interpolation model used EPA’s AQS ambient data for the 2013–2017 time period.
Linear regression with mixed effects (random and fixed) was used to develop spatial-temporal models of daily concentrations for PAH456, EC, and NO2, incorporating air pollution measurement data from 2002–2015 (Noth et al. 2011, 2020). In doing so, we make the assumption that we can use the spatial and temporal relationships observed in our air pollution measurement data and apply those to unsampled locations and times. Making this assumption is necessary to improve spatial predictions where high regional variability in pollutant levels can result in exposure misclassification bias in epidemiology studies (Özkaynak et al. 2013). Sampling location and date were treated as random effects in order to simultaneously capture the temporal and spatial components. Covariates considered for each exposure model include the continuously measured daily pollutant concentrations (pPAH, BC, NO2) at fixed sites, relative humidity, temperature, wind speed, atmospheric stability, distance to nearest freeway, Caline4 dispersion model estimates of concentrations from local traffic, distance to nearest rail lines, and amount of rail yard, urban, and open space land use within 1 km radius circular buffers (Supplemental Table 1). The inclusion criteria for covariates were their statistical significance and percentage of variance explained (i.e., improvement in explanatory power). Using the between and within sampling location and date covariance estimates, the models for PAH456, EC, and NO2 explain 53%, 95%, and 99% of the observed temporal variance, and 74%, 88%, and 74% of the observed spatial variance.
2.4.3. Individual-level air pollution exposure estimates
During their visit to the study center, parents or guardians were asked to report the street address, city and state of all residences at which the child participant had lived since birth. Only addresses at which the child had lived for at least 1 month were recorded. Each address was geocoded using ESRI Software (Redlands, CA) and/or Google Earth to create a lifetime, residential history for each participant.
Daily exposures for each participant for each day during the year prior to the study visit were estimated using the residential location on the day of interest (taken from the reported residential histories) and either the interpolated daily value of PM2.5 or, for PAH456, EC and NO2, the daily spatial-temporal parameters in each pollutant model (details in Supplemental Table 1). Using daily exposure estimates at the primary residential location, we assigned exposures for each participant for 1-day lag, and aggregated daily exposures to 1-week, 1-month, 3-month, 6-month, and 1-year averages prior to each participant’s visit date.
2.5. Statistical analysis
Model covariates were based on a directed acyclic graph (Supplemental Figure 1). Race/ethnicity, age, sex and socioeconomic status (SES) were likely to impact exposure as well the outcome and so were included in all models. Because our air pollution exposures are based on the residential locations of our study participants, we considered race/ethnicity because racist historical housing practices in Fresno such as redlining and racially restrictive covenants, (Zuk 2013; Nardone et al. 2020) may have resulted in the uneven distribution of race/ethnicity groups across residential locations in Fresno and hence, differential proximity to heavily trafficked roadways and rail lines and thus traffic-related air pollution (TRAP) exposure. Age (months) and sex were included as they might be related to a participant’s outside activity and therefore to TRAP exposure. SES was included because it might also be related to differential TRAP exposure; SES was modeled using annual family income <$15,000 (28% of the study population). Weight was not included in the models because it was considered to be on the causal pathways from exposure to several of the outcomes. Logged values for adiponectin, leptin, BMI-percentile95, and 8-isoprostane were used because the distributions of the residual values for these outcomes were skewed; the other outcome variables (HbA1c, WHR, percent body fat, SBP, DBP) were used untransformed. For blood pressure models, height was added to the models as it is strongly associated with blood pressure in children. Descriptive statistics for outcomes, pollutants, model covariates were calculated using SAS 9.4 (Cary, NC: SAS Institute).
Next, we used generalized additive models (mgcv package in R) with a smooth term for study day (maximum degrees of freedom=15) to estimate associations between each air pollutant exposure and each outcome (anthropometric index (log BMI-percentile95, waist-to-height ratio (WHR), percent body fat percentage), HbA1c, log serum adipokines (leptin and adiponectin), log urinary 8-isoprostane (creatinine adjusted), and blood pressure (systolic and diastolic)). The smooth term for study day allowed us to adjust for non-monotonic changes over time and secular trends in the outcome.
To compare results across different pollutants, findings are presented as change in the outcome associated with an interquartile range change (IQR) in each pollutant, using the IQR calculated for that particular averaging period. For non-logged outcomes (WHR, percent body fat, HbA1c, and systolic and diastolic blood pressure), effect estimates represent the expected change to the absolute value of the outcome with an IQR-unit change. For logged outcomes (leptin, adiponectin, creatinine-adjusted 8-isoprostane, and BMI-percentile95), effect estimates represent the expected percent change to the outcome with an IQR-unit change derived using the ß coefficient and standard error (SE) as follows:
Analyses were restricted to exposures of 3-month, 6-month and 1-year averages for HbA1c, BMI-percentile95, WHR, percent body fat, adiponectin and leptin since these outcomes change slowly over time. Even though HbA1c is present in red blood cells, which turnover in approximately 115 days, their production could be influenced by inflammation occurring on a longer scale, and thus timeframes longer than 3 months were also considered. For SBP, DBP and 8-isoprostane, we also analyzed associations with 1-day lag, and 1-week and 1-month average exposures because short-term exposures were thought to be important for these outcomes. Estimated effects are presented in supplemental tables to the thousandths place for all models to balance consistency in our reporting, while estimates in the body of the paper are presented with an appropriate number of significant digits based on measurement accuracy. We fit a separate model for each averaging time for each pollutant. We did not adjust for multiple comparisons, but rather chose to interpret the results of pollutant-specific models in the context of trends by considering findings in groups. We interpret the findings for a single time frame across the entire group of traffic-related pollutants or for a single pollutant across all time frames, rather than focusing on results from individual models.
3. RESULTS
3.1. Descriptive Statistics
The study population consisted of 299 children. The sociodemographic characteristics of the participating children are shown in Table 1. The potential participants were screened at ages 6–8 and seen for their baseline visit at ages 6–9. The population was 53% male, 80% Hispanic/Latinx, and 11% Black. The remaining participants were Non-Hispanic White (6.0%) and Asian/Pacific Islander (3.0%). Nearly 80% of the study population lived in rented homes and nearly 30% was from a family with <$15,000 annual household income. Using CDC criteria, 25% of the children were obese and another 16% were overweight.
Table 1.
Demographic characteristics of study participants (n=299)
| Characteristic | Mean (SD) | % |
|---|---|---|
| Age (months) | 95.6 (7.0) | |
| Weight (kg) | 31.5 (8.8) | |
| Male | 53.2 | |
| Race/Ethnicity | ||
| Hispanic/Latinx | 79.6 | |
| Black | 11.4 | |
| Non-Hispanic White | 6.0 | |
| Asian/Pacific Islander | 3.0 | |
| Primarily Spanish-speaking | 17.4 | |
| Renter* | 78.0 | |
| Annual household income <$15K* | 28.0 | |
| Obese** | 24.8 | |
| Overweight** | 16.1 |
Responses were refused, not applicable or unknown for n=3 for each of the home renting and household income questions
Using age-and sex-specific percentiles of the 2000 CDC growth charts, obese was defined as BMI ≥ 95th percentile and overweight was defined as BMI 85th to <95th percentiles.
Table 2 shows summary outcome data (HbA1c, leptin, adiponectin, urinary 8-isoprostane, systolic and diastolic blood pressure, WHR, percent body fat, and BMI-percentile95), as well as the number of children with data available for each outcome. The Q3 values of 95.0 and 100.0 for BMI for age and sex and BMI for age and sex relative to the 95th percentile, respectively, correspond to the 25% of our cohort which is obese. Ten percent of the children had severe obesity (BMI-percentile95 ≥ 120, data not shown). Twenty-nine percent of the children had HbA1c values ≥ 5.7, a level that is an indicator of pre-diabetes (American Diabetes Association 2010). Using the ratio of blood pressure to height, a simplified method for screening children for their potential risk for hypertension in adulthood (Ma et al. 2016; Xi et al. 2016), 39% had high SBP, 20% had high DBP, and 15% had both high SBP and DBP (data not shown). A correlation matrix of the outcomes is shown in Supplemental Table 3a.
Table 2.
Descriptive statistics for measures and biomarkers of metabolic syndrome
| Measure or Biomarker | N | Mean | Median (Q1, Q3) |
|---|---|---|---|
| Glycosylated hemoglobin A1C (%) | 275 | 5.5 | 5.5 (5.4, 5.7) |
| Leptin (ng/ml)* | 271 | 1.6 | 0.9 (0.7, 1.6) |
| Adiponectin (μg/ml)* | 276 | 25.7 | 20.3 (9.9, 34.2) |
| Urinary 8-isoprostane (creatinine-adjusted) (ng/mg)* | 290 | 5.5 | 4.4 (2.8, 6.6) |
| Systolic blood pressure (mmHg) | 296 | 105.9 | 104.5 (98.0, 113.5) |
| Diastolic blood pressure (mmHg) | 296 | 64.9 | 64.5 (59.5, 69.5) |
| BMI-percentile for age and sex | 299 | 71.0 | 77.9 (53.4, 95.0) |
| BMI for age and sex relative to the 95th percentile** | 299 | 92.1 | 85.9 (78.7, 100.0) |
| Waist-to-height ratio | 244 | 0.5 | 0.5 (0.5, 0.6) |
| Percent body fat | 299 | 27.8 | 28.3 (20.9, 35.6) |
Leptin, adiponectin, and 8-isoprostane were not normally distributed and were logged for analysis.
This was the measure used in our models, based on CDC recommendations if a large proportion of children in the study population have severe obesity (BMI-percentile95 ≥ 120).
Summary data for modeled pollutant concentrations (EC, PAH456, NO2, and PM2.5) for each of the averaging times are presented in Supplemental Table 2. A correlation matrix of the exposure periods is shown in Supplemental Table 3b.
3.2. Associations between air pollutant exposures and metabolic outcomes
The associations between traffic-related air pollutants (TRAP) and all longer-term anthropometric measures and biomarkers (i.e., HbA1C, WHR, percent body fat, BMI-percentile95, leptin and adiponectin) can be seen in Supplemental Table 4 and select measures/biomarkers are shown in Figure 1, while the associations between traffic-related air pollutants and anthropometric measures and biomarkers that may vary over both short-term and longer-term periods (i.e., SBP, DBP, and 8-isoprostane) can be seen in Figure 1 and Supplemental Table 5. The patterns in these results are described below.
Figure 1.
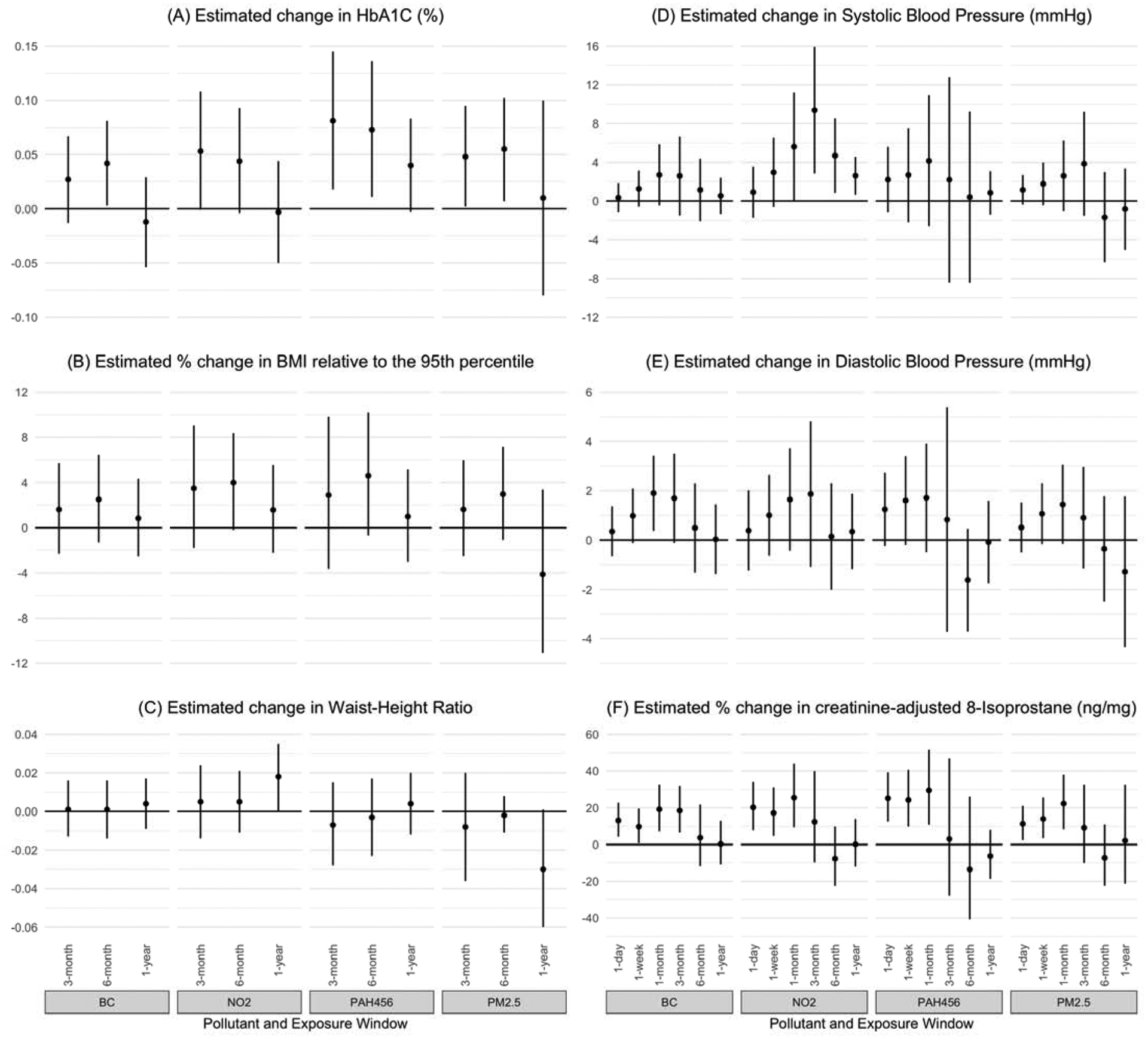
Effects of Traffic-Related Air Pollutants on Anthropometric Measures and Biomarkers of Metabolic Syndrome associated with an IQR increase in exposure
3.2.1. Longer-term metabolic outcome measures
When considering concentrations over the prior 6 months, TRAP is associated with increases in HbA1c (Figure 1A). While these results can be seen in detail in Supplemental Table 4, HbA1c increased approximately 0.04–0.07% per IQR increase in each of the pollutants. For EC, a 0.23 μg/m3 increase in the 6-month average was associated with a 0.042% increase in HbA1c (95% CI: 0.003, 0.081). For NO2, a 3.42 ppb increase in the 6-month average was associated with a 0.044% increase in HbA1c (95% CI: −0.004, 0.093). For PAH456, a 4.42ng/m3 increase in the 6-month average was associated with a 0.073% increase in HbA1c (95% CI: 0.011, 0.136). For PM2.5, a 3.62 μg/m3 increase in the 6-month average was associated with a 0.055% increase in HbA1c (95% CI: 0.007, 0.102). A similar pattern of increases was seen for 3-month average exposures, though fewer of the pollutant relationships met statistical significance at this time frame. Averaging exposure over 1 year, there is no longer a consistent pattern of effects between TRAP and HbA1c.
Associations between TRAP and longer-term anthropometric measures other than HbA1c were less notable. However, small increases in BMI were consistently, but not significantly, associated with increased TRAP across pollutants and exposure windows, especially at 3- and 6-month average exposures (Figure 1B and Supplemental Table 4). Though significance was reached in one isolated finding (1-year average NO2 and WHR; Figure 1C), there was no clear pattern relating TRAP exposure to WHR or percent body fat (Supplemental Table 4). There were no significant associations between TRAP and levels of leptin or adiponectin at 3, 6, or 1-year average exposures (Supplemental Table 4).
3.2.2. Short- and longer-term metabolic outcome measures
While not always achieving statistical significance, we observed small increases in blood pressure measures (both SBP and DBP) associated with pollutant exposure over short periods, with the effect increasing in magnitude across all TRAPs from the 1-day lag up to the 1- or 3-month average exposure, and then decreasing in magnitude down to the 1-year average exposure (Figures 1D and 1E and Supplemental Tables 4 and 5). Statistical significance of effect was only consistently seen for the association between NO2 and SBP – NO2 was associated with increased SBP for medium- and longer-term (1-month, 3-month, 6-month and 1-year) average exposures. The largest estimate was for 3-month average NO2, where a 6.2 ppb increase was associated with a 9.4 mmHg increase in SBP (95% CI: 2.8, 15.9). The average short-term (1-day and 1-week) exposures to NO2 were not significantly different than zero in their relationship to SBP, suggesting that the effects are more strongly related to medium- and longer-term exposures (Figure 1D).
Short-term average TRAP exposure (1-day, 1-week and 1-month) was consistently and significantly associated with creatinine-adjusted urinary 8-isoprostane (Figure 1F and Supplemental Table 5). Estimated percent changes ranged from a 9.8% increase in 8-isoprostane for a 0.37μg/m3 increase in 1-week EC (95% CI: 0.7–19.6), to a 29.6% increase in 8-isoprostane for an 8.15 ng/m3 increase in 1-month PAH456 (95% CI: 10.8–51.5). There were no associations between longer-term TRAP exposures and 8-isoprostane.
4. DISCUSSION
In a well-characterized cohort of young children, we found that estimated average ambient residential exposure to several traffic-related air pollutants was associated with a marker of potential risk for metabolic syndrome (HbA1c), as well as oxidative stress (urinary 8-isoprostane), a hypothesized mediator of air pollution-related health effects. The associations with HbA1c were seen for 3- and 6-month average pollutant exposures, as expected based on the known half-life of HbA1c, while those for urinary 8-isoprostane were observed with shorter averaging times (i.e., 1-day lag, and 1-week and 1-month average pollutant exposures).
Animal studies have shown that 8-isoprostane levels increase quickly (within hours) in response to oxidative stress, yet urinary levels are relatively stable day to day, making this a reliable biomarker (Roberts and Morrow, 2000). The known quick response of 8-isoprostane fits with our findings that air pollution exposures were related to this biomarker over the short term (up to one month or 3 months, depending on the pollutant), but no effects were seen for longer exposure windows. There were also consistent patterns in the associations between traffic-related air pollutants and other outcomes, even when individual effects did not reach statistical significance. Blood pressure was associated with TRAP, with the largest effect for the 3-month exposure window and small increases in BMI were consistently associated with increased TRAP.
Both human exposure and observational studies have demonstrated that air pollution can increase blood pressure in adults (Yang et al. 2018; Li et al. 2020); though data are sparse in children, we anticipated that there may be similar effects (Zeng et al. 2017). Because the mechanisms that affect adult blood pressure likely behave similarly in children, we expected potential short-term effects of pollutants on blood pressure (mechanistically these could occur via acute changes in endothelial function, autonomic regulation, inflammation and oxidative stress; Brook et al. 2009), and we also expected that there could be strong long-term effects (related to vascular remodeling and other adaptation or maladaptation to chronic exposures). It may simply be that air pollutant effects on long-term blood pressure are of smaller magnitude than effects on short-term blood pressure (especially given that there is more short-term variability in blood pressure compared to long-term). However, seasonality effects may also mask the relationships at the 6-month and 1-year intervals. In Fresno, there are functionally two seasons: a hot/dry season (March through October) and a cold/wet season (November through February). Thus, the longer exposure windows are likely to cross seasons and may obscure an effect that would be apparent either within a single season or across multiple complete season cycles.
Motor vehicle and rail line emissions are the likely source of the pollutants for which we report associations – PAHs, NO2, EC, and PM2.5 – in the Fresno area. To our knowledge, this is the first study to show associations between ambient PAH concentrations and increased levels of HbA1c and 8-isoprostane. These results support measurement of ambient PAHs as another marker of the TRAP mixture.
Although there is controversy whether the designation of metabolic syndrome can be applied to young children, certain biomarkers for this condition are present in this age group, and the antecedents of metabolic syndrome in adults likely begin in childhood (Faienza et al. 2016). For example, elevated HbA1c in children predicts risk for type 2 diabetes (Vijayakumar et al. 2017). Similarly, childhood blood pressure has been shown to predict both hypertension and metabolic syndrome in adulthood (Sun et al. 2007; Chen et al. 2008), as well as carotid intima-media thickness, a measure of the progression of atherosclerotic disease (Koskinen et al. 2019). A report from Saudi Arabia suggested that exposure to ambient PAHs was associated with brachial artery distensibility and blood pressure in adolescent males (Trasande et al. 2015). The outcomes assessed in our study were selected to represent indices of metabolic syndrome.
It has been specifically shown that childhood exposures to secondhand tobacco smoke can increase risks for adverse cardiovascular outcomes in adults (Chen et al. 2015; Gall et al. 2014). Because secondhand smoke and ambient air pollution share an exposure route, many chemical constituents, and mechanisms of toxicity, such as oxidative stress and elevated chronic systemic inflammation, childhood exposures to outdoor air pollution may also contribute substantially to the risk of metabolic syndrome later in life (McConnell et al. 2015).
Evidence that air pollution can contribute to type 2 diabetes in adults has been emerging over the past decade (Thiering and Heinrich 2015; Eze et al. 2015; Li et al. 2015; Li et al. 2016a), although the data are less clear for obesity (Chiu et al. 2017; An et al. 2018). More recently, several studies have reported associations between air pollution and both diabetes and obesity in children. Of particular interest, a study in New York City reported associations between maternal exposures to ambient PAHs during pregnancy and increased BMI and body fat composition in the offspring at age 7 (Rundle et al. 2012). A study from Iran showed associations between urinary concentrations of PAH metabolites and obesity in children ages 6–18 (Poursafa et al. 2018). A more recent study in New York City reported that higher prenatal and perinatal exposures to PM2.5 are associated with increased HbA1c (Moody et al. 2019). With regard to pre-diabetes, two reports in overweight and obese Los Angeles children of color showed higher fasting insulin levels, lower insulin sensitivity, higher acute insulin response to glucose, decreased β-cell function, and higher fasting glucose levels with long-term exposures to NO2 and PM2.5 (Alderete et al. 2017; Toledo-Corral et al. 2018). These findings suggest potential mechanisms by which TRAP exposures impact type 2 diabetes risk. Long-term air pollution exposure may both decrease insulin-dependent glucose uptake leading to insulin resistance and impair β-cell function resulting in reduced insulin secretion (Park 2017). Experimental animal data suggest upstream mechanistic pathways linking traffic-related air pollution exposure to insulin resistance and β-cell dysfunction that include oxidative stress, systemic inflammation, and adipose tissue inflammation (Rajagopalan and Brook 2012). However, to date, these pathways have not been adequately studied in children.
Chronic systemic inflammation is known to be associated with insulin resistance that is characterized by abnormal production of adipokines such as leptin and adiponectin (Rajagopalan and Brook 2012; Piya et al. 2013). Leptin upregulation is associated with chronic systemic inflammation; whereas, adiponectin is associated with anti-inflammatory functions. We hypothesized exposures to traffic-related air pollutants would induce inflammation that would, in turn, affect levels of these adipokines in our study children. However, we did not find significant associations between either short-term or longer-term exposure to our air pollutants of interest and adipokine levels. Mean adiponectin levels were much higher in both boys and girls aged 7 and 8 in our cohort compared with European children in the IDEFICS cohort (Erhardt et al. 2014). However, the mean adiponectin values for our study population were lower than those for a cohort of demographically similar 9-year-old Mexican-American children from Salinas, CA (Volberg et al. 2013). Mean leptin values in our cohort were lower for both boys and girls than in the IDEIFCS cohort and lower than both boys and girls in the Salinas cohort (Erhardt et al. 2014; Volberg et al. 2013).
A paper from the Framingham Heart Study reported that living near a major roadway and exposure to traffic-related air pollution were associated with glucose dysregulation, but there was no such association with either leptin or adiponectin (Li et al. 2018). Our pattern of findings with significant relationships between HbA1c and TRAP exposures fits with these prior results. Other studies of air pollution exposure and adipokines, both experimental animal and epidemiological, have reported mixed results (Sun et al. 2009; Wang et al. 2014; Wolf et al. 2016).
Oxidative stress is one pathway by which exposure to ambient air pollutants can induce systemic inflammation. We and others have previously used 8-isoprostane, a marker of lipid peroxidation, to assess air pollution-induced oxidative stress (Chen et al. 2007; Li et al. 2016b), though some animal evidence suggest that it may be produced from other sources of inflammation (van’t Erve et al 2016). The mean urinary 8-isoprostane level in our cohort, adjusted for creatinine, was higher than the mean value from the 9-year-old Salinas cohort (Tran et al. 2017). Importantly, short-term exposures to all four traffic-related air pollutants (PAH456, PM2.5, EC, and NO2) were significantly associated with elevated urinary concentrations of 8-isoprostane, supporting oxidative stress and possibly inflammation as mediators of the adverse metabolic effects of traffic-related air pollution.
This study has multiple strengths, including high-quality, individual-level exposure assessment for traffic-related air pollutants, careful outcome assessment for several biomarkers of pre-metabolic syndrome, and a study population of vulnerable children of color. In particular, our ability to assign exposures to ambient PAHs for our study participants is unique. The combination of measures of obesity, glucose dysregulation, and oxidative stress, and measurement of blood pressure provide a battery of biomarkers that together likely represent risk for metabolic syndrome later in life. Our study population of almost 90% Latinx or Black children who have high prevalence of increased BMI, HbA1c, and blood pressure, constitutes a high-risk group.
Despite these strengths, our study has some limitations. Because the analysis is cross-sectional, the temporality of exposure-response cannot be assessed. The cross-sectional nature of our analysis also may obscure within-person variability in our measured outcomes, such as blood pressure, where variation may not be well captured using single point-in-time measurements. We are currently following our cohort of children longitudinally, and future longitudinal analyses will be able to address some of these limitations of our current cross-sectional analysis.
In addition, our results could be affected by residual confounding. Although we considered inclusion of a number of variables in our models, our adjustment for socioeconomic factors (using household income) may not have been sufficient to account for all other relevant social and environmental factors. We also performed a large number of statistical tests, examining nine outcomes and four pollutants with multiple exposure periods (n=144 comparisons in total). While performing these multiple comparisons increases the likelihood of incorrectly rejecting a null hypothesis, we have been careful here not to draw attention to any one particular association. Instead, we describe consistent patterns in the relationships between several highly correlated traffic-related air pollutants and multiple markers of metabolic syndrome, and these trends appear to be robust.
We also do not have sufficient data on lipid metabolism and markers of systemic inflammation to allow a more complete characterization of the antecedents of metabolic syndrome in our child participants. We are in the process of collecting longitudinal data for this cohort at ages 9–11 and will be able to better characterize participants as well as better address temporality. It is curious that 1-year averages of PAH456, PM2.5, and EC were generally not associated with the outcomes we examined. It is possible that this is because the vast majority of our participants are from a relatively small geographic area – the part of Fresno served by FUSD – and thus the spatial and temporal variability in annual averages may not have been sufficient to detect associations with these longer-term exposures. Future analyses using distributed lag models to flexibly model yearly exposures without averaging are planned.
In conclusion, the results of this study suggest that estimated individual-level outdoor residential exposures to several traffic-related air pollutants, including ambient PAHs, are associated with measures and biomarkers of metabolic syndrome (increased HbA1c and blood pressure) and of oxidative stress (increased urinary 8-isoprostane) in a well-characterized cohort primarily consisting of low-income children of color. Our results provide evidence that exposure to traffic-related air pollution may contribute to risk of obesity and glucose dysregulation in high-risk children of color, of whom there are many in the United States.
Supplementary Material
HIGHLIGHTS.
Traffic-related air pollution was associated with metabolic outcomes in children
Children were ages 6–8 and predominantly low-income and of color (Latinx or Black)
Air pollutants included polycyclic aromatic hydrocarbons and elemental carbon
Outcomes included HbA1c, systolic blood pressure, and oxidative stress
ACKNOWLEDGEMENTS:
The authors would like to thank the UCSF Fresno research team (Griselda Aguilar, Christian Bonilla, Karina Corona, Cynthia Cortez, Alexa Lopez, Carolina Orozco and Janna Blaauw) for their hard work in conducting the clinical visits, Brian Nguyen and Kelly Nabaglo for their assistance with blood and urine analyses in the Holland laboratory, Barune Thapa and Beth MacDonald for general assistance with study management, and all of the participating children and families for their patience and dedication to the study.
FUNDING:
This research was supported by the Children’s Health and Air Pollution Study (CHAPS), an NIH/EPA-funded Children’s Environmental Health Research Center (EPA: RD83543501, NIH: ES022849) and an additional grant (NIH: F31ES0277510). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Gwen Tindula received a Student/New Investigator Travel Award of $750.00 to attend and present at the 2019 Environmental Mutagenesis and Genomics Society (EMGS) meeting in Washington DC from September 19-23.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CREDIT AUTHOR STATEMENT
JKM and LL co-equally led the data collection, data management and data analysis and made major contributions to the interpretation of the data and writing of the manuscript; SMH assisted with data analysis, data interpretation manuscript revision; HGM, AMN, EAE and SC assisted with data analysis and interpretation; TT, MP, and KN assisted with data collection; NH and GT performed the biomarker assays; EMN, FL, and SKH conducted the air pollution exposure assessment; JRB conceived the study, supervised the overall conduct of the study, and led the writing of the manuscript. All authors reviewed drafts and contributed to the writing of the manuscript.
The authors report no conflicts of interest.
REFERENCES
- Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC Jr. 2009. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 120(16):1640–1645, 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- Alderete TL, Habre R, Toledo-Corral CM, Berhane K, Chen Z, Lurmann FW, Weigensberg MJ, Goran MI, Gilliland FD. 2017. Longitudinal associations between ambient air pollution with insulin sensitivity, β-cell function, and adiposity in Los Angeles Latino children. Diabetes 66(7):1789–1796, 10.2337/db16-1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Diabetes Association. 2010. Diagnosis and classification of diabetes mellitus. Diabetes Care 33(Suppl. 1):S62–S69, 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An R, Ji M, Yan H, Guan C. 2018. Impact of ambient air pollution on obesity: a systematic review. Int J Obes (Lond) 42(6):1112–1126, 10.1038/s41366-018-0089-y. [DOI] [PubMed] [Google Scholar]
- Babey SH, Wolstein J, Diamant AL, Goldstein H. 2016. Prediabetes in California: nearly half of California adults on path to diabetes. Policy Brief UCLA Cent Health Policy Res Mar;(PB2016–1):1–8, http://healthpolicy.ucla.edu/publications/search/pages/detail.aspx?pubID=1472. [PubMed] [Google Scholar]
- Brook RD, Urch B, Dvonch JT, Bard RL, Speck M, Keeler G, Morishita M, Marsik FJ, Kamal AS, Kaciroti N, Harkema J, Corey P, Silverman F, Gold DR, Wellenius G, Mittleman MA, Rajagopalan S, Brook JR. 2009. Insights into the mechanisms and mediators of the effects of air pollution exposure on blood pressure and vascular function in healthy humans. Hypertension 54(3):659–67, 10.1161/HYPERTENSIONAHA.109.130237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- California Department of Education. 2017. California School Dashboard District Performance Overview: Fresno Unified. https://www.caschooldashboard.org/reports/10621660000000/2017 (accessed 12/7/2020).
- Centers for Disease Control and Prevention. 2011a. National Health and Nutrition Examination Survey Anthropometry Procedures Manual. https://wwwn.cdc.gov/nchs/data/nhanes/2011-2012/manuals/Anthropometry_Procedures_Manual.pdf (accessed 1/28/2019).
- Centers for Disease Control and Prevention. 2011b. National Health and Nutrition Examination Survey Physician Physical Examination Procedures Manual. https://www.cdc.gov/nchs/data/nhanes/nhanes_11_12/Physician_Exam_Manual.pdf (accessed 1/28/2019).
- Centers for Disease Control and Prevention. 2016. A SAS Program for the 2000 CDC Growth Charts (ages 0 to <20 years). https://www.cdc.gov/nccdphp/dnpao/growthcharts/resources/sas.htm (accessed 1/29/2019).
- Chen C, Arjomandi M, Balmes J, Tager I, Holland N. 2007. Effects of chronic and acute ozone exposure on lipid peroxidation and antioxidant capacity in healthy young adults. Environ Health Perspect 115(12):1732–1737, 10.1289/ehp.10294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Yun M, Fernandez C, Li S, Sun D, Lai CC, Hua Y, Wang F, Zhang T, Srinivasan SR, Johnson CC, Berenson GS. 2015. Secondhand smoke exposure is associated with increased carotid artery intima-media thickness: the Bogalusa Heart Study. Atherosclerosis 240(2):374–379, 10.1016/j.atherosclerosis.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Wang Y. 2008. Tracking of blood pressure from childhood to adulthood: a systematic review and meta-regression analysis. Circulation 117(25):3171–3180, 10.1161/CIRCULATIONAHA.107.730366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu YM, Hsu HL, Wilson A, Coull BA, Pendo MP, Baccarelli A, Kloog I, Schwartz J, Wright RO, Taveras EM, Wright RJ. 2017. Prenatal particulate air pollution exposure and body composition in urban preschool children: examining sensitive windows and sex-specific associations. Environ Res 158:798–805, 10.1016/j.envres.2017.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erhardt E, Foraita R, Pigeot I, Barba G, Veidebaum T, Tornaritis M, Michels N, Eiben G, Ahrens W, Moreno LA, Kovács E, Molnár D; IDEFICS consortium. 2014. Reference values for leptin and adiponectin in children below the age of 10 based on the IDEFICS cohort. Int J Obes (Lond) 38 Suppl 2:S32–8. 10.1038/ijo.2014.133. [DOI] [PubMed] [Google Scholar]
- Eze IC, Hemkens LG, Bucher HC, Hoffmann B, Schindler C, Künzli N, Schikowski T, Probst-Hensch NM. 2015. Association between ambient air pollution and diabetes mellitus in Europe and North America: systematic review and meta-analysis. Environ Health Perspect 123(5):381–389, 10.1289/ehp.1307823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faienza MF, Wang DQ, Frühbeck G, Garruti G, Portincasa P. 2016. The dangerous link between childhood and adulthood predictors of obesity and metabolic syndrome. Intern Emerg Med 11(2):175–182, 10.1007/s11739-015-1382-6. [DOI] [PubMed] [Google Scholar]
- Gall S, Huynh QL, Magnussen CG, Juonala M, Viikari JS, Kähönen M, Dwyer T, Raitakari OT, Venn A. 2014. Exposure to parental smoking in childhood or adolescence is associated with increased carotid intima-media thickness in young adults: evidence from the Cardiovascular Risk in Young Finns study and the Childhood Determinants of Adult Health Study. Eur Heart J 35(36):2484–2491, 10.1093/eurheartj/ehu049. [DOI] [PubMed] [Google Scholar]
- Goran MI, Kaskoun MC, Carpenter WH, Poehlman ET, Ravussin E, Fontvieille AM. 1993. Estimating body composition of young children by using bioelectrical resistance. J Appl Physiol (1985) 75(4):1776–1780, 10.1152/jappl.1993.75.4.1776. [DOI] [PubMed] [Google Scholar]
- Huang PL. 2009. A comprehensive definition for metabolic syndrome. Dis Model Mech 2(5–6): 231–237, 10.1242/dmm.001180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutcheson R, Rocic P. 2012. The metabolic syndrome, oxidative stress, environment, and cardiovascular disease: the great exploration. Exp Diabetes Res 2012:271028, 10.1155/2012/271028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koskinen J, Juonala M, Dwyer T, Venn A, Petkeviciene J, Čeponienė I, Bazzano L, Chen W, Sabin MA, Burns TL, Viikari JSA, Woo JG, Urbina EM, Prineas R, Hutri-Kähönen N, Sinaiko A, Jacobs DR Jr, Steinberger J, Daniels S, Raitakari O, Magnussen CG. 2019. Utility of different blood pressure measurement components in childhood to predict adult carotid intima-media thickness. Hypertension 73(2):335–341, 10.1161/HYPERTENSIONAHA.118.12225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Qian Z, Vaughn M, Boutwell B, Ward P, Lu T, Lin S, Zhao Y, Zeng XW, Liu RQ, Qin XD, Zhu Y, Chen W, Dong GH. 2015. Sex-specific difference of the association between ambient air pollution and the prevalence of obesity in Chinese adults from a high pollution range area: 33 Communities Chinese Health Study. Atmos Environ 117:227–33, 10.1016/j.atmosenv.2015.07.029. [DOI] [Google Scholar]
- Li N, Chen G, Liu F, Mao S, Liu Y, Liu S, Mao Z, Lu Y, Wang C, Guo Y, Xiang H, Li S. 2020. Associations between long-term exposure to air pollution and blood pressure and effect modifications by behavioral factors. Environ Res 182:109109, 10.1016/j.envres.2019.109109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Dorans KS, Wilker EH, Rice MB, Schwartz J, Coull BA, Koutrakis P, Gold DR, Fox CS, Mittleman MA. 2016a. Residential proximity to major roadways, fine particulate matter, and adiposity: The Framingham heart study. Obesity (Silver Spring) 24(12):2593–2599, https://doi.org/0.1002/oby.21630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Wilker EH, Dorans KS, Rice MB, Schwartz J, Coull BA, Koutrakis P, Gold DR, Keaney JF Jr, Lin H, Vasan RS, Benjamin EJ, Mittleman MA. 2016b. Short-term exposure to air pollution and biomarkers of oxidative stress: The Framingham Heart Study. J Am Heart Assoc 5(5) pii: e002742, 10.1161/JAHA.115.002742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Dorans KS, Wilker EH, Rice MB, Kloog I, Schwartz JD, Koutrakis P, Coull BA, Gold DR, Meigs JB, Fox CS, Mittleman MA. 2018. Ambient air pollution, adipokines, and glucose homeostasis: The Framingham Heart Study. Environ Int 111(2):14–22, 10.1016/j.envint.2017.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim CC, Thurston GD. 2019. Air pollution, oxidative stress, and diabetes: a life course epidemiologic perspective. Curr Diab Rep 19(8):58, 10.1007/s11892-019-1181-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C, Kelishadi R, Hong YM, Bovet P, Khadilkar A, Nawarycz T, Krzywińska-Wiewiorowska M, Aounallah-Skhiri H, Zong X, Motlagh ME, Kim HS, Khadilkar V, Krzyżaniak A, Ben Romdhane H, Heshmat R, Chiplonkar S, Stawińska-Witoszyńska B, El Ati J, Qorbani M, Kajale N, Traissac P, Ostrowska-Nawarycz L, Ardalan G, Parthasarathy L, Zhao M, Xi B. 2016. Performance of eleven simplified methods for the identification of elevated blood pressure in children and adolescents. Hypertension 68(3):614–620, 10.1161/HYPERTENSIONAHA.116.07659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis HG, Mann JK, Lurmann FW, Mortimer KM, Balmes JR, Hammond SK, Tager IB. 2009. Altered pulmonary function in children with asthma associated with highway traffic near residence. Int J Environ Health Res 19(2):139–55, 10.1080/09603120802415792. [DOI] [PubMed] [Google Scholar]
- McConnell R, Shen E, Gilliland FD, Jerrett M, Wolch J, Chang CC, Lurmann F, Berhane K. 2015. A longitudinal cohort study of body mass index and childhood exposure to secondhand tobacco smoke and air pollution: the Southern California Children’s Health Study. Environ Health Perspect 123(4):360–366, 10.1289/ehp.1307031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody EC, Cantoral A, Tamayo-Ortiz M, Pizano-Zárate ML, Schnaas L, Kloog I, Oken E, Coull B, Baccarelli A, Téllez-Rojo MM, Wright RO, Just AC. 2019. Association of prenatal and perinatal exposures to particulate matter with changes in hemoglobin A1c Levels in children aged 4 to 6 years. JAMA Netw Open 2;2(12):e1917643, 10.1001/jamanetworkopen.2019.17643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardone A, Casey JA, Morello-Frosch R, Mujahid M, Balmes JR, Thakur N. 2020. Associations between historical residential redlining and current age-adjusted rates of emergency department visits due to asthma across eight cities in California: an ecological study. Lancet Planet Health 4(1):e24–e31. 10.1016/S2542-5196(19)30241-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noth EM, Hammond SK, Biging GS and Tager IB. 2011. A spatial-temporal regression model to predict daily outdoor residential PAH concentrations in an epidemiologic study in Fresno, CA. Atmos Environ 45(11): 2394–2403, 10.1016/j.atmosenv.2011.02.014. [DOI] [Google Scholar]
- Noth EM, Lurmann F, Northcross A, Perrino C, Vaughn D, Hammond SK. 2016. Spatial and temporal distribution of polycyclic aromatic hydrocarbons and elemental carbon in Bakersfield, California. Air Qual Atmos Health 9(8):899–908, 10.1007/s11869-016-0399-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noth EM, Lurmann F, Perrino C, Vaughn D, Minor HA, Hammond SK. 2020. Decrease in Ambient Polycyclic Aromatic Hydrocarbon Concentrations in California’s San Joaquin Valley 2000–2019. Atmos Environ (1994) 242:117818., 10.1016/j.atmosenv.2020.117818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Kit BK, Flegal KM. 2012. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999–2010. JAMA 307(5):483–490, 10.1001/jama.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Özkaynak H, Baxter LK, Dionisio KL, Burke J. 2013. Air pollution exposure prediction approaches used in air pollution epidemiology studies. J Expo Sci Environ Epidemiol 23(6):566–72, 10.1038/jes.2013.15. [DOI] [PubMed] [Google Scholar]
- Park SK. 2017. Ambient air pollution and type 2 diabetes: do the metabolic effects of air pollution start early in life? Diabetes 66(7):1755–1757, 10.2337/dbi17-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piya MK, McTernan PG, Kumar S. 2013. Adipokine inflammation and insulin resistance: the role of glucose, lipids and endotoxin. J Endocrinol 216(1):T1–T15, 10.1530/JOE-12-0498. [DOI] [PubMed] [Google Scholar]
- Poursafa P, Dadvand P, Amin MM, Hajizadeh Y, Ebrahimpour K, Mansourian M, Pourzamani H, Sunyer J, Kelishadi R. 2018. Association of polycyclic aromatic hydrocarbons with cardiometabolic risk factors and obesity in children. Environ Int 118:203–210, 10.1016/j.envint.2018.05.048. [DOI] [PubMed] [Google Scholar]
- Rajagopalan S, Brook RD. 2012. Air pollution and type 2 diabetes: mechanistic insights. Diabetes 61:3037–3045, 10.2337/db12-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts LJ, Morrow JD. 2000. Measurement of F(2)-isoprostanes as an index of oxidative stress in vivo. Free Radic Biol Med 28(4):505–13, 10.1016/s0891-5849(99)00264-6. [DOI] [PubMed] [Google Scholar]
- Rundle A, Hoepner L, Hassoun A, Oberfield S, Freyer G, Holmes D, Reyes M, Quinn J, Camann D, Perera F, Whyatt R. 2012. Association of childhood obesity with maternal exposure to ambient air polycyclic aromatic hydrocarbons during pregnancy. Am J Epidemiol 175(11):1163–72, 10.1093/aje/kwr455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q, Yue P, Deiuliis JA, Lumeng CN, Kampfrath T, Mikolaj MB, Cai Y, Ostrowski MC, Lu B, Parthasarathy S, Brook RD, Moffatt-Bruce SD, Chen LC, Rajagopalan S. 2009. Ambient air pollution exaggerates adipose inflammation and insulin resistance in a mouse model of diet-induced obesity. Circulation 119(4):538–546, 10.1161/CIRCULATIONAHA.108.799015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun SS, Grave GD, Siervogel RM, Pickoff AA, Arslanian SS, Daniels SR. 2007. Systolic blood pressure in childhood predicts hypertension and metabolic syndrome later in life. Pediatrics 119(2):237–246, 10.1016/j.jpeds.2007.07.055. [DOI] [PubMed] [Google Scholar]
- Tager IB, Balmes J, Lurmann F, Ngo L, Alcorn S, Künzli N. 2005. Chronic exposure to ambient ozone and lung function in young adults. Epidemiology 16(6):751–9, 10.1097/01.ede.0000183166.68809.b0. [DOI] [PubMed] [Google Scholar]
- Thiering E, Heinrich J. 2015. Epidemiology of air pollution and diabetes. Trends Endocrinol Metab 26(7):384–394, 10.1016/j.tem.2015.05.002. [DOI] [PubMed] [Google Scholar]
- Toledo-Corral CM, Alderete TL, Habre R, Berhane K, Lurmann FW, Weigensberg MJ, Goran MI, Gilliland FD. 2018. Effects of air pollution exposure on glucose metabolism in Los Angeles minority children. Pediatr Obes 13(1):54–62, 10.1111/ijpo.12188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran V, Tindula G, Huen K, Bradman A, Harley K, Kogut K, Calafat AM, Nguyen B, Parra K, Ye X, Eskenazi B, Holland N. 2017. Prenatal phthalate exposure and 8-isoprostane among Mexican-American children with high prevalence of obesity. J Dev Orig Health Dis 8(2):196–205, 10.1017/S2040174416000763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trasande L, Urbina EM, Khoder M, Alghamdi M, Shabaj I, Alam MS, Harrison RM, Shamy M. 2015. Polycyclic aromatic hydrocarbons, brachial artery distensibility and blood pressure among children residing near an oil refinery. Environ Res 136(1):133–140, 10.1016/j.envres.2014.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van’t Erve TJ, Lih FB, Jelsema C, et al. 2016. Reinterpreting the best biomarker of oxidative stress: The 8-iso-prostaglandin F2α/prostaglandin F2α ratio shows complex origins of lipid peroxidation biomarkers in animal models. Free Radic Biol Med 95:65–73, 10.1016/j.freeradbiomed.2016.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayakumar P, Nelson RG, Hanson RL, Knowler WC, Sinha M. 2017. HbA1c and the prediction of type 2 diabetes in children and adults. Diabetes Care 40(1):16–21, 10.2337/dc16-1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volberg V, Harley KG, Aguilar RS, Rosas LG, Huen K, Yousefi P, Davé V, Phan N, Lustig RH, Eskenazi B, Holland N. 2013. Associations between perinatal factors and adiponectin and leptin in 9-year-old Mexican-American children. Pediatr Obes 8(6):454–463, 10.1111/j.2047-6310.2012.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Eliot MN, Kuchel GA, Schwartz J, Coull BA, Mittleman MA, Lipsitz LA, Wellenius GA. 2014. Long-term exposure to ambient air pollution and serum leptin in older adults: results from the MOBILIZE Boston study. J Occup Environ Med 56(9):e73–77, 10.1097/JOM.0000000000000253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf K, Popp A, Schneider A, Breitner S, Hampel R, Rathmann W, Herder C, Roden M, Koenig W, Meisinger C, Peters A, KORA-Study Group. 2016. Association between long-term exposure to air pollution and biomarkers related to insulin resistance, subclinical inflammation, and adipokines. Diabetes 65(11):3314–3326, 10.2337/db15-1567. [DOI] [PubMed] [Google Scholar]
- Xi B, Zhang T, Zhang M, Liu F, Zong X, Zhao M, Wang Y. 2016. Trends in Elevated Blood Pressure Among US Children and Adolescents: 1999–2012. Am J Hypertens 29(2):217–225, 10.1093/ajh/hpv091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang BY, Qian Z, Howard SW, Vaughn MG, Fan SJ, Liu KK, Dong GH. 2018. Global association between ambient air pollution and blood pressure: A systematic review and meta-analysis. Environ Pollut 235(4):576–588, 10.1016/j.envpol.2018.01.001. [DOI] [PubMed] [Google Scholar]
- Zeng XW, Qian ZM, Vaughn MG, Nelson EJ, Dharmage SC, Bowatte G, Perret J, Chen DH, Ma H, Lin S, de Foy B, Hu LW, Yang BY, Xu SL, Zhang C, Tian YP, Nian M, Wang J, Xiao X, Bao WW, Zhang YZ, Dong GH. 2017. Positive association between short-term ambient air pollution exposure and children blood pressure in China-Result from the Seven Northeast Cities (SNEC) study. Environ Pollut 224:698–705, 10.1016/j.envpol.2017.02.054. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Dong S, Wang H, Tao S, Kiyama R. 2016. Biological impact of environmental polycyclic aromatic hydrocarbons (ePAHs) as endocrine disruptors. Environ Pollut 213:809–824. 10.1016/j.envpol.2016.03.050. [DOI] [PubMed] [Google Scholar]
- Zuk MZ. 2013. Health equity in a new urbanist environment: land use planning and community capacity building in Fresno, CA (Doctoral dissertation, University of California, Berkeley; ), http://escholarship.org/uc/item/4pq5p68j. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


