Abstract
Purpose
Hypertension is associated with incident atrial fibrillation (AF) and AF-related complications. We investigated the associations between average systolic blood pressure (SBP) and outcomes in a nationwide cohort of Asian patients with non-valvular atrial fibrillation (NVAF).
Patients and Methods
A multicenter nationwide registry of patients with NVAF in Thailand was conducted during 2014–2017. Clinical data, including blood pressure, were recorded at baseline and then every 6 months. Average SBP was calculated from the average of SBP from every visit. Cox regression models were used to calculate the rate of clinical outcomes of interest, ie ischemic stroke or transient ischemic attack (TIA), intracerebral hemorrhage (ICH), and all-cause death. Average SBP was categorized into three groups: <120, 120–140, and ≥140 mmHg.
Results
A total of 3402 patients were included, and the mean age was 67.4±11.3 years. The mean (±SD) baseline and average SBPs were 128.5±18.5 and 128.0±13.4 mmHg, respectively. The mean follow-up duration was 25.7±10.6 months. The median rate of ischemic stroke/TIA, ICH, and all-cause death was 1.43 (1.17–1.74), 0.70 (0.52–0.92), and 3.77 (3.33–4.24) per 100 person-years, respectively. The rate of ischemic stroke/TIA and ICH was lowest in patients with average SBP <120 mmHg, and highest among those with average SBP ≥140 mmHg. The death rates were consistent with a J-curve effect, being lowest in patients with an average SBP 120–140 mmHg. Sustained SBP control is more important than the SBP from a single visit.
Conclusion
Sustained control of SBP was significantly associated with a reduction in adverse clinical outcomes in patients with NVAF.
Keywords: atrial fibrillation, ischemic stroke, intracerebral hemorrhage, blood pressure, hypertension
Introduction
Non-valvular atrial fibrillation (NVAF) is the most common sustained arrhythmia,1 with a greater prevalence and incidence in the elderly.2 More than half of patients with NVAF have hypertension,3 which is a risk factor for both ischemic and hemorrhagic stroke.4 Indeed, treatment of hypertension can reduce stroke and bleeding risk.5 The annual rate of major bleeding and intracerebral hemorrhage (ICH) in patients who were on warfarin was approximately 1–2% and 0.5%, respectively.6 Although the non-vitamin K antagonist oral anticoagulants (NOAC) have a lower rate of ICH compared to warfarin, these drugs are prescribed less than warfarin in Thailand due to their comparatively high cost and the reimbursement policies of the national healthcare coverage schemes in Thailand.7 The presence of uncontrolled hypertension can lead to bleeding, especially in patients who are on oral anticoagulants (OAC).8
The J-curve effect has been demonstrated in patients with diabetes9 and coronary artery disease.10 Results of the SPRINT trial showed that the rate of cardiovascular events and death was lower among patients with a systolic blood pressure less than 120 mmHg.11 Thus, the results of the SPRINT trial do not support the J-curve theory leading to a guideline recommendation of a lower blood pressure target, especially in the presence of hypertensive target organ damage.12,13 There has been no specific recommendation that the treatment of hypertension in NVAF should be different from the treatment of hypertension in non-NVAF patients.5,12
The aim of this study was to investigate the associations between average systolic blood pressure (SBP) and clinical outcomes in a nationwide cohort of Asian patients with NVAF using prospective data from a multicenter nationwide NVAF registry in Thailand.
Methods
Study Population, Study Protocol and Data Collection
The COhort of antithrombotic use and Optimal INR Level in patients with non-valvular Atrial Fibrillation in Thailand (COOL-AF) registry is a prospective multicenter registry of patients with NVAF that was conducted from 2014 to 2017.7 We enrolled patients with NVAF aged older than 18 years. Patients who had the following conditions were excluded: 1) rheumatic valvular disease; 2) mechanical heart valve; 3) hematologic conditions that increased bleeding risk, such as thrombocytopenia and myeloproliferative disorders; 4) NVAF from transient reversible cause, such as pneumonia; 5) life expectancy less than 3 years; 6) ischemic stroke within 3 months; 7) could not attend follow-up visits; 8) pregnancy; and/or 9) refusal to participate. For this study, patients needed to have at least 6 months of follow-up data. Details of study population and study protocol were previously described.7 The protocol for this study was approved by the Institutional Review Board (IRB) of Faculty of Medicine, Siriraj Hospital, Mahidol University, Faculty of Medicine, Chulalongkorn University, Faculty of Medicine Ramathibodi Hospital, Mahidol University, Faculty of Medicine, Chiang Mai University, Police General Hospital, Phramongkutklao College of Medicine, Faculty of Medicine Vajira Hospital, Navamindradhiraj University, Central Chest Institute of Thailand, Faculty of Medicine, Prince of Songkla University, Faculty of Medicine, Thammasat University, Rangsit Campus, Faculty of Medicine, Naresuan University, Faculty of Medicine, Khon Kaen University, Golden Jubilee Medical Center, Charoen Krung Pracha Rak Hospital, Lampang Hospital, Nakornping Hospital, Prapokklao Hospital (Chanthaburi), Maharat Nakhon Ratchasima Hospital, Suratthani Hospital, Chonburi Hospital, Buddhachinaraj Hospital, Sapphasitthiprasong Hospital, Ratchaburi Hospital, Chiangrai Prachanukroh Hospital, Udonthani Hospital, Queen Savang Vadhana Memorial Hospital, Surin Hospital. All patients gave written informed consent prior to participation.
The following baseline data were recorded: 1) demographic data; 2) weight and height; 3) blood pressure and pulse rate; 4) risk factors (diabetes, hypertension, smoking, dyslipidemia); 5) the components of the CHA2DS2-VASc and HAS-BLED scores; 6) comorbidities (heart failure, coronary artery disease, stroke); 7) laboratory data; 8) cardiac investigations (ECG, echocardiogram); and 9) medications, including anticoagulants and antiplatelets. Investigators were instructed to measure blood pressure according to guideline recommendation.12 To improve accuracy of blood pressure measurement in patients with AF, investigator were encouraged to take three blood pressure measurements and recording the average.14 All required data were entered into a web-based system. Site monitoring was performed at every study site to confirm data quality and to ensure that the study was conducted according to good clinical practice (GCP) guideline.
Outcomes
The following outcomes were collected: 1) death and cause of death; 2) ischemic stroke or transient ischemic attack (TIA); 3) major bleeding, including intracerebral hemorrhage (ICH); 4) heart failure; and, 5) myocardial infarction. For this study, we focused on ischemic stroke/TIA, ICH, and all-cause death. The occurrence and date of each clinical outcome was recorded in the case record form and the web-based system. For all outcomes, investigators had to upload the source documents used to support the outcomes into the web-based system. Source documents were sent to adjudication committee members to conclude the presence or absence of clinical outcomes. Queries seeking additional information or clarification were made when needed.
Definitions of Outcomes
Ischemic stroke was defined as acute onset of neurological deficit lasting for at least 24 hours. The diagnosis was confirmed by the physician after a review of clinical information and imaging data. TIA was defined similar to ischemic stroke, but the duration of the neurological deficit is less than 24 hours. ICH was defined as acute onset of neurological deficit caused by bleeding within the central nervous system that was confirmed by imaging.
Statistical Analysis
Continuous data are described as mean plus/minus standard deviation (SD), and categorical data are presented as number and percentage. Average SBP was calculated by adding all of the SBP values from all visits, and then divided that sum by the number of visits. Patients were categorized into the three following groups based on average SBP: Group 1, <120 mmHg; Group 2, 120–140 mmHg; and Group 3, ≥140 mmHg. Comparisons of continuous data among three groups were performed by analysis of variance (ANOVA) test with Bonferroni post hoc analysis. Comparisons of categorical data among three groups were performed by chi-square test with Bonferroni post hoc analysis. Occurrence of clinical outcomes is described by rate per 100 person-years and 95% confidence interval (CI). The results of survival analysis of clinical outcomes compared among the three average SBP groups were compared using Log rank test. Cubic spline graph was used to calculate the hazard ratio and 95% CI of clinical outcomes with average SBP being considered as a continuous data. Cox proportional hazards model was used to perform univariate and multivariate analysis to determine the effect of average SBP on each clinical outcome. Multivariate analysis was then performed to determine the effect of average SBP on each clinical outcome with two models of adjustment: 1) adjustment for baseline covariates, including age, gender, risk factors, and comorbidities, and antithrombotic medications and 2) adjustment for time varying covariates for the factors mentioned in model 1. Cox model was also used to calculate the rate of clinical outcome with “death without event” considered to be a competing risk. Generalized estimating equation (GEE) with exchangeable correlation structure was used to determine the effect of the average SBP groups on clinical outcomes with the adjustment of time varying covariates. Sensitivity analysis was performed for the assessment of the relationship between the three average SBP groups and clinical outcomes using Cox proportional hazards model and cubic spline graph in which average SBP was treated as a continuous variable. Sensitivity analysis was also performed for the assessment of the interaction test among focused subgroups, ie, 1) with and without history of hypertension, 2) with and without antihypertensive drugs, 3) with and without OAC, 4) with and without CKD, and 5) with and without exclusion of type 2 diabetes or eGFR <20 mL/min/1.73 m2 mimicking exclusion population of SPRINT trial. All analyses were performed using SPSS statistical software version 18.0 (SPSS, Inc., Chicago, IL, USA) and R version 3.6.3 (www.r-project.org).
Results
Baseline Characteristics
There were a total of 3402 patients (mean age was 67.4±11.3 years; 58.2% male) were included. The mean ±SD baseline and average SBPs were 128.5±18.5 and 128.0±13.4 mmHg, respectively. The study population was categorized into three groups according to average SBP, as follows: Group 1 (average SBP <120 mmHg) 922 patients, Group 2 (average SBP ≥120, but <140 mmHg) 1866 patients, and Group 3 (average SBP ≥140 mmHg) 614 patients. Baseline characteristics of the entire study population, including a comparison among the three average SBP groups, are shown in Table 1. Overall, Group 3 had more elderly subjects and females; as well as more paroxysmal AF, diabetes mellitus, dyslipidemia and antiplatelet therapy use; as well as higher mean CHA2DS2-VASc and HAS-BLED scores; and were less likely to have heart failure.
Table 1.
Baseline Demographic and Clinical Characteristics Among the Three Average SBP Groups
| Characteristics | All Patients (N=3402) | SBP Average <120 (n=922) | SBP Average 120 to <140 (n=1866) | SBP Average ≥140 (n=614) | p-value |
|---|---|---|---|---|---|
| Age (years) | 67.4±11.3 | 65.6±12.4 | 67.5±10.8 | 69.5±10.5 | <0.001 |
| Female gender | 1422 (41.8%) | 368 (39.9%) | 760 (40.7%) | 294 (47.9%) | 0.003 |
| Time after diagnosis of AF (years) | 3.36±4.33 | 3.43±4.03 | 3.46±4.58 | 2.95±3.91 | 0.022 |
| Atrial fibrillation | 0.002 | ||||
| - Paroxysmal | 1148 (33.7%) | 271 (29.4%) | 640 (34.3%) | 237 (38.6%) | |
| - Persistent | 643 (18.9%) | 189 (20.5%) | 336 (18.0%) | 118 (19.2%) | |
| - Permanent | 1611 (47.4%) | 462 (50.1%) | 890 (47.7%) | 259 (42.2%) | |
| Symptomatic AF | 2618 (77.0%) | 728 (79.0%) | 1430 (76.6%) | 460 (74.9%) | 0.163 |
| History of heart failure | 912 (26.8%) | 320 (34.7%) | 436 (23.4%) | 156 (25.4%) | <0.001 |
| History of coronary artery disease | 547 (16.1%) | 167 (8.1%) | 279 (15.0%) | 101 (16.4%) | 0.098 |
| Cardiac implantable electronic device | 341 (10.0%) | 104 (11.3%) | 189 (10.1%) | 48 (7.8%) | 0.084 |
| History of ischemic stroke/TIA | 592 (17.4%) | 173 (18.8%) | 322 (17.3%) | 97 (15.8%) | 0.314 |
| Diabetes mellitus | 839 (24.7%) | 165 (17.9%) | 483 (25.9%) | 191 (31.1%) | <0.001 |
| History of hypertension | 2328 (68.4%) | 466 (50.6%) | 1330 (71.3%) | 532 (86.6%) | <0.001 |
| Smoking | 678 (19.9%) | 205 (22.2%) | 370 (19.8%) | 103 (16.8%) | 0.032 |
| Dyslipidemia | 1915 (56.3%) | 468 (50.8%) | 1084 (58.1%) | 363 (59.1%) | <0.001 |
| CKD (n = 2538) | 1594 (62.8%) | 429 (62.2%) | 879 (63.3%) | 286 (62.3%) | 0.860 |
| Renal replacement therapy | 40 (1.2%) | 7 (0.8%) | 23 (1.2%) | 10 (1.6%) | 0.285 |
| Dementia | 29 (0.9%) | 8 (0.9%) | 16 (0.9%) | 5 (0.8%) | 0.993 |
| History of bleeding | 323 (9.5%) | 89 (9.7%) | 181 (9.7%) | 53 (8.6%) | 0.722 |
| CHA2DS2-VASc score | <0.001 | ||||
| − 0 | 196 (5.8%) | 68 (7.4%) | 114 (6.1%) | 14 (2.3%) | |
| − 1 | 422 (12.4%) | 462 (50.1%) | 890 (47.7%) | 259 (42.2%) | |
| - ≥2 | 2784 (81.8%) | 711 (77.1%) | 1525 (81.7%) | 548 (89.3%) | |
| HAS-BLED score | <0.001 | ||||
| − 0 | 490 (14.4%) | 155 (16.8%) | 270 (14.5%) | 65 (10.6%) | |
| − 1–2 | 2373 (69.8%) | 634 (98.8%) | 1319 (70.7%) | 420 (68.4%) | |
| - ≥3 | 539 (15.8%) | 133 (14.4%) | 277 (14.8%) | 129 (21.0%) | |
| Antiplatelet | 890 (26.2%) | 253 (27.4%) | 458 (24.5%) | 179 (29.2%) | 0.046 |
| Anticoagulant | 2566 (75.4%) | 695 (75.4%) | 1419 (76.0%) | 452 (73.6%) | |
| - Warfarin | 2338 (68.7%) | 638 (69.2%) | 1288 (69.0%) | 412 (67.1%) | |
| - NOACs | 228 (6.7%) | 57 (6.2%) | 131 (7.0%) | 40 (6.5%) | |
| Antihypertensive agents | 3091 (90.9%) | 842 (91.3%) | 1681 (90.1%) | 568 (92.5%) | 0.166 |
| -Beta-blockers | 2476 (72.8%) | 727 (78.9%) | 1318 (70.6%) | 431 (70.2%) | <0.001* |
| -Calcium channel blockers | 934 (27.5%) | 141 (15.3%) | 558 (29.9%) | 235 (38.3%) | <0.001* |
| -ACEI | 758 (22.3%) | 230 (24.9%) | 382 (20.5%) | 146 (23.8%) | 0.017* |
| -ARB | 814 (23.9%) | 159 (17.2%) | 449 (24.1%) | 206 (33.6%) | <0.001* |
| -Aldosterone blockade | 280 (8.2%) | 149 (16.2%) | 101 (5.4%) | 30 (4.9%) | <0.001* |
| -Diuretics | 1030 (30.3%) | 358 (38.8%) | 506 (27.1%) | 166 (27.0%) | <0.001* |
Notes: Data presented as mean ± standard deviation or number and percentage. *A p-value<0.05 indicates statistical significance.
Abbreviations: SBP, systolic blood pressure; AF, atrial fibrillation; TIA, transient ischemic attack; NOAC, non-vitamin K antagonist oral anticoagulant; ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker.
Average SBP and Rate of Clinical Outcomes
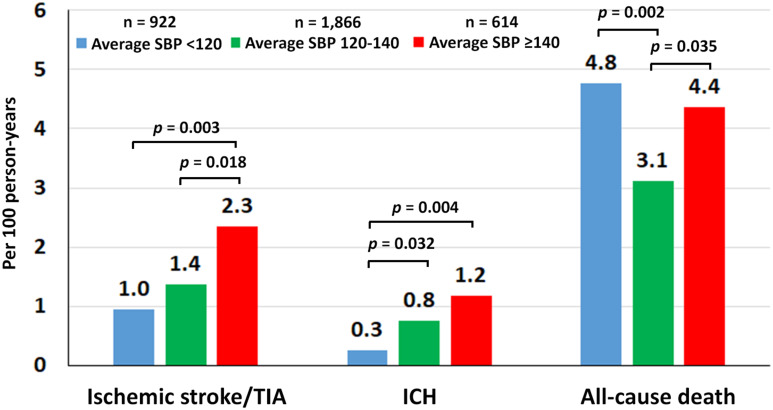
The mean follow-up duration was 25.7±10.6 months or 7192.6 person-years. The incidence rate of ischemic stroke/TIA, ICH, and all-cause death per 100 person-years in Group 1, Group 2, and Group 3 (Table 2). The median (range) overall rate of ischemic stroke/TIA, ICH, and all-cause death was 1.43 (1.17–1.74), 0.70 (0.52–0.92), and 3.77 (3.33–4.24) per 100 person-years, respectively. The bar graph in Figure 1 shows comparisons of the event rates among the three average SBP groups. The rate of ischemic stroke/TIA was lowest in Group 1, and highest in Group 3. The death rate was lowest in Group 2 compared to Group 1 and Group 3.
Table 2.
Rate of Clinical Outcomes Among the Tthree Different Baseline SBP Groups and Among the Three Different Average SBP Groups
| SBP Groups | Number of Patients | Number of Events | 100 Person-Years | Rate per 100 Person-Years (95% CI) | Absolute 2-Year Risk (95% CI) (Death as Competing Risk) |
|---|---|---|---|---|---|
| Ischemic Stroke/TIA | |||||
| Baseline SBP (mmHg) | |||||
| <120 | 1097 | 29 | 22.64 | 1.28 (0.86–1.84) | 2.32 (1.68–3.12) |
| 120–140 | 1426 | 38 | 30.41 | 1.25 (0.88–1.72) | 2.86 (2.28–3.54) |
| ≥140 | 879 | 36 | 18.87 | 1.91 (1.34–2.64) | 3.52 (2.46–4.85) |
| Average SBP (mmHg) | |||||
| <120 | 922 | 18 | 18.88 | 0.95 (0.57–1.51) | 2.41 (1.80–3.16) |
| 120–140 | 1866 | 55 | 40.21 | 1.37 (1.03–1.78) | 3.00 (2.36–3.76) |
| ≥140 | 614 | 30 | 12.83 | 2.34 (1.58–3.34) | 3.73 (2.45–5.40) |
| ICH | |||||
| Baseline SBP (mmHg) | |||||
| <120 | 1097 | 7 | 22.64 | 0.31 (0.12–0.64) | 1.15 (0.70–1.79) |
| 120–140 | 1426 | 22 | 30.41 | 0.72 (0.45–1.10) | 1.41 (1.02–1.90) |
| ≥140 | 879 | 21 | 18.87 | 1.11 (0.69–1.70) | 1.73 (1.02–2.78) |
| Average SBP (mmHg) | |||||
| <120 | 922 | 5 | 18.88 | 0.26 (0.09–0.62) | 1.28 (0.84–1.86) |
| 120–140 | 1866 | 30 | 40.21 | 0.75 (0.50–1.07) | 1.44 (1.02–2.00) |
| ≥140 | 614 | 15 | 12.83 | 1.17 (0.65–1.93) | 1.63 (0.84–2.88) |
| Death | |||||
| Baseline SBP (mmHg) | |||||
| <120 | 1097 | 85 | 22.64 | 3.75 (3.00–4.64) | |
| 120–140 | 1426 | 102 | 30.41 | 3.35 (2.73–4.07) | |
| ≥140 | 879 | 84 | 18.87 | 4.45 (3.55–5.51) | |
| Average SBP (mmHg) | |||||
| <120 | 922 | 90 | 18.88 | 4.77 (3.83–5.86) | |
| 120–140 | 1866 | 125 | 40.21 | 3.11 (2.59–3.70) | |
| ≥140 | 614 | 56 | 12.83 | 4.37 (3.30–5.57) | |
Abbreviations: SBP, systolic blood pressure; CI, confidence interval; TIA, transient ischemic attack; ICH, intracranial hemorrhage.
Figure 1.
Rate of ischemic stroke/transient ischemic attack (TIA), intracerebral hemorrhage (ICH), and death compared among the three average systolic blood pressure (SBP) groups (<120, 120–140, and ≥140 mmHg).
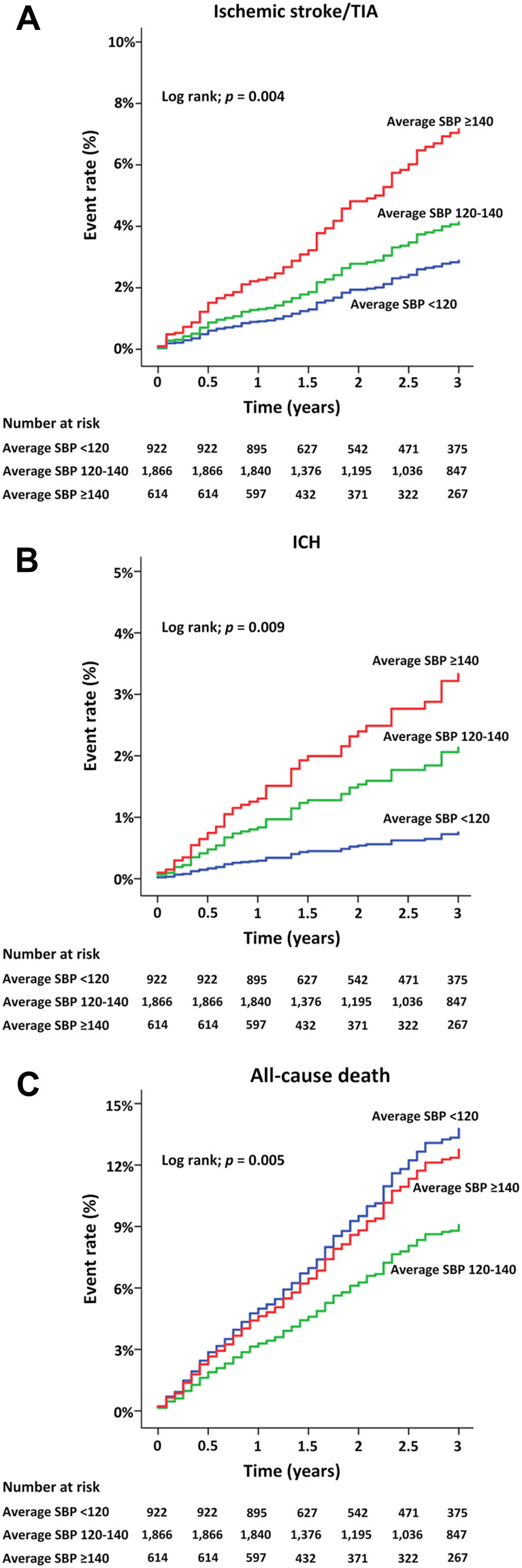
The cumulative event rates of ischemic stroke/TIA, ICH, and all-cause death in Groups 1, 2, and 3 over time during follow-up are shown in Figure 2. A comparison among groups by Log rank test showed similar trends as that described in Figure 1.
Figure 2.
(A) Cumulative event rate over time for ischemic stroke/transient ischemic attack (TIA), (B) intracerebral hemorrhage (ICH), and (C) death (C) among the three average systolic blood pressure (SBP) groups (<120, 120–140, and ≥140 mmHg).
Univariate and Multivariate Analysis
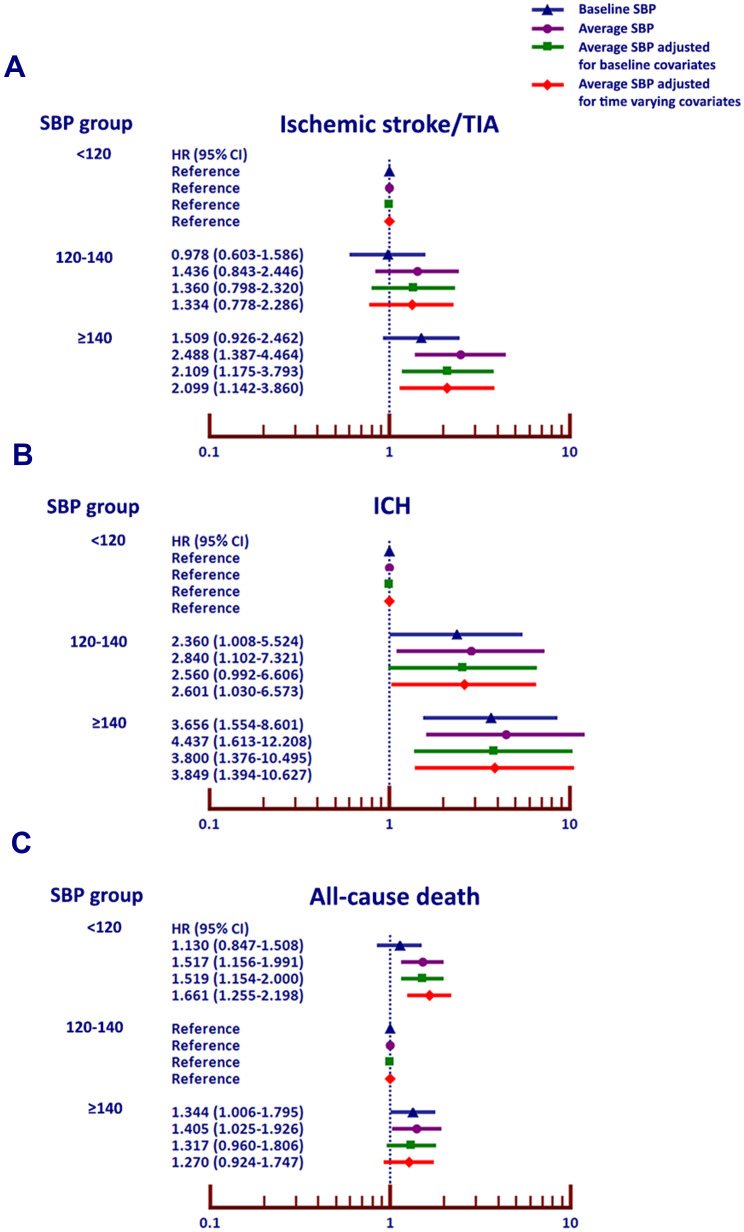
Univariate and multivariate analysis was performed to determine the effect of baseline and average SBP on the clinical outcomes. Figure 3 shows forest plots of unadjusted hazard ratio (HR) and 95% confidence interval (CI) for the effect of baseline and average SBP on ischemic stroke/TIA, ICH, and all-cause death, with Group 1 as a reference group for ischemic stroke/TIA and ICH, and Group 2 as the reference group for all-cause death. For each clinical outcome, the effect of average SBP was stronger than the effect of baseline SBP alone.
Figure 3.
Hazard ratio (HR) and 95% confidence interval (CI) for (A) ischemic stroke/transient ischemic attack (TIA), (B) intracerebral hemorrhage (ICH), and (C) death (C) among the three average systolic blood pressure (SBP) groups (<120, 120–140, and ≥140 mmHg) with and without adjustment for baseline and time-varying covariates.
Figure 3 shows multivariate analysis of the effect of SBP on ischemic stroke/TIA, ICH, and all-cause death based on 1) baseline SBP 2), average SBP 3) average SBP adjusted for baseline covariates and 4) average SBP adjusted for time varying covariates. The rate of ischemic stroke/TIA and ICH was lowest in patients with average SBP <120 mmHg, and highest among those with average SBP ≥140 mmHg. The death rates were consistent with a J-curve effect, being lowest in patients with an average SBP 120–140 mmHg. Sustained SBP control is more important than the SBP from a single visit. Baseline SBP was not significantly predict ischemic stroke/TIA, whereas average SBP with or without adjustment of covariates significantly predict ischemic stroke/TIA. For ICH, the outcome was associated with both baseline SBP and average SBP. For death, average SBP also associated with outcome more than baseline SBP. When we analyzed the causes of death, we found that the proportions of cardiovascular and non-cardiovascular death were not different among the three groups of average SBP. Since CKD data was based on laboratory results, it was available in 2538 patients (74.6%). Mean eGFR was 55.9±21.3 mL/min/1.73 m2. CKD stage 3–5 was present in 1594 patients (62.8%). Since CKD data had a significant proportion of missing data, we did not decide to use CKD for multivariate analysis despite the fact that imputation may be performed. For univariate analysis, CKD significantly increased risk of Ischemic stroke/TIA and all-cause death with HR and 95% CI of 2.21 (1.27–3.84, p=0.005) and 1.56 (1.16–2.10, p=0.003) respectively but did not increase risk of ICH, HR (95% CI) = 1.19 (0.63–2.25, p=0.601).
The significant effect of average SBP on clinical outcomes remained after adjustment for both baseline covariates and time varying covariates. Left ventricular ejection fraction (LVEF) was lowest in patients with an average SBP lower than 120 mmHg, whereby the mean LVEF values in patients with an average SBP <120, 120–140, and ≥140 mmHg were 54.8±16.6%, 61.3±13.4%, and 63.4±13.5%, respectively (p<0.001). The rate of cardiovascular causes of death was not significantly different among the three groups of average SBP (4.0%, 3.1%, and 4.4% in patients with average SBP <120, 120–140, and ≥140 mmHg, respectively).
Sensitivity Analysis
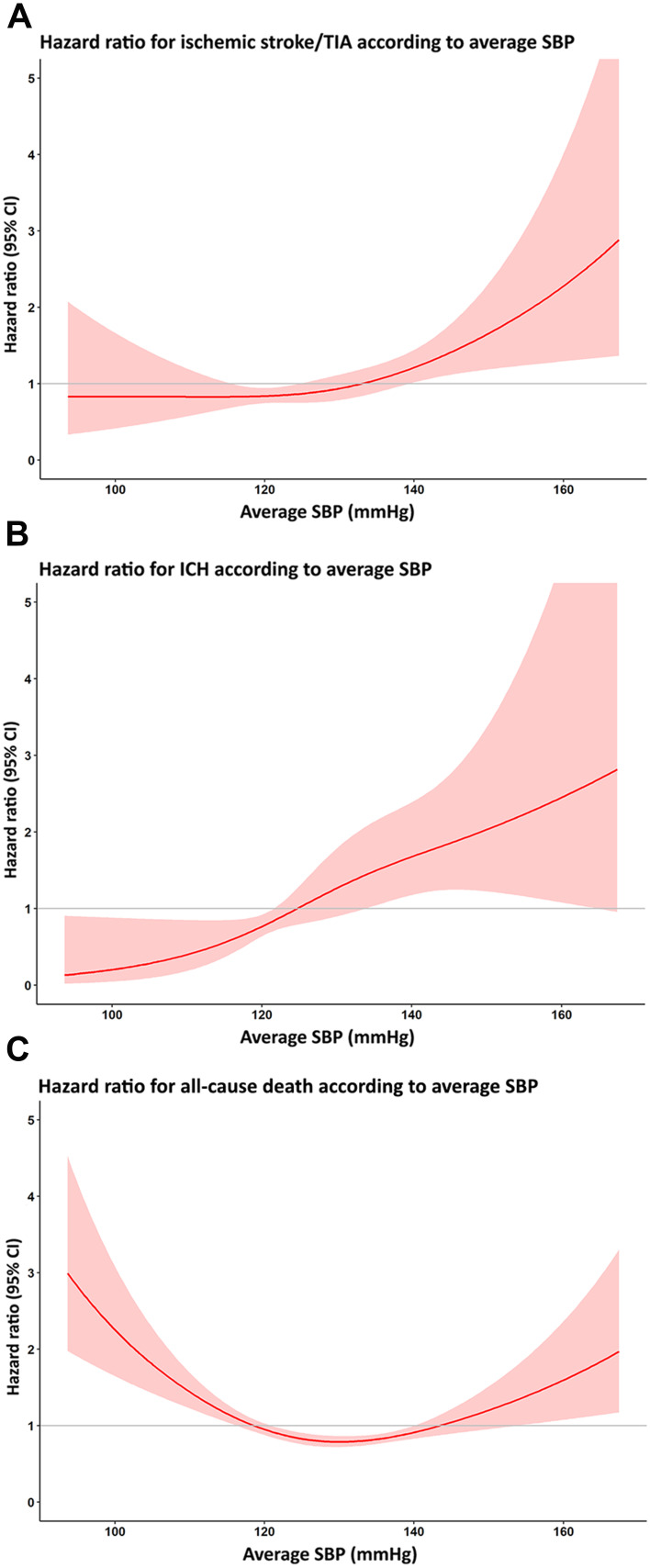
We analyzed the relationship between the three average SBP groups and clinical outcomes using Cox proportional hazards model in which average SBP was treated as a continuous variable. The results of that analysis are shown as a cubic spline graph in Figure 4. The risk of ischemic stroke/TIA and ICH increased, as shown by an HR greater than 1 when the average SBP was greater than 130 mmHg and 125 mmHg, respectively. Regarding all-cause death, the risk of all-cause death increased when the average SBP was greater than 140 or lower than 120 mmHg.
Figure 4.
Cubic spline graph showing the adjusted hazard ratio (HR) and 95% confidence interval (CI) for (A) ischemic stroke/transient ischemic attack (TIA), (B) intracerebral hemorrhage (ICH), and (C) death relative to average systolic blood pressure (SBP) as a continuous variable.
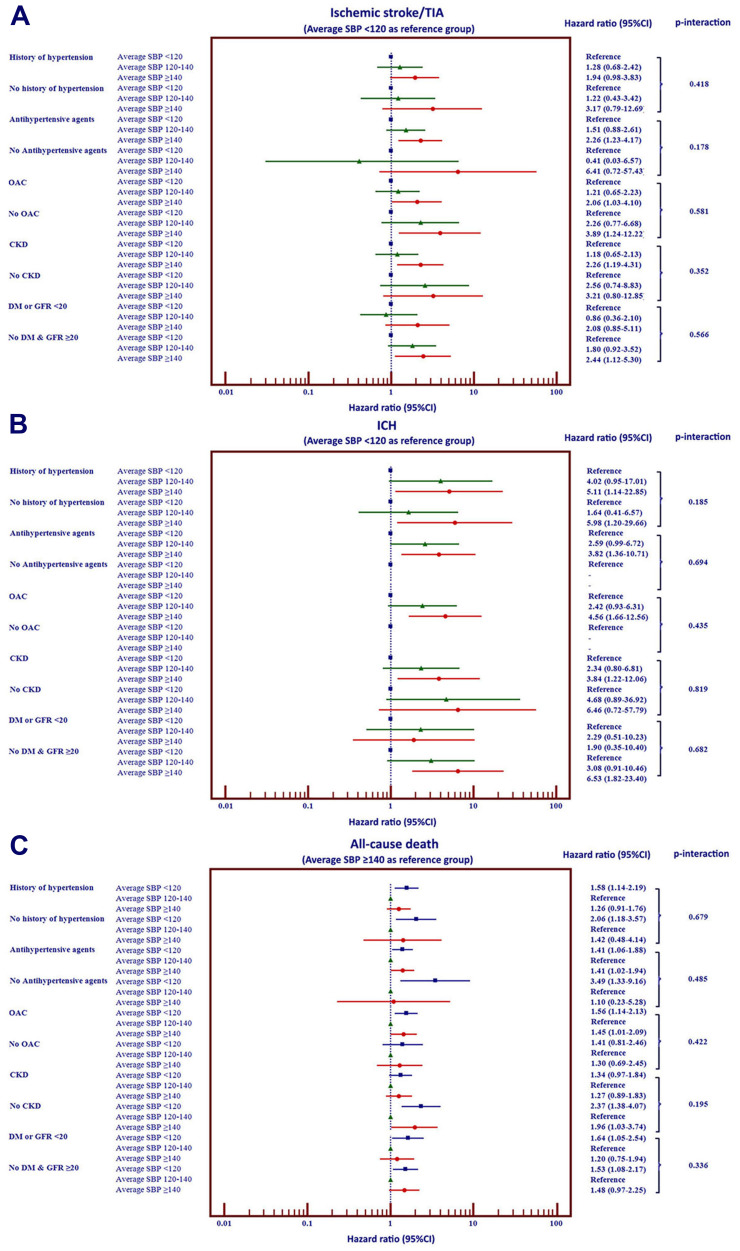
We performed interaction tests to determine whether there is a difference in the impact of average SBP and clinical outcomes between patients 1) with and without history of hypertension, 2) with and without antihypertensive drugs, 3) with and without OAC, 4) with and without CKD, and 5) with and without exclusion of type 2 diabetes or eGFR <20 mL/min/1.73 m2 mimicking exclusion population of SPRINT trial. The results of interaction test We found no significant interaction among patients with and without these subgroups on the effect of average SBP on ischemic stroke/TIA, ICH, and all-cause death (Figure 5).
Figure 5.
Forest plot of Hazard ratio and 95% confidence interval selected subgroups of patients with atrial fibrillation on clinical outcomes (A–C).
Discussion
In this prospective multicenter nationwide NVAF registry our principal findings are as follows: 1) the risk of ischemic stroke/TIA and ICH increased as the average SBP increased; 2) the rate of ischemic stroke/TIA and ICH was lowest in patients with average SBP <120 mmHg, and highest among those with average SBP ≥140 mmHg; 3) the death rates were consistent with a J-curve effect, being lowest in patients with an average SBP 120–140 mmHg; and 4) sustained SBP control is more important than the SBP from a single visit.
Previous studies observed a J-curve effect of blood pressure lowering, which means that the rate of adverse outcomes increased when the SBP become too low after treatment.10,15 Other data from meta-analysis have demonstrated that intensive BP lowering can reduce the risk of cardiovascular events,16 which is consistent with the results of the SPRINT study, which targeted SBP control at 120 mmHg.11 Thus, it has been recommended that the SBP should not be lower than 120 mmHg during hypertension treatment.12 However, the data in patients with NVAF are limited. It has been shown that both oscillometric and automated blood pressure measurements were accurate and reliable in patients with NVAF.14,17,18 Recent data from the RELY study showed that achievement of blood pressure control was related to clinical outcomes,19 whereby the lowest event rates for death, ischemic stroke, and bleeding were achieved at an SBP of 120–140 mmHg. The event rates of patients with an achieved SBP greater than 140 or lower than 120 mmHg were greater than in those with an achieved SBP of 120–140 mmHg.
Data from the Action to Control Cardiovascular Risk in Diabetes (ACCORD) Study demonstrated that all-cause mortality had a trend towards an increased risk in type 2 diabetes patients with tight control of SBP to below 120 mmHg.9 Similarly, The prospective observational longitudinal registry of patients with stable coronary artery disease (CLARIFY) showed that among patients with stable CAD, the risk of the composite outcome including cardiovascular death, myocardial infarction, and stroke increased when the SBP was below 120 mmHg.10 For patients with combined type 2 diabetes and CAD, results from the International Verapamil SR-Trandolapril Study (INVEST) indicated that tight control group targeted at SBP below 130 mmHg had an increased risk of composite outcome of all-cause death, myocardial infarction, and ischemic stroke.20 It has been postulated that when the SBP becomes too low, perfusion to the heart and brain may be inadequate, which results in an increased risk of adverse events.21 The SPRINT study demonstrated that subjects with a tight control strategy (SBP aim less than 120 mmHg) had a lower rate of clinical outcomes compared to the usual strategy, which aimed for a SBP less than 140 mmHg.11 The results of the SPRINT trial informed guideline changes in recommended target SBP levels.12,13 We analyzed the association of antihypertensive drugs used and average SBP below 120 mmHg. The results showed that use of antihypertensive drugs was not different in patients with average SBP <120 versus ≥120 mmHg (91.3% vs 90.7%, p=0.566). Therefore, the differences in clinical outcomes among different average SBP groups should not be related to antihypertensive drugs use. Besides, we did not find any significant interaction between the use of antihypertensive drugs and the three clinical outcomes, namely ischemic stroke/TIA, ICH, and all-cause death (interaction p-value 0.178, 0.694, and 0.485, respectively).
The results of our cohort study are different from those reported from the RELY study in that we could not demonstrate a J-curve effect for ischemic stroke and ICH in our population. The rate of ischemic stroke and ICH in our study was lowest in patients with an average SBP lower than 120 mmHg, and highest in those with an SBP >140 mmHg. However, the J-curve effect was present for the death outcome in our study. We showed that the average SBP to be superior to single-visit SBP or baseline SBP for predicting clinical outcomes. One previous study showed mean achieved SBP to be more important than baseline SBP for predicting death and major bleeding.19 This indicates that sustained SBP adherence should be recommended to improve clinical outcomes.
We showed that the average SBP to be superior to single-visit SBP or baseline SBP for predicting clinical outcomes. Although the results of our study suggest that the ischemic stroke and ICH outcomes to be lowest in patients with an average SBP lower than 120 mmHg, this group had an increased risk of all-cause death compared to those with an average SBP 120–140 mmHg. Although the observed difference in LVEF may play a role in the increased death rate among patients with an average SBP <120 mmHg, we could not demonstrate that cardiovascular causes of death were increased in these patients. The average SBP of our study was 128 mmHg, which is relatively within normal range. Antihypertensive drugs were used in 90.9%, which may be one of the reasons why the SBP was not high. The sensitivity analysis of our data demonstrated that there was no significant interaction among focused subgroups based on history of hypertension, use of antihypertensive agents, use of OAC, presence of CKD, and SPRINT mimicked population, which reflected that the results of our study are relatively consistent among subgroups.
Our results support the data from the Korean National Health Insurance Service database from 246,459 NVAF patients during 2005–2015, which is data from Asian population like our study population. They showed that long-term strict control of SBP was associated with a reduction in ischemic stroke risk.22 Their results support the control of SBP target to less than 120 mmHg. With our nationwide multicenter registry design, we could control the quality of data via the use of a well-planned prospective registry design. We also monitored for data quality at every study site. Moreover, we included other outcomes, such as ICH and all-cause death. Data from the FUSHIMI study, which enrolled 3713 Japanese patients with NVAF, demonstrated that history of hypertension was not a predictor of ischemic stroke or major bleeding; however, uncontrolled hypertension defined as a baseline SBP ≥150 mmHg was found to be a predictor of ischemic stroke and major bleeding.23 We also demonstrated that average SBP, which reflects sustained control of SBP, is more important than baseline SBP for predicting clinical outcome. This finding supports the theory of integrated AF management, ie the “C” of the ABC (Atrial fibrillation Better Care) pathway recommended in guidelines, which refers to cardiovascular and comorbidity risk optimization.5,24
Limitations
Our study has some limitations. First, our study population was mainly enrolled from large community hospitals or university hospitals. Because we did not include patients from all care level settings, the generalizability of our findings could be limited. Second, the majority of NVAF patients received OAC, and OAC might affect clinical outcomes. Therefore, the results of this study may not reflect the effect of SBP control in NVAF per se. In our study 75.4% of the patients were on OAC, and NOACs were used in 8.9% of those who were on OAC. Third, not all patients in our study had hypertension. Hypertension was present in 68.4% of the patients in our study. Therefore, the results of our study should not be interpreted as the target SBP in patients with AF and hypertension. The results of our study reflect a comparison of clinical outcomes among patients with NVAF who had different average SBP levels. We also analyzed the interaction of specific subgroups such as history of hypertension versus no history of hypertension, OAC versus no OAC, etc. We found no significant interaction between these subgroups on the effect of average SBP on ischemic stroke/TIA, ICH, and all-cause death. Although CKD has been considered as a high risk factor of patients with NVAF,25 we did not include CKD in multivariate analysis due to a significant proportion of missing data.
Conclusion
Average SBP is related to ischemic stroke/TIA, ICH, and all-cause death in patients with NVAF. The rate of ischemic stroke/TIA and ICH was lowest in patients with average SBP <120 mmHg, and highest in those with average SBP ≥140 mmHg. In contrast, the death rate demonstrated a J-curve effect, being lowest in patients with average SBP 120–140 mmHg. Sustained control of SBP was significantly associated with lower adverse clinical outcomes in patients with NVAF.
Acknowledgment
This study was funded by grants from the Health Systems Research Institute (HSRI) (grant no. 59-053) and the Heart Association of Thailand under the Royal Patronage of H.M. the King. Neither of the aforementioned funding sources influenced any aspect of this study or the decision of the authors to submit this manuscript for publication.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
GYHL: Consultant for Bayer/Janssen, BMS/Pfizer, Medtronic, Boehringer Ingelheim, Novartis, Verseon and Daiichi-Sankyo. Speaker for Bayer, BMS/Pfizer, Medtronic, Boehringer Ingelheim, and Daiichi-Sankyo. No fees are directly received personally. Other authors declare no personal or professional conflicts of interest, and no financial support from the companies that produce and/or distribute the drugs, devices, or materials described in this report.
References
- 1.Best JG, Bell R, Haque M, Chandratheva A, Werring DJ. Atrial fibrillation and stroke: a practical guide. Pract Neurol. 2019;19(3):208–224. doi: 10.1136/practneurol-2018-002089 [DOI] [PubMed] [Google Scholar]
- 2.Tse HF, Wang YJ, Ahmed Ai-Abdullah M, et al. Stroke prevention in atrial fibrillation–an Asian stroke perspective. Heart Rhythm. 2013;10(7):1082–1088. doi: 10.1016/j.hrthm.2013.03.017 [DOI] [PubMed] [Google Scholar]
- 3.Kakkar AK, Mueller I, Bassand JP, et al. Risk profiles and antithrombotic treatment of patients newly diagnosed with atrial fibrillation at risk of stroke: perspectives from the international, observational, prospective GARFIELD registry. PLoS One. 2013;8(5):e63479. doi: 10.1371/journal.pone.0063479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137(2):263–272. doi: 10.1378/chest.09-1584 [DOI] [PubMed] [Google Scholar]
- 5.Hindricks G, Potpara T, Dagres N, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS) The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42:373–498. [DOI] [PubMed] [Google Scholar]
- 6.Chiang CE, Wang KL, Lip GY. Stroke prevention in atrial fibrillation: an Asian perspective. Thromb Haemost. 2014;111(5):789–797. doi: 10.1160/TH13-11-0948 [DOI] [PubMed] [Google Scholar]
- 7.Krittayaphong R, Winijkul A, Methavigul K, et al. Risk profiles and pattern of antithrombotic use in patients with non-valvular atrial fibrillation in Thailand: a multicenter study. BMC Cardiovasc Disord. 2018;18(1):174. doi: 10.1186/s12872-018-0911-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lip GY, Andreotti F, Fauchier L, et al. Bleeding risk assessment and management in atrial fibrillation patients: a position document from the European Heart Rhythm Association, endorsed by the European Society of Cardiology Working Group on Thrombosis. Europace. 2011;13(5):723–746. doi: 10.1093/europace/eur126 [DOI] [PubMed] [Google Scholar]
- 9.Cushman WC, Evans GW, Byington RP, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362(17):1575–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vidal-Petiot E, Ford I, Greenlaw N, et al. Cardiovascular event rates and mortality according to achieved systolic and diastolic blood pressure in patients with stable coronary artery disease: an international cohort study. Lancet. 2016;388(10056):2142–2152. doi: 10.1016/S0140-6736(16)31326-5 [DOI] [PubMed] [Google Scholar]
- 11.Wright JT, Williamson JD, Whelton PKet al.; SPRINT Research Group. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373(22):2103–2116. doi: 10.1056/NEJMoa1511939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018;39(33):3021–3104. [DOI] [PubMed] [Google Scholar]
- 13.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71(19):e127–e248. [DOI] [PubMed] [Google Scholar]
- 14.Pagonas N, Schmidt S, Eysel J, et al. Impact of atrial fibrillation on the accuracy of oscillometric blood pressure monitoring. Hypertension. 2013;62(3):579–584. doi: 10.1161/HYPERTENSIONAHA.113.01426 [DOI] [PubMed] [Google Scholar]
- 15.Weber MA, Bakris GL, Hester A, et al. Systolic blood pressure and cardiovascular outcomes during treatment of hypertension. Am J Med. 2013;126(6):501–508. doi: 10.1016/j.amjmed.2013.01.007 [DOI] [PubMed] [Google Scholar]
- 16.Ettehad D, Emdin CA, Kiran A, et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet. 2016;387(10022):957–967. doi: 10.1016/S0140-6736(15)01225-8 [DOI] [PubMed] [Google Scholar]
- 17.Clark CE, McDonagh STJ, McManus RJ. Accuracy of automated blood pressure measurements in the presence of atrial fibrillation: systematic review and meta-analysis. J Hum Hypertens. 2019;33(5):352–364. doi: 10.1038/s41371-018-0153-z [DOI] [PubMed] [Google Scholar]
- 18.Stergiou GS, Kyriakoulis KG, Stambolliu E, et al. Blood pressure measurement in atrial fibrillation: review and meta-analysis of evidence on accuracy and clinical relevance. J Hypertens. 2019;37(12):2430–2441. doi: 10.1097/HJH.0000000000002201 [DOI] [PubMed] [Google Scholar]
- 19.Bohm M, Brueckmann M, Eikelboom JW, et al. Cardiovascular outcomes, bleeding risk, and achieved blood pressure in patients on long-term anticoagulation with the thrombin antagonist dabigatran or warfarin: data from the RE-LY trial. Eur Heart J. 2020;41(30):2848–2859. doi: 10.1093/eurheartj/ehaa247 [DOI] [PubMed] [Google Scholar]
- 20.Cooper-DeHoff RM, Gong Y, Handberg EM, et al. Tight blood pressure control and cardiovascular outcomes among hypertensive patients with diabetes and coronary artery disease. JAMA. 2010;304(1):61–68. doi: 10.1001/jama.2010.884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruland S, Aiyagari V. Cerebral autoregulation and blood pressure lowering. Hypertension. 2007;49(5):977–978. doi: 10.1161/HYPERTENSIONAHA.107.087502 [DOI] [PubMed] [Google Scholar]
- 22.Kim TH, Yang PS, Yu HT, et al. Effect of hypertension duration and blood pressure level on ischaemic stroke risk in atrial fibrillation: nationwide data covering the entire Korean population. Eur Heart J. 2019;40(10):809–819. doi: 10.1093/eurheartj/ehy877 [DOI] [PubMed] [Google Scholar]
- 23.Ishii M, Ogawa H, Unoki T, et al. Relationship of hypertension and systolic blood pressure with the risk of stroke or bleeding in patients with atrial fibrillation: the Fushimi AF registry. Am J Hypertens. 2017;30(11):1073–1082. doi: 10.1093/ajh/hpx094 [DOI] [PubMed] [Google Scholar]
- 24.Lip GYH. The ABC pathway: an integrated approach to improve AF management. Nat Rev Cardiol. 2017;14(11):627–628. doi: 10.1038/nrcardio.2017.153 [DOI] [PubMed] [Google Scholar]
- 25.Agarwal MA, Potukuchi PK, Sumida K, et al. Clinical outcomes of warfarin initiation in advanced chronic kidney disease patients with incident atrial fibrillation. JACC Clin Electrophysiol. 2020;6(13):1658–1668. doi: 10.1016/j.jacep.2020.06.036 [DOI] [PMC free article] [PubMed] [Google Scholar]