Abstract
Purpose
This study aimed to determine the minimum inhibitory concentrations (MICs) of ertapenem on Neisseria gonorrhoeae collected from eight Chinese provinces in 2018.
Methods
The MICs of ertapenem on 503 Neisseria gonorrhoeae isolates (415 isolates selected randomly and 88 isolates selected with preference) were measured using the agar dilution method. For comparison, the MICs of ceftriaxone and azithromycin were detected.
Results
Among 415 randomly selected isolates, the MIC range for ertapenem was from ≤0.008 mg/L to 0.5 mg/L. The corresponding MIC50 and MIC90 were 0.06 and 0.125 mg/L, respectively. Twelve of 415 isolates (2.9%) exhibited MIC values ≥0.25 mg/L, and only one isolate (0.2%) had a MIC of 0.5 mg/L. By comparing all 503 tested isolates, a correlation of r = 0.487 (P <0.001) between ertapenem and ceftriaxone MIC was observed, and the correlation between MICs of ertapenem and azithromycin was low (r = −0.12, P = 0.007). In 24 ceftriaxone-decreased susceptibility isolates, four isolates (16.7%) showed a MIC ≥0.25 mg/L for ertapenem. In 85 azithromycin resistant isolates, three isolates (3.5%) showed a MIC ≥0.25 mg/L for ertapenem.
Conclusion
The in vitro results suggest that ertapenem has satisfactory susceptibility in isolates collected from eight provinces in China; hence, it might be a promising treatment option for resistant gonococcal infections.
Keywords: Neisseria gonorrhoeae, treatment, ertapenem, antimicrobial resistance
Introduction
Gonorrhea, caused by Neisseria gonorrhoeae, is one of the commonly seen sexually transmitted diseases with a total global incidence of approximately 86.9 million per year.1 N. gonorrhoeae can cause urogenital infection and extragenital infection. It usually leads to dysuria and urethral discharge in men and frequently causes asymptomatic genital tract infection in women. If left untreated, it may lead to severe sequelae such as pelvic inflammatory disease and infertility.2 Moreover, it can even facilitate the risk of HIV acquisition and transmission.3 To prevent complications and control the transmission, successful treatment is of great significance.
Ceftriaxone and azithromycin in various doses are recommended as first-line treatments in guidelines in America,4 Australia,5 Canada,6 Europe,7 New Zealand,8 and by the World Health Organization (WHO).9 Monotherapy with ceftriaxone (1 g intramuscularly in a single dose) for uncomplicated gonococcal infections is recommended as the first-line treatment according to the current guidelines in China.10 However, N. gonorrhoeae has developed resistance to all previously used antimicrobial therapies and shows decreased susceptibility to ceftriaxone (DSC, defined as isolates with ceftriaxone MIC ≥0.25 mg/L in China currently) which poses a significant global healthcare burden.11–13 The emergence of N. gonorrhoeae isolates with a multidrug resistant or extremely drug-resistant profile highlights the importance of considering alternate therapy. Noteworthy, one treatment failure with 1 g ceftriaxone was reported in UK, but that infection was cleared with intravenous ertapenem 1 g once daily for 3 days.14
Ertapenem belongs to the class of carbapenem antibiotics, which interacts with penicillin binding proteins (PBPs) located on the bacterial cell wall. In particular, its inhibitory effect to PBPs 2 and 3 disturbs the final transpeptidation step in the synthesis of peptidoglycan, an essential component of the bacterial cell wall. Subsequently, dysfunctional cell wall causes cell death of Gram-positive and Gram-negative aerobic and anaerobic pathogens. According to the elimination half-life of ertapenem in healthy volunteers (3.8 hours), it is suitable for both intramuscular injection and intravenous.15 Besides its stability for DHP-1 (dehydropeptidase-1), one injection per day without combination of cilastatin is sufficient in clinical practice. Furthermore, there has been research proving that ertapenem can be regarded as a promising alternative for the treatment of ceftriaxone resistance both in vitro and in vivo. For instance, in the UK and Australia, ertapenem has been successfully applied to patients with multidrug resistant gonorrhea.14,15 Additionally, in vitro studies have shown that isolates resistant to ceftriaxone, including isolates with high levels of resistance to ceftriaxone, were still sensitive to ertapenem.16,17
Based on successful clinical treatment cases and data of previous in vitro studies,16,17 in this study, we analyzed the susceptibility of ertapenem of 503 N. gonorrhoeae isolates. Those isolates were collected in 2018 from eight different Chinese provinces.
Materials and Methods
Gonococcal Isolates
The ethics approval for the China Gonococcal Resistance Surveillance Programme (China-GRSP) was obtained from the Medical Ethics Committee at the Institute of Dermatology, the Chinese Academy of Medical Sciences & Peking Union Medical College and the National Center for Sexually Transmitted Disease Control at Nanjing (2014-LS-026) for the use of specimens collected annually from patients attending local dermatology or STD clinics. Participants no less than 18 years of age who signed an informed consent to provide urine, vaginal and rectal swabs were enrolled in the study. The isolates detected in our study were randomly selected from the China-GRSP sample bank collected in 2018. Samples were inoculated, identified, preserved, and transferred as previously described.18
Clinical gonococcal isolates from eight provinces (Beijing, Guangdong, Guangxi, Liaoning, Sichuan, Shaanxi, Tianjin and Yunnan) were used. In total, there are 503 isolates, which were all collected from the urogenital tract. Among them, 415 isolates were selected out of random sampling for MIC distribution analysis in different provinces, and the other 88 isolates were additionally selected with preference (isolates with relatively high MICs of ceftriaxone or azithromycin) for cross resistance analysis.
Antimicrobial Susceptibility Tests by Agar Dilution
Antimicrobial susceptibility tests to ertapenem, ceftriaxone, and azithromycin were determined for all isolates by the agar dilution method as previously described.19 For ertapenem, the concentrations were 0.008, 0.015, 0.03, 0.06, 0.125, 0.25, 0.5, and 1 mg/L, for ceftriaxone they were 0.008, 0.015, 0.03, 0.06, 0.125, 0.25, 0.5, and 1 mg/L, and for azithromycin they were 0.03, 0.06, 0.125, 0.25, 0.5, 1, 2, and 4 mg/L. The lowest antibiotic concentration that inhibited growth was defined as the MIC of isolates. Resistance to azithromycin (RTA) was defined as MIC ≥ 1.0 mg/L, and DSC was defined as MIC ≥ 0.25 mg/L. So far, no standardized criteria for ertapenem to N. gonorrhoeae has been published from EUCAST and Clinical and Laboratory Standards Institute (CLSI). Quality assurance was taken using WHO N. gonorrhoeae reference isolates (G, J, K, L, and P) and ATCC 49226.20
Data Analysis
The MIC of the reference strain was within the reference range whereas the batch of results was included in the analysis. 415 isolates from eight provinces were used to evaluate the MIC distribution in various province in China. Other analyses were carried out using all 503 isolates. Here, we describe the eight provinces’ MICs distribution of collected N. gonorrhoeae isolates to ertapenem. Correlation between MICs of ertapenem and ceftriaxone or azithromycin was calculated using log2-transformed MIC data by linear regression among 503 isolates. For MICs detected as ≤ or ≥ a specific value, these specific values were used for the calculation of log2-transformed MIC data. A p-value <0.05 was considered statistically significant. Statistical analyses were carried out utilizing SPSS software (IBM, New York, USA), and Excel (Microsoft, Washington, USA). Figures were made using R (GNU System).
Results
Antimicrobial Susceptibility Test of 415 Isolates
Geographically, the 415 isolates were collected from Beijing, Guangdong, Guangxi, Liaoning, Sichuan, Shaanxi, Tianjin, and Yunnan. The specific numbers of isolates for each province were 40, 59, 53, 38, 67, 52, 48, and 58, respectively.
All isolates were determined for their MIC values to ertapenem, ceftriaxone, and azithromycin. MIC values obtained from the WHO N. gonorrhoeae reference strains were identical or within 1 MIC dilution of those previously reported in every batch of tests.20 It supported the reliability of the MIC values to those three antimicrobials.
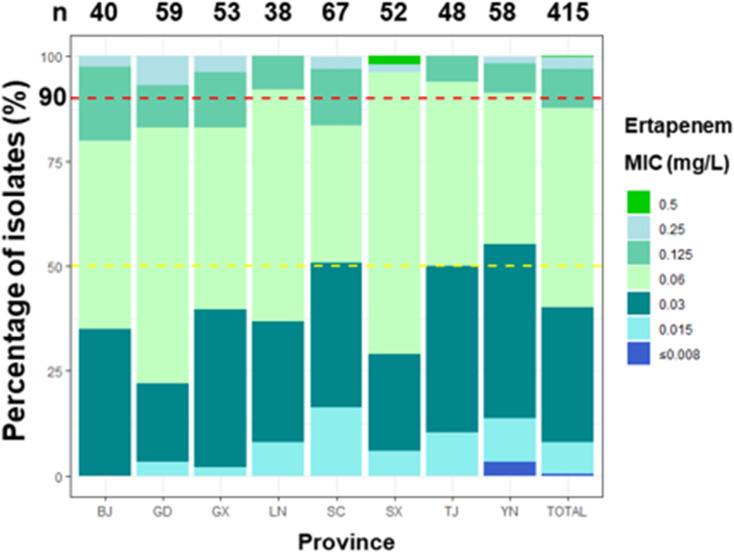
Ertapenem MICs ranged from ≤0.008 to 0.5 mg/L. The MIC proportions of ertapenem in different provinces is shown in Figure 1. Strains (12 out of 415, 2.9%) with MIC greater than 0.25 mg/L were found in six provinces. Among them, the largest proportion (4 out of 12) were strains from Guangdong Province. Meanwhile, no strains with a MIC ≥ 0.25 mg/L were found in Liaoning and Tianjin (Table 1). In addition, there was one strain with MIC greater than 0.5 mg/L found in Shaanxi province (Table 1 and Figure 1). Overall, the MIC50 of the eight provinces was 0.06 mg/L, with the exception of Sichuan and Yunnan provinces, those of which were both 0.03 mg/L (Table 1). While the overall MIC90 of the eight provinces was 0.125 mg/L, although those of Liaoning, Shaanxi, Tianjin and Yunnan were 0.06 mg/L, the other four provinces were consistent with the overall value (Table 1). The difference between MIC50 and MIC90 values in different provinces suggested that the MIC distribution of ertapenem could be inconsistent among regions.
Figure 1.
The percentage of N. gonorrhoeae isolates with different MICs (mg/L) for ertapenem. The MIC50 (yellow-dotted line), MIC90 (red-dotted line) and number of isolates per province are shown.
Abbreviations: BJ, Beijing; GD, Guangdong; GX, Guangxi; LN, Liaoning; SC, Sichuan; SX, Shaanxi; TJ, Tianjin; YN, Yunnan; n, number.
Table 1.
The Proportions of the Isolates with Different MICs of Ertapenem by Provinces
| Province | Isolates Detected | MIC (mg/L) | MIC ≥ 0.25 mg/L | |||
|---|---|---|---|---|---|---|
| Range | MIC50 | MIC90 | n (%) | P-value | ||
| Beijing | 40 | 0.03–0.25 | 0.06 | 0.125 | 1 (2.5%) | 1.0000 |
| Guangdong | 59 | 0.015–0.25 | 0.06 | 0.125 | 4 (6.8%) | 0.1252 |
| Guangxi | 53 | 0.015–0.25 | 0.06 | 0.125 | 2 (3.8%) | 0.6655 |
| Liaoning | 38 | 0.015–0.125 | 0.06 | 0.06 | 0 | 0.6109 |
| Sichuan | 67 | 0.015–0.25 | 0.03 | 0.125 | 2 (3.0%) | 1.0000 |
| Shaanxi | 52 | 0.015–0.5 | 0.06 | 0.06 | 2 (3.9%) | 0.6617 |
| Tianjin | 48 | 0.015–0.125 | 0.03 | 0.06 | 0 | 0.6230 |
| Yunnan | 58 | ≤0.008–0.25 | 0.03 | 0.06 | 1 (1.8%) | 1.0000 |
| Total | 415 | ≤0.008–0.5 | 0.06 | 0.125 | 12 (2.9%) | |
Notes: P-value was the chi-square of isolates with MIC ≥ 0.25 mg/L in different provinces to total.
Correlation Between MICs of Ertapenem and Ceftriaxone or Azithromycin
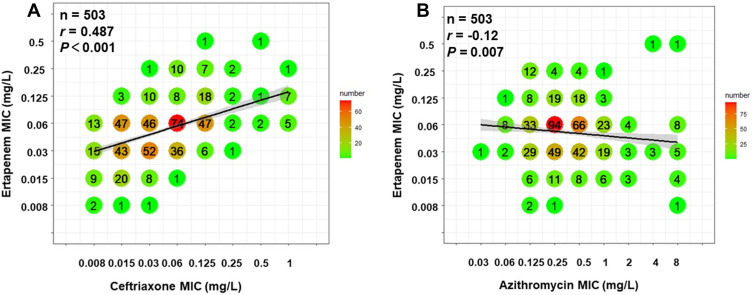
MIC distribution and correlation analyses for ertapenem with ceftriaxone and azithromycin are shown in Figure 2. A correlation of r = 0.487 (P <0.001) was observed between ertapenem and ceftriaxone (Figure 2A). In comparison, the MIC values of ertapenem and azithromycin correlated poorly with r = −0.12 (P = 0.007) (Figure 2B). These results indicated a cross resistance between ertapenem and ceftriaxone. However, azithromycin resistance might have limited impact on ertapenem susceptibility.
Figure 2.
Correlation between MICs of ertapenem and ceftriaxone or azithromycin. Each symbol represents one or multiple isolates, which is also shown with specific numbers and colors. The regression line was calculated using log2-transformed MIC data. For the MICs ≤ or ≥ a specific value, log2-transformed this specific value was used for calculating linear regression. (A) Correlation between MICs of ertapenem and ceftriaxone, r = 0.487, P <0.001. (B) Correlation between MICs of ertapenem and azithromycin, r = −0.12, P = 0.007.
MICs Distribution of Ertapenem in Isolates with Decreased Susceptibility to Ceftriaxone or Resistance to Azithromycin
Out of all 503 tested isolates, 24 isolates showed decreased susceptibility to ceftriaxone (DSC), 85 isolates were resistant to azithromycin (RTA), three isolates possessed a resistance to both ceftriaxone and azithromycin. Ertapenem MICs distributions for DSC or RTA isolates are shown in Table 2. Among the 24 DSC isolates, only four isolates showed a MIC ≥ 0.25 mg/L in ertapenem. Regarding RTA isolates, only three out of 85 showed MIC ≥ 0.25 mg/L for ertapenem.
Table 2.
Ertapenem MICs Distributions for Decreased Susceptibility to Ceftriaxone (DSC) or Azithromycin-Resistant (RTA) N. gonorrhoeae Isolates
| Ertapenem MIC (mg/L) | DSC | RTA | ||||
|---|---|---|---|---|---|---|
| Number | Percentage | Cumulative % | Number | Percentage | Cumulative % | |
| ≤0.008 | 0 | 0 | 0 | 1 | 1.2 | 1.2 |
| 0.015 | 0 | 0 | 0 | 13 | 15.3 | 16.5 |
| 0.03 | 1 | 4.2 | 4.2 | 30 | 35.3 | 51.8 |
| 0.06 | 9 | 37.5 | 41.7 | 35 | 41.2 | 92.9 |
| 0.125 | 10 | 41.7 | 83.3 | 3 | 3.5 | 96.5 |
| 0.25 | 3 | 12.5 | 95.8 | 1 | 1.2 | 97.6 |
| 0.5 | 1 | 4.2 | 100.0 | 2 | 2.4 | 100.0 |
| Sum | 24 | 100.0 | 85 | 100.0 | ||
Discussion
The antimicrobial resistance of N. gonorrhoeae remains a severe issue for public health. Ceftriaxone-resistant isolates have been reported in many countries over the world,2,3,12,13,21,22 which suggests the importance of finding alternative treatments. The recommended strategies from the World Health Organization include “increasing the therapeutic dose”, “new antimicrobial drugs development”, “recycle “old” antimicrobial agents”, etc. The gonorrhea treatment guidelines in China suggest an increase of ceftriaxone dose in the treatment of gonorrhea (from 250 mg in 2014 to 1 g in 2020). However, this can only temporarily alleviate the dilemma of gonorrhea treatment. With the evolution of gonococcal drug resistance, highly ceftriaxone-resistant isolates may continue to emerge. Therefore, it is urgent to find other effective therapeutic drugs.
For the time being, new antibiotics are also under intensive development, such as zoliflodacin and gepotidacin.23,24 However, the development of new drugs is known to be extremely time and cost-consuming. It is estimated that the research and development of new antimicrobials requires high socio-economic costs from target to product, which may take 15 years and $2.5 billion.25 Compared with the drug R & D returns of other non-infectious and chronic diseases, the R & D of antimicrobials development has more market risks. Therefore, the reassessment of developed drugs is of great significance. It is not only one of the alternative treatment strategies recommended by the WHO, but also the fastest treatment for gonococcal resistance.
Studies have shown that ertapenem may be a good alternative to ceftriaxone-resistant isolates both in vitro and in vivo. In vitro studies showed that ertapenem was still sensitive to ceftriaxone-resistant isolates (including highly ceftriaxone-resistant isolates).16 In addition, there are reports about ertapenem successfully curing multidrug-resistant gonorrhea patients.15 However, N. gonorrhoeae can easily evolve antimicrobial resistant variants. Therefore, we suggest a parallel antimicrobial resistance surveillance of ertapenem if it is used in a larger scale.
In this study, we found that ertapenem demonstrated a low MIC value (MIC range: ≤0.008–0.5 mg/L) in isolates collected from eight provinces in China. Using the PK-PD (non-species related) breakpoints reported in EUCAST clinical breakpoint of ertapenem (MIC ≤ 0.5 mg/L, susceptible), all isolates were sensitive to ertapenem, which indicated that ertapenem might be a potential therapeutic agent in gonorrhea treatment. Moreover, the MIC90 of ertapenem (0.125 mg/L) equaled the value of ceftriaxone in our study, but higher than that (MIC90 0.06 mg/L) reported by Yang et al., who had detected that level with 504 isolates collected from Zhejiang province from 2011–2012 and 2015–2017.26 This variation might be related to the time periods of the selected isolates, or the differences between provinces, as we found that the MIC90 in different provinces ranges from 0.06 to 0.125 mg/L in our study revealed the existence of variations in different provinces. The proportions of MIC ≥ 0.25 mg/L ranged from 0 (Liaoning and Tianjin) to 6.8% (Guangdong). Although this difference was not statistically significant, it could indicate the importance of antimicrobial susceptibility surveillance. Especially before prescribing antibiotics to patients, an antimicrobial sensitivity test is vitally important.
Since both ertapenem and ceftriaxone are located to β-Lactams, we analyzed the correlation between ertapenem and ceftriaxone MIC to evaluate the cross resistance. The MICs of ertapenem and ceftriaxone showed a correlation of r = 0.487, which indicated a cross resistance between ertapenem and ceftriaxone. But the MIC ranges of ertapenem in DSC isolates were lower than that of ceftriaxone, and in 24 DSC isolates, only four isolates showed ertapenem MIC ≥ 0.25 mg/L. Two of the 503 isolates had a MIC value of 0.5 mg/L. Whether these isolates will evolve into ertapenem-resistant isolates in the future will need further monitoring. Accordingly, ertapenem had in vitro advantages over ceftriaxone for isolates with ceftriaxone resistance. Generally clinicians are cautious about the use of carbapenem antibiotics, as they are usually used for serious infections in clinical practice. But facing high dose ceftriaxone treatment failure or persistent gonococcal infection patients, the clinicians may draw their attention to the application of ertapenem.
Our in vitro results suggested that ertapenem might be an effective treatment option for gonorrhea, particularly for the DSC isolates currently identified, which could serve as a foundation for further clinical trials in vivo. This study is the first multi-province research reporting the N. gonorrhoeae susceptibility to ertapenem in China. Moreover, our results provide a reference for antimicrobial susceptibility data of ertapenem in vitro for gonococcal infections in China. Nevertheless, there are some limitations in this study: the number of isolates accounts for only 0.3% (415/133,156) of the reported cases of gonorrhea in China for 2018, and lacking extragenital isolates in this study may also lead to a potential bias. At the same time, when interpreting the susceptibility data, we refer to the susceptibility break points of PK-PD (non-species related) reported in EUCAST, and there is no break point of antimicrobial susceptibility of ertapenem to N. gonorrhoeae, which may also lead to bias in the analysis of results. All these deficiencies may limit the generalizability of this study in China.
Conclusion
The study evaluated ertapenem MICs of N. gonorrhoeae isolates in 2018 from eight provinces in China and the correlation was analyzed to see the potential cross resistance between ertapenem and ceftriaxone or azithromycin. The antimicrobial effect of ertapenem on isolates with DSC and resistant to azithromycin were also evaluated. Our results suggest that ertapenem has low MIC values with isolates collected from eight provinces in China. Overall, this study provides new scope for ertapenem in the treatment of gonorrhea, although its effectiveness in vivo needs further study.
Acknowledgments
This work was supported by the National Science and Technology Major Project (2018ZX101010 01-004-003), the Nanjing Incubation Program for National Clinical Research Center (2019060001), and Jiangsu Province Youth Fund Project (BK20180156). The authors thank the patients who provided the specimens and acknowledge LF Yuan, WL Cao, BY Zhu, ZG Zhang, G Yong, CS Zhu, ZJ Zheng and L Bai for their contribution in isolates collecting.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Rowley J, Vander Hoorn S, Korenromp E, et al. Chlamydia, gonorrhoea, trichomoniasis and syphilis: global prevalence and incidence estimates, 2016. Bull World Health Organ. 2019;97(8):548P–562P. doi: 10.2471/BLT.18.228486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Skerlev M, Čulav-košćak I. Gonorrhea: new challenges. Clin Dermatol. 2014;32(2):275–281. doi: 10.1016/j.clindermatol.2013.08.010 [DOI] [PubMed] [Google Scholar]
- 3.Cohen MS, Hoffman IF, Royce RA, et al. Reduction of concentration of HIV-1 in semen after treatment of urethritis: implications for prevention of sexual transmission of HIV-1. Lancet 1996;349(9069):1868–1873. doi: 10.1016/S0140-6736(97)02190-9 [DOI] [PubMed] [Google Scholar]
- 4.Workowski KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm Rep. 2021;70(4):1–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Australian Sexual Health Alliance. Australian STI management guidelines for use in primary care; 2019. Available from: http://www.sti.guidelines.org.au/sexually-transmissible-infections/gonorrhoea#management. Accessed September 27, 2020.
- 6.Public Health Agency of Canada. Canadian guidelines on sexually transmitted infections; 2017. Available from: http://publications.gc.ca/collections/collection_2017/aspc-phac/HP40-1-2017-2-eng.pdf. Accessed September 27, 2020.
- 7.Unemo M, Ross J, Serwin AB, Gomberg M, Cusini M, Jensen JS. 2020 European guideline for the diagnosis and treatment of gonorrhoea in adults. Int J STD AIDS. 2020;956462420949126. doi: 10.1177/0956462420949126 [DOI] [PubMed] [Google Scholar]
- 8.The New Zealand Sexual Health Society. New Zealand guideline for the management of gonorrhoea, 2014, and response to the threat of antimicrobial resistance; 2014. Available from: https://www.nzshs.org/docman/guidelines/best-practice-guidelines/142-new-zealand-guideline-for-the-management-of-gonorrhoea-2014-and-response-to-the-threat-of-antimicrobial-resistance/file. Accessed September 27, 2020.
- 9.World Health Organization. WHO Guidelines for the Treatment of Neisseria Gonorrhoeae. Geneva: WHO; 2016. [PubMed] [Google Scholar]
- 10.National Center for STD Control, Chinese Center for Disease Control and Prevention; Venereology Group, Chinese Society of Dermatology; Subcommittee on Venereology, China Dermatologist Association. Guidelines for diagnosis and treatment of syphilis, gonorrhea and genital Chlamydia trachomatis infection (2020). Chin J Dermatol. 2020;53(3):168–179. [Google Scholar]
- 11.Wi T, Lahra MM, Ndowa F, et al. Antimicrobial resistance in Neisseria gonorrhoeae: global surveillance and a call for international collaborative action. PLoS Med. 2017;14(7):e1002344. doi: 10.1371/journal.pmed.1002344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lefebvre B, Martin I, Demczuk W, et al. Ceftriaxone-resistant Neisseria gonorrhoeae, Canada, 2017. Emerg Infect Dis. 2018;24(2):381–383. doi: 10.3201/eid2402.171756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lahra MM, Ryder N, Whiley DM. A new multidrug-resistant strain of Neisseria gonorrhoeae in Australia. N Engl J Med. 2014;371(19):1850–1851. doi: 10.1056/NEJMc1408109 [DOI] [PubMed] [Google Scholar]
- 14.Eyre DW, Sanderson ND, Lord E, et al. Gonorrhoea treatment failure caused by a Neisseria gonorrhoeae strain with combined ceftriaxone and high-level azithromycin resistance, England, February 2018. Euro Surveill. 2018;23(27). doi: 10.2807/1560-7917.ES.2018.23.27.1800323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lewis DA. New treatment options for Neisseria gonorrhoeae in the era of emerging antimicrobial resistance. Sex Health. 2019;16(5):449–456. doi: 10.1071/SH19034 [DOI] [PubMed] [Google Scholar]
- 16.Unemo M, Golparian D, Limnios A, et al. In vitro activity of ertapenem versus ceftriaxone against Neisseria gonorrhoeae isolates with highly diverse ceftriaxone MIC values and effects of ceftriaxone resistance determinants: ertapenem for treatment of gonorrhea? Antimicrob Agents Chemother. 2012;56(7):3603–3609. doi: 10.1128/AAC.00326-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eyre DW, Town K, Street T, et al. Detection in the United Kingdom of the Neisseria gonorrhoeae FC428 clone, with ceftriaxone resistance and intermediate resistance to azithromycin, October to December 2018. Eurosurveillance. 2019;24(10):1900147. doi: 10.2807/1560-7917.ES.2019.24.10.1900147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yin YP, Han Y, Dai XQ, et al. Susceptibility of Neisseria gonorrhoeae to azithromycin and ceftriaxone in China: a retrospective study of national surveillance data from 2013 to 2016. PLoS Med. 2018;15(2):e1002499. doi: 10.1371/journal.pmed.1002499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zheng XL, Xu WQ, Liu JW, et al. Evaluation of drugs with therapeutic potential for susceptibility of Neisseria gonorrhoeae isolates from 8 provinces in China from 2018. Infect Drug Resist. 2020;13:4475–4486. doi: 10.2147/IDR.S278020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Unemo M, Golparian D, Sánchez-Busó L, et al. The novel 2016 WHO Neisseria gonorrhoeae reference strains for global quality assurance of laboratory investigations: phenotypic, genetic and reference genome characterization. J Antimicrob Chemother. 2016;71(11):3096–3108. doi: 10.1093/jac/dkw288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dong Y, Yang Y, Wang Y, Martin I, Demczuk W, Gu W. Shanghai Neisseria gonorrhoeae isolates exhibit resistance to extended-spectrum cephalosporins and clonal distribution. Front Microbiol. 2020;11:580399. doi: 10.3389/fmicb.2020.580399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang H, Wang Y, Yong G, et al. Emergence and genomic characterization of the ceftriaxone-resistant Neisseria gonorrhoeae FC428 clone in Chengdu, China. J Antimicrob Chemother. 2020;75(9):2495–2498. doi: 10.1093/jac/dkaa123 [DOI] [PubMed] [Google Scholar]
- 23.Bradford PA, Miller AA, O’Donnell J, Mueller JP. Zoliflodacin: an oral spiropyrimidinetrione antibiotic for the treatment of Neisseria gonorrheae, including multi-drug-resistant isolates. ACS Infect Dis. 2020;6(6):1332–1345. doi: 10.1021/acsinfecdis.0c00021 [DOI] [PubMed] [Google Scholar]
- 24.Flamm RK, Farrell DJ, Rhomberg PR, Scangarella-Oman NE, Sader HS. Gepotidacin (GSK2140944) in vitro activity against gram-positive and gram-negative bacteria. Antimicrob Agents Chemother. 2017;61(7):e00468–17. doi: 10.1128/AAC.00468-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DiMasi JA, Grabowski HG, Hansen RW. Innovation in the pharmaceutical industry: new estimates of R&D costs. J Health Econ. 2016;47:20–33. doi: 10.1016/j.jhealeco.2016.01.012 [DOI] [PubMed] [Google Scholar]
- 26.Yang F, Yan J, Zhang J, van der Veen S. Evaluation of alternative antibiotics for susceptibility of gonococcal isolates from China. Int J Antimicrob Agents. 2020;55(2):105846. doi: 10.1016/j.ijantimicag.2019.11.003 [DOI] [PubMed] [Google Scholar]