Abstract
Objective
To develop a practical thromboelastograph guided (TEG) anticoagulation protocol to guide the management of COVID-19 critically ill patients.
Design
An inter disciplinary team reviewed the current literature on hypercoagulability in critically ill COVID-19 patients, clinical management practices and challenges with high rates of thrombotic events despite anticoagulant therapies.
Setting
The largest tertiary care hospital within the Northwell Health System in New York.
Patients
COVID-19 invasively mechanically ventilated patients in Medical Intensive Care Unit Settings.
Methods
TEG was monitored in critically ill COVID-19 patients. Patterns were reviewed to guide the development of a treatment protocol leveraging TEG parameters to select anticoagulant therapy. Three patients are reported to highlight TEG profiles that led to the development of the algorithm. Clinical trajectory and treatment decisions were extracted retrospectively from the Electronic Health Record, with input from the intensivists. Anticoagulant use, laboratory and TEG values, and venous/arterial lower extremity (LE) ultrasound results were recorded.
Main Results
These patients demonstrated hypercoagulable TEG results despite prophylactic or therapeutic dosages of unfractionated heparin or low-molecular-weight heparin (LMHW). TEG surveillance identified functional fibrinogen and maximum amplitude in high-risk patients with hyper inflammatory markers. Anticoagulation assessment, TEG parameters, and LE ultrasound monitoring for venous and arterial thrombus were used to construct an algorithm to guide and escalate anticoagulant therapy.
Conclusions
TEG provides patient-specific evidence for a hypercoagulable state in patients receiving all types of anticoagulant therapy. The proposed TEG algorithm guides anticoagulation management decisions to maintain or escalate anticoagulant dose and/or change choice of anticoagulant. A TEG algorithm may help negotiate the potential harm/benefit balance of full-dose anticoagulation in critically ill COVID-19 patients, by allowing for a more individualized approach that goes beyond the review of activated partial thromboplastin time (aPTT) levels.
Keywords: anticoagulants, COVID-19, critical care, respiratory distress syndrome, thromboelastography, thrombosis
Introduction
Coronavirus disease 2019 (COVID-19) infection is associated with hypercoagulability and thrombotic risks, which have prompted worldwide clinical trials investigating the best anticoagulation strategies to minimize thromboembolic risk while balancing risk of bleeding.
Autopsy findings in COVID-19 patients have demonstrated venous thromboembolic events (TE) in up to 58% of cases, diffuse alveolar damage, endothelial damage and pulmonary microthrombi.1 , 2 SARS Coronavirus 2 (SARS-CoV-2) cell entry causes a pro-inflammatory cascade through increased cytokines and angiotensin II effects following downregulation of angiotensin-converting enzyme 2.3 , 4 This complex path of SARS-CoV2 infection involves an immuno-thrombotic effect, leading to endothelial dysfunction and a vasculopathy, on a multi-organ level. Elevated D-dimer and fibrinogen levels are associated with increased thrombosis despite routine thrombo-prophylactic dosing with either unfractionated heparin (UFH) or low-molecular-weight heparin (LMWH).5, 6, 7, 8 Limited evidence is emerging regarding optimal thromboprophylaxis anticoagulant dosing; however, the thrombotic risk clearly is evident based on pro-coagulant parameters and clinical outcomes.9 The authors' institution's standard of care had adopted prophylactic-to-intermediate dosing for prevention of DVT in hospitalized COVID-19 patients.10 In the recently published multicenter trials REMAP-CAP, ACTIV-4a and ATTACC, therapeutic-dose anticoagulation with heparin in non-critically ill patients with COVID-19 increased survival to hospital discharge, with reduced use of cardiovascular or respiratory organ support compared to the thromboprophylactic group. These benefits were not replicated in critically ill COVID-19 patients, however. The question still exists as to whether select ICU patient populations may benefit from therapeutic anticoagulation.11 , 12 A targeted approach to anticoagulation may help to identify those critically ill patients who would benefit from escalated anticoagulation. Published protocolized guides have been implemented in many healthcare institutions to address the growing concern for thrombotic complications in the critically ill. Some examples of such protocols were guided by d-dimer and thromboelastography (TEG).13 , 14 Hranjec et al found increased risk for mechanical ventilation, acute kidney injury, dialysis and death in critically-ill COVID-19 patients managed without algorithm-TEG guidance compared to patients managed with such an algorithm.14 TEG profiles consistently demonstrated extreme hypercoagulable states, while absent fibrinolysis, coupled with elevated D-dimer, have been predictive of TE and hemodialysis in critically ill COVID-19 patients.8 , 15, 16, 17, 18
Viscoelastic testing has been used as a measure of viscosity and clot formation since the end of the 19th century. The original viscometers and the early thromboelastograph were bedside monitors that utilized a “cup-in-pin” platform. The cup was filled with whole blood, while the motion of a pin inside the cup was measured as either the pin or the cup oscillated. The motion of the pin then was coupled to an oscillograph from which signature tracings evolved. The signature tracings have become well-described throughout the literature as diagnostic of certain disease states. Platelet dysfunction, fibrinogen dysfunction and other hypocoagulable states are diagnosed by a certain tracing appearance. Less frequently adopted but equally important is the use of the viscoelastic test in the detection and identification of hypercoagulable states. Patients with a higher maximum amplitude (MA), or clot strength, immediately after non-cardiac surgery, may have a higher occurrence of thrombotic complications and perioperative myocardial infarction.19 Kashuk et al showed that patients with a high MA in the surgical intensive care unit had higher incidences of postoperative TE.20 Gurbel and colleagues studied the intracoronary stent population and found that patients with a higher baseline MA prior to stent had a higher incidence of stent occlusion and ischemic events.21 , 22 These same investigators also studied platelet reactivity indices for six months after stent placement and found a correlation between higher MA and ischemic events throughout the study period. Many investigators have studied the high reactivity of platelets by using MA response to adenosine diphosphate (MA-ADP) in the Platelet Mapping ® assay. High MA-ADP has been associated with post-stent ischemic events.23
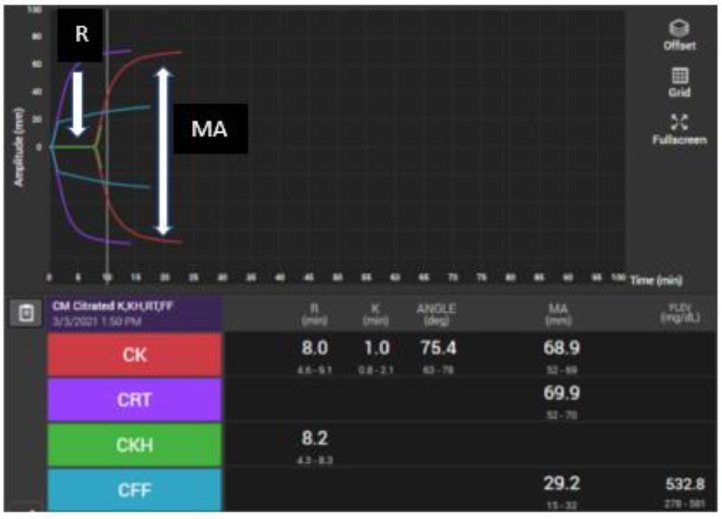
Viscoelastic testing has evolved throughout many iterations. The original TEG tracing had a characteristic shape and output parameters. A typical tracing is shown in Figure 1 . The cup-in-pin technology of viscoelastic testing has been replaced with newer ultrasonic technology in a cartridge-based system, the TEG 6S®. The TEG 6S ® is a point-of-care instrument that uses such cartridge technology to simultaneously measure the parameters of clot formation in four channels. The test outputs that once were available by running four different cup-in-pin channels now are available from a single cartridge that houses four channels. The clinically relevant parameters that the authors used from these four channels are described in Table 1 .
Figure 1.
Thromboelastograph from TEG 6S® displaying four tracings of whole blood exposed to various agents and activators. Reaction (R) time is the time to the “split”, the first detectable clot formation. The K time is the time to an amplitude of 20 mm. The alpha angle (α), is the angle between the horizontal axis of the R time and the tangent to the curve at the point where the amplitude is 20 mm wide. K time and alpha angle are parameters that measure kinetics of clot formation such as fibrinogen turnover. They have not been used widely in clinical diagnosis or algorithms. The maximal clot formation (MA) is the widest amplitude on the tracing, representing the clot strength based on platelet and fibrinogen interaction. When kaolin is the activator used in citrated (C) whole blood, this amplitude is referred to as “citrated-kaolin MA” (CK MA). CK (red) = citrated specimen with kaolin activator; CRT (purple) = Rapid TEG which tests a citrated specimen with tissue factor; CKH (green) = citrated specimen with kaolin and heparinase; CFF (blue) is a citrated specimen with tissue factor plus abciximab, a glycoprotein IIb/IIIa inhibitor that will remove platelet binding; thus, the resultant amplitude represents fibrinogen function alone.
Table 1.
| Parameter-all in citrate | Full Name | Additives | Interpretation | Clinical use |
|---|---|---|---|---|
| CK- R | Reaction time | Kaolin | Coagulation function | Anticoagulation or coagulopathy |
| CK-MA | Maximum amplitude | Kaolin | Platelets and fibrinogen clot strength | Sensitive to prolonged R time |
| CRT-MA | Maximum amplitude | Tissue factor (TF) | Platelets and fibrinogen clot strength | Rapid resulting; less sensitive to prolonged R time |
| CKH-R | Reaction time | Heparinase | Coagulation with heparin neutralized | Diagnose heparin effect |
| CFF-MA | Functional fibrinogen | TF+Abciximab | Fibrinogen function | Rapid resulting, specific |
| Platelet mapping MA-ADP | Maximum amplitude to ADP | ADP | Platelet response to ADP agonist | Clopidogrel and other P2Y12 blockers |
| Platelet mapping % inhibition | % inhibition | ADP | Calcuated from MA-ADP | Anti-platelet therapies |
Table 2 summarizes TEG patterns seen under different states of coagulopathy and platelet inhibition.
Table 2.
Thromboelastograph Patterns Under Various Conditions of Coagulopathy and Platelet Inhibition
| Conditions | R time [4.6 – 9.1 min] | CK MA[52 – 69 mm] | Heparinase R time[4.3 – 8.3 min] | Rapid TEG MA[52 – 70 mm] | FF[15 – 32 mm] |
|---|---|---|---|---|---|
| HYPER-coagulable | ↓ | ↑↑ | ↓ | ↑↑ | ↑↑ |
| HYPO-coagulable | ↑ | ↓ | ↑ | ↓ | ↓ |
| UFH infusion | ↑ | ↓/normal | normal | ||
| Argatroban infusion | ↑ | ↓/normal | ↑ | ||
| Conditions |
MA-ADP [45 – 69 mm] |
% inhibition [0 – 17 mm] |
|||
| Inadequate platelet inhibition | normal, ↑ | < 30% | |||
| Adequate platelet inhibition | ↓ (< 45 mm) | ↑↑ (> 30%) | |||
R = reaction time; CK MA = citrate-kaolin maximum amplitude; Rapid TEG MA = maximum amplitude under tissue factor activator; FF = functional fibrinogen; UFH = unfractionated heparin, ADP = adenosine diphosphate. MA-ADP represents clot strength in the presence of P2Y12 adenosine diphosphate-receptor blocker (e.g., clopidogrel, prasugrel).
Patients
The authors used TEG 6s® viscoelastic testing to study the hypercoagulable state in COVID-19 illness, focusing on the parameters of R time, CK MA, Rapid TEG MA and Functional Fibrinogen (FF) to help guide anticoagulation management. After studying the tracings of a number of critically ill patients, the authors identified similar patterns of hypercoagulability that led them to create an algorithm for anticoagulant treatment of patients with severe COVID illness. They also used the Platelet Mapping® assay to guide anti platelet therapy decision-making. The following three cases are examples of the hypercoagulable TEG tracings observed in critically ill COVID-19 patients, which led to the development of a critical care COVID-19 anticoagulation algorithm for this institution.
Patient 1 – A 67-year-old female, with history of hypertension, type 2 diabetes mellitus (T2DM), coronary artery disease (CAD) on aspirin and clopidogrel, chronic kidney disease, and obesity (BMI 53.1 kg/m2), was admitted for COVID-19 and acute respiratory distress syndrome (ARDS). SpO2 was 86% on non invasive ventilation (NIV) including positive airway pressure (PAP) and high-flow nasal cannula (HFNC) 100% FIO2, 60 LPM. Hospital day one inflammatory markers were elevated: d-dimer was 6,069 ng/mL [ref <= 229 ng/mL], C-reactive protein (CRP) of 33.64 mg/dL [ref 0.00 – 0.40 mg/dL]. Hospital medications included UFH 7,500 units every eight hours as thromboprophylaxis, ASA 81 mg daily, clopidogrel 75 mg daily, and dexamethasone. Remdesivir was not given due to low creatinine clearance. On day two, d-dimer and CRP decreased to 3,506 ng/mL and 19.44 mg/dL, respectively; however, the patient required invasive mechanical ventilation (IMV). Partial arterial pressure of oxygen-to-fraction of inspired oxygen ratio (PaO2/ FIO2) ratio (PaO2/FIO2) was 117. LE ultrasound was negative for DVT. Echocardiogram showed right ventricular enlargement and large pericardial effusion without tamponade. CT chest angiogram (CTA) was not performed due to patient instability. TEG drawn during prophylactic dosed UFH demonstrated an excessively high functional fibrinogen and high Rapid TEG MA, consistent with a hypercoagulable state [Table 3 , Figure 2 A and 2B]. The TEG reflected lack of therapeutic heparinization, as evidenced by normal R time and elevated CK MA. The patient expired on day five, following progressive multi-organ failure.
Table 3.
Thromboelastogram Results of Cases
| Patient | Anticoagulant and/or anti platelet medication | R time [4.6 – 9.1 min] | CK MA [52 – 69 mm] | Heparinase R time [4.3 – 8.3 min] | Rapid TEG MA [52 – 70 mm] | FF MA [15 – 32 mm] |
|---|---|---|---|---|---|---|
| 1 | Prophylactic heparin, ASA 81 mg, Clopidogrel 75 mg | 7.7 | 73.5 | 6.2 | > 75 | > 52 |
| 2 | UFH infusion | > 17 | 65.1 | 8.7 | > 75 | > 52 |
| 3 | UFH infusion | 12.3 | 69.1 | 8.6 | 71.9 | > 52 |
| 3 | Argatroban infusion | > 17 | 60.6 | > 17 | > 75 | > 52 |
R = reaction time; CK MA = citrate-kaolin maximum amplitude; Rapid TEG MA = maximum amplitude under tissue factor activator.
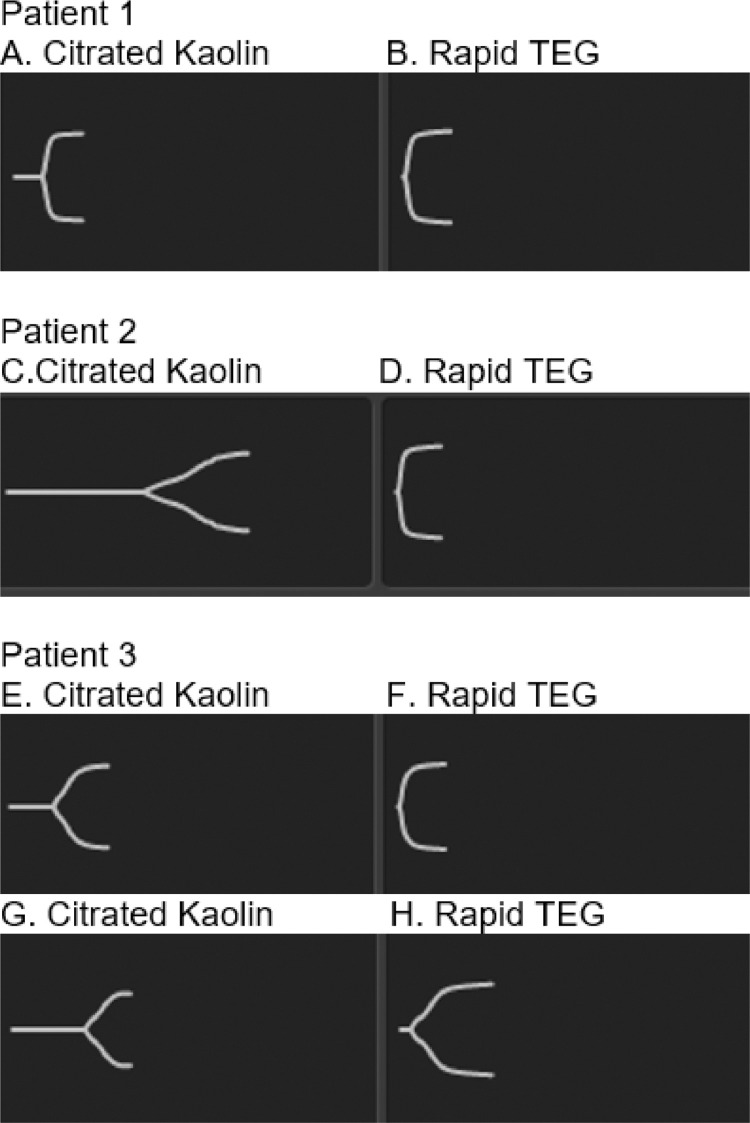
Figure 2.
TEG tracings for patient cases. Patient 1 receiving prophylactic-dose UFH, with TEG tracings shown in A and B. Tracing A depicts normal R time of 7.7 min (normal range 4.6 - 9.1 min), and high CK MA of 73.5 mm (normal range 52 - 69 mm). Tracing A visually is like Tracing B, which depicts high Rapid TEG MA > 75 mm (normal range 52 - 70 mm) and is consistent with a hypercoagulable state, suggesting that prophylactic UFH dose was insufficient to reduce coagulopathy. TEG tracings for patient 2 while receiving therapeutic UFH infusion are shown in C and D. Tracing C has a long R time of > 17 min, and normal CK MA of 65 mm consistent with effective anticoagulation with UFH. Tracing D depicts a high Rapid TEG MA > 75 mm, consistent with the elevated functional fibrinogen (FF), and a hypercoagulable state in patient 2. TEG tracings for patient 3 while receiving therapeutic UFH (E and F) and argatroban infusions (G and H) are shown. Tracing E has a long R time of 12.3 min and a high CK MA of 69.1 mm, indicative of a hypercoagulable state despite therapeutic UFH. Tracing F displays the Rapid TEG MA of 71.9 mm. Tracing G shows a long R time > 17 min and a lower MA of 60.4 mm in the setting of therapeutic argatroban anticoagulation, consistent with a lessened degree of hypercoagulopathy. Tracing H shows a persistently hypercoagulable state related to FF.
Patient 2 - A 72-year-old male with history of T2DM, transient ischemic attack, and obesity (BMI of 53.4 kg/m2) was admitted for COVID-19-related respiratory failure. Noted were elevated d-dimer of 499 ng/mL, C-reactive protein (CRP) of 28.42 mg/dL, and ferritin of 680 ng/mL [400 ng/mL]. CTA was negative for pulmonary embolus. He received NIV with 100% FIO2, dexamethasone and enoxaparin 40 mg SQ twice daily (BID). On day five, d-dimer increased to 9,330 ng/mL and enoxaparin was increased empirically to 1 mg/kg BID, although Dopplers were negative for thrombus. On day ten, the patient developed altered mental status, left hemiparesis, worsening hypoxemia, and subsequently was intubated for ARDS during a code stroke. CT of the head demonstrated acute right middle cerebral artery and posterior cerebral artery infarcts without hemorrhagic transformation. PaO2/FIO2 was 200 on IMV. Enoxaparin was changed to UFH infusion due to concerns for enoxaparin failure given the new embolic strokes. TEG profile while on therapeutic UFH infusion demonstrated a high R time and normal CK MA; however, Rapid TEG MA and FF were elevated, indicative of a persistent hypercoagulable state [Table 3, Figure 2C and 2D]. The patient developed worsening kidney injury requiring dialysis; however, his family decided against further invasive measures and the patient expired on hospital day 21.
Patient 3 – A 73-year-old male with history of chronic obstructive pulmonary disease, T2DM, and CAD was diagnosed with COVID-19 twelve days prior to hospitalization and presented with diabetic ketoacidosis and mild hypoxemia (SpO2 of 88% on room air). Noted laboratory results were: d-dimer 200 ng/mL, CRP 9.47 mg/dL, and ferritin 947 ng/mL. The patient received prophylactic-dose enoxaparin, dexamethasone and remdesivir. On day nine, d-dimer increased to 7,328 ng/mL, prompting therapeutic dosing of enoxaparin (1 mg /kg BID). CTA and LE Doppler were negative for TE; however, ARDS ensued and on day 13, the patient required IMV. Enoxaparin was changed to UFH infusion due to concerns for enoxaparin failure. TEG showed hypercoagulable state, with elevation of CK MA, FF and Rapid TEG MA [Table 3, Figure 2E and 2F]. Arterial Doppler demonstrated flow-limiting stenosis of the right superficial femoral artery and posterior tibial artery, and peroneal artery occlusion. UFH infusion was changed to argatroban to enhance anti-thrombin and anti-platelet activity. Repeat TEG on argatroban showed normalized CK MA but persistently high FF and Rapid TEG MA. [Table 3, Figure 2G, 2H]. PaO2/FIO2 remained in the low 100s and progressive AKI led to dialysis by day 21. Unfortunately, the patient expired on day 22 due to multi-organ failure.
These cases are a sample of difficult decision-making in COVID-19 critical care. The incidence of TE and the persistence of elevated functional fibrinogen activity on TEG, despite anticoagulation, prompted a multidisciplinary task force to generate a protocol to guide decision-making concerning anticoagulation.
Results
TEG Algorithm
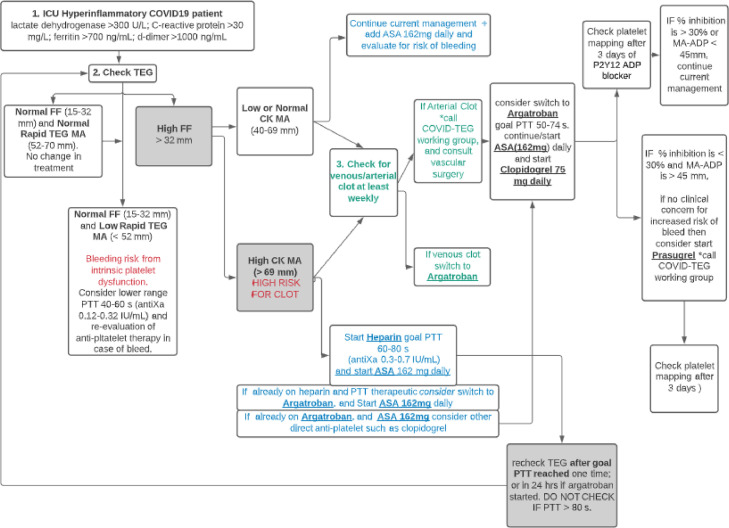
Hospitalized COVID-19 patients were placed on prophylactic anticoagulation with UFH or LMWH based on BMI and creatinine clearance as directed by the institutional protocol. It became apparent with TEG evaluation that a hallmark of the hypercoagulable state was extremely high FF and Rapid TEG MA, which also persisted despite large doses of anticoagulants and even anti-platelet agents. The authors determined that patients with hyperinflammatory markers (lactate dehydrogenase > 300 U/L, C-reactive protein > 30 mg/L, ferritin > 700 ng/mL, d-dimer > 1000 ng/mL) should trigger evaluation via TEG. The branch point of the TEG algorithm (Figure 3 ) hinged upon the finding of a high FF. Patients with a normal FF and Rapid TEG MA were not put through the hypercoagulable algorithm. Patients with a normal FF and low Rapid TEG MA were suspected to have platelet dysfunction and were evaluated for bleeding risk. Alongside a high FF, overall clot strength signified by CK MA was reviewed. With a low/normal CK MA, current anticoagulation was maintained, and patients were monitored for venous or arterial thrombosis weekly or sooner as per the clinical protocol. If CK MA (sensitive to anticoagulation) was high while on anticoagulation, the patient would be at high risk for thrombosis, prompting an accelerated anticoagulant regimen from the current regimen, provided that there was no bleeding risk. For example, a patient on prophylactic anticoagulation would be switched to therapeutic dosing or a patient already receiving therapeutic UFH would be switched to argatroban. Aspirin would be added at this time if appropriate. Recommended monitoring parameters for UFH include activated partial thromboplastin time (aPTT) range of 60 – 80 seconds or anti factor Xa (anti-Xa) range of 0.3 – 0.7 IU/mL. TEG analysis after an anticoagulation change is not used to assess the efficacy of the anticoagulation; that was done with aPTT or anti-Xa level. TEG analysis would be performed to assess if the patient still had thrombotic risk based upon the FF and CK-MA. This would determine if further acceleration of therapy was warranted with argatroban or anti-platelet therapy. Despite ubiquitously elevated FF, this working group decided against thrombolysis due to bleeding concerns. Instead, the team opted for antiplatelet agents to be added to anticoagulants. In patients for whom P2Y12 ADP receptor blockers were added due to continued hypercoagulabilitly, the Platelet Mapping® ADP cartridge was used to assess adequacy of platelet inhibition.
Figure 3.
TEG Algorithm. A high FF indicating high fibrinogen activity is used in combination with CK MA to assess COVID-19 patient hypercoagulable state. Presence of clot may be treated with argatroban with/without anti-platelet therapy. FF = functional fibrinogen; Rapid TEG MA = Rapid TEG maximum amplitude indicative of platelet function + fibrinogen after extrinsic activation; CK MA = citrate-kaolin maximum amplitude; MA-ADP = maximum amplitude in the presence of P2Y12 adenosine diphosphate-receptor blocker.
The knowledge acquired through TEG monitoring in critically ill COVID-19 patients with hyperinflammatory states informed the understanding of the pathophysiology of hypercoagulable states in this patient population. Routine clinical parameters, along with the proposed TEG algorithm, served as a guide to the intensivist in complex clinical scenarios, such as anticoagulant management in the setting of clinical deterioration, but without the feasibility of definitively ruling out a thrombus in unstable patients.
Discussion
Intensivists commonly need to balance clinical judgment with a paucity of evidence when addressing anticoagulation needs for critically ill COVID-19 patients. Of particular concern is the risk of harm from empiric anticoagulation versus the benefit of mitigating thromboembolism. A further concern is whether the conventionally used markers of adequate anticoagulation are misleading in some patients. The authors proposed this TEG protocol as a guide to anticoagulation management in critically ill COVID-19 patients; however, many gaps in the understanding of COVID-19 thrombotic risk and anti-thrombotic therapies still exist. The next step is to retrospectively study outcomes of thrombotic events, major bleeding and mortality in critically ill COVID-19 patients managed with the TEG-guided protocol versus those not guided by TEG. The authors hope to determine if there is utility in implementing the TEG algorithm in guiding anticoagulation decisions.
Further studies are needed to address what is (1) the anticoagulant of choice and dosing in ward versus critically ill COVID-19 patients; and (2) the most effective monitoring strategies to ensure that goal anticoagulant dosing is achieved. In COVID-19 adult patients admitted to the ICU, the INSPIRATION randomized clinical trial did not find a significant difference between intermediate versus prophylactic doses of enoxaparin in the primary outcome of a composite of venous or arterial thrombosis, treatment with extracorporeal membrane oxygenation (ECMO), or 30-day mortality.24 However, absent laboratory monitoring for enoxaparin was a study limitation and, perhaps, intermediate dose was insufficient to achieve a significant outcome. Enoxaparin use is not checked routinely with anti-Xa level and target goals may vary. Standard dosing of LMWH was associated with suboptimal anti-Xa levels in 30% of COVID-19 ward patients.25 Despite meeting anti-Xa targets in ICU COVID-19 patients treated with higher-dose LMWH as thromboprophylaxis, TEG profiles remained hypercoagulable.26 Argatroban, a direct inhibitor of free and clot-bound thrombin, works independently of antithrombin levels and may be a promising anticoagulant with anti-inflammatory, antiviral, and anti-platelet properties.27 In a cohort of ten ICU COVID-19 patients with confirmed thrombosis despite prophylactic dose LMWH, full-dose heparin failed to achieve therapeutic anti-Xa levels, which was attributed to laboratory evidence of antithrombin deficiency. For this reason, the anticoagulant was changed to argatroban, which improved thrombotic complications in renal replacement and ECMO circuits.28
Heparin resistance, defined as requiring more than 35,000 units/day of UFH, occurs in COVID-19 ICU patients, raising the concern as to the optimal aPTT goal or monitoring parameter.29 Antithrombin deficiency can result in heparin resistance in which case argatroban may be a better anticoagulant. TEG provides a viscoelastic assessment of thrombotic versus bleeding risk and may guide decisions around escalation versus maintaining anticoagulation. In the context of hypercoagulable TEG profiles without an overt bleeding risk, the algorithm may serve to select out critically ill patients for consideration of escalation to full-dose anticoagulation with UFH or argatroban. Antiplatelet therapy with aspirin and/or clopidogrel also is a consideration, particularly in the treatment of arterial thrombus. Fibrinolysis at 30 minutes (LY30) is another TEG parameter for consideration that the authors did not measure in their patient population. One report showed that complete lack of LY30 and d-dimer > 2,600 ng/mL were associated with a venous TE rate of 50% compared to 0% in patients without either parameter.18 COVID-19-associated inflammation is known to increase levels of plasminogen activator inhibitor 1 (PAI-1), an inhibitor of fibrinolysis. This causes fibrinolysis shutdown, thus increasing thrombotic risk in COVID-19 illness.30 LY30 may have a role in guiding the use of thrombolytics as a potential therapeutic in severe COVID-19 ARDS patients.31
Anti-platelet agents may have favorable effects when combined with anticoagulation for thromboprophylaxis by means of reducing clot strength and attenuating consequences of endothelial damage in systemic and pulmonary vasculature in COVID-19.32 Clopidogrel with higher doses of anticoagulation achieved improved viscoelastic parameters and reduced levels of d-dimer and fibrinogen compared to baseline hypercoagulable values.33 Higher oxygenation improvements in COVID-19 patients treated with a regimen of tirofiban, acetylsalicylic acid, clopidogrel and fondaparinux were observed in a case-control study.34 The anti platelet agent, dipyridamole, also may inhibit SARS-CoV-2 replication and have anti inflammatory properties.35
Conclusion
Hypercoagulable TEG profiles are observed in COVID-19 ICU patients. The proposed TEG algorithm serves as a guide to assess hypercoagulable states in COVID-19 ICU patients in a manner that addresses individual thrombosis profiles. Bleeding risks also can be monitored using TEG profiles. TEG provides objective, patient-specific evidence for the pathologic coagulation status in critically ill COVID-19 patients.
Footnotes
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors, Drs. Janice Wang and Negin Hajizadeh were Investigators in a multicenter clinical trial funded by Genentech investigating the use of tissue plasminogen activator in COVID-19 Acute Respiratory Distress Syndrome. Thromboelastography was utilized during the study however, cases described in the manuscript are not subjects enrolled in this trial. Additionally, this study has been closed during the writing of this manuscript.
All authors, Janice Wang, MD, Negin Hajizadeh, MD, and Linda Shore-Lesserson, MD do not have any declarations of interest.
References
- 1.Wichmann D, Sperhake JP, Lütgehetmann M, et al. Autopsy findings and venous thromboembolism in patients with COVID-19: A prospective cohort study. Ann Intern Med. 2020;173(4):268–277. doi: 10.7326/M20-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ackermann M, Verleden SE, Kuehnel M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020 Jul 9;383(2):120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu PP, Blet A, Smyth D, Li H. The science underlying COVID-19: Implications for the cardiovascular system. Circulation. 2020 Jul 7;142(1):68–78. doi: 10.1161/CIRCULATIONAHA.120.047549. [DOI] [PubMed] [Google Scholar]
- 4.Shang J, Wan Y, Luo C, et al. Cell entry mechanisms of SARS-CoV-2. Proc Natl Acad Sci U S A. 2020 May 26;117(21):11727–11734. doi: 10.1073/pnas.2003138117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Helms J, Tacquard C, Severac F, et al. High risk of thrombosis in patients in severe SARS-CoV-2 infection: a multicenter prostpective cohort study. Intensive Care Medicine. 2020 doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bilaloglu S, Aphinyanaphongs Y, Jones S, et al. Thrombosis in hospitalized patients with COVID-19 in a New York City health system. JAMA. 2020 Aug 25;324(8):799–801. doi: 10.1001/jama.2020.13372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Poissy J, Goutay J, Caplan M, et al. Pulmonary embolism in patients with COVID-19: awareness of an increased prevalence. Circulation. 2020;142:184–186. doi: 10.1161/CIRCULATIONAHA.120.047430. [DOI] [PubMed] [Google Scholar]
- 8.Yuriditsky E, Horowitz JM, Merchan C, et al. Thromboelastography profiles of critically ill patients with coronavirus disease 2019. Crit Care Med. 2020;48(9):1319–1326. doi: 10.1097/CCM.0000000000004471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bikdeli B, Madhavan MV, Jimenez D, et al. COVID-19 and thrombotic or thromboembolic disease: Implications for prevention, antithrombotic therapy, and follow-Up: JACC State-of-the-Art Review. J Am Coll Cardiol. 2020 Jun 16;75(23):2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spyropoulos AC, Levy JH, Ageno W, et al. Clinical guidance on the diagnosis, prevention, and treatment of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. 2020 Aug; 18;(8):1859–1865. doi: 10.1111/jth.14929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.REMAP-CAP Investigators; ACTIV-4a Investigators; ATTACC Investigators Therapeutic Anticoagulation with Heparin in Critically Ill Patients with Covid-19. N Engl J Med. 2021 Aug 26;385(9):777–789. doi: 10.1056/NEJMoa2103417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.ATTACC Investigators; ACTIV-4a Investigators; REMAP-CAP Investigators Therapeutic Anticoagulation with Heparin in Noncritically Ill Patients with Covid-19. N Engl J Med. 2021 Aug 26;385(9):790–802. doi: 10.1056/NEJMoa2105911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tassiopoulos AK, Mofakham S, Rubano JA, et al. D-dimer-driven anticoagulation reduces mortality in intubated COVID-19 Patients: A cohort study with a propensity-matched analysis. Front Med (Lausanne) 2021;8 doi: 10.3389/fmed.2021.631335. Published 2021 Feb 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hranjec T, Estreicher M, Rogers B, et al. Integral use of thromboelastography with platelet mapping to guide appropriate treatment, avoid complications, and improve survival of patients with coronavirus disease 2019–related coagulopathy. Critical Care Explorations: December 2020;2(12):e0287. doi: 10.1097/CCE.0000000000000287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mortus JR, Manek SE, Brubaker LS, et al. Thromboelastographic results and hypercoagulability syndrome in patients with coronavirus disease 2019 who are critically ill. JAMA Netw Open. 2020;3(6) doi: 10.1001/jamanetworkopen.2020.11192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Panigada M, Bottino N, Tagliabue P, et al. Hypercoagulability of COVID-19 patients in intensive care unit: A report of thromboelastography findings and other parameters of hemostasis. J Thromb Haemost. 2020 Jul; 18;(7):1738–1742. doi: 10.1111/jth.14850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salem N, Atallah B, El Nekidy WS, et al. Thromboelastography findings in critically ill COVID-19 patients. J Thromb Thrombolysis. 2020 Oct 4:1–5. doi: 10.1007/s11239-020-02300-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wright FL, Vogler TO, Moore EE, et al. Fibrinolysis shutdown correlation with thromboembolic events in severe COVID-19 infection. J Am Coll Surg. 2020 Aug; 231;(2) doi: 10.1016/j.jamcollsurg.2020.05.007. 193-203.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McGrath DJ, Cerboni E, Frumento RJ, et al. Thromboelastography maximum amplitude predicts postoperative thrombotic complications including myocardial infarction. Anesth Analg. 2005;100:1576–1583. doi: 10.1213/01.ANE.0000155290.86795.12. [DOI] [PubMed] [Google Scholar]
- 20.Kashuk JL, Moore EE, Sabel A, et al. Rapid thrombelastography (r-TEG) identifies hypercoagulability and predicts thromboembolic events in surgical patients. Surgery. 2009;146:764–774. doi: 10.1016/j.surg.2009.06.054. [DOI] [PubMed] [Google Scholar]
- 21.Gurbel PA, Bliden KP, Guyer K, et al. Platelet reactivity in patients and recurrent events post-stenting: Results of the PREPARE POST-STENTING Study. J. Am. Coll. Cardiol. 2005;46:1820–1826. doi: 10.1016/j.jacc.2005.07.041. [DOI] [PubMed] [Google Scholar]
- 22.Gurbel PA, Bliden KP, Kreutz RP, et al. The link between heightened thrombogenicity and inflammation: Pre-procedure characterization of the patient at high risk for recurrent events after stenting. Platelets. 2009;20:97–104. doi: 10.1080/09537100802687666. [DOI] [PubMed] [Google Scholar]
- 23.Bliden KP, DiChiara J, Tantry US, et al. Increased risk in patients with high platelet aggregation receiving chronic clopidogrel therapy undergoing percutaneous coronary intervention, Is the current antiplatelet therapy adequate? Journal of the American College of Cardiology. 2007;49:657–666. doi: 10.1016/j.jacc.2006.10.050. [DOI] [PubMed] [Google Scholar]
- 24.INSPIRATION Investigators Effect of intermediate-dose vs standard-dose prophylactic anticoagulation on thrombotic events, extracorporeal membrane oxygenation treatment, or mortality among patients with COVID-19 admitted to the intensive care unit: The INSPIRATION Randomized Clinical Trial. JAMA. 2021 doi: 10.1001/jama.2021.4152. Published online March 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dutt T, Simcox D, Downey C, et al. Thromboprophylaxis in COVID-19: Anti-FXa-the missing factor? Am J Respir Crit Care Med. 2020;202(3):455–457. doi: 10.1164/rccm.202005-1654LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vlot E, Van den Hackeng C, et al. Anti Xa activity after high dose LMWH thrombosis prophylaxis in covid 19 patients at the intensive care unit. Thromb Res. 2020;196:1–3. doi: 10.1016/j.thromres.2020.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aliter KF, Al-Horani RA. Thrombin inhibition by argatroban: potential therapeutic benefits in COVID-19. Cardiovasc Drugs Ther. 2020:1–9. doi: 10.1007/s10557-020-07066-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arachchillage DJ, Remmington C, Rosenberg A, et al. Anticoagulation with argatroban in patients with acute antithrombin deficiency in severe COVID-19. Br J Haematol. 2020 Sep;190(5):e286–e288. doi: 10.1111/bjh.16927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.White D., MacDonald S., Bull T., et al. Heparin resistance in COVID-19 patients in the intensive care unit. J Thromb Thrombolysis. 2020;50:287–291. doi: 10.1007/s11239-020-02145-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Loo J, Spittle DA, Newnham M. COVID-19, immunothrombosis and venous thromboembolism: biological mechanisms. Thorax. 2021;76:412–420. doi: 10.1136/thoraxjnl-2020-216243. [DOI] [PubMed] [Google Scholar]
- 31.Moore HB, Barrett CD, Moore EE, et al. STudy of Alteplase for Respiratory failure in SARS-Cov2/COVID-19: Study design of the phase IIa STARS trial. Res Pract Thromb Haemost. 2020 May 21;4(6):984–996. doi: 10.1002/rth2.12395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kow CS, Hasan SS. The use of antiplatelet agents for arterial thromboprophylaxis in COVID-19. Rev Esp Cardiol (Engl Ed) 2021;74(1):114–115. doi: 10.1016/j.rec.2020.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ranucci M, Ballotta A, Di Dedda U, et al. The procoagulant pattern of patients with COVID-19 acute respiratory distress syndrome. J Thromb Haemost. 2020 Jul;18(7):1747–1751. doi: 10.1111/jth.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Viecca M., Radovanovic D., Forleo G.B., Santus P. Enhanced platelet inhibition treatment improves hypoxemia in patients with severe Covid-19 and hypercoagulability. A case control, proof of concept study. Pharmacol Res. 2020;158 doi: 10.1016/j.phrs.2020.104950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu X, Li Z, Liu S, et al. Potential therapeutic effects of dipyridamole in the severely ill patients with COVID-19. Acta Pharm Sin B. 2020 Jul;10(7):1205–1215. doi: 10.1016/j.apsb.2020.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]