Abstract
Comorbid depression and insomnia are ubiquitous mental complaints among women going through the perimenopausal stage of life and can result in major decline in quality of life. Antidepressive agents combined with/without hypnotics, and/or hormone therapy are currently the most common treatment for perimenopausal depression (PMD) and insomnia (PMI). Balancing the benefits of these pharmacotherapies against the risk of adverse events (AEs) is a difficult task for both clinicians and women. There has been a growing body of research regarding the utilization of acupuncture for treatment of PMD or PMI, whereas no studies of acupuncture for comorbid PMD and PMI have appeared. In this review, we summarize the clinical and preclinical evidence of acupuncture as a treatment for PMD or PMI, and then discuss the potential mechanisms involved and the role of acupuncture in helping women during this transition. Most clinical trials indicate that acupuncture ameliorates not only PMD/PMI but also climacteric symptoms with minimal AEs. It also regulates serum hormone levels. The reliability of trials is however limited due to methodological flaws in most studies. Rodent studies suggest that acupuncture prolongs total sleep time and reduces depression-like behavior in PMI and PMD models, respectively. These effects are possibly mediated through multiple mechanisms of action, including modulating sex hormones, neurotransmitters, hypothalamic-pituitary-adrenal axis/hypothalamic-pituitary-ovary axis, oxidative stress, signaling pathways, and other cellular events. In conclusion, acupuncture is a promising therapeutic strategy for comorbid depression and insomnia during perimenopause. Neuroendocrine modulation is likely to play a major role in mediating those effects. High-quality trials are required to further validate acupuncture’s effectiveness.
Keywords: acupuncture, perimenopause, insomnia, depression, comorbid, mechanisms
Background
Perimenopause is a universal phenomenon that marks a mid-life transition from fertility to infertility in women’s lives.1 It is a stage during which women are particularly vulnerable to the onset of depression,2–4 even without any previous history of depressive disorders.2 A large quantity of studies also link both insomnia and vasomotor symptoms (hot flushes and night sweating) to perimenopausal depression (PMD),3,5,6 while insomnia is correlated more strongly and consistently with depression than vasomotor symptoms are.7–9 The prevalence of comorbid depression and insomnia in perimenopause is approximately 31.5%.9
The Comorbidity of Perimenopausal Insomnia and Depression is More Than a “Domino” Effect
The interaction between depression and insomnia, and its contribution to psychosomatic impairments, is clear.10 In comparison to women without sleep disturbances, women with sleep disturbance are 10 times more likely to experience depression; and women with depression are more vulnerable to insomnia, particularly waking during the night and having difficulty falling asleep again.11,12 In various insomnia patterns, depression is highly associated with difficulty falling asleep and waking up earlier than desired.10 Women in the menopausal transition are at increased risk for sleep-disordered breathing, which can also contribute to both depressed mood and poor sleep.3 In light of the actigraphy data, perimenopausal insomniacs with comorbid depression show longer sleep onset latency, shorter sleep duration, and lower sleep efficiency (but not number of awakenings or wakefulness after sleep onset), in comparison to women with insomnia only.10 Depression and insomnia are not only a mental burden for perimenopausal women but they also increase the difficulty of treating menopause-related somatic issues such as cardiovascular disease and osteoporosis.13 Anticipation of symptom self-healing might be futile as untreated PMD can increase medical morbidity after menopause, including risks of endocrine and cardiovascular disease.14
It is challenging to find an established theoretical basis for interpreting intertwined phenomenon of insomnia and depression as women transit menopause, although the two are commonly co-encountered.4,11 Two kinds of “domino” theory are sometimes asserted to partially explain this bidirectionality, or even link this bidirectionality to vasomotor symptoms: 1) PMD due to climacteric vasomotor symptoms or other psychosocial risk factors decreases sleep quality and quantity and 2) frequent night hot flushes may be tied to disrupted sleep patterns, and in turn, trigger the development of depressive symptoms.4,5 The complicated interrelationships between these three symptoms (depression, insomnia, and vasomotor complaints), however, may far exceed the “domino” explanation, because not all women with hot flushes develop depression, insomnia, or both; and many perimenopausal women suffer from depression and/or insomnia in the absence of hot flushes.10
Current Management Strategies for Comorbid Depression and Insomnia During Perimenopause
It is imperative for women in mid-life to find effective strategies to manage comorbid depression and insomnia, and maximize their well-being. Because of the co-occurrence or overlap, interdependent and interactional relationship,6,15 management of insomnia is also recommended as part of treatment for PMD, in “Guidelines for the Evaluation and Treatment of Perimenopausal Depression” issued by the Board of Trustees for The North American Menopause Society (NAMS) and Mood Disorders Task Force of the National Network of Depression Centers (NNDC).6 According to NAMS and NNDC, antidepressants and cognitive behavioral therapy (CBT) are the front-line antidepressant treatments for PMD.6 However, antidepressant medications have some relatively minor adverse events (AEs) that include but are not limited to dryness of mouth, digestive upsets, drowsiness, and fatigue, and some more major AEs such as rapid weight gain and sexual dysfunction.5,16–18 Similarly, sedatives and hypnotics have side effects that patients may find uncomfortable19 and are not recommended for longer term treatment of insomnia. CBT is highly recommended for the treatment of both depression and insomnia, but an impediment to its wider utilization is a lack of suitably trained psychologists.20 Hormone replacement therapy (HRT) can be an effective treatment for perimenopausal syndrome, while it is not FDA-approved to treat perimenopausal mood disturbance.6
Complementary and alternative medicine (CAM) has many proponents and supporters3 and has been sought to treat depression16 and insomnia19 associated with perimenopause. As previously reported, during peri- to post-menopause up to 50% of the women worldwide seek assistance from CAM approaches, including herbal medicine, yoga, massage therapy, dietotherapy, as well as acupuncture for symptomatic relief.3,21 Acupuncture has a long tradition of usage for the treatment of various menopause-related symptoms, including PMI and PMD, dating back thousands of years.22 Its clinical efficacy has been investigated for natural menopause and chemical-/surgery-induced menopause.22 It has also been reported that menopausal women sustaining insomnia as a major complaint tend to select CAM approaches, particularly body therapies, as their first choice of remedy.23,24 A menopausal epidemiological study carried out in the US indicated that in women who primarily used alternative therapies including acupuncture to manage menopause-related depression, 1.0% were completely symptom free, 84.6% made symptoms get a little to a lot better, 12.5% showed no change in symptoms, and 1.9% reported the worsening of symptoms, which was slightly less effective than the conditions in women who primarily used anti-depressive or anti-anxiety agents for menopause-related depression (5.6% were completely symptom free, 88.6% made symptoms get a little to a lot better, 3.9% showed no change in symptoms, and 1.9% reported the worsening of symptoms).25 However, due to the limited evidence and uncertainty about its effectiveness and mechanism of action in physiological terms, acupuncture is still striving for general scientific recognition and support.
This review aims to summarize and assess existing clinical and preclinical evidence of acupuncture as a treatment for perimenopausal insomnia and depression, as well as discuss the potential mechanisms involved. We aim to provide an evidence-base for decision-making for clinical practitioners to recommend (or for perimenopausal to women select) acupuncture as a potential therapy.
Effects and Safety of Acupuncture on Depression and Insomnia
Acupuncture is one of the simplest, popular, and safest CAM procedures.19,26,27 It is derived from Traditional Chinese Medicine (TCM) and has a history of use in medicine of at least 4000 years under the influence of oriental philosophical theories.28 It is a clinical treatment modality in which targeted proposed specific locations of the body, the acupuncture points (also called acupoints) have thin, solid, metallic needles inserted deeply for therapeutic purposes. The therapeutic efficacy is more effectively manifested when the pierced needles are manipulated manually with slight rotation (back-and-forth motion) and/or pistoned (up-and-down motion) or other complex combinations (manual acupuncture, MA), or are stimulated by the electric current via an impulse generator (electro-acupuncture, EA).27–30 As illustrated in neuroimaging reports, acupuncture can cause a wide array of central nervous system (CNS) responses involving the hippocampus, amygdala, cerebellum, hypothalamus, and other limbic structures.31 Dysfunction or disorder in these cerebral regions has been previously implicated in the development of depression32,33 and/or insomnia.34,35 Both clinical and basic studies indicate these two forms of acupuncture (MA and EA) are different with regard to outcomes and underlying physiological mechanisms, it is however difficult to conclude which one is more efficacious.29 Furthermore, patterns of usage of MA or EA usually vary by condition treated, stimulus demand, and/or acupuncturist preference.29
Patients are attracted to acupuncture in part by its reputation for being low-risk. Several prospective surveys with large sample sizes have suggested that acupuncture-related AEs mainly involve minor local reactions at the site of needling, such as bruising, pain, or minor bleeding, and the incidence of these events is generally very low (no more than 3%).36,37 No serious AEs requiring hospital visits or resulting in permanent disability or death have been reported from acupuncture.36,37 Twelve prospective studies involving several Asian and European countries validated that the incidence of the acupuncture-related serious AEs (ie, life threatening, hospital admission required or prolongation of existing hospital stay, persistent or significant disability or incapacity, death, etc) was estimated to be 0.55 per 10,000 patients, and 0.05 per 10,000 treatment sessions.32,38 Given its non-pharmacological basis, acupuncture also obviates the concerns with respect to toxicity and AEs that commonly occur when using hypnotics/sedatives, such as hangover, tolerance, increased alertness, and even endocrinological, hematological or cardiovascular events,39 and concerns regarding common side effects of contemporary antidepressive agents, such as dry mouth, cardiovascular/gastrointestinal side effects, seizure, bleeding, sexual dysfunction, weight gain, and even suicidality.40 Acupuncture also does not increase the metabolic burden of the liver and kidney. It is thus a potentially safe, promising and attractive remedy in the management of comorbid depression and insomnia associated with perimenopause. But, the question remains as to whether it is really effective?
As of March 2019, there were at least 31 systematic reviews and/or meta-analyses (SRs/MAs) regarding acupuncture treatment for depression.41 As of September 2018, there were at least 34 SRs/MAs regarding acupuncture treatment for insomnia.30 Although the included primary randomized controlled trials (RCTs) are less than well-powered and have some methodological flaws, the available evidence is in favor of acupuncture’s effectiveness and safety, and supports it as a monotherapy or adjuvant therapy for patients with depression or insomnia, particularly those who are intolerant to Western medications.30,41 In China, acupuncture has even been added as a routine remedy in the latest Guidelines for the Diagnosis and Treatment of Insomnia formulated by the Chinese Sleep Research Society (CSRS).30 The clinical guidelines issued by the Canadian Network for Mood and Anxiety Treatments (CANMAT) Depression Work Group recommended acupuncture as a third-line treatment with “Level 2 Evidence” for the adjunctive treatment of mild-to-moderate depression.41,42
How is Acupuncture for Perimenopausal Depression and Insomnia Conceptualised in TCM
TCM looks upon human life as a holistic, dynamic, spiritual, and functional unity, and the development of disease mainly as the result of a disordered state of the human functional balance (imbalance in the forces between Yin and Yang).26,28,43 Therefore, the basic protocol of preventing and/or treating disease in TCM is to restore the imbalance state and achieve Yin-Yang harmony via various remedies (eg, herbal medicine, acupuncture, moxibustion, Tui-na, etc) depending upon the individual and the malady.26,28,43
In clinical setting, acupuncture is ubiquitously applied based on the basic theory of TCM, which emphasizes the concept of “syndrome pattern”.44 “Treatment based on Syndrome Differentiation” is the essential principle of TCM. That is, patients are classified into different TCM syndrome patterns according to their clinical symptoms and signs, and then corresponding treatments, including acupuncture, are prescribed.44,45 According to the concept and theory of TCM gynecology, all perimenopausal diseases and disorders including PMI/PMD can be classified into the main category of “Juejing-Qianhou-Zhuzheng” (绝经前后诸症, meaning “symptoms and signs associated with perimenopause”, which is similar to “perimenopausal syndrome” in Western medicine) for treatment.46 In light of different symptoms, patients with PMI/PMD are generally further divided into different syndrome patterns within the scope of perimenopausal syndrome, and then receive corresponding TCM therapies. This strategy is the embodiment and process of “Treatment based on Syndrome Differentiation”. PMI/PMD is usually classified into different syndrome patterns in different published studies.47,48 Based on bibliometric analysis,47,48 we listed the top six syndromes of each of these two disorders and the proportion of patients in each pattern, respectively (see Table 1; organs in the syndrome pattern correspond to the TCM understanding). It is interesting to identify that in three out of nine patterns, PMI and PMD overlap. The pattern with the highest proportion of patients in both conditions is consistent, that is, the pattern of “depression of Liver and deficiency of kidney”. Given that TCM prescriptions (eg, acupuncture, herbal medicine, etc) are highly dependent on patterns, establishing standardized pattern classifications of PMI/PMD is essential for research and improved clinical practice.
Table 1.
Common TCM Syndrome Patterns of PMI and PMD
| Syndrome Patterns | Major Symptoms | Tongue | Pulse | Ratio | |
|---|---|---|---|---|---|
| PMI | PMD | ||||
| Depression of liver and deficiency of kidney | Insomnia or dreamful sleep, depressed mood, fatigue, chest distress and preference for sighing, hot flushes and sweating, frequent urination or dribbling urination | Red tongue with little coating | Stringy and thready, or stringy and rapid | 60.78% | 30.42% |
| Liver depression and spleen deficiency | Depression or irritability, poor sleep, poor appetite, abdominal distension, loose stool | White coated tongue | Stringy and slow | 24.02% | – |
| Stagnation of Qi due to depression of liver | Depression or irritability, preference for sighing, hypochondrium distress and/or pain, distending pain of breasts | Thin whitish coating of the tongue | Stringy | – | 18.80% |
| Imbalance between heart-Yang and kidney-Yin | Irritability and insomnia, palpitation, dizziness and tinnitus, loss of memory, dry mouth, soreness and weakness of waist and knees | Red tongue with little coating | Thready and rapid | – | 13.46% |
| Spleen–kidney Yang deficiency | Insomnia due to nocturia, soreness and weakness of waist and knees, chilly sensation and the cold limbs, diarrhea before dawn or loose stools, edema in the extremities | Pale and slight corpulent tongue with whitish and slippery fur | Deep and retarded without strength | 4.31% | – |
| Deficiency of both kidney Yin and kidney Yang | Poor sleep, sometimes hot flushes and sweating and sometimes chilly sensation and the cold limbs, soreness and weakness of waist and knees, dizziness and tinnitus | Pale tongue with thin coating | Stringy and thready | 4.31% | – |
| Heart-Gallbladder Qi deficiency | Palpitation, timidity, feeling fearful, difficulty in falling asleep, tendency to sleep lightly, nightmares, depressed mood, short of breath, spontaneous perspiration | Pale tongue with whitish coating | Stringy and thready | 3.70% | 6.72% |
| Heart–spleen deficiency | Insomnia, dreamful sleep, worry beyond measure, palpitation, poor appetite, loose stool, fatigue, sallow complexion, loss of memory | Pale tongue with whitish coating | Thin and weak | 2.87% | 6.11% |
| Hyperactivity of fire due to Yin deficiency | Vexation, irritable and depressed mood, dry mouth, tidal reddening of the cheeks, dysphoria in chest palms-soles | Red tongue with little coating | Stringy and rapid | – | 4.65% |
Table 1 is not a direct quote from any paper, but is compiled by us from data in two published papers (Ref 47 & 48 of this manuscript); Similarly, Table 3 is compiled by us from data in two published papers (Ref 19 & 50 of this manuscript); The rest of the tables and figures are original and do not require reference. 47 and 48.
Despite a lack of standardized pattern classification, perimenopausal symptoms including PMI/PMD are closely related to liver and kidney in TCM clinical practice.47,49 Hence, herbal medicines (or acupoints) associated with regulating liver (or Liver Meridian of Foot-Jueyin) and kidney (or Kidney Meridian of Foot-Shaoyin) are often selected for treating depression and insomnia associated with perimenopause.47,49 Smoothing liver (smooths liver and regulates Qi) and nourishing kidney (nourishes kidney and enriches essence) are regarded as the general principles for perimenopausal disorders.47,49
Evidence from Clinical Trials
Does the existing clinical evidence support acupuncture as a safe and effective therapy for comorbid PMI and PMD, and is the evidence reliable? To answer this question, a comprehensive literature search with no restrictions for research types was carried out. Unfortunately, no RCTs, cohort studies or case studies within this theme were retrieved, reflecting a significant research and practice gap. Our team thereby is currently carrying out an RCT focusing on this theme. This trial has been registered in the Chinese Clinical Trial Registry (No. ChiCTR2100043054), and the results are expected to be published in 2022. However, we retrieved many RCTs and some SRs/MAs focusing on acupuncture treatment for PMD alone or PMI alone. Those SRs/MAs involved several modified modalities of acupuncture for which mechanisms of action differ from those for MA/EA and were not discussed in this review; therefore, only eligible original RCTs in those SRs/MAs were extracted.
Our team has published two SRs/MAs19,50 within related fields (15 RCTs in SR/MA of PMI; 25 RCTs in SR/MA of PMD) where we covered our retrieval strategy in detail (retrieval in 2020), as well as our quality evaluation and interpretation of the evidence. Based on updated retrieval (July 2021), we have included more RCTs recently published (22 RCTs of PMI; 25 RCTs of PMD). The critical information and major findings of each RCT were extracted and summarized in Table 2. The results of meta-analyses cited from our SRs/MAs are displayed in Table 3, and detailed analysis process can be referred to in our published articles.19,50 Given that this review is not an SR, a narrative summary on the findings followed by comments on the methodology and implications for future research of these studies is provided. We expect that these summaries will help clinicians and policymakers judge the role of acupuncture, and its potential and feasibility for the management of depression and insomnia associated with perimenopause.
Table 2.
Summary of Clinical Trials Investigating the Effects and Safety of Acupuncture on PMI and PMD
| Author/Year | Study Groups/No. of Participants | Disease (Diagnostic System) | TCM Syndrome Pattern | Acupuncture Interventions | Acupoints | Controls | Outcome Measures | Results (Compared with the Control Group) at Post-Treatment | Follow-Up | Adverse Events |
|---|---|---|---|---|---|---|---|---|---|---|
| Fu et al 201751 | - MA/n=37 - placebo-MA/n=37 |
PMI (ICSD-3) | NR | 20 min/day for 10 sessions | BL18, BL23, GB25, LR14 | Streitberger placebo-needle control | PSQI, ISI, PSG (SOL, TST, WASO, SE, Arl, N1%, N2%, N3%, REM%) | (i) Lower PSQI and ISI in MA group (ii) Shorter SOL and WASO, longer TST, higher SE, lower Arl, lower N1%, and higher REM% in MA group; no differences in N2% and N3% between two groups |
No follow-up | - MA/n=0 - placebo-MA/n=4 (worsening of insomnia) |
| Wang 201552 | - EA/n=30 - placebo-EA/n=30 |
PMI (ICSD-3) | NR | 30 min/day, 3 days/week for 8 weeks | BL15, BL18, BL23, CV3, CV4, CV6, CV12, EX-HN3, GV3, GV4, GV14, GV20, HT7, KI3, LR3, PC6, SP6, ST36 | Streitberger placebo-needle control | PSQI, MRS, Men-QoL | No differences in PSQI, MRS and MEN-QoL between two groups | (1) No difference in PSQI and Men-QoL between two groups at 4-week follow-up (2) Lower MRS in EA group at 4-week follow-up |
No adverse events |
| Lin et al 201753 | - MA/n=33 - waitlist-control/n=32 |
PMI (CCMD-3, CDTE-TCM) | NR | 30 min/day, 3 days/week for 4 weeks | CV4, CV6, CV12, EX-HN3, GV20, GV24, HT7, PC6, SP6, ST36 | Waitlist | PSQI, KI | Lower PSQI and KI in MA group | No follow-up | NR |
| Li et al 2020a54 | - EA/n=42 - placebo-EA/n=42 |
PMI (ICSD-3) | Kidney Yin/Yang deficiency | − 30 min/day for 18 days (3 days/week for 4 weeks + 2 day/week for 2 weeks + 1 day/week for 2 weeks) - continuous wave, 2.5 Hz, 4–5 mA |
BL23, CV4, CV6, EX, EX-HN3, GV4, GV20, GV24, HT7, KI3, KI7, SP6 | Streitberger placebo-needle control | PSQI, MEN-QoL, Actigraphy (TST, WASO, SE, ATs, AA), ISI, SAS, SDS | (i) Lower PSQI, ISI, MEN-QoL, SAS in EA group; no differences in SDS between two groups (ii) Longer TST, higher SE, and less AA in EA group; no differences in ATs and WASO between two groups |
Lower PSQI and Men-QoL in EA group at 4- and 12- week follow-ups | - EA/n=2 [mild bleeding (1); mild pain (1)] - placebo-EA/n=1 (mild pain) |
| Wang et al 201555 | - MA/n=33 - sham-MA/n=33 |
PMD (CCMD-3) | NR | 30 min/day, 3 days/week for 8 weeks | KI6, LU7, PC6, SP4 | Sham-MA [shallow puncture (2.5–5 mm), no De-qi, no retention of the needle] | SDS, Men-QoL | Lower SDS and Men-QoL in MA group | (i) Lower SDS in MA group at 4-week follow-up (ii) no difference in Men-QoL between two groups |
NR |
| Li 2015a56 | - EA/n=30 - sham-EA/n=30 - Escitalopram/n=30 |
PMD (ICD-10) | NR | − 30 min/day, 3 days/week for 12 weeks - dense-sparse wave, 10/50 Hz, 0.5–1 mA |
CV4, EX-CA1, EX-HN3, GV20, LI4, LR3, SP6, ST25 | (i) Sham-MA [shallow puncture (3 mm), no De-qi, no current output] (ii) Escitalopram 10 mg/day for 12 weeks |
HAMD, Men-QoL, FSH, E2, LH | (i) Lower HAMD and Men-QoL in EA group, compared with sham-EA; no differences in HAMD and Men-QoL between EA and escitalopram groups (ii) No differences in FSH, LH and E2 levels between two groups (vs sham-EA or vs escitalopram) |
(i) Lower HAMD in EA group in 4-week and 12-week follow-ups (vs sham-EA); no difference in HAMD between EA and escitalopram groups in 4-week and 12-week follow-ups (ii) Lower Men-QoL in EA in 4-week follow-up (vs sham-EA or vs escitalopram); Men-QoL from low to high (EA < escitalopram < sham-EA) at 8-week and 12-week follow-ups |
- EA/n=2 (hematoma) - sham-EA/n=0 - Escitalopram/n=25 [fatigue (17); headache (2); sleep disturbance (7); dizziness (7); palpitation (4); sweating (10); dry mouth (14); constipation (8)] |
| Ma 201797 | - EA/n=37 - Progynova + Medroxyprogesterone acetate/n=36 |
PMI (CCMD-2-R) | NR | − 30 min/day, 3 days/week for 12 weeks - sparse-dense wave, 2/15 Hz |
CV4, EX-CA1, EX-HN3, HT7, SP6, ST25 | A total of 3 treatment cycles. Each treatment cycle includes Progynova 1mg daily for 21 consecutive days (with Medroxyprogesterone acetate 10mg daily added from day 14 to day 21) and then stop medication for 7 days | PSQI, KI, Men-QoL, FSH, E2 | (i) Lower PSQI in EA group; no differences in KI and Men-QoL between two groups (ii) No differences in FSH level between two groups; higher E2 level in EA group |
(i) Lower PSQI in EA group at 12-week follow-up (ii) No differences in KI MENQOL, and FSH and E2 levels between two groups |
- EA/n=3 [hematoma (2); mild dizziness (1)] - Progynova + Medroxyprogesterone acetate/n=4 [breast tenderness (2); mild headache (1); colporrhagia (1)] |
| Chen et al 201357 | - EA/n=38 - Alprazolam/n=32 |
PMI (DSM-IV) | NR | − 30 min/day for 20 days (7 days off every 10 days) - continuous wave, 0.7 Hz |
EX, GV20, HT7, KI3, KI7, KI10, LR3, PC6, SP6, SP9, SP10 | Alprazolam 0.4 mg/day for 20 days | AIS | Lower AIS in EA group | No follow-up | - EA/n=0 - Estazolam/n=8 (development of drug dependence after treatment) |
| Luo 202058 | - MA/n=30 - Estazolam/n=30 |
PMI (CCMD-3, CDTE-TCM) | Incoordination between heart and kidney | 30 min/day, 5 days/week for 12 weeks | BL15, BL23, BL62, EX, EX-HN1, GV20, KI3, KI6, PC6, SP6 | Estazolam 1 mg/day, 3 days/week for 12 weeks | PSQI | No differences in PSQI between two groups | No follow-up | - MA/n=0 - Estazolam/n=5 [dizziness (2); nausea and vomiting (2); skin rashes (1)] |
| Du et al 201759 | - EA/n=41 - Estazolam/n=41 |
PMI (CCMD-3) | NR | − 30 min/day, 6 days/week for 4 weeks - continuous wave, >50 Hz |
PC6, SP6, Sishenzhen (1.5 Cun apart from GV20), Dingshenzhen (0.5 Cun up to EX-HN3, and 0.5 Cun up to GB14) | Estazolam 1 mg/day, 7 days/week for 4 weeks | PSQI, KI, WHOQOL-BREF, FSH, E2 | (i) Lower PSQI and KI, and higher WHOQOL-BREF in EA group (ii) Higher E2 levels, and lower FSH levels in EA group |
No follow-up | - EA/n=6 (mild tension before EA) - Estazolam/n=26 [dizziness (26); daytime sleepiness (26)] |
| Kang 201560 | - MA/n=31 - Estazolam/n=33 |
PMI (CCMD-3) | Heart and Gallbladder Qi deficiency | 40 min/day, 6 days/week for 4 weeks | EX, EX-HN1, GB13, GB15, GV16, GV20, GV24, scalp acupoint (1 Cun up to GB15) | Estazolam 1 mg/day, 7 days/week for 4 weeks | PSQI, KI | Lower PSQI and KI in MA group | No follow-up | - MA/n=0 - Estazolam/n=1 (mild nausea) |
| Lai 201661 | - MA/n=34 - Eszopiclone/n=33 |
PMI (CCMD-3) | Incoordination between heart and kidney | 30 min/day, 6 days/week for 3 weeks (acupuncture on specific time) | BL62, KI6, LU7, SI3 | Eszopiclone 3 mg/day, 7 days/week for 3 weeks | PSQI, KI | (i) Lower KI in MA group; no differences in PSQI between two groups | No follow-up | - MA/n=2 (hematoma) - Eszopiclone/n=3 [dizziness (1); dry mouth (2)] |
| Li 201462 | - MA/n=120 - Estazolam/n=120 |
PMI (CCMD-3) | NR | 30 min/day for 30 days | SP6, SP8, Shenguan (Tianhuangfuxue) | Estazolam 2 mg/day for 30 days | PSQI | Lower PSQI in MA group | No follow-up | NR |
| Li et al 2018a63 | - MA/n=60 - Alprazolam/n=62 |
PMI (CDTE-TCM) | NR | 30–40 min/day, 5 days/week for 9 weeks | BL13, BL15, BL17, BL18, BL20, BL23, HT7 | Alprazolam 0.4–0.8 mg/day, 7 days/week for 9 weeks | PSQI, FSH, E2, LH | (i) Lower PSQI in MA group (ii) Higher E2 levels, and lower FSH and LH levels in MA group |
Follow-up 30 days; NR for valid data | NR |
| Lu et al 201449 | - MA/n=52 - Estazolam/n=52 |
PMI (CCMD-3, ICD-10) | NR | 30 min/day for 30 days | CV12, EX-HN1, GB20, GV20, HT7, LR3, LR14, SP6, SP15 | Estazolam 1 mg/day for 30 days | PSQI | Lower PSQI in MA group | No follow-up | NR |
| Ma 201464 | - EA/n=45 - Estazolam/n=45 |
PMI (CCMD-2) | Any TCM syndrome pattern according to CDTE-TCM | − 30 min/day, 3 days/week for 4 weeks - continuous wave, >50 Hz |
PC6, SP6, Sishenzhen (1.5 Cun apart from GV20), Dingshenzhen (0.5 Cun up to EX-HN3, and 0.5 Cun up to GB14) | Estazolam 1 mg/day, 7 days/week for 4 weeks | PSQI, HAMD | Lower PSQI and HAMD in EA group | No follow-up | No adverse events |
| Qin 201865 | - MA/n=34 - Estazolam/n=33 |
PMI (CCMD-3, ICD-10) | Deficiency of kidney and hyperactivity of liver | 30 min/day, 5 days/week for 4 weeks | BL17, BL18, BL23, EX, EX-HN1, GV20, KI3, LR3 | Estazolam 1–2 mg/day, 7 days/week for 4 weeks | PSQI, HAMA MSMSMS (REM%) | (i) Lower PSQI in MA group (ii) Higher REM% in MA group |
No follow-up | - MA/n=3 (hematoma) - Estazolam/n=7 [dizziness (2); daytime sleepiness (2); fatigue (3)] |
| Yang et al 201766 | - MA/n=81 - Estazolam/n=81 |
PMI (CCMD-2) | Liver and kidney Yin deficiency | 30 min/day, 15 days/month (one treatment every other day) for 3 months | CV12, HT7, KI3, PC6, ST36, ST40, four scalp acupoints (middle 1/3 of frontal apical band, posterior 1/3 of frontal apical band, anterior 1/3 of skull base band, middle 1/3 of skull base band) | Estazolam 1 mg/day, 10 days/month for 3 months | PSQI, FSH, E2, LH | (i) Lower PSQI in MA group (ii) Higher FSH levels, and lower E2 and LH levels in MA group |
No follow-up | NR |
| Zhang et al 201767 | - MA/n=31 - Estazolam/n=30 |
PMI (DTICA, CDTE-TCM) | Six syndrome patterns with liver as the core | 30 min/day, 5 days/week for 4 weeks | BL17, BL18, EX, EX-HN1, GV20, LR3 | Estazolam 1 mg/day, 7 days/week for 4 weeks | PSQI, KI, HAMA, HAMD | Lower PSQI, KI, HAMA and HAMD in MA group | No follow-up | - MA/n=1 (hematoma) - Estazolam/n=4 [dizziness (2); fatigue and daytime sleepiness (2); memory loss (2)] |
| Yan et al 202168 | - MA/n=42 - Estazolam/n=43 |
PMI (CCMD-3) | NR | 30 min/day, 7 days/week for 12 weeks | CV4, CV6, GV20, GV24, HT7, SP6 | Estazolam 1 mg/day, 7 days/week for 12 weeks | PSQI, Men-QoL | Lower PSQI and Men-QoL in MA group | No follow-up | - MA/n=4 [dizziness (3); hematoma (1)] - Estazolam/n=3 [dizziness (1); fatigue and daytime sleepiness (2)] |
| Li et al 2018b69 | - EA/n=116 - Escitalopram/n=105 |
PMD (DSM-V, ICD-10) | NR | − 30 min/day, 3 days/week for 12 weeks - dilatational wave, 50 Hz, 0.5–1 mA |
CV4, EX-CA1, EX-HN3, GV20, LI4, LR3, SP6, ST25 | Escitalopram 10mg/day for 12 weeks | HAMD, Men-QoL, FSH, E2, LH | (i) No differences in HAMD and Men-QoL between two groups (ii) No differences in FSH, E2 and LH levels between two groups |
(i) Lower HAMD in EA group at 4-and 12-week follow-ups (ii) Lower Men-QoL in EA group in 4-, 8-and 12-week follow-ups |
- EA/n=14 (hematoma) - Escitalopram/n=18 (dizziness, palpitation, stomachache) |
| Chi et al 201170 | - MA/n=30 - Fluoxetine/n=30 |
PMD (CCMD-3) | NR | 30 min/day for 4 weeks | EX-HN1, EX-HN3, GV20, KI3, LR3, LR14, SP6, ST36 | Fluoxetine 20 mg/day for 4 weeks | HAMD | Lower HAMD in MA group | No follow-up | - MA/n=0 - Fluoxetine/n=3 [dizziness (1); nausea (2)] |
| Deng 200871 | - MA/n=29 - Deanxit/n=29 |
PMD (ICD-10) | NR | 20–30 min/day, after 3 consecutive days of treatment, once treatment every 3 days for total 4 weeks | CV3, CV4, CV6, CV10, CV12, KI17, Qipang (0.5 Cun beside CV6), Xiafengshidian (1 Cun below and beside ST26) | Deanxit 20mg/day for 4 weeks | HAMD, KI, 5-HT | (i) No differences in HAMD and KI between two groups (ii) No differences in 5-HT levels between two groups |
(i) Lower HAMD in MA at 2-week follow-up (ii) No difference in HAMD between two groups at 4-week follow-up (iii) No difference in KI between two groups at 2-and 4-week follow-ups |
- MA/n=3 [changes of character of stool (2); palpitation (1)] - Deanxit/n=32 [changes of character of stool (6); dry mouth and halitosis (9); dysphoria (6); dreaminess (6); breast distending pain (5)] |
| Dong 201585 | - MA/n=30 - Nilestriol + Fluoxetine/n=30 |
PMD (CCMD-3, CDTE-TCM) | NR | 30 min/day for 30 days | BL13, BL15, BL17, BL18, BL20, BL21, BL23 | Nilestriol 2mg/15days for 30 days + Fluoxetine 20mg/day for 30 days | HAMD | Lower HAMD in MA group | No follow-up | NR |
| Li 2015b72 | - MA/n=32 - Fluoxetine/n=32 |
PMD (CCMD-3) | Liver stagnation and kidney deficiency | 30 min/day, 6 days/week for 12 weeks | BL15, BL18, BL23, EX-HN1, EX-HN3, GV20, GV24, PC6 | Fluoxetine 20mg/day for 12 weeks | HAMD, KI | Lower HAMD and KI in MA group | Follow-up for 12 weeks; no data of HAMD and KI global scores for follow-up | - MA/n=0 - Fluoxetine/n=8 [nausea and vomiting (2); dry mouth (1); indigestion (1); diarrhea (1); dizziness (1); headache (1)] |
| Ma et al 200973 | - MA/n=30 - Fluoxetine/n=30 |
PMD (CCMD-3, CDTE-TCM) | NR | 30 min/day, 5 days/week for 8 weeks | EX-HN1, EX-HN3, GV20, HT7, PC6, PC7, SP6, ST36 | Fluoxetine 20mg/day for 8 weeks | HAMD | No differences in HAMD between two groups | No follow-up | - MA/n=0 - Fluoxetine/n=6 [nausea (2); dizziness (2)] |
| Niu et al 201774 | - MA/n=41 - Fluoxetine/n=41 |
PMD (CCMD-3) | Stagnation of liver Qi | 30 min/day, 5 days/week for 6 weeks | BL13, BL15, BL17, BL18, BL20, BL23 | Fluoxetine 20mg/day for 6 weeks | HAMD | Lower PSQI in MA | No follow-up | - MA/n=7 [dizziness (2); palpitation (1); dry mouth (1); nausea (3)] - Fluoxetine/n=6 [dizziness (1); palpitation (2); dry mouth (2); nausea (1)] |
| Qian et al 200775 | - MA/n=33 - Fluoxetine/n=30 |
PMD (CCMD-3) | NR | 25 min/day, 5 days/week for 6 weeks | BL13, BL15, BL17, BL18, BL20, BL23 | Fluoxetine 20mg/day for 6 weeks | HAMD | No differences in HAMD between two groups | No follow-up | - MA/n=2 [dizziness (1); palpitation (1)] - Fluoxetine/n=9 [insomnia (1); akathisia (1); dry mouth (1); nausea (1); palpitation (1); skin symptom (1); excitation and agitation (2)] |
| Qiang 200876 | - MA/n=30 - Fluoxetine/n=30 |
PMD (CCMD-3) | NR | 25 min/day, 5 days/week for 6 weeks | BL15, BL18, BL23, EX-HN1, GB20 | Fluoxetine 20mg/day for 6 weeks | HAMD | No differences in HAMD between two groups | No follow-up | NR |
| Shi et al 201877 | - EA/n=30 - Escitalopram/n=30 |
PMD (DSM-V) | NR | − 30 min/day, 3 days/week for 12 weeks - dense-sparse wave, 10/50Hz, 0.5–1.0mA |
CV4, EX-CA1, EX-HN3, GV20, LI4, LR3, SP6, ST25 | Escitalopram 10mg/day for 12 weeks | HAMD | Lower HAMD in EA group | Lower HAMD in EA at 4- and 12- week follow-ups | NR |
| Sun et al 201578 | - EA/n=21 - Escitalopram/n=21 |
PMD (DSM-V) | NR | − 30 min/day, 3 days/week for 12 weeks - dense-sparse wave, 10/50Hz, 0.5–1.0mA |
CV4, EX-CA1, EX-HN3, GV20, LI4, LR3, SP6, ST25 | Escitalopram 10mg/day for 12 weeks | HAMD | Lower HAMD in EA group | No follow-up | NR |
| Wang et al 201079 | - MA/n=21 - Deanxit/n=21 |
PMD (CCMD-3) | NR | 30 min/day, after 3 consecutive days of treatment, once treatment every 3 days for total 4 weeks | CV3, CV4, CV6, CV10, CV12, KI17 | Deanxit 10 mg/day for 4 weeks | HAMD | No differences in HAMD between two groups | Lower HAMD in MA at 2- and 4- week follow-ups | - MA/n=3 [changes of character of stool (2); palpitation (1)] - Deanxit/n=15 [dry mouth and halitosis (9); dysphoria, dreaminess, or breast distending pain (6)] |
| Zhang 201086 | - EA/n=44 - Nilestriol+ Fluoxetine/n=46 |
PMD (CCMD-3) | NR | − 30 min/day, 5 days/week for 12 weeks - dilatational wave, 8–9 mA, 6V |
BL13, BL15, BL17, BL20, BL23, GV20, KI3, LR3, PC6, SP6 | Nilestriol 2mg/14 days for 30 days + Fluoxetine 20mg/day for 12 weeks | HAMD, KI, FSH, E2, LH | (i) No differences in HAMD and KI between two groups (ii) No differences in FSH, LH and E2 levels between two groups |
No follow-up | - EA/n=5 (sweating, dizziness, vomiting) - Nilestriol+ Fluoxetine/n=23 [dry mouth and halitosis (5); nausea (6); dysphoria (2); constipation (6); dreaminess (2); breast distending pain (2)] |
| Zhang 201387 | - MA/n=94 - Premarin + Provera + Fluoxetine/n=94 |
PMD (CCMD-3) | NR | 30 min/day, 7 days/week for 12 weeks | EX-HN1, GB13, GV20, GV24, HT7 | Premarin 0.625mg/day and Provera 6mg/day + Fluoxetine 20mg/day for 12 weeks | HAMD, FSH, E2, LH | (i) Lower HAMD in MA group (ii) No differences in FSH, LH and E2 levels between two groups |
No follow-up | - MA/n=2 (feeling pain when inserting needle) - Premarin + Provera + Fluoxetine/n=12 [dizziness (5); nausea and vomiting (4); hypersomnia (3)] |
| Zheng et al 201088 | - MA/n=60 - Premarin + Provera + Fluoxetine/n=60 |
PMD (CCMD-3) | NR | 30 min/day, 7 days/week for 12 weeks [needle retaining time for 8 hour in three acupoints (BL8, GV19, GV21) per session] |
BL8, BL18, BL23, GV19, GV21, KI3, LR3, SP6 | Premarin 0.625mg/day for 20 days + and Provera 6mg/day + Fluoxetine 20mg/day for 12 weeks | HAMD, KI, FSH, E2, LH | (i) No differences in HAMD and KI between two groups (ii) No differences in FSH and LH levels between two groups; Lower E2 level in MA group |
Lower HAMD and KI in MA at 24-week follow-up | - MA/n=2 (feeling pain when inserting needle) - Premarin + Provera + Fluoxetine/n=18 [loss of appetite (5); dizziness (4); diarrhea (3); breast distending pain (3); leukorrhagia (2); spasmus (1)] |
| Ding et al 200780 | - MA/n=39 - Fluoxetine/n=39 |
PMD (CCMD-2-R) | NR | 30 min/day, 6 days/week for 4 weeks | BL15, BL18, BL20, BL23, GV20, HT7, LR3, SP6 | Fluoxetine 20mg/day for 4 weeks | HAMD, KI | No differences in HAMD between two groups; Lower KI in MA group | No follow-up | NR |
| Li et al 2020b81 | - EA/n=30 - Fluoxetine/n=30 |
PMD (CDTE-TCM) | NR | − 25 min/day, 3 days/week for 6 weeks - dilatational wave, 15 Hz, 1 mA |
EX-HN1, EX-HN3, GV20, HT7, LI4, PC6, SP6, ST36 | Fluoxetine 20mg/day for 6 weeks | HAMD, HAMA | No differences in HAMD between two groups; Lower HAMA in EA group | No follow-up | NR |
| Zhang 201582 | - MA/n=29 - Deanxit/n=29 |
PMD (CCMD-3) | NR | 30 min/day, after 3 consecutive days of treatment, once treatment every 3 days for total 4 weeks | CV3, CV4, CV6, CV10, CV12, KI17 | Deanxit 20 mg/day for 4 weeks | HAMD | No differences in HAMD between two groups | Lower HAMD in MA at 2- and 4- week follow-ups | NR |
| Xing 201183 | - MA/n=120 - Fluoxetine/n=120 |
PMD (CCMD-3) | Stagnation of liver Qi, heart and spleen deficiency; liver depression and phlegm-heat | 20 min/day, 7 days/week for 6 weeks | GV26, PC5 | Fluoxetine 20mg/day for 6 weeks | HAMD | No differences in HAMD between two groups | No follow-up | NR |
| Zhou et al 200784 | - MA/n=30 - Fluoxetine/n=28 |
PMD (CCMD-3) | Liver and kidney Yin deficiency, spleen and kidney Yang deficiency, stagnated Qi transforming into fire, stagnation of phlegm and Qi | 30 min/day, 6 days/week for 6 weeks | BL15, BL18, BL23, EX-HN1, GB13, GV24, SP6, ST36 | Fluoxetine 20mg/day for 6 weeks | HAMD, 5-HIAA, NE, DA | (i) Lower HAMD in MA group (ii) No differences in 5-HIAA and NE levels between two groups; Lower DA level in MA group |
No follow-up | NR |
| Gao et al 201489 | - MA + Estazolam/n=32 - Estazolam/n=32 |
PMI (CCMD-3) | NR | 20 min/day, 6 days/week for 4 weeks | EX-B2 | Estazolam 2 mg/day for 4 weeks | PSQI | Lower PSQI in MA + Estazolam group | No follow-up | NR |
| Xu 202090 | - MA + Alprazolam/n=50 - Alprazolam/n=50 |
PMI (CDTE-TCM) | NR | 30 min/day, 7 days/week for 9 weeks | EX-HN1, GV24, HT7, KI3, LR3, PC6, SP6, ST36 | Alprazolam 0.4–0.8 mg/day, 7 days/week for 9 weeks | PSQI | Lower PSQI in MA + Alprazolam group | No follow-up | NR |
| Ma 201691 | - MA + Estazolam/n=35 - Estazolam/n=35 |
PMI (CCMD-3) | NR | 7 days/week for 4 weeks | EX, HT7, KI3, KI7, KI10, LR3, SP6, SP10, ST36 | Estazolam 2 mg/day, 7 days/week for 4 weeks | PSQI, FSH, E2 | (i) Lower PSQI in MA + Estazolam group (ii) Higher E2 levels, and lower FSH levels in MA + Estazolam group |
No follow-up | NR |
| Zhu et al 201692 | - MA + Estazolam/n=37 - Estazolam/n=37 |
PMI (CCMD-3) | Heart and Spleen deficiency | 20 min/day, 5 days/week for 4 weeks | CV12, EX, EX-HN1, GV20, GV24, HT7, KI3, LR3, SP9, ST25 | Estazolam 1 mg/day, 5 days/week for 4 weeks | PSQI | No differences in PSQI between two groups | No follow-up | NR |
| Ma et al 201195 | - EA + Paroxetine/n=55 - Paroxetine/n=50 |
PMD (CCMD-3) | NR | − 45 min/day, 7 days/week for 6 weeks - dilatational wave, 8–9 mA |
EX-HN3, GV20, LI4, PC6, ST36 | Paroxetine 10mg/day for 6 weeks | HAMD | No differences in HAMD between two groups | No follow-up | - EA + Paroxetine/n=2 [dizziness (1); nausea (1)] - Paroxetine/n=3 [dizziness (1); elevated blood pressure (2)] |
| Liu et al 201993 | - MA + Sertraline/n=40 - Sertraline/n=40 |
PMD (CCMD-3, ICD-10) | NR | 30 min/day, 3 days/week for 12 weeks | BL23, CV4, HT7, KI3, LI4, LR3, SP6 | Sertraline 50mg/day for 6 weeks | HAMD, FSH, E2, 5-HT, GABA | (i) Lower HAMD in MA + Sertraline group (ii) Lower FSH level in MA + Sertraline group; higher E2, 5-HT and GABA levels in MA + Sertraline group |
No follow-up | NR |
| Ning 201594 | - MA + Nilestriol + Fluoxetine/n=45 - Nilestriol + Fluoxetine/n=45 |
PMD (Psychiatry textbook) | NR | 30 min/day, 7 days/week for 12 weeks | BL13, BL15, BL18, BL20, BL23, GV20, HT7, KI3, LI4, LR3 | Nilestriol 2mg/15days + Fluoxetine 20mg/day for 12 weeks | HAMD, TESS | Lower HAMD and TESS in MA + Nilestriol + Fluoxetine group | No follow-up | NR |
Abbreviations: NR, no report; MA, manual acupuncture; EA, electroacupuncture; PMI, perimenopausal insomnia; PMD, perimenopausal depression; DSM-IV, Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition); ICSD-3, International classification of sleep disorders (Third Edition); CCMD-2-R, revised Chinese Classification of Mental Disorders (Second Edition); CCMD-3, Chinese Classification of Mental Disorders (Third Edition); ICD-10, International Classification of Diseases (10th edition); DTICA, Guidelines for Diagnosis and Treatment of Insomnia in Chinese Adults (2012 Edition); CDTE-TCM, Criteria of Diagnosis and Therapeutic Effect of Diseases and Syndromes in TCM; PSQI, Pittsburgh Sleep Quality Index; ISI, Insomnia Severity Index; AIS, Athens Insomnia Scale; KI, Kupperman Index; MRS, Menopause Rating Scale; Men-QoL, Menopause-Specific Quality of Life; HAMA, Hamilton Anxiety Scale; HAMD, Hamilton Depression Scale; SAS, Self-Rating Anxiety Scale; SDS, Self-Rating Depression Scale; WHOQOL-BREF, World Health Organization’s quality of life scale-brief form questionnaire; TESS, Treatment Emergent Symptom Scale; PSG, polysomnography; MSMSMS, micromovement sensitive mattress sleep monitoring system; SOL, sleep onset latency; WASO, wake after sleep onset; TST, total sleep time; SE, sleep efficiency; ATs, awakening times; AA, average awakening; ArI, arousal index; REM, rapid eye movement; N1(2, 3, 4), 1st(2nd, 3rd, 4th) period of non-rapid eye movement sleep (NREM); FSH, follicle-stimulating hormone; LH, luteinizing hormone; E2, estradiol; 5-HT, 5-hydroxy tryptamine; NE, norepinephrine; DA, dopamine; GABA, gamma-aminobutyric acid; six syndrome patterns with liver as the core [liver stagnation (stasis); excessive liver fire due to emotional suppression; Disturbance of liver Yang; deficiency of kidney and hyperactivity of liver; liver depression invading the stomach; liver depression invading the heart]; five TCM syndrome patterns according to CDTE-TCM [excessive liver fire due to emotional suppression; disturbance of Heart due to phlegm heat; Yin deficiency leading to excessive fire; Heart and Spleen deficiency; Heart and Gallbladder Qi deficiency]; BL8, Luoque; BL13, Feishu; BL15, Xinshu; BL17, Geshu; BL18, Ganshu; BL20, Pishu; BL21, Weishu; BL23, Shenshu; BL62, Shenmai; CV3, Zhongji; CV3, Zhongji; CV4, Guanyuan; CV6, Qihai; CV10, Xiawan; CV12, Zhongwan; EX, Anmian; EX-B2, Jiaji; EX-CA1, Zigong; EX-HN1, Sishencong; EX-HN3, Yintang; GB13, Benshen; GB14, Yangbai; GB15, Toulinqi; GB20, Fengchi; GB25, Jingmen; GV3, Yaoyangguan; GV4, Mingmen; GV14, Dazhui; GV16, Fengfu; GV19, Houding; GV20, Baihui; GV21, Qianding; GV24, Shenting; GV26, Shuigou; HT7, Shenmen; KI3, Taixi; KI6, Zhaohai; KI7, Fuliu; KI10, Yingu; KI13, Qixue; KI17, Shangqu; LI4, Hegu; LR3, Taichong; LR14, Qimen; LU7, Lieque; PC5, Jianshi; PC6, Neiguan; PC7, Daling; SI3, Houxi; SP4, Gongsun; SP6, Sanyinjiao; SP8, Diji; SP9, Yinlingquan; SP10, Xuehai; SP15, Daheng; ST25, Tianshu; ST26, Wailing; ST36, Zusanli; ST40, Fenglong.
Table 3.
Results of Meta-Analyses for Existing RCTs
| Comparison | Study (n); Participants (n) | Outcomes | Mean/Std. Mean Difference IV, Random, 95% CI | p |
|---|---|---|---|---|
| Acupuncture vs waitlist-control or placebo-/sham- acupuncture | RCTs (n = 4); PMI women (n = 283) | PSQI | MD = −4.03, 95% CI (−6.85, −1.21) | <0.01 |
| RCTs (n < 3, no meta-analysis); PMD women (n = 126) | - | - | - | |
| Acupuncture vs hypnotic | RCTs (n = 12); PMI women (n = 1204) | PSQI | MD = −2.24, 95% CI (−3.13, −1.36) | <0.01 |
| RCTs (n = 4); PMI women (n = 274) | KI | MD = −5.95, 95% CI (−10.68, −1.21) | 0.01 | |
| Acupuncture vs antidepressant/antidepressant + HRT | RCTs (n = 21); PMD women (n = 1842) | HAMD at post-treatment | SMD = −0.54, 95% CI (−0.91, −0.16) | <0.01 |
| RCTs (n = 3); PMD women (n = 169) | HAMD at 2-week follow-up | SMD= −0.64, 95% CI (−0.95, −0.33) | 0.01 | |
| RCTs (n = 6); PMD women (n = 504) | HAMD at 4-week follow-up | SMD = −1.36, 95% CI (−2.72, 0.00) | 0.05 | |
| RCTs (n = 3); PMD women (n = 341) | HAMD at 12-week follow-up | SMD = −2.73, 95% CI (−6.14, 0.67) | 0.12 | |
| RCTs (n = 5); PMD women (n = 410) | KI | MD= −2.80, 95% CI (−5.60, −0.01) | 0.05 | |
| Acupuncture + hypnotics vs hypnotics | RCTs (n = 4); PMI women (n = 308) | PSQI | MD = −2.80, 95% CI (−4.23, −1.37) | <0.01 |
| Acupuncture + antidepressant/antidepressant + HRT vs antidepressant or antidepressant + HRT | RCTs (n = 3); PMD women (n = 275) | HAMD at post-treatment | SMD = −0.82, 95% CI (−1.07, −0.58) | <0.01 |
Note: Data from references 19 and 50.
Abbreviations: PSQI, Pittsburgh Sleep Quality Index; KI, Kupperman Index; HAMD, Hamilton Depression Scale; HRT, hormone replacement therapy.
Effectiveness and Efficacy of Acupuncture for PMI and PMD
We classified the retrieved RCTs into two categories: acupuncture as an independent or as an adjuvant to standard care in the management of PMI/PMD.
In the first category, six RCTs confirmed significant improvements acupuncture produced on sleep duration and quality51–54 as well as depressed mood,55,56 when compared with waitlist or placebo controls. Thirteen RCTs compared the effects between acupuncture and various hypnotics including Estazolam, Alprazolam, and Eszopiclone.49,57–68 All these studies reported that acupuncture reduced global scores of Pittsburgh Sleep Quality Index (PSQI)/Athens Insomnia Scale (AIS) more effectively than hypnotics, except for two trials58,61 that reported the sleep-promoting effects between the two therapies were equivalent. Twelve out of these 13 trials employed the PSQI as an outcome measure, and the results of meta-analysis favored acupuncture [MD = −2.24, 95% CI (−3.13, −1.36), p< 0.01]. Twenty-one RCTs compared the effects between acupuncture and antidepressants including escitalopram, fluoxetine, and deanxit,56,69–84 or the effects between acupuncture and antidepressants combined with HRT.85–88 All trials adopted the Hamilton Depression Scale (HAMD) as an outcome measure. The results of meta-analysis favored acupuncture in reducing the HAMD global scores [SMD= −0.54, 95% CI (−0.91, −0.16), p< 0.01]. Some of these RCTs also reported that the effects of acupuncture remained no weaker than those of Western medications at 2-, 4-, and 12-week follow-ups, suggesting an intermediate- to long-term antidepressive efficacy of acupuncture (Table 3).
In the second category, the meta-analysis pooled the effects of four RCTs89–92 and supported a better effect of acupuncture combined with hypnotics in reducing PSQI global score, in comparison with hypnotics alone [MD = −2.80, 95% CI (−4.23, −1.37), p < 0.01]. Two RCTs93,94 indicated that acupuncture combined with antidepressants produced lower HAMD scores than antidepressants alone. Although another RCT95 found no statistically significant differences in HAMD scores between the two therapies at post-treatment, the clinical effectiveness rate (calculated based on the HAMD reduction rate) favored the combination therapy (acupuncture + Paroxetine 90.91% vs Paroxetine alone 78.00%). The meta-analysis based on these three RCTs favored the combination therapy in reducing HAMD global scores as well [SMD = −0.82, 95% CI (−1.07, −0.58), p < 0.01] (Table 3).
Safety of Acupuncture for PMI and PMD
The incidence of AEs associated with each intervention in the included RCTs is summarized in Appendix 1 (in order of highest % for acupuncture). Consistent with the previous studies that acupuncture is safe to manipulate,36,37 there appears to be few risks associated with utilizing acupuncture in the management of PMI/PMD as the AEs caused by acupuncture were minimal and very mild. Hematoma (7.72%), the most frequent complaint, usually faded within a few days.19,50 The incidence of other acupuncture-related AEs was less than 4%, with the exception of sweating (6.76%). The latter however is likely to be related to menopause rather than acupuncture as this is a common symptom for perimenopausal women.13,96 The incidence and severity of AEs associated with psychotropic agents (hypnotics or antidepressants) far exceeded those of acupuncture. The most common of these were daytime sleepiness and/or fatigue (29.38%), breast tenderness (22%) and night-time sleep disturbance (18.18%). These findings are noteworthy given the problem of PMI/PMD and suggest that some of these treatments may exacerbate rather than alleviate the symptoms. Only one RCT97 used HRT alone as a control but still identified at least three types of AEs.
These findings are highly supportive of the use of acupuncture as a therapeutic strategy in PMI and/or PMD. The data suggest that it does not matter whether the therapeutic effect of acupuncture is better or only equivalent to that of HRT and/or hypnotics/antidepressants, acupuncture can be supported as a front-line, first-choice therapeutic because of its exemplary safety profile.
Implications for Clinical Practice
This section discusses the meaningfulness and feasibility of acupuncture for PMI and PMD. In addition to improving sleep or depression outcomes, acupuncture also showed positive effects on indices of climacteric symptoms (ie, Kupperman Index, Menopause Rating Scale, etc), quality of life in menopause (ie, Menopause-Specific Quality of Life, World Health Organization’s quality of life scale-brief form questionnaire, etc), and/or anxiety symptoms (ie, Hamilton Anxiety Scale, etc). (Tables 2 and 3) These findings suggest that women with PMI/PMD as their chief complaint benefited from acupuncture in a broad range of signs and symptoms associated with life change. This finding is consistent with the “holistic medicine” concept highlighted in the TCM, which views the body as a complete entirety.43,44,98 “Healthy” in TCM does not simply mean “disease free”; it means that all Zang and Fu (organs), meridians and collaterals are working in harmony, and the flow of Qi, blood and body fluids is at ease, and emotions and spiritual state are in balance.26,28,43 In terms of TCM theory, a diseased condition is not only a problem in a local part of the body but a local reflection of disharmony of the entire body.43,44 Hence, TCM therapies generally address a diseased condition (condition with an imbalance between Yin and Yang) by regulating and mobilizing the entire body rather than just regulating a single factor (one symptom or one part/organ of the body).43,44,98 As suggested, any potential climacteric condition (eg, nocturnal hot flushes, chronic pain, neuropsychiatric problems, etc) that may adversely affect sleep and/or mood should be considered when menopausal women seek medical advice.23 This circumstance is in line with the “holistic medicine” theory of TCM, which makes acupuncture more worthy of being recommended.
Limited evidence suggests acupuncture is likely to have an add-on effect to hypnotic and/or antidepressive drugs. However, in seven trials focusing on acupuncture combined with standard care, only one study95 reported the AEs; no trials included follow-up (Tables 2 and 3). Consequently, the safety and long-term effects of this integrative remedy is still less well understood. Consumers who are not familiar with acupuncture may not be willing to give up drug-based medicines immediately.19 Whether reduced use of conventional drugs can be made up by the addition of acupuncture is a topic with important clinical significance because reduction of dosage in conventional drugs means fewer risks of side effects. Likewise, this add-on effect of acupuncture is also of importance in personalized medicine. Given the efficacy for all therapies may be hampered by the fact that women respond differently to each, acupuncture is ideal to be recommended as the first therapy in a layered approach, so that if acupuncture is ineffective then lower dose Western medication (eg, HRT, psychotropic agents, etc) could be tried in combination with the acupuncture prior to direct usage of pharmacotherapy or higher dose of pharmacotherapy. This treatment strategy may be particularly suitable for those women with PMI/PMD who are intolerant of drugs.
Acupoint selection is one of the decisive factors affecting the clinical effectiveness of acupuncture.99 As illustrated in Table 2, the three most commonly used acupoints for PMI were Sanyinjiao (SP6), Baihui (GV20), and Shenmen (HT7), while the three most commonly used acupoints for PMD were GV20, SP6, and Yintang (EX-HN3). According to “Indications of Acupuncture Points [GB/T 30233–2013]” (National Standard of People’s Republic of China, 2013 Version),100 GV20, HT7, and EX-HN3 are classic acupoints for the treatment of psychiatric and psychological disorders. SP6 is the preferred acupoint for gynaecological disorders, and together with HT7, it promotes sleep. Based on TCM syndrome patterns, for any deficient patterns, the Back-shu are used as these acupoints where the Qi of the internal organs is accumulated, and are used to strengthen the corresponding organs.100 Ganshu (BL18), the Back-shu of liver, as well as Shenshu (BL23), the Back-shu of kidney, were selected in many trials (Table 2), which is consistent with the aforementioned findings that smoothing liver and nourishing kidney is the general principle for all perimenopausal disorders. In treating comorbid PMI and PMD, practitioners are hence recommended to select Back-shu of liver and kidney, and/or acupoints of Liver Meridian of Foot-Jueyin and Kidney Meridian of Foot-Shaoyin, in addition to the classical acupoints utilized for mental disease/disorders. Both MA and EA showed significant clinical benefits for PMI/PMD (Table 2). However, no study has compared MA with EA. The differences in clinical effects and underlying mechanisms of MA and EA should be explored in future clinical trials and animal studies.
Our comprehensive retrieval of literature failed to identify any RCT carried outside of China, reflecting the extremely inadequate awareness and attention paid to acupuncture in PMI/PMD management among Western researchers, despite well-documented evidence supporting the growing widespread use of CAM therapy for psychiatric complaints among Western populations.23,101 Meanwhile, some surveys alluded that most Western women may also underestimate the value and role of acupuncture as a management strategy for PMI and/or PMD. As reported, only 4.8% women in Australia have visited an acupuncturist due to menopausal symptoms;102 In the UK, 6.4% climacteric women utilize aromatherapy, reflexology, or acupuncture to reduce symptoms.103 This review thereby is expected to evoke awareness of the potential of acupuncture among both perimenopausal women and clinicians working in this field (ie, gynaecologists, psychiatrists, and naturopaths) in Western countries. Given all the participants in the included RCTs were Chinese women, generalizing the currently optimistic results to women of other races requires some further evidence.104 According to the differences in awareness, perception, and tolerance of acupuncture between Westerners and Chinese, limited modification to the nature acupuncture prescription/protocol summarized in published Chinese studies may also enhance the acceptance and applicability of acupuncture among Western perimenopausal women.
Another issue that has not yet been addressed is whether interventions can be provided during pre-menopause to prevent or reduce the occurrence of PMI and/or PMD. Women with a history of depression105 or experiencing stressful events6 at pre-menopause are at high risk for PMD. Women with a history of severe premenstrual syndrome and/or psychological disturbances may benefit from preventive treatments for reducing the likelihood of PMI or ameliorating the symptoms.10 These women may be a target group for preventive acupuncture (also called “acupuncture pre-treatment”), which is in line with the idea of “preventive treatment of disease” (治未病, prevent individuals from being trapped by diseases) in TCM theory.44 Despite the lack of direct evidence, acupuncture pre-treatment may have positive preventive effects for perimenopausal symptoms. Most research in this area has been based on animal models. Acupuncture pre-treatment was observed to reduce oxidative stress levels,106,107 inhibit the hyperactivity of hypothalamic-pituitary-ovary (HPO) axis,106 as well as regulate the inflammatory response and disorders of the immune system108 in either ageing female rats or rats that were ovariectomized to mimic menopause. Li et al reported that EA pre-treatment (EA was provided 10 days before chronic stress) effectively prevented the depression-like behavior of rats after undergoing chronic unpredicted mild stress (CUMS).109
Implications for Research
The data from the few clinical trials discussed here appears to be promising, it is however too early to draw any definitive conclusions. Many RCTs have missing components in their methodology, which dilutes and weakens the quality of evidence, and may hinder the development of acupuncture as a form of evidence-based healthcare for comorbid depression and insomnia during perimenopause.
We have summarized the common deficiencies among these RCTs and listed the potential negative consequences in Table 4. Detailed assessment and analysis on quality of evidence is described in our published SRs/MAs.19,50 As reported, poor-quality acupuncture studies usually show a higher proportion of positive results than high-quality studies.104 Well-designed trials with robust methodology and high-quality reporting quality are warranted in future research.
Table 4.
Common Defects of Study Design and Reporting Quality Among Current RCTs
| Items | Limitations | Potential Negative Consequences |
|---|---|---|
| Study design | Lack of appropriate sample size estimation | Diminishment of credibility of the results; reduction in likelihood of finding an effect of the treatment |
| Lack of sham-/placebo- acupuncture as parallel control in trials comparing acupuncture with Western medications | Potential bias and uncertainty on the placebo effects of acupuncture | |
| Without appropriate statistics (ie intention-to-treat analysis, etc) for data of those withdrawal/dropout patients | Increased risk of an overestimation/underestimation of the efficacy of acupuncture or Western medications | |
| Lack of follow-ups | Unable to evaluate medium- and long- term effects/safety of acupuncture | |
| Objective indices (ie, polysomnography, actigraphy, etc) is seldom used in PMI studies | (i) Unable to understand the effects of acupuncture on sleep architecture (ii) A biased judgement or even a question on acupuncture’s real efficacy due to a mismatch between the subjective and objective sleep-wake duration |
|
| No discussion of the effects of acupuncture responding to specific/each TCM syndrome pattern (all TCM syndrome patterns are mixed for efficacy assessment) | Difficult to judge the real therapeutic effect of acupuncture | |
| Reporting quality | Lack of reports on if or how allocation concealment and blinding of patients/outcome assessments are performed | Inadequate blinding is associated with an exaggeration of the estimated efficacy |
| Lack of description and in-depth analysis of the specific causes of withdrawal/dropout | Unable to understand patient’s compliance and acceptance of acupuncture, placebo or Western medications | |
| Protocols and/or registration Information are not always available | Greatly weakens the reliability of the evidence | |
| Incomplete descriptions of needling details and/or treatment regimen, as well as acupuncturists’ background | (i) Poor reproducibility of the trial results (ii) Hinder the clinical promotion of effective acupuncture prescription |
Underlying Mechanisms of Acupuncture’s Effects Against Depression and Insomnia During Perimenopause
It is challenging to explore the mechanisms underlying the effects of acupuncture on depression and insomnia during the transition to menopause in only RCTs. To enhance our understanding, investigation via animal studies is a valuable option. Together with clinical findings, animal studies can shed light on the mechanisms of action of acupuncture (Figure 1) as well as give an insight into the direction of further research. Findings of animal studies are summarized in Table 5.
Figure 1.
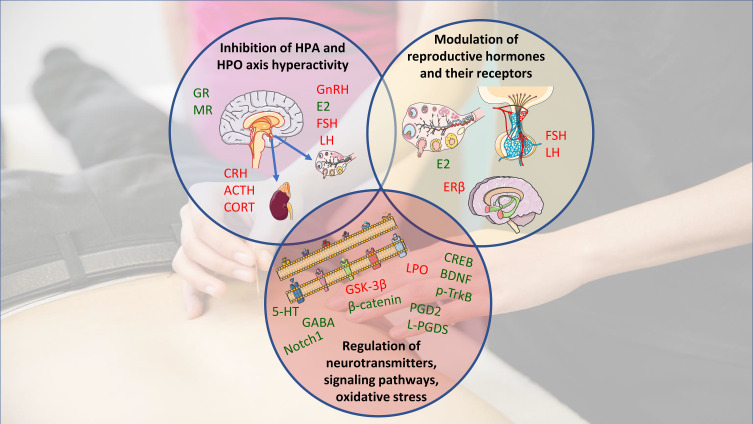
Potential mechanisms by which acupuncture may improve perimenopausal depression and insomnia. Manual- and/or electro-acupuncture may alter hypothalamic-pituitary-adrenal and -ovarian hyperactivity; modulate reproductive hormones and their receptors, or regulate neurotransmitters, signaling pathways, and oxidative stress. Green indicates an upregulation/increase by acupuncture (↑); red indicates a downregulation/decrease (↓). Acupuncture may also improve perimenopausal depression and insomnia by improving vasomotor symptoms, while this indirect effect is not shown. Images are adapted from Servier Medical Art under Creative Commons CC-BY license.
Abbreviations: HPA, hypothalamic-pituitary-adrenal; HPO, hypothalamic-pituitary-ovary; GR, glucocorticoid receptor; MR, mineralocorticoid receptor; GnRH, gonadotropin-releasing hormone; E2, estradiol; FSH, follicle-stimulating hormone; LH, luteinizing hormone; CRH, corticotropin-releasing hormone; ACTH, adrenocorticotropic hormone; CORT, cortisol; ERβ, estrogen receptor beta; 5-HT, 5-hydroxytryptamine; GABA, gamma-amino butyric acid; CREB, cAMP-response element-binding protein; BDNF, brain-derived neurotrophic factor; p-TrkB, phosphorylated-TrkB; PGD-2, prostaglandin D2; L-PGDS, lipocalin-type prostaglandin D synthase; LPO, lipid peroxidation; GSK-3β, glycogen synthase kinase-3 beta.
Table 5.
Summary of Animal Studies Determining the Mechanisms Underlying Acupuncture on PMI and PMD
| Author/Year | Animals | Disease Models | Methods of Modeling | Study Groups/No. of Participants | Interventions | Acupuncture Protocol | Acupoints | Outcome Measures | Results |
|---|---|---|---|---|---|---|---|---|---|
| Cao et al 2019152 | SD rats, 280–320g, 12 months | PMI | (i) OVX (ii) Electrical stimulation |
- EA/n= 12 - Model/n= 12 - Sham-surgery/n= 12 |
- EA/OVX + electrical stimulation + EA - Model/OVX + electrical stimulation - Sham-surgery/sham-OVX + electrical stimulation |
− 20 min/day for 14 days - Dense wave, 50Hz |
SP6 | (i) MER (REM, SWS) (ii) 5-HT, 5-HIAA, 5-HT1A in hippocampus |
(i) EA increased REM and SWS (ii) EA increased 5-HT and 5-HT1A levels; no significant change in 5-HIAA level |
| Cao et al 2020136 | Wistar rats, 230–270g, 5–7 months | PMI | (i) OVX (ii) Electrical stimulation |
- EA/n= 10 - Model/n= 10 - Sham-surgery/n= 10 |
- EA/OVX + electrical stimulation + EA - Model/OVX + electrical stimulation - Sham-surgery/sham-OVX + electrical stimulation |
− 15 min/day for 21 days - Dense wave, 50Hz |
EX-HN1, HT7 | (i) MER (TST, REM, SWS, LS) (ii) L-PGDS, PGD2, PGE2 in CSF (iii) GABA in VLPO |
(i) EA increased REM, SWS; no significant change in TST and LS (ii) EA increased L-PGDS and PGD2 levels; no significant change in PGE2 level (iii) EA increased GABA level |
| Jin et al 2010189 | SD rats, 230–270g, 3 months | PMI | (i) OVX (ii) Electrical stimulation |
- EA/n= 30 - Model/n= 30 - Sham-surgery/n= 30 |
- EA/OVX + electrical stimulation + EA - Model/OVX + electrical stimulation - Sham-surgery/sham-OVX + electrical stimulation |
− 30 min/day for 7 days - Continuous wave, 15Hz, 2mA |
BL23, SP6 | (i) SOL and TST (ii) Heat-resistant time (iii) Energy (iv) Times of locomotor activity |
(i) EA increased TST and reduced SOL (ii) EA increased heat-resistant time (iii) EA increased energy (iv) No significant change in times of locomotor activity (v) The earlier the intervention of EA in the perimenopausal period, the better the effect |
| Jin et al 2011190 | SD rats, 150–180g | PMI | (i) OVX (ii) Electrical stimulation |
- EA/n= 30 - Model/n= 30 - Sham-surgery/n= 30 |
- EA/OVX + electrical stimulation + EA - Model/OVX + electrical stimulation - Sham-surgery/sham-OVX + electrical stimulation |
− 30 min/day for 7 days - Continuous wave, 15Hz, 2mA |
BL23, SP6 | (i) SOL and TST (ii) Heat-resistant time (iii) Energy |
(i) EA increased TST and reduced SOL (ii) EA increased heat-resistant time (iii) EA increased energy (iv) The earlier the intervention of EA in the perimenopausal period, the better the effect |
| Xie 2013116 | SD rats, 216–245.8g, 6–8 weeks | PMI | OVX | - EA/n= 25 - Model/n= 25 - Sham-surgery/n= 25 - Sham-EA/n= 25 |
- EA/OVX + EA - Model/OVX - Sham-surgery/sham-OVX -Sham-EA/OVX + sham-EA |
− 15 min/day for 7 days - Continuous wave, 2Hz |
BL23, EX | (i) EEG/EMG recording systems for rats and mice (WASO, REM, NREM) (ii) Serum FSH, E2 (iii) Estrogen receptor in VLPO and TMN (iv) Weight |
(i) EA increased NREM, and reduced WASO and REM (ii) EA increased E2 and decreased FSH levels (iii) No significant change in estrogen receptor (iv) EA reduced weight |
| Yu 2012117 | SD rats, 230–270g, 6–8 weeks | PMI | OVX | - EA/n= 12 - Model/n= 12 - Sham-surgery/n= 12 - Sham-EA/n= 12 |
- EA/OVX + EA - Model/OVX - Sham-surgery/sham-OVX - Sham-EA/OVX + sham-EA |
− 15 min every other day for 7 days - Continuous wave, 2Hz |
EX | (i) EEG/EMG recording systems for rats and mice (WASO, REM, NREM) (ii) Serum FSH, E2, LH |
(i) EA increased NREM and reduced WASO; no significant change in REM; EA alleviated sleep fragmentation during the daytime (ii) EA increased E2 and reduced FSH levels; no significant change in LH level |
| Yang 2017121 | SD rats, 230–270g, 6–8 weeks | PMI | OVX | - EA/n= 40 - Model/n= 40 - Sham-surgery/n= 40 - Sham-EA/n= 40 |
- EA/OVX + EA - Model/OVX - Sham-surgery/sham-OVX - Sham-EA/OVX + sham-EA |
− 15 min/day for 7 days - Continuous wave, 15Hz |
BL23, EX | (i) EEG/EMG recording systems for rats and mice (WASO, REM, NREM) (ii) serum E2 and 5-HT (iii) Adrenocortical E2 (iv) Hypothalamic 5-HT, NE, ER-α mRNA, ER-β mRNA |
(i) EA increased NREM, and reduced WASO and REM; EA alleviated sleep fragmentation during the daytime (ii) EA increased E2 and 5-HT levels (iii) EA increased E2 level (iv) EA increased 5-HT, and decreased NE and ERβmRNA; no significant change in ERαmRNA |
| Jing et al 2020174 | SD rats, 190–210g, 56–62days | PMD | (i) OVX (ii) CUMS |
- EA/n= 10 - Control/n= 10 - Model/n= 10 - Sham-surgery/n= 10 - Clomipramine/n= 10 |
- EA/OVX + CUMS + EA - Control/no treatment - Model/OVX + CUMS - Sham-surgery/sham-OVX + CUMS - Clomipramine/OVX + CUMS + Clomipramine |
− 20 min/day for 28 days-isperse-dense wave, 4/20Hz, 18V | BL23, GV20, SP6 | (i) Behavioral tests (OFT, SPT) (ii) Serum E2, LH, GnRH (iii) GSK-3β mRNA, β-catenin mRNA in hippocampus (iv) β-Catenin protein, p-β-catenin protein in hippocampus |
(i) EA improved rats’ depression-like behavior in behavioral tests (ii) EA increased E2, and decreased LH and GnRH levels (iii) EA decreased GSK-3β mRNA and increased β-catenin mRNA expression (iii) EA increased β-catenin protein and decreased p-β-catenin protein expression |
| Guo et al 2019145 | KM mice, 18–22g | PMD | (i) OVX (ii) CUMS |
- EA/n= 10 - Control/n= 10 - Model/n= 10 - Clomipramine/n= 10 |
- EA/OVX + CUMS + EA - Control/no treatment - Model/OVX + CUMS - Clomipramine/OVX + CUMS + Clomipramine |
− 20 min/day for 28 days- disperse-dense wave, 2/10Hz, 18V |
BL23, GV20, SP6 | (i) Behavioral tests (FST, IAT, ADE, TSE) (ii) Serum E2, FSH, LH (iii) 5-HT, NE, DA in hippocampus |
(i) EA improved mice’ depression-like behavior in behavioral tests (ii) EA increased E2 and decreased LH and FSH levels (ii) EA increased 5-HT, NE and DA levels |
| Seo et al 2018170 | SD rats, 250–300g | PMD | OVX | - EA/n= NR - Model/n= NR - Sham-surgery/n= NR -Sham-EA/n= NR |
- EA/OVX + EA - Model/OVX - Sham-surgery/sham-OVX - Sham-EA/OVX + sham-EA |
60 seconds/day for 4 days | SP6 | (i) Behavioral tests (FST, OFT, EPM) (ii) E2 and estrogen receptor expression in plasma and hippocampus (iii) BDNF and p-TrkB receptor in hippocampus (iv) NPY in hippocampus |
(i) EA improved rats’ depression-like behavior in behavioral tests (ii) no significant change in E2 level or estrogen receptor expression (iii) EA increased BDNF and p-TrkB receptor levels (iv) EA increased NPY levels |
| Deng et al 2017185 | SD rats, 190–210g, 56–62days | PMD | (i) OVX (ii) CUMS |
- EA/n= 12 - Control/n= 12 - Model/n= 12 - Clomipramine/n= 12 |
- EA/OVX + CUMS + EA - Control/no treatment - Model/OVX + CUMS - Clomipramine/OVX + CUMS + Clomipramine |
− 20 min/day for 28 days- disperse-dense wave, 4/20Hz, 18V |
BL23, GV20, SP6 | (i) Behavioral tests (FST, OFT) (ii) MAP-2, Notch1, Jagged1, Hes1 in hippocampus (iii) MAP-2 mRNA, Notch1 mRNA, Jagged1 mRNA, Hes1 mRNA in hippocampus |
(i) EA improved rats’ depression-like behavior in behavioral tests (ii) EA increased MAP-2 protein and Notch1 protein levels, and decreased Jagged1 protein and Hes1 protein levels (ii) EA increased MAP-2 mRNA and Notch1 mRNA expression, and decreased Jagged1 mRNA and Hes1 mRNA expression |
| Deng 2017177 | SD rats, 190–210g, 56–62days | PMD | (i) OVX (ii) CUMS |
- EA/n= 10 - Control/n= 10 - Model/n= 10 - Sham-surgery/n= 10 - Clomipramine/n= 10 |
- EA/OVX + CUMS + EA - Control/no treatment - Model/OVX + CUMS - Sham-surgery/sham-OVX + CUMS - Clomipramine/OVX + CUMS + Clomipramine |
− 20 min/day for 28 days- disperse-dense wave, 4/20Hz, 18V |
BL23, GV20, SP6 | (i) Behavioral tests (FST, OFT, SPT) (ii) GSK-3β, β-catenin in hippocampus (iii) β-catenin protein, p-β-catenin protein in hippocampus (iv) GSK-3β mRNA, β-catenin mRNA in hippocampus |
(i) EA improved rats’ depression-like behavior in behavioral tests (ii) EA decreased GSK-3β and increased β-catenin contents (iii) EA increased β-catenin protein expression; no significant change in p-β-catenin protein expression (iv) EA decreased GSK-3β mRNA and increased β-catenin mRNA expression |
| Huangfu 2018129 | SD rats, 190–210g, 56–62days | PMD | (i) OVX (ii) CUMS |
- EA/n= 10 - Control/n= 10 - Model/n= 10 - Clomipramine/n= 10 |
- EA/OVX + CUMS + EA - Control/no treatment - Model/OVX + CUMS - Clomipramine/OVX + CUMS + Clomipramine |
− 20 min/day for 28 days- disperse-dense wave, 4/20Hz, 18V |
BL23, GV20, SP6 | (i) Behavioral tests (FST, OFT, SPT, TSE) (ii) Glucocorticoid receptor, mineralocorticoid receptor in hippocampus |
(ii) EA improved rats’ depression-like behavior in behavioral tests (ii) EA increased glucocorticoid receptor and mineralocorticoid receptor levels |
| Jiang 2017124 | SD rats, 190–210g, 56–62days | PMD | (i) OVX (ii) CUMS |
- EA/n= 12 - Control/n= 12 - Model/n= 12 - Sham-surgery/n= 12 - Clomipramine/n= 12 |
- EA/OVX + CUMS + EA - Control/no treatment - Model/OVX + CUMS - Sham-surgery/sham-OVX + CUMS - Clomipramine/OVX + CUMS + Clomipramine |
− 20 min/day for 28 days- disperse-dense wave, 4/20Hz, 18V |
BL23, GV20, SP6 | (i) Behavioral tests (FST, SPT, TSE) (ii) Serum E2, FSH, LH, GnRH, CRH, ACTH, CORT, β-EP |
(i) EA improved rats’ depression-like behavior in behavioral tests (ii) EA increased E2 andβ-EP, and decreased FSH, LH, GnRH, CORT, ACTH and CRH levels |
| Jiang et al 2017127 | SD rats, 190–210g, 8–9 weeks | PMD | (i) OVX (ii) CUMS |
- EA/n= 12 - Control/n= 12 - Model/n= 12 - Sham-surgery/n= 12 - Clomipramine/n= 12 |
- EA/OVX + CUMS + EA - Control/no treatment - Model/OVX + CUMS - Sham-surgery/sham-OVX + CUMS - Clomipramine/OVX + CUMS + Clomipramine |
− 20 min/day for 28 days- disperse-dense wave, 4/20Hz, 18V |
BL23, GV20, SP6 | (i) Behavioral test (SPT) (ii) Serum E2, LH, GnRH, β-EP |
(i) EA improved rats’ depression-like behavior in behavioral test (ii) EA increased E2 andβ-EP, and decreased LH and GnRH levels |
| Jing et al 2018178 | SD rats, 180–220g, 17 weeks | PMD | (i) OVX (ii) CUMS |
- EA/n= 12 - Control/n= 12 - Model/n= 12 - Clomipramine/n= 12 |
- EA/OVX + CUMS + EA - Control/no treatment - Model/OVX + CUMS - Clomipramine/OVX + CUMS + Clomipramine |
− 20 min/day for 28 days- disperse-dense wave, 2/10Hz, 18V |
BL23, GV20, SP6 | (i) Behavioral test (TSE) (ii) E2, NE in hippocampus (iii) DKK1, LRP-5, LRP-6 in hippocampus |
(i) EA improved rats’ depression-like behavior in behavioral test (ii) EA increased E2 and NE levels (iii) EA decreased DKK1, and decreased LRP-5 and LRP-6 expression |
| Zhou et al 2015146 | SD rats, 220–260g | PMD | (i) OVX (ii) CUMS |
- MA/n= 9 - Model-1/n= 9 - Model-2/n= 8 - Sham-surgery/n= 6 - Nilestriol + Fluoxetine/n= 9 |
- MA/OVX + CUMS + MA - Model-1/OVX + CUMS - Model-2/OVX - Sham-surgery/sham-OVX - Nilestriol + Fluoxetine/OVX + CUMS + Nilestriol + Fluoxetine |
20 min/day for 21 days | BL23, GV20, SP6 | (i) Behavioral test (OFT) (ii) 5-HT, NE, DA in hypothalamus |
(i) MA improved rats’ depression-like behavior in behavioral test (ii) MA increased 5-HT level; no significant changes in NE and DA levels |
| Shi et al 2012147 | SD rats, 300–350g, 4 months | PMD | (i) OVX (ii) CUMS |
- MA/n= 10 - Model/n= 5 - Sham-surgery/n= 5 - Estradiol benzoate/n= 10 |
- MA/OVX + CUMS + MA - Model/OVX + CUMS - Sham-surgery/sham-OVX + CUMS - Estradiol benzoate/OVX + CUMS + Estradiol benzoate |
30 min/session, once session every 2 days for total 30 sessions | CV4, CV6, LI4, LR3, PC6, SP6, ST36 | (i) Serum E2 (ii) 5-HT in hypothalamus |
(i) EA increased E2 level (ii) EA increased 5-HT level |
| Sun 2009118 | (i) SD rats, 250–300g, 11–15 months (n= 45) (ii) Young SD rats, 150–200g, 4 months (n= 10) |
PMD | CUMS | - EA/n= 15 - Model/n= 15 - Clomipramine/n= 15 - Young/n= 10 |
- EA/CUMS + EA - Model/CUMS - Clomipramine/CUMS + Clomipramine - Young/CUMS |
− 20 min/day for 7 days- disperse-dense wave, 4/20Hz, 2V |
BL18, BL23, GV20, SP6 | (i) Behavioral tests (OFT) (ii) Serum E2, NE |
(i) EA improved rats’ depression-like behavior in behavioral test (ii) EA increased E2 and NE levels |
| Wang et al 2016171 | SD rats, 220–260g, 17weeks | PMD | (i) OVX (ii) CUMS |
- EA/n= 10 - Control/n= 10 - Model/n= 10 - Clomipramine/n= 10 |
- EA/OVX + CUMS + EA - Control/no treatment - Model/OVX + CUMS - Clomipramine/OVX + CUMS + Clomipramine |
− 20 min/day for 28 days- disperse-dense wave, 2/10Hz |
BL23, GV20, SP6 | (i) Behavioral test (OFT) (ii) CREB, BDNF in hippocampus |
(i) EA improved rats’ depression-like behavior in behavioral test (ii) EA increased BDNF and CREB levels |
| Wu 2012148 | (i) SD rats, 260–471g, 11–13 months (n= 73) (ii) Young SD rats, 140–191g, 4 months (n= 20) |
PMD | CUMS | - EA/n= 18 - Model/n= 19 - Clomipramine/n= 18 - Herbal medicine/n= 18 - Young/n= 20 |
- EA/CUMS + EA - Model/CUMS - Clomipramine/CUMS + Clomipramine - Herbal medicine/ CUMS + XiaoYao-Pill - Young/CUMS |
− 20 min/day for 10 days- disperse-dense wave, 2/15Hz, 2V |
BL18, BL23, GV20, SP6, ST36 | (i) Behavioral test (OFT) (ii) Serum E2 (iii) 5-HT2A mRNA, p-ERK 1/2 in hippocampus |
(i) EA improved rats’ depression-like behavior in behavioral test (ii) EA increased E2 level (ii) EA decreased 5-HT2A mRNA expression; EA increased p-ERK 1/2 expression |
| Zhang 2009128 | (i) SD rats, 250–300g, 11–15 months (n= 45) (ii) Young SD rats, 150–200g, 4 months (n= 10) |
PMD | CUMS | - EA/n= 15 - Model/n= 15 - Clomipramine/n= 15 - Young/n= 10 |
- EA/CUMS + EA - Model/CUMS - Clomipramine/CUMS + Clomipramine - Young/CUMS |
− 20 min/day for 7 days- continuous wave, 20Hz, 2V |
BL18, BL23, GV20, SP6 | (i) Behavioral test (OFT) (ii) Serum E2 (iii) β-EP in hippocampus |
(i) EA improved rats’ depression-like behavior in behavioral test (ii) EA increased E2 level (ii) EA increased β-EP level |
| Zhao et al 2011149 | (i) SD rats, 260–471g, 11–13 months (n= 66) (ii) Young SD rats, 140–191g, 4 months (n= 20) |
PMD | CUMS | - EA/n= 15 - Model/n= 18 - Clomipramine/n= 17 - Herbal medicine/n= 16 - Young/n= 20 |
- EA/CUMS + EA - Model/CUMS - Clomipramine/CUMS + Clomipramine - Herbal medicine/ CUMS + XiaoYao-Pill - Young/CUMS |
− 20 min/day for 28 days | BL18, BL23, GV20, SP6, ST36 | (i) Behavioral test (OFT) (ii) Serum 5-HT, HDL |
(i) EA improved rats’ depression-like behavior in behavioral test (ii) EA increased 5-HT and HDL levels |
| Song et al 2021119 | KM mice, 18–22g, 5–6 weeks | PMD | (i) OVX (ii) Ice-water swimming stimulation (I-WSS) |
- EA/n= 10 - Control/n= 10 - Model/n= 10 |
- MA/OVX + I-WSS + MA - Control/I-WSS - Model/OVX + I-WSS |
− 30 min/day, 6 days/week for 4 weeks | CV4, KI3, KI15, SP6, SP10, ST36 | Serum E2 | EA increased E2 level |
Abbreviations: NR, no report; PMI, perimenopausal insomnia; PMD, perimenopausal depression; MA, manual acupuncture; EA, electroacupuncture; OVX, ovariectomy; CUMS, chronic unpredictable mild stress; MER, multichannel electrophysiological recordings; SOL, sleep onset latency; TST, total sleep time; WASO, wake after sleep onset; REM, rapid eye movement; NREM, non-rapid eye movement; LS, light sleep; SWS, slow wave sleep; FSH, follicle-stimulating hormone; LH, luteinizing hormone; E2, estradiol; CSF, cerebrospinal fluid; VLPO, ventrolateral preoptic; TMN, tuberomammillary nucleus; PGD2, prostaglandin D2; PGE2, prostaglandin E2; L-PGDS, lipocalin-type prostaglandin D synthase; GABA, gamma-amino butyric acid; 5-HT, 5-hydroxytryptamine; 5-HIAA, 5-hydroxyindole acetic acid; NE, norepinephrine; DA, dopamine; ER-α, Estrogen receptor-α; ER-β, Estrogen receptor-β; GnRH, gonadotropin-releasing hormone; GSK-3β, glycogen synthase kinase-3β; p-β-catenin, phosphorylated β-catenin; BDNF, brain-derived neurotrophic factor; NPY, neuropeptide Y; MAP-2, microtubule- associated protein-2; CRH, corticotropin releasing hormone; ACTH, adrenocorticotropic hormone; CORT, cortisol; β-EP, beta-endorphin; DKK1, Dickkopf1; LRP-5, low density lipoprotein receptor related protein 5; LRP-6, low density lipoprotein receptor related protein 6; CREB, cyclic-AMP response binding protein; p-ERK 1/2, phosphorylated-extracellular regulated kinase 1/2; HDL, high-density lipoprotein; CUMS, chronic unpredictable mild stress; SPT, sucrose preference test; OFT, open field test; FST, forced swimming test; IAT, independent activity test; ADE, avoiding dark experiment; TSE, tail suspension experiment; EPM, elevated plus maze; BL18, Ganshu; BL23, Shenshu; CV4, Guanyuan; CV6, Qihai; EX, Anmian; EX-HN1, Sishencong; GV20, Baihui; HT7, Shenmen; KI3, Taixi; KI15, Zhongzhu; LI4, Hegu; LR3, Taichong; PC6, Neiguan; SP6, Sanyinjiao; SP10, Xuehai; ST36, Zusanli.
Modulation of Reproductive Hormones and Their Receptors
The established association between fluctuating hormonal milieu and sleep as well as mood is one explanation for the development of insomnia and/or depression during perimenopause.2–4,13,110 The endocrine system is regarded as one of the principal intrinsic factors affecting women’s sleep and mood during their reproductive life span.1,14,96 As estrogen is involved in the regulation and stabilization of the circadian rhythm system, its physiological fluctuation as well as a decreased sensitivity to estrogens in the hypothalamus during perimenopause contributes to circadian rhythm disturbances, and possibly the subsequent development of PMI.4 Woods et al investigated 286 women approaching menopause by establishing a mixed effects model, and found that in addition to age, crucial factors influencing sleep included the usage of exogenous hormones and women’s endogenous reproductive hormone levels.111 The former can be used to explain the improvement that HRT produces in terms of PMI; and the latter may be a pathway to understand the possible mechanism underlying the benefits of acupuncture. That is, acupuncture moderates estrogen level in perimenopausal women as shown in Table 2. Likewise, the changing hormonal milieu also contributes to the depressive symptoms in women who are in menopausal transition.112 Some longitudinal analyses have suggested the correlation between reproductive hormones [eg, estradiol (E2), follicle-stimulating hormone (FSH), luteinizing hormone (LH), etc] levels and sleep disturbances1 or depressive symptoms112 in perimenopause. Increased FSH levels are correlated with trouble staying asleep, while decreased E2 levels are correlated with both difficulty in falling and staying asleep.1 A strong linkage between frequent nocturnal awakenings and altered sex hormone levels (increments in FSH, and decrements in E2 and estrone) were reported in both the Study of Women’s Health Across the Nation (SWAN) and the Seattle Midlife Women’s Health Study (SMWHS).10 The alteration of reproductive hormone levels due to ovarian aging also contributes to vasomotor symptoms via at least three pathways: 1) slight increases in core body temperature; 2) altered thermal threshold/resetting and narrowing of the thermoregulatory system; and 3) a decreased thermoneutral zone in the brain.13,110 The latter two alterations can reduce women’s ability to tolerate nocturnal hot flushes and subsequently cause sleep disruption.110 An interesting finding is the inconsistent results in the links between different sleep indexes and FSH. Data from SWAN showed a relationship between the increased FSH over time and more slow wave sleep (SWS) recorded by polysomnography (PSG), which contrasts with findings correlating hormone fluctuation with subjective sleep indexes.10 Among women with PMI, Fu et al also observed that acupuncture induced reductions in both wake after sleep onset (WASO) and arousal index (ArI),51 which were negatively correlated with E2 levels but positively correlated with FSH.113 Estrogen is a mood-elevator as well, and its effect on emotional functions is partly mediated by the amygdala and hypothalamus.114 New onset of PMD was strikingly associated with increased FSH and LH levels.112 Decreasing E2 levels that occur approaching menopause are weakly linked to more depressive symptoms.115 Exogenous estrogen (in the form of E2) has also been shown to be related to the reduction of PMD.114 Among women without a history of depression, high variability in levels of FSH, LH, and E2 is linked to increased prevalence of PMD.3
Many clinical and animal studies cited in this review have confirmed the regulation of serum sex hormone levels by acupuncture (Tables 2 and 5). Despite the lack of correlational analysis, almost all studies showed a consistent trend— the amelioration of PMI and/or PMD produced by acupuncture, along with increments in circulating E2 and/or decrements in FSH and LH levels. Researchers thereby inferred that the improvement of PMI/PMD by acupuncture might be achieved via the modulation of expression of one or more of these three hormones.116–119
The estrogen receptors (ERα and ERβ) are closely related to the pathophysiology of climacteric-related neurological and psychiatric illness,120 and their aberrant expression has been linked to a variety of cancers, particularly breast and ovarian cancers.120 Yang reported that successful PMI modeling resulted in an increased expression of ERβ in the hypothalamus, which was prevented by acupuncture.121 This kind of aberrant expression of estrogen receptors are observed when rodents exposed to ovariectomy (OVX), modelling menopause, followed by CUMS, to induce depressive-like behaviours, as well.120 These findings suggest that acupuncture may have brain-predominant effects in modulating estrogen receptors, with potential protective benefits underlying its sleep-promoting and antidepressive effects.
Inhibition of Hyperactivity of HPA and HPO Axes
Hyperactivity of the hypothalamic-pituitary-adrenal (HPA) axis is a significant biological marker of depressive disorder.122 A previous SR suggested that EA could reduce cortisol (CORT) release from the adrenal glands and thereby modify the HPA axis by downregulating the expression of corticotropin-releasing hormone (CRH) and adrenocorticotropic hormone (ACTH) in the hypothalamus and pituitary gland, respectively, as well as decreasing the expression of CRH mRNA in the hypothalamus.123 This hypothesis was tested in a PMD model by Jiang et al.124 In this study, EA showed antidepressant-like effects that were no weaker than clomipramine in PMD rats, along with significant reductions in CRH, ACTH and CORT. Jiang also reported an increase in E2 and a decrease in FSH and LH levels in the rats.124 The response of HPA axis can be modulated by estrogen.122 Hence, Jiang’s findings further support the idea that acupuncture’s regulation of estrogen also contributes to its inhibition of hyperfunction of the HPA axis. This theory also partially explains the benefits of acupuncture on PMI. Since increased expression of ACTH and CORT causes an awakening effect, insomnia closely links to the hyperactivity of HPA axis as well.125 Xi et al reported significant symptom-relief in chronic insomniacs who underwent acupuncture, accompanied by downregulation in serum CRH, ACTH and CORT expression.126 A reciprocal relationship has been established between the HPO and HPA axes.122 Acupuncture appears to suppress these two axes synchronously. In three animal studies,124,127,128 EA’s effects on HPO axis-related hormones was observed, including normalizing the reduced E2 and beta-endorphin as well as the increased LH and gonadotropin-releasing hormone (GnRH) in a PMD model. The sleep-promoting benefit of acupuncture may be further explained as well because there is also an interaction between the sleep-wake cycle system and the HPO axis.113 There is also an association between HPO axis and the orexin system, which is involved in the regulation of arousal and plays a cardinal role in sleep and wakefulness.113
Huangfu et al have provided further prominent evidence that the inhibition of the HPA axis by EA might also be achieved via its modulation of the glucocorticoid receptor (GR) and mineralocorticoid receptor (MR).129 Disruption of GR signaling is proposed to underlie HPA axis dysregulation observed in stress-related psychiatric illness.130 Huangfu reported a significant decline of both GR and MR in the hippocampus of PMD rats, which was reversed with 28-day EA (20 min per day). Along with the improvement of depression-like behavior, EA also increased the number and functional activity of neurons in the hippocampal CA3 region in this group of rats.129
Regulation of Neurotransmitters
At the neurochemical level, several mechanisms of sleep-wakefulness control and emotion regulation have been identified. Coordination of various neurotransmitters appears to play a vital role in this regulated alternation state.131 For instance, the positive regulation of estrogen in sleep and mood disorders associated with menopause114,132 can be partially explained by its wide involvement in the metabolism of gamma-amino butyric acid (GABA), serotonin [5-hydroxytryptamine (5-HT)], norepinephrine, and acetylcholine.132
It is well known that GABA is the dominant inhibitory transmitter.133 Decreased GABAergic activity links to a weakening of the sleep-promotion system or a strengthening of the arousal system.134 As the first-line hypnotics, benzodiazepines and benzodiazepine receptor agonists (Z-drugs) act by increasing the intrinsic activity of the GABA via binding to the receptor binding region of the GABAA receptor, which in turn exerts an inhibitory and suppressing effect on the outputs to all of the major cell groups in the brainstem and hypothalamus that promote hyperarousal.134 Acupuncture appears to be able to ameliorate PMI via a similar pathway. The ventrolateral preoptic nucleus (VLPO) is a main cerebral region containing cluster of neurons essential for the initiation and maintenance of sleep.135 Cao et al observed that acupuncture upregulated the GABA levels in the VLPO in a PMI rat, which was positively correlated with prolonged REM sleep and SWS.136 The same study also reported the effects of acupuncture on prostaglandin (PG) D2 system in cerebrospinal fluid. There were significant increments in lipocalin-type PGD synthase and PGD2 levels, and exiguity decrements in PGE2 levels, indicative of the multi-targeted effect of the intervention. Although acupuncture’s regulation of GABA is rarely investigated in PMI-related clinical trials, many trials have reported that acupuncture up-regulated serum GABA levels in patients with primary insomnia and chronic insomnia.137,138 Estrogen receptors are found throughout the brain, so estrogen shows modulatory effects on the neurotransmitter systems.5 One of the interpretations for the increased risk of PMD posits that depression is caused by the impacts of E2 fluctuations on neurotransmitter systems in cerebral regions that regulate mood and emotion.8,14 Dysregulation of the GABA balance between GABAA and GABAB due to hormone fluctuations also contributes to the increased vulnerability to depression during perimenopause.96 Among women with PMD, the decreased GABA concentrations were found mainly in the anterior cingulate cortex, which is usually activated by diverse tasks including emotion processing and regulation and is closely associated with the occurrence and development of depression.139 The GABAergic system and GABAergic neurosteroids show potential involvements in the etiology of PMD, as women whose GABARAR receptor fails to adjust to the rapid change in neurosteroid levels may be more prone to PMD.133 The effect of acupuncture in reinstating reduced hippocampal GABA content in rats with depression has been widely investigated and confirmed.140,141 It is worthwhile noting that the GABA system also plays a key role in restraining the HPA axis at the level of the paraventricular nucleus of the hypothalamus.133 The close relationship between the hyperactivity of the HPA and HPO axes and PMI and/or PMD has been mentioned in the earlier section. Furthermore, in many animal experiments cited in this review (Table 5), ovarian hormone deprivation due to OVX evoked significantly increased serum FSH and LH levels as well as reduced serum E2 level, suggesting ovarian failure results in the exhausted hypothalamic GABA contents. The acupuncture-induced reversal of these disrupted hormone and neurotransmitter levels thereby seems to modulate the upstream factors of the HPO axis and inhibit the HPA-axis hyperactivity, potentially by reinstating the hypothalamic GABAergic neuronal function.
As with GABA system, a dysfunctional serotonergic system is another factor contributing to the development of PMD, a mechanism that is receiving increasing attention.133,142 The available evidence suggests there are lower serum 5-HT levels in postmenopausal women than in regularly menstruating women;143 and in comparison to the natural menopause in humans, the decline of 5-HT levels following ovariectomy is more drastic.143 Selective serotonin reuptake inhibitors are one of the most frequently utilized non-hormonal substitutes for menopausal symptoms,144 suggesting that serotonin is widely involved in multiple symptoms during the menopausal stage. Both the odds of PMD and the severity of PMD symptoms are negatively correlated with serotonin levels.142 Both human clinical71,84,93 and animal studies145–149 have revealed the increases in serum 5-HT and/or hippocampal 5-HT and its major metabolites caused by acupuncture, along with decreased PMD symptoms or depression-like behaviors. In addition to the enhancement of 5-HT synthesis and the increase of 5-HT and its metabolite content in the brain and synaptic gaps, previous studies also suggested that acupuncture restored synaptic plasticity by regulating serotonin expression in the hippocampus resulting in relieved depression symptoms.123,150 This process involves acupuncture-induced bidirectional regulation of the expression of serotonin transporters (5-HTTs) and serotonin-1A (5-HT1A), which then increases the expression of hippocampal galanin (Gal) and tryptophan hydroxylase (TPH) as well as restores or reverses the impairments in synaptic plasticity.123 Given existing animal studies mainly focused on hippocampus, future studies within in field might benefit from some investigations of other cerebral regions important for depressive-like symptoms including amygdala and prefrontal cortex. Serotonin also plays an integral part in the process of sleep preparation, initiation, and maintenance.151 In both serum and hippocampus of PMI rats,121,152 researchers observed that acupuncture treatment led to increments in serotonin and its chief end-product of metabolism, accompanied by significant increases in REM and SWS. This finding is also meaningful for PMD, because these two sleep outcomes are usually shown to be less in women with depression compared with the healthy women.11 Estrogen affects the serotonin system in several ways, including promoting the synthesis of serotonin by increasing the rate of degradation of monoamine oxidase (the enzyme that catabolizes serotonin), and inhibiting the reuptake of serotonin by affecting intraneuronal serotonin transport.114,153 E2, for instance, is able to stimulate the production of neurotransmitters such as serotonin and to enhance serotonergic function in depression and its treatment.114 In the previous section, we displayed both clinical and animal evidence that acupuncture increased E2 levels. It is hence reasonable to summarize that acupuncture can directly elevate serotonin levels, but also indirectly strengthen the benign regulation of serotonin in sleep and mood with this increased E2.
Reduction of Oxidative Stress Levels
Oxidative stress plays a critical part in the pathophysiology of sleep disturbance, as brain-free radicals accumulate during wakefulness and are removed during sleep.154 Compared with good sleepers, insomniacs generally have much higher oxidative stress parameters.155 Meanwhile, oxidative stress is a pivotal factor in ageing.156 Menopause is an aging process, in which oxidative stress plays a crucial role in developing a diversity of symptoms.156 The decline of estrogen during menopause results in the massive release of reactive oxygen species (ROS) and creates a pro-oxidant state,157,158 which explains the association between increased oxidative stress and climacteric symptoms due to insufficient estrogen. Treatment with antioxidant estrogen has been shown to lead to a reduction in the occurrence of sleep disturbances, implying that the improvement of oxygen metabolism may be a potential management strategy for climacteric-related sleep disorders.13,159 Recent study identified an evident imbalance between lipid peroxidation (LPO) and the antioxidant defense system among the menopausal insomniacs,160 providing a pathogenic rationale for including drugs that inhibit LPO activation in the synthetical therapy of these patients.161 A cross-sectional study involving 187 perimenopausal women also suggested that there was a positive correlation between insomnia score, menopause rating score, and LPO.157 Acupuncture has been found to normalize disordered LPO processes, and prevent formation and accumulation of LPO products in sleep deprivation-associated regions of the brain, particularly hippocampus.162,163 The association between menopause-related depression and oxidative stress has been identified as well. Hirose et al reported that depressive symptoms among middle-aged women were independently associated with high urinary 8-OHdG, a biomarker indicator of oxidatively damaged DNA reflecting the severity of oxidative stress.164 Although there is no direct evidence investigating the effect of acupuncture on 8-OHdG in PMD populations, MA was found to improve cognitive function and mood in patients with vascular dementia, along with significant decline in urinary 8-OHdG.165 The mechanisms linking PMD to oxidative stress appear to be bidirectional: high oxidative stress may lower norepinephrine and serotonin and levels, which in turn results in depression; depression may contribute to ROS production as well as weaken the antioxidant defense system.164 Tian observed striking increases in serum malondialdehyde (MDA) which is the LPO product as well as striking decreases in serum superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) which are the two major intracellular antioxidant enzymes in rats that underwent OVX, which were reversed by acupuncture.106 A recent RCT revealed that EA significantly decreased level of lipid hydroperoxides, a byproduct of oxidative stress, among insomniacs.166 Acupuncture was also reported to retard the drastic decline of SOD and nitric oxide synthase (NOS) levels in plasma and uterine tissues of rats naturally entering menopause.107 Besides the increment of GSH-Px and SOD contents, the upregulation in expression of CuZnSOD mRNA and protein is another pathway by which acupuncture may ameliorate oxidative injuries.162 These findings suggest that the antioxidant mechanism underlying acupuncture may be associated with its effects on a cluster of oxidative stress-related enzymes inducing a nonspecific response. Oxidative stress levels are also positively correlated with climacteric hot flushes,167,168 a potential contributor of PMI169 and PMD5 as mentioned. Among women aged 45–55 years, those with hot flushes were found to show lower levels of plasma total antioxidant activity compared with women free of this symptom.168 Therefore, acupuncture may also reduce hot flushes via resisting the oxidation effects, which in turn reduces PMI and PMD.
Promotion of Signaling Pathways
The effects of acupuncture on signaling pathways have also been widely studied, providing a new perspective to understand the clinical efficacy of acupuncture at a molecular level.123 We identified six animal studies involving three signal transduction pathways that revealed possible mechanisms implicated in the improvement of PMD by acupuncture.
Brain-derived neurotrophic factor (BDNF) in the hippocampus is a crucial target for the treatment of depression,170 and it may be linked with the development of depression by cAMP-response element-binding protein (CREB) and its upstream pathways.140 Many conventional antidepressive agents mediate antidepressant-like effects via increasing synthesis and signal transduction of cerebral BDNF expression.123,170 Acupuncture appears to work on the same signaling pathway. Two studies demonstrated that EA altered depression-like behavior in PMD rats by normalizing the downregulated expression of hippocampal CREB, BDNF, and phosphorylated-TrkB (p-TrkB) which is a high-affinity receptor of BDNF.170,171 Activation of BDNF/TrkB/CREB pathway is thereby suggested as a potential mechanism implicated in the beneficial effects of acupuncture on PMD. This pathway may also link to the improvements in PMI by acupuncture. Although there is no direct evidence, Liu reported that EA improved both sleep and cognitive functions (learning and memory) in sleep-deprived rats by activating the PKA-Cβ/CREB/BDNF/TrkB signaling pathway.172
Activation of the Wnt/β-catenin signaling pathway has moderation effect on multiple aspects of hippocampal neurogenesis, which has been linked to depressive symptoms.173 Glycogen synthase kinase-3β (GSK-3β) and β-catenin are key proteins of Wnt signaling, which comprise a degradation complex to phosphorylated (p)-β-catenin to be ubiquitined.174 These critical regulatory molecules particularly GSK-3β and β-catenin are suggested to be targets and/or action modifiers of antidepressants such as lithium175 and fluoxetine.176 Interestingly, regulation of these proteins was also observed in two studies in which acupuncture improved depressive-like symptoms in PMD rats.174,177 As reported, hippocampal mRNA and protein expression of GSK-3β were decreased, while mRNA and protein expression of β-catenin were increased after 28-days EA treatment. Another study showed that acupuncture reduced the expression of Dickkopf 1 (DKK1) and low-density lipoprotein receptor-related protein (LRP) 5/6 in the hippocampus of PMD rats.178 As an endogenous inhibitor, DKK1 binds to LRP 5/6 and results in the inhibition of canonical Wnt signaling pathway.179 These findings suggest that activation of the Wnt/β-catenin signaling pathway may be another mechanism by which acupuncture mediates antidepressant-like effects. We identified no studies investigating PMI in association with the Wnt/β-catenin signaling pathway, highlighting a significant gap in the literature, but Qu et al have reported that activation of this pathway enhanced hippocampal neurogenesis and improved learning and memory in sleep-deprived mice.180
The Notch signaling pathway is involved in the promotion of neurogenesis and brain plasticity.181,182 Notch1 is the principal Notch receptor,183 and it can interact with ligands such as Jagged1 and then trigger the release of the Notch intracellular domain.182 As the major downstream target genes of Notch signaling, Hes is found to take part in the inhibition of neuronal differentiation.184 Emerging evidence has shown that Notch signaling is associated with the depression-like performance in mid-aged female rats undergoing chronic restraint stress.182 Deng et al established PMD model by chronic stress and reported decreased expression in hippocampal Notch1 protein and mRNA, as well as increased expression in both hippocampal Jagged1 protein and mRNA as well as hippocampal Hes1 protein and mRNA in PMD rats, which were reversed by EA treatment.185 These results imply that acupuncture can improve PMD performance, possibly by increased neurogenesis and promoted neuronal differentiation via activating the Notch signaling pathway.
Attenuation of Vasomotor and Other Perimenopausal Symptoms
Krystal et al suggested that PMI is a vasomotor-initiated sleep disruption as it is usually triggered initially by night sweats.169 Some longitudinal investigations and SRs/MAs of perimenopause have also indicated the positive correlation between vasomotor complaints and poor sleep and/or depressive symptoms.1,2 In comparison with those without vasomotor symptoms, women suffering from more night sweats weekly are with more likelihood to report sleep difficulties.1 Translated, women with vasomotor symptoms are more at risk for insomnia.186 However, there is a theory that vasomotor symptoms are not a necessary prerequisite for PMD,13 while perimenopausal women with hot flushes are indeed at higher risk of developing depression.5 Moreover, women with PMD have a higher likelihood of being irritable from vasomotor symptoms and report greater severity of their hot flushes. They are also most likely to seek help from primary health care services.5 Some recent evidence reveals a bidirectional relationship between PMD and vasomotor complaints.8,122 It is thought that this relationship may not be simply explained by the mediating effect of sleep disruption,8,122 whereas PMD and perimenopausal anxiety due to vasomotor symptoms do contribute to the impaired sleep.110 Attenuation of vasomotor symptoms by acupuncture thereby partially explains why it promotes the PMI relief.186 Plenty of clinical data cited in our review have shown that acupuncture significantly lowered the Kupperman Index global scores (Table 2). Hot flushes and sweating are the critical and most weighted items of the Kupperman Index scale.187 Although the frequency of hot flushes and night sweats were not directly assessed in our included clinical papers, acupuncture is sure to be promising to mitigate menopausal-associated vasomotor symptoms.188 As denoted in the umbrella systematic review performed by Befus et al in 2018, evidence from RCTs supports the utilization of acupuncture as an adjunctive or stand-alone remedy option for reducing vasomotor symptoms.188 Additionally, two included animal studies189,190 reported that acupuncture significantly increased total sleep time and reduced sleep onset latency in PMI rats, along with enhancement of the rats’ heat-resistant ability (prolongation of heat-resistant time). Accumulating evidence suggests that acupuncture-induced temperature control and reduction in hot flushes among climacteric women is achieved by elevating beta-endorphin level and suppressing GnRH secretion.191 These benefits of acupuncture were validated again in PMD rats and were accompanied by a significant reduction in depression-like behavior.124,127,128 Interestingly, serotonin regulates thermoregulation and peripheral vasculature.153 The effect of acupuncture on serotonin may therefore explain the reduced vasomotor symptoms as well. Although there is debate available regarding acupuncture treatment for hot flushes, the controversy focuses only on its placebo effects. A comprehensive review by Ee et al suggested that acupuncture did not show better effects in comparison with sham-acupuncture;192 another network meta-analysis exhibited opposite conclusion.193 In comparison with no treatment, however, the benefits of acupuncture or placebo-acupuncture are clear, according to both papers.192,193 In summary, reducing the sleep disruption and/or depressive symptoms by relieving hot flushes is a possible explanation for acupuncture’s benefits on PMI and PMD, although further research is required.
The interdependent and interactional relationship between anxiety, depression, and insomnia has been established in women with perimenopausal complaints.6,13,15 However, this relationship appears to be underestimated and does not receive adequate attention in the current research, as only 4 of the 22 PMI RCTs included emotion-related indicators54,64,65,67 and none of the 25 PMD RCTs included sleep-related indicators. According to the domino theory mentioned in the previous section, the improvement of insomnia caused by acupuncture may also be one of the mechanisms of PMD remission, and vice versa. Future trials hence should be more comprehensive in their outcome tools. For instance, scales/questionnaires should cover sleep, mood, vasomotor symptoms as well as quality of life, and even consider introducing PSG/actigraphy and neuropsychological tests. With these data we will be able to appraise intricate associations between menopausal characteristics, sleep, mood, lifestyle factors, and women’s expectation and experience of acupuncture, which will further our understanding of the true role of acupuncture in perimenopause management. Animal experiments are also required to investigate the mechanisms behind acupuncture on a larger scale.
Conclusion
Our review demonstrates that acupuncture has a positive and broad effect on PMI and PMD, being beneficial on mood, sleep, and vasomotor symptoms. Consistent data from clinical trials and animal studies support that those effects possibly are mediated through a number of mechanisms, from regulation of reproductive hormones and neurotransmitters, and inhibitions in oxidative stress and HPA and HPO axis hyperactivity, to activation and deactivation of key proteins in various signaling pathways. Neuroendocrine modulation is likely to play a major role in mediating those effects. Despite some methodological deficiencies, acupuncture could be an adjunct therapy or viable and safe option for women with comorbid depression and insomnia during perimenopause. The multi-faceted effects of acupuncture on, and the TCM syndrome patterns of, PMI and PMD provide an innovative direction for future research.
Funding Statement
This work was sponsored by RMIT Research Stipend Scholarship, RMIT University, Australia, and University’s scientific research project, Shanghai Sanda University [2021zz02-yj] to FYZ; and Three-year Action Plan for Public Health 2020–2022 (Key discipline construction-TCM psychology/TCM psychiatry), Shanghai Municipal Health Commission [GWV-10.1-XK20], Project Management and Technical Specifications of Insomnia Treatment Service Key Promotion, Shanghai Municipal Health Commission [ZY(2018–2020)-ZWB-1001-FWB-07)] to WJZ.
Disclosure
None of the authors have any conflicts of interest to declare in this study.
References
- 1.Kravitz HM, Joffe H. Sleep during the perimenopause: a SWAN story. Obstet Gynecol Clin North Am. 2011;38(3):567–586. doi: 10.1016/j.ogc.2011.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Augoulea A, Moros M, Lykeridou A, Kaparos G, Lyberi R, Panoulis K. Psychosomatic and vasomotor symptom changes during transition to menopause. Prz Menopauzalny. 2019;18(2):110–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raglan GB, Schulkin J, Micks E. Depression during perimenopause: the role of the obstetrician-gynecologist. Arch Womens Ment Health. 2020;23(1):1–10. doi: 10.1007/s00737-019-0950-6 [DOI] [PubMed] [Google Scholar]
- 4.Caruso D, Masci I, Cipollone G, Palagini L. Insomnia and depressive symptoms during the menopausal transition: theoretical and therapeutic implications of a self-reinforcing feedback loop. Maturitas. 2019;123:78–81. doi: 10.1016/j.maturitas.2019.02.007 [DOI] [PubMed] [Google Scholar]
- 5.Gyllstrom ME, Schreiner PJ, Harlow BL. Perimenopause and depression: strength of association, causal mechanisms and treatment recommendations. Best Pract Res Clin Obstet Gynaecol. 2007;21(2):275–292. doi: 10.1016/j.bpobgyn.2006.11.002 [DOI] [PubMed] [Google Scholar]
- 6.Maki PM, Kornstein SG, Joffe H, et al. Board of trustees for the North American Menopause Society (NAMS) and the Women and Mood Disorders Task Force of the National Network of Depression Centers. Guidelines for the evaluation and treatment of perimenopausal depression: summary and recommendations. Menopause. 2018;25(10):1069–1085. [DOI] [PubMed] [Google Scholar]
- 7.Ayaki M, Tsubota K, Kawashima M, Kishimoto T, Mimura M, Negishi K. Sleep disorders are a prevalent and serious comorbidity in dry eye. Invest Ophthalmol Vis Sci. 2018;59(14):DES143–DES150. doi: 10.1167/iovs.17-23467 [DOI] [PubMed] [Google Scholar]
- 8.Joffe H, Petrillo LF, Koukopoulos A, et al. Increased estradiol and improved sleep, but not hot flashes, predict enhanced mood during the menopausal transition. J Clin Endocrinol Metab. 2011;96(7):E1044–E1054. doi: 10.1210/jc.2010-2503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Terauchi M, Obayashi S, Akiyoshi M, Kato K, Matsushima E, Kubota T. Insomnia in Japanese peri- and postmenopausal women. Climacteric. 2010;13(5):479–486. doi: 10.3109/13697130903353478 [DOI] [PubMed] [Google Scholar]
- 10.Baker FC, de Zambotti M, Colrain IM, Bei B. Sleep problems during the menopausal transition: prevalence, impact, and management challenges. Nat Sci Sleep. 2018;10:73–95. doi: 10.2147/NSS.S125807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morssinkhof MWL, van Wylick DW, Priester-Vink S, et al. Associations between sex hormones, sleep problems and depression: a systematic review. Neurosci Biobehav Rev. 2020;118:669–680. doi: 10.1016/j.neubiorev.2020.08.006 [DOI] [PubMed] [Google Scholar]
- 12.Im EO, Yang YL, Liu J, Chee W. The association of depressive symptoms to sleep-related symptoms during menopausal transition: racial/ethnic differences. Menopause. 2020;27(11):1315–1321. doi: 10.1097/GME.0000000000001611 [DOI] [PubMed] [Google Scholar]
- 13.Santoro N, Epperson CN, Mathews SB. Menopausal symptoms and their management. Endocrinol Metab Clin North Am. 2015;44(3):497–515. doi: 10.1016/j.ecl.2015.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller LJ, Girgis C, Gupta R. Depression and related disorders during the female reproductive cycle. Womens Health (Lond). 2009;5(5):577–587. doi: 10.2217/WHE.09.44 [DOI] [PubMed] [Google Scholar]
- 15.Krystal AD. Depression and insomnia in women. Clin Cornerstone. 2004;6(1):S19–S28. doi: 10.1016/S1098-3597(04)80022-X [DOI] [PubMed] [Google Scholar]
- 16.Xiao X, Zhang J, Jin Y, Wang Y, Zhang Q. Effectiveness and safety of acupuncture for perimenopausal depression: a systematic review and meta-analysis of randomized controlled trials. Evid Based Complement Alternat Med. 2020;2020:5865697. doi: 10.1155/2020/5865697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Las Cuevas C, Peñate W, Sanz EJ. Risk factors for non-adherence to antidepressant treatment in patients with mood disorders. Eur J Clin Pharmacol. 2014;70(1):89–98. doi: 10.1007/s00228-013-1582-9 [DOI] [PubMed] [Google Scholar]
- 18.Ashton AK, Jamerson BD, Weinstein L, Wagoner C. Antidepressant-related adverse effects impacting treatment compliance: results of a patient survey. Curr Ther Res Clin Exp. 2005;66(2):96–106. doi: 10.1016/j.curtheres.2005.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao FY, Fu QQ, Kennedy GA, et al. Comparative utility of acupuncture and Western medication in the management of perimenopausal insomnia: a systematic review and meta-analysis. Evid Based Complement Alternat Med. 2021;2021:5566742. doi: 10.1155/2021/5566742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Asnis GM, Thomas M, Henderson MA. Pharmacotherapy treatment options for insomnia: a primer for clinicians. Int J Mol Sci. 2015;17(1):50. doi: 10.3390/ijms17010050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dodin S, Blanchet C, Marc I, et al. Acupuncture for menopausal hot flushes. Cochrane Database Syst Rev. 2013;(7):CD007410. doi: 10.1002/14651858.CD007410.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baumelou A, Liu B, Wang XY, Nie GN. Perspectives in clinical research of acupuncture on menopausal symptoms. Chin J Integr Med. 2011;17(12):893–897. doi: 10.1007/s11655-011-0930-9 [DOI] [PubMed] [Google Scholar]
- 23.Hachul H, Oliveira DS, Bittencourt LR, Andersen ML, Tufik S. The beneficial effects of massage therapy for insomnia in postmenopausal women. Sleep Sci. 2014;7(2):114–116. doi: 10.1016/j.slsci.2014.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Newton KM, Buist DS, Keenan NL, Anderson LA, LaCroix AZ. Use of alternative therapies for menopause symptoms: results of a population-based survey. Obstet Gynecol. 2002;100(1):18–25. [DOI] [PubMed] [Google Scholar]
- 25.Williams RE, Kalilani L, DiBenedetti DB, Zhou X, Fehnel SE, Clark RV. Healthcare seeking and treatment for menopausal symptoms in the United States. Maturitas. 2007;58(4):348–358. doi: 10.1016/j.maturitas.2007.09.006 [DOI] [PubMed] [Google Scholar]
- 26.Cheuk DKL, Yeung WF, Chung KF, Wong V. Acupuncture for insomnia. Cochrane Database Syst Rev. 2012;9(9):CD005472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yeung WF, Chung KF, Leung YK, Zhang SP, Law AC. Traditional needle acupuncture treatment for insomnia: a systematic review of randomized controlled trials. Sleep Med. 2009;10(7):694–704. doi: 10.1016/j.sleep.2008.08.012 [DOI] [PubMed] [Google Scholar]
- 28.Chon TY, Lee MC. Acupuncture. Mayo Clin Proc. 2013;88(10):1141–1146. doi: 10.1016/j.mayocp.2013.06.009 [DOI] [PubMed] [Google Scholar]
- 29.Langevin HM, Schnyer R, MacPherson H, et al.; Executive Board of the Society for Acupuncture Research. Manual and electrical needle stimulation in acupuncture research: pitfalls and challenges of heterogeneity. J Altern Complement Med. 2015;21(3):113–128. doi: 10.1089/acm.2014.0186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.He W, Li M, Zuo L, et al. Acupuncture for treatment of insomnia: an overview of systematic reviews. Complement Ther Med. 2019;42:407–416. doi: 10.1016/j.ctim.2018.12.020 [DOI] [PubMed] [Google Scholar]
- 31.Smith CA, Armour M, Lee MS, Wang LQ, Hay PJ. Acupuncture for depression. Cochrane Database Syst Rev. 2018;3(3):CD004046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Femenía T, Gómez-Galán M, Lindskog M, Magara S. Dysfunctional hippocampal activity affects emotion and cognition in mood disorders. Brain Res. 2012;1476:58–70. doi: 10.1016/j.brainres.2012.03.053 [DOI] [PubMed] [Google Scholar]
- 33.Hamidi M, Drevets WC, Price JL. Glial reduction in amygdala in major depressive disorder is due to oligodendrocytes. Biol Psychiatry. 2004;55(6):563–569. doi: 10.1016/j.biopsych.2003.11.006 [DOI] [PubMed] [Google Scholar]
- 34.Li C, Dong M, Yin Y, Hua K, Fu S, Jiang G. Abnormal whole-brain functional connectivity in patients with primary insomnia. Neuropsychiatr Dis Treat. 2017;13:427–435. doi: 10.2147/NDT.S128811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O’Byrne JN, Berman Rosa M, Gouin JP, Dang-Vu TT. Neuroimaging findings in primary insomnia. Pathol Biol (Paris). 2014;62(5):262–269. doi: 10.1016/j.patbio.2014.05.013 [DOI] [PubMed] [Google Scholar]
- 36.MacPherson H, Thomas K, Walters S, Fitter M. The York acupuncture safety study: prospective survey of 34 000 treatments by traditional acupuncturists. BMJ. 2001;323(7311):486–487. doi: 10.1136/bmj.323.7311.486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.White A. The safety of acupuncture–evidence from the UK. Acupunct Med. 2006;24(1S):53–57. doi: 10.1136/aim.24.Suppl.53 [DOI] [Google Scholar]
- 38.White A. A cumulative review of the range and incidence of significant adverse events associated with acupuncture. Acupunct Med. 2004;22(3):122–133. doi: 10.1136/aim.22.3.122 [DOI] [PubMed] [Google Scholar]
- 39.European Medicines Agency. Guideline on Medicinal Products for the Treatment of Insomnia. London: European Medicines Agency; 2011:14–16. [Google Scholar]
- 40.Wang SM, Han C, Bahk WM, et al. Addressing the side effects of contemporary antidepressant drugs: a comprehensive review. Chonnam Med J. 2018;54(2):101–112. doi: 10.4068/cmj.2018.54.2.101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li M, Niu J, Yan P, et al. The effectiveness and safety of acupuncture for depression: an overview of meta-analyses. Complement Ther Med. 2020;50:102202. doi: 10.1016/j.ctim.2019.102202 [DOI] [PubMed] [Google Scholar]
- 42.Ravindran AV, Balneaves LG, Faulkner G, et al.; CANMAT Depression Work Group. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder: section 5. Complementary and alternative medicine treatments. Can J Psychiatry. 2016;61(9):576–587. doi: 10.1177/0706743716660290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sun DZ, Li SD, Liu Y, Zhang Y, Mei R, Yang MH. Differences in the origin of philosophy between Chinese medicine and Western medicine: exploration of the holistic advantages of Chinese medicine. Chin J Integr Med. 2013;19:706–711. doi: 10.1007/s11655-013-1435-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zheng HX. The Basic Theory of Traditional Chinese Medicine (Tenth Edition) [Article in Chinese]. Beijing, China: China Press of Traditional Chinese Medicine; 2016. [Google Scholar]
- 45.Jiang L, Liu B, Xie Q, et al. Investigation into the influence of physician for treatment based on syndrome differentiation. Evid Based Complement Alternat Med. 2013;2013:587234. doi: 10.1155/2013/587234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Volker S. Globalising Chinese medical understandings of menopause. East Asian Sci Technol Soc. 2008;2:485–506. doi: 10.1215/s12280-009-9069-6 [DOI] [Google Scholar]
- 47.Zhuo ZW, Hu L, Chen QL, Liang WN, Li CD. Study on syndrome elements and literature laws in patients with perimenopausal depression [article in Chinese]. Trad Chin Med J. 2019;18(1):53–58. [Google Scholar]
- 48.Zhang Y, Huang JS, Wu Song Y, et al. The distribution and characteristics of TCM syndromes of perimenopausal insomnia [article in Chinese]. Trad Chin Med J. 2013;54(18):1574–1576. [Google Scholar]
- 49.Lu C, Yang XJ, Hu J. Efficacy comparison between acupuncture smoothing-Liver and regulating-Spleen method and regulating Governor-Vessel method for perimenopausal insomnia [article in Chinese]. Chin Acup Moxib. 2014;34(8):759–762. [PubMed] [Google Scholar]
- 50.Zhao FY, Fu QQ, Kennedy GA, Conduit R, Zhang WJ, Zheng Z. Acupuncture as an independent or adjuvant management to standard care for perimenopausal depression: a systematic review and meta-analysis. Front Psychiatry. 2021;12:666988. doi: 10.3389/fpsyt.2021.666988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fu C, Zhao N, Liu Z, et al. Acupuncture improves perimenopausal insomnia: a randomized controlled trial. Sleep. 2017;40(11):zsx153. doi: 10.1093/sleep/zsx153 [DOI] [PubMed] [Google Scholar]
- 52.Wang WD. Effect Comparison Between Electroacupuncture with Yinyang-Tiaoheng-Touci Needling Method and Placebo-Electroacupuncture in Treating Perimenopausal Insomnia [Article in Chinese] [Master thesis]. Shanghai University of Traditional Chinese Medicine; 2015. [Google Scholar]
- 53.Lin WX, Yin P, Xu SF. Clinical efficacy evaluation of Tiaoren-Tongdu needling for perimenopausal insomnia [article in Chinese]. Shanghai J Acup Moxib. 2017;36(8):900–904. [Google Scholar]
- 54.Li S, Wang Z, Wu H, et al. Electroacupuncture versus sham acupuncture for perimenopausal insomnia: a randomized controlled clinical trial. Nat Sci Sleep. 2020a;12:1201–1213. doi: 10.2147/NSS.S282315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang C, Bai YF, Fu WB, He YH. Clinical effect of acupuncture at four acupoints of Qijing on perimenopausal depression [article in Chinese]. Chin J Gerontol. 2015;35(12):3390–3392. [Google Scholar]
- 56.Li ZF. Clinical Study on the Effect of Electroacupuncture on Patients with Mild to Moderate Depression in Perimenopausal Period [Article in Chinese] [Doctorate thesis]. Guangzhou University of Chinese Medicine; 2015a. [Google Scholar]
- 57.Chen XL, Xu K, Qin XH. Clinical study on electroacupuncture for perimenopausal insomnia. J Acupunct Tuina Sci. 2013;11(6):336–338. doi: 10.1007/s11726-013-0722-1 [DOI] [Google Scholar]
- 58.Luo L. Clinical Observation on the Therapeutic Effect of Acupuncture with Tonifying Kidney and Regulating Heart Method on the Patients with Perimenopausal Insomnia [Article in Chinese] [Master thesis]. Nanjing Univeity of Chinese Medicine; 2020. [Google Scholar]
- 59.Du JL, Fan WJ, Du HJ. Clinical observation of Jin’s three-needle combined with Jiaweiwumei-pill in the treatment of perimenopausal insomnia [article in Chinese]. China Pharm. 2017;28(8):1104–1107. [Google Scholar]
- 60.Kang H. Clinical Observation of Scalp Acupuncture on the Treatment of Insomnia with Heart and Gallbladder Qi Deficiency in Perimenopausal Period [Article in Chinese] [Master thesis]. Heilongjiang University of Chinese Medicine; 2015. [Google Scholar]
- 61.Lai XJ. The Clinical Study of Using the Xu’s Feitengbafa on Shenmai and Zhaohai Point on Time in Treating Perimenopausal Insomnia (Disharmony Between the Heart and Kidney) [Article in Chinese] [Master thesis]. Chengdu University of Traditional Chinese Medicine; 2016. [Google Scholar]
- 62.Li YN. Effect of acupuncture “Xiasanhuang” acupoints on 120 cases of perimenopausal insomnia [article in Chinese]. China Prac Med. 2014;9(19):244–246. [Google Scholar]
- 63.Li OJ, Wang F. Acupuncture at back-shu points of five Zang, Geshu (BL 17) and Shenmen (HT 7) for the treatment of perimenopausal insomnia [article in Chinese]. Chin Acup Moxib. 2018a;38(5):469–472. [DOI] [PubMed] [Google Scholar]
- 64.Ma GG. Clinical Research on Perimenopausal Insomnia Treated with Jin Three-Needle Therapy [Article in Chinese] [Doctorate thesis]. Guangzhou University of Chinese Medicine; 2014. [Google Scholar]
- 65.Qin YY. Clinical Study of Acupuncture in Treating Perimenopausal Insomnia with Liver Hyperactivity and Kidney Deficiency Syndrome Pattern Based on the Theory of Treating Insomnia Based on Liver [Article in Chinese] [Master thesis]. Chengdu University of Traditional Chinese Medicine; 2018. [Google Scholar]
- 66.Yang JR, Xu HY, Bai JM, Tang ZG, Lu R, Wang ZY. Scalp and body acupuncture in treating 81 cases of perimenopausal insomnia [Article in Chinese]. West J Trad Chin Med. 2017;30(2):4–6. [Google Scholar]
- 67.Zhang W, Pi Y, Chen T, Wang WW, Yang WF, Wang ZY. Clinical observation of treating perimenopausal insomnia by acupuncture based on the theory of Liver [article in Chinese]. J Sichuan Trad Chin Med. 2017;35(9):152–155. [Google Scholar]
- 68.Yan B, Ma XM, Zhou WX, Yu HB, Yang ZX. Effect of acupuncture with Tongren-Tiaodu method on perimenopausal insomnia [article in Chinese]. Hubei J TCM. 2021;43(5):40–42. [Google Scholar]
- 69.Li S, Li ZF, Wu Q, et al. A multicenter, randomized, controlled trial of electroacupuncture for perimenopause women with mild-moderate depression. Biomed Res Int. 2018b;2018:5351210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chi H, Zou W. Yishen-tiaoan acupuncture therapy in the treatment of perimenopausal depression: an observation of 30 cases [article in Chinese]. JCAM. 2011;27(7):4–7. [Google Scholar]
- 71.Deng AJ. A Randomized Controlled Clinical Trial of Bo’s Abdominal Acupuncture in the Treatment of Perimenopausal Depression [Article in Chinese] [Master thesis]. Guangzhou University of Chinese Medicine; 2008. [Google Scholar]
- 72.Li HB. Clinical Observation of Acupuncture Treatment on Perimenopausal Depression with Kidney Deficiency and Liver Stagnation [Article in Chinese] [Master thesis]. Heilongjiang University of Traditional Chinese Medicine; 2015b. [Google Scholar]
- 73.Ma J, Liu Z. A clinical study of acupuncture treatment on perimenopausal depression [article in Chinese]. J Psychiatr. 2009;22(4):276–278. [Google Scholar]
- 74.Niu XS, Wang P. Clinical study of acupuncture on Wangshi-Wuzangshu combined Geshu in the treatment of perimenopausal depression with stagnation of Liver Qi syndrome pattern [article in Chinese]. Int J Trad Chin Med. 2017;39(11):999–1002. [Google Scholar]
- 75.Qian J, Zhang J, Pei Y, Cheng J. Clinical observation of acupuncture on Wangshi Wuzangshu combined Geshu for perimenopausal depression [article in Chinese]. Beijing J TCM. 2007;26:491–492. [Google Scholar]
- 76.Qiang BQ. Acupuncture treatment on 30 cases of perimenopause depression [article in Chinese]. J Shaanxi TCM. 2008;29(7):871–872. [Google Scholar]
- 77.Shi J, Huang DM, Feng QF, Tang NL. Clinical observation of electroacupuncture treatment for mild to moderate depression in perimenopausal period [article in Chinese]. China J Mod Med. 2018;28(20):52–56. [Google Scholar]
- 78.Sun YJ, Tan Z, Jiang CY, Li SY. Clinical observation of electroacupuncture in the treatment of mild and moderate perimenopausal depression [article in Chinese]. J Guangxi Univ Chin Med. 2015;18(4):13–15. [Google Scholar]
- 79.Wang XY, Li XY, Deng AJ. Comparative study on abdominal acupuncture and Western medicine for treatment of perimenopause depression [article in Chinese]. Chin Acup Moxib. 2010;30(11):913–917. [PubMed] [Google Scholar]
- 80.Ding L, Liu B. Treatment of perimenopausal depression by acupuncture with Nourishing-Kidney-Regulating-Liver-Strengthening-Spleen-Tranquilizing-Heart method [article in Chinese]. Chin Archiv Trad Chin Med. 2007;25(5):1066–1067. [Google Scholar]
- 81.Li P, Dai W. Clinical observation of electroacupuncture in the treatment of perimenopause depression [article in Chinese]. Clin J Trad Chin Med. 2020b;32(3):555–558. [Google Scholar]
- 82.Zhang J. Comparative study on the efficacy of abdominal acupuncture and Western medicine in treating perimenopause depression [article in Chinese]. World Latest Med Inf. 2015;64:145. [Google Scholar]
- 83.Xing K. Clinical observation of 120 cases of perimenopausal depression treated by Xingshen-Jieyu acupuncture [article in Chinese]. Matern Child Health Care China. 2011;26(34):5373–5375. [Google Scholar]
- 84.Zhou SH, Wu FD. Therapeutic effect of acupuncture on perimenopausal depression and its effects on DA, NE and 5-HIAA contents [article in Chinese]. Chin Acup Moxib. 2007;27(5):317–321. [PubMed] [Google Scholar]
- 85.Dong Y. Clinical observation of acupuncture on beishu acupoint in the treatment of perimenopausal depression [article in Chinese]. Jilin J Trad Chin Med. 2015;35(3):306–308. [Google Scholar]
- 86.Zhang YL. Effect of Electroacupuncture on Hamilton Score and Sex Hormone Levels in Patients with Perimenopausal Depression [Article in Chinese] [Master thesis]. Shaanxi University of Chinese Medicine; 2010. [Google Scholar]
- 87.Zhang YQ. Clinical study of acupuncture treatment on 94 cases of perimenopausal depression [article in Chinese]. Inner Mongol J Trad Chin Med. 2013;32(11):41–42. [Google Scholar]
- 88.Zheng SH, Wu YT, Liao JR, Xu MZ, Hu ZN, Chen LH. Clinical study of Sishen-needle treatment on perimenopausal depression [article in Chinese]. Liaoning J Trad Chin Med. 2010;37(4):726–728. [Google Scholar]
- 89.Gao L, Niu HY. A preliminary study on clinical effects of Panlong-needling on perimenopausal insomnia [article in Chinese]. Jilin J Tradit Chin Med. 2014;34(1):88–90. [Google Scholar]
- 90.Xu HB. Effect of acupuncture on perimenopausal insomnia and its impact on PSQI [article in Chinese]. Chin Commun Doctors. 2020;36(31):109–110. [Google Scholar]
- 91.Ma WL. Effect of acupuncture combined with medication on perimenopausal insomnia and the impacts on FSH and E2 levels [article in Chinese]. Acta Chin Med Pharmacol. 2016;44(2):89–91. [Google Scholar]
- 92.Zhu SP, Li PP, Zhu XL. Observation of the effect of Tiaodu-Anshen acupuncture on perimenopausal insomnia [article in Chinese]. Mod J Integr Trad Chin West Med. 2016;25(26):2885–2888. [Google Scholar]
- 93.Liu HF, Chen GZ. Effect of acupuncture combined with Sertraline on hormone levels and neurotransmitters in women with perimenopausal depression–clinical data of 40 cases [article in Chinese]. Jiangsu J Trad Chin Med. 2019;51(2):70–72. [Google Scholar]
- 94.Ning Y. Clinical study on acupuncture treatment of perimenopausal depression [article in Chinese]. J Sichuan Trad Chin Med. 2015;33(1):154–155. [Google Scholar]
- 95.Ma YB, Guo YM, Li XJ. Therapeutic effect of electroacupuncture combined with paroxetine on 55 cases of perimenopausal depression [article in Chinese]. Med J Chin Peoples Health. 2011;23(11):1426–1428. [Google Scholar]
- 96.Santoro N, Roeca C, Peters BA, Neal-Perry G. The menopause transition: signs, symptoms, and management options. J Clin Endocrinol Metab. 2021;106(1):1–15. doi: 10.1210/clinem/dgaa764 [DOI] [PubMed] [Google Scholar]
- 97.Ma RJ, Feng SS, He KL, Yang HT. Clinical Effect of Electroacupuncture on Perimenopausal Insomnia [Article in Chinese]. Proceedings of 2017 World Acupuncture Conference/ 2017 Annual Meeting of the Chinese Acupuncture Society; 2017: 302–303. [Google Scholar]
- 98.Wang YH, Zheng CL, Huang C, et al. Systems pharmacology dissecting holistic medicine for treatment of complex diseases: an example using cardiocerebrovascular diseases treated by TCM. Evid Based Complement Alternat Med. 2015;980190. doi: 10.1155/2015/980190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhao FY, Fu QQ, Zheng Z, Lao LX, Song HL, Shi Z. Verum- versus sham-acupuncture on Alzheimer’s Disease (AD) in animal models: a preclinical systematic review and meta-analysis. Biomed Res Int. 2020;2020:5901573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.China National Standardization Administration. China International Standardization Administration. GB/T 30233-2013, Indications of Acupuncture Points. Beijing: Chinese Standard Press; 2013. [Google Scholar]
- 101.van der Watt G, Laugharne J, Janca A. Complementary and alternative medicine in the treatment of anxiety and depression. Curr Opin Psychiatry. 2008;21(1):37–42. doi: 10.1097/YCO.0b013e3282f2d814 [DOI] [PubMed] [Google Scholar]
- 102.van der Sluijs CP, Bensoussan A, Liyanage L, Shah S. Women’s health during mid-life survey: the use of complementary and alternative medicine by symptomatic women transitioning through menopause in Sydney. Menopause. 2007;14(3 Pt 1):397–403. doi: 10.1097/01.gme.0000236937.36078.f4 [DOI] [PubMed] [Google Scholar]
- 103.Gentry-Maharaj A, Karpinskyj C, Glazer C, et al. Prevalence and predictors of complementary and alternative medicine/non-pharmacological interventions use for menopausal symptoms within the UK Collaborative Trial of Ovarian Cancer Screening. Climacteric. 2017;20(3):240–247. doi: 10.1080/13697137.2017.1301919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhu Y, Yang Y, Li J. Does acupuncture help patients with spasticity? A narrative review. Ann Phys Rehabil Med. 2019;62(4):297–301. doi: 10.1016/j.rehab.2018.09.010 [DOI] [PubMed] [Google Scholar]
- 105.Clayton AH, Ninan PT. Depression or menopause? Presentation and management of major depressive disorder in perimenopausal and postmenopausal women. Prim Care Companion J Clin Psychiatry. 2010;12(1):PCC.08r00747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tian SL. Effect of Pre-Acupuncture on Guanyuan and Sanyinjiao on HPO Axis of Ovariectomized Rats and Its Antioxidant Effect [Article in Chinese] [Master thesis]. Beijing University of Chinese Medicine; 2007. [Google Scholar]
- 107.Zhang H, Zhang LF, Li XH, et al. Pre-acupuncture at Guanyuan acupoint in the regulation of superoxide dismutase and nitric oxide synthase activities in the natural aging rat model [article in Chinese]. J Clin Rehabil Tis Eng Res. 2005;9(31):147–149. [Google Scholar]
- 108.Li XH, Wang HB, Xu LL, et al. Effect of pre-acupuncture at Guanyuan on the expression of HSP 70 and HSP 70 mRNA in spleen and the contents of serum IL-2, TNF-α in perimenopausal model rat [article in Chinese]. Acupunct Res. 2009;34(2):83–88. [PubMed] [Google Scholar]
- 109.Li YJ, Mo YP, Deng XF, et al. Expression of 5-HT, 5-HTT in hippocampus of chronic unpredictable mild stress-induced depressive rats treated by electroacupuncture pretreatment [article in Chinese]. J Clin Acupunct Moxib. 2014;30(7):57–60. [Google Scholar]
- 110.Ciano C, King TS, Wright RR, Perlis M, Sawyer AM. Longitudinal study of insomnia symptoms among women during perimenopause. J Obstet Gynecol Neonatal Nurs. 2017;46(6):804–813. doi: 10.1016/j.jogn.2017.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Woods NF, Mitchell ES. Sleep symptoms during the menopausal transition and early postmenopause: observations from the Seattle Midlife Women’s Health Study. Sleep. 2010;33(4):539–549. doi: 10.1093/sleep/33.4.539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Freeman EW, Sammel MD, Lin H, Nelson DB. Associations of hormones and menopausal status with depressed mood in women with no history of depression. Arch Gen Psychiatry. 2006;63(4):375–382. doi: 10.1001/archpsyc.63.4.375 [DOI] [PubMed] [Google Scholar]
- 113.de Zambotti M, Colrain IM, Baker FC. Interaction between reproductive hormones and physiological sleep in women. J Clin Endocrinol Metab. 2015;100(4):1426–1433. doi: 10.1210/jc.2014-3892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Douma SL, Husband C, O’Donnell ME, Barwin BN, Woodend AK. Estrogen-related mood disorders: reproductive life cycle factors. ANS Adv Nurs Sci. 2005;28(4):364–375. doi: 10.1097/00012272-200510000-00008 [DOI] [PubMed] [Google Scholar]
- 115.Freeman EW, Sammel MD, Liu L, Gracia CR, Nelson DB, Hollander L. Hormones and menopausal status as predictors of depression in women in transition to menopause. Arch Gen Psychiatry. 2004;61(1):62–70. doi: 10.1001/archpsyc.61.1.62 [DOI] [PubMed] [Google Scholar]
- 116.Xie C. Experimental Study of Electroacupuncture Interving Rat’s Sleep-Wake During Perimenopause by Adjusting VLPO and TMN [Article in Chinese] [Master thesis]. Shanghai University of Traditional Chinese Medicine; 2013. [Google Scholar]
- 117.Yu XT. Experimental Study of Electroacupuncture for Sleep-Wake Regulation on Rats with Insomnia During Perimenopause [Article in Chinese] [Master thesis]. Shanghai University of Traditional Chinese Medicine; 2012. [Google Scholar]
- 118.Sun Y. Experimental Study on the Mechanism of Electroacupuncture in the Treatment of Perimenopausal Depression [Article in Chinese] [Master thesis]. Liaoning University of Traditional Chinese Medicine; 2009. [Google Scholar]
- 119.Song L, Li WC, Li HF, et al. Effects of acupuncture with YangXueRouGan technique on estrogen level in perimenopausal depression mice [article in Chinese]. Occup Health. 2021;37(7):902–904. [Google Scholar]
- 120.Zhou XD, Yang XJ, Zheng Y, et al. Jie-Yu Pill, a proprietary herbal medicine, ameliorates mood disorder-like behavior and cognitive impairment in estrogen-deprived mice exposed to chronic unpredictable mild stress: implication for a potential therapy of menopause syndrome. Front Psychiatry. 2020;11:579995. doi: 10.3389/fpsyt.2020.579995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yang WJ. Study on the Neuroendocrine Mechanism of Acupuncture in the Treatment of Perimenopausal Insomnia Based on Estrogen and Its Receptor [Article in Chinese] [Doctorate thesis]. Shanghai University of Traditional Chinese Medicine; 2017. [Google Scholar]
- 122.Natari RB, Clavarino AM, McGuire TM, Dingle KD, Hollingworth SA. The bidirectional relationship between vasomotor symptoms and depression across the menopausal transition: a systematic review of longitudinal studies. Menopause. 2018;25(1):109–120. doi: 10.1097/GME.0000000000000949 [DOI] [PubMed] [Google Scholar]
- 123.Han X, Gao Y, Yin X, et al. The mechanism of electroacupuncture for depression on basic research: a systematic review. Chin Med. 2021;16(1):10. doi: 10.1186/s13020-020-00421-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Jiang XR. Study on the Correlation Between HPO Axis-and HPA-Axis in Perimenopausal Depression Model Rats Treated by Electroacupuncture [Article in Chinese] [Master thesis]. Liaoning University of Traditional Chinese Medicine; 2017. [Google Scholar]
- 125.Liu C, Zheng S, Wu W, et al. Effects of acupuncture on the hypothalamus-pituitary-adrenal axis in chronic insomnia patients: a study protocol for a randomized controlled trial. Trials. 2019;20(1):810. doi: 10.1186/s13063-019-3964-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Xi HQ, Wu WZ, Liu CY, et al. Effect of Tongdu-Tiaoshen acupuncture in treatment of chronic insomnia by regulating the hypothalamus-pituitary-adrenal axis [article in Chinese]. Acupunct Res. 2020;45(7):552–556. [DOI] [PubMed] [Google Scholar]
- 127.Jiang XR, Ren L, Li CR. Effect of electroacupuncture on HPO axis in rats with perimenopausal depression [article in Chinese]. Acupunct Res. 2017;42(1):45–49. [PubMed] [Google Scholar]
- 128.Zhang JB. Effect of Electroacupuncture on the Behavior and Brain β-Endorphin and Other Indices of Rats with Perimenopausal Depression [Article in Chinese] [Master thesis]. Liaoning University of Traditional Chinese Medicine; 2009. [Google Scholar]
- 129.Huangfu WL. Effect of Electroacupuncture on Hippocampus and Internal Environment of Perimenopausal Depression Model Rats [Article in Chinese] [Master thesis]. Liaoning University of Traditional Chinese Medicine; 2018. [Google Scholar]
- 130.Hartmann J, Bajaj T, Klengel C, et al. Mineralocorticoid receptors dampen glucocorticoid receptor sensitivity to stress via regulation of FKBP5. Cell Rep. 2021;35(9):109185. doi: 10.1016/j.celrep.2021.109185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Oh J, Petersen C, Walsh CM, Bittencourt JC, Neylan TC, Grinberg LT. The role of co-neurotransmitters in sleep and wake regulation. Mol Psychiatry. 2019;24(9):1284–1295. doi: 10.1038/s41380-018-0291-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Eichling PS, Sahni J. Menopause related sleep disorders. J Clin Sleep Med. 2005;1(3):291–300. doi: 10.5664/jcsm.26347 [DOI] [PubMed] [Google Scholar]
- 133.Schweizer-Schubert S, Gordon JL, Eisenlohr-Moul TA, et al. Steroid hormone sensitivity in reproductive mood disorders: on the role of the GABAA receptor complex and stress during hormonal transitions. Front Med (Lausanne). 2021;7:479646. doi: 10.3389/fmed.2020.479646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Riemann D, Nissen C, Palagini L, Otte A, Perlis ML, Spiegelhalder K. The neurobiology, investigation, and treatment of chronic insomnia. Lancet Neurol. 2015;14(5):547–558. doi: 10.1016/S1474-4422(15)00021-6 [DOI] [PubMed] [Google Scholar]
- 135.Saito YC, Maejima T, Nishitani M, et al. Monoamines inhibit GABAergic neurons in ventrolateral preoptic area that make direct synaptic connections to hypothalamic arousal neurons. J Neurosci. 2018;38(28):6366–6378. doi: 10.1523/JNEUROSCI.2835-17.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Cao H, Feng MG, Hou S, Wei KX. Effects of electroacupuncture at HT7 and EX-HN1 on sleep phases and PGD2 system of CSF in ovariectomized rats [article in Chinese]. JCAM. 2020;36(05):71–75. [Google Scholar]
- 137.Pan Y, Luo J, Zhang HL. Study on the effect of acupuncture at Sishencong and Baihui on the serum amino acids neurotransmitters of insomnia patients. WJAM. 2017;27(1):23–27. [Google Scholar]
- 138.Xi HQ, Wu WZ, Liu CY, et al. Effect of acupuncture at Tiaoshen acupoints on hyperarousal state in chronic insomnia [article in Chinese]. Chin Acup Moxib. 2021;41(3):263–267. [DOI] [PubMed] [Google Scholar]
- 139.Wang D, Wang X, Luo MT, Wang H, Li YH. Gamma-aminobutyric acid levels in the anterior cingulate cortex of perimenopausal women with depression: a magnetic resonance spectroscopy study. Front Neurosci. 2019;13:785. doi: 10.3389/fnins.2019.00785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Duan DM, Tu Y, Liu P, Jiao S. Antidepressant effect of electroacupuncture regulates signal targeting in the brain and increases brain-derived neurotrophic factor levels. Neural Regen Res. 2016;11(10):1595–1602. doi: 10.4103/1673-5374.193238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Liu HJ, He XF, Chen XH. Effects of acupuncture on behavior and hippocampal amino-acid neurotransmitters in rats with depression [article in Chinese]. J Med Res. 2010;39(3):79–81. [Google Scholar]
- 142.Schneider-Matyka D, Grochans E, Lubkowska A, Panczyk M, Szkup M. The effect of tryptophan and serotonin levels on the severity of depressive and climacteric symptoms in perimenopausal women. Eur Rev Med Pharmacol Sci. 2021;25(9):3425–3431. [DOI] [PubMed] [Google Scholar]
- 143.Gonzales GF, Carrillo C. Blood serotonin levels in postmenopausal women: effects of age and serum oestradiol levels. Maturitas. 1993;17(1):23–29. doi: 10.1016/0378-5122(93)90120-7 [DOI] [PubMed] [Google Scholar]
- 144.Albertazzi P. A review of non-hormonal options for the relief of menopausal symptoms. Treat Endocrinol. 2006;5(2):101–113. doi: 10.2165/00024677-200605020-00004 [DOI] [PubMed] [Google Scholar]
- 145.Guo X, Pan F, Wang B, Li W, Xia C, Ju Z. Effect of electroacupuncture on mice model of permenopausal depression. Saudi J Biol Sci. 2019;26(8):2030–2036. doi: 10.1016/j.sjbs.2019.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zhou SH, Sun FJ, Chen Z, Kong NN. Regulation of acupuncture with Kidney-tonifying method on monoamine neurotransmitter in perimenopausal depression model rats [article in Chinese]. Lishizhen Med Materia Medica Res. 2015;26(9):2299–2301. [Google Scholar]
- 147.Shi MQ, Han K. Mechanism study of Kidney-Tonifying-and-Liver-Soothing therapy in regulating estradiol and 5-HT levels in ovariectomized rats [article in Chinese]. J Liaoning Univ TCM. 2012;14(2):185–188. [Google Scholar]
- 148.Wu GF. Study on the Effect and Mechanism of Electroacupuncture on Animal Model of Perimenopausal Depression with Liver-Stagnation Syndrome Pattern [Article in Chinese] [Master thesis]. Liaoning University of Traditional Chinese Medicine; 2012. [Google Scholar]
- 149.Zhao SZ, Ren L. Effect of electroacupuncture on high-density lipoprotein in rat model of perimenopausal depression [article in Chinese]. Heilongjiang Med J. 2011;24(3):395–397. [Google Scholar]
- 150.Duan D, Tu Y, Yang X, Liu P. Electroacupuncture restores 5-HT system deficit in chronic mild stress-induced depressed rats. Evid Based Complement Alternat Med. 2016;2016:7950635. doi: 10.1155/2016/7950635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Cespuglio R. Serotonin: its place today in sleep preparation, triggering or maintenance. Sleep Med. 2018;49:31–39. doi: 10.1016/j.sleep.2018.05.034 [DOI] [PubMed] [Google Scholar]
- 152.Cao H, Feng MG, Hou S, Teng JY. Effects and mechanism of electroacupuncture at SP6 on sleep phase in rats with perimenopausal insomnia [article in Chinese]. JCAM. 2019;35(08):80–83. [Google Scholar]
- 153.Rybaczyk LA, Bashaw MJ, Pathak DR, Moody SM, Gilders RM, Holzschu DL. An overlooked connection: serotonergic mediation of estrogen-related physiology and pathology. BMC Womens Health. 2005;5:12. doi: 10.1186/1472-6874-5-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Palagini L, Geoffroy PA, Miniati M, et al. Insomnia, sleep loss, and circadian sleep disturbances in mood disorders: a pathway toward neurodegeneration and neuroprogression? A theoretical review. CNS Spectr. 2021;1–11. doi: 10.1017/S1092852921000018 [DOI] [PubMed] [Google Scholar]
- 155.Gulec M, Ozkol H, Selvi Y, et al. Oxidative stress in patients with primary insomnia. Prog Neuropsychopharmacol Biol Psychiatry. 2012;37(2):247–251. doi: 10.1016/j.pnpbp.2012.02.011 [DOI] [PubMed] [Google Scholar]
- 156.Doshi SB, Agarwal A. The role of oxidative stress in menopause. J Midlife Health. 2013;4(3):140–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sánchez-Rodríguez MA, Zacarías-Flores M, Arronte-Rosales A, Correa-Muñoz E, Mendoza-Núñez VM. Menopause as risk factor for oxidative stress. Menopause. 2012;19(3):361–367. doi: 10.1097/gme.0b013e318229977d [DOI] [PubMed] [Google Scholar]
- 158.Agarwal A, Aponte-Mellado A, Premkumar BJ, Shaman A, Gupta S. The effects of oxidative stress on female reproduction: a review. Reprod Biol Endocrinol. 2012;10:49. doi: 10.1186/1477-7827-10-49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Hachul de Campos H, Brandão LC, D’Almeida V, et al. Sleep disturbances, oxidative stress and cardiovascular risk parameters in postmenopausal women complaining of insomnia. Climacteric. 2006;9(4):312–319. doi: 10.1080/13697130600871947 [DOI] [PubMed] [Google Scholar]
- 160.Kolesnikova LL, Semenova NV, Solodova EI, Madaeva LM. Oxidative stress in women with insomnia in different stages of menopause [article in Russian]. Ter Arkh. 2017;89(8):50–56. [DOI] [PubMed] [Google Scholar]
- 161.Kolesnikova LL, Madaeva LM, Semenova NV, Solodova EI, Grebenkina LA, Darenskaya MA. Evaluation of lipid peroxidation - antioxidant protection in perimenopausal women with sleep disorders [article in Russian]. Vestn Ross Akad Med Nauk. 2014;69(11–12):11–16. doi: 10.15690/vramn.v69i11-12.1177 [DOI] [PubMed] [Google Scholar]
- 162.Lin YF, Liu ZD, Ma W, Shen WD. Hazards of insomnia and the effects of acupuncture treatment on insomnia. J Integr Med. 2016;14(3):174–186. doi: 10.1016/S2095-4964(16)60248-0 [DOI] [PubMed] [Google Scholar]
- 163.Wu BF. An Experimental Study on the Mechanism of Electroacupuncture on Sleep Deprivation and Its Induced Oxidative Stress in Rats [Article in Chinese] [Doctorate thesis]. Heilongjiang University of Chinese Medicine; 2008. [Google Scholar]
- 164.Hirose A, Terauchi M, Akiyoshi M, Owa Y, Kato K, Kubota T. Depressive symptoms are associated with oxidative stress in middle-aged women: a cross-sectional study. Biopsychosoc Med. 2016;10:12. doi: 10.1186/s13030-016-0066-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Liu CZ, Shi GX, Huang YL, Zhu H, Wang LP. Effect of acupuncture on urinary biomarkers of oxidative stress in vascular dementia patients [article in Chinese]. Chin J Inf Tradit Chin Med. 2011;18(7):18–20. [Google Scholar]
- 166.Yeung WF, Yu BYM, Yuen JWM, et al. Semi-individualized acupuncture for insomnia disorder and oxidative stress: a randomized, double-Blind, sham-controlled trial. Nat Sci Sleep. 2021;13:1195–1207. doi: 10.2147/NSS.S318874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Sánchez-Rodríguez MA, Zacarías-Flores M, Arronte-Rosales A, Mendoza-Núñez VM. Association between hot flashes severity and oxidative stress among Mexican postmenopausal women: a cross-sectional study. PLoS One. 2019;14(9):e0214264. doi: 10.1371/journal.pone.0214264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Miquel J, Ramírez-Boscá A, Ramírez-Bosca JV, Alperi JD. Menopause: a review on the role of oxygen stress and favorable effects of dietary antioxidants. Arch Gerontol Geriatr. 2006;42(3):289–306. doi: 10.1016/j.archger.2005.08.005 [DOI] [PubMed] [Google Scholar]
- 169.Krystal AD, Edinger J, Wohlgemuth W, Marsh GR. Sleep in peri-menopausal and post-menopausal women. Sleep Med Rev. 1998;2(4):243–253. doi: 10.1016/S1087-0792(98)90011-9 [DOI] [PubMed] [Google Scholar]
- 170.Seo SY, Moon JY, Kang SY, et al. An estradiol-independent BDNF-NPY cascade is involved in the antidepressant effect of mechanical acupuncture instruments in ovariectomized rats. Sci Rep. 2018;8(1):5849. doi: 10.1038/s41598-018-23824-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wang G, Ren L. Effect of electroacupuncture on hippocampal BDNF and CREB in rats with perimenopausal depression [article in Chinese]. Liaoning J TCM. 2016;43(4):858–860. [Google Scholar]
- 172.Liu YZ. Comparative Effects Between Electroacupuncture at Single Acupoint and at Group of Acupoints on Learning and Memory Ability of Sleep-Deprived Rats [Article in Chinese] [Master thesis]. Changchun University of Chinese Medicine; 2019. [Google Scholar]
- 173.Xiao Z, Cao Z, Yang J, et al. Baicalin promotes hippocampal neurogenesis via the Wnt/β-catenin pathway in a chronic unpredictable mild stress-induced mouse model of depression. Biochem Pharmacol. 2021;190:114594. doi: 10.1016/j.bcp.2021.114594 [DOI] [PubMed] [Google Scholar]
- 174.Jing Q, Ren L, Deng X, et al. Electroacupuncture promotes neural proliferation in hippocampus of perimenopausal depression rats via Wnt/β-Catenin signaling pathway. J Acupunct Meridian Stud. 2020;13(3):94–103. doi: 10.1016/j.jams.2020.03.065 [DOI] [PubMed] [Google Scholar]
- 175.Wexler EM, Geschwind DH, Palmer TD. Lithium regulates adult hippocampal progenitor development through canonical Wnt pathway activation. Mol Psychiatry. 2008;13(3):285–292. doi: 10.1038/sj.mp.4002093 [DOI] [PubMed] [Google Scholar]
- 176.Dai J, Pan JY, Liao N, et al. Influence of miR-155 on behaviors of depression mice through regulating Wnt/β-catenin signaling pathway. Eur Rev Med Pharmacol Sci. 2020;24(3):1398–1407. [DOI] [PubMed] [Google Scholar]
- 177.Deng X. Effect of Electroacupuncture on the Proliferation and Differentiation of Hippocampal Neural Stem Cells and Wnt Pathway in a Rat Model of Perimenopausal Depression [Article in Chinese] [Doctorate thesis]. Liaoning University of Traditional Chinese Medicine; 2017. [Google Scholar]
- 178.Jing Q, Lin H, Ren L. Effect of electroacupuncture on Wnt/β-Catenin signaling pathway in hippocampus of perimenopausal depression rats model [article in Chinese]. Chin Archiv TCM. 2018;36(6):1347–1350. [Google Scholar]
- 179.Park BM, Kim EJ, Nam HJ, et al. Cyclized oligopeptide targeting LRP5/6-DKK1 interaction reduces the growth of tumor burden in a multiple myeloma mouse model. Yonsei Med J. 2017;58(3):505–513. doi: 10.3349/ymj.2017.58.3.505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Qu DJ, Huan Y, Zhao YY, et al. Involvement of Wnt/β-catenin signaling mediated hippocampal neurogenesis in sleep-deprivation induced memory impairment in mice [article in Chinese]. Chin J Neuroanat. 2019;35(02):134–140. [Google Scholar]
- 181.Ables JL, Breunig JJ, Eisch AJ, Rakic P. Not(ch) just development: notch signalling in the adult brain. Nat Rev Neurosci. 2011;12(5):269–283. doi: 10.1038/nrn3024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Shen J, Lin L, Liao L, et al. The involvement of Notch1 signaling pathway in mid-aged female rats under chronic restraint stress. Neurosci Lett. 2020;738:135313. doi: 10.1016/j.neulet.2020.135313 [DOI] [PubMed] [Google Scholar]
- 183.Tian H, Li X, Tang Q, et al. Yi-nao-jie-yu prescription exerts a positive effect on neurogenesis by regulating Notch signals in the hippocampus of post-stroke depression rats. Front Psychiatry. 2018;9:483. doi: 10.3389/fpsyt.2018.00483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Jalali A, Bassuk AG, Kan L, et al. HeyL promotes neuronal differentiation of neural progenitor cells. J Neurosci Res. 2011;89(3):299–309. doi: 10.1002/jnr.22562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Deng X, Ren L. Effect of electroacupuncture on expression of hippocampal Notch signaling pathway in rats with perimenopausal depression [article in Chinese]. Liaoning J TCM. 2017;44(3):595–601. [Google Scholar]
- 186.Mathews SB, Epperson CN. Clinical management of menopause-related sleep disturbance. In: Pal L, Sayegh R, editors. Essentials of Menopause Management. Cham: Springer; 2017:105–127. [Google Scholar]
- 187.Davis SR. The Kupperman Index undressed. Maturitas. 2019;126:90–91. doi: 10.1016/j.maturitas.2019.04.219 [DOI] [PubMed] [Google Scholar]
- 188.Befus D, Coeytaux RR, Goldstein KM, et al. Management of menopause symptoms with acupuncture: an umbrella systematic review and meta-analysis. J Altern Complement Med. 2018;24(4):314–323. doi: 10.1089/acm.2016.0408 [DOI] [PubMed] [Google Scholar]
- 189.Jin YB, Yang ZY, Jin HF, Mi HQ. Discussion of the effect on ethology of perimenopausal syndrome rats by electroacupuncture in different periods [article in Chinese]. CJTCMP. 2010;25(10):1689–1693. [Google Scholar]
- 190.Jin YB, Mi HQ, Jin HF. Discussion of the best intervention time for electroacupuncture in treating rats with perimenopausal syndrome. J Acupunct Tuina Sci. 2011;9(5):273–277. doi: 10.1007/s11726-011-0530-4 [DOI] [Google Scholar]
- 191.Ebrahimi A, Tayebi N, Fatemeh A, Akbarzadeh M. Investigation of the role of herbal medicine, acupressure, and acupuncture in the menopausal symptoms: an evidence-based systematic review study. J Fam Med Prim Care. 2020;9(6):2638–2649. doi: 10.4103/jfmpc.jfmpc_1094_19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Ee C, French SD, Xue CC, Pirotta M, Teede H. Acupuncture for menopausal hot flashes: clinical evidence update and its relevance to decision making. Menopause. 2017;24(8):980–987. doi: 10.1097/GME.0000000000000850 [DOI] [PubMed] [Google Scholar]
- 193.Kim TH, Lee MS, Alraek T, Birch S. Acupuncture in sham device controlled trials may not be as effective as acupuncture in the real world: a preliminary network meta-analysis of studies of acupuncture for hot flashes in menopausal women. Acupunct Med. 2020;38(1):37–44. doi: 10.1136/acupmed-2018-011671 [DOI] [PMC free article] [PubMed] [Google Scholar]