Abstract
Information about blood arterial oxygen saturation (SpO2) is crucial in critical care settings or home health monitoring during the COVID-19 pandemic. Also, we need to identify the factors that affect the SpO2 measurement. In this paper, the effect of compression of the cuff during noninvasive blood pressure (NIBP) measurement on the SpO2 results was investigated. A custom-made system was used for simultaneous measurement of NIBP and SpO2. The study was conducted on 213 subjects aged between 21 and 93, with a systolic blood pressure of (94 to 194) mmHg, diastolic blood pressure of (52–98) mmHg, and 994 NIBP readings were used for the analysis. During the NIBP measurement, momentary changes in SpO2 can reach ±17% and are in most cases positive (mean 2.9%). The change was not correlated with sex, age, height, body weight, BMI, HR and blood pressure. The obtained results show that frequent NIBP measurements may lead to wrong conclusions about SpO2. In our study, pressure measurements mainly caused the increase of blood oxygenation level.
Keywords: Blood oxygen saturation, Pulse oximeter, Blood pressure monitor, Photoplethysmography, Physiological parameters, Measurement accuracy
1. Introduction
Pulse oximetry is a non-invasive method of monitoring blood arterial oxygen saturation (SpO2). This method is based on difference in light absorption between oxygenated and non-oxygenated hemoglobin. Although, pulse oximetry has revolutionized the monitoring of oxygenation due to its low-cost, continuous and non-invasive measurement capabilities [1], it also has accuracy limitations due to current technology [2]. However, the limitations of the SpO2 measurement are not as significant as the advantages of pulse oximeters. For example, information about SpO2 is vital in critical care settings [3] and other many cases. Due to the big threat caused by the COVID-19 through its influence on the respiratory system of an infected person, monitoring SpO2 has gained even more importance. Using saturation monitoring devices could help with early detection of “silent hypoxemia” in COVID-19 patients [4], [5], [6]. Moreover, there may be no additional immediate respiratory sings related to “silent hypoxemia” except a very low SpO2 level [5]. Responding to the needs of the market due to the COVID-19 situation and growth of home health monitoring, a variety of pulse oximeters are available at different prices for personal or medical use. Depending on the final application, we can divide pulse oximeters into consumer grade (portable fingertip pulse oximeters) and medical grade. In [7] researchers compared three commercially available portable pulse oximeters with a bedside monitor and confirmed their clinical usefulness. In turn, a comparison of pulse oximeters in terms of accuracy was presented in [8] and failure time was presented in [9]. Apart from differences in accuracy between the available pulse oximeters, the conditions under which measurements are performed are also important [1], [10]. The most important factors influencing the SpO2 measurement are motion artifacts, inappropriate placement of a probe and the measurement site [10], [11], [12]. Motion artifacts like tremors or convulsions can cause low SpO2 readings (even below 50%) [1]. More information about measurement sites can be read in [12] where authors investigated values of saturation depending on different measurement sites (finger, toe, forehead and earlobe). They suggested use of an earlobe probe in intensive care patients with heart surgery because of higher agreement ration with blood gas analysis. Another factor is pressure on the blood vessels caused by, for example, cuff-based oscillometric noninvasive blood pressure (NIBP) measurement or by inappropriate body positioning. Such problems may occur in particular during continuous measurements lasting for hours. The general problem of NIBP influence on SpO2 results were discussed in previous studies [9], [13], [14]. However, these studies do not provide detailed information on how the NIBP measurement affects the SpO2 result. In this article, the authors investigated the effect of NIBP measurement on the SpO2 result and determined whether these changes were dependent on other factors i.e. sex, age, body mass index (BMI), mean heart rate (HR) and blood pressure.
2. Principle of blood oxygen saturation measurement
2.1. Basis of SpO2 measurement
The gold standard for SpO2 measurement is arterial blood gas analysis [15]. It is an invasive method which is inconvenient, painful, time consuming, costly and used only in hospital care units. A blood gas analyzer uses the sample of the subject’s blood through an arterial puncture or an indwelling arterial catheter [16]. This method makes the oxygen saturation results intermittent and, because of the delay between sampling and test result, it has fewer diagnostic possibilities than commonly used non-invasive method based on photoplethysmography (PPG) [17].
The PPG method is based on a difference in absorption of various light waves by blood depending on the level of oxygenation. It mostly uses two different wavelengths: 660 nm (Red light) and 940 nm (Infrared light). The more oxygenated blood is, the more infrared light is absorbed and the less red light is being absorbed [1]. Absorption graph is presented in Fig. 1 . Oxygenated hemoglobin is marked as HbO2, deoxygenated as Hb, water is represented by H2O and vertical lines indicates Red and Infrared light.
Fig. 1.
Absorption spectrum of Hb and HbO2.
The Hb and HbO2 are characterized by different absorption throughout the spectral range with the exception of the crossing point (about 800 nm) where the absorption coefficients are equal. For the wavelengths of less than the crossing point, Hb has a higher absorption coefficient as compared to HbO2, while the opposite is true for the wavelengths above the crossing point. Significant differences in absorption for Hb and HbO2 occur at wavelengths of 660 nm and 940 nm, which are those used for determination of SpO2.
Two methods can be used to measure PPG signal i.e. transmission (Fig. 2 (a)) and reflectance (Fig. 2(b)) [18], [19].
Fig. 2.
Measurement of PPG signals methods: (a) transmission (b) reflectance.
In the transmission method the light transmitted by the source is detected by a photodetector on the side opposite to the light source. In the reflectance method the photodetector receives light that is back-scattered or reflected by tissues, bones or blood vessels. The transmission mode gives a relatively good signal but is limited in the number of possible measurement sites (i.e. thickness of tissue the light has to go through e.g. earlobe or fingertip). In comparison, the reflectance method is not limited to measurement sites but, in this, case measurement is affected by pressure disturbances and motion artifacts.
The ratio R of absorbance of red and infrared wavelengths is calculated and calibrated against direct measurements of arterial oxygen saturation. Based on the R ratio and calibration curve, the SpO2 value is calculated by a mathematical relationship [1], [2]. New methods for SpO2 measurements are also being investigated. The bi-level pulsed frequency-division excitation method, which relies on exciting two LEDs by bi-level pulse of 200 Hz and 400 Hz and synchronously obtaining a dual-wavelength photoplethysmogram by utilizing the digital demodulation algorithm, is described in [20]. Moreover noncontact SpO2 measurement method based on imaging photoplethysmography and Eulerian video magnification, where the main carriers of information are the changes of skin color due to the heart cycle was presented in [21].
2.2. Measurement artifacts
PPG signals can be affected by many factors: the wavelength of light, measurement site, contact force, motion artifacts, skin color (racial), henna pigmentation, nail varnish ambient light intensity, ambient temperature [10], [11]. Among these, motion artifact is one of the most important. If the subject moves the part of the body where a sensor is located or there is a need to correct the location of the probe, it will have an impact on signal measurement, and it will cause a variability of SpO2 results. The reason is a change of the optical length path [22]. The motion artifact can be reduced by appropriate location of the probe which also changes signal quality [18]. Data fusion of signals from PPG and the accelerometer is also applied. The reduction of motion artifact is performed by usage of stopband filters set on motion frequencies [23]. Measurement of SpO2 is based on the absorption of light, so every factor which influences the absorption will also disturb the signal. One of these artifacts will be the color of skin [24]. If a patient has a dark skin, his SpO2 could be overestimated by up to 2% [25]. Also nail varnish can be an artifact when the measurement is conducted on his or her fingers [26]. Application of gel nail polish could result statistically significant change and overestimations in the SpO2 value [27]. Likewise, intravenous dyes in which isosulfan blue dye absorbs much light and causes interference and significantly lower results [28]. Dyshemoglobinemias change blood color, thus also absorption spectrum which influences the saturation results [25].
As mentioned in the introduction, an important factor affecting the SpO2 result is pressure on the blood vessels. This is the case, for example, when measuring NIBP and SpO2 on one limb. During the NIBP measurement the blood vessels close which affects the SpO2 result [13], [14]. In pulse oximeters this may cause a temporary lack of measurement results. The return to the “normal” state can take even several seconds. Since the time of an NIBP measurement with the cuff-base auscultation method can be up to one minute, the obtained SpO2 result may be misleading to medical personnel [9].
3. Methods
3.1. Measurement system
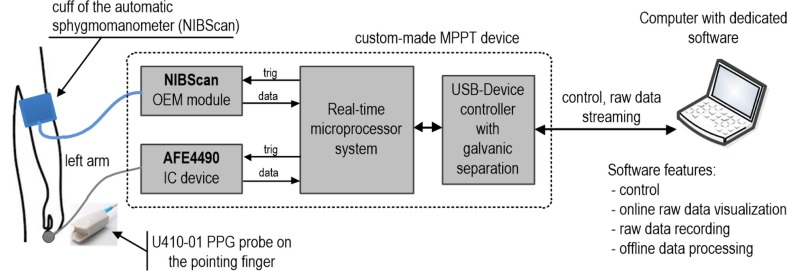
For the oxygen saturation measurement, we used a custom-made system called the MPPT, described in detail in [29]. The MPPT is a precise, multi-site system for the simultaneous, real-time, synchronous measurement of PPG and electrocardiographic signals with simultaneous NIBP assessment. For PPG measurement a transmission probe type U410-01 was used on the left pointing finger. This probe has one RED diode (wavelength 660 nm) and one IR diode (wavelength 905 nm). NIBP measurements were performed with a NIBScan blood pressure device. NIBScan is an OEM (Original Equipment Manufacturer) device and therefore it is possible to fully control the measuring process. The cuff of this device was placed on the left arm. The block diagram of the measurement system used for this study is shown in Fig. 3 .
Fig. 3.
Block diagram of the measurement system used for this study.
The most important elements of the measurement system used in this work are: an automatic sphygmomanometer (NIBScan with cuff), a specialized AFE4490 analog-front-end integrated circuit (IC) with a U410-01 probe for SpO2 measurement, real-time microprocessor system based on an STM32F746 microcontroller, a USB-Device controller with galvanic separation and a computer with appropriate software. For synchronous NIBP and PPG measurements, the NIBScan and AFE4490 were triggered by the microcontroller. The sampling frequency of PPG signals (RED and IR LED diodes) was 1 kHz. In order to maintain constant PPG measurement conditions, no adaptive change of the amplification and LED diodes current were used in the PPG measurement channel. The NIBP data was also supplemented with time stamps representing the beginning and end of the measurement process. The PPG signal was measured continuously, while the NIBP measurement was triggered every 3 min. The synchronization error between the PPG and NIBP signal was less than 1 s. Raw (unprocessed) data of PPG and NIBP were directly and in real-time transferred to the computer. In the computer, the raw data was shown online and saved to a file. Signal processing and calculation of SpO2 were performed after completing all measurements (offline mode).
The SpO2 calculation was calibrated with use of a Rigel Uni-SiM Patient Simulator (as described in the Data processing Section). The accuracy of the oxygen saturation calculation was tested by comparing our device with a commercial pulse oximeter (Contec CMS50D-BT) which is FDA and CE certified. The correlation plot and Bland-Altman methods were used to establish the level of agreement between the two devices. The results are shown in Fig. 4 .
Fig. 4.
Comparison our device with commercial pulse oximeters: (a) correlation plot (b) Bland-Altman plot.
Pearson correlation coefficient (r) between our custom-made device and the commercial device which was found to be 0.99 (Fig. 4(a)). Mean difference was 1.1% what is within the measuring error of the commercial device. The standard deviation (std) was 0.76% (Fig. 4(b)) (n – number of valid data pairs, RMSE – root mean squared error).
Although a very good agreement between our device and the commercial one was obtained, other errors can appear in the entire measurement chain. These errors can be related to the artifacts mentioned in Section 2.2. However, in our study, the subject population (Section 3.2) and protocol (Section 3.3) make these errors insignificant compared to the SpO2 measurements interference caused by cuff pressures of automatic sphygmomanometers.
3.2. Study population
The study was conducted on 213 subjects aged between 21 and 93, in the period from December 2018 to February 2020, following the approval of the Bioethics Committee of the National Institute of Geriatrics, Rheumatology and Rehabilitation in Warsaw. The subjects had been informed of the purpose and procedure of the study prior to the measurements. Before inclusion in the study, all the participants were made to provide a written informed consent. Characteristics of the study group are shown in Table 1 .
Table 1.
Characteristics of the study group.
| Variable | mean (std) [min–max] or number |
|---|---|
| Number of subjects | 213 |
| Sex: male/female | 99/114 |
| Age | 61.5 (22.6) [21–93] |
| Height, cm | 167 (12) [141–196] |
| Weight, kg | 76 (15) [46–118] |
| Body Mass Index - BMI, kg/m2 | 27 (5) [18–45] |
| Heart rate (HR), bpm | 63 (8) [41–86] |
| Systolic blood pressure (SBP), mmHg | 123 (16) [94–194] |
| Diastolic blood pressure (DBP), mmHg | 72 (9) [52–98] |
| Mean arterial pressure (MAP), mmHg | 93 (13) [65–144] |
| Pulse pressure (PP), mmHg | 51 (13) [28–129] |
mean – mean value, std – standard deviation, min – minimum value, max – maximum value.
MAP, or mean arterial pressure, is defined as average pressure in a patient’s arteries during one cardiac cycle. Pulse pressure (PP) is the difference between systolic and diastolic blood pressure. The parameters HR, SBP, DBP, MAP and PP were calculated as the mean value of all measurements for subjects.
The study population included different subjects of different ages. In contrast to the study presented in [13], critically ill subjects requiring intensive care and those with extremely low or high blood pressure were not studied. Our study looked at the typical blood pressure values found in many subjects who were able to move independently.
3.3. Study protocol
The tests were carried out during the day, in a separate and quiet room, with ambient temperature of about 22–24 °C. Measurements were made in the supine position after about 15 min of supine rest on a medical settee. All measurements were performed by the same operator. The PPG signals were measured continuously for 15 min. At the same time, from 3 to 5 NIBP measurements were taken (usually 5 measurements), each took from 26 s to 86 s. The number of NIBP measurements depended on the quality of the signal which was automatically evaluated through the NIBScan device. For all 213 subjects 994 NIBP readings were used for analysis. During the tests, the ECG and PPG signals were also recorded from other sites, but they were not further used for the analysis performed in this study.
3.4. Data processing
Data processing was conducted with MATLAB software (MathWorks, R2020b) using the built-in functions for digital signal processing according to the algorithm shown in the Fig. 5 . A low-pass filter was applied to process the PPG signal. The cutoff frequency was set to 30 Hz which allows to remove ambient light [30] and power line interference [31]. Small changes in signal were eliminated using the moving average function. Then, a high-pass filter was used with cutoff frequencies at 0.1 Hz. This allowed for removal of the DC component (baseline signal). Next, the first derivative function of the obtained signal was calculated. This, in turn, allowed to find the beginning of the PPG pulse (foot of the signal [32]) and points for which the function reaches maximum.
Fig. 5.
Algorithm of SpO2 calculation.
To execute the last step, which is the calculation of the SpO2, the R ratio must be determined according to the Eq. (1) [33].
| (1) |
where:
ACRED and ACIR are amplitudes of the PPG pulse (pulsatile component),
DCRED and DCIR are average values of the PPG pulse (non-pulsatile or “direct current” component).
The value of R ratio was inserted into equation of a first degree polynomial calculated during calibration procedure, which is presented in a formula (2).
| (2) |
Calibration was performed with a Rigel Uni-SiM Patient Simulator with a PULS-R SpO2 Finger Simulator. It consisted in recording the signal from the finger probe when simulating a signal of a specific SpO2. Next, from the obtained data the R ratio was calculated, and the calibration curve for the R ratio and SpO2 was determined. In addition, a lookup table had been designated and degree 2 and 3 polynomials were tested but most adequate was first degree polynomial. In order to show momentary changes, the SpO2 was calculated with a resolution of 0.1%. The calculated SpO2 during the NIBP measurement either exceeded the maximum value of 100% or achieved values under 80%. To keep the information about the trend, the algorithm inserted NaN (Not a Number in MATLAB) values if SpO2 was under 80% or above 110%. Also the perfusion index (PI) as the ratio of AC to DC components was calculated. The PI carries information about the pulsatile strength [34]. The higher the value of the PI, the more accurate the measurement become.
4. Results and discussion
4.1. SpO2 measurement results
Sample of SpO2 and PI results outside NIBP measurement are presented in Figs. 6 (a) and 7 (a). It can be observed that SpO2 and PI values are not fixed, but have some fluctuations.
Fig. 6.
Sample of SpO2 and PI measurement for subject ID1: (a) without NIBP measurement (b) with NIBP measurement.
Fig. 7.
Sample of SpO2 and PI measurement for subject ID2: (a) without NIBP measurement (b) with NIBP measurement.
In Figs. 6(b) and 7(b) the results of a 13-minute measurement with NIBP operation for two subjects were presented. It can be observed that during measurement by pressure cuff, which are marked by green fields, SpO2 and PI change rapidly.
4.2. Artifacts due to NIBP measurement
Relative values of SpO2 and PI for every 994 instances of NIBP measurement are presented in Fig. 8 . The pressure measurement lasted from 26 s to 86 s, depending on the subject.
Fig. 8.
Relative values changing during the NIBP measurement: (a) for SpO2 (b) for PI.
Fig. 8(a) presents relative changes of SpO2 during the pressure cuff operation. It can be noticed that in most cases there is a growth tendency during the NIBP measurement. PI presented in Fig. 8(b) decreases which means a fall in the pulsatile strength. The AC component represents smaller and smaller percentage of the DC component. During the measurement there occurred instances when it was necessary to inflate the NIBP cuff with an additional amount of air, which extended the time with low PI.
4.3. Influence of NIBP measurement on SpO2 results
4.3.1. Determining the difference in SpO2 values
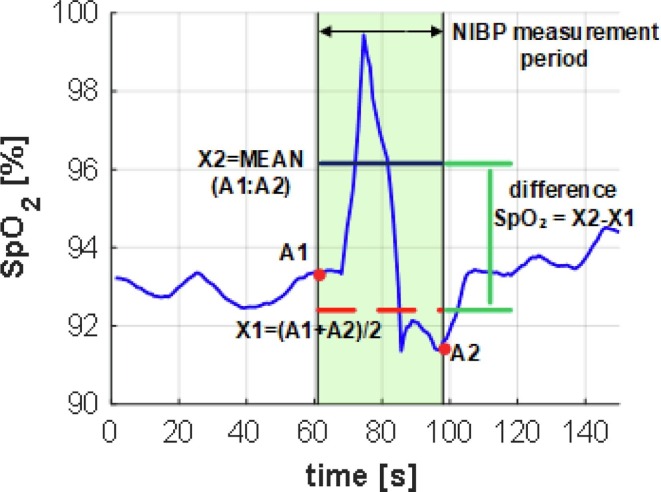
A method of calculating the influence of a NIBP measurement on SpO2 is shown in Fig. 9 . In this case A1 and A2 SpO2 values were determined as the last before and the first after the NIBP measurement. Then the mean value (X1) of A1 and A2 was used as a reference value. The average value of the range in which NIBP had been measured was calculated (X2). The difference in SpO2 between X2 and X1 was the measure of trends during operation of the pressure cuff was.
Fig. 9.
Determining the difference in SpO2 values outside and during NIBP measurement.
4.3.2. Results
In each of 213 subjects, the measurement of NIBP had influence on the value of oxygen saturation. For each of the NIBP measurements (n = 994), the relative difference of SpO2 change was determined in accordance with Fig. 9. The results of its impact are presented in Fig. 10 (a). According to the histogram the NIBP measurement caused the increase of SpO2 value. The average change was 1.4% of oxygen saturation with the standard deviation 1.4%. A histogram of the maximum, momentary deviations occurring during the NIBP measurement was also created. The results are shown in Fig. 10(b).
Fig. 10.
Histogram of SpO2 changing during and after NIBP measurements: (a) difference of SpO2 average values (X2-X1) (b) maximum deviations (according to Fig. 8(a)).
4.3.3. Influence of sex
The impact of sex (categorical variable) on difference SpO2 was calculated by using of statistic tests. The number of participants in each group (men, women), mean, standard deviation and median of difference SpO2 are included in the Fig. 11 .
Fig. 11.
Influence of sex on SpO2 difference.
For a statistical comparison of those groups we first calculated the probability of normal distribution using the Lilliefors test. In result, the data did not constitute a normal distribution. Therefore, for two groups the Mann-Whitney test was used. These two groups did not differ statistically with the p-value 0.79.
4.3.4. Influence of other variables
The Pearson’s correlation coefficient r between SpO2 difference and other continuous variables was examined. Results are presented in Table 2 .
Table 2.
Summary comparison of difference SpO2 for other continuous variables.
| Variable | r | RMSE [%] |
|---|---|---|
| Age | 0.06 | 1.49 |
| Height | 0.00 | 1.50 |
| Weight | 0.08 | 1.49 |
| BMI | 0.10 | 1.49 |
| HR | –0.09 | 1.49 |
| SBP | –0.20 | 1.47 |
| DBP | –0.11 | 1.49 |
| MAP | –0.17 | 1.48 |
| PP | –0.16 | 1.48 |
Obtained r with age, height, weight, BMI, HR, systolic and diastolic blood pressure, mean arterial pressure and pulse pressure indicates a lack of dependency on the SpO2 difference. The root mean square error (RMSE) remains around 1.47–1.50.
4.4. Discussion
Pulse oximeters are well known and commonly-used devices. However, they are very sensitive to measurement site conditions as well other factors and artifacts. For this reason it is difficult to obtain a signal of good quality. With the COVID-19 pandemic and the popularity of in-home measurements, knowledge of the SpO2 measurement artifact is essential. In the hospital, SpO2 is often measured simultaneously with other vital parameters and, very often, continuously. One example is the measurement of blood pressure the common use of the cuff NIBP technique. Considering this, the study of influence of cuff pressure on blood vessels on arterial oxygen saturation is both useful and needed. The study of NIBP influence on SpO2 results is discussed in the papers [9], [13], [14]. However, these studies focused on failure times [9] of pulse oximeters during NIBP measurements, detection of a systolic pressure threshold for reliable SpO2 readings [13] and during cuff-inducted hypoperfusion [14]. In our work we provide information on how the NIBP measurement affects the momentary SpO2 results. Such tests were possible thanks to the simultaneous measurement of NIBP with a fully controllable OEM device (NIBScan module) and a custom-made SpO2 device. Thus, there were no delays in SpO2 calculation during NIBP measurements. In commercial pulse oximeters (e.g. used in [9], [13], [26]), the results presented are averaged, which takes even to a dozen or so seconds, so measurements of this type are difficult. However, we did not precisely investigate the phases (inflation, constant pressure and deflation) of cuff pressure operations. In addition, the impact on SpO2 results may vary depending on the device used for NIBP measurements. The reason for this can be different algorithms of inflation and deflation phases used in commercial blood pressure measuring devices. This is the limitation of this work.
In this paper data collected from the PPG finger probe during a 15-minute test with simultaneously several (from 3 to 5) NIBP measurements were used. Accurate, up to one second, the start and end of the NIBP measurement had been determined. Moreover, SpO2 values were determined with a delay of up to one second and an accuracy of 0.1% from the recorded raw RED and IR diode PPG signals based on Formula (2). Considering the obtained results, with the large group of volunteers of different ages and both sexes taking part in the study, every NIBP measurement had an influence on oxygen saturation. The important factor influencing the results is the way the cuff-based pressure device works. Various duration of pressure measurement, caused e.g. by adding air to the cuff, can also influence the results. On the average, a subject's SpO2 measurements overstated the results by 1.4% for all the subjects, but the maximum changes were as high as −4.27% and +7.16% (Fig. 10(a)). Moreover, if each of the momentary (beat-to-beat) SpO2 measurements is treated independently, then the mean of momentary overstatements is 2.87% and the maximum changes are −17.43% and +20.42%, respectively (Fig. 10(b)). The maximum perfusion index momentary changes can be up to +22% and drops by up to −26% (Fig. 8(b)). During the NIBP measurement, the value of the perfusion index usually dropped, but it also increased in some cases. An inaccurate SpO2 readout value and the perfusion index may be particularly misleading for the user or medical staff. The study showed, that change of blood oxygen saturation was not correlated with sex, age, height, weight, BMI, HR and blood pressure (Fig. 11 and Table 2).
Our findings are slightly different than those in other similar studies. Hinkelbein et al. [13] compared the results of oxygen saturation from pulse oximeter and arterial blood gas analysis during blood pressure measurement. They have obtained up to 45% lower results from pulse oximeter than from reference. However, it should be noted that only mechanically ventilated and critically ill patients with a reduced SBP were examined. Also the number of subjects was only of 25. Moreover, the study [13] was mainly concerned with establishing the low SBP threshold for reliable reading of SpO2. Furthermore, the range of testing SBP in the study [13] it was (25–101) mmHg and in our work (94–194) mmHg. Values close to 25 mmHg are critically low. It should be noted that our results also show a decrease of SpO2 (maximum to −17.43%, Fig. 10b), but for a smaller number of readings. In another similar work, Kyriacou et al. [14] conducted research on commercial (transmittance) and custom made (reflectance) pulse oximeters. They indicated that both oximeters showed gradual decrease of saturations during cuff-induced hypoperfusion. However, in the work [14] a small group (only 14) of healthy male and most of the young volunteers was examined. Furthermore, the above-mentioned study lacks information on the applied methods of calculating SpO2, averaging and the operating of the pulse oximeter at low perfusion. Although the results obtained in the cited works give a slightly different conclusion, it should be noted that the study group was significantly smaller and different than ours. In addition, our results also show many decreases in SpO2 but, nevertheless, more frequent increases.
5. Conclusions
The pressure on blood vessels which is caused by blood pressure monitor cuff significantly reduces the perfusion index level and causes incorrect (usually overstated) reading of SpO2. Momentary changes in SpO2 can reach ±17% and are positive in most cases (mean 2.9%). The obtained results show that frequent NIBP measurements or other long-persistent pressure on the vessels may lead to wrong conclusions about increase of oxygen saturation. In turn, an increase in SpO2 means improved health. Thus, situations may arise in which we misinterpret an inaccurate value in measured blood oxygenation. This is important in respiratory monitoring, especially for people suffering from “silent hypoxemia” caused by the COVID-19.
Funding
This work was supported by the Military University of Technology under Grant GBMON/13-996/2018/WAT.
CRediT authorship contribution statement
Tadeusz Sondej: Conceptualization, Methodology, Software, Validation, Formal analysis, Investigation, Resources, Data curation, Writing – original draft, Writing – review & editing, Visualization, Project administration, Supervision, Funding acquisition. Sylwia Zawadzka: Software, Validation, Formal analysis, Investigation, Resources, Writing – original draft, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Chan E.D., Chan M.M., Chan M.M. Pulse oximetry: understanding its basic principles facilitates appreciation of its limitations. Respir. Med. 2013;107(6):789–799. doi: 10.1016/j.rmed.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 2.Nitzan M., Romem A., Koppel R. Pulse oximetry: fundamentals and technology update. Med. Devices (Auckl) 2014;7:231–239. doi: 10.2147/MDER.S47319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jubran A. Pulse oximetry. Crit. Care. 2015;19(1) doi: 10.1186/s13054-015-0984-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simonson T.S., Baker T.L., Banzett R.B., Bishop T., Dempsey J.A., Feldman J.L., Guyenet P.G., Hodson E.J., Mitchell G.S., Moya E.A., Nokes B.T., Orr J.E., Owens R.L., Poulin M., Rawling J.M., Schmickl C.N., Watters J.J., Younes M., Malhotra A. Silent hypoxaemia in COVID-19 patients. J. Physiol. 2021;599(4):1057–1065. doi: 10.1113/tjp.v599.410.1113/JP280769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chauhan A., Kaur R., Chakrbarti P., Pal A. “Silent hypoxemia” leads to vicious cycle of infection, coagulopathy and cytokine storm in COVID-19: can prophylactic oxygen therapy prevent it? Indian J. Clin. Biochem. 2021;36(4):468–472. doi: 10.1007/s12291-021-00967-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jouffroy R., Jost D., Prunet B. Prehospital pulse oximetry: a red flag for early silent hypoxemia in COVID-19 patients. Crit. Care. 2020;24:313. doi: 10.1186/s13054-020-03036-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schrading W.A., McCafferty B., Grove J., Page D.B. Portable, consumer-grade pulse oximeters are accurate for home and medical use: implications for use in the COVID-19 pandemic and other resource-limited environments. J. Am. Coll. Emerg. Physicians Open. 2020;1(6):1450–1458. doi: 10.1002/emp2.v1.610.1002/emp2.12292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith R.N., Hofmeyr R. Perioperative comparison of the agreement between a portable fingertip pulse oximeter v. a conventional bedside pulse oximeter in adult patients (COMFORT trial) S. Afr. Med. J. 2019;109:154–158. doi: 10.7196/SAMJ.2019.v109i3.13633. [DOI] [PubMed] [Google Scholar]
- 9.Kawagishi T., Kanaya N., Nakayama M., Kurosawa S., Namiki A. A comparison of the failure times of pulse oximeters during blood pressure cuff-induced hypoperfusion in volunteers. Anesthesia & Analgesia. 2004;99(3):793–796. doi: 10.1213/01.ANE.0000130343.66453.37. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization, Using the Pulse Oximeter, https://www.who.int/patientsafety/safesurgery/pulse_oximetry/who_ps_pulse_oxymetry_tutorial2_advanced_en.pdf?ua=1, 2011 (accessed 12.04.2021).
- 11.Tamura T. Current progress of photoplethysmography and SPO2 for health monitoring. Biomed. Eng. Lett. 2019;9(1):21–36. doi: 10.1007/s13534-019-00097-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seifi S., Khatony A., Moradi G., Abdi A., Najafi F. Accuracy of pulse oximetry in detection of oxygen saturation in patients admitted to the intensive care unit of heart surgery: comparison of finger, toe, forehead and earlobe probes. BMC Nurs. 2018;17:15. doi: 10.1186/s12912-018-0283-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hinkelbein J., Genzwuerker H.V., Fiedler F. Detection of a systolic pressure threshold for reliable readings in pulse oximetry. Resuscitation. 2005;64(3):315–319. doi: 10.1016/j.resuscitation.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 14.Kyriacou P.A., Shafqat K., Pal S.K. Pilot investigation of photoplethysmographic signals and blood oxygen saturation values during blood pressure cuff-induced hypoperfusion. Measurement. 2009;42(7):1001–1005. doi: 10.1016/j.measurement.2009.02.005. [DOI] [Google Scholar]
- 15.Plüddemann A., Thompson M., Heneghan C., Price C. Pulse oximetry in primary care: primary care diagnostic technology update. Br. J. Gen. Pract. 2011;61(586):358–359. doi: 10.3399/bjgp11X572553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mendelson Y. Pulse oximetry: theory and applications for noninvasive monitoring. Clin. Chem. 1992;38:1601–1607. doi: 10.1093/clinchem/38.9.1601. [DOI] [PubMed] [Google Scholar]
- 17.Allen J. Photoplethysmography and its application in clinical physiological measurement. Physiol. Meas. 2007;28(3):R1–R39. doi: 10.1088/0967-3334/28/3/R01. [DOI] [PubMed] [Google Scholar]
- 18.Tamura T., Maeda Y., Sekine M., Yoshida M. Wearable photoplethysmographic sensors—past and present. Electronics. 2014;3(2):282–302. doi: 10.3390/electronics3020282. [DOI] [Google Scholar]
- 19.Sondej T., Różanowski K., Sondej D. Reflectance method for a wearable photoplethysmographic sensor – experimental evaluation. Pol. J. Aviat. Med. Bioeng. Psychol. 2017;22(1):5–20. doi: 10.13174/pjambp.30.12.2016.01. [DOI] [Google Scholar]
- 20.Song S., Jiang F., Hao L., Xu L., Yi X., Li G., Lin L. Use of bi-level pulsed frequency-division excitation for improving blood oxygen saturation precision. Measurement. 2018;129:523–529. doi: 10.1016/j.measurement.2018.07.076. [DOI] [Google Scholar]
- 21.de Fatima Galvao Rosa A., Betini R.C. Noncontact SpO2 measurement using Eulerian video magnification. IEEE Trans. Instrum. Measur. 2020;69(5):2120–2130. doi: 10.1109/TIM.2019.2920183. [DOI] [Google Scholar]
- 22.Clarke G.W.J., Chan A.D.C., Adler A. Effects of motion artifact on the blood oxygen saturation estimate in pulse oximetry. IEEE MeMeA. 2014:1–4. doi: 10.1109/MeMeA.2014.6860071. [DOI] [Google Scholar]
- 23.Nabavi S., Bhadra S. A Robust Fusion Method for Motion Artifacts Reduction in Photoplethysmography Signal. IEEE Trans. Instrum. Measur. 2020;69(12):9599–9608. doi: 10.1109/TIM.2020.3006636. [DOI] [Google Scholar]
- 24.Sjoding M.W., Dickson R.P., Iwashyna T.J., Gay S.E., Valley T.S. Racial bias in pulse oximetry measurement. N. Engl. J. Med. 2020;383(25):2477–2478. doi: 10.1056/NEJMc2029240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.K.D. Torp, P. Modi, L.V. Simon, Pulse Oximetry, StatPearls Publishing, 2021 https://pubmed.ncbi.nlm.nih.gov/29262014/ (accessed 09.04.2021). [PubMed]
- 26.Hakverdioğlu Yönt G., Akin Korhan E., Dizer B. The effect of nail polish on pulse oximetry readings. Intensive & Crit Care Nurs. 2014;30(2):111–115. doi: 10.1016/j.iccn.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 27.Yek J.L.J., Abdullah H.R., Goh J.P.S., Chan Y.W. The effects of gel-based manicure on pulse oximetry. Singapore Med. J. 2019;60:432–435. doi: 10.11622/smedj.2019031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Piñero A., Illana J., García-Palenciano C., Cañizarese F., Canteras M., Cañadillas V., Durán E., Parilla P. Effect on oximetry of dyes used for sentinel lymph node biopsy. Arch. Surg. 2004;139:1204–1207. doi: 10.1001/archsurg.139.11.1204. [DOI] [PubMed] [Google Scholar]
- 29.Sondej T., Sieczkowski K., Olszewski R., Dobrowolski A. Simultaneous multi-site measurement system for the assessment of pulse wave delays. Biocybern. Biomed. Eng. 2019;39(2):488–502. doi: 10.1016/j.bbe.2019.01.001. [DOI] [Google Scholar]
- 30.Li K., Warren S. A wireless reflectance pulse oximeter with digital baseline control for unfiltered photoplethysmograms. IEEE T. BIOMED. CIRC. S. 2012;6(3):269–278. doi: 10.1109/TBCAS.2011.2167717. [DOI] [PubMed] [Google Scholar]
- 31.Liu S.-H., Liu H.-C., Chen W., Tan T.-H. Evaluating quality of photoplethymographic signal on wearable forehead pulse oximeter with supervised classification approaches. IEEE Access. 2020;8:185121–185135. doi: 10.1109/Access.628763910.1109/ACCESS.2020.3029842. [DOI] [Google Scholar]
- 32.Hemon M.C., Phillips J.P. Comparison of foot finding methods for deriving instantaneous pulse rates from photoplethysmographic signals. J. Clin. Monit. Comput. 2016;30(2):157–168. doi: 10.1007/s10877-015-9695-6. [DOI] [PubMed] [Google Scholar]
- 33.Davies H.J., Williams I., Peters N.S., Mandic D.P. In-Ear SpO2: A Tool for Wearable, Unobtrusive Monitoring of Core Blood Oxygen Saturation. Sensors (Basel). 2020;20:4879. doi: 10.3390/s20174879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Masimo Corporation, Clinical Applications of Perfusion Index, https://www.masimo.co.uk/siteassets/uk/documents/pdf/clinical-evidence/whitepapers/lab3410f_whitepapers_perfusion_index.pdf, 2007 (accessed 13.04.2021).