Abstract
Persistent myofibroblast activation distinguishes pathological fibrosis from physiological wound healing, suggesting that therapies selectively inducing myofibroblast apoptosis could prevent progression and potentially reverse established fibrosis in diseases such as scleroderma, a heterogeneous autoimmune disease characterized by multiorgan fibrosis. We demonstrate that fibroblast-to-myofibroblast differentiation driven by matrix stiffness increases the mitochondrial priming (proximity to the apoptotic threshold) of these activated cells. Mitochondria in activated myofibroblasts, but not quiescent fibroblasts, are primed by death signals such as the proapoptotic BH3-only protein BIM, which creates a requirement for tonic expression of the antiapoptotic protein BCL-XL to sequester BIM and ensure myofibroblast survival. Myofibroblasts become particularly susceptible to apoptosis induced by “BH3 mimetic” drugs inhibiting BCL-XL such as ABT-263. ABT-263 displaces BCL-XL binding to BIM, allowing BIM to activate apoptosis on stiffness-primed myofibroblasts. Therapeutic blockade of BCL-XL with ABT-263 (navitoclax) effectively treats established fibrosis in a mouse model of scleroderma dermal fibrosis by inducing myofibroblast apoptosis. Using a BH3 profiling assay to assess mitochondrial priming in dermal fibroblasts derived from patients with scleroderma, we demonstrate that the extent of apoptosis induced by BH3 mimetic drugs correlates with the extent of their mitochondrial priming, indicating that BH3 profiling could predict apoptotic responses of fibroblasts to BH3 mimetic drugs in patients with scleroderma. Together, our findings elucidate the potential efficacy of targeting myofibroblast antiapoptotic proteins with BH3 mimetic drugs in scleroderma and other fibrotic diseases.
INTRODUCTION
Activated myofibroblasts orchestrate tissue repair by integrating complex signals from the wound microenvironment. Although the mechanisms driving myofibroblast activation have received much attention, less is known about the resolution of the repair program, which likely involves apoptosis of activated myofibroblasts or reversion of these cells to a quiescent phenotype (1-5). Failure of myofibroblasts to undergo apoptosis or revert to a quiescent phenotype may contribute to the excessive production, remodeling, and cross-linking of extracellular matrix (ECM) that characterizes progressive fibrotic diseases (6-8) and drives disease progression to end-organ failure and death (7). A major insight into tissue repair and fibrosis pathogenesis has been the discovery that the biophysical properties of tissues undergoing repair, increased matrix stiffness, can participate in a positive feedback loop with myofibroblast activation and persistence (9, 10). In this loop, increased matrix stiffness promotes myofibroblast differentiation and activation (9). The increased deposition and cross-linking of ECM proteins produced by these activated myofibroblasts further increases matrix stiffness, completing this amplification loop. However, how this amplification loop is terminated during normal wound healing but persists in progressive fibrotic disease is unclear. We and others have recently demonstrated that interrupting mechanical signaling pathways (mechanotransduction) reduces tissue fibrosis and induces apoptosis of stiffness-activated myofibroblasts (6, 11-15). In contrast, inhibition of mechanotransduction pathways in nonactivated fibroblasts in normal tissue environments does not induce their apoptosis. These findings imply that stiffness-activated myofibroblasts, traditionally viewed as apoptosis-resistant cells, depend on continual mechanical signals for survival. The mechanistic basis for this phenomenon is largely unknown.
Matrix stiffness induces fibroblast-to-myofibroblast differentiation by modulating the activity of fibroblast integrins, which transmit mechanical force from the matrix to the actin cytoskeleton through multiprotein complexes called focal adhesions. Focal adhesion–associated proteins such as focal adhesion kinase (FAK) initiate biochemical signaling that stimulates Rho-associated protein kinase (ROCK)–dependent actin remodeling. Stiffness-induced actin polymerization drives nuclear translocation of two sets of transcriptional coactivators: myocardin-related transcription factor-A (MRTF-A) and MRTF-B, and yes-associated protein (YAP) and transcriptional coactivator with PDZ-binding motif (TAZ) (16, 17). When nuclear, these coactivators induce profibrotic gene expression that drives fibroblast-to-myofibroblast differentiation (18, 19). Recent data showed that target genes regulated by matrix stiffness also include the BCL-2 gene (11). BCL-2 encodes the protein BCL-2 (B cell leukemia/lymphoma 2), which belongs to the BCL-2 family of proteins and has previously been described as a major regulator of the mitochondrial pathway of apoptosis. The BCL-2 protein family members are divided by function into four subfamilies: effector, activator, antiapoptotic, and sensitizer proteins (20, 21). Structurally, BCL-2 family members contain one or more of the four conserved BCL-2 homology (21, 22) domains, designated BH1, BH2, BH3, and BH4. Activation of the proapoptotic multidomain “effectors” BAK and BAX initiates apoptosis through the pore-forming activities of these proteins, which induce mitochondrial outer membrane permeabilization (MOMP). MOMP results in the release of caspase-activating factors such as cytochrome c into the cytoplasm, leading to cell death. BAX and BAK activation requires the binding of the proapoptotic BH3-only “activator” protein BIM or BID. More recently, the BH3-only protein PUMA has also been shown to have activator functions (23). The ability of BAX and BAK to produce MOMP can be prevented by “antiapoptotic” multidomain BCL-2 family members, such as BCL-2 itself, BCL-XL, BCL-W, MCL-1, and A1 (Bfl-1), which can directly bind and block both effector and activator proteins. Last, a fourth subset of BCL-2 proteins, BH3-only “sensitizers” including BAD, NOXA, HRK, PUMA, and BMF, indirectly promote apoptosis by binding antiapoptotic family members and displacing activator proteins from them. The freed activators can then initiate apoptosis by activating BAX and BAK. The observation that survival of stiffness-activated myofibroblasts is dependent on continual mechanotransduction suggests that pro- and antiapoptotic BCL-2 family members are differentially regulated during fibroblast-to-myofibroblast differentiation. Thus, we hypothesize that the expression of proapoptotic BCL-2 family members is increased during myofibroblast differentiation driven by matrix stiffness, a mechanism that would predispose myofibroblasts to be rapidly eliminated upon completion of tissue repair. As a result, stiffness-activated myofibroblasts are exquisitely sensitive to death after a decrease in prosurvival mechanical signaling (11). Such a system would explain the dependence of stiffness-activated myofibroblasts on continuous mechanotransduction. This model provides a mechanism for the resolution of tissue repair. Furthermore, dysregulation of this system may lead to persistence of activated myofibroblasts, which contributes to progressive tissue fibrosis.
We have previously defined the term “mitochondrial priming” as a measure of the proximity of mitochondria to the threshold of apoptosis (24-26). The closer a cell’s mitochondria are to the activation threshold that initiates permeabilization of the outer mitochondrial membrane, the more primed that cell is. The balance between proapoptotic and antiapoptotic BCL-2 family members thus defines a cell’s mitochondrial priming. Mitochondria loaded with proapoptotic proteins are more predisposed toward apoptosis, a cellular state we call “primed for death” (27). Cells primed for death are highly dependent on the expression and function of specific antiapoptotic BCL-2 family members to block pro-death signaling and ensure their survival (25), and blockade of these antiapoptotic protein(s) in highly primed cells results in a rapid induction of apoptosis (24, 28, 29). Using BH3 profiling, a functional assay that we developed to measure cell mitochondrial priming (25, 30), here, we demonstrate that fibroblast-to-myofibroblast differentiation driven by matrix stiffness increases overall mitochondrial apoptotic priming (proximity to apoptosis) in these activated cells, which become primed for death and “addicted” to BCL-XL expression to ensure survival. Therapeutic blockade of BCL-XL by the BH3 mimetic drug ABT-263 (navitoclax) induced myofibroblast apoptosis and effectively treated established dermal fibrosis in a mouse model of scleroderma. Our studies also demonstrate that the extent of mitochondrial priming assessed by BH3 profiling predicts apoptotic responses to BH3 mimetic drugs in fibrotic fibroblasts from patients with scleroderma. Together, our findings elucidate the potential therapeutic efficacy of targeting myofibroblast antiapoptotic proteins with BH3 mimetic drugs in scleroderma and other fibrotic diseases.
RESULTS
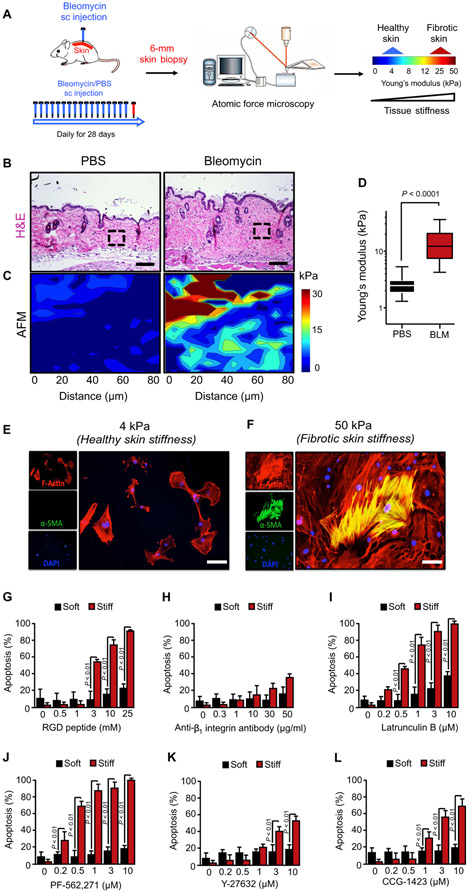
Fibrosis increases dermal stiffness in a mouse model of scleroderma
We and others have shown that inhibiting the ability of activated myofibroblasts to sense or respond to physical signals delivered to them by stiff conditions in vitro induces their apoptosis (9, 11, 31). To confirm the relevance of these findings in the development of dermal fibrosis in vivo, we first measured dermal tissue stiffness in a mouse model of scleroderma fibrosis induced by subcutaneous injections of bleomycin (32). The development of dermal fibrosis in this model is noted by increased dermal thickness and hydroxyproline content (33, 34). To characterize the changes in tissue stiffness associated with the development of dermal fibrosis, we used atomic force microscopy (AFM) microindentation to map local elastic properties of thin slices of fresh mouse skin samples, harvested after 28 daily subcutaneous injections of either phosphate-buffered saline (PBS) or bleomycin (Fig. 1, A and B). Elastographs from AFM microindentation mapping of 80 × 80 μm areas demonstrated marked differences in the range and distribution of tissue stiffness in the dermis of fibrotic skin compared to control (Fig. 1, C and D). Saline-challenged skin had a median Young’s modulus of 2.45 kPa (n = 695, from five PBS-treated mice), with few areas demonstrating stiffness of >3 kPa. Bleomycin-challenged skin exhibited an overall increase in tissue Young’s modulus to a median of 14.20 kPa (n = 1339, from five bleomycin-treated mice), with localized increases in stiffness to >30 kPa in the skin dermis (Fig. 1D).
Fig. 1. Matrix stiffness sensitizes myofibroblasts to apoptosis induced by inhibition of their mechanotransduction pathways.
(A) Schematic diagram of atomic force microscopy (AFM) for microindentation on skin tissues. AFM was applied to map local elastic properties of thin slices of fresh mouse skin samples, harvested after 28 daily subcutaneous (sc) injections of either saline or bleomycin (BLM). (B) Representative hematoxylin and eosin (H&E)–stained images from 28-day saline-versus bleomycin-treated mice. Scale bars, 100 μm. n = 6 for all groups. (C) Representative elastographs from AFM microindentation of tissue stiffness in normal and fibrotic skin. The color bar indicates Young’s modulus, which is quantified in (D). Maps were made from tissue in the respective regions of interest (boxes) identified in (B). Data are means ± SD of stiffness measurements pooled from five animals each for normal and fibrotic groups in two independent bleomycin injection experiments. P value was determined by Student’s t test. (E and F) Effect of matrix stiffness on α-smooth muscle actin (α-SMA) protein expression as assessed by immunofluorescence. Human dermal fibroblasts (HDFs) were cultured on collagen-coated polyacrylamide hydrogels that recapitulated the stiffness of normal (4 kPa) (E) or densely fibrotic (50 kPa) (F) skin for 24 hours. Myofibroblasts were identified by staining for α-SMA (green). Fibroblasts were costained with phalloidin (red) to visualize F-actin and 4′,6-diamidino-2-phenylindole (DAPI; blue) to visualize nuclei. Scale bars, 50 μm. (G to L) Effect of matrix stiffness on the susceptibility of primary HDFs to apoptosis induced by inhibition of mechanotransduction pathways. HDFs were cultured on collagen-coated polyacrylamide hydrogels that recapitulated the stiffness of normal (4 kPa) or densely fibrotic (50 kPa) skin for 24 hours and treated with or without the agents indicated for an additional 48 hours. Apoptosis was assessed by annexin V staining. Data are means ± SD from three independent experiments. P value was determined by Student’s t test.
Increased matrix stiffness sensitizes myofibroblasts to apoptosis induced by inhibition of mechanotransduction pathways
To examine the effects of augmented matrix stiffness on susceptibility of primary human dermal fibroblasts (HDFs) to apoptosis induced by inhibition of mechanotransduction, we cultured these cells on collagen-coated polyacrylamide hydrogels of 4 kPa (soft) and 50 kPa (stiff) Young’s modulus, representing the stiffness observed in normal and fibrotic skin, respectively. Exposure of HDFs to increased stiffness induced their differentiation into myofibroblasts, characterized by increased α-smooth muscle actin (α-SMA) expression (Fig. 1, E and F), as we previously described (9). We then inhibited mechanotransduction in HDFs using a panel of agents to target different components of mechanotransduction pathways, including (i) RGD peptides and an anti–β1 integrin subunit blocking antibody to target adhesion molecules that bind the matrix, (ii) the FAK inhibitor PF-562,271 and the ROCK inhibitor Y-27632 to target mechanosensitive signaling enzymes, (iii) latrunculin B to target actin dynamics, and (iv) the MRTF inhibitor CCG-1423 to target actin polymerization–sensitive transcriptional coactivators. Each agent, except latrunculin B, has previously demonstrated efficacy in mouse models of fibrotic diseases in vivo (11, 14, 31, 35, 36), although the mechanisms of their antifibrotic activity in these models are not yet fully understood. We found markedly increased HDF apoptosis induced by each of these agents when cells were cultured on stiff matrix (Fig. 1, G to L). These results indicate that interruption of mechanotransduction pathways causes the apoptosis of myofibroblasts exposed to pathologically increased (50 kPa) but not physiologically normal (4 kPa) matrix stiffness.
Matrix stiffness induces myofibroblast mitochondrial priming for apoptosis
To elucidate the molecular mechanisms that increase the susceptibility of stiffness-exposed myofibroblasts to apoptosis induced by inhibition of mechanotransduction, we first investigated whether mechanotransduction inhibition induced apoptosis through the mitochondrial (intrinsic) or the receptor-dependent (extrinsic) pathway. Treatment of stiffness-exposed myofibroblasts with the pan-caspase inhibitor Z-VAD-FMK protected these cells from apoptosis induced by inhibition of FAK with PF-562,271 (fig. S1A). To investigate the intrinsic mitochondrial pathway, we treated HDFs with PF-562,271 and looked for the induction of MOMP. MOMP in cells can be inferred from collapse of their mitochondrial transmembrane potential (ΔΨm). PF-562,271 treatment of HDFs on stiff but not soft matrices induced MOMP (fig. S1B), indicating that inhibition of mechanotransduction in stiffness-exposed myofibroblasts activates the mitochondrial pathway of apoptosis. The mitochondrial pathway of apoptosis can also be activated through the extrinsic apoptotic pathway via caspase-8–mediated generation of truncated BID (tBid), an activator of BAX/BAK. Pharmacological inhibition of caspase-8 with emricasan showed no effect on the ability of PF-562,271 to induce MOMP and apoptosis in HDFs (fig. S1, A and B), indicating that inhibition of mechanotransduction induces apoptosis of stiffness-exposed myofibroblast by activating the mitochondrial (intrinsic) pathway of apoptosis.
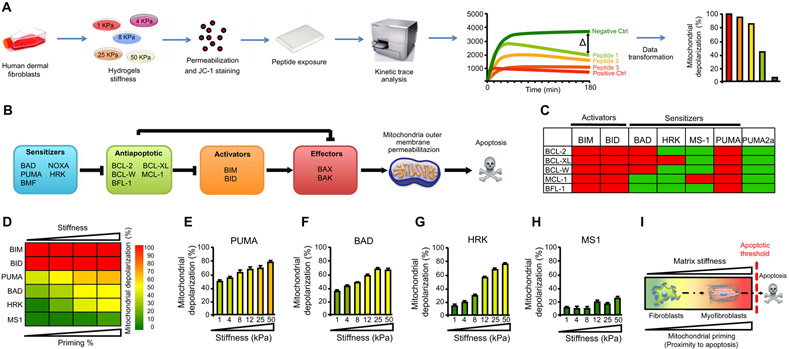
On the basis of these data, we hypothesized that increased susceptibility of stiffness-exposed myofibroblasts to apoptosis induced by inhibition of mechanotransduction is due to a high state of mitochondrial priming of these cells. BH3 profiling of HDFs cultured on hydrogels of defined stiffness ranging from 4 to 50 kPa measured the proximity of cells to the apoptotic threshold or their mitochondrial priming (24-26). In our BH3 profiling assay, HDFs are exposed to a panel of peptides derived from the BH3 domains of BCL-2 family activators or sensitizers, which mimic the proapoptotic activities of these BH3-only proteins (Fig. 2A). Different peptides in the panel give information on the different aspects of the mitochondrial pathway assessed based on the molecular interactions between the different BCL-2 protein subfamilies that control mitochondrial apoptosis (Fig. 2B). The predicted binding of the various peptides used in our BH3 profiling assay is summarized in Fig. 2C. Mitochondrial depolarization induced by these peptides is used as a surrogate indicator of MOMP in this assay and is measured with the fluorescent dye JC-1, which is sensitive to changes in the mitochondrial electropotential gradient across the inner mitochondrial membrane. We have previously shown that JC-1 fluorescence provides a good surrogate for MOMP (30) and that this fluorescence can track mitochondrial depolarization in cells using the proton ionophore carbonyl cyanide p-trifluoromethoxyphenylhydrazone (FCCP) as a positive control, which dissipates the mitochondrial transmembrane potential gradient in these cells.
Fig. 2. Increased mitochondrial apoptotic priming In stiffness-induced myofibroblasts.
(A) Schematic diagram of BH3 profiling assay. HDFs were cultured on matrices with different stiffnesses, permeabilized, and exposed to an array of peptides derived from different proapoptotic BH3-only proteins, and MOMP was measured indirectly using the mitochondrial JC-1 dye (fluorescent dye sensitive to the electropotential gradient across the inner mitochondrial membrane). The decay of DYm was measured as fluorescence at 590 nm over 180 min. (B) Schematic illustrating how BCL-2 family interactions govern MOMP. The terminal execution phase of apoptosis is controlled by BCL-2 family members BAX and BAK, which are required to initiate apoptosis by causing MOMP through their pore-forming activities. MOMP releases caspase-activating factors (cytochrome c) into the cytoplasm, which results in collapse of the DYm and caspase activation. MOMP activation is directly guarded by prosurvival proteins [BCL-2, BCL-XL, BCL-W, MCL-1, and A1 (Bfl-1)], which directly bind and block proapoptotic BAX/BAK. BH3-only activator proteins convey signals to initiate apoptosis by direct activation of BAX/BAK (BIM and BID) or indirectly by binding and inhibiting their prosurvival relatives (PUMA, BAD, NOXA, and HRK), which are known as sensitizers. (C) Interaction map for BH3 peptides and the antiapoptotic BCL-2 members. Red boxes indicate tight binding with a Kd (dissociation constant) of less than 100 nM;green boxes indicate weak or absent interaction, as determined by fluorescence polarization. Diagrams from (A) to (C) are adapted with permission from (23). (D) Heat map of mitochondrial depolarization induced by peptides derived from different proapoptotic BH3-only proteins. HDFs cultured for 48 hours on matrices with stiffness of 1, 4, 8, 25, and 50 kPa were subjected to BH3 profiling assay using BIM and BID (activate proapoptotic effectors BAX and BAK), PUMA (block prosurvival proteins), BAD (selective BCL-2, BCL-XL, and BCL-W sensitizer), and HRK (binds BCL-XL and MS1 and blocks MCL-1) peptides. The concentration of peptides in the assays was 100 μM except for MS1 (10 μM). Data are means ± SD of three replicate wells for each peptide. (E to H) Mitochondrial depolarization caused by the PUMA (E), BAD (F), HRK (G), and MS1 (H) peptides in HDFs cultured on matrices with increasing matrix stiffness. (I) Working model. Matrix stiffness–induced fibroblast-to-myofibroblast transformation increases mitochondrial priming.
The BH3 peptides derived from the activators BIM and BID directly bind and activate the proapoptotic effectors BAX and BAK (Fig. 2, B and C). If these effectors are present and functional in the cells being profiled, then saturating concentrations of BIM- and BID-derived peptides should induce complete mitochondrial depolarization. We found that BIM- and BID-derived peptides induced complete mitochondrial depolarization of HDFs at all stiffnesses tested (Fig. 2D), indicating that mitochondrial cell death effectors BAX and BAK are expressed and fully operational in these fibroblasts across this stiffness range.
To measure overall mitochondrial priming of HDFs cultured on different stiffnesses, we used a BH3 peptide derived from PUMA, which binds to and inhibits all BCL-2 antiapoptotic subfamily proteins but does not directly activate BAX or BAK in BH3 profiling assay. Because PUMA blocks all BCL-2 antiapoptotic proteins, the extent of mitochondrial depolarization induced by the PUMA-derived BH3 peptide de pends only on the amount of proapoptotic activator proteins (BIM or BID) expressed in these cells. In this context, antiapoptotic proteins are blocked by PUMA-derived BH3 peptide, and freed proapoptotic activators can now bind and activate effectors BAK and BAX to induce MOMP: the more mitochondrial depolarization induced by the PUMA-derived BH3 peptide, the higher the amount of proapoptotic activators in the studied mitochondria. In our studies, the PUMA-derived BH3 peptide induced increasing mitochondrial depolarization in HDFs cultured on increasingly stiff matrices, providing evidence that fibroblasts exposed to a stiff fibrotic microenvironment increase their mitochondrial priming (Fig. 2, D and E). Our results suggest that elevated matrix stiffness in tissue fibrosis induces fibroblast-to-myofibroblast differentiation, which increases overall mitochondrial apoptotic priming (proximity to apoptosis) in these activated cells, a state we call primed for death.
BCL-XL preserves mitochondria outer membrane integrity in stiffness-primed myofibroblasts
Our BH3 profiling assay can also assess which antiapoptotic proteins do cells that are primed for death become addicted to for their survival. To determine which antiapoptotic proteins protect stiffness-primed HDFs from apoptosis, we exposed these cells to BH3 peptides derived from the sensitizers BAD and HRK and the peptide MS1. In contrast to PUMA, BAD and HRK bind and block only certain antiapoptotic proteins: BAD binds BCL-2, BCL-XL, or BCL-W, whereas HRK binds only BCL-XL. The MS1 peptide specifically blocks MCL-1 (37). Consequently, BAD-derived peptides will induce mitochondrial depolarization of primed cells that depend on BCL-2, BCL-XL, and/or BCL-W, but not MCL-1 or Bfl-1, for their survival (Fig. 2C). In contrast, HRK-derived peptides will induce mitochondrial depolarization only in primed cells that depend on BCL-XL for their survival, and the peptide induces mitochondrial depolarization only in primed cells that are dependent on MCL-1 (Fig. 2C). Our results revealed that exposure to BAD peptides induced increasing mitochondrial depolarization as matrix stiffness increased, indicating that survival on stiff matrices is maintained by any combination of the three prosurvival members targeted by BAD (Fig. 2, D and F). When we exposed HDFs to HRK peptides, which inhibit BCL-XL but not BCL-2 or BCL-W, mitochondrial depolarization was induced on increasingly stiff matrices to the same extent as BAD peptides, indicating that BCL-XL rather than BCL-2 or BCL-W, is responsible for HDF survival on stiff matrices (Fig. 2, D and G). In contrast, treatment of HDFs with MS1 peptides had no effect on mitochondrial depolarization on any matrix stiffness tested (Fig. 2, D and H), indicating that MCL-1, the prosurvival target of MS1, did not contribute to survival of HDFs exposed to increased stiffness. Together, these results indicate that, as increasing matrix stiffness primes HDFs for death (Fig. 2I), these cells become specifically dependent on BCL-XL for their survival.
The BIM/BCL-XL axis regulates susceptibility of stiffness-primed myofibroblasts to apoptosis
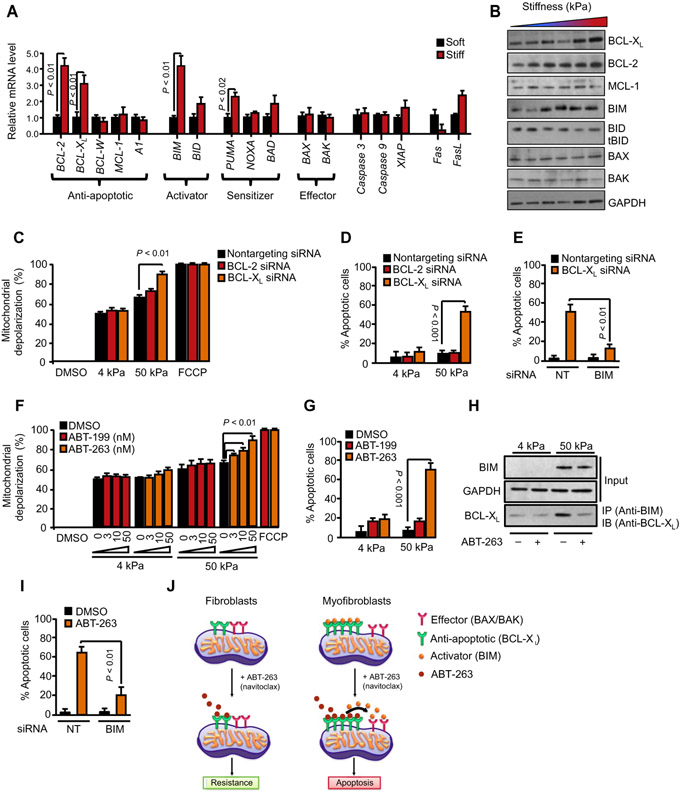
Our functional studies using BH3 profiling indicated that increased matrix stiffness increases mitochondrial priming in myofibroblasts and that these primed cells become dependent on BCL-XL to survive in stiff fibrotic tissues. To investigate the molecular determinants of increased mitochondrial priming produced by increased stiffness, we quantified the expression of apoptosis-related genes in HDFs that were plated on soft (4 kPa) or stiff (50 kPa) hydrogels. Our HDF expression profiling demonstrated that matrix stiffness did not increase mRNA and protein expression of the proapoptotic effector BAX or BAK (Fig. 3, A and B), suggesting that changes in the amount of these effector proteins do not contribute to priming. In contrast, high stiffness increased mRNA and protein expression of the proapoptotic BH3-only activator BIM (Fig. 3, A and B). We did not observed increased expression of activator BID mRNA or increased cleaved/truncated BID (tBID) protein with increasing stiffness, indicating that matrix stiffness does not activate the extrinsic pathway of apoptosis in myofibroblasts (Fig. 3, A and B). Together, these results suggest that matrix stiffness–induced BIM expression could be responsible for the increased mitochondrial priming observed in stiffness-exposed myofibroblasts.
Fig. 3. Pharmacological or genetic inhibition of BCL-XL induces apoptosis of stiffness-primed myofibroblasts.
(A) Effect of matrix stiffness on gene expression of BCL-2 family members in HDFs (n = 4 for each condition). Gene expression was analyzed by quantitative polymerase chain reaction (qPCR), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a housekeeping gene. Data are means ± SD from three independent experiments. P value was determined by Student’s t test. (B) Effect of matrix stiffness on BCL-2, BCL-XL, MCL-1, BIM, BID, BAX, and BAK protein expression as demonstrated by Western blotting. GAPDH was used as a loading control. (C and D) Effect of small interfering RNA (siRNA)–mediated BCL-2 or BCL-XL knockdown on mitochondrial priming (C) and apoptosis (D) of HDFs exposed to 4 or 50 kPa matrix stiffness. Nontargeting siRNA was used as control. Mitochondrial priming was determined by BH3 profiling using the PUMA-derived peptide (10 mM). Carbonyl cyanide p-trifluoromethoxyphenylhydrazone (FCCP) was used as a positive control for mitochondrial depolarization. Apoptosis was assessed by annexin V staining. Data are means ± SD from three independent experiments. P value was determined by Student’s t test. (E) Effect of siRNA-mediated BIM knockdown on BCL-XL knockdown–induced apoptosis of HDFs. Nontargeting siRNA was used as a control. Apoptosis was assessed by annexin V staining. Data are means ± SD from three independent experiments. P value was determined by Student’s t test. (F and G) Effect of BH3 mimetic drugs ABT-199 (BCL-2 inhibitor) and ABT-263 (BCL-XL inhibitor) on mitochondrial priming (F) and apoptosis (G) of HDFs exposed to 4 or 50 kPa matrix stiffness. Dimethyl sulfoxide (DMSO) was used as a control. Mitochondrial priming was determined by BH3 profiling using the PUMA-derived peptide (10 μM). FCCP is used as a positive control for mitochondrial depolarization. Apoptosis was assessed by annexin V staining. Data are means ± SD of three replicate wells for each peptide. P value was determined by Student’s t test. (H) BIM is sequestered by BCL-XL in stiffness-exposed myofibroblasts and displaced by antagonism of BCL-XL by ABT-263. Anti-BIM immunoprecipitation of whole–cell lysates (50 μg) from HDFs exposed to 4 or 50 kPa matrix stiffness for 24 hours and then treated with or without ABT-263 (1 μm) for 6 hours. BIM and BCL-XL were immunoblotted. (I) Effect of siRNA-mediated BIM knockdown on ABT-263–induced apoptosis of HDFs exposed to 50 kPa matrix stiffness. Nontargeting siRNA was used as a control. Apoptosis was assessed by annexin V staining. Data are means ± SD from three independent experiments. P value was determined by Student’s t test. (J) Model of ABT-263–induced myofibroblast apoptosis. Prosurvival BCL-XL is sequestered by pro-death BIM in stiffness-exposed myofibroblasts. Upon treatment with ABT-263, BIM is displaced and BCL-XL becomes occupied by ABT-263. Freed BIM then interacts with BAX or BAK, which initiates apoptosis in myofibroblasts.
Our HDF expression profiling further demonstrated that matrix stiffness increased mRNA and protein expression of the antiapoptotic proteins BCL-2 and BCL-XL, but not MCL-1, BCL-W, or A1 (Fig. 3, A and B). These results suggest that BCL-2 and/or BCL-XL up-regulation on stiff matrices could prevent the apoptosis of stiffness-primed fibroblasts. To distinguish the functional contributions of these two anti-apoptotic proteins to the survival of stiffness-primed HDFs, we blocked stiffness-induced BCL-2 or BCL-XL protein expression by small interfering RNA (siRNA). Western blots revealed greater than 90% reduction in target mRNA and protein expression after 48 hours of siRNA transfection (fig. S2). The effects of these knockdowns were then determined by BH3 profiling, using PUMA-derived peptides, to assess the extent of mitochondrial apoptotic priming in HDFs. For HDFs grown on 50 kPa matrices, BCL-XL knockdown markedly increased HDF mitochondrial depolarization in response to PUMA-derived peptides to 87%, compared to 64% of fibroblasts transfected with nontargeting control siRNA (Fig. 3C). In contrast, BCL-2 knockdown had no effect on HDF mitochondrial depolarization induced by PUMA-derived peptides compared with nontargeting control siRNA. For HDFs grown on 4 kPa matrices, neither BCL-XL nor BCL-2 knockdown had marked effects on HDF mitochondrial depolarization in response to PUMA-derived peptides. These results suggest that increased expression of BCL-XL prevents stiffness-primed myofibroblasts to undergo apoptosis.
To test this hypothesis, we measured apoptosis ofsiRNA-transfected HDFs after culture on soft (4 kPa) or stiff (50 kPa) matrices for 96 hours. For HDFs grown on 50 kPa matrices, BCL-XL knockdown markedly increased apoptosis to 56%, compared to 9% of HDFs transfected with nontargeting siRNA controls (Fig. 3D). In contrast, BCL-2 knockdown had no significant effect on HDF apoptosis on stiff matrices. For HDFs grown on 4 kPa matrices, neither BCL-XL nor BCL-2 knockdowns had significant effects on apoptosis. To demonstrate that BIM-mediated mitochondrial priming drives HDF apoptosis on stiff matrices, we cotransfected HDFs with siRNAs targeting BCL-XL (or nontargeting control) and the proapoptotic activator BIM (or nontargeting control). As demonstrated in Fig. 3E, BIM knockdown rescued HDFs on stiff matrices from the increased apoptosis induced by BCL-XL knockdown. Together, the BIM/BCL-XL axis regulates susceptibility of stiffness-primed myofibroblasts to apoptosis.
Inhibition of BCL-XL function with the BH3 mimetic drug ABT-263 induces apoptosis of stiffness-primed myofibroblasts
To complement our studies genetically knocking down BCL-XL expression in stiffness-primed myofibroblasts, we performed studies blocking BCL-XL function with BH3 mimetic drugs (small molecules designed to block the function of antiapoptotic BCL-2 proteins). We first tested the effects of ABT-263 (38), an orally bioavailable derivative of the prototype BH3 mimetic ABT-737, which binds to the hydrophobic BH3-binding groove of BCL-XL and blocks its ability to bind and sequester the BH3-only protein BIM (39). ABT-263 also blocks the antiapoptotic activities of BCL-2 and BCL-W. For HDFs grown on 50 kPa but not 4 kPa matrices, ABT-263 treatment markedly increased mitochondrial depolarization in response to PUMA-derived BH3 peptide, compared to fibroblasts treated with vehicle only, in a dose-dependent manner (Fig. 3F). In contrast, ABT-199, a BH3 mimetic that selectively inhibits only BCL-2 (40), had no marked effect on HDF mitochondrial depolarization induced by PUMA-derived BH3 peptide. Consistent with our knockdown experiments described above, ABT-263 markedly increased apoptosis assessed after 48 hours in HDFs grown on 50 kPa matrices to 71 ± 7%, compared to 7 ± 4% of HDFs treated with vehicle control (Fig. 3G). In contrast, ABT-199 had no marked effect on HDF apoptosis on stiff matrices. For HDFs grown on 4 kPa matrices, neither ABT-263 nor ABT-199 had marked effects on apoptosis of these cells. We and others have previously shown that the mechanism of action underlying ABT-263 proapoptotic activities relies on releasing BIM from complexes with BCL-XL and/or BCL-2 (24, 27). To further assess the mechanism of ABT-263 proapoptotic responses in myofibroblasts, we performed coimmunoprecipitation of BIM/BCL-XL complexes from whole-cell lysates extracted from HDFs cultured on soft (4 kPa) or stiff (50 kPa) matrices. ABT-263 treatment led to loss of BIM binding to BCL-XL in stiffness-exposed myofibroblasts, which is consistent with the notion that ABT-263 induces myofibroblast apoptosis by releasing proapoptotic BIM from BCL-XL (Fig. 3H). Accordingly, the increased apoptosis of stiffness-exposed HDFs induced by ABT-263 was abrogated by siRNA-mediated knockdown of BIM (Fig. 3I). Together, our data support a model where (i) increased stiffness of fibrotic tissues induces fibroblast-to-myofibroblast transformation, which primes these cells for death by increasing the expression of the proapoptotic activator BH3-only protein BIM; (ii) integrity of the mitochondrial outer membrane requires up-regulation of the antiapoptotic protein BCL-XL, which binds and inhibits BIM to ensure myofibroblast survival; and (iii) pharmacological blockade or knockdown of BCL-XL releases BIM, which directly binds and activates BAX and BAK to induce myofibroblast apoptosis on stiff, but not soft, matrices (Fig. 3J).
ABT-263 reduces dermal fibrosis and promotes myofibroblast apoptosis in vivo
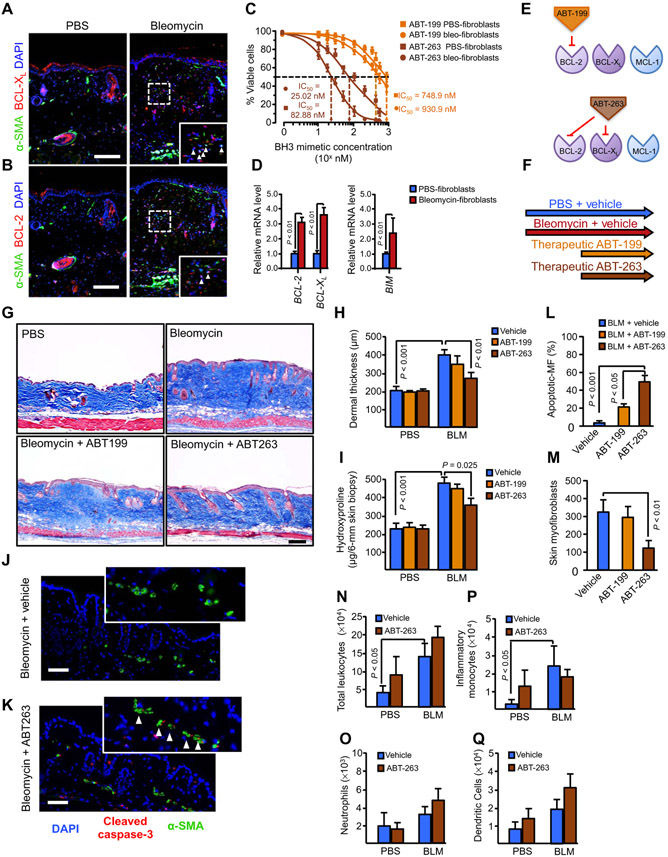
On the basis of our findings, we hypothesized that therapeutic targeting of BCL-XL with ABT-263 could treat established dermal fibrosis by inducing apoptosis of stiffness-primed myofibroblasts in vivo. Immunofluorescence staining revealed that myofibroblasts in the fibrotic skin of bleomycin-challenged mice had increased abundance of both BCL-XL and BCL-2 compared to saline-challenged controls (Fig. 4, A and B). Mouse dermal fibroblasts (MDFs) isolated from bleomycin-challenged skin demonstrated markedly increased sensitivity to apoptosis induced by ABT-263 compared to saline-treated skin (Fig. 4C). In contrast, the median inhibitory concentration (IC50) of ABT-199 for apoptosis induction was not significantly different for bleomycin-challenged versus saline-challenged MDFs. Quantitative polymerase chain reaction (qPCR) analysis confirmed that BIM and BCL-XL mRNA were up-regulated in MDFs isolated from bleomycin-challenged versus saline-challenged skin, consistent with increases in mitochondrial priming in these cells (Fig. 4D).
Fig. 4. Pharmacological BCL-XL inhibition attenuates bleomycin-induced skin fibrosis.
(A and B) Paraffin-embedded skin sections from mice treated with saline or bleomycin stained for BCL-2 or BCL-XL (red), α-SMA (green), and DAPI (blue) to visualize the expression of BCL-2 or BCL-XL, myofibroblasts, and nuclei, respectively. Arrows in higher-magnification views (insets, enlarged fivefold) denote overexpression of BCL-2 and BCL-XL in α-SMA + myofibroblasts in vivo. Scale bars, 100 μm. n = 4 for all groups. (C) Viability of mouse dermal fibroblasts (MDFs) explanted from saline- or bleomycin-treated mice (n = 3 per condition) and exposed to serial dilutions of either ABT-263 or ABT-199 for 48 hours. Data are means ± SD. P value was determined by Student’s t test. IC50, median inhibitory concentration. (D) Gene expression analysis of prosurvival and proapoptotic members in MDFs explanted from saline- or bleomycin-treated mice (n = 4 for each cell line). Gene expression was analyzed by qPCR, and GAPDH was used as a housekeeping gene. P value was determined by Student’s t test. (E) Schematic showing BH3 mimetics (small-molecule inhibitors that mimic the activity of proapoptotic BH3-only proteins, counteracting prosurvival proteins). ABT-737 and its orally bioavailable derivate ABT-263 (navitoclax) bind and neutralize the prosurvival proteins BCL-2 and BCL-XL but not MCL-1 or A1.ABT-199 is a highly potent, orally bioavailable BCL-2–selective inhibitor. (F to I) Therapeutic effects of BH3 mimetic drugs ABT-263 and ABT-199 on bleomycin-induced dermal fibrosis in vivo as assessed by Masson’s trichrome stain (G), dermal thickness (H), and hydroxyproline content (I). n = 8 for all groups. Graphs are means ± SD. P value was determined by two-way analysis of variance (ANOVA). Scale bar, 100 μm. (J and K) Paraffin-embedded skin sections from mice treated with bleomycin in combination with saline vehicle (J) or ABT-263 (K) stained for cleaved (active) caspase (red), α-SMA (green), and DAPI (blue) to visualize apoptotic cells, myofibroblasts, and nuclei, respectively. Arrows in higher-magnification views (enlarged fivefold) indicate dual cleaved caspase-3+ α-SMA+ myofibroblasts in vivo. n = 4 for all groups. Scale bars, 50 μm. (L and M) Quantification of number of total α-SMA+ myofibroblasts (MF) and apoptotic myofibroblasts (α-SMA+ caspase-3+) inskinsections from mice treated with bleomycin in combination with saline vehicle (L) or ABT-263 (M). Three hundred cells were counted per condition. Data are means ± SD (n = 4 for all groups). P value was determined by two-way ANOVA. (N to Q) Effect of ABT-263 or ABT-199 treatment on immune cell populations in mice treated with bleomycin or saline control, (N) total number of leukocytes, (O) neutrophils, (P) inflammatory monocytes, and (Q) dendritic cells. n = 4 for all groups. Graphs are means ± SD. P value was determined by two-way ANOVA.
We then examined the therapeutic potential of BH3 mimetic drugs to treat established fibrosis in the bleomycin dermal fibrosis model (Fig. 4, E and F). When administered orally once daily at 100 mg/kg in a therapeutic regimen beginning 14 days after the onset of daily bleomycin challenges, ABT-263 markedly reduced bleomycin-induced dermal fibrosis compared to vehicle treatment, as assessed at day 28 by trichrome staining of the skin for collagen, measurement of skin dermal thickness, and whole-skin hydroxyproline content (Fig. 4, G to I). Day 28 dermal thickness was reduced by 58% in bleomycin-challenged, ABT-263–treated mice compared to bleomycin-challenged, vehicle-treated mice; day 28 skin hydroxyproline was reduced by 46% with the ABT-263 treatment. In contrast, administration of ABT-199 demonstrated no marked effects on dermal fibrosis (Fig. 4, G to I).
To gain insight into the mechanism of action of ABT-263 in the dermal fibrosis model, we assessed myofibroblast apoptosis in situ by concurrent α-SMA and cleaved (active) caspase-3 staining of skin sections. Consistent with our in vitro studies, ABT-263 increased the number of caspase-3+ apoptotic myofibroblasts in fibrotic skin by 64%, reducing the total number of myofibroblasts by 74% (Fig. 4, J to M). In contrast, ABT-199 had no marked effects on dermal myofibroblast apoptosis or numbers (Fig. 4, L and M). To evaluate the effect of ABT-263 treatment on immune cells (neutrophils, inflammatory monocytes, and dendritic cells), which also play a pathological role in myofibroblast activation and fibrosis (41), we performed flow cytometry analysis of skin tissues from mice treated with or without ABT-263 after saline or bleomycin challenge. Our data showed that ABT-263 treatment did not affect the total number of leukocytes that increased in bleomycin-treated animals (Fig. 4N). There was no significant difference in the number of neutrophils, inflammatory monocytes, or dendritic cells that were present in the skin of bleomycin-challenged, ABT-263–treated mice compared to bleomycin-challenged, vehicle-treated mice (Fig. 4, O to Q). Together, these results suggest that ABT-263 promotes myofibroblast apoptosis in vivo without affecting the inflammatory response.
Forced expression of α-SMA is sufficient to increase mitochondrial priming in HDFs
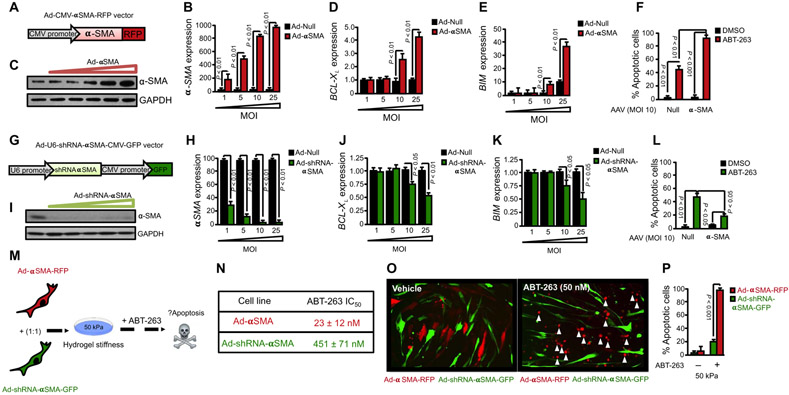
Our results indicate that mitochondrial priming is increased in stiffness-exposed myofibroblasts; however, it still remains unknown whether increased mitochondrial priming results from environmental stresses of the fibrotic tissue such as matrix stiffening or as a consequence of the phenotypic transformation from quiescent fibroblast to activated α-SMA–positive myofibroblasts driven by stiffness. We generated a nonreplicative recombinant adenovirus expressing human α-SMA fused to red fluorescent protein (Ad-α-SMA-RFP) to investigate whether forced expression of α-SMA is sufficient to prime HDFs for death. Adenovirus-mediated overexpression of α-SMA-RFP resulted in dose-dependent increase in α-SMA mRNA and protein expression compared to null adenovirus (Fig. 5, A to C). Forced expression of α-SMA markedly increased both BIM and BCL-XL mRNA expression, indicating that increased amount of the α-SMA protein is sufficient to augment mitochondrial priming in HDFs (Fig. 5, D and E). Accordingly, HDFs transduced with α-SMA-RFP–overexpressing adenovirus increased sensitivity of HDFs to ABT-263–induced apoptosis up to 73%, compared to 17% of HDFs transduced with null adenovirus [Fig. 5F; Ad-null ABT-263 IC50 = 389 ± 26 nM; Ad-α-SMA-RFP ABT-263 IC50 = 28 ± 17 nM]. We also investigated the converse, whether mitochondrial priming and sensitivity to ABT-263–induced apoptosis is modulated in α-SMA–negative fibroblasts, by generating a nonreplicative recombinant adenovirus expressing both short hairpin RNA (shRNA) against α-SMA and green fluorescent protein (Ad-U6-shRNA-α-SMA-CMV-GFP). Adenovirus-mediated overexpression of α-SMA-shRNA resulted in a dose-dependent decrease in α-SMA mRNA and protein expression compared to null adenovirus (Fig. 5, G to I). Accordingly, knockdown of α-SMA resulted in decreased BIM and BCL-XL mRNA expression, and decreased sensitivityto ABT-263–induced apoptosis of HDFs plated on stiff matrix (Fig. 5, J to L; Ad-null ABT-263 IC50 = 377 ± 57 nM; Ad-GFP-U6-α-SMA-shRNA ABT-263 IC50 = 480 ± 27 nM). To further analyze the role of α-SMA in controlling fibroblast mitochondrial priming, we investigated sensitivity to ABT-263–induced apoptosis within a heterogeneous population of fibroblasts composed of α-SMA–positive and α-SMA–negative cells. HDFs transduced with α-SMA-RFP–overexpressing adenovirus were mixed with HDFs transduced with α-SMA shRNA/GFP–overexpressing adenovirus before plating on stiff matrices (Fig. 5M). Treatment of mixed fibroblast cultures with ABT-263 (50 nM) resulted in marked apoptosis of α-SMA-RFP–overexpressing fibroblasts (95 ± 5%) compared to α-SMA shRNA/GFP–overexpressing fibroblasts (18 ± 9%) [Fig. 5, N to P (Ad-α-SMA-RFP ABT-263 IC50 = 23 ± 12 nM; Ad-shRNA-α-SMA/GFP ABT-263 IC50 = 451 ± 71 nM)]. Together, our data suggest that increased mitochondrial priming and sensitivity to ABT-263–induced apoptosis results from increased α-SMA expression in myofibroblasts, indicating that the phenotypic shift involved in fibroblast-to-myofibroblast differentiation induced by matrix stiffness is responsible for the decreased apoptotic threshold observed in myofibroblasts.
Fig. 5. Forced expression of α-SMA increases mitochondrial priming and sensitivity of dermal myofibroblasts to ABT-263–induced apoptosis.
(A) Schematic diagram of adenoviral vector design for overexpression of human α-SMA fused to red fluorescent protein (Ad-CMV-αSMA-RFP). (B and C) α-SMA mRNA (B) and protein (C) expression in HDFs transduced with either control null adenovirus or Ad-CMV-αSMA-RFP with increasing multiplicities of infection (MOIs). GAPDH was used as a housekeeping gene and loading control. Data are means ± SD from three independent experiments. P value was determined by Student’s t test. (D and E) Gene expression analysis of BCL-XL and BIM in HDFs transduced with either control null adenovirus or Ad-CMV-αSMA-RFP with increasing MOI. GAPDH was used as a housekeeping gene. Data are means ± SD from three independent experiments. P value was determined by Student’s t test. (F) Effect of ABT-263 (BCL-XL inhibitor; 300 nM) on apoptosis of HDFs transduced with Ad-Null or Ad-CMV-αSMA-RFP. Apoptosis was assessed by annexin V staining. Data are means ± SD from three independent experiments. P value was determined by Student’s t test. (G) Schematic diagram of adenoviral vector design for overexpression of green fluorescent protein (GFP) and shRNA against human α-SMA (Ad-U6-shRNA-αSMA-CMV-GFP). (H and I) α-SMA mRNA and protein expression in HDFs transduced with either control null adenovirus or Ad-U6-shRNA-αSMA-CMV-GFP with increasing MOI. α-SMA expression was quantified by qPCR and Western blotting. GAPDH was used as a housekeeping gene and loading control. Data are means ± SD from three independent experiments. P value was determined by Student’s t test. (J and K) Gene expression analysis of BCL-XL (J) and BIM (K) in HDFs transduced with either control null adenovirus or Ad-U6-shRNA-αSMA-CMV-GFP with increasing MOI. GAPDH was used as a housekeeping gene. Data are means ± SD from three independent experiments. P value was determined by Student’s t test. (L) Effect of ABT-263 (300 nM) on apoptosis of HDFs transduced with Ad-Null or Ad-CMV-αSMA-RFP. Apoptosis was assessed by annexin V staining. Data are means ± SD from three independent experiments. P value was determined by Student’s t test. (M) Schematic showing experimental design. HDFs were transduced with Ad-CMV-αSMA-RFP or Ad-U6-shRNA-αSMA-CMV-GFP, and then cells were mixed at a 1:1 ratio and plated on 50 kPa hydrogels for 24 hours. Cells were treated with ABT-263 for 12 hours before assessment of cell apoptosis. (N) Table showing calculation of ABT-263 IC50 for each cell line. Data are means ± SD from three independent experiments. (O) Representative image of ABT-263–induced apoptosis of fibroblast transduced with either Ad-CMV-αSMA-RFP or Ad-U6-shRNA-αSMA-CMV-GFP as assessed by fluorescence live cell imaging. White arrows indicate apoptotic cells. (P) Quantification of cell apoptosis in (O) by annexin V staining. Data are means ± SD from three independent experiments. P value was determined by Student’s t test.
Mechanosensitive YAP/TAZ signaling controls BCL-XL expression in stiffness-primed myofibroblasts
We and others have previously shown that myofibroblast differentiation induced by matrix stiffness is controlled by mechanosensitive transcriptional coactivators YAP and TAZ (18, 42). In culture, matrix stiffness drives accumulation of YAP/TAZ in the nuclei of human fibroblasts, which is required to induce the expression of profibrotic genes including α-SMA. A recent study has shown that YAP signaling promotes human lung cancer cell survival by up-regulating BCL-XL protein expression in these cells (43); thus, we reasoned that mechanosensitive YAP/TAZ signaling may similarly promote survival of primed myofibroblasts by controlling BCL-XL expression. Our studies showed that knockdown of YAP expression by siRNA markedly increased apoptosis to 52% in HDFs primed for death by forced adenoviral α-SMA overexpression, compared to 8% of unprimed HDFs transduced with null adenovirus (fig. S3, A and C). Similarly, TAZ knockdown significantly increased apoptosis to 46% in primed HDFs, compared to 5% of unprimed HDFs (fig. S3, B and C). Moreover, dual YAP/TAZ knockdown further increased apoptosis to 82% of primed HDFs compared to 18% of unprimed HDFs. Mechanistically, YAP/TAZ knockdowns resulted in decreased mRNA expression of prosurvival BCL-XL (fig. S3D), indicating that both YAP and TAZ control mitochondrial priming and survival of primed for death HDFs.
To complement our studies involving genetic knockdown of YAP/TAZ expression in primed HDFs, we studied whether forced induction of YAP or TAZ activation is sufficient to protect fibroblasts from ABT-263–induced apoptosis, using NIH3T3 fibroblasts expressing doxycycline-inducible mutant YAP or TAZ constructs in which the serine residues in the cytoplasmic retention signal are substituted with alanine (hereafter referred to as YAP5SA or TAZ4SA cells), allowing YAP or TAZ to constitutively accumulate in the nucleus of these cells (18). Forced induction of YAP or TAZ activation promoted fibroblast resistance to apoptosis induced by ABT-263 treatment by increasing BCL-XL (fig. S3, E and F). Together, our results indicate that YAP/TAZ signaling directly controls mitochondrial priming and survival of primed HDFs by inducing the expression of prosurvival BCL-XL.
Mitochondrial priming predicts responsiveness of scleroderma fibroblasts to BH3 mimetics
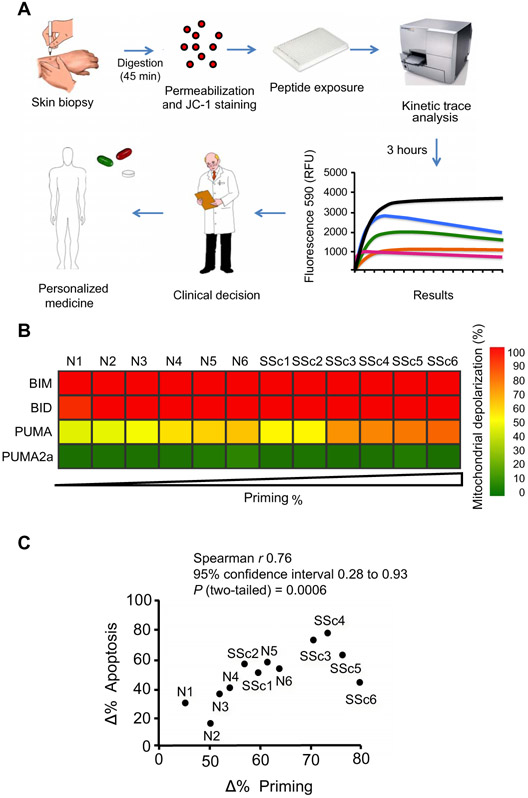
Using BH3 profiling, we have previously shown that the extent of mitochondrial priming predicts clinical responses to antitumor therapy and prognosis in cancer cells (44-46). We hypothesized that BH3 profiling might similarly guide treatment decisions in human scleroderma, a heterogeneous disease for which functional tests to predict clinical responses to novel antifibrotic therapies are lacking. BH3 profiling of HDFs isolated from skin biopsies from scleroderma patients (n = 6) and healthy controls (n = 6) was used to assess mitochondrial priming (Fig. 6A). Using PUMA-derived BH3 peptide, four of the six sets of scleroderma HDFs (SSc3 to SSc6) showed increased mitochondrial priming (reduced apoptotic thresholds) compared to the six sets of normal control cells (N1 to N6) (Fig. 6B). We then investigated whether differences in HDF mitochondrial priming would predict the sensitivity of these cells to apoptosis induced by 48 hours of ABT-263 treatment. Considering scleroderma patient and control subject HDFs together, we found that the extent of apoptosis induced by ABT-263 in HDFs markedly correlated with the extent of mitochondrial priming of these cells (Fig. 6C), indicating that mitochondrial priming predicts cell apoptotic responses to BH3 mimetics.
Fig. 6. Mitochondrial priming predicts response to ABT-263 in scleroderma fibroblasts.
(A) Schematic diagram of BH3 profiling in scleroderma clinical practice. Single-cell suspension of fibroblasts obtained from scleroderma cell lines (passage 2 to 4) or from fresh skin primary biopsy from scleroderma patients is subjected to BH3 profiling assays. Diagram adapted with permission from (27). RFU, relative fluorescence units. (B) Heat map of mitochondrial depolarization caused by the BIM, BID, and PUMA peptides (measurement of overall “priming”) in dermal fibroblasts isolated from six patients with scleroderma (SSc) compared to six healthy controls (N). Individual patient codes are shown along the x axis, and samples are ordered according to increasing depolarization by peptide. The concentration of peptides in the assays was 100 μM except for MS1 (10 mμM). PUMA2a peptide was used as a negative control, and FCCP was used as a positive control for mitochondrial depolarization. (C) Mitochondrial depolarization caused by the HRK peptide in scleroderma patient samples compared with their responses to ABT-263–induced cell apoptosis. Plot showing correlation between Δ% priming induced by HRK peptide and Δ% cell death at 48 hours upon ABT-263 treatment. Data are means ± SD from three independent experiments. P value was determined by two-tailed Spearman rank test.
Scleroderma fibroblasts depend on different antiapoptotic BCL-2 proteins for survival
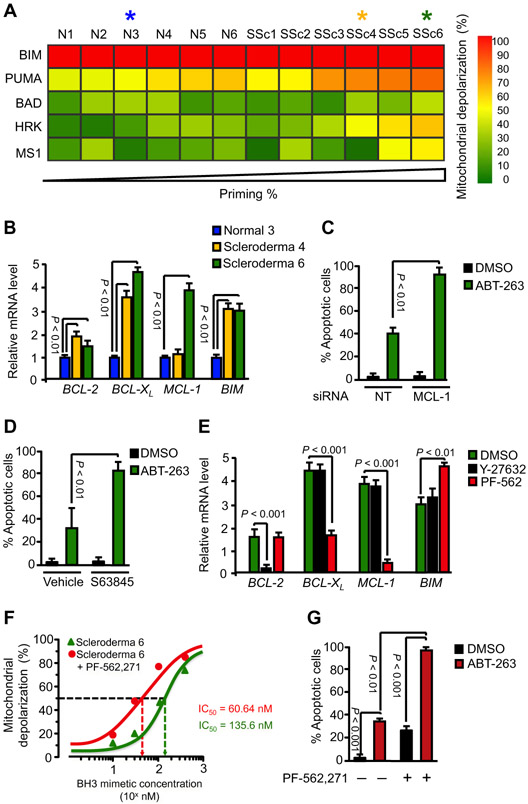
Despite the significant overall correlation we observed between mitochondrial priming and apoptosis induced by ABT-263 in scleroderma HDFs, highly primed scleroderma HDFs isolated from different patients exhibited substantial variability in their responsiveness to this BH3 mimetic. Two sets of scleroderma fibroblasts, SSc5 and SSc6, responded poorly to ABT-263 treatment despite being highly primed (Fig. 6C). Because ABT-263 only blocks BCL-XL, BCL-2, and BCL-W, we hypothesized that other BCL-2 prosurvival members might be responsible for resistance to ABT-263–induced apoptosis in these subsets of scleroderma fibroblasts. To test this hypothesis, we performed BH3 profiling with BAD-derived, HRK-derived, and MS1 peptides to functionally identify which antiapoptotic BCL-2 proteins these cells depend on for their survival. Whereas the HRK BH3 peptide induced mitochondrial depolarization in SSc3, SSc4, SSc5, and SSc6 HDFs, depolarization was also induced in SSc5 and SSc6 by the MS1 peptide (Fig. 7A and fig. S4). These results indicate that whereas BCL-XL promotes the survival of HDFs from each of these four scleroderma subjects, MCL-1 also contributes to the survival of SSc5 and SSc6 HDFs. Consistent with these BH3 profiling studies, gene expression profiling confirmed that SSc6 HDFs display high expression of both BCL-XL and MCL-1 mRNA compared to N3 HDFs, whereas in SSc4 HDFs only BCL-XL was elevated (Fig. 7B). These data are consistent with increased MCL-1 expression in SSc6 HDFs mediating the resistance of these cells to ABT-263. To support this conclusion, we investigated whether siRNA knockdown of MCL-1 would augment the ability of ABT-263 to induce apoptosis of SSc6 HDFs. ABT-263–induced apoptosis increased from 39 to 86% upon MCL-1 depletion (Fig. 7C). Similarly, pharmacological inhibition of MCL-1 with S63845, an MCL-1–selective BH3 mimetic, restored sensitivity of SSc6 HDFs to ABT-263–induced apoptosis (Fig. 7D). Elevated cancer cell MCL-1 expression has been shown to be a major mechanism of resistance to BH3 mimetics targeting other antiapoptotic BCL-2 proteins in certain cancer patients (47), and our results here suggest that increased myofibroblast MCL-1 expression could similarly be an important resistance factor to BH3 mimetics such as ABT-263 in subsets of scleroderma patients.
Fig. 7. Mitochondrial priming and expression of BCL-2 family proteins is controlled by mechanotransduction pathways in scleroderma fibroblasts.
(A) BH3 profiling. Heat map of mitochondrial depolarization caused by the BIM, PUMA, BAD, HRK, and MS1 peptides in dermal fibroblasts isolated from six patients with scleroderma compared to six healthy controls. (B) Gene expression analysis of prosurvival and proapoptotic members in HDFs from healthy volunteer “Normal 3” and from patients with scleroderma: “Scleroderma 4” and “Scleroderma 6.” Gene expression was analyzed by qPCR, and GAPDH was used as a housekeeping gene. Data are means ± SD from three independent experiments. (C and D) Effect of siRNA-mediated knockdown of MCL-1 (C) or S63845 (D), an MCL-1-selective BH3 mimetic, on ABT-263–induced apoptosis in Scleroderma 6 fibroblasts. Nontargeting siRNA or vehicle was used as a control. Apoptosis was assessed by annexin V staining. Data are means ± SD from three independent experiments. P value was determined by Student’s t test. (E) Effect of inhibition of mechanotransduction pathways on the expression of pro- and antiapoptotic family members in Scleroderma 6 fibroblasts. Cells were treated for 24 hours with the FAK inhibitor PF-562,271 or the ROCK inhibitor Y-27632, and gene expression was analyzed by qPCR. GAPDH was used as a housekeeping gene. Data are means ± SD from three independent experiments. P value was determined by Student’s t test. (F) Effect of PF-562,271 before treatment for 24 hours on ABT-263–induced mitochondrial depolarization in Scleroderma 6 fibroblasts. DMSO was used as a vehicle control. Data are means ± SD from three independent experiments. (G) Effect of PF-562,271 before treatment for 24 hours on ABT-263–induced apoptosis in Scleroderma 6 fibroblasts. DMSO was used as a vehicle control. Data are means ± SD from three independent experiments. P value was determined by Student’s t test.
Mitochondrial priming and expression of BCL-2 family proteins is controlled by mechanotransduction pathways in scleroderma fibroblasts
To further investigate the mechanism through which inhibition of mechanotransduction induces the apoptosis of stiffness-primed HDFs, we investigated the effects of inhibitors of two mechanosensitive signaling enzymes, FAK and ROCK, on the mitochondrial priming of these cells. Using BH3 profiling, we found that FAK inhibition with PF-562,271 markedly increased mitochondrial priming of stiffness-primed HDFs (fig. S5A). Similarly, inhibition of ROCK signaling with Y-27632 also increased mitochondrial priming of these cells (fig. S5A), albeit to a lesser extent than the FAK inhibitor PF-562,271. These results correlate with the extent of apoptosis induced by these two inhibitors in stiffness-primed HDFs (Fig. 1, J and K). To delineate the molecular mechanism underlying these findings, we investigated the effect of FAK and ROCK inhibition on the expression of BCL-2 family members in stiffness-exposed fibroblasts. FAK inhibition diminished mRNA and protein expression of both BCL-XL and MCL-1 compared to vehicle, but not BCL-2 (fig. S5, B to D). In contrast, ROCK inhibition markedly reduced mRNA expression of BCL-2 compared to vehicle control, but not of BCL-XL or MCL-1 (fig. S5B). These results indicate that antiapoptotic BCL-XL expression on stiffness-primed myofibroblasts is controlled by prosurvival FAK signaling rather than ROCK signaling, and that inhibition of FAK signaling with PF-562,271 reduces anti-apoptotic BCL-XL protein in stiffness-primed myofibroblasts, increasing the mitochondrial priming of these cells up to the apoptotic threshold and initiating the mitochondrial pathway of apoptosis.
Excessive mechanosensitive signaling, including FAK and ROCK activation, appears to be essential for the profibrotic activities of scleroderma fibroblasts (48-50). Because FAK and ROCK signaling also control the expression of different BCL-2 antiapoptotic proteins in stiffness-activated myofibroblasts, we investigated whether manipulation of these mechanosensitive pathways in scleroderma fibroblasts would similarly affect mitochondrial priming in these cells. We focused our studies on SSc6 HDF line, which relies on BCL-XL and MCL-1, but not on BCL-2, for survival. As noted, MCL-1 overexpression in SSc6 HDFs is responsible for the resistance to apoptosis induced by the BCL-XL inhibitor ABT-263 (Fig. 7, C and D). Because FAK signaling also controls MCL-1 expression in stiffness-primed myofibroblasts, we rationalized that pharmacological inhibition of FAK with PF-562,271 would similarly reduce MCL-1 expression in SSc6 HDFs and would therefore sensitize these cells to ABT-263–induced apoptosis. We confirmed that FAK inhibition diminished mRNA expression of both BCL-XL and MCL-1, but not of BCL-2, in SSc6 HDFs, whereas ROCK inhibition reduced mRNA expression of BCL-2, but not of BCL-XL or MCL-1, in the same cells (Fig. 7E). Functional studies demonstrated that pretreatment of SSc6 HDFs with low doses of PF-562,271 (20 nM) markedly increased the sensitivity of these cells to ABT-263–induced mitochondrial depolarization. The IC50 of ABT-263 for mitochondrial depolarization of SSc6 HDFs markedly decreased from 135.6 nM for ABT-263 alone to 60.64 nM for ABT-263 plus PF-562,271 (Fig. 7F). Finally, we demonstrated that pretreatment of SSc6 HDF with PF-562,271 restored the sensitivity of these cells to ABT-263–induced apoptosis, consistent with MCL-1 down-regulation by FAK inhibition (Fig. 7G). Together, these results indicate that mechanotransduction pathways control myofibroblast mitochondrial priming and that inhibitors of these pathways can be exploited therapeutically, and in a patient-specific fashion, to induce apoptosis of primed myofibroblasts from patients with scleroderma.
DISCUSSION
The increased stiffness of fibrotic tissues, rather than simply being the consequence of fibrosis, is now appreciated to actively contribute to fibrosis progression. We and others have demonstrated that increased stiffness contributes to myofibroblast activation and persistence (9, 10). Paradoxically, increased tissue stiffness also gives drugs that target mechanotransduction pathways the ability to induce myofibroblast apoptosis (6, 11, 31, 51, 52), potentially increasing the therapeutic index of these agents in fibrotic diseases. The mechanism(s) responsible for this effect of increased tissue stiffness, however, had not been fully elucidated. By adapting our BH3 profiling assay to evaluate the sensitivity of fibroblasts to undergo apoptosis, we present evidence that fibroblast-to-myofibroblast differentiation induced by matrix stiffness increases mitochondrial priming of myofibroblasts (the proximity to initiate the intrinsic pathway of apoptosis) (24-26). On the molecular level, mitochondrial priming is determined by the relative balance between proapoptotic and antiapoptotic BCL-2 family members. As noted, mitochondria of stiffness-activated myofibroblasts are “primed” with proapoptotic BH3 activator proteins, a cellular state we previously called primed for death (27). In this state, cells require up-regulation of prosurvival proteins for survival. We found that mitochondrial priming in stiffness-activated myofibroblasts results from up-regulation of the proapoptotic BH3-only activator protein BIM. Cells primed for death are highly dependent on the expression and function of specific anti-apoptotic BCL-2 family members to block pro-death signaling and ensure their survival (25). We found that the mitochondrial integrity of stiffness-primed myofibroblasts is maintained by concomitant up-regulation of BCL-XL and that stiffness-primed dermal myofibroblasts are addicted to the ability of BCL-XL to sequester BIM and block its proapoptotic function. Our findings indicate that BCL-XL expression in stiffness-primed myofibroblasts is controlled by integrinmediated mechanotransduction pathways including FAK signaling. Here, we show that targeting FAK signaling with PF-562,271 rapidly suppresses the expression of BCL-XL and induces apoptosis of stiffness-primed myofibroblasts, providing new insight into the mechanistic basis behind the potent antifibrotic effects of FAK inhibitors (12, 14, 48, 49, 53-57). Integrin-mediated FAK mechanosignaling drives nuclear accumulation of transcriptional coactivators YAP and TAZ to promote cell survival (58). Consistent with these findings, our results show that targeting YAP/TAZ-mediated mechanotransduction at the transcriptional level profoundly impaired survival of stiffness-primed myofibroblast by suppressing BCL-XL expression in these cells. Our data are consistent with recent findings demonstrating that survival of tumor cells with oncogenic BRAF mutation depends on YAP-mediated BCL-XL expression (43). Together, the dependence of stiffness-primed cells on prosurvival BCL-2 family proteins such as BCL-XL creates a therapeutic opportunity to treat fibrosis in scleroderma patients, by inducing apoptosis of their myofibroblasts with drugs that control BCL-XL expression including FAK or YAP/TAZ inhibitors.
Our study also unveils a new therapeutic strategy for the treatment of fibrosis based on induction of myofibroblast apoptosis by BH3 mimetic drugs, which have been developed to directly block and inhibit the function of prosurvival proteins such as BCL-XL. We have previously shown that increased mitochondrial priming in cancer cells is the basis for clinical responses to traditional chemotherapies in ovarian cancer, multiple myeloma, acute lymphocytic leukemia, and acute myelogenous leukemia (29, 44-46). BH3 mimetic agents such as ABT-263, which targets BCL-XL, have been developed to selectively induce apoptosis of primed cancer cells (20, 59). Here, we show that increased mitochondrial priming of myofibroblasts in dermal fibrosis can provide a similar therapeutic opportunity in scleroderma, making these cells similarly susceptible to apoptosis by BH3 mimetics targeting BCL-XL, such as ABT-263. Our data suggest that, in scleroderma, fbroblast-to-myofbroblast differentiation induced by matrix stiffness, rather than a genetic mutation in malignant cells, causes activated myofibroblasts to be susceptible to BH3 mimetic therapy, where quiescent fibroblasts in tissues with normal stiffness are not susceptible. Genetic knockdown of BCL-XL, or its pharmacological inhibition with ABT-263, induced apoptosis of stiffness-primed myofibroblasts in vitro, and ABT-263 treatment reduced dermal fibrosis in the bleomycin mouse model of scleroderma, when administration was initiated after fibrosis was already established, by inducing myofibroblast apoptosis in vivo (fig. S6). Agents able to induce the regression of established fibrosis would represent a substantial addition to currently available antifibrotic therapies, which only slow the progression of fbrotic diseases (37, 38). ABT-263 is an orally bioavailable drug with demonstrated clinical efficacy in hematological malignancies, but drug development for this indication was shifted to ABT-199 when ABT-263 was found to induce platelet apoptosis and dose-limiting thrombocytopenia due to platelet dependence on BCL-XL, but not BCL-2, for survival (60). Evidence that solid tumors more frequently up-regulate BCL-XL than BCL-2 has maintained interest in targeting BCL-XL, and the risk of thrombocytopenia could be manageable if the clinical efficacy of a BH3 mimetic targeting BCL-XL for solid tumors, or fibrotic diseases, was high enough (60). A recent study has shown that cancer-associated fibroblasts are similarly primed for death and undergo apoptosis in response to treatment with ABT-263 (61), consistent with the notion that acquisition of an activated phenotype in fibroblasts increases the mitochondrial priming of these cells.
The clinical manifestations of scleroderma are extremely heterogeneous, which has complicated the evaluation of candidate drugs in clinical trials. Patients are currently stratified into disease subgroups based on the extent of cutaneous disease involvement and autoantibody profile. The molecular basis ofscleroderma appears to be heterogeneous even within these clinical subgroups, suggesting that antifibrotic therapies targeting different molecular pathways may be required by different individual scleroderma patients. Understanding the molecular heterogeneity of scleroderma will be critical for this type of “personalized” medicine approach. Our results presented here suggest that BH3 profiling of dermal fibroblasts in skin biopsies may help stratify scleroderma patients and facilitate their individualized treatment. As we have demonstrated, BH3 profiling may be able to predict which scleroderma patients would respond well to BH3 mimetics by assessing the extent of dermal fibroblast mitochondrial priming. Our studies also suggest that BH3 profiling is a useful tool for detecting which antiapoptotic protein is more critical for survival of scleroderma fibroblasts, and thus, BH3 profiling may predict which specific BH3 mimetics and/or which specific inhibitors of mechanotransduction pathways individual scleroderma patients would respond to best. Together, we propose a personalized medicine approach to fibrotic diseases such as scleroderma, in which BH3 profiling is used to stratify scleroderma patients into more homogeneous cohorts based on their myofibroblast BH3 profile signature.
MATERIALS AND METHODS
Study design
The main goal of our study was to evaluate the BH3 mimetic drug ABT-263, a BCL-XL inhibitor, as a therapeutic agent for scleroderma fibrosis. Using BH3 profiling to assess mitochondrial priming (proximity of cells to their apoptotic threshold) in fibroblasts from scleroderma patients, we demonstrated that mitochondria from these cells are primed by death signals such as the proapoptotic BH3-only protein BIM, which creates a requirement for tonic expression of the anti-apoptotic protein BCL-XL to sequester BIM and ensure survival. We hypothesized that BCL-XL promotes scleroderma fibroblast survival and that pharmacological inhibition of BCL-XL would induce apoptosis of these cells. Pharmacological inhibition of BCL-XL with the BH3 mimetic drug ABT-263 and siRNA-mediated BCL-XL knockdown experiments were performed in scleroderma fibroblasts to test our hypothesis in vitro. For experiments in vivo, the bleomycin-induced skin fibrosis model was chosen as a well-established animal model of scleroderma. Sample sizes were calculated by power analysis. In each experiment evaluating the effects of inhibition of BCL-XL by ABT-263 on the extent of dermal fibrosis produced in mice, n ≥ 8 mice per group were used to achieve statistical significance and account for the inherent variability in the fibrotic response of mice. Mice were randomly assigned to treatment groups. To test whether ABT-263 may hold therapeutic potential in skin fibrosis, ABT-263 was administered in a therapeutic regimen beginning 14 days after the onset of daily bleomycin challenges. ABT-263 injection and skin fibrosis measurements were double-blinded until statistical analysis. Skin fibrosis was quantified by Masson’s trichrome staining on skin sections and hydroxyproline content in 6-mm skin biopsies. All sample measurements were blinded, and no animals were excluded from analysis. Each in vivo and in vitro experiment was performed in triplicate and repeated three times.
Mouse model of skin scleroderma
Adult sex- and age-matched C57BL/6N mice at 6 to 8 weeks of age were purchased from the National Cancer Institute–Frederick Mouse Repository (Frederick, MD) and used throughout this study. All experiments were performed in accordance with National Institutes of Health guidelines and protocols approved by the Massachusetts General Hospital Subcommittee on Research Animal Care, and all mice were maintained in a specific pathogen–free environment certified by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC). Skin fibrosis was induced by daily subcutaneous injection of bleomycin (100 μl from a stock of 10 μg/ml) for 28 days, as previously described (33). Sterile saline was used as control. ABT-263 and ABT-199 were administered daily by oral gavage in 10% ethanol, 30% polyethylene glycol 400, and 60% Phosal 50 PG at a dosage of 100 mg/kg body weight. At the conclusion of experiments, mice were sacrificed, and full-thickness 6-mm punch biopsies were obtained for histologic and immuno-histochemical studies as well as hydroxyproline analysis.
Atomic force microscopy
Mouse skin fresh tissue strips were mechanically characterized using an atomic force microscope (MFP-3D; Asylum Research) by performing microindentation using a sphere-tipped probe (Novascan) with a diameter of 5 μm and a nominal spring constant of 60 pN/nm. The cantilever spring constant was further confirmed by the thermal fluctuation method. The AFM system was calibrated by following the manufacturer’s instruction before each indentation measurement. Force-indentation profiles were acquired at an indentation rate of 20 μm/s separated by 5 μm spatially in a 16 × 16 sample grid covering an area of 80 × 80 μm. Shear modulus at each point on the grid was calculated from fitting force-indentation data using a Hertz sphere model (62), and the resulting shear modulus data were plotted in contour maps. For skin tissue, a Poisson’s ratio of 0.4 (63) was used to convert elastic modulus (E) to shear modulus (G), using the relationship E = 2 × (1 + ν) × G.
Antibodies and reagents
Antibodies used were as follows: α-SMA (1A4, Sigma-Aldrich); cleaved caspase-3 (5A1E, Cell Signaling); MCL-1, BCL-XL, BAK, BAK, BIM, BID, tubulin, and GAPDH (glyceraldehyde-3-phosphate dehydrogenase) (Cell Signaling); and BCL-2 (Bcl-2-100, Life Technologies). Secondary antibodies were obtained from Invitrogen [Alexa Fluor 488 goat anti-mouse immunoglobulin G2a (IgG2a) and Alexa Fluor 555 goat anti-rabbit IgG1]. F-actin and nuclei were stained with Alexa Fluor 546–phalloidin and 4′,6-diamidino-2-phenylindole (DAPI) (Invitrogen), respectively.
Reagents used included PF-562,271 (Selleckchem), S63845 (ApexBio), ABT-263 (Active Biochem), ABT-199 (Active Biochem), and Y-27632, RGD peptide, CCG-1423, and anti–integrin β1 antibody (Sigma). siRNA duplexes targeting human BCL-2, human BCL-XL, human MCL-1, human BIM, human YAP, and human TAZ were obtained from Dharmacon Inc. using ON-TARGETplus SMARTpool (Thermo Scientific).
Nonreplicative recombinant adenovirus expressing human α-SMA fused to red fluorescent protein (Ad-α-SMA-RFP) or shRNA against α-SMA and GFP (Ad-GFP-U6-α-SMA-shRNA) were generated, amplified, and purified by Vector Biolabs. Table S1 lists the primer and siRNA sequences used in this study.
Cell lines and treatments
Human foreskin fibroblasts (ATCC SCRC-1041) and primary normal dermal fibroblasts (ATCC PCS-201-010) were purchased from the American Type Culture Collection (ATCC) and cultured according to the vendor’s protocol. Primary MDFs were isolated by outgrowth from explanted control and bleomycin-treated mouse skin and cultured in Dulbecco’s modified Eagle’s medium (DMEM) with 10% fetal bovine serum (FBS; Lonza), penicillin (100 U/ml), and streptomycin (100 μg/ml) at 37°C in a humidified atmosphere of 5% CO2. Cells were used between the second and sixth passages. YAP5SA- and TAZ4SA-expressing fibroblasts were generated and cultured as previously described (18).
To test the effect of matrix stiffness on cell apoptosis, cells were seeded at 50 cells/mm2 on collagen-coated polyacrylamide hydrogels with different stiffnesses (Young’s modulus, 0.2, 0.5, 1.2, 4, 8, 12, 25, and 50 kPa). Cells were cultured in DMEM with 1% FBS for 48 hours before fixation for immunostaining. For drug treatments, PF-562,271, ABT-263, or ABT-199 was added after 48 hours of initial attachment, and cells were then incubated with inhibitors for 24 hours. For infection with recombinant adenoviruses, cells were transduced with control adenovirus (Ad-Null), Ad-α-SMA-RFP, or Ad-GFP-U6-α-SMA-shRNA for 6 hours using the indicated multiplicity of infection and incubated for 48 hours.
Cell viability assays
Fibroblasts plated on different stiffnesses were treated with the indicated drugs for 48 hours. Cells were stained with fluorescent conjugates of annexin V (Sigma) and/or propidium iodide (PI) and analyzed on a FACSCanto machine (BD Biosciences). Viable cells were annexin V–negative and PI-negative, and cell death was expressed as 100% – % viable cells. Analysis was done with FlowJo software.
Flow cytometry
Single-cell suspensions were isolated from 6-mm skin biopsies using Liberase Blendzyme (final concentration, 0.14 U/ml; Roche) and deoxyribonuclease I (final concentration, 60 μg/ml; Sigma) for 45 min at 37°C. The number of cells isolated from each sample was counted with a hemocytometer. Cells were incubated with FcRII and FcRIII blocking antibody (BioLegend, clone 93) for 10 min at 4°C followed by staining with the following fluorophore-conjugated antibody panels: Alexa Fluor 700–conjugated anti-CD45.2 (BioLegend, clone 104), phycoerythrin-conjugated anti–Siglec-F (BD Biosciences, clone E50–2440), Brilliant Violet 605–conjugated anti-CD11c (BioLegend, clone N418), phycoerythrin–cyanine 7–conjugated anti-MHC (major histocompatibility complex) class II (BioLegend, clone M5/114.15.2), Brilliant Violet 421–conjugated anti-CD103 (BioLegend, clone 2E7), Brilliant Ultraviolet 395–conjugated anti-CD11b (BD Biosciences, clone M1/70), allophycocyanin-conjugated anti-CD64 (BioLegend, X54-5/7.1), peridinin chlorophyll–cyanine 5.5–conjugated anti-Ly6C (BioLegend, clone HK1.4), and fluorescein isothiocyanate–conjugated anti-Ly6G (BioLegend, clone 1A8). eFluor 780 fixable viability dye (eBioscience) staining was performed to exclude dead cells. Flow cytometry was performed using a BD LSRFortessaX-20 cell analyzer, and FlowJo software was used for analysis.
Normal human and scleroderma fibroblasts
Six-millimeter punch biopsies from the forearm of healthy individuals and scleroderma patients with clinical cutaneous involvement were performed under protocols approved by the Institutional Ethics Committee (Centre de Recherche du Centre Hospitalier de l’Université de Montréal, Montreal); all scleroderma subjects were part of the registry of the Canadian Scleroderma Research Group and provided written informed consent. These biopsies were used for isolation and culture of skin fibroblasts. The biopsies were extracted from six normal female donors (control subject ages ranged from 49 to 65 years old) and six female scleroderma patients with clinical cutaneous involvement of the biopsied site (patient ages ranged from 45 to 63 years old). Cells were cultured in DMEM with 10% FBS (Lonza) and used between the second and fifth passages.
BH3 peptide synthesis
Peptides were synthesized and high-performance liquid chromatography–purified at the Tufts University Physiology Core Facility. A total of 10 mM peptide solutions in dimethyl sulfoxide (DMSO) were stored at −80°C.
ΔΨm BH3 profile
HDFs were cultured on matrices with different stiffnesses for 24 hours and then trypsinized and suspended in T-EB buffer [300 mM trehalose, 10 mM Hepes-KOH (pH 7.7), 80 mM KCl, 1 mM EGTA, 1 mM EDTA, 0.1% bovine serum albumin, 5 mM succinate]. Single-cell suspensions were washed in T-EB before being resuspended at four times their final density. Cells were then added to an equal volume of 4× staining mastermix [4 μM JC-1, oligomycin (40 μg/ml), 20 mM 2-mercaptoethanol, and 0.02% digitonin in T-EB buffer]. A total of 15 μl of the 2× cell/dye mix was added to wells containing 15 μl of peptides at 2× final concentration in T-EB to yield the final profiling plate with 20,000 cells per well in a black 384-well plate (BD Falcon no. 353285). BIM, BID, BAD, PUMA, PUMA2a, and HRK BH3 peptides were used at 100 μM; MS1 peptide was used at 10 μM in T-EB. Fluorescence at 590 nm was monitored using 545-nm excitation on a Tecan Safire 2 at a controlled temperature of 30°C with automated readings every 5 min. Individual analyses were performed using triplicates for DMSO, FCCP, and the different BH3 peptides used, and the expressed values represent the average of three different readings. The area under each peptide response curve was calculated using GraphPad Prism, and these areas were normalized to the internal FCCP and DMSO controls as Depolarization (%) = 1 – ([sample-FCCP]/[DMSO-FCCP]).
Statistical analysis
Sample sizes were calculated by power analysis. In each experiment evaluating the effects of inhibition of BCL-XL by ABT-263 on the extent of dermal fibrosis produced in mice, n ≥ 8 mice per group were used to achieve statistical significance. Sample sizes were estimated based on an 80% power to detect a 50% reduction in the amount of fibrosis present in mice treated with ABT-263 compared with wild-type control mice, accepting a type I error rate of 0.05. Animals were distributed into groups of equal body weight. No animals were excluded from the analysis. Experimental data were analyzed by unpaired Student’s t test for differences between each of the experimental conditions, two-way analysis of variance (ANOVA) for overall condition effects, two-tailed Spearman rank for correlation, or nonlinear dose-response curve fitting for EC50 calculation using GraphPad Prism 5.0 software. The P values obtained are indicated in the figures and figure legends when statistically significant. P < 0.05 was considered significantly different between groups. All data displayed a normal distribution. Data are reported as means ± SD.
Supplementary Material
Fig. S1. Effect of pan-caspase inhibitor Z-VAD and caspase-8 inhibitor emricasan on myofibroblast apoptosis induced by FAK inhibition.
Fig. S2. siRNA transfection efficiency and gene expression of BCL-2, BCL-XL, and MCL-1.
Fig. S3. Effect of YAP/TAZ knockdown and overexpression on fibroblast mitochondrial priming and survival.
Fig. S4. BH3 profiling of Normal 3, Scleroderma 4, and Scleroderma 6 fibroblasts.
Fig. S5. Effect of FAK and ROCK inhibition on mitochondrial priming and antiapoptotic BCL-2 members.
Fig. S6. Targeted apoptosis of myofibroblast reverses established fibrosis.
Table S1. Reverse transcription PCR primer and siRNA sequences.
Acknowledgments:
We thank A. Franklin and J. W. Griffith (both from the Pulmonary and Critical Care Division at the Massachusetts General Hospital) for their technical assistance with mouse breeding and flow cytometry, respectively.
Funding:
This work was supported by a Scleroderma Foundation New Investigator Grant and an American Thoracic Society Foundation/Pulmonary Fibrosis Foundation Research Grant to D.L., a Scleroderma Research Foundation Investigator-Initiated Research Grant and NIH grant R01 HL108975 to A.M.T., and NIH grant R01 HL092961 to D.J.T.
Footnotes
Competing interests: D.L. is paid consultant to Blade Therapeutics Inc. and Unity Biotechnology. A.L., J.R., and J.M. are inventors on patent WO2014047342 A1 held by Dana-Farber Cancer Institute that covers the invention of dynamic BH3 profiling, which provides methods of predicting cell sensitivity or resistance to a therapeutic agent.
Data and materials availability: Data are available from the corresponding author upon reasonable request.
REFERENCES AND NOTES
- 1.Gurtner GC, Werner S, Barrandon Y, Longaker MT, Wound repair and regeneration. Nature 453, 314–321 (2008). [DOI] [PubMed] [Google Scholar]
- 2.Desmoulière A, Factors influencing myofibroblast differentiation during wound healing and fibrosis. Cell Biol. Int 19, 471–476 (1995). [DOI] [PubMed] [Google Scholar]
- 3.Desmoulière A, Redard M, Darby I, Gabbiani G, Apoptosis mediates the decrease in cellularity during the transition between granulation tissue and scar. Am. J. Pathol 146, 56–66 (1995). [PMC free article] [PubMed] [Google Scholar]
- 4.Kisseleva T, Cong M, Paik Y, Scholten D, Jiang C, Benner C, Iwaisako K, Moore-Morris T, Scott B, Tsukamoto H, Evans SM, Dillmann W, Glass CK, Brenner DA, Myofibroblasts revert to an inactive phenotype during regression of liver fibrosis. Proc. Natl. Acad. Sci. U.S.A 109, 9448–9453 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Troeger JS, Mederacke I, Gwak G-Y, Dapito DH, Mu X, Hsu CC, Pradere J-P, Friedman RA, Schwabe RF, Deactivation of hepatic stellate cells during liver fibrosis resolution in mice. Gastroenterology 143, 1073–1083.e22 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ho YY, Lagares D, Tager AM, Kapoor M, Fibrosis—A lethal component of systemic sclerosis. Nat. Rev. Rheumatol 10, 390–402 (2014). [DOI] [PubMed] [Google Scholar]
- 7.Rockey DC, Bell PD, Hill JA, Fibrosis—A common pathway to organ injury and failure. N. Engl. J. Med 372, 1138–1149 (2015). [DOI] [PubMed] [Google Scholar]
- 8.Varga J, Abraham D, Systemic sclerosis: A prototypic multisystem fibrotic disorder. J. Clin. Invest 117, 557–567 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu F, Mih JD, Shea BS, Kho AT, Sharif AS, Tager AM, Tschumperlin DJ, Feedback amplification of fibrosis through matrix stiffening and COX-2 suppression. J. Cell Biol 190, 693–706 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parker MW, Rossi D, Peterson M, Smith K, Sikström K, White ES, Connett JE, Henke CA, Larsson O, Bitterman PB, Fibrotic extracellular matrix activates a profibrotic positive feedback loop. J. Clin. Invest 124, 1622–1635 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou Y, Huang X, Hecker L, Kurundkar D, Kurundkar A, Liu H, Jin T-H, Desai L, Bernard K, Thannickal VJ, Inhibition of mechanosensitive signaling in myofibroblasts ameliorates experimental pulmonary fibrosis. J. Clin. Invest 123, 1096–1108 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wong VW, Rustad KC, Akaishi S, Sorkin M, Glotzbach JP, Januszyk M, Nelson ER, Levi K, Paterno J, Vial IN, Kuang AA, Longaker MT, Gurtner GC, Focal adhesion kinase links mechanical force to skin fibrosis via inflammatory signaling. Nat. Med 18, 148–152 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henderson NC, Arnold TD, Katamura Y, Giacomini MM, Rodriguez JD, McCarty JH, Pellicoro A, Raschperger E, Betsholtz C, Ruminski PG, Griggs DW, Prinsen MJ, Maher JJ, Iredale JP, Lacy-Hulbert A, Adams RH, Sheppard D, Targeting of αv integrin identifies a core molecular pathway that regulates fibrosis in several organs. Nat. Med 19, 1617–1624 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lagares D, Busnadiego O, García-Fernández RA, Kapoor M, Liu S, Carter DE, Abraham D, Shi-Wen X, Carreira P, Fontaine BA, Shea BS, Tager AM, Leask A, Lamas S, Rodríguez-Pascual F, Inhibition of focal adhesion kinase prevents experimental lung fibrosis and myofibroblast formation. Arthritis Rheum. 64, 1653–1664 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barry-Hamilton V, Spangler R, Marshall D, McCauley S, Rodriguez HM, Oyasu M, Mikels A, Vaysberg M, Ghermazien H, Wai C, Garcia CA, Velayo AC, Jorgensen B, Biermann D, Tsai D, Green J, Zaffryar-Eilot S, Holzer A, Ogg S, Thai D, Neufeld G, Van Vlasselaer P, Smith V, Allosteric inhibition of lysyl oxidase–like-2 impedes the development of a pathologic microenvironment. Nat. Med 16, 1009–1017 (2010). [DOI] [PubMed] [Google Scholar]
- 16.Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, Zanconato F, Le Digabel J, Forcato M, Bicciato S, Elvassore N, Piccolo S, Role of YAP/TAZ in mechanotransduction. Nature 474, 179–183 (2011). [DOI] [PubMed] [Google Scholar]
- 17.Vartiainen MK, Guettler S, Larijani B, Treisman R, Nuclear actin regulates dynamic subcellular localization and activity of the SRF cofactor MAL. Science 316, 1749–1752 (2007). [DOI] [PubMed] [Google Scholar]
- 18.Liu F, Lagares D, Choi KM, Stopfer L, Marinković A, Vrbanac V, Probst CK, Hiemer SE, Sisson TH, Horowitz JC, Rosas IO, Fredenburgh LE, Feghali-Bostwick C, Varelas X, Tager AM, Tschumperlin DJ, Mechanosignaling through YAP and TAZ drives fibroblast activation and fibrosis. Am.J. Physiol. Lung Cell. Mol. Physiol 308, L344–L357 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sakai N, Chun J, Duffield JS, Wada T, Luster AD, Tager AM, LPA1-induced cytoskeleton reorganization drives fibrosis through CTGF-dependent fibroblast proliferation. FASEB J. 27, 1830–1846 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lessene G, Czabotar PE, Colman PM, BCL-2 family antagonists for cancer therapy. Nat. Rev. Drug Discov 7, 989–1000 (2008). [DOI] [PubMed] [Google Scholar]
- 21.Youle RJ, Strasser A, The BCL-2 protein family: Opposing activities that mediate cell death. Nat. Rev. Mol. Cell Biol 9, 47–59 (2008). [DOI] [PubMed] [Google Scholar]
- 22.Hinchcliff M, Huang C-C, Wood TA, Matthew Mahoney J, Martyanov V, Bhattacharyya S, Tamaki Z, Lee J, Carns M, Podlusky S, Sirajuddin A, Shah SJ, Chang RW, Lafyatis R, Varga J, Whitfield ML, Molecular signatures in skin associated with clinical improvement during mycophenolate treatment in systemic sclerosis. J. Invest. Dermatol 133, 1979–1989 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dai H, Pang Y-P, Ramirez-Alvarado M, Kaufmann SH, Evaluation of the BH3-only protein Puma as a direct Bak activator. J. Biol. Chem 289, 89–99 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Del Gaizo Moore V, Brown JR, Certo M, Love TM, Novina CD, Letai A, Chronic lymphocytic leukemia requires BCL2 to sequester prodeath BIM, explaining sensitivity to BCL2 antagonist ABT-737. J. Clin. Invest 117, 112–121 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deng J, Carlson N, Takeyama K, Dal Cin P, Shipp M, Letai A, BH3 profiling identifies three distinct classes of apoptotic blocks to predict response to ABT-737 and conventional chemotherapeutic agents. Cancer Cell 12, 171–185 (2007). [DOI] [PubMed] [Google Scholar]
- 26.Ryan JA, Brunelle JK, Letai A, Heightened mitochondrial priming is the basis for apoptotic hypersensitivity of CD4+ CD8+ thymocytes. Proc. Natl. Acad. Sci. U.S.A 107, 12895–12900 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Certo M, Del Gaizo Moore V, Nishino M, Wei G, Korsmeyer S, Armstrong SA, Letai A, Mitochondria primed by death signals determine cellular addiction to antiapoptotic BCL-2 family members. Cancer Cell 9, 351–365 (2006). [DOI] [PubMed] [Google Scholar]
- 28.Brunelle JK, Ryan J, Yecies D, Opferman JT, Letai A, MCL-1–dependent leukemia cells are more sensitive to chemotherapy than BCL-2–dependent counterparts. J. Cell Biol 187, 429–442 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davids MS, Deng J, Wiestner A, Lannutti BJ, Wang L, Wu CJ, Wilson WH, Brown JR, Letai A, Decreased mitochondrial apoptotic priming underlies stroma-mediated treatment resistance in chronic lymphocytic leukemia. Blood 120, 3501–3509 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ryan J, Letai A, BH3 profiling in whole cells byfluorimeter or FACS. Methods 61, 156–164 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sisson TH, Ajayi IO, Subbotina N, Dodi AE, Rodansky ES, Chibucos LN, Kim KK, Keshamouni VG, White ES, Zhou Y, Higgins PDR, Larsen SD, Neubig RR, Horowitz JC, Inhibition of myocardin-related transcription factor/serum response factor signaling decreases lung fibrosis and promotes mesenchymal cell apoptosis. Am. J. Pathol 185, 969–986 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamamoto T, Takagawa S, Katayama I, Yamazaki K, Hamazaki Y, Shinkai H, Nishioka K, Animal model of sclerotic skin. I: Local injections of bleomycin induce sclerotic skin mimicking scleroderma. J. Invest. Dermatol 112, 456–462 (1999). [DOI] [PubMed] [Google Scholar]
- 33.Lagares D, García-Fernández RA, Jiménez CL, Magán-Marchal N, Busnadiego O, Lamas S, Rodríguez-Pascual F, Endothelin 1 contributes to the effect of transforming growth factor β1 on wound repair and skin fibrosis. Arthritis Rheum. 62, 878–889 (2010). [DOI] [PubMed] [Google Scholar]
- 34.Castelino FV, Seiders J, Bain G, Brooks SF, King CD, Swaney JS, Lorrain DS, Chun J, Luster AD, Tager AM, Amelioration of dermal fibrosis by genetic deletion or pharmacologic antagonism of lysophosphatidic acid receptor 1 in a mouse model of scleroderma. Arthritis Rheum. 63, 1405–1415 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kotoh K, Nakamuta M, Kohjima M, Fukushima M, Morizono S, Kobayashi N, Enjoji M, Nawata H, Arg-Gly-Asp (RGD) peptide ameliorates carbon tetrachloride-induced liver fibrosis via inhibition of collagen production and acceleration of collagenase activity. Int. J. Mol. Med 14, 1049–1053 (2004). [PubMed] [Google Scholar]
- 36.Liu S, Kapoor M, Denton CP, Abraham DJ, Leask A, Loss of β1 integrin in mouse fibroblasts results in resistance to skin scleroderma in a mouse model. Arthritis Rheum. 60, 2817–2821 (2009). [DOI] [PubMed] [Google Scholar]
- 37.Foight GW, Ryan JA, Gullá SV, Letai A, Keating AE, Designed BH3 peptides with high affinity and specificity for targeting Mcl-1 in cells. ACS Chem. Biol 9, 1962–1968 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tse C, Shoemaker AR, Adickes J, Anderson MG, Chen J, Jin S, Johnson EF, Marsh KC, Mitten MJ, Nimmer P, Roberts L, Tahir SK, Xiao Y, Yang X, Zhang H, Fesik S, Rosenberg SH, Elmore SW, ABT-263: A potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res. 68, 3421–3428 (2008). [DOI] [PubMed] [Google Scholar]
- 39.Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA, Bruncko M, Deckwerth TL, Dinges J, Hajduk PJ, Joseph MK, Kitada S, Korsmeyer SJ, Kunzer AR, Letai A, Li C, Mitten MJ, Nettesheim DG, Ng S, Nimmer PM, O’Connor JM, Oleksijew A, Petros AM, Reed JC, Shen W, Tahir SK, Thompson CB, Tomaselli KJ, Wang B, Wendt MD, Zhang H, Fesik SW, Rosenberg SH, An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature 435, 677–681 (2005). [DOI] [PubMed] [Google Scholar]
- 40.Souers AJ, Leverson JD, Boghaert ER, Ackler SL, Catron ND, Chen J, Dayton AD, Ding H, Enschede SH, Fairbrother WJ, Huang DCS, Hymowitz SG, Jin S, Khaw SL, Kovar PJ, Lam LT, Lee J, Maecker HL, Marsh KC, Mason KD, Mitten MJ, Nimmer PM, Oleksijew A, Park CH, Park C-M, Phillips DC, Roberts AW, Sampath D, Seymour JF, Smith ML, Sullivan GM, Tahir SK, Tse C, Wendt MD, Xiao Y, Xue JC, Zhang H, Humerickhouse RA, Rosenberg SH, Elmore SW, ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat. Med 19, 202–208 (2013). [DOI] [PubMed] [Google Scholar]
- 41.Wynn TA, Ramalingam TR, Mechanisms of fibrosis: Therapeutic translation for fibrotic disease. Nat. Med 18, 1028–1040 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li CX, Talele NP, Boo S, Koehler A, Knee-Walden E, Balestrini JL, Speight P, Kapus A, Hinz A, MicroRNA-21 preserves the fibrotic mechanical memory of mesenchymal stem cells. Nat. Mater 16, 379–389 (2017). [DOI] [PubMed] [Google Scholar]
- 43.Lin L, Sabnis AJ, Chan E, Olivas V, Cade L, Pazarentzos E, Asthana S, Neel D, Yan JJ, Lu X, Pham L, Wang MM, Karachaliou N, Cao MG, Manzano JL, Ramirez JL, Torres JMS, Buttitta F, Rudin CM, Collisson EA, Algazi A, Robinson E, Osman I, Muñoz-Couselo E, Cortes J, Frederick DT, Cooper ZA, McMahon M, Marchetti A, Rosell R, Flaherty KT, Wargo JA, Bivona TG, The Hippo effector YAP promotes resistance to RAF- and MEK-targeted cancer therapies. Nat. Genet 47, 250–256 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ni Chonghaile T, Sarosiek KA, Vo T-T, Ryan JA, Tammareddi A, Del Gaizo Moore V, Deng J, Anderson KC, Richardson P, Tai Y-T, Mitsiades CS, Matulonis UA, Drapkin R, Stone R, Deangelo DJ, McConkey DJ, Sallan SE, Silverman L, Hirsch MS, Carrasco DR, Letai A, Pretreatment mitochondrial priming correlates with clinical response to cytotoxic chemotherapy. Science 334, 1129–1133 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Montero J, Sarosiek KA, DeAngelo JD, Maertens O, Ryan J, Ercan D, Piao H, Horowitz NS, Berkowitz RS, Matulonis U, Jänne PA, Amrein PC, Cichowski K, Drapkin R, Letai A, Drug-induced death signaling strategy rapidly predicts cancer response to chemotherapy. Cell 160, 977–989 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vo T-T, Ryan J, Carrasco R, Neuberg D, Rossi DJ, Stone RM, Deangelo DJ, Frattini MG, Letai A, Relative mitochondrial priming of myeloblasts and normal HSCs determines chemotherapeutic success in AML. Cell 151, 344–355 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Harrison LRE, Micha D, Brandenburg M, Simpson KL, Morrow CJ, Denneny O, Hodgkinson A, Yunus Z, Dempsey C, Roberts D, Blackhall F, Makin G, Dive C, Hypoxic human cancer cells are sensitized to BH-3 mimetic-induced apoptosis via downregulation of the Bcl-2 protein Mcl-1. J. Clin. Invest 121, 1075–1087 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mimura Y, Ihn H, Jinnin M, Asano Y, Yamane K, Tamaki K, Constitutive phosphorylation of focal adhesion kinase is involved in the myofibroblast differentiation of scleroderma fibroblasts. J. Invest. Dermatol 124, 886–892 (2005). [DOI] [PubMed] [Google Scholar]
- 49.Shi-wen X, Thompson K, Khan K, Liu S, Murphy-Marshman H, Baron M, Denton CP, Leask A, Abraham DJ, Focal adhesion kinase and reactive oxygen species contribute to the persistent fibrotic phenotype of lesional scleroderma fibroblasts. Rheumatology 51, 2146–2154 (2012). [DOI] [PubMed] [Google Scholar]
- 50.Akhmetshina A, Dees C, Pileckyte M, Szucs G, Spriewald BM, Zwerina J, Distler O, Schett G, Distler JHW, Rho-associated kinases are crucial for myofibroblast differentiation and production of extracellular matrix in scleroderma fibroblasts. Arthritis Rheum. 58, 2553–2564 (2008). [DOI] [PubMed] [Google Scholar]
- 51.Hinz B, Phan SH, Thannickal VJ, Galli A, Bochaton-Piallat M-L, Gabbiani G, The myofibroblast: One function, multiple origins. Am. J. Pathol 170, 1807–1816 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Van De Water L, Varney S, Tomasek JJ, Mechanoregulation of the myofibroblast in wound contraction, scarring, and fibrosis: Opportunities for new therapeutic intervention. Adv. Wound Care 2, 122–141 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lagares D, Busnadiego O, García-Fernández RA, Lamas S, Rodríguez-Pascual F, Adenoviral gene transfer of endothelin-1 in the lung induces pulmonary fibrosis through the activation of focal adhesion kinase. Am. J. Respir. Cell Mol. Biol 47, 834–842 (2012). [DOI] [PubMed] [Google Scholar]
- 54.Lagares D, Kapoor M, Targeting focal adhesion kinase in fibrotic diseases. BioDrugs 27, 15–23 (2013). [DOI] [PubMed] [Google Scholar]
- 55.Jiang H, Hegde S, Knolhoff BL, Zhu Y, Herndon JM, Meyer MA, Nywening TM, Hawkins WG, Shapiro IM, Weaver DT, Pachter JA, Wang-Gillam A, DeNardo DG, Targeting focal adhesion kinase renders pancreatic cancers responsive to checkpoint immunotherapy. Nat. Med 22, 851–860 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhao X-K, Yu L, Cheng M-L, Che P, Lu Y-Y, Zhang Q, Mu M, Li H, Zhu L-L, Zhu J-J, Hu M, Li P, Liang Y-D, Luo X-H, Cheng Y-J, Xu Z-X, Ding Q, Focal adhesion kinase regulates hepatic stellate cell activation and liver fibrosis. Sci. Rep 7, 4032 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang J, Fan G, Zhao H, Wang Z, Li F, Zhang P, Zhang J, Wang X, Wang W, Targeted inhibition of focal adhesion kinase attenuates cardiac fibrosis and preserves heart function in adverse cardiac remodeling. Sci. Rep 7, 43146 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim N-G, Gumbiner BM, Adhesion to fibronectin regulates Hippo signaling via the FAK–Src–PI3K pathway. J. Cell Biol 210, 503–515 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Czabotar PE, Lessene G, Strasser A, Adams JM, Control of apoptosis by the BCL-2 protein family: Implications for physiology and therapy. Nat. Rev. Mol. Cell Biol 15, 49–63 (2014). [DOI] [PubMed] [Google Scholar]
- 60.Mullard A, Pioneering apoptosis-targeted cancer drug poised for FDA approval. Nat. Rev. Drug Discov 15, 147–149 (2016). [DOI] [PubMed] [Google Scholar]
- 61.Rizvi S, Mertens JC, Bronk SF, Hirsova P, Dai H, Roberts LR, Kaufmann SH, Gores GJ, Platelet-derived growth factor primes cancer-associated fibroblasts for apoptosis. J. Biol. Chem 289, 22835–22849 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Richert L, Engler AJ, Discher DE, Picart C, Elasticity of native and cross-linked polyelectrolyte multilayer films. Biomacromolecules 5, 1908–1916 (2004). [DOI] [PubMed] [Google Scholar]
- 63.Butler JP, Nakamura M, Sasaki H, Sasaki T, Takishima T, Poissons’ ratio of lung parenchyma and parenchymal interaction with bronchi. Jpn. J. Physiol 36, 91–106 (1986). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Effect of pan-caspase inhibitor Z-VAD and caspase-8 inhibitor emricasan on myofibroblast apoptosis induced by FAK inhibition.
Fig. S2. siRNA transfection efficiency and gene expression of BCL-2, BCL-XL, and MCL-1.
Fig. S3. Effect of YAP/TAZ knockdown and overexpression on fibroblast mitochondrial priming and survival.
Fig. S4. BH3 profiling of Normal 3, Scleroderma 4, and Scleroderma 6 fibroblasts.
Fig. S5. Effect of FAK and ROCK inhibition on mitochondrial priming and antiapoptotic BCL-2 members.
Fig. S6. Targeted apoptosis of myofibroblast reverses established fibrosis.
Table S1. Reverse transcription PCR primer and siRNA sequences.