Summary:
Alcohol, a muscle relaxant, can potentially worsen obstructive sleep apnea (OSA) but the literature on the effects of alcohol on OSA is conflicting. This systematic review and meta-analysis of randomized controlled trials examined the impact of alcohol on breathing parameters during sleep. Ovid Medline, Embase and PsycINFO databases were queried through November 1, 2017 for studies that reported objective measures of breathing during sleep, prior to and after alcohol administration. Weighted mean differences (WMD) and 95% confidence intervals (CI) were calculated for apnea-hypopnea index (AHI) and mean oxyhemoglobin saturation (SpO2). Secondary outcome measures were examined where available. The meta-analysis of 14 eligible studies (n=422; 71.9% male) found that AHI increased significantly after alcohol administration (WMD=2.33; 95%CI=1.41 to 3.25, I2=62%) and mean SpO2 was significantly reduced (WMD=−0.60; 95% CI=−0.72 to −0.49, I2=0%). The increase in AHI was greater in snorers (WMD = 4.20; 95% CI = 1.19 to 6.50, I2 = 0%) and those with a diagnosis of OSA (WMD = 7.10; 95% CI = 3.59 to 10.61, I2 = 0%). Additionally, a significant increase in respiratory event duration (WMD=0.86; 95% CI=0.18 to 1.55, I2=19%) and decrease in nadir SpO2 (WMD=−1.25; 95% CI=−2.00 to −0.50, I2=25%) were noted. Alcohol is a modifiable risk factor that can result in the development or worsening of OSA.
Keywords: Alcohol, sleep apnea, oxygen saturation
Introduction:
Obstructive sleep apnea (OSA) is characterized by repeated, partial or complete closure of the upper airway occurring during sleep. It is a highly prevalent condition that is associated with multiple adverse neuropsychological and cardiovascular complications (1). OSA has been shown to increase the risk of incident hypertension, cardiac arrhythmias, ischemic heart disease and heart failure (2–5). While smoking is a recognized modifiable risk factor for OSA, the impact of alcohol on OSA is not as clear, with studies yielding conflicting results on the effect of alcohol consumption on sleep disordered breathing parameters (6–10). Alcohol, a central nervous system depressant with peripheral muscle relaxant effects, could theoretically exert an important moderating effect on the incidence and severity of OSA. Current American Academy of Sleep Medicine (AASM) guidelines recommend educating patients about the possible deleterious effects of alcohol on OSA (11).
Alcohol consumption is common in the United States with up to 56% of individuals ≥ 18 years of age reporting alcohol use within the past month according to the 2015 National Survey on Drug use and Health (12). A recent study demonstrated that there has been a significant rise in the proportion of adults consuming alcohol in the general population (13). There is some evidence to suggest that moderate alcohol use, defined as up to one drink per day for women and up to two drinks per day for men, is associated with a reduced risk of heart disease and ischemic stroke (14, 15). However, alcohol has been shown to reduce genioglossal muscle activity; this may in turn worsen sleep apnea and conceivably lead to heightened morbidity and mortality risks (16).
While current guidelines recommend against use of alcohol in patients with OSA, the extent of its effect on sleep-related breathing parameters in subjects with OSA is unclear. Furthermore, the impact of alcohol on sleep-related breathing parameters in those without OSA is uncertain. We performed a systematic review and meta-analysis of randomized controlled trials to examine the impact of alcohol consumption on respiratory parameters during sleep in all subjects regardless of comorbidity. The primary outcomes of interest were the apnea-hypopnea index (AHI) and mean oxyhemoglobin saturation (SpO2) in sleep. Subgroup analyses based on the presence of OSA and snoring status were also performed to examine the differential impact of alcohol on sleep-related breathing parameters in normal subjects and high-risk groups.
Methods:
The reporting of this manuscript follows the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines (Record ID: 84086) (17).
Search strategy and selection criteria:
A systematic search of the literature was performed by an independent librarian who verified the strategy prior to conducting the search. OVID Medline (1946 to July 31, 2016), Embase (1988 to July 31, 2016) and PsycINFO (1967 to July 31, 2016) databases were queried utilizing search terms of alcohol/ethanol and sleep apnea/disturbance/polysomnogram (further details of search terms and results are provided in the Supplement). The literature search was subsequently updated to November 1, 2017 to ensure that no recent articles were missed. Additionally, references of research articles and review articles were manually reviewed. Any study of human subjects that reported objective measurement(s) of breathing parameters (AHI, apnea index (AI), hypopnea index (HI), mean and/or minimum oxyhemoglobin saturation) and provided data on the same subjects, both prior to and following the consumption of alcohol i.e., reported at least one alcohol challenge night and one placebo night, were eligible for inclusion.
Study selection and data extraction:
All titles and abstracts were initially reviewed to identify articles of interest by two of the authors independently (MF and FS). Any discrepancy was resolved by discussion with a third author (BPK). Following this initial review, full texts of the included abstracts were examined to assess for eligibility. The data from the articles that met full inclusion criteria were extracted by authors MF and FS. For studies where the methodology indicated that details regarding breathing parameters were likely captured, authors were contacted and requested to share any unpublished data if possible.
Outcomes:
The primary outcomes of interest were AHI and/or mean SpO2 in sleep. Secondary outcomes were assessed when available, and included obstructive apnea index, central apnea index, oxyhemoglobin desaturation index, lowest (nadir) SpO2 and respiratory event duration.
Risk of bias assessment:
The Cochrane Collaboration Risk of Bias tool was used to assess the risk of bias within and between the individual studies. Bias was assessed based on subject selection, randomization to placebo/alcohol, blinding of personnel and subjects, and incomplete reporting.
Statistical Analysis:
Since both primary outcomes and secondary outcomes were continuous measures, weighted mean differences (WMD) and 95% confidence intervals were calculated. In studies where subjects received varying quantities of alcohol on different nights and/or included different patient subgroups, a single combined mean and standard deviation for breathing parameters was computed using the formulae provided in the Cochrane Handbook for Systematic Reviews of Interventions (18). The D-L random effects model was utilized to pool WMDs of the included studies. Additional analyses where results were expressed in terms of standard mean differences (SMD) were also performed. Additional data to allow for subgroup analysis was only available for categorization based on the participants’ snoring status. Sensitivity analyses were performed using the “trim-and-fill” method which included trimming and filling the studies that exclusively recruited subjects with chronic obstructive pulmonary disease (COPD) and/or OSA. Publication bias was assessed by visually inspecting a funnel plot. Heterogeneity was assessed using I2 statistic. All statistical analyses were performed utilizing the RevMan™ version 5.3.
Results:
Search results:
The initial literature search yielded 1463 articles (Fig. 1). After review of the abstracts, full texts of 45 articles were considered. Of these, 14 articles met full inclusion criteria; eight studies provided data on both AHI and mean SpO2, and three studies each provided data on either AHI or mean SpO2 alone (9, 10, 19–29). Detailed review of references did not yield any further articles. An updated search contributed 114 new articles, none of which met inclusion criteria. Additional data was made available by the authors of one study, which were incorporated in the meta-analysis (30).
Figure 1.
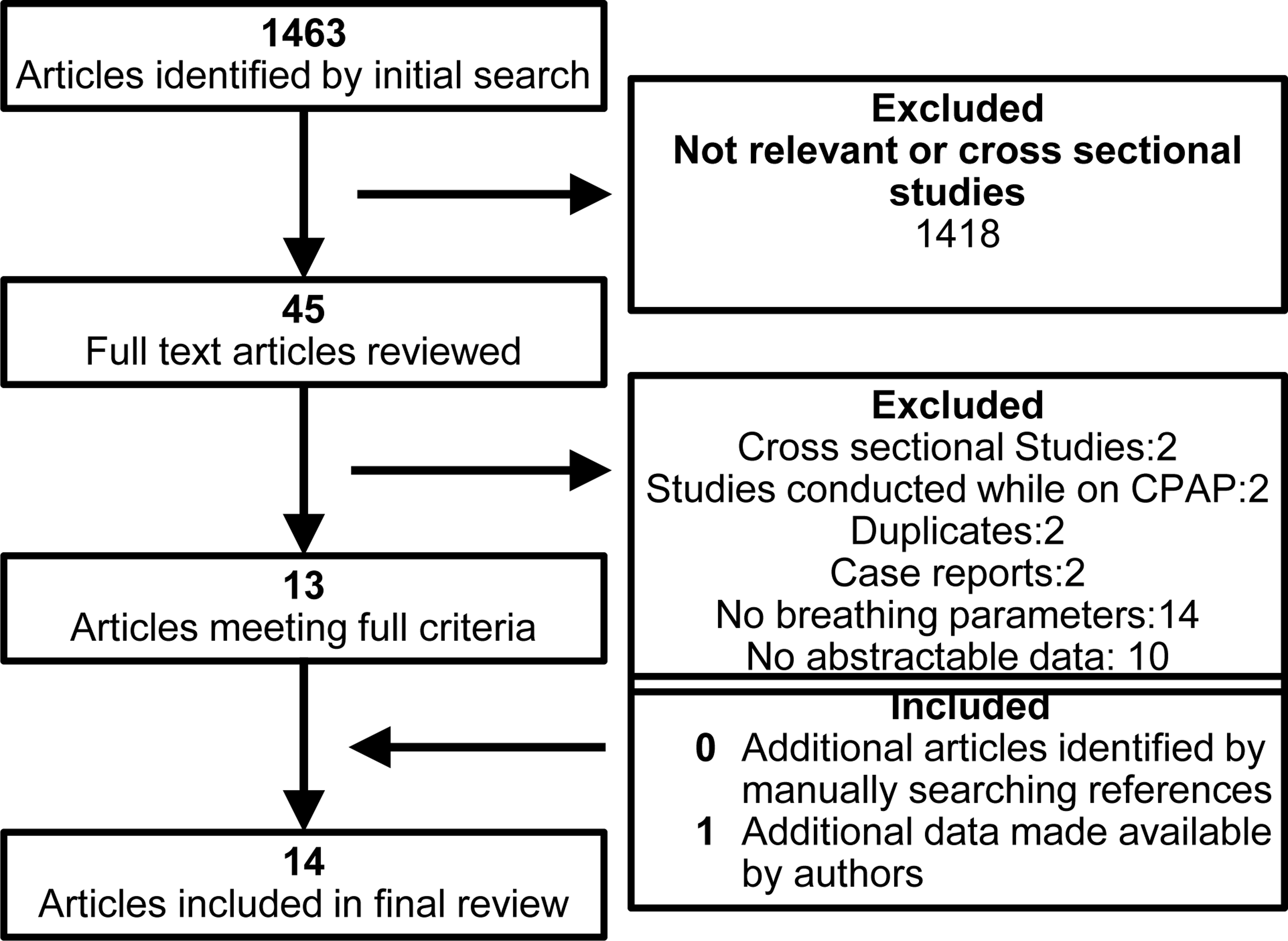
Flow diagram of studies included in the meta-analyses
Characteristics of included studies:
The characteristics of the included studies are outlined in Table 1. All studies were cross-over randomized controlled trials of alcohol versus placebo. A total of 422 subjects were included, 71.9% were male. The overall risk of bias was considered to be moderate. The majority of investigations recruited asymptomatic healthy male volunteers. Two studies exclusively recruited patients with COPD (9, 21). Two other studies recruited patients with a prior diagnosis of OSA (22, 29). One of these studies examined the impact of alcohol on continuous positive airway pressure (CPAP) requirements. In this study, only data comparing breathing parameters during sleep on the control night to the night when alcohol was consumed without the use of CPAP were included in the analyses (29). The estimated number of standard drinks consumed per subject, assuming a body weight of 70 kg and 14 g of alcohol per standard drink, varied between 1.5 and six per night, with the majority of the studies utilizing two to three standard drinks per night (31). Expressed in terms of grams per kilogram (g/kg) of body weight, this ranged from 0.325 g/kg to 1.2 g/kg with a median dose of 0.5 g/kg.
Table 1.
Characteristics of studies included in the meta-analysis
| Author and date of Publication | N ()* | Patient characteristics | Study design | Blinding status | Amount and type of alcohol consumed | AHI- Baseline (SD) | AHI-After alcohol consumption (SD) | Mean oxygen saturation in sleep-Baseline (SD) | Mean oxygen saturation in sleep-After alcohol consumption (SD) |
|---|---|---|---|---|---|---|---|---|---|
| Arnedt-2017 | 93 | Healthy adults | Cross-over randomized clinical trial | Double-blind | 1.2 g/kg for men and 1.1 g/kg for women | 2.59(3.17) | 2.84(4.23) | 96.88 (1.08) | 96.14 (1.05) |
| Block-1986 | 78 | Healthy subjects | Cross-over randomized clinical trial | None | 1 ml/kg vodka | Not available | Not available | 95.32 (6.78) | 95.37 (5.06) |
| Block-1987 | 17 (34) | Healthy subjects | Cross-over randomized clinical trial | None | 1.1 ml/kg vodka/0.95 scotch | Not available | Not available | 95.9 (1.3) | 95.7 (1.4) |
| Brander-1992 | 9 | Male patients with advanced COPD | Cross-over randomized clinical trial | None | 0.5 g/Kg | Not available | Not available | 89.1 (1.6) | 88.4 (2) |
| Collop-1994 | 13 | Men with OSA | Cross-over clinical trial | None | 1 ml/kg of 100-proof vodka | 9.6 (5.3) | 20.2 (16) | 91.8 (1) | 91.8 (1) |
| Herzog-2004 | 21 (42) | 8 snorers and 13 non-snorers | Cross-over randomized clinical trial | None | Blood alcohol concentrations (BAC) of 0.5 and 0.8%. | 2.76 (2.18) | 5.4 (4.78) | 95.86 (0.25) | 95.23 (0.48) |
| Holmendahl-2015 | 26 | Patients with COPD | Cross-over randomized clinical trial | None | 0.5ml/kg of 96% vol ethanol | 9.7 (10.2) | 10.4 (16.4) | 93.4 (4.7) | 93.2 (5.7) |
| Izumi-2005 | 23 | Healthy men | Cross-over randomized clinical trial | None | 0.7 g/kg varied | 2.9 (4.5) | 7.8 (8.2) | 95.7 (1.3) | 95.2 (1.3) |
| Kido-2016 | 18 | Healthy subjects | Cross-over randomized clinical trial | None | 40 g total- traditional Japanese alcohol beverages | 2 (1) | 5.6 (4.8) | Not available | Not available |
| Reimann-2010 | 20 (40) | 20 men (10 snorers and 10 non-snorers) | Cross-over randomized clinical trial | None | Blood alcohol concentrations (BAC) of 0.5 and 0.8%. | 3.15 (2.98) | 6 (6.65) | 95.4 (3.76) | 94.57 (6.05) |
| Scanlan-2000 | 21 | Men- habitual snorers | Cross-over randomized clinical trial | None | 0.5 g/kg White wine | 7.1 (1.9) | 9.7 (2.1) | 95.9 (0.4) | 95.3 (0.4) |
| Scrima-1989 | 62 (186) | 30 snorers and 32 non-snorers | Cross-over randomized clinical trial | Double Blind | 0.325, 0.65, and 0.8 1 g /kg | 13.32 (9.72) | 14.26 (11) | 95.95 (2.6) | 95.34 (3.16) |
| Taasan-1981 | 20 | Healthy men | Cross-over randomized clinical trial | Blinding attempted but broken | 2 g/kg vodka | 0.7 (0.75) | 2.33 (1) | Not available | Not available |
| Teschler-1996 | 14 | Men with OSA | Cross-over randomized clinical trial | Investigators blinded to alcohol status | 0.5 g/kg vodka | 44.1 (7.2) | 50.6 (0.8) | Not available | Not available |
BMI ¼ body mass index, kg/m2; SpO2 ¼ oxyhemoglobin saturation; AHI ¼ apnea-hypopnea index; OSA ¼ Obstructive sleep apnea; COPD ¼ chronic obstructive pulmonary disease.
() = Number of subjects included in meta-analysis if multiple doses of alcohol were compared against placebo in the study.∞ Number of drinks estimated assuming body weight of 70 kg and 14 g alcohol per average drink.
In two of the 14 studies, both subjects and investigators were blinded to alcohol status (10, 30). One group attempted to blind subjects and investigators to alcohol status but reported that the blinding was easily broken (28). Another study reported that only the investigators were blinded to alcohol status (29). The remainder of the studies either did not attempt to blind subjects/investigators or did not provide any information regarding blinding. The order in which subjects received alcohol/placebo was a random in all studies except one, where subjects received alcohol on the second night of recording (22).
Eight out of the 14 studies reported total sleep times on alcohol and placebo nights (9, 19, 20, 22, 25, 27, 29, 30). Only two studies found a significant difference in the sleep duration with subjects obtaining more sleep on the nights that they received alcohol (20, 22). One study reported differences in breathing parameters between rapid eye movement (REM) and non-REM sleep (10). In this study, the number of disordered breathing events per hour of REM and non-REM sleep was similar following the administration of alcohol and placebo. Four studies reported differences based on the time of the night (21, 22, 24, 29). Two studies reported that mean SpO2 following alcohol consumption was lower in the initial part of the night compared to placebo (21, 24). There was no difference in mean SpO2 between the two conditions in the latter part of the night. The findings with regard to the differences in AHI based on the time of the night were conflicting. One study reported a significant difference following alcohol administration in the initial 3 h of sleep while another reported differences only in the latter part of the night (22, 24). A third study reported no differences in AHI between alcohol and placebo nights in the initial 2 h of sleep (29) (Table 2).
Table 2.
Additional details from included studies
| Author and date of Publication | AHI Definition | Changes in Sleep Duration following alcohol use TST (SD) Alcohol Night Placebo Night P value of difference |
Differences based on Sleep Stage REM AHIR(SD) NREM AHI N(SD) P value of difference |
Differences based on hours following alcohol consumption Hours from sleep onset AHIA/SpO2A alcohol(SD) AHIp/SpO2p placebo(SD) P value of difference |
|---|---|---|---|---|
| Arnedt et al 2017 30 | NA | 425.25(42.60) 436.69(31.02) NS |
NA | NA |
| Block et al 1986 19 | Apnea: cessation of flow at the nose and mouth for ≥10 s Hypopnea: Decreased flow and chest movement with oxygen desaturation Desaturation was thought to be clinically significant when a decrease of 4 >% from the preceding baseline occurred |
319.48 (61.01) 338.31 (70.59) NS |
NA | NA |
| Block et al 1987 20 | Apnea: cessation of flow at the nose and mouth for ≥10 s Hypopnea: Decreased flow and chest movement with oxygen desaturation Desaturation was thought to be clinically significant when a decrease of >4% from the preceding baseline occurred |
357 (34) 281.5 (59.82) P <0.05 |
NA | NA |
| Brander et al 1992 21 | No AHI reported | NA | NA | SpO2 was lower in the first 2 h after alcohol consumption* |
| Collop et al 1994 22 | Apnea: cessation of airflow at the mouth and nose for ≥10 s Hypopnea: 50% diminution in airflow accompanied by oxygen desaturation of ≥4% |
403.7 (24) 346.1 (75.7) P <0.05 |
NA | 1st 3 hours: AHIA=23.8 (7.7) AHIP=7.37 (1.7) P <0.05 |
| Herzog et al 2004 23 | Apnea: 20% reduction to complete cessation of respiratory airflow for >10 s Hypopnea: 50% reduction in airflow |
NA | NA | NA |
| Herzog et al 2004 23 | Apnea: cessation of nasal pressure recording for ≥10 s Hypopnea: ≥30% reduction of nasal pressure for ≥10 s with ≥4% desaturation from baseline, with ≥90% of the event’s duration meeting the amplitude reduction criteria for hypopnea |
354 (62.5) 354 (65.3) NS |
||
| Izumi et al 2005 24 | Apnea: >10 second pause in ventilation Hypopnea: ≥30% reduction in airflow |
NA | NA | In the latter half of the night: AHIA=5.5(5.5) AHIP=1.3(1.6) P<0.001 In the early half of the night: SpO2A=94.8(1.4) SpO2P=95.7(1.3) P<0.001∞ |
| Kido et al 2016 25 | Not Available | 482.9 (41.3) 474.1 (38.2) NS |
NA | NA |
| Reimann et al 2010 26 | NA | NA | NA | NA |
| Scanlan et al 2000 27 | Apnea: absence of oronasal airflow for >10 s despite continued out-of-phase chest and abdominal movements Hypopnea: reduction in oronasal airflow for >10 s associated with a >2% fall in SpO2 despite continued out-of-phase chest and abdominal movements, increased submental EMG activity, or snoring |
399 (8) 400 (11) NS |
NA | NA |
| Scrima et al 1989 10 | No AHI reported, only DBEs. Apnea: thermistor tracing indicated cessation of breathing for >10 s accompanied by rhythmical bursts of intercostal EMG activity Hypopnea: discernible decrease but not cessation in ventilation for >10 s, accompanied by increased respiratory effort and a decline in SpO2 to 92% or below |
NA | No difference in DBEs in REM and NREM sleep following alcohol consumption compared to placebo | NA |
| Taasan et al 1981 28 | Apnea: cessation of nasal or oral flow ≥ 10 s Hypopnea: desaturation (≥4%) and simultaneous decrease in air flow or chest movement |
NA | NA | NA |
| Teschler et al 1996 29 | Apnea: episodes of cessation of airflow lasting >10 s associated with paradoxical movements of the chest wall and abdomen Hypopnea: episodes other than apneas lasting >10 seconds during which the thermistor signal was reduced to <50% of its magnitude during normal unobstructed breathing and SpO2 dropped by ≥4% |
320 (6) 333 (5) NS |
First 2 hours of the night: AHIA= 41.0(7.8) AHIP= 45.6(6.2) NS |
AHI= Apnea hypopnea index NA=Not available NS= Not significant AHIA=AHI on alcohol night AHIP=AHI on placebo night SpO2A=SpO2 on alcohol night SpO2P=SpO2 on placebo night DBE=disordered breathing events.
Numerical values not provided.∞ No significant difference in AHI in the early part of the night and SpO2 in the latter part of the night
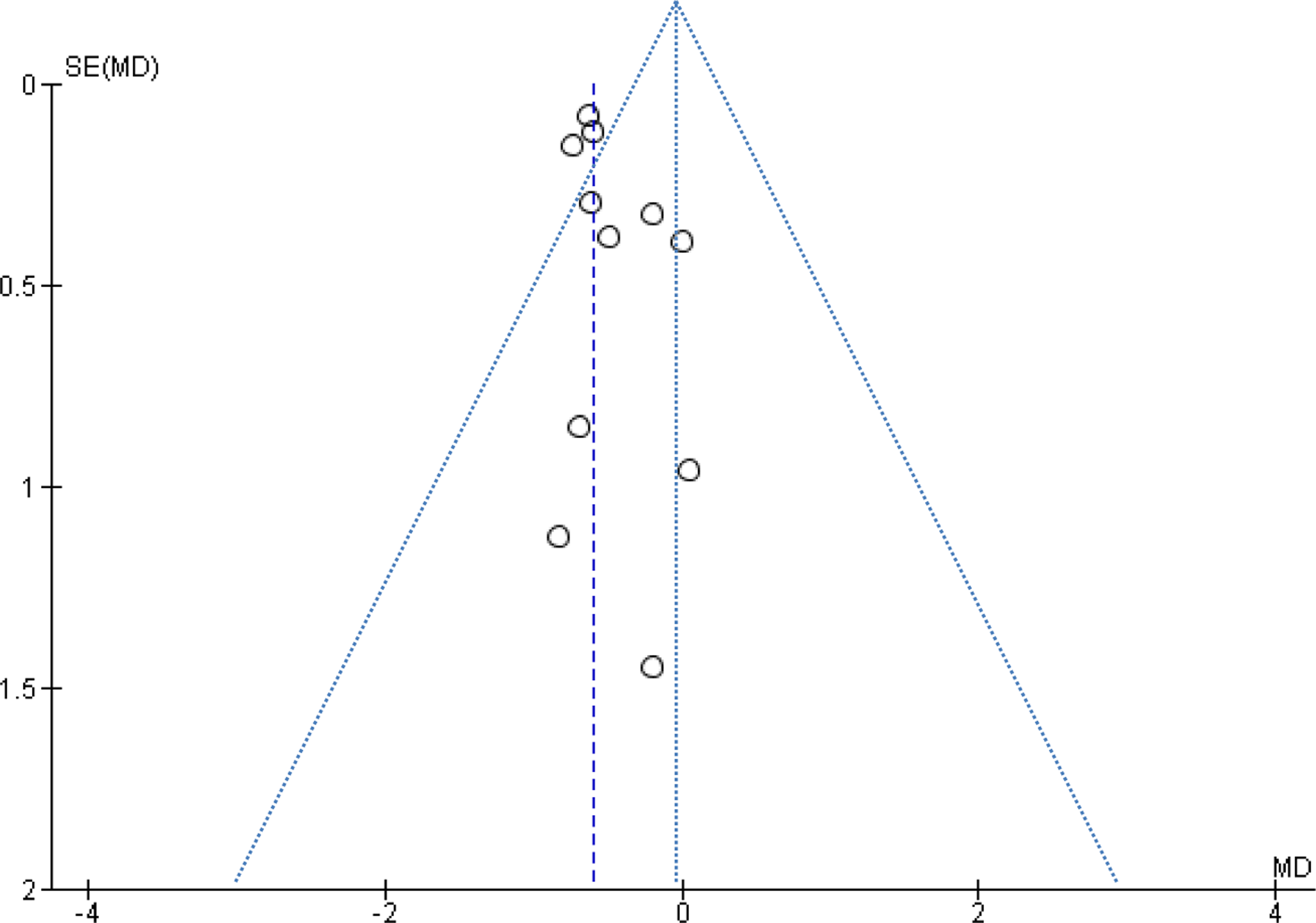
A funnel plot indicated potential publication bias with fewer small negative studies (Fig. 2a,b). Overall, risk of bias of the included investigations was moderate. There was moderate heterogeneity among the studies that reported data on AHI (I2 =62%). Evaluation of the heterogeneity among the articles that reported data on mean SpO2 was not possible (I2=0%).
Figure 2.
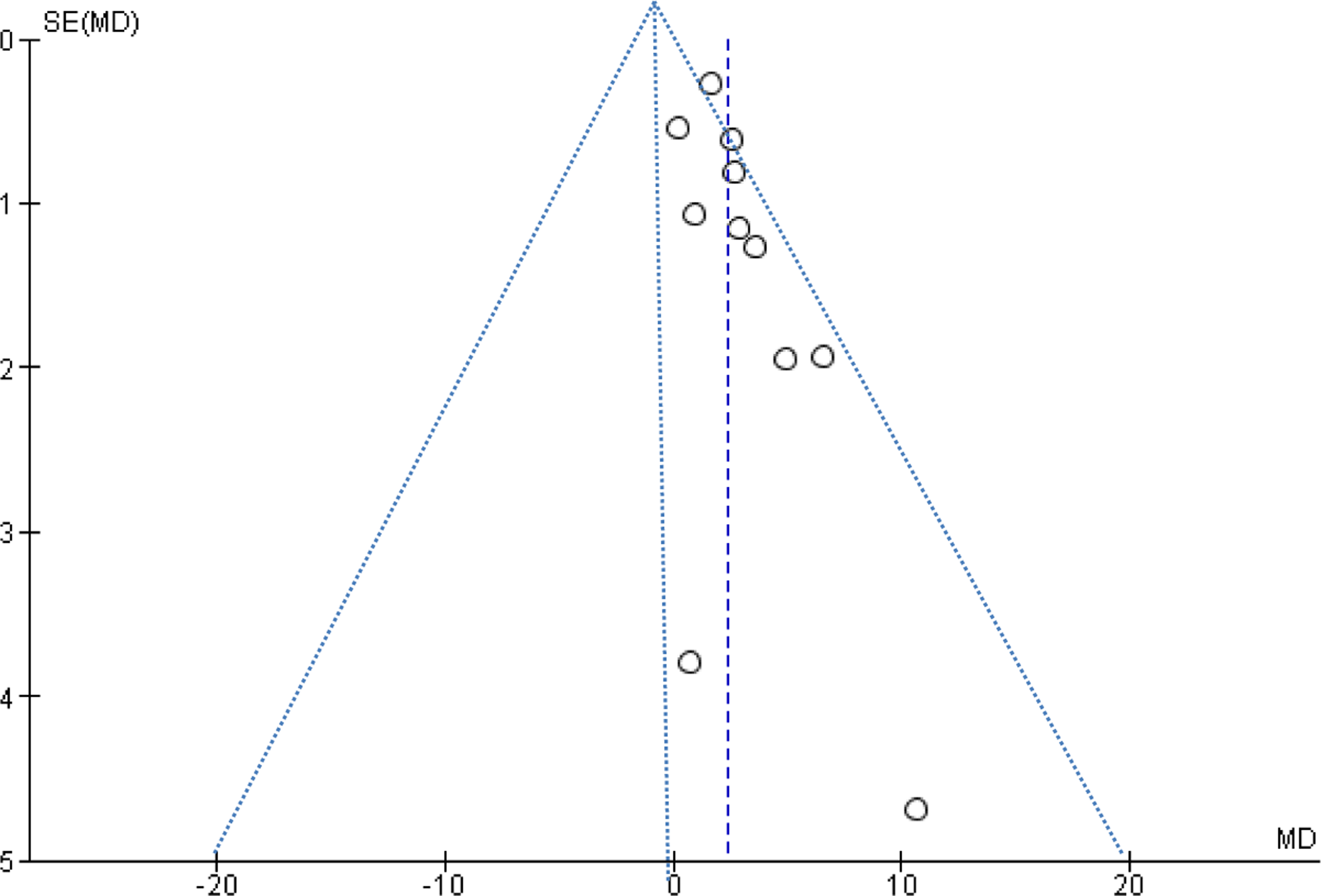
a. Funnel plot of studies comparing AHI
Figure 2 b. Funnel plot of studies comparing mean SpO2
Impact of alcohol on AHI and oxygenation status:
A total of 11 studies provided data regarding the change in AHI as well as mean SpO2 in sleep following the consumption of alcohol versus placebo. Alcohol intake resulted in significant increase in AHI (WMD=2.33 events/hour; 95% CI=1.41 to 3.25, I2=62%). The use of alcohol also resulted in significant decrease in mean SpO2 in sleep (WMD=−0.60; 95% CI=−0.72 to −0.49, I2=0%) (Figs 3 and 4). Additional analyses were performed after excluding subjects with a diagnosis of COPD and those with COPD and OSA; alcohol use continued to be associated with significant worsening of AHI and mean SpO2 in sleep (Figures S1–4 in the Supplement). Analyses reporting results in terms of SMD are presented in the online supplement (Figures S5 and 6 in the Supplement).
Figure 3.
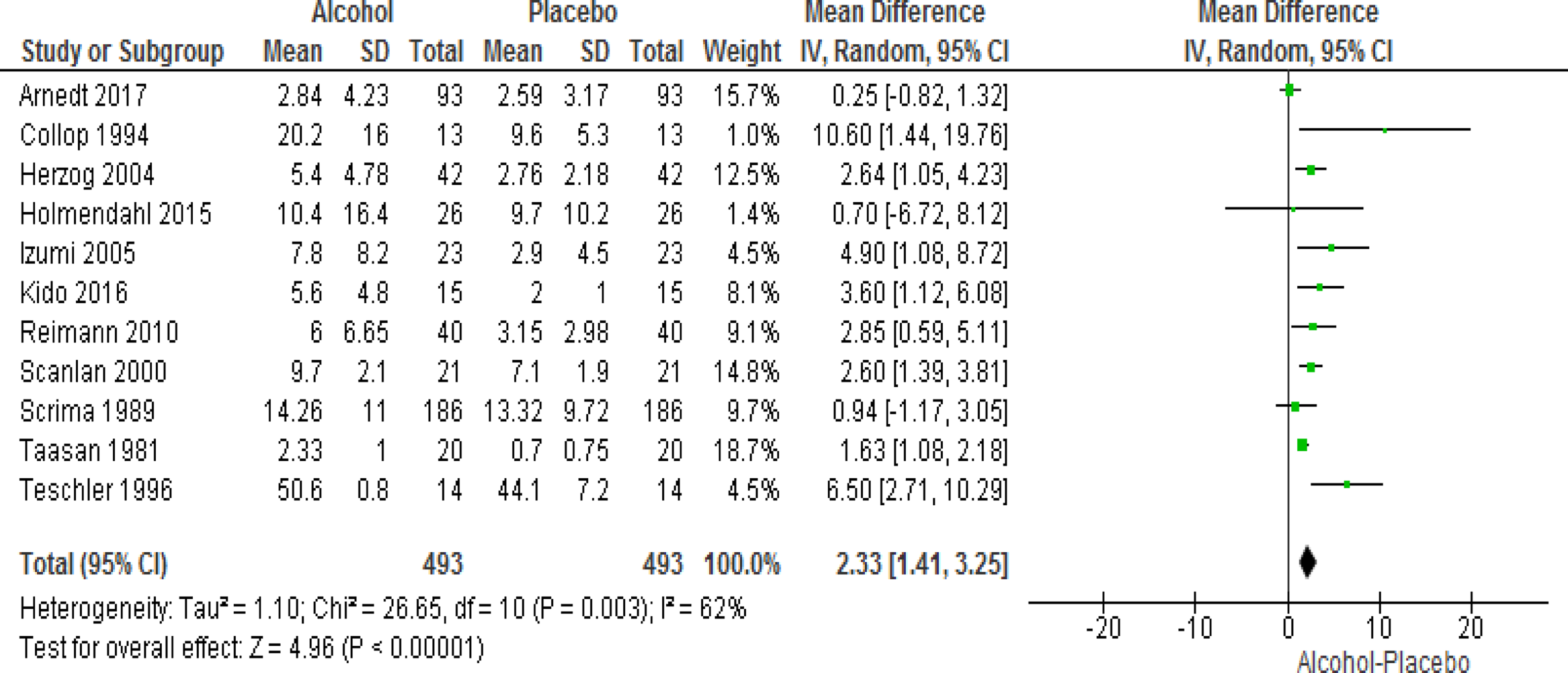
Forest plot of mean difference in AHI on alcohol exposed nights V. Placebo nights
Figure 4.
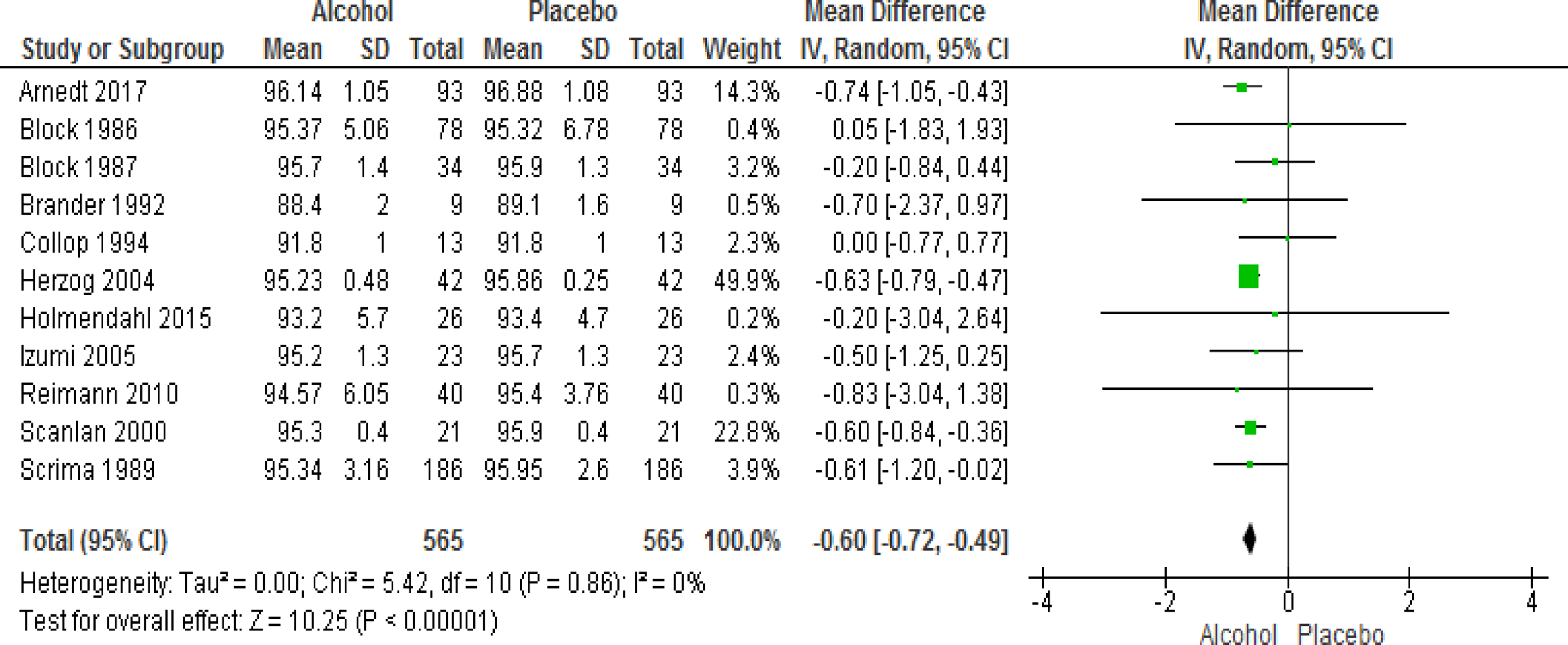
Forest plot of mean difference in mean SpO2 on alcohol exposed nights V. Placebo nights
Effects of alcohol on AHI and oxygenation by snoring status:
Three studies reported on the effect of alcohol on respiratory parameters in snorers versus non-snorers (10, 23, 26). Subgroup analysis examining the impact of alcohol on breathing parameters in subjects divided by snoring status indicated that the AHI (WMD=4.20 events/hour; 95% CI=1.19 to 6.50, I2=0%) and mean SpO2 in sleep (WMD=−0.92; 95% CI=−1.07 to −0.78, I2=0%) worsened with alcohol consumption compared to placebo in subjects who snored. This effect on the AHI was not noted in subjects without a history of snoring (WMD=0.45 events/hour; 95%CI=−0.22 to 1.12, I2=91%). However, a significant reduction in mean SpO2 in sleep was seen after alcohol consumption even in the non-snoring group (WMD=−0.45; 95% CI= −0.49 to −0.41, I2=0%) (Figures S7 and 8 in the Supplement).
Effects of alcohol on AHI and oxygenation by sleep apnea status:
Two studies reported the effects of alcohol on AHI in subjects with a prior diagnosis of OSA. One study included subjects with mild OSA (mean AHI = 7.3 ± 1.7) (22). The severity of OSA in individual subjects was not categorized in the other study (mean AHI = 44.1 ± 7.2) (29). Subgroup analysis examining the impact of alcohol on breathing parameters indicated that AHI (WMD=7.10 events/hour; 95% CI=3.59 to 10.61, I2=0%) worsened significantly in subjects with OSA (Fig. 5). This increase in AHI was much more substantial when compared to those without a diagnosis of OSA (WMD = 1.97 events/hour; 95% CI = 1.18 to 2.76, I2 = 53%). A subanalysis of the impact on alcohol on SpO2 in subjects with OSA could not be conducted as one of the two studies did not report data on the effect of alcohol on SpO2. The overall impact of alcohol on oxygenation status was unchanged after excluding the single study of subjects with OSA that reported changes in SpO2 (WMD= −0.62; 95% CI= −0.74 to −0.50, I2 = 0%) (Figures S9–10 in the Supplement).
Figure 5.
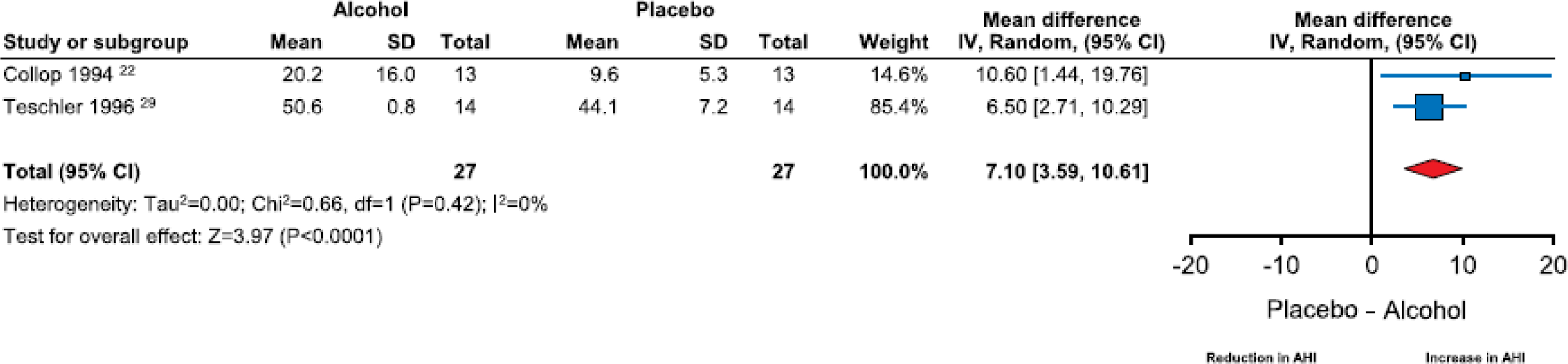
Forest plot of mean difference in apnea-hypopnea index on alcohol-exposed nights versus placebo nights in subjects with OSA.
Effects of alcohol on other breathing parameters during sleep:
Overall, alcohol use did not appear to worsen the apnea index (WMD=0.60 events/hour; 95% CI= −1.54 to 2.75, I2=5%) or hypopnea index (WMD=1.30 events/hour; 95% CI= −0.06 to 2.67, I2=0%), where this was reported separately. Alcohol consumption was associated with a significant lowering of the nadir SpO2 (WMD= −1.25; 95% CI=−2.00 to −0.50, I2=25%) as well as an increase in respiratory event duration (WMD=0.86 s; 95% CI=0.18 to 1.55, I2=19%) and oxyhemoglobin desaturation index (WMD=1.08 events/hour; 95% CI=0.69 to 1.48, I2=0%) versus placebo (Figures S11–15 in the Supplement).
Discussion:
This systematic review and meta-analysis identified 14 crossover randomized controlled trials that examined the impact of alcohol versus placebo on AHI and/or mean SpO2 during sleep. Consumption of alcohol prior to sleep onset resulted in a significant increase in AHI. Sub-group analysis indicated that this effect was particularly evident in patients with OSA and those with a history of snoring. In addition, a reduction in the mean SpO2 with alcohol compared to placebo was noted, however, the clinical relevance of this reduction is likely minimal.
Previous cross-sectional and epidemiological surveys have shown an association between OSA and alcohol consumption (32). This investigation is the first systematic review and meta-analysis of randomized controlled trials reporting objective measures of sleep disordered breathing in subjects prior to and following the consumption of alcohol. The results clearly demonstrate that alcohol consumption worsens sleep-related breathing parameters, particularly in those with a prior diagnosis of OSA and a history of snoring.
OSA is associated with significant cardiovascular risk and the prevalence of OSA has been steadily increasing (2, 3, 33). Lifestyle measures such as weight loss and avoiding smoking are routinely recommended to patients with OSA. Data from the current systematic review and meta-analysis indicate that alcohol consumption can also significantly exacerbate OSA. The changes noted in mean SpO2, although statistically significant, are unlikely to be of much clinical significance. While overall increases in AHI were also small (2.33 events/hour), alcohol consumption in subjects with a history of snoring or a diagnosis of OSA resulted in a change in AHI that was much more substantial (4.2 and 7.1 events/hour respectively).
In subjects with OSA, an increase in AHI by 7.1 events/hour could result in a change from the mild to moderate sleep apnea category. Mild OSA has not been found to be associated with significant cardiovascular risk, and therefore, treatment decisions in this group of patients are generally based on the presence of symptoms such as sleepiness (34). Increasing AHI has been associated with adverse cardiovascular outcomes and neurocognitive effects (3, 4, 35, 36). In regular drinkers with mild OSA, this increase in AHI following alcohol consumption could result in reclassification to moderate OSA and potentially impact long-term risk requiring reassessment of treatment options (37). Patients with OSA should be counseled appropriately about these risks (6, 11). This discussion is particularly important in light of the increasing rates of alcohol consumption in the general population and the perception that moderate alcohol use is associated with cardiovascular benefits (13).
Snoring is a significant risk factor for OSA. There is evidence to suggest that an increase in snoring intensity is associated with a greater severity of sleep disordered breathing (38). Two studies examined the impact of alcohol use on snoring intensity. In one study, the use of alcohol resulted in a rise in the frequency and loudness of snoring in subjects with a prior history of snoring but did not influence either outcome in non-snorers (26). In contrast, another study of habitual snorers showed no effect of alcohol on the intensity of snoring (27). Our sub-group analysis comparing the impact of alcohol in snorers versus non-snorers indicated that although the mean SpO2 in sleep was reduced in both groups, alcohol use worsened the AHI in snorers alone. These results appear to indicate that subjects who snore may be particularly vulnerable to the impact of alcohol and should also be counseled accordingly.
The vast majority of studies included in the meta-analyses utilized between 2 and 3 standard drinks; importantly, this is within the current recommended limits for alcohol consumption (39, 40). Three studies examined the impact of varying amounts of alcohol on breathing parameters during sleep. In one study, increasing levels of alcohol consumption were associated with lower nadir SpO2, particularly in snorers, but not with AHI (10). Another study reported that higher doses of alcohol were associated with an increase in AHI and reduction in mean SpO2 in both snorers and nonsnorers (23). The third study reported that higher alcohol doses were associated with a rise in AHI in snorers and a decrease in mean SpO2 in non-snorers (26). Overall, these findings appear to suggest that higher doses of alcohol may result in an increase in the severity of sleep disordered breathing in a dose-response fashion, particularly in snorers.
Our initial review identified three studies that examined the impact of alcohol consumption on continuous positive airway pressure (CPAP) requirements; only one of these studies was eligible for inclusion in the current meta-analysis (29). In this study as well as the two remaining studies that examined the effect of alcohol in patients with a prior diagnosis of OSA utilizing CPAP, the use of alcohol did not appear to change the required effective pressure to control sleep disordered breathing events (29, 41, 42). Only pressures required to control snoring in the first 2 h following sleep onset appeared to be increased following alcohol consumption (41). One study speculated that the upper airway was already collapsible in patients with OSA and the addition of alcohol did not significantly alter this in patients receiving CPAP therapy to maintain airway patency (42). Thus, the results of these studies with a total of 30 subjects imply that patients with OSA already on CPAP are unlikely to require pressure adjustment following alcohol consumption.
Two studies included in this review comprised of patients with COPD (9, 21). In both of these investigations, a moderate amount of alcohol was consumed and did not influence the mean SpO2 in sleep overall, although, in one of these studies, the mean spO2 was significantly reduced in the initial 2 h following sleep onset (21). Taken together, these results of these studies appear to indicate that moderate alcohol consumption in patients with stable COPD does not significantly affect mean SpO2 in sleep, but it should be noted that only a limited number of patients with COPD were assessed. Sensitivity analysis performed after excluding these two studies indicated that alcohol use resulted in an increase in AHI and a drop in mean SpO2 during sleep in subjects without COPD.
The average metabolic rate of alcohol is about 7 g per hour which translates to about half a drink per hour (43). Subjects in this review, on average, consumed 2–3 standard drinks which could take 4–6 h to be completely eliminated. In addition, alcohol concentrations would be higher in the initial part of the night. Thus there is the potential for alcohol to have a greater impact on breathing parameters in the early part of the night. While two studies did show that the SpO2 was lower only in the early part of the night after alcohol consumption, the results with regard to the change in AHI were conflicting (21, 24). When alcohol and placebo nights were compared; one study demonstrated a reduction in AHI in the early part of the night, another, a reduction in the latter part of the night, while a third showed no change (22, 24, 29). These studies were inconsistent in terms of time of the night that was evaluated. Finally, low to moderate doses of alcohol, as consumed by a majority of subjects in this study, can result in a reduction in REM sleep percentage and delay onset to REM sleep but the differential impact of alcohol consumption on AHI in REM sleep compared to non-REM sleep needs further study (44). In the one study included in this review that reported these differences, the number of disordered breathing events was similar between alcohol and placebo nights in both REM and non-REM sleep.
This systematic review and meta-analysis should be considered in light of some limitations. Based on the funnel plot, there was evidence of possible publication bias, with fewer small negative studies. A moderate degree of heterogeneity of studies reporting AHI data was noted but we attempted to account for this by performing subgroup and sensitivity analyses. Studies included in this meta-analysis were published between 1981 and 2017; during this time there have been significant changes in equipment and scoring criteria which may contribute to heterogeneity of results. Furthermore, the majority of subjects recruited healthy male adults, limiting the ability to generalize these findings to women. The subgroup analysis demonstrating a difference between snorers and non-snorers should be treated as hypothesis-generating and further studies to confirm these findings are warranted. Finally, only a limited number of studies reported additional sleep-related breathing variables such as nadir SpO2, respiratory event duration and oxyhemoglobin desaturation indices, hence, exploratory analyses examining these outcomes should be considered preliminary in nature.
Conclusions:
Consumption of alcohol resulted in significant worsening of the AHI and mean SpO2 in sleep. This was especially notable in subjects with a history of OSA and snoring. Alcohol also appeared to result in prolongation of respiratory event duration and lowering of the minimum SpO2 in sleep. Patients with a diagnosis of OSA and those at risk for this condition, particularly snorers, should be made aware of the potential impact of alcohol on breathing in sleep. Alcohol use should be considered a modifiable risk factor for OSA. Among patients with OSA on CPAP treatment, alcohol consumption did not seem to influence the effective pressure required to control disordered breathing events.
Supplementary Material
Practice Points.
Consumption of alcohol prior to falling asleep results in significant increase in AHI
Alcohol intake prior to sleep also results in reduction of the mean SpO2 but the clinical relevance of this change is likely to be minimal
Subjects with OSA and those with a history of snoring are particularly vulnerable to these effects of alcohol
Alcohol consumption does not appear to influence CPAP pressures required to maintain airway patency
Research Agenda.
Future research must examine the modulating effect of the timing of alcohol consumption prior to sleep onset on sleep-related breathing parameters
Sex differences in the effect of alcohol on breathing parameters during sleep need be evaluated
The amount of alcohol consumed must be measured in a standardized fashion in future studies examining the impact of alcohol on breathing parameters
Acknowledgements:
MPM is the principal investigator on a research grant funded by ResMed Corporation and a benefactor-sponsored career development award at Mayo Clinic, Rochester, Minnesota, that are unrelated to the current study. The authors would like to thank Patricia Erwin, MLS, for her assistance with designing the search strategy and performing the literature search. The authors would also like to thank J. Todd Arnedt, PhD, for providing additional data for inclusion in the meta analyses.
Abbreviations
- AHI
Apnea-hypopnea index
- AI
Apnea index
- CI
Confidence interval
- COPD
Chronic obstructive pulmonary disease
- CPAP
Continuous positive airway pressure
- HI
Hypopnea index
- OSA
Obstructive sleep apnea
- SpO2
Oxyhemoglobin saturation
- WMD
Weighted mean difference
Footnotes
Conflicts of interest: The other authors have no conflict of interest or funding to report.
References:
- 1.Jordan AS, McSharry DG, Malhotra A. Adult obstructive sleep apnoea. Lancet. 2014;383(9918):736–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342(19):1378–84. [DOI] [PubMed] [Google Scholar]
- 3.Punjabi NM, Caffo BS, Goodwin JL, Gottlieb DJ, Newman AB, O’Connor GT, et al. Sleep-disordered breathing and mortality: a prospective cohort study. PLoS medicine. 2009;6(8):e1000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gottlieb DJ, Yenokyan G, Newman AB, O’Connor GT, Punjabi NM, Quan SF, et al. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the sleep heart health study. Circulation. 2010;122(4):352–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cadby G, McArdle N, Briffa T, Hillman DR, Simpson L, Knuiman M, et al. Severity of OSA is an independent predictor of incident atrial fibrillation hospitalization in a large sleep-clinic cohort. Chest. 2015;148(4):945–52. [DOI] [PubMed] [Google Scholar]
- 6.Krishnan V, Dixon-Williams S, Thornton JD. Where There Is Smoke…There Is Sleep Apnea: Exploring the Relationship Between Smoking and Sleep Apnea. Chest. 2014;146(6):1673–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Young T, Skatrud J, Peppard PE. Risk factors for obstructive sleep apnea in adults. Jama. 2004;291(16):2013–6. [DOI] [PubMed] [Google Scholar]
- 8.Mason M, Cates CJ, Smith I. Effects of opioid, hypnotic and sedating medications on sleep-disordered breathing in adults with obstructive sleep apnoea. The Cochrane database of systematic reviews. 2015(7):Cd011090. [DOI] [PubMed] [Google Scholar]
- 9.Holmedahl NH, Øverland B, Fondenes O, Ellingsen I, Hardie JA. Alcohol at bedtime induces minor changes in sleep stages and blood gases in stable chronic obstructive pulmonary disease. Sleep and Breathing. 2015;19(1):307–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scrima L, Hartman PG, Hiller FC. Effect of three alcohol doses on breathing during sleep in 30–49 year old nonobese snorers and nonsnorers. Alcohol Clin Exp Res. 1989;13(3):420–7. [DOI] [PubMed] [Google Scholar]
- 11.Epstein LJ, Kristo D, Strollo PJ Jr., Friedman N, Malhotra A, Patil SP, et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5(3):263–76. [PMC free article] [PubMed] [Google Scholar]
- 12.[cited 2017 November 1]. Available from: https://www.samhsa.gov/data/sites/default/files/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015.htm#tab2-41b.
- 13.Grant BF, Chou S, Saha TD, et al. Prevalence of 12-month alcohol use, high-risk drinking, and dsm-iv alcohol use disorder in the united states, 2001–2002 to 2012–2013: Results from the national epidemiologic survey on alcohol and related conditions. JAMA Psychiatry. 2017;74(9):911–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Tol A, Hendriks HFJ. Moderate alcohol consumption: effects on lipids and cardiovascular disease risk. Current Opinion in Lipidology. 2001;12(1):19–23. [DOI] [PubMed] [Google Scholar]
- 15.Agarwal DP. Carioprotective effects of light-moderate consumption of alcohol: A review of putative mechanisms. Alcohol and Alcoholism. 2002;37(5):409–15. [DOI] [PubMed] [Google Scholar]
- 16.Krol RC, Knuth SL, Bartlett D Jr. Selective reduction of genioglossal muscle activity by alcohol in normal human subjects. Am Rev Respir Dis. 1984;129(2):247–50. [PubMed] [Google Scholar]
- 17.Moher D, Liberati A, Tetzlaff J, Altman DG, The PG. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS medicine. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.[cited 2017 November 1]. Available from: http://handbook-5-1.cochrane.org/. [Google Scholar]
- 19.Block AJ, Hellard DW, Slayton PC. Effect of alcohol ingestion on breathing and oxygenation during sleep. Analysis of the influence of age and sex. Am J Med. 1986;80(4):595–600. [DOI] [PubMed] [Google Scholar]
- 20.Block AJ, Hellard DW. Ingestion of either scotch or vodka induces equal effects on sleep and breathing of asymptomatic subjects. Arch Intern Med. 1987;147(6):1145–7. [PubMed] [Google Scholar]
- 21.Brander PE, Kuitunen T, Salmi T, Partinen M. Nocturnal oxygen saturation in advanced chronic obstructive pulmonary disease after a moderate dose of ethanol. Eur Respir J. 1992;5(3):308–12. [PubMed] [Google Scholar]
- 22.Collop NA. Medroxyprogesterone acetate and ethanol-induced exacerbation of obstructive sleep apnea. Chest. 1994;106(3):792–9. [DOI] [PubMed] [Google Scholar]
- 23.Herzog M, Riemann R. Alcohol ingestion influences the nocturnal cardio-respiratory activity in snoring and non-snoring males. Eur Arch Otorhinolaryngol. 2004;261(8):459–62. [DOI] [PubMed] [Google Scholar]
- 24.Izumi I, Nasermoaddeli A, Sekine M, Kagamimori S. Effect of moderate alcohol intake on nocturnal sleep respiratory parameters in healthy middle-aged men. Environ. 2005;10(1):16–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kido M, Asakawa A, Koyama KK, Takaoka T, Tajima A, Takaoka S, et al. Acute effects of traditional Japanese alcohol beverages on blood glucose and polysomnography levels in healthy subjects. PeerJ. 2016;2016(4):e1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Riemann R, Volk R, Muller A, Herzog M. The influence of nocturnal alcohol ingestion on snoring. Eur Arch Otorhinolaryngol. 2010;267(7):1147–56. [DOI] [PubMed] [Google Scholar]
- 27.Scanlan MF, Roebuck T, Little PJ, Redman JR, Naughton MT. Effect of moderate alcohol upon obstructive sleep apnoea. Eur Respir J. 2000;16(5):909–13. [DOI] [PubMed] [Google Scholar]
- 28.Taasan VC, Block AJ, Boysen PG, Wynne JW. Alcohol increases sleep apnea and oxygen desaturation in asymptomatic men. Am J Med. 1981;71(2):240–5. [DOI] [PubMed] [Google Scholar]
- 29.Teschler H, Berthon-Jones M, Wessendorf T, Meyer HJ, Konietzko N. Influence of moderate alcohol consumption on obstructive sleep apnoea with and without AutoSet nasal CPAP therapy. Eur Respir J. 1996;9(11):2371–7. [DOI] [PubMed] [Google Scholar]
- 30.Arnedt JT, Rohsenow DJ, Almeida AB, Hunt SK, Gokhale M, Gottlieb DJ, et al. Sleep Following Alcohol Intoxication in Healthy, Young Adults: Effects of Sex and Family History of Alcoholism. Alcoholism: Clinical and Experimental Research. 2011;35(5):870–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.[Available from: https://www.rethinkingdrinking.niaaa.nih.gov/How-much-is-too-much/what-counts-as-a-drink/whats-A-Standard-drink.aspx.
- 32.Stradling JR, Crosby JH . Predictors and prevalence of obstructive sleep apnoea and snoring in 1001 middle aged men. Thorax. 1991;46(2):85–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Senaratna CV, Perret JL, Lodge CJ, Lowe AJ, Campbell BE, Matheson MC, et al. Prevalence of obstructive sleep apnea in the general population: A systematic review. Sleep Med Rev.34:70–81. [DOI] [PubMed] [Google Scholar]
- 34.Chowdhuri S, Quan SF, Almeida F, Ayappa I, Batool-Anwar S, Budhiraja R, et al. An Official American Thoracic Society Research Statement: Impact of Mild Obstructive Sleep Apnea in Adults. Am J Respir Crit Care Med. 2016;193(9):e37–54. [DOI] [PubMed] [Google Scholar]
- 35.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342(19):1378–84. [DOI] [PubMed] [Google Scholar]
- 36.Bucks RS, Olaithe M, Eastwood P. Neurocognitive function in obstructive sleep apnoea: a meta-review. Respirology. 2013;18(1):61–70. [DOI] [PubMed] [Google Scholar]
- 37.Kendzerska T, Mollayeva T, Gershon AS, Leung RS, Hawker G, Tomlinson G. Untreated obstructive sleep apnea and the risk for serious long-term adverse outcomes: a systematic review. Sleep Med Rev. 2014;18(1):49–59. [DOI] [PubMed] [Google Scholar]
- 38.Maimon N, Hanly PJ. Does Snoring Intensity Correlate with the Severity of Obstructive Sleep Apnea? Journal of Clinical Sleep Medicine : JCSM : Official Publication of the American Academy of Sleep Medicine. 2010;6(5):475–8. [PMC free article] [PubMed] [Google Scholar]
- 39.Kerr WC, Stockwell T. Understanding standard drinks and drinking guidelines. Drug Alcohol Rev. 2012;31(2):200–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.: NIAAA; [cited 2017 November 15]. Available from: https://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/what-standard-drink.
- 41.Mitler MM, Dawson A, Henriksen SJ, Sobers M, Bloom FE. Bedtime ethanol increases resistance of upper airways and produces sleep apneas in asymptomatic snorers. Alcohol Clin Exp Res. 1988;12(6):801–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Berry RB, Desa MM, Light RW. Effect of ethanol on the efficacy of nasal continuous positive airway pressure as a treatment for obstructive sleep apnea. Chest. 1991;99(2):339–43. [DOI] [PubMed] [Google Scholar]
- 43.Cederbaum AI. Alcohol Metabolism. Clin Liver Dis. 2012;16(4):667–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ebrahim Irshaad O, Shapiro Colin M, Williams Adrian J, Fenwick Peter B. Alcohol and Sleep I: Effects on Normal Sleep. Alcoholism: Clinical and Experimental Research. 2013;37(4):539–49. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


