Abstract
Environmental pollution from microplastics (MPs) in air is a matter of growing concern because of human health implications. Airborne MPs can be directly and continuously inhaled in air environments. Especially high MPs contributions can be found in indoor air due to the erosion and breakage of consumer, domestic and construction products, although there is little information available on their sources and concentrations and the risks they might pose. This is in part due to the fact that sampling and analysis of airborne MPs is a complex and multistep procedure where techniques used are not yet standardized. In this study, we provide an overview on the presence of MPs in indoor air, potential health impacts, the available methods for their sampling and detection and implications from the use of face masks during the COVID-19 pandemic.
Keywords: Particulate matter, Air pollution, Indoor air, Health risk, Face masks
Graphical abstract
1. Introduction
Plastics are synthetic polymers typically prepared by polymerization of monomers derived from oil or gas with the addition of different chemical additives (Thompson et al., 2009). They are one of the most universally-used and multipurpose materials in the global economy (Plastics Europe, 2020) due to their extraordinary properties such as versatility, lightweight, strength, durability, corrosion resistivity, high thermal and electrical insulation (Halden, 2010; Thompson et al., 2009). Plastic production has correspondingly increased globally from 1.7 to 360 million tonnes annually within the last 70 years (Fig. 1 ), expanding their use across a myriad of consumer and construction products, notably in packaging, building and construction, and the automotive industry. The polymers mostly deployed are polypropylene (19.4%), polyethylene (low (17.4%) and high (12.4%) density), polyvinyl chloride (10%), polyurethane (7.9%), and polyethylene terephthalate (7.9%) (Plastics Europe, 2020). These polymers are not biodegradable, so they accumulate in landfills or in the natural environment (Barnes et al., 2009). As a consequence, plastic pollution is already a huge environmental problem that is expected to increase: annual waste production is projected to rise to 3.4 billion million tonnes in the next 30 years (Kaza et al., 2018).
Fig. 1.
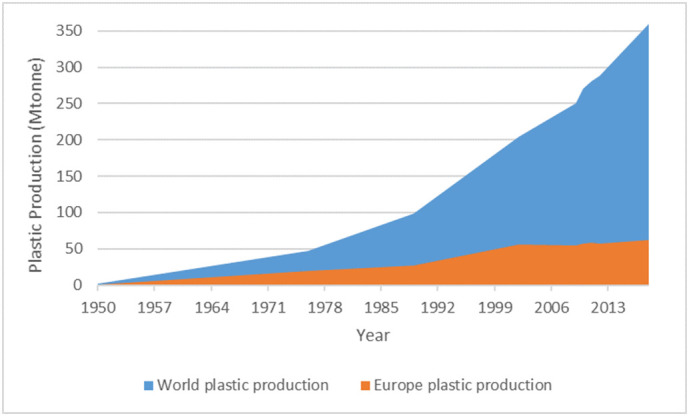
Plastic production's growing from 1950 to 2018 (data adapted from Plastics Europe, Association of Plastic Manufacturers: Brussels).
Once released to the environment, plastics are eroded and weathered, breaking to progressively smaller fragments over time. The term ‘microplastic’ was firstly coined in 2004 to describe small plastic particles (from millimetre to sub-millimetre-sized particles) (Thompson et al., 2004), but it was not until 2008 when they were defined by The National Oceanic and Atmospheric Administration (NOAA) as plastic particles smaller than 5 mm (Arthur et al., 2009). A more recent definition places the lower limit of the size range as 1 μm (Hartmann et al., 2019) whereas other authors have defined microplastics (MPs) as particles with diameter < 10 mm (Graham and Thompson, 2009), between 2 and 6 mm (Thompson, 2006), < 2 mm or < 1 mm (Browne et al., 2008; Claessens et al., 2011). Today, there is as yet no universally accepted definition of the relevant particle size range.
Microplastics are heterogeneous in terms of chemical composition, diameter, shape, specific density, and colour (Amato-Lourenço et al., 2020). They include 1-D fibres (one larger dimension), 2-D fragments (flat particles) and 3-D spherules (Dris et al., 2015), and can be either primary or secondary in origin. Primary MPs are purposefully produced and enter the environment as particles/powder (typically less than 0.5 mm) used for example as abrasives in cosmetic products or ‘scrubbers’ used to blast clean surfaces (Bergmann et al., 2015; GESAMP, 2015). In contrast, secondary MPs originate from the fragmentation of larger plastic litter present in the environment (UNEP, 2015). The most common process of generating secondary MPs is weathering which typically occurs when plastic is exposed to solar UV radiation (GESAMP, 2015) that catalyses the oxidative degradation of polymers (free-radical mediated oxidation reaction) (Andrady et al., 1996; Celina, 2013). During this degradation plastic normally discolours, loses mechanical integrity, gets weaker, and develops surface cracks (Cooper and Corcoran, 2010; GESAMP, 2015; Pegram and Andrady, 1989). In addition to UV radiation there are other mechanisms (e.g., mechanical stress by wind and waves, heat, hydrolysis, and the enzymatic processes of microorganisms) aiding plastic degradation and fragmentation (Andrady, 2011; GESAMP, 2015).
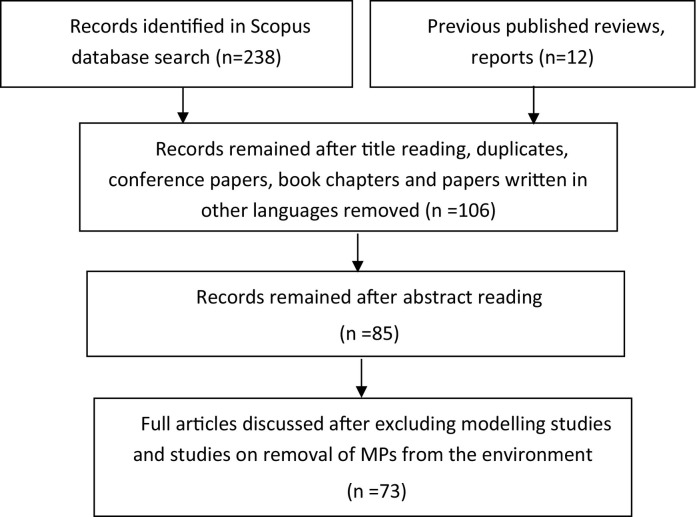
Microplastics are globally spread throughout the environment (Rocha-Santos and Duarte, 2015) and their negative impact is enhanced by their ability to adsorb organic pollutants and heavy metals (Wang et al., 2021), both considered priority pollutant vectors in the Stockholm and Basel Convention (Gallo et al., 2018). They have been shown capable of decreasing photosynthesis and microalgal growth (Sjollema et al., 2016), releasing harmful plastic additives (Hermabessiere et al., 2017; Verla et al., 2019) and inducing the growth and transport of pathogenic bacteria (GESAMP, 2015). Moreover, they are easily transferred and bioaccumulated through the food chain and thus transferred to humans (Chen et al., 2020a). Microplastic pollution in aquatic environments in particular has attracted the scientific community, with the majority of research to date focussing on MP in surface waters, shorelines, continental waters including remote places such as Polar Regions (González-Pleiter et al., 2020) or deep sea (Zhang D. et al., 2020), as well as in soils and sediments at a global scale (Boucher and Friot, 2017; Claessens et al., 2011; Efimova et al., 2018; GESAMP, 2015). During the last decade, attention has increasingly been paid to other environmental compartments, such as air (Dris et al., 2016; Evangeliou et al., 2020; Zhang Q. et al., Q. 2020). However, the analysis of MPs in air is in its beginning. Sampling and analysis of airborne MPs is a complex and multistep procedure where techniques used significantly diverge between studies. Thus, physicochemical properties of airborne MPs are not well characterized; and consequently health effects of inhaled MPs are poorly understood. With this study, we aim to provide an overview on the presence of MPs both in outdoor and indoor air, their potential health impacts, and the available methods for their sampling and detection. Although previous published reviews (e.g. Chen et al., 2020a, Chen et al., 2020b and Zhang et al., Y. 2020) discuss most of the abovementioned issues, in this paper we additionally emphasize in the implications from the use of large plastic products and face masks during the COVID-19 pandemic. Finally, we suggest ways to study the risk due to inhalation of MPs released by the face masks. We performed a literature search of Scopus online database without year restriction using the following keywords: microplastics, and nanoplastics, in combination with the terms: airborne, particulate matter, aerosols, indoor air, outdoor air, COVID-19, face masks, inhalation risk. Previously published reviews and reports were also consulted and studied. Conference papers and articles written in other language than English were excluded. Modeling studies focusing on atmospheric transport of microplastics and studies discussing removal methods from the environment were also excluded. Studies discussed in the present work were first identified from the study title, then from the abstract and last from the full paper. A total of 73 papers were considered (Fig. 2).
Fig. 2.
Flow chart showing the identification, screening and selection process performed in the current study.
2. Microplastics in air
Interest in the presence of MPs in air is on the increase because airborne particles can be directly and continuously inhaled into the human body (Prata, 2018). The distribution and behaviour of MPs suspended in the atmosphere is like those of other airborne pollutants: their concentration, transport, dispersion and removal depend on the emission sources, meteorological conditions, and long-range transport among other factors. Recent studies have demonstrated the presence of MPs both in outdoor and indoor air (Table 1 ). Concentrations of MPs in outdoor air largely vary depending on the sampling site (urban, industrial, remote); additionally, the heterogeneous ways of expressing results make comparisons difficult (Table 1). Higher levels of MPs were observed in urban sites than those found in rural areas. Dris et al. (2016) compared the levels of MPs in atmospheric fallout at an urban site and at a sub-urban site in the Greater area of Paris, France. The sub-urban site systematically showed less MPs than the urban one. The average atmospheric flux of total fibres was 110 and 53 particles/m2/day on the urban and sub-urban site, respectively. This difference was attributed to intensified anthropogenic activities and higher population density in the urban site (Dris et al., 2016). Similarly, Liao et al. (2021) found higher abundance of airborne MPs at an urban transit station (287 ± 72 MPs/m3), than at rural farmland (137 ± 57 MPs/m3), wetland (97 ± 33 MPs/m3) and mountain (70 ± 18 MPs/m3) site. However, contradicting results were reported by Klein and Fischer (2019) that compared MPs concentrations in atmospheric deposition in 3 urban sites and 3 rural/forest sites. The mean MPs concentration was 137 and 396 particles/m2/day for the urban and rural sites respectively. The main explanation for the higher concentration of MPs in the rural/forest sites is the comb-out effect, the ability of plants to filter particles from the dry atmospheric deposition. In a precipitation event during the sampling period, the particles got washed off the leaves and added to the number of particles in the bulk samplers (Klein and Fischer, 2019). Studying MPs morphology could help to infer their origin: fibres are related with clothing or upholstery production whereas fragments could come from plastics degradation (Liu et al., 2019). Size and diameter are also critical characterization parameters due to its influence in MPs interaction with the media: diameter have high influence in persistence and toxicity (Gasperi et al., 2018). Moreover, studies which report chemical composition (Liu et al., 2019; Gaston et al., 2020; Wright et al., 2020) show that polyethylene terephthalate (PET) and polyethylene (PE) are present in all outdoor samples. Characterization and quantification of MPs smaller than 10 μm, particularly those smaller than 2 μm, would be very helpful in informing human and environmental risk assessments and aligning with air quality guidelines (Wright et al., 2021).
Table 1.
Available data on characteristics, analysis and concentrations of MPs in both outdoor and indoor air.
| Sampling zone (continent) | Air type | Sampling method | Filter (pore size) | Identification method | Physical characterization |
Levels detected | References | ||
|---|---|---|---|---|---|---|---|---|---|
| Majoritarian shape/form | Size (μm) | Polymer types | |||||||
| Greater Paris, France (Europe) | Outdoor | Atmospheric deposition | Glass fibre (1.6 μm) | VO | Fibres | 1000 - 5000 | N/A | 118 particles/m2/day | Dris et al., 2015 |
| Greater Paris, France (Europe) | Outdoor | Atmospheric deposition | Quartz fibre (1.6 μm) |
VO | Fibres | >50 | PE, PET | 2-355 particles/m2/day |
Dris et al., 2016 |
| Hamburg, Germany (Europe) | Outdoor | Atmospheric deposition | Cellulose | Fluorescence microscope + μRaman | Fragments | < 63 | EVA, PTFE, PVA, PET, PE | 275.0 MPs/m2/day | Klein and Fischer, 2019 |
| Shanghai, China (Asia) | Outdoor | Active sampler | Glass filter (1.6 μm) |
μFT-IR (+VO) | Particles | 23-100 | PET, PE, PES, PAN, PAA, EVA, RY, EP | 0–4.18 MPs/m3 | Liu et al., 2019 |
| Asaluyeh, Iran (Asia) | Outdoor | Active pump sampler | PTFE filter (0.2 μm) |
Microscopic techniques (fluorescence, polarized light, SEM) | Fibres | 2 - 100 | N/A | 0.3–1.1 MP/m3 | Abbasi et al., 2020 |
| California, USA (North America) | Outdoor Indoor |
Active pump sampler | Glass microfibre filter (1.6 μm) | μFT-IR (+VO) | Fibres, fragments | Outdoor: 104.8 -616 Indoor: 58.6 - 641 |
PS, PET, PE, PVC, PC, PA, ABS | Outdoor: 0.6–5.6 MPs/m3 Indoor: 3.3–12.6 MPs/m3 |
Gaston et al., 2020 |
| Beijing, China (Asia) | Outdoor | Active pump sampler | Mixed cellulose ester (0.8 μm) | SEM - EDX | Fibres | 5-20 | N/A | 5.1–7.2·10-3 f/mL | Li et al., 2020 |
| London, U.K. (Europe) | Outdoor | Atmospheric deposition | Alumina-based membrane filters (0.2 μm) | FTIR (+VO) | Fibres, Particles | 75-100 | PAN, PET, PA, PUR, PS, PVC, PVA, PES, PP, PE | Fibres: 712 ± 162 MP/m2/day Particles: 59 ± 32 MP/m2/day |
Wright et al., 2020 |
| Paris, France (Europe) | Indoor | Active pump sampler | Quartz fibre filters (1.6 μm) | ATR-FTIR | Fibre | 50–3250 | PP, PE | 5.4 fibres/m3 | Dris et al., 2017 |
| Aarhus, Denmark (Europe) | Indoor | Breathing Thermal Manikin | Cut silver membranes (0.8 μm) |
FPA-μFTIR-Imaging | Fibres, fragments | 37–177 | N/A | 9.3 ± 5.8 MPs/m3/d | Vianello et al., 2019 |
| Australia (Oceania) | Indoor | Active pump sampler | Glass filter (1.6 μm) |
FTIR and Pyr-GC/MS (+VO) | Fibre | 19–3948 | N/A | 1.6 ± 1.8 fibres/m3 | O’Brien et al., 2020 |
| Aveiro, Portugal (Europe) | Indoor | N/A | N/A | ATR-FTIR | Fibre, fragments | 17–3669 | N/A | 5 MPs/m3 | Prata et al., 2020a |
| Shanghai, China (Asia) | Indoor | Passive sampling | Cellulose membranes (5 μm) |
μFT-IR (+VO) | Fibres | 50–2000 | PS, PP, PA, PE, RY | 2.1·103 − 2.9·104 MPs/m2/d | Zhang Q. et al., 2020 |
| Hull East Riding Yorkshire, U.K (Europe) |
Indoor | Passive sampling | Mixed cellulose esters membrane (5 μm) |
Stereomicroscope + μFTIR | Fibres | 5-5000 | PET, PA, PP | 0–5412 MPs m− 2 day− 1 | Jenner et al., 2021 |
| Wenzhou, China (Asia) |
Outdoor Indoor |
Active sampler | Glass fibre (0.7 μm) |
Fluorescence microscope + μFTIR | Fragments | <100 | PE, PP, PES PES, PA, PP |
189 ± 85 MPs/m3 1583 ± 1181 MPs/m3, |
Liao et al., 2021 |
N/A not available/reported, VO visual observation. PP polypropylene, PA polyamide, PET polyethylene terephthalate, PTFE teflon, PE polyethylene, PES polyester, PAN polyacrylonitrile, PAA poly(N-methylacrylamide), PVA poly(vinyl acetate), PUR polyurethane, RY rayon, EVA ethylene vinyl acetate, PMMA poly(methyl methacrylate), PS polystyrene, PVC polyvinyl chloride, ABS acrylonitrile butadiene styrene, PE polyethylene, PC polycarbonates, EP ethylene propylene.
In indoor air, MPs are present in higher concentrations than outdoors (Gasperi et al., 2015), creating an increased concern as people spend an average 70-90% of their time inside (Alzona et al., 1967). In indoor areas, MPs behaviour is governed by room distribution, ventilation, and airflows (Prata, 2018). Therefore, low rates of air renovation generally result in high concentrations of indoor MPs. Besides, the use of synthetic materials contributes to the total MPs concentration: furniture, traditional cleaning habits and activities are permanently producing MPs (Catarino et al., 2018; Chen et al., 2020a; Wang et al., 2019). The main source of MPs in indoor air is considered to be synthetic textiles given that small fibres easily tear from clothes and other fibre products during wearing, cleaning and drying (Chen et al., 2020a; Dris et al., 2016; Dris et al., 2017; Liu et al., 2019; O’Brien et al., 2020). Other daily activities have been reported as MPs sources, such as the opening of plastic packaging (Sobhani et al., 2020) or using a 3D printer (Zhang et al., 2017). Concentrations reported are between 1.6 and 12.6 MPs/m3 and it is remarkable how all of them use infrared spectroscopy, IR – based analysis techniques (Table 1). PS, PE, PES, PP and PA seem to be the most abundant polymers, although some authors do not report the polymers found. However, it would be useful when trying to identify potential sources of indoor MPs.
Nevertheless, information about airborne MPs in both indoor and outdoor air is still very limited (Enyoh et al., 2019), in particular in urban and industrial environments. Generally, there is a high variability in the chemical composition of the samples and further research is needed to harmonize analytical methods, establish correlations between sampling locations and compare the chemical compositions and concentrations reported.
3. Sampling and analysis of MPs in air
Monitoring studies are being implemented to better understand the abundance of MPs in the atmosphere and their health impacts. However, standardized sampling and quantitative analytical methods for MPs in the atmosphere have not yet been validated (Chen et al., 2020b). In this context it is particularly important to consider the sampling methodology. There are two main methods used for sampling airborne MPs: passive atmospheric deposition and active pumped samplers (Dris et al., 2015; Dris et al., 2017). Passive sampling uses MPs fallout by gravitational, inertial, or diffusive mechanisms to estimate MPs levels in the air (Dris et al., 2016). The fallout is collected through glass funnel and is stored in a glass bottle. This method is usually preferred for sampling in remote areas with no access to power or for long-term continuous sample collection. On the other hand, active pumped samplers allow to rapidly collect MPs in outdoor and indoor air with high efficiency (Chen et al., 2020b). This method is easily replicable because the intake flow rate of the pump can be adjusted and MPs abundance can be expressed as number of MPs/m3 (Chen et al., 2020b; Zhang et al., Q. 2020). Different filters have been used in active air samplers as collection substrates for MPs: quartz filters, cellulose, glass fibre filters, alumina, and silver membranes. Wright et al. (2019) found that inhalable MPs are not visibly detectable against quartz or spectroscopically detectable against polytetrafluoroethylene (PTFE) and alumina-based filters but, considering Raman Spectral Imaging, the greatest intensities for MPs were observed against the silver membrane filter.
Several methodologies are proposed for sample pre-treatment, but once again there is no standardization currently available. Early research identified microplastics through visual techniques but this was only possible for large fragments. Sample preparation has become necessary to analyse <500 μm MPs basically because organic matter may interfere in the analysis by increasing background noise (Zhang Y. et al., 2020). To eliminate the organic matter, samples are treated typically with a 30% hydrogen peroxide solution or sodium hypochlorite (Chen et al., 2020b; Klein et al., 2019), although recent research has identified Fenton's reagent (mix of hydrogen peroxide and ferrous ion) as more efficient at digesting organic matter (Prata et al., 2019). In this step, it is critical to avoid artificial changes of sample composition. Most polymers have been shown to resist degradation associated with hydrogen peroxide, but little is known about the influence of chemical treatments on weathered plastics (Xu et al., 2019a). After removing the organic matter, the MPs must be separated from other atmospheric particles. The method most commonly used is density separation (Chen et al., 2020b). To date, zinc chloride solutions with a density of 1.6 - 1.7 g/cm3 is considered to be the most effective method for separating multiple microplastic particles (Chen et al., 2020b; Dris et al., 2017).
Microplastic particle identification takes place by stereomicroscope and different analytical methods. Firstly, it is common to perform a morphological analysis (abundance, size, shape, and colour) by visual observation. Microscopes (e.g., stereomicroscopes) controlled by powerful software programs for image analysis enable rapid counting of a large number of MPs, although results may be affected by human bias, microscopy quality, sample matrix and MPs size limitations. Scanning electron microscopy (SEM) is also commonly used in MPs identification (Fries et al., 2013). A high-intensity electron beam scans the surface by interaction between the electrons beam and the sample. As a result, high resolution images (<0.5 nm resolution) of the surface are obtained (Rocha-Santos and Duarte, 2015). Moreover, this can be combined with energy-dispersive X-ray spectroscopy (SEM-EDS) providing information on the elemental composition (Fries et al., 2013). However, it is a time-consuming method, so it is not suitable for analysing large number of MPs in a sample(Chen et al., 2020b).
As a second step, accurate instrumental analysis is needed for further identification of the polymeric composition of MPs, particularly for sizes <500 μm (Chen et al., 2020b; Verla et al., 2019; Zhang et al., Y. 2020). Until now the most common procedures are spectroscopy-based methods such as Fourier transform infrared spectroscopy (FTIR), Raman spectroscopy (Elert et al., 2017) or pyrolysis gas chromatography–mass spectrometry (Pyr-GC/MS) (Zhang Y. et al., 2020). FTIR is the most common technique used in the identification of the polymeric composition of MPs (Chen et al., 2020b). FTIR determines composition (molecular structure) through examination using an IR wavelength range of 400–4000/cm. A proportion of the wavelengths are absorbed by the particle being analysed. This absorption is transformed using the Fourier Transform function, creating a spectrum (Zhang et al., Y. 2020). Comparing the target spectrum with those of known materials in libraries, the type of polymer composing the MPs can be directly identified (Wang and Wang, 2018). There are three different operating modes for MPs analysis in FTIR: transmission, reflection, and attenuated total-reflectance (ATR). Transmission and ATR are the most popular modes for MPs analysis (Xu et al., 2019a). In transmission mode, light passes through the sample and is collected afterwards. Therefore, the filter for the tested MPs requires transparency and it cannot be used for high absorption materials with colour because no light is transmitted to the detector. In reflection mode, the incident beam passes back through the sample by reflection on an IR reflective substrate. The main problem of this mode is that the reflected signal is often disturbed by reflection errors caused by light scattering and depends on the morphology of the MP particle. Finally, in ATR the sample is placed in optical contact with a material of high refractive index (ATR crystal) and the surface is irradiated with an evanescent wave. This ATR crystal can degrade over time with surface scratching and cracking (Xu et al., 2019a). FTIR techniques have several advantages such as non-destructiveness, low sample amount requirement, possibility for high throughput screening and environmental friendliness (Araujo et al., 2018). Additionally, FTIR can be combined with a confocal microscope (μFTIR) and a Mercury Cadmium Telluride (MCT) detector to reduce the practical particle size down toward the diffraction limit (~10 μm as the whole wavelength must pass through the material) (Zhang Y. et al., 2020). It also exists the possibility of coupling chemical imaging, enabling the collection simultaneously of both spatial and spectral information. (This is especially interesting because MPs determination is a four-dimensional analysis (chemical composition, size, shape, and abundance of each polymer particle) (Xu et al., 2019a). However, there are also some limitations to consider (e.g., expensive instrumentations, time-consuming, need of well-trained operators) (Chen et al., 2020b; Rocha-Santos and Duarte, 2015).
Raman spectroscopy requires only small quantities of sample to produce high reliable results (Araujo et al., 2018). A laser with a single wavelength is directed onto a target sample, different types of excitation are produced and detected due to the reflection, scatter, and absorption by a sample (Chen et al., 2020a, Chen et al., 2020b). The fingerprints of chemical structures obtained allow the identification of the components present in the sample (Käppler et al., 2018). Combining Raman with a spectral imaging equipment gives the possibility to detect MPs down to 1 μm, a resolution which cannot be achieved by other methods (Chen et al., 2020b; Lenz et al., 2015). However, the wavelengths used for Raman causes the particles to fluoresce so normally there is high background fluorescence (Zhang et al., Y. 2020). Organic matter removal should be nearly perfect to avoid these interferences (Chen et al., 2020b). Moreover, it is relatively new to MPs research so the libraries of polymers are not yet well developed and the presence of additives easily affect the accuracy of the spectral results (Zhang et al., Y. 2020).
Finally, Pyr-GC/MS has not been undertaken on atmospheric samples, but it is widely used in studies of MPs in other environments (Fries et al., 2013). In this technique the chemical composition of MPs can be identified by analysing their thermal monomeric products and comparing the results with a program database (Chen et al., 2020b; Fries et al., 2013). It is a fairly straight forward method as each chromatographic analysis takes around 30 min and several polymers can be detected in a single run. Although the results are not affected by the additives contained in MPs, it has some limitations: analyse only a portion of the filter due to the relatively small capacity of the support to place the filter and being a destructive method (Käppler et al., 2018).
4. Health impact from MPs inhalation
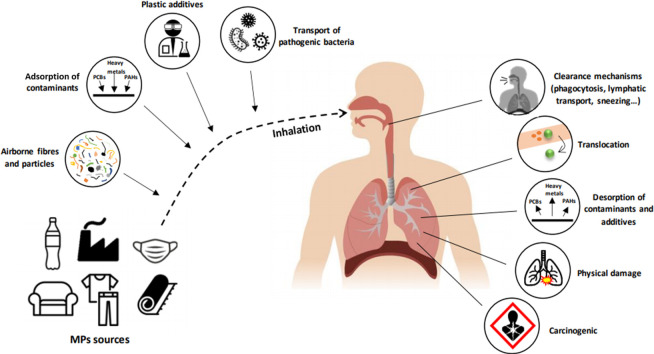
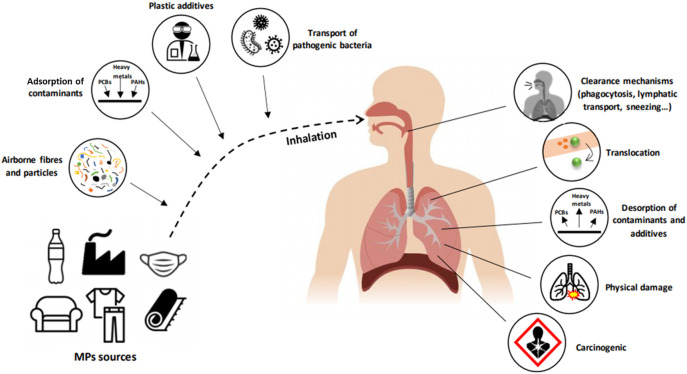
Although the presence of MPs in air is a fact, human health risks due to their inhalation remain unclear (Wright et al., 2020). Many questions remain unanswered in this field such as how naturally weathered inhaled MPs might contribute to the pathogenesis of different pulmonary diseases, whether inhaled MPs can translocate to the blood, whether deleterious health outcomes may be linked to the desorption of contaminants in the respiratory system (Amato-Lourenço et al., 2020), whether MP particles can produce physical damage to the tissues, or to what extent MPs act as a carrier of organic pollutants or pathogens (Fig. 3 ). In 1998 it was revealed that 97% of malignant lung specimens contained microfibres (Pauly et al., 1998). After being inhaled, MPs deposition occurs, this greatly depending on the aerodynamic equivalent diameter of the particle (influenced by density and diameter of the particle) (Gasperi et al., 2018; Prata, 2018). Physical mechanisms such as sedimentation, impaction, interception or diffusion are involved in deposition of MPs in terminal bronchioles, alveolar ducts, and alveoli (Amato-Lourenço et al., 2020; Prata, 2018). Nevertheless, the human body has defence mechanical methods to prevent MPs deposition such as sneezing, mucociliary escalator, phagocytosis by macrophages or lymphatic transport to avoid biopersistence of MPs (Bank and Hansson, 2019; Gasperi et al., 2018). However, -these clearance mechanisms cannot exclude the occurrence of inflammatory lesions (Pauly et al., 1998) caused by interconnected mechanisms: dust overload, oxidative stress, cytotoxicity, and traslocation (Prata, 2018). Dust overloading explains the effects of accumulation of inert particles in the respiratory system. In this case, clearance is avoided by losing mobility of alveolar macrophages due to high particle accumulation or high surface particles which induce high chemotactic gradients that prevent macrophage migration (Morrow, 1992; Tran et al., 2000).
Fig. 3.
Implications of MPs inhalation and possible consequences in human respiratory system. (PCBs: polychlorinated biphenyls, PAHs: polycyclic aromatic hydrocarbons).
Oxidative stress is produced when MPs generate reactive oxygen species (ROS) in some species (Chen et al., 2020a, Chen et al., 2020b). Organisms respond releasing pro-inflammatory cytokines and fibrogenic mediators as a result of antioxidant protective response saturation (Donaldson and Lang Tran, 2002; Prata, 2018). Oxidative stress induction could be due to transporting oxidizing species (e.g., adsorbed metals) or to the interaction of their high surface area with biological systems (Kelly and Fussell, 2012). Finally, MPs can translocate (depending on their hydrophilicity) and reach the circulation, especially during inflammation due to the increase in tissue permeability (Browne et al., 2008). The chronic irritation and inflammation produced by the mechanisms described above might promote cancer. This is due to the inefficient removal of particles by macrophages, DNA damage caused by oxidative stress, evasion of detection by the immune system and gene mutation favours the formation and progression of malignant cells (Prata, 2018).
Catarino et al. (2018) made an estimation of human ingestion of MPs during evening meals based on fallout, but no approximation has been made yet to human exposure through inhalation. Assuming a conservative level of 1 MP/m3 and an adult breathing of 17.40 m3 (U.S. EPA, 2011) of air per day, about 6351 MPs would be being inhaled each year. Over and above the possible (and generally unknown) chronic effects of long-term background MPs inhalation at low concentrations, there are already several studies on the health impacts of being exposed to high concentrations of MP materials. Thus, airborne MPs are known to cause occupational hazard disease in industrial workers, with for example the inhalation of synthetic fibres being linked to respiratory lesions and chronic bronchitis (Goldberg and Thériault, 1994). “Flock workers lung” is an occupational interstitial lung disease (pulmonary fibrosis) caused by inhalation of flock fibres typically comprising rotary-cut polyamide (nylon), although other microplastic particles such as polypropylene and polyethylene may also be present (Kern et al., 1998). Similarly, industrial exposure to polyvinyl chloride can increase restrictive lung disease (e.g., pneumoconiosis) (Studnicka et al., 1995). Excessive inhalation of fibrous particles is well known to present increased carcinogenic risk due to possible chemical, mechanical (irritation), immune (autoimmune response), and genotoxic (genogenic mutations) impacts (Prata et al., 2018; Siegel et al., 2020), following the classic studies on asbestiform silicates (chrysolite, amosite, crocidolite, tremolite, actinolite, and anthophyllite) once used as construction and fire hazard materials (Nielsen et al., 2014; Furuya et al., 2018). Microplastic fibres lack the complex inorganic chemistry of asbestiform silicates, but they share the fibrous form implicated in frustrated phagocytosis and generation of damaging reactive oxygen species within the lung tissue (Riediker et al., 2019). People in a normal environment do not acquire these occupational diseases, but the precautionary principle advises that this does not absolve the chronic inhalation of airborne fibrous MPs from any health risk (Chen et al., 2020a).
Size, density, hydrophobicity, and surface charge of particles can all influence the deposition and absorption of MPs via the respiratory system. Smaller and lighter particles would reach deeper into the lungs (Rist et al., 2018) with translocation to other organs and traversing cell membranes being likely very efficient with nano-sized plastic particles (Zarus et al., 2021). A recent study found that the repeated inhalation of nano polystyrene particles (100 nm diameter) caused alterations in several endpoints related to physiological, serum biochemical, hematological, and respiratory function markers (Lim et al., 2021). Schirinzi et al. (2017) have studied the cytotoxicity of commonly used nanomaterials and MPs on cerebral and epithelial human cells. They found that polystyrene particles caused higher oxidative stress than polyethylene particles and they attributed this to the smaller size of polystyrene compared to polyethylene particles. Wang et al. (2020) observed increasing uptake rates of polystyrene particles from colonic cancer Caco-2 cells with decreasing particle size. The uptake rates of nanoplastics were 73%, and 71% for 300 nm and 500 nm sized polysterene particles respectively. Lower uptake rates were observed for microplastics, equal to 49%, 43%, and 30% for 1 μm, 3 μm, and 6 μm sized polystyrene particles, respectively. Similarly, Xu et al. (2019b) evaluated the effects of polystyrene nanoparticles of two different sizes: 25 nm and 70 nm diameter on the human alveolar epithelial A549 cell line including internalization, cell viability, cell cycle, apoptosis, and associated gene transcription and protein expression. Their results showed that 25 nm polystyrene was internalized more rapidly and efficiently into the cytoplasm than 70 nm polystyrene particles.
5. Microplastics and the COVID-19 pandemic
Several proposals have recently emerged to decrease the quantity of MPs discharged into the environment: reducing, reusing and recycling plastics, use of alternative materials (biodegradable plastic, bioplastics) or the improvement of legislations such as the European regulation ‘Strategy for Plastics in the Circular Economy’ that aim to restrict the use of plastics (European Comission, 2018; Kaza et al., 2018).
However, COVID-19 respiratory disease has postponed remediation actions and plastic bans. In December 2019, a novel type of coronavirus (SARS-CoV-2) led to a global sanitary, political, economic and environmental crisis. Several measures have been adopted worldwide to contain the virus: lockdowns, travel restrictions, social distancing, or personal protection equipment's (PPE) use are some of the more effective preventive measures. In this context, single-use-plastic's (SUP) demand has increased due to COVID-19 high contagiousness. As a result, many governments have delayed SUP bans and have encouraged population to use them in order to avoid cross-contamination (Patrício Silva et al., 2020). In many countries PPE (including facial masks and gloves) are mandatory for all citizens indoors and outdoors, so there is a boost in production (e.g. China produced 200 million face masks a day (June 2020) which is twenty times the amount they made at the start of February 2020 (Aragaw, 2020).
Indiscriminate use of masks worldwide leads to a monthly consumption of 129 billion masks approximately (Prata et al., 2020b), considering 7.8 billion inhabitants (Worldometers.info, 2021). Different kinds of masks are being used: surgical, KN95, FFP2, FFP3, cotton, fashion, or activated carbon masks are some of the most popular. However, surgical masks are the most used. These masks should be worn for a few hours (e.g., 4 h) and adequately discarded. Generally, mask wastes are discarded without precautionary measures which means that high amounts of contaminated plastic are ending up in the environment and streets. If we consider an incorrect disposal of only 1%, about 10 million masks are being introduced in the environment monthly which is near to 30–40,000 kg of plastic (Fadare and Okoffo, 2020; Silva et al., 2020). Consequently, vast portions of MPs would be produced and remain in the environment indefinitely. Abbasi et al. (2020) reported peaks of MPs from the principal masks’ components (polypropylene, polyethylene) in marine ecosystems suggesting high accumulations within short time. Thus, the fraction of MPs coming from masks is expected to increase the following years.
Apart from the environmental impact of masks, it would be interesting to evaluate their impact on human health. Reusing masks or using them for long times can generate micro/nanoplastics (Aragaw, 2020; Fadare and Okoffo, 2020). Masks are disposed over mouth and nose what creates a perfect atmosphere to inhale the MPs generated. A recent study conducted by Li et al. (2021) showed that inhalation risks of fibre MPs increased with improper use of masks, although all masks reduce the inhalation risks of MPs particles (except when using disinfection pre-treatments). This study pointed that using poor – quality masks can pose higher MPs inhalation risk compared with good – quality ones. Another recent study (Fernandez- Arribas et al., 2021) examined the organophosphate ester content in different types of face masks used for COVID-19 prevention. Organophosphate esters are widely used as plasticizers and flame retardants. The highest levels of organophosphate esters were found in KN95 masks (mean value of 11.6 μg/mask), while the lowest values were those of surgical masks (mean value of 237 ng/mask).
Breathing simulation experiments with masks are useful to estimate MPs emissions of masks, but there could be other ways to obtain information. For instance, nasal lavages could be used as a tool for monitoring human exposure to MPs. The nose is the first region of the respiratory tract to be in contact with airborne pollutants (Koren et al., 1990). Some studies showed that most particulate matter is deposited in the anterior third of the nasal cavity, but deposition would depend on the size and density of the particles (Stuart, 1984). This deposition is mainly due to the nasal structure and mucus generated by calceiform cells: they generate a turbulence in the airflow, so airborne pollutants are trapped. If the trapped particles are respiratory irritants, our bodies would response to them quickly (Koren et al., 1990).
Nasal lavages is a simple technique which allows the measurement of the irritation response to the particles deposited on the mucosal surface. Normally, medical biomarkers are measured after the lavage such as inflammatory cell influx, eicosanoid mediators, neuropeptide release, or nasal glandular products (Peden, 1996). Previous works report satisfactory results using nasal lavages on different fields: measurement the effect of allergens (Kaliner and Lemanske, 1992), tools to control air quality (Norbäck et al., 2000) or assessment of inflammations produced by ozone or volatile organic compounds (VOCs) (Laumbach et al., 2005). Only a recent study (Velázquez-Gómez and Lacorte, 2019) used nasal lavages to monitor and evaluate direct inhalation of organic pollutants. Their results showed that nasal lavages can be used to assess exposure to contaminants which are widely distributed in indoor and outdoor environments. In the COVID-19 context, MPs originating from face masks may be accumulated in the nasal cavity so nasal lavages would permit a direct evaluation of human exposure to this and other sources of microplastics.
Although MPs inhalation is a minor problem compared with COVID-19, it would be interesting to make further research on this area to fully understand current information.
6. Conclusions and recommendations for future research
Airborne microplastics are now attracting scientific attention. Lately, several studies have reported different MPs concentrations both indoor and outdoor air. Outdoor and, specially, indoor airborne MPs represent a relatively neglected but significant pathway for human plastic exposure and further studies should be carried out to understand their implications to human health. There is an urgent need to standardize methods for sampling and analysis airborne MPs because it would help to compare results of different scenarios and have a global knowledge of current airborne MPs status. Moreover, better quality data would be reported. In the COVID-19 context, special attention should be given to the increase of global plastic waste and inhalation of MPs due to face masks use. Face masks have become indispensable in society, so future research should investigate health risks related to short and long-term inhalation of MPs.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
A.Karanasiou, acknowledges funding by the Ramón y Cajal Fellowship (RYC-2014-16885) awarded by the Spanish Ministry of Science, Innovation and Universities co-funded by the European Social Fund. This work was supported by the Spanish Ministry of Science and Innovation (Excelencia Severo Ochoa, Project CEX2018-000794-S) within the project MOMIA and project PID2019-105732GB-C21 from the Spanish Ministry of Science, Innovation and Universities.
Editor: Anastasia Paschalidou
References
- Abbasi S.A., Khalil A.B., Arslan M. Extensive use of face masks during COVID-19 pandemic: (micro-)plastic pollution and potential health concerns in the Arabian Peninsula. Saudi J. Biol. Sci. 2020;27(12):3181–3186. doi: 10.1016/j.sjbs.2020.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzona J., Cohen B.L., Rudoplh H., Jow H.N., Frohliger J.O. Indoor-outdoor relationships for airborne particulate matter of outdoor origin. Atmos. Environ. 1967;13(1):55–60. doi: 10.1016/0004-6981(79)90244-0. [DOI] [Google Scholar]
- Amato-Lourenço L.F., dos Santos Galvão L., de Weger L.A., Hiemstra P.S., Vijver M.G., Mauad T. An emerging class of air pollutants: potential effects of microplastics to respiratory human health? Sci. Total Environ. 2020;749 doi: 10.1016/j.scitotenv.2020.141676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrady A.L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011;62(8):1596–1605. doi: 10.1016/j.marpolbul.2011.05.030. [DOI] [PubMed] [Google Scholar]
- Andrady A.L., Pegram J.E., Searle N.D. Wavelength sensitivity of enhanced photodegradable polyethylenes, ECO, and LDPE/MX. J. Appl. Polym. Sci. 1996;62(9):1457–1463. doi: 10.1002/(SICI)1097-4628(19961128)62:9<1457::AID-APP15>3.0.CO;2-W. [DOI] [Google Scholar]
- Aragaw T.A. Surgical face masks as a potential source for microplastic pollution in the COVID-19 scenario. Mar. Pollut. Bull. 2020;159 doi: 10.1016/j.marpolbul.2020.111517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araujo C.F., Nolasco M.M., Ribeiro A.M.P., Ribeiro-Claro P.J.A. Identification of microplastics using raman spectroscopy: latest developments and future prospects. Water Res. 2018;142:426–440. doi: 10.1016/j.watres.2018.05.060. [DOI] [PubMed] [Google Scholar]
- Arthur C., Baker J., Bamford H. 2009. Proceedings of the International Research Workshop on the Occurrence, Effects and Fate of Microplastic Marine Debris. Sept 9–11, 2008. NOAA Technical Memorandum NOS-OR&R-30. [Google Scholar]
- Bank M.S., Hansson S.V. The plastic cycle: a novel and holistic paradigm for the anthropocene. Environ. Sci. Technol. 2019;53(13):7177–7179. doi: 10.1021/acs.est.9b02942. [DOI] [PubMed] [Google Scholar]
- Barnes D.K.A., Galgani F., Thompson R.C., Barlaz M. Accumulation and fragmentation of plastic debris in global environments. Phil. Trans. R. Soc. B. 2009;1526:1985–1998. doi: 10.1098/rstb.2008.0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann M., Lars G., Klages M. Marine Anthropogenic Litter. 2015. Microplastics; pp. 185–200. [Google Scholar]
- Boucher J., Friot D. Primary Microplastics in the Oceans: A Global Evaluation of Sources. IUCN; Gland, Switzerland: 2017. p. 43. [Google Scholar]
- Browne M.A., Dissanayake A., Galloway T.S., Lowe D.M., Thompson R.C. Ingested microscopic plastic translocates to the circulatory system of the mussel, Mytilus edulis (L) Environ. Sci. Technol. 2008;42(13):5026–5031. doi: 10.1021/es800249a. [DOI] [PubMed] [Google Scholar]
- Catarino A.I., Macchia V., Sanderson W.G., Thompson R.C., Henry T.B. Low levels of microplastics (MP) in wild mussels indicate that MP ingestion by humans is minimal compared to exposure via household fibres fallout during a meal. Environ. Pollut. 2018;237:675–684. doi: 10.1016/j.envpol.2018.02.069. [DOI] [PubMed] [Google Scholar]
- Celina M.C. Review of polymer oxidation and its relationship with materials performance and lifetime prediction. Polym. Degrad. Stab. 2013;98(12):2419–2429. [Google Scholar]
- Chen G., Feng Q., Wang J. Mini-review of microplastics in the atmosphere and their risks to humans. Sci. Total Environ. 2020;703 doi: 10.1016/j.scitotenv.2019.135504. [DOI] [PubMed] [Google Scholar]
- Chen G., Fu Z., Yang H., Wang J. An overview of analytical methods for detecting microplastics in the atmosphere. TrAC - Trends in Anal. Chem. 2020;130 doi: 10.1016/j.trac.2020.115981. [DOI] [Google Scholar]
- Claessens M., De Meester S., Van Landuyt K., De Clerck K., Janssen C.R. Occurrence and distribution of microplastics in marine sediments along the Belgian coast. Mar. Pollut. Bull. 2011;62(10):2199–2204. doi: 10.1016/j.marpolbul.2011.06.030. [DOI] [PubMed] [Google Scholar]
- Cooper D.A., Corcoran P.L. Effects of mechanical and chemical processes on the degradation of plastic beach debris on the island of Kauai, Hawaii. Mar. Poll. Bull. 2010;60(5):650–654. doi: 10.1016/j.marpolbul.2009.12.026. [DOI] [PubMed] [Google Scholar]
- Donaldson K., Lang Tran C. Inflammation caused by particles and fibers. Inhal. Toxicol. 2002;14(1):5–27. doi: 10.1080/089583701753338613. [DOI] [PubMed] [Google Scholar]
- Dris R., Gasperi J., Rocher V., Saad M., Renault N., Tassin B. Microplastic contamination in an urban area: a case study in greater Paris. Environ. Chem. 2015;12(5):592–599. doi: 10.1071/EN14167. [DOI] [Google Scholar]
- Dris R., Gasperi J., Saad M., Mirande C., Tassin B. Synthetic fibers in atmospheric fallout: a source of microplastics in the environment? Mar. Pollut. Bull. 2016;104(1–2):290–293. doi: 10.1016/j.marpolbul.2016.01.006. [DOI] [PubMed] [Google Scholar]
- Dris R., Gasperi J., Mirande C., Mandin C., Guerrouache M., Langlois V., Tassin B. A first overview of textile fibers, including microplastics, in indoor and outdoor environments. Environ. Pollut. 2017;221:453–458. doi: 10.1016/j.envpol.2016.12.013. [DOI] [PubMed] [Google Scholar]
- Efimova I., Bagaeva M., Bagaev A., Kileso A., Chubarenko I.P. Secondary microplastics generation in the sea swash zone with coarse bottom sediments: laboratory experiments. Front. Mar. Sci. 2018;5(SEP) doi: 10.3389/fmars.2018.00313. [DOI] [Google Scholar]
- Elert A.M., Becker R., Duemichen E., Eisentraut P., Falkenhagen J., Sturm H., Braun U. Comparison of different methods for MP detection: what can we learn from them, and why asking the right question before measurements matters? Environ. Pollut. 2017;231:1256–1264. doi: 10.1016/j.envpol.2017.08.074. [DOI] [PubMed] [Google Scholar]
- Enyoh C.E., Verla A.W., Verla E.N., Ibe F.C., Amaobi C.E. Airborne microplastics: a review study on method for analysis, occurrence, movement and risks. Environ. Mon. Assess. 2019;191(11) doi: 10.1007/s10661-019-7842-0. [DOI] [PubMed] [Google Scholar]
- European Comission . A European Strategy for Plastics in a Circular Economy. 2018. pp. 1–18.https://ec.europa.eu/environment/circular-economy/pdf/plastics-strategy-brochure.pdf [Google Scholar]
- Evangeliou N., Grythe H., Klimont Z., Heyes C., Eckhardt S., Lopez – Aparicio S., Stohl Atmospheric transport is a major pathway of microplastics to remote regions. Nat. Commun. 2020;11:3381. doi: 10.1038/s41467-020-17201-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadare O.O., Okoffo E.D. Covid-19 face masks: a potential source of microplastic fibers in the environment. Sci. Total Environ. 2020;737 doi: 10.1016/j.scitotenv.2020.140279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries E., Dekiff J.H., Willmeyer J., Nuelle M.T., Ebert M., Remy D. Identification of polymer types and additives in marine microplastic particles using pyrolysis-GC/MS and scanning electron microscopy. Environ. Sci.: Process. Impacts. 2013;15(10):1949–1956. doi: 10.1039/c3em00214d. [DOI] [PubMed] [Google Scholar]
- Furuya S., Chimed-Ochir O., Takahashi K., David A., Takala J. Global Asbestos disaster. Int. J. Environ. Res. Public Health. 2018;15:1000. doi: 10.3390/ijerph15051000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo F., Fossi C., Weber R., Santillo D., Sousa J., Ingram I., Nadal A., Romano D. Marine litter plastics and microplastics and their toxic chemicals components: the need for urgent preventive measures. Environ. Sci. Eur. 2018;30(1) doi: 10.1186/s12302-018-0139-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasperi J., Dris R., Mandin C., Tassin B. 15th EuCheMS International Conference on Chemistry and the Environment, September, Leipzig, Germany. 2015. First overview of microplastics in indoor and outdoor air. [Google Scholar]
- Gasperi J., Wright S.L., Dris R., Collard F., Mandin C., Guerrouache M., Langlois V., Kelly F.J., Tassin B. Microplastics in air: are we breathing it in? Curr. Opin. Environ. Sci. Health. 2018;1:1–5. doi: 10.1016/j.coesh.2017.10.002. [DOI] [Google Scholar]
- Gaston E., Woo M., Steele C., Sukumaran S., Anderson S. Microplastics differ between indoor and outdoor air masses: insights from multiple microscopy methodologies. Appl. Spectrosc. 2020;74(9):1079–1098. doi: 10.1177/0003702820920652. [DOI] [PubMed] [Google Scholar]
- GESAMP . Sources, Fate and Effects of MP in the Marine Environment: A Global Assessment. Rep. Stud. Vol. 90. 2015. p. 96.https://ec.europa.eu/environment/marine/good-environmental-status/descriptor-10/pdf/GESAMP_microplastics%20full%20study.pdf [Google Scholar]
- Goldberg M.S., Thériault G. Retrospective cohort study of workers of a synthetic textiles plant in Quebec: II. Colorectal cancer mortality and incidence. Am. J. Ind. Med. 1994;25(6):909–922. doi: 10.1002/ajim.4700250613. [DOI] [PubMed] [Google Scholar]
- González-Pleiter M., Velázquez D., Edo C., Carretero O., Gago J., Barón-Sola Á., Hernández L.E., Yousef I., Quesada A., Leganés F., Rosal R., Fernández-Piñas F. Fibers spreading worldwide: microplastics and other anthropogenic litter in an Arctic freshwater lake. Sci. Total Environ. 2020;722 doi: 10.1016/j.scitotenv.2020.137904. [DOI] [PubMed] [Google Scholar]
- Graham E.R., Thompson J.T. Deposit- and suspension-feeding sea cucumbers (Echinodermata) ingest plastic fragments. J. Exp. Mar. Biol. Ecol. 2009;368(1):22–29. doi: 10.1016/j.jembe.2008.09.007. [DOI] [Google Scholar]
- Halden R.U. Plastics and health risks. Annu. Rev. Public Health. 2010;31:179–194. doi: 10.1146/annurev.publhealth.012809.103714. [DOI] [PubMed] [Google Scholar]
- Hartmann N.B., Hüffer T., Thompson R.C., Hassellöv M., Verschoor A., Daugaard A.E., Rist S., Karlsson T., Brennholt N., Cole M., Herrling M.P., Hess M.C., Ivleva N.P., Lusher A.L., Wagner M. Are we speaking the same language? Recommendations for a definition and categorization framework for plastic debris. Environ. Sci. Technol. 2019;53(3):1039–1047. doi: 10.1021/acs.est.8b05297. [DOI] [PubMed] [Google Scholar]
- Hermabessiere L., Dehaut A., Paul-Pont I., Lacroix C., Jezequel R., Soudant P., Duflos G. Occurrence and effects of plastic additives on marine environments and organisms: a review. Chemosphere. 2017;182:781–793. doi: 10.1016/j.chemosphere.2017.05.096. [DOI] [PubMed] [Google Scholar]
- Jenner L.C., Sadofsky L.R., Danopoulos E., Rotchell J.M. Household indoor microplastics within the Humber region (United Kingdom): quantification and chemical characterisation of particles present. Atmos. Environ. 2021;259 doi: 10.1016/j.atmosenv.2021.118512. [DOI] [Google Scholar]
- Kaliner M., Lemanske R. Rhinitis and asthma. J. Am. Med. Assoc. 1992;268(20):2807–2829. doi: 10.1001/jama.1992.03490200059007. [DOI] [PubMed] [Google Scholar]
- Käppler A., Fischer M., Scholz-Böttcher B.M., Oberbeckmann S., Labrenz M., Fischer D., Eichhorn K.J., Voit B. Comparison of µ-ATR-FTIR spectroscopy and py-GCMS as identification tools for microplastic particles and fibers isolated from river sediments. Anal. Bioanal. Chem. 2018;410(21):5313–5327. doi: 10.1007/s00216-018-1185-5. [DOI] [PubMed] [Google Scholar]
- Kaza S., Yao L.C., Bhada-Tata P., Van Woerden F. World Bank©. World Bank. License: CC BY 3.0 IGO; Washington, DC: 2018. What a Waste 2.0: A Global Snapshot of Solid Waste Management to 2050. Urban Development.https://openknowledge.worldbank.org/handle/10986/30317 [Google Scholar]
- Kelly F.J., Fussell J.C. Size, source and chemical composition as determinants of toxicity attributable to ambient particulate matter. Atmos. Environ. 2012;60:504–526. doi: 10.1016/j.atmosenv.2012.06.039. [DOI] [Google Scholar]
- Kern D.G., Crausman R.S., Durand K.T.H., Nayer Ali, Kuhn Charles., III Flock worker's lung: chronic interstitial lung disease in the nylon flocking industry. Ann. Intern. Med. 1998;129:261–272. doi: 10.7326/AWED202001070. [DOI] [PubMed] [Google Scholar]
- Klein M., Fischer E.K. Microplastic abundance in atmospheric deposition within the metropolitan area of Hamburg, Germany. Sci. Total Environ. 2019;685:96–103. doi: 10.1016/j.scitotenv.2019.05.405. [DOI] [PubMed] [Google Scholar]
- Koren H.S., Hatch G.E., Graham D.E. Nasal lavage as a tool in assessing acute inflammation in response to inhaled pollutants. Toxicology. 1990;60(1–2):15–25. doi: 10.1016/0300-483X(90)90159-E. [DOI] [PubMed] [Google Scholar]
- Laumbach R.J., Fiedler N., Gardner C.R., Laskin D.L., Fan Z.H., Zhang J., Weschler C.J., Lioy P.J., Devlin R.B., Ohman-Strickland P., Kelly-McNeil K., Kipen H.M. Nasal effects of a mixture of volatile organic compounds and their ozone oxidation products. J. Occup. Environ. Med. 2005;47(11):1182–1189. doi: 10.1097/01.jom.0000183338.95778.f0. [DOI] [PubMed] [Google Scholar]
- Lenz R., Enders K., Stedmon C.A., MacKenzie D.M.A., Nielsen T.G. A critical assessment of visual identification of marine microplastic using Raman spectroscopy for analysis improvement. Mar. Pollut. Bull. 2015;100(1):82–91. doi: 10.1016/j.marpolbul.2015.09.026. [DOI] [PubMed] [Google Scholar]
- Li Y., Shao L., Wang W., Zhang M., Feng X., Li W., Zhang D. Airborne fiber particles: types, size and concentration observed in Beijing. Sci. Total Environ. 2020;705 doi: 10.1016/j.scitotenv.2019.135967. [DOI] [PubMed] [Google Scholar]
- Li L., Zhao X., Li Z., Song K. COVID-19: performance study of microplastic inhalation risk posed by wearing masks. J. Hazard. Mater. 2021;411:1–9. doi: 10.1016/j.jhazmat.2020.124955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Z., Ji X., Ma Y., Lv Baoqiang, Huang Wei, Zhu Xuan, Fang Mingzhu, Wang Qi, Wang Xuedong, Dahlgren Randy, Shang Xu. Airborne microplastics in indoor and outdoor environments of a coastal city in Eastern China. J. Hazard. Mater. 2021;417 doi: 10.1016/j.jhazmat.2021.126007. [DOI] [PubMed] [Google Scholar]
- Lim D., Jeong J., Song Kyung Seuk, Sung Jae Hyuck, Oh Seung Min, Choi Jinhee. Inhalation toxicity of polystyrene micro(nano)plastics using modified OECD TG 412. Chemosphere. 2021;262 doi: 10.1016/j.chemosphere.2020.128330. [DOI] [PubMed] [Google Scholar]
- Liu K., Wang X., Fang T., Xu P., Zhu L., Li D. Source and potential risk assessment of suspended atmospheric microplastics in Shanghai. Sci. Total Environ. 2019;675:462–471. doi: 10.1016/j.scitotenv.2019.04.110. [DOI] [PubMed] [Google Scholar]
- Morrow P.E. Dust overloading of the lungs: update and appraisal. Toxicol. Appl. Pharmacol. 1992;113(1):1–12. doi: 10.1016/0041-008X(92)90002-A. [DOI] [PubMed] [Google Scholar]
- Nielsen L.S., Baelum J., Rasmussen J., Dahl S., Olsen K.E., Albin M., Hansen N.C., Sherson D. Occupational asbestos exposure and lung cancer – a systematic review of the literature. Arch. Environ. Occup. Health. 2014;69(4):191–206. doi: 10.1080/19338244.2013.863752. [DOI] [PubMed] [Google Scholar]
- Norbäck D., Wålinder R., Wieslander G., Smedje G., Erwall C., Venge P. Indoor air pollutants in schools: nasal patency and biomarkers in nasal lavage. Allergy. 2000;55(2):163–170. doi: 10.1034/j.1398-9995.2000.00353.x. [DOI] [PubMed] [Google Scholar]
- O’Brien S., Okoffo E.D., O’Brien J.W., Ribeiro F., Wang X., Wright S.L., Samanipour S., Rauert C., Toapanta T.Y.A., Albarracin R., Thomas K.V. Airborne emissions of microplastic fibres from domestic laundry dryers. Sci. Total Environ. 2020;747 doi: 10.1016/j.scitotenv.2020.141175. [DOI] [PubMed] [Google Scholar]
- Patrício Silva A.L., Prata J.C., Walker T.R., Campos D., Duarte A.C., Soares A.M.V.M., Barcelò D., Rocha-Santos T. Rethinking and optimising plastic waste management under COVID-19 pandemic: policy solutions based on redesign and reduction of single-use plastics and personal protective equipment. Sci. Total Environ. 2020;742 doi: 10.1016/j.scitotenv.2020.140565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauly J.L., Stegmeier S.J., Allaart H.A., Cheney R.T., Zhang P.J., Mayer A.G., Streck R.J. Inhaled cellulosic and plastic fibers found in human lung tissue. Cancer Epidemiol. Biomark. Prev. 1998;7(5):419–428. [PubMed] [Google Scholar]
- Peden D.B. The use of nasal lavage for objective measurement of irritant-induced nasal inflammation. Regul. Toxicol. Pharm. 1996;24(1):76–78. doi: 10.1006/rtph.1996.0081. [DOI] [PubMed] [Google Scholar]
- Pegram J.E., Andrady L. Outdoor weathering of selected polymeric materials under marine exposure conditions. Polym. Degrad. Stab. 1989;26(4):333–345. doi: 10.1016/0141-3910(89)90112-2. [DOI] [Google Scholar]
- Plastics Europe . 2020. Plastics - The Facts 2020, an Analysis of European Latest Plastics Production, Demand and Waste Data.https://www.plasticseurope.org/en/resources/publications/4312-plastics-facts-2020 [Google Scholar]
- Prata J.C. Airborne microplastics: consequences to human health? Environ. Pollut. 2018;234:115–126. doi: 10.1016/j.envpol.2017.11.043. [DOI] [PubMed] [Google Scholar]
- Prata Joana C., Castro J.L., da Costa J.P., Duarte A.C., Rocha-Santos T., Cerqueira M. The importance of contamination control in airborne fibers and microplastic sampling: experiences from indoor and outdoor air sampling in Aveiro, Portugal. Mar. Pollut. Bull. 2020;159 doi: 10.1016/j.marpolbul.2020.111522. [DOI] [PubMed] [Google Scholar]
- Prata Joana C., da Costa J.P., Girão A.V., Lopes I., Duarte A.C., Rocha-Santos T. Identifying a quick and efficient method of removing organic matter without damaging microplastic samples. Sci. Total Environ. 2019;686:131–139. doi: 10.1016/j.scitotenv.2019.05.456. [DOI] [PubMed] [Google Scholar]
- Prata Joana C., Silva A.L.P., Walker T.R., Duarte A.C., Rocha-Santos T. COVID-19 pandemic repercussions on the use and management of plastics. Environ. Sci. Technol. 2020;54(13):7760–7765. doi: 10.1021/acs.est.0c02178. [DOI] [PubMed] [Google Scholar]
- Riediker M., Zink D., Kreyling W., Oberdörster G., Elder A., Graham U., Lynch I., Duschl A., Ichihara G., Ichihara S., Kobayashi T., Hisanaga N., Umezawa M., Cheng Tsun-Jen, Handy R., Gulumian M., Tinkle S., Cassee F. Particle toxicology and health – wehere are we ? Part. Fibre Toxicol. 2019;16(1):19. doi: 10.1186/s12989-019-0302-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rist S., Almroth B., Hartmann N., Karlsson T. A critical perspective on early communications concerning human health aspects of microplastics. Sci. Total Environ. 2018;626:720–726. doi: 10.1016/j.scitotenv.2018.01.092. [DOI] [PubMed] [Google Scholar]
- Rocha-Santos T., Duarte A.C. A critical overview of the analytical approaches to the occurrence, the fate and the behavior of microplastics in the environment. TrAC - Trends Anal. Chem. 2015;65:47–53. doi: 10.1016/j.trac.2014.10.011. [DOI] [Google Scholar]
- Schirinzi G.F., Pérez-Pomeda I., Sanchís J., Rossini C., Farré M., Barceló D. Cytotoxic effects of commonly used nanomaterials and microplastics on cerebral and epithelial human cells. Environ. Res. 2017;159:579–587. doi: 10.1016/j.envres.2017.08.043. [DOI] [PubMed] [Google Scholar]
- Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2020. J. Clin. 2020:1–24. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- Sjollema S.B., Redondo-Hasselerharm P., Leslie H.A., Kraak M.H.S., Vethaak A.D. Do plastic particles affect microalgal photosynthesis and growth? Aqua. Toxicol. 2016;170:259–261. doi: 10.1016/j.aquatox.2015.12.002. [DOI] [PubMed] [Google Scholar]
- Sobhani Z., Lei Y., Tang Y., Wu L., Zhang X., Naidu R., Megharaj M., Fang C. Microplastics generated when opening plastic packaging. Sci. Rep. 2020;10(1):1–7. doi: 10.1038/s41598-020-61146-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart B.O. Deposition and clearance of inhaled particles. Environ. Health Perspect. 1984;55:369–390. doi: 10.1289/ehp.8455369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studnicka M.J., Menzinger G., Drlicek M., Maruna H., Neumann M.G. Pneumoconiosis and systemic sclerosis following 10 years of exposure to polyvinyl chloride dust. Thorax. 1995;50:583–585. doi: 10.1136/thx.50.5.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson R.C. Federal Agency for Nature Conservation; 2006. Plastic Debris in the Marine Environment: Consequences and Solution, Marine Nature Conservation in Europe. [Google Scholar]
- Thompson R.C., Olson Y., Mitchell R.P., Davis A., Rowland S.J., John A.W.G., McGonigle D., Russell A.E. Lost at sea: where is all the Plastic? Sci. 2004;304(5672):838. doi: 10.1126/science.1094559. [DOI] [PubMed] [Google Scholar]
- Thompson R.C., Swan S.H., Moore C.J., Vom Saal F.S. Our plastic age. Phil. Trans. R. Soc. B. 2009;364(1526):1973–1976. doi: 10.1098/rstb.2009.0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran C.L., Buchanan D., Cullen R.T., Searl A., Jones A.D., Donaldson K. Inhalation of poorly soluble particles. II. Influence of particle surface area on inflammation and clearance. Inhal. Toxicol. 2000;12(12):1113–1126. doi: 10.1080/08958370050166796. [DOI] [PubMed] [Google Scholar]
- U.S. EPA . Exposure Factors Handbook 2011 Edition (Final Report) U.S. Environmental Protection Agency; Washington, DC: 2011. Chapter 6: inhalation rates. EPA/600/R-09/052F. [Google Scholar]
- UNEP . 2015. Plastic in Cosmetics. [Google Scholar]
- Velázquez-Gómez M., Lacorte S. Nasal lavages as a tool for monitoring exposure to organic pollutants. Environ. Res. 2019;178:108726. doi: 10.1016/j.envres.2019.108726. [DOI] [PubMed] [Google Scholar]
- Verla A.W., Enyoh C.E., Verla E.N., Nwarnorh K.O. Microplastic–toxic chemical interaction: a review study on quantified levels, mechanism and implication. SN Appl. Sci. 2019;1(11):1–30. doi: 10.1007/s42452-019-1352-0. [DOI] [Google Scholar]
- Vianello A., Jensen R.L., Liu L., Vollertsen J. Simulating human exposure to indoor airborne microplastics using a breathing thermal manikin. Sci. Rep. 2019;9(1):1–11. doi: 10.1038/s41598-019-45054-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Wang J. Investigation of microplastics in aquatic environments: an overview of the methods used, from field sampling to laboratory analysis. Trends Anal. Chem. 2018;108:195–202. doi: 10.1016/j.trac.2018.08.026. [DOI] [Google Scholar]
- Wang T., Li B., Zou X., Wang Y., Li Y., Xu Y., Mao L., Zhang C., Yu W. Emission of primary microplastics in mainland China: invisible but not negligible. Wat. Res. 2019;162:214–224. doi: 10.1016/j.watres.2019.06.042. [DOI] [PubMed] [Google Scholar]
- Wang Q., Bai J., Ning B., Fan L., Sun T., Fang Y., Wu J., Li S., Duan C., Zhang Y., Liang J., Gao Z. Effects of bisphenol a and nanoscale and microscale polystyrene plastic exposure on particle uptake and toxicity in human Caco-2 cells. Chemosphere. 2020;254 doi: 10.1016/j.chemosphere.2020.126788. [DOI] [PubMed] [Google Scholar]
- Wang Y., Wang X., Li Y., Li J., Liu Y., Xia S., Zhao J. Effects of exposure of polyethylene microplastics to air, water and soil on their adsorption behaviors for copper and tetracycline. Chem. Eng. J. 2021;404 doi: 10.1016/j.cej.2020.126412. [DOI] [Google Scholar]
- Worldometers.info . Dover; Delaware, U.S.A: 2021. Worldometer.https://www.worldometers.info/world-population/ From: [Google Scholar]
- Wright Stephanie L., Levermore J.M., Kelly F.J. Raman spectral imaging for the detection of inhalable microplastics in ambient particulate matter samples. Environ. Sci. Technol. 2019;53(15):8947–8956. doi: 10.1021/acs.est.8b06663. [DOI] [PubMed] [Google Scholar]
- Wright S.L., Ulke J., Font A., Chan K.L.A., Kelly F.J. Atmospheric microplastic deposition in an urban environment and an evaluation of transport. Environ. Int. 2020;136 doi: 10.1016/j.envint.2019.105411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S.L., Gouin T., Koelmans A.A., et al. Development of screening criteria for microplastic particles in air and atmospheric deposition: critical review and applicability towards assessing human exposure. Micropl.&Nanopl. 2021;1:6. doi: 10.1186/s43591-021-00006-y. [DOI] [Google Scholar]
- Xu M., Halimu G., Zhang Q., Song Y., Fu X., Li Y., Li X., Zhang H. Internalization and toxicity: a preliminary study of effects of nanoplastic particles on human lung epithelial cell. Sci. Total Environ. 2019;694 doi: 10.1016/j.scitotenv.2019.133794. [DOI] [PubMed] [Google Scholar]
- Xu J.L., Thomas K.V., Luo Z., Gowen A.A. FTIR and raman imaging for microplastics analysis: state of the art, challenges and prospects. Trends Anal. Chem. 2019;119 doi: 10.1016/j.trac.2019.115629. [DOI] [Google Scholar]
- Zarus G.M., Muianga C., Hunter C.M., Pappas R.S. A review of data for quantifying human exposures to micro and nanoplastics and potential health risks. Sci. Total Environ. 2021;756 doi: 10.1016/j.scitotenv.2020.144010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Kang S., Allen S., Allen D., Gao T., Sillanpää M. Atmospheric microplastics: a review on current status and perspectives. Earth-Sci. Rev. 2020;203 doi: 10.1016/j.earscirev.2020.103118. [DOI] [Google Scholar]
- Zhang D., Liu X., Huang W., Li J., Wang C., Zhang D., Zhang C. Microplastic pollution in deep-sea sediments and organisms of the Western Pacific Ocean. Environ. Pollut. 2020;259 doi: 10.1016/j.envpol.2020.113948. [DOI] [PubMed] [Google Scholar]
- Zhang Qian, Wong J.P.S., Davis A.Y., Black M.S., Weber R.J. Characterization of particle emissions from consumer fused deposition modeling 3D printers. Aer. Sci. Technol. 2017;51(11):1275–1286. doi: 10.1080/02786826.2017.1342029. [DOI] [Google Scholar]
- Zhang Qun, Zhao Y., Du F., Cai H., Wang G., Shi H. Microplastic fallout in different indoor environments. Environ. Sci. Technol. 2020;54(11):6530–6539. doi: 10.1021/acs.est.0c00087. [DOI] [PubMed] [Google Scholar]