Abstract
Objectives:
The incidence of gastric cancer continues to decrease globally, approaching levels that in some populations could define it as a rare disease. To explore this on a wider scale, we predict its future burden in 34 countries with long-standing population-based data.
Methods:
Data on gastric cancer incidence by year of diagnosis, sex and age were extracted for 92 cancer registries in 34 countries included in Cancer Incidence in Five Continents Plus. Numbers of new cases and age-standardised incidence rates (ASR per 100,000) were predicted up to 2035 by fitting and extrapolating age-period-cohort models.
Results:
Overall gastric cancer incidence rates are predicted to continue falling in the future in the majority of countries, including high-incidence countries such as Japan (ASR 36 in 2010 vs. ASR 30 in 2035) but also low-incidence countries such as Australia (ASR 5.1 in 2010 vs. ASR 4.6 in 2035). A total of 16 countries are predicted to fall below the rare disease threshold (defined as 6 per 100,000 person-years) by 2035, while the number of newly diagnosed cases remains high and is predicted to continue growing. In contrast, incidence increases were seen in younger age groups (below age 50 years) in both low- and high-incidence populations.
Conclusions:
While gastric cancer is predicted to become a rare disease in a growing number of countries, incidence levels remain high in some regions and increasing risks have been observed in younger generations. The predicted growing number of new cases highlights that gastric cancer remains a major challenge to public health on a global scale.
Keywords: gastric cancer, epidemiology, helicobacter pylori
Introduction
Six decades ago, gastric cancer was one of the most commonly diagnosed cancers worldwide. Today, with over 1 million estimated new cases in 2018, gastric cancer remains the 5th most commonly diagnosed malignancy worldwide that, in terms of incidence rates, ranks 9th in high-income countries and 7th in low-income countries.[1] On the other hand, due to its advanced-stage diagnosis, excess mortality from this cancer is high, making gastric cancer the 3rd most common cause of cancer-related death with close to 800,000 deaths globally.[1]
Steady declines in gastric cancer incidence and mortality rates have been observed consistently across world regions, with decreases evident in both sexes. Part of these declines have been attributed to better food preservation practices linked to refrigeration during the transport and storage of food. At the same time, the risk of infection with Helicobacter pylori (H. pylori), a major confirmed cause of gastric cancer, is enhanced by overcrowding and poor hygiene and thus better living conditions associated with economic development will have contributed to the reduction of the prevalence of this bacterium.[2] In Japan and the Republic of Korea (Korea), countries with some of the highest levels of gastric cancer incidence in the world, national screening programmes using endoscopic and/or radiographic methods have been implemented over the last few decades, with some success in achieving mortality reductions.[3, 4]
In view of the historical and ongoing decreases in the incidence of gastric cancer worldwide, this study examines data from high quality population-based cancer registries to predict future trends and determine whether gastric cancer is on its way to becoming a rare disease.
Methods
Data sources
Data on gastric cancer incidence (ICD10, C16) by year of diagnosis (1953–2012), sex and 5-year age group were extracted from national and regional population-based cancer registries available from the Cancer Incidence in Five Continents Plus (CI5plus) database.[5] The specific requirements for the inclusion of a registry were at least 15 consecutive years of data available, alongside inclusion in the last three volumes of the CI5 series (with 2012 as last year of diagnosis). These criteria are indicative of each registry’s data quality over time given that the editorial process involves a detailed assessment of the comparability, completeness and validity of the incidence data. Data from 92 registries satisfied these criteria and allowed for robust model-based predictions in 34 countries, representing 10 world regions. Where applicable, data from two or more regional registries were aggregated to obtain a proxy of the (unknown) national incidence. Population predictions (based on the United Nations, U.N., medium-fertility variant) were obtained from the U.N World Population Prospects 2017 Revision, by country, year (up to 2035), sex and age.[6]
Statistical analysis
Incidence rates were calculated by 5-year period of diagnosis and 5-year age group for all ages. Age-standardized incidence rates (ASR) per 100,000 person-years were calculated using the world standard population.[7] To predict the numbers of new cases and incidence rates from 2012 to 2035 by country, sex and age, a log-linear age-period-cohort model that levels off exponential growth and limits linear trend projection, was fitted to recent trends. The model, implemented in R through the package NORDPRED [8], has been shown to perform well empirically in projecting current trends in cancer incidence into the future.[8, 9] The three or four most recent five-year observed periods (depending on data availability) were extrapolated using a power function to level off the growth, with a projection of the recent linear trend for the last ten years that was attenuated (or accentuated in the case of negative trends) by 25%, 50% and 75% in the second, third and fourth prediction periods, respectively. The numbers of new cases were predicted for the year 2035 by taking a weighted average of the projected incidence rates for the last two prediction periods, centering on 2035 and then applying the rates to the U.N. national population forecasts available for each country for that year. Observed and predicted ASRs were then analyzed in light of the incidence threshold of 6 per 100,000 person-years, below which a cancer can be considered rare.[10] The numbers of predicted cases due to changes in demographics (population growth and ageing) were estimated using the 2010 rates applied to the projected populations, and the numbers predicted due to risk change were estimated as the difference between the total and those due to demographic changes.[9]
Stata 14 (Stata Corp, College Station, TX, U.S.) was used for data management and plotting the observed and modelled trends. The modelling analyses were performed using R 2.15 and the functions available in Epi package version 1.1.36, R Studio and the NORDPRED package (http://www.kreftregisteret.no/en/Research/Projects/Nordpred/Nordpred-software/).
While results for 12 populations in both sexes combined are presented graphically in the main paper, all 36 included populations from 34 countries are shown for both sexes combined and by sex in the supplementary material (Annex Tables 1–2, Annex Figures 1–4).
Patient and Public Involvement
As this work is a retrospective study involving examination of secondary cancer data only, patients were not involved in the design and conduct of this research.
Results
Gastric cancer incidence varies more than eight-fold across countries, with the highest rates for 2010 observed in Eastern Asia namely Japan and Korea (up to 41 per 100,000 person-years) and lowest rates in Northern America and Europe (below 5 per 100,000) (Figure 1). Historically, incidence rates have been falling in all included countries with the exception of some high-incidence countries (above 20 per 100,000) in Asia, for example Korea where rates have been stable over the past years (Figure 3, Annex Figure 2). The overall trend of decreasing rates is predicted to continue into the future, with reductions in most countries, including high-incidence countries such as Japan (ASR 36 in 2010 vs. ASR 30 in 2035) or Belarus (ASR 19 in 2010 vs. ASR 16 in 2035) but also low-incidence countries such as Australia (ASR 5.1 in 2010 vs. ASR 4.6 in 2035) or the UK (ASR 5.2 in 2010 vs. ASR 4.7 in 2035). While overall incidence rates were substantially and consistently higher in males than in females (generally about double), trends were similar although reductions in incidence were less pronounced in females and slight increases in rates were predicted in some populations (e.g. United States Blacks, Poland and France (Annex Figures 1, 3–4). Using the incidence threshold of 6 per 100,000, 16 out of 34 countries will have reached this threshold in 2035 compared to 13 countries in 2010.
Figure 1.
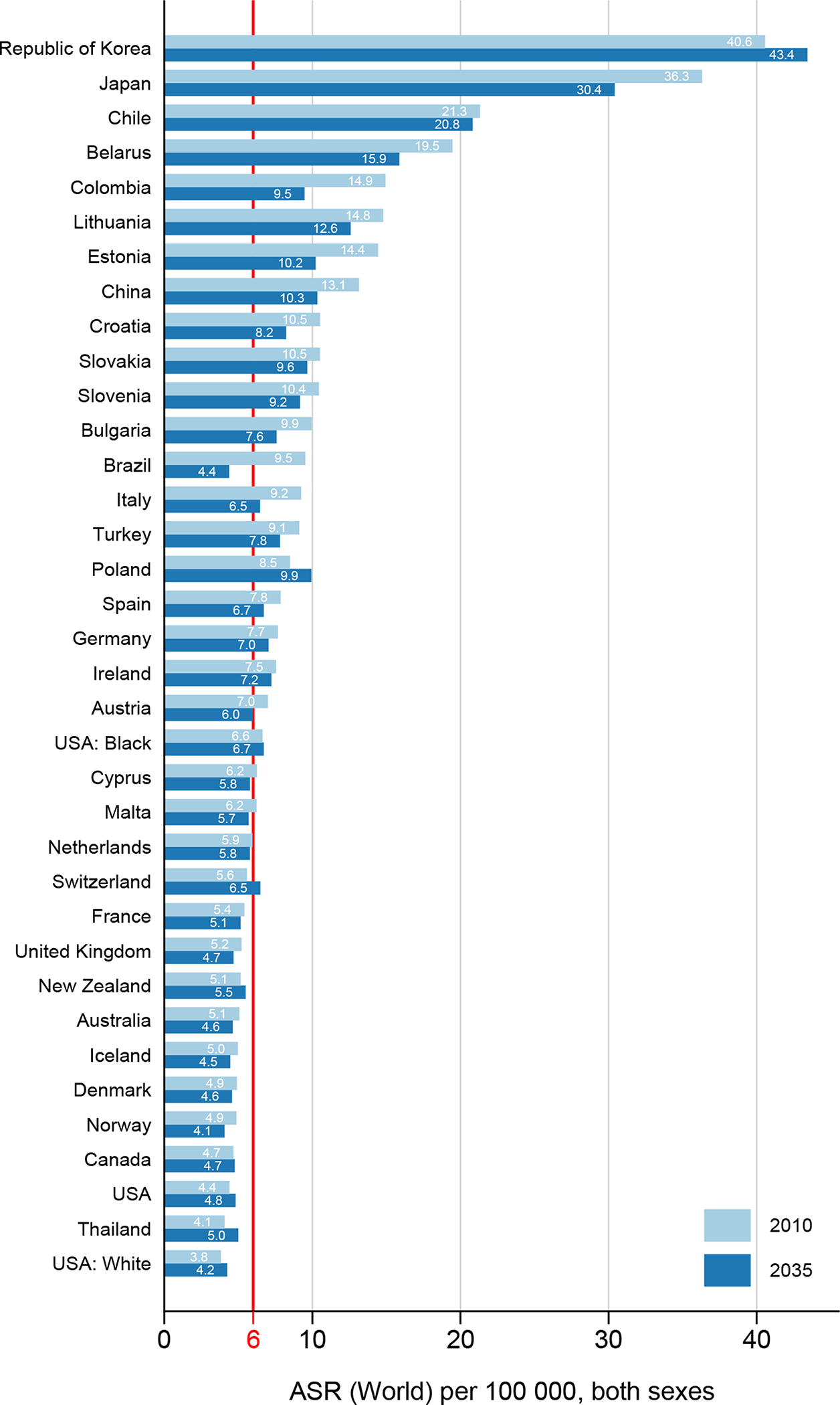
Age-standardized gastric cancer incidence rates (ASR) in 2010 and predicted rates in 2035, both sexes combined.
Figure 3.
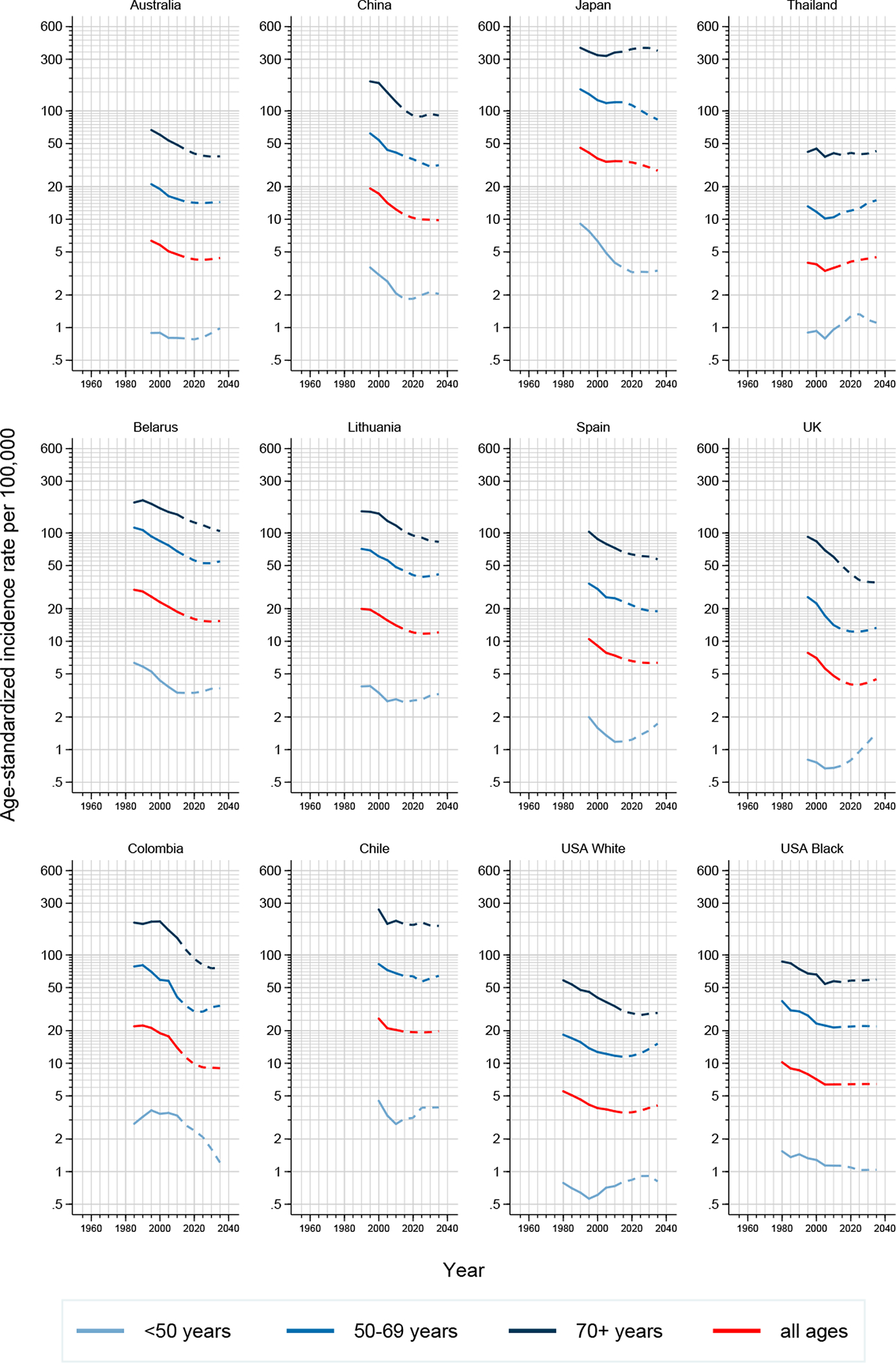
Trends in (truncated) age-standardized gastric cancer incidence rates in 12 selected countries by age: observed (solid lines) and predicted (dashed lines), both sexes combined
Even though incidence rates were found to continue decreasing, the absolute number of new gastric cancer cases is expected to further increase in the majority of countries (Table 1). The number of new cases is expected to more than double in Canada, Cyprus, Korea, Slovakia, and Thailand, while a handful of other countries (such as Bulgaria and Lithuania) can expect slight decreases in the number of new cases. This development is mainly related to changes in the population size and structure but also partly due to changes in the prevalence of risk factors, especially in those aged below 50 years (Table 1, Annex Table 3).
Table 1:
Number of new gastric cancer cases, age-standardized incidence rates and percentage change in cases due to population and risk, 2010 and 2035, both sexes.
| Population (annual, million) | Number of new cases | Age-standardized rate | Total change (%) | Change due to population (%) | Change due to risk (%) | ||||
|---|---|---|---|---|---|---|---|---|---|
| 2010 | 2035 | 2010 | 2035 | 2010 | 2035 | ||||
| South America | |||||||||
| Brazil† | 196.8 | 229.2 | 18406 | 18005 | 9.5 | 4.4 | −2.2 | 114.0 | −116.2 |
| Chile† | 17.0 | 20.1 | 4649 | 9067 | 21.3 | 20.8 | 95.0 | 104.9 | −9.9 |
| Colombia† | 45.9 | 54.1 | 6462 | 9123 | 14.9 | 9.5 | 41.2 | 133.5 | −92.3 |
| Northern America | |||||||||
| Canada | 26.0 | 41.9 | 2238 | 4738 | 4.7 | 4.7 | 111.7 | 144.4 | −32.7 |
| U.S.† | 308.6 | 365.0 | 22988 | 36546 | 4.4 | 4.8 | 59.0 | 64.4 | −5.4 |
| U.S.: Black† | 42.2 | 50.7 | 3399 | 6749 | 6.6 | 6.7 | 98.6 | 93.8 | 4.8 |
| U.S.: White† | 245.7 | 272.0 | 17278 | 26531 | 3.8 | 4.2 | 53.6 | 55.6 | −2.0 |
| Eastern Asia | |||||||||
| China† | 1359.8 | 1433.5 | 225895 | 345087 | 13.1 | 10.3 | 52.8 | 99.9 | −47.2 |
| Japan† | 128.6 | 118.5 | 124550 | 152914 | 36.3 | 30.4 | 22.8 | 24.7 | −2.0 |
| Republic of Korea† | 49.6 | 52.8 | 29220 | 63613 | 40.6 | 43.4 | 117.7 | 95.0 | 22.7 |
| South-Eastern Asia | |||||||||
| Thailand† | 67.2 | 69.2 | 3684 | 8927 | 4.1 | 5.0 | 142.3 | 110.6 | 31.7 |
| Western Asia | |||||||||
| Turkey† | 72.3 | 90.9 | 6679 | 12298 | 9.1 | 7.8 | 84.1 | 107.5 | −23.4 |
| Eastern Europe | |||||||||
| Belarus | 9.6 | 9.0 | 3298 | 3034 | 19.5 | 15.9 | −8.0 | 19.2 | −27.2 |
| Bulgaria | 7.5 | 6.2 | 1651 | 1248 | 9.9 | 7.6 | −24.4 | 1.6 | −26.1 |
| Poland† | 38.3 | 35.7 | 5651 | 7622 | 8.5 | 9.9 | 34.9 | 41.9 | −7.1 |
| Slovakia | 3.3 | 5.3 | 583 | 1306 | 10.5 | 9.6 | 124.2 | 155.2 | −30.9 |
| Northern Europe | |||||||||
| Denmark | 5.5 | 6.1 | 549 | 739 | 4.9 | 4.6 | 34.5 | 44.6 | −10.1 |
| Estonia | 1.3 | 1.2 | 401 | 322 | 14.4 | 10.2 | −19.6 | 17.1 | −36.7 |
| Iceland | 0.3 | 0.4 | 29 | 40 | 5.0 | 4.5 | 39.2 | 86.4 | −47.3 |
| Ireland | 4.6 | 5.4 | 539 | 984 | 7.5 | 7.2 | 82.6 | 94.1 | −11.5 |
| Lithuania | 3.2 | 2.6 | 950 | 774 | 14.8 | 12.6 | −18.6 | 5.5 | −24.1 |
| Norway | 4.9 | 6.2 | 475 | 594 | 4.9 | 4.1 | 25.0 | 70.3 | −45.3 |
| United Kingdom† | 63.3 | 71.9 | 7446 | 7863 | 5.2 | 4.7 | 5.6 | 52.4 | −46.8 |
| Southern Europe | |||||||||
| Croatia | 4.4 | 3.8 | 1035 | 873 | 10.5 | 8.2 | −15.6 | 16.7 | −32.3 |
| Cyprus | 0.8 | 1.3 | 90 | 227 | 6.2 | 5.8 | 153.3 | 123.0 | 30.3 |
| Italy† | 59.7 | 57.5 | 14926 | 14292 | 9.2 | 6.5 | −4.2 | 39.1 | −43.4 |
| Malta | 0.4 | 0.4 | 54 | 73 | 6.2 | 5.7 | 33.9 | 59.0 | −25.1 |
| Slovenia | 2.1 | 2.0 | 486 | 517 | 10.4 | 9.2 | 6.5 | 29.7 | −23.2 |
| Spain† | 46.8 | 45.9 | 7843 | 9541 | 7.8 | 6.7 | 21.6 | 51.0 | −29.4 |
| Western Europe | |||||||||
| Austria | 8.4 | 9.0 | 1306 | 1583 | 7.0 | 6.0 | 21.2 | 52.7 | −31.4 |
| France† | 63.0 | 68.9 | 7399 | 9737 | 5.4 | 5.1 | 31.6 | 46.5 | −14.9 |
| Germany† | 80.9 | 81.7 | 14939 | 17395 | 7.7 | 7.0 | 16.4 | 33.7 | −17.2 |
| Netherlands | 16.6 | 17.7 | 2021 | 2646 | 5.9 | 5.8 | 30.9 | 59.4 | −28.6 |
| Switzerland† | 7.8 | 9.4 | 888 | 1668 | 5.6 | 6.5 | 87.9 | 61.0 | 26.9 |
| Oceania | |||||||||
| Australia | 22.0 | 29.5 | 2002 | 3044 | 5.1 | 4.6 | 52.0 | 82.2 | −30.2 |
| New Zealand | 4.4 | 5.4 | 376 | 591 | 5.1 | 5.5 | 57.1 | 78.5 | −21.4 |
regional registries: Brazil (Goiana), Chile (Valdivia), China (Hong Kong, Jiashan, Shanghai), Colombia (Cali), France (Bas-Rhin, Calvados, Doubs, Haut-Rhin, Herault, Isere, Loire-Atlantique, Manche, Martinique, Somme), Germany (Hamburg, Saarland), Italy (Biella, Ferrara, Modena, Naples, Parma, Ragusa, Romagna, Veneto), Japan (Miyagi, Nagasaki, Osaka), Poland (Kielce), Republic of Korea (Busan, Gwangju, Incheon, Seoul, Ulsan), Spain (Albacete, Basque Country, Canary Islands, Cuenca, Girona, Granada, Murcia, Navarra, Tarragona), Switzerland (Geneva, Neuchatel, St Gall-Appenzell, Ticino, Valais, Vaud), Thailand (Chiang Mai, Khon Kaen, Lampang, Songkhla), Turkey (Antalya, Izmir), UK (East England, East Midlands, London, North East, North West, South East, South West, West Midlands, Yorkshire-Humber, Northern Ireland, Scotland), U.S. (SEER9, that includes the states of Connecticut, Hawaii, Iowa, New Mexico and Utah, and the areas of Detroit in Michigan, San Francisco in California, Atlanta in Georgia, and Seattle in Washington)
Observed trends differed across age groups. While generally decreases or stabilization of incidence rates were observed in those aged 50 years and above, this was not always the case in the younger age groups (Figures 2–3). Increases in incidence in those younger than 50 years were predicted in 15 out of 34 both low- and high-incidence countries, including Belarus, Chile, The Netherlands, Canada and the UK. This pattern was less pronounced among females.
Figure 2.
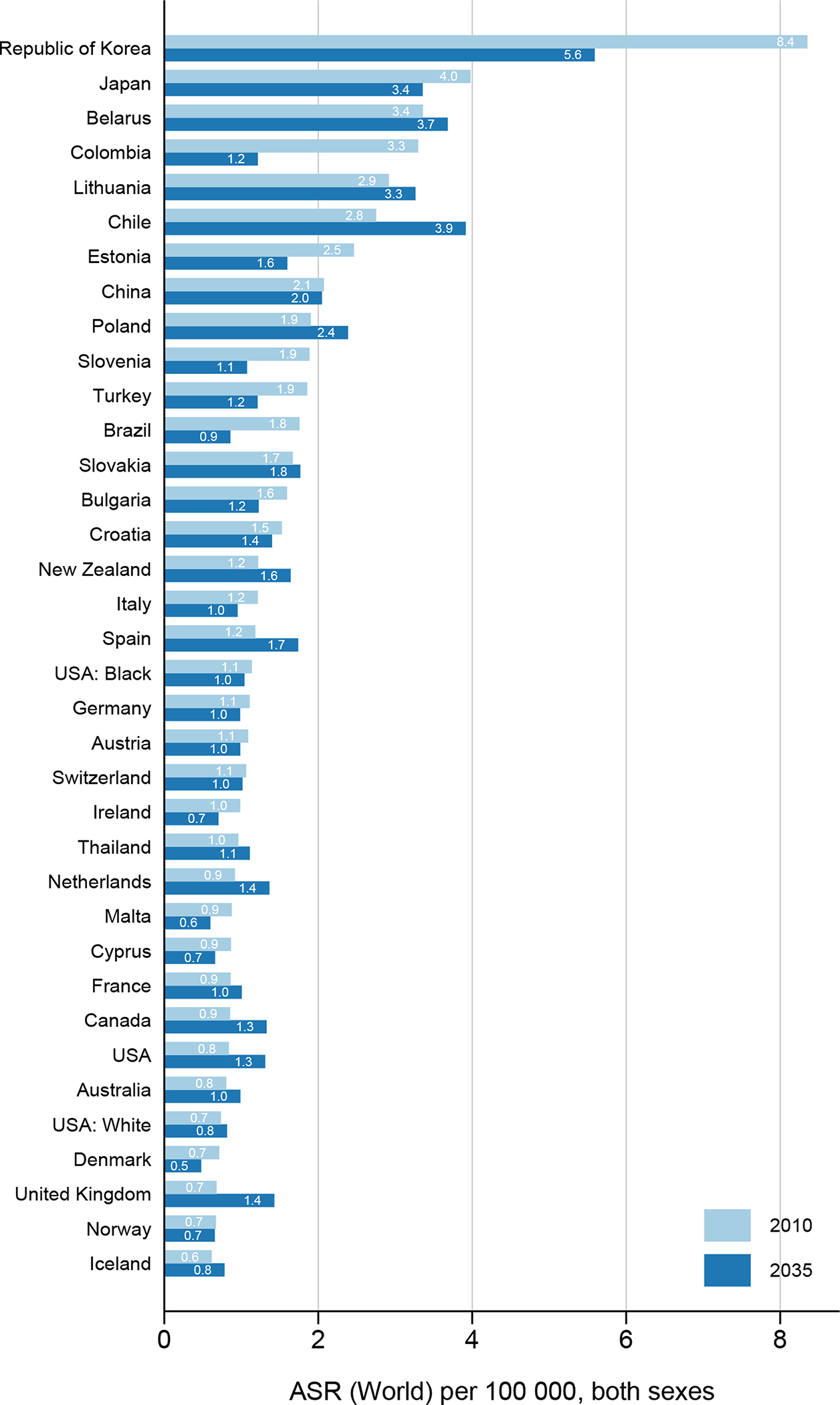
Age-standardized gastric cancer incidence rates (ASR) in 2010 and predicted rates in 2035, both sexes combined, age <50 years.
Discussion
Large geographic variation continues to exist in gastric cancer burden across world regions, yet incidence rates continue to fall (or remain stable) in most countries and are predicted to follow this trend into the future, largely irrespective of underlying incidence levels. Rare cancer thresholds could be reached – or already have been reached – in over half of the countries examined by 2035 but, despite this, the number of new gastric cancer cases is set to remain substantial in most countries in the future mainly due to population growth and ageing. A new and contrasting observation is the recent incidence increases in those aged below 50 years in both low- and high-incidence countries such as the U.K. and the U.S. or Chile and Belarus, particularly in males.
The epidemiology of gastric cancer is changing and whether or not gastric cancer will become a rare disease globally is yet to be determined. To-date, gastric cancer can already be considered rare in several (mainly high-income) countries and is set to decrease towards this threshold in many others. Yet, three observations speak against the rare cancer hypothesis. First, despite decreasing trends in most high-incidence countries (except Korea), rates remain relatively high in those countries, for example exceeding 20 per 100,000 person-years in Japan or Chile. Second, the absolute burden of the disease (number of new cases) is set to stay stable, if not increase, even in population where decreases in incidence rates have been observed. Third, increases in incidence rates in younger generations, especially those aged below 50 years today, could indicate the beginning of a cohort effect that may continue (as those cohorts grow older) into the future and warrant monitoring.
The finding that gastric cancer rates are declining is certainly not new. A trend that, some 30 years ago, was termed “an unplanned triumph” of cancer prevention, because this occurred in the absence of active primary prevention programmes and, outside of Japan and Korea, population-based screening.[11] At the same time, improvements in survival, which might impact on mortality rates, have also been extremely limited, and thus there is no basis for believing that either secondary prevention or treatment are responsible for the secular trends. Evidence from a variety of epidemiological studies including migration and family studies, indicates that the observed time trends as well as the extensive geographic variation in the burden of gastric cancer are most likely due to changes in behavioural and environmental risk factors. These include improvements in sanitation, changes in diet (increases in the consumption of fruit and vegetables and decreases in the consumption of salt), widespread antibiotic use, and, predominantly, the decreasing prevalence of H. pylori infection.[12] A recent study on the estimation of global trends in the burden from gastric cancer [13] confirmed these observations and showed that most of the decline in the incidence of gastric cancer was associated with economic development.
In contrast to the overall declining rates, we observed increases in those aged younger than 50 years, especially in low-incidence countries such as the U.K. and the U.S. – populations with typically a low prevalence of H. pylori infection.[2] This phenomenon has been confirmed by previous studies indicating an increase of gastric cancer of both the cardia (the proximal region of the stomach) and corpus (a subsite of the distal, non-cardia, stomach) especially in younger generations in the U.S. [14, 15]. While increases in distal gastric cancer in this age group have been postulated to be linked with dysbiosis of the gastric microbiome associated with modern lifestyles (as opposed to H. pylori, the more traditional cause of distal gastric cancer), increases in cardia gastric cancer have been associated with increasing levels of overweight and obesity.[16] The U.S. and the U.K. belong to the high-income countries with the highest obesity rates in adulthood, childhood and adolescence, which continues to constitute a real concern to public health.[17] Yet, the importance of each of these modifiable risk factors has been less clearly established. Intake of food preserved in salt, alcohol drinking, and body fatness (for cardia gastric cancer) have been identified as stronger risk factors for gastric cancer.[16] In addition, there is some evidence that consumption of processed meat, grilled or barbecued meat and fish and low fruit and vegetable intake as well as cigarette smoking might increase the risk of gastric cancer, while intake of citrus fruit has been postulated to have beneficial effects on risk.[16, 18] Changes in the prevalence of some of these lifestyle factors may have contributed to increases shown in more recent generations.
Globally, distal gastric cancer remains the most common form of gastric cancer, with chronic H. pylori infection being considered the principal cause and thus making it highly amenable to prevention. [19, 20] According to recent meta-analyses [2, 21] large variation exists in the prevalence of H. pylori across countries, yet this information is lacking from the majority of countries in transition. Capturing comparable prevalence data on the population-level remains challenging due to differences in testing modalities, age groups and time periods. Randomized controlled trials indicate that H. pylori treatment lowers gastric cancer risk [22] and eradication of the bacterium appears to be cost-effective for gastric cancer prevention.[23] However, these trials did not report overall benefits and the results may not be widely generalizable as the trials were conducted mainly in East Asia. In addition, there is remaining uncertainty about possible adverse consequences of H. pylori eradication, such as increases in antibiotic resistance, antibiotic-induced alterations in microbiota [24], increases in oesophageal adenocarcinoma and gastroesophageal reflux disease (GERD) risk [25], weight gain [26, 27] and a loss of beneficial effects of H. pylori infection on immunological reactivity [28, 29]. Ongoing H. pylori eradication clinical trials should address some of these remaining questions.[30, 31, 32]
Nationwide gastric cancer screening programmes have been ongoing in Korea and Japan over several decades.[33, 34] In Korea, the only high-incidence country with predicted increases in incidence, gastric cancer screening is carried out with either upper gastrointestinal series (UGI) or upper endoscopy every 2 years to individuals aged 40 and older.[4] The participation rate was close to 50% in 2011, with 6 million participants undergoing gastric cancer screening out of 13 million people who were invited to the program.[35] These efforts have led to an increasing number of cases diagnosed at an early, curable stage and 5-year survival of 76% in 2012–2016.[36] It remains to be determined as to how much of these survival increases are attributable to lead time and detection bias, which might also in parts explain the increasing incidence rates we predict. A recent nested case-control study showed an approximately 50% reduced risk of death from gastric cancer with upper endoscopy (OR=0.53, 95% CI=0.51–0.56) in Korea.[4] In Japan, gastric cancer secondary prevention programmes have primarily focused on early detection using upper gastrointestinal X-ray series (photofluorography).[37] However, screening has not been widely accepted by the target population with less than 10% of participation rate with the radiographic screening.[3, 37] National emphasis and efforts have been shifting towards treating H. pylori in patients with endoscopically diagnosed chronic gastritis.[38]
Our study has several strengths, but also shortcomings. Following several prediction studies on the national or regional level [39, 40, 41], this is to our knowledge the first attempt to characterize future trends in gastric cancer incidence from a global perspective. Using longitudinal high-quality data, we examined whether gastric cancer might become a “rare” disease (here set at less than 6 cases per 100,000 population). This rare cancer threshold, defined during previous studies on cervical cancer [42] and the RARECARE project [10], might however not be appropriate for global use and should be revised based on in-depth knowledge of the complete natural disease history that continues to be lacking today. We included data from 36 populations (in 34 countries) where long-term high quality cancer registry data are available. The interpretation of our findings might therefore not pertain to countries where data are absent or insufficient and where incidence may be high, specifically in low-income settings. Furthermore, while the distinction between cardia and distal gastric cancers is crucial from an etiological point of view, current data do not allow for sub-site specific analyses of incidence trends. This is partly to due to changes in diagnostic and registration practice, with growing awareness of cardia gastric cancer representing a different entity in more recent years, and historically high proportions of cancers registered with unspecified topography within the stomach (coded as C16.9), which together render subsite-specific trends unreliable and impossible to interpret. Ideally, prediction models should accommodate information on risk factor prevalence and trends. [43] Given that comparable population-level data on the prevalence of H. pylori infection is often limited or unavailable, and that other risk factors such as obesity are confined to few population where proximal gastric cancer is more common, we were not able to take into account information on risk factor prevalence. Finally, trend-based predictions of incidence rates as applied in this study are by definition based on the assumption that incidence trends observed in the past will continue into the future and thus involve a certain degree of uncertainty [43]. Yet, in favor of the method employed, the NORDPRED age-period-cohort model has previously been shown to provide among the best estimates of future cancer burden [8]. By using a drift model that attenuates predicted change, in this paper we have chosen a conservative approach, meaning that true reduction in gastric cancer incidence might be even greater. When comparing our predicted incidence rates (from 2012 onwards) with those already observed in for example Denmark and Australia until 2015, these seem fairly close (e.g., observed ASR 6.6 in Danish males in 2015 vs. 6.4 predicted [44]; observed ASR 4.9 in Australia in 2015 vs. 4.7 predicted [45]). An exception was Korea, where the observed ASR in 2015 was somewhat lower than our predicted rate (33.8 vs 40.2), indicating that our prediction model is of limited use in settings where screening has had a big impact on incidence rates in recent years. [46]
In summary, while overall gastric cancer incidence rates are predicted to continue declining and the disease is set out to become ‘rare’ in a growing number of countries, geographical variation in incidence levels remains high and some countries will continue to experience high rates in the future. This development seems to be accompanied by an increase in incidence in younger generations residing in particular in low-incidence countries. Future studies examining the roles of the microbiome and modern lifestyle are needed to elucidate the drivers of early-onset gastric cancer. At the same time, the absolute number of new gastric cancer cases will likely further increase as a result of population growth and aging, making gastric cancer a major challenge to public health in some world regions. These changes in the epidemiology of gastric cancer warrant further research and action in terms of cancer control, with primary and secondary prevention being the principal goal given the generally poor prognosis in many parts of the world.
Supplementary Material
SUMMARY BOX.
- What is already known about this subject?
- Incidence rates of gastric cancer have been declining for several decades in most parts of the world in the absence of active primary and secondary prevention measures. It is yet unclear whether gastric cancer is set out to become rare in the future.
- What are the new findings?
- Overall gastric cancer incidence rates are predicted to continue declining in the future and the disease is set out to become ‘rare’ in a growing number of countries.
- Geographical variation in gastric cancer incidence remains high and some countries will continue to experience high rates in the future.
- Recent increases in incidence in younger ages residing in high-income countries suggest generational transitions in disease risk and the epidemiology of gastric cancer.
- How might it impact on clinical practice in the foreseeable future?
- Despite declining incidence rates in most countries, clinicians can expect to see more gastric cancer cases in the future due to ageing and growth of high-risk populations.
- Surveillance of ongoing transitions in the epidemiology of gastric cancer is therefore highly relevant to future cancer control and clinical practice.
Acknowledgments
The authors gratefully acknowledge all cancer registries and their staff who have contributed in sharing their data needed for this study.
Where authors are identified as personnel of the International Agency for Research on Cancer / World Health Organization, the authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policy or views of the International Agency for Research on Cancer / World Health Organization.
Abbreviations:
- ASR
age-standardized rate
- GERD
gastroesophageal reflux disease
- H pylori
helicobacter pylori
- UGI
upper gastrointestinal series
- U.K.
United Kingdom
- U.N.
United Nations
- U.S.
United States of America
Footnotes
Competing interests: The authors declare no relevant competing interests.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 2.Hooi JKY, Lai WY, Ng WK, Suen MMY, Underwood FE, Tanyingoh D, et al. Global Prevalence of Helicobacter pylori Infection: Systematic Review and Meta-Analysis. Gastroenterology 2017;153:420–9. [DOI] [PubMed] [Google Scholar]
- 3.Hamashima C, Systematic Review G, Guideline Development Group for Gastric Cancer Screening G. Update version of the Japanese Guidelines for Gastric Cancer Screening. Jpn J Clin Oncol 2018;48:673–83. [DOI] [PubMed] [Google Scholar]
- 4.Jun JK, Choi KS, Lee HY, Suh M, Park B, Song SH, et al. Effectiveness of the Korean National Cancer Screening Program in Reducing Gastric Cancer Mortality. Gastroenterology 2017;152:1319–28 e7. [DOI] [PubMed] [Google Scholar]
- 5.Ferlay J, Colombet M, Bray F. Cancer Incidence in Five Continents, CI5plus: IARC CancerBase No. 9. Lyon, France: International Agency for Research on Cancer, 2018. [Google Scholar]
- 6.World Population Prospects. The 2017 revision. New York: United Nations, Department of Economics and Social Affairs, Population Division, 2017. [Google Scholar]
- 7.Doll R, Payne P, Waterhouse J. Cancer Incidence in Five Continents: A Technical Report. Berlin, 1966. [Google Scholar]
- 8.Moller B, Fekjaer H, Hakulinen T, Sigvaldason H, Storm HH, Talback M, et al. Prediction of cancer incidence in the Nordic countries: empirical comparison of different approaches. Stat Med 2003;22:2751–66. [DOI] [PubMed] [Google Scholar]
- 9.Moller B, Fekjaer H, Hakulinen T, Tryggvadottir L, Storm HH, Talback M, et al. Prediction of cancer incidence in the Nordic countries up to the year 2020. Eur J Cancer Prev 2002;11 Suppl 1:S1–96. [PubMed] [Google Scholar]
- 10.Gatta G, van der Zwan JM, Casali PG, Siesling S, Dei Tos AP, Kunkler I, et al. Rare cancers are not so rare: the rare cancer burden in Europe. Eur J Cancer 2011;47:2493–511. [DOI] [PubMed] [Google Scholar]
- 11.Howson CP, Hiyama T, Wynder EL. The decline in gastric cancer: epidemiology of an unplanned triumph. Epidemiol Rev 1986;8:1–27. [DOI] [PubMed] [Google Scholar]
- 12.Karimi P, Islami F, Anandasabapathy S, Freedman ND, Kamangar F. Gastric cancer: descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol Biomarkers Prev 2014;23:700–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Collaborators GBDSC. The global, regional, and national burden of stomach cancer in 195 countries, 1990–2017: a systematic analysis for the Global Burden of Disease study 2017. Lancet Gastroenterol Hepatol 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anderson WF, Rabkin CS, Turner N, Fraumeni JF Jr., Rosenberg PS, Camargo MC. The Changing Face of Noncardia Gastric Cancer Incidence Among US Non-Hispanic Whites. J Natl Cancer Inst 2018;110:608–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Camargo MC, Anderson WF, King JB, Correa P, Thomas CC, Rosenberg PS, et al. Divergent trends for gastric cancer incidence by anatomical subsite in US adults. Gut 2011;60:1644–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diet, Nutrition, Physical activity, and Stomach Cancer. Continuous Update Project Report. World Cancer Research Fund / American Institute for Cancer Research, 2016. [Google Scholar]
- 17.Collaboration NCDRF. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet 2017;390:2627–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Praud D, Rota M, Pelucchi C, Bertuccio P, Rosso T, Galeone C, et al. Cigarette smoking and gastric cancer in the Stomach Cancer Pooling (StoP) Project. Eur J Cancer Prev 2018;27:124–33. [DOI] [PubMed] [Google Scholar]
- 19.Colquhoun A, Arnold M, Ferlay J, Goodman KJ, Forman D, Soerjomataram I. Global patterns of cardia and non-cardia gastric cancer incidence in 2012. Gut 2015;64:1881–8. [DOI] [PubMed] [Google Scholar]
- 20.Plummer M, Franceschi S, Vignat J, Forman D, de Martel C. Global burden of gastric cancer attributable to Helicobacter pylori. Int J Cancer 2015;136:487–90. [DOI] [PubMed] [Google Scholar]
- 21.Peleteiro B, Bastos A, Ferro A, Lunet N. Prevalence of Helicobacter pylori infection worldwide: a systematic review of studies with national coverage. Dig Dis Sci 2014;59:1698–709. [DOI] [PubMed] [Google Scholar]
- 22.Ford AC, Forman D, Hunt RH, Yuan Y, Moayyedi P. Helicobacter pylori eradication therapy to prevent gastric cancer in healthy asymptomatic infected individuals: systematic review and meta-analysis of randomised controlled trials. BMJ 2014;348:g3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Areia M, Carvalho R, Cadime AT, Rocha Goncalves F, Dinis-Ribeiro M. Screening for gastric cancer and surveillance of premalignant lesions: a systematic review of cost-effectiveness studies. Helicobacter 2013;18:325–37. [DOI] [PubMed] [Google Scholar]
- 24.Blaser M Antibiotic overuse: Stop the killing of beneficial bacteria. Nature 2011;476:393–4. [DOI] [PubMed] [Google Scholar]
- 25.Yaghoobi M, Farrokhyar F, Yuan Y, Hunt RH. Is there an increased risk of GERD after Helicobacter pylori eradication?: a meta-analysis. Am J Gastroenterol 2010;105:1007–13; quiz 6, 14. [DOI] [PubMed] [Google Scholar]
- 26.Lane JA, Murray LJ, Harvey IM, Donovan JL, Nair P, Harvey RF. Randomised clinical trial: Helicobacter pylori eradication is associated with a significantly increased body mass index in a placebo-controlled study. Alimentary pharmacology & therapeutics 2011;33:922–9. [DOI] [PubMed] [Google Scholar]
- 27.Francois F, Roper J, Joseph N, Pei Z, Chhada A, Shak JR, et al. The effect of H. pylori eradication on meal-associated changes in plasma ghrelin and leptin. BMC Gastroenterol 2011;11:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blaser MJ. Equilibria of humans and our indigenous microbiota affecting asthma. Proc Am Thorac Soc 2012;9:69–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holster IL, Vila AM, Caudri D, den Hoed CM, Perez-Perez GI, Blaser MJ, et al. The impact of Helicobacter pylori on atopic disorders in childhood. Helicobacter 2012;17:232–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pan KF, Zhang L, Gerhard M, Ma JL, Liu WD, Ulm K, et al. A large randomised controlled intervention trial to prevent gastric cancer by eradication of Helicobacter pylori in Linqu County, China: baseline results and factors affecting the eradication. Gut 2016;65:9–18. [DOI] [PubMed] [Google Scholar]
- 31.Choi JM, Kim SG, Choi J, Park JY, Oh S, Yang HJ, et al. Effects of Helicobacter pylori eradication for metachronous gastric cancer prevention: a randomized controlled trial. Gastrointest Endosc 2018;88:475–85 e2. [DOI] [PubMed] [Google Scholar]
- 32.Leja M, Park JY, Murillo R, Liepniece-Karele I, Isajevs S, Kikuste I, et al. Multicentric randomised study of Helicobacter pylori eradication and pepsinogen testing for prevention of gastric cancer mortality: the GISTAR study. BMJ open 2017;7:e016999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee KS, Oh DK, Han MA, Lee HY, Jun JK, Choi KS, et al. Gastric cancer screening in Korea: report on the national cancer screening program in 2008. Cancer Res Treat 2011;43:83–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakamura Y, Takeshita M, Hirota Y, Ueda K, Yao T, Kohzuki T, et al. An evaluation of gastric cancer screening program in Hisayama, Japan. Gastroenterol Jpn 1977;12:427–34. [DOI] [PubMed] [Google Scholar]
- 35.Lee S, Jun JK, Suh M, Park B, Noh DK, Jung KW, et al. Gastric cancer screening uptake trends in Korea: results for the National Cancer Screening Program from 2002 to 2011: a prospective cross-sectional study. Medicine (Baltimore) 2015;94:e533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jung KW, Won YJ, Kong HJ, Lee ES. Cancer Statistics in Korea: Incidence, Mortality, Survival, and Prevalence in 2016. Cancer Res Treat 2019;51:417–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Asaka M, Kato M, Graham DY. Strategy for eliminating gastric cancer in Japan. Helicobacter 2010;15:486–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Asaka M, Mabe K. Strategies for eliminating death from gastric cancer in Japan. Proc Jpn Acad Ser B Phys Biol Sci 2014;90:251–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bray F, Pineros M. Cancer patterns, trends and projections in Latin America and the Caribbean: a global context. Salud Publica Mex 2016;58:104–17. [DOI] [PubMed] [Google Scholar]
- 40.Joliat GR, Hahnloser D, Demartines N, Schafer M. Future development of gastrointestinal cancer incidence and mortality rates in Switzerland: a tumour registry- and population-based projection up to 2030. Swiss Med Wkly 2015;145:w14188. [DOI] [PubMed] [Google Scholar]
- 41.Mistry M, Parkin DM, Ahmad AS, Sasieni P. Cancer incidence in the United Kingdom: projections to the year 2030. Br J Cancer 2011;105:1795–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Simms KT, Steinberg J, Caruana M, Smith MA, Lew JB, Soerjomataram I, et al. Impact of scaled up human papillomavirus vaccination and cervical screening and the potential for global elimination of cervical cancer in 181 countries, 2020–99: a modelling study. Lancet Oncol 2019;20:394–407. [DOI] [PubMed] [Google Scholar]
- 43.Bray F, Moller B. Predicting the future burden of cancer. Nat Rev Cancer 2006;6:63–74. [DOI] [PubMed] [Google Scholar]
- 44.Engholm G, Ferlay J, Christensen N, Kejs AMT, Johannesen TB, Khan S, et al. NORDCAN: Cancer Incidence, Mortality, Prevalence and Survival in the Nordic Countries, Version 8.1 (28.06.2018). Association of the Nordic Cancer Registries. Danish Cancer Society. http://www.ancr.nu, 2018. [Google Scholar]
- 45.Cancer Data in Australia; Australian Cancer Incidence and Mortality (ACIM) books: stomach cancer Canberra: Australian Institute of Health and Welfare (AIHW), 2018. [Google Scholar]
- 46.Jung KW, Won YJ, Kong HJ, Lee ES, Community of Population-Based Regional Cancer R. Cancer Statistics in Korea: Incidence, Mortality, Survival, and Prevalence in 2015. Cancer Res Treat 2018;50:303–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


