Abstract
Objective: Male infertility is involved in about half of the casess of infertility and the only sole reason for infertility in 20%-30% of the cases. Following the recent interest in the use of medicinal plants, scientists have sought to clarify their effects on male fertility. This review aimed to summarize the results of studies available to determine the effectiveness, safety and mechanism of herbal treatments in the improvement of male fertility.
Materials and methods: Medline/PubMed, Scopus, Science Direct, and the Cochrane Central Register of Controlled Trials (Central) databases were searched for randomized controlled trials (RCTs) published during 2000-2020. Studies were only included if they adhered to the CONSORT checklist. The methodological quality of the selected studies was assessed using the Cochrane risk of bias tool.
Results: Finally, 20 studies recruiting a total of 1519 individuals were reviewed. These studies compared the effects of eleven different medicinal plants, i.e. ginseng, saffron, Nigella sativa, palm pollen, ADOFON, TOPALAF, sesame, and Mucuna pruriens, on male fertility with those of placebo. All studies (except one) confirmed the beneficial effects of medicinal plants on the improvement of sperm and reproductive parameters and thus male infertility.
Conclusion: The existing RCTs indicated the positive effects of medicinal plants on male fertility. Therefore, in order to develop a novel approach to the treatment of male infertility, further clinical trials are warranted to determine the maximum dosage and duration of treatment with herbal medicines and evaluate any potential side effects of such interventions.
Key Words: Male Infertility, Medicinal Plants, Fertility
Introduction
With a prevalence rate of 2.5%-12%, male infertility affects over 30 million men around the world. It is the sole reason and the contributing factor for 20%-30% and half of all cases of infertility, respectively (1, 2). Since male fertility largely depends on sperm count, quality, motility, and morphology, defects in any of these factors can lead to infertility (3). A large percentage (up to 90%) of all infertile couples have to deal with low sperm count and/or quality (4). Under development of the testicles, reproductive system diseases, elevated scrotal temperature, immune system and endocrine disorders, and lifestyle, along with environmental and nutritional factors have been reported to negatively affect sperm parameters and cause male infertility (5).
Complementary therapies for infertility have received growing attention during recent years and various antioxidants, nutritional approaches, and medicinal plants have been proposed for the treatment of fertility problems in infertile and sub fertile couples (6). Several medicinal plants with antifertility or fertility boosting effects have been traditionally used to either decrease or increase male fertility throughout the world. Fertility-related properties of these plants have also been the subject of interest in modern scientific research (7).Following men’s increasing interest in effective herbal treatments of infertility, complementary approaches to infertility treatment have received growing attention (8). The European Association of Urology has recently reported the use of complementary treatments based on traditional medicine as a multidimensional integrative approach to male infertility treatment (9). The World Health Organization (WHO) encourages the use of medicinal plants, and suggested researchers to define the rational use of medicinal plants as a source of new treatments (10). Such a growing interest in medicinal plants has inspired scientists to clarify their effects on male fertility. Based on the available literature, some of these plants increase sperm count and motility while a number of others alter the secretion of hormones by the testicles (11). The flavonoids and phenolic compounds in some plants serve as potent antioxidants against oxygenated free radicals. They actually protect the sperms against free radicals and improve sperm quality and fertility parameters (12). However, according to the World Health Organization (WHO), despite the wide use of herbal medicines, scientific knowledge on their properties is still scarce. It is hence essential to evaluate the effects of biologically active herbal compounds on male fertility and to identify natural compounds with estrogenic and anti-estrogenic properties(5). Therefore, this review aimed to summarize the results of studies available to determining the effectiveness, safety and mechanism of herbal treatments in the improvement of male fertility.
Materials and methods
Search strategy : Medline/PubMed, Scopus, Science Direct, and the Cochrane Central Register of Controlled Trials (Central) databases were searched for English articles published during 2000-2017 which contained a number of key terms including “male fertility”, “medicinal plants”, and “male infertility". The keywords in each group were combined using the Boolean operator “OR”. All searches containing at least one keyword from each group were combined using the Boolean operator “AND”. The search process was completed in September 2020.
Inclusion and exclusion criteria : This review only included randomized controlled trials (RCTs) evaluating the effects of medicinal plants (irrespective of treatment duration and route of administration) on improving sperm parameters, testicular function, and male reproductive system disorders and comparing these effects with those of no treatment, treatment with placebo, and treatment with other non-herbal agents. Studies without a control group and those without numerical outcome data were excluded. Observational and qualitative studies, case reports, and papers presenting a subgroup analysis of another primary study were also excluded. No language limitations were imposed during the search process.
Study selection : Two authors (FA and NR) evaluated the titles and abstracts of the search results, retrieved the full texts of potentially relevant RCTs, and examined the eligibility of the extracted articles. Studies were only included if they contained all the required information based on the 25-item Consolidated Standards of Reporting Trials (CONSORT) checklist (13). Cases of disagreement about article eligibility were resolved through consensus.
Data extraction : The same two authors independently extracted and recorded study characteristics including the name of the administered medicinal plant, the participants and their grouping, the interventions and their duration, measured outcomes, and effectiveness parameters. The extracted data were then compared and cases of disagreement were resolved through consensus.
Risk of bias assessment in the selected studies : The mentioned authors used the Cochrane Risk of Bias Assessment Tool (14) to independently assess the risk of bias by evaluating the randomization method, allocation concealment, blinding processes, incomplete outcome data, and selective outcome reporting (Table 1). Then the results were compared and cases of disagreement were resolved through consensus.
Table 1.
Summarized results of the studies
| Authors (years) | Plant name | Participants |
Intervention
(daily dose) |
Control
group |
Duration |
Study outcome
measures |
Improvements | Side effect |
Consort
score |
|---|---|---|---|---|---|---|---|---|---|
| Heidary (2008), (15) | Saffron | 52 nonsmoker infertile men |
50mg | Placebo | 3 months | Semen parameters | Sperm morphology and motility(p<0.001) |
None reported |
16 |
| Safarinejad (2011), (16) |
Saffron | 260 infertile men |
60 mg | Placebo | 26 weeks | Semen parameters and total seminal plasma antioxidant capacity |
No improvement | Safe | 19 |
| Shamsa (2009), (17) | Saffron | 20 male patients with ED |
200mg | Placebo | 10 days | RigiScan parameters and sexual function |
Rigidity, tumescence, erectile function, sexual satisfaction, orgasm, sexual desire and overall satisfaction (p<0.001) |
None reported |
20 |
| Rasekh (2015), (18) | Palm pollen | 50 infertile men |
120 mg/ kg | Placebo | 2 months | Sperm parameters | Sperm motility, morphology and forward progressive motility (p<0.05) |
None reported |
17 |
| Saeed (2020), (19) | Date Palm pollen | 40 subfertile men |
500mg | Control | 3months | Semen parameters and serum hormone levels |
sperm motility in oligoasthenozoosper mic patients (p<0.05) |
Safe | 17 |
| Khoradmehr (2015), (20) |
TOPALAF | 62 infertile men |
12.5 mg | Placebo | 3months | Sperm parameters | Count and sperm motility(p<0.05) |
None reported |
16 |
| Ramezani (2015), (21) |
ADOFON | 62 infertile men |
12.5 mg | Placebo | 3months | Sperm parameters | Count and sperm motility(p<0.05) |
None reported |
16 |
| Kim (2002), (22) | Ginseng | 80 patients with abnormal semen |
2.4 gm | Placebo | 2 months | Sperm parameters | Count and Semen volume (p<0.05) |
None reported |
17 |
| Park (2016), (23) | Ginseng | 80infertile men |
1.5-g | Placebo | 3 months | Sperm analysis was performed and hormonal levels |
Sperm concentrations, motility, morphology, and viability(p<0.05) |
None reported |
21 |
| Khani (2013), (24) | Sesame | 25 infertile men |
0.5 mg/kg | Placebo | 3months | Sperm parameters | Sperm count and motility(p<0.05) |
No major side effect was reported. |
18 |
| Kolahdooz (2014), (25) |
Nigella sativa | 34 Iranian infertile men |
5 ml | Placebo | 2 months | Sperm parameters | Sperm count, motility and morphology and semen volume, pH and round cells (p<0.05) |
Safe | 22 |
| Marbat (2013), (26) | Nigella sativa | 50 infertile men |
2g | Placebo | 3 months | Sperm analysis was performed and hormonal levels |
Sperm count, motility, viability, morphology, serum FSH, LH and testosterone levels (p<0.01) |
None reported |
15 |
| Ambiye (2013), (27) | Ashwagandha | 68infertile men |
675 mg | Placebo | 3 months | Semen parameters and serum hormone levels |
Sperm count, volume, motility, testosterone and LH levels (p<0.05) |
Safe | 20 |
| Ahmad (2010), (28) | Ashwagandha | 75 normal healthy fertile men |
5 g | Placebo | 3 months | Seminal plasma biochemical parameters, antioxidant vitamins, and hormone levels |
Sperm count, motility, seminal plasma levels of antioxidant enzymes, vitamins A, C, and E, testosterone and LH levels (p<0.05) |
Safe | 17 |
| Mahdi (2011), (29) | Ashwagandha | 121infertile men |
5 g | Placebo | 3 months | Seminal plasma biochemical parameters, antioxidant vitamins, and hormone levels |
Sperm count, motility, seminal plasma levels of antioxidant enzymes, testosterone and LH levels (p<0.05) |
None reported |
17 |
| Mares (2012), (30) | Ashwagandha | 75 infertile men |
No reported | Placebo | 3 months | Semen parameters and serum hormone levels |
Sperm count, motility, viability, morphology, volume, serum glutathione, serum FSH, LH and testosterone levels(p<0.05) |
None reported |
15 |
| Gupta (2013), (31) | Ashwagandha | 180 infertile men |
5 g | Placebo | 3 months | Semen parameters and serum hormone levels |
Sperm count, motility, disturbed concentrations of lactate, alanine, citrate, GPC, histidine, and phenylalanine in seminal plasma (p<0.05) |
None reported |
18 |
| Shukla (2009), (32) | Mucunapruriens | 75 infertile men |
5 g | Placebo | 3 months | Semen parameters and serum hormone levels |
Sperm count and motility, and LH, dopamine, adrenaline, and noradrenaline levels(p<0.05) |
None reported |
18 |
| Mkrtchyan (2005), (33) |
Andrographis paniculata | 50 infertile men |
60 mg | ginseng | 13 days | Semen parameters | Sperm volume, and count (p<0.05) |
Safe | 17 |
| Kolangi (2019), (34) | Alpinia officinarum | 60 infertile men |
300 mg | Placebo | 3 months | Sperm parameters | Sperm count and morphology(p<0.05) |
Safe | 22 |
Results
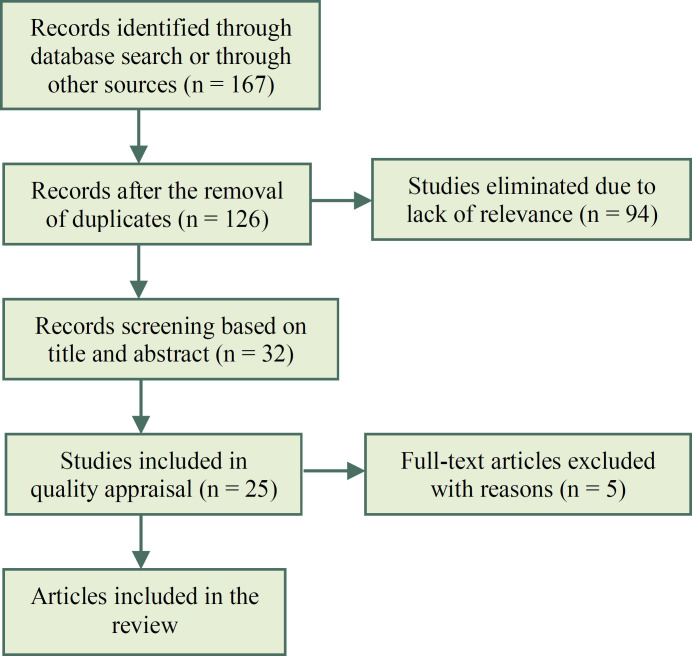
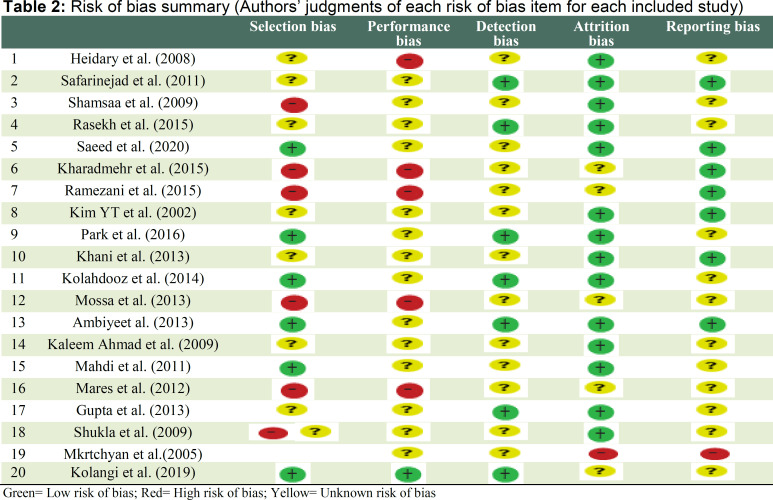
After the primary search, the information provided in 167 papers was individually assessed. Duplicate articles were then excluded and the abstracts and titles of the remaining 126 papers were reviewed. In the next stage, 94 unrelated papers were excluded. Five papers that lacked specific data were also excluded. Evaluating the full texts of the remaining articles confirmed the eligibility of only 20 papers. Figure 1 shows the flow diagram of the literature review process. Afterward, the main findings of the selected papers were summarized and the articles were scored based on their adherence to CONSORT checklist (Table 2). The studies were then evaluated in terms of bias (Table 1). The selected studies were performed on 20-260 participants and a total of 1519 individuals were included in the 20 eligible studies. The selected RCTs were placebo, ginseng and pretest controlled. They implemented a variety of interventions and used various medicinal plants including ashwagandha (n = 5), ginseng (n = 2), saffron (n = 3), Nigella sativa (n = 2), palm pollen (n = 2), ADOFON (n = 1), TOPALAF (n = 1), sesame (n = 1), Andrographis paniculata (n = 1), Alpinia officinarum (n = 1) and Mucuna pruriens (n = 1).
Figure 1.
Flow diagram of the search process
The interventions lasted for 10 days to 26 weeks (three months in 14 studies). In most selected papers, the main measured outcome was the effectiveness of the intervention which was determined in terms of sperm and semen parameters, serum hormone levels, total seminal plasma antioxidant capacity, RigiScan parameters, sexual function, seminal plasma biochemical parameters, and antioxidant vitamins.
All studies (except one) confirmed the beneficial effects of the administered plants on male infertility, sperm parameters and sexual function. The majority of the selected papers did not find any adverse effects following the use of medicinal plants. Moreover, 8 studies directly reported the safety of the plants. Most studies (n = 15) highlighted improved sperm parameters, specifically sperm count and motility, after the interventions. Also in one study, intervention affected sexual function among participation.
We assessed bias in all studies by using the Cochrane tool. Attrition bias was rated low in all studies (72%). None of the studies had a high risk of detection bias. There was an unknown risk in performance bias (72%) in most studies. Across studies, the risk of bias was generally unknown.
Discussion
Due to the significance of male infertility, this systematic review summarized the results of 20 high-quality studies with a low risk of bias to clarify the effects of medicinal plants on male fertility. Based on the reviewed RCTs, medicinal plants improved semen volume and sperm quality in infertile men.
Efforts to identify the possible mode of action of herbs against infertility showed that the use of these natural products by infertile men significantly regulated not only the levels of male sex hormones but also sperm motility and concentration. While the reviewed studies generally confirmed the efficacy of medicinal plants in promoting male fertility, they were not always in agreement in cases of effectiveness and side effects.
Differences in the daily dose of the medical plants might have been responsible for such controversies. In other words, the effects of medicinal plants on male fertility were determined by type of plant and content, the use dosage, and the duration of the interventions.
Several potential approaches for infertility have been investigated over a long time including genetic, hormonal, chemical and immunological approaches. According to some studies, oligosaccharides, i.e. active constituents of medicinal plants with fertility-boosting effects, were able to protect the DNA of human sperm against damage (35). The results of the present study showed medicinal plants modulate estrogen, progesterone, FSH, LH, and GnRH levels.
Shukla et al. showed that the administration of M. pruriens in infertile men significantly increased testosterone, luteinizing hormone (LH), dopamine, adrenaline, and noradrenaline levels but decreased follicle-stimulating hormone (FSH) and prolactin levels. Moreover, the treatment restored sperm count and motility to their normal ranges (32).
Another research highlighted the potential role of micro-elements isolated from palm pollen, e.g. estrogen and sterols, in enhancing male fertility and improving sperm motility and viability, acrosome reaction, and lipid peroxidation (36). Moreover, by boosting the immune system, saffron not only reduces oxidative stresses, but also increases the lifespan of spermatozoa and the number of viable spermatozoa (37). The compounds in saffron stimulate FSH, LH, and testosterone hormones and thus promote epithelial cell proliferation in seminiferous tubules, enhance the activity of Leydig cells, increase the number of spermatocytes, and ultimately promote spermatogenesis (38).
According to previous research, some medicinal plants prevent free radicals, lipid peroxidation, and sperm damage by improving antioxidant activity. They also increase the number of testicular veins, the number and lifespan of spermatozoa, protect germ cells, and enhance the activity of the hypothalamic-pituitary-gonadal axis (4).
Many studies on the use of medicinal plants for the treatment of male infertility have focused on Withania somnifera. Treatment with W. somnifera was found to restore LH, FSH, and prolactin levels which are directly correlated with semen quality. Furthermore, the aqueous extract of this plant promoted gonadotropin secretion, improved epididymis sperm pattern, and stimulated testicular development and spermatogenesis through its direct effects on seminiferous tubules (28). Owing to the alkaloids, ergostane steroids, amino acids (including tryptophan), central nervous system inhibitors, centrally acting hypotensive agents, and gamma-aminobutyric acid (GABA) and serotonin agonists available in its roots, W. somnifera can treat stress-induced infertility (29). As reported by two clinical trials and several animal studies, ginseng enhanced spermatogenesis by promoting the expression of glial cell-derived neurotrophic factor (GDNF). GDNF is a growth factor involved in the communication between sertoli cells and spermatogonia. Ginseng can also upregulate C21-steroid hormone metabolism via Cyp11a1 and thus cause anti-aging effects in senescent rat testes (23). The beneficial effects of Nigella sativa on sperm and reproductive parameters have also been documented in various studies. This study showed many beneficial effects of medicinal plants on male infertility treatment are associated with antioxidant effects. This suggests that antioxidants have been to improve spermatogenesis of male reproduction system.
Although the exact mechanisms of these effects are still unknown, the antioxidant properties of N. sativa have been suggested to be responsible. While the antioxidants in semen can normally neutralize reactive oxygen species (ROS), decreased antioxidant property or increased ROS content of the semen would elevate the oxidative stress levels and damage sperm parameters (39).
Despite the existing controversies over the exact effects of medicinal plants on male infertility, in vitro and animal studies have confirmed the benefits of a number of herbal medicines in promoting men fertility. These benefits have been justified by the effects of medicinal plants on both the central nervous system and the reproductive tract. Nevertheless, further in-depth studies are required to elucidate such effects.
Conclusion
In conclusion, previous RCTs have highlighted the fertility-boosting effects of medicinal plants in men. However, in order to develop a new approach toward the treatment of male fertility, further RCTs are warranted to confirm the mentioned effects, determine the maximum dosage and duration of treatment with herbal medicines, and evaluate any potential side effects of such treatments. Like any other study, this review had a number of limitations. First, some of the selected studies had low methodological quality and very small sample size. Furthermore, articles in languages other than English were not included. Lack of information about the use of novel herbal therapies in men's infertility was another limitation of our review.
Acknowledgments
The authors wish to express their gratitude to the reviewer comments.
Conflict of Interests
Authors have no conflict of interests.
Notes:
Citation: Roozbeh N, Amirian A, Abdi F, Haghdoost S. A Systematic Review on Use of Medicinal Plants for Male Infertility Treatment. J Fam Reprod Health 2021; 15(2): 74-81.
References
- 1.Agarwal A, Roychoudhury S, Bjugstad KB, Cho C-L. Oxidation-reduction potential of semen: what is its role in the treatment of male infertility? Ther Adv Urol. 2016;8:302–18. doi: 10.1177/1756287216652779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hosseini H, Abdi F. Experiences of vasectomy: A phenomenological study. N Am J Med Sci. 2012;4:619–23. doi: 10.4103/1947-2714.104311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oyeyemi M. O, Olukole S. G, Esan O. Sperm morphological studies of West African Dwarf Bucks treated with pumpkin plant (Cucurbita pepo). Int J Morphol. 2008;26:121–6. [Google Scholar]
- 4.Roozbeh N, Rostami S, Abdi F. A Review on herbal medicine with Fertility and Infertility characteristics in Males. IJOGI. 2016;19:18–32. (Persian) [Google Scholar]
- 5.Marbeen MI, AL-Snafi AE, Marbut MM, Allahwerdy IY. The probable therapeutic effects of date palm pollen in the treatment of male infertility. Tikrit Journal Pharmaceutical Sciences. 2005;1:30–5. [Google Scholar]
- 6.Abdi F, Roozbeh N, Mortazavian AM. Effects of date palm pollen on fertility: research proposal for a systematic review. BMC Res Notes. 2017;10:363. doi: 10.1186/s13104-017-2697-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jain S, Choudhary GP, Jain DK. Medicinal plants with potential anti-fertility activity: A review. International Journal of Green Pharmacy. 2015;9:223–8. [Google Scholar]
- 8.Yao DF, Mills JN. Male infertility: lifestyle factors and holistic, complementary, and alternative therapies. Asian J androl . 2016;18:410–8. doi: 10.4103/1008-682X.175779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nejatbakhsh F, Shirbeigi L, Rahimi R, Abolhassani H. Review of local herbal compounds found in the Iranian traditional medicine known to optimise male fertility. Andrologia. 2016;48:850–9. doi: 10.1111/and.12675. [DOI] [PubMed] [Google Scholar]
- 10.Nantia E, Moundipa PF, Monsees TK, Carreau S. Medicinal plants as potential male anti-infertility agents: a review. Androl . 2009;19:148–58. [Google Scholar]
- 11.Khaki A, Fathiazad F, Nouri M, Khaki A, Ozanci CC, Ghafari-Novin M, et al. The effects of Ginger on spermatogenesis and sperm parameters of rat. Iranian Journal of Reproductive Medicine. 2009;7:7–12. [Google Scholar]
- 12.Rekka EA, Kourounakis AP, Kourounakis PN. Investigation of the effect of chamazulene on lipid peroxidation and free radical processes. Res Commun Mol Pathol Pharmacol. 1996;92:361–4. [PubMed] [Google Scholar]
- 13.Abdi F, Kazemi F, Ramezani Tehrani F, Roozbeh N. Protocol for systematic review and meta-analysis: hop (Humulus lupulus L.) for menopausal vasomotor symptoms. BMJ Open. 2016;6:e010734. doi: 10.1136/bmjopen-2015-010734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heidary M, Vahhabi S, Reza Nejadi J, Delfan B, Birjandi M, Kaviani H, et al. Effect of saffron on semen parameters of infertile men. Urol J. 2008;5:255–9. [PubMed] [Google Scholar]
- 16.Safarinejad MR, Shafiei N, Safarinejad S. A prospective double‐blind randomized placebo‐controlled study of the effect of saffron (Crocus sativus Linn.) on semen parameters and seminal plasma antioxidant capacity in infertile men with idiopathic oligoasthenoteratozoospermia. Phytother Res. 2011;25:508–16. doi: 10.1002/ptr.3294. [DOI] [PubMed] [Google Scholar]
- 17.Shamsa A, Hosseinzadeh H, Molaei M, Shakeri MT, Rajabi O. Evaluation of Crocus sativus L.(saffron) on male erectile dysfunction: a pilot study. Phytomedicine. 2009;16:690–3. doi: 10.1016/j.phymed.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 18.Rasekh A, Jashni HK, Rahmanian K, Jahromi AS. Effect of palm pollen on sperm parameters of infertile man. Pak J Biol Sci. 2015;18:196. doi: 10.3923/pjbs.2015.196.199. [DOI] [PubMed] [Google Scholar]
- 19.Saeed HS, Osman B, El-Hadiyah TMH, Mohamed MS, Osman WJA, Abdoon IH, et al. Date Palm Pollen Grains as a Potential Manager for Male Sub-fertility: A Clinical Trial. Journal of Pharmaceutical Research International. 2020;32:83–95. [Google Scholar]
- 20.Khoradmehr A, Khalili MA, Ramezani M, Vahidi S, Moein MR. Improvement of sperm physiological parameters in patients with fertility problems after taking the herbal medicine "TOPALAF". Iranian Journal of Medicinal and Aromatic Plants. 2014;30:275–81. [Google Scholar]
- 21.Ramezani M, Khalili M, Khoradnehr The Study Of Sperm Quality After Consumption Of A Traditional Herbal Compound (Adofon) By Infertile Men. Animal Biology. 2014;6:37–44. [Google Scholar]
- 22.Kim YT, Lee HL, Lee SC, Shin KH, Han KH, Lee SC, Jang H, Kim TH, Kim WJ. Effect of Panax Ginseng Water Extract for Treatment of Male Infertility. Korean J Androl. 2002;20:94–9. [Google Scholar]
- 23.Park HJ, Choe S, Park NC. Effects of Korean red ginseng on semen parameters in male infertility patients: a randomized, placebo-controlled, double-blind clinical study. Chinese journal of integrative medicine. 2016;22:490–5. doi: 10.1007/s11655-015-2139-9. [DOI] [PubMed] [Google Scholar]
- 24.Khani B, Bidgoli SR, Moattar F, Hassani H. Effect of sesame on sperm quality of infertile men. J Res Med Sci. 2013;18:184–7. [PMC free article] [PubMed] [Google Scholar]
- 25.Kolahdooz M, Nasri S, Modarres SZ, Kianbakht S, Huseini HF. Effects of Nigella sativa L. seed oil on abnormal semen quality in infertile men: a randomized, double-blind, placebo-controlled clinical trial. Phytomedicine. 2014;21:901–5. doi: 10.1016/j.phymed.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 26.Marbat M, Ali M, Hadi AM. The use of Nigella sativa as a single agent in treatment of male infertility. Tikrit J Pharma Sci. 2013;9:19–29. [Google Scholar]
- 27.Ambiye VR, Langade D, Dongre S, Aptikar P, Kulkarni M, Dongre A. Clinical evaluation of the spermatogenic activity of the root extract of Ashwagandha (Withania somnifera) in oligospermic males: a pilot study. Evid Based Complement Alternat Med. 2013;2013:571420. doi: 10.1155/2013/571420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ahmad MK, Mahdi AA, Shukla KK, Islam N, Rajender S, Madhukar D, et al. Withania somnifera improves semen quality by regulating reproductive hormone levels and oxidative stress in seminal plasma of infertile males. Fertil Steril. 2010;94:989–96. doi: 10.1016/j.fertnstert.2009.04.046. [DOI] [PubMed] [Google Scholar]
- 29.Mahdi AA, Shukla KK, Ahmad MK, Rajender S, Shankhwar SN, Singh V, et al. Withania somnifera improves semen quality in stress-related male fertility. Evid Based Complement Alternat Med. 2009;2011:576962. doi: 10.1093/ecam/nep138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mares WAAK, Najam WS. The effect of Ginger on semen parameters and serum FSH, LH & testosterone of infertile men. Tikrit Medical Journal. 2012;18:322–9. [Google Scholar]
- 31.Gupta A, Mahdi AA, Shukla KK, Ahmad MK, Bansal N, Sankhwar P, et al. Efficacy of Withania somnifera on seminal plasma metabolites of infertile males: a proton NMR study at 800MHz. J Ethnopharmacol. 2013;149:208–14. doi: 10.1016/j.jep.2013.06.024. [DOI] [PubMed] [Google Scholar]
- 32.Shukla KK, Mahdi AA, Ahmad MK, Shankhwar SN, Rajender S, Jaiswar SP. Mucuna pruriens improves male fertility by its action on the hypothalamus-pituitary-gonadal axis. Fertil Steril. 2009;92:1934–40. doi: 10.1016/j.fertnstert.2008.09.045. [DOI] [PubMed] [Google Scholar]
- 33.Mkrtchyan A, Panosyan V, Panossian A, Wikman G, Wagner H. A phase I clinical study of Andrographis paniculata fixed combination Kan Jang versus ginseng and valerian on the semen quality of healthy male subjects. Phytomedicine. 2005;12:403–9. doi: 10.1016/j.phymed.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 34.Kolangi F, Shafi H, Memariani Z, Kamalinejad M, Bioos S, Jorsaraei SGA, et al. Effect of Alpinia officinarum Hance rhizome extract on spermatogram factors in men with idiopathic infertility: A prospective double‐blinded randomised clinical trial. Andrologia. 2019;51:e13172. doi: 10.1111/and.13172. [DOI] [PubMed] [Google Scholar]
- 35.Chen DL, Li N, Lin L, Long HM, Lin H, Chen J, et al. Confocal mirco-Raman spectroscopic analysis of the antioxidant protection mechanism of the oligosaccharides extracted from Morinda officinalis on human sperm DNA. J Ethnopharmacol. 2014;153:119–24. doi: 10.1016/j.jep.2014.01.021. [DOI] [PubMed] [Google Scholar]
- 36.Bahmanpour S, Talaei T, Vojdani Z, Panjehshahin MR, Poostpasand A, Zareei S, et al. Effect of Phoenix dactylifera pollen on sperm parameters and reproductive system of adult male rats. Iran J Med Sci. 2006;31:208–212. [Google Scholar]
- 37.Cao L, Leers-Sucheta S, Azhar S. Aging alters the functional expression of enzymatic and non-enzymatic anti-oxidant defense systems in testicular rat Leydig cells. J Steroid Biochem Mol Biol. 2004;88:61–7. doi: 10.1016/j.jsbmb.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 38.Nazem H, Modaresi M, Messripour M, Marghmaleki MA, Ebadi AG. Effect of Saffron Extract on Pituitary-Testis Axis in Mice. Asian Journal of Chemistry. 2009;21:1616–18. [Google Scholar]
- 39.Walczak–Jedrzejowska R, Wolski JK, Slowikowska–Hilczer J. The role of oxidative stress and antioxidants in male fertility. Cent European J Urol. 2013;66:60–7. doi: 10.5173/ceju.2013.01.art19. [DOI] [PMC free article] [PubMed] [Google Scholar]