Abstract
Objective: Recently, sildenafil as a drug effective in relaxing smooth muscles can be used as an adjunct to delay the onset of uterine contractions and therefore the occurrence of preterm labor. The aim of this study was to evaluate the effect of nifedipine combination with sildenafil on preterm delivery compared with nifedipine alone.
Materials and methods: This randomized double-blinded clinical trial was performed on pregnant women with a gestational age of 26-34 weeks with singleton pregnancy and symptoms of preterm delivery. The mothers were randomly assigned into two groups receiving nifedipine plus sildenafil or those receiving nifedipine alone. The time of delivery, maternal and neonatal complications were compared between the two groups.
Results: Mothers who received the combination therapy experienced significantly lower preterm delivery within 72 hours of intervention compared to nifedipine alone (4.5% versus 27.3%, p = 0.002). The rate of delivery during the first 7 days after discharge was 7.6% and 31.8% in nifedipine plus sildenafil and nifedipine alone, respectively (P = 0.001). The prevalence of neonatal respiratory distress syndrome (RDS) as well as mean birth weight was higher in the nifedipine group alone. Treatment protocol with nifedipine and sildenafil compared with nifedipine alone was associated with a significant increase in preterm delivery delay (beta =-5.819, p = 0.001).
Conclusion: The use of sildenafil in addition to nifedipine causes more delay in delivery in cases of preterm labor, followed by lower risk for RDS, reduces neonatal intensive care unit (NICU) admission, and preserves neonatal birth weight.
Key Words: Sildenafil Citrate, Nifedipine, Premature Obstetric Labor
Introduction
One of the most important issues in obstetrics and gynecology is preterm labor because caring and treating the complications of premature infants might cause irreparable psychological damage to families. In fact, the birth of a healthy and uncomplicated newborn is the main goal of pregnancy. Preterm labor cannot be stopped, but it can be delayed for a few days. This delay can have a profound effect on the consequences of preterm delivery, including mortality and disability of premature infants.
In recent years, special attention has been paid to nifedipine, a calcium channel blocker, as a first-line tocolytic drug (1). Nifedipine is a potent vasodilator that is 90% orally absorbed from the gastrointestinal tract and excreted in the feces after being metabolized in the liver. The mechanism of action of nifedipine is inhibition of calcium flow through slow calcium channels into smooth muscle cells. Oral nifedipine can be used as a safe and effective drug as a tocolytic in patients at risk of preterm delivery with shortened cervical length (2). Various studies have shown that nifedipine, like other tocolytic drugs, can cause uterine muscle relaxation (3). Some researchers have suggested nifedipine as the first choice in the treatment of preterm labor (4).
Sildenafil has been used for many years for erectile dysfunction in men. This drug shows strong vasodilator effects by acting on nitric oxide and thus inhibiting phosphodiesterase (5). Therefore, studies focused on this drug are used to treat many disorders, especially those related to smooth muscle contractions (6, 7).
Childbirth is no exception to this rule, and this drug has been tested in the treatment of uterine-umbilical artery disorders and uterine muscle contractions and has led to an improved vascular exchange on the one hand and modulation of uterine contractions on the other (8-10). The growing use of sildenafil in the treatment of vascular or contractile disorders during pregnancy has now been raised (11-14). Today, the benefits of using this drug in the treatment of preeclampsia as well as fetal growth restriction disorder have been confirmed (15-18).
Also, some strong evidence are available in its beneficial effects on modulation of uterine muscle relaxation, and thus it seems to be beneficial in delaying preterm delivery (19-21). However, its effect alone has not been confirmed and seems to be effective in combination with first-line drugs of choice for delaying labor. The aim of this study was to evaluate the effect of nifedipine combination with sildenafil on preterm delivery compared with nifedipine alone.
Materials and methods
Study setting and patients' allocation : This randomized double-blinded clinical trial was performed on pregnant women with a gestational age of 26-34 weeks with singleton pregnancy and diagnosis of preterm delivery referred to our medical centre in Tehran in 2020. Diagnosis of preterm labor was based upon clinical criteria of regular uterine contractions (≥4 every 20 minutes or ≥8 in 1 hour) accompanied by cervical dilation. The exclusion criteria were cervical dilatation greater than 4 cm, premature membrane rupture, suspected vaginal bleeding, fetal death or distress, intra uterine growth retardation (IUGR), life-threatening fetal abnormalities on ultrasound, history of trauma, severe preeclampsia, suspected chorioamnionitis, maternal heart rate < 100/min or blood pressure less than 90/50 mm-Hg.
The patients enrolled in the study after explaining the study and reassuring the mothers about the confidentiality of their information. Demographic data were collected by filling questionnaires for each patient, obtaining relevant data including demographic information (age, gestational age, body mass index (BMI), parity, history of preterm labor). The randomization, treatment and follow‐up of participants are shown in Figure 1.
Figure 1.
The randomisation and follow‐up of participants
*Excluded due to cervical dilatation greater than 4 cm, premature membrane rupture, suspected vaginal bleeding, fetal death or distress, intra uterine growth retardation (IUGR), history of trauma, severe preeclampsia, suspected chorioamnionitis, maternal heart rate < 100/min, patient's unwillingness to participate in the study, **Didn’t receive Immediate fetal distress, ***Adverse drug reaction: hypotension
Study clinical protocol: The mothers were randomly (using the block randomization method) assigned into two groups including the mothers receiving nifedipine (10 mg every 6 to 8 hours, orally) plus sildenafil (25mg every 8 hours, vaginally) or those who receiving nifedipine alone. This medication lasted 48 to 72 hours. During treatment, mothers (with regard to heart rate, blood pressure, uterine contractions) and fetuses (with regard to heart rate) were monitored every 15 to 30 minutes for the first 4 hours. The time of delivery along with maternal and neonatal complications were compared between the two groups.
Statistical analysis : For statistical analysis, results were presented as mean ± standard deviation (SD) for quantitative variables and were summarized by frequency (percentage) for categorical variables. Continuous variables were compared using t-test or Mann-Whitney test whenever the data did not appear to have normal distribution or when the assumption of equal variances was violated across the study groups. Categorical variables were, on the other hand, compared using either chi-square test or fisher’s exact test. P values of ≤ 0.05 were considered statistically significant. For the statistical analysis, the statistical software SPSS version 23.0 for windows (IBM, Armonk, New York) was used.
Ethical considerations: In this study, the information of the subjects remained confidential; no change was made in the diagnostic and treatment process of the patients, and no cost was imposed on the patients.
The study protocol was verified by the Ethics Committee of Iran University of Medical Sciences (IR.IUMS.FMD.REC 1398.389). All case subjects signed the informed consent forms.
Results
In the present study, 66 patients in the nifedipine plus sildenafil group and 66 patients in the nifedipine alone group were analyzed. As shown in Table 1, the two groups were matched in baseline characteristics including mean age, mean BMI, gestational age, mean of cervical length, number of parity, and history of preterm labor.
Table 1.
Comparison of demographic characteristics in the both groups
| Characteristics |
Nifedipine and
sildenafil (n=66) |
Nifedipine
alone (n=66) |
Comparison
test |
P value |
Power of
analysis |
|---|---|---|---|---|---|
| age, year (mean± sd) | 31.95±5.07 | 32.39±4.72 | T-test | 0.607 | 70.8% |
| body mass index, kg/m2 (mean± sd) | 29.95±3.46 | 30.23±3.54 | T-test | 0.656 | 35.8% |
| gestational age, week (mean± sd) | 29.64±2.91 | 29.76±2.65 | T-test | 0.423 | 10.5% |
| cervical length, cm (mean± sd) | 28.62±3.90 | 29.50±3.51 | T-test | 0.176 | 99.9% |
| parity | Chi square test | 0.784 | 99.9% | ||
| Nulliparous (n (%)) | 7 (10.6) | 8 (12.1) | |||
| Multiparous (n (%)) | 59 (89.4) | 58 (87.9) | |||
| History of preterm labor (n (%)) | 14 (21.2) | 6 (9.1) | Chi square test | 0.052 | 96.0% |
Data are expressed as number (%) and mean±sd.
In both groups, the rate of delivery within 24 hours after admission was 4.5% and 0.3%, respectively, which the difference was not statistically significant. (fisher’s exact P-value = 0.446, power :99.9%). The rate of delivery within 48 hours of admission was 6.1% and 7.6%, respectively, with no significant difference (fisher’s exact P-value = 0.223, power 99.9%). But those who received the combination therapy experienced significantly lower delivery within 72 hours of intervention (4.5% versus 27.3%, chi square p-value = 0.002, power 18.4%).
Also, the frequency of cases of non-delivery during hospitalization was lower in the combined treatment group as compared to another group (62.1% versus 84.8%, chi square p-value = 0.001 power 80.8%). The frequency of delivery during the first 7 days after discharge was 7.6% and 31.8%, respectively, which was lower in the group treated with the combined protocol (chi square p-value = 0.001 power:99.9%).
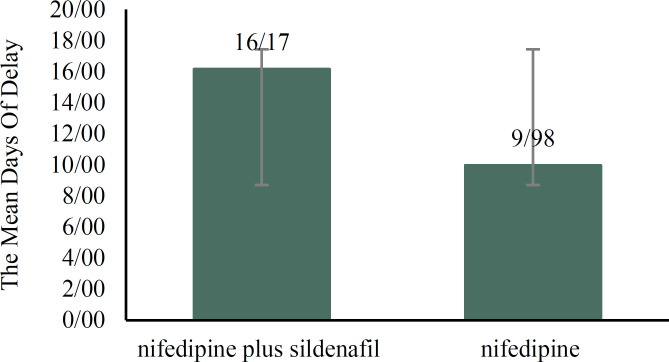
The mean days of delay in delivery in nifedipine plus sildenafil group and nifedipine alone group were 16.17 ± 5.14 days and 9.98 ± 3.50 days, respectively, which was significantly longer in the combination therapy (t-test P-value = 0.001 power:99.9%) (Figure 2). Comparison of neonatal complications between the two study groups (Table 2) showed higher prevalence rate of neonatal respiratory distress syndrome (RDS) as well as lower mean birth weight in the group that received nifedipine alone.
Figure 2.
Comparison of mean days of delay in delivery between the two groups
Table 2.
Comparison of pregnancy outcomes in the both groups
| Characteristics |
Nifedipine and
sildenafil (n=66) |
Nifedipine
alone (n=66) |
Comparison test | P value |
Power of
analysis |
|---|---|---|---|---|---|
| Preterm rupture of membrane (n (%)) | 7 (10.6%) | 6 (9.1%) | Chi square test | 0.770 | 99.9% |
| Neurodevelopmental defect (n (%)) | 6 (9.1%) | 8 (12.1%) | Chi square test | 0.572 | 99.9% |
| Neonatal infection (n (%).) | 5 (7.6%) | 4 (6.1%) | Fisher’s exact test | 0.999 | 99.9% |
| Neonatal death (n (%)) | 5 (7.6%) | 3 (4.5%) | Fisher’s exact test | 0.718 | 99.9% |
| Respiratory distress syndrome (n(%)) | 5 (7.6) | 17 (25.8) | Chi square test | 0.005 | 58.8% |
| Needing nicu admission (n (%)) | 24 (36.4) | 44 (66.7) | Chi square test | 0.001 | 99.9% |
| Mean birth weight, kg(mean± 2sd) | 2154.5±221.3 | 1609.0±204.3 | t-test | 0.001 | 99.9% |
Data are expressed as number (%) and mean±sd
Based on multivariate linear regression model (Table 3) and among all underlying and confounding factors, first, the treatment protocol with nifedipine and sildenafil compared to the use of nifedipine alone was associated with a significant increase in delayed labor (beta =-5.819, p = 0.001). In addition to the type of treatment protocol, cervical length was another factor influencing the rate of delay in delivery (beta =-0.295, p = 0.005).
Table 3.
Factors associated with delayed delivery
| Characteristics | Beta |
Standard
error |
P value |
|---|---|---|---|
| Combination therapy | -5.819 | 0.760 | 0.001 |
| Age | -0.010 | 0.077 | 0.898 |
| Parity | -1.017 | 1.189 | 0.394 |
| Body mass index | -0.143 | 0.108 | 0.191 |
| History of preterm labor | -0.761 | 1.056 | 0.472 |
| Gestational age | 0.141 | 0.136 | 0.302 |
| Cervical length | -0.295 | 0.103 | 0.005 |
Discussion
Delaying preterm birth prevents neonatal complications and adverse maternal outcomes by providing an opportunity for fetal development. For this purpose, the use of various tocolytic drugs has always been considered. In this regard, the use of nifedipine can be mentioned. Recently, sildenafil as an effective drug in relaxing smooth muscles can be used as an adjunct to delay the onset of uterine contractions and therefore the occurrence of preterm labor.
In the present study, the effectiveness of the combined protocol of nifedipine and sildenafil in comparison with nifedipine alone in the treatment of preterm labor and delaying delivery was evaluated. We found that the use of this combination therapy was associated with reduced uterine contractions and therefore delayed delivery in cases with preterm labor. In our study, these effects were quite evident 72 hours after the intervention. Certainly, such a clinical delay in the onset of preterm labor has allowed the fetus to develop further, especially respiratory system development, which will ultimately reduce the risk of neonatal RDS and therefore less need for NICU admission. Also, this delay in delivery will provide the conditions for the fetus to gain weight and thus reduce neonatal complications.
To date, no comprehensive study has been performed on the efficacy of sildenafil in preterm birth prevention and improving the outcomes of preterm delivery. In the only clinical trial study conducted by Maher et al in 2019, combination therapy with nifedipine and sildenafil was compared with nifedipine alone in delaying preterm delivery. Similar to ours, the cases of non-preterm delivery in combination therapy were more than single drug therapy (81.8% vs. 68.6%). Also, the need for NICU hospitalization in the first group was significantly lower than in the second group. In addition, fetal weight gain was higher in the combination therapy group (22).
A study by Chiossi et al. was hypothesized whether sildenafil could potentiate the effects of nifedipine. Myometrial biopsies were collected from 22 pregnant women undergoing elective cesarean delivery. Tissue strips were incubated with sildenafil. The study showed that, first, nifedipine inhibited spontaneous and oxytocin-induced myometrial contractility. Second, Preincubation with sildenafil enhanced the inhibitory effect of nifedipine (23).
Conclusion
In conclusion, the use of sildenafil as a supplement to nifedipine causes more delay in delivery in cases of preterm labor than the exclusive use of nifedipine, which induces the possibility of further development of the respiratory system, prevention of RDS, reduced NICU admission, and increased neonatal birth weight.
Acknowledgments
The authors would like to thank the Shahid Akbarabadi Clinical Research Development Unit (ShACRDU), Iran University of Medical Sciences (IUMS), Tehran, Iran and to all patients who participated in this research.
Conflict of Interests
Authors have no conflict of interests.
Notes:
Citation: Mohammadi E, Noei Teymoordash S, Norouzi AR, Norouzi F, Norouzi HR. Comparison of the Effect of Nifedipine Alone and the Combination of Nifedipine and Sildenafil in Delaying Preterm Labor: A Randomized Clinical Trial. J Fam Reprod Health 2021; 15(2): 112-7.
References
- 1.Tsatsaris V, Papatsonis D, Goffinet F, Dekker G, Carbonne B. Tocolysis with nifedipine or beta-adrenergic agonists: a meta-analysis. Obstet Gynecol. 2001;97:840–7. doi: 10.1016/s0029-7844(00)01212-6. [DOI] [PubMed] [Google Scholar]
- 2.King JF, Flenady V, Papatsonis D, Dekker G, Carbonne B. Calcium channel blockers for inhibiting preterm labour; a systematic review of the evidence and a protocol for administration of nifedipine. Aust N Z J Obstet Gynaecol. 2003;43:192–8. doi: 10.1046/j.0004-8666.2003.00074.x. [DOI] [PubMed] [Google Scholar]
- 3.Simhan HN, Caritis SN. Prevention of preterm delivery. N Engl J Med. 2007;357:477–87. doi: 10.1056/NEJMra050435. [DOI] [PubMed] [Google Scholar]
- 4.King JF, Flenady VJ, Papatsonis DN, Dekker GA, Carbonne B. Calcium channel blockers for inhibiting preterm labour. Cochrane Database Syst Rev. 2003;(1):CD002255. doi: 10.1002/14651858.CD002255. [DOI] [PubMed] [Google Scholar]
- 5.Terrett NK, Bell AS, Brown D, Ellis P. Sildenafil (Viagra™), a potent and selective inhibitor of type 5 cGMP phosphodiesterase with utility for the treatment of male erectile dysfunction. Bioorganic & Medicinal Chemistry Letters. 1996;6:1819–24. [Google Scholar]
- 6.Tan K, Krishnamurthy MB, O’Heney JL, Paul E, Sehgal A. Sildenafil therapy in bronchopulmonary dysplasia-associated pulmonary hypertension: a retrospective study of efficacy and safety. Eur J Pediatr. 2015;174:1109–15. doi: 10.1007/s00431-015-2515-7. [DOI] [PubMed] [Google Scholar]
- 7.Rodway GW, Lovelace AJ, Lanspa MJ, McIntosh SE, Bell J, Briggs B, et al. Sildenafil and Exercise Capacity in the Elderly at Moderate Altitude. Wilderness Environ Med. 2016;27:307–15. doi: 10.1016/j.wem.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 8.Maharaj CH, O'Toole D, Lynch T, Carney J, Jarman J, Higgins BD, et al. Effects and mechanisms of action of sildenafil citrate in human chorionic arteries. Reprod Biol Endocrinol. 2009;7:34. doi: 10.1186/1477-7827-7-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gillis EE, Mooney JN, Garrett MR, Granger JP, Sasser JM. Sildenafil Treatment Ameliorates the Maternal Syndrome of Preeclampsia and Rescues Fetal Growth in the Dahl Salt-Sensitive Rat. Hypertension. 2016;67:647–53. doi: 10.1161/HYPERTENSIONAHA.115.06071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yallampalli C, Garfield RE. Inhibition of nitric oxide synthesis in rats during pregnancy produces signs similar to those of preeclampsia. Am J Obstet Gynecol. 1993;169:1316–20. doi: 10.1016/0002-9378(93)90299-x. [DOI] [PubMed] [Google Scholar]
- 11.Lacassie HJ, Germain AM, Valdés G, Fernández MS, Allamand F, López H. Management of Eisenmenger syndrome in pregnancy with sildenafil and L-arginine. Obstet Gynecol. 2004;103:1118–20. doi: 10.1097/01.AOG.0000125148.82698.65. [DOI] [PubMed] [Google Scholar]
- 12.Panda S, Das A, Md Nowroz H. Sildenafil citrate in fetal growth restriction. J Reprod Infertil. 2014;15:168–9. [PMC free article] [PubMed] [Google Scholar]
- 13.Reynolds LP, Caton JS, Redmer DA, Grazul-Bilska AT, Vonnahme KA, Borowicz PP, et al. Evidence for altered placental blood flow and vascularity in compromised pregnancies. J Physiol. 2006;572:51–8. doi: 10.1113/jphysiol.2005.104430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun X, Wang K, Wang W, Li B. [Clinical study on sildenafil in treatment of pregnant women with pulmonary arterial hypertension] Zhonghua Fu Chan Ke Za Zhi. 2014;49:414–8. [PubMed] [Google Scholar]
- 15.Block-Abraham DM, Turan OM, Doyle LE, Kopelman JN, Atlas RO, Jenkins CB, et al. First-trimester risk factors for preeclampsia development in women initiating aspirin by 16 weeks of gestation. Obstet Gynecol. 2014;123:611–7. doi: 10.1097/AOG.0000000000000118. [DOI] [PubMed] [Google Scholar]
- 16.Burke SD, Zsengellér ZK, Khankin EV, Lo AS, Rajakumar A, DuPont JJ, et al. Soluble fms-like tyrosine kinase 1 promotes angiotensin II sensitivity in preeclampsia. J Clin Invest. 2016;126:2561–74. doi: 10.1172/JCI83918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gathiram P, Moodley J. Pre-eclampsia: its pathogenesis and pathophysiolgy. Cardiovasc J Afr. 2016;27:71–8. doi: 10.5830/CVJA-2016-009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rolnik DL, Wright D, Poon LCY, Syngelaki A, O'Gorman N, de Paco Matallana C, et al. ASPRE trial: performance of screening for preterm pre-eclampsia. Ultrasound Obstet Gynecol. 2017;50:492–5. doi: 10.1002/uog.18816. [DOI] [PubMed] [Google Scholar]
- 19.El-Sayed MA, Saleh SA, Maher MA, Khidre AM. Utero-placental perfusion Doppler indices in growth restricted fetuses: effect of sildenafil citrate. J Matern Fetal Neonatal Med. 2018;31:1045–50. doi: 10.1080/14767058.2017.1306509. [DOI] [PubMed] [Google Scholar]
- 20.Sharp A, Cornforth C, Jackson R, Harrold J, Turner MA, Kenny LC, et al. Maternal sildenafil for severe fetal growth restriction (STRIDER): a multicentre, randomised, placebo-controlled, double-blind trial. Lancet Child Adolesc Health. 2018;2:93–102. doi: 10.1016/S2352-4642(17)30173-6. [DOI] [PubMed] [Google Scholar]
- 21.Maher MA, Sayyed TM, Elkhouly N. Sildenafil Citrate Therapy for Oligohydramnios: A Randomized Controlled Trial. Obstet Gynecol. 2017;129:615–20. doi: 10.1097/AOG.0000000000001928. [DOI] [PubMed] [Google Scholar]
- 22.Maher MA, Sayyed TM, El-Khadry SW. Nifedipine alone or combined with sildenafil citrate for management of threatened preterm labour: a randomised trial. BJOG. 2019;126:729–35. doi: 10.1111/1471-0528.15503. [DOI] [PubMed] [Google Scholar]
- 23.Chiossi G, Costantine MM, Betancourt A, Hankins GD, Longo M, Saade GR, et al. Does sildenafil citrate affect myometrial contractile response to nifedipine in vitro? Am J Obstet Gynecol. 2010;203:252. doi: 10.1016/j.ajog.2010.05.007. e1-5. [DOI] [PubMed] [Google Scholar]