Abstract
Background
Trials of the Pfizer-BioNTech BNT162b2 mRNA vaccine showed 95% efficacy in preventing symptomatic disease; however, the trials excluded immunocompromised patients (ICPs). We aim at analyzing antibody response in ICPs.
Methods
A prospective cohort study was conducted at Sheba Medical Center, Israel, between January and April 2020, in 1274 participants who received the vaccine, including 1002 ICPs and 272 immunocompetent healthcare workers (HCWs). Antibodies were measured two-four weeks after vaccination by SARS-CoV-2 anti–receptor binding domain IgG antibodies (RBD IgG) and pseudo-virus neutralization assays. Multivariable logistic regression analyses were used to identify factors associated with vaccine-induced antibody response. Adverse events (AEs) were monitored.
Findings
RBD-IgG antibodies were detected in 154/156 (98.7%) of patients with HIV, 75/90 (83.3%) with solid malignancies, 149/187 (79.7%) with myeloma, 83/111 (74.8%) following hematopoietic stem cell transplants, 25/36 (69.4%) following liver transplantation, 26/43 (60.5%) with myelodysplastic syndrome, 96/188 (51.0%) with chronic lymphocytic leukemia/non-Hodgkin's lymphoma, 50/110 (45.5%) following kidney transplantation, 15/80 (18.8%) following heart transplantation, and 269/272 (98.9%) in controls. There was a significant correlation r = 0.74 (95%CI 0.69,0.78) between RBD-binding IgG and neutralizing antibodies in all groups. Multivariate logistic regression analysis showed that age > 65 years (OR 0.41,95%CI 0.30,0.57) and underlying immunosuppression (OR 0.02,95%CI 0.01,0.07) were significantly associated with a non-reactive response of IgG antibodies. HIV patients showed a similar immunological response as healthy adults. The vaccine was safe without any episodes of rejection, graft-versus-host disease (GVHD) or allergy. Immunocompetent HCWs experienced significantly more AEs than ICPs.
Interpretation
Antibody response to the Pfizer-BioNTech vaccine was highly variable among different ICPs; thus, individual recommendations should be provided for the different immunosuppression states.
Research in context.
Evidence before this study
The Pfizer-BioNTech BNT162b2 mRNA vaccine clinical trials excluded immunocompromised patients (ICPs), as their immune response to vaccination is usually blunted. The American Society of Transplantation and multiple oncology organizations have recommended vaccinating transplant recipients as well as patients with cancer against SARS-CoV-2 despite lack of data regarding efficacy in these populations. Several studies demonstrated low rates of antibody response to the BNT162b2 vaccine among solid organ transplant recipients.
Added value of this study
Younger patients – particularly those with HIV infection, those with solid malignancies being treated with immunochemotherapy, those with multiple myeloma, HSCT recipients six months post-transplant without GVHD, liver transplant patients and probably other transplant patients not receiving antimetabolite maintenance immunosuppression – are more likely to develop antibody responses, and vaccination should be encouraged for those patients. In contrast, older patients, particularly those after heart and kidney transplants, are less likely to develop antibody response and should be warned to follow strict infection control measures, particularly vaccination of all other household members.
Implications of all the available evidence
Antibody response to the BNT162b2 mRNA vaccine is highly variable among different immunosuppressed patients, and thus individual recommendations should be provided for the different immunosuppressed patients.
Alt-text: Unlabelled box
1. Introduction
In December 2019, COVID-19 emerged in Wuhan, China and has subsequently infected over 194 million people and is responsible for over 4.1 million deaths globally (as of 24 July 2021) [1]. Older adults, persons with certain coexisting conditions, and front-line workers are at the highest risk for COVID-19 and its complications [2].
The incidence of COVID-19 among patients receiving active treatment for cancer is variable, with most available evidence suggesting that incidence rates lie between 1 and 4% [3]. Immunocompromised patients (ICPs), mainly those with hematologic malignancies and lung cancer are at a higher risk, vs. the non-compromised population, for severe COVID-19 outcomes, including intensive care unit admission, invasive ventilation, and death [4]. Overall, COVID-19 has had devastating effects on patients with cancer, with large numbers of missed diagnoses and delayed treatments due to health systems under pressure and patient reluctance to seek medical care [5].
The Pfizer-BioNTech BNT162b2 mRNA vaccine clinical trials showed that the vaccine has 95% efficacy in preventing symptomatic laboratory-confirmed COVID-19. However, the trials excluded ICPs, as their immune response to vaccination is usually blunted [6]. Nonetheless, the Israel Ministry of Health approved vaccination of patients on immunosuppressive therapy or biological response modifiers associated with any malignancy, patients after solid organ or stem cell transplantation or splenectomy, and patients with HIV or primary immune deficiency [7].
Although randomized clinical trials with a placebo arm are considered the gold standard for evaluating the efficacy of a vaccine, it seemed unethical to perform such a study in subpopulations of high-risk patients, e.g., in ICPs, especially following the excellent results of the mRNA vaccine trials. In addition, an evaluation of vaccine efficacy through comparison between the rates of infections in different periods is not informative due to the relatively small numbers and the different incidence rates in different periods. Given the strong correlation between vaccine efficacy and the production of protective antibodies that has emerged from human vaccine studies [8], it seemed appropriate to rely on this parameter to define the efficacy of the vaccine in different subpopulations at risk. Finally, several studies have shown a significant immune response, including receptor binding domain immunoglobulin G (RBD-IgG) and SARS-CoV-2 neutralizing titers in sera following the first vaccine dose and more so after the second dose [8], [9], [10], [11], [12], [13], [14].
Here, we present the first large-scale study evaluating the antibody response in 1002 ICPs with diverse underlying diseases and assessing correlates of antibody-mediated immunity following vaccination with the Pfizer-BioNTech vaccine.
2. Methods
Cohort: As soon as BNT162b2 mRNA vaccination was authorized in Israel, we recommended that the adult ICPs (> 18 years) in our care should be vaccinated. In the first three months thereafter, we offered all ICPs who were scheduled for routine clinic or daycare visits the opportunity to participate in a prospective study at Sheba Medical Center, the largest tertiary medical center in Israel (1600 beds). ICPs who consented to be vaccinated and to participate in the study, and for whom there was serology test result 2–4 weeks after the second dose of the vaccine, were included in the study.
The study commenced with 1265 ICPs treated at the Sheba Medical Center, but 240 patients were lost to the study because there were no blood samples available 2 to 4 weeks after the second vaccine, and 23 patients refused to participate. The final study population thus consisted of 1002 ICPs treated at nine different daycare and outpatient clinics. The patients had various active malignancies, solid organ transplants (SOTs), allogeneic hematopoietic stem cell transplants (HSCTs), and human immunodeficiency virus (HIV), treated as detailed in Table S1: chronic lymphocytic leukemia and non-Hodgkin's lymphoma (CLL/NHL) were treated mainly with Bruton's tyrosine kinase inhibitors or a BCL2 inhibitor; multiple myeloma was treated with several lines of therapies including immunomodulatory drugs, proteasome inhibitors, anti CD-38 and autologous bone marrow transplantation; solid malignancies were treated with various combination chemo/biologic/hormonal/immunotherapy protocols; and myelodysplastic syndrome (MDS) was treated mostly with azacitidine. HSCT patients were included six months following transplantation; these patients were treated mainly with low-dose cyclosporine A and prednisone. Following SOT, patients were treated with different combinations of immunosuppressive medications, including calcineurin inhibitors, anti-metabolites, prednisone and mTOR inhibitors. HIV patients were treated with combination antiretroviral therapy (ART), mainly an integrase inhibitor-based treatment. One drug common to most regimens was some form of glucocorticosteroid. Patients receiving anti CD20 monoclonal antibodies during the six months prior vaccination and patients with acute graft vs. host disease (GVHD) were excluded, as were patients hospitalized for induction therapy for acute leukemia, lymphoma or transplantation. Patients with a positive SARS-CoV-2 PCR test before or after the first vaccination and during the first week after the second vaccination were also excluded.
Our cohort did not include immunocompromised patients associated with rheumatologic, autoimmune, inflammatory bowel diseases or other chronic diseases requiring immunosuppressive therapy.
Controls were 272 immunocompetent healthcare workers, who were tested for the antibody response two-four weeks following the second vaccine, and who were not infected with SARS-CoV-2.
The vaccine was administered to all patients and controls at the standard recommended two doses 21 days apart.
Ethical approval and patient consent: IRB approval was obtained from the ethical review boards of the Sheba Medical Center (7982–20-SMC for ICP and 8008–20-SMC for immunocompetent HCW). Written informed consent was obtained from all participants.
Serology assays: Samples from participants were tested with an enzyme-linked immunosorbent assay that detects IgG antibodies against the RBD-SARS-CoV-2 [15]. Titers ≥ 1.1 were defined positive. A SARS-CoV-2 pseudo-virus neutralization assay was performed using a propagation-competent VSV-spike similar to that previously published, which was kindly provided by Gert Zimmer, University of Bern, Switzerland (Supplementary Appendix) [16]. Sera not capable of reducing viral replication by 50% at a 1 in 8 dilution or below were considered non-neutralizing. All samples that were positive for RBD-IgG were tested for neutralizing antibodies (NA). Samples with negative RBD-IgG tests were not tested for NAs, since we have found that negative IgG-RBD tests yielded negative NA tests.
Safety: All adverse events (AE), including local and systemic reactions, were monitored and recorded. Both solicited and unsolicited events were recorded up to four weeks after the second injection. Each patient was specifically requested to report local reactions (pain, tenderness, erythema, induration, and lymphadenopathy) and systemic reactions (fatigue, headache, fever, myalgia, paresthesia and any other reactions).
Statistical analysis: Continuous variables were assessed for normality by Kolmogorov–Smirnov test and are presented as means ± SD or medians with interquartile range (IQR), where appropriate. Titers are presented as geometric mean (GMT) and 95% confidence intervals (CI). Categorical variables are presented as frequencies and percentages. Only samples with NA titers above the cutoff (> 8) were included in the analysis for GMT-NA. For group comparisons, nonparametric statistical tests (Kruskall–Wallis test) were used, for continuous variables and χ2 test for categorical variables, with adjustment for multiple comparisons according to Tukey. Multivariable logistic and linear regression analyses were used to identify factors associated with vaccine-induced antibody response in the entire cohort (ICP and immunocompetent controls). The whole group was adjusted in the statistical models for timing of serology after the second vaccine dose, age and sex. Results are presented as odds ratio (OR), CIs, and P-values. All P-values reflect the results of two-sided tests. All data analyses were performed with SAS 9.4 software (Cary, NC, USA).
A scatter plot of log-transformed IgG and NA was obtained using GraphPad Prism 5.0 (GraphPad Software, Inc., San Diego, CA). The correlation between IgG and log-transformed NA was analyzed using Spearman's correlation by two-tailed parametric t-test means with 95% CIs [17]. STROBE checklist was followed [18].
Role of funding source: The study did not receive funding. The data was available to all authors and GR decided to submit for publication.
3. Results
The study cohort consisted of 1274 individuals, comprising nine ICP cohorts (1002 patients) compared to an immunocompetent cohort (272 individuals). The cohort included (Table 1): 188 patients with CLL (140)/NHL(48), of which 67% were not on active immunosuppressive therapy at the times of vaccination; 187 with multiple myeloma, of which 39% were not on immunosuppressive treatment during vaccination, however all were treated in the course of their disease by different lines of therapies; 43 with MDS; 90 with solid malignancies (36 gastrointestinal, 19 breast, 14 malignant melanoma,10 lung cancer, 11 with various malignant solid tumors, of these 66 had metastatic disease and 24 local disease), all on immunosuppressive treatment; 111 following HSCT (median time from transplant was 3.4 [IQR 2.0–6.3] years, 40% had chronic GVHD, 45% were no longer on immunosuppressive treatment and 15% were treated for their underlying disease, in order to prevent relapse: azacitidine for MDS/AML, TKI for Philadelphia positive (pH+) leukemia, FLT3 inhibitors for FLT3 positive AML); 227 following SOT (111 kidney transplantation, 80 heart transplantation, 36 liver transplantation, with a median time from transplant of 3.1 [IQR 1.0–9.2], 7.4 [IQR 3.3–15.1], 7.0 [IQR 4.0–16.0] years, respectively), with all patients following SOT being on immunosuppressive therapy; 156 patients with HIV, and of these 95% with an undetectable viral load, with a mean CD4+ T cell count of 700 cells per microliter. The different treatments modalities are presented in Table S2.
Table 1.
Underlying diseases and demographic characteristics of the study cohort.
| Underlying disease | Study population N (%) | Age at vaccination Years, median (IQR) | M/F (% M) | Days from 2nd Vx to serology median (IQR) |
|---|---|---|---|---|
| All patients | 1002 | 63.0 (51.0–72.0) | 654/348 (65.3) | 19.0 (14.0–25.0) |
| CLL/NHL | 188 (18.8) | 69.0 (61.0–74.0) | 102/86 (54.3) | 18.0 (15.0–27.0) |
| Multiple myeloma | 187 (18.7) | 66.0 (59.0–73.0) | 117/70 (62.6) | 18.0 (15.0–23.0) |
| HIV | 156 (15.6) | 49.0 (42.0–57.0) | 137/19 (87.8) | 19.0 (14.0–21.0) |
| HSCT | 111 (11.1) | 62.0 (49.0–70.0) | 70/41 (63.1) | 21.0 (17.0–28.0) |
| Kidney transplant | 111 (11.1) | 60.0 (49.0–70.0) | 88/23 (79.3) | 22.0 (15.0–33.0) |
| Solid malignancies | 90 (9) | 64.0 (53.0–73.0) | 44/45 (49.4) | 15.0 (12.0–20.0) |
| Heart transplant | 80 (8) | 61.5 (50.0–68.0) | 55/25 (68.8) | 15.0 (14.0–26.5) |
| Myelodysplastic disorders | 43 (4.3) | 73.0 (66.0–80.0) | 22/21 (51.2) | 26.0 (17.0–35.0) |
| Liver transplant | 36 (3.6) | 68.0 (51.0–71.0) | 19/17 (52.8) | 15.0 (13.0–26.5) |
| Control after 2nd dose | 272 | 57.0 (44.0–67.0) | 66/206 (24.3) | 26.0 (24.0–27.0) |
SD – standard deviation, M/F – males/females, Vx – vaccination, CLL - chronic lymphocytic leukemia, NHL - non-Hodgkin's lymphoma, HIV-human immunodeficiency virus, HSCT - hematopoietic stem cell transplantation.
The median age of the patients was 63.0 years (IQR 51.0–72.0), and the age was statistically different between the controls and patients with multiple myeloma, CLL and MDS (p < 0.0001), patients with solid malignancies (p = 0.0002) and patients with HIV (p = 0.0001). The median age was not statistically different between the controls and patients who had undergone heart, kidney, liver or bone marrow transplantation (Table S3). Of the patients, 65.3% were males (49.4% with solid malignancies and 87.8% with HIV). The gender distribution was statistically different between the control group and all the different groups (Table S4). All the participants in the study were of Caucasian origin, The median number of days from the second vaccine to serology was 19.0 days (IQR 14.0–25.0). Data on the control group is summarized in Table 1.
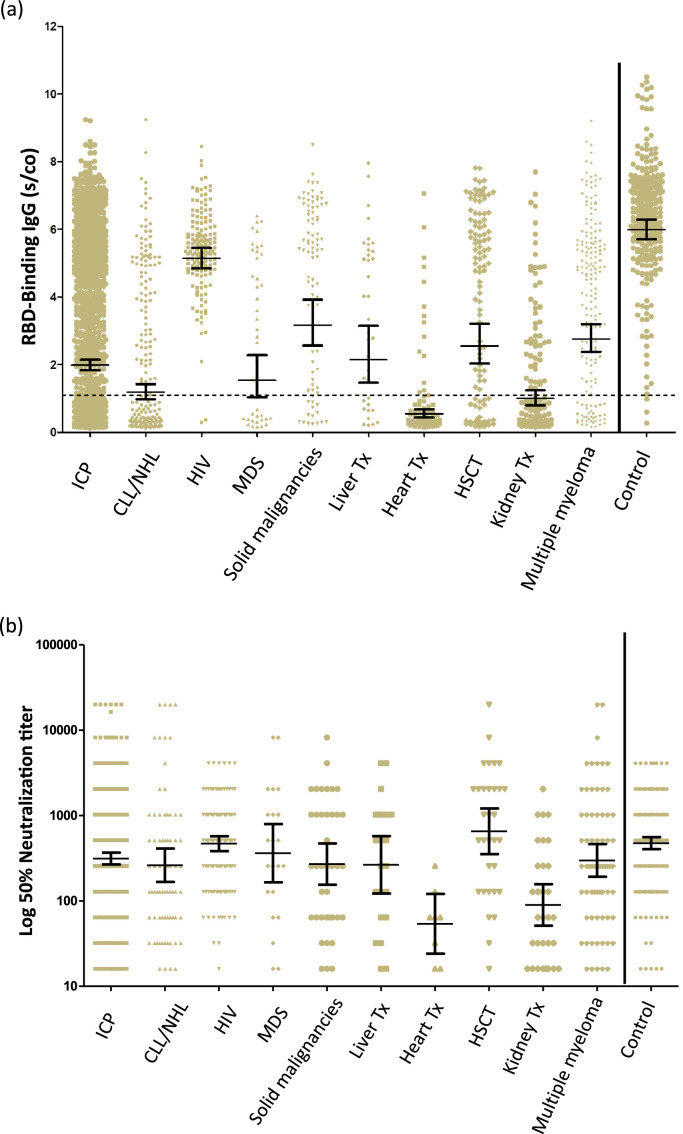
The antibody response two to four weeks after the second vaccine dose is summarized in Fig. 1a and Table 2. The highest response rate was observed in the HIV patients:154/156 (98.7%) developed antibodies, with a GMT of 5.14 (95% CI 4.84,5.46). Patients with solid malignancies also showed a remarkable response: 75/90 (83.3%) developed antibodies, with a GMT of 3.17 (95% CI 2.56,3.92). The remainder of the patients also developed antibodies, albeit with lower titers in the following order: multiple myeloma 79.7%, HSCT 74.8%, liver transplantation 69.4%, MDS 60.5%, CLL/NHL 51.0%, kidney transplantation 45.0%, and heart transplantation only 18.8%. For the control group, 269/272 (98.9%) developed antibodies with a GMT of 5.98 (95% CI 5.70, 6.28). Statistical comparison in RBD-IgG GMT between groups is presented in Table S5.
Fig. 1.
Quantitation of IgG following the second dose of the BNT162b2 vaccine in immunocompromised patients and immunocompetent health care workers.
(A) RBD-IgG levels. (B) Neutralizing antibodies above the cutoff. The dotted black line indicates the limit level of positive antibodies. The short black line indicates GMT and 95% CI.
RBD - receptor binding domain, S/CO - sample/cutoff ratio, ICP - immunocompromised patients, CLL - chronic lymphocytic leukemia, NHL - non-Hodgkin's lymphoma, HIV - human immunodeficiency virus, MDS - myelodysplastic syndrome, HSCT - hematopoietic stem cell transplantation, Tx – transplantation.
Table 2.
RBD-IgG and neutralizing antibodies (NA) following the second vaccine dose, GMT.
| Underlying disease | RBD-IgG N | Positive RBD-IgG after 2nd Vx n/N (%) | RBD-IgG GMT (95% CI) | NA* GMT (95% CI) |
|---|---|---|---|---|
| Controls** | 272 | 269/272 (98.9) | 5.98 (5.70–6.28) | 474.0 (403.2–557.3) |
| HIV | 156 | 154/156 (98.7) | 5.14 (4.84–5.46) | 467.60 (382.5–571.7) |
| Solid malignancies | 90 | 75/90 (83.3) | 3.17 (2.56–3.92) | 270.0 (154.8–471.0) |
| Multiple myeloma | 187 | 149/187 (79.7) | 2.76 (2.38–3.20) | 297.7 (191.8–462.1) |
| HSCT | 111 | 83/111 (74.8) | 2.55 (2.03–3.21) | 653.8 (353.3–1210) |
| Liver transplant | 36 | 25/36 (69.4) | 2.14 (1.46–3.14) | 264.6 (121.8–574.9) |
| Myelodysplastic disorders | 43 | 26/43 (60.5) | 1.54 (1.04–2.28) | 362.0 (164.9–795.0) |
| CLL/NHL | 188 | 96/188 (51.0) | 1.18 (0.97–1.43) | 261.4 (166.8–409.6) |
| Kidney transplant | 111 | 50/111 (45.0) | 1.00 (0.80–1.24) | 89.5 (51.1–156.7) |
| Heart transplant | 80 | 15/80 (18.8) | 0.55 (0.44–0.68) | 53.8 (24.1–120.4) |
| All patients | 1002 | 673/1002 (67.2) | 1.98 (1.83–2.14) | 313.2 (267.2–367.1) |
Only samples with neutralizing antibody titers above the cutoff (> 8) were included in the analysis for GMT-NA, ** Controls – immunocompetent healthcare workers, RBD - receptor binding domain, Vx - vaccination, GMT- geometric mean titer, CI - confidence interval, CLL - chronic lymphocytic leukemia, NHL - non-Hodgkin's lymphoma, HIV - human immunodeficiency virus, HSCT - hematopoietic stem cell transplantation.
Following the second vaccine dose, HSCT and HIV RBD-IgG positive patients developed high NA titers (GMT 653.8 and 467.6, respectively). In contrast, heart and kidney transplant patients developed low NA titers (GMT 53.8 and 89.5, respectively) (Table 2, Fig. 1b). The GMT for NAs in the control group was 474 (403.2–557.3). Interestingly, among patients after HSCT and those with solid malignancies, a bimodal distribution of the antibody response was observed (Fig. 1a,b); some patients had a minimal response, while others developed an almost normal response. It is important to note that those patients after HSCT who did develop an antibody response were no longer on immunosuppressive treatment. Statistical comparison in Neutralizing antibodies GMT between groups is presented in Table S6.
Multivariable logistic regression analysis was used to determine the influence of age, gender, and the underlying immunosuppressive disease on the magnitude of response to the second dose of the vaccine in the entire cohort (Tables 3a and S7). Older age (OR 0.41, 95% CI 0.3–0.57) and underlying immunosuppression were significantly associated with a non-reactive response of IgG antibodies. HIV patients demonstrated a similar antibody response to the vaccine to that of healthy adults; C statistic = 0.857. The OR for the whole ICP group except HIV to respond to the vaccine compared to the controls was 0.02 (95% CI, 0.01,0.07), p < 0.001 (Tables 3b and S8).
Table 3a.
Multivariate logistic regression analysis of predictors for positive IgG antibodies following the second dose of vaccine in immunocompromised patients (1002) and immunocompetent controls (272); N = 1274.
| Effect | Odds ratio | Lower 95% CI | Upper 95% CI | P-value |
|---|---|---|---|---|
| Gender, F vs. M | 1.23 | 0.89 | 1.69 | 0.21 |
| Age, > 65 y vs. < 65y | 0.41 | 0.30 | 0.57 | < 0.0001 |
| Days after 2nd vaccine | 1.02 | 1.01 | 1.04 | 0.0064 |
| HIV | 0.80 | 0.13 | 4.90 | 0.81 |
| Solid malignancies | 0.08 | 0.02 | 0.27 | < 0.0001 |
| Multiple myeloma | 0.06 | 0.02 | 0.19 | < 0.0001 |
| HSCT | 0.04 | 0.01 | 0.13 | < 0.0001 |
| Liver transplant | 0.03 | 0.01 | 0.12 | < 0.0001 |
| Myelodysplastic disorders | 0.02 | 0.01 | 0.08 | < 0.0001 |
| CLL/NHL | 0.02 | 0.00 | 0.05 | < 0.0001 |
| Kidney transplant | 0.01 | 0.00 | 0.03 | < 0.0001 |
| Heart transplant | 0.00 | 0.00 | 0.01 | < 0.0001 |
Table 3b.
Multivariate logistic regression analysis for predictors to positive IgG antibodies following the second dose of vaccine in immunocompromised patients (846), patients with HIV (156) and immunocompetent controls (272); N = 1274.
| Effect | Odds ratio | Lower 95% CI | Upper 95% CI | P-value |
|---|---|---|---|---|
| Age, > 65 yr vs. < 65yr | 0.54 | 0.41 | 0.72 | < 0.0001 |
| Gender, F vs. M | 1.34 | 1.00 | 1.79 | 0.04 |
| Days after 2nd vaccine | 1.38 | 1.03 | 1.84 | 0.031 |
| HIV vs. controls | 0.90 | 0.15 | 5.53 | 0.91 |
| All ICP except HIV vs. controls | 0.02 | 0.01 | 0.07 | < 0.001 |
CI – confidence interval. F - female, M- male, HSCT - hematopoietic stem cell transplantation, CLL - chronic lymphocytic leukemia, NHL - non-Hodgkin's lymphoma, HIV - human immunodeficiency virus.
Linear regression analysis of predictors for RBD-IgG levels and NA titers following the second vaccine demonstrated that age and underlying immunosuppression were predictors for lower titers of antibodies (Tables 4a and 4b). There was a high correlation (r = 0.74 [95% CI 0.69,0.78; p < 0.0001]) between RBD-binding IgG and NAs (Fig. S1).
Table 4a.
Multivariate linear regression analysis of predictors for RBD-IgG levels following the second dose of the vaccine in the study population (1002) vs. immunocompetent controls (272); N = 1274.
| Effect | Ratio of mean | Lower 95% CI | Upper 95% CI | P-value |
|---|---|---|---|---|
| Gender, male | 0.97 | 0.86 | 1.09 | 0.58 |
| Age | 0.99 | 0.98 | 0.99 | < 0.0001 |
| HIV | 0.83 | 0.67 | 1.02 | 0.070 |
| Solid malignancies | 0.60 | 0.47 | 0.76 | < 0.0001 |
| Multiple myeloma | 0.53 | 0.44 | 0.64 | < 0.0001 |
| HSCT | 0.45 | 0.36 | 0.56 | < 0.0001 |
| Liver transplant | 0.38 | 0.27 | 0.54 | < 0.0001 |
| Myelodysplastic disorders | 0.31 | 0.23 | 0.43 | < 0.0001 |
| CLL/NHL | 0.23 | 0.19 | 0.28 | < 0.0001 |
| Kidney transplant | 0.18 | 0.14 | 0.22 | < 0.0001 |
| Heart transplant | 0.10 | 0.08 | 0.12 | < 0.0001 |
Table 4b.
Multivariate linear regression analysis of predictors for positive neutralizing antibody levels following the second dose of vaccine in 617 participants, 420 ICPs and 197 immunocompetent controls.
| Effect | Ratio of mean | Lower 95% CI | Upper 95% CI | P-value |
|---|---|---|---|---|
| Gender, male | 0.83 | 0.65 | 1.08 | 0.16 |
| Age | 0.98 | 0.98 | 0.99 | 0.0002 |
| HIV | 0.91 | 0.62 | 1.32 | 0.61 |
| Solid malignancies | 0.57 | 0.34 | 0.98 | 0.04 |
| Multiple myeloma | 0.69 | 0.45 | 1.07 | 0.09 |
| HSCT | 1.35 | 0.77 | 2.36 | 0.29 |
| Liver transplant | 0.54 | 0.27 | 1.06 | 0.07 |
| Myelodysplastic disorders | 0.95 | 0.50 | 1.82 | 0.88 |
| CLL/NHL | 0.58 | 0.38 | 0.87 | 0.008 |
| Kidney transplant | 0.19 | 0.11 | 0.34 | < 0.0001 |
| Heart transplant | 0.11 | 0.04 | 0.31 | < 0.0001 |
RBD – receptor binding domain, CI – confidence interval, HIV - human immunodeficiency virus, HSCT - hematopoietic stem cell transplant, CLL – chronic lymphatic leukemia, NHL – non-Hodgkin's lymphoma, ICPs – Immunocompromised patients.
Vaccine-related serious AEs were not observed in the study (Table S9). No rejection episodes or GVHD were observed at a mean follow up of 30 days following the second dose. Allergic responses were not observed. The frequency of local AEs following the first vaccine varied between 9.9% in HSCT recipients to 40.6% in HIV patients (Table S3, Fig S2). The frequency of local AEs following the second dose was similar to that reported after the first dose, except in HIV patients for whom local AEs decreased. The most common local reaction was pain at the injection site, which was mild in most cases and subsided within 24 h. Systemic AEs were more common following the second vaccine in all groups and included mainly fatigue and headache. Immunocompetent HCW experienced significantly more local AEs than all ICPs groups (p < 0.0001). Systemic AEs were also more common in the controls than in patients with CLL/NHL, HSCT and solid malignancies.
4. Discussion
We recruited 1002 ICPs to participate in a study testing RBD-IgG and NAs following two doses of the BNT162b2 mRNA vaccine. The proportion of participants achieving an effective antibody response two to four weeks following the second vaccine varied from 18.8% and 45% after heart and kidney transplantation, respectively, to 74.8% following HSCT, 79.7% with multiple myeloma, 83.3% with solid malignancies and 98.7% in patients with HIV, the last of these being similar to the 98.9% response in immunocompetent controls. Multivariate logistic regression analysis revealed that older age and underlying immunosuppression were significantly associated with IgG non-reactive response, except in HIV patients who demonstrated a similar antibody response to that of the controls. Furthermore, linear regression analysis demonstrated that the same variables, namely, age and underlying immunosuppression (except HIV), were predictors for lower titers of antibodies measured by RBD-IgG and neutralizing assays.
The spectrum of ICPs is heterogeneous with different degrees of immune impairment. ICPs have higher rates of mortality from COVID-19 compared to other populations due to the immunosuppression associated with the underlying disease and its treatment [19]. An additional concern is that ICPs exhibit prolonged shedding of the virus, which may cause increased transmission, leading to prolonged isolation of the patient and thus delaying chemotherapy or transplantation. Furthermore, persistent infection of SARS-CoV-2 within immunocompromised hosts could serve as a reservoir for the generation of mutations and the subsequent emergence of novel strains with the potential to evade immune responses [20]. Our results raise the possibility that weak antibody response in ICP contributes substantially to these outcomes.
In this study, patients with HIV mounted a humoral response to the vaccine that was similar to the response of the healthy controls. This finding is in keeping with previous studies demonstrating that in the era of ART, pneumococcal vaccines are immunogenic in HIV patients, particularly if ART is initiated immediately after HIV diagnosis, but the durability of the protection remains unknown [21]. The enhanced humoral response in our HIV cohort is probably related to their well-preserved immunological status, with an undetectable viral load and mean CD4 of 700 cells/mL at the time of vaccination. To date, the responses to SARS-CoV-2 vaccines have not been fully characterized in people living with HIV, but a recent study on 12 patients demonstrated a robust immune response [22].
In contrast to the HIV patients, patients following SOTs responded significantly less favorably to the vaccine, with a response ranging from as little as 18.8% in heart transplants, through 45% in kidney transplants, to 69.4% in liver transplants. In keeping with these findings, NA-GMT in liver transplant recipients was significantly higher than that in kidney and heart transplant recipients. The degree of immunosuppression for SOT recipients is based on risk assessment for rejection [23] versus infection and is determined by multiple factors, such as the transplant type, degree of sensitization to human leukocyte antigens, prior allograft rejection, age, and comorbidities. The lower response of heart transplant recipients was not related to age, as those patients were not older than the kidney or liver transplant subpopulations. It is also not related to increased rejection rates, as patients exhibiting rejection were excluded from the study. Our preliminary results on heart transplant recipients suggest that the type of immunosuppression impacts the ability to mount an immune response; notably, mycophenolate use was independently associated with a reduced likelihood of generating an antibody response (OR = 0.09, P = 0.021) [24]. A recent study on the efficacy of the vaccine among kidney transplant recipients found that only 51/136 (37.5%) developed antibodies. Variables associated with non responsiveness were older age, high-dose corticosteroids in the last 12 months, maintenance with triple immunosuppression and regimen that includes mycophenolate [25]. Anti-SARS-CoV-2 antibodies were detected in 47.5% of patients following liver transplantation; predictors for negative response were older age, lower eGFR, and treatment with high dose steroids and mycophenolate [26].
Immunological responses to the vaccine in recipients of SOTs should therefore be further assessed in terms of the duration and dosage of immunosuppressive therapy. Surprisingly, 74.8% of patients following HSCT developed RBD-IgG antibodies, and, furthermore, GMT-NA among those with positive RBD-IgG was the highest among all immunocompromised subpopulations with titers similar to those of the controls. Since lymphocytes require several months to mature sufficiently to produce an effective vaccine response, the optimal timing for vaccinating after HSCT is difficult to determine. Mean time from transplant to vaccination in our cohort was 3.4 (IQR 2.0–6.3) years, which may explain lymphocyte maturation and production of antibodies. The effects of GVHD and immunosuppressive treatment might delay the process of immune reconstitution and limit the effectiveness of vaccination. Recent guidelines recommend starting vaccination against influenza, pneumococcal infection, and Haemophilus influenzae type b as early as three months after HSCT [27]. We chose to vaccinate HSCT patients > 6 months following transplantation and to vaccinate only those without GVHD, which may explain the enhanced immunity conferred by the vaccine in this subpopulation. We observed bimodal distributions of the antibody response in patients following HSCT, namely, those with minimal responses and those with almost normal responses. The latter group consisted of the 45% of HSCT patients who were no longer on immunosuppressive treatment. A recent study on 66 patients after allogeneic HSCT found evidence of a humoral and/or cellular response to the vaccine in 75% [28].
More than 80% of patients with solid malignancies developed a serological response to the vaccine. Treatment for many cancers has progressed significantly in recent years, resulting in improved patient outcomes and prolonged survival, but only a few studies on immunity and vaccination have been published. The patients in our cohort had malignancies of the gastrointestinal tract, breast, and lung and melanoma. Treatment modalities covered a range of chemotherapies, biological targeted therapies, and hormonal treatments, sometimes given in combination, but most of the frequently used treatments were not intensely immunocompromising [29]. A bimodal distribution of the humoral immune response was also observed in this group. A recent study of 102 patients with cancer revealed a serological response in 90%; in that study, treatment with chemotherapy plus immunotherapy was associated with reduced antibody titers [30]. In the future, additional analyses with all risk factors should be done to elucidate these different immune responses.
Patients with hematologic malignancies tended to be more immunocompromised than those with solid tumors, but antibodies did develop in 79.7% of patients with multiple myeloma, in 51.0% with CLL/NHL and in 60.5% with MDS. The enhanced response in patients with multiple myeloma was unexpected in the face of the immunoparesis of the uninvolved immunoglobulins in a significant proportion of patients at the time of diagnosis and the additional decrease due to immunosuppressive treatment. It has been demonstrated that BNT162b2 is immunogenic in 78.6% of multiple myeloma patients, while those on anti-CD38-based treatment responded significantly less well [31].
A recent study of 167 patients with CLL found an antibody response rate of 39.5%, with the highest response in patients in clinical remission after treatment (79.2%), followed by 55.2% in treatment-naïve patients. In patients treated with either BTK inhibitors or venetoclax, with or without anti-CD20 antibody, response rates were 16.0% and 13.6%, respectively. None of the patients exposed to anti-CD20 antibodies < 12 months prior to vaccination responded [32].
We found that the BNT162b2 mRNA vaccine was safe, without any episode of rejection, GVHD or allergy. While concern has been raised that vaccination might trigger rejection, numerous trials have shown no causal association between vaccination and organ rejection [23]. The immunocompetent controls in our study experienced significantly more AEs than the ICPs.
The limitations of the study include differences in demographic characteristics within ICPs and also between ICPs and the control group, although statistical models were used to control for these differences. Unfortunately, BMI was not available for all patients in the study. Another limitation is the lack of treatment subgroup analysis, which is beyond the scope of this study. Our nine ICP groups were treated with many different treatment protocols, with various combination of chemo-immunotherapy and biological treatments. Further analysis on each immunosuppressed group should be done so as to explore the specific risk factors for non-immunogenicity. In addition, the durability of the protection conferred by the BNT162b2 mRNA vaccine remains unknown and must be addressed in future research. Yet another limitation was that pre-vaccination serology and/or PCR were not systematically evaluated and hence previous infection with COVID-19 status is uncertain; however, all patients denied having any symptoms or a positive PCR test before enrollment in the study. We can therefore assume that the occurrence of prior undiagnosed COVID-19 was negligible in ICPs, as patients and their caregivers are usually strict about social distancing measures and mask wearing. Furthermore, the antibody response is only one component of the immune response to vaccines. Cell-mediated immunity is a critical determinant of protection. Indeed, loss of antibodies does not necessarily imply loss of clinical protection and immune memory can persist, even in individuals with low antibody concentrations.
We demonstrated that antibody response to the BNT162b2 mRNA vaccine is highly variable among different immunosuppressed patients, and thus individual recommendations should be provided for different immunosuppressed patients. Younger patients – particularly those with HIV infection, those with solid malignancies being treated with immunochemotherapy, those with multiple myeloma, HSCT recipients six months post-transplant without GVHD, liver transplant patients and probably other transplant patients not receiving antimetabolite maintenance immunosuppression – are more likely to develop antibody responses, and vaccination should be encouraged for those patients. In contrast, older patients, particularly those after heart and kidney transplants, are less likely to develop antibody response and should be warned to follow strict infection control measures, particularly vaccination of all other household members. Further studies should be conducted to explore the cellular immune response to the vaccine in ICPs and the effect of additional booster doses of the vaccine.
Declaration of Competing Interest
OB has received lectures and consulting fees from AbbVie, Janssen and AstraZeneca, GRY has received Grant from Pfizer, lectures and consulting fees from Pfizer, Teva and MSD. All the other authors declare that they have nothing to disclose.
Acknowledgments
Funding
The study did not receive funding.
Contributors
Galia Rahav: design, supervisory role, data collection analysis and interpretation, writing; Yaniv Lustig: Laboratory work, data analysis and interpretation, writing; Jacob Lavee: data collection; Ohad Benjamini: data collection; Hila Magen: data collection; Tammy Hod: data collection; Noga Shem-Tov: data collection; Einat Shacham Shmueli:, data collection; Drorit Merkel: data collection; Ziv Ben-Ari: data collection; Rebecca Halperin: data collection, coordinator; Victoria Indenbaum: laboratory work; Liraz Olmer: data analysis; Amit Huppert: data analysis; Eytan Mor: data collection; Gili Regev-Yochay: supervisory role; Carmit Cohen: data collection; Anat Wieder-Finesod: supervisory role; Itzchak Levy, data collection, analysis and interpretation, writing.
Data sharing statement
The authors declare that all the data was collected through the electronic clinical files of patients treated at the Sheba Medical Center and is available upon request to the corresponding author.
Acknowledgement
The authors gratefully acknowledge the support of Prof. Yitshak Kreiss, Director General of the Sheba Medical Center and Ms. Inez Mureinik for language editing.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2021.101158.
Appendix. Supplementary materials
References
- 1.Johns Hopkins University Coronavirus Resource Center. COVID-19 dashboard by the center for systems science and engineering (CSSE) at Johns Hopkins University. 2020 (https://coronavirus.jhu.edu/map.html. opens in new tab).
- 2.Centers for Disease Control and Prevention. COVID-19 information page (https://www.cdc.gov/coronavirus/2019-ncov/index.html. opens in new tab) 2021.
- 3.Wang Q., Berger N.A., Xu R. Analyses of risk, racial disparity, and outcomes among US patients with cancer and COVID-19 infection. JAMA Oncol. 2021;7(2):220. doi: 10.1001/jamaoncol.2020.6178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liang W., Guan W., Chen R. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21(3):335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Riera R., Bagattini Â.M., Pacheco R.L., Pachito D.V., Roitberg F., Ilbawi A. Delays and disruptions in cancer health care due to COVID-19 pandemic: systematic review. JCO Glob Oncol. 2021;7:311–323. doi: 10.1200/GO.20.00639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Polack F.P., Thomas S.J., Kitchin N. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Israel Ministry of Health. Coronavirus (COVID-10) vaccines https://www.health.gov.il/UnitsOffice/HD/PH/epidemiology/td/docs/365_Corona.pdf. 2021
- 8.Plotkin S.A. Correlates of protection induced by vaccination. Clin Vaccine Immunol. 2010;17:1055–1065. doi: 10.1128/CVI.00131-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McKay P.F., Hu K., Blakney A.K. Self-amplifying RNA SARS-CoV-2 lipid nanoparticle vaccine candidate induces high neutralizing antibody titers in mice. Nat Commun. 2020;11:3523–3529. doi: 10.1038/s41467-020-17409-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walsh E.E., Frenck R.W., Falsey A.R. Safety and immunogenicity of two RNA-based COVID-19 vaccine candidates. N Engl J Med. 2020;383:2439–2450. doi: 10.1056/NEJMoa2027906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sahin U., Muik A., Derhovanessian E. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature. 2020;586:594–599. doi: 10.1038/s41586-020-2814-7. [DOI] [PubMed] [Google Scholar]
- 12.Prendecki M., Clarke C., Brown J. Effect of previous SARS-CoV-2 infection on humoral and T-cell responses to single-dose BNT162b2 vaccine. Lancet. 2021;397:1178–1181. doi: 10.1016/S0140-6736(21)00502-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manisty C., Otter A.D., Treibel T.A. Antibody response to first BNT162b2 dose in previously SARS-CoV-2-infected individuals. Lancet. 2021;397:1057–1058. doi: 10.1016/S0140-6736(21)00501-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krammer F. Correlates of protection from SARS-CoV-2 infection. Lancet. 2021;397(10283):1421–1423. doi: 10.1016/S0140-6736(21)00782-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oved K., Olmer L., Shemer-Avni Y. Multi-center nationwide comparison of seven serology assays reveals a SARS-CoV-2 non-responding seronegative subpopulation. EClinicalMedicine. 2020;29-30 doi: 10.1016/j.eclinm.2020.100651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dieterle M.E., Haslwanter D., Bortz R.H. A replication-competent vesicular stomatitis virus for studies of SARS-CoV-2 spike-mediated cell entry and its inhibition. Cell Host Microbe. 2020;28(3):486–496. doi: 10.1016/j.chom.2020.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lustig Y., Sapir E., Regev-Yochay G. BNT162b2 COVID-19 vaccine and correlates of humoral immune responses and dynamics: a prospective, single-centre, longitudinal cohort study in health-care workers. Lancet Respir Med. 2021;9:999–1009. doi: 10.1016/S2213-2600(21)00220-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The strengthening the reporting of observational studies in epidemiology (STROBE) Statement: guidelines for reporting observational studies (2008). [DOI] [PubMed]
- 19.Azzi Y., Bartash R., Scalea J., Loarte-Campos P., Akalin E. COVID-19 and solid organ transplantation: a review article. Transplantation. 2021;105(1):37–55. doi: 10.1097/TP.0000000000003523. [DOI] [PubMed] [Google Scholar]
- 20.Truong T.T., Ryutov A., Pandey U. Persistent SARS-CoV-2 infection and increasing viral variants in children and young adults with impaired humoral immunity. MedRxiv. 2021 doi: 10.1016/j.ebiom.2021.103355. Mar 2:2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garcia Garrido H.M., Schnyder J.L., Tanck M.W.T. Immunogenicity of pneumococcal vaccination in HIV infected individuals: a systematic review and meta-analysis. EClinicalMedicine. 2020;29-30 doi: 10.1016/j.eclinm.2020.100576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Woldemeskel B.A., Karaba A.H., Garliss C.C. BNT162b2 mRNA vaccine elicits robust humoral and cellular immune responses in people living with HIV. Clin Infect Dis. 2021:ciab648. doi: 10.1093/cid/ciab648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cordero E., Bulnes-Ramos A., Aguilar-Guisado M. effect of influenza vaccination inducing antibody mediated rejection in solid organ transplant recipients. Front Immunol. 2020;11:1917. doi: 10.3389/fimmu.2020.01917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peled Y., Ram E., Lavee J. BNT162b2 vaccination in heart transplant recipients: clinical experience and antibody response. J Heart Lung Transplant. 2021;40:759–762. doi: 10.1016/j.healun.2021.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grupper A., Rabinowich L., Schwartz D. Reduced humoral response to mRNA SARS-CoV-2 BNT162b2 vaccine in kidney transplant recipients without prior exposure to the virus. Am J Transplant. 2021;21:2719–2726. doi: 10.1111/ajt.16615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rabinowich L., Grupper A., Baruch R. Low immunogenicity to SARS-CoV-2 vaccination among liver transplant recipients. J Hepatol. 2021;75:435–438. doi: 10.1016/j.jhep.2021.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cordonnier C., Einarsdottir S., Cesaro S. Vaccination of haemopoietic stem cell transplant recipients: guidelines of the 2017 European conference on infections in leukaemia (ECIL 7) Lancet Infect Dis. 2019;19:e200–e212. doi: 10.1016/S1473-3099(18)30600-5. [DOI] [PubMed] [Google Scholar]
- 28.Ram R., Hagin D., Kikozashvilli N. Safety and immunogenicity of the BNT162b2 mRNA COVID-19 vaccine in patients after allogeneic HCT or CD19-based CART therapy-A single-center prospective cohort study. Transplant Cell Ther. 2021;27:788–794. doi: 10.1016/j.jtct.2021.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vollaard A., Schreuder I., Slok-Raijmakers L. Influenza vaccination in adult patients with solid tumours treated with chemotherapy. Eur J Cancer. 2017;76:134–143. doi: 10.1016/j.ejca.2017.02.012. [DOI] [PubMed] [Google Scholar]
- 30.Massarweh A., Eliakim-Raz N., Stemmer A. Evaluation of seropositivity following BNT162b2 messenger RNA vaccination for SARS-CoV-2 in patients undergoing treatment for cancer. JAMA Oncol. 2021 doi: 10.1001/jamaoncol.2021.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pimpinelli F., Marchesi F., Piaggio G. Fifth-week immunogenicity and safety of anti-SARS-CoV-2 BNT162b2 vaccine in patients with multiple myeloma and myeloproliferative malignancies on active treatment: preliminary data from a single institution. J Hematol Oncol. 2021;14(1):81. doi: 10.1186/s13045-021-01090-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Herishanu Y., Avivi I., Aharon A. Efficacy of the BNT162b2 mRNA COVID-19 vaccine in patients with chronic lymphocytic leukemia. Blood. 2021;137:3165–3173. doi: 10.1182/blood.2021011568. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.