ABSTRACT
The Autophagy, Inflammation and Metabolism (AIM) Center organized a globally accessible, virtual eSymposium during the COVID-19 pandemic in 2020. The conference included presentations from scientific leaders, as well as a career discussion panel, and provided a much-needed platform for early-career investigators (ECIs) to showcase their research in autophagy. This Perspective summarizes the science presented by the ECIs during the event and discusses the lessons learned from a virtual meeting of this kind during the pandemic. The meeting was a learning experience for all involved, and the ECI participants herein offer their thoughts on the pros and cons of virtual meetings as a modality, either as standalone or hybrid events, with a view towards the post-pandemic world.
Summary: We report on the Autophagy, Inflammation and Metabolism Center eSymposium 2020 for early career investigators (ECIs), describe its impact on ECI careers and provide a post-pandemic world meetings formula.
Introduction
In 2016, the Nobel Prize in Physiology or Medicine was awarded to Dr Yoshinori Ohsumi for the discovery and mechanistic understanding of the cellular recycling pathway named autophagy. This tightly regulated nutrient-sensing process is utilized by a variety of organisms and induced in diverse contexts (Hansen et al., 2018). In brief, a double-membrane structure engulfs cytoplasmic material, forming a vesicle-like autophagosome. Subsequently, autophagosomes fuse with acidic compartments, the lysosomes, where pH-optimized enzymes break down the content of the autophagosome to recycle building blocks like amino acids, nucleotides and metabolites (Dikic and Elazar, 2018). The autophagy machinery also supports a range of vital, parallel functions, including pathogen targeting, phagocytosis and secretion (Nieto-Torres et al., 2021b). Owing to the complex regulation of the pathway, its roles in diverse cellular processes, including metabolism, infection, immunity, cell division and cell death, as well as its implication in a variety of diseases, such as neurodegeneration and cancer (Dikic and Elazar, 2018), the scientific field of autophagy is extremely broad and continues to expand every year.
The autophagy research community actively shares findings in diverse and popular specialized meetings. As a result of the COVID-19 global pandemic, these events were postponed or canceled in both 2020 and 2021. To circumvent this situation and provide a platform for young investigators to share their work, the Autophagy, Inflammation and Metabolism (AIM) Center, Albuquerque, NM, USA (Deretic et al., 2018), supported by Journal of Cell Science, hosted an international eSymposia seminar series. This series started in August 2020 and consisted of a total of 24 hours of meeting time spread out over four months. The seminars, held once a month, were hosted by Drs Nikolaos Mellios and Vojo Deretic from the AIM Center and featured two established leaders in the fields of autophagy, inflammation and metabolism. In the fall, three additional sessions were hosted by Drs Malene Hansen (Sanford Burnham Prebys Medical Discovery Institute, La Jolla, CA, USA), Sharon Tooze (Francis Crick Institute, London, UK) and Carmine Settembre (Telethon Institute of Genetics and Medicine, Naples, Italy), and were devoted to early-career investigators (ECIs), allowing them to share their findings and network with peers in what constituted the ECI eSymposium. These sessions featured short talks and flash talks selected by the symposium organizers, as well as poster presentations and a career development panel discussion. This Perspective article focuses on summarizing and evaluating the ECI eSymposium, with comments from both the ECIs and the meeting organizers.
ECI eSymposium – short talk presenters
Nine ECIs, at different professional stages, presented their research related to several aspects of the autophagy process that fall into the following categories.
Molecular basis of autophagy

Dr Carlos Guardia
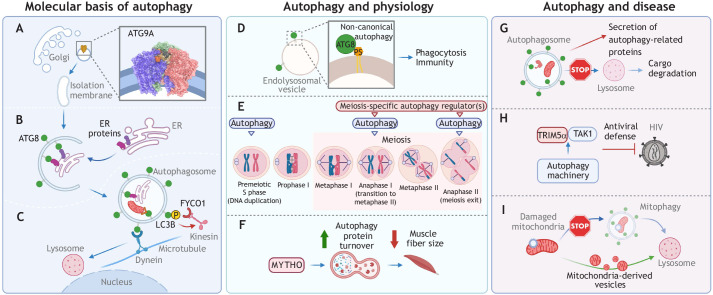
Dr Carlos Guardia has been a postdoctoral fellow at the NIH National Institute of Child Health and Human Development in the USA under the supervision of Dr Juan Bonifacino for almost seven years. Carlos studies autophagosome formation and the role of lysosome movement and activity in autophagy. During the meeting, Carlos presented his latest research on the first high-resolution structure of human ATG9A, the only transmembrane protein of the core autophagy machinery, whose function is not fully understood (Yamamoto et al., 2012). Using state-of-the-art cryogenic electron microscopy (cryo-EM) techniques, his work has revealed that ATG9A forms a homotrimer with multiple internal cavities (Fig. 1A) and defined membrane-association and protein–protein interaction domains as parts of the molecular repertoire of ATG9A that are relevant for autophagosome formation (Guardia et al., 2020). Subsequently, two other groups have confirmed that ATG9 is a lipid scramblase (Maeda et al., 2020; Matoba et al., 2020), validating that ATG9 proteins function as lipid transfer proteins during autophagosome expansion. Carlos has recently been hired as a Stadtman Tenure-Track Investigator at the National Institute of Environmental Health Sciences at NIH, where he will continue to explore the different molecular mechanisms of autophagy in new cell and organism models.
Fig. 1.
Graphical abstracts of the research findings discussed by the ECIs during their short talks at the AIM International eSymposium 2020. The nine talks were organized under three different topics: molecular basis of autophagy (A–C), autophagy and physiology (D–F), and autophagy and disease (G–I). See main text for details about the talks.
Dr Paolo Grumati

Dr Paolo Grumati was recruited as an assistant investigator at the Telethon Institute of Genetics and Medicine (TIGEM) in Italy in January 2019. Over the past two years, he has established his own laboratory and is now investigating the selective degradation of cellular components via autophagy (Johansen and Lamark, 2020). Paolo discussed how the endoplasmic reticulum (ER) is not only a source for autophagosome membrane formation but is itself degraded via a selective type of autophagy named ER-phagy (Stolz and Grumati, 2019) (Fig. 1B). Moreover, several ER-resident proteins function as ER-phagy receptors. Among them are some ER morphogens, such as FAM134B (also known as RETREG1) and RTN3, that can intrinsically shape ER membranes in order to fragment the ER network into smaller vesicles that can be engulfed inside autophagosomes (Grumati et al., 2017; Khaminets et al., 2015). This work showed that other members of the FAM134 protein family, namely FAM134A (RETREG2) and FAM134C (RETREG3), despite their poor homology with FAM134B, also participate in ER protein homeostasis in an autophagy-dependent manner. These findings shed light on the regulation of ER homeostasis, and may have implications for human diseases in which ER-phagy does not operate properly.
Dr Jose L. Nieto-Torres

Dr Jose L. Nieto-Torres has been a postdoctoral fellow at Sanford Burnham Prebys Medical Discovery Institute in the USA since 2016, where he is mentored by Dr Malene Hansen. Jose is interested in elucidating the molecular pathways that regulate the canonical degradative functions and non-canonical functions of the autophagy machinery. Jose described that phosphorylation of the autophagy protein LC3B (also known as MAP1LC3B, a member of the ATG8 protein family) facilitates autophagy via regulation of subcellular transport of autophagosomes, an essential step of autophagy (Søreng et al., 2018). Specifically, LC3B phosphorylation decreases the association of the transport-related protein FYCO1 to autophagosomes to promote proficient transport of autophagosomes toward the perinuclear area and facilitate autophagosome–lysosome fusion (Fig. 1C). In the absence of LC3B phosphorylation, autophagosomes show an aberrant increased transport to the cell periphery, ultimately compromising autophagosome–lysosome fusion and autophagy completion (Nieto-Torres et al., 2021a). Overall, Jose described a novel regulatory axis controlling directional autophagosome transport within cells that is key for the successful completion of autophagy, with potential implications in both physiology and disease. Jose is the recipient of an NIH K99/R00 Pathway to Independence Award and is currently exploring opportunities to start his own academic lab.
Autophagy and physiology

Dr Jo Durgan
Dr Jo Durgan joined the Babraham Institute in the UK in 2014 as a Marie Curie and L'Oreal for Women in Science fellow, studying cell cannibalism. She then transitioned to a long-term, senior role, researching autophagy and chairing the Green Labs, a sustainability program focused on improving environmental impact in a range of areas, including energy, transport, waste, food and biodiversity. During the pandemic, Jo was also homeschooling her kids, aged 7 and 9, so the AIM ECI eSymposium provided a perfect opportunity to present to an autophagy audience in a family-friendly and environmentally responsible format. She described a novel molecular mechanism related to the parallel functions of the autophagy machinery in non-canonical autophagy (Durgan et al., 2021). Specifically, Jo showed that during non-canonical autophagy induced by drugs, phagocytosis or influenza virus infection, ATG8 proteins are alternatively conjugated to the lipid phosphatidylserine (PS) at PS-enriched endolysosomal membranes (Fig. 1D). This specific lipidation pattern is dependent on the WD40 domain of the autophagy protein ATG16L1, which directs lipidation of ATG8 to the target membrane. This work provides an important new insight into autophagy signaling, as ATG8–PS lipidation may provide a specific ‘molecular signature’ for non-canonical autophagy, thus uncovering a novel means of detecting and monitoring this emerging pathway, with key roles in phagocytosis and immunity (Florey et al., 2011; Heckmann and Green, 2019).
Dr Fei Wang

Dr Fei Wang has been an assistant professor in the Department of Cell Biology at UT Southwestern Medical Center in the USA for four years. His research goal is to reveal how autophagy facilitates gametogenesis (meiosis), one of the most complex cellular processes. Meiosis mistakes, such as aneuploidy or polyploidy, are major causes of human miscarriages and lead to devastating human disorders with origins that are difficult to reveal. The role of autophagy during meiosis remains largely unclear. Fei's presentation revealed that the autophagy activity appears to be regulated at multiple meiotic stages (Fig. 1E) for different purposes, including to enable DNA replication (during premeiotic S phase), to facilitate cell transition to metaphase II (during anaphase I) and to assure proper chromosome segregation and meiosis exit (during anaphase II). The underlying molecular mechanism of autophagy regulation and cargo selection at each of these meiotic stages is under investigation; this work may have important implications in the biology of reproduction.
Dr Anais Franco-Romero

Dr Anais Franco-Romero has been a postdoctoral fellow in Professor Marco Sandri's laboratory at the University of Padova in Italy since 2019. Anais introduced the relevance of protein turnover in the regulation of muscle mass, in which autophagy plays a critical role. In particular, the Forkhead box O (FoxO) protein is a critical transcription factor that regulates genes from the autophagic machinery pathway, such as LC3 proteins, p62 (SQSTM1), BNIP3 and GABARAPL1, during muscle atrophy (Milan et al., 2015). Her latest work presented at the meeting identifies a novel FoxO-dependent gene, named MYTHO, that promotes autophagic activity, protein degradation and muscle fiber atrophy (Fig. 1F). MYTHO, together with the muscle catabolism regulatory pathway, may also have important implications in aging and age-related diseases. Anais’s upcoming career goals include securing funding via international or national postdoctoral fellowships, and then applying for independent academic positions.
Autophagy and disease

Dr Andrew M. Leidal
Dr Andrew M. Leidal is a postdoctoral fellow at the Department of Pathology, University of California, San Francisco in the USA, in the lab of Jayanta Debnath, M.D. Andrew's research has been focused on non-canonical secretory functions of the autophagy machinery with roles in physiology and diseases (Ponpuak et al., 2015). The connection between the degradative and secretory functions shared by the autophagy machinery is currently unknown. Andrew recently discovered a previously unknown secretory pathway involving small extracellular vesicles and the autophagy machinery (Leidal et al., 2020), and presented his latest finding on how inhibition of the latest stages of degradative autophagy affects the protein composition of the autophagy-mediated secretome. In summary, upon compromising lysosome acidification or autophagosome–lysosome fusion, the secretome is enriched in autophagy proteins and autophagy receptors (Fig. 1G), indicating a potential redirection of cargo toward secretion. This could have important implications in diseases where degradative autophagy is compromised. Andrew is currently applying for positions to become an independent investigator in academia.
Dr Michael Mandell

Dr Michael Mandell has been an assistant professor at the University of New Mexico in the USA for five years. Michael's research aims to uncover the roles of TRIM proteins in autophagy, inflammation and antiviral defense. Michael presented findings indicating a non-degradative role for the autophagy machinery in the antiretroviral actions of the HIV restriction factor TRIM5α. He showed that proteins involved in autophagy initiation, autophagosome formation and autophagosome–lysosome fusion all contribute to TRIM5α-dependent type I interferon responses. The effect of the autophagy machinery is tied to assembly of active TRIM5α signaling complexes consisting of TRIM5α and the kinase TAK1 (also known as MAP3K7) (Fig. 1H) (Saha et al., 2020). These findings may have direct implications for the treatment of infectious diseases.
Dr Christina Towers

Dr Christina Towers is a new assistant professor at the Salk Institute for Biological Studies in the USA and is also a recipient of an NIH K99/R00 Pathway to Independence Award. Christina's work focuses on the nutrient recycling pathways that cancer cells use to survive. One of the most promising clinical applications for autophagy manipulation is in cancer patients, where there are currently over 60 clinical trials utilizing lysosomal inhibitors to block the autophagy pathway and decrease tumor growth (Levy et al., 2017). Christina described her recent work that utilized a unique CRISPR/Cas9 assay to identify cancer cells that are highly dependent on autophagy for survival (Towers et al., 2019, 2020). Christina discovered that rare clones within these autophagy-dependent populations can adapt to the loss of core autophagy genes and develop new mechanisms to circumvent the canonical autophagy machinery. In focusing on how these autophagy-adapted cells can maintain mitochondrial homeostasis, she discovered the cells had acquired dependencies on alternative mitochondrial pathways, including mitochondrial dynamics and mitochondrial-derived vesicles (Fig. 1I). These findings may have implications for the design of more efficient autophagy-targeting therapies against cancer.
The ECI eSymposium as a career development opportunity
Presenting research findings at a conference is an effective strategy to share knowledge with peers and gain exposure in the research field. This may be particularly appealing to ECIs as, in many cases, they need to navigate the transition from training to independent positions – a time-consuming process that can delay the publication of results. The AIM ECI eSymposium presented a special career development opportunity for the ECIs for several reasons. Firstly, all AIM ECI eSymposium speakers, selected from submitted abstracts, were given a separate session in the overall program. Thus, the opportunities for ECIs to give a talk were greatly increased. Simultaneously, comments and advice on their research could be directly provided by a broader audience, including senior researchers who represented around 20% of a total of 90–100 attendees during the eSymposium. Such a process is pivotal to help establish ECIs in their respective fields. Secondly, given that the eSymposium focused on ECIs, networking sessions could ignite discussions directly related to career development, including strategies to develop independent research directions, the challenges of running a lab or the opportunities that industry could offer (see section ‘Panel discussion on scientific careers – academia, industry and publishing’). Thirdly, senior researchers were able to advise ECIs on research ideas, approaches and career experience. In addition, managers and researchers from industry were able to demonstrate that companies could offer opportunities, different to those in academia, for scientists to develop their skills and build their careers (see section ‘Panel discussion on scientific careers – academia, industry and publishing’). Finally, the virtual meeting format of the AIM ECI eSymposium also eliminated several restrictions linked to economic or geopolitical limitations that would otherwise hamper such early-career development discussions (see section ‘Pros and cons of online meetings’).
Examples of tangible benefits of this ECI-centered eSymposium can be found among the authors of this Perspective. Fei described his experience as being tremendously fruitful, as he received plenty of insightful comments. Paolo was able to present his first research project, which was submitted to a peer-reviewed journal, as a young independent principal investigator (PI). Carlos, now recruited as a tenure-track investigator, was actively applying for PI jobs, and the AIM ECI eSymposium offered a unique exposure platform for him. It was also a great opportunity for Anais to present her recent work in front of a specialized autophagy audience only one year after obtaining her PhD. The eSymposium allowed Anais to expand her scientific network, which now includes the lab of professor Malene Hansen, who helped Anais move her research efforts forward by discussing several experimental approaches and procedures. For Jo, the AIM ECI series was a valuable opportunity to present to the autophagy field for the first time, without the time burden or carbon footprint of long-haul travel (see section ‘Pros and cons of online meetings’). For Jose, the ECI eSymposium constituted an excellent platform to disseminate his research among his peers, which may facilitate his future transition toward an independent academic position. And for Christina, the ECI series was a fantastic opportunity to showcase her trajectory as an independent scientist and publicize the recruitment of trainees to her new, officially open lab.
Panel discussion on scientific careers – academia, industry and publishing
A key part of career development for ECIs is being informed about the career choices available to them as they progress from PhD student through postdocs to become an ECI. The AIM ECI eSymposium organizers selected panelists for their ability to speak about the most popular career options for scientists. Professor Eric H. Baehrecke (University of Massachusetts Medical School, USA), Dr Leon Murphy (Chief Scientific Officer, Casma Therapeutics, UK) and Dr Sharon Ahmad (Executive Editor, Journal of Cell Science) participated in a brief but wide-ranging discussion on scientific careers, which highlighted both the pros and cons of their chosen careers in academia, industry and publishing, as well as specific skills and traits important for each career track. To that end, the three panelists were each asked to share one piece of advice relevant to their career. For academia, the advice was to be a thoughtful leader; for industry, to show a broad interest in science; and for publishing, to understand the importance of networking.
Pros and cons of online meetings
As was the case with most scientific conferences during 2020, the AIM eSymposium was an online event designed to accomplish the goal of fostering scientific interaction without exacerbating a worldwide public health crisis. Following their participation, the ECIs considered the strengths and weaknesses of COVID-era virtual scientific meetings, and these are summarized in alphabetical order below.
Diversity and inclusion
Virtual meetings are substantially more affordable to attend, with no-to-low travel or registration costs, creating a more inclusive opportunity to experience cutting-edge talks and present to peers. However, in-person conferences provide important opportunities for further exposure and networking, which may particularly benefit underrepresented groups, raising visibility.
Environment
The unfolding climate crisis presents a profound threat to humanity (Masson-Delmotte et al., 2021). The substantial carbon footprint of our collective conference travel is one area that scientists must consider carefully (Glausiusz, 2021; Hamant et al., 2019; Klöwer et al., 2020). Had an equivalent ECI event run in person, with the same 47 international ECI scientists (i.e. the number of abstracts submitted) traveling to New Mexico, the travel emissions alone would have amounted to an equivalent of ∼80 metric tons of CO2 (estimated using the carbonfootprint.com calculator and based on economy-class round-trip flights of average occupancy between each participant's home location and Albuquerque, including a radiative forcing factor of 1.891 to account for high altitude). This would equal the daily electricity emissions of over 30,000 UK households (assuming average UK household usage of 3700 kWh/year, as per UK Government Department for Business, Energy and Industrial Strategy figures, and a conversion factor of 0.2532 kg/kWh, as recommended by carbonfootprint.com).
Equality
Virtual meetings are significantly less burdensome on scientists with family commitments, enabling more equal participation. However, the online experience can feel less immersive, as it runs alongside normal daily life, limiting the chance to get away and focus.
Lack of interaction
Two major drawbacks of online events are reduced networking opportunities and a lack of sustained, in-depth discussions of science. This particularly impacts ECIs, who must build their scientific network, trade technical and career advice, and forge lasting collaborations. Online networking is unlikely to rival building relationships in person over shared meals and in new places.
Participation
Poster sessions, which in the AIM ECI eSymposium consisted of prerecorded presentations of the poster that could be watched prior to attending the virtual poster discussion room, did not translate easily online, as not all attendees watch the poster presentation beforehand, therefore missing context of the study. Also, for some speakers, the virtual presentation felt a little flat compared to speaking in person. Nevertheless, the dynamics of a virtual meeting may encourage participation in other ways; for instance, typing a question into the chat may feel less intimidating for some than asking in person, which was reflected in highly dynamic Q and A sessions during the AIM ECI eSymposium, in which senior researchers and other ECIs attending this event actively participated.
Reduced exposure
Online meetings provide fewer opportunities to increase exposure and visibility, which is of critical importance not only to ECIs, but also to underrepresented groups.
Time commitment
The reduced time load associated with virtual meeting participation can help secure high-profile speakers, whose schedules might otherwise prohibit attendance. This can elevate the scientific content available for all participants.
Time zone
Virtual meetings with an international audience are challenging because participants frequently live in different time zones. The AIM ECI eSymposium had attendees from virtually all over the world. Although starting at 8 am in the Pacific Time Zone worked well for most people attending from the USA and Europe, it was challenging for people attending from East Asia. Recording the sessions is a potential solution, but this comes with concerns regarding unpublished data, as discussed below.
Unpublished data
There is an understandable reluctance on the part of presenters, at all career stages, to show unpublished data in a recorded, and somewhat anonymous, format that could be instantaneously shared worldwide without their consent.
How should the scientific conferences of the future be designed?
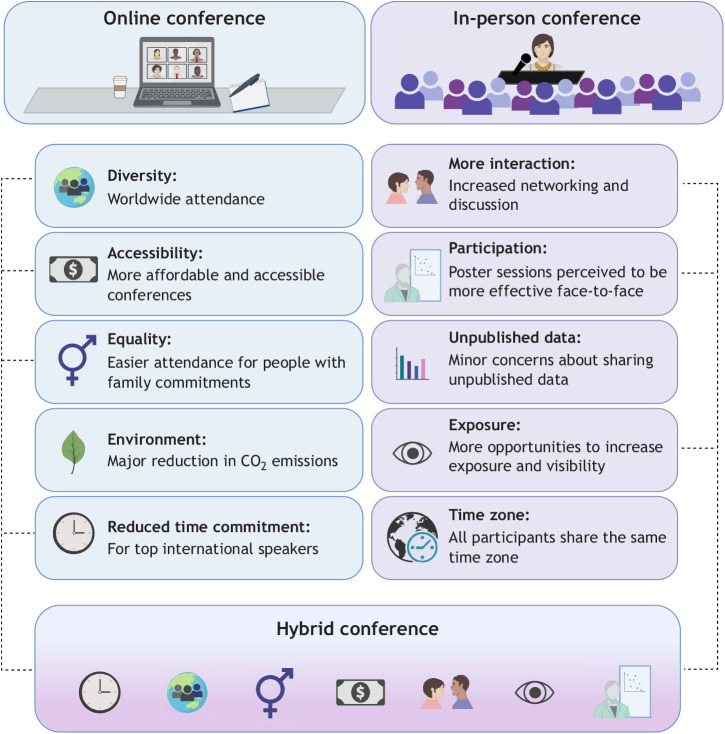
The authors of this Perspective next asked what a ‘Symposium of 2022’ should look like and felt that some hybrid form of in-person and virtual meeting is both inevitable and desirable (Fig. 2). The AIM eSymposium provided a valuable pilot run, and some useful guidelines have emerged. Based on the experiences and discussions of the authors of this Perspective with the meeting organizers, and the ideas of other peers (Bottanelli et al., 2020), we have identified some key areas for consideration by future conference organizers and attendees.
Fig. 2.
Advantages of online and in-person conferences with a view towards hybrid meetings, the potential scientific conference of the future.
Hybrid meetings
A hybrid conferencing model can combine the benefits of both online and in-person meetings (Fig. 2). This can be achieved using mixed-format meetings, an alternating approach, or even multi-center events using regional hubs, with virtual links to enjoy the talks online, an alternative that is being considered in several research fields and could also be tried in autophagy meetings. Links to recorded talks could also be considered to deal with time zone differences, whenever speakers are comfortable with this format. ECIs, in most cases, are not among the invited speakers and thus need to cover traveling costs using their start-up budget. Thus, hybrid meetings might represent a valuable opportunity to maximize the number of conferences they can attend while limiting the costs involved.
Flash talk presentations
The AIM eSymposium program included three flash talks per session that were presented after the ECI short talks. The flash talk presenters were selected from the abstracts considered for poster presentations in order to help introduce the poster topic to a higher number of attendees. In a virtual format, we suggest that traditional poster sessions should be replaced or supplemented with an even larger number of single-slide flash talk presentations. This could be combined with subsequent virtual breakout rooms with access for presenters to show and discuss their poster and receive feedback on their data, without requiring that the attendees watch prerecorded presentations beforehand.
Networking
Well-facilitated breakout rooms, or speed-networking sessions, could further enhance online networking, depending on participation from senior scientists, which was excellent during the eSymposium. To ensure and encourage the participation of senior scientists in other events, a piece of advice from the eSymposium meeting organizers is to involve other senior colleagues in order to facilitate reaching their peers. Furthermore, creating an email database of senior researchers invited to participate in this kind of event at other meetings would be a way to facilitate this task.
Travel quota
As individuals, we could all opt to reduce our travel footprints by attending a smaller number of in-person conferences and complementing them with additional, more environmentally responsible, online events each year. Choosing when to attend in person would depend on a range of personal factors, including project stage, career requirements and budget, but the collective impact of choosing to reduce our air miles where reasonable would be substantial.
Conclusions
The 2020 AIM eSymposium met the needs of scientific communities during the pandemic and was an attempt to provide opportunities to ECIs to present their work at a time when many scheduled conferences had been canceled. The effort included a large number of investigators, both as organizers and presenters, from around the globe, and by that measure alone was a success, as it kept scientific communities connected. The post-meeting analysis presented here has identified the strengths of conducting such meetings virtually, as well as areas for improvement. As we move towards post-pandemic planning, we suggest that virtual meetings might continue to play an important role in scientific conferencing and that a hybrid format can integrate the benefits of both online and in-person meetings.
Acknowledgements
We thank the AIM director Dr Vojo Deretic and Dr Nikolaos Mellios for organizing the 2020 AIM eSymposium, as well as Drs Malene Hansen, Sharon Tooze and Carmine Settembre for organizing and hosting the AIM ECI eSymposium. We also thank all AIM leadership and staff, in particular Sally Ann Garcia, Shaina Aguirre and Lee Allers for the organization under pandemic conditions, and to all speakers and participants of the 2020 AIM eSymposium. The AIM Center is supported by National Institutes of Health grant P20GM121176. The main text of this piece was generated by ECI participants and was curated and edited by Drs Malene Hansen, Sharon Tooze and Carmine Settembre, the co-organizers of the 2020 AIM ECI eSymposium, who provided helpful discussions and critical reading of this manuscript. Figs 1 and 2 were created with BioRender.com.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Funding
J.L.N.-T. is supported by a Fundación Ramón Areces Postdoctoral Fellowship and a National Institutes of Health K99/R00 pathway to independence grant (K99AG062774). A.F.-R is supported by an EMBO short-term fellowship (ASTF 7874-2019). F.W. is supported by grants from the National Institutes of Health (R01GM133899) and from the Welch Foundation (I-2019-20190330), as well as funding from Nancy Cain and Jeffrey A. Marcus Scholar in Medical Research, in Honor of Dr Bill S. Vowell. M.A.M. is supported by grants from the National Institutes of Health (1P20GM121176-02 and 1R21AI131964). J.D. is supported by grants from the Biotechnology and Biological Sciences Research Council, (BB/P013384/1, BBS/E/B/000C0432 and BBS/E/B/000C0434). P.G. is supported by Fondazione Telethon and the Roche Foundation. C.G.T. is supported by a National Institutes of Health K99/R00 pathway to independence award (K99CA245187) and a Cancer League of Colorado fellowship. A.M.L. is supported by a Banting Postdoctoral Fellowship from the Government of Canada (201409BPF-335868) and a Cancer Research Society Scholarship for Next Generation of Scientists. Deposited in PMC for release after 12 months.
References
- Bottanelli, F., Cadot, B., Campelo, F., Curran, S., Davidson, P. M., Dey, G., Raote, I., Straube, A. and Swaffer, M. P. (2020). Science during lockdown – from virtual seminars to sustainable online communities. J. Cell Sci. 133, jcs249607. 10.1242/jcs.249607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deretic, V., Prossnitz, E., Burge, M., Campen, M. J., Cannon, J., Liu, K. J., Sklar, L. A., Allers, L., Garcia, S. A., Baehrecke, E. H.et al. (2018). Autophagy, Inflammation, and Metabolism (AIM) center of biomedical research excellence: supporting the next generation of autophagy researchers and fostering international collaborations. Autophagy 14, 925-929. 10.1080/15548627.2018.1465784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dikic, I. and Elazar, Z. (2018). Mechanism and medical implications of mammalian autophagy. Nat. Rev. Mol. Cell Biol. 19, 349-364. 10.1038/s41580-018-0003-4 [DOI] [PubMed] [Google Scholar]
- Durgan, J., Lystad, A. H., Sloan, K., Carlsson, S. R., Wilson, M. I., Marcassa, E., Ulferts, R., Webster, J., Lopez-Clavijo, A. F., Wakelam, M. J.et al. (2021). Non-canonical autophagy drives alternative ATG8 conjugation to phosphatidylserine. Mol. Cell 81, 2031-2040.e8. 10.1016/j.molcel.2021.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florey, O., Kim, S. E., Sandoval, C. P., Haynes, C. M. and Overholtzer, M. (2011). Autophagy machinery mediates macroendocytic processing and entotic cell death by targeting single membranes. Nat. Cell Biol. 13, 1335-1343. 10.1038/ncb2363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glausiusz, J. (2021). Rethinking travel in a post-pandemic world. Nature 589, 155-157. 10.1038/d41586-020-03649-8 [DOI] [PubMed] [Google Scholar]
- Grumati, P., Morozzi, G., Hölper, S., Mari, M., Harwardt, M.-L. I. E., Yan, R., Müller, S., Reggiori, F., Heilemann, M. and Dikic, I. (2017). Full length RTN3 regulates turnover of tubular endoplasmic reticulum via selective autophagy. eLife 6, e25555. 10.7554/eLife.25555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guardia, C. M., Tan, X.-F., Lian, T., Rana, M. S., Zhou, W., Christenson, E. T., Lowry, A. J., Faraldo-Gómez, J. D., Bonifacino, J. S., Jiang, J.et al. (2020). Structure of human ATG9A, the only transmembrane protein of the core autophagy machinery. Cell Rep. 31:107837. 10.1016/j.celrep.2020.107837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamant, O., Saunders, T. and Viasnoff, V. (2019). Seven steps to make travel to scientific conferences more sustainable. Nature 573, 451-452. 10.1038/d41586-019-02747-6 [DOI] [PubMed] [Google Scholar]
- Hansen, M., Rubinsztein, D. C. and Walker, D. W. (2018). Autophagy as a promoter of longevity: insights from model organisms. Nat. Rev. Mol. Cell Biol. 19, 579-593. 10.1038/s41580-018-0033-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckmann, B. L. and Green, D. R. (2019). LC3-associated phagocytosis at a glance. J. Cell Sci. 132, jcs231472. 10.1242/jcs.231472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen, T. and Lamark, T. (2020). Selective autophagy: ATG8 family proteins, LIR motifs and cargo receptors. J. Mol. Biol. 432, 80-103. 10.1016/j.jmb.2019.07.016 [DOI] [PubMed] [Google Scholar]
- Khaminets, A., Heinrich, T., Mari, M., Grumati, P., Huebner, A. K., Akutsu, M., Liebmann, L., Stolz, A., Nietzsche, S., Koch, N.et al. (2015). Regulation of endoplasmic reticulum turnover by selective autophagy. Nature 522, 354-358. 10.1038/nature14498 [DOI] [PubMed] [Google Scholar]
- Klöwer, M., Hopkins, D., Allen, M. and Higham, J. (2020). An analysis of ways to decarbonize conference travel after COVID-19. Nature 583, 356-359. 10.1038/d41586-020-02057-2 [DOI] [PubMed] [Google Scholar]
- Leidal, A. M., Huang, H. H., Marsh, T., Solvik, T., Zhang, D., Ye, J., Kai, F. B., Goldsmith, J., Liu, J. Y., Huang, Y.-H.et al. (2020). The LC3-conjugation machinery specifies the loading of RNA-binding proteins into extracellular vesicles. Nat. Cell Biol. 22, 187-199. 10.1038/s41556-019-0450-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy, J. M. M., Towers, C. G. and Thorburn, A. (2017). Targeting autophagy in cancer. Nat. Rev. Cancer 17, 528-542. 10.1038/nrc.2017.53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda, S., Yamamoto, H., Kinch, L. N., Garza, C. M., Takahashi, S., Otomo, C., Grishin, N. V., Forli, S., Mizushima, N. and Otomo, T. (2020). Structure, lipid scrambling activity and role in autophagosome formation of ATG9A. Nat. Struct. Mol. Biol. 27, 1194-1201. 10.1038/s41594-020-00520-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masson-Delmotte, V., Zhai, P., Pirani, A., Connors, S. L., Péan, C., Berger, S., Caud, N., Chen, Y., Goldfarb, L., Gomis, M. I.et al. (2021). IPCC, 2021: Summary for policymakers. In Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assesment Report of the Intergovernmental Panel on Climate Change (ed. Masson-Delmotte V., Zhai P., Pirani A., Connors S. L., Péan C., Berger S., Caud N., Chen Y., Goldfarb L., Gomis M. I., Huang M., Leitzell K., Lonnoy E., Matthews J. B. R., Maycock T. K., Waterfield T., Yelekçi O., Yu R. and Zhou B.). Cambridge, UK: Cambridge University Press; (In Press). https://www.ipcc.ch/report/ar6/wg1/downloads/report/IPCC_AR6_WGI_SPM.pdf [Google Scholar]
- Matoba, K., Kotani, T., Tsutsumi, A., Tsuji, T., Mori, T., Noshiro, D., Sugita, Y., Nomura, N., Iwata, S., Ohsumi, Y.et al. (2020). Atg9 is a lipid scramblase that mediates autophagosomal membrane expansion. Nat. Struct. Mol. Biol. 27, 1185-1193. 10.1038/s41594-020-00518-w [DOI] [PubMed] [Google Scholar]
- Milan, G., Romanello, V., Pescatore, F., Armani, A., Paik, J.-H., Frasson, L., Seydel, A., Zhao, J., Abraham, R., Goldberg, A. L.et al. (2015). Regulation of autophagy and the ubiquitin-proteasome system by the FoxO transcriptional network during muscle atrophy. Nat. Commun. 6, 6670. 10.1038/ncomms7670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto-Torres, J., Shanahan, S.-L., Chassefeyre, R., Chaiamarit, T., Zaretski, L., Landeras-Bueno, S., Verhelle, A., Encalada, S. and Hansen, M. (2021a). LC3B phosphorylation regulates FYCO1 binding and directional transport of autophagosomes. Curr. Biol. 31, 3440-3449.E7. 10.1016/j.cub.2021.05.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto-Torres, J. L., Leidal, A. M., Debnath, J. and Hansen, M. (2021b). Beyond autophagy: the expanding roles of ATG8 proteins. Trends Biochem. Sci. 46, 673-686. 10.1016/j.tibs.2021.01.004 S0968-0004(21)00006-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponpuak, M., Mandell, M. A., Kimura, T., Chauhan, S., Cleyrat, C. and Deretic, V. (2015). Secretory autophagy. Curr. Opin. Cell Biol. 35, 106-116. 10.1016/j.ceb.2015.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha, B., Chisholm, D., Kell, A. M. and Mandell, M. A. (2020). A non-canonical role for the autophagy machinery in anti-retroviral signaling mediated by TRIM5α. PLoS Pathog. 16, e1009017. 10.1371/journal.ppat.1009017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Søreng, K., Neufeld, T. P. and Simonsen, A. (2018). Membrane trafficking in autophagy. In International Review of Cell and Molecular Biology, Vol. 336 (Ed. Galluzzi L.), pp. 1-92. Academic Press. 10.1016/bs.ircmb.2017.07.001 [DOI] [PubMed] [Google Scholar]
- Stolz, A. and Grumati, P. (2019). The various shades of ER-phagy. FEBS J. 286, 4642-4649. 10.1111/febs.15031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towers, C. G., Fitzwalter, B. E., Regan, D., Goodspeed, A., Morgan, M. J., Liu, C.-W., Gustafson, D. L. and Thorburn, A. (2019). Cancer cells upregulate NRF2 signaling to adapt to autophagy inhibition. Dev. Cell 50, 690-703.e6. 10.1016/j.devcel.2019.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towers, C. G., Wodetzki, D., Thorburn, J., Smith, K. R., Caino, M. C. and Thorburn, A. (2020). Alternate mitochondrial pathways compensate for loss of LC3-mediated mitophagy. SSRN Electron. J. 10.2139/ssrn.3728139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto, H., Kakuta, S., Watanabe, T. M., Kitamura, A., Sekito, T., Kondo-Kakuta, C., Ichikawa, R., Kinjo, M. and Ohsumi, Y. (2012). Atg9 vesicles are an important membrane source during early steps of autophagosome formation. J. Cell Biol. 198, 219-233. 10.1083/jcb.201202061 [DOI] [PMC free article] [PubMed] [Google Scholar]