ABSTRACT
Mitochondria, which resemble their α-proteobacteria ancestors, are a major cellular asset, producing energy ‘on the cheap’ through oxidative phosphorylation. They are also a liability. Increased oxidative phosphorylation means increased oxidative stress, and damaged mitochondria incite inflammation through release of their bacteria-like macromolecules. Mitophagy (the selective macroautophagy of mitochondria) controls mitochondria quality and number to manage these risky assets. Parkin, BNIP3 and NIX were identified as being part of the first mitophagy pathways identified in mammals over a decade ago, with additional pathways, including that mediated by FUNDC1 reported more recently. Loss of Parkin or PINK1 function causes Parkinson's disease, highlighting the importance of mitophagy as a quality control mechanism in the brain. Additionally, mitophagy is induced in idiopathic Parkinson's disease and Alzheimer's disease, protects the heart and other organs against energy stress and lipotoxicity, regulates metabolism by controlling mitochondrial number in brown and beige fat, and clears mitochondria during terminal differentiation of glycolytic cells, such as red blood cells and neurons. Despite its importance in disease, mitophagy is likely dispensable under physiological conditions. This Review explores the in vivo roles of mitophagy in mammalian systems, focusing on the best studied examples – mitophagy in neurodegeneration, cardiomyopathy, metabolism, and red blood cell development – to draw out common themes.
KEY WORDS: PRKN, Park2, Park6, Mitochondria quality control, Neurodegeneration
Summary: This Review explores the in vivo roles of mitophagy in mammals, focusing on the best-studied examples – mitophagy in neurodegeneration, cardiomyopathy, metabolism and red blood cell development – to draw out common themes.
Introduction
Mitophagy is the selective degradation of mitochondria by macroautophagy (hereafter, autophagy), the process by which intracellular components are delivered to the lysosome for degradation. During mitophagy, a mitochondrion is selectively captured in a double-membraned structure called an autophagosome (Box 1). The outer membrane of the autophagosome subsequently fuses with an acidic lysosome (forming a mito-lysosome), and the inner membrane of the autophagosome and the captured mitochondrion are degraded. Free lipids and amino acids are exported from the lysosome to the cytosol, where they may be used for energy or as building blocks, depending on the overall cellular state (Youle and Narendra, 2011; Onishi et al., 2021). The study of mitophagy in vivo has been greatly aided by the development of new mitophagy reporters (Box 2).
Box 1. The autophagy machinery.
Mitophagy depends on the cellular machinery that generates autophagosomes for both non-selective bulk autophagy and selective autophagy. Many of the 18 core autophagy-related genes (ATGs) regulating this process were discovered initially in yeast by Yoshinori Ohusmi, for which he was awarded the 2016 Nobel prize (reviewed in Morishita and Mizushima, 2019). These fall into six functional groups:
(1) ULK complex (ULK1 and/or ULK2, ATG101, ATG13 and FIP200)
(2) ATG9
(3) ATG14–PtdIns3 kinase (PI3K) complex (VPS34, VPS15, BECN1, ATG14)
(4) ATG2–ATG18/WIPI complex (ATG2A and/or ATG2B, WIPI2, CMP1, TMEM41B)
(5) ATG12 conjugation system (ATG5, ATG7, ATG10, ATG12 and ATG16)
(6) ATG8/LC3 conjugation system (ATG3, ATG4, ATG7 and ATG8).
These can be distinguished into early (1 to 4) and late (5 and 6) complexes. The early complexes are involved in autophagophore nucleation (1 and 2) and expansion (3 and 4). The late complexes (5 and 6) form the late conjugation systems. One of the main functions of the late conjugation systems is to anchor LC3B (or one of its five orthologs) into the autophagosome by conjugation of LC3B to phosphatidylethanolamine, forming LC3-II. LC3-II, which migrates separately from unconjugated LC3-I on an SDS-PAGE gel, serves as a useful marker of autophagosome abundance in canonical autophagy. Autophagosomes can still form in the absence of the late conjugation systems, but the resulting autophagosome may be smaller and fuse less efficiently with lysosomes (Nguyen et al., 2016; Tsuboyama et al., 2016). For some forms of autophagy (termed alternative autophagy), the late conjugation system is not involved or is not rate-limiting for substrate clearance (Nishida et al., 2009). This appears to be the case for mitophagy in developing erythrocytes, which depends on early complexes, such as ULK and PI3K complexes, but not on the late conjugations systems, as discussed in detail below (Kundu et al., 2008; Zhang et al., 2009; Matsui et al., 2006; Honda et al., 2014).
Box 2. Measuring mitophagy in vivo.
Detection of mitophagy in vivo has been aided by the development of fluorescent protein (FP)-based mitophagy reporters. The most widely used reporters rely on differential fluorescence of the reporter in a basic and acidic environment to distinguish free mitochondria from mito-lysosomes. This is achieved by using a FP, such as Keima, which undergoes a pH-dependent spectral shift (Allen et al., 2013; Katayama et al., 2011, 2020), or the combination of an acid-sensitive (e.g. GFP or YPET) and an acid-insensitive FP (e.g. mCherry or TOLLES) fused in tandem.
The best characterized reporter mice are mt-Keima (with Keima directed to the mitochondrial matrix) and mito-QC (with tandem GFP–mCherry targeted to the OMM by the FIS1 anchor) (McWilliams et al., 2016; Sun et al., 2015). In a recent variation of the mito-QC reporter, GFP–mCherry is targeted to the OMM by the anchor of OMP25 rather than FIS1 (Yamashita et al., 2021). With this GFP–mCherry–OMP25 reporter, mitophagic flux can be followed as the ratio of monomeric mCherry to tandem GFP–mCherry–OMP25 on an immunoblot, as well as by fluorescence microscopy (Yamashita et al., 2021).
mt-Keima is sensitive for mitophagy but requires in vivo or ex vivo imaging in non-fixed tissues (Katayama et al., 2011; Sun et al., 2015), whereas mito-QC and mito-SRAI can be fixed (Katayama et al., 2020; McWilliams et al., 2016). A recent report has suggested that mito-QC might be less sensitive than mt-Keima in cultured cells following PINK1-Parkin activation and in reporter mice subjected to exhaustive exercise (Liu et al., 2021b), although it has been speculated that differences in mouse strain background could have affected the in vivo results in this study (for comment and response, see Ganley et al., 2021; Liu et al., 2021c). Mito-QC was also reported to be less sensitive than the recently described mito-SRAI, which targets an optimized FP pair TOLLES-YPET to the mitochondrial matrix, in assays of the PINK1-Parkin pathway in cultured cells (Katayama et al., 2020). Other reporters include mito-Timer, which depends on an irreversible spectral shift driven by ROS (Wilson et al., 2019). Additionally, mitophagy can be inferred from mitochondrial protein or DNA turnover, using stable isotope labeling (Collins et al., 2003; Vincow et al., 2013, 2019).
In vivo, mitophagy serves different purposes. In the liver, it frees amino acids for gluconeogenesis (Ashford and Porter, 1962; Ezaki et al., 2011). During development, mitophagy clears mitochondria as part of a metabolic shift (e.g. from oxidative phosphorylation to glycolysis) (Schweers et al., 2007; Sandoval et al., 2008; Esteban-Martínez et al., 2017). Finally, it is a mechanism of quality control, curbing by-products of dysfunctional mitochondria, such as excessive reactive oxygen and nitrogen species (ROS and RNS) and inflammation-inciting damage-associated molecular patterns (DAMPs) (Matheoud et al., 2019; Narendra et al., 2008; Sliter et al., 2018). Given these diverse functions, it is not surprising that multiple (and sometimes overlapping) pathways mediate mitophagy in vivo.
The focus of this Review is on functions of mitophagy in vivo, with an emphasis on the best-studied examples in mammalian models – mitophagy in neurodegeneration, cardiomyopathy, metabolism, and red blood cell development. Findings from yeast, other model systems and cultured cells are addressed only as they relate to mammalian systems in vivo.
Mitophagy receptors
The selectivity of mitophagy for mitochondria is usually mediated by mitophagy receptors, which target some component of the autophagy machinery to mitochondria (reviewed in Gubas and Dikic, 2021). The mitophagy receptors are of two general types, (1) direct (i.e. ubiquitin-independent) and (2) ubiquitin-dependent (Fig. 1A).
Fig. 1.
Ubiquitin-dependent and -independent mitophagy receptors. (A) Mitophagy is mediated by ubiquitin-independent mitophagy receptors, which include NIX (BNIP3L), BNIP3 and FUNDC1. All three have an LC3-interacting region (LIR) in the N-terminus. In the case of NIX and likely BNIP3, the LIR and the BH3 domains are not necessary for their activity. Instead, the minimal essential region (MER) and their transmembrane domain (TM) are required. In ubiquitin-dependent mitophagy, PINK1, which contains a mitochondrial-targeting signal (MTS), is stabilized on the outer mitochondrial membrane (OMM) of impaired mitochondria. Through its C-terminal serine/threonine kinase domain, it phosphorylates its substrate ubiquitin (Ub), which is attached to the OMM proteins, as well as the ubiquitin-like domain (UBL) of Parkin. Parkin contains four RING domains (R0–R3) that collectively form a RING-between-RING E3 ubiquitin ligase domain. Parkin ubiquitylates several OMM proteins; these then recruit ubiquitin-dependent autophagy receptors, such as optineurin (OPTN) and NDP52 (also known as CALCOCO2), which bind ubiquitin with their zinc finger (ZF) and/or UBAN domains. OPTN recruits ATG9 proteins through its LZ domain and NDP52 recruits the ULK1 complex through its SKICH domains to promote mitophagy. OPTN and NDP52 additionally contain coiled-coils (CCs). (B) Key steps of the ubiquitin-dependent mitophagy pathway mediated by PINK1 and Parkin are shown. In the first stage, an impaired mitochondrion is recognized in a series of steps involving, firstly, PINK1 stabilization on the OMM. Parkin is then recruited to the OMM from the cytosol by PINK1 and ubiquitylates OMM proteins. Following this, ubiquitin recruits the autophagy adaptors and NDP52. Through specific interactions with autophagy machinery including between OPTN and ATG9, NDP52 and the ULK1 complex, and OPTN–NDP52 and LC3, autophagy adaptors promote both the formation of an autophagosome and its capture of the mitochondrion, which then fuses with a lysosome wherein it is degraded with the aid of luminal hydrolases. CS, conjugating system.
The direct mitophagy receptors are expressed on the outer mitochondrial membrane (OMM) and are believed to directly interact with the autophagy machinery to achieve selectivity. The best studied in vivo are BCL2-interacting protein 3 (BNIP3), Nip3-like protein X (NIX; also known as BNIP3L) and FUN14 domain containing 1 (FUNDC1) (Liu et al., 2012; Sandoval et al., 2008; Schweers et al., 2007; Zhang et al., 2008). These mediate mitophagy at least in part by binding to LC3 proteins (herein LC3 refers to the LC3 and GABARAP proteins) through their LC3-interacting region (LIR) (Liu et al., 2012; Novak et al., 2010), although for NIX, and likely BNIP3, this interaction is not necessary for mitophagy in vivo, which is mediated instead by a minimal essential region (Zhang et al., 2012). Direct mitophagy receptors are regulated through their level of expression on the OMM, post-translational modifications, such as phosphorylation (as proposed for NIX) or dephosphorylation (as proposed for FUNDC1) (Schweers et al., 2007; Zhang et al., 2008; Liu et al., 2012; Yuan et al., 2017), and dimerization in the case of NIX (Marinković et al., 2021). The best studied ubiquitin-dependent mitophagy pathway is the PINK1-Parkin pathway, which marks dysfunctional mitochondria with ubiquitin for recognition by selective autophagy receptors (Box 3 and Fig. 1B) (Geisler et al., 2010; Matsuda et al., 2010; Narendra et al., 2008, 2010; Vives-Bauza et al., 2010).
Box 3. The PINK1-Parkin mitophagy pathway.
In response to mitochondrial distress (e.g. membrane potential loss), PINK1 is selectively stabilized on the OMM (Geisler et al., 2010; Matsuda et al., 2010; Narendra et al., 2010; Vives-Bauza et al., 2010). PINK1 activates Parkin by phosphorylating ubiquitin (Ub) conjugated to OMM proteins and the ubiquitin-like domain of Parkin, both on serine 65 (S65) (Kondapalli et al., 2012; Kane et al., 2014; Kazlauskaite et al., 2014; Koyano et al., 2014; Ordureau et al., 2014). Parkin activation requires both direct phosphorylation and phospho-ubiquitin binding. Crystal structures (PDB 6GLC, for Parkin in active conformation with pUb bound, and PDB 4BM9, for auto-inhibited Parkin) demonstrate that binding to pS65-Ub stabilizes Parkin in its active, open conformation rather than its closed conformation, in which its active site is sterically blocked from the E2 Ub-conjugating enzyme (Gladkova et al., 2018; Trempe et al., 2013). Once activated, Parkin ubiquitylates additional OMM proteins, some of which are phosphorylated by PINK1 in an amplification loop (Ordureau et al., 2014). Most of the Ub species (whether phosphorylated or not) exist as mono-Ub or short Ub chains (Swatek et al., 2019). Parkin-mediated ubiquitylation either leads to the degradation of the OMM proteins by the ubiquitin-proteasome system, which is the primary fate of the four GTPases regulating mitochondrial dynamics, MFN1, MFN2, RHOT1 (also known as MIRO1) and RHOT2 (also known as MIRO2), and/or recruits ubiquitin-dependent autophagy receptors, resulting in mitophagy (Gegg et al., 2010; Poole et al., 2010; Tanaka et al., 2010; Wang et al., 2011; Ziviani et al., 2010). While there are at least five autophagy receptors that are recruited in this manner, only the autophagy receptors optineurin (OPTN) and NDP52 efficiently promote mitophagy (Heo et al., 2015; Lazarou et al., 2015; Wong and Holzbaur, 2014). Both OPTN and NDP52 contain a LIR, which can bind LC3 proteins on mature autophagophores (Heo et al., 2015; Lazarou et al., 2015; Wong and Holzbaur, 2014). Additionally, OPTN and NDP52 can stimulate autophagophore formation close to the mitochondrial surface by recruiting early autophagy complexes (Itakura et al., 2012; Vargas et al., 2019; Yamano et al., 2020). OPTN directly interacts with ATG9 (Yamano et al., 2020), and NDP52 directly interacts with the ULK1 complex (Vargas et al., 2019).
While autophagy and mitochondrial dynamics are essential for life, mitophagy, likely, is not under physiological conditions. In contrast to the non-redundant canonical autophagy genes (reviewed in Kuma et al., 2017) and the GTPases governing mitochondrial fission (DRP1; also known as DNM1L) and fusion (MFN1, MFN2 and OPA1) (Chen et al., 2003; Davies et al., 2007; Ishihara et al., 2009; Wakabayashi et al., 2009), mouse knockouts of key mediators of mitophagy are viable. These include NIX (Sandoval et al., 2008; Schweers et al., 2007), BNIP3 (Diwan et al., 2007), Parkin (Goldberg et al., 2003), PINK1 (Kitada et al., 2007), FUNDC1 (Zhang et al., 2016), the NIX-Parkin double knockout (DKO) (Yuan et al., 2017), and PINK1-Parkin DKO (Kitada et al., 2009). This may reflect both redundancy among the mitophagy receptors and the fact that mitophagy is often a stress-evoked response.
The PINK1-Parkin pathway in neurodegeneration
The most common causes of recessive Parkinson's disease (PD) are loss of function mutations in PRKN (encoding the protein Parkin) and Pink1 (Kitada et al., 1998; Valente et al., 2004). The link of PRKN and Pink1 to a common pathway was initially established in the fruit fly (Park et al., 2006; Clark et al., 2006, Yang et al., 2006). Parkin was subsequently discovered to promote mitophagy in cultured mammalian cells (Narendra et al., 2008), and PINK1 shown to function upstream of Parkin in the pathway (Geisler et al., 2010; Matsuda et al., 2010; Narendra et al., 2010; Vives-Bauza et al., 2010).
Although loss of the PINK1-Parkin pathway in mice (through single PINK1 or Parkin KO or DKO) does not cause loss of DA neurons or parkinsonism (Kitada et al., 2009), Parkin KO leads to a more typical parkinsonian phenotype on genetic backgrounds with high levels of mitochondrial DNA mutations (Pickrell et al., 2015; Song et al., 2017a). Recent studies from either PINK1 or Parkin KO mice suggest that inflammation triggered by damaged mitochondria may be responsible for the DA neuronal loss and parkinsonism in these models. A recent study from the Youle laboratory found that DA neuronal loss in the Parkin KO/mutator model is caused by inflammation induced by the cGAS-STING1 pathway, likely in response to mitochondrial DNA (mtDNA) released from damaged mitochondria (Sliter et al., 2018). In PINK1-PD and PRKN-PD patients, this model is supported by a correlation between plasma levels of free mtDNA and IL-6, a marker of inflammation observed to be STING-dependent in the PINK1 KO mouse (Borsche et al., 2020; Sliter et al., 2018). Similarly, challenging PINK1 KO mice to a bacterial infection increases inflammation, but in this case by increasing the presentation of mitochondrial antigens. Here, the presented mitochondrial antigens establish cytotoxic CD8+ T-cells directed at mitochondria in DA neuronal axons, leading to terminal DA loss (Matheoud et al., 2016, 2019). Together these studies suggest that inflammation resulting from loss of PINK1-Parkin mitochondrial quality control may drive neurodegeneration in PD.
The most specific biomarker of PINK1 activity is pS65-ubiquitin (Ub), the levels of which can be measured by mass spectrometry, immunoblotting and ELISA (Kane et al., 2014; Kazlauskaite et al., 2014; Koyano et al., 2014; Pickrell et al., 2015; Sliter et al., 2018). Basal pS65-Ub levels are reduced to near undetectable levels in PINK1 KO mice, demonstrating the specificity of pS65-Ub as a PINK1 biomarker and supporting the view that PINK1 is the primary ubiquitin kinase in mammals (at least directed at the S65 site) (Sliter et al., 2018; Watzlawik et al., 2020).
Using the pS65-Ub biomarker, PINK1-Parkin pathway activity was found to increase in the brain with normal aging and was further increased in sporadic forms of the two most common neurodegenerative disorders, PD and Alzheimer's disease (AD). In sporadic PD and related disorders (such as dementia with Lewy bodies), pS65-Ub-positive structures are observed more frequently than in age-matched controls, particularly in the SNpc (Hou et al., 2018; Shiba-Fukushima et al., 2017). pS65-Ub is seen in cells with no or early Lewy bodies, but not in cells with mature dense-core Lewy bodies, suggesting that the PINK1-Parkin pathway is likely activated early in the pathogenesis of sporadic PD (Hou et al., 2018).
As in PD, brain regions affected in AD exhibit increased pS65-Ub structures compared to age-matched controls (Hou et al., 2021). Comparing a series of AD brains with varying degrees of TAU or amyloid β (Aβ) pathology, pS65-Ub-positive structures correlate strongly with the tauopathy but do not independently correlate with Aβ pathology (Hou et al., 2021). A similar correlation between tauopathy and pS65-Ub-positive structures is observed in PD and related Lewy body disorders (Hou et al., 2018). Consistent with this, pS65-Ub-positive structures accumulate in a transgenic mouse model of tauopathy (rTg4510) but not in a transgenic Aβ mouse model (APP/PS1) (Hou et al., 2021). Notably, previous studies have demonstrated greater tauopathy or Aβ pathology in the absence of Parkin or PINK1 (Du et al., 2017; Guerrero et al., 2009; Rodríguez-Navarro et al., 2008), as well as partial suppression of these pathologies upon overexpression of Parkin or PINK1 or activation of mitophagy with small molecules (Hong et al., 2014; Du et al., 2017; Fang et al., 2019), suggesting that PINK1-Parkin-mediated mitophagy most likely represents a protective response in AD. Together these findings suggest that PINK1-Parkin-mediated mitophagy is activated in the AD hippocampus predominately by TAU pathology and is likely protective.
In summary, there is strong evidence for a role of PINK1-Parkin-mediated mitophagy protecting against common neurodegenerative disorders, such as PD and AD.
Mitophagy in cardiac and skeletal muscle
The heart has high concentrations of mitochondria that provide energy needed for repetitive muscle contractions (Pagliarini et al., 2008). Together with mitochondrial fission and fusion, mitophagy helps the heart adapt to stress.
Mitophagy in neonatal heart development
The developing heart undergoes an abrupt metabolic shift in the first days of life as it transitions from glycolytic metabolism to oxidative phosphorylation fueled by fatty acid oxidation (Lopaschuk et al., 1991). This is accompanied by reshaping of the mitochondrial network, driven at least in part by mitochondrial biogenesis (Lai et al., 2008) and mitochondrial dynamics (Ishihara et al., 2015; Kageyama et al., 2014; Papanicolaou et al., 2012).
Mitophagy has also been suggested to play a role in mitochondrial remodeling during this critical neonatal period, although there is conflicting evidence. Using the mito-QC mitophagy reporter mouse (see Box 2), mitophagy was shown to be active in the heart during late embryonic development (E17.5) (McWilliams et al., 2016), although it is not known whether this extends into the first week of life, when mitochondria undergo rapid proliferation. Constitutive Parkin KO mice have normal heart function until at least 1 year of life and Parkin/PINK1 DKO mice have a normal lifespan (Kitada et al., 2009; Kubli et al., 2013). However, Parkin conditional KO (cKO) from the heart at P1 (mediated by the MerCreMer system) causes neonatal lethality in most but not all animals (Gong et al., 2015). This was interpreted by the authors to suggest that Parkin-dependent mitophagy is essential for neonatal heart development, and that its loss may be compensated (by an unidentified mechanism) in the constitutive but not the conditional KO.
Arguing against a role for Parkin-mediated mitophagy in neonatal development, however, is the fact that all tested autophagy genes are dispensable for heart function until at least 38 weeks of age. These include heart cKOs of the ULK1 complex [ULK1 and ULK2 cDKO, or FIP200 (also known as Rb1cc1) cKO] (Li et al., 2021; Wang et al., 2019), the phosphoinositide 3-kinase (PI3K) complex [PI3K3C (also known as Vps34) cKO] (Li et al., 2021), and the late conjugation systems (Atg5 or Atg7 cKO) (Nakai et al., 2007; Saito et al., 2019). As these complexes are essential for conventional autophagy, as well as described forms of alternative autophagy, the viability of adult mice lacking autophagy in the heart suggests that mitophagy is not essential in the neonatal heart. Therefore, it is unclear whether the neonatal lethality in the Parkin cKO reflects loss of mitophagy, loss of a different Parkin function, or, possibly, the reported cardiotoxicity related to the tamoxifen-dependent activation of MerCreMer (Bersell et al., 2013).
Similarly, a form of mitophagy dependent on the mitochondrial fission protein DRP1 has been suggested to be critical for neonatal heart development (Kageyama et al., 2014). Here, Parkin was found to partially compensate for DRP1 cKO in this neonatal period, leading the authors to conclude that parallel Parkin-dependent and -independent mitophagy pathways are activated during neonatal heart development (Kageyama et al., 2014). The argument for a DRP1-dependent mitophagy pathway in the heart rests largely on the appearance of mitophagy intermediates, in the form of p62 (also known as SQSTM1)-labeled mitochondria. However, it is not clear from the data in this paper whether these mitophagy intermediates accumulate in the heart due to loss of mitophagy or as a response to mitochondrial damage incurred by loss of mitochondrial fission. Notably, loss fission in the neonatal heart leads to poorly distributed mtDNA and respiratory complexes, which correlate with mitochondrial damage (Ishihara et al., 2015). In this context, Parkin may partially compensate for DRP1 cKO through mitophagy or through its evolutionarily conserved role in degrading the mitochondrial fusion proteins MFN1 and MFN2 (Poole et al., 2010; Ziviani et al., 2010), as has been observed in the mouse liver upon DRP1 KO (Yamada et al., 2018). Inhibiting mitochondrial fusion may compensate for loss of mitochondrial fission by restoring mitochondrial morphology, albeit at the cost of decreased mixing of mitochondrial components. Such partial rescue has been observed in yeast, mouse heart and mouse liver (Sesaki and Jensen, 2001; Chen et al., 2015; Song et al., 2017b). Thus, the dependence on DRP1 in the neonatal heart may reflect its role in mitochondrial fission for biogenesis rather than mitophagy.
Considered together, while mitochondrial biogenesis and mitochondrial dynamics are essential for neonatal heart development, autophagy appears dispensable, arguing against a critical role for mitophagy in this process.
Mitophagy protects against ischemia in the heart
Tissue ischemia results from decreased blood flow and can lead to infarction if protracted. Mitophagy protects against ischemia in the heart (Diwan et al., 2007; Huang et al., 2011; Kubli et al., 2013), kidney (Tang et al., 2018), and brain (Yuan et al., 2017), although it has been best studied in the heart.
The Parkin-dependent mitophagy pathway has been shown to protect against ischemic injury in the heart induced by vessel ligation (Huang et al., 2011; Kubli et al., 2013). Ischemia leads to increased levels of LC3-II (lipidated LC3 proteins, see Box 1) and Parkin in the borderzone of the infarct, along with decreased levels of mitochondrial proteins (Kubli et al., 2013). Parkin KO mice suffer larger myocardial infarcts than WT mice (Kubli et al., 2013) and gain smaller benefit from ischemic preconditioning (Huang et al., 2011), suggesting that Parkin protects against ischemia by eliminating dysfunctional or vulnerable mitochondria (Huang et al., 2011; Kubli et al., 2013). While Parkin-dependent mitophagy does not appear to play a role during reperfusion injury to the heart (Huang et al., 2011), BNIP3-dependent mitophagy appears to be protective (Diwan et al., 2007).
Similar to ischemia induced by vessel ligation, exhaustive exercise, which induces demand ischemia in the heart, leads to a PINK1-dependent two-fold increase in mitophagy (measured with the mt-Keima reporter) (Liu et al., 2021b; Sliter et al., 2018) and increased levels of the PINK1-Parkin pathway biomarker pS65-Ub in the heart (Sliter et al., 2018). Inhibition of Usp30, which opposes ubiquitylation by Parkin (Bingol et al., 2014), similarly increases mitophagy by about two-fold in the heart (Luo et al., 2021).
More recently, a Parkin- and LC3-independent pathway was identified that is also activated by ischemia and other forms of energy stress (Saito et al., 2019). Using the mt-Keima reporter mouse and transmission electron microscopy, mitophagy in the heart was shown to be upregulated following both starvation and ischemia in a manner that depended on ULK1 and Rab9 phosphorylation (on S179) but not on Atg7. In Rab S179A knock-in (KI) mice, ischemia induced less mitophagy and caused a larger infarct, demonstrating that ULK1 and Rab9 mitophagy protects against ischemia. Mechanistically, ULK1 is phosphorylated at the known S555 activating site by AMP-activated protein kinase (AMPK), suggesting that AMPK senses the energy stress to activate ULK1 in the pathway. ULK1 then assembles in a complex with Rab9, Rip1 and DRP1 to induce mitophagy (Saito et al., 2019). The same group also found that DRP1-dependent mitophagy is activated in response to thoracic aortic constriction (modeling cardiac hypertrophy in response to high systemic blood pressure) (Shirakabe et al., 2016) and cardiac-reperfusion injury (Ikeda et al., 2015), although the latter finding was not reproduced by a separate group (Bouche et al., 2021). Whether a specific mitophagy receptor is involved in this form of alternative mitophagy has yet to be established. Notably, BNIP3 was recently found to mediate hypoxia-induced mitophagy in the zebrafish heart and might be a candidate (Wrighton et al., 2021), as hypoxia has also been found to stimulate the DRP1-dependent pathway in the mouse heart (Saito et al., 2019).
FUNDC1-dependent mitophagy also protects against ischemia but through its effects on platelet function rather than on the ischemic tissues (Zhang et al., 2016). Platelets help seal the injured endothelium of blood vessels to prevent bleeding but can also block blood flow and cause ischemia when excessively or inappropriately activated. FUNDC1 was previously observed to induce mitophagy in cultured cells following hypoxia through a mechanism that involved its dephosphorylation at Tyr18 to increase its binding to LC3-II on autophagophores (Liu et al., 2012). To assess its in vivo function, the same group generated a FUNDC1 KO mouse and assessed mitophagy in response to hypoxia (Zhang et al., 2016). The largest effect was observed in platelets, with smaller effects seen in liver, skeletal muscle and heart. In platelets, hypoxia reduced mitochondrial protein levels by ∼90% with a concomitant increase in LC3-II levels. Both changes depended fully on FUNDC1 and Atg5. Failure of FUNDC1-mediated mitophagy in platelets eliminated the typical downregulation of oxidative phosphorylation during hypoxia and led to abnormal platelet aggregation basally and a failure to increase aggregation in response to hypoxia. Aberrant platelet activation led to greater ischemia-reperfusion injury in the hearts of FUNDC1 KO mice (Zhang et al., 2016).
When comparing FUNDC1 to PINK1-Parkin- and NIX-dependent pathways in vivo, it is striking that FUNDC1 KO blocks the stress-induced accumulation of LC3-II (Zhang et al., 2016), which is not observed with KO of the other mitophagy pathways (Kubli et al., 2013; Tang et al., 2018; Yuan et al., 2017). This suggests that FUNDC1 may be required for the upstream induction of autophagy, as well as having selectivity for mitochondria in hypoxic platelets. FUNDC1 is thought to mediate mitophagy through an interaction with LC3 proteins (Liu et al., 2012); however, this does not explain why FUNDC1 is also required to generate LC3-II in platelets, suggesting that only part of the mechanism is understood.
Together, these findings suggest that redundant mitophagy pathways are likely activated in response to ischemia in the heart to protect the tissue from damaged mitochondria and subsequent infarction.
Regulation of metabolism by mitophagy
Regulating metabolism in mammals depends, in part, on insulin and temperature-responsive organs, including the liver, fat, and skeletal muscle. Failure to adapt metabolism appropriately to excess calories may predispose patients to the metabolic syndrome and associated obesity, type II diabetes, non-alcoholic fatty liver disease (NAFLD) and cardiomyopathy (Eckel et al., 2005), while failure to adapt metabolism to cold or warm environments may led to hypothermia or hyperthermia, respectively. Through regulation of mitochondrial number and quality in these tissues, mitophagy mediated by the PINK1-Parkin and FUNDC1 pathways helps adapt metabolism to changes in diet and external temperature.
Mitophagy in the liver may promote gluconeogenesis
A major metabolic role of the liver in mammals is to produce glucose from amino acids and fatty acids by gluconeogenesis to maintain steady blood glucose levels between feedings. This is promoted by counterregulatory hormones, including glucagon, that increase in response to low blood glucose. Mitophagy may facilitate this response, as first elucidated in a classic study of liver ultrastructure following liver perfusion with glucagon (Ashford and Porter, 1962). At 4 h after glucagon perfusion, lysosomes increase three-fold and nearly all contain a structurally intact mitochondrion or mitochondrial remnants. This process was subsequently coined ‘cellular autophagy’ (Deter et al., 1967). Later on, it was observed that bioenergetically intact mitochondria are selectively degraded by autophagy following glucagon treatment plus nutrient deprivation in primary rat or mouse hepatocytes (Kim et al., 2007; Rodriguez-Enriquez et al., 2006). Autophagy is critical for maintaining plasma glucose and amino acid levels during starvation (triggered by falling insulin in the setting of stable glucagon) (Ezaki et al., 2011). Whether degradation of mitochondria per se is critical for the maintenance of glucose and amino acid levels and how selective degradation of mitochondria is achieved in response to glucagon or starvation is not known.
Mitophagy in the liver and heart under lipid overload
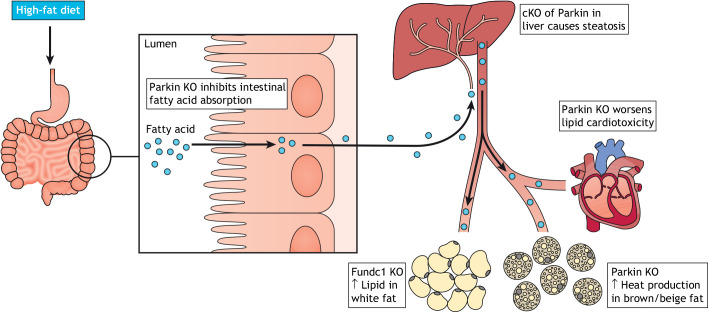
NAFLD and cardiomyopathy both develop as a consequence of excess energy intake and are modeled in mice by feeding with a high-fat diet. The liver is the metabolic hub for fatty acid metabolism, and in conditions of lipid excess, lipid accumulates in hepatocytes (a condition known as steatosis), which can lead to fibrosis, scarring (cirrhosis), type II diabetes through insulin resistance, and hepatocellular carcinoma (Friedman et al., 2018). The heart, as a heavy consumer of fatty acids, is also subjected to increased lipid flux on a high-fat diet. Excess lipids (particularly, in the form of free diacylglycerols and ceramides) cause lipid toxicity and cardiomyopathy. This toxicity can be mitigated by storage of excess fatty acids in lipid droplets and through increased fatty-acid oxidation within mitochondria (reviewed in Olzmann and Carvalho, 2019). Mitophagy may also mitigate against the ill effects of lipid overload (Fig. 2).
Fig. 2.
Effects of Parkin and FUNDC1 on lipid metabolism. Parkin has pleotropic effects on lipid metabolism. Parkin KO diminishes lipid absorption by the small intestine, resulting in reduced lipid accumulating in the liver and adipose in response to a high-fat diet. Conditional KO (cKO) of Parkin in the liver, however, exacerbates lipid overload in response to a high-fat diet. Additionally, Parkin KO exacerbates lipid toxicity in the heart. Parkin also promotes the heat-generating activity of brown and beige fat after cold exposure, as brown and beige fat cells fail to decrease mitochondrial numbers appropriately through mitophagy. FUNDC1 KO affects lipid handling in white fat, resulting in increased weight gain and signs of the metabolic syndrome when animals are placed on a high-fat diet.
Parkin has multiple effects on lipid metabolism. Perhaps counterintuitively, Parkin KO mice resist weight gain on a high-fat diet and are protected from liver steatosis and insulin resistance (Kim et al., 2011). However, the apparent protection of the liver in the Parkin KO mice is due to decreased intestinal lipid absorption (by an unclear mechanism), resulting in a reduced dose of fatty acids seen by the liver (Costa et al., 2016). If the intestinal absorption defect is circumvented by Parkin cKO in the liver, steatosis and insulin resistance develop more readily in Parkin cKO mice than in wild-type mice (Edmunds et al., 2020). Consistent with this, Parkin KO mice are more vulnerable to steatosis in the setting of alcohol abuse, which shares pathophysiological features with damage from excess lipids (Williams et al., 2015).
Similar to what is seen in Parkin liver cKO animals, FUNDC1 KO animals develop increased steatosis and insulin resistance on a high-fat diet, which appears to be due to failed mitophagy in white adipose tissue rather than due to a direct effect of FUNDC1 in the liver (Wu et al., 2019). Thus, Parkin and FUNDC1 protect the liver against steatosis and insulin resistance likely through their promotion of mitophagy in the liver and white adipose tissue, respectively.
Parkin-dependent mitophagy similarly protects the heart against damage from lipid excess. On initiation of a high-fat diet, mitophagy measured with mt-Keima transiently increases in the heart over 6 weeks and then decreases, as the excess lipid state becomes chronic (Tong et al., 2019). This transient increase in mitophagy depends on Atg7 and to a lesser extent on Parkin. Atg7 cKO and Parkin KO mice exhibit decreased mitochondrial function and fail to upregulate β-fatty acid oxidation appropriately, leading to the accumulation of lipid droplets and total triglycerides in the tissue (Tong et al., 2019). Preemptively upregulating mitochondrial fatty acid β-oxidation through cKO of acetyl coenzyme A carboxylase 2 (ACC2; also known as ACACB) prevents mitochondrial dysfunction and damage induced by chronic administration of a high-fat diet (Shao et al., 2020). Additionally, increased fatty acid β-oxidation restores mitophagy (Shao et al., 2020), perhaps by preventing the downregulation of Parkin levels in the chronic phase of high-fat diet administration. Together, these findings suggest that Parkin-dependent mitophagy likely protects against lipid excess in the heart, particularly early during pathology. It also points to a potential vicious cycle whereby a mitophagy deficit leaves mitochondria vulnerable to fatty acid toxicity, which decreases fatty acid β-oxidation, leading to increased tissue fatty acids, which further inhibit mitophagy.
The PINK1-Parkin pathway in brown and beige fat
Brown and beige adipocytes are mitochondria-rich fat cells that produce heat to maintain thermal neutrality and contribute substantially to overall energy balance. In response to cold exposure or β3-adrenergic agonists (which mimic cold exposure), brown fat is activated, and white fat transdifferentiates into beige fat. Following cold exposure, the tissues deacclimate with brown tissue producing less heat and beige fat transdifferentiating back to white fat (Cairó et al., 2019; Lu et al., 2018).
Recent studies demonstrate that Parkin-dependent mitophagy is suppressed during cold acclimation to allow mitochondrial proliferation, and activated during de-acclimatization to clear excess mitochondria from both brown and beige fat cells (Lu et al., 2018; Cairó et al., 2019). In brown fat activated by cold exposure or β3-adrenergic agonists, Parkin is downregulated transcriptionally by a mechanism that depends on lipolysis and Pparδ (Cairó et al., 2019). Consistent with this, Parkin KO increases heat production by brown fat both basally and following cold exposure, and Parkin KO blocks the typical mitochondrial protein reduction that occurs after cold-exposed animals are returned to warmth (Cairó et al., 2019). The increased brown fat activity in Parkin KO animals may protect against weight gain and insulin resistance on a high-fat diet, although decreased intestinal fat absorption in the Parkin KO likely also contributes to these phenotypes (as discussed above) (Costa et al., 2016).
Parkin similarly mediates mitochondrial clearance by mitophagy during the conversion of beige fat back into white fat, when animals are returned to warmth after cold exposure (or this transition is mimicked pharmacologically) (Lu et al., 2018). The reduction in mitochondria coincides with increased mitophagic flux as measured with the mt-Keima reporter and depends on Atg12 and Atg5 (Altshuler-Keylin et al., 2016). Thus, Parkin is downregulated in response to cold exposure to allow mitochondrial proliferation and upregulated when returning from cold to warmth to mediate mitochondrial clearance by mitophagy.
Somewhat counterintuitively, FUNDC1-dependent mitophagy has recently been proposed to be upregulated in brown fat concurrently with cold exposure, when mitochondrial biogenesis is increased (Liu et al., 2021a). However, FUNDC1 KO also blocked mitochondrial biogenesis and Ucp1 expression in response to cold (Liu et al., 2021a,b,c). Thus, these findings could be due to failure of mitochondrial biogenesis and not primarily a failure of mitophagy as the authors suggested. Additional work is needed to clarify why mitochondrial biogenesis depends on FUNDC1 in brown fat.
Mitophagy in skeletal muscle
Skeletal muscle relies on mitophagy following both acute and chronic stresses. Acute stresses include immobilization of a limb or denervation of a muscle, leading to disuse or denervation muscle atrophy, respectively. A week after hindlimb immobilization, mitophagy increases approximately two-fold in the soleus muscle, in conjunction with an increase in oxidative stress (Yamashita et al., 2021). Increased expression of autophagy- and mitophagy-related genes occurs in two waves, with Ulk1 and Nix levels peaking around days 1 to 3 and Prkn peaking around days 3 to 7 (Yamashita et al., 2021). A similar response is seen following denervation of the soleus muscle with an increase in autophagosomes and Prkn expression at 7 days following sciatic nerve transection (Furuya et al., 2014). In both Atg7 cKO and Parkin KO mice, the muscle atrophy that normally occurs by day 7 is delayed, respiratory complex function is reduced and oxidative stress is increased (Furuya et al., 2014). This leads to increased cell death at 14 days after denervation (Furuya et al., 2014), suggesting that mitophagy helps adapt the muscle to its denervated state by reducing mitochondrial numbers and/or clearing damaged mitochondria. Whether NIX-dependent mitophagy also plays a protective role at an early stage in immobilization or denervation muscle injury is currently unknown.
Additionally, upregulating mitophagy may enhance normal muscle performance and improve muscle function in the setting of muscular dystrophy. The natural compound urolithin A (UA) has been identified as stimulating mitochondrial function and lifespan by promoting mitophagy in the round worm (Caenorhabditis elegans) (Ryu et al., 2016). In mice, UA enhances muscle function in the wild-type mice and extends the lifespan of a Duchene's muscular dystrophy model mouse, with improved muscle function and decreased fibrosis in the heart, leg muscle and diaphragm (Luan et al., 2021). Although the precise mechanism of action has not been identified, UA restores expression of Parkin and PINK1 in round worm and mouse models of muscular dystrophy, and PINK1 and Parkin are required for the beneficial effect of UA in round worms (Luan et al., 2021; Ryu et al., 2016). As Parkin levels and mitophagy have been observed to decrease with chronic stress in several models (including high-fat diet and pressure overload in the heart) (Shao et al., 2020; Shirakabe et al., 2016; Tong et al., 2019), derepressing Parkin expression in these pathological conditions might be beneficial for several disorders. Notably, UA was recently found to be well-tolerated in a first-in-human trial for a mitophagy-targeted therapy, with demonstration of target engagement in the muscle (Andreux et al., 2019).
Together these findings suggest that mitophagy is important in protecting muscle against injury (e.g. from denervation) and that upregulation of mitophagy may be beneficial in the treatment of muscular dystrophy or aging-associated muscular atrophy.
Programmed mitochondrial clearance by mitophagy
In programmed mitochondrial clearance, mitochondria are degraded as part of a developmental program. In the most extreme cases, such as in erythrocytes (mature red blood cells) (Tooze and Davies, 1965) and the lens of the eye (Bassnett, 2002), mitochondria are eliminated completely. This allows tight packing of hemoglobin in erythrocytes and clears the lens to refract light.
Programmed mitochondrial clearance is mediated by both NIX-dependent mitophagy and autophagy-independent pathways. NIX-dependent mitophagy mediates programmed mitochondrial clearance in developing erythrocytes (as discussed in mechanistic detail below) and might also mediate (in a small part) programmed mitochondrial clearance from the lens (Brennan et al., 2018). NIX-dependent mitophagy was also recently found to partially clear mitochondria in developing retinal ganglion cells, as they transition to a predominately glycolytic metabolism (Esteban-Martínez et al., 2017). Thus, a principal function of NIX-induced mitophagy is elimination of mitochondria during development, particularly in those tissues that rely primarily on glycolysis in their terminally differentiated state.
Another form of programmed mitochondrial clearance occurs shortly after fertilization, as paternal mitochondria are selectively eliminated from the zygote, ensuring that only maternal mtDNA is passed on. In mammals, these mitochondria are marked by ubiquitin, likely by the PINK1-Parkin pathway, and degraded by mitophagy (with the ubiquitin ligase MUL1 playing a partially redundant role with Parkin) (Rojansky et al., 2016; Sutovsky et al., 1999).
In addition to mitophagy, autophagy-independent mechanisms also contribute to programmed mitochondrial clearance. Both early and late autophagy complexes are largely dispensable for mitochondrial clearance from the lens, which instead depends on degradation of mitochondrial membranes by the phospholipase A and acyltransferase (PLAAT) phospholipases, in an autophagy-independent pathway (Morishita et al., 2013, 2021). Likewise, while mitophagy is needed to efficiently clear mitochondria from developing erythrocytes, most adult erythrocytes still eliminate their mitochondria in its absence (Schweers et al., 2007; Sandoval et al., 2008; Kundu et al., 2008). Whether a redundant non-autophagy mechanism exists in reticulocytes, such as that mediated by PLAAT phospholipases in the lens, has yet to be established.
Thus, mitophagy serves as one (but not the only) mechanism mediating programmed mitochondrial clearance during development.
Mitochondrial elimination by the NIX-ULK1 pathway during red blood cell maturation
Perhaps the best-studied example of mitophagy in mammals is the elimination of mitochondria from reticulocytes as they terminally differentiate into erythrocytes with a purely glycolytic metabolism (Sandoval et al., 2008; Schweers et al., 2007). Mitochondria, which are required to synthesize heme in the oxygen-carrying hemoglobin, are the last major organelle to be eliminated from reticulocyte as it matures. This transition coincides with a transcriptionally driven increase in NIX (Schweers et al., 2007), a protein on the OMM that contains both a minimal essential region for mitophagy and a separate LIR motif (Novak et al., 2010; Zhang et al., 2012). Consistent with there being a specific role for NIX in mitochondrial clearance, ∼40% of erythrocytes from adult NIX KO mice retain their mitochondria in vivo (Sandoval et al., 2008; Schweers et al., 2007).
Autophagosome formation also increases during reticulocyte development and is critical for mitophagy. Interestingly, here, mitophagy depends on ULK1 (the sole ULK1/2 paralog expressed in reticulocytes) but appears to be independent of the late conjugation systems, as ULK1 KO but not Atg5 cKO mice retain mitochondria to a similar extent to NIX KO mice (Honda et al., 2014; Joo et al., 2011; Kundu et al., 2008; Matsui et al., 2006). Additionally, although LC3-II-coated vesicles form, they do not appear to associate with mitochondria (Honda et al., 2014), and Atg7 cKO, which disrupts both late conjugation systems, only weakly blocks mitochondrial clearance (Zhang et al., 2009). Based on the ULK1 dependence and independence of Atg5 and/or Atg7, mitophagy in reticulocyte maturation has been suggested to occur by a form of alternative autophagy, differentiating it from the LC3-dependent conventional autophagy (Honda et al., 2014). Like other forms of alternative autophagy, mitophagy in reticulocytes is additionally blocked by PI3K complex inhibitors (Kundu et al., 2008; Honda et al., 2014; Zhu et al., 2014).
Phosphorylation of ULK1 at S555 by AMPK is at least partially required for ULK1 activity in reticulocytes, as KO of Prkaa1, the catalytic subunit of AMPK, in mice also decreases clearance of mitochondria from reticulocytes (although to a lesser extent than ULK1 KO) (Zhu et al., 2014). In the absence of phosphorylation, ULK1 exhibits reduced association with Fip200 in the ULK1 complex, and the PI3K complex does not fully assemble (Zhu et al., 2014). As with many serine/threonine kinases, ULK1 is also dependent on Hsp90A family proteins and their co-chaperone Cdc37 for full kinase activity (Joo et al., 2011). ULK1-dependent autophagy appears to be particularly important for programmed mitochondrial clearance after adult acute anemic stress induced with the haemolytic agent phenylhydrazine and in fetal erythrocyte maturation, but is less important in adult maintenance and embryonic erythrocyte maturation (Honda et al., 2014). This points to redundant (and potentially autophagy independent) mechanisms for programmed mitochondrial clearance in the adult and embryo.
Electron microscopy shows that in animals lacking NIX or ULK1, autophagic membranes form and appear to loosely associate with mitochondria (Honda et al., 2014; Kundu et al., 2008; Sandoval et al., 2008; Schweers et al., 2007). However, mitochondria are not captured within the autophagic membranes, thereby eluding delivery to the lysosome/vacuole or the extracellular space. The autophagic membranes have a different appearance in NIX KO and ULK1 KO animals. In ULK1 KO animals, an extended phagophore (a long flattened tubular structure) develops but does not enclose the attached mitochondria to form a spherical autophagosome (Honda et al., 2014; Kundu et al., 2008). By contrast, in NIX KO animals, an autophagosome forms but mitochondria attach to the cytosolic rather than the luminal surface, giving the appearance in thin section of medallions on a necklace (Sandoval et al., 2008; Schweers et al., 2007). Thus, NIX is not required for autophagosome formation per se but rather guides mitochondrial capture, whereas ULK1 is required to form a mature autophagosome.
The mechanism by which NIX functions as a mitophagy receptor is not entirely clear. NIX has an LIR motif that directly binds to LC3 proteins in vitro (Novak et al., 2010). However, this motif is not strictly required for its function (Zhang et al., 2012). Additionally, as NIX can mediate mitophagy in the absence of LC3 conjugation (e.g. in Atg5 or Atg7 cKO mice), an interaction with LC3 proteins must be dispensable for NIX-mediated mitophagy (Matsui et al., 2006). Instead, the minimal essential region (MER) of NIX comprises a small region C-terminal to the LIR (spanning residues 70 to 86 with an essential function for L74) (Zhang et al., 2012). This region and anchoring to the mitochondria are the only clear requirements for NIX-dependent mitophagy in developing erythrocytes. Additionally, the NIX ortholog BNIP3 can substitute for NIX in mouse reticulocytes, suggesting that BNIP3 and NIX likely promote mitophagy by a shared mechanism at least in reticulocytes (Zhang et al., 2012). However, BNIP3-mediated compensation of NIX has not been observed in all systems (Esteban-Martínez et al., 2017; Lampert et al., 2019; Yazdankhah et al., 2021). Together these findings suggest a model in which NIX mediates mitophagy by connecting the mitochondria to the autophagosome as a receptor. What NIX binds to on the autophagosome, however, is not clear, as LC3 binding is dispensable.
Concluding remarks
Since NIX, BNIP3 and Parkin were described as the first components of pathways mediating mammalian mitophagy over a decade ago, our understanding of mitophagy in vivo has greatly expanded. These pathways have been shown to maintain mitochondrial quality in response to stress and to reduce mitochondrial numbers when they are not needed. Additional mitophagy receptors, such as FUNDC1, have been reported to mediate mitophagy in vivo. Mitophagy has been shown to protect against neurodegeneration, cardiomyopathy, metabolic syndrome and anemia. Some initial assumptions have been challenged and, as a result, have generated remaining questions for the field (Box 4). Moreover, it is notable that the first mitophagy-targeted therapies have entered human trials with the hope that upregulating mitophagy might help manage these risky assets (Andreux et al., 2019).
Box 4. Outstanding questions.
How do mitophagy receptors mediate mitophagy? Although the presence of LIRs was initially used to identify many mitophagy receptors, binding to LC3 proteins may not be essential for their action. Indeed, OPTN and NDP52 directly recruit early autophagy complexes independently of LC3 binding (Vargas et al., 2019; Yamano et al., 2020). Are similar mechanisms also mediating the action of NIX, which does not need its LIR for mitophagy in reticulocytes, and FUNDC1, which promotes not only mitophagy but also LC3-II conjugation following hypoxia in platelets (Zhang et al., 2012, 2016)?
Why do some forms of mitophagy depend on late conjugation systems but others do not? The PINK1-Parkin pathway appears to rely on the late conjugation systems in vivo, whereas NIX does not in reticulocytes (Honda et al., 2014; Matsui et al., 2006; Zhang et al., 2009). Is this a general feature of NIX or are reticulocytes a special case?
Which non-autophagy pathways mediate mitochondrial clearance? Surprisingly, in the lens of the eye, autophagy is dispensable for mitochondrial clearance (Morishita et al., 2013). Instead, PLAAT phospholipases clear mitochondria through degradation of their membranes (Morishita et al., 2021). Could a similar mechanism account for mitochondrial clearance in autophagy-deficient reticulocytes? Are these non-autophagy mechanisms of mitochondrial clearance deployed only when all mitochondria must be eliminated, or might they function in other tissues?
What injures a tissue when mitophagy is impaired? Is the primary injury due to energy failure, oxidative stress, or, as has been recently highlighted (Matheoud et al., 2016, 2019; Sliter et al., 2018), DAMPs that cause secondary damage by triggering inflammation?
Finally, can mitophagy be safely upregulated while maintaining its selectivity for impaired mitochondria? Several strategies hold therapeutic promise. Parkin activity has been upregulated in the heart with a USP30 inhibitor (Luo et al., 2021). The Parkin-PINK1 pathway has been derepressed in muscle with UA treatment (Luan et al., 2021; Ryu et al., 2016). Small peptides derived from BECLIN1 and FUNDC1 have increased mitophagy in the heart and platelets (Shirakabe et al., 2016; Zhang et al., 2016). Will one or more of these strategies prove safe and effective in patients?
Acknowledgements
I thank Yi-Ting Ling for help with initial planning of the Review, and Richard Youle and Julia Thayer for a careful reading of and insightful comments on the manuscript. Fig. 2 was created with Biorender.com.
Footnotes
Competing interests
The author declares no competing or financial interests.
Funding
My work is supported by the Intramural Research Program of the NINDS, National Institutes of Health. Deposited in PMC for release after 12 months.
References
- Allen, G. F. G., Toth, R., James, J. and Ganley, I. G. (2013). Loss of iron triggers PINK1/Parkin-independent mitophagy. EMBO Rep. 14, 1127-1135. 10.1038/embor.2013.168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altshuler-Keylin, S., Shinoda, K., Hasegawa, Y., Ikeda, K., Hong, H., Kang, Q., Yang, Y., Perera, R. M., Debnath, J. and Kajimura, S. (2016). Beige adipocyte maintenance is regulated by autophagy-induced mitochondrial clearance. Cell Metab. 24, 402-419. 10.1016/j.cmet.2016.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreux, P. A., Blanco-Bose, W., Ryu, D., Burdet, F., Ibberson, M., Aebischer, P., Auwerx, J., Singh, A. and Rinsch, C. (2019). The mitophagy activator urolithin A is safe and induces a molecular signature of improved mitochondrial and cellular health in humans. Nat. Metab. 1, 595-603. 10.1038/s42255-019-0073-4 [DOI] [PubMed] [Google Scholar]
- Ashford, T. P. and Porter, K. R. (1962). Cytoplasmic components in hepatic cell lysosomes. J. Cell Biol. 12, 198-202. 10.1083/jcb.12.1.198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassnett, S. (2002). Lens organelle degradation. Exp. Eye Res. 74, 1-6. 10.1006/exer.2001.1111 [DOI] [PubMed] [Google Scholar]
- Bersell, K., Choudhury, S., Mollova, M., Polizzotti, B. D., Ganapathy, B., Walsh, S., Wadugu, B., Arab, S. and Kühn, B. (2013). Moderate and high amounts of tamoxifen in αMHC-MerCreMer mice induce a DNA damage response, leading to heart failure and death. Dis. Model. Mech. 6, 1459-1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingol, B., Tea, J. S., Phu, L., Reichelt, M., Bakalarski, C. E., Song, Q., Foreman, O., Kirkpatrick, D. S. and Sheng, M. (2014). The mitochondrial deubiquitinase USP30 opposes parkin-mediated mitophagy. Nature 510, 370-375. 10.1038/nature13418 [DOI] [PubMed] [Google Scholar]
- Borsche, M., König, I. R., Delcambre, S., Petrucci, S., Balck, A., Brüggemann, N., Zimprich, A., Wasner, K., Pereira, S. L., Avenali, M.et al. (2020). Mitochondrial damage-associated inflammation highlights biomarkers in PRKN/PINK1 parkinsonism. Brain J. Neurol. 143, 3041-3051. 10.1093/brain/awaa246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouche, L., Kamel, R., Tamareille, S., Garcia, G., Villedieu, C., Pillot, B., Gueguen, N., Chehaitly, A., Chao de la Barca, J. M., Beaumont, J.et al. (2021). DRP1 haploinsufficiency attenuates cardiac ischemia/reperfusion injuries. PloS One 16, e0248554. 10.1371/journal.pone.0248554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan, L. A., McGreal-Estrada, R., Logan, C. M., Cvekl, A., Menko, A. S. and Kantorow, M. (2018). BNIP3L/NIX is required for elimination of mitochondria, endoplasmic reticulum and Golgi apparatus during eye lens organelle-free zone formation. Exp. Eye Res. 174, 173-184. 10.1016/j.exer.2018.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairó, M., Campderrós, L., Gavaldà-Navarro, A., Cereijo, R., Delgado-Anglés, A., Quesada-López, T., Giralt, M., Villarroya, J. and Villarroya, F. (2019). Parkin controls brown adipose tissue plasticity in response to adaptive thermogenesis. EMBO Rep 20, e46832. 10.15252/embr.201846832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, H., Detmer, S. A., Ewald, A. J., Griffin, E. E., Fraser, S. E. and Chan, D. C. (2003). Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J. Cell Biol. 160, 189-200. 10.1083/jcb.200211046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, H., Ren, S., Clish, C., Jain, M., Mootha, V., McCaffery, J. M. and Chan, D. C. (2015). Titration of mitochondrial fusion rescues Mff-deficient cardiomyopathy. J. Cell Biol. 211, 795-805. 10.1083/jcb.201507035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark, I. E., Dodson, M. W., Jiang, C., Cao, J. H., Huh, J. R., Seol, J. H., Yoo, S. J., Hay, B. A. and Guo, M. (2006). Drosophila pink1 is required for mitochondrial function and interacts genetically with parkin. Nature 441, 1162-1166. 10.1038/nature04779 [DOI] [PubMed] [Google Scholar]
- Collins, M. L., Eng, S., Hoh, R. and Hellerstein, M. K. (2003). Measurement of mitochondrial DNA synthesis in vivo using a stable isotope-mass spectrometric technique. J. Appl. Physiol. 94, 2203-2211. [DOI] [PubMed] [Google Scholar]
- Costa, D. K., Huckestein, B. R., Edmunds, L. R., Petersen, M. C., Nasiri, A., Butrico, G. M., Abulizi, A., Harmon, D. B., Lu, C., Mantell, B. S.et al. (2016). Reduced intestinal lipid absorption and body weight-independent improvements in insulin sensitivity in high-fat diet-fed Park2 knockout mice. Am. J. Physiol. Endocrinol. Metab. 311, E105-E116. 10.1152/ajpendo.00042.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies, V. J., Hollins, A. J., Piechota, M. J., Yip, W., Davies, J. R., White, K. E., Nicols, P. P., Boulton, M. E. and Votruba, M. (2007). Opa1 deficiency in a mouse model of autosomal dominant optic atrophy impairs mitochondrial morphology, optic nerve structure and visual function. Hum. Mol. Genet. 16, 1307-1318. 10.1093/hmg/ddm079 [DOI] [PubMed] [Google Scholar]
- Deter, R. L., Baudhuin, P. and de Duve, C. (1967). Participation of lysosomes in cellular autophagy induced in rat liver by glucagon. J. Cell Biol. 35, C11-C16. 10.1083/jcb.35.2.C11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diwan, A., Krenz, M., Syed, F. M., Wansapura, J., Ren, X., Koesters, A. G., Li, H., Kirshenbaum, L. A., Hahn, H. S., Robbins, J.et al. (2007). Inhibition of ischemic cardiomyocyte apoptosis through targeted ablation of Bnip3 restrains postinfarction remodeling in mice. J. Clin. Invest. 117, 2825-2833. 10.1172/JCI32490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du, F., Yu, Q., Yan, S., Hu, G., Lue, L.-F., Walker, D. G., Wu, L., Yan, S. F., Tieu, K. and Yan, S. S. (2017). PINK1 signalling rescues amyloid pathology and mitochondrial dysfunction in Alzheimer's disease. Brain J. Neurol. 140, 3233-3251. 10.1093/brain/awx258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckel, R. H., Grundy, S. M. and Zimmet, P. Z. (2005). The metabolic syndrome. Lancet Lond. Engl. 365, 1415-1428. 10.1016/S0140-6736(05)66378-7 [DOI] [PubMed] [Google Scholar]
- Edmunds, L. R., Xie, B., Mills, A. M., Huckestein, B. R., Undamatla, R., Murali, A., Pangburn, M. M., Martin, J., Sipula, I., Kaufman, B. A.et al. (2020). Liver-specific Prkn knockout mice are more susceptible to diet-induced hepatic steatosis and insulin resistance. Mol. Metab. 41, 101051. 10.1016/j.molmet.2020.101051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteban-Martínez, L., Sierra-Filardi, E., McGreal, R. S., Salazar-Roa, M., Mariño, G., Seco, E., Durand, S., Enot, D., Graña, O., Malumbres, M.et al. (2017). Programmed mitophagy is essential for the glycolytic switch during cell differentiation. EMBO J. 36, 1688-1706. 10.15252/embj.201695916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezaki, J., Matsumoto, N., Takeda-Ezaki, M., Komatsu, M., Takahashi, K., Hiraoka, Y., Taka, H., Fujimura, T., Takehana, K., Yoshida, M.et al. (2011). Liver autophagy contributes to the maintenance of blood glucose and amino acid levels. Autophagy 7, 727-736. 10.4161/auto.7.7.15371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang, E. F., Hou, Y., Palikaras, K., Adriaanse, B. A., Kerr, J. S., Yang, B., Lautrup, S., Hasan-Olive, M. M., Caponio, D., Dan, X.et al. (2019). Mitophagy inhibits amyloid-β and tau pathology and reverses cognitive deficits in models of Alzheimer's disease. Nat. Neurosci. 22, 401-412. 10.1038/s41593-018-0332-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman, S. L., Neuschwander-Tetri, B. A., Rinella, M. and Sanyal, A. J. (2018). Mechanisms of NAFLD development and therapeutic strategies. Nat. Med. 24, 908-922. 10.1038/s41591-018-0104-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuya, N., Ikeda, S.-I., Sato, S., Soma, S., Ezaki, J., Oliva Trejo, J. A., Takeda-Ezaki, M., Fujimura, T., Arikawa-Hirasawa, E., Tada, N.et al. (2014). PARK2/Parkin-mediated mitochondrial clearance contributes to proteasome activation during slow-twitch muscle atrophy via NFE2L1 nuclear translocation. Autophagy 10, 631-641. 10.4161/auto.27785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganley, I. G., Whitworth, A. J. and McWilliams, T. G. (2021). Comment on “mt-Keima detects PINK1-PRKN mitophagy in vivo with greater sensitivity than mito-QC”. Autophagy. 10.1080/15548627.2021.1907269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gegg, M. E., Cooper, J. M., Chau, K.-Y., Rojo, M., Schapira, A. H. V. and Taanman, J.-W. (2010). Mitofusin 1 and mitofusin 2 are ubiquitinated in a PINK1/parkin-dependent manner upon induction of mitophagy. Hum. Mol. Genet. 19, 4861-4870. 10.1093/hmg/ddq419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler, S., Holmström, K. M., Skujat, D., Fiesel, F. C., Rothfuss, O. C., Kahle, P. J. and Springer, W. (2010). PINK1/Parkin-mediated mitophagy is dependent on VDAC1 and p62/SQSTM1. Nat. Cell Biol. 12, 119-131. 10.1038/ncb2012 [DOI] [PubMed] [Google Scholar]
- Gladkova, C., Maslen, S. L., Skehel, J. M. and Komander, D. (2018). Mechanism of parkin activation by PINK1. Nature 559, 410-414. 10.1038/s41586-018-0224-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg, M. S., Fleming, S. M., Palacino, J. J., Cepeda, C., Lam, H. A., Bhatnagar, A., Meloni, E. G., Wu, N., Ackerson, L. C., Klapstein, G. J.et al. (2003). Parkin-deficient mice exhibit nigrostriatal deficits but not loss of dopaminergic neurons. J. Biol. Chem. 278, 43628-43635. 10.1074/jbc.M308947200 [DOI] [PubMed] [Google Scholar]
- Gong, G., Song, M., Csordas, G., Kelly, D. P., Matkovich, S. J. and Dorn, G. W. (2015). Parkin-mediated mitophagy directs perinatal cardiac metabolic maturation in mice. Science 350, aad2459. 10.1126/science.aad2459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubas, A. and Dikic, I. (2021). A guide to the regulation of selective autophagy receptors. FEBS J. 10.1111/febs.15824 [DOI] [PubMed] [Google Scholar]
- Guerrero, R., Navarro, P., Gallego, E., Garcia-Cabrero, A. M., Avila, J. and Sanchez, M. P. (2009). Hyperphosphorylated tau aggregates in the cortex and hippocampus of transgenic mice with mutant human FTDP-17 Tau and lacking the PARK2 gene. Acta Neuropathol. 117, 159-168. [DOI] [PubMed] [Google Scholar]
- Heo, J.-M., Ordureau, A., Paulo, J. A., Rinehart, J. and Harper, J. W. (2015). The PINK1-PARKIN mitochondrial ubiquitylation pathway drives a program of OPTN/NDP52 recruitment and TBK1 activation to promote mitophagy. Mol. Cell 60, 7-20. 10.1016/j.molcel.2015.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda, S., Arakawa, S., Nishida, Y., Yamaguchi, H., Ishii, E. and Shimizu, S. (2014). ULK1-mediated Atg5-independent macroautophagy mediates elimination of mitochondria from embryonic reticulocytes. Nat. Commun. 5, 4004. 10.1038/ncomms5004 [DOI] [PubMed] [Google Scholar]
- Hong, X., Liu, J., Zhu, G., Zhuang, Y., Suo, H., Wang, P., Huang, D., Xu, J., Huang, Y., Yu, M.et al. (2014). Parkin overexpression ameliorates hippocampal long-term potentiation and β-amyloid load in an Alzheimer's disease mouse model. Hum. Mol. Genet. 23, 1056-1072. 10.1093/hmg/ddt501 [DOI] [PubMed] [Google Scholar]
- Hou, X., Fiesel, F. C., Truban, D., Castanedes Casey, M., Lin, W.-L., Soto, A. I., Tacik, P., Rousseau, L. G., Diehl, N. N., Heckman, M. G.et al. (2018). Age- and disease-dependent increase of the mitophagy marker phospho-ubiquitin in normal aging and Lewy body disease. Autophagy 14, 1404-1418. 10.1080/15548627.2018.1461294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou, X., Watzlawik, J. O., Cook, C., Liu, C.-C., Kang, S. S., Lin, W.-L., DeTure, M., Heckman, M. G., Diehl, N. N. and Al-Shaikh, F. S. H. (2021). Mitophagy alterations in Alzheimer's disease are associated with granulovacuolar degeneration and early tau pathology. Alzheimers Dement. 17, 417-430. 10.1002/alz.12198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, C., Andres, A. M., Ratliff, E. P., Hernandez, G., Lee, P. and Gottlieb, R. A. (2011). Preconditioning involves selective mitophagy mediated by Parkin and p62/SQSTM1. PloS One 6, e20975. 10.1371/journal.pone.0020975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda, Y., Shirakabe, A., Maejima, Y., Zhai, P., Sciarretta, S., Toli, J., Nomura, M., Mihara, K., Egashira, K., Ohishi, M.et al. (2015). Endogenous Drp1 mediates mitochondrial autophagy and protects the heart against energy stress. Circ. Res. 116, 264-278. 10.1161/CIRCRESAHA.116.303356 [DOI] [PubMed] [Google Scholar]
- Ishihara, N., Nomura, M., Jofuku, A., Kato, H., Suzuki, S. O., Masuda, K., Otera, H., Nakanishi, Y., Nonaka, I., Goto, Y.et al. (2009). Mitochondrial fission factor Drp1 is essential for embryonic development and synapse formation in mice. Nat. Cell Biol. 11, 958-966. 10.1038/ncb1907 [DOI] [PubMed] [Google Scholar]
- Ishihara, T., Ban-Ishihara, R., Maeda, M., Matsunaga, Y., Ichimura, A., Kyogoku, S., Aoki, H., Katada, S., Nakada, K., Nomura, M.et al. (2015). Dynamics of mitochondrial DNA nucleoids regulated by mitochondrial fission is essential for maintenance of homogeneously active mitochondria during neonatal heart development. Mol. Cell. Biol. 35, 211-223. 10.1128/MCB.01054-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itakura, E., Kishi-Itakura, C., Koyama-Honda, I. and Mizushima, N. (2012). Structures containing Atg9A and the ULK1 complex independently target depolarized mitochondria at initial stages of Parkin-mediated mitophagy. J. Cell Sci. 125, 1488-1499. [DOI] [PubMed] [Google Scholar]
- Joo, J. H., Dorsey, F. C., Joshi, A., Hennessy-Walters, K. M., Rose, K. L., McCastlain, K., Zhang, J., Iyengar, R., Jung, C. H., Suen, D.-F.et al. (2011). Hsp90-Cdc37 chaperone complex regulates ULK1- and Atg13-mediated mitophagy. Mol. Cell 43, 572-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kageyama, Y., Hoshijima, M., Seo, K., Bedja, D., Sysa-Shah, P., Andrabi, S. A., Chen, W., Höke, A., Dawson, V. L., Dawson, T. M.et al. (2014). Parkin-independent mitophagy requires Drp1 and maintains the integrity of mammalian heart and brain. EMBO J. 33, 2798-2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane, L. A., Lazarou, M., Fogel, A. I., Li, Y., Yamano, K., Sarraf, S. A., Banerjee, S. and Youle, R. J. (2014). PINK1 phosphorylates ubiquitin to activate Parkin E3 ubiquitin ligase activity. J. Cell Biol. 205, 143-153. 10.1083/jcb.201402104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama, H., Kogure, T., Mizushima, N., Yoshimori, T. and Miyawaki, A. (2011). A sensitive and quantitative technique for detecting autophagic events based on lysosomal delivery. Chem. Biol. 18, 1042-1052. 10.1016/j.chembiol.2011.05.013 [DOI] [PubMed] [Google Scholar]
- Katayama, H., Hama, H., Nagasawa, K., Kurokawa, H., Sugiyama, M., Ando, R., Funata, M., Yoshida, N., Homma, M., Nishimura, T.et al. (2020). Visualizing and modulating mitophagy for therapeutic studies of neurodegeneration. Cell 181, 1176-1187.e16. 10.1016/j.cell.2020.04.025 [DOI] [PubMed] [Google Scholar]
- Kazlauskaite, A., Kondapalli, C., Gourlay, R., Campbell, D. G., Ritorto, M. S., Hofmann, K., Alessi, D. R., Knebel, A., Trost, M. and Muqit, M. M. K. (2014). Parkin is activated by PINK1-dependent phosphorylation of ubiquitin at Ser65. Biochem. J. 460, 127-139. 10.1042/BJ20140334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, I., Rodriguez-Enriquez, S. and Lemasters, J. J. (2007). Selective degradation of mitochondria by mitophagy. Arch. Biochem. Biophys. 462, 245-253. 10.1016/j.abb.2007.03.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, K.-Y., Stevens, M. V., Akter, M. H., Rusk, S. E., Huang, R. J., Cohen, A., Noguchi, A., Springer, D., Bocharov, A. V., Eggerman, T. L.et al. (2011). Parkin is a lipid-responsive regulator of fat uptake in mice and mutant human cells. J. Clin. Invest. 121, 3701-3712. 10.1172/JCI44736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitada, T., Asakawa, S., Hattori, N., Matsumine, H., Yamamura, Y., Minoshima, S., Yokochi, M., Mizuno, Y. and Shimizu, N. (1998). Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature 392, 605-608. 10.1038/33416 [DOI] [PubMed] [Google Scholar]
- Kitada, T., Pisani, A., Porter, D. R., Yamaguchi, H., Tscherter, A., Martella, G., Bonsi, P., Zhang, C., Pothos, E. N. and Shen, J. (2007). Impaired dopamine release and synaptic plasticity in the striatum of PINK1-deficient mice. Proc. Natl. Acad. Sci. USA 104, 11441-11446. 10.1073/pnas.0702717104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitada, T., Tong, Y., Gautier, C. A. and Shen, J. (2009). Absence of nigral degeneration in aged parkin/DJ-1/PINK1 triple knockout mice. J. Neurochem. 111, 696-702. 10.1111/j.1471-4159.2009.06350.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondapalli, C., Kazlauskaite, A., Zhang, N., Woodroof, H. I., Campbell, D. G., Gourlay, R., Burchell, L., Walden, H., Macartney, T. J., Deak, M.et al. (2012). PINK1 is activated by mitochondrial membrane potential depolarization and stimulates Parkin E3 ligase activity by phosphorylating Serine 65. Open Biol. 2, 120080. 10.1098/rsob.120080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyano, F., Okatsu, K., Kosako, H., Tamura, Y., Go, E., Kimura, M., Kimura, Y., Tsuchiya, H., Yoshihara, H., Hirokawa, T.et al. (2014). Ubiquitin is phosphorylated by PINK1 to activate parkin. Nature 510, 162-166. 10.1038/nature13392 [DOI] [PubMed] [Google Scholar]
- Kubli, D. A., Zhang, X., Lee, Y., Hanna, R. A., Quinsay, M. N., Nguyen, C. K., Jimenez, R., Petrosyan, S., Murphy, A. N. and Gustafsson, A. B. (2013). Parkin protein deficiency exacerbates cardiac injury and reduces survival following myocardial infarction. J. Biol. Chem. 288, 915-926. 10.1074/jbc.M112.411363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuma, A., Komatsu, M. and Mizushima, N. (2017). Autophagy-monitoring and autophagy-deficient mice. Autophagy 13, 1619-1628. 10.1080/15548627.2017.1343770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundu, M., Lindsten, T., Yang, C.-Y., Wu, J., Zhao, F., Zhang, J., Selak, M. A., Ney, P. A. and Thompson, C. B. (2008). Ulk1 plays a critical role in the autophagic clearance of mitochondria and ribosomes during reticulocyte maturation. Blood 112, 1493-1502. 10.1182/blood-2008-02-137398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai, L., Leone, T. C., Zechner, C., Schaeffer, P. J., Kelly, S. M., Flanagan, D. P., Medeiros, D. M., Kovacs, A. and Kelly, D. P. (2008). Transcriptional coactivators PGC-1α and PGC-lβ control overlapping programs required for perinatal maturation of the heart. Genes Dev. 22, 1948-1961. 10.1101/gad.1661708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampert, M. A., Orogo, A. M., Najor, R. H., Hammerling, B. C., Leon, L. J., Wang, B. J., Kim, T., Sussman, M. A. and Gustafsson, Å. B. (2019). BNIP3L/NIX and FUNDC1-mediated mitophagy is required for mitochondrial network remodeling during cardiac progenitor cell differentiation. Autophagy 15, 1182-1198. 10.1080/15548627.2019.1580095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarou, M., Sliter, D. A., Kane, L. A., Sarraf, S. A., Wang, C., Burman, J. L., Sideris, D. P., Fogel, A. I. and Youle, R. J. (2015). The ubiquitin kinase PINK1 recruits autophagy receptors to induce mitophagy. Nature 524, 309-314. 10.1038/nature14893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, D., Vogel, P., Li-Harms, X., Wang, B. and Kundu, M. (2021). ATG14 and RB1CC1 play essential roles in maintaining muscle homeostasis. Autophagy. 10.1080/15548627.2021.1911549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, L., Feng, D., Chen, G., Chen, M., Zheng, Q., Song, P., Ma, Q., Zhu, C., Wang, R., Qi, W.et al. (2012). Mitochondrial outer-membrane protein FUNDC1 mediates hypoxia-induced mitophagy in mammalian cells. Nat. Cell Biol. 14, 177-185. 10.1038/ncb2422 [DOI] [PubMed] [Google Scholar]
- Liu, L., Li, Y., Wang, J., Zhang, D., Wu, H., Li, W., Wei, H., Ta, N., Fan, Y., Liu, Y.et al. (2021a). Mitophagy receptor FUNDC1 is regulated by PGC-1α/NRF1 to fine tune mitochondrial homeostasis. EMBO Rep. 22, e50629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y.-T., Sliter, D. A., Shammas, M. K., Huang, X., Wang, C., Calvelli, H., Maric, D. S. and Narendra, D. P. (2021b). Mt-Keima detects PINK1-PRKN mitophagy in vivo with greater sensitivity than mito-QC. Autophagy. 10.1080/15548627.2021.1896924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y.-T., Sliter, D. A., Shammas, M. K., Huang, X., Wang, C., Calvelli, H., Maric, S. D. and Narendra, D. P. (2021c). Comment on “mt-Keima detects PINK1-PRKN mitophagy in vivo with greater sensitivity than mito-QC”. Autophagy. 10.1080/15548627.2021.1907269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopaschuk, G. D., Spafford, M. A. and Marsh, D. R. (1991). Glycolysis is predominant source of myocardial ATP production immediately after birth. Am. J. Physiol. 261, H1698-H1705. [DOI] [PubMed] [Google Scholar]
- Lu, X., Altshuler-Keylin, S., Wang, Q., Chen, Y., Henrique Sponton, C., Ikeda, K., Maretich, P., Yoneshiro, T. and Kajimura, S. (2018). Mitophagy controls beige adipocyte maintenance through a Parkin-dependent and UCP1-independent mechanism. Sci. Signal. 11, eaap8526. 10.1126/scisignal.aap8526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan, P., D'Amico, D., Andreux, P. A., Laurila, P.-P., Wohlwend, M., Li, H., Imamura de Lima, T., Place, N., Rinsch, C., Zanou, N.et al. (2021). Urolithin A improves muscle function by inducing mitophagy in muscular dystrophy. Sci. Transl. Med. 13, eabb0319. 10.1126/scitranslmed.abb0319 [DOI] [PubMed] [Google Scholar]
- Luo, H., Krigman, J., Zhang, R., Yang, M. and Sun, N. (2021). Pharmacological inhibition of USP30 activates tissue-specific mitophagy. Acta Physiol. Oxf. Engl. 232. e13666. 10.1111/apha.13666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinković, M., Šprung, M. and Novak, I. (2021). Dimerization of mitophagy receptor BNIP3L/NIX is essential for recruitment of autophagic machinery. Autophagy 17, 1232-1243. 10.1080/15548627.2020.1755120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matheoud, D., Sugiura, A., Bellemare-Pelletier, A., Laplante, A., Rondeau, C., Chemali, M., Fazel, A., Bergeron, J. J., Trudeau, L.-E., Burelle, Y.et al. (2016). Parkinson's disease-related proteins PINK1 and Parkin repress mitochondrial antigen presentation. Cell 166, 314-327. 10.1016/j.cell.2016.05.039 [DOI] [PubMed] [Google Scholar]
- Matheoud, D., Cannon, T., Voisin, A., Penttinen, A.-M., Ramet, L., Fahmy, A. M., Ducrot, C., Laplante, A., Bourque, M.-J., Zhu, L.et al. (2019). Intestinal infection triggers Parkinson's disease-like symptoms in Pink1−/− mice. Nature 571, 565-569. 10.1038/s41586-019-1405-y [DOI] [PubMed] [Google Scholar]
- Matsuda, N., Sato, S., Shiba, K., Okatsu, K., Saisho, K., Gautier, C. A., Sou, Y.-S., Saiki, S., Kawajiri, S., Sato, F.et al. (2010). PINK1 stabilized by mitochondrial depolarization recruits Parkin to damaged mitochondria and activates latent Parkin for mitophagy. J. Cell Biol. 189, 211-221. 10.1083/jcb.200910140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui, M., Yamamoto, A., Kuma, A., Ohsumi, Y. and Mizushima, N. (2006). Organelle degradation during the lens and erythroid differentiation is independent of autophagy. Biochem. Biophys. Res. Commun. 339, 485-489. 10.1016/j.bbrc.2005.11.044 [DOI] [PubMed] [Google Scholar]
- McWilliams, T. G., Prescott, A. R., Allen, G. F. G., Tamjar, J., Munson, M. J., Thomson, C., Muqit, M. M. K. and Ganley, I. G. (2016). mito-QC illuminates mitophagy and mitochondrial architecture in vivo. J. Cell Biol. 214, 333-345. 10.1083/jcb.201603039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishita, H. and Mizushima, N. (2019). Diverse cellular roles of autophagy. Annu. Rev. Cell Dev. Biol. 35, 453-475. 10.1146/annurev-cellbio-100818-125300 [DOI] [PubMed] [Google Scholar]
- Morishita, H., Eguchi, S., Kimura, H., Sasaki, J., Sakamaki, Y., Robinson, M. L., Sasaki, T. and Mizushima, N. (2013). Deletion of autophagy-related 5 (Atg5) and Pik3c3 genes in the lens causes cataract independent of programmed organelle degradation. J. Biol. Chem. 288, 11436-11447. 10.1074/jbc.M112.437103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishita, H., Eguchi, T., Tsukamoto, S., Sakamaki, Y., Takahashi, S., Saito, C., Koyama-Honda, I. and Mizushima, N. (2021). Organelle degradation in the lens by PLAAT phospholipases. Nature 592, 634-638. 10.1038/s41586-021-03439-w [DOI] [PubMed] [Google Scholar]
- Nakai, A., Yamaguchi, O., Takeda, T., Higuchi, Y., Hikoso, S., Taniike, M., Omiya, S., Mizote, I., Matsumura, Y., Asahi, M.et al. (2007). The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress. Nat. Med. 13, 619-624. 10.1038/nm1574 [DOI] [PubMed] [Google Scholar]
- Narendra, D., Tanaka, A., Suen, D.-F. and Youle, R. J. (2008). Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J. Cell Biol. 183, 795-803. 10.1083/jcb.200809125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narendra, D. P., Jin, S. M., Tanaka, A., Suen, D.-F., Gautier, C. A., Shen, J., Cookson, M. R. and Youle, R. J. (2010). PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS Biol. 8, e1000298. 10.1371/journal.pbio.1000298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen, T. N., Padman, B. S., Usher, J., Oorschot, V., Ramm, G. and Lazarou, M. (2016). Atg8 family LC3/GABARAP proteins are crucial for autophagosome-lysosome fusion but not autophagosome formation during PINK1/Parkin mitophagy and starvation. J. Cell Biol. 215, 857-874. 10.1083/jcb.201607039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida, Y., Arakawa, S., Fujitani, K., Yamaguchi, H., Mizuta, T., Kanaseki, T., Komatsu, M., Otsu, K., Tsujimoto, Y. and Shimizu, S. (2009). Discovery of Atg5/Atg7-independent alternative macroautophagy. Nature 461, 654-658. 10.1038/nature08455 [DOI] [PubMed] [Google Scholar]
- Novak, I., Kirkin, V., McEwan, D. G., Zhang, J., Wild, P., Rozenknop, A., Rogov, V., Löhr, F., Popovic, D., Occhipinti, A.et al. (2010). Nix is a selective autophagy receptor for mitochondrial clearance. EMBO Rep. 11, 45-51. 10.1038/embor.2009.256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olzmann, J. A. and Carvalho, P. (2019). Dynamics and functions of lipid droplets. Nat. Rev. Mol. Cell Biol. 20, 137-155. 10.1038/s41580-018-0085-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onishi, M., Yamano, K., Sato, M., Matsuda, N. and Okamoto, K. (2021). Molecular mechanisms and physiological functions of mitophagy. EMBO J. 40, e104705. 10.15252/embj.2020104705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordureau, A., Sarraf, S. A., Duda, D. M., Heo, J.-M., Jedrykowski, M. P., Sviderskiy, V., Olszewski, J. L., Koerber, J. T., Xie, T., Beausoleil, S. A.et al. (2014). Quantitative proteomics reveal a feed-forward model for mitochondrial PARKIN translocation and UB chain synthesis. Mol. Cell 56, 360-375. 10.1016/j.molcel.2014.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliarini, D. J., Calvo, S. E., Chang, B., Sheth, S. A., Vafai, S. B., Ong, S.-E., Walford, G. A., Sugiana, C., Boneh, A., Chen, W. K.et al. (2008). A mitochondrial protein compendium elucidates complex I disease biology. Cell 134, 112-123. 10.1016/j.cell.2008.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papanicolaou, K. N., Kikuchi, R., Ngoh, G. A., Coughlan, K. A., Dominguez, I., Stanley, W. C. and Walsh, K. (2012). Mitofusins 1 and 2 are essential for postnatal metabolic remodeling in heart. Circ. Res. 111, 1012-1026. 10.1161/CIRCRESAHA.112.274142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, J., Lee, S. B., Lee, S., Kim, Y., Song, S., Kim, S., Bae, E., Kim, J., Shong, M., Kim, J.-M.et al. (2006). Mitochondrial dysfunction in Drosophila PINK1 mutants is complemented by parkin. Nature 441, 1157-1161. 10.1038/nature04788 [DOI] [PubMed] [Google Scholar]
- Pickrell, A. M., Huang, C.-H., Kennedy, S. R., Ordureau, A., Sideris, D. P., Hoekstra, J. G., Harper, J. W. and Youle, R. J. (2015). Endogenous Parkin preserves dopaminergic substantia nigral neurons following mitochondrial DNA mutagenic stress. Neuron 87, 371-381. 10.1016/j.neuron.2015.06.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole, A. C., Thomas, R. E., Yu, S., Vincow, E. S. and Pallanck, L. (2010). The mitochondrial fusion-promoting factor mitofusin is a substrate of the PINK1/parkin pathway. PloS One 5, e10054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Enriquez, S., Kim, I., Currin, R. T. and Lemasters, J. J. (2006). Tracker dyes to probe mitochondrial autophagy (mitophagy) in rat hepatocytes. Autophagy 2, 39-46. 10.4161/auto.2229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Navarro, J. A., Gómez, A., Rodal, I., Perucho, J., Martinez, A., Furió, V., Ampuero, I., Casarejos, M. J., Solano, R. M., de Yébenes, J. G.et al. (2008). Parkin deletion causes cerebral and systemic amyloidosis in human mutated tau over-expressing mice. Hum. Mol. Genet. 17, 3128-3143. 10.1093/hmg/ddn210 [DOI] [PubMed] [Google Scholar]
- Rojansky, R., Cha, M.-Y. and Chan, D. C. (2016). Elimination of paternal mitochondria in mouse embryos occurs through autophagic degradation dependent on PARKIN and MUL1. eLife 5, e17896. 10.7554/eLife.17896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu, D., Mouchiroud, L., Andreux, P. A., Katsyuba, E., Moullan, N., Nicolet-Dit-Félix, A. A., Williams, E. G., Jha, P., Lo Sasso, G., Huzard, D.et al. (2016). Urolithin A induces mitophagy and prolongs lifespan in C. elegans and increases muscle function in rodents. Nat. Med. 22, 879-888. [DOI] [PubMed] [Google Scholar]
- Saito, T., Nah, J., Oka, S.-I., Mukai, R., Monden, Y., Maejima, Y., Ikeda, Y., Sciarretta, S., Liu, T., Li, H.et al. (2019). An alternative mitophagy pathway mediated by Rab9 protects the heart against ischemia. J. Clin. Invest. 129, 802-819. 10.1172/JCI122035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoval, H., Thiagarajan, P., Dasgupta, S. K., Schumacher, A., Prchal, J. T., Chen, M. and Wang, J. (2008). Essential role for Nix in autophagic maturation of erythroid cells. Nature 454, 232-235. 10.1038/nature07006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweers, R. L., Zhang, J., Randall, M. S., Loyd, M. R., Li, W., Dorsey, F. C., Kundu, M., Opferman, J. T., Cleveland, J. L., Miller, J. L.et al. (2007). NIX is required for programmed mitochondrial clearance during reticulocyte maturation. Proc. Natl. Acad. Sci. USA 104, 19500-19505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesaki, H. and Jensen, R. E. (2001). UGO1 encodes an outer membrane protein required for mitochondrial fusion. J. Cell Biol. 152, 1123-1134. 10.1083/jcb.152.6.1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao, D., Kolwicz, S. C., Wang, P., Roe, N. D., Villet, O., Nishi, K., Hsu, Y.-W. A., Flint, G. V., Caudal, A., Wang, W.et al. (2020). Increasing fatty acid oxidation prevents high-fat diet-induced cardiomyopathy through regulating Parkin-mediated mitophagy. Circulation 142, 983-997. 10.1161/CIRCULATIONAHA.119.043319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiba-Fukushima, K., Ishikawa, K.-I., Inoshita, T., Izawa, N., Takanashi, M., Sato, S., Onodera, O., Akamatsu, W., Okano, H., Imai, Y.et al. (2017). Evidence that phosphorylated ubiquitin signaling is involved in the etiology of Parkinson's disease. Hum. Mol. Genet. 26, 3172-3185. [DOI] [PubMed] [Google Scholar]
- Shirakabe, A., Zhai, P., Ikeda, Y., Saito, T., Maejima, Y., Hsu, C.-P., Nomura, M., Egashira, K., Levine, B. and Sadoshima, J. (2016). Drp1-dependent mitochondrial autophagy plays a protective role against pressure overload-induced mitochondrial dysfunction and heart failure. Circulation 133, 1249-1263. 10.1161/CIRCULATIONAHA.115.020502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sliter, D. A., Martinez, J., Hao, L., Chen, X., Sun, N., Fischer, T. D., Burman, J. L., Li, Y., Zhang, Z., Narendra, D. P.et al. (2018). Parkin and PINK1 mitigate STING-induced inflammation. Nature 561, 258-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, L., McMackin, M., Nguyen, A. and Cortopassi, G. (2017a). Parkin deficiency accelerates consequences of mitochondrial DNA deletions and Parkinsonism. Neurobiol. Dis. 100, 30-38. 10.1016/j.nbd.2016.12.024 [DOI] [PubMed] [Google Scholar]
- Song, M., Franco, A., Fleischer, J. A., Zhang, L. and Dorn, G. W. (2017b). Abrogating mitochondrial dynamics in mouse hearts accelerates mitochondrial senescence. Cell Metab. 26, 872-883.e5. 10.1016/j.cmet.2017.09.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, N., Yun, J., Liu, J., Malide, D., Liu, C., Rovira, I. I., Holmström, K. M., Fergusson, M. M., Yoo, Y. H., Combs, C. A.et al. (2015). Measuring in vivo mitophagy. Mol. Cell 60, 685-696. 10.1016/j.molcel.2015.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutovsky, P., Moreno, R. D., Ramalho-Santos, J., Dominko, T., Simerly, C. and Schatten, G. (1999). Ubiquitin tag for sperm mitochondria. Nature 402, 371-372. 10.1038/46466 [DOI] [PubMed] [Google Scholar]
- Swatek, K. N., Usher, J. L., Kueck, A. F., Gladkova, C., Mevissen, T. E. T., Pruneda, J. N., Skern, T. and Komander, D. (2019). Insights into ubiquitin chain architecture using Ub-clipping. Nature 572, 533-537. 10.1038/s41586-019-1482-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka, A., Cleland, M. M., Xu, S., Narendra, D. P., Suen, D.-F., Karbowski, M. and Youle, R. J. (2010). Proteasome and p97 mediate mitophagy and degradation of mitofusins induced by Parkin. J. Cell Biol. 191, 1367-1380. 10.1083/jcb.201007013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, C., Han, H., Yan, M., Zhu, S., Liu, J., Liu, Z., He, L., Tan, J., Liu, Y., Liu, H.et al. (2018). PINK1-PRKN/PARK2 pathway of mitophagy is activated to protect against renal ischemia-reperfusion injury. Autophagy 14, 880-897. 10.1080/15548627.2017.1405880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong, M., Saito, T., Zhai, P., Oka, S.-I., Mizushima, W., Nakamura, M., Ikeda, S., Shirakabe, A. and Sadoshima, J. (2019). Mitophagy is essential for maintaining cardiac function during high fat diet-induced diabetic cardiomyopathy. Circ. Res. 124, 1360-1371. 10.1161/CIRCRESAHA.118.314607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooze, J. and Davies, H. G. (1965). Cytolysomes in amphibian erythrocytes. J. Cell Biol. 24, 146-150. 10.1083/jcb.24.1.146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trempe, J.-F., Sauvé, V., Grenier, K., Seirafi, M., Tang, M. Y., Ménade, M., Al-Abdul-Wahid, S., Krett, J., Wong, K., Kozlov, G.et al. (2013). Structure of Parkin reveals mechanisms for ubiquitin ligase activation. Science 340, 1451-1455. 10.1126/science.1237908 [DOI] [PubMed] [Google Scholar]
- Tsuboyama, K., Koyama-Honda, I., Sakamaki, Y., Koike, M., Morishita, H. and Mizushima, N. (2016). The ATG conjugation systems are important for degradation of the inner autophagosomal membrane. Science 354, 1036-1041. 10.1126/science.aaf6136 [DOI] [PubMed] [Google Scholar]
- Valente, E. M., Abou-Sleiman, P. M., Caputo, V., Muqit, M. M. K., Harvey, K., Gispert, S., Ali, Z., Del Turco, D., Bentivoglio, A. R., Healy, D. G.et al. (2004). Hereditary early-onset Parkinson's disease caused by mutations in PINK1. Science 304, 1158-1160. 10.1126/science.1096284 [DOI] [PubMed] [Google Scholar]
- Vargas, J. N. S., Wang, C., Bunker, E., Hao, L., Maric, D., Schiavo, G., Randow, F. and Youle, R. J. (2019). Spatiotemporal control of ULK1 activation by NDP52 and TBK1 during selective autophagy. Mol. Cell 74, 347-362.e6. 10.1016/j.molcel.2019.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincow, E. S., Merrihew, G., Thomas, R. E., Shulman, N. J., Beyer, R. P., MacCoss, M. J. and Pallanck, L. J. (2013). The PINK1–Parkin pathway promotes both mitophagy and selective respiratory chain turnover in vivo. Proc. Natl. Acad. Sci. 110, 6400-6405. 10.1073/pnas.1221132110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincow, E. S., Thomas, R. E., Merrihew, G. E., Shulman, N. J., Bammler, T. K., MacDonald, J. W., MacCoss, M. J. and Pallanck, L. J. (2019). Autophagy accounts for approximately one-third of mitochondrial protein turnover and is protein selective. Autophagy 15, 1592-1605. 10.1080/15548627.2019.1586258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vives-Bauza, C., Zhou, C., Huang, Y., Cui, M., de Vries, R. L. A., Kim, J., May, J., Tocilescu, M. A., Liu, W., Ko, H. S.et al. (2010). PINK1-dependent recruitment of Parkin to mitochondria in mitophagy. Proc. Natl. Acad. Sci. USA 107, 378-383. 10.1073/pnas.0911187107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi, J., Zhang, Z., Wakabayashi, N., Tamura, Y., Fukaya, M., Kensler, T. W., Iijima, M. and Sesaki, H. (2009). The dynamin-related GTPase Drp1 is required for embryonic and brain development in mice. J. Cell Biol. 186, 805-816. 10.1083/jcb.200903065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, X., Winter, D., Ashrafi, G., Schlehe, J., Wong, Y. L., Selkoe, D., Rice, S., Steen, J., LaVoie, M. J. and Schwarz, T. L. (2011). PINK1 and Parkin target Miro for phosphorylation and degradation to arrest mitochondrial motility. Cell 147, 893-906. 10.1016/j.cell.2011.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, B., Maxwell, B. A., Joo, J. H., Gwon, Y., Messing, J., Mishra, A., Shaw, T. I., Ward, A. L., Quan, H., Sakurada, S. M.et al. (2019). ULK1 and ULK2 Regulate Stress Granule Disassembly Through Phosphorylation and Activation of VCP/p97. Mol. Cell 74, 742-757.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watzlawik, J. O., Hou, X., Fricova, D., Ramnarine, C., Barodia, S. K., Gendron, T. F., Heckman, M. G., DeTure, M., Siuda, J., Wszolek, Z. K.et al. (2020). Sensitive ELISA-based detection method for the mitophagy marker p-S65-Ub in human cells, autopsy brain, and blood samples. Autophagy 10.1080/15548627.2020.1834712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams, J. A., Ni, H.-M., Ding, Y. and Ding, W.-X. (2015). Parkin regulates mitophagy and mitochondrial function to protect against alcohol-induced liver injury and steatosis in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 309, G324-G340. 10.1152/ajpgi.00108.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson, R. J., Drake, J. C., Cui, D., Zhang, M., Perry, H. M., Kashatus, J. A., Kusminski, C. M., Scherer, P. E., Kashatus, D. F., Okusa, M. D.et al. (2019). Conditional MitoTimer reporter mice for assessment of mitochondrial structure, oxidative stress, and mitophagy. Mitochondrion 44, 20-26. 10.1016/j.mito.2017.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong, Y. C. and Holzbaur, E. L. F. (2014). Optineurin is an autophagy receptor for damaged mitochondria in parkin-mediated mitophagy that is disrupted by an ALS-linked mutation. Proc. Natl. Acad. Sci. 111, E4439-E4448. 10.1073/pnas.1405752111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrighton, P. J., Shwartz, A., Heo, J.-M., Quenzer, E. D., LaBella, K. A., Harper, J. W. and Goessling, W. (2021). Quantitative intravital imaging in zebrafish reveals in vivo dynamics of physiological-stress-induced mitophagy. J. Cell Sci. 134, jcs256255. 10.1242/jcs.256255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, H., Wang, Y., Li, W., Chen, H., Du, L., Liu, D., Wang, X., Xu, T., Liu, L. and Chen, Q. (2019). Deficiency of mitophagy receptor FUNDC1 impairs mitochondrial quality and aggravates dietary-induced obesity and metabolic syndrome. Autophagy 15, 1882-1898. 10.1080/15548627.2019.1596482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada, T., Murata, D., Adachi, Y., Itoh, K., Kameoka, S., Igarashi, A., Kato, T., Araki, Y., Huganir, R. L., Dawson, T. M.et al. (2018). Mitochondrial stasis reveals p62-mediated ubiquitination in Parkin-independent mitophagy and mitigates nonalcoholic fatty liver disease. Cell Metab. 28, 588-604.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamano, K., Kikuchi, R., Kojima, W., Hayashida, R., Koyano, F., Kawawaki, J., Shoda, T., Demizu, Y., Naito, M., Tanaka, K.et al. (2020). Critical role of mitochondrial ubiquitination and the OPTN-ATG9A axis in mitophagy. J. Cell Biol. 219, e201912144. 10.1083/jcb.201912144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita, S.-I., Kyuuma, M., Inoue, K., Hata, Y., Kawada, R., Yamabi, M., Fujii, Y., Sakagami, J., Fukuda, T., Furukawa, K.et al. (2021). Mitophagy reporter mouse analysis reveals increased mitophagy activity in disuse-induced muscle atrophy. J. Cell. Physiol. 10.1002/jcp.30404 [DOI] [PubMed] [Google Scholar]
- Yang, Y., Gehrke, S., Imai, Y., Huang, Z., Ouyang, Y., Wang, J.-W., Yang, L., Beal, M. F., Vogel, H. and Lu, B. (2006). Mitochondrial pathology and muscle and dopaminergic neuron degeneration caused by inactivation of Drosophila Pink1 is rescued by Parkin. Proc. Natl. Acad. Sci. USA 103, 10793-10798. 10.1073/pnas.0602493103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazdankhah, M., Ghosh, S., Shang, P., Stepicheva, N., Hose, S., Liu, H., Chamling, X., Tian, S., Sullivan, M. L. G., Calderon, M. J.et al. (2021). BNIP3L-mediated mitophagy is required for mitochondrial remodeling during the differentiation of optic nerve oligodendrocytes. Autophagy 10.1080/15548627.2020.1871204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youle, R. J. and Narendra, D. P. (2011). Mechanisms of mitophagy. Nat. Rev. Mol. Cell Biol. 12, 9-14. 10.1038/nrm3028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan, Y., Zheng, Y., Zhang, X., Chen, Y., Wu, X., Wu, J., Shen, Z., Jiang, L., Wang, L., Yang, W.et al. (2017). BNIP3L/NIX-mediated mitophagy protects against ischemic brain injury independent of PARK2. Autophagy 13, 1754-1766. 10.1080/15548627.2017.1357792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, H., Bosch-Marce, M., Shimoda, L. A., Tan, Y. S., Baek, J. H., Wesley, J. B., Gonzalez, F. J. and Semenza, G. L. (2008). Mitochondrial autophagy is an HIF-1-dependent adaptive metabolic response to hypoxia. J. Biol. Chem. 283, 10892-10903. 10.1074/jbc.M800102200 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Zhang, J., Randall, M. S., Loyd, M. R., Dorsey, F. C., Kundu, M., Cleveland, J. L. and Ney, P. A. (2009). Mitochondrial clearance is regulated by Atg7-dependent and -independent mechanisms during reticulocyte maturation. Blood 114, 157-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, J., Loyd, M. R., Randall, M. S., Waddell, M. B., Kriwacki, R. W. and Ney, P. A. (2012). A short linear motif in BNIP3L (NIX) mediates mitochondrial clearance in reticulocytes. Autophagy 8, 1325-1332. 10.4161/auto.20764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, W., Ren, H., Xu, C., Zhu, C., Wu, H., Liu, D., Wang, J., Liu, L., Li, W., Ma, Q.et al. (2016). Hypoxic mitophagy regulates mitochondrial quality and platelet activation and determines severity of I/R heart injury. eLife 5, e21407. 10.7554/eLife.21407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, H., Foretz, M., Xie, Z., Zhang, M., Zhu, Z., Xing, J., Leclerc, J., Gaudry, M., Viollet, B. and Zou, M.-H. (2014). PRKAA1/AMPKα1 is required for autophagy-dependent mitochondrial clearance during erythrocyte maturation. Autophagy 10, 1522-1534. 10.4161/auto.29197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziviani, E., Tao, R. N. and Whitworth, A. J. (2010). Drosophila parkin requires PINK1 for mitochondrial translocation and ubiquitinates mitofusin. Proc. Natl. Acad. Sci. USA 107, 5018-5023. 10.1073/pnas.0913485107 [DOI] [PMC free article] [PubMed] [Google Scholar]