Abstract
Background
People with end‐stage kidney disease (ESKD) have high rates of cardiovascular events. Randomised controlled trials (RCTs) of homocysteine‐lowering therapies have not shown reductions in cardiovascular event rates in the general population. However, people with kidney disease have higher levels of homocysteine and may have different mechanisms of cardiovascular disease. We performed a systematic review of the effect of homocysteine‐lowering therapies in people with ESKD.
Objectives
To evaluate the benefits and harms of established homocysteine lowering therapy (folic acid, vitamin B6, vitamin B12) on all‐cause mortality and cardiovascular event rates in patients with ESKD.
Search methods
We searched Cochrane Kidney and Transplant's Specialised Register to 25 January 2016 through contact with the Information Specialist using search terms relevant to this review.
Selection criteria
Studies conducted in people with ESKD that reported at least 100 patient‐years of follow‐up and assessed the effect of therapies that are known to have homocysteine‐lowering properties were included.
Data collection and analysis
Two authors independently extracted data using a standardised form. The primary outcome was cardiovascular mortality. Secondary outcomes included all‐cause mortality, incident cardiovascular disease (fatal and nonfatal myocardial infarction and coronary revascularisation), cerebrovascular disease (stroke and cerebrovascular revascularisation), peripheral vascular disease (lower limb amputation), venous thromboembolic disease (deep vein thrombosis and pulmonary embolism), thrombosis of dialysis access, and adverse events. The effects of homocysteine‐lowering therapies on outcomes were assessed with meta‐analyses using random‐effects models. Prespecified subgroup and sensitivity analyses were conducted.
Main results
We included six studies that reported data on 2452 participants with ESKD. Interventions investigated were folic acid with or without other vitamins (vitamin B6, vitamin B12). Participants' mean age was 48 to 65 years, and proportions of male participants ranged from 50% to 98%.
Homocysteine‐lowering therapy probably leads to little or no effect on cardiovascular mortality (4 studies, 1186 participants: RR 0.93, 95% CI 0.70 to 1.22). There was no evidence of heterogeneity among the included studies (I² = 0%). Homocysteine‐lowering therapy had little or no effect on all‐cause mortality or any other of this review's secondary outcomes. All prespecified subgroup and sensitivity analyses demonstrated little or no difference. Reported adverse events were mild and there was no increase in the incidence of adverse events from homocysteine‐lowering therapies (3 studies, 1248 participants: RR 1.12, 95% CI 0.51 to 2.47; I2 = 0%). Overall, studies were assessed as being at low risk of bias and there was no evidence of publication bias.
Authors' conclusions
Homocysteine‐lowering therapies were not found to reduce mortality (cardiovascular and all‐cause) or cardiovascular events among people with ESKD.
Plain language summary
Interventions for lowering plasma homocysteine levels in dialysis patients
Background
People with advanced kidney disease frequently develop heart disease, which is the most common cause of deaths in these people. An increased level of the amino acid (homocysteine) in the blood is a risk factor for heart disease in people with advanced kidney disease. Therapies that reduce homocysteine levels (e.g. folic acid, vitamins B6 and B12) are often used, but the benefits and harms of their use are unclear. We aimed to assess the benefits and harms of homocysteine‐lowering therapies in people with advanced kidney disease who were on dialysis.
Study characteristics
From a search of the literature in January 2016, we identified six randomised controlled trials that involved 2452 participants aged between 48 and 65 years to be analysed.
Key results
We found that homocysteine‐lowering therapies had no benefits for heart health in people with advanced kidney disease who were on dialysis. These therapies did not achieve any reduction in rates of heart disease‐related death. However, homocysteine‐lowering therapies were generally well tolerated, and had a mild side effect profile.
Quality of the evidence
Overall, studies were assessed as high quality.
Background
Description of the condition
Dialysis‐dependent end‐stage kidney disease (ESKD) patients have reduced life expectancy, with annual mortality of 14.8% in Australia (McDonald 2007), and 16.7% in the USA (USRDS 2007). Chronic kidney disease (CKD) is an independent and powerful risk factor for cardiovascular disease (Go 2004; Weiner 2004). Cardiovascular mortality is significant in CKD (de Jager 2009). For dialysis‐dependent ESKD patients, death from cardiovascular disease is 10 to 100 times higher than in age and sex‐matched controls (Foley 1998).
The increased prevalence of cardiovascular disease among ESKD patients is not completely accounted for by the presence of traditional risk factors such as hypertension, dyslipidaemia, diabetes mellitus, smoking, and left ventricular hypertrophy (Baigent 2000). Thus, there has been increasing investigation of nontraditional risk factors such as anaemia, hyperparathyroidism and hyperhomocysteinaemia.
Description of the intervention
Homocysteine is an amino acid produced from the metabolism of methionine. It is believed to play a role in the pathogenesis of atherosclerosis by damaging the endothelium and promoting clotting (Eikelboom 1999). Epidemiological studies have shown homocysteine to be an independent risk factor for cardiovascular disease (HSC 2002). Elevated homocysteine level is a predictor of vascular disease including stroke, myocardial infarction (MI, heart attack), atherosclerosis (thickening of artery walls), arterial and venous thrombosis (clotting) and cardiovascular death in the general population (HSC 2002; Wald 2002) and in people with ESKD (Chauveau 1993; Moustapha 1998; Robinson 1996).
In contrast to the well‐documented association between homocysteine levels and vascular events in the general population, the association between homocysteine levels and risk for atherothrombotic disease is not consistent among the ESKD population. Some investigators have described an inverse association between homocysteine levels and clinical outcomes in the ESKD patients with low levels of homocysteine being associated with worse outcomes (Kalantar‐Zadeh 2004; Wrone 2001). However, even a mild increase in the homocysteine level appears to be a vascular risk factor in the general population and there is high prevalence of hyperhomocysteinaemia in ESKD. Thus, the paradoxical reverse association between homocysteine and clinical outcome in ESKD patients does not as such refute a possible role for homocysteine in the vascular pathogenesis. Furthermore, it has also been shown that association of hyperhomocysteinaemia and worse clinical outcomes persists in ESKD patients without chronic inflammation‐malnutrition state (Ducloux 2006). These authors postulate that the burden of chronic inflammation‐malnutrition might mask the true relationship between hyperhomocysteinaemia and cardiovascular risk among dialysis‐dependent ESKD patients.
How the intervention might work
Because kidney metabolism is the primary means by which homocysteine is cleared, plasma homocysteine has a strong inverse correlation with estimated glomerular filtration rate (Freidman 2001). Among patients with ESKD, the prevalence of hyperhomocysteinaemia is 85% to 100% (Bostom 1999).
In classical homocystinuria, plasma total homocysteine concentrations are very high (100 to 400 μmol/L), and untreated patients die prematurely from venous thromboembolism and malignant arterial disease. Long‐term treatment aimed at lowering homocysteine levels has been extremely effective in reducing the potentially life‐threatening vascular risk in these patients modified by agents that lower homocysteine such as folic acid and vitamins B6 and B12. Of the homocysteine‐lowering therapies, folic acid is the most effective and consistent in reducing homocysteine levels in ESKD (van Guldener 2006).
Why it is important to do this review
A limited number of studies of homocysteine‐lowering therapy in ESKD patients have been conducted which individually have been unable to find a statistically significant effect on surrogate markers of cardiovascular disease, cardiovascular events or all‐cause mortality (ASFAST 2004; HOST Study 2004; Vianna 2007; Wrone 2004). The picture is confused by the inability of studies to normalise homocysteine levels in most patients, despite the administration of larger doses of folic acid than in studies of other population groups (ASFAST 2004; Gonin 2005).
Although there is no RCT evidence to support folic acid or vitamin B interventions, multivitamin supplementation for patients with ESKD is commonly practiced. The K/DOQI Clinical Practice Guidelines for Cardiovascular Disease in Dialysis Patients promote the administration of folate, vitamins B6 and B12 to compensate for dialysate losses and prevent elevation of homocysteine on the grounds of cardiovascular risk (K/DOQI 2005). The group also acknowledged the lack of evidence in the area and state that further data are required regarding the effect of vitamin therapy on clinical outcomes. Furthermore potential harms associated with folic acid supplements have not been excluded (Zoccali 2010).
In light of the burden of cardiovascular disease for patients with ESKD, the effectiveness or otherwise of any potentially effective interventions needs to be clearly established. This review aimed to assess the benefits and harms of homocysteine lowering therapy among people with ESKD to guide decision making and improve outcomes for patients.
Objectives
To evaluate the benefits and harms of established homocysteine lowering therapy (folic acid, vitamin B6, vitamin B12) on all‐cause mortality and cardiovascular event rates in patients with ESKD.
Methods
Criteria for considering studies for this review
Types of studies
RCTs and quasi‐RCTs (RCTs in which allocation to treatment was obtained by alteration, use of alternate medical records, date of birth or other predictable methods) looking at the use of established homocysteine lowering therapy, with a minimum of 100 patient years were included to reduce the risk of reporting or publication bias. Sequential and cross‐over studies were excluded.
Types of participants
We included adults (aged over 18 years) with ESKD defined as those requiring maintenance dialysis. Patients with functioning kidney transplants were excluded.
Types of interventions
Studies randomising patients to therapies which have proven efficacy in lowering homocysteine levels were included. Studies of regimens in which the main mechanism of action is thought not to be homocysteine lowering were excluded (e.g. simvastatin plus folic acid, N‐acetyl cysteine). We investigated the following comparisons.
Homocysteine‐lowering therapy versus placebo or usual care
Higher dose homocysteine‐lowering therapy versus lower dose homocysteine‐lowering therapy
Any schedule of treatment
Any route of treatment.
Types of outcome measures
Primary outcomes
Cardiovascular mortality
Secondary outcomes
All‐cause mortality
-
Cardiovascular disease
Fatal and nonfatal MI
Coronary revascularisation
-
Cerebrovascular disease
Stroke
Cerebrovascular revascularisation
-
Peripheral vascular disease and venous thromboembolic disease
Lower limb amputation
Deep vein thrombosis (DVT) and pulmonary embolism (PE)
-
Kidney‐specific outcomes
Thrombosis of dialysis access
-
Adverse events from folic‐based therapy
Gastrointestinal events
Dermatological events
Neurological events
Malignancy incidence and mortality
Any self‐reported adverse events.
Search methods for identification of studies
Electronic searches
We searched Cochrane Kidney and Transplant's Specialised Register to 25 January 2016 through contact with the Information Specialist using search terms relevant to this review. The Specialised Register contains studies identified from the following sources.
Quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL)
Weekly searches of MEDLINE OVID SP
Handsearching of kidney‐related journals and the proceedings of major kidney conferences
Searching of the current year of EMBASE OVID SP
Weekly current awareness alerts for selected kidney journals
Searches of the International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov.
Studies contained in the Specialised Register are identified through search strategies for CENTRAL, MEDLINE, and EMBASE based on the scope of Cochrane Kidney and Transplant. Details of these strategies as well as a list of handsearched journals, conference proceedings and current awareness alerts are available in the Specialised Register section of information about the Cochrane Kidney and Transplant.
See Appendix 1 for search terms used in strategies for this review.
Searching other resources
Reference lists of review articles, relevant studies and clinical practice guidelines.
Letters seeking information about unpublished or incomplete studies to investigators known to be involved in previous studies.
Data collection and analysis
Steps in data collection and analyses are outlined below.
Selection of studies
The search strategy described was used to obtain titles and abstracts of studies that may be relevant to the review. Titles and abstracts were screened independently by two authors, and studies that were not applicable were discarded. However, studies and reviews that might include relevant data or information on studies were retained initially. Two authors independently assessed retrieved abstracts and the full text as necessary of these studies to determine which studies satisfy the inclusion criteria.
Data extraction and management
Data extraction was carried out independently by two authors using standard data extraction forms. Studies reported in non‐English language journals were translated before assessment. Where more than one publication of one RCT existed, only the publication with the most complete data were to be included; however, we did not find any duplicate publications. Disagreements were resolved by consultation.
Assessment of risk of bias in included studies
The following items were independently assessed by two authors using the risk of bias assessment tool (Higgins 2011) (see Appendix 2).
Was there adequate sequence generation (selection bias)?
Was allocation adequately concealed (selection bias)?
-
Was knowledge of the allocated interventions adequately prevented during the study?
Participants and personnel (performance bias)
Outcome assessors (detection bias)
Were incomplete outcome data adequately addressed (attrition bias)?
Are reports of the study free of suggestion of selective outcome reporting (reporting bias)?
Was the study apparently free of other problems that could put it at a risk of bias?
Measures of treatment effect
For dichotomous outcomes (all‐cause mortality, MI, coronary revascularisation, cardiovascular death, stroke, cerebrovascular revascularisation, lower limb amputation, thrombosis of arteriovenous (AV) access, DVT, PE), results were expressed as risk ratio (RR) with 95% confidence intervals (CI). Data were pooled using the random‐effects model but the fixed‐effect model was also analysed to ensure robustness of the model chosen and susceptibility to outliers.
Dealing with missing data
Any further information required from the original author was to be requested by written correspondence and any relevant information obtained in this manner was included in the review. Attrition rates, such as drop‐outs, losses to follow‐up and withdrawals were to be investigated. Issues of missing data and imputation methods (e.g. last‐observation‐carried‐forward) were to be critically appraised (Higgins 2011).
Assessment of heterogeneity
Heterogeneity was analysed using a Chi2 test on N‐1 degrees of freedom, with an alpha of 0.05 used for statistical significance and with the I² test (Higgins 2003). I2 values of 25%, 50% and 75% correspond to low, medium and high levels of heterogeneity.
Assessment of reporting biases
Publication bias was assessed using a funnel plot (Egger 1997). Attrition bias was assessed using the loss/event ratio.
Data synthesis
Data were pooled using the random‐effects model but the fixed‐effect model was also analysed to ensure robustness of the model chosen and susceptibility to outliers.
Subgroup analysis and investigation of heterogeneity
Subgroup analyses were conducted to explore possible sources of heterogeneity using inverse variance according to the following characteristics.
Study intervention
Exposure to folic acid among control group participants
Proportion of participants with diabetes mellitus
Proportion of participants with cardiac disease
Study duration follow‐up
Study event number.
We aimed to analyse the ability of interventions to normalise homocysteine levels in a subgroup of studies that enrolled patients with elevated homocysteine levels. Plausible explanations for variations in treatment effect were explored using subgroup analyses based on study quality and length of follow‐up.
Sensitivity analysis
Sensitivity analyses were conducted to ensure conclusions are robust to decisions made during the review process such as inclusion criteria and imputing of missing data. Where outcomes sought were reported in insufficient detail to allow meta‐analysis and further information was not forthcoming from triallists, we planned to summarise these outcomes and assess with descriptive techniques.
We planned to calculate the number of persons needed to treat to avoid one cardiovascular death if adequate data were available; however, our non‐significant results did not permit making this calculation.
Results
Description of studies
Detailed description of study search results and description of studies is outlined below.
Results of the search
We searched Cochrane Kidney and Transplant's Specialised Register to 25 January 2016 through contact with the Information Specialist using search terms relevant to this review. Additionally, reference lists of review articles, relevant studies and clinical practice guidelines were also searched. This comprehensive search yielded 336 records. The titles and abstracts of these 336 records were assessed and 110 articles were excluded since they did not meet the prespecified review criteria. The full texts of the remaining 220 records were reviewed; six studies (34 records) met our review criteria (Figure 1). Prior to publication three additional studies were identified and these will be assessed in a future update of this review (NCT00004495; Soleimani 2011; Tayebi‐Khosroshahi 2013).
1.
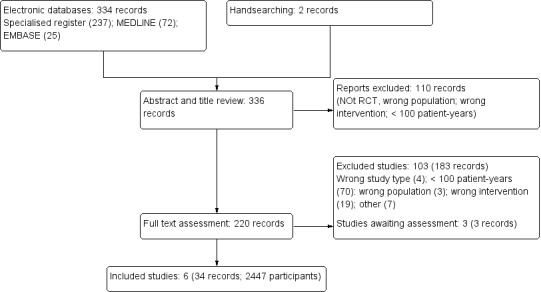
Flow diagram showing study selection
Included studies
We included six studies that involved 2452 participants with ESKD requiring dialysis (ASFAST 2004; Heinz 2009; HOST Study 2004; Righetti 2003; Vianna 2007; Wrone 2004). All studies were reported between 2003 and 2009.
Design
All included studies were parallel RCTs (ASFAST 2004; Heinz 2009; HOST Study 2004; Righetti 2003; Vianna 2007; Wrone 2004). Five studies were double‐blinded (ASFAST 2004; Heinz 2009; HOST Study 2004; Vianna 2007; Wrone 2004) and one study followed an open‐label design (Righetti 2003).
Sample sizes
Sample sizes of included studies ranged from 88 participants (Righetti 2003) to 761 participants (HOST Study 2004).
Setting
The included studies were conducted in outpatient dialysis units (ASFAST 2004; Heinz 2009; HOST Study 2004; Righetti 2003; Vianna 2007; Wrone 2004). Two were single centre studies (Righetti 2003; Vianna 2007) and four were multicentre studies (ASFAST 2004; Heinz 2009; HOST Study 2004; Wrone 2004). Studies were conducted in the USA (HOST Study 2004; Wrone 2004), Australia and New Zealand (ASFAST 2004), Germany (Heinz 2009), Brazil (Vianna 2007) and Italy (Righetti 2003). Mandatory folate fortification was present only in the USA at the time the studies were conducted. Three studies included ESKD patients on maintenance haemodialysis (Heinz 2009; Righetti 2003; Vianna 2007) only. Three studies included ESKD patients on both maintenance haemodialysis and peritoneal dialysis (ASFAST 2004; HOST Study 2004; Wrone 2004). Proportions of peritoneal dialysis patients were 28% (ASFAST 2004), 8.2% (Wrone 2004) or not reported (HOST Study 2004).
Participants
The mean age of study participants was between 48 and 65 years (Characteristics of included studies). The proportion of study participants who were male ranged from 50% to 98%, the proportion with a diagnosis of diabetes mellitus ranged from 19% to 55%, and the proportion with a history of cardiac disease ranged from 11% to 58%. Follow‐up ranged from 2.0 to 3.6 years. Participants consisted entirely of people with ESKD in four studies (Heinz 2009; Righetti 2003; Vianna 2007; Wrone 2004) and both ESKD and CKD in two studies (ASFAST 2004; HOST Study 2004). Specific outcome data on ESKD patients were obtained from the study authors of ASFAST 2004.
Interventions
Interventions investigated were folic acid with or without other vitamins (vitamin B6, vitamin B12). The dose of folic acid was variable from the equivalent daily dose of 5 mg to 40 mg. Wrone 2004 compared placebo versus low dose (5 mg) versus high dose (15 mg) folate therapy with the latter two arms combined for the purposes of this review. Comparator treatment was placebo (ASFAST 2004; HOST Study 2004; Vianna 2007), low dose folic acid (Heinz 2009; Wrone 2004) or usual care (Righetti 2003).
Outcomes
For ASFAST 2004, the primary outcomes were change in rate of progression of mean maximum carotid artery intimal media thickness, composite of MI, stroke, and death from cardiovascular cause. Secondary outcomes were all fatal and nonfatal cardiovascular events including MI, stroke, unstable angina, revascularisation, and peripheral vascular disease.
The primary outcome in Heinz 2009 was overall mortality. Secondary outcomes were occurrence of first fatal or nonfatal cardiovascular event (MI, unstable angina, coronary vascularisation procedure, sudden cardiac death, stroke, peripheral artery disease, PE, and thromboses). Shunt thromboses were not regarded as an end point.
The primary outcome in HOST Study 2004 was time to death from any cause. Secondary outcomes were time to MI, stroke, amputation of all or part of a lower extremity, and a composite of these three plus all‐cause mortality.
For Righetti 2003, the primary outcome was a composite cardiovascular end point (typical history of angina with abnormal myocardial scintigraphy or coronarography, fatal and nonfatal MI, symptomatic extracranial carotid stenosis resulting in carotid endarterectomy, fatal and nonfatal stroke and sudden cardiac arrest). There were no secondary outcomes.
The primary outcome in Vianna 2007 was a composite of new major cardiovascular events, including death from cardiovascular causes, nonfatal MI, cardiac arrhythmias, angina, heart failure, and cerebral vascular accident. Secondary outcome was the evaluation of the intima‐media thickness of the common carotid arteries.
For Wrone 2004, the primary outcomes were cardiovascular events (coronary artery intervention, MI, stroke, transient ischaemic attack, carotid endarterectomy, limb amputation) and mortality. The secondary outcome was vascular access thrombosis (among those with AV fistulae).
Excluded studies
Excluded studies are described (Characteristics of excluded studies). Reasons for exclusion were: wrong study design (4 studies); less than 100 patient‐years follow‐up (70 studies); wrong study population (3 studies); not homocysteine‐lowering interventions (19 studies); outcome data were not extractable (7 studies).
Risk of bias in included studies
A risk of bias graph and summary are presented in Figure 2 and Figure 3. Risk of selection bias related to random sequence generation and allocation concealment was low in 50% of the included studies. Risks attributed to performance, attrition, detection, and reporting biases were low in more than 65% of included studies.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Method of sequence generation was assessed as being at low risk of bias in three studies (ASFAST 2004; HOST Study 2004; Righetti 2003) and unclear in three studies (Heinz 2009; Vianna 2007; Wrone 2004).
Allocation concealment was assessed as being at low risk of bias in three studies (ASFAST 2004; Righetti 2003; Wrone 2004) and unclear in three studies (Heinz 2009; HOST Study 2004; Vianna 2007).
Blinding
Performance bias (participants and study staff) was assessed as low in five studies (ASFAST 2004; Heinz 2009; HOST Study 2004; Vianna 2007; Wrone 2004) and high in one study (Righetti 2003).
Detection bias (outcome assessors) was assessed as low in four studies (ASFAST 2004; Heinz 2009; HOST Study 2004; Vianna 2007) and unclear in two studies (Righetti 2003; Wrone 2004).
Incomplete outcome data
Attrition bias was assessed as low in all six studies.
Selective reporting
Reporting bias was assessed as low in five studies (ASFAST 2004; Heinz 2009; HOST Study 2004; Vianna 2007; Wrone 2004) and unclear in one study (Righetti 2003).
Other potential sources of bias
Righetti 2003 was assessed as being at high risk of other bias due to the imbalance between the groups in the baseline data; all other studies were assessed as being at low risk of other potential biases.
Funnel plot analysis did not show publication bias (Figure 4).
4.

Funnel plot of comparison: 2 Secondary outcomes, outcome: 2.1 all‐cause mortality
Effects of interventions
Primary outcomes
Cardiovascular mortality
Four studies (ASFAST 2004; Heinz 2009; Righetti 2003; Vianna 2007) reported 173 cardiovascular mortality events among 1186 participants. Homocysteine‐lowering therapy had no overall effect on cardiovascular mortality (Analysis 1.1 (4 studies, 1186 participants): RR 0.93, 95% CI 0.70 to 1.22; I2 = 0%). There was no evidence of heterogeneity.
1.1. Analysis.

Comparison 1 Primary outcome, Outcome 1 Cardiovascular mortality.
We planned to calculate the number of persons needed to treat to avoid one cardiovascular death if adequate data were available; however, our non‐significant results did not enable this calculation to be made.
Secondary outcomes
All‐cause mortality
The six included studies reported 719 all‐cause mortality events among 2447 participants. Homocysteine‐lowering therapy had no overall effect on all‐cause mortality (Analysis 2.1 (6 studies, 247 participants): RR 1.00, 95% CI 0.89 to 1.11; I2 = 0%). There was no evidence of heterogeneity.
2.1. Analysis.

Comparison 2 Secondary outcomes, Outcome 1 All‐cause mortality.
Fatal and nonfatal myocardial infarction
Four studies (ASFAST 2004; Heinz 2009; Righetti 2003; Wrone 2004) reported 75 fatal and nonfatal MI events among 1510 participants. Homocysteine‐lowering therapy had no overall effect on MI (Analysis 2.2 (4 studies; 1510 participants): RR 1.04, 95% CI 0.66 to 1.62; I2 = 0%). There was no evidence of heterogeneity.
2.2. Analysis.

Comparison 2 Secondary outcomes, Outcome 2 Myocardial infarction (fatal and non fatal).
Coronary revascularisation
Heinz 2009 and Wrone 2004 reported 44 coronary revascularisation events among 1160 participants. Homocysteine‐lowering therapy had no overall effect on coronary revascularisation (Analysis 2.3 (2 studies, 1160 participants): RR 0.83, 95% CI 0.22 to 3.14; I2 = 74%). Significant heterogeneity was observed.
2.3. Analysis.

Comparison 2 Secondary outcomes, Outcome 3 Coronary revascularisation.
Stroke
Four studies (ASFAST 2004; Heinz 2009; Righetti 2003; Wrone 2004) reported 77 stroke events among 1510 participants. Homocysteine‐lowering therapy had no overall effect on stroke (Analysis 2.4 (4 studies, 1510 participants): RR 0.89, 95% CI 0.57 to 1.40; I2 = 0%)). There was no evidence of heterogeneity.
2.4. Analysis.

Comparison 2 Secondary outcomes, Outcome 4 Stroke.
Cerebrovascular revascularisation
Adequate data on this outcome were not available in published reports and further information was not forthcoming from the triallists.
Lower limb amputation
Adequate data on this outcome were not available in published reports and further information was not forthcoming from the triallists.
Deep vein thrombosis and pulmonary embolism
Heinz 2009 reported 13 DVT and PE events among 650 participants. Homocysteine‐lowering therapy had no overall effect on DVT and PE (Analysis 2.5: RR 1.15, 95% CI 0.39 to 3.39).
2.5. Analysis.

Comparison 2 Secondary outcomes, Outcome 5 Deep venous thrombosis and pulmonary embolism.
Thrombosis of dialysis access
HOST Study 2004 and Wrone 2004 reported 533 dialysis access thrombosis events among 1261 participants. Homocysteine‐lowering therapy had no overall effect dialysis access thrombosis (Analysis 2.6 (2 studies, 1261 participants): RR 1.00, 95% CI 0.88 to 1.14; I2 = 0%). There was no evidence of heterogeneity.
2.6. Analysis.

Comparison 2 Secondary outcomes, Outcome 6 Thrombosis of dialysis access.
Adverse events from folic acid‐based therapy
Adverse event rates were reported in three studies (Heinz 2009; Righetti 2003; Wrone 2004, 1248 participants) where homocysteine‐lowering therapy had no effect on adverse event rates (Analysis 2.7 (3 studies, 1248 participants): (RR 1.12, 95% CI 0.51 to 2.47; I2 = 0%). Reported adverse events included nausea, abdominal discomfort, hunger and weight gain, skin rashes, headaches, fatigue, and paraesthesia. We were unable to complete planned separate analyses of gastrointestinal, dermatological, neurological and malignant events due to the small number of studies reporting these outcomes. Rate of withdrawal from study treatment due to adverse events was less than 1% among the included studies.
2.7. Analysis.

Comparison 2 Secondary outcomes, Outcome 7 Adverse events.
Subgroup analyses
Subgroup analyses were conducted to explore possible sources of heterogeneity according to the following characteristics.
Study intervention
The impact of intervention on cardiovascular events did not differ significantly (P = 0.649) when studies of folic acid alone (ASFAST 2004; Vianna 2007) were compared with studies of folic acid plus B group vitamins (Heinz 2009; Righetti 2003).
Exposure to folic acid among control group participants
The impact of intervention on cardiovascular events did not differ significantly (P = 0.557) when studies of low dose exposure in control group participants (Heinz 2009) were compared with studies of no low dose exposure in control group participants (ASFAST 2004; Righetti 2003; Vianna 2007).
Proportion of participants with diabetes mellitus
The impact of intervention on cardiovascular events did not differ significantly (P = 0.557) when studies that included more than a third of participants with diabetes mellitus (Heinz 2009) were compared with studies that included fewer than a third of participants with diabetes mellitus (ASFAST 2004; Righetti 2003; Vianna 2007).
Proportion of participants with cardiac disease
The impact of intervention on cardiovascular events did not differ significantly (P = 1.000) when studies that included more than a third of participants with cardiac disease (Heinz 2009; Righetti 2003) were compared with studies that included fewer than a third of participants with cardiac disease (ASFAST 2004; Vianna 2007).
Study follow‐up duration
The impact of intervention on cardiovascular events did not differ significantly (P = 0.649) when studies with more than 30 month follow‐up (ASFAST 2004) were compared with those that had with less than 30 months follow‐up (Heinz 2009; Vianna 2007; Righetti 2003).
Study event number
The impact of interventions on cardiovascular events did not differ significantly (P = 0.557) when studies with more than 150 events (Heinz 2009) were compared with those that had fewer than 150 events (ASFAST 2004; Vianna 2007; Righetti 2003).
We aimed to analyse the ability of interventions to normalise homocysteine level in a subgroup of studies that enrolled patients with elevated homocysteine levels. However, there was only one study (Vianna 2007) in which a significant proportion of patients in the active arm achieved normalisation of homocysteine levels. In this study, there was no difference in cardiovascular mortality between active and control arm participants.
Sensitivity analyses
The same results were observed with fixed‐effects and random‐effects models.
Plausible explanations for variations in treatment effect for primary outcome were explored using subgroup analyses based on study quality and length of follow‐up.
The impact of intervention on cardiovascular mortality did not differ significantly (P = 0.649 for heterogeneity) when studies with and without blinding of participants and study staff (ASFAST 2004; Heinz 2009; Vianna 2007) were compared.
The impact of interventions on cardiovascular mortality did not differ significantly (P = 0.657) when studies with median follow‐up over three years (ASFAST 2004) were compared with median follow‐up of fewer than three years.
Discussion
Summary of main results
In this systematic review including 2452 participants with ESKD, randomisation to folic acid‐based homocysteine‐lowering therapy did not affect cardiovascular mortality, all‐cause mortality, and incidence of cardiovascular events including MI, stroke, venous or access thrombosis. Null results were consistently observed across all prespecified subgroups and sensitivity analyses. Reported adverse events from these therapies were mild and rates of adverse events were variable across the studies. Overall, there was no evidence of harm defined by rates of adverse events in this population.
Overall completeness and applicability of evidence
Guidelines for ESKD patients routinely suggest supplementation with folic acid and B group vitamins (homocysteine‐lowering therapies) without lack of clear evidence (K/DOQI 2005), and many people with ESKD appear to be receiving folic acid supplementation (Andreucci 2004). Our findings suggest that folic acid‐based homocysteine‐lowering should not be used for cardiovascular prevention in people with kidney disease, a population in whom medication burden is often high.
Quality of the evidence
The strengths of our systematic review we that an important clinical question has been investigated focusing on a study population with ESKD, using rigorous methodology and study search strategy techniques. We found consistent null results in primary, secondary, subgroup and sensitivity analyses.
These findings have direct implications for millions of people with ESKD globally who are currently taking homocysteine‐lowering medications.
Risk of selection bias related to random sequence generation and allocation concealment was low in 50% of the included studies. Risks attributed to performance, attrition, detection, and reporting biases were low in more than 65% of included studies.
Potential biases in the review process
Limitations of our systematic review include reliance on tabular rather than individual patient level data. We focused on cardiovascular mortality as our primary outcome; however, this outcome was reported in only four of the six included studies. Competing risks for cardiovascular mortality were not analysed. Although funnel plot analysis suggested a low possibility of publication bias, this cannot be excluded based on the small number of included studies. We could not conduct separate analyses for peritoneal and haemodialysis patients since adequate data were not available. Overall, participants from the included studies were younger than the average ESKD population and this may limit generalisability.
Data on genetic polymorphisms that determine homocysteine‐lowering effects of therapies (Klerk 2002) were not available; however, these polymorphisms have been shown to have no effects among dialysis patients (Aucella 2005). Nevertheless, although lowering of homocysteine levels were noted in all studies, normalisation of homocysteine levels was rare, pointing to the possibility of folic acid resistance in ESKD patients (Robinson 1996) and possible need for higher doses. However, in studies where high dose (> 5 mg daily) folic acid was administered, similar null effects on outcomes were noted (ASFAST 2004; HOST Study 2004; Wrone 2004).
Recent data indicate that there may be non‐cardiovascular benefits of homocysteine lowering in the general population (such as reduced fracture risk) (Yang 2012) and our review did not evaluate effects on such outcomes.
Agreements and disagreements with other studies or reviews
Our findings were consistent with a recent meta‐analysis conducted in the general population for cardiovascular mortality, all‐cause mortality and MI (Huang 2012); however, our findings were discordant in terms of effects on stroke risk reduction.
Huang 2012 pooled data from 19 studies that included 47,921 participants and observed approximately 12% reduction in stroke risk in the general population. Although the analyses by Huang 2012 suffer from heterogeneity of patient populations in the pooled studies, other possible reasons for differences in stroke risk reduction between general and ESKD populations may relate to differences in biological risk factors for cerebrovascular disease in dialysis versus general populations (Kanbay 2010).
VITATOPS 2010 was conducted in patients who had experienced a recent stroke or transient ischaemic attack and found no significant difference for cardiovascular events (RR 0.91, 95% CI 0.82 to 1.00, P = 0.05) or stroke (RR 0.92, 95% CI 0.81 to 1.06).
There have been two recent meta‐analyses that evaluated the effects of homocysteine lowering therapies on cardiovascular events in patients with kidney disease. Jardine 2012 included 11 studies comprising 4389 patients with CKD, 2452 with ESKD, and 4110 with functioning kidney transplants. In Jardine 2012, folic acid‐based therapy did not reduce cardiovascular event incidence (RR 0.97, 95% CI 0.92 to 1.03, P = 0.326). Pan 2012 pooled 10 studies involving 4836 participants with CKD or ESKD. The investigators noted that the estimated relative risks were not significantly different for any cardiovascular outcomes and all‐cause mortality.
Our findings were consistent with these findings. In addition, we specifically derived outcome data on ESKD patients from studies that involved both CKD and ESKD patients (ASFAST 2004; HOST Study 2004). Considering these differences, we believe that the findings of our review are likely to be a better estimate of the true effect of folic acid‐based homocysteine‐lowering on cardiovascular outcomes in ESKD patients. In combination with results from Jardine 2012 and Pan 2012 we feel confident regarding our conclusions on null effect from homocysteine‐lowering therapies in ESKD patients and further solidify futility of such treatments as discussed in a recent editorial (Haynes 2012).
Recent data regarding associations between hyperhomocysteinaemia and cardiovascular risk (Menon 2006; Suliman 2007) have questioned the earlier findings that linked hyperhomocysteinaemia with increased cardiovascular risk (Robinson 1996) and thus further strengthen our conclusions. In fact, hyperhomocysteinaemia in ESKD may be an illustration of reverse epidemiology with some authors finding elevated homocysteine levels associated with reduced cardiovascular risk (Kalantar‐Zadeh 2004) as has been described for factors such as obesity (Kalantar‐Zadeh 2005) in the population with ESKD. Regardless of the nature of the association reported by observational studies, our results clearly indicate that interventions to lower homocysteine levels in people with ESKD do not offer cardiovascular risk reduction and are likely to be futile. We found one study listed at clinicaltrials.gov with unknown recruitment status on this topic (NCT00004495); however, the enrolment goal for this study is listed as 84 and it is unlikely to significantly affect results of our analyses.
Authors' conclusions
Implications for practice.
In patients with ESKD, homocysteine‐lowering therapies do not reduce mortality (cardiovascular and all‐cause) or cardiovascular events. Homocysteine‐lowering therapies should not be used in the ESKD population for cardiovascular risk reduction.
Implications for research.
From this review and meta‐analyses, we conclude that there is no benefit in terms of cardiovascular health and mortality from homocysteine‐lowering therapies in ESKD patients. The lack of benefit is consistent across various subgroups and in sensitivity analyses. Future research efforts in ESKD should focus on alternative therapies for the prevention of cardiovascular events.
History
Protocol first published: Issue 1, 2004 Review first published: Issue 5, 2016
| Date | Event | Description |
|---|---|---|
| 11 December 2008 | New citation required and major changes | New authors |
| 1 September 2008 | Amended | Converted to new review format. |
Acknowledgements
We would like to thank the referees for their comments and feedback during the preparation of this review.
Appendices
Appendix 1. Electronic search strategies
| Database | Search terms |
| CENTRAL |
|
| MEDLINE |
|
| EMBASE |
|
Appendix 2. Risk of bias assessment tool
| Potential source of bias | Assessment criteria |
|
Random sequence generation Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence |
Low risk of bias: Random number table; computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots; minimisation (minimisation may be implemented without a random element, and this is considered to be equivalent to being random). |
| High risk of bias: Sequence generated by odd or even date of birth; date (or day) of admission; sequence generated by hospital or clinic record number; allocation by judgement of the clinician; by preference of the participant; based on the results of a laboratory test or a series of tests; by availability of the intervention. | |
| Unclear: Insufficient information about the sequence generation process to permit judgement. | |
|
Allocation concealment Selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment |
Low risk of bias: Randomisation method described that would not allow investigator/participant to know or influence intervention group before eligible participant entered in the study (e.g. central allocation, including telephone, web‐based, and pharmacy‐controlled, randomisation; sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes). |
| High risk of bias: Using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or non‐opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure. | |
| Unclear: Randomisation stated but no information on method used is available. | |
|
Blinding of participants and personnel Performance bias due to knowledge of the allocated interventions by participants and personnel during the study |
Low risk of bias: No blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding; blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding; blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Blinding of outcome assessment Detection bias due to knowledge of the allocated interventions by outcome assessors. |
Low risk of bias: No blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding; blinding of outcome assessment ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding; blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Incomplete outcome data Attrition bias due to amount, nature or handling of incomplete outcome data. |
Low risk of bias: No missing outcome data; reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias); missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size; missing data have been imputed using appropriate methods. |
| High risk of bias: Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size; ‘as‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation; potentially inappropriate application of simple imputation. | |
| Unclear: Insufficient information to permit judgement | |
|
Selective reporting Reporting bias due to selective outcome reporting |
Low risk of bias: The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way; the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon). |
| High risk of bias: Not all of the study’s pre‐specified primary outcomes have been reported; one or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. sub‐scales) that were not pre‐specified; one or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); one or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis; the study report fails to include results for a key outcome that would be expected to have been reported for such a study. | |
| Unclear: Insufficient information to permit judgement | |
|
Other bias Bias due to problems not covered elsewhere in the table |
Low risk of bias: The study appears to be free of other sources of bias. |
| High risk of bias: Had a potential source of bias related to the specific study design used; stopped early due to some data‐dependent process (including a formal‐stopping rule); had extreme baseline imbalance; has been claimed to have been fraudulent; had some other problem. | |
| Unclear: Insufficient information to assess whether an important risk of bias exists; insufficient rationale or evidence that an identified problem will introduce bias. |
Data and analyses
Comparison 1. Primary outcome.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cardiovascular mortality | 4 | 1186 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.70, 1.22] |
Comparison 2. Secondary outcomes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause mortality | 6 | 2447 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.89, 1.11] |
| 2 Myocardial infarction (fatal and non fatal) | 4 | 1510 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.66, 1.62] |
| 3 Coronary revascularisation | 2 | 1160 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.22, 3.14] |
| 4 Stroke | 4 | 1510 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.57, 1.40] |
| 5 Deep venous thrombosis and pulmonary embolism | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6 Thrombosis of dialysis access | 2 | 1261 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.88, 1.14] |
| 7 Adverse events | 3 | 1248 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.51, 2.47] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
ASFAST 2004.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | RCT with random sequence generation |
| Allocation concealment (selection bias) | Low risk | Study medication dispensed in identical containers with neither study staff nor participant aware of treatment allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind RCT |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All clinical end points were subject to an independent adjudication process by an endpoint monitoring committee. Carotid intimal‐medial thickness measurements were performed in the same study laboratory in each major city centre and a single reference laboratory performed all image analyses. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on mortality and morbidity available for 99% of participants; data analysed per intention to treat |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes reported |
| Other bias | Low risk | The study appears to be free of other bias |
Heinz 2009.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | RCT but sequence generation unclear |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind RCT |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome assessment not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Mortality data available on all patients |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes reported |
| Other bias | Low risk | The study appears to be free of other bias |
HOST Study 2004.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random permuted block design of varying block size |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind RCT |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | For primary outcome all patients were counted. For secondary outcome analyses, 32 ESKD patients (from 751) were censored due to study withdrawal |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes reported |
| Other bias | Low risk | The study appears to be free of other bias |
Righetti 2003.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Independent person performed randomisation using a box containing blind numbers |
| Allocation concealment (selection bias) | Low risk | Independent person performed randomisation using a box containing blind numbers |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open RCT |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data complete |
| Selective reporting (reporting bias) | Unclear risk | Composite outcome reported |
| Other bias | High risk | No wash out period for group A, significant differences in baseline variables |
Vianna 2007.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind RCT with participants and personnel blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded fashion interpretation of secondary outcomes mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No patient lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes reported |
| Other bias | Low risk | The study appears to be free of other bias |
Wrone 2004.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Low risk | Randomisation codes were kept in a separate, locked file |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | To maintain double‐blind status, neither the person performing the randomisation nor the person preparing study medication for distribution to clinical coordinators had direct contact with participants. Patients, clinicians, and study staff with patient contact did not have access to any information that could identify treatment arm |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 18 patients of 588 did not receive intervention and were not analysed |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes reported |
| Other bias | Low risk | The study appears to be free of other bias |
AV ‐ arteriovenous; CAPD ‐ continuous ambulatory peritoneal dialysis; CKD ‐ chronic kidney disease; CrCl ‐ creatinine clearance; ESKD ‐ end‐stage kidney disease; HD ‐ haemodialysis; IMT ‐ intima‐media wall thickness; MI ‐ myocardial infarction; PD ‐ peritoneal dialysis; PE ‐ pulmonary embolism; RCT ‐ randomised controlled trial; SCr ‐ serum creatinine; SD ‐ standard deviation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ali 2003 | Intervention's primary action is not homocysteine lowering |
| Alvares Delfino 2007 | < 100 patient‐years |
| Anderson 2006 | < 100 patient‐years |
| Ardalan 2003 | < 100 patient‐years |
| Ardalan 2003a | Kidney transplant not dialysis patients |
| Armada 2003 | < 100 patient‐years |
| Arnadottir 2003 | < 100 patient‐years |
| ATIC Study 2005 | Multiple interventions and < 100 patient‐years |
| Azadibakhsh 2007 | < 100 patient‐years |
| Beavers 2008 | Not homocysteine lowering |
| Bennett‐Richards 2002 | Cross‐over or sequential design |
| Bostom 1996 | < 100 patient‐years |
| Bostom 2000 | < 100 patient‐years |
| Branley 2000 | < 100 patient‐years |
| Brensing 2002 | < 100 patient‐years |
| Brensing 2003 | < 100 patient‐years |
| Chang 2007 | < 100 patient‐years |
| Chiu 2009 | < 100 patient‐years |
| Cianciolo 2008 | Compares different formulations |
| Cutler 2009 | < 100 patient‐years |
| De Angelis 2007 | < 100 patient‐years |
| De Vecchi 2001 | < 100 patient‐years |
| De Vriese 2003 | Sequential or cross‐over design |
| Del Pozo 2005 | < 100 patient‐years |
| Dierkes 1999 | < 100 patient‐years |
| Dierkes 2001 | < 100 patient‐years |
| DIVINe Study 2010 | Population: diabetic nephropathy |
| Dobronravov 2008 | < 100 patient‐years |
| Ducloux 2002 | < 100 patient‐years |
| Elian 2002 | < 100 patient‐years |
| Friedman 2003 | < 100 patient‐years |
| Galli 2003 | < 100 patient‐years |
| Gonin 2003 | < 100 patient‐years |
| Gonin 2003a | < 100 patient‐years |
| Hauser 2001 | < 100 patient‐years |
| Henning 2001 | < 100 patient‐years |
| Hoffer 2005 | < 100 patient‐years |
| Hoffer 2005a | < 100 patient‐years |
| HOPE‐2 Study 2006 | Population: CKD |
| House 1999 | < 100 patient‐years |
| House 2004 | Not folic acid‐based homocysteine lowering |
| Imani 2009 | Not folic acid‐based homocysteine lowering |
| Isbel 2003 | < 100 patient‐years |
| ISRCTN22151635 | Not folic acid‐based homocysteine lowering |
| Jara 2001 | < 100 patient‐years |
| Kazory 2008 | Not folic acid‐based homocysteine lowering |
| Klemm 2004 | Not proven homocysteine lowering treatment |
| Kooshki 2011 | < 100 patient‐years |
| Koyama 2002 | < 100 patient‐years |
| Koyama 2010 | < 100 patient‐years |
| Kuhlmann 2004 | < 100 patient‐years |
| Kumar 2007b | Cross‐over study |
| Kuo 2001a | < 100 patient‐years |
| LANDMARK Study 2006 | Multiple interventions |
| Libetta 2004 | < 100 patient‐years |
| Madsen 2011 | Primary mechanism is not homocysteine lowering |
| Manns 2001 | < 100 patient‐years |
| Mazdeh 2005 | < 100 patient‐years |
| McGregor 2000a | < 100 patient‐years |
| Mudge 2005 | < 100 patient‐years |
| Mueller 2001 | < 100 patient‐years |
| Muller 2001 | < 100 patient‐years |
| Nakamura 2003 | Not an intervention study |
| Nakhoul 2004 | < 100 patient‐years |
| Nascimento 2010 | No pre‐specified outcome data |
| OPACH Study 2006 | Intervention with other effects other than homocysteine lowering |
| Ossareh 2009 | < 100 patient‐years |
| Pakfetrat 2013 | < 100 patient‐years |
| Pastore 2006 | < 100 patient‐years |
| Peng 2005 | < 100 patient‐years |
| Polkinghorne 2003 | < 100 patient‐years |
| Poulia 2011 | < 100 patient‐years |
| Sanchez Alvarez 2005 | < 100 patient‐years |
| Scholze 2004 | N‐acetylcysteine study: intervention with multiple other effects other than homocysteine lowering |
| Seo 2003 | < 100 patient‐years |
| Sepe 1999 | < 100 patient‐years |
| Shemin 2001 | Review article |
| Signorelli 2006 | Intervention with no proven effects on homocysteine lowering |
| Skoutakis 1975 | Not homocysteine lowering study |
| Stavrianaki 2002 | < 100 patient‐years |
| Tamadon 2011 | < 100 patient‐years |
| Tayyebi‐Khosroshahi 2010 | Primary mechanism is not homocysteine lowering |
| Tepel 2003 | N‐acetylcysteine study: intervention with multiple other effects other than homocysteine lowering |
| Thaha 2006 | < 100 patient‐years |
| Thaha 2008 | < 100 patient‐years |
| Thaha 2009 | Cross‐over study |
| Thambyrajah 2000 | < 100 patient‐years |
| Tobe 1999 | < 100 patient‐years |
| Tochihara 2008 | No pre‐specified outcomes |
| Treleaven 2001 | No pre‐specified outcomes |
| Tremblay 2000 | < 100 patient‐years |
| Trimarchi 2002 | < 100 patient‐years |
| Tungkasereerak 2006 | < 100 patient‐years |
| Urquhart 2008 | < 100 patient‐years |
| van Guldener 1998 | No pre‐specified outcomes |
| Van Tellingen 2001 | No pre‐specified outcomes |
| VIENNA Study 2000 | No pre‐specified outcomes |
| Vrentzos 2001 | No pre‐specified outcomes |
| Vychytil 2003 | < 100 patient‐years |
| Westphal 2001 | Intervention with multiple effects in addition to homocysteine lowering |
| Yango 2001 | < 100 patient‐years |
| Zeman 2006 | < 100 patient‐years |
| Zuo 2001 | < 100 patient‐years |
CKD ‐ chronic kidney disease
Characteristics of studies awaiting assessment [ordered by study ID]
NCT00004495.
| Methods | This is an RCT. Patients are stratified according to pre‐study homocysteine levels (above or below average). Patients are randomised to receive placebo or one of two doses of oral folic acid, with or without pyridoxine and cyanocobalamin. Arm I: Patients receive oral placebo daily. Arm II: Patients receive oral pyridoxine, cyanocobalamin, and oral placebo daily. Arm III: Patients receive oral pyridoxine, cyanocobalamin, and folic acid daily. Arm IV: Patients receive oral pyridoxine and cyanocobalamin plus a higher dose of folic acid daily. Arm V: Patients receive oral placebo and oral folic acid daily. Arm VI: Patients receive oral placebo and higher dose folic acid daily. Treatment continues for 8 weeks |
| Participants | Ages eligible for study: 21 to 89 years Genders eligible for study: both Accepts healthy volunteers: no Entry criteria Disease characteristics: diagnosis of ESKD requiring regular HD treatment 3 times weekly; baseline predialysis total homocysteine concentration in plasma greater than 16 µmol/L; no prior or concurrent pernicious anaemia; no blood smear examination showing unexplained macrocytosis Prior/concurrent therapy Chemotherapy: no concurrent chemotherapy for cancer Other: no concurrent levodopa or carbidopa; no concurrent penicillamine or trimethoprim‐sulphonamide combination; no concurrent antiviral therapy No concurrent anticonvulsants Patient characteristics Hematopoietic: HCT at least 25% Other: not pregnant or nursing Negative pregnancy test; fertile patients must use effective contraception; no Parkinson's disease; no convulsions or epilepsy requiring treatment; no lactose intolerance or allergy to milk products; no history of allergic sensitization following administration of folic acid, pyridoxine (vitamin B6), or cyanocobalamin (vitamin B12); no vitamin B12 concentration below lower limit of normal (150 pmol/L); no untreated hypothyroidism or psoriasis |
| Interventions | Patients are randomised to receive placebo or one of two doses of oral folic acid, with or without pyridoxine and cyanocobalamin Arm I: Patients receive oral placebo daily Arm II: Patients receive oral pyridoxine, cyanocobalamin, and oral placebo daily Arm III: Patients receive oral pyridoxine, cyanocobalamin, and folic acid daily Arm IV: Patients receive oral pyridoxine and cyanocobalamin plus a higher dose of folic acid daily Arm V: Patients receive oral placebo and oral folic acid daily Arm VI: Patients receive oral placebo and higher dose folic acid daily Treatment continues for 8 weeks |
| Outcomes | Objectives I. Compare the efficacy of two doses of folic acid in normalizing plasma total homocysteine concentration in patients with ESKD receiving regular HD therapy resulting in hyperhomocysteinaemia II. Determine the requirement of co‐supplementation with extra pyridoxine (vitamin B6) and cyanocobalamin (vitamin B12) daily in these patients III. Assess the safety and tolerability of this therapy in these patients |
| Notes |
Soleimani 2011.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Tayebi‐Khosroshahi 2013.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
ESKD ‐ end‐stage kidney disease; HCT ‐ haematocrit; HD ‐ haemodialysis
Differences between protocol and review
In the protocol, we mentioned N‐acetyl cysteine as an intervention that lowers serum homocysteine levels to be considered for this review. Although it has this effect there are significant other effects including anti‐oxidant effects from this intervention and since this review is focused on interventions that primarily reduce homocysteine, we decided to not include studies that evaluated N‐acetyl cysteine in the ESKD setting in this review. Our search identified only four such studies (Ali 2003; Nascimento 2010; Scholze 2004; Tepel 2003; Thaha 2006).
Contributions of authors
Draft the protocol: AC, MG, MJ, AK, SK, SDN, SN, TN, VP
Study selection: MJ, AK, SK, SDN, SN, VP,
Extract data from studies: MJ, SK, SDN, SN, VP
Enter data into RevMan: MJ, SN
Carry out the analysis: MJ, SK, SDN, SN, TN, VP
Interpret the analysis: MJ, SK, SDN, SN, TN, VP, GS, SZ
Draft the final review: MJ, SK, SDN, SN, TN, VP
Disagreement resolution: AC, MG, MJ, AK, SK, SDN, SN, TN, VP, GS, SZ
Update the review: AC, MG, MJ, AK, SK, SDN, SN, TN, VP, GS, SZ
Sources of support
Internal sources
No sources of support supplied
External sources
-
Clinical Scientist in Nephrology award from the American Kidney Fund, USA.
Sagar U Nigwekar
Declarations of interest
Sagar U Nigwekar: none known
Amy Kang: none known
Sophia Zoungas: I have received speaker honoria from Servier, MSD, Novo Nordisk, Sanofi Aventis, Johnson and Johnson and Astra Zeneca/BMS. I have served on external advisory boards for MSD, Amgen, AbbVie, Novo Nordisk, Novartis, Takeda, Sanofi Aventis and Astra Zeneca.
Alan Cass: The Menzies School of Health Research has received unconditional research funding from AMGEN, Merck and Novartis for research in chronic kidney disease in Indigenous populations
Martin P Gallagher: Martin Gallagher has received competitive research funding from the Royal Australasian College of Physicians and the Australian National Health and Medical Research Council in the last 36 months.
Satyarth Kulshrestha: none known
Sankar D Navaneethan: none known
Vlado Perkovic: none known
Giovanni FM Strippoli: Institutional support from AIfa‐italian medicines agenda for Cedose trial funding (ESA dose); employment by Diaverum, renal service provider for dialysis
Meg J Jardine: is supported by a NHMRC Career Development Fellowship and National Heart Foundation Future Leader Fellowship.
New
References
References to studies included in this review
ASFAST 2004 {published data only}
- McGrath B, Zoungas S, McNeil J, Branley P, Kerr P, ASFAST Investigators. The atherosclerosis and folic acid supplementation trial in CRF (ASFAST): rationale, design and baseline results [abstract no: 65]. Nephrology 2002;7(Suppl 3):A17. [CENTRAL: CN‐00856590] [Google Scholar]
- McGrath B, Zoungas S, McNeil J, Kerr PG. The atherosclerosis and folic acid supplementation trial in CRF (ASFAST): rationale, design and baseline results [abstract]. Journal of the American Society of Nephrology 2001;12(Program & Abstracts):228A. [CENTRAL: CN‐00446685] [Google Scholar]
- Polkinghorne K, Kerr PG, Muske C, McGrath BP, Zoungas S, The ASFAST Investigators. The effect of lowering homocysteine on haemodialysis vascular access outcomes: the ASFAST randomised placebo controlled trial [abstract no: SU‐FC110]. Journal of the American Society of Nephrology 2007;18(Abstracts):92A. [CENTRAL: CN‐00716085] [Google Scholar]
- Zoungas S, Branley P, Kerr PG, Ristevski S, Muske C, Demos L, et al. Atherosclerosis and folic acid supplementation trial in chronic renal failure: baseline results. Nephrology 2004;9(3):130‐41. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Zoungas S, Cameron JD, Kerr PG, Wolfe R, Muske C, McNeil JJ, et al. Association of carotid intima‐medial thickness and indices of arterial stiffness with cardiovascular disease outcomes in CKD. American Journal of Kidney Diseases 2007;50(4):622‐30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Zoungas S, Kerr P, Lui M, Teede H, McNeil J, McGrath B, et al. Chronic kidney disease, cardiovascular events and the effect of diabetes: post hoc analyses from ASFAST [abstract no: 128]. Nephrology 2008;13(Suppl 3):A133. [Google Scholar]
- Zoungas S, Lui M, Kerr PG, Teede HJ, McNeil JJ, McGrath BP, et al. Advanced chronic kidney disease, cardiovascular events and the effect of diabetes: data from the Atherosclerosis and Folic Acid Supplementation Trial. Internal Medicine Journal 2011;41(12):825‐32. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Zoungas S, McGrath BP, Branley P, Kerr PG, Muske C, Wolfe R, et al. Cardiovascular morbidity and mortality in the Atherosclerosis and Folic Acid Supplementation Trial (ASFAST) in chronic renal failure: a multicenter, randomized, controlled trial. Journal of the American College of Cardiology 2006;47(6):1108‐16. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Heinz 2009 {published data only}
- Heinz J, Dierkes J, Domrose U, Neumann KH, Luley C. Influence of a supplementation with vitamins on morbidity and mortality of ESRD patients: a multi‐center randomly designed double blinded intervention trial; Prismavit ‐ prospective intervention study from Magdeburg with vitamins [abstract]. XIV Lipid Meeting; 2003 Dec 5‐7; Leipzig, Germany. 2003:89. [CENTRAL: CN‐00740532]
- Heinz J, Domrose U, Luley C, Westphal S, Kropf S, Neumann KH, et al. Influence of a supplementation with vitamins on cardiovascular morbidity and mortality in patients with end‐stage renal disease: design and baseline data of a randomized clinical trial. Clinical Nephrology 2009;71(3):363‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Heinz J, Kropf S, Domrose U, Westphal S, Borucki K, Luley C, et al. B vitamins and the risk of total mortality and cardiovascular disease in end‐stage renal disease: results of a randomized controlled trial. Circulation 2010;121(12):1432‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
HOST Study 2004 {published data only}
- Brady CB, Gaziano JM, Cxypoliski RA, Guarino PD, Kaufman JS, Warren SR, et al. Homocysteine lowering and cognition in CKD: the Veterans Affairs homocysteine study. American Journal of Kidney Diseases 2009;54(3):440‐9. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamison R, Hartigan P, Gaziano M, Kaufman J, Goldfarb DS, Warren S, et al. High plasma homocysteine (Hcy) and cardiovascular (CV) disease in end stage (ESRD) and advanced chronic (ACKD) kidney disease. The VA Coop. study program homocysteine study (HOST) progress report [abstract no: PUB272]. Journal of the American Society of Nephrology 2003;14(Nov):832A. [CENTRAL: CN‐00550589] [Google Scholar]
- Jamison RL, Hartigan P, Gaziano JM, Fortmann SP, Goldfarb DS, Haroldson JA, et al. Design and statistical issues in the homocysteinemia in kidney and end stage renal disease (HOST) study. Clinical Trials 2004;1(5):451‐60. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Jamison RL, Hartigan P, Gaziano M, Kaufman J, Goldfarb DS, Warren S, et al. Cardiovascular disease in patients with advanced chronic kidney disease (ACKD) and hyperhomocysteinemia. The VA Coop. Studies Program Homocysteine Study (HOST) [abstract no: F‐PO300]. Journal of the American Society of Nephrology 2004;15(Oct):132A. [CENTRAL: CN‐00550590] [Google Scholar]
- Jamison RL, Hartigan P, Kaufman JS, Goldfarb DS, Warren SR, Guarino P, et al. Homocysteine lowering effect on mortality and vascular disease in advanced chronic kidney disease and end stage renal disease [abstract no: M‐FC‐041]. 4th World Congress of Nephrology.19th International Congress of the International Society of Nephrology (ISN); 2007 Apr 21‐25; Rio de Janeiro, Brazil. 2007:211.
- Jamison RL, Hartigan P, Kaufman JS, Goldfarb DS, Warren SR, Guarino PD, et al. Effect of homocysteine lowering on mortality and vascular disease in advanced chronic kidney disease and end‐stage renal disease: a randomized controlled trial.[Erratum appears in JAMA. 2008 Jul 9;300(2):170]. JAMA 2007;298(10):1163‐70. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Jamison RL, Shih MC, Humphries DE, Guarino PD, Kaufman JS, Goldfarb DS, et al. Effect of the MTHFR C677T and A1298C polymorphisms on survival in patients with advanced CKD and ESRD: a prospective study. American Journal of Kidney Diseases 2009;53(5):779‐89. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Jovanovich A, Chonchol M, Cheung AK, Kaufman JS, Greene T, Roberts WL, et al. Racial differences in markers of mineral metabolism in advanced chronic kidney disease. Clinical Journal of the American Society of Nephrology: CJASN 2012;7(4):640‐7. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendrick J, Cheung AK, Kaufman JS, Greene T, Roberts WL, Smits G, et al. Associations of plasma 25‐hydroxyvitamin D and 1,25‐dihydroxyvitamin D concentrations with death and progression to maintenance dialysis in patients with advanced kidney disease. American Journal of Kidney Diseases 2012;60(4):567‐75. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendrick JB, Cheung AK, Kaufman JS, Greene T, Roberts W, Smits G, et al. Fibroblast growth factor‐23 and progression to dialysis in patients with advanced kidney disease [abstract no: F‐PO1879]. Journal of the American Society of Nephrology 2009;20:542A. [CENTRAL: CN‐00740513] [Google Scholar]
- Kendrick JB, Cheung AK, Kaufman JS, Greene T, Roberts W, Smits G, et al. Fibroblast growth factor‐23 and the risk of death and cardiovascular events among patients with chronic kidney disease [abstract no: F‐PO1873]. Journal of the American Society of Nephrology 2009;20:540A. [CENTRAL: CN‐00740525] [Google Scholar]
- Kendrick JB, Cheung AK, Kaufman JS, Greene T, Roberts W, Smits G, et al. Higher intact parathyroid hormone levels are not associated with all‐cause mortality or cardiovascular events in patients with advanced kidney disease [abstract no: F‐PO1902]. Journal of the American Society of Nephrology 2009;20:547A. [CENTRAL: CN‐00740514] [Google Scholar]
- Kendrick JB, Cheung AK, Kaufman JS, Greene T, Roberts W, Smits G, et al. Low serum levels of 1,25‐dihydroxyvitamin D, not 25‐hydroxyvitamin D, is associated with all‐cause mortality in chronic kidney disease [abstract no: TH‐PO656]. Journal of the American Society of Nephrology 2009;20:265A. [CENTRAL: CN‐00740559] [Google Scholar]
- Kendrick JB, Cheung AK, Kaufman JS, Greene T, Roberts W, Smits G, et al. Low serum levels of 1,25‐dihydroxyvitamin D, not 25‐hydroxyvitamin D, is associated with progression to dialysis in patients with advanced kidney disease [abstract no: TH‐PO647]. Journal of the American Society of Nephrology 2009;20:263A. [CENTRAL: CN‐00740517] [Google Scholar]
- Montford JR, Chonchol M, Cheung AK, Kaufman JS, Greene T, Roberts WL, et al. Low body mass index and dyslipidemia in dialysis patients linked to elevated plasma fibroblast growth factor 23. American Journal of Nephrology 2013;37(3):183‐90. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafeq Z, Roh JD, Guarino P, Kaufman J, Joseph J. Adverse myocardial effects of B‐vitamin therapy in subjects with chronic kidney disease and hyperhomocysteinaemia. Nutrition Metabolism & Cardiovascular Diseases 2013 Sep;23(9):836‐42. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Righetti 2003 {published data only}
- Righetti M. Homocysteine‐lowering vitamin B treatment decreases cardiovascular events in hemodialysis patients. Clinical Chemistry & Laboratory Medicine 2007;45(12):1586‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Righetti M, Ferrario GM, Milani S, Serbelloni P, Rosa L, Uccellini M, et al. Effects of folic acid treatment on homocysteine levels and vascular disease in hemodialysis patients. Medical Science Monitor 2003;9(4):19‐24. [MEDLINE: ] [PubMed] [Google Scholar]
- Righetti M, Ferrario GM, Milani S, Serbelloni P, Rosa L, Uccellini M, et al. Long‐term effects of oral folic acid therapy on homocysteinemia and vascular disease in haemodialysis patients [abstract no: T237]. Nephrology Dialysis Transplantation 2002;17(Suppl 1):255. [CENTRAL: CN‐00509435] [Google Scholar]
- Righetti M, Serbelloni P, Milani S, Ferrario G. Homocysteine‐lowering vitamin B treatment decreases cardiovascular events in hemodialysis patients. Blood Purification 2006;24(4):379‐86. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Vianna 2007 {published data only}
- Vianna AC, Mocelin AJ, Matsuo T, Morais‐Filho D, Largura A, Delfino VA, et al. Uremic hyperhomocysteinemia: a randomized trial of folate treatment for the prevention of cardiovascular events. Hemodialysis International 2007;11(2):210‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wrone 2004 {published data only}
- Wrone E, Hornberger J, Zehnder J, McCann L, Coplon N, Fortmann S. Randomized trial of folic acid for homocysteine reduction in end‐stage renal disease [abstract]. American Journal of Kidney Diseases 2002;39(4):A34. [CENTRAL: CN‐00403143] [Google Scholar]
- Wrone EM, Hornberger JM, Zehnder JL, McCann LM, Coplon NS, Fortmann SP. Randomized trial of folic acid for prevention of cardiovascular events in end‐stage renal disease. Journal of the American Society of Nephrology 2004;15(2):420‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Ali 2003 {published data only}
- Ali Z, Idham C, Effendi I. The effect of n‐acetylcysteine on homocysteine level in chronic hemodialysis patients: randomized control trial [abstract]. Nephrology 2003;8(Suppl 3):A62. [CENTRAL: CN‐00671778] [Google Scholar]
Alvares Delfino 2007 {published data only}
- Alvares Delfino V, Andrade Vianna AC, Mocelin AJ, Barbosa DS, Mise RA, Matsuo T. Folic acid therapy reduces plasma homocysteine levels and improves plasma antioxidant capacity in hemodialysis patients. Nutrition 2007;23(3):242‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Anderson 2006 {published data only}
- Anderson TJ, Sun YH, Hubacek J, Hyndman ME, Verma S, Shewchuk L, et al. Effects of folinic acid on forearm blood flow in patients with end‐stage renal disease. Nephrology Dialysis Transplantation 2006;21(7):1927‐33. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ardalan 2003 {published data only}
- Ardalan MR, Etemadi J, Mortazavi M, Majidi J. Hyperhomocysteinaemia therapy in haemodialysis patients with combination of Vitamin B6, B12 and folic acid [abstract no: W467]. Nephrology Dialysis Transplantation 2003;18(4):696. [CENTRAL: CN‐00716091] [Google Scholar]
Ardalan 2003a {published data only}
- Ardalan MR, Mortazavi M, Etemadi J. Hyperhomocysteinemia and its therapy in renal transplant recipients with combination of: Vit B6, Vit B12 and folic acid [abstract]. Nephrology Dialysis Transplantation 2003;18(Suppl 4):509‐10. [CENTRAL: CN‐00444217] [Google Scholar]
Armada 2003 {published data only}
- Armada E, Perez C, Otero A, Esteban J, Camba M, Gayoso P, et al. Neither folic nor folinic acid normalize homocysteine levels in hemodialysis patients. Clinical Nephrology 2003;60(3):168‐75. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Arnadottir 2003 {published data only}
- Arnadottir M, Hultberg B. The effect of vitamin B12 on total plasma homocysteine concentration in folate‐replete hemodialysis patients. Clinical Nephrology 2003;59(3):186‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
ATIC Study 2005 {published data only}
- Nanayakkara PW, Kiefte‐De Jong JC, Stehouwer CD, Ittersum FJ, Olthof MR, Kok RM, et al. Association between global leukocyte DNA methylation, renal function, carotid intima‐media thickness and plasma homocysteine in patients with stage 2‐4 chronic kidney disease. Nephrology Dialysis Transplantation 2008;23(8):2586‐92. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Nanayakkara PW, Kiefte‐de Jong JC, ter Wee PM, Stehouwer CD, Ittersum FJ, Olthof MR, et al. Randomized placebo‐controlled trial assessing a treatment strategy consisting of pravastatin, vitamin E, and homocysteine lowering on plasma asymmetric dimethylarginine concentration in mild to moderate CKD. American Journal of Kidney Diseases 2009;53(1):41‐50. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Nanayakkara PW, Teerlink T, Stehouwer CD, Allajar D, Spijkerman A, Schalkwijk C, et al. Plasma asymmetric dimethylarginine (ADMA) concentration is independently associated with carotid intima‐media thickness and plasma soluble vascular cell adhesion molecule‐1 (sVCAM‐1) concentration in patients with mild‐to‐moderate renal failure. Kidney International 2005;68(5):2230‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Nanayakkara PW, Guldener C, ter Wee PM, Scheffer PG, Ittersum FJ, Twisk JW, et al. Effect of a treatment strategy consisting of pravastatin, vitamin E, and homocysteine lowering on carotid intima‐media thickness, endothelial function, and renal function in patients with mild to moderate chronic kidney disease: results from the Anti‐Oxidant Therapy in Chronic Renal Insufficiency (ATIC) Study. Archives of Internal Medicine 2007;167(12):1262‐70. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Nanayakkara PW, Guldener C, Ittersum F, Stehouwer CD, ter Wee PM. Effect of an oxidative‐stress‐reducing strategy on carotid intima‐media thickness, endothelial function and renal function in patients with mild‐to‐moderate chronic kidney disease [abstract no: F‐PO145]. Journal of the American Society of Nephrology 2006;17(Abstracts):367A. [CENTRAL: CN‐00602094] [Google Scholar]
- Nanayakkara PW, Ittersum FJ, Fouque D, Charrie A, Stehouwer CD, ter Wee PM. Plasma adiponectin is inversely associated with renal function in patients with Stage 3 and 4 chronic kidney disease [abstract no: F‐PO146]. Journal of the American Society of Nephrology 2006;17(Abstracts):367A. [CENTRAL: CN‐00602093] [Google Scholar]
- Thijs A, Nanayakkara PW, ter Wee PM, Huijgens PC, Guldener C, Stehouwer CD. Mild‐to‐moderate renal impairment is associated with platelet activation: a cross‐sectional study. Clinical Nephrology 2008;70(4):325‐31. [MEDLINE: ] [PubMed] [Google Scholar]
- Veringa SJ, Nanayakkara PW, Ittersum FJ, Vegting IL, Guldener C, Smulders YM, et al. Effect of a treatment strategy consisting of pravastatin, vitamin E, and homocysteine lowering on arterial compliance and distensibility in patients with mild‐to‐moderate chronic kidney disease. Clinical Nephrology 2012;78(4):263‐72. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Azadibakhsh 2007 {published data only}
- Azadibakhsh N, Hosseini RS, Atabak S, Nateghiyan N, Golestan B, Rad AH. Efficacy of folate and vitamin B12 in lowering homocysteine concentrations in hemodialysis patients. Saudi Journal of Kidney Diseases & Transplantation 2009;20(5):779‐88. [MEDLINE: ] [PubMed] [Google Scholar]
- Azadibakhsh N, Hosseini RS, Atabak S, Nateghiyan N, Golestan B, Rad AH. The effect of folate and vitamin B12 supplementation on homocysteine concentrations: a study in hemodialysis patients. Tehran University Medical Journal 2007;65(8):49‐56. [CENTRAL: CN‐00796293] [Google Scholar]
Beavers 2008 {published data only}
- Beavers KM, Beavers DP, Bowden RG, Wilson RL, Gentile M. Effect of over‐the‐counter fish‐oil administration on plasma Lp(a) levels in an end‐stage renal disease population. Journal of Renal Nutrition 2009;19(6):443‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Beavers KM, Beavers DP, Bowden RG, Wilson RL, Gentile M. Omega‐3 fatty acid supplementation and total homocysteine levels in end‐stage renal disease patients. Nephrology 2008;13(4):284‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bennett‐Richards 2002 {published data only}
- Bennett‐Richards K, Kattenhorn M, Donald A, Oakley G, Varghese Z, Rees L, et al. Does oral folic acid lower total homocysteine levels and improve endothelial function in children with chronic renal failure?. Circulation 2002;105(15):1810‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Bennett‐Richards K, Taylor M, Oakley G, Varghese Z, Deanfield J, Rees L. Does oral folic acid lower total homocystine levels and improve endothelial function in children with CRF? [abstract]. Pediatric Nephrology 2001;16(8):C167. [CENTRAL: CN‐00444396] [DOI] [PubMed] [Google Scholar]
Bostom 1996 {published data only}
- Bostom AG, Shemin D, Lapane KL, Hume AL, Yoburn D, Nadeau MR, et al. High dose‐B‐vitamin treatment of hyperhomocysteinemia in dialysis patients. Kidney International 1996;49(1):147‐52. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bostom 2000 {published data only}
- Bostom AG, Shemin D, Bagley P, Massy ZA, Zanabli A, Christopher K, et al. Controlled comparison of L‐5‐methyltetrahydrofolate versus folic acid for the treatment of hyperhomocysteinemia in hemodialysis patients.[Erratum appears in Circulation 2000 Aug 1;102(5):598]. Circulation 2000;101(24):2829‐32. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Bostom AG, Shemin D, Bagley P, Massy ZA, Zanabli A, Spiegel P, et al. Reduced folates are an expensive treatment for hyperhomocysteinemia in hemodialysis patients with no greater efficacy than folic acid: results of a controlled comparison study [abstract]. Journal of the American Society of Nephrology 2000;11(Sept):257A. [CENTRAL: CN‐00550713] [Google Scholar]
- Bostom AG, Shemin D, Gohh RY, Beaulieu AJ, Bagley P, Massy ZA, et al. Treatment of hyperhomocysteinemia in hemodialysis patients and renal transplant recipients. Kidney International ‐ Supplement 2001;59(Suppl 78):S246‐52. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Bostom AG, Shemin D, Gohh RY, Beaulieu AJ, Jacques PF, Dworkin L, et al. Treatment of mild hyperhomocysteinemia in renal transplant recipients versus hemodialysis patients. Transplantation 2000;69(10):2128‐31. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Branley 2000 {published data only}
- Branley P, D'Intini V, Thomson N, Fraenkel M, McNeil JJ. Randomised placebo controlled clinical trial of supplementary folic acid in CRF [abstract]. Nephrology 2000;5(3):A79. [CENTRAL: CN‐00400364] [Google Scholar]
Brensing 2002 {published data only}
- Brensing KA, Kaschell D, Hages M, Lutjohann D, Sigit J, Pietrizik K, et al. Improved hyperhomocysteinemia in hemodialysed ESRD‐patients by intravenous folate or folinic acid: a randomized controlled trial [abstract no: O100]. Nephrology Dialysis Transplantation 2002;17(Suppl 1):30. [CENTRAL: CN‐00509104] [Google Scholar]
Brensing 2003 {published data only}
- Brensing KA, Hages M, Raab P, Pietrzik K, Bergmann K, Frotscher U, et al. Vitamin B12 and folic‐acid as intravenous high‐dose vitamin therapy for hyperhomocysteinemia in hemodialysed ESRD‐patients: data of a prospective randomized controlled study [abstract no: SU‐PO1048]. Journal of the American Society of Nephrology 2003;14(Nov):766A. [CENTRAL: CN‐00644257] [Google Scholar]
Chang 2007 {published data only}
- Chang TY, Chou KJ, Tseng CF, Chung HM, Fang HC, Hung YM, et al. Effects of folic acid and vitamin B complex on serum C‐reactive protein and albumin levels in stable hemodialysis patients. Current Medical Research & Opinion 2007;23(8):1879‐86. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Chiu 2009 {published data only}
- Chiu YW, Chang JM, Hwang SJ, Tsai JC, Chen HC. Pharmacological dose of vitamin B12 is as effective as low‐dose folinic acid in correcting hyperhomocysteinemia of hemodialysis patients. Renal Failure 2009;31(4):278‐83. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cianciolo 2008 {published data only}
- Cianciolo G, Manna G, Coli L, Donati G, D'Addio F, Persici E, et al. 5‐methyltetrahydrofolate administration is associated with prolonged survival and reduced inflammation in ESRD patients. American Journal of Nephrology 2008;28(6):941‐8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cutler 2009 {published data only}
- Cutler MJ, Urquhart BL, Freeman DJ, Spence JD, House AA. Mesna for the treatment of hyperhomocysteinemia in hemodialysis patients. Blood Purification 2009;27(3):306‐10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
De Angelis 2007 {published data only}
- Angelis S, Noce A, Dessi M, Pastore A, Scaccia F, Trombetta M, et al. Hyperhomocysteinemia and alternate vitamin supplementation [Iperomocisteinemia nei pazienti uremici: effetti della supplementazione alternata di acido folico e vitamina B 12]. Giornale Italiano di Nefrologia 2007;24(1):51‐5. [MEDLINE: ] [PubMed] [Google Scholar]
- Dessi M, Giovamberardino G, Pieri M, Noce A, Zenobi R, Daniele N, et al. Influence of dialysis techniques and alternate vitamin supplementation on homocysteine levels in patients with known MTHFR genotypes. Clinical & Experimental Nephrology 2015;19(1):140‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Del Pozo 2005 {published data only}
- Pozo C, Alvarez L, Lopez‐Menchero R, Torregrosa I, Sanchez L, Albero MD. Optimization of a therapy schedule with folinic acid to treat hyperhomocysteinemia in patients on hemodialysis [abstract no: SP261]. Nephrology Dialysis Transplantation 2005;20(Suppl 5):v106. [CENTRAL: CN‐00671828] [Google Scholar]
De Vecchi 2001 {published data only}
- Vecchi AF, Patrosso C, Novembrino C, Finazzi S, Colucci P, Franceschi M, et al. Folate supplementation in peritoneal dialysis patients with normal erythrocyte folate: effect on plasma homocysteine. Nephron 2001;89(3):297‐302. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
De Vriese 2003 {published data only}
- Vriese AS, Langlois M, Bernard D, Geerolf I, Stevens L, Boelaert JR, et al. Effect of dialyser membrane pore size on plasma homocysteine levels in haemodialysis patients. Nephrology Dialysis Transplantation 2003;18(12):2596‐600. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Dierkes 1999 {published data only}
- Dierkes J, Domrose U, Ambrosch A, Bosselmann HP, Neumann KH, Luley C. Response of hyperhomocysteinemia to folic acid supplementation in patients with end‐stage renal disease. Clinical Nephrology 1999;51(2):108‐15. [MEDLINE: ] [PubMed] [Google Scholar]
Dierkes 2001 {published data only}
- Dierkes J, Domrose U, Bosselmann KP, Neumann KH, Luley C. Homocysteine lowering effect of different multivitamin preparations in patients with end‐stage renal disease. Journal of Renal Nutrition 2001;11(2):67‐72. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
DIVINe Study 2010 {published data only}
- House AA, Ehasziw M, Cattran DC, Churchill DN, Oliver MJ, Fine A, et al. Vitamin therapy for homocysteine accelerates loss of renal function in diabetic kidney disease [abstract no: SA‐FC343]. Journal of the American Society of Nephrology 2009;20:80A. [CENTRAL: CN‐00740523] [Google Scholar]
- House AA, Eliasziw M, Cattran DC, Churchill DN, Oliver MJ, Fine A, et al. Effect of B‐vitamin therapy on progression of diabetic nephropathy: a randomized controlled trial. JAMA 2010;303(16):1603‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Dobronravov 2008 {published data only}
- Dobronravov V, Ardebili M, Golubev R, Galkina O, Nabokow A, Kliem V, et al. The effect of IV administration of sodium 2,3‐dimercaptopropane‐1‐sulfonate (DMPS) and 8‐week high‐dosed IV vitamin B therapy on total plasma homocysteine (tHcy) in patients undergoing chronic hemodialysis (HD) [abstract no: PUB584]. Journal of the American Society of Nephrology 2008;19(Abstracts Issue):943A. [CENTRAL: CN‐00758243] [Google Scholar]
Ducloux 2002 {published data only}
- Ducloux D, Aboubakr A, Motte G, Toubin G, Fournier V, Chalopin JM, et al. Hyperhomocysteinaemia therapy in haemodialysis patients: folinic versus folic acid in combination with vitamin B6 and B12. Nephrology Dialysis Transplantation 2002;17(5):865‐70. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ducloux D, Aboubakr A, Motte G, Toubin G, Fournier V, Chalopin JM, et al. Hyperhomocysteinemia therapy in hemodialysis (HD) patients: folinic versus folic acid in combination with vitamin B6 and B12 [abstract]. Journal of the American Society of Nephrology 2001;12(Program & Abstracts):354A‐5A. [CENTRAL: CN‐00445156] [DOI] [PubMed] [Google Scholar]
Elian 2002 {published data only}
- Elian KM, Hoffer LJ. Hydroxocobalamin reduces hyperhomocysteinemia in end‐stage renal disease. Metabolism: Clinical & Experimental 2002;51(7):881‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Friedman 2003 {published data only}
- Friedman AN, Bostom AG, Laliberty P, Selhub J, Shemin D. The effect of N‐acetylcysteine on plasma total homocysteine levels in hemodialysis: a randomized, controlled study. American Journal of Kidney Diseases 2003;41(2):442‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Galli 2003 {published data only}
- Galli F, Benedetti S, Buoncristiani U, Piroddi M, Conte C, Canestrari F, et al. The effect of PMMA‐based protein‐leaking dialyzers on plasma homocysteine levels. Kidney International 2003;64(2):748‐55. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Galli F, Benedetti S, Floridi A, Canestrari F, Piroddi M, Buoncristiani E, et al. Glycoxidation and inflammatory markers in patients on treatment with PMMA‐based protein‐leaking dialyzers. Kidney International 2005;67(2):750‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gonin 2003 {published data only}
- Gonin JM, Nguyen H, Gonin R, Sarna A, Michels A, Masri‐Imad F, et al. Controlled trials of very high dose folic acid, vitamins B12 and B6, intravenous folinic acid and serine for treatment of hyperhomocysteinemia in ESRD. Journal of Nephrology 2003;16(4):522‐34. [MEDLINE: ] [PubMed] [Google Scholar]
- Gonin JM, Sarna A, Loya A, Gonin R, Chassaing C, Wainer I, et al. A double‐blind, controlled study of folate and vitamin therapy for hyperhomocysteinemia in hemodialysis [abstract]. Journal of the American Society of Nephrology 2000;11(Sept):269A. [CENTRAL: CN‐00755224] [Google Scholar]
Gonin 2003a {published data only}
- Gonin J, Nguyen H, Michels A, Santos A, Gonin R, Cary D, et al. Evaluation of folinic acid and serine in hyperhomocysteinemia in ESRD: a prospective placebo‐controlled randomized study [abstract]. Journal of the American Society of Nephrology 2001;12(Program & Abstracts):356A. [CENTRAL: CN‐00445513] [Google Scholar]
- Gonin JM, Nguyen H, Gonin R, Sarna A, Michels A, Masri‐Imad F, et al. Controlled trials of very high dose folic acid, vitamins B12 and B6, intravenous folinic acid and serine for treatment of hyperhomocysteinemia in ESRD. Journal of Nephrology 2003;16(4):522‐34. [MEDLINE: ] [PubMed] [Google Scholar]
Hauser 2001 {published data only}
- Hagen W, Hauser A, Rehak PH, Buchmayer H, Fodinger M, Horl WH, et al. Comparison of intravenous folinic acid with intravenous folic acid for the treatment of hyperhomocysteinemia in hemodialysis patients. A randomized, double blinded trial [abstract]. Journal of the American Society of Nephrology 2000;11(Sept):148A. [CENTRAL: CN‐00583332] [Google Scholar]
- Hagen W, Kletzmayr J, Fodinger M, Hauser AC, Horl WH, Sunder‐Plassmann G. Effect of intravenous folic acid or folinic acid therapy on mean arterial pressure and pulse pressure in hemodialysis patients [abstract]. Journal of the American Society of Nephrology 2001;12(Program & Abstracts):483A. [CENTRAL: CN‐00583333] [Google Scholar]
- Hauser AC, Hagen W, Rehak PH, Buchmayer H, Fodinger M, Papagiannopoulos M, et al. Efficacy of folinic versus folic acid for the correction of hyperhomocysteinemia in hemodialysis patients. American Journal of Kidney Diseases 2001;37(4):758‐65. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Henning 2001 {published data only}
- Henning BF, Zidek W, Riezler R, Graefe U, Tepel M. Homocyst(e)ine metabolism in hemodialysis patients treated with vitamins B6, B12 and folate. Research in Experimental Medicine 2001;200(3):155‐68. [MEDLINE: ] [PubMed] [Google Scholar]
Hoffer 2005 {published data only}
- Hoffer LJ, Saboohi F, Golden M, Barre PE. Cobalamin dose regimen for maximum homocysteine reduction in end‐stage renal disease. Metabolism: Clinical & Experimental 2005;54(6):835‐40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hoffer 2005a {published data only}
- Hoffer LJ, Djahangirian O, Bourgouin PE, Eid J, Saboohi F. Comparative effects of hydroxocobalamin and cyanocobalamin on plasma homocysteine concentrations in end‐stage renal disease. Metabolism: Clinical & Experimental 2005;54(10):1362‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
HOPE‐2 Study 2006 {published data only}
- Lonn E, Held C, Arnold JM, Probstfield J, McQueen M, Micks M, et al. Rationale, design and baseline characteristics of a large, simple, randomized trial of combined folic acid and vitamins B6 and B12 in high‐risk patients: the Heart Outcomes Prevention Evaluation (HOPE)‐2 trial. Canadian Journal of Cardiology 2006;22(1):47‐53. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonn E, Yusuf S, Arnold MJ, Sheridan P, Pogue J, Micks M, et al. Homocysteine lowering with folic acid and B vitamins in vascular disease.[erratum appears in N Engl J Med. 2006 Aug 17;355(7):746]. New England Journal of Medicine 2006;354(15):1567‐77. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mann JF, Sheridan P, McQueen MJ, Held C, Arnold JM, Fodor G, et al. Homocysteine lowering with folic acid and B vitamins in people with chronic kidney disease‐‐results of the renal Hope‐2 study. Nephrology Dialysis Transplantation 2008;23(2):645‐53. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ray JG, Kearon C, Yi Q, Sheridan P, Lonn E, Heart Outcomes Prevention Evaluation (HOPE‐2). Homocysteine‐lowering therapy and risk for venous thromboembolism: a randomized trial.[Summary for patients in Ann Intern Med. 2007 Jun 5;146(11):I22; PMID: 17470823]. Annals of Internal Medicine 2007;146(11):761‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Righetti M. Hopeful for a second HOPE‐2 post hoc analysis. Nephrology Dialysis Transplantation 2008;23(7):2428‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Saposnik G, Ray JG, Sheridan P, McQueen M, Lonn E. Homocysteine‐lowering therapy and stroke risk, severity, and disability: additional findings from the HOPE 2 trial. Stroke 2009;40(4):1365‐72. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Sawka AM, Ray JG, Yi Q, Josse RG, Lonn E. Randomized clinical trial of homocysteine level lowering therapy and fractures. Archives of Internal Medicine 2007;167(19):2136‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
House 1999 {published data only}
- House A, Freeman D. Larger intradialytic effect of high flux vs low flux polysulfone on homocysteine levels, but not on lipids or apo AI/CIII ratio [abstract]. Journal of the American Society of Nephrology 2001;12(Program & Abstracts):209A. [CENTRAL: CN‐00445778] [Google Scholar]
- House AA, Donnelly JG. Effect of multivitamins on plasma homocysteine and folate levels in patients on hemodialysis. ASAIO Journal 1999;45(1):94‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- House AA, Donnelly JG. Impact of methylenetetrahydrofolate reductase (MTHFR) genotype on folate status and homocysteine (hcy) in hemodialysis patients [abstract]. Journal of the American Society of Nephrology 1999;10(Program & Abstracts):168A. [CENTRAL: CN‐00756935] [Google Scholar]
- House AA, Wells GA, Donnelly JG, Nadler SP, Hebert PC. Effect of high flux vs low flux hemodialysis on homocysteine, lipoprotein(a) and lipid profiles: a prospective randomized trial [abstract]. Journal of the American Society of Nephrology 1998;9(Program & Abstracts):211A. [CENTRAL: CN‐00401331] [Google Scholar]
- House AA, Wells GA, Donnelly JG, Nadler SP, Hebert PC. Randomized trial of high‐flux vs low‐flux haemodialysis: effects on homocysteine and lipids. Nephrology Dialysis Transplantation 2000;15(7):1029‐34. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
House 2004 {published data only}
- House AA, Eliasziw M, Urquhart BL, Freeman DJ, Spence JD. Dimercaptosuccinic acid for the treatment of hyperhomocysteinemia in hemodialysis patients: a placebo‐controlled, double‐blind, randomized trial. American Journal of Kidney Diseases 2004;44(4):689‐94. [MEDLINE: ] [PubMed] [Google Scholar]
Imani 2009 {published data only}
- Imani H, Tabibi H, Atabak S, Rahmani L, Ahmadinejad M, Hedayati M. Effects of soy consumption on oxidative stress, blood homocysteine, coagulation factors, and phosphorus in peritoneal dialysis patients. Journal of Renal Nutrition 2009;19(5):389‐95. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Tabibi H, Imani H, Hedayati M, Atabak S, Rahmani L. Effects of soy consumption on serum lipids and apoproteins in peritoneal dialysis patients: a randomized controlled trial. Peritoneal Dialysis International 2010;30(6):611‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Isbel 2003 {published data only}
- Isbel NM, Reiger K, Hayley CM, Campbell SB, Burke J, Bofinger A, et al. Vitamin B12 is effective in the treatment of hyperhomocysteinaemia in patients with ESRD [abstract no: 58]. Nephrology 2003;8(Suppl 3):A69. [CENTRAL: CN‐00758625] [Google Scholar]
ISRCTN22151635 {published data only}
- ISRCTN22151635. Assessment of the lipid lowering agents on plasma homocysteine concentration and renal function. controlled‐trials.com/ISRCTN22151635 (accessed 4 February 2016).
Jara 2001 {published data only}
- Jara A, Morales M. Intravenous folinic acid versus oral folic acid for the treatment of hyperhomocysteinemia in hemodialysis patients [abstract]. Journal of the American Society of Nephrology 2001;12(Program & Abstracts):357A. [CENTRAL: CN‐00445900] [Google Scholar]
Kazory 2008 {published data only}
- Kazory A, Kribs M, Fournier V, Motte G, Guy C, Devillard N, et al. Homocysteine lowering effect of adsorptive dialysis membranes: a multicenter, randomized, open study [abstract no: 118]. American Journal of Kidney Diseases 2008;51(4):A57. [CENTRAL: CN‐00756882] [Google Scholar]
Klemm 2004 {published data only}
- Klemm A, Franke C, Busch M, Muller A, Franke S, Lang D, et al. Influence of hemodialysis membrane permeability on serum levels of advanced glycation end products (AGEs) and homocysteine metabolites. Clinical Nephrology 2004;61(3):191‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kooshki 2011 {published data only}
- Kooshki A, Taleban FA, Tabibi H, Hedayati M. Effects of marine omega‐3 fatty acids on serum systemic and vascular inflammation markers and oxidative stress in hemodialysis patients. Annals of Nutrition & Metabolism 2011;58(3):197‐202. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kooshki A, Taleban FA, Tabibi H, Hedayati M. Effects of omega‐3 fatty acids on serum lipids, lipoprotein (a), and hematologic factors in hemodialysis patients. Renal Failure 2011;33(9):892‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Koyama 2002 {published data only}
- Koyama K, Usami T, Takeuchi O, Morozumi K, Kimura G. Efficacy of methylcobalamin on lowering total homocysteine plasma concentrations in haemodialysis patients receiving high‐dose folic acid supplementation. Nephrology Dialysis Transplantation 2002;17(5):916‐22. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Koyama 2010 {published data only}
- Koyama K, Ito A, Ichikawa T, Miura T, Ono M. Short term regimen of homocysteine‐lowering therapy reduces serum concentration of asymmetric dimethylarginine in patients on chronic hemodialysis [abstract no: SaP317]. Nephrology Dialysis Transplantation 2007;22(Suppl 6):vi339. [CENTRAL: CN‐00671829] [Google Scholar]
- Koyama K, Ito A, Yamamoto J, Nishio T, Ichikawa T. Co‐administration of methylcobalamin and folic acid normalizes hyperhomocysteinemia and reduces arterial stiffness in hemodialsyis patients [abstract no: PUB074]. Journal of the American Society of Nephrology 2005;16:798A. [CENTRAL: CN‐00676039] [Google Scholar]
- Koyama K, Ito A, Yamamoto J, Nishio T, Kajikuri J, Dohi Y, et al. Randomized controlled trial of the effect of short‐term coadministration of methylcobalamin and folate on serum ADMA concentration in patients receiving long‐term hemodialysis. American Journal of Kidney Diseases 2010;55(6):1069‐78. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kuhlmann 2004 {published data only}
- Kuhlmann MK, Obeid R, Herrmann W. Normalization of plasma homocystein (Hcy) levels in hyperhomocysteinemic hemodialysis patients by intravenous dosage of vitamins B6, B12 and folic acid. [abstract no SA‐PO345]. Journal of the American Society of Nephrology 2004;15(Oct):377A. [CENTRAL: CN‐00757352] [Google Scholar]
Kumar 2007b {published data only}
- Kumar R, Vincent L, Perkins NJ, Tobe SW. Impact of folic acid on homocysteine levels in haemodialysis patients, a randomized double blinded placebo controlled cross over trial [abstract no: SA‐PO788]. Journal of the American Society of Nephrology 2007;18(Abstracts Issue):516A. [CENTRAL: CN‐00716086] [Google Scholar]
Kuo 2001a {published data only}
- Kuo HT, Tsai JC, Chen LN, Chen HC, Guh JY, Hwang SJ, et al. Intravenous methylcobalamin further improves hyperhomocysteinemia in hemodialysis patients under maintenance folic acid therapy: role of methyletetrahydrofolate reductase (mthfr) genotype [abstract]. Nephrology Dialysis Transplantation 2001;16(6):A87. [CN‐00509297] [Google Scholar]
LANDMARK Study 2006 {published data only}
- Isbel N, Rakhit D, Armstrong K, Leano R, Hawley C, Campbell S, et al. Rapid progression of myocardial ischaemia in patients with chronic kidney disease is not altered by risk factor modification [abstract no: PS107]. Nephrology 2005;10(Suppl 3):A408. [CENTRAL: CN‐00583922] [Google Scholar]
- Isbel NM, Haluska B, Johnson DW, Beller E, Hawley C, Marwick TH. Increased targeting of cardiovascular risk factors in patients with chronic kidney disease does not improve atheroma burden or cardiovascular function. American Heart Journal 2006;151(3):745‐53. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Isbel NM, Reiger K, Hawley CM, Campbell SB, Haluska B, Short L, et al. Aggressive intervention in traditional cardiovascular risk factors in patients with chronic renal failure does not improve atheroma burden, cardiovascular function or outcome [abstract]. Nephrology 2004;9(Suppl 1):A1‐2. [CENTRAL: CN‐00509247] [Google Scholar]
- Johnson DW, Armstrong K, Campbell SB, Mudge DW, Hawley CM, Coombes JS, et al. Metabolic syndrome in severe chronic kidney disease: Prevalence, predictors, prognostic significance and effects of risk factor modification. Nephrology 2007;12(4):391‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Rakhit DJ, Marwick TH, Armstrong KA, Johnson DW, Leano R, Isbel NM. Effect of aggressive risk factor modification on cardiac events and myocardial ischaemia in patients with chronic kidney disease. Heart 2006;92(10):1402‐8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Libetta 2004 {published data only}
- Libetta C, Corbetta R, Zucchi M, Gori E, Villa G, Bezoari G, et al. Treatment of hyperhomocysteinemia in hemodialysis patients reduce progressive atherosclerosis: a long‐term controlled study [abstract no: SA‐PO343]. Journal of the American Society of Nephrology 2004;15(Oct):376A‐7A. [CENTRAL: CN‐00626028] [Google Scholar]
- Libetta C, Corbetta R, Zucchi M, Gori E, Villa G, Sepe V, et al. Long‐term treatment of hyperhomocysteinemia prevents carotid artery atherosclerosis in haemodialysed patients [abstract]. 41st Congress. European Renal Association. European Dialysis and Transplantation Association; 2004 May 15‐18; Lisbon, Portugal. 2004:4. [CENTRAL: CN‐00604353]
- Libetta C, Villa G, Gori E, Zucchi M, Pisacco P, Bezoari G, et al. Vitamins supplementation effectiveness on hyperhomocysteinemia in hemodialyzed patients: a two‐year single‐blind randomized study [abstract no: SA‐PO344]. Journal of the American Society of Nephrology 2004;15(Oct):377A. [CENTRAL: CN‐00626027] [Google Scholar]
- Libetta C, Villa G, Gori E, Zucchi M, Sepe V, Pisacco P, et al. Effect of folate or folate plus vitamins B6 and B12 on hyperhomocysteinemia in hemodialyzed patients: a 2‐year controlled randomized study [abstract]. 41st Congress. European Renal Association. European Dialysis and Transplantation Association; 2004 May 15‐18; Lisbon, Portugal. 2004:126. [CENTRAL: CN‐00636150]
Madsen 2011 {published data only}
- Madsen T, Christensen JH, Toft E, Aardestrup I, Lundbye‐Christensen S, Schmidt EB. Effect of intravenous omega‐3 fatty acid infusion and hemodialysis on fatty acid composition of free fatty acids and phospholipids in patients with end‐stage renal disease. Jpen: Journal of Parenteral & Enteral Nutrition 2011;35(1):97‐106. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Manns 2001 {published data only}
- Hyndman ME, Manns BJ, Snyder FF, Bridge PJ, Scott‐Douglas NW, Fung E, et al. Vitamin B12 decreases, but does not normalize, homocysteine and methylmalonic acid in end‐stage renal disease: a link with glycine metabolism and possible explanation of hyperhomocysteinemia in end‐stage renal disease. Metabolism: Clinical & Experimental 2003;52(2):168‐72. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Manns B, Hyndman E, Burgess E, Parsons H, Schaefer J, Snyder F, et al. Oral vitamin B(12) and high‐dose folic acid in hemodialysis patients with hyper‐homocyst(e)inemia. Kidney International 2001;59(3):1103‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mazdeh 2005 {published data only}
- Abolghasemi R, Mazdeh MM, Khatami M, Lessan‐Pezeshki M, Ahmadi F, Seifi S, et al. Hyperhomocysteinemia therapy in haemodialysis patients: folinic versus folic acid in combination with vitamin B6 and B12 [abstract no: MP318]. Nephrology Dialysis Transplantation 2005;20(Suppl 5):v307. [DOI] [PubMed] [Google Scholar]
- Mazdeh MM, Abolghasemi R, Khatami M, Lessan‐Pezeshki M, Ahmadi F, Seifi S, et al. Hyperhomocysteinemia therapy in hemodialysis patients: folinic versus folic acid in combination with vitamin B6 and B12 [abstract no: W‐PO40014]. Nephrology 2005;10(Suppl 1):A283. [CENTRAL: CN‐00756890] [Google Scholar]
McGregor 2000a {published data only}
- McGregor D, Shand B, Lynn K. A controlled trial of the effect of folate supplements on homocysteine, lipids and hemorheology in end‐stage renal disease. Nephron 2000;85(3):215‐20. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mudge 2005 {published data only}
- Mudge D, Rogers R, Hollett P, Law B, Reiger K, Petrie JJP, et al. Plasma homocysteine levels with standard high flux haemodialysis compared with FX class dialysers [abstract]. Nephrology 2004;9(Suppl 1):A16. [CENTRAL: CN‐00509369] [Google Scholar]
- Mudge DW, Rogers R, Hollett P, Law B, Reiger K, Petrie JJ, et al. Randomized trial of FX high flux vs standard high flux dialysis for homocysteine clearance. Nephrology Dialysis Transplantation 2005;20(10):2178‐85. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mueller 2001 {published data only}
- Mueller T, Mueller N, Moser R, Stief T, Celle D, Pietrizk K. Reduced folates have an anti‐inflammatory effect in hemodialysis patients [abstract]. Nephrology Dialysis Transplantation 2003;18(Suppl 4):700. [CENTRAL: CN‐00446856] [Google Scholar]
- Mueller TF, Mueller N, Moser R, Stief T, Nau H, Lange H, et al. Effect of folate therapy on the inflammatory burden in hemodialysis patients [abstract no: SA‐FC185]. Journal of the American Society of Nephrology 2002;13(September, Program & Abstracts):38A. [CENTRAL: CN‐00446855] [Google Scholar]
- Mueller TF, Mueller N, Moser R, Stief T, Ziegler A, Nau H, et al. Effect of oral substitution of reduced folates on homocysteine, neopterin, and lipid levels in patients treated with hemodialysis [abstract]. Journal of the American Society of Nephrology 2001;12(Program & Abstracts):360A‐1A. [CENTRAL: CN‐00756385] [Google Scholar]
Muller 2001 {published data only}
- Muller A, Busch M, Stein G, Hein GE, Funfstuck R. L‐methionine loading test for the prediction of increased total homocysteine levels due to long term l‐methionine treatment [abstract]. Journal of the American Society of Nephrology 2001;12(Program & Abstracts):734A. [CENTRAL: CN‐00757145] [Google Scholar]
Nakamura 2003 {published data only}
- Nakamura T, Hirayama H, Tajiri M, Saionji K, Hata A. Methylenetetrahydrofolate reductase (MTHFR) gene polymorphism influences the efficacy of folic acid (FA) and vitamin B12 (B12) on hyperhomocyteinemia in hemodialysis (HD) patients [abstract]. Nephrology Dialysis Transplantation 2003;18(Suppl 4):699‐700. [CENTRAL: CN‐00446896] [Google Scholar]
Nakhoul 2004 {published data only}
- Nakhoul F, Abassi Z, Plawner M, Khankin E, Ramadan R, Lanir N, et al. Comparative study of response to treatment with supraphysiologic doses of B‐vitamins in hyperhomocysteinemic hemodialysis patients. Israel Medical Association Journal ‐ Imaj 2004;6(4):213‐7. [MEDLINE: ] [PubMed] [Google Scholar]
- Plawner M, Nakhoul F, Khankin E, Laneer N, Brenner B, Green J. Folic acid and varying doses of B‐vitamines in the treatment of hyperhomocystinemia in hemodialysis patients [abstract]. Journal of the American Society of Nephrology 2000;11(Sept):295A. [CENTRAL: CN‐00644331] [Google Scholar]
Nascimento 2010 {published data only}
- Nascimento MM, Suliman ME, Anderstam B, Silva MM, Hayashi S, Pecoits‐Filho R, et al. Effect of N‐acetylcysteine supplementation on inflammatory and oxidative stress markers in peritoneal dialysis patients: a randomized placebo controlled study [abstract no: F‐PO769]. Journal of the American Society of Nephrology 2007;18(Abstracts):270A. [CENTRAL: CN‐00756376] [Google Scholar]
- Nascimento MM, Suliman ME, Silva M, Chinaglia T, Marchioro J, Hayashi SY, et al. Effect of oral N‐acetylcysteine treatment on plasma inflammatory and oxidative stress markers in peritoneal dialysis patients: a placebo‐controlled study. Peritoneal Dialysis International 2010;30(3):336‐42. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
OPACH Study 2006 {published data only}
- Harving F, Svensson M, Flyvbjerg A, Schmidt EB, Jorgensen KA, Eriksen HH, et al. n‐3 polyunsaturated fatty acids and adiponectin in patients with end‐stage renal disease. Clinical Nephrology 2015;83(5):279‐85. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kirkegaard E, Svensson M, Strandhave C, Schmidt EB, Jorgensen KA, Christensen JH. Marine n‐3 fatty acids, atrial fibrillation and QT interval in haemodialysis patients. British Journal of Nutrition 2012;107(6):903‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Rasmussen LE, Svensson M, Jorgensen KA, Schmidt EB, Christensen JH. The content of docosahexaenoic acid in serum phospholipid is inversely correlated with plasma homocysteine levels in patients with end‐stage renal disease. Nutrition Research 2010;30(8):535‐40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Svensson M, Christensen JH, Jorgensen KA, Schmidt EB. Omacor as secondary prevention against cardiovascular disease in patients undergoing chronic hemodialysis: a randomised, placebo controlled intervention study [abstract no: F‐PO606]. Journal of the American Society of Nephrology 2005;16:466A. [CENTRAL: CN‐00583663] [DOI] [PubMed] [Google Scholar]
- Svensson M, Frobert O, Schmidt EB, Jorgensen KA, Simonsen U, Christensen JH. The effect of n‐3 fatty acids on levels of methylarginines in patients with end‐stage renal disease. Journal of Nephrology 2010;23(4):459‐64. [MEDLINE: ] [PubMed] [Google Scholar]
- Svensson M, Schmidt EB, Jorgensen KA, Christensen JH. The effect of n‐3 fatty acids on heart rate variability in patients treated with chronic hemodialysis. Journal of Renal Nutrition 2007;17(4):243‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Svensson M, Schmidt EB, Jorgensen KA, Christensen JH. The effect of n‐3 fatty acids on lipids and lipoproteins in patients treated with chronic haemodialysis: a randomized placebo‐controlled intervention study. Nephrology Dialysis Transplantation 2008;23(9):2918‐24. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Svensson M, Schmidt EB, Jorgensen KA, Christensen JH, OPACH Study Group. N‐3 fatty acids as secondary prevention against cardiovascular events in patients who undergo chronic hemodialysis: A randomized, placebo‐controlled intervention trial. Clinical Journal of the American Society of Nephrology: CJASN 2006;1(4):780‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ossareh 2009 {published data only}
- Ossareh S, Shayan‐Moghaddam H, Salimi A, Asgari M, Farrokhi F. Different doses of oral folic acid for homocysteine‐lowering therapy in patients on hemodialysis: a randomized controlled trial.[Erratum appears in Iran J Kidney Dis. 2010 Jan;4(1):88 Note: Salimi, Ashraf [added]; Asgari, Mojgan [added]]. Iranian Journal of Kidney Diseases 2009;3(4):227‐33. [MEDLINE: ] [PubMed] [Google Scholar]
Pakfetrat 2013 {published data only}
- Pakfetrat M, Shahroodi JR, Zolgadr AA, Larie HA, Nikoo MH, Malekmakan L. Effects of zinc supplement on plasma homocysteine level in end‐stage renal disease patients: a double‐blind randomized clinical trial. Biological Trace Element Research 2013;153(1‐3):11‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pastore 2006 {published data only}
- Pastore A, Angelis S, Casciani S, Ruggia R, Giovamberardino G, Noce A, et al. Effects of folic acid before and after vitamin B12 on plasma homocysteine concentrations in hemodialysis patients with known MTHFR genotypes. Clinical Chemistry 2006;52(1):145‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Peng 2005 {published data only}
- Peng L, Liu Q. A prospective, randomized, placebo‐controlled study to evaluate the effect of combined treatment of folic acid and intravenous B‐complex vitamins on hyperhomocysteinemia in hemodialysis patients [abstract no: W‐PO40032]. Nephrology 2005;10(Suppl 1):A288. [CENTRAL: CN‐00671827] [Google Scholar]
Polkinghorne 2003 {published data only}
- Polkinghorne KR, Zoungas S, Branley P, Villanueva E, McNeil JJ, Atkins RC, et al. A randomised placebo controlled trial of intramuscular vitamin B12 (hydroxyocobalamin) for the treatment of hyperhomocysteinemia in haemodialysis patients [abstract]. Kongres Nasional VIII Annual Meeting Perhimpunan Nefrologi Indonesia; 2002 Oct; Surabaya, Indonesia. 2002:2.5. [CENTRAL: CN‐00447252]
- Polkinghorne KR, Zoungas S, Branley P, Villanueva E, McNeil JJ, Atkins RC, et al. A randomized placebo controlled trial of intramuscular vitamin B12 (hydroxyocobalamin) for the treatment of hyperhomocysteinemia in haemodialysis patients [abstract no: SU‐FC267]. Journal of the American Society of Nephrology 2002;13(September, Program & Abstracts):55A. [CENTRAL: CN‐00447251] [Google Scholar]
- Polkinghorne KR, Zoungas S, Branley P, Villanueva E, McNeil JJ, Atkins RC, et al. Randomized, placebo‐controlled trial of intramuscular vitamin B12 for the treatment of hyperhomocysteinaemia in dialysis patients. Internal Medicine Journal 2003;33(11):489‐94. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Polkinghorne KR, Zoungas S, Branley P, Villnueva E, McNeil J, Atkins RC, et al. A randomised placebo controlled trial of intramuscular vitamin B12 (hydroxyocobalamin) for the treatment of hyperhomocysteinemia in haemodialysis patients [abstract]. Nephrology 2002;7(Suppl 3):A68. [Google Scholar]
Poulia 2011 {published data only}
- Poulia KA, Panagiotakos DB, Tourlede E, Rezou A, Stamatiadis D, Boletis J, et al. Omega‐3 fatty acids supplementation does not affect serum lipids in chronic hemodialysis patients. Journal of Renal Nutrition 2011;21(6):479‐84. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sanchez Alvarez 2005 {published data only}
- Sanchez Alvarez JE, Perez Tamajon L, Hernandez D, Alvarez Gonzalez A, Delgado P, Lorenzo V. Efficacy and safety of two vitamin supplement regimens on homocysteine levels in hemodialysis patients. Prospective, randomized clinical trial. Nefrologia 2005;25(3):288‐96. [MEDLINE: ] [PubMed] [Google Scholar]
- Sanchez JE, Molina E, Vega MJ, Hernandez D, Perez L, Garcia R, et al. Sustained effect of two vitamin supplement regimens on hyperhomocysteinemia in maintenance hemodialysis (MHD) patients [abstract no: F‐PO773]. Journal of the American Society of Nephrology 2003;14(Nov):231A. [CENTRAL: CN‐00583425] [Google Scholar]
Scholze 2004 {published data only}
- Scholze A, Rinder C, Beige J, Riezler R, Zidek W, Tepel M. Acetylcysteine reduces plasma homocysteine concentration and improves pulse pressure and endothelial function in patients with end‐stage renal failure. Circulation 2004;109(3):369‐74. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Scholze A, Rinder C, Beige J, Riezler R, Zidek W, Tepel M. Reduced plasma homocysteine concentration and improved pulse pressure in hemodialysis patients with acetylcysteine [abstract]. 41st Congress. European Renal Association. European Dialysis and Transplantation Association; 2004 May 15‐18; Lisbon, Portugal. 2004:127. [CENTRAL: CN‐00509467]
Seo 2003 {published data only}
- Seo MD, Yoon S, Jeong JC, Choi SS, Kim SY, Son I, et al. Effects of folic acid treatment on homocysteine levels and echocardiographic findings in ESRD [abstract no: SA‐PO900]. Journal of the American Society of Nephrology 2003;14(Nov):497A. [CN‐00756462] [Google Scholar]
Sepe 1999 {published data only}
- Sepe V, Patrucco G, Santagostine A, Bolis V, Caminiti R, Cecere P, et al. Hyperhomocysteinemia [HHcy] is not associated with elevated platelet annexin V [plt a‐v] expression in maintenance haemodialysis [HD] patients [abstract]. Nephrology Dialysis Transplantation 1999;14(9):A192. [CENTRAL: CN‐00485810] [Google Scholar]
Shemin 2001 {published data only}
- Shemin D, Bostom AG, Selhub J. Treatment of hyperhomocysteinemia in end‐stage renal disease. American Journal of Kidney Diseases 2001;38(4 Suppl 1):S91‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Signorelli 2006 {published data only}
- Signorelli SS, Fatuzzo P, Rapisarda F, Neri S, Ferrante M, Oliveri CG, et al. A randomised, controlled clinical trial evaluating changes in therapeutic efficacy and oxidative parameters after treatment with propionyl L‐carnitine in patients with peripheral arterial disease requiring haemodialysis. Drugs & Aging 2006;23(3):263‐70. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Signorelli SS, Fatuzzo P, Rapisarda F, Neri S, Ferrante M, Oliveri CG, et al. Propionyl‐L‐carnitine therapy: effects on endothelin‐1 and homocysteine levels in patients with peripheral arterial disease and end‐stage renal disease. Kidney & Blood Pressure Research 2006;29(2):100‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Skoutakis 1975 {published data only}
- Skoutakis VA, Acchiardo SR, Meyer MC, Hatch FE. Folic acid dosage for chronic hemodialysis patients. Clinical Pharmacology & Therapeutics 1975;18(2):200‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Stavrianaki 2002 {published data only}
- Stavrianaki D, Bagiatoudi G, Salpigidis K, Sarris E, Siakotos M. Treatment efficacy of folic or folinic acid in lowering total plasma homocysteine levels in patients on hemodialysis [abstract]. Nephrology Dialysis Transplantation 2002;17(Suppl 12):256. [CENTRAL: CN‐00758491] [Google Scholar]
Tamadon 2011 {published data only}
- Tamadon MR, Jamshidi L, Soliemani A, Ghorbani R, Malek F, Malek M. Effect of different doses of folic acid on serum homocysteine level in patients on hemodialysis. Iranian Journal of Kidney Diseases 2011;5(2):93‐6. [MEDLINE: ] [PubMed] [Google Scholar]
Tayyebi‐Khosroshahi 2010 {published data only}
- Tayyebi‐Khosroshahi H, Houshyar J, Tabrizi A, Vatankhah AM, Razzagi ZN, Dehghan‐Hesari R. Effect of omega‐3 fatty acid on oxidative stress in patients on hemodialysis. Iranian Journal of Kidney Diseases 2010;4(4):322‐6. [MEDLINE: ] [PubMed] [Google Scholar]
Tepel 2003 {published data only}
- Tepel M, Giet M, Statz M, Jankowski J, Zidek W. The antioxidant acetylcysteine reduces cardiovascular events in patients with end‐stage renal failure: a randomized, controlled trial. Circulation 2003;107(7):992‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Thaha 2006 {published data only}
- Thaha M, Widodo, Yogiantoro M, Tomino Y. Reduction of pulse pressure by intravenous N‐acetylcyseine administration in chronic hemodialysis patients [abstract no: 1620]. Nephrology 2006;11(Suppl 2):A33. [CENTRAL: CN‐00653775] [Google Scholar]
- Thaha M, Yogiantoro M, Tomino Y. Intravenous N‐acetylcysteine during haemodialysis reduces the plasma concentration of homocysteine in patients with end‐stage renal disease. Clinical Drug Investigation 2006;26(4):195‐202. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Thaha M, Yogiantoro M, Tomino Y. Intravenous N‐acetylcysteine during haemodialysis reduces the plasma concentration of homocysteine in patients with end‐stage renal disease [abstract no: 1560]. Nephrology 2006;11(Suppl 2):A18. [DOI] [PubMed] [Google Scholar]
Thaha 2008 {published data only}
- Thaha M, Widodo B, Pranawa M, Yogiantoro HM, Tomino Y. Intravenous N‐acetylcysteine during hemodialysis reduces homocysteine and asymmetric dimethylarginine in ESRD patients: a randomized double blind controlled trial [abstract no: 1159]. Nephrology 2007;12(Suppl 2):A40. [CENTRAL: CN‐00740521] [DOI] [PubMed] [Google Scholar]
- Thaha M, Widodo, Pranawa W, Yogiantoro M, Tomino Y. Intravenous N‐acetylcysteine during hemodialysis reduces asymmetric dimethylarginine level in end‐stage renal disease patients. Clinical Nephrology 2008;69(1):24‐32. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Thaha 2009 {published data only}
- Thaha M, Mohani CI, Yogiantoro HM, Tomino Y. Oral n‐acetyl cysteine to treat non‐diabetic chronic kidney disease stage 1‐4 with albuminuria [abstract no: SA‐PO2242]. Journal of the American Society of Nephrology 2009;20:622A. [CENTRAL: CN‐00740560] [Google Scholar]
Thambyrajah 2000 {published data only}
- Thambyrajah J, Landray MJ, McGlynn FJ, Jones HJ, Wheeler DC, Townend JN. Does folic acid decrease plasma homocysteine and improve endothelial function in patients with predialysis renal failure?. Circulation 2000;102(8):871‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Thambyrajah J, Landray MJ, Townend JN, Wheeler DC. Folic acid decreases plasma homocysteine but does not improve endothelial function in chronic renal failure [abstract]. Journal of the American Society of Nephrology 2000;11(Sept):372A. [CENTRAL: CN‐00583252] [Google Scholar]
Tobe 1999 {published data only}
- Tobe SW, Helmers K, Naimark DMJ, Perkins N, Baker B. Impact on homocysteine levels of folic acid and adherance in haemodialysis patients. A randomized double blind placebo cross over trial [abstract no: A1332]. Journal of the American Society of Nephrology 2000;11(Sept):253A. [CENTRAL: CN‐00583286] [Google Scholar]
- Tobe SW, Naimark DN, Helmers KF, Perkins NJ, Baker B. Plasma homocysteine (hcy) levels in patients on chronic haemodialysis are inversely correlated with folate and B12 levels [abstract]. Journal of the American Society of Nephrology 1999;10(Program & Abstracts):306A. [CENTRAL: CN‐00583285] [Google Scholar]
Tochihara 2008 {published data only}
- Tochihara Y, Whiting MJ, Barbara JA, Mangoni AA. Effects of pre‐ vs. intra‐dialysis folic acid on arterial wave reflections and endothelial function in patients with end‐stage renal disease. British Journal of Clinical Pharmacology 2008;66(5):717‐22. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Treleaven 2001 {published data only}
- Treleaven D, Gough J, Lonn E, Strickland D, Kitching A, Churchill D, et al. High dose folic acid in hemodialysis patients results in frequent side effects without improvement in plasma homocysteine levels [abstract]. Journal of the American Society of Nephrology 2001;12(Program & Abstracts):365A. [CENTRAL: CN‐00583283] [Google Scholar]
Tremblay 2000 {published data only}
- Tremblay R, Bonnardeaux A, Geadah D, Busque L, Lebrun M, Ouimet D, et al. Hyperhomocysteinemia in hemodialysis patients: effects of 12‐month supplementation with hydrosoluble vitamins. Kidney International 2000;58(2):851‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Trimarchi 2002 {published data only}
- Trimarchi H, Schiel A, Freixas E, Diaz M. Randomized trial of methylcobalamin and folate effects on homocysteine in hemodialysis patients. Nephron 2002;91(1):58‐63. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tungkasereerak 2006 {published data only}
- Tungkasereerak P, Chaiyasoot W, Taruangsri P, Leowattana W, Vasuvattakul S, Vareesangthip K, et al. Can folate and vitamin B supplementation reduce total homocysteine levels and atherosclerosis in hemodialysis patients? [abstract no: SA‐PO472]. Journal of the American Society of Nephrology 2004;15(Oct):406A. [CENTRAL: CN‐00583721] [Google Scholar]
- Tungkasereerak P, Ong‐ajyooth L, Chaiyasoot W, Ong‐ajyooth S, Leowattana W, Vasuvattakul S, et al. Effect of short‐term folate and vitamin B supplementation on blood homocysteine level and carotid artery wall thickness in chronic hemodialysis patients. Journal of the Medical Association of Thailand 2006;89(8):1187‐93. [MEDLINE: ] [PubMed] [Google Scholar]
Urquhart 2008 {published data only}
- Urquhart BL, Freeman DJ, Cutler MJ, Mainra R, Spence JD, House AA. Mesna for treatment of hyperhomocysteinemia in hemodialysis patients: a placebo‐controlled, double‐blind, randomized trial. Clinical Journal of the American Society of Nephrology: CJASN 2008;3(4):1041‐7. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
van Guldener 1998 {published data only}
- Guldener C, Janssen M, Lambert J, ter Wee P, Donker A, Stehonwer C. Treatment of hyperhomocysteinaemia with folic acid does not ameliorate endothelial function in chronic haemodialysis patients [abstract]. Nephrology Dialysis Transplantation 1997;12(9):A114. [CENTRAL: CN‐00261358] [Google Scholar]
- Guldener C, Janssen MJ, Lambert J, ter Wee PM, Donker AJ, Stehouwer CD. Folic acid treatment of hyperhomocysteinemia in peritoneal dialysis patients does not ameliorate endothelial function [abstract]. Journal of the American Society of Nephrology 1997;8(Program & Abstracts):274A. [CENTRAL: CN‐00448137] [Google Scholar]
- Guldener C, Janssen MJ, Lambert J, ter Wee PM, Donker AJ, Stehouwer CD. Folic acid treatment of hyperhomocysteinemia in peritoneal dialysis patients: no change in endothelial function after long‐term therapy. Peritoneal Dialysis International 1998;18(3):282‐9. [MEDLINE: ] [PubMed] [Google Scholar]
- Guldener C, Janssen MJ, Lambert J, ter Wee PM, Jakobs C, Donker AJ, et al. No change in impaired endothelial function after long‐term folic acid therapy of hyperhomocysteinaemia in haemodialysis patients. Nephrology Dialysis Transplantation 1998;13(1):106‐12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Guldener C, Janssen MJ, Stehouwer CD, Ter Wee PM, Donker AJ. Treatment of hyperhomocysteinemia in chronic dialysis patients with folic acid and betaine [abstract no: A1266]. Journal of the American Society of Nephrology 1996;7(9):1502. [CENTRAL: CN‐00758506] [Google Scholar]
- Guldener C, Janssen MJ, Stehouwer CD, ter Wee PM, Donker AJ. Folic acid and betaine in the treatment of hyperhomocysteinaemia in haemodialysis patients [abstract]. Netherlands Journal of Medicine 1996;48(5):A67. [CENTRAL: CN‐00416837] [Google Scholar]
- Guldener C, Janssen MJ, Meer K, Donker AJ, Stehouwer CD. Effect of folic acid and betaine on fasting and postmethionine‐loading plasma homocysteine and methionine levels in chronic haemodialysis patients. Journal of Internal Medicine 1999;245(2):175‐83. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Guldener C, Lambert J, ter Wee PM, Donker AJ, Stehouwer CD. Carotid artery stiffness in patients with end‐stage renal disease: no effect of long‐term homocysteine‐lowering therapy. Clinical Nephrology 2000;53(1):33‐41. [MEDLINE: ] [PubMed] [Google Scholar]
Van Tellingen 2001 {published data only}
- Tellingen A, Grooteman MP, Bartels PC, Limbeek J, Guldener C, Wee PM, et al. Long‐term reduction of plasma homocysteine levels by super‐flux dialyzers in hemodialysis patients. Kidney International 2001;59(1):342‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
VIENNA Study 2000 {published data only}
- Sunder‐Plassmann G, Fodinger M, Buchmayer H, Papagiannopoulos M, Wojcik J, Kletzmayr J, et al. Effect of high dose folic acid therapy on hyperhomocysteinemia in hemodialysis patients: results of the Vienna multicenter study. Journal of the American Society of Nephrology 2000;11(6):1106‐16. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Sunder‐Plassmann G, Fodinger M, Buchmayer H, Wolfl G, Papagiannoupoulos M, Wojzik J, et al. High dose folic acid therapy in hemodialysis (HD) patients. The Vienna multicenter study [abstract]. Journal of the American Society of Nephrology 1999;10(Program & Abstracts):269A. [CENTRAL: CN‐00756862] [DOI] [PubMed] [Google Scholar]
Vrentzos 2001 {published data only}
- Vrentzos G, Ganotakis E, Vardakis K, Maliaraki N, Stratigis S, Stilianou K, et al. Effect of vitamin B12 supplementation in serum total homocysteine and folate in patients with end‐stage renal failure [abstract]. Atherosclerosis Supplements 2001;2(2):107. [CENTRAL: CN‐00671820] [Google Scholar]
Vychytil 2003 {published data only}
- Vychytil A, Fodinger M, Pleiner J, Mullner M, Konner P, Skoupy S, et al. Acute effect of amino acid peritoneal dialysis solution on vascular function. American Journal of Clinical Nutrition 2003;78(5):1039‐45. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Vychytil A, Fodinger M, Pleiner J, Mullner M, Wolzt M, Sunder‐Plassmann G. Acute effect of amino acid peritoneal dialysis (PD) solution on vascular function ‐ no association with HCY [abstract]. Journal of Inherited Metabolic Disease 2003;26(Suppl 1):94. [CENTRAL: CN‐00677763] [Google Scholar]
- Vychytil A, Foedinger M, Pleiner J, Muellner M, Konner P, Skoupy S, et al. Acute effect of amino acid peritoneal dialysis solution on vascular function [abstract]. Nephrology Dialysis Transplantation 2003;18(Suppl 4):215. [DOI] [PubMed] [Google Scholar]
- Vychytil A, Foedinger M, Pleiner J, Muellner M, Konner P, Skoupy S, et al. Acute effect of amino acid peritoneal dialysis solution on vascular function. [abstract no: SU‐PO935]. Journal of the American Society of Nephrology 2003;14(Nov):741A. [DOI] [PubMed] [Google Scholar]
Westphal 2001 {published data only}
- Westphal S, Dierkes J, Luley C. Effects of fenofibrate and gemfibrozil on plasma homocysteine. Lancet 2001;358(9275):39‐40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Yango 2001 {published data only}
- Ghandour H, Bagley PJ, Shemin D, Hsu N, Jacques PF, Dworkin L, et al. Distribution of plasma folate forms in hemodialysis patients receiving high daily doses of L‐folinic or folic acid. Kidney International 2002;62(6):2246‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Yango A, Shemin D, Hsu N, Jacques PF, Dworkin L, Selhub J, et al. Rapid communication: L‐folinic acid versus folic acid for the treatment of hyperhomocysteinemia in hemodialysis patients. Kidney International 2001;59(1):324‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Zeman 2006 {published data only}
- Zeman M, Zak A, Vecka M, Tvrzicka E, Pisarikova A, Stankova B. N‐3 fatty acid supplementation decreases plasma homocysteine in diabetic dyslipidemia treated with statin‐fibrate combination. Journal of Nutritional Biochemistry 2006;17(6):379‐84. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Zuo 2001 {published data only}
- Zuo L, Wang M, Feng L, Wei H, Wang H. Can high dose of folic acid normalize fast total plama homocysteine (thcy) level in maintenance hemodialysis patients? [abstract]. Journal of the American Society of Nephrology 2001;12(Program & Abstracts):421A. [CENTRAL: CN‐00689429] [Google Scholar]
References to studies awaiting assessment
NCT00004495 {published data only}
- Wilcox CS. Randomized study of folic acid therapy for hyperhomocysteinemia in patients with end stage renal disease receiving hemodialysis. clinicaltrials.gov/ct2/show/NCT00004495 (accessed 18 February 2014).
Soleimani 2011 {published data only}
- Soleimani A, Usefzadeh M, Mianehsaz E, Foroozanfard F, Nikoueinejad H, Moraveji SA, et al. Comparison of oral folic acid and folinic acid on blood homocysteine level of patients on hemodialysis. Iranian Journal of Kidney Diseases 2011;5(1):45‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Tayebi‐Khosroshahi 2013 {published data only}
- Tayebi‐Khosroshahi H, Dehgan R, Habibi AB, Safaian A, Panahi F, Estakhri R, et al. Effect of omega‐3 supplementation on serum level of homocysteine in hemodialysis patients. Iranian Journal of Kidney Diseases 2013;7(6):479‐84. [MEDLINE: ] [PubMed] [Google Scholar]
Additional references
Andreucci 2004
- Andreucci VE, Fissell RB, Bragg‐Gresham JL, Ethier J, Greenwood R, Pauly M, et al. Dialysis Outcomes and Practice Patterns Study (DOPPS) data on medications in hemodialysis patients. American Journal of Kidney Diseases 2004;44(5 Suppl 2):61‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Aucella 2005
- Aucella F, Margaglione M, Grandone E, Vigilante M, Gatta G, Forcella M, et al. The C677T methylenetetrahydrofolate reductase gene mutation does not influence cardiovascular risk in the dialysis population: results of a multicentre prospective study. Nephrology Dialysis Transplantation 2005;20(2):382‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Baigent 2000
- Baigent C, Burbury K, Wheeler D. Premature cardiovascular disease in chronic renal failure. Lancet 2000;356(9224):147‐52. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bostom 1999
- Bostom AG, Culleton BF. Hyperhomocysteinemia in chronic renal disease. Journal of the American Society of Nephrology 1999;10(4):891‐900. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Chauveau 1993
- Chauveau P, Chadefaux B, Coude M, Aupetit J, Hannedouche T, Kamoun P, et al. Hyperhomocysteinemia, a risk factor for atherosclerosis in chronic uremic patients. Kidney International ‐ Supplement 1993;41:S72‐7. [MEDLINE: ] [PubMed] [Google Scholar]
de Jager 2009
- Jager DJ, Grootendorst DC, Jager KJ, Dijk PC, Tomas LM, Ansell D, et al. Cardiovascular and noncardiovascular mortality among patients starting dialysis. JAMA 2009;302(16):1782‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ducloux 2006
- Ducloux D, Klein A, Kazory A, Devillard N, Chalopin JM. Impact of malnutrition‐inflammation on the association between homocysteine and mortality. Kidney International 2006;69(2):331‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey‐Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple graphical test. BMJ 1997;315(7109):629‐34. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Eikelboom 1999
- Eikelboom JW, Lonn E, Genest J Jr, Hankey G, Yusuf S. Homocyst(e)ine and cardiovascular disease: a critical review of the epidemiologic evidence. Annals of Internal Medicine 1999;131(5):363‐75. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Foley 1998
- Foley RN, Parfrey PS, Sarnak MJ. Epidemiology of cardiovascular disease in chronic renal disease. Journal of the American Society of Nephrology 1998;9(12 Suppl):S16‐23. [MEDLINE: ] [PubMed] [Google Scholar]
Freidman 2001
- Friedman AN, Bostom AG, Selhub J, Levey AS, Rosenberg IH. The kidney and homocysteine metabolism. Journal of the American Society of Nephrology 2001;12(10):2181‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Go 2004
- Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization.[Erratum appears in N Engl J Med. 2008;18(4):4]. New England Journal of Medicine 2004;351(13):1296‐305. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gonin 2005
- Gonin JM. Folic acid supplementation to prevent adverse events in individuals with chronic kidney disease and end stage renal disease. Current Opinion in Nephrology & Hypertension 2005;14(3):277‐81. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Haynes 2012
- Haynes R, Clarke R. Homocysteine, the kidney, and vascular disease. BMJ 2012;344:e3925. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
HSC 2002
- Homocysteine Studies Collaboration. Homocysteine and risk of ischemic heart disease and stroke: a meta‐analysis. JAMA 2002;288(16):2015‐22. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Huang 2012
- Huang T, Chen Y, Yang B, Yang J, Wahlqvist ML, Li D. Meta‐analysis of B vitamin supplementation on plasma homocysteine, cardiovascular and all‐cause mortality. Clinical Nutrition 2012;31(4):448‐54. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Jardine 2012
- Jardine MJ, Kang A, Zoungas S, Navaneethan SD, Ninomiya T, Nigwekar SU, et al. The effect of folic acid based homocysteine lowering on cardiovascular events in people with kidney disease: systematic review and meta‐analysis. BMJ 2012;344:e3533. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
K/DOQI 2005
- K/DOQI Workgroup. K/DOQI clinical practice guidelines for cardiovascular disease in dialysis patients. American Journal of Kidney Diseases 2005;45(4 Suppl 3):S1‐153. [MEDLINE: ] [PubMed] [Google Scholar]
Kalantar‐Zadeh 2004
- Kalantar‐Zadeh K, Block G, Humphreys MH, McAllister CJ, Kopple JD. A low, rather than a high, total plasma homocysteine is an indicator of poor outcome in hemodialysis patients. Journal of the American Society of Nephrology 2004;15(2):442‐53. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kalantar‐Zadeh 2005
- Kalantar‐Zadeh K, Abbott KC, Salahudeen AK, Kilpatrick RD, Horwich TB. Survival advantages of obesity in dialysis patients. American Journal of Clinical Nutrition 2005;81(3):543‐54. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kanbay 2010
- Kanbay M, Afsar B, Gusbeth‐Tatomir P, Covic A. Arterial stiffness in dialysis patients: where are we now?. International Urology & Nephrology 2010;42(3):741‐52. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Klerk 2002
- Klerk M, Verhoef P, Clarke R, Blom HJ, Kok FJ, Schouten EG, et al. MTHFR 677C‐‐>T polymorphism and risk of coronary heart disease: a meta‐analysis. JAMA 2002;288(16):2023‐31. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
McDonald 2007
- McDonald S, Excell L, Livingston B. ANZDATA Registry 2007 Report. Chapter 3 Deaths. www.anzdata.org.au/anzdata/AnzdataReport/30thReport/Ch03Deaths.pdf 2007; Vol. (accessed 19 January 2016).
Menon 2006
- Menon V, Sarnak MJ, Greene T, Wang X, Pereira AA, Beck GJ, et al. Relationship between homocysteine and mortality in chronic kidney disease. Circulation 2006;113(12):1572‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Moustapha 1998
- Moustapha A, Naso A, Nahlawi M, Gupta A, Arheart KL, Jacobsen DW, et al. Prospective study of hyperhomocysteinemia as an adverse cardiovascular risk factor in end‐stage renal disease.[Erratum appears in Circulation 1998 Feb 24;97(7):711]. Circulation 1998;97(2):138‐41. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pan 2012
- Pan Y, Guo LL, Cai LL, Zhu XJ, Shu JL, Liu XL, et al. Homocysteine‐lowering therapy does not lead to reduction in cardiovascular outcomes in chronic kidney disease patients: a meta‐analysis of randomised, controlled trials. British Journal of Nutrition 2012;108(3):400‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Robinson 1996
- Robinson K, Gupta A, Dennis V, Arheart K, Chaudhary D, Green R, et al. Hyperhomocysteinemia confers an independent increased risk of atherosclerosis in end‐stage renal disease and is closely linked to plasma folate and pyridoxine concentrations. Circulation 1996;94(11):2743‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Suliman 2007
- Suliman ME, Lindholm B, Bárány P, Qureshi AR, Stenvinkel P. Homocysteine‐lowering is not a primary target for cardiovascular disease prevention in chronic kidney disease patients. Seminars in Dialysis 2007;20(6):523‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
USRDS 2007
- U.S. Renal Data System, USRDS 2007 Annual Data Report: Atlas of End‐Stage Renal Disease in the United States, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 2007. www.usrds.org/atlas07.aspx (accessed January 2016).
van Guldener 2006
- Guldener C. Why is homocysteine elevated in renal failure and what can be expected from homocysteine‐lowering?. Nephrology Dialysis Transplantation 2006;21(5):1161‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
VITATOPS 2010
- VITATOPS Trial Study Group. B vitamins in patients with recent transient ischaemic attack or stroke in the VITAmins TO Prevent Stroke (VITATOPS) trial: a randomised, double‐blind, parallel, placebo‐controlled trial. Lancet Neurology 2010;9(9):855‐65. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wald 2002
- Wald DS, Law M, Morris JK. Homocysteine and cardiovascular disease: evidence on causality from a meta‐analysis. BMJ 2002;325(7374):1202. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Weiner 2004
- Weiner DE, Tighiouart H, Amin MG, Stark PC, MacLeod B, Griffith JL, et al. Chronic kidney disease as a risk factor for cardiovascular disease and all‐cause mortality: a pooled analysis of community‐based studies. Journal of the American Society of Nephrology 2004;15(5):1307‐15. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wrone 2001
- Wrone EM, Zehnder JL, Hornberger JM, McCann LM, Coplon NS, Fortmann SP. An MTHFR variant, homocysteine, and cardiovascular comorbidity in renal disease. Kidney International 2001;60(3):1106‐13. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Yang 2012
- Yang J, Hu X, Zhang Q, Cao H, Wang J, Liu B. Homocysteine level and risk of fracture: A meta‐analysis and systematic review. Bone 2012;51(3):376‐82. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Zoccali 2010
- Zoccali C, Jager KJ. Hyperhomocysteinemia: a renal and cardiovascular risk factor?. Nature Reviews Nephrology 2010;6(12):695‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Nigwekar 2009
- Nigwekar SU, Cass A, Gallagher MP, Jardine MJ, Kang A, Kulshrestha S, et al. Interventions for lowering plasma homocysteine levels in dialysis patients. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD004683.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]


