Abstract
Background
Preterm birth remains the major risk factor for the development of intraventricular haemorrhage, an injury that occurs in 25% of very low birth weight infants. Intraventricular haemorrhage is thought to be venous in origin and intrinsic thromboses in the germinal matrix are likely to play a triggering role. Heparin activates antithrombin and promotes the inactivation of thrombin and other target proteinases. The administration of anticoagulants such as heparin may offset the increased risk of developing intraventricular haemorrhage and may also reduce the risk of developing parenchymal venous infarct, a condition known to complicate intraventricular haemorrhage.
Objectives
To assess whether the prophylactic administration of heparin reduces the incidence of germinal matrix‐intraventricular haemorrhage in very preterm neonates when compared to placebo, no treatment, or other anticoagulants.
Search methods
We searched the Cochrane Central Register of Controlled Trials (The Cochrane Library 2015), MEDLINE (1996 to 22 November 2015), EMBASE (1980 to 22 November 2015) and CINAHL (1982 to 22 November 2015), applying no language restrictions. We searched the abstracts of the major congresses in the field (Perinatal Society of Australia and New Zealand and Pediatric Academic Societies) from 2000 to 2015.
Selection criteria
Randomised controlled trials, quasi‐randomised controlled trials and cluster trials comparing the administration of early, i.e. within the first 24 hours of life, heparin in very preterm infants (gestational age < 32 weeks).
Data collection and analysis
For each of the included trials, two authors independently extracted data (e.g. number of participants, birth weight, gestational age, dose of heparin, mode of administration, and duration of therapy, etc.) and assessed the risk of bias (e.g. adequacy of randomisation, blinding, completeness of follow up). The primary outcomes considered in this review are intraventricular haemorrhage, severe intraventricular haemorrhage and neonatal mortality.
Main results
Two randomised controlled trials enrolling a total of 155 infants met the inclusion criteria of this review. Both trials compared low‐dose heparin to the same solution without heparin in very preterm newborns requiring umbilical catheterisation. No trials were identified that specifically studied the use of heparin in infants at risk of germinal matrix‐intraventricular haemorrhage.
We found no differences in the rates of intraventricular haemorrhage (typical RR 0.93, 95% CI 0.61 to 1.41; typical RD −0.03, 95% CI −0.17 to 0.12; 2 studies, 155 infants; I² = 57% for RR and I² = 65% for RD), severe intraventricular haemorrhage (typical RR 1.01, 95% CI 0.46 to 2.23; typical RD 0.00, 95% CI −0.11 to 0.11; 2 studies, 155 infants; I² = 0% for RR and I² = 0% for RD) and neonatal mortality (typical RR 0.69, 95% CI 0.28 to 1.67; typical RD −0.04, 95% CI −0.14 to 0.06; 2 studies, 155 infants; I² = 28% for RR and I² = 50% for RD). We judged the quality of the evidence supporting these findings as very low (rates of intraventricular haemorrhage) and low (severe intraventricular haemorrhage and neonatal mortality) mainly because of limitations in the study designs and the imprecision of estimates. We found very few data on other relevant outcomes, such as bronchopulmonary dysplasia, pulmonary haemorrhage and patent ductus arteriosus; and no study assessing long‐term outcomes (e.g. neurodevelopmental disability).
Authors' conclusions
There is very limited data on the effect of prophylactic administration of heparin on the incidence and severity of IVH in very preterm neonates. Both the identified trials used heparin in the context of maintaining umbilical line patency and not specifically as an agent to prevent germinal matrix‐intraventricular haemorrhage. Given the imprecision of our estimates, the results of this systematic review are consistent with either a benefit or a detrimental effect of heparin and do not provide a definitive answer to the review question. Limited evidence is available on other clinically relevant outcomes.
Plain language summary
The use of the anticoagulant heparin to reduce the risk of intraventricular haemorrhage (i.e. bleeding in the brain) in very preterm infants
Review question: Does heparin reduce the risk of intraventricular haemorrhage (i.e. bleeding in the brain) and mortality in very preterm infants?
Background: Heparin is a drug that modulates blood coagulation together with other factors. On the basis of an observational study in very preterm infants, it has been suggested that the administration of drugs that prevent clotting (anticoagulants) such as heparin may reduce the risk of intraventricular haemorrhage and progression of intraventricular haemorrhage, a frequent complication of preterm neonates. This systematic review synthesises the available evidence on the effectiveness of heparin in preventing intraventricular haemorrhage in very preterm neonates.
Study characteristics: We included two trials for a total of 155 very preterm newborn infants comparing low‐dose heparin with the same solution without heparin.
Results: The use of heparin does not reduce the risks of bleeding in the brain, mortality or any other relevant outcomes in very preterm neonates when compared to solution without heparin.
Conclusions: The results of this systematic review are consistent with either a benefit or a detrimental effect of heparin and do not provide a definitive answer to the review question.
Summary of findings
Summary of findings for the main comparison. Heparin compared to placebo/no treatment for the prevention of intraventricular haemorrhage in preterm infants.
| Heparin compared to no treatment for the prevention of intraventricular haemorrhage in preterm infants | ||||||
|
Patient or population: preterm infants at risk of intraventricular haemorrhage
Setting: Neonatal Intensive Care Intervention: Heparin Comparison: no treatment | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| No treatment | Heparin | |||||
| Any Intraventricular Haemorrage (IVH) | Study population | RR 0.93 (0.61 to 1.41) | 155 (2 studies) | ⊕⊝⊝⊝ very low1,2,3 | ||
| 383 per 1000 | 356 per 1000 (233 to 540) | |||||
| Severe IVH | Study population | RR 1.01 (0.46 to 2.23) | 155 (2 studies) | ⊕⊕⊝⊝ low1,3 | ||
| 136 per 1000 | 137 per 1000 (62 to 303) | |||||
| Neonatal mortality (all‐cause) | Study population | RR 0.69 (0.28 to 1.67) | 155 (2 studies) | ⊕⊕⊝⊝ low1,3 | ||
| 136 per 1000 | 94 per 1000 (38 to 227) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
The assumed risk is the risk of the control arm.
1 Limitations in study design: unclear risk of bias for allocation concealment and reporting bias. 2 Inconsistency: moderate heterogeneity 3 Imprecision: small number of patients, few events
Background
Description of the condition
Preterm birth remains the largest risk factor for the development of germinal matrix‐intraventricular haemorrhage (GM‐IVH), an injury that occurs in 25% of very low birth weight infants (Vermont Oxford Network 2013), often in the first day of life (Dolfin 1983). The classification of GM‐IVH was originally developed for the interpretation of computerised tomography (CT) scans and thereafter adapted by ultrasonographers and neonatologists (Papile 1978). The grading proposed by Papile is widely used, although magnetic resonance imaging (MRI) has been shown to be more sensitive than brain ultrasound or CT scans in detecting small‐sized cerebral haemorrhages (Nandigam 2009; Parodi 2013). The classification consists of four grades: grade 1 haemorrhage is confined to the subependymal GM; grade 2 is a small haemorrhage within the ventricular lumen; grade 3 is a large haemorrhage that expands the ventricle; and grade 4 is characterised by parenchymal haemorrhagic venous infarction (Papile 1978; Volpe 2008).
The aetiology of GM‐IVH is multifactorial, not completely elucidated, and involves the intrinsic vulnerability of the germinal matrix (Perlman 1983). This haemorrhagic disorder is thought to be venous in origin (Volpe 2008); and venous vessels are known to be more vulnerable to thrombotic phenomena compared to arteries. Moreover, in the absence of significant environmental fluctuations in cerebral blood flow, thromboses in the tiniest vessels of the germinal matrix are likely to play a crucial and triggering role (Ghazi‐Birry 1997; Paneth 1994; Ramenghi 2005). Sinovenous thrombosis in the deep venous system as a nosological entity is known to determine late appearance of GM‐IVH in premature infants also in the absence of other risk factors (Kersbergen 2011; Ramenghi 2002). A retrospective analysis investigating the association between thrombophilia and increased risk of developing GM‐IVH estimated an absolute risk of IVH (grade 2 to 4) of 80% for the preterm newborns with a point mutation of the factor V gene, as compared to 14% in absence of the mutation (Petäjä 2001). The combined frequency of established thrombophilic abnormalities in infants with GM‐IVH was 32% (Petäjä 2001). The presence of genetic prothrombotic factors significantly increases the risk of developing GM‐IVH to a similar degree as other factors such as low Apgar score or the use of inotropic agents (Ramenghi 2011). Moreover, a systematic review of observational studies estimated the impact of thrombophilia on risk of cerebral sinovenous thrombosis in neonates and children (Kenet 2010). The development of the haemostatic system in neonates is an age‐dependent process and preterm infants have low levels of both pro‐coagulants and anti‐coagulants factors (Tripodi 2008).
Description of the intervention
Heparin is produced by basophils and mast cells (Guyton 2006). It activates antithrombin by an allosteric conformational change mechanism that specifically enhances factor Xa inactivation and, by a ternary complex bridging mechanism, promotes the inactivation of thrombin and other target proteinases (Björk 1982; Chuang 2001). Thus, the activated antithrombin inactivates proteases involved in blood clotting, such as thrombin and factor Xa. While antifactor Xa activity requires only the pentasaccharide binding site, the activity of heparin against thrombin is size‐dependent (Petitou 1999). For this reason, low‐molecular‐weight heparin (LMWH) was introduced in order to target factor Xa instead of antithrombin. LMWH has an improved therapeutic index, with a reduced risk of osteoporosis and thrombocytopenia in adults (Garcia 2012) and children (Newall 2009), respectively. It may change coagulation profiles (O'Neill 1974); and may cause heparin‐induced thrombocytopenia in users (Potter 1992), including newborns (Spadone 1992). Neonatal overdose, which can be fatal (Monagle 2012b), may be reversed with protamine sulphate (Wiernikowski 2007). Other potential side effects of heparin include elevation of aminotransferase levels (Carlson 2001) and hypokalaemia (Cho 2013).
Heparin can be administered either as a liquid, intravenously or subcutaneously, or released by specific catheters whose lumens contain the anticoagulant. In addition, it may be added either directly to the infusate or to fluids used to intermittently flush catheters. Heparin should be given frequently or as a continuous infusion because of its short half‐life, i.e. less than one hour for heparin (McDonald 1981), three to four hours for LMWH (Streif 2003). High doses of heparin have a longer half‐life, as endothelial cell binding becomes saturated, reducing renal clearance (Weitz 2010). One unit of heparin, known as a 'Howell unit' after the physiologist who nearly a century ago reported on heparin as an anticoagulant (Howell 1918), corresponds to approximately 2 mcg of heparin, which is the amount to keep 1 mL of blood fluid for 24 hours at 0°C. Many concentrations of heparin are available and range from 1 to 20,000 units/mL. Daily flushes of 10 units/mL heparin are used to maintain patency of central catheters in neonates; flushing is required more frequently (e.g. every 6 to 8 hours) for capped polyvinyl chloride catheters and peripheral heparin locks (Monagle 2012a). The volume of heparin flush is usually 0.5 to 1 mL, similar to the volume of the catheter; the dose of heparin flush used should not approach therapeutic unit/kg dose (Monagle 2012a). In preterm neonates receiving larger volumes per body weight of total parenteral nutrition (TPN) solutions, a lower dose of 0.5 units/mL of heparin may be preferable in order to avoid approaching therapeutic amounts (Monagle 2012a). In this population, the same concentration should be used for arterial lines.
How the intervention might work
The exact mechanism or the unique 'primum movens' explaining all cases of GM‐IVH are not known although pathogenesis is likely to differ according to severity and timing of this intracranial haemorrhage. GM‐IVH can derive from catastrophic and sudden haemodynamic changes in the venous flow in those very early and severe cases while a sequence of events seems more probable in less severe cases occurring later in more stable babies, after the first few hours of life (Ramenghi 2015). There is histological evidence that among the potential triggers of GM‐IVH, thrombosis in the microvasculature of the germinal matrix plays a significant role (Paneth 1994). Once venous haemorrhage is initiated within the germinal‐matrix there is a further increase in tissue pressure and venous congestion aggravating venous stasis and venous thrombosis (Ghazi‐Birry 1997).
The presence of hypercoagulability in the first hours of life has been shown to increase the risk of developing GM‐IVH (McDonald 1984) and this is consistent with the above mentioned cascade of events. Although we cannot establish the proportion of thrombosis exclusively triggered by inherited thrombophilic disorders the evidence of thrombosis per se within germinal matrix microvasculature remains an important key factor in the pathogenesis of GM‐IVH (Ghazi‐Birry 1997; Paneth 1994). Therefore, the early administration of anticoagulants such as heparin may offset this risk of GM‐IVH at least in those babies with congenital thrombophilia aggravated by the hypercoagulable state. Moreover, anticoagulants may prove useful in reducing the risk of developing parenchymal venous infarct, a thrombotic occlusive condition known to complicate grade 4 IVH (Volpe 2008). Beneficial effects of heparin might vary in specific populations, e.g. neonates with complex coagulation disorders, sepsis with disseminated intravascular coagulation, transient neonatal protein C deficiency. Low cord blood levels of protein C (< 0.1 unit/mL) may reflect delayed maturation or increased turnover in certain infants and appear to convey an independent risk of thrombosis (Manco‐Johnson 1991). Taken together, the developmental immaturity of the haemostasis system as well as the frequent need for medical interventions in critically ill preterm infants increase the risk for thromboembolism. These newborns might benefit from heparin treatment by avoiding thrombus formation. On the other hand, retrospective studies have reported an association between heparin exposure and increased risk of GM‐IVH (Lesko 1986; Malloy 1995), although the true risk could not be determined because of confounding factors inherent in the study design.
Why it is important to do this review
Three Cochrane Reviews have been published regarding the use of heparin specifically in neonates: 1) "Continuous heparin infusion to prevent thrombosis and catheter occlusion in neonates with peripherally placed percutaneous central venous catheters" (Shah 2008); 2) "Heparin for prolonging peripheral intravenous catheter use in neonates" (Shah 2005); and 3) "Umbilical artery catheters in the newborn: effects of heparin" (Barrington 2000a). Barrington also conducted another Cochrane review on umbilical artery catheters from another perspective: the effects of catheter materials, including the presence of heparin in the catheter (Barrington 2000b). A fifth Cochrane review assessed the effect of heparin‐bonded central venous catheters on the duration of their patency in children, including neonates (Shah 2014).
Each of these reviews included only infants with catheters. The present review has a broader scope and includes the use of heparin for any reason in the first 24 hours of life, including use for prolonging catheter patency. It focuses on very preterm infants, i.e. less than 32 weeks' gestational age, a population where the prevention of GM‐IVH is a major concern. Taking into account the pathophysiological mechanisms of GM‐IVH development, the controversial findings from retrospective studies on GM‐IVH incidence during heparin administration and the increasing use of heparin for catheter patency in preterm newborns, a systematic approach of available evidence is needed.
Objectives
To assess whether the prophylactic administration of heparin reduces the incidence of germinal matrix‐intraventricular haemorrhage in very preterm neonates when compared to placebo, no treatment, or other anticoagulants.
We planned subgroup analyses according to gestational age, birth weight, requirement for assisted ventilation, presence of GM‐IVH, indication for administration, route of administration, and comparison group, i.e. placebo or no treatment (see Subgroup analysis and investigation of heterogeneity).
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled clinical trials (RCTs), quasi‐randomised trials and cluster trials. We excluded cross‐over trials.
Types of participants
We included newborn infants of less than 32 weeks' gestational age and any birth weight. We also planned to include studies enrolling infants with existing GM‐IVH and to assess the extension of haemorrhage in a subgroup of infants.
Types of interventions
Comparison 1: heparin compared to placebo or no treatment (specific subgroup analyses for each comparison are described in Subgroup analysis and investigation of heterogeneity);
Comparison 2: heparin compared to other anticoagulant treatments, e.g. factor Xa inhibitors and direct thrombin inhibitors. We excluded trials comparing heparin with antithrombin as they are included in a separate Cochrane review (Bruschettini 2015).
We included trials testing any dose, mode of administration, and duration of heparin therapy. In addition, we included trials where heparin was administered specifically to prevent GM‐IVH, as well as those where heparin was used to prevent thrombosis (in catheters and other locations). We excluded trials if heparin was:
started beyond the first 24 hours of life (as the aim of the review is to assess the ability of heparin to prevent GM‐IVH);
administered to achieve systemic heparinisation, i.e. during extracorporeal membrane oxygenation or for the treatment of thrombosis (regardless of the dosage of heparin);
administered through the use of heparin‐bonded catheters.
Types of outcome measures
Primary outcomes
1. Germinal matrix‐intraventricular haemorrhage (GM‐IVH):
Any IVH (grade 1 to 4 according to Papile classification) (Papile 1978).
Severe IVH (grade 3 and 4 according to Papile classification) (Papile 1978).
2. Neonatal all‐cause mortality (first 28 days).
Secondary outcomes
Death during initial hospitalisation (all‐cause mortality).
Bronchopulmonary dysplasia (BPD)/Chronic lung disease (CLD): 28 days (NIH 1979); 36 weeks postmenstrual age (Jobe 2001); physiological definition (Walsh 2004).
Pneumothorax (on chest x‐ray).
Duration of mechanical intermittent positive pressure ventilation (IPPV; days).
Duration of respiratory support (IPPV or continuous positive airway pressure, days).
Duration of oxygen therapy (days).
Duration of hospital stay (days).
Retinopathy of prematurity: any and severe (stage 3 or greater; ICROP 1984).
Necrotising enterocolitis (any grade and requiring surgery).
Need for blood transfusion.
Need for medical or surgical treatment for persistent ductus arteriosus (PDA).
Pulmonary haemorrhage.
Clinically apparent bleeding during treatment in the first week of life.
Central catheter (umbilical line or peripherally inserted central catheter) thrombosis (along the length of, or at the tip of, the catheter) as determined by Doppler ultrasonography or contrast venography.
Central catheter occlusion, identified by inability to infuse fluids.
Peripheral intravenous catheter occlusion.
Cerebellar haemorrhage on brain ultrasound in the first month of life.
Cystic periventricular leukomalacia on brain ultrasound in the first month of life.
Brain MRI abnormalities at term equivalent age (yes/no), defined as: white matter lesions, i.e. cavitations (Rutherford 2010) and punctate lesions (Cornette 2002); GM‐IVH (Parodi 2013); cerebellar haemorrhage (Fumagalli 2009; Limperopoulos 2007).
Cerebral haemodynamics impairment, based on cerebral near‐infrared spectroscopy (NIRS).
Long‐term neurodevelopmental outcome (yes/no): cerebral palsy on physician assessment, developmental delay, i.e. IQ two standard deviations (SD) below the mean on validated assessment tools e.g. Bayley Mental Developmental Index (Bayley 1993; Bayley 2006).
Major neurodevelopmental disability: cerebral palsy, developmental delay (Bayley Mental Developmental Index (Bayley 1993; Bayley 2006) or Griffiths Mental Development Scale assessment (Griffiths 1954) more than two SD below the mean), intellectual impairment (IQ more than two SD below mean), blindness (vision < 6/60 in both eyes), or sensorineural deafness requiring amplification (Jacobs 2013). We planned to evaluate each of these components as a separate outcome and to extract data on this long‐term outcome from studies that evaluated children after 18 months of chronological age. Data on children aged 18 to 24 months and those aged three to five years were to be assessed separately.
Search methods for identification of studies
Electronic searches
We used the criteria and standard methods of Cochrane and the Cochrane Neonatal Review Group (see the Cochrane Neonatal Group search strategy for specialized register).
We undertook a comprehensive search including:
the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library, 2015;
MEDLINE (1996 to November 2015);
EMBASE (1980 to November 2015);
CINAHL (1982 to November 2015);
Australian New Zealand Clinical Trials Registry (PSANZ; 2005 to November 2015);
abstracts of the Pediatric Academic Societies (PAS; 2000 to November 2015).
Appendix 1 reports the full search strategies for each database.
We applied no language restrictions. We searched the reference lists of any cited articles.
Searching other resources
We searched clinical trial registries for ongoing or recently completed trials (clinicaltrials.gov and controlled‐trials.com).
Data collection and analysis
We used the standard methods of the Cochrane Neonatal Group. Two review authors (RB, SZ) independently selected the included studies, extracted their data and assessed their risk of bias. At each stage, we resolved disagreements by consensus or by discussion with a third review author (MGC).
Selection of studies
Two review authors (RB, SZ) independently screened the titles and abstracts retrieved by the literature search to identify eligible trials that met the inclusion criteria. The review authors then retrieved the full texts of all potentially relevant articles and independently confirmed their eligibility using an eligibility form designed in accordance with the review inclusion criteria.
Data extraction and management
Two reviewers (MG, SZ) independently undertook data abstraction using a standardised data extraction form developed ad hoc and integrated with a modified version of the Cochrane Effective Practice and Organisation of Care Group (EPOC) data collection checklist (EPOC 2015).
We extracted the following characteristics from each included trial:
Administrative details: author(s); publication status; year the trial was conducted and year of publication; details of other relevant papers cited.
Details of the trial: study design; type, duration, and completeness of follow‐up; country and setting of the trial; informed consent and ethics approval.
Details of participants: birth weight, gestational age, and number of participants.
Details of intervention: type of heparin, dose of heparin, duration of therapy, mode of administration.
Details of outcomes, as listed in Types of outcome measures.
In the case of on‐going trials, we planned to collect data on first author, research question(s), methods, outcome measures, and estimate of the reporting date.
We intended to contact the authors of included trials to clarify eligibility and data extraction and to retrieve additional information. One author (MGC) entered the data in the Review Manager 5 software (RevMan 2014) and a second one (SZ) checked their accuracy.
Assessment of risk of bias in included studies
Two authors (RB, SZ) independently assessed the risk of bias of each of the included trials by using the Cochrane's tool for assessing risk of bias (Higgins 2011). We resolved disagreements by consensus or, if necessary, by adjudication with a third review author (MGC). We used 'Risk of bias' graphs to illustrate risk across trials.
We appraised the following items :
1. Selection bias (random sequence generation and allocation concealment)
1a. Random sequence generation
For each included trial, we categorised the risk of selection bias related to random sequence generation as:
Low risk: the investigators describe a random component in the sequence generation process such as referring to a random number table, using a computer random number generator, coin tossing, shuffling cards or envelopes, throwing dice, drawing of lots, or minimisation;
High risk: the investigators describe a non‐random component in the sequence generation process such as sequence generated by odd or even date of birth, sequence generated by some rule based on date or day of admission, sequence generated by some rule based on hospital or clinic record number, allocation by judgment of the clinician, allocation by preference of the participant, allocation based on the results of a laboratory test or a series of tests, or allocation by availability of the intervention;
Unclear risk: no or unclear information provided.
1b. Allocation concealment
For each included trial, we categorised the risk of selection bias related to allocation concealment as:
Low risk: participants and investigators enrolling participants could not foresee assignment because one of the following, or an equivalent method, was used to conceal allocation: central allocation (including telephone, web‐based, and pharmacy‐controlled randomisation), sequentially numbered drug containers of identical appearance, sequentially numbered sealed opaque envelopes;
High risk: participants and investigators enrolling participants could possibly foresee assignments and thus introduce selection bias, such as allocation based on open random allocation schedule (e.g. a list of random numbers), unsealed or non‐opaque envelopes, alternation or rotation, date of birth, or case record number;
Unclear risk: no or unclear information provided.
2. Blinding (performance bias)
For each included trial, we categorised the risk of performance bias related to the methods used to blind study personnel from knowledge of which intervention a participant received as:
Low risk: no blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding; blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken;
High risk: no blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding; blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding;
Unclear risk: no or unclear information provided.
3. Blinding (detection bias)
For each included trial, we categorised the risk of detection bias related to the methods used to blind outcome assessors from knowledge of which intervention a participant received as:
Low risk: no blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding; blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken;
High risk: no blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding; blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding;
Unclear risk: no or unclear information provided.
4. Incomplete outcome data (attrition bias)
For each included trial and for each outcome, we described the risk of attrition bias related to the completeness of data including attrition and exclusions from the analysis as:
Low risk:
No missing outcome data;
Reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to introduce bias);
Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups;
For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate;
For continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size;
Missing data have been imputed using appropriate methods.
High risk:
Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups;
For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate;
For continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size;
'As‐treated' analysis done with substantial departure of the intervention received from that assigned at randomisation;
Potentially inappropriate application of simple imputation.
Unclear risk: no or unclear information provided.
5. Selective reporting (reporting bias)
For each included trial, we described how we investigated the risk of selective outcome reporting bias and what we found. We searched trial registries (clinicaltrials.gov; controlled‐trials.com; and who.int/ictrp) and we intended to contact the study author to have access to the full protocol.
We assessed the risk of bias related to the reporting method as:
Low risk: the study protocol is available and all of the study's pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way; or the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon).
High risk: not all of the study’s pre‐specified primary outcomes have been reported; one or more primary outcomes is reported using measurements, analysis methods, or subsets of the data (e.g. subscales) that were not pre‐specified; one or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); one or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis; or the study report fails to include results for a key outcome that would be expected to have been reported for such a study.
Unclear risk: no or unclear information provided (the study protocol was not available).
6. Other potential sources of bias (other bias)
For each included trial, we described any important concerns we have about other possible sources of bias (for example, whether there was a potential source of bias related to the specific study design used).
We assessed the risk of bias related to other problems as:
Low risk: the study appears to be free of other sources of bias;
High risk: the trial has at least one important risk of bias (e.g. the trial has a potential source of bias related to the specific study design used or has been claimed to have been fraudulent or had some other problem);
Unclear risk: there may be a risk of bias, but there is either insufficient information to assess whether an important risk of bias exists or insufficient rationale or evidence that an identified problem may introduce bias.
Measures of treatment effect
We followed the standard methods of the Cochrane Neonatal Group for data synthesis. We extracted categorical data for each intervention group and calculated relative risks (RRs), relative risk reduction, and absolute risk differences (RDs). For each measure of effect, we provided the corresponding 95% confidence intervals (CIs). If RDs were statistically significant, we would have further calculated numbers needed to treat for an additional beneficial outcome (NNTBs) and numbers needed to treat for an additional harmful outcome (NNTHs). This review does not include outcomes assessed with continuous measures.
Unit of analysis issues
In cluster trials, groups of individuals are randomly allocated to study arms, and outcomes are then measured based on the individual cluster members. Under such circumstances, it is necessary to adjust the results to account for the fact that the randomisation was performed on the clusters rather than the individuals. As many cluster‐randomised trials fail to report appropriate analyses, corrections for clustering are needed before they are included in a meta‐analyses.
To calculate adjusted (inflated) CIs that account for the clustering, we planned to follow the approach suggested by the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), i.e. to multiply the standard error of the effect estimate (from an analysis ignoring clustering) by the square root of the design effect. The design effect is calculated from the average cluster size and the intra‐cluster correlation coefficient. We planned to derive intra‐cluster correlation coefficient(s) from similar studies and only include trials in meta‐analyses if corrections were possible.
Dealing with missing data
We recorded drop‐out rate for each trial. A drop‐out rate equal to or greater than the event rate of the control group was considered as significant and additional information was sought by the trial author(s) to facilitate an intention‐to‐treat analysis. When this was not possible, we performed a complete case analysis.
Assessment of heterogeneity
We planned to assess clinical heterogeneity by comparing the distribution of important participant factors between trials (for example, age) and trial factors (allocation concealment, blinding of outcome assessment, losses to follow‐up, treatment type, co‐interventions). We assessed statistical heterogeneity by examining the I² statistic (Higgins 2011), a quantity that describes the proportion of variation in a point estimate that is due to variability across studies rather than sampling error. We interpreted the I² statistic as described by Higgins 2003:
< 25% – no heterogeneity;
25% to 49% – low heterogeneity;
50% to 74% – moderate heterogeneity; and
≥ 75% – high heterogeneity.
In addition, we planned to do a Chi² test of homogeneity to determine the strength of evidence that heterogeneity was genuine.
Assessment of reporting biases
If more than 10 trials had been included, we would have explored publication bias using funnel plots (Egger 1997; Higgins 2011).
Data synthesis
We summarised data using Review Manager 5 (RevMan 2014). We used the standard methods of the Cochrane Neonatal Review Group to synthesise data using RRs, RDs, NNTBs, NNTHs, weighted mean differences (WMDs), and 95% CIs. We preferred a fixed‐effect model to perform meta‐analyses. In case of moderate or high heterogeneity among the trials, we also conducted and reported meta‐analyses using a random‐effects model.
Although this was not planned in the review protocol (see Differences between protocol and review), we summarised the evidence of this review in a 'Summary of findings' table. We used the control arm data to calculate the 'assumed risk' values and select incidence and severity of IVH and all‐cause mortality as critical outcomes.
We assessed the overall quality of the evidence for each outcome using the GRADE (Grading of Recommendations Assessment, Development and Evaluation) approach, as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We considered the following quality domains: study design and limitations, consistency of results, directness (generalisability), precision (sufficient data), and reporting of the results across all studies that measure that particular outcome. The quality starts at high when high quality RCTs provide results for the outcome, and reduces by a level for each of the factors not met.
High quality evidence: there are consistent findings among at least 75% of RCTs with no limitations of the study design, consistent, direct and precise data, and no known or suspected publication biases. Further research is unlikely to change either the estimate or our confidence in the results.
Moderate quality evidence: one of the domains is not met. Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low quality evidence: two of the domains are not met. Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low quality evidence: three of the domains are not met. We are very uncertain about the results.
No evidence: no RCTs were identified that addressed this outcome.
Subgroup analysis and investigation of heterogeneity
We planned the following subgroup analyses:
Gestational age (with two subgroups: < 28 weeks versus ≥ 28 weeks).
Birth weight (with two subgroups: < 1000 grams versus ≥ 1000 grams).
Infants requiring assisted ventilation versus infants not requiring assisted ventilation.
With or without GM‐IVH (any grade) at trial entry.
Trials that used heparin for GM‐IVH prevention (regardless of the dosage) versus other indications, e.g. line patency.
Route of administration, e.g. intravenous versus subcutaneous.
Heparin compared to placebo versus heparin compared to no treatment.
Sensitivity analysis
If enough trials had been included, we would have conducted sensitivity analyses to explore the effect of the methodological quality of the trials, checking to ascertain if trials with a high risk of bias overestimate the effect of treatment.
Results
Description of studies
See Characteristics of included studies and Characteristics of excluded studies.
Results of the search
The literature search run in November 2015 identified 407 references (Figure 1). After screening, we assessed for eligibility six full‐text articles and included two RCTs (Chang 1997; Stec 1993).
1.
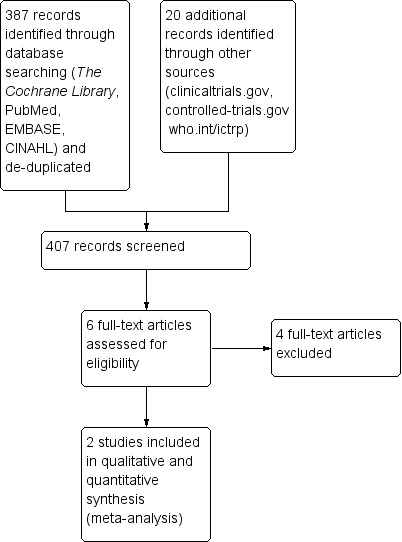
Study flow diagram.
We did not find other relevant trials by searching clinical trials registries (clinicaltrials.gov; controlled‐trials.com; and who.int/ictrp).
Included studies
Two trials recruiting 155 infants met the inclusion criteria (Chang 1997; Stec 1993). Both trials compared low‐dose heparin (1 unit per mL) with the same solution without heparin in very preterm infants with umbilical catheter. Heparin was administered continuously to the umbilical catheter infusate in Chang 1997 and intermittently in the flush solution in Stec 1993. Study's hypothesis in both trials was that the use of heparin would increase, not prevent, the incidence of GM‐IVH. We identified no trials comparing heparin to other anticoagulants.
Details are described in Characteristics of included studies.
Chang 1997 was a two‐centre randomised controlled trial enrolling 118 newborns (57 in the heparin group, 61 in the control group) of less than 31 weeks gestation with umbilical catheter, either venous or arterial lines (both venous and arterial lines were studied in this trial). Five infants (two in the heparin group, three in the control group) were randomised but excluded from the analysis as they contravened inclusion criteria (two had chromosomal anomalies, one had a congenital diaphragmatic hernia, one had a congenital cyanotic heart lesion; one was assessed to have been born at greater than 31 weeks' gestation).
Within each gestational age block (< 28 and ≥ 28 weeks), randomisation cards were prepared in blocks of 10. Gestational age and birth weight were similar in the two groups, i.e. 27.4 weeks ± 1.8 and 1074 ± 300 grams in the heparin group and 27.1 weeks ± 1.9 and 1065 ± 352 grams in the control group. Less than 40% of the infants were treated with antenatal steroids. All infants received vitamin K intramuscularly. Solutions used to flush all indwelling catheters did not contain heparin. Infants in the intervention group (n = 55) received 1 unit of heparin per mL in their umbilical catheter infusate, whereas control group (n = 58) received the same solution without heparin. All investigators and staff members caring for the infants remained masked, except the pharmacist. The study was conducted from the first through the fifth day of life. The trial ended before the fifth day of life if all umbilical catheters were removed or if a catheter necessitating heparin‐containing infusate was inserted (e.g. a percutaneous intravenous central catheter). All cranial sonograms were performed at one week of life and interpreted by a masked paediatric neuroradiologist.
Stec 1993 was a single‐centre randomised controlled trial enrolling 42 infants with birth weight less than 2000 grams and requirement for umbilical artery catheterisation (arterial lines), defined as FiO₂ > 0.4 to maintain PaO₂ > 60 torr. Gestational age and birth weight were similar in the two groups, i.e. 28 weeks ± 3 and 1096 ± 350 grams in the heparin group and 28 weeks ± 3 and 1000 ± 415 grams in the control group. The intervention group of infants (n = 19) received a flush solution with one unit of heparin per mL, while the control group (n = 23) received the same solution without heparin. Solutions were prepared by the hospital pharmacy; staff members caring for the infants remained masked. The flush solutions were used to clear the umbilical artery catheters after routine blood samples (2 to 3 cc of blood to clear the catheter, obtaining the blood specimen, and returning the non‐sample blood to the infant). The catheters were then reconnected to a non‐heparinised intravenous infusion. Cranial sonograms were performed on the first, fourth and seventh postnatal days and reviewed by a masked paediatric radiologist or a neuroradiologist.
Excluded studies
We excluded three trials because heparin administration was started beyond 24 hours of life (Birch 2010; Kamala 2002; Treas 1992); and one trial because the gestational age of infants was greater than 32 weeks (Brown 1999); (details are reported in Characteristics of excluded studies).
Risk of bias in included studies
Figure 2 and Figure 3 summarise the risk of bias of the included trial.
2.
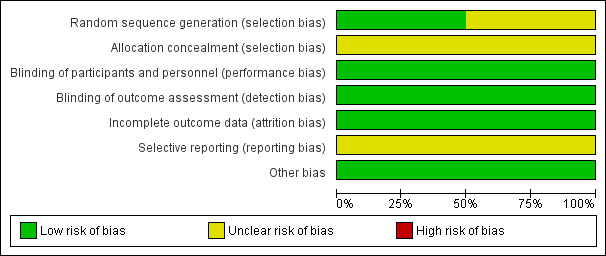
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
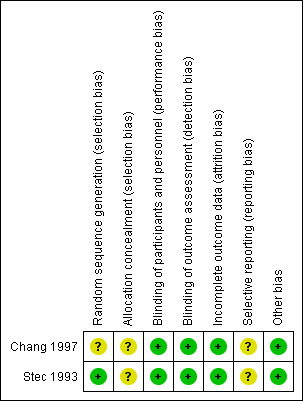
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Chang 1997 did not provide sufficient information on how the randomisation sequence was generated and concealed and was then judged at unclear risk of selection bias.
Stec 1993 provide information on the allocation sequence generation but not on its concealment.
Blinding
The two included trials were designed as blinded. Participants, investigators, and outcome assessors were not aware of the allocated study treatments.
Incomplete outcome data
In Chang 1997, five infants (two in the heparin group, three in the control group) were randomised but excluded from the analysis as they contravened inclusion criteria (two had chromosomal anomalies, one had a congenital diaphragmatic hernia, one had a congenital cyanotic heart lesion; one was assessed to have been born at greater than 31 weeks' gestation). Moreover, five infants died before a sonogram could be obtained and one infant did not have cranial ultra‐sonography performed (two of these infants were in the heparin group, four in the 'no heparin' group). Thus, data on incidence and severity of IVH were available on 107 infants (53 infants in the heparin group, 54 infants in the control group). Overall, 11 out of 118 randomised infants (9.3%) did not complete the study.
Stec 1993 reported outcomes for all randomised infants (no drop‐outs).
Selective reporting
The included studies were not registered. We were not able to contact the authors of these studies to retrieve the study protocols. Thus, we judged them at unclear risk of bias.
Other potential sources of bias
The trials appeared free of other biases.
Effects of interventions
See: Table 1
Heparin versus placebo or no treatment (comparison 1)
Two trials (Chang 1997; Stec 1993), which included a total of 155 infants, met eligibility criteria. Both trials compared heparin to the same solution without heparin (see Table 1).
Primary outcomes
Any GM‐IVH (grade 1 to 4) (Outcome 1.1): Both studies (n = 155 infants) reported this outcome. Prophylactic administration of heparin did not alter the risk of developing any GM‐IVH (typical RR 0.93, 95% CI 0.61 to 1.41; typical RD −0.03, 95% CI −0.17 to 0.12; 2 studies, 155 infants; I² = 57% for RR and I² = 65% for RD). See:Analysis 1.1Figure 4
1.1. Analysis.
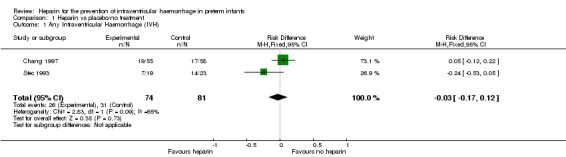
Comparison 1 Heparin vs placebo/no treatment, Outcome 1 Any Intraventricular Haemorrhage (IVH).
4.
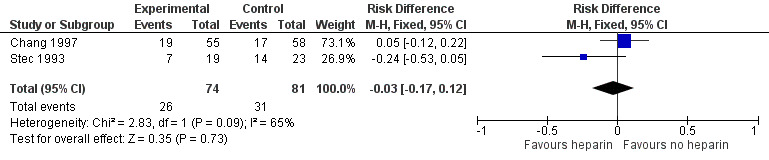
Forest plot of comparison: 1 Heparin vs no treatment, outcome: 1.1 Any IVH.
Severe IVH (grade 3 and 4) (Outcome 1.2): Both trials reported this outcome. Prophylactic administration of heparin did not alter the risk of developing any severe IVH (typical RR 1.01, 95% CI 0.46 to 2.23; typical RD 0.00, 95% CI −0.11 to 0.11; 2 studies, 155 infants; I² = 0% for RR and I² = 0% for RD). See:Analysis 1.2Figure 5
1.2. Analysis.
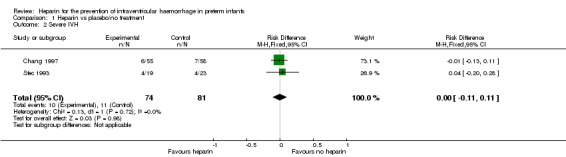
Comparison 1 Heparin vs placebo/no treatment, Outcome 2 Severe IVH.
5.
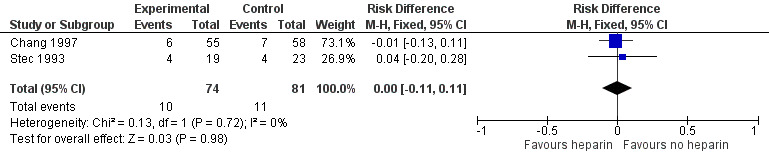
Forest plot of comparison: 1 Heparin vs no treatment, outcome: 1.2 Severe IVH.
Neonatal all‐cause mortality (Outcome 1.3): Both trials (n = 155) reported this outcome. Prophylactic administration of heparin did not alter the risk of neonatal mortality (typical RR 0.69, 95% CI 0.28 to 1.67; typical RD −0.04, 95% CI −0.14 to 0.06; 2 studies, 155 infants; I² = 28% for RR and I² = 50% for RD). In Chang 1997, 10 infants in the control group and five in the heparin group died during the study. In Stec 1993, two deaths occurred in the heparin group and one in the control group. See:Analysis 1.3Figure 6
1.3. Analysis.
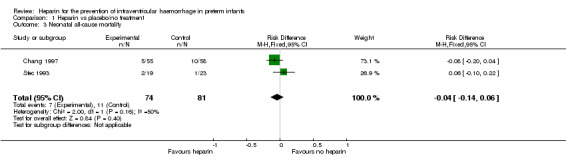
Comparison 1 Heparin vs placebo/no treatment, Outcome 3 Neonatal all‐cause mortality.
6.
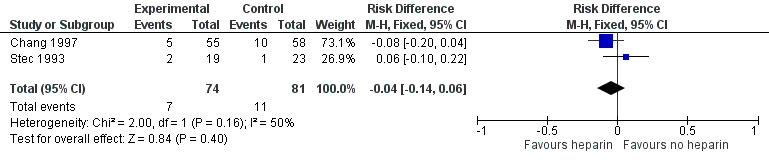
Forest plot of comparison: 1 Heparin vs no treatment, outcome: 1.3 Neonatal all‐cause death.
Secondary outcomes
Bronchopulmonary dysplasia (Outcome 1.4): One trial reported that bronchopulmonary dysplasia defined as the need for supplemental oxygen at 28 days of age was diagnosed in 21 infants in the heparin group (21/55) versus 18 infants in the control group (18/58) (Chang 1997); this difference was not significant (RR 1.23, 95% CI 0.74 to 2.05; RD 0.07, 95% CI −0.10 to 0.25). The test for heterogeneity was not applicable. See:Analysis 1.4
1.4. Analysis.
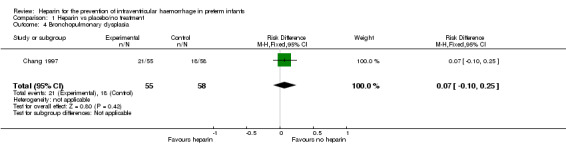
Comparison 1 Heparin vs placebo/no treatment, Outcome 4 Bronchopulmonary dysplasia.
Pneumothorax (Outcome 1.5): Both trials (n = 155) reported this outcome (typical RR 0.50, 95% CI 0.17 to 1.53; typical RD −0.05, 95% CI −0.14 to 0.03; I² = 57% for RR and I² = 5% for RD) (Analysis 1.5). See:Analysis 1.5
1.5. Analysis.
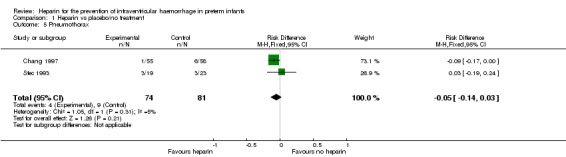
Comparison 1 Heparin vs placebo/no treatment, Outcome 5 Pneumothorax.
Patent ductus arteriosus (Outcome 1.6); One trial reported on PDA as diagnosed by physical examination or echocardiogram (RR 0.79, 95% CI 0.54 to 1.16; RD −0.12, 95% CI −0.30 to 0.07) (Chang 1997). The test for heterogeneity was not applicable. Need for medical or surgical treatment for PDA was not specified. See:Analysis 1.6
1.6. Analysis.
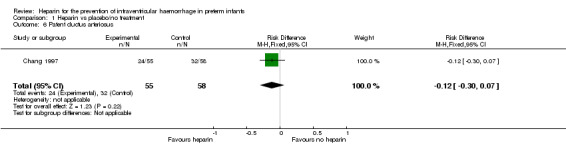
Comparison 1 Heparin vs placebo/no treatment, Outcome 6 Patent ductus arteriosus.
Pulmonary haemorrhage (Outcome 1.7); One trial reported on this outcome (RR 0.45, 95% CI 0.19 to 1.09; RD −0.13, 95% CI −0.27 to 0.01) (Chang 1997). The test for heterogeneity was not applicable. See:Analysis 1.7
1.7. Analysis.
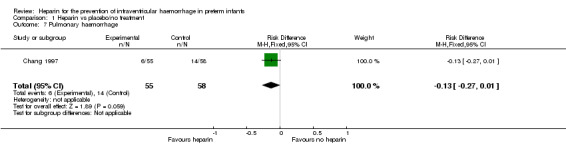
Comparison 1 Heparin vs placebo/no treatment, Outcome 7 Pulmonary haemorrhage.
Central catheter occlusion (Outcome 1.8): One trial reported on this outcome (RR 0.40, 95% CI 0.13 to 1.28; RD −0.23, 95% CI −0.49 to 0.02) (Stec 1993). The test for heterogeneity was not applicable. See:Analysis 1.8
1.8. Analysis.
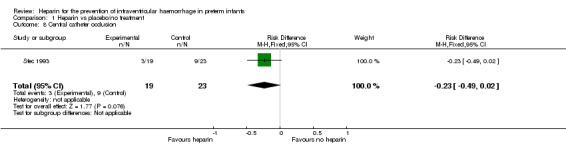
Comparison 1 Heparin vs placebo/no treatment, Outcome 8 Central catheter occlusion.
We did not find any data on the following outcomes:
Death during initial hospitalisation (all‐cause mortality); duration of mechanical ventilation; duration of respiratory support; duration of oxygen therapy; duration of hospital stay; retinopathy of prematurity; necrotising enterocolitis; need for blood transfusion; clinically apparent bleeding during treatment; central catheter thrombosis; peripheral intravenous catheter occlusion; cerebellar haemorrhage; cystic periventricular leukomalacia; brain MRI abnormalities at term equivalent age; cerebral haemodynamics impairment; long‐term neurodevelopmental outcome; major neurodevelopmental disability.
Subgroup Analysis
We were unable to conduct any of the planned subgroup analyses, as only two trials met the inclusion criteria for this review.
Heparin versus other anticoagulant treatments (comparison 2)
We did not find trials comparing heparin to other anticoagulants.
Discussion
Summary of main results
We evaluated the efficacy of the prophylactic administration of heparin in the prevention of GM‐IVH (germinal matrix‐intraventricular haemorrhage) in very preterm infants. Only two trials (Chang 1997; Stec 1993), for a total of 155 preterm infants, met the inclusion criteria of this review. Heparin was not better than solution or infusate without heparin in terms of incidence or severity of intraventricular haemorrhage and neonatal mortality, the primary outcomes of this review. Heterogeneity was low to moderate for two of our primary outcomes. We could not formally explore the reasons for this heterogeneity due to the small number of included trials, however a possible explanation could be that the two studies used different type of infusion. In terms of secondary outcomes, such as bronchopulmonary dysplasia, pneumothorax, pulmonary haemorrhage and patent ductus arteriosus, we did not find evidence for a benefit.
Overall completeness and applicability of evidence
Both trials compared a low dose of heparin (1 unit per mL) with the same solution without heparin in very preterm infants with umbilical catheter. Importantly, heparin was administered continuously to the umbilical catheter infusate in Chang 1997 and intermittently in the flush solution in Stec 1993. However, even when administered continuously as in Chang 1997, heparin doses received by the infant averaged 4.3 U/kg/h and did not result in clinically significant changes in antithrombin levels. In addition, this review includes trials conducted more than 20 years ago, when the use of antenatal steroid was low (< 40% of the infants enrolled in the more recent trial, i.e. Chang 1997; not reported in Stec 1993). We did not find trials comparing heparin to other anticoagulants.
Quality of the evidence
According to the GRADE approach, we rated the overall quality of the evidence for clinically relevant outcomes as "low" and "very low" (see Table 1). We downgraded the overall quality of the evidence for the critical outcomes because of 1) limitations in the study design (i.e. selection bias due to an unclear allocation concealment and reporting bias), and 2) the imprecision of results (a small number of participants) that could be a source of random error risk. The random error is closely related to the imprecision as the results of smaller studies are subject to greater sampling variation and hence are less precise (Higgins 2011). The estimate of the primary outcome incidence of IVH was also affected by a moderate heterogeneity, thus we further downgraded the overall quality of the evidence supporting this outcome (Guyatt 2011).
Potential biases in the review process
As the aim of the present review is to assess the efficacy of heparin to prevent GM‐IVH which mostly occurs in the first three days of life, we excluded trials where the intervention was started beyond the first 24 hours of life, such as Birch 2010 and Brown 1999. However, when evaluating the progression of the bleeding, interventions started beyond 24 hours of life may play a relevant role. Importantly, in Birch 2010 the continuous infusion of 0.5 IU/mL of heparin started at a mean age of two days of life resulted in significant reduction of the progression of IVH in preterm infants weighing less than 850 g (0/26 IVH progressions in the heparin group versus 4/28 in the control group).
Agreements and disagreements with other studies or reviews
Evidence supports the use of heparin in neonates with peripherally placed percutaneous central venous catheters (Shah 2008) and umbilical artery catheters (Barrington 2000a), but not for peripheral intravenous catheter (Shah 2005). The dose and method of administration of heparin varies widely, with concentrations ranging from 0.1 to 10 U/mL of heparin administered as either intermittent flush solution or as an additive in parenteral nutrition solutions (Shah 2005). Though not corroborated by any high‐quality study, it has been suggested that a low dose of 0.5 units/mL of heparin may be preferable in preterm neonates receiving larger volumes per body weight of total parenteral nutrition (Monagle 2012a).
The present review differs from the reviews of Shah and Barrington in the objective and in the inclusion criteria, and consequently in the included trials (Barrington 2000a; Barrington 2000b; Shah 2005; Shah 2008; Shah 2014). We aimed to investigate the effects of early (i.e. within 24 hours of life) administration of heparin for any reason, including promotion of catheter patency. In both included trials heparin was used in infants with umbilical catheter. We will update with new studies if any are published on this topic.
Authors' conclusions
Implications for practice.
There is very limited data on the effect of prophylactic administration of heparin on the incidence and severity of IVH in very preterm neonates. Both the identified trials used heparin in the context of maintaining umbilical line patency and not specifically as an agent to prevent germinal matrix‐intraventricular haemorrhage. Given the imprecision of our estimates, the results of this systematic review are consistent with either a benefit or a detrimental effect of heparin and do not provide a definitive answer to the review question. Limited evidence is available on other clinically relevant outcomes.
Implications for research.
Heparin may reduce the risk of developing parenchymal venous infarct, a thrombotic occlusive condition known to complicate grade 4 IVH. Future trials might therefore evaluate both occurrence and progression of existing GM‐IVH. Moreover, efficacy and safety of different doses, formulations, and routes of administration of heparin might deserve to be investigated.
Acknowledgements
We thank Roger Soll for his valuable advice; and Yolanda Brosseau and Colleen Ovelman for their kind and efficient support.
Appendices
Appendix 1. Search strategy
The Cochrane Library: Search Terms: heparin AND (infant or newborn or neonate or neonatal or premature or very low birth weight or low birth weight or VLBW or LBW)
MEDLINE: (heparin[MeSH] OR heparin) AND ((infant, newborn[MeSH] OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW or infan* or neonat*) AND (randomised controlled trial [pt] OR controlled clinical trial [pt] OR Clinical Trial[ptyp] OR randomised [tiab] OR placebo [tiab] OR clinical trials as topic [mesh: noexp] OR randomly [tiab] OR trial [ti]) NOT (animals [mh] NOT humans [mh]))
EMBASE: (heparin and (hemorrhage or haemorrhage or bleeding) and (infant, newborn or newborn or neonate or neonatal or premature or very low birth weight or low birth weight or VLBW or LBW or Newborn or infan* or neonat*) and (human not animal) and (randomised controlled trial or controlled clinical trial or randomised or placebo or clinical trials as topic or randomly or trial or clinical trial)).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword]
CINAHL: heparin AND (infant, newborn OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW or Newborn or infan* or neonat*) AND (randomized controlled trial OR controlled clinical trial OR randomized OR placebo OR clinical trials as topic OR randomly OR trial OR PT clinical trial)
abstractsonline of the Pediatric Academic Societies (PAS) from 2000‐2015: heparin AND infant
clinicaltrials.govcontrolled‐trials.com and Australian New Zealand Clinical Trials Registry: heparin AND infant
Data and analyses
Comparison 1. Heparin vs placebo/no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Any Intraventricular Haemorrhage (IVH) | 2 | 155 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.03 [‐0.17, 0.12] |
| 2 Severe IVH | 2 | 155 | Risk Difference (M‐H, Fixed, 95% CI) | 0.00 [‐0.11, 0.11] |
| 3 Neonatal all‐cause mortality | 2 | 155 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.04 [‐0.14, 0.06] |
| 4 Bronchopulmonary dysplasia | 1 | 113 | Risk Difference (M‐H, Fixed, 95% CI) | 0.07 [‐0.10, 0.25] |
| 5 Pneumothorax | 2 | 155 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.05 [‐0.14, 0.03] |
| 6 Patent ductus arteriosus | 1 | 113 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.12 [‐0.30, 0.07] |
| 7 Pulmonary haemorrhage | 1 | 113 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.13 [‐0.27, 0.01] |
| 8 Central catheter occlusion | 1 | 42 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.23 [‐0.49, 0.02] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Chang 1997.
| Methods | Double‐blinded randomised controlled trial. Two centres: Prentice Women's Hospital, Chicago, Illinois: Evanston Hospital, Evanston, Illinois, USA, between August 1992 and June 1994. Randomisation in blocks of 10 within each gestational age block (< 28 and ≥ 28 weeks). |
|
| Participants | 118 newborns (57 in the heparin group, 61 in the control group) < 31 weeks' gestation with umbilical catheter (both venous and arterial lines were studied in this trial). Five infants (two in the heparin group, three in the control group) were randomised but excluded from the analysis as they contravened inclusion criteria (two had chromosomal anomalies, one had a congenital diaphragmatic hernia, one had a congenital cyanotic heart lesion; one was assessed to have been born at greater than 31 weeks' gestation). Exclusion criteria: multiple congenital anomalies, chromosomal abnormalities, platelet count < 100 × 109/L at birth, or transferred from an outside hospital. Gestational age and birth weight were similar in the two groups, i.e. 27.4 weeks ± 1.8 and 1074 ± 300 g in the heparin group and 27.1 weeks ± 1.9 and 1065 ± 352 grams in the control group. All infants received vitamin K intramuscularly. Solutions used to flush all indwelling catheters without heparin. |
|
| Interventions | Intervention group: 1 unit of heparin per mL in their umbilical catheter infusate. Control group: same infusate without heparin. All investigators and staff members caring for the infants remained masked, except the pharmacist. The trial was conducted from the first through the fifth day of life. The trial ended before the fifth day of life if all umbilical catheters were removed or if a catheter necessitating heparin‐containing infusate was inserted (e.g. a percutaneous intravenous central catheter). All cranial sonograms were performed at one week of life and interpreted by a masked paediatric neuroradiologist. |
|
| Outcomes | Primary outcome: GM‐IVH. Secondary outcomes: severe IVH; coagulation profile; surfactant use; use of inotropic agents; pneumothorax; pulmonary haemorrhage; patent ductus arteriosus; bronchopulmonary dysplasia; death. |
|
| Notes | Lack of power of the study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The pharmacist was the only person who knew the randomisation assignment. All investigators and staff members caring for the infants remained masked. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All cranial sonograms were interpreted by a masked paediatric neuroradiologist. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Five infants (two in the heparin group, three in the control group) were randomised but excluded from the analysis as they contravened inclusion criteria (two had chromosomal anomalies, one had a congenital diaphragmatic hernia, one had a congenital cyanotic heart lesion; one was assessed to have been born at greater than 31 weeks' gestation). Moreover, five infants died before a sonogram could be obtained and one infant did not have cranial ultra‐sonography performed (two of these infants were in the heparin group, four in the 'no heparin' group). Thus, data on incidence and severity of IVH were available on 107 infants (53 infants in the heparin group, 54 infants in the control group). Overall, 11 out of 118 randomised infants (9.3%) did not complete the study. |
| Selective reporting (reporting bias) | Unclear risk | The trial was not registered and no protocol was available. We could not ascertain if there were deviations from the original protocol in the final publication. |
| Other bias | Low risk | Appears free of other bias. |
Stec 1993.
| Methods | Double‐blinded randomised controlled trial. Single‐centre: St. Peter's Medical Center Intensive Care Nursery, New Brunswick, New Jersey, USA, between 1 June 1 1987 and 30 August 30 1989. |
|
| Participants | 42 inborn newborns (19 in the heparin group, 23 in the control group) with birth weight < 2000 grams, requirement for umbilical artery catheterisation and informed consent obtained from the parents. Gestational age and birth weight were similar in the two groups, i.e. 28 weeks ± 3 and 1096 ± 350 grams in the heparin group and 28 weeks ± 3 and 1000 ± 415 grams in the control group. |
|
| Interventions | Intervention group: flush solution (5% dextrose/0.2 normal saline) with 1 unit of heparin per mL. Control group: same flush solution without heparin. |
|
| Outcomes | Primary outcome: GM‐IVH. Secondary outcomes: severe IVH; complications of the umbilical artery catheters (hypertension, color changes of the extremities, and clotting of the catheter); need for respiratory support; pneumothorax; acidosis; sepsis; need for volume expanders; platelet count; death. |
|
| Notes | Lack of power of the study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Infants were randomised by a random number table. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The flush solutions were prepared by the hospital pharmacy and the vials' contents were unknown to the clinical staff caring for the infants. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The ultrasound studies were reviewed by a paediatric radiologist or a neuroradiologist who were unaware of the infants' clinical course. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes reported for all randomised infants (no drop‐outs). |
| Selective reporting (reporting bias) | Unclear risk | The trial was not registered and no protocol was available. We could not ascertain if there were deviations from the original protocol in the final publication. |
| Other bias | Low risk | Appears free of other bias. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Birch 2010 | Timing of intervention (at a mean age > 2 days of life) beyond the review's inclusion criteria (i.e. heparin started within 24 hours of life). |
| Brown 1999 | Gestational age (mean 36 weeks and 5 days) greater than that specified in the review's inclusion criteria (i.e. < 32 weeks). |
| Kamala 2002 | Timing of intervention (at a mean age > 3 days of life) beyond the review's inclusion criteria (i.e. heparin started within 24 hours of life). |
| Treas 1992 | Timing of intervention beyond the review's inclusion criteria; Gestational age range: 28 to 43 weeks. |
Differences between protocol and review
To better clarify that this review includes trials irrespective of the reason for the use of heparin, we have specified that we included trials specifically aimed to prevent GM‐IVH as well as those attempting to prevent thrombosis (in catheters and other locations) (see Types of interventions).
When we prepared the protocol of this review, we did not plan to summarise the review results in a 'Summary of findings' table, which was included at the review stage to be consistent with Cochrane's and the Cochrane Neonatal Review Group's guidelines (see Table 1).
Contributions of authors
Study concept: MB, OR, LAR Selection of studies: SZ, RB, MGC Acquisition of data: SZ, MGC 'Risk of bias' assessment: SZ, RB Analysis of data: SZ, MGC, MB, OR. Drafting of the manuscript: MB, OR Interpretation and critical revision of the manuscript for important intellectual content: MB, OR, SZ, RB, LAR, MGC.
Sources of support
Internal sources
-
Institute for Clinical Sciences, Lund University, Lund, Sweden.
MB and OR are employed by this organization
-
Istituto Giannina Gaslini, Genoa, Italy.
LAR and MGC are employed by this organization
External sources
-
Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services, USA.
Editorial support of the Cochrane Neonatal Review Group has been funded with Federal funds from this organization under Contract No. HHSN275201100016C
Declarations of interest
MB, OR, SZ, RB, LAR and MGC declare to have no known conflicts of interest.
New
References
References to studies included in this review
Chang 1997 {published data only}
- Chang GY, Lueder FL, DiMichele DM, Radkowski MA, McWilliams LJ, Jansen RD. Heparin and the risk of intraventricular hemorrhage in premature infants. Journal of Pediatrics 1997;131(3):362‐6. [PUBMED: 9329410] [DOI] [PubMed] [Google Scholar]
Stec 1993 {published data only}
- Stec T, Metcalf M, Anwar M, Hiatt M, Hegyi T. The role of heparin in the etiology of intraventricular hemorrhage: a controlled trial. The Journal of Maternal‐Fetal & Neonatal Medicine 1993;2(6):283‐7. [Google Scholar]
References to studies excluded from this review
Birch 2010 {published data only}
- Birch P, Ogden S, Hewson M. A randomised, controlled trial of heparin in total parenteral nutrition to prevent sepsis associated with neonatal long lines: The Heparin in Long Line Total Parenteral Nutrition (HILLTOP) trial. Archives of Disease in Childhood. Fetal and Neonatal Edition 2010;95(4):F252‐7. [PUBMED: 20530110] [DOI] [PubMed] [Google Scholar]
Brown 1999 {published data only}
- Brown K, Tay‐Uyboco JS, McMillan DD. Heparin is not required for peripheral intravenous locks in neonates. Paediatrics & Child Health 1999;4(1):39‐42. [PUBMED: 20212988] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kamala 2002 {published data only}
- Kamala F, Boo NY, Cheah FC, Birinder K. Randomized controlled trial of heparin for prevention of blockage of peripherally inserted central catheters in neonates. Acta Paediatrica 2002;91(12):1350‐6. [PUBMED: 12578294] [DOI] [PubMed] [Google Scholar]
Treas 1992 {published data only}
- Treas LS, Latinis‐Bridges B. Efficacy of heparin in peripheral venous infusion in neonates. Journal of Obstetric, Gynecologic, and Neonatal Nursing 1992;21(3):214‐9. [PUBMED: 1640278] [DOI] [PubMed] [Google Scholar]
Additional references
Barrington 2000a
- Barrington KJ. Umbilical artery catheters in the newborn: effects of heparin. Cochrane Database of Systematic Reviews 2000, Issue 2. [DOI: 10.1002/14651858.CD000507] [DOI] [PMC free article] [PubMed] [Google Scholar]
Barrington 2000b
- Barrington KJ. Umbilical artery catheters in the newborn: effects of catheter materials. Cochrane Database of Systematic Reviews 2000, Issue 2. [DOI: 10.1002/14651858.CD000949] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bayley 1993
- Bayley N. Bayley Scales of Infant Development. 2nd Edition. San Antonio, TX: The Psychological Corporation, 1993. [Google Scholar]
Bayley 2006
- Bayley N. Bayley Scales of Infant and Toddler Development. San Antonio, Texas: Harcourt Assessment, 2006. [Google Scholar]
Björk 1982
- Björk I, Lindahl U. Mechanism of the anticoagulant action of heparin. Molecular and Cellular Biochemistry 1982;48(3):161‐82. [PUBMED: 6757715] [DOI] [PubMed] [Google Scholar]
Bruschettini 2015
- Bruschettini M, Romantsik O, Zappettini S, Banzi R, Ramenghi LA, Calevo MG. Antithrombin for the prevention of intraventricular hemorrhage in very preterm infants. Cochrane Database of Systematic Reviews 2015, Issue 4. [DOI: 10.1002/14651858.CD011636] [DOI] [PubMed] [Google Scholar]
Carlson 2001
- Carlson MK, Gleason PP, Sen S. Elevation of hepatic transaminases after enoxaparin use: case report and review of unfractionated and low‐molecular‐weight heparin‐induced hepatotoxicity. Pharmacotherapy 2001;21(1):108‐13. [PUBMED: 11191729] [DOI] [PubMed] [Google Scholar]
Cho 2013
- Cho R, Leclaire M, Kempainen R. Heparin‐induced hyperkalemia in a patient diagnosed with thyroid storm. Annals of Pharmacotherapy 2013;47(9):1213‐7. [PUBMED: 24259739] [DOI] [PubMed] [Google Scholar]
Chuang 2001
- Chuang YJ, Swanson R, Raja SM, Olson ST. Heparin enhances the specificity of antithrombin for thrombin and factor Xa independent of the reactive center loop sequence. Evidence for an exosite determinant of factor Xa specificity in heparin‐activated antithrombin. Journal of Biological Chemistry 2001;276(18):14961–71. [PUBMED: 11278930] [DOI] [PubMed] [Google Scholar]
Cornette 2002
- Cornette LG, Tanner SF, Ramenghi LA, Miall LS, Childs AM, Arthur RJ, et al. Magnetic resonance imaging of the infant brain: anatomical characteristics and clinical significance of punctate lesions. Archives of Disease in Childhood. Fetal and Neonatal Edition 2002;86(3):F171‐7. [PUBMED: 11978747] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dolfin 1983
- Dolfin T, Skidmore MB, Fong KW, Hoskins EM, Shennan AT. Incidence, severity, and timing of subependymal and intraventricular hemorrhages in preterm infants born in a perinatal unit as detected by serial real‐time ultrasound. Pediatrics 1983;71(4):541‐6. [PUBMED: 6835737] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ (Clinical Research Ed.) 1997;315(7109):629‐34. [PUBMED: 9310563] [DOI] [PMC free article] [PubMed] [Google Scholar]
EPOC 2015
- Effective Practice, Organisation of Care (EPOC). Cochrane EPOC Review Group data collection checklist. EPOC resources for review authors. Oslo: Norwegian Knowledge Centre for the Health Services; 2015. Available at: http://epoc.cochrane.org/sites/epoc.cochrane.org/files/uploads/datacollectionchecklist.pdf.
Fumagalli 2009
- Fumagalli M, Ramenghi LA, Righini A, Groppo M, Bassi L, Carli A, et al. Cerebellar haemorrhages and pons development in extremely low birth weight infants. Frontiers in Bioscience 2009;1:537‐41. [PUBMED: 19482668] [DOI] [PubMed] [Google Scholar]
Garcia 2012
- Garcia DA, Baglin TP, Weitz JI, Samama MM, American College of Chest Physicians. Parenteral anticoagulants: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians evidence‐based clinical practice guidelines. Chest 2012;141(2 Suppl):e24S‐43S. [PUBMED: 22315264] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ghazi‐Birry 1997
- Ghazi‐Birry HS, Brown WR, Moody DM, Challa VR, Block SM, Reboussin DM. Human germinal matrix: venous origin of hemorrhage and vascular characteristics. American Journal of Neuroradiology 1997;18(2):219‐29. [PUBMED: 9111655] [PMC free article] [PubMed] [Google Scholar]
Griffiths 1954
- Griffiths R. The abilities of babies: a study in mental measurement. New York, NY: McGraw‐Hill Book Co. Inc, 1954. [Google Scholar]
Guyatt 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction‐GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [PUBMED: 21195583] [DOI] [PubMed] [Google Scholar]
Guyton 2006
- Guyton AC, Hall JE. Textbook of Medical Physiology. Vol. 11, Philadelphia: Elsevier Saunders, 2006. [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [PUBMED: 12958120] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Howell 1918
- Howell WH, Holt E. Two new factors in blood coagulation ‐ heparin and pro‐antithrombin. American Journal of Physiology 1918;47:328‐41. [Google Scholar]
ICROP 1984
- ICROP. An International Classification of Retinopathy of Prematurity. Pediatrics 1984;74(1):127‐33. [PUBMED: 6547526] [PubMed] [Google Scholar]
Jacobs 2013
- Jacobs SE, Berg M, Hunt R, Tarnow‐Mordi WO, Inder TE, Davis PG. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database of Systematic Reviews 2013, Issue 1. [DOI: 10.1002/14651858.CD003311.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jobe 2001
- Jobe AH, Bancalari E. Bronchopulmonary Dysplasia. American Journal of Respiratory and Critical Care Medicine 2001;163(7):1723‐9. [PUBMED: 11401896] [DOI] [PubMed] [Google Scholar]
Kenet 2010
- Kenet G, Lütkhoff LK, Albisetti M, Bernard T, Bonduel M, Brandao L, et al. Impact of thrombophilia on risk of arterial ischemic stroke or cerebral sinovenous thrombosis in neonates and children: a systematic review and meta‐analysis of observational studies. Circulation 2010;121(16):1838‐47. [DOI] [PubMed] [Google Scholar]
Kersbergen 2011
- Kersbergen KJ, Groenendaal F, Benders MJ, Straaten HL, Niwa T, Nievelstein RA, et al. The spectrum of associated brain lesions in cerebral sinovenous thrombosis: relation to gestational age and outcome. Archives of Disease in Childhood. Fetal and Neonatal Edition 2011;96(6):F404‐9. [PUBMED: 21317440] [DOI] [PubMed] [Google Scholar]
Lesko 1986
- Lesko SM, Mitchell AA, Epstein MF, Louik C, Giacoia GP, Shapiro S. Heparin use as a risk factor for intraventricular hemorrhage in low‐birth‐weight infants. New England Journal of Medicine 1986;314(18):1156‐60. [PUBMED: 3960090] [DOI] [PubMed] [Google Scholar]
Limperopoulos 2007
- Limperopoulos C, Bassan H, Gauvreau K, Robertson RL Jr, Sullivan NR, Benson CB, et al. Does cerebellar injury in premature infants contribute to the high prevalence of long‐term cognitive, learning, and behavioral disability in survivors?. Pediatrics 2007;120(3):584‐93. [PUBMED: 17766532] [DOI] [PubMed] [Google Scholar]
Malloy 1995
- Malloy MH, Cutter GR. The association of heparin exposure with intraventricular hemorrhage among very low birth weight infants. Journal of Perinatology 1995;15(3):185‐91. [PUBMED: 7666265] [PubMed] [Google Scholar]
Manco‐Johnson 1991
- Manco‐Johnson MJ, Abshire TC, Jacobson LJ, Marlar RA. Severe neonatal protein C deficiency: prevalence and thrombotic risk. Journal of Pediatrics 1991;119(5):793‐8. [PUBMED: 1834822] [DOI] [PubMed] [Google Scholar]
McDonald 1981
- McDonald MM, Jacobson LJ, Hay WW Jr, Hathaway WE. Heparin clearance in the newborn. Pediatric Research 1981;15(7):1015‐18. [PUBMED: 7254945] [DOI] [PubMed] [Google Scholar]
McDonald 1984
- McDonald MM, Johnson ML, Rumack CM, Koops BL, Guggenheim MA, Babb C, et al. Role of coagulopathy in newborn intracranial hemorrhage. Pediatrics 1984;74(1):26‐31. [PUBMED: 6739216] [PubMed] [Google Scholar]
Monagle 2012a
- Monagle P, Chan AK, Goldenberg NA, Ichord RN, Journeycake JM, Nowak‐Göttl U, et al. Antithrombotic therapy in neonates and children: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence‐based clinical practice guidelines. Chest 2012;141(2 Suppl):e737S‐801S. [PUBMED: 22315277] [DOI] [PMC free article] [PubMed] [Google Scholar]
Monagle 2012b
- Monagle P, Studdert DM, Newall F. Infant deaths due to heparin overdose: time for a concerted action on prevention. Journal of Paediatrics and Child Health 2012;48(5):380‐1. [PUBMED: 21679338] [DOI] [PubMed] [Google Scholar]
Nandigam 2009
- Nandigam RN, Viswanathan A, Delgado P, Skehan ME, Smith EE, Rosand J. MR imaging detection of cerebral microbleeds: effect of susceptibility‐weighted imaging, section thickness, and field strength. American Journal of Neuroradiology 2009;30(2):338‐43. [PUBMED: 19001544] [DOI] [PMC free article] [PubMed] [Google Scholar]
Newall 2009
- Newall F, Johnston L, Ignjatovic V, Monagle P. Unfractionated heparin therapy in infants and children. Pediatrics 2009;123(3):e510‐8. [PUBMED: 19221154] [DOI] [PubMed] [Google Scholar]
NIH 1979
- National Institutes of Health. Report of Workshop on Bronchopulmonary Dysplasia. NIH Publication No. 80‐1660. Washington, DC: National Institutes of Health. 1979.
O'Neill 1974
- O'Neill TJ, Tierney LM Jr, Proulx RJ. Heparin lock‐induced alterations in the activated partial thromboplastin time. The Journal of the American Medical Association 1974;227(11):1297‐8. [PUBMED: 4405979] [PubMed] [Google Scholar]
Paneth 1994
- Paneth N, Rudelli R, Kazam E, Monte W. The pathology of germinal matrix intraventricular hemorrhage. Brain damage in the preterm infant. Clinics in developmental medicine. 1st Edition. Vol. 131, London, UK: Mac Keith Press, 1994:23–53. [Google Scholar]
Papile 1978
- Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. Journal of Pediatrics 1978;92(4):529‐34. [PUBMED: 305471] [DOI] [PubMed] [Google Scholar]
Parodi 2013
Perlman 1983
- Perlman JM, McMenamin JB, Volpe JJ. Fluctuating cerebral blood‐flow velocity in respiratory‐distress syndrome. Relation to the development of intraventricular hemorrhage. New England Journal of Medicine 1983;309(4):204‐9. [PUBMED: 6866033] [DOI] [PubMed] [Google Scholar]
Petitou 1999
- Petitou M, Herault JP, Bernat A, Driguez PA, Duchaussoy P, Lormeau JC, et al. Synthesis of thrombin inhibiting heparin mimetics without side effects. Nature 1999;398(6726):417‐22. [PUBMED: 10201371] [DOI] [PubMed] [Google Scholar]
Petäjä 2001
- Petäjä J, Hiltunen L, Fellman V. Increased risk of intraventricular hemorrhage in preterm infants with thrombophilia. Pediatric Research 2001;49(5):643‐6. [PUBMED: 11328946] [DOI] [PubMed] [Google Scholar]
Potter 1992
- Potter C, Gill JC, Scott JP, McFarland JG. Heparin‐induced thrombocytopenia in a child. Journal of Pediatrics 1992;121(1):135‐8. [PUBMED: 1625071] [DOI] [PubMed] [Google Scholar]
Ramenghi 2002
- Ramenghi LA, Gill BJ, Tanner SF, Martinez D, Arthur R, Levene MI. Cerebral venous thrombosis, intraventricular haemorrhage and white matter lesions in a preterm newborn with factor V (Leiden) mutation. Neuropediatrics 2002;33(2):97‐9. [PUBMED: 12075492] [DOI] [PubMed] [Google Scholar]
Ramenghi 2005
- Ramenghi LA, Mosca F, Counsell S, Rutherford MA. Magnetic resonance imaging of the brain in preterm infants. In: Tortori‐Donati P, Rossi A editor(s). Pediatric Neuroradiology. 1st Edition. Heidelberg, Germany: Springer, 2005:199‐234. [Google Scholar]
Ramenghi 2011
- Ramenghi LA, Fumagalli M, Groppo M, Consonni D, Gatti L, Bertazzi PA, et al. Germinal matrix hemorrhage: intraventricular hemorrhage in very‐low‐birth‐weight infants: the independent role of inherited thrombophilia. Stroke 2011;42(7):1889‐93. [PUBMED: 21597013] [DOI] [PubMed] [Google Scholar]
Ramenghi 2015
- Ramenghi LA. Germinal matrix ‐ intraventricular haemorrhage: still a very important brain lesion in premature infants!. The Journal of Maternal‐Fetal & Neonatal Medicine 2015;28(Suppl 1):2259‐60. [PUBMED: 26365359] [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rutherford 2010
- Rutherford MA, Supramaniam V, Ederies A, Chew A, Bassi L, Groppo M, et al. Magnetic resonance imaging of white matter diseases of prematurity. Neuroradiology 2010;52(6):505‐21. [PUBMED: 20422407] [DOI] [PubMed] [Google Scholar]
Shah 2005
- Shah PS, Ng E, Sinha AK. Heparin for prolonging peripheral intravenous catheter use in neonates. Cochrane Database of Systematic Reviews 2005, Issue 4. [DOI: 10.1002/14651858.CD002774.pub2] [DOI] [PubMed] [Google Scholar]
Shah 2008
- Shah PS, Shah VS. Continuous heparin infusion to prevent thrombosis and catheter occlusion in neonates with peripherally placed percutaneous central venous catheters. Cochrane Database of Systematic Reviews 2008, Issue 2. [DOI: 10.1002/14651858.CD002772.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shah 2014
- Shah PS, Shah N. Heparin‐bonded catheters for prolonging the patency of central venous catheters in children. Cochrane Database of Systematic Reviews 2014, Issue 2. [DOI: 10.1002/14651858.CD005983.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Spadone 1992
- Spadone D, Clark F, James E, Laster J, Hoch J, Silver D. Heparin‐induced thrombocytopenia in the newborn. Journal of Vascular Surgery 1992;15(2):306‐11. [PUBMED: 1735891] [DOI] [PubMed] [Google Scholar]
Streif 2003
- Streif W, Goebel G, Chan AK, Massicotte MP. Use of low molecular mass heparin (enoxaparin) in newborn infants: a prospective cohort study of 62 patients. Archives of Disease in Childhood ‐ Fetal and Neonatal Edition 2003;88(5):F365‐70. [PUBMED: 12937038] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tripodi 2008
- Tripodi A, Ramenghi LA, Chantarangkul V, Carli A, Clerici M, Groppo M, et al. Normal thrombin generation in neonates in spite of prolonged conventional coagulation tests. Haematologica 2008;93(8):1256‐9. [PUBMED: 18403390] [DOI] [PubMed] [Google Scholar]
Vermont Oxford Network 2013
- Database of Very Low Birth Weight Infants Born in 2012. Burlington, VT: Vermont Oxford Network, 2013. Nightingale Internet Reporting System, accessed April 4, 2014.
Volpe 2008
- Volpe JJ. Neurology of the Newborn. 5th Edition. Philadelphia, PA: Saunders, 2008. [Google Scholar]
Walsh 2004
- Walsh MC, Yao Q, Gettner P, Hale E, Collins M, Hensman A, et al. Impact of a physiologic definition on bronchopulmonary dysplasia rates. Pediatrics 2004;114(5):1305‐11. [PUBMED: 15520112] [DOI] [PubMed] [Google Scholar]
Weitz 2010
- Weitz DS, Weitz JI. Update on heparin: what do we need to know?. Journal of Thrombosis and Thrombolysis 2010;29(2):199–207. [PUBMED: 19882363] [DOI] [PubMed] [Google Scholar]
Wiernikowski 2007
- Wiernikowski JT, Chan A, Lo G. Reversal of anti‐thrombin activity using protamine sulfate. Experience in a neonate with a 10‐fold overdose of enoxaparin. Thrombosis Research 2007;120(2):303‐5. [PUBMED: 17079003] [DOI] [PubMed] [Google Scholar]


