Abstract
Background
Steroid‐sparing strategies have been attempted in recent decades to avoid morbidity from long‐term steroid intake among kidney transplant recipients. Previous systematic reviews of steroid withdrawal after kidney transplantation have shown a significant increase in acute rejection. There are various protocols to withdraw steroids after kidney transplantation and their possible benefits or harms are subject to systematic review. This is an update of a review first published in 2009.
Objectives
To evaluate the benefits and harms of steroid withdrawal or avoidance for kidney transplant recipients.
Search methods
We searched the Cochrane Kidney and Transplant Specialised Register to 15 February 2016 through contact with the Information Specialist using search terms relevant to this review.
Selection criteria
All randomised and quasi‐randomised controlled trials (RCTs) in which steroids were avoided or withdrawn at any time point after kidney transplantation were included.
Data collection and analysis
Assessment of risk of bias and data extraction was performed by two authors independently and disagreement resolved by discussion. Statistical analyses were performed using the random‐effects model and dichotomous outcomes were reported as relative risk (RR) and continuous outcomes as mean difference (MD) with 95% confidence intervals.
Main results
We included 48 studies (224 reports) that involved 7803 randomised participants. Of these, three studies were conducted in children (346 participants). The 2009 review included 30 studies (94 reports, 5949 participants). Risk of bias was assessed as low for sequence generation in 19 studies and allocation concealment in 14 studies. Incomplete outcome data were adequately addressed in 22 studies and 37 were free of selective reporting.
The 48 included studies evaluated three different comparisons: steroid avoidance or withdrawal compared with steroid maintenance, and steroid avoidance compared with steroid withdrawal. For the adult studies there was no significant difference in patient mortality either in studies comparing steroid withdrawal versus steroid maintenance (10 studies, 1913 participants, death at one year post transplantation: RR 0.68, 95% CI 0.36 to 1.30) or in studies comparing steroid avoidance versus steroid maintenance (10 studies, 1462 participants, death at one year after transplantation: RR 0.96, 95% CI 0.52 to 1.80). Similarly no significant difference in graft loss was found comparing steroid withdrawal versus steroid maintenance (8 studies, 1817 participants, graft loss excluding death with functioning graft at one year after transplantation: RR 1.17, 95% CI 0.72 to 1.92) and comparing steroid avoidance versus steroid maintenance (7 studies, 1211 participants, graft loss excluding death with functioning graft at one year after transplantation: RR 1.09, 95% CI 0.64 to 1.86). The risk of acute rejection significantly increased in patients treated with steroids for less than 14 days after transplantation (7 studies, 835 participants: RR 1.58, 95% CI 1.08 to 2.30) and in patients who were withdrawn from steroids at a later time point after transplantation (10 studies, 1913 participants, RR 1.77, 95% CI 1.20 to 2.61). There was no evidence to suggest a difference in harmful events, such as infection and malignancy, in adult kidney transplant recipients. The effect of steroid withdrawal in children is unclear.
Authors' conclusions
This updated review increases the evidence that steroid avoidance and withdrawal after kidney transplantation significantly increase the risk of acute rejection. There was no evidence to suggest a difference in patient mortality or graft loss up to five year after transplantation, but long‐term consequences of steroid avoidance and withdrawal remain unclear until today, because prospective long‐term studies have not been conducted.
Keywords: Adult, Child, Humans, Kidney Transplantation, Kidney Transplantation/mortality, Graft Rejection, Graft Rejection/immunology, Graft Rejection/prevention & control, Graft Survival, Graft Survival/drug effects, Graft Survival/immunology, Immunosuppression Therapy, Immunosuppressive Agents, Immunosuppressive Agents/administration & dosage, Immunosuppressive Agents/adverse effects, Randomized Controlled Trials as Topic, Steroids, Steroids/administration & dosage, Steroids/adverse effects, Withholding Treatment
Plain language summary
Steroid avoidance or withdrawal for kidney transplant recipients
What is the issue?
Each year more than 28,000 kidney transplants are performed globally. Kidney transplantation is the treatment of choice for eligible people who have lost kidney function. Most kidney transplant recipients receive corticosteroids as part of their immunosuppression treatment. Steroids are effective in preventing acute rejection, which is a major problem in the early period after kidney transplantation. However, steroids can also lead to serious side effects when taken long‐term. This review looked at two strategies to reduce steroid administration after kidney transplantation: either discontinuing steroids soon after transplantation (within 14 days) or stopping steroid treatment later.
What did we do?
We searched the literature up to February 2016 and identified 48 studies (7803 patients) that were evaluated in this review. Only three studies included children. This is an update of a review that was last published in 2009.
What did we find?
Our review looked at data relating to 7803 kidney transplant recipients. We assessed the risk of bias in all studies and found that most were unblinded, about half did not report funding sources or how they randomised and allocated study participants.
We found that the risk of acute rejection significantly increased with both steroid‐reducing treatments among adults who received kidney transplants. There was no little or no difference in the numbers of deaths or loss of transplanted kidneys for both steroid‐reducing strategies within five years after kidney transplantation. Side effects, such as infection, cancer or diabetes after transplantation did not differ between groups of patients whose steroids were discontinued compared with those who continued to take steroids. The effect of steroid withdrawal in children is unclear.
Conclusions
There was no evidence to suggest a difference in patient mortality or graft loss up to five year after transplantation, but longer‐term consequences of steroid avoidance and withdrawal still remain unclear.
Summary of findings
Summary of findings for the main comparison. Steroid withdrawal versus steroid maintenance for kidney transplant recipients.
| Steroid withdrawal versus steroid maintenance for kidney transplant recipients | |||||
| Patient or population: kidney transplant recipients Intervention: steroid withdrawal Comparison: steroid maintenance | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Steroid maintenance | Steroid withdrawal | ||||
| Mortality Follow‐up: 1 year | 22 per 1000 | 15 per 1000 (8 to 29) | RR 0.68 (0.36 to 1.3) | 1913 (10) | ⊕⊕⊝⊝ low1,2 |
| Graft loss (excluding death) Follow‐up: 1 year | 32 per 1000 | 38 per 1000 (23 to 62) | RR 1.17 (0.72 to 1.92) | 1817 (8) | ⊕⊕⊝⊝ low2,3 |
| Acute rejection Follow‐up: 1 year | 152 per 1000 | 268 per 1000 (182 to 396) | RR 1.77 (1.2 to 2.61) | 1913 (10) | ⊕⊕⊕⊝ moderate1 |
| NODAT Follow‐up: 5 years | 57 per 1000 | 44 per 1000 (28 to 69) | RR 0.77 (0.49 to 1.21) | 1439 (6) | ⊕⊕⊝⊝ low2,4 |
| CMV infection Follow‐up: 5 years | 100 per 1000 | 104 per 1000 (80 to 137) | RR 1.04 (0.8 to 1.36) | 1758 (5) | ⊕⊕⊝⊝ low2,5 |
| *The assumed risk is the baseline risk in the control group treated with steroid maintenance. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; NODAT: new‐onset diabetes after transplantation; CMV ‐ cytomegalovirus | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1 Most studies were unblinded (9 studies) and did not report details about random sequence generation or allocation concealment or both (8 studies). One study had inappropriate random sequence generation. Four studies were industry sponsored. ITT analysis was unclear in four. 2 Total number of events were fewer than 300. 3 Most studies were unblinded (7 studies) and did not report details about random sequence generation or allocation concealment or both (6 studies). One study had inappropriate random sequence generation. Four studies were industry sponsored. ITT analysis was unclear in two. 4 Most studies were unblinded (5 studies) and did not report details about random sequence generation or allocation concealment or both (5 studies). Three studies were industry sponsored. ITT analysis was unclear in three studies. One study had selective outcome reporting. 5 Most studies were unblinded (4 studies) and did not report details about random sequence generation or allocation concealment or both (4 studies). Three studies were industry sponsored. ITT analysis was unclear in two studies. One study had selective outcome reporting.
Summary of findings 2. Steroid avoidance versus steroid maintenance for kidney transplant recipients.
| Steroid avoidance versus steroid maintenance for kidney transplant recipients | ||||||
| Patient or population: kidney transplant recipients Intervention: steroid avoidance Comparison: steroid maintenance | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Steroid avoidance versus steroid maintenance | |||||
| Mortality Follow‐up: 1 year | 31 per 1000 | 30 per 1000 (16 to 56) | RR 0.96 (0.52 to 1.8) | 1462 (10) | ⊕⊕⊝⊝ low1,2 | |
| Graft loss (excluding death) Follow‐up: 1 year | 42 per 1000 | 46 per 1000 (27 to 79) | RR 1.09 (0.64 to 1.86) | 1211 (7) | ⊕⊕⊝⊝ low2,3 | |
| Acute rejection Follow‐up: 1 year | 204 per 1000 | 323 per 1000 (221 to 470) | RR 1.58 (1.08 to 2.3) | 835 (7) | ⊕⊕⊕⊝ moderate4 | |
| NODAT Follow‐up: 5 years | 107 per 1000 | 80 per 1000 (54 to 117) | RR 0.75 (0.51 to 1.1) | 1618 (9) | ⊕⊕⊝⊝ low2,5 | |
| CMV Infection Follow‐up: 5 years | 106 per 1000 | 101 per 1000 (74 to 138) | RR 0.96 (0.7 to 1.31) | 1454 (6) | ⊕⊕⊝⊝ low2,6 | |
| *The assumed risk is the baseline risk in the control group treated with steroid maintenance. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; NODAT: new‐onset diabetes after transplantation; CMV ‐ cytomegalovirus | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate | ||||||
1 All studies were unblinded. Six studies were industry sponsored. In six studies random sequence generation or allocation concealment or both was unclear. In two studies ITT was either not performed or unclear. One study had selective outcome reporting 2 Total number of events was fewer than 300 3 All studies were unblinded. Five studies were industry sponsored. In four studies random sequence generation or allocation concealment or both was unclear. ITT was unclear in one study. One study had selective outcome reporting 4 All studies were unblinded. Five studies were industry sponsored. In four studies random sequence generation or allocation concealment or both was unclear. In three studies ITT was either not performed or unclear. One study had selective outcome reporting 5 Most studies were unblinded (8 studies). Five studies were industry sponsored. In four studies random sequence generation or allocation concealment or both was unclear. One study had selective outcome reporting 6 Most studies were unblinded (5 studies). Four studies were industry sponsored. One study had unclear ITT
Background
Description of the condition
Patients with end‐stage kidney disease (ESKD) have to undergo renal replacement therapy which is available either as dialysis or kidney transplantation. Kidney transplantation is the preferred treatment for eligible patients with ESKD, because it offers a nearly normal life and is associated with better survival and quality of life compared to dialysis treatment. More than 16,000 kidney transplants are currently performed annually in the USA (OPTN/SRTR 2014) and more than 12,000 in Europe (ERA‐EDTA 2013). Despite kidney transplants from live donors, organ demand exceeds organ availability worldwide and the number of patients wait listed for kidney transplantation continues to rise (ANZDATA 2012; ERA‐EDTA 2013; OPTN/SRTR 2014).
Although short‐term outcomes of kidney transplantation have continuously improved since the 1980s, long‐term results have only marginally improved until today. Death with a functioning graft and chronic allograft nephropathy are the most important causes of graft loss (Pascual 2002). Thus, strategies that prolong patient survival and graft patency have become a priority in kidney transplantation.
One of the key factors that influence transplant outcomes is immunosuppression which prohibits progressive immune mediated injury of the allograft. Standard immunosuppressive protocols nowadays consist of an initial induction treatment followed by a maintenance regimen. Immunosuppression is induced by an intensive treatment for the initial days after transplantation either with higher dosages of the immunosuppressive drugs or by adding an additional immunosuppressive agent, such as anti‐T‐cell antibodies or interleukin 2 receptor antibodies. Maintenance immunosuppression usually comprises a combination of three drug groups: calcineurin inhibitors, such as cyclosporin (CsA) or tacrolimus (TAC), anti‐proliferative agents, such as azathioprine (AZA) or mycophenolate mofetil (MMF) and corticosteroids, such as prednisolone.
Corticosteroids are long known for their anti‐inflammatory and immunosuppressive properties and have been used to prevent rejection since the early days of kidney transplantation. Although steroids are effective in preventing acute rejection, chronic steroid use may be an important cause of morbidity and mortality (Opelz 2005). Steroids exhibit a wide range of adverse effects, such as skin fragility, bodyweight gain, osteoporosis and cataracts, can adversely affect important cardiovascular and metabolic risk factors including hypertension, hyperglycaemia and dyslipidaemia and may contribute to an increased risk of infection (Coutinho 2011; Czock 2005; Matas 2005; Patel 2001). A literature review on the safety of low dose glucocorticoid treatment in rheumatoid arthritis suggested that the toxicity of steroids is overestimated, because adverse effects of chronic low dose steroid treatment (≤ 10 mg/d prednisolone equivalent) were found to be modest and rarely statistically significantly different from placebo (Da Silva 2006).
Description of the intervention
With the aim to reduce the adverse effects of long‐term corticosteroid therapy, there has been much effort to limit the exposure of kidney transplant recipients to steroids. Lessening exposure to steroids can be achieved by either steroid avoidance or steroid withdrawal. In steroid avoidance, steroids are either avoided completely or withdrawn within the first days after kidney transplantation and steroid withdrawal refers to discontinuation of steroids at a certain time point in the later post‐transplant phase. This review evaluated all steroid avoidance or withdrawal strategies in kidney transplant recipients.
How the intervention might work
Steroids show adverse cardiovascular and metabolic effects and therefore discontinuing steroid treatment may take effect by a decrease in this accelerated cardiovascular risk. However, while steroid avoidance and withdrawal potentially reduces post‐transplant atherosclerosis, ischaemic heart disease, post‐transplant diabetes and death, it may significantly increase the risk of acute rejection. Acute rejection is associated with late graft loss, especially if rejection episodes are severe, followed by impaired kidney function, occur late and affect arteries (Basadonna 1993; Massy 1996). The new immunosuppressants TAC and MMF have led to important declines in the incidence of acute rejection and may provide a more potent substrate to attempt safe steroid‐free immunosuppression or steroid withdrawal.
Why it is important to do this review
It is important to reduce the cardiovascular risk in kidney transplant recipients, who area population at increased cardiovascular risk, but at the same time it is important to avoid rejection and graft loss. Steroids have been associated with increased cardiovascular risk in kidney transplant recipients, but long‐term benefits and harms of steroid discontinuation have not yet been established with controlled long‐term data (Knight 2010). Prednisone was perceived as the least effective and least favoured immunosuppressive drug compared to calcineurin inhibitors, MMF and AZA in a survey among Canadian kidney transplant recipients and the majority of US transplant physicians and surgeons stated that steroid‐free immunosuppression was a goal for future organ transplant recipients (Hricik 2002; Prasad 2003). Steroid use varies largely in clinical practice around the globe. While steroids are discontinued in many centres worldwide, they are at the same time frequently used for long‐term treatment in kidney transplant recipients to protect the allograft. There is no consensus whether discontinuation of steroids is safe, what type of patients benefit from steroid discontinuation and at what time point after transplantation steroids are best stopped. A number of RCTs evaluating steroid avoidance or withdrawal at various time‐points after kidney transplantation with different immunosuppressive regimes have been performed during the last decades and were first systematically reviewed in 2009 (Pascual 2009). Steroid avoidance and steroid withdrawal strategies in kidney transplantation were not associated with increased patient mortality or graft loss, despite an overall higher incidence of acute rejection for steroid withdrawal strategies compared with steroid maintenance. The aim of this review was to update the benefits and harms of steroid withdrawal and avoidance in kidney transplant recipients with new evidence from RCTs.
Objectives
To evaluate the benefits and harms of steroid withdrawal or avoidance for kidney transplant recipients.
Methods
Criteria for considering studies for this review
Types of studies
All RCTs or quasi‐RCTs (in which allocation to treatment was obtained by alternation, use of alternate medical records, date of birth or other predictable methods), whether published or unpublished, in which steroids were avoided or withdrawn at any time point after kidney transplantation were eligible for inclusion. RCTs evaluating any other steroid‐sparing strategy (i.e. dose reduction) or attempting other interventions in addition to steroid withdrawal (i.e. switch from AZA to MMF, induction treatment in addition to steroid withdrawal) were excluded in this review.
Types of participants
Adult and paediatric recipients of a first or subsequent kidney transplant from a cadaveric or living donor. Recipients of multiorgan transplants (kidney‐pancreas, kidney‐liver, kidney‐heart) were excluded.
Types of interventions
Steroid avoidance, defined as steroid use during less than 14 days after kidney transplantation versus steroid maintenance
Steroid withdrawal, defined as steroid use for more than 14 days after transplantation versus steroid maintenance
Steroid avoidance versus steroid withdrawal.
Types of outcome measures
Outcome measures used by transplant registries to report patient and graft survival were selected for this review. Outcome events were assessed within the first year and up to five years after kidney transplantation. A secondary outcome looking at infection has been amended for this update to specify cytomegalovirus (CMV) infection.
Primary outcomes
All‐cause mortality
Graft loss or death with a functioning graft; and graft loss censored for death with a functioning graft (loss of graft function resulting in either return to dialysis or retransplantation)
Acute rejection (clinically suspected and treated) and biopsy‐proven acute rejection.
Secondary outcomes
Cardiovascular events
New‐onset diabetes after transplantation (NODAT)
Malignancy
Infection and CMV infection
Kidney function measures (serum creatinine (mg/dL); creatinine clearance (mL/min)).
Search methods for identification of studies
Electronic searches
We searched the Cochrane Kidney and Transplant Specialised Register up to 15 February 2016 through contact with the Information Specialist using search terms relevant to this review. The Specialised Register contains studies identified from several sources.
Monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL)
Weekly searches of MEDLINE OVID SP
Handsearching of kidney‐related journals and the proceedings of major kidney conferences
Searching of the current year of EMBASE OVID SP
Weekly current awareness alerts for selected kidney journals
Searches of the International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov.
Studies contained in the Specialised Register are identified through search strategies for CENTRAL, MEDLINE, and EMBASE based on the scope of Cochrane Kidney and Transplant. Details of these strategies, as well as a list of handsearched journals, conference proceedings and current awareness alerts, are available in the Specialised Register section of information about Cochrane Kidney and Transplant.
See Appendix 1 for search terms used in strategies for this review.
Searching other resources
Reference lists of review articles, relevant studies and clinical practice guidelines.
Letters seeking information about unpublished or incomplete studies to investigators known to be involved in previous studies.
Data collection and analysis
Selection of studies
The search strategies described was used to obtain title and abstracts of studies relevant to this review. Three authors independently screened titles and abstracts, and discarded reports that were not applicable. Studies and reviews that might include relevant data or information on studies were retained initially and two authors independently assessed retrieved abstracts and, if necessary the full text, of these studies to determine which studies satisfied the inclusion criteria. Disagreement about inclusion was resolved by discussion with a third author.
Data extraction and management
Two authors independently carried out data extraction using standard data extraction forms. Studies reported in non‐English language journals will be translated before assessment. Where more than one report of a study existed, reports were grouped together and the publication with the most complete data was used in the analyses. We examined any prior or subsequent report for supplementary outcomes or data to ensure the inclusion of all relevant information. If data were unclear, ambiguous or missing, authors were contacted for further information and any provided additional data was included in the review. Whenever necessary, disagreements were resolved by discussion.
Assessment of risk of bias in included studies
Two authors independently assessed the following items using the risk of bias assessment tool (Higgins 2011) (see Appendix 2).
Was there adequate sequence generation (selection bias)?
Was allocation adequately concealed (selection bias)?
-
Was knowledge of the allocated interventions adequately prevented during the study?
Participants and personnel (performance bias)
Outcome assessors (detection bias)
Were incomplete outcome data adequately addressed (attrition bias)?
Are reports of the study free of suggestion of selective outcome reporting (reporting bias)?
Was the study apparently free of other problems that could put it at a risk of bias?
Measures of treatment effect
For dichotomous outcomes results were expressed as risk ratio (RR) with 95% confidence intervals (CI). Where continuous scales of measurement were used to assess the effects of treatment, the mean difference (MD) was used, or the standardised mean difference (SMD) if different scales had been used.
Unit of analysis issues
The unit of analysis was the study participant and not the events; that is the number of study participants with an acute rejection rather than the number of episodes of acute rejection.
Dealing with missing data
Any further information required from the original author was requested by written correspondence (e.g. emailing corresponding author) and any relevant information obtained in this manner was to be included in the review. Evaluation of important numerical data such as screened, randomised patients as well as intention‐to‐treat, as‐treated and per‐protocol population will be carefully performed. Attrition rates, for example drop‐outs, losses to follow‐up and withdrawals were investigated. Issues of missing data and imputation methods (for example, last‐observation‐carried‐forward) were critically appraised (Higgins 2011). If standard deviation was not available, it was estimated using standard error (if provided) (Higgins 2011).
Assessment of heterogeneity
Heterogeneity was analysed using a Chi2 (on N‐1 degrees of freedom, with an alpha of 0.05 used for statistical significance) and with the I2 statistic, calculated to measure the proportion of total variation in the estimates of treatment effect that was due to heterogeneity beyond chance (Higgins 2003). I2 values of 25%, 50% and 75% correspond to low, medium and high levels of heterogeneity.
Assessment of reporting biases
We assessed publication bias by constructing funnel plots for primary outcomes if there was sufficient data available to enable this analysis (at least 10 included studies in the meta‐analysis).
Data synthesis
Data were pooled for summary estimates using the random‐effects model but the fixed‐effect model was also to be used to ensure robustness of the model chosen and susceptibility to outliers. Results reported used the random‐effects model because this is more conservative in the presence of known or unknown heterogeneity (Deeks 2001).
Subgroup analysis and investigation of heterogeneity
Subgroup analyses were used to explore possible sources of heterogeneity and potential effect modifiers were defined a priori. The main source of heterogeneity among participants could be related to age, therefore adults and children who were kidney transplant recipients were analysed separately. Heterogeneity in treatments could be related to duration of steroid therapy and concomitant immunosuppressants. Therefore subgroup analysis was undertaken using stratified meta‐analysis for type of calcineurin inhibitor, type of antimetabolite and whether an induction treatment was administered.
Sensitivity analysis
Sensitivity analysis was performed to demonstrate that final results did not vary where low quality studies were included or excluded. Low quality studies were defined based on publication type (conference abstract or peer reviewed journal) and methodological conduct (whether intention‐to‐treat analysis was assessed as adequate or inadequate/unclear).
Results
Description of studies
Results of the search
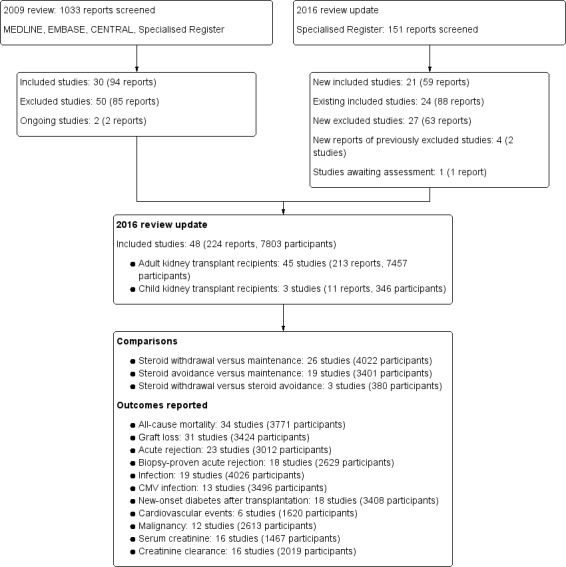
A search in 15 February 2016 identified 151 reports. Additionally three previously excluded studies were re‐evaluated and included; these had been incorrectly excluded for reasons of insufficient data (Aswad 1998; Kacar 2004; Pisani 2001). All three are published as abstract only. Pisani 2001 contributed data for the meta‐analysis. We also re‐evaluated three previously included studies and excluded them because they had been incorrectly included despite a wrong co‐intervention (CARMEN Study 2005; Tarantino 1991; ter Meulen 2002). In CARMEN Study 2005 and ter Meulen 2002 induction treatment with daclizumab was only given to patients in the steroid withdrawal group and in Tarantino 1991 AZA was given solely to patients in the steroid maintenance group. We included 21 new studies (59 reports) that involved 1854 participants, two of these new studies (seven reports) concerned children. We found that 88 new reports were additional reports of previously included studies. This update includes 48 studies (224 reports) that involved 7803 participants, including three studies (11 reports) that involved 346 children. See Figure 1.
1.
Study flow diagram.
Included studies
See Characteristics of included studies.
The 48 included studies were published in 22 different journals and seven had preliminary abstract data only available (Aswad 1998; Burke 2000; del Castillo 2005; INFINITY Study 2013; Kacar 2004; Kim 2002; Pisani 2001).The effect of steroid withdrawal compared versus steroid maintenance was investigated in 26 studies (4022 participants) and the effect of steroid avoidance compared versus steroid maintenance was investigated in 19 studies (3401 participants). We identified three studies (380 participants) that evaluated the effect of steroid avoidance compared versus steroid withdrawal. Numbers of participants per study varied from 21 (Aswad 1998) to 560 patients (THOMAS Study 2002). It is noteworthy that 25 studies randomised fewer than 100 participants, 15 studies included between 100 and 300 participants, and eight studies randomised more than 300 participants.
Trials in adult kidney transplant recipients
This update included 45 studies (208 reports, 7457 participants) of steroid withdrawal or avoidance in adult kidney transplant recipients.
Participants
Trials recruited participants who were older than 18 years of age, except two studies which recruited participants older than 12 years (Stiller 1983) or between five and 62 years (Nagib 2015). In 14 studies the age range was not further specified (Albert 1985; Aswad 1998; Gulanikar 1991; INFINITY Study 2013; Isoniemi 1990; Johnson 1989a; Kacar 2004; Kim 2002; Ratcliffe 1993; Schulak 1989; Smak Gregoor 1999; Sola 2002; THOMAS Study 2002; Zhu 2008a).The majority of studies included cadaveric and living kidney transplant recipients (25 studies: Ahsan 1999; ATLAS Study 2005; Boletis 2001; Boots 2002; Burke 2000; DOMINOS Study 2012; EVIDENCE Study 2014; Farmer 2006; FREEDOM Study 2008; Gulanikar 1991; Jankowska‐Gan 2009; Kim 2002; Kumar 2005; Laftavi 2005; Lebranchu 1999; Matl 2000; Montagnino 2005; Nott 1985; Pelletier 2006; Schulak 1989; Stiller 1983; Smak Gregoor 1999; THOMAS Study 2002; Vincenti 2003a; Woodle 2005). Kidney transplantation was limited to cadaveric donor sources in 11 studies (Bouma 1996; De Vecchi 1986; FRANCIA Study 2007; Isoniemi 1990; Johnson 1989a; Maiorca 1988; Ponticelli 1997; Ratcliffe 1993; Sandrini 2009; Sola 2002; Zhu 2008a) and to living donors in four studies (Aswad 1998; Nagib 2015; Nematalla 2007; Park 1994) In 17 studies first or subsequent kidney transplant recipients were eligible (Boots 2002; Bouma 1996; DOMINOS Study 2012; EVIDENCE Study 2014; Farmer 2006; Gulanikar 1991; Johnson 1989a; Lebranchu 1999; Montagnino 2005; Nott 1985; Pisani 2001; Ponticelli 1997; Ratcliffe 1993; Schulak 1989; Stiller 1983; THOMAS Study 2002; Woodle 2005), while in 19 studies limited participants to recipients of first kidney transplants (Ahsan 1999; ATLAS Study 2005; Boletis 2001; Burke 2000; del Castillo 2005; FRANCIA Study 2007; FREEDOM Study 2008; INFINITY Study 2013; Isoniemi 1990; Kumar 2005; Laftavi 2005; Maiorca 1988; Matl 2000; Nagib 2015; Nematalla 2007; Park 1994; Pelletier 2006; Sandrini 2009; Vincenti 2003a).
Study comparisons
The 45 included studies evaluated three different comparisons in adults.
Steroid withdrawal compared versus steroid maintenance was investigated in 24/45 studies in adult patients (Ahsan 1999; Albert 1985; Aswad 1998; Boletis 2001; Bouma 1996; Burke 2000; del Castillo 2005; EVIDENCE Study 2014; Farmer 2006; Gulanikar 1991; Isoniemi 1990; Jankowska‐Gan 2009; Kacar 2004; Lebranchu 1999; Maiorca 1988; Matl 2000; Park 1994; Pelletier 2006; Pisani 2001; Ratcliffe 1993; Smak Gregoor 1999; Sola 2002; THOMAS Study 2002; Zhu 2008a). Steroids were withdrawn three months after transplantation in eight studies (Ahsan 1999; EVIDENCE Study 2014; Gulanikar 1991; Isoniemi 1990; Lebranchu 1999; Park 1994; Sola 2002; THOMAS Study 2002); six months after transplantation in eight studies (Albert 1985; Aswad 1998; Boletis 2001; Burke 2000; del Castillo 2005; Pisani 2001; Smak Gregoor 1999; Zhu 2008a); one year after transplantation in one study (Matl 2000), and beyond one year after transplantation in six studies (Bouma 1996; Farmer 2006; Jankowska‐Gan 2009; Kacar 2004; Maiorca 1988; Ratcliffe 1993). In one study, steroids were withdrawn at different time points after transplantation and the time point of withdrawal was not reported, but all patients had steroids for more than 14 days (Pelletier 2006).
Steroid avoidance compared versus steroid maintenance was investigated in 18/45 studies in adult kidney transplant recipients (ATLAS Study 2005; De Vecchi 1986; FRANCIA Study 2007; FREEDOM Study 2008; Nott 1985; INFINITY Study 2013; Johnson 1989a; Kim 2002; Kumar 2005; Laftavi 2005; Stiller 1983; Montagnino 2005; Nagib 2015; Nematalla 2007; Ponticelli 1997; Schulak 1989; Vincenti 2003a; Woodle 2005). In two studies steroids were not given at any time point before, during or after transplantation (FREEDOM Study 2008; Stiller 1983). Steroids were withdrawn until day seven after transplantation in 12 studies (ATLAS Study 2005; De Vecchi 1986; FRANCIA Study 2007; Nott 1985; Johnson 1989a; Kim 2002; Kumar 2005; Laftavi 2005; Montagnino 2005; Nematalla 2007; Ponticelli 1997; Vincenti 2003a) and between day 8 and day 14 in two studies (Schulak 1989; Woodle 2005).
Steroid avoidance was compared versus steroid withdrawal in 3/45 studies with adults (Boots 2002; DOMINOS Study 2012; Sandrini 2009). In all of these three studies, steroids were withdrawn until day seven after transplantation in the avoidance group and between three to six months after transplantation in the withdrawal group.
Immunosuppression
CsA was used in 34 studies evaluating steroid withdrawal or steroid avoidance (Ahsan 1999; Albert 1985; Boletis 2001; Bouma 1996; Burke 2000; del Castillo 2005; De Vecchi 1986; DOMINOS Study 2012; EVIDENCE Study 2014; Farmer 2006; FRANCIA Study 2007; FREEDOM Study 2008; Gulanikar 1991; INFINITY Study 2013; Isoniemi 1990; Jankowska‐Gan 2009; Johnson 1989a; Kim 2002; Kumar 2005; Lebranchu 1999; Maiorca 1988; Matl 2000; Montagnino 2005; Nott 1985; Park 1994; Pelletier 2006; Pisani 2001; Ponticelli 1997; Ratcliffe 1993; Sandrini 2009; Schulak 1989; Smak Gregoor 1999; Vincenti 2003a). TAC was used in 10 studies investigating steroid withdrawal or steroid avoidance (Aswad 1998; ATLAS Study 2005; Boots 2002; Laftavi 2005; Nagib 2015; Nematalla 2007; Sola 2002; THOMAS Study 2002; Woodle 2005; Zhu 2008a). One study provided no information about the baseline immunosuppression used (Kacar 2004). Of the three studies comparing steroid avoidance with steroid withdrawal, two used a CsA‐based immunosuppression (DOMINOS Study 2012; Sandrini 2009) and one used a TAC‐based immunosuppression (Boots 2002).
Five studies investigated steroid withdrawal compared versus steroid maintenance in patients without an additional antiproliferative immunosuppressant (either MMF or enteric‐coated mycophenolate sodium or AZA or mTOR‐inhibitor) (Albert 1985; Bouma 1996; Gulanikar 1991; Maiorca 1988; Park 1994) and five studies investigated steroid avoidance compared versus steroid maintenance without an additional antiproliferative (De Vecchi 1986; Johnson 1989a; Nott 1985; Stiller 1983; Ponticelli 1997). Steroid avoidance compared versus steroid withdrawal in patients without an antiproliferative was investigated in Boots 2002. An immunosuppressive regimen including an additional antiproliferative agent was used in 18 studies that investigated steroid withdrawal compared versus steroid maintenance (Ahsan 1999; Aswad 1998; Boletis 2001; Burke 2000; del Castillo 2005; EVIDENCE Study 2014; Farmer 2006; Isoniemi 1990; Jankowska‐Gan 2009; Lebranchu 1999; Matl 2000; Pelletier 2006; Pisani 2001; Ratcliffe 1993; Smak Gregoor 1999; Sola 2002; THOMAS Study 2002; Zhu 2008a). Of these 18 studies, 12 used MMF (Ahsan 1999; Boletis 2001; del Castillo 2005; Burke 2000; Jankowska‐Gan 2009; Pelletier 2006; Pisani 2001; Smak Gregoor 1999; Sola 2002; THOMAS Study 2002; Lebranchu 1999; Zhu 2008a), five used AZA (Aswad 1998; Farmer 2006; Isoniemi 1990; Matl 2000; Ratcliffe 1993), and one used Everolimus (EVIDENCE Study 2014). Steroid avoidance compared versus steroid maintenance using an additional antiproliferative immunosuppressant was used in 13 studies (ATLAS Study 2005; FRANCIA Study 2007; FREEDOM Study 2008; INFINITY Study 2013; Kim 2002; Kumar 2005; Laftavi 2005; Montagnino 2005; Nagib 2015; Nematalla 2007; Schulak 1989; Vincenti 2003a; Woodle 2005). Of these, nine used MMF (ATLAS Study 2005; FRANCIA Study 2007; Kim 2002; Kumar 2005; Laftavi 2005; Nagib 2015Nematalla 2007; Vincenti 2003a; Woodle 2005), two used enteric‐coated mycophenolate sodium (FREEDOM Study 2008; INFINITY Study 2013), one used AZA (Schulak 1989), and one used everolimus (Montagnino 2005). Steroid avoidance compared versus steroid withdrawal in patients treated with an additional antiproliferative was investigated in two studies (DOMINOS Study 2012; Sandrini 2009). One study used enteric‐coated mycophenolate sodium (DOMINOS Study 2012) and one used sirolimus (Sandrini 2009) as the third immunosuppressant.
Induction treatment was administered in 17 studies with adult kidney transplant recipients in three studies comparing steroid withdrawal with steroid maintenance (EVIDENCE Study 2014; Pelletier 2006; Pisani 2001), in 12 studies comparing steroid avoidance with steroid maintenance (FRANCIA Study 2007; FREEDOM Study 2008; INFINITY Study 2013; Kim 2002; Kumar 2005; Laftavi 2005; Montagnino 2005; Nagib 2015; Nematalla 2007; Schulak 1989; Vincenti 2003a; Woodle 2005), and in two studies comparing steroid avoidance with steroid withdrawal (DOMINOS Study 2012; Sandrini 2009). In 12 studies an IL‐2 receptor antagonist was used for induction treatment (DOMINOS Study 2012; EVIDENCE Study 2014; FREEDOM Study 2008; INFINITY Study 2013; Kim 2002; Kumar 2005; Montagnino 2005; Nagib 2015; Nematalla 2007; Pisani 2001; Sandrini 2009; Vincenti 2003a), in three studies an anti‐lymphocytic depleting antibodies was used (FRANCIA Study 2007; Laftavi 2005; Schulak 1989) and two studies allowed the type of induction treatment to be chosen by the investigator (Pelletier 2006; Woodle 2005).
Studies in child kidney transplant recipients
This update included three studies (11 reports, 346 participants) of steroid withdrawal or avoidance in child kidney transplant recipients (Benfield 2005; Höcker 2009; Mericq 2013).
Participants
Studies recruited participants who were younger than 20 years of age. All three studies included cadaveric and living kidney transplant recipients. In Benfield 2005 and Mericq 2013 only first kidney transplant recipients were eligible; in Höcker 2009 first or subsequent kidney transplantation was included.
Study comparisons
The three studies evaluated two different comparisons in children. Benfield 2005 and Höcker 2009 investigated steroid withdrawal versus steroid maintenance; Mericq 2013 investigated steroid avoidance versus steroid withdrawal.
Immunosuppression
All three studies used a calcineurin inhibitor‐based immunosuppressive regimen including an additional antiproliferative agent. Höcker 2009 used CsA and MMF, Benfield 2005 allowed either CsA or TAC to be used with sirolimus and Mericq 2013 used TAC in combination with MMF. Benfield 2005 and Mericq 2013 also used basiliximab for induction treatment, but Benfield 2005 was terminated early when the Data Safety Monitoring Board noted an excess risk of post‐transplant lymphoproliferative disease in both treatment groups.
Reported outcome measures
The reporting of outcome measures varied across studies. Of the 45 included studies, 34 reported patient mortality and 23 reported acute rejection (see Figure 1). Reporting of harms was more limited and inconsistent among studies (six studies reported cardiovascular events with varying definitions of cardiovascular events or definitions not reported). Frequently, studies reported incomplete data for harm outcomes or expressed their results as 'episodes', which complicated meaningful use of such data in the meta‐analysis.
Excluded studies
We excluded a total of 48 studies because studies: were not randomised (12), concerned ineligible populations (3), involved ineligible interventions ( 11) or ineligible co‐interventions (22).
Risk of bias in included studies
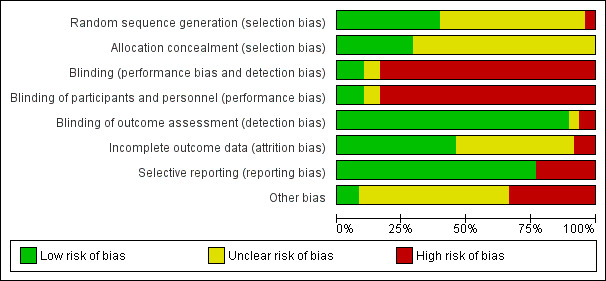
Reporting of details of study methodology regarding design and conduct of the study was incomplete in most studies. The assessment of risk of bias is shown in Figure 2 and Figure 3. Figure 2 shows the risk of bias indicators for individual studies. Figure 3 shows the proportion of studies assessed as low, high or unclear risk of bias for each risk of bias indicator.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study
3.
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
Allocation
Random sequence generation was judged to be at low risk of bias in 19 studies (Ahsan 1999; ATLAS Study 2005; Benfield 2005; DOMINOS Study 2012; EVIDENCE Study 2014; FRANCIA Study 2007; FREEDOM Study 2008; Gulanikar 1991; Höcker 2009; Johnson 1989a; Kumar 2005; Laftavi 2005; Mericq 2013; Montagnino 2005; Nematalla 2007; Ponticelli 1997; Schulak 1989; Stiller 1983; Woodle 2005) and considered at high risk in two studies (Aswad 1998; Matl 2000). Randomisation methods were not reported in 27 studies (Albert 1985; Boletis 2001; Boots 2002; Bouma 1996; Burke 2000; del Castillo 2005; De Vecchi 1986; Farmer 2006; INFINITY Study 2013; Isoniemi 1990; Jankowska‐Gan 2009; Kacar 2004; Kim 2002; Lebranchu 1999; Maiorca 1988; Nagib 2015; Nott 1985; Park 1994; Pelletier 2006; Pisani 2001; Ratcliffe 1993; Sandrini 2009; Smak Gregoor 1999; Sola 2002; THOMAS Study 2002; Vincenti 2003a; Zhu 2008a).
Allocation concealment was assessed to be at low risk of bias in 14 studies (ATLAS Study 2005; Boots 2002; De Vecchi 1986; DOMINOS Study 2012; Farmer 2006; FRANCIA Study 2007; Gulanikar 1991; Isoniemi 1990; Mericq 2013; Montagnino 2005; Nematalla 2007; Smak Gregoor 1999; Stiller 1983; Woodle 2005); no study was judged to be at high risk of bias. Methods used for allocation concealment were unclear in the remaining 34 studies (Ahsan 1999; Albert 1985; Aswad 1998; Benfield 2005; Boletis 2001; Bouma 1996; Burke 2000; del Castillo 2005; EVIDENCE Study 2014; FREEDOM Study 2008; Höcker 2009; INFINITY Study 2013; Jankowska‐Gan 2009; Johnson 1989a; Kacar 2004; Kim 2002; Kumar 2005; Laftavi 2005; Lebranchu 1999; Maiorca 1988; Matl 2000; Nagib 2015; Nott 1985; Park 1994; Pelletier 2006; Pisani 2001; Ponticelli 1997; Ratcliffe 1993; Sandrini 2009; Schulak 1989; Sola 2002; THOMAS Study 2002; Vincenti 2003a; Zhu 2008a).
Blinding
Participants and investigators were blinded in only five studies (Ahsan 1999; Benfield 2005; Burke 2000; Gulanikar 1991; Woodle 2005). The absence of blinding was judged as high risk of bias because clinical management could be influenced by knowledge of treatment group. Blinding of outcome assessment was considered as low risk of bias because outcomes were objective and therefore more robust against influence by knowledge of treatment group (e.g. death, graft loss, serum creatinine).
Incomplete outcome data
Incomplete outcome data was judged to be at low risk of bias in 22 studies (Ahsan 1999; ATLAS Study 2005; Benfield 2005; Boots 2002; Bouma 1996; del Castillo 2005; DOMINOS Study 2012; FRANCIA Study 2007; FREEDOM Study 2008; Gulanikar 1991; Höcker 2009; Isoniemi 1990; Kumar 2005; Matl 2000; Montagnino 2005; Ponticelli 1997; Ratcliffe 1993; Schulak 1989; Smak Gregoor 1999; THOMAS Study 2002; Vincenti 2003a; Woodle 2005). Exclusion of participants after randomisation and attrition were considered at high risk in four studies (Boletis 2001; Burke 2000; De Vecchi 1986; Nagib 2015). Methods for addressing incomplete outcome data remained unclear in 22 studies (Albert 1985; Aswad 1998; EVIDENCE Study 2014; Farmer 2006; INFINITY Study 2013; Jankowska‐Gan 2009; Johnson 1989a; Kacar 2004; Kim 2002; Laftavi 2005; Lebranchu 1999; Maiorca 1988; Mericq 2013; Nematalla 2007; Nott 1985; Park 1994; Pelletier 2006; Pisani 2001; Sandrini 2009; Sola 2002; Stiller 1983; Zhu 2008a).
Selective reporting
Selective outcome reporting was judged as low risk in 37 studies (Ahsan 1999; Aswad 1998; ATLAS Study 2005; Benfield 2005; Boots 2002; Bouma 1996; del Castillo 2005; De Vecchi 1986; DOMINOS Study 2012; EVIDENCE Study 2014; FRANCIA Study 2007; FREEDOM Study 2008; Höcker 2009; INFINITY Study 2013; Isoniemi 1990; Jankowska‐Gan 2009; Kacar 2004; Kumar 2005; Lebranchu 1999; Maiorca 1988; Matl 2000; Mericq 2013; Montagnino 2005; Nagib 2015; Nematalla 2007; Park 1994; Pelletier 2006; Pisani 2001; Ponticelli 1997; Sandrini 2009; Schulak 1989; Smak Gregoor 1999; Sola 2002; Stiller 1983; THOMAS Study 2002; Vincenti 2003a; Woodle 2005). Eleven studies did not report all hard clinical outcomes that were considered primary outcomes for this review and were assessed as high risk of bias for selective outcome reporting (Albert 1985; Boletis 2001; Burke 2000; Farmer 2006; Gulanikar 1991; Nott 1985; Johnson 1989a; Kim 2002; Laftavi 2005; Ratcliffe 1993; Zhu 2008a).
Other potential sources of bias
Funding from academic independent sources was considered as low risk of bias in four studies (De Vecchi 1986; Isoniemi 1990; Matl 2000; Mericq 2013). In 16 studies a pharmaceutical company was reported as funding source, which was judged as high risk of bias (Ahsan 1999; ATLAS Study 2005; Benfield 2005; Bouma 1996; DOMINOS Study 2012; FRANCIA Study 2007; FREEDOM Study 2008; Kumar 2005; Montagnino 2005; Gulanikar 1991; Smak Gregoor 1999; Stiller 1983; THOMAS Study 2002; Vincenti 2003a). In 27 studies funding sources were not disclosed (Albert 1985; Aswad 1998; Boletis 2001; Boots 2002; Burke 2000; del Castillo 2005; EVIDENCE Study 2014; Farmer 2006; Höcker 2009; INFINITY Study 2013; Jankowska‐Gan 2009; Johnson 1989a; Kacar 2004; Kim 2002; Laftavi 2005; Lebranchu 1999; Maiorca 1988; Nematalla 2007; Nott 1985; Park 1994; Pelletier 2006; Pisani 2001; Ponticelli 1997; Ratcliffe 1993; Sandrini 2009; Schulak 1989; Sola 2002; Woodle 2005; Zhu 2008a). Publication bias was assessed by constructing funnel plots for three comparisons that included at least 10 studies in the meta‐analysis (death and acute rejection for steroid withdrawal versus steroid maintenance and acute rejection for steroid avoidance versus steroid maintenance). All funnel plots are symmetric and do not indicate publication bias (see Figure 4).
4.

Funnel plot of comparisons that included at least 10 studies in the meta‐analysis
Effects of interventions
Studies in adults with kidney transplant recipients
Steroid withdrawal versus steroid maintenance
Steroid withdrawal may lead to little of no difference in patient mortality at either one year (Analysis 1.1.1 (10 studies, 1913 participants): RR 0.68, 95% CI 0.36 to 1.30; I2 = 0%) or one to five years post transplantation (Analysis 1.1.2 (7 studies, 1118 participants): RR 1.26, 95% CI 0.73 to 2.17; I2 = 0%). Likewise steroid withdrawal may lead to little or no difference in graft loss excluding death at either one year (Analysis 1.1.5 (8 studies, 1817 participants): RR 1.17, 95% CI 0.72 to 1.92; I2 = 0%) or one to five years post transplantation (Analysis 1.1.6 (7 studies, 1092 participants): RR 1.61, 95% CI 0.98 to 2.64; I2 = 0%).
1.1. Analysis.
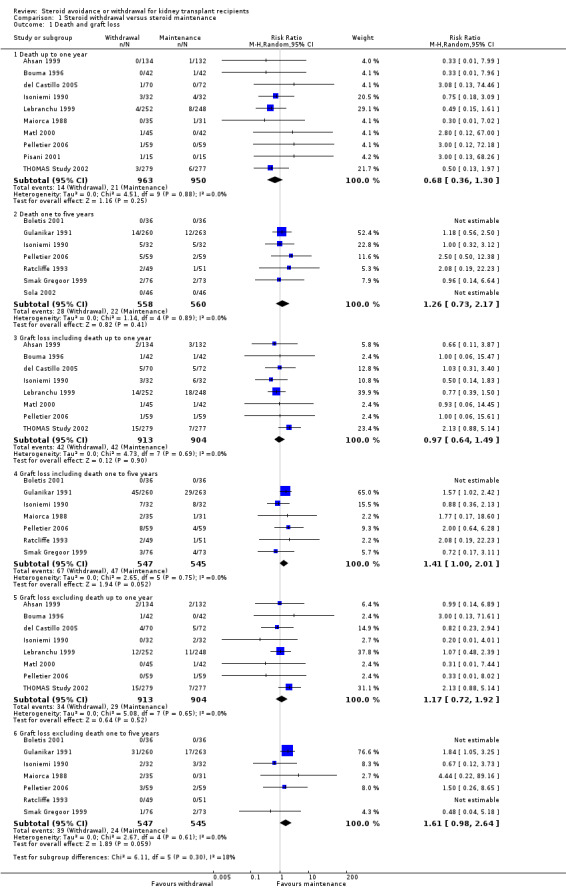
Comparison 1 Steroid withdrawal versus steroid maintenance, Outcome 1 Death and graft loss.
The risk of acute rejection significantly increased by 77% in patients withdrawn from steroids compared versus patients maintained on steroids within the first year after transplantation (Analysis 1.2.1 (10 studies, 1913 participants): RR 1.77, 95% CI 1.20 to 2.61; I2 = 54%), but there was no difference in the incidence of biopsy‐proven acute rejection (Analysis 1.2.2 (5 studies, 1292 participants): RR 1.32, 95% CI 0.78 to 2.22; I2 = 65%).
1.2. Analysis.

Comparison 1 Steroid withdrawal versus steroid maintenance, Outcome 2 Rejection.
The incidence of NODAT (Analysis 1.3.1 (6 studies, 1439 participants): RR 0.77, 95% CI 0.49 to 1.21; I2 = 0%) as well as the incidence of cardiovascular events (Analysis 1.3.2 (2 studies, 607 participants): RR 0.98, 95% CI 0.42 to 2.33; I2 = 0%) up to five years after transplantation were not significantly different between groups, mainly because of the low number of studies reporting these rarely occurring outcomes. Likewise data was sparse for harmful events, such as infection (Analysis 1.4.1 (5 studies, 1819 participants): RR 1.02, 95% CI 0.84 to 1.22; I2 = 30%), CMV infection (Analysis 1.4.2 (RR 1.04, 95% CI 0.80 to 1.36; participants = 1758; studies = 5; I2 = 0%), and malignancy (Analysis 1.4.3 (3 studies, 756 participants): RR 0.77, 95% CI 0.41 to 1.46; I2 = 0%) and a difference in these outcomes could not be demonstrated up to five years after transplantation. There was also no evidence of difference in kidney function as determined by measurement of serum creatinine and creatinine clearance up to one as well as up to five years after transplantation (Analysis 1.5) (See also Table 1).
1.3. Analysis.

Comparison 1 Steroid withdrawal versus steroid maintenance, Outcome 3 New‐onset diabetes after transplantation and cardiovascular events.
1.4. Analysis.
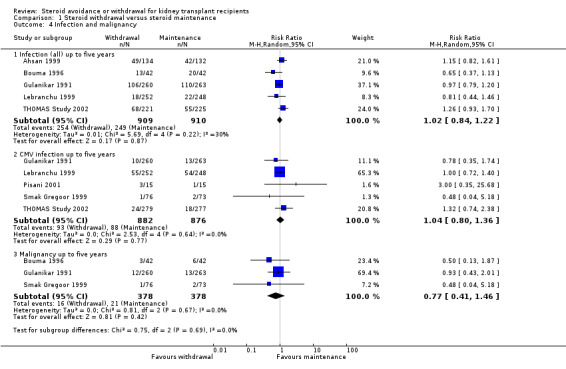
Comparison 1 Steroid withdrawal versus steroid maintenance, Outcome 4 Infection and malignancy.
1.5. Analysis.

Comparison 1 Steroid withdrawal versus steroid maintenance, Outcome 5 Kidney function.
Sensitivity and subgroup analyses for steroid withdrawal versus steroid maintenance studies
Results of the sensitivity and subgroup analyses are summarised in Table 3.
1. Steroid withdrawal versus steroid maintenance ‐ stratified subgroup and sensitivity analysis for death, graft loss and acute rejection up to one year after transplantation.
| Death | Graft loss | Acute rejection | Biopsy‐proven acute rejection | |||||||||
| Studies | RR | 95% CI | Studies | RR | 95% CI | Studies | RR | 95% CI | Studies | RR | 95% CI | |
| Publication status | ||||||||||||
| Peer reviewed journal | 8 | 0.60 | 0.31 to 1.17 | 7 | 1.25 | 0.73 to 2.13 | 8 | 2.02 | 1.23 to 3.23 | 4 | 1.32 | 0.66 to 2.66 |
| Abstract only | 2 | 3.04 | 0.33 to 28.29 | 1 | 0.82 | 0.23 to 2.94 | 2 | 1.25 | 0.67 to 2.32 | 1 | 1.37 | 0.70 to 2.69 |
| ITT analysis | ||||||||||||
| ITT analysis used | 6 | 0.69 | 0.30 to 1.61 | 6 | 1.31 | 0.69 to 2.46 | 6 | 2.07 | 1.10 to 3.91 | 3 | 1.37 | 0.64 to 2.94 |
| ITT analysis not used/unclear | 4 | 0.67 | 0.25 to 1.81 | 2 | 1.00 | 0.46 to 2.17 | 4 | 1.65 | 0.81 to 3.36 | 2 | 1.04 | 0.24 to 4.59 |
| Calcineurin inhibitor | ||||||||||||
| CsA | 9 | 0.75 | 0.36 to 1.54 | 7 | 0.90 | 0.50 to 5.14 | 9 | 2.08 | 1.29 to 3.35 | 4 | 1.60 | 0.87 to 2.92 |
| TAC | 1 | 0.50 | 0.13 to 1.97 | 1 | 2.13 | 0.88 to 5.14 | 1 | 1.11 | 0.82 to 1.51 | 1 | 0.89 | 0.61 to 1.30 |
| Antimetabolite | ||||||||||||
| MMF or EC‐MPS | 6 | 0.67 | 0.31 to 1.47 | 5 | 1.25 | 0.75 to 2.08 | 6 | 1.41 | 1.02 to 1.94 | 3 | 1.27 | 0.81 to 2.00 |
| AZA | 2 | 0.93 | 0.26 to 3.40 | 2 | 0.25 | 0.03 to 2.18 | 2 | 2.61 | 0.62 to 10.91 | 1 | 0.33 | 0.04 to 2.56 |
| MMF or EC‐MPS or AZA | 8 | 0.73 | 0.38 to 1.43 | 7 | 1.15 | 0.70 to 1.89 | 8 | 1.46 | 1.07 to 1.98 | 4 | 1.19 | 0.75 to 1.90 |
| none | 2 | 0.31 | 0.03 to 2.95 | 1 | 3.00 | 0.13 to 71.61 | 2 | 5.80 | 2.16 to 15.57 | 1 | 9.00 | 1.19 to 67.93 |
| Induction treatment | ||||||||||||
| Induction (yes) | 2 | 3.00 | 0.32 to 27.87 | 1 | 0.33 | 0.01 to 8.02 | 2 | 0.80 | 0.22 to 2.91 | NA | ‐‐ | ‐‐ |
| Induction (no) | 8 | 0.60 | 0.31 to 1.17 | 7 | 1.21 | 0.74 to 1.99 | 8 | 1.93 | 1.26 to 2.94 | NA | ‐‐ | ‐‐ |
AZA ‐ azathioprine; CI ‐ confidence interval; CsA ‐ cyclosporin A; EC‐MPS ‐ enteric‐coated mycophenolate sodium; ITT ‐ intention to treat; MMF ‐ mycophenolate mofetil; NA ‐ not available; RR ‐ risk ratio; TAC ‐ tacrolimus
We have performed sensitivity analysis to assess the impact of publication status and use of intention‐to‐treat‐analysis on primary endpoints (mortality, death censored graft loss, acute rejection and biopsy‐proven acute rejection) using data from studies reporting these outcomes at any time point within the first year after transplantation. There was no evidence to suggest a difference in effect estimates of mortality, graft loss and biopsy‐proven acute rejection for studies depending on whether they have performed intention‐to‐treat analysis or whether the study was published in a peer‐reviewed journal. The significant increase in risk for acute rejection in patients withdrawn from steroids compared versus those maintained on steroids was further increased in studies published in a peer‐reviewed journal (8 studies, 1741 participants: RR 2.02, 95% CI 1.26 to 3.23) and in studies that applied intention‐to‐treat analysis (6 studies, 1199 participants: RR 2.07, 95% CI 1.10 to 3.91), but was lost in studies published as abstract‐only and in studies where intention‐to‐treat analysis was either not used or unclear.
We performed subgroup analysis stratified by calcineurin‐inhibitor type, type of antimetabolite and induction treatment on primary endpoints (mortality, death censored graft loss, acute rejection and biopsy‐proven acute rejection) using data from studies reporting these outcomes at any time point within the first year after transplantation. There was no difference in mortality and graft loss in any of the subgroups. The risk of acute rejection after steroid withdrawal was further increased in patients treated with CsA (9 studies, 1357 participants: RR 2.08, 95% 1.29 to 3.35), especially among those who did not receive an additional antimetabolite (2 studies, 150 participants: RR 5.80, 95% CI 2.16 to 15.57) and in patients who did not receive induction treatment (8 studies, 1765 participants: RR 1.93, 95% CI 1.26 to 2.94), but was decreased in patients who received either MMF or enteric‐coated mycophenolate sodium (6 studies, 1612 participants: RR 1.41, 95% CI 1.02 to 1.94) or any type of antimetabolite (8 studies, 1763 participants: RR 1.46, 95% CI 1.07 to 1.98).
Steroid avoidance versus steroid maintenance
Results are summarised in Table 2.
Steroid avoidance did not show a significant effect on patient mortality at either one year (Analysis 2.1.1 (10 studies, 1462 participants): RR 0.96, 95% CI 0.52 to 1.80; I2 = 0%) or one to five years post transplantation (Analysis 2.1.2 (7 studies, 1201 participants): RR 0.57, 95% CI 0.32 to 1.01; I2 = 0%). Likewise steroid avoidance did not show any significant effects on graft loss excluding death at either one year (Analysis 2.1.5 (7 studies, 1211 participants): RR 1.09, 95% CI 0.64 to 1.86; I2 = 0%) or one to five years post transplantation (Analysis 2.1.6 (7 studies, 1245 participants): RR 0.98, 95% CI 0.66 to 1.45; I2 = 0%).
2.1. Analysis.

Comparison 2 Steroid avoidance versus steroid maintenance, Outcome 1 Death and graft loss.
Steroid avoidance significantly increased the risk of acute rejection within the first year after transplantation by 58% compared versus patients maintained on steroids (Analysis 2.2.1 (7 studies, 835 participants): RR 1.58, 95% CI 1.08 to 2.30; I2 = 63%). This effect of steroid avoidance was also demonstrated for biopsy‐proven acute rejection with a risk increase of 94% within the first year after transplantation (Analysis 2.2.2 (6 studies, 1073 participants): RR 1.94, 95% CI 1.26 to 2.98; I2 = 45%).
2.2. Analysis.

Comparison 2 Steroid avoidance versus steroid maintenance, Outcome 2 Rejection.
There was no evidence of difference in the occurrence of NODAT, cardiovascular events, infection, CMV infection and malignancy between groups up to five years after transplantation (Analysis 2.3; Analysis 2.4). Kidney function determined as serum creatinine and creatinine clearance up to one year as well as up to five years after transplantation was not different for patients treated with steroids for less than 14 days compared versus patients maintained on steroids (Analysis 2.5).
2.3. Analysis.
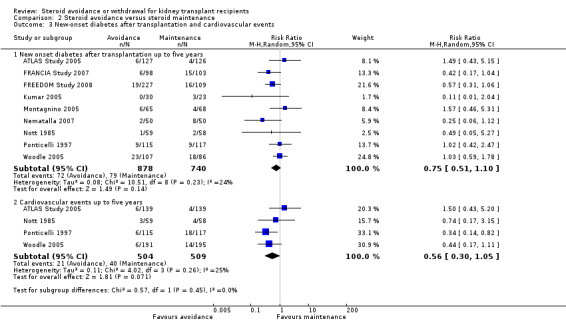
Comparison 2 Steroid avoidance versus steroid maintenance, Outcome 3 New‐onset diabetes after transplantation and cardiovascular events.
2.4. Analysis.
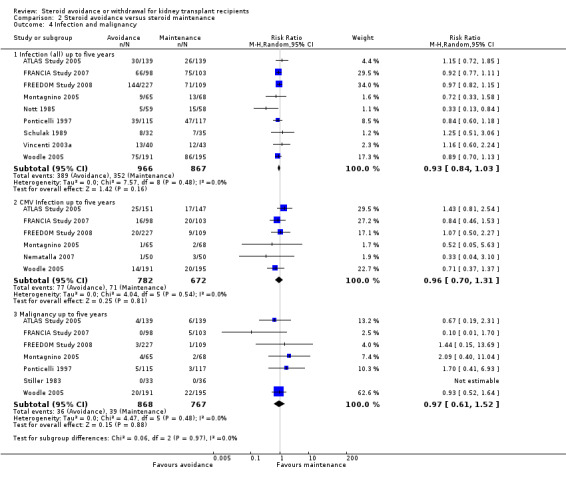
Comparison 2 Steroid avoidance versus steroid maintenance, Outcome 4 Infection and malignancy.
2.5. Analysis.
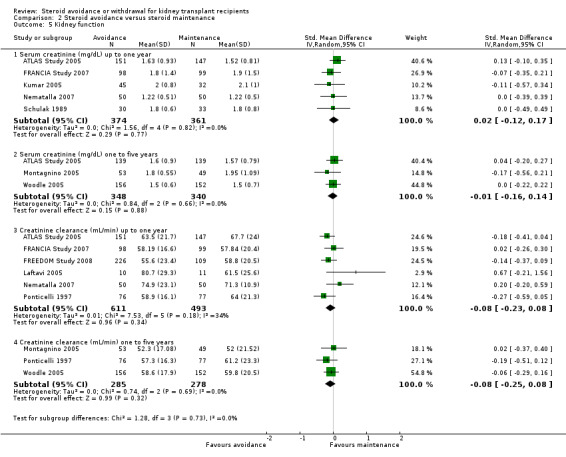
Comparison 2 Steroid avoidance versus steroid maintenance, Outcome 5 Kidney function.
Sensitivity and subgroup analysis for steroid avoidance versus steroid maintenance ‐ studies
We performed sensitivity analysis to assess the impact of use of intention‐to‐treat‐analysis on primary endpoints (mortality, death censored graft loss, acute rejection and biopsy‐proven acute rejection) using data from studies reporting these outcomes at any time point within the first year after transplantation. There was no study investigating steroid avoidance compared versus steroid maintenance that was published as abstract only, consequently the influence of publication status on the effect estimates could not be tested. There was no evidence to suggest a difference in effect estimates of mortality and graft loss for studies depending on whether they have performed intention‐to‐treat analysis. The increased risk for acute rejection and biopsy‐proven acute rejection in patients treated with steroids for less than 14 days after kidney transplantation compared versus those maintained on steroids was further increased in studies that applied intention‐to‐treat analysis (acute rejection: 4 studies, 655 participants: RR 1.92, 95% CI 1.18 to 3.14; biopsy‐proven acute rejection: 4 studies, 918 participants: RR 2.31, 95% CI 1.47 to 3.63), but lost significance in studies where intention‐to‐treat analysis was either not used or unclear.
We have performed subgroup analysis stratified by type of calcineurin inhibitor, type of antimetabolite and induction treatment on primary endpoints (mortality, death censored graft loss, acute rejection and biopsy‐proven acute rejection) using data from studies reporting these outcomes at any time point within the first year after transplantation. Stratified analysis did not reveal any difference in patient mortality and graft loss. The significant increase in risk for biopsy‐proven acute rejection persisted in patients treated with CsA (3 studies, 615 participants: RR 1.89, 95% CI 1.29 to 2.79), while patients treated with TAC did not have an increased risk for biopsy‐proven acute rejection (See Table 4).
2. Steroid avoidance versus steroid maintenance ‐ stratified subgroup and sensitivity analysis for death, graft loss and acute rejection up to one year after transplantation.
| Death | Graft loss | Acute rejection | Biopsy‐proven acute rejection | |||||||||
| Studies | RR | 95% CI | Studies | RR | 95% CI | Studies | RR | 95% CI | Studies | RR | 95% CI | |
| ITT analysis | ||||||||||||
| ITT analysis used | 7 | 1.16 | 0.48 to 2.83 | 5 | 1.09 | 0.56 to 2.11 | 4 | 1.92 | 1.18 to 3.14 | 4 | 2.31 | 1.47 to 3.63 |
| ITT analysis not used/unclear | 3 | 0.51 | 0.07 to 3.83 | 2 | 1.11 | 0.46 to 2.67 | 3 | 1.24 | 0.97 to 1.59 | 2 | 1.05 | 0.49 to 2.23 |
| Calcineurin inhibitor | ||||||||||||
| CsA | 8 | 0.88 | 0.47 to 1.66 | 5 | 1.08 | 0.59 to 1.99 | 5 | 1.31 | 1.05 to 1.63 | 3 | 1.89 | 1.29 to 2.79 |
| TAC | 2 | 6.82 | 0.36 to 130.81 | 2 | 1.14 | 0.39 to 3.3 | 2 | 2.40 | 1.05 to 5.49 | 3 | 1.81 | 0.66 to 4.99 |
| Antimetabolite | ||||||||||||
| MMF or EC‐MPS | 6 | 1.15 | 0.36 to 3.69 | 6 | 1.09 | 0.56 to 2.11 | 5 | 1.87 | 1.20 to 2.91 | 6 | 1.94 | 1.26 to 2.98 |
| AZA | 1 | 1.64 | 0.29 to 9.2 | NA | ‐‐ | ‐‐ | NA | ‐‐ | ‐‐ | NA | ‐‐ | ‐‐ |
| MMF or EC‐MPS or AZA | 8 | 1.16 | 0.48 to 2.83 | NA | ‐‐ | ‐‐ | NA | ‐‐ | ‐‐ | NA | ‐‐ | ‐‐ |
| None | 2 | 0.51 | 0.07 to 3.83 | 1 | 1.11 | 0.46 to 2.67 | 2 | 1.26 | 0.95 to 1.65 | NA | ‐‐ | ‐‐ |
| Induction treatment | ||||||||||||
| Induction (yes) | 7 | 0.97 | 0.38 to 2.48 | 5 | 1.06 | 0.45 to 2.46 | 4 | 1.50 | 0.97 to 2.32 | 5 | 1.67 | 1.19 to 2.36 |
| Induction (no) | 3 | 0.92 | 0.17 to 5.01 | 2 | 1.12 | 0.57 to 2.2 | 3 | 1.72 | 0.89 to 3.32 | 1 | 3.89 | 2.16 to 7.03 |
AZA ‐ azathioprine; CI ‐ confidence interval; CsA ‐ cyclosporin A; EC‐MPS ‐ enteric‐coated mycophenolate sodium; ITT ‐ intention to treat; MMF ‐ mycophenolate mofetil; NA ‐ not available; RR ‐ risk ratio; TAC ‐ tacrolimus
Steroid avoidance versus steroid withdrawal
Only three studies investigating the effect of steroid avoidance compared versus steroid withdrawal were identified, wherefore data is specifically sparse for this comparison. There is no evidence to suggest a difference in any outcome (death: Analysis 3.1; rejection: Analysis 3.2; NODAT, infection, malignancy: Analysis 3.3; kidney function: Analysis 3.4). Sensitivity and subgroup analysis could not be performed due to the small number of studies identified.
3.1. Analysis.
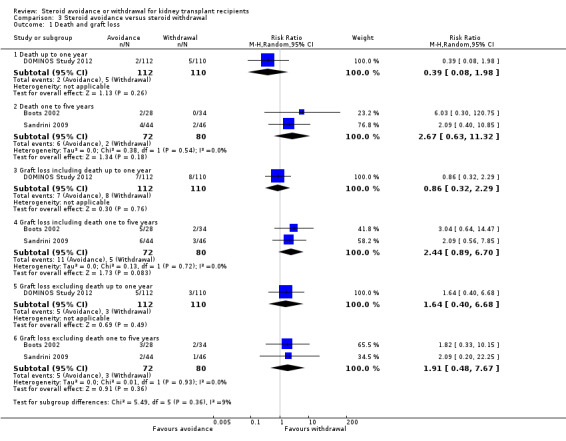
Comparison 3 Steroid avoidance versus steroid withdrawal, Outcome 1 Death and graft loss.
3.2. Analysis.
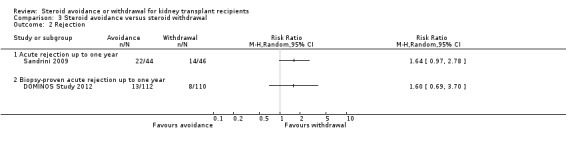
Comparison 3 Steroid avoidance versus steroid withdrawal, Outcome 2 Rejection.
3.3. Analysis.
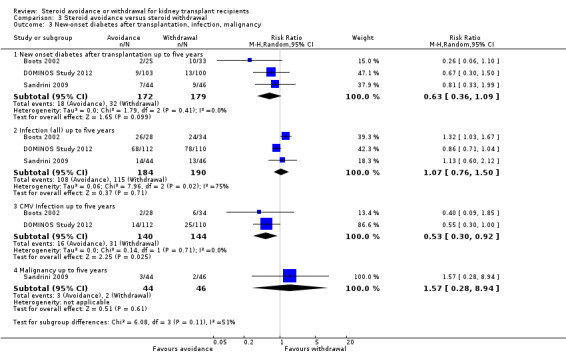
Comparison 3 Steroid avoidance versus steroid withdrawal, Outcome 3 New‐onset diabetes after transplantation, infection, malignancy.
3.4. Analysis.
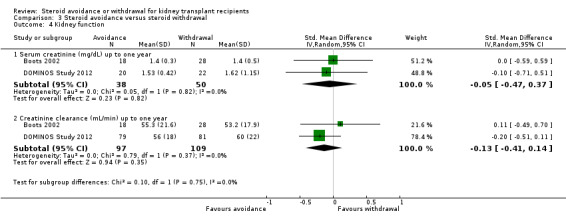
Comparison 3 Steroid avoidance versus steroid withdrawal, Outcome 4 Kidney function.
Studies in children with kidney transplant recipients
Steroid withdrawal versus steroid maintenance
We identified only two studies that investigated the effect of steroid withdrawal compared versus steroid maintenance in children (Benfield 2005; Höcker 2009). Death and graft loss at five years were significantly lower for children withdrawn from steroids, but these results were drawn from Benfield 2005 only, since neither death nor graft loss were observed in Höcker 2009 (Analysis 4.1.2: RR 0.16, 95% CI 0.02 to 1.35). The effect of steroid withdrawal on acute rejection is unclear due to the small number of studies and wide confidence intervals (Analysis 4.2). Kidney function was reported in Höcker 2009 only and was not significantly different between groups (Analysis 4.3).
4.1. Analysis.

Comparison 4 Steroid withdrawal versus maintenance in children, Outcome 1 Death and graft loss.
4.2. Analysis.

Comparison 4 Steroid withdrawal versus maintenance in children, Outcome 2 Rejection, malignancy.
4.3. Analysis.
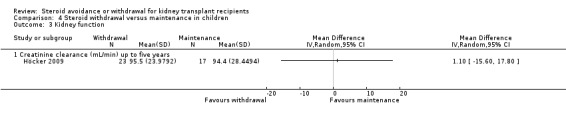
Comparison 4 Steroid withdrawal versus maintenance in children, Outcome 3 Kidney function.
Benfield 2005 was terminated early due to an unanticipated high incidence of post‐transplant lymphoproliferative disease. Of the 274 enrolled participants, 19 developed post‐transplant lymphoproliferative disease, 10 before randomisation. Sensitivity and subgroup analysis could not be performed due to the small number of studies identified.
Steroid avoidance versus steroid maintenance
Only Mericq 2013 investigated the effect of steroid avoidance compared versus steroid maintenance in children. Neither death nor graft loss was observed in this study, and due to sparse data, a difference in biopsy‐proven acute rejection could not be demonstrated. Kidney function was not reported. Sensitivity and subgroup analysis could not be performed on a single study.
Discussion
Summary of main results
The aim of this review was to provide updated evidence addressing the benefits and harms of steroid avoidance and withdrawal in kidney transplant recipients. All identified studies concerned one of the three comparisons defined for this review. The majority of studies compared steroid withdrawal versus steroid maintenance (24 adult studies, 2 studies in children). Steroid avoidance was compared versus steroid maintenance in 19 studies, one of which was conducted in child kidney transplantation. Of the three studies that compared steroid avoidance versus steroid withdrawal, none involved children. In adult kidney transplantation meta‐analysis could be carried out for all three comparisons, but data was particularly scarce for the comparison of steroid avoidance with steroid withdrawal. The low number of studies with child kidney transplant recipients did not enable data synthesis through meta‐analysis.
We were unable to demonstrate clear beneficial effects, such as a reduction in mortality or NODAT within five years after transplantation for steroids withdrawal or avoidance in adult kidney transplant recipients. Both steroid withdrawal and steroid avoidance showed little or no effect on mortality, graft loss, and CMV infection. The risk of acute rejection did significantly increase by 77% after steroid withdrawal and by 58% after steroid avoidance compared to steroid maintenance (see Table 1, Table 2).
The effect of steroid withdrawal in children is uncertain. The available data allowed only one meta‐analysis for acute rejection in children, which found no significant difference. Death and graft loss had not been observed in one of the two studies in children and outcomes such as biopsy‐proven acute rejection and malignancy were only reported in one of the two studies which further reduced the quantity of the available data. Only one study investigated the effect of steroid avoidance compared versus steroid maintenance in children, thus a meta‐analysis was not possible.
Overall completeness and applicability of evidence
An extensive literature review was performed to identify studies that assessed the benefits and harms of steroid withdrawal or avoidance in kidney transplant recipients. In general, two parameters are particularly relevant for assessing benefits and harms of steroid withdrawal in kidney transplant recipients: firstly, the time‐point of steroid withdrawal after kidney transplantation and secondly, the duration of follow‐up to observe outcome events in kidney transplant patients.
Steroids are withdrawn at various time points after kidney transplantation in clinical practice and this fact was reflected by the variety of time points used to investigate the effects of steroid withdrawal in clinical studies. We used a cut‐off of 14 days after transplantation to discriminate between steroid withdrawal and steroid avoidance. With this approach we were able to combine different time points for steroid withdrawal within these clinically relevant time frames. The majority of steroid avoidance studies used steroids for seven days or less, and the majority of the steroid withdrawal studies withdrew steroids between three to six months after transplantation. Thus, our findings may not be applicable for patients who are withdrawn from steroids at other time‐points after transplantation.
Most studies had between one and three years of follow‐up after either steroid avoidance or withdrawal which constitutes a major limitation for conclusions regarding long‐term consequences for patient and graft survival. Acute rejection is a major risk factor for reduced long‐term graft survival and typically occurs within the first year after transplantation. The impact of acute rejection on long‐term graft outcomes depends on the severity, recurrence and treatment of the acute rejection. While particularly severe and recurrent rejections increase the risk of graft loss, a single early acute rejection with complete functional recovery after treatment appears to be less harmful for long‐term graft outcomes. Most of the acute rejections reported in the included studies occurred early after transplantation and were mild and easily controlled with steroids which could be an argument to conclude that an increased risk of long‐term graft loss after steroid withdrawal is unlikely. However, recognizing that potential harms arising from steroid withdrawal may remain hidden for up to five years after steroid withdrawal (Gulanikar 1991); follow‐up periods of the included studies were too short to determine long‐term graft survival. Furthermore, it is important to stress that only half of the studies reported acute rejection. Consequently, potential harmful effects of steroid withdrawal on long‐term graft survival cannot be ruled out with this review with sufficient confidence.
Reporting of harmful events was especially limited and inconsistent. More than half of the studies did not report adverse events such as infection and CMV infection and less than a third of the studies reported malignancy and cardiovascular events. Even though we did not find evidence to suggest a difference in harmful events, it is important to point out that the absence of evidence does not mean there is evidence for absence of effect. It is unclear which outcomes occurred in the studies that provided no data. Although we believe this is the most comprehensive evidence summary on this topic, interpretation of our findings must consider the limitations of available data from this cohort. The value of increasing available evidence of potential harms associated with interventions has been widely recognised and is also not a problem peculiar to this review, but is common to many randomised studies and systematic reviews (Cuervo 2003; Tunis 2003).
Only one study investigating steroid avoidance included an mTOR‐inhibitor as baseline immunosuppression. Consequently, we cannot extrapolate the safety of steroid avoidance or withdrawal to protocols including mTOR‐inhibitors.
The inclusion and exclusion criteria for participation in the included studies may mean that our findings are not generalizable to all kidney transplant recipients. Eight studies did not specify any exclusion criteria, of which four did not specify any inclusion criteria. In three studies only recipients of a living kidney transplant were included and 11 studies included solely recipients of a cadaveric kidney transplant. Seventeen studies limited participation for patients who received their first kidney transplant and 16 studies excluded kidney transplant recipients who had experienced previous acute rejection. Kidney transplant recipients with a PRA > 50% were excluded in 13 studies. It is unclear whether the findings of this review apply to kidney transplant recipients with a higher immunologic transplant risk.
Although almost all studies included participants of a wide range of adult ages, none of the studies reported results for different age groups. Therefore we were unable to determine whether there is any difference in results depending on age. Due to the low number of studies in child kidney transplantation, our findings need to be interpreted with great caution in the light of a clear lack of evidence in children.
Quality of the evidence
The quality of the included studies was rather variable. The main limitations in the quality of the studies were allocation concealment, incomplete outcome data, blinding of participants and personnel and disclosure of funding. Of the 48 included studies only five studies blinded participants and personnel. This was considered a high risk of bias because clinical decision making could be influenced by knowledge of the treatment, such as for example that patients withdrawn from steroids were more closely monitored for signs of acute rejection. Adequate allocation concealment was reported in 14 studies and 19 studies demonstrated adequate sequence generation. The lack of adequate sequence generation and allocation concealment can lead to biased estimates of treatment effects in the original study and thus in a systematic review (Hollis 1999; Juni 1999; Moher 1998; Schulz 1995). All hard clinical outcomes (mortality, graft loss, acute rejection) were reported in 37 studies, but incomplete reporting of relevant data for a meta‐analysis in many studies hampered use of the provided data in our analysis. Comparison of kidney function was only possible in a limited number of studies because frequently either the number of participants in whom kidney function was measured or a measure of variability of the effect estimate were not provided. It might be more informative to compare the number of patients at risk of graft loss with a low creatinine clearance rather than assessing mean data. However, these data were not provided in any of the studies. Similarly dichotomous outcomes, especially infection and acute rejection were frequently reported as rates or episodes which complicated the use of such data for meta‐analysis. For disclosure of funding sources, 16 studies reported receiving of funding from pharmaceutical companies and 28 studies did not disclose their sponsor. We found that blinding of outcome assessors was adequate in 43 studies where the primary outcome were hard‐clinical endpoints (mortality, graft loss, acute rejection) and considered unlikely to be influenced by lack of blinding.
Potential biases in the review process
We searched multiple databases without language restriction in attempt to reduce publication bias. The Cochrane Kidney and Transplant's Specialised Register contains handsearched reports of studies presented at conferences and meetings, but there is a possibility that we missed unpublished data presented at smaller conferences or studies published in foreign language journals and low impact journals. Studies may have been added since our last search of the register. Not all included studies reported all outcomes which may have affected the results of the meta‐analysis.
Agreements and disagreements with other studies or reviews
Several previous systematic reviews have addressed steroid avoidance and withdrawal after kidney transplantation. The first review included three steroid withdrawal and four steroid avoidance studies in patients on CsA with or without AZA and showed a significant increase in acute rejection with an incidence of acute rejection of 48% in those withdrawn from steroids versus 30% in those maintained on steroids (P = 0.012) (Hricik 1993). The review published seven years later (Kasiske 2000) included 10 studies and showed an increased proportion of patients with acute rejection by 0.14 (95% CI 0.10 to 0.17; P < 0.001) and an increase in graft failure after steroid withdrawal by 40% (RR 1.40, 95% CI 1.09 to 1.70; P = 0.012). Most studies included in this meta‐analysis used CsA‐based immunosuppression with either no anti‐metabolite added or in combination with AZA. Only two studies with MMF were included and subgroup analysis showed similar results for these studies compared versus those that did not include MMF. A review of six studies of steroid withdrawal in kidney transplant recipients on triple therapy with calcineurin inhibitors and MMF showed an increase in acute rejection and no difference in graft failure (Pascual 2004). Due to the relative short follow‐up in these six studies long‐term consequences for graft survival given the observed increase in acute rejection after steroid withdrawal is unclear. A meta‐analysis published in 2012 by Knight 2010 found an increased risk of acute rejection and a reduced cardiovascular risk after steroid withdrawal or avoidance, but these findings resulted from a combined analysis of all steroid withdrawal or avoidance time points and were based on surrogate outcomes such as hypercholesterolaemia, hypertension and NODAT. Another review (Pascual 2012) with nine studies comparing steroid avoidance to steroid maintenance in kidney transplant recipients who received an immunosuppressive regimen consisting of antibody induction, either CsA or TAC and MMF reported that the increased risk of acute rejection in steroid avoidance was lost when patients received TAC‐based immunosuppression.
Authors' conclusions
Implications for practice.
Steroid avoidance and steroid withdrawal after kidney transplantation significantly increased the risk of acute rejection. We found no evidence to suggest a difference in patient and graft survival up to five years after transplantation, but the data to support the absence of harm is limited due to the low number of events observed in rather small studies. Follow‐up periods were too short to draw any conclusions on long‐term outcomes in kidney transplant recipients after steroid withdrawal or avoidance. In child kidney transplant recipients data is very limited and does not allow any conclusions about steroid withdrawal, but caution is warranted with induction treatment that may increase the risk of post‐transplant lymphoproliferative disease in children.
Implications for research.
Proving that steroid avoidance or withdrawal after kidney transplantation is safe and beneficial requires demonstration of beneficial effects, such as a reduction in patient mortality or cardiovascular events while at the same time graft survival is not reduced in the long‐term. Until now, only short‐term data exist that demonstrate an increased risk of acute rejection and the absence of evidence of harm, but there is no long‐term data to draw any conclusions about the harms and benefits of steroid avoidance or withdrawal beyond five years after transplantation. Long‐term RCTs are needed to determine whether steroid withdrawal and avoidance after kidney transplantation are safe and beneficial. Child kidney transplant recipients constitute a target population in a clear need of well‐conducted steroid withdrawal studies.
What's new
| Date | Event | Description |
|---|---|---|
| 15 February 2016 | New search has been performed | New studies included (20) |
| 15 February 2016 | New citation required and conclusions have changed | New studies added; paediatric studies included |
History
Protocol first published: Issue 1, 2006 Review first published: Issue 1, 2009
| Date | Event | Description |
|---|---|---|
| 14 July 2008 | Amended | Converted to new review format. |
Acknowledgements
The authors would like to acknowledge Gail Higgins, Information Specialist for Cochrane Kidney and Transplant, for performing the literature searches.
Appendices
Appendix 1. Electronic search strategies
| Database | Search terms |
| CENTRAL | 1. Kidney Transplantation [MESH] 2. kidney transplant* 3. 1 or 2 4. (avoid* or minim* or free* or withdraw* or spar* or discontinu* or taper* or conversion* or convert*) near25 (predniso* or corticosteroid* or steroid*) 5. 3 and 4 |
| MEDLINE | 1. Kidney Transplantation/ 2. ((avoid$ or minim$ or free$ or withdraw$ or spar$ or discontinu$ or taper$ or conversion$ or convert$) adj25 (predniso$ or corticosteroid$ or steroid$)).tw. 3. and/1‐2 |
| EMBASE | 1. exp kidney transplantation/ 2. Drug Withdrawal/ 3. ((avoid$ or minim$ or free$ or withdraw$ or spar$ or discontinu$ or taper$ or conversion$ or convert$) adj25 (predniso$ or corticosteroid$ or steroid$)).tw. 4. or/2‐3 5. and/1,4 |
Appendix 2. Risk of bias assessment tool
| Potential source of bias | Assessment criteria |
|
Random sequence generation Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence |
Low risk of bias: Random number table; computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots; minimization (minimization may be implemented without a random element, and this is considered to be equivalent to being random). |
| High risk of bias: Sequence generated by odd or even date of birth; date (or day) of admission; sequence generated by hospital or clinic record number; allocation by judgement of the clinician; by preference of the participant; based on the results of a laboratory test or a series of tests; by availability of the intervention. | |
| Unclear: Insufficient information about the sequence generation process to permit judgement. | |
|
Allocation concealment Selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment |
Low risk of bias: Randomisation method described that would not allow investigator/participant to know or influence intervention group before eligible participant entered in the study (e.g. central allocation, including telephone, web‐based, and pharmacy‐controlled, randomisation; sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes). |
| High risk of bias: Using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or non‐opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure. | |
| Unclear: Randomisation stated but no information on method used is available. | |
|
Blinding of participants and personnel Performance bias due to knowledge of the allocated interventions by participants and personnel during the study |
Low risk of bias: No blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding; blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding; blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Blinding of outcome assessment Detection bias due to knowledge of the allocated interventions by outcome assessors. |
Low risk of bias: No blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding; blinding of outcome assessment ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding; blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Incomplete outcome data Attrition bias due to amount, nature or handling of incomplete outcome data. |
Low risk of bias: No missing outcome data; reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias); missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size; missing data have been imputed using appropriate methods. |
| High risk of bias: Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size; ‘as‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation; potentially inappropriate application of simple imputation. | |
| Unclear: Insufficient information to permit judgement | |
|
Selective reporting Reporting bias due to selective outcome reporting |
Low risk of bias: The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way; the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon). |
| High risk of bias: Not all of the study’s pre‐specified primary outcomes have been reported; one or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not pre‐specified; one or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); one or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis; the study report fails to include results for a key outcome that would be expected to have been reported for such a study. | |
| Unclear: Insufficient information to permit judgement | |
|
Other bias Bias due to problems not covered elsewhere in the table |
Low risk of bias: The study appears to be free of other sources of bias. |
| High risk of bias: Had a potential source of bias related to the specific study design used; stopped early due to some data‐dependent process (including a formal‐stopping rule); had extreme baseline imbalance; has been claimed to have been fraudulent; had some other problem. | |
| Unclear: Insufficient information to assess whether an important risk of bias exists; insufficient rationale or evidence that an identified problem will introduce bias. |
Data and analyses
Comparison 1. Steroid withdrawal versus steroid maintenance.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death and graft loss | 15 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Death up to one year | 10 | 1913 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.36, 1.30] |
| 1.2 Death one to five years | 7 | 1118 | Risk Ratio (M‐H, Random, 95% CI) | 1.26 [0.73, 2.17] |
| 1.3 Graft loss including death up to one year | 8 | 1817 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.64, 1.49] |
| 1.4 Graft loss including death one to five years | 7 | 1092 | Risk Ratio (M‐H, Random, 95% CI) | 1.41 [1.00, 2.01] |
| 1.5 Graft loss excluding death up to one year | 8 | 1817 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.72, 1.92] |
| 1.6 Graft loss excluding death one to five years | 7 | 1092 | Risk Ratio (M‐H, Random, 95% CI) | 1.61 [0.98, 2.64] |
| 2 Rejection | 11 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Acute rejection up to one year | 10 | 1913 | Risk Ratio (M‐H, Random, 95% CI) | 1.77 [1.20, 2.61] |
| 2.2 Biopsy‐proven acute rejection up to one year | 5 | 1292 | Risk Ratio (M‐H, Random, 95% CI) | 1.32 [0.78, 2.22] |
| 3 New‐onset diabetes after transplantation and cardiovascular events | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 New onset diabetes after transplantation up to five years | 6 | 1439 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.49, 1.21] |
| 3.2 Cardiovascular events up to five years | 2 | 607 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.42, 2.33] |
| 4 Infection and malignancy | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Infection (all) up to five years | 5 | 1819 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.84, 1.22] |
| 4.2 CMV infection up to five years | 5 | 1758 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.80, 1.36] |
| 4.3 Malignancy up to five years | 3 | 756 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.41, 1.46] |
| 5 Kidney function | 8 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 Serum creatinine (mg/dL) up to one year | 4 | 644 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.21, 0.13] |
| 5.2 Serum creatinine (mg/dL) one to five years | 5 | 762 | Std. Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.06, 0.23] |
| 5.3 Creatinine clearance (mL/min) up to one year | 2 | 215 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.35, 0.21] |
| 5.4 Creatinine clearance (mL/min) one to five years | 3 | 669 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.56, 0.13] |
Comparison 2. Steroid avoidance versus steroid maintenance.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death and graft loss | 13 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Death up to one year | 10 | 1462 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.52, 1.80] |
| 1.2 Death one to five years | 7 | 1201 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.32, 1.01] |
| 1.3 Graft loss including death up to one year | 7 | 1211 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.72, 1.62] |
| 1.4 Graft loss including death one to five years | 7 | 1245 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.53, 1.18] |
| 1.5 Graft loss excluding death up to one year | 7 | 1211 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.64, 1.86] |
| 1.6 Graft loss excluding death one to five years | 7 | 1245 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.66, 1.45] |
| 2 Rejection | 9 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Acute rejection up to one year | 7 | 835 | Risk Ratio (M‐H, Random, 95% CI) | 1.58 [1.08, 2.30] |
| 2.2 Biopsy‐proven acute rejection up to one year | 6 | 1073 | Risk Ratio (M‐H, Random, 95% CI) | 1.94 [1.26, 2.98] |
| 3 New‐onset diabetes after transplantation and cardiovascular events | 9 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 New onset diabetes after transplantation up to five years | 9 | 1618 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.51, 1.10] |
| 3.2 Cardiovascular events up to five years | 4 | 1013 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.30, 1.05] |
| 4 Infection and malignancy | 11 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Infection (all) up to five years | 9 | 1833 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.84, 1.03] |
| 4.2 CMV Infection up to five years | 6 | 1454 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.70, 1.31] |
| 4.3 Malignancy up to five years | 7 | 1635 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.61, 1.52] |
| 5 Kidney function | 10 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 Serum creatinine (mg/dL) up to one year | 5 | 735 | Std. Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.12, 0.17] |
| 5.2 Serum creatinine (mg/dL) one to five years | 3 | 688 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.16, 0.14] |
| 5.3 Creatinine clearance (mL/min) up to one year | 6 | 1104 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.23, 0.08] |
| 5.4 Creatinine clearance (mL/min) one to five years | 3 | 563 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.25, 0.08] |
Comparison 3. Steroid avoidance versus steroid withdrawal.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death and graft loss | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Death up to one year | 1 | 222 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.08, 1.98] |
| 1.2 Death one to five years | 2 | 152 | Risk Ratio (M‐H, Random, 95% CI) | 2.67 [0.63, 11.32] |
| 1.3 Graft loss including death up to one year | 1 | 222 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.32, 2.29] |
| 1.4 Graft loss including death one to five years | 2 | 152 | Risk Ratio (M‐H, Random, 95% CI) | 2.44 [0.89, 6.70] |
| 1.5 Graft loss excluding death up to one year | 1 | 222 | Risk Ratio (M‐H, Random, 95% CI) | 1.64 [0.40, 6.68] |
| 1.6 Graft loss excluding death one to five years | 2 | 152 | Risk Ratio (M‐H, Random, 95% CI) | 1.91 [0.48, 7.67] |
| 2 Rejection | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.1 Acute rejection up to one year | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Biopsy‐proven acute rejection up to one year | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 New‐onset diabetes after transplantation, infection, malignancy | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 New onset diabetes after transplantation up to five years | 3 | 351 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.36, 1.09] |
| 3.2 Infection (all) up to five years | 3 | 374 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.76, 1.50] |
| 3.3 CMV Infection up to five years | 2 | 284 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.30, 0.92] |
| 3.4 Malignancy up to five years | 1 | 90 | Risk Ratio (M‐H, Random, 95% CI) | 1.57 [0.28, 8.94] |
| 4 Kidney function | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Serum creatinine (mg/dL) up to one year | 2 | 88 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.47, 0.37] |
| 4.2 Creatinine clearance (mL/min) up to one year | 2 | 206 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.41, 0.14] |
Comparison 4. Steroid withdrawal versus maintenance in children.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death and graft loss | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Death up to five years | 2 | 174 | Risk Ratio (M‐H, Random, 95% CI) | 0.16 [0.02, 1.35] |
| 1.2 Graft loss including death up to five years | 2 | 174 | Risk Ratio (M‐H, Random, 95% CI) | 0.09 [0.01, 0.69] |
| 1.3 Graft loss excluding death up to five years | 2 | 174 | Risk Ratio (M‐H, Random, 95% CI) | 0.09 [0.00, 1.64] |
| 2 Rejection, malignancy | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Acute rejection up to one year | 2 | 174 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.13, 1.02] |
| 2.2 Biopsy‐proven acute rejection up to one year | 1 | 42 | Risk Ratio (M‐H, Random, 95% CI) | 0.17 [0.01, 3.27] |
| 2.3 Malignancy (PTLD) up to five years | 1 | 132 | Risk Ratio (M‐H, Random, 95% CI) | 1.89 [0.51, 6.98] |
| 3 Kidney function | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1 Creatinine clearance (mL/min) up to five years | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 5. Publication bias.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Funnel plots | 20 | 5288 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.36 [1.15, 1.62] |
| 1.1 Death, steroid withdrawal versus maintenance | 10 | 1913 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.39, 1.29] |
| 1.2 Acute rejection steroid withdrawal versus maintenance | 10 | 1913 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.55 [1.28, 1.87] |
| 1.3 Death, steroid avoidance versus maintenance | 10 | 1462 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.52, 1.63] |
5.1. Analysis.
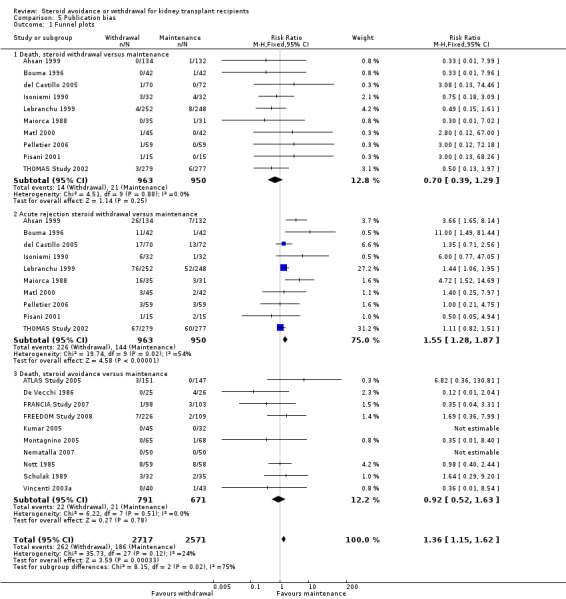
Comparison 5 Publication bias, Outcome 1 Funnel plots.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ahsan 1999.
| Methods |
|
|
| Participants |
|
|
| Interventions | Withdrawal group
Maintenance group
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'Randomization was stratified by centre and was done centrally to maintain a 1:1 ratio at each centre' |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind placebo controlled. Stated 'After randomisation, recipients received blister packs containing tablets for their 'prednisone' dose. Neither recipients nor physicians knew whether a randomised patient was in the withdrawal group' |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind placebo controlled. Stated 'After randomisation, recipients received blister packs containing tablets for their 'prednisone' dose. Neither recipients nor physicians knew whether a randomised patient was in the withdrawal group' |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind placebo controlled. Outcomes are objective hard endpoints |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis performed; all participants were followed for the primary endpoint until study closure on 22 July 1998 |
| Selective reporting (reporting bias) | Low risk | Primary outcomes for this review reported |
| Other bias | High risk | The study was supported by Roche Laboratories |
Albert 1985.
| Methods |
|
|
| Participants |
|
|
| Interventions | Avoidance group
Withdrawal group
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated 'randomised' but no further information provided |
| Allocation concealment (selection bias) | Unclear risk | not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes are objective hard endpoints |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear whether ITT analysis performed, total number of patients by group analysed not reported, results presented as percentages/rates |
| Selective reporting (reporting bias) | High risk | Acute rejection not reported |
| Other bias | Unclear risk | Funding sources not reported |
Aswad 1998.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Stated 'randomly assigned' but no further information provided |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes are objective hard endpoints |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number of patients by group not reported for outcomes; unclear if ITT analysis performed |
| Selective reporting (reporting bias) | Low risk | Primary outcomes for this review reported |
| Other bias | Unclear risk | Funding source not reported Abstract‐only publication |
ATLAS Study 2005.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stated 'Randomization was performed with a 1:1 ratio stratified by centre. The randomization list was generated by the Data Operation Department of Fujisawa GmbH. Each centre received a unique sequence of patient numbers and a set of sealed envelopes.' |
| Allocation concealment (selection bias) | Low risk | Stated 'sealed envelopes' |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes are objective hard endpoints |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis performed; all patients followed up or accounted for |
| Selective reporting (reporting bias) | Low risk | Primary outcomes for this review have been reported |
| Other bias | High risk | Sponsored by a grant from Fujisawa GmbH The investigator‐initiated 1‐year follow‐up was supported by an unrestricted grant from Astellas, Munich, Germany |
Benfield 2005.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stated 'centrally randomised' but no further information provided. |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Stated 'in a placebo controlled double‐blinded fashion' but no further information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Stated 'in a placebo controlled double‐blinded fashion' but no further information provided |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Stated 'in a placebo controlled double‐blinded fashion' but no further information provided. Outcomes are objective hard endpoints |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Total number of patients by group not reported for outcomes; ITT analysis performed |
| Selective reporting (reporting bias) | Low risk | Primary outcomes for this review have been reported |
| Other bias | High risk | High drop‐out rate before randomisation (52%) Choice of calcineurin inhibitor was centre specific (TAC or CsA) Support provided by NIH UO1‐A1‐46135 and Wyeth Pharmaceuticals The study was terminated early due to an unanticipated high incidence of PTLD |
Boletis 2001.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated 'randomly assigned' but no further information provided |
| Allocation concealment (selection bias) | Unclear risk | not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes are objective hard endpoints |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Number of patients in whom the outcome were measured is ambiguous (two reports with different number of patients in each group); 14% failed to comply with follow‐up protocol; unclear if ITT analysis performed |
| Selective reporting (reporting bias) | High risk | Death and graft loss are only reported in one of the two published reports, but number of participants in each group vary between reports |
| Other bias | Unclear risk | Unclear whether informative censoring is present, because the two published reports are different in regard to number of participants and time period of study Funding source not reported |
Boots 2002.
| Methods |
|
|
| Participants |
|
|
| Interventions | Avoidance group
Withdrawal group
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation was performed by opening a closed opaque numbered envelope |
| Allocation concealment (selection bias) | Low risk | Stated 'closed opaque envelopes' |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes are objective hard endpoints |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients followed up or accounted for; ITT analysis performed ('Analyses were made on an ITT basis.' |
| Selective reporting (reporting bias) | Low risk | Primary outcomes for this review have been reported |
| Other bias | Unclear risk | Funding source not reported |
Bouma 1996.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated 'randomised' but no further information provided |
| Allocation concealment (selection bias) | Unclear risk | not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes are objective hard endpoints |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis performed; all patients followed up or accounted for |
| Selective reporting (reporting bias) | Low risk | Primary outcomes for this review reported |
| Other bias | High risk | This study was supported by a grant from Sandoz, The Netherlands |
Burke 2000.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated 'all patients were randomised' but no further information provided. |
| Allocation concealment (selection bias) | Unclear risk | not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind placebo controlled, but partially unblinded for interim analysis |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind placebo controlled, but partially unblinded for interim analysis |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind placebo controlled, outcomes are objective hard endpoints |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 43% of patients were withdrawn from the study for various reasons; patients who died/lost their graft were excluded from the study; unclear if ITT analysis performed |
| Selective reporting (reporting bias) | High risk | Primary endpoints for this review not reported, primarily surrogate outcomes reported |
| Other bias | Unclear risk | Abstract data only available Funding source not reported |
De Vecchi 1986.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated 'randomly assigned' but no further information provided |
| Allocation concealment (selection bias) | Low risk | Stated 'assigned by sealed envelopes' |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes are objective hard endpoints |
| Incomplete outcome data (attrition bias) All outcomes | High risk | ITT analysis not performed; 6 patients in treatment group and 5 patients in control group excluded because of switch to different immunosuppression |
| Selective reporting (reporting bias) | Low risk | Primary outcomes for this review reported |
| Other bias | Low risk | Funded by grant of the Consiglio Nazionale delle Richerche |
del Castillo 2005.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated 'randomised' but no further information provided |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes are objective hard endpoints |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis performed; all patients followed‐up or accounted for |
| Selective reporting (reporting bias) | Low risk | Primary outcomes for this review reported |
| Other bias | Unclear risk | Funding source not reported; abstract data only |
DOMINOS Study 2012.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'Patients were randomised using a block size of 4 with no stratification by the contract research organization using a validated automated system.' |
| Allocation concealment (selection bias) | Low risk | 'With sealed envelopes distributed to the participating centers...opened after randomization by the investigator.' |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes are objective hard endpoints |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis performed, all patients followed up or accounted for |
| Selective reporting (reporting bias) | Low risk | Primary outcomes for this review reported |
| Other bias | High risk | The study was funded by Novartis Pharma SAS, Rueil‐Malmaison, France The manuscript was prepared with editorial support from a freelance medical writer funded by Novartis Pharma SAS |
EVIDENCE Study 2014.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | '...eligible patients were randomised 1:1 to 1 of the treatment arms. Randomization was stratified according to centre, recipient age at transplantation (<60 and 60 years) and creatinine clearance at month 3 (55 and >55 mL/min), according to a biased coin design.' |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | ITT analysis for primary analysis, but total number of patients by group for outcomes not reported |
| Selective reporting (reporting bias) | Low risk | Primary outcomes for this review reported |
| Other bias | High risk | Difference in CsA levels between groups (higher levels in treatment group) The study was sponsored by Novartis according to ClinicalTrials.gov. 'Editorial assistance was provided by Mary Hines, Springer Healthcare Communications, and funded by Novartis Farma, Italy.' |
Farmer 2006.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated 'randomised' but no further information provided. |
| Allocation concealment (selection bias) | Low risk | 'Using sealed envelopes'. |
| Blinding (performance bias and detection bias) All outcomes | High risk | 'Patients were informed to which arm of the trial they had been allocated.' |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "The patients randomised to the withdrawal group were followed with more frequent serum creatinine estimation." A rise in serum creatinine prompted kidney biopsy to detect biopsy proven acute cellular rejection which is the primary endpoint of this study |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Total number of patients by group for outcomes not reported. Number of patients who were lost to follow up is unclear |
| Selective reporting (reporting bias) | High risk | Patient and graft survival are not reported |
| Other bias | Unclear risk | Time lead bias, because follow up started with date steroids were completely withdrawn in treatment group but with randomisation for control group Funding source not reported |
FRANCIA Study 2007.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'Eligible patients were assigned to CS or non‐CS treatment at a 1:1 ratio using block randomization with stratification according to the recipient's age and cold ischaemia time.' |
| Allocation concealment (selection bias) | Low risk | 'Treatment codes were provided in sealed envelopes'. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes are objective hard endpoints. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis performed; 4 patients in control group excluded from analysis for acute rejection but included for patient and graft survival analysis. |
| Selective reporting (reporting bias) | Low risk | Primary outcomes for this review reported. |
| Other bias | High risk | TAC, SRL, EVL, AZA could be introduced according to centre practice Steroid dosing after 6 months according to centre practice, unclear whether patients were withdrawn from steroids or maintained on steroids Study was sponsored by the Nantes University Hospital Statistical analysis of study data was supported by Fresenius Biotech GmbH, Germany |
FREEDOM Study 2008.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Control group
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stated 'Randomization was undertaken in a 1:1:1 ratio using a validated system that automates the random assignment of treatment groups to randomization numbers.' |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes are objective hard endpoints |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis performed; all patients followed up or accounted for |
| Selective reporting (reporting bias) | Low risk | Primary endpoints for this review reported |
| Other bias | High risk | The study was funded by Novartis Pharma AG |
Gulanikar 1991.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Baseline Immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stated 'randomised blocks of various sizes were generated and used to attain a balanced, restricted randomization according to treatment centre. The order of randomization did not have a repeating sequence' |
| Allocation concealment (selection bias) | Low risk | Stated 'Physicians did not know the randomization number until the patient was enrolled, and the code was not broken until the analysis' |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Stated '...the code was not broken until the analysis. Patients were randomly assigned at 90 days to receive either a placebo or prednisone by means of a process that prevented prior knowledge of their treatment group' |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Stated 'The study was doubly blinded. The placebo and prednisone were prepared in an indistinguishable form and dispensed as coded therapy' |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blinded placebo controlled, outcomes are objective hard endpoints |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Stated 'No patients were excluded after entry (as distinct from withdrawals in the survival analysis) or lost to follow‐up.'; ITT analysis performed |
| Selective reporting (reporting bias) | High risk | Acute rejection not reported |
| Other bias | High risk | This work was supported by Sandoz Ltd., Basel, Switzerland, Sandoz Canada Inc., Dorval, Que., Upjohn Ltd., Kalamazoo, Mich., the Richard and Jean Ivey Fund, London, Ont., the Michael Fung Endowment Fund, London, Ont., the Claudine Keown Endowment Fund, London, Ont., the University Hospital Transplant Research Fund, London, Ont., Robarts Research Institute endowment funds and the City of London, Ont |
Höcker 2009.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stated 'central randomization by the principal investigator', stated 'block randomization stratified by pubertal status'. |
| Allocation concealment (selection bias) | Unclear risk | Stated 'concealed allocation' but not further information provided |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes are objective hard endpoints |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis performed; all patients followed up or accounted for |
| Selective reporting (reporting bias) | Low risk | Primary outcomes for this review reported |
| Other bias | Unclear risk | 'Because recruitment of patients for this study was more difficult than anticipated (because some patient's parents and covering physicians had a strong bias pro or con steroid withdrawal, we performed an interim analysis, which revealed a significant difference in growth between both groups. Hence, the study was finished prematurely.' Funding source not reported |
INFINITY Study 2013.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear if ITT analysis conducted |
| Selective reporting (reporting bias) | Low risk | Primary outcomes for this review reported |
| Other bias | High risk | Funding source not reported but authors disclose 'Grant/Research Support, Novartis (Myfortic)', Co‐authors affiliated with Novartis Pharma SAS, Rueil‐Malmaison, France Abstract data only Lack of important information regarding design and conduct of study |
Isoniemi 1990.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated 'randomised' but no further information provided. |
| Allocation concealment (selection bias) | Low risk | Stated 'using the sealed envelope method' |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes are objective hard endpoints |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis performed; all patients followed up or accounted for |
| Selective reporting (reporting bias) | Low risk | Primary outcomes for this review reported |
| Other bias | Low risk | 'The study was supported by a grant from the Sigrid Juselius Foundation.' AZA dose was increased during and after steroid withdrawal in treatment group while it remained unchanged in maintenance group |
Jankowska‐Gan 2009.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated 'randomised' but no further information provided |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes are objective hard endpoints |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear whether ITT analysis performed; number of patients by group not reported for outcomes |
| Selective reporting (reporting bias) | Low risk | Primary outcomes for this review reported |
| Other bias | Unclear risk | Funding source not reported Patients in treatment group were enrolled later after transplantation compared to control group |
Johnson 1989a.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'The recipient was entered into the trial by drawing a card to determine immunosuppressive therapy.' |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes are objective hard endpoints |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear if ITT analysis performed; total number of patients by group not reported for outcomes, results presented as rates and percentages |
| Selective reporting (reporting bias) | High risk | Acute rejection not reported |
| Other bias | Unclear risk | Funding sources not reported 465 patients included in first publication (1989), 700 patients included in second publication in (1990). Patients in third arm (AZA + steroids) remained equal in size, while the treatment group (steroid avoidance = CsA monotherapy) gained most of the additional patients, which was the group with the better outcomes in first publication. Immunosuppressive protocol differs between these two publications with lower CsA target levels and more steroids in 2nd publication. |
Kacar 2004.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated 'randomised' but no further information provided |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes are objective hard endpoints |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear whether ITT analysis performed; total number of patients by group for outcomes not reported |
| Selective reporting (reporting bias) | Low risk | Primary outcomes for this review reported |
| Other bias | Unclear risk | Funding source not reported Abstract‐only publication |
Kim 2002.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated 'randomised 1:1 ratio' but no further information provided |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes are objective hard endpoints |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | One patient lost to follow up in withdrawal group (8%), unlikely to affect results; unclear if ITT analysis performed |
| Selective reporting (reporting bias) | High risk | Graft loss not reported |
| Other bias | Unclear risk | Funding source not reported Abstract‐only publication |
Kumar 2005.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'Randomization was completed using the first generator plan from randomization.com.' |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes are objective hard endpoints |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis performed; all patients followed up or accounted for |
| Selective reporting (reporting bias) | Low risk | Primary outcomes for this review reported |
| Other bias | High risk | First 17 patients (38%) in withdrawal group received steroids until day 7 and two additional doses of basiliximab, the remaining 28 patients (62%) received steroids until day 2 and no additional basiliximab 'The study was funded internally by clinical revenue. The manuscript was support by an unrestricted educational grant from Novartis Pharm. Corp.' |
Laftavi 2005.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'Patients were randomised by a blinded nurse coordinator according to random numbers.' |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | 'A single pathologist who was blinded to the treatment arms, evaluated biopsy specimens for severity of rejection and fibrosis.' |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear whether ITT analysis performed. In treatment group 16 of 32 patients and in control group 14 of 28 patients completed 1 year follow‐up |
| Selective reporting (reporting bias) | High risk | Mortality and graft loss are not reported |
| Other bias | Unclear risk | Funding source not reported Unclear whether groups were similar at baseline, because 'steroid withdrawal patients were at greater risk for rejection, having a higher average number of HLA mismatches and a greater number of African American patients' |
Lebranchu 1999.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated 'Patients were randomly assigned to one of two treatment groups in a 1:1 ratio, with stratification by cadaveric/ living related donor transplant recipient and by type of cyclosporine' but random sequence generation unclear |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Stated 'Treatment continued in a blinded fashion for 6 months, after which the study was to be unblinded during a further 6 months, for a total study length of 1 year' |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Stated 'Treatment continued in a blinded fashion for 6 months, after which the study was to be unblinded during a further 6 months, for a total study length of 1 year' |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes are objective hard endpoints |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear whether ITT analysis performed; stated 'At 12 months 17% in the control group and 25% in the treatment group were prematurely withdrawn from the study' |
| Selective reporting (reporting bias) | Low risk | Primary outcomes for this review reported |
| Other bias | Unclear risk | Funding source not reported |
Maiorca 1988.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated' randomised' but no further information provided |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes are objective hard endpoints |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | All patients followed up or accounted for; unclear if ITT analysis performed |
| Selective reporting (reporting bias) | Low risk | Primary outcomes for this review reported |
| Other bias | Unclear risk | Funding source not reported |
Matl 2000.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Patients were randomised according to the month of birth |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes are objective hard endpoints |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients followed up or accounted for; ITT analysis performed |
| Selective reporting (reporting bias) | Low risk | Primary outcomes for this review reported |
| Other bias | Low risk | The study was supported by grant N°3631‐3 awarded by the Internal Grant Agency of the Ministry of Health of the Czech Republic |
Mericq 2013.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stated 'central randomization by the principle investigator'. |
| Allocation concealment (selection bias) | Low risk | Stated 'stratified treatment allocation on the basis of block randomization carried out by a statistician who was not participating in this study using numbered containers by a computerized statistical program'. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes are objective hard endpoints |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear whether ITT analysis performed; outcomes for prepubertal patients only reported. Number of events and per group not reported |
| Selective reporting (reporting bias) | Low risk | Primary outcomes for this review reported |
| Other bias | Low risk | This study was supported by Fondecyt 1080166 (National Fund for Scientific and Technological Development) |
Montagnino 2005.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centralised randomisation by a randomisation list, stratified within centres using an interactive voice‐response system |
| Allocation concealment (selection bias) | Low risk | 'The sequence was concealed until interventions were assigned.' |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes are objective hard endpoints |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients followed up or accounted for; ITT analysis performed ('All the analyses considered all the randomised patients, grouped originally by randomised treatment as per ITT concept.') |
| Selective reporting (reporting bias) | Low risk | Primary outcomes for this review reported |
| Other bias | High risk | Supported by grant from Novartis |
Nagib 2015.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | '...patients were randomised to receive...' but no further information provided. |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Unclear if ITT analysis performed; total number of patients by group for outcomes not reported |
| Selective reporting (reporting bias) | Low risk | Primary outcomes for this review reported |
| Other bias | Unclear risk | Funding source not reported |
Nematalla 2007.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | '100 similar closed opaque envelopes were made, each containing a slip of opaque paper with the type of maintenance immunosuppression. Therefore, 50 envelopes were with steroid and the rest were without. All envelopes were kept closed until the morning of the transplant day, when one envelope was selected for each patient'. |
| Allocation concealment (selection bias) | Low risk | 'Similar closed opaque envelopes, each containing a slip of opaque paper'. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes are objective hard endpoints |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear whether ITT analysis performed; number of patients in groups varies slightly between reports |
| Selective reporting (reporting bias) | Low risk | Primary outcomes for this review reported |
| Other bias | Unclear risk | Different protocol between groups for steroid dosing before withdrawal Funding source not reported |
Nott 1985.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation was achieved by drawing a card |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes are objective hard endpoints |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Total number of patients by group not reported for outcomes; ITT analysis performed |
| Selective reporting (reporting bias) | High risk | Acute rejection not reported |
| Other bias | Unclear risk | Immunosuppressive protocol differs between publications No patient characteristics shown, unclear whether the groups were similar at baseline Funding source not reported |
Park 1994.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated 'randomised' but no further information provided. |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes are objective hard endpoints |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number of patients in which the outcome was measured are not reported, survival only reported as rates; unclear if ITT analysis performed |
| Selective reporting (reporting bias) | Low risk | Primary outcomes for this review reported |
| Other bias | Unclear risk | Funding source not reported There's a substantial difference between number of participants in first published report (1994) (294) and second report (1998) (68) which is not explained |
Pelletier 2006.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated 'randomised' but no further information provided. |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes are objective hard endpoints |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear if ITT analysis performed |
| Selective reporting (reporting bias) | Low risk | Primary outcomes for this review reported |
| Other bias | Unclear risk | Funding source not reported Steroids have been withdrawn at different time points after transplantation and the time point of steroid withdrawal is unclear Different induction treatments used, 14% of patients did not receive any induction treatment |
Pisani 2001.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated 'randomised' but no further information provided. |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes are objective hard endpoints |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear whether ITT analysis was performed; number of patients per group and in total vary between reports |
| Selective reporting (reporting bias) | Low risk | Primary outcomes for this review reported |
| Other bias | Unclear risk | Funding source not reported Abstract‐only data Lack of important information regarding design and conduct of study |
Ponticelli 1997.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'Random assignments were made according to a randomization list balanced per centre through a telephone call to the coordinating centre.' |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes are objective hard endpoints |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis performed; all patients followed up or accounted for |
| Selective reporting (reporting bias) | Low risk | Primary outcomes for this review reported |
| Other bias | Unclear risk | Funding source not reported Sandoz Prodotti Farmaceutici SpA provided logistic support for the SIMTRE group meetings |
Ratcliffe 1993.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated 'randomised' but no further information provided. |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes are objective hard endpoints. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients followed up or accounted for; ITT analysis performed ("Unless otherwise stated, data were analysed with groups assigned on the basis of "intention‐to‐treat") |
| Selective reporting (reporting bias) | High risk | Acute rejection is not reported |
| Other bias | Unclear risk | Funding source not reported |
Sandrini 2009.
| Methods |
|
|
| Participants |
|
|
| Interventions | Avoidance group
Withdrawal group
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated 'randomised' but no further information provided |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label, primary study endpoint was the percentage of patients who could be successfully withdrawn from steroids at 1 and 4 years after transplantation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Stated ' The results were analyzed on an ITT basis' but patients were excluded from analysis due to protocol violation; reasons for loss to follow‐up not reported |
| Selective reporting (reporting bias) | Low risk | Primary outcomes for this review reported |
| Other bias | Unclear risk | Funding source not reported Lack of important information regarding design and conduct of study High percentage of protocol failure (38% in avoidance group and 33% in withdrawal group not withdrawn from steroids at 1 year after transplantation) |
Schulak 1989.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'Patients were randomised using a table of random numbers' |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes are objective hard endpoints |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients followed up or accounted for; ITT analysis performed |
| Selective reporting (reporting bias) | Low risk | Primary outcomes for this review reported |
| Other bias | Unclear risk | Groups at baseline were different regarding gender, race and causes of kidney failure with more females, less African‐Americans, more diabetics in the steroid avoidance group Funding source not reported |
Smak Gregoor 1999.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated 'Patients were randomly assigned to one of the three treatment groups in a 1:1:1 ratio, with stratification for cadaveric/living related transplant, for centre, and for the number of acute rejections during the first 6 mo after transplantation' but random sequence generation not reported |
| Allocation concealment (selection bias) | Low risk | Stated 'Randomization was carried out by opening a sealed envelope with the lowest available study number' |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes are objective hard endpoints |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis performed; all patients followed up or accounted for |
| Selective reporting (reporting bias) | Low risk | Primary outcomes for this review reported |
| Other bias | High risk | The study was supported by Roche Pharmaceuticals, Mijdrecht, the Netherlands |
Sola 2002.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Baseline Immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated 'randomised' but no further information provided |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes are objective hard endpoints |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number of events and number of patients analysed not reported; unclear if ITT analysis performed |
| Selective reporting (reporting bias) | Low risk | Primary outcomes for this review reported |
| Other bias | Unclear risk | Funding source not reported |
Stiller 1983.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'A computer‐derived randomised blocks of varying size was generated and noted in a series of opaque envelopes held by the research pharmacist at each participating centre.' |
| Allocation concealment (selection bias) | Low risk | Stated 'opaque envelopes' |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes are objective hard endpoints |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear whether ITT analysis performed and whether all patients have been followed up or accounted for |
| Selective reporting (reporting bias) | Low risk | Primary outcomes for this review reported |
| Other bias | High risk | The study was supported by Medical Research Council of Canada; Richard and Jean Ivey Fund, London, Ontario; Sandoz Ltd, Basel; the Micheal Fung Endowment Fund, London, Ontario; the University Hospital Transplant Research Fund, London, Ontario |
THOMAS Study 2002.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated 'Randomization (1:1:1) was stratified by centre and donor type' but random sequence generation not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Stated 'The investigators were blinded with respect to randomization until the month‐3 visit.' which is the time before start of the intervention, but thereafter investigators were unblinded, thus this is an open‐label study |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Stated 'The investigators were blinded with respect to randomization until the month‐3 visit.' which is the time before start of the intervention, but thereafter investigators were unblinded, thus this is an open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes are objective hard endpoints |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis performed; all patients followed‐up or accounted for |
| Selective reporting (reporting bias) | Low risk | Primary outcomes for this review reported |
| Other bias | High risk | Unclear whether the rather short follow‐up period allows sufficient time for endpoints to occur This study was supported by Fujisawa GmbH, Munich, Germany |
Vincenti 2003a.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated 'randomised' but no further information provided |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes are objective hard endpoints |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis performed; all patients followed up or accounted for |
| Selective reporting (reporting bias) | Low risk | Primary outcomes for this review reported |
| Other bias | High risk | The study was supported by Novartis Pharmaceuticals Corporation, East Hanover, NJ |
Woodle 2005.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stated 'Randomization was based on a permuted block design with block sizes of 6 within each clinical site. Randomization was performed using a central randomization service at the EMMES Corporation (Potomac, Md, US). Patients were randomised 1:1 stratified by race and donor type' |
| Allocation concealment (selection bias) | Low risk | Stated 'The EMMES Corporation generated the allocation sequence and maintained the allocation code. The randomization order did not have a repeating sequence, and the randomization code was not broken or revealed to patients/investigators until subjects completed study' |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Stated 'Patients received a blinded study drug beginning on posttransplant day 8' |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Stated 'Study subjects, investigators, study personnel, and those assessing outcomes remained blinded throughout 5‐year duration of the study, unless medical necessity to unblind occurred' |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Stated 'Study subjects, investigators, study personnel, and those assessing outcomes remained blinded throughout 5‐year duration of the study, unless medical necessity to unblind occurred' |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis performed; all patients followed up or accounted for |
| Selective reporting (reporting bias) | Low risk | Primary outcomes for this review reported |
| Other bias | Unclear risk | Funding source not reported |
Zhu 2008a.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Baseline Immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated 'randomised' but no further information provided |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes are objective hard endpoints |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number of patients and number of events per group not reported; unclear whether ITT analysis was performed. Number of patients lost to follow up not reported |
| Selective reporting (reporting bias) | High risk | Graft loss not reported |
| Other bias | Unclear risk | Funding source not reported It was not reported how many of the participants were randomised to either group, whether the timing of outcome assessment is similar in all groups, whether the groups were similar at baseline, whether co‐interventions were avoided or similar Important information on design and conduct of study not reported |
ALG ‐ anti‐lymphocyte globulin; ATG ‐ anti‐thymocyte globulin; AZA ‐ azathioprine; CMV ‐ cytomegalovirus; CNI ‐ calcineurin inhibitor; CrCl ‐ creatinine clearance; CsA ‐ cyclosporin; EC‐MPS ‐ enteric‐coated mycophenolate sodium; eGFR ‐ estimated glomerular filtration rate; EVL ‐ everolimus; GFR ‐ glomerular filtration rate; HBsAG ‐ hepatitis B surface antigen; HCT ‐ haematocrit; HIV ‐ human immunodeficiency virus; HLA ‐ human leukocyte antigen; HTLV‐1 ‐ human T‐lymphotropic virus type 1; IL‐2RA ‐ interleukin 2 receptor antagonist; ITT ‐ intention‐to‐treat analysis; IV ‐ intravenous; MMF ‐ mycophenolate mofetil; NODAT ‐ new‐onset diabetes post transplant; PO ‐ oral; PRA ‐ panel reactive antibodies; PTLD ‐ Post‐transplant lymphoproliferative disease; RCT ‐ randomised controlled trial; SCr ‐ serum creatinine; SD ‐ standard deviation; SRL ‐ sirolimus; TAC ‐ tacrolimus; WCC ‐ white cell count
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Alexander 2006 | Wrong co‐intervention |
| Anil Kumar 2005 | Wrong co‐intervention |
| Axelrod 2005 | Not RCT |
| Berney 2004 | Pancreatic islet transplantation |
| Birkeland 1998b | Not RCT |
| Birkeland 2002 | Pancreatic islet transplantation |
| Budde 2001 |
Wrong co‐intervention PLEASE ADD REASON FOR EXCLUSION |
| CAMPASIA Study 2005 | Wrong co‐intervention |
| CARMEN Study 2005 | Wrong co‐intervention |
| Citterio 2002 | Wrong co‐intervention |
| CORRETA Study 2008 | No steroid withdrawal or avoidance |
| Curtis 1982 | No steroid withdrawal or avoidance |
| Daniel 1985 | No steroid withdrawal or avoidance |
| De Backer 1992 | No steroid withdrawal or avoidance |
| de Sandes Freitas 2011 | Wrong co‐intervention |
| Delucchi 2006 | Difference in co‐intervention |
| ECSEL Study 2008 | Wrong co‐intervention |
| Hibbs 2010 | Not RCT |
| Hilbrands 1993 | Not RCT |
| Hodson 1989 | Not RCT |
| Hricik 1993a | Not RCT |
| Hricik 1993b | Not RCT |
| John 2005 | Not RCT |
| Juarez 2006 | Not steroid withdrawal or avoidance |
| Kim 2004 | Wrong co‐intervention |
| Kim 2005 | Not RCT |
| Lehmann 2004 | Pancreatic islet transplantation |
| Li 2011a | Not steroid withdrawal or avoidance |
| Morris 1982 | Not steroid withdrawal or avoidance |
| MYSS Study 2004 | Wrong co‐intervention |
| NCT00089947 | Wrong co‐intervention |
| Nori 2008 | Wrong co‐intervention |
| Paczek 2003a | Wrong co‐intervention |
| Papadakis 1982 | Not steroid withdrawal or avoidance |
| Reed 1991 | Wrong co‐intervention |
| Remport 2001 | Not steroid withdrawal or avoidance |
| Robertson 1980 | Not RCT |
| Sarwal 2012 | Wrong co‐intervention |
| SENIOR Study 2009 | Wrong co‐intervention |
| Shapiro 1993 | Wrong co‐intervention |
| Silverstein 2005 | Not RCT |
| SOCRATES Study 2014 | Not steroid withdrawal or avoidance |
| Tarantino 1991 | Wrong co‐intervention |
| Teplan 2003 | Not RCT |
| ter Meulen 2002 | Wrong co‐intervention |
| TRIMS Study 2010 | Wrong co‐intervention |
| TWIST Study 2010 | Wrong co‐intervention |
| Weimert 2008 | Wrong co‐intervention |
RCT ‐ randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
Newstead 1989.
| Methods | Unclear if this was a RCT |
| Participants | Kidney transplant recipients not further specified, unclear time frame, but before 1989 |
| Interventions | Steroid withdrawal versus steroid maintenance plus CsA |
| Outcomes | Serum creatinine and acute rejection |
| Notes | Abstract‐only data; unable to contact authors |
CsA ‐ cyclosporin; RCT ‐ randomised controlled trial
Differences between protocol and review
In the earlier version of this review (Pascual 2009), we did not specifically include CMV infection as an outcome. Recent publications include reporting of CMV infection as a specific outcome, and this has been translated to our review.
Since this review was last published (in 2009), the Cochrane risk of bias tool has been updated, and the current tool has been used in assessments for this update.
Contributions of authors
MCH: performed study selection, data extraction, risk of bias assessment, data entry, data analyses and wrote the manuscript.
AR: performed study selection, data extraction, risk of bias assessment, reviewed results and manuscript.
EVN: performed study selection, resolved disagreement, reviewed results and manuscript and provided senior methodological support.
JP: resolved disagreement, reviewed results and manuscript and provided senior expert support.
ACW: resolved disagreement, reviewed results and manuscript and provided senior expert support.
Sources of support
Internal sources
No sources of support supplied
External sources
-
ERBP‐Fellowship to assist guideline development process (Ghent University, Renal Division, Belgium), Belgium.
Dr Maria C. Haller and Dr Evi V. Nagler are ERBP research fellows. European Renal Best Practice (ERBP) is the official guidance issuing body of the European Renal Association – European Dialysis and Transplant Association (ERA‐EDTA)
Declarations of interest
MCH: none known
AR: none known
EVN: none known
JP: none known
ACW: none known
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Ahsan 1999 {published data only}
- Ahsan N, Hricik D, Matas A, Rose S, Tomlanovich S, Wilkinson A, et al. Prednisone withdrawal in kidney transplant recipients on cyclosporine and mycophenolate mofetil ‐ a prospective randomized study. Steroid Withdrawal Study Group. Transplantation 1999;68(12):1865‐74. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Matas A, Ewell M, Cooperative Clinical Trials in Adult Transplantation Group. Prednisone withdrawal in kidney transplant recipients on CSA/MMF ‐ a prospective randomized study [abstract no: 4]. Transplantation 1999;67(9):S543. [CENTRAL: CN‐00765998] [DOI] [PubMed] [Google Scholar]
Albert 1985 {published data only}
- Albert FW, Schmidt U. Cyclosporin A (Cy A) therapy with or without steroids in cadaveric kidney transplantation. A prospective randomized one‐center study [abstract]. Kidney International 1985;28(4):708. [CENTRAL: CN‐00550573] [Google Scholar]
- Albert FW, Schmidt U. Cyclosporine therapy with or without steroids in cadaveric kidney transplantation ‐ A prospective randomized one‐center study. Transplantation Proceedings 1985;17(6):2669‐70. [EMBASE: 1986055112] [Google Scholar]
Aswad 1998 {published data only}
- Aswad S, Zapanta R, Wu L, Bogaard T, Asai P, Khetan U, et al. Steroid withdrawal in living related kidney transplant patients receiving FK506 [abstract]. 35th Congress. European Renal Association. European Dialysis and Transplantation Association; 1998 Jun 6‐9; Rimini, Italy. 1998:394. [CENTRAL: CN‐00483058]
ATLAS Study 2005 {published data only}
- Klinger M, Vitko S, Salmela K, Wlodarczyk Z, Tyden G, ATLAS Study Group. Large, prospective study evaluating steroid‐free immunosuppression with tacrolimus/basiliximab and tacrolimus/MMF compared with tacrolimus/MMF/steroids in renal transplantation [abstract no: W748]. Nephrology Dialysis Transplantation 2003;18(Suppl 4):788‐9. [CENTRAL: CN‐00446121] [Google Scholar]
- Kramer BK, Klinger M, Salmela K, Wlodarczyk Z, Tyden G, Vitko S. Two steroid‐free immunosuppressive regimens (basiliximab/tacrolimus and tacrolimus/MMF) in comparison to tacrolimus/MMF/steroid therapy after renal transplantation [abstract no: F‐FC039]. Journal of the American Society of Nephrology 2003;14(Nov):9A. [CENTRAL: CN‐00583329] [Google Scholar]
- Kramer BK, Klinger M, Vitko S, Glyda M, Midtvedt K, Stefoni S, et al. Tacrolimus‐based, steroid‐free regimens in renal transplantation: 3‐year follow‐up of the ATLAS trial. Transplantation 2012;94(5):492‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kramer BK, Klinger M, Wlodarczyk Z, Ostrowski M, Midvedt K, Stefoni S, et al. Tacrolimus combined with two different corticosteroid‐free regimens compared with a standard triple regimen in renal transplantation: one year observational results. Clinical Transplantation 2010;24(1):E1‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kramer BK, Kruger B, Hoffmann U, Wlodarcyzk Z, Tyden G, Senatorski G, et al. 1‐year‐follow‐up of two steroid‐free immunosuppressive regimens ‐ basiliximab/tacrolimus and tacrolimus/MMF ‐ in comparison to tacrolimus/MMF/steroids after renal transplantation [abstract no: F‐PO1026]. Journal of the American Society of Nephrology 2004;15(Oct):289A. [CENTRAL: CN‐00688822] [Google Scholar]
- Kramer BK, Kruger B, Mack M, Obed A, Banas B, Paczek L, et al. Steroid withdrawal or steroid avoidance in renal transplant recipients: focus on tacrolimus‐based immunosuppressive regimens. Transplantation Proceedings 2005;37(4):1789‐91. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kramer BK, Margreiter R, Hoffmann U, Wlodarcyzk Z, Tyden G, Senatorski G, et al. 1‐year‐follow‐up of two steroid‐free immunosuppressive regimens ‐ basiliximab/tacrolimus and tacrolimus/MMF ‐ compared to tacrolimus/MMF/steroids after renal transplantation [abstract no: 216]. 3rd International Congress on Immunosuppression; 2004 Dec 8‐11; San Diego (CA). 2004.
- Vitko S, Klinger M, Salmela K, Wlodarczyk Z, Tyden G, Senatorski G, et al. Two corticosteroid‐free regimens‐tacrolimus monotherapy after basiliximab administration and tacrolimus/mycophenolate mofetil‐in comparison with a standard triple regimen in renal transplantation: results of the Atlas study. Transplantation 2005;80(12):1734‐41. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Vitko S, Klinger M, Salmela KW, Tyden G, ATLAS Study Group. Comparison of two steroid‐free regimens ‐ basiliximab/tacrolimus and tacrolimus/MMF ‐ with tacrolimus/MMF/steroid therapy after renal transplantation [abstract]. American Journal of Transplantation 2003;3(Suppl 5):312. [CENTRAL: CN‐00433656] [Google Scholar]
Benfield 2005 {published data only}
- Benfield MR, Bartosh S, Ikle D, Warshaw B, Bridges N, Morrison Y, et al. A randomized double‐blind, placebo controlled trial of steroid withdrawal after pediatric renal transplantation. American Journal of Transplantation 2010;10(1):81‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Benfield MR, Munoz R, Warshaw BL, Bartosh SM, Stablein DM, McIntosh MJ, et al. A randomized controlled double‐blind trial of steroid withdrawal in pediatric renal transplantation: a study of the Cooperative Clinical trials in pediatric transplantation (CCTPT) [abstract no: 966]. American Journal of Transplantation 2005;5(Suppl 11):402. [CENTRAL: CN‐00676069] [Google Scholar]
- McDonald RA, McIntosh M, Stablein D, Grimm P, Wyatt R, Lirenman D, et al. Increased incidence of PTLD in pediatric renal transplant recipients enrolled in a randomized controlled trial of steroid withdrawal: a study of the CCTPT [abstract no: 1028]. American Journal of Transplantation 2005;5(Suppl 11):418. [Google Scholar]
- McDonald RA, Smith JM, Ho M, Lindblad R, Ikle D, Grimm P, et al. Incidence of PTLD in pediatric renal transplant recipients receiving basiliximab, calcineurin inhibitor, sirolimus and steroids. American Journal of Transplantation 2008;8(5):984‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Boletis 2001 {published data only}
- Boletis JN, Konstadinidou I, Chelioti H, Theodoropoulou H, Avdikou K, Kostakis A, et al. Successful withdrawal of steroid after renal transplantation. Transplantation Proceedings 2001;33(1‐2):1231‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Boletis JN, Konstadinidou I, Chelioti H, Theodoropoulou H, Avdikou K, Kostakis A, et al. Successful withdrawal of steroids after renal transplantation [abstract]. XVIII International Congress of the Transplantation Society; 2000 Aug 27‐Sep 1; Rome, Italy. 2000. [CENTRAL: CN‐00444474]
- Boletis JN, Konstadinidou I, Darema M, Psimenou E, Chiras T, Kostakis A, et al. Steroids withdrawal in renal transplant recipients: a randomized controlled study [abstract no: T447]. Nephrology Dialysis Transplantation 2002;17(Suppl 1):312. [CENTRAL: CN‐00509095] [Google Scholar]
Boots 2002 {published data only}
- Boots JM, Christaiaans MH, Duijnhoven EM, Suylen RJ, Hooff JP. Early steroid withdrawal in renal transplant recipients with tacrolimus dual therapy [abstract]. XIXth International Congress of the Transplantation Society; 2002 Aug 25‐30; Miami (FL). 2002. [CENTRAL: CN‐00415308]
- Boots JM, Christiaans MH, Duijnhoven EM, Suylen RJ, Hooff JP. Early steroid withdrawal in renal transplantation with tacrolimus dual therapy: a pilot study. Transplantation 2002;74(12):1703‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Boots JM, Christiaans MH, Duijnhoven EM, Suylen R, Hooff JP. Early steroid withdrawal in renal transplant recipients with tacrolimus double therapy immunosuppression [abstract]. Journal of the American Society of Nephrology 2001;12(Program & Abstracts):878A. [CENTRAL: CN‐00550471] [Google Scholar]
- Boots JM, Christiaans MH, Duijnhoven EM, Suylen RJ, Hooff JP. Early steroid withdrawal in renal transplantation with tacrolimus dual therapy: a pilot study. Transplantation Proceedings 2002;34(5):1698‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bouma 1996 {published data only}
- Bouma GJ, Hollander DA, Meer‐Prins EM, Bree SP, Rood JJ, Woude FJ, et al. In vitro sensitivity to prednisolone may predict kidney rejection after steroid withdrawal. Transplantation 1996;62(10):1422‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Bouma GJ, Hollander DJ, Doxiadis IN, Meer‐Prins EM, Bree SP, Rood JJ, et al. In vitro study: prediction of graft rejection after withdrawal of steroids [In vitro‐untersuchungen zur voraussage von transplantatabstossungen nach steroid‐sbsetzung: eine pilot‐studie]. Transplantationsmedizin: Organ Der Deutschen Transplantationsgesellschaft 1996;8(2):79‐83. [EMBASE: 1996240201] [Google Scholar]
- Hollander AA, Hene RJ, Hermans J, Es LA, Woude FJ. Late prednisone withdrawal in cyclosporine‐treated kidney transplant patients: a randomized study. Journal of the American Society of Nephrology 1997;8(2):294‐301. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hollander AA, Hene RJ, Es LA, Woude FJ. Late prednisone withdrawal in renal transplant patients [abstract no: A3323]. Journal of the American Society of Nephrology 1996;7(9):1911. [DOI] [PubMed] [Google Scholar]
- Hollander AA, Hene RJ, Es LA, Woude FJ. Late prednisone withdrawal in renal transplant patients [abstract]. Nephrology Dialysis Transplantation 1996;11(6):A276. [CENTRAL: CN‐00261334] [Google Scholar]
Burke 2000 {published data only}
- Burke J, Francos BB, Francos GC. Double‐blind, placebo‐controlled trial of steroid withdrawal in kidney transplant recipients with a cyclosporine/mycophenolate regimen‐three year follow up [abstract]. American Journal of Transplantation 2001;1(Suppl 1):296. [CENTRAL: CN‐00764555] [Google Scholar]
- Burke JF, Francos GC, Francos BB, Michael B, Gaughan WJ. A double‐blind, placebo‐controlled, three‐year study of steroid withdrawal using a neoral‐based immunosuppressive regimen in primary renal transplant recipients: an interim report [abstract no: 426]. Transplantation 2000;69(8 Suppl):S224. [CENTRAL: CN‐00444589] [Google Scholar]
- Francos GC, Frankel CJ, Dunn SR, Francos BB, Burke JF. Double‐blind, placebo‐controlled, 3 year study of steroid withdrawal using a neoral and mycophenolate mofetil (MMF)‐based immunosuppressive regimen in primary renal transplant recipients [abstract no: 137]. American Journal of Transplantation 2002;2(Suppl 3):172. [CENTRAL: CN‐00415671] [Google Scholar]
del Castillo 2005 {published data only}
- Castillo D, Franco A, Tabernero JM, Errasti P, Valdes F, García C, et al. Prospective, multicenter, randomized, open‐label study of myfortic with steroid withdrawal vs myfortic with standard steroid regimen to prevent acute rejection in de novo kidney transplantation [abstract no: 136]. American Journal of Transplantation 2005;5(Suppl 11):191. [CENTRAL: CN‐00644130] [Google Scholar]
De Vecchi 1986 {published data only}
- Vecchi A, Tarantino A, Montagnino G, Aroldi A, Aniasi A, Vegeto A, et al. Ciclosporin alone or combined with steroid in the treatment of cadaveric renal transplant recipients. Clinical Transplantation 1987;1:198‐202. [CENTRAL: CN‐00764108] [Google Scholar]
- Vecchi A, Tarantino A, Rivolta E, Egidi F, Montagnino G, Berardinelli L, et al. Ciclosporin alone or associated with steroid for immunosuppression of cadaveric renal transplants?. Contributions to Nephrology 1986;51:88‐90. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Vecchi A, Tarantino A, Rivoltan E, Egidi F, Ponticelli C. Need for steroid in cyclosporine (Cy) treated cadaveric renal transplant recipients (pts) [abstract]. Kidney International 1985;28(2):394. [CENTRAL: CN‐00550392] [Google Scholar]
DOMINOS Study 2012 {published data only}
- Thierry A, Mourad G, Buchler M, Kamar N, Villemain F, Heng AE, et al. Steroid avoidance with early intensified dosing of enteric‐coated mycophenolate sodium: a randomized multicentre trial in kidney transplant recipients. Nephrology Dialysis Transplantation 2012;27(9):3651‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Touchard G, Mourad G, Lebranchu Y, Rostaing L, Villemain F. Multicenter, randomized, comparative, open‐label study to evaluate efficacy and safety a combination of anti‐IL2R, intensified dose of enteric‐coated mycophenolate sodium (EC‐MPS) for 6 weeks, ciclosporine micro‐emulsion (CsA‐ME), with or without steroids, in adult kidney de novo transplant recipients (TxR) [abstract no: 1679]. American Journal of Transplantation 2010;10(Suppl 4):515. [Google Scholar]
- Touchard G, Mourad G, Lebranchu Y, Rostaing L, Villemain F, Heng AE, et al. Intensified dose of enteric‐coated mycophenolate sodium (EC‐MPS) for steroids avoidance, in combination with ciclosporine micro‐emulsion (CsA‐ME): multicenter, randomized, open label, comparative study in de novo kidney transplantation (DOMINOS) [abstract no: P‐557]. Transplant International 2009;22(Suppl 2):232‐3. [Google Scholar]
EVIDENCE Study 2014 {published data only}
- Carmellini M, Todeschini P, Manzia TM, Valerio F, Messina M, Sghirlanzoni MC, et al. Twelve‐month outcomes from EVIDENCE trial (everolimus once‐a‐day regimen with cyclosporine versus corticosteroid elimination) in adult kidney transplant recipients [abstract no: O193]. Transplant International 2013;26(Suppl 2):100. [EMBASE: 71359334] [Google Scholar]
- Ponticelli C, Carmellini M, Tisone G, Sandrini S, Segoloni G, Rigotti P, et al. A randomized trial of everolimus and low‐dose cyclosporine in renal transplantation: with or without steroids?. Transplantation Proceedings 2014;46(10):3375‐82. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Farmer 2006 {published data only}
- Farmer C, Abbs I, Hilton R, et al. What is the value of short synacthen tests in predicting the ease of steroid withdrawal in renal transplant recipients? A randomised controlled trial [abstract]. American Journal of Transplantation 2001;1(Suppl 1):190. [CENTRAL: CN‐00767027] [Google Scholar]
- Farmer CK, Hampson G, Abbs IC, Hilton RM, Koffman CG, Fogelman I, et al. Late low‐dose steroid withdrawal in renal transplant recipients increases bone formation and bone mineral density. American Journal of Transplantation 2006;6(12):2929‐36. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
FRANCIA Study 2007 {published data only}
- Albano L, Cantarovich D, Rostaing L, Kamar N, Ducloux D, Mourad G, et al. Corticosteroid avoidance in adult kidney transplant recipients receiving ATG Fresenius induction: 5 years results of a prospective and randomized study [abstract no: P497]. Transplant International 2013;26(Suppl 2):285. [EMBASE: 71360109] [Google Scholar]
- Cantarovich D. Steroid avoidance in adult kidney transplant recipients: 5‐year results of a prospective and randomized multicenter study [abstract no: 234]. American Journal of Transplantation 2013;13(Suppl S5):101‐2. [EMBASE: 71056810] [Google Scholar]
- Cantarovich D, FRANCIA French Study Group. ATG‐Fresenius induction and immunosuppression without steroids following renal transplantation: a prospective and randomized study [abstract no: P189]. Transplant International 2007;20(Suppl 2):141. [CENTRAL: CN‐00740578] [Google Scholar]
- Cantarovich D, FRANCIA Study Group. Acute renal rejections are increased in the absence of corticosteroids but are not detrimental at one year in the context of ATG induction [abstract no: 515]. American Journal of Transplantation 2010;10(Suppl 4):191. [EMBASE: 70463876] [Google Scholar]
- Cantarovich D, Rostaing L, Kamar N, Ducloux D, Saint‐Hillier Y, Mourad G, et al. Early corticosteroid avoidance in kidney transplant recipients receiving ATG‐F induction: 5‐year actual results of a prospective and randomized study. American Journal of Transplantation 2014;14(11):2556‐64. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Cantarovich D, Rostaing L, Kamar N, Saint‐Hillier Y, Ducloux D, Mourad G, et al. Corticosteroid avoidance in adult kidney transplant recipients under rabbit anti‐T‐lymphocyte globulin, mycophenolate mofetil and delayed cyclosporine microemulsion introduction. Transplant International 2010;23(3):313‐24. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Cantarovich D, Rostaing L, Mourad G. Steroid avoidance after renal transplantation: results of a prospective and randomized trial using Fresenius ATG [abstract no: 711]. Transplantation 2008;86(2 Suppl):249. [CENTRAL: CN‐00740579] [Google Scholar]
- Louis S, Audrain M, Cantarovich D, Schaffrath B, Hofmann K, Janssen U, et al. Long‐term cell monitoring of kidney recipients after an antilymphocyte globulin induction with and without steroids. Transplantation 2007;83(6):712‐21. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
FREEDOM Study 2008 {published data only}
- Chadban S, Walker R, Russ G, Kanellis J. Renal functions and rejection incidence in de novo renal transplant patients randomised to steroid avoidance, steroid withdrawal or standard steroids [abstract]. Immunology & Cell Biology 2007;85(4):A24. [Google Scholar]
- Schena FP, Vincenti F, Paraskevas S, Hauser I, FREEDOM Study Group. Renal function and rejection incidence in de novo renal transplant patients randomized to steroid avoidance, steroid withdrawal or standard steroids [abstract no: F‐FC153]. Journal of the American Society of Nephrology 2006;17(Abstracts):69A. [CENTRAL: CN‐00601969] [Google Scholar]
- Schena FP, Vincenti F, Paraskevas S, Hauser I, Grinyo J, FREEDOM Study Group. 12‐month results of a prospective, randomized trial of steroid avoidance, steroid withdrawal or standard steroids in de novo renal transplant patients receiving cyclosporine, enteric‐coated mycophenolate sodium (EC‐MPS, myfortic®) and basiliximab [abstract no: 54]. American Journal of Transplantation 2006;6(Suppl 2):84‐5. [CENTRAL: CN‐00644263] [Google Scholar]
- Vincenti F, Schena F, Walker R, Pescovitz M, Shoker A. 3 months interim results of a 12‐month study with enteric‐coated mycophenolate sodium (EC‐MPS, Myfortic®), basiliximab, and neoral C‐2 comparing different steroid protocols in de novo kidney recipients [abstract no: TH‐PO544]. Journal of the American Society of Nephrology 2005;16(Oct):236A. [CENTRAL: CN‐00583476] [Google Scholar]
- Vincenti F, Schena FP, Paraskevas S, Hauser I, FREEDOM Study Group. Comparison of metabolic parameters in renal transplant patients randomized to steroid avoidance, steroid withdrawal or standard steroids with a 12‐month, randomized, multicenter trial [abstract no: F‐PO1076]. Journal of the American Society of Nephrology 2006;17(Abstracts):562A. [CENTRAL: CN‐00602097] [Google Scholar]
- Vincenti F, Schena FP, Paraskevas S, Hauser I, Grinyo J. Metabolic effects of steroid avoidance or early steroid withdrawal: 12‐month results of a randomized trial in de novo renal transplant patients receiving cyclosporine, enteric‐coated mycophenolate sodium (EC‐MPS) and basiliximab [abstract no: 1232]. American Journal of Transplantation 2006;6(Suppl 2):483. [CENTRAL: CN‐00765654] [Google Scholar]
- Vincenti F, Schena FP, Paraskevas S, Hauser IA, Walker RG, Grinyo J, et al. A randomized, multicenter study of steroid avoidance, early steroid withdrawal or standard steroid therapy in kidney transplant recipients.[Erratum appears in Am J Transplant.2008 May;8(5):1080]. American Journal of Transplantation 2008;8(2):307‐16. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Vincenti F, Schena FP, Walker R, Pescovitz MD, Shoker A, Grinyo J, et al. Preliminary 3‐month results comparing immunosuppressive regimens of enteric‐coated mycophenolate sodium (EC‐MPS) without steroids vs short‐term use of steroids vs standard steroid treatment including basiliximab, and neoral C‐2 in de novo kidney recipients [abstract no: 1542]. American Journal of Transplantation 2005;5(Suppl 11):548. [CENTRAL: CN‐00644288] [Google Scholar]
- Walker R, Campbell S, Chadban S, Kanellis J, Pilmore H, Russ G. Preliminary results of a 12‐month study with enteric‐coated mycophenolate sodium (EC‐MPS), basiliximab, and neoral C‐2 comparing a regimen without steroids or short‐term use of steroids with standard steroid treatment in de novo kidney recipients [abstract no: 34]. Transplantation Society of Australia & New Zealand (TSANZ). 24th Annual Scientific Meeting; 2006 Mar 29‐31; Canberra, Australia. 2006:52. [CENTRAL: CN‐00583481]
- Walker R, Chadban S, Russ G, et al. Comparison of metabolic parameters in renal transplant patients randomised to steroid avoidance, steroid withdrawal or standard steroids within a 12 month randomised multicentre trial [abstract]. Transplantation Society of Australia & New Zealand (TSANZ). 25th Annual Scientific Meeting; 2007 Mar 28‐30; Canberra (ACT). 2007:32. [CENTRAL: CN‐00764330]
- Walker R, Vincenti F, Schena FP, Pescovitz MD, Shoker A, Grinyo J, et al. Preliminary results of a 12‐month study with enteric‐coated mycophenolate sodium (EC‐MPS), basiliximab, and neoral C‐2 comparing two investigational steroid regimens (without steroids or short‐term use of steroids) with standard steroid treatment in de novo kidney recipients [abstract no: T‐PO50027]. Nephrology 2005;10(Suppl):A214. [CENTRAL: CN‐00583480] [Google Scholar]
Gulanikar 1991 {published data only}
- Gulanikar AC, Belitsky P, MacDonald AS, Cohen A, Bitter‐Suermann H. Randomized controlled trial of steroids versus no steroids in stable cyclosporine‐treated renal graft recipients. Transplantation Proceedings 1991;23(1 Pt 2):990‐1. [MEDLINE: ] [PubMed] [Google Scholar]
- Sinclair NR. Low‐dose steroid therapy in cyclosporine‐treated renal transplant recipients with well‐functioning grafts. The Canadian Multicentre Transplant Study Group. CMAJ Canadian Medical Association Journal 1992;147(5):645‐57. [MEDLINE: ] [PMC free article] [PubMed] [Google Scholar]
Höcker 2009 {published data only}
- Hoecker B, Weber LT, Feneberg R, Drube J, John U, Fehrenbach H, et al. Prospective randomized trial on late steroid withdrawal in pediatric renal transplant recipients under CsA and MMF: 2‐year data [abstract no: 605]. American Journal of Transplantation 2009;9(Suppl 2):366. [EMBASE: 70010478] [DOI] [PubMed] [Google Scholar]
- Höcker B, Weber LT, Feneberg R, Drube J, John U, Fehrenbach H, et al. Improved growth and cardiovascular risk after late steroid withdrawal: 2‐year results of a prospective, randomised trial in paediatric renal transplantation. Nephrology Dialysis Transplantation 2010;25(2):617‐24. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Höcker B, Weber LT, Feneberg R, Drube J, John U, Fehrenbach H, et al. Prospective, randomized trial on late steroid withdrawal in pediatric renal transplant recipients under cyclosporine microemulsion and mycophenolate mofetil. Transplantation 2009;87(6):934‐41. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Tönshoff B, Weber L, Höcker B. Prospective randomized multicenter trial on withdrawal of steroids in pediatric renal transplant recipients with stable graft function on cyclosporin A (CsA) and mycophenolate mofetil (MMF) [abstract no: 240 (SY)]. Pediatric Nephrology 2007;22(9):1429. [Google Scholar]
- Weber LT, Hoecker B, Feneberg R, Drube J, John U, Fehrenbach H, et al. Late steroid withdrawal in pediatric renal transplant recipients under cyclosporin microemulsion and mycophenolate mofetil [abstract no: SA‐PO2535]. Journal of the American Society of Nephrology 2008;19(Abstracts Issue):679A. [Google Scholar]
- Weber LT, Hoecker B, Feneberg R, Drube J, John U, Fehrenbach H, et al. Prospective randomized trial on late steroid withdrawal in pediatric renal transplant recipients under CSA and MMF: 2‐year data [abstract no: SAT‐M‐124]. Pediatric Nephrology 2009;24(7):1876. [DOI] [PubMed] [Google Scholar]
INFINITY Study 2013 {published data only}
- Thierry A, Mourad G, Buchler M, Kamar N, Villemain F, Heng A, et al. Three‐year safety and efficacy outcomes in kidney transplant patients randomized to steroid avoidance or maintenance steroids with early intensified dosing of enteric‐coated mycophenolate sodium: The INFINITY Study [abstract no: B1099]. American Journal of Transplantation 2013;13(Suppl S5):358. [EMBASE: 71057675] [Google Scholar]
Isoniemi 1990 {published data only}
- Isoniemi H. Renal allograft immunosuppression V: Glucose intolerance occurring in different immunosuppressive treatments. Clinical Transplantation 1991;5(3):268‐72. [EMBASE: 1991187379] [Google Scholar]
- Isoniemi H. Renal allograft immunosuppression. III. Triple therapy versus three different combinations of double drug treatment: two year results in kidney transplant patients. Transplant International 1991;4(1):31‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Isoniemi H, Ahonen J, Eklund B, Hockerstedt K, Salmela K, Willebrand E, et al. Renal allograft immunosuppression. II. A randomized trial of withdrawal of one drug in triple drug immunosuppression. Transplant International 1990;3(3):121‐7. [MEDLINE: ] [PubMed] [Google Scholar]
- Isoniemi H, Ahonen J, Krogerus L, Eklund B, Hockerstedt K, Salmela K, et al. Chronic rejection of renal allografts with four immunosuppressive regimens. Transplantation Proceedings 1992;24(6):2716‐7. [MEDLINE: ] [PubMed] [Google Scholar]
- Isoniemi H, Eklund B, Hockerstedt K, Korsback C, Salmela K, Willebrand E, et al. Discontinuation of one drug in triple drug treatment of renal allograft patients: 1‐year results. Transplantation Proceedings 1990;22(4):1365‐6. [MEDLINE: ] [PubMed] [Google Scholar]
- Isoniemi H, Krogerus L, Willebrand E, Taskinen E, Gronhagen‐Riska C, Ahonen J, et al. Renal allograft immunosuppression. VI. Triple drug therapy versus immunosuppressive double drug combinations: histopathological findings in renal allografts. Transplant International 1991;4(3):151‐6. [MEDLINE: ] [PubMed] [Google Scholar]
- Isoniemi H, Tikkanen M, Hayry P, Eklund B, Hockerstedt K, Salmela K, et al. Lipid profiles with triple drug immunosuppressive therapy and with double drug combinations after renal transplantation and stable graft function. Transplantation Proceedings 1991;23(1 Pt 2):1029‐31. [MEDLINE: ] [PubMed] [Google Scholar]
- Isoniemi H, Tikkanen MJ, Ahonen J, Hayry P. Renal allograft immunosuppression. IV. Comparison of lipid and lipoprotein profiles in blood using double and triple immunosuppressive drug combinations. Transplant International 1991;4(3):130‐5. [MEDLINE: ] [PubMed] [Google Scholar]
- Isoniemi H, Willebrand E, Ahonen J, Eklund B, Hockerstedt K, Krogerus L, et al. Late histopathological findings in renal allografts with four immunosuppressive regimens. Transplant International 1992;5 Suppl 1:S6‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Isoniemi HM, Ahonen J, Tikkanen MJ, Willebrand EO, Krogerus L, Eklund BH, et al. Long‐term consequences of different immunosuppressive regimens for renal allografts.[Erratum appears in Transplantation 1998 Sep 15;66(5):678]. Transplantation 1993;55(3):494‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Isoniemi HM, Krogerus L, Willebrand E, Taskinen E, Ahonen J, Hayry P. Histopathological findings in well‐functioning, long‐term renal allografts. Kidney International 1992;41(1):155‐60. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Jankowska‐Gan 2009 {published data only}
- Jankowska‐Gan E, Sollinger HW, Pirsch JD, Cai J, Pascual J, Haynes LD, et al. Successful reduction of immunosuppression in older renal transplant recipients who exhibit donor‐specific regulation. Transplantation 2009;88(4):533‐41. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Johnson 1989a {published data only}
- Johnson RW, Mallick NP, Bakran A, Pearson RC, Scott PD, Dyer P, et al. Cadaver renal transplantation without maintenance steroids. Transplantation Proceedings 1989;21(1 Pt 2):1581‐2. [MEDLINE: ] [PubMed] [Google Scholar]
- Johnson RW, Mallick NP, Scott PD, Riad H, Pearson RC, Dyer P, et al. Prospective trials with cyclosporine monotherapy in cadaver renal transplantation. Journal of Nephrology 1990;3(4 Suppl 1):47‐9. [EMBASE: 1992097616] [Google Scholar]
Kacar 2004 {published data only}
- Kacar S, Gurkan A, Karaoglan M, Akman F, Varilsuha C, Karaca C, et al. Steroid withdrawal protocol in renal transplantation [abstract]. 41st Congress. European Renal Association. European Dialysis and Transplantation Association; 2004 May 15‐18; Lisbon, Portugal. 2004:401. [CENTRAL: CN‐00509258]
Kim 2002 {published data only}
- Kim EH, Gohh R, Morrissey P, Simpson M, Monaco A, Yango A. Rapid steroid withdrawal versus standard steroid treatment in patients treated with basiliximab, cyclosporine, and mycophenolate mofetil for the prevention of acute rejection in kidney transplantation: a 2‐year follow‐up [abstract no: 1029]. American Journal of Transplantation 2002;2(Suppl 3):397. [CENTRAL: CN‐00416017] [Google Scholar]
Kumar 2005 {published data only}
- Fa K, Kode RK, Lu Q, Kumar MS, Laftavi MR, Pankewycz OG. Value of one month protocol biopsies combined with a molecular analysis in predicting efficacy of rapid steroid withdrawal after renal transplantation [abstract no: 132]. American Journal of Transplantation 2002;2(Suppl 3):171. [CENTRAL: CN‐00415620] [Google Scholar]
- Fa K, Laftavi MR, Ferry E, Kumar AM, Fyfe B, Pankewycz OG. The predictive value of subclinical rejection in a steroid free immunosuppressive regimen [abstract no: 1282]. American Journal of Transplantation 2003;3(Suppl 5):480. [CENTRAL: CN‐00445267] [Google Scholar]
- Kumar MS, Hahn J, Adams C, Fa K, Fyfe B, Damask A, et al. Steroid avoidance (SA) in kidney transplant recipients treated with simulect (BMAB), neoral (CSA) and cellcept (MMF) ‐ a randomized prospective controlled clinical trial [abstract]. American Journal of Transplantation 2002;2(Suppl 3):393. [Google Scholar]
- Kumar MS, Hahn J, Adams C, Fa K, Fyfe B, Damask A, et al. Steroid avoidance (SA) in kidney transplant recipients treated with simulect (BMAB), neoral (CSA) and cellcept (MMF)‐a randomized prospective controlled clinical trial [abstract no: 2440]. XIXth International Congress of the Transplantation Society; 2002 Aug 25‐30; Miami (FL). 2002. [CENTRAL: CN‐00416079]
- Kumar MS, Heifets M, Moritz MJ, Parikh MH, Saeed MI, Lingaraju R, et al. Basiliximab induction in African American recipients (AA) of cadaver kidneys (CAD) facilitates steroid avoidance, reduces acute rejection (AR) and prolongs survival [abstract no: 1627]. American Journal of Transplantation 2005;5(Suppl 11):570. [CENTRAL: CN‐00644172] [Google Scholar]
- Kumar MS, Heifets M, Moritz MJ, Saeed MI, Khan SM, Fyfe B, et al. Safety and efficacy of steroid withdrawal two days after kidney transplantation: analysis of results at three years. Transplantation 2006;81(6):832‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kumar MS, Xiao SG, Fyfe B, Sierka D, Heifets M, Moritz MJ, et al. Steroid avoidance in renal transplantation using basiliximab induction, cyclosporine‐based immunosuppression and protocol biopsies. Clinical Transplantation 2005;19(1):61‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Laftavi 2005 {published data only}
- Laftavi M, Stefanick B, Stephan R, Kohli R, Min I, Sridhar N, et al. The significance of protocol biopsy in immunominimization protocol: a prospective study of steroid withdrawal [abstract no: O229]. Transplantation 2004;78(2 Suppl):89. [CENTRAL: CN‐00509305] [Google Scholar]
- Laftavi MR, Leca N, Dagher F, Kilmer L, Stephanick B, Kohli R, et al. Steroid withdrawal is associated with more chronic allograft nephropathy (CAN) following kidney transplantation [abstract no: 518]. American Journal of Transplantation 2005;5(Suppl 11):288. [Google Scholar]
- Laftavi MR, Stephan R, Stefanick B, Kohli R, Dagher F, Applegate M, et al. Randomized prospective trial of early steroid withdrawal compared with low‐dose steroids in renal transplant recipients using serial protocol biopsies to assess efficacy and safety. Surgery 2005;137(3):364‐71. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Pankewycz OG, Stephan R, Stefanick B, Dagher F, Applegate M, Kohli R, et al. The clinical benefits of early steroid withdrawal (7 days) and utility of protocol biopsies at 1, 6 and 12 months in guiding steroid‐free immunosuppressive therapy after renal transplantation [abstract no: 1534]. American Journal of Transplantation 2004;4(Suppl 8):579. [CENTRAL: CN‐00509401] [Google Scholar]
- Pankewyez O, Stephan R, Stefanick B, Rubino A, Kohli R, Min I, et al. Induction immunosuppression for renal transplantation using thymoglobulin, FK506 and mycophenolate mofetil allows for safe steroid withdrawal and eliminates the need for early protocol biopsies [abstract no: 1271]. American Journal of Transplantation 2003;3(Suppl 5):478. [CENTRAL: CN‐00447092] [Google Scholar]
Lebranchu 1999 {published data only}
- Hene RJ, M55002 Study Group. A randomized, double‐blind, multi‐center trial comparing two corticosteroid regimens in combination with mycophenolate mofetil (MMF) and cyslosporine (CYA) in renal transplant recipients [abstract no: 420]. Transplantation 1998;65(12):S107. [CENTRAL: CN‐00763818] [Google Scholar]
- Lebranchu Y. Comparison of two corticosteroid regimens in combination with CellCept and cyclosporine A for prevention of acute allograft rejection: 12 month results of a double‐blind, randomized, multi‐center study. M 55002 Study Group. Transplantation Proceedings 1999;31(1‐2):249‐50. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lebranchu Y, Aubert P, Bayle F, Bedrossian J, Berthoux F, Bourbigot B, et al. Could steroids be withdrawn in renal transplant patients sequentially treated with ATG, cyclosporine, and cellcept? One‐year results of a double‐blind, randomized, multicenter study comparing normal dose versus low‐dose and withdrawal of steroids. M 55002 French Study Group. Transplantation Proceedings 2000;32(2):396‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lebranchu Y, M55002 Study Group. A comparison of two corticosteroid regimens in kidney transplanted patients treated with ATG/OKT3 induction, mycophenolate mofetil (MMF) and cyclosporine A (CyA) for prevention of acute allograft rejection. 12 months results of a double‐blind, randomized, multi‐center study [abstract no: 932]. Transplantation 1999;67(7):S239. [CENTRAL: CN‐00763351] [DOI] [PubMed] [Google Scholar]
- Nowacka‐Cieciura E, Durlik M, Cieciura T, Kukula K, Lewandowska D, Baczkowska T, et al. Elevated serum immunoglobulins after steroid withdrawal in renal allograft recipients. Transplantation Proceedings 2002;34(2):564‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Nowacka‐Cieciura E, Durlik M, Cieciura T, Lewandowska D, Baczkowska T, Kukula K, et al. Steroid withdrawal after renal transplantation‐‐risks and benefits. Transplantation Proceedings 2002;34(2):560‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Nowacka‐Cieciura E, Durlik M, Cieciura T, Talalaj M, Kukula K, Lewadowska D, et al. Positive effect of steroid withdrawal on bone mineral density in renal allograft recipients [abstract no: P0568W]. XVIII International Congress of the Transplantation Society; 2000 Aug 27‐Sept 1; Rome, Italy. 2000. [CENTRAL: CN‐00446978] [DOI] [PubMed]
- Nowacka‐Cieciura E, Durlik M, Cieciura T, Talalaj M, Kukula K, Lewandowska D, et al. Positive effect of steroid withdrawal on bone mineral density in renal allograft recipients. Transplantation Proceedings 2001;33(1‐2):1273‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Nowacka‐Cieciura E, Soluch L, Cieciura T, Lewandowska D, Durlik M, Shaibani B, et al. Effect of glucocorticoid‐free immunosuppressive protocol on serum lipids in renal transplant patients. Transplantation Proceedings 2000;32(6):1339‐43. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Puig i Mari JM. Induction treatment with mycophenolate mofetil, cyclosporine, and low‐dose steroids with subsequent early withdrawal in renal transplant patients: results of the Spanish Group. Spanish Group of the CellCept Study. Transplantation Proceedings 1999;31(6):2256‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Vanrenterghem Y, Lebranchu Y, Hene R, Oppenheimer F, Ekberg H. Double‐blind comparison of two corticosteroid regimens plus mycophenolate mofetil and cyclosporine for prevention of acute renal allograft rejection. Transplantation 2000;70(9):1352‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Maiorca 1988 {published data only}
- Cristinelli L, Brunori G, Manganoni AM, Manganoni A, Setti G, Maiorca R. Controlled study of steroid withdrawal after 6 months in renal transplant patients treated with ciclosporin. Contributions to Nephrology 1986;51:91‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Cristinelli L, Brunori G, Setti G, Manganoni A, Manganoni AM, Scolari F, et al. Withdrawal of methylprednisolone at the sixth month in renal transplant recipients treated with cyclosporine. Transplantation Proceedings 1987;19(1 Pt 3):2021‐3. [MEDLINE: ] [PubMed] [Google Scholar]
- Cristinelli L, Brunori G, Setti G, Scolari F, Scaini P, Manganoni S, et al. Controlled randomised trial of methylprednisolone withdrawal at the sixth month in renal transplant recipients treated with cyclosporin [abstract]. Nephrology Dialysis Transplantation 1986;1(2):139. [CENTRAL: CN‐00260301] [Google Scholar]
- Maiorca R, Cristinelli L, Brunori G, Setti G, Salerni B, Nobili U, et al. Prospective controlled trial of steroid withdrawal after six months in renal transplant patients treated with cyclosporine. Transplantation Proceedings 1988;20(3 Suppl 3):121‐5. [MEDLINE: ] [PubMed] [Google Scholar]
Matl 2000 {published data only}
- Matl I, Lacha J, Lodererova A, Simova M, Teplan V, Lanska V, et al. Withdrawal of prednisone from a triple combination of immunosuppressive agents after kidney transplantation [Vysazení prednisonu z trojkombinace imunosupresiv u nemocných po transplantaci ledviny]. Casopis Lekaru Ceskych 2000;139(4):115‐9. [MEDLINE: ] [PubMed] [Google Scholar]
- Matl I, Lacha J, Lodererova A, Simova M, Teplan V, Lanska V, et al. Withdrawal of steroids from triple‐drug therapy in kidney transplant patients. Nephrology Dialysis Transplantation 2000;15(7):1041‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Matl I, Lacha J, Lodererova A, Simova M, Vitko S. Withdrawal of steroids from triple drug therapy in renal transplant patients [abstract]. 35th Congress. European Renal Association. European Dialysis and Transplantation Association; 1998 Jun 6‐9; Rimini, Italy. 1998:351. [CENTRAL: CN‐00485004] [DOI] [PubMed]
Mericq 2013 {published data only}
- Mericq V, Salas P, Pinto V, Cano F, Reyes L, Brown K, et al. Steroid withdrawal in pediatric kidney transplant allows better growth, lipids and body composition: a randomized controlled trial. Hormone Research in Pædiatrics 2013;79(2):88‐96. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Montagnino 2005 {published data only}
- Montagnino G, Sandrini S, Casciani C, Schena FP, Carmellini M, Civati G, et al. A randomized trial of steroid avoidance in renal transplant patients treated with everolimus and cyclosporine. Transplantation Proceedings 2005;37(2):788‐90. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Montagnino G, Sandrini S, Iorio B, Schena FP, Carmellini M, Rigotti P, et al. A randomized exploratory trial of steroid avoidance in renal transplant patients treated with everolimus and low‐dose cyclosporine. Nephrology Dialysis Transplantation 2008;23(2):707‐14. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ponticelli C, Sandrini S, Casciani C, Schena FP, STAR Group. A randomized trial of steroid avoidance in renal transplant patients treated with everolimus and cyclosporine. [abstract no: O432]. Transplantation 2004;78(2 Suppl):170. [CENTRAL: CN‐00509422] [DOI] [PubMed] [Google Scholar]
Nagib 2015 {published data only}
- Nagib AM, Abbas MH, Abu‐Elmagd MM, Denewar AA, Neamatalla AH, Refaie AF, et al. Long‐term study of steroid avoidance in renal transplant patients: a single‐center experience. Transplant Proceedings 2015;47(4):1099‐104. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Nagib AM, Neamatalla AH, Bakr MA, Refaie AF, Abo‐Elmagd MM, Wafa IW. Long term study of steroid avoidance in renal transplant patients: A single center experience [abstract]. Experimental & Clinical Transplantation 2014;12:100. [EMBASE: 71976229] [DOI] [PubMed] [Google Scholar]
Nematalla 2007 {published data only}
- Gheith OA, Nematalla AH, Bakr MA, Refaie A, Shokeir AA, Ghoneim MA. Cost‐benefit of steroid avoidance in renal transplant patients: a prospective randomized study. Scandinavian Journal of Urology & Nephrology 2010;44(3):175‐82. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Gheith OA, Nematalla AH, Bakr MA, Refaie A, Shokeir AA, Ghoneim MA. Steroid avoidance reduce the cost of morbidities after live‐donor renal allotransplants: a prospective, randomized, controlled study. Experimental & Clinical Transplantation: Official Journal of the Middle East Society for Organ Transplantation 2011;9(2):121‐7. [MEDLINE: ] [PubMed] [Google Scholar]
- Neamatalla A, Bakr A, Agroudy A, Shehawy E, Shokier A, Ghoneim M. Improving quality of life after steroid avoidance immunosuppression regimen in live donor renal allotransplant recipients ‐ a prospective randomized controlled study single center experience (two year follow up) [abstract no: FP222]. Nephrology Dialysis Transplantation 2007;22(Suppl 6):vi93. [PubMed] [Google Scholar]
- Neamatalla AH, Bakr MA, Agroudy AE, Shehawy EL, Shokier AA. Improving quality of life after steroid avoidance immunosuppression regimen in live donor renal allotransplant recipients ‐ a prospective randomized controlled study single center experience (two year follow up) [abstract no: P163]. Transplant International 2007;20(Suppl 2):134. [CENTRAL: CN‐00653762] [PubMed] [Google Scholar]
- Nematalla AH, Bakr MA, Agroudy A, Shehawy E, Abdel Rahman ME. Steroid free immunosuppression regimen in live donor renal allotransplant recipients ‐ a prospective randomized study (single center experience) [abstract no: PO‐466]. Transplant International 2005;18(Suppl 1):157. [Google Scholar]
- Nematalla AH, Bakr MA, Agroudy AE, Shehawy E, Salim M, Shokier AA. Steroid avoidance immunosuppression regimen in live donor renal allotransplant recipients ‐ a prospective randomized controlled study single center experience (one year follow up) [abstract no: SP734]. Nephrology Dialysis Transplantation 2006;21(Suppl 4):iv263. [CENTRAL: CN‐00653763] [PubMed] [Google Scholar]
- Nematalla AH, Bakr MA, Gheith OA, Akl A, Agroudy AE, Shahawy M, et al. Steroid avoidance immunosuppression: long term evaluation in live donor renal allotransplant recipients [abstract no: P66]. British Transplantation Society (BTS).11th Annual Congress; 2008 Apr 16‐18; Glasgow, UK. 2008. [CENTRAL: CN‐00766492]
- Nematalla AH, Bakr MA, Gheith OA, Akl AE. Steroid avoidance immunosuppressive protocol: long term evaluation of prospective randomized study after live donor renal allotransplant [abstract: O‐323]. Transplant International 2009;22(Suppl 2):86‐7. [Google Scholar]
- Nematalla AH, Bakr MA, Gheith OA, Agroudy AE, El Shahawy, Aghoneim M. Steroid‐avoidance immunosuppression regimen in live‐donor renal allotransplant recipients: a prospective, randomized, controlled study. Experimental & Clinical Transplantation: Official Journal of the Middle East Society for Organ Transplantation 2007;5(2):673‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Nott 1985 {published data only}
- Griffin PJ, Costa CA, Salaman JR. A controlled trial of steroids in cyclosporine‐treated renal transplant recipients. Transplantation 1987;43(4):505‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Griffin PJ, Gomes da Costa CA, Salaman JR. Renal transplantation without steroids: a controlled clinical trial. Transplantation Proceedings 1986;18(4):797‐8. [EMBASE: 1986223826] [Google Scholar]
- Nott D, Griffin PJ, Salaman JR. Low‐dose steroids do not augment cyclosporine immunosuppression but do diminish cyclosporine nephrotoxicity. Transplantation Proceedings 1985;17(1 II):1289‐90. [EMBASE: 1985078719] [Google Scholar]
- Salaman JR, Gomes Da Costa CA, Griffin PJ. Renal transplantation without steroids. Journal of Pediatrics 1987;111(6 Pt 2):1026‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Park 1994 {published data only}
- Kim HC, Chang KJ, Kwon JK, Park SB, Cho WH, Park CH. Long‐term results of cyclosporine monotherapy in renal transplantation. Transplantation Proceedings 1998;30(7):3539‐40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Park K, Kim ST, Lee SR, Koh YB, Kim HC. A 1‐year prospective randomized study in Korean living donor kidney transplant recipients: comparing cyclosporine monotherapy and cyclosporine/prednisolone during the maintenance phase of immunosuppression. Transplantation Proceedings 1994;26(4):1985‐6. [MEDLINE: ] [PubMed] [Google Scholar]
Pelletier 2006 {published data only}
- Akin B, Ferguson RM, Pelletier RP. Five year follow‐up after steroid withdrawal demonstrates no evidence of worsening renal function [abstract]. American Journal of Transplantation 2004;4(Suppl 8):298. [CENTRAL: CN‐00509047] [Google Scholar]
- Ing SW, Sinnott LT, Davies EA, Pelletier RP. Bone mineral density changes in a prospective, randomized trial of steroid withdrawal in kidney transplant recipients [abstract no: 621]. American Journal of Transplantation 2007;7(Suppl 2):310. [CENTRAL: CN‐00764017] [Google Scholar]
- Ing SW, Sinnott LT, Donepudi S, Davies EA, Pelletier RP, Lane NE. Change in bone mineral density at one year following glucocorticoid withdrawal in kidney transplant recipients. Clinical Transplantation 2011;25(2):E113‐23. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier RP, Akin B, Ferguson RM. Prospective, randomized trial of steroid withdrawal in kidney recipients treated with mycophenolate mofetil and cyclosporine. Clinical Transplantation 2006;20(1):10‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Pelletier RP, Davies EA, Elkhammas EA, Bumgardner GL, Henry ML, Ferguson RM. Randomized, prospective trial of prednisone withdrawal in stable renal transplant recipients [abstract]. Transplantation 2000; Vol. 69, issue 8 Suppl:S260. [CENTRAL: CN‐00447152]
Pisani 2001 {published data only}
- Coppelli A, Buonomo O, Iaria G, Pisani F, Pollicita S, Rizzello A. Preliminary results of a prospective randomized study of basiliximab and steroid withdrawal in kidney transplantation [abstract no: 1617]. 2001 A Transplant Odyssey; 2001 Aug 20‐23; Istanbul, Turkey. 2001. [CENTRAL: CN‐00400600]
- Pisani F, Buonomo O, Iaria G, Tisone G, Mazzarella V, Pollicita S, et al. Preliminary results of a prospective randomized study of basiliximab in kidney transplantation. Transplantation Proceedings 2001;33(1‐2):2032‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ponticelli 1997 {published data only}
- Aroldi A, Tarantino A, Montagnino G, Cesana B, Cocucci C, Ponticelli C. Effects of three immunosuppressive regimens on vertebral bone density in renal transplant recipients: a prospective study. Transplantation 1997;63(3):380‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Montagnino G, Tarantino A, Maccario M, Elli A, Cesana B, Ponticelli C. Long‐term results with cyclosporine monotherapy in renal transplant patients: a multivariate analysis of risk factors. American Journal of Kidney Diseases 2000;35(6):1135‐43. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Montagnino G, Tarantino A, Segoloni GP, Cambi V, Rizzo G, Altieri P, et al. Long‐term results of a randomized study comparing three immunosuppressive schedules with cyclosporine in cadaveric kidney transplantation. Journal of the American Society of Nephrology 2001;12(10):2163‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ponticelli C. Steroid withdrawal in organ transplant recipients [abstract no: 0351]. XVIII International Congress of the Transplantation Society; 2000 Aug 27‐Sept 1; Rome, Italy. 2000. [CENTRAL: CN‐00583616]
- Ponticelli C, Aroldi A. Osteoporosis after organ transplantation. Lancet 2001;357(9268):1623. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ponticelli C, Tarantino A, Montagnino G. Steroid withdrawal in renal transplant recipients. Transplantation Proceedings 2001;33(1‐2):987‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ponticelli C, Tarantino A, Segoloni GP, Cambi V, Rizzo G, Altieri P, et al. A randomized study comparing cyclosporine alone vs double and triple therapy in renal transplants. The Italian Multicentre Study Group for Renal Transplantation (SIMTRe). Transplantation Proceedings 1997;29(1‐2):290‐1. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ponticelli C, Tarantino A, Segoloni GP, Cambi V, Rizzo G, Altieri P, et al. A randomized study comparing three cyclosporine‐based regimens in cadaveric renal transplantation. Italian Multicentre Study Group for Renal Transplantation (SIMTRe). Journal of the American Society of Nephrology 1997;8(4):638‐46. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Tarantino A, Italian Multicentre Study Group for Renal Transplantation (SIMTRe). Is cylosporine (CsA, Sandimmun) monotherapy an effective and safe immunosuppressant in renal transplant recipients? [abstract]. 16th Annual Meeting. American Society of Transplant Physicians (ASTP); 1997 May 10‐14; Chicago (ILL). 1997:128. [CENTRAL: CN‐00509501]
- Tarantino A, Segoloni GP, Cambi V, Rizzo G, Altieri P, Mastrangelo F, et al. A randomized study comparing three cyclosporine‐based regimens in cadaveric renal transplantation: results at 7 years. Transplantation Proceedings 1998;30(5):1729‐31. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Tarantino A, per il Gruppo SIMTRE. Long‐term results of a randomized trial of 3 cyclosporine (CSA) regimens in cadaveric kidney allografts (TXC) [abstract]. Nephrology Dialysis Transplantation 2000;15(9):A250. [CENTRAL: CN‐00461836] [Google Scholar]
Ratcliffe 1993 {published data only}
- Dudley CR, Ratcliffe PJ, et al. Effect of steroid withdrawal on graft function in renal transplant recipients [abstract]. Nephrology Dialysis Transplantation 1994;9(11):1672. [CENTRAL: CN‐00261076] [Google Scholar]
- Ratcliffe PJ, Dudley CR, Higgins RM, Firth JD, Smith B, Morris PJ. Randomised controlled trial of steroid withdrawal in renal transplant recipients receiving triple immunosuppression. Lancet 1996;348(9028):643‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ratcliffe PJ, Firth JD, Higgins RM, Smith B, Gray DW, Morris PJ. Randomized controlled trial of complete steroid withdrawal in renal transplant patients receiving triple immunosuppression. Transplantation Proceedings 1993;25(1 Pt 1):590. [MEDLINE: ] [PubMed] [Google Scholar]
Sandrini 2009 {published data only}
- Sandrini S, Setti G, Bossini N, Chiappini R, Valerio F, Mazzola G, et al. Early (fifth day) vs. late (sixth month) steroid withdrawal in renal transplant recipients treated with Neoral plus Rapamune: four‐yr results of a randomized monocenter study. Clinical Transplantation 2010;24(5):669‐77. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Sandrini S, Setti G, Bossini N, Maffei C, Iovinella L, Tognazzi N, et al. Steroid withdrawal five days after renal transplantation allows for the prevention of wound‐healing complications associated with sirolimus therapy. Clinical Transplantation 2009;23(1):16‐22. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schulak 1989 {published data only}
- Hricik DE, Mayes JT, Schulak JA. Independent effects of cyclosporine and prednisone on posttransplant hypercholesterolemia. American Journal of Kidney Diseases 1991;18(3):353‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hricik DE, Moritz C, Mayes JT, Schulak JA. Association of the absence of steroid therapy with increased cyclosporine blood levels in renal transplant recipients. Transplantation 1990;49(1):221‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hricik DE, Whalen CC, Lautman J, Bartucci MR, Moir EJ, Mayes JT, et al. Withdrawal of steroids after renal transplantation‐‐clinical predictors of outcome. Transplantation 1992;53(1):41‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Schulak JA, Mayes JT, Moritz CE, Hricik DE. A prospective randomized trial of prednisone versus no prednisone maintenance therapy in cyclosporine‐treated and azathioprine‐treated renal transplant patients. Transplantation 1990;49(2):327‐32. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Schulak JA, Moritz CE, Hricik DE. Renal transplantation without prednisolone: effects of bone marrow tolerance azathioprine. Transplantation Proceedings 1989;21(1 Pt 2):1709‐11. [MEDLINE: ] [PubMed] [Google Scholar]
Smak Gregoor 1999 {published data only}
- Roodnat J, Hilbrands LB, Hene RJ, Sevaux RG, Gregoor PJ, Gestel JA, et al. 15 year follow‐up of a multicentre, randomised, calcineurin inhibitor (CNI) withdrawal study in kidney transplantation [abstract no: BO156]. Transplant International 2013;26(Suppl 2):83‐4. [EMBASE: 71359271] [Google Scholar]
- Roodnat JI, Hilbrands LB, Hene RJ, Sevaux RG, Smak Gregoor PJ, Kal‐van Gestel JA, et al. 15‐year follow‐up of a multicenter, randomized, calcineurin inhibitor withdrawal study in kidney transplantation. Transplantation 2014;98(1):47‐53. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Smak Gregoor PJ, Sevaux RG, Ligtenberg G, et al. A prospective randomised study of withdrawal of cyclosporine or prednisone in renal transplant recipients treated with mycophenolate mofetil, cyclosporine, and prednisone: 18 months follow‐up data [abstract]. American Journal of Transplantation 2001;1(Suppl 1):246. [CENTRAL: CN‐00763745] [Google Scholar]
- Smak Gregoor PJ, Sevaux RG, Hene RJ, Hesse CJ, Hilbrands LB, Vos P, et al. Effect of cyclosporine on mycophenolic acid trough levels in kidney transplant recipients. Transplantation 1999;68(10):1603‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Smak Gregoor PJ, Sevaux RG, Ligtenberg G, Hoitsma AJ, Hene RJ, Weimar W, et al. Withdrawal of cyclosporine or prednisone six months after kidney transplantation in patients on triple drug therapy: a randomized, prospective, multicenter study. Journal of the American Society of Nephrology 2002;13(5):1365‐73. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Smak Gregoor PJ, Gelder T, IJzermans JN, Weimar W. Long‐term results of a randomized, prospective study after withdrawal of cyclosporine or prednisone in renal transplant recipients treated with mycophenolate mofetil, cyclosporine, and prednisone [abstract]. American Journal of Transplantation 2003;3(Suppl 5):217. [CENTRAL: CN‐00447777] [Google Scholar]
- Gelder T, Sevaux R, Hene R, Weimar W, Hoitsma A, Ligtenberg G, et al. Discontinuation of cyclosporine or prednisone 6 months after kidney transplantation: a randomized trial [abstract]. Journal of the American Society of Nephrology 2001;12(Program & Abstracts):920A. [CENTRAL: CN‐00583812] [Google Scholar]
- Sevaux RGL, Gregoor P, Smak JH, Hene RJ, Weimar W, Hoitsma AJ, et al. Withdrawal of cyclosporine or prednisone in renal transplant recipients treated with mycophenolate mofetil, cyclosporine, and prednisone: a randomized study [abstract no: 935]. Transplantation 1999;67(7):S240. [CENTRAL: CN‐00767038] [Google Scholar]
Sola 2002 {published data only}
- Sola E, Alférez MJ, Cabello M, Burgos D, González MM. Low‐dose and rapid steroid withdrawal in renal transplant patients treated with tacrolimus and mycophenolate mofetil. Transplantation Proceedings 2002;34(5):1689‐90. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Stiller 1983 {published data only}
- Canadian Transplant Study Group. The requirements for maintenance steroids in cyclosporine‐treated renal transplant recipients [abstract]. Kidney International 1984;25(6):998. [Google Scholar]
- MacDonald AS, Daloze P, Dandavino R, Jindal S, Bear L, Dossetor JB, et al. A randomized study of cyclosporine with and without prednisone in renal allograft recipients. Canadian Transplant Group. Transplantation Proceedings 1987;19(1 Pt 3):1865‐6. [MEDLINE: ] [PubMed] [Google Scholar]
- Stiller C. The requirements for maintenance steroids in cyclosporine‐treated renal transplant recipients. Transplantation Proceedings 1983;15(4 Suppl 1‐2):2490‐4. [EMBASE: 1984089082] [Google Scholar]
THOMAS Study 2002 {published data only}
- Boots JM, Ham EC, Christiaans MH, Hooff JP. Risk of adrenal insufficiency with steroid maintenance therapy in renal transplantation. Transplantation Proceedings 2002;34(5):1696‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Budde K, Salmela K, Pascual J, Rigotti P, THOMAS Follow‐up Study Group. Steroid‐withdrawal in tacrolimus‐treated renal transplant recipients: results of a 3‐year follow‐up study [abstract]. 3rd International Congress on Immunosuppression; 2004 Dec 8‐11; San Diego (CA). 2004. [CENTRAL: CN‐00550470]
- Jindal RM, Salmela K, Vanrentergheim Y, Hooff JP, Squifflet JP. Reduction of high cholesterol levels by early withdrawal of steroids from a tacrolimus‐based triple regimen [abstract no: 206]. American Journal of Transplantation 2002;2(Suppl 3):190. [CENTRAL: CN‐00520347] [Google Scholar]
- Pascual J, Hooff JP, Salmela K, Lang P, Rigotti P, Budde K. Three‐year observational follow‐up of a multicenter, randomized trial on tacrolimus‐based therapy with withdrawal of steroids or mycophenolate mofetil after renal transplant. Transplantation 2006;82(1):55‐61. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Pascual J, Vanrenterghem Y, Hooff JP, Squifflet JP, Salmela K, Rigotti P. Safe withdrawal of MMF or steroids following 3‐months of tacrolimus triple therapy: results of a large, prospective, multicentre study [abstract]. XIXth International Congress of the Transplantation Society; 2002 Aug 25‐30; Miami (FL). 2002. [CENTRAL: CN‐00416426]
- Pascual J, Hooff JP, Salmela K, Budde K, Rigotti P, Lang P. Long‐term efficacy and safety of steroid‐withdrawal in tacrolimus treated renal transplant recipients: results of a 3 year follow‐up [abstract no: 1524]. American Journal of Transplantation 2004;4(Suppl 8):576‐7. [CENTRAL: CN‐00615852] [Google Scholar]
- Rigotti P, European Tacrolimus/MMF Transplantation Study Group. Patients with high cholesterol levels benefit most from early withdrawal of corticosteroids. Transplantation Proceedings 2002;34(5):1797‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Squifflet JP, Vanrenterghem Y, Hooff JP, Salmela K, Rigotti P, European Tacrolimus/MMF Transplantation Study Group. Safe withdrawal of corticosteroids or mycophenolate mofetil: results of a large, prospective, multicenter, randomized study. Transplantation Proceedings 2002;34(5):1584‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Vanrenterghem Y, Hooff JP, Squifflet JP, Salmela K, Rigotti P, Jindal RM, et al. Minimization of immunosuppressive therapy after renal transplantation: results of a randomized controlled trial. American Journal of Transplantation 2005;5(1):87‐95. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hooff JP, European Tacrolimus/MMF Transplantation Study Group. Effect of controlled steroid withdrawal on glucose levels in a tacrolimus‐based immunosuppression regimen [abstract]. 3rd International Congress on Immunosuppression; 2004 Dec 8‐11; San Diego (CA). 2004. [CENTRAL: CN‐00550420]
- Hooff JP, Salmela K, Budde K, Pascual J, Rigotti P, Lang P, et al. Long‐term efficacy and safety of steroid‐withdrawal in tacrolimus‐treated patients: results of a long‐term follow‐up study in renal transplant recipients [abstract no: 99]. 11th Congress of the European Society for Transplantation (ESOT); 2003 Sept 20‐24; Venice, Italy. 2003. [CENTRAL: CN‐00653800]
- Hooff JP, Vanrenterghem Y, Squifflet JP, Salmela K, Ancona E, European Tacrolimus/MMF Transplantation Study Group. First, large, prospective study of a controlled withdrawal of steroids or MMF following three months of tacrolimus/MMF/steroid therapy [abstract]. Journal of the American Society of Nephrology 2001;12(Program & Abstracts):920A‐1A. [CENTRAL: CN‐00550422] [Google Scholar]
- Ham EC, Kooman JP, Christiaans MH, Hooff JP. The influence of early steroid withdrawal on body composition in renal transplant patients [abstract]. Journal of the American Society of Nephrology 2000;11(Sept):711A. [CENTRAL: CN‐00550623] [Google Scholar]
- Ham EC, Kooman JP, Christiaans MH, Hooff JP. The influence of early steroid withdrawal on bone mineral density in renal transplant patients [abstract]. Journal of the American Society of Nephrology 2000;11(Sept):711A. [CENTRAL: CN‐00550623] [Google Scholar]
- Ham EC, Kooman JP, Christiaans ML, Hooff JP. The influence of early steroid withdrawal on body composition and bone mineral density in renal transplantation patients. Transplant International 2003;16(2):82‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Vincenti 2003a {published data only}
- Painter PL, Topp KS, Krasnoff JB, Adey D, Strasner A, Tomlanovich S, et al. Health‐related fitness and quality of life following steroid withdrawal in renal transplant recipients. Kidney International 2003;63(6):2309‐16. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Topp KS, Painter PL, Walcott S, Krasnoff JB, Adey D, Sakkas GK, et al. Alterations in skeletal muscle structure are minimized with steroid withdrawal after renal transplantation. Transplantation 2003;76(4):667‐73. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Vincenti F, Monaco A, Grinyo J, Kinkhabwala M, Neylan J, Roza A, et al. A multicenter randomized trial of rapid steroid withdrawal vs standard steroid treatment in patients treated with Simulect, Neoral and Cellcept for the prevention of acute rejection in renal transplantation [abstract no: 583]. Transplantation 1999;67(7):S152. [CENTRAL: CN‐00403005] [Google Scholar]
- Vincenti F, Monaco A, Grinyo J, Kinkhabwala M, Neylan J, Roza A, et al. Rapid steroid withdrawal versus standard steroid therapy in patients treated with basiliximab, cyclosporine, and mycophenolate mofetil for the prevention of acute rejection in renal transplantation. Transplantation Proceedings 2001;33(1‐2):1011‐2. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Vincenti F, Monaco A, Grinyo J, Kinkhabwala M, Roza A. Multicenter randomized prospective trial of steroid withdrawal in renal transplant recipients receiving basiliximab, cyclosporine microemulsion and mycophenolate mofetil. American Journal of Transplantation 2003;3(3):306‐11. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Vincenti F, Monaco A, Grinyo J, Kinkhabwala M, Roza A, Neylan J, et al. Rapid steroid withdrawal versus standard steroid treatment in patients treated with simulect, neoral, and cellcept for the prevention of acute rejection in renal transplantation: a multicenter, randomized trial [abstract]. Transplantation 2000;69(8 Suppl):S133. [CENTRAL: CN‐00448209] [Google Scholar]
Woodle 2005 {published data only}
- Alloway R, Woodle ES, Gaber AO, Pirsch J, Shihab F, Veldhuisen P, et al. Multivariate analysis of risk factors for acute rejection with early (7 day) corticosteroid withdrawal: results from a randomized, double blind, placebo‐controlled trial [abstract no: 567]. American Journal of Transplantation 2006;6(Suppl 2):257. [Google Scholar]
- Gaber AO, Moore LW, Alloway RR, Woodle ES, Pirsch J, Shihab F, et al. Acute rejection characteristics from a prospective, randomized, double‐blind, placebo‐controlled multicenter trial of early corticosteroid withdrawal. Transplantation 2013;95(4):573‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Gaber AO, Moore LW, Pirsch J, Shihab F, Woodle ES, Astellas Steroid Withdrawal Study Group. Characteristics of rejection in renal allografts following early corticosteroid withdrawal in a randomized controlled clinical trial: results of 3 year followup [abstract no: 330]. American Journal of Transplantation 2006;6(Suppl 2):178. [CENTRAL: CN‐00764094] [Google Scholar]
- Lin S, Henning AK, Akhlaghi F, Reisfield R, Vergara‐Silva A, First MR. Interleukin‐2 receptor antagonist therapy leads to increased tacrolimus levels after kidney transplantation. Therapeutic Drug Monitoring 2015;37(2):206‐13. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Pirsch J, Woodle ES, Shihab F, Gaber AO, Veldhuisen P, Gao J, et al. Effect of steroid withdrawal on new onset diabetes after transplant: results of a randomized double‐blind placebo controlled trial [abstract no: 856]. American Journal of Transplantation 2006;6(Suppl 2):355. [Google Scholar]
- Pirsch JD, Henning AK, First MR, Fitzsimmons W, Gaber AO, Reisfield R, et al. New‐onset diabetes after transplantation: results from a double‐blind early corticosteroid withdrawal trial. American Journal of Transplantation 2015;15(7):1982‐90. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Shihab F, Woodle ES, Gaber AO, Pirsch J, Gao J, Veldhuisen P, et al. Effect of corticosteroid withdrawal on tacrolimus blood trough levels and dosing: results from a randomized double‐blind placebo controlled trial [abstract no: 336]. American Journal of Transplantation 2006;6(Suppl 2):180. [Google Scholar]
- Shihab F, Woodle ES, Gaber AO, Pirsch J, Veldhuisen P, Gao J, et al. Leukopenia limits mycophenolic mofetil dosing following early corticosteroid withdrawal: results from a randomized double‐blinded placebo controlled trial [abstract no: 747]. American Journal of Transplantation 2006;6(Suppl 2):318. [Google Scholar]
- Shihab FS, Lee ST, Smith LD, Woodle ES, Pirsch JD, Gaber AO, et al. Effect of corticosteroid withdrawal on tacrolimus and mycophenolate mofetil exposure in a randomized multicenter study. American Journal of Transplantation 2013;13(2):474‐84. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Woodle ES. A multicenter, randomized, double blind, placebo‐controlled trial of early corticosteroid cessation: final five year report [abstract no: 863]. Transplantation 2008;86(2 Suppl):301. [Google Scholar]
- Woodle ES, Astellas Steroid Withdrawal Study Group. A randomized double blind, placebo‐controlled trial of early corticosteroid cessation versus chronic corticosteroids: five year results [abstract no: 453]. American Journal of Transplantation 2008;8(Suppl 2):300. [CENTRAL: CN‐00644265] [Google Scholar]
- Woodle ES, Astellas Steroid Withdrawal Study Group. A randomized double blind, placebo‐controlled trial of early corticosteroid cessation versus chronic corticosteroids: four year results [abstract no: 1704]. American Transplant Congress; 2007 May 5‐9; San Francisco (CA). 2007. [CENTRAL: CN‐00671799]
- Woodle ES, Astellas Steroid Withdrawal Study Group. A randomized, double‐blind placebo controlled trial of early corticosteroid cessation versus chronic corticosteroids: three year results [abstract no: 326]. American Journal of Transplantation 2006;6(Suppl 2):177. [CENTRAL: CN‐00644264] [Google Scholar]
- Woodle ES, First MR, Pirsch J, Shihab F, Gaber AO, Veldhuisen P, et al. A prospective, randomized, double‐blind, placebo‐controlled multicenter trial comparing early (7 day) corticosteroid cessation versus long‐term, low‐dose corticosteroid therapy. Annals of Surgery 2008;248(4):564‐77. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Woodle ES, Fujisawa Corticosteroid Withdrawal Study Group. A prospective, randomized, multicenter, double‐blind study of early corticosteroid cessation versus long‐term maintenance of corticosteroid therapy with tacrolimus and mycophenolate mofetil in primary renal transplant recipients: one year report. Transplantation Proceedings 2005;37(2):804‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Woodle ES, Fujisawa Steroid Withdrawal Study Group. A prospective, randomized, double blind multicenter study of early (7 day) corticosteroid cessation vs. long term low dose corticosteroid therapy under tacrolimus and mycophenolate mofetil therapy with antibody induction in renal transplant recipients [abstract no: P732]. Transplantation 2004;78(2 Suppl):457. [Google Scholar]
- Woodle ES, Fujisawa Steroid Withdrawal Study Group. A prospective, randomized, double blind multicenter study of early (7 day) corticosteroid cessation vs. long term low dose corticosteroid therapy under tacrolimus and mycophenolate mofetil therapy with antibody induction in renal transplant recipients [abstract]. American Journal of Transplantation 2004;4(Suppl 8):578. [CENTRAL: CN‐00509566] [Google Scholar]
- Woodle ES, Fujisawa Steroid Withdrawal Study Group. A prospective, randomized, double blind, multicenter trial of early (7 day) corticosteroid cessation vs long term low dose corticosteroid therapy under tacrolimus and mycophenolate mofetil therapy with antibody induction [abstract no: 183]. American Journal of Transplantation 2003;3(Suppl 5):198. [CENTRAL: CN‐00448416] [Google Scholar]
- Woodle ES, Fujisawa Steroid Withdrawal Study Group. A prospective, randomized, double blind, placebo controlled multicenter study of early (7 day) corticosteroid cessation vs. long term low dose corticosteroid therapy under tacrolimus and mycophenolate mofetil therapy with antibody induction in renal transplant recipients [abstract]. 3rd International Congress on Immunosuppression; 2004 Dec 8‐11; San Diego (CA). 2004. [CENTRAL: CN‐00550702]
- Woodle ES, Fujisawa Steroid Withdrawal Study Group. A randomized, double blinded, placebo controlled trial of early corticosteroid cessation versus chronic corticosteroid maintenance therapy [abstract no: 1511]. American Journal of Transplantation 2005;5(Suppl 11):540. [Google Scholar]
- Woodle ES, Gaber AO, Shihab F, Pirsch J, First MR, Fitzsimmons W, et al. Comparison of T cell depleting and non‐T cell depleting antibody induction therapy for early corticosteroid withdrawal regimens [abstract no: 849]. American Journal of Transplantation 2006;6(Suppl 2):353. [CENTRAL: CN‐00764352] [Google Scholar]
- Woodle ES, Pirsch J, Alloway R, Shihab F, Gaber AO, Veldhuisen P, et al. Long term effects of early corticosteroid withdrawal and chronic corticosteroid therapy on posttransplant weight gain [abstract no: 287]. American Journal of Transplantation 2006;6(Suppl 2):163. [Google Scholar]
- Woodle ES, Pirsch J, Gaber AO, Shihab F, Alloway R, First MR, et al. African Americans experience different risks and benefits from early corticosteroid withdrawal than non‐African Americans: results from a three year, double blind, randomized, placebo controlled trial [abstract no: 325]. American Journal of Transplantation 2006;6(Suppl 2):176. [Google Scholar]
Zhu 2008a {published data only}
- Zhu QG, Zhao YK, Liu W, Luo H, Qiu Y, Gao ZZ. Two‐year observation of a randomized trial on tacrolimus‐based therapy with withdrawal of steroids or mycophenolate mofetil after renal transplantation. Chinese Medical Sciences Journal 2008;23(4):244‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Alexander 2006 {published data only}
- Alexander JW, Goodman HR, Cardi M, Austin J, Goel S, Safdar S, et al. Simultaneous corticosteroid avoidance and calcineurin inhibitor minimization in renal transplantation. Transplant International 2006;19(4):295‐302. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Anil Kumar 2005 {published data only}
- Anil Kumar MS, Fyfe B, Sierka D, Heifets M, Saeed MI, Parikh MH. Comparison of efficacy and safety of sirolimus (SLR) and mycophenolate mofetil (MMF) as adjunct to calcineurin inhibitor (CNIi) based steroid free immunosuppression in kidney transplantation [abstract]. American Journal of Transplantation 2004;4(Suppl 8):578. [CENTRAL: CN‐00509056] [Google Scholar]
- Anil Kumar MS, Heifets M, Fyfe B, Saaed MI, Moritz MJ, Parikh MH, et al. Comparison of steroid avoidance in tacrolimus/mycophenolate mofetil and tacrolimus/sirolimus combination in kidney transplantation monitored by surveillance biopsy. Transplantation 2005;80(6):807‐14. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Anil Kumar MS, Heifets M, Fyfe B, Sierka D, Saeed MI, Parekh M, et al. A prospective randomized study to compare the efficacy and safety of sirolimus (SLR) and mycophenolate mofetil (MMF) monitored by protocol biopsies in tacrolimus (TAC) based steroid free immunosuppression [abstract]. American Journal of Transplantation 2004;4(Suppl 8):216. [CENTRAL: CN‐00509296] [Google Scholar]
- Kumar A, Lee D, Xiao SG, Moritz MJ, Fyfe B, Heifets M, et al. Comparison of tacrolimus (FK506) and sirolimus (SRL) combination with FK506 and mycophenolate mofetil (MMF) in kidney transplant recipients with steroid avoidance [abstract]. American Journal of Transplantation 2003;3(Suppl 5):350. [CENTRAL: CN‐00446223] [Google Scholar]
Axelrod 2005 {published data only}
- Axelrod D, Leventhal JR, Gallon LG, Parker MA, Kaufman DB. Reduction of CMV disease with steroid‐free immunosuppression in simultaneous pancreas‐kidney transplant recipients. American Journal of Transplantation 2005;5(6):1423‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Berney 2004 {published data only}
- Berney T, Bucher P, Mathe Z, Andres A, Bosco D, Mage R, et al. Islet of langerhans allogeneic transplantation at the University of Geneva in the steroid free era in islet after kidney and simultaneous islet‐kidney transplantations. Transplantation Proceedings 2004;36(4):1121‐2. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Birkeland 1998b {published data only}
- Birkeland SA, Larsen KE, Rohr N. Pediatric renal transplantation without steroids. Pediatric Nephrology 1998;12(2):87‐92. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Birkeland 2002 {published data only}
- Birkeland SA, Beck‐Nielsen H, Rohr N, Bertuzzi F, Secchi A, Shapiro J, et al. Steroid‐free immunosuppression in kidney‐islet transplantation: a long‐term follow‐up. Transplantation 2002;73(9):1527. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Budde 2001 {published data only}
- Budde K, Diekmann F, Fritsche L, Geissler S, Hallebach G, Neumayer H. Steroid withdrawal in long‐term cyclosporine treated patients using mycophenolate mofetil: a prospective randomized pilot study [abstract no: 1233]. A Transplant Odyssey; 2001 Aug 20‐23; Istanbul, Turkey. 2001. [CENTRAL: CN‐00644340] [DOI] [PubMed]
- Budde K, Fritsche L, Geissler S, Hallebach G, Diekmann F, Mai I, et al. Steroid withdrawal in long‐term cyclosporine A treated patients using mycophenolate mofetil: a prospective randomized pilot study. Transplantation Proceedings 2001;33(7‐8):3250‐2. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Budde K, Geissler S, Hallebach G, Fritsche L, Waiser J, Neumayer H. Prospective randomized trial of steroid withdrawal in mycophenolate mofetil (MMF) and cyclosporine (CYA) treated patients (PTS) [abstract]. Journal of the American Society of Nephrology 1998;9(Program & Abstracts):668A. [CENTRAL: CN‐00444574] [Google Scholar]
- Budde K, Geissler S, Hallebach G, Waiser J, Fritsche L, Bohler T, et al. Prospective randomized pilot study of steroid withdrawal with mycophenolate mofetil in long‐term cyclosporine‐treated patients: 4‐year follow‐up. Transplantation Proceedings 2002;34(5):1703‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
CAMPASIA Study 2005 {published data only}
- Munoz AS, Cabanayan‐Casasola CB, Danguilan RA, Padua FB, Ona ET. Campath‐1H (alemtuzumab) as an induction agent for the prevention of graft rejection and preservation of renal function in kidney transplant patients: Philippine 3‐year follow‐up. Transplantation Proceedings 2008;40(7):2230‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Vathsala A, CAMPASIA Study Group. Safety and efficacy of campath‐1h (mabcampath®) with low dose cyclosporine monotherapy in patients receiving kidney transplants ‐ 6 month analysis of the pilot randomised controlled [abstract]. Transplantation 2004;78(2 Suppl):56. [CENTRAL: CN‐00509538] [Google Scholar]
- Vathsala A, Campasia Study Group. One year results of a pilot randomised controlled trial of the effectiveness of alemtuzumab as an induction agent for prevention of graft rejection and preservation of renal function in patients receiving kidney transplants [abstract no: T‐PO50029]. Nephrology 2005;10(Suppl):A215. [CENTRAL: CN‐00583367] [Google Scholar]
- Vathsala A, Ona ET, Tan S, Suresh S, Chan Y, Lou H, et al. CAMPASIA: a pilot randomised controlled trial of the effectiveness of campath‐1h (mabcampath®) as an induction agent for prevention of graft rejection and preservation of renal function in patients receiving kidney transplants [abstract]. American Journal of Transplantation 2004;4(Suppl 8):406. [CENTRAL: CN‐00509539] [Google Scholar]
- Vathsala A, Ona ET, Tan SY, Suresh S, Lou HX, Cabanayan Casasola CB, et al. Lymphocyte recovery after depletion by alemtuzumab in renal transplant recipients: impact on outcome [abstract no: 1015]. American Journal of Transplantation 2005;5(Suppl 11):415. [CENTRAL: CN‐00644284] [Google Scholar]
- Vathsala A, Ona ET, Tan SY, Suresh S, Lou HX, Casasola CB, et al. Induction therapy with alemtuzumab together with low dose cyclosporine monotherapy permits steroid‐free immunosuppression, mitigates drug‐related, non‐immune toxicities and improves quality of life [abstract no: TH‐PO550]. Journal of the American Society of Nephrology 2006;17(Abstracts):224A. [CENTRAL: CN‐00644285] [Google Scholar]
- Vathsala A, Ona ET, Tan SY, Suresh S, Lou HX, Casasola CB, et al. Randomized trial of Alemtuzumab for prevention of graft rejection and preservation of renal function after kidney transplantation. Transplantation 2005;80(6):765‐74. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
CARMEN Study 2005 {published data only}
- Budde K, Neumayer HH, Rostaing L, Catarovich D, Mourad G, Rigotti P, et al. Steroid‐free immunosuppression with daclizumab, tacrolimus and MMF is efficacious and improves cholesterol, glucose and bone mineral density ‐ the CARMEN study [abstract]. Transplantation 2004;78(2 Suppl):168. [CENTRAL: CN‐00509111] [Google Scholar]
- Cantarovich D, Rostaing L, Mourad G, Neumayer HH, Rigotti P, Tacrolimus Steroid Withdrawal Study Group. The combination of Daclizumab, Tacrolimus, and MMF is an effective and safe steroid‐free immunosuppressive regimen after renal transplantation. Results of a large multicentre trial [abstract]. Nephrology Dialysis Transplantation 2003;18(Suppl 4):788. [CENTRAL: CN‐00444672] [Google Scholar]
- Kramer BK, Kruger B, Mack M, Obed A, Banas B, Paczek L, et al. Steroid withdrawal or steroid avoidance in renal transplant recipients: focus on tacrolimus‐based immunosuppressive regimens. Transplantation Proceedings 2005;37(4):1789‐91. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mourad G, Rostaing L, Cantarovich D, Neumayer H, Rigotti P, Tacrolimus Steroid Withdrawal Study Group. Immunosuppression without steroids: daclizumab/tacrolimus/MMF vs. tacrolimus/MMF/steroids in renal transplantation [abstract no: 12]. 11th Congress of the European Society for Transplantation (ESOT); 2003 Sept 20‐24; Venice, Italy. 2003. [CENTRAL: CN‐00653705]
- Pascual J, Rigotti P, Vialtel P, Sanchez‐Fructuoso A, Escuin F, Bone Mineral Density Study Group. Immunosuppression without steroids: a daclizumab, tacrolimus and MMF regimen prevents loss of bone mass following renal transplantation [abstract no: 369]. 11th Congress of the European Society for Transplantation (ESOT); 2003 Sept 20‐24; Venice, Italy. 2003. [CENTRAL: CN‐00653764]
- Rigotti P, Vialtel P, Pascual J, Sanchez‐Fructuoso A, Escuin F, Bone Mineral Density Study Group. Immunosuppression without maintenance steroids prevents decline of bone mineral density following renal transplantation [abstract]. American Journal of Transplantation 2003;3(Suppl 5):199. [CENTRAL: CN‐00447406] [Google Scholar]
- Rostaing L, Cantarovich D, Mourad G, Budde K, Rigotti P, Mariat C, et al. Corticosteroid‐free immunosuppression with tacrolimus, mycophenolate mofetil, and daclizumab induction in renal transplantation. Transplantation 2005;79(7):807‐14. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Rostaing L, Catarovich D, Mourad G, Neumayer HH, Rigotti P, CARMEN Study Group. Steroid‐free immunosuppression with a combination of Daclizumab, Tacrolimus and MMF is efficacious and safe: results of a large multicenter trial in renal transplantation [abstract]. American Journal of Transplantation 2003;3(Suppl 5):312. [CENTRAL: CN‐00447473] [Google Scholar]
- Zaoui P, Vialtel P, Rigotti P, Pascual J, Sanchez‐Fructuoso A, Escuin F, et al. A steroid‐free immunosuppressive regimen of Daclizumab, Tacrolimus and MMF prevents loss of bone mass following renal transplantation [abstract no: T670]. Nephrology Dialysis Transplantation 2003;18(Suppl 4):495. [CENTRAL: CN‐00448519] [Google Scholar]
Citterio 2002 {published data only}
- Citterio F, Baldan N, Tondolo E, Marchini F, Castagneto M, Rigotti P. Medium term results of steroid withdrawal in tacrolimus treated renal transplant recipients [abstract]. 3rd International Congress on Immunosuppression; 2004 Dec 8‐11; San Diego (CA). 2004. [CENTRAL: CN‐00550659]
- Citterio F, Baldan N, Tondolo V, Marchini F, Romagnoli J, Furian L, et al. Five years prospective study of steroid withdrawal in renal transplant recipients [abstract no: 340]. American Journal of Transplantation 2005;5(Suppl 11):242. [CENTRAL: CN‐00644339] [Google Scholar]
- Citterio F, Rigotti P, Scata MC, Baldan N, Marchini F, Castagneto M. Steroid withdrawal in renal transplant patients immunosuppressed with tacrolimus [abstract no: 135]. American Journal of Transplantation 2002;2(Suppl 3):172. [CENTRAL: CN‐00415437] [DOI] [PubMed] [Google Scholar]
- Citterio F, Rigotti P, Scata MC, Romagnoli J, Baldan N, Marchini F, et al. Steroid withdrawal from tacrolimus‐based therapy in renal transplant patients. Transplantation Proceedings 2002;34(5):1707‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
CORRETA Study 2008 {published data only}
- Garcia VD, Carvalho DB, Goncalves RT, Cavalcanti RL, Campos HH, Abbud‐Filho M, et al. CORRETA trial (corticosteroid reduction with tacrolimus): prospective Brazilian multicenter, randomized trial of early corticosteroid reduction vs regular corticosteroid dosage maintenance on a tacrolimus (Prograf®) and mycophenolate mofetil (Cellcept®) immunosuppression regimen in kidney transplant recipients ‐ interim analysis [abstract no: P146]. Transplant International 2007;20(Suppl 2):130‐1. [CENTRAL: CN‐00724889] [DOI] [PubMed] [Google Scholar]
- Garcia VD, Carvalho DB, Goncalves RT, Cavalcanti RL, Campos HH, Abbud‐Filho M, et al. Corticosteroid reduction with tacrolimus (CORRETA) TRIAL: a prospective Brazilian multicenter, randomized trial of early corticosteroid reduction versus regular corticosteroid dosage maintenance on a tacrolimus (Prograf) and mycophenolate mofetil (Cellcept) immunosuppression regimen in kidney transplant recipients: interim analysis. Transplantation Proceedings 2008;40(3):689‐92. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Garcia VD, Carvalho DB, Goncalves RT, Cavalcanti RL, Campos HH, Abbud‐Filho M, et al. Randomized trial of early corticosteroid reduction vs. regular‐dose corticosteroid maintenance in combination with tacrolimus and mycophenolate mofetil in living donor kidney transplant recipients: the Brazilian CORRETA trial. Clinical Transplantation 2010;24(4):E109‐15. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Curtis 1982 {published data only}
- Curtis JJ, Galla JH, Woodford SY, Lucas BA, Luke RG. Effect of alternate‐day prednisone on plasma lipids in renal transplant recipients. Kidney International 1982;22(1):42‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Daniel 1985 {published data only}
- Daniel V, Opelz G, Dreikorn K. Lymphocyte subpopulations in kidney transplant patients with different types of immunosuppression. Transplantation Proceedings 1985;17(6):2254‐7. [EMBASE: 1986058424] [Google Scholar]
- Dreikorn K, Horsch R, Rohl L. A randomized trial with different immunosuppressive regimens after renal transplantation. Transplantation Proceedings 1985;17(6):2663‐5. [EMBASE: 1986055110] [Google Scholar]
De Backer 1992 {published data only}
- Backer D, Abramowicz D, Goldman M, Pauw L, Viseur P, Vanherweghem JL, et al. High or low dose steroid therapy for acute renal transplant rejection after prophylactic OKT3 treatment: a prospective randomized study. Transplant International 1992;5 Suppl 1:S437‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Delucchi 2006 {published data only}
- Delucchi B A, Valenzuela A M, Ferrario B M, Lillo D AM, Guerrero G JL, Rodriguez S E, et al. Early steroid withdrawal in pediatric renal transplantation [Retiro precoz de esteroides en la inmunosupresion del trasplante renal pediatrico]. Revista Medica de Chile 2006;134(11):1393‐401. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
de Sandes Freitas 2011 {published data only}
- Sandes Freitas TV, Harada KM, Felipe CR, Galante NZ, Sampaio EL, Ikehara E, et al. Steroid or tacrolimus withdrawal in renal transplant recipients using sirolimus. International Urology & Nephrology 2011;43(4):1221‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
ECSEL Study 2008 {published data only}
- Smith MP, Newstead CG, Ahmad N, Lewington AJ, Tibble S, Lodge JP, et al. Poor tolerance of sirolimus in a steroid avoidance regimen for renal transplantation. Transplantation 2008;85(4):636‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Welberry‐Smith MP, Gone K, Tibble S, Littler D, Newstead CG, et al. Poor tolerance of sirolimus in a steroid avoidance regime [abstract no: P37]. British Transplantation Society (BTS). 9th Annual Congress; 2006 Mar 29‐31; Edinburgh, UK. 2006.
Hibbs 2010 {published data only}
- Hibbs J, Hamdallah O, Cannon R, Ravindra K, Ouseph R, Gleason J, et al. Alemtuzumab induction with rapid corticosteroid elimination is associated with comparable outcomes in high immunologic risk and non high risk renal transplant recipients [abstract no: 976]. American Journal of Transplantation 2010;10(Suppl 4):322. [EMBASE: 70464352] [Google Scholar]
Hilbrands 1993 {published data only}
- Hilbrands LB, Demacker PN, Hoitsma AJ. Cyclosporin and serum lipids in renal transplant recipients. Lancet 1993;341(8847):765‐6. [MEDLINE: ] [PubMed] [Google Scholar]
- Hilbrands LB, Demacker PN, Hoitsma AJ, Stalenhoef AF, Koene RA. The effects of cyclosporine and prednisone on serum lipid and (APO)lipoprotein levels in renal transplant recipients. Journal of the American Society of Nephrology 1995;5(12):2073‐81. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hilbrands LB, Hoitsma AJ, Koene KA. Randomized, prospective trial of cyclosporine monotherapy versus azathioprine‐prednisone from three months after renal transplantation. Transplantation 1996;61(7):1038‐46. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hilbrands LB, Hoitsma AJ, Koene RA. Costs of drugs used after renal transplantation. Transplant International 1996;9 Suppl 1:S399‐402. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hilbrands LB, Hoitsma AJ, Koene RA. Effect of immunosuppressive therapy on quality of life after renal transplantation [abstract]. Journal of the American Society of Nephrology 1994;5(3):1011. [CENTRAL: CN‐00615875] [Google Scholar]
- Hilbrands LB, Hoitsma AJ, Koene RA. Medication compliance after renal transplantation. Transplantation 1995;60(9):914‐20. [MEDLINE: ] [PubMed] [Google Scholar]
- Hilbrands LB, Hoitsma AJ, Koene RA. The effect of immunosuppressive drugs on quality of life after renal transplantation. Transplantation 1995;59(9):1263‐70. [MEDLINE: ] [PubMed] [Google Scholar]
Hodson 1989 {published data only}
- Hodson EM, Knight JF, Sheil AG, Roy LP. Cyclosporin A as sole immunosuppressive agent for renal transplantation in children: effect on catch‐up growth. Transplantation Proceedings 1989;21(1 Pt 2):1687‐92. [MEDLINE: ] [PubMed] [Google Scholar]
Hricik 1993a {published data only}
- Hricik DE, Schulak JA. Metabolic effects of steroid withdrawal in adult renal transplant recipients. Kidney International ‐ Supplement 1993;43:S26‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Hricik 1993b {published data only}
- Hricik DE, O'Toole MA, Schulak JA, Herson J. Steroid‐free immunosuppression in cyclosporine‐treated renal transplant recipients: a meta‐analysis. Journal of the American Society of Nephrology 1993;4(6):1300‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
John 2005 {published data only}
- John E, Lumpaopang A, Oberholzer J, Testa G, Sankary H, Benedetti E. Superior outcomes in growth and renal function with early steroid discontinuation in pediatric kidney transplantation: 1 1/2 years follow‐up [abstract no: 1329]. American Journal of Transplantation 2005;5(Suppl 11):494. [Google Scholar]
Juarez 2006 {published data only}
- Juarez FJ, Barrios Y, Cano L, Lopez E, Martinez J, Limones M, et al. A randomized trial comparing two corticosteroid regimens combined with mycophenolate mofetil and cyclosporine for prevention of acute renal allograft rejection. Transplantation Proceedings 2006;38(9):2866‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kim 2004 {published data only}
- Kim B, Huh W, Baek HJ, Lim YH, Yeo HM, Kim JA, et al. Randomized trial of tacrolimus versus cyclosporine in steroid withdrawal in living donor renal transplant recipients [abstract no: SU‐PO523]. Journal of the American Society of Nephrology 2003;14(Nov):648A. [CENTRAL: CN‐00626065] [Google Scholar]
- Kim B, Huh W, Kim MO, Yeo HM, Kim HJ, Kim JA, et al. Randomized trial of tacrolimus versus cyclosporine in steroid withdrawal in living donor renal transplant recipients. Korean Journal of Nephrology 2004;23(5):785‐92. [CENTRAL: CN‐01044957] [DOI] [PubMed] [Google Scholar]
- Kim SJ, Lee KW, Lee DS, Lee HH, Lee SK, Kim B, et al. Randomized trial of tacrolimus versus cyclosporine in steroid withdrawal in living donor renal transplant recipients. Transplantation Proceedings 2004;36(7):2098‐100. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kim 2005 {published data only}
- Kim JS, Aviles DH, Silverstein DM, Leblanc PL, Matti Vehaskari V. Effect of age, ethnicity, and glucocorticoid use on tacrolimus pharmacokinetics in pediatric renal transplant patients. Pediatric Transplantation 2005;9(2):162‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lehmann 2004 {published data only}
- Lehmann R, Weber M, Berthold P, Zullig R, Pfammatter T, Moritz W, et al. Successful simultaneous islet‐kidney transplantation using a steroid‐free immunosuppression: two‐year follow‐up. American Journal of Transplantation 2004;4(7):1117‐23. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Li 2011a {published data only}
- Li S, Wang W, Hu X, Ren L, Yin H, Yang X, et al. The effects of early rapid corticosteroid reduction on cell‐mediated immunity in kidney transplant recipients. Transplant Immunology 2011;24(2):127‐30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Li SH, Wang W, Hu XP, Yin H, Ren L, Yang XY, et al. Monitoring immune function after rapid corticosteroid reduction in kidney transplant recipients. Chinese Medical Journal 2011;124(5):679‐82. [MEDLINE: ] [PubMed] [Google Scholar]
Morris 1982 {published data only}
- Morris PJ, Chan L, French ME, Ting A. Low dose oral prednisolone in renal transplantation. Lancet 1982;1(8271):525‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
MYSS Study 2004 {published data only}
- Gotti E, Perico N, Gaspari F, Cattaneo D, Lesti MD, Ruggenenti P, et al. Blood cyclosporine level soon after kidney transplantation is a major determinant of rejection: insights from the Mycophenolate Steroid‐Sparing Trial. Transplantation Proceedings 2005;37(5):2037‐40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Perico N, Ruggenenti P, Gotti E, Gaspari F, Cattaneo D, Valente U, et al. In renal transplantation blood cyclosporine levels soon after surgery act as a major determinant of rejection: insights from the MY.S.S. trial. Kidney International 2004;65(3):1084‐90. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Perico N, Ruggenenti P, Gotti E, Gaspari F, Cattaneo D, Valente U, et al. In renal transplantation low blood cyclosporine levels soon after surgery is a determinant of rejection: insights from the MY.S.S. trial [abstract]. Journal of the American Society of Nephrology 2003;14(Nov):11A. [CENTRAL: CN‐00601980] [Google Scholar]
- Remuzzi G, Cravedi P, Costantini M, Lesti M, Ganeva M, Gherardi G, et al. Mycophenolate mofetil versus azathioprine for prevention of chronic allograft dysfunction in renal transplantation: the MYSS follow‐up randomized, controlled clinical trial. Journal of the American Society of Nephrology 2007;18(6):1973‐85. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Remuzzi G, Lesti M, Gotti E, Ganeva M, Dimitrov BD, Ene‐Iordache B, et al. Mycophenolate mofetil versus azathioprine for prevention of acute rejection in renal transplantation (MYSS): a randomised trial. Lancet 2004;364(9433):503‐12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
NCT00089947 {published data only}
- NCT00089947. Randomized, prospective, phase 2 study comparing thymoglobulin in a rapid discontinuation of corticosteroids protocol with standard corticosteroid therapy in living donor renal transplantation using mycophenolate mofetil and tacrolimus maintenance therapy. www.clinicaltrials.gov/ct2/show/NCT00089947 (accessed 15 February 2016).
Nori 2008 {published data only}
- Nori US, Pesavento TE, Davies EA, Visger J, Miller BS, Ferguson RM. Randomized, prospective prednisone (P) withdrawal trial in kidney transplant patients treated with sirolimus (S) vs microemulsified cyclosporine (CsA) based regimens [abstract no: 458]. American Journal of Transplantation 2008;8(Suppl 2):301. [CENTRAL: CN‐00725014] [Google Scholar]
Paczek 2003a {published data only}
- Paczek L, Wlodarczyk Z, Perner F, Vitko S, Ostrowski M, Bachleda P, et al. Absence of rejection and stable serum creatinine are excellent criteria for steroid‐withdrawal in kidney transplant patients receiving tacrolimus treatment [abstract]. Nephrology Dialysis Transplantation 2003;18(Suppl 4):787‐8. [CENTRAL: CN‐00447076] [Google Scholar]
Papadakis 1982 {published data only}
- Papadakis J, Brown CB, Cameron JS, Adu D, Bewick M, Donaghey R, et al. High versus "low" dose corticosteroids in recipients of cadaveric kidneys: prospective controlled trial. British Medical Journal Clinical Research Ed 1983;286(6371):1097‐100. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadakis JT, Bewick M, Cameron JS, Rudge C, Ogg CS, Brown CB, et al. Low dose steroids in renal transplantation. Lancet 1982;1(8277):916‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Reed 1991 {published data only}
- Reed A, Pirsch JD, Armbrust MJ, Burlingham WJ, Knechtle SJ, D'Alessandro AM, et al. A comparison of donor‐specific and random transfusions in living‐related renal transplantation and their effect on steroid withdrawal. Transplantation Proceedings 1991 Feb;23(1 Pt 2):1321‐2. [MEDLINE: ] [PubMed] [Google Scholar]
Remport 2001 {published data only}
- Remport A, Sasvari I, Borka P, Toronyi E, Sarvary E, Weszelits W, et al. Comparative analysis of mycophenolate‐mofetil‐cyclosporin immunosuppression of kidney transplantation recipients with two different corticosteroid doses and conventional cyclosporin‐corticosteroid therapy [abstract]. XVIII International Congress of the Transplantation Society; 2000 Aug 27‐Sept 1; Rome, Italy. 2000. [CENTRAL: CN‐00447378]
- Remport A, Sasvari I, Borka P, Toronyi E, Sarvary E, Weszelits W, et al. Evaluation of the effect of different corticosteroid doses in combination with mycophenolate‐mofetil‐cyclosporin immunosuppression in kidney transplanted recipients [abstract]. Nephrology Dialysis Transplantation 2000;15(9):A256. [CENTRAL: CN‐00461586] [Google Scholar]
- Remport A, Sasvari I, Toronyi E, Borka P, Lazar N, Jaray J, et al. Mycofenolate mofetil‐cyclosporine immunosuppression of kidney transplantation recipients with two different corticosteroid doses. Transplantation Proceedings 2001;33(3):2302‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Robertson 1980 {published data only}
- Robertson AJ, Gibbs J, Potts R, Brown RA, Browning MC, Beck JS. Renal transplant rejection after gradual withdrawal of prednisolone. British Medical Journal 1980;281(6235):305‐6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sarwal 2012 {published data only}
- Chaudhuri A, Ozawa M, Everly MJ, Ettenger R, Dharnidharka V, Benfield M, et al. The clinical impact of humoral immunity in pediatric renal transplantation. Journal of the American Society of Nephrology 2013;24(4):655‐64. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kambham N, Naesens M, Sigdel T, Waskerwitz J, Salvatierra O, Sarwal M. A protocol biopsy analysis from an NIH multicenter pediatric renal transplant trial reveals no adverse effect of steroid avoidance on the histological evolution of chronic graft injury [abstract no: LB01]. American Journal of Transplantation 2008;8(Suppl 2):334. [Google Scholar]
- Naesens M, Kambham N, Sigdel T, Waskerwitz J, Salvatierra O, Sarwal M. A protocol biopsy analysis from an NIH multicenter pediatric renal transplant trial revels no adverse effect of steroid avoidance on the histological evolution of chronic graft injury [abstract no: 54]. Transplantation 2008;86(2S):18. [Google Scholar]
- Naesens M, Salvatierra O, Benfield M, Ettenger RB, Dharnidharka V, Harmon W, et al. Subclinical inflammation and chronic renal allograft injury in a randomized trial on steroid avoidance in pediatric kidney transplantation. American Journal of Transplantation 2012;12(10):2730‐43. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarwal M, Benfield M, Ettenger R, Dharnidharka V, Mathias R, McDonald R, et al. One year results of a prospective, randomized, multicenter trial of steroid avoidance in pediatric renal transplantation [abstract no: 52]. American Journal of Transplantation 2008;8(Suppl 2):192. [Google Scholar]
- Sarwal M, Chadhuri A, Ozawa M, Everly M, Ettenger R, Dharnidharka V, et al. The clinical impact and evolution of humoral immunity in a randomized multicenter trial of steroid avoidance in pediatric renal transplantation [abstract no: D1767]. American Journal of Transplantation 2013;13(Suppl S5):552. [EMBASE: 71058343] [Google Scholar]
- Sarwal MM, Ettenger RB, Dharnidharka V, Benfield M, Mathias R, Portale A, et al. Complete steroid avoidance is effective and safe in children with renal transplants: a multicenter randomized trial with three‐year follow‐up. American Journal of Transplantation 2012;12(10):2719‐29. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
SENIOR Study 2009 {published data only}
- Andres A, Budde K, Clavien PA, Becker T, Kessler M, Pisarski P, et al. A randomized trial comparing renal function in older kidney transplant patients following delayed versus immediate tacrolimus administration. Transplantation 2009;88(9):1101‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Shapiro 1993 {published data only}
- Shapiro R, Jordan M, Scantlebury V, Vivas C, Fung J, McCauley J, et al. A prospective, randomized trial of FK‐506 in renal transplantation‐‐a comparison between double‐ and triple‐drug therapy. Clinical Transplantation 1994;8(6):508‐15. [MEDLINE: ] [PMC free article] [PubMed] [Google Scholar]
- Shapiro R, Jordan ML, Scantlebury VP, Fung JJ, Jensen C, Vivas C, et al. Randomized trial of FK 506/prednisone vs FK 506/azathioprine/prednisone after renal transplantation: preliminary report. Transplantation Proceedings 1993;25(1 Pt 1):669‐72. [MEDLINE: ] [PMC free article] [PubMed] [Google Scholar]
- Shapiro R, Jordan ML, Scantlebury VP, Vivas C, Fung JJ, McCauley J, et al. A prospective randomized trial of FK506‐based immunosuppression after renal transplantation. Transplantation 1995;59(4):485‐90. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro R, Jordan ML, Scantlebury VP, Vivas C, Fung JJ, McCauley J, et al. A prospective, randomized trial of FK 506/prednisone vs FK 506/azathioprine/prednisone in renal transplant patients. Transplantation Proceedings 1995;27(1):814‐7. [MEDLINE: ] [PMC free article] [PubMed] [Google Scholar]
- Shapiro R, Jordan ML, Scantlebury VP, Vivas C, Gitsch HA, McCauley J, et al. The outcome after steroid withdrawal in renal transplant patients receiving tacrolimus‐based immunosuppression [abstract no: 188]. 16th Annual Meeting. American Society of Transplant Physicians (ASTP); 1997 May 10‐14; Chicago (ILL). 1997:131. [CENTRAL: CN‐00509473]
- Shapiro R, Jordan ML, Scantlebury VP, Vivas C, Gritsch HA, McCauley J, et al. Outcome after steroid withdrawal in renal transplant patients receiving tacrolimus‐based immunosuppression. Transplantation Proceedings 1998;30(4):1375‐7. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro R, Jordan ML, Scantlebury VP, Vivas C, Gritsch HA, McCauley J, et al. Tacrolimus in renal transplantation. Transplantation Proceedings 1996;28(4):2117‐8. [MEDLINE: ] [PMC free article] [PubMed] [Google Scholar]
Silverstein 2005 {published data only}
- Silverstein DM, Aviles DH, LeBlanc PM, Jung FF, Vehaskari VM. Results of one‐year follow‐up of steroid‐free immunosuppression in pediatric renal transplant patients. Pediatric Transplantation 2005;9(5):589‐97. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
SOCRATES Study 2014 {published data only}
- Chadban SJ, Eris JM, Kanellis J, Pilmore H, Lee PC, Lim SK, et al. A randomized, controlled trial of everolimus‐based dual immunosuppression versus standard of care in de novo kidney transplant recipients. Transplant International 2014;27(3):302‐11. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russ G, Eris J, Kanellis J, Hutchison B, Hibberd A, Pilmore H, et al. Multicentre RCT of early switch to everolimus plus steroids or everolimus plus CSA versus CSA, MPA and steroids in de novo kidney transplant recipients: 12 month analysis [abstract no: 93]. Transplantation Society of Australia & New Zealand (TSANZ). 30th Annual Scientific Meeting; 2012 Jun 27‐29; Canberra (ACT). 2012:103.
Tarantino 1991 {published data only}
- Tarantino A, Aroldi A, Stucchi L, Montagnino G, Mascaretti L, Vegeto A, et al. A randomized prospective trial comparing cyclosporine monotherapy with triple‐drug therapy in renal transplantation. Transplantation 1991;52(1):53‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Teplan 2003 {published data only}
- Teplan V, Schuck O, Stollova M, Vitko S. Obesity and hyperhomocysteinaemia after kidney transplantation. Nephrology Dialysis Transplantation 2003;18 Suppl 5:v71‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
ter Meulen 2002 {published data only}
- Hendrikx TK, Klepper M, Ijzermans J, Weimar W, Baan CC. Clinical rejection and persistent immune regulation in kidney transplant patients. Transplant Immunology 2009;21(3):129‐35. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hesselink DA, Ngyuen H, Wabbijn M, Gregoor PJ, Steyerberg EW, Riemsdijk IC, et al. Tacrolimus dose requirement in renal transplant recipients is significantly higher when used in combination with corticosteroids. British Journal of Clinical Pharmacology 2003;56(3):327‐30. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesselink DA, Ngyuen H, Wabbijn M, Smak Gregoor PJH, Steyerberg EW, Riemsdijk IC, et al. Tacrolimus dose requirement in renal transplant recipients is significantly higher when used in combination with corticosteroids [abstract]. American Journal of Transplantation 2003;3(Suppl 5):482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ter Meulen CG, Goertz JH, Klasen IS, Verweij CM, Hilbrands LB, Wetzels JF, et al. Decreased renal excretion of soluble interleukin‐2 receptor alpha after treatment with daclizumab. Kidney International 2003;64(2):697‐703. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- ter Meulen CG, Hilbrands LB, Bergh JP, Hermus AR, Hoitsma AJ. The influence of corticosteroids on quantitative ultrasound parameters of the calcaneus in the 1st year after renal transplantation. Osteoporosis International 2005;16(3):255‐62. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- ter Meulen CG, Riemsdijk I, Hene RJ, Christiaans MH, Borm GF, Corstens FH, et al. No important influence of limited steroid exposure on bone mass during the first year after renal transplantation: a prospective, randomized, multicenter study. Transplantation 2004;78(1):101‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- ter Meulen CG, Riemsdijk I, Hene RJ, Christiaans MH, Borm GF, Gelder T, et al. Steroid‐withdrawal at 3 days after renal transplantation with anti‐IL‐2 receptor alpha therapy: a prospective, randomized, multicenter study. American Journal of Transplantation 2004;4(5):803‐10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- ter Meulen CG, Riemsdijk IC, Hene RJ, Christiaans MH, Gelder T, Hilbrands LB, et al. A prospective randomized trial comparing steroid‐free immunosuppression with limited steroid exposure on bone mineral density in the first year after renal transplantation [abstract no: 0344]. XIXth International Congress of the Transplantation Society; 2002 Aug 25‐30; Miami (FL). 2002. [CENTRAL: CN‐00402832]
- Gelder T, ter Meulen CG, Hene RJ, Christiaans MH, Borm GF, Riemsdijk IC, et al. Steroid withdrawal at three days after renal transplantation with anti IL‐2 receptor therapy: a prospective randomized multicenter trial [abstract]. American Journal of Transplantation 2004;4(Suppl 8):578. [CENTRAL: CN‐00509529] [DOI] [PubMed] [Google Scholar]
- Riemsdijk IC, Termeulen RG, Christiaans MH, Hene RJ, Hoitsma AJ, Hooff JP, et al. Anti‐CD25 prophylaxis allows steroid‐free renal transplantation in tacrolimus‐based immunosuppression [abstract no: 133]. American Journal of Transplantation 2002;2(Suppl 3):171. [CENTRAL: CN‐00402963] [Google Scholar]
TRIMS Study 2010 {published data only}
- Woodle ES, Peddi VR, Tomlanovich S, Mulgaonkar S, Kuo PC, TRIMS Study Investigators. A prospective, randomized, multicenter study evaluating early corticosteroid withdrawal with Thymoglobulin in living‐donor kidney transplantation. Clinical Transplantation 2010;24(1):73‐83. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Woodle ES, TRIMS Study Group. A randomized, prospective, multicenter comparative study evaluating a thymoglobulin‐based early corticosteroid cessation regime in renal transplantation [abstract no: 673]. American Journal of Transplantation 2006;6(Suppl 2):294. [CENTRAL: CN‐00716028] [Google Scholar]
- Woodle ES, TRIMS Study Group. A randomized, prospective, multicenter study of thymoglobulin in renal transplantation for induction and minimization of steroids (TRIMS) [abstract no: 1632]. American Journal of Transplantation 2005;5(Suppl 11):571. [CENTRAL: CN‐00716027] [Google Scholar]
TWIST Study 2010 {published data only}
- Billing H, Hoecker B, Fichtner A, Damme‐Lombaerts R, Friman S, Jaray J, et al. Single nucleotide polymorphism of CYP3A5 influences the exposure to tacrolimus in pediatric renal transplant recipients: A pharmacogenetic substudy of the TWIST trial [abstract]. Transplantation 2014;98(Suppl 1):147. [EMBASE: 71543993] [Google Scholar]
- Billing H, Sander A, Susal C, Ovens J, Feneberg R, Hocker B, et al. Soluble CD30 and ELISA‐detected human leukocyte antigen antibodies for the prediction of acute rejection in pediatric renal transplant recipients. Transplant International 2013 Mar;26(3):331‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Feneberg R. Single nucleotide polymorphisms of CYP3A5, but not of other genes, influence the exposure to tacrolimus in paediatric renal transplant recipients: a pharmacognetic substudy of the Twist Study [abstract no: SAT‐M‐130]. Transplantation Society of Australia & New Zealand (TSANZ). 27th Annual Meeting; 2009 Jun 17‐19; Canberra (ACT). 2009:121.
- Grenda R, Watson A, Trompeter R, Tonshoff B, Jaray J, Fitzpatrick M, et al. A randomized trial to assess the impact of early steroid withdrawal on growth in pediatric renal transplantation: the TWIST study. American Journal of Transplantation 2010;10(4):828‐36. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Trompeter RS, Grenda R, Watson A. Improved growth in pediatric kidney recipients after early steroid withdrawal: daclizumab, tacrolimus (TAC) and mycophenolate mofetil (MMF) versus TAC, MMF and steroids (TWIST Study) [abstract no: 604]. American Journal of Transplantation 2009;9(Suppl 2):365. [EMBASE: 70010477] [Google Scholar]
- Watson AR, Grenda R, Trompeter RS. Reduced complications after early steroid withdrawal in paediatric kidney recipients: daclizumab (DAC), tacrolimus (TAC) and mycophenolate mofetil (MMF) versus TAC, MMF and steroids (Twist Study) [abstract no: OC053]. Pediatric Nephrology 2009;24(9):1799. [Google Scholar]
- Webb N, Douglas S, Rajai A, Roberts S, Grenda R, Marks SD, et al. Corticosteroid free immunosuppression is associated with continuing improved growth in young children following kidney transplantation: long term follow‐up results from the TWIST randomised controlled trial [abstract no: O76]. Pediatric Nephrology 2014;29(9):1683. [EMBASE: 71662390] [Google Scholar]
- Webb NJ, Douglas SE, Rajai A, Roberts SA, Grenda R, Marks SD, et al. Corticosteroid‐free kidney transplantation improves growth: 2‐year follow‐up of the TWIST randomized controlled trial. Transplantation 2015;99(6):1178‐85. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Weimert 2008 {published data only}
- Weimert N, Alloway R, Vinks A, Rike A, Young S, Cardi M, et al. A 12‐month, prospective, randomized, single center, open label pilot study to evaluate the safety and efficacy of Myfortic® in combination with tacrolimus and thymoglobulin® in early corticosteroid withdrawal [abstract no: 102]. Transplantation 2008;86(2 Suppl):36. [Google Scholar]
References to studies awaiting assessment
Newstead 1989 {published data only}
- Newstead C, Moore R, et al. Renal transplant function after steroid withdrawal from triple immunosuppression [abstract]. Nephrology Dialysis Transplantation 1989;4:518. [CENTRAL: CN‐00260463] [Google Scholar]
Additional references
ANZDATA 2012
- Clayton P, Campbell S, Chadban S, McDonald S, Hurst K. ANZDATA Registry Report 2012. Adelaide, South Australia: Australia and New Zealand Dialysis and Transplant Registry, 2012. [Google Scholar]
Basadonna 1993
- Basadonna GP, Matas AJ, Gillingham KJ, Payne WD, Dunn DL, Sutherland DE, et al. Early versus late acute renal allograft rejection: Impact on chronic rejection. Transplantation 1993;55(5):993‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Coutinho 2011
- Coutinho AE, Chapman KE. The anti‐inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Molecular & Cellular Endocrinology 2011;335(1):2‐13. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cuervo 2003
- Cuervo LG, Clarke M. Balancing benefits and harms in health care. BMJ 2003;327(7406):65‐6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Czock 2005
- Czock D, Keller F, Rasche FM, Haussler U. Pharmacokinetics and pharmacodynamics of systemically administered glucocorticoids. Clinical Pharmacokinetics 2005;44(1):61‐98. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Da Silva 2006
- Silva JA, Jacobs JW, Kirwan JR, Boers M, Saag KG, Inês LB, et al. Safety of low dose glucocorticoid treatment in rheumatoid arthritis: published evidence and prospective trial data. Annals of the Rheumatic Diseases 2006;65(3):285‐93. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2001
- Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta‐analysis. In: Egger M, Davey Smith G, Altman DG editor(s). Systematic reviews in health care: meta‐analysis in context. 2nd Edition. London: BMJ Publishing Group, 2001:285–312. [Google Scholar]
ERA‐EDTA 2013
- ERA‐EDTA Registry. ERA‐EDTA Registry Annual Report 2011. Amsterdam: Academic Medical Center, Department of Medical Informatics, 2013. [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hollis 1999
- Hollis S, Campbell F. What is meant by intention to treat analysis? Survey of published randomised controlled trials. BMJ 1999;319(7221):670‐4. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hricik 1993
- Hricik DE, O'Toole MA, Schulak JA, Herson J. Steroid‐free immunosuppression in cyclosporine‐treated renal transplant recipients: a meta‐analysis. Journal of the American Society of Nephrology 1993;4(6):1300‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hricik 2002
- Hricik DE. Steroid‐free immunosuppression in kidney transplantation: an editorial review. American Journal of Transplantation 2002;2(1):19‐24. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Juni 1999
- Juni P, Witschi A, Bloch R, Egger M. The hazards of scoring the quality of clinical trials for meta‐analysis. JAMA 1999;282(11):1054–60. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kasiske 2000
- Kasiske BL, Chakkera HA, Louis TA, Ma JZ. A meta‐analysis of immunosuppression withdrawal trials in renal transplantation. Journal of the American Society of Nephrology 2000;11(10):1910‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Knight 2010
- Knight SR, Morris PJ. Steroid avoidance or withdrawal after renal transplantation increases the risk of acute rejection but decreases cardiovascular risk. A meta‐analysis. Transplantation 2010;89(1):1‐14. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Massy 1996
- Massy ZA, Guijarro C, Kasiske BL. Clinical predictors of chronic renal allograft rejection. Kidney International ‐ Supplement 1996;52:S85‐8. [MEDLINE: ] [PubMed] [Google Scholar]
Matas 2005
- Matas AJ, Kandaswamy R, Gillingham KJ, McHugh L, Ibrahim H, Kasiske B, et al. Prednisone‐free maintenance immunosuppression‐a 5‐year experience. American Journal of Transplantation 2005;5(10):2473‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Moher 1998
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M. Does the quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses?. Lancet 1998;352(9128):609‐13. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Opelz 2005
- Opelz G, Dohler B, Laux G, Collaborative Transplant Study. Long‐term prospective study of steroid withdrawal in kidney and heart transplant recipients. American Journal of Transplantation 2005;5(4 Pt 1):720‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
OPTN/SRTR 2014
- Department of Health and Human Services, Health Resources and Services Administration, Healthcare Systems Bureau, Division of Transplantation, United Network for Organ Sharing. 2012 Annual Report of the U.S. Organ Procurement and Transplantation Network and the Scientific Registry of Transplant Recipients: Transplant Data 1994‐2013. Ann Arbor, USA: HHS/HRSA/OSP/DOT and UNOS, 2012. [Google Scholar]
Pascual 2002
- Pascual M, Theruvath T, Kawai T, Tolkoff‐Rubin N, Cosimi AB. Strategies to improve long‐term outcomes after renal transplantation. New England Journal of Medicine 2002;346(8):580‐90. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pascual 2004
- Pascual J, Quereda C, Zamora J, Hernandez D. Steroid withdrawal in renal transplant patients on triple therapy with a calcineurin inhibitor and mycophenolate mofetil: a meta‐analysis of randomized controlled trials. Transplantation 2004;78(10):1548‐56. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pascual 2012
- Pascual J, Royuela A, Galeano C, Crespo M, Zamora J. Very early steroid withdrawal or complete avoidance for kidney transplant recipients: a systematic review. Nephrology Dialysis Transplantation 2012;27(2):825‐32. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Patel 2001
- Patel S, Kwan JT, McCloskey E, McGee G, Thomas G, Johnson D, et al. Prevalence and causes of low bone density and fractures in kidney transplant patients. Journal of Bone & Mineral Research 2001;16(10):1863‐70. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Prasad 2003
- Prasad GV, Nash MM, McFarlane PA, Zaltzman JS. Renal transplant recipient attitudes toward steroid use and steroid withdrawal. Clinical Transplantation 2003;17(2):135‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tunis 2003
- Tunis SR, Stryer DB, Clancy CM. Practical clinical trials; increasing the value of clinical research for decision making in clinical and health policy. JAMA 2003;290(12):1624‐32. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Pascual 2006
- Pascual Santos J, Quereda C, Zamora J. Steroid avoidance or withdrawal for kidney transplant recipients. Cochrane Database of Systematic Reviews 2006, Issue 1. [DOI: 10.1002/14651858.CD005632] [DOI] [PubMed] [Google Scholar]
Pascual 2009
- Pascual J, Zamora J, Galeano C, Royuela A, Quereda C. Steroid avoidance or withdrawal for kidney transplant recipients. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD005632.pub2] [DOI] [PubMed] [Google Scholar]


