Abstract
Background
Seizures are common following perinatal asphyxia and may exacerbate secondary neuronal injury. Barbiturate therapy has been used for infants with perinatal asphyxia in order to prevent seizures. However, barbiturate therapy may adversely affect neurodevelopment leading to concern regarding aggressive use in neonates.
Objectives
To determine the effect of administering prophylactic barbiturate therapy on death or neurodevelopmental disability in term and late preterm infants following perinatal asphyxia.
Search methods
We used the standard search strategy of the Cochrane Neonatal Review group to search the Cochrane Central Register of Controlled Trials (CENTRAL, 2015, Issue 11), MEDLINE via PubMed (1966 to 30 November 2015), EMBASE (1980 to 30 November 2015), and CINAHL (1982 to 30 November 2015). We also searched clinical trials databases, conference proceedings, and the reference lists of retrieved articles for randomized controlled trials (RCT) and quasi‐RCTs.
Selection criteria
We included all RCTs or quasi‐RCTs of prophylactic barbiturate therapy in term and late preterm infants without clinical or electroencephalographic evidence of seizures compared to controls following perinatal asphyxia.
Data collection and analysis
Three review authors independently selected, assessed the quality of, and extracted data from the included studies. We assessed methodologic quality and validity of studies without consideration of the results. The review authors independently extracted data and performed meta‐analyses using risk ratios (RR) and risk differences (RD) for dichotomous data and mean difference for continuous data with 95% confidence intervals (CI). For significant results, we calculated the number needed to treat for an additional beneficial outcome (NNTB) or for an additional harmful outcome (NNTH).
Main results
In this updated review, we identified nine RCTs of any barbiturate therapy in term and late preterm infants aged less than three days old with perinatal asphyxia without evidence of seizures. Eight of these studies compared prophylactic barbiturate therapy to conventional treatment (enrolling 439 infants) and one study compared barbiturate therapy to treatment with phenytoin (enrolling 17 infants).
Prophylactic barbiturate therapy versus conventional treatment: one small trial reported a decreased risk of death or severe neurodevelopmental disability for barbiturate therapy (phenobarbital) versus conventional treatment (RR 0.33, 95% CI 0.14 to 0.78; RD ‐0.55, 95% CI ‐0.84 to ‐0.25; NNTB 2, 95% CI 1 to 4; 1 study, 31 infants) (very low quality evidence).
Eight trials comparing prophylactic barbiturate therapy with conventional treatment following perinatal asphyxia demonstrated no significant impact on the risk of death (typical RR 0.88, 95% CI 0.55 to 1.42; typical RD ‐0.02, 95% CI ‐0.08 to 0.05; 8 trials, 429 infants) (low quality evidence) and the one small trial noted above reported a significant decrease in the risk of severe neurodevelopmental disability (RR 0.24, 95% CI 0.06 to 0.92; RD ‐0.43, 95% CI ‐0.73 to ‐0.13; NNTB 2, 95% CI 1 to 8; 1 study, 31 infants) (very low quality evidence).
A meta‐analysis of the six trials reporting on seizures in the neonatal period demonstrated a statistically significant reduction in seizures in the prophylactic barbiturate group versus conventional treatment (typical RR 0.62, 95% CI 0.48 to 0.81; typical RD ‐0.18, 95% CI ‐0.27 to ‐0.09; NNTB 5, 95% CI 4 to 11; 6 studies, 319 infants) (low quality evidence). There were similar results in subgroup analyses based on type of barbiturate and Sarnat score.
Prophylactic barbiturate therapy versus other prophylactic anticonvulsant therapy: one study reported on prophylactic barbiturate versus prophylactic phenytoin. There was no significant difference in seizure activity in the neonatal period between the two study groups (RR 0.89, 95% CI 0.07 to 12.00; 1 trial, 17 infants).
Authors' conclusions
We found only low or very low quality evidence addressing the use of prophylactic barbiturates in infants with perinatal asphyxia. Although the administration of prophylactic barbiturate therapy to infants following perinatal asphyxia did reduce the risk of seizures, there was no reduction seen in mortality and there were few data addressing long‐term outcomes. The administration of prophylactic barbiturate therapy for late preterm and term infants in the immediate period following perinatal asphyxia cannot be recommended for routine clinical practice. If used at all, barbiturates should be reserved for the treatment of seizures. The results of the current review support the use of prophylactic barbiturate therapy as a promising area of research. Future studies should be of sufficient size and duration to detect clinically important reductions in mortality and severe neurodevelopmental disability and should be conducted in the context of the current standard of care, including the use of therapeutic hypothermia.
Plain language summary
Use of prophylactic barbiturates to prevent death or serious developmental problems in term or late preterm infants following birth asphyxia
Review question
Does the use of prophylactic barbiturate therapy reduce the possibility of dying or having severe developmental problems in term and late preterm infants following birth asphyxia?
Background
Seizures (fits) are common following birth asphyxia (where the infant did not get enough oxygen during birth). These seizures may worsen brain injury caused by birth asphyxia. In theory, treatment with barbiturates (a medicine that causes relaxation and sleepiness and is used to treat seizures) given to babies soon after birth asphyxia may improve outcomes by preventing seizures and protecting the brain. Such treatment is called prophylaxis because it is given to prevent seizures rather than treat them once they have happened. Barbiturate therapy has some side effects and there are concerns that barbiturates might impair brain development.
Study characteristics
We identified nine clinical trials for inclusion in this review by searching medical databases in November 2015. We found only low or very low quality evidence of prophylactic barbiturates in infants with perinatal asphyxia. The studies in this review included small numbers of babies and only a few studies looked at the health of infants over a long period of time.
Key results
At present, there is not enough information to recommend giving barbiturate therapy to newborn babies soon after birth asphyxia but before seizures start.
Conclusion
It is unclear whether giving barbiturates to newborn babies soon after birth asphyxia but before seizures start is safe or effective. More studies are needed.
Summary of findings
Summary of findings for the main comparison. Barbiturate treatment compared to placebo or no treatment.
| Barbiturate treatment compared to placebo or no treatment | ||||||
| Patient or population: infants with perinatal asphyxia Setting: neonatal intensive care Intervention: prophylactic barbiturate Comparison: no treatment or selective barbiturate treatment | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with selective barbiturate treatment | Risk with prophylactic barbiturate | |||||
| Death or major neurodevelopmental disability follow‐up: > 12 months | Study population | RR 0.33 (0.14 to 0.78) | 31 (1 RCT) | Very low quality 1, 2 |
Risk of bias: unblinded study; concern regarding performance bias, detection bias, and incomplete follow‐up. Imprecision: 95% CI were wide and imprecise | |
| 813 per 1000 | 268 per 1000 (114 to 634) | |||||
| Death | Study population | RR 0.88 (0.55 to 1.42) | 429 (8 RCTs) | Low quality 1, 2 |
Risk of bias: unblinded studies; concern regarding performance bias and detection bias. Imprecision: 95% CI were wide and imprecise | |
| 145 per 1000 | 128 per 1000 (80 to 206) | |||||
| Major neurodevelopmental disability follow‐up: > 12 months | Study population | RR 0.24 (0.06 to 0.92) | 31 (1 RCT) | Very low quality 1, 2 |
Risk of bias: unblinded study; concern regarding performance bias, detection bias, and incomplete follow‐up. Imprecision: 95% CI were wide and imprecise | |
| 563 per 1000 | 135 per 1000 (34 to 518) | |||||
| Cerebral palsy | Study population | RR 0.58 (0.19 to 1.70) | 69 (2 RCTs) | Very low quality 1, 2, 3 |
Risk of bias: unblinded studies; concern regarding performance bias and detection bias. Imprecision: 95% CI were wide and imprecise. Inconsistency: clinically important heterogeneity noted | |
| 242 per 1000 | 141 per 1000 (46 to 412) | |||||
| Developmental delay | Study population | RR 0.54 (0.24 to 1.19) | 63 (2 RCTs) | Very low quality 1, 2 |
Risk of bias: unblinded studies; concern regarding performance bias and detection bias. Imprecision: 95% CI were wide and imprecise | |
| 355 per 1000 | 192 per 1000 (85 to 422) | |||||
| Seizures (clinical or electrographic) | Study population | RR 0.62 (0.48 to 0.81) | 319 (6 RCTs) | Low quality 1, 4 |
Risk of bias: unblinded studies; concern regarding performance bias and detection bias. Inconsistency: statistical heterogeneity noted | |
| 474 per 1000 | 294 per 1000 (228 to 384) | |||||
| Abnormal neurologic exam at discharge | Study population | RR 0.85 (0.67 to 1.08) | 245 (4 RCTs) | Low quality 1, 5 |
Risk of bias: unblinded studies; concern regarding performance bias and detection bias. Inconsistency: statistical heterogeneity noted | |
| 432 per 1000 | 367 per 1000 (290 to 467) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio; RCT: randomized controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Downgraded for possible performance and detection bias.
2 Imprecision: wide CIs. Sample size below optimal information size.
3 Clinically important heterogeneity noted.
4 I2 = 68%.
5 I2 = 76%.
Background
Seizures are common following perinatal asphyxia and may exacerbate secondary neuronal injury. Barbiturate therapy has been used for infants with perinatal asphyxia in order to prevent seizures. However, barbiturate therapy may adversely affect neurodevelopment leading to concern regarding aggressive use in neonates.
Description of the condition
Perinatal asphyxia resulting in hypoxic‐ischemic encephalopathy (HIE) remains an important cause of mortality and severe long‐term neurologic and developmental disability in children worldwide.
Paramount in the treatment of HIE is the early identification of infants whose encephalopathy may be subject to treatment. The management of these infants has traditionally been supportive, with the goal of restoring and maintaining cerebral perfusion, maintaining glucose homeostasis, treating other organ dysfunction, and treating seizures when present. Currently, there are no targeted neuroprotective interventions that have been shown to be effective in human trials, with the notable exception of therapeutic hypothermia (Jacobs 2013). Multiple trials have shown that initiation of therapeutic hypothermia, both whole body and selective head cooling, within six hours of age reduces mortality without increasing major morbidity in survivors (Jacobs 2013). Although there are multiple etiologies for neonatal encephalopathy, the use of therapeutic hypothermia is restricted to infants with HIE due to perinatal asphyxia. Typically, the highest risk infants are able to be identified shortly after birth by a constellation of clinical findings including:
evidence of a sentinel event during labor (i.e. fetal heart rate abnormality);
a severely depressed infant (low extended Apgar score);
the need for resuscitation in the delivery room (i.e. intubation, chest compressions with or without epinephrine);
evidence of severe fetal acidemia (cord umbilical artery pH less than 7.00 or base deficit greater than 16 mEq/L (16 mmol/L), or both);
evidence of an early abnormal neurologic examination or abnormal assessment of cerebral function, or both (Perlman 1996; Perlman 2006).
The therapeutic window that exists to minimize secondary injury and improve overall outcome is short (thought to be less than six hours) (Gunn 1998). Following a reversible hypoxic‐ischemic insult, neuronal cell death occurs in two phases (Gluckman 1992; Lorek 1994; Penrice 1996). Phase one, the initial anoxic event, results in primary neuronal injury and cell death due to cellular hypoxia with exhaustion of cellular energy stores (Hossman 1983). Phase two, the reperfusion phase, occurs following a latent period and results in delayed neuronal injury during the recovery period following initial resuscitation (Williams 1991). Mechanisms thought to be important in the second phase of neuronal injury include production of oxygen free radicals (McCord 1985), intracellular calcium influx (Siesjo 1992), increase in excitatory neurotransmitters, cerebral edema, and active cell death or apoptosis (Buttke 1994). Clinically, this second phase of injury is associated with encephalopathy and increased seizure activity. It is the severity of this second phase that correlates with mortality and adverse neurodevelopmental outcomes at one and four years of age (Roth 1992; Roth 1997).
A continuum of severity exists among infants with HIE. In an attempt to help estimate the risk of adverse outcomes, Sarnat and Sarnat developed the original scoring system for categorizing encephalopathy in infants after perinatal distress. This system, with some modifications, is the approach used by most authors/researchers today and includes Stage I (Sarnat 1) ‐ mild, Stage II (Sarnat 2) ‐ moderate, Stage III (Sarnat 3) ‐ severe (Sarnat 1976).
Description of the intervention
Seizures are a common feature of HIE (Sarnat 1976), occurring in up to 60% of infants with moderate to severe HIE (Gluckman 2005; Pfister 2012; Shankaran 2005). When present, seizures substantially increase cerebral metabolic demand (Younkin 1986), cause the release of excitatory neurotransmitters such as glutamate (McDonald 1990), lead to fluctuations in systemic arterial pressure (Clozel 1985), and result in hypoxia and hypercapnia. Though the degree to which seizures are associated with an increased risk of death and neurodevelopmental disability in this population is unclear (Glass 2009; Kwon 2011; McBride 2000; Wyatt 2007), seizures are thought to contribute to ongoing neuronal injury following asphyxia (Wirrell 2001; Yager 2002). The potential benefits of preventing further neuronal injury through the treatment and prevention of seizures following asphyxia has prompted the widespread use of anticonvulsants in this population. Barbiturates, specifically phenobarbital, remain the preferred drugs of choice for the management of neonatal seizures, including seizures that occur following perinatal asphyxia.
How the intervention might work
Barbiturates are a class of anticonvulsants that facilitate gamma‐aminobutyric acid (GABA)‐mediated opening of chloride channels and in so doing enhance the effectiveness of GABA. The anticonvulsant effect of barbiturates is seen when GABA activation of the postsynaptic GABAA receptor results in an influx of chloride, and thereby an inhibitory effect as seen in mature neurons. However, this effect is less consistent in the developing brain (Cherubini 1991; Rivera 1999; Yamada 2004). In addition to the potential anticonvulsant effects, barbiturates are also known to decrease central nervous system (CNS) metabolic rate when given in high doses (Nilsson 1971), reduce calcium entry postischemia, and scavenge free radicals (Demopoulos 1977). Thus, barbiturates may theoretically attenuate the cascade of damaging processes initiated by the hypoxic‐ischemic insult and reduce secondary neuronal injury. For these reasons, many clinicians have used barbiturates, specifically phenobarbital, prophylactically with or without therapeutic hypothermia, in cases of HIE. While there are data to suggest that seizure duration and evidence of brain injury on magnetic resonance imaging (MRI) may be reduced when either clinical or subclinical seizure patterns are treated (van Rooij 2010), animal models in the developing brain have been conflicting, demonstrating abnormal neuronal development both following phenobarbital exposure (Bittigau 2002) and seizures (Holmes 1998).
Why it is important to do this review
Although therapeutic hypothermia has led to improvement in outcomes for infants with HIE, there remains a significant degree of morbidity and mortality among these infants. The establishment of safe and effective adjunct therapies is important to provide further neuroprotection and improve neurodevelopmental outcomes in this population. While the theoretical benefits of prophylactic phenobarbital administration seem to support its use, the potential risks associated with such use cannot be overlooked. These risks include, potential fluctuations in systemic blood pressure, mainly hypotension following administration, and the potential negative cognitive developmental effects suggested by animal studies.
Objectives
To determine the effect of administering prophylactic barbiturate therapy on death or neurodevelopmental disability in term and late preterm infants following perinatal asphyxia.
Methods
Criteria for considering studies for this review
Types of studies
All published or unpublished randomized controlled trials (RCTs) or quasi‐RCTs of prophylactic barbiturate use in asphyxiated late preterm and term infants.
Types of participants
Term infants (37 weeks or greater) and late preterm infants (34 to 36+6 weeks' gestation) three days of age or less with perinatal asphyxia.
-
Evidence of perinatal asphyxia, characterized by evidence of neonatal or fetal distress with each enrolled infant satisfying at least one of the following criteria:
Cord gas or postnatal blood gas (within the first hour of life) with pH 7.0 or less or base deficit 12 mEq/L or greater;
Apgar score 5 or less at 10 minutes;
Need for mechanical ventilation or resuscitation at 10 minutes of life;
-
with or without evidence of encephalopathy (moderate or severe) according to Sarnat staging (Sarnat 1976):
Stage 1 (mild): hyperalertness, hyper‐reflexia, dilated pupils, tachycardia, absence of seizures;
Stage 2 (moderate): lethargy, hyper‐reflexia, miosis, bradycardia, seizures, hypotonia with weak suck, and Moro;
Stage 3 (severe): stupor, flaccidity, small‐to‐mid position pupils that react poorly to light, decreased stretch reflexes, hypothermia, and absent Moro.
No evidence of seizures.
No major congenital abnormalities recognizable at birth.
Seizures were considered present if suspected clinically or identified electroencephalographically via electroencephalograph (EEG) or amplitude‐integrated electroencephalography (aEEG).
(Modified from Jacobs 2013.)
Types of interventions
Barbiturates administered in the early neonatal period (within the first three days of life) with the intention of preventing seizures or improving neurodevelopmental outcomes and mortality (or both) following perinatal asphyxia.
Types of outcome measures
Primary outcomes
Death or major neurodevelopmental disability assessed at 12 months of age or greater (defined as cerebral palsy, developmental delay (Bayley or Griffith assessment more than two standard deviations (SD) below the mean) or intellectual impairment (intelligence quotient (IQ) more than two SD below mean), blindness (vision less than 6/60 in both eyes) or sensorineural deafness requiring amplification).
Secondary outcomes
Death (at any time).
Major neurodevelopmental disability assessed at 12 months of age or greater (defined as cerebral palsy, developmental delay (Bayley or Griffith assessment more than two SD below the mean) or intellectual impairment (intelligence quotient (IQ) more than two SD below mean), blindness (vision less than 6/60 in both eyes) or sensorineural deafness requiring amplification).
-
Each component of major neurodevelopmental disability:
cerebral palsy;
-
developmental delay:
Bayley or Griffith assessment more than two SD below the mean;
Neuromotor development (Bayley Scales of Infant Development ‐ Psychomotor Development Index (BSID PDI)) assessed in survivors;
Cognitive development (Bayley Scales of Infant Development ‐ Mental Development Index (BSID MDI)) assessed in survivors;
intellectual impairment (IQ more than two SD below mean);
blindness (vision less than 6/60 in both eyes);
sensorineural deafness requiring amplification.
-
Seizures (suspected clinically or identified by EEG or aEEG):
seizures during initial neonatal period;
seizures or need for anticonvulsants at follow‐up 12 months of age or greater.
-
Incidence of adverse effects of barbiturate administration:
respiratory depression requiring intubation;
hypotension requiring inotropic support;
significant rash (i.e. exfoliative dermatitis, Stevens‐Johnson syndrome).
Post hoc outcomes
Abnormal neurologic exam at discharge.
Search methods for identification of studies
Electronic searches
Appendix 1 shows the original search strategy in 2007.
For the 2015 update, we used the criteria and standard methods of Cochrane and the Cochrane Neonatal Review Group (see the Cochrane Neonatal Group search strategy for specialized register).
We conducted a comprehensive search including: Cochrane Central Register of Controlled Trials (CENTRAL, 2015, Issue 11); MEDLINE via PubMed (1996 to 30 November 2015); EMBASE (1980 to 30 November 2015); and CINAHL (1982 to 30 November 2015) using the following search terms: (phenobarbital OR anticonvulsant) AND (asphyxia OR encephalopathy), plus database‐specific limiters for RCTs and neonates (see Appendix 2 for the full search strategies for each database). We applied no language restrictions.
We searched clinical trials registries for ongoing or recently completed trials (clinicaltrials.gov; the World Health Organization's International Trials Registry and Platform www.whoint/ictrp/search/en/, and the ISRCTN Registry).
Searching other resources
Published abstracts: we handsearched the abstracts of the Society for Pediatric Research (USA) (published in Pediatric Research) for the years 1985 to 1999 using the following key words: {phenobarbital, anticonvulsant} AND {asphyxia, encephalopathy}. We searched abstracts for 2000 to 2014 electronically through the Pediatric Academic Societies' website.
Data collection and analysis
Selection of studies
The review authors (LY, MB, RS) screened the title and abstract of studies identified by the above search strategy. We reviewed the full‐text reports of each study identified as potentially relevant. We included studies meeting any of the prespecified inclusion criteria and evaluated them for inclusion and methodologic quality without consideration of results. Review authors were not blinded to authorship, institution, or journal.
Data extraction and management
Two review authors (LY, RS) separately extracted, assessed, and coded all data for each study using a form that was designed specifically for this review. For each included study, we collected information regarding the method of randomization, blinding, drug intervention, stratification, and whether the trial was conducted at a single center or multiple centers. We noted information regarding inclusion criteria, including gestational age, postnatal age at the time of treatment, and disease severity criteria. We collected information on clinical outcomes including death, neurodevelopmental disability assessed at 12 months of age or greater (defined as cerebral palsy, developmental delay or intellectual impairment, blindness (vision less than 6/60 in both eyes), or sensorineural deafness requiring amplification), seizure in the neonatal period, continued need for anticonvulsant therapy or persistent seizures at follow‐up 12 months of age or greater, hypotension requiring inotropic support, respiratory depression requiring intubation, and significant rash following administration of anticonvulsant.
Seizures were either apparent clinically or detected by EEG or aEEG.
For each study, one review author entered final data into Review Manager 5 and a second review author checked them (RevMan 2014). A third review author addressed any disagreements.
Assessment of risk of bias in included studies
We used the standard method of the Cochrane Neonatal Review Group to assess risk of bias. The review authors independently assessed the risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreements by discussion.
We assessed methodologic quality of the studies using the following criteria.
-
Sequence generation (checking for possible selection bias): for each included study, we categorized the method used to generate the allocation sequence as:
adequate (any truly random process, e.g. random number table; computer random number generator);
inadequate (any nonrandom process, e.g. odd or even date of birth; hospital or clinic record number);
unclear.
-
Allocation concealment (checking for possible selection bias): for each included study, we categorized the method used to conceal the allocation sequence as:
adequate (e.g. telephone or central randomization; consecutively numbered sealed opaque envelopes);
inadequate (open random allocation; unsealed or nonopaque envelopes, alternation; date of birth);
unclear.
-
Blinding (checking for possible performance bias): for each included study, we categorized the methods used to blind study participants and personnel from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes. We categorized the methods as:
adequate, inadequate, or unclear for participants;
adequate, inadequate, or unclear for personnel;
adequate, inadequate, or unclear for outcome assessors.
-
Incomplete outcome data (checking for possible attrition bias through withdrawals, drop‐outs, protocol deviations): for each included study and for each outcome, we described the completeness of data including attrition and exclusions from the analysis. We noted whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomized participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported or supplied by the trial authors, we re‐included missing data in the analyses. We categorized the methods as:
adequate (less than 20% missing data);
inadequate (20% or greater missing data);
unclear.
-
Selective reporting bias: for each included study, we described how we investigated the possibility of selective outcome reporting bias and what we found. We assessed the methods as:
adequate (where it was clear that all of the study's prespecified outcomes and all expected outcomes of interest to the review were reported);
inadequate (where not all the study's prespecified outcomes were reported; one or more reported primary outcomes were not prespecified; outcomes of interest were reported incompletely and so could not be used; study did not include results of a key outcome that would have been expected to have been reported);
unclear.
Other sources of bias: for each included study, we described any important concerns we had about other possible sources of bias (e.g. whether there was a potential source of bias related to the specific study design or whether the trial was stopped early due to some data‐dependent process). We assessed whether each study was free of other problems that could put it at risk of bias as: yes; no; unclear.
Measures of treatment effect
We examined the treatment effects of individual trials by comparing groups allocated to the treatment under study (prophylactic barbiturate therapy) versus placebo with conventional treatment (including anticonvulsants for the treatment of seizures) or conventional treatment alone. We compared data relating to the primary and secondary outcomes (Primary outcomes; Secondary outcomes). Where relevant and if possible, we calculated risk ratio (RR) and risk difference (RD) for dichotomous data and mean difference (MD) for continuous data, with 95% confidence intervals (CI) for all analyses. For significant results, we calculated the number needed to treat for an additional beneficial outcome (NNTB) or additional harmful outcome (NNTH). Analysis of outcome data was by intention to treat.
Assessment of heterogeneity
We examined treatment effects of individual trials and heterogeneity between trials by inspecting the forest plots and quantifying the impact of heterogeneity using the I2 statistic. If we detected statistical heterogeneity, we planned to explore the possible causes (e.g. differences in study quality, participants, intervention regimens, or outcome assessments) using post hoc subgroup analyses.
Data synthesis
Where relevant, we performed meta‐analyses using the fixed‐effect 'assumption free' model. Where relevant and if possible, we examined heterogeneity between trial results using the I2 test for dichotomous outcomes.
Quality of evidence
We assessed the quality of evidence for the main comparison at the outcome level using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach (Guyatt 2011a). This methodologic approach considers evidence from RCTs as high quality that may be downgraded based on consideration of any of five areas: design (risk of bias), consistency across studies, directness of the evidence, precision of estimates, and presence of publication bias (Guyatt 2011a). The GRADE approach results in an assessment of the quality of a body of evidence in one of four grades:
high: we are very confident that the true effect lies close to that of the estimate of the effect;
moderate: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different;
low: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect;
very low: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect (Schünemann 2013).
The review authors assessed the quality of the evidence found for outcomes identified as critical or important for clinical decision making. These outcomes were defined post hoc and included: death or major neurodevelopmental disability assessed at 12 months of age or greater (defined as cerebral palsy, developmental delay (Bayley or Griffith assessment more than two SD below the mean) or intellectual impairment (IQ more than two SD below mean), blindness (vision less than 6/60 in both eyes) or sensorineural deafness requiring amplification), as well as death (at any time), major neurodevelopmental disability assessed at 12 months of age or greater, and cerebral palsy or developmental delay. In addition, we included seizures (suspected clinically or identified by EEG or aEEG) and abnormal neurologic exam at discharge.
In cases where we considered the risk of bias arising from inadequate concealment of allocation, randomized assignment, complete follow‐up, or blinded outcome assessment to reduce our confidence in the effect estimates, we downgraded the quality of evidence accordingly (Guyatt 2011b). We evaluated consistency by similarity of point estimates, extent of overlap of CIs and statistical criteria including measurement of heterogeneity (I2 statistic). We downgraded the quality of evidence when large and unexplained inconsistency across studies results was present (i.e. some studies suggested important benefit and other studies suggested no effect or harm without a clinical explanation) (Guyatt 2011c). We assessed precision according with the 95% CI around the pooled estimation (Guyatt 2011d). When trials were conducted in populations other than the target population, we downgraded the quality of evidence because of indirectness (Guyatt 2011e).
We entered data (i.e. pooled estimates of the effects and corresponding 95% CI) and explicit judgments for each of the above aspects assessed into the Guideline Development Tool, the software used to create 'Summary of findings' tables (GRADEpro 2008). We explained all judgments involving the assessment of the study characteristics described above in footnotes or comments in the 'Summary of findings' table.
Subgroup analysis and investigation of heterogeneity
Barbiturate treatment compared to placebo or no treatment
Subgroup analyses:
gestational age (late preterm (34 to 36+6 weeks' gestation) versus term (37 weeks' gestation or greater));
type of barbiturate (phenobarbital or thiopentone);
severity of HIE (Sarnat score 2 or greater);
cooling therapy;
studies in low‐ and middle‐income countries.
Barbiturate treatment compared to other barbiturate treatment
Subgroup analyses:
gestational age (late preterm (34 to 36+6 weeks' gestation) versus term (37 weeks' gestation or greater));
type of barbiturate (phenobarbital or thiopentone);
severity of HIE (Sarnat score 2 or greater);
cooling therapy;
studies in low‐ and middle‐income countries.
Barbiturate treatment compared to other nonbarbiturate anticonvulsant treatment
Subgroup analyses:
gestational age (late preterm (34 to 36+6 weeks' gestation) versus term (37 weeks' gestation or greater));
type of anticonvulsant;
severity of HIE (Sarnat score 2 or greater);
cooling therapy;
studies in low‐ and middle‐income countries.
Results
Description of studies
The search identified 11 RCTs or quasi‐RCTs using neonatal barbiturate therapy following perinatal asphyxia, including eight previously identified studies (Goldberg 1986; Hall 1998; Ruth 1991; Singh 2004; Vargas‐Origel 2004; Vela 1987; Ruth 1988; Wilkinson 1989), and three new studies (Avasiloaiei 2013; Gathwala 2011; Velaphi 2013) (Figure 1). Eight studies compared barbiturate treatment to placebo or no treatment (Avasiloaiei 2013; Gathwala 2011; Goldberg 1986; Hall 1998; Ruth 1991; Singh 2004; Vargas‐Origel 2004; Velaphi 2013). One study compared barbiturate treatment to other nonbarbiturate therapy (Vela 1987). No studies compared barbiturate treatment to other barbiturate.
1.
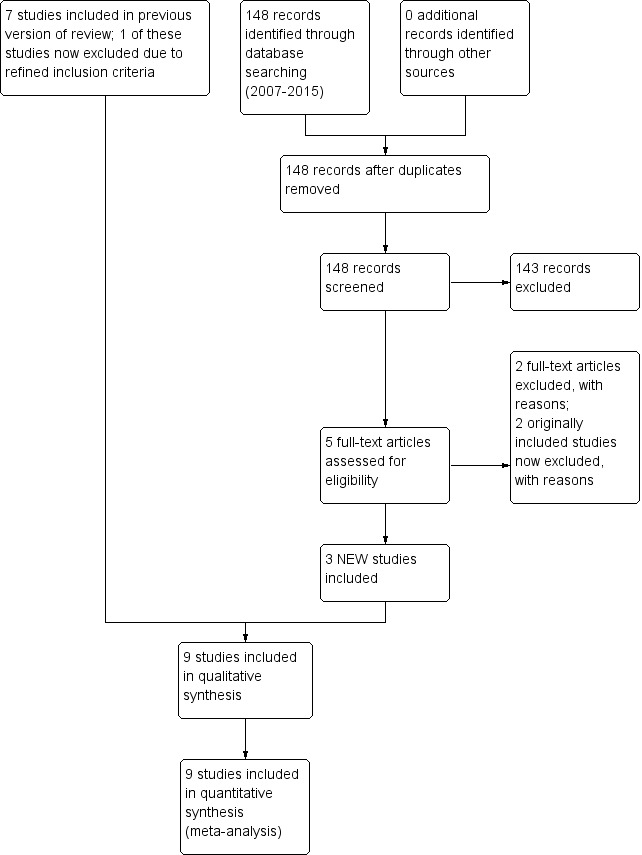
Study flow diagram: review update.
We excluded two studies using barbiturate therapy in neonates following perinatal asphyxia (Ruth 1988 included preterm, very low birth weight infants and Wilkinson 1989 used anticonvulsants for treatment of seizures, not prophylaxis). In addition, we excluded one previously included study not identified through our search (Kuzemko 1972, as it did not evaluate barbiturate therapy).
Results of the search
We updated the search in May 2014. Nine studies met criteria for inclusion, including three new studies.
We also identified additional data for a previously included study, Singh 2004 (from a 2005 publication of the same study population).
Included studies
The individual details for each study are noted below and in the Characteristics of included studies table.
Barbiturate treatment compared to placebo or no treatment
Eight studies compared barbiturate therapy to conventional therapy with or without placebo (Avasiloaiei 2013; Gathwala 2011; Goldberg 1986; Hall 1998; Ruth 1991; Singh 2004; Vargas‐Origel 2004; Velaphi 2013).
Four studies recorded long‐term neurodevelopmental outcome assessed at 12 months of age or greater (Avasiloaiei 2013; Goldberg 1986; Hall 1998; Ruth 1991). The remaining studies reported only short‐term neonatal outcomes (death, seizures in the neonatal period, and abnormal neurologic status) (Gathwala 2011; Singh 2004; Vargas‐Origel 2004; Velaphi 2013).
Goldberg 1986 reported seizure activity and developmental outcome at 12 months of age or greater for 32 severely asphyxiated term neonates with signs of HIE and requiring mechanical ventilation within the first hour of life (15 infants randomized to conventional therapy (control) and 17 infants randomized to prophylactic thiopental plus conventional therapy) in a single‐center RCT. Participants were term infants (37 weeks' gestation or greater) with severe perinatal asphyxia who had neurologic signs of HIE (decreased or absent response to noxious stimuli, seizure activity, or increased irritability) and required mechanical ventilation within first hour of life; plus two of the following three criteria: perinatal distress (abnormal fetal heart pattern or requiring prolonged neonatal resuscitation); Apgar score of 4 or less at five minutes; and base deficit 15 mEq/L or greater within first hour of life. Thirty‐two infants met the inclusion criteria and were randomized. The RCT excluded six infants prior to randomization for intractable hypotension, congenital intrauterine infection, complex congenital malformations, and maternal chorioamnionitis.
The thiopental treatment group (17 infants) received conventional therapy plus thiopental loading dose (15 mg/kg intravenous (IV) over 30 minutes), followed by a constant infusion of thiopental (10 mg/kg/hour for 90 minutes, 5 mg/kg/hour for 60 minutes, 3 mg/kg/hour for eight hours, 1.5 mg/kg/hour for six hours and 0.75 mg/kg/hour for six hours). The conventional therapy group (15 infants) received fluid restriction, mechanical ventilation, and treatment with phenobarbital or phenytoin for clinical apparent seizure activity. The primary outcome measured was reduction of intracranial pressure to the normal range. Secondary outcomes were hypotension requiring inotropes, overall effect on neurologic outcome, seizure activity over first 72 hours of life, death, and long‐term neurodevelopmental outcome. Neurodevelopmental assessments were performed at three, six, nine, 12, 18, 24, and 36 months. Developmental outcome was measured using the Bayley scales of infant development (normal 84 or greater, questionable 68 to 83, abnormal 67 or less) through two years of age, after which Stanford‐Binet assessments were performed.
Ruth 1991 reported on death before neurodevelopmental assessment and neurodevelopmental outcome at six years of age for 38 term neonates with evidence of perinatal asphyxia (17 controls randomized to conventional therapy and 21 infants randomized to prophylactic phenobarbital therapy) in a single‐center RCT. Eligible infants were term (37 weeks' gestation or greater) with evidence of perinatal asphyxia (five‐minute Apgar score 3 or less or need for ventilator assistance beyond 30 minutes of life). Thirty‐eight infants met the inclusion criteria and were randomized. The phenobarbital treatment group (21 infants) received conventional therapy plus a phenobarbital loading dose (30 mg/kg IV) prior to four hours of age, followed by a second dose of phenobarbital (15 mg/kg) four hours after the initial dose. These infants then received 5 mg/kg/day for five days. The control group (17 infants) received conventional therapy. Measured outcomes included death before neurodevelopmental assessment and disability (stated as cerebral palsy). Six‐year follow‐up included cognitive assessment (Wechsler Intelligence Scale for Children ‐ revised (WISC‐r)), expressed as IQ; neuropsychological tests (copying design (Visual‐Motor Integration (VMI) test), attention and confrontation naming (Developmental NEuroPSYchological (NEPSY) assessment)), and quality of life scale.
Hall 1998 reported on seizures in the newborn period, death before neurodevelopmental evaluation, neurologic outcome at three years and serum and cerebrospinal fluid (CSF) enzyme levels for 40 severely asphyxiated term and post term infants (20 controls randomized to conventional therapy and 20 infants randomized to prophylactic phenobarbital) in a single‐center RCT. Eligible infants were 37 weeks' gestation or greater with severe perinatal asphyxia (initial arterial pH 7 or less with base deficit 15 mEq/L or greater; five‐minute Apgar 3 or less; or failure to initiate spontaneous respirations at 10 minutes of life). Infants were excluded if they had any condition that was abnormal unrelated to asphyxia. Forty infants met inclusion criteria and were randomized. Thirty‐one infants (15 in the phenobarbital group and 16 in the conventional therapy group) completed the trial. Study failures included inability to obtain successful lumbar puncture, protocol violations, and infants lost to follow‐up. All infants were outborn and transferred to the neonatal intensive care unit within the first day of life. The phenobarbital treatment group (n = 20) received conventional therapy plus a single phenobarbital dose (40 mg/kg IV over 60 minutes) immediately following trial entry. The control group (n = 20) received conventional therapy. Conventional therapy for both groups included phenobarbital administration for the treatment of clinical seizures. This trial evaluated reduction in the incidence of clinical seizures as a primary outcome. Secondary outcomes included total lactate dehydrogenase (LDH), creatine kinase (CK) and the presence or absence of CK‐BB isoenzymes in CSF obtained day one and day two. Deaths before neurodevelopmental assessment and neurodevelopmental outcomes at three years of age were also evaluated. Neurodevelopmental assessments were completed at six, 12, 24, and 36 months of age using the Gessell, Bayley, or Stanford‐Binet tests. Adverse effects of phenobarbital administration were evaluated including hypotension and respiratory depression.
Singh 2004 reported on CSF levels of lipid peroxides, antioxidant enzymes, and serum levels of antioxidant vitamins in relation to adverse outcomes (death or abnormal neurologic exam at discharge) for 45 asphyxiated late preterm and term infants with evidence of HIE (abnormalities in tone or level of consciousness, or both) within the first six hours of life (20 controls randomized to conventional therapy and 25 infants randomized to prophylactic phenobarbital therapy). Singh and colleagues published a follow‐up report (Singh 2005, see Singh 2004), on adverse outcomes (death or neurologic abnormality at discharge) and secondary outcome variables including seizures, need for ventilation, and multiorgan dysfunction for the same 45 asphyxiated late preterm and term infants with evidence of HIE in this single‐center RCT. Eligible infants for this trial were 34 weeks' gestation or greater with perinatal asphyxia and showed evidence of HIE in the first six hours of life. Infants were included if, in the setting of a one‐minute Apgar score of 5 or less and evidence of fetal distress (fetal bradycardia, meconium‐stained amniotic fluid or arterial cord pH 7.15 or less), they developed abnormalities in tone or level of consciousness (or both) within the first six hours of life. Sixty infants met inclusion criteria and were randomized. Following randomization, 15 infants were excluded because of CSF findings suggestive of meningitis (one infant) or intracranial bleed/traumatic lumbar puncture (14 infants), leaving 45 infants (20 infants in the control group and 25 infants in the phenobarbital group). In addition, infants were excluded if they had major congenital malformations or if their mothers received phenobarbital during the last week prior to delivery. The phenobarbital group received conventional therapy plus phenobarbital (20 mg/kg IV over 20 minutes) within the first six hours of life. The control group received conventional therapy. Conventional therapy was as per unit protocol for management of HIE and included phenobarbital administration for seizures diagnosed clinical. Measured outcomes included primary outcome of death or abnormal neurologic exam at discharge (exam performed as described by Amiel‐Tison). Secondary outcomes included seizures, need for ventilation, and multiorgan dysfunction (Singh 2005, see Singh 2004). CSF levels of malondialdehyde (MDA), superoxide dismutase (SOD), glutathione peroxidase (GPx), and plasma levels of vitamins A and E were also evaluated at 10 to 12 hours of life and evaluated in relation to adverse outcomes (death or abnormal neurologic exam at discharge) (Singh 2004). Cranial ultrasounds were also obtained on day one, day three and day seven. Neonatologists who were blinded to group allocation performed discharge neurologic exam and cranial ultrasounds. In addition, evidence of adverse effects of phenobarbital administration included hypotension, need for ventilation, presence myocardial dysfunction, and excessive sleepiness.
Vargas‐Origel 2004 reported the frequency of HIE (according to Sarnat classification), clinical seizure activity, short‐term neurologic abnormalities, postasphyxial nonbrain complications, and adverse effects associated with phenobarbital administration for 73 term and post‐term infants with perinatal asphyxia (36 controls randomized to conventional treatment and 37 infants randomized to prophylactic phenobarbital) in an RCT. Eligible infants were term and post‐term infants (37 weeks' gestation or greater) with perinatal asphyxia (cord or arterial pH 7.0 or less within 15 minutes of life plus one or more of the following, base deficit 15 mEq/L or greater, Apgar score 3 or less at five minutes of life, or no spontaneous respiration by 15 minutes of life). Infants were excluded if they died within the first 48 hours of life; had major congenital abnormalities, congenital infection, or trauma during delivery; or exposure to maternal medications that would result in neonatal depression. The phenobarbital group received conventional therapy plus a phenobarbital loading dose (40 mg/kg IV over 60 minutes) administered within the first hour of life. The control group received placebo and conventional therapy, including phenobarbital at standard dosing for clinically evident seizure activity. Short‐term neonatal outcomes including clinical seizure activity, neurologic status at discharge, and postasphyxia nonbrain complications (renal, respiratory, cardiovascular, gastrointestinal, and metabolic) were measured. HIE grading according to Sarnat classification was performed to estimate frequency and severity of HIE.
Gathwala 2011 reported on levels of lipid peroxides and antioxidant enzymes in CSF, death, and neurologic outcome at one‐month follow‐up for 72 inborn term neonates with severe perinatal asphyxia (36 controls randomized to conventional therapy and 36 infants randomized to prophylactic phenobarbital) in a single‐center RCT. Eligible participants were term infants (37 weeks' gestation or greater) with severe perinatal asphyxia (cord blood pH less than 7 and Apgar score 5 or less at five minutes). Seventy‐two infants met the inclusion criteria and were randomized. Four infants were excluded prior to randomization for obvious congenital malformation. The phenobarbital treatment group received conventional therapy per unit protocol for HIE plus a phenobarbital loading dose (40 mg/kg IV infusion over 60 minutes) within the first two hours of life. Heart rate, oxygen saturation, respiration, and mean arterial pressure were monitored continuously during administration. The conventional therapy group received conventional therapy for HIE per unit protocol. Primary outcomes were CSF levels of lipid peroxides (MDA) and antioxidant enzymes (SOD and GPx) at 12 ± 2 hours of life. Additional outcomes included death, seizure activity, neurologic exam at discharge and neurologic outcome at one‐month follow‐up (based on neurologic exam, MRI, and electroencephalogram (EEG) results) were also evaluated. HIE staging was assessed for each infant according to Sarnat staging. Cranial ultrasounds were also obtained on day three and day seven of life. Adverse effects of phenobarbital administration were evaluated including hypotension and respiratory depression.
Velaphi 2013 reported on seizure activity, death, and neurologic outcome at hospital discharge for 94 term and late preterm infants with perinatal asphyxia (44 infants randomized to placebo plus conventional therapy and 50 infants randomized to prophylactic phenobarbital plus conventional therapy) in a single‐center RCT. Eligible infants were 34 weeks' gestation or greater or 2000 g or greater (or both) with evidence of perinatal asphyxia (base deficit greater than 16 mEq/L measured within one hour of life and Apgar score 6 or less at five minutes or requiring resuscitation for more than five minutes). Ninety‐four infants met the inclusion criteria and were randomized. Exclusion criteria included presence of congenital abnormalities, inability to randomize by six hours of life, or lack of spontaneous respirations within 20 minutes of life with or without bradycardia. The prophylactic phenobarbital group received conventional therapy plus phenobarbital loading dose (40 mg/kg IV infused over 60 minutes) within the first six hours of life. The control group received conventional therapy plus placebo (normal saline 1 mL/kg IV infused over 60 minutes) within the first six hours of life. Conventional therapy for both groups included regular monitoring of vital signs and administration of dextrose containing IV fluids and antibiotics. Clinical seizures were treated with phenytoin with or without clonazepam. If seizures persisted despite treatment with phenytoin and clonazepam, the code was broken and phenobarbital was administered if the infant had been in the control group. If the infant was in the phenobarbital group, a blood phenobarbital level was obtained and phenobarbital was administered if the level was below the therapeutic range. Measured outcomes included seizures, death, and neurologic outcome at discharge (improving or worsening HIE stage as compared to Sarnat stage at time of enrollment). Neonatal consultants uninvolved the care of the study infants performed neurologic exams according to Sarnat staging in all infants as 12 to 24 hours, two to three days, and five to seven days after birth and on discharge. In addition, possible adverse effects of phenobarbital administration were monitored for including hypotension and respiratory depression.
Avasiloaiei 2013 reported on red blood cell activity of antioxidant enzymes (SOD and GPx), total serum antioxidant status (TAS), lipid peroxidation (using MDA), mortality, seizures, neurologic abnormalities at greater than 72 hours of life and long‐term neurologic outcome at 12 months of age or greater for 67 term neonates with perinatal asphyxia (23 controls randomized to supportive care, 22 infants randomized to prophylactic phenobarbital, and 22 infants randomized to erythropoietin) in a single‐center RCT. Participants included term infants 37 weeks' gestation or greater with severe perinatal asphyxia. Infants were determined to have perinatal asphyxia when three of the four criteria of the American Academy of Pediatrics and the American College of Obstetricians and Gynecologists were met (umbilical artery blood pH less than 7.0, base deficit 12 mEq/L or greater, Apgar 3 or less at more than five minutes of life, neonatal neurologic sequelae (i.e. seizures, coma, hypotonia), multiple organ involvement (i.e. kidney, lungs, liver, heart, intestines), or a combination of these. Infants less than 37 weeks' gestation, with major congenital malformations, and with hemolytic disease due to rhesus (Rh) incompatibility were excluded from the study. Sixty‐seven infants met the inclusion criteria and were randomized. The prophylactic phenobarbital group received conventional supportive therapy plus phenobarbital loading dose (40 mg/kg IV) during the first four hours after birth. The erythropoietin group received conventional supportive therapy plus subcutaneous erythropoietin (1000 IU/kg per day) for three days. The supportive therapy group received conventional supportive therapy including oxygen, volume expanders, inotrope, diuretics, antibiotics, and anticonvulsants for clinically suspected seizures confirmed by aEEG. Serum levels of antioxidant enzymes (SOD and GPx), TAS, and lipid peroxidation (using MDA levels) were measured at four, 24, 48, and 72 hours and at seven days of life. Mortality, presence or absence of seizures (confirmed by aEEG), neurologic abnormalities on exam at greater than 72 hours of life and at the time of discharge (using Amiel‐Tison assessment), and long‐term neurologic outcome (including assessment for motor disability and disorders of receptive language, expressive language, and cognitive development) at three, six, nine, 12, and 18 months of age (using Bayley II assessment) were evaluated.
Barbiturate treatment compared to other barbiturate treatment
We found no trials comparing barbiturate versus other barbiturate.
Barbiturate treatment compared to other nonbarbiturate anticonvulsant treatment
One RCT compared barbiturate versus other nonbarbiturate anticonvulsant therapy.
Vela 1987 reported on seizure activity for 17 term neonates with perinatal asphyxia randomized to prophylactic treatment with phenobarbital plus conventional therapy (nine infants) or phenytoin plus conventional therapy (eight infants) in a single‐center RCT. Eligible infants included term infants with perinatal asphyxia (defined as three of the following criteria: multiple late fetal heart rate decelerations, fetal bradycardia for more than 20 minutes, meconium‐stained fluid, Apgar score of less than 8 at five minutes, requiring mechanical ventilation, requiring IV bicarbonate therapy). Seventeen infants met the inclusion criteria and were randomized. The phenobarbital treatment group (nine infants) received conventional therapy plus phenobarbital (12 mg/kg intramuscular (IM) on day one, followed by 6 mg/kg/day through day seven). The phenytoin group (eight infants) received conventional therapy plus phenytoin (12 mg/kg IM on day one, followed by 6 mg/kg/day through day seven). The primary outcome measured was development of seizure activity. Adverse effects of medication administration (i.e. drowsiness and bradycardia) were evaluated.
Excluded studies
We excluded four RCTs (see Characteristics of excluded studies table).
Ruth 1988 randomized premature, very low birth weight infants to receive phenobarbital or placebo following birth; perinatal asphyxia was not an eligibility criterion.
Wilkinson 1989 compared four anticonvulsants in an RCT. The anticonvulsants were used for treatment (not prophylaxis) of EEG‐apparent seizures of any etiology, not exclusively perinatal asphyxia. Infants were a mixture of term and premature neonates. It was not possible to extract the data relating only to term infants following asphyxia.
Kuzemko 1972 randomized infants to receive chloral hydrate or diazepam, agents that were not of interest in the current meta‐analysis.
van Rooij 2010 randomized infants to receive treatment (not prophylaxis) for both clinical and subclinical seizures or blinding of aEEG registration and treatment of clinical seizures only.
Risk of bias in included studies
Selection bias
Four studies achieved randomization/allocation concealment by means of random number table (Gathwala 2011; Hall 1998; Velaphi 2013), or computer‐generated random numbers (Singh 2004), concealed in opaque envelopes and opened at the time of randomization. In three studies, the method of randomization was by random‐draw numerical assignment (Avasiloaiei 2013), and "list of random numbers" (Goldberg 1986), or "random numbers" (Vargas‐Origel 2004) with method of allocation concealment not specified. Two studies did not specify the methods for both randomization and allocation concealment (Ruth 1991; Vela 1987).
Performance bias
In six studies, there was no blinding or no blinding specified and no use of placebo (Avasiloaiei 2013; Gathwala 2011; Goldberg 1986; Hall 1998; Ruth 1991; Singh 2004). In two of these studies (Goldberg 1986; Singh 2004), though initial blinding did not occur, long‐term follow‐up was performed by developmentalists and neurologists blinded to the infant's treatment regimen (Goldberg 1986), CSF samples were coded before sending to the laboratory to ensure blinding (Singh 2004), and discharge neurologic exams and cranial ultrasounds were performed by neonatologists blinded to group allocation (Singh 2004). Three studies administered a placebo (Vargas‐Origel 2004; Velaphi 2013) or second study drug (Vela 1987). Blinding was clearly stated in only one study (Vela 1987), and implied in two (Vargas‐Origel 2004; Velaphi 2013).
Potential bias arising from co‐intervention remained a possibility in the six studies that did not use caretaker blinding (Avasiloaiei 2013; Gathwala 2011; Goldberg 1986; Hall 1998; Ruth 1991; Singh 2004). In Avasiloaiei 2013, 13/23 (56.5%) infants in the supportive care group received phenobarbital for clinically suspected seizure activity confirmed by aEEG. In Goldberg 1986, 14 infants in the group treated with thiopental and 12 infants in the control group received phenobarbital for the treatment of clinical seizures. Hall 1998 compared phenobarbital with conventional treatment for the prevention of seizures following asphyxia. The control group received a mean of 27 mg/kg of phenobarbital as treatment for seizures, compared to a mean of 39 mg/kg in the experimental group. The clinicians caring for the infants were aware of treatment allocation, and may have had a lower threshold to diagnose and treat clinical seizures in the control group (co‐intervention bias). Three RCTs did not clearly state the number of infants treated with additional anticonvulsants (Gathwala 2011; Ruth 1991; Singh 2004).
Attrition bias
There was a postrandomization loss of 23% in the study by Hall 1998; 6% of living infants in Goldberg 1986; 3% of living infants in Gathwala 2011; and 0% in Avasiloaiei 2013, Singh 2004, Vargas‐Origel 2004, Velaphi 2013, and Vela 1987. There were no data to calculate attrition in Ruth 1991.
Detection bias
Only four studies had blind assessment of outcome (Goldberg 1986; Singh 2004; Vela 1987; Velaphi 2013), and only one of these assessed outcomes outside the neonatal period (Goldberg 1986). Vela 1987 utilized a double‐blind study design. Goldberg 1986 used a blinded outcome assessment at one to three years but was not blinded for short‐term outcomes.
Effects of interventions
See: Table 1
Nine RCTS enrolled 456 term and late preterm infants aged less than three days old with perinatal asphyxia without evidence of seizures to determine the effect of administering prophylactic barbiturate therapy on death (Avasiloaiei 2013; Gathwala 2011; Goldberg 1986; Hall 1998; Ruth 1991; Singh 2004; Vargas‐Origel 2004; Velaphi 2013), short‐term medical outcomes (Gathwala 2011; Goldberg 1986; Hall 1998), short‐term neurologic outcomes (Avasiloaiei 2013; Goldberg 1986; Hall 1998; Singh 2004; Vargas‐Origel 2004; Vela 1987; Velaphi 2013), and longer‐term neurodevelopmental outcomes (Avasiloaiei 2013; Goldberg 1986; Hall 1998; Ruth 1991; Vargas‐Origel 2004).
Data presented allowed for analysis according to subgroups for type of barbiturate, severity of HIE, and studies in low‐ and middle‐income countries.
Barbiturate treatment compared to placebo or no treatment
Primary outcomes
Death or severe neurodevelopmental disability (outcome 1.1)
One trial permitted the assessment of the effect of barbiturate versus control on the composite outcome of death or severe neurodevelopmental disability (Hall 1998). There were 31 infants included in the results of this trial, of whom 17 either died or had severe neurodevelopmental disability. This study demonstrated a significant reduction in the combined outcome of death or severe neurodevelopmental disability in infants treated with phenobarbital (RR 0.33, 95% CI 0.14 to 0.78; RD ‐0.55, 95% CI ‐0.84 to ‐0.25; NNTB 2, 95% CI 1 to 4; 1 study, 31 infants) (very low quality evidence) (Analysis 1.1). Heterogeneity of the effect was not applicable.
1.1. Analysis.
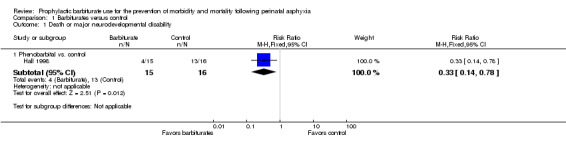
Comparison 1 Barbiturates versus control, Outcome 1 Death or major neurodevelopmental disability.
Secondary outcomes
Death (at any time) (outcome 1.2)
Eight trials comparing barbiturates with conventional therapy reported on death (Avasiloaiei 2013; Gathwala 2011; Goldberg 1986; Hall 1998; Ruth 1991; Singh 2004; Vargas‐Origel 2004; Velaphi 2013). No studies found a statistically significant difference in mortality rates between the experimental and control groups. The meta‐analysis of all eight trials demonstrated no significant difference in death between the two groups (typical RR 0.88, 95% CI 0.55 to 1.42; 8 trials, 429 infants) (low quality evidence) (Analysis 1.2.1). There was no evidence of heterogeneity of effect (I2 = 0%).
1.2. Analysis.
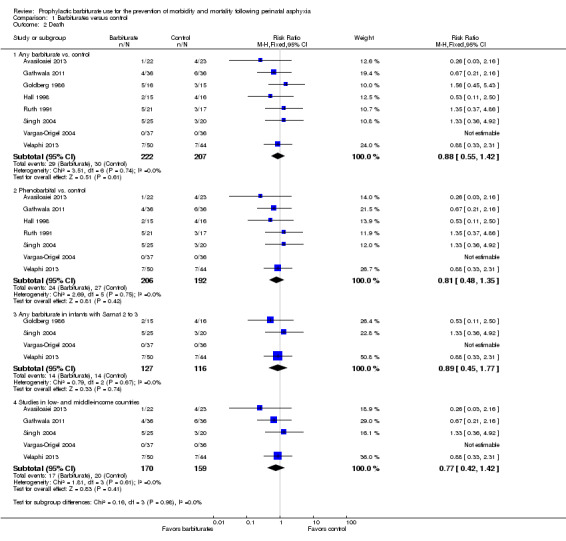
Comparison 1 Barbiturates versus control, Outcome 2 Death.
Seven trials compared phenobarbital versus control (Avasiloaiei 2013; Gathwala 2011; Hall 1998; Ruth 1991; Singh 2004; Vargas‐Origel 2004; Velaphi 2013). Meta‐analysis showed no statistically significant effect on mortality (typical RR 0.81, 95% CI 0.48 to 1.35; 7 trials, 398 infants) (Analysis 1.2.2). There was no evidence of heterogeneity of effect (I2 = 0%).
The one study that compared thiopentone versus control also showed no difference in mortality (RR 1.47, 95% CI 0.42 to 5.14; 1 trial, 31 infants) (Goldberg 1986).
Four trials presented results for the subset of infants with Sarnat 2 or greater for barbiturate versus control (Goldberg 1986; Singh 2004; Vargas‐Origel 2004; Velaphi 2013). Meta‐analysis showed no difference in mortality (typical RR 0.89, 95% CI 0.45 to 1.77; 4 trials, 243 infants) (Analysis 1.2.3). There was no evidence of heterogeneity of effect (I2 = 0%).
Five trials were conducted in low‐ or middle‐income countries (Avasiloaiei 2013; Gathwala 2011; Singh 2004; Vargas‐Origel 2004; Velaphi 2013). Meta‐analysis showed no difference in mortality (typical RR 0.77, 95% CI 0.42 to 1.42; 5 trials, 329 infants) (Analysis 1.2.4). There was no evidence of heterogeneity of effect (I2 = 0%).
Severe neurodevelopmental disability in survivors examined (outcome 1.3)
One trial including 31 infants comparing prophylactic barbiturate therapy with conventional therapy reported data on the effect on major neurodevelopmental disability in survivors (Hall 1998). Of the 31 infants in this trial, 11 had severe neurodevelopmental disability. This study demonstrated a significant reduction in severe neurodevelopmental disability in infants treated with phenobarbital (RR 0.24, 95% CI 0.06 to 0.92; RD ‐0.43, 95% CI ‐0.73 to ‐0.13; NNTB 2, 95% CI 1 to 8; 1 study, 31 infants) (very low quality evidence) (Analysis 1.3). Heterogeneity of effect was not applicable.
1.3. Analysis.
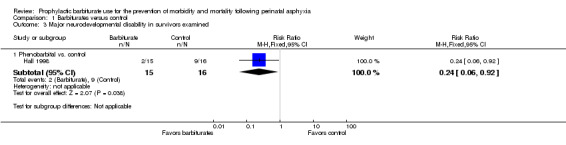
Comparison 1 Barbiturates versus control, Outcome 3 Major neurodevelopmental disability in survivors examined.
Each component of major neurodevelopmental disability
Cerebral palsy in survivors assessed (outcome 1.4)
Two trials comparing barbiturates (both used phenobarbital) with conventional therapy reported on the long‐term outcome of cerebral palsy (Hall 1998; Ruth 1991). There were 69 infants, of these 13 were identified as having cerebral palsy. Neither study found a statistically significant difference in rates of cerebral palsy between the experimental and control groups. The meta‐analysis of the two trials demonstrated no significant difference in cerebral palsy between the two groups (typical RR 0.58, 95% CI 0.19 to 1.70; 2 trials, 69 infants) (very low quality evidence) (Analysis 1.4). There was moderate heterogeneity of effect (I2 = 67%).
1.4. Analysis.
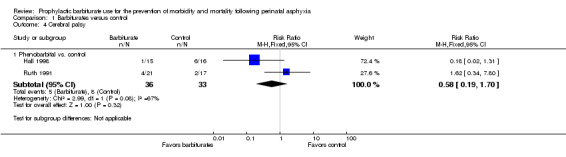
Comparison 1 Barbiturates versus control, Outcome 4 Cerebral palsy.
Developmental delay or intellectual impairment in survivors assessed (outcome 1.5)
Two trials comparing barbiturates with conventional therapy reported on developmental delay in survivors (Avasiloaiei 2013; Goldberg 1986). There were 63 infants, of these 17 were identified as having developmental delay. Neither study found a statistically significant difference in the rates of developmental delay between the experimental and control groups. Meta‐analysis of the two trials found no statistically significant difference in developmental delay between the two groups (typical RR 0.54, 95% CI 0.24 to 1.19; 2 trials, 63 infants) (very low quality evidence) (Analysis 1.5). There was moderate heterogeneity of effect (I2 = 50%).
1.5. Analysis.
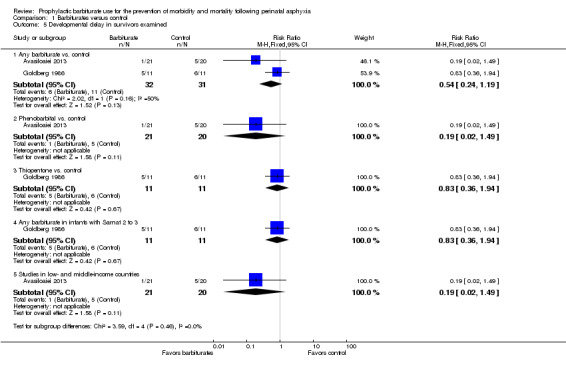
Comparison 1 Barbiturates versus control, Outcome 5 Developmental delay in survivors examined.
The one trial comparing phenobarbital versus conventional therapy showed no difference in developmental delay between the groups (RR 0.19, 95% CI 0.02 to 1.49; 1 trial, 41 infants) (Analysis 1.5.2) (Avasiloaiei 2013). The one trial that compared thiopentone versus control also showed no difference in developmental delay (RR 0.83, 95% CI 0.36 to 1.94; 1 trial, 31 infants) (Analysis 1.5.3) (Goldberg 1986).
One trial identified results for the subset of infants with Sarnat 2 or greater for barbiturate versus control (Goldberg 1986). This trial showed no difference in developmental delay between the two groups for this subset of infants (RR 0.83, 95% CI 0.36 to 1.94); 1 trial, 31 infants) (Analysis 1.5.4).
One trial was conducted in a low or middle income country (Avasiloaiei 2013), this trial did not demonstrate a difference in developmental delay between the groups (RR 0.19, 95% CI 0.02 to 1.49; 1 trial, 41 infants) (Analysis 1.5.5). Heterogeneity of effect was not applicable.
IQ in survivors (outcome 1.6)
One trial comparing barbiturate with conventional therapy reported on IQ in survivors (Ruth 1991). There was no significant difference in the IQ of survivors at six years of age, treated with phenobarbital versus controls (MD ‐3.00, 95% CI ‐21.57 to 15.57; 1 trial, 30 infants) (Analysis 1.6).
1.6. Analysis.
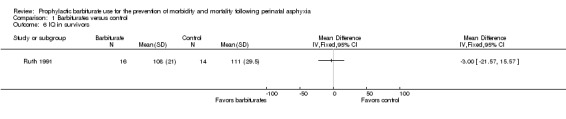
Comparison 1 Barbiturates versus control, Outcome 6 IQ in survivors.
Blindness vision (less than 6/60 in both eyes)
We found no trials reporting blindness vision.
Sensorineural deafness requiring amplification
We found no trials reporting sensorineural deafness requiring amplification.
Seizures within the neonatal period (outcome 1.7)
Six trials comparing barbiturates with conventional therapy reported on seizures in the neonatal period (Avasiloaiei 2013; Goldberg 1986; Hall 1998; Singh 2004; Vargas‐Origel 2004; Velaphi 2013). There were 319 infants, of these 121 infants developed seizures in the neonatal period. One study found a statistically significant reduction in seizures for the experimental group compared to controls (Singh 2004). Three studies showed a nonsignificant reduction in seizures in favor of the experimental group (Avasiloaiei 2013; Hall 1998; Velaphi 2013). Meta‐analysis of all six trials demonstrated a statistically significant reduction in seizures for the experimental group compared to controls (typical RR 0.62, 95% CI 0.48 to 0.81; typical RD ‐0.18, 95% CI ‐0.27 to ‐0.09; NNTB 5, 95% CI 4 to 11; 6 studies, 319 infants) (low quality evidence) (Analysis 1.7.1) (Figure 2). There was moderate heterogeneity of effect (I2 = 68%).
1.7. Analysis.
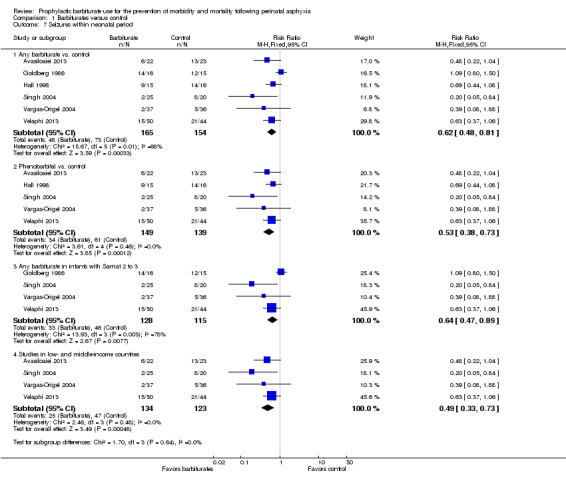
Comparison 1 Barbiturates versus control, Outcome 7 Seizures within neonatal period.
2.
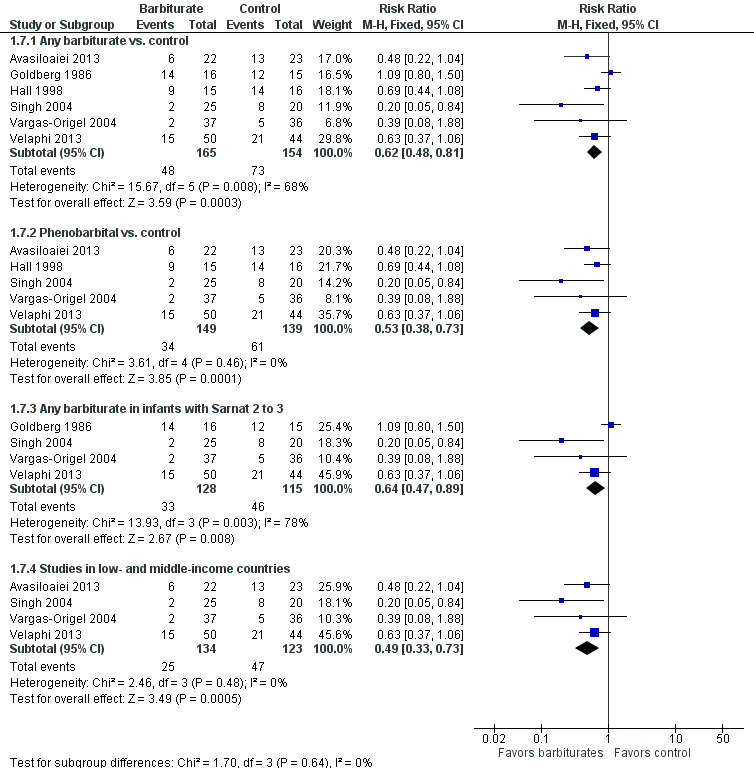
Forest plot of comparison: 1 Barbiturates versus control, outcome: 1.7 Seizures within neonatal period.
Meta‐analysis of the five trials that compared phenobarbital versus control showed a statistically significant difference in seizures between the two groups in favor of the experimental group (typical RR 0.53, 95% CI 0.38 to 0.73; typical RD ‐0.20, 95% CI ‐0.30 to ‐0.11; NNTB 5, 95% CI 3 to 9; 5 studies, 288 infants) (Analysis 1.7.2) (Avasiloaiei 2013; Hall 1998; Singh 2004; Vargas‐Origel 2004; Velaphi 2013). There was no evidence of heterogeneity of effect (I2 = 0%). The one study that compared thiopentone versus control showed no difference in seizures between the two groups (RR 1.09, 95% CI 0.80 to 1.50; 1 trial, 31 infants) (Goldberg 1986).
Meta‐analysis of the four trials that identified results for the subset of infants with Sarnat 2 or greater for barbiturate versus control also showed a significant reduction in seizures in the experimental group compared to the control group (typical RR 0.64, 95% CI 0.47 to 0.89; typical RD ‐0.14, 95% CI ‐0.25 to ‐0.04; NNTB 7, 95% CI 4 to 25; 4 studies, 243 infants) (Analysis 1.7.3) (Goldberg 1986; Singh 2004; Vargas‐Origel 2004; Velaphi 2013). There was substantial heterogeneity of effect (I2 = 78%).
Meta‐analysis of the four trials conducted in low‐ or middle‐income countries showed a significant reduction in seizures in the experimental group compared to the control group (typical RR 0.49, 95% CI 0.33 to 0.73; typical RD ‐0.20, 95% CI ‐0.30 to ‐0.09; NNTB 5, 95% CI 3 to 11; 4 studies, 257 infants) (Analysis 1.7.4) (Avasiloaiei 2013; Singh 2004; Vargas‐Origel 2004; Velaphi 2013). There was no evidence of heterogeneity of effect (I2 = 0%).
Adverse effects of barbiturate administration
Respiratory depression (outcome 1.8)
Two trials comparing barbiturate (both phenobarbital) with conventional therapy reported on respiratory depression requiring intubation (Gathwala 2011; Hall 1998). There were 93 infants, of whom seven required intubation for respiratory depression. All of these infants were from the same trial (Hall 1998). There was no difference in respiratory depression requiring intubation between these two groups (typical RR 0.75, 95% CI 0.18 to 3.11; typical RD ‐0.02, 95% CI ‐0.14 to 0.09; 2 trials, 93 infants) (Analysis 1.8).
1.8. Analysis.
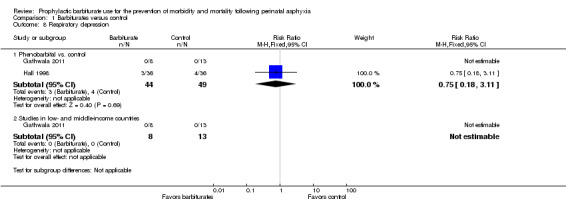
Comparison 1 Barbiturates versus control, Outcome 8 Respiratory depression.
Hypotension requiring inotropic support (outcome 1.9)
Only one trial comparing thiopentone with conventional therapy reported on hypotension requiring inotropic support (Goldberg 1986). There were 31 infants, of whom 21 required inotropic support. There was a statistically significant difference between the experimental and control groups in favor of the control (RR 1.88, 95% CI 1.06 to 3.32; RD 0.41, 95% CI 0.11 to 0.71; NNTH 7, 95% CI 1 to 9; 1 trial, 31 infants) (Analysis 1.9). Heterogeneity of effect was not applicable.
1.9. Analysis.
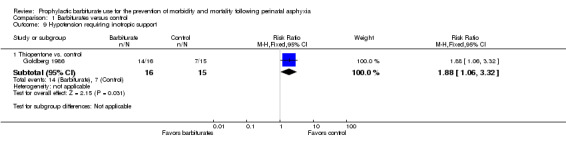
Comparison 1 Barbiturates versus control, Outcome 9 Hypotension requiring inotropic support.
Post hoc outcomes
Abnormal neurologic exam (outcome 1.10)
Four trials comparing phenobarbital with conventional therapy reported on abnormal neurologic exam at discharge (Avasiloaiei 2013; Singh 2004; Vargas‐Origel 2004; Velaphi 2013). There were 245 infants, of whom 97 had an abnormal neurologic exam at discharge. None of the studies found a statistically significant difference between the experimental and control groups. Meta‐analysis of the four trials demonstrated a nonsignificant trend in favor of the experimental group compared to the control (typical RR 0.85, 95% CI 0.67 to 1.08; 4 trials, 245 infants) (very low quality evidence) (Analysis 1.10.1). There was substantial heterogeneity of effect (I2 = 76%).
1.10. Analysis.
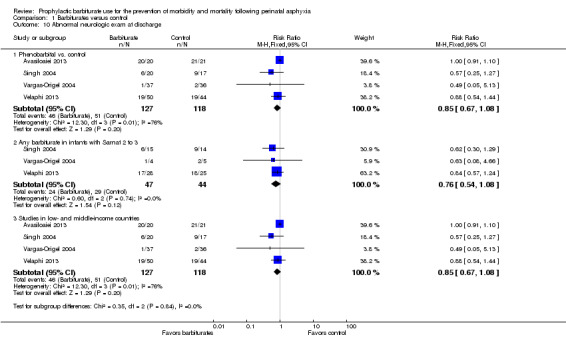
Comparison 1 Barbiturates versus control, Outcome 10 Abnormal neurologic exam at discharge.
Meta‐analysis of the three trials that identified results for the subset of infants with Sarnat 2 or greater for barbiturate versus control also showed a nonsignificant trend in favor of the experimental group compared to the control group (typical RR 0.76, 95% CI 0.54 to 1.08; 3 studies, 91 infants) (Analysis 1.10.2) (Singh 2004; Vargas‐Origel 2004; Velaphi 2013). There was no heterogeneity of effect (I2 = 0%).
Meta‐analysis of the four trials conducted in low‐ and middle‐income countries demonstrated a nonsignificant trend in favor of the experimental group compared to the control (typical RR 0.85, 95% CI 0.67 to 1.08; 4 trials, 245 infants) (Analysis 1.10.3) (Avasiloaiei 2013; Singh 2004; Vargas‐Origel 2004; Velaphi 2013). There was substantial heterogeneity of effect (I2 = 76%)
Barbiturate treatment compared to other barbiturate treatment
We found no trials comparing barbiturate versus other barbiturate.
Barbiturate treatment compared to other nonbarbiturate anticonvulsant treatment
We found one RCT comparing barbiturate therapy to other nonbarbiturate anticonvulsant therapy, which compared barbiturate therapy to phenytoin (Vela 1987).
Primary outcomes
Death or severe neurodevelopmental disability
The trial did not report death or severe neurodevelopmental disability.
Secondary outcomes
Seizures within the neonatal period (outcome 2.1)
The trial comparing barbiturate therapy to other nonbarbiturate anticonvulsant therapy reported seizures in the neonatal period (Vela 1987). There were 17 infants, of whom two had seizures in the neonatal period. There was no significant difference in seizure activity in the neonatal period between the two study groups (RR 0.89, 95% CI 0.07 to 12.00; 1 trial, 17 infants) (Analysis 2.1). Heterogeneity of effect was not applicable.
2.1. Analysis.
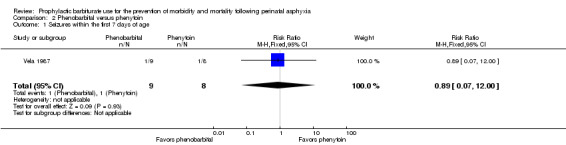
Comparison 2 Phenobarbital versus phenytoin, Outcome 1 Seizures within the first 7 days of age.
The trial reported no other relevant outcomes for this comparison.
Discussion
Outcome of infants with moderate‐to‐severe HIE has improved with the introduction of mild hypothermia (Jacobs 2013). Despite these advances, perinatal asphyxia remains an important cause of death and neurodevelopmental disability. The exploration of additional neuroprotective strategies remains an important clinical issue.
In this review, we identified nine RCTs of prophylactic barbiturates for the prevention of morbidity and mortality in full term and late preterm infants with perinatal asphyxia. Eight of these studies compared prophylactic barbiturate therapy to conventional treatment (enrolling 439 infants) and one study compared barbiturate therapy to treatment with phenytoin (enrolling 17 infants).
Prophylactic barbiturate treatment versus conventional treatment
Only one study reported on the composite primary outcome of mortality or severe neurodevelopmental disability (Hall 1998). This trial, which compared phenobarbital to conventional therapy, was small and did not blind allocation of the intervention. Hall 1998 reported a significant reduction in the combined outcome of death or severe neurodevelopmental disability in infants treated with phenobarbital.
Regarding the components of our primary outcome, eight trials comparing barbiturates with conventional therapy reported death (Avasiloaiei 2013; Gathwala 2011; Goldberg 1986; Hall 1998; Ruth 1991; Singh 2004; Vargas‐Origel 2004; Velaphi 2013). No studies found a statistically significant difference in mortality rates between the experimental and control groups and the meta‐analysis did not support any difference in mortality.
Only one trial reported on major neurodevelopmental disability in survivors (Hall 1998). There were 31 infants in this trial, of whom 11 had severe neurodevelopmental disability. This study reported a significant reduction in severe neurodevelopmental disability in infants treated with phenobarbital.
In the current review, the outcome of abnormal neurologic exam at the time of discharge was added post hoc. Though not statistically significant, meta‐analysis of the four trials that reported on this outcome showed a trend towards a reduction in the risk of an abnormal exam with the use of prophylactic barbiturate therapy compared to conventional therapy. This trend is of interest as an abnormal neurologic exam at discharge has been shown to be associated with a greater risk of death or disability (Gunn 2008; Sarnat 1976; Shankaran 2012), and served as a better predictor of death or disability at 18 months of age than did the treatment group allocation (control versus hypothermia) in a secondary analysis of the National Institute of Child Health and Human Development (NICHD) trial of whole body hypothermia (Shankaran 2012).
Most studies included in this review reported seizures within the neonatal period. Meta‐analysis of these trials demonstrated a significant reduction in the risk of seizures in infants treated with barbiturates soon after perinatal asphyxia when compared to those who received conventional therapy. In the overall analysis, we noted substantial heterogeneity (I2 = 68%); however, this heterogeneity was not noted in the a priori subgroup analysis of the five trials that used phenobarbital.
Though the reduction in seizures was significant, the clinical importance of this remains unclear. This reduction does not appear, from the limited data available in the current review, to translate into a reduction in the risk of developmental delay. In infants with HIE, clinical seizures may be associated with worse neurodevelopmental outcome, independent of HIE severity (Glass 2009). In addition, post hoc analysis of data from the Cool‐Cap trial revealed that absence of seizures represented an independent predictor of better 18‐month outcome in asphyxiated neonates (Wyatt 2007). However, it is unknown whether the successful pharmacologic treatment of seizures can lead to improved neurodevelopmental outcome.
The one trial that compared barbiturate therapy to other nonbarbiturate anticonvulsant therapy did not show a significant difference in the risk of seizures.
Prophylactic barbiturate treatment versus other prophylactic nonbarbiturate anticonvulsant treatment
There were no data reported for the primary outcome for the comparison of barbiturate therapy to other nonbarbiturate anticonvulsant therapy. One study reported on prophylactic barbiturate versus prophylactic phenytoin. There was no significant difference in seizure activity in the neonatal period between the two study groups.
Limitations
A sizable limitation to the current review is that none of the available trials included therapeutic hypothermia as part of standard or conventional management of these infants. Therapeutic hypothermia has been proven to decrease the risk of mortality and neurodevelopmental delay in infants with early moderate‐to‐severe encephalopathy and has become the standard of care (Jacobs 2013). This limits our ability to generalize to this population of infants who will receive therapeutic hypothermia. Issues to consider are the pharmacokinetics of phenobarbital during therapeutic hypothermia and the potential for exacerbation of the potential negative effects of phenobarbital administration. However, it may also be that phenobarbital, in the context of therapeutic hypothermia, may provide additional benefit. Phenobarbital administration prior to cooling is associated with an altered temperature profile during induction, with infants reaching goal temperature more quickly compared to infants who did not receive phenobarbital (Sant'Anna 2012).
Only one study utilized newer bedside technologies for the diagnosis and management of seizures (Avasiloaiei 2013). Use of aEEG has been increasing as it is readily available at the bedside for immediate assessment of infants and is more readily interpreted without specialized expertise. This use is controversial as it is subject to artefact, and has fewer electrodes than full channel EEG, resulting in potential underdiagnosis of seizure burden (Shellhaas 2007; Wusthoff 2009). Nonetheless, given that infants with suspected seizure are routinely monitored by aEEG, use of this modality should be included in studies assessing seizure burden and barbiturate use.
In addition, no uniform definition of perinatal asphyxia was used between studies. In the absence of a valid practical single marker of a hypoxic‐ischemic insult (with potential to cause significant cerebral injury), researchers should strive to use consensus based diagnostic criteria and endeavor to collect data relating to the markers in the neonatal period known at present to be associated with a poor neurodevelopmental outcome (e.g. MRI, EEG data, etc.). It is also important for future research to identify other early and reliable markers associated with greatest risk of asphyxial cerebral injury, in order that potential neuroprotective strategies, commenced soon after birth, can be assessed.
Authors' conclusions
Implications for practice.
Although the administration of prophylactic barbiturate therapy to infants following perinatal asphyxia did reduce the risk of seizures, there was no reduction in mortality and there remains a paucity of data for long‐term outcomes. At the present time, the administration of prophylactic barbiturate therapy to late preterm and term infants in the immediate period following perinatal asphyxia cannot be recommended for routine clinical practice.
Implications for research.
Given the frequent use of phenobarbital in this population, trials of prophylactic barbiturate therapy remain an important area for further investigation. Future trials should be of high quality, randomized controlled trials with blinding of allocation, performance, and outcome assessment. These studies should be sufficient size to detect clinically important reductions in mortality and severe neurodevelopmental disability and should be conducted in the context of therapeutic hypothermia, as there is currently no data for infants in this setting. The next generation of studies should use 'state of the art' diagnosis and management of seizures in neonates, including either use of amplitude‐integrated electroencephalography or continuous video monitoring electroencephalography in the management of these high risk infants.
Potential adverse effects, such as hypotension and respiratory depression following barbiturate therapy, should be prospectively defined and reported. Given the relatively low incidence of perinatal asphyxia, future studies should include a collaborative effort between centers.
What's new
| Date | Event | Description |
|---|---|---|
| 15 March 2016 | New citation required and conclusions have changed | Based on the change in scope and new studies identified for inclusion, results have changed. |
| 14 March 2016 | New search has been performed | The new search identified 3 new studies for inclusion (Avasiloaiei 2013; Gathwala 2011; Velaphi 2013) and further information was identified on the study of Singh 2004. |
| 16 February 2014 | New search has been performed | 1. scope limited to barbiturate therapy 2. additional subgroup analyses 3. late preterm infants included |
History
Protocol first published: Issue 3, 1998 Review first published: Issue 3, 1998
| Date | Event | Description |
|---|---|---|
| 2 February 2014 | Amended | Converted to new review format. |
| 8 March 2007 | New search has been performed | This review updates the existing review "Anticonvulsants for preventing mortality and morbidity in full term newborns with perinatal asphyxia", first published in The Cochrane Library, Issue 3, 1998 and updated in Issue 2, 2001 (Evans 1998, Evans 2001). Our search was updated in January 2007. Two additional studies have been incorporated in the review since 2001. |
| 8 March 2007 | New citation required but conclusions have not changed | New citation ‐ conclusions not changed |
Acknowledgements
None.
Appendices
Appendix 1. Search strategy through 2007
We used the standard search strategy of the Cochrane Neonatal Review Group as outlined in The Cochrane Library (2007, Issue 2). This included searches of the Oxford Database of Perinatal Trials, the Cochrane Central Register of Controlled Trials (CENTRAL, 2007, Issue 2), MEDLINE, previous reviews including cross‐references, abstracts, conferences, symposia proceedings, expert informants, and journal handsearching. We did not apply any language restrictions.
The original search strategy for MEDLINE and the Cochrane Controlled Trials Registry is given below. We searched the abstracts of the Society for Pediatric Research and European Society for Pediatric Research, published in the Journal of Pediatric Research from January 1980 to January 2007. We searched cited references from retrieved articles for additional studies. We reviewed abstracts and letters to the editor to identify randomized controlled trials that had not been published and editorials, indicating expert opinion, to identify any further studies, not already included in the review.
MEDLINE search
Dates: 1966 to November 2006
Strategy
#1 explode 'Asphyxia‐Neonatorum' / all subheadings #2 asphyx$ #3 hypox$ ADJ ischaemi$ #4 hypox$ ADJ ischemi$ #5 encephalopath$ #6 #1 or #2 or #3 or #4 or #5 #7 explode 'Anticonvulsants‐' / all subheadings #8 anticonvuls$ #9 explode 'Seizures‐' / all subheadings #10 convulsi$ #11 seiz$ #12 #7 or #8 or #9 or #10 or #11 #13 #6 and #12
This output was combined with the search filter for randomized controlled trials (see Appendix 5c of the Cochrane Handbook for Systematic Reviews of Interventions; Higgins 2011).
Cochrane Controlled Trials Registry Search
Date: 2006, Issue 3
Strategy
#1 ASPHYXIA‐NEONATORUM*:ME #2 ASPHYX* #3 HYPOX* #4 ENCEPH* #5 (((#1 or #2) or #3) or #4) #6 ANTICONVULSANTS*:ME #7 SEIZURES*:ME #8 CONVULSI* #9 SEIZ* #10 ANTICONVULS* #11 ((((#6 or #7) or #8) or #9) or #10) #12 (#5 and #11)
Appendix 2. Standard search methodology 2015 update
Cochrane Central Register of Controlled Trials (CENTRAL, 2015, Issue 11)
(infant or newborn or neonate or neonatal or premature or very low birth weight or low birth weight or VLBW or LBW)
MEDLINE via PubMed
((infant, newborn[MeSH] OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW or infan* or neonat*) AND (randomized controlled trial [pt] OR controlled clinical trial [pt] OR Clinical Trial[ptyp] OR randomized [tiab] OR placebo [tiab] OR clinical trials as topic [mesh: noexp] OR randomly [tiab] OR trial [ti]) NOT (animals [mh] NOT humans [mh]))
EMBASE
(infant, newborn or newborn or neonate or neonatal or premature or very low birth weight or low birth weight or VLBW or LBW or Newborn or infan* or neonat*) AND (human not animal) AND (randomized controlled trial or controlled clinical trial or randomized or placebo or clinical trials as topic or randomly or trial or clinical trial)
CINAHL
(infant, newborn OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW or Newborn or infan* or neonat*) AND (randomized controlled trial OR controlled clinical trial OR randomized OR placebo OR clinical trials as topic OR randomly OR trial OR PT clinical trial)
Data and analyses
Comparison 1. Barbiturates versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death or major neurodevelopmental disability | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Phenobarbital vs. control | 1 | 31 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.14, 0.78] |
| 2 Death | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Any barbiturate vs. control | 8 | 429 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.55, 1.42] |
| 2.2 Phenobarbital vs. control | 7 | 398 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.48, 1.35] |
| 2.3 Any barbiturate in infants with Sarnat 2 to 3 | 4 | 243 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.45, 1.77] |
| 2.4 Studies in low‐ and middle‐income countries | 5 | 329 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.42, 1.42] |
| 3 Major neurodevelopmental disability in survivors examined | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Phenobarbital vs. control | 1 | 31 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.06, 0.92] |
| 4 Cerebral palsy | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Phenobarbital vs. control | 2 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.19, 1.70] |
| 5 Developmental delay in survivors examined | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Any barbiturate vs. control | 2 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.24, 1.19] |
| 5.2 Phenobarbital vs. control | 1 | 41 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.02, 1.49] |
| 5.3 Thiopentone vs. control | 1 | 22 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.36, 1.94] |
| 5.4 Any barbiturate in infants with Sarnat 2 to 3 | 1 | 22 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.36, 1.94] |
| 5.5 Studies in low‐ and middle‐income countries | 1 | 41 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.02, 1.49] |
| 6 IQ in survivors | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7 Seizures within neonatal period | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Any barbiturate vs. control | 6 | 319 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.48, 0.81] |
| 7.2 Phenobarbital vs. control | 5 | 288 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.38, 0.73] |
| 7.3 Any barbiturate in infants with Sarnat 2 to 3 | 4 | 243 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.47, 0.89] |
| 7.4 Studies in low‐ and middle‐income countries | 4 | 257 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.33, 0.73] |
| 8 Respiratory depression | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 Phenobarbital vs. control | 2 | 93 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.18, 3.11] |
| 8.2 Studies in low‐ and middle‐income countries | 1 | 21 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Hypotension requiring inotropic support | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 Thiopentone vs. control | 1 | 31 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.88 [1.06, 3.32] |
| 10 Abnormal neurologic exam at discharge | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 Phenobarbital vs. control | 4 | 245 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.67, 1.08] |
| 10.2 Any barbiturate in infants with Sarnat 2 to 3 | 3 | 91 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.54, 1.08] |
| 10.3 Studies in low‐ and middle‐income countries | 4 | 245 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.67, 1.08] |
Comparison 2. Phenobarbital versus phenytoin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Seizures within the first 7 days of age | 1 | 17 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.07, 12.00] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Avasiloaiei 2013.
| Methods | Single‐center randomized controlled trial in Romania | |
| Participants | Included 67 term neonates with severe perinatal asphyxia (defined as having 3 of 4 criteria (umbilical artery blood pH < 7.0 with or without base deficit ≥ 12 mEq/L, Apgar ≤ 3 at > 5 minutes of life, neonatal neurologic sequelae (i.e. seizures, coma, hypotonia) or multiple organ involvement (i.e. kidney, lungs, liver, heart, intestines)) without major congenital malformations or hemolytic disease due to Rh incompatibility | |
| Interventions | Randomized into 1 of 3 groups Phenobarbital group (n = 22) received conventional supportive therapy plus a phenobarbital loading dose (40 mg/kg IV) during the first 4 hours after birth Erythropoietin group (n = 22) received conventional supportive therapy plus subcutaneous erythropoietin (1000 IU/kg per day) for 3 days Supportive therapy group (n = 23) received conventional supportive therapy including oxygen, volume expanders, inotropes, diuretics, antibiotics, and anticonvulsants for clinically suspected seizures confirmed by aEEG |
|
| Outcomes | Serum levels of antioxidant enzymes (SOD and GPx), TAS, and lipid peroxidation (using MDA levels) at 4, 24, 48, and 72 hours and at 7 days of life Death, seizures (confirmed by aEEG), neurologic abnormalities on examination at > 72 hours of life and at the time of discharge (exam as per Amiel‐Tison) and long‐term neurologic outcome (assessed at 3, 6, 9, 12, and 18 months of age using Bayley II assessment) |
|
| Notes | Long‐term neurodevelopmental data were reported as percentages, unpublished incidence data were requested, provided, and included. Seizure data were reported as number of infants at a given time, unpublished incidence data were requested, provided, and included | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random‐draw numerical assignment |
| Allocation concealment (selection bias) | High risk | Treatment team was not blinded to allocation |
| Blinding (performance bias and detection bias) All outcomes | High risk | No blinding of the treatment team or assessors. No placebo used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data was complete to 100% |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported Long‐term neurodevelopmental data were reported as percentages, unpublished incidence date were requested, provided, and included Seizure data were reported as number of infants at a given time, unpublished incidence data were requested, provided, and included |
Gathwala 2011.
| Methods | Single‐center open label, randomized controlled trial in India | |
| Participants | Included 72 full term neonates (all inborn) with severe perinatal asphyxia (cord blood pH < 7 and Apgar score ≤ 5 at 5 minutes) without obvious congenital malformation | |
| Interventions | Phenobarbital group (n = 36) received a phenobarbital loading dose (40 mg/kg IV infusion over 60 minutes) within the first 2 hours of life, followed by conventional therapy per unit protocol for HIE. Heart rate, oxygen saturation, respiration, and mean arterial pressure were monitored continuously during administration of phenobarbital Control group (n = 36) were managed with conventional therapy per unit protocol for the management of HIE |
|
| Outcomes | Primary outcome: CSF levels of lipid peroxides (MDA) and antioxidant enzymes (SOD and GPx) at 12 ± 2 hours of life Secondary outcomes: death, seizure activity, neurologic exam at discharge and neurologic outcome at 1‐month follow‐up (based on neurologic exam, MRI and EEG results) HIE staging for each infant according to the criteria of Sarnat and Sarnat Cranial ultrasounds on day 3 and day 7 of life Adverse effects of phenobarbital administration |
|
| Notes | Reported on seizure duration. Incidence data was requested without response from the authors There were no specific data given for the number of infants from each group with neonatal seizures. The authors stated that the incidence of neonatal seizures was comparable in the phenobarbital group and the control group |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Low risk | Random number sequences were placed in sealed envelopes and opened following infants resuscitation if selection criteria met |
| Blinding (performance bias and detection bias) All outcomes | High risk | There was no blinding of the treatment team or assessors. No placebo was used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1‐month follow‐up: 2/32 (6%) living infants missing from the intervention group, 0/30 (0%) living infants missing for the control group |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
Goldberg 1986.
| Methods | Single‐center randomized controlled trial in the US | |
| Participants | Included 32 consecutively admitted term infants with severe perinatal asphyxia [defined as having 2 of 3 criteria (perinatal distress (abnormal fetal heart pattern or requiring prolonged neonatal resuscitation); Apgar score of ≤ 4 at 5 minutes; and base deficit ≥ 15 mEq/L within first hour of life), who had neurologic signs of HIE (decreased or absent response to noxious stimuli, seizure activity, or increased irritability) and required mechanical ventilation within first hour of life without congenital intrauterine infection, complex congenital malformations, or maternal chorioamnionitis | |
| Interventions | Thiopental treatment group (n = 17) received a thiopental loading dose (15 mg/kg IV over 30 minutes), followed by a constant infusion of thiopental (10 mg/kg/hour for 90 minutes, 5 mg/kg/hour for 60 minutes, 3 mg/kg/hour for 8 hours, 1.5 mg/kg/hour for 6 hours, and 0.75 mg/kg/hour for 6 hours) plus conventional therapy Control group (n = 15) received conventional therapy (including fluid restriction, mechanical ventilation, and treatment with phenobarbital or phenytoin for clinical seizure activity) |
|
| Outcomes | Primary outcome: reduction of intracranial pressure to the normal range Secondary outcomes: hypotension requiring inotropes, overall effect on neurologic outcome, clinical seizure activity over first 72 hours of life, death, and long‐term neurodevelopmental outcome. Neurodevelopmental assessments were performed at 3, 6, 9, 12, 18, 24, and 36 months using the Bayley Scales of Infant Development and the Stanford‐Binet Intelligence Scale |
|
| Notes | Co‐intervention: 14 experimental and 12 control infants received phenobarbital for seizures For follow‐up, both the developmentalist and neurologist were unaware of the infant's treatment regimen |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Assigned using list of random numbers |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not specified |
| Blinding (performance bias and detection bias) All outcomes | High risk | There was no blinding of the treatment team or assessors during hospitalization and no placebo used The follow‐up assessment was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Developmental follow‐up: 1/12 (8%) living infants missing from the intervention group, 1/12 (8%) living infants missing from the control group Neurologic follow‐up: 1/12 (8%) living infants missing from intervention group, 0/12 (0%) living infants missing from the control group |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
Hall 1998.
| Methods | Single‐center randomized controlled trial in the US | |
| Participants | Included 40 term or post‐term infants with severe perinatal asphyxia (initial arterial pH ≤ 7 with base deficit ≥ 15 mEq/L; 5‐minute Apgar ≤ 3; or failure to initiate spontaneous respirations at 10 minutes of life) without congenital abnormality | |
| Interventions | Phenobarbital treatment group (n = 20) received a phenobarbital loading dose (40 mg/kg IV over 60 minutes) immediately following trial entry plus conventional therapy Control group (n = 20) received conventional therapy Conventional therapy included phenobarbital administration for the treatment of clinical seizures |
|
| Outcomes | Primary outcome: incidence of clinical seizures Secondary outcomes: total LDH, CK and the presence or absence of CK‐BB isoenzymes in CSF (obtained on day 1 and day 2), death before neurodevelopmental assessment, and neurodevelopmental outcome at 3 years of age (Gessell, Bayley, or Stanford‐Binet) Neurodevelopmental assessments were completed at 6, 12, 24, and 36 months of age Adverse effects of phenobarbital administration were also evaluated |
|
| Notes | Co‐intervention: control group received mean of 27 mg/kg of phenobarbital in first 24 hours, compared to a mean of 39 mg/kg in the experimental group All infants were outborn and transferred to the neonatal intensive care unit within the first day of life. Absence of seizure activity prior to transfer is inferred but not specifically stated Results were only reported for the 31 infants (15 in the phenobarbital treatment group and 16 in the control group) that completed the study |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Low risk | Allocation concealment was by sealed envelope |
| Blinding (performance bias and detection bias) All outcomes | High risk | There was no blinding of the treatment team or the assessors and no placebo used |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Postrandomization: 5/20 (25%) infants lost from intervention group (2 unable to obtain LP, 1 protocol violation, 2 lost to follow‐up), 4/20 (20%) of infants lost from the control group (1 unable to obtain LP, 3 lost to follow‐up) |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
Ruth 1991.
| Methods | Single‐center randomized controlled trial in Finland | |
| Participants | Included 38 term infants with evidence of perinatal asphyxia (5‐minute Apgar score ≤ 3 or need for mechanical ventilation > 30 minutes after birth) | |
| Interventions | Phenobarbital group (n = 21) received conventional therapy plus a phenobarbital loading dose (30 mg/kg IV) prior to 4 hours of age, followed by a second dose of phenobarbital (15 mg/kg) 4 hours after the initial dose. These infants then received 5 mg/kg/day for 5 days Control group (n = 17) received conventional therapy |
|
| Outcomes | Death before neurodevelopmental assessment Severe neurodevelopmental disability (stated as cerebral palsy) 6‐year neurodevelopmental outcome (assessed using WISC‐r expressed as IQ; neuropsychological tests (VMI, NEPSY) and quality of life scale) |
|
| Notes | Co‐intervention: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomization was not specified |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not specified |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Blinding of the treatment team and assessors was not specified |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 6‐year follow‐up: unclear how many infants were included, means presented for "testable infants" |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
Singh 2004.
| Methods | Single‐center randomized controlled trial in India | |
| Participants | Included 45 neonates term and late preterm infants (≥ 34 weeks' gestation) with perinatal asphyxia (Apgar ≤ 5 at 1 minute plus fetal distress (fetal bradycardia, meconium‐stained amniotic fluid or arterial cord pH ≤ 7.15) and evidence of HIE (abnormalities in tone or level of consciousness, or both)) in the first 6 hours of life Infants were excluded if they had major congenital malformations, meningitis, intracranial hemorrhage, or if their mothers received phenobarbital during the last week prior to delivery |
|
| Interventions | Phenobarbital group (n = 25) received phenobarbital (20 mg/kg IV over 20 minutes) within the first 6 hours of life plus conventional therapy Control group (n = 20) received conventional therapy Conventional therapy was as per unit protocol for management of HIE and included phenobarbital administration for clinically diagnosed seizures |
|
| Outcomes | Primary outcome: death or abnormal neurologic examination at discharge (exam as per Amiel‐Tison) Secondary outcomes: seizures, need for mechanical ventilation, and multiorgan dysfunction CSF levels of MDA, SOD, GPx, and plasma levels of vitamins A and E (evaluated at 10‐12 hours of life) were evaluated in relation to adverse outcomes (death or abnormal neurologic exam at discharge) Cranial ultrasounds were obtained on day 1, day 3, and day 7 Adverse effects of phenobarbital administration were evaluated Post hoc analysis was done for the group of infants with stage II and III HIE |
|
| Notes | Co‐intervention: 8 infants in the control group received phenobarbital for seizures (5.6 ± 0.6 hours). 1 of these infants required a dose of phenytoin for seizure management | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers |
| Allocation concealment (selection bias) | Low risk | Assignment was concealed in serially numbered opaque envelopes opened at the time of randomization |
| Blinding (performance bias and detection bias) All outcomes | High risk | There was no blinding of the treatment team and no placebo was used Samples were coded before sending to the lab to ensure blinding Discharge neurologic exam and cranial ultrasounds were performed by neonatologists blinded to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Postrandomization: 15/60 (25%) infants were excluded (1 due to CSF findings consistent with meningitis and 14 due to traumatic LP). Assessment otherwise complete |
| Selective reporting (reporting bias) | Low risk | All outcomes were resulted |
Vargas‐Origel 2004.
| Methods | Single‐center randomized controlled trial in Mexico | |
| Participants | Included 73 term (≥ 37 weeks' gestation) and post‐term (≥ 43 weeks' gestation) infants with perinatal asphyxia (cord or arterial pH ≤ 7.0 within 15 minutes of life plus ≥ 1 of the following, base deficit ≥15 mEq/L, Apgar score < 3 at 5 minutes of life, or no spontaneous respiration by 15 minutes of life) without major congenital abnormalities, congenital infection, trauma during delivery, or exposure to maternal medications that would result in neonatal depression Infants were also excluded if they died within the first 48 hours of life |
|
| Interventions | Phenobarbital group (n = 37) received a phenobarbital loading dose (40 mg/kg IV over 60 minutes) within the first hour of life plus conventional therapy Control group (n = 36) received conventional therapy. Received phenobarbital at conventional doses, only if there was clinical evidence of seizures Conventional therapy included administration of phenobarbital at standard dosing for clinically evident seizure activity |
|
| Outcomes | Short‐term outcomes: death, seizures in the neonatal period, neurologic status at discharge, and postasphyxia nonbrain complications (renal, respiratory, cardiovascular, gastrointestinal, and metabolic) Frequency and severity of HIE was estimated using Sarnat classification |
|
| Notes | Co‐intervention: 5/36 infants in control group given phenobarbital (mean dose 26 mg/kg, mean time 9.2 hours. Experimental group given phenobarbital 40 mg/kg (mean time 0.88 hours) Infants in the trial had a relatively low rate of HIE grade II or III (12% overall: 11% experimental, 14% control) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Infants were divided by random numbers |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment is not specified |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Blinding of the treatment team is implied but not specifically described. A placebo was administered |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data were complete to 100% |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
Vela 1987.
| Methods | Single‐center double‐blind randomized controlled trial in Spain | |
| Participants | Included 17 term infants with perinatal asphyxia (defined as 3 of the following criteria: multiple late fetal heart rate decelerations, fetal bradycardia for > 20 minutes, meconium‐stained amniotic fluid, Apgar score at 5 minutes of ≤ 7, requiring mechanical ventilation, requiring IV bicarbonate therapy) | |
| Interventions | Phenobarbital group (n = 9) received conventional therapy plus phenobarbital (12 mg/kg IM, followed by 6 mg/kg/day for 7 days) Phenytoin group (n = 8) received conventional therapy plus phenytoin (12 mg/kg IM, followed by 6 mg/kg/day for 7 days) |
|
| Outcomes | Primary outcome: seizures Secondary outcomes: adverse effects of medication administration (i.e. drowsiness, bradycardia) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method for randomization not stated |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not specified |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Referred to as a double‐blind trial, blinding of treatment team or assessors (or both) not clearly stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data was complete to 100% |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
Velaphi 2013.
| Methods | Single‐center randomized controlled trial in South Africa | |
| Participants | Included 94 infants ≥ 34 weeks' gestation or ≥ 2000 g weight (or both) with evidence of perinatal asphyxia (base deficit > 16 mEq/L measured within 1 hour of life and Apgar score at 5 minutes ≤ 6 or requiring resuscitation for > 5 minutes) Infants were excluded if there was evidence of congenital abnormalities, inability to randomize by 6 hours of life, or lack of spontaneous respirations within 20 minutes of life with or without bradycardia |
|
| Interventions | Phenobarbital group (n = 50) received phenobarbital loading dose (40 mg/kg IV infused over 60 minutes) within the first 6 hours of life plus conventional therapy Control group (n = 44) received placebo (normal saline 1 mL/kg IV infused over 60 minutes) within the first 6 hours of life plus conventional therapy Conventional therapy for both groups included, regular monitoring of vital signs, administration of dextrose containing IV fluids and antibiotics Clinical seizures were treated with phenytoin with or without clonazepam. If seizures persisted despite treatment, the code was broken and phenobarbital was administered for infants in the control group. For infants in the phenobarbital group, a phenobarbital level was obtained and phenobarbital was administered if the level was below the therapeutic range |
|
| Outcomes | Short‐term outcomes: seizures, death, and neurologic outcome at discharge compared to Sarnat stage at time of enrollment Neurologic exam according to Sarnat staging were performed at 12‐24 hours, 2‐3 days, 5‐7 days of life, and on discharge Adverse effects of phenobarbital administration were evaluated (including hypotension and respiratory depression) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Low risk | Sealed serially numbered opaque envelopes that were opened at the time of randomization |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Blinding of the treatment team is implied but not clearly stated. A placebo was administered Neonatal consultants uninvolved the care of the study infants performed neurologic exams |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data was complete to 100% |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
aEEG: amplitude‐integrated electroencephalography; CK: creatine kinase; CSF: cerebrospinal fluid; EEG: electroencephalography; GPx: glutathione peroxidase; HIE: hypoxic‐ischemic encephalopathy; IQ: intelligence quotient; IM: intramuscular; IU: international unit; IV: intravenous; LDH: lactate dehydrogenase; LP: lumbar puncture; MDA: malondialdehyde; MRI: magnetic resonance imaging; n: number of infants; NEPSY: Developmental NEuroPSYchological; Rh: rhesus; SOD: superoxide dismutase; TAS: total serum antioxidant status; VMI: Visual‐Motor Integration; WISC‐r: Wechsler Intelligence Scale for Children ‐ revised.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Kuzemko 1972 | Anticonvulsants used (chloral hydrate and diazepam) were not agents of interest in the current meta‐analysis |
| Ruth 1988 | Randomized premature, very low birth weight infants to receive phenobarbital or placebo following birth; perinatal asphyxia was not an eligibility criterion |
| Srinivasakumar 2015 | Randomized controlled trial evaluating the impact of treating electrographic seizures in hypoxic ischemic encephalopathy. Neonates ≥ 36 weeks' gestation with moderate or severe hypoxic ischemic encephalopathy were randomly assigned to either treatment of electrographic seizures alone or treatment of clinical seizures. Conventional electroencephalography video was monitored in both groups for up to 96 hours. Cumulative electrographic seizure burden was calculated in seconds and converted to log units for analysis. Magnetic resonance imaging scans were scored for severity of brain injury. Infants underwent neurodevelopmental evaluation at 18‐24 months |
| van Rooij 2010 | Randomized infants to receive treatment (not prophylaxis) for both clinical and subclinical seizures compared to blinding of amplitude‐integrated electroencephalography registration and treatment of clinical seizures only |
| Wilkinson 1989 | Anticonvulsants were used for treatment (not prevention) of electroencephalographically apparent seizures of any etiology, not exclusively perinatal asphyxia. Infants were a mixture of term and premature neonates. It was not possible to extract the data relating only to term infants following asphyxia |
Characteristics of ongoing studies [ordered by study ID]
IRCT2014090919101N1.
| Trial name or title | The Effect of Phenobarbital on the Outcome of Early Ischemic Encephalopathy |
| Methods | Randomized unblinded |
| Participants | Target sample size: 30 infants Inclusion criteria: 38‐42 weeks' gestation; 5‐minute Apgar score < 3 at birth or delay in breathing for > 5 minutes after birth; lethargy; seizures; abnormal neonatal reflexes; hypotonia Exclusion criteria: twins; congenital malformations; chromosomal abnormalities; congenital infection; or chorioamnionitis |
| Interventions | Phenobarbital 20 mg/kg IV within 6 hours after birth versus control |
| Outcomes | |
| Starting date | 23 September 2014 |
| Contact information | Panahandeh Gholamreza Shahrekord University of Medical Sciences, Shahrekord, Islamic Republic of Iran gholamrezapanahandeh@gmail.com |
| Notes | Recruitment complete |
Differences between protocol and review
There are major differences between the original review and this update. We changed the scope to address only prophylactic use of barbiturates. We added the post hoc comparison 'abnormal neurologic exam at discharge'. We added the methodology and plan for 'Summary of findings' tables and GRADE recommendations, which were not included in the original protocol or in the originally published review.
Contributions of authors
David Evans and Malcolm Levene conducted the original review in 1999 and update in 2001.
For the 2007 update, Maria Tsakmakis implemented the search and arranged for translation of papers. DE and MT appraised the new studies and DE updated the analyses and text of the review.
For the current review, LY, MB and RS appraised the new studies, RS and LY updated the analysis, and LY updated text of the review.
Sources of support
Internal sources
No sources of support supplied
External sources
-
Eunice Kennedy Shriver National Institute of Child Health and Human Development National Institutes of Health, Department of Health and Human Services, USA.
Editorial support of the Cochrane Neonatal Review Group was funded with Federal funds from the Eunice Kennedy Shriver National Institute of Child Health and Human Development National Institutes of Health, Department of Health and Human Services, USA, under Contract No. HHSN275201100016C.
Declarations of interest
None.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Avasiloaiei 2013 {published and unpublished data}
- Avasiloaiei A, Dimitriu C, Moscalu M, Paduraru L, Stamatin M. High‐dose phenobarbital or erythropoietin for the treatment of perinatal asphyxia in term newborns. Pediatrics International 2013;55(5):589‐93. [PUBMED: 23659666] [DOI] [PubMed] [Google Scholar]
Gathwala 2011 {published data only}
- Gathwala G, Marwah A, Gahlaut V, Marwah P. Effect of high‐dose phenobarbital on oxidative stress in perinatal asphyxia: an open label randomized controlled trial. Indian Pediatrics 2011;48(8):613‐7. [PUBMED: 21169640] [DOI] [PubMed] [Google Scholar]
Goldberg 1986 {published data only}
- Goldberg RN, Moscoso P, Bauer CR, Bloom FL, Curless RG, Burke B, et al. Use of barbiturate therapy in severe perinatal asphyxia: a randomized controlled trial. Journal of Pediatrics 1986;109(5):851‐6. [PUBMED: 3534201] [DOI] [PubMed] [Google Scholar]
Hall 1998 {published data only}
- Hall RT, Hall FK, Daily DK. High‐dose phenobarbital therapy in term newborn infants with severe perinatal asphyxia: a randomized, prospective study with three‐year follow‐up. Journal of Pediatrics 1998;132(2):345‐8. [PUBMED: 9506654] [DOI] [PubMed] [Google Scholar]
Ruth 1991 {published data only}
- Ruth V, Korkman M, Liikanen A, Paetau R. High‐dose phenobarbitol treatment to prevent postasphyxial brain damage: a 6‐year follow up [abstract]. Pediatric Research 1991;30:638. [DOI: 10.1203/00006450-199112000-00091] [DOI] [Google Scholar]
Singh 2004 {published data only}
- Singh D, Kumar P, Majumdar S, Narang A. Effect of phenobarbital on free radicals in neonates with hypoxic ischemic encephalopathy ‐ a randomized controlled trial. Journal of Perinatal Medicine 2004;32(3):278‐81. [PUBMED: 15188805] [DOI] [PubMed] [Google Scholar]
- Singh D, Kumar P, Narang A. A randomized controlled trial of phenobarbital in neonates with hypoxic ischemic encephalopathy. Journal of Maternal‐Fetal and Neonatal Medicine 2005;18(6):391‐5. [PUBMED: 16390805] [DOI] [PubMed] [Google Scholar]
Vargas‐Origel 2004 {published data only}
- Vargas‐Origel A, Espinosa‐Garcia JOG, Muniz‐Quezada E, Vargas‐Nieto MA, Aguilar‐Garcia G. Prevention of hypoxic‐ischemic encephalopathy with high‐dose, early phenobarbital therapy [Prevencion de la encefalopatia hipoxico‐isquemica mediante Fenobarbital en forma temprana y a dosis alta]. Gaceta Medica de Mexico 2004;140(2):147‐53. [MEDLINE: ] [PubMed] [Google Scholar]
Vela 1987 {published data only}
- Vela F, Duran JA, Chunga F, Serrano JS, Valls A. Preventive treatment of convulsions in perinatal asphyxia [Tratamiento profilactico de convulsiones en asfixia perinatal]. Anales Espanoles de Pediatria 1987;27(2):95‐9. [PUBMED: 3662273] [PubMed] [Google Scholar]
Velaphi 2013 {published data only}
- Velaphi S, Mokhachane M, Mphahlele R, Beckh‐Arnold E. Effect of prophylactic phenobarbital on seizures, encephalopathy and mortality in neonates with perinatal asphyxia. South African Journal of Child Health 2013;7(1):17‐21. [Google Scholar]
References to studies excluded from this review
Kuzemko 1972 {published data only}
- Kuzemko JA, Hartley S. Treatment of cerebral irritation in the newborn: double‐blind trial with chloral hydrate and diazepam. Developmental Medicine and Child Neurology 1972;14(6):740‐6. [PUBMED: 4566107] [DOI] [PubMed] [Google Scholar]
Ruth 1988 {published data only}
- Ruth V, Virkola K, Paetau R, Raivio KO. Early high‐dose phenobarbital treatment for prevention of hypoxic‐ischemic brain damage in very low birth weight infants. Journal of Pediatrics 1988;112(1):81‐6. [PUBMED: 2447257] [DOI] [PubMed] [Google Scholar]
Srinivasakumar 2015 {published data only}
- Srinivasakumar P, Zempel J, Trivedi S, Wallendorf M, Rao R, Smith B, et al. Treating EEG seizures in hypoxic ischemic encephalopathy: a randomized controlled trial. Pediatrics 2015;136(5):e1302‐9. [PUBMED: 26482675] [DOI] [PubMed] [Google Scholar]
van Rooij 2010 {published data only}
- Rooij LG, Toet MC, Huffelen AC, Groenendaal F, Laan W, Zecic A, et al. Effect of treatment of subclinical neonatal seizures detected with aEEG: randomized, controlled trial. Pediatrics 2010;125(2):e358‐66. [PUBMED: 20100767] [DOI] [PubMed] [Google Scholar]
Wilkinson 1989 {published data only}
- Rochefort MJ, Wilkinson AR. The safety and efficacy of alternative anticonvulsant regimes to control newborns seizures [abstract]. Early Human Development 1989;19:218. [Google Scholar]
- Wilkinson AR, Rochefort MJ. Phenytoin reduces frequency and duration of neonatal seizures in the newborn: a randomised trial of four anticonvulsants [abstract]. Pediatric Research 1989;26:522. [Google Scholar]
References to ongoing studies
IRCT2014090919101N1 {published data only}
- Gholamreza P. The effect of phenobarbital on the outcome of early ischemic encephalopathy. www.irct.ir/searchresult.php?id=19101&number=1 (accessed 27 March 2016).
Additional references
Bittigau 2002
- Bittigau P, Sifringer M, Genz K, Reith E, Pospischil D, Govindarajalu S, et al. Antiepileptic drugs and apoptotic neurodegeneration in the developing brain. Proceedings of the National Academy of Sciences of the United States of America 2002;99(23):15089‐94. [PUBMED: 12417760] [DOI] [PMC free article] [PubMed] [Google Scholar]
Buttke 1994
- Buttke TM, Sandstrom PA. Oxidative stress as a mediator of apoptosis. Immunology Today 1994;15(1):7‐10. [PUBMED: 8136014] [DOI] [PubMed] [Google Scholar]
Cherubini 1991
- Cherubini E, Gaiarsa JL, Ben‐Ari Y. GABA: an excitatory transmitter in early postnatal life. Trends in Neurosciences 1991;14(12):515‐9. [PUBMED: 1726341] [DOI] [PubMed] [Google Scholar]
Clozel 1985
- Clozel M, Daval JL, Monin P, Debruc C, Morselli PL, Vert P. Regional cerebral blood flow during bicuculline‐induced seizures in the newborn piglet: effect of phenobarbital. Developmental Pharmacology and Therapeutics 1985;8(3):189‐99. [PUBMED: 4006653] [DOI] [PubMed] [Google Scholar]
Demopoulos 1977
- Demopoulos HG, Flamm ES, Seligman MC, Jorgensen E, Ransohoff J. Antioxidant effects of barbiturates in model membranes undergoing free radical damage. Acta Neurologica Scandinavica 1977;64:152‐3. [PUBMED: 268766] [PubMed] [Google Scholar]
Glass 2009
- Glass HC, Glidden D, Jeremy RJ, Barkovich AJ, Ferriero DM, Miller SP. Clinical neonatal seizures are independently associated with outcome in infants at risk for hypoxic‐ischemic brain injury. Journal of Pediatrics 2009;155(3):318‐23. [PUBMED: 19540512] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gluckman 1992
- Gluckman PD, Williams CE. When and why do brain cells die?. Developmental Medicine and Child Neurology 1992;34(11):1010‐4. [PUBMED: 1358734] [DOI] [PubMed] [Google Scholar]
Gluckman 2005
- Gluckman PD, Wyatt JS, Azzopardi D, Ballard R, Edwards AD, Ferriero DM, et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet 2005;365(9460):663‐70. [PUBMED: 15721471] [DOI] [PubMed] [Google Scholar]
GRADEpro 2008 [Computer program]
- Brozek J, Oxman A, Schünemann H. GRADEpro [Version 3.2 for Windows]. McMaster University, 2008.
Gunn 1998
- Gunn AJ, Gunn TR, Gunning MI, Williams CE, Gluckman PD. Neuroprotection with prolonged head cooling started before postischemic seizures in fetal sheep. Pediatrics 1998;102(5):1098‐106. [PUBMED: 9794940] [DOI] [PubMed] [Google Scholar]
Gunn 2008
- Gunn AJ, Wyatt JS, Whitelaw A, Barks J, Azzopardi D, Ballard R, et al. Therapeutic hypothermia changes the prognostic value of clinical evaluation of neonatal encephalopathy. Journal of Pediatrics 2008;152(1):55‐8. [PUBMED: 18154900] [DOI] [PubMed] [Google Scholar]
Guyatt 2011a
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction‐GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [PUBMED: 21195583] [DOI] [PubMed] [Google Scholar]
Guyatt 2011b
- Guyatt GH, Oxman AD, Vist G, Kunz R, Brozek J, Alonso‐Coello P, et al. GRADE guidelines: 4. Rating the quality of evidence ‐ study limitations (risk of bias). Journal of Clinical Epidemiology 2011;64(4):407‐15. [PUBMED: 21247734] [DOI] [PubMed] [Google Scholar]
Guyatt 2011c
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 7. Rating the quality of evidence ‐ inconsistency. Journal of Clinical Epidemiology 2011;64(12):1294‐302. [PUBMED: 21803546] [DOI] [PubMed] [Google Scholar]
Guyatt 2011d
- Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso‐Coello P, Rind D, et al. GRADE guidelines 6. Rating the quality of evidence ‐ imprecision. Journal of Clinical Epidemiology 2011;64(12):1283‐93. [PUBMED: 21839614] [DOI] [PubMed] [Google Scholar]
Guyatt 2011e
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 8. Rating the quality of evidence ‐ indirectness. Journal of Clinical Epidemiology 2011;64(12):1303‐10. [PUBMED: 21802903] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Greene S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Holmes 1998
- Holmes GL, Ben‐Ari Y. Seizures in the developing brain: perhaps not so benign after all. Neuron 1998;21(6):1231‐4. [PUBMED: 9883716] [DOI] [PubMed] [Google Scholar]
Hossman 1983
- Hossman KA. Neuronal survival and revival during and after cerebral ischaemia. American Journal of Emergency Medicine 1983;1(2):191‐7. [PUBMED: 6680620] [DOI] [PubMed] [Google Scholar]
Jacobs 2013
- Jacobs SE, Berg M, Hunt R, Tarnow‐Mordi WO, Inder TE, Davis PG. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database of Systematic Reviews 2013, Issue 1. [DOI: 10.1002/14651858.CD003311.pub3; PUBMED: 23440789] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kwon 2011
- Kwon JM, Guillet R, Shankaran S, Laptook AR, McDonald SA, Ehrenkranz RA, et al. Clinical seizures in neonatal hypoxic‐ischemic encephalopathy have no independent impact on neurodevelopmental outcome: secondary analyses of data from the neonatal research network hypothermia trial. Journal of Child Neurology 2011;26(3):322‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lorek 1994
- Lorek A, Takei Y, Cady EB, Wyatt JS, Penrice J, Edwards AD, et al. Delayed (+ACI‐secondary+ACI‐) cerebral energy failure after acute hypoxia‐ischemia in the newborn piglet: continuous 48‐hour studies by phosphorus magnetic resonance spectroscopy. Pediatric Research 1994;36(6):699‐706. [PUBMED: 7898977] [DOI] [PubMed] [Google Scholar]
McBride 2000
- McBride MC, Laroia N, Guillet R. Electrographic seizures in neonates correlate with poor neurodevelopmental outcome. Neurology 2000;55(4):506‐13. [PUBMED: 10953181] [DOI] [PubMed] [Google Scholar]
McCord 1985
- McCord JM. Oxygen‐derived free radicals in postischemic tissue injury. New England Journal of Medicine 1985;312(3):159‐63. [PUBMED: 2981404] [DOI] [PubMed] [Google Scholar]
McDonald 1990
- McDonald JW, Johnston MV. Physiological and pathophysiological roles of excitatory amino acids during central nervous system development. Brain Research. Brain Research Reviews 1990;15(1):41‐70. [PUBMED: 2163714] [DOI] [PubMed] [Google Scholar]
Nilsson 1971
- Nilsson L. The influence of barbiturate anaesthesia upon the energy state and upon acid‐base parameters of the brain in arterial hypotension and in asphyxia. Acta Neurologica Scandinavica 1971;47(2):233‐53. [PUBMED: 5566570] [DOI] [PubMed] [Google Scholar]
Penrice 1996
- Penrice J, Cady EB, Lorek A, Wylezinska M, Amess PN, Aldridge RF, et al. Proton magnetic resonance spectroscopy of the brain in normal preterm and term infants, and early changes after perinatal hypoxia‐ischemia. Pediatric Research 1996;40(1):6‐14. [PUBMED: 8798238] [DOI] [PubMed] [Google Scholar]
Perlman 1996
- Perlman JM, Risser R. Can asphyxiated infants at risk for neonatal seizures be rapidly identified by current high‐risk markers?. Pediatrics 1996;97(4):456‐62. [PUBMED: 8632928] [PubMed] [Google Scholar]
Perlman 2006
Pfister 2012
- Pfister RH, Bingham P, Edwards EM, Horbar JD, Kenny MJ, Inder T, et al. The Vermont Oxford Neonatal Encephalopathy Registry: rational, methods, and initial results. BMC Pediatrics 2012;12:84. [PUBMED: 22726296] [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rivera 1999
- Rivera C, Voipio J, Payne JA, Ruusuvuori E. The K+/Cl‐ co‐transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature 1999;397(6716):251‐5. [PUBMED: 9930699] [DOI] [PubMed] [Google Scholar]
Roth 1992
- Roth SC, Edwards AD, Cady EB, Delpy DT, Wyatt JS, Azzopardi D, et al. Relation between cerebral oxidative metabolism following birth asphyxia, and neurodevelopmental outcome and brain growth at one year. Developmental Medicine and Child Neurology 1992;34(4):285‐95. [PUBMED: 1572514] [DOI] [PubMed] [Google Scholar]
Roth 1997
- Roth SC, Baudin J, Cady E, Johal K, Townsend JP, Wyatt JS, et al. Relation of deranged neonatal cerebral oxidative metabolism with neurodevelopmental outcome and head circumference at 4 years. Developmental Medicine and Child Neurology 1997;39(11):718‐25. [PUBMED: 9393884] [DOI] [PubMed] [Google Scholar]
Sant'Anna 2012
- Sant'Anna G, Laptook AR, Shankaran S, Bara R, McDonald SA, Higgins RD, et al. Phenobarbital and temperature profile during hypothermia for hypoxic‐ischemic encephalopathy. Journal of Child Neurology 2012;27(4):451‐7. [PUBMED: 21960671] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sarnat 1976
- Sarnat HB, Sarnat MS. Neonatal encephalopathy following fetal distress. Archives of Neurology 1976;33:696‐705. [DOI] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A (editors). GRADE handbook for grading quality of evidence and strength of recommendations. Updated October 2013. Available from http://gdt.guidelinedevelopment.org/central_prod/_design/client/handbook/handbook.html.
Shankaran 2005
- Shankaran S, Laptook AR, Ehrenkranz RA, Tyson JE, McDonald SA, Donovan EF, et al. Whole‐body hypothermia for neonates with hypoxic‐ischemic encephalopathy. New England Journal of Medicine 2005;353(15):1574‐84. [PUBMED: 16221780] [DOI] [PubMed] [Google Scholar]
Shankaran 2012
- Shankaran S, Laptook AR, Tyson JE, Ehrenkranz RA, Bann CM, Das A, et al. Evolution of encephalopathy during whole body hypothermia for neonatal hypoxic‐ischemic encephalopathy. Journal of Pediatrics 2012;160(4):567‐72. [PUBMED: 22050871] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shellhaas 2007
- Shellhaas RA, Soaita AI, Clancy RR. Sensitivity of amplitude‐integrated electroencephalography for neonatal seizure detection. Pediatrics 2007;120(4):770‐7. [PUBMED: 17908764] [DOI] [PubMed] [Google Scholar]
Siesjo 1992
- Siesjo BK. Pathophysiology and treatment of focal cerebral ischaemia. Part II: Mechanisms of damage and treatment. Journal of Neurosurgery 1992;77(3):337‐54. [PUBMED: 1506880] [DOI] [PubMed] [Google Scholar]
Williams 1991
- Williams CE, Gunn A, Gluckman PD. Time course of intracellular edema and epileptiform activity following prenatal cerebral ischemia in sheep. Stroke 1991;22(4):516‐21. [PUBMED: 2024281] [DOI] [PubMed] [Google Scholar]
Wirrell 2001
- Wirrell EC, Armstrong EA, Osman LD, Yager JY. Prolonged seizures exacerbate perinatal hypoxic‐ischemic brain damage. Pediatric Research 2001;50(4):445‐54. [PUBMED: 11568286] [DOI] [PubMed] [Google Scholar]
Wusthoff 2009
- Wusthoff CJ, Shellhaas RA, Clancy RR. Limitations of single‐channel EEG on the forehead for neonatal seizure detection. Journal of Perinatology 2009;29(3):237‐42. [PUBMED: 19052554] [DOI] [PubMed] [Google Scholar]
Wyatt 2007
- Wyatt JS, Gluckman PD, Liu PY, Azzopardi D, Ballard RA, Edwards AD, et al. Determinants of outcomes after head cooling for neonatal encephalopathy. Pediatrics 2007;119(5):912‐21. [PUBMED: 17473091] [DOI] [PubMed] [Google Scholar]
Yager 2002
- Yager JY, Armstrong EA, Miyashita H, Wirrell EC. Prolonged neonatal seizures exacerbate hypoxic‐ischemic brain damage: correlation with cerebral energy metabolism and excitatory amino acid release. Developmental Neuroscience 2002;24(5):367‐81. [PUBMED: 12640175] [DOI] [PubMed] [Google Scholar]
Yamada 2004
- Yamada J, Okabe A, Toyoda H, Kilb W, Luhmann HJ, Fukuda A. Cl‐ uptake promoting depolarizing GABA actions in immature rat neocortical neurones is mediated by NKCC1. Journal of Physiology 2004;557(Pt 3):829‐41. [PUBMED: 15090604] [DOI] [PMC free article] [PubMed] [Google Scholar]
Younkin 1986
- Younkin DP, Delivoria‐Papadopoulos M, Maris J, Donlon E, Clancy R, Chance B. Cerebral metabolic effects of neonatal seizures measured with in vivo 31P NMR spectroscopy. Annals of Neurology 1986;20(4):513‐9. [PUBMED: 3789667] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Evans 1998
- Evans DJ, Levene MI. Anticonvulsants for preventing mortality and morbidity in full term newborns with perinatal asphyxia. Cochrane Database of Systematic Reviews 1998, Issue 3. [DOI: 10.1002/14651858.CD001240; PUBMED: 10796257] [DOI] [PubMed] [Google Scholar]
Evans 2001
- Evans DJ, Levene MI. Anticonvulsants for preventing mortality and morbidity in full term newborns with perinatal asphyxia. Cochrane Database of Systematic Reviews 2001, Issue 2. [DOI: 10.1002/14651858.CD001240.pub2; PUBMED: 11686984] [DOI] [PubMed] [Google Scholar]


