Abstract
The overarching goal of this study was to simultaneously model the dynamic relationships among statin exposure, statin discontinuation, and potentially statin‐related myopathic outcomes. We extracted data from the Indiana Network of Patient Care for 134,815 patients who received statin therapy between January 4, 2004, and December 31, 2008. All individuals began statin treatment, some discontinued statin use, and some experienced myopathy and/or rhabdomyolysis while taking the drug or after discontinuation. We developed a militate model to characterize 12 transition probabilities among six different states defined by use or discontinuation of statin and its associated myopathy or rhabdomyolysis. We found that discontinuation of statin therapy was common and frequently early, with 44.4% of patients discontinuing therapy after 1 month, and discontinuation is a strong indicator for statin‐induced myopathy (risk ratio, 10.8; p < 0.05). Women more likely than men (p < 0.05) and patients aged 65 years and older had a higher risk than those aged younger than 65 years to discontinue statin use or experience myopathy. In conclusion, we introduce an innovative multistate model that allows clear depiction of the relationship between statin discontinuation and statin‐induced myopathy. For the first time, we have successfully demonstrated and quantified the relative risk of myopathy between patients who continued and discontinued statin therapy. Age and sex were two strong risk factors for both statin discontinuation and incident myopathy.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
Statins demonstrate good efficacy as primary and secondary tools to prevent atherosclerotic disease. However, their adverse effects can be severe and cause low adherence to the prescription. Quantitative models are necessary to describe the relationships among statin initiation, discontinuation, and adverse effects.
WHAT QUESTION DID THIS STUDY ADDRESS?
Does the probability of discontinuation continue to increase after stain initiation? Does discontinuation of statin therapy indicate adverse effect? What factors influence the risk of statin‐induced adverse effects or discontinuation?
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
Statin discontinuation happens often and early, but the probability of discontinuation decreases after 1 year; discontinuation is a strong indicator for statin‐induced myopathy. Female and older patients are at higher risk to develop myopathy and/or rhabdomyolysis and to discontinue statin use than male and younger patients.
HOW MIGHT THIS CHANGE DRUG DISCOVERY, DEVELOPMENT, AND/OR THERAPEUTICS?
A multistate transition model provides a comprehensive, dynamic, and quantitative view of the relationships among medications, patient adherence, and side effects, allowing a more precise analysis of risk factors.
INTRODUCTION
Statins are a class of drug that inhibit the synthesis of biocholesterol by binding to the 3‐hydroxy‐3‐methyl‐glutaryl‐coenzyme A reductase enzyme. 1 These drugs are widely used as safe and effective treatments to lower atherogenic cholesterol and prevent atherosclerotic cardiovascular disease (ASCVD). 2 Although the benefits of statin therapy are well established, 3 , 4 the frequency of statin discontinuation is high. Statin discontinuation has been reported within 1 year in 40% to 75% of patients in a community sample and in approximately 30% in the general population within settings of primary and secondary ASCVD prevention. 5 , 6 Statin‐associated muscle symptoms (SAMS) include reported muscle pain (myalgias) or weakness (myopathy) that a patient suspects is attributed to statin therapy. 7 Objective evidence of muscle inflammation (myositis), whether or not accompanied by myalgias or myopathy, is most often measured by creatine kinase (CK) level elevation in blood samples and is generally felt to represent a more severe adverse outcome associated with statin use. 8 Myonecrosis involves evidence of the significant breakdown of skeletal muscle (e.g., moderate myonecrosis has been defined as CK elevation ≥10‐fold above the upper limit of normal), and if accompanied by myoglobinuria or renal failure, termed rhabdomyolysis. 9 One observational study has reported a near 20% incidence of myalgia, 10 and another group has noted approximately four cases of rhabdomyolysis per 100,000 person‐years. 11 SAMS are well‐known determinants of statin discontinuation, but description of the quantitative relationship is challenging. To date, to the authors’ knowledge, no statistical model has yet characterized the frequency of both muscle symptoms and CK level elevation induced by undertaking statin therapy that continue even after discontinued use. 12
Currently, the risk of medication‐induced side effects is most commonly assessed using either logistic regression or Cox proportional hazard models. 13 , 14 Logistic regression fits data generated from cross‐sectional studies in which drug exposure and side effects are captured in a snapshot of a patient, whereas the Cox model can represent the longitudinal relationship between drug exposure and side effects. However, in studying the relationship among drug exposure, drug discontinuation, and the onset of side effects, both drug and side effect status are time‐dependent and can recur. This process involves intermediate events and competing risk, which cannot be solved by logistic regression or Cox proportional hazard models directly. Thus, a multistate transition model may be a superior choice versus a Cox proportional hazard model for this evaluation. 15 Such a model has been used to characterize breast cancer recurrence including two intermittent transition states (distant relapse and locoregional relapse) and two competing end states (death from cancer and death from other cause) 16 and in a different application to model long‐term medication adherence in a patient population with type 2 diabetes. 17
In this article, we describe our experience using a multistate transition model we have developed to characterize the relationships among statin drug exposure, discontinuation, myopathy, and rhabdomyolysis and further examine whether and how these relationships differ in various patient population demographics.
METHODS
Data
Following the Observational Health Data Sciences and Informatics Version 5.0 guideline for the construction of a common data model (http://www.ohdsi.org/data‐standardization), we applied the myopathy concept definition (Table S1) to electronic medical record data extracted from the Indiana Network of Patient Care database for patients who took statins at any time between January 4, 2004, and December 31, 2008. Our study population comprised 134,815 patients who had begun therapy with statins, including simvastatin, atorvastatin, fluvastatin, lovastatin, pitavastatin, pravastatin, or rosuvastatin, continued or discontinued the therapy, and whether they experienced myopathy or rhabdomyolysis during or after statin use. We defined discontinuation as a gap exceeding 30 days between two statin prescription dates, 18 and we defined the primary side effects of statin use as myopathy (mild outcome), including any disorder of skeletal muscle, muscle pain, such as myalgia and myositis, muscle weakness, and polymyositis, and rhabdomyolysis (severe outcome), constituting muscle, ligament, and fascia disorders or myoglobinuria. 19 Demographic variables noted were sex and older age (patients younger than 65 years vs. those aged 65 years or older). 20
Multistate transition model
Our data involved two processes: (1) whether patients were taking their statin medication and (2) whether patients were experiencing any adverse muscle weakness symptoms, mild or severe, during or after discontinuing therapy. Patients were considered to be taking the medication if the time gap between two prescriptions was 30 days or less, but a gap exceeding 30 days indicated their discontinued use. If patients experienced myopathy and rhabdomyolysis during the same time period, their side effect was defined as rhabdomyolysis.
Consideration of these two processes yields the following six possible states: (1) taking statin without adverse muscle, (2) discontinuing statin without adverse muscle effect, (3) taking statin and experiencing myopathy, (4) discontinuing statin and experiencing myopathy, (5) discontinuing statin and experiencing rhabdomyolysis, and (6) taking statin and experiencing rhabdomyolysis. However, the sixth state is not realistic because a patient experiencing the severe side effect of rhabdomyolysis would be very unlikely to continue statin use. We have therefore formulated a five‐state model with state space (see Figure 1) that could potentially include 20 possible transitions, but our multistate model actually has only 12 transitions because we allow for only one transition event at a time. For example, the model does not provide for direct transition from taking statin without side effect to statin discontinuation with myopathy. That is, it does not allow for simultaneous change in side effect and medication status. Table 1 outlines the transition matrix, and Figure 1 displays the model. We used the R package, mstate, 21 to fit the data and estimate the transition probabilities. A Cox model was used to compare the risk between different transitions.
FIGURE 1.
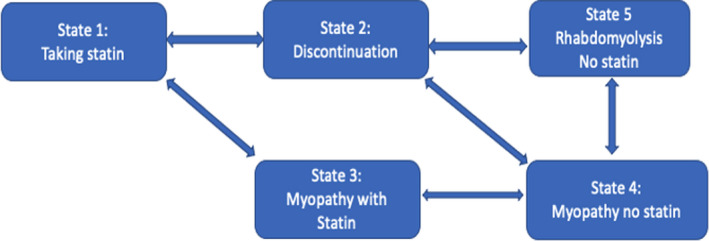
Five‐state transition model with 12 total transitions
TABLE 1.
Transition matrix of the multistate transition model
| Taking statin without adverse muscle effect | Discontinuing statin without adverse muscle effect | Taking statin and experiencing myopathy | Experiencing myopathy after discontinuing statin | Experiencing rhabdomyolysis after discontinuing statin | |
|---|---|---|---|---|---|
| Taking statin without adverse muscle effect | a | 1 | 2 | NA | NA |
| Discontinuing statin without adverse muscle effect | 3 | a | NA | 4 | 5 |
| Taking statin and experiencing myopathy | 6 | NA | a | 7 | NA |
| Experiencing myopathy after discontinuing statin | NA | 8 | 9 | a | 10 |
| Experiencing rhabdomyolysis after discontinuing statin | NA | 11 | NA | 12 | a |
NA indicates that the transition cannot happen.
Abbreviation: NA, not applicable.
The diagonal part of the intensity matrix can be calculated by the sum of other transition intensities in the same row.
The calculation of the transition probability matrix in an interval is based on the Aalen–Johansen estimator, 22
where I is a identity matrix, and is the estimated transition intensity estimate matrix at the time . The off diagonal element of the intensity matrix is estimated by
which is the Nelson–Aalen estimator derived by a counting process. 23 The diagonal element is estimated by the negative summation of other transition intensities in the same row. is the total number of patients at risk who stay at state at time . is the transition frequency or change in patient number from state to state at time . The elemet of transition probability matrix will be interpreted as the the probability of transitioning from state to state in the time interval .
Let denote the transition probability from state to state between Day 0 to Day n, which is the elemet of transition probability matrix . The transition probability from Day 0 to Day (n+1) will be calculated based on the following recursive equations.
Transition probability calculation between two directly connected states:
The calculations of probabilities for other direct connected transitions can be found in Supplementary Text S1.
Transition probability calculation between two indirectly connected states:
Model validation
The difference between observed and expected prevalence will be investigated. The expected prevalence at time is estimated as follows:
where is the initital state distribution. The observed and expected prevalence will be calculated monthly, that is, . Meanwhile, a comparison between the Kaplan–Meier estimator and the Aalen–Johansen estimator for showing the advantage of a multistate model over a survival analysis that ignores intermediate events is provided in the Supplementary Text S2.
RESULTS
The difference between observed and expected prevalence is small
Figure 2 shows, with the prevalence in percentages for five states, that the mapping of expected to observed is good. The observed survival can be captured accurately. It can be used to predict the prevalence for all five states only based on the baseline data. Besides goodness of fit, the prevalence for five states during the study time are clearly described in Figure 2. In the long term, the prevalence for patients taking statins and discontinuation was about 50% and 45%, respectively. The prevalence for State 4 (myopathy no statin) and State 5 (rhabdomyolysis no statin) increase over time. At the end of the study, 4% patients had myopathy, and the percentage of patients developing rhabdomyolysis was less than 0.5%. The proportion of patients with myopathy who kept taking statins decreased over time at <0.1%.
FIGURE 2.
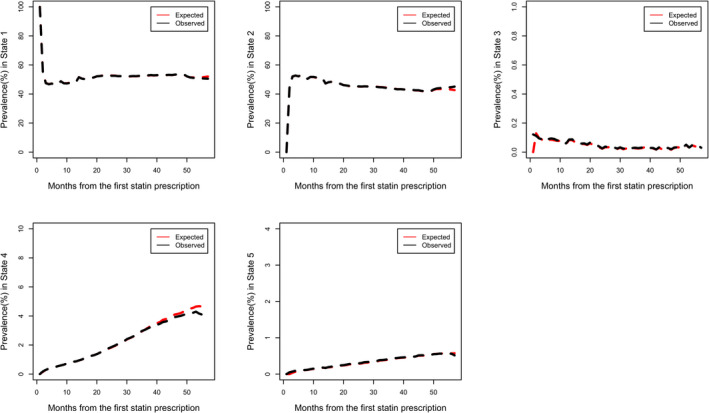
Comparison between observed and expected prevalence among five states
Statin discontinuation happens often and early
Most statin discontinuations happened after the first month (Figure 3a,b). Frequency of discontinuation at the end of the first month was 381 times greater than the average frequency thereafter. Figure 3b shows that approximately 44.4% (44.1%, 44.7%) of patients discontinued statin use immediately after the first month; that number rose to 53.3% (53.0%, 53.6%) within the first 6 months, and the proportion of discontinuation decreased slightly thereafter. During the entire observational period, the overall proportion of discontinuation ranged between 44% and 54%.
FIGURE 3.
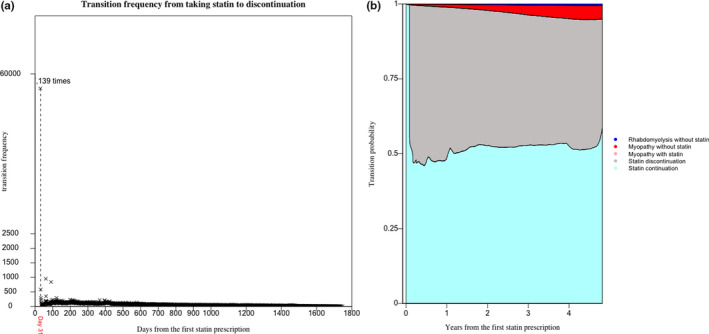
(a)Transition frequency from State 1 (taking statin) to State 2 (discontinuation without side effect) over time. (b) Stacked transition probabilities from taking statin to the other states. The state myopathy with statin is imperceptible in the plot for low frequency
After the first 6 months, both the probability of statin continuation and the probabilities of transition from taking statin to experiencing myopathy and rhabdomyolysis (with or without statin) increased, and the transition probability for statin discontinuation decreased.
Discontinuation is a strong indicator for statin‐induced myopathy
Careful scrutiny of Figure 3b reveals an almost invisible probability of transition between statin use and the development of myopathy (with statin use) compared with the probabilities of transition between statin use and the development of myopathy or rhabdomyolysis after discontinuation. Therefore, we further compared two transition probabilities‒from statin use to myopathy with its use and from statin use to myopathy without statin use (Figure 4). We observed an apparent 10.8‐times greater risk of developing myopathy among patients who discontinued statin use compared with those who continued on statins at the end of the first year(p < 0.05), and in the course of time, the probability of myopathy after discontinuation increased compared with those who kept taking stains with myopathy.
FIGURE 4.
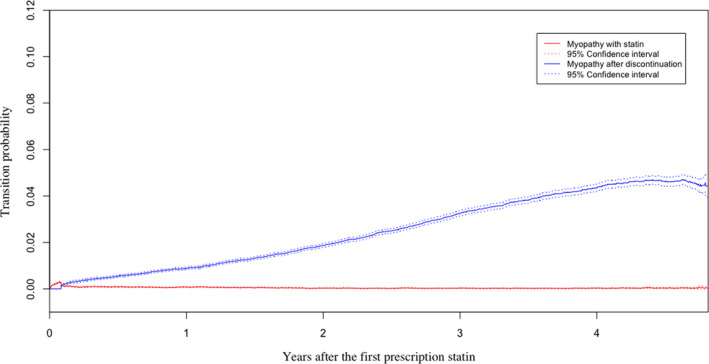
Transition probabilities from taking statin to taking statin and developing myopathy and from statin discontinuation to the development of myopathy without statin
Sex and age are two important risk factors in predicting statin discontinuation and the development of myopathy and/or rhabdomyolysis
We compared the risk factors of sex and age, 65 years and older versus younger than 65 years, among transition probabilities starting from State 1 in patients taking statins. Because all patients are in State 1 at the beginning of the study, the prevalence of the other four states can be easily captured during the whole course of time. Figure 5 shows the effects of sex on the transition probabilities from taking statins to statin discontinuation, experiencing myopathy with statin use and following its discontinuation, and experiencing rhabdomyolysis without statin use. After the first 6 months, the probability of discontinuation decreased by time in both sex groups. The proportion for male and female patients taking stain with myopathy decreases after six months. The overall trends in both sex groups for myopathy or rhabdomyolysis after discontinuation increased with time. We observed a greater likelihood of both statin discontinuation and the development of myopathy following discontinuation among women, evidence that was statistically significant because their 95% confidence intervals did not overlap. However, sex demonstrated no statistically significant effect on the transition probabilities from taking statins and either developing myopathy while taking the medication or developing rhabdomyolysis after its discontinuation.
FIGURE 5.
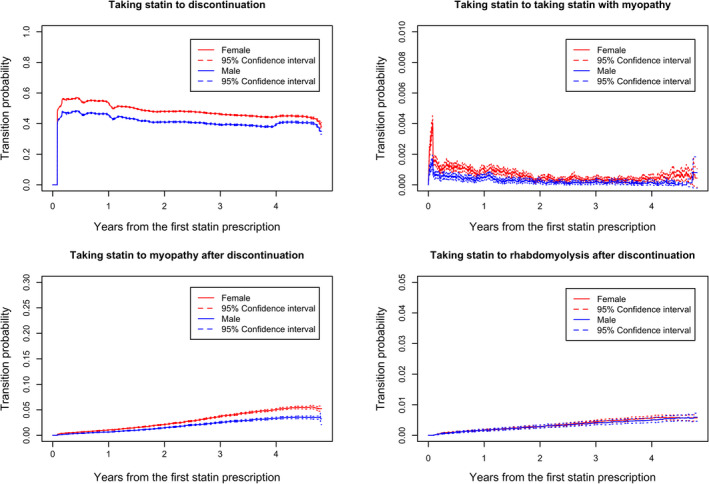
Sex comparisons of transition probabilities from taking statin to other states
Figure 6 shows the effects of younger or older age on the transition probabilities. The overall trends for the four transitions probabilities changing were similar to the sex group. Patients younger than 65 years appeared more likely to discontinue statin use, but patients 65 years or older were more likely to develop myopathy and/or rhabdomyolysis after discontinuing statin use. This evidence was statistically significant because their 95% confidence intervals did not overlap, but no evidence suggested statistically significant difference between the two age groups with regard to developing myopathy while taking the medication.
FIGURE 6.
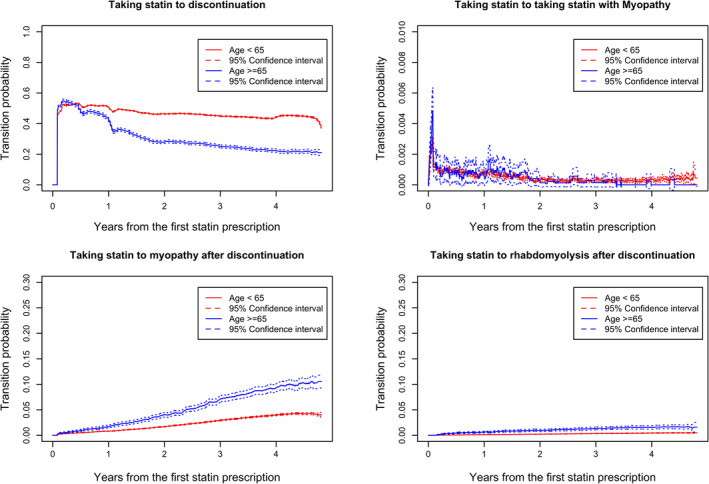
Age comparison of transition probabilities from taking statin to other states
CONCLUSION AND DISCUSSION
Statin discontinuation happens often and early. Our data suggested that approximately 45% of patients discontinued statin use 1 month after starting their prescriptions, and 50% discontinued the medication within 1 year, results resembling those of a cohort study that showed an abrupt increase in statin discontinuation after one prescription and an approximately 50% discontinuation rate at 1 year. 24 The observed abruptly high statin discontinuation rate at 1 month was largely attributed to our assumption of 1 month statin prescription between two prescriptions. In our data analysis, even if we increased the prescription time to 3 months, the same trend holds, that is, the same abruptly high discontinuation rate at 3 months.
Nevertheless, whereas they showed a continuing increase in the probability of discontinuation, we noted a decreasing trend in the probability of discontinuation after the first 6 months. We believe this was attributable to the fact that those investigators based their data analysis on the traditional Cox proportional hazard regression model, which considered two states, the initiation and discontinuation of statin use, but their model discarded cases of retaking statin after discontinuation. In contrast, our multistate transition model allows for patients to take statins after discontinuation. Consequently, we found a decrease in the probability of discontinuation after the first 6 months, which seems to suggest that if patients take statins long enough, they will be less likely to discontinue use.
We also observed a much higher risk for myopathy to develop among patients who discontinued statin use compared with those who continued the medication. This may result from a long‐lasting myopathy effect of statin use in some patients even after they discontinue its use, 12 or it may stem from patients’ discontinuing the medication after experiencing myopathy immediately after starting statin therapy without their early myopathy symptoms being noted in their electronic medical records before they stopped taking the statin. Another alternative interpretation is that some patients who discontinued the statin may take other medications, such as pain management, and continue on experiencing myopathy afterward. These confounding variables are not yet justified in our current multistate model. Adding more confounding variables to extend the research scope will be our future aim. Nevertheless, the data suggest that statin discontinuation is a strong indicator for statin‐induced myopathy.
Both sex and age are strong risk factors in predicting statin discontinuation and myopathy. We observed that women were more likely than men to discontinue statin therapy as well as to develop myopathy, which is a finding supported by other studies. One group reported a myopathy risk ratio of 1.52 (1.37–1.66, 95% confidence interval [CI]) for women compared with men. 25 Another group noted a rhabdomyolysis risk ratio of 2.53 (0.91–7.32) for women compared with men, 26 and a study of adherence to statin therapy observed an odds ratio of 1.10 (1.07–1.13, 95% CI) between women and men for statin nonadherence. 27
We observed that older patients were less likely than younger patients to discontinue statin use and demonstrated higher risks of developing myopathy or rhabdomyolysis. With regard to medication discontinuation, Perreault et al. reported a hazard ratio between older and younger patients of 0.85 (0.81–0.90, 95% CI), and Halava and colleagues (2016) reported a ratio of 0.81 (0.68–0.98, 95% CI). 24 , 28 With respect to the likelihood to develop rhabdomyolysis, Schech et al. noted a four‐times higher risk among older patients. 26
Our multistate transition model framework permits the characterization of the relationship between statin discontinuation and the risks of statin‐induced myopathy and/or rhabdomyolysis. Analysis of this relationship has been impossible with other statistical models, such as logistic regression and Cox proportional hazard regression, because they cannot depict transition probabilities among various mutually exclusive states and do not allow recurrence. This is the primary strength we describe. Our proposed multistate transition model can be broadly applied to pharmaco‐epidemiological studies and will provide an improved approach in the identification of subpopulations for the evaluation of drug efficacy and/or adverse drug effects.
CONFLICT OF INTEREST
The authors declared no conflict of interest.
AUTHOR CONTRIBUTIONS
Y.Z., L.L., and M.W.M. wrote the manuscript. L.L., G.B., D.Z., W.C., and M.D. designed the research. Y.Z. and L.L. perfomed the research. Y.Z., C.C., L.W., and P.Z. analyzed the data. Y.Z. and L.L. contributed new reagents/analytical tools.
Supporting information
Supplementary Material
ACKNOWLEDGMENTS
The authors gratefully acknowledge support by R01GM124104 awarded by the National Institutes of Health and National Science Foundation Grant NSF1622526.
Zhu Y, Chiang C‐W, Wang L, et al. A multistate transition model for statin‐induced myopathy and statin discontinuation. CPT Pharmacometrics Syst Pharmacol. 2021;10:1236–1244. 10.1002/psp4.12691
Funding information
Funding was provided by National Institutes of Health Grant R01GM124104 and National Science Foundation Grant NSF1622526.
REFERENCES
- 1. Hope HF, Binkley GM, Fenton S, Kitas GD, Verstappen SMM. Systematic review of the predictors of statin adherence for the primary prevention of cardiovascular disease. PLoS One. 2019;14:e0201196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nguyen KA, Li L, Lu D, et al. A comprehensive review and meta‐analysis of risk factors for statin‐induced myopathy. Eur J Clin Pharmacol. 2018;74:1099‐1109. [DOI] [PubMed] [Google Scholar]
- 3. Ho PM, Spertus JA, Masoudi FA, et al. Impact of medication therapy discontinuation on mortality after myocardial infarction. Arch Intern Med. 2006;166:1842‐1847. [DOI] [PubMed] [Google Scholar]
- 4. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2889‐2934. [DOI] [PubMed] [Google Scholar]
- 5. Kamal‐Bahl SJ, Burke T, Watson D, Wentworth C. Discontinuation of lipid modifying drugs among commercially insured United States patients in recent clinical practice. Am J Cardiol. 2007;99:530‐534. [DOI] [PubMed] [Google Scholar]
- 6. Banach M, Stulc T, Dent R, Toth PP. Statin non‐adherence and residual cardiovascular risk: there is need for substantial improvement. Int J Cardiol. 2016;225:184‐196. [DOI] [PubMed] [Google Scholar]
- 7. Jacobson TA. NLA task force on statin safety‐2014 update. J Clin Lipidol. 2014;8:S1‐S4. [DOI] [PubMed] [Google Scholar]
- 8. Abd TT, Jacobson TA. Statin‐induced myopathy: a review and update. Exp Opin Drug Saf. 2011;10:373‐387. [DOI] [PubMed] [Google Scholar]
- 9. Iwere RB, Hewitt J. Myopathy in older people receiving statin therapy: a systematic review and meta‐analysis. Br J Clin Pharmacol. 2015;80:363‐371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Selva‐O’Callaghan A, Alvarado‐Cardenas M, Pinal‐Fernández I, et al. Statin‐induced myalgia and myositis: an update on pathogenesis and clinical recommendations. Exp Rev Clin Immunol. 2018;14:215‐224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Law M, Rudnicka AR. Statin safety: a systematic review. Am J Cardiol. 2006;97:S52‐S60. [DOI] [PubMed] [Google Scholar]
- 12. Echaniz‐Laguna A, Mohr M, Tranchant C. Neuromuscular symptoms and elevated creatine kinase after statin withdrawal. N Engl J Med. 2010;362:564‐565. [DOI] [PubMed] [Google Scholar]
- 13. Rowan CG, Brunelli SM, Munson J, et al. Clinical importance of the drug interaction between statins and CYP3A4 inhibitors: a retrospective cohort study in The Health Improvement Network. Pharmacoepidemiol Drug Saf. 2012;21:494‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Carr D, O’Meara H, Jorgensen AL, et al. SLCO1B1 genetic variant associated with statin‐induced myopathy: a proof‐of‐concept study using the clinical practice research datalink. Clin Pharmacol Ther. 2013;94:695‐701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sarkar K, Chowdhury R, Dasgupta A. Analysis of survival data: challenges and algorithm‐based model selection. J Clin Diagn Res. 2017;11:LC14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rueda OM, Sammut S‐J, Seoane JA, et al. Dynamics of breast‐cancer relapse reveal late‐recurring ER‐positive genomic subgroups. Nature. 2019;567:399‐404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jensen ML, Jørgensen ME, Hansen EH, Aagaard L, Carstensen B. A multistate model and an algorithm for measuring long‐term adherence to medication: a case of diabetes mellitus type 2. Value Health. 2014;17:266‐274. [DOI] [PubMed] [Google Scholar]
- 18. Fung V. Patients’ perspectives on nonadherence to statin therapy: a focus‐group study. Perm J. 2010;14:4‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Han X, Quinney SK, Wang Z, et al. Identification and mechanistic investigation of drug–drug interactions associated with myopathy: a translational approach. Clin Pharmacol Ther. 2015;98:321‐327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mortensen MB, Falk E. Primary prevention with statins in the elderly. J Am Coll Cardiol. 2018;71:85‐94. [DOI] [PubMed] [Google Scholar]
- 21. de Wreede LC, Fiocco M, Putter H. mstate: an R package for the analysis of competing risks and multi‐state models. J Stat Softw. 2011;38:1‐30. [Google Scholar]
- 22. Andersen PK, Borgan O, Gill RD, Keiding N. Statistical Models Based on Counting Processes. Berlin/Heidelberg, Germany: Springer Science & Business Media; 2012. [Google Scholar]
- 23. Aalen O. A model for nonparametric regression analysis of counting processes. In Mathematical Statistics and Probability Theory. Springer; 1980: 1‐25. [Google Scholar]
- 24. Perreault S, Blais L, Lamarre D, et al. Persistence and determinants of statin therapy among middle‐aged patients for primary and secondary prevention. Br J Clin Pharmacol. 2005;59:564‐573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Skilving I, Eriksson M, Rane A, Ovesjö M‐L. Statin‐induced myopathy in a usual care setting—a prospective observational study of gender differences. Eur J Clin Pharmacol. 2016;72:1171‐1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schech S, Graham D, Staffa J, et al. Risk factors for statin‐associated rhabdomyolysis. Pharmacoepidemiol Drug Saf. 2007;16:352‐358. [DOI] [PubMed] [Google Scholar]
- 27. Lewey J, Shrank WH, Bowry ADK, Kilabuk E, Brennan TA, Choudhry NK. Gender and racial disparities in adherence to statin therapy: a meta‐analysis. Am Heart J. 2013;165:665‐678.e1. [DOI] [PubMed] [Google Scholar]
- 28. Halava H, Huupponen R, Pentti J, Kivimäki M, Vahtera J. Predictors of first‐year statin medication discontinuation: a cohort study. J Clin Lipidol. 2016;10:987‐995. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


