Abstract
Rivaroxaban has been investigated in the EINSTEIN‐Jr program for the treatment of acute venous thromboembolism (VTE) in children aged 0 to 18 years and in the UNIVERSE program for thromboprophylaxis in children aged 2 to 8 years with congenital heart disease after Fontan‐procedure. Physiologically‐based pharmacokinetic (PBPK) and population pharmacokinetic (PopPK) modeling were used throughout the pediatric development of rivaroxaban according to the learn‐and‐confirm paradigm. The development strategy was to match pediatric drug exposures to adult exposure proven to be safe and efficacious. In this analysis, a refined pediatric PopPK model for rivaroxaban based on integrated EINSTEIN‐Jr data and interim PK data from part A of the UNIVERSE phase III study was developed and the influence of potential covariates and intrinsic factors on rivaroxaban exposure was assessed. The model adequately described the observed pediatric PK data. PK parameters and exposure metrics estimated by the PopPK model were compared to the predictions from a previously published pediatric PBPK model for rivaroxaban. Ninety‐one percent of the individual post hoc clearance estimates were found within the 5th to 95th percentile of the PBPK model predictions. In patients below 2 years of age, however, clearance was underpredicted by the PBPK model. The iterative and integrative use of PBPK and PopPK modeling and simulation played a major role in the establishment of the bodyweight‐adjusted rivaroxaban dosing regimen that was ultimately confirmed to be a safe and efficacious dosing regimen for children aged 0 to 18 years with acute VTE in the EINSTEIN‐Jr phase III study.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
Rivaroxaban is under development in two pediatric indications: treatment of acute venous thromboembolism (VTE) in children in the age range 0 and 18 years and thromboprophylaxis in children aged 2 to 8 years with congenital heart disease, with an intensive application of physiologically‐based (PBPK) and population pharmacokinetic (PopPK) modeling and simulation.
WHAT QUESTION DID THIS STUDY ADDRESS?
A refined pediatric PopPK model has been developed and the ability of PBPK modeling to predict the exposure of rivaroxaban in pediatric patients with VTE was retrospectively assessed.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
The underlying dataset is to our knowledge one of the broadest datasets for a single compound compiled from controlled clinical trials in children to date including patients aged 0 to 18 years with a bodyweight range between 2.7 kg and 194 kg.
HOW MIGHT THIS CHANGE DRUG DISCOVERY, DEVELOPMENT, AND/OR THERAPEUTICS?
The application of PBPK and PopPK modeling in this rivaroxaban study serves as an example for model‐informed pediatric drug development.
INTRODUCTION
Rivaroxaban, an oral direct inhibitor of factor Xa, is efficacious for the treatment and prevention of venous thromboembolism (VTE) in adults and has demonstrated to have a lower risk of major bleeding in adult patients with VTE, as compared with the traditional combination of heparin followed by a vitamin K antagonist. 1 , 2 , 3 The approved adult doses for treatment of deep vein thrombosis (DVT) and prevention of recurrent DVT and pulmonary embolism (PE) are 15 mg bi‐daily (b.i.d.) for 3 weeks followed by 20 mg once daily (o.d.) as maintenance dose, and the dose for prevention of VTE in adult patients undergoing elective hip or knee replacement is 10 mg o.d. The EINSTEIN‐Jr program investigated the use of rivaroxaban for the treatment of VTE in children in the age range between 0 and 18 years through phases I to III and targeted an exposure similar to that observed in young adults with VTE treated with rivaroxaban 20 mg once daily. 4 Currently, rivaroxaban is being developed for thromboprophylaxis in children aged 2 to 8 years with congenital heart disease (CHD) after the Fontan‐procedure in the UNIVERSE phase III study. The target in UNIVERSE is to match the exposure of 10 mg rivaroxaban total daily dose in adults. 5
Modeling and simulation were intensively applied during rivaroxaban development in adults and children. Prior to the start of clinical studies in pediatric patients with VTE, a physiologically‐based pharmacokinetic (PBPK) model for children was developed 6 based on a pre‐existing PBPK model for adults using a generic physiological scaling approach that is in detail described in previous publications. 7 , 8 This pediatric PBPK model was used to establish a bodyweight‐adjusted dosing table, which was tested in a single‐dose phase I study that aimed to characterize pharmacodynamics (PD) and pharmacokinetics (PK) of rivaroxaban in children aged 0.5 to less than 18 years. 9 PK observations in this phase I study informed the first pediatric population PK (PopPK) model of rivaroxaban that delivered individual PK post hoc estimates and confirmed the applicability of the initial rivaroxaban pediatric PBPK model to further guide dosing recommendations. 10 During phase II of the EINSTEIN‐Jr program, the PBPK and PopPK models were continued to be used to refine the dose strengths and dosing regimen of rivaroxaban with the goal to achieve similar rivaroxaban exposure as that in an adult reference population treated for DVT with 20 mg o.d. 11 Furthermore, the models informed the switches from the o.d. regimen to b.i.d. and thrice‐daily (t.i.d.) rivaroxaban administration, which were introduced in children with bodyweight below 30 and 12 kg, respectively. 11 The bodyweight adjusted o.d., b.i.d., and t.i.d. regimens were further confirmed in the EINSTEIN‐Jr phase III study, 4 which showed that children with acute VTE who were treated with rivaroxaban according to the modeling‐informed dosing scheme had a similarly low VTE recurrent VTE risk and reduced thrombotic burden without increased bleeding, as compared with standard anticoagulants. 12 , 13
Despite the usefulness of the PBPK model in guiding the dose selection, there still exists a need to develop a suitable pediatric PopPK model, which can be used to describe the pediatric PK of rivaroxaban and to explore the impact of covariates. The aim of this paper is to report a refined pediatric PopPK model for rivaroxaban that is based on integrated EINSTEIN‐Jr data and first PK data from part A of the UNIVERSE program and to investigate the influence of potential covariates and intrinsic factors on rivaroxaban exposure. A second aim is to assess the ability of the PBPK model to predict exposure of rivaroxaban in pediatric patients with VTE retrospectively and to discuss the role of PopPK and PBPK modeling throughout the pediatric development of rivaroxaban.
DATA AND METHODS
PopPK dataset
The final dataset consisted of 1988 rivaroxaban concentration measurements that were valid for PK analysis from 524 pediatric patients across the different study phases (Table 1). Five hundred twelve (97.7%) of these patients participated in one of the EINSTEIN‐Jr studies and contributed 1916 (96.4%) PK samples. Twelve pediatric post‐Fontan patients (2.3%) from the PK part (part A) of the UNIVERSE study contributed 72 PK samples (3.6%). Eighty‐six of the 524 patients (16.4%) were below 2 years of age. The dataset contained PK data after single or multiple rivaroxaban doses and different dose levels and regimens (o.d., b.i.d., or t.i.d.). PK sampling was generally sparse in children. In the single dose EINSTEIN‐Jr phase I studies, the maximum number of PK samples that were collected was five in adolescents and two in children aged 6 months to 2 years. In the EINSTEIN‐Jr multiple dose studies during phases I/II, II, and III, one or two PK samples were taken per child on different study days after a rivaroxaban dose (Table S1). Details about the number of available PK samples per age group, the PK sampling windows per study phase and information about the handling of missing covariate information are provided in the Supplementary Information.
TABLE 1.
Data used for the pediatric PopPK analysis
| PROGRAM | EINSTEIN‐Jr | UNIVERSE | TOTAL | |||||
|---|---|---|---|---|---|---|---|---|
| Phase | I | I | I/II | II | II | III | III | I–III |
| Age range |
12–<18 years 6–<12 years 2 – <6 years 0.5 – <2 years |
2 month–<12 years | birth–<0.5 years | 6–<18 years | 0.5–<6 years | birth–<18 years. | 2–<8 years. | Birth – <18 years |
| Dosing regimen | Single dose | Single dose | Multiple doses, b.i.d., t.i.d. | Multiple doses, o.d., b.i.d. | Multiple doses, b.i.d. | Multiple doses, o.d., b.i.d., t.i.d. | Multiple doses, b.i.d. | Single and multiple doses, o.d., b.i.d., t.i.d. |
| Dose range |
BW adjusted, low (0.4 mg–10 mg) and high (0.8 mg–20 mg) doses |
BW adjusted, 0.6 mg–10 mg |
BW adjusted, 1.0 mg–8.7 mg daily dose |
BW adjusted, 5.0 mg–20 mg daily dose |
BW adjusted, 1.4 mg–15 mg daily dose |
BW adjusted, 2.4 mg–20 mg daily dose |
BW adjusted, 2.2 mg–5.0 mg daily dose |
BW adjusted, 0.4 mg–20 mg |
| Formulation | Tablet or ready‐to‐use oral suspension (undiluted or diluted) | Granules for oral suspension | Ready‐to‐use oral suspension (diluted) or granules for oral suspension | Tablet or ready‐to‐use oral suspension (diluted) | Ready‐to‐use oral suspension (diluted) | Tablets or granules for oral suspension | Granules for oral suspension | Tablet or ready‐to‐use oral suspension (undiluted or diluted), or granules for oral suspension |
| No. of subjects (%) | 59 (11.3%) | 45 (8.6%) | 10 (1.9%) | 42 (8.0%) | 40 (7.6%) | 316 (60.3%) | 12 (2.3%) | 524 (100%) |
| No. PK samples (%) | 199 (10.0%) | 132 (6.6%) | 37 (1.9%) | 168 (8.5%) | 148 (7.4%) | 1.232 (62.0%) | 72 (3.6%) | 1.988 (100%) |
| No. of subjects birth–<2 years (%) | 10 (11.6%) | 18 (20.9%) | 10 (11.6%) | 0 (0.0%) | 13 (15.1%) | 35 (40.7%) | 0 (0.0%) | 86 (100%) |
Abbreviations: BW, body weight; PK, pharmacokinetic; PopPK, population pharmacokinetic.
PopPK model development
The previously published PopPK model for rivaroxaban in children was used as a starting point. This model was based on 199 plasma concentrations observed in 59 children that participated in the initial EINSTEIN‐Jr phase I study. 10 Structurally, the model consists of two compartments with first‐order absorption and first‐order elimination from the central compartment and is described by the following set of parameters: the rate constant for oral absorption (ka), the relative oral bioavailability (F1), the clearance from the central compartment (CL), the volumes of the central (Vc) and peripheral compartments (Vp), and the intercompartmental clearance (Q).
PopPK analyses were performed by means of nonlinear mixed‐effect modeling, including fixed effects (structural parameters) as well as random effects (stochastic parameters). The details of the software that was used and the criteria that were applied during model development and for acceptance of a final model are summarized in the Supplementary Information.
Covariate analysis
Prior knowledge about rivaroxaban PK in adults 14 , 15 , 16 , 17 , 18 combined with the outcome of the covariate analysis in the recently published integrated PopPK analysis in adults 19 were used to pre‐define and select potentially relevant covariates for investigation in children:
Bodyweight (considered to be time‐varying in children): allometric relationships were tested on CL, Q, Vc, and Vp.
Age: age was tested in addition to bodyweight as a potential covariate for CL and F1.
Dose: dose was tested as a covariate on F1 because rivaroxaban (Biopharmaceutics Classification System class 2) is known to be subject to solubility‐limited oral absorption at therapeutic doses in adults. 18 , 19
Formulation: three different formulations that were used throughout the EINSTEIN‐Jr program (tablets, a ready‐to‐use oral suspension being administered either undiluted, i.e., directly into the mouth, or diluted through mixing with a defined volume of non‐sparkling liquid, and granules for oral suspension; Table 1) were tested as covariates on ka.
Renal function: four different approaches were tested: (i) estimated glomerular filtration rate (eGFR) based on height and serum creatinine according to the Schwartz‐equation, 20 (ii) ratio of individual serum creatinine and the upper limit of normal, (iii) a categorical score (serum creatinine is equal or below the upper limit of normal vs. serum creatinine is above the upper limit of normal), and (iv) eGFR calculated by the formula of Rhodin et al. 21 using individual body size measures and postmenstrual age (independent of serum creatinine).
Comedications: as in adults, 19 five selected comedication categories were tested: CYP3A4 inhibitors (classified as weak, moderate, or strong), CYP3A4 inducers, and P‐gp inhibitors. Due to the small numbers of such comedication use, the effect could only exploratively be assessed in children.
Fontan: categorial covariate (Fontan yes/no) was tested for a potential influence of the patient population (post‐Fontan patients vs. patients with VTE) on PK.
After a graphical exploration of potential covariate effects (Figures S1 and S2), all selected covariates were included in a statistical evaluation of the relationship between the individual estimates of the random effects and the covariate values. The covariate analysis was done in a forward inclusion‐backward deletion procedure.
Intrinsic factors
To further assess potential influences of intrinsic factors on rivaroxaban PK, subgroups of interest were defined and graphically highlighted in plots showing rivaroxaban exposure versus age or bodyweight. Extremes in bodyweight (underweight and obese children, for definitions, see Supplementary Information), gender, race/ethnicity, as well as the presence of two types of potentially relevant medical disorders common in the pediatric study population, namely concurrent functional gastrointestinal (GI) disorders (e.g., diarrhea and malabsorption conditions) and certain malignant diseases (acute leukemias and Hodgkin's lymphomas) were explored. These graphical assessments were performed using the pooled dataset from the multiple dose EINSTEIN‐Jr phase I, II, and III studies only.
RESULTS
PopPK development and covariate analysis
Table 2 summarizes the parameter estimates of the refined pediatric PopPK model reported in this paper. Selected goodness‐of‐fit plots and visual predictive checks are available in Supplementary Information Figures S3 and S4 demonstrating that the model adequately described the central trend as well as the interindividual variability of the observed pediatric rivaroxaban PK data.
TABLE 2.
Model parameters estimated for rivaroxaban in children
| Parameter | Unit | Value a | SE b | CV (%) c | LLCI d | ULCI e | |
|---|---|---|---|---|---|---|---|
| Fixed effects | |||||||
| ka for tablets, granules and diluted suspension | h−1 | 0.799 | 0.0736 | 9.21 | 0.655 | 0.944 | |
| ka for undiluted suspension | h−1 | 0.226 | 0.0365 | 16.2 | 0.154 | 0.297 | |
| CL for subject with BW of 82.48 kg f | L h−1 | 8.02 | 0.252 | 3.14 | 7.53 | 8.51 | |
| Exponent to scale CL on BW | ‐ | 0.481 | 0.0238 | 4.96 | 0.434 | 0.527 | |
| Vc for subject with BW of 82.48 kg f | L | 53.2 | 3.07 | 5.77 | 47.2 | 59.3 | |
| Vp for subject with BW of 82.48 kg f | L | 59.1 | 15.3 | 25.9 | 29.1 | 89.1 | |
| Exponent to scale Vc and Vp on BW | ‐ | 0.821 | 0.0308 | 3.75 | 0.760 | 0.881 | |
| Q for subjects with BW of 82.48 kg f | L h−1 | 2.50 | 0.414 | 16.6 | 1.69 | 3.31 | |
| Exponent to scale Q on BW | ‐ | 0.761 | 0.102 | 13.4 | 0.561 | 0.961 | |
| Random effects: Interindividual variability | |||||||
|
|
0.0705 (27.0) g | 0.0128 | 18.2 | 0.0453 | 0.0957 | ||
|
|
0.0612 (25.1) g | 0.0105 | 17.2 | 0.0407 | 0.0818 | ||
| Random effects: residual error | |||||||
|
|
0.220 (46.9) h | 0.00918 | 4.18 | 0.202 | 0.238 | ||
Abbreviations: BW, body weight; CL, clearance; Ka, rate constant for oral absorption; Q, intercompartmental clearance; Vc, volume of the central compartment; Vp, volume of the peripheral compartment.
Reported by NONMEM.
Standard error of parameter estimate, reported by NONMEM.
Coefficient of variation (CV), calculated as SE/Value*100%.
Lower limit of 95% confidence interval (LLCI).
Upper limit of 95% confidence interval (ULCI).
Mean weight of the integrated pharmacokinetic analysis in adults used as reference.
The population variation is calculated using the following equation: popvar =sqrt{exp(ω2)‐1}*100.
The population variation is calculated using the following equation: sigvar =sqrt{σ2}*100.
The median bodyweight of the pooled pediatric population was 29.5 kg (range 2.7 to 194 kg). Bodyweight was found to have a significant effect on CL, Q, V c, and V p. CL was estimated to be 8.02 L/h (95% confidence interval [CI]: 7.53–8.51 L/h) at a reference bodyweight of 82.48 kg (the median bodyweight of the integrated PopPK analysis in adults) 19 and allometrically scaled with bodyweight with an exponent of 0.481 (95% CI: 0.434–0.527). Q was estimated to be 2.50 L/h (95% CI: 1.69–3.31 L/h) and scaled with an allometric exponent of 0.761 (95% CI: 0.561–0.961). V c and V p were estimated to be 53.2 L (95% CI: 47.2–59.3 L) and 59.1 L (95% CI: 29.1–89.1 L), respectively, in a subject with bodyweight of 82.48 kg and were allometrically scaled with an exponent of 0.821 (95% CI: 0.760–0.881, assumed to be identical for V c and V p).
Across all studies, bodyweight‐normalized single rivaroxaban doses ranged between ~ 0.1 to 0.5 mg/kg and absolute single/daily doses in the EINSTEIN‐Jr program ranged between 0.4 and 20 mg, as shown in Figure 1. Relative oral bioavailability was found to decrease with increasing dose per bodyweight in children. This dose‐dependency was adequately described using the previously reported F1 function in adults, 19 after replacing the absolute dose in mg by dose per bodyweight in mg/kg (Figure 2). In the absence of data on absolute bioavailability of rivaroxaban in children, relative oral bioavailability was set to 100% for a pediatric dose of 0.12 mg/kg (corresponding to 10 mg in an 82.48 kg subject) and decreased gradually to 79.1% at 0.30 mg/kg and 68.1% at 0.50 mg/kg. A lower k a was estimated for the undiluted ready‐to‐use oral suspension (0.226 1/h, 95% CI: 0.154–0.297 1/h) when compared to the other tested formulations (i.e., tablet, granules for oral suspension, and diluted ready‐to‐use oral suspension, k a = 0.799 1/h, 95% CI: 0.655–0.944 1/h).
FIGURE 1.

Evolution of bodyweight adjusted single or daily doses as a function of study phase in the EINSTEIN‐Jr program. (*) In Japan, children with bodyweight greater than or equal to 50 kg receive 15 mg o.d., the dose for adult Japanese patients with VTE
FIGURE 2.

Relationship between relative oral bioavailability and bodyweight‐normalized dose. Symbols represent individual estimates for children, the solid red line represents the function derived for adults 19 that was also applied in the PopPK model for children
The median age of the pooled pediatric population was 9.0 years (range 0 to 18 years), 16.4% pediatric patients were younger than 2 years (Table 1). No effect of age on CL or F1 could be identified by the model.
The eGFR according to the Schwartz‐formula of the pediatric patients ranged from 43.8 to 456 ml/min/1.73 m2 with a median of 150 ml/min/1.73 m2. None of the four methods to test for an influence of renal function led to a significant improvement of the objective function.
Only a small fraction of the valid concentration measurements was obtained under the influence of relevant comedications (Table 3). Because the use of strong inhibitors of both CYP3A4 and P‐gp was excluded per protocol, no PK data was available in children while receiving strong CYP3A4 inhibitors or P‐gp inhibitors concomitantly. Likewise, concomitant use of strong inducers of CYP3A4 was not allowed per protocol. The exploratory evaluation of potential effects of concomitant medication of weak or moderate CYP3A4 inhibitors or CYP3A4 inducers on either CL or F1 revealed no significant effects.
TABLE 3.
Number of subjects and PK observations while on comedication
| Comedication | Subjects with valid PK measurements | Valid PK measurements | ||
|---|---|---|---|---|
| Number | Percentage | Number | Percentage | |
| CYP3A4 inducers | ||||
| No use | 508 | 96.9% | 1954 | 98.2% |
| Any use | 16 | 3.1% | 36 | 1.8% |
| Weak CYP3A4 inhibitors | ||||
| No use | 483 | 92.2% | 1867 | 93.8% |
| Any use | 41 | 7.8% | 123 | 6.2% |
| Moderate CYP3A4 inhibitors | ||||
| No use | 506 | 96.6% | 1940 | 97.5% |
| Any use | 18 | 3.4% | 50 | 2.5% |
| Strong CYP3A4 inhibitors | ||||
| No use | 524 | 100% | 1990 | 100% |
| Any use | 0 | 0% | 0 | 0% |
| P‐gp inhibitors (with narrow scope) | ||||
| No use | 524 | 100% | 1990 | 100% |
| Any use | 0 | 0% | 0 | 0% |
Abbreviation: PK, pharmacokinetic.
The covariate “Fontan” showed a small but statistically significant drop in the objective function when applied to CL in the univariate forward inclusion step. However, in the backward elimination step with a more stringent criterion, the change in minimum value of the objective function was not statistically significant and this covariate was therefore not included in the final model.
Interindividual variability (IIV) was identified for CL and F1. All fixed‐effects and random‐effects parameters of the final model could be identified with high precision. The degree of η‐shrinkage for CL was acceptable (23.5%) whereas F1 had a larger shrinkage of 33.2%. Although shrinkage was slightly above the commonly accepted threshold of 30%, 22 this was accepted because leaving out IIV on F1 would significantly worsen the model fit. The ε‐shrinkage for the residual error was 9.74%.
Intrinsic factors
Plots highlighting subgroups with intrinsic factors of interest are shown in the Supplementary Information Figures S5 to S11. The available data did not indicate any influence of underweight or obesity (Figure S5), gender (Figure S6), Japanese, Chinese, or Asian (outside of Japan and China) origin (Figures S7–S9), the presence of acute or chronic GI disorders (Figure S10), acute lymphocytic or myeloid leukemias or Hodgkin's lymphoma (Figure S11) on PK. In summary, no obvious trends for a clustering of any subgroup of interest at either the upper or lower end of the exposure range could be observed.
Comparison with PBPK model predictions
Figure 3 shows a comparison of the individual clearance estimates derived from the PopPK model with the initial PBPK predictions for total plasma clearance 6 as a function of age. Ninety‐one percent of the individual CL estimates are found within the 5th to 95th percentile of the PBPK predictions (light gray shaded area) and 100% of the estimates are within the enlarged expected range (dark gray shaded area, indicating the range from 0.5 × the 5th percentile to 1.5 times the 95th percentile of the PBPK predictions) that was introduced to account for uncertainties in the estimation of physiological parameters relevant for the pediatric PBPK model. 6 , 19 Both the central trend for CL versus age as well as the IIV were very well‐predicted by the PBPK model down to an age of ~ 2 years. Below this age, the clearance was underpredicted by the PBPK model and most of the individual clearance estimates are located between the 50th and 95th percentile of the PBPK model.
FIGURE 3.
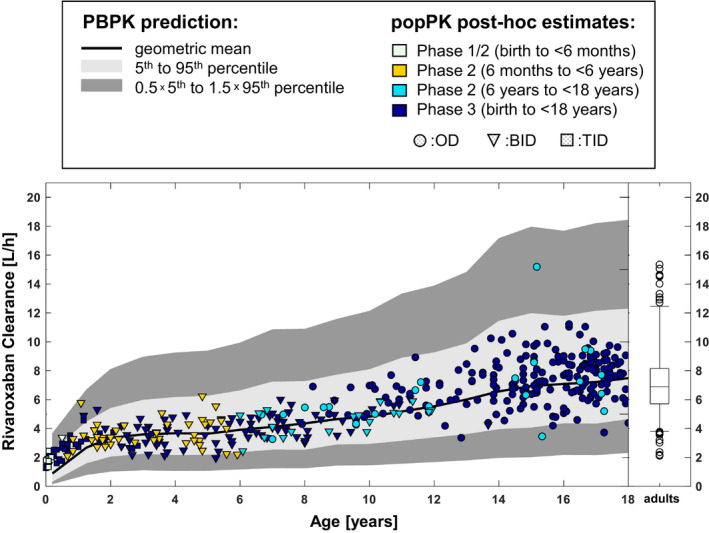
Total rivaroxaban plasma clearance as a function of age. Prospective PBPK predictions 6 are shown as black line and gray shaded areas, symbols represent individual post hoc estimates derived for the children using the PopPK model. Clearance estimates for an adult reference population (adult patients with VTE ≤45 years receiving 20 mg o.d., N = 203) are shown on the right for comparison. PBPK, physiologically‐based pharmacokinetic; PopPK, population pharmacokinetic; VTE, venous thromboembolism
Figure 4 compares distributions of the estimated steady‐state exposure metrics (area under the concentration curve AUC(0–24),ss, maximum plasma concentration Cmax ,ss, and trough plasma concentration Ctrough ,ss) by age group with the corresponding exposure metrics simulated with the PBPK model 6 for the phase III study (for tabulated data, see Table S2 in the Supplementary Information). Consistent with the observed relationship between clearance and age, the agreement between simulated and observed exposure metrics is very good for children between 18 and 2 years. In the lowest age group from birth to less than 2 years, the PBPK model tended to overpredict the exposure of rivaroxaban.
FIGURE 4.

Comparison of PopPK post hoc estimates for AUC(0‐24h),ss, Cmax,ss, and Ctrough,ss in the EINSTEIN‐Jr phase III study with corresponding PBPK simulations for the phase III doses (geoMean and geoSD) by age groups. AUC( 0–24h),ss, area under the concentration curve from zero to 24 hours, under steady‐state; Cmax,ss, maximum plasma concentration under steady‐state; Ctrough,ss, trough plasma concentration under steady‐state; PBPK, physiologically‐based pharmacokinetic; PopPK, population pharmacokinetic
DISCUSSION
Modeling and simulation were intensively applied during the pediatric development program of rivaroxaban following the well‐known and previously applied “learn‐and‐confirm” paradigm. 23 , 24 , 25 The basis of this paradigm is a continuous integration of knowledge and data into decision making and an iterative refinement of models.
PBPK modeling was mainly applied in a predictive mode to support bodyweight‐adjusted doses and dosing regimens. In the first‐in‐children study, a cautious dosing approach was used, and the daily rivaroxaban doses were stepwise increased during the course of pediatric development up to the final bodyweight adjusted dosing scheme, as shown in Figure 1. After clinical data from the phase I study became available in children between 0.5 and 18 years of age, 9 the first pediatric PopPK model was established to quantitatively assess the single‐dose rivaroxaban PK in children. 10 Although PK data collection was sparse per subject, the density of the sampling was sufficient to inform a two‐compartmental PopPK model. 10 The comparison of observed plasma concentration‐time data and model‐derived post hoc PK parameters and exposure metrics with corresponding PBPK predictions confirmed the applicability of the rivaroxaban pediatric PBPK model for prediction purposes, 10 although the data were still limited at that time. In the EINSTEIN‐Jr phase I study, 9 , 10 only 10 subjects below 2 years of age were included (Table 1). During phase II, b.i.d. and t.i.d. dosing regimens were introduced for children below 30 kg and 12 kg, respectively, supported by PBPK and PopPK modeling. 11
PopPK modeling was conducted in a cumulative and iterative fashion in EINSTEIN‐Jr. Whenever a study of the pediatric program was completed, the newly available PK data were added to the data pool and the PopPK model version was updated. The PopPK model presented in this paper is based on a database that integrates final PK data collected in the phase I, II, and III studies of EINSTEIN‐Jr and preliminary PK data observed in 12 post‐Fontan patients from the UNIVERSE study (Table 1). This database is to our knowledge one of the broadest datasets for a single compound compiled from controlled clinical trials in children to date. Nevertheless, the heterogeneity of the demographic parameters is considerable given that patients aged 0 to 18 years with a bodyweight range between 2.7 kg and 194 kg are included. One of the guiding principles of PopPK modeling throughout the pediatric development of rivaroxaban—in‐line with the learn‐and‐confirm‐paradigm—was to make use of prior knowledge, whenever reasonable, from previous quantitative analyses in children 10 and adults. 14 , 15 , 16 , 17 , 18 , 19 , 26 , 27 , 28 It is, therefore, not surprising that the main outcomes of this PopPK model are qualitatively consistent with the results of the previously reported PopPK model. 10
Bodyweight‐adjusted low and high doses (approximately equivalent to 10 and 20 mg in adults) were analyzed in the previous PopPK model and F1 in the high‐dose group was found to be 64.8% of F1 in the low‐dose group. 10 The broad range of doses per bodyweight in the pediatric PopPK dataset allowed for a continuous assessment of the dose‐dependency of F1 in the current model (Figure 2). The observed decline of F1 with increasing dose per bodyweight was consistent with the decline of F1 versus (absolute) dose that was identified in the integrated PopPK analysis in adults. 19 Therefore, the functional relationship established for adults was considered prior knowledge and the previously established adult dose‐dependency of F1 was applied to children after replacing the absolute dose‐by‐dose per bodyweight and normalization to F1 = 100% at a dose of 10 mg/82.48 kg (the median bodyweight of the integrated PopPK analysis in adults 19 ).
A lower rate of absorption of the undiluted ready‐to‐use suspension was found in this model (consistent with the previous pediatric PopPK model 10 ) but the extent of absorption is not affected by ka and, thus, AUC(0–24h),ss is independent of the formulation. More importantly, the current model demonstrated that k a does not differ for the tablet formulation and the granules for oral suspension formulation used in phase III. With the granules for oral suspension formulation, the need for a dilution step for the ready‐to‐use suspension could be overcome. The dilution step was introduced for the ready‐to‐use suspension after the delayed oral absorption became obvious, but it added undesired complexity and an increased risk for dosing errors.
Total plasma clearance for a subject with bodyweight of 82.48 kg was estimated to be 8.02 L/h in the refined model, which is similar to the value obtained in the first PopPK model for children (7.26 L/h for a subject with a bodyweight of 70 kg 10 ) and in the integrated PopPK model in adults (6.58 L/h 19 ). The allometric clearance exponent in this model was 0.481 (0.323 in the first model 10 ). This value is considerably lower than the theoretical value of 0.75, but this is not an uncommon finding for orally administered drugs. 29 Notably, inclusion of age as an additional covariate for CL did not improve the model fit, indicating that no age‐related maturation function was required to describe rivaroxaban clearance in children over the entire age range. The allometric exponent for the volume parameters Vc and Vp was 0.821 in this model, which is reasonably close to the theoretical value of 1.0. No efforts were undertaken to fix the fitted allometric exponents to theoretical or published values, nor were efforts undertaken to apply other body size parameters (such as lean bodyweight), as the ultimate goal was a model that most adequately describes the data in order to assess possible age‐related differences in the PK of rivaroxaban in pediatric patients.
Renal function is known to affect rivaroxaban clearance in adults, 15 , 18 , 19 , 30 but it did not show up as a significant covariate in the first PopPK model in children (unreported result). Four different approaches to include measures of renal function as either continuous or categorical covariate were tested in the current model. None of the four tested methods could improve the model fit. The likely reason for that is that most children in the rivaroxaban studies had either normal or only moderately impaired kidney functions. Except for one child with a calculated eGFR according to the Schwartz‐formula of 43.8 ml/min/1.73 m2, all estimated eGFR values in the pooled pediatric dataset were above 50 ml/min/1.73 m2.
A quantitative assessment of potential comedication effects was hampered by the low number of PK observations under the influence of relevant comedications (Table 3). In contrast to the integrated rivaroxaban PopPK model for adults, 19 no significant effect of any tested comedication on the PK in children could be identified based on the available pediatric dataset.
The influence of other intrinsic factors, such as gender, race/ethnicity, or hepatic function on rivaroxaban PK, had been investigated in detail in adults. 14 , 17 , 19 , 26 , 27 , 30 , 31 Within the pediatric population, exploratory graphical assessments were performed for extremes in bodyweight, gender, race/ethnicity, and two pathophysiological conditions, namely the presence of GI disorders and the presence of malignant diseases (acute leukemias and Hodgkin’s lymphomas). No data were available in children with hepatic diseases. The selection of these intrinsic factors was driven by clinical considerations and discussions that came up during the conduction of the pediatric studies. None of the investigated intrinsic factors had a relevant impact on rivaroxaban exposure in children, confirming that the bodyweight‐adjusted dosing scheme established in the EINSTEIN‐Jr program can be applied regardless of these factors with the exception of Japanese patients. To maintain consistency with the adult label, 32 a maximum dose of 15 mg (instead of 20 mg) o.d. has been proposed for pediatric Japanese patients with VTE with a bodyweight greater than or equal to 50 kg.
Last, a retrospective comparison of estimated PK parameters and exposure metrics and their initial PBPK predictions was performed. This comparison showed that the PBPK model could reliably predict the dose‐exposure relationship of rivaroxaban in children down to an age of ~ 2 years and, thus, confirmed the usefulness of PBPK modeling to guide dosing decisions in pediatric patients. 33 , 34 , 35 , 36 , 37 In the age group below 2 years (corresponding to a bodyweight of approximately 12 kg), however, the rivaroxaban PBPK model tended to underestimate clearance and, thus, overpredict exposure (Figures 3 and 4). This tendency became evident when PK data of the first five neonates (who received rivaroxaban in a b.i.d. regimen), were available, but it was not obvious from the PK data of the first 10 children aged between 0.5 and less than 2 years in phase I. 10 As a consequence, the dosing regimen was changed for children with a bodyweight below 12 kg to a t.i.d. schedule with the same individual dose that was previously administered twice daily, leading to a 50% increase in AUC(0‐24h),ss and also an increase in Ctrough,ss. 11 Relevant available prior knowledge that should consider a change in clearance over age, such as validated maturation functions of CYP3A as well as the ontogenies of glomerular filtration and tubular secretion, was considered in the pediatric PBPK model for rivaroxaban. 6 Taken together, these clearance processes account for ~ 54% of rivaroxaban elimination according to adult mass balance data. 6 , 18 For rivaroxaban metabolization processes with unknown maturation information, namely CYP2J2 (14%) and CYP‐independent hydrolysis (14%; 7% were excreted unchanged via feces and 11% were unaccounted in the adult mass balance study), 6 , 18 the PBPK model conservatively assumed the same enzyme activity per gram tissue as in adults (i.e., no maturation of enzyme activity), but these assumptions could not predict the apparent high rivaroxaban clearance in pediatric patients below 2 years. This finding could neither be explained by the sample size nor by renal function nor by the weight‐by‐age relationship, which was similar in the actual study population and the virtual population of the PBPK model. A contribution of CYP3A7 or of age‐dependent changes in plasma protein binding to this observation could also be excluded based on historic and recently repeated in vitro analyses (unpublished results, data on file). Thus, the physiological nature of the unexpectedly high clearance and rapid rivaroxaban elimination in young children below 2 years remains unclear.
In summary, modeling and simulation were applied throughout the pediatric development of rivaroxaban according to the learn‐and‐confirm paradigm. PBPK modeling was predominantly applied in the early phases of the pediatric program to predict first‐in‐children doses and to refine the bodyweight adjusted doses, whereas PopPK modeling, established after phase I, was used to derive post hoc estimates for PK parameters and exposure metrics based on individually observed plasma concentrations of the pediatric patients and to guide dosing decisions and adjustments of dose strength or dosing regimen during phase II and III of pediatric development. The combination of PBPK and PopPK modeling and simulation played a major role for the establishment of a bodyweight‐adjusted dosing scheme for rivaroxaban that ultimately led to a safe and efficacious dosing regimen for children aged 0 to 18 years with acute VTE as demonstrated in the EINSTEIN‐Jr phase III study. 12 The pediatric development program of rivaroxaban can, thus, be seen as a realization of the model‐informed drug development (MIDD) paradigm. 25
CONFLICT OF INTEREST
S.W., K.C., Y.Z., H.M., I.I., K.T., D.K., A.W.A.L., W.M., and J.L. are employees and potential share owners of Bayer AG. E.M. and H.J.D. are employees of LAP&P and were paid consultants for Bayer during the conduct of the analysis. H.Y. and P.Z. are former employees of Janssen Pharmaceuticals.
AUTHOR CONTRIBUTIONS
S.W. wrote the manuscript. S.W., Y.Z., K.T., D.K., A.W.A.L., W.M., and J.L. designed the research. S.W., K.C., Y.Z., H.M., I.I., E.M., K.T., D.K., A.W.A.L., H.Y., P.Z., H.J.D., and J.L. performed the research and analyzed the data.
Supporting information
Supplementary Material
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank Matthias Frede for skillful modelling and simulation work in the early stages of the EINSTEIN‐Jr program and many unnamed colleagues of the EINSTEIN‐Jr and UNIVERSE programs for continuous support.
Willmann S, Coboeken K, Zhang Y, et al. Population pharmacokinetic analysis of rivaroxaban in children and comparison to prospective physiologically‐based pharmacokinetic predictions. CPT Pharmacometrics Syst Pharmacol. 2021;10:1195–1207. 10.1002/psp4.12688
Funding information
No funding was received for this work.
REFERENCES
- 1. Bauersachs R, Berkowitz SD, Brenner B, et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. 2010;363:2499‐2510. [DOI] [PubMed] [Google Scholar]
- 2. Büller HR, Prins MH, Lensin AW, et al. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med. 2012;366:1287‐1297. [DOI] [PubMed] [Google Scholar]
- 3. Prins MH, Lensing AW, Bauersachs R, et al. EINSTEIN Investigators: Oral rivaroxaban versus standard therapy for the treatment of symptomatic venous thromboembolism: a pooled analysis of the EINSTEIN‐DVT and PE randomized studies. Thromb J. 2013;11:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lensing AWA, Male C, Young G, et al. Rivaroxaban versus standard anticoagulation for acute venous thromboembolism in childhood. Design of the EINSTEIN‐Jr phase III study. Thrombosis J. 2018;16:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pina LM, Dong X, Zhang L, et al. Rivaroxaban, a direct Factor Xa inhibitor, versus acetylsalicylic acid as thromboprophylaxis in children post‐Fontan procedure: rationale and design of a prospective, randomized trial (the UNIVERSE study). Am Heart J. 2019;213:97‐104. [DOI] [PubMed] [Google Scholar]
- 6. Willmann S, Becker C, Burghaus R, et al. Development of a paediatric population‐based model of the pharmacokinetics of rivaroxaban. Clin Pharmacokinet. 2014;53:89‐102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Edginton AN, Schmitt W, Voith B, Willmann S. A mechanistic approach for the scaling of clearance in children. Clin Pharmacokinet. 2006;45:683‐704. [DOI] [PubMed] [Google Scholar]
- 8. Edginton AN, Schmitt W, Willmann S. Development and evaluation of a generic physiologically based pharmacokinetic model for children. Clin Pharmacokinet. 2006;45:1013‐1034. [DOI] [PubMed] [Google Scholar]
- 9. Kubitza D, Willmann S, Becka M, et al. Exploratory evaluation of pharmacodynamics, pharmacokinetics and safety of rivaroxaban in children and adolescents: an EINSTEIN‐Jr phase I study. Thromb J. 2018;16:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Willmann S, Thelen K, Kubitza D, et al. Pharmacokinetics of rivaroxaban in children using physiologically based and population pharmacokinetic modelling: an EINSTEIN‐Jr phase I study. Thromb J. 2018;16:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Monagle P, Lensing AWA, Thelen K, et al. EINSTEIN‐Jr Phase 2 Investigators. Bodyweight‐adjusted rivaroxaban for children with venous thromboembolism (EINSTEIN‐Jr): results from three multicentre, single‐arm, phase 2 studies. Lancet Haematol. 2019;6:e500‐e509. [DOI] [PubMed] [Google Scholar]
- 12. Male C, Lensing AWA, Palumbo JS, et al. EINSTEIN‐Jr Phase 3 Investigators.: Rivaroxaban compared with standard anticoagulants for the treatment of acute venous thromboembolism in children: a randomised, controlled, phase 3 trial. Lancet Haematol. 2020;7:e18‐e27. [DOI] [PubMed] [Google Scholar]
- 13. Young G, Lensing AWA, Monagle P, et al. Phase 3 Investigators*: Rivaroxaban for treatment of pediatric venous thromboembolism. An Einstein‐Jr Phase 3 dose‐exposure‐response evaluation. J Thromb Haemost. 2020;18:1672‐1685. [DOI] [PubMed] [Google Scholar]
- 14. Mueck W, Borris LC, Dahl OE, et al. Population pharmacokinetics and pharmacodynamics of once‐ and twice‐daily rivaroxaban for the prevention of venous thromboembolism in patients undergoing total hip replacement. Thromb Haemost. 2008;100:453‐461. [PubMed] [Google Scholar]
- 15. Mueck W, Lensing AW, Agnelli G, Decousus H, Prandoni P, Misselwitz F. Rivaroxaban: population pharmacokinetic analyses in patients treated for acute deep‐vein thrombosis and exposure simulations in patients with atrial fibrillation treated for stroke prevention. Clin Pharmacokinet. 2011;50:675‐686. [DOI] [PubMed] [Google Scholar]
- 16. Xu XS, Moore K, Burton P, et al. Population pharmacokinetics and pharmacodynamics of rivaroxaban in patients with acute coronary syndromes. Br J Clin Pharmacol. 2012;74:86‐97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Girgis IG, Patel MR, Peters GR, et al. Population pharmacokinetics and pharmacodynamics of rivaroxaban in patients with non‐valvular atrial fibrillation: results from ROCKET AF. J Clin Pharmacol. 2014;54:917‐927. [DOI] [PubMed] [Google Scholar]
- 18. Mueck W, Stampfuss J, Kubitza D, Becka M. Clinical pharmacokinetic and pharmacodynamic profile of rivaroxaban. Clin Pharmacokinet. 2014;53:1‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Willmann S, Zhang L, Frede M, et al. Integrated Population Pharmacokinetic Analysis of Rivaroxaban Across Multiple Patient Populations. CPT Pharmacometrics Syst Pharmacol. 2018;7:309‐320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schwartz GJ, Muñoz A, Schneider MF, et al. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20:629‐637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rhodin MM, Anderson BJ, Peters AM, et al. Human renal function maturation: a quantitative description using weight and postmenstrual age. Pediatr Nephrol. 2009;24:67‐76. [DOI] [PubMed] [Google Scholar]
- 22. Savic RM, Karlsson MO. Importance of shrinkage in empirical Bayes estimates for diagnostics: problems and solutions. AAPS J. 2009;11:558‐569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sheiner LB. Learning versus confirming in clinical drug development. Clin Pharmacol Ther. 1997;61:275‐291. [DOI] [PubMed] [Google Scholar]
- 24. Chien JY, Friedrich S, Heathman MA, de Alwis DP, Sinha V. Pharmacokinetics/Pharmaco‐dynamics and the stages of drug development: role of modeling and simulation. AAPS J. 2005;7:E544‐E559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. EFPIA MID3 Workgroup , Marshall SF, Burghaus R, et al. Good practices in model‐informed drug discovery and development: practice, application, and documentation. CPT Pharmacometrics Syst. Pharmacol. 2016;5(3):93‐122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhao X, Sun P, Zhou Y, et al. Safety, pharmacokinetics and pharmacodynamics of single/multiple doses of the oral, direct Factor Xa inhibitor rivaroxaban in healthy Chinese subjects. Br J Clin Pharmacol. 2009;68:77‐88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tanigawa T, Kaneko M, Hashizume K, et al. Model‐based dose selection for phase III rivaroxaban study in Japanese patients with non‐valvular atrial fibrillation. Drug Metab Pharmacokinet. 2013;28(1):59‐70. [DOI] [PubMed] [Google Scholar]
- 28. Kubitza D, Roth A, Becka M, et al. Effect of hepatic impairment on the pharmacokinetics and pharmacodynamics of a single dose of rivaroxaban, an oral, direct Factor Xa inhibitor. Br J Clin Pharmacol. 2013;76(1):89‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. McLeay SC, Morrish GA, Kirkpatrick CMJ, Green B. The relationship between drug clearance and body size: systematic review and meta‐analysis of the literature published from 2000 to 2007. Clin Pharmacokinet. 2012;51:319‐330. [DOI] [PubMed] [Google Scholar]
- 30. Kubitza D, Becka M, Mueck W, et al. Effects of renal impairment on the pharmacokinetics, pharmacodynamics and safety of rivaroxaban, an oral, direct Factor Xa inhibitor. Br J Clin Pharmacol. 2010;70:703‐712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Speed V, Green B, Roberts LN, et al. Fixed dose rivaroxaban can be used in extremes of bodyweight: A population pharmacokinetic analysis. J Thromb Haemost. 2020;18(9):2296‐2307. [DOI] [PubMed] [Google Scholar]
- 32. Yamada N, Hirayama A, Maeda H, et al. Oral rivaroxaban for Japanese patients with symptomatic venous thromboembolism ‐ the J‐EINSTEIN DVT and PE program. Thromb J. 2015;13:2. Erratum in: Thromb J. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jorga K, Chavanne C, Frey N, et al. Bottom‐up meets top‐down: complementary physiologically based pharmacokinetic and population pharmacokinetic modeling for regulatory approval of a dosing algorithm of valganciclovir in very young children. Clin Pharmacol Ther. 2016;100:761‐769. [DOI] [PubMed] [Google Scholar]
- 34. Mehrotra N, Bhattaram A, Earp JC, et al. Role of quantitative clinical pharmacology in pediatric approval and labeling. Drug Metab Dispos. 2016;44(7):924‐933. [DOI] [PubMed] [Google Scholar]
- 35. Grimstein M, Yang Y, Zhang X, et al. Physiologically based pharmacokinetic modeling in regulatory science: an update from the U.S. Food and Drug Administration's Office of Clinical Pharmacology. J Pharm Sci. 2019;108(1):21‐25. [DOI] [PubMed] [Google Scholar]
- 36. Bi Y, Liu J, Li L, et al. Role of model‐informed drug development in pediatric drug development, regulatory evaluation, and labeling. J Clin Pharmacol. 2019;59(Suppl 1):S104‐S111. [DOI] [PubMed] [Google Scholar]
- 37. Ince I, Solodenko J, Frechen S, et al. Predictive pediatric modeling and simulation using ontogeny information. J Clin Pharmacol. 2019;59(Suppl 1):S95‐S103. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Supplementary Material


