Abstract
Background
To successfully initiate and maintain breastfeeding for a longer duration, the World Health Organization's Ten Steps to Successful Breastfeeding recommends total avoidance of artificial teats or pacifiers for breastfeeding infants. Concerns have been raised that offering the pacifier instead of the breast to calm the infant may lead to less frequent episodes of breastfeeding and as a consequence may reduce breast‐milk production and shorten duration of breastfeeding.
Objectives
To assess the effect of restricted versus unrestricted pacifier use in healthy full‐term newborns whose mothers have initiated breastfeeding and intend to exclusively breastfeed, on the duration of breastfeeding, other breastfeeding outcomes and infant health.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (30 June 2016) and reference lists of retrieved studies.
Selection criteria
Randomised and quasi‐randomised controlled trials comparing restricted versus unrestricted pacifier use in healthy full‐term newborns who have initiated breastfeeding.
Data collection and analysis
Two review authors independently assessed trials for inclusion and risk of bias, extracted data and checked them for accuracy. The quality of the evidence was assessed using the GRADE approach.
Main results
We found three trials (involving 1915 babies) for inclusion in the review, but have included only two trials (involving 1302 healthy full‐term breastfeeding infants) in the analysis. Meta‐analysis of the two combined studies showed that pacifier use in healthy breastfeeding infants had no significant effect on the proportion of infants exclusively breastfed at three months (risk ratio (RR) 1.01; 95% confidence interval (CI) 0.96 to 1.07, two studies, 1228 infants), and at four months of age (RR 1.01; 95% CI 0.94 to 1.09, one study, 970 infants, moderate‐quality evidence), and also had no effect on the proportion of infants partially breastfed at three months (RR 1.00; 95% CI 0.98 to 1.02, two studies, 1228 infants), and at four months of age (RR 0.99; 95% CI 0.97 to 1.02, one study, 970 infants). None of the included trials reported data on the other primary outcomes, i.e. duration of partial or exclusive breastfeeding, or secondary outcomes: breastfeeding difficulties (mastitis, cracked nipples, breast engorgement); infant's health (dental malocclusion, otitis media, oral candidiasis; sudden infant death syndrome (SIDS)); maternal satisfaction and level of confidence in parenting. One study reported that avoidance of pacifiers had no effect on cry/fuss behavior at ages four, six, or nine weeks and also reported no effect on the risk of weaning before age three months, however the data were incomplete and so could not be included for analysis.
Authors' conclusions
Pacifier use in healthy term breastfeeding infants, started from birth or after lactation is established, did not significantly affect the prevalence or duration of exclusive and partial breastfeeding up to four months of age. Evidence to assess the short‐term breastfeeding difficulties faced by mothers and long‐term effect of pacifiers on infants' health is lacking.
Keywords: Female; Humans; Infant; Infant, Newborn; Lactation; Motivation; Breast Feeding; Breast Feeding/psychology; Breast Feeding/statistics & numerical data; Pacifiers; Pacifiers/adverse effects; Pacifiers/statistics & numerical data; Randomized Controlled Trials as Topic; Time Factors
Plain language summary
Effect of restricted pacifier use on duration of breastfeeding in full‐term infants
What is the issue and why is it important?
A pacifier, used to calm an infant, has become a cultural norm in many parts of the world. Unlimited pacifier use might cause nipple confusion in newborn and hence early termination of breastfeeding. We wanted to explore the effect of restricting the use of a pacifier on the duration of breastfeeding.
What evidence did we find?
We updated the search on 30 June 2016. We identified three studies, with a total of 1915 babies. One study could not be included in the analysis and so findings are based on two studies involving 1302 infants. The mothers in the studies were motivated to breastfeed recruited immediately after birth and at two weeks of life, respectively. We found that unrestricted use of a pacifier did not affect the proportion of infants exclusive or partial breastfeeding at three and four months. The studies were remarkably consistent. We judged this to be moderate‐quality evidence. There was no information on the effect of pacifier use on any breastfeeding difficulties experienced by the mothers, maternal satisfaction, infant crying and fussing and infant problems such as otitis media and dental malocclusion.
What does this mean?
In motivated mothers, there is moderate‐quality evidence that pacifier use in healthy term breastfeeding infants before and after lactation is established does not reduce the duration of breastfeeding up to four months of age. However, there is insufficient information on the potential harms of pacifiers on infants and mothers. Until further information becomes available on the effects of pacifiers on the infant, mothers who are well‐motivated to breastfeed should be encouraged to make a decision on the use of a pacifier based on personal preference.
Summary of findings
Summary of findings for the main comparison. Restriction of pacifier use versus no restriction for increasing duration of breastfeeding.
| Pacifier use versus pacifier restriction for increasing duration of breastfeeding | ||||||
|
Patient or population: healthy full‐term newborns whose mothers have initiated breastfeeding and intend to exclusively breastfeed
Settings: multi‐centre trial carried out at 5 tertiary centres in Argentina
Intervention: restricted pacifier use Comparison: no restriction in pacifier use | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Pacifier use versus pacifier restriction | |||||
| Proportion of infants exclusively breastfed at 4‐6 months | Study population | RR 1.01 (0.94 to 1.09) | 970 (1 study) | ⊕⊕⊕⊝ moderate1 | Not downgraded for study limitations (lack of blinding of the intervention as there was blinding of the outcome assessor and outcome is objective) | |
| 743 per 1000 | 751 per 1000 (699 to 810) | |||||
| Duration of full or exclusive breastfeeding | Outcome not reported | |||||
| Breastfeeding difficulties | Outcome not reported | |||||
| Maternal satisfaction and level of confidence in parenting | Outcome not reported | |||||
| Infant otitis media | Outcome not reported | |||||
| Infant dental malocclusion | Outcome not reported | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) taken from the included studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Evidence obtained from only one study and so downgraded for imprecision
Background
Description of the condition
The World Health Organization (WHO) Expert Consultation recommends that infants be exclusively breastfed (the infant receives only breast milk with no other liquids including water or solids) up to the first six months of life and as a dietary supplement thereafter. In order to successfully initiate and maintain breastfeeding for a longer duration, and avoid supplementary feeding, the WHO's Ten Steps to Successful Breastfeeding recommends artificial teats or pacifiers should not be given for breastfeeding infants (WHO 1998).
The pacifier, a non‐nutritive sucking device, which is also called the dummy or teether is a smooth rubber or plastic object that is given to an infant to suck on, in an attempt to provide comfort and to stop crying. Its use has been documented since 1000 B.C. (Kramer 2001; Levin 1971). Infants have a biological need to suck, which includes non‐nutritive sucking (NNS) on fingers, thumbs, and pacifiers (Neifert 1995). NNS is considered normal for infants and it often starts in the womb. The prevalence of NNS in a society depends on ethnic and social‐economic factors and childcare practices and has become a cultural norm in many parts of the world as a device used to calm the infant (Barros 1995).
Pacifiers are often believed to be harmless or even necessary and beneficial for infants' development (Victora 1997), especially for preterm infants. They provide a calming effect and have been used for pain and anxiety prevention. A meta‐analysis of seven case‐control studies (Hauck 2005) demonstrated an association between pacifier use and a reduction in the risk of sudden infant death syndrome (SIDS). Many hospitals have traditionally provided pacifiers at birth.
Some observational studies (Levi 2002; Ullah 2003; Vogel 2001) suggest that early infant exposure to a pacifier may interfere with breast‐milk production and lead to early discontinuation of breastfeeding by three to six months (Boccolini 2015; Mascarenhas 2006) and overall breastfeeding by 12 months (Scott 2005). This is perhaps due to less frequent episodes of breastfeeding, ineffective sucking on the breasts which may lead to increased breastfeeding difficulty and thus, decreased maternal motivation to breastfeed.
Description of the intervention
For this review, the intervention is the restriction of pacifiers in breastfeeding infants. With restricted pacifier use, mothers should be advised to initiate breastfeeding early, breastfeed their infant on demand and to avoid offering the pacifier or artificial teats unless medically indicated for a short duration. Information might be given on the possible effects of pacifier use on breast‐milk production, including the possibility of nipple confusion and the effect of reduced sucking at the breast. Mothers could be taught alternative methods to manage their infants' fuss and cry instead of using a pacifier to calm and soothe them. Using a pacifier between the feeds sparingly or occasionally for a short duration to calm the infant from pain or anxiety when other effort has failed or for the control of procedural pain is thought to be unlikely to affect the frequency of breastfeeding (Vogel 2001), or cause breastfeeding difficulty (Ullah 2003) and hence, it should be left to the mother's discretion to decide on their infant's need after ensuring that breastfeeding frequency is not compromised. The control intervention is unrestricted pacifier use where a pacifier could be offered liberally to the infant to suck on for many hours a day between the feeds without any clear medical reason.
How the intervention might work
It has been suggested that avoidance or restricted daily usage of pacifier in breastfeeding infants, especially in the first few weeks of life until breastfeeding is fully established, is beneficial in increasing the duration of breastfeeding (Boccolini 2015; Levi 2002) as it allow infants to be exclusively breastfed without interference. Studies have shown that breast‐milk production and supply are maintained by frequent suckling of the breast and nipple stimulation by the infant (Aarts 1999; Neville 1988). In order to breastfeed successfully, infants must learn to attach and suckle properly at the breast. Effective breast sucking technique requires the infant to have a wide open mouth, with the tongue under the areola and requires slow and deep sucks, whereas sucking on a pacifier is superficial sucking, with short and fast sucks using minimal effort (Gomes 2006; Righard 1992).
The difference in oral dynamics between sucking on the breasts and sucking on a pacifier might cause 'nipple confusion', which might lead to ineffective sucking of breast milk (Gomes 2006; Neifert 1995). Incorrect latching onto the breasts and superficial sucking on the mother's nipple may lead to a cracked nipple and mastitis, which might further impede breastfeeding. In addition, frequent and prolonged use of pacifier might lead to the development of a preference for an artificial teat instead of the mother's nipple. As a consequence, it would not only reduce a mother's breast‐milk production causing early weaning of breastfeeding (Howard 1999; Righard 1998), but it might also increase the fuss and cry due to inadequate breast‐milk supply, which might result in the mother supplementing her infant with formula milk.
Interestingly, there is evidence to suggest that the pacifiers may have a positive effect on breastfeeding. This might be because they may help to take the infant off the breast and thereby increase the interval between feedings and possibly increase breast‐milk intake by the infant (Victora 1997). Observational evidence also indicates that occasional use of the pacifier has no effect on breastfeeding duration compared to daily pacifier use (Ullah 2003; Vogel 2001) and thus it remains unclear whether pacifiers are an independent causal factor for reducing breastfeeding duration.
Why it is important to do this review
Conventional wisdom and that derived from observational studies holds that pacifiers interfere with breastfeeding and significantly decrease breastfeeding duration, but this association has not been confirmed by high‐quality studies (Kair 2013; O'Connor 2009).
There is some evidence that pacifiers may have a beneficial effect in preventing SIDS including in breastfed infants (Hauck 2005) and many practitioners and hospitals recommend their use during sleep time. However, some breastfeeding advocates have expressed concern that promotion of pacifier use to be protective against SIDS, is inconsistent with promotion of breastfeeding. A more recent meta‐analysis of 18 case‐control studies (Hauck 2011) suggested that breastfeeding itself might also be protective against SIDS, especially when breastfeeding is exclusive. Other studies have suggested an association between long‐term sucking on the pacifier and increased risk of recurrent acute otitis media (Jackson 1999), oral candidiasis (Darwazeh 1995) and dental malocclusion (Caglar 2005). However a systematic review (Pinelli 2000) reported that decreasing the use of the pacifier around age two and discontinuing by age four might minimise the development of malocclusion.
Nevertheless, pacifier use is a cultural norm and a lifestyle choice. The current available evidence is not yet clear yet on the impact of pacifier use on breastfeeding duration or exclusivity. It also remains unclear whether early breastfeeding cessation and a maternal intention to wean the infant from exclusive breastfeeding precedes the use of a pacifier or vice versa. It is possible that a mother may have experienced breastfeeding difficulties early and intended to stop breastfeeding by introducing the pacifier to the infant in preparation to take on bottle feeding.
Therefore, the aim of this review is to study the effect of restricted pacifier exposure in healthy infants whose mothers have initiated breastfeeding and intend to exclusively breastfeed, on the duration of breastfeeding and infant health.
Objectives
To assess the effect of restricted pacifier use versus unrestricted pacifier use in healthy full‐term newborns whose mothers have initiated breastfeeding and intend to exclusively breastfeed, on the duration of breastfeeding, other breastfeeding outcomes and infant health.
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled trials including quasi‐randomised trials and cluster‐randomised trials. Cross‐over trials were not eligible for inclusion.
Types of participants
Healthy full‐term newborns whose mothers have initiated breastfeeding and intend to exclusively breastfeed regardless of whether they were born at home or in hospital. We planned to exclude studies including newborns exposed to bottle feeding prior to enrolment.
Types of interventions
Advice against pacifier use (restricted) compared with unrestricted or actively encouraged use of a pacifier in breastfeeding infants from postpartum period till six months of age.
Types of outcome measures
Definition of breastfeeding and partial breastfeeding
Full or exclusive breastfeeding is defined as no food (solid or liquid including water) other than breast milk. Almost exclusive breastfeeding allows infrequent supplemental liquids, other than milk formula, and in partial breastfeeding other milk supplements are regularly given along with breastfeeding (Labbok 1990).
Primary outcomes
Duration of breastfeeding as measured by one of the following.
Prevalence or proportion of infants being fully or partially breastfed at three, four and six months of age.
Duration of full or exclusive breastfeeding (months) as defined by Labbok 1990.
Duration of any or partial breastfeeding (months).
Secondary outcomes
Breastfeeding difficulties (cracked nipples, breast engorgement, mastitis).
Maternal satisfaction and level of confidence in parenting.
Episodes/frequency of infant crying and fussing per day.
Incidence of sudden infant death syndrome (SIDS).
Infant oral candidiasis.
Infant otitis media.
Infant dental malocclusion.
Search methods for identification of studies
The following methods section of this review is based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting their Information Specialist (30 June 2016).
The Register is a database containing over 22,000 reports of controlled trials in the field of pregnancy and childbirth. For full search methods used to populate the Pregnancy and Childbirth Group’s Trials Register including the detailed search strategies for CENTRAL, MEDLINE, Embase and CINAHL; the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service, please follow this link to the editorial information about the Cochrane Pregnancy and Childbirth Group in the Cochrane Library and select the ‘Specialized Register ’ section from the options on the left side of the screen.
Briefly, the Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by their Information Specialist and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE (Ovid);
weekly searches of Embase (Ovid);
monthly searches of CINAHL (EBSCO);
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Search results are screened by two people and the full texts of all relevant trial reports identified through the searching activities described above are reviewed. Based on the intervention described, each trial report is assigned a number that corresponds to a specific Pregnancy and Childbirth Group review topic (or topics), and is then added to the Register. The Information Specialist searches the Register for each review using this topic number rather than keywords. This results in a more specific search set which has been fully accounted for in the relevant review sections (Included studies; Excluded studies).
Searching other resources
We searched the reference lists of retrieved studies.
We did not apply any language or date restrictions.
Data collection and analysis
For methods used in previous versions of this review, seeJaafar 2011; Jaafar 2012.
For this update, we planned to use the following methods to assess the one report identified as a result of the updated search. Unfortunately, no new studies were included in this update.
The following methods section of this review is based on a standard template used by Cochrane Pregnancy and Childbirth.
Selection of studies
Two review authors independently assessed for inclusion the potential study identified as a result of the search strategy. We resolved any disagreement through discussion or, if required, we consulted the third review author.
Data extraction and management
We designed a form to extract data. In the previous version of this review (Jaafar 2012), for eligible studies, two review authors extracted the data using the agreed form. We resolved discrepancies through discussion or, if required, we consulted the third review author. Data were entered into Review Manager software (RevMan 2014) and checked for accuracy.
When information regarding any of the above was unclear, we planned to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
In the previous version of this review (Jaafar 2012), two review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Any disagreement was resolved by discussion or by involving a third assessor.
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we planned to re‐include missing data in the analyses which we undertook.
We assessed methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for each included study any important concerns we had about other possible sources of bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Handbook (Higgins 2011). With reference to (1) to (6) above, we planned to assess the likely magnitude and direction of the bias and whether we considered it is likely to impact on the findings. In future updates, we will explore the impact of the level of bias through undertaking sensitivity analyses ‐ seeSensitivity analysis.
Assessment of the quality of the evidence using the GRADE approach
For this update (2016) the quality of the evidence was assessed using the GRADE approach as outlined in the GRADE handbook in order to assess the quality of the body of evidence relating to the following outcomes for the main comparisons of unrestricted pacifier versus no pacifier use in breastfeeding infants.
Primary outcomes
Prevalence or proportion of infants fully breastfed four to six months of age.
Duration of full or exclusive breastfeeding (months).
Secondary outcomes
Breastfeeding difficulties such as cracked nipples, breast engorgement, mastitis.
Maternal satisfaction and level of confidence in parenting.
Infant otitis media.
Infant dental malocclusion.
We used GRADEpro Guideline Development Tool to import data from Review Manager 5.3 (RevMan 2014) in order to create a ’Summary of findings’ table. A summary of the intervention effect and a measure of quality for each of the above outcomes was produced using the GRADE approach. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome. The evidence can be downgraded from 'high quality' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio with 95% confidence intervals.
Continuous data
Had we encountered continuous outcomes, we would have reported the mean difference if outcomes were measured in the same way between trials. We would have used the standardised mean difference to combine trials that measured the same outcome, but used different methods.
Unit of analysis issues
Cluster‐randomised trials
Had we identified any cluster‐randomised trials, we would have included them in the analyses along with individually‐randomised trials. We would have adjusted their sample sizes or standard errors using the methods described in the Handbook [Section 16.3.4 or 16.3.6] using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population. If we had used ICCs from other sources, we would have reported this and conducted sensitivity analyses to investigate the effect of variation in the ICC. If we had identified both cluster‐randomised trials and individually‐randomised trials, we planned to synthesise the relevant information. We would have considered it reasonable to combine the results from both if there was little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit was considered to be unlikely.
We would have also acknowledged heterogeneity in the randomisation unit and performed a sensitivity analysis to investigate the effects of the randomisation unit.
Cross‐over trials
Cross‐over trials were not eligible for inclusion.
Other unit of analysis issues
Had the included studies recruited twins, we would have reported the proportion of twins in the study and described how these were dealt with in the randomisation process.
Dealing with missing data
For included studies, levels of attrition were noted. In future updates, if more eligible studies are included, the impact of including studies with high levels of missing data in the overall assessment of treatment effect will be explored by using sensitivity analysis.
For all outcomes, analyses were carried out, as far as possible, on an intention‐to‐treat basis i.e. we attempted to include all participants randomised to each group in the analyses. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the Tau², I² and Chi² statistics. We regarded heterogeneity as substantial if an I² was greater than 30% and either the Tau² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity. Had we identified substantial heterogeneity (above 30%), we planned to explore it by pre‐specified subgroup analysis.
Assessment of reporting biases
In future updates, if there are 10 or more studies in the meta‐analysis, we will investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually. If asymmetry is suggested by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2014). We used fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect: i.e. where trials were examining the same intervention, and the trials’ populations and methods were judged sufficiently similar.
If there was clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or if substantial statistical heterogeneity was detected, we planned to use random‐effects meta‐analysis to produce an overall summary, if an average treatment effect across trials was considered clinically meaningful. The random‐effects summary would have been treated as the average of the range of possible treatment effects and we would have discussed the clinical implications of treatment effects differing between trials. If the average treatment effect was not clinically meaningful, we planned not to combine trials. If we had used random‐effects analyses, the results would have been presented as the average treatment effect with 95% confidence intervals, and the estimates of Tau² and I².
Subgroup analysis and investigation of heterogeneity
Had we identified substantial heterogeneity, we would have investigated it using subgroup analyses and sensitivity analyses. We would have considered whether an overall summary was meaningful, and if it was, we would have used random‐effects analysis to produce it.
We planned to carry out the following subgroup analyses:
primiparous versus multiparous mother;
vaginal delivery versus cesarean section.
We planned to use the following outcomes in subgroup analyses:
duration of full breastfeeding (months);
duration of any or partial breastfeeding (months);
prevalence or proportion of infants being fully or partially breastfed at three, four and six months of age.
However, we were unable to carry out subgroup analysis in this update due to lack of data.
In future updates, if subgroup analysis is possible, we will assess subgroup differences by interaction tests available within RevMan (RevMan 2014). We will report the results of subgroup analyses quoting the Chi² statistic and P value, and the interaction test I² value.
Sensitivity analysis
We planned to carry out sensitivity analyses to explore the effect of trial quality assessed by concealment of allocation, high attrition rates, or both, with poor quality studies being excluded from the analyses in order to assess whether this makes any difference to the overall result. However, we were unable to carry out sensitivity analysis due to lack of data in this update.
Results
Description of studies
Results of the search
For the previous version of this review (Jaafar 2012), we identified nine reports of five randomised controlled trials (RCTs). We included three studies and excluded two. For this update we identified one new trial report, which we excluded (Feldens 2013).
Included studies
SeeCharacteristics of included studies. We included three studies involving 1915 babies (Jenik 2009; Kramer 2001; Schubiger 1997). However, only two of these studies (involving 1302 babies: Jenik 2009; Kramer 2001) contribute data to the analyses.
Jenik 2009: a multicentre trial evaluated pacifier use in breastfeeding infants once lactation was well‐established to see whether it reduced the prevalence or duration of breastfeeding. A total of 1021 mothers highly motivated to breastfeed were recruited and randomly assigned to whether pacifier was offered (n = 528) or not offered (n = 493). The study was designed as a non‐inferiority trial and only mothers who were already successfully breastfeeding at two weeks and who indicated their intention to continue to do so for at least three months were enrolled. Mothers with breast problems that could interfere with breastfeeding (sore nipples, mastitis, inverted nipples, breast surgery) were not included. Participating mothers were interviewed at one, two, three, four, five, six, eight, 10 and 12 months after birth or until breastfeeding ended. Interviews were conducted by a research assistant using a structured questionnaire designed to assess exclusive or any breastfeeding prevalence, duration of breastfeeding and whether the baby had used a pacifier. The primary outcome was prevalence of exclusive breastfeeding at three months. The main secondary outcomes were the prevalence of exclusive and any breastfeeding and duration of any breastfeeding. Primary analysis was by intention‐to‐treat. Comparison between the two groups in the study did not show any difference in the baseline characteristics namely the infant birthweight, mode of delivery, maternal age and education, and onset of breastfeeding.
Kramer 2001: a double‐blind RCT, examined whether or not regular pacifier use is related to weaning by three months of age. A total of 281 healthy breastfeeding women who intended to breastfeed their infant longer and their healthy term singleton infants were recruited in the immediate postnatal period prior to discharge from hospital and randomised to one of two counselling interventions provided by a trained research nurse. In the experimental group (n = 140) the mother was asked to avoid pacifier use when the infant cried or fussed and to first offer the breast and, failing that, to try carrying or rocking the infant. In the control group (n = 141) all options were discussed for calming the infant, including breastfeeding, carrying, rocking and pacifier use. To ascertain the outcome, mothers were asked to complete a validated behaviour diary on three consecutive days, when their infants were four, six and nine weeks of age. Study mothers were interviewed at three months by a research assistant who was blinded to the intervention status of the mother. A total of 258 (91.8%) mother‐infant pairs completed three months follow‐up.
Schubiger 1997: a multicentre prospective randomised trial evaluating whether avoidance of bottles and pacifiers in the first five days of life affected long‐term breastfeeding performance. In order to participate, hospitals were required to have established functioning breastfeeding programmes with early initiation of breastfeeding, lactation consultants, unrestricted rooming‐in and a policy of restricted infant formula supplements. A total of 602 healthy term infants of mothers who intended to stay in the hospital for five days postpartum and planned to breastfeed for three months or more were selected and randomly assigned to the experimental group (n = 294) where breastfeeding was encouraged and pacifiers and all forms of artifical teats were forbidden, and to the control group (n = 308) where pacifiers were offered without restriction to breastfeeding infants. In both groups, the fluid supplements during the first five days, consisting of a 10% dextrin‐maltose solution, were allowed when medically indicated and it was given by cup or spoon in the experimental group and by bottle in the control group. Upon discharge from hospital it was left to the mothers of both groups to decide whether or not to use pacifier and/or bottle. Questionnaires were sent to the mothers at two, four and six months to request feedback on breastfeeding, introduction of supplementary nutrition and use of pacifiers. For the hospital outcomes at five days of life only 180 in the experiment group were analysed after 114 (39%) were excluded for protocol violations, of which 70 were due to use of pacifier. In the control group there were 17 (5.5%) exclusions for protocol violations at five days of life. Follow‐up data at two, four and six months was collected from the 70 infants who had protocol violations for use of pacifier and were included in the analysis. In addition, loss to follow‐up of 23 (restricted pacifier) and 13 (controls) was reported. It is not clear whether this applies to all three time points.
In the previous version of the review, we excluded Schubiger 1997 from the analysis due to it being at high risk of bias for incomplete outcome data, with incomplete outcome data of nearly 40% in the intervention group, which exceeded our pre‐specified 20% attrition limit. However, in this update we have updated the methods and re‐assessed this study. We decided we would now include this study in the analysis and examine the effect of high attrition by conducting sensitivity analysis. However, there were insufficient data for our pre‐specified outcomes to be included in the analysis due to unclear denominators for the four‐ and six‐month time points. We have contacted the authors for clarification of these data.
Excluded studies
We excluded three studies from the review (Collins 2004; Feldens 2013; Howard 2003). One study (Collins 2004) compared the use of bottles and pacifiers versus cup feeding in preterm breastfeeding infants who wanted to breastfeed their infant. Howard 2003 compared the effect of early versus late pacifier use in term infants on duration of breastfeeding. The intervention for the other excluded study identified during this update, (Feldens 2013) was home visits to advise mothers about breastfeeding and pacifier use and the outcome was the risk of pacifier use. For further information, seeCharacteristics of excluded studies.
Risk of bias in included studies
See Figure 1 for a 'Risk of bias' graph and Figure 2 for a 'Risk of bias' summary.
1.
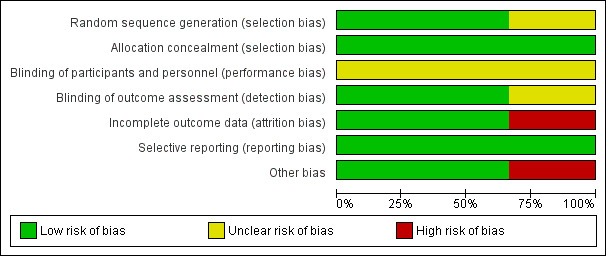
Figure 1: 'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
2.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Two included studies employed computerised central randomisation (Jenik 2009; Kramer 2001). Both studies used consecutively numbered, opaque sealed envelopes but in the third trial (Schubiger 1997), the method of randomisation was not described and we rated this trials as 'unclear'. All three studies were at low risk of allocation concealment.
Blinding
Two studies reported blinding of research nurse and outcome assessors (Jenik 2009; Kramer 2001). In both studies blinding of the care‐giver was not mentioned. It would not be feasible to blind participants to the intervention. One study did not mention whether there was any blinding (Schubiger 1997).
Incomplete outcome data
Overall, the dropout rate was less than 10% from both arms, i.e. 4.9% versus 4.5% in Jenik 2009, 9.3% versus 7.1% in Kramer 2001, respectively. However, in Schubiger 1997, the total dropout rate (lost to follow‐up and protocol violations) was 22% versus 9.7%, respectively. We judged this imbalance to be high risk of bias.
Selective reporting
We detected no selective reporting and all expected outcomes were reported.
Other potential sources of bias
There were no other potential sources of bias identified for two studies (Jenik 2009; Kramer 2001). We judged Schubiger 1997 to be at high risk of bias in this domain because we had to impute the figures for the primary outcomes from percentages and others from a graph. In addition, the exact denominators for the primary outcomes are unclear.
Effects of interventions
See: Table 1
Comparison: Restricted or no pacifier use versus unrestricted pacifier use
Primary outcomes
We included two out of three RCTs enrolling 1302 healthy full‐term breastfeeding infants for meta‐analysis (Jenik 2009; Kramer 2001). Both of the trials contributed to at least one of the primary outcomes, i.e. proportion of infants partially or exclusively breastfed at three and four months of age. Comparison between restricted pacifier use (intervention) and unrestricted pacifier use (control) revealed that there was no difference in the proportion of infants exclusively breastfed at three months (risk ratio (RR) 1.01; 95% confidence interval (CI) 0.96 to 1.07, two studies, 1228 babies, I² = 0%, (Analysis 1.1)) and at four months of age (RR 1.01; 95% CI 0.94 to 1.09, one study, 970 babies, moderate‐quality evidence (Analysis 1.3)). There was also no difference in the proportion of infants partially breastfed at three months (RR 1.00; 95%; CI 0.98 to 1.02, two studies, 1228 babies, I² = 0%, (Analysis 1.2)), or at four months (RR 0.99; 95% CI 0.97 to 1.02, one study, 970 babies (Analysis 1.4)). Thus, restricted or no pacifier use in full‐term breastfeeding infants after birth or after the establishment of lactation did not significantly affect the prevalence or duration of exclusive or partial breastfeeding up to the age of four months.
1.1. Analysis.
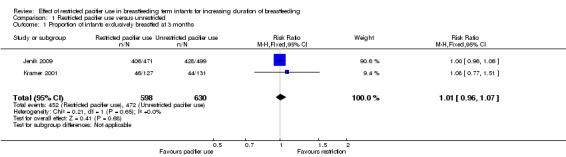
Comparison 1 Restricted pacifier use versus unrestricted, Outcome 1 Proportion of infants exclusively breastfed at 3 months.
1.3. Analysis.
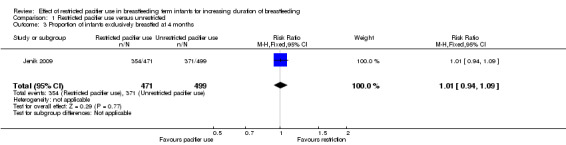
Comparison 1 Restricted pacifier use versus unrestricted, Outcome 3 Proportion of infants exclusively breastfed at 4 months.
1.2. Analysis.
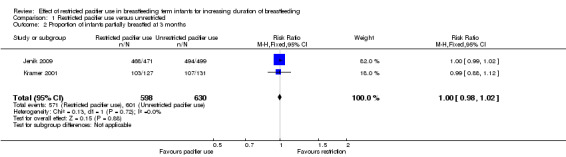
Comparison 1 Restricted pacifier use versus unrestricted, Outcome 2 Proportion of infants partially breastfed at 3 months.
1.4. Analysis.
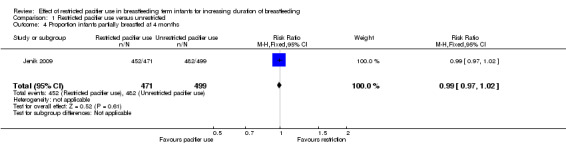
Comparison 1 Restricted pacifier use versus unrestricted, Outcome 4 Proportion infants partially breastfed at 4 months.
None of the included studies reported data on the other primary outcomes, i.e. duration of partial or exclusive breastfeeding.
Secondary outcomes
Kramer 2001 reported that avoidance of pacifiers had no effect on cry/fuss behavior at ages four, six, or nine months and had no effect on the risk of weaning before age three months. However, the data were incomplete for analysis. None of the included studies reported data on breastfeeding difficulties (mastitis, cracked nipples, breast engorgement); infant's health (dental malocclusion, otitis media, oral candidiasis, sudden infant death syndrome (SIDS)); and maternal satisfaction and level of confidence in parenting.
Discussion
Summary of main results
We identified three randomised controlled trials (RCTs) (involving 1915 babies) for inclusion in the review. We were only able to include two multicentre RCTs in the analysis. These two trials involved six tertiary hospitals from two different countries, enrolling a total of 1302 women. Meta‐analysis of the two trials showed that pacifier use in healthy breastfeeding infants had no significant effect on the proportion of infants exclusively breastfed at three months, and at four months of age, and also had no effect on the proportion of infants partially breastfed at three months and at four months of age. All of these effect estimates are very close to 1, the line of no effect, and the confidence intervals (CIs) are all remarkably narrow suggesting true evidence of no effect.
Our review suggests that, in highly‐motivated mothers, pacifier use was not associated with a reduction in the rate or duration of exclusive or partial breastfeeding, regardless of whether the pacifier was introduced before or after lactation was established.
Overall completeness and applicability of evidence
Mothers enrolled into these trials were highly motivated to continue breastfeeding. Jenik 2009 used extremely restrictive inclusion criteria resulting in inclusion of mothers who had successfully established breastfeeding after two weeks, and excluding those with problems that could interfere with breastfeeding, while Kramer 2001 enrolled mothers after childbirth before lactation was established. These differences in inclusion criteria could partly explain the differences in breastfeeding rates seen at the end of the intervention period. This difference i.e. 34% in Kramer 2001 compared with more that 85% in Jenik 2009, suggests that the effect of the intervention would be similar across a range of breastfeeding rates. The findings of our review suggest that pacifier use before or after breastfeeding is established does not affect duration of breastfeeding when mothers are motivated to breastfeed their infants. The finding of this review, however, may not apply to mothers who are less motivated or who have no desire to breastfeed their infants longer.
This review was unable to evaluate any of our pre‐specified secondary outcomes (the effect of pacifier use on breastfeeding difficulties faced by the mothers, and the effect of pacifier on long‐term infant health, e.g. dental malocclusion, otitis media, dental caries and sudden infant death syndrome (SIDS)). Further trials are needed to evaluate these effects.
The WHO 'Ten Steps To Successful Breastfeeding' are valuable guidelines for hospitals. Some recommendations however are based on observational studies. The use of pacifier is a common practice in many populations and thus, without having a solid scientific evidence of its impact on breastfeeding duration, this recommendation should also incorporate evidence from randomised controlled trials. It should be noted that our review provides evidence on which to base recommendations for women who are motivated to breastfeed. The WHO Ten Steps To Successful Breastfeeding of necessity needs to make recommendations taking into account all levels of motivation of women using a birthing facility. The American Academy of Pediatrics task force on SIDS recommends that parents might consider the use of a pacifier at nap and bedtime after breastfeeding is firmly established (AAP 2011). Our evidence does not contradict this recommendation.
Quality of the evidence
Our primary outcome was the proportion of infants with partial or exclusive breastfeeding at three, four or six months. Of these, we judged the longer durations of breastfeeding to be more important and although there were data from two included studies, for the outcome exclusive breastfeeding at four to six months, there was only one study. We therefore rated the quality of evidence as moderate for this outcome. However we noted that the primary outcome of duration of breastfeeding reported in four ways (exclusive and partial breastfeeding at three and four months), consistently showed evidence of no effect with extremely narrow confidence intervals. We did not downgrade for the lack of blinding of the intervention because in both studies there was blinding of the outcome assessor. In addition, we judged duration of breastfeeding to be an objective outcome that would not be influenced by the lack of blinding. We were unable to assess other GRADE outcomes because they were not reported in the trials: duration of full breastfeeding (months); breastfeeding difficulties such as cracked nipples, breast engorgement, mastitis; infant otitis media; infant dental malocclusion.
Potential biases in the review process
We were able to conduct an extensive search according to the methods of Cochrane Pregnancy and Childbirth. Since pacifier use is a non‐pharmacological intervention we believe there is a low risk of publication bias, although as noted in feedback previously posted, there is evidence of industry involvement in one of our included studies, (seeFeedback). A potential bias of this review is that we analysed only two (1302 participants) of our three included studies involving 1915 participants. In an earlier version we pre‐specified we would not include studies in our analysis if there was an attrition rate of 20% or higher because we considered such studies to be an extreme risk of attrition bias. For this update the criteria for attrition bias have been updated according to the current recommendations for dealing with attrition (Higgins 2011).
An earlier version of this review aimed to assess the effect of pacifier use compared with no pacifier use. After receiving feedback (Feedback 1) on this version it became apparent that our question would be better stated as it is currently, to assess the effect of restricted pacifier use. This eliminated a potential issue of contamination between the groups which was raised in the feedback. This post hoc change could be regarded as a potential bias of the review.
Agreements and disagreements with other studies or reviews
The proposed mechanism for the relationship between reduced breastfeeding and pacifier use is that when infants use pacifiers they tend to suck on the breast less, and as a result the milk supply is reduced, and subsequently fails. Our review contradicts the finding of a meta‐analysis of 31 cross‐sectional and cohort studies (Karabulut 2009) enrolling several thousand infants that reported the use of pacifiers was associated with shortened duration of exclusive and of any breastfeeding before six months of age (RR 2.02; 95% CI 1.62 to 2.51 and RR 2.76; 95% CI 2.08 to 3.7, respectively). However, the Karabulut 2009 review did not include any randomised controlled trials, including the two studies in this review.
Authors' conclusions
Implications for practice.
In motivated mothers, there is moderate‐quality evidence that pacifier use in healthy term breastfeeding infants before and after lactation is established does not reduce the duration of breastfeeding up to four months of age. However, there is insufficient information on the potential harms of pacifiers on infants and mothers. In the light of the current review, until further information becomes available on the effects of pacifiers on the infant, mothers who are well‐motivated to breastfeed should be encouraged to make a decision on the use of a pacifier based on personal preference.
Implications for research.
Further research is recommended to address the effect of pacifier use on duration of breastfeeding that include less‐motivated women. We also recommend well‐designed randomised controlled trials to assess the rate of breastfeeding difficulties faced by mothers associated with pacifier use and the long‐term effect of pacifier use on mother and infant health.
Feedback
Di Mario, 6 July 2011
Summary
Pacifier use and breastfeeding [1] is an issue that is highly relevant to health professionals and families, for example this topic was the most accessed among Evidence Updates registrants (http://plus.mcmaster.ca/EvidenceUpdates) and it is relevant to one of the ten steps to successful breastfeeding of the WHO‐UNICEF Baby Friendly Hospital Initiative [2].
We believe that this Cochrane review, stating that pacifier does not reduce breastfeeding rates, is severely flawed and biased and therefore should be promptly revised. Here below is our criticism in detail.
The analysis is based only on two randomized controlled trials (RCTs) [3,4]. Validity of the review authors conclusions is limited as they have excluded from the review a third RCT which shows an association between pacifier use and breastfeeding discontinuation at four weeks [5]. The reason for this exclusion is reported as being that both groups were exposed to pacifier. Actually, the intervention group was exposed to pacifier soon after birth while the control group was advised to avoid pacifiers up to five weeks of life of the newborn. Therefore, data comparing breastfeeding practice before five weeks of life could have been appropriately included in the review, or at least commented on.
In addition, the two studies included in the review were not designed to answer the clinical question about the effect of pacifier use for healthy full‐term newborns whose mothers have initiated breastfeeding and intend to exclusively breastfeed, on the duration of breastfeeding. These two trials assessed the effects on breastfeeding of interventions aimed at reducing the use of pacifiers; they did not assess the effect of pacifiers on breastfeeding. Mothers in the pacifier group used it in 71% of cases, while mothers in not pacifier group used it in 44% of cases (overall rates). Contamination between two treatment arms points to no difference or inconclusive results. Your conclusions of a null effect of pacifier on breastfeeding success based only on two studies with high contamination rate are therefore falsely reassuring.
Major problems of the studies included in the review are insufficiently discussed. The larger of the two included studies (1021 infants out of a total of 1302) [3], has exclusion and inclusion criteria so strict that the population observed is extremely selected, limiting the external validity of the conclusions, which is not even mentioned. For example, participating hospitals had established breastfeeding programs, with early initiation of breastfeeding, lactation consultants, and unrestricted rooming‐in. Mothers were encouraged to avoid pacifier use until breastfeeding was well established. At term healthy infants, exclusively breastfeeding, whose mothers reported an intention to breastfeed for at least three months, not using pacifiers and with lactation well established at the age of 2 weeks were included. Exclusion criteria were breast problems that could interfere with breastfeeding (persistently sore nipples, mastitis, earlier breast surgery, and severely flat or inverted nipples). Mothers who communicated a preference in the introduction or not of a pacifier were also excluded. Further evidence that this study assessed an extremely selected population of women is the remarkably high rate of exclusive breastfeeding at three months for both groups (> 85%), much higher than the rate of exclusive breastfeeding at three months commonly seen in Europe (e.g. 47% in Italy in 2008, and in Sweden ranging between 68% at four months and 79% at two months in 2002) [6,7]. Finally the authors of the study powered the sample to perform an analysis based on intention to treat, but as the trial was non‐inferiority, the ‘according to protocol’ analysis would have been more appropriate [8]. Unfortunately, as the authors admit, the study sample was not sufficiently large to adequately perform this analysis.
The second RCT included in the review also suggests that the null effect of pacifier on breastfeeding could be a false conclusion [4]. As there was a high contamination rate, results are presented based on actual exposure (observational analysis) in addition to the analysis based on randomized groups. This observational analysis showed a significant difference between pacifier users and not users for weaning by 3 months (RR: 1.9; 95%CI: 1.1, 3.3). Although observational studies are not reliable for assessing the association between pacifier use and breastfeeding practice, due to residual confounding and reverse causality, we think that RCTs with low compliance and high contamination, as in this study, cannot provide a valuable answer, especially when no differences among groups are detected. None of these issues were adequately discussed in this Cochrane review.
Finally, we believe that a potentially very relevant conflict of interest in one of the trials included [3] was not mentioned: the authors of the study report as a funding source an association (the International Children Medical Research Association) whose characteristics are unclear, since it is not possible to find any information on it in the web. The only other citation of this association we have traced is a letter by Dr Peter PW Weiss to Pediatrics [9] criticizing a paper that reported a relationship between reduced pacifier use and reduced acute otitis media incidence. Is he maybe the same Peter Weiss, consultant for a manufacturer of pacifiers, that appears in the acknowledgment section of the trial report [3]? A Dr Peter Weiss is also the vice‐president (the president is unknown) of the International Children Medical Research Society, which is, maybe, another name of the International Children Medical Research Association, created in Switzerland by a company founded by the same manufacturers of pacifiers. Should this be made clear to the readers of the Cochrane review?
Our view is that these issues raise questions about the validity of the conclusions of this Cochrane review. Considering that Cochrane reviews represent a seal of quality among health professionals and the public, we think that it is responsibility of the Cochrane Collaboration to scrutinize the evidence selection, its critical appraisal and the validity of the conclusions, specially for a hot topic relevant for public health, as is the case for breastfeeding.
Simona Di Mario1, Adriano Cattaneo2, Vittorio Basevi1, Nicola Magrini1
1 NHS CeVEAS, NHS Centre for the Evaluation of the Effectiveness of Health Care, WHO Collaborating Centre for Evidence‐based Research Synthesis and Guideline Development in Reproductive Health, Emilia‐Romagna, V. le L. Muratori 201, Modena, Italy, 41100 2 Unit for Health Services Research and International Health, WHO Collaborating Centre for Mother and Child Health, Institute of Child Health, IRCCS Burlo Garofolo, Trieste, Italy
References
1. Jaafar SH, et al. Pacifier use versus no pacifier use in breastfeeding term infants for increasing duration of breastfeeding. Cochrane Database Syst Rev. 2011 Mar 16;3:CD007202. 2. Kramer MS, et al; PROBIT Study Group (Promotion of Breastfeeding Intervention Trial). Promotion of Breastfeeding Intervention Trial (PROBIT): a randomized trial in the Republic of Belarus. JAMA 2001;285:413‐20. 3. Jenik AG, et al; Pacifier and Breastfeeding Trial Group. Does the recommendation to use a pacifier influence the prevalence of breastfeeding? J Pediatr 2009;155:350‐4.e1. 4. Kramer MS, et al. Pacifier use, early weaning, and cry/fuss behavior: a randomized controlled trial. JAMA 2001;286:322‐6. 5. Howard CR, et al. Randomized clinical trial of pacifier use and bottle‐feeding or cupfeeding and their effect on breastfeeding. Pediatrics 2003;111:511‐8. 6. Cuoghi C, et al. Prevalence of breastfeeding in Emilia‐Romagna Region in 2008. [Prevalenza dell'allattamento al seno in Emilia‐Romagna. Ricerca anno 2008] Fifth Edition. Bologna: Regione Emilia‐Romagna; 2010 [Italian]. 7. Statistics Health and Diseases. Breast‐feeding, children born 2002. The National Board of Health and Welfare. Centre for epidemiology. Stockholm 2004 [Swedish, English summary]. 8. Gøtzsche PC. Lessons from and cautions about noninferiority and equivalence randomized trials. JAMA 2006;295:1172‐4 9. Weiss PP, Nowak AJ. Pacifier as a risk factor for acute otitis media. Pediatrics 2002;109:351‐2.
Reply
We thank De Mario et al for their comments, and have responded in the order they made their comments.
We disagree that our review is ‘severely flawed and biased’. The protocol and review have been prepared according to Cochrane methods.
The study by Howard et al is excluded because the study population do not meet our inclusion criteria, as it included women who did not intend to breastfeed. Also, the results for breastfeeding duration are presented as adjusted odds ratios and the primary data are not reported. Finally, our review did not have an outcome ‘breastfeeding at five weeks, as this is too short a duration to be clinically meaningful. Whilst preparing this response we noticed that the text in ‘types of participants’ was not as explicit as the text in our objectives. We have therefore modified ‘types of participants’ so that it matches the objectives.
We agree our review is about the effect of recommending restricted pacifier use. For clarification we have modified the title of the review, and the background text. This clarification also means that contamination between the two intervention groups is no longer an issue. In addition, we disagree that contamination could have been the reason for the null effect. If the high baseline rate of pacifier use had any diluting effect on the final pooled results it would be very small, as the relative risks were consistently close to 1.00 with extremely tight confidence intervals. This is now clarified in the discussion.
Di Mario, Cattaneo, Basevi and Magrini wrote: Major problems of the studies included in the review are insufficiently discussed. The larger of the two included studies (1021 infants out of a total of 1302) [3] has exclusion and inclusion criteria so strict that the population observed is extremely selected, limiting the external validity of the conclusions, which is not even mentioned. For example, participating hospitals had established breastfeeding programs, with early initiation of breastfeeding, lactation consultants, and unrestricted rooming‐in. Mothers were encouraged to avoid pacifier use until breastfeeding was well established. At term healthy infants, exclusively breastfeeding, whose mothers reported an intention to breastfeed for at least three months, not using pacifiers and with lactation well established at the age of two weeks were included. Exclusion criteria were breast problems that could interfere with breastfeeding (persistently sore nipples, mastitis, earlier breast surgery, and severely flat or inverted nipples). Mothers who communicated a preference in the introduction or not of a pacifier were also excluded. Further evidence that this study assessed an extremely selected population of women is the remarkably high rate of exclusive breastfeeding at three months for both groups (> 85%), much higher than the rate of exclusive breastfeeding at three months commonly seen in Europe (e.g. 47% in Italy in 2008, and in Sweden ranging between 68% at four months and 79% at two months in 2002) [6,7]. Finally the authors of the study powered the sample to perform an analysis based on intention to treat, but as the trial was non‐inferiority, the ‘according to protocol’ analysis would have been more appropriate [8]. Unfortunately, as the authors admit, the study sample was not sufficiently large to adequately perform this analysis.
The report of Jenik 2009 does state this was a non‐inferiority trial. Whilst an ‘according to protocol analysis’ might have been appropriate for this trial, that is not relevant for this review as we specified we would use intention‐to‐treat analysis, which complies with Cochrane methods. We have included a paragraph in the discussion about external validity of the included trials.
We agree that the high contamination between intervention groups in Kramer 2001 may have had a diluting effect, and have included this in the discussion.
Jenik 2009 states that the sponsors had no role in any part of the study. However, the report does acknowledge helpful advice from Peter Weiss. We agree this may be the same Peter Weiss who is the vice president of the funding body, as well as a consultant for a pacifier company. We have now included this information in the Table of ‘Characteristics of Included Studies’.
Contributors
Jacqueline J Ho, Sharifah Halimah Jaafar, Shayesteh Jahanfar
Paulussen, 21 March 2012
Summary
There is a mistake in this review. In the table of characteristics of included studies, Kramer 2001 is stated to have 140 women in the intervention group (avoid pacifiers), and 141 women in the control group (use a pacifier). Under incomplete data it states data were available for 127 women in the intervention group (avoid pacifiers) and 131 in the control group (use a pacifier). In the data analysis, data for this study from the intervention and control group are switched. It should be 44/131 for the use pacifier group (not 46/127) and 46/127 for the avoid pacifier group (not 44/131). This means that the total and risk ratio are also incorrect.
[Feedback received from Valérie Paulussen, 7 February 2012]
Reply
We thank Valérie Paulussen for pointing out this mistake, which we have now corrected in this update. The correction does not alter or change the finding and conclusion of the review.
Contributors
Sharifah Halimah Jaafar
What's new
| Date | Event | Description |
|---|---|---|
| 30 June 2016 | New search has been performed | Search updated 30 June 2016 and one new study was identified and excluded. We updated and rearranged the Background under the standard headings. We have also rearranged the 'Risk of bias' assessment under standard headings. This resulted in a decision to move one study previously in our qualitative analysis, to our quantitative analysis. We selected outcomes for inclusion in a 'Summary of findings' table and included the 'Summary of findings' table in this updated version of the review. We rewrote the Plain language summary in a structured format. We also corrected a typographical error in our feedback to Di Mario et al. |
| 30 June 2016 | New citation required but conclusions have not changed | For this update we identified one new trial report, which we excluded (Feldens 2013). |
History
Protocol first published: Issue 3, 2008 Review first published: Issue 3, 2011
| Date | Event | Description |
|---|---|---|
| 1 May 2012 | New citation required but conclusions have not changed | Review updated and title changed from "Pacifier use versus no pacifier use in breastfeeding term infants for increasing duration of breastfeeding" to "Effect of restricted pacifier use on breastfeeding duration", as detailed in the authors' response to feedback ‐ see Feedback 1. |
| 1 May 2012 | Feedback has been incorporated | Authors' response to feedback from Di Mario added (seeFeedback 1); and feedback from Paulussen and authors' response added (seeFeedback 2). |
| 14 March 2012 | New search has been performed | Search updated. No new trial reports identified. |
| 22 December 2011 | Feedback has been incorporated | Comments from Simona Di Mario, Adriano Cattaneo, Vittorio Basevi and Nicola Magrini added ‐ see Feedback. |
Acknowledgements
We would like to acknowledge the contributions of the SEA‐ORCHID group and members of the Cochrane Australasian Centre for their role in the development of the protocol and first version of the review.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
This research was supported by a grant from the Evidence and Programme Guidance Unit, Department of Nutrition for Health and Development, World Health Organization. The findings, interpretations and conclusions expressed in this paper are entirely those of the authors and should not be attributed in any manner whatsoever to WHO.
Data and analyses
Comparison 1. Restricted pacifier use versus unrestricted.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Proportion of infants exclusively breastfed at 3 months | 2 | 1228 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.96, 1.07] |
| 2 Proportion of infants partially breastfed at 3 months | 2 | 1228 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.98, 1.02] |
| 3 Proportion of infants exclusively breastfed at 4 months | 1 | 970 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.94, 1.09] |
| 4 Proportion infants partially breastfed at 4 months | 1 | 970 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.97, 1.02] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Jenik 2009.
| Methods | A multicentre, non‐inferiority, RCT. The randomisation was carried out centrally with consecutively numbered, sealed, opaque envelopes containing random‐generated numbers constructed by an independent statistician. | |
| Participants | 1021 mothers highly motivated to breastfeed their term newborns of birthweight 2500 g or more and who regained weight by 15 days postpartum, were assigned to offer or not to offer pacifiers as part of the advice given on how to comfort crying infants. Mothers with breast problems that could interfere with breastfeeding were not included in the study. The study did not state whether twins were included. | |
| Interventions | The group offered pacifiers (n = 528) received a package containing 6 silicone pacifiers and a written guide for parents. They were also informed that other pacifiers could be use according to their preference. The group that were not offered pacifier use (n = 493) received a guide with other alternatives for comforting a crying baby. At the 3‐month assessment, complete data for 499 mother‐infants pairs in the group offered pacifiers and 471 in the group not offered pacifiers were available for the main outcome analysis. |
|
| Outcomes | Primary outcomes: the prevalence of exclusive breastfeeding at 3 months. Secondary outcomes: prevalence of exclusive and any breastfeeding at specified ages and duration of any breastfeeding. |
|
| Notes | The study was carried out at 5 tertiary centres in Argentina. The author stated that the sponsor (International Children Medical Research Association, Switzerland) had no role in any part of the study. However, they acknowledge helpful advice from Peter Weiss, a consultant from a pacifier manufacturer who may be the same Peter Weiss who is the vice president of the funding body. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | It is reported that the randomisation was carried out centrally with random generation conducted by an independent statistician. |
| Allocation concealment (selection bias) | Low risk | Consecutively numbered, sealed opaque envelopes were used to conceal a randomly‐generated assignment. A series of 500 envelopes was given to research assistants at each participating hospital with instructions to open the envelopes in numerical sequence and to assign the dyads to the corresponding group. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. Comment: participant binding is not feasible. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were 'blinded to the group assignment'. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4.9% (26/528) participants in 'offer pacifier' group and 4.5% (22/493) in the non‐offer pacifier group were lost to follow‐up due to various reasons. |
| Selective reporting (reporting bias) | Low risk | None detected. |
| Other bias | Low risk | None detected. |
Kramer 2001.
| Methods | Double‐blinded RCT. | |
| Participants | A total of 281 healthy breastfeeding women who were motivated to breastfeed and their healthy term singleton infants recruited in the immediate postpartum period prior to hospital discharge. | |
| Interventions | Participants were randomly allocated to 1 of 2 counselling interventions provided by a research nurse trained in lactation counselling. A basic breastfeeding promotion package was included in both the intervention and control groups. The intervention group (n = 140) were "asked to avoid pacifiers when the infant cried or fussed" and suggested alternative ways to provide comfort. The control group (n = 141) "all options were discussed for calming an infant" including pacifier use. |
|
| Outcomes | Mothers were asked to complete a validated behaviour diary on 3 consecutive days, at 4, 6 and 9 weeks of age. Study mothers were interviewed at 3 months. Primary outcome measures: rate of early weaning at 3 months, 72‐hour infant behaviour logs detailing frequency and duration of crying and fussing and pacifier use at 4, 6, 9 weeks. |
|
| Notes | The trial was carried out from January 1998 to August 1999 on women giving birth at the Royal Victoria Hospital, a McGill University‐affiliatted maternity hospital in Montreal, Quebec. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisation within each stratum was accomplished using computer‐generated random numbers in blocks of 4." "Women were stratified by parity and if multiparous according to whether they had breastfed previously." |
| Allocation concealment (selection bias) | Low risk | "The assigned allocation was contained in an opaque envelope opened by a research nurse after the consent was obtained." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. Comment: blinding of the participants is not feasible. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Study mothers were interviewed at 3 months by a research assistant who was blinded to the intervention status of the mother." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 8.2% (23/281) participants, i.e. 13/140 from pacifier‐avoidance group, 10/141 from pacifier‐advised group lost to follow‐up and did not complete the trial. |
| Selective reporting (reporting bias) | Low risk | None detected. |
| Other bias | Low risk | None detected. |
Schubiger 1997.
| Methods | Multicentre prospective randomised trial (from 10 centres). | |
| Participants | A total of 602 healthy full‐term infants (> 37 weeks of gestation, birthweight 2750 g to 4200 g) of mothers who intended to stay in the hospital for 5 days postpartum and planned to breastfeed for more than 3 months. The study did not state whether twins were included. | |
| Interventions | UNICEF group (n = 294): "bottles, teats and pacifiers were strictly forbidden"; "supplements if medically indicated were administered by cup or spoon". Standard group (n = 308): "pacifiers were offered to all infants without restriction. Supplements were conventionally offered by bottle after breastfeeding". In both groups, the fluid supplements during the first few days consisted of a 10% dextrin‐maltose solution. Fluid supplements were considered to be medically indicated in the following situations: babies agitated or screaming after breastfeeding; signs of dehydration (no urine output over 4 hours after day 1); symptoms of hypoglycaemia with blood glucose < 2 mmol/L. In the standard group fluids were more liberally offered. About 180 participants in the UNICEF group and 291 participants in the standard group completed the protocol. Almost 40% of the participants in the UNICEF group violated protocol during the first 5 days in the hospital. Upon discharge from the hospital, it was left to the mothers of both groups to decide whether to use a pacifier and/or bottle. |
|
| Outcomes | Incidence of breastfeeding at day 5, and at 2, 4, 6 months, proportion of fully or partially breastfeeding on day 5, sucking behaviour (good, mediocre, insufficient), incidence of fever, incidence of phototherapy. Questionaires administered to mothers at 2, 4, and 6 months were used to collect breastfeeding outcomes after hospital discharge. | |
| Notes | Study conducted in Switzerland. Results were reported in 2 separate publications with slight differences in the presentation of results. This study however was not included for analysis due to high attrition bias (almost 40% loss of participants in the intervention group) due to protocol violation in the first weeks of the study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Sealed protocol forms were centrally randomised." Comment: The method of random sequence generation is not described. |
| Allocation concealment (selection bias) | Low risk | "Sealed protocol forms were centrally randomised." Comment: Allocation concealment incompletely described but likely to have been present. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Randomising participants in the same room or ward rather than comparing routines of one ward to another ‐ not feasible to blind participants. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of clinician and outcome assessor is not described. However it is unlikely that they were blinded to the intervention. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The rate protocol violators is approximately 15% and 5.5% respectively and the rate of lost to follow‐up is 7.8% and 4.2%. Thus, the total dropout rate is 22% versus 9.7%, respectively after 70 protocol violaters due to pacifier use included into the analysis. The other protocol violaters were due to bottle feeding, failure to spoon/cup feed, early discharge and others. |
| Selective reporting (reporting bias) | Low risk | Protocol not available. All expected outcomes reported. |
| Other bias | High risk | Primary outcome data have to be imputed from percentages and exact denominators at 4 and 6 months follow‐up are unclear. |
RCT: randomised controlled trial
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Collins 2004 | This RCT aimed to determine the effect of artificial teats and cup on breastfeeding in preterm infants and not term infants, our pre‐specified inclusion criteria. |
| Feldens 2013 | This was an RCT examining the effect of home visits for the purpose of giving breastfeeding advice as well as advice about pacifier use. The control group treatment was not described. The primary outcome was pacifier use. |
| Howard 2003 | This RCT evaluated the effect of bottle feeding and pacifier use versus cup feeding and delayed pacifier use in breastfeeding infants. Infants in both the intervention and the control group used pacifiers and hence there is no comparison between pacifier use and non‐pacifier use in breastfeeding infants. The study is excluded because the study population do not meet our inclusion criteria, as it included women who did not intend to breastfeed. Furthermore, the results for breastfeeding duration are presented as adjusted odds ratios and the primary data are not reported. Additionally, our review did not have an outcome 'breastfeeding at 5 weeks', as this is too short a duration to be clinically meaningful. |
RCT: randomised controlled trial
Differences between protocol and review
The methods have been updated to reflect the latest Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) and the Cochrane Pregnancy and Childbirth Group's methodological guidelines. We are no longer excluding studies from analysis based on high attrition rates, but are instead planning on conducting sensitivity analysis to explore the effects of high attrition.
In this update, it has been clarified that the intervention is "restricted pacifier use" and the control is "unrestricted pacifier use".
The title has been changed from "Pacifier use versus no pacifier use in breastfeeding term infants for increasing duration of breastfeeding" to "Effect of restricted pacifier use on breastfeeding duration", as detailed in the authors' response to feedback ‐ see Feedback 1.
Contributions of authors
Sharifah Halimah is the main author and guarantor for the review. She wrote the first draft of the protocol; provided a clinical and policy perspective as well as providing general advice on the development of the protocol. For the review she assessed studies for inclusion, assessed trial quality and extracted and analysed the data, and wrote the review. For this update she rewrote the background and updated other sections of the review.
Jacqueline Ho provided general comments and advice from the protocol development to the completion of the review and provided extensive input for this update. She assessed trial quality where disagreement arose in the decision to include or exclude trials. She revised the Plain language summary for the update and prepared the 'Summary of findings' table.
Shayesteh Jahanfar provided input into the protocol development as well as the review. She independently assessed the quality of the trials, extracted and analysed the data. She also wrote the Plain language summary of the review. She critically evaluated the update and made recommendations.
Mubashir Angolkar provided general comment, proof read the draft of the protocol as well as the review.
Sources of support
Internal sources
University Kuala Lumpur Royal College of Medicine Perak, Malaysia.
Penang Medical College, Malaysia.
Jawaharlal Nehru Medical College Campus, Belgaum, India.
Ipoh Specialist Hospital, Perak, Malaysia.
External sources
SEA ORCHID, Malaysia.
Evidence and Programme Guidance Unit, Department of Nutrition for Health and Development, World Health Organization, Switzerland.
Declarations of interest
None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Jenik 2009 {published data only}
- Jenik A. Influence of pacifiers on breastfeeding duration. ClinicalTrials.gov (http://clinicaltrials.gov/) (accessed 20 February 2008).
- Jenik A, Vain N, Gorenstein A, Jacobi N, the Pacifier & Breastfeeding Study Group. Does the recommendation to use a pacifier influence the prevalence of breastfeeding? Results of a multicenter randomized clinical trial. Breastfeeding Medicine 2008;3(3):200. [Google Scholar]
- Jenik AG, Vain NE, Gorestein AN, Jacobi NE, for the Pacifier and Breastfeeding Trial Group. Does the recommendation to use a pacifier influence the prevalence of breastfeeding?. Journal of Pediatrics 2009;155(3):350‐4. [DOI] [PubMed] [Google Scholar]
Kramer 2001 {published data only}
- Kramer MS, Barr RG, Dagenais S, Yang H, Jones P, Ciofani L, et al. Pacifier use, early weaning, and cry/fuss behavior: a randomized controlled trial. JAMA 2001;286(3):322‐6. [DOI] [PubMed] [Google Scholar]
- Kramer MS, Barr RG, Jane R, Yang H, Dagenais S, Jones P, et al. Pacifier use, breastfeeding, and infant CRY/FUSS behavior: a randomized trial. Pediatric Research 2000;47(4):203A. [Google Scholar]
Schubiger 1997 {published data only}
- Kind C, Schubiger G, Schwarz U, Tonz O. Provision of supplementary fluids to breast fed infants and later breast feeding success. Advances in Experimental Medicine & Biology 2000;478:347‐54. [DOI] [PubMed] [Google Scholar]
- Schubiger G, Schwarz U, Tonz O. UNICEF/WHO baby‐friendly hospital initiative: Does the use of bottles and pacifiers in the neonatal nursery prevent successful breastfeeding?. European Journal of Pediatrics 1997;156:874‐7. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Collins 2004 {published data only}
- Collins CT, Ryan P, Crowther CA, McPhee AJ, Paterson S, Hiller JE. Effect of bottles, cups, and dummies on breast feeding in preterm infants: a randomised controlled trial. BMJ 2004;329(7459):193‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Feldens 2013 {published data only}
- Feldens CA, Ardenghi TM, Cruz LN, Scalco GP, Vitolo MR. Advising mothers about breastfeeding and weaning reduced pacifier use in the first year of life: a randomized trial. Community Dentistry & Oral Epidemiology 2013;41(4):317‐26. [DOI] [PubMed] [Google Scholar]
Howard 2003 {published data only}
- Howard CR, Howard FM, Lanphear B, Eberly S, deBlieck EA, Oakes D, et al. Randomized clinical trial of pacifier use and bottle‐feeding or cupfeeding and their effect on breastfeeding. Pediatrics 2003;111(3):511‐8. [DOI] [PubMed] [Google Scholar]
Additional references
AAP 2011
- Task Force on Sudden Infant Death Syndrome, Moon RY. SIDS and other sleep‐related infant deaths: expansion of recommendations for a safe infant sleeping environment. Pediatrics 2011;128(5):1030‐9. [PUBMED: 22007004] [DOI] [PubMed] [Google Scholar]
Aarts 1999
- Aarts C, Hornell A, Kylberg E, Hofyander Y, Gebre‐Medhin M. Breastfeeding patterns in relation to thumb sucking and pacifier use. Pediatrics 1999;104(4):e50. [DOI] [PubMed] [Google Scholar]
Barros 1995
- Barros FC, Victora CG, Semer TG, Tonioli Filho S, Tomasi E, Weiderpass E. Use of pacifiers is associated with decreased breastfeeding duration. Pediatrics 1995;95(4):497‐9. [PubMed] [Google Scholar]
Boccolini 2015
- Boccolini CS, Lazaro de Carvalho M, Couto de Oliveira MI. Factors associated with exclusive breastfeeding in the first six months of life in Brazil: a systematic review. Revista de Saude Publica 2015;49(91):1‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Caglar 2005
- Caglar E, Larsson E, Andersson EM, Hauge MS, Ogaard B, Bishara S, et al. Feeding, artificial sucking habits, and malocclusion in 3‐year‐old girls in different regions of the world. Journal of Dentition in Children 2005;72(1):25‐30. [PubMed] [Google Scholar]
Darwazeh 1995
- Darwazeh AM, Al Bashir A. Oral candidal flora in healthy infants. Journal of Oral Pathological Medicine 1995;24(8):361‐4. [DOI] [PubMed] [Google Scholar]
Gomes 2006
- Gomes CF, Trezza EM, Murade EC, Padovani CR. Surface electromyography of facial muscles during natural and artificial feeding of infants. Journal of Pediatrics 2006;82(2):85‐6. [DOI] [PubMed] [Google Scholar]
Hauck 2005
- Hauck FR, Omojokun OO, Siadaty MS. Do pacifiers reduce the risk of sudden infant death syndrome? A meta‐analysis. Pediatrics 2005;116(5):716‐23. [DOI] [PubMed] [Google Scholar]
Hauck 2011
- Hauck FR, Thompson JM, Tanabe KO, Moon RY Vennemann MM. Breastfeeding and reduced risk of sudden infant death syndrome: a meta‐analysis. Pediatrics 2011;128(1):103‐10. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Howard 1999
- Howard CR, Howard FM, Lanphear B, DeBlieck EA, Eberly S, Lawrence RA. The effects of early pacifier use on breastfeeding duration. Pediatrics 1999;103(3):e133. [DOI] [PubMed] [Google Scholar]
Jackson 1999
- Jackson JM, Mourino AP. Pacifier use and otitis media in infants twelve months of age or younger. Pediatric Dentition 1999;21(4):255‐60. [PubMed] [Google Scholar]
Kair 2013
- Kair LR, Kenron D, Etheredge K, Jaffe AC, Phillipi C. Pacifier restriction and exclusive breastfeeding. Pediatrics 2013;131(4):e1101‐06. [DOI] [PubMed] [Google Scholar]
Karabulut 2009
- Karabulut E, Yalcin SS, Ozdemi‐ Geyik P, Karaağaoğlu E. Effect on pacifier use on exclusive and any breastfeeding: meta‐analysis. Turkish Journal of Paediatrics 2009;51(1):35‐43. [PubMed] [Google Scholar]
Labbok 1990
- Labbok M, Kosovec K. Towards consistency in breastfeeding definition. Studies in Family Planning 1990;21(4):226‐30. [PubMed] [Google Scholar]
Levi 2002
- Levy SM, Slager SL, Warren JJ, Levy BT, Nowak AJ. Associations of pacifier use, digit sucking, and child care attendance with cessation of breastfeeding. Journal of Family Practice 2002;51(5):465. [PubMed] [Google Scholar]
Levin 1971
- Levin S. Dummies. South African Medical Journal 1971;45:237‐40. [PubMed] [Google Scholar]
Mascarenhas 2006
- Mascarenhas ML, Albernaz EP, Silva MB, Silviera RB. Prevalence of exclusive breastfeeding and its determiners in the first 3 months of life in South Brazil. Journal de Pediatria 2006;82(4):289‐94. [DOI] [PubMed] [Google Scholar]
Neifert 1995
- Neifert M, Lawrence RA, Seacat J. Nipple confusion: toward a formal definition. Journal of Pediatrics 1995;126:S125‐S129. [DOI] [PubMed] [Google Scholar]
Neville 1988
- Neville MC, Keller R, Seacat J, Lutes V, Neifert M, Casey C, et al. Studies in human lactation: milk outputs in lactating women during the onset of lactation and full lactation. American Journal of Clinical Nutrition 1988;48(6):1375‐86. [DOI] [PubMed] [Google Scholar]
O'Connor 2009
- O’Connor NR, Tanabe KO, Siadaty MS, Hauck FR. Pacifiers and breastfeeding: a systematic review. Archives of Pediatrics & Adolescent Medicine 2009;163(4):378‐82. [DOI] [PubMed] [Google Scholar]
Pinelli 2000
- Pinelli J, Symington A. How rewarding can a pacifier be? A systematic review of nonnutritive sucking in preterm infants. Neonatal Network : NN 2000;19:41‐8. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Righard 1992
- Righard L, Alade MO. Sucking technique and its effect on success of breastfeeding. Birth 1992;19(4):185‐9. [DOI] [PubMed] [Google Scholar]
Righard 1998
- Righard L. Are breastfeeding problems related to incorrect breastfeeding technique and the use of pacifiers and bottles?. Birth 1998;25(1):40‐4. [DOI] [PubMed] [Google Scholar]
Scott 2005
- Scott JA, Binns CW, Oddy WH, Graham KI. Predictors of breastfeeding duration: evidence from a cohort study. Pediatrics 2005;117:e646‐e655. [DOI] [PubMed] [Google Scholar]
Ullah 2003
- Ullah S, Griffiths P. Does the use of pacifiers shorten breastfeeding duration in infants. British Journal of Community Nursing 2003;8(10):458‐63. [DOI] [PubMed] [Google Scholar]
Victora 1997
- Victora CG, Behague DP, Barros FC, Olinto MT, Weiderpass E. Pacifier use and short breastfeeding duration: cause, consequence, or coincidence?. Pediatrics 1997;99(3):445‐53. [DOI] [PubMed] [Google Scholar]
Vogel 2001
- Vogel AM, Hutchison BL, Mitchell EA. The impact of pacifier use on breastfeeding: a prospective cohort study. Journal of Paediatrics and Child Health 2001;37(1):58‐3. [DOI] [PubMed] [Google Scholar]
WHO 1998
- WHO. World Health Organization. Evidence for the Ten Steps to Successful Breastfeeding. WHO/CHD/98.9. Geneva: WHO, 1998. [Google Scholar]
References to other published versions of this review
Jaafar 2011
- Jaafar SH, Jahanfar S, Angolkar M, Ho JJ. Pacifier use versus no pacifier use in breastfeeding term infants for increasing duration of breastfeeding. Cochrane Database of Systematic Reviews 2011, Issue 3. [DOI: 10.1002/14651858.CD007202.pub2] [DOI] [PubMed] [Google Scholar]
Jaafar 2012
- Jaafar SH, Jahanfar S, Angolkar M, Ho JJ. Effect of restricted pacifier use in breastfeeding term infants for increasing duration of breastfeeding. Cochrane Database of Systematic Reviews 2012, Issue 7. [DOI: 10.1002/14651858.CD007202.pub3] [DOI] [PubMed] [Google Scholar]


