Abstract
Our overall objective is to synthesize mast-seeding data on North American Pinaceae to detect characteristic features of reproduction (i.e. development cycle length, serotiny, dispersal agents), and test for patterns in temporal variation based on weather variables. We use a large dataset (n = 286 time series; mean length = 18.9 years) on crop sizes in four conifer genera (Abies, Picea, Pinus, Tsuga) collected between 1960 and 2014. Temporal variability in mast seeding (CVp) for 2 year genera (Abies, Picea, Tsuga) was higher than for Pinus (3 year), and serotinous species had lower CVp than non-serotinous species; there were no relationships of CVp with elevation or latitude. There was no difference in family-wide CVp across four tree regions of North America. Across all genera, July temperature differences between bud initiation and the prior year (ΔT) was more strongly associated with reproduction than absolute temperature. Both CVp and ΔT remained steady over time, while absolute temperature increased by 0.09°C per decade. Our use of the ΔT model included a modification for Pinus, which initiates cone primordia 2 years before seedfall, as opposed to 1 year. These findings have implications for how mast-seeding patterns may change with future increases in temperature, and the adaptive benefits of mast seeding.
This article is part of the theme issue ‘The ecology and evolution of synchronized seed production in plants’.
Keywords: conifer, CVp, mast seeding, weather, serotiny, dispersal
1. Introduction
Mast seeding is the spatially synchronous and highly temporally variable production of seed crops by a population of perennial plants [1–5], and is widespread both taxonomically and geographically [6,7]. In the most variable species, very large amounts of seed are produced infrequently, with few seeds produced in other years [8]. Strong temporal variation in seed-crop production has important consequences for forest regeneration [9] and pulses of seed production have cascading effects in ecosystems [10,11]. For instance, mast-seeding pulses in New Zealand lead to increases in non-native mammal populations that prey on native birds [11]; and obligate seed-eating birds in North America show widespread irruptions as a response to a lack of conifer seed during years of poor seed production [12]. Despite the critical role of mast seeding for an array of taxa, there is still a limited understanding of how mast seeding varies geographically and across species.
There are multiple hypotheses for the adaptive benefits of mast seeding (e.g. predator satiation and pollination efficiency) that suggest species with specific life-history strategies will have greater temporal variability in seed crops [3]. High temporal variability is hypothesized to satiate seed predators when seed production is high, enhancing pre-germination survivorship and reduce seed predator population numbers in the interval between resource pulses [13–15]. Conversely, species that are animal pollinated or animal dispersed are hypothesized to have lower temporal variability (measured as the coefficient of variation of the time series, CVp) to ensure sufficient populations of animal pollinators or dispersers [16,17]. Serotiny is a common life-history strategy among fire-adapted conifers that also influences the adaptive benefits of mast seeding. Serotinous species retain their cones for years after seed maturation and their population persistence requires prompt regeneration after episodic, stand-replacing fire [18]. Because these species store their seeds aerially, with viable seeds as old as 20 years, there are fewer adaptive benefits of high CVp owing to animal dispersal or to seed predation [19], and reproduction in serotinous species might vary owing to resource matching [16].
It is generally agreed that the primary proximate cause of mast seeding is related to meteorological variables [20–24], especially temperature, and thus the temporal variability in seed crops may vary in relation to weather patterns in a given region (see also [25]). Specifically, for plants with a 2 year reproduction cycle, seed crop size may not actually be driven by absolute summer temperature, but rather by the difference in temperature from the two previous summers, referred to as the ΔT model [21]. In this model, the cue determining the number of initiated reproductive buds is the difference in temperature between the summer before the crop (year ‘t − 1’) and the temperature in the preceding summer (year ‘t − 2’), with the resulting crop size (in year ‘t’) a response to the sign and magnitude of the difference in those two earlier summer temperatures [21,26,27]. Simply, for those 2 year reproductive cycle species that are cued by the temperature difference, a cool summer followed by a very warm summer induces a larger seed crop 1 year later [21,26,27]. The cues appear to be different for species in arid climates such as the United States southwest, with cool and wet summers during bud initiation associated with large, subsequent seed crops in dryland forested ecosystems [28–30]; i.e. absolute temperature-regime matters [31]. Whether absolute temperature values (T) or relative temperature compared to the previous year (ΔT) drive reproduction is critical to forecasting future mast events under a warming climate; if ΔT is the main driver then mast-seeding variability is not expected to change [21], whereas direct climate warming would lead to an increased cueing frequency that may induce a breakdown in mast-seeding patterns over time [32]. While temperature appears to play a key role, the depletion of endogenous resources by a large reproductive event diminishes the number of reproductive buds the following year even when the conditions are favourable, such that high levels of reproduction do not happen in consecutive years [26,33,34].
Much of our knowledge on mast-seeding patterns comes from studies on one or a few species at relatively local scales. Compilations of mast-seeding databases have allowed for data across large areas and over long time periods to be used to answer questions about spatio-temporal dynamics [15,16,26,27,35,36], with massive global databases used to examine patterns broadly based on phylogenetic relationships and/or processes related to global change [6,7,37,38]. Global-scale studies have been useful in identifying broad patterns of mast seed production of plants, such as latitudinal gradients and associations with pollination and dispersal traits [7,16]. However, by combining information from such disparate plant taxa, these studies struggle to understand the consequences of other plant traits. As a result, a more focused analysis of seed production among closely related species can help for understanding how plant life-history strategies influence spatio-temporal patterns of mast seeding [7]. Here, we focus on North American conifers to identify broad patterns of seed production. In doing so, we limit our analyses to a geographical area and a phylogenetically related collection of species to facilitate understanding of life-history strategies, including serotiny, types of seed dispersal and the timing of seed-development.
Pinaceae are common in North America across a range of habitats and environmental conditions, from hot and humid to cold and dry, and span a wide range of latitudes, and altitudes [39]. Pinaceae dominate the boreal forest and high altitudes, regions anticipated to be particularly vulnerable to climate change, as well as sandy, fire-prone terrain in the southeast, and the coastal region of the Pacific northwest. Conifer seeds are a key food resource for numerous species of animals, including birds, insects and small mammals [40–42]. Most members of this family lack any capacity to asexually recruit from dormant basal buds following the death of the crown, and thus we suspect that selection for higher or lower temporal variation in crop production will lead to stronger associations between traits and a measure of that variation. There are large numbers of long-term datasets on conifer reproduction [6] as these species are key players in the forestry sector of the economy, and thus climate trends affecting temporal variability in crop production may be discerned.
Our overall objective is to synthesize mast-seeding data on North American Pinaceae to: (i) quantify relationships between CVp (the coefficient of variation of conifer reproduction data for the time series of a population) with life-history strategies and location; (ii) to test for relationships between CVp between genera and tree regions; (iii) assess changes in CVp over time across genera; and (iv) examine the generality of the ΔT model relative to the absolute T model for Pinaceae in North America. We restrict the analysis to four genera with a large number of records (Pinus, Tsuga, Picea and Abies), leading to a resulting dataset of 286 time series (mean = 18.9 years of data) spanning much of the continent of North America across 55 years (1960–2014). This dataset has a broader spatial distribution of sites, approximately 2000 more years of data, and is an increase of nearly 50% in the number of continuous datasets since the last synthetic studies on mast seeding in multiple genera of conifers [37,43].
Variation in CVp has been predicted to either increase at higher latitudes [16], or to peak and then decline at higher latitudes [7]. The elevation is also predicted to impact plant reproduction, with high elevation sites reducing reproductive potential and leading to lower CVp [23]. In addition to variability in CVp as a function of geographical location, we predict that CVp will vary among plants with different life-history strategies, with animal-dispersed species and serotinous species predicted to have a lower CVp. We also compare CVp across four tree regions of North America (northern forests, Pacific coast, Rocky and western mountains, southeast coast [44]) and predict that more northern areas will have higher CVp. Given our data compilation extends back to 1960, we will also look for temporal trends in CVp. We also extend the ΔT model to temporal patterns of mast seeding for groups with contrasting seed developmental timing, and to groups within Pinus that occur in hot (low altitude/southern latitude) environments versus cool (high altitude/northern latitude) environments. For plant species with a 2 year cycle of development, reproductive bud primordia are initiated in year t − 1, with the sequence of pollination through to seed maturation completed in year t. By contrast, Pinus establishes the bud primordia in the summer of t − 2, with pollination in t − 1 and completion of seed maturation in year t. Thus, we reframe the ΔT model for Pinus with a suitable modification of the predictor variable (the temperature difference now based on t − 2 minus t − 3) to determine if this will lead to results consistent with 2 year species. We tested the prediction that if the ΔT model holds, then rising temperatures over time will result in no change in ΔT, and subsequently, there will be no change in mast-seeding variability [21] (except insofar as the slightly increased variation in local inter-annual temperatures is expected to increase slightly) [45–47].
2. Methods
(a) . Mast seeding data
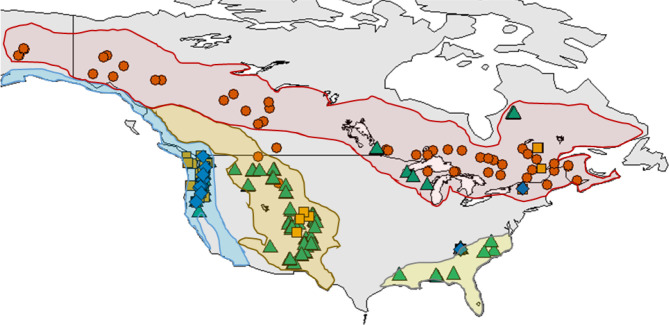
Data on annual seed production were obtained from a mast-seeding database [6], augmented with data from searches of the literature and data repositories and data from co-authors. All data included in analyses met the criteria that they: (i) had at least 6 years of mast-seeding data for a species of coniferous tree in North America, (ii) data were collected on a continuous scale (based on seed traps, visual cone counts, or cone scars), and (iii) occurred between 1960 and 2014. Additionally, (iv) for a taxon to be included in the study, there was a minimum of 10 separate time series at the level of the genus. Data from distinct sites or on different species were included separately. Based on these requirements, we compiled a total of 286 mast-seeding datasets with a mean time series length of 18.9 years and a total of 5398 years of data, including four genera (Abies (n = 54), Picea (n = 87), Pinus (n = 126) and Tsuga (n = 19)) and 25 species (figure 1; electronic supplementary material, table S1). This dataset includes a broader distribution of sites with data, approximately 2000 more years of data, and is an increase of nearly 50% in the number of continuous datasets since the last synthetic studies on mast seeding in multiple genera of conifers [37,43].
Figure 1.
Mast-seeding time series of North American conifers spanning 55 years (1960–2014). These data include 286 time series with a minimum of 6 years of data, four genera: (a) Abies (orange squares; n = 55), (b) Picea (vermillion circles; n = 87), (c) Pinus (bluish-green triangles; n = 128), and (d) Tsuga (blue diamonds; n = 20). Shaded areas represent the tree regions of northern forests (red), Pacific coast (blue), Rocky and western mountain (orange), and southeast coast (yellow) (adapted from Sakai & Weiser [44]). One Picea dataset in Medicine Hat, Alberta, Canada was included with northern forests for analysis, as it was a characteristically boreal forest species (Picea glauca). (Online version in colour.)
Data locations ranged across the continent and included four major forest regions of North America, northern forests, the Pacific coast region, the Rocky and western mountain region and the southeast coast region [44] (figure 1). The 286 datasets cover 3769 km in latitude and 3456 m in elevation.
(b) . Life-history attributes
Attributes of each conifer species that are predicted to influence temporal variability in conifer reproduction were assigned to each species. These life-history attributes included: cone serotiny (no serotiny = 0; semi-serotiny or serotiny = 1), whether animals are recognized as a key dispersal agent for seeds (no = 0; yes = 1) based on having wingless or functionally wingless seeds [48], and the number of years required for seed development, from bud primordia to seed maturity (2 years or 3 years) (electronic supplementary material, table S2). Attributes were assigned based on the predominant status identified for a species (e.g. our dataset includes several records on the characteristically serotinous species Pinus contorta, however, there may be some variability among populations). In a future paper, we will show that non-serotinous and serotinous populations of both P. contorta and Pinus clausa have similar CVp values.
(c) . Climate data
We obtained climate data using ClimateNA v6.30, a software application that provides point-location climate data on a monthly, seasonal and annual basis for a given year or time period over the entire continent of North America [49]. ClimateNA is based on the extraction and downscaling of gridded (4 × 4 km) monthly climate data for the reference normal period (1961–1990) based on PRISM [50] and WorldClim [51], and then using that as a baseline to downscale historical and future climate variables between 1901 and 2100. Climate data for specific point locations is accomplished through a combination of interpolation along with an elevation adjustment. We obtained monthly climate data for all mast-seeding dataset locations between 1958 (2 years prior to our first year of data) and 2014.
(d) . Analysis
Prior to analysis, we standardized each dataset to values between 0 and 100 based on the range of seed set values within each record because units of seed set varied among studies [6,26]. We calculated CVp for each dataset using annual values of reproduction across the duration of the data (standard deviation/overall mean). We built models to test hypotheses of whether CVp was driven by life-history characteristics or elements of geography (latitude, elevation). Life-history characteristics included serotiny of cones, animal dispersal of seeds and seed-development time (as assigned above) at the species level (electronic supplementary material, table S2). We built linear mixed-effects models with life-history characteristics, latitude and elevation and their interaction, and included random intercepts for site and mast-seeding data collection method (e.g. visual cone counts, seed traps, cone scars) to account for potential differences in the intercepts of CVp. The global model had five fixed effects and an interaction as described above, and we compared all possible models of fixed effects and a null model using Akaike information criteria corrected for small sample size (AICc) model comparisons. For each model, we weighted the contribution of each dataset by the number of years data were collected for, as CVp can be influenced by the length of the time series. We ran this analysis for all 286 conifer datasets combined and the best model(s) was determined using AICc model selection and AICc weights to construct a 90% confidence model set [52] and model-averaging using the MuMin package [53]. All statistical analyses were conducted in RStudio using R v. 4.0.2.
We tested for variation in CVp across genera using a likelihood-ratio test between linear mixed-effects models with and without genera, with site and sampling method as random effects using the lmtest package [54]. We computed estimated marginal means for each group and did post hoc pairwise comparisons using the emmeans package [55]. We also tested for variation in CVp across tree regions using a likelihood-ratio test between linear mixed-effects models with and without tree region, with site and sampling method as random effects and weights for the number of years of data available for each dataset.
To test for changes in CVp over time, we split the 55-year span of the entire database into three-time intervals (1960–1977, 1978–1995, 1996–2014; interval length = 18 years, 18 years, 19 years; electronic supplementary material, figure S1). We chose three-time intervals because this approach provided an interval length similar to the 20-year intervals used in [6]. For each time interval, we calculated CVp for each dataset that had at least 6 years of data during that interval. We built a linear mixed-effects model with CVp during each time interval as the response variable, time interval, genus and their interaction as independent variables, and we included random intercepts for site and mast-seeding data collection method, as above. We also conducted this analysis across tree regions with the same model set-up (tree region replaced genus). We determined the best model(s) from all possible models using the MuMin package [53] and AICc and AICc weights for model comparison [52].
We tested for the influence of weather conditions and lags in reproduction on standardized reproduction. We reviewed conifer species profiles and found that July has been identified as a key month both across conifer species and across broad geographical locations within species [39]. Across North America, July has the hottest mean monthly temperature [56]. For genera with a 2-year seed-development time (Abies, Picea and Tsuga), we used ΔT calculated from July temperatures in year t − 1 minus July temperatures in year t − 2 (ΔT1), while for Pinus (3-year seed development, with cone primordia laid 2 years before seedfall) we modified ΔT to be based on July temperatures in year t − 2 minus July temperatures in year t − 3 (ΔT2). We tested for general patterns in the influence of summer temperatures and lags in reproduction on annual reproduction for each genus separately, and for all 2-year seed-development time genera together. We built linear mixed-effects models using standardized reproduction over the full datasets as the response variable. Models for genera with seed-development times of 2 years included each of July temperature in t − 1 and ΔT1, both alone and with reproduction in the previous year (a 1-year lag in reproduction), the 1-year lag in reproduction alone and a null model.
For Pinus, the genus with a seed-development time of 3 years, we first divided up the mast-seeding records for species based on their general climate conditions, with species located in ‘hot’ climates (Pinus edulis, Pinus palustris, Pinus ponderosa subsp. scopulorum and Pinus taeda) analysed separately from Pinus species located in ‘cool’ climates (see the electronic supplementary material, table S1), identified based on [39]. This was done as we hypothesized that the relationship between temperature and reproduction may vary among hot and cool pines, with hot pines expected to be negatively associated with temperature [28,29]. The Pinus models included a term for reproduction 2 years prior (a 2-year lag in reproduction), July temperature in t − 2 and both ΔT2 and ΔT1. For all the above models, we included random intercepts for site, species and year (for Tsuga, species was not included as a random effect because having only three species led to a singular fit), and weights based on the number of years in a dataset. For each level of analysis, we determined the best model(s) using the MuMin package [53] and AICc and AICc weights for model comparison [52]. We calculated variable importance values and model-averaged parameter estimates (and 95% confidence intervals (CIs)) for standardized conifer reproduction patterns over time from the 90% AICc confidence model set for each of set of models run.
Lastly, we tested for temporal changes in July temperature and ΔT at each site for the duration of the study period. We built linear mixed-effects models for each of mean July temperature and ΔT, with year, geographical region, and their interaction as response variables, and site as a random effect. We compared these full models to models based on subsets of these terms and a null model using AICc model comparisons.
3. Results
Across the 286 datasets, the interannual variability in seed production within a given population (CVp) had a mean of 1.57 (median CVp = 1.52). There was a wide distribution in CVp values, with a minimum of 0.54 and a maximum of 3.56 (figure 2).
Figure 2.
Distribution of CVp across mast-seeding datasets by North American conifer tree species during 1960–2014 (n = 286 datasets).
(a) . CVp: relationships with life-history attributes and location
Seed-development time and serotiny were associated with CVp: the top model for CVp across all mast-seeding data included the fixed effects of seed-development time and serotiny, which had an AICc weight of 0.723 (electronic supplementary material, table S3). The next best model included fixed effects of seed-development time, serotiny and animal dispersal (ΔAICc of 4.21, wi = 0.088). Variable importance values for life-history attributes terms across all of the candidate models were 1.00 for seed-development time, 0.96 for serotiny and 0.12 for animal dispersal. Importance values for location-based terms were 0.13 for elevation, 0.05 for latitude and less than 0.01 for their interaction. The CVp of mast-seeding datasets with a 3-year seed-development time were 0.37 lower than those with a 2-year development time, and serotiny resulted in a lower CVp by 0.47 (table 1). The 95% CI of model-averaged parameter estimates for animal dispersal, elevation and latitude all overlapped zero; there was no relationship between animal dispersal, elevation or latitude with CVp.
Table 1.
Model-averaged parameter estimates (and 95% confidence intervals (CIs)) for CVp of North American conifer trees from the 90% confidence model set (see the electronic supplementary material, table S3). (The estimates represent the expected change in CVp when moving from one value of the life-history attribute to the nexta.)
| term | estimate (95% CI) | |
|---|---|---|
| life-history attributes | seed-development time (2 or 3 years) | −0.37 (−0.53, −0.21) |
| serotiny (0 = no; 1 = yes) | −0.47 (−0.81, −0.13) | |
| animal dispersal (0 = no; 1 = yes) | −0.01 (−0.11, 0.09) | |
| location | elevation (100 m) | 0.001 (−0.007, 0.010) |
| latitudeb | −0.0005 (−0.0053, 0.0043) |
aFor example, the CVp associated with a 3-year seed-development time is estimated to be 0.37 lower than the CVp associated with a 2-year seed-development time.
bLatitude scale is in decimal degrees.
(b) . CVp: relationships across genera and tree regions
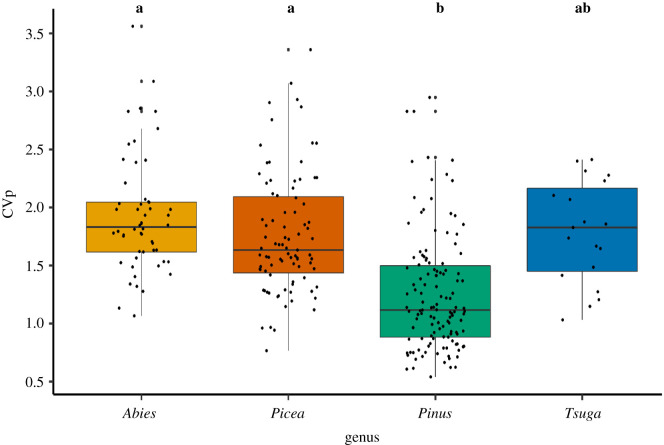
There was a significant difference in mean CVp across conifer genera (χ23 = 24.28, p < 0.001) with the mean CVp of Pinus spp. (1.26 ± 0.50) being significantly lower than both Abies spp. (1.93 ± 0.50; t = 4.47, p < 0.001) and Picea spp (1.76 ± 0.52; t = 5.24, p < 0.001; figure 3). The mean CVp of Pinus spp. was not significantly different than Tsuga spp. (1.79 ± 0.44; t = 2.24, p = 0.11), and there were no significant differences in mean CVp between other genera. There were no significant differences in mean CVp across four tree regions of North America (χ23 = 2.84, p = 0.42; table 2).
Figure 3.
Variability in mast-seeding patterns of North American conifers quantified as the coefficient of variation. These data include 286 time series with a minimum of 6 years of data, spanning 55 years (1960–2014). Letters indicate groups based on significant post hoc tests for differences between group means; colours for genera match figure 1. (Online version in colour.)
Table 2.
Mast-seeding variability (CVp) within four tree regions of North America (as defined by [44] between 1960 and 2014. (Number of datasets (n) and means ± standard deviation are shown.)
| region | n | CVp |
|---|---|---|
| northern forests | 84 | 1.53 ± 0.54 |
| Pacific coast | 68 | 1.75 ± 0.45 |
| Rocky and western mountain | 112 | 1.44 ± 0.62 |
| southeast coast | 22 | 1.86 ± 0.64 |
(c) . CVp: changes over time
We found no evidence for a change in CVp over time at the genus level or across tree regions (figure 4; electronic supplementary material, table S4), suggesting that there was no directional change in temporal variability in mast seeding between 1960 and 2014. AICc model comparisons, with time interval, genus, and their interactions as independent variables, showed that the null model was the top model (AICc weight = 0.810; electronic supplementary material, table S4). AICc model comparisons based on tree regions, with time interval and their interaction as independent variables also showed that the null model was the top model (AICc weight of 0.793; electronic supplementary material, table S4). There was no difference in these conclusions (no change over time detected) when the 55-year timeframe was separated into five-time intervals of 11 years, compared to the three longer time intervals shown here.
Figure 4.
Patterns of CVp over time for four genera, (a) Abies, (b) Picea, (c) Pinus and (d) Tsuga. The entire time period (1960–2014) was split into three-time intervals. (Online version in colour.)
(d) . Modelling mast-seeding dynamics: influence of temperature and lag-reproduction
Across all genera, ΔT variables were most strongly associated with standardized reproduction, suggesting that ΔT rather than absolute temperature most strongly drives reproduction. For Abies, Picea and Tsuga reproduction was associated with ΔT1 and reproduction the prior year (Reproductiont−1): these two variables were in the most parsimonious model (AICc weight = 1.000 for Abies, 0.942 for Picea and 0.715 for Tsuga (electronic supplementary material. table S5)). For Tsuga reproduction, the second-best model only included ΔT1 (AICc weight = 0.283) (electronic supplementary material, table S5). For all 2-year development genera combined, the most parsimonious model was ΔT1 + Reproductiont−1 (AICc weight = 1.000). Regarding the direction of effects of the independent variables on standardized reproduction, for all 2-year genera (i.e. Tsuga, Abies and Picea), ΔT1 had a positive association with reproduction in year ‘t’, while the lag in reproduction from the previous year (Reproductiont−1) had a negative association (table 3). For Abies, Picea, and the analysis of all 2-year genera combined, the 95% CIs of the parameter estimates did not overlap zero, which for Tsuga, the 95% CI for Reproductiont−1 overlapped zero (table 3).
Table 3.
Model importance values and model-averaged parameter estimates (and 95% confidence intervals (CIs)) for standardized conifer reproduction patterns over time from the 90% AICc confidence model set for each of four genera. (Models for genera with seed-development times of 2 years included a 1-year lag in reproduction and July temperature and ΔT1 (temperature in July of year t − 1 minus July of year t − 2). When there was only one top model, there are no importance values for terms. For the seed-development time of 3 years, the models included 1 and 2-year lags in reproduction and July temperature and both ΔT1 and ΔT2 (temperature in July of year t − 2 minus July of year t − 3).)
| genus | seed-development time | term | importance | estimate (95% CI) |
|---|---|---|---|---|
| Abies | 2 years | ΔT1 | n.a. | 2.18 (1.48, 2.88) |
| Reproductiont−1 | n.a. | −0.17 (−0.22, −0.13) | ||
| Picea | 2 years | ΔT1 | n.a. | 2.64 (2.03, 3.25) |
| Reproductiont−1 | n.a. | −0.10 (−0.15, −0.05) | ||
| Tsuga | 2 years | ΔT1 | 1.00 | 3.21 (2.23, 4.18) |
| Reproductiont−1 | 0.72 | −0.09 (−0.21, 0.04) | ||
| all 2-year genera | ΔT1 | n.a. | 2.82 (2.47, 3.17) | |
| Reproductiont−1 | n.a. | −0.14 (−0.17, −0.11) | ||
| Pinus (cool) | 3 years | Reproductiont−2 | 0.99 | 0.17 (0.09, 0.25) |
| ΔT2 | 0.78 | −1.49 (−3.32, 0.35) | ||
| ΔT1 | 0.32 | 0.10 (−0.63, 0.85) | ||
| Temperaturet−2 | 0.21 | −0.34 (−1.67, 0.99) | ||
| Pinus (hot) | 3 years | ΔT2 | 0.98 | −2.72 (−4.22, −1.22) |
| ΔT1 | 0.50 | −0.38 (−1.75, 0.99) | ||
| Reproductiont−2 | 0.14 | −0.005 (−0.044, 0.034) |
For Pinus, the genus with a 3-year seed-development time, reproduction of both cool and hot species was associated with ΔT2 (the difference in July temperature during the year of cone initiation (2 years prior to seed maturation) and the year prior); the importance value of ΔT2 was 0.78 for cool species and 0.98 for hot species. Contrary to the patterns in the 2-year development time genera, for both hot and cool Pinus species, increasing values of ΔT2 had negative effects on standardized reproduction in year ‘t’ (table 3). In other words, a hot summer prior to cone initiation followed by a cool summer during the year of cone initiation leads to high reproduction of Pinus, whereas the reverse is true for the other genera. For the hot group, multiple cooling years may be important (both ΔT2 and ΔT1 are in the top models; electronic supplementary material, table S5). For cool groups, the top models have both Reproductiont−1 and ΔT2; note that for the cool group all 95% CIs overlapped zero (table 3). For the hot group, only ΔT2 did not overlap zero.
(e) . Mean July temperature and ΔT: changes over time
Mixed models indicated mean July temperature across study sites has increased over time (1960–2014). The top model (weight = 1.000) included only tree region and year as fixed effects, with no significant region * year interaction. Overall, temperatures increased by an estimated 0.5°C during the study, at a rate of 0.091 ± 0.008°C (mean ± s.e.) per decade. The southeast region was warmest, with the northern tree region being the coolest, with the Pacific coast being similar to the northern region, while the Rocky and western mountain region had the widest range of temperatures (electronic supplementary material, figure S2). For ΔT, model selection based on AICc indicated that the null model was the most parsimonious (weight = 0.974) suggesting no change in ΔT over time in any of the tree regions. The next best model included year and had a ΔAICc of 7.25 and a weight of 0.026 (electronic supplementary material, figure S3).
4. Discussion
The North American conifer species analysed here displayed nearly as much variability in CVp values (0.54–3.56) as analyses based on mast-seeding datasets around the world [7]. Across four genera representing 25 species of conifers in North America and 286 datasets, temporal variability in mast seeding was related to seed-development periods, serotiny and genus. All species of Pinus included in this study take 3 years for their seeds to develop, and had a lower CVp compared to the other genera with 2-year seed-development periods; Abies, Picea and Tsuga. In addition to highlighting differences in temporal variability in mast seeding among genera and life-history strategies, our study documented widespread support for the ΔT model across genera as opposed to absolute temperature and found that both CVp and ΔT remained steady over the time period examined (1960–2014) while absolute temperatures increased. These findings have implications for how mast-seeding patterns may change with future projected increases in temperature and the adaptive benefits of mast seeding.
Serotinous species had lower temporal variability in mast seeding. Rather than selecting for high CVp to satiate seed predators, these serotinous species are instead more likely to have temporal patterns of reproduction that reflect resource matching [16], and selection for enhanced cone and seed defences to reduce seed predation [57]. We had lower support for animal dispersal in driving CVp patterns across species, which differs from other studies that found seed dispersal by animals was related to lower CVp [7,17]. Our classification of a species as animal dispersed was based on reports of successful dispersal and growth related to animals and if the wing was absent or so short to allow for wind dispersal [48]. Other studies have separated endozoochorus seed dispersal (seeds that are commonly passed through the gut of animals) and those seeds dispersed by animals that commonly consume cached seeds (dyszoochorus) [7].
We found no support that CVp varied geographically in relation to elevation and latitude. While CVp has been predicted to increase with latitude, both Kelly & Sork [16] and Pearse et al. [7] found a humped-shaped relationship that peaked at approximately 40° latitude and decreasing towards the poles, our data probably fit the declining portion of that trend. While Pinus had CVp values significantly lower than the other genera, there were no significant differences in CVp across regions. This suggests that mast-seeding patterns of conifers in northern forests, which includes the boreal forest of Canada and Alaska, are no more or less variable on average than in forests in the south, which included data from Florida, USA.
We found that, as predicted by Kelly et al. [21] there have been no changes in CVp for North American conifers over the timeframe of 1960–2014. By contrast, Pearse et al. [6] used 20-year intervals from 1900 to 2014, and with a worldwide dataset found that CVp increased over time, for the whole dataset and with only Pinaceae. Partly this difference in conclusions might be traced to the longer time span available to them or their wider spatial coverage. We do point out, however, that extending their temporal record back twice as far as ours, entailed risks: e.g. there would be only 27 data points (five authors; five species; different sites) in the first 40 years, and 14% of these data (far higher than for the rest of the record) were for a serotinous species, which, as demonstrated here, has a reduction in CVp of 0.47 compared to non-serotinous species. Thus, the documented increase in CVp may be a result of the limited data available that tended to favour a lower CVp in the early part of the record. In fitting models of standardized reproduction over time, we found that ΔT based on July temperatures was included in top models, and that ΔT1 was in the most parsimonious model for each of the 2-year genera separately and for all of them combined. Similarly, for Pinus the 3-year genera, ΔT2 has importance values of 0.78 for the cool species and 0.98 for the hot species. With ΔT being a highly important variable, particularly when compared to the actual absolute July temperature one or 2 years prior to seed maturity, our finding of no change in CVp or ΔT over time is internally consistent. In addition to ΔT, a 1-year lag in reproduction had high importance in reproduction models for Abies, Picea and Tsuga, with a negative impact on conifer reproduction. By contrast, for Pinus, there was a slight positive effect of reproduction lags 2 years prior in cool species, and a very small negative effect of lagged reproduction in hot species. Pinus data showed lower variability across years compared to the other genera, suggesting perhaps that some individuals in those populations may be reproducing most years. The lag effect of reproduction would be expected to be stronger in those species with higher CVp.
For all of the 2-year development time genera, warmer ΔT1 values were related to greater levels of standardized reproduction. This finding is consistent with other research within these genera [26,58]. For Pinus, we created a modification of the ΔT model, because the cone primordia are differentiated 2 years before seedfall as opposed to the usual 1 year prior; while ΔT2 had high importance in model selection; for both the cool and hot locations, higher ΔT2 had a negative impact on the amount of standardized reproduction. In other words, cool years at the time of cone initiation that were preceded by hot years lead to high reproductive years, which is the opposite to what we observed with Abies, Picea and Tsuga. For pine species adapted for hotter climates, we had hypothesized this negative relationship given these species are more likely to be water-limited rather than energy-limited, and prior research has found negative relationships with hot temperatures during cone initiation and reproduction within this group of species [28–30]. Yet for the cooler pines we had hypothesized a positive relationship, similar to what we observed with all other genera. The observed negative relationship of these cool Pinus species similar to the hot Pinus species, may be that the adaptive cue to signal reproduction was developed early into the evolution, as Pinus tended to occupy seasonally dry environments [59]. Abnormally hot July temperatures in the year prior to reproductive bud induction that lead to greater vapour pressure deficits [60] may thus increase water stress and, therefore, signal the tree to promote higher cone set for this more drought-adapted genus.
While the data used in our analyses included nearly 300 datasets and spanned vast distances, from Florida to Alaska (over 3700 km of latitude) and 3450 m of elevation, there are areas where mast-seeding coverage in conifers is sparse to non-existent, which is a limitation of this study (that being said, much of the interior United States and part of Canada are defined as prairie ecosystems without dense forests). Different methods of data collection and sampling regimes (e.g. number of samples, seed trap size, size of trees sampled) influence CVp values where too small seed traps will rarely catch seed, and small trees that rarely reproduce will both result in false zeros and elevated CVp values [61,62] and efforts could be made to standardize sampling. Future data collection should involve increasing the spatial spread of mast-seeding data collection in both the United States and Canada, particularly for Abies, Pinus and Tsuga. However, even for Picea, while the coverage may appear better, there are gaps in the data and some data are historical and are no longer being collected. Also, there are some species within the genera presented here that were not included owing to lack of data, as well as some genera with only a few records that we could find. Note that there is confounding between the genus Pinus and 3-year development times.
The goal of this study was to examine Pinaceae generally, there may be dramatic interspecific and intraspecific variation to be explored. For example, in a hot Pinus species, P. edulis, CVp is highest in more climatically stressful sites (high water deficits and low monsoonal precipitation) [63]. Thus while we detected no broad patterns in CVp across regions, more localized patterns may exist within species [26,64]. In addition, increasing data collection across elevational gradients at different latitudes in mountainous areas will allow for documenting mast-seeding patterns over time and testing hypotheses related to climate change (see also [34]). While temperature increases are predicted to be substantial at high latitudes, other components of climate change include frequency and severity of fires, insect attacks and extreme weather events [65], and increasing CO2 has been shown to experimentally impact conifer seed production [66] and could impact long-term mast-seeding patterns.
We found that there were similar temperature and lag-reproduction drivers in genera with the same seed-development times. This suggests that there could be interspecific synchrony between species that overlap in their spatial distribution, leading to widespread mast years (or, alternatively, poor crops) across regions that would have a much greater effect on vertebrate seed predator abundances than would the seed production dynamics of a single species. Across all genera examined, we found much greater support for the ΔT model, rather than absolute temperature, driving reproduction. In addition, we found no evidence of change in ΔT for the study duration, despite increases in mean July temperature. This suggests that recent and projected increases in temperature will not result in subsequent increases in reproductive output among Picea, Tsuga and Abies, or declines in reproductive output among Pinus (but see [29]). This is also consistent with our finding that variation in mast-seeding patterns (CVp) has not changed over time for the four genera included here, which has implications for the wide array of wildlife species whose population dynamics are linked to mast-seeding patterns [11,15,40,67,68].
Acknowledgements
We thank all of those who have collected mast-seeding data which make a study such as this possible, and Jessica Barton for contributing to the compilation of data. We also thank Ian Pearse and Walt Koenig for their efforts to compile global mast-seeding data, for many interesting discussions about mast seeding, and for generous and valuable feedback on an early version of this manuscript. We appreciate the feedback from Dave Kelly and two anonymous reviewers.
Data accessibility
The datasets supporting this article are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.612jm643z [69].
Authors' contributions
J.M.L. led the overall study, conducted preliminary data analysis and drafted an initial manuscript. All authors compiled or contributed data, substantially participated in group discussions about research ideas and provided considerable and meaningful contributions to writing the manuscript. A.P.W. conducted data analysis on the weather variables. All authors gave final approval for publication.
Competing interests
We declare we have no competing interests
Funding
This work was supported by the National Science Foundation grants DEB-1745496 and DEB-1926341 awarded to J.M.L., USDA National Institute of Food and Agriculture, McIntire Stennis project 1022908 awarded to M.D.R. and funding from the McIntire–Stennis programme and a series (2005–2014) of NSERC grants to D.F.G.
References
- 1.Silvertown JW. 1980. The evolutionary ecology of mast seeding in trees. Biol. J. Linn. Soc. 14, 235-250. [Google Scholar]
- 2.Janzen DH. 1976. Why bamboos wait so long to flower. Annu. Rev. Ecol. Syst. 7, 347-391. [Google Scholar]
- 3.Kelly D. 1994. The evolutionary ecolgy of mast seeding. Trends Ecol. Evol. 9, 465-470. [DOI] [PubMed] [Google Scholar]
- 4.Kelly D, Turnbull MH, Pharis RP, Sarfati MS. 2008. Mast seeding, predator satiation, and temperature cues in Chionochloa (Poaceae). Popul. Ecol. 50, 343-355. ( 10.1007/s10144-008-0109-1) [DOI] [Google Scholar]
- 5.Koenig WD. 2021. A brief history of masting research. Phil. Trans. R. Soc. B 376, 20200423. ( 10.1098/rstb.2020.0423) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pearse IS, LaMontagne JM, Koenig WD. 2017. Inter-annual variation in seed production has increased over time (1900–2014). Proc. R. Soc. B 284, 20171666. ( 10.1098/rspb.2017.1666) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pearse IS, LaMontagne JM, Lordon M, Hipp AL, Koenig WD. 2020. Biogeography and phylogeny of masting: do global patterns fit functional hypotheses? New Phytol. 227, 1557-1567. ( 10.1111/nph.16617) [DOI] [PubMed] [Google Scholar]
- 8.LaMontagne JM, Boutin S. 2009. Quantitative methods for defining mast-seeding years across species and studies. J. Veg. Sci. 20, 745-753. ( 10.1111/j.1654-1103.2009.01068.x) [DOI] [Google Scholar]
- 9.Rossi S, Morin H, Gionest F, Laprise D. 2012. Episodic recruitment of the seedling banks in balsam fir and white spruce. Am. J. Bot. 99, 1942-1950. ( 10.3732/ajb.1200267) [DOI] [PubMed] [Google Scholar]
- 10.Ostfeld RS, Keesing F. 2000. Pulsed resources and community dynamics of consumers in terrestrial ecosystems. Trends Ecol. Evol. 15, 232-237. [DOI] [PubMed] [Google Scholar]
- 11.Kelly D, Koenig WD, Liebhold AM. 2008. An intercontinental comparison of the dynamic behavior of mast seeding communities. Pop. Ecol. 50, 329-342. ( 10.1007/s10144-008-0114-4) [DOI] [Google Scholar]
- 12.Koenig WD, Knops JMH. 2001. Seed-crop size and eruptions of North American boreal seed-eating birds. J. Anim. Ecol. 70, 609-620. ( 10.1046/j.1365-2656.2001.00516.x) [DOI] [Google Scholar]
- 13.Janzen DH. 1971. Seed predation by animals. Annu. Rev. Ecol. Syst. 2, 465-492. ( 10.1146/annurev.es.02.110171.002341) [DOI] [Google Scholar]
- 14.Fletcher QE, Boutin S, Lane JE, LaMontagne JM, Mcadam AG, Krebs CJ, Humphries MM. 2010. The functional response of a hoarding seed predator to mast seeding. Ecology 91, 2673-2683. ( 10.1890/09-1816.1) [DOI] [PubMed] [Google Scholar]
- 15.Bogdziewicz M, Kelly D, Tanentzap AJ, Thomas PA, Lageard J, Hacket-Pain A. 2020. Climate change strengthens phenotypic selection for mast seeding in European beech. Curr. Biol. 30, 3477-3483.e2. ( 10.1016/j.cub.2020.06.056) [DOI] [PubMed] [Google Scholar]
- 16.Kelly D, Sork VL. 2002. Mast seeding in perennial plants: why, how, where? Annu. Rev. Ecol. Syst. 33, 427-447. ( 10.1146/annurev.ecolsys.33.020602.095433) [DOI] [Google Scholar]
- 17.Wang Y, Zhang J, LaMontagne JM, Lin F, Li B, Ye J, Yuan Z, Wang X, Hao Z. 2017. Variation and synchrony of tree species mast seeding in an old-growth temperate forest. J. Veg. Sci. 28, 413-423. ( 10.1111/jvs.12494) [DOI] [Google Scholar]
- 18.Owens JN. 1986. Cone and seed biology. In Proc. Conifer Tree Seed in the Inland Mountain West Symp., Missoula, Montana, August 5–6, 1985 (ed. Shearer RC), pp. 14-31. Ogden, Utah: US Department of Agriculture, Forest Service. [Google Scholar]
- 19.Halvorson C. 1986. Influences of vertebrates on conifer seed production. In Proc. Conifer Tree Seed in the Inland Mountain West Symp., Missoula, Montana, August 5–6, 1985 (ed. Shearer RC), pp. 201-222. Odgen, Utah: US Department of Agriculture, Forest Service. [Google Scholar]
- 20.Pearse IS, Koenig WD, Kelly D. 2016. Mechanisms of mast seeding: resources, weather, cues, and selection. New Phytol. 212, 546-562. ( 10.1111/nph.14114) [DOI] [PubMed] [Google Scholar]
- 21.Kelly D, et al. 2013. Of mast and mean: differential-temperature cue makes mast seeding insensitive to climate change. Ecol. Lett. 16, 90-98. ( 10.1111/ele.12020) [DOI] [PubMed] [Google Scholar]
- 22.Schauber EM, et al. 2002. Masting by eighteen New Zealand plant species: the role of temperature as a synchronizing cue. Ecology 83, 1214-1225. [Google Scholar]
- 23.Roland CA, Schmidt JH, Johnstone JF. 2014. Climate sensitivity of reproduction in a mast-seeding boreal conifer across its distributional range from lowland to treeline forests. Oecologia 174, 665-677. ( 10.1007/s00442-013-2821-6) [DOI] [PubMed] [Google Scholar]
- 24.Krebs CJ, LaMontagne JM, Kenney AJ, Boutin S. 2012. Climatic determinants of white spruce cone crops in the boreal forest of southwestern Yukon. Botany 90, 113-119. ( 10.1139/B11-088) [DOI] [Google Scholar]
- 25.Wion AP, Pearse IS, Rodman KC, Veblen TT, Redmond MD. 2021. The effects of ENSO and the North American monsoon on mast seeding in two Rocky Mountain conifer species. Phil. Trans. R. Soc. B 376, 20200378. ( 10.1098/rstb.2020.0378) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.LaMontagne JM, Pearse IS, Greene DF, Koenig WD. 2020. Mast seeding patterns are asynchronous at a continental scale. Nat. Plants 6, 460-465. ( 10.1038/s41477-020-0647-x) [DOI] [PubMed] [Google Scholar]
- 27.Vacchiano G, Hacket-Pain A, Turco M, Motta R, Maringer J, Conedera M, Drobyshev I, Ascoli D. 2017. Spatial patterns and broad-scale weather cues of beech mast seeding in Europe. New Phytol. 215, 595-608. ( 10.1111/nph.14600) [DOI] [PubMed] [Google Scholar]
- 28.Mooney KA, Linhart YB, Snyder MA. 2011. Masting in ponderosa pine: comparisons of pollen and seed over space and time. Oecologia 165, 651-661. ( 10.1007/s00442-010-1742-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Redmond MD, Forcella F, Barger NN. 2012. Declines in pinyon pine cone production associated with regional warming. Ecosphere 3, art120. ( 10.1890/es12-00306.1) [DOI] [Google Scholar]
- 30.Parmenter RR, Zlotin RI, Moore DI, Myers OB. 2018. Environmental and endogenous drivers of tree mast production and synchrony in piñon–juniper–oak woodlands of New Mexico. Ecosphere 9, e02360. ( 10.1002/ecs2.2360) [DOI] [Google Scholar]
- 31.Moreira X, Abdala-Roberts L, Linhart YB, Mooney KA. 2015. Effects of climate on reproductive investment in a masting species: assessment of climatic predictors and underlying mechanisms. J. Ecol. 103, 1317-1324. ( 10.1111/1365-2745.12434) [DOI] [Google Scholar]
- 32.Bogdziewicz M, Hacket-Pain A, Kelly D, Thomas PA, Lageard J, Tanentzap AJ. 2021. Climate warming causes mast seeding to break down by reducing sensitivity to weather cues. Glob. Chang. Biol. 27, 1952-1961. ( 10.1111/gcb.15560) [DOI] [PubMed] [Google Scholar]
- 33.Sala A, Hopping K, Mcintire EJB, Delzon S, Crone EE. 2012. Masting in whitebark pine (Pinus albicaulis) depletes stored nutrients. New Phytol. 196, 189-199. [DOI] [PubMed] [Google Scholar]
- 34.Hacket-Pain A, Bogdziewicz M. 2021. Climate change and plant reproduction: trends and drivers of mast seeding change. Phil. Trans. R. Soc. B 376, 20200379. ( 10.1098/rstb.2020.0379) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ascoli D, Hacket-Pain A, LaMontagne JM, Cardil A, Conedera M, Maringer J, Motta R, Pearse IS, Vacchiano G. 2020. Climate teleconnections synchronize Picea glauca masting and fire disturbance: evidence for a fire-related form of environmental prediction. J. Ecol. 108, 1186-1198. ( 10.1111/1365-2745.13308) [DOI] [Google Scholar]
- 36.Ascoli D, Vacchiano G, Turco M, Maringer J, Motta R, Hacket-pain A, Conedera M, Drobyshev I. 2017. Inter-annual and decadal changes in teleconnections drive continental-scale synchronization of tree reproduction. Nat. Commun. 8, 2205. ( 10.1038/s41467-017-02348-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koenig WD, Knops JMH. 2000. Patterns of annual seed production by northern hemisphere trees: a global perspective. Am. Nat. 155, 59-69. [DOI] [PubMed] [Google Scholar]
- 38.Fernández-Martínez M, et al. 2019. Nutrient scarcity as a selective pressure for mast seeding. Nat. Plants 5, 1222-1228. ( 10.1038/s41477-019-0549-y) [DOI] [PubMed] [Google Scholar]
- 39.Burns R, Honkala B. 1990. Silvics of North America: 1. Conifers; 2. Hardwoods. Agriculture handbook 654, vol. 2. Washington, DC: US Department of Agriculture, Forest Service. [Google Scholar]
- 40.Strong C, Zuckerberg B, Betancourt JL, Koenig WD.. 2015. Climatic dipoles drive two principal modes of North American boreal bird irruption. Proc. Natl Acad. Sci. USA 112, 2795-2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.LaMontagne JM, Williams CT, Donald JL, Humphries MM, McAdam AG, Boutin S. 2013. Linking intraspecific variation in territory size, cone supply, and survival of North American red squirrels. J. Mammal. 94, 1048-1058. ( 10.1644/12-MAMM-A-245.1) [DOI] [Google Scholar]
- 42.Shearer RC.1986. Proceedings - conifer tree seed in the Inland Mountain West symposium, Missoula, Montana, August 5–6, 1985. Gen. Tech. Rep. ( ) [DOI]
- 43.Koenig WD, Knops JMH. 1998. Scale of mast-seeding and tree-ring growth. Nature 396, 225-226. [Google Scholar]
- 44.Sakai A, Weiser CJ. 1973. Freezing resistance of trees in North America with reference to tree regions. Ecology 54, 118-126. [Google Scholar]
- 45.Holland EP, James A. 2014. Assessing the efficacy of population-level models of mast seeding. Theor. Ecol. 8, 121-132. ( 10.1007/s12080-014-0238-4) [DOI] [Google Scholar]
- 46.Pearse IS, Koenig WD, Knops JMH. 2014. Cues versus proximate drivers: testing the mechanism behind masting behavior. Oikos 123, 179-184. ( 10.1111/j.1600-0706.2013.00608.x) [DOI] [Google Scholar]
- 47.Monks A, Monks JM, Tanentzap AJ. 2016. Resource limitation underlying multiple masting models makes mast seeding sensitive to future climate change. New Phytol. 210, 419-430. ( 10.1111/nph.13817) [DOI] [PubMed] [Google Scholar]
- 48.Tomback DF, Linhart YB. 1990. The evolution of bird-dispersed pines. Evol. Ecol. 4, 185-219. ( 10.1007/BF02214330) [DOI] [Google Scholar]
- 49.Wang T, Hamann A, Spittlehouse D, Carroll C. 2016. Locally downscaled and spatially customizable climate data for historical and future periods for North America. PLoS ONE 11, e0156720. ( 10.1371/journal.pone.0156720) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Daly C, Halbleib M, Smith J, Gibson W, Doggett M, Taylor G, Curtis J, Pasteris P. 2008. Physiographically sensitive mapping of climatological temperature and precipitation across the conterminous United States. Int. J. Climatol. 28, 2031-2064. ( 10.1002/joc.1688) [DOI] [Google Scholar]
- 51.Hijmans RJ, Cameron S, Parra J, Jones P, Jarvis A. 2005. Very high resolution interpolated climate surfaces for global land area. Int. J. Climatol. 25, 1965-1978. ( 10.1002/joc.1276) [DOI] [Google Scholar]
- 52.Burnham K, Anderson D. 2002. Model selection and multimodel interference. New York, NY: Springer-Verlag. [Google Scholar]
- 53.Barton K. 2019. MuMIn: multi-model inference. R package version 1.43.6. See https://cran.r-project.org/package=MuMIn.
- 54.Zeileis A, Hothorn T. 2002. Diagnostic checking in regression relationships. R News 2, 7-10. [Google Scholar]
- 55.Lenth RV, Buerkner P, Herve M, Love J, Singmann H. 2020. emmeans: estimated marginal means, aka least-squares means. R package version 1.5.3. (doi:10.1080/00031305.1980.10483031>.License)
- 56.Régnière J, St-Amant R. 2007. Stochastic simulation of daily air temperature and precipitation from monthly normals in North America north of Mexico. Int. J. Biometeorol. 51, 415-430. ( 10.1007/s00484-006-0078-z) [DOI] [PubMed] [Google Scholar]
- 57.Parker AL, Benkman CW. 2020. Enhanced seed defenses potentially relax selection by seed predators against serotiny in lodgepole pine. Ecol. Evol. 10, 6001-6008. ( 10.1002/ece3.6339) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Krebs CJ, Donoghue MO, Taylor S, Kenney AJ, Hofer EJ, Boutin S. 2017. Predicting white spruce cone crops in the boreal forests of southern and central Yukon. Can. J. For. Res. 52, 47-52. ( 10.1139/cjfr-2016-0180) [DOI] [Google Scholar]
- 59.Axelrod D. 1986. Cenozoic history of some western American Pines. Ann. MO. Bot. Gard. 73, 565-641. [Google Scholar]
- 60.Anderson D. 1936. Relative humidity or vapor pressure deficit. Ecology 17, 277-282. [Google Scholar]
- 61.Bogdziewicz M, Szymkowiak J, Calama R, Crone EE, Espelta JM. 2020. Does masting scale with plant size? High reproductive variability and low synchrony in small and unproductive individuals. Ann. Bot. 126, 971-979. ( 10.1093/aob/mcaa118) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kelly D, Harrison AL, Lee WG, Payton IJ, Wilson PR, Schauber EM. 2000. Predator satiation and extreme mast seeding in 11 species of Chionochloa (Poaceae). Oikos 90, 477-488. [Google Scholar]
- 63.Wion AP, Weisberg PJ, Pearse IS, Redmond MD. 2020. Aridity drives spatiotemporal patterns of masting across the latitudinal range of a dryland conifer. Ecography (Cop.) 43, 569-580. ( 10.1111/ecog.04856) [DOI] [Google Scholar]
- 64.Crone EE, McIntire EJB, Brodie J. 2011. What defines mast seeding? Spatio-temporal patterns of cone production by whitebark pine. J. Ecol. 99, 438-444. ( 10.1111/j.1365-2745.2010.01790.x) [DOI] [Google Scholar]
- 65.Gauthier S, Bernier P, Burton PJ, Edwards J, Isaac K, Isabel N, Jayen K, Le Goff H, Nelson EA.. 2014. Climate change vulnerability and adaptation in the managed Canadian boreal forest. Environ. Rev. 22, 256-285. ( 10.1139/er-2013-0064) [DOI] [Google Scholar]
- 66.Way DA, Ladeau SL, McCarthy HR, Clark JS, Oren R, Finzi AC, Jackson RB. 2010. Greater seed production in elevated CO2 is not accompanied by reduced seed quality in Pinus taeda L. Glob. Chang. Biol. 16, 1046-1056. ( 10.1111/j.1365-2486.2009.02007.x) [DOI] [Google Scholar]
- 67.Tachiki Y, Iwasa Y. 2013. Coevolution of mast seeding in trees and extended diapause of seed predators. J. Theor. Biol. 339, 129-139. ( 10.1016/j.jtbi.2013.05.026) [DOI] [PubMed] [Google Scholar]
- 68.Clotfelter ED, Pedersen AB, Cranford JA, Ram N, Snajdr EA, Nolan V, Ketterson ED. 2007. Acorn mast drives long-term dynamics of rodent and songbird populations. Oecologia 154, 493-503. ( 10.1007/s00442-007-0859-z) [DOI] [PubMed] [Google Scholar]
- 69.LaMontagne JM, Redmond MD, Wion AP, Greene DF. 2021. Data from: An assessment of temporal variability in mast seeding of North American Pinaceae. Dryad Digital Repository. ( 10.5061/dryad.612jm643z) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets supporting this article are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.612jm643z [69].